Abstract
FRS2 is a lipid-anchored docking protein that plays an important role in linking fibroblast growth factor (FGF) and nerve growth factor receptors with the Ras/mitogen-activated protein (MAP) kinase signaling pathway. In this report, we demonstrate that FRS2 forms a complex with the N-terminal SH2 domain of the protein tyrosine phosphatase Shp2 in response to FGF stimulation. FGF stimulation induces tyrosine phosphorylation of Shp2, leading to the formation of a complex containing Grb2 and Sos1 molecules. In addition, a mutant FRS2 deficient in both Grb2 and Shp2 binding induces a weak and transient MAP kinase response and fails to induce PC12 cell differentiation in response to FGF stimulation. Furthermore, FGF is unable to induce differentiation of PC12 cells expressing an FRS2 point mutant deficient in Shp2 binding. Finally, we demonstrate that the catalytic activity of Shp2 is essential for sustained activation of MAP kinase and for potentiation of FGF-induced PC12 cell differentiation. These experiments demonstrate that FRS2 recruits Grb2 molecules both directly and indirectly via complex formation with Shp2 and that Shp2 plays an important role in FGF-induced PC12 cell differentiation.
The family of fibroblast growth factors (FGFs) consists of at least 10 different growth factors that control cellular processes such as growth, differentiation, and cell migration (reviewed in reference 2). FGFs induce their biological responses by binding to and activating a family of cell surface receptors with intrinsic protein tyrosine kinase activity (reviewed in reference 12). By contrast to other growth factors such as platelet-derived growth factor (PDGF) or epidermal growth factor, acidic FGF (aFGF; also called FGF1) binds to the FGF receptor (FGFR1) monovalently, and FGFR dimerization and activation are mediated by multivalent interactions between heparin sulfate proteoglycans and FGF (reviewed in reference 26).
Upon activation, receptor tyrosine kinases undergo rapid autophosphorylation on numerous tyrosine residues. Autophosphorylation sites located within the catalytic domain are crucial for stimulation of kinase activity, while autophosphorylation sites located in other regions are usually involved in the recruitment of cellular target proteins (21). FGFR1 (encoded by flg) contains at least seven autophosphorylation sites. Two are located in the catalytic domain (Y653 and Y654) and are essential for kinase activation (17). One phosphorylation site in the C-terminal tail (Y766) functions as a high-affinity binding site for the SH2 domain of phospholipase C-γ (19). Phosphorylation of Y766 is essential for phosphatidylinositol hydrolysis but not for FGF-induced DNA synthesis in myoblasts or differentiation of PC12 cells, indicating that these biological responses are mediated by different FGF-dependent signaling pathways (18, 22). Interestingly, elimination of all known tyrosine autophosphorylation sites on FGFR1 by site-directed mutagenesis (except the two sites in the catalytic domain) does not impair FGF-induced mitogen-activated protein (MAP) kinase activation, mitogenesis, or PC12 cell differentiation (17).
The Ras/MAP kinase signaling pathway plays an important role in signaling via FGF receptors (1, 20). It is well established that the adapter protein Grb2 (6, 16) links receptor tyrosine kinases with the Ras signaling pathway by binding to the guanine nucleotide-releasing factor Sos through its SH3 domains and to tyrosine-phosphorylated receptors or docking molecules via its SH2 domain (25). We have recently identified a lipid-anchored docking protein, termed FRS2, that links FGFR molecules with the Ras/MAP kinase signaling pathway (14). We demonstrated that FRS2 is tyrosine phosphorylated and forms a complex with Grb2 and Sos in response to FGF stimulation (14). In this report, we demonstrate that in addition to the direct interactions with Grb2, tyrosine-phosphorylated FRS2 forms a complex with the SH2 domain-containing protein tyrosine phosphatase Shp2. This interaction results in tyrosine phosphorylation of Shp2 and complex formation between Shp2 and Grb2. Moreover, an FRS2 mutant impaired in Grb2 and Shp2 binding induces weak and transient MAP kinase response and fails to induce neuronal differentiation of PC12 cells. In addition, the catalytic activity of Shp2 is essential for a sustained MAP kinase response and for potentiation of FGF-induced neurite outgrowth in PC12 cells. These experiments demonstrate that FRS2 and Shp2 play an important role in FGF-induced MAP kinase activation and FGF-induced PC12 cell differentiation.
MATERIALS AND METHODS
Cell lines.
PC12 cells were grown in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS) and 10% horse serum. NIH 3T3 cells were grown in DMEM with 10% calf serum, and 293 cells were grown in DMEM containing 10% FCS as previously described (14).
Antibodies.
Antiphosphotyrosine (anti-pY), anti-FRS2, anti-Erk1 (MAP kinase), and anti-FGFR1 antibodies were previously described (14). Anti-Shp2, anti-Grb2, and anti-Sos1 antibodies were purchased from Santa Cruz Biotechnology Inc., and phosphoprotein-specific MAP kinase antibodies which recognize catalytically activated Erk1 and Erk2 were purchased from BioLabs, Inc.
Generation of GST–N-SH2 and GST–C-SH2 fusion proteins and in vitro binding assay.
Glutathione S-transferase (GST)–N-SH2 and GST–C-SH2 fusion proteins, containing the amino-terminal (amino acids 4 to 105) and carboxy-terminal (amino acids 112 to 213), respectively, SH2 domains of Shp2, were constructed. The primers used for amplification of the amino-terminal SH2 domain of Shp2 were constructed. The primers used for amplification of the amino-terminal SH2 domain of Shp2 were 5′-GGGGGATCCCGGAGATGGTTTCACCCAAAT-3′ and 5′-GGGGAATTCCTGCACAGTTCAGAGGATATTT-3′; primers used for the amplification of the carboxy-terminal SH2 domain of Shp2 were 5′-GGGGGATCCTGGTTTCATGGACATCTCTCT-3′ and 5′-GGGGAATTCCTTGAGTTGTAGTACTGTACC-3′. The amplified DNAs encoding for these SH2 domains were cloned into pGEX-2T. GST fusion proteins were purified over a glutathione-agarose column. Far-Western immunoblotting was performed as previously described (15).
Immunoprecipitation and immunoblotting analysis.
Cells were lysed in 25 mM HEPES–150 mM NaCl–1 mM EDTA–1% Triton X-100–40 mM β-glycerophosphate–10 mM pyrophosphate–1 mM VO3–5 μg of aprotinin per ml–5 μg of leupeptin per ml–1 mM phenylmethylsulfonyl fluoride, pH 7.5 (lysis buffer). Transfected 293 cells overexpressing the various expression vectors were starved overnight in DMEM containing 0.1% FCS and then solubilized with lysis buffer. PC12 or NIH 3T3 cells were starved overnight, then stimulated with aFGF (100 ng/ml) for 5 min at 37°C, and solubilized with lysis buffer. The cell lysates were subjected to immunoprecipitation with protein A-Sepharose and immunoblotting with different antibodies according to published procedures (3, 17).
Expression of FRS2 and FRS2 mutants.
Human 293 cells were used for transient expression studies according to published procedures (10). Site-directed mutagenesis was performed by using a QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. Generation of the FRS2 4F mutant, in which tyrosine residues at positions Y196, Y306, Y349, and Y392 (Grb2 binding sites) were replaced by phenylalanine residues, was previously described (14). The FRS2 1F point mutant, in which Shp2 binding site at position Y436 was replaced by a phenylalanine residue, was generated by using the overlapping primers set 5′-GAACATAGGCAACTCAATTTTATACAGGTGGATTTGG-3′ and 5′-CCAAATCCACCTGTATAAAATTGAGTTGCCTATGTT-3′. In the FRS2 5F mutant, all five tyrosine phosphorylation sites were replaced by phenylalanine residues. The mutations in FRS2 were confirmed by DNA sequencing (Sequenase version 2.0; Amersham).
Generation of Cys/Ser catalytically inactive mutant Shp2.
Site-directed mutagenesis was performed by using a QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. The Cys/Ser catalytically inactive mutant Shp2 was generated by using the overlapping primers set 5′-GTGGTGCACAGCAGTGCTGGTATTG-3′ and 5′-CAATTCCAGCACTGCTGTGCACCAC-3′. The Cys/Ser mutation in Shp2 was confirmed by DNA sequencing (Sequenase version 2.0; Amersham).
Generation of PC12 cells stably expressing FRS2 or Shp2 constructs.
The cDNAs for FRS2 and Shp2 were cloned into the pLXSN expression vector, and a high-titer stock of virus was produced (14). Parental PC12 cells were infected with a virus that combined FRS2 or Shp2 constructs and the neomycin resistance gene. Cells were selected for 2 weeks in medium supplemented with Geneticin (500 μg/ml). Pools of selected cultures were used in the studies.
MAP kinase assay.
PC12 cells overexpressing wild-type or mutant FRS2 or Shp2 were stimulated with aFGF (100 ng/ml), and cell extracts were prepared in lysis buffer. Total cell lysates (100 μg of protein) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8% gel, transferred to a nitrocellulose filter, and immunoblotted with phosphoprotein-specific anti-MAP kinase antibodies. The amount of MAP kinase was determined by stripping the blots and then reblotting with anti-Erk1 antibodies. Densitometry measurements of both phosphoprotein-specific MAP kinase antibodies and Erk1 immunoblots were performed for quantitation of MAP kinase activation.
Quantitation of neurite outgrowth.
PC12 cells overexpressing FRS2 or Shp2 mutants were grown in the presence or absence of aFGF (100 ng/ml) and heparin (5 μg/ml). Neurite outgrowth was quantitated by scoring the number of cells with neurites longer than the size of two cell bodies. The length of the neurites was measured, and the average neurite length was calculated for each cell line.
RESULTS
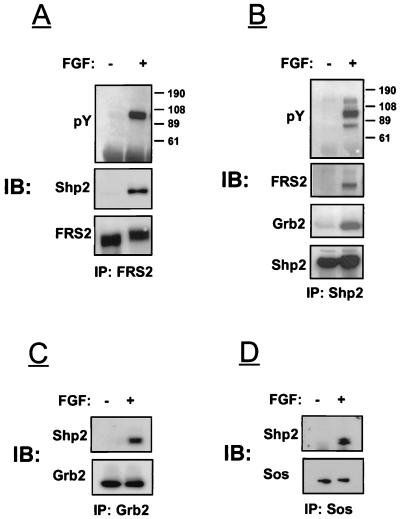
We have previously demonstrated that upon tyrosine phosphorylation, FRS2 can directly interact with Grb2 molecules (14). Inspection of the primary structure of FRS2 reveals the presence of a potential binding site at Y436 (YIXV) for the amino-terminal SH2 domain of the protein tyrosine phosphatase Shp2 (28). We next examined the possibility that Shp2 and FRS2 form a complex in response to FGF stimulation. NIH 3T3 cells were stimulated with FGF, and lysates from stimulated or unstimulated cells were subjected to immunoprecipitation with anti-FRS2 antibodies followed by immunoblotting with either anti-Shp2 or anti-pY antibodies. The experiment presented in Fig. 1A shows that Shp2 coimmunoprecipitates with FRS2 after FGF stimulation. In another experiment (Fig. 1B), cell lysates were first subjected to immunoprecipitation with anti-Shp2 antibodies followed by immunoblotting with anti-pY antibodies. In this experiment, three tyrosine-phosphorylated proteins (p72, p90, and p120) were immunoprecipitated by anti-Shp2 antibodies. The 72- and 90-kDa polypeptides represent tyrosine-phosphorylated Shp2 and FRS2, respectively, while the nature of the 120-kDa phosphoprotein is unknown. A similar experiment was performed to examine the possibility of whether the closely related SH2 domains containing protein tyrosine phosphatase Shp1 also binds to FRS2 in response to FGF stimulation. By contrast, Shp1 was not found to be bound to FRS2 in lysates from FGF-stimulated or unstimulated cells (data not shown).
FIG. 1.
FGF induces association between FRS2 and Shp2 in NIH 3T3 cells. NIH 3T3 cells were starved overnight, stimulated with aFGF, and extracted with lysis buffer. Lysates from stimulated or unstimulated cells were immunoprecipitated with anti-FRS2 (A), anti-Shp2 (B), anti-Grb2 (C), or anti-Sos1 (D) antibodies. Samples were analyzed by immunoblotting (IB) with the indicated antibodies.
Binding of Grb2 to Shp2 in response to FGF stimulation.
We have previously demonstrated that PDGF stimulation leads to tyrosine phosphorylation of Shp2 and formation of a ternary Shp2-Grb2-Sos complex bound to a tyrosine autophosphorylation site in the carboxy-terminal tail of the PDGF receptor (15). We therefore examined the possibility that Grb2 can be recruited to FRS2 by means of Shp2 following FGF stimulation. In this experiment, NIH 3T3 cells were stimulated with FGF and lysates from stimulated or unstimulated cells were subjected to immunoprecipitation with anti-Shp2 antibodies. The experiment presented in Fig. 1B reveals coimmunoprecipitation of Shp2 with Grb2 only in lysates from FGF-stimulated cells. Conversely, Shp2 was also found to be associated with Grb2 in Grb2 immunoprecipitates (Fig. 1C) and with Sos1 in Sos1 immunoprecipitates (Fig. 1D). On the basis of these experiments, we conclude that FGF stimulation leads to tyrosine phosphorylation of Shp2 and association with the Grb2-Sos complex.
Identification of Shp2 binding site on FRS2.
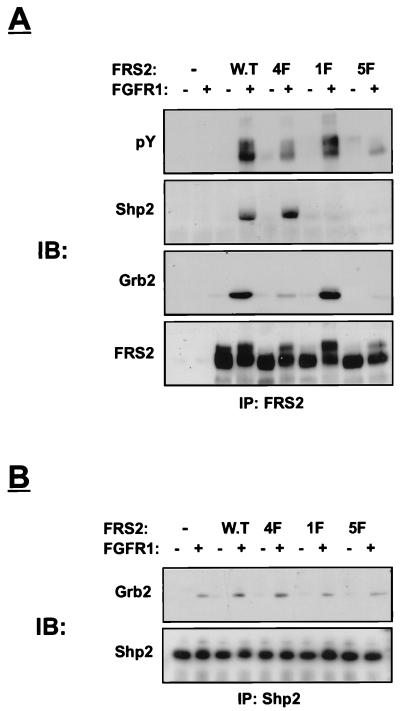
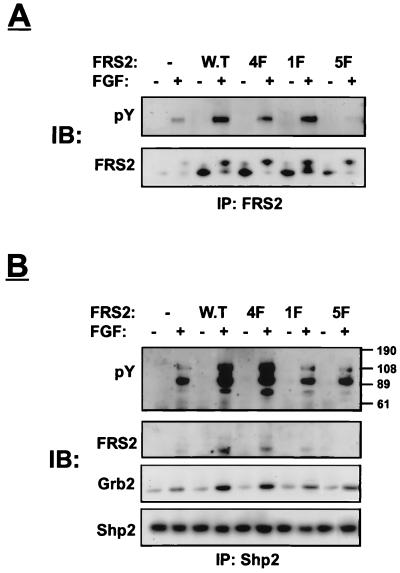
We next explored the possibility that Y436 functions as a binding site for Shp2. We previously described an FRS2 mutant in which the four Grb2 binding sites at Y196, Y306, Y349, and Y392 were replaced by phenylalanine residues (4F mutant) (14). FRS2 with mutated Y436 was prepared by site-directed mutagenesis and designated the 1F mutant, and FRS2 mutated in all five tyrosine residues is designated the 5F mutant. Human 293 cells were transfected with expression vectors that direct the synthesis of FGFR1 together with either the wild type or the 1F, 4F, or 5F mutant. Lysates from unstimulated or stimulated cells were subjected to immunoprecipitation with anti-FRS2 antibodies followed by immunoblotting with anti-pY antibodies. As expected, this experiment demonstrated strong tyrosine phosphorylation of wild-type FRS2 and somewhat reduced tyrosine phosphorylation of the 1F mutant (Fig. 2A). A weak tyrosine phosphorylation of the 4F mutant was observed, while the tyrosine phosphorylation of the 5F mutant was undetectable. This experiment demonstrates that Y196, Y306, Y349, Y392, and Y436 are the major tyrosine phosphorylation sites of FRS2. Furthermore, FRS2 is probably not phosphorylated on additional tyrosine residues, or phosphorylation on other tyrosines occurs with very low stoichiometry. Figure 2A also shows anti-Shp2 immunoblots of the same anti-FRS2 immunoprecipitates. Clearly, wild-type FRS2 and the 4F mutant form a complex with Shp2 whereas the 1F and the 5F mutants do not. Anti-Grb2 immunoblots of the same immunoprecipitates revealed the presence of Grb2 in wild-type- or 1F-derived cell lysates but not in lysates derived from 5F-transfected cells. The amount of Grb2 found in lysates from 4F-transfected cells was significantly reduced but was still detectable, probably due to the existence of a population of Grb2 molecules bound to FRS2 indirectly via Shp2. Finally, the cell lysates were subjected to immunoprecipitation with anti-Shp2 antibodies followed by immunoblotting with anti-Grb2 antibodies. Strong association between Shp2 and Grb2 was detected in lysates prepared from cells expressing either wild-type FRS2 or the 4F mutant in response to FGF stimulation. However, the association between Shp2 and Grb2 in lysates from 1F or 5F mutant-transfected cells was similar to the association detected for these two proteins in control cells transfected with vector alone. This background association is most likely due to the presence of endogenous FRS2 in 293 cells (14).
FIG. 2.
Phosphorylated tyrosine 436 on FRS2 binds to Shp2. Human 293 cells were transiently transfected with expression vectors for wild-type (W.T) FRS2 or FRS2 mutants (1 μg) together with FGFR1 expression vector (0.1 μg). After 36 h, the cells were lysed and subjected to immunoprecipitation with anti-FRS2 (A) or anti-Shp2 (B) antibodies. Samples were resolved by SDS-PAGE (10% gel), transferred to a nitrocellulose filter, and immunoblotted (IB) with the indicated antibodies.
These experiments show that various binary complexes composed of FRS2-Grb2, FRS2-Shp2, and Shp2-Grb2 are formed in response to FGF stimulation. We would like to propose that a subpopulation of the Shp2-Grb2 complex is bound to tyrosine 436 on FRS2, leading to the formation of a ternary FRS2-Shp2-Grb2 complex. The exact stoichiometries of the various binary and ternary complexes cannot be determined with the methodology applied in this study.
Shp2 binds to tyrosine-phosphorylated FRS2 via its amino-terminal SH2 domain.
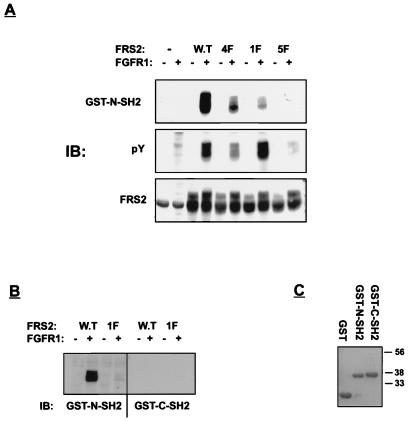
The abilities of the N- and C-terminal SH2 domains of Shp2 to bind to FRS2 were compared. In this experiment, human 293 cells were transfected with expression vectors for FGFR1 together with expression vectors that direct the synthesis of either wild-type or mutant FRS2. GST fusion proteins containing the N- or C-terminal SH2 domain of Shp2 were tested for the ability to bind to wild-type or mutant FRS2, using a far-Western immunoblotting procedure. The experiment presented in Fig. 3 shows that the N-terminal SH2 domain of Shp2 binds to tyrosine-phosphorylated FRS2. However, the association between the N-terminal SH2 domain and the 1F mutant was strongly reduced, and the association between the N-terminal SH2 domain and the 5F mutant was essentially eliminated (Fig. 3A). The association between the N-terminal SH2 domain and the 4F mutant was also reduced in comparison to the association detected for wild-type FRS2 but was significantly stronger than the association detected for the 1F mutant. It is possible that under conditions of forced overexpression, the N-terminal SH2 domain of Shp2 can also bind to the noncognate Grb2 binding sites. Finally, the GST fusion protein containing the C-terminal SH2 domain of Shp2 did not bind to either wild-type or 1F mutant FRS2 (Fig. 3B) or to GST alone (data not shown). These experiments demonstrate that Shp2 binds to tyrosine-phosphorylated FRS2 by means of its N-terminal SH2 domain.
FIG. 3.
Shp2 binds to tyrosine-phosphorylated FRS2 via its amino-terminal SH2 domain. Human 293 cells were transiently transfected with expression vectors for wild-type (W.T) FRS2 and FRS2 mutants as described in legend to Fig. 2. (A) Total cell lysates were resolved by SDS-PAGE (10% gel), transferred to a nitrocellulose filter, and immunoblotted (IB) with the indicated antibodies. The nitrocellulose filter was incubated with GST–N-SH2 (3 μg/ml) together with monoclonal anti-GST antibodies as described in Materials and Methods. After washing, the nitrocellulose filter was blotted with anti-mouse horseradish peroxidase-conjugated antibodies. (B) Total cell lysates of cells transfected with wild-type or 1F FRS2 (same samples as in panel A) were immunoblotted with either GST–N-SH2 or GST–C-SH2 fusion protein. (C) GST alone (3 μg/ml) or GST–N-SH2 and GST–C-SH2 fusion proteins were purified over a glutathione agarose column, resolved by SDS-PAGE (10% gel), and stained with Coomassie blue.
Activation of MAP kinase by FRS2 mutants.
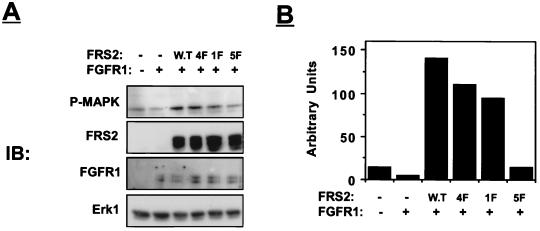
We next examined the effects of wild-type FRS2 and FRS2 mutants on FGF-induced MAP kinase activation in 293 cells. Human 293 cells were transfected with expression vectors that direct the synthesis of FGFR1 and MAP kinase (Erk1) together with either wild-type FRS2 or the 1F, 4F, or 5F mutant. The activity of MAP kinase was determined by immunoblotting cell lysates with antibodies which specifically recognize the activated form of MAP kinase (13). We have previously demonstrated that overexpression of wild-type FRS2 leads to strong potentiation of FGF-induced MAP kinase activation whereas overexpression of 4F mutant induces a partial MAP kinase response (Fig. 4 and reference 14). The experiment presented in Fig. 4 shows that the 1F mutant induces a compromised MAP kinase response compared to MAP kinase activation induced by wild-type FRS2 in these cells. After subtraction of background activation of MAP kinase (probably due to tyrosine phosphorylation of endogenous FRS2 and Shc) (14), the 4F and the 1F mutants induced approximately 70 and 60%, respectively, of the MAP kinase response that was detected in cells overexpressing wild-type FRS2. By contrast, overexpression of the 5F mutant induced only a background MAP kinase response similar to the stimulation detected in control cells. This experiment demonstrates that MAP kinase activation mediated by FRS2 is totally dependent on the phosphorylation of Y196, Y306, Y349, Y392, and Y436. The partial activation of MAP kinase observed in cells transfected with the 4F or 1F mutant demonstrates that both direct recruitment of Grb2 and indirect recruitment via Shp2 are necessary for a complete MAP kinase response. An alternative avenue for activation of the Ras/MAP kinase pathway in response to FGF stimulation may be via tyrosine phosphorylation of Shc (29). However, FRS2 probably plays a prominent role in linking activated FGFR with the Ras/MAP kinase signaling pathway since FGF is unable to activate MAP kinase in cells expressing the 5F mutant of FRS2 under conditions that cause tyrosine phosphorylation of Shc.
FIG. 4.
Activation of MAP kinase in cells overexpressing FRS2 mutants. Human 293 cells were transiently transfected with expression vectors for wild-type (W.T) FRS2 and FRS2 mutants (0.2 μg) together with FGFR1 (0.01 μg) and Erk1 (2 μg) expression vectors. After 36 h, the cells were lysed, and cellular proteins were resolved by SDS-PAGE (10% gel), transferred to a nitrocellulose filter, and immunoblotted (IB) with the indicated antibodies (A). Quantitation of MAP kinase (MAPK) activity was carried out with a phosphorimager (B). Similar results were obtained in three different experiments.
Neurite outgrowth in PC12 cells overexpressing FRS2 mutants.
PC12 cells were transfected with expression vectors that direct the synthesis of wild-type and mutant FRS2. Stably expressing cell lines matched for similar level of expression of FRS2 mutants were further characterized. These cells were treated with aFGF, lysed, subjected to immunoprecipitation with anti-FRS2 antibodies, and immunoblotted with anti-pY antibodies. As expected, wild-type FRS2 was strongly phosphorylated on tyrosine residues in response to FGF stimulation (Fig. 5A), while tyrosine phosphorylation of the 5F mutant was barely detectable. Interestingly, although the 5F mutant was not tyrosine phosphorylated, it still exhibited slower mobility in SDS-gels in response to FGF stimulation (Fig. 5A). This retarded mobility may be due to Ser/Thr phosphorylation of FRS2 or other posttranslational modifications induced by FGF stimulation.
FIG. 5.
Analysis of PC12 cells overexpressing FRS2 or FRS2 mutants. Constructs encoding for wild-type (W.T) FRS2 or FRS2 mutants were cloned into the pLXSN expression vector, and a high-titer stock of viruses was produced. Parental PC12 cells were infected with the different viruses. Cells were selected for 2 weeks in medium supplemented with Geneticin (0.5 mg/ml). Pools of selected cultures were used in this experiment. The cells were starved overnight and treated with aFGF (100 ng/ml for 5 min). Cell lysates derived from stimulated or unstimulated cells were subjected to immunoprecipitation with anti-FRS2 antibodies (A) or anti-Shp2 antibodies (B). The samples were resolved by SDS-PAGE (10%), transferred to a nitrocellulose filter, and immunoblotted (IB) with the indicated antibodies.
Figure 5 depicts a detailed immunoprecipitation/immunoblotting analysis of FRS2 with lysates from PC12 cells similar to the experiments performed with NIH 3T3 or 293 cells (Fig. 2 and 3). Anti-Shp2 immunoprecipitates from FGF-stimulated PC12 cells also contained three major tyrosine-phosphorylated proteins: p72 (Shp2), p90 (FRS2), and p120 (unknown). Overall, similar results were obtained irrespective whether the experiments were performed with NIH 3T3 cells expressing endogenous proteins, stably transfected PC12 cells, or transiently transfected 293 cells; FGF stimulation led to the formation of various binary complexes, and probably a subpopulation of Shp2-Grb2 was bound to FRS2 in a ternary complex.
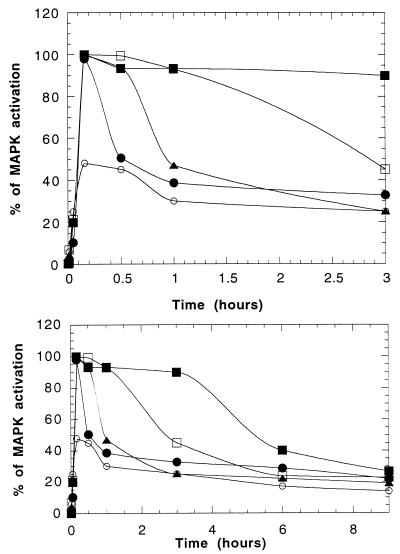
Next we compared the capacity of wild-type and mutant FRS2 to induce MAP kinase response in transfected PC12 cells. In control PC12 cells, transfected with the vector alone, maximal activation of MAP kinase was observed after 10 min of FGF stimulation, followed by rapid decline (Fig. 6). By contrast, MAP kinase response in PC12 cells overexpressing wild-type FRS2 was sustained for 3 h. In cells overexpressing the 4F mutant, MAP kinase activation lasted for approximately 1 h, and in cells overexpressing the 1F mutant, activation of MAP kinase was transient (30 min). A very weak and transient activation of MAP kinase was detected in PC12 cells overexpressing the 5F mutant (Fig. 6). These results demonstrate that overexpression of wild-type FRS2 leads to sustained activation of MAP kinase, while elimination of binding sites for Grb2 and Shp2 leads to transient and weak MAP kinase response.
FIG. 6.
Kinetics of MAP kinase activation in PC12 cells transfected with vector alone (•), wild-type FRS2 (▪), 4F mutant (□), 1F mutant (▴), and 5F mutant (○). The upper and lower panels show the early and late time points of MAP kinase (MAPK) activation, respectively.
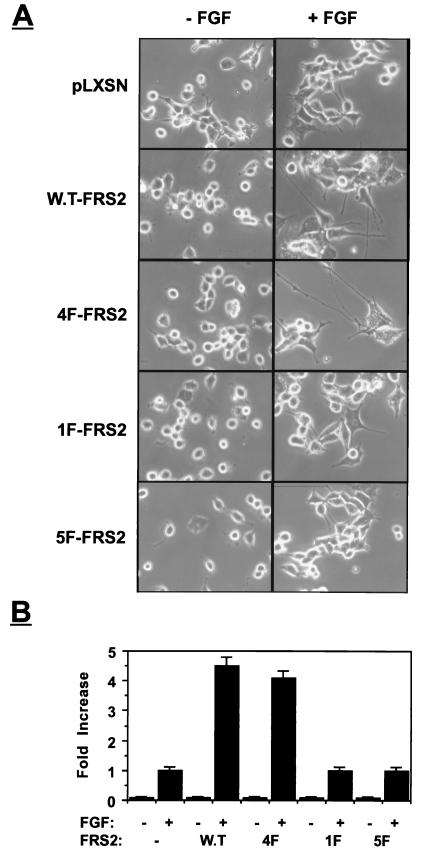
We next compared the PC12-derived cell lines for the ability to undergo neuronal differentiation in response to FGF stimulation. These cells were incubated with low concentrations of aFGF and heparin that induce weak, barely detectable neurite outgrowth. The experiment presented in Fig. 7 demonstrates that overexpression of the wild type or the 4F mutant leads to strong potentiation of FGF-induced neurite outgrowth. By contrast, overexpression of 1F or 5F mutants did not potentiate FGF-induced neurite outgrowth in these cells (Fig. 7). These experiments reveal a good correlation between the duration of MAP kinase activation and potentiation of neurite outgrowth.
FIG. 7.
Neurite outgrowth in PC12 cells induced by overexpression of FRS2 and FRS2 mutants. PC12 cells expressing pLXSN vector alone or cells overexpressing wild-type FRS2 (W.T-FRS2) or FRS2 mutants were grown in the presence or absence of aFGF (100 ng/ml) and heparin (5 μg/ml). Neurite outgrowth was detected (A) and quantitated (B) following induction for 72 h. Neurite outgrowth was quantitated by scoring the number of cells with neurites longer then the size of two cell bodies. The length of the neurites was measured, and the average neurite length was calculated for each cell line. Similar results were obtained in four different experiments.
Overexpression of wild-type and catalytically inactive mutant Shp2 in PC12 cells.
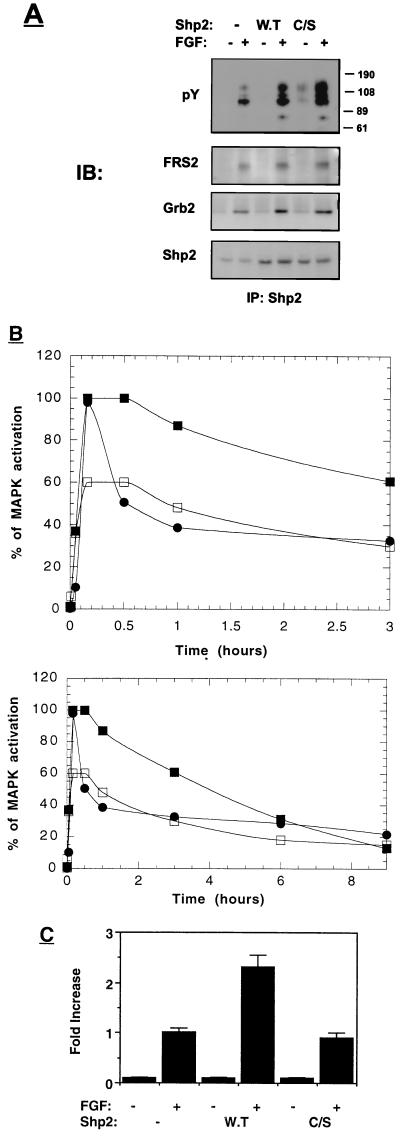
To further investigate the role of Shp2 in FGF signal transduction pathway, PC12 cells were transfected with wild-type or catalytically inactive Cys/Ser mutant Shp2. Stably transfected cell lines overexpressing either wild-type or mutant Shp2 were treated with aFGF, lysed, and subjected to immunoprecipitation with anti-Shp2 antibodies followed by immunoblotting with anti-pY antibodies (Fig. 8A). In immunoprecipitates derived from transfected cells, both the wild type and the Shp2 point mutant were strongly phosphorylated on tyrosine residues in response to FGF stimulation compared to mock-transfected cells with vector alone (Fig. 8A). Furthermore, a strong association between Shp2 and FRS2 as well as between Shp2 and Grb2 was detected in lysates prepared from these cells (Fig. 8A). This experiment clearly demonstrates that expression of the Cys/Ser inactive mutant Shp2 does not influence the recruitment of Shp2 by FRS2, the tyrosine phosphorylation of Shp2, and the association between Shp2 and Grb2.
FIG. 8.
MAP kinase activation and neurite outgrowth in cells overexpressing wild-type or catalytically inactive Shp2. (A) PC12 cells were stably transfected with an expression vector that directs the synthesis of wild-type (W.T) or Cys/Ser inactive mutant (C/S) Shp2. The cells were starved overnight, stimulated with aFGF (100 ng/ml for 5 min), and extracted with lysis buffer. Lysates from stimulated or unstimulated cells were immunoprecipitated with anti-Shp2 antibodies and immunoblotted (IB) with the indicated antibodies. (B) PC12 cells expressing vector alone (•) or wild-type (▪) or Cys/Ser inactive mutant (□) Shp2 were stimulated with aFGF (100 ng/ml) for different time periods. The upper and lower panels show the early and late time points of MAP kinase (MAPK) activation, respectively. Quantitation of MAP kinase activation was determined as described in Materials and Methods. (C) PC12 cells expressing the pLXSN vector alone (control) and cells overexpressing wild-type or Cys/Ser inactive mutant Shp2 were grown in the absence or presence of aFGF (100 ng/ml) and heparin (5 μg/ml). Neurite outgrowth was quantitated following induction for 72 h. Quantification of neurite outgrowth was calculated as described in Materials and Methods.
The kinetics of MAP kinase activation in response to aFGF stimulation was next determined in the transfected cell lines. In PC12 cells overexpressing wild-type Shp2, the activation of MAP kinase was sustained for more than 1 h (Fig. 8B). However, MAP kinase response in cells overexpressing the catalytically inactive Shp2 phosphatase was both transient and weaker in comparison to the MAP kinase response induced by overexpression of wild-type Shp2. This experiment demonstrates that the catalytic activity of Shp2 is essential for sustained MAP kinase activation in response to FGF stimulation.
We also analyzed the neuronal differentiation of these PC12 cells in response to aFGF stimulation. The experiment presented in Fig. 8C demonstrates that overexpression of wild-type Shp2 leads to potentiation of FGF-induced neurite outgrowth. By contrast, overexpression of the Cys/Ser inactive mutant Shp2 did not potentiate FGF-induced neurite outgrowth in these cells (Fig. 8C). As previously shown, a good correlation exists between the duration of MAP kinase response and PC12 cell differentiation. Moreover, the catalytic activity of Shp2 is essential for sustained MAP kinase response and for neurite outgrowth in response to FGF stimulation.
DISCUSSION
Following FGF or nerve growth factor stimulation, the lipid-anchored docking protein FRS2 is phosphorylated on multiple tyrosine residues (Y196, Y306, Y349, and Y392) that function as docking sites for Grb2-Sos complexes (14). In this report, we demonstrate that FRS2 contains an additional phosphorylation site at Y436 that functions as a docking site for the N-terminal SH2 domain of the protein tyrosine phosphatase Shp2. FGF-induced tyrosine phosphorylation of Shp2 leads to recruitment of an additional Grb2-Sos1 complex by FRS2. The experiments presented in this report demonstrate that FRS2 has five binding sites for Grb2; four molecules bind directly, and at least one Grb2 molecule is recruited by an indirect mechanism mediated by the protein tyrosine phosphatase Shp2.
We have explored the biological outcome of Grb2-Sos recruitment by FRS2 by investigating the properties of FRS2 proteins with mutations in tyrosine phosphorylation sites responsible for Grb2 binding (4F mutant), Shp2 binding (1F mutant), and both Grb2 and Shp2 binding (5F mutant). We surmise that FRS2 does not have additional major tyrosine phosphorylation sites since the 5F mutant is not tyrosine phosphorylated in response to FGF stimulation. We have previously demonstrated that overexpression of wild-type FRS2 leads to strong potentiation of FGF-induced MAP kinase activation and neuronal differentiation of PC12 cells (14). Both processes were blocked by a dominant interfering mutant of Ras (RasN17), indicating that FRS2 acts upstream of Ras in mediating these cellular responses (14). In this report, we demonstrate that FGF-induced MAP kinase activation is compromised but not prevented in cells expressing either the 4F or the 1F mutant. By contrast, the 5F mutant failed to potentiate FGF-induced MAP kinase activation, indicating that this response is mediated by both direct and indirect recruitment of Grb2-Sos complexes.
Most growth factor receptors are able to recruit Grb2 by more than one mechanism. The reason for the existence of multiple mechanisms for the recruitment of Grb2-Sos by receptor tyrosine kinases and by tyrosine-phosphorylated docking proteins is unknown. Shc appears to be a promiscuous target for tyrosine phosphorylation in response to activation of a broad range of cell surface receptors, including receptor tyrosine kinases, lymphokine receptors, and G-protein-coupled receptors, as well as T-cell and B-cell antigen receptors (3, 5, 27, 33). Since FRS2 is tyrosine phosphorylated in response to a more restricted set of cell surface stimuli, we have proposed that this docking protein may participate in the control of specific responses of FGF or nerve growth factor (14).
We have previously demonstrated that overexpression of wild-type FRS2 potentiates FGF-induced neuronal differentiation of PC12 cells (14). In this report, we demonstrate that the 4F mutant is capable of inducing both MAP kinase activation and PC12 cell differentiation. However, a sustained MAP kinase response was detected in cells overexpressing wild-type FRS2, while the duration of the MAP kinase response was shorter in cells overexpressing the 4F mutant. Moreover, MAP kinase response in cells overexpressing the 1F mutant was transient, and this mutant was unable to potentiate neurite outgrowth in response to FGF stimulation. A transient and weak MAP kinase response was observed in cells overexpressing the 5F mutant; this mutant was unable to potentiate FGF-induced neurite outgrowth. These results indicate that recruitment of both Grb2 and Shp2 by FRS2 is essential for sustained activation of the Ras/MAP kinase signaling pathway. We also present evidence that the catalytic activity of Shp2 is essential for sustained MAP kinase response and for potentiation of FGF-induced neuronal differentiation of PC12 cells. Taken together, these experiments demonstrate that recruitment of Shp2 by FRS2 and the catalytic activity of Shp2 are crucial for sustained MAP kinase response and for neuronal differentiation of PC12 cells. Experiments presented in this report reveal a good correlation between the duration of MAP kinase response induced by an extracellular stimulus and PC12 cell differentiation. These results are consistent with earlier studies demonstrating that sustained activation and nuclear translocation of MAP kinase are essential for PC12 cell differentiation (7, 9, 32).
Protein tyrosine phosphatase can play both a positive and a negative role in the control of cell growth and differentiation (31). Genetic studies in Drosophila and biochemical studies with mammalian cells suggest that Shp2 and its Drosophila homolog Corkscrew play a positive role in the control of MAP kinase activation, cell growth, and differentiation (4, 11, 23, 24, 30, 34). These studies do not provide conclusive answer to the question whether Corkscrew and Shp2 function as components of the Ras/MAP kinase signaling pathway. However, all of these studies, including the present report, indicate that the protein tyrosine phosphatase Shp2 has a positive role in the Ras/MAP kinase signaling pathway. It was demonstrated that targeted disruption of the FGFR1 gene in mice leads to embryonal lethality since FGFR1 is essential for normal gastrulation (8). A similar defect in gastrulation was observed in mice homozygous for a mutant Shp2 that lacks a functional N-terminal SH2 domain (24). The close similarity in the FGFR1 and Shp2 mutant mice suggests that the phenotype of Shp2 mutant embryos results from a defect in FGFR signaling. Moreover, it was demonstrated that Shp2 is required for full and sustained activation of MAP kinase following FGF stimulation of fibroblasts derived from Shp2-mutated mice (24).
The experiments presented in this report clearly demonstrate that Shp2 plays an important positive role in the control of the Ras/MAP kinase signaling pathway activated by FGF stimulation. At least two mechanisms can be envisioned for how Shp2 can positively regulate signaling via FGFR. It is clear that Shp2 can function as a docking protein that recruits Grb2-Sos complex leading to activation of the MAP kinase signaling pathway. In this report, we show that the catalytic activity of Shp2 plays a role in activation of the MAP kinase signaling pathway. It is noteworthy that the N-terminal SH2 domain of Shp2 is responsible for binding to FRS2. The C-terminal SH2 domain of Shp2, on the other hand, does not interact with FRS2 and therefore could be involved in the recruitment of a tyrosine-phosphorylated protein that may play a role in control of neurite outgrowth. The molecular identification of this putative phosphoprotein will provide additional clues concerning the cellular circuitry that relays information from the cell surface to the nucleus to control cell growth and differentiation.
ACKNOWLEDGMENT
Y. R. Hadari was supported by a long-term fellowship from the International Human Frontier Science Program Organization.
ADDENDUM IN PROOF
An additional binding site at pTyr471 on FRS2 was identified for Shp2.
REFERENCES
- 1.Bar-Sagi D, Feramisco J R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 2.Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 3.Batzer A G, Rotin D, Urena J M, Skolnik E Y, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett A M, Hausdorff S F, O’Reilly A M, Freeman R M, Neel B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Grall D, Salcini A E, Pelicci P G, Pouyssegur J, Van-Obberghen-Schilling E. Shc adaptor proteins are key transducers of mitogenic signaling mediated by the G protein-coupled thrombin receptor. EMBO J. 1996;15:1037–1044. [PMC free article] [PubMed] [Google Scholar]
- 6.Clark S G, Stern M J, Horvitz H R. C. elegans cell-signaling gene Sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- 7.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 8.Deng C X, Wynshaw-Boris A, Shen M M, Daugherty C, Ornitz D M, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 9.Dikic I, Schlessinger J, Lax I. PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr Biol. 1994;4:702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 10.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 11.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 12.Jaye M, Schlessinger J, Dionne C A. Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochim Biophys Acta. 1992;1135:185–199. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signaling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 14.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Lax I, Schlessinger J. A lipid-anchored Grb2 binding protein that links FGF receptor activation to the Ras/MAP kinase signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi M, Dikic I, Sorokin A, Burgess W H, Jaye M, Schlessinger J. Identification of six novel autophosphorylation sites on fibroblast growth factor receptor 1 and elucidation of their importance in receptor activation and signal transduction. Mol Cell Biol. 1996;16:977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadi M, Dionne C A, Li W, Li N, Spivak T, Honegger A M, Jaye M, Schlessinger J. Point mutation in FGF receptor eliminated phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992;358:681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi M, Honegger A M, Rotin D, Fischer R, Bellot F, Li W, Dionne C A, Jaye M, Rubinstein M, Schlessinger J. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol Cell Biol. 1991;11:5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda M, Ko M, Ogura A, Liu D G, Amano T, Takano T, Ikawa Y. Sarcoma viruses carrying ras oncogene induce differentiation-associated properties in a neuronal cell line. Nature. 1985;318:73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- 21.Pawson T, Schlessinger J. SH2 and SH3 domains. Curr Biol. 1993;3:434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- 22.Peters K G, Marie J, Wilson E, Ives H E, Escobedo J, Del Rosario M, Mirda D, Williams L T. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992;358:678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- 23.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maroy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 24.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev Biol. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 26.Schlessinger J, Lax I, Lemmon M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 1995;83:357–360. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 27.Skolnik E Y, Batzer A, Li N, Lee C H, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 28.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantly L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 29.Spivak-Kroizman T, Mohammadi M, Hu P, Jaye M, Schlessinger J, Lax I. Point mutation in the fibroblast growth factor receptor eliminates phosphatidylinositol hydrolysis without affecting neuronal differentiation of PC12 cells. J Biol Chem. 1994;269:14419–14423. [PubMed] [Google Scholar]
- 30.Tang T L, Freeman R M, Jr, O’Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 31.Tonks N K, Neel B G. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 32.Traverse S, Seedorf K, Paterson H, Marshall C J, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 33.van Biesen T, Luttrell L M, Hawes B E, Lefkowitz R J. Mitogenic signaling via G protein-coupled receptors. Endocrine Rev. 1996;17:698–713. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 34.Wright J H, Drueckes P, Bartoe J, Zhao Z, Shen S, Krebs E G. A role for SHP-2 tyrosine phosphatase in nerve growth factor-induced PC12 cell differentiation. Mol Biol Cell. 1997;8:1575–1585. doi: 10.1091/mbc.8.8.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]