Abstract
Background
Cervical cancer remains a threat to female health due to high mortality. Clarification of the long-term trend of survival rate over time and the associated risk factors would be greatly informative to improve the prognosis of cervical cancer patients.
Methods
This retrospective study was based on data extracted from the Surveillance, Epidemiology, and End Results (SEER) database of the United States. The 3-year and 5-year overall survival rates of patients with cervical cancer during 2002–2006, 2007–2011, and 2012–2016 were analyzed. Period analysis was used to assess the variation in survival rate stratified by age, race, and socioeconomic status during the 15-year study period and then predicted the relative survival rate in the following period from 2017 to 2021.
Results
During 2002–2016, the 3-year relative survival rate of cervical cancer patients increased from 73.1% to 73.5% with a high jump between 2007 and 2011. This upward trend is expected to continue to 74.3% between 2017 and 2021. Patients older than 60 years, black ethnicity, or medium and high poverty status were likely to have a lower relative survival rate.
Conclusion
This study confirmed the increased relative survival rate of cervical cancer patients over years and identified relevant risk factors. Targeted initiatives for elderly and socially underprivileged individuals may be able to mitigate inequality.
Keywords: cervical cancer; Surveillance, Epidemiology, and End Results database; period analysis; survival rates; prediction
Plain language summary
Why was the study conducted? Cervical cancer is one of the most common cancers endangering global women’s health. Although there are currently relevant screening methods and vaccines, cervical cancer still leads to a higher risk of death in infected women and poses a serious threat to women’s health. Therefore, it would be informative for future policy making if the risk factors affecting prognosis were assessed and the trend of long-term survival rate of patients with cervical cancer over time was predicted.
What did the researchers do? We extracted data on cervical cancer patients from the Surveillance, Epidemiology, and End Results (SEER) database between 2002 and 2016 and used a model-based period analysis to assess the characteristics of the 3- and 5-year relative survival rates of cervical cancer patients stratified by age, race, and socioeconomic status. The relative survival rate for the period from 2017 to 2021 was projected.
What did the researchers find? Our study found that the 3-year relative survival rate for cervical cancer patients increased from 73.1% to 73.5% between 2002 and 2016, with a jump between 2007 and 2011. Patients older than 60 years, those of black ethnicity, or those with medium and high poverty status were more likely to have a low relative survival rate.
What do the findings mean? Our study confirms that the relative survival rate of cervical cancer patients has increased in recent years and has maintained an overall upward trend. Our findings suggest that age, race, and socioeconomic status are relevant risk factors. These findings would help us to predict future trends, better allocate medical resources, and optimize health policies to improve the prognosis of cervical cancer, such as targeting the elderly and other vulnerable groups.
Introduction
Cervical cancer ranks the fourth most common cancer in women worldwide, with an estimated 604,000 new cases and 342,000 deaths in 2020, 1 with a worse disease burden in some medical resource-limited countries. 2 In 2018, the World Health Organization called for action including scaling up preventive, screening, and treatment interventions toward achieving the global elimination of cervical cancer, 3 which requires baseline data on the survival status to inform the determination of cervical cancer prevention strategy.
The United States has implemented large-scale cervical cancer screening programs for almost half a century, and mortality rates have declined quite rapidly in the United States. 4 In addition to the introduction of the HPV vaccine against human papillomavirus (HPV) infection, organized cervical screening for precancerous and cancerous lesions has been implemented. The updated recommendation of screening for cervical cancer from the United States Preventive Services Task Force (USPSTF) refers to screening for cervical cancer every 3 years with cervical cytology alone in women aged 21–29 years, every 3 years with cervical cytology alone, every 5 years with HPV testing along with high-risk HPV testing along with cytology (co-testing) in women aged 30–65 years. 5
Precise monitoring of long-term patterns in cervical cancer survival rates and identification of risk factors associated with cervical cancer survival outcomes would favor the elimination of cervical cancer. 6 In addition to high-risk HPV persistent infection, multiple sociodemographic factors such as age, race, socioeconomic status, different geographic regions, early sexual initiation, number of sexual partners, and availability and quality of cytology tests may also affect the survival of cervical cancer patients.7,8 Long-term survival is a key prognostic indicator in monitoring the effectiveness of cancer treatment and management strategies and is highly informative for the improvement of clinical interventions for patient treatment and care. 9 Period analysis is a new and effective method for assessing long-term survival and stratifying newly diagnosed cancer patients by social determinants, cancer sites, and histopathological types.10,11
In our study, we used period analysis to evaluate 3- and 5-year long-term relative survival rates of cervical cancer patients based on the SEER database, aiming to explore the changing trend of the long-term survival rate of cervical cancer and relevant risk factors.
Methods
Data Source
This study is retrospective, and the data were obtained from the Incidence-SEER Research Data, 18 registries, Nov 2019 Sub (2000–2017) in the SEER database. This database assembles and reports well-qualitied data on cancer cases from 18 states representing approximately 27.8% of the US population. 11 The SEER database is sponsored by the National Cancer Institute and is the only well-qualified population-based cancer registry in the US, including the most comprehensive data on cancer incidence, treatment survival, demographics, and socioeconomic characteristics. 12
SEER*Stat software (version 8.3.9) was used to extract data from patients diagnosed with ICD-O-3 (site codes C53.0–53.9 and histology codes 8010–8671 and 8940–8941 during 2002–2016, restrict to females and microscopically confirmed) between 2002 and 2016. 13 Cases of cervical cancer were included if they were aged 20–100 years, and the time at diagnosis was between 2002 and 2016. Cases were excluded if cancers were reported through only a death certificate or autopsy, lacked any follow-up information, had uncertain/invalid date at diagnosis, were of unclear race, or had unclear socioeconomic status (Supplementary Figure 1).
This study conforms to the principles outlined in the 1964 Declaration of Helsinki and its subsequent amendments. Approval was waived by the local ethics committee because SEER data are publicly available and de-identified.
Data Sorting and Variable Selection
Variables extracted from the SEER database included birth date, diagnostic date, last follow-up date, vital status, race, and socioeconomic status. Age at diagnosis was divided into six groups: 20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years. The cases were stratified by race into three groups: whites, blacks, and other races (American Indian/AK Native and Asian/Pacific Islander). Socioeconomic status was determined by the family poverty rate in the residing area, which is the percentage of families living below the national poverty line as measured by the U.S. Census Bureau. 14 In this study, the patients were divided into four groups stratified by socioeconomic status: rich (<5.34%), low-poverty (≥ 5.34% and < 7.70%), medium-poverty (≥ 7.70% and < 12.69%), and high-poverty (≥ 12.69%) areas. The diagnosis time was divided into three independent observation periods: 2002–2006, 2007–2011, and 2012–2016.
Statistical Analysis
The relative survival rate of cervical cancer patients was estimated using the data of the existing complete cancer registration system, and the variation trend was analyzed. Relative survival refers to the ratio of the proportion of observed survivors in a cohort to the proportion of expected survivors, reflecting the probability of survival from the specific cancer rather than the overall probability of survival. This parameter considers the expected non-cancer deaths, thus minimizing the effects of age and loss of follow-up on survival.15–17 The relative survival rate can be expressed as
In the above formula, and represent the observed and expected survival rates, respectively. 18
The relative survival rate of cervical cancer patients during 2002–2006, 2007–2011, and 2012–2016 was estimated by period analysis based on a generalized linear model. We used this model to predict the 3- and 5-year relative survival rates for patients diagnosed in 2017–2021. All analyses were performed using the PeriodR software package.
Results
General Characteristics of Cervical Cancer Patients
The baseline characteristics of cervical cancer cases registered in the SEER database during the three observation periods are listed in Table 1. During 2002–2016, 23,121 cervical cancer patients were included in this study, and the absolute number of cases tended to stably decrease over the 15-year study period, from 8488 cases in the first five years to 7793 cases in the second five years, and then to 6840 cases in the third five years. The age at diagnosis is mostly between 30 and 59 years for each period. The number of cases remarkably varied by race, with far more white than black and other races. In stratified socioeconomic status subgroups, a heavy burden of cases was observed in poverty, particularly in high poverty.
Table 1.
Basic Characteristics Related With Cervical Cancer From Year 2002 to 2016 in SEER Database.
| Characteristics | Absolute Cases and Proportions | ||
|---|---|---|---|
| 2002–2006 n (%) | 2007–2011 n (%) | 2012–2016 n (%) | |
| Overall | 8488 | 7793 | 6840 |
| Age (years) | |||
| 20–29 | 454 (5.3) | 401 (5.1) | 364 (5.3) |
| 30–39 | 1788 (21.1) | 1615 (20.7) | 1333 (19.5) |
| 40–49 | 2276 (26.8) | 2007 (25.8) | 1655 (24.2) |
| 50–59 | 1676 (19.7) | 1582 (20.3) | 1522 (22.3) |
| 60–69 | 1069 (12.6) | 1111 (14.3) | 1065 (15.6) |
| 70+ | 1225 (14.4) | 1077 (13.8) | 901 (13.2) |
| Race | |||
| White | 6310 (74.3) | 5741 (73.7) | 4894 (71.5) |
| Black | 1042 (12.3) | 974 (12.5) | 866 (12.7) |
| Other races | 1136 (13.4) | 1078 (13.8) | 1080 (15.8) |
| Socioeconomic status | |||
| Rich | 1814 (21.4) | 1754 (22.5) | 1518 (22.2) |
| Low poverty | 1834 (21.6) | 1809 (23.3) | 1700 (24.9) |
| Medium poverty | 1467 (17.3) | 1315 (16.9) | 1183 (17.3) |
| High poverty | 3373 (39.7) | 2915 (37.4) | 2439 (35.7) |
Note. Not all columns under the same category add up to 100% because of rounding.
Changing Trend in the Survival Rate by Age Group
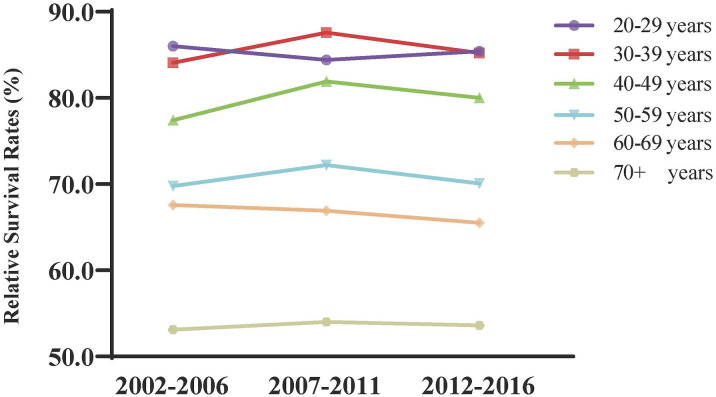
The 3- and 5-year relative survival rates of cervical cancer patients are shown in Table 2, and changing trends are presented in Figure 1. The 3-year and 5-year relative survival rates of cervical cancer patients tended to increase from 2002 to 2016, especially with a sharp jump between 2007 and 2011. Generalized linear modeling predicted that this mounting pattern will continue between 2017 and 2021, with 3- and 5-year survival rates of 74.3% and 64.1%, respectively. Additionally, as the age at diagnosis increased, the relative survival rate of patients decreased. For instance, during the years 2002–2006, the 3-year survival rate fell from 86.0% in 20–29 years to 53.1% in 70 years and older group. Despite an overall improvement in the survival rate over the past 15 years, age-specific improvements in the survival rate are clearly inequitable, pointing to the need to transfer advanced medical inventions to older patients.
Table 2.
3-Year and 5-Year Relative Survival Rates of Cervical Cancer Patients by Age Groups From 2002 to 2016 and Prediction of the Relative Survival Rates of Cervical Cancer Patients by Age Groups From 2017 to 2021.
| Age Groups (year) | Survival Rates | |||
|---|---|---|---|---|
| 2002–2006 (%) | 2007–2011 (%) | 2012–2016 (%) | 2017–2021 (%) | |
| 3 year Survival | ||||
| Total | 73.1 ± 0.6 | 75.5 ± 0.5 | 73.5 ± 0.5 | 74.3 |
| 20–29 | 86.0 ± 1.9 | 84.4 ± 1.8 | 85.4 ± 1.8 | 85.2 |
| 30–39 | 84.1 ± 1.1 | 87.6 ± 0.8 | 85.2 ± 1.0 | 87.7 |
| 40–49 | 77.4 ± 1.1 | 81.9 ± 0.9 | 80.0 ± 1.0 | 83.3 |
| 50–59 | 69.8 ± 1.4 | 72.2 ± 1.2 | 70.1 ± 1.2 | 72.0 |
| 60–69 | 67.6 ± 1.8 | 66.9 ± 1.5 | 65.5 ± 1.5 | 65.2 |
| 70+ | 53.1 ± 2.0 | 54.0 ± 1.8 | 53.6 ± 1.9 | 47.3 |
| 5-year survival | ||||
| Total | 65.9 ± 0.9 | 70.1 ± 0.6 | 67.6 ± 0.6 | 64.1 |
| 20–29 | 85.5 ± 2.0 | 82.3 ± 1.9 | 82.4 ± 2.0 | 80.2 |
| 30–39 | 77.9 ± 1.7 | 84.6 ± 0.9 | 81.3 ± 1.1 | 81.0 |
| 40–49 | 69.5 ± 1.7 | 77.2 ± 1.0 | 74.9 ± 1.1 | 78.4 |
| 50–59 | 58.8 ± 2.3 | 64.0 ± 1.3 | 61.5 ± 1.3 | 60.0 |
| 60–69 | 61.3 ± 2.6 | 59.9 ± 1.6 | 59.2 ± 1.6 | 53.9 |
| 70+ | 47.3 ± 2.8 | 46.9 ± 1.9 | 45.5 ± 2.0 | 34.1 |
Note. Data are means ± standard error of the means.
Figure 1.
Trend and prediction of survival rate in patients with cervical cancer of different ages.
Changing Trends in Survival Rate by Race
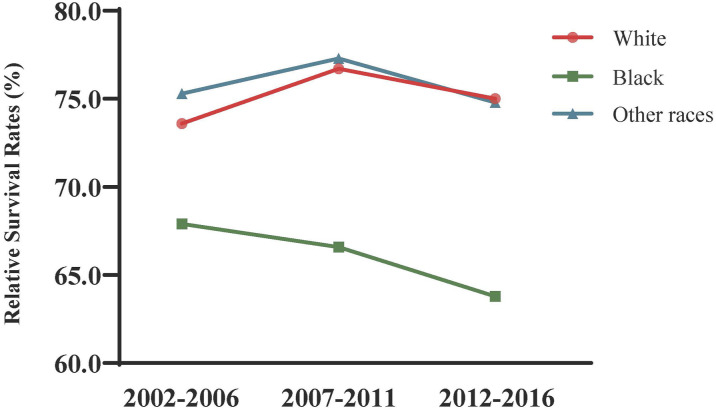
The relative survival of patients with cervical cancer varied by race (Table 3). Race-specific changing trends in the survival rate over years are displayed in Figure 2. Black American patients had worse 3- and 5-year relative survival rates from 2002 to 2016 than those among white patients and other races. In addition, the gap in the survival rate of cervical cancer between black Americans and white Americans widened over time from a 3-year survival rate of 5.7% in 2002–2006, to 10.1% in 2007–2011, and up to 11.2% in 2012–2016. This predicted gap in 2017–2021 was even wider, reaching 14.4% in the 3-year survival rate, with 76.5% in white Americans and 62.1% in black Americans. In contrast to the rising survival rate among white Americans, no obvious improvements were observed in black Americans.
Table 3.
3-Year and 5-Year Relative Survival Rates of Cervical Cancer Patients by Race From 2002 to 2016 and Prediction of the Relative Survival Rates of Cervical Cancer Patients by Race From 2017 to 2021.
| Race | Survival Rates | |||
|---|---|---|---|---|
| 2002–2006 (%) | 2007–2011 (%) | 2012–2016 (%) | 2017–2021 (%) | |
| 3 year Survival Rates | ||||
| White | 73.6 ± 0.7 | 76.7 ± 0.6 | 75.0 ± 0.7 | 76.5 |
| Black | 67.9 ± 1.8 | 66.6 ± 1.6 | 63.8 ± 1.6 | 62.1 |
| Other races | 75.3 ± 1.7 | 77.3 ± 1.4 | 74.8 ± 1.4 | 74.1 |
| 5 year survival rates | ||||
| White | 67.0 ± 1.0 | 71.5 ± 0.6 | 69.5 ± 0.7 | 66.5 |
| Black | 59.1 ± 2.7 | 59.6 ± 1.7 | 55.9 ± 1.7 | 51.1 |
| Other races | 66.2 ± 2.8 | 72.1 ± 1.5 | 68.3 ± 1.5 | 63.8 |
Note. Survival rates is relative survival rates; data are means ± standard error of the means.
Figure 2.
Trend and prediction of survival rate in patients with cervical cancer by different races.
Changing Trends in Survival Rates by Socioeconomic Status
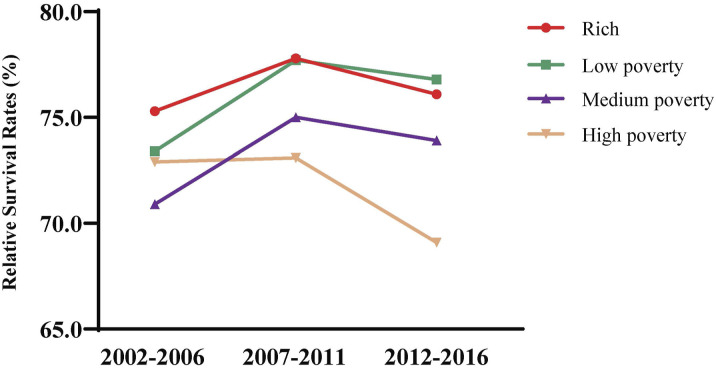
The significant impact of socioeconomic status on the 3- and 5-year relative survival rates of cervical cancer patients is shown in Table 4, and the changing trends over time are displayed in Figure 3. In all periods from 2007 to 2016, the improvement in socioeconomic status was positively related to a gradual increase in the relative survival rate. Patients in poverty status, particularly those with high poverty, were prone to have lower 3- and 5-year relative survival rates than those in superior economic status. However, the survival rate of all patients, except those in high poverty status, has improved during 2012-2016 compared to the period of 2002-2006. Accordingly, the predicted relative survival rates of cervical cancer patients in rich status were 76.4% for 3-year survival and 67.2% for 5-year survival, whereas those in high-poverty areas were 68.8% for 3-year survival and 58.5% for 5-year survival.
Table 4.
3-Year and 5-Year Relative Survival Rates of Cervical Cancer Patients by Socioeconomic Status From 2002 to 2016 and Prediction of the Relative Survival Rates of Cervical Cancer Patients by Socioeconomic Status From 2017 to 2021.
| Socioeconomic Status | Survival Rates | |||
|---|---|---|---|---|
| 2002–2006 (%) | 2007–2011 (%) | 2012–2016 (%) | 2017–2021 (%) | |
| 3 year Survival Rates | ||||
| Rich | 75.3 ± 1.3 | 77.8 ± 1.1 | 76.1 ± 1.1 | 76.4 |
| Low poverty | 73.4 ± 1.3 | 77.7 ± 1.0 | 76.8 ± 1.0 | 78.6 |
| Medium poverty | 70.9 ± 1.5 | 75.0 ± 1.3 | 73.9 ± 1.3 | 75.7 |
| High poverty | 72.9 ± 1.0 | 73.1 ± 0.9 | 69.1 ± 1.0 | 68.8 |
| 5 year survival rates | ||||
| Rich | 70.4 ± 1.9 | 72.6 ± 1.2 | 70.3 ± 1.2 | 67.2 |
| Low poverty | 65.2 ± 2.1 | 72.4 ± 1.1 | 72.0 ± 1.1 | 68.6 |
| Medium poverty | 63.4 ± 2.0 | 68.7 ± 1.4 | 67.1 ± 1.4 | 64.7 |
| High poverty | 65.1 ± 1.5 | 67.7 ± 0.9 | 62.6 ± 1.0 | 58.5 |
Note. data are mean ± standard error of the mean.
Figure 3.
Trend and prediction of survival rate in patients with cervical cancer by different socioeconomic status.
Discussion
Period analysis allows a more comprehensive assessment of long-term progress in cancer prognosis from a perspective view. 19 Our findings based on the SEER database indicated that the relative survival rate of cervical cancer increased over time within the three observation periods from 2002 to 2016, which may benefit from the rapid progress of medical prevention and treatment technologies, the enhancement of health awareness, the continuously deepening understanding of pathogenesis, and the implementation of large-scale cervical cancer screening programs. These variations in survival rates were closely dependent on the age at diagnosis of patients and social determinants of health framework, including race and socioeconomic status.
The relative survival rate of cervical cancer patients decreased with age in all three observation periods in our study, suggesting that age at diagnosis was one of the key risk factors for cancer prognosis. This finding was consistent with previous research,20,21 which added that older patients had lower survival rates than younger patients. The aging immune system against the relapse of cervical cancer among older patients might partly explain their lower survival rate. 22 In addition, some elderly cancer patients are reluctant to accept aggressive treatment because of the toxicity of radiotherapy and chemotherapy. 20 The inability of older women to adhere to screening recommendations for breast cancer is also a challenging issue. 23 Compared with young women, older women are more reluctant to attend a cervical smear screening; therefore, once cervical cancer is diagnosed, older women are more likely to be found at an advanced stage and die from this cancer within three years of diagnosis. 24 Consequently, elderly patients have the lowest survival rate with the least improvement.
Black women were found to have lower survival rates of cervical cancer than whites and other races in our study. These findings may indicate the inequity of social health determinants, partly because of the lower access of Black women to health care, including screening programs and timely diagnosis and treatment, than White women.25,26 It was reported that Black women had higher odds of receiving a diagnosis of advanced stage cervical cancer than White women. 27 Moreover, barriers other than access to health care need to be identified because Blacks are reported to be significantly less likely to receive surgical resection compared with whites, even with apparently identical health care coverage.28,29 Ethnicity (i.e., Hispanic vs non-Hispanic), incorporating races, might be more informative in analyzing the complex effect of social determinants of health framework on the survival of cervical cancer patients.
In addition, socioeconomic factors influenced the survival rate of patients with cervical cancer. Our study found that socioeconomic status was negatively associated with survival rate, which was consistent with the findings of Mundt et al 30 and Morgan et al. 31 This association is partly due to inferior treatment opportunities from economic barriers to groups with lower socioeconomic status. 32 Patients in poverty may have worse survival outcomes owing to challenges in medical and health care services, a lack of social aid, and delayed medical treatment. Patients may also have lower levels of education and an inability to grasp medical knowledge.33,34
The findings in this study, based on the American Cancer Research database SEER’s extensive sample of representative American cancer patients from different regions of the United States, may be extrapolated to other countries with similar medical settings. However, caution should be taken when generalizing the ages, races, and socioeconomic status associated with survival rate in other settings due to a gap in treatment strategies, social health determinants, and follow-up measures of patients in and out of SEER and non-SEER regions. Stage at diagnosis, as one of the risk factors affecting the survival of cancer patients, was not evaluated in the present study because of the varied definition of cancer stage among stage datasets during 2006–2016. Therefore, the clinical stage and pathological subtypes of cervical cancer associated with the survival rate need to be considered when strategies are made to improve the survival rate. Due to the inability to provide a detailed classification of “other” races in the SEER database, specific races such as American Indian/AK Native and Asian/Pacific Islander associated with the relative survival rate were not evaluated. In addition, cancer survival prediction based on previous data might have some disparities from the real world, especially in the context of unpredictable events such as COVID-19 epidemics. The use of multiple drastic measures to stop the spread of the virus might limit women’s access to cervical cancer screening and diagnosis, leading to a short-term decline in cancer screening rates and delayed diagnosis 35 and subsequently negatively affect the future survival rate of cervical cancer to some extent. 36
Conclusion
We found that the relative survival rate of patients with cervical cancer increased from 2002 to 2016. It is worth noticing that the 3- and 5-year survival rates of elderly patients with cervical cancer (>70) were still very low, and the differences among various socioeconomic statuses gradually increased over time. Our prediction results showed that the survival rate from 2017 to 2021 would continue to rise. 32 These findings would be informative for predicting future trends along with better assignment of medical resources and optimizing health policies to improve the prognosis of cervical cancer.
Supplemental Material
Supplemental Material for Evaluation and Prediction Analysis of 3- and 5-Year Relative Survival Rates of Patients with Cervical Cancer: A Model-Based Period Analysis by Xiaodong Fan, MD, Wei He, MD, Qing Zhang, MD, Binjie Zhang, MD, Li Dong, PhD, Li Li, MD, and Xiaochun Liu, PhD in Cancer Control.
Acknowledgments
The author sincerely thanks Waqas Farooq and Wanqing Pang for their help to us.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (82273718 and 81971365) and Key Research and development program of Shanxi province (201903D421060).
Supplemental Material: Supplemental material for this article is available online.
Ethical Statement
Ethical Approval
Our institution does not require ethical approval for reporting public dataset-based study. SEER data is publicly available and de-identified, and ethical approval was waived by the local ethics committee.
ORCID iD
Xiaodong Fan https://orcid.org/0009-0002-7230-5412
Data Availability Statement
The datasets in this study are available in the SEER database (https://seer.cancer.gov/).
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [DOI] [PubMed] [Google Scholar]
- 2.Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whop LJ, Cunningham J, Garvey G, Condon JR. Towards global elimination of cervical cancer in all groups of women. Lancet Oncol. 2019;20(5):e238. [DOI] [PubMed] [Google Scholar]
- 4.Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. 2020;112(2):229-232. [DOI] [PubMed] [Google Scholar]
- 5.Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US preventive services Task Force recommendation statement. JAMA. 2018;320(7):674-686. [DOI] [PubMed] [Google Scholar]
- 6.Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889-899. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros de Azevedo PR, Bezerra-Rocha J, Araújo de Medeiros Fernandes TA, Veríssimo-Fernandes J. Analysis of cervical cancer mortality rate trends in Natal-RN, Brazil, between 2000 and 2012. Rev Salud Publica. 2019;21(2):161-167. [DOI] [PubMed] [Google Scholar]
- 8.Smick AH, Holbert M, Neff R. Association of physician densities and gynecologic cancer outcomes in the United States. Obstet Gynecol. 2022;140(5):751-757. [DOI] [PubMed] [Google Scholar]
- 9.Allemani C, Matsuda T, Di Carlo V. et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner H, Gefeller O. An alternative approach to monitoring cancer patient survival. Cancer. 1996;78(9):2004-2010. [PubMed] [Google Scholar]
- 11.Jiang X, Wang L, Cheng Y, Tang H, Chen T. Assessment of long-term survival of cancer patients using cancer registry data from eastern China: period analysis is superior to traditional methods. Int J Cancer. 2020;147(4):996-1005. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Li Y, Liu Q. et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13(1):57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International classification of diseases (ICD) [Internet]. https://www.who.int/classifications/icd/en
- 14.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holleczek B, Gondos A, Brenner H. periodR - an R package to calculate long-term cancer survival estimates using period analysis. Methods Inf Med. 2009;48(2):123-128. [DOI] [PubMed] [Google Scholar]
- 16.Henson DE, Ries LA. The relative survival rate. Cancer. 1995;76(10):1687-1688. [DOI] [PubMed] [Google Scholar]
- 17.Brenner H, Hakulinen T. Up-to-date and precise estimates of cancer patient survival: model-based period analysis. Am J Epidemiol. 2006;164(7):689-696. [DOI] [PubMed] [Google Scholar]
- 18.Brenner H, Gondos A, Arndt V. Recent major progress in long-term cancer patient survival disclosed by modeled period analysis. J Clin Oncol. 2007;25(22):3274-3280. [DOI] [PubMed] [Google Scholar]
- 19.Brenner H, Spix C. Combining cohort and period methods for retrospective time trend analyses of long-term cancer patient survival rates. Br J Cancer. 2003;89(7):1260-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert A, Lee A, Allbright R, Vijayakumar S. Impact of age on receipt of curative treatment for cervical cancer: an analysis of patterns of care and survival in a large, national cohort. J Geriatr Oncol. 2019;10(3):465-474. [DOI] [PubMed] [Google Scholar]
- 21.Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019;18(1):29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster AD, Sivarapatna A, Gress RE. The aging immune system and its relationship with cancer. Aging Health. 2011;7(5):707-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard RA, O'Meara ES, Henderson LM. et al. Multilevel factors associated with long-term adherence to screening mammography in older women in the U.S. Prev Med. 2016;89:169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawaya G, Sung H, Kearney K. et al. Advancing age and cervical cancer screening and prognosis. J Am Geriatr Soc. 2001;49(11):1499-1504. [DOI] [PubMed] [Google Scholar]
- 25.Spencer JC, Kim JJ, Tiro JA. et al. Racial and ethnic disparities in cervical cancer screening from three U.S. Healthcare settings. Am J Prev Med. 2023;65:667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford S, Tarraf W, Williams KP, Roman LA, Leach R. Differences in cervical cancer screening and follow-up for black and white women in the United States. Gynecol Oncol. 2021;160(2):369-374. [DOI] [PubMed] [Google Scholar]
- 27.Holt HK, Peterson CE, MacLaughlan David S, et al. Mediation of racial and ethnic inequities in the diagnosis of advanced-stage cervical cancer by insurance status. JAMA Netw Open. 2023;6(3):e232985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers SO, Ray WA, Smalley WE. A population-based study of survival among elderly persons diagnosed with colorectal cancer: does race matter if all are insured? (United States). Cancer Causes Control. 2004;15(2):193-199. [DOI] [PubMed] [Google Scholar]
- 29.Matz M, Weir HK, Alkhalawi E, Coleman MP, Allemani C, US CONCORD Working Group . Disparities in cervical cancer survival in the United States by race and stage at diagnosis: an analysis of 138,883 women diagnosed between 2001 and 2014 (CONCORD-3). Gynecol Oncol. 2021;163(2):305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mundt A, Connell P, Campbell T, Hwang J, Rotmensch J, Waggoner SJG. Race and clinical outcome in patients with carcinoma of the uterine cervix treated with radiation therapy. Gynecol Oncol. 1998;71(2):151-158. [DOI] [PubMed] [Google Scholar]
- 31.Morgan M, Behbakht K, Benjamin I, Berlin M, King S, Rubin SC. Gynecology: racial differences in survival from gynecologic cancer. Obstet Gynecol. 1996;88(6):914-918. [DOI] [PubMed] [Google Scholar]
- 32.Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang RJ, Shah SC, Camargo MC, Palaniappan L, Hwang JH. County rurality and socioeconomic deprivation is associated with reduced survival from gastric cancer in the United States. Gastroenterology. 2020;159(4):1555-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Qurayshi Z, Randolph GW, Srivastav S, Kandil E. Outcomes in endocrine cancer surgery are affected by racial, economic, and healthcare system demographics. Laryngoscope. 2016;126(3):775-781. [DOI] [PubMed] [Google Scholar]
- 35.Sasidharanpillai S, Ravishankar N. The short-term impact of COVID-19 pandemic on cervical cancer screening: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2022;23(5):1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller MJ, Xu L, Qin J, et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21-65 Years in a large integrated health care system - southern California, january 1-september 30, 2019, and january 1-september 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(4):109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Evaluation and Prediction Analysis of 3- and 5-Year Relative Survival Rates of Patients with Cervical Cancer: A Model-Based Period Analysis by Xiaodong Fan, MD, Wei He, MD, Qing Zhang, MD, Binjie Zhang, MD, Li Dong, PhD, Li Li, MD, and Xiaochun Liu, PhD in Cancer Control.
Data Availability Statement
The datasets in this study are available in the SEER database (https://seer.cancer.gov/).