Abstract
Background
Adhesive capsulitis (also termed frozen shoulder) is a common condition characterised by spontaneous onset of pain, progressive restriction of movement of the shoulder and disability that restricts activities of daily living, work and leisure. Electrotherapy modalities, which aim to reduce pain and improve function via an increase in energy (electrical, sound, light, thermal) into the body, are often delivered as components of a physical therapy intervention. This review is one in a series of reviews which form an update of the Cochrane review 'Physiotherapy interventions for shoulder pain'.
Objectives
To synthesise the available evidence regarding the benefits and harms of electrotherapy modalities, delivered alone or in combination with other interventions, for the treatment of adhesive capsulitis.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, CINAHL Plus and the ClinicalTrials.gov and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) clinical trials registries up to May 2014, unrestricted by language, and reviewed the reference lists of review articles and retrieved trials to identify any other potentially relevant trials.
Selection criteria
We included randomised controlled trials (RCTs) and controlled clinical trials using a quasi‐randomised method of allocation that included adults with adhesive capsulitis and compared any electrotherapy modality to placebo, no treatment, a different electrotherapy modality, or any other intervention. The two main questions of the review focused on whether electrotherapy modalities are effective compared to placebo or no treatment, or if they are an effective adjunct to manual therapy or exercise (or both). The main outcomes of interest were participant‐reported pain relief of 30% or greater, overall pain, function, global assessment of treatment success, active shoulder abduction, quality of life, and the number of participants experiencing any adverse event.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted the data, performed a risk of bias assessment, and assessed the quality of the body of evidence for the main outcomes using the GRADE approach.
Main results
Nineteen trials (1249 participants) were included in the review. Four trials reported using an adequate method of allocation concealment and six trials blinded participants and personnel. Only two electrotherapy modalities (low‐level laser therapy (LLLT) and pulsed electromagnetic field therapy (PEMF)) have been compared to placebo. No trial has compared an electrotherapy modality plus manual therapy and exercise to manual therapy and exercise alone. The two main questions of the review were investigated in nine trials.
Low quality evidence from one trial (40 participants) indicated that LLLT for six days may result in improvement at six days. Eighty per cent (16/20) of participants reported treatment success with LLLT compared with 10% (2/20) of participants receiving placebo (risk ratio (RR) 8.00, 95% confidence interval (CI) 2.11 to 30.34; absolute risk difference 70%, 95% CI 48% to 92%). No participants in either group reported adverse events.
We were uncertain whether PEMF for two weeks improved pain or function more than placebo at two weeks because of the very low quality evidence from one trial (32 participants). Seventy‐five per cent (15/20) of participants reported pain relief of 30% or more with PEMF compared with 0% (0/12) of participants receiving placebo (RR 19.19, 95% CI 1.25 to 294.21; absolute risk difference 75%, 95% CI 53% to 97%). Fifty‐five per cent (11/20) of participants reported total recovery of joint function with PEMF compared with 0% (0/12) of participants receiving placebo (RR 14.24, 95% CI 0.91 to 221.75; absolute risk difference 55%, 95% CI 31 to 79).
Moderate quality evidence from one trial (63 participants) indicated that LLLT plus exercise for eight weeks probably results in greater improvement when measured at the fourth week of treatment, but a similar number of adverse events, compared with placebo plus exercise. The mean pain score at four weeks was 51 points with placebo plus exercise, while with LLLT plus exercise the mean pain score was 32 points on a 100 point scale (mean difference (MD) 19 points, 95% CI 15 to 23; absolute risk difference 19%, 95% CI 15% to 23%). The mean function impairment score was 48 points with placebo plus exercise, while with LLLT plus exercise the mean function impairment score was 36 points on a 100 point scale (MD 12 points, 95% CI 6 to 18; absolute risk difference 12%, 95% CI 6 to 18). Mean active abduction was 70 degrees with placebo plus exercise, while with LLLT plus exercise mean active abduction was 79 degrees (MD 9 degrees, 95% CI 2 to 16; absolute risk difference 5%, 95% CI 1% to 9%). No participants in either group reported adverse events. LLLT's benefits on function were maintained at four months.
Based on very low quality evidence from six trials, we were uncertain whether therapeutic ultrasound, PEMF, continuous short wave diathermy, Iodex phonophoresis, a combination of Iodex iontophoresis with continuous short wave diathermy, or a combination of therapeutic ultrasound with transcutaneous electrical nerve stimulation (TENS) were effective adjuncts to exercise. Based on low or very low quality evidence from 12 trials, we were uncertain whether a diverse range of electrotherapy modalities (delivered alone or in combination with manual therapy, exercise, or other active interventions) were more or less effective than other active interventions (for example glucocorticoid injection).
Authors' conclusions
Based upon low quality evidence from one trial, LLLT for six days may be more effective than placebo in terms of global treatment success at six days. Based upon moderate quality evidence from one trial, LLLT plus exercise for eight weeks may be more effective than exercise alone in terms of pain up to four weeks, and function up to four months. It is unclear whether PEMF is more or less effective than placebo, or whether other electrotherapy modalities are an effective adjunct to exercise. Further high quality randomised controlled trials are needed to establish the benefits and harms of physical therapy interventions (that comprise electrotherapy modalities, manual therapy and exercise, and are reflective of clinical practice) compared to interventions with evidence of benefit (for example glucocorticoid injection or arthrographic joint distension).
Keywords: Adult, Humans, Bursitis, Bursitis/therapy, Electric Stimulation Therapy, Electric Stimulation Therapy/methods, Randomized Controlled Trials as Topic, Shoulder Pain, Shoulder Pain/therapy
Plain language summary
Electrotherapy modalities for adhesive capsulitis (frozen shoulder)
Background
Frozen shoulder is a common cause of shoulder pain and stiffness. The pain and stiffness can last up to two to three years before going away, and in the early stages it can be very painful.
Electrotherapy modalities (also known as electrophysical agents) are types of physical therapy that aim to reduce pain and improve function via an increase in energy (electrical, sound, light, thermal) into the body. Examples include therapeutic ultrasound, low‐level laser therapy (LLLT), interferential current, transcutaneous electrical nerve stimulation (TENS), and pulsed electromagnetic field therapy (PEMF). Electrotherapy modalities are delivered by various clinicians, including physiotherapists, chiropractors and osteopaths. In practice, patients with frozen shoulder seldom receive a single electrotherapy modality in isolation from other components of physical therapy treatment (for example manual therapy, exercise).
Study characteristics
This summary of an updated Cochrane review presents what we know from research about the benefits and harms of electrotherapy modalities in people with frozen shoulder. After searching for all relevant studies published up to May 2014, we included 19 trials (1249 participants). Of the included participants, 61% were women, the average age was 55 years, and the average duration of the condition was 5.5 months. The average duration of delivery of electrotherapy interventions was four weeks.
Key results ‐ LLLT and exercise compared to placebo and exercise
Pain (higher scores mean worse pain)
People who received LLLT and exercise had less pain than people who had placebo plus exercise ‐ pain was 19 points less (ranging from 15 to 23 points less) at the fourth week of treatment (19% absolute improvement, ranging from 15% to 23% improvement).
‐ People who had LLLT and exercise rated their pain score as 32 points on a scale of 0 to 100 points.
‐ People who had placebo and exercise rated their pain score as 51 points on a scale of 0 to 100 points.
Function impairment (higher scores mean worse function impairment)
People who received LLLT and exercise had less function impairment than people who had placebo and exercise ‐ function impairment was 12 points less (ranging from 6 to 18 points less) at the fourth week of treatment (12% absolute improvement, ranging from 6% to 18% improvement).
‐ People who had LLLT and exercise rated their function impairment as 36 points on a scale of 0 to 100 points.
‐ People who had placebo and exercise rated their function impairment as 48 points on a scale of 0 to 100 points.
Active shoulder abduction (higher degrees of movement mean greater shoulder abduction)
People who received LLLT and exercise had greater active shoulder abduction than people who had placebo and exercise ‐ active shoulder abduction was 9 degrees more (ranging from 2 to 16 degrees more) at the fourth week of treatment (5% absolute improvement, ranging from 1% to 9% improvement).
‐ People who had LLLT and exercise had active shoulder abduction of 79 degrees.
‐ People who had placebo and exercise had active shoulder abduction of 70 degrees.
Side effects
No person in either group reported any side effects.
Participant‐reported pain relief of 30% or greater, global assessment of treatment success, and quality of life
These were not measured in this trial.
Quality of the evidence
There was low quality evidence that LLLT for six days may improve global assessment of treatment success more than placebo, when measured at six days. Further research is likely to change the estimate.
We are very uncertain about whether PEMF for two weeks improves pain or function any more than placebo because of the very low quality evidence from one trial.
There was moderate quality evidence that LLLT plus exercise for eight weeks may improve pain, up to four weeks, and function, up to four months, more than placebo plus exercise. Further research may change the estimate.
We are very uncertain about whether therapeutic ultrasound, PEMF, Iodex phonophoresis, continuous short wave diathermy, a combination of Iodex iontophoresis with continuous short wave diathermy, or a combination of therapeutic ultrasound with transcutaneous electrical nerve stimulation (TENS) are effective adjuncts to exercise.
Summary of findings
Summary of findings for the main comparison. Low‐level laser therapy (LLLT) compared to placebo for adhesive capsulitis (frozen shoulder).
| Low‐level laser therapy (LLLT) compared to placebo for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: physical therapy clinic in high‐income country Intervention: LLLT Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | LLLT | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Overall pain | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Function | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Global assessment of treatment success 'Excellent' or 'good' result (self‐rated) Follow‐up: end of 6 days treatment | Study population1 | RR 8.00 (2.11 to 30.34) | 40 (1 study) | ⊕⊕⊕⊝ low2 | Absolute risk difference 70% (48% to 92% more); relative per cent change 700% (111% to 2934% more) NNTB = 1 (1 to 2) |
|
| 100 per 1000 | 800 per 1000 (211 to 1000) | |||||
| Active shoulder abduction | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Adverse events | See comment | See comment | Not estimable | 40 (1 study) | ⊕⊕⊕⊝ low2 | No participant in either group reported experiencing any adverse event |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of treatment success in the placebo group in Taverna 1990 used as the assumed control group risk.
2 Sample size is small, yielding a very wide 95% CI. Outcome measured at the end of six days of treatment, so effect may not be generalisable to a later time point (e.g. up to six weeks).
Summary of findings 2. Pulsed electromagnetic field therapy (PEMF) compared to placebo for adhesive capsulitis (frozen shoulder).
| Pulsed electromagnetic field therapy (PEMF) compared to placebo for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: physical therapy clinic in high‐income country Intervention: Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PEMF | |||||
|
Participant‐reported pain relief ≥ 30% Complete resolution of SPADI pain Follow‐up: end of 15 days treatment |
Study population1 |
RR 19.19 (1.25 to 294.21) |
32 (1 study) |
⊕⊝⊝⊝ very low2 | Absolute risk difference 75% (53% to 97% more); relative per cent change 1819% (25% to 29321% more) NNTB = 1 (1 to 2) |
|
| 83 per 1000 |
1000 per 1000 (104 to 1000) |
|||||
| Overall pain | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Function Total recovery of joint function Follow‐up: end of 15 days treatment | Study population1 |
RR 14.24 (0.91 to 221.75) |
32 (1 study) | ⊕⊝⊝⊝ very low2 | Absolute risk difference 55% (31% to 79% more); relative per cent change 1324% (9% fewer to 22075% more) NNTB not applicable. |
|
| 83 per 1000 |
1000 per 1000 (76 to 1000) |
|||||
| Global assessment of treatment success | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Active shoulder abduction | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of treatment success in placebo group in Battisti 2007 used as the assumed control group risk. 2 High risk of attrition bias because a high proportion of the placebo group withdrew due to lack of response to treatment, which is likely to bias the results of the trial in favour of the active treatment group; 95% CI very wide.
Summary of findings 3. Low‐level laser therapy (LLLT) plus exercise compared to exercise for adhesive capsulitis (frozen shoulder).
| Low‐level laser therapy (LLLT) plus exercise compared to placebo plus exercise for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: physical therapy clinic in high‐income country Intervention: LLLT plus exercise Comparison: placebo laser therapy plus exercise | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo laser therapy plus exercise | LLLT plus exercise | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
|
Overall pain 0‐100 visual analogue scale (lower score = less pain) Follow‐up: at 4th week of treatment |
The mean overall pain in the control group was 51 points | The mean overall pain in the intervention group was 19 points lower (23 to 15 lower) | 63 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference 19% (23% to 15% fewer); relative per cent change2 28% (34% to 22% fewer) NNTB = 1 (1 to 2) |
|
| Function Shoulder Disabilty Questionnaire 0‐100 (lower scores = better function) Follow‐up: at 4th week of treatment | The mean function in the control group was 48 points | The mean function in the intervention group was 12 points lower (18 to 6 lower) | 63 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference 12% (18% to 6% fewer); relative per cent change3 19% (29% to 10% fewer) NNTB = 2 (2 to 5) |
|
| Global assessment of treatment success | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
|
Active shoulder abduction Degrees Follow‐up: 4 weeks |
The mean active shoulder abduction in the control group was 70 degrees | The mean active shoulder abduction in the intervention group was 9 degrees higher (2 to 16 higher) | 63 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference 5% (1% to 9% more); relative per cent change415% (3% to 27% more) | |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Adverse events | See comment | See comment | Not estimable | 63 (1 study) | ⊕⊕⊕⊝ moderate1 | No participant reported experiencing any adverse event |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Sample size is small, yielding wide 95% CIs.
2 Baseline mean overall pain score of placebo group was 67.
3 Baseline mean function score of placebo group was 62.
4 Baseline mean active abduction of placebo group was 59.
Background
Description of the condition
This review is one in a series of reviews aiming to determine the evidence of the benefits and safety of common interventions for shoulder pain. This series of reviews form the update of an earlier Cochrane review of physiotherapy for shoulder disorders (Green 2003). Since our original review, many new clinical trials studying a diverse range of interventions have been performed. To improve usability of the review, we have subdivided the review by type of shoulder disorder and type of intervention as patients within different diagnostic groupings may respond differently to interventions. This review focuses on electrotherapy modalities for adhesive capsulitis (frozen shoulder). Separate reviews of (i) manual therapy and exercise for adhesive capsulitis (Page 2014), (ii) manual therapy and exercise for rotator cuff disorders, and (iii) electrotherapy modalities for rotator cuff disorders are currently underway.
Adhesive capsulitis (also termed frozen shoulder, painful stiff shoulder or periarthritis) is a common condition characterised by spontaneous onset of pain, progressive restriction of movement of the shoulder, and disability that restricts activities of daily living, work and leisure (Codman 1934; Neviaser 1987; Reeves 1975). There is an acknowledged lack of specific diagnostic criteria for the condition. Reviews of the diagnostic criteria used in clinical trials of adhesive capsulitis have found that all trialists reported that restricted movement must be present but the amount of restriction, whether the restriction had to be active or passive, or both, and the direction of restriction were inconsistently defined (Green 1998; Schellingerhout 2008). The cumulative incidence of adhesive capsulitis has been reported as 2.4 per 1000 people per year (95% confidence interval (CI) 1.9 to 2.9) based on presentations to Dutch general practice (van der Windt 1995). Adhesive capsulitis has been reported to affect slightly more women than men (Tekavec 2012; Walker 2004) and occurs most commonly in middle age, with an increased frequency in people with diabetes. Most studies indicate that it is a self‐limiting condition lasting up to two to three years (Reeves 1975), although some people may have residual clinically detectable restriction of movement and disability beyond this time point (Binder 1984a; Hazelman 1972). The largest case series (269 shoulders in 223 people) found that at a mean follow‐up of 4.4 years (range 2 to 20 years) 41% had ongoing symptoms (Hand 2008).
Description of the intervention
Electrotherapy modalities (also known as electrophysical agents) are types of physical therapy that aim to reduce pain and improve function via an increase in energy (electrical, sound, light, thermal) into the body (Watson 2008a; Watson 2010). Several electrotherapy modalities exist, including low‐level laser therapy (LLLT), therapeutic ultrasound, interferential current and transcutaneous electrical nerve stimulation (TENS). The use of particular electrotherapy modalities in physical therapy practice has varied over time. Between 1990 and 2010, use of therapeutic ultrasound has increased in several countries, LLLT continues to enjoy consistent use, and use of TENS and interferential current has increased in the UK but declined in Australia (Shah 2012). Patients seeking treatment for musculoskeletal conditions seldom receive a single electrotherapy modality in isolation; other physical therapy interventions such as manual therapy and exercise are commonly delivered as co‐interventions (Hanchard 2011). A brief description of the electrotherapy modalities investigated in this review, and their presumed mechanisms of action, are outlined as follows.
Low‐level laser therapy (LLLT) generates a beam of light with a particular wavelength which has the potential to deliver light energy to tissue depths below the dermis (Basford 1989; Bjordal 2010; Peplow 2010). Studies suggest that LLLT contributes to pain relief by reducing pro‐inflammatory cytokines and increasing anti‐inflammatory growth factors and cytokines (Bjordal 2006; Peplow 2010; Sakurai 2000). Systematic reviews of randomised controlled trials (RCTs) have found that LLLT is more effective than placebo in the short‐term for neck pain (Chow 2009), although findings are inconclusive for non‐specific low‐back pain (Yousefi‐Nooraie 2008). The effects of LLLT are considered to be dependent on dosage, wavelength, site and duration of treatment, and researchers have argued that previous RCTs of LLLT with inconclusive findings may have delivered dosages that are below that expected to achieve a biological response (Bjordal 2006; Bjordal 2010).
Therapeutic ultrasound delivers energy to deep tissue sites through ultrasonic waves (at 1 or 3 MHz frequency and intensities between 0.1 watts/cm2 and 3 watts/cm2) using a crystal sound head. Treatment can be delivered in two forms, continuous (non‐stop ultrasonic waves) and pulsed (intermittent ultrasonic waves) (Allen 2006; Watson 2008b). The purpose of treatment is to increase tissue temperature and induce non‐thermal physiological changes (such as cell permeability and cell growth), which are believed to promote soft tissue healing and muscle relaxation (O'Brien 2007; Watson 2008b). However, previous Cochrane reviews have found no high quality evidence to support the use of therapeutic ultrasound for chronic low‐back pain (Ebadi 2014), osteoarthritis (Rutjes 2010), carpal tunnel syndrome (Page 2013b) or acute ankle sprains (van den Bekerom 2011).
Interferential current involves crossing two medium frequency currents (most commonly 4000 Hz), which reportedly generates a low‐frequency 'beating' (amplitude‐modulated) effect at between 0 and 150 Hz in the deep tissues (Beatti 2010). These beat frequencies are believed to decrease pain, increase circulation and block nerve conduction. Two recent systematic reviews have found insufficient evidence to support the use of interferential current over placebo, or as an adjunct to other interventions, for a range of musculoskeletal conditions (Beatti 2010; Fuentes 2010).
Transcutaneous electrical nerve stimulation (TENS) delivers electrical stimulation via electrodes placed over the intact skin surface near the source of pain to activate underlying nerves (Jones 2009; Sluka 2003). Several types of TENS applications exist, the most common are conventional TENS (high frequency and low intensity, which is sufficient to produce a comfortable tingling sensation) and acupuncture‐like TENS (low frequency and high intensity, which is sufficient to elicit muscle twitching) (Johnson 2008). The development of TENS was based on the Gate Control Theory of Pain (Melzack 1965), which suggests that there is a 'gating' mechanism in the dorsal horn of the spinal cord that regulates the amount of incoming painful stimuli via small diameter afferent nerve fibres and that stimulation of large diameter afferent nerve fibres using other stimuli (such as TENS) can 'close the gate' and reduce the perception of pain (Walsh 2009). Evidence from animal studies suggests that TENS reduces ongoing nociceptive cell activity and inhibits pain facilitatory pathways (DeSantana 2008; Jones 2009). However, previous Cochrane reviews have found no high quality evidence to support the use of TENS for chronic low‐back pain (Khadilkar 2008), knee osteoarthritis (Rutjes 2009) or acute pain associated with medical procedures or rib fractures (Walsh 2009).
Pulsed electromagnetic field therapy (PEMF) involves the delivery of pulsing (that is 'on‐off') low‐frequency magnetic fields through the body, which is believed to provide temporary pain relief by influencing tissue generation and cell proliferation (Gordon 2007; Markov 2007). Moderate quality evidence from a previous Cochrane review suggests that PEMF is more effective than placebo in terms of reducing osteoarthritis pain, but not on function or quality of life (Li 2013).
Continuous short wave diathermy is the delivery of a constant stream of short wave (wavelength 3 to 30 m, frequency 10 to 100 MHz) electromagnetic radiation to produce deep heating within tissues (Allen 2006; Shields 2001). Short wave diathermy is designed to produce heat at deeper tissue levels than superficial agents (such as a hot pack). The deep tissue heating is believed to induce an increase in metabolic activity, blood flow, collagen extensibility and nerve conduction, which are thought to encourage healing and relieve pain (Allen 2006; Shields 2001). A systematic review of continuous short wave diathermy for knee osteoarthritis found small effects on pain immediately post‐treatment but no clinically important effect on function (Laufer 2012).
Two electrotherapy modalities are designed to facilitate delivery of topical medication through the skin (that is transdermal delivery). Phonophoresis is administered using a therapeutic ultrasound device (Machet 2002; Watson 2008b), and iontophoresis is administered using a low‐intensity electrical current (Batheja 2006; Roustit 2014). The therapeutic ultrasound device used in phonophoresis is believed to enhance the absorption of the topically applied medication (Machet 2002). The iontophoretic device is believed to induce electromigration and electro‐osmosis, which are thought to facilitate the movement of positively and negatively charged drugs into the skin (Roustit 2014). Previous Cochrane reviews have found very low quality evidence suggesting that phonophoresis results in better quality of life scores than therapeutic ultrasound in people with chronic low‐back pain (Ebadi 2014), but that iontophoresis is no more effective than placebo for neck pain (Kroeling 2013).
Why it is important to do this review
The previous version of this review (Green 2003) included three trials investigating the efficacy of electrotherapy modalities for adhesive capsulitis (Leclaire 1991; Lee 1973; Taverna 1990). Leclaire 1991 and Lee 1973 concluded that there was little evidence to either support or refute the benefits of PEMF or infrared irradiation, respectively, while Taverna 1990 reported that LLLT was more effective than placebo laser. Other recently published systematic reviews of interventions for adhesive capsulitis (Favejee 2011; Maund 2012) have identified several new trials. Therefore, there is a need to synthesise the most up‐to‐date evidence on the efficacy of electrotherapy modalities for adhesive capsulitis.
Objectives
To synthesise the available evidence regarding the benefits and harms of electrotherapy modalities, delivered alone or in combination with other interventions, for the treatment of adhesive capsulitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any design (for example parallel, cross‐over, factorial) and controlled clinical trials using a quasi‐randomised method of allocation, such as by alternation or date of birth. Reports of trials were eligible regardless of the language or date of publication.
Types of participants
We included trials that enrolled adults (> 16 years of age) with adhesive capsulitis (as defined by the trialists) for any duration. We included trials enrolling participants with various soft tissue disorders only if the results for the participants with adhesive capsulitis were presented separately or if 90% or more of participants in the trial had adhesive capsulitis. We excluded trials including participants with a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulders, and pain in the shoulder region as part of a complex myofacial neck/shoulder/arm pain condition.
Types of interventions
We included trials comparing any electrotherapy modality to placebo, no treatment, a different electrotherapy modality, or any other intervention. Examples of eligible electrotherapy modalities included therapeutic ultrasound, LLLT, TENS, PEMF, interferential current, phonophoresis, iontophoresis, and continuous short wave diathermy. Trials primarily evaluating the effect of a manual therapy or exercise intervention were excluded and are included in a separate Cochrane review.
Types of outcome measures
We did not consider outcomes as part of the eligibility criteria.
Adhesive capsulitis is characterised by pain and global loss of range of movement. Given the mechanism by which electrotherapy modalities work, we determined reduction of pain to be the main aim of treatment. Considerable variation has been noted in the outcome measures reported in clinical trials of interventions for pain. However, there is general agreement that the outcome measures of greatest importance to patients should be considered. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has published consensus recommendations for determining clinically important changes in outcome measures in clinical trials of interventions for chronic pain (Dworkin 2008). Reductions in pain intensity of ≥ 30% and ≥ 50% reflect moderate and substantial clinically important differences, respectively, and it is recommended that the proportion of patients who respond with these degrees of pain relief should be reported.
Continuous outcome measures used in pain trials, such as mean change on a 100 mm visual analogue scale (VAS), may not follow a Gaussian distribution. Often a bimodal distribution is seen instead, where patients tend to report either very good or very poor pain relief (Moore 2010). This creates difficulty in interpreting the meaning of average changes in continuous pain measures. For this reason, a dichotomous outcome measure (the proportion of participants reporting ≥ 30% pain relief) may or may not also be clinically relevant for trials of adhesive capsulitis.
The original review determined that no trials had included a dichotomous outcome for pain, in keeping with the recognition that it has been the practice in most trials of interventions for chronic pain to report continuous measures only. We therefore also included a continuous measure of overall pain.
A global rating of treatment success such as the Patient Global Impression of Change scale (PGIC), which provides an outcome measure that integrates pain relief, changes in function and adverse events into a single, interpretable measure, is also recommended by IMMPACT and was included as a main outcome measure (Dworkin 2008).
Main outcomes
Participant‐reported pain relief of 30% or greater (a moderate clinically important difference)
Overall pain (mean or mean change measured by VAS, numerical or categorical rating scales)
Function. Where trialists reported outcome data for more than one function scale we extracted data on the scale that was highest on the following a priori defined list: (1) Shoulder Pain and Disability Index (SPADI); (2) Croft Shoulder Disability Questionnaire; (3) Constant Score; (4) Short Form‐36 (SF‐36) Physical Component Score; (5) Health Assessment Questionnaire; (6) any other function scale
Global assessment of treatment success as defined by the trialists (for example proportion of participants with significant overall improvement)
Active shoulder abduction (measured in degrees or other)
Quality of life as measured by generic measures (such as components of the SF‐36) or disease‐specific tools
Number of participants experiencing any adverse events
Other outcomes
Night pain measured by VAS, numerical or categorical rating scales
Pain on motion measured by VAS, numerical or categorical rating scales
Other range of motion (ROM) measures for example flexion, external rotation and internal rotation (measured in degrees or other such as hand behind back distance in centimetres). Where trialists reported outcome data for both active and passive ROM measures we extracted the data on active ROM only
Work disability
Requiring surgery, for example manipulation under anaesthesia, arthroscopy
Timing of outcome assessment
We extracted outcome measures that assessed benefits of treatment (for example pain or function) at the following time points:
up to three weeks;
longer than three and up to six weeks (this was the main time point);
longer than six weeks and up to six months; and
longer than six months.
If data were available in a trial at multiple time points within each of the above periods (for example at four, five, and six weeks) we only extracted data at the latest possible time point of each period. We extracted adverse events at all time points.
We collated the main results of the review into summary of findings (SoF) tables, which provide key information concerning the quality of evidence and the magnitude and precision of the effect of the interventions. We included the main outcomes (see above) in the SoF tables with results at, or nearest, the main time point (six weeks) presented.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (to Issue 4, 2014 in The Cochrane Library), MEDLINE (January 1966 to May 2014), EMBASE (January 1980 to May 2014), and CINAHL Plus (January 1937 to May 2014). The complete search strategies are presented in Appendix 1. The search terms used included clinical terms relevant to adhesive capsulitis, rotator cuff disorders and manual therapy and exercise interventions as the current review and Cochrane reviews of (i) manual therapy and exercise for adhesive capsulitis, (ii) manual therapy and exercise for rotator cuff disorders, and (iii) electrotherapy modalities for rotator cuff disorders were conducted simultaneously.
Searching other resources
We searched for ongoing trials and protocols of published trials in the clinical trials register that is maintained by the US National Institute of Health (http://clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/). We also reviewed the reference lists of the included trials and any relevant review articles retrieved from the electronic searches to identify any other potentially relevant trials.
Data collection and analysis
Selection of studies
Two review authors (MJP and BM) independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups.
Possibly relevant: studies that met the inclusion criteria and studies from which it was not possible to determine whether they met the criteria either from their title or abstract.
Excluded: studies clearly not meeting the inclusion criteria.
If a title or abstract suggested that the study was eligible for inclusion, or we could not tell, we obtained a full text version of the article and two review authors (MJP and BM) independently assessed it to determine whether the study met the inclusion criteria. The review authors resolved discrepancies through discussion or adjudication by a third author (SG or RB).
Data extraction and management
Two review authors (MJP and either SK or RJ) independently extracted data using a standard data extraction form developed for this review. The authors resolved any discrepancies through discussion or adjudication by a third author (SG or RB) until consensus was reached. We pilot tested the data extraction form and modified it accordingly before use. In addition to items for assessing risk of bias and numerical outcome data, we also recorded the following characteristics:
trial characteristics, including type (for example parallel or cross‐over), country, source of funding, and trial registration status (with registration number recorded if available);
participant characteristics, including age, sex, duration of symptoms, and inclusion and exclusion criteria;
intervention characteristics, including type of electrotherapy modality, duration of treatment, use of co‐interventions;
outcomes reported, including the measurement instrument used and timing of outcome assessment.
One author (MJP) compiled all comparisons and entered the outcome data into Review Manager 5.2.
For a particular systematic review outcome there may be a multiplicity of results available in the trial reports (for example multiple scales, time points and analyses). To prevent selective inclusion of data based on the results (Page 2013a), we used the following a priori defined decision rules to select data from trials:
where trialists reported both final values and change from baseline values for the same outcome, we extracted final values;
where trialists reported both unadjusted and adjusted values for the same outcome, we extracted unadjusted values;
where trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (for example per‐protocol, as‐treated), we extracted ITT‐analysed data;
for cross‐over RCTs, we preferentially extracted data from the first period only.
Where trials did not include a measure of overall pain but included one or more other measures of pain, for the purpose of combining data for the primary analysis of overall pain we combined overall pain with other types of pain in the following hierarchy: unspecified pain; pain with activity; daytime pain.
Assessment of risk of bias in included studies
Two review authors (MJP and either SK or RJ) independently assessed the risk of bias in the included trials using The Cochrane Collaboration's tool for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The following domains were assessed:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment (assessed separately for self‐reported and objectively assessed outcomes);
incomplete outcome data;
selective reporting;
other sources of bias (for example baseline imbalance).
Each item was rated as being at 'Low risk', 'Unclear risk' or 'High risk' of bias. We resolved any discrepancies through discussion or adjudication by a third author (SG or RB).
Measures of treatment effect
We used The Cochrane Collaboration's statistical software, Review Manager 5.2, to perform data analysis. We expressed dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous outcomes as mean differences (MDs) with 95% CIs if different trials used the same measurement instrument to measure the same outcome. Alternatively, we analysed continuous outcomes using the standardised mean difference (SMD) when trials measured the same outcome but employed different measurement instruments. To enhance interpretability of dichotomous outcomes, risk differences and the number needed to treat to benefit (NNTB) or the number needed to treat to harm (NNTH) were calculated. To enhance interpretability of continuous outcomes, pooled SMDs of overall pain and function were back‐transformed to an original 0 to 100 mm VAS by multiplying the SMD and 95% CI by a representative pooled standard deviation (SD) at the baseline of one of the included trials.
Unit of analysis issues
The unit of analysis was the participant. Two trials included a small number of participants with bilateral adhesive capsulitis. In these trials we analysed data based on the number of participants, not the number of shoulders, in order to produce conservative estimates of effect.
Dealing with missing data
Where required, we contacted trialists via email (twice, separated by three weeks) to retrieve missing information about trial design, outcome data, or attrition rates such as dropouts, losses to follow‐up and post‐randomisation exclusions in the included trials. For continuous outcomes with no standard deviations (SD) reported, we calculated SDs from standard errors (SEs), 95% CIs or P values. If no measures of variation were reported and SDs could not be calculated, we planned to impute SDs from other trials in the same meta‐analysis, using the median of the other SDs available (Ebrahim 2013). Where data were imputed or calculated (for example SDs calculated from SEs, 95% CIs or P values, or imputed from graphs or from SDs in other trials) we reported this in the tables Characteristics of included studies.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials. We assessed statistical heterogeneity using the Chi2 statistic and the I2 statistic (Higgins 2002). We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% may not be important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess publication bias, we planned to generate funnel plots if at least 10 trials examining the same intervention comparison were included in the review, and comment on whether any asymmetry in the funnel plot was due to publication bias or methodological or clinical heterogeneity of the trials (Sterne 2011). To assess outcome reporting bias, we compared the outcomes specified in trial protocols with the outcomes reported in the corresponding trial publications; if trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the trial publications (Dwan 2011; Norris 2013). We generated an Outcome Reporting Bias In Trials (ORBIT) Matrix (http://ctrc.liv.ac.uk/orbit/) using the ORBIT classification system (Kirkham 2010). We compared the fixed‐effect model estimate against the random‐effects model estimate to assess the possible presence of small sample bias in the published literature (that is where the intervention effect is more beneficial in smaller studies). In the presence of small sample bias, the random‐effects model estimate of the intervention effect is generally more beneficial than the fixed‐effect model estimate (Sterne 2011).
Data synthesis
For this review update, a large number of trials that investigated a diverse range of interventions were identified. To define the most clinically important questions to investigate in the review, after completing data extraction one author (MJP) sent the list of all possible trial comparisons to both of the original primary authors of this review, who are both clinicians (SG, physiotherapist and RB, rheumatologist). After reviewing the list of possible trial comparisons, both authors discussed and drafted a list of clinically important review questions and categorised each trial comparison under the review question to which it fitted best. This process was conducted iteratively until all trial comparisons were allocated to a review question and was conducted without knowledge of the results of any outcomes. The following questions were defined.
Is an electrotherapy modality effective compared to placebo or no treatment?
Is an electrotherapy modality combined with manual therapy or exercise (or both) effective compared to manual therapy or exercise (or both) alone?
Is an electrotherapy modality effective compared to another active intervention (for example glucocorticoid injection, oral non‐steroidal anti‐inflammatory drugs (NSAIDs))?
Is one type of electrotherapy modality more effective than another?
Is a combination of an electrotherapy modality with manual therapy or exercise (or both) effective compared to placebo, no treatment, or another active intervention?
Is a combination of an electrotherapy modality with manual therapy or exercise (or both) and another active intervention more effective than the other active intervention alone?
Is a combination of an electrotherapy modality with manual therapy or exercise (or both) and another active intervention more effective than placebo or no treatment?
The first two questions were considered the main questions of the review.
We combined the results of trials with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of benefits and harms. Where we could not combine data, we have summarised effect estimates and 95% CIs of each trial narratively. We planned to combine results using a random‐effects meta‐analysis model based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an impact on the results.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses.
Sensitivity analysis
We planned to perform a sensitivity analysis to investigate the robustness of the treatment effect (of the main outcomes) to allocation concealment and participant blinding by removing the trials that reported inadequate or unclear allocation concealment and lack of participant blinding from the meta‐analysis to see if this changed the overall treatment effect.
Summary of findings tables
We presented the results of the most important comparisons of the review in summary of findings (SoF) tables, which summarise the quality of evidence, the magnitude of effect of the interventions examined, and the sum of the available data on the outcomes as recommended by The Cochrane Collaboration (Schünemann 2011a). The SoF tables include an overall grading of the evidence related to each of the main outcomes, using the GRADE approach (Schünemann 2011b).
In the comments column of the SoF tables, we reported the absolute per cent difference, the relative per cent change from baseline, and the number needed to treat (NNT) (the NNT was only provided when the outcome showed a statistically significant difference).
For dichotomous outcomes (pain relief of 30% or greater, global assessment, adverse events) the absolute risk difference was calculated using the risk difference statistic in RevMan. The result was expressed as a percentage, and the relative per cent change was calculated as the risk ratio (RR) ‐ 1 and expressed as a percentage. For continuous outcomes (overall pain, function, active shoulder abduction, quality of life) the absolute risk difference was calculated as the improvement in the intervention group minus the improvement in the control group, in the original units (that is MD from RevMan divided by the units in the original scale), expressed as a percentage. The relative per cent change was calculated as the absolute change (or MD) divided by the baseline mean of the control group, expressed as a percentage.
In addition to the absolute and relative magnitude of effect provided in the SoF tables, for dichotomous outcomes the number needed to treat to benefit (NNTB) or the number needed to treat to harm (NNTH) was calculated from the control group event rate and the RR using the Visual Rx NNT calculator (Cates 2004). For the continuous outcomes, overall pain and function, the NNT was calculated using the Wells calculator software available at the Cochrane Musculoskeletal Review Group (CMSG) editorial office (www.cochranemsk.org). We assumed a minimal clinically important difference (MCID) of 1.5 points on a 10 point scale (or 15 points on a 100 point scale) for pain (Hawker 2011), and 10 points on a 100 point scale for function or disability (for example SPADI, Constant‐Murley, Disabilities of the Arm, Shoulder and Hand (DASH)) for input into the calculator (Angst 2011; Roy 2009; Roy 2010).
Results
Description of studies
Results of the search
The search, conducted up to May 2014, yielded 3471 records across the four databases. Three additional records were identified from other sources (for example screening reference lists of previous systematic reviews and included trials). After removal of duplicates, 2627 unique records remained. Of these, 311 were retrieved for further scrutiny based on the title and abstract. Based on full text screening, 19 trials were deemed eligible for inclusion (Battisti 2007; Bumin 2001; Calis 2006; Carette 2003; Cheing 2008; Dewan 2011; Dogru 2008; Ghosh 2012; Guler‐Uysal 2004; Kanai 2006; Leclaire 1991; Lee 1973; Leung 2008; Maryam 2012; Pajareya 2004; Rigato 2002; Ryans 2005; Stergioulas 2008; Taverna 1990). One trial was only available as a conference abstract and is awaiting assessment (Alicicco 2000), and one ongoing trial was identified in a clinical trials registry (ACTRN12611000680965). A flow diagram of the study selection process is presented in Figure 1.
1.
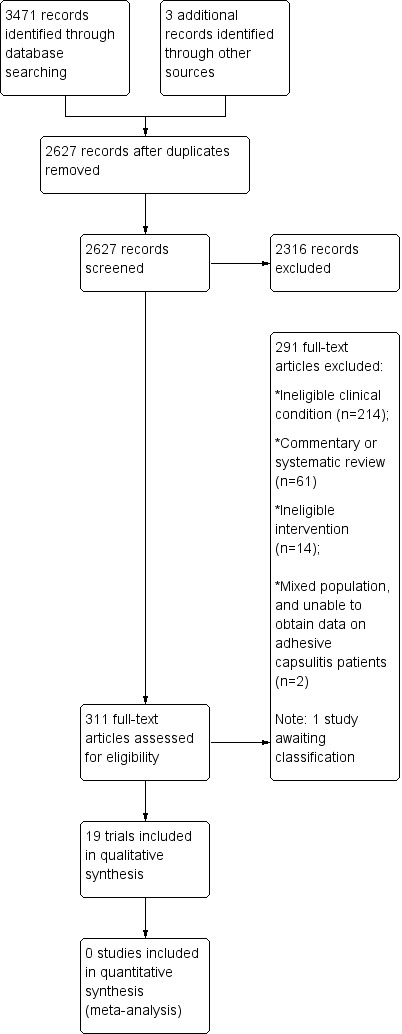
Study flow diagram.
Included studies
A full description of all included trials is provided in the table of Characteristics of included studies. We contacted the authors of 17 trials to retrieve either (a) information about the study design, participants, interventions, and outcomes in the trial; (b) information required to complete the risk of bias assessments; or (c) missing data for unreported or partially reported outcomes. We received replies from six trialists (Carette 2003; Dogru 2008; Maryam 2012; Pajareya 2004; Ryans 2005; Stergioulas 2008).
Design
All trials were described as RCTs, and all trials used a parallel group design. Eight trials included two intervention arms (Dewan 2011; Dogru 2008; Guler‐Uysal 2004; Kanai 2006; Leclaire 1991; Pajareya 2004; Stergioulas 2008; Taverna 1990), seven included three arms (Battisti 2007; Bumin 2001; Cheing 2008; Ghosh 2012; Leung 2008; Maryam 2012; Rigato 2002), and four included four arms (Calis 2006; Carette 2003; Lee 1973; Ryans 2005).
Participants
A total of 1249 participants were included in the 19 trials, with the number of participants per trial ranging from 30 to 122. The median of the mean age of participants in each trial was 55 years, and the median of the mean duration of symptoms was 5.5 months. Sixty‐one per cent of participants were female. Diagnostic criteria or definitions of adhesive capsulitis varied in regards to the type, amount and direction of shoulder restriction, and ranged from undefined (Taverna 1990) to very specific (for example painful and limited passive glenohumeral mobility, with more restricted lateral rotation (< 8 °) relative to abduction and medial rotation) (Stergioulas 2008). Trials were conducted in Turkey (n = 4); Italy (n = 3); Canada, Hong Kong, India and United Kingdom (n = 2 each); and Greece, Iran, Japan and Thailand (n = 1 each).
Interventions
The characteristics of the electrotherapy modalities are summarised in Table 4. The trials evaluated physical therapy interventions comprising therapeutic ultrasound (four trials: Calis 2006; Carette 2003; Dogru 2008; Ghosh 2012), TENS (four trials: Calis 2006; Carette 2003; Dewan 2011; Maryam 2012), continuous short wave diathermy (four trials: Bumin 2001; Guler‐Uysal 2004; Leung 2008; Pajareya 2004), PEMF (three trials: Battisti 2007; Leclaire 1991; Rigato 2002), interferential current (three trials: Cheing 2008; Dewan 2011; Ryans 2005), LLLT (two trials: Stergioulas 2008; Taverna 1990), Iodex phonophoresis (one trial: Bumin 2001), Iodex iontophoresis (one trial: Bumin 2001), polarity exchangeable permanent magnet (one trial: Kanai 2006), and infrared irradiation (one trial: Lee 1973). The median duration of electrotherapy was four weeks (range 1 to 12) with a median of three treatment sessions delivered per week (range 1 to 15) and a median of 10 treatment sessions provided in total across the treatment period (range 1 to 36). Several trials did not report important components of the electrotherapy modality, including duration of each treatment session, and frequency and intensity of the intervention. Five trials evaluated the efficacy of an electrotherapy modality delivered in isolation, testing: PEMF (Battisti 2007; Rigato 2002), LLLT (Taverna 1990), TENS (Dewan 2011), interferential current (Dewan 2011), and polarity exchangeable permanent magnet (Kanai 2006). The comparators also varied considerably comprising no treatment, placebo electrotherapy, glucocorticoid injection, manual therapy, exercises, hot pack, and oral NSAIDs.
1. Electrotherapy intervention characteristics.
| Electrotherapy modality | Study ID | Frequency/Intensity | Session duration | # electrotherapy sessions per week | # weeks of electrotherapy | Total # electrotherapy sessions |
| Therapeutic ultrasound | Calis 2006 | Frequency: not reported; Intensity: 1.5 W/cm2 | 5 mins | 5 | 2 | 10 |
| Carette 2003 | Not reported | Not reported | 3 | 4 | 12 | |
| Dogru 2008 | Frequency: 3 MHz; Intensity: 1.5 W/cm2 | 10 mins | 5 | 2 | 10 | |
| Ghosh 2012 | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Continuous short wave diathermy | Bumin 2001 | Not reported | 20 mins | 1 | 10 | 10 |
| Guler‐Uysal 2004 | Frequency: 27.12 MHz | 20 mins | 5 | 2 | 10 | |
| Leung 2008 | Frequency: 27.12 MHz; Intensity: adjusted to patient's feeling of comfortable warmth | 20 mins | 3 | 4 | 12 | |
| Pajareya 2004 | Not reported | 20 mins | 3 | 3 | 9 | |
| Pulsed electromagnetic field therapy | Battisti 2007 | Frequency: 100 Hz | 30 mins | 7 | 2 | 14 |
| Leclaire 1991 | Frequency: range from 10 to 30 Hz | 30 mins | 3 | 12 | 36 | |
| Rigato 2002 | Frequency: 100 Hz | 30 mins | 7 | 2 | 14 | |
| Interferential current | Cheing 2008 | Current swept from 80 to 120 Hz | 20 mins | 2.5 | 4 | 10 |
| Dewan 2011 | Current swept from 80 to 120 Hz | 20 mins | 2.5 | 4 | 10 | |
| Ryans 2005 | Not reported | Not reported | 2 | 4 | 8 | |
| TENS | Calis 2006 | Intensity: patient's tolerance | 20 mins | 5 | 2 | 10 |
| Carette 2003 | Not reported | Not reported | 3 | 4 | 12 | |
| Dewan 2011 | Frequency: High; Intensity: tolerance level just below pain threshold | 20 mins | 2.5 | 4 | 10 | |
| Maryam 2012 | Not reported | Not reported | 1 | 6 | 6 | |
| Low‐level laser therapy | Stergioulas 2008 | 810‐nm Galium‐Aluminum‐Arsenide (Ga‐Al‐As) laser with a continuous output of 60 mW applied to eight of the most painful points for 30 seconds each | 4 mins | 1.5 | 8 | 12 |
| Taverna 1990 | Frequency 1000 Hz and power 24 mW applied to painful points, points of greater access, and trigger points | 15 to 20 mins | 15 | 1 | 15 | |
| Iodex iontophoresis | Bumin 2001 | Intensity: 2 mA | 20 mins | 1 | 10 | 10 |
| Iodex phonophoresis | Bumin 2001 | Intensity: 1.5 W/cm2 | 5 mins | 1 | 10 | 10 |
| Polarity exchangeable permanent magnet | Kanai 2006 | Not reported | 24 hours | 1 | 1 | 1 |
| Infra‐red irradiation | Lee 1973 | Not reported | 10 mins | 1 | 6 | 6 |
Outcomes
An Outcome Reporting Bias In Trials (ORBIT) matrix, which presents the level of reporting of each outcome in each trial (rated as fully reported, partially reported, measured but not reported, unclear if measured, or not measured), is presented in Table 5. Of the main outcomes, two trials measured participant‐reported pain relief of 30% or greater, 14 measured overall pain (mean or mean change), 13 measured function, four measured global assessment of treatment success, four measured active shoulder abduction, three measured quality of life, and five measured adverse events. Overall pain was most commonly measured using a 0 to 10 or 0 to 100 VAS. Function was most commonly measured using the SPADI, followed by the Constant Score. Of the other outcomes, 12 trials measured other measures of range of motion (ROM), two measured night pain, and four measured pain on motion. No trial explicitly measured work disability or requiring surgery. Partial reporting of outcomes occurred in eight trials. We contacted the authors of these eight trials to retrieve missing outcome data, and we obtained data from one (Stergioulas 2008).
2. Outcome Reporting Bias In Trials (ORBIT) matrix.
| Study ID | Main outcomes | Other outcomes | ||||||||||
| Participant‐reported pain relief ≥30% | Overall pain | Function | Global assessment | Active shoulder abduction | QoL | Adverse events | Night pain | Pain on motion | Other ROM | Work disability | Requiring surgery | |
| Battisti 2007 | Full | Full | Full | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Bumin 2001 | ? | Full | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Calis 2006 | ? | Partial | Full | ? | ? | ? | ? | ? | ? | Full | ? | ? |
| Carette 2003 | ? | Full | Full | ? | Measured | Full | ? | ? | ? | Full | ? | ? |
| Cheing 2008 | ? | Full | Full | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Dewan 2011 | ? | Partial | Partial | ? | ? | ? | ? | ? | ? | Partial | ? | ? |
| Dogru 2008 | ? | Full | Full | ? | ? | Full | ? | ? | Full | Full | ? | ? |
| Ghosh 2012 | ? | ? | ? | Full | ? | ? | ? | ? | ? | ? | ? | ? |
| Guler‐Uysal 2004 | ? | Full | ? | Full | ? | ? | ? | Full | Full | Full | ? | ? |
| Kanai 2006 | ? | Partial | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Leclaire 1991 | ? | Measured | Measured | ? | ? | ? | Full | ? | Full | Full | ? | ? |
| Lee 1973 | ? | ? | ? | ? | Partial | ? | ? | ? | ? | Partial | ? | ? |
| Leung 2008 | ? | ? | Full | ? | ? | ? | ? | ? | ? | Full | ? | ? |
| Maryam 2012 | Not measured | Full | Full | Not measured | Not measured | Not measured | Not measured | Not measured | Not measured | Full | Not measured | Not measured |
| Pajareya 2004 | ? | ? | Full | Full | ? | ? | Full | ? | ? | Full | ? | ? |
| Rigato 2002 | Partial | Full | Full | ? | ? | ? | Full | ? | ? | ? | ? | ? |
| Ryans 2005 | ? | Full | Full | ? | Measured | Measured | ? | ? | ? | Full | ? | ? |
| Stergioulas 2008 | ? | Full | Full | ? | Full | ? | Full | Full | Full | Full | ? | ? |
| Taverna 1990 | ? | ? | ? | Full | ? | ? | Full | ? | ? | ? | ? | ? |
'Full'= sufficient data for inclusion in a meta‐analysis was reported (e.g. mean, standard deviation, and sample size per group for continuous outcomes)
'Partial' = insufficient data for inclusion in a meta‐analysis was reported (e.g. means only, with no measures of variation)
'Measured' = outcome was measured but no outcome data was reported
'Not measured' = outcome was not measured by the trialists
'?' = unclear whether the outcome was measured or not (as a trial protocol was unavailable)
Excluded studies
Of the 311 full text records retrieved for further scrutiny, the majority (n = 275) were excluded because they were studies or commentaries focused on shoulder pain due to conditions other than adhesive capsulitis (that is rotator cuff disorders or mixed shoulder pain conditions). We have listed 16 adhesive capsulitis studies in the table Characteristics of excluded studies. The reasons for their exclusion were that the intervention was ineligible (for example an electrotherapy modality was provided to all groups with or without a co‐intervention (n = 14)), or the trial included a mixed population of participants with either adhesive capsulitis or lateral epicondylitis and data could not be obtained on the subgroup of adhesive capsulitis participants (n = 2).
Risk of bias in included studies
A summary of the risk of bias in the included trials is presented in Figure 2 and Figure 3.
2.
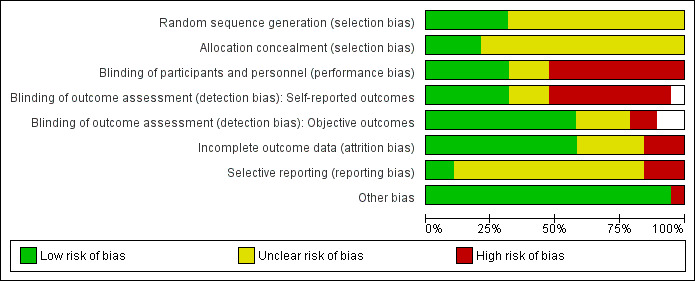
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
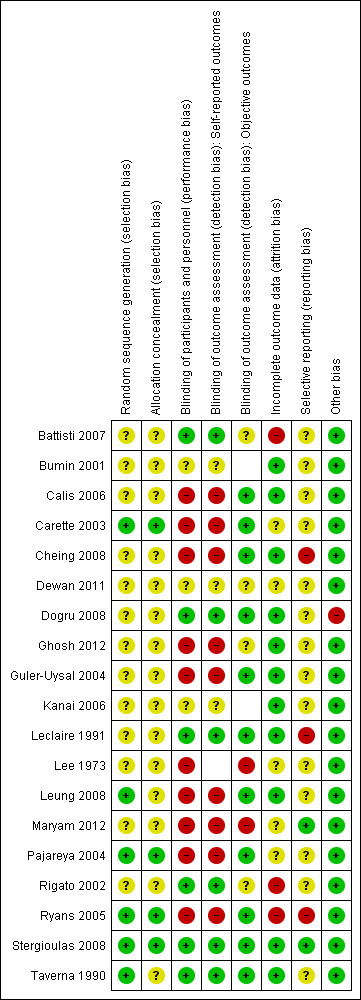
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six trials (Carette 2003; Leung 2008; Pajareya 2004; Ryans 2005; Stergioulas 2008; Taverna 1990) reported using an adequate method to generate a random allocation sequence, while only four trials (Carette 2003; Pajareya 2004; Ryans 2005; Stergioulas 2008) reported using an adequate method of allocation concealment. Thirteen trials did not report how the allocation sequence was generated, and 15 trials did not report how the allocation sequence was concealed, so the risk of selection bias in these trials was unclear.
Blinding
Six trials (Battisti 2007; Dogru 2008; Leclaire 1991; Rigato 2002; Stergioulas 2008; Taverna 1990) were rated at low risk of performance bias due to successful blinding of participants. This was achieved by delivering a placebo intervention to the control group or not informing participants of the type of electrotherapy they would receive. Three trials were rated at unclear risk of performance bias because participants received different types of electrotherapy, but it was unclear whether they were provided with any information that would make them perceive the type of electrotherapy they received as superior or inferior to the alternative type of electrotherapy (Bumin 2001; Dewan 2011; Kanai 2006). The remaining 10 trials were rated at high risk of performance bias as the participants were not blinded and may have had different expectations about the benefits of each intervention. Of 18 trials assessing self‐reported outcomes, the same six trials that blinded participants were rated at low risk of detection bias for self‐reported outcomes, three were rated at unclear risk of detection bias due to unclear participant blinding (Bumin 2001; Dewan 2011; Kanai 2006), and the remaining nine trials were rated at high risk of detection bias for self‐reported outcomes due to lack of participant blinding. Of 17 trials measuring objectively‐rated outcomes (for example ROM), 11 trials (Calis 2006; Carette 2003; Cheing 2008; Dogru 2008; Guler‐Uysal 2004; Leclaire 1991; Leung 2008; Pajareya 2004; Ryans 2005; Stergioulas 2008; Taverna 1990) reported blinding of outcome assessors and were thus rated at low risk of detection bias for objective outcomes. Two trials (Lee 1973; Maryam 2012) failed to blind the assessors of objective outcomes, so the risk of detection bias for objective outcomes was high; whereas four trials (Battisti 2007; Dewan 2011; Ghosh 2012; Rigato 2002) did not report whether such blinding was done, so the risk of detection bias for objective outcomes was unclear.
Incomplete outcome data
Eleven trials (Bumin 2001; Calis 2006; Cheing 2008; Dogru 2008; Ghosh 2012; Guler‐Uysal 2004; Kanai 2006; Leclaire 1991; Leung 2008; Stergioulas 2008; Taverna 1990) either had no dropouts, losses to follow‐up or exclusions, or had a small amount of incomplete data that was deemed unlikely to bias the results. These trials were rated at low risk of attrition bias. Three trials (Battisti 2007; Rigato 2002; Ryans 2005) reported differential dropouts across the groups, with the reasons appearing to be related to the treatments received, and were thus rated at high risk of attrition bias. The remaining five trials did not report either the amount of or the reasons for incomplete outcome data and so had an unclear risk of attrition bias (Carette 2003; Dewan 2011; Lee 1973; Maryam 2012; Pajareya 2004).
Selective reporting
Two trials (Maryam 2012; Stergioulas 2008) were rated at low risk of selective reporting bias because all outcomes specified in the trial registry entry were fully reported in the trial publications or were provided by the trialist on request. Three trials were rated at high risk of selective reporting bias because some of the outcomes that were reported in either the trial registry entry or in the methods section of the publication were not reported at all in the results section (Cheing 2008; Leclaire 1991; Ryans 2005). The remaining 14 trials were rated at unclear risk of selective reporting bias because either (a) the outcome data were completely reported for all outcomes specified in the methods section of the publication, but none of these trials were registered in a trials registry or had an available trial protocol so it was unclear whether other outcomes were measured but not reported based on the results; or (b) the outcome data were incompletely reported (for example reporting means without any measures of variation) but it was unclear whether the data were incompletely reported based on the statistical significance, magnitude or direction of the results, or not.
Other potential sources of bias
All trials except one (Dogru 2008) were rated as being free from other potential sources of bias. Dogru 2008 reported that participants in the therapeutic ultrasound plus home exercises group had worse pre‐treatment values and lower compliance with the home exercises than participants in the placebo ultrasound plus home exercises group, which may have biased the results towards the null.
Effects of interventions
See: Table 1; Table 2; Table 3
Due to heterogeneity of the interventions, comparators and outcomes, we were unable to conduct any meta‐analyses. Non‐synthesised summary data and effect estimates (with 95% CIs) of all outcomes were presented either in the Data and analyses or Additional tables sections (we have also reported effect estimates and 95% CIs for the main outcomes at all time points for comparisons falling under questions 1 and 2 in the following section). We have reported all time points as post‐randomisation. Unless otherwise stated, differences between groups in overall pain and function that were reported as 'significant' meant that the effect estimate met our criteria for a minimal clinically important difference and the 95% CI did not include the null value.
1) Is an electrotherapy modality effective compared to placebo or no treatment?
No trial compared therapeutic ultrasound, interferential current, infrared irradiation, continuous short wave diathermy, iontophoresis, TENS or multiple electrotherapy modalities to placebo or no treatment. Three trials compared an electrotherapy modality to placebo: one trial compared LLLT to placebo (Taverna 1990), and two trials compared PEMF to placebo (Battisti 2007; Rigato 2002).
LLLT
See Table 6; Table 1. Taverna 1990 compared LLLT to placebo for six days in 40 participants. Apart from an unclear risk of selection bias (the trialists did not report the method of allocation sequence) all other risk of bias domains were at low risk. The trialists found that participants receiving LLLT were statistically significantly more likely to be rated as having global treatment success at six days than participants receiving placebo (RR 8.00, 95% CI 2.11 to 30.34). No participant in either group reported any adverse events. Overall, based on low quality evidence, LLLT may be more effective than placebo at the end of six days of treatment.
3. Taverna 1990: LLLT (intervention) versus placebo (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||
| Events | Total | Events | Total | Risk ratio (95% CI) | |
| Global assessment of treatment success ("excellent" or "good" result) at 6 days | 16 | 20 | 2 | 20 | 8.00 [2.11, 30.34] |
PEMF
See Table 7; Table 2. Two trials compared PEMF to placebo for two weeks (Battisti 2007; Rigato 2002), but no outcome data were available for the placebo group in Rigato 2002 (none were reported in the publication and the trialist no longer had access to the data). Battisti 2007 (60 participants) was a three‐arm trial comparing low‐frequency (100 MHz) PEMF to Therapeutic Application of a Musically Modulated Electromagnetic Field (TAMMEF) and to placebo, and assessed outcomes at two weeks. The TAMMEF intervention is not a standard type of PEMF that can be applied by physical therapists, so no data for this group were included in the review. Participants and outcome assessors were blinded but there was a high risk of attrition bias because a high proportion of the placebo group withdrew due to lack of response to treatment, which was likely to bias the results of the trial in favour of the active treatment groups. The trialists found that statistically significantly more participants receiving low‐frequency (100 Hz) PEMF reported pain relief of 30% or greater compared to participants receiving placebo, at two weeks (RR 19.19, 95% CI 1.25 to 294.21) but there was no statistically significant difference between groups in terms of total recovery of joint function (RR 14.24, 95% CI 0.91 to 221.75). The precision of these effect estimates was very low, so there was a large degree of uncertainty in these results. Overall, based on very low quality evidence, we are uncertain whether PEMF is more or less effective than placebo.
4. Battisti 2007: PEMF (low frequency 100 Hz) (intervention) versus placebo (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||
| Events | Total | Events | Total | Risk ratio (95% CI) | |
| Overall pain (complete resolution of SPADI pain) at 15 days | 15 | 20 | 0 | 12 | 19.19 [1.25, 294.21] |
| Function (total recovery of joint function) at 15 days | 11 | 20 | 0 | 12 | 14.24 [0.91, 221.75] |
2) Is an electrotherapy modality combined with manual therapy or exercise (or both) effective compared to manual therapy or exercise (or both) alone?
No trial compared an electrotherapy modality plus manual therapy to manual therapy alone. No trial compared an electrotherapy modality plus manual therapy and exercise to manual therapy and exercise alone. Six trials compared an electrotherapy modality plus exercise to exercise alone (Bumin 2001; Calis 2006; Dogru 2008; Leclaire 1991; Leung 2008; Stergioulas 2008). Figure 4 presents non‐synthesised data for all trials reporting overall pain, and Figure 5 presents non‐synthesised data for all trials reporting function (the data were presented as SMDs because the trials used different measurement instruments). Data for other outcomes are reported in the tables indicated below. A SoF table was created for the comparison LLLT plus exercise versus placebo plus exercise because, of all the trials falling under this review question, the trial investigating this comparison reported the largest number of our main review outcomes.
4.

Forest plot of comparison: 1 Electrotherapy modality plus manual therapy or exercise (or both) versus manual therapy or exercise (or both), outcome: 1.1 Overall pain.
5.
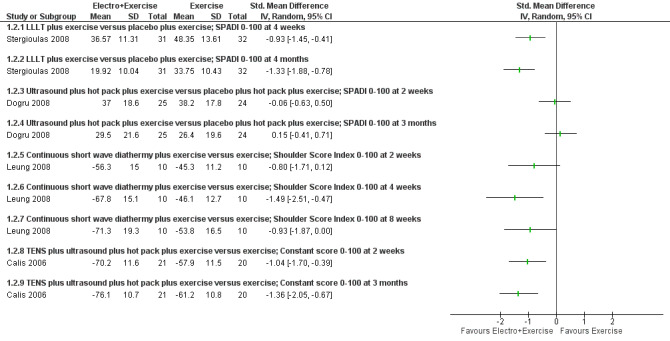
Forest plot of comparison: 1 Electrotherapy modality plus manual therapy or exercise (or both) versus manual therapy or exercise (or both), outcome: 1.2 Function.
LLLT
See Table 8; Table 3. One trial (63 participants) compared LLLT plus home exercises to placebo plus home exercises for eight weeks (Stergioulas 2008). All risk of bias domains were rated at low risk. The trialists found that, compared to placebo plus exercise, participants receiving LLLT plus exercise had clinically and statistically significantly lower overall pain at the fourth week of treatment (MD ‐18.81, 95% CI ‐22.68 to ‐14.94, 100 point scale) and statistically (but not clinically) significantly lower pain at four months (MD ‐12.68, 95% CI ‐15.95 to ‐9.41, 100 point scale); clinically and statistically significantly less disability at four weeks (MD ‐11.78, 95% CI ‐17.95 to ‐5.61, 100 point scale) and four months (MD ‐13.83, 95% CI ‐18.88 to ‐8.78, 100 point scale); and greater active abduction at four weeks (MD 8.99, 95% CI 2.41 to 15.57) but not at four months (MD 5.20, 95% CI ‐1.60 to 12.00). All these 95% CIs included non‐clinically important differences as possible estimates of effect. In terms of other outcomes, the LLLT group had statistically significantly lower night pain and pain on motion at four weeks and four months, but other measures of active ROM (flexion and external rotation) did not significantly differ between groups at either time point. No participant in either group reported any adverse events. Overall, based on moderate quality evidence, LLLT is probably an effective adjunct to home exercises in terms of pain up to four weeks and function up to four months.
5. Stergioulas 2008: LLLT plus exercise (intervention) versus placebo plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) at 4 weeks | 32.34 | 7.44 | 31 | 51.15 | 8.22 | 32 | ‐18.81 [‐22.68, ‐14.94] |
| Overall pain (VAS 0‐100) at 4 months | 23.92 | 6.11 | 31 | 36.6 | 7.09 | 32 | ‐12.68 [‐15.95, ‐9.41] |
| Function (SPADI 0‐100) at 4 weeks | 36.57 | 11.31 | 31 | 48.35 | 13.61 | 32 | ‐11.78 [‐17.95, ‐5.61] |
| Function (SPADI 0‐100) at 4 months | 19.92 | 10.04 | 31 | 33.75 | 10.43 | 32 | ‐13.83 [‐18.88, ‐8.78] |
| Night pain (VAS 0‐100) at 4 weeks | 41.42 | 7.69 | 31 | 55.67 | 8.49 | 32 | ‐14.25 [‐18.25, ‐10.25] |
| Night pain (VAS 0‐100) at 4 months | 19.38 | 5.77 | 31 | 42.35 | 7.57 | 32 | ‐22.97 [‐26.29, ‐19.65] |
| Pain on motion (VAS 0‐100) at 4 weeks | 45.57 | 8.27 | 31 | 67.75 | 8.03 | 32 | ‐22.18 [‐26.21, ‐18.15] |
| Pain on motion (VAS 0‐100) at 4 months | 22.54 | 6.02 | 31 | 39.78 | 7.65 | 32 | ‐17.24 [‐20.63, ‐13.85] |
| Active flexion (degrees) at 4 weeks | 101.07 | 14.42 | 31 | 98.22 | 14.14 | 32 | 2.85 [‐4.20, 9.90] |
| Active flexion (degrees) at 4 months | 102.55 | 14.78 | 31 | 97.72 | 14.01 | 32 | 4.83 [‐2.29, 11.95] |
| Active abduction (degrees) at 4 weeks | 78.67 | 13.76 | 31 | 69.68 | 12.87 | 32 | 8.99 [2.41, 15.57] |
| Active abduction (degrees) at 4 months | 85.63 | 13.95 | 31 | 80.43 | 13.58 | 32 | 5.20 [‐1.60, 12.00] |
| Active external rotation (degrees) at 4 weeks | 35.33 | 9.91 | 31 | 33.56 | 9.12 | 32 | 1.77 [‐2.94, 6.48] |
| Active external rotation (degrees) at 4 months | 42.72 | 10.05 | 31 | 38.53 | 9.9 | 32 | 4.19 [‐0.74, 9.12] |
Therapeutic ultrasound
See Table 9. One trial (49 participants) compared therapeutic ultrasound plus hot pack and exercise to placebo ultrasound plus hot pack and exercise for two weeks (Dogru 2008). Participants and outcome assessors were blinded but those in the ultrasound group had worse pre‐treatment values and lower compliance with home exercises than participants in the placebo ultrasound group, which may have biased results towards the null. Therapeutic ultrasound plus hot pack and exercise was not significantly different to placebo ultrasound plus hot pack and exercise in terms of overall pain at two weeks (MD 4.50, 95% CI ‐4.62 to 13.62, 100 point scale) and three months (MD 5.80, 95% CI ‐4.93 to 16.53, 100 point scale), function at two weeks (MD ‐1.20, 95% CI ‐11.39 to 8.99, 100 point scale) and three months (MD 3.10, 95% CI ‐8.44 to 14.64, 100 point scale), quality of life at three months (SF‐36 Physical Component Summary (PCS) MD ‐0.40, 95% CI ‐5.22 to 4.42, 100 point scale; SF‐36 Mental Component Sumamry (MCS) MD 1.00, 95% CI ‐5.19 to 7.19, 100 point scale), or pain on motion and passive ROM at two weeks and three months. Overall, based on very low quality evidence, we are uncertain whether therapeutic ultrasound is an effective adjunct to hot packs and exercise.
6. Dogru 2008: Therapeutic ultrasound plus hot pack plus exercise (intervention) versus placebo ultrasound plus hot pack plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (SPADI 0‐100) at 2 weeks | 40.1 | 18.6 | 25 | 35.6 | 13.7 | 24 | 4.50 [‐4.62, 13.62] |
| Overall pain (SPADI 0‐100) at 3 months | 31 | 20 | 25 | 25.2 | 18.3 | 24 | 5.80 [‐4.93, 16.53] |
| Function (SPADI 0‐100) at 2 weeks | 37 | 18.6 | 25 | 38.2 | 17.8 | 24 | ‐1.20 [‐11.39, 8.99] |
| Function (SPADI 0‐100) at 3 months | 29.5 | 21.6 | 25 | 26.4 | 19.6 | 24 | 3.10 [‐8.44, 14.64] |
| Pain on motion (VAS 0‐100) at 2 weeks | 39.6 | 25.3 | 25 | 40.7 | 20.3 | 24 | ‐1.10 [‐13.92, 11.72] |
| Pain on motion (VAS 0‐100) at 3 months | 24.8 | 29.9 | 25 | 23.6 | 25.5 | 24 | 1.20 [‐14.34, 16.74] |
| Passive abduction (degrees) at 2 weeks | 142.8 | 25.9 | 25 | 146 | 26.2 | 24 | ‐3.20 [‐17.79, 11.39] |
| Passive abduction (degrees) at 3 months | 147.8 | 30.1 | 25 | 148 | 26.5 | 24 | ‐0.20 [‐16.06, 15.66] |
| Passive flexion (degrees) at 2 weeks | 162.6 | 12.4 | 25 | 165.4 | 15 | 24 | ‐2.80 [‐10.52, 4.92] |
| Passive flexion (degrees) at 3 months | 163.7 | 16.5 | 25 | 168.5 | 13 | 24 | ‐4.80 [‐13.10, 3.50] |
| Passive internal rotation (degrees) at 2 weeks | 52.2 | 15.7 | 25 | 58.3 | 15.5 | 24 | ‐6.10 [‐14.84, 2.64] |
| Passive internal rotation (degrees) at 3 months | 57.4 | 13.8 | 25 | 60.9 | 15.3 | 24 | ‐3.50 [‐11.67, 4.67] |
| Passive external rotation (degrees) at 2 weeks | 58 | 16.6 | 25 | 71.3 | 14.9 | 24 | ‐13.30 [‐22.12, ‐4.48] |
| Passive external rotation (degrees) at 3 months | 65.7 | 19.4 | 25 | 75.4 | 15.5 | 24 | ‐9.70 [‐19.51, 0.11] |
| Quality of life (SF‐36 PCS 0‐100) at 3 months | 44.2 | 8.4 | 25 | 44.6 | 8.8 | 24 | ‐0.40 [‐5.22, 4.42] |
| Quality of life (SF‐36 MCS 0‐100) at 3 months | 44.8 | 11.5 | 25 | 43.8 | 10.6 | 24 | 1.00 [‐5.19, 7.19] |
Phonophoresis
See Table 10. One trial (30 participants) compared Iodex phonophoresis plus exercise to placebo ultrasound plus exercise (Bumin 2001). Participants and outcome assessors were blinded but the risk of selection bias was unclear. Iodex phonophoresis plus exercise resulted in significantly less overall pain at the end of 10 treatment sessions than placebo ultrasound plus exercise (MD ‐2.40, 95% CI ‐3.48 to ‐1.32, 10 point scale), though the time point was unclear as the trialists did not report how many sessions were delivered per week. Overall, based on very low quality evidence, we are uncertain whether Iodex phonophoresis is an effective adjunct to exercise.
7. Bumin 2001: Phonophoresis plus exercise (intervention) versus placebo ultrasound plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at the end of 10 sessions | 2.6 | 1.3 | 15 | 5 | 1.69 | 15 | ‐2.40 [‐3.48, ‐1.32] |
PEMF
See Table 11. One trial (47 participants) compared PEMF plus hot pack and exercise to placebo electrotherapy plus hot pack and exercise for 12 weeks (Leclaire 1991). The participants and outcome assessors were blinded but there was an unclear risk of selection bias. There was no significant difference between groups in pain on motion or ROM (unclear if active or passive) at four or eight weeks. Overall, based on very low quality evidence, we are uncertain whether PEMF is an effective adjunct to hot packs and exercise.
8. Leclaire 1991: PEMF plus hot pack plus exercise (intervention) versus placebo plus hot pack plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Pain at rest (4‐point ordinal scale) ar 12 weeks | 1.5 | 0.61 | 22 | 1.4 | 0.65 | 25 | Not estimable (outcome is not continuous) |
| Pain on movement (4‐point ordinal scale) ar 12 weeks | 2.2 | 0.76 | 22 | 2.2 | 0.7 | 25 | Not estimable (outcome is not continuous) |
| Flexion (degrees) at 4 weeks (unclear if active or passive) | 149 | 15.4 | 22 | 154 | 9.8 | 25 | ‐5.00 [‐12.49, 2.49] |
| Flexion (degrees) at 8 weeks (unclear if active or passive) | 163 | 17.1 | 22 | 171 | 11.9 | 25 | ‐8.00 [‐16.53, 0.53] |
| Abduction (degrees) at 4 weeks (unclear if active or passive) | 115 | 17.3 | 22 | 120 | 13.2 | 25 | ‐5.00 [‐13.89, 3.89] |
| Abduction (degrees) at 8 weeks (unclear if active or passive) | 135 | 19.8 | 22 | 142 | 13.1 | 25 | ‐7.00 [‐16.74, 2.74] |
| External rotation (degrees) at 4 weeks (unclear if active or passive) | 57 | 22.4 | 22 | 62 | 16.8 | 25 | ‐5.00 [‐16.44, 6.44] |
| External rotation (degrees) at 8 weeks (unclear if active or passive) | 71 | 20.3 | 22 | 80 | 14.5 | 25 | ‐9.00 [‐19.21, 1.21] |
| Internal rotation (degrees) at 4 weeks (unclear if active or passive) | 33 | 10.3 | 22 | 36 | 10 | 25 | ‐3.00 [‐8.82, 2.82] |
| Internal rotation (degrees) at 8 weeks (unclear if active or passive) | 38 | 9.9 | 22 | 40 | 4 | 25 | ‐2.00 [‐6.42, 2.42] |
Continuous short wave diathermy
See Table 12. One trial (30 participants) compared continuous short wave diathermy plus exercise to exercise alone for four weeks (Leung 2008). Given the inability to blind participants and personnel, the trial had a high risk of performance bias and detection bias for the self‐reported outcomes. The participants in the continuous short wave diathermy and exercise group had significantly better function scores than participants receiving exercise alone at four weeks (MD 21.70, 95% CI 9.47 to 33.93, 100 point scale) and eight weeks (MD 17.50, 95% CI 1.76 to 33.24, 100 point scale) and had statistically significantly greater external rotation and less hand‐behind‐back distance than the exercise alone group, though flexion did not significantly differ between the groups. Overall, based on very low quality evidence, we are uncertain whether continuous short wave diathermy is an effective adjunct to exercise.
9. Leung 2008: Short wave diathermy plus exercise (intervention) versus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Shoulder Score Index 0‐100) at 2 weeks | 56.3 | 15 | 10 | 45.3 | 11.2 | 10 | 11.00 [‐0.60, 22.60] |
| Function (Shoulder Score Index 0‐100) at 4 weeks | 67.8 | 15.1 | 10 | 46.1 | 12.7 | 10 | 21.70 [9.47, 33.93] |
| Function (Shoulder Score Index 0‐100) at 8 weeks | 71.3 | 19.3 | 10 | 53.8 | 16.5 | 10 | 17.50 [1.76, 33.24] |
| Flexion (degrees) at 2 weeks (unclear if active or passive) | 146.9 | 13.5 | 10 | 134.7 | 16.6 | 10 | 12.20 [‐1.06, 25.46] |
| Flexion (degrees) at 4 weeks (unclear if active or passive) | 146.9 | 14.2 | 10 | 132.1 | 25.7 | 10 | 14.80 [‐3.40, 33.00] |
| Flexion (degrees) at 8 weeks (unclear if active or passive) | 148.2 | 14.4 | 10 | 137.6 | 20.8 | 10 | 10.60 [‐5.08, 26.28] |
| External rotation (degrees) at 2 weeks (unclear if active or passive) | 59.3 | 19.8 | 10 | 39.5 | 20.6 | 10 | 19.80 [2.09, 37.51] |
| External rotation (degrees) at 4 weeks (unclear if active or passive) | 60.9 | 14.5 | 10 | 43.3 | 22.6 | 10 | 17.60 [0.96, 34.24] |
| External rotation (degrees) at 8 weeks (unclear if active or passive) | 62.1 | 11.5 | 10 | 41.1 | 23.2 | 10 | 21.00 [4.95, 37.05] |
| Hand behind back distance (cm) at 2 weeks (unclear if active or passive) | 7.2 | 6.1 | 10 | 14.7 | 8.1 | 10 | ‐7.50 [‐13.78, ‐1.22] |
| Hand behind back distance (cm) at 4 weeks (unclear if active or passive) | 7.6 | 5.7 | 10 | 14.7 | 8 | 10 | ‐7.10 [‐13.19, ‐1.01] |
| Hand behind back distance (cm) at 8 weeks (unclear if active or passive) | 6 | 7.3 | 10 | 13 | 6.7 | 10 | ‐7.00 [‐13.14, ‐0.86] |
Multiple electrotherapy modalities
See Table 13. One trial (30 participants) compared Iodex iontophoresis plus continuous short wave diathermy plus exercise to placebo ultrasound plus exercise (Bumin 2001). Participants and outcome assessors were blinded but the risk of selection bias was unclear. The trialists found that Iodex iontophoresis plus continuous short wave diathermy plus exercise resulted in significantly lower overall pain at the end of 10 treatment sessions compared to placebo ultrasound and exercise (MD ‐2.60, 95% CI ‐3.77 to ‐1.43, 10 point scale), though the time point was unclear as the trialists did not report how many sessions were delivered per week. Overall, based on very low quality evidence, we are uncertain whether Iodex iontophoresis plus continuous short wave diathermy is an effective adjunct to exercise.
10. Bumin 2001: Iontophoresis plus short wave diathermy plus exercise (intervention) versus placebo ultrasound plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at the end of 10 sessions | 2.4 | 1.59 | 15 | 5 | 1.69 | 15 | ‐2.60 [‐3.77, ‐1.43] |
See Table 14. One trial (41 participants) compared a combination of therapeutic ultrasound, TENS, hot pack and home exercises to home exercises alone for two weeks (Calis 2006). Given the inability to blind participants and personnel, the trial had a high risk of performance bias and detection bias for the self‐reported outcomes. In the multiple electrotherapies group, functional ability scores were significantly higher (that is better) than the home exercises alone group at two weeks (MD 12.30, 95% CI 5.23 to 19.37, 100 point scale) and three months (MD 14.90, 95% CI 8.32 to 21.48, 100 point scale). However, the 95% CIs included non‐clinically important differences as possible estimates of effect. In addition, the multiple electrotherapies group had statistically significantly greater passive abduction and external rotation than the home exercise alone group. Overall, based on very low quality evidence, we are uncertain whether a combination of therapeutic ultrasound, TENS and hot packs is an effective adjunct to exercise.
11. Calis 2006: Therapeutic ultrasound plus TENS plus hot pack plus home exercises (intervention) versus home exercises (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant score 0‐100) at 2 weeks | 70.2 | 11.6 | 21 | 57.9 | 11.5 | 20 | 12.30 [5.23, 19.37] |
| Function (Constant score 0‐100) at 3 months | 76.1 | 10.7 | 21 | 61.2 | 10.8 | 20 | 14.90 [8.32, 21.48] |
| Passive abduction (degrees) at 2 weeks | 145.4 | 19.2 | 21 | 125 | 20.1 | 20 | 20.40 [8.36, 32.44] |
| Passive abduction (degrees) at 3 months | 158.4 | 18.3 | 21 | 133.5 | 15.3 | 20 | 24.90 [14.59, 35.21] |
| Passive external rotation (degrees) at 2 weeks | 63.8 | 11.7 | 21 | 52.7 | 9.3 | 20 | 11.10 [4.65, 17.55] |
| Passive external rotation (degrees) at 3 months | 73.8 | 10.4 | 21 | 55 | 8.1 | 20 | 18.80 [13.11, 24.49] |
3) Is an electrotherapy modality effective compared to another active intervention, for example glucocorticoid injection, oral non‐steroidal anti‐inflammatory drugs (NSAIDs)?
Five trials compared an electrotherapy modality to another active intervention (Calis 2006; Cheing 2008; Guler‐Uysal 2004; Lee 1973; Leung 2008).
Interferential current
See Table 15. One trial (47 participants) compared interferential current plus home exercises to electroacupuncture plus home exercises and to home exercises alone for four weeks (Cheing 2008). Given the inability to blind participants and personnel, the trial had a high risk of performance bias and detection bias for the self‐reported outcomes. Also, no outcome data were reported for the group receiving home exercises alone. There was no statistically significant difference between interferential current plus exercise and electroacupuncture plus exercise in terms of overall pain at four weeks (MD ‐0.10, 95% CI ‐1.19 to 0.99, 10 point scale), four months, or seven months; or function at four weeks (MD ‐1.10, 95% CI ‐5.85 to 3.65, 100 point scale), four months, or seven months. Overall, based on very low quality evidence, we are uncertain whether interferential current is more or less effective than electroacupuncture.
12. Cheing 2008: Interferential current plus exercise (intervention) versus electroacupuncture plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 4 weeks | 3.4 | 1.9 | 23 | 3.5 | 1.9 | 24 | ‐0.10 [‐1.19, 0.99] |
| Overall pain (VAS 0‐10) at 4 months | 2 | 1.5 | 23 | 2.4 | 2.2 | 24 | ‐0.40 [‐1.47, 0.67] |
| Overall pain (VAS 0‐10) at 7 months | 1.3 | 1.4 | 23 | 1.7 | 2.3 | 24 | ‐0.40 [‐1.48, 0.68] |
| Function (Constant score 0‐100) at 4 weeks | 84.9 | 8.4 | 23 | 86 | 8.2 | 24 | ‐1.10 [‐5.85, 3.65] |
| Function (Constant score 0‐100) at 4 months | 90.2 | 9.7 | 23 | 93.3 | 6 | 24 | ‐3.10 [‐7.73, 1.53] |
| Function (Constant score 0‐100) at 7 months | 95.5 | 4.1 | 23 | 93.8 | 6.4 | 24 | 1.70 [‐1.36, 4.76] |
Infrared irradiation
One trial (80 participants) compared infrared irradiation plus home exercises to glucocorticoid injection plus home exercises and to analgesics plus home exercises for six weeks (Lee 1973). Insufficient data were reported for the only outcome reported in the trial paper, ROM.
Continuous short wave diathermy
Two trials compared continuous short wave diathermy to another active intervention (Guler‐Uysal 2004; Leung 2008). Given the inability to blind participants and personnel, both trials had a high risk of performance bias and detection bias for the self‐reported outcomes.
See Table 16. One trial (30 participants) compared continuous short wave diathermy plus exercise to hot pack plus exercise for four weeks (Leung 2008). There was no significant difference between continuous short wave diathermy and exercise compared to hot pack and exercise in terms of function at four weeks (MD 11.30, 95% CI ‐1.50 to 24.10, 100 point scale) or eight weeks. However, in terms of ROM, the continuous short wave diathermy and exercise group had statistically significantly greater flexion and external rotation and less hand‐behind‐back distance than the hot pack and exercise group (it was unclear whether the ROM was active or passive in this trial).
13. Leung 2008: Short wave diathermy plus exercise (intervention) versus hot pack plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Shoulder Score Index 0‐100) at 2 weeks | 56.3 | 15 | 10 | 54.2 | 15.4 | 10 | 2.10 [‐11.22, 15.42] |
| Function (Shoulder Score Index 0‐100) at 4 weeks | 67.8 | 15.1 | 10 | 56.5 | 14.1 | 10 | 11.30 [‐1.50, 24.10] |
| Function (Shoulder Score Index 0‐100) at 8 weeks | 71.3 | 19.3 | 10 | 57.8 | 16.3 | 10 | 13.50 [‐2.16, 29.16] |
| Flexion (degrees) at 2 weeks (unclear if active or passive) | 146.9 | 13.5 | 10 | 120.2 | 21 | 10 | 26.70 [11.23, 42.17] |
| Flexion (degrees) at 4 weeks (unclear if active or passive) | 146.9 | 14.2 | 10 | 122 | 20.9 | 10 | 24.90 [9.24, 40.56] |
| Flexion (degrees) at 8 weeks (unclear if active or passive) | 148.2 | 14.4 | 10 | 124.7 | 20.3 | 10 | 23.50 [8.07, 38.93] |
| External rotation (degrees) at 2 weeks (unclear if active or passive) | 59.3 | 19.8 | 10 | 27.6 | 18.7 | 10 | 31.70 [14.82, 48.58] |
| External rotation (degrees) at 4 weeks (unclear if active or passive) | 60.9 | 14.5 | 10 | 32.6 | 21.1 | 10 | 28.30 [12.43, 44.17] |
| External rotation (degrees) at 8 weeks (unclear if active or passive) | 62.1 | 11.5 | 10 | 32.6 | 21.7 | 10 | 29.50 [14.28, 44.72] |
| Hand behind back distance (cm) at 2 weeks (unclear if active or passive) | 7.2 | 6.1 | 10 | 22.2 | 11.5 | 10 | ‐15.00 [‐23.07, ‐6.93] |
| Hand behind back distance (cm) at 4 weeks (unclear if active or passive) | 7.6 | 5.7 | 10 | 18.5 | 8.9 | 10 | ‐10.90 [‐17.45, ‐4.35] |
| Hand behind back distance (cm) at 8 weeks (unclear if active or passive) | 6 | 7.3 | 10 | 18.3 | 7.5 | 10 | ‐12.30 [‐18.79, ‐5.81] |
See Table 17. One trial (42 participants) compared continuous short wave diathermy, hot pack and exercise to deep friction massage (Cyriax approach) and exercise for two weeks (Guler‐Uysal 2004). There was no significant difference between groups in terms of overall pain at two weeks. In contrast, those receiving continuous short wave diathermy were statistically significantly less likely to be rated as global treatment successes, had higher pain on motion, and had less passive internal and external rotation at two weeks. Differences in night pain and passive abduction and flexion at two weeks were not statistically significant.
14. Guler‐Uysal 2004: Short wave diathermy plus exercises (intervention) versus manual therapy plus exercises (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐100) at 2 weeks | 21.2 | 17.9 | 20 | 15.2 | 18.5 | 20 | 6.00 [‐5.28, 17.28] |
| Night pain (VAS 0‐100) at 2 weeks | 42 | 25.6 | 20 | 39.1 | 28.1 | 20 | 2.90 [‐13.76, 19.56] |
| Pain on motion (VAS 0‐100) at 2 weeks | 62.5 | 12.6 | 20 | 50.4 | 24.5 | 20 | 12.10 [0.03, 24.17] |
| Passive internal rotation (degrees) at 2 weeks | 56.1 | 14.7 | 20 | 66.7 | 10 | 20 | ‐10.60 [‐18.39, ‐2.81] |
| Passive external rotation (degrees) at 2 weeks | 52.8 | 24.3 | 20 | 74.4 | 14.2 | 20 | ‐21.60 [‐33.93, ‐9.27] |
| Passive abduction (degrees) at 2 weeks | 145.3 | 28.5 | 20 | 157.7 | 21.6 | 20 | ‐12.40 [‐28.07, 3.27] |
| Passive flexion (degrees) at 2 weeks | 146.4 | 22.7 | 20 | 155.5 | 14.2 | 20 | ‐9.10 [‐20.83, 2.63] |
| Events | Total | Events | Total | Risk ratio (95% CI) | |||
| Global assessment of treatment success (reaching 80% of normal ROM) at 2 weeks | 13 | 20 | 19 | 20 | 0.68 [0.49, 0.96] | ||
Overall, based on low quality evidence from two small trials, continuous short wave diathermy may not be more effective than hot packs or deep friction massage.
Multiple electrotherapy modalities
See Table 18 and Table 19. One trial (70 participants) compared a combination of therapeutic ultrasound, TENS, hot pack and home exercises to (1) sodium hyaluronate injection plus home exercises, and (2) glucocorticoid injection plus home exercises for two weeks (Calis 2006). Given the inability to blind participants and personnel, the trial had a high risk of performance bias and detection bias for self‐reported outcomes. In the multiple electrotherapies group, functional ability scores (100 point scale) were significantly higher (that is better) than the sodium hyaluronate injection group at two weeks but not at three months, and were not significantly different to the glucocorticoid injection group at either time point. However, the 95% CIs for the significant differences included non‐clinically important differences as possible estimates of effect. In addition, the multiple electrotherapies group had statistically significantly greater passive abduction and external rotation than the sodium hyaluronate injection group but, compared to glucocorticoid injection, only passive external rotation was greater in the multiple electrotherapies group. Overall, based on the very low quality evidence, we are uncertain whether a combination of therapeutic ultrasound, TENS and hot pack is more or less effective than sodium hyaluronate injection or glucocorticoid injection.
15. Calis 2006: Therapeutic ultrasound plus TENS plus hot pack plus home exercises (intervention) versus sodium hyaluronate injection plus home exercises (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant score 0‐100) at 2 weeks | 70.2 | 11.6 | 21 | 58.4 | 11 | 24 | 11.80 [5.17, 18.43] |
| Function (Constant score 0‐100) at 3 months | 76.1 | 10.7 | 21 | 70.1 | 10.3 | 24 | 6.00 [‐0.16, 12.16] |
| Passive abduction (degrees) at 2 weeks | 145.4 | 19.2 | 21 | 127.2 | 19 | 24 | 18.20 [7.01, 29.39] |
| Passive abduction (degrees) at 3 months | 158.4 | 18.3 | 21 | 145.9 | 21 | 24 | 12.50 [1.02, 23.98] |
| Passive external rotation (degrees) at 2 weeks | 63.8 | 11.7 | 21 | 52.9 | 10.7 | 24 | 10.90 [4.31, 17.49] |
| Passive external rotation (degrees) at 3 months | 73.8 | 10.4 | 21 | 63.3 | 11.4 | 24 | 10.50 [4.13, 16.87] |
16. Calis 2006: Therapeutic ultrasound plus TENS plus hot pack plus home exercises (intervention) versus glucocorticoid injection plus home exercises (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Function (Constant score 0‐100) at 2 weeks | 70.2 | 11.6 | 21 | 66.5 | 11.6 | 25 | 3.70 [‐3.03, 10.43] |
| Function (Constant score 0‐100) at 3 months | 76.1 | 10.7 | 21 | 70.3 | 9.9 | 25 | 5.80 [‐0.20, 11.80] |
| Passive abduction (degrees) at 2 weeks | 145.4 | 19.2 | 21 | 135.1 | 23.4 | 25 | 10.30 [‐2.01, 22.61] |
| Passive abduction (degrees) at 3 months | 158.4 | 18.3 | 21 | 150.3 | 19.6 | 25 | 8.10 [‐2.87, 19.07] |
| Passive external rotation (degrees) at 2 weeks | 63.8 | 11.7 | 21 | 54.8 | 10.5 | 25 | 9.00 [2.52, 15.48] |
| Passive external rotation (degrees) at 3 months | 73.8 | 10.4 | 21 | 63 | 10.8 | 25 | 10.80 [4.66, 16.94] |
4) Is one type of electrotherapy modality more effective than another?
Two trials compared one type of electrotherapy modality to another (Bumin 2001; Dewan 2011).
See Table 20. One trial (30 participants) compared Iodex iontophoresis plus continuous short wave diathermy plus exercise to Iodex phonophoresis plus exercise (Bumin 2001). It was unclear whether participants would be able to tell the difference between the electrotherapy modalities, and the risk of selection bias was unclear. The trialists found that participants receiving Iodex iontophoresis plus continuous short wave diathermy plus exercise did not have statistically significantly lower overall pain at the end of 10 treatment sessions compared to the Iodex phonophoresis and exercise group (though the time point was unclear as the trialists did not report how many sessions were delivered per week). Based on very low quality evidence, we are uncertain whether Iodex iontophoresis plus continuous short wave diathermy is more or less effective than Iodex phonophoresis (when delivered with exercise).
17. Bumin 2001: Iontophoresis plus short wave diathermy plus exercise (intervention) versus phonophoresis plus exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at the end of 10 sessions | 2.4 | 1.59 | 15 | 2.6 | 1.3 | 15 | ‐0.20 [‐1.24, 0.84] |
See Table 21. One trial (50 participants) compared interferential current to TENS for four weeks (Dewan 2011). The sample size on which each analysis was based was unclear, so no effect sizes were estimable.
18. Dewan 2011: Interferential current (intervention) versus TENS (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95% CI) | |
| Overall pain (VAS 0‐10) at 4 weeks | 2.15 | 0.75 | ? | 5.1 | 0.85 | ? | Not estimable (sample size unknown) |
| Range of flexion (degrees) at 4 weeks (unclear if active or passive) | 148.5 | 12.99 | ? | 99 | 18.04 | ? | Not estimable (sample size unknown) |
| Range of abduction (degrees) at 4 weeks (unclear if active or passive) | 154 | 14.29 | ? | 104 | 16.35 | ? | Not estimable (sample size unknown) |
| Range of external rotation (degrees) at 4 weeks (unclear if active or passive) | 65.5 | 8.09 | ? | 34 | 12.42 | ? | Not estimable (sample size unknown) |
One trial (64 participants) compared a polarity exchangeable permanent magnet to a non‐polarity exchangeable permanent magnet for 24 hours (Kanai 2006). No effect estimates were reported in a format that was suitable for extraction and analysis.
5) Is a combination of an electrotherapy modality with manual therapy or exercise (or both) effective compared to placebo, no intervention or another active intervention?
No trial compared a combination of an electrotherapy modality with manual therapy or exercise (or both) to placebo or no treatment. Four trials compared a combination of an electrotherapy modality with manual therapy or exercise (or both) to another active intervention (Carette 2003; Ghosh 2012; Maryam 2012; Ryans 2005). All trials had a high risk of performance and detection bias for the self‐reported outcomes. The outcome data for these trials are presented in the companion review of manual therapy and exercise for adhesive capsulitis (Page 2014). Overall, based on low quality evidence from these four trials, we are uncertain whether a combination of an electrotherapy modality with manual therapy or exercise (or both) is more or less effective than glucocorticoid injection, placebo injection or manipulation under anaesthesia.
6) Is a combination of an electrotherapy modality with manual therapy or exercise (or both) and another active intervention more effective than the other active intervention alone?
Four trials compared a combination of an electrotherapy modality with manual therapy or exercise (or both) and another active intervention to the other active intervention alone (Carette 2003; Maryam 2012; Pajareya 2004; Ryans 2005). All trials had a high risk of performance and detection bias for the self‐reported outcomes. The outcome data for these trials are presented in the companion review of manual therapy and exercise for adhesive capsulitis (Page 2014). Overall, based on low quality evidence from these four trials, we are uncertain whether a combination of an electrotherapy modality with manual therapy or exercise (or both) is an effective adjunct to glucocorticoid injection or oral NSAIDs.
7) Is a combination of an electrotherapy modality with manual therapy or exercise (or both) and another active intervention more effective than placebo or no treatment?
Two trials compared a combination of an electrotherapy modality with manual therapy, exercise and glucocorticoid injection to placebo injection (Carette 2003; Ryans 2005). Both trials had a high risk of performance bias and detection bias for the self‐reported outcomes. The outcome data for these trials are presented in the companion review of manual therapy and exercise for adhesive capsulitis (Page 2014). Overall, based on low quality evidence from these two trials, the multi‐component intervention may be more effective than placebo injection at six weeks, but not at six or 12 months.
Sensitivity analyses and assessment of publication bias
Due to the inability to conduct any meta‐analyses, we did not undertake any of our planned sensitivity analyses or formal investigations of publication bias (that is using funnel plots).
Discussion
Summary of main results
Overall, based on the results of 19 trials involving 1249 participants, there is limited evidence from which to draw firm conclusions about the efficacy or safety of several electrotherapy modalities, delivered either in isolation, with manual therapy or exercise, or with manual therapy, exercise and another active intervention (for example glucocorticoid injection), in terms of patient‐relevant outcomes such as pain, function, global assessment of treatment success, active shoulder abduction and quality of life. Only five trials measured adverse events, with one reporting statistically non‐significant differences between groups (Pajareya 2004) and four reporting no adverse events in any group (Leclaire 1991; Rigato 2002; Stergioulas 2008; Taverna 1990).
The two main questions of the review, which focus on whether electrotherapy modalities are (1) effective compared to placebo or no treatment, or (2) an effective adjunct to manual therapy or exercise (or both), were investigated in nine trials (Battisti 2007; Bumin 2001; Calis 2006; Dogru 2008; Leclaire 1991; Leung 2008; Rigato 2002; Stergioulas 2008; Taverna 1990). The overall impression from these trials is that only one electrotherapy modality, LLLT, has evidence of benefit when compared to placebo or when used as an adjunct to exercise. Low quality evidence from one trial suggests that LLLT was more effective than placebo in terms of global assessment of treatment success at the end of six days of treatment (Taverna 1990). Moderate quality evidence from another trial suggests that LLLT plus exercise was more effective than placebo plus exercise in terms of overall pain reduction and active abduction at four weeks and improved function at four weeks and four months (Stergioulas 2008). Very low quality evidence from another trial suggests that PEMF was more effective than placebo in terms of participant‐reported pain relief of 30% or greater and function at two weeks, but the 95% CIs were very wide leading to uncertainty in this result. Based on single trials, it is unclear whether therapeutic ultrasound (Dogru 2008), PEMF (Leclaire 1991), Iodex phonophoresis (Bumin 2001), continuous short wave diathermy (Leung 2008), a combination of Iodex iontophoresis with continuous short wave diathermy (Bumin 2001) or a combination of therapeutic ultrasound with TENS (Calis 2006) are an effective adjunct to exercise.
Regarding the other questions of the review, the majority of the differences between groups were not statistically or clinically significant. Any statistically significant differences (favouring either the electrotherapy or other intervention group) that were detected in these trials are likely to be exaggerated due to the high risk of performance and detection bias resulting from non‐blinding of participants and personnel.
Overall completeness and applicability of evidence
The diagnostic criteria for (or definitions of) adhesive capsulitis varied across the trials in regards to the type, amount and direction of shoulder restriction (as has been found in previous reviews, for example Green 1998; Schellingerhout 2008). Despite this variation in diagnosis, the study populations in all trials appeared to be representative of patients seen in routine care, and the age, gender ratio and symptom duration were similar across trials. Also, trials were conducted in a range of high and low‐middle income countries. The median duration of electrotherapy was four weeks (range 1 to 12), with a median of three treatment sessions delivered per week (range 1 to 15), though this differed by type of modality (see Table 4). Several trials did not report important components of the electrotherapy modality, such as the frequency and intensity of the intervention and the duration of the session, which makes it difficult to draw implications for clinical practice from these trials. For example, the trial comparing LLLT to placebo (Taverna 1990) reported the power of the laser (24 mW) but not the wavelength or device used (for example Gallium‐Arsenide (GaAs) or Galium‐Aluminum‐Arsenide (GaAlAs)).
There are several comparisons that are relevant to clinical practice which have not yet been undertaken in this field. Only two electrotherapy modalities (LLLT and PEMF) have been compared to placebo. No trial has compared an electrotherapy modality plus manual therapy to manual therapy alone. No trial has compared an electrotherapy modality plus manual therapy and exercise to manual therapy and exercise alone. The only modality with evidence of benefit when compared to placebo (that is LLLT) has not been compared to any active intervention with evidence of benefit, for example glucocorticoid injection or arthrographic joint distension (Buchbinder 2008; Favejee 2011). No trial has compared any electrotherapy modality to arthrographic joint distension, oral steroids or NSAIDs. Few trials have compared different electrotherapy modalities to one another, and no trial has compared different variants of the same modality (for example LLLT at one dosage versus another dosage). It is unclear whether factors such as dosage, wavelength, site and duration of treatment impact on the effect of specific electrotherapy modalities for adhesive capsulitis.
There was considerable variation in the outcomes measured across the included trials. Only two trials (11%) measured pain using a dichotomous measure, as recommended by IMMPACT (Dworkin 2008). The proportion of trials measuring other main outcomes of the review were overall pain (mean or mean change) (74%), function (68%), global assessment of treatment success (21%), active shoulder abduction (21%), quality of life (16%) and adverse events (26%). Development of a core set of outcomes for trials of adhesive capsulitis and other shoulder disorders would improve our ability to synthesise the evidence.
Quality of the evidence
We used the GRADE approach to assess the quality of all included trials (Schünemann 2011b). Most trials were downgraded to low or very low quality based on three factors: (1) the risk of selection bias was unclear because trialists did not report whether the allocation sequence was concealed, (2) the risk of performance and detection bias was high for self‐reported outcomes because participants were not blinded, and (3) the 95% CIs of the effect estimates were imprecise (due to small sample sizes). Trials with unclear allocation concealment have been found to overestimate treatment effects by 7% (ratio of odds ratios 0.93, 95% credible interval 0.87 to 0.99), and unblinded assessment of self‐reported outcomes (such as pain and function) is estimated to exaggerate the treatment benefit by about 22% (ratio of odds ratios 0.78, 95% credible interval 0.65 to 0.92) (Savovic 2012). Thus, given that most trials included in our review had unclear allocation concealment and unblinded assessment of self‐reported outcomes, further high quality trials may show even smaller effect estimates than those summarised in this review. Only one trial was not downgraded to low or very low quality, Stergioulas 2008 was downgraded to moderate quality due to imprecision.
Potential biases in the review process
Upon completion of a thorough search of all major databases with no language restrictions, it is likely that all relevant trials were identified. Two review authors independently assessed the trials for inclusion in the review, extracted data and assessed the risk of bias, and a third review author adjudicated whenever there was any discrepancy. Defining of review comparisons of interest was conducted with full knowledge of all comparisons undertaken within the trials but no knowledge of the results. We used a priori defined decision rules to select data from trials when multiple measurement scales, time points and analyses were reported to prevent selective inclusion of results (Page 2013a).
Agreements and disagreements with other studies or reviews
Our companion review of manual therapy and exercise for adhesive capsulitis reached the conclusion that the effects of physical therapy interventions for adhesive capsulitis are uncertain (Page 2014). Based on 32 clinically heterogeneous trials, the companion review found that a combination of manual therapy and exercise may not be as effective as glucocorticoid injection at seven weeks. However, it is unclear whether (a) a combination of manual therapy, exercise and electrotherapy is an effective adjunct to glucocorticoid injection or oral NSAIDs, (b) manual therapy or exercise are effective compared to other active interventions when not delivered together, and (c) one type of manual therapy or exercise is more effective than another.
We are aware of two other relevant systematic reviews of interventions for adhesive capsulitis published within the last five years (Favejee 2011; Maund 2012). Both reviews examined a range of conservative and surgical interventions. Of the 14 trials included in our review that investigated the primary or adjunct effect of an electrotherapy modality (that is trials not falling under questions five to seven), Favejee 2011 included seven (Calis 2006; Cheing 2008; Guler‐Uysal 2004; Kanai 2006; Lee 1973; Stergioulas 2008; Taverna 1990) and Maund 2012 included four (Calis 2006; Dogru 2008; Leung 2008; Stergioulas 2008). Despite including more trials, we reached a similar conclusion to both reviews, that there is no or only limited evidence to determine the effectiveness of a range of electrotherapy modalities.
Authors' conclusions
Implications for practice.
Of the various electrotherapy modalities, only LLLT and PEMF have been compared to placebo in randomised controlled trials. Also, there are no trials that have compared an electrotherapy modality plus manual therapy to manual therapy alone, or an electrotherapy modality plus manual therapy and exercise to manual therapy and exercise alone. Based on the best currently available data, LLLT may be more effective than placebo in terms of global treatment success at six days; and may be an effective adjunct to exercise in terms of pain up to four weeks, and function up to four months, although its long‐term effect has not been investigated. It is unclear whether PEMF is more or less effective than placebo. It is unclear whether therapeutic ultrasound, PEMF, Iodex phonophoresis, continuous short wave diathermy, a combination of Iodex iontophoresis with continuous short wave diathermy, or a combination of therapeutic ultrasound with TENS are effective adjuncts to exercise.
Implications for research.
Further high quality randomised controlled trials are needed to establish the benefits and harms of physical therapy interventions (that comprise electrotherapy modalities, manual therapy and exercise, and are reflective of clinical practice) for adhesive capsulitis. In particular, future trials should compare a combination of LLLT, manual therapy and exercise to interventions with evidence of benefit (for example glucocorticoid injection or arthrographic joint distension). Adhesive capsulitis can last for several years, although most of the previous trials have only assessed outcomes during treatment or in the weeks following treatment cessation. Assessment of longer‐term outcomes, for example up to six to 12 months, would be worthwhile in future trials. Trials could also explore the impact of factors such as dosage, wavelength, site and duration of treatment on the effect of electrotherapy modalities (particularly LLLT). Trials should include strategies designed to minimise the potential for bias, including adequate allocation concealment and blinding of participants and outcome assessors. Development of a core set of outcomes for trials of adhesive capsulitis and other shoulder disorders would enhance this endeavour and improve our ability to synthesise the evidence.
History
Review first published: Issue 10, 2014
| Date | Event | Description |
|---|---|---|
| 1 May 2008 | Amended | Converted to RM5. CMSG ID C067‐R |
| 24 February 2003 | Amended | This review is based on the original review of 'Interventions for shoulder pain'. Please see published notes for further details. |
| 24 February 2003 | New citation required and conclusions have changed | Substantive amendment |
Notes
The original review, 'Physiotherapy interventions for shoulder pain' was split into four reviews upon updating: 'Manual therapy and exercise for adhesive capsulitis (frozen shoulder)', 'Electrotherapy modalities for adhesive capsulitis (frozen shoulder)', 'Manual therapy and exercise for rotator cuff disorders'. and 'Electrotherapy modalities for rotator cuff disorders'. The review has also been broadened by including all randomised and quasi‐randomised clinical trials regardless of whether outcome assessment was blinded.
Acknowledgements
We thank Steve McDonald from the Australasian Cochrane Centre for his help with the search strategy. We thank Sibel Basaran, Simon Carette, Mobini Maryam, Kingkaew Pajareya, Ian Ryans and Apostolos Stergioulas for providing us with unpublished trial data.
Appendices
Appendix 1. Search strategies
Search strategy for CENTRAL:
MeSH descriptor: [Shoulder Pain] explode all trees
MeSH descriptor: [Shoulder Impingement Syndrome] explode all trees
MeSH descriptor: [Rotator Cuff] explode all trees
MeSH descriptor: [Bursitis] explode all trees
((shoulder* in All Text or rotator* in All Text) and (bursitis in All Text or frozen in All Text or impinge* in All Text or tendonitis in All Text or tendonitis in All Text or tendinopathy in All Text or pain* in All Text))
"rotator cuff" in All Text
"adhesive capsulitis" in All Text
#1 or #2 or #3 or #4 or #5 or #6 or #7
MeSH descriptor: [Rehabilitation] explode all trees
MeSH descriptor: [Physical Therapy Modalities] explode all trees
MeSH descriptor: [Exercise Movement Techniques] explode all trees
MeSH descriptor: [Ultrasonography, Interventional] explode all trees
rehabilitat* in All Text or physiotherapy* in All Text or "physical therap*" in All Text or "manual therap*" in All Text or exercis* in All Text
(ultrasound in All Text or ultrasonograph* in All Text or tns in All Text or tens in All Text or shockwave in All Text or electrotherap* in All Text or mobili* in All Text)
#9 or #10 or #11 or #12 or #13 or #14
#8 and #15
Search strategy for MEDLINE:
shoulder pain/
shoulder impingement syndrome/
rotator cuff/
exp bursitis/
((shoulder$ or rotator cuff) adj5 (bursitis or frozen or impinge$ or tendinitis or tendonitis or tendinopathy or pain$)).mp.
rotator cuff.mp.
adhesive capsulitis.mp.
or/1‐7
exp rehabilitation/
exp physical therapy techniques/
exp musculoskeletal manipulations/
exp exercise movement techniques/
exp ultrasonography, interventional/
(rehabilitat$ or physiotherap$ or physical therap$ or manual therap$ or exercis$ or ultrasound or ultrasonograph$ or TNS or TENS or shockwave or electrotherap$ or mobili$). mp.
or/9‐14
clinical trial.pt
random$.mp.
((single or double) adj (blind$ or mask$)).mp.
placebo$.mp.
or/16‐19
8 and 15 and 20
Search strategy for EMBASE:
‘shoulder pain’/exp
‘shoulder impingement syndrome’/exp
‘rotator cuff’/exp
‘bursitis’/exp
((shoulder* OR rotator*) AND (‘bursitis’/de OR frozen OR impinge* OR ‘tendonitis’/de OR ‘tendinitis’/de OR ‘tendinopathy’/de OR pain*))
‘rotator cuff’
‘adhesive capsulitis’
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
‘rehabilitation’/exp
‘physiotherapy’/exp
‘kinesiotherapy’/exp
‘endoscopic echography’/exp
rehabilitat* OR physiotherapy* OR ‘physical therapy’ OR ‘manual therapy’ OR kinesiotherap* OR exercis*
‘ultrasound’/de OR ultrasonograph* OR ‘transcutaneous nerve stimulation’ OR ‘transcutaneous electrical nerve stimulation’ OR shockwave OR electrotherap* OR mobili*
#9 OR #10 OR #11 OR #12 OR #13 OR #14
‘randomized controlled trial’/exp
#8 AND #15 AND #16
Search strategy for CINAHL Plus:
S1 MH “shoulder pain”
S2 MH “shoulder impingement syndrome”
S3 MH “rotator cuff”
S4 MH bursitis+
S5 TX (shoulder* N5 bursitis) or TX(shoulder* N5 frozen) or TX(shoulder* N5 impinge*) or TX(shoulder* N5 tend?nitis) or TX(shoulder* N5 tendinopathy) or TX(shoulder* N5 pain*)
S6 TX (rotator cuff N5 bursitis) or TX(rotator cuff N5 frozen) or TX(rotator cuff N5 impinge*) or TX(rotator cuff N5 tend?nitis) or TX(rotator cuff N5 tendinopathy) or TX(rotator cuff N5 pain*)
S7 TX rotator cuff
S8 TX adhesive capsulitis
S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8
S10 MH Rehabilitation+
S11 MH physical therapy+
S12 MH Manual Therapy+
S13 MH Therapeutic Exercise+
S14 MHUltrasonography+
S15 TX rehabilitat* or physiotherapy* or physical therap* or manual therap* or exercise* or ultrasound or ultrasonograph* or TNS or TENS or shockwave or electrotherapy* or mobili*
S16 S10 or S11 or S12 or S13 or S14 or S15
S17 PT clinical trial
S18 TX random*
S19 TX(single blind*) or TX(single mask*)
S20 TX(double blind*) or TX(double mask*)
S21 placebo*
S22 S17 or S18 or S19 or S20 or S21
S23 S9 and S16 and S22
Data and analyses
Comparison 1. Electrotherapy modality plus manual therapy or exercise (or both) versus manual therapy or exercise (or both).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall pain | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 LLLT plus exercise versus placebo plus exercise; VAS 0‐100 at 4 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 LLLT plus exercise versus placebo plus exercise; VAS 0‐100 at 4 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Ultrasound plus hot pack plus exercise versus placebo plus hot pack plus exercise; SPADI 0‐100 at 2 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Ultrasound plus hot pack plus exercise versus placebo plus hot pack plus exercise; SPADI 0‐100 at 3 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Phonophoresis plus exercise versus placebo plus exercise; VAS 0‐10 at end of 10 sessions | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Iontophoresis plus continuous short wave diathermy plus exercise versus placebo plus exercise; VAS 0‐10 at end of 10 sessions | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Function | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 LLLT plus exercise versus placebo plus exercise; SPADI 0‐100 at 4 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 LLLT plus exercise versus placebo plus exercise; SPADI 0‐100 at 4 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Ultrasound plus hot pack plus exercise versus placebo plus hot pack plus exercise; SPADI 0‐100 at 2 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Ultrasound plus hot pack plus exercise versus placebo plus hot pack plus exercise; SPADI 0‐100 at 3 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Continuous short wave diathermy plus exercise versus exercise; Shoulder Score Index 0‐100 at 2 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Continuous short wave diathermy plus exercise versus exercise; Shoulder Score Index 0‐100 at 4 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Continuous short wave diathermy plus exercise versus exercise; Shoulder Score Index 0‐100 at 8 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 TENS plus ultrasound plus hot pack plus exercise versus exercise; Constant score 0‐100 at 2 weeks | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 TENS plus ultrasound plus hot pack plus exercise versus exercise; Constant score 0‐100 at 3 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
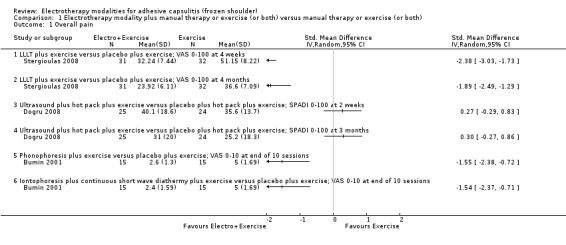
Comparison 1 Electrotherapy modality plus manual therapy or exercise (or both) versus manual therapy or exercise (or both), Outcome 1 Overall pain.
1.2. Analysis.
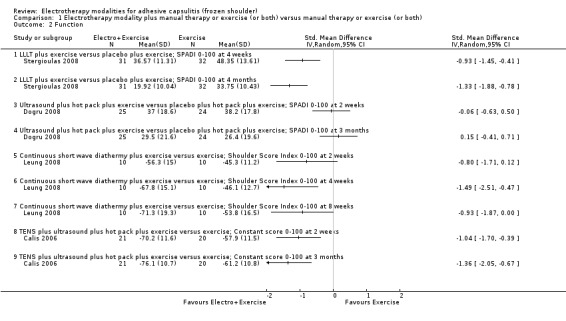
Comparison 1 Electrotherapy modality plus manual therapy or exercise (or both) versus manual therapy or exercise (or both), Outcome 2 Function.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battisti 2007.
| Methods |
Design: Parallel group, three‐arm, single‐blind randomised controlled trial (Italy) Interventions: Low‐frequency (100 Hz) pulsed electromagnetic field therapy (PEMF) or Therapeutic Application of a Musically Modulated Electromagnetic Field (TAMMEF) or simulated (placebo) electromagnetic field, each while listening to music Sample size calculation: Not reported Analysis: Per protocol analysis Source of funding: Fondazione Monte dei Paschi di Siena (non‐industry) |
|
| Participants |
Number of participants: 60 (20 per group) Baseline characteristics: Basline characteristics by group were not reported Mean (SD; range) age = 47.6 (7.3; 37‐66) years; Male:Female = 32:28 Mean (SD) duration of symptoms = 1.4 (1.9) months Inclusion criteria: 1. Affected by shoulder periarthritis for less than three months 2. Stopped taking analgesic anti‐inflammatory drugs 15 days prior to electromagnetic therapy 3. Had never had infiltrative steroid therapy Exclusion criteria: Not reported |
|
| Interventions |
Low‐frequency (100 Hz) pulsed electromagnetic field therapy while listening to music (N=20) Components of intervention: Extremely low‐frequency (100 Hz) electromagnetic field therapy was delivered by applying magnets to the shoulder while the participant listened to music Dosage: 30 minutes Frequency of administration: Daily for 15 days (15 sessions) Provider: Physicist Therapeutic Application of a Musically Modulated Electromagnetic Field (TAMMEF) while listening to music (N=20)* Components of intervention: TAMMEF was delivered by applying magnets to the shoulder while the participant listened to music. The electromagnetic field parameters (frequency, intensity, waveform) were modified in time, randomly varying within the respective ranges, so that all the possible codes can occur during a single application Dosage: 30 minutes Frequency of administration: Daily for 15 days (15 sessions) Provider: Physicist Simulated (placebo) electromagnetic field while listening to music (N=20) Components of intervention: A simulated (placebo) electromagnetic field was delivered by applying magnets to the shoulder while the participant listened to music Dosage: 30 minutes Frequency of administration: Daily for 15 days (15 sessions) Provider: Physicist |
|
| Outcomes | Outcomes measured at baseline, day 7, day 15 (end of treatment), and day 45 (30 days post‐treatment cessation). No primary outcome was specified by the trialists 1. Shoulder pain and disability index (SPADI) (0‐100 scale where a higher score indicates worse pain and/or disability) 2. Joint function, rated as 0 = absence of functional limitation; 1 = slight limitation; 2 = moderate limitation; 3 = severe limitation |
|
| Notes | *This intervention is not a standard type of pulsed electromagnetic field therapy that can be applied by physical therapists, so no data for this group was included in the review. Article is in Italian. MP used Google Translate to translate into English. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We examined 60 subjects, aged between 37 and 66 years, 28 women and 32 men, suffering from painful shoulder easier to less than 3 months, who were randomly divided into three groups: A = 20 patients undergoing TAMMEF, B = 20 patients undergoing ELF and C = 20 patients undergoing simulated field, listening to music" (Google Translate translation of Italian article) Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "This study was conducted in a blinded fashion" (Google Translate translation of Italian article) Comment: The trialists did not specify who was blind to treatment in this study (participants, personnel, or outcome assessors, or more than one of these parties), but given the nature of the interventions, it is likely that participants were blinded |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | "This study was conducted in a blinded fashion" (Google Translate translation of Italian article) Comment: Participants self‐reported pain and disability, and were probably blind to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | "This study was conducted in a blinded fashion" (Google Translate translation of Italian article) Comment: The trialists did not specify who was blind to treatment in this study (participants, personnel, or outcome assessors, or more than one of these parties), and while participants were likely to have been blinded, it is unclear whether assessors of joint function were |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All patients in groups A and B have completed the course of therapy, without the occurrence of noteworthy local or systemic effects that would require the suspension of such treatment" (Google Translate translation of Italian article) Quote: "After the first week of treatment, 8 patients (40%) in group C had to stop treatment because of ineffective applications. The remaining 12 patients (60%) completed the cycle in the manner already described" (Google Translate translation of Italian article) Comment: A high proportion of the placebo group withdrew due to lack of response to treatment, which is likely to bias the results of the study in favour of the two active treatment groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: Data for most outcomes listed in the methods section were present in the results section of the report (except for improvement at day 45, in which data was not reported for the simulated electromagnetic field therapy group). Also, without a trial protocol it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Bumin 2001.
| Methods |
Design: Parallel group, three‐arm randomised controlled trial (Turkey) Interventions: Iodex iontophoresis plus continuous short wave diathermy plus exercise or iodex phonophoresis plus exercise or placebo ultrasound plus exercise Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 45 (15 per group) Baseline characteristics: Duration of symptoms was not reported Iodex iontophoresis, continuous short wave diathermy and exercise group: Mean (SD) age = 53.67 (3.03) years; Male:Female = 6:9 Iodex phonophoresis and exercise group: Mean (SD) age = 51.8 (3.86) years; Male:Female = 7:8 Placebo ultrasound and exercise group: Mean (SD) age = 50.93 (3.87) years; Male:Female = 10:5 Inclusion criteria: 1. Diagnosis of shoulder periarthritis Exclusion criteria: Not reported |
|
| Interventions | Ten sessions of exercises (not specified) were done by all groups Iodex iontophoresis plus continuous short wave diathermy (N=15) Components of intervention: The pomade used was a mixture of 4.8% methyl salicylate and 4.7% iodine. In order to increase ion penetration, continuous short wave diathermy application with three dosages was applied for 20 minutes following the iontophoresis application. Direct current with a maximum intensity of 2 mA was applied for 20 minutes Dosage: 20 minutes Frequency of administration: 10 sessions (number of sessions per week not reported) Provider: Physical therapist Iodex phonophoresis (N=15) Components of intervention: Before application, iodex pomade was applied to the area and then direct ultrasound was applied with a 1.5 watt/cm2 dosage for five minutes Dosage: 5 minutes Frequency of administration: 10 sessions (number of sessions per week not reported) Provider: Physical therapist Placebo ultrasound (N=15) Components of intervention: Placebo ultrasound application Dosage: 5 minutes Frequency of administration: 10 sessions (number of sessions per week not reported) Provider: Physical therapist |
|
| Outcomes | Outcome assessed before and at the end of 10 sessions of treatment. No primary outcome was reported by the trialists 1. Pain measured using a visual analogue scale (10 point scale from 0 = no pain to 10 = severe pain) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Forty five cases who had shoulder periarthritis were randomly divided into three equal groups (n = 15)." Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different electrotherapy modalities, but it is unclear whether they were provided any information that would make them perceive the type of electrotherapy they received as superior or inferior to the alternative type of electrotherapy |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain, but it is unclear whether they were provided any information that would make them perceive the electrotherapy they received as superior or inferior to the alternative type of electrotherapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No drop‐outs or losses to follow‐up were reported, and the analysis is reported as being based on the total number of participants randomised |
| Selective reporting (reporting bias) | Unclear risk | Comment: Pain was the only reported outcome. Without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Calis 2006.
| Methods |
Design: Parallel group, four‐arm randomised controlled trial (Turkey) Interventions: Electrotherapy modalities (ultrasound, transcutaneous electrical nerve stimulation) plus hot pack plus exercises or sodium hyaluronate injection plus exercises or triamsinolone acetonide injection plus exercises or exercises alone Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 90 (21, 24, 25 and 20 in each respective group) Baseline characteristics: Duration of symptoms was not reported Electrotherapy modalities (ultrasound, TENS) plus hot pack group: Mean (SD) age = 52.33 (10.1) years; Male:Female = 8:13 Sodium hyaluronate injection group: Mean (SD) age = 59.7 (9.81) years; Male:Female = 10:14 Triamsinolone acetonide injection group: Mean (SD) age = 56.36 (11.3) years; Male:Female = 9:16 Stretching and Codman exercises alone group: Mean (SD) age = 59.25 (6.8) years; Male:Female = 6:14 Inclusion criteria: 1. At least a one‐month history of pain 2. Limited active and passive shoulder movement 3. Decreased passive range of motion of 20% or more, in at least three movements, according to the American Medical Association guide for the evaluation of permanent impairment 4. No previous injection in the involved shoulder 5. No history of allergy to local anaesthetics, steroids or sodium hyaluronate 6. Absence of coagulation disease 7. Absence of cervical radiculopathy, fracture, dislocation, and rotator cuff laceration 8. Absence of hematological, infectious, endocrine, neurological, and malignant disease, severe osteoporosis, cardiovascular disease, hepatic, and renal disorders 9. Subacromial impingement injection test negativity Exclusion criteria: See inclusion criteria |
|
| Interventions | All groups were recommended stretching and Codman exercises to do at home for two weeks Electrotherapy modalities (ultrasound, TENS) plus hot pack group (N=21) Components of intervention: ‐ Electrotherapy: Ultrasonic therapy at 1.5 W/cm2, and TENS at the patient’s tolerance ‐ Other: hot pack Dosage: ‐ Electrotherapy: Ultrasonic therapy for five minutes; TENS for 20 minutes ‐ Hot pack: 20 minutes Frequency of administration: Daily for 10 days (10 sessions) Provider: Physiatrist Sodium hyaluronate injection (N=24) Components of intervention: Sodium hyaluronate 30 mg (Orthovisc 30 mg) was injected into the shoulder joint by the posterior approach. The injection was done with a 22‐gauge needle as follows: while the participant was sitting, the index finger of the operator's free hand was placed on the tip of the coracoid process with the thumb at the angle of the acromion and the spine of the scapula. The needle punctured the skin near operator's thumb and was aimed just laterally to the tip of the index finger Dosage: N/A Frequency of administration: Once a week for two weeks Provider: Rheumatologist Triamsinolone acetonide injection (N=25) Components of intervention: A 40 mg dose of triamsinolone asetonide (Kenakort‐A) was injected into the shoulder joint by the posterior approach. The injection was done with a 22‐gauge needle as follows: while the participant was sitting, the index finger of the operator's free hand was placed on the tip of the coracoid process with the thumb at the angle of the acromion and the spine of the scapula. The needle punctured the skin near operator's thumb and was aimed just laterally to the tip of the index finger Dosage: N/A Frequency of administration: Once Provider: Rheumatologist Stretching and Codman exercises alone (N=20) |
|
| Outcomes | Outcomes assessed at baseline, day 15, and the third month after the initial visit. No primary outcome was reported by the trialists 1. Pain using a horizontal visual analogue scale (scale units not reported) 2. Passive range of motion in abduction and external rotation using a goniometry 3. Constant score (0‐100 scale where a higher score indicates better functional ability) |
|
| Notes | One participant in the electrotherapy modalities group, three in the sodium hyaluronate injection group, and one in the triamsinolone asetonide injection group had bilateral adhesive capsulitis and contributed both shoulders to the trial. The unit of analysis reported was shoulders, not participants. Trialists did not report adjusting for the bilateral involvement, or how bilateral shoulders were randomised (i.e. whether both shoulders received the same or different interventions is unclear). As a conservative estimate of the treatment effect, we entered the number of participants per group as the sample sizes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of the four treatment groups" Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported pain and some components of the Constant score |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The same specialist (MC) determined the diagnosis and treatment protocol in all patients. All the patients were evaluated by another physiatrist (SU) who was blinded to groups" Comment: The outcome assessor of range of motion was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: No dropouts or losses to follow‐up reported, and the analyses are reported as being based on the total number of randomised shoulders |
| Selective reporting (reporting bias) | Unclear risk | Comment: No numerical outcome data was reported for pain. Instead, mean endpoint values (with no measures of variation) were presented in Figure format. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Carette 2003.
| Methods |
Design: Parallel group, four‐arm, single‐blind randomised controlled trial (Canada) Interventions: Supervised physiotherapy (transcutaneous electrical nerve stimulation or ultrasound, mobilisation techniques, active ROM exercises, ice application) plus corticosteroid injection (triamcinolone hexacetonide 40 mg) or corticosteroid injection alone or supervised physiotherapy plus saline injection or saline injection alone Sample size calculation: 36 participants per group were estimated to be needed based upon detecting a clinically relevant difference of ≥10 points in the Shoulder Pain and Disability Index (SPADI) (SD≤15) at the 5% level of statistical significance with 80% power Analysis: Intention‐to‐treat analysis (analysing all participants randomised, using a last observation carried forward analysis) Source of funding: Arthritis Society of Canada (non‐industry) |
|
| Participants |
Number of participants: 93 (21, 23, 26, and 23 in each respective group) Baseline characteristics: Supervised physiotherapy plus corticosteroid injection group: Mean (SD) age = 54.9 (10.5) years; Male:Female = 7:14 Mean (SD) duration of symptoms: 22.1 (14.9) weeks Corticosteroid injection alone group: Mean (SD) age = 55.4 (10) years; Male:Female = 8:15 Mean (SD) duration of symptoms: 21.2 (11) weeks Supervised physiotherapy plus saline injection group: Mean (SD) age = 54.2 (8.3) years; Male:Female = 14:12 Mean (SD) duration of symptoms: 20.8 (11.2) weeks Saline injection alone group: Mean (SD) age = 56.5 (9.4) years; Male:Female = 9:14 Mean (SD) duration of symptoms: 20.3 (7.3) weeks Inclusion criteria: 1. Age 18 years or older 2. Had been symptomatic for <1 year (defined as the presence of shoulder pain with limitation of both active and passive movements of the glenohumeral joint of ≥25% in at least 2 directions (abduction, flexion, external rotation, internal rotation), as compared with the contralateral shoulder or with normal values 3. A total score of ≥30 on the Shoulder Pain and Disability Index (SPADI) Exclusion criteria: 1. Adhesive capsulitis was secondary to another cause, including inflammatory, degenerative, metabolic, or infectious arthritis, cerebrovascular accident, or fracture 2. Had a known blood coagulation disorder or an allergy to radiologic contrast material |
|
| Interventions | All participants were taught a 10‐minute exercise program consisting of active and auto‐assisted ROM exercises in the planes of flexion, abduction, external rotation, and internal rotation (hand behind back) to be done at home twice daily for 3 months Supervised physiotherapy plus glucocorticoid injection (N=21) Components of physiotherapy intervention: ‐ Electrotherapy: TENS (for acute adhesive capsulitis); therapeutic ultrasound (for chronic adhesive capsulitis) ‐ Manual therapy: Mobilisation techniques (not specified) ‐ Supervised exercise: Active ROM exercises (for acute adhesive capsulitis); active and auto‐assisted ROM exercises and isometric strengthening exercises (for chronic adhesive capsulitis) ‐ Other: Ice application Dosage: 1 hour overall Frequency of administration: Three times a week for four weeks (12 sessions) Provider: Physiotherapist Components of glucocorticoid injection: Under fluoroscopic guidance, a 21‐gauge needle, 2.5–3" long, was directed into the shoulder joint space. Aqueous contrast material (Omnipaque; Sanofi‐Winthrop, Markham, Ontario, Canada) was injected to confirm the correct location of the needle in the joint. This was followed by injection of 40 mg triamcinolone hexacetonide (2 ml) Glucocorticoid injection alone (N=23) The same injection method as described above was delivered Supervised physiotherapy plus placebo injection (N=26) The same injection and supervised physiotherapy methods as described above were delivered, except that isotonic saline (2 ml) was injected into the shoulder joint space Placebo injection alone (N=23) The same injection method as described above was delivered, except that isotonic saline (2 ml) was injected into the shoulder joint space |
|
| Outcomes | Outcomes assessed at 6 weeks, 3 months, 6 months and 1 year post‐randomisation Primary outcome: 1. Shoulder pain and disability index (SPADI) (0‐100 scale where a higher score indicates worse pain and/or disability) Secondary outcomes: 2. General health status measured using the SF‐36 3. Active and passive range of motion in flexion, abduction, and external rotation, assessed using a goniometer with the participant in a supine position |
|
| Notes | Trialists reported the following protocol violation: "Five patients (2 in the combination group and 1 in each of the other groups) received, in addition to their assigned injection, a glucocorticoid injection (triamcinolone hexacetonide, 20 mg) after randomization, and 1 patient in the saline group underwent rotator cuff repair 8 months after enrolment. All of these injections were prescribed by study investigators who were blinded to the original treatment assignment, and all were done under fluoroscopic guidance. The patient in the placebo group and the patient in the physiotherapy group each received the injection after the 6‐week visit; the 3 patients in the corticosteroid and combination group received it after the 3‐month or 6‐month visits". Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The assignment scheme was generated from a table of random numbers. Random assignments to the treatment groups were stratified according to study center and balanced after every 12 assignments" Comment: An adequate method to generate the allocation sequence was used |
| Allocation concealment (selection bias) | Low risk | Quote: "The opaque prenumbered envelopes containing the assignments were kept by the hospital pharmacist at each center" Comment: An adequate method to conceal the allocation sequence was probably used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The syringes containing the triamcinolone hexacetonide or saline were prepared by the hospital pharmacist and covered with aluminum foil so the radiologist administering the injections and the patient were not aware of the treatment" Comment: Participants and personnel were blind to the injection component of the intervention, but not the physiotherapy component. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Participants self‐reported their SPADI and general health scores, and were not blind to whether they had received physiotherapy or not. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Each subject was assessed by the same physiotherapist throughout the trial, with a few exceptions. The physiotherapists involved in these assessments were unaware of the treatment allocation and did not normally work in the clinics where the physiotherapy was administered" Comment: Outcome assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The primary analysis was based on an intent‐to‐treat principle, and all subjects were included in the analysis. In the case of subjects lost to followup, the data from the last available assessment were imputed to all subsequent evaluations." Quote: "Of the remaining 93 patients, 2 in the combination group, 9 in the corticosteroid group, 4 in the physiotherapy group, and 1 in the placebo group did not return for all visits." Comment: There was a higher amount of loss to follow‐up in the glucocorticoid injection group compared to the other three groups, but it is unclear if the reasons for loss to follow‐up were related to treatment received (or whether they were balanced across the groups) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Cheing 2008.
| Methods |
Design: Parallel group, three‐arm, single‐blind randomised controlled trial (Hong Kong) Interventions: Interferential current plus home exercises or electroacupuncture plus home exercises or no treatment Sample size calculation: Not reported Analysis: Per protocol analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 74 (25, 24, and 25 in each respective group) Baseline characteristics: Sex of participants was reported as 22 males and 48 females. Age range for all participants was reported as 33‐90 years Interferential current plus home exercises group: Mean (SD) duration of treatment = 6.7 (6.05) months Electroacupuncture plus home exercises group: Mean (SD) duration of treatment = 6.71 (6.5) months No treatment group: Mean (SD) duration of treatment = 8.26 (7.94) months Inclusion criteria: 1. Patients who reported localized pain over one shoulder, experienced night pain and had restricted active and passive shoulder motions Exclusion criteria: 1. History of trauma, fractures, previous shoulder surgery, cervical or thoracic pain syndrome, complex regional pain syndrome, malignancies, on anti‐coagulant therapy 2. Had received acupuncture treatment to the painful shoulder in the past six months |
|
| Interventions |
Interferential current plus home exercises (N=25) Components of intervention: ‐ Interferential current: An interferential electrotherapy machine (a Phyaction Guidance E unit) delivered a current swept from 80 to 120 Hz, and 4 suction‐type electrodes were placed around the shoulder region in a coplanar arrangement. The intensity of the stimulation was adjusted to just below the pain threshold and the stimulation lasted for 20 minutes ‐ Home exercises: Participants were instructed to follow a chart and perform a standard set of shoulder mobilisation exercises five times a day, which included four directions: (i) forward flexion – with the help of an overhead pulley system; (ii) external rotation – keeping the arm close to trunk, using a small bamboo to externally rotate the shoulder through pushing against the palm; (iii) horizontal adduction – pressing a horizontally adducted arm against the chest with the other arm to achieve horizontal adduction; and (iv) internal rotation – placing the affected arm behind the back and grasping one end of a towel, the other hand then pulling the opposite end of the towel to achieve maximum internal rotation Dosage: ‐ Interferential current: 20 minutes ‐ Home exercises: Not reported Frequency of administration: ‐ Interferential current: 10 sessions over four weeks ‐ Home exercises: Five times a day for six months Provider: Physiotherapist Electroacupuncture plus home exercises (N=25) Components of intervention: ‐ Electrotheracupuncture: Sterile stainless steel acupuncture needles were inserted 15–25 mm intramuscularly into three acupoints including one trigger point, one local point (LI 15: Jianyu), and one distal point (ST38: Tiaokou) (14). Trigger points were identified by areas of greatest tenderness around the painful shoulder that were determined on an individual basis. The two needles in the shoulder region (trigger point and LI 15) were connected to an electroacupuncture device (Model: ES‐160, ITO Co. Ltd, 3‐3‐3 Tpupta, al‐M inami, Nerima‐ku, Tokyo 176‐86 05, Japan) and stimulated with an alternating frequency of 2–100 Hz at a pulse duration of 100– 400 μs for 20 minutes. The intensity of the stimulation was adjusted to a tolerance level of just below the pain threshold. The needle that was applied at the distal point S T38 (Tiaokou) was retained for 20 minutes and was manually lifted and thrusted every 10 minutes ‐ Home exercises: See above Dosage: ‐ Electroacupuncture: 40 minutes ‐ Home exercises: Not reported Frequency of administration: ‐ Electroacupuncture: Two to three times a week for four weeks (10 sessions in total) ‐ Home exercises: Five times a day for six months Provider: Physiotherapist No treatment (N=25) |
|
| Outcomes | Outcomes assessed at the end of 4 weeks treatment and at 1, 3 and 6 months follow‐up for Group 1 and 2, but only at the end of 4 weeks treatment for Group 3 No primary outcome was reported by the trialists 1. Constant score (0‐100 scale where a higher score indicates better functional ability) 2. Pain severity at the moment of assessment, measured using a 10cm visual analogue scale, with "No pain" anchored at the left and "Pain as bad as it could be" anchored at the right |
|
| Notes | No outcome data for the no treatment group was reported in the trial publication, so we could not analyse the comparison between interferential current and home exercises versus no treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly allocated into: (i ) the EA group (n = 24); (ii) IFE group (n = 23); or (iii) control group (n = 23)" Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was a double‐blind, randomized, controlled clinical trial. An independent assessor was blind to the group allocation." Comment: Despite reporting this trial as "double‐blind", given the nature of the interventions (electrotherapy versus no treatment), participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported pain and some components of the Constant score |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The study was a double‐blind, randomized, controlled clinical trial. An independent assessor was blind to the group allocation." Comment: Outcome assessors of some components of the Constant score were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One participant dropped out of each of the electroacupuncture group and interferential electrotherapy group, both because of time conflict, and two participants dropped out of the no treatment group because they experienced no improvement." Comment: While drop‐out is related to treatment in the no treatment group, the number of dropouts is small and unlikely to affect the function and pain outcomes |
| Selective reporting (reporting bias) | High risk | Comment: The trialists reported the mean (SD) scores for the Constant Murley Assessment scale and VAS pain at the end of four weeks treatment for the electroacupuncture and interferential current groups, but not for the no treatment group, because the no treatment group did not have a statistically significant improvement from baseline. Also, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Dewan 2011.
| Methods |
Design: Parallel group, two‐arm, randomised controlled trial (India) Interventions: Interferential current or TENS Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 50 (25 per group) Baseline characteristics: No baseline characteristics reported Inclusion criteria: 1. Aged 40‐60 years 2. Reported localised pain over one shoulder, experienced night pain and had restricted active and passive shoulder motions Exclusion criteria: 1. Aged below 40 or above 60 years; 2. History of trauma, fractures, previous shoulder surgery, cervical or thoracic pain syndrome, complex regional pain syndrome, malignancies, on anticoagulant therapy, psychic patient, hypermobile joint, or had received acupuncture treatment to the painful shoulder in the past six months. |
|
| Interventions |
Interferential current (N=25) Components of intervention: The participant was positioned comfortably and the skin was prepared, washed and any skin lesion insulated with petroleum jelly. An interferential electrotherapy machine delivered current swept from 80 to 120 Hz, and 4 suction‐type electrodes were placed around the shoulder region used in two pairs (quadripolar technique was applied), each pair being indicated by the colorings of the wire from the machine. The electrodes of each pair were placed diagonally opposite one another in such a way that the interference effect was produced in the tissues where it was required, which was very deep. The participant was warned that he or she would feel a tingling sensation which should not be too uncomfortable or burning. The intensity of the stimulation was adjusted to just below the pain threshold Dosage: 20 minutes Frequency of administration: Two to three sessions per week for four weeks (10 sessions) Provider: Physiotherapist Transcutaneous electrical nerve stimulation (TENS) (N=25) Components of intervention: The skin in the treatment area was first sterilized with an isopropyl alcohol skin wipe. Conductive rubber electrodes covered with a conductive gel in order to gain good skin contact were placed on the participant's skin. The electrodes could be bandaged onto the participant or fixed with adhesive tape. Four electrodes were placed. High frequency TENS was used. The intensity of the stimulation was adjusted to a tolerance level of just below the pain threshold. Pulses of around 0.2 ms at about 100Hz were given at intensities that provoke gentle contraction. The participant should have felt a tingling pins and needle sensation. It was applied to acupuncture points but was sometimes applied to motor points of muscles. The intensity of the stimulation was adjusted to a tolerance level of just below the pain threshold. The needle was retained for 20 minutes, and was manually lifted and thrusted every 10 minutes Dosage: 20 minutes Frequency of administration: Two to three sessions per week for four weeks (10 sessions) Provider: Physiotherapist |
|
| Outcomes | Outcomes assessed at the end of four weeks treatment. No primary outcome was reported by the trialists 1. Range of motion in flexion, abduction and external ration, using a goniometer (not reported whether active or passive) 2. Constant score (0‐100 scale where a higher score indicates better functional ability) 3. Pain using a 10cm visual analogue scale, anchored as 1="No pain" and 10="Severe pain" |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly allocated into: (i) the IFE group (n = 25); (ii) TENS group (n = 25)" Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different electrotherapy modalities, but it is unclear whether they were provided any information that would make them perceive the type of electrotherapy they received as superior or inferior to the alternative type of electrotherapy |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain and function, but it is unclear whether they were provided any information that would make them perceive the electrotherapy they received as superior or inferior to the alternative type of electrotherapy |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: Trialists did not report whether assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Twenty‐five participants were randomly allocated to each group. No drop‐outs, losses to follow‐up or exclusions were reported, but it is clear that the outcome data reported was not based on the total number of randomised participants. The sample sizes on which each outcome was based were not reported in tables. However SDs and SEs per group for each outcome were. When calculating the sample size (based on the SD and SE), none of the SEs matched the SDs when a sample size of 25 per group was assumed (in some cases, an assumed sample size of 16 lead to the calculation of the correct SE and SD. Therefore, data was not collected on all participants, and the number of dropouts and reasons for drop‐out were unclear |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trialists fully reported post‐treatment data for both groups for pain and range of motion, but reported post‐treatment Constant Score means and SDs for the interferential current group only (no measures of variation were reported for the Constant Score in the TENS group). However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Dogru 2008.
| Methods |
Design: Parallel group, two‐arm, double‐blind randomised controlled trial (Turkey) Interventions: Ultrasound plus hot pack and exercise or placebo ultrasound plus hot pack and exercise Sample size calculation: 34 participants per group were estimated to be needed based upon detecting a clinically relevant difference of 10.7 points in the Shoulder Pain and Disability Index (SPADI) (SD=14) at the 5% level of statistical significance with 80% power including a 15% rate of loss at follow‐up Analysis: Per protocol analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 50 (25 per group) Baseline characteristics: Ultrasound plus hot pack plus exercise group: Mean (SD; range) age = 53.9 (7.8; 41‐72) years; Male:Female = 11:14 Mean (SD; range) duration of symptoms = 6.3 (3.5; 3‐12) months Placebo ultrasound plus hot pack plus exercise group: Mean (SD; range) age = 56.8 (7.3; 46‐70) years; Male:Female = 10:14 Mean (SD; range) duration of symptoms = 5.2 (2.9; 3‐12) months Inclusion criteria: 1. Shoulder pain of minimum three months duration with no major trauma 2. ≥25% loss of shoulder motion in all planes 3. Pain with motion with a minimum visual analogue scale (VAS) score of 40 mm 4. Normal findings on radiographs of the glenohumeral joint 5. Absence of arthritis, malignancy, and medical conditions such as cardiac diseases, infections and coagulation disorders Exclusion criteria: 1. Patients with adhesive capsulitis due to rotator cuff tears, fractures, dislocations and reflex sympathetic dystrophy |
|
| Interventions |
Ultrasound plus hot pack plus exercise (N=25) Components of intervention: ‐ Ultrasound: Continuous ultrasound with 3 MHz frequency and 1.5 W/cm2 intensity (Intelect® Mobile Ultrasound device, Chattanooga Group) with a transducer head of 5 cm2 was delivered. After coating the skin with an aquasonic gel, ultrasound was delivered by moving the applicator over the anterior, superior and posterior regions of the target joint in slow, overlapping strokes ‐ Hot pack: Superficial heat was administered by use of hot packs (60 °C) ‐ Supervised exercise: The exercise program consisted of Codman's exercises and wall climbing followed by glenohumeral joint stretching exercises to the patient's tolerance ‐ Home exercise: Consisting of Codman's exercises, active range of motion and stretching exercises Dosage: ‐ Ultrasound: 10 minutes ‐ Hot pack: 20 minutes ‐ Supervised exercise: 20 minutes ‐ Home exercise: Not reported Frequency of administration: Every day for two weeks except weekends (10 sessions) for the ultrasound, hot pack and supervised exercise program; after these two weeks, home exercises were conducted for three months Provider: Physical therapist Placebo ultrasound plus hot pack plus exercise (N=25) Participants received the same interventions as described above, except that for the ultrasound component, the skin was covered with an aquasonic gel and ultrasound was applied in the same manner except the device was not switched to "on" |
|
| Outcomes | Outcomes assessed at the end of two weeks treatment, and at three months from baseline Primary outcome 1. Shoulder pain and disability index (SPADI) (0‐100 scale where a higher score indicates worse pain and/or disability) Secondary outcomes: 2. Passive range of motion in abduction, flexion, inner and outer rotation using a goniometer 3. Pain on motion using a 0‐100 visual analogue scale 4. General health status using the SF‐36. Both the Physical Component Score and Mental Component score were reported (scores range between 0 and 100 and lower scores represent worse health status) |
|
| Notes | Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifty patients were numbered sequentially and assigned to either the ultrasound (US) group or placebo (sham US) group by another physician (second author)." Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Participants were likely blind to treatment |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain, SPADI and SF‐36 |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All patients were assessed by the same physician who was blind to the treatment groups (first author)." Quote: "The first author was blind to the treatment groups. She only evaluated the patients according to a standardized form including physical examination and supervised patients while they are filling in the questionnaires" (personal communication) Comment: Trialists reported via personal communication that the outcome assessor was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Fifty patients were numbered sequentially and assigned to either the ultrasound (US) group or placebo (sham US) group by another physician (second author). One patient from the sham US group discontinued the intervention at the beginning of the first week due to personnel reasons. Twenty‐five patients in the US group and 24 patients in the sham US group were assessed for final evaluation." Comment: Only one participant dropped out (from the control group) for personal reasons. This is unlikely to have affected the outcomes |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | High risk | Quote: "Effectiveness of US might be masked by worse pre‐treatment values of the US group and higher exercise compliance of the sham US group." Quote: "The percentage of exercise compliance was calculated from the charts given to the patients on the control evaluation. Exercise compliance of the sham US group was significantly higher than the US group (76.6±15.2 vs. 67.1±14.9 respectively, p = 0.04)." Comment: Participants in the ultrasound group had worse pre‐treatment values and lower compliance with home exercises than participants in the sham ultrasound group. These may have biased results towards the null |
Ghosh 2012.
| Methods |
Design: Parallel group, three‐arm randomised controlled trial (India) Interventions: Ultrasound plus active and passive mobilisation exercises plus shoulder wheel and pulley exercises or manipulation under anaesthesia or glucocorticoid injection (all received home exercises) Sample size calculation: Not reported Analysis: Per‐protocol analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 72 (24 per group) Baseline characteristics: Baseline characteristics by group were not reported. Sex was not reported Age range: 40‐73 years Duration of symptoms: 0‐2 months (N=33), 2‐4 months (N=23), 4‐6 months (N=16) Inclusion criteria: 1. Pain and stiffness of shoulder for six months or less 2. Mild osteoporosis Exclusion criteria: 1. Diabetes mellitus, rheumatoid arthritis, hyperthyroidism, locked posterior and anterior dislocation, sub‐acromial impingement syndrome or rotator cuff lesion 2. Disease duration more than 6 months |
|
| Interventions | All participants were advised to perform active shoulder mobilisation exercises at home Therapeutic ultrasound plus mobilisation exercises plus shoulder wheel and pulley exercises (N=24) Components of intervention: ‐ Electrotherapy: Ultrasound ‐ Supervised exercises: Active and passive shoulder mobilisation exercises plus shoulder wheel and pulley exercises Dosage: Not reported Frequency of administration: For six months (number of sessions per week not reported) Provider: Physiotherapist Manipulation under anaesthesia (N=24) Components of intervention: After general anaesthesia manipulations were done in the sequence of flexion, extension, abduction, adduction, external rotation and internal rotation. Analgesics were given post‐manipulation period for two to three days and shoulder mobilisation exercises started three to four days after manipulation which were taught previously Dosage: Not reported Frequency of administration: Once Provider: Not reported Glucocorticoid injection (N=24) Components of intervention: An injection of methylprednisolone in 40 mg dosage was given intra‐articularly by the anterior approach under strict aseptic preparation Dosage: See above Frequency of administration: An average of three doses with three week interval Provider: Not reported |
|
| Outcomes | Outcome assessed at the end of six months treatment 1. Clinical improvement rated as "Good" (no pain, no tenderness present, ROM is equal or comparable with normal limb, and no muscle wasting present), "Fair" (mild pain and tenderness may or may not be present, mild restriction of ROM still present even after 6 months, and muscle wasting may or may not be present), or "Poor" (gross restriction of movement is still present, with or without pain). |
|
| Notes | To analyse the "treatment success" outcome we dichotomised participants into those who had a clinical improvement rating of "Good" versus those who had a rating of "Fair" or "Poor". Trialists reported that participants in the study had "almost equal right and left sided affection with one having bilateral affection". However, the group that the bilaterally affected participant was allocated to was not reported, nor was any mention of controlling for the correlation between shoulders (but this is unlikely to have affected the results substantially given the dichotomous 'clinical improvement' outcome used). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These patients were randomly allocated in 3 groups" Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported pain and tenderness |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information about whether assessors of muscle atrophy and range of motion were blind to treatment was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Only one participant was lost to follow‐up (in the glucocorticoid injection group). This is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: The results of the single outcome reported in the methods section of the publication (treatment success) were fully reported, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Guler‐Uysal 2004.
| Methods |
Design: Parallel group, two‐arm, single blind randomised controlled trial (Turkey) Interventions: Continuous short wave diathermy application, hot pack, stretching exercises and home exercises or Cyriax approach of deep friction massage, stretching exercises and home exercises Sample size calculation: 20 participants per group were estimated to be needed based upon detecting a 40% increase in the number of patients treated successfully in the Cyriax group at the 5% level of statistical significance with 80% power Analysis: Per protocol analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 42 (21 per group) Baseline characteristics: Continuous short wave diathermy application and hot pack group: Mean (SD; range) age = 58.4 (9.7; 44‐82) years; Male:Female = 7:13 Median (SD; range) duration of symptoms: 5.6 (3.9; 2‐12) months Cyriax approach of deep friction massage group: Mean (SD; range) age = 53.6 (6.9; 43‐70) years; Male:Female = 5:15 Median (SD; range) duration of symptoms: 7.6 (3.9; 2‐12) months Inclusion criteria: 1. Shoulder pain of minimum 2 months duration with no major shoulder trauma 2. Marked loss of active and passive shoulder motion 3. Pain with motion with a minimum visual analogue scale (VAS) score of 30 mm 4. Normal findings on anteroposterior and axillary lateral radiographs of the glenohumeral joint 5. Absence of polyarthritis or neurological diseases or cervical neuropathy 6. Absence of medical conditions such as cardiac disease, Infections, coagulation disorders Exclusion criteria: 1. Patients who had adhesive capsulitis secondary to shoulder dislocation, fractures, reflex sympathetic dystrophy and rotator cuff tears |
|
| Interventions | Both groups received active stretching and pendulum exercises at the end of each treatment session, and were also instructed in a standardised home exercise program consisting of passive ROM and pendulum exercises to be performed every day Continuous short wave diathermy application and hot pack (N=21) Components of intervention: ‐ Continuous short wave diathermy: Continuous short wave diathermy with 220 V/50 Hz power source and 27.12 MHz oscillation frequency was applied to the therapy region for deep heating while the participants were lying supine (Short wave Diathermy KSF Model equipment ITO, Tokyo‐Japan) ‐ Hot pack: Wrapped in towelling and placed on the target shoulder for superficial heating Dosage: ‐ Continuous short wave diathermy: 20 minutes ‐ Hot pack: 20 minutes Frequency of administration: Every day except weekends for two weeks (10 sessions) Provider: Physiotherapist Cyriax approach of deep friction massage (N=21) Components of intervention: ‐ Manual therapy: Cyriax approach of deep friction massage ‐ Supervised exercises: Mobilisation exercises Dosage: One hour Frequency of administration: Three times per week for two weeks (six sessions) Provider: Physiotherapist |
|
| Outcomes | Outcomes assessed at the end of the first and second week of treatment Primary outcome: 1. Number of participants who reached 80% of normal range of motion of the shoulder at the end of the second week of treatment Secondary outcomes: 2. Pain (spontaneous pain, night pain, and pain with motion) using a 100mm visual analogue scale 3. Passive range of motion in flexion, abduction, inner rotation, outer rotation using a goniometer |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "42 patients were randomised for enrolment in the study. The patients were numbered sequentially and allocated to two groups (the Cyriax group and the physical therapy group)." Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "42 patients were randomised for enrolment in the study. The patients were numbered sequentially and allocated to two groups (the Cyriax group and the physical therapy group)." Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The pre‐treatment evaluation of shoulder pain and ROM was carried out by a blinded observer at the beginning of the study." Comment: Outcome assessors of range of motion were probably blind to treatment (though it is unclear how blinding of pain was achieved, given it was self‐reported by unblinded participants) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in the CYR group were excluded from the study due to poor compliance and one from the PT group discontinued the intervention due to attacks of unstable hypertension in the first week." Comment: The number of drop‐outs or exclusions was low and equal between groups, and reasons are unlikely to influence the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data was fully reported for all outcomes specified in the methods section. However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Kanai 2006.
| Methods |
Design: Parallel group, two‐arm, randomised controlled trial (Japan) Interventions: Polarity exchangeable permanent magnet (PEPM) device (which emits a magnetic field with an alternating north and south pole) or a non‐polarity exchangeable permanent magnet (N‐PEPM) device Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 64 (32 per group) Baseline characteristics: Polarity exchangeable permanent magnet (PEPM) group: Mean (SD) age not reported, but 3 were aged between 20‐29, 7 between 30‐39, 13 between 40‐49, 5 between 50‐59, and 4 between 60‐69 years; Male:Female = 16:16 Mean (SD duration of symptoms not reported, but 21 had a contraction period between 1‐6 months, 5 between 7‐12 months, 2 between 13‐24 months, 3 between 25‐48 months, and 1 >49 months Non‐polarity exchangeable permanent magnet (N‐PEPM) group: Mean (SD) age not reported, but 4 were aged between 20‐29, 8 between 30‐39, 12 between 40‐49, 6 between 50‐59, and 2 between 60‐69 years; Male:Female = 16:16 Mean (SD duration of symptoms not reported, but 14 had a contraction period between 1‐6 months, 7 between 7‐12 months, 7 between 13‐24 months, 3 between 25‐48 months, and 1 >49 months Inclusion criteria: 1. Had frozen shoulder 2. Had not received any medication to reduce pain within the week before enrolment Exclusion criteria: 1. Were concurrently being treated for hyperthermia, massage or acupuncture 2. Presence of a severe disorder such as cancer, hypertension, diabetes mellitus, an inflammatory disease or a cardiac disease 3. Presence of a cardiac pacemaker or other metallic implants |
|
| Interventions |
Polarity exchangeable permanent magnet (PEPM) (N=32) Components of intervention: Polarity exchangeable permanent magnet (PEPM) device applied to the area of frozen shoulder pain for 24 hours. The device consisted of a cylindrical magnet that rotated 180 degrees every second and had north and south poles that came into contact with the patient's skin in an alternating fashion. The area that was exposed to the magnetic field from the PEPM device was four times wider than that from the N‐PEPM device Dosage: 24 hours Frequency of administration: One day Provider: Not reported Non‐polarity exchangeable permanent magnet (N‐PEPM) (N=32) Components of intervention: Non‐polarity exchangeable permanent magnet (N‐PEPM) device applied to the area of frozen shoulder pain for 24 hours. The device consisted of a cylindrical magnet of the same size as that in the PEPM device but the magnet in the N‐PEPM device did not rotate Dosage: 24 hours Frequency of administration: One day Provider: Not reported |
|
| Outcomes | Outcomes assessed at three hours after treatment started, at the end of 24 hours treatment and at 24 hours follow‐up (i.e. 48 hours from baseline). No primary outcome was reported by the trialists 1. Overall pain, calculated by summing the score of four 0‐10 visual analogue scales (measuring spontaneous pain, limited range of motion, pain to palpation, and night pain) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned to receive treatment with a PEPM device (n = 32) or an N‐PEPM device (n = 32)" Comment: No information about how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: In contrast to the N‐PEPM device, the PEPM device rotated and the area of the shoulder that was covered by the PEPM device was four times larger than the area covered by the N‐PEPM device. However, it is unclear whether participants were provided any information that would make them perceive the type of electrotherapy they received as superior or inferior to the alternative type of electrotherapy |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain, but it is unclear whether they were provided any information that would make them perceive the type of electrotherapy they received as superior or inferior to the alternative type of electrotherapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no dropouts, losses to follow‐up or exclusions reported, and outcome data was reported as being based on the number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trialists reported percentage change from baseline (with no measures of variation) in overall pain at 3 and 24 hours. Trialists also reported percentage change from baseline (with standard errors) in overall pain at 3, 24, and 48 hours in Figure format. Therefore, no data suitable for meta‐analysis was reported. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Leclaire 1991.
| Methods |
Design: Parallel group, two‐arm, triple blind randomised controlled trial (Canada) Interventions: Pulsed electromagnetic field therapy (PEMF) plus hot pack applications plus passive manual stretching and pulley exercises or placebo electromagnetic field therapy plus hot pack applications plus passive manual stretching and pulley exercises Sample size calculation: Trialists reported that "…the power of this study was 90% to show a change of 37 degrees in mean total range of motion recorded for the placebo group" (pg 286). However, this was reported in the Discussion section and could be a post hoc power calculation Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 47 (22 and 25 in each respective group) Baseline characteristics: Baseline characteristics by group were not reported Mean (SD) age = 58 (6.9) years; Male:Female = 18:29 Mean (SD) duration of symptoms = 17 (4.1) weeks Inclusion criteria: 1. Shoulder pain for more than two months 2. Limited active and passive shoulder movement 3. Pain on resisted abduction, internal and external rotation, and impaired glenohumeral joint motion 4. Decreased passive range of motion of 20% or more, in at least three movements, according to the American Medical Association guide for the evaluation of permanent impairment, i.e. flexion <144 degrees, extension <32 degrees, abduction <120 degrees, adduction <24 degrees, external rotation <72 degrees, and internal rotation <32 degrees Exclusion criteria: 1. Have arthritis, bone or neurologic disease, unstable heart disease, or haemostatic disorder 2. Have rotator cuff rupture, x‐ray calcification >2mm, or severe adhesive capsulitis defined as a limitation of flexion to 100 degrees, abduction to 90 degrees, or global rotations by 20 degrees or more 3. Currently receiving anticoagulants or anti‐inflammatory drugs, or have received steroid injection in the shoulder previously |
|
| Interventions |
Pulsed electromagnetic field therapy (PEMF) plus hot pack plus exercise (N=22) Components of intervention: ‐ PEMF: The schedule was: 30 Gauss, 10 Hz for sessions 1 to 6; 40 Gauss, 15 Hz for sessions 7 to 16; and 60 Gauss, 30 Hz for sessions 17 and beyond ‐ Hot pack ‐ Supervised exercise: Passive glenohumeral joint stretching exercises to the participants tolerance plus standardised pulley exercises ‐ Home exercise: Active non‐assisted exercises using a wooden stick Dosage: ‐ PEMF: 30 minutes ‐ Hot pack: 30 minutes ‐ Supervised exercise: 5 minutes (stretching) and 10 minutes (pulley) ‐ Home exercise: 20 minutes Frequency of administration: Three times a week up to a maximum of 12 weeks (36 sessions); home exercises only conducted on the days in which physical therapy was not received Provider: Not reported Placebo electromagnetic field therapy (N=25) Participants received the same interventions as described above except that placebo electromagnetic field therapy was applied |
|
| Outcomes | Outcomes assessed weekly for 12 weeks. No primary outcome was reported by the trialists 1. Range of motion in flexion, extension, abduction, adduction, external rotation, internal rotation measured at week 4, 8 and 12 (not reported whether passive or active) 2. Pain intensity at rest, on motion, and lying down, using a 4‐point ordinal scale rated as 1=absence of pain, 2=light pain, 3=moderate pain, and 4=severe pain 3. Pain intensity using a 100mm visual analogue scale 4. Disability (interference with daily activities) using a 100mm visual analogue scale 5. Adverse events |
|
| Notes | Trialists did not report any outcome data for VAS pain and VAS disability. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Consenting participants were then randomised". Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A separate individual was provided the randomization code and controlled the concealed switch." Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study used a triple‐blind parallel group design. Subjects received either (1) electromagnetic field therapy or sham therapy…The patient, therapist, and investigator were blind to the procedure. A separate individual was provided the randomization code and controlled the concealed switch." Comment: Participants and personnel were blind to treatment |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Quote: "The patient, therapist, and investigator were blind to the procedure." Comment: Blinded participants self‐reported pain and disability |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The patient, therapist, and investigator were blind to the procedure." Comment: Range of motion was assessed by blinded a outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "…and all completed the study according to the protocol." Comment: There were no dropouts, exclusions, or losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: Outcome data was fully reported for all outcomes specified in the methods section of the publication, except for VAS pain and VAS disability (which appear to have been incompletely reported because there was no statistically significant difference between groups on these outcomes). Also, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Lee 1973.
| Methods |
Design: Parallel group, four‐arm randomised controlled trial (United Kingdom) Interventions: Infrared irradiation plus active exercises or intra‐articular injection of hydrocortisone acetate 25 mg (anterior approach, below the coracoid process) plus active exercises or intra‐articular injection of hydrocortisone acetate 25 mg into the synovial sheath surrounding the bicipital tendon of the bicipital groove of the humerus plus active exercises or analgesics only Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 80 (20 per group) Baseline characteristics: Age, sex, and duration of symptoms not reported Inclusion criteria: 1. Pain in the shoulder associated with limitation of passive movement of the shoulder joint Exclusion criteria: 1. Participants with a known cause of arthritis, bone or neurological disease, determined by full clinical, haematological, and radiographic examination |
|
| Interventions | All participants received a program of graduated active exercises according to the participants tolerance for six weeks. The exercises were divided into two categories: (1) Free active exercises, which were given to work the flexors and extensors of the shoulder joint, the abductors, and the medial and lateral rotators. A progression was followed using gravity, firstly to assist the movement, then with its effect eliminated, and finally with its effect resisting the action. The participants were asked to practice these exercises three times daily for 10 minutes each session, specifically in the morning, at midday, and in the evening; (2) Manual resistance, using proprioceptive neuromuscular facilitation techniques Infrared irradiation (N=20) Components of intervention: Infrared irradiation to both the anterior and posterior aspects of the shoulder region Dosage: 10 minutes Frequency of administration: Not reported Provider: Physiotherapist Intra‐articular injection of hydrocortisone acetate 25 mg (anterior approach, below the coracoid process) (N=20) Components of intervention: Intra‐articular injection of hydrocortisone acetate 25 mg (anterior approach, below the coracoid process) Dosage: N/A Frequency of administration: Not reported Provider: Rheumatologist Intra‐articular injection of hydrocortisone acetate 25 mg into the synovial sheath surrounding the bicipital tendon of the bicipital groove of the humerus (N=20) Components of intervention: Intra‐articular injection of hydrocortisone acetate 25 mg into the synovial sheath surrounding the bicipital tendon of the bicipital groove of the humerus Dosage: N/A Frequency of administration: Not reported Provider: Rheumatologist Analgesics (N=20) Components of intervention: Analgesics such as paracetamol, aspirin, codeine, or dihydrocodeine Dosage: As required Frequency of administration: Six weeks Provider: N/A |
|
| Outcomes | Outcomes assessed weekly for six weeks. No primary outcome was reported by the trialists 1. Range of motion (active abduction of the coronal plane, passive abduction of the coronal plane, active lateral rotation with the arm by the side, active medial rotation with the arm by the side) using a goniometer |
|
| Notes | Trialists reported that since there was high positive correlation between the four range of motion measures, component analysis was used to produce a single measure. The results of this measure were presented in Figure format as means with no measures of variation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Consecutive patients were allocated to one of the four treatment groups according to a randomised plan unknown to the referring clinician" Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the nature of the trial it was impossible for it to be double blind in construction, but it was strictly controlled" Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "Due to the nature of the trial it was impossible for it to be double blind in construction, but it was strictly controlled" Comment: Outcome assessors were not blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, but it was unclear whether the outcome data reported was based on the total number of randomised participants (as sample sizes were not reported in data tables) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trialists reported that since there was high positive correlation between the four range of motion measures, component analysis was used to produce a single measure. The results of this measure were presented in Figure format as means with no measures of variation. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Leung 2008.
| Methods |
Design: Parallel group, three‐arm, single‐blind randomised controlled trial (Hong Kong) Interventions: Continuous short wave diathermy plus stretching exercises or superficial heating (hot pack) plus stretching exercises or stretching exercises alone Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 30 (10 per group) Baseline characteristics: Duration of symptoms not reported Continuous short wave diathermy plus stretching exercises group: Mean (SD) age = 59.8 (12.87) years; Male:Female = 5:5 Hot pack plus stretching exercises group: Mean (SD) age = 62.5 (12.13) years; Male:Female = 2:8 Stretching exercises alone group: Mean (SD) age = 57.3 (13.1) years; Male:Female = 2:8 Inclusion criteria: 1. Experienced shoulder pain and limited shoulder movement for at least eight weeks Exclusion criteria: 1. History of trauma to the shoulder 2. Acute signs of inflammation over the shoulder 3. Intrinsic shoulder pathology 4. Taking analgesic or anti‐inflammatory drugs 5. Had metal implants 6. Impaired sensation of hot and cold 7. Pregnant 8. Had a cardiac pacemaker |
|
| Interventions | All participants received four stretching exercises in the following fixed sequence: stretching in external rotation, in flexion, followed by stretching in hand behind the back and cross‐body abduction. Participants were asked to repeat the stretches four times. Each stretch was sustained for 30 seconds, with 10 seconds rest between each stretch. The participants were asked to perform the stretching exercises at home every day for four weeks Continuous short wave diathermy (N=10) Components of intervention: A continuous shortwave diathermy machine (Curapuls 419, Enraf Nonius, the Netherlands) with an operating frequency of 27.12 MHz was used to deliver deep heating treatment. A pair of disc electrodes was placed on the anterior–posterior aspects of the affected glenohumeral joint, separated by a hand's‐breadth from the surface of the body. The intensity of the current was adjusted according to the participants' subjective feeling of comfortable warmth. If the level of perceived heating changed during the application, the machine's output was adjusted to maintain the sensation of comfortable warmth throughout the treatment Dosage: 20 minutes Frequency of administration: Three times a week for four weeks (12 sessions) Provider: Physiotherapist Hot pack (N=10) Components of intervention: An electrical hot pack sized 35.5 x 68.5 cm was used to deliver superficial heating. The temperature was set to 63 degrees Centigrade. The participants were informed that the only purpose of the heating was to produce a feeling of comfortable warmth. If they felt that the heat was excessive, the temperature of the electrical hot pack was adjusted immediately to ensure that the heat remained at a comfortably warm level only throughout the treatment Dosage: 20 minutes Frequency of administration: Three times a week for four weeks (12 sessions) Provider: Physiotherapist Stretching exercises only (N=10) See description of exercises above |
|
| Outcomes | Outcomes assessed before the intervention at sessions 6 (week 2) and 12 (end of 4 weeks treatment), and at four weeks post‐treatment cessation. No primary outcome was reported by the trialists 1. Shoulder score index, which combines self‐reported scores for pain (using a 10cm visual analogue scale) and function (using a 10‐item questionnaire addressing activities of daily living, each scored on a 4‐point ordinal scale of level of difficulty: 0=unable to do; 1=very difficult to do; 2=somewhat difficult; 3=not difficult). Both the pain and function score were weighted equally (50 points each) and combined for a total score of 100 points, which a higher score indicating better function. This combined score is calculated as (10 – VAS pain score) x 5 + (5/3 x cumulative activities of daily living score) 2. Range of motion in flexion, cross‐body adduction, external rotation with the arm by the side, external rotation with the arm in 90 degrees abduction, and hand‐behind‐back using a goniometer (not reported whether passive or active) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using an online randomization plane (http:/www.randomization.com)" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "A single‐blinded, randomized controlled study was conducted. The rater was blinded to the group allocation" Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "A single‐blinded, randomized controlled study was conducted. The rater was blinded to the group allocation" Comment: Assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "None of the participants in any of the treatment groups dropped out throughout the study period." Comment: Data for the complete sample of randomised participants was reported for each outcome |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data was fully reported for all outcomes specified in the methods section of the publication, but it is unclear why pain and function sub‐scores of the shoulder index were not reported, and without a trial protocol it is unclear whether any other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Maryam 2012.
| Methods |
Design: Parallel group, three‐arm, single blind randomised controlled trial (Iran) Interventions: Physiotherapy (transcutaneous electrical nerve stimulation, active range of motion exercises, and ice application) or glucocorticoid injection or physiotherapy plus glucocorticoid injection Sample size calculation: 35 participants per group were estimated to be needed based upon detecting a clinically relevant difference at the 5% level of statistical significance with 80% power (outcome used in power calculation not reported) Analysis: Per‐protocol analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 87 (27, 31, and 29 in each respective group) Baseline characteristics: Physiotherapy group: Mean (SD) age = 53.73 (7.49) years; Male:Female = 1:26 Mean (SD) duration of symptoms: 4.48 (3.37) months Glucocorticoid injection group: Mean (SD) age = 53.33 (7.49) years; Male:Female = 4:25 Mean (SD) duration of symptoms: 6.83 (3.75) months Physiotherapy plus glucocorticoid injection group: Mean (SD) age = 53.71 (6.69) years; Male:Female = 4:27 Mean (SD) duration of symptoms: 6.21 (3.95) months Inclusion criteria: 1. 18 years or older 2. Duration of symptoms were <1 year 3. Frozen shoulder defined as the presence of shoulder pain with limitation of both active and passive range of motion in glenohumeral joint ≤ 25% in at least 2 directions: flexion, abduction, external and internal rotation, as compared with normal values or contra lateral shoulder 4. Total score of ≥30 on Shoulder Pain and Disability Index (SPADI) Exclusion criteria: 1. Disorder was secondary to inflammatory, degenerative, metabolic (except for diabetes mellitus), trauma, septic arthritis and cerebrovascular accident 2. Had been treated with injection or physiotherapy in last six months |
|
| Interventions |
Physiotherapy (N=27) Components of intervention: ‐ Electrotherapy: TENS ‐ Supervised exercises: Active range of motion exercises ‐ Other: Ice application Dosage: Not reported Frequency of administration: 10 sessions (number of sessions per week not reported) Provider: Physiotherapist Glucocorticoid injection (N=31) Components of intervention: Cortiosteroid injection included as 60 milligrams triamcinolone acetonide and 3 cc lidocaine in shoulder joint with posterior approach and 20 milligrams triamcinolone acetonide and 1.5 cc lidocaine in subacromial bursa Dosage: See above Frequency of administration: Once Provider: Rheumatologist Physiotherapy plus glucocorticoid injection (N=29) Physiotherapy (as above) one week after glucocorticoid injection (as above) |
|
| Outcomes | Outcomes assessed at six weeks and six months. No primary outcome was reported by the trialists 1. Shoulder pain and disability index (SPADI) (0‐100 scale where a higher score indicates worse pain and/or disability) 2. Passive range of motion in flexion, abduction, external rotation, and distance of hand behind back using a goniometer |
|
| Notes | Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request. Trial registered in the Iranian Registry of Clinical Trials (http://www.irct.ir/searchresult.php?id=1828&number=1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After taking written informed consent, the patients were randomized to 1 of the following 3 groups" Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After taking written informed consent, the patients were randomized to 1 of the following 3 groups" Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "Evaluations of SPADI score were done by an observer blind to treatment allocation." Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some components of the SPADI |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Comment: Trialists confirmed via personal communication that the assessor of range of motion was not blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Eight patients in physiotherapy group, 7 in combination therapy group and 3 in injection group did not continue, so statistical analysis was done on 69 remaining patients." Quote: "About 36 patients have been reevaluated in 24 weeks (Table‐III). However we cannot consider this stage of study because of a high number of missed patients, but we can see a more subjective improvement during 6 months in physiotherapy group." Comment: Trialists did not report the reasons for participants not continuing (and did not provide this information when requested), so it is unclear whether the reasons were balanced between groups and related to the treatment received |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data was fully reported for all outcomes specified in the trial registry entry |
| Other bias | Low risk | Comment: No other sources of bias identified |
Pajareya 2004.
| Methods |
Design: Parallel group, two‐arm single‐blind randomised Interventions: Physical therapy (continuous short wave diathermy, mobilisation and passive glenohumeral joint stretching exercises) plus ibuprofen or ibuprofen alone Sample size calculation: 60 participants per group were estimated to be needed based upon detecting a difference in success rate (measured by improvement in a global pain and disability index) of 25% at the 5% level of statistical significance with 80% power Analysis: Per‐protocol analysis (reported that intention‐to‐treat analysis was used to test statistical significance, but outcome data presented in tables was reported as based on the number of participants completing assessments at each week) Source of funding: Department of Research Promotion, Faculty of Medicine, Siriraj Hospital, Mahidol University and partially supported by Thailand Research Fund (non‐industry) |
|
| Participants |
Number of participants: 122 (61 per group) Baseline characteristics: Baseline characteristics reported for the participants who completed the week 3 assessment (N=119) Physical therapy plus ibuprofen group: Mean (SD) age = 56.3 (10.6) years; Male:Female = 14:45 Duration of symptoms: No. participants with duration <6 weeks (N=6), between 6‐11 weeks (N=20), and 12 or more weeks (N=33) Ibuprofen alone group: Mean (SD) age = 57.7 (10) years; Male:Female = 24:36 Duration of symptoms: No. participants with duration <6 weeks (N=13), between 6‐11 weeks (N=20), and 12 or more weeks (N=27) Inclusion criteria: 1. Had shoulder pain and limitation of a passive range of shoulder motion in all directions that interfered with their activities of daily living Exclusion criteria: 1. Secondary adhesive capsulitis 2. Intrinsic causes of shoulder problems such as a history of fracture, or dislocation or extrinsic causes such as neuromuscular disorders (stroke, parkinsonism), generalised arthritis, bilateral involvement, contraindication for NSAIDs 3. Bleeding tendencies |
|
| Interventions | Both groups received ibuprofen 400 mg three times daily for three weeks, and general advice (an information sheet containing advice on protection of the shoulder from vigorous activities such as pushing and pulling, and encouragement to use their arms in a normal fashion for reaching and other activities of daily life) Physical therapy plus ibuprofen (N=61) Components of intervention: ‐ Electrotherapy: Continuous short wave diathermy ‐ Manual therapy: Mobilisation. If, during the passive movements the patients felt pain before the therapist reached the end of the range, exercise was not attempted ‐ Supervised exercise: Passive glenohumeral joint stretching exercises up to the participant's tolerance, based on Cyriax ‐ Home exercise: Pulley exercises (actively assisted exercises for five minutes) and active non‐assisted exercises using a towel and wall (five minutes after applying a hot pack for 20 minutes) Dosage: ‐ Electrotherapy: 20 minutes ‐ Manual therapy: Not reported ‐ Supervised exercise: Not reported ‐ Home exercise: 10 minutes Frequency of administration: ‐ Electrotherapy: Three times a week for three weeks (9 sessions) ‐ Manual therapy: Three times a week for three weeks (9 sessions) ‐ Supervised exercise: Three times a week for three weeks (9 sessions) ‐ Home exercise: Four days a week for three weeks (on the days they did not receive the hospital‐based physical therapy program) Provider: Physical therapist Ibuprofen (N=61) See above |
|
| Outcomes | All outcomes assessed at the end of three weeks treatment (except for "success", which was also assessed at six, 12 and 24 weeks) Primary outcome: 1. "Success", measured by participants rating themselves as having disappearance of shoulder complaints or some pain/limitation which does not interfere with everyday life (on a global pain and disability index with a 5‐point Likert scale with response options "disappearance of shoulder complaints", "some pain or limitation but which does not interfere with everyday life", "minimal inconvenience to everyday life", "moderate inconvenience", and "marked inconvenience") Secondary outcomes: 2. Shoulder pain and disability index (SPADI) (0‐100 scale where a higher score indicates worse pain and/or disability) 3. Passive range of motion (abduction, external rotation, internal rotation quantified by measuring the distance between thumb and tip of C7 spine in hand behind back position) using a goniometer 4. Adverse events recorded for the physical therapy group by asking "Do you have pain that persisted more than 2 hours after treatment or more disability the next morning or not?", and by asking all patients, "Have the trial drugs and/or treatment program upset you in any way?" and examining the patient for any signs of echymosis or burn during range of motion evaluation |
|
| Notes | Adverse events due to ibuprofen were not reported separately per group: "During the 3‐week period, the patients in the study group reported a total of 10 episodes of pain that persisted more than 2 hours after treatment from 4 subjects.There were no other complications recorded. Regarding NSAIDs, 15 subjects (12.6%) had gastrointestinal side effects; the number of those who had severe dyspepsia and had to stop NSAIDs was 6 (4.2%). There were 2 reports of severe oedema and 1 case with a severe headache, which rapidly subsided after the drug was discontinued" (pg 477 of trial publication). Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients who gave informed written consent were randomly allocated to a 3‐week treatment protocol by simple randomisation using a random numbers table and allocation concealed within an opaque envelope." Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients who gave informed written consent were randomly allocated to a 3‐week treatment protocol by simple randomisation using a random numbers table and allocation concealed within an opaque envelope." Personal communication: "I prepared opaque envelopes before hand. Within each envelope, I put the letter "I" or "C". The series of "I" and "C" came from the random number table. I didn't remember any part of the series" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported a global pain and disability index and the SPADI |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Moreover, at each follow‐up, an investigator, blinded to treatment modality asked all patients "Have the trial drugs and/or treatment program upset you in any way?" and examined the patient for any signs of echymosis or burn during range of motion evaluation." Personal communication: "The range of motion assessor was blinded. I had told all of the participants that "Please don't tell the assessor about the treatment you have"" Comment: Assessors of adverse events and range of motion were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "At the end of the 3rd week, 2 subjects dropped out from the study; 1 from the control group and 1 from the study group. The total number of cases included in the analysis was 59 in the control and 60 in the study group. By the end of the 24th week, a total of 12 cases (10.1%) had withdrawn from the study (Fig. 1). All of them lost to follow‐up for unknown reasons and the investigators could not contact them." Quote: "The results were analysed by intention to treat analysis even though the treatments actually received were modified from the protocol, because it was found that the reasons for modifying the treatment were strongly related to the results of allocated interventions." Comment: It is unclear whether reasons for losses to follow‐up were related to the interventions received |
| Selective reporting (reporting bias) | Unclear risk | Quote: Outcome data was fully reported for all outcomes specified in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Quote: "About three‐quarters of the subjects of both groups received NSAIDs as prescribed. The reasons why some patients received fewer NSAIDs than the others was due to gastrointestinal discomfort, forgetting to take them or a misunderstanding about the schedule. In the study group, 7 cases (11.7%) received fewer than 6 sessions of hospital‐based PT, 5 cases (8.3%) performed the home programme exercises fewer than 6 sessions. Two cases from the control group reported that they had additional treatment; 1 had Chinese herbal medicine and 1 received analgesics from a private clinic. No patient in the control group had hospital‐based PT or home exercise therapy for their shoulder." Quote: "The deviation from the protocol in the present study might not reverse the results. On the contrary, the differences of the outcomes at the end of the study should be elicited more easily if there was no protocol deviation. Because the patients in the study group received fewer treatments than the schedule determined (six cases had fewer than 6 sessions of hospital‐based PT and 6 cases performed home exercise fewer than 6 sessions), while the subjects in the control group received more treatment than the schedule (one case had Chinese herbal medicine and 1 case had analgesics from a private clinic)." Comment: Protocol violations are unlikely to have influenced the results |
Rigato 2002.
| Methods |
Design: Parallel group, three‐arm, single‐blind randomised controlled trial (Italy) Interventions: Low‐frequency (100 Hz) pulsed electromagnetic field therapy (PEMF) or Therapeutic Application of a Musically Modulated Electromagnetic Field (TAMMEF) or simulated (placebo) electromagnetic field therapy Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 49 (18, 17, and 14 in each respective group) Baseline characteristics: Age and duration of symptoms not reported. Sex by group was not reported Male:Female = 20:29 Inclusion criteria: 1. Unilateral non‐calcified shoulder periarthritis Exclusion criteria: Not reported |
|
| Interventions |
Low‐frequency (100 Hz) pulsed electromagnetic field (N=17) Components of intervention: Low‐frequency (100 Hz) electromagnetic field therapy was delivered by applying magnets to the shoulder Dosage: 30 minutes Frequency of administration: Daily for 15 days (15 sessions) Provider: Physicist Therapeutic Application of a Musically Modulated Electromagnetic Field (TAMMEF) (N=18)* Components of intervention: TAMMEF was delivered by applying magnets to the shoulder. The electromagnetic field parameters (frequency, intensity, waveform) were modified in time, randomly varying within the respective ranges, so that all the possible codes can occur during a single application Dosage: 30 minutes Frequency of administration: Daily for 15 days (15 sessions) Provider: Physicist Simulated (placebo) electromagnetic field (N=14) Components of intervention: A simulated (placebo) electromagnetic field was delivered by applying magnets to the shoulder Dosage: 30 minutes Frequency of administration: Daily for 15 days (15 sessions) Provider: Physicist |
|
| Outcomes | Outcomes assessed at day 7, day 15 (end of treatment) and day 45 (i.e. 30 days post‐treatment cessation). No primary outcome was reported by the trialists. 1. Pain using a visual analogue scale rated from 0=absence of pain to 10=maximum intensity 2. Articular functionality by executing semeiological manoeuvres to define the functionality of single regions affected, expressed as 0=absence of functional limitation, 1=slight limitation, 2=moderate limitation, and 3=serious limitation 3. Local or systemic side effects |
|
| Notes | *This intervention is not a standard type of pulsed electromagnetic field therapy that can be applied by physical therapists, so no data for this group was included in the review This RCT included participants with shoulder periarthritis or cervical spondylosis. Pain and articular functionality outcome data was reported separately per cervical spondylosis and shoulder periarthritis participants in the two active intervention groups at the end of 15 days treatment, but not at 30 days follow‐up, and was not reported separately at any time point for the placebo group. Unpublished data was requested but was unable to be provided by the trialist as he no longer had access to the data (had changed place of work). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into three groups" Comment: No information on how the allocation sequence was generated was reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Specifically, they knew that they would be subjected to an experimental treatment based on low‐frequency electromagnetic fields; they also knew of the therapeutic objectives and the previously obtained results. However, for obvious experimental reasons, they were not informed about the difference between the two treatments and the consequent division into groups." Comment: Participants (but probably not personnel) were probably blind to treatment |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information on whether articular functionality was assessed by blinded outcome assessors was reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All the patients of groups A and B completed the therapeutic cycle, without appreciable local or systemic side‐effects that might have required suspension of the treatment."
Quote: "After the first week of treatment, application of the simulated magnetic field had to be suspended in 20 group C patients (40%) because of its ineffectiveness. The remaining 30 patients (60%) completed the cycle according to the procedure described above." Comment: There were no dropouts in the two active intervention groups and 40% dropout in the placebo group which was related to the treatment received (this 40% comprises participants with cervical spondylosis or shoulder periarthritis; the number of shoulder periarthritis participants who were randomised to and who dropped out of this group was not reported) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data was reported separately per cervical spondylosis and shoulder periarthritis participants in the two active intervention groups at the end of 15 days treatment, but not at 30 days follow‐up, and was not reported separately at any time point for the placebo group. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Ryans 2005.
| Methods |
Design: Parallel group, four‐arm, single blind randomised controlled trial (United Kingdom) Interventions: Physiotherapy (interferential current, proprioceptive neuromuscular facilitation, Maitland mobilizations and active exercise) plus glucocorticoid injection or glucocorticoid injection alone or physiotherapy plus placebo injection or placebo injection alone Sample size calculation: 20 participants per group were estimated to be needed based upon detecting a difference of 1.04 points on a 5‐point pain scale (SD=1.6) at 4 weeks at the 5% level of statistical significance with 82% power Analysis: Per protocol analysis Source of funding: Arthritis Research Campaign (non‐industry) |
|
| Participants |
Number of participants: 80 (20 per group) Baseline characteristics: Physiotherapy plus glucocorticoid injection group: Mean (SD) age = 56.3 (6.4) years; Male:Female = 11:9 Mean (SD) duration of symptoms: 14.2 (4.4) weeks Glucocorticoid injection alone group: Mean (SD) age = 52.3 (9.3) years; Male:Female = 6:13 Mean (SD) duration of symptoms: 12.2 (5.3) weeks Physiotherapy plus placebo injection group: Mean (SD) age = 52.6 (7.7) years; Male:Female = 6:14 Mean (SD) duration of symptoms: 14.4 (4.4) weeks Placebo injection alone group: Mean (SD) age = 55.2 (9.4) years; Male:Female = 9:10 Mean (SD) duration of symptoms: 14.9 (3.7) weeks Inclusion criteria: 1. Aged 18 years or older 2. A painful shoulder, in the fifth cervical (C5) dermatome distribution, of more than four weeks and less than six months duration 3. Limitation of active and passive range of movement greater than 25% in abduction and external rotation compared with the other shoulder Exclusion criteria: 1. Pain was less than four weeks duration 2. Symptoms of more than six months duration 3. Had a previous intra‐articular injection or prior physiotherapy for this episode of shoulder pain 4. Presence of restriction of active and passive range of movement in external rotation only or glenohumeral abduction only 5. Had evidence of glenohumeral osteoarthritis on plain X‐ray 6. Had clinical evidence of a complete rotator cuff tear (i.e. positive drop‐off sign or weakness of the rotator cuff muscles) 7. Had clinical evidence of significant cervical spine disease, history of significant trauma to the shoulder or a history of inflammatory joint disease or of a cerebrovascular accident affecting the study shoulder 8. Had bilateral adhesive capsulitis 9. Had a contraindication to triamcinolone injection |
|
| Interventions | All participants were provided with 50x500mg paracetamol tablets with suggestions to take one or two tablets 4‐ to 6‐hourly as required for pain, taking no more than a maximum of eight tablets daily. All participants were also instructed by a physiotherapist in an identical home exercise programme using a video and home exercise instruction sheet Physiotherapy plus glucocorticoid injection (N=20) Components of physiotherapy intervention: ‐ Electrotherapy: Standardised interferential current ‐ Manual therapy: Maitland mobilizations which were progressed as the condition improved, and proprioceptive neuromuscular facilitation ‐ Supervised exercise: Active exercise therapy with gym equipment Dosage: Not reported Frequency of administration: Twice a week for four weeks (eight sessions) Provider: Physiotherapist Components of glucocorticoid injection: Injections of triamcinolone 20mg (1 ml) and normal saline 2 ml plus physiotherapy for four weeks. Injections were given (without imaging guidance) by a combined approach to the shoulder: half the solution (1.5 ml) was injected by an anterior approach and half (1.5 ml) by a lateral approach Glucocorticoid injection alone (N=20) The same injection method as described above was delivered Physiotherapy plus placebo injection (N=20) The same injection and physiotherapy method as described above was delivered, except that normal saline 3 ml was injected into the shoulder Placebo injection alone (N=20) The same injection method as described above was delivered, except that normal saline 3 ml was injected into the shoulder |
|
| Outcomes | Outcomes assessed at 6 and 16 weeks post‐randomisation Primary outcome: 1. Croft Shoulder Disability Questionnaire (0‐22 score range, where a score of 0 indicates no disability and a score of 5 and over represents significant disability) Secondary outcomes: 1. General health status using the SF‐36 (assessed at 16 weeks post‐randomisation only) 2. Passive and active range of motion in forward flexion, abduction, external rotation, internal rotation using a goniometer 3. Daytime pain at rest using a 100mm visual analogue scale 4. Global function using a 100mm visual analogue scale |
|
| Notes | *Outcome data fully reported only for these outcomes. No outcome data reported for other outcomes. Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request. Trial registered in ISRCTN but outcomes not provided at time of registration (http://www.controlled‐trials.com/ISRCTN25152388). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly allocated in permuted blocks of four using random number tables to one of four treatments. The randomization process took place in the hospital pharmacy department. Allocations were placed in sealed envelopes which were opened by the physiotherapist teaching the home exercise programme" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: See quote above. An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Injections were provided in opaque syringes, and the investigator measuring outcomes (IR) was not present at the time of randomization or injection and was blinded to all study interventions. Both patients and the physiotherapist were blinded to the nature of the injection. Clearly, it was impossible to blind subjects regarding physiotherapy but subjects were asked not to reveal if they were having physiotherapy treatment." Comment: Participants and personnel were blind to the injection component of the intervention, but not the physiotherapy component. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Participants self‐reported pain, general health status and function, and were not blind to whether they had received physiotherapy or not. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Injections were provided in opaque syringes, and the investigator measuring outcomes (IR) was not present at the time of randomization or injection and was blinded to all study interventions. Both patients and the physiotherapist were blinded to the nature of the injection. Clearly, it was impossible to blind subjects regarding physiotherapy but subjects were asked not to reveal if they were having physiotherapy treatment." Comment: Assessors of objective outcomes were blind to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eighty subjects were recruited and randomly assigned to four groups. One subject was randomized twice and another failed to attend for intervention after randomization; 78 subjects were therefore available for analysis. Twenty subjects were enrolled in Group A (steroid injection and physiotherapy), 19 in Group B (steroid injection and no physiotherapy), 20 in Group C (placebo injection and physiotherapy) and 19 in Group D (placebo injection and no physiotherapy). Six subjects did not return for all follow‐up visits: three in Group A, one in Group B, one in Group C and one in Group D. Fifteen subjects withdrew from the study due to failure of the study treatment. Six patients withdrew from Group B, three from Group C and six from Group D" Quote: "We also looked to see if there were significant differences in numbers dropping out in each group due to failure of treatment. Significantly more patients dropped out in Group D (placebo injection and no physiotherapy) and in Group B (steroid injection and no physiotherapy (Pearson chi‐square = 8.72, P=0.033). No subjects dropped out of Group A (steroid injection and physiotherapy)." Comment: The was differential drop‐out across the groups and the reasons appear to be related to the treatments received |
| Selective reporting (reporting bias) | High risk | Quote: "Secondary outcome measures were...range of movement as measured by passive external rotation. External rotation was chosen as the indicator range of movement as restriction in this range has been described as the most severely restricted plane of movement in shoulder capsulitis" Quote: "Analysis of improvement in the range of movement in abduction and internal rotation (thumb–C7 distance) revealed no significant association with either steroid injection or physiotherapy." Comment: Trialists reported measuring passive and active range of motion (forward flexion, abduction, external rotation, internal rotation) using a goniometer. However, outcome data was only reported for passive external rotation. The decision not to report outcome data for the other measures of range of motion appears to be related to the statistical significance of the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Stergioulas 2008.
| Methods |
Design: Parallel group, two‐arm, triple‐blind randomised controlled trial (Greece) Interventions: Low‐level laser therapy (LLLT) plus home exercises or placebo laser therapy plus home exercises Sample size calculation: Not reported Analysis: Per protocol analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 74 (37 per group) Baseline characteristics: Low‐level laser therapy plus exercises group: Mean (SD) age = 55.51 (5.84) years; Male:Female = 19:12 Mean (SD) duration of symptoms: 26.5 (12.8) weeks Placebo laser therapy plus exercises group: Mean (SD) age = 56.83 (6.82) years; Male:Female = 21:11 Mean (SD) duration of symptoms: 27.1 (13.6) weeks Inclusion criteria: 1. Painful and limited passive glenohumeral mobility 2. More restricted lateral rotation (<8°) relative to abduction and medial rotation 3. No clear signs (e.g. painful arc, positive resistance testing, or loss of power) that the shoulder pain was caused by another condition Exclusion criteria: 1. Insulin‐dependent diabetes mellitus 2. Bilateral symptoms 3. Systemic inflammatory joint disease (such as rheumatoid arthritis or polymyalgia rheumatica) 4. Treatment with corticosteroid injections or physiotherapy during the preceding six months 5. Serious infection 6. Uncontrolled hypertension 7. Peptic ulceration for which oral steroids are contraindicated 8. Surgery, dislocation, or fracture(s) of the shoulder 9. Calcification about the shoulder joint 10. Pregnancy 11. A complete rotator cuff tear |
|
| Interventions | All patients were instructed to execute pendulum and pain‐free active exercises at home Low‐level laser therapy (N=37) Components of intervention: Low‐level laser therapy with a 810‐nm Galium‐Aluminum‐Arsenide (Ga‐Al‐As) laser with a continuous output of 60 mW applied to eight of the most painful points on the capsule of the glenohumeral joint (as indicated by the participant and checked with an algesiometer) for 30 seconds each, for a total dose of 1.8 J per point and 14.4 J per session Dosage: 4 minutes Frequency of administration: Two sessions per week from week 1‐4 and one session per week from week 5‐8 (12 sessions) Provider: Physical therapist Placebo laser therapy (N=37) Participants received the same interventions as described above, except that placebo laser therapy was provided |
|
| Outcomes | Outcomes assessed at the end of four and eight weeks treatment, and at eight weeks follow‐up (16 weeks post‐randomisation). No primary outcome was reported by the trialists 1. Overall, night, and activity‐related pain using a 100mm visual analogue scale, with end points marked "no pain" at one end and "worst pain" at the other 2. Shoulder pain and disability index (SPADI) (0‐100 scale where a higher score indicates worse pain and/or disability) 3. Croft shoulder disability questionnaire, which includes 22 items which participants answer each as "yes" or 'no", and the number of positive responses is summed to give a score ranging from 0‐22 with higher scores indicating more severe disability 4. Disability of the Arm, Shoulder, and Hand (DASH) questionnaire, for which subjects gave their answers to each of 30 items. The DASH score is expressed as a percentage 5. Heath Assessment Questionnaire (HAQ), which is a 19‐item, arthritis‐specific functional assessment measure. Patients were asked to rate two or three items each in eight areas of daily life. Each item on the HAQ is scored on a scale from 0 (no disability) to 3 (greatest disability) 6. Active range of motion in flexion, abduction, and external rotation using an inclinometer 7. Adverse events |
|
| Notes | Unpublished numerical outcome data and information regarding study design (required for risk of bias assessment) provided by trialist on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An assistant at the center randomized subjects into one of two groups by asking them to select one of 74 identical opaque sealed envelopes. The envelopes contained a study number and a group number: 1 (placebo) or 2 (laser). The group number corresponded to the settings on a switch on the laser unit" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "An assistant at the center randomized subjects into one of two groups by asking them to select one of 74 identical opaque sealed envelopes. The envelopes contained a study number and a group number: 1 (placebo) or 2 (laser). The group number corresponded to the settings on a switch on the laser unit" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the assistant of the center, the treating physiotherapists, nor the patients had any knowledge of which group was receiving the active laser treatment." Comment: Participants and personnel were blind to treatment |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Quote: "Neither the assistant of the center, the treating physiotherapists, nor the patients had any knowledge of which group was receiving the active laser treatment." Comment: Blinded participants self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "A physical therapist at the center, who was unaware of the treatment type being received by each patient, performed the clinical assessments at baseline and at weeks 4, 8, and 16." Comment: Blinded outcome assessors measured range of motion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Eleven patients (six from the experimental group and five from the control group) left the study to seek another treatment method because they still had symptoms after six treatments. The study was completed with 63 patients." Comment: The number of dropouts (and reasons for this) were similar between the groups and are unlikely to have biased the results |
| Selective reporting (reporting bias) | Low risk | Comment: Numerical outcome data was fully reported for overall pain, night pain, and activity‐related pain. Data for all other outcomes was reported in Figures as means with unlabelled error bars and an indication of whether differences between groups were statistically significant (P<0.05) or not. However, complete numerical data for these partially reported outcomes was provided by the trialist on request |
| Other bias | Low risk | Comment: No other sources of bias identified |
Taverna 1990.
| Methods |
Design: Parallel group, two‐arm double‐blind randomised controlled trial (Italy) Interventions: Low‐level laser therapy (LLLT) or placebo laser therapy Sample size calculation: Not reported Analysis: Intention‐to‐treat analysis Source of funding: Not reported |
|
| Participants |
Number of participants: 40 (20 per group) Baseline characteristics: Age, sex and duration of symptoms not reported Inclusion criteria: 1. Diagnosed with scapulohumeral periarthritis Exclusion criteria: Not reported |
|
| Interventions |
Laser therapy (N=20) Components of intervention: Low‐level laser therapy (1000Hz, 24mW). Trialists irradiated painful points (where the pain occurs spontaneously and with a ratio more or less closely with the damaged structures), the points of greater access (points which may also not evoke a painful response, or even pressure, but where the emitted beam can penetrate better into the tissues and effectively reach treatment areas) and to a lesser extent the trigger points (points that, when excited, trigger pain in a target area that never corresponds to the trigger point) Dosage: 15 to 20 minutes Frequency of administration: Daily for six days Provider: Orthopaedic physician Placebo laser therapy (N=20) Participants received the same interventions as described above, except that placebo laser therapy was provided |
|
| Outcomes | Outcomes assessed at the end of six days treatment. No primary outcome was reported by the trialists 1. Patient‐reported improvement in pain and function, rated as "excellent result" = improvement of 80% or more; "good result" = improvement between 60% to 80%; "reasonable result" = improvement between 40% to 60%; or "insufficient result" = improvement less than 40% 2. Adverse events |
|
| Notes | Article is written in Italian. MP used Google Translate to translate into English. Quality of translation was good. There were 40 additional participants in this RCT who had cervical osteoarthritis (their data has not be included in this table). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For each type of pathology we divided the patients, using the table of random numbers, into two groups: treated and untreated with IR laser..." (Google Translate translation of Italian article) Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on how the allocation sequence was concealed was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "... and all were subjected to the same number of sessions and the same application diagrams with the apparatus of laser emission both cases "in function", with the same sounds (acoustic marks bearer of power is on) and bright light (pointing), a subgroup was actually treated while the other was used as a control being turned OFF prior to the application through the laser diode removed from the handpiece" (Google Translate translation of Italian article) Comment: Participants, but not personnel, were blind to treatment |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Comment: Blinded participants self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The evaluation was conducted before treatment and at the end of the same, and the results were evaluated by one of A. not aware of the subgroup to which the patient belonged (treated or placebo)" (Google Translate translation of Italian article) Comment: Assessors of adverse events were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, and outcome data was reported as being based on the number of randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data was fully reported for all outcomes specified in the methods section of the publication, but without a trial protocol it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arslan 2001 | Ineligible intervention: randomised controlled trial of glucocorticoid injection versus physical therapy plus non‐steroidal anti‐inflammatory drug. Not able to separate out the effect of physical therapy. Included in Cochrane Review of corticosteroid injection for shoulder disorders |
| Buchbinder 2007 | Ineligible intervention: placebo ultrasound was provided to one group (and compared to other physical therapies) |
| Celik 2010 | Ineligible intervention: TENS was provided to both groups (along with a physical therapy) |
| Fang 2006 | Ineligible intervention: trial compared transcutaneous electrical point stimulation to electroacupuncture, each applied to various acupuncture points (which would not be able to be delivered by a manual therapist/physical therapist/physiotherapist) |
| Grossi 1986 | Seventy‐three patients with either lateral epicondylitis or adhesive capsulitis (numbers of each individual diagnosis not given). Not possible to separate lateral epicondylitis and adhesive capsulitis data |
| Johnson 2007 | Ineligible intervention: therapeutic ultrasound was provided to all groups (along with a physical therapy) |
| Koh 2013 | Ineligible intervention: TENS provided to all groups (with or without bee venom acupuncture) |
| Ma 2013 | Ineligible intervention: therapeutic ultrasound and interferential current provided to all groups (with or without cryotherapy) |
| Morgan 1996 | Ineligible intervention: RCT of the use of TENS to control pain during a painful intervention for shoulder disorder, not an intervention for the disorder |
| Nellutla 2009 | Ineligible intervention: therapeutic ultrasound was provided to all groups (with or without a co‐intervention) |
| Sharad 2011 | Ineligible intervention: therapeutic ultrasound was provided to all groups (with or without a co‐intervention) |
| Sirajuddin 2010 | Ineligible intervention: therapeutic ultrasound was provided to all groups (with or without a co‐intervention) |
| Vecchini 1984 | Adhesive capsulitis data not presented separately. Twelve of the 24 subjects in the study had adhesive capsulitis, while the remaining 12 had lateral epicondylitis of the elbow |
| Wen 2009 | Ineligible intervention: interferential current was provided to all groups (with or without a co‐intervention) |
| Yang 2012 | Ineligible intervention: therapeutic ultrasound was provided to all groups (with or without a co‐intervention) |
| Zhu 2004 | Ineligible intervention: trial compared exercises plus Chinese medicine iontophoresis to pain block therapy. A manual therapist/physical therapist/physiotherapist would be unable to deliver the Chinese medicine components |
Characteristics of studies awaiting assessment [ordered by study ID]
Alicicco 2000.
| Methods | Currently only available as a conference abstract |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611000680965.
| Trial name or title | The rehabilitation of glenohumeral Range of Motion in Patients with Frozen Shoulder: A Comparison Between Conventional Therapy, Placebo and 'SCENAR' Electrical Stimulation Therapy. |
| Methods | Parallel group, two‐arm double‐blind randomised controlled trial (Australia) |
| Participants | Inclusion criteria: Patients must present with frozen shoulder Exclusion criteria: Pregnancy, Pacemakers, Tumours, Any cognitive impairment, intellectual disability or mental illness that affects their ability to understand written and verbal instructions Age minimum: 18 years Age maximum: 65 years Gender: Both males and females |
| Interventions | Self Controlled Energy Neurological Adaptive Device (SCENAR) electrical stimulation therapy 1 X 30 minute treatment sessions on the shoulder joint per week for 12 weeks. SCENAR is administered in a setting similar to massage therapy, with the patient sitting or lying on a massage table. The device is then placed on the patients skin and moved around the area of the injury. During this the patient may feel a slight tingling sensation SCENAR Placebo stimulation therapy 1 X 30 minute treatment sessions on the shoulder joint per week for 12 weeks. This treatment will be exactly the same as SCENAR therapy, excpet that the patient will not feel a slight tingling sensation, this is a custom made placebo device that turns on but does not emit any electrical signal. The patients be assured that some people are more sensitive than others and may or may not feel anything during treatment |
| Outcomes |
Primary outcomes: Shoulder range of motion The Constant Shoulder Score and the Shoulder Assessment Form will be used Secondary outcomes: To measure changes in pain and quality of life during recovery using, SF‐36 PIQ (Pain Impact Questionnaire)‐6 |
| Starting date | 1st June 2011 |
| Contact information | Name: Dr Dale Lovell Address: University of the Sunshine Coast, Sippy Downs Drive, Sippy Downs, QLD, 4556, Australia Email: dlovell@usc.edu.au |
| Notes | ACTRN12611000680965 |
Differences between protocol and review
The original review outcomes were pain, range of motion (active and passive), function or disability and quality of life, strength, return to work, participant perception of overall effect, global preference, physician preference and adverse events. The outcomes reported in this review have been modified from the original review to make them as consistent as possible with other Cochrane reviews on shoulder disorders and other chronic pain conditions. To improve the succinctness of the review, we only included one measurement instrument per outcome and one time point per outcome category. We assessed study risk of bias using The Cochrane Collaboration's 'Risk of bias' tool in this update of the review. We have included a 'summary of findings' table and an ORBIT outcome matrix.
Contributions of authors
MJP was responsible for writing the review, performing the searches, selecting trials, performing risk of bias assessment, data extraction, analysing the data and interpreting the results of the updated review.
SG was responsible for performing the searches, selecting trials and performing the data extraction and quality assessment for the initial review, defining the review comparisons and outcomes of interest of the initial and updated review, analysing and interpreting the results, and contributing to writing both the initial and updated review.
SK was responsible for performing risk of bias assessment, data extraction and contributing to writing the manuscript for the updated review.
RJ was responsible for performing risk of bias assessment, data extraction and contributing to writing the manuscript for the updated review.
BM was responsible for selecting trials and contributing to writing the manuscript for the updated review.
RB was responsible for performing the data extraction and quality assessment for the initial review, defining the review comparisons and outcomes of interest of both the initial and updated review, analysing and interpreting the results, and contributing to writing both the initial and updated review.
Sources of support
Internal sources
Australasian Cochrane Centre, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Australia.
External sources
No sources of support supplied
Declarations of interest
RB is Joint Co‐ordinating Editor and RJ is Managing Editor of the Cochrane Musculoskeletal Group. To avoid bias, they excluded themselves from the editorial and publication process for this review.
SG is a practicing physiotherapist in part‐time private physiotherapy practice (self employed) and as such receives remuneration for the delivery of physiotherapy interventions.
BM is a practicing physiotherapist in private physiotherapy practice and as such receives remuneration for the delivery of physiotherapy interventions.
New
References
References to studies included in this review
Battisti 2007 {published data only}
- Battisti E, Bianciardi L, Albanese A, Piazza E, Rigato M, Galassi G, et al. The new magnetic therapy TAMMEF in the treatment of simple shoulder pain. La Clinica Terapeutica 2007;158:397‐401. [PubMed] [Google Scholar]
Bumin 2001 {published data only}
- Bumin G, Can F. Effects of iontophoresis and phonophoresis methods on pain in cases with shoulder periarthritis. Pain Clinic 2001;13:159‐62. [Google Scholar]
Calis 2006 {published data only}
- Calis M, Demir H, Ulker S, Kirnap M, Duygulu F, Calis HT. Is intraarticular sodium hyaluronate injection an alternative treatment in patients with adhesive capsulitis?. Rheumatology International 2006;26:536‐40. [DOI] [PubMed] [Google Scholar]
Carette 2003 {published data only}
- Carette S, Moffet H, Tardif J, Bessette L, Morin F, Frémont P, et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo‐controlled trial. Arthritis and Rheumatism 2003;48:829‐38. [DOI] [PubMed] [Google Scholar]
Cheing 2008 {published data only}
- Cheing GLY, So EML, Chao CYL. Effectiveness of electroacupuncture and interferential electrotherapy in the management of frozen shoulder. Journal of Rehabilitation Medicine 2008;40:166‐70. [DOI] [PubMed] [Google Scholar]
Dewan 2011 {published data only}
- Dewan A, Sharma R. Effectiveness of transcutaneous electrical nerve stimulation and interferential electrotherapy in adhesive capsulitis. Pb Journal of Orthopaedics 2011;12(1):64‐71. [Google Scholar]
Dogru 2008 {published data only}
- Dogru H, Basaran S, Sarpel T. Effectiveness of therapeutic ultrasound in adhesive capsulitis. Joint, Bone, Spine 2008;75:445‐50. [DOI] [PubMed] [Google Scholar]
Ghosh 2012 {published data only}
- Ghosh TK, Bera AK, Hossain ME, Sarkar PS. Comparison of results of three different methods of treatment for adhesive capsulitis of shoulder. Journal of the Indian Medical Association 2012;110(11):827‐8. [PubMed] [Google Scholar]
Guler‐Uysal 2004 {published data only}
- Guler‐Uysal F, Kozanoglu E. Comparison of the early response to two methods of rehabilitation in adhesive capsulitis. Swiss Medical Weekly 2004;134:353‐8. [DOI] [PubMed] [Google Scholar]
Kanai 2006 {published data only}
- Kanai S, Taniguchi N. Effect of polarity exchangeable permanent magnet on frozen shoulder pain. Pain Clinic 2006;18:37‐45. [Google Scholar]
Leclaire 1991 {published data only}
- Leclaire R, Bourgouin J. Electromagnetic treatment of shoulder periarthritis: a randomized controlled trial of the efficiency and tolerance of magnetotherapy. Archives of Physical Medicine and Rehabilitation 1991;72:284‐7. [PubMed] [Google Scholar]
Lee 1973 {published data only}
- Lee M, Haq AM, Wright V, Longton EB. Periarthritis of the shoulder: a controlled trial of physiotherapy. Physiotherapy 1973;59:312‐5. [PubMed] [Google Scholar]
Leung 2008 {published data only}
- Leung MS, Cheing GL. Effects of deep and superficial heating in the management of frozen shoulder. Journal of Rehabilitation Medicine 2008;40:145‐50. [DOI] [PubMed] [Google Scholar]
Maryam 2012 {published data only}
- Maryam M, Zahra K, Adeleh B, Morteza Y. Comparison of corticosteroid injections, physiotherapy, and combination therapy in treatment of frozen shoulder. Pakistan Journal of Medical Sciences 2012;28:648‐51. [Google Scholar]
Pajareya 2004 {published data only}
- Pajareya K, Chadchavalpanichaya N, Painmanakit S, Kaidwan C, Puttaruksa P, Wongsaranuchit Y. Effectiveness of physical therapy for patients with adhesive capsulitis: a randomized controlled trial. Journal of the Medical Association of Thailand 2004;87:473‐80. [PubMed] [Google Scholar]
Rigato 2002 {published data only}
- Rigato M, Battisti E, Fortunato M, Giordano N. Comparison between the analgesic and therapeutic effects of a musically modulated electromagnetic field (TAMMEF) and those of a 100 Hz electromagnetic field: blind experiment on patients suffering from cervical spondylosis or shoulder periarthritis. Journal of Medical Engineering & Technology 2002;26:253‐8. [DOI] [PubMed] [Google Scholar]
Ryans 2005 {published data only}
- Ryans I, Montgomery A, Galway R, Kernohan WG, McKane R. A randomized controlled trial of intra‐articular triamcinolone and/or physiotherapy in shoulder capsulitis. Rheumatology 2005;44:529‐35. [DOI] [PubMed] [Google Scholar]
Stergioulas 2008 {published data only}
- Stergioulas A. Low‐power laser treatment in patients with frozen shoulder: preliminary results. Photomedicine and Laser Surgery 2008;26:99‐105. [DOI] [PubMed] [Google Scholar]
Taverna 1990 {published data only}
- Taverna E, Parrini M, Cabitza P. Laser therapy versus placebo in the treatment of some bone and joint pathology. Minerva Ortopedica e Traumatologica 1990;41:631‐6. [Google Scholar]
References to studies excluded from this review
Arslan 2001 {published data only}
- Arslan S, Celiker R. Comparison of the efficacy of local corticosteroid injections and physiotherapy for the treatment of adhesive capsulitis. Rheumatology International 2001;21:20‐3. [DOI] [PubMed] [Google Scholar]
Buchbinder 2007 {published data only}
- Buchbinder R, Youd JM, Green S, Stein A, Forbes A, Harris A, et al. Efficacy and cost‐effectiveness of physiotherapy following glenohumeral joint distension for adhesive capsulitis: a randomized trial. Arthritis and Rheumatism 2007;57:1027‐37. [DOI] [PubMed] [Google Scholar]
Celik 2010 {published data only}
- Celik D. Comparison of the outcomes of two different exercise programs on frozen shoulder. Acta Orthopaedica et Traumatologica Turcica 2010;44:285‐92. [DOI] [PubMed] [Google Scholar]
Fang 2006 {published data only}
- Fang J‐Q, Zhang Y, Xuan L‐H, Liu K‐Z, Chen L. Observation on clinical therapeutic effect of transcutaneous point electric stimulation on periarthritis of shoulder at different stages. Zhongguo Zhenjiu 2006;26:11‐4. [PubMed] [Google Scholar]
Grossi 1986 {published data only}
- Grossi E, Monza GC, Pollavini S, Bona L. NSAID ionisation in the management of soft‐tissue rheumatism: Role played by the drug, electrical stimulation and suggestion. Clinical and Experimental Rheumatology 1986;4:265‐7. [PubMed] [Google Scholar]
Johnson 2007 {published data only}
- Johnson AJ, Godges JJ, Zimmerman GJ, Ounanian LL. The effect of anterior versus posterior glide joint mobilization on external rotation range of motion in patients with shoulder adhesive capsulitis. Journal of Orthopaedic and Sports Physical Therapy 2007;37:88‐99. [DOI] [PubMed] [Google Scholar]
Koh 2013 {published data only}
- Koh PS, Seo BK, Cho NS, Park HS, Park DS, Baek YH. Clinical effectiveness of bee venom acupuncture and physiotherapy in the treatment of adhesive capsulitis: a randomized controlled trial. Journal of Shoulder and Elbow Surgery 2013;22(8):1053‐62. [DOI] [PubMed] [Google Scholar]
Ma 2013 {published data only}
- Ma S‐Y, Je HD, Jeong JH, Kim H‐Y, Kim H‐D. Effects of whole‐body cryotherapy in the management of adhesive capsulitis of the shoulder. Archives of Physical Medicine & Rehabilitation 2013;94(1):9‐16. [DOI] [PubMed] [Google Scholar]
Morgan 1996 {published data only}
- Morgan B, Jones AR, Mulcahy KA, Finlay DB, Collett B. Transcutaneous electric nerve stimulation (TENS) during distension shoulder arthrography: a controlled trial. Pain 1996;64:265‐7. [DOI] [PubMed] [Google Scholar]
Nellutla 2009 {published data only}
- Nellutla M, Gin P. Comparative study between efficacy of PNF movement patterns versus conventional free exercises on functional activities among patients with chronic peri‐arthritis of shoulder. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:62‐7. [Google Scholar]
- Nellutla M, Giri P, M'Kumbuzi VRP, Patel HC. PNF movement patterns compared to the use of conventional free exercises to improve joint ROM in chronic peri‐arthritis of the shoulder. Indian Journal of Physiotherapy & Occupational Therapy 2009;3:31‐4. [Google Scholar]
Sharad 2011 {published data only}
- Sharad KS. A comparitive study on the efficacy of end range mobilization techniques in treatment of adhesive capsulitis of shoulder. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:28‐31. [Google Scholar]
Sirajuddin 2010 {published data only}
- Sirajuddin M, Quddus N, Grover D. Comparison of anterior versus posterior glide mobilisation techniques for improving internal rotation range of motion in shoulder adhesive capsulitis. Indian Journal of Physiotherapy & Occupational Therapy 2010;4:152‐7. [Google Scholar]
Vecchini 1984 {published data only}
- Vecchini L, Grossi E. Ionisation with diclofenac sodium in rheumatic disorders: A double blind placebo controlled trial. Journal of International Medical Research 1984;12:346‐50. [DOI] [PubMed] [Google Scholar]
Wen 2009 {published data only}
- Wen L. Analysis on effect of functional exercises to promote rehabilitation of patients with periarthritis of shoulder. Chinese Nursing Research 2009;23:1925‐6. [Google Scholar]
Yang 2012 {published data only}
- Yang JL, Jan MH, Chang CW, Lin JJ. Effectiveness of the end‐range mobilization and scapular mobilization approach in a subgroup of subjects with frozen shoulder syndrome: a randomized control trial. Manual Therapy 2012;17:47‐52. [DOI] [PubMed] [Google Scholar]
Zhu 2004 {published data only}
- Zhu ZZ, Liu CM, Feng WX. Analgesic effects of traditional Chinese medicine iontophoresis and rehabilitation training on periarthritis of shoulder: a randomized controlled study. Chinese Journal of Clinical Rehabilitation 2004;8:2698‐9. [Google Scholar]
References to studies awaiting assessment
Alicicco 2000 {published data only}
- Alicicco E, Chinni LM, Castellano V, Aparo UL. Hyperthermia in scapulohumeral periarthritis due to overuse in athletes. Journal of Sports Sciences 2000;18:489. [Google Scholar]
References to ongoing studies
ACTRN12611000680965 {published data only}
- http://www.anzctr.org.au/ACTRN12611000680965.aspx. The rehabilitation of glenohumeral range of motion in patients with frozen shoulder: a comparison between conventional therapy, placebo and 'SCENAR' electrical stimulation therapy.
Additional references
Allen 2006
- Allen RJ. Physical agents used in the management of chronic pain by physical therapists. Physical Medicine and Rehabilitation Clinics of North America 2006;17:315‐45. [DOI] [PubMed] [Google Scholar]
Angst 2011
- Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care and Research 2011;63:S174‐88. [DOI] [PubMed] [Google Scholar]
Basford 1989
- Basford JR. Low‐energy laser therapy: controversies and new research findings. Lasers in Surgery and Medicine 1989;9:1‐5. [DOI] [PubMed] [Google Scholar]
Batheja 2006
- Batheja P, Thakur R, Michniak B. Transdermal iontophoresis. Expert Opinion on Drug Delivery 2006;3:127‐38. [DOI] [PubMed] [Google Scholar]
Beatti 2010
- Beatti A, Raynor A, Souvlis T, Chipchase L. The analgesic effect of interferential therapy on clinical and experimentally induced pain. Physical Therapy Reviews 2010;15:243‐52. [Google Scholar]
Binder 1984a
- Binder AI, Bulgen DY, Hazelman BL, Roberts S. Frozen shoulder: a long‐term prospective study. Annals of the Rheumatic Diseases 1984;43:361‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjordal 2006
- Bjordal JM, Johnson MI, Iverson V, Aimbire F, Lopes‐Martins RAB. Photoradiation in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo controlled trials. Photomedicine and Laser Surgery 2006;24:158‐68. [DOI] [PubMed] [Google Scholar]
Bjordal 2010
- Bjordal JM, Lopes‐Martins RAB, Joensen J, Iversen VV. The anti‐inflammatory mechanism of low level laser therapy and its relevance for clinical use in physiotherapy. Physical Therapy Reviews 2010;15:286‐93. [Google Scholar]
Buchbinder 2008
- Buchbinder R, Green S, Youd JM, Johnston RV, Cumpston M. Arthrographic distension for adhesive capsulitis (frozen shoulder). Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD007005] [DOI] [PubMed] [Google Scholar]
Cates 2004 [Computer program]
- Dr Chris Cates EBM website. Available from http://www.nntonline.net/. Visual Rx NNT Calculator. Dr Chris Cates EBM website. Available from http://www.nntonline.net/, 2004.
Chow 2009
- Chow R, Johnson MI, Lopes‐Martins RAB, Bjordal JM. Efficacy of low‐level laser therapy in the management of neck pain: a systematic review and meta‐analysis of randomised placebo or active‐treatment controlled trials. Lancet 2009;374:1897‐908. [DOI] [PubMed] [Google Scholar]
Codman 1934
- Codman EA. The Shoulder. Boston: Thomas Toddog, 1934. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
DeSantana 2008
- DeSantana J, Walsh DM, Vance C, Rakel B, Sluka KA. Effectiveness of Transcutaneous Electrical Nerve Stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports 2008;10:492‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Ebadi 2014
- Ebadi S, Henschke N, Nakhostin Ansari N, Fallah E, Tulder MW. Therapeutic ultrasound for chronic low‐back pain. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD009169.pub2] [DOI] [PubMed] [Google Scholar]
Ebrahim 2013
- Ebrahim S, Akl EA, Mustafa RA, Sun X, Walter SD, Heels‐Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2013;66(9):1014‐21. [DOI] [PubMed] [Google Scholar]
Favejee 2011
- Favejee MM, Huisstede BMA, Koes BW. Frozen shoulder: the effectiveness of conservative and surgical interventions‐‐systematic review. British Journal of Sports Medicine 2011;45:49‐56. [DOI] [PubMed] [Google Scholar]
Fuentes 2010
- Fuentes JP, Armijo Olivo S, Magee DJ, Gross DP. Effectiveness of interferential current therapy in the management of musculoskeletal pain: a systematic review and meta‐analysis. Physical Therapy 2010;90:1219–38. [DOI] [PubMed] [Google Scholar]
Gordon 2007
- Gordon GA. Designed electromagnetic pulsed therapy: clinical applications. Journal of Cellular Physiology 2007;212:579–82. [DOI] [PubMed] [Google Scholar]
Hanchard 2011
- Hanchard NCA, Goodchild L, Thompson J, O'Brien T, Davison D, Richardson C. A questionnaire survey of UK physiotherapists on the diagnosis and management of contracted (frozen) shoulder. Physiotherapy 2011;97:115‐25. [DOI] [PubMed] [Google Scholar]
Hand 2008
- Hand C, Clipsham K, Rees JL, Carr AJ. Long‐term outcome of frozen shoulder. Journal of Shoulder and Elbow Surgery 2008;17:231‐6. [DOI] [PubMed] [Google Scholar]
Hawker 2011
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care and Research 2011;63:S240‐52. [DOI] [PubMed] [Google Scholar]
Hazelman 1972
- Hazleman BL. The painful stiff shoulder. Rheumatology and Physical Medicine 1972;11(8):413‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Johnson 2008
- Johnson M. Transcutaneous Electrical Nerve Stimulation. In: Watson T editor(s). Electrotherapy: Evidence based practice. 12th Edition. Edinburgh: Churchill Livingstone, 2008. [Google Scholar]
Jones 2009
- Jones I, Johnson MI. Transcutaneous electrical nerve stimulation. Continuing Education in Anaesthesia, Critical Care & Pain 2009;9:130‐5. [Google Scholar]
Khadilkar 2008
- Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low‐back pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003008.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Kroeling 2013
- Kroeling P, Gross A, Graham N, Burnie SJ, Szeto G, Goldsmith CH, et al. Electrotherapy for neck pain. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004251.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laufer 2012
- Laufer Y, Dar G. Effectiveness of thermal and athermal short‐wave diathermy for the management of knee osteoarthritis: a systematic review and meta‐analysis. Osteoarthritis and Cartilage 2012;20:957‐66. [DOI] [PubMed] [Google Scholar]
Li 2013
- Li S, Yu B, Zhou D, He C, Zhuo Q, Hulme JM. Electromagnetic fields for treating osteoarthritis. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD003523.pub2] [DOI] [PubMed] [Google Scholar]
Machet 2002
- Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. International Journal of Pharmaceuticals 2002;243:1‐15. [DOI] [PubMed] [Google Scholar]
Markov 2007
- Markov MS. Expanding use of pulsed electromagnetic field therapies. Electromagnetic Biology and Medicine 2007;26:257–74. [DOI] [PubMed] [Google Scholar]
Maund 2012
- Maund E, Craig D, Suekarran S, Neilson Ar, Wright K, Brealey S, et al. Management of frozen shoulder: a systematic review and cost‐effectiveness analysis. Health Technology Assessment 2012;16:1‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain Mechanisms: a new theory. Science 1965;150:971‐9. [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain‐establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Neviaser 1987
- Neviaser JS. Adhesive capsulitis of the shoulder: a study of the pathological findings in periarthritis of the shoulder. Journal of Bone and Joint Surgery 1945;27:211‐22. [Google Scholar]
Norris 2013
- Norris SL, Moher D, Reeves BC, Shea B, Loke Y, Garner S, et al. Issues relating to selective reporting when including non‐randomized studies in systematic reviews on the effects of healthcare interventions. Research Synthesis Methods 2013;4:36‐47. [DOI] [PubMed] [Google Scholar]
O'Brien 2007
- O’Brien WD. Ultrasound ‐ biophysics mechanisms. Progress in Biophysics and Molecular Biology 2007;93:212‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2013a
- Page MJ, McKenzie JE, Forbes A. Many scenarios exist for selective inclusion and reporting of results in randomized trials and systematic reviews. Journal of Clinical Epidemiology 2013;66(5):524‐37. [DOI] [PubMed] [Google Scholar]
Page 2013b
- Page MJ, O'Connor D, Pitt V, Massy‐Westropp N. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD009601.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2014
- Page MJ, Green S, Kramer S, Johnston RV, McBain B, Chau M, Buchbinder R. Manual therapy and exercise for adhesive capsulitis (frozen shoulder). Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peplow 2010
- Peplow PV, Chung T‐Y, Baxter D. Application of low‐level laser technologies for pain relief and wound healing. Physical Therapy Reviews 2010;15:253‐85. [Google Scholar]
Reeves 1975
- Reeves B. The natural history of the frozen shoulder syndrome. Scandinavian Journal of Rheumatology 1975;4:193‐6. [DOI] [PubMed] [Google Scholar]
Roustit 2014
- Roustit M, Blaise S, Cracowski JL. Trials and tribulations of skin iontophoresis in therapeutics. British Journal of Clinical Pharmacology 2014;77:63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2009
- Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis and Rheumatism 2009;61:623‐32. [DOI] [PubMed] [Google Scholar]
Roy 2010
- Roy JS, MacDermid JC, Woodhouse LJ. A systematic review of the psychometric properties of the Constant‐Murley score. Journal of Shoulder and Elbow Surgery 2010;19:157‐64. [DOI] [PubMed] [Google Scholar]
Rutjes 2009
- Rutjes AWS, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2010
- Rutjes AWS, Nüesch E, Sterchi R, Jüni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003132.pub2] [DOI] [PubMed] [Google Scholar]
Sakurai 2000
- Sakurai Y, Yamaguchi M, Abiko Y. Inhibitory effect of low‐level laser irradiation on LPS‐stimulated prostaglandin E2 production and cyclooxygenase‐2 in human gingival fibroblasts. European Journal of Oral Sciences 2000;1081:29‐34. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schellingerhout 2008
- Schellingerhout JM, Verhagen AP, Thomas S, Koes BW. Lack of uniformity in diagnostic labeling of shoulder pain: time for a different approach. Manual Therapy 2008;13:478‐83. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Shah 2012
- Shah SGS, Farrow A. Trends in the availability and usage of electrophysical agents in physiotherapy practices from 1990 to 2010: a review. Physical Therapy Reviews 2012;17:207‐26. [Google Scholar]
Shields 2001
- Shields N, Gormley J, O'Hare. Short‐wave diathermy: a review of existing clinical trials. Physical Therapy Reviews 2001;6:101‐18. [Google Scholar]
Sluka 2003
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. Journal of Pain 2003;4:109‐21. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Tekavec 2012
- Tekavec E, Joud A, Rittner R, Mikoczy Z, Nordander C, Petersson IF, Englund M. Population‐based consultation patterns in patients with shoulder pain diagnoses. BMC Musculoskeletal Disorders 2012;13:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Bekerom 2011
- Bekerom MPJ, Windt DAWM, ter Riet G, Heijden GJ, Bouter LM. Therapeutic ultrasound for acute ankle sprains. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD001250.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Windt 1995
- Windt DA, Koes BW, Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Annals of the Rheumatic Diseases 1995;54:959‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2004
- Walker‐Bone K, Palmer KT, Reading I, Coggon D, Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Care and Research 2004;51:642‐51. [DOI] [PubMed] [Google Scholar]
Walsh 2009
- Walsh DM, Howe TE, Johnson MI, Moran F, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006142.pub2] [DOI] [PubMed] [Google Scholar]
Watson 2008a
- Watson T. Electrotherapy: Evidence based practice. 12th Edition. Edinburgh: Churchill Livingstone, 2008. [Google Scholar]
Watson 2008b
- Watson T. Ultrasound in contemporary physiotherapy practice. Ultrasonics 2008;48:321‐9. [DOI] [PubMed] [Google Scholar]
Watson 2010
- Watson T. Key concepts with electrophysical agents. Physical Therapy Reviews 2010;15:351‐9. [Google Scholar]
Yousefi‐Nooraie 2008
- Yousefi‐Nooraie R, Schonstein E, Heidari K, Rashidian A, Pennick V, Akbari‐Kamrani M, et al. Low level laser therapy for nonspecific low‐back pain. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005107.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Green 1998
- Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment and efficacy. BMJ 1998;316:354‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2003
- Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004258] [DOI] [PMC free article] [PubMed] [Google Scholar]


