Abstract
Background:
Cervical cancer is a malignant neoplasm that originates in the cervix, and it is a leading cause of mortality, with 270,000 deaths every year globally. Of these, 85% occur in developing countries, including Ethiopia. Routine cervical cancer screening and early treatment can prevent up to 80% of cervical cancers. Health professionals are expected to screen for and be screened for cervical cancer. However, there is limited information about the uptake of cervical cancer screening among health professionals in the study area.
Objective:
This study aimed to determine the magnitude of cervical cancer screening uptake and identify its barriers among health professionals.
Methods:
A multicenter cross-sectional study design was conducted among health professionals from December 01 to 30, 2022. A total of 164 respondents were included in the study, and simple random sampling was used to select the respondents. Variables with a p-value of <0.05 at 95% confidence interval (CI) were considered significantly associated with the outcome variable.
Results:
Of the total respondents, 112 (68.3%) were younger than the age of 30 years, with a mean age of 29.4 years ranging from 21 to 45 years. Seventy-nine of the respondents (48.2%) have work experience of 6–10 years, and 103 (62.8%) are nurses in profession. In this study, the magnitude of cervical cancer screening uptake was 28.1% (95% CI: 27.7%–35.6%). Moreover, attitude (adjusted odds ratio [AOR] = 3.3, 95% CI: 2.1–5.1), age at first sexual intercourse (AOR = 2.1, 95% CI: 1.3–3.4), having history of sexually transmitted infections (STIs; AOR = 3.6, 95% CI: 1.5–11.6), knowing someone who had been screened (AOR = 2.9, 95% CI: 1.8–4.8), and cervical cancer screening training (AOR = 1.6, 95% CI: 1.1–2.9) were significantly associated with cervical cancer screening.
Conclusion:
Generally, this study reported that the magnitude of cervical cancer screening uptake was low. The study also indicated that attitude, age at first sexual intercourse, history of STIs, knowing someone who had been screened, and training of cervical cancer screening were independent predictors of uptake of cervical cancer screening.
Keywords: cervical cancer, cervical cancer screening, health professionals, public hospitals
Background
Cervical cancer is the fourth-most frequent cancer in women, with an estimated 570,000 new cases representing 6.6% of all female cancers globally.1 It is also a leading cause of mortality, with 270,000 deaths every year; of these, 85% are in developing countries.2 It is a relatively rare disease in countries that have national screening programs and quality control with appropriate monitoring and evaluation.3 In the United States, deaths from cervical cancer reduced by 50% between 1975 and 2016, due to earlier detection of the cervical cancer.4
Each year, 348 new cases of cervical cancer are diagnosed in sub-Saharan Africa, and 225 women die from the disease per 1,000,000 women, accounting for 22% of all global cervical cancer.5 In Ethiopia, 7000 new cases and 4884 deaths of cervical cancer occur annually.6,7 An annual report compiled by Tikur Anbessa specialized referral hospital showed that cervical cancer accounts for around 30.3% of all cancer cases diagnosed in the hospital.7
Routine cervical cancer screening, and early treatment can prevent up to 80% of cervical cancers if abnormalities are identified at stages when they can be easily treated.8,9 PAP smear test is a screening test that checks the presence of cancer or precancerous cells in the cervix.10 More than 80% of cancers in sub-Saharan Africa are detected at a late stage, which is associated with low survival rates after surgery or radiotherapy; it is also associated with lack of and/or limited treatment modalities, too expensive and inaccessible for many women in low-resource countries, including Ethiopia.11
Studies revealed that women with a diagnosis of cervical cancer experienced physical, psychosocial, financial, and emotional burdens. This is due to surgical morbidity and chemotherapy toxicity, loss of fertility, changes in body image, sexual concerns, and altered relationships.12,13 Radiotherapy as part of treatment has the highest risk of long-term dysfunction of the bladder, bowel, sexual dysfunction, and psychosocial consequences.14 In terms of economy, the study shows that the mean outpatient cost per patient for cervical cancer is $407.2.1,15
Investigating the uptake of cervical cancer screening among health professionals is very important to show the gap in screening and to identify its barriers before investigating it among the community at large. Unless we investigate it among health professionals first and apply all the necessary measures based on the possible findings, it is difficult to say that health professionals have a good level of knowledge, positive attitude, and increased uptake of cervical cancer screening for themselves, which can also help them to increase the knowledge, change the attitude, and enhance the uptake of cervical cancer screening for the community at large to prevent and treat it early when it occurs.
However, there was limited information about the uptake of cervical cancer screening and its barriers among health professionals in the area, and the country at large. Therefore, this study aimed to assess the uptake of cervical cancer screening and its barriers among health professionals working in public hospitals in South Gondar Zone, Northcentral Ethiopia, 2022.
Objective
To determine the magnitude of cervical cancer screening uptake and identify its barriers among female health professionals working in public hospitals, South Gondar Zone, Northcentral Ethiopia, 2022.
Methods
Study design, area, and period
A multicenter cross-sectional study design was conducted among female health professionals working in public hospitals, South Gondar Zone from December 01 to 30, 2022.
Source and study population
All female health professionals working in all public hospitals in South Gondar Zone were the source population, whereas all female health professionals working in the three selected public hospitals were the study population.
Inclusion and exclusion criteria
Female health professionals whose age is ≥20 years were included in the study, whereas those who had a history of cervical cancer, hysterectomy, and who had no history of sexual intercourse were excluded from the study.
Sample size determination and sampling procedure/technique
The sample size (n) was calculated by computer-based Epi Info 7 software using a single-population proportion at 95% confidence interval (CI), with a 5% margin of error, and by assuming the magnitude of cervical cancer screening uptake to be 11.4%.2
Based on this assumption, the sample size (n) for the study was calculated as follows:
where n = the minimum sample size required for the study; Z = standard normal distribution (Z = 1.96) with 95% CI; P = magnitude of cervical cancer screening uptake (11.4% = 0.114); and d = tolerable margin of error (d = 5% = 0.05).
n = 156. Then, by adding a 10% (0.1) nonresponse rate, the final sample size (n) was calculated to be 172 for this study.
Three public hospitals (Debre Tabor comprehensive specialized hospital [DTCSH], Addis Zemen primary hospital, and Mekane-Eyesus primary hospital) were selected among the eight public hospitals (one referral hospital, and seven primary hospitals) purposively for this study. Then, the final sample size was allocated for each hospital proportionally (DTCSH = 114, Addis Zemen = 30, and Mekane-Eyesus = 28). The respondents were also selected using a simple random sampling technique from each hospital.
Dependent variable
Cervical cancer screening uptake.
Independent variables
Sociodemographic factors (age, marital status, educational status, profession, work experience, workplace, and income), knowledge (a total of 10 questions), and attitude of the respondents (with a total of 24 Likert scale questions [strongly disagree = 1, disagree = 2, neutral = 3, agree = 4, and strongly agree = 5]). The total score of Likert scale questions was calculated, and then, the mean score was also computed to determine the level of attitude.
Sexual and reproductive health (RH) factors (early sexual intercourse, multiple sexual partners, sexually transmitted infections [STIs], and oral contraceptive pills).
Environmental factors (working facility, availability of screening service).
Information-related factors (know someone who has been screened).
Operational definitions
Cervical cancer screening uptake
Those respondents who have ever been screened for cervical cancer within the past 5 years were regarded as having cervical cancer screening uptake, whereas those who have never been screened within the past 5 years were regarded as having no screening uptake.16,17
Knowledge
Respondents who had answered ≥70% (≥7/10) of the given knowledge-related questions were said to have good knowledge, whereas those who have answered <70% (≤7/10) of the given knowledge-related questions were said to have poor knowledge.16,17
Attitude
Respondents who scored ≥ the mean score (47.2) of Likert-scale questions that used to assess nurses' attitude were said to have a positive attitude, whereas those who scored < the mean score (47.2) were said to have a negative attitude toward cervical cancer screening.16,17
Data collection tool and procedures
A structured and pretested self-administered questionnaire was used to collect the data. The questionnaire was adapted by reviewing different literatures,2,6,16–19 and it was prepared in English language. The questionnaire contains questions related to sociodemographic characteristics, sexual and RH factors, environmental factors, knowledge, and attitude. Reliability of the tool was also established with a reliability coefficient (Cronbach's alpha score) of 0.82 for knowledge-related questions and 0.86 for standardized Likert-scale questions to assess the attitude of respondents. Before data collection, training was given for both the data collectors and supervisors. Before giving the questionnaire, the data collectors have informed the respondents about the aims/purposes, risks, and possible benefits of the study, the rights and refusals to participate in the study, and the collected information would be kept confidential.
Those who were willing and had signed the informed voluntary consent form were requested to fill out the questionnaire. The data collection was held from December 01 to 30, 2022.
Data quality control, processing, and analysis
Five percent of the questionnaires were pretested in Koladiba primary hospital to assess the reliability, clarity, sequence, consistency, understandability, and the total time that it could take to finish the questionnaire before the actual data collection. Then, the necessary comments and feedback were incorporated in the final tool to improve its quality. Two trained degree nurses were involved in the coordination of the data collection process.
Training was given for both data collectors and supervisors regarding the objective of the study, data collection tools, ways of data collection, checking the completeness of the data collection tool, and how to maintain confidentiality. Proper coding and categorization of data were maintained for the quality of the data to be analyzed.
The collected data were checked for completeness, accuracy, cleaned and coded manually, and then entered into Epi-Data version 4.2. A double data entry was done to check its validity and compare it with the original data, and then exported to Stata version 14 for analysis. Outliers had also been checked, and simple frequencies and cross-tabulations were done for missing values and variables.
A descriptive analysis was conducted to summarize the data, and the final result was interpreted in the form of text, figure, and tables. Binary logistic regression was used to identify the barriers to cervical cancer screening uptake. Bivariate and multivariable analyses were done to see the association between the outcome variable and each independent variable. The assumptions of binary logistic regression were checked, and the goodness of fit was tested by Hosmer–Lemeshow statistic and Omnibus tests.
All variables with a p-value of <0.2 in the bivariate analysis were entered into the final multivariable analysis model to control all possible confounders, and the variables were selected by the enter method. An adjusted odds ratio (AOR) along with 95% CI was estimated to identify the barriers to cervical cancer screening uptake among the respondents using multivariable analysis. Variables with a p-value of <0.05 were considered significantly associated with the outcome variable.
Ethical consideration
Ethical clearance was obtained from Debre Tabor University, College of Health Sciences, Ethics Review Board. All the respondents were informed about the purpose of the study, their right to refuse, and written and signed voluntary consent was obtained from all respondents before data collection. The participants were told that the information obtained from them would be treated with complete confidentiality and would not cause any harm.
Results
Of the total of 172 respondents, 164 were included in the final analysis, giving a response rate of 95.4%.
Sociodemographic characteristics
Of the total respondents, 112 (68.3%) were younger than the age of 30 years, with the mean age of 29.4 years ranging from 21 to 45 years. Moreover, 79 (48.2%) of the respondents have work experience of 6–10 years; and about two-third, 103 (62.8%), are nurses in profession (Table 1).
Table 1.
Sociodemographic Characteristics of the Respondents Working in Public Hospitals in South Gondar Zone, Northcentral Ethiopia, 2022 (n = 164)
| Variables | Category | Frequency | Percentage (%) |
|---|---|---|---|
| Age | <30 | 112 | 68.3 |
| 30–35 | 27 | 16.5 | |
| ˃35 | 25 | 15.2 | |
| Work experience | 1–5 Years | 58 | 35.4 |
| 6–10 Years | 79 | 48.2 | |
| >10 Years | 27 | 16.4 | |
| Profession | Nurse | 103 | 62.8 |
| Midwife | 22 | 13.4 | |
| Others | 39 | 23.8 | |
| Educational status | Diploma | 67 | 40.9 |
| BSc | 91 | 55.5 | |
| MSc | 6 | 3.6 | |
| Husband's educational status | Diploma and below | 9 | 5.5 |
| Degree and above | 155 | 94.5 | |
| Husband's job | Government employee | 123 | 75.0 |
| Private | 41 | 25.0 |
Others, physicians, laboratory technicians, anesthetists, dentists, and radiographers.
Magnitude of cervical cancer screening uptake
In this study, the magnitude of cervical cancer screening uptake among health professionals was 46 (28.1%; 95% CI: 27.7%–35.6%) (Table 2).
Table 2.
Magnitude of Cervical Cancer Screening Uptake Among Health Professionals Working in Public Hospitals in South Gondar Zone, Northcentral Ethiopia, 2022 (n = 164)
| Variables | Category | Distribution of screening uptake |
Cervical cancer screening uptake |
||
|---|---|---|---|---|---|
| No (%) | Yes (%) | No (%) | Yes (%) | ||
| Age | <30 | 85 (75.9) | 27 (24.1) | 85 (72.0) | 27 (58.7) |
| 30–35 | 19 (70.4) | 8 (29.6) | 19 (16.1) | 8 (17.4) | |
| ≥35 | 14 (56.0) | 11 (44.0) | 14 (11.9) | 11 (23.9) | |
| Work experience | 1–5 | 51 (87.9) | 7 (12.1) | 51 (43.2) | 7 (15.2) |
| 6–10 | 57 (72.2) | 22 (27.8) | 57 (48.3) | 22 (47.8) | |
| ≥10 | 10 (37.0) | 17 (63.0) | 10 (8.5) | 17 (37.0) | |
| Profession | Nurse | 69 (67.0) | 34 (33.0) | 69 (58.5) | 34 (73.9) |
| Midwife | 17 (77.3) | 5 (22.7) | 17 (14.4) | 5 (10.9) | |
| Others | 32 (82.1) | 7 (17.9) | 32 (27.1) | 7 (15.2) | |
| Knowledge | Poor knowledge | 65 (85.5) | 11 (14.5) | 65 (55.1) | 11 (23.9) |
| Good knowledge | 53 (60.2) | 35 (39.8) | 53 (44.9) | 35 (76.1) | |
| Attitude | Negative attitude | 61 (78.2) | 17 (21.8) | 61 (51.7) | 17 (37.0) |
| Positive attitude | 57 (66.3) | 29 (33.7) | 57 (48.3) | 29 (63.0) | |
| Age at first sexual intercourse | <20 | 36 (52.9) | 32 (47.1) | 36 (30.5) | 32 (69.6) |
| ≥20 | 82 (85.4) | 14 (14.6) | 82 (69.5) | 14 (30.4) | |
| Use of oral contraceptives | Yes | 48 (65.8) | 25 (34.2) | 48 (40.7) | 25 (54.3) |
| No | 70 (76.9) | 21 (23.1) | 70 (59.3) | 21 (45.7) | |
| History of STI | Yes | 3 (50.0) | 3 (50.0) | 3 (2.5) | 3 (6.5) |
| No | 115 (72.8) | 43 (27.2) | 115 (97.5) | 43 (93.5) | |
| Knowing someone screened | Yes | 30 (57.7) | 22 (42.3) | 30 (25.4) | 22 (47.8) |
| No | 88 (78.6) | 24 (21.4) | 88 (74.6) | 24 (52.2) | |
| Cervical cancer screening training | Yes | 9 (60.0) | 6 (40.0) | 9 (7.6) | 6 (13.0) |
| No | 109 (73.2) | 40 (26.8) | 109 (92.4) | 40 (87.0) | |
Others, physicians, laboratory technicians, anesthetists, dentists, and radiographers.
STI, sexually transmitted infection.
Of those who had been screened, 35 (76.1%) had been screened once, and 43 (93.5%) and 3 (6.5%) had negative and positive results, respectively. In addition, among those who had been screened, the majority, 29 (63.0%), of them did not know the methods of cervical cancer screening.
Barriers of cervical cancer screening uptake
Of the total respondents, 88 (53.7%) had a good level of knowledge. Similarly, 85 (51.8%) also had a positive attitude toward cervical cancer screening practice. On the contrary, the majority of respondents, 149 (90.9%), did not get cervical cancer screening training (Table 3).
Table 3.
Barriers of Cervical Cancer Screening Uptake Among Health Professionals Working in Public Hospitals in South Gondar Zone, Northcentral Ethiopia, 2022 (n = 164)
| Variables | Category | Frequency | Percentage (%) |
|---|---|---|---|
| Knowledge | Poor knowledge | 76 | 46.3 |
| Good knowledge | 88 | 53.7 | |
| Attitude | Negative attitude | 79 | 48.2 |
| Positive attitude | 85 | 51.8 | |
| Age at first sexual intercourse | <20 | 68 | 41.5 |
| ≥20 | 96 | 58.5 | |
| Use of oral contraceptives | Yes | 73 | 44.5 |
| No | 91 | 55.5 | |
| History of STI | Yes | 6 | 3.7 |
| No | 158 | 96.3 | |
| Knowing someone screened | Yes | 52 | 31.7 |
| No | 112 | 68.3 | |
| Cervical cancer screening training | Yes | 15 | 9.1 |
| No | 149 | 90.9 |
Reasons for not being screened for cervical cancer
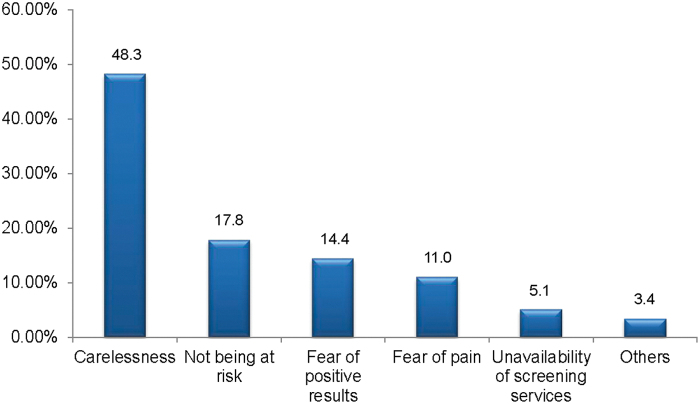
The most common reasons for not being screened were carelessness, 57 (48.3%), and perceiving that they are not at risk, 21 (17.8%) (Fig. 1).
FIG. 1.
Reasons for not being screened for cervical cancer among health professionals working in public hospitals in South Gondar Zone, Northcentral Ethiopia, 2022 (n = 164).
The association between cervical cancer screening uptake and independent variables
A total of nine variables (work experience, profession, knowledge, attitude, age at first sexual intercourse, use of oral contraceptives, history of STI, knowing someone who had been screened, and cervical cancer screening training) were included in the final multivariable analysis. In the multivariable analysis, respondents with a positive attitude toward cervical cancer screening uptake were 3.3 times more likely to be screened than those with a negative attitude (AOR = 3.3, 95% CI: 2.1–5.1).
Likewise, respondents who had first sexual intercourse at the age of <20 years were also 2.1 times more likely to be screened for cervical cancer compared with respondents who had sexual intercourse at the age of 20 years or older (AOR = 2.1, 95% CI: 1.3–3.4). Similarly, respondents with a history of STI were 3.6 times more likely to be screened than those who did not have a history of STI (AOR = 3.6, 95% CI: 1.5–11.6).
Moreover, those respondents who knew someone who had screened for cervical cancer were also 2.9 times more likely to be screened for cervical cancer than those who did not know (AOR = 2.9, 95% CI: 1.8–4.8). Likewise, those respondents who had received cervical cancer screening training were 1.6 times more likely to be screened for cervical cancer compared with respondents who had not received cervical cancer screening training (AOR = 1.6, 95% CI: 1.1–2.9) (Table 4).
Table 4.
Showing the Association Between Independent Variables with Cervical Cancer Screening Uptake Among Health Professionals Working in Public Hospitals in South Gondar Zone, Northcentral Ethiopia, 2022 (n = 164)
| Variables | Category | Uptake |
OR (95% CI) |
p | ||
|---|---|---|---|---|---|---|
| No | Yes | COR | AOR | |||
| Age | <30 | 85 | 27 | 1 | 1 | |
| 30–35 | 19 | 8 | 1.1 (0.7–1.9) | 0.9 (0.5–1.8) | ||
| ≥35 | 14 | 11 | 2.7 (1.6–4.4) | 1.7 (0.8–3.4) | ||
| Work experience | 1–5 | 51 | 7 | 1 | 1 | |
| 6–10 | 57 | 22 | 1.4 (0.9–2.2) | 1.2 (0.7–2.0) | ||
| ≥10 | 10 | 17 | 2.6 (1.5–4.5) | 1.5 (0.7–3.4) | ||
| Profession | Nurse | 69 | 34 | 0.6 (0.4–1.7) | 0.6 (0.3–1.6) | |
| Midwife | 17 | 5 | 0.7 (0.4–1.3) | 0.7 (0.3–1.4) | ||
| Others | 32 | 7 | 1 | 1 | ||
| Level of knowledge | Poor knowledge | 65 | 11 | 1 | 1 | |
| Good knowledge | 53 | 35 | 0.5 (0.3–1.2) | 0.5 (0.3–1.1) | ||
| Level of attitude | Negative attitude | 61 | 17 | 1 | 1 | |
| Positive attitude | 57 | 29 | 3.5 (2.4–5.3) | 3.3 (2.1–5.1) | 0.001 | |
| Age at first sexual intercourse | <20 | 36 | 32 | 1.6 (1.1–2.3) | 2.1 (1.3–3.4) | 0.002 |
| ≥20 | 82 | 14 | 1 | 1 | ||
| Use of oral contraceptives | Yes | 48 | 25 | 1.7 (0.9–1.4) | 1.6 (0.7–1.3) | |
| No | 70 | 21 | 1 | 1 | ||
| History of STI | Yes | 3 | 3 | 2.7 (1.1–6.8) | 3.6 (1.5–11.6) | 0.007 |
| No | 115 | 43 | 1 | 1 | ||
| Knowing someone screened | Yes | 30 | 22 | 4.3 (2.9–6.3) | 2.9 (1.8–4.8) | 0.001 |
| No | 88 | 24 | 1 | 1 | ||
| Cervical cancer screening training | Yes | 9 | 6 | 1.5 (1.2–3.2) | 1.6 (1.1–2.9) | 0.001 |
| No | 109 | 40 | 1 | 1 | ||
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odd ratio.
Discussion
Investigating the uptake of cervical cancer screening among health professionals is very important to show the gap of screening service utilization and its barriers before investigating it among the community at large. Unless we investigate it among professionals and implement all the necessary measures based on the possible findings to scale-up the uptake of cervical cancer screening by intervening at the barriers, such as changing their level of knowledge and attitude and providing cervical cancer screening training, we will not be able to scale-up the uptake of cervical cancer screening.
This study revealed that the magnitude of cervical cancer screening uptake among health professionals was 28.1%. The study also found that attitude toward cervical cancer screening uptake, age at first sexual intercourse, history of STI, knowing someone who had been screened for cervical cancer, and training of cervical cancer screening were significantly associated with the uptake of cervical cancer screening.
In this study, the magnitude of cervical cancer screening uptake among health professionals was 28.1%. This finding is lower than the Federal Ministry of Health-Ethiopia, National cervical cancer prevention strategic plan (80%).20 However, it was higher than the studies conducted among health professionals in Saudi Arabia (20.6%),21 Uganda (19%),22 Mekele city (10.7%),16 Southern Ethiopia (11.4%),2 and Addis Ababa (17%).23 The reason for the difference in cervical cancer rates could be because of the varying study periods and the amount of information provided through different media channels. The government is taking notice of this and is creating national policies and strategies to prevent and control cervical cancer.
This study showed that those respondents who had a positive attitude were 3.26 times more likely to be screened compared with those who had a negative attitude toward cervical cancer screening. This finding is similar to studies conducted in Nepal,18 Mekele,16 and Debre Markos.23 This might be due to the fact that having a positive attitude toward cervical cancer screening may trigger or initiate for cervical cancer screening uptake.
This study also indicated that respondents who had first sexual intercourse at the age of <20 years were 2.1 times more likely to be screened for cervical cancer compared with respondents who had sexual intercourse at the age of 20 years or older. This study is in line with studies conducted in Montero,8 Rwanda,24 Japan,25 and India.26 The reason could be some people might be at a higher risk of sexually transmitted infections (STIs) due to engaging in sexual activity at an early age without being married. This can lead to having multiple sexual partners and an increased likelihood of contracting an STI. As a result, these individuals may choose to get screened for STIs.
Similarly, this study indicated that respondents with a history of STIs were 3.6 times more likely to be screened than those who did not have. This finding is supported by studies conducted in India, Turkey, and Debre Markos, Ethiopia.18,21,27 This might be due to the fact that respondents who had been exposed to STI and become symptomatic are more likely to receive medical care, and by being in the medical care system, their likelihood of screening would be higher. On the contrary, this study showed that those respondents who knew someone who had been screened were also 2.9 times more likely to be screened for cervical cancer than those who did not know. This report is similar to studies conducted in Uganda9 and Debre Markos.18 This might be due to information sharing, which might have, in turn, triggered them to be screened.
Moreover, this study reported that those respondents who received cervical cancer screening training were 40% more likely to be screened for cervical cancer; this might be due to the fact that the training could have changed the attitude, knowledge toward cervical cancer screening, and the magnitude of uptake among the health professionals easily.
Limitations of the study
Since the design was cross-sectional, it cannot be used to analyze the uptake over a period of time. In addition, the respondents might also be subjected to recall and social desirability biases.
Conclusion
Generally, the findings of this study reported that the magnitude of cervical cancer screening uptake among female health professionals was low. The study also indicated that attitude toward cervical cancer screening, age at first sexual intercourse, history of STI, knowing someone who had been screened for cervical cancer, and cervical cancer screening training were independent predictors of cervical cancer screening uptake among female health professionals working in South Gondar Zone public hospitals.
Recommendations
-
(1)
To detect and manage cervical cancer early, the Ministry of Health, Amhara Regional Health Bureau, Zonal Health Department, nongovernmental organizations (NGOs), and other responsible stakeholders must increase their emphasis on cervical cancer screening uptake among female health professionals, and the community at large.
-
(2)
Those female health professionals shall improve their knowledge, attitude, and uptake of cervical cancer screening to detect and manage cervical cancer at the early stage.
-
(3)
Cervical cancer screening experts and mass media shall strengthen their roles to make health professionals, and the community at large, to be well informed about cervical cancer and its screening.
-
(4)
Other researchers should conduct further studies using different tools and study designs to show the gaps clearly and identify additional factors.
Consent for Publication
Not applicable.
Data Availability
All the data used for the study were included in the article.
Acknowledgments
First and foremost, we would like to extend our deepest gratitude to Debre Tabor University, College of Health Sciences, for giving us the opportunity to conduct this study. Second, we would like to thank the hospital administrators for their permission and unlimited support. Last, we also give our heartfelt thanks to all the health professionals who have participated in this study.
Abbreviations Used
- AOR
adjusted odds ratio
- CI
confidence interval
- DTCSH
Debre Tabor comprehensive specialized hospital
- RH
reproductive health
- STI
sexually transmitted infection
Authors' Contributions
T.M.A. wrote the research proposal, conducted the study, and did the data entry and analysis. Y.T.K. was involved in data entry and analysis. S.D.K. was involved in proposal development and data analysis.
Author Disclosure Statement
We declare that there are no conflicts of interest.
Funding Information
No funding was received for this article.
Cite this article as: Aytenew TM, Kassie YT, Kebede SD (2024) Uptake of cervical cancer screening and its barriers using health belief model among health professionals working in public hospitals in South Gondar Zone, northcentral Ethiopia: multicenter cross-sectional study, Women's Health Reports 5:1, 152–160, DOI: 10.1089/whr.2023.0030.
References
- 1. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2018;68(4):297–316; doi: 10.3322/caac.21446 [DOI] [PubMed] [Google Scholar]
- 2. Dulla D, Daka D, Wakgari N. Knowledge about cervical cancer screening and its practice among female health care workers in southern Ethiopia: A cross-sectional study. Int J Womens Health 2017;9:365–372; doi: 10.2147/IJWH.S132202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi H, Fang X, Li Y, et al. High expression of serine hydroxymethyltransferase 2 indicates poor prognosis of gastric cancer patients. Med Sci Monit 2019;25:7430; doi: 10.12659/MSM.917435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73(1):17–48; doi: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 5. Loke AY, Chan ACO, Wong YT. Facilitators and barriers to the acceptance of human papillomavirus (HPV) vaccination among adolescent girls: A comparison between mothers and their adolescent daughters in Hong Kong. BMC Res Notes 2017;10(1):1–13; doi: 10.1186/s13104-017-2734-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heena H, Durrani S, AlFayyad I, et al. Knowledge, attitudes, and practices towards cervical cancer and screening amongst female healthcare professionals: A cross-sectional study. J Oncol 2019;2019:5423130; doi: 10.1155/2019/5423130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abate SM. Trends of cervical cancer in Ethiopia. Cervical Cancer 2015;1(1):1–4; doi: 10.4172/2475-3173.1000103 [DOI] [Google Scholar]
- 8. Legemaat M, Carr PJ, van Rens RM, et al. Peripheral intravenous cannulation: Complication rates in the neonatal population: A multicenter observational study. J Vasc Access 2016;17(4):360–365; doi: 10.5301/jva.50005 [DOI] [PubMed] [Google Scholar]
- 9. Ndejjo R, Mukama T, Musabyimana A, et al. Uptake of cervical cancer screening and associated factors among women in rural Uganda: A cross sectional study. PLoS One 2016;11(2):e0149696; doi: 10.1371/journal.pone.0149696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Web Annex A. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention. World Health Organization; 2021. Available from: https://europepmc.org/books/n/who342365/?extid=23762963&src=med [Last accessed: January 30, 2023]. [PubMed]
- 11. Abate Z, Woldemariam W. Mathiwos Wondu-Yeethiopia Cancer Society role in implementation of community-based cervical cancer control projects across three regions in Ethiopia. J Glob Oncol 2018;4:144; doi: 10.1200/jgo.18.18600 [DOI] [Google Scholar]
- 12. Westin SN, Bustillos D, Gano JB, et al. Social factors affecting treatment of cervical cancer: Ethical issues and policy implications. Obstet Gynecol 2008;111(3):747–751; doi: 10.1097/AOG.0b013e318165f1a9 [DOI] [PubMed] [Google Scholar]
- 13. Tabano M, Condosta D, Coons M. Symptoms affecting quality of life in women with gynecologic cancer. Semin Oncol Nurs 2002;18(3):223–230; doi: 10.1053/sonu.2002.34084 [DOI] [PubMed] [Google Scholar]
- 14. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: Long-term quality of life and social support. Clin Ther 2015;37(1):39–48; doi: 10.1016/j.clinthera.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hailu A, Mariam DH. Patient side cost and its predictors for cervical cancer in Ethiopia: A cross sectional hospital based study. BMC Cancer 2013;13:1–8; doi: 10.1186/1471-2407-13-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gebreegziabher M, Asefa NG, Berhe S. Factors affecting the practices of cervical cancer screening among female nurses at public health institutions in Mekelle town, Northern Ethiopia, 2014: A cross-sectional study. J Cancer Res 2016;2016:1–7; doi: 10.1155/2016/4743075 [DOI] [Google Scholar]
- 17. Nigussie T, Admassu B, Nigussie A. Cervical cancer screening service utilization and associated factors among age-eligible women in Jimma town using health belief model, South West Ethiopia. BMC Womens Health 2019;19(1):1–10; doi: 10.1186/s12905-019-0826-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jassim G, Obeid A, Al Nasheet HA. Knowledge, attitudes, and practices regarding cervical cancer and screening among women visiting primary health care centres in Bahrain. BMC Public Health 2018;18(1):1–6; doi: 10.1186/s12889-018-5023-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Javaeed A, Shoukat S, Hina S, et al. Knowledge, attitude, and practices related to cervical cancer among adult women in Azad Kashmir: A hospital-based cross-sectional study. Cureus 2019;11(3):e4234; doi: 10.7759/cureus.4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Federal Ministry of Health Ethiopia. National Cancer Control Plan 2016–2020. Addis Ababa Disease Prevention and Control Directorate, Editor Directorate dpac. 2015. Available from: https://NCCP Ethiopia Final 261015.pdf (iccp-portal.org) [Last accessed: February 30, 2023].
- 21. Ifemelumma C, Anikwe C, Okorochukwu B, et al. Cervical cancer screening: Assessment of perception and utilization of services among health workers in low resource setting. Int J Reprod Med 2019;2019:6505482; doi: 10.1155/2019/6505482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mutyaba T, Mmiro FA, Weiderpass E. Knowledge, attitudes and practices on cervical cancer screening among the medical workers of Mulago Hospital, Uganda. BMC Med Educ 2006;6(1):1–4; doi: 10.1186/1472-6920-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asseffa NA. Cervical cancer: Ethiopia's outlook. J Gynecol Womens Health 2017;5(2):555660; doi: 10.19080/JGWH.2017.05.555660 [DOI] [Google Scholar]
- 24. Makuza JD, Nsanzimana S, Muhimpundu MA, et al. Prevalence and risk factors for cervical cancer and pre-cancerous lesions in Rwanda. Pan Afr Med J 2015;22(1):26; doi: 10.11604/pamj.2015.22.26.7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaneko N. Factors associated with cervical cancer screening among young unmarried Japanese women: Results from an internet-based survey. BMC Womens Health 2018;18:1–9; doi: 10.1186/s12905-018-0623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oba S, Toyoshima M, Ogata H. Association of cervical cancer screening with knowledge of risk factors, access to health related information, health profiles, and health competence beliefs among community-dwelling women in Japan. Asian Pac J Cancer Prev 2017;18(8):2115; doi: 10.22034/APJCP.2017.18.8.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rahman H, Kar S. Knowledge, attitudes and practice toward cervical cancer screening among Sikkimese nursing staff in India. Indian J Med Paediatr Oncol 2015;36(2):105–110; doi: 10.4103/0971-5851.158840 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used for the study were included in the article.