Abstract
Background
Sevoflurane is an inhaled volatile anaesthetic that is widely used in paediatric anaesthetic practice. Since its introduction, postoperative behavioural disturbance known as emergence agitation (EA) or emergence delirium (ED) has been recognized as a problem that may occur during recovery from sevoflurane anaesthesia. For the purpose of this systematic review, EA has been used to describe this clinical entity. A child with EA may be restless, may cause self‐injury or may disrupt the dressing, surgical site or indwelling devices, leading to the potential for parents to be dissatisfied with their child's anaesthetic. To prevent such outcomes, the child may require pharmacological or physical restraint. Sevoflurane may be a major contributing factor in the development of EA. Therefore, an evidence‐based understanding of the risk/benefit profile regarding sevoflurane compared with other general anaesthetic agents and adjuncts would facilitate its rational and optimal use.
Objectives
To compare sevoflurane with other general anaesthetic (GA) agents, with or without pharmacological or non‐pharmacological adjuncts, with regard to risk of EA in children during emergence from anaesthesia. The primary outcome was risk of EA; secondary outcome was agitation score.
Search methods
We searched the following databases from the date of inception to 19 January 2013: CENTRAL, Ovid MEDLINE, Ovid EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost), Evidence‐Based Medicine Reviews (EBMR) and the Web of Science, as well as the reference lists of other relevant articles and online trial registers.
Selection criteria
We included all randomized (or quasi‐randomized) controlled trials investigating children < 18 years of age presenting for general anaesthesia with or without surgical intervention. We included any study in which a sevoflurane anaesthetic was compared with any other GA, and any study in which researchers investigated adjuncts (pharmacological or non‐pharmacological) to sevoflurane anaesthesia compared with no adjunct or placebo.
Data collection and analysis
Two review authors independently searched the databases, decided on inclusion eligibility of publications, ascertained study quality and extracted data. They then resolved differences between their results by discussion. Data were entered into RevMan 5.2 for analyses and presentation. Comparisons of the risk of EA were presented as risk ratios (RRs) with 95% confidence intervals (CIs). Sevoflurane is treated as the control anaesthesia in this review. Sensitivity analyses were performed as appropriate, to exclude studies with a high risk of bias and to investigate heterogeneity.
Main results
We included 158 studies involving 14,045 children. Interventions to prevent EA fell into two broad groups. First, alternative GA compared with sevoflurane anaesthesia (69 studies), and second, use of an adjunct with sevoflurane anaesthesia versus sevoflurane without an adjunct (100 studies). The overall risk of bias in included studies was low. The overall Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE) assessment of the quality of the evidence was moderate to high. A wide range of EA scales were used, as were different levels of cutoff, to determine the presence or absence of EA. Some studies involved children receiving potentially inadequate or no analgesia intraoperatively during painful procedures.
Halothane (RR 0.51, 95% CI 0.41 to 0.63, 3534 participants, high quality of evidence) and propofol anaesthesia were associated with a lower risk of EA than sevoflurane anaesthesia. Propofol was effective when used throughout anaesthesia (RR 0.35, 95% CI 0.25 to 0.51, 1098 participants, high quality of evidence) and when used only during the maintenance phase of anaesthesia after sevoflurane induction (RR 0.59, 95% CI 0.46 to 0.76, 738 participants, high quality of evidence). No clear evidence was found of an effect on risk of EA of desflurane (RR 1.46, 95% CI 0.92 to 2.31, 408 participants, moderate quality of evidence) or isoflurane (RR 0.76, 95% CI 0.46 to 1.23, 379 participants, moderate quality of evidence) versus sevoflurane.
Compared with no adjunct, effective adjuncts for reducing the risk of EA during sevoflurane anaesthesia included dexmedetomidine (RR 0.37, 95% CI 0.29 to 0.47, 851 participants, high quality of evidence), clonidine (RR 0.45, 95% CI 0.31 to 0.66, 739 participants, high quality of evidence), opioids, in particular fentanyl (RR 0.37, 95% CI 0.27 to 0.50, 1247 participants, high quality of evidence) and a bolus of propofol (RR 0.58, 95% CI 0.38 to 0.89, 394 participants, moderate quality of evidence), ketamine (RR 0.30, 95% CI 0.13 to 0.69, 231 participants, moderate quality of evidence) or midazolam (RR 0.57, 95% CI 0.41 to 0.81, 116 participants, moderate quality of evidence) at the end of anaesthesia. Midazolam oral premedication (RR 0.81, 95% CI 0.59 to 1.12, 370 participants, moderate quality of evidence) and parental presence at emergence (RR 0.91, 95% CI 0.51 to 1.60, 180 participants, moderate quality of evidence) did not reduce the risk of EA.
One or more factors designated as high risk of bias were noted in less than 10% of the included studies. Sensitivity analyses of these studies showed no clinically relevant changes in the risk of EA. Heterogeneity was significant with respect to these comparisons: halothane; clonidine; fentanyl; midazolam premedication; propofol 1 mg/kg bolus at end; and ketamine 0.25 mg/kg bolus at end of anaesthesia. With investigation of heterogeneity, the only clinically relevant changes to findings were seen in the context of potential pain, namely, the setting of adenoidectomy/adenotonsillectomy (propofol bolus; midazolam premedication) and the absence of a regional block (clonidine).
Authors' conclusions
Propofol, halothane, alpha‐2 agonists (dexmedetomidine, clonidine), opioids (e.g. fentanyl) and ketamine reduce the risk of EA compared with sevoflurane anaesthesia, whereas no clear evidence shows an effect for desflurane, isoflurane, midazolam premedication and parental presence at emergence. Therefore anaesthetists can consider several effective strategies to reduce the risk of EA in their clinical practice. Future studies should ensure adequate analgesia in the control group, for which pain may be a contributing or confounding factor in the diagnosis of EA. Regardless of the EA scale used, it would be helpful for study authors to report the risk of EA, so that this might be included in future meta‐analyses. Researchers should also consider combining effective interventions as a multi‐modal approach to further reduce the risk of EA.
Keywords: Child; Humans; Anesthesia Recovery Period; Adjuvants, Anesthesia; Adjuvants, Anesthesia/adverse effects; Akathisia, Drug‐Induced; Akathisia, Drug‐Induced/etiology; Akathisia, Drug‐Induced/prevention & control; Anesthesia, General; Anesthetics, Inhalation; Anesthetics, Inhalation/adverse effects; Clonidine; Clonidine/adverse effects; Desflurane; Dexmedetomidine; Dexmedetomidine/adverse effects; Halothane; Halothane/adverse effects; Isoflurane; Isoflurane/adverse effects; Isoflurane/analogs & derivatives; Methyl Ethers; Methyl Ethers/adverse effects; Midazolam; Midazolam/adverse effects; Propofol; Propofol/adverse effects; Sevoflurane
Plain language summary
Agitation in children after sevoflurane anaesthesia
Review question
We reviewed the evidence looking at how often children wake up agitated after a sevoflurane general anaesthetic compared with other general anaesthetics. We also reviewed evidence looking at the effects of other treatments (e.g. a medication given during the anaesthetic, the presence of a parent when a child wakes up) on how often children wake up agitated after receiving a sevoflurane anaesthetic.
Background
Sevoflurane is a commonly used anaesthetic gas for children because it can be breathed in by face mask and works very quickly in getting children off to sleep. Sevoflurane is given continuously during an operation to keep the child asleep, and it is turned off when it is time for the child to wake up. It is very common for children, especially preschool children, to wake up restless, agitated, delirious or thrashing around after receiving a sevoflurane anaesthetic. We call this "emergence agitation." It can occur even when no pain is present and usually resolves within 30 minutes of waking up. Children with emergence agitation may injure themselves, bump the operation wound and pull out drips or wound drains. Emergence agitation can be distressing for parents and caregivers. We wanted to discover whether the rate of emergence agitation is lowered when different anaesthetics are used. We also wanted to know whether treatments can be given to reduce the rate of emergence agitation when sevoflurane is used.
Study characteristics
The evidence is current to January 2013. We found a total of 158 studies involving 14,045 children. A total of 69 studies compared a sevoflurane anaesthetic with a different anaesthetic, and 100 studies looked at treatments to reduce the rate of emergence agitation with a sevoflurane anaesthetic. Most of these treatments were medications that were compared with a dummy treatment (placebo) or with no medication. We reran the search in April 2014 and will address identified studies of interest when we update the review.
Key results
The medications propofol, halothane, alpha‐2 agonists (dexmedetomidine, clonidine), opioids (e.g. fentanyl) and ketamine reduce the rate of emergence agitation, whereas no clear evidence of an effect was found for the anaesthetic gases desflurane and isoflurane, the premedication midazolam and parental presence when a child wakes up from anaesthesia.
Quality of the evidence
Overall the evidence is of moderate to high quality. Researchers should consider combining effective interventions to see whether the risk of EA can be reduced further.
Summary of findings
Summary of findings for the main comparison. Other GA/adjunct versus sevoflurane anaesthesia for reducing risk of emergence agitation.
| Other GA/adjunct versus sevoflurane anaesthesia for reducing risk of emergence agitation | ||||||
| Patient or population: patients with emergence agitation Settings: hospital setting (postanaesthesia care unit) Intervention: other GA/adjunct versus sevoflurane anaesthesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Other GA/adjunct versus sevoflurane anaesthesia | |||||
| Emergence agitation—Propofol induction and maintenance | Study populationa | RR 0.35 (0.25 to 0.51) | 1098 (14 studies) | ⊕⊕⊕⊕ highb | ||
| 314 per 1000 | 110 per 1000 (78 to 160) | |||||
| Moderatea | ||||||
| 368 per 1000 | 129 per 1000 (92 to 188) | |||||
| Emergence agitation—Dexmedetomidine | Study populationa | RR 0.37 (0.29 to 0.47) | 851 (12 studies) | ⊕⊕⊕⊕ highb | ||
| 434 per 1000 | 160 per 1000 (126 to 204) | |||||
| Moderatea | ||||||
| 368 per 1000 | 136 per 1000 (107 to 173) | |||||
| Emergence agitation—Fentanyl | Study populationa | RR 0.37 (0.27 to 0.5) | 1247 (15 studies) | ⊕⊕⊕⊕ high | ||
| 442 per 1000 | 164 per 1000 (119 to 221) | |||||
| Moderatea | ||||||
| 368 per 1000 | 136 per 1000 (99 to 184) | |||||
| Emergence agitation—Clonidine | Study populationa | RR 0.45 (0.31 to 0.66) | 739 (9 studies) | ⊕⊕⊕⊕ high | ||
| 436 per 1000 | 196 per 1000 (135 to 288) | |||||
| Moderatea | ||||||
| 368 per 1000 | 166 per 1000 (114 to 243) | |||||
| Emergence agitation—Halothane | Study populationa | RR 0.51 (0.41 to 0.63) | 3534 (34 studies) | ⊕⊕⊕⊕ highc | ||
| 294 per 1000 | 150 per 1000 (121 to 186) | |||||
| Moderatea | ||||||
| 368 per 1000 | 188 per 1000 (151 to 232) | |||||
| Emergence agitation—Isoflurane | Study populationa | RR 0.76 (0.46 to 1.23) | 379 (6 studies) | ⊕⊕⊕⊝ moderated | ||
| 328 per 1000 | 249 per 1000 (151 to 403) | |||||
| Moderatea | ||||||
| 368 per 1000 | 280 per 1000 (169 to 453) | |||||
| Emergence agitation—Desflurane | Study populationa | RR 1.46 (0.92 to 2.31) | 408 (6 studies) | ⊕⊕⊕⊝ moderated | ||
| 176 per 1000 | 258 per 1000 (162 to 408) | |||||
| Moderatea | ||||||
| 368 per 1000 | 537 per 1000 (339 to 850) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aWe provided 1 typical risk value for the outcome of emergence agitation and assigned this as "moderate risk." We used the mean control group risk of emergence agitation for all control group participants across all comparisons for this purpose. bLarge effect with precise result of appreciable benefit. cSensitivity analyses of studies assessed as having high risk of bias did not change the results. Large effect with precise result of appreciable benefit. dDowngraded for possible imprecision as the result of wide confidence intervals.
Background
Sevoflurane is an inhaled volatile anaesthetic that is widely used in paediatric anaesthetic practice. First described in 1975 (Wallin 1975), its use commenced in Japan in 1992 and became widespread in 1995 (Holzki 1999). Sevoflurane is a non‐pungent, insoluble agent that facilitates smooth, rapid induction and emergence (Holzki 1999; Johr 2002). However, since its introduction, postoperative behavioural disturbance, observed predominantly in the paediatric population (Cole 2002; Voepel‐Lewis 2003), has become an important clinical issue. Some studies suggest that sevoflurane is associated with the highest risk of behavioural disturbance of all current general anaesthetics, while others show conflicting results (Abbotts 2006; Foesel 2001; Johr 2002; Vlajkovic 2006).
These behavioural changes have been described in the literature using a variety of descriptive terms, such as emergence agitation (EA), emergence delirium (ED) and postanaesthetic excitation. No consensus has been reached regarding a definition (Cole 2002; Vlajkovic 2006); however the condition has been described as a mental disturbance during recovery from general anaesthesia that may consist of hallucinations, delusions and confusion manifested by moaning, restlessness, involuntary physical activity and thrashing about in the bed (Sikich 2004). Emergence delirium appears to represent a subset of EA, as not all agitated children are truly delirious (Bajwa 2010; Malarbi 2011). For the purpose of this systematic review, the term 'emergence agitation' will be used to encompass this clinical entity. Until recently, no reliable and validated scale has been available to measure EA. Concern has been expressed as to the reliability of research results and the ease of comparing studies; this probably played a part in the development in 2004 of the Pediatric Anesthesia Emergence Delirium (PAED) scale (Sikich 2004). Although the PAED scale is now the most frequently used scale in research studies, at least one investigator has described a PAED scale modification (Locatelli 2013), and some study authors are now reporting EA by using two scales simultaneously—typically PAED plus one other scale (Patel 2010; Li 2011; Na 2013; Salik 2011). In addition, some trial authors are using different PAED scores as cutoffs for the presence of EA (Characteristics of included studies). This suggests that no one scale currently fulfils all relevant requirements in determination of EA (Bajwa 2010). The potential adverse effects of EA are mostly short lived (Veyckemans 2001). It would be unusual for children with EA to be discharged from the postanaesthetic care unit (PACU), as a restless child may cause self‐injury, the dressing or surgical site may be disrupted and indwelling devices have the potential to become dislodged. To prevent such outcomes, the child may require pharmacological or physical restraint. Pharmacological management provides the disadvantage of exposing the child to medications such as opioids and sedatives. These drugs themselves could have adverse effects, and their administration could delay discharge from the PACU or hospital. The psychological and long‐term consequences of EA are largely unknown, but it has been suggested that maladaptive behaviours, for example, withdrawal, sleeping and eating problems, may be associated with EA (Holzki 1999; Kain 2004). Extra care is required to manage a patient with EA, and this might strain already limited nursing resources. Caregivers are at risk of injury when managing these children and may feel dissatisfied with the quality of available anaesthetic care. Parents who witness EA may become concerned regarding future anaesthetic experiences for their child (Houck 2005). Additional costs and potential delays to discharge may be significant. The exact aetiology of EA remains unclear; however research to date suggests numerous predisposing factors (Voepel‐Lewis 2003). Anaesthetic factors may include rapid emergence and the intrinsic characteristics of the anaesthetic. The newer volatile anaesthetic agents, such as sevoflurane, allow faster emergence, which potentially results in early manifestation of acute pain and anxiety (Wells 1999). Some authors have suggested that sevoflurane exerts a stimulating or even neurotoxic effect on the central nervous system (Constant 1999; Johr 2002; Vlajkovic 2006). Patient‐related factors include age, preoperative anxiety and the temperament of the child. The risk of EA is highest in preschoolers, potentially because of psychological immaturity in this age group (Voepel‐Lewis 2003). Surgery‐related factors include pain and type of surgery. Pain may increase the risk of EA (Lynch 1998), and the behaviour of a child in pain may mimic EA. Otorhinolaryngology and ophthalmological procedures carry an increased risk of EA; however this phenomenon has been observed even after non‐painful imaging procedures (Voepel‐Lewis 2003).
Sevoflurane may be a major contributor to the development of EA. Therefore, an evidence‐based understanding of the risk/benefit profile regarding sevoflurane compared with other general anaesthetic (GA) agents and adjuncts would facilitate its rational and optimal use.
Description of the condition
As stated above, although the condition described by the authors of included studies included terms such as ED, EA and postoperative excitation, we have used the term EA throughout this review to describe postoperative behavioural disturbance during emergence from anaesthesia.
Description of the intervention
Alternative anaesthetic agent to sevoflurane or adjunct with sevoflurane anaesthesia. Sevoflurane is treated as the control anaesthesia in this review.
How the intervention might work
Interventions may delay emergence, allowing for washout of sevoflurane before emergence occurs or modifying emergence effects on the brain in some other way yet to be determined.
Why it is important to do this review
Approximately one‐third of children experience EA after sevoflurane anaesthesia if a preventative intervention is not used (Bajwa 2010).
Objectives
To compare sevoflurane with other general anaesthetic agents, with or without pharmacological or non‐pharmacological adjuncts, with regard to risk of EA in children during emergence from anaesthesia. The primary outcome was risk of EA; the secondary outcome was agitation score.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized and quasi‐randomized, published and unpublished controlled clinical studies.
Types of participants
We included children younger than 18 years of age presenting for general anaesthesia with or without surgical intervention.
Types of interventions
We have included any sevoflurane anaesthetic with or without nitrous oxide compared with any other general anaesthetic.
Types of general anaesthetics included were other volatile anaesthetics, for example, isoflurane, desflurane and halothane; and any other general anaesthetics, for example, propofol or ketamine.
Pharmacological adjuncts such as fentanyl or other opioids, propofol, midazolam, ketamine, dexmedetomidine or clonidine or non‐pharmacological adjuncts such as parental presence.
Types of outcome measures
Primary outcomes
Emergence agitation (EA) was defined as the number of participants with postoperative behavioural disturbance, as measured by the authors of included studies.
Secondary outcomes
Postoperative behavioural disturbance or agitation scores such as PAED (Sikich 2004).
Number of participants with EA during the postoperative period after leaving the PACU, as measured by the authors of included studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 1; see Appendix 1) and the following electronic medicine, nursing, psychology and medical databases: MEDLINE (Ovid SP, 1966 to January 2013; see Appendix 2), EMBASE (Ovid SP, 1980 to January 2013; see Appendix 3), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost, 1982 to January 2013; see Appendix 4), Evidence‐Based Medicine Reviews (EBMR) and the Web of Science (1954 to January 2013; see Appendix 5).
We searched MEDLINE using medical subject headings (MeSH) and text words. We combined this search with the Cochrane highly sensitive search strategy, phases one and two, as provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005).
We adapted the other databases as appropriate.
We searched for ongoing clinical trials and unpublished studies via Internet searches on the following sites.
We reran the search in April 2014 and will address identified studies of interest when we update the review.
Searching other resources
Trials were also identified by:
manual searching: Relevant conference proceedings abstracts will be searched;
snowballing: Reference lists of relevant articles will be checked; and
contacts: Relevant trial authors will be contacted by e‐mail to identify additional studies.
We did not apply language or publication restrictions.
Data collection and analysis
Selection of studies
Review authors identified titles and abstracts of studies during the initial search. Potentially relevant studies were retrieved in full‐text version and were evaluated for inclusion by two review authors working independently.
Data extraction and management
Two review authors independently extracted data from relevant studies using a standardized data collection form. We resolved disagreements by discussion. If additional information was required, we contacted the authors of the relevant study.
Two review authors independently assessed the following.
Randomization.
Allocation concealment, which was graded according to blinding of participants and personnel according to the standard scheme of The Cochrane Collaboration.
Blinding of outcome assessments.
Losses to follow‐up and treatment of withdrawals.
Selective reporting.
Sensitivity analysis was performed to assess major differences with regard to methodological quality. Excluded studies are listed in the Characteristics of excluded studies table, and reasons for exclusion are given.
Measures of treatment effect
Sevoflurane is treated as the control anaesthesia in this review. We have presented the results from dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs); categorical scales of EA were reported in the text. We performed, as applicable, meta‐analyses on subgroups of trials that used similar outcome reporting strategies, for example, all those reporting number of children with postoperative EA in each group.
Unit of analysis issues
We identified no unit of analysis issues.
Dealing with missing data
We contacted authors of studies to ask for missing data; this was reported in the notes section of the Characteristics of included studies table. For example in one study, EA was reported only in the intervention group, and study authors provided control group data for this outcome upon request (Le Berre 2001). Included studies with > 15% withdrawals were assessed as having high risk of attrition bias.
Assessment of heterogeneity
We applied the I2 statistic (Higgins 2002) to test for heterogeneity among the studies. We used the random‐effects model to limit the effects of heterogeneity between trials, but when substantial inconsistency (I2 > 40%) was found, we explored the reasons for this.
Assessment of reporting biases
Outcomes stated in the methods section and not reported by trial authors were noted and stated in the Characteristics of included studies table or the Main results section. We sought publication bias by using funnel plots.
Data synthesis
We used the Review Manager software of The Cochrane Collaboration (RevMan 5.2) to perform quantitative analysis. The different scales and definitions of EA were managed by using measures of EA as defined by the authors of included studies. We calculated dichotomous data by using risk ratios (RRs) and 95% confidence intervals (CIs). When pooled analyses were not possible, we reported the trial results of individual studies separately. Other data were presented as reported in the original trial. Summary of findings tables were prepared by using the GRADE process and software.
Subgroup analysis and investigation of heterogeneity
Originally, subgroup analyses were preplanned to be undertaken to compare different age groups, different types of surgery—emergency or elective, premedication adjuncts, intraoperative adjuncts, nitrous oxide or air, intravenous fluids or no fluids and surgical versus non‐surgical procedures such as magnetic resonance imaging (MRI), when sufficient numbers of studies were identified. It became clear during performance of this review that these planned analyses were not feasible, so no subgroup analyses were performed. We structured our review to include different interventions for EA, presented as subgroups.
In exploring heterogeneity when three or more trials were found and I2 > 40%, we found potentially inadequate analgesia in the control group; multiple routes of adjunct administration such as intravenous (IV) or caudal (e.g. for clonidine as an adjunct) and adenotonsillectomy/adenoidectomy (as painful procedures known to have a high risk of EA) versus other procedures were potential candidates for subgroup analysis in future updates.
Sensitivity analysis
Sensitivity analyses were performed in those studies in which one or more factors indicated a high risk of bias, when compared with all studies.
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies table (below).
Results of the search
Our search yielded 158 included studies investigating 14,045 children and excluded 30 trials (Figure 1). We reran the search in April 2014 and found a further 120 citations with 29 studies of interest. We will address identified studies of interest when we update the review.
1.
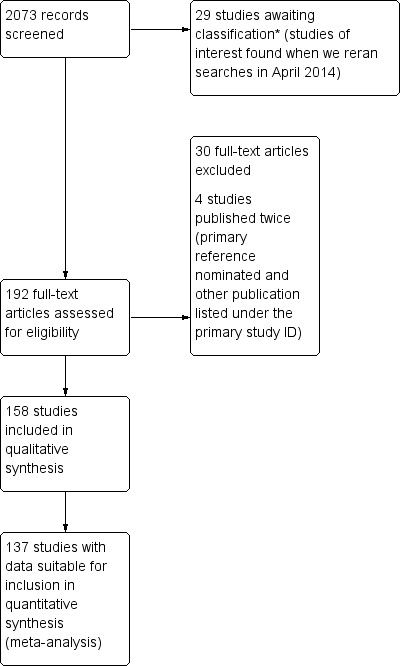
Study flow diagram. We reran the search in April 2014. We found a further 29 studies of interest, which we will assess for eligibility when we update the review.*
Included studies
Interventions to prevent EA fell into two broad groups. First, alternative GA compared with sevoflurane anaesthesia (69 studies, of which 65 reported risk of EA), and second, use of an adjunct with sevoflurane anaesthesia versus sevoflurane without an adjunct (100 studies, of which 75 reported risk of EA). Eleven studies investigated both an alternative GA and an adjunct.
All studies included preschool‐aged children, and most studies (57%) limited inclusion to children eight years of age or younger. Only 12 studies (7.5%) included children beyond the age of 12 years. A total of 25 studies included infants younger than one year of age. Nearly all studies excluded American Society of Anesthesiology (ASA) status classification III, IV and V children and those with a history of chronic illness, developmental delay or preexisting behavioural problems.
Among studies in which a sedative premedication was not the intervention, 56 involved administration of a sedative premedication to all children (mostly midazolam), and one study allowed sedative premedication at the discretion of the attending anaesthetist.
Three main categories of procedures were noted in the included studies: children having non‐painful investigations (such as MRI) under general anaesthesia; those undergoing surgery with effective (as defined by study authors) regional analgesia; and children having surgery with or without intraoperative analgesia, in which pain is likely to be a contributing factor to emergence agitation. Some studies involved children receiving potentially inadequate or no analgesia intraoperatively during painful procedures.
Postoperative behavioural disturbance during emergence from anaesthesia was most commonly termed emergence agitation, although the terms emergence delirium and excitation were also used. Numerous definitions of EA during emergence from anaesthesia were based on a wide range of scales. Furthermore, different researchers used a range of cutoffs to determine the presence or absence of EA. Most but not all studies reported the risk of EA, and this measure was the primary outcome for this review. Most studies used a three‐ or five‐point categorical scale to define a cutoff for EA. More recent studies have tended to report the PAED scale as an outcome for EA. At least three studies have reported on both of these outcomes.
Our secondary outcome—"number of participants with EA in the postoperative period after leaving the PACU, as measured by the authors of included studies"—was not reported in any of the included studies.
Although we had planned to pool continuous data when the same EA scale was used, in practice we found no scales suitable for this form of meta‐analysis.
Further details are given in the Characteristics of included studies table.
Excluded studies
Of the 30 excluded studies, 11 (37%) failed to report EA as an outcome (Akinci 2008; Ariffin 1997; Delvi 2007; El‐Hennawy 2009; Funk 2000; Greenspun 1995; Ingelmo 2007; Isik 2006b; Piat 1994; Sarner 1995; Wagner 2003).
Two studies (Hung 2005; Ibrahim 2001) (7%) included adult participants.
Twelve studies (Almenrader 2007; Chen 2010; Choi 2011; Ertugrul 2006; Kawaai 2008; Kawaraguchi 2002; Malmgren 2004; Mckay 2011; Ozer 2003; Shaban 2008; Steinmetz 2007; Uysal 2011) (40%) failed to compare EA versus a control group.
Three studies (Cole 2002; Kain 1999; Mizrak 2011) (10%) did not investigate sevoflurane, and in one study (Kain 2007), it was unclear whether sevoflurane was used.
One article (Mayer 2006) was retracted after publication.
Further details are given in the Characteristics of excluded studies table.
Risk of bias in included studies
2.
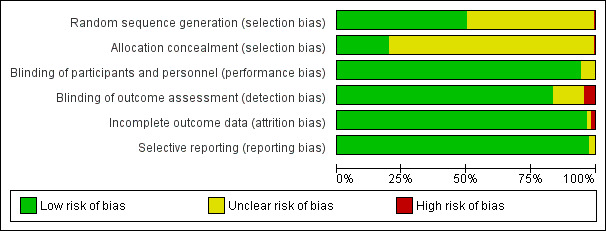
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One study was quasi‐randomized (Murray 2002). All but three (Erden 2012; Mikawa 2002; Shin 2001) of the remaining 157 included studies reported that the study was randomized, but only 79 studies (50%) reported the method of random sequence generation applied. Allocation concealment was adequately reported in only 30 trials (19%).
Blinding
Given that a vast majority of children were preschoolers, with the exception of parental presence studies, all children were effectively blinded to the intervention of interest. Most trials (133 studies) reported blinding of the outcome assessors. The seven studies in which it was clear that the outcome assessor was not blinded were assessed as having high risk of bias (Aguilera 2003; Johannesson 1995; Michalek‐Sauberer 1998; Milic 2010; Sury 1996; Tripi 2004; Villani 1998).
Incomplete outcome data
One hundred two studies (65%) reported no withdrawals of study participants after randomization. Only three studies (Chandler 2012; Keaney 2004; Kulka 2001b) had > 15% withdrawals and were assessed as having high risk of attrition bias.
Selective reporting
All included studies reported either risk of EA or an EA score, except for three studies (Erden 2012; Jacob 2012; Salik 2011), which failed to report actual data but provided a P value for differences between groups.
Other potential sources of bias
Some evidence of asymmetry can be seen in both funnel plots (Figure 4 and Figure 5). Trials may be missing from the small negative trials corner (i.e. in favour of sevoflurane and not in favour of the other drugs), which suggests possible publication bias.
4.
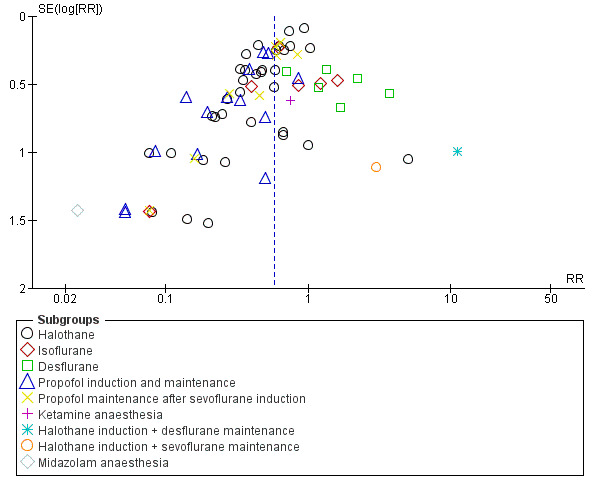
Funnel plot of comparison: 1 Any other GA versus sevoflurane anaesthesia, outcome: 1.1 Emergence agitation.
5.
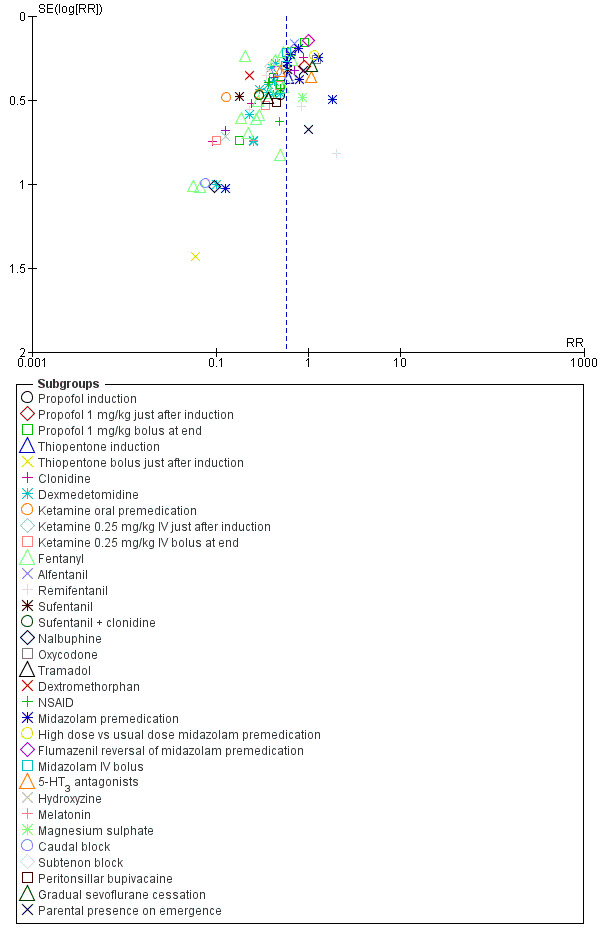
Funnel plot of comparison: 2 Adjunct versus placebo/No adjunct during sevoflurane anaesthesia, outcome: 2.1 Emergence agitation.
Effects of interventions
See: Table 1
Comparison 1. Any other GA versus sevoflurane anaesthesia Studies investigating risk of EA when comparing alternative GAs with sevoflurane are summarized in Analysis 1.1. Results of different individual agents compared with sevoflurane are reported below.
1.1. Analysis.
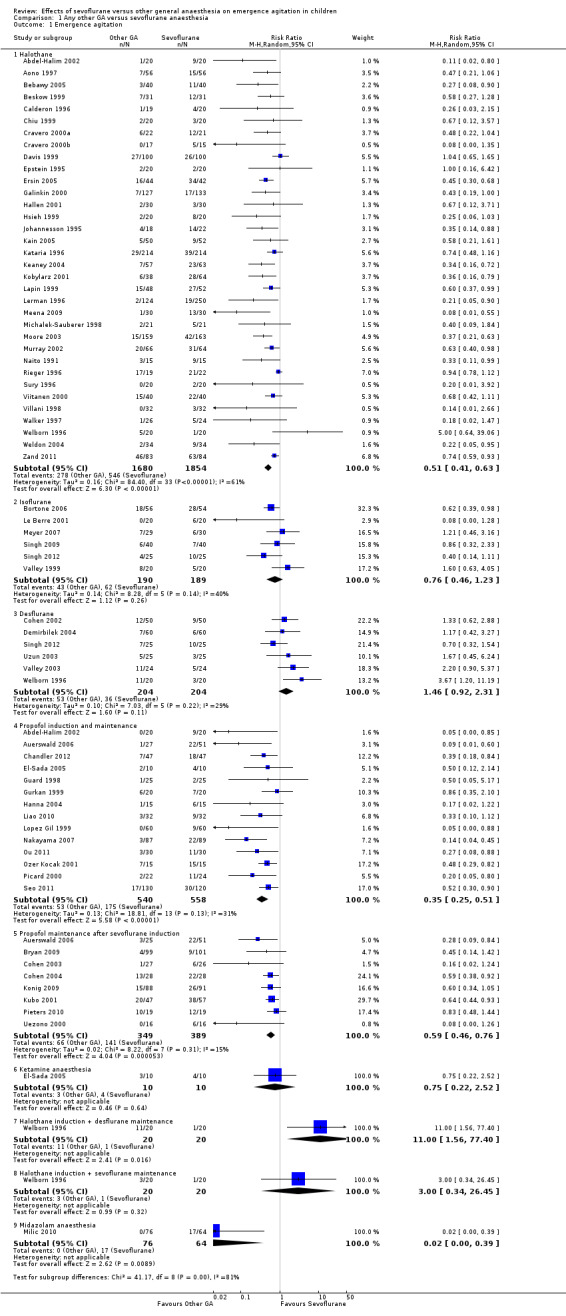
Comparison 1 Any other GA versus sevoflurane anaesthesia, Outcome 1 Emergence agitation.
Sevoflurane versus halothane
All studies (34 trials) for this comparison reported risk of EA and show that halothane has a lower risk of EA when compared with sevoflurane (RR 0.51, 95% CI 0.41 to 0.63).
Sevoflurane versus isoflurane
All six studies (Bortone 2006; Le Berre 2001; Meyer 2007; Singh 2009; Singh 2012; Valley 1999) investigating this comparison reported risk of EA. No difference in EA was found.
Sevoflurane versus desflurane
Six studies (Cohen 2002; Demirbilek 2004; Singh 2012; Uzun 2003; Valley 2003; Welborn 1996) investigating this comparison reported risk of EA. No difference in EA was found. One study (Ahishakiye 2011) used a five‐point scale and reported no difference in agitation scores.
Sevoflurane versus propofol induction and maintenance (including total intravenous anaesthesia [TIVA])
Fourteen studies (Abdel‐Halim 2002; Auerswald 2006; Chandler 2012; El‐Sada 2005; Guard 1998; Gurkan 1999; Hanna 2004; Liao 2010; Lopez Gil 1999; Nakayama 2007; Ou 2011; Ozer Kocak 2001; Picard 2000; Seo 2011) investigated risk of EA for this comparison and found that propofol anaesthesia reduced risk of EA (RR 0.35, 95% CI 0.25 to 0.51). It should be noted that in one of these studies (Ou 2011), the TIVA arm included ketamine 1.5 mg/kg at induction of anaesthesia, and that another study (Hanna 2004) compared TIVA with sevoflurane anaesthesia with 1 mg/kg of propofol at the end of anaesthesia, yet showed TIVA propofol to be superior in reducing EA.
One additional study reported lower mean PAED scores in the propofol group (Kol 2008).
Sevoflurane versus propofol maintenance (after sevoflurane induction)
Eight studies (Auerswald 2006; Bryan 2009; Cohen 2003; Cohen 2004; Konig 2009; Kubo 2001; Pieters 2010; Uezono 2000) reported risk of EA and found that the propofol group had a lower risk of EA (RR 0.59, 95% CI 0.46 to 0.76). Two further studies (Ahishakiye 2011; Jacob 2012) found lower EA scores in the propofol group. One study (Ahishakiye 2011) used a five‐point scale, and another small study reported that PAED scores indicated a greater frequency of EA in the sevoflurane group without reporting the risk (Jacob 2012).
Sevoflurane versus ketamine anaesthesia
One small study (El‐Sada 2005) included only 20 participants and showed no difference in risk of EA.
Sevoflurane versus midazolam anaesthesia
One study (Milic 2010) found lower risk of EA, although it should be noted that the outcome assessor was not blinded.
Sevoflurane versus other GA combinations
Two further comparisons were reported in one small study (Welborn 1996).
Comparison 2. Adjuncts to sevoflurane anaesthesia
Studies investigating the effectiveness of an adjunct for EA while sevoflurane anaesthesia was administered are summarized in Analysis 2.1. Results of different individual adjuncts compared with no adjunct or placebo are reported below.
2.1. Analysis.
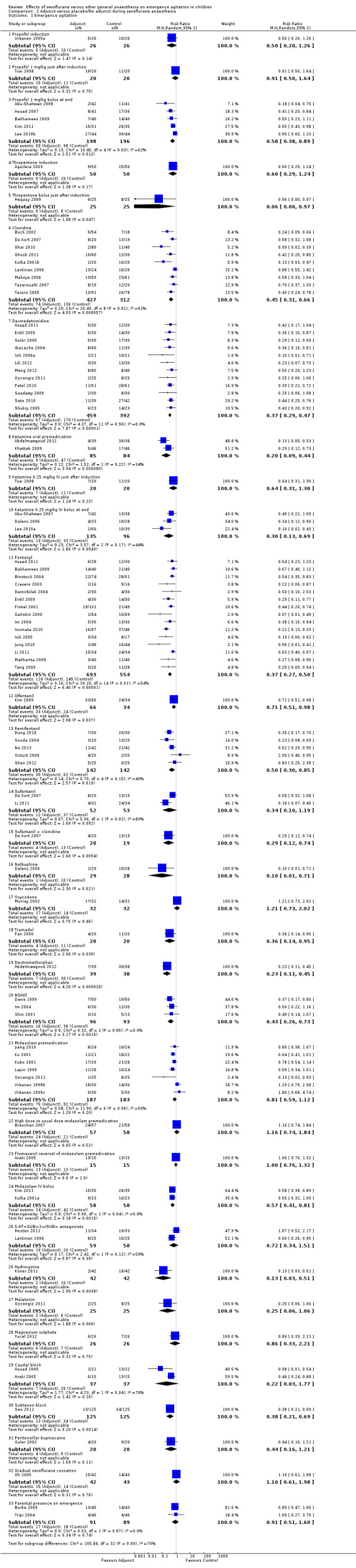
Comparison 2 Adjunct versus placebo/No adjunct during sevoflurane anaesthesia, Outcome 1 Emergence agitation.
Propofol bolus
At induction
One study (Viitanen 1999a) investigated propofol 3 mg/kg induction and showed no effect on risk of EA, whilst another study (Bal 2006) investigated 2 to 2.5 mg/kg propofol induction with no difference in EA scores between groups. Two studies (Bal 2006; Tsai 2008) investigated 1 mg/kg of propofol just after sevoflurane induction. The former showed no difference in scores for EA whilst the latter showed no effect on risk of EA.
At the end of anaesthesia
Five studies (Abu‐Shahwan 2008; Aouad 2007; Bakhamees 2009; Kim 2011; Lee 2010b) investigating the effect of 1 mg/kg of propofol administered at the end of anaesthesia showed decreased risk of EA (RR 0.58, 95% CI 0.38 to 0.89).
Two further studies (Chiba 2003; Sun 2010) reported only EA scores. Sun 2010 gave propofol 1 mg/kg and found lower PAED scores, whilst Chiba 2003 compared propofol 1 mg/kg versus 2 mg/kg vs intralipid placebo and found no difference in mean EA scores across the three groups.
In view of I2 > 40%, we investigated heterogeneity and found that when we removed the trial with an MRI setting, I2 decreased from 62% to 49%, and EA was still significantly reduced (RR 0.66, 95% CI 0.46 to 0.93). When only the two studies of adenotonsillectomy were analysed, the reduction in EA was no longer evident (RR 0.74, 95% CI 0.42 to 1.32) (I2 = 52%).
Thiopentone
One study (Aguilera 2003) reported no difference in risk of EA following thiopentone induction, and another study (Hegazy 2009) administered 2 to 3 mg/kg thiopentone after sevoflurane induction and found reduced risk of EA and lower PAED scores in children undergoing MRI scans with mean duration of less than 20 minutes.
Clonidine
Nine studies (Bock 2002; De Kort 2007; Ghai 2010; Ghosh 2011; Kulka 2001b; Lankinen 2006; Malviya 2006; Tazeroualti 2007; Tesoro 2005) investigated risk of EA and showed an overall reduction in risk of EA (RR 0.45, 95% CI 0.31 to 0.66) (I2 = 61%).
Bergendahl 2004 compared midazolam and clonidine premedication and showed reduced EA scores in children younger than five years of age receiving clonidine. Mikawa 2002 investigated oral clonidine premedication 4 mcg/kg and found that it was more effective when compared with clonidine 2 mcg/kg, midazolam 0.5 mg/kg, diazepam 0.4 mg/kg or placebo, without influencing discharge readiness.
In view of I2 > 40%, we investigated heterogeneity with respect to studies with regional block analgesia versus systemic analgesia and route of administration (IV vs caudal). I2 for these analyses remained unaffected, as did the effectiveness of this intervention. When clonidine (all routes) was used in conjunction with a regional block (seven studies), it was effective in reducing EA (RR 0.37, 95% CI 0.23 to 0.59) (I2 = 55%), whereas in the two systemic analgesia studies (one of which was an adenoidectomy study), this effect was no longer evident (RR 0.74, 95% CI 0.49 to 1.12) (I2 = 19%).
Dexmedotomidine
Twelve studies (Asaad 2011; Erdil 2009; Guler 2005; Ibacache 2004; Isik 2006a; Lili 2012; Meng 2012; Ozcengiz 2011; Patel 2010; Saadawy 2009; Sato 2010; Shukry 2005) investigating this intervention found a large overall reduction in risk of EA, with I2 = 0 (RR 0.37, 95% CI 0.29 to 0.47). An additional four studies reported lower EA scores for this intervention (Al‐Zaben 2010; Anand 2011; Mizrak 2013; Salik 2011).
Ketamine
Oral premedication
Two studies (Abdelmawgoud 2012; Khattab 2009) have shown this to be an effective intervention with an overall reduction in risk of EA.
Ketamine bolus after induction
One study showed no reduction in risk of EA compared with placebo (Analysis 2.1.9), although study authors reported lower risk of EA (Tsai 2008).
Ketamine 0.25 mg/kg bolus at end of anaesthesia
Three studies (Abu‐Shahwan 2007; Dalens 2006; Lee 2010a) show an overall reduction in risk of EA (RR 0.30, 95% CI 0.13 to 0.69).
In view of I2 > 40%, we investigated heterogeneity with respect to potentially inadequate analgesia for painful surgery versus any other setting such as MRI, or painful surgery with analgesia. When the study in which no analgesia was reported to be administered to the control group undergoing adenotonsillectomy was removed (Lee 2010a), I2 decreased from 44% to 0%, yielding RRs of 0.30 (95% CI 0.13 to 0.69) and 0.43 (95% CI 0.22 to 0.81), respectively.
Fentanyl
Fifteen included studies showed an overall decrease in risk of EA (RR 0.37, 95% CI 0.27 to 0.50) (I2 = 54%). One further study investigating intranasal fentanyl 1 mcg/kg (Rampersad 2010) reported no difference in risk of EA (but did not present the risk data) or mean EA scores (data were reported).
In view of I2 > 40%, we investigated heterogeneity with respect to potentially inadequate analgesia for painful surgery versus any other setting such as MRI, or painful surgery with analgesia or route of administration.
When the three studies of adenotonsillectomy/adenoidectomy (Bakhamees 2009; Demirbilek 2004; Erdil 2009) in which limited (rectal paracetamol only) or no analgesia was reported to be administered to the control group were removed, I2 remained high at 61% but still showed fentanyl to be an effective means of decreasing EA (RR 0.34, 95% CI 0.23 to 0.49).
Analysis of IV fentanyl versus non‐IV fentanyl (intranasal, transmucosal) studies showed that this intervention was still effective in the 12 IV fentanyl studies (RR 0.35, 95% CI 0.24 to 0.51) (I2 = 55%) (Asaad 2011; Bakhamees 2009; Cravero 2003; Demirbilek 2004; Erdil 2009; Im 2004; Inomata 2010; Isik 2005; Jung 2010; Li 2011; Makharita 2009; Tazeroualti 2007). The three non‐IV studies (Binstock 2004; Finkel 2001; Galinkin 2000) had an RR of 0.42 (95% CI 0.22 to 0.77) (I2 = 59%).
Other opioids (including tramadol)
Five studies (Dong 2010; Gouda 2004; Na 2013; Ozturk 2009; Shen 2012) show an overall reduction in risk of EA when remifentanil is administered (RR 0.50, 95% CI 0.30 to 0.85).
Two studies (De Kort 2007; Li 2011) show no overall reduction in risk of EA when sufentanil is administered.
One study (Kim 2009) administered alfentanil and reduced the risk of EA.
One study (Dalens 2006) found nalbuphine to be effective in reducing the risk of EA.
One study (Abdelmawgoud 2012) administered dextromethorphan and found it to be effective in reducing the risk of EA.
One study (Murray 2002) found that oxycodone premedication was ineffective in reducing the risk of EA.
Two studies investigated tramadol. One showed reduced risk of EA (Fan 2000), and the other (Sun 2009) found lower PAED scores.
Midazolam
Oral midazolam premedication
Seven studies (Jiang 2010; Ko 2001; Kubo 2001; Lapin 1999; Ozcengiz 2011; Viitanen 1999b; Viitanen 1999c) showed no overall reduction in risk of EA (RR 0.81, 95% CI 0.59 to 1.12) (I2 = 50%).
Two further studies (Arai 2005; Kazak 2010) found no difference in EA scores.
One study (Breschan 2007) compared 0.5 mg/kg versus 1 mg/kg oral premedication and found no difference in the risk of EA.
In view of I2 > 40%, we investigated heterogeneity with respect to midazolam dose; studies with regional block analgesia versus systemic or no analgesia and adenoidectomy versus other procedures. The only difference was found when the two adenoidectomy studies were removed, resulting in I2 = 4% and an overall reduction in EA (RR 0.68, 95% CI 0.53 to 0.87).
Flumazenil reversal of midazolam premedication
This comparison showed no difference in the risk of EA in one study (Araki 2005).
Midazolam IV at induction of anaesthesia
One study (Byon 2012) showed no difference in PAED scores.
Midazolam IV at the end of anaesthesia
Two studies (Kim 2011; Kulka 2001a) reduced the risk of EA.
One study (Bae 2010) showed a reduction in EA scores.
Non‐steroidal anti‐inflammatory drugs (NSAIDs)
Three studies were suitable for meta‐analysis. Two investigated ketorolac (Davis 1999; Im 2004), and one, ibuprofen premedication (Shin 2001); these studies showed a decrease in risk of EA (RR 0.43, 95% CI 0.26 to 0.73). One further study investigating ketorolac (Rampersad 2010) reported no difference in risk of EA (but did not present the risk data) nor mean EA scores (data were reported), and another, investigating diclofenac, showed lower PAED scores (Sun 2009).
Regional blocks (local anaesthetic)
Caudal
Two studies (Aouad 2005; Araki 2005) showed reduced risk of EA.
Subtenon
One study (Seo 2011) showed reduced risk of EA but provided no analgesia to the control group.
Peritonsillar bupivacaine
One small study (Guler 2002) showed no difference in risk of EA.
5‐HT3 antagonists
Three studies investigated this intervention—two with ondansetron (Erden 2012; Hosten 2011) and one with tropisetron (Lankinen 2006)—with no overall effect in EA. Erden 2012 reported no difference in agitation scores but did not report the risk of EA, so this study does not appear in Analysis 2.1.25.
Hydroxyzine
One study (Koner 2011), which added this intervention to a midazolam premedication, showed reduced risk of EA.
Melatonin
One study (Ozcengiz 2011) showed no reduction in risk of EA with melatonin compared with placebo (Analysis 2.1.28), although study authors reported lower risk of EA.
Magnesium sulphate
One study (Yucel 2012) showed no effect on risk of EA or PAED scores, whilst Apan 2010 also showed no difference in agitation scores.
Gradual cessation of sevoflurane
One study (Oh 2005) showed no difference in risk of EA when compared with rapid cessation of sevoflurane.
Sevoflurane concentration
One study (Liao 2011) showed no difference in PAED scores with lower concentrations of sevoflurane titrated to BIS (Bispectral Index).
Nitrous oxide washout
One study (Shibata 2005) showed lower agitation scores when inhaled nitrous oxide concentration was maintained at the end of surgery until BIS had reached 80 to wash out sevoflurane.
Diazepam
Diazepam 0.25 mg/kg added to midazolam 0.25 mg/kg for premedication showed decreased EA scores compared with midazolam 0.5 mg/kg alone and placebo (Arai 2005).
Sufentanil + clonidine (multi‐modal)
One study (De Kort 2007) investigated a multi‐modal approach to EA and found that sufentanil and clonidine in combination (four of 20 participants experienced EA) decreased the risk of EA further than when either drug was used alone (eight of 20 with EA) or against placebo (13 of 19 with EA).
Parental presence on emergence
Two studies (Burke 2009; Tripi 2004) showed no difference in the risk of EA.
Acupuncture
One study (Lin 2009) reported lower EA scores with acupuncture after induction of anaesthesia for children undergoing bilateral myringotomy and tympanostomy tube insertion.
Parental presence at induction of anaesthesia (PPIA)
PPIA showed lower EA scores when all participants were premedicated with midazolam (Arai 2007). Another study (Kazak 2010) using a lower dose of midazolam premedication (0.25 mg/kg) in the PPIA group compared with the non‐PPIA group, which received 0.5 mg/kg midazolam, showed no effect on EA scores.
Airway management
One study (Lee 2011) showed lower risk of EA when a laryngeal mask airway (LMA) was removed deep compared with an endotracheal tube (ETT) removed awake. No difference between ETT deep versus LMA deep extubation was reported. However no LMA awake group was included; therefore no comparison was performed with ETT awake versus LMA awake or LMA deep versus LMA awake.
Discussion
Strengths of the review
This review is the most comprehensive to date examining the effects of interventions used to prevent EA associated with sevoflurane anaesthesia in children.
Limitations
The main limitation with a review of this type is outcome definition. In this case, we designated EA as the universal term for our outcome of interest, irrespective of the term used by study authors, provided their description reflected this outcome. A degree of variability in scales and definitions was noted, along with varying cutoffs for what might be considered EA by different authors. For example, investigators using the PAED scale sometimes regarded > 10 as the cutoff for EA, whilst others used ≥ 16 as the cutoff for EA. As with a hypotension review (Cyna 2006), we used the definition of EA provided by study authors to pool these data in our meta‐analyses.
In several studies, analgesia was stated to be an effective means of reducing EA when the effect of pain on the child's behaviour was a potential confounder in determining this outcome. Some authorities express the opinion that undertreated pain contributes to EA (Rosen 2013). This was a reason for using potentially inadequate analgesia in our sensitivity analyses, where it was shown to be an important factor in generating heterogeneity between trials.
We note that several studies are awaiting assessment and acknowledge that there will be a lag time in assessing and incorporating these studies in future reviews. However, it appears unlikely that these studies will impact our key findings.
Summary of main results
As can be seen from the Results section and from the meta‐analyses, we have found a large number of well‐designed trials of adequate size with low risk of bias, generating outcomes of interest that are likely to be relevant to clinical practice.
Our key findings were that propofol, halothane, alpha‐2 agonists (dexmedetomidine, clonidine), opioids (e.g. fentanyl) and ketamine reduce the risk of EA (Main results; Summary of findings table 1). No clear evidence showed an effect of desflurane, isoflurane, midazolam premedication and parental presence at emergence in reducing the risk of EA.
Overall completeness and applicability of evidence
This review is very likely to represent research findings to date and to be applicable to clinical practice. In view of the funnel plot findings detailed in the Results section, we suggest some caution about the magnitude of the findings in favour of other GAs/adjuncts, but not the direction of effect.
Sensitivity analyses of adenotonsillectomy/adenoidectomy studies seem to show that this setting is more refractory to reductions in EA with propofol bolus, midazolam premedication and clonidine. In contrast, fentanyl and ketamine remain effective, irrespective of this context.
Quality of the evidence
This review shows good evidence of benefit or lack of benefit for several interventions, as stated above. Eleven studies had one or more factors designated as causing high risk of bias, but sensitivity analyses in the nine studies in which this was possible (Chandler 2012; Johannesson 1995; Keaney 2004; Kulka 2001b; Michalek‐Sauberer 1998; Murray 2002; Sury 1996; Tripi 2004; Villani 1998) showed no difference in findings. The other two were single studies for which sensitivity analyses were impossible (Aguilera 2003; Milic 2010).
We have noted some significant heterogeneity, using a random‐effects model, with some of the comparisons, namely, halothane, clonidine, fentanyl, midazolam premedication, propofol 1 mg/kg bolus at end and ketamine 0.25 mg/kg bolus at end. Sensitivity analysis showed minimal changes in overall findings.
Potential biases in the review process
See "Limitations" at the beginning of the Discussion.
Agreements and disagreements with other studies or reviews
Our findings are consistent with those described in the halothane versus sevoflurane review by Kuratani 2008, and we have included all of the 23 studies in their review plus an additional 11 studies investigating EA. Dahmani 2010 reviewed pharmacological interventions for sevoflurane and desflurane anaesthesia, such as propofol, midazolam, ketamine, fentanyl, alpha‐2 agonists and perioperative analgesia, for preventing EA; the only indication of disagreement with the current review was a statement that IV fentanyl was ineffective in preventing EA. This discrepancy appears to be due to inclusion of fewer studies in this previous review (only two IV fentanyl studies vs 12 in the current review). One meta‐analysis of randomized controlled trials compared propofol anaesthesia (induction and maintenance, or maintenance after sevoflurane induction) versus sevoflurane for reducing EA in children (Sun 2008). Although we included more studies and separated the analyses for propofol maintenance after sevoflurane induction from those for propofol for induction and maintenance, our findings were consistent.
Authors' conclusions
Implications for practice.
Propofol, halothane, alpha‐2 agonists (dexmedetomidine, clonidine), opioids (e.g. fentanyl) and ketamine have been shown to reduce the risk of EA when compared with sevoflurane anaesthesia. No clear evidence shows an effect in reducing the risk of EA with desflurane, isoflurane, midazolam premedication and parental presence at emergence.
Implications for research.
Data on the effects of halothane versus sevoflurane on EA were adequate, and further research is unlikely to generate useful additional findings with respect to EA risk in children. Further investigation of dexmedetomidine and use of propofol‐based anaesthesia, as described in the included studies, is also unlikely to generate further useful data, as these interventions have been shown to be clearly effective. It could be useful to apply a multi‐modal approach by using two or more of the effective interventions reported in this review.
Future studies should ensure adequate analgesia in the control group, for which pain may be a confounding factor for the diagnosis of EA.
Regardless of the EA scale used, it would be helpful for study authors to report the risk of EA, so that this might be included in future meta‐analyses.
Our secondary outcome, "number of participants with EA in the postoperative period after leaving the PACU, as measured by the authors of included studies," was not reported in any of the included studies and is probably of limited clinical relevance. Therefore it will be excluded as an outcome in future reviews.
In view of the likely different mechanisms in new‐onset maladaptive behaviours (e.g. sleep or eating disturbance, poor concentration) in the postoperative period after discharge from hospital, we have excluded and will be excluding maladaptive behaviours after the immediate emergence period in future updates of this review.
Acknowledgements
We would like to thank Dr Anna Lee (content editor); Munib Haroon, Robert F Seal, Nai Ming Lai and William B. McIlvaine (peer reviewers); Janet Wale, Durhane Wong‐Rieger and Anne Lyddiatt (consumers); and the Cochrane Child Health Field for help and editorial advice provided during preparation of the original protocol.
We would like to thank Anna Lee (content editor); Nathan Pace (statistical editor); Robert Seal, Nai Ming Lai and William McIlvaine (peer reviewers); and Tracey Lloyd (consumer referee) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 sevofluran* #2 MeSH descriptor Propofol explode all trees #3 MeSH descriptor Halothane explode all trees #4 MeSH descriptor Isoflurane explode all trees #5 MeSH descriptor Anesthetics, General explode all trees #6 MeSH descriptor Enflurane explode all trees #7 MeSH descriptor Anesthetics explode all trees #8 MeSH descriptor Receptors, GABA‐A explode all trees #9 MeSH descriptor Anesthesia, General explode all trees #10 MeSH descriptor Anesthetics, Intravenous explode all trees #11 MeSH descriptor Nitrous Oxide explode all trees #12 MeSH descriptor Thiopental explode all trees #13 MeSH descriptor Analgesics, Opioid explode all trees #14 Propofol or Halothane or Isoflurane or an?esth* or Enflurane or "Nitrous Oxide" or Thiopental or (Opioid near analg*) #15 (#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR # OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #16 MeSH descriptor Psychomotor Agitation explode all trees #17 MeSH descriptor Postoperative Complications explode all trees #18 MeSH descriptor Anesthesia Recovery Period explode all trees #19 MeSH descriptor Confusion explode all trees #20 MeSH descriptor Delirium explode all trees #21 (emergence near (agitation or excit* or delirium or confusion)) #22 ((postoperative or postan?esthetic) near (agitation or confusion or behavio?ral change*)) #23 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22) #24 (#1 AND #15 AND #23) #25 Child* or Adolescent or Infant* #26 adult* #27 (#26 AND NOT ( #25 AND #26 )) #28 (#24 AND NOT #27)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. sevofluran*.mp. 2. exp Propofol/ or exp Halothane/ or exp Isoflurane/ or exp Anesthetics, General/ or exp Enflurane/ or exp Anesthetics/ or exp Receptors, GABA‐A/ or exp Anesthesia, General/ or exp Anesthetics, Intravenous/ or exp Nitrous Oxide/ or exp Thiopental/ or exp Analgesics, Opioid/ 3. exp Psychomotor Agitation/ or exp Postoperative Complications/ or exp Anesthesia Recovery Period/ or exp confusion/ or exp delirium/ or (emergence adj3 (agitation or excit$ or delirium or confusion)).mp. or ((postoperative or postan?esthetic) adj3 (agitation or confusion or behavio?ral change$)).mp. 4. exp Child/ or exp Adolescent/ or exp Infant/ or child*.mp. 5. exp Adult/ or adult*.mp. 6. 5 not (4 and 5) 7. (1 and 2 and 3) not 6 8. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 9. 8 and 7
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp sevoflurane/ or sevofluran*.mp. 2. exp propofol/ or exp halothane/ or exp isoflurane/ or exp anesthetic agent/ or exp enflurane/ or exp 4 aminobutyric acid a receptor/ or exp general anaesthesia/ or exp intravenous anesthetic agent/ or exp nitrous oxide/ or exp thiopental/ or exp narcotic analgesic agent/ 3. exp restlessness/ or exp postoperative complication/ or exp anesthetic recovery/ or exp confusion/ or exp delirium/ or (emergence adj3 (agitation or excit$ or delirium or confusion)).mp. or ((postoperative or postan?esthetic) adj3 (agitation or confusion or behavio?ral change$)).mp. 4. exp child/ or exp adolescent/ or exp infant/ or child*.mp. 5. exp adult/ or adult*.mp. 6. 5 not (4 and 5) 7. (1 and 2 and 3) not 6 8. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animal* not (human* and animal*)).sh. 9. 8 and 7
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 ("Propofol") or (MM "Propofol") or ("Halothane") or (MM "Halothane") or ("Isoflurane") or (MM "Isoflurane") or (MH "Anesthetics, General") or "Enflurane" or (MH "Anesthetics") or (MH "Anesthetics, General") or (MH "Anesthetics, Intravenous") or (MH "GABA Agents") or (MH "Nitrous Oxide") or ("Thiopental") or (MM "Thiopental") or (MH "Analgesics, Opioid") S2 (MH "Psychomotor Agitation+") or (MH "Postoperative Complications") or (MH "Anesthesia Recovery") or (MH "Confusion+") or (MM "Delirium") or TX emergence and TX ( agitation or excit* or delirium or confusion ) or (TX ( postoperative or postan?esthetic ) and TX ( agitation or confusion or behavio?ral change*)) S3 Child* or Adolescent or Infant* S4 adult* S5 S4 not (S3 and S4) (S1 and S2 and sevofluran*) not S5
Appendix 5. Search strategy for ISI Web of Science
#1 TS=sevofluran* #2 TS=(Propofol or Halothane or Isoflurane or an?esth* or Enflurane or GABA‐A or Nitrous Oxide or Thiopental or Opioid*) #3 TS=Psychomotor Agitation or TS=Anesthesia Recovery Period or TS=(confusion or delirium) or TS=(emergence SAME (agitation or excit* or delirium or confusion)) or TS=((postoperative or postanesthetic or postanaesthetic) SAME(agitation or confusion or behavioral change*)) #4 #3 AND #2 AND #1 #5 TS=(Child* or Adolescent or Infant*) #6 TS=adult* #7 #4 not (#6 not (#5 and #6))
Data and analyses
Comparison 1. Any other GA versus sevoflurane anaesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Emergence agitation | 65 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Halothane | 34 | 3534 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.41, 0.63] |
| 1.2 Isoflurane | 6 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.23] |
| 1.3 Desflurane | 6 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.92, 2.31] |
| 1.4 Propofol induction and maintenance | 14 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.51] |
| 1.5 Propofol maintenance after sevoflurane induction | 8 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.46, 0.76] |
| 1.6 Ketamine anaesthesia | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.22, 2.52] |
| 1.7 Halothane induction + desflurane maintenance | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [1.56, 77.40] |
| 1.8 Halothane induction + sevoflurane maintenance | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.34, 26.45] |
| 1.9 Midazolam anaesthesia | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.39] |
Comparison 2. Adjunct versus placebo/No adjunct during sevoflurane anaesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Emergence agitation | 75 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Propofol induction | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.20, 1.26] |
| 1.2 Propofol 1 mg/kg just after induction | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.50, 1.64] |
| 1.3 Propofol 1 mg/kg bolus at end | 5 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.38, 0.89] |
| 1.4 Thiopentone induction | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.29, 1.24] |
| 1.5 Thiopentone bolus just after induction | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.97] |
| 1.6 Clonidine | 9 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.31, 0.66] |
| 1.7 Dexmedetomidine | 12 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.29, 0.47] |
| 1.8 Ketamine oral premedication | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.44] |
| 1.9 Ketamine 0.25 mg/kg IV just after induction | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.31, 1.30] |
| 1.10 Ketamine 0.25 mg/kg IV bolus at end | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.69] |
| 1.11 Fentanyl | 15 | 1247 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.27, 0.50] |
| 1.12 Alfentanil | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.51, 0.98] |
| 1.13 Remifentanil | 5 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.30, 0.85] |
| 1.14 Sufentanil | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.10, 1.19] |
| 1.15 Sufentanil + clonidine | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.74] |
| 1.16 Nalbuphine | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.71] |
| 1.17 Oxycodone | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.73, 2.02] |
| 1.18 Tramadol | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.95] |
| 1.19 Dextromethorphan | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.11, 0.45] |
| 1.20 NSAID | 3 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.73] |
| 1.21 Midazolam premedication | 7 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.59, 1.12] |
| 1.22 High dose vs usual dose midazolam premedication | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.74, 1.84] |
| 1.23 Flumazenil reversal of midazolam premedication | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.76, 1.32] |
| 1.24 Midazolam IV bolus | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.81] |
| 1.25 5‐HT3 antagonists | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.34, 1.51] |
| 1.26 Hydroxyzine | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.03, 0.51] |
| 1.27 Melatonin | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.06] |
| 1.28 Magnesium sulphate | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.33, 2.21] |
| 1.29 Caudal block | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.77] |
| 1.30 Subtenon block | 1 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.69] |
| 1.31 Peritonsillar bupivacaine | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.21] |
| 1.32 Gradual sevoflurane cessation | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.61, 1.98] |
| 1.33 Parental presence on emergence | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.51, 1.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Halim 2002.
| Methods | Randomized controlled trial | |
| Participants | 2‐10 years, ASA I‐II, bone marrow aspiration, intrathecal aspiration of CSF Exclusion criteria: none stated Recruitment: 60 children (20 in each of 3 groups) Location: Turkey |
|
| Interventions | Halothane group: halothane induction and maintenance in oxygen Propofol group: propofol induction and maintenance Control group: sevoflurane induction and maintenance in oxygen |
|
| Outcomes | EA defined as "crying or doing abnormal excitatory movements on emergence" Other outcomes: somnolence, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded to maintenance technique but not induction technique (but would not be viable to blind child between IV and inhalational induction), anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Abdelmawgoud 2012.
| Methods | Randomized controlled trial | |
| Participants | 4‐10 years, ASA I, adenotonsillectomy Exclusion criteria: history of cardiovascular, pulmonary or neurological disease, chronic cough, bronchial asthma, coagulation defects, allergy to study drugs or recent URTI in past 2 weeks Recruitment: 120 children randomly assigned, 116 analysed (38 control group, 39 dextromethorphan group, 39 ketamine group) Location: Egypt |
|
| Interventions | Dextromethorphan group: dextromethorphan 1 mg/kg oral premedication 60 minutes before surgery Ketamine group: ketamine 5 mg/kg oral premedication 60 minutes before surgery Control group: placebo oral premedication 60 minutes before surgery All participants: parental presence at induction if separation from parents "unsuccessful" (separation score of 3 or 4), sevoflurane 8% induction with 50% nitrous oxide, IV insertion, intubation, rectal paracetamol 15 mg/kg, maintenance with sevoflurane 3%‐4% and 50% nitrous oxide, gauze soaked with lidocaine 2% applied to tonsillar bed by surgeon |
|
| Outcomes | EA defined as score of 4 or 5 on the following 5‐point scale: 1 = obtunded with no response to stimuli 2 = asleep but responsive to movement and stimuli 3 = awake and appropriately responsive 4 = crying and difficult to console 5 = wild thrashing behaviour that requires restraint Other outcomes: separation scores before induction of anaesthesia, cooperation score at induction, duration of emergence, time in PACU, vital signs and side effects in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "closed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | anaesthetist and child blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients excluded because of bleeding in the surgical region after extubation: 2 from control group, 1 from ketamine group, 1 from dextromethorphan group |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Abu‐Shahwan 2007.
| Methods | Randomized controlled trial | |
| Participants | 4‐7 years, ASA I‐II, dental repair Exclusion criteria: behavioural problems, developmental delay Recruitment: 85 children randomly assigned, 80 analysed (42 in intervention group, 38 in control group) Location: Canada |
|
| Interventions | Ketamine group: ketamine 0.25 mg/kg IV 10 minutes before end of surgery Control group: saline placebo IV 10 minutes before end of surgery All participants: premedication with acetaminophen 30 mg/kg and midazolam 0.5 mg/kg, sevoflurane with nitrous oxide for induction and maintenance, mivacurium 0.25 mg/kg and ketorolac 1 mg/kg IV before intubation |
|
| Outcomes | EA defined as PAED score ≥ 16/20 at any time during first 30 minutes in PACU Other outcomes: CHEOPS 10‐point scale for pain, adverse anaesthetic or surgical outcomes, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomized into a placebo‐controlled, double‐blinded study," block randomization in blocks of 10 participants, method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | anaesthetist and child blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 children excluded because of intraoperative opioid administration (no details of which groups they were from) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Abu‐Shahwan 2008.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I‐II, MRI examination Exclusion criteria: mental retardation, need for sedative premedication Recruitment: 84 children randomly assigned, 83 analysed (42 intervention group, 41 control group) Location: Canada |
|
| Interventions | Propofol group: 1 mg/kg propofol (maximum 30 mg) at completion of diagnostic procedure Control group: inhalation induction with sevoflurane/nitrous oxide and sevoflurane/nitrous oxide maintenance. Saline instead of propofol at completion of diagnostic procedure |
|
| Outcomes | EA defined as PAED score ≥ 16/20 at any time during first 30 minutes in PACU Other outcomes: time in PACU, adverse anaesthetic or surgical outcome |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "each patient was allocated to one of the two groups using a concealed random number generator" |
| Allocation concealment (selection bias) | Unclear risk | "each patient was allocated to one of the two groups using a concealed random number generator" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | anaesthetist and child blinded (anaesthesia assistant prepared and administered study drug) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 of the children from the control group excluded because of propofol administration during induction |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Aguilera 2003.
| Methods | Randomized controlled trial | |
| Participants | 2‐14 years, ASA I‐II, ENT surgery Exclusion criteria: history of sleep apnoea, parental preference for a particular type of induction, not correctly premedicated, contraindication to study drug Recruitment: 110 children randomly assigned, 100 analysed (50 in each group) Location: United Kingdom |
|
| Interventions | Intervention group: thiopentone IV induction followed by mivacurium 0.2 mg/kg Control group: inhalational induction with sevoflurane and nitrous oxide All participants: midazolam 0.5 mg/kg premedication, sevoflurane/nitrous oxide maintenance, morphine 0.1 mg/kg IV given intraoperatively when indicated |
|
| Outcomes | "Emergence anxiety" defined as 1 or 2 on the following 4‐point scale (measured 30 minutes after arrival to recovery room): 1 = crying or distressed, uncooperative 2 = anxious but cooperative 3 = awake and calm 4 = asleep Other outcomes: anxiety on arrival to anaesthetic room and on induction of anaesthesia (same scale as above) and late behavioural changes assessed through questionnaire to parents after 1 week, induction time, recovery time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | anaesthetist not blinded (not viable), not viable to blind child between IV and inhalational induction; however midazolam premedicated at the time of induction |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | outcome assessor not blinded to anaesthesia technique for emergence assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 children excluded because of protocol violations or non‐evaluability of study parameters: errors in giving premedication to 4 children, 3 children refused the induction method they had been allocated (2 from IV group, 1 from inhalational group), 1 child had "dysphoric reaction during the recovery period precluding satisfactory evaluation of the degree of anxiety and the recovery time," 2 children allocated to IV group had to be anaesthetized by inhalation route because of difficulty locating suitable veins |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ahishakiye 2011.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, elective tonsillectomy and adenoidectomy Recruitment: 266 analysed (93 propofol group, 85 desflurane group, 88 control group) Location: Belgium |
|
| Interventions | Propofol group: propofol maintenance Desflurane group: desflurane maintenance Control group: sevoflurane maintenance All participants: sevoflurane induction, standardized analgesic treatment |
|
| Outcomes | EA assessed using a 5‐point scale (scale not described) and reported mean (SD) scores Other outcomes: time to extubation, time in PACU, sedation scores, parental interview 24 hours postoperatively regarding agitation and combative behaviour at home |
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | report the number who "completed the study" but not the number randomly assigned |
| Selective reporting (reporting bias) | Low risk | reported EA scores |
Al‐Zaben 2010.
| Methods | Randomized controlled trial | |
| Participants | 1‐12 years, ASA I, hypospadias repair Exclusion criteria: history of allergy to dexmedetomidine, renal or hepatic dysfunction, cardiac disease, behavioural disturbances or mental retardation, need for sedative or analgesic premedication Recruitment: 48 children (24 in each group) Location: Jordan |
|
| Interventions | Dexmedetomidine loading dose 1 mcg/kg IV over 10 minutes after induction of anaesthesia, then infusion at 0.7 mcg/kg/h until end of surgery Control group: saline placebo All participants: sevoflurane induction with 50% nitrous oxide in oxygen, fentanyl 2 mcg/kg and atracurium 0.5 mg/kg IV, tracheal intubation, maintenance with 2% sevoflurane (adjusted to maintain BIS 40‐60) with 60% nitrous oxide. Increases in BP or HR > 20% baseline treated with IV fentanyl 1 mcg/kg, decreases in BP or HR < 20% baseline treated with fluid boluses, ephedrine, atropine as necessary |
|
| Outcomes | Behaviour scores measured in PACU up to 120 minutes after arrival, using scale as described by Watcha: 0 = child asleep 1 = calm 2 = crying but can be consoled 3 = crying and cannot be consoled 4 = agitated and thrashing around Report mean (SD) behaviour scores, do not report incidence of EA Other outcomes: additional intraoperative fentanyl requirements, sevoflurane requirement, HR and BP intraoperatively and in PACU, time to extubation, pain scores, time to first analgesic requirement, number of participants requiring analgesia (morphine) in PACU, morphine dose in PACU, adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | "computer‐generated randomization table used to assign each patient," method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child binded, syringe containing saline or dexmedetomidine prepared "by an anaesthesiologist not involved in anaesthetic management of the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported postoperative behaviour scores |
Anand 2011.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐6 years, ASA I‐II, lower abdominal surgeries Exclusion criteria: known allergy to study drugs, suspected coagulopathy, infection at site of caudal block, history of developmental delay, neurological diseases and skeletal deformity Recruitment: 60 children (30 in each group) Location: India |
|
| Interventions | Intervention group: caudal 0.25% ropivacaine 1 mL/kg + dexmedetomidine 2 mcg/kg Control group: caudal 0.25% ropivacaine 1 mL/kg + 0.5 mL normal saline All participants: oral midazolam premedication 0.5 mg/kg 1 hour before induction with 8% sevoflurane and 50% nitrous oxide in oxygen, laryngeal mask airway, maintenance with 3% sevoflurane and 50% nitrous oxide with sevoflurane concentration adjusted to maintain haemodynamic changes within 30% of baseline |
|
| Outcomes | Emergence behaviour assessed using the following 4‐point scale and reported mean (SD) emergence behaviour scores: 1 = calm 2 = not calm but could be easily calmed 3 = not easily calmed, moderately agitated or restless 4 = combative, excited, disoriented. Incidence of EA not reported Other outcomes: time to emergence, sedation scores, pain scores, heart rate, blood pressure, analgesic duration |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "simple randomized sampling was done by lottery method" |
| Allocation concealment (selection bias) | Unclear risk | "simple randomized sampling was done by lottery method," method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child and anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported emergence behaviour scores |
Aono 1997.
| Methods | Randomized controlled trial | |
| Participants | Healthy boys aged 3‐10 years undergoing circumcision (aged 3‐5 years) or inguinal hernia repair (aged 6‐10 years) Exclusion criteria: agitation or crying before or during anaesthesia induction, > 10% increase in preoperative stable value of heart rate or systolic blood pressure after skin incision, participants reporting pain during recovery period Recruitment: 116 boys randomly assigned, 112 analysed (56 in each group) Location: Japan |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: diazepam 0.2 mg/kg premedication, maintenance with 1 MAC of anaesthetic agent, caudal block 0.25% bupivacaine |
|
| Outcomes | EA defined as grade 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = not easily calmed, moderately agitated or restless 4 = combative, excited, disoriented Other outcomes: time to extubation, time to emergence |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | dice throw |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded to agent used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 exclusions from control group: 2 for signs of incomplete pain relief, 1 for agitation before induction. 1 exclusion from intervention group for signs of incomplete pain relief |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Aouad 2005.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, inguinal hernia repair Exclusion criteria: developmental delay, neurological disease, indication of RSI, contraindication for caudal analgesia, failed caudal block, preop agitation Recruitment: 48 children randomly assigned, 44 analysed (22 in each group) Location: Lebanon |
|
| Interventions | Intervention group: caudal block 1 mL/kg 0.25% bupivacaine Control group: 1 mcg/kg fentanyl IV before skin incision with further 1 mcg/kg fentanyl if required All participants: sevoflurane induction and maintenance |
|
| Outcomes | EA defined as score of 3 or 4 on following 4‐point scale: 1 = calm 2 = not calm but could be easily consoled 3 = moderately agitated or restless and not easily calmed 4 = combative, excited or disoriented, thrashing around Other outcomes: number of children requiring analgesia in recovery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random numbers table |
| Allocation concealment (selection bias) | Unclear risk | "randomized," method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 exclusions: 2 in fentanyl group due to preop agitation, 2 in caudal group due to inadequate caudal block |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Aouad 2007.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I‐II, strabismus surgery Exclusion criteria: mental disease, neurological disease, treatment with sedatives, full stomach, indication for rapid sequence induction Recruitment: 80 randomly assigned, 77 analysed (36 control, 41 intervention) Location: Lebanon |
|
| Interventions | Intervention group: 1 mg/kg propofol at completion of surgery Control group: saline bolus at completion of surgery All participants: midazolam 0.5 mg/kg oral premedication, inhalational induction with sevoflurane, 1 mg/kg IV lidocaine, 15 mg/kg IV paracetamol, 1 mg/kg IV dexamethasone (maximum 16 mg), sevoflurane maintenance with 60% nitrous oxide |
|
| Outcomes | 2 scales used to assess EA: PAED scores measured (means ± SD reported) but EA defined as grade 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = not easily calmed, moderately agitated or restless 4 = combative, excited, disoriented Highest scores from time of LMA arrival until calm were reported. Different assessors for each scale Other outcomes: time to LMA removal, time to emergence, discharge time from PACU, parental satisfaction |
|
| Notes | PAED scale scores were correlated with dichotomous outcomes on the 4‐point scale, with study authors concluding that a threshold PAED scale score > 10 was the best discriminator between presence and absence of EA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random numbers generated by a computer |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded from the saline group because of incomplete data collection |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA and PAED scores |
Apan 2010.
| Methods | Randomized controlled trial | |
| Participants | 3‐16 years; ASA I; adenoidectomy, tonsillectomy or both with or without myringotomy and pressure equalization tube placement Exclusion criteria: preexisting renal disease, cardiovascular dysfunction, neurological or psychological disorders or any conditions that may influence co‐operation, respiratory disease, known allergy to study drugs, receipt of any analgesic or sedatives within 2 days before surgery, parent refusal Recruitment: 118 randomly assigned, 110 analysed (55 in each group) Location: Turkey |
|
| Interventions | Intervention: magnesium sulphate 30 mg/kg IV infusion over last 10 minutes of sevoflurane anaesthesia Control: normal saline infusion of same volume (20 mL) over last 10 minutes of sevoflurane anaesthesia All participants: EMLA cream and awake IV access, midazolam 0.1 mg/kg IV premedication, propofol 2‐2.5 mg/kg IV induction, vecuronium 0.1 mg/kg, fentanyl 1 mcg/kg, then intubation, maintenance with sevoflurane at 1 MAC with nitrous oxide in oxygen (35%), reversal with neostigmine and atropine |
|
| Outcomes | EA assessed using Aono's 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = moderately agitated or restless 4 = combative, excited, disoriented Other outcomes: pain/discomfort scores, time to extubation, time to eye opening, time to emergence, time to full Aldrete score, time to interaction, time to discharge from PACU, magnesium levels preinfusion and post infusion, laryngospasm, nausea and vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization procedure was performed using a computer‐generated number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded, "independent observer assessed the agitation level" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 withdrawals: 4 in study group and 3 in control group due to "unexpected reasons or parent refusal" and 1 in study group due to "inappropriate data collection" (presumably this last one was actually from the control group, as investigators report 55 patients from each group completing the study) |
| Selective reporting (reporting bias) | Low risk | reported agitation scores |
Arai 2005.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years, ASA I‐II, adenotonsillectomy Exclusion criteria: long‐term therapy with hepatic enzyme‐inducing drugs and dysfunction of the central nervous or cardiovascular system, refusal to take the whole dose of premedication Recruitment: 42 children (14 in each of 3 groups) Location: Japan |
|
| Interventions | Mi group: midazolam 0.5 mg/kg oral premedication Mi + Di group: midazolam 0.25 mg/kg and diazepam 0.25 mg/kg oral premedication Control group: no premedication All participants: study premedications given 45 minutes before induction, sevoflurane induction and maintenance in 100% oxygen, tracheal intubation, local anaesthetic infiltration of the tonsillar fossae |
|
| Outcomes | Emergence behaviour assessed on the following 5‐point scale: 1 = obtunded with no response to stimulation 2 = asleep but responsive to movement or stimulation 3 = awake and responsive 4 = inconsolable crying 5 = thrashing behaviour requiring restraint Reported median (range) scores for emergence behaviour, not incidence of EA Other outcome: sedation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | children blinded between 2 active premeds but investigators did not describe using a placebo premed for the control group, anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported emergence scores |
Arai 2007.
| Methods | Randomized controlled trial | |
| Participants | 1‐3 years, ASA I, minor plastic surgery Exclusion criteria: history of chronic illness, prematurity, developmental delay, previous surgery, parental insistence on a particular study group Recruitment: 60 children randomly assigned, 58 analysed (19 midazolam group, 20 PPIA group, 20 midazolam + PPIA group) Location: Japan |
|
| Interventions | Midazolam group: midazolam 0.5 mg/kg PO premedication 40 minutes before induction PPIA group: parental presence (mother) at induction of anaesthesia Midazolam + PPIA group: both of the above All participants: sevoflurane induction in 100% oxygen, fentanyl 1 mcg/kg and vecuronium 0.1 mg/kg to facilitate tracheal intubation, sevoflurane maintenance 1.5%‐2.5% in 60% oxygen and fentanyl 4 mcg/kg |
|
| Outcomes | Emergence behaviour assessed on the following 5‐point scale: 1 = obtunded with no response to stimulation 2 = asleep but responsive to movement or stimulation 3 = awake and responsive 4 = inconsolable crying 5 = thrashing behaviour requiring restraint Reported median (range) scores for emergence behaviour, not incidence of agitation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | not viable to blind child and anaesthetist or to parental presence |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 children excluded as a result of refusal to take oral midazolam (1 from midazolam group and 1 from midazolam + PPIA group) |
| Selective reporting (reporting bias) | Low risk | reported emergence behaviour scores |
Araki 2005.
| Methods | Randomized controlled trial | |
| Participants | 1‐8 years, ASA I‐II, elective herniorrhaphy Exclusion criteria: allergy to drugs involved, previous adverse anaesthesia experience, mental retardation, long‐term medication that could react with midazolam Recruitment: 60 children recruited (15 in each of 4 groups) Location: Japan |
|
| Interventions | Group 1: no caudal and IV saline placebo at the end of surgery Group 2: no caudal and IV flumazenil 0.02 mg/kg at the end of surgery Group 3: caudal with 1 mL/kg 0.25% bupivacaine and IV saline placebo at the end of surgery Group 4: caudal with 1 mL/kg 0.25% bupivacaine and IV flumazenil 0.02 mg/kg at the end of surgery All children received 0.5 mg/kg midazolam premedication, induced with 5% sevoflurane with 66% nitrous oxide in oxygen, maintained with 3% sevoflurane with 66% nitrous oxide in oxygen |
|
| Outcomes | EA defined as a score of 2 or 3 on the following scale: 1 = asleep, calm or mildly agitated but easily consolable 2 = moderately agitated or restless but inconsolable 3 = hysterical, crying inconsolably, thrashing Other outcomes: effect of oral midazolam on entering the operating room |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned," "computer‐generated random number" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Asaad 2011.
| Methods | Randomized controlled trial | |
| Participants | 5‐10 years, ASA I, elective surgery of expected duration 30‐60 minutes (e.g. inguinal hernia repair, hydrocoele, circumcision) under general anaesthesia with caudal block Exclusion criteria: chronic or acute intake of any sedative or analgesic drug, known adverse effects of study drugs, failure of caudal block (defined as an increase in heart rate or mean arterial blood pressure > 10% of preincision value) Recruitment: 90 children randomly assigned, 88 analysed (28 in fentanyl group, 30 in each of the other 2 groups) Location: Egypt |
|
| Interventions | Fentanyl group: 1 mcg/kg IV fentanyl (in 10 mL over 10 minutes) Dexmedetomidine group: 0.15 mcg/kg IV dexmedetomidine (in 10 mL over 10 minutes) Control group: 10 mL saline placebo IV over 10 minutes All participants: sevoflurane induction and maintenance with 50% nitrous oxide in oxygen, spontaneous ventilation, intubation without relaxant, caudal block with 0.5 mL 0.25% bupivacaine |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = moderately agitated or restless 4 = combative, excited, disoriented Other outcomes: pain scores (CHIPPS), modified Aldrete scores, haemodynamics values, time to eye opening, time to discharge from PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned by a concealed envelope" |
| Allocation concealment (selection bias) | Low risk | "concealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child and anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals from fentanyl group due to meeting definition of failed caudal block |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Auerswald 2006.
| Methods | Randomized controlled trial | |
| Participants | 1‐5 years, ASA I‐III, adenoidectomy Exclusion criteria: none described Recruitment: 103 children in 4 groups (24 group 1, 27 group 2, 25 group 3, 27 group 4) Location: Germany |
|
| Interventions | Group 1: sevoflurane induction in oxygen, sevoflurane maintenance in 60% nitrous oxide with oxygen Group 2: sevoflurane induction in oxygen, sevoflurane maintenance in air/oxygen with alfentanil IV 20 mcg/kg, then 10‐mcg/kg boluses as required (maximum 100 mcg/kg/h) Group 3: sevoflurane induction in oxygen, propofol + alfentanil maintenance Group 4: TIVA with propofol + alfentanil All participants: premedication with 0.5 mg/kg midazolam 30 minutes preoperatively and EMLA cream/paracetamol 40 mg/kg 60 minutes preoperatively |
|
| Outcomes | EA defined as score of 0 on the following scale: 0 = very agitated, not consoled by nurse 1 = light agitation, easily consoled 2 = not agitated, sleeping Other outcomes: effect of premedication at induction, pain on injection with propofol, intubating conditions, postoperative vomiting, pain scores |
|
| Notes | English abstract, full paper in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized by drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | anaesthetist not blinded, children not blinded between IV induction group and inhalational induction groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether PACU nurse performing agitation assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12% of participants randomly assigned to group 4 (TIVA) were reassigned to group 3 protocol (sevo induction followed by IV maintenance) as uncooperative for IV insertion |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Bae 2010.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I, strabismus surgery Exclusion criteria: none described Recruitment: 60 children (15 in each of 4 groups) Location: Korea |
|
| Interventions | Group I (control): normal saline 2 mL IV at cessation of sevoflurane Group II: midazolam 0.025 mg/kg IV at cessation of sevoflurane Group III: midazolam 0.05 mg/kg IV at cessation of sevoflurane Group IV: normal saline 2 mL IV at cessation of sevoflurane + treatment with IV midazolam if agitation score of 4 or 5 occurred All participants: no premedication; induction with IV thiopental sodium, vecuronium and atropine; intubation; maintenance with sevoflurane 2%‐3% and 50% nitrous oxide; ketorolac 0.8 mg/kg; reversal with glycopyrrolate and pyridostigmine; parental presence in PACU |
|
| Outcomes | EA assessed on the following scale: 1 = asleep 2 = calm 3 = crying but can be consoled 4 = crying and cannot be consoled 5 = agitated and thrashing around Reported mean (SD) agitation scores, not incidence of EA Other outcomes: preinduction agitation scores, time to extubation, duration of emergence agitation, time to discharge from PACU, effect of IV midazolam in PACU to treat emergence agitation in group IV participants who had an emergence agitation score of 4 or 5 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly placed in 4 groups," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | "randomly placed in 4 groups," method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | children blinded, not stated whether treating anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported agitation scores |
Bakhamees 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I, adenotonsillectomy ± insertion of grommets Exclusion criteria: history of previous anaesthesia, bad hospital experience, known allergy to study drugs, diagnosed psychological or behavioural problems, participants who required propofol or fentanyl at any stage of anaesthesia other than planned Recruitment: 120 children (40 in each group) Location: Kingdom of Saudi Arabia |
|
| Interventions | Group F: fentanyl 1.5 mcg/kg before start of surgery and 0.1 mL/kg saline at the end of surgery Group FP: fentanyl 1.5 mcg/kg before start of surgery and propofol 1 mg/kg (maximum 30 mg) at the end of surgery Group C (control): 0.1 mL/kg saline at end of procedure All participants: midazolam 0.5 mg/kg oral premedication, sevoflurane induction and maintenance with 50% nitrous oxide, rectal paracetamol 40 mg/kg |
|
| Outcomes | EA was measured on a 10‐point scale: 1 = calm and sleepy 2‐4 = mild agitation 5‐7 = medium agitation 8‐10 = severe agitation Reported incidence of "any agitation" (used in forest plot) and incidence of agitation score of 5 or higher for longer than 5 minutes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Bal 2006.
| Methods | Randomized controlled trial | |
| Participants | 2‐10 years, ASA I‐II, elective adenotonsillectomy and adenoidectomy Exclusion criteria: cardiac, pulmonary, hepatic, renal diseases and known psychiatric disorder Recruitment: 120 children recruited (40 in each group) Location: Turkey |
|
| Interventions | Group P: propofol induction Group SP: sevoflurane induction followed by propofol 1 mg/kg Group S: sevoflurane induction All participants: no premedication, EMLA cream, parents not present at induction, induction as per group allocation, vecuronium 0.1 mg/kg, intubation, fentanyl 1 mcg/kg, sevoflurane 2%‐4% maintenance with 50% nitrous oxide, metamizole 15‐20 mg/kg, metoclopramide 0.25 mg/kg |
|
| Outcomes | "Anxiety" at 30 minutes after arrival in PACU was measured on a 4‐point scale: 1 = agitated, crying, uncooperative 2 = anxious but cooperative 3 = awake and calm 4 = asleep Reported median score and mean score ± SD Other outcomes: anxiety on arrival to anaesthetic room or at induction of anaesthesia and postoperative behavioural disturbances in the following week |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated using random number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | anaesthetist and child not binded to IV versus inhalational induction (not feasible) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported "anxiety" scores |
Bebawy 2005.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I‐II, inguinal hernia repair Exclusion criteria: contraindication to NSAIDs; history of sleep apnoea, developmental delay or psychological disorders Recruitment: 80 children (40 in each group) Location: Egypt |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: premedication with oral midazolam 0.5 mg/kg and rectal diclofenac 2 mg/kg, nitrous oxide during induction and maintenance, IV fentanyl 1 mcg/kg, local infiltration with ropivacaine 3 mg/kg |
|
| Outcomes | EA defined as 3 or 4 on the following scale: 1 = awake, calm, cooperative 2 = crying, requires consoling 3 = irritable, restless, screaming, inconsolable 4 = combative, disoriented EA measured on admission to PACU, then every 5 minutes Other outcomes: modified Yale Preoperative Anxiety Scale, a separation scale, an induction scale, objective pain scale (0‐10 inch), emergence and recovery times |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned," computer‐generated table |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned," method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded (not feasible) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Bergendahl 2004.
| Methods | Randomized controlled trial | |
| Participants | 1‐11 years, ASA I, adenoidectomy and/or tonsillectomy Exclusion criteria: none stated Recruitment: 104 children randomly assigned, 100 analysed (48 clonidine group, 52 midazolam group) Location: Sweden |
|
| Interventions | Intervention group: rectal clonidine premedication 5 mcg/kg 30‐60 minutes preinduction Control group: rectal midazolam premedication 300 mcg/kg 30‐60 minutes preinduction All participants: rectal atropine 40 mcg/kg included with the study drug premedication, sevoflurane induction in oxygen, IV fentanyl 2 mcg/kg, intubation, maintenance in sevoflurane 2%‐4% with nitrous oxide 65%, rectal paracetamol 20 mg/kg, awake extubation |
|
| Outcomes | Emergence delirium defined as a "confusion" score ≥ 3 on the following scale: 1 = calm 2 = could be easily calmed 3 = could not be easily calmed 4 = combative, excited, disoriented "Confusion" scores assessed on arrival in PACU and every 30 minutes for first 2 postoperative hours and reported sum of scores Other outcomes: Objective Pain Scale (OPS) scores for first 2 hours, number of rescue doses of fentanyl (for OPS ≥ 5), sedation, shivering, vomiting, parental assessment of analgesia, sedation and sleeping pattern over 24 hours |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was performed according to a computer‐generated random list" |
| Allocation concealment (selection bias) | Low risk | "both patients and investigators were blinded with respect to the randomized treatment and the study code was opened once the entire study was concluded" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "both patients and investigators were blinded with respect to the randomized treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "investigators were blinded with respect to the randomized treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 exclusions due to refusal to accept rectal premedication, major unexpected haemorrhage, severe bronchospasm after intubation necessitating cancellation of surgery and refusal to accept mask induction |
| Selective reporting (reporting bias) | Unclear risk | reported sum of "confusion" scores, but not incidence of delirium |
Beskow 1999.
| Methods | Randomized controlled trial | |
| Participants | 8 months‐18 years, ASA I‐II, minor surgery Exclusion criteria: infection, emergence surgery requiring rapid sequence induction, mental retardation, need for preoperative opioids, history of marked agitation after anaesthesia Recruitment: 62 children (31 in each group) Location: Sweden |
|
| Interventions | Intervention group: halothane maintenance Control group: sevoflurane maintenance All participants: rectal premedication with midazolam 0.4 mg/kg and atropine 0.02 mg/kg, local anaesthetic ointment before IV cannulation, parental presence at induction, IV induction with propofol 3 mg/kg (4 children in halothane group and 6 children in sevoflurane group had inhalational inductions caused by cannulation difficulties or fear of needles), maintenance study agent with 65% nitrous oxide, caudal block or local infiltration with bupivacaine 0.5‐1 mL/kg 0.25%, when appropriate |
|
| Outcomes | EA defined as a score greater than 5 on a 10‐point visual analogue scale (VAS) with VAS scores assessed upon arrival in PACU and at 15, 30, 45, 60, 75, 90 and 120 minutes after arrival Other outcomes: number of children with pain (pain assessed with VAS), vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned," method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "assessment was done by a specially trained nurse anaesthetist who was unaware of the agent given" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Binstock 2004.
| Methods | Randomized controlled trial | |
| Participants | 2‐10 years, ASA I‐II, day case surgery Exclusion criteria: none stated Recruitment: 129 children randomly assigned, 125 analysed (23 group 1, 27 group 2, 25 group 3, 26 group 4, 24 group 5) Location: USA |
|
| Interventions | 5 groups to study the effects of 2 different doses of oral transmucosal fentanyl (OTFC) premedication with or without ondansetron: Group 1: OTFC normal dose (10‐15 mcg/kg) premedication + ondansetron 0.1 mg/kg IV Group 2: OTFC normal dose (10‐15 mcg/kg) premedication + placebo Group 3: placebo + ondansetron 0.1 mg/kg IV Group 4: placebo + placebo Group 5: OTFC low dose (100 mcg fixed dose) premedication + placebo All participants: sevoflurane with nitrous oxide for induction and maintenance, caudal blocks with 0.125% bupivacaine for surgery below umbilicus |
|
| Outcomes | Postoperative "anxiety/agitation" defined as not "calm" but "clinging or apprehensive" or "crying" in PACU. Time point used in forest plot is upon arrival in PACU Other outcomes: number of participants requiring analgesia in PACU, caregiver and parental satisfaction, PONV, respiratory events, time to emergence, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all study drugs and placebos were prepared by the OR pharmacy and labelled with only the patients' name and hospital numbers" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "patients were monitored after anesthesia by an independent blinded observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 withdrawals reported for the following reasons:
|
| Selective reporting (reporting bias) | Low risk | reported incidence of "anxiety/agitation" |
Bock 2002.
| Methods | Randomized controlled trial | |
| Participants | 3‐8 years, ASA I‐II, minor surgery with caudal block Exclusion criteria: known endocrine disease, family history of malignant hyperthermia, aortic stenosis, signs of infection, preoperative agitation Recruitment: 80 children randomly assigned, 72 analysed (18 in each of 4 groups) Location: Germany |
|
| Interventions | Group I: clonidine 1 mcg/kg added to caudal + saline placebo IV Group II: clonidine 3 mcg/kg added to caudal + saline placebo IV Group III: no clonidine added to caudal + clonidine 3 mcg/kg IV Group IV: no clonidine added to caudal + saline placebo IV All participants: oral midazolam 0.4 mg/kg premedication, sevoflurane with 70% nitrous oxide induction and maintenance, atropine 0.1 mg/kg and atracurium 0.3 mg/kg IV, intubation, caudal block with 0.175% bupivacaine 1 mL/kg, caudal block judged to be effective if end‐tidal sevoflurane concentration could be reduced to 1.0% or lower without movement or increase in heart rate/blood pressure (> 15% of presurgical level) with surgical stimulus, parental presence at emergence |
|
| Outcomes | EA defined as total score ≥ 3 at any time point for items 3‐5 of the Pain/Discomfort Scale (as described by Hanallah et al. Anesthesiology 1987;66:832‐4) Other outcomes: nausea and vomiting, hypotension, bradycardia, time to PACU discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized into four groups according to a computer‐generated code" |
| Allocation concealment (selection bias) | Unclear risk | "randomized into four groups," method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "all of the drug combinations in the study were prepared before induction of anesthesia, leaving the observer unaware of group assignment," but not stated whether treating anaesthetist was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "one blinded observer... recorded all data" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants withdrawn because of preoperative agitation, and 6 participants withdrawn because the end‐tidal concentration of sevoflurane could not be reduced to below the predetermined threshold |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Bortone 2006.
| Methods | Randomized controlled trial | |
| Participants | 1‐6 years, ASA I‐II, elective subumbilical surgery suitable for regional block Exclusion criteria: chronic illness, developmental delay, parents not able to collaborate during induction, premedication, ineffective regional block Recruitment: 110 children randomly assigned (54 sevoflurane group, 56 isoflurane group) Location: Italy |
|
| Interventions | Intervention group: isoflurane maintenance at 1‐1.5 MAC Control group: sevoflurane maintenance at 1‐1.5 MAC All participants: un‐premedicated, induction with sevoflurane up to 8% in air/oxygen 50:50 mix with parental presence, LMA with sevo 3%‐5% maintenance until regional block (penile, caudal ilioinguinal) performed with 0.25% bupivacaine, rectal paracetamol 20 mg/kg, randomization to study maintenance agent once block deemed effective |
|
| Outcomes | EA defined as 3 or 4 on the following scale: 1 = calm child: no kind of intervention 2 = consolable child: requires only physical contact with parents 3 = agitated child: a screaming and crying child 4 = aggressive child: must be physically restrained to avoid harm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "children were randomly allocated using a computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "children, their parents as well as the researcher who gathered outcome data, were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals after randomization |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Breschan 2007.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐5 years, scheduled minor surgery Exclusion criteria: regional block not working Recruitment: 115 children (57 children in intervention group, 58 children in control group) Location: Austria |
|
| Interventions | Intervention group: rectal midazolam 1 mg/kg 10‐15 minutes before surgery Control group: rectal midazolam 0.5 mg/kg 10‐15 minutes before surgery All participants: general anaesthesia induced with propofol or sevoflurane and maintained with 1.5% sevoflurane, appropriate regional block (caudal, penile or local infiltration) |
|
| Outcomes | EA defined as a score of 3 measured on following 3 point scale: 1 = calm and cooperative 2 = mildly anxious and agitated but consolable 3 = severely agitated, totally out of control and inconsolable Other outcomes: sedation scores, time to awakening |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomisation list," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor (recovery room nurse) blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Bryan 2009.
| Methods | Randomized controlled trial | |
| Participants | 18 months‐7 years, ASA I‐II, MRI brain Exclusion criteria: inpatients, tracheostomy, family history of malignant hyperpyrexia, allergy to propofol, diagnostic or therapeutic procedures in addition to MRI Recruitment: 201 children randomly assigned, 200 analysed (101 in GAS group, 99 in GAP group) Location: USA |
|
| Interventions | GAP group: propofol maintenance (i.e. following sevoflurane induction, a propofol bolus of 2 mg/kg given, then 200 mcg/kg/min infusion maintained) without LMA GAS (control) group: sevoflurane 2% maintenance with LMA All participants: no premedication, sevoflurane induction, maintenance agent increased if movement occurred and decreased if apnoea occurred |
|
| Outcomes | EA defined as PAED score > 10 Also reported median PAED scores with interquartile ranges Other outcomes: pausing of MRI scan, respiratory complications, timeliness of care (including time in PACU) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A block‐randomized plan stratified by age was used to assign the patients to the two treatment groups" "In addition, block randomization with block size 8 was used to ensure that the allocation ratio of the number of subjects in each treatment was 1:1 after every 8th randomized participant in each age‐treatment stratum" method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor (research co‐ordinator) blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded from the final analysis (not stated which group) because a radiologist added sequences of the spine after scanning the brain, although stated that "the analysis population was the intent‐to‐treat population" |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA and complete PAED score data |
Burke 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I‐III, neurologically intact children, elective MRI, with sevoflurane induction and isoflurane maintenance Exclusion criteria: children requiring preoperative sedation Recruitment: 88 children randomly assigned (45 in parent present group, 43 in parent absent group) Location: USA |
|
| Interventions | Parent present (intervention) group: parent reunited with child before emergence in PACU Parent absent (control) group: parent reunited per routine practice after arousal of patient in PACU at discretion of PACU nurse |
|
| Outcomes | EA scored on the following 4‐point scale: None = quiet, calm, cooperative Mild = restless/crying, quiets with reassurance Moderate = agitated, restless, non‐purposeful, difficult to control Severe = agitated, thrashing, kicking, non‐purposeful, incoherent, inconsolable Children scoring moderate or severe also scored on PAED scale Other outcomes: duration of agitation, parent and nurse survey, modified Post‐Hospitalization Behaviour Questionaire on day 1 and day 7 (parents telephoned) |
|
| Notes | Sevoflurane only used at induction and isoflurane used for maintenance in all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | child and anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor (trained observer) blinded as to purpose of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals after randomization |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Byon 2012.
| Methods | Randomized controlled trial | |
| Participants | 4‐12 years, ASA I‐II, strabismus surgery Exclusion criteria: history of motion sickness, previous PONV, gastrointestinal tract disorders or administration of antiemetic in the 24 hours before surgery Recruitment: 446 children randomly assigned, 405 analysed (203 in midazolam group, 202 in control group), 41 withdrawals described in risk of bias table Location: Republic of Korea |
|
| Interventions | Intervention group: midazolam 0.1 mg/kg IV before induction of anaesthesia Control group: no midazolam All participants: no premedication, ramosetron 6 mcg/kg IV before induction of anaesthesia, induced with thiopental 6 mg/kg and atropine 0.02 mg/kg, sevoflurane 8% after loss of consciousness, LMA insertion, maintenance with sevoflurane 2%‐2.5% with nitrous oxide 50% |
|
| Outcomes | EA (secondary outcome) assessed with PAED scale on arrival to PACU and after 30 minutes in PACU. Report mean (SD) PAED scores at these 2 time points. Do not report incidence of EA Other outcomes: incidence of nausea, retching or vomiting (primary outcome); number of participants requiring postoperative analgesics |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "41 patients were excluded because they were lost to follow‐up" (22 from midazolam group, 19 from control group) |
| Selective reporting (reporting bias) | Low risk | reported PAED scores |
Calderon 1996.
| Methods | Randomized controlled trial | |
| Participants | 3 months‐10 years, ASA I‐II, day case infraumbilical or ENT procedures < 3 hours' surgical time Exclusion criteria: pneumonia in the previous year, anticipated difficult intubation, CVS or CNS disease, congenital cardiac or respiratory anomalies, renal or hepatic insufficiency, family history of muscle disorders (malignant hyperthermia), general anaesthesia in past 2 weeks Recruitment: 39 participants (20 in control group, 19 in intervention group) Location: Spain |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: midazolam premedication 0.2 mg/kg intranasally, nitrous oxide during induction and maintenance, caudal or ilioinguinal block for infraumbilical procedures, fentanyl 2 mcg/kg IV for ENT procedures, postoperative analgesia with paracetamol 10 mg/kg |
|
| Outcomes | Reported emergence agitation rate but no specific scale or definition used Other outcomes: time until awakening, recovery (Aldrete scale) scores, adverse effects during recovery period |
|
| Notes | English abstract, full paper in Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | report incidence of EA |
Chandler 2012.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I‐II, elective strabismus repair Exclusion criteria: not stated Recruitment: 112 participants randomly assigned, 94 analysed (47 in each group, 18 exclusions described in risk of bias table) Location: Canada |
|
| Interventions | Intervention group: TIVA propofol and remifentanil Control group: sevoflurane and nitrous oxide induction and maintenance All participants: oral premedication of paracetamol 20 mg/kg and ibuprofen 10 mg/kg, LMA |
|
| Outcomes | Emergence delirium defined as PAED score ≥ 10 (PAED scores recorded 5‐minutely from LMA removal) Other outcomes: induction behaviour (PACBIS), pain scores (FLACC scale), time to LMA removal, time in PACU |
|
| Notes | Abstract only (conference proceedings) Full paper published in Pediatric Anesthesia 2013;23(4):309‐15 (outside 19 January 2013 search date) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child not blinded to induction technique, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "masked investigator assessed ED" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18 exclusions (1 failed IV, 17 protocol deviations) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Chiba 2003.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years, short (< 1 hour) general anaesthesia (urology, ENT, paediatric surgery, ophthalmology, orthopaedics, plastics) Exclusion criteria: none stated Recruitment: 90 children (30 in each of 3 groups) Location: Japan |
|
| Interventions | Group P1: propofol 1 mg/kg 5 minutes before end of operation Group P2: propofol 2 mg/kg 5 minutes before end of operation Control group: intralipid 0.2 mL/kg 5 minutes before end of operation All participants: no premedication, sevoflurane 7% induction, sevoflurane maintenance 2.5%‐3.5%, sevoflurane ceased 5 minutes before end of operation |
|
| Outcomes | EA assessed with new scoring system, which investigators devised (scoring range ‐4 to 10), and report mean scores for each group Other outcomes: time to extubation, time to emergence |
|
| Notes | English abstract, full paper in Japanese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | report EA scores |
Chiu 1999.
| Methods | Randomized controlled trial | |
| Participants | 1‐10 years, ASA I‐II, < 25 kg, elective urology surgery < 1 hour Exclusion criteria: none stated Recruitment: 40 children (20 in each group) Location: Malaysia |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance |
|
| Outcomes | Restlessness and agitation evaluated using the 3 subjective components of the Objective Pain Scale. If the child was crying inconsolably and was thrashing and hysterical, he was reported to be agitated Other outcomes: emergence time, PONV |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "....recorded by a second investigator blinded to the agents used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Cohen 2002.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I‐II, adenoidectomy ± myringotomy tubes Exclusion criteria: sleep apnoea, developmental delay, psychological disorders Recruitment: 100 children (50 in each group) Location: USA |
|
| Interventions | Intervention group: desflurane maintenance 4%‐6% Control group: sevoflurane maintenance 1.5%‐2% All participants: no premedication, sevoflurane induction in nitrous oxide/oxygen 70:30 mix, mivacurium for ETT, nitrous oxide during maintenance, fentanyl 2.5 mcg/kg, ondansetron 0.1 mg/kg |
|
| Outcomes | EA measured on following 3‐point scale with score of 3 defined as severe agitation: 1 = calm 2 = agitated but consolable 3 = severely agitated, inconsolable Other outcomes: pain scores (Objective Pain Scale), time until discharge (Steward recovery score); incidence of agitation, pain, vomiting at home 24 hours after surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | report incidence of EA |
Cohen 2003.
| Methods | Randomized controlled trial | |
| Participants | 2‐36 months, ASA I‐II, elective ambulatory surgery Exclusion criteria: < 40 weeks postconceptional age, signs of developmental delay, procedures expected to last < 30 minutes Recruitment: 53 children (26 sevoflurane group, 27 propofol group) Location: USA |
|
| Interventions | Intervention group: propofol maintenance (200 mcg/kg/h) Control group: sevoflurane maintenance All participants: sevoflurane induction, 60% nitrous oxide during induction and maintenance; IV fentanyl 2 mcg/kg or caudal block with 0.25% bupivacaine according to surgical procedure |
|
| Outcomes | EA defined as 3 on the following 3‐point scale: 1 = calm 2 = agitated but consolable 3 = severely agitated, inconsolable Other outcomes: time to emergence, recovery and discharge from PACU, agitation and pain at home 24 hours postoperatively |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "an observer blinded to anesthetic technique recorded degree of agitation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Cohen 2004.
| Methods | Randomized controlled trial | |
| Participants | < 3 years (2‐33 months), ASA I‐II, ambulatory surgical procedures Exclusion criteria: none stated Recruitment: 56 children (28 in each group) Location: USA |
|
| Interventions | Intervention group: propofol maintenance 200 mcg/kg/min Control group: sevoflurane 1.5%‐2.5% maintenance All participants: sevoflurane 8% induction with nitrous oxide 60%, mivacurium to facilitate intubation, nitrous oxide 60% during maintenance, caudal block 1 mL 0.25% bupivacaine for infraumbilical procedures, fentanyl 1 mcg/kg IV for other procedures |
|
| Outcomes | "Recovery assessments" performed and reported, along with number of participants requiring additional opioids or midazolam to control pain or agitation during recovery Other outcomes: ionized calcium (primary outcome) and magnesium concentrations, haemodynamics, recovery times, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "assessment was performed by investigator blinded to the anaesthetic technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported an agitation outcome |
Cravero 2000a.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐10 years, ASA I‐II, bilateral pressure equalization tube insertion with same surgeon Exclusion criteria: none stated Recruitment: 43 children (22 intervention group, 21 control group) Location: USA |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: nitrous oxide throughout anaesthesia, rectal paracetamol 25 mg/kg |
|
| Outcomes | EA defined as "the child thrashing and requiring restraint for > 3 min" Other outcomes: nausea and vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated blocks of 8 participants |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded observer....recorded the level of agitation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Cravero 2000b.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐10 years, ASA I‐II, MRI scans Exclusion criteria: emotional disorders, cognitive delays, neurological conditions affecting communication Recruitment: 32 children (17 intervention, 15 control) Location: USA |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: no premedication, nitrous oxide throughout anaesthesia |
|
| Outcomes | EA assessed with the following 5‐point scale: 1 = obtunded with no response to stimulation 2 = asleep but responsive to movement or stimulation 3 = awake and responsive 4 = crying 5 = thrashing behaviour that requires restraint High threshold EA definition = level 5 for > 3 minutes Low threshold EA definition = level 4 for > 3 minutes High threshold definition used for this review Other outcomes: time to discharge from PACU and secondary recovery unit, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random assignment in blocks of five" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a blinded observer ...recorded the level of agitation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Cravero 2003.
| Methods | Randomized controlled trial | |
| Participants | 18 months‐10 years, ASA I‐II, MRI scans Exclusion criteria: midazolam premedication, neurological condition limiting communication, defined psychological/emotional disorder, cognitive delay Recruitment: 32 participants (16 intervention, 16 control) Location: USA |
|
| Interventions | Intervention group: fentanyl 1 mcg/kg IV 10 minutes before end of anaesthesia Control group: saline IV 10 minutes before end of anaesthesia All participants: sevoflurane induction with nitrous oxide, sevoflurane maintenance in 100% oxygen |
|
| Outcomes | EA defined as a score ≥ 4 for ≥ 5 minutes despite all calming efforts by the child's parents/guardians and nursing personnel using the following 5‐point scale: 1 = obtunded with no response to stimulation 2 = asleep but responsive to movement or stimulation 3 = awake and responsive 4 = crying 5 = thrashing behaviour that requires restraint Other outcomes: duration of anaesthesia, duration of agitation, itching or vomiting, time to hospital discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number assignment" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether treating anaesthetist was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "one trained observer, blinded to patient group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Dalens 2006.
| Methods | Randomized placebo‐controlled trial | |
| Participants | 6 months‐8 years, MRI scans Exclusion criteria: haemodynamic/respiratory unstable participants, severe mental retardation/physical disabilities, sedative/anticonvulsant treatment, emergency MRI scans, extremely distressed/uncooperative children, parental refusal Recruitment: 90 participants (28 control, 33 group K, 29 group N) Location: Canada |
|
| Interventions | Group K: ketamine 0.25 mg/kg IV at end of procedure Group N: nalbuphine 0.1 mg/kg IV at end of procedure Control group: saline at end of procedure All participants: sevoflurane induction in 100% oxygen, sevoflurane maintenance in air/oxygen, 10 mL/kg Ringer's solution followed by maintenance, LMA |
|
| Outcomes | EA defined as a score ≥ 4 for ≥ 5 minutes despite all calming efforts by the child's parents/guardians and nursing personnel using the following 5‐point scale: 1 = obtunded with no response to stimulation 2 = asleep but responsive to movement or stimulation 3 = awake and responsive 4 = crying 5 = thrashing behaviour that requires restraint |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random list was established by the department of Epidemiology and Human Statistics of our institution" |
| Allocation concealment (selection bias) | Low risk | "The vials used for the study were prepared by the pharmacy of our institution. All vials were identical and contained the same volume of transparent and unidentifiable fluid. The patient's anesthesiologist and the nursing staff were unaware of the drug administered" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | as above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Davis 1999.
| Methods | Randomized controlled trial | |
| Participants | 1‐5 years, ASA I‐II, ambulant surgery for bilateral myringotomy Exclusion criteria: none stated Recruitment: 200 participants (50 in each of 4 groups) Location: USA |
|
| Interventions | Group 1: halothane + IV ketorolac 1 mg/kg Group 2: halothane + IV saline placebo Group 3: sevoflurane + IV ketorolac 1 mg/kg Group 4: sevoflurane + IV saline placebo All participants: intranasal midazolam 0.2 mg/kg premedication, induction and maintenance with study anaesthetic agent with nitrous oxide |
|
| Outcomes | EA defined as a score of 2 or 3 on the following 3‐point scale: 1 = asleep, calm, or mildly agitated but easily consolable 2 = moderately agitated or restless but inconsolable 3 = hysterical, crying inconsolably, or thrashing Other outcomes: vomiting, number of participants requiring rescue analgesia in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number code" |
| Allocation concealment (selection bias) | Low risk | "injections were prepared by the hospital pharmacist and placed in specially labelled syringes. Patients, physicians, and the research nurse were blinded to the syringes contents" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | as above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
De Kort 2007.
| Methods | Randomized controlled trial | |
| Participants | 2‐8 years, ASA I‐II, circumcision, orchidopexy or inguinal hernia repair Exclusion criteria: insufficient block suspected by haemodynamic variables Recruitment: 80 children randomly assigned, 79 analysed (19 placebo group, 20 in each of the other 3 groups) Location: Belgium |
|
| Interventions | Group 1: saline placebo Group 2: sufentanil 0.1 mg/kg Group 3: clonidine 2 mcg/kg Group 4: sufentanil 0.1 mg/kg + clonidine 2 mcg/kg All participants: sevoflurane induction and maintenance, caudal or penile block, then randomly assigned to treatments above |
|
| Outcomes | EA assessed on the following 5‐point scale: 1 = obtunded with no response to stimulation 2 = asleep but responsive to movement/stimulation 3 = awake and responsive 4 = crying 5 = thrashing behaviour that requires restraint Incidence of score ≥ 4 used as incidence of EA for this review |
|
| Notes | We have assumed that study authors reported clonidine dosage of 2 mg/kg as a typographical error, and that the actual dosage was 2 mcg/kg Abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly in a double‐blind fashion," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "blinded solution of sufentanil and/or clonidine" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "blinded solution of sufentanil and/or clonidine" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant from placebo group discontinued from the study because of inadequate penile block |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Demirbilek 2004.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I, adenotonsillectomy ± grommets Exclusion criteria: "none of the patients had a history of sleep apnoea, developmental delay or psychological disorders" Recruitment: 120 children (30 in each of 4 groups) Location: Turkey |
|
| Interventions | Group 1: sevoflurane + saline Group 2: sevoflurane + fentanyl 2.5 mcg/kg during induction Group 3: desflurane + saline Group 4: desflurane + fentanyl 2.5 mcg/kg during induction All children: oral midazolam 0.5 mg/kg premedication, thiopental induction, atracurium to facilitate endotracheal intubation, maintenance with study anaesthetic agent, rectal acetaminophen 30 mg/kg |
|
| Outcomes | EA defined as a score of 3 on the following 3‐point scale: 1 = asleep/calm 2 = agitated but consolable 3 = severely agitated/inconsolable Other outcomes: pain scores, vomiting, emergence time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, treating anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded anesthesiologist also assessed the degree of agitation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Dong 2010.
| Methods | Randomized controlled trial | |
| Participants | 3‐7 years, ASA I‐II, adenotonsillectomy Exclusion criteria: psychological or emotional disorders, abnormal cognitive development, developmental delay, known history of allergy to drugs in the protocol Recruitment: 60 participants (30 in each group) Location: China |
|
| Interventions | Intervention group: remifentanil infusion 1 mcg/kg/min intraoperatively Control group: no remifentanil All participants: IM atropine 0.01 mg/kg 30 minutes preoperatively, IV induction fentanyl 3 mcg/kg and propofol 2.5 mg/kg, intubation after vecuronium 0.1 mg/kg, maintenance with sevoflurane 1.5%‐2.5% with 50% nitrous oxide, sevoflurane concentration adjusted to maintain stable haemodynamics |
|
| Outcomes | Emergence agitation defined as PAED scale score ≥ 10 PAED score assessed 5‐minutely for first 30 minutes in PACU Other outcomes: time to eye opening, time to extubation, haemodynamics, SpO2 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "concealed random number generator" |
| Allocation concealment (selection bias) | Unclear risk | "concealed random number generator" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "scored by two nurses who were blinded to the anaesthetic used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
El‐Sada 2005.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years, ASA I‐II, surgery for congenital esotropia, paralytic or myogenic strabismus Exclusion criteria: disturbed neuromuscular transmission, treatment with aminoglycosides Recruitment: 30 participants (10 in each group) Location: Egypt |
|
| Interventions | Propofol group : propofol TIVA (2.5 mg/kg induction, then 10 mg/kg/h) S‐ketamine group: IV induction and maintenance with S‐ketamine (0.5 mg/kg, then 1 mg/kg/h) Control group: sevoflurane induction and maintenance, nitrous oxide All participants premedicated with 0.2 mg/kg midazolam and 0.02 mg/kg atropine IM |
|
| Outcomes | EA defined as "crying, difficult to console, or wild thrashing behaviour that require restraint" Other outcomes: PONV, respiratory depression |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "randomized to one of the three groups using sealed envelope assignment, concealing the randomization from the attending anaesthesia staff until directly before induction of anaesthesia" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child not blinded to induction technique but blinded to maintenance, attending anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor in PACU blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Epstein 1995.
| Methods | Randomized controlled trial | |
| Participants | 9 months‐16 years, ASA I‐II, elective inpatient otorhinolaryngological or orthopaedic procedures Exclusion criteria: none stated Recruitment: 40 participants (20 in each group) Location: USA |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: 2:1 nitrous oxide/oxygen mixture, vecuronium 0.1 mg/kg, intubation, maintained at 1.5 MAC for first 20 minutes then 0.75 MAC until end of surgery, fentanyl 1 mcg/kg 15 minutes before end, reversed with atropine/neostigmine, extubated awake |
|
| Outcomes | Reported "excitement" on emergence Other outcomes: haemodynamics, induction and emergence times, coughing, breath‐holding |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor of emergence was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | report incidence of "excitement" on emergence |
Erden 2012.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, infraumbilical surgery with caudal block Exclusion criteria: none stated Recruitment: 40 participants (20 in each group) Location: Turkey |
|
| Interventions | Intervention group: ondansetron 0.1 mg/kg IV Control group: placebo All participants: oral midazolam 0.5 mg/kg premedication, sevoflurane anaesthesia, caudal block |
|
| Outcomes | EA assessed with PAED scale at 10, 20, 30, 60, 120, 180 minutes. Reported comparison of agitation scores between groups (P value) but not the scores Other outcomes: induction quality, parental separation, sedation and pain scores |
|
| Notes | Abstract only (conference proceeding) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "assigned to two groups," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | reported comparison of agitation scores between groups (P value) but not scores or incidence of EA |
Erdil 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I, adenoidectomy ± myringotomy/insertion of tubes Exclusion criteria: sleep apnoea, developmental delay, psychological disorders Recruitment: 90 children (30 in each of 3 groups) Location: Turkey |
|
| Interventions | Group F: fentanyl 2.5 mcg/kg IV after tracheal intubation Group D: dexmedetomidine 0.5 mcg/kg IV after tracheal intubation Control group: saline All participants: sevoflurane induction with 50% nitrous oxide, atracurium 0.6 mg/kg for tracheal intubation, dexamethasone 0.5 mg/kg for pain and vomiting, sevoflurane maintenance in oxygen |
|
| Outcomes | EA assessed on following 5‐point scale with severe agitation defined as ≥ 4: 1 = sleeping 2 = awake, calm 3 = irritable, crying 4 = inconsolable, crying 5 = severe restlessness, disorientation, thrashing around Other outcomes: haemodynamics, pain, PONV, emergence times |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | study drugs and saline were "prepared by a team member not involved in data recording." All given as 10 mL volume via syringe labelled with child's study number |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ersin 2005.
| Methods | Randomized controlled trial | |
| Participants | Children with intellectual disability (mean age 11 years), dental treatment under GA Exclusion criteria: none stated Recruitment: 86 participants (44 intervention, 42 control) Location: Turkey |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: midazolam premedication 0.5‐0.75 mg/kg oral or PR, nitrous oxide, atracurium 0.5 mg/kg and atropine 10‐20 mcg/kg IV, diclofenac 2‐2.5 mg/kg PR, local infiltration with lignocaine for extractions |
|
| Outcomes | EA defined as VAS > 5 (0‐10 100‐mm scale) Agitation distinguished from pain by the fact that the child was uncooperative, screaming, could not be consoled and tried to leave his/her bed Other outcomes: pain (VAS), PONV, bleeding, laryngospasm, bronchospasm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly selected by use of a computer‐generated table to receive either..." |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "specially trained recovery nurse, who was unaware of the anesthetic method used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Fan 2000.
| Methods | Randomized controlled trial | |
| Participants | 1‐8 years, ASA I, inguinal surgery Exclusion criteria: signs of infection, emergency surgery, mental retardation, GA within past 3 months, contraindication to use of tramadol Recruitment: 40 children (20 in each group) Location: Taiwan |
|
| Interventions | Intervention group: tramadol 1 mg/kg IV when suturing Control group: no tramadol All participants: EMLA 1 hour before surgery, premedication with 0.01 mg/kg of atropine orally, thiamylal 4 mg/kg IV induction, sevoflurane maintenance in oxygen, lignocaine infiltration applied before skin incision |
|
| Outcomes | EA measured on a visual analague scale (VAS). Reported number of children with VAS > 5 and mean VAS scores Other outcomes: pain, coughing, nausea and vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | child blinded, not stated whether treating anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "emergence agitation was recorded....by a blinded anesthesiologist in the PACU" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Finkel 2001.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐5 years, ASA I‐II, bilateral myringotomy Exclusion criteria: none stated Recruitment: 150 participants (51 fentanyl 1 mcg/kg, 50 fentanyl 2 mcg/kg, 49 control group) Location: USA |
|
| Interventions | Intervention groups: intranasal fentanyl 1 mcg/kg or 2 mcg/kg Control group: intranasal saline All participants: sevoflurane with 60% nitrous oxide induction and maintenance, PR paracetamol 40 mg/kg |
|
| Outcomes | EA defined as 3 or more on the following 4‐point scale: 1 = calm 2 = crying but can be consoled 3 = crying and cannot be consoled 4 = agitated and thrashing around Other outcomes: vomiting, pain (Objective Pain Scale) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the study drug was prepared in a 1‐mL syringe for each patient by a member of the research team who was not involved in the care or observation of the patient being studied" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Galinkin 2000.
| Methods | Randomized controlled trial | |
| Participants | 9 months‐6 years, weight < 25 kg, ASA I‐II, bilateral myringotomy Exclusion criteria: allergy to fentanyl or midazolam, history suggestive of increased risk of malignant hyperthermia, risk of airway obstruction, neurodevelopmental delay, cardiopulmonary disease, active history of untreated gastro‐oesophageal reflux Recruitment: 265 children randomly assigned, 260 analysed (64, 69, 66 and 61 in each group) Location: USA |
|
| Interventions | 4 groups: Sevoflurane + nasal fentanyl 2 mcg/kg Sevoflurane + nasal saline placebo Halothane + nasal fentanyl 2 mcg/kg Halothane + nasal saline placebo All participants: oral premedication with acetaminophen 10 mg/kg and midazolam 0.5 mg/kg, study inhalation agent with nitrous oxide for induction and maintenance |
|
| Outcomes | Severe EA defined as a score of 4 on the following 4‐point scale: 1 = calm 2 = not calm but consolable 3 = not easily calmed, moderate agitation, restlessness 4 = combative, excited, disoriented, thrashing Other outcomes: pain (CHEOPS), vomiting, adverse respiratory events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "anesthesiologist, research observer, patient, parents and PACU nurse were all blinded to the intranasal solution administered, which was drawn up by a third party with no further involvement in the study" "research observer, patient, parents and PACU nurse were all blinded to the inhalation agent used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals due to protocol violations (unclear to which groups participants were assigned) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ghai 2010.
| Methods | Randomized controlled trial | |
| Participants | 1‐6 years, ASA I‐II, cataract surgery Exclusion criteria: history of allergy to midazolam, clonidine or bupivacaine; seizure disorder; developmental delay; any neurological disease potentially associated with symptoms of agitation; family history of malignant hyperthermia; or ineffective sub‐Tenon block during surgery (defined as rise in heart rate or blood pressure of 20% above baseline or requirement of > 1.5% sevoflurane) Recruitment: 124 children randomly assigned, 120 analysed (39 group C1, 41 group C2, 40 control group) Location: India |
|
| Interventions | Group C1 = clonidine 1 mcg/kg IV Group C2 = clonidine 2 mcg/kg IV Control group: normal saline IV All participants: oral midazolam premedication 0.5 mg/kg, sevoflurane 8% induction in 40:60 mix of oxygen/nitrous oxide, maintenance with sevoflurane 1%‐1.5% with 33% oxygen in nitrous oxide, spontaneous ventilation with LMA, sub‐Tenon block by surgeon 0.08‐0.1 mL/kg 0.5% bupivacaine after proparacaine eyedrops, IV paracetamol 10 mg/kg |
|
| Outcomes | EA defined as Pain/Discomfort Score (PDS) ≥ 3 using items 3‐5 only. PDS measured at 15‐minute intervals for 90 minutes. Severe agitation defined as PDS > 6 Other outcomes: parental separation scores, induction scores, time to discharge, incidence of cardiovascular and respiratory depression, nausea and vomiting, intraoperative heart and systolic blood pressure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random number" |
| Allocation concealment (selection bias) | Low risk | "kept in opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child, anaesthetist, surgeon all blinded: "the envelope was opened by an independent anaesthetist not involved in the study, who prepared the study drugs" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 exclusions: 1 excluded from group C1 as subtenon block could not be administered because of compromised sclera, 2 excluded from group C2 because of incomplete postoperative data, 1 excluded from control group because of incomplete postoperative data |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ghosh 2011.
| Methods | Randomized controlled trial | |
| Participants | 1‐5 years, ASA I‐II, elective urogenital and lower limb surgery Exclusion criteria: history of allergic reaction to local anaesthetics, aspirin ingestion in the preceding 4 weeks, history of bleeding disorder, preexisting neurological and spinal disorder and infection at the local site Recruitment: 90 (30 in each of 3 groups) Location: India |
|
| Interventions | Clonidine 1 mcg/kg in local anaesthetic caudal block Clonidine 0.75 mcg/kg in local anaesthetic caudal block Control group: no clonidine in local anaesthetic caudal block All participants: no premedication, sevoflurane 4%‐5% induction with 50% nitrous oxide, intubation after rocuronium 1 mg/kg, maintenance with sevoflurane 1% with oxygen/nitrous oxide in 1:2 ratio, no opioids given, caudal block 0.75 mL/kg of 0.25% bupivacaine, reversal with neostigmine/atropine at end of surgery |
|
| Outcomes | EA defined as Pain/Discomfort Score (PDS) ≥ 3 using items 3‐5 only Other outcomes: hypotension, bradycardia, respiratory compromise |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A resident doctor who did not take part in the care of the children prepared the study drugs. The study investigators, as well as the children and their parents were unaware of the contents of the study drug" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The same investigator, who was blind to the randomisation process, observed all of the patients in PACU" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Gouda 2004.
| Methods | Randomized controlled trial | |
| Participants | 3‐8 years, ASA I‐II, elective myringotomy Exclusion criteria: any neurological condition, medication that might affect anaesthesia or recovery Recruitment: 40 participants (20 in each group) Location: Egypt |
|
| Interventions | Remifentanil 0.25 mcg/kg IV at conclusion of surgical procedure Control group: saline placebo 10 mL IV at conclusion of surgical procedure All participants: sevoflurane with nitrous oxide induction and maintenance, PR acetaminophen 15 mg/kg |
|
| Outcomes | EA defined as a score ≥ 4 for ≥ 5 minutes on the following 5‐point scale, despite all calming efforts by the child's parents or guardians and nursing personnel: 1 = no response to stimuli 2 = responsive to stimuli but asleep 3 = awake and responsive 4 = crying and difficult to console 5 = wild thrashing, behaviour requiring restraint Other outcomes: time in PACU, nausea/vomiting, 4‐point pain scale (1 = none, 2 = mild, 3 = moderate, 4 = severe) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised into 2 groups", method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "nurse blinded to patient group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Guard 1998.
| Methods | Randomized controlled trial | |
| Participants | 2‐8 years, ASA I‐II, elective urological procedures as outpatients Exclusion criteria: none stated Recruitment: 50 children (25 in each group) Location: Canada |
|
| Interventions | Intervention group: propofol induction and maintenance Control group: sevoflurane induction and maintenance All participants: no premedication, 70% nitrous oxide before IV access then continued throughout, rocuronium and reversal, lumbar or caudal epidural block ± catheter insertion |
|
| Outcomes | "Excitation" during emergence reported Other outcomes: haemodynamics, time in PACU, number of participants requiring analgesia in PACU, number of participants with delayed discharge from hospital, adverse anaesthetic or surgical outcomes, caregiver and parental satisfaction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number tables" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | children blinded (all received 70% nitrous oxide initially), anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "research nurse who was blinded to the anaesthetic assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of "excitation" during emergence |
Guler 2002.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria: 3‐6 years, ASA I, adenotonsillectomy Exclusion criteria: none stated Recruitment: 40 children (20 in each group) Location: Turkey |
|
| Interventions | Intervention group: peritonsillar infiltration of 0.25% bupivacaine 0.5 mL/kg beforesurgical procedure Control group: peritonsillar infiltration of 0.9% saline 0.5 mL/kg before surgical procedure All participants: premedication (medication not stated), sevoflurane 8% induction with 60% nitrous oxide, sevoflurane 2%‐3% maintenance, rocuronium 0.6 mg/kg, intubation, atropine and neostigmine reversal |
|
| Outcomes | EA defined as score of 3 or 4 on following 4‐point scale: 1 = calm 2 = crying but can be consoled 3 = crying, cannot be consoled 4 = agitated and thrashing Other outcomes: pain scores, analgesic requirement, vomiting, haemodyamics |
|
| Notes | English abstract, full paper in Turkish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist or surgeon blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Guler 2005.
| Methods | Randomized controlled trial | |
| Participants | 3‐7 years, ASA I, adenotonsillectomy Exclusion criteria: midazolam premedication due to anxiety Recruitment: 60 participants (30 in each group) Location: Turkey |
|
| Interventions | Dexmedetomidine 0.5 mcg/kg IV 5 minutes before end of surgery Control group: saline placebo IV 5 minutes before end of surgery All participants: oral acetaminophen 15 mg/kg premedication, sevoflurane with nitrous oxide induction and maintenance, tracheal intubation facilitated by vecuronium 0.1 mg/kg IV, reversal with 0.05 mg/kg neostigmine and 0.02 mg/kg atropine IV |
|
| Outcomes | Severe EA defined as score ≥ 4 on the following 5‐point scale: 1 = sleeping 2 = awake, calm 3 = irritable, crying 4 = inconsolable crying 5 = severe restlessness, disorientation, thrashing around Other outcomes: vomiting, pain (5‐point scale: 1 = no pain, asleep; 2 = mild pain but without distress; 3 = stating that the throat was very painful but not distressed or moderately tearful and consolable; 4 = considerable distress; 5 = screaming and struggling violently) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization program" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "anesthesiologist was blinded to the drug," both given as 5‐mL volume |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded research observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Gurkan 1999.
| Methods | Randomized controlled trial | |
| Participants | 3‐15 years, ASA I, strabismus surgery Exclusion criteria: allergy to any of the drugs to be used, symptomatic medical illness Recruitment: 40 children (20 in each group) Location: Turkey |
|
| Interventions | Intervention group: propofol induction and maintenance Control group: sevoflurane induction and maintenance All participants: nitrous oxide 66% throughout surgery, vecuronium 0.1 mg/kg for intubation, neostigmine and atropine for reversal, acetaminophen 10 mg/kg oral or rectal in PACU for pain |
|
| Outcomes | Reported incidence of EA but did not give a definition of EA Other outcomes: incidence of oculocardiac reflex, PONV |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child not blinded to induction technique, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded nurse" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Hallen 2001.
| Methods | Randomized controlled trial | |
| Participants | 3‐8 years, ASA I, elective myringotomy Exclusion criteria: none stated Recruitment: 60 participants (30 in each group) Location: Sweden |
|
| Interventions | Halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: premedication with rectal midazolam 0.3 mg/kg and paracetamol 25 mg/kg, nitrous oxide throughout |
|
| Outcomes | Postoperative excitation defined as 2 or higher on the following scale: 0 = quiet, calm 1 = crying, not restless 2 = crying and restless 3 = crying, restless, uncontrollable Other outcomes: PONV, pain (4‐point scale: 1 = none, 2 = mild, 3 = moderate, 4 = severe) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor (experienced nurse) blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of postoperative excitation |
Hanna 2004.
| Methods | Randomized controlled trial | |
| Participants | 4‐10 years, ASA I‐II, adjustable strabismus surgery Exclusion criteria: history of neurological disease, mental retardation, muscle disease or previous history of pseudocholinesterase deficiency Recruitment: 30 participants (15 in each group) Location: Egypt |
|
| Interventions | Intervention group: propofol induction and maintenance for placement of adjustable sutures, allowed to briefly emerge, then propofol 1 mg/kg for adjustment of sutures Control group: sevoflurane induction and maintenance for placement of adjustable sutures, allowed to briefly emerge, then propofol 1 mg/kg for adjustment of sutures All participants: 50% nitrous oxide in oxygen, mivacurium bolus then infusion, remifentanil 0.5 mcg/kg/min infusion |
|
| Outcomes | EA defined as "combative, excited and disoriented behaviour that requires transient physical restraint." Assessment performed after adjustment of sutures Other outcomes: extubation time, PONV |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into two equal groups," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child not blinded to induction technique, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "independent observer, blinded to the nature of the anaesthetic used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Hegazy 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐8 years, ASA I‐II, elective cranial MRI Exclusion criteria: diagnostic imaging for trauma, those for whom any of the agents investigated were contraindicated, upper respiratory infection, known gastro‐oesophageal disease, craniofacial malformation, intracranial bleeding, hypertension, full stomach Recruitment: 50 children (25 in each group) Location: Egypt |
|
| Interventions | Intervention group: thiopental 2‐3 mg/kg IV given after sevoflurane induction Control group: no thiopental All participants: no premedication, sevoflurane 8% induction, IV access, LMA insertion, sevoflurane 1.5%‐2% maintenance in 100% oxygen |
|
| Outcomes | EA defined as PAED score ≥ 16 at any time in the first 15 minutes after emergence. PAED scores assessed upon emergence and every 5 minutes for first 15 minutes and mean (SD) peak PAED scores reported Other outcomes: recovery times, heart rate, blood pressure, SpO2, nausea, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "patients were blinded to group assignment," anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "staff collecting data were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Hosten 2011.
| Methods | Randomized controlled trial | |
| Participants | 1‐6 years, ASA I, minor surgery below umbilicus (inguinal hernia repair, undescended testis, hypospadias, circumcision) Exclusion criteria: neurological or psychiatric disease, attention deficit disorder, emergency procedure, medical contraindication to performance of caudal block or a previous anaesthesia experience, caudal block judged inadequate (heart rate and/or blood pressure increase > 10% baseline on skin incision, mCHEOPS pain score > 4 in recovery room) Recruitment: 70 children randomly assigned, 67 analysed (34 ondansetron, 33 control) Location: Turkey |
|
| Interventions | Ondansetron 0.1 mg/kg IV up to maximum of 4 mg Control group: placebo All participants: premedication with rectal midazolam 0.5 mg/kg 30 minutes previously, sevoflurane 8% induction with 50% nitrous oxide in oxygen, LMA insertion, randomization to ondansetron or placebo, caudal block 0.8 mL/kg 0.25% levobupivacaine with skin incision 15 minutes after block completed, maintenance with sevoflurane 1%‐2% (corrected for stable intraoperative haemodynamics) in 60:40 nitrous oxide/oxygen mixture, awake LMA removal |
|
| Outcomes | EA defined as score > 5 on 10‐point scale (TPS) ranging from 1 (calm or asleep) to 10 (worst possible and inconsolable agitation). TPS assessed every 10 minutes for first 30 minutes in recovery room Other outcomes: mYPAS preoperative anxiety scores, mCHEOPS pain scores in recovery, time to eye opening, time to discharge readiness, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomisation was performed immediately before anaesthetic induction by drawing prepared numbers from closed envelopes" |
| Allocation concealment (selection bias) | Low risk | "closed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "independent observer blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 exclusions: 1 from each group for inadequate caudal block intraoperatively (received fentanyl following skin incision), and 1 exclusion from placebo group for mCHEOPS pain score > 4 in PACU |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Hsieh 1999.
| Methods | Randomized controlled trial | |
| Participants | 3 months‐1 year, 5‐11 kg, ASA I‐II, day case urology Exclusion criteria: history of cardiac, respiratory, hepatic, renal, neurological or neuromuscular disease Recruitment: 40 participants (20 in each group) Location: Taiwan |
|
| Interventions | Halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: nitrous oxide, atropine 0.01 mg/kg and atracurium 0.5 mg/kg IV after induction, ilioinguinal and iliohypogastric blocks before surgery |
|
| Outcomes | Postoperative "restlessness and agitation" reported without a formal definition Other outcomes: cough, laryngospasm, breath‐holding and vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number tables" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "observer who was blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ibacache 2004.
| Methods | Randomized controlled trial | |
| Participants | 1‐10 years, ASA I, subumbilical surgery Exclusion criteria: chronic or acute intake of sedative or analgesic drug, adverse effect to any study drug, previous anaesthesia, failure of caudal block Recruitment: 90 participants (30 in each of 3 groups) Location: Chile |
|
| Interventions | Dexmedetomidine 0.15 mcg/kg IV Dexmedetomidine 0.30 mcg/kg IV Control group: saline placebo All participants: sevoflurane with nitrous oxide induction and maintenance, caudal block 0.5‐1 mL/kg 0.25% bupivacaine, study drug given after induction |
|
| Outcomes | EA defined as 3 or higher on the following 4‐point scale: 1 = calm 2 = not calm but easily calmed 3 = not easily calmed, moderately agitated or restless 4 = combative, excited, disoriented Other outcomes: haemodynamics, emergence and recovery times, pain scores (CHIPPS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random numbers generated by a computer" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "administration of anesthesia and study drugs....were made by two investigators blinded as to the study drugs" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "evaluated by a blinded nurse" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Im 2004.
| Methods | Randomized controlled trial | |
| Participants | 3‐10 years, ASA I‐II, tonsillectomy and adenoidectomy Exclusion criteria: developmental delay, epilepsy, obstructive sleep apnoea, previous history of emergence agitation Recruitment: 60 children (15 in each of 4 groups) Location: Korea |
|
| Interventions | Group 1: nil Group 2: ketorolac 0.5 mg/kg Group 3: fentanyl 1 mcg/kg Group 4: ketorolac 0.5 mg/kg + fentanyl 1 mcg/kg All participants: thiopentone 5 mg/kg IV induction, rocuronium 0.6 mg/kg, atropine 0.02 mg/kg, sevoflurane maintenance in oxygen/nitrous oxide, study drug given IV after intubation |
|
| Outcomes | EA defined as a score of 3 on following scale: 0 = asleep 1 = calm, crying, can be consoled 2 = cannot be consoled 3 = hysterical, agitated, thrashing around Other outcomes: severe pain on objective pain/discomfort scale, faces scale |
|
| Notes | English abstract, full paper in Korean | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blinded design" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a blinded observer evaluated each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Inomata 2010.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I‐II, elective minor surface surgery Exclusion criteria: airway malformation, clinical evidence of a difficult airway, asthma, any sign of upper respiratory infection, participants taking CNS depressants, CNS disorders including spinal cord dysfunction, developmental delay, autism, participants who received rescue doses of fentanyl after surgery (i.e. complained of pain after surgery or tried to remove dressing after surgery) Recruitment: 150 children randomly assigned, 139 analysed (45 group F1, 48 group F2, 46 control group) Location: Japan |
|
| Interventions | Group F1: fentanyl 1 mcg/kg bolus IV, then infusion 0.5 mcg/kg/h Group F2: fentanyl 2 mcg/kg bolus IV, then infusion 1 mcg/kg/h Control group: normal saline All participants: no premedication, sevoflurane 5% induction in oxygen, intubation after bolus of study drug, sevoflurane 1.5%‐2.5% maintenance (titrated to maintain haemodynamics within 20% baseline) in oxygen, local anaesthetic (ropivacaine) field block, awake extubation |
|
| Outcomes | PAED scores assessed continuously for 15 minutes after emergence, with the score that lasted the longest considered the agitation score. Reported mean (SD) PAED scores. Also reported frequency of emergence agitation (PAED score > 10) for the 2 intervention groups Other outcomes: intubating conditions, time to extubation, time to recovery, haemodynamics |
|
| Notes | Successful correspondence with study author to obtain frequency of agitation (PAED score > 10) in the control group (unpublished data) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated to one of three groups using computer‐generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, attending anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "assessed by an anaesthesiologist blinded to the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 exclusions: 4 control group participants, 5 group F1 participants and 2 group F2 participants excluded after administration of fentanyl rescue doses |
| Selective reporting (reporting bias) | Low risk | reported PAED scores and incidence of EA |
Isik 2005.
| Methods | Randomized placebo‐controlled trial | |
| Participants | 18 months‐10 years, ASA I‐II, cranial MRI Exclusion criteria: none stated Recruitment: 51 children (17 in each of 3 groups) Location: Turkey |
|
| Interventions | Group Fi: fentanyl 1 mcg/kg IV at induction of anaesthesia (2 mL saline 10 minutes before end anaesthesia) Group Fe: fentanyl 1 mcg/kg IV 10 minutes before end anaesthesia (2 mL saline at induction of anaesthesia) Group P: placebo control group (2 mL saline at induction and 10 minutes before end of anaesthesia) All participants: no premedication, sevoflurane induction and maintenance with 50% nitrous oxide, IV insertion, LMA |
|
| Outcomes | EA defined as a score of 4 or 5 on a 5‐point agitation scale (in Turkish). EA assessed every 5 minutes for first 25 minutes Other outcomes: recovery times, side effects |
|
| Notes | English abstract, full paper inTurkish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into three groups," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Isik 2006a.
| Methods | Randomized controlled trial | |
| Participants | 18 months‐10 years, cranial MRI, no premedication Exclusion criteria: psychological disorder, cognitive delay, chronic or acute sedative drug intake, neurological condition limiting communication Recruitment: 42 participants (21 in each group) Location: Turkey |
|
| Interventions | Group D: dexmedetomidine 1 mcg/kg IV Group P: saline placebo All participants: sevoflurane with 50% nitrous oxide induction and maintenance, insertion of IV catheter then study drug given over 2 minutes Location: Turkey |
|
| Outcomes | EA assessed on the following 5‐point scale and delirium defined as a score ≥ 4 for ≥ 5 minutes despite calming efforts of parents: 1 = sleeping 2 = awake/calm 3 = irritable, crying 4 = inconsolable crying 5 = severe restlessness, disorientation Other outcomes: haemodynamics, emergence and recovery times, PONV, bronchospasm |
|
| Notes | Reported incidence of EA and incidence of delirium. EA incidence used in forest plot of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomization list," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "administration of anesthesia and study drugs....were made by investigators blinded to the study drugs" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Jacob 2012.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, MRI Exclusion criteria: none stated Recruitment: 21 children (10 in sevoflurane group, 11 in propofol group) Location: USA |
|
| Interventions | Intervention group: propofol maintenance with supplemental oxygen Control group: sevoflurane maintenance with LMA All participants: sevoflurane induction |
|
| Outcomes | EA assessed with PAED scale but no report of EA incidence or PAED scores (stated that "PAED score revealed that ED was more frequent in children in Group S") Other outcomes: in vivo proton MR spectroscopy (1H‐MRS) to detect cerebral metabolites |
|
| Notes | Abstract only (conference proceeding) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | EA incidence or PAED scores not reported |
Jiang 2010.
| Methods | Randomized controlled trial | |
| Participants | Children undergoing cataract surgery; ASA I‐II; normal development; normal cardiovascular, respiratory, liver, kidney and neurological symptoms; no sedatives within 24 hours of surgery; mean (SD) ages: 2.4 (1.3) years in midazolam group and 2.7 (1.7) years in control group Recruitment: 48 children (24 in each group) Location: China |
|
| Interventions | Oral midazolam premedication 0.5 mg/kg 30 minutes before operation Control group: oral placebo 30 minutes before operation All participants: sevoflurane 8% induction with nitrous oxide 60%, spontaneous ventilation via LMA, maintenance with sevoflurane 2%‐3% with nitrous oxide 50% |
|
| Outcomes | EA defined as a score of 4 or higher on the following 5‐point scale: 1 = asleep 2 = awake and calm 3 = agitated and crying 4 = inconsolable 5 = delirious and inconsolable Other outcomes: emergence time, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomization numbers |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | report incidence of EA |
Johannesson 1995.
| Methods | Randomized controlled trial | |
| Participants | Children, ASA I, ENT surgery, median (range) age: group H 4.2 (1.1‐7.3) years, group S 4.0 (2.1‐7.5) years Exclusion criteria: none stated Recruitment: 40 participants (18 in halothane group, 22 in sevoflurane group) Location: Sweden |
|
| Interventions | Group H: halothane induction and maintenance Group S: sevoflurane induction and maintenance All participants: rectal midazolam and atropine premedication, nitrous oxide, intubation, paracetamol 10 mg/kg |
|
| Outcomes | Immediate behaviour during emergence from anaesthesia was evaluated according to the following scale: calm; cooperative; crying unhappily; or being turbulent. Those who were crying unhappily and were evaluated as turbulent were classified as excited Other outcomes: haemodynamics, PONV |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "open randomized study," not stated whether outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Jung 2010.
| Methods | Multi‐centre randomized controlled trial | |
| Participants | 3‐10 years, ASA I, surgery for strabismus or entropion Exclusion criteria: previous anaesthesia; cardiovascular, pulmonary or neurological disease; current upper respiratory tract infection Recruitment: 95 children "studied," 93 analysed (25 group 1, 23 group 2, 24 group 3, 21 group 4) Location: Korea |
|
| Interventions | Group 1: ketamine 1.5 mg/kg IV induction + fentanyl 1.5 mcg/kg IV after induction Group 2: ketamine 1.5 mg/kg IV induction + no fentanyl Group 3: thiopental sodium 5 mg/kg IV induction + fentanyl 1.5 mcg/kg IV after induction Group 4: thiopental sodium 5 mg/kg IV induction + no fentanyl All participants: no premedication, rocuronium 0.4 mg/kg, intubation, maintenance with 2%‐3% sevoflurane (adjusted to maintain stable haemodynamics and adequate anaesthesia) with 50% nitrous oxide in oxygen, ketorolac 0.5 mg/kg, ondansetron 0.1 mg/kg, pyridostigmine/glycopyrrolate reversal, awake extubation |
|
| Outcomes | EA defined as score of 3 on following 3‐point scale: 1 = asleep, calm or mildly agitated 2 = moderately agitated or restless but consolable 3 = hysterical, crying inconsolable, or thrashing Other outcomes: preoperative anxiety, time to extubation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned to one of four groups," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the state of emergence was evaluated by an anesthesiologist who was blind to the anesthetic technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95 children "studied" but outcome data reported for only 93; reasons for exclusion of 2 participants not stated |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Kain 2005.
| Methods | Randomized controlled trial | |
| Participants | 3‐10 years, ASA I‐II, outpatient surgery Exclusion criteria: chronic illness, prematurity, developmental delay Recruitment: 102 participants (52 control group, 50 halothane group) Location: USA |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: induction with study inhalation agent with nitrous oxide, procedure‐specific analgesia protocols |
|
| Outcomes | "Emergence behaviour was rated based on a scale developed and validated by Keegan et al" (no other explanation) and reported incidence of ED Other outcomes: postoperative maladaptive behavioural changes (PHBQ, primary outcome), sleep, recovery inventory, pain (Paediatric Pain Measure for Parents) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child and parents blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all research assistants who gathered outcome data were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Kataria 1996.
| Methods | Randomized controlled trial | |
| Participants | 0‐18 years, ASA I‐II, surgery > 1 hour Exclusion criteria: significant cardiac or respiratory abnormalities; renal, central nervous system or hepatic disease; history of unusual response to halogenated anaesthetics; had received a general anaesthesia in previous 2 weeks; taking medication known to affect MAC, hepatic or renal function Recruitment: 431 participants randomly assigned, 428 analysed (214 in each group) Location: 12 institutions from 6 nations (1 from Austria, 1 from Belgium, 1 from United Kingdom, 2 from Switzerland and 6 from USA) |
|
| Interventions | Halothane induction and maintenance Control group: sevoflurane induction and maintenance Preoperative medication (midazolam or fentanyl) was standardized at each centre, study agent was administered with 60%‐70% nitrous oxide, muscle relaxants were used when needed, opioids (fentanyl) were permitted but standardized at each site for both groups, morphine was given postoperatively when needed for pain |
|
| Outcomes | Reported incidence of "excitement" on emergence (no definition given) Other outcomes: emergence and recovery times, plasma fluoride levels, adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "open‐label," "trained, independent observers" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 children in the halothane groups were discontinued from the study because of the appearance of cardiac dysrhythmia (supraventricular ectopies and ventricular bigeminy). Administration of halothane was discontinued, and the anaesthetic was changed to isoflurane in both cases, with resolution of dysrhythmia |
| Selective reporting (reporting bias) | Low risk | reported incidence of "excitement" |
Kazak 2010.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I, routine procedures of less than 90 minutes Exclusion criteria: use of sedatives or hypnotics within the previous month, use of theophylline or hepatic enzyme‐inducing drugs, presence of severe central nervous system dysfunction, increased intracranial pressure, malformation of cardiovascular system, hypertonus, hyperthyroidism Recruitment: 60 children (20 in each of 3 groups) Location: Turkey |
|
| Interventions | Group M: midazolam oral premedication 0.5 mg/kg orally Group MP: midazolam oral premedication 0.25 mg/kg + parental presence at induction Group P: placebo oral premedication + parental presence at induction All participants: sevoflurane 7% induction in oxygen, rocuronium 0.15 mg/kg, LMA insertion, sevoflurane 1.5%‐2.5% maintenance with 50% nitrous oxide in oxygen, paracetamol 15‐20 mg/kg rectally before surgery started, reversal of muscle relaxant, awake LMA removal |
|
| Outcomes | Postanaesthetic recovery was evaluated using FLACC score, Anxiety Scale score, visual analogue scale (VAS) pain score and Observer Pain Scale (OPS) score every 10 minutes Other outcomes: sedation scores preoperatively and postoperatively, Anxiety Scale scores preoperatively, parental satisfaction, parental interviews at 1 and 14 days postoperatively for changes in child's behaviour |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated into three groups by sealed envelope method," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded (placebo premedication), not stated whether anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "physician in the postanaesthesia care unit blinded to the study groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported postoperative behavioural scores |
Keaney 2004.
| Methods | Randomized controlled trial | |
| Participants | Children, ASA I‐II, day cases, mean (SD) age in months: 39.9 (31.3) sevoflurane, 47.7 (35.3) halothane Exclusion criteria: previous GA, central nervous system or psychological disorders Recruitment: 197 children randomly assigned, 120 analysed (63 sevoflurane group, 57 halothane group) Location: Ireland |
|
| Interventions | Halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: nitrous oxide, analgesia (PR diclofenac 1 mg/kg and local anaesthesia by regional technique or local infiltration) was administered as appropriate and normally practised |
|
| Outcomes | "Distress on emergence" measured as a score of 3 or higher on following 4‐point scale: 1 = calm and cooperative 2 = mildly anxious but consolable 3 = tearful and inconsolable 4 = totally out of control Other outcomes: Post‐Hospital Behavioural Questionnaire (PHBQ) for behavioural disturbance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the emergence score was documented by the recovery room nursing staff, who were blinded to the anesthetic agent" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 77 withdrawals from study (i.e. 39% of those randomly assigned): 22 excluded because of breach of protocol (not mentioned how many from each group); 55 excluded because of failure to return questionnaire (halothane group n = 25, sevoflurane group n = 30) |
| Selective reporting (reporting bias) | Low risk | reported emergence scores |
Khattab 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I‐II, elective dental surgery Exclusion criteria: developmental problems, cerebral palsy, Down syndrome, inborn errors of metabolism, history of epileptic fits, body weight less than 10 kg or greater than 26 kg, known allergy to any medications used, procedures lasting less than 30 minutes or longer than 2 hours Recruitment: 95 children randomly assigned, 46 analysed in each group, 3 exclusions described in risk of bias table Location: Qatar |
|
| Interventions | Group M: oral midazolam 0.5 mg/kg premedication Group KM: oral midazolam 0.5 mg/kg + ketamine 2 mg/kg premedication All participants: premedication with study drugs mixed with ibuprofen suspension 10 mg/kg, sevoflurane 8% induction in 100% oxygen, atropine 0.02 mg/kg, fentanyl 1 mcg/kg, cisatracurium 0.15 mg/kg, intubation then maintenance with sevoflurane 1.5%‐2% in 35:65 oxygen/nitrous oxide mix, IV paracetamol 15 mg/kg, neostigmine/atropine reversal, awake extubation |
|
| Outcomes | EA assessed with the following 5‐point scale: 1 = obtunded with no response to stimuli 2 = asleep but responsive to movement and stimuli 3 = awake and appropriately responsive 4 = crying and difficult to console 5 = wild thrashing behaviour that requires restraint. EA defined as an agitation score ≥ 4 for longer than 5 minutes despite all calming efforts by the parents Other outcomes: time to emergence, postoperative fentanyl consumption, time to hospital discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned...via computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "study drugs were prepared by a trained nurse (who was not involved in any other part of the study) into identical 5 ml‐syringes," "the anesthetist... was blind to the premedication" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all scores and observations were recorded by the anesthesia technician, who was unaware of the type of premedication" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 exclusions for procedure duration exceeding 2 hours (2 from group M and 1 from group KM) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Kim 2009.
| Methods | Randomized controlled trial | |
| Participants | 3‐10 years, ASA I‐II, adenotonsillectomy Exclusion criteria: airway disease, sleep apnoea, developmental delay, psychological disorder Recruitment: 105 children randomly assigned, 100 analysed (32 group A10, 34 group A20, 34 control group) Location: Korea |
|
| Interventions | Group A10: alfentanil 10 mcg/kg Group A20: alfentanil 20 mcg/kg Control group: saline All participants: sevoflurane 8% with nitrous oxide induction and maintenance, ketorolac 1 mg/kg, dexamethasone 0.1 mg/kg, study drug IV after induction |
|
| Outcomes | "Severe agitation" defined as 3 or 4 on 4‐point Aono's scale: 1 = calm 2 = not calm but could be easily consoled 3 = moderately agitated 4 = combative, excited or disoriented, thrashing around PAED scale also assessed, with mean and range PAED scores reported Other outcomes: emergence and recovery times, pain scores (CHIPPS), number of participants requiring analgesia in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated using a sealed envelope system," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "an independent researcher prepared the study syringe for each patient" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the anaesthesiologist who recorded the result was blinded to the patient group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants excluded: 1 from control group because of breath‐holding, 3 from group A10 (1 because of postoperative bleeding, 1 as the result of systemic epinephrine absorption, 1 as the result of breath‐holding), 1 from Group A20 because of postoperative bleeding |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA and PAED scores |
Kim 2011.
| Methods | Randomized controlled trial | |
| Participants | 1‐13 years, ASA I‐II, strabismus surgery Exclusion criteria: refusal by parents, neurological disease, developmental delay, history of previous surgery, ASA III‐IV, airway disease Recruitment: 101 children (35 group M, 31 group P, 35 group S) Location: Korea |
|
| Interventions | Group M: midazolam 0.05 mg/kg IV at end of anaesthesia Group P: propofol 1 mg/kg IV at end of anaesthesia Group S: saline IV at end of anaesthesia (control group) All participants: atropine 0.01 mg/kg IM premedication, IV induction thiopentone 5 mg/kg, rocuronium 0.6 mg/kg, intubation, maintenance with sevoflurane 2%‐3% with 50% nitrous oxide in oxygen, IV paracetamol 10 mg/kg, awake extubation, parental presence in PACU; 5 minutes before end of surgery, sevoflurane and nitrous oxide were discontinued and study drugs were given |
|
| Outcomes | EA defined as score of 3 or 4 over 10 minutes during PACU stay using the following scale: 1 = calm 2 = not calm but could be easily calmed 3 = not easily calmed, moderately agitated or restless 4 = excited or disoriented Other outcomes: time to emergence, number of participants needing fentanyl to treat severe agitation (score of 4), laryngospasm, nausea/vomiting, desaturation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned by means of random numbers generated by a computer" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "in the PACU, a nurse who was blinded to the patient's study group recorded the degree of emergence agitation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ko 2001.
| Methods | Initially randomized controlled trial but no longer randomized after 1 month (all children in intervention group) | |
| Participants | 12‐110 months, ASA I‐II, elective outpatient surgery Recruitment: during randomized phase of trial, 43 children randomly assigned (22 control, 21 midazolam). Total of 88 children in study, including non‐randomized phase (22 control, 66 midazolam). Location: Taiwan |
|
| Interventions | Midazolam 0.2 mg/kg oral premedication at least 10 minutes before induction Control group: placebo premedication All participants: sevoflurane with nitrous oxide induction and maintenance, atropine 0.01 mg/kg before intubation |
|
| Outcomes | EA defined as > 3 on a 10‐point scale (0 = none, 5 = moderate, 10 = worst) If score ≥ 5, IV meperidine 0.5 mg/kg was given. If 10 minutes after M1, agitation still persisted with a score ≥ 5, a second dose of meperidine (0.5 mg/kg) was given. After another 10 minutes, if agitation continued to persist, IV propofol 1 mg/kg was given Other outcomes: time in PACU, parental and PACU nurse satisfaction |
|
| Notes | No longer RCT after 1 month; as the authors believed the midazolam group was doing so much better, they decided to give everyone midazolam. Correspondence with study authors revealed that the number of participants in the midazolam group was 21 and the incidence of EA was 11 cases before randomization ceased Entered data for randomized phase only in forest plot |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | study changed from double‐blind (initially both anaesthetist and outcome assessor were blinded, as was major nursing caregiver) to single‐blind part‐way through the study (only outcome assessors remained blinded) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded throughout study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals for randomized portion of the study (randomized part of study was stopped early because of high incidence of emergence agitation in control group) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Kobylarz 2001.
| Methods | Randomized controlled trial | |
| Participants | Inclusion criteria: 2 weeks‐14 years, ASA I‐II, various surgeries except cardiothoracic and neurosurgical Recruitment: 102 children (38 halothane, 64 sevoflurane) Location: Poland |
|
| Interventions | Intervention group: halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: analgesia or regional anaesthesia technique, subgroups received midazolam/atropine premedication versus no premedication |
|
| Outcomes | EA defined as the need for constant care of the emerging child with 1‐to‐1 nursing | |
| Notes | English abstract, full paper in Polish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Kol 2008.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I‐II, cranial MRI for diagnosis of convulsion, epilepsy and headache Exclusion criteria: upper respiratory infection, known gastro‐oesophageal disease, craniofacial malformation, intracranial bleeding, hypertension, full stomach Recruitment: 88 children randomly assigned, 79 analysed (37 propofol, 42 sevoflurane) Location: Turkey |
|
| Interventions | Intervention group: propofol induction and maintenance Control group: sevoflurane induction and maintenance All participants: parental presence on induction, no premedication, EMLA cream applied, IV cannula inserted awake, randomization after insertion of IV cannula |
|
| Outcomes | EA assessed with PAED scale and reported mean (SD) PAED scores Other outcomes: haemodynamics, MRI interruption, emergence times, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random allocations in a ratio of 1:1 in balanced blocks of 8" |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered, opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 in the propofol group and 2 in the sevoflurane group were excluded from data analysis because of protocol violations resulting from lack of recording of clinical data |
| Selective reporting (reporting bias) | Low risk | reported PAED scores |
Koner 2011.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years, ASA I‐II, infraumbilical day case surgery Exclusion criteria: allergy to study drugs, psychological or emotional disorders, developmental delay, contraindication to caudal block, caudal block failure (heart rate increase at skin incision) Recruitment: 95 children randomly assigned, 84 analysed (42 in each group) Location: Turkey |
|
| Interventions | Group MH: midazolam 0.5 mg/kg and hydroxyzine 1 mg/kg oral premedication 30 minutes before Group M: midazolam 0.5 mg/kg oral premedication 30 minutes before All participants: no parental presence at induction, induction with sevoflurane 7% with nitrous oxide 60% in oxygen, maintenance sevoflurane 2% with 60% nitrous oxide, IV alfentanil 10 mcg/kg, LMA, caudal block 0.25% bupivacaine (0.8 mL/kg up to 16 mL), parental presence in PACU |
|
| Outcomes | PAED scale score assessed every 5 minutes for first 30 minutes in PACU EA defined as PAED score ≥ 16 at any time during the 30‐minute follow‐up period Median PAED scores for each group also reported Other outcomes: parental separation score, induction quality, sedation quality (before induction), Ramsay sedation score at 30 minutes in PACU, pain scores in PACU (CHIPPS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation and allocation....was performed using computerised numbers" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 withdrawals from group M (5 did not take premedication, 2 delayed transfer to operating room) and 4 from group MH (3 did not take premedication, 1 had caudal block failure) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Konig 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐12 years, ambulatory dental surgery under general anaesthesia Exclusion criteria: psychiatric, behavioural or developmental history; medical contraindication to any of the study drugs; history of malignant hyperpyrexia; ASA III or higher Recruitment: 184 children randomly assigned, 179 analysed (91 in sevoflurane group, 88 in propofol group) Location: USA |
|
| Interventions | Intervention group: propofol maintenance Control group: sevoflurane maintenance All children: 20 mg/kg paracetamol preoperatively, midazolam 0.5 mg/kg at the discretion of the anaesthetist, sevoflurane induction, nitrous oxide during maintenance, boluses of fentanyl if clinically indicated by heart rate/blood pressure criteria |
|
| Outcomes | EA measured on PAED scale every 5 minutes after waking for 30 minutes. Highest score during this period for each of the 5 behaviours was used to compute the final PAED score Reported median PAED scores with interquartile ranges and number of participants with PAED score > 10. Number of participants with PAED score > 10 used for this review Other outcomes: PONV, number of nursing interventions in the recovery room, time to discharge readiness, parental satisfaction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned by a computer‐generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "trained observers were blinded to the anesthetic techniques and intraoperative drugs" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants were excluded from the data analysis (1 from sevoflurane group and 4 from propofol group). One exclusion was the result of equipment failure, and 4 exclusions were due to airway concerns on the part of the responsible anaesthesiologist, leading to the decision not to extubate in a deep plane of anaesthesia |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA and PAED scores |
Kubo 2001.
| Methods | Randomized controlled trial | |
| Participants | 3 months‐6 years, ASA I‐II, hernia day surgery Exclusion criteria: allergies Recruitment: 110 children randomly assigned, 104 analysed (28 group S, 23 group P, 29 group MS, 24 group MP), 6 withdrawals described in risk of bias table Location: Japan |
|
| Interventions | Group S: sevoflurane 2% maintenance with 70% nitrous oxide until end of surgery Group P: propofol maintenance (2.5 mg/kg, then 20 mg/kg/h for 10 minutes, then 10 mg/kg/h, then 5 mg/kg/h when closing wound, ceased at end of surgery) Group MS: as for group S and oral premedication with midazolam 0.5 mg/kg and famotidine 1 mg/kg 30 minutes preinduction Group MP: as for group P and oral premedication with midazolam 0.5 mg/kg and famotidine 1 mg/kg 30 minutes preinduction All participants: sevoflurane induction with 70% nitrous oxide, regional block 0.5 mg/kg bupivacaine 0.25% |
|
| Outcomes | EA assessed on the following 3‐point scale: 1 = none—child is lying quietly with no crying 2 = mild—occasional movement and/or crying; no need for restraint 3 = marked—thrashing and/or needs restraint and/or constant crying Other outcomes: recovery scores (Steward scores), pain scores, PONV, time to oral intake, time to discharge home |
|
| Notes | English abstract, full paper in Japanese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 excluded because they required "top‐up" medication for light anaesthesia (1 from group S, 5 from group P) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Kulka 2001a.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years , ASA I‐II, penile operations Exclusion criteria: mental retardation, neurological disease, insufficient pain therapy with penile block in previous operations or in PACU, behavioural difficulties Recruitment: 46 children randomly assigned, 40 analysed (20 in each group) Location: Germany |
|
| Interventions | Intervention group: midazolam 0.1 mg/kg IV before termination of anaesthesia Control group: saline placebo All participants: sevoflurane induction and maintenance in 2:1 nitrous oxide in oxygen, penile block, paracetamol 15 mg/kg PR |
|
| Outcomes | Agitaton defined as non‐purposeful movement that requires intervention from staff. Severe agitation defined as requirement for midazolam in PACU Other outcomes: PONV, pain scores |
|
| Notes | English abstract, full paper in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind placebo controlled" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 exclusions from each group due to insufficient penile block |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Kulka 2001b.
| Methods | Randomized controlled trial | |
| Participants | Aged 2‐7 years, ASA I‐II, undergoing circumcision Exclusion criteria: mental retardation, history of agitation, any other neurological disease, inappropriate pain therapy Recruitment: 49 children randomly assigned, 40 analysed (20 in each group) Location: Germany |
|
| Interventions | Intervention group: clonidine 2 mcg/kg IV 5 minutes after start of surgery Control group: saline placebo All participants: midazolam premedication 0.5 mg/kg orally 30 minutes before induction, sevoflurane with nitrous oxide induction and maintenance penile block (0.1 mL/kg 0.5% bupivacaine), paracetamol 15 mg/kg suppository |
|
| Outcomes | EA defined as "unpurposeful movement requiring restraint." If midazolam required, episode was classed as severe Other outcomes: PONV, pain and discomfort scores |
|
| Notes | Correspondence with study author confirmed that 20 participants were analysed in each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind," "the anesthesiologist was blinded to the drug" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "evaluation was performed by a second investigator, who was blinded to the patient's treatment schedule" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 participants excluded from analysis (6 from clonidine group and 3 from placebo group). Reasons for exclusion were increase in heart rate and blood pressure, indicating ineffective regional penile block (5 clonidine group, 3 placebo group); and reported pain in the area that had been operated on (1 from clonidine group) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Lankinen 2006.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years old, ASA I‐II, outpatient adenoidectomy Exclusion criteria: allergy to study medications, asthma, cardiovascular disease Recruitment: 75 children (26 control group, 25 tropisetron group, 24 clonidine group) Location: Finland |
|
| Interventions | Tropisetron 0.1 mg/kg IV Clonidine 1.5 mcg/kg IV Control group: saline placebo All participants: sevoflurane induction and maintenance in 100% oxygen, alfentanil 20 mcg/kg IV, rectal diclofenac 1 mg/kg, study drug given after induction |
|
| Outcomes | EA defined as a score of 3 or higher on the Modified Pain/Discomfort scale Other outcomes: emergence and discharge times, number of participants requiring analgesia, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number assignment" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the study was double‐blind and placebo‐controlled" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all observers, as well as the children and their parents, were unaware of the contents of the study drug" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Lapin 1999.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐6 years, ASA I‐II, myringotomy and tube insertion Exclusion criteria: none stated Recruitment: 104 children randomly assigned, 100 analysed (28 in sevomidaz group, 24 in each of the other 3 groups) Location: USA |
|
| Interventions | Halo group: halothane Halomidaz group: oral midazolam 0.5 mg/kg + halothane Sevo group: sevoflurane Sevomidaz group: oral midazolam 0.5 mg/kg + sevoflurane All participants: induction and maintenance with study inhalational agent with nitrous oxide, rectal paracetamol 15‐30 mg/kg |
|
| Outcomes | EA defined as PACU nurse answering "yes" to the question: "Did this patient become uncontrollable to the point that you had to restrain the patient and you could not perform any other functions?" Other outcomes: recovery and discharge home times |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the recovery room nurses and discharging nurses were blinded to the anesthetic technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants excluded because of problems with premedication |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Le Berre 2001.
| Methods | Randomized controlled trial | |
| Participants | 2 months‐6 years, ASA I‐II, non‐ambulatory elective surgical procedures below umbilicus, orthopaedic surgery of lower limb and surgery requiring completion time of at least 90 minutes Exclusion criteria: none stated Recruitment: 40 children (20 in each group) Location: France |
|
| Interventions | Intervention group: isoflurane maintenance Control group: sevoflurane maintenance All participants: rectal midazolam 0.2 mg/kg 60 minutes before induction, sevoflurane induction with 60% nitrous oxide, caudal block for analgesia |
|
| Outcomes | EA defined as "disoriented behaviour characterised by inconsolable agitation" Other outcomes: recovery and discharge times, postoperative vomiting |
|
| Notes | Correspondence with study author to clarify how many participants in isoflurane group experienced agitation. Study author replied that no participants who received isoflurane showed agitation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a single‐blinded observer, who was not informed about the halogenated agent the patient had received" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported EA incidence |
Lee 2010a.
| Methods | Randomized controlled trial | |
| Participants | 2‐14 years, ASA I‐II, adenotonsillectomy or adenoidectomy Exclusion criteria: cognitive or developmental disorders Recruitment: 93 children randomly assigned, 90 analysed (30 in each of 3 groups) Location: Republic of Korea |
|
| Interventions | Group K0.25: ketamine 0.25 mg/kg IV 10 minutes before end of surgery Group K0.5: ketamine 0.5 mg/kg IV 10 minutes before end of surgery Control group: saline 10 minutes before end of surgery All participants: atropine 0.01 mg/kg IM premedication 30 minutes before, thiopentone 5 mg/kg IV induction, rocuronium 0.6 mg/kg, intubation, sevoflurane 1.5%‐3% with 50% nitrous oxide maintenance, pyridostgimine 0.2 mg/kg glycopyrrolate 0.008 mg/kg reversal, awake extubation |
|
| Outcomes | EA assessed on arrival to PACU, then 5‐minutely on the following 4‐point scale: 1 = asleep 2 = awake but calm 3 = agitated but consolable 4 = severely agitated and difficult to console Highest score during PACU stay recorded. Study authors have not defined EA, but we have used incidence of a score of 4 in the forest plot of this review Other outcomes: extubation time, pain scores (modified CHEOPS), PONV, laryngospasm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly to one of three groups," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the experimental drugs were administered by physicians not participating in the anesthesia" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "emergence agitation was observed by nurses who were blinded to the patients' groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 withdrawals (1 from each group) for laryngospasm after extubation |
| Selective reporting (reporting bias) | Low risk | reported EA scores |
Lee 2010b.
| Methods | Randomized controlled trial | |
| Participants | 3‐8 years, ASA I, adenotonsillectomy without myringotomy Exclusion criteria: mental or neurological disease, use of sedative medication Recruitment: 90 children randomly assigned, 88 analysed (44 in each group) Location: Republic of Korea |
|
| Interventions | Propofol group: propofol 1 mg/kg at end of surgery Control group: saline 0.1 mL/kg at end of surgery All participants: thiopental sodium 1 mg/kg IV before entering theatre, induction with thiopental sodium 5 mg/kg IV and atracurium 0.5 mg/kg, intubation, maintenance with sevoflurane 2%‐2.5% with 50% nitrous oxide, ketorolac 1 mg/kg IV, awake extubation, parental presence in PACU |
|
| Outcomes | EA assessed with 2 scales simultaneously (Aono's 4‐point scale and PAED scale) at 5, 15 and 30 minutes after emergence. EA defined as score of 3 or 4 on the 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = moderately agitated or restless and not easily calmed 4 = combative, excited or disoriented, thrashing around Time point of 5 minutes used in forest plot PAED scale used to assess severity of EA and reported mean (SEM) PAED scores at 3 time points Other outcomes: pain scores (CHIPP scale), Ramsay sedation scores after emergence, emergence time, time to discharge from PACU, incidence of PONV |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization" |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes which were opened on the day of the scheduled operation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 exclusion from propofol group due to intraoperative ST depression on EKG, 1 exclusion from saline group due to bleeding in the surgical region after extubation |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA and PAED scores |
Lee 2011.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I‐II, elective subumbilical surgery (inguinal herniorrhaphy, hydrocoelectomy or orchidopexy) Exclusion criteria: known history of a neurological or psychological disorder, history of previous anaesthesia, presence of sleep apnoea, participants in whom intubation or LMA insertion failed on the first attempt Recruitment: 168 children (56 in each of 3 groups) Location: Republic of Korea |
|
| Interventions | Interventions: ETT removed awake, ETT removed deep, LMA removed deep All participants: no premedication, PPIA, sevoflurane induction 8% in nitrous oxide/oxygen (3/1) mixture, IV access, glycopyrrolate 0.004 mg/kg, insertion of airway device as per randomization, fentanyl 2 mcg/kg IV, ondansetron 0.1 mg/kg, maintenance with sevoflurane in air/oxygen mixture to maintain HR and BP within 20% of preinduction values and BIS of 40‐60, PPEA |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = not easily calmed, moderately agitated or restless 4 = combative, excited or disoriented Other outcomes: behaviour on induction (3‐point scale), duration of anaesthesia (induction to eye opening), length of stay in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, treating anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "emergence agitation was evaluated by one anaesthesiologist, who was unaware of each patient's group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Lerman 1996.
| Methods | Randomized controlled trial | |
| Participants | 1‐12 years, ASA I‐II, elective ambulatory surgery Exclusion criteria: none stated Recruitment: 375 children randomly assigned, 374 analysed (124 halothane, 250 sevoflurane) Location: USA and Canada, multi‐centre (5 centres) phase 3 trial for sevoflurane |
|
| Interventions | Halothane induction and maintenance Control group: sevoflurane induction and maintenance All participants: un‐premedicated, 60% nitrous oxide, caudal block or local anaesthetic wound infiltration, fentanyl given according to haemodynamic/respiratory parameters but not within 30 minutes of the conclusion of surgery |
|
| Outcomes | EA defined as involuntary movement of 1 or more extremities during recovery from anaesthesia Other outcomes: modified Aldrete score, Objective Pain Discomfort score, induction and emergence characteristics, child or caregiver satisfaction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned to receive either sevoflurane or halothane in a 2:1 ratio using a computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "recorded by a nurse, who was blinded to the treatment administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 child from halothane group excluded from analysis because of severe airway obstruction and hypoxia caused by a mucous plug |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Li 2011.
| Methods | Randomized controlled trial | |
| Participants | 3‐11 years, ASA I‐II, adenotonsillectomy Exclusion criteria: history of airway disease, sleep apnoea, developmental delay, psychological disorders, those crying on arrival to the operating theatre Recruitment: 105 children randomly assigned,100 analysed (32 sufentanil, 34 fentanyl, 34 control) Location: China |
|
| Interventions | Sufentanil 0.2 mcg/kg IV Fentanyl 2 mcg/kg IV Control group: saline placebo All participants: sevoflurane 8% induction with 60% nitrous oxide, study drug given 1 minute after loss of the eyelash reflex, intubation, sevoflurane maintenance 1.5%‐2.5%, tramadol 2 mg/kg, dexamethasone 0.1 mg/kg |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale (Aono's scale): 1 = calm 2 = not calm but could be easily consoled 3 = moderately agitated or restless and not easily calmed 4 = combative, excited or disoriented, thrashing around Also measured and reported median (range) PAED scores Other outcomes: time to extubation, time in PACU, pain scores (CHIPPS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated using a sealed envelope system," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "an independent researcher prepared the syringe for each patient and the anesthesiologist who recorded the result was blinded to the patient group" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "an independent researcher prepared the syringe for each patient and the anesthesiologist who recorded the result was blinded to the patient group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals after randomization (3 from sufentanil group—1 postoperative bleeding, 1 systemic epinephrine absorption, 1 breath‐holding; 1 from fentanyl group—postoperative bleeding; 1 from control group—breath‐holding) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA and PAED scores |
Liao 2010.
| Methods | Randomized controlled trial | |
| Participants | 1‐4 years, ASA I‐II, undergoing rigid bronchoscopy for removal of tracheal or bronchial foreign body Exclusion criteria: known allergy to any anaesthetic agent, family history of malignant hyperthermia, coagulopathy, legal guardian's refusal to sign consent Recruitment: 64 children (32 in each group) Location: China |
|
| Interventions | Group TIVA: propofol target controlled‐infusion (TCI) with remifentanil 0.05‐1 mcg/kg/min Group VIMA: sevoflurane induction 8% and maintenance 2.5%‐3.5% All participants: no premedication, BIS 40‐60, lignocaine 4% spray to airway, dexamethasone 0.1 mg/kg |
|
| Outcomes | "Excitement after procedure" was recorded Other outcomes: haemodynamics throughout procedure, induction and emergence times, intubating conditions, breath‐holding, cough, laryngospasm, desaturation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child not blinded to induction technique but blinded to maintenance, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Liao 2011.
| Methods | Randomized controlled trial | |
| Participants | 3‐12 years, ASA I‐II, elective urological outpatient surgery Exclusion criteria: history of premature delivery; reported developmental delay; deafness; significant cardiovascular, respiratory, neurological disease; receiving medication known to affect the central nervous system Recruitment: 160 children (54 in SP group, 52 in BIS group, 54 in AAI group) Location: Taiwan |
|
| Interventions | BIS (bispectral index) group: sevoflurane anaesthesia controlled by BIS 40‐60 AAI (A‐line autoregressive index) group: sevoflurane anaesthesia controlled by AAI 15‐30 SP (standard practice) group: sevoflurane anaesthesia controlled by clinical parameters All participants: no premedication, PPIA, sevoflurane induction with 50% nitrous oxide, LMA insertion, sevoflurane maintenance (concentration control determined by group allocation) with spontaneous ventilation with 50% oxygen in air, fentanyl 1 mcg/kg 5 minutes before incision |
|
| Outcomes | PAED scores, assessed every 5 minutes after awakening for 30 minutes Other outcomes: recovery time (primary outcome), incidence of PONV, parental satisfaction, sevoflurane consumption |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "allocated randomly by computer‐generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded (monitors on all participants), trained investigators (first and second anaesthesiologists) blinded to anaesthetic techniques and grouping of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "third investigator, who was also unaware of the grouping of the patient, was responsible for the assessment of the patient during the emergence" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported PAED scores |
Lili 2012.
| Methods | Randomized controlled trial | |
| Participants | 3‐7 years, ASA I‐II, vitreoretinal surgery Exclusion criteria: asthma, cardiovascular disease, abnormal renal or hepatic function, any known sensitivity to study medication, previous sedative or analgesic medication use, history of past anaesthesia Recruitment: 60 children (30 in each group) Location: China |
|
| Interventions | Dexmedetomidine 0.5 mcg/kg IV Control group: saline placebo All participants: no premedication, sevoflurane 8% induction in oxygen, IV insertion, atropine 0.01 mg/kg IV, propofol 2 mg/kg, remifentanil 0.5 mcg/kg, cistracurium 0.15 mg/kg to facilitate intubation, study drug given over a 10‐minute period after induction of anaesthesia, maintenance of anaesthesia with sevoflurane 1%‐2% and remifentanil 0.2 mcg/kg/min in oxygen with BIS maintained at 40‐60 |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = calm but could be easily calmed 3 = not easily calmed, moderately agitated or restless 4 = combative, excited, or disoriented Other outcomes: pain (CHIPPS scale), extubation time, emergence time, intraoperative blood pressure and heart rate, intraocular pressure, airway events and incidence/severity of coughing after extubation, oxygen desaturation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "allocated randomly (by computer‐generated random numbers)" |
| Allocation concealment (selection bias) | Low risk | "study drugs were prepared by the hospital pharmacy in identical containers marked with consecutive numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "patients and investigators were unaware which was dexmedetomidine or normal saline (double‐blind)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Lin 2009.
| Methods | Randomized controlled trial | |
| Participants | 1‐6 years, ASA I‐II, bilateral myringotomy and tube placement as a first procedure Exclusion criteria: neurological disease, developmental delay, analgesia or sedatives within 36 hours before surgery Recruitment: 60 children (30 in each group) Location: USA |
|
| Interventions | Intervention group: acupuncture at 2 points: LI‐4(he‐gu) and HT‐7(shen‐men) points. Manual stimulation for 10 seconds and needles kept in situ for 10 minutes Control group: no acupuncture All participants: no premedication, sevoflurane induction with 70% nitrous oxide in oxygen followed by addition of sevoflurane |
|
| Outcomes | EA measured on the following 4‐point scale and reported median (range) scores: 1 = asleep/calm 2 = mildly agitated but easily consolable 3 = moderately agitated or restless and inconsolable 4 = hysterical, crying inconsolably or thrashing Other outcomes: pain scores (CHEOPS), number of participants requiring analgesia in PACU, vomiting, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | blinded observer in PACU evaluating pain and agitation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported EA scores |
Lopez Gil 1999.
| Methods | Randomized controlled trial | |
| Participants | 6‐144 months, ASA I‐II, surgery below umbilicus Exclusion criteria: risk of aspiration, requiring high‐pressure ventilation Recruitment: 120 participants (60 in each group) Location: Spain |
|
| Interventions | Propofol induction (3 mg/kg) and maintenance (5 mg/kg/h) Control group: sevoflurane induction and maintenance All participants: premedication with oral midazolam 0.5 mg/kg, IV access before induction, nitrous oxide during induction and maintenance, fentanyl bolus 3 mcg/kg followed by an infusion 1 mcg/kg/h. If surgery to last longer than 30 minutes, atracurium 0.5 mg/kg and pressure‐controlled ventilation. Postoperative analgesia with metimazole 40 mg/kg |
|
| Outcomes | Reported number of participants who were "agitated" on emergence. No definition of EA given Other outcomes: time to LMA insertion/removal, haemodynamics, oxygen saturation, emergence times, number of participants requiring analgesia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | child not blinded to induction technique, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | outcome assessor of EA blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Makharita 2009.
| Methods | Randomized controlled trial | |
| Participants | 3‐8 years, ASA I‐II, bilateral myringotomy and tube placement Exclusion criteria: known allergy to study medicines; bronchial asthma, cardiovascular disease, psychological disorder or cognitive delay or any neurological condition that would limit a parent's ability to communicate with, or understand, nursing personnel; treatment with sedatives or anticonvulsants; extremely distressed and uncooperative children; parental refusal Recruitment: 80 children (40 in each group) EA Location: Egypt |
|
| Interventions | Fentanyl 1 mcg/kg IV 10 minutes before end of anaesthesia Control group: saline placebo All participants: rectal acetaminophen 40 mg/kg 90‐120 minutes before induction of anaesthesia, parental presence at induction, sevoflurane induction and maintenance |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but easily calmed 3 = moderately agitated or restless 4 = excited or disoriented Other outcomes: recovery time, time to hospital discharge, adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number assignment" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind," "the patients randomly received the prepared syringe (either IV saline or fentanyl) in a blinded fashion, the researcher did not know the solution nature" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a research observer who was blinded to the treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Malviya 2006.
| Methods | Randomized controlled trial | |
| Participants | 2‐10 years, healthy children, otological or pulse dye laser procedures Exclusion criteria: cardiovascular disease or neurological impairment, sensitivity to clonidine or ketorolac or requirement for premedication with midazolam Recruitment: 133 children randomly assigned, 120 analysed (59 clonidine group, 61 placebo group) |
|
| Interventions | Clonidine 2 mcg/kg IV Control group: saline placebo All participants: oral acetaminophen preoperatively, sevoflurane with nitrous oxide induction, maintenance with isoflurane, IV placement after induction and then random assignment to receive clonidine or placebo, ketorolac given for analgesia Location: USA |
|
| Outcomes | EA assessed on the following 4‐point scale: 0 = quiet, calm 1 = mildly agitated but consolable 2 = moderately agitated, non‐purposeful and inconsolable 4 = severely agitated PACU nurse was asked to subjectively qualify whether the agitation was indicative of delirium Other outcomes: blood pressure, emergence time, time in PACU; parent telephone interview regarding sleepiness, agitation, aggression, anxiety, fear or nightmares in first 24 hours after surgery |
|
| Notes | Sevoflurane for induction only, maintenance with isoflurane | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "all care providers were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all care providers were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 cases were excluded (unclear from which groups they came)—6 required premedication; 2 had medical exclusions; in 3 cases, IV cannulation was not obtained; 1 procedure was more extensive than planned and 1 was cancelled (Note: 5 participants in the clonidine group and 6 participants in the placebo group refused oral acetaminophen preoperatively but were included in the final analysis) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Meena 2009.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐3 years, ASA I‐II undergoing cleft lip/palate repair Exclusion criteria: other associated congenital anomalies, major systemic illness, previous history of agitation following anaesthesia, hypersensitivity to anaesthetic drugs Recruitment: 60 children (30 in each group) Location: India |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: oral midazolam 0.5 mg/kg premedication 1 hour before the procedure, induction and maintenance with study agent and 60% nitrous oxide, fentanyl 1 mcg/kg IV, rocuronium and reversal |
|
| Outcomes | Reported incidence of postoperative "excitement/restlessness" Other outcomes: haemodynamics, recovery times and scores, number of participants requiring analgesia in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random number code" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "observed in the post‐operative period by an independent observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Meng 2012.
| Methods | Randomized controlled trial | |
| Participants | 5‐14 years, ASA I‐II, tonsillectomy Exclusion criteria: known history of allergy to study drugs, history of previous surgery; participants receiving other analgesics such as non‐steroidal anti‐inflammatory drugs; participants receiving α‐methyldopa, clonidine or a beta‐blocker; psychiatric disorder, neurological or neuromuscular disorders; those who had used drug, alcohol or tobacco 2 weeks before the study began Recruitment: 120 children (40 in each of 3 groups) Location: China |
|
| Interventions | Dexmedetomidine 0.5 mcg/kg IV loading dose, then infusion 0.2 mcg/kg/h Dexmedetomidine 1 mcg/kg IV loading dose, then infusion 0.4 mcg/kg/h Control group: lactated Ringer's placebo All participants: midazolam 40 mcg/kg IV premedication, IV induction with propofol 1.2‐2 mg/kg, sufentanil 0.4 mcg/kg, cisatracurium 0.15 mg/kg, intubation, maintenance with sevoflurane 1.5%‐2.5% and remifentanil infusion, BIS maintained 45‐55, tropisetron 0.1 mg/kg, neostigmine and atropine reversal |
|
| Outcomes | EA defined as a score of 3 or 4 on the following scale: 1 = awake and calm, cooperative 2 = crying, requiring consoling 3 = irritable, restless, screaming, inconsolable 4 = combative, disoriented, thrashing. EA score recorded at 5‐minute intervals for first 30 minutes, then at 10‐minute intervals until 60 minutes. Data in forest plot represent time point 5 minutes after extubation Other outcomes: heart rate, blood pressure, extubation time, time in PACU, pain scores (VAS), sedation scores |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized using a computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind," "all study drugs were prepared by an anesthesiologist, who was blinded to the details of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all postoperative observations and scores were performed by the same anesthesiologist, who was blinded to the group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Meyer 2007.
| Methods | Randomized controlled trial | |
| Participants | 1‐6 years, ASA I‐II, minor surgery below umbilicus Exclusion criteria: neurological disease, psychiatric disease and attention deficit disorder Recruitment: 63 children randomly assigned, 59 analysed (30 sevoflurane group, 29 isoflurane group) Location: USA |
|
| Interventions | Isoflurane maintenance Control group: sevoflurane maintenance All participants: oral midazolam 0.4 mg/kg premedication 30 minutes preoperatively; IV induction with thiopentone, alfentanil and mivacurium; caudal block; rectal paracetamol; awake extubation. If IV access failed after 2 attempts, inhalational induction performed (2 children in isoflurane group and 4 in sevoflurane group) |
|
| Outcomes | EA defined as > 5 on a 10‐point scale and ED as > 7 on a 10‐point scale where 1 = calm/asleep and 10 = worst possible/inconsolable Other outcomes: postoperative anxiety, time in PACU, number of participants requiring analgesia, caregiver and parental satisfaction, PONV |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "according to randomization," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "independent observer blinded for the group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants excluded (not specified which groups)—2 because of insufficient caudal anaesthesia, 1 because of fentanyl given on induction and 1 as the result of additional surgical intervention on the face |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Michalek‐Sauberer 1998.
| Methods | Randomized controlled trial | |
| Participants | 2‐16 years, ASA I‐III, elective surgical procedure > 1 hour duration Exclusion criteria: none stated Recruitment: 42 children (21 in each group) Location: Austria |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: midazolam premedication (1 mg/kg rectal or 0.01 mg/kg IV for older children) 15 minutes before induction, induction and maintenance with study agent with nitrous oxide, vecuronium used to facilitate intubation at the discretion of anaesthetist, analgesia provided in IV increments of fentanyl 2‐3 mcg/kg or by a regional anaesthetic technique |
|
| Outcomes | Reported incidence of mild, moderate and severe excitement on emergence (moderate/severe excitement on emergence used in forest plot in this review) Other outcomes: haemodynamics, emergence times, modified Aldrete scores, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "a sealed envelope was opened to allocate patients randomly," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "patients were observed by an independent, non‐blinded observer throughout the study period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Mikawa 2002.
| Methods | Randomized controlled trial | |
| Participants | 2‐11 years, undergoing minor (urological, ophthalmological, otological and orthopaedic) surgery Exclusion criteria: none stated Recruitment: 175 children (35 in each of 5 groups) Location: Japan |
|
| Interventions | Group CLO‐2: oral clonidine 2 mcg/kg (up to 150 mcg) Group CLO‐4: oral clonidine 4 mcg/kg (up to 150 mcg) Group MID: 0.5 mg/kg (up to 10 mg) Group DIA: 0.4 mg/kg (up to 10 mg) Control group: placebo All participants: premedication with study drug 30‐60 minutes before anaesthesia, sevoflurane with nitrous oxide induction and maintenance, rectal diclofenac 12.5 mg or 25 mg given immediately after induction of anaesthesia |
|
| Outcomes | EA assessed on following 4‐point scale: 0 = asleep 1 = calm 2 = agitated but consolable 3 = severely agitated and inconsolable |
|
| Notes | Attempted to contact study authors regarding randomization, no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The word "randomized" was not reported, but prospective study with equal numbers in each group, ethics approval and informed participant consent, so randomization assumed |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "we blindly recorded agitation score" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals reported |
| Selective reporting (reporting bias) | Low risk | reported agitation scores |
Milic 2010.
| Methods | Randomized controlled trial | |
| Participants | All children scheduled for elective cleft lip and palate surgical repair (3 months‐10 years) Exclusion criteria: contraindications for surgery, infection, anaemia, asthma, severe heart defects, mental retardation Recruitment: 140 children randomly assigned (76 midazolam anaesthesia group, 64 sevoflurane anaesthesia group) Location: Croatia |
|
| Interventions | Midazolam anaesthesia group: IV induction with midazolam 0.05 mg/kg, fentanyl 0.005 mg/kg, vecuronium 0.1 mg/kg, then maintenance with IV boluses of midazolam (0.025 mg/kg every 45 minutes), fentanyl (0.001 mg/kg depending on heart rate) and vecuronium (0.01 mg/kg every 40 minutes, or if increase in ETCO2), reversal with atropine and neostigmine Sevoflurane anaesthesia group: sevoflurane induction 5%‐8% and sevoflurane maintenance 0.8%‐1% in oxygen/air mixture, IV fentanyl 0.005 mg/kg, then additional boluses of 0.001 mg/kg depending on heart rate All participants: oral midazolam premedication, sevoflurane 5%‐8% "preinduction" for IV cannulation, then sevoflurane ceased and randomly assigned to either group, 2% lidocaine infiltration by surgeon |
|
| Outcomes | EA assessed over 2 hours in PACU on following scale: 0 = child sleeps peacefully 1 = agitation Study authors stated: "only cases in which agitation persisted after the administration of analgesics were included in the analysis" (agitation that disappeared after analgesia was considered pain‐related) Other outcomes: intraoperative complications (difficult intubation, ventricular asystole, bronchospasm), PONV, perioperative oxygen saturation, heart rate, haemoglobin and haematocrit |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number list" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "it was not possible to achieve postoperative blinding," "the anesthesiologists carried out the anesthesia and collected the data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Mizrak 2013.
| Methods | Randomized controlled trial | |
| Participants | 5‐15 years, ASA I‐II, adenotonsillectomy Exclusion criteria: hypertension, psychiatric disorders, drug allergy, cardiovascular and clotting disorders, peptic ulcers, those who could not use visual analogue scales, adenoidectomy alone Recruitment: 60 children (30 in each group) Location: Turkey |
|
| Interventions | Intervention group: dexmedetomidine IV 0.5 mcg/kg over 10 minutes after induction of anaesthesia Control group: saline placebo All participants: midazolam 0.05 mg/kg IM premedication 45 minutes before induction, sevoflurane induction and maintenance with 50% nitrous oxide, rocuronium 0.5 mg/kg, intubation |
|
| Outcomes | EA assessed on the following 5‐point scale with median (standard error of the mean) scores reported at the 15th minute postoperatively: 1 = sleeping 2 = awake and calm 3 = irritable and crying 4 = inconsolable crying 5 = severe restlessness and disorientation, purposelessly wanting to get out of bed, wanting to stand on the bed, shouting, crying or mumbling aloud Other outcomes: haemodynamics, intraoperative blood loss, operation time, recovery time, pain scores (VAS), analgesic requirement, sedation scores, Hb, INR, APTT |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind," "syringes prepared by another anesthetist" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported agitation scores |
Moore 2003.
| Methods | Randomized controlled trial | |
| Participants | 3‐12 years, day case general or ENT surgery Exclusion criteria: history of allergic or other serious adverse experiences with anaesthesia, severe cardiovascular/metabolic or CNS disease, anticipated airway difficulties, if regimen was expected to include succinylcholine Recruitment: 347 children randomly assigned, 322 analysed (163 control group, 159 intervention group) Location: United Kingdom |
|
| Interventions | Intervention group: halothane maintenance (after propofol induction) Control group: sevoflurane maintenance (after sevoflurane induction) All participants: no premedication, nitrous oxide, rectal diclofenac 12.5‐25 mg, paracetamol if unsuitable for diclofenac, IV opioids/local infiltration/regional block depending on procedure |
|
| Outcomes | Reported incidence of "recovery mental state" according to the following 3 categories:
Other outcomes: PONV, adverse events during induction and recovery, recovery and discharge times |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number sequence" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded to maintenance agent, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "recovery room nurse was asked to judge the patients mental state in recovery," not stated whether they were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 withdrawals: 15 for protocol violation, 5 for cancellation of operation, 5 for withdrawal of consent |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Murray 2002.
| Methods | Quasi‐randomized controlled trial | |
| Participants | < 7 years old, myringotomy tube placement Exclusion criteria: none stated Recruitment: 130 children (33 in halothane/oxycodone and halothane groups, 32 in sevoflurane/oxycodone and sevoflurane groups) Location: USA |
|
| Interventions | Halothane/oxycodone group: halothane anaesthesia with oxycodone 0.1 mg/kg preinduction Halothane group: halothane anaesthesia with no oxycodone Sevoflurane/oxycodone group: sevoflurane anaesthesia with oxycodone 0.1 mg/kg preinduction Sevoflurane group: sevoflurane anaesthesia with no oxycodone |
|
| Outcomes | EA defined as score of 4 or 5 on following 5‐point scale: 1 = sleeping 2 = awake/calm 3 = irritable 4 = inconsolable 5 = agitated, combative, restless Other outcomes: number of children with EA for > 10 minutes, PONV, discharge times |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "assigned to receive either no premedication or oxycodone," "then alternately assigned to either a sevoflurane or halothane inhalation anaesthetic" |
| Allocation concealment (selection bias) | High risk | "alternately assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "nurse scoring the patient's behaviour was blinded to the preoperative medication and the inhalation anaesthetic" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Na 2013.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, ASA I‐II, adenotonsillectomy Exclusion criteria: sleep apnoea, developmental delay, psychological disorder, any neurological disorder Recruitment: 84 children (42 in each group) Location: Republic of Korea |
|
| Interventions | Intervention group: maintenance with sevoflurane 1% and remifentanil (1 mcg/kg bolus at induction, then infusion 0.5 mcg/kg/min until intubated, then 0.25 mcg/kg/min) Control group: maintenance with sevoflurane 2%‐3% without remifentanil All participants: no premedication, IV induction with thiopental 5 mg/kg and rocuronium 0.6 mg/kg, ketorolac 1 mg/kg, dexamethasone 0.15 mg/kg, nitrous oxide 60% during maintenance, peritonsillar 2% lignocaine injection |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but easily calmed 3 = moderately agitated or restless 4 = combative, excited, disoriented Also assessed EA with PAED scale and reported median (IQR) PAED scores and incidence of PAED scores ≥ 10 (also used as a second definition of EA by authors) Other outcomes: duration of EA, time to emergence, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated, randomised group allocation" |
| Allocation concealment (selection bias) | Low risk | "sealed‐envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "in the PACU, one anaesthesiologist blinded to the patient group evaluated EA" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Naito 1991.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years, pulse dye laser for port wine stain Exclusion criteria: none stated Recruitment: 30 children (15 in each group) Location: Japan |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: induction and maintenance with study agent and nitrous oxide |
|
| Outcomes | Incidence of postoperative restlessness and agitation was reported. No further definition given Other outcomes: emergence and recovery times, vomiting, complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated randomly," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "assessed by the nurse, who was not informed which anaesthetic had been used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Nakayama 2007.
| Methods | Randomized controlled trial | |
| Participants | 2‐11 years, ASA I‐II, otorhinolaryngological procedures Exclusion criteria: known allergy to study drugs, sleep apnoea, developmental delay, psychological disorders Recruitment: 186 children randomly assigned, 176 analysed (89 sevoflurane, 87 propofol) Location: Japan |
|
| Interventions | Control group: sevoflurane induction and maintenance Intervention group: propofol induction and maintenance |
|
| Outcomes | EA defined as a score of 3 or 4 on following 4‐point scale: 1 = calm, 2 = not calm but could easily be consoled 3 = not easily calmed, moderately agitated or restless 4 = combative, excited or disoriented Other outcomes: emergence time, time in PACU, haemodynamics, PONV, laryngospasm, preschool versus school‐aged incidence of EA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "during the recovery period, an independent anesthesiologist, who was blinded to the anaesthetic used, recorded all the observations and measurements" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 children excluded because of insufficient analgesia (6 propofol, 4 sevoflurane) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Oh 2005.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years, ASA I‐II, elective urological surgery Exclusion criteria: history of sleep apnoea, developmental delay, psychological disorder, any neurological disorder Recruitment: 85 children (43 in group G, 42 in group I) Location: Republic of Korea |
|
| Interventions | Group G: gradual cessation of sevoflurane at end of surgery (0.1% per minute) Group I (control): immediate cessation of sevoflurane at end of surgery All participants: no premedication; IV induction with thiopental, atropine, rocuronium; sevoflurane maintenance 2%‐2.5% with 50% nitrous oxide, pyridostigmine and glycopyrrolate reversal |
|
| Outcomes | EA defined as grade 3 or 4 on following 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = moderately agitated or restless 4 = combative, excited or disoriented Other outcomes: time to BIS 70/80/90, time to first movement, time to extubation, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "an observer blinded to the patient group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ou 2011.
| Methods | Randomized controlled trial | |
| Participants | 2‐11 years, ASA I‐II, strabismus surgery Exclusion criteria: none stated Recruitment: 60 children (30 in each group) Location: China |
|
| Interventions | Intervention group (TIVA): IV induction ketamine 1.5 mg/kg, midazolam 0.1 mg/kg, propofol 1 mg/kg, vecuronium 0.1 mg/kg, remifentanil 2 mcg/kg, intubation, maintenance with propofol 3‐6 mg/kg/h with remifentanil 0.1‐0.125 mcg/kg/min Control group: sevoflurane induction, midazolam 0.1 mg/kg IV, vecuronium 0.1 mg/kg IV, intubation, sevoflurane maintenance 2%‐3% |
|
| Outcomes | Reported incidence of EA but no definition for EA Other outcomes: time to extubation, time to emergence, incidence of oculocardiac reflex, PONV |
|
| Notes | English abstract, full paper in Chinese No ketamine in control (sevoflurane) group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ozcengiz 2011.
| Methods | Randomized controlled trial | |
| Participants | 3‐9 years, ASA I‐II, oesophageal dilatation procedures Exclusion criteria: lack of consent; known adverse reactions to dexmedetomidine, midazolam or melatonin; mental retardation, developmental delay, neurological or psychiatric illness that may be associated with agitation Recruitment: 100 children (25 in each group) Location: Turkey |
|
| Interventions | Group MD: midazolam 0.5 mg/kg oral premedication Group D: dexmedetomidine 2.5 mcg/kg oral premedication Group ML: melatonin 0.1 mg/kg oral premedication Group P: placebo oral premedication All participants: premedication (according to randomization) given with paracetamol to be easily drinkable 40‐45 minutes before induction, sevoflurane 8% induction and 2%‐4% maintenance with 50% nitrous oxide, IV vecuronium for intubation, no supplemental analgesic, atropine and neostigmine reversal |
|
| Outcomes | EA defined as score of 3 or 4 on the following scale: 1 = awake and calm, cooperative 2 = crying, requiring consoling 3 = irritable/restless, screaming, inconsolable 4 = combative, disoriented, thrashing EA scale assessed on arrival to PACU and at 5, 10, 15, 30 and 60 minutes after arrival to PACU Other outcomes: heart rate, blood pressure, oxygen saturation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization was performed using a table random method" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "for the purpose of creating double‐blind conditions, neither the researcher who attended clinical applications and observations nor the parents were informed as to which drug was administered" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all patients were evaluated by the same researcher who was unaware which drug was administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ozer Kocak 2001.
| Methods | Randomized controlled trial | |
| Participants | Children aged 6.3 ± 1.6 years, ASA I‐II, adenoidectomy with bilateral myringotomy and insertion of tubes and/or tonsillectomy Exclusion criteria: none stated Recruitment: 30 children (15 in each group) Location: Turkey |
|
| Interventions | Group I: TIVA propofol (3 mg/kg/h) and remifentanil (0.5 mcg/kg/min) with 50% oxygen in air/oxygen mix Group II: sevoflurane 2.5%‐3% maintenance with 50% nitrous oxide in oxygen All participants: IV cannula inserted awake after EMLA cream, IV midazolam premedication 0.03‐0.05 mg/kg, IV induction with remifentanil 1 mcg/kg and propofol 2 mg/kg, cisatracurium, intubation, rectal paracetamol, neostigmine and atropine reversal |
|
| Outcomes | EA assessed on the following 3‐point scale: 1 = asleep or calm 2 = mildly agitated, crying but consolable 3 = hysterical, crying inconsolably |
|
| Notes | Inconsistencies in data between abstract and results section of full paper. Emailed study author to clarify with no response. In the forest plot of this review, used incidence of EA reported in the abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "nurse, blinded to the anaesthetic technique, assessed the quality of emergence and postoperative agitation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Ozturk 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐6 years, elective fibreoptic bronchoscopy Exclusion criteria: haemoptysis, previous fibreoptic bronchoscopy Recruitment: 50 children (25 in each group) Location: Turkey |
|
| Interventions | Remifentanil 1 mcg/kg bolus over 2 minutes, then infusion 0.15 mcg/kg/min Control group: no remifentanil All participants: premedication with oral midazolam 0.5 mg/kg and nebulized 4% lignocaine 4 mg/kg, sevoflurane induction and maintenance in 100% oxygen, IV access after induction, then given IV atropine 10 mcg/kg |
|
| Outcomes | EA defined as a score ≥ 4 on the following scale: 1 = sleeping 2 = awake, calm and cooperative 3 = crying, requiring consoling 4 = irritable/restless, screaming, inconsolable 5 = combative, disoriented, thrashing Measured at emergence, 5, 10, 15 minutes Other outcomes: thoracic wall rigidity during bolus, coughing, emergence and recovery times |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized by sealed envelope," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "persons scoring the children for agitation and cough were unaware of group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Patel 2010.
| Methods | Randomized controlled trial | |
| Participants | 2‐10 years, ASA II‐III, elective tonsillectomy and adenoidectomy Exclusion criteria: known allergy to alpha‐2 agonists, developmental delay, cardiac and craniofacial abnormalities, anxiety disorder, chronic disabilities or pain syndrome and use of psychotherapeutic medications, beta‐blockers, digoxin, cimetidine, alpha‐2 agonists, anticonvulsants or psychotropic medications Recruitment: 137 children randomly assigned, 122 analysed (61 in each group) Location: USA |
|
| Interventions | Intervention group: dexmedetomidine IV 2 mcg/kg over 10 minutes (as soon as IV access obtained) followed by 0.7 mcg/kg/h until 5 minutes before end of surgery Control group: fentanyl IV 1 mcg/kg as soon as IV access obtained All participants: no premedication, sevoflurane 8% induction with 60% nitrous oxide in oxygen, IV access, rocuronium 0.6 mg/kg to facilitate intubation, maintenance with 1 MAC sevoflurane and 60% nitrous oxide as long as BIS remained below 60 (if BIS reached 60 or higher, sevoflurane increased to reduce BIS to below 60), IV dexamethasone 0.5 mg/kg (maximum 10 mg), rectal acetaminophen 30‐40 mg/kg (maximum 1000 mg) before start of surgery, fentanyl 0.5‐1 mcg/kg for increase in heart rate or systolic blood pressure to above 30% baseline, glycopyrrolate and neostigmine reversal |
|
| Outcomes | EA evaluated on 2 scales (5‐point scale described by Cole, PAED scale) at the same time intervals (arrival in PACU, every 5 minutes for first 15 minutes, then every 15 minutes for next 2 hours). "Severe EA" was defined as a score of 4 or 5 on the 5‐point scale. Incidence of "severe EA" on arrival to PACU used in the forest plot of this review. Also reported percentage of participants with PAED score 10 or above Other outcomes: intraoperative heart rate and blood pressure, time to emergence, time to extubation, Objective Pain Scale (OPS) score, morphine rescue in PACU (incidence, dosage), desaturation episodes below SpO2 95% |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number table was used to assign subjects" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "anesthesiologists and data collectors in operating room (OR) were not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "observers in the postanesthesia care unit (PACU) were blinded to treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 withdrawals for the following reasons: surgery was cancelled for 2 participants, 1 refused to participate after enrolling, 1 had an intraoperative complication, 11 participants who completed the study had deviation from study protocol or incomplete data |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Picard 2000.
| Methods | Randomized controlled trial | |
| Participants | 3‐10 years old, ASA I‐II, elective tonsillectomy Exclusion criteria: children screaming before arrival to operating room Recruitment: 50 children randomly assigned, 46 analysed (24 control group, 22 propofol group) Location: Switzerland |
|
| Interventions | Propofol induction and maintenance Control group: sevoflurane induction and maintenance All participants: nitrous oxide 60% during maintenance, alfentanil 20 mcg/kg and atracurium 0.5 mg/kg to facilitate intubation, rectal paracetamol 20 mg/kg and ibuprofen 10 mg/kg, local infiltration with bupivacaine 2 mg/kg |
|
| Outcomes | EA defined as score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm 3 = moderate agitation, restless 4 = combative, excited, disoriented Other outcomes: emergence time, time in PACU, PONV, laryngospasm, bronchospasm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a random numbers table was used to assign children" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child not blinded to induction technique but blinded to maintenance, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "all observations and measurements were recorded by an independent anaesthesiologist, who was blinded to the anaesthetic given" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 withdrawals: 2 excluded because of postoperative bleeding and 2 because of missing data |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Pieters 2010.
| Methods | Randomized controlled trial | |
| Participants | 3‐7 years, ASA I‐II, elective tonsillectomy and adenoidectomy Exclusion criteria: presence of a genetic syndrome, allergy to study medications, behavioural disorders, use of psychiatric medications, non‐English speaking families, participants requiring sedative medication before going to the operating room Recruitment: 42 children randomly assigned, 38 analysed (19 in each group) Location: USA |
|
| Interventions | Intervention group: propofol maintenance 150‐300 mcg/kg/h Control group: sevoflurane maintenance 1.5%‐4% All participants: sevoflurane induction with nitrous oxide, fentanyl 2 mcg/kg IV, then intubation, mechanical ventilation with air/oxygen mix, maintenance agent titrated to clinical effect, ondansetron 0.1 mg/kg, dexamethasone 0.2 mg/kg, awake extubation |
|
| Outcomes | PAED scores assessed 1 minute after awake extubation, then every 10 minutes, for a total of 30 minutes. Emergence delirium defined as PAED score ≥ 16. Dichotomous data in forest plot are for PAED score ≥ 16 at any time in PACU. Also reported median (range) and maximum PAED scores Other outcomes: pain scores (CHEOPS), extubation time, time in PACU, fentanyl dose in PACU, Aldrete score post extubation, nursing and parental satisfaction, PONV, hospital length of stay, anaesthetic complications, subsequent emergency room admissions |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated numbers in blocks of four" |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered opaque envelopes opened immediately prior to entering OR" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "postoperatively agitation and pain were measured by blinded study personnel" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 excluded from sevoflurane group after recruitment because of administration of preoperative midazolam; 2 excluded from propofol group—1 because of administration of diphenhydramine in the PACU after a cutaneous reaction to an antibiotic, and another because of loss of the IV cannula in the PACU |
| Selective reporting (reporting bias) | Low risk | reported incidence of emergence delirium |
Rampersad 2010.
| Methods | Randomized controlled trial | |
| Participants | 1‐5 years, ASA I‐II, bilateral myringotomy procedures Exclusion criteria: any procedure in addition to the bilateral myringotomy procedure, chronic medical issues (ASA III or higher), developmental delay making agitation and pain scoring harder, any allergy or contraindication to study medications, receiving opioid medications or NSAIDs on a long‐term basis Recruitment: 229 randomly assigned, 228 analysed (75 group AF, 76 group AK, 77 group A) Location: USA |
|
| Interventions | Group AF: intranasal fentanyl 1 mcg/kg after induction but before start of surgery Group AK: intramuscular ketorolac 1 mg/kg after induction but before start of surgery Group A (control): no fentanyl or ketorolac All participants: oral midazolam 0.5 mg/kg premedication, sevoflurane and nitrous oxide for induction and maintenance by mask, rectal acetaminophen 40 mg/kg after induction but before start of surgery |
|
| Outcomes | EA defined as score ≥ 3 on the following 4‐point scale: 1 = calm 2 = crying but can be consoled 3 = crying, cannot be consoled 4 = agitated and thrashing around Study authors state that no statistically significant differences were noted between the 3 groups for the incidence of EA at any time point during recovery, but they do not report the incidence of EA in each of the 3 groups (therefore no forest plot data for this study) Other outcomes: pain scores (CHEOPS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "standard randomization was carried out by the pharmacy," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sequentially numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "observer remained blinded as to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant withdrawn from study before the start of surgery, as treating surgeon determined that an additional procedure was required |
| Selective reporting (reporting bias) | Low risk | reported EA scores |
Rieger 1996.
| Methods | Randomized controlled trial | |
| Participants | 2‐10 years, ASA I‐II, adenoidotomy, otomicroscopy and myringotomy Exclusion criteria: fever > 38 or purulent nasal secretions (all children had a mild upper respiratory infection) Recruitment: 41 children (19 halothane group, 22 sevoflurane group) Location: Germany |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: rectal premedication with midazolam 0.5 mg/kg and atropine 0.02 mg/kg, inhalational induction and maintenance with study agent, endotracheal intubation following suxamethonium 1.5 mg/kg |
|
| Outcomes | Reported incidence of "excitation" on emergence Other outcomes measured: pain scores, time in PACU, modified Aldrete score, complications and side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned, by a computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "recovery was characterized and evaluated by an independent observer," not stated whether observers were blinded to anaesthetic agent |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Saadawy 2009.
| Methods | Randomized controlled trial | |
| Participants | 1‐6 years, male, ASA I, having unilateral hernia/orchidopexy Exclusion criteria: contraindication to caudal, such as bleeding diathesis, preexisting neurological/spinal surgery or abnormality of sacrum, local anaesthetic allergy or conduction problems Recruitment: 60 children (30 in each group) Locations: Egypt and Saudi Arabia |
|
| Interventions | Intervention group: caudal block with bupivacaine + dexmedetomidine 1 mcg/kg Control group: caudal block with bupivacaine All participants: propofol 3‐4 mg/kg induction, LMA insertion, sevoflurane with nitrous oxide maintenance |
|
| Outcomes | EA defined as score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but easily calmed 3 = not easily calmed, moderately agitated or restless 4 = combative, excited or disoriented Other outcomes: haemodynamics, sedation scores, postoperative analgesia requirement, side effects such as vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "the study drugs were randomly prepared by an anesthetist, who was not involved in the study, in an unlabelled syringe, and handed to the anesthesiologist, who was blind to the identity of the drug" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "observer‐blinded study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Salik 2011.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐13 years, ASA I‐III, neurointerventional radiology procedures Exclusion criteria: none stated Recruitment: 28 participants (14 in each group) Location: USA |
|
| Interventions | Dexmedetomidine 1 mg/kg loading dose over 10 minutes, followed by infusion 0.4‐0.7 mg/kg/h beginning 1 hour before end of procedure and continuing for 1 hour of recovery in the PACU/PICU Control group: normal saline placebo All participants: sevoflurane and nitrous oxide induction, maintenance with sevoflurane in oxygen/air mix with remifentanil IV infusion, IV hydromorphone before extubation |
|
| Outcomes | EA assessed using 5‐point scale (Cole) and PAED scale. Do not report incidence of EA or agitation/PAED scores, so no data suitable for forest plots. "Statistical significance was seen at the P < 0.05 level for the Cole scale between 10 and 30 minutes, the period of greatest EA" Other outcomes: heart rate, blood pressure, excessive sedation |
|
| Notes | Abstract only (conference publication) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | do not report actual incidence of EA nor agitation scores |
Sato 2010.
| Methods | Randomized controlled trial | |
| Participants | 1‐9 years, ASA I‐II, same‐day or overnight stay surgery Exclusion criteria: mental retardation, neurological or heart disease, uncontrollable asthma, any type of acute illness Recruitment: 81 children (39 intervention group, 42 control group) Location: Japan |
|
| Interventions | Intervention group: IV dexmedetomidine 0.3 mcg/kg after induction in 5 mL saline over 10 minutes Control group: saline placebo All participants: no premedication, parental presence during sevoflurane 8% induction in oxygen, IV access, LMA insertion, sevoflurane 2%‐5% maintenance in air/oxygen mix to provide stable heart rate, blood pressure and spontaneous ventilation, rectal acetaminophen 40 mg/kg or diclofenac 1 mg/kg, ropivacaine infiltration (except for laser irradiation or myringotomy with tube insertion), deep LMA removal, parental presence in PACU |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale: 1 = calm 2 = not calm but could be easily calmed 3 = not easily calmed, moderately agitated, or restless 4 = combative, excited, or disoriented Other outcomes: heart rate, blood pressure, pain (CHIPPS), parental satisfaction, adverse events, modified Aldrete score, time to PACU discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned...using a randomization list" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "attending anesthesiologists, surgeons and nurses were blinded to the treatment assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "observer blinded to the patient assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Seo 2011.
| Methods | Randomized controlled trial | |
| Participants | 4‐10 years, ASA I‐II, extraocular muscle surgery for strabismus Exclusion criteria: intense preanaesthetic anxiety or crying Recruitment: 260 randomly assigned, 250 analysed (60 group SS, 60 group SL, 65 group BS, 65 group BL), 10 withdrawals described in risk of bias table Location: Republic of Korea |
|
| Interventions | Group SS: sevoflurane, subtenon saline injection Group SL: sevoflurane, subtenon lignocaine injection Group BS: propofol/remifentanil, subtenon saline injection Group BL: propofol/remifentanil, subtenon lignocaine injection All participants: no oral premedication, IV thiopental sodium 2‐3 mg/kg sedation in the preanaesthetic waiting room just before parental separation, IV induction with propofol 2‐2.5 mg/kg, rocuronium 0.8 mg/kg, intubation, adjustment of sevoflurane or propofol/remifentanil to maintain heart rate and blood pressure within 20% baseline during maintenance, nitrous oxide during maintenance |
|
| Outcomes | EA defined as score of 4 or higher on the following 5‐point scale (Cole): 1 = asleep 2 = awake and calm 3 = irritable or consolable crying 4 = inconsolable crying 5 = severe restlessness EA assessed on arrival to PACU and at 10‐minute intervals for first 30 minutes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐derived randomization list" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "two well trained [observers], who were blinded to which group the patient was assigned" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 withdrawals because of incomplete data collection (5 from group SS, 5 from group SL) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Shen 2012.
| Methods | Randomized controlled trial | |
| Participants | 2‐5 years, ASA I‐II, elective electronic cochlear implantation Exclusion criteria: current upper respiratory tract infection, asthma, mental disease, congenital disease Recruitment: 50 children (25 in each group) Location: China |
|
| Interventions | Remifentanil 0.02‐0.05 mcg/kg/min (titrated to maintain adequate spontaneous ventilation) Control group: saline All participants: no premedication, sevoflurane 8% induction in oxygen, fentanyl 2 mcg/kg with propofol 1 mg/kg to facilitate intubation, ondansetron 0.15 mg/kg, dexamethasone 0.2 mg/kg, sevoflurane adjusted to MAC of 1.3 for maintenance of anaesthesia, extubation at sevoflurane MAC 1.3 in control group whereas sevoflurane reduced to 1.0 MAC 10 minutes before extubation in remifentanil group |
|
| Outcomes | EA defined as PAED score > 10 Other outcomes: respiratory rate, end‐tidal CO2, incidence of use of oral airway after deep extubation, time to emergence, time in PACU, PONV, coughing |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "anesthesia assistant blinded to the group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Shibata 2005.
| Methods | Randomized controlled trial | |
| Participants | Children, ASA I, minor otolaryngological surgery, mean (SD) ages: 6 (2) and 7 (2) years in 2 groups Exclusion criteria: none stated Recruitment: 20 children (10 in each group) Location: Japan |
|
| Interventions | Nitrous oxide washout group: sevoflurane discontinued at completion of surgery but nitrous oxide continued until BIS reached 80 Control group: sevoflurane and nitrous oxide discontinued at completion of surgery All participants: no premedication, sevoflurane and nitrous oxide induction and maintenance, local anaesthesia on the surgical field |
|
| Outcomes | EA measured on a 3‐point scale: 1 = asleep or calm 2 = mildly agitated, crying but consolable, restless 3 = hysterical, crying inconsolably, thrashing |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded nurse observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported EA scores |
Shin 2001.
| Methods | Randomized controlled trial | |
| Participants | 4‐7 years, tonsillectomy Exclusion criteria: none stated Recruitment: 29 children (16 ibuprofen group, 13 placebo group) Location: Republic of Korea |
|
| Interventions | Intervention group: oral ibuprofen 5 mg/kg 60 minutes preoperatively Control group: oral placebo All participants: oral chloral hydrate 50 mg/kg premedication 60 minutes preoperatively, sevoflurane with nitrous oxide induction and maintenance, vecuronium 0.1 mg/kg, fentanyl 1 mcg/kg after intubation |
|
| Outcomes | Incidence of EA reported, with EA defined as "frenzy, tumultuous, very worried or upset and showing this in their behaviour, movement or voice" (Korean translation) Other outcomes: pain scores (VAS 1‐5), Aldrete scores, vomiting, postoperative bleeding |
|
| Notes | English abstract, full paper in Korean | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "assigned into two groups," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Shukry 2005.
| Methods | Randomized controlled trial | |
| Participants | 1‐10 years old, ASA I‐II, elective outpatient surgical procedure Exclusion criteria: known adverse reaction to dexmedetomidine, mental retardation, developmental delay, neurological/psychiatric illness Recruitment: 50 children randomly assigned, 46 analysed (23 in each group) |
|
| Interventions | Dexmedetomidine infusion 0.2 mcg/kg/h (commenced 5 minutes after securing airway and ceased 15 minutes after arrival to PACU) Control group: saline placebo infusion All participants: sevoflurane induction and maintenance in oxygen to achieve BIS of 40‐60, intraoperative fentanyl at blinded anaesthetist's discretion, parental presence on emergence Location: USA |
|
| Outcomes | ED defined as a score of 3 or 4 on the following 4‐point scale for > 3 minutes: 0 = asleep 1 = calm 2 = crying, consolable 3 = crying, unconsolable 4 = agitated, thrashing Other outcomes: haemodynamics, time to extubation, time in PACU, pain scores |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized by a computer‐generated program" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "blinded anaesthesia team" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 withdrawals: 1 due to breach of protocol, 1 due to IV dislodgement, 1 due to history of severe ED, 1 due to history of seizures |
| Selective reporting (reporting bias) | Low risk | reported incidence of ED |
Singh 2009.
| Methods | Randomized controlled trial | |
| Participants | 1‐12 years, ASA I‐II, elective surgery for spinal dysraphism Exclusion criteria: previous spinal surgery; cardiac, respiratory, renal, hepatic disease; hydrocephalus, Chiari malformation, history of seizures Recruitment: 80 children (40 in each group) Location: India |
|
| Interventions | Isoflurane maintenance Control group: sevoflurane maintenance All participants: sevoflurane with nitrous oxide and maintenance, rocuronium 1 mg/kg, fentanyl 2 mcg/kg IV with further 1 mcg/kg repeated every hour and last dose about 15 minutes before end of surgery |
|
| Outcomes | EA was evaluated by using 3 points of the Objective Pain Scale. "If the child was crying inconsolably, thrashing, and was hysterical, he or she was reported to be 'agitated'" Ohter outcomes: haemodynamics, emergence times, modified Aldrete scores, nausea/vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized according to computer‐generated sequence of numbers" |
| Allocation concealment (selection bias) | Low risk | "sealed envelope technique" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Singh 2012.
| Methods | Randomized controlled trial | |
| Participants | 4 months‐7 years, ASA I‐II, elective subumbilical surgery Exclusion criteria: active airway disease, sleep apnoea, developmental delay, psychological disorder, neurological disorder, cardiovascular abnormality or requirement of postoperative ventilation Recruitment: 75 children (25 in each of 3 groups) Location: India |
|
| Interventions | Group I: isoflurane maintenance Group D: desflurane maintenance Group S (control group): sevoflurane maintenance All participants: no premedication, sevoflurane 8% induction in 100% oxygen, IV access, fentanyl 2 mcg/kg IV, atracurium 500 mcg/kg IV, dexamethasone 0.5 mg/kg IV (maximum 8 mg), maintenance with study drug (1.0‐1.2 MAC) with nitrous oxide, caudal block 0.2% bupivacaine and rectal paracetamol 30 mg/kg before surgical incision. If heart rate/blood pressure rose by 20%, then caudal block considered ineffective and excluded from study |
|
| Outcomes | EA defined as PAED score ≥ 16 Other outcomes: time to extubation, time to emergence, time in PACU, time to readiness for discharge home, pain scores (FLACC), adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Sun 2009.
| Methods | Randomized controlled trial | |
| Participants | 4‐7 years, ASA I‐II, adenotonsillectomy Exclusion criteria: sensitive to aspirin Recruitment: 45 children (15 in each of 3 groups) Location: China |
|
| Interventions | Intervention groups: Group 1: diclofenac 12.5 mg rectal immediately after intubation Group 2: diclofenac 12.5 mg rectal at end of surgery Control group: no diclofenac All participants: oral midazolam 0.5 mg/kg premedication, IV induction with midazolam 0.1 mg/kg, atropine, ketamine 2 mg/kg, remifentanil 0.5 mcg/kg, vecuronium 100 mcg/kg, intubation, sevoflurane maintenance (1 MAC) with nitrous oxide 50% |
|
| Outcomes | EA assessed with PAED scale at 10, 20, 30 minutes after arrival to PACU and report median (range) PAED scores at each time point. Do not report incidence of EA Other outcomes: extubation time, time in PACU, pain scores, modified Aldrete scores |
|
| Notes | English abstract, full paper in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, not stated whether anaesthetist blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported PAED scores |
Sun 2010.
| Methods | Randomized controlled trial | |
| Participants | Children, ASA I‐II, adenotonsillectomy, mean (SD) ages: 5.8 (1.5), 6.1 (1.5), 6.5 (1.6) years in 3 groups Exclusion criteria: none stated Recruitment: 90 children (30 in each of 3 groups) Location: China |
|
| Interventions | Intervention groups: Tramadol group: tramadol 1 mg/kg IV 30 minutes before end of operation Tramadol + propofol group: tramadol 1 mg/kg IV 30 minutes before end of operation and propofol 1 mg/kg at end of operation Control group: saline 0.1 mL/kg IV 30 minutes before end of operation All participants: oral midazolam premedication 0.5 mg/kg (maximum 15 mg) 20‐30 minutes preoperatively, sevoflurane 7% induction with 60% nitrous oxide, IV access, vecuronium 0.1 mg/kg, intubation, sevoflurane 1.5%‐2% maintenance with nitrous oxide 50% |
|
| Outcomes | EA assessed with PAED scale every 10 minutes after arrival to PACU and report median (range) PAED scores. Do not report incidence of EA Other outcomes: time to extubation, time in PACU, modified Aldrete scores, pain scores (CHIPPS), PONV |
|
| Notes | English abstract, full paper in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not stated whether outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported PAED scores |
Sury 1996.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐6 years, ASA I‐II, day case general surgical, urological or orthopaedic Exclusion criteria: history of epilepsy Recruitment: 40 children (20 in each group) Location: United Kingdom |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: no sedative or anxiolytic premedication, induction and maintenance with study agent and nitrous oxide, analgesia appropriate to surgery soon after induction (local anaesthetic block and/or diclofenac suppository) |
|
| Outcomes | Incidence of "excitement" on emergence reported Other outcomes: emergence and recovery times, complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "the observer had been present during induction and was therefore not blinded to the choice of vapour" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Tang 2009.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I‐II, cleft lip and/or palate surgery Exclusion criteria: weight deviating by more than 20% from average, obstructive sleep apnoea, psychological disorders, any congenital problem, difficult airway, developmental delay Recruitment: 54 children (26 intervention group, 28 control group) Location: China |
|
| Interventions | Intervention group: "multimodal analgesia" with fentanyl 0.5 mcg/kg 10 minutes before end of surgery and rectal paracetamol (2‐4 years given 120 mg, 5‐7 years given 325 mg) just after intubation Control group: no fentanyl 10 minutes before end and no paracetamol All participants: no premedication, sevoflurane 5% induction with 50% nitrous oxide, IV access, fentanyl 2 mcg/kg IV, atracurium 0.6 mg/kg, intubation, local anaesthesia (bupivacaine + lidocaine) to surgical site by surgeon at beginning of surgery, sevoflurane 2%‐2.5% maintenance with 50% nitrous oxide, ondansetron 0.1 mg/kg |
|
| Outcomes | EA defined as a score of 4 or 5 on the following scale: 1 = slow, not responding to stimulus 2 = drowsy but responds 3 = awake, calm, cooperative 4 = crying, difficult to console 5 = agitated, needing restraint Other outcomes: sedation, Objective Pain Scale, time to readiness for PACU discharge, adverse effects |
|
| Notes | English abstract, full paper in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "an observer who was blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Tazeroualti 2007.
| Methods | Randomiszed controlled trial | |
| Participants | 1‐6 years, ASA I‐II, circumcision Exclusion criteria: family history of malignant hyperthermia, mental retardation, any neurological disease with potential for agitation Recruitment: 68 children randomly assigned, 60 analysed (20 midazolam group, 21 clonidine 2 mcg/kg, 19 clonidine 4 mcg/kg) Location: Belgium |
|
| Interventions | Clonidine 2 mcg/kg oral premedication Clonidine 4 mcg/kg oral premedication Control group: midazolam 0.5 mg/kg oral premedication All participants: sevoflurane with nitrous oxide for induction and maintenance, penile block (bupivacaine 0.5% 0.3 mL/kg), rectal paracetamol 30 mg/kg |
|
| Outcomes | Modified Objective Pain Scale used to measure agitation: 3 of 5 items used to assess agitation, with total score ≥ 3 defined as agitation Other outcomes: emergence time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...drew the sealed envelope," i.e. drawing lots |
| Allocation concealment (selection bias) | Low risk | "an anaesthesiologist not involved in the clinical protocol prepared the randomization envelopes," "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "the anaesthetist who administered the premedication was excluded from the perioperative management and postoperative assessment of the child' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "independent observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 children excluded (unclear to which groups assigned): 7 for ineffective penile block at the time of incision and 1 because of a study protocol violation |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Tesoro 2005.
| Methods | Randomized controlled trial | |
| Participants | ASA I‐II, day surgery cases, mean (SD) ages: 3.2 (1.4) years (no age limit set) Exclusion criteria: history of seizures or mental illness Recruitment: 171 randomly assigned, 169 analysed (91 clonidine group, 78 control group) Location: Italy |
|
| Interventions | Intervention group: clonidine 2 mcg/kg IV just before start of surgery Control group: saline placebo All participants: oral midazolam 0.5 mg/kg premedication, sevoflurane induction in air, LMA, regional/central block at beginning or local infiltration at end with 2.5 mg/kg ropivacaine, rectal paracetamol 30 mg/kg at end of surgery |
|
| Outcomes | EA defined as "nonpurposeful movements that could be calmed and did not require restraint" for ≥ 5 minutes. Severe EA defined as "nonpurposeful movements that could not be calmed and required restraint" for ≥ 5 minutes Other outcomes: emergence time, number of participants requiring analgesia in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned to a treatment group," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, "the anesthesiologist caring for the child was blinded to the patients' group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a second anesthesiologist also blinded to the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals: 1 inadequate caudal block, 1 participant with seizures |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Tripi 2004.
| Methods | Randomized controlled trial | |
| Participants | 18 months‐9 years, ASA I‐II, inguinal or penile surgery Exclusion criteria: none stated Recruitment: 100 randomly assigned, 92 analysed (46 in each group) Location: USA |
|
| Interventions | Intervention group: parental presence at emergence Control group: no parental presence at emergence All participants: oral midazolam premedication 0.5‐0.75 mg/kg mixed with acetaminophen, sevoflurane and nitrous oxide induction, maintenance with isoflurane and nitrous oxide via face mask or LMA, caudal block 1 mL/kg 0.125% bupivacaine, no opioids or muscle relaxants |
|
| Outcomes | Emergence behaviours recorded using 2 measures: the Operating Room Behaviour Rating Scale and the Operating Room Cooperation Rating Scale (1 = total co‐operation, 7 = total lack of co‐operation). Severe emergence distress defined as co‐operation score of 5 or greater Other outcomes: parental survey of child's temperament, parent anxiety, parent satisfaction, postoperative negative behavioural changes 1 and 4 weeks following surgery |
|
| Notes | Sevoflurane used only at induction and isoflurane used for maintenance for all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | child and anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "one research assistant was responsible for collecting all data, however she was not blinded to the presence or absence of a parent during emergence" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 withdrawals because of deviations from the protocol (4 from each group): 7 received IV fentanyl, 1 received sevoflurane maintenance |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Tsai 2008.
| Methods | Randomized controlled trial | |
| Participants | 12‐110 months, ASA I‐II, elective outpatient surgeries Exclusion criteria: none stated Recruitment: 60 children (20 in each of 3 groups) Location: Taiwan |
|
| Interventions | Propofol 1 mg/kg just after induction Ketamine 0.25 mg/kg just after induction Control group: saline placebo All participants: midazolam 0.2 mg/kg premedication, sevoflurane induction followed by IV insertion and administration of study drug, sevoflurane maintenance |
|
| Outcomes | EA defined as score > 3 when measured on a 10‐point visual analogue scale (VAS) Other outcomes: severity of EA (VAS scores), duration of PACU stay, parent and nurse satisfaction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "the i.v. agents and the i.v. set were covered to ensure that all personnel were blinded to the treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "PACU nurse blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Uezono 2000.
| Methods | Randomized 2‐period cross‐over study | |
| Participants | 1‐5 years, ASA I‐II, eye examination for retinoblastoma Exclusion criteria: history of neurological disorder, series of radiation therapy Recruitment: 18 participants, 16 of whom received each of the 2 types of anaesthesia on separate occasions (8 received sevoflurane maintenance first and 8 received propofol maintenance first) Location: Japan |
|
| Interventions | Propofol maintenance (0.1‐0.4 mcg/kg/min after a 2‐mg/kg bolus) Control group: sevoflurane maintenance All participants: oral midazolam 0.5 mg/kg as premedication, sevoflurane induction in air/oxygen, rectal paracetamol 3 mg/kg, intubation facilitated by vecuronium 0.1 mg/kg |
|
| Outcomes | EA defined as "inconsolable crying, combative behavior, or thrashing" Other outcomes: time in PACU, PONV, parental satisfaction, number of participants with pain |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, treating anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded anesthesiologist" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals due to not having repeat procedure within 6 months of the first |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Uzun 2003.
| Methods | Randomized controlled trial | |
| Participants | 4‐12 years, ASA I‐II, tonsillectomy and/or adenoidectomy, adenoidectomy and/or tube insertion, tonsillectomy and/or tube insertion Exclusion criteria: long‐term sedative medication, central nervous system disease, history of allergy to drugs Recruitment: 50 children (25 in each group) Location: Turkey |
|
| Interventions | Desflurane 6%‐7% maintenance Control group: sevoflurane 2%‐2.5% maintenance All participants: oral midazolam 0.5 mg/kg premedication, propofol 2‐2.5 mg/kg IV induction, alfentanil 10 mcg/kg, cisatracurium 0.1 mg/kg, rectal paracetamol 20 mg/kg, dexamethasone 150 mcg/kg, maintenance with study volatile and 60% nitrous oxide in oxygen |
|
| Outcomes | EA defined as a score of 3 on following 3‐point scale: 1 = asleep or calm 2 = mildly agitated, crying but consolable, restless 3 = hysterical, crying inconsolably, thrashing Other outcomes: emergence time, recovery time, perioperative heart rate and blood pressure |
|
| Notes | English abstract, full paper in Turkish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Valley 1999.
| Methods | Randomized controlled trial | |
| Participants | 4 months‐14 years, ASA I‐II, elective surgery below the umbilicus Exclusion criteria: history of airway disease Recruitment: 40 children (20 in each group) Location: USA |
|
| Interventions | Isoflurane maintenance Control group: sevoflurane maintenance All participants: sevoflurane induction or propofol IV induction, maintenance with study agent and nitrous oxide, ETT placement facilitated by muscle relaxants, regional or local anaesthesia depending on procedure |
|
| Outcomes | Emergence delirium defined as "disoriented behaviour characterized by inconsolable agitation" Other outcomes: vomiting, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random assignment," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "research nurse, blinded to the assigned group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of emergence delirium |
Valley 2003.
| Methods | Randomized controlled trial | |
| Participants | 6 months‐13 years, ASA I‐II, elective surgery below the umbilicus Exclusion criteria: history of airway disease Recruitment: 48 children (24 in each group) Location: USA |
|
| Interventions | Desflurane maintenance Control group: sevoflurane maintenance All participants: premedication with oral midazolam 0.5 mg/kg at anaesthetist's discretion, sevoflurane or propofol induction at anaesthetist's discretion, maintenance with study agent with nitrous oxide, regional or local block at end of procedure |
|
| Outcomes | EA defined as "disoriented behavior characterized by inconsolable agitation" Other outcomes: number of participants needing analgesia in PACU, vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "research nurse, blinded to the assigned group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Viitanen 1999a.
| Methods | Randomized controlled trial | |
| Participants | 1‐3 years, ASA I‐II, ambulatory adenoidectomy with or without myringotomy Exclusion criteria: none stated Recruitment: 52 children (26 in each group) Location: Finland |
|
| Interventions | Propofol induction 3 mg/kg IV Control group: sevoflurane induction All participants: no sedating premedication, EMLA cream, IV atropine 10 mcg/kg and IV alfentanil 10 mcg/kg before induction, sevoflurane and nitrous oxide maintenance, mivacurium to facilitate intubation, rectal paracetamol 20 mg/kg |
|
| Outcomes | Emergence delirium defined as total score > 3 on the pain/discomfort scale described by Hannallah et al (Hanallah 1994) Other outcomes: intubating conditions, recovery times, PONV, laryngospasm, number of participants requiring pain relief |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned, by computer‐based random numbers listing" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child not blinded to induction technique; however all children underwent IV cannulation before induction, inducing anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "recovery was assessed by a specially trained nurse, who was unaware of the induction method" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of emergence delirium |
Viitanen 1999b.
| Methods | Randomized controlled trial | |
| Participants | 1‐3 years, ASA I‐II, ambulatory adenoidectomy Exclusion criteria: allergic to drugs used, taking medication that would interact with midazolam (e.g antibiotics, antiepileptics, sedatives) Recruitment: 60 children (30 in each group) Location: Finland |
|
| Interventions | Midazolam 0.5 mg/kg oral premedication 30 minutes before induction of anaesthesia Control group: placebo All participants: IV cannula inserted before induction, atropine 0.01 mg/kg and alfentanil 10 mcg/kg IV, sevoflurane and nitrous oxide induction and maintenance, rectal acetaminophen 20 mg/kg after intubation |
|
| Outcomes | "Postanesthetic excitement" defined as total score > 3 using the 3 objective components (crying, movement and agitation) of the pain/discomfort scale reported by Hannallah et al (Hanallah 1994) Other outcomes: number of children requiring analgesia in PACU, time to emergence, vomiting, laryngospasm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "all children were randomized according to a computer‐generated random numbers program in a double‐blinded fashion" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all observers, as well as the children and their parents, were unaware of the contents of the premedicant" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals for EA outcome |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Viitanen 1999c.
| Methods | Randomized controlled trial | |
| Participants | 1‐3 years, ASA I‐II, ambulatory adenoidectomy Exclusion criteria: allergy to drugs used, recent or long‐term medication that could interact with midazolam Recruitment: 60 children (30 in each group) Location: Finland |
|
| Interventions | Midazolam 0.5 mg/kg oral premedication 30 minutes before induction Control group: placebo All participants: IV cannula inserted before induction after EMLA cream, atropine 10 mcg/kg and alfentanil 10 mcg/kg given IV, lignocaine 10 mg IV with propofol induction of 3 mg/kg with increments of 0.5 mg/kg to achieve acceptance of face mask, mivacurium 0.2 mg/kg to facilitate tracheal intubation, maintenance with sevoflurane and 70% nitrous oxide, rectal acetaminophen 20 mg/kg after intubation |
|
| Outcomes | "Arousal distress" defined as total score > 3 on a pain/discomfort scale based on that of Hannallah et al (Hanallah 1994). Total numbers for incidence of "arousal distress" used in forest plot Other outcomes: time to emergence, time to discharge, vomiting, airway difficulty, number needing postoperative pain relief in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numbers listing" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all observers, as well as the children and their parents, were unaware of the contents of the oral premedicant" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Viitanen 2000.
| Methods | Randomized controlled trial | |
| Participants | 1‐3 years, ASA I‐II, adenoidectomy with or without myringotomy Exclusion criteria: none stated Recruitment: 40 children (20 in each group) Location: Finland |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: no sedating premedication, induction and maintenance with study agent and 70% nitrous oxide, tracheal intubation, then rectal diclofenac 12.5 mg |
|
| Outcomes | "Post‐anaesthetic excitement" defined as total score > 3 using a modified pain/discomfort scale based on that used by Hannallah et al (Hanallah 1994) Other outcomes: laryngospasm, PONV, number of participants requiring analgesia postoperatively |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated by use of a computer‐generated table" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "recovery of all children was evaluated by the same trained recovery nurse, who was blinded to the anaesthetic method used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Villani 1998.
| Methods | Randomized controlled trial | |
| Participants | 3‐12 years, ASA I‐II, elective urological, abdominal and orthopaedic surgery Exclusion criteria: ASA III or greater; pulmonary, heart, CNS, neuromuscular or renal disease; obese participants (> 30% of ideal body weight) Recruitment: 64 children (32 in each group) Location: Spain |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: oral flunitrazepam 0.05 mg/kg premedication, induction and maintenance with study agent |
|
| Outcomes | Reported "agitation during emergence" without a further definition Other outcomes: hypotension during induction, laryngospasm, vomiting, renal function tests (urea, creatinine) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "using sealed envelopes, patients were randomly selected," method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "all observations and measurements were made and recorded by a trained observer, who was not blind to the choice of vapour" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Walker 1997.
| Methods | Randomized controlled trial | |
| Participants | 2‐13 years, ASA I‐II, day case surgery Exclusion criteria: history of significant cardiac, respiratory, renal, hepatic or musculoskeletal abnormalities; participants receiving drugs that potentially affect renal or hepatic function, recent general anaesthesia, expected duration of surgery > 3 hours Recruitment: 52 children randomly assigned, 50 analysed (26 halothane group, 24 sevoflurane group) Location: Australia |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: premedication with oral midazolam 0.5 mg/kg (maximum 15 mg), oral paracetamol preinduction or rectal paracetamol post induction, induction and maintenance with study agent and nitrous oxide, regional block when appropriate with bupivacaine 2.5 mg/kg |
|
| Outcomes | Incidence of "excitement" on emergence reported Other outcomes: induction and emergence times; objective pain/discomfort scores; haemodynamics, respiratory events, shivering, nausea and vomiting on emergence; blood and urine biochemistry |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | "the drug allocation was contained in a sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "emergence events were noted and graded in severity by the nurse observer," not stated whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals from sevoflurane group: 1 because of problems with vaporizer and 1 as the result of cancelled surgery |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Welborn 1996.
| Methods | Randomized controlled trial | |
| Participants | 1‐7 years, ASA I‐II, adenoidectomy with bilateral myringotomy and insertion of tubes Exclusion criteria: none stated Recruitment: 80 children (20 in each of 4 groups) Location: USA |
|
| Interventions | Group 1: sevoflurane induction and maintenance Group 2: halothane induction and sevoflurane maintenance Group 3: halothane induction and maintenance Group 4: halothane induction and desflurane maintenance All participants: oral midazolam 0.5 mg/kg premedication, 60% nitrous oxide, no opioids given in theatre |
|
| Outcomes | "Postoperative agitation" defined as child was crying inconsolably, thrashing and hysterical Other outcomes: number of participants requiring analgesia in PACU, postoperative vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned via a computer‐generated random numbers table" |
| Allocation concealment (selection bias) | Low risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a single‐blinded, independent observer evaluated each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Weldon 2004.
| Methods | Randomized controlled trial | |
| Participants | 12 months‐6 years, ASA I, inguinal hernia repair Exclusion criteria: emergency procedures, medical contraindication to placement of a block, mental retardation, developmental delay, attention‐deficit/hyperactivity disorder, psychiatric illness, history of paradoxical excitation with sedatives or previous episode of emergence delirium Recruitment: 80 children randomly assigned, 68 analysed (34 in each group) Location: USA |
|
| Interventions | Halothane anaesthesia Control group: sevoflurane anaesthesia All participants: oral premedication with midazolam 0.5 mg/kg mixed with ibuprofen 10 mg/kg, induction and maintenance with study agent and nitrous oxide, caudal block (1 mL/kg of a mixture of equal volumes of 0.25% bupivacaine and 1% lignocaine), caudal block judged inadequate if the child's heart rate increased by > 20% within 60 seconds of skin incision |
|
| Outcomes | EA defined as 3 or 4 on the following 4‐point scale (assessed on arrival to PACU and at 5‐minute intervals): 1 = awake and calm 2 = crying, requiring consoling 3 = irritable/restless, screaming, inconsolable 4 = combative, disoriented, thrashing Severe EA defined as a score that remained > 3 for 5 minutes after arrival of a parent Data in forest plot are from the 5‐minute time point Other outcomes: Yale Preoperative Anxiety Scale (YPAS) score, separation scale, induction scale, parental satisfaction scores |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization program" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | child blinded, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "trained observer blinded to the inhaled anesthetic group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 children (6 from each group) excluded because of heart rate response at skin incision (suspected ineffective block) |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Yucel 2012.
| Methods | Randomized controlled trial | |
| Participants | 3‐7 years, ASA I‐II, adenotonsillectomy with or without myringotomy Exclusion criteria: known allergy to study drugs, sensitivity or contraindication to opioids or local anaesthetic, renal or liver impairment, acute pharyngeal infection, history of asthma, clotting disorder Recruitment: 52 children (26 in each group) Location: Turkey |
|
| Interventions | Magnesium sulphate 15 mg/kg IV (commenced 2 minutes after intubation in 20 mL saline over 20 minutes) Control group: saline placebo All participants: midazolam 0.5 mg/kg oral premedication and rectal paracetamol 30‐40 mg/kg 1 hour before induction, parental presence at induction if agitated despite premedication, sevoflurane 8% induction with nitrous oxide 50%, IV insertion, cisatracurium 0.2 mg/kg IV, alfentanil 20 mg/kg IV, intubation, maintenance with sevoflurane 1.5%‐2.5% with nitrous oxide 60%, nesostigmine and atropine reversal |
|
| Outcomes | EA defined as PAED score ≥ 16 Other outcomes: preinfusion and postinfusion magnesium levels, time to extubation, time to eye opening, pain scores (Oucher Visual Analog Pain Scale), adverse events, haemodynamics |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization program" |
| Allocation concealment (selection bias) | Low risk | numbered, identical syringes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "neither the treatment assignment or the contents of the syringe were known to anaesthesia staff" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "research observer who was blinded to the treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no withdrawals |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Zand 2011.
| Methods | Randomized controlled trial | |
| Participants | 2‐7 years, ASA I‐II, anaesthesia for short (less than 0.5 hour) outpatient subumbilical surgery, compatible with peripheral nerve block Exclusion criteria: history of chronic illness or developmental delay, mental retardation, attention‐deficit/hyperactivity disorder, psychiatric illness or paradoxical excitation with sedatives Recruitment: 169 randomly assigned, 167 analysed (44 group S, 40 group SM, 41 group H, 42 group HM) Location: Iran |
|
| Interventions | Group S: sevoflurane with parental presence at induction and no premedication Group SM: sevoflurane with oral midazolam 0.5 mg/kg premedication Group H: halothane with parental presence at induction and no premedication Group HM: halothane with oral midazolam 0.5 mg/kg premedication All participants: induction and maintenance with study volatile and 60% nitrous oxide via face mask, rectal diclofenac 2 mg/kg, peripheral field block by surgeon with 0.25% bupivacaine |
|
| Outcomes | EA defined as a score of 3 or 4 on the following 4‐point scale: 1 = awake and calm, cooperative 2 = crying, requiring consoling 3 = irritable/restless, screaming, inconsolable 4 = combative, disoriented, thrashing Other outcomes: emergence time, time in PACU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization program" |
| Allocation concealment (selection bias) | Unclear risk | method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | child not blinded to parental presence or premedication, anaesthetist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "one recovery room nurse blinded to the group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant each from group H and group SM was excluded because of ventricular arrhythmia and regurgitation of gastric content, respectively |
| Selective reporting (reporting bias) | Low risk | reported incidence of EA |
Abbreviations used in tables:
AAI = A‐line autoregressive index.
APTT = activated partial thromboplastin time.
ASA = American Society of Anesthesiologists.
BIS = Bispectral Index.
BP = blood pressure.
CHEOPS = Children's Hospital Eastern Ontario Pain Scale.
CHIPPS = Children's and Infants’ Postoperative Pain Scale.
CNS = central nervous system.
CSF = cerebrospinal fluid.
CVS = cardiovascular system.
EA = emergence agitation.
ED = emergence delirium.
EKG = electrocardiogram.
ENT = ear, nose and throat.
ETCO2 = end‐tidal carbon dioxide.
ETT = endotracheal tube.
FLACC = face, legs, activity, cry, consolability.
Hb = haemoglobin.
HR = heart rate.
INR = international normalized ratio.
IQR = interquartile range.
IV = intravenous.
LMA = laryngeal mask airway.
MAC = minimum alveolar concentration.
mCHEOPS = modified Children's Hospital Eastern Ontario Pain Scale.
mYPAS = modified Yale Preoperative Anxiety Scale.
MRI = magnetic resonance imaging.
NSAID = non‐steroidal anti‐inflammatory drug.
OPS = Objective Pain Scale.
OTFC = oral transmucosal fentanyl citrate.
OR = operating room.
PACBIS = Perioperative Adult Child Behavioral Interaction Scale.
PAED = Pediatric Anesthesia Emergence Delirium.
PACU = postanaesthesia care unit.
PICU = paediatric intensive care unit.
PDS = pain/discomfort score.
PHBQ = Post‐Hospital Behavioural Questionnaire.
PONV = postoperative nausea and vomiting.
PPIA = parental presence at induction of anaesthesia.
PPEA = parental presence at emergence from anaesthesia.
PR = per rectum.
RCT = randomized controlled trial.
RSI = rapid sequence induction.
SD = standard deviation.
SEM = standard error of the mean.
SpO2 = peripheral capillary oxygen saturation.
TCI = target‐controlled infusion.
TPS = ten‐point scale.
TIVA = total intravenous anaesthesia.
URTI = upper respiratory tract infection.
VAS = visual analogue scale.
YPAS = Yale Preoperative Anxiety Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akinci 2008 | EA not studied |
| Almenrader 2007 | No control group |
| Ariffin 1997 | EA not studied |
| Chen 2010 | No control group (comparison of midazolam, propofol and ketamine) |
| Choi 2011 | No control group (comparison of remifentanil and nitrous oxide during maintenance) |
| Cole 2002 | Study drugs halothane and isoflurane, not sevoflurane |
| Delvi 2007 | EA not studied |
| El‐Hennawy 2009 | EA not studied. Aim of study to measure analgesic effects of clonidine and dexmedetomidine in caudal blocks with the FLACC (face, legs, activity, cry, consolability) pain scale used for this purpose |
| Ertugrul 2006 | No control group (comparison of ketamine, meperidine and tramadol) |
| Funk 2000 | EA not studied and researchers have not controlled for sevoflurane |
| Greenspun 1995 | EA not studied |
| Hung 2005 | Includes adult participants |
| Ibrahim 2001 | Adult participants only |
| Ingelmo 2007 | EA not studied |
| Isik 2006b | EA not studied |
| Kain 1999 | Sevoflurane not used (halothane induction with isoflurane maintenance) |
| Kain 2007 | Unclear whether all participants were maintained with sevoflurane. Attempted to contact study author for clarification but no reply |
| Kawaai 2008 | No control group |
| Kawaraguchi 2002 | No control group |
| Malmgren 2004 | No control group |
| Mayer 2006 | Article retracted in 2011 by editor‐in‐chief of Anesthesia & Analgesia for "unethical conduct of research," as IRB approval could not be confirmed |
| Mckay 2011 | No standardized sevoflurane control group (switched mid‐study from halothane maintenance to sevoflurane maintenance when halothane became unavailable) |
| Mizrak 2011 | No sevoflurane anaesthesia |
| Ozer 2003 | No control group (comparison of tramadol and meperidine) |
| Piat 1994 | EA not studied |
| Sarner 1995 | EA not studied |
| Shaban 2008 | No control group |
| Steinmetz 2007 | Fentanyl dose not controlled between the 2 comparison groups |
| Uysal 2011 | No control group |
| Wagner 2003 | All participants received isoflurane maintenance after sevoflurane induction with no reported risk of EA |
Abbreviations used:
EA = emergence agitation.
IRB = Institutional Review Board.
Characteristics of studies awaiting assessment [ordered by study ID]
Abdelhalim 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Abdulatif 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Ali 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Aydin 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Celebi 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Chandler 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Chen 2013a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Chen 2013b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Cheng 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Elgebaly 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Gai 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Gupta 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Hasanein 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Hasani 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
He 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Khalifa 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Kim 2013a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Kim 2013b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Kim 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Kodra 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Li 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Locatelli 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Oofuvong 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Park 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Pedersen 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Rashad 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Rizk 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Sethi 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Sheta 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not yet assessed |
Differences between protocol and review
The title of the published protocol (Stephens 2008) has been edited to include updated terminology for our primary outcome—emergence agitation (EA).
Minor rewording of Objective revised. "To compare sevoflurane with other general anaesthetic agents, with or without pharmacological or non‐pharmacological adjuncts, with regard to risk of EA in children during emergence from anaesthesia." Change in terminology of our primary outcome to risk of EA.
A new secondary outcome of agitation score has been added because EA scores have been developed and used since the writing of the original protocol for this review. Protocol Methods updated to make future reviews more relevant to clinical practice. We removed subgroup analyses for different age groups; different types of surgery—emergency or elective surgery; other premedication adjuncts, especially other preemptive analgesics; use of different opioids; use of other intraoperative analgesic adjuncts; and surgical versus less invasive techniques, when sufficient numbers of studies are identified. These were replaced with more relevantly worded subgroups.
Added "agitation scores such as PAED" as a secondary outcome in light of this measure of EA used in more recent studies.
Removal of "Number of participants with other, new‐onset maladaptive behaviours (e.g. sleep or eating disturbance, poor concentration) during the postoperative period, as measured by the authors of included studies" as a secondary outcome, as multiple factors other than sevoflurane will be influencing this outcome, which are quite separate from those influencing our primary outcome of EA.
Sensitivity and subgroup analyses have been revised so that future versions of this review will incorporate those most likely to influence clinical practice.
'Types of interventions' has been modified and clarified further since publication of the Protocol to include "any sevoflurane anaesthetic with or without nitrous oxide compared with any other general anaesthetic. The types of general anaesthetics included were other volatile anaesthetics, for example, isoflurane, desflurane and halothane; and any other general anaesthetics, for example, propofol or ketamine. Pharmacological adjuncts such as use of fentanyl or other opioids, propofol, midazolam, ketamine, dexmedetomidine or clonidine or non‐pharmacological adjuncts such as parental presence."
The published protocol stated that primary outcomes were "The number of participants with postoperative behavioural disturbance as measured by the authors of included studies. Emergence delirium will be defined as stated above: a mental disturbance during recovery from general anaesthesia consisting of hallucinations, delusions and confusion manifested by moaning, restlessness, involuntary physical activity and thrashing about in the bed. The review now states, "Emergence agitation (EA) was defined as the number of participants with postoperative behavioural disturbance as measured and/or reported by the authors of included studies.This updated primary outcome reflects changes in nomenclature and understanding of EA since the time the protocol was written.
Similarly, we have updated both secondary outcomes. The published protocol stated, "Secondary outcomes, Number of participants with other, new‐onset maladaptive behaviours (e.g. sleep or eating disturbance, poor concentration) during the postoperative period, as measured by the authors of included studies. Number of participants with emergence delirium during the postoperative period after leaving the PACU, as measured by the authors of included studies." Our review now states the secondary outcomes as "Postoperative behavioural disturbance or agitation scores such as PAED (Sikich 2004) and Number of participants with EA during the postoperative period after leaving the PACU, as measured by the authors of included studies."
New authors were added to the review team since the protocol was published in April 2008: David Costi joined in 2008, very soon after the protocol was published; Samira Ahmed and James Ellwood joined in 2010; Cheryl Chooi and Laura Burgoyne joined in 2012.
Contributions of authors
David Costi (DC), Allan M Cyna (AMC), Samira Ahmed (SA), Kate Stephens (KS), Penny Strickland (PS), James Ellwood (JE), Jessica N Larsson (JNL), Cheryl Chooi (CC), Laura L Burgoyne (LLB), Philippa Middleton (PM)
Conceiving of the review: AMC, KS, PS. Co‐ordinating the review: DC, AMC. Undertaking manual searches: DC, KS, PS, JNL. Screening search results: DC, KS, PS, JNL. Organizing retrieval of papers: DC, KS, PS, JNL. Screening retrieved papers against inclusion criteria: DC, SA, KS, PS, JE, JNL, CC, LLB, AMC. Appraising quality of papers: DC, PM, SA, KS, PS, JE, JNL, CC, LLB, AMC. Abstracting data from papers: DC, SA, KS, PS, JE, JNL, CC, LLB, AMC. Writing to authors of papers to ask for additional information: DC, JE. Obtaining and screening data on unpublished studies: N/A. Managing data for the review: DC, PM, AMC. Entering data into Review Manager (RevMan 5.2): DC, SA. Analysing RevMan statistical data: PM, DC, AMC. Performing other statistical analyses not using RevMan: N/A. Performing double entry of data: (data entered by person one): N/A. Interpreting data: DC, AMC, PM. Making statistical inferences: PM. Writing the review: DC, AMC, PM, KS, PS. Securing funding for the review: N/A. Performing previous work that was the foundation of the present study: N/A. Serving as guarantor for the review (one author): DC. Taking responsibility for reading and checking the review before submission: AMC, DC.
Sources of support
Internal sources
Child Youth and Women's Health Service (CYWHS), Australia.
External sources
No sources of support supplied
Declarations of interest
David Costi: received a SPANZA research grant (The Society for Paediatric Anaesthesia in New Zealand and Australia) for a randomized controlled study of EA in 2010.
Allan M Cyna: received travel and accommodation expenses for speaking at the New Zealand Society of Hypnosis ASM in 2013.
Samira Ahmed: none known.
Kate Stephens: none known.
Penny Strickland: none known.
James Ellwood: none known.
Jessica N Larsson: none known.
Cheryl Chooi: none known.
Laura L Burgoyne: none known.
Philippa Middleton: none known.
New
References
References to studies included in this review
Abdel‐Halim 2002 {published data only}
- Abdel‐Halim JMK, Azer MS, El‐Awady GA. Comparison of induction and recovery characteristics of sevoflurane, halothane and propofol in pediatric outpatients. Journal of the Egyptian National Cancer Institute 2002;14(4):319‐23. [Google Scholar]
Abdelmawgoud 2012 {published data only}
- Abdelmawgoud A, Mohy A. Effect of oral dextromethorphan versus oral ketamine on sevoflurane related emergence agitation in children undergoing adenotonsillectomy. Egyptian Journal of Anaesthesia 2012;8:243‐8. [DOI: 10.1016/j.egja.2012.05.005] [DOI] [Google Scholar]
Abu‐Shahwan 2007 {published data only}
- Abu‐Shahwan I, Chowdary K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Pediatric Anesthesia 2007;17(9):846‐50. [PUBMED: 7683402] [DOI] [PubMed] [Google Scholar]
Abu‐Shahwan 2008 {published data only}
- Abu‐Shahwan I. Effect of propofol on emergence behavior in children after sevoflurane general anesthesia. Pediatric Anesthesia 2008;18(1):55‐9. [PUBMED: 18095967] [DOI] [PubMed] [Google Scholar]
Aguilera 2003 {published data only}
- Aguilera IM, Patel D, Meakin GH, Masterson J. Perioperative anxiety and postoperative behavioural disturbances in children undergoing intravenous or inhalation induction of anaesthesia. Pediatric Anesthesia 2003;13(6):501‐7. [PUBMED: 12846706] [DOI] [PubMed] [Google Scholar]
Ahishakiye 2011 {published data only}
- Ahishakiye D, Najafi N, Velde A, Poelaert J. A randomized controlled trial to evaluate the incidence of emergence agitation in children: a comparison of intravenous versus inhalational anesthesia. European Journal of Anaesthesiology 2011;28(Suppl 48):149. [Google Scholar]
Al‐Zaben 2010 {published data only}
- Al‐Zaben KR, Qudaisat IY, Al‐Ghanem SM, Massad IM, Al‐Mustafa MM, Al‐Oweidi AS, et al. Intraoperative administration of dexmedetomidine reduces the analgesic requirements for children undergoing hypospadius surgery. European Journal of Anaesthesiology 2010;27(3):247‐52. [PUBMED: 19952754] [DOI] [PubMed] [Google Scholar]
Anand 2011 {published data only}
- Anand VG, Kannan M, Thavamani A, Bridgit MJ. Effects of dexmedetomidine added to caudal ropivacaine in paediatric lower abdominal surgeries. Indian Journal of Anaesthesia 2011;55(4):340‐6. [PUBMED: 22013248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aono 1997 {published data only}
- Aono J, Ueda W, Mamiya K, Takimoto E, Manabe M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology 1997;87(6):1298‐300. [PUBMED: 9416712] [DOI] [PubMed] [Google Scholar]
Aouad 2005 {published data only}
- Aouad MT, Kanazi GE, Siddik‐Sayyid SM, Gerges FJ, Rizk LB, Baraka AS. Preoperative caudal block prevents emergence agitation in children following sevoflurane anesthesia. Acta Anaesthesiologica Scandinavica 2005;49(3):300‐4. [PUBMED: 15752392] [DOI] [PubMed] [Google Scholar]
Aouad 2007 {published data only}
- Aouad MT, Yazbeck‐Karam VG, Nasr VG, El‐Khatib MF, Kanazi GE, Bleik JH. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology 2007;107(5):733‐8. [PUBMED: 18073548] [DOI] [PubMed] [Google Scholar]
Apan 2010 {published data only}
- Apan A, Aykaç E, Kazkayasi M, Doganci N, Tahran FD. Magnesium sulphate infusion is not effective on discomfort or emergence phenomenon in paediatric adenoidectomy/tonsillectomy. International Journal of Pediatric Otorhinolaryngology 2010;74(12):1367‐71. [PUBMED: 20880596] [DOI] [PubMed] [Google Scholar]
Arai 2005 {published data only}
- Arai YCP, Fukunaga K, Hirota S. Comparison of a combination of midazolam and diazepam and midazolam alone as oral premedication on preanesthetic and emergence condition in children. Acta Anaesthesiologica Scandinavica 2005;49(5):698‐701. [PUBMED: 15836687] [DOI] [PubMed] [Google Scholar]
Arai 2007 {published data only}
- Arai YCP, Ito H, Kandatsu N, Kurokawa S, Kinugasa S, Komatsu T. Parental presence during induction enhances the effect of oral midazolam on emergence behavior of children undergoing general anesthesia. Acta Anaesthesiologica Scandinavica 2007;51(7):858‐61. [PUBMED: 17578463] [DOI] [PubMed] [Google Scholar]
Araki 2005 {published data only}
- Araki H, Fujiwara Y, Shimada Y. Effect of flumazenil on recovery from sevoflurane anesthesia in children premedicated with oral midazolam before undergoing herniorrhaphy with or without caudal analgesia. Journal of Anesthesia 2005;19(3):204‐7. [PUBMED: 16032447] [DOI] [PubMed] [Google Scholar]
Asaad 2011 {published data only}
- Asaad OM, Hafez M, Mohamed MY, El‐Mahgoup SS. Comparative study between prophylactic single dose of fentanyl and dexmedetomidine in the management of agitation after sevoflurane anesthesia in children. Egyptian Journal of Anaesthesia 2011;27:31‐7. [DOI: 10.1016/j.egja.2010.12.005] [DOI] [Google Scholar]
Auerswald 2006 {published data only}
- Auerswald K, Behrends K, Burkhardt U, Olthoff D. Propofol for paediatric patients in ear, nose and throat surgery. Practicability, quality and cost‐effectiveness of different anaesthesia procedures for adenoidectomy in infants [Propofoleinsatz für Hals‐Nasen‐Ohren‐Eingriffe im Kindesalter]. Der Anaesthesist 2006;8:846‐53. [PUBMED: 16773342] [DOI] [PubMed] [Google Scholar]
Bae 2010 {published data only}
- Bae JH, Koo BW, Kim SJ, Lee DH, Lee ET, Kang CJ. The effects of midazolam administered postoperatively on emergence agitation in pediatric strabismus surgery. Korean Journal of Anesthesiology 2010;58(1):45‐9. [PUBMED: 20498811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakhamees 2009 {published data only}
- Bakhamees HS, Mercan A, El‐Halafawy YM. Combination effect of low dose fentanyl and propofol on emergence agitation in children following sevoflurane anesthesia. Saudi Medical Journal 2009 Apr;30(4):500‐3. [PUBMED: 19370275] [PubMed] [Google Scholar]
Bal 2006 {published data only}
- Bal N, Saricaoglu F, Uzun S, Dal D, Celebi N, Celiker V, et al. Perioperative anxiety and postoperative behavioural disturbances in children: comparison between induction techniques. European Journal of Anaesthesiology 2006;23:470‐5. [PUBMED: 16507189] [DOI] [PubMed] [Google Scholar]
Bebawy 2005 {published data only}
- Bebawy NW, Quesny KM, Greiss HF, Said AM. The effect of balanced analgesia on the incidence and severity of emergence agitation in children after sevoflurane versus halothane anaesthesia. Egyptian Journal of Anaesthesia 2005;21(2):177‐80. [Google Scholar]
Bergendahl 2004 {published data only}
- Bergendahl HTG, Lonnqvist PA, Eksborg S, Ruthstrom E, Nordenberg L, Zetterqvist H, et al. Clonidine vs. midazolam as premedication in children undergoing adeno‐tonsillectomy: a prospective, randomized, controlled clinical trial. Acta Anaesthesiologica Scandinavica 2004;48(10):1292‐300. [PUBMED: 15504191] [DOI] [PubMed] [Google Scholar]
Beskow 1999 {published data only}
- Beskow A, Westrin P. Sevoflurane causes more postoperative agitation in children than does halothane. Acta Anaesthesiologica Scandinavica 1999;43(5):536‐41. [PUBMED: 10342001] [DOI] [PubMed] [Google Scholar]
- Westrin P, Beskow A. Sevoflurane causes more postoperative agitation in children than does halothane. Anesthesiology 1997;87:A1061. [DOI] [PubMed] [Google Scholar]
Binstock 2004 {published data only}
- Binstock W, Rubin R, Bachman C, Kahana M, McDade W, Lynch JP. The effect of premedication with OTFC, with or without ondansetron, on postoperative agitation, and nausea and vomiting in pediatric ambulatory patients. Paediatric Anaesthesia 2004;14(9):759‐67. [PUBMED: 15330959] [DOI] [PubMed] [Google Scholar]
Bock 2002 {published data only}
- Bock M, Kunz P, Schreckenberger R, Graf BM, Martin E, Motsch J. Comparison of caudal and intravenous clonidine in the prevention of agitation after sevoflurane in children. British Journal of Anaesthesia 2002;88(6):790‐6. [PUBMED: 12173195] [DOI] [PubMed] [Google Scholar]
Bortone 2006 {published data only}
- Bortone L, Ingelmo P, Grossi S, Grattagliano C, Bricchi C, Barantani D, et al. Emergence agitation in preschool children: double‐blind, randomized, controlled trial comparing sevoflurane and isoflurane anesthesia. Paediatric Anaesthesia 2006;16(11):1138‐43. [PUBMED: 17040302] [DOI] [PubMed] [Google Scholar]
Breschan 2007 {published data only}
- Breschan C, Platzer M, Jost R, Stettner H, Likar R. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatric Anaesthesia 2007;17(4):347‐52. [PUBMED: 17359403] [DOI] [PubMed] [Google Scholar]
Bryan 2009 {published data only}
- Bryan YF, Hoke LK, Taghon TA, Nick TG, Wang Y, Kennedy SM, et al. A randomized trial comparing sevoflurane and propofol in children undergoing MRI scans. Pediatric Anesthesia 2009;19(7):672‐81. [PUBMED: 19638112] [DOI] [PubMed] [Google Scholar]
- Hoke LK, Taghon TA, Wang Y, Kennedy S, Bryan YF. Use of PAED scale in children undergoing MRI scans with sevoflurane and propofol. Anesthesiology 2007;107:A1401. [DOI] [PubMed] [Google Scholar]
Burke 2009 {published data only}
- Burke CN, Voepel‐Lewis T, Hadden S, DeGrandis M, Skotcher S, D'Agostino R, et al. Parental presence on emergence: effect on postanesthesia agitation and parent satisfaction. Journal of Perianesthesia Nursing 2009;24(4):216‐21. [PUBMED: 19647657] [DOI] [PubMed] [Google Scholar]
Byon 2012 {published data only}
- Byon HJ, Lee SJ, Kim JT, Kim HS. Comparison of the antiemetic effect of ramosetron and combined ramosetron and midazolam in children: a double‐blind, randomised clinical trial. European Journal of Anaesthesiology 2012;29:192‐6. [PUBMED: 22273828] [DOI] [PubMed] [Google Scholar]
Calderon 1996 {published data only}
- Calderón E, Torres LM. Comparative study of recovery from general anesthesia with halothane or sevoflurane in pediatrics. Revista Española de Anestesiología y Reanimación 1996;43(8):272‐5. [PUBMED: 9011896] [PubMed] [Google Scholar]
Chandler 2012 {published data only}
- Chandler JR, Myers D, Mehta D, Whyte E, Misse M, Montgomery CJ, et al. Emergence delirium in children, a randomized, double‐blind trial of total intravenous anesthesia vs sevoflurane. Pediatric Anesthesia 2012;22:918‐9. [DOI: 10.1111/j.1460-9592.2012.03913.x] [DOI] [Google Scholar]
Chiba 2003 {published data only}
- Chiba S, Shima T, Murakami N, Kato M. Effect of propofol on sevoflurane agitation in children [Japanese]. Masui ‐ Japanese Journal of Anesthesiology 2003;52(6):611‐5. [PUBMED: 12854475] [PubMed] [Google Scholar]
Chiu 1999 {published data only}
- Chiu CL, Chan YK, Ong G, Delilkan AE. A comparison of the induction and emergence characteristics of sevoflurane and halothane in children. The Medical Journal of Malaysia 1999;54(3):346‐51. [PUBMED: 11045061] [PubMed] [Google Scholar]
Cohen 2002 {published data only}
- Cohen IT, Finkel JC, Hannallah RS, Hummer KA, Patel KM. The effect of fentanyl on the emergence characteristics after desflurane or sevoflurane anesthesia in children. Anesthesia and Analgesia 2002;94(5):1178‐81. [PUBMED: 11973185] [DOI] [PubMed] [Google Scholar]
Cohen 2003 {published data only}
- Cohen IT, Finkel JC, Hannallah RS, Hummer KA, Patel KM. Rapid emergence does not explain agitation following sevoflurane anaesthesia in infants and children: a comparison with propofol. Paediatric Anaesthesia 2003;13(1):63‐7. [PUBMED: 12535042] [DOI] [PubMed] [Google Scholar]
Cohen 2004 {published data only}
- Cohen IT, Finkel JC, Hannallah RS, Goodale DB. Clinical and biochemical effects of propofol EDTA vs sevoflurane in healthy infants and young children. Pediatric Anesthesia 2004;14(2):135‐42. [PUBMED: 14962329] [DOI] [PubMed] [Google Scholar]
Cravero 2000a {published data only}
- Cravero JP, Beach M, Dodge CP, Whalen K. Emergence characteristics of sevoflurane compared to halothane in pediatric patients undergoing bilateral pressure equalization tube insertion. Journal of Clinical Anesthesia 2000;12(5):397‐401. [PUBMED: 11025242] [DOI] [PubMed] [Google Scholar]
Cravero 2000b {published data only}
- Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatric Anaesthesia 2000;10(4):419‐24. [PUBMED: 10886700] [DOI] [PubMed] [Google Scholar]
Cravero 2003 {published data only}
- Cravero JP, Beach M, Thyr B, Whalen K. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesthesia and Analgesia 2003;97(2):364‐7. [PUBMED: 12873918] [DOI] [PubMed] [Google Scholar]
Dalens 2006 {published data only}
- Dalens BJ, Pinard AM, Létourneau DR, Albert NT, Truchon RJY. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesthesia and Analgesia 2006;102(4):1056‐61. [PUBMED: 16551898] [DOI] [PubMed] [Google Scholar]
Davis 1999 {published data only}
- Davis PJ, Greenberg JA, Gendelman M, Fertal K. Recovery characteristics of sevoflurane and halothane in preschool‐aged children undergoing bilateral myringotomy and pressure equalization tube insertion. Anesthesia and Analgesia 1999;88(1):34‐8. [PUBMED: 9895062] [DOI] [PubMed] [Google Scholar]
De Kort 2007 {published data only}
- Kort R, Vandevelde A, Lauwers M, Camu F. Comparison of sufentanil and clonidine in the prevention of agitation in children after sevoflurane anesthesia. Acta Anaesthesiologica Belgica 2007;58(2):148. [Google Scholar]
Demirbilek 2004 {published data only}
- Demirbilek S, Togal T, Cicek M, Aslan U, Sizanli E, Ersoy MO. Effects of fentanyl on the incidence of emergence agitation in children receiving desflurane or sevoflurane anaesthesia. European Journal of Anaesthesiology 2004;21(7):538‐42. [PUBMED: 15318465] [DOI] [PubMed] [Google Scholar]
Dong 2010 {published data only}
- Dong YX, Meng LX, Wang Y, Zhang JJ, Zhao GY, Ma CH. The effect of remifentanil on the incidence of agitation on emergence from sevoflurane anaesthesia in children undergoing adenotonsillectomy. Anaesthesia and Intensive Care 2010;38(4):718‐22. [PUBMED: 20715737] [DOI] [PubMed] [Google Scholar]
El‐Sada 2005 {published data only}
- El‐Sada M, Gamal H, Kassem R. Sevoflurane, propofol and S‐ketamine anaesthesia for intraocular muscle injection of Botulinum toxin to correct strabismus in children. Egyptian Journal of Anaesthesia 2005;21(1):41‐5. [Google Scholar]
Epstein 1995 {published data only}
- Epstein RH, Mendel HG, Guarnieri KM, Staudt SR, Lessin JB, Marr AT. Sevoflurane versus halothane for general anesthesia in pediatric patients: a comparative study of vital signs, induction, and emergence. Journal of Clinical Anesthesia 1995;7:237‐44. [PUBMED: 7669316] [DOI] [PubMed] [Google Scholar]
Erden 2012 {published data only}
- Erden O, Koner O, Temur S, Menda F, Bilgen S. Effects of ondansetron on sevoflurane induced paediatric emergence agitation. British Journal of Anaesthesia 2012;108(Suppl 2):ii301. [Google Scholar]
Erdil 2009 {published data only}
- Erdil F, Demirbilek S, Begec Z, Ozturk E, Ulger MH, Ersoy MO. The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesthesia and Intensive Care 2009;37(4):571‐6. [PUBMED: 19681413] [DOI] [PubMed] [Google Scholar]
Ersin 2005 {published data only}
- Ersin NK, Onçag O, Cogulu D, Ciçek S, Balcioglu ST, Cökmez B. Postoperative morbidities following dental care under day‐stay general anesthesia in intellectually disabled children. Journal of Oral and Maxillofacial Surgery 2005;63(12):1731‐6. [PUBMED: 16297693] [DOI] [PubMed] [Google Scholar]
Fan 2000 {published data only}
- Fan KT, Lee TH, Yu KL, Tang CS, Lu DV, Chen PY, et al. Influences of tramadol on emergence characteristics from sevoflurane anesthesia in pediatric ambulatory surgery. The Kaohsiung Journal of Medical Sciences 2000;16(5):255‐60. [PUBMED: 10969521] [PubMed] [Google Scholar]
Finkel 2001 {published data only}
- Finkel JC, Cohen IT, Hannallah RS, Patel KM, Kim MS, Hummer KA, et al. The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral myringotomy tube placement. Anesthesia and Analgesia 2001;92(5):1164‐8. [PUBMED: 11323340] [DOI] [PubMed] [Google Scholar]
Galinkin 2000 {published data only}
- Galinkin JL, Fazi LM, Cuy RM, Chiavacci RM, Kurth CD, Shah UK, et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology 2000;93(6):1378‐83. [PUBMED: 11149429] [DOI] [PubMed] [Google Scholar]
Ghai 2010 {published data only}
- Ghai B, Ram J, Chauhan S, Wig J. Effects of clonidine on recovery after sevoflurane anaesthesia in children undergoing cataract surgery. Anaesthesia and Intensive Care 2010;38(3):530‐7. [PUBMED: 20514964] [DOI] [PubMed] [Google Scholar]
Ghosh 2011 {published data only}
- Ghosh SM, Agarwala RB, Pandey M, Vajifdar H. Efficacy of low‐dose caudal clonidine in reduction of sevoflurane‐induced agitation in children undergoing urogenital and lower limb surgery: a prospective randomised double‐blind study. European Journal of Anaesthesiology 2011;28(5):329‐33. [PUBMED: 21150631] [DOI] [PubMed] [Google Scholar]
Gouda 2004 {published data only}
- Gouda N. The effects of single bolus dose of remifentanil on the emergence characteristics of paediatric patients undergoing myringotomy after sevoflurane anaesthesia. Egyptian Journal of Anaesthesia 2004;20(1):63‐6. [Google Scholar]
Guard 1998 {published data only}
- Guard BC, Sikich N, Lerman J, Levine M. Maintenance and recovery characteristics after sevoflurane or propofol during ambulatory surgery in children with epidural blockade. Canadian Journal of Anaesthesia 1998;45(11):1072‐8. [PUBMED: 10021955] [DOI] [PubMed] [Google Scholar]
Guler 2002 {published data only}
- Guler G, Akin A, Tosun Z, Bicer C, Esmaoglu A, Boyaci A. The effect of peritonsillar infiltration with bupivacaine for paediatric tonsillectomy on pain and agitation after sevoflurane anaesthesia. Turkish Journal of Anaesthesiology and Reanimation 2002;30:463‐7. [Google Scholar]
Guler 2005 {published data only}
- Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single‐dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Pediatric Anesthesia 2005;15(9):762‐6. [PUBMED: 16101707] [DOI] [PubMed] [Google Scholar]
Gurkan 1999 {published data only}
- Gürkan Y, Kiliçkan L, Toker K. Propofol‐nitrous oxide versus sevoflurane‐nitrous oxide for strabismus surgery in children. Paediatric Anaesthesia 1999;9(6):495‐9. [PUBMED: 10597552] [DOI] [PubMed] [Google Scholar]
Hallen 2001 {published data only}
- Hallén J, Rawal N, Gupta A. Postoperative recovery following outpatient pediatric myringotomy: a comparison between sevoflurane and halothane. Journal of Clinical Anesthesia 2001;13(3):161‐6. [PUBMED: 11377152] [DOI] [PubMed] [Google Scholar]
Hanna 2004 {published data only}
- Hanna MG, Said HA. Comparison of two rapid recovery anaesthetic techniques using propofol versus sevoflurane in adjustable strabismus surgery. Egyptian Journal of Anaesthesia 2004;20(1):59‐62. [Google Scholar]
Hegazy 2009 {published data only}
- Hegazy MM, Elkhouly AH, Azer MS. Sevoflurane anaesthesia in pediatrics during cranial magnetic resonance image: is single dose thiopental of value in prevention of emergence agitation?. Egyptian Journal of Anaesthesia 2009;25:327‐34. [Google Scholar]
Hosten 2011 {published data only}
- Hosten T, Solak M, Elemen L, Ozgun M, Toker K. Ondansetron does not modify emergence agitation in children. Anaesthesia and Intensive Care 2011;39(4):640‐5. [PUBMED: 21823383] [DOI] [PubMed] [Google Scholar]
Hsieh 1999 {published data only}
- Hsieh YC, Chu YC, Lin SM, Tsou MY, Tsai SK, Lee TY. Comparison of recovery characteristics of sevoflurane and halothane for outpatient surgery in infants. Chinese Medical Journal (Taipei) 1999;62(11):801‐6. [PUBMED: 10575809] [PubMed] [Google Scholar]
Ibacache 2004 {published data only}
- Ibacache ME, Muñoz HR, Brandes V, Morales AL. Single‐dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesthesia and Analgesia 2004;98(1):60‐3. [PUBMED: 14693585] [DOI] [PubMed] [Google Scholar]
Im 2004 {published data only}
- Im MJ, Kim DY, Kim CH, Kim YJ. The effect of ketorolac and fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing tonsillectomy and adenoidectomy. Korean Journal of Anesthesiology 2004;46:29‐34. [Google Scholar]
Inomata 2010 {published and unpublished data}
- Inomata S, Maeda, T Shimizu T, Satsumae T, Tanaka M. Effects of fentanyl infusion on tracheal intubation and emergence agitation in preschool children anaesthetized with sevoflurane. British Journal of Anaesthesia 2010;105(3):361‐7. [PUBMED: 20627877] [DOI] [PubMed] [Google Scholar]
Isik 2005 {published data only}
- Isik B, Arslan M, Dogan AT, Kurtipek O. The effect of fentanyl administration at different periods on emergence agitation [Farkli zamanlarda uygulanan fentanilin derlenme ajitasyonu uzerine etkisi]. Turk Anesteziyoloji ve Reanimasyon Dernegi Dergisi 2005;33:311‐7. [Google Scholar]
Isik 2006a {published data only}
- Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Pediatric Anaesthesia 2006;16(7):748‐53. [PUBMED: 16879517] [DOI] [PubMed] [Google Scholar]
Jacob 2012 {published data only}
- Jacob Z, Li H, Zhang S, Jambawalikar S, Makaryus R, Benveniste H. Brain metabolites discriminate sevoflurane from propofol anesthesia in children. Anesthesia and Analgesia 2011;112(Suppl):S351. [Google Scholar]
Jiang 2010 {published data only}
- Jiang XY, Li TZ, Li L. Premedication with oral midazolam in children receive sevoflurane general anesthesia. National Medical Journal of China 2010;90(37):2633‐5. [PUBMED: 21162931] [PubMed] [Google Scholar]
Johannesson 1995 {published data only}
- Johannesson GP, Florén M, Lindahl SGE. Sevoflurane for ENT‐surgery in children. A comparison with halothane. Acta Anaesthesiologica Scandinavica 1995;39(4):546‐50. [PUBMED: 7676795] [DOI] [PubMed] [Google Scholar]
Jung 2010 {published data only}
- Jung HJ, Kim JB, Im KS, Oh SH, Lee JM. Effect of ketamine versus thiopental sodium anesthetic induction and a small dose of fentanyl on emergence agitation after sevoflurane anesthesia in children undergoing brief ophthalmic surgery. Korean Journal of Anesthesiology 2010;58(2):148‐52. [PUBMED: 20498793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kain 2005 {published data only}
- Kain ZN, Caldwell‐Andrews AA, Weinberg ME, Mayes LC, Wang SM, Gaal D, et al. Sevoflurane versus halothane: postoperative maladaptive behavioral changes: a randomized, controlled trial. Anesthesiology 2005;102(4):720‐6. [PUBMED: 15791099] [DOI] [PubMed] [Google Scholar]
Kataria 1996 {published data only}
- Kataria B, Epstein R, Bailey A, Schmitz M, Backus WW, Schoeck D, et al. A comparison of sevoflurane to halothane in paediatric surgical patients: results of a multicentre international study. Paediatric Anaesthesia 1996;6(4):283‐92. [PUBMED: 8827744] [DOI] [PubMed] [Google Scholar]
Kazak 2010 {published data only}
- Kazak Z, Sezer GB, Yilmaz AA, Ates Y. Premedication with oral midazolam with or without parental presence. European Journal of Anaesthesiology 2010;27(4):347‐52. [PUBMED: 20306569] [DOI] [PubMed] [Google Scholar]
Keaney 2004 {published data only}
- Keaney A, Diviney D, Harte S, Lyons B. Postoperative behavioral changes following anesthesia with sevoflurane. Pediatric Anaesthesia 2004;14(10):866‐70. [PUBMED: 15385017] [DOI] [PubMed] [Google Scholar]
Khattab 2009 {published data only}
- Khattab AM, El‐Seify ZA. Sevoflurane‐emergence agitation: effect of supplementary low‐dose oral ketamine premedication in preschool children undergoing dental surgery. Saudi Journal of Anaesthesia 2009;3(2):61‐6. [PUBMED: 20532105] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab AM, El‐Seify ZA, Shaaban A, Radojevic D, Jankovic I. Sevoflurane‐emergence agitation: effect of supplementary low‐dose oral ketamine premedication in preschool children undergoing dental surgery. European Journal of Anaesthesiology 2010;27(4):353‐8. [PUBMED: 20035226] [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim JY, Chang YJ, Lee JY, Park HY, Kwak HJ. Post‐induction alfentanil reduces sevoflurane‐associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiologica Scandinavica 2009;53(5):678‐81. [PUBMED: 19419364] [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim YH, Yoon SZ, Lim HJ, Yoon SM. Prophylactic use of midazolam or propofol at the end of surgery may reduce the incidence of emergence agitation after sevoflurane anaesthesia. Anaesthesia and Intensive Care 2011;39(5):904‐08. [PUBMED: 21970137] [DOI] [PubMed] [Google Scholar]
Ko 2001 {published and unpublished data}
- Ko YP, Huang CJ, Hung YC, Su NY, Tsai PS, Chen CC, et al. Premedication with low‐dose oral midazolam reduces the incidence and severity of emergence agitation in pediatric patients following sevoflurane anesthesia. Acta Anaesthesiologica Sinica 2001;39(4):169‐77. [PUBMED: 11840583] [PubMed] [Google Scholar]
Kobylarz 2001 {published data only}
- Kobylarz K, Kolaczyk D, Stanczyk M. Sevoflurane in paediatric practice—our own experience [Sevofluran w praktyce pediatrycznej—doswiadczenia wlasne]. Folia Medica Cracoviensia 2001;42(4):211‐6. [PUBMED: 12815781] [PubMed] [Google Scholar]
Kol 2008 {published data only}
- Kol IO, Egilmez H, Kaygusuz K, Gursoy S, Mimaroglu C. Open‐label, prospective, randomized comparison of propofol and sevoflurane for laryngeal mask anesthesia for magnetic resonance imaging in pediatric patients. Clinical Therapeutics 2008;30(1):175‐81. [PUBMED: 18343254] [DOI] [PubMed] [Google Scholar]
Koner 2011 {published data only}
- Koner O, Ture H, Mercan A, Menda F, Sozubir S. Effects of hydroxyzine‐midazolam premedication on sevoflurane‐induced paediatric emergence agitation: a prospective randomised clinical trial. European Journal of Anaesthesiology 2011;28(9):640‐5. [PUBMED: 21822077] [DOI] [PubMed] [Google Scholar]
Konig 2009 {published data only}
- König MW, Varughese AM, Brennen KA, Barclay S, Shackleford TM, Samuels PJ, et al. Quality of recovery from two types of general anesthesia for ambulatory dental surgery in children: a double‐blind, randomized trial. Pediatric Anesthesia 2009;19(8):748‐55. [PUBMED: 19538532] [DOI] [PubMed] [Google Scholar]
Kubo 2001 {published data only}
- Kubo S, Kinouchi K, Taniguchi A, Fukumitsu K, Kitamura S. Recovery characteristics of propofol anesthesia in pediatric outpatients; comparison with sevoflurane anesthesia. Japanese Journal of Anesthesiology [Masui] 2001;50(4):371‐7. [PUBMED: 11345748] [PubMed] [Google Scholar]
Kulka 2001a {published data only}
- Kulka PJ, Bressem M, Wiebalck A, Tryba M. Prevention of "post‐sevoflurane delirium" with midazolam. Anaesthetist 2001;50:401‐5. [PUBMED: 11458720] [DOI] [PubMed] [Google Scholar]
Kulka 2001b {published data only}
- Kulka PJ, Bressem M, Tryba M. Clonidine prevents sevoflurane‐induced agitation in children. Anesthesia and Analgesia 2001;93(2):335‐8. [PUBMED: 11473855] [PubMed] [Google Scholar]
Lankinen 2006 {published data only}
- Lankinen U, Avela R, Tarkkila P. The prevention of emergence agitation with tropisetron or clonidine after sevoflurane anesthesia in small children undergoing adenoidectomy. Anesthesia and Analgesia 2006;102(5):1383‐6. [PUBMED: 16632814] [DOI] [PubMed] [Google Scholar]
Lapin 1999 {published data only}
- Lapin SL, Auden SM, Goldsmith LJ, Reynolds AM. Effects of sevoflurane anaesthesia on recovery in children: a comparison with halothane. Paediatric Anaesthesia 1999;9(4):299‐304. [PUBMED: 10411764] [DOI] [PubMed] [Google Scholar]
Le Berre 2001 {published and unpublished data}
- Berre PY, Wodey E, Joly A, Carré P, Ecoffey C. Comparison of recovery after intermediate duration of anaesthesia with sevoflurane and isoflurane. Paediatric Anaesthesia 2001;11(4):443‐8. [PUBMED: 11442862] [DOI] [PubMed] [Google Scholar]
Lee 2010a {published data only}
- Lee YS, Kim WY, Choi JH, Son JH, Kim JH, Park YC. The effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesia. Korean Journal of Anesthesiology 2010;58(5):440‐5. [PUBMED: 20532051] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010b {published data only}
- Lee CJ, Lee SE, Oh MK, Shin CM, Kim YJ, Choe YK, et al. The effect of propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy. Korean Journal of Anesthesiology 2010;59(2):75‐81. [PUBMED: 20740210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee YC, Kim JM, Ko HB, Lee SR. Use of laryngeal mask airway and its removal in a deeply anaesthetized state reduces emergence agitation after sevoflurane anaesthesia in children. The Journal of International Medical Research 2011;39(6):2385‐92. [PUBMED: 22289558] [DOI] [PubMed] [Google Scholar]
Lerman 1996 {published data only}
- Lerman J, Davis PJ, Welborn LG, Orr RJ, Rabb M, Carpenter R, et al. Induction, recovery, and safety characteristics of sevoflurane in children undergoing ambulatory surgery. A comparison with halothane. Anesthesiology 1996;84(6):1332‐40. [PUBMED: 8669674] [DOI] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li J, Huang ZL, Zhang XT, Luo K, Zhang ZQ, Mao Y, et al. Sufentanil reduces emergence agitation in children receiving sevoflurane anesthesia for adenotonsillectomy compared with fentanyl. Chinese Medical Journal 2011;124:3682‐5. [PUBMED: 22340224] [PubMed] [Google Scholar]
Liao 2010 {published data only}
- Liao R, Li JY, Liu GY. Comparison of sevoflurane volatile induction/maintenance anaesthesia and propofol‐remifentanil total intravenous anaesthesia for rigid bronchoscopy under spontaneous breathing for tracheal/bronchial foreign body removal in children. European Journal of Anaesthesiology 2010;27(11):930‐4. [PUBMED: 20683333] [DOI] [PubMed] [Google Scholar]
Liao 2011 {published data only}
- Liao WW, Wang JJ, Wu GJ, Kuo CD. The effect of cerebral monitoring on recovery after sevoflurane anesthesia in ambulatory setting in children: a comparison among bispectral index, A‐line autoregressive index, and standard practice. Journal of the Chinese Medical Association 2011;74(1):28‐36. [PUBMED: 21292200] [DOI] [PubMed] [Google Scholar]
Lili 2012 {published data only}
- Lili X, Jianjun S, Haiyan Z. The application of dexmedetomidine in children undergoing vitreoretinal surgery. Journal of Anesthesia 2012;26:556‐61. [PUBMED: 22415678] [DOI] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin YC, Tassone RF, Jahng S, Rahbar R, Holzman RS, Zurakowski D, et al. Acupuncture management of pain and emergence agitation in children after bilateral myringotomy and tympanostomy tube insertion. Paediatric Anaesthesia 2009;19(11):1096‐101. [PUBMED: 19709377] [DOI] [PubMed] [Google Scholar]
Lopez Gil 1999 {published data only}
- Lopéz Gil ML, Brimacombe J, Clar B. Sevoflurane versus propofol for induction and maintenance of anaesthesia with the laryngeal mask airway in children. Paediatric Anaesthesia 1999;9(6):485‐90. [PUBMED: 10597550] [DOI] [PubMed] [Google Scholar]
Makharita 2009 {published data only}
- Makharita MY, Ahmady M, El‐Morsy G, El‐Emam H, Raouf AA, Eldeek BS. Prevention of pediatric emergence agitation after sevoflurane anaesthesia using preoperative rectal acetaminophen and intraoperative IV fentanyl. Egyptian Journal of Anaesthesia 2009;25:319‐25. [Google Scholar]
Malviya 2006 {published data only}
- Malviya S, Voepel‐Lewis T, Ramamurthi RJ, Burke C, Tait AR. Clonidine for the prevention of emergence agitation in young children: efficacy and recovery profile. Paediatric Anaesthesia 2006;16(5):554‐9. [PUBMED: 16677266] [DOI] [PubMed] [Google Scholar]
Meena 2009 {published data only}
- Meena K, Pandy M, Jain A. Comparison of induction and recovery characteristics of sevoflurane versus halothane in pre‐school children undergoing cleft lip palate repair. Journal of Anaesthesiology and Clinical Pharmacology 2009;25(2):171‐4. [Google Scholar]
Meng 2012 {published data only}
- Meng QT, Xia ZY, Luo T, Wu Y, Tang LH, Zhao B, et al. Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case‐control study. International Journal of Pediatric Otorhinolaryngology 2012;76(7):1036‐41. [PUBMED: 22537843] [DOI] [PubMed] [Google Scholar]
Meyer 2007 {published data only}
- Meyer RR, Münster P, Werner C, Brambrink AM. Isoflurane is associated with a similar incidence of emergence agitation/delirium as sevoflurane in young children‐a randomized controlled study. Paediatric Anaesthesia 2007;17(1):56‐60. [PUBMED: 17184433] [DOI] [PubMed] [Google Scholar]
Michalek‐Sauberer 1998 {published data only}
- Michalek‐Sauberer A, Wildling E, Pusch F, Semsroth M. Sevoflurane anaesthesia in paediatric patients: better than halothane?. European Journal of Anaesthesiology 1998;15(3):280‐6. [PUBMED: 9649985] [DOI] [PubMed] [Google Scholar]
Mikawa 2002 {published data only}
- Mikawa K, Nishina K, Shiga M. Prevention of sevoflurane‐induced agitation with oral clonidine premedication. Anesthesia and Analgesia 2002;94(6):1675‐6. [PUBMED: 12032063] [DOI] [PubMed] [Google Scholar]
Milic 2010 {published data only}
- Milic M, Goranovic T, Knezevic P. Complications of sevoflurane‐fentanyl versus midazolam‐fentanyl anesthesia in pediatric cleft lip and palate surgery: a randomized comparison study. International Journal of Oral and Maxillofacial Surgery 2010;39(1):5‐9. [PUBMED: 19854614] [DOI] [PubMed] [Google Scholar]
Mizrak 2013 {published data only}
- Mizrak A, Karatas E, Saruhan R, Kara F, Oner U, Saricicek V, et al. Does dexmedetomidine affect intraoperative blood loss and clotting tests in pediatric adenotonsillectomy patients?. The Journal of Surgical Research 2013;179(1):94‐8. [PUBMED: 23122669] [DOI] [PubMed] [Google Scholar]
Moore 2003 {published data only}
- Moore JK, Moore EW, Elliott RA, Leger AS, Payne K, Kerr J. Propofol and halothane versus sevoflurane in paediatric day‐case surgery: induction and recovery characteristics. British Journal of Anaesthesia 2003;90(4):461‐6. [PUBMED: 12644418] [DOI] [PubMed] [Google Scholar]
Murray 2002 {published data only}
- Murray DJ, Cole JW, Shrock CD, Snider RJ, Martini JA. Sevoflurane versus halothane: effect of oxycodone premedication on emergence behaviour in children. Paediatric Anaesthesia 2002;12(4):308‐12. [PUBMED: 11982836] [DOI] [PubMed] [Google Scholar]
Na 2013 {published data only}
- Na HS, Song IA, Hwang JW, Do SH, Oh AY. Emergence agitation in children undergoing adenotonsillectomy: a comparison of sevoflurane vs. sevoflurane‐remifentanil administration. Acta Anaesthesiologica Scandinavica 2013;57:100‐5. [PUBMED: 23110746] [DOI] [PubMed] [Google Scholar]
Naito 1991 {published data only}
- Naito Y, Tamai S, Shingu K, Fujimori R, Mori K. Comparison between sevoflurane and halothane for paediatric ambulatory anaesthesia. British Journal of Anaesthesia 1991 Oct;67(4):387‐9. [PUBMED: 1931394] [DOI] [PubMed] [Google Scholar]
Nakayama 2007 {published data only}
- Nakayama S, Furukawa H, Yanai H. Propofol reduces the incidence of emergence agitation in preschool‐aged children as well as in school‐aged children: a comparison with sevoflurane. Journal of Anesthesia 2007;21(1):19‐23. [PUBMED: 17285408] [DOI] [PubMed] [Google Scholar]
Oh 2005 {published data only}
- Oh AY, Seo KS, Kim SD, Kim CS, Kim HS. Delayed emergence process does not result in a lower incidence of emergence agitation after sevoflurane anesthesia in children. Acta Anaesthesiologica Scandinavica 2005;49(3):297‐9. [PUBMED: 15752391] [DOI] [PubMed] [Google Scholar]
Ou 2011 {published data only}
- Ou M, Zhang XY, Wang JP, Li KH, Du Z. Application of sevoflurane inhalation anesthesia on strabismus operation in children. Internationational Journal of Ophthalmology 2011;11:1853‐5. [Google Scholar]
Ozcengiz 2011 {published data only}
- Ozcengiz D, Gunes Y, Ozmete O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. Journal of Anesthesia 2011;25(2):184‐8. [PUBMED: 21327805] [DOI] [PubMed] [Google Scholar]
Ozer Kocak 2001 {published data only}
- Ozer Kocak Z, Altunkan AA, Atici S, Cinel I, Oral U. Comparison of remifentanil‐propofol and sevoflurane for preventing cardiovascular response and quality of recovery in paediatric otolaryngologic surgery. Turkish Journal of Medical Sciences 2001;31:559‐64. [Google Scholar]
Ozturk 2009 {published data only}
- Ozturk T, Erbuyun K, Keles GT, Ozer M, Yuksel H, Tok D. The effect of remifentanil on the emergence characteristics of children undergoing FBO for bronchoalveolar lavage with sevoflurane anaesthesia. European Journal of Anaesthesiology 2009 Apr;26(4):338‐42. [PUBMED: 19401665] [DOI] [PubMed] [Google Scholar]
Patel 2010 {published data only}
- Patel A, Davidson M, Tran MCJ, Quraishi H, Schoenberg C, Sant M, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesthesia and Analgesia 2010;111(4):1004‐10. [PUBMED: 20705788] [DOI] [PubMed] [Google Scholar]
Picard 2000 {published data only}
- Picard V, Dumont L, Pellegrini M. Quality of recovery in children: sevoflurane versus propofol. Acta Anaesthesiologica Scandinavica 2000;44(3):307‐10. [PUBMED: 10714845] [DOI] [PubMed] [Google Scholar]
Pieters 2010 {published data only}
- Pieters BJ, Penn E, Nicklaus P, Bruegger D, Mehta B, Weatherly R. Emergence delirium and postoperative pain in children undergoing adenotonsillectomy: a comparison of propofol vs sevoflurane anesthesia. Pediatric Anesthesia 2010;20(10):944‐50. [PUBMED: 20735801] [DOI] [PubMed] [Google Scholar]
Rampersad 2010 {published data only}
- Rampersad S, Jimenez N, Bradford H, Seidel K, Lynn A. Two‐agent analgesia versus acetaminophen in children having bilateral myringotomies and tubes surgery. Pediatric Anesthesia 2010;20(11):1028‐35. [PUBMED: 20964769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rieger 1996 {published data only}
- Rieger A, Schröter G, Philippi W, Hass I, Eyrich K. A comparison of sevoflurane with halothane in outpatient adenotomy in children with mild upper respiratory tract infections. Journal of Clinical Anesthesia 1996;8(3):188‐97. [PUBMED: 8703451] [DOI] [PubMed] [Google Scholar]
Saadawy 2009 {published data only}
- Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiologica Scandinavica 2009;53(2):251‐6. [PUBMED: 19076110] [DOI] [PubMed] [Google Scholar]
Salik 2011 {published data only}
- Salik I, Narang J, Brous P. Dexmedetomidine in emergence agitation in children undergoing general anesthesia for interventional radiology. Journal of Neurosurgical Anesthesiology 2011;23(4):436. [Google Scholar]
Sato 2010 {published data only}
- Sato M, Shirakami G, Tazuke‐Nishimura M, Matsuura S, Tanimoto K, Fukuda K. Effect of single‐dose dexmedetomidine on emergence agitation and recovery profiles after sevoflurane anesthesia in pediatric ambulatory surgery. Journal of Anesthesia 2010;24(5):675‐82. [PUBMED: 20585813] [DOI] [PubMed] [Google Scholar]
Seo 2011 {published data only}
- Seo IS, Seong CR, Jung G, Park SJ, Kim SY, Kim MM. The effect of sub‐tenon lidocaine injection on emergence agitation after general anaesthesia in paediatric strabismus surgery. European Journal of Anaesthesiology. 2011;28(5):334‐9. [PUBMED: 21206277] [DOI] [PubMed] [Google Scholar]
Shen 2012 {published data only}
- Shen X, Hu C, Li W. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of sevoflurane and sevoflurane in combination with low‐dose remifentanil. Pediatric Anesthesia 2012;22:1179‐84. [PUBMED: 22755543] [DOI] [PubMed] [Google Scholar]
Shibata 2005 {published data only}
- Shibata S, Shigeomi S, Sato W, Enzan K. Nitrous oxide administration during washout of sevoflurane improves postanesthetic agitation in children. Journal of Anesthesia 2005;19(2):160‐3. [PUBMED: 15875135] [DOI] [PubMed] [Google Scholar]
Shin 2001 {published data only}
- Shin M, Park T, Kim T, Kim I. Ibuprofen potentiates an analgesic effect and hastens a recovery after tonsillectomy in children. Korean Journal of Anesthesiology 2001;45:555‐9. [Google Scholar]
Shukry 2005 {published data only}
- Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane‐based general anesthesia?. Pediatric Anesthesia 2005;15(12):1098‐104. [PUBMED: 16324031] [DOI] [PubMed] [Google Scholar]
Singh 2009 {published data only}
- Singh D, Rath GP, Dash HH, Bithal PK. Sevoflurane provides better recovery as compared with isoflurane in children undergoing spinal surgery. Journal of Neurosurgical Anesthesiology 2009;21(3):202‐6. [PUBMED: 19542996] [DOI] [PubMed] [Google Scholar]
Singh 2012 {published data only}
- Singh R, Kharbanda M, Sood N, Mahajan V, Chatterji C. Comparative evaluation of incidence of emergence agitation and post‐operative recovery profile in paediatric patients after isoflurane, sevoflurane and desflurane anaesthesia. Indian Journal of Anaesthesia 2012;56:156‐61. [PUBMED: 22701207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2009 {published data only}
- Sun Y, Xu W‐Y, Hu J, Wang Y‐T, Bai J . Effects of diclofenac sodium suppositories on emergence agitation after sevoflurane maintenance in children undergoing adenotonsillectomy. Journal of Shanghai Jiaotong University (Medical Science) 2009;29:842‐4. [Google Scholar]
Sun 2010 {published data only}
- Sun Y, Hu J, Xu W‐Y, Bai J, Zhang M‐Z. Combination effect of tramadol and low dose propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy procedure. Journal of Shanghai Jiaotong University (Medical Science) 2010;30:73‐5. [Google Scholar]
Sury 1996 {published data only}
- Sury MR, Black A, Hemington L, Howard R, Hatch DJ, Mackersie A. A comparison of the recovery characteristics of sevoflurane and halothane in children. Anaesthesia 1996;51(6):543‐6. [PUBMED: 8694205] [DOI] [PubMed] [Google Scholar]
Tang 2009 {published data only}
- Tang YF, Chen F, Wang BF, Li HF, Fuzaylov G, Li J, et al. Analgesic and sedative effects of multimodal analgesia in stage of emergence after general anesthesia for cleft lip and/or palate prosthesis [Article in Chinese]. National Medical Journal of China 2009;89(13):906‐8. [PUBMED: 19671292] [PubMed] [Google Scholar]
Tazeroualti 2007 {published data only}
- Tazeroualti N, Groote F, Hert S, Villé A, Dierick A, Linden P. Oral clonidine vs midazolam in the prevention of sevoflurane‐induced agitation in children. a prospective, randomized, controlled trial. British Journal of Anaesthesia 2007;98(5):667‐71. [PUBMED: 17416907] [DOI] [PubMed] [Google Scholar]
Tesoro 2005 {published data only}
- Tesoro S, Mezzetti D, Marchesini L, Peduto VA. Clonidine treatment for agitation in children after sevoflurane anesthesia. Anesthesia and Analgesia 2005;101(6):1619‐22. [PUBMED: 16301230] [DOI] [PubMed] [Google Scholar]
Tripi 2004 {published data only}
- Tripi PA, Palermo TM, Thomas S, Goldfinger MM, Florentino‐Pineda I. Assessment of risk factors for emergence distress and postoperative behavioural changes in children following general anaesthesia. Paediatric Anaesthesia 2004;14(3):235‐40. [PUBMED: 14996262] [DOI] [PubMed] [Google Scholar]
Tsai 2008 {published data only}
- Tsai PS, Hsu YW, Lin CS, Ko YP, Huang CJ. Ketamine but not propofol provides additional effects on attenuating sevoflurane‐induced emergence agitation in midazolam premedicated pediatric patients. Pediatric Anesthesia 2008;18(11):1114‐5. [PUBMED: 18950343] [DOI] [PubMed] [Google Scholar]
Uezono 2000 {published data only}
- Uezono S, Goto T, Terui K, Ichinose F, Ishguro Y, Nakata Y, et al. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesthesia and Analgesia 2000;91(3):563‐6. [PUBMED: 10960377] [DOI] [PubMed] [Google Scholar]
Uzun 2003 {published data only}
- Uzun S, Tuncer S, Tavlan A, Reisli R, Sarkilar G, Ökesli S. Comparison of maintenance and recovery characteristics of desflurane and sevoflurane in children [Çocuklarda Desfluran‐Sevofluran Anestezisinin Idame ve Derlenme Üzerine Olan Etkilerinin Karsilastirilmasi]. Turkish Journal of Anaesthesiology and Reanimation 2003;31:415‐21. [Google Scholar]
Valley 1999 {published data only}
- Valley RD, Ramza JT, Calhoun P, Freid EB, Bailey AG, Kopp VJ, et al. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of isoflurane and sevoflurane. Anesthesia and Analgesia 1999;88(4):742‐5. [PUBMED: 10195515] [DOI] [PubMed] [Google Scholar]
Valley 2003 {published data only}
- Valley RD, Freid EB, Bailey AG, Kopp VJ, Georges LS, Fletcher J, et al. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of desflurane and sevoflurane. Anesthesia and Analgesia 2003;96(5):1320‐4. [PUBMED: 12707126] [DOI] [PubMed] [Google Scholar]
Viitanen 1999a {published data only}
- Viitanen H, Tarkkila P, Mennander S, Viitanen M, Annila P. Sevoflurane‐maintained anesthesia induced with propofol or sevoflurane in small children: induction and recovery characteristics. Canadian Journal of Anaesthesia 1999;46(1):21‐8. [PUBMED: 10078398] [DOI] [PubMed] [Google Scholar]
Viitanen 1999b {published data only}
- Viitanen H, Annila P, Viitanen M, Tarkkila P. Premedication with midazolam delays recovery after ambulatory sevoflurane anesthesia in children. Anesthesia and Analgesia 1999;89(1):75‐9. [PUBMED: 10389782] [DOI] [PubMed] [Google Scholar]
Viitanen 1999c {published data only}
- Viitanen H, Annila P, Viitanen M, Yli‐Hankala A. Midazolam premedication delays recovery from propofol‐induced sevoflurane anesthesia in children 1‐3 yr. Canadian Journal of Anaesthesia 1999;46(8):766‐71. [PUBMED: 10451136] [DOI] [PubMed] [Google Scholar]
Viitanen 2000 {published data only}
- Viitanen H, Baer G, Annila P. Recovery characteristics of sevoflurane or halothane for day‐case anaesthesia in children aged 1‐3 years. Acta Anaesthesiologica Scandinavica 2000;44(1):101‐6. [PUBMED: 10669280] [DOI] [PubMed] [Google Scholar]
Villani 1998 {published data only}
- Villani A, Zuccoli P, Rovella C, Laviani R, Gulli E, Guddo AM, et al. A prospective, randomized clinical comparison of sevoflurane and halothane in children. Minerva Anestesiologica 1998;64(9 Suppl 3):3‐10. [10731735] [PubMed] [Google Scholar]
Walker 1997 {published data only}
- Walker SM, Haugen RD, Richards A. A comparison of sevoflurane with halothane for paediatric day case surgery. Anaesthesia and Intensive Care 1997;25(6):643‐9. [PUBMED: 9452847] [DOI] [PubMed] [Google Scholar]
Welborn 1996 {published data only}
- Welborn LG, Hannallah RS, Norden JM, Ruttimann UE, Callan CM. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesthesia and Analgesia 1996;83(5):917‐20. [PUBMED: 8895263] [DOI] [PubMed] [Google Scholar]
Weldon 2004 {published data only}
- Weldon BC, Abele A, Simeon R, Erkman C, Kosa J, Roth P, et al. Postoperative agitation: sevoflurane vs halothane. Anesthesiology 1997;87(3A):A1057. [Google Scholar]
- Weldon BC, Bell M, Craddock T. The effect of caudal analgesia on emergence agitation in children after sevoflurane versus halothane anesthesia. Anesthesia and Analgesia 2004;98(2):321‐6. [PUBMED: 14742362] [DOI] [PubMed] [Google Scholar]
Yucel 2012 {published data only}
- Yucel A, Begec Z, Ozgul U, Aydogan MS, Gulhas N, Ersoy MO. The effect of magnesium on emergence agitation in children undergoing adenotonsillectomy under sevoflurane general anesthesia: a prospective randomised clinical trial. HealthMED 2012;6:1605‐10. [Google Scholar]
Zand 2011 {published data only}
- Zand F, Allahyary E, Hamidi AR. Postoperative agitation in preschool children following emergence from sevoflurane or halothane anesthesia: a randomized study on the forestalling effect of midazolam premedication versus parental presence at induction of anesthesia. Acta Anaesthesiologica Taiwanica 2011;49:96‐9. [PUBMED: 21982170] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akinci 2008 {published data only}
- Akinci SB, Kose EA, Ocal T, Aypar U. The effects of maternal presence during anesthesia induction on the mother's anxiety and changes in children's behavior. Turkish Journal of Pediatrics 2008;50(6):566‐71. [PUBMED: 19227421] [PubMed] [Google Scholar]
Almenrader 2007 {published data only}
- Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Premedication in children: a comparison of oral midazolam and oral clonidine. Pediatric Anaesthesia 2007;17(12):1143‐9. [PUBMED: 17986032] [DOI] [PubMed] [Google Scholar]
Ariffin 1997 {published data only}
- Ariffin SA, Whyte A, Malins AF, Cooper GM. Comparison of induction and recovery between sevoflurane and halothane supplementation of anaesthesia in children undergoing outpatient dental extractions. British Journal of Anaesthesia 1997;78:157‐9. [PUBMED: 9068332] [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen J, Li W, Hu X, Wang D. Emergence agitation after cataract surgery in children: a comparison of midazolam, propofol and ketamine. Pediatric Anesthesia 2010;20(9):873‐9. [PUBMED: 20716081] [DOI] [PubMed] [Google Scholar]
Choi 2011 {published data only}
- Choi HR, Cho JK, Lee S, Yoo BH, Yon JH, Kim KM. The effect of remifentanil versus N2O on postoperative pain and emergence agitation after pediatric tonsillectomy/adenoidectomy. Korean Journal of Anesthesiology 2011;61(2):148‐53. [PUBMED: 21927686] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2002 {published data only}
- Cole JW, Murray DJ, McAllister JD, Hirschberg GE. Emergence behaviour in children: defining the incidence of excitement and agitation following anaesthesia. Paediatric Anaesthesia 2002;12:442‐7. [PUBMED: 12060332] [DOI] [PubMed] [Google Scholar]
Delvi 2007 {published data only}
- Delvi MB, Samarkandi A, Zahrani T, Faden A. Recovery profile for magnetic resonance imaging in pediatric daycase‐‐sevoflurane vs. isoflurane. Middle East Journal of Anesthesiology 2007;19(1):205‐11. [PUBMED: 17511194] [PubMed] [Google Scholar]
El‐Hennawy 2009 {published data only}
- El‐Hennawy AM, Abd‐Elwahab AM, Abd‐Elmaksoud AM, El‐Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. British Journal of Anaesthesia 2009 Aug;103(2):268‐74. [PUBMED: 19541679] [DOI] [PubMed] [Google Scholar]
Ertugrul 2006 {published data only}
- Ertugrul F, Akbas M, Karsli B, Kayacan N, Bulut F, Trakya A. Pain relief for children after adenotonsillectomy. The Journal of International Medical Research 2006;34(6):648‐54. [PUBMED: 17294997] [DOI] [PubMed] [Google Scholar]
Funk 2000 {published data only}
- Funk W, Jakob W, Riedl T, Taeger K. Oral preanaesthetic medication for children: double‐blind randomized study of a combination of midazolam and ketamine vs midazolam or ketamine alone. British Journal of Anaesthesia 2000;84(3):335‐40. [PUBMED: 10793592] [DOI] [PubMed] [Google Scholar]
Greenspun 1995 {published data only}
- Greenspun JCF, Hannallah RS, Welborn LG, Norden JM. Comparison of sevoflurane and halothane anesthesia in children undergoing outpatient ear, nose and throat surgery. Journal of Clinical Anesthesia 1995;7:398‐402. [PUBMED: 7576676] [DOI] [PubMed] [Google Scholar]
Hung 2005 {published data only}
- Hung WT, Chen CC, Liou CM, Tsai WY. The effects of low‐dose fentanyl on emergence agitation and quality of life in patients with moderate developmental disabilities. Journal of Clinical Anaesthesia 2005;17:494‐8. [PUBMED: 16297747] [DOI] [PubMed] [Google Scholar]
Ibrahim 2001 {published data only}
- Ibrahim AE, Ghoneim MM, Kharasch ED, Epstein RH, Groudine SB, Ebert TJ, et al. Speed of recovery and side‐effect profile of sevoflurane sedation compared with midazolam. Anesthesiology 2001;94(1):87‐94. [PUBMED: 11135727] [DOI] [PubMed] [Google Scholar]
Ingelmo 2007 {published data only}
- Ingelmo PM, Bendall EJ, Frawley G, Locatelli BG, Milan B, Lodetti D, et al. Bupivacaine caudal epidural anesthesia: assessing the effect of general anesthetic technique on block onset. Pediatric Anesthesia 2007;17(3):255‐62. [PUBMED: 17263741] [DOI] [PubMed] [Google Scholar]
Isik 2006b {published data only}
- Isik Y, Goksu S, Kocoglu H, Oner U. Low flow desflurane and sevoflurane anaesthesia in children. European Journal of Anaesthesiology 2006;23:60‐4. [PUBMED: 16390568] [DOI] [PubMed] [Google Scholar]
Kain 1999 {published data only}
- Kain ZN, Mayes LC, Wang SM, Hofstander MB. Postoperative behavioural outcomes in children: effects of sedative premedication. Anesthesiology 1999;90(3):758‐65. [PUBMED: 10078677] [DOI] [PubMed] [Google Scholar]
Kain 2007 {published data only}
- Kain ZN, Caldwell‐Andrews AA, Mayes LC, Weinberg ME, Wang SM, MacLaren JE, et al. Family‐centered preparation for surgery improves perioperative outcomes in children: a randomized controlled trial. Anesthesiology 2007;106(1):65‐74. [PUBMED: 17197846] [DOI] [PubMed] [Google Scholar]
Kawaai 2008 {published data only}
- Kawaai H, Sato J, Watanabe M, Ito H, Ogawa S, Shimamura K, et al. Dexmedetomidine for postoperative management after sevoflurane anesthesia in children. Journal of Japanese Dental Society of Anesthesiology 2008;36:269‐77. [Google Scholar]
Kawaraguchi 2002 {published data only}
- Kawaraguchi Y, Miyamoto Y, Fukumitsu K, Taniguchi A, Hirao O, Kitamura S, et al. The effect of ketamine on reducing postoperative agitation after sevoflurane anesthesia in pediatric strabismus surgery. Japanese Journal of Anesthesiology [Masui] 2002;51(12):1343‐8. [PUBMED: 12607270] [PubMed] [Google Scholar]
Malmgren 2004 {published data only}
- Malmgren W, Akeson J. Similar excitation after sevoflurane anaesthesia in young children given rectal morphine or midazolam as premedication. Acta Anaesthesiologica Scandinavica 2004;48(10):1277‐82. [PUBMED: 15504188] [DOI] [PubMed] [Google Scholar]
Mayer 2006 {published data only}
- Mayer J, Boldt J, Röhm KD, Scheuermann K, Suttner SW. Desflurane anesthesia after sevoflurane inhaled induction reduces severity of emergence agitation in children undergoing minor ear‐nose‐throat surgery compared with sevoflurane induction and maintenance. Anesthesia and Analgesia 2006;102(2):400‐4. [PUBMED: 16428532] [DOI] [PubMed] [Google Scholar]
Mckay 2011 {published data only}
- Mckay WP, Derdall K, Brahmania M, Nagle CL, Hamilton I, Teekasingh MA. Three adjuncts to anesthesia to prevent emergence agitation in pediatric dentistry; a pilot study. Internet Journal of Anesthesiology 2011;29(1):1. [Google Scholar]
Mizrak 2011 {published data only}
- Mizrak A, Erbagci I, Arici T, Avci N, Ganidagli S, Oner U. Dexmedetomidine use during strabismus surgery in agitated children. Medical Principles and Practice 2011;20(5):427‐32. [PUBMED: 21757931] [DOI] [PubMed] [Google Scholar]
Ozer 2003 {published data only}
- Ozer Z, Görür K, Altunkan AA, Bilgin E, Camdeviren H, Oral U. Efficacy of tramadol versus meperidine for pain relief and safe recovery after adenotonsillectomy. European Journal of Anaesthesiology 2003;20(11):920‐4. [PUBMED: 14649346] [DOI] [PubMed] [Google Scholar]
Piat 1994 {published data only}
- Piat V, Dubois MC, Johanet S, Murat I. Induction and recovery characteristics and hemodynamic responses to sevoflurane and halothane in children. Anesthesia and Analgesia 1994;79:840‐4. [PUBMED: 7978397] [DOI] [PubMed] [Google Scholar]
Sarner 1995 {published data only}
- Sarner JB, Levine M, Davis PJ, Lerman J, Cook R, Motoyama EK. Clinical characteristics of sevoflurane in children—a comparison with halothane. Anesthesiology 1995;82(1):38‐46. [PUBMED: 7832332] [DOI] [PubMed] [Google Scholar]
Shaban 2008 {published data only}
- Shaban MM, Asida SM. Oral midazolam with low dose ketamine, fentanyl, or ketoprofen for the prevention of emergence agitation after pediatric ambulatory surgery. Egyptian Journal of Anaesthesia 2008;24(1):27‐33. [Google Scholar]
Steinmetz 2007 {published data only}
- Steinmetz J, Holm‐Knudsen R, Sorensen MK, Eriksen K, Rasmussen LS. Hemodynamic differences between propofol‐remifentanil and sevoflurane anesthesia for repair of cleft lip and palate in infants. Pediatric Anesthesia 2007;17(1):32‐7. [PUBMED: 17184429] [DOI] [PubMed] [Google Scholar]
Uysal 2011 {published data only}
- Uysal HY, Takmaz SA, Yaman F, Baltaci B, Basar H. The efficacy of intravenous paracetamol versus tramadol for postoperative analgesia after adenotonsillectomy in children. Journal of Clinical Anesthesia 2011;23(1):53‐7. [PUBMED: 21296248] [DOI] [PubMed] [Google Scholar]
Wagner 2003 {published data only}
- Wagner D, Pandit U, Voepel‐Lewis T, Weber M. Dolasetron for the prevention of postoperative vomiting in children undergoing strabismus surgery. Paediatric Anaesthesia 2003;13(6):522‐6. [PUBMED: 12846709] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdelhalim 2013 {published data only}
- Abdelhalim AA, Alarfaj AM. The effect of ketamine versus fentanyl on the incidence of emergence agitation after sevoflurane anesthesia in pediatric patients undergoing tonsillectomy with or without adenoidectomy. Saudi Journal of Anaesthesia 2013;7:392‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abdulatif 2013 {published data only}
- Abdulatif M, Ahmed A, Mukhtar A, Badawy S. The effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesia. Anaesthesia 2013;68:1045‐52. [DOI] [PubMed] [Google Scholar]
Ali 2013 {published data only}
- Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi Journal of Anaesthesia 2013;7:296‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aydin 2013 {published data only}
- Aydin G, Apan A, Kose EA, Oz G. Pre‐ versus postoperative tramadol instillation: both are effective for decreasing pain and/or agitation in pediatric adenotonsillectomy. Turkish Journal of Medical Sciences 2013;43:899‐904. [Google Scholar]
Celebi 2013 {published data only}
- Celebi S, Topak M, Develioglu ON, Caglar E, Yalcin E, Unsel M, et al. Effect of thermal welding tonsillectomy on emergence agitation. Journal of Craniofacial Surgery 2013;24:1844‐8. [DOI] [PubMed] [Google Scholar]
Chandler 2013 {published data only}
- Chandler JR, Myers D, Mehta D, Whyte E, Groberman MK, Montgomery CJ, et al. Emergence delirium in children: a randomized trial to compare total intravenous anesthesia with propofol and remifentanil to inhalational sevoflurane anesthesia. Pediatric Anesthesia 2013;23:309‐15. [DOI] [PubMed] [Google Scholar]
Chen 2013a {published data only}
- Chen J‐Y, Jia J‐E, Liu T‐J, Qin M‐J, Li W‐X. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Canadian Journal of Anesthesia 2013;60:385‐92. [DOI] [PubMed] [Google Scholar]
Chen 2013b {published data only}
- Chen L, Yu L, Fan Y, Manyande A. A comparison between total intravenous anaesthesia using propofol plus remifentanil and volatile induction/maintenance of anaesthesia using sevoflurane in children undergoing flexible fibreoptic bronchoscopy. Anaesthesia and Intensive Care 2013;41:742‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2014 {published data only}
- Cheng X, Huang Y, Zhao Q, Gu E. Comparison of the effects of dexmedetomidine‐ketamine and sevoflurane‐sufentanil anesthesia in children with obstructive sleep apnea after uvulopalatopharyngoplasty: an observational study. Journal of Anaesthesiology and Clinical Pharmacology 2014;30:31‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elgebaly 2013 {published data only}
- Elgebaly AS. Subtenon's lidocaine injection improves emergence agitation after general anaesthesia in paediatric ocular surgery. Southern African Journal of Anaesthesia and Analgesia 2013;19:114‐9. [Google Scholar]
Gai 2013 {published data only}
- Gai C‐A, Zhu Z‐R, Hu Z‐Y, Jiang Y‐L. Clinical comparison of sevoflurane and propofol anesthesia with propofol and remifentanil anesthesia for children with cleft lip and palate repair surgery. National Medical Journal of China 2013;93:1819‐21. [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- Gupta N, Rath GP, Prabhakar H, Dash HH. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. Journal of Neurosurgical Anesthesiology 2013;25:271‐8. [DOI] [PubMed] [Google Scholar]
Hasanein 2013 {published data only}
- Hasanein R, El‐Sayed W. The effect of nebulized lidocaine hydrochloride on emergence from sevoflurane anesthesia in children undergoing tonsillectomy. Egyptian Journal of Anaesthesia 2013;29:351‐6. [Google Scholar]
Hasani 2013 {published data only}
- Hasani A, Gecaj‐Gashi A, Llullaku S, Jashari H. Postoperative analgesia in children after propofol versus sevoflurane anesthesia. Pain Medicine 2013;14:442‐6. [DOI] [PubMed] [Google Scholar]
He 2013 {published data only}
- He L, Wang X, Zheng S, Shi Y. Effects of dexmedetomidine infusion on laryngeal mask airway removal and postoperative recovery in children anaesthetised with sevoflurane. Anaesthesia and Intensive Care 2013;41:328‐33. [DOI] [PubMed] [Google Scholar]
Khalifa 2013 {published data only}
- Khalifa OSM, Hassanin AAM. Melatonin, ketamine and their combination in half doses for management of sevoflurane agitation in children undergoing adenotonsillectomy. Egyptian Journal of Anaesthesia 2013;29:337‐41. [Google Scholar]
Kim 2013a {published data only}
- Kim D, Doo AR, Lim H, Son J‐S, Lee J‐R, Han Y‐J, et al. Effect of ketorolac on the prevention of emergence agitation in children after sevoflurane anesthesia. Korean Journal of Anesthesiology 2013;64:240‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013b {published data only}
- Kim MS, Moon B‐E, Kim H, Lee J‐R, Hemmings HC. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. British Journal of Anaesthesia 2013;110:274‐80. [DOI] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim NY, Kim SY, Yoon HJ, Kil HK. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Medical Journal 2014;55:209‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kodra 2013 {published data only}
- Kodra N, Gani H, Beqiri V, Bedalli F, Naco M, Doko P. Comparison of oral premedication between midazolam and clonidine on children that undergo urology surgery. European Journal of Anaesthesiology 2013;30:157. [Google Scholar]
Li 2013 {published data only}
- Li X, Zhang Y, Zhou M, Xia Q, Li W, Lu Q. The effect of small dose sufentanil on emergence agitation in preschool children following sevoflurane anesthesia for elective repair of unilateral inguinal hernia. Saudi Medical Journal 2013;34:40‐5. [PubMed] [Google Scholar]
Locatelli 2013 {published data only}
- Locatelli BG, Ingelmo PM, Emre S, Meroni V, Minardi C, Frawley G, et al. Emergence delirium in children: a comparison of sevoflurane and desflurane anesthesia using the Paediatric Anesthesia Emergence Delirium scale. Pediatric Anesthesia 2013;23:301‐8. [DOI] [PubMed] [Google Scholar]
Oofuvong 2014 {published data only}
- Oofuvong M, Siripruekpong S, Naklongdee J, Hnookong R, Lakateb C. Comparison of the incidence of emergence agitation between sevoflurane and desflurane after pediatric ambulatory urologic surgery. Journal of the Medical Association of Thailand 2013;96:1470‐5. [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park JH, Lim BG, Kim HZ, Kong MH, Lim SH, Kim NS, et al. Comparison of emergence agitation between sevoflurane/nitrous oxide administration and sevoflurane administration alone in children undergoing adenotonsillectomy with preemptive ketorolac. Korean Journal of Anesthesiology 2014;66:34‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 2013 {published data only}
- Pedersen NA, Jensen AG, Kilmose L, Olsen KS. Propofol‐remifentanil or sevoflurane for children undergoing magnetic resonance imaging? A randomised study. Acta Anaesthesiologica Scandinavica 2013;57:988‐95. [DOI] [PubMed] [Google Scholar]
Rashad 2014 {published data only}
- Rashad MM, Soud DEM. The effect of different drugs on sevoflurane emergence agitation in pediatric patients undergoing hypospadias repair surgery. Egyptian Journal of Anaesthesia 2014;30:123‐7. [Google Scholar]
Rizk 2014 {published data only}
- Rizk SN, Samir EM. Use of ketofol to control emergence agitation in children undergoing adenotonsillectomy. Egyptian Journal of Anaesthesia 2014;30:13‐9. [Google Scholar]
Sethi 2013 {published data only}
- Sethi S, Ghai B, Ram J, Wig J. Postoperative emergence delirium in pediatric patients undergoing cataract surgery—A comparison of desflurane and sevoflurane. Pediatric Anesthesia 2013;23:1131‐7. [DOI] [PubMed] [Google Scholar]
Sheta 2014 {published data only}
- Sheta SA, Al‐Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double‐blinded randomized controlled trial. Pediatric Anesthesia 2014;24:181‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Abbotts 2006
- Sevoflurane product information. Abbotts Pharmacology 2006.
Bajwa 2010
- Bajwa SA, Costi D, Cyna AM. A comparison of emergence delirium scales following general anesthesia in children. Pediatric Anesthesia 2010;20:704‐11. [DOI] [PubMed] [Google Scholar]
Constant 1999
- Constant D, Dubois M, Piat V, Moutard M, McCue M, Murat I. Changes in the electroencephalogram and autonomic cardiovascular activity during induction of anesthesia with sevoflurane compared with halothane in children. Anesthesiology 1999;91:1604‐15. [DOI] [PubMed] [Google Scholar]
Cyna 2006
- Cyna AM, Andrew M, Emmett RS, Middleton P, Simmons SW. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD002251.pub2] [DOI] [PubMed] [Google Scholar]
Dahmani 2010
- Dahmani S, Stany I, Brasher C, Lejeune C, Bruneau B, Wood C, et al. Pharamcological prevention of sevoflurane‐ and desflurane‐related emergence agitation in children: a meta‐analysis of published studies. British Journal of Anaesthesia 2010;104:216‐23. [DOI] [PubMed] [Google Scholar]
Foesel 2001
- Foesel T, Reisch H. Postoperative behavioural changes in children: comparison between halothane and sevoflurane. Paediatric Anaesthesia 2001;11:719‐23. [DOI] [PubMed] [Google Scholar]
Hanallah 1994
- Hannallah RS, Britton JT, Schafer PG, Patel RI, Norden JM. Propofol anaesthesia in paediatric ambulatory patients: a comparison with thiopentone and halothane. Canadian Journal of Anaesthesia 1994;41:12‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics In Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Holzki 1999
- Holzki J, Kretz F. Changing aspects of sevoflurane in paediatric anaesthesia: 1975‐99. Paediatric Anaesthesia 1999;9:283‐6. [DOI] [PubMed] [Google Scholar]
Houck 2005
- Houck CS. Wild Child in the PACU: Update on Emergence Agitation. SPA Pediatric Anesthesiology Meeting. 2005. [http://www.pedsanesthesia.org/meetings/2005winter/man/RC1.pdf]
Johr 2002
- Johr M. Postanaesthesia excitation. Paediatric Anaesthesia 2002;12:293‐5. [DOI] [PubMed] [Google Scholar]
Kain 2004
- Kain Z, Caldwell‐Andrews A, Maranets I, McClain B, Gaal D, Mayes L, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviours. Anaesthesia and Analgesia 2004;99:1648‐54. [DOI] [PubMed] [Google Scholar]
Kuratani 2008
- Kuratani N, Yumiko O. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane. Anesthesiology 2008;109:225‐32. [DOI] [PubMed] [Google Scholar]
Lynch 1998
- Lynch E, Lazor M, Gellis J, Orav J, Goldman L, Marcantonio E. The impact of postoperative pain on the development of postoperative delirium. Anesthesia and Analgesia 1998;86:781‐5. [DOI] [PubMed] [Google Scholar]
Malarbi 2011
- Malarbi S, Stargatt R, Howard K, Davidson A. Characterizing the behavior of children emerging with delirium from general anesthesia. Pediatric Anesthesia 2011;21(9):942‐50. [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Rosen 2013
- Rosen HD, Cravero JP. Research on emergence agitation in children. Canadian Journal of Anesthesia 2013;60:822‐3. [DOI] [PubMed] [Google Scholar]
Sikich 2004
- Sikich N, Lerman J. Development and psychometric evaluation of the paediatric anaesthesia emergence delirium scale. Anesthesiology 2004;100(5):1138‐45. [DOI] [PubMed] [Google Scholar]
Sun 2008
- Sun J, Han N, Wu X. Sevoflurane maintenance in children: a systematic review. Chinese Journal of Evidence‐based Medicine 2008;8:988‐96. [Google Scholar]
Veyckemans 2001
- Veyckemans F. Excitation phenomena during sevoflurane anaesthesia in children. Current Opinion in Anaesthesiology 2001;14:339‐43. [DOI] [PubMed] [Google Scholar]
Vlajkovic 2006
- Vlajkovic G, Sindjelic R. Emergence delirium in children: many questions, few answers. Anesthesia and Analgesia 2007;104(1):84‐91. [DOI] [PubMed] [Google Scholar]
Voepel‐Lewis 2003
- Voepel‐Lewis T, Malviya S, Tait A. A prospective cohort study of emergence agitation in the paediatric postanesthetic recovery. Anesthesia and Analgesia 2003;96:1625‐30. [DOI] [PubMed] [Google Scholar]
Wallin 1975
- Wallin R, Regan B, Napoli M, Stern I. Sevoflurane: a new inhalational anesthetic agent. Anesthesia and Analgesia 1975;54(6):758‐66. [DOI] [PubMed] [Google Scholar]
Wells 1999
- Wells L, Rasch D. Emergence "delirium" after sevoflurane anesthesia: a paranoid delusion?. Anesthesia and Analgesia 1999;88:1308‐10. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Stephens 2008
- Stephens K, Strickland P, Larsson JN, Middleton P, Cyna AM. Sevoflurane versus other general anaesthesia on postoperative behaviour disturbance in children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007084] [DOI] [PMC free article] [PubMed] [Google Scholar]


