Abstract
Background
Polygenic effects have been proposed to account for some disease phenotypes; these effects are calculated as a polygenic risk score (PRS). This score is correlated with Alzheimer’s disease (AD)-related phenotypes, such as biomarker abnormalities and brain atrophy, and is associated with conversion from mild cognitive impairment (MCI) to AD. However, the AD PRS has been examined mainly in Europeans, and owing to differences in genetic structure and lifestyle, it is unclear whether the same relationships between the PRS and AD-related phenotypes exist in non-European populations. In this study, we calculated and evaluated the AD PRS in Japanese individuals using genome-wide association study (GWAS) statistics from Europeans.
Methods
In this study, we calculated the AD PRS in 504 Japanese participants (145 cognitively unimpaired (CU) participants, 220 participants with late mild cognitive impairment (MCI), and 139 patients with mild AD dementia) enrolled in the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) project. In order to evaluate the clinical value of this score, we (1) determined the polygenic effects on AD in the J-ADNI and validated it using two independent cohorts (a Japanese neuropathology (NP) cohort (n = 565) and the North American ADNI (NA-ADNI) cohort (n = 617)), (2) examined the AD-related phenotypes associated with the PRS, and (3) tested whether the PRS helps predict the conversion of MCI to AD.
Results
The PRS using 131 SNPs had an effect independent of APOE. The PRS differentiated between CU participants and AD patients with an area under the curve (AUC) of 0.755 when combined with the APOE variants. Similar AUC was obtained when PRS calculated by the NP and NA-ADNI cohorts was applied. In MCI patients, the PRS was associated with cerebrospinal fluid phosphorylated-tau levels (β estimate = 0.235, p value = 0.026). MCI with a high PRS showed a significantly increased conversion to AD in APOE ε4 noncarriers with a hazard rate of 2.22. In addition, we also developed a PRS model adjusted for LD and observed similar results.
Conclusions
We showed that the AD PRS is useful in the Japanese population, whose genetic structure is different from that of the European population. These findings suggest that the polygenicity of AD is partially common across ethnic differences.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01414-x.
Keywords: Polygenic risk score, Alzheimer’s disease, Mild cognitive impairment
Background
Alzheimer’s disease (AD) is a neurodegenerative disease caused by environmental and genetic factors [1, 2]. Environmental factors, which are acquired and modifiable, associated with AD include smoking status, alcohol consumption, diet, and physical activity [3]. On the other hand, the heritability of AD is approximately 70%, and genetic factors are inborn and nonmodifiable [4, 5]. However, knowing one’s genetic risk early in life can motivate one to improve modifiable factors. Indeed, sharing genetic test results with carriers of genetic risk for disease may promote behavioural changes rather than increase psychological distress [6, 7]. Thus, knowledge of the individual genetic risk of AD is expected to contribute to delaying the onset of AD and early therapeutic intervention.
The largest genetic risk factor for AD is the ε4 allele of the apolipoprotein E (APOE) gene, but APOE ε4 explains only approximately 10% of AD cases based on heritability [4, 5]. In addition, even when other AD-associated genetic variants found in previous genome-wide association studies (GWAS) are also considered, they do not explain all the genetic variance in AD patients [8], suggesting the existence of additional unknown AD-related genetic variants. To clarify this “missing heritability”, polygenic effects that aggregate the small effects of many alleles have been proposed to underlie AD.
Polygenic risk score (PRS) is a measure to quantify the combined effect of genetic variants on an individual’s risk for disease. The combination of the APOE ε4 allele dose and PRS has been shown to improve disease prediction accuracy in the European population [9]. Moreover, the PRS is associated with AD-related phenotypes, such as brain volumes [10–12], brain amyloid-beta (Aβ) burden [11, 12], and plasma phosphorylated tau [13], and has been reported to be useful in predicting conversion from mild cognitive impairment (MCI) to AD [14, 15].
However, the clinical application of the PRS must be approached with caution. One of several concerns is that the effects of the PRS are not consistent across different ancestries [16, 17]. This is because genetic structures, such as linkage disequilibrium (LD) blocks, are different across populations and because the GWAS summary statistics used as a weight for each single-nucleotide polymorphism (SNP) to calculate the PRS are based primarily on people of European ancestry. Taking a PRS calculation method based on GWAS summary statistics from European individuals and applying it to non-European individuals compromises prediction accuracy since the genetic risk of that population may not be reflected properly [18]. Therefore, for future clinical application of the AD PRS, it is necessary to evaluate the utility of this score in populations of different ancestry. In addition, harmonization of protocols such as inclusion and exclusion criteria is critical for rigorous comparisons between different cohorts.
Therefore, in this study, we calculated the AD PRS in 504 Japanese participants (145 cognitively unimpaired participants, 220 participants with late MCI, and 139 patients with mild AD dementia) enrolled in the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) project and evaluated its effectiveness in the North American ADNI (NA-ADNI) cohort including North American 1070 participants. The J-ADNI study used a harmonized protocol to the NA-ADNI study. The previous comparative study of AD dementia between the US and Japan in the ADNI projects reported that MCI in the Japanese population shows similar progression profile as MCI in North America in terms of cognitive function [19]. We moreover validated the AD PRS using independent genomic data from 565 Japanese individuals with a neuropathological diagnosis by autopsy. Furthermore, we also examined the AD endophenotypes in association with PRS and tested whether the PRS is useful for predicting conversion from MCI to AD.
Materials and methods
Japanese participants from the J-ADNI cohort
Data used in the preparation of this article were obtained from the J-ADNI database deposited in the National Bioscience Database Center Human Database, Japan (Research ID: hum0043.v1, 2016) [19]. This database enrolled cognitively unimpaired (CU) participants, participants with late MCI, and patients with mild AD dementia (ADD) using criteria consistent with those of the North American ADNI (NA-ADNI) [20]. The J-ADNI was launched in 2007 as a public–private partnership led by Principal Investigator Takeshi Iwatsubo, MD. The J-ADNI was aimed to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of late MCI and mild ADD in the Japanese population. The J-ADNI did not recruit participants with early MCI. The ethics committees of the University of Tokyo, Osaka University and Niigata University approved the study.
A total of 715 volunteer participants between the ages of 60 and 84 years were diagnosed with late MCI or mild ADD or were CU and considered for inclusion in the J-ADNI. Of the 715 participants assessed for study eligibility, 537 met the criteria and were enrolled. Of these 537 participants, 508 (CU, 147; MCI, 221; ADD, 140) underwent genotyping analysis. Participants were evaluated every 6 or 12 months over a period of 36 months for CU and MCI participants and over a period of 24 months for participants with ADD, as in the NA-ADNI. As detailed below, the J-ADNI collected various imaging, clinical and neuropsychological data from these participants in addition to the genomic data. These data were obtained from the database described above.
Japanese neuropathological cohort
An independent neuropathological (NP) cohort composed of 577 brain donors was used for PRS validation [21]. Of these donors, 365 control donors had little pathological findings associated with AD and 212 case donors had those consistent with AD. All ADD patients were neuropathologically diagnosed by senile plaque and neurofibrillary tangle. No neuropathological features of other neurodegenerative disorders such as dementia with Lewy body disease, frontotemporal lobal degeneration, and Parkinson’s disease, were observed. Control individuals did not show the typical neuropathological hallmarks of AD. As no clinical diagnosis is provided in this cohort, the term case or control is used in this study. As shown below, 565 brain donors (358 controls and 207 cases) passed QC. The demographic data of all the participants from the NP cohort are shown in Table S1.
Genotyping, quality control, and imputation
Whole blood samples from 508 participants in the J-ADNI cohort and post-mortem frontal cortices from 577 donors in the NP cohort were genotyped using the Infinium Asian Screening Array (Illumina), containing 657,490 SNPs. APOE genotypes in each participant were determined by haplotypes derived from rs7412 and rs429358, which were genotyped using TaqMan Assays (Applied Biosystems). We excluded SNPs that (i) had duplicated genomic positions, (ii) had low call rates (< 5%), (iii) deviated from Hardy–Weinberg equilibrium compared to controls (p < 1 × 10−5), or (iv) had low minor allele frequency (< 0.01). For QC purposes, we excluded participants who (i) had sex inconsistencies, (ii) had autosomal heterozygosity deviation (|Fhet|≥ 0.2), (iii) had < 99% of their genotypes called, or (iv) were in the same family according to pi-hat (> 0.2). Furthermore, we used principal component analysis to remove outliers based on the 1000 Genomes Project samples (Phase3 v5) [22]. Finally, 451,713 autosomal SNPs and the samples, including 504 participants from the J-ADNI cohort and 565 brain donors from the NP cohort passed the QC procedures.
Next, we performed phasing with Eagle v2.4.1 [23] and imputation with Minimac4 [24] using the whole-genome sequencing data of 3541 participants obtained from the BioBank Japan Project [25] and the 1000 Genomes Project [22] as reference genome data. After repeating the above QC procedure for the imputed SNP markers, we excluded SNPs with poor imputation quality (r2 ≤ 0.3). Finally, we obtained 7,633,670 SNPs and the samples, including the 504 participants from the J-ADNI (CU, 145; MCI, 220; and ADD, 139) and 565 brain donors from the NP cohort (control, 358; case, 207).
The NA-ADNI genetic data
The independent cohort data used in this study were obtained from the NA-ADNI [26]. The NA-ADNI was launched in 2003 as a public–private partnership led by Principal Investigator Michael W. Weiner, MD. The NA-ADNI was aimed to test whether serial MRI and PET data and the analysis of other biological markers and clinical and neuropsychological assessments can be combined to characterize the progression of MCI and early ADD.
SNP data from the NA-ADNI project were available for 1674 participants across ADNI 1 and ADNI GO/2. Genotyping was conducted using three different platforms: Human610-Quad, HumanOmniExpress and Omni 2.5 M (Illumina) [27]. The SNP data were imputed using the TOPMeD imputation server after identical marker QC and sample QC as was used for the J-ADNI was performed. The SNP data analysed on each of the three platforms were imputed separately. After repeating the QC for the imputed SNP markers, we excluded SNPs with poor imputation quality (r2 ≤ 0.3). If a participant was genotyped on more than one genotyping array, the dataset with the fewest missing values was selected.
According to the following procedures, we selected participants with predicted central European ancestry and self-reported white non-Hispanic ethnicity. For predicted ancestry, we used SNPweights software to infer genetic ancestry from genotyped SNPs [28]. The reference panel comprised European, West African, East Asian and Native American ancestral populations. Participants with predicted central European ancestry of 80% or more were retained. We obtained self-reported ethnicity information from the NA-ADNI database. The clinical diagnosis at the final visit was used to categorize the data. Furthermore, four participants who had significant memory concerns but no cognitive impairment were excluded. Finally, 1482 participants (CU, 377; MCI, 481; and ADD, 624) remained.
Of the 1482 participants, 412 participants were participants in the Alzheimer’s Disease Genetics Consortium (ADGC) and were included in the meta-analysis of AD GWAS used as SNP weights in the PRS calculation described below. We analysed a set of 1070 participants (CU, 257; MCI, 453; and ADD, 360), excluding the 412 participants to avoid overfitting. The demographic data of all the participants from the NA-ADNI cohort are shown in Table S2.
Calculation of the PRS and prediction accuracy
The PRS was calculated for each individual and is expressed as the following weighted sum:
where PRSi is the PRS for individual i; M is the total number of SNPs used in the calculation; βj is the weight of SNPj, defined according to the effect size calculated by an independent GWAS; and xi,j is the number of minor alleles of SNPj that individual i has, thus has a value of 0, 1, or 2. In other words, the more minor alleles that are strongly associated with the disease, the higher the PRS.
SNPs included in the PRS were determined by the clumping and thresholding (C + T) method, the most common and supported method in AD studies [29, 30]. We used PRSice software implementing the C + T method to calculate the PRS [31]. The clumping method preferentially retains markers most strongly associated with disease from correlated markers in the same LD block. The thresholding method removes variants with GWAS p values greater than the selected p value threshold (pT) (p > pT). To determine the optimal pT, we tested pT values of 5 × 10−8, 1 × 10−6, 1 × 10−5, 1 × 10−4, 1 × 10−3, 1 × 10−2, 0.05, 0.5, and 1.0. SNPs were weighted by their effect sizes (beta coefficient) from the AD GWAS in the European population [32].
The ability of the PRS to accurately classify CU participants and ADD patients was estimated in terms of (1) Nagelkerke’s R2, the proportion of the variance explained by the regression model and (2) the area under the receiver operator characteristic curve (AUC). To calculate Nagelkerke’s R2, we constructed a logistic regression model, including the PRS and the first two components from the multidimensional scaling (MDS) analysis (full model), and compared it to a model with only the first two MDS components (null model). We assessed the difference in Nagelkerke’s R2 between the full and null models (R2 = R2Full − R2Null) and used the pT corresponding to the highest value of Nagelkerke’s R2. The Nagelkerke’s R2 was calculated by PRSice software using default parameters [31]. To avoid potential overfitting due to differences in LD between the European and Japanese populations, we used the LD score (R2) of the EUR population of 1000 Genomes in the LDpop Tool [33] to exclude SNPs suspected of LD using the criterion of R2 > 0.5. In this analysis, when adjacent SNPs had R2 > 0.5, one SNP with a lower GWAS p-value was selected to calculate PRS and the other was excluded. When more than one SNP was observed between two SNPs with R2 > 0.5, all of them may be in the same LD block, and the SNP showing the lowest GWAS p-value was selected from this LD block.
The AUC was calculated based on the prediction results of the logistic regression model using the J-ADNI cohort as a test cohort. We also performed fivefold cross validation (CV) to evaluate a predictive performance in a test cohort. We estimated the 95% credible intervals by using the ci.auc function from the R package “pROC”. DeLong’s test was conducted to assess potential significant differences between curves using the roc.test function from the R package “pROC”.
CSF biomarkers
In the J-ADNI cohort, cerebrospinal fluid (CSF) samples were assayed for Aβ(1–42), total tau (tTau), and phosphorylated tau (pTau) by using a multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with an Innogenetics (INNO-BIA AlzBio3; Ghent, Belgium) immunoassay kit-based reagent [34]. Of the 504 participants who underwent genotyping, 192 participants (CU, 52; MCI, 85; ADD, 55) also underwent CSF biomarker measurements at baseline.
Structural MRI and PET imaging
All participants in the J-ADNI cohort underwent a structural MRI scan at a signal strength of 1.5 Tesla using a three-dimensional magnetization-prepared rapid-acquisition gradient-echo sequence according to a standardized protocol [35]. Cross-sectional and longitudinal processing streams in FreeSurfer, version 5.3, were used to estimate the atrophic changes in specific regions; we also evaluated the cortical thickness extracted in the longitudinal analysis. Of the 504 participants who underwent genotyping, the entorhinal cortex and hippocampus of 443 participants (CU, 133; MCI, 196; ADD, 114) was assessed by the FreeSurfer longitudinal stream. Each cortical thickness value was adjusted by the total intracranial volume.
Of the 504 participants, 315 and 162 individuals underwent a positron emission tomography (PET) scan using 18F-2-fluoro-2-deoxy-D-glucose (FDG) and 11C-Pittsburgh compound B (PiB), respectively. The PET scanning protocol was standardized to minimize the inter-site and inter-scanner variability [36]. All PET images went through the J-ADNI PET QC process [36]. The FDG PET images were classified into seven categories based on the criteria of Silverman et al. [37]. We analysed only PET images of 110 participants classified as having a normal pattern (N1 pattern) and 161 participants classified as having an AD pattern (P1 pattern). For PiB PET, the visual interpretation of four cortical areas on each side (frontal lobe, lateral temporal lobe, lateral parietal lobe, and precuneus/posterior cingulate gyrus) was evaluated by classifying PiB uptake in each cortical region as positive, equivocal, or negative. Cases with one or more positive cortical areas were defined as amyloid scan positive, and those with negative results in all four cortical regions were defined as amyloid scan negative. Other cases were considered equivocal. We analysed 65 negative and 87 positive amyloid scans, excluding 10 participants who were judged to be equivocal.
Neuropsychological tests
All participants in the J-ADNI cohort underwent the following neuropsychological tests: Mini–Mental State Examination (MMSE), Functional Assessment Questionnaire (FAQ), Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), and AD Assessment Scale–Cognitive Subscale (ADAS-Cog).
Statistical analyses
Gene functional enrichment analysis of the closest genes around SNPs included in the PRS was performed using the Metascape database (http://metascape.org/) [38].
For the association analyses between the PRS and endophenotypes, we compared slopes with zero by linear regression model analyses. The covariates included age at baseline examination, sex, years of education, the first two principal components (PCs), and doses of APOE ε4 and ε2 alleles. P values were adjusted by false discovery rate (FDR) to avoid type I error.
Cox proportional hazards models using months of follow-up as a time scale were used to analyse the effects of PRSs on incident AD, presented as hazard ratios (HRs) and 95% confidence intervals (CIs) derived from a model with the following covariates: age at baseline examination, sex, years of education, the first two PCs, and dose of APOE ε4 and ε2 alleles. We analysed 208 MCI participants over a follow-up period of ≥ 12 months. Nonconverters were censored at the end of follow-up. Log-rank test was performed to examine the difference in conversion to AD between two PRS groups. This test was performed using only the PRS without covariates because the covariates other than PRS could affect the differences between the groups. Cox proportional hazard model analyses and log-rank tests were performed using the coxph and survdiff functions from the R package “survival”, respectively.
Results
The PRS successfully distinguish ADD patients and CU individuals in the J-ADNI cohort
After quality control of the genotyping data, the J-ADNI cohort included the 504 participants. The group with ADD had a higher mean age (p value < 0.001), a lower mean length of education (p value < 0.001), and a higher frequency of APOE ɛ4 carriers (p value < 0.001) than the CU group, whereas no differences were found in sex (p value = 0.429) or the frequency of APOE ɛ2 carriers (p value = 0.292) (Table 1).
Table 1.
Summary of the J-ADNI participants
| CU | MCI | ADD | p value | |
|---|---|---|---|---|
| N | 145 | 220 | 139 | - |
| Age in years, mean ± SE | 67.8 ± 0.472 | 72.8 ± 0.397 | 73.8 ± 0.563 | < 2.00 × 10−16a |
| Sex (M:F) | 72:73 | 106:114 | 59:80 | 0.429b |
| Years of education, mean ± SE | 13.8 ± 0.230 | 13.0 ± 0.189 | 12.5 ± 0.266 | 8.69 × 10−4 a |
| APOEε4 alleles (0:1:2) | 109:34:2 | 106:97:17 | 55:62:22 | 2.31 × 10−10 c |
| APOEε2 alleles (0:1:2) | 134:11:0 | 211:9:0 | 133:6:0 | 0.323 c |
Abbreviations: CU Cognitively unimpaired, MCI Mild cognitive impairment, ADD Alzheimer’s disease dementia, APOE Apolipoprotein E, SE Standard error, ANOVA analysis of variance
aOne-way ANOVA
bChi-squared test
cFisher’s exact test
We investigated whether the PRSs that were calculated using the statistics from the AD GWAS in the European population [32] are useful for discriminating between patients with ADD and CU individuals in the Japanese population. We calculated PRSs for 145 CU participants and 139 patients with ADD from the J-ADNI cohort. Our model using 173 SNPs showed the highest predictive power at pT < 1 × 10−5 and had a Nagelkerke’s R2 of 0.167 (left side of Table 2), indicating that it explained more than 15% of the variance between the CU and ADD groups.
Table 2.
Nagelkerke's R2 at differenct p value thresholds
| PRS (All SNPs) | PRS.noAPOE (except APOE region) | |||||
|---|---|---|---|---|---|---|
| pT | Nagelkerke's R2 | p value | #SNPs | Nagelkerke's R2 | p value | #SNPs |
| p < 5 × 10–8 | 0.148 | 5.92 × 10–8 | 81 | 0.081 | 4.57 × 10–5 | 44 |
| p < 1 × 10–6 | 0.143 | 9.91 × 10–8 | 107 | 0.067 | 2.07 × 10–4 | 70 |
| p < 1 × 10–5 | 0.167 | 1.40 × 10–8 | 173 | 0.085 | 3.18 × 10–5 | 131 |
| p < 1 × 10–4 | 0.091 | 1.53 × 10–5 | 410 | 0.029 | 0.013 | 364 |
| p < 1 × 10–3 | 0.034 | 6.85 × 10–3 | 1,696 | 0.014 | 0.077 | 1,642 |
| p < 1 × 10–2 | 0.004 | 0.324 | 10,101 | 0.007 | 0.213 | 10,041 |
| p < 0.05 | 0.000 | 0.929 | 33,065 | 0.000 | 0.979 | 32,999 |
| p < 0.5 | 0.005 | 0.288 | 121,721 | 0.004 | 0.332 | 121,647 |
| p < 1.0 | 0.006 | 0.262 | 158,289 | 0.005 | 0.303 | 158,208 |
The highest accuracy was highlighted in bold
P value was calculated by Wald test
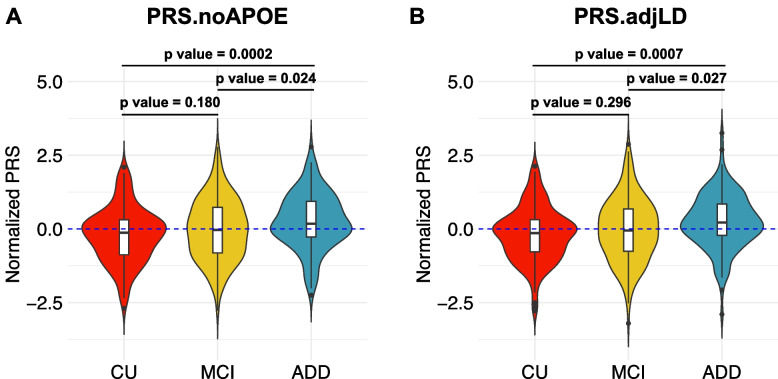
Given the known predictive power of SNPs in the APOE region for AD, we next removed this region from our PRS calculation to evaluate the predictive power of other loci. To exclude the effect of APOE, we excluded ± 500 kb around APOE (Figure S1). This PRS, referred to as the PRS.noAPOE, was used in subsequent analyses. The predictive power of the PRS.noAPOE was the highest for pT < 1 × 10−5, with a Nagelkerke’s R2 of 0.085 (right side of Table 2). To remove the effect of APOE regions completely, we also validated PRS.nochr19 excluding SNPs located on chromosome 19. The predictive power of the PRS.nochr19 was the highest for pT < 1 × 10−5, with a Nagelkerke’s R2 of 0.082 (Table S3). To further avoid potential overfitting due to differences in LD between the European and Japanese populations, we excluded 18 SNPs with suspected LD in the European population from PRS.noAPOE (see “Methods”). We referred to this PRS adjusted for LD as the PRS.adjLD. A Nagelkerke’s R2 of the PRS.adjLD was 0.075 (p value = 9.31 × 10−5). We analysed the PRS.noAPOE and PRS.adjLD in this study. The normalized values of the PRS.noAPOE and PRS.adjLD of the ADD patients were significantly higher than those of the CU and MCI participants (p value < 0.05, Tukey’s honestly significant difference (HSD) test; Fig. 1), while there were no significant difference between the CU and MCI participants (p value = 0.180 in PRS.noAPOE, p value = 0.296 in PRS.adjLD, Tukey’s HSD test; Fig. 1). These results suggest that the PRS contribute to distinguish between ADD patients and CU individuals in J-ADNI cohort even when the APOE region is excluded.
Fig. 1.
The PRS.noAPOE in the ADD group was significantly higher than those in the CU and MCI groups. The PRS.noAPOEs (A) or PRS.adjLD (B) in each group were represented by violin plots (CU, n = 145; MCI, n = 220; ADD, n = 139). Each violin plot includes the kernel probability density of the data at different values and the box plots with the median value and the interquartile range. Tukey’s HSD test was used to perform multiple comparisons of PRSs among each group. We normalized the PRS distribution to have a mean of 0 and an SD of 1. CN = cognitively normal; MCI = mild cognitive impairment; ADD = Alzheimer’s disease dementia
The PRS in combination with the APOE alleles improves predictive power
Next, we examined whether the PRSs and the characteristics of the participants independently influence the predictive power in J-ADNI cohort. The PRS.noAPOE and PRS.adjLD were not correlated with sex, years of education, age at baseline examination, or the dose of the APOE ε4 or ε2 allele, even when participants were stratified into CU, MCI, and ADD groups (p value > 0.05; Figures S2 and S3). These results suggest that these factors contribute independently to the discrimination of AD and that combinations of these factors improve discrimination accuracy. We constructed models including only the PRS.noAPOE or PRS.adjLD and doses of APOE ε4 and ε2 alleles. These models showed predictive performance of AUC = 0.755 in the model including PRS.noAPOE (95% CI = 0.695–0.807) and AUC = 0.748 in the model including PRS.adjLD (95% CI = 0.687–0.800) (Table 3). The predictive performance of a monogenic model of only APOE alleles without the PRS.noAPOE was AUC = 0.696 (95% CI = 0.640–0.751) (Table 3). The addition of polygenic effects significantly improved the predictive accuracy of the monogenic model using only APOE (p value = 9.36 × 10−4 in the PRS.noAPOE model, p value = 2.59 × 10−3 in the PRS.adjLD model, DeLong test). Additionally, the PRS model incorporating APOE alleles independently (PRS.noAPOE + APOE doses) has higher accuracy than the PRS model that includes SNPs in the APOE region (PRS.incAPOE) (AUC = 0.706; 95% CI = 0.643–0.764; p value = 0.049, DeLong test). Therefore, we constructed a predictive model including the PRS.noAPOE, sex, years of education, age at baseline examination, and doses of APOE ε4 and ε2 alleles. This model showed discriminative performance of AUC = 0.855 in distinguishing between the ADD patients and CU individuals in the J-ADNI cohort (95% CI = 0.808–0.898) (Table 3). This tendency was conserved even when LD effects were adjusted (AUC = 0.853; 95% CI = 0.806–0.897). These predictive performances showed the similar tendencies when evaluated by fivefold CV (Table S4). Taken together, these results showed that the PRS based on European GWAS statistics was useful in discriminating between patients with ADD and CU participants in the Japanese population. Furthermore, the PRS had an effect independent of APOE alleles, and their combination improved predictive accuracy.
Table 3.
Predictive accuracy of each model
| Training cohort | Validation cohort | |||||
|---|---|---|---|---|---|---|
| J-ADNI | NP | NA-ADNI | ||||
| Model | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI |
| PRS and APOE alleles | ||||||
| APOE ε4 | 0.693 | 0.638–0.747 | 0.691 | 0.654–0.730 | 0.701 | 0.665–0.735 |
| APOE ε4 + ε2 | 0.696 | 0.640–0.751 | 0.698 | 0.659–0.737 | 0.712 | 0.675–0.750 |
| PRS.noAPOE | 0.640 | 0.576–0.704 | 0.550 | 0.500–0.599 | 0.602 | 0.559–0.649 |
| PRS.adjLD | 0.639 | 0.574–0.704 | 0.541 | 0.493–0.589 | 0.594 | 0.552–0.640 |
| PRS.incAPOE | 0.706 | 0.643–0.764 | 0.628 | 0.590–0.625 | 0.679 | 0.639–0.720 |
| PRS.noAPOE + APOE ε4 + ε2 | 0.755 | 0.695–0.807 | 0.731 | 0.686–0.773 | 0.730 | 0.692–0.767 |
| PRS.adjLD + APOE ε4 + ε2 | 0.748 | 0.687–0.800 | 0.728 | 0.680–0.771 | 0.731 | 0.693–0.769 |
| PRS and all covariates | ||||||
| Age + Sex + (Education) + APOE ε4 | 0.837 | 0.788–0.883 | 0.725 | 0.681–0.770 | 0.710 | 0.670–0.750 |
| Age + Sex + (Education) + APOE ε4 + ε2 | 0.838 | 0.789–0.883 | 0.723 | 0.679–0.768 | 0.706 | 0.665–0.746 |
| Age + Sex + (Education) + APOE ε4 + ε2 + PRS.noAPOE | 0.855 | 0.808–0.898 | 0.737 | 0.693–0.780 | 0.722 | 0.683–0.761 |
| Age + Sex + (Education) + APOE ε4 + ε2 + PRS.adjLD | 0.853 | 0.806–0.897 | 0.733 | 0.690–0.777 | 0.718 | 0.678–0.757 |
Abbreviations: Age, age at examination; Education, years of education. Years of education were not provided in the NP cohort
The effect of our PRS model is replicated in the independent cohorts
To examine the predictive accuracy of PRS.noAPOE and PRS.adjLD in independent cohorts, we calculated the PRS values for 565 brain donors in the NP cohort (control, 358; case, 207) and 617 participants (CU, 257; ADD, 360) in the NA-ADNI using our PRS models. We note that the samples from the NP cohort received a definitive diagnosis based on the typical neuropathological hallmarks of AD using autopsy brains. The logistic regression model constructed in the J-ADNI cohort was applied to each cohort to assess discrimination accuracy. The predictive performance of PRS.noAPOE for the NP cohort was lower than that for the J-ADNI cohort (AUC = 0.550 (95% CI = 0.500–0.599) in the PRS.noAPOE; AUC = 0.541 (95% CI = 0.493–0.589) in the PRS.adjLD), but when APOE alleles were added, the predictive performance was replicated (AUC = 0.731 (95% CI = 0.686–0.773) in the PRS.noAPOE model; AUC = 0.728 (95% CI = 0.680–0.771) in the PRS.adjLD model) (Table 3).
We also analysed the NA-ADNI cohort to verify the transferability of PRS.noAPOE in different ancestries. In the NA-ADNI cohort, the imputed genotyping data included 130 of the 131 SNPs used in the PRS.noAPOE. The PRS.adjLD model used all 113 SNPs. A similar analysis in the NA-ADNI cohort also showed that the predictive performance of PRS.noAPOE or PRS.adjLD in combination with APOE alleles were similar to that of the NP cohort (AUC = 0.730 (95% CI = 0.692–0.767) in the PRS.noAPOE model; AUC = 0.731 (95% CI = 0.693–0.769) in the PRS.adjLD model). These analyses showed the reproducibility of our PRS model in independent cohorts.
ADD in the J-ADNI shows the polygenicity related to immune pathway
In order to examine the polygenicity of our PRS, we compared a model including only the PRS.noAPOE with a single-variable model for each of the 131 SNPs comprising the PRS.noAPOE. The single models with individual SNPs showed AUCs of 0.499 to 0.605 (median AUC = 0.515), while the model including only the PRS.noAPOE showed an AUC of 0.640 (95% CI = 0.576–0.704) (Table 3 and S5), suggesting that the PRS.noAPOE reflects a polygenic effect. Here, SNPs with AUCs of less than 0.5 indicate protection rather than risk in our data.
We examined the genes closest to 131 SNPs included in the PRS.noAPOE. We found the 96 closest genes located within ± 100 kb around the SNPs (Table S6). These genes were associated with leukocyte-mediated immunity (FDR = 3.78 × 10−5), haematopoietic cell lineage (FDR = 4.45 × 10−5), the amyloid precursor protein (APP) catabolic process (FDR = 5.16 × 10−5), regulation of transferase activity (FDR = 3.57 × 10−4), and glial cell proliferation (FDR = 5.60 × 10−3) (Table S7). The 89 closest genes in the PRS.adjLD also contained basically similar pathways (Tables S6 and S8). Overall, we found that the integrated scores of multiple SNPs around genes mainly associated with immune pathways may explain the Japanese AD traits.
The PRS associates with AD-related phenotypes
To examine whether our PRS associates with clinical characteristics, we next investigated the correlation between the PRS.noAPOE or PRS.adjLD and AD-related phenotypes, namely CSF biomarker data and FDG and PiB PET brain imaging data. We performed linear regression model analyses based on three models controlling for seven covariates: age at baseline examination, sex, years of education, the first two PCs, and the doses of APOE ε4 and ε2 alleles. Model 1 controlled only age at baseline examination, sex, years of education, and the first two PCs. Models 2 and 3 took into the dose of APOE ε4 allele in addition to Model 1. Model 3 also added the dose of APOE ε2 allele as a full model.
The CSF tTau/Aβ42 and pTau/Aβ42 ratios were significantly associated with the PRS.noAPOE and PRS.adjLD values. These associations were basically maintained in all models (FDR < 0.05, Wald test; Table 4a and Fig. 2) and reflected the influences of tTau and pTau levels but not Aβ42 levels (Table S9).
Table 4.
Associations between PRS and AD-related phenotypes
| Model 1 (Age, Sex, Education, PC1, PC2) |
Model 2 (Age, Sex, Education, PC1, PC2, APOEε4) |
Model 3 (Age, Sex, Education, PC1, PC2, APOEε4, APOEε2) |
||||
|---|---|---|---|---|---|---|
| Beta (noAPOE, adjLD) | p value (noAPOE, adjLD) | Beta (noAPOE, adjLD) | p value (noAPOE, adjLD) | Beta (noAPOE, adjLD) | p value (noAPOE, adjLD) | |
| 4a CSF biomarker | ||||||
| All subjects | ||||||
| tTau/Aβ42 | 0.167, 0.159 | 0.017a, 0.017a | 0.149, 0.146 | 0.058, 0.053 | 0.163, 0.156 | 0.040a, 0.039a |
| pTau/Aβ42 | 0.200, 0.168 | 0.004b, 0.011b | 0.188, 0.154 | 0.013a, 0.033a | 0.189, 0.155 | 0.013a, 0.032a |
| CU subjects | ||||||
| tTau/Aβ42 | -0.082, 0.204 | 0.804, 0.522 | -0.050, 0.265 | 0.888, 0.435 | -0.131, 0.194 | 0.717, 0.579 |
| pTau/Aβ42 | -0.143, -0.010 | 0.488, 0.960 | -0.134, 0.014 | 0.545, 0.948 | -0.178, -0.029 | 0.430, 0.895 |
| MCI subjects | ||||||
| tTau/Aβ42 | 0.292, 0.277 | 0.025a, 0.032a | 0.294, 0.290 | 0.043a, 0.043a | 0.295, 0.292 | 0.044a, 0.044a |
| pTau/Aβ42 | 0.363, 0.318 | 0.004b, 0.011a | 0.396, 0.355 | 0.006a, 0.013a | 0.396, 0.357 | 0.007a, 0.014a |
| ADD patients | ||||||
| tTau/Aβ42 | -0.054, -0.042 | 0.665, 0.703 | -0.053, -0.043 | 0.682, 0.703 | 0.026, 0.006 | 0.854, 0.959 |
| pTau/Aβ42 | 0.066, 0.018 | 0.577, 0.866 | 0.070, 0.019 | 0.565, 0.862 | 0.084, 0.028 | 0.485, 0.796 |
| 4b Brain volume | ||||||
| All subjects | ||||||
| Entorhinal | -0.014, -0.024 | 0.776, 0.622 | -0.008, -0.018 | 0.869, 0.714 | -0.010, -0.019 | 0.836, 0.699 |
| Hippocampus | -0.109, -0.115 | 0.042, 0.033 | -0.101, -0.106 | 0.087, 0.073 | -0.101, -0.106 | 0.088, 0.073 |
| CU subjects | ||||||
| Entorhinal | 0.032, 0.024 | 0.512, 0.626 | 0.033, 0.024 | 0.509, 0.626 | 0.036, 0.033 | 0.495, 0.529 |
| Hippocampus | 0.041, -0.010 | 0.758, 0.943 | 0.041, -0.010 | 0.760, 0.942 | 0.043, -0.016 | 0.754, 0.908 |
| MCI subjects | ||||||
| Entorhinal | -0.355, -0.357 | 0.150, 0.156 | -0.335, -0.344 | 0.180, 0.176 | -0.332, -0.341 | 0.184, 0.180 |
| Hippocampus | -0.064, -0.082 | 0.505, 0.407 | -0.041, -0.071 | 0.694, 0.505 | -0.043, -0.073 | 0.684, 0.497 |
| ADD patients | ||||||
| Entorhinal | 0.108, -0.078 | 0.745, 0.811 | 0.084, -0.089 | 0.801, 0.788 | 0.112, -0.070 | 0.739, 0.832 |
| Hippocampus | -0.043, -0.055 | 0.755, 0.688 | -0.080, -0.072 | 0.574, 0.609 | -0.069, -0.064 | 0.633, 0.652 |
| 4c PET imaging | ||||||
| All subjects | ||||||
| FDG (positive) | 0.180, 0.193 | 0.189, 0.149 | 0.162, 0.174 | 0.260, 0.218 | 0.162, 0.172 | 0.262, 0.224 |
| PiB (positive) | 0.386, 0.359 | 0.024a, 0.030 | 0.446, 0.417 | 0.025a, 0.030 | 0.442, 0.412 | 0.027, 0.033 |
| CU subjects | ||||||
| FDG (positive) | -0.119, -0.070 | 0.729, 0.839 | -0.109, -0.064 | 0.753, 0.852 | -0.109, -0.061 | 0.755, 0.860 |
| PiB (positive) | 0.037, 0.068 | 0.923, 0.858 | 0.072, 0.221 | 0.874, 0.620 | 0.059, 0.194 | 0.899, 0.671 |
| MCI subjects | ||||||
| FDG (positive) | 0.020, 0.053 | 0.942, 0.845 | 0.038, 0.077 | 0.892, 0.787 | 0.040, 0.076 | 0.888, 0.791 |
| PiB (positive) | 0.201, 0.175 | 0.489, 0.552 | 0.299, 0.320 | 0.432, 0.407 | 0.303, 0.327 | 0.433, 0.405 |
| ADD patients | ||||||
| FDG (positive) | -0.395, -0.232 | 0.357, 0.542 | -0.390, -0.232 | 0.366, 0.545 | -0.352, -0.195 | 0.417, 0.611 |
| PiB (positive) | -0.716, -0.777 | 0.313, 0.196 | -0.747, -0.935 | 0.314, 0.133 | -0.716, -0.915 | 0.338, 0.146 |
| 4d Neuropsychological test | ||||||
| All subjects | ||||||
| ADAS | 0.171, 0.127 | 0.001b, 0.008b | 0.163, 0.117 | 0.002b, 0.019a | 0.164, 0.116 | 0.002b, 0.019a |
| CDRSB | 0.188, 0.167 | 6.29 × 10−5b, 0.001b | 0.182, 0.160 | 1.78 × 10−4b, 0.003b | 0.182, 0.160 | 1.84 × 10−4b, 0.003b |
| FAQ | 0.166, 0.168 | 3.93 × 10−4b, 3.58 × 10−4b | 0.159, 0.161 | 0.001b, 0.001b | 0.159, 0.161 | 0.001b, 0.001b |
| MMSE | -0.160, -0.149 | 0.001b, 0.002b | -0.152, -0.141 | 0.002b, 0.005b | -0.152, -0.141 | 0.002b, 0.005b |
| CU subjects | ||||||
| ADAS | -0.118, 0.045 | 0.592, 0.837 | -0.119, 0.045 | 0.591, 0.837 | -0.111, 0.057 | 0.617, 0.795 |
| CDRSB | -1.178, -0.835 | 0.152, 0.305 | -1.208, -0.883 | 0.152, 0.290 | -1.217, -0.896 | 0.150, 0.284 |
| FAQ | 0.291, 0.359 | 0.473, 0.368 | 0.294, 0.359 | 0.470, 0.369 | 0.287, 0.349 | 0.483, 0.385 |
| MMSE | -0.153, -0.228 | 0.454, 0.258 | -0.153, -0.229 | 0.459, 0.258 | -0.140, -0.212 | 0.503, 0.303 |
| MCI subjects | ||||||
| ADAS | 0.194, 0.193 | 0.098, 0.107 | 0.176, 0.185 | 0.147, 0.136 | 0.182, 0.192 | 0.133, 0.123 |
| CDRSB | 0.146, 0.162 | 0.289, 0.251 | 0.144, 0.160 | 0.297, 0.256 | 0.149, 0.166 | 0.280, 0.241 |
| FAQ | 0.119, 0.145 | 0.239, 0.161 | 0.121, 0.146 | 0.233, 0.159 | 0.118, 0.143 | 0.246, 0.168 |
| MMSE | -0.065, -0.062 | 0.604, 0.626 | -0.055, -0.056 | 0.661, 0.664 | -0.045, -0.046 | 0.720, 0.722 |
| ADD patients | ||||||
| ADAS | -0.048, -0.026 | 0.719, 0.844 | -0.057, -0.029 | 0.671, 0.824 | -0.057, -0.029 | 0.672, 0.826 |
| CDRSB | 0.102, 0.063 | 0.290, 0.501 | 0.109, 0.066 | 0.260, 0.485 | 0.097, 0.060 | 0.318, 0.528 |
| FAQ | 0.078, 0.056 | 0.327, 0.472 | 0.093, 0.062 | 0.248, 0.430 | 0.088, 0.060 | 0.275, 0.450 |
| MMSE | 0.036, 0.045 | 0.794, 0.734 | 0.031, 0.044 | 0.823, 0.746 | 0.033, 0.045 | 0.810, 0.740 |
β estimates and p value were calculated by a linear regression model. P value was corrected in each subject group. Statistically significance was highlighted in bold
aFDR < 0.05
bFDR < 0.01
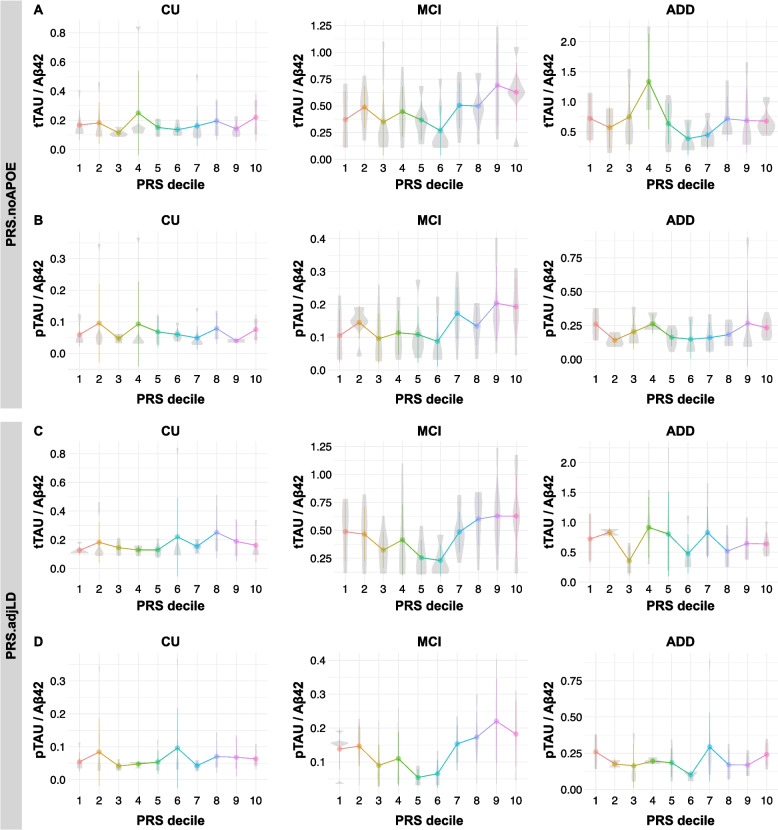
Fig. 2.
The PRS.noAPOE and PRS.adjLD correlated with CSF Tau/Aβ42 ratios in the MCI. CSF tTau/Aβ42 (A, C) and pTau/Aβ42 (B, D) ratios by decile of PRS are shown in each diagnostic group. The participants were divided into ten groups based on the PRS.noAPOE, ranging from the lowest group (1st decile) to the highest group (10th decile). CN = cognitively normal; MCI = mild cognitive impairment; ADD = Alzheimer’s disease dementia
To investigate the PRS effects to brain atrophy, we first tested the associations between the PRS and the volumes of the entorhinal cortex and hippocampus. Hippocampal volume showed a significant association with the PRSs in Model 1 that did not include APOE alleles, but this association did not remain significance after FDR correction (p value = 0.042 in the PRS.noAPOE, p value = 0.033 in the PRS.adjLD, Wald test; Table 4b). We investigated whether the PRSs contribute to the discrimination between the normal pattern (N1 pattern) and the AD pattern (P1 pattern) in FDG PET imaging and between negative and positive amyloid scans in PiB PET imaging. As a result, the PRSs were associated only with PiB PET imaging (p value = 0.024 in the PRS.noAPOE, p value = 0.030 in the PRS.adjLD, Wald test; Table 4c).
We also investigated the correlations between the PRSs and cognitive functions. The neuropsychological tests, including the ADAS-Cog, CDR-SB, FAQ, and MMSE, were significantly associated in all models (FDR < 0.01, Wald test; Table 4d).
We next stratified the participants into the CU, MCI and ADD groups and examined the association between the PRS.noAPOE or PRS.adjLD and each phenotype. Significant positive correlations between the PRSs and CSF tTau/Aβ and between the PRSs and pTau/Aβ42 ratios were observed in only the MCI participants (FDR < 0.05, Wald test; Table 4a; Fig. 2). In contrast, these ratios remained stable or reached a plateau relative to the PRSs in the CU and ADD participants (Fig. 2), suggesting that the polygenic burden beyond APOE explains some of the heterogeneity in MCI, especially in terms of tau-related biomarker.
APOE ε4 non-carriers with high PRS are at high risk of AD conversion
Finally, we examined difference in conversion to AD in the participants with MCI stratified by PRS. We divided MCI participants into three groups based on the PRS.noAPOE or PRS.adjLD distribution of all participants. We compared the conversion to AD of MCI participants in the 1st tertile, referred to as the low-PRS group, and of MCI participants in the 3rd tertile, noted as the high-PRS group. We performed Cox proportional hazard model analysis controlling seven covariates: age at baseline examination, sex, years of education, the first two PCs, and the doses of APOE ε4 and ε2 alleles. We did not find significantly different conversion patterns between the high- and low-PRS groups (p value = 0.202 in the PRS.noAPOE, p value = 0.236 in the PRS.adjLD, log-rank test; Table 5a and Fig. 3).
Table 5.
Polygenic risk of conversion of MCI to AD
| 5a PRS.noAPOE | |||||||||
| All MCI subjects | MCI subjects without APOE ε4 | MCI subjects with APOE ε4 | |||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| PRS (High group) | 1.301 | 0.847–1.998 | 0.230 | 2.216 | 1.058–4.643 | 0.035 | 0.985 | 0.770–1.259 | 0.902 |
| Age | 1.070 | 0.846–1.353 | 0.574 | 1.069 | 0.725–1.578 | 0.736 | 0.961 | 0.687–1.344 | 0.816 |
| Sex (Male) | 0.858 | 0.532–1.383 | 0.529 | 0.589 | 0.257–1.353 | 0.212 | 0.983 | 0.539–1.793 | 0.956 |
| Education year | 0.791 | 0.605–1.035 | 0.087 | 0.745 | 0.452–1.229 | 0.249 | 0.856 | 0.610–1.202 | 0.37 |
| PC1 | 0.939 | 0.769–1.146 | 0.534 | 0.769 | 0.541–1.093 | 0.143 | 1.034 | 0.795–1.343 | 0.806 |
| PC2 | 0.954 | 0.753–1.209 | 0.697 | 1.028 | 0.713–1.481 | 0.884 | 0.900 | 0.647–1.251 | 0.529 |
| APOE ε4 alleles | 1.604 | 1.153–2.230 | 0.005 | NA | NA | NA | 1.108 | 0.516–2.382 | 0.793 |
| APOE ε2 alleles | 1.447 | 0.440–4.755 | 0.543 | 1.665 | 0.492–5.635 | 0.412 | NA | NA | NA |
| Log-rank test (high vs low PRS) | 0.202 | 0.031 | 0.292 | ||||||
| 5b PRS.adjLD | |||||||||
| All MCI subjects | MCI subjects without APOE ε4 | MCI subjects with APOE ε4 | |||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| PRS (High group) | 1.161 | 0.752–1.792 | 0.500 | 1.547 | 0.727–3.296 | 0.258 | 1.030 | 0.812–1.305 | 0.809 |
| Age | 1.137 | 0.902–1.433 | 0.278 | 1.251 | 0.819–1.912 | 0.301 | 1.011 | 0.742–1.379 | 0.943 |
| Sex (Male) | 0.736 | 0.445–1.219 | 0.234 | 0.571 | 0.234–1.394 | 0.219 | 0.808 | 0.425–1.538 | 0.516 |
| Education year | 0.927 | 0.714–1.205 | 0.573 | 0.873 | 0.531–1.435 | 0.592 | 0.962 | 0.689–1.344 | 0.821 |
| PC1 | 0.909 | 0.738–1.119 | 0.368 | 0.682 | 0.460–1.011 | 0.057 | 1.042 | 0.801–1.355 | 0.758 |
| PC2 | 0.927 | 0.733–1.173 | 0.529 | 1.006 | 0.697–1.452 | 0.975 | 0.910 | 0.663–1.249 | 0.558 |
| APOE ε4 alleles | 1.560 | 1.102–2.209 | 0.012 | NA | NA | NA | 1.201 | 0.543–2.658 | 0.651 |
| APOE ε2 alleles | 0.708 | 0.169–2.965 | 0.636 | 0.709 | 0.163–3.085 | 0.647 | NA | NA | NA |
| Log-rank test (high vs low PRS) | 0.236 | 0.174 | 0.650 | ||||||
Abbreviations: HR Hazard ratio, 95% CI 95% confidence interval, APOE apolipoprotein E
Cox proportional hazard model: Conversion/follow-up = PRS (Low = 0, High = 1) + Age + Sex (Female = 0, Male = 1) + Education year + PC1 + PC2 + APOE ε4 alleles + APOE ε2 alleles
HR, 95% CI, and p-value were calculated by a Cox proportional hazard model controlling age at examination, sex, education years, the first two PCs, and the doses of APOE ε4 and ε2 alleles. Statistically significant was highlighted in bold
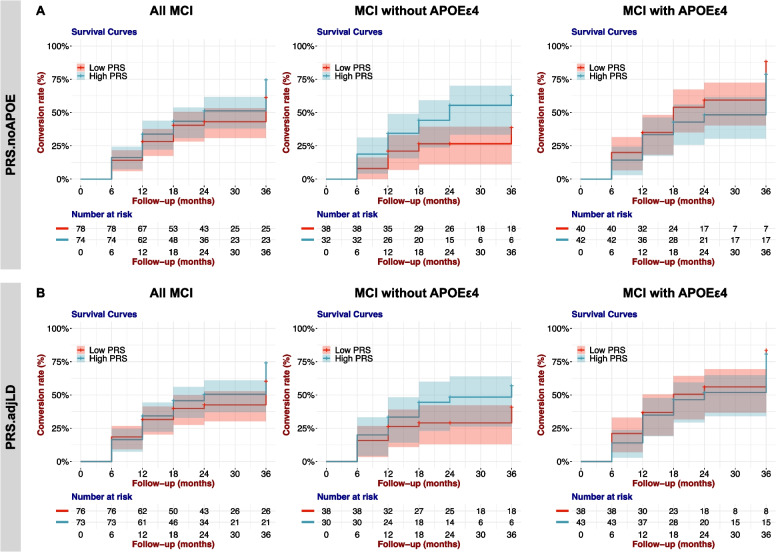
Fig. 3.
The high-PRS group was more likely to convert to AD than the low-PRS group in the APOE ε4 non-carrier individuals with MCI. Kaplan–Meier survival curves for conversion rates of MCI to AD in the low-PRS group (1st tertile) and the high-PRS group (3rd tertile). The shaded area represents the 95% confidence interval
When we examined the contribution of each variable, we found that the dose of the APOE ε4 allele significantly affected the conversion to AD (HR = 1.604, 95% CI = 1.153–2.230, and p value = 0.005 in the PRS.noAPOE; HR = 1.560, 95% CI = 1.102–2.209, and p value = 0.012 in the PRS.adjLD, Wald test; Table 5a), suggesting that this difference in conversion between the two PRS groups was influenced by the APOE ε4 allele dose. Therefore, we stratified MCI participants into those with and without APOE ε4. In that analysis, we found that in the PRS.noAPOE, among MCI participants without APOE ε4, the high-PRS group showed a significantly higher conversion to AD than the low-PRS group (p value = 0.031, log-rank test; Table 5a and Fig. 3A). Moreover, the PRS.noAPOE significantly contributed to the difference in AD conversion between the two groups (HR = 2.216; 95% CI = 1.058–4.643; p value = 0.035, Wald test; Table 5a). We also found no difference in AD conversion among MCI participants with APOE ε4 (p value = 0.292, log-rank test; Table 5a and Fig. 3A). In the PRS.adjLD, no significance was observed (Table 5b and Fig. 3B). These results suggested that polygenic effects increase the risk of AD conversion, particularly in MCI subjects without APOE ε4.
On the other hand, in APOE ε4 carriers, a single factor, namely, APOE ε4, may explain much of the AD conversion risk. As expected, there was no significant difference between the APOE ε4 noncarrier group with high-PRS and the APOE ε4 carrier group (p value = 0.595 in the PRS.noAPOE, p value = 0.345 in the PRS.adjLD, log-rank test; Figure S4). Although age differences between the groups compared in the above analysis could have affected the results, there were no differences in age at baseline examination between the low- and high-PRS groups or between the converted and nonconverted participants (p value > 0.05, Wilcoxon rank-sum test; Figure S5). These results suggest that the PRS contributes to the conversion to AD in participants without APOE ε4.
Discussion
In this study, we evaluated the utility of the PRS for AD in a Japanese cohort. The results showed that the PRS had an effect independent of APOE and showed relatively high predictive accuracy when combined with APOE ε4. In addition, this effect was replicated in the cohort with a neuropathological diagnosis and the protocol-harmonized independent NA-ADNI cohort. The PRS was significantly associated with CSF tau levels in MCI participants, and MCI with a high PRS was associated with an elevated risk of AD conversion in APOE ε4 noncarriers.
Despite the difference in genetic structure between the European and Japanese populations [39], the PRS developed in this study, PRS.noAPOE, showed meaningful predictive accuracy. We also developed PRS.adjLD, which avoids overfitting due to differences between European and Japanese LD blocks, and showed that PRS.adjLD had similar accuracy. Such predictive accuracy may be achieved because all participants were diagnosed according to unified inclusion and exclusion criteria and harmonized standardized diagnostic criteria using the same neuropsychological tests (MMSE, CDR-SB, and Wechsler Memory Scale Logical Memory II). The optimal p value threshold for the PRS excluding the APOE region was also similar to that reported in previous studies, pT < 1 × 10−5 [5, 10, 40]. Moreover, while dozens of SNPs were incorporated into these previous PRSs, 131 or 113 SNPs were included to calculate the PRS in our study. This difference in the number of SNPs is likely due to differences in genetic structure such as LD blocks. Hence, even if there are ancestral differences, adding a few dozen SNPs may preserve accuracy.
We also examined potential overfitting due to differences in LD between European and Japanese populations, which may cause a small reduction in predictive accuracy. On the other hand, it is possible that SNPs in the same LD in Japanese are independent (i.e. linkage equilibrium) in European population. In this case, underfitting may occur and the actual predictive accuracy may be underestimated. To solve this issue, a larger AD GWAS data derived from Japanese population will be needed, and this warrants further investigation.
There is no consensus on the number of SNPs that should be included in the AD PRS. According to a systematic review of PRS studies in AD, PRSs of AD can be organized into two groups: PRSs containing relatively large numbers of SNPs, ranging from 4431 to 359,500, and PRSs containing relatively small numbers, ranging from 5 to 31 [41]. The latter group is referred to as the oligogenic effect, in contrast to the polygenic effect [42]. From this perspective, our PRS apparently represents an oligogenic effect. Notably, a relatively small number of SNPs has the advantage of providing an inexpensive gene panel. In addition, a PRS composed of many SNPs may be sensitive to geographic differences in genetic structure, whereas a PRS composed of a few dozen SNPs is robust to population bias [43, 44]. However, we should note that our PRS may reflect ancestral differences due to the use of European GWAS statistics. In the future, more robust polygenic effects could be verified by using GWAS statistics for large groups of East Asians, including Japanese individuals.
In our study, the genes contributing to the PRS.noAPOE or PRS.adjLD were associated with APP degradation, immunity, and glial cell proliferation. Genetic variants found in a recent AD GWAS were associated with the APP catabolic process and tau protein binding [45]. In addition, many of the genes affected by their genetic variants are expressed in microglia [45]. An analysis of cognitively healthy centenarians in addition to ADD patients and healthy controls revealed that the PRS associated with the immune system was lower in the centenarian group independent of APOE ε4, indicating that immune system function is involved in AD resistance [46]. Therefore, our results suggest that common factors related to AD may be shared in the vulnerability of clearance mechanisms and neuroimmune surveillance in the brain among different population.
In our study, the PRS.noAPOE and PRS.adjLD showed significant correlations with CSF tTau/Aβ42 and pTau/Aβ42 ratios only in individuals with MCI. Tau but not Aβ42 strongly influenced this result even controlling APOE effect. CU and AD are relatively homogeneous in terms of AD-related biomarker changes. However, MCI is a heterogeneous condition, in which CSF biomarkers are highly variable with dynamic changes. Because of this variation in CSF biomarkers, significant correlations with PRS were observed in MCI group. Interestingly, NA-ADNI studies have shown that the PRS is associated beyond APOE with CSF tau but not CSF Aβ42 [44, 47]. From the above, independent studies in different ancestry groups have confirmed that polygenic effects are associated with tau-related biomarkers, especially in individuals with MCI.
Although our results are noteworthy, we must approach the clinical application of our PRS with caution at this stage because the predictive accuracy of our PRS alone is not very high. Similar to currently available PRSs, few biomarkers can perfectly distinguish disease or not; most markers bear some uncertainty. AD and MCI are explained not only by genetic aspects such as PRS, but also by anatomic aspects such as MRI and PET imaging and biological aspects such as CSF biomarkers [48], suggesting that combining multiple biomarkers could compensate for each other’s weaknesses in predictive performance. PRS will allow individuals’ disease risk to be assessed at a relatively early stage, leading to future lifestyle modification and disease prevention.
There were several limitations to this study. First, the CU participants included in the J-ADNI were relatively young. We acknowledge that these CU participants include potential patients who will develop AD in the future. Considering the average age of onset of AD and the allele frequency of APOE ε4 in the Japanese population, future work should ideally include CU participants that are over 70 years old [49]. Second, because the number of participants available for the study was small, there was limited power to identify relationships between the PRS and some phenotypes. Larger studies are needed to validate the results of this study. Therefore, combining samples from multiple East Asian cohorts, including cohorts from Japan, is necessary for analysis.
Conclusion
This study demonstrated that the AD PRS showed a relatively high performance in the Japanese population, despite differences in genetic structure from the European population. Furthermore, this PRS was replicated in the independent Japanese and European cohorts. The AD PRS correlated with phenotypes such as CSF tau levels in MCI. The AD PRS predicted the development of AD in MCI participants without APOE ε4. The application of the PRS will allow us to know an individuals’ disease risk at a relatively early life stage, which may lead to future lifestyle modification and disease prevention.
Supplementary Information
Additional file 1: Figure S1. The excluded region around the APOE gene. We removed the APOE region, consisting of ±500 kb, from around the top-hit SNP rs1160985 (chr19:45403412) in our data. Each data point indicates GWAS p values from Jansen et al. [32] used as SNP weights in the PRS calculation. Figure S2. Associations between the PRS and covariates. Age at baseline examination and years of education were examined by Spearman correlation. Sex and doses of APOE ε4 and ε2 alleles were analysed by t tests or ANOVAs. CN = cognitively normal; MCI = mild cognitive impairment; ADD = Alzheimer's disease dementia. Figure S3. Associations between the PRS.adjLD and covariates. Age at examination and years of education were examined by Spearman correlations. Sex and dose of APOE ε4 and ε2 alleles were analysed by t tests or ANOVAs. Figure S4. Comparison of AD conversion between the APOE ε4 carriers and the APOE ε4 noncarriers with high PRS. Kaplan–Meier survival curves for conversion rates of MCI to AD in the APOE ε4 carriers and the APOE ε4 noncarriers with high PRS values. p-values were calculated by log-rank test. Figure S5. Age differences between the low- and high-PRS groups and between the nonconverters and converters. Baseline ages were compared between groups using the Wilcoxon rank-sum test. Each violin plot includes the kernel probability density of the data at different values and the box plots with the median value and the interquartile range.
Acknowledgements
We thank all the participants and staff of the J-ADNI and NA-ADNI, and the donors and facility staff for providing autopsy brains. The J-ADNI was supported by the following funding sources: the Translational Research Promotion Project from the New Energy and Industrial Technology Development Organization of Japan; Research on Dementia, Health Labor Sciences Research Grant; the Life Science Database Integration Project of Japan Science and Technology Agency; the Research Association of Biotechnology (Astellas Pharma Inc., Bristol-Myers Squibb, Daiichi-Sankyo, Eisai, Eli Lilly and Company, Merck-Banyu, Mitsubishi Tanabe Pharma, Pfizer Inc., Shionogi & Co., Ltd., Sumitomo Dainippon, and Takeda Pharmaceutical Company), Japan; and a grant from an anonymous foundation. The reference genome data used for this research were originally obtained by participants in the Tailor-made Medical Treatment Program (BioBank Japan: BBJ), led by Prof. Michiaki Kubo; these data are available at the website of the NBDC Human Database/the Japan Science and Technology Agency (JST).
Consortia
The Alzheimer’s Disease Neuroimaging Initiative (ADNI)
Michael W. Weiner13, Sara S. Mason13, Colleen S. Albers13, David Knopman13, Kris Johnson13, Paul Aisen14, Ronald Petersen15, Clifford R. Jack16, William Jagust17, John Q. Trojanowki18, Arthur W. Toga19, Lon S. Schneider19, Sonia Pawluczyk19, Mauricio Beccera19, Liberty Teodoro19, Bryan M. Spann19, Laurel Beckett20, Robert C. Green21, John Morris22, Leslie M. Shaw22, Beau Ances22, John C. Morris22, Maria Carroll22, Mary L. Creech22, Erin Franklin22, Mark A. Mintun22, Stacy Schneider22, Angela Oliver22, Jeffrey Kaye23, Joseph Quinn23, Lisa Silbert23, Betty Lind23, Raina Carter23, Sara Dolen23, James Brewer24, Helen Vanderswag24, Adam Fleisher24,63, Judith L. Heidebrink25, Joanne L. Lord25, Rachelle S. Doody26, Javier Villanueva-Meyer26, Munir Chowdhury26, Susan Rountree26, Mimi Dang26, Yaakov Stern27, Lawrence S. Honig27, Karen L. Bell27, Daniel Marson28, Randall Griffith28, David Clark28, David Geldmacher28, John Brockington28, Erik Roberson28, Marissa Natelson Love28, Hillel Grossman29, Effie Mitsis29, Raj C. Shah30, Leyla deToledo-Morrell30, Ranjan Duara31, Daniel Varon31, Maria T. Greig31, Peggy Roberts31, Marilyn Albert32, Chiadi Onyike32, Daniel D'Agostino32, Stephanie Kielb32, James E. Galvin33, Brittany Cerbone33, Christina A. Michel33, Dana M. Pogorelec33, Henry Rusinek33, Mony J. de Leon33, Lidia Glodzik33, Susan De Santi33, P. Murali Doraiswamy34, Jeffrey R. Petrella34, Salvador Borges-Neto34, Terence Z. Wong34, Edward Coleman34, Charles D. Smith35, Greg Jicha35, Peter Hardy35, Partha Sinha35, Elizabeth Oates35, Gary Conrad35, Anton P. Porsteinsson36, Bonnie S. Goldstein36, Kim Martin36, Kelly M. Makino36, M. Saleem Ismail36, Connie Brand36, Ruth A. Mulnard37, Gaby Thai37, Catherine Mc-Adams-Ortiz37, Kyle Womack38, Dana Mathews38, Mary Quiceno38, Allan I. Levey39, James J. Lah39, Janet S. Cellar39, Jeffrey M. Burns40, Russell H. Swerdlow40, William M. Brooks40, Liana Apostolova41, Martin R. Farlow41, Ann Marie Hake41, Brandy R. Matthews41, Jared R. Brosch41, Scott Herring41, Cynthia Hunt41, Kathleen Tingus42, Ellen Woo42, Daniel H. S. Silverman42, Po H. Lu42, George Bartzokis42, Neill R. Graff-Radford43, Francine Parfitt43, Tracy Kendall43, Heather Johnson43, Christopher H. van Dyck44, Richard E. Carson44, Martha G. MacAvoy44, Pradeep Varma44, Howard Chertkow45, Howard Bergman45, Chris Hosein45, Sandra Black46, Bojana Stefanovic46, Curtis Caldwell46, Ging-Yuek Robin Hsiung47, Howard Feldman47, Benita Mudge47, Michele Assaly47, Elizabeth Finger48, Stephen Pasternack48, Irina Rachisky48, Dick Trost48, Andrew Kertesz48, Charles Bernick49, Donna Munic49, Marek Marsel Mesulam50, Kristine Lipowski50, Sandra Weintraub50, Borna Bonakdarpour50, Diana Kerwin50, Chuang-Kuo Wu50, Nancy Johnson50, Carl Sadowsky51, Teresa Villena51, Raymond Scott Turner52, Kathleen Johnson52, Brigid Reynolds52, Reisa A. Sperling53, Keith A. Johnson53, Gad Marshall53, Jerome Yesavage54, Joy L. Taylor54, Barton Lane54, Allyson Rosen54, Jared Tinklenberg54, Marwan N. Sabbagh55, Christine M. Belden55, Sandra A. Jacobson55, Sherye A. Sirrel55, Neil Kowall56, Ronald Killiany56, Andrew E. Budson56, Alexander Norbash56, Patricia Lynn Johnson56, Thomas O. Obisesan57, Saba Wolday57, Joanne Allard57, Alan Lerner58, Paula Ogrocki58, Curtis Tatsuoka58, Parianne Fatica58, Evan Fletcher59, Pauline Maillard59, John Olichney59, Charles DeCarli59, Owen Carmichael59, Smita Kittur60, Michael Borrie61, T.-Y. Lee61, Rob Bartha61, Sterling Johnson62, Sanjay Asthana62, Cynthia M. Carlsson62, Steven G. Potkin37, Adrian Preda37, Dana Nguyen37, Pierre Tariot63, Anna Burke63, Nadira Trncic63, Stephanie Reeder63, Vernice Bates64, Horacio Capote64, Michelle Rainka64, Douglas W. Scharre65, Maria Kataki65, Anahita Adeli65, Earl A. Zimmerman66, Dzintra Celmins66, Alice D. Brown66, Godfrey D. Pearlson67, Karen Blank67, Karen Anderson67, Laura A. Flashman68, Marc Seltzer68, Mary L. Hynes68, Robert B. Santulli68, Kaycee M. Sink69, Leslie Gordineer69, Jeff D. Williamson69, Pradeep Garg69, Franklin Watkins69, Brian R. Ott70, Henry Querfurth70, Geoffrey Tremont70, Stephen Salloway71, Paul Malloy71, Stephen Correia71, Howard J. Rosen72, Bruce L. Miller72, David Perry72, Jacobo Mintzer73, Kenneth Spicer73, David Bachman73, Nunzio Pomara74, Raymundo Hernando74, Antero Sarrael74, Norman Relkin75, Gloria Chaing75, Michael Lin75, Lisa Ravdin75, Amanda Smith76, Balebail Ashok Raj76, Kristin Fargher.76
13Magnetic Resonance Unit at the VA Medical Center and Radiology, Medicine, Psychiatry and Neurology, University of California, San Francisco, CA, USA. 14UC San Diego School of Medicine, University of California, La Jolla, CA, USA. 15Neurology, Mayo Clinic, Rochester, MN, USA. 16Radiology, Mayo Clinic, Rochester, MN, USA. 17University of California, Berkeley, Berkeley, CA, USA. 18University of Pennsylvania, Philadelphia, PA, USA. 19University of Southern California, Los Angeles, CA, USA. 20University of California, Davis, Davis, CA, USA. 21Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA. 22Washington University, St. Louis, MO, USA. 23Oregon Health and Science University, Portland, OR, USA. 24University of California, San Diego, San Diego, CA, USA. 25University of Michigan, Ann Arbor, MI, USA. 26Baylor College of Medicine, Houston, TX, USA. 27Columbia University Medical Center, New York, NY, USA. 28University of Alabama, Birmingham, AL, USA. 29Mount Sinai School of Medicine, New York, NY, USA. 30Rush University Medical Center, Chicago, IL, USA. 31Wien Center, Miami Beach, FL, USA. 32Johns Hopkins University, Baltimore, MD, USA. 33New York University, New York, NY, USA. 34Duke University Medical Center, Durham, NC, USA. 35University of Kentucky, Lexington, KY, USA. 36University of Rochester Medical Center, Rochester, NY, USA. 37University of California, Irvine, Irvine, CA, USA. 38University of Texas Southwestern Medical School, Dallas, TX, USA. 39Emory University, Atlanta, GA, USA. 40University of Kansas Medical Center, Kansas City, KS, USA. 41Indiana University, Bloomington, IN, USA. 42Universityof California, Los Angeles, Los Angeles, CA, USA. 43Mayo Clinic, Jacksonville, FL, USA. 44Yale University School of Medicine, New Haven, CT, USA. 45McGill University and Jewish General Hospital, Montreal, Quebec, Canada. 46Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada. 47UBC Clinic for Alzheimer Disease and Related Disorders, Vancouver, British Columbia, Canada. 48Cognitive Neurology-St. Joseph's, London, Ontario, Canada. 49Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, USA. 50Northwestern University, Evanston, IL, USA. 51Premiere Research Institute and Palm Beach Neurology, West Palm Beach, FL, USA. 52Georgetown University Medical Center, Washington, DC, USA. 53Brigham and Women's Hospital, Boston, MA, USA. 54Stanford University, Stanford, CA, USA. 55Banner Sun Health Research Institute, Sun City, AZ, USA. 56Boston University, Boston, MA, USA. 57Howard University, Boston, DC, USA. 58Case Western Reserve University, Cleveland, OH, USA. 59UC Davis School of Medicine, Sacramento, CA, USA. 60Neurological Care of CNY, Syracuse, NY, USA. 61Parkwood Hospital, Philadelphia, PA, USA. 62University of Wisconsin, Madison, WI, USA. 63Banner Alzheimer's Institute, Phoenix, AZ, USA. 64DENT Neurologic Institute, New York, NY, USA. 65Ohio State University, Columbus, OH, USA. 66Albany Medical College, Albany, NY, USA. 67Hartford Hospital, Olin Neuropsychiatry Research Center, Hartford, CT, USA. 68Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA. 69Wake Forest University Health Sciences, Winston-Salem, NC, USA. 70Rhode Island Hospital, Providence, RI, USA. 71Butler Hospital, Providence, RI, USA. 72University of California, San Francisco, San Francisco, CA, USA. 73Medical University of South Carolina, Charleston, SC, USA. 74Nathan Kline Institute, Orangeburg, NY, USA. 75Cornell University, Ithaca, NY, USA. 76USF Health Byrd Alzheimer's Institute, University of South Florida, Tampa, FL, USA.
The Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI)
Takeshi Iwatsubo12, Takashi Asada77,119, Hiroyuki Arai78,105, Morihiro Sugishita79, Hiroshi Matsuda80,129, Noriko Sato80,148, Hajime Sato80, Kengo Ito81, Teruhiko Kachi81, Kenji Toba81, Michio Senda82, Kenji Ishii83,141, Ryozo Kuwano11, Takeshi Ikeuchi3, Shun Shimohama84, Masaki Saitoh84, Rika Yamauchi84, Takashi Hayashi84, Chiyoko Takanami84, Seiju Kobayashi85, Norihito Nakano86, Junichiro Kanazawa87, Takeshi Ando88, Masato Hareyama89, Masamitsu Hatakenaka90, Eriko Tsukamoto91, Shinji Ochi92, Mikio Shoji93, Etsuro Matsubara93, Takeshi Kawarabayashi93, Yasuhito Wakasaya93, Takashi Nakata93, Naoko Nakahata93, Shuichi Ono94, Yoshihiro Takai94, Satoshi Takahashi95, Hisashi Yonezawa95, Junko Takahashi95, Masako Kudoh95, Kuniko Ueno95, Hiromi Sakashita95, Kuniko Watanabe95, Makoto Sasaki96, Yutaka Matsumura97, Yohsuke Hirata97, Tsuyoshi Metoki97, Susumu Hayakawa97, Yuichi Sato97,100, Masayuki Takeda97, Koichiro Sera97, Kazunori Terasaki97, Toshiaki Sasaki98, Yoshihiro Saitoh99, Shoko Goto99, Ken Nagata100, Tetsuya Maeda100, Yasushi Kondoh100, Takashi Yamazaki100, Daiki Takano100, Mio Miyata100, Hiromi Komatsu100, Mayumi Watanabe100, Tomomi Sinoda100, Rena Muraoka100, Kayoko Kikuchi101, Hitomi Ito102, Aki Sato102, Toshibumi Kinoshita103, Hideyo Toyoshima103, Kaoru Sato103, Shigeki Sugawara103, Isao Ito104, Fumiko Kumagai104, Katsutoshi Furukawa105, Masaaki Waragai105, Naoki Tomita105, Mari Ootsuki105, Katsumi Sugawara105, Satomi Sugawara105, Nobuyuki Okamura106, Shunji Mugikura107, Atsushi Umetsu107, Takanori Murata107, Tatsuo Nagasaka107, Yukitsuka Kudo108, Manabu Tashiro109, Shoichi Watanuki109, Masatoyo Nishizawa110, Takayoshi Tokutake110, Saeri Ishikawa111, Emiko Kishida111, Nozomi Sato111, Mieko Hagiwara112, Kumi Yamanaka112, Takeyuki Watanabe112, Taeko Takasugi112, Shoichi Inagawa113, Kenichi Naito113, Masanori Awaji113, Tsutomu Kanazawa113, Kouiti Okamoto114, Masaki Ikeda114, Yuiti Tasiro114, Syunn Nagamine114, Sathiko Kurose114, Tsuneo Yamazaki115, Shiori Katsuyama115, Sayuri Fukushima116, Etsuko Koya116, Makoto Amanuma117, Kouiti Ujita117, Kazuhiro Kishi117, Kazuhisa Tuda117, Noboru Oriuti118, Katsuyoshi Mizukami119, Tetsuaki Arai119, Etsuko Nakajima119, Katsumi Miyamoto120, Tomoya Kobayashi120, Saori Itoya120, Jun Ookubo120, Toshiya Akatsu120, Yoshiko Anzai120, Junya Ikegaki120, Yuuichi Katou120, Kaori Kimura120, Hajime Saitou120, Kazuya Shinoda120, Satoka Someya120, Hiroko Taguchi120, Kazuya Tashiro120, Masaya Tanaka120, Tatsuya Nemoto120, Ryou Wakabayashi120, Daisuke Watanabe120, Kousaku Saotome121, Ryou Kuchii121, Harumasa Takano122, Tetsuya Suhara122, Hitoshi Shinoto122, Hitoshi Shimada122,126, Makoto Higuchi122, Takaaki Mori122, Hiroshi Ito123, Takayuki Obata123, Yoshiko Fukushima124, Kazuko Suzuki124, Izumi Izumida124, Katsuyuki Tanimoto125, Takahiro Shiraishi125, Hitoshi Shinotoh126, Junko Shiba126, Hiroaki Yano126, Miki Satake126, Aimi Nakui126, Yae Ebihara126, Tomomi Hasegawa126, Yasumasa Yoshiyama127, Mami Kato127, Yuki Ogata127, Hiroyuki Fujikawa127, Nobuo Araki128, Yoshihiko Nakazato128, Takahiro Sasaki128, Tomokazu Shimadu128, Kimiko Yoshimaru128, Etsuko Imabayashi129, Asako Yasuda129, Keiko Ozawa129, Etuko Yamamoto130, Natsumi Nakamata130, Noriko Miyauchi130, Rieko Hashimoto131, Taishi Unezawa131, Takafumi Ichikawa131, Hiroki Hayashi131, Masakazu Yamagishi131, Tunemichi Mihara131, Masaya Hirano131, Shinichi Watanabe131, Junichiro Fukuhara132, Hajime Matsudo132, Nobuyuki Saito133, Atsushi Iwata134, Hisatomo Kowa134, Toshihiro Hayashi134, Ryoko Ihara134, Toji Miyagawa134, Mizuho Yoshida134, Yuri Koide134, Eriko Samura134, Kurumi Fujii134, Kaori Watanabe135, Nagae Orihara135, Toshimitsu Momose136, Miwako Takahashi136, Takuya Arai136, Yoshiki Kojima136, Akira Kunimatsu137, Harushi Mori137, Masami Goto138, Takeo Sarashina138, Syuichi Uzuki138, Seiji Katou138, Yoshiharu Sekine138, Yukihiro Takauchi138, Chiine Kagami139, Kazutomi Kanemaru140, Yasushi Nishina140, Maria Sakaibara140, Yumiko Okazaki140, Rieko Okada140, Maki Obata140,146, Shigeo Murayama4,5, Masaki Takao141,258, Yuko Iwata142,147,157, Mizuho Minami142,157, Yasuko Hanabusa142,147, Hanae Shingyouji142,147, Kyoko Tottori142, Aya Tokumaru143, Makoto Ichinose143, Kazuya Kume143, Syunsuke Kahashi143, Kunimasa Arima144, Shin Tanaka144, Yuko Nagahusa144, Masuhiro Sakata144, Mitsutoshi Okazaki144, Yuko Saito4, Maki Yamada144, Tadashi Tukamoto145, Tiine Kodama146, Tomoko Takeuchi146, Keiichiro Ozawa146, Yoshiko Kawaji147, Kyouko Tottori147,157, Yasuhiro Nakata148, Satoshi Sawada148, Makoto Mimatsu148, Daisuke Nakkamura148, Takeshi Tamaru148, Shunichirou Horiuchi148, Heii Arai149, Tsuneyoshi Ota149, Aiko Kodaka149, Yuko Tagata149, Tomoko Nakada149, Eizo Iseki150, Kiyoshi Sato150, Hiroshige Fujishiro151, Norio Murayama152, Masaru Suzuki153, Satoshi Kimura153, Masanobu Takahashi153, Haruo Hanyu154, Hirofumi Sakurai154, Takahiko Umahara154, Hidekazu Kanetaka154, Kaori Arashino154, Mikako Murakami154, Ai Kito154, Seiko Miyagi155, Kaori Doi155, Kazuyoshi Sasaki155, Mineo Yamazaki156, Akiko Ishiwata156, Yasushi Arai156, Akane Nogami156, Sumiko Fukuda156, Koichi Kozaki158, Yukiko Yamada158, Sayaka Kimura158, Ayako Machida158, Kuninori Kobayashi159, Hidehiro Mizusawa160, Nobuo Sanjo160, Mutsufusa Watanabe160, Takuya Ohkubo160, Hiromi Utashiro160, Yukiko Matsumoto160, Kumiko Hagiya160, Yoshiko Miyama160, Hitoshi Shibuya160, Isamu Ohashi160, Akira Toriihara160, Takako Shinozaki161, Haruko Hiraki161, Shinichi Ohtani162, Toshifumi Matsui163, Tomomi Toyama163, Hideki Sakurai163, Kumiko Sugiura163, Yu Hayasaka164, Hirofumi Taguchi165, Shizuo Hatashita166, Akari Imuta167, Akiko Matsudo167, Daichi Wakebe168, Hideki Hayakawa168, Mitsuhiro Ono168, Takayoshi Ohara168, Yukihiko Washimi169, Yutaka Arahata169, Akinori Takeda169, Akiko Yamaoka169, Masashi Tsujimoto169, Takiko Kawai169, Ai Honda169, Yoko Konagaya170, Hideyuki Hattori171, Kenji Yoshiyama171,212, Rina Miura171, Takashi Sakurai172,228, Miura Hisayuki173, Hidetoshi Endou174, Syousuke Satake175, Young Jae Hong175, Katsunari Iwai176, Masaki Suenaga176, Sumiko Morita176, Kengo Itou177, Takashi Kato177, Ken Fujiwara177, Rikio Katou178, Mariko Koyama178, Naohiko Fukaya178, Akira Tsuji178, Hitomi Shimizu178, Hiroyuki Fujisawa178, Tomoko Nakazawa178, Satoshi Koyama178, Takanori Sakata178, Masahito Yamada179, Mitsuhiro Yoshita179, Miharu Samuraki179, Kenjiro Ono179, Moeko Shinohara179, Yuki Soshi179, Kozue Niwa179, Chiaki Doumoto179, Mariko Hata180, Miyuki Matsushita180, Mai Tsukiyama180, Nozomi Takeda181, Sachiko Yonezawa181, Ichiro Matsunari181, Osamu Matsui182, Fumiaki Ueda182, Yasuji Ryu182, Masanobu Sakamoto183, Yasuomi Ouchi183,184, Yumiko Fujita183, Madoka Chita185, Rika Majima186, Hiromi Tsubota187, Umeo Shirasawa188, Masashi Sugimori188, Wataru Ariya188, Yuuzou Hagiwara188, Yasuo Tanizaki188, Hidenao Fukuyama189, Shizuko Tanaka-Urayama189, Shin-Ichi Urayama189, Ryosuke Takahashi190, Kengo Uemura190, Hajime Takechi191, Chihiro Namiki192, Takeshi Kihara193, Hiroshi Yamauchi194, Emiko Maeda195, Natsu Saito196, Shiho Satomi197, Konomi Kabata198, Tomohisa Okada199, Koichi Ishizu200, Shigeto Kawase201, Satoshi Fukumoto201, Masanori Nakagawa202, Masaki Kondo202, Fumitoshi Niwa202, Toshiki Mizuno202, Yoko Oishi202, Mariko Yamazaki202, Daisuke Yamaguchi202, Takahiko Tokuda203, Kyoko Ito204, Yoku Asano204, Chizuru Hamaguchi204, Kei Yamada205, Chio Okuyama205, Kentaro Akazawa205, Shigenori Matsushima205, Takamasa Matsuo206, Toshiaki Nakagawa206, Takeshi Nii206, Takuji Nishida206, Kuniaki Kiuchi207, Masami Fukusumi208, Hideyuki Watanabe209, Toshiaki Taoka210, Akihiro Nogi211, Masatoshi Takeda212, Toshihisa Tanaka212, Hiroaki Kazui212, Takashi Kudo212, Masayasu Okochi212, Takashi Morihara212, Shinji Tagami212, Masahiko Takaya212, Tamiki Wada212, Mikiko Yokokoji212, Hiromichi Sugiyama212, Daisuke Yamamoto212, Keiko Nomura212, Mutsumi Tomioka212, Naoyuki Sato213, Noriyuki Hayashi214, Shuko Takeda215, Eiichi Uchida216, Yoshiyuki Ikeda216, Mineto Murakami216, Takami Miki217, Hiroyuki Shimada217, Suzuka Ataka217, Akitoshi Takeda217, Yuki Iwamoto217, Motokatsu Kanemoto218, Jun Takeuchi219, Rie Azuma220, Naomi Tagawa221, Junko Masao221, Yuka Matsumoto221, Yuko Kikukawa221, Hisako Fujii221, Junko Matsumura221, Susumu Shiomi222, Joji Kawabe222, Yoshihiro Shimonishi223, Mitsuji Higashida223, Tomohiro Sahara223, Takashi Yamanaga223, Yukio Miki224, Shinichi Sakamoto224, Hiroyuki Tsushima225, Kiyoshi Maeda226, Yasuji Yamamoto226, Kazuo Sakai226, Haruhiko Oda226, Yoshihiko Tahara226, Toshio Kawamata227, Taichi Akisaki228, Mizuho Adachi229, Masako Kuranaga229, Sachi Takegawa229, Seishi Terada230, Yuki Kishimoto230, Naoya Takeda230, Nao Imai230, Mayumi Yabe230, Reiko Wada230, Takeshi Ishihara231, Hajime Honda232, Osamu Yokota232, Kentaro Ida233, Daigo Anami234, Seiji Inoue234, Toshi Matsushita234, Shinsuke Hiramatsu235, Hiromi Tonbara236, Reiko Yamamoto236, Kenji Nakashima237, Kenji Wada-Isoe237, Saori Yamasaki237, Eijiro Yamashita238, Yu Nakamura239, Ichiro Ishikawa239, Sonoko Danjo239, Tomomi Shinohara239, Yuka Kashimoto239, Miyuki Ueno240, Yoshihiro Nishiyama241, Yuka Yamamoto241, Narihide Kimura241, Kazuo Ogawa242, Yasuhiro Sasakawa242, Takashi Ishimori242, Yukito Maeda242, Tatsuo Yamada243, Shinji Ouma243, Aika Fukuhara-Kaneumi243, Nami Sakamoto244, Rie Nagao244, Kengo Yoshimitsu245, Yasuo Kuwabara245, Ryuji Nakamuta246, Minoru Tanaka246, Manabu Ikeda247, Yuusuke Yatabe247, Mamoru Hashimoto248, Keiichirou Kaneda248, Kazuki Honda248, Naoko Ichimi248, Mariko Morinaga248, Miyako Noda248, Fumi Akatuka249, Mika Kitajima250, Toshinori Hirai251, Shinya Shiraishi251, Naoji Amano252, Shinsuke Washizuka252, Tetsuya Hagiwara252, Yatsuka Okada252, Tomomi Ogihara252, Toru Takahashi253, Shin Inuzuka253, Nobuhiro Sugiyama253, Takehiko Yasaki253, Minori Kitayama253, Tomonori Owa253, Akiko Ryokawa253, Rie Takeuchi254, Satoe Goto254, Keiko Yamauchi254, Mie Ito254, Tomoki Kaneko255, Hitoshi Ueda256, Shuichi Ikeda257, Ban Mihara258, Hirofumi Kubo258, Akiko Takano258, Gou Yasui258, Masami Akuzawa258, Kaori Yamaguchi258, Toshinari Odawara259, Naomi Oota259, Megumi Shimamura260, Mikiko Sugiyama260, Atsushi Watanabe261, Shigeo Takebayashi262, Yoshigazu Hayakawa262, Mitsuhiro Idegawa262, Noriko Toya262, Kazunari Ishii.263
77Tsukuba University, Tsukuba, Japan. 78Tohoku University, Sendai, Japan. 79Institute of Brain and Blood Vessels, Isesaki, Japan. 80National Center of Neurology and Psychiatry, Kodaira, Japan. 81National Center of Geriatrics and Gerontology, Tokyo, Japan. 82Institute of Biomedical Research and Innovation, Kobe, Japan. 83Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Tokyo, Japan. 84Department of Neurology, Sapporo Medical University, Sapporo, Japan. 85Department of Neuropsychiatry, Sapporo Medical University, Sapporo, Japan. 86Department of Clinical Psychology, School of Psychological Science, Health Sciences University of Hokkaido, Sapporo, Japan. 87School of Psychological Science, Health Sciences University of Hokkaido, Sapporo, Japan. 88Graduate School of Psychological Science, Health Sciences University of Hokkaido, Sapporo, Japan. 89Department of Radiology, Sapporo Medical University, Sapporo, Japan. 90Division of Diagnostic Radiology, Sapporo Medical University, Sapporo, Japan. 91Social Medical Corporation Teishinkai Central CI Clinic, Sapporo, Japan. 92Radiology Department, Social Medical Corporation Teishinkai Central CI Clinic, Sapporo, Japan. 93Department of Neurology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan. 94Department of Radiology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan. 95Division of Neurology and Gerontology, Department of Internal Medicine, Iwate Medical University, Morioka, Japan. 96Division of Ultrahigh Field MRI, Institute for Biomedical Sciences, Iwate Medical University, Morioka, Japan. 97Center for Radiological Sciences, Iwate Medical University, Morioka, Japan. 98Cyclotron Research Center, Iwate Medical University, Morioka, Japan. 99Nishina Memorial Cyclotron Center, Japan Radioisotope Association, Takizawa, Japan. 100Departmetnt of Neurology, Research Institute for Brain and Blood Vessels-Akita, Akita, Japan. 101Pharmacy, Research Institute for Brain and Blood Vessels-Akita, Akita, Japan. 102Medical Support Department, Research Institute for Brain and Blood Vessels-Akita, Akita, Japan. 103Department of Radiology, Research Institute for Brain and Blood Vessels-Akita, Akita, Japan. 104Clinical Laboratory, Research Institute for Brain and Blood Vessels-Akita, Akita, Japan. 105Geriatrics and Gerontology, Tohoku University Hospital, Sendai, Japan. 106Pharamacology, Tohoku University Hospital, Sendai, Japan. 107RadiologyTohoku University Hospital, Sendai, Japan. 108Neuroimaging, Tohoku University Hospital, Sendai, Japan. 109Nuclear Medicine, Tohoku University Hospital, Sendai, Japan. 110Department of Neurology, Brain Research Institute, Niigata University, Niigata, Japan. 111Department of Rehabilitation, Niigata University Medical and Dental Hospital, Niigata, Japan. 112Clinical Research Center, Niigata University Medical and Dental Hospital, Niigata, Japan. 113Department of Radiology, Niigata University Medical and Dental Hospital, Niigata, Japan. 114Department of Neurology, Gunma University Hospital, Maebashi, Japan. 115Department of Health of Rehabilitation, Gunma University Hospital, Maebashi, Japan. 116Clinical Trial Unit, Gunma University Hospital, Maebashi, Japan. 117Department of Radiology, Gunma University Hospital, Maebashi, Japan. 118Department of Nuclear Medicine Image, Gunma University Hospital, Maebashi, Japan. 119Department of Psychiatry Division of Clinical Medicine Faculty of Medicine, University of Tsukuba, Tsukuba, Japan. 120Department of Radiological Technology, Tsukuba Medical Center Hospital, Tsukuba, Japan. 121Former Department of Radiological Technology, Tsukuba Medical Center Hospital, Tsukuba, Japan. 122Molecular Imaging Center Molecular Neuroimaging Program, National Institute of Radiological Sciences, Chiba, Japan. 123Molecular Imaging Center Biophysics Program, National Institute of Radiological Sciences, Chiba, Japan. 124Molecular Imaging Center Planning and Promotion Unit Clinical Research Support Section, National Institute of Radiological Sciences, Chiba, Japan. 125Hospital Research Center for Charged Particle Therapy Radiological Technology Section, National Institute of Radiological Sciences, Chiba, Japan. 126Asahi Hospital for Neurological Diseases and Rehabilitation, Matsudo, Japan. 127National Hospital Organization Chiba East National Hospital, Chiba, Japan. 128Department of Neurology, Stroke Care Unit, Saitama Medical University Hospital, Moroyama, Japan. 129Department of Nuclear Medicine, Saitama Medical University Hospital, Moroyama, Japan. 130Department of Rehabilitation, Saitama Medical University Hospital, Moroyama, Japan. 131Central Radiology Division, Saitama Medical University Hospital, Moroyama, Japan. 132Pharmacy Division, Saitama Medical University Hospital, Moroyama, Japan. 133SHI Accelerator Service Ltd. (SAS), Tokyo, Japan. 134Department of Neurology, The University of Tokyo Hospital, Tokyo, Japan. 135Clinical Research Center, The University of Tokyo Hospital, Tokyo, Japan. 136Department of Nuclear Medicine, The University of Tokyo Hospital, Tokyo, Japan. 137Department of Diagnostic Radiology, The University of Tokyo Hospital, Tokyo, Japan. 138Radiological Center, The University of Tokyo Hospital, Tokyo, Japan. 139Neuroscience, Faculty of Medicine, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan. 140Department of Neurology, Tokyo Metropolitan Geriatric Hospital, Tokyo, Japan. 141Tokyo Metropolitan Institute of Gerontology, Tokyo, Japan. 142Clinical Support Corporation, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Tokyo, Japan. 143Department of Radiology, Tokyo Metropolitan Geriatric Hospital, Tokyo, Japan. 144Department of Psychiatry, National Center Hospital National Center of Neurology and Psychiatry, Kodaira, Japan. 145Department of Neurology, National Center Hospital National Center of Neurology and Psychiatry, Kodaira, Japan. 146Administration Office of J-ADNI, National Center Hospital National Center of Neurology and Psychiatry, Kodaira, Japan. 147Clinical Support Corporation, National Center Hospital National Center of Neurology and Psychiatry, Kodaira, Japan. 148Department of Radiology, National Center Hospital National Center of Neurology and Psychiatry, Tokyo, Japan. 149Juntendo University Hospital, Mental Clinic, Tokyo, Japan. 150PET/CT Dementia Research Center, Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan. 151PET/CT Dementia Research Center, Department of Neuro-Psychiatry, Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan. 152Department of Neuro-Psychiatry, Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan. 153Radiology, Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan. 154Department of Geriatric Medicine, Tokyo Medical University, Tokyo, Japan. 155Tokyo Medical University, Tokyo, Japan. 156Division of Neurology, Department of Internal Medicine, Nippon Medical School, Tokyo, Japan. 157Clinical Support Corporation, Nippon Medical School Hospital, Japan. 158Geriatric Medicine, Kyorin University, Mitaka, Japan. 159Radiation Medicine, Kyorin University, Mitaka, Japan. 160Tokyo Medical and Dental University Graduate school of Medicine and Dental Sciences, Tokyo, Japan. 161Clinical Research Center, Tokyo Medical and Dental University Hospital Faculty of Medicine, Tokyo, Japan. 162Department of Radiology, Tokyo Medical and Dental University Hospital Faculty of Medicine, Tokyo, Japan. 163Clinical Center for Neurodegenerative, National Hospital Organization Kurihama Medical, Addiction Center, Yokosuka, Japan. 164Department of Psychiatry, Kitasato University School of Medicine, Sagamihara, Japan. 165Department of Radiology, National Hospital Organization Kurihama Medical, Addiction Center, Yokosuka, Japan. 166Neurosurgery, Shonan-atsugi Hospital, Atsugi, Japan. 167Clinical Research Center, Shonan-atsugi Hospital, Atsugi, Japan. 168Radiology, Shonan-atsugi Hospital, Atsugi, Japan. 169Department of Cognitive Disorders, National Center of Geriatrics and Gerontology, Obu, Japan. 170Dementia Care Research and Training Center, National Center of Geriatrics and Gerontology, Obu, Japan. 171Department of Psychiatry, National Center of Geriatrics and Gerontology, Obu, Japan. 172Center for Comprehensive Care and Research on Memory Disorders, National Center of Geriatrics and Gerontology, Obu, Japan. 173Department of Home Medical Care Support, National Center of Geriatrics and Gerontology, Obu, Japan. 174Department of Comprehensive Geriatric Medicine, National Center of Geriatrics and Gerontology, Obu, Japan. 175Department of Geriatrics, National Center of Geriatrics and Gerontology, Obu, Japan. 176Department of Neurology, National Center of Geriatrics and Gerontology, Obu, Japan. 177Department of Clinical and Experimental Neuroimaging, National Center of Geriatrics and Gerontology, Obu, Japan. 178Department of Radiology, National Center of Geriatrics and Gerontology, Obu, Japan. 179Department of Neurology and Neurobiology of Aging, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan. 180Center for Clinical Research Management, Kanazawa University Hospital, Kanazawa, Japan. 181The Medical and Pharmacological Research Center Foundation, Kanazawa, Japan. 182Department of Radiology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan. 183Department of Neurology, Hamamatsu Medical Center, Hamamatsu, Japan. 184Hamamatsu University School of Medicine, Hamamatsu, Japan. 185Hamamatsu Medical Center, Hamamatsu, Japan. 186Department of Psychiatry, Hamamatsu Medical Center, Hamamatsu, Japan. 187Department of Clinical Research Management, Hamamatsu Medical Center, Hamamatsu, Japan. 188Department of Radiological Technology, Hamamatsu Medical Center, Hamamatsu, Japan. 189Human Brain Research Center, Kyoto University Graduate School of Medicine, Kyoto, Japan. 190Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto, Japan. 191Department of Geriatric Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan. 192Eli Lilly Japan K.K Medical Science, Kobe, Japan. 193Rakuwakai Misasagi Hospital, Kyoto, Japan. 194Shiga Medical Center Research Institute, Moriyama, Japan. 195Takemura Clinic, Kyoto, Japan. 196Tonami General Hospital, Tonami, Japan. 197University Hospital, Kyoto Prefectural University of Medicine, Kyoto, Japan. 198Department of Diagnostic Pathology, Kyoto University Hospital, Kyoto, Japan. 199Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan. 200Department of Human Health Sciences, Kyoto University Graduate School of Medicine, Kyoto, Japan. 201Department of Radiology and Nuclear Medicine Service, Kyoto University Hospital, Kyoto, Japan. 202Department of Neurology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan. 203Department of Molecular Pathobiology of Brain Diseases (Department of Neurology), Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan. 204Tokyo Yakuriken Co., Ltd, Tokyo, Japan. 205Department of Radiology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan. 206Kyoto Prefectural University of Medicine Hospital, Kyoto, Japan. 207Department of Psychiatry, Nara Medical University, Kashihara, Japan. 208Faculty of Psychology, Doshisha University, Kyotanabe, Japan. 209Heartland Shigisan Hospital, Sango, Japan. 210Department of Radiology, Nara Medical University, Kashihara, Japan. 211Central Radiology, Nara Medical University, Kashihara, Japan. 212Psychiatry, Department of Integrated Medicine, Division of Internal Medicine, Osaka University Graduate School of Medicine, Suita, Japan. 213Department of Clinical Gene Therapy, Department of Geriatric Medicine, Osaka University Graduate School of Medicine, Suita, Japan. 214Department of Complementary and Alternative Medicine, Osaka University Graduate School of Medicine, Suita, Japan. 215Japan Society for the Promotion of Science, Tokyo, Japan. 216Uchida Clinic/Department of radiology, Osaka University Graduate School of Medicine, Ibaraki/Suita, Japan. 217Department of Geriatrics and Neurology, Osaka City University Graduate School of Medicine, Osaka, Japan. 218Osaka Municipal Kohsaiin Hospital, Suita, Japan. 219Kishiwada City Hospital, Kishiwada, Japan. 220Department of Psychiatry, Kosaka Hospital, Higashiosaka, Japan. 221Osaka City University Hospital Center for Drug and Food Clinical Evaluation, Osaka, Japan. 222Department of Nuclear Medicine, Osaka City University Graduate School of Medicine, Osaka, Japan. 223Department of Radiology, Osaka City University Hospital, Osaka, Japan. 224Department of Radiology, Osaka City University Graduate School of Medicine, Osaka, Japan. 225Department of Radiological Science, Ibaraki Prefectural University of Health Sciences, Ami, Japan. 226Department of Psychiatry, Kobe University Graduate School of Medicine, Kobe, Japan. 227Kobe University Graduate School of Health Sciences, Kobe, Japan. 228Department of General Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan. 229Derartment of Psychiatry and Neurology, Kobe University Hospital, Kobe, Japan. 230Department of Psychiatry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical, Okayama, Japan. 231Department of Psychiatry, Kawasaki Hospital, Kurashiki, Japan. 232Department of Psychiatry, Okayama University Hospital, Okayama, Japan. 233Department of Imaging Diagnosis, Okayama Diagnostic Imaging Center, Okayama, Okayama, Japan. 234Department of Imaging Technology, Okayama Diagnostic Imaging Center, Okayama, Okayama, Japan. 235Department of Administration, Okayama Diagnostic Imaging Center, Okayama, Japan. 236Okayama University Hospital, Okayama, Japan. 237Division of Neurology, Department of Brain and Neurosciences, Faculty of Medicine, Tottori University, Yonago, Japan. 238Division of Clinical Radiology, Tottori University Hospital, Yonago, Japan. 239Department of Neuropsychiatry Kagawa University School of Medicine, Kagawa, Japan. 240Kagawa University Hospital Cancer center, Kagawa, Japan. 241Department of Radiology, Faculty of Medicine, Kagawa University, Kagawa, Japan. 242Department of Clinical Radiology, Kagawa University Hospital, Kagawa, Japan. 243Department of Neurology, Fukuoka University, Fukuoka, Japan. 244Neues Corporation, Tokyo, Japan. 245Department of Radiology, Fukuoka University, Fukuoka, Japan. 246Radiology Center, Fukuoka University Hospital, Fukuoka, Japan. 247Department of Psychiatry and Neuropathobiology, Faculty of Life Science, Kumamoto University, Kumamoto, Japan. 248Department of Neuropsychiatry, Kumamoto University Hospital, Kumamoto, Japan. 249A Center of Support for Child Development, Kumamoto University Hospital, Kumamoto, Japan. 250Department of Medical Imaging, Kumamoto University, Kumamoto, Japan. 251Department of Diagnostic Imaging, Faculty of Life Science, Kumamoto University, Kumamoto, Japan. 252Department of Psychiatry, Shinshu University School of Medicine, Matsumoto, Japan. 253Department of Psychiatry, Shinshu University Hospital, Matsumoto, Japan. 254Clinical Trial Research Center, Shinshu University Hospital, Matsumoto, Japan. 255Department of Radiology, Shinshu University School of Medicine, Matsumoto, Japan. 256Radiology Division, Shinshu University Hospital, Matsumoto, Japan. 257Department of Medicine (Neurology and Rheumatology), Shinshu University School of Medicine, Matsumoto, Japan. 258Mihara Memorial Hospital, Isesaki, Japan. 259Psychiatric Center, Yokohama City University Medical Center, Yokohama, Japan. 260Neurology, Yokohama City University Medical Center, Yokohama, Japan. 261Yokohama City University Medical Center, Yokohama, Japan. 262Radiology, Yokohama City University Medical Center, Yokohama, Japan. 263Kindai University, Sayama, Japan.
Abbreviations
- PRS
Polygenic risk score
- AD
Alzheimer’s disease
- J-ADNI
Japanese Alzheimer’s Disease Neuroimaging Initiative
- APOE
Apolipoprotein E
- Aβ
Amyloid-beta
- MCI
Mild cognitive impairment
- GWAS
Genome-wide association study
- MRI
Magnetic resonance imaging
- CU
Cognitively unimpaired
- ADD
Alzheimer’s disease dementia
- NA-ADNI
North American Alzheimer’s Disease Neuroimaging Initiative
- C + T
Clumping and thresholding
- AUC
Area under the receiver operator characteristic curve
- MDS
Multidimensional scaling
- CSF
Cerebrospinal fluid
- tTau
Total tau
- pTau
Phosphorylated tau
- PET
Positron emission tomography
- FDG
18F-2-fluoro-2-deoxy-D-glucose
- PiB
11C-Pittsburgh compound B
- MMSE
Mini–Mental State Examination
- FAQ
Functional Assessment Questionnaire
- CDR
Clinical Dementia Rating
- CDR-SB
CDR–Sum of Boxes
- ADAS-Cog
AD Assessment Scale–Cognitive Subscale
- FDR
False discovery rate
- HR
Hazard ratio
- CI
Confidence interval
Authors’ contributions
MK: Study design, analysis and interpretation of data, and manuscript draft. AM and NH: Genotyping analysis, interpretation of data, and manuscript revision. YS, SM, AK, HA: Provision of autopsy brains and manuscript revision. KK, KO, SN, RK, TIwatsubo, and AN: Interpretation of data and manuscript revision. TIkeuchi: Study design, interpretation of data, and manuscript draft. All authors read and approved the final manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (grant numbers 20K15778 to MK, 21K07271 and 21H03537 to AM, and 22H04923 to YS) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), by grants from the Japan Agency for Medical Research and Development (AMED) (grant numbers JP21dk0207045 and JP23dk0207060 to MK, AM, KO, SN, and TI, JP23wm0525019 to MK and TI, and JP21wm0425019 to YS), by Grants-in Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan (grant number 20FC1049 to YS). The funders had no role in the study design, data collection, decision to publish, or preparation of the manuscript.
Availability of data and materials
All the J-ADNI data except for the genome data and the reference genome data were obtained from the NBDC Human Database/the Japan Science and Technology Agency (JST) (https://humandbs.biosciencedbc.jp/en/hum0043-v1), (https://humandbs.biosciencedbc.jp/en/hum0014-latest#JGAS000114rp). GWAS statistics were obtained from the Center for Neurogenomics and Cognitive Research (https://ctg.cncr.nl/software/summary_statistics). The J-ADNI genome data are available on request.
The data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (adni.loni.usc.edu). Thus, the investigators within the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in the analysis or the writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_ apply/ADNI_Acknowledgement_List.pdf.
Data used in preparation of this article were obtained from the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) database within the National Bioscience Database Center Human Database, Japan (Research ID: hum0043.v1, 2016). Thus, the investigators within J-ADNI contributed to the design and implementation of J-ADNI and/or provided data but did not participate in the analysis or the writing of this report. A complete listing of J-ADNI investigators can be found at https://humandbs.biosciencedbc.jp/en/hum0043-j-adni-authors.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committees of the University of Tokyo, Osaka University and Niigata University. For both ADNI and J-ADNI, ethics approval was obtained from the review boards of the participating institutions. Informed consent was obtained from every participant prior to enrolment.
Consent for publication
Consent for publication has been granted by J-ADNI administrators.
Competing interests
The authors declare no competing interests.
Footnotes
The original version of this article was revised: the authors corrected the error in Figure 2 (Figures 2C and D are the same figures as Figures 2A and B in the original publication).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/10/2024
A Correction to this paper has been published: 10.1186/s13195-024-01514-8
Contributor Information
Masataka Kikuchi, Email: kikuchi@edu.k.u-tokyo.ac.jp.
Takeshi Ikeuchi, Email: ikeuchi@bri.niigata-u.ac.jp.
the Alzheimer’s Disease Neuroimaging Initiative:
Michael W. Weiner, Sara S. Mason, Colleen S. Albers, David Knopman, Kris Johnson, Paul Aisen, Ronald Petersen, Clifford R. Jack, William Jagust, John Q. Trojanowki, Arthur W. Toga, Lon S. Schneider, Sonia Pawluczyk, Mauricio Beccera, Liberty Teodoro, Bryan M. Spann, Laurel Beckett, Robert C. Green, John Morris, Leslie M. Shaw, Beau Ances, John C. Morris, Maria Carroll, Mary L. Creech, Erin Franklin, Mark A. Mintun, Stacy Schneider, Angela Oliver, Jeffrey Kaye, Joseph Quinn, Lisa Silbert, Betty Lind, Raina Carter, Sara Dolen, James Brewer, Helen Vanderswag, Adam Fleisher, Judith L. Heidebrink, Joanne L. Lord, Rachelle S. Doody, Javier Villanueva-Meyer, Munir Chowdhury, Susan Rountree, Mimi Dang, Yaakov Stern, Lawrence S. Honig, Karen L. Bell, Daniel Marson, Randall Griffith, David Clark, David Geldmacher, John Brockington, Erik Roberson, Marissa Natelson Love, Hillel Grossman, Effie Mitsis, Raj C. Shah, Leyla deToledo-Morrell, Ranjan Duara, Daniel Varon, Maria T. Greig, Peggy Roberts, Marilyn Albert, Chiadi Onyike, Daniel D’Agostino, Stephanie Kielb, James E. Galvin, Brittany Cerbone, Christina A. Michel, Dana M. Pogorelec, Henry Rusinek, Mony J. de Leon, Lidia Glodzik, Susan De Santi, P. Murali Doraiswamy, Jeffrey R. Petrella, Salvador Borges-Neto, Terence Z. Wong, Edward Coleman, Charles D. Smith, Greg Jicha, Peter Hardy, Partha Sinha, Elizabeth Oates, Gary Conrad, Anton P. Porsteinsson, Bonnie S. Goldstein, Kim Martin, Kelly M. Makino, M. Saleem Ismail, Connie Brand, Ruth A. Mulnard, Gaby Thai, Catherine Mc-Adams-Ortiz, Kyle Womack, Dana Mathews, Mary Quiceno, Allan I. Levey, James J. Lah, Janet S. Cellar, Jeffrey M. Burns, Russell H. Swerdlow, William M. Brooks, Liana Apostolova, Martin R. Farlow, Ann Marie Hake, Brandy R. Matthews, Jared R. Brosch, Scott Herring, Cynthia Hunt, Kathleen Tingus, Ellen Woo, Daniel H. S. Silverman, Po H. Lu, George Bartzokis, Neill R. Graff-Radford, Francine Parfitt, Tracy Kendall, Heather Johnson, Christopher H. van Dyck, Richard E. Carson, Martha G. MacAvoy, Pradeep Varma, Howard Chertkow, Howard Bergman, Chris Hosein, Sandra Black, Bojana Stefanovic, Curtis Caldwell, Ging-Yuek Robin Hsiung, Howard Feldman, Benita Mudge, Michele Assaly, Elizabeth Finger, Stephen Pasternack, Irina Rachisky, Dick Trost, Andrew Kertesz, Charles Bernick, Donna Munic, Marek Marsel Mesulam, Kristine Lipowski, Sandra Weintraub, Borna Bonakdarpour, Diana Kerwin, Chuang-Kuo Wu, Nancy Johnson, Carl Sadowsky, Teresa Villena, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Reisa A. Sperling, Keith A. Johnson, Gad Marshall, Jerome Yesavage, Joy L. Taylor, Barton Lane, Allyson Rosen, Jared Tinklenberg, Marwan N. Sabbagh, Christine M. Belden, Sandra A. Jacobson, Sherye A. Sirrel, Neil Kowall, Ronald Killiany, Andrew E. Budson, Alexander Norbash, Patricia Lynn Johnson, Thomas O. Obisesan, Saba Wolday, Joanne Allard, Alan Lerner, Paula Ogrocki, Curtis Tatsuoka, Parianne Fatica, Evan Fletcher, Pauline Maillard, John Olichney, Charles DeCarli, Owen Carmichael, Smita Kittur, Michael Borrie, T.-Y. Lee, Rob Bartha, Sterling Johnson, Sanjay Asthana, Cynthia M. Carlsson, Steven G. Potkin, Adrian Preda, Dana Nguyen, Pierre Tariot, Anna Burke, Nadira Trncic, Stephanie Reeder, Vernice Bates, Horacio Capote, Michelle Rainka, Douglas W. Scharre, Maria Kataki, Anahita Adeli, Earl A. Zimmerman, Dzintra Celmins, Alice D. Brown, Godfrey D. Pearlson, Karen Blank, Karen Anderson, Laura A. Flashman, Marc Seltzer, Mary L. Hynes, Robert B. Santulli, Kaycee M. Sink, Leslie Gordineer, Jeff D. Williamson, Pradeep Garg, Franklin Watkins, Brian R. Ott, Henry Querfurth, Geoffrey Tremont, Stephen Salloway, Paul Malloy, Stephen Correia, Howard J. Rosen, Bruce L. Miller, David Perry, Jacobo Mintzer, Kenneth Spicer, David Bachman, Nunzio Pomara, Raymundo Hernando, Antero Sarrael, Norman Relkin, Gloria Chaing, Michael Lin, Lisa Ravdin, Amanda Smith, Balebail Ashok Raj, and Kristin Fargher
the Japanese Alzheimer’s Disease Neuroimaging Initiative:
Takashi Asada, Hiroyuki Arai, Morihiro Sugishita, Hiroshi Matsuda, Noriko Sato, Hajime Sato, Kengo Ito, Teruhiko Kachi, Kenji Toba, Michio Senda, Kenji Ishii, Shun Shimohama, Masaki Saitoh, Rika Yamauchi, Takashi Hayashi, Chiyoko Takanami, Seiju Kobayashi, Norihito Nakano, Junichiro Kanazawa, Takeshi Ando, Masato Hareyama, Masamitsu Hatakenaka, Eriko Tsukamoto, Shinji Ochi, Mikio Shoji, Etsuro Matsubara, Takeshi Kawarabayashi, Yasuhito Wakasaya, Takashi Nakata, Naoko Nakahata, Shuichi Ono, Yoshihiro Takai, Satoshi Takahashi, Hisashi Yonezawa, Junko Takahashi, Masako Kudoh, Kuniko Ueno, Hiromi Sakashita, Kuniko Watanabe, Makoto Sasaki, Yutaka Matsumura, Yohsuke Hirata, Tsuyoshi Metoki, Susumu Hayakawa, Yuichi Sato, Masayuki Takeda, Koichiro Sera, Kazunori Terasaki, Toshiaki Sasaki, Yoshihiro Saitoh, Shoko Goto, Ken Nagata, Tetsuya Maeda, Yasushi Kondoh, Takashi Yamazaki, Daiki Takano, Mio Miyata, Hiromi Komatsu, Mayumi Watanabe, Tomomi Sinoda, Rena Muraoka, Kayoko Kikuchi, Hitomi Ito, Aki Sato, Toshibumi Kinoshita, Hideyo Toyoshima, Kaoru Sato, Shigeki Sugawara, Isao Ito, Fumiko Kumagai, Katsutoshi Furukawa, Masaaki Waragai, Naoki Tomita, Mari Ootsuki, Katsumi Sugawara, Satomi Sugawara, Nobuyuki Okamura, Shunji Mugikura, Atsushi Umetsu, Takanori Murata, Tatsuo Nagasaka, Yukitsuka Kudo, Manabu Tashiro, Shoichi Watanuki, Masatoyo Nishizawa, Takayoshi Tokutake, Saeri Ishikawa, Emiko Kishida, Nozomi Sato, Mieko Hagiwara, Kumi Yamanaka, Takeyuki Watanabe, Taeko Takasugi, Shoichi Inagawa, Kenichi Naito, Masanori Awaji, Tsutomu Kanazawa, Kouiti Okamoto, Masaki Ikeda, Yuiti Tasiro, Syunn Nagamine, Sathiko Kurose, Tsuneo Yamazaki, Shiori Katsuyama, Sayuri Fukushima, Etsuko Koya, Makoto Amanuma, Kouiti Ujita, Kazuhiro Kishi, Kazuhisa Tuda, Noboru Oriuti, Katsuyoshi Mizukami, Tetsuaki Arai, Etsuko Nakajima, Katsumi Miyamoto, Tomoya Kobayashi, Saori Itoya, Jun Ookubo, Toshiya Akatsu, Yoshiko Anzai, Junya Ikegaki, Yuuichi Katou, Kaori Kimura, Hajime Saitou, Kazuya Shinoda, Satoka Someya, Hiroko Taguchi, Kazuya Tashiro, Masaya Tanaka, Tatsuya Nemoto, Ryou Wakabayashi, Daisuke Watanabe, Kousaku Saotome, Ryou Kuchii, Harumasa Takano, Tetsuya Suhara, Hitoshi Shinoto, Hitoshi Shimada, Makoto Higuchi, Takaaki Mori, Hiroshi Ito, Takayuki Obata, Yoshiko Fukushima, Kazuko Suzuki, Izumi Izumida, Katsuyuki Tanimoto, Takahiro Shiraishi, Hitoshi Shinotoh, Junko Shiba, Hiroaki Yano, Miki Satake, Aimi Nakui, Yae Ebihara, Tomomi Hasegawa, Yasumasa Yoshiyama, Mami Kato, Yuki Ogata, Hiroyuki Fujikawa, Nobuo Araki, Yoshihiko Nakazato, Takahiro Sasaki, Tomokazu Shimadu, Kimiko Yoshimaru, Etsuko Imabayashi, Asako Yasuda, Keiko Ozawa, Etuko Yamamoto, Natsumi Nakamata, Noriko Miyauchi, Rieko Hashimoto, Taishi Unezawa, Takafumi Ichikawa, Hiroki Hayashi, Masakazu Yamagishi, Tunemichi Mihara, Masaya Hirano, Shinichi Watanabe, Junichiro Fukuhara, Hajime Matsudo, Nobuyuki Saito, Atsushi Iwata, Hisatomo Kowa, Toshihiro Hayashi, Ryoko Ihara, Toji Miyagawa, Mizuho Yoshida, Yuri Koide, Eriko Samura, Kurumi Fujii, Kaori Watanabe, Nagae Orihara, Toshimitsu Momose, Miwako Takahashi, Takuya Arai, Yoshiki Kojima, Akira Kunimatsu, Harushi Mori, Masami Goto, Takeo Sarashina, Syuichi Uzuki, Seiji Katou, Yoshiharu Sekine, Yukihiro Takauchi, Chiine Kagami, Kazutomi Kanemaru, Yasushi Nishina, Maria Sakaibara, Yumiko Okazaki, Rieko Okada, Maki Obata, Masaki Takao, Yuko Iwata, Mizuho Minami, Yasuko Hanabusa, Hanae Shingyouji, Kyoko Tottori, Aya Tokumaru, Makoto Ichinose, Kazuya Kume, Syunsuke Kahashi, Kunimasa Arima, Shin Tanaka, Yuko Nagahusa, Masuhiro Sakata, Mitsutoshi Okazaki, Maki Yamada, Tadashi Tukamoto, Tiine Kodama, Tomoko Takeuchi, Keiichiro Ozawa, Yoshiko Kawaji, Kyouko Tottori, Yasuhiro Nakata, Satoshi Sawada, Makoto Mimatsu, Daisuke Nakkamura, Takeshi Tamaru, Shunichirou Horiuchi, Heii Arai, Tsuneyoshi Ota, Aiko Kodaka, Yuko Tagata, Tomoko Nakada, Eizo Iseki, Kiyoshi Sato, Hiroshige Fujishiro, Norio Murayama, Masaru Suzuki, Satoshi Kimura, Masanobu Takahashi, Haruo Hanyu, Hirofumi Sakurai, Takahiko Umahara, Hidekazu Kanetaka, Kaori Arashino, Mikako Murakami, Ai Kito, Seiko Miyagi, Kaori Doi, Kazuyoshi Sasaki, Mineo Yamazaki, Akiko Ishiwata, Yasushi Arai, Akane Nogami, Sumiko Fukuda, Koichi Kozaki, Yukiko Yamada, Sayaka Kimura, Ayako Machida, Kuninori Kobayashi, Hidehiro Mizusawa, Nobuo Sanjo, Mutsufusa Watanabe, Takuya Ohkubo, Hiromi Utashiro, Yukiko Matsumoto, Kumiko Hagiya, Yoshiko Miyama, Hitoshi Shibuya, Isamu Ohashi, Akira Toriihara, Takako Shinozaki, Haruko Hiraki, Shinichi Ohtani, Toshifumi Matsui, Tomomi Toyama, Hideki Sakurai, Kumiko Sugiura, Yu Hayasaka, Hirofumi Taguchi, Shizuo Hatashita, Akari Imuta, Akiko Matsudo, Daichi Wakebe, Hideki Hayakawa, Mitsuhiro Ono, Takayoshi Ohara, Yukihiko Washimi, Yutaka Arahata, Akinori Takeda, Akiko Yamaoka, Masashi Tsujimoto, Takiko Kawai, Ai Honda, Yoko Konagaya, Hideyuki Hattori, Kenji Yoshiyama, Rina Miura, Takashi Sakurai, Miura Hisayuki, Hidetoshi Endou, Syousuke Satake, Young Jae Hong, Katsunari Iwai, Masaki Suenaga, Sumiko Morita, Kengo Itou, Takashi Kato, Ken Fujiwara, Rikio Katou, Mariko Koyama, Naohiko Fukaya, Akira Tsuji, Hitomi Shimizu, Hiroyuki Fujisawa, Tomoko Nakazawa, Satoshi Koyama, Takanori Sakata, Masahito Yamada, Mitsuhiro Yoshita, Miharu Samuraki, Kenjiro Ono, Moeko Shinohara, Yuki Soshi, Kozue Niwa, Chiaki Doumoto, Mariko Hata, Miyuki Matsushita, Mai Tsukiyama, Nozomi Takeda, Sachiko Yonezawa, Ichiro Matsunari, Osamu Matsui, Fumiaki Ueda, Yasuji Ryu, Masanobu Sakamoto, Yasuomi Ouchi, Yumiko Fujita, Madoka Chita, Rika Majima, Hiromi Tsubota, Umeo Shirasawa, Masashi Sugimori, Wataru Ariya, Yuuzou Hagiwara, Yasuo Tanizaki, Hidenao Fukuyama, Shizuko Tanaka-Urayama, Shin-Ichi Urayama, Ryosuke Takahashi, Kengo Uemura, Hajime Takechi, Chihiro Namiki, Takeshi Kihara, Hiroshi Yamauchi, Emiko Maeda, Natsu Saito, Shiho Satomi, Konomi Kabata, Tomohisa Okada, Koichi Ishizu, Shigeto Kawase, Satoshi Fukumoto, Masanori Nakagawa, Masaki Kondo, Fumitoshi Niwa, Toshiki Mizuno, Yoko Oishi, Mariko Yamazaki, Daisuke Yamaguchi, Takahiko Tokuda, Kyoko Ito, Yoku Asano, Chizuru Hamaguchi, Kei Yamada, Chio Okuyama, Kentaro Akazawa, Shigenori Matsushima, Takamasa Matsuo, Toshiaki Nakagawa, Takeshi Nii, Takuji Nishida, Kuniaki Kiuchi, Masami Fukusumi, Hideyuki Watanabe, Toshiaki Taoka, Akihiro Nogi, Masatoshi Takeda, Toshihisa Tanaka, Hiroaki Kazui, Takashi Kudo, Masayasu Okochi, Takashi Morihara, Shinji Tagami, Masahiko Takaya, Tamiki Wada, Mikiko Yokokoji, Hiromichi Sugiyama, Daisuke Yamamoto, Keiko Nomura, Mutsumi Tomioka, Naoyuki Sato, Noriyuki Hayashi, Shuko Takeda, Eiichi Uchida, Yoshiyuki Ikeda, Mineto Murakami, Takami Miki, Hiroyuki Shimada, Suzuka Ataka, Akitoshi Takeda, Yuki Iwamoto, Motokatsu Kanemoto, Jun Takeuchi, Rie Azuma, Naomi Tagawa, Junko Masao, Yuka Matsumoto, Yuko Kikukawa, Hisako Fujii, Junko Matsumura, Susumu Shiomi, Joji Kawabe, Yoshihiro Shimonishi, Mitsuji Higashida, Tomohiro Sahara, Takashi Yamanaga, Yukio Miki, Shinichi Sakamoto, Hiroyuki Tsushima, Kiyoshi Maeda, Yasuji Yamamoto, Kazuo Sakai, Haruhiko Oda, Yoshihiko Tahara, Toshio Kawamata, Taichi Akisaki, Mizuho Adachi, Masako Kuranaga, Sachi Takegawa, Seishi Terada, Yuki Kishimoto, Naoya Takeda, Nao Imai, Mayumi Yabe, Reiko Wada, Takeshi Ishihara, Hajime Honda, Osamu Yokota, Kentaro Ida, Daigo Anami, Seiji Inoue, Toshi Matsushita, Shinsuke Hiramatsu, Hiromi Tonbara, Reiko Yamamoto, Kenji Nakashima, Kenji Wada-Isoe, Saori Yamasaki, Eijiro Yamashita, Yu Nakamura, Ichiro Ishikawa, Sonoko Danjo, Tomomi Shinohara, Yuka Kashimoto, Miyuki Ueno, Yoshihiro Nishiyama, Yuka Yamamoto, Narihide Kimura, Kazuo Ogawa, Yasuhiro Sasakawa, Takashi Ishimori, Yukito Maeda, Tatsuo Yamada, Shinji Ouma, Aika Fukuhara-Kaneumi, Nami Sakamoto, Rie Nagao, Kengo Yoshimitsu, Yasuo Kuwabara, Ryuji Nakamuta, Minoru Tanaka, Manabu Ikeda, Yuusuke Yatabe, Mamoru Hashimoto, Keiichirou Kaneda, Kazuki Honda, Naoko Ichimi, Mariko Morinaga, Miyako Noda, Fumi Akatuka, Mika Kitajima, Toshinori Hirai, Shinya Shiraishi, Naoji Amano, Shinsuke Washizuka, Tetsuya Hagiwara, Yatsuka Okada, Tomomi Ogihara, Toru Takahashi, Shin Inuzuka, Nobuhiro Sugiyama, Takehiko Yasaki, Minori Kitayama, Tomonori Owa, Akiko Ryokawa, Rie Takeuchi, Satoe Goto, Keiko Yamauchi, Mie Ito, Tomoki Kaneko, Hitoshi Ueda, Shuichi Ikeda, Ban Mihara, Hirofumi Kubo, Akiko Takano, Gou Yasui, Masami Akuzawa, Kaori Yamaguchi, Toshinari Odawara, Naomi Oota, Megumi Shimamura, Mikiko Sugiyama, Atsushi Watanabe, Shigeo Takebayashi, Yoshigazu Hayakawa, Mitsuhiro Idegawa, Noriko Toya, and Kazunari Ishii
References
- 1.Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hypponen E, Kuzma E, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437. doi: 10.1001/jama.2019.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Licher S, Ahmad S, Karamujic-Comic H, Voortman T, Leening MJG, Ikram MA, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25(9):1364–1369. doi: 10.1038/s41591-019-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 5.Karlsson IK, Escott-Price V, Gatz M, Hardy J, Pedersen NL, Shoai M, et al. Measuring heritable contributions to Alzheimer's disease: polygenic risk score analysis with twins. Brain Commun. 2022;4(1):fcab308. doi: 10.1093/braincomms/fcab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frieser MJ, Wilson S, Vrieze S. Behavioral impact of return of genetic test results for complex disease: systematic review and meta-analysis. Health Psychol. 2018;37(12):1134–1144. doi: 10.1037/hea0000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit AK, Allen M, Beswick B, Butow P, Dawkins H, Dobbinson SJ, et al. Impact of personal genomic risk information on melanoma prevention behaviors and psychological outcomes: a randomized controlled trial. Genet Med. 2021;23(12):2394–2403. doi: 10.1038/s41436-021-01292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridge PG, Hoyt KB, Boehme K, Mukherjee S, Crane PK, Haines JL, et al. Assessment of the genetic variance of late-onset Alzheimer's disease. Neurobiol Aging. 2016;41(200):e13–e20. doi: 10.1016/j.neurobiolaging.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, et al. Common polygenic variation enhances risk prediction for Alzheimer's disease. Brain. 2015;138(Pt 12):3673–3684. doi: 10.1093/brain/awv268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabuncu MR, Buckner RL, Smoller JW, Lee PH, Fischl B, Sperling RA, et al. The association between a polygenic Alzheimer score and cortical thickness in clinically normal subjects. Cereb Cortex. 2012;22(11):2653–2661. doi: 10.1093/cercor/bhr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mormino EC, Sperling RA, Holmes AJ, Buckner RL, De Jager PL, Smoller JW, et al. Polygenic risk of Alzheimer disease is associated with early- and late-life processes. Neurology. 2016;87(5):481–488. doi: 10.1212/WNL.0000000000002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge T, Sabuncu MR, Smoller JW, Sperling RA, Mormino EC, Alzheimer's Disease Neuroimaging I Dissociable influences of APOE epsilon4 and polygenic risk of AD dementia on amyloid and cognition. Neurology. 2018;90(18):e1605–e12. doi: 10.1212/WNL.0000000000005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zettergren A, Lord J, Ashton NJ, Benedet AL, Karikari TK, Lantero Rodriguez J, et al. Association between polygenic risk score of Alzheimer's disease and plasma phosphorylated tau in individuals from the Alzheimer's disease neuroimaging initiative. Alzheimers Res Ther. 2021;13(1):17. doi: 10.1186/s13195-020-00754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daunt P, Ballard CG, Creese B, Davidson G, Hardy J, Oshota O, et al. Polygenic risk scoring is an effective approach to predict those individuals most likely to decline cognitively due to Alzheimer's disease. J Prev Alzheimers Dis. 2021;8(1):78–83. doi: 10.14283/jpad.2020.64. [DOI] [PubMed] [Google Scholar]
- 15.Pyun JM, Park YH, Lee KJ, Kim S, Saykin AJ, Nho K, et al. Predictability of polygenic risk score for progression to dementia and its interaction with APOE epsilon4 in mild cognitive impairment. Transl Neurodegener. 2021;10(1):32. doi: 10.1186/s40035-021-00259-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wand H, Lambert SA, Tamburro C, Iacocca MA, O'Sullivan JW, Sillari C, et al. Improving reporting standards for polygenic scores in risk prediction studies. Nature. 2021;591(7849):211–219. doi: 10.1038/s41586-021-03243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polygenic Risk Score Task Force of the International Common Disease A Responsible use of polygenic risk scores in the clinic: potential benefits, risks and gaps. Nat Med. 2021;27(11):1876–84. doi: 10.1038/s41591-021-01549-6. [DOI] [PubMed] [Google Scholar]
- 18.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51(4):584–591. doi: 10.1038/s41588-019-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwatsubo T, Iwata A, Suzuki K, Ihara R, Arai H, Ishii K, et al. Japanese and North American Alzheimer's disease neuroimaging initiative studies: harmonization for international trials. Alzheimers Dement. 2018;14(8):1077–1087. doi: 10.1016/j.jalz.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. Recent publications from the Alzheimer's disease neuroimaging initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017;13(4):e1–e85. doi: 10.1016/j.jalz.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen Y, Miyashita A, Kitamura N, Tsukie T, Saito Y, Hatsuta H, et al. SORL1 is genetically associated with neuropathologically characterized late-onset Alzheimer's disease. J Alzheimers Dis. 2013;35(2):387–394. doi: 10.3233/JAD-122395. [DOI] [PubMed] [Google Scholar]
- 22.Genomes Project C. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh PR, Danecek P, Palamara PF, Fuchsberger C, Reshef YA, Finucane HK, et al. Reference-based phasing using the Haplotype reference consortium panel. Nat Genet. 2016;48(11):1443–8. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada Y, Momozawa Y, Sakaue S, Kanai M, Ishigaki K, Akiyama M, et al. Deep whole-genome sequencing reveals recent selection signatures linked to evolution and disease risk of Japanese. Nat Commun. 2018;9(1):1631. doi: 10.1038/s41467-018-03274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, et al. The Alzheimer's disease neuroimaging initiative. Neuroimaging Clin N Am. 2005;15(4):869–77. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, et al. Alzheimer's disease neuroimaging initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010;6(3):265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CY, Pollack S, Hunter DJ, Hirschhorn JN, Kraft P, Price AL. Improved ancestry inference using weights from external reference panels. Bioinformatics. 2013;29(11):1399–1406. doi: 10.1093/bioinformatics/btt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prive F, Vilhjalmsson BJ, Aschard H, Blum MGB. Making the most of clumping and thresholding for polygenic scores. Am J Hum Genet. 2019;105(6):1213–1221. doi: 10.1016/j.ajhg.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonenko G, Baker E, Stevenson-Hoare J, Sierksma A, Fiers M, Williams J, et al. Identifying individuals with high risk of Alzheimer's disease using polygenic risk scores. Nat Commun. 2021;12(1):4506. doi: 10.1038/s41467-021-24082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Euesden J, Lewis CM, O'Reilly PF. PRSice: Polygenic Risk Score software. Bioinformatics. 2015;31(9):1466–1468. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. 2019;51(3):404–413. doi: 10.1038/s41588-018-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander TA, Machiela MJ. LDpop: an interactive online tool to calculate and visualize geographic LD patterns. BMC Bioinform. 2020;21(1):14. doi: 10.1186/s12859-020-3340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasuga K, Kikuchi M, Tsukie T, Suzuki K, Ihara R, Iwata A, et al. Different AT(N) profiles and clinical progression classified by two different N markers using total tau and neurofilament light chain in cerebrospinal fluid. BMJ Neurol Open. 2022;4(2):e000321. doi: 10.1136/bmjno-2022-000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujishima M, Kawaguchi A, Maikusa N, Kuwano R, Iwatsubo T, Matsuda H, et al. Sample size estimation for Alzheimer's disease trials from Japanese ADNI serial magnetic resonance imaging. J Alzheimers Dis. 2017;56(1):75–88. doi: 10.3233/JAD-160621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikari Y, Nishio T, Makishi Y, Miya Y, Ito K, Koeppe RA, et al. Head motion evaluation and correction for PET scans with 18F-FDG in the Japanese Alzheimer's disease neuroimaging initiative (J-ADNI) multi-center study. Ann Nucl Med. 2012;26(7):535–544. doi: 10.1007/s12149-012-0605-4. [DOI] [PubMed] [Google Scholar]
- 37.Silverman DH, Small GW, Chang CY, Lu CS, De Kung Aburto MA, Chen W, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–7. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, et al. Genetic structure of human populations. Science. 2002;298(5602):2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 40.Najar J, van der Lee SJ, Joas E, Wetterberg H, Hardy J, Guerreiro R, et al. Polygenic risk scores for Alzheimer's disease are related to dementia risk in APOE varepsilon4 negatives. Alzheimers Dement (Amst) 2021;13(1):e12142. doi: 10.1002/dad2.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stocker H, Mollers T, Perna L, Brenner H. The genetic risk of Alzheimer's disease beyond APOE epsilon4: systematic review of Alzheimer's genetic risk scores. Transl Psychiatry. 2018;8(1):166. doi: 10.1038/s41398-018-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Sidorenko J, Couvy-Duchesne B, Marioni RE, Wright MJ, Goate AM, et al. Risk prediction of late-onset Alzheimer's disease implies an oligogenic architecture. Nat Commun. 2020;11(1):4799. doi: 10.1038/s41467-020-18534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerminen S, Martin AR, Koskela J, Ruotsalainen SE, Havulinna AS, Surakka I, et al. Geographic variation and bias in the polygenic scores of complex diseases and traits in Finland. Am J Hum Genet. 2019;104(6):1169–1181. doi: 10.1016/j.ajhg.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altmann A, Scelsi MA, Shoai M, de Silva E, Aksman LM, Cash DM, et al. A comprehensive analysis of methods for assessing polygenic burden on Alzheimer's disease pathology and risk beyond APOE. Brain Commun. 2020;2(1):047. doi: 10.1093/braincomms/fcz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellenguez C, Kucukali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, et al. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet. 2022;54(4):412–436. doi: 10.1038/s41588-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesi N, van der Lee SJ, Hulsman M, Jansen IE, Stringa N, van Schoor NM, et al. Immune response and endocytosis pathways are associated with the resilience against Alzheimer's disease. Transl Psychiatry. 2020;10(1):332. doi: 10.1038/s41398-020-01018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leonenko G, Shoai M, Bellou E, Sims R, Williams J, Hardy J, et al. Genetic risk for alzheimer disease is distinct from genetic risk for amyloid deposition. Ann Neurol. 2019;86(3):427–435. doi: 10.1002/ana.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kikuchi M, Kobayashi K, Itoh S, Kasuga K, Miyashita A, Ikeuchi T, et al. Identification of mild cognitive impairment subtypes predicting conversion to Alzheimer's disease using multimodal data. Comput Struct Biotechnol J. 2022;20:5296–5308. doi: 10.1016/j.csbj.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunomura A, Chiba S, Eto M, Saito M, Makino I, Miyagishi T. Apolipoprotein E polymorphism and susceptibility to early- and late-onset sporadic Alzheimer's disease in Hokkaido, the northern part of Japan. Neurosci Lett. 1996;206(1):17–20. doi: 10.1016/0304-3940(96)12415-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The excluded region around the APOE gene. We removed the APOE region, consisting of ±500 kb, from around the top-hit SNP rs1160985 (chr19:45403412) in our data. Each data point indicates GWAS p values from Jansen et al. [32] used as SNP weights in the PRS calculation. Figure S2. Associations between the PRS and covariates. Age at baseline examination and years of education were examined by Spearman correlation. Sex and doses of APOE ε4 and ε2 alleles were analysed by t tests or ANOVAs. CN = cognitively normal; MCI = mild cognitive impairment; ADD = Alzheimer's disease dementia. Figure S3. Associations between the PRS.adjLD and covariates. Age at examination and years of education were examined by Spearman correlations. Sex and dose of APOE ε4 and ε2 alleles were analysed by t tests or ANOVAs. Figure S4. Comparison of AD conversion between the APOE ε4 carriers and the APOE ε4 noncarriers with high PRS. Kaplan–Meier survival curves for conversion rates of MCI to AD in the APOE ε4 carriers and the APOE ε4 noncarriers with high PRS values. p-values were calculated by log-rank test. Figure S5. Age differences between the low- and high-PRS groups and between the nonconverters and converters. Baseline ages were compared between groups using the Wilcoxon rank-sum test. Each violin plot includes the kernel probability density of the data at different values and the box plots with the median value and the interquartile range.
Data Availability Statement
All the J-ADNI data except for the genome data and the reference genome data were obtained from the NBDC Human Database/the Japan Science and Technology Agency (JST) (https://humandbs.biosciencedbc.jp/en/hum0043-v1), (https://humandbs.biosciencedbc.jp/en/hum0014-latest#JGAS000114rp). GWAS statistics were obtained from the Center for Neurogenomics and Cognitive Research (https://ctg.cncr.nl/software/summary_statistics). The J-ADNI genome data are available on request.
The data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (adni.loni.usc.edu). Thus, the investigators within the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in the analysis or the writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_ apply/ADNI_Acknowledgement_List.pdf.
Data used in preparation of this article were obtained from the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) database within the National Bioscience Database Center Human Database, Japan (Research ID: hum0043.v1, 2016). Thus, the investigators within J-ADNI contributed to the design and implementation of J-ADNI and/or provided data but did not participate in the analysis or the writing of this report. A complete listing of J-ADNI investigators can be found at https://humandbs.biosciencedbc.jp/en/hum0043-j-adni-authors.