Abstract
Background
Antipsychotic drugs are the core treatment for schizophrenia. Treatment guidelines state that there is no difference in efficacy between antipsychotic compounds, however, low‐potency antipsychotic drugs are often clinically perceived as less efficacious than high‐potency compounds, and they also seem to differ in their side‐effects.
Objectives
To review the effects in clinical response of haloperidol and low‐potency antipsychotics for people with schizophrenia.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (July 2010).
Selection criteria
We included all randomised trials comparing haloperidol with first‐generation low‐potency antipsychotic drugs for people with schizophrenia or schizophrenia‐like psychosis.
Data collection and analysis
We extracted data independently. For dichotomous data, we calculated risk ratios (RR) and their 95% confidence intervals (CI) on an intention‐to‐treat basis based on a random‐effects model. For continuous data, we calculated mean differences (MD), again based on a random‐effects model.
Main results
The review currently includes 17 randomised trials and 877 participants. The size of the included studies was between 16 and 109 participants. All studies were short‐term with a study length between two and 12 weeks. Overall, sequence generation, allocation procedures and blinding were poorly reported. We found no clear evidence that haloperidol was superior to low‐potency antipsychotic drugs in terms of clinical response (haloperidol 40%, low‐potency drug 36%, 14 RCTs, n = 574, RR 1.11, CI 0.86 to 1.44 lowquality evidence). There was also no clear evidence of benefit for either group in acceptability of treatment with equivocal difference in the number of participants leaving the studies early due to any reason (haloperidol 13%, low‐potency antipsychotics 17%, 11 RCTs, n = 408, RR 0.82, CI 0.38 to 1.77, low quality evidence). Similar equivocal results were found between groups for experiencing at least one adverse effect (haloperidol 70%, low‐potency antipsychotics 35%, 5 RCTs n = 158, RR 1.97, CI 0.69 to 5.66, very low quality evidence ). More participants from the low‐potency drug group experienced sedation (haloperidol 14%, low‐potency antipsychotics 41%, 2 RCTs, n = 44, RR 0.30, CI 0.11 to 0.82, moderate quality evidence), orthostasis problems (haloperidol 25%, low‐potency antipsychotics 71%, 1 RCT, n = 41, RR 0.35, CI 0.16 to 0.78) and weight gain (haloperidol 5%, low‐potency antipsychotics 29%, 3 RCTs, n = 88, RR 0.22, CI 0.06 to 0.81). In contrast, the outcome 'at least one movement disorder' was more frequent in the haloperidol group (haloperidol 72%, low‐potency antipsychotics 41%, 5 RCTs, n = 170, RR 1.64, CI 1.22 to 2.21, low quality evidence). No data were available for death or quality of life. The results of the primary outcome were robust in several subgroup and sensitivity analyses.
Authors' conclusions
The results do not clearly show a superiority in efficacy of haloperidol compared with low‐potency antipsychotics. Differences in adverse events were found for movement disorders, which were more frequent in the haloperidol group, and orthostatic problems, sedation and weight gain, which were more frequent in the low‐potency antipsychotic group. The quality of studies was low, and the quality of evidence for the main outcomes of interest varied from moderate to very low, so more newer studies would be needed in order to draw a definite conclusion about whether or not haloperidol is superior or inferior to low‐potency antipsychotics.
Keywords: Humans; Antipsychotic Agents; Antipsychotic Agents/adverse effects; Antipsychotic Agents/therapeutic use; Dyskinesia, Drug‐Induced; Haloperidol; Haloperidol/adverse effects; Haloperidol/therapeutic use; Patient Dropouts; Patient Dropouts/statistics & numerical data; Randomized Controlled Trials as Topic; Schizophrenia; Schizophrenia/drug therapy; Weight Gain
Plain language summary
Haloperidol versus first‐generation low‐potency antipsychotic drugs for schizophrenia
Drugs called antipsychotics are the main treatment for schizophrenia. Schizophrenia is a serious mental illness where sufferers experience both positive symptoms of delusions and hallucinations and negative symptoms such as apathy, lack of drive, disorganisation of behaviour and thought. This review examined whether a high‐potency antipsychotic, haloperidol is more effective than low‐potency antipsychotics. The classification into high‐potency and low‐potency medication means that for low‐potency antipsychotic drugs, higher doses are necessary to obtain the same effect and response in patients. Haloperidol is the most frequently used antipsychotic drug in many countries and, along with other high‐potency antipsychotics is often considered more effective than low‐potency antipsychotics. Typical examples of low‐potency antipsychotic drugs are chlorpromazine, chlorprothixene, thioridazine or levomepromazine. High‐ and low‐potency antipsychotics also seem to differ in their side‐effects. Low‐potency drugs cause sedation and poor muscle strength, whereas high‐potency drugs produce side‐effects such as movement disorders (the inability to sit still, uncontrollable shaking and difficulty in walking). The review is based on results of a search run in 2010 and includes 17 studies with a total of 877 participants comparing haloperidol with low‐potency antipsychotic drugs.The results do not clearly show a superiority of haloperidol compared with low‐potency antipsychotics. However, more participants from the low‐potency drug group experienced weight gain and sedation. The experience of at least one movement disorder was significantly higher with haloperidol. The number as well as the quality of studies is low, for the main outcomes of interest the authors rated quality of evidence for as moderate quality for two of them, two as low quality and one as very low quality. So the evidence is not strong and more newer studies would be needed in order to draw a conclusion about whether or not haloperidol is superior to low‐potency antipsychotics. For people with schizophrenia it is important to know that there is moderate quality evidence that haloperidol and low‐potency antipsychotics are approximately equal in their effectiveness, but there is lower quality evidence that they clearly differ in side‐effects (such as weight gain and movement disorders).
This plain language summary has been written by a consumer Benjamin Gray: Service User and Service User Expert, Rethink Mental Illness, Email: ben.gray@rethink.org
Summary of findings
Summary of findings for the main comparison. HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS for schizophrenia.
| HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS for schizophrenia | ||||||
| Patient or population: patients with schizophrenia Settings: Intervention: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS | |||||
| Clinical response to treatment Follow‐up: 2‐12 weeks | Study population | RR 1.11 (0.86 to 1.44) | 574 (14 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 361 per 1000 | 401 per 1000 (310 to 520) | |||||
| Moderate | ||||||
| 334 per 1000 | 371 per 1000 (287 to 481) | |||||
| Leaving the study early ‐ Due to any reason Follow‐up: 1‐3 months | Study population | RR 0.82 (0.38 to 1.77) | 408 (11 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 164 per 1000 | 135 per 1000 (62 to 291) | |||||
| Moderate | ||||||
| 136 per 1000 | 112 per 1000 (52 to 241) | |||||
| Adverse effects ‐ at least one adverse effect | Study population | RR 1.97 (0.69 to 5.66) | 158 (5 studies) | ⊕⊝⊝⊝ very low1,3,4 | ||
| 346 per 1000 | 681 per 1000 (239 to 1000) | |||||
| Moderate | ||||||
| 235 per 1000 | 463 per 1000 (162 to 1000) | |||||
| Adverse effects ‐ movement disorders ‐ At least one movement disorder Follow‐up: 1‐3 months | Study population | RR 1.64 (1.22 to 2.21) | 170 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 414 per 1000 | 679 per 1000 (505 to 914) | |||||
| Moderate | ||||||
| 375 per 1000 | 615 per 1000 (458 to 829) | |||||
| Adverse effects ‐ other ‐ Sedation Follow‐up: 6‐12 weeks | Study population | RR 0.3 (0.11 to 0.82) | 44 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 500 per 1000 | 150 per 1000 (55 to 410) | |||||
| Moderate | ||||||
| 420 per 1000 | 126 per 1000 (46 to 344) | |||||
| Death | See comment | See comment | Not estimable | 0 (0) | See comment | |
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias: rated 'serious' ‐ many studies did not report the methods for sequence generation and/or allocation concealment, missing or unclear results for incomplete outcome data and selective reporting. 2 Imprecision: rated 'serious' ‐ the total number of events is less than 300 and the estimate of effect includes appreciable benefit/harm. 3 Inconsistency: rated 'serious' ‐ the P value for heterogeneity was statistically significant and the I‐square higher than 80%, the direction of the effect was not the same for all the studies. 4 Imprecision: rated 'serious' ‐ only a few studies contributed to this event and the confidence interval was large.
Background
Description of the condition
Schizophrenia is often a chronic and disabling psychiatric disorder. It afflicts approximately one per cent of the population world‐wide with little gender differences (Berger 2003). The typical manifestations of schizophrenia are 'positive' symptoms such as fixed, false beliefs (delusions) and perceptions without cause (hallucinations), 'negative' symptoms such as apathy and lack of drive, disorganisation of behaviour and thought, and catatonic symptoms such as mannerisms and bizarre posturing (Carpenter 1994). The degree of suffering and disability is considerable with 80% to 90% not working (Marvaha 2004) and up to 10% dying by suicide (Tsuang 1978).
Description of the intervention
Antipsychotic drugs are the core treatment for schizophrenia. All antipsychotic drugs block, to a greater or lesser extent, D2‐receptors in the brain. They can be classified according to their biochemical structure (e.g. butyrophenones, phenothiazines, thioxanthenes etc.), their risk of producing movement disorders ('atypical' versus 'typical' antipsychotics) and the doses necessary for an antipsychotic effect (high‐potency versus low‐potency antipsychotics). The classification into high‐potency and low‐potency medication means that for low‐potency antipsychotic drugs higher doses are necessary to obtain the same dopamine receptor occupancy and efficacy (Seeman 1975). In this context, haloperidol belongs to the high‐potency antipsychotic drug group. It is the most frequently used conventional antipsychotic drug in many countries including Germany (Kaye 2003; Lohse 2005; Paton 2003). Haloperidol is mostly indicated in schizophrenia, acute psychosis and delirium.
Low‐potency antipsychotic drugs will be the comparator drugs in this review. Typical examples of low‐potency antipsychotic drugs are chlorpromazine, chlorprothixene, thioridazine or levomepromazine. It is an old psychiatric dogma that can be found in textbooks and guidelines that ‐ with the exception of clozapine ‐ there is no difference in efficacy between any antipsychotic compounds (Gaebel 2006; Lehman 2004). Nevertheless, low‐potency antipsychotic drugs are often clinically perceived as less efficacious than high‐potency compounds, and high‐ and low‐potency antipsychotics also seem to differ in their adverse effects. Low‐potency drugs have a high incidence of sedation or hypotonia, whereas high‐potency drugs produce most extrapyramidal adverse effects.
How the intervention might work
The theory is that schizophrenia is a chronic disorder caused by hyper‐dopaminergic states in the limbic system (Berger 2003). All antipsychotic drugs block dopamine receptors. Haloperidol was discovered by Paul Janssen and developed in 1957. With regard to dopamine receptor blockade, haloperidol is approximately 50 times more potent than chlorpromazine. It is a butyrophenone antipsychotic that is effective against delusions and hallucinations. It is a strong dopamine (mainly D2) receptor antagonist with antipsychotic properties and antiemetic properties. Haloperidol effectively blocks receptors in the limbic system of the brain. It has a rapid onset of action lasting three to six hours and a bioavailability of 60%. The elimination half‐life period is about 12 to 36 hours. However, the dopaminergic action is blocked in the nigrostriatal pathways and this blockage can lead to extrapyramidal side‐effects.
Low‐potency medications have a lower affinity for dopamine receptors so that a higher dose is required to effectively treat symptoms of schizophrenia. In addition, they block other than dopamine receptors, such as cholinergic or histaminergic receptors. This also explains the occurrence of adverse effects, which are potentially less frequent with high‐potency drugs, such as sedation or hypotonia. The cut‐off between high‐ and low‐potency drugs is not clear, but attempts have been made to express their relationship in terms of dose equivalence. The most frequently applied concept is based on chlorpromazine equivalents according to Davis 1974 or Haase 1983 and provides data about comparable doses of various antipsychotic drugs to achieve a therapeutic effect similar to 100 mg chlorpromazine.
Why it is important to do this review
Systematic reviews on the comparative efficacy of high‐potency versus low‐potency antipsychotic drugs are not available. Cochrane reviews on the effects of specific conventional antipsychotic drugs have been published, but they compared the effects of one antipsychotic drug versus any other antipsychotic drugs (e.g. Pimozide versus any other antipsychotic drug, Fenton 2007) and thus did not consider the important classification in high‐potency and low‐potency antipsychotics. Due to this lack of evidence, treatment guidelines make statements such as “all conventional antipsychotics if adequately dosed have comparable efficacy” (German national schizophrenia guideline (Gaebel 2006, also see the guideline of the World Federations of Societies of Biological Psychiatry (Falkai 2005)). These guidelines contrast with the clinical impression that low‐potency conventional antipsychotic drugs are less efficacious than high‐potency conventional antipsychotic drugs. The clinical consequences in following these guidelines are considerable, because high‐potency and low‐potency antipsychotics differ clearly in side‐effects. High‐potency antipsychotics often produce extrapyramidal symptoms, low‐potency antipsychotics on the other hand have strong sedating properties and often also lead to hypotension.
Conventional antipsychotic drugs are still the mainstay of treatment in countries that can not afford newer, expensive 'atypical' or 'second‐generation' antipsychotic drugs and even in some industrialised countries such as Germany, conventional antipsychotic medications still account for 50% of the market‐share (Lohse 2005). Recent studies about these more expensive second‐generation antipsychotics have also called into question their superiority (Jones 2006; Leucht 2009a; Lieberman 2005). Therefore, research on older conventional agents is essential and has been asked for (Leucht 2009b).
For a list of related reviews, please seeTable 2.
1. Series of similar reviews.
| Title | Reference |
| Haloperidol versus first‐generation antipsychotics for schizophrenia | Dold 2012 |
| Perphenazine versus low‐potency antipsychotic drugs | Tardy 2011b |
| Fluphenazine versus low‐potency antipsychotic drugs | Tardy 2011c |
| Trifluoperazine versus low‐potency antipsychotic drugs | Tardy 2011d |
| Flupenthixol versus low‐potency antipsychotic drugs | Tardy 2011e |
Objectives
To review the effects of the high‐potency antipsychotic drug haloperidol versus low‐potency antipsychotic drugs. Haloperidol is sometimes perceived to be more efficacious than low‐potency antipsychotics and we tested this hypothesis in our review.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled (parallel group or cross‐over) trials that had a minimum follow‐up duration of three weeks. We included trials that were described as randomised or where randomisation was implied. We excluded quasi‐randomised trials, such as those that used alternation, an open list of random numbers, or any other method of recruitment where allocation to interventions was predictable.
Types of participants
People with schizophrenia and schizophrenia‐like psychoses (schizophreniform and schizoaffective disorders). There is no clear evidence that the schizophrenia‐like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994). We included studies irrespective of the diagnostic criteria used. Diagnostic criteria, such as ICD 10 (International Classification of Diseases) or DSM‐IV (Diagnostic and Statistical Manual ‐ Version IV), are not routinely used in clinical practice and restricting inclusion to trials that used operationally defined diagnostic criteria would have reduced generalisation and representativeness.
We were interested in making sure that information is as relevant to the current care of people with schizophrenia as possible so proposed to clearly highlight the current clinical state (acute, early post‐acute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent) and as to whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Intervention: Haloperidol
Any dose of oral mode of administration (no depots, no short‐acting parenteral forms of administration). We a priori decided that haloperidol would be the intervention because it is sometimes perceived to be more efficacious than low‐potency drugs. Therefore, our hypothesis was that haloperidol is more efficacious and was thus chosen as the intervention drug.
2. Low‐potency antipsychotic drugs
The control interventions were low‐potency conventional antipsychotic drugs, any oral form of administration and any dose. We used the dose equivalence tables by Davis (Davis 1974) and/or Haase (Haase 1983) to define drugs as low‐potency with a chlorpromazine equivalence roughly equal or higher than chlorpromazine. The chlorpromazine equivalence dose of sulpiride is often estimated to be approximately 100. However, it has similar properties as amisulpride, which is an atypical antipsychotic and thus not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation, which is an important characteristic of low‐potency antipsychotics. Therefore, we decided to cover sulpiride in another Cochrane review on haloperidol (Dold 2012).
Types of outcome measures
Primary outcomes
1. Clinical response
Response to treatment as defined by the original studies
Secondary outcomes
1. Mental state: symptoms of schizophrenia
1.1 Overall symptoms ‐ average score/change in mental state 1.2 Positive symptoms ‐ average score/change in positive symptoms 1.3 Negative symptoms ‐ average score/change in negative symptoms
2. Global state: average score/change in global state
3. Relapse ‐ as defined by each of the studies
4. Leaving the study early
4.1 Acceptability of treatment ‐ leaving the study early due to any reason 4.2 Leaving the study early due to inefficacy of treatment 4.3 Leaving the study early due to side‐effects
5. Service use
5.1 Rehospitalisation 5.2 Days in hospital 5.3 Healthy days
6. Death
6.1 Death (all causes) 6.2 Suicide
7. Adverse effects
7.1 At least one adverse effect 7.2 Extrapyramidal/movement disorders 7.3 Cardiac effects 7.4 Hypotension 7.5 Sedation 7.6 Weight gain 7.7 Other
8. Quality of life
9. Participant's/carer`s satisfaction with care
10. Economic outcomes
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
We searched the ‘Cochrane Schizophrenia Group Trials Register’ for relevant studies (July 2010) using the phrase:
[(*haloperidol* in intervention of STUDY) OR (*haloperidol* in title, abstract and index terms of REFERENCE entered >=01/05/10)]
This register is compiled by systematic searches of major databases, handsearches and conference proceedings (seeGroup Module).
Searching other resources
1. Reference searching
We inspected the references of all identified studies for more trials.
2. Previous reviews
We searched previous conventional reviews (Davis 1989; Klein 1969).
3. Personal contact
We contacted the first author of each included study for missing information and for the existence of further studies.
4. Drug companies
We contacted the original manufacturer of haloperidol (Janssen) and asked them for further relevant studies and for missing information on identified studies.
Data collection and analysis
Selection of studies
Two review authors (MT, MH) independently inspected all abstracts identified in the searches. Disagreement was resolved by discussion and where doubt still remained, we acquired the full article for further inspection. Once the full articles were obtained, at least two review authors (MT, MH) independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we resolved it with a third review author (SL) or sought further information from the study authors.
Data extraction and management
1. Extraction
Two review authors (MT, MH) independently extracted data from all selected trials. Any disagreement was discussed, decisions documented and, if necessary, authors of studies were contacted for clarification. With remaining problems SL helped clarify issues and those final decisions were documented. Attempts were made to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary.
2. Management
2.1 Forms
We extracted data onto simple standard forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a. the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument was not written or modified by one of the trialists for that particular trial.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data and only use change data if the former were not available. Endpoint and change data were combined in the analysis as we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011, Cochrane Handbook for Systematic Reviews of Interventions Chapter 9.4.5.2 ).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion: a) standard deviations (SD) and means were reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution), (Altman 1996); c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) Kay 1986), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. Skewed data from studies of less than 200 participants would have been entered as other data within the Data and analyses section rather than into a statistical analysis. Skewed data pose less of a problem when looking at means if the sample size is large and data from studies with over 200 participants would have been entered into statistical syntheses. When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not and skewed change data were entered into statistical analysis.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, efforts were made to convert outcome measures to dichotomous data. This could be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It was generally assumed that if there had been a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for haloperidol. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not improved'), we did report data where the left of the line indicates an unfavourable outcome. This is noted in the relevant graphs.
2.8 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and GRADE profiler (GRADE) to import data from RevMan 5 (Review Manager) to create a 'Summary of findings' table. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes rated as important to patient‐care and decision making. We have selected the following long‐term main outcomes for inclusion in the summary of findings table.
We anticipated including the following short or medium‐term outcomes in a 'Summary of findings' table.
Response to treatment
Leaving the study early due to any reason
Adverse effect ‐ at least one adverse effect
Adverse effects ‐ movement disorders ‐ at least one movement disorder
Adverse effects ‐ sedation
Death
Quality of life
Assessment of risk of bias in included studies
Again working independently, two authors assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases.
The risk of bias in each domain and overall were assessed and categorised into:
A. Low risk of bias: plausible bias unlikely to seriously alter the results (categorised as 'Yes' in 'Risk of bias' table) B. High risk of bias: plausible bias that seriously weakens confidence in the results (categorised as 'No' in 'Risk of bias' table) C. Unclear risk of bias: plausible bias that raises some doubt about the results (categorised as 'Unclear' in 'Risk of bias' table)
If the raters disagreed, the final rating was made by consensus with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies to obtain further information. We reported non‐concurrence in quality assessment.
Measures of treatment effect
1. Dichotomous data
For binary outcomes we calculated a standard estimation of the random‐effects risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. Where possible, efforts were made to convert outcome measures to dichotomous data. This could be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It was generally assumed that if there had been a 50% reduction in a scale‐derived score such as the BPRS (Overall 1962) or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2. Continuous data
For continuous outcomes we estimated a mean difference (MD) between groups using the random‐effects model as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We did not calculate standardised mean differences (SMD) measures. There was one exception to this rule, however. In the case of where scales were of such similarity to allow pooling we calculated the SMD and, whenever possible, transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Had we identified any cluster randomised trials and clustering had not been accounted for in primary studies, we planned to present data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. Where clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect. If a cluster study had been appropriately analysed taking into account intra‐class correlation co‐efficient and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique, where the natural logarithm of the effect estimate (and standard errors) for all included trials for that outcome would be calculated and entered into RevMan along with the log of the effect estimate (and standard errors) from the cluster randomised trial(s). We would have used methods described in section 7.7.7.2 and 7.7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to obtain standard errors.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, randomised cross‐over studies were eligible but we used only data up to the point of first cross‐over.
3. Studies with multiple treatment groups
If we had included any studies involving more than two treatment arms, if relevant, the additional treatment arms would have been presented in comparisons. If data were binary these would simply have been added and combined within the two‐by‐two table. If data were continuous, we would have combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, the data would not have been reproduced.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). The loss to follow‐up in randomised schizophrenia trials is often considerable calling the validity of the results into question. Nevertheless, it is unclear which degree of attrition leads to a high degree of bias. We did not exclude trials from outcomes on the basis of the percentage of participants completing them. We, however, used the 'Risk of bias' tool described above to indicate potential bias when more than 25% of the participants from the haloperidol group and low‐potency drug group left the studies prematurely (Xia 2009), when the reasons for attrition differed between the intervention and the control group, and when no appropriate imputation strategies were applied.
2. Dichotomous data
Data were presented on a 'once‐randomised‐always‐analyse' basis, assuming an intention‐to‐treat (ITT) analysis. If the authors applied such a strategy, we used their results. If the original authors presented only the results of the per‐protocol or completer population, we assumed that those participants lost to follow‐up would have had the same percentage of events as those who remained in the study.
3. Continuous data
3.1 Attrition
Intention‐to‐treat (ITT) was used when available. We anticipated that in some studies, in order to do an ITT analysis, the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leon 2006). Therefore, where LOCF data were used in the analysis, it was indicated in the review.
3.2 Standard deviations
We first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data but an exact standard error (SE) and confidence interval were available for group means, and either 'P' value or 't' value were available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): When only the SE is reported, standard deviations (SDs) are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we calculated the SDs according to a validated imputation method, which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study's outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies without any comparison group to judge clinical heterogeneity.
We simply inspected all studies for clearly outlying situations or people which we had not predicted would arise and discussed them fully, if such situations or participants arose..
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise and discussed them if they were evident.
3. Statistical
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies was investigated by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2011). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a confidence interval for I2).
An I2 estimate of 50% to 90% accompanied by a statistically significant Chi2 statistic, may represent substantial heterogeneity (Section 9.5.2 Cochrane Handbook for Systematic Reviews of Interventions ‐ Higgins 2011) and reasons for heterogeneity were explored. If the inconsistency was high and clear reasons were found, data were presented separately.
Assessment of reporting biases
1. Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011). If necessary, we tried to locate protocols of included randomised trials. If the protocol was available, outcomes in the protocol and in the published report were compared. If the protocol was not available, outcomes listed in the methods section of the trial report were compared with actually reported results.
2. Funnel plot
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are again described in Section 10 of the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In addition to the usual inspection of funnel plots, we used the trim‐and‐fill method by Duval and Tweedy (Duval 2000) to examine whether missing studies would have changed the mean effect size to an important extent.
Data synthesis
We employed a random‐effects model for analyses (Der‐Simonian 1986). We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. Therefore, the random‐effects model is usually more conservative in terms of statistical significance, although as a disadvantage it puts added weight onto smaller studies, which can either inflate or deflate the effect size. We examined in a secondary analysis whether using a fixed‐effect model markedly changed the results of the primary outcome.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analysis
All subgroup analyses were applied only to the primary outcome.
1.1 Different low‐potency drugs
Subgrouping was performed by comparing haloperidol with each single low‐potency antipsychotic separately.
1.2 Clinical state, stage or problem
We tried to report data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, this was reported. First, we investigated whether data had been entered correctly. Second, if data had been correct, the graph was visually inspected and outlying studies were successively removed to see if heterogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, data were presented. If not, data were not pooled and issues discussed. We know of no supporting research for this 10% cut‐off but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcome we included these studies and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then all data were employed from these studies.
2. Implication of non double‐blind trials
We aimed to include trials in a sensitivity analysis if participants and treating psychiatrists were not blinded. For the primary outcome we included these studies and if there was no substantive difference when the non double‐blind studies were added to the double‐blind studies, then all data were employed from these studies.
3. Assessment of dosage
We aimed to include trials in a sensitivity analysis if doses between haloperidol and low‐potency antipsychotics were clearly discrepant by our judgement based on the chlorpromazine equivalence tables (Davis 1974; Haase 1983; Andreasen 2010). If there was no substantive difference when studies with discrepant doses were added, then we used all data from these studies.
Results
Description of studies
For substantive description of studies please seeCharacteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The search strategy in the "Cochrane Schizophrenia Group Trials Register" generated 2239 reports of which 73 publications on 58 studies were closely inspected (Figure 1). Seventeen studies (877 participants) met the inclusion criteria. No further studies were identified by reference search or by contacting the major manufacturer of haloperidol.
1.
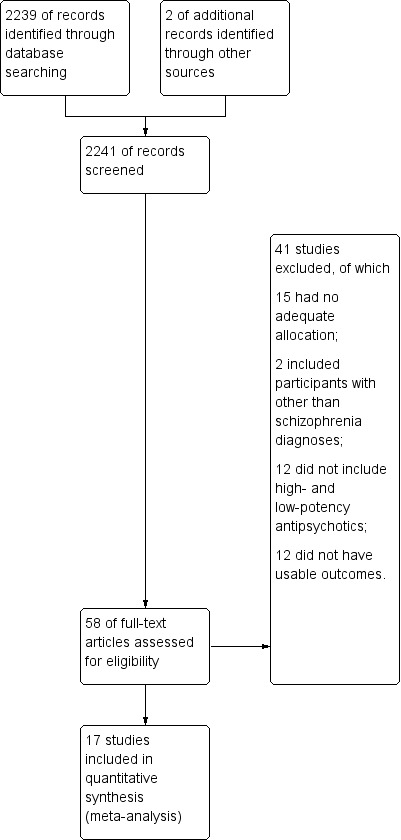
Study flow diagram.
Included studies
1. Length of trials
Of the included studies, nine had durations of up to one month (Bi 1994; Blin 1996; Fox 1964; Gallant 1967; Klimke 1993; Nishizono 1994; Schmidt 1982; Shalev 1993; White 1981). Two studies lasted six weeks (Borison 1989; Dufresne 1993) and six studies lasted between seven weeks and three months (Clark 1969; McCreadie 1977; Prasad 1966; Rompel 1978; Serafetinides 1972; Weston 1973). Thus, all studies fell into the short‐term category.
2. Participants
In seven studies, participants were diagnosed according to clinical criteria (Clark 1969, Fox 1964; Gallant 1967; Prasad 1966; Rompel 1978; Serafetinides 1972; Weston 1973). Three studies used the Diagnostic and Statistical Manual Version III (DSM‐III) (APA 1980) (Borison 1989; Dufresne 1993; Shalev 1993) and one study the Diagnostic and Statistical Manual Version III Revised (DSM‐III‐R) (APA 1987) (Blin 1996). One study used the Feighner´s criteria (Feighner 1972) (White 1981) and one Schneiderian first rank symptoms (Schneider 1959) (McCreadie 1977). Two studies used International Classification of Diseases, ninth revision (ICD‐9) (WHO 1978) (Klimke 1993; Schmidt 1982) and one ICD‐10 (WHO 1992) (Nishizono 1994). One study (Bi 1994) diagnosed participants according to the Research Diagnositic Criteria (RDC) (Spitzer 1978) and the Chinese classification of mental disorders (CCMD‐2).
The mean age was 39.1 years (reported by 13 studies) and the mean age at onset was 28.3 years. The mean duration of illness was 11.3 years (as indicated by seven studies) and the mean number of previous hospitalisations was three (based on four studies). Four studies reported information on severity of illness at baseline (Borison 1989; Dufresne 1993; Klimke 1993; White 1981), with scores between 35 and 59.9 on the Brief Psychiatric Rating Scale (BPRS) (Overall 1962).
3. Setting
Thirteen studies were conducted in hospitals (Blin 1996; Clark 1969; Fox 1964; Gallant 1967; Klimke 1993; McCreadie 1977; Prasad 1966; Rompel 1978; Schmidt 1982; Serafetinides 1972; Shalev 1993; Weston 1973; White 1981). Four studies (Bi 1994; Borison 1989; Dufresne 1993; Nishizono 1994) did not report on setting.
4. Study size
Borison 1989 was the smallest study, randomising only 16 participants. Twelve studies (Bi 1994; Clark 1969; Dufresne 1993; Fox 1964; Gallant 1967; McCreadie 1977; Prasad 1966; Rompel 1978; Schmidt 1982; Serafetinides 1972; Shalev 1993; White 1981) randomised between 20 and 40 participants. Four studies randomised more than 40 participants, Blin 1996 (41 participants), Klimke 1993 (50 participants), Weston 1973 (86 participants) and Nishizono 1994 as the largest study with 109 participants.
5. Interventions
In most studies flexible doses of antipsychotic drugs could be prescribed. The dose range for haloperidol was between 2 mg/day and 100 mg/day. Eight studies (Bi 1994; Clark 1969; Fox 1964; Gallant 1967; McCreadie 1977; Nishizono 1994; Rompel 1978; Serafetinides 1972) used chlorpromazine as the comparator antipsychotic with doses between 50 to 1800 mg/day. Two studies (Blin 1996; Shalev 1993) used levomepromazine with doses range between 50 to 379 mg/day. One study (White 1981) used 100 to 800 mg/day mesoridazine. Two studies (Klimke 1993; Schmidt 1982) used 300 to 900 mg/day perazine and four studies (Borison 1989; Dufresne 1993; Prasad 1966; Weston 1973) used thioridazine with doses between 60 and 800 mg/day.
6. Outcomes
6.1 Response to treatment
Our primary outcome was response to treatment as defined by the original studies. An at least 20% reduction of the Positive and Negative Syndrome Scale (PANSS) total score from baseline was used as the threshold of clinically significant response by Blin 1996. Dufresne 1993 defined response as an at least 20% reduction on the BPRS. A 50% reduction of the BPRS total score was used as a response cut‐off by Nishizono 1994.
Four further studies (Bi 1994; Borison 1989; Klimke 1993; White 1981) also based response on the BPRS, but did not give detailed response criteria. Clark 1969 based response both on the BPRS as well as the Clinical Global Impression Scale (CGI), but did not indicate the cut‐offs. The CGI was used by Schmidt 1982 and Serafetinides 1972. All other studies based response on clinical judgement (Fox 1964; Gallant 1967McCreadie 1977; Prasad 1966; Rompel 1978; Shalev 1993; Weston 1973).
Of the 17 included studies, 13 studies reported useable data for this primary outcome (Bi 1994; Blin 1996; Dufresne 1993; Fox 1964; Gallant 1967; McCreadie 1977; Nishizono 1994; Prasad 1966; Rompel 1978; Schmidt 1982; Serafetinides 1972; Shalev 1993; Weston 1973).
6.2 Mental and global state
Average scores in mental state were assessed with the BPRS (Overall 1962), the PANSS Kay 1986), and the CGI scale (Guy 1976). Altogether five studies provided data on these continuous outcome measures (Bi 1994; Blin 1996; Borison 1989; McCreadie 1977; White 1981).
6.3 Relapse
Only five studies (Borison 1989; Klimke 1993; McCreadie 1977; Schmidt 1982; Serafetinides 1972) reported relapse rates with various definitions.
6.4 Leaving the study early
The number of participants leaving the study early were recorded for the categories any reason, adverse events and lack of efficacy. Eleven studies (Bi 1994; Blin 1996; Borison 1989; Clark 1969; Dufresne 1993; Klimke 1993; McCreadie 1977; Prasad 1966; Schmidt 1982; Serafetinides 1972; Weston 1973) reported on leaving the study early due to any reason and leaving the study early due to adverse events. Ten studies (Bi 1994; Blin 1996; Borison 1989; Clark 1969; Klimke 1993; McCreadie 1977; Prasad 1966; Schmidt 1982; Serafetinides 1972; Weston 1973) reported data on leaving the study early due to inefficacy.
6.5 Service use
None of the included studies reported data on this outcome.
6.6. Adverse effects
Scales that provided usable data are described below.
6.6.1 Abnormal Involuntary Movement Scale ‐ AIMS (Guy 1976)
This scale has been used to assess tardive dyskinesia, a long‐term, drug‐induced movement disorder and short‐term movement disorders such as tremor. A high score indicates severe dyskinesia (score from zero to four).
6.6.2 Asberg Rating Scale of side‐effects (Asberg 1970)
This 10‐item scale, with a scoring system of zero to four for each item, measures drug‐induced parkinsonism, a short‐term drug‐induced movement disorder. A low score indicates low levels of parkinsonism.
6.6.3 Extrapyramidal Symptom Rating Scale ‐ ESRS (Chouinard 1980)
This scale assess four types of medication induced extrapyramidal side‐effects: parkinsonism, akathisia, dystonia, and tardive dyskinesia. Low scores indicate low levels of these side‐effects.
6.6.4 Reversible Extrapyramidal Symptom Rating Scale ‐ ERS (DiMascio 1976)
This 10‐item scale, with a scoring system of zero to four for each item, measures drug‐induced parkinsonism, a short‐term drug‐induced movement disorder. A low score indicates low levels of parkinsonism.
6.6.5 Simpson Angus Scale ‐ SAS (Simpson 1970)
This 10‐item scale, with a scoring system of zero to four for each item, measures drug‐induced parkinsonism, a short‐term drug‐induced movement disorder. A low score indicates low levels of extrapyramidal side‐effects.
6.6.6 Treatment Emergent Symptom Rating Scale ‐ TESS (NIMH 1985)
This six‐item scale measures various medication side‐effects, it was developed by the National Institute of Mental Health.
6.6.7 Other adverse effects
The following adverse events were reported in a dichotomous manner in terms of the number of participants with such a side‐effect: 'at least one adverse event', at least one movement disorder, akathisia, dyskinesia, dystonia, rigor, tremor, use of antiparkinson medication, hypotension, sedation, weight gain, allergic reactions, amenorrhoea, ankle oedema, asthenia, blurring of vision, confusion, constipation, dizziness, drooling, dry mouth, excitement, galactorrhoea, headache, loss of associated movement, micturition disturbances, nausea, oculogyric crisis, orthostatic symptoms, palpitations, photosensitivity, prolactin increase, rash, repercussions on sexual life, somnolence, sleep disturbances, sweating, syncope, tongue changes, urinary retention, weight loss. None of the included studies reported data on the following predefined important adverse effects: death, suicide and cardiac effects.
6.7 Quality of life and participant´s/carer´s satisfaction with care
None of the included studies reported data on this outcome.
6.8 Economic outcomes
None of the included studies reported data on this outcome.
Excluded studies
Forty‐one studies were excluded from the analysis. Fifteen were not randomised (Azima 1960; Blum 1969; Eitan 1992; Gerlach 1978; Gillis 1977; Hayano 1989; Horodnicki 1985; Liu 1996; Mechri 2006; Minami 1990; Mori 1990; Singh 1975; Terminska 1989; Wang 2000; Zuoning 1999). Gonier 1970 randomised participants, but later described that randomisation did not work. Dubin 1985 and Harris 1992 included participants with diagnoses other than schizophrenia. Altogether, 12 studies compared haloperidol against medications not relevant for this review, because they were not low‐potency first‐generation antipsychotics. Of these, seven compared haloperidol with sulpiride (Cassano 1975; Guazzelli 1995; Mori 1989; Munk‐Andersen 1984; Okuda 1979; Rama 1981; Ropert 1989); two studies used placebo as a comparator (Crow 1986; Garry 1962); Giordana 1984 compared with pipotiazine; Nahunek 1982 with oxyprothepine and placebo; and Smith 1985 compared different haloperidol dosages without a comparator drug. de Lima 2005 and Palma 1997 did not have results for each single drug separately. Nine studies met all the inclusion criteria, however, they did not present any usable data (Bagne 1992; Cosar 1999; Davies 2007; Fux 1991; Hogan 1992; Lempérière 1962; Marjerrison 1971; Shvartsburd 1984; Teja 1975).
Risk of bias in included studies
For graphical representations of our judgements of risk of bias please refer to Figure 2 and Figure 3. Full details of judgements are seen in the 'Risk of bias' tables.
2.
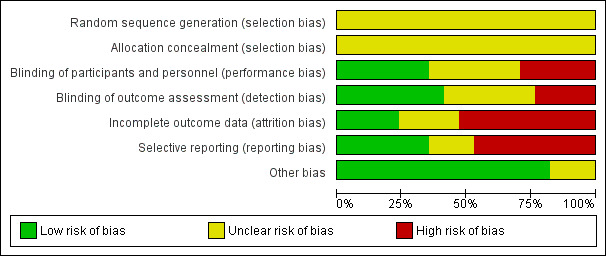
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
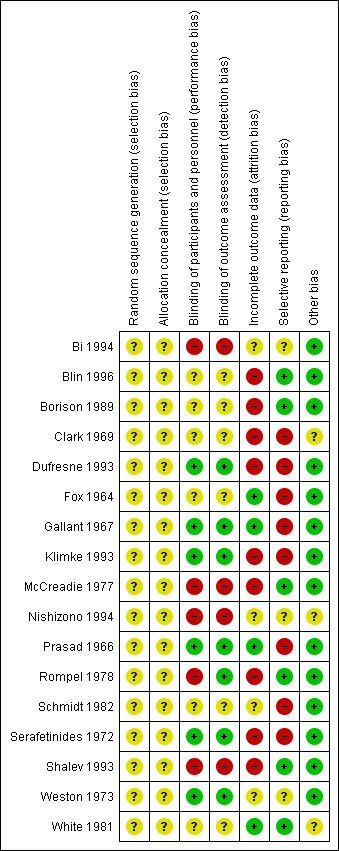
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In all studies, random sequence generation was unclear. Among these, 14 studies were described as randomised without providing further details about random sequence generation. Three further studies did not provide any information about sequence generation, but they were double‐blind, therefore we assumed randomisation (Clark 1969; Prasad 1966; White 1981).
Only one of the 17 included randomised studies provided details on allocation concealment (Shalev 1993). Therefore, it was unclear for all the other studies whether adequate allocation concealment methods were used.
Blinding
Bi 1994, Shalev 1993 and Nishizono 1994 were probably open studies and were thus rated to have a high risk of bias in terms of blinding. One study was rated with a high risk of bias (McCreadie 1977), because psychiatrists could guess correctly in eight participants out of 20 which antipsychotic they had been given. Six studies were described as double‐blind but did not provide even a minimal description of the blinding methods. These studies were rated as unclear with regards to blinding (Blin 1996; Borison 1989; Clark 1969; Fox 1964; Schmidt 1982; White 1981). Seven studies provided at least some description of the blinding process. Five studies described that they used identical capsules and were rated to have a low risk of bias (Dufresne 1993; Gallant 1967; Prasad 1966; Serafetinides 1972; Weston 1973). Klimke 1993 made an explicit statement about blinding and was therefore also rated to have a low risk of bias. Rompel 1978 was single‐blind study. It seems that nurses know the treatment, but they were not allowed to tell raters and patients. Therefore, there might have been a performance bias, but no detection bias.
Incomplete outcome data
In four studies risk of bias for incomplete outcome data was rated as adequate (Fox 1964; Gallant 1967; Prasad 1966; White 1981). Four studies provided insufficient information to permit judgement about incomplete outcome data and were rated as unclear (Bi 1994; Nishizono 1994; Schmidt 1982; Weston 1973). Weston 1973 described that there were no drop‐outs in the haloperidol group, yet there were two participants missing from global assessment. It is not clear what happened with these two participants. Nine studies were rated to have a high risk of bias. Of these, six studies had an attrition rate between 10% and 25% and most of these studies only analysed study completers (Borison 1989; Clark 1969; Dufresne 1993; McCreadie 1977; Rompel 1978; Serafetinides 1972). Two studies had a high attrition rate of > 25% and drop‐outs were not equally distributed between groups (Blin 1996; Klimke 1993). Another reason for high risk of bias was incomplete reporting of the exact number of drop‐outs for each drug group (Shalev 1993).
Selective reporting
We judged six studies to be free of selective reporting (Blin 1996; Borison 1989; McCreadie 1977; Rompel 1978; Shalev 1993; White 1981). Three studies did not provide enough information to permit a reasonable judgement and were rated as unclear (Bi 1994; Nishizono 1994; Weston 1973). We judged eight studies to have a high risk of bias. The following seven studies did not report sufficiently on predefined outcomes: Clark 1969; Dufresne 1993; Fox 1964; Gallant 1967; Prasad 1966; Schmidt 1982; Serafetinides 1972. One study presented results only for subgroups or independently of treatment condition (Klimke 1993).
Other potential sources of bias
Fourteen studies were free of other potential sources of bias and in three studies this was unclear (Clark 1969; Nishizono 1994; White 1981).
Effects of interventions
See: Table 1
For dichotomous data, we calculated risk ratios (RR) and for continuous data, we calculated mean differences (MD), both with 95% confidence intervals (CI).
1. Haloperidol versus low‐potency antipsychotic drugs
1.1 Response to treatment
There was no significant difference between haloperidol and low‐potency antipsychotics in terms of response to treatment as defined by the original studies (haloperidol 40%, low‐potency antipsychotics 36%, 14 RCTs, n = 574, RR 1.11, CI 0.86 to 1.44, Analysis 1.1).
1.1. Analysis.
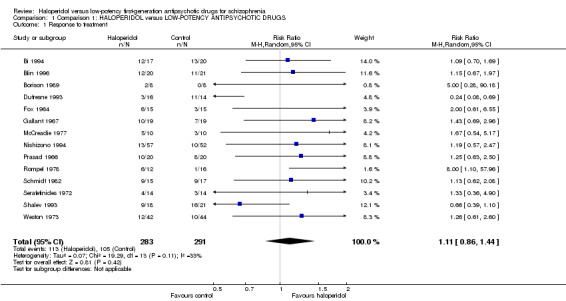
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 1 Response to treatment.
1.2 Mental state
There was no significant difference between groups for total BPRS scoring (4 RCTs, n = 133, MD ‐0.09, CI ‐2.15 to 1.96, Analysis 1.2) or PANSS scores (1 RCT, n = 41, MD ‐4.00, CI ‐20.69 to 12.69, Analysis 1.3).
1.2. Analysis.
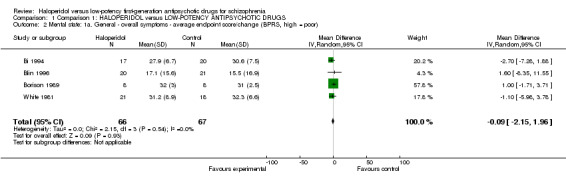
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 2 Mental state: 1a. General ‐ overall symptoms ‐ average endpoint score/change (BPRS, high = poor).
1.3. Analysis.
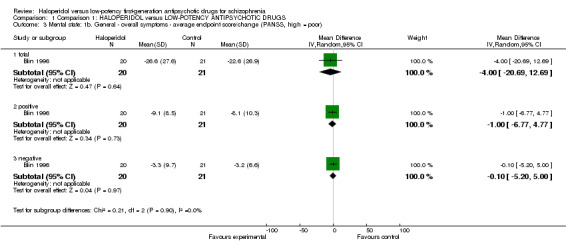
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 3 Mental state: 1b. General ‐ overall symptoms ‐ average endpoint score/change (PANSS, high = poor).
Subscores of PANSS positive symptom reduction were also not significant (1 RCT, n = 41, MD ‐1.00, CI ‐6.77 to 4.77), neither were negative symptom scores (1 RCT, n = 41, MD ‐0.10, CI ‐5.20 to 5.00, Analysis 1.3).
1.3 Global state
We found no significant difference in CGI scores between drugs (3 RCTs, n = 117, MD 0.09, CI ‐0.31 to 0.50, Analysis 1.4).
1.4. Analysis.
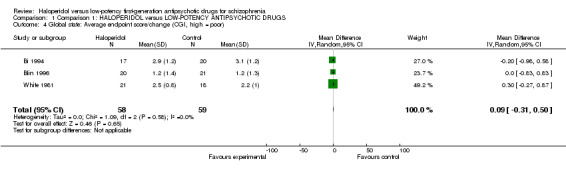
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 4 Global state: Average endpoint score/change (CGI, high = poor).
1.4 Relapse
There was no significant difference between drugs (haloperidol 7%, low‐potency drug 14%, 5 RCTs, n = 141, RR 0.64, CI 0.26 to 1.57, Analysis 1.5).
1.5. Analysis.
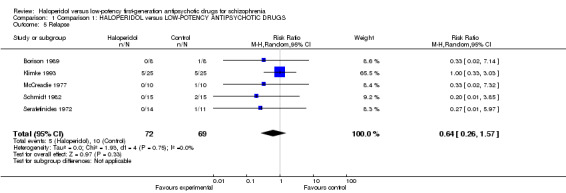
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 5 Relapse.
1.5 Leaving the study early
There was no significant difference in the number of participants leaving the study early due to any reason (haloperidol 13%, low‐potency antipsychotics 17%, 11 RCTs, n = 408, RR 0.82, CI 0.38 to 1.77, Analysis 1.6). Similar findings were evident for the reason of leaving because of adverse effects (haloperidol 2%, low‐potency antipsychotics 7%, 11 RCTs, n = 408, RR 0.44, CI 0.16 to 1.20) or inefficacy of treatment (haloperidol 4.3%, low‐potency antipsychotics 8%, 10 RCTs, n = 378, RR 0.63, CI 0.31 to 1.29).
1.6. Analysis.
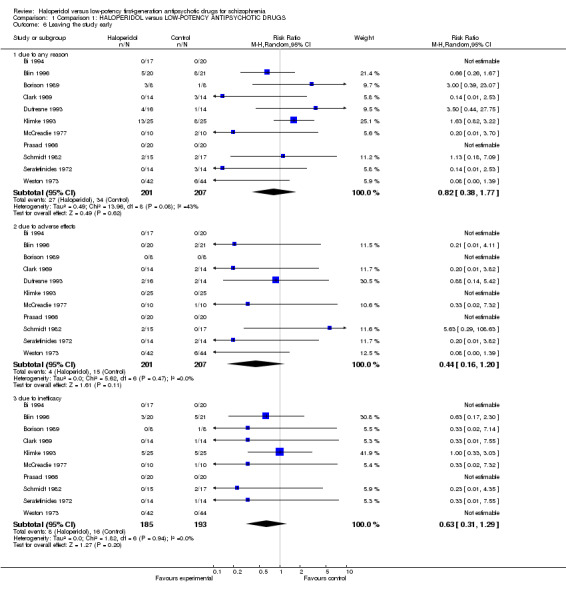
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 6 Leaving the study early.
1.6 Adverse effects
1.6.1 General
There was no significant difference for the general outcome of 'at least one adverse effect' (haloperidol 70%, low‐potency antipsychotics 35%, 5 RCTs, n = 158, RR 1.97, CI 0.69 to 5.66, Analysis 1.7).
1.7. Analysis.
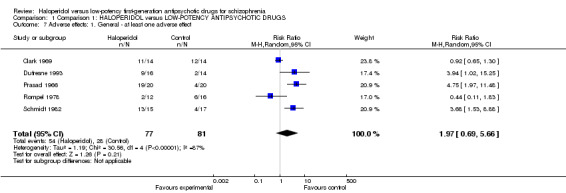
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 7 Adverse effects: 1. General ‐ at least one adverse effect.
1.6.2 Specific ‐ movement disorder
When it came to movement disorders (Analysis 1.8), there was a significant difference in favour of low‐potency antipsychotics for the outcome of 'at least one movement disorder adverse effect' (haloperidol 72%, low‐potency antipsychotics 41%, 5 RCTs, n = 170, RR 1.64, CI 1.22 to 2.21).
1.8. Analysis.
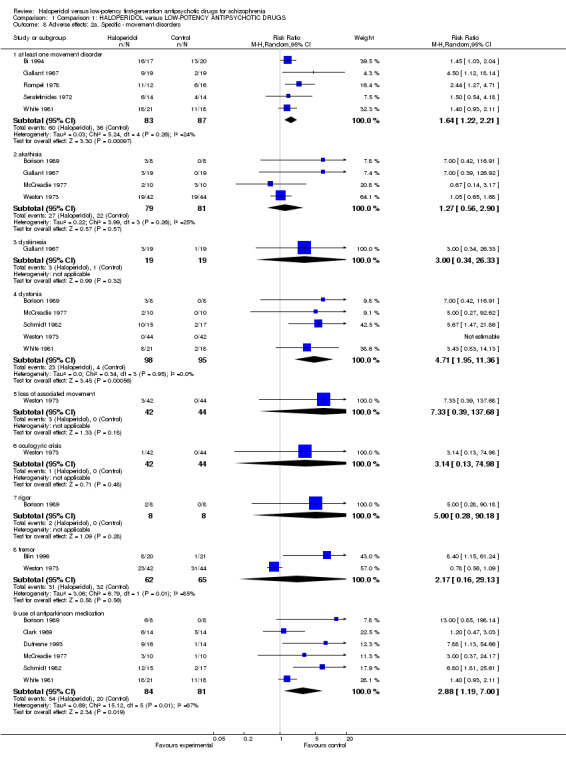
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 8 Adverse effects: 2a. Specific ‐ movement disorders.
There was no significant difference for 'akathisia' (haloperidol 34%, low‐potency antipsychotics 27%, 4 RCTs, n = 160, RR 1.27, CI 0.56 to 2.90) or dyskinesia (haloperidol 16%, low‐potency antipsychotics 5%, 1 RCT, n = 38, RR 3.00, CI 0.34 to 26.33). For dystonia, there was a significant difference in favour of low‐potency antipsychotics (haloperidol 23%, low‐potency antipsychotics 4%, 5 RCTs, n = 193, RR 4.71, CI 1.95 to 11.36) but not for 'loss of associated movement', (haloperidol 7%, low‐potency antipsychotics 0%, 1 RCT, n = 86, RR 7.33,CI 0.39 to 137.68), oculogyric crisis (haloperidol 2%, low‐potency antipsychotics 0%, 1 RCT, n = 86, RR 3.14, CI 0.13 to 74.98), or 'rigor' (haloperidol 25%, low‐potency antipsychotics 0%, 1 RCT, n = 16, RR 5.00, CI 0.28 to 90.18). For tremor, there was no significant difference (haloperidol 50%, low‐potency antipsychotics 49%, 2 RCTs, n = 127, RR 2.17, CI 0.16 to 29.13) but for the less specific 'use of antiparkinson medication' there was (haloperidol 64%, low‐potency antipsychotics 25%, 6 RCTs, n = 165, RR 2.88, CI 1.19 to 7.00). There was, however, significant heterogeneity of the study results (P < .01, I2 = 67%), but the antiparkinson medication rates were always lower in the low‐potency drug group than in the haloperidol group. Thus, the heterogeneity expresses a difference in the magnitude of the superiority rather than in the direction of the effect which is less problematic.
For rating scales, there was there a significant difference in favour of low‐potency antipsychotic drugs (2 RCTs, n = 78, MD 2.01, CI 1.35 to 2.68, Analysis 1.9).
1.9. Analysis.
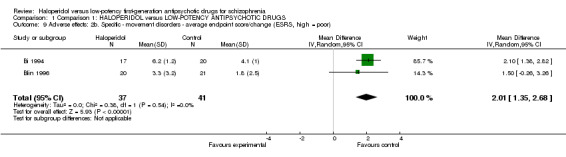
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 9 Adverse effects: 2b. Specific ‐ movement disorders ‐ average endpoint score/change (ESRS, high = poor).
1.6.3 Specific ‐ others
a. Allergy
There was no significant difference for 'allergic reactions' (haloperidol 2%, low‐potency antipsychotics 0%, 1 RCT, n = 86, RR 3.14, CI 0.13 to 74.98, Analysis 1.10) and for 'rash' (haloperidol 5%, low‐potency antipsychotics 0%, 1 RCT, n = 38, RR 3.00, CI 0.13 to 69.31). Based on a single study, there was a significant difference in favour of haloperidol for 'tongue reactions' (haloperidol 26%, low‐potency antipsychotics 52%, 1 RCT, n = 86, RR 0.50, CI 0.28 to 0.90 NNH 4 CI 2‐17).
1.10. Analysis.
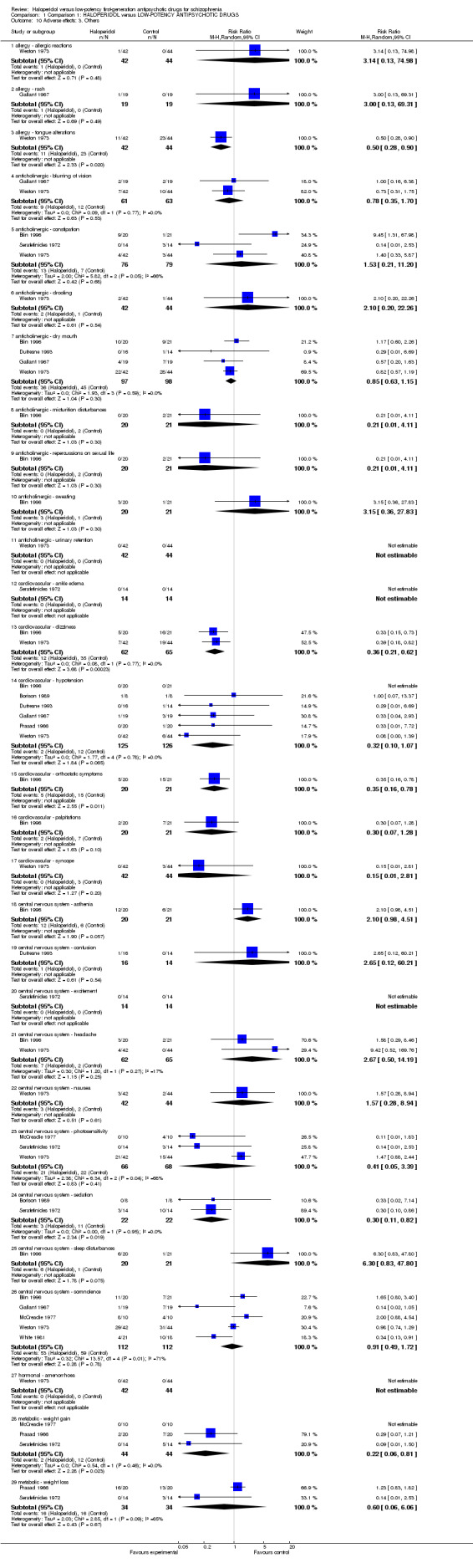
Comparison 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, Outcome 10 Adverse effects: 3. Others.
b. Anticholinergic
There was no significant difference for 'blurring of vision' (haloperidol 15%, low‐potency antipsychotics 19%, 2 RCTs, n = 124, RR 0.78, CI 0.35 to 1.70), constipation (haloperidol 17%, low‐potency antipsychotics 9%, 3 RCTs, n = 155, RR 1.53, CI 0.21 to 11.20), drooling (haloperidol 5%, low‐potency antipsychotics 2%, 1 RCT, n = 86, RR 2.10, CI 0.20 to 22.26), dry mouth (haloperidol 37%, low‐potency antipsychotics 46%, 4 RCTs, n = 195, RR 0.85, CI 0.63 to 1.15), micturition disturbances (haloperidol 0%, low‐potency antipsychotics 10%, 1 RCT, n = 41, RR 0.21, CI 0.01 to 4.11), repercussions on sexual life (haloperidol 0%, low‐potency antipsychotics 10%, 1 RCT, n = 41, RR 0.21, CI 0.01 to 4.11), sweating (haloperidol 15%, low‐potency antipsychotics 5%, 1 RCT, n = 41, RR 3.15, CI 0.36 to 27.83) or urinary retention (haloperidol 0%, low‐potency antipsychotics 0%, 1 RCT, n = 86, RR not estimable).
c. Cardiovascular
There was no significant difference for 'ankle oedema' (haloperidol 0%, low‐potency antipsychotics 0%, 1 RCT, n = 28, RR not estimable), palpitations (haloperidol 10%, low‐potency antipsychotics 33%, 1 RCT, n = 41, RR 0.30, CI 0.07 to 1.28), or syncope (haloperidol 0%, low‐potency antipsychotics 7%, 1 RCT, n = 86, RR 0.15, CI 0.01 to 2.81). There was a significant difference in favour of haloperidol for dizziness (haloperidol 19%, low‐potency antipsychotics 54%, 2 RCTs, n = 127, RR 0.36, CI 0.21 to 0.62), and orthostatic symptoms (haloperidol 25%, low‐potency antipsychotics 71%, 1 RCT, n = 41, RR 0.35, CI 0.16 to 0.78). There was a trend in favour haloperidol in terms of hypotension but the difference was not statistically significant (haloperidol 2%, low‐potency antipsychotics 46%, 6 RCTs, n = 251, RR 0.32, CI 0.10 to 1.07).
d. Central nervous system
There was a trend in favour of low‐potency antipsychotics for 'asthenia' but the difference was not statistically significant (haloperidol 60%, low‐potency antipsychotics 29%, 1 RCT, n = 41, RR 2.10, CI 0.98 to 4.51). There was no significant difference for 'confusion' (haloperidol 6%, low‐potency antipsychotics 0%, 1 RCT, n = 30, RR 2.65, CI 0.12 to 60.21), excitement (haloperidol 0%, low‐potency antipsychotics 0%, 1 RCT, n = 28, RR not estimable), headache (haloperidol 11%, low‐potency antipsychotics 4%, 2 RCTs, n = 127, RR 2.67, CI 0.50 to 14.19), nausea (haloperidol 7%, low‐potency antipsychotics 5%, 1 RCT, n = 86, RR 1.57, CI 0.28 to 8.94), photosensitivity (haloperidol 32%, low‐potency antipsychotics 32%, 3 RCTs, n = 134, RR 0.41, CI 0.05 to 3.39), somnolence (haloperidol 47%, low‐potency antipsychotics 53%, 5 RCTs, n = 224, RR 0.91, CI 0.49 to 1.72), and sleep disturbances (haloperidol 30%, low‐potency antipsychotics 5%, 1 RCT, n = 41, RR 6.30, CI 0.83 to 47.80). There was a significant difference in favour of haloperidol in terms of sedation (haloperidol 14%, low‐potency antipsychotics 41%, 2 RCTs, n = 44, RR 0.30, CI 0.11 to 0.82).
e. Hormonal
For amenorrhoea there was no significant difference (haloperidol 0%, low‐potency antipsychotics 0%, 1 RCT, n = 86, RR not estimable).
f. Metabolic
There was a significant difference in the number of participants with weight gain in favour of haloperidol (haloperidol 5%, low‐potency antipsychotics 29%, 3 RCTs, n = 88, RR 0.22, CI 0.06 to 0.81). There was no significant difference regarding weight loss (haloperidol 40%, low‐potency antipsychotics 40%, 2 RCTs, n = 68, RR 0.60, CI 0.06 to 6.06).
1.13 Missing outcomes
There were no data on potentially important outcomes such as death, hospital admission, cost, satisfaction with care, quality of life or employment.
2. Subgroup analyses
All subgroup analyses were conducted only on the primary outcome response to treatment as defined by the original studies.
2.1 Different low‐potency drugs
Seven studies compared haloperidol with chlorpromazine and did not find a significant difference in the primary outcome response to treatment (haloperidol 39%, chlorpromazine 27%, 7 RCTs, n = 290, RR 1.31, CI 0.97 to 1.77). The comparisons with levomepromazine (haloperidol 55%, levomepromazine 64%, 2 RCTs, n = 80, RR 0.86, CI 0.50 to 1.49), perazine (haloperidol 60%, perazine 53%, 1 RCT, n = 32, RR 1.13, CI 0.62 to 2.08) and thioridazine (haloperidol 32%, thioridazine 37%, 3 RCTs, n = 156, RR 0.78, CI 0.31 to 1.97) also did not find any significant difference of these low‐potency first‐generation antipsychotic drugs compared with haloperidol.
2.2 Clinical state, stage or problem
Two studies included only participants who were treatment‐resistant and did not show a superiority of haloperidol compared with low‐potency antipsychotics (drug 50%, low‐potency antipsychotics 61%, 2 RCTs, n = 59, RR 0.91, CI 0.38 to 2.17). There was no significant difference compared with the rest of the studies (test for subgroup differences: Chi² = 0.27, df = 1 (P = 0.60), I² = 0%).
3. Sensitivity analyses
All sensitivity analyses were conducted only on the primary outcome "response to treatment as defined by the original studies".
3.1 Fixed‐effect model
When a fixed‐effect model was applied, haloperidol was again not significantly different from low‐potency drugs (haloperidol 40%, low‐potency 37%, 13 RCTs, n = 558, RR 1.11, CI 0.91 to 1.36).
3.2 Exclusion of studies for which randomisation was implied because they were double‐blind
There was one study which was not explicitly described as randomised (Prasad 1966). Excluding this study did not change overall results. Haloperidol was still not significantly different from the low‐potency drugs (haloperidol 40%, low‐potency 37%, 12 RCTs, n = 518 RR 1.09, CI 0.82 to 1.44).
3.3 Exclusion of studies which were not blinded
There were two studies which were probably open (Bi 1994; Nishizono 1994). Excluding these two studies did not change overall results (haloperidol 43%, low‐potency 39%, 11 RCTs, n = 412, RR 1.11, CI 0.80 to 1.54).
3.4 Investigation of heterogeneity
There was one outlying study (Dufresne 1993). Excluding this study removed heterogeneity, but did not change the overall results (haloperidol 42%, low‐potency antipsychotics 35%, 12 RCTs, n = 528, RR 1.13, CI 0.93 to 1.39).
3.4 Assessment of dosage
There were no clearly discrepant doses between haloperidol and low‐potency antipsychotics in the included studies, therefore this sensitivity analysis was not performed.
4. Other results
4.1 Publication bias
The funnel plot of the primary outcome response to treatment as defined by the original studies was slightly asymmetrical (seeFigure 4), however only two studies were not within the scope of the funnel plot. Duval's and Tweedy's (Duval 2000) trim‐and‐fill method also did not suggest a publication bias, because the point estimate did not change much (RR 1.02, CI 0.77‐1.34).
4.
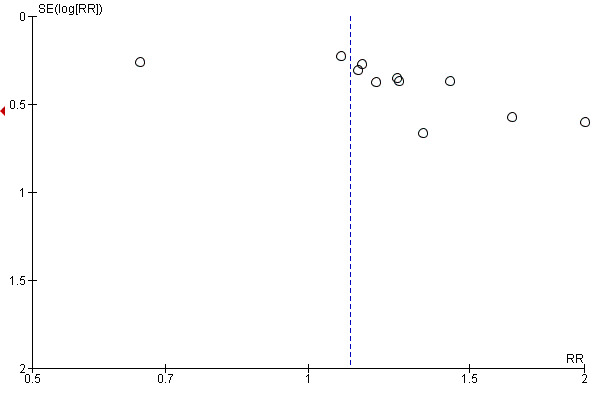
Funnel plot of comparison: 1 Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS, outcome: 1.1 Response to treatment.
4.2 'Summary of findings' table
The results of the outcomes clinically significant response, leaving the study early, at least one adverse effect, at least one movement disorder, sedation, death and quality of life were inspected in the 'Summary of findings' table. Based on this tool, we considered the quality of the results for the outcomes sedation to be moderate, for response to treatment, leaving the study due to any reason and at least one movement disorder to be low and at least one adverse effect to be very poor. Moreover, no data on the outcome death as well as quality of life were available. The judgements derived from this instrument were used for the discussion section of the review (seeDiscussion ‐ Summary of main results).
Discussion
Summary of main results
1. General
Conventional antipsychotic drugs are still used in the treatment of schizophrenia, both in poorer as well as in richer countries. This review compared haloperidol with low‐potency antipsychotics, including 17 trials with 877 participants. No superiority of haloperidol versus low‐potency antipsychotics or vice versa was found for the primary outcome 'response to treatment'. This finding is in line with the statements of treatment guidelines that low‐potency drugs are as efficacious as high‐potency antipsychotics such as haloperidol (e.g. Falkai 2005). This finding contrasts with a clinical impression that low‐potency conventional antipsychotic drugs are less efficacious than high‐potency conventional antipsychotic drugs. However, this review has general limitations. The included studies were old and small, and all studies except one (Nishizono 1994), randomised fewer than 100 participants. Current studies for registration of new antipsychotics usually include several hundreds of participants (e.g. Kane 2011). Also, for studies conducted in the 1960s and 1970s operationalised diagnostic criteria such as DSM‐III or its more recent versions were not available. The studies often did not report on the primary outcome response to treatment, used different cut‐offs of rating scale reductions to define it, and the methods of sequence generation and blinding were often poorly reported. Important outcomes such as hospital admission, death, quality of life, employment, cost of care, have not been reported at all. All in all, the data obtained are not ideal for making conclusions about the relative tolerability and efficacy of antipsychotics with differing potency, which is also reflected by the low quality of most outcomes in the 'Summary of findings' table (seeTable 1).
2. Treatment effects
2.1 Response to treatment
The overall results of the outcome response to treatment do not suggest any difference in efficacy between haloperidol and low‐potency antipsychotic drugs. This applies to the primary dichotomous outcome as well as continuous measures, although data on the latter were scarce. This result supports early narrative work, which was not based on meta‐analytic methods (Davis 1989; Klein 1969), and does not confirm the clinical perception that low‐potency antipsychotic drugs are less efficacious than haloperidol. However, the criteria and cut‐offs used for the primary outcome 'response to treatment' varied, although Furukawa 2011 showed that this is not so much of a problem as long as relative risks and odds ratios are used as effect sizes.
2.2 Leaving the study early
There was no significant difference between haloperidol and low‐potency antipsychotics in the outcome leaving the studies early due to any reason. As 'leaving the studies early for any reason' combines inefficacy of treatment and overall tolerability, it suggests that haloperidol and low‐potency antipsychotics are not different in their overall acceptability for people with schizophrenia. We also found no significant difference for leaving the study early due to adverse events as well as leaving early due to inefficacy. However, only 11 studies with 400 participants reported data on these outcomes, which is relatively little and more data would be needed to strengthen this interpretation.
2.3 Adverse effects
In those studies that reported on adverse events, high‐potency antipsychotics produced more movement disorders in terms of at least one movement disorder, dystonia and use of antiparkinson medication. Low‐potency antipsychotics produced significantly more dizziness, orthostatic symptoms, sedation and weight gain, but only a few studies reported data on these important outcomes. Weight gain can have particularly serious consequences, such as diabetes and associated mortality, and it can also have a negative effect on treatment adherence. The findings on adverse effects are in line with today's knowledge that high‐potency and low‐potency antipsychotics have different affinities and binding properties to dopamine and other receptors, and that they thus differ in the nature of adverse events (Kane 1996).
2.4 Missing outcomes
None of the included studies reported on service use, death, quality of life, participants'/carers' satisfaction with care or economic outcomes. These outcomes may be more important for afflicted people and policy makers than conventional measures of efficacy and tolerability. It is therefore disappointing that they are not available.
3. Publication bias
The funnel plot of the primary outcome response to treatment as defined by the original studies was slightly asymmetrical (seeFigure 4), so it is possible that unpublished studies we are not aware of exist. However, Egger´s test (Egger 1997) was not significant (intercept 1.11, P value 0.24, df 11) and when Duval's and Tweedy's (Duval 2000) trim‐and‐fill method was used, the relative risk did not change much. However, both are imperfect methods to test for unpublished studies, therefore, a publication bias can not be ruled out with certainty.
4. Subgroup analyses and investigation of heterogeneity
The effects of haloperidol versus each single low‐potency antipsychotic drug did not show significant differences between groups, but the results are clearly limited by the small number of trials assigned to each single low‐potency drug. There was also no difference in effects between studies with treatment‐resistant participants and the remaining studies. When the one study that accounted for heterogeneity was excluded, haloperidol was still not different from low‐potency antipsychotics in terms of clinically important response to treatment.
5. Sensitivity analyses
The exclusion of studies that were not described as randomised or double‐blind did not change the overall results of the primary outcome. Also, the results of the primary outcome were not different when a fixed‐effect model instead of a random‐effects model was applied. Therefore, the results were robust towards the sensitivity analyses.
Overall completeness and applicability of evidence
1. Completeness
Of the 17 included studies, 13 studies reported sufficient data for the primary outcome response to treatment. Therefore, we believe that the evidence on the primary outcome is quite complete. Nevertheless, several limitations, which are relevant for the conclusions of this systematic review, must be considered. The classification of high‐ and low‐potency antipsychotics is not clear cut and there were not data on all low‐potency antipsychotics. For example, there were no data for low‐potency antipsychotics such as mesoridazine, chlorprothixene or promazine, so that the overall evidence of the effectiveness of haloperidol compared with low‐potency antipsychotics is incomplete. The evidence on adverse events is particularly incomplete, as none of the included studies reported predefined adverse events such as death, suicide or cardiac effects, and for example, only three studies reported on weight gain. Finally, all studies fell in to the short‐term category. The long‐term effects are thus unclear.
2. Applicability
Half of the studies were from the 1960s and 1970s and diagnosed participants according to clinical criteria, because at that time operationalised diagnostic criteria such as DSM‐III or its more recent versions were not available. Thus it is possible that those older studies included participants who nowadays would have another diagnosis than schizophrenia. The application of the findings for people diagnosed with schizophrenia nowadays must therefore be made with caution. Furthermore, most of the included studies were characterised by small sample sizes (< 100 participants). It has been estimated that approximately 1000 participants need to be included in psychiatric meta‐analyses for the results to be robust (Trikalinos 2004). However, this meta‐analyses included altogether only 677 participants and the primary outcome response to treatment was based on only 574 participants.
Quality of the evidence
None of the included studies described the exact randomisation and allocation concealment methods and had to be rated as unclear in this regard. Most of the included studies were described as double‐blind but did not report many details on the methods and success of blinding. Lack of blinding is not necessarily a problem for objective outcomes, but problematic for subjective outcomes such as response to treatment or side‐effects. One could also argue that studies could also have unblinded by differences in side‐effects. We feel that the authors who used identical capsules probably did what they could, but we would recommend that the success of blinding should be verified in future trials. Most of the studies were not free of selective reporting because they failed to report on previously defined outcomes or on standard deviations for continuous outcomes. Incomplete outcome data and selective reporting can have an influence on the estimates of effect. There were also studies which did not report on the number of patients involved in the group or reported results independent of the treatment condition. Thus, a quarter of the included studies could not be used for the main outcome and even less so for other outcomes such as adverse events. In summary, the overall quality of the studies according to these criteria was rather low (also seeTable 1).
Potential biases in the review process
We pooled all low‐potency antipsychotics in one group for all outcomes except for the primary outcome response to treatment, for which we also performed a subgroup analysis of each single low‐potency antipsychotic drug. However, the number of studies available for each individual drug was so small that analyses of most results would not have been meaningful and the "high‐potency versus low‐potency antipsychotic" classification is frequently used. Moreover, the search was based on Cochrane Schizophrenia Trials Register and the last search date was 2010, so it is possible that there are unpublished trials that we are not aware of. There is a possibility of publication bias, although the funnel plot may also be slightly asymmetrical due to other factors.
We decided post‐hoc to include all outcomes reported by a study, not only the predefined outcomes. This change to the protocol was made on the basis that other outcomes (e.g. non‐prespecified adverse effects) might be important as well and did not affect review authors´ biases.
Agreements and disagreements with other studies or reviews
We are not aware of other reviews on the efficacy of haloperidol versus low‐potency antipsychotic drugs.
Authors' conclusions
Implications for practice.
1. For clinicians
Clinicians should know that we did not find any clear differences in efficacy between haloperidol and low‐potency first‐generation antipsychotics. However, haloperidol produced more movement disorders, while low‐potency antipsychotics produced more weight gain, dizziness and orthostatic symptoms. However, due to the limited number of studies and participants, and due to an overall low quality of the included studies, the results have to be interpreted with caution.
2. For people with schizophrenia
For people with schizophrenia, it is important to know that there is moderate quality evidence that haloperidol and low‐potency antipsychotics are approximately equal in their effects on treatment response, and that there is evidence of lower quality and that they clearly differ in side‐effects (such as weight gain and movement disorders). They might tell their doctors that they want to be involved in the choice of the antipsychotic that is best for them.
3. For managers/policy makers
There were no data on re‐hospitalisation, economic outcomes, healthy days or quality of life, which can be considered very important outcomes for decision makers. Thus, it is not possible to make any recommendations apart from the fact that all of the examined drugs in this review have lost their patents and are therefore rather inexpensive.
Implications for research.
1. General
The outcome reporting about the effects of haloperidol versus low‐potency antipsychotics was insufficient. Few data were available, and long‐term effects were not at all reported. Strict adherence to the CONSORT statement (Moher 2010) would make such studies much more informative.
2. Specific
2.1 Reviews
Studies we have had to exclude because they were not directly relevant, however, do still show how this compound has been evaluated in other ways. Some of these remain clinically relevant and may merit further systematic reviews (Table 3).
2. Comparisons suggested by excluded studies.
| Comparison | Excluded study tag | Current relevant Cochrane review |
| Amitriptyline for schizophrenia | Teja 1975 | Whitehead 2002 |
| Antipsychotic (various) withdrawal versus continuation for schizophrenia | Crow 1986 | ‐ |
| Benztropine plus antipsychotic combinations for schizophrenia | Singh 1975 | ‐ |
| Haloperidol dose for schizophrenia | Smith 1985 | Donnelly 2013 |
| Haloperidol versus mid/high‐potency antipsychotic drugs for schizophrenia | Giordana 1984 (pipotiazine), Nahunek 1982 (oxyprothepine) | ‐ |
| Haloperidol versus placebo | Garry 1962, Marjerrison 1971, Nahunek 1982, Teja 1975 | Adams 2013 |
| Olanzapine versus first‐generation antipsychotic drugs for schizophrenia | de Lima 2005 | Duggan 2005 |
| Sulpiride for schizophrenia | Cassano 1975, Guazzelli 1995, Mori 1989, Munk‐Andersen 1984, Okuda 1979, Rama 1981, Ropert 1989 | Omori 2009, Soares 1999 |
2.2 Trials
The number of studies providing data adverse events was low, as well as the overall quality of the included studies. Thus it would be of interest to have newer and longer studies examining the difference between haloperidol and low‐potency antipsychotic drugs, as first‐generation antipsychotic drugs are still frequently prescribed, not only in developing countries but also in some industrial nations such as Germany. Table 4 presents an outline for a study design.
3. Design of a future study.
| Methods | Allocation: randomised ‐ clearly described generation of sequence and concealment of allocation. Blinding: double ‐ described and tested. Duration: 6 months. |
| Participants | People with schizophrenia or schizophrenia‐like disorder. N = 500. Age: any. Sex: both. History: any. |
| Interventions | 1. High‐potency antipsychotic drug (haloperidol). 2. Low‐potency antipsychotic drugs. |
| Outcomes | Response (primary outcome) Global state (number of participants improved) Leaving the study early (including specific causes) Death (natural and unnatural causes) Quality of life Satisfaction with care Days in hospital Number of healthy days Side‐effects Employment and other measures of functioning |
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the methods sections of their reviews. We have used this text as the basis of what appears here and adapted it as required.
Data and analyses
Comparison 1. Comparison 1: HALOPERIDOL versus LOW‐POTENCY ANTIPSYCHOTIC DRUGS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to treatment | 14 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.86, 1.44] |
| 2 Mental state: 1a. General ‐ overall symptoms ‐ average endpoint score/change (BPRS, high = poor) | 4 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐2.15, 1.96] |
| 3 Mental state: 1b. General ‐ overall symptoms ‐ average endpoint score/change (PANSS, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 total | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐20.69, 12.69] |
| 3.2 positive | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐6.77, 4.77] |
| 3.3 negative | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐5.20, 5.00] |
| 4 Global state: Average endpoint score/change (CGI, high = poor) | 3 | 117 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.31, 0.50] |
| 5 Relapse | 5 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.26, 1.57] |
| 6 Leaving the study early | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 due to any reason | 11 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.38, 1.77] |
| 6.2 due to adverse effects | 11 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.20] |
| 6.3 due to inefficacy | 10 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.31, 1.29] |
| 7 Adverse effects: 1. General ‐ at least one adverse effect | 5 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.69, 5.66] |
| 8 Adverse effects: 2a. Specific ‐ movement disorders | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 at least one movement disorder | 5 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.22, 2.21] |
| 8.2 akathisia | 4 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.56, 2.90] |
| 8.3 dyskinesia | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.34, 26.33] |
| 8.4 dystonia | 5 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 4.71 [1.95, 11.36] |
| 8.5 loss of associated movement | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 7.33 [0.39, 137.68] |
| 8.6 oculogyric crisis | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 8.7 rigor | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.28, 90.18] |
| 8.8 tremor | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.16, 29.13] |
| 8.9 use of antiparkinson medication | 6 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [1.19, 7.00] |
| 9 Adverse effects: 2b. Specific ‐ movement disorders ‐ average endpoint score/change (ESRS, high = poor) | 2 | 78 | Mean Difference (IV, Random, 95% CI) | 2.01 [1.35, 2.68] |
| 10 Adverse effects: 3. Others | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 allergy ‐ allergic reactions | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 10.2 allergy ‐ rash | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.31] |
| 10.3 allergy ‐ tongue alterations | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 10.4 anticholinergic ‐ blurring of vision | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.35, 1.70] |
| 10.5 anticholinergic ‐ constipation | 3 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.21, 11.20] |
| 10.6 anticholinergic ‐ drooling | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.20, 22.26] |
| 10.7 anticholinergic ‐ dry mouth | 4 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.15] |
| 10.8 anticholinergic ‐ micturition disturbances | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.11] |
| 10.9 anticholinergic ‐ repercussions on sexual life | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.11] |
| 10.10 anticholinergic ‐ sweating | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.36, 27.83] |
| 10.11 anticholinergic ‐ urinary retention | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.12 cardiovascular ‐ ankle edema | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.13 cardiovascular ‐ dizziness | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.21, 0.62] |
| 10.14 cardiovascular ‐ hypotension | 6 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.10, 1.07] |
| 10.15 cardiovascular ‐ orthostatic symptoms | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.16, 0.78] |
| 10.16 cardiovascular ‐ palpitations | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.07, 1.28] |
| 10.17 cardiovascular ‐ syncope | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 2.81] |
| 10.18 central nervous system ‐ asthenia | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.98, 4.51] |
| 10.19 central nervous system ‐ confusion | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.12, 60.21] |
| 10.20 central nervous system ‐ excitement | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.21 central nervous system ‐ headache | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.50, 14.19] |
| 10.22 central nervous system ‐ nausea | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.28, 8.94] |
| 10.23 central nervous system ‐ photosensitivity | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.05, 3.39] |
| 10.24 central nervous system ‐ sedation | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.11, 0.82] |
| 10.25 central nervous system ‐ sleep disturbances | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 6.30 [0.83, 47.80] |
| 10.26 central nervous system ‐ somnolence | 5 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.49, 1.72] |
| 10.27 hormonal ‐ amenorrhoea | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.28 metabolic ‐ weight gain | 3 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.81] |
| 10.29 metabolic ‐ weight loss | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.06, 6.06] |
Comparison 2. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to treatment ‐ each low‐potency antipsychotic separately | 13 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.85, 1.42] |
| 1.1 versus chlorpromazine | 7 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.97, 1.77] |
| 1.2 versus levomepromazine | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.50, 1.49] |
| 1.3 versus perazine | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.08] |
| 1.4 versus thioridazine | 3 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.97] |
| 2 Response to treatment ‐ treatment resistance | 13 | 558 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.85, 1.42] |
| 2.1 not treatment resistant | 11 | 499 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.89, 1.51] |
| 2.2 treatment resistant | 2 | 59 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.38, 2.17] |
2.1. Analysis.
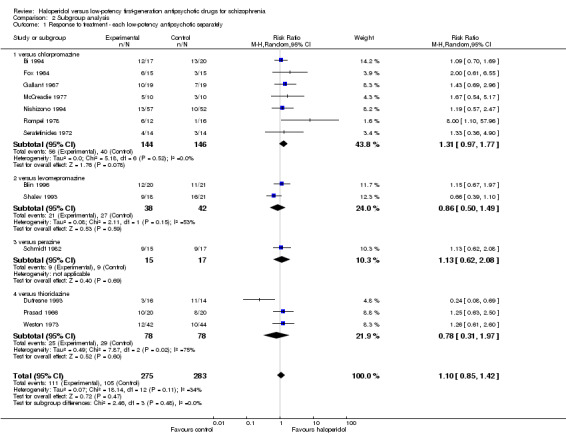
Comparison 2 Subgroup analysis, Outcome 1 Response to treatment ‐ each low‐potency antipsychotic separately.
2.2. Analysis.
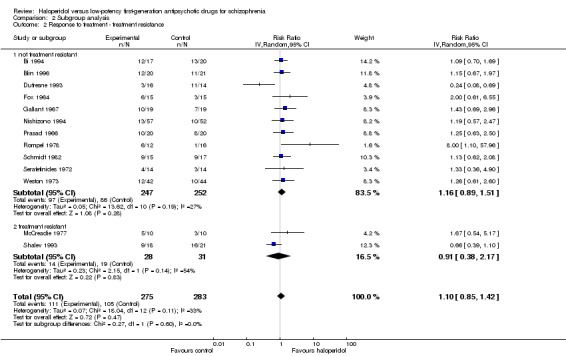
Comparison 2 Subgroup analysis, Outcome 2 Response to treatment ‐ treatment resistance.
Comparison 3. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to treatment ‐ fixed‐effect model | 13 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.36] |
| 2 Response to treatment ‐ exclusion of studies in which randomisation was assumed from blinding | 12 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.82, 1.44] |
| 3 Response to treatment ‐ exclusion of non double‐blind studies | 11 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.80, 1.54] |
| 4 Response to treatment ‐ exclusion of outlier study leading to heterogeneity (Dufresne 1993) | 12 | 528 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.93, 1.39] |
3.1. Analysis.
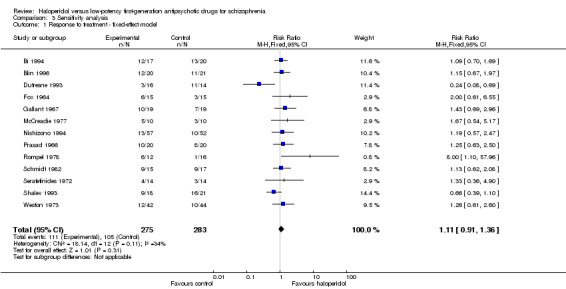
Comparison 3 Sensitivity analysis, Outcome 1 Response to treatment ‐ fixed‐effect model.
3.2. Analysis.
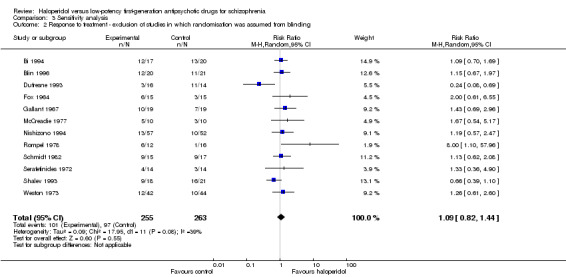
Comparison 3 Sensitivity analysis, Outcome 2 Response to treatment ‐ exclusion of studies in which randomisation was assumed from blinding.
3.3. Analysis.
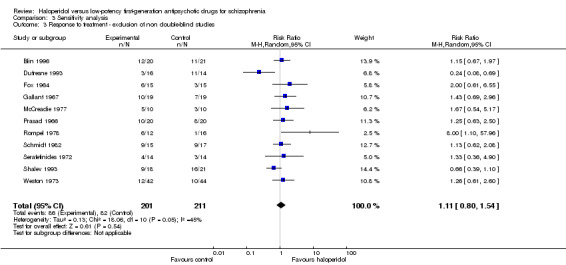
Comparison 3 Sensitivity analysis, Outcome 3 Response to treatment ‐ exclusion of non double‐blind studies.
3.4. Analysis.
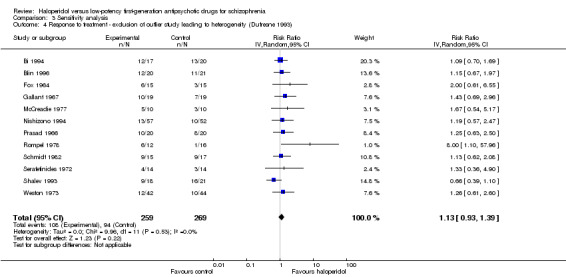
Comparison 3 Sensitivity analysis, Outcome 4 Response to treatment ‐ exclusion of outlier study leading to heterogeneity (Dufresne 1993).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bi 1994.
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: n.i., probably open. Duration: 4 weeks. Design: parallel. Location: n.i.. Setting: n.i.. | |
| Participants | Diagnosis: schizophrenia (Research Diagnostic Criteria RDC) and (CCMD‐2, Chinese classification of mental disorders). N = 37. Gender: 24 M, 26 F. Age: 18‐45 years. History: duration stable ‐ n.i., duration ill ‐ n.i., number of previous hospitalisations ‐ n.i., age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i.. | |
| Interventions | 1. Haloperidol: fixed/flexible dose n.i., allowed dose range 5 to 100 mg/day, mean dose n.i.. N = 17. 2. Chlorpromazine: fixed/flexible dose n.i., allowed dose range 50 to 500 mg/day, mean dose n.i.. N = 20. Other medication: n.i.. | |
| Outcomes | Response to treatment: BPRS (no clear definition).
Leaving the study early.
Mental state: BPRS. Global state: CGI. Adverse effects: at least one movement disorder, average score change in EPS. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not indicated, probably open. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not indicated, probably open. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement about incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment about selective reporting. |
| Other bias | Low risk | No clear other bias. |
Blin 1996.
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double, no further details. Duration: 4 weeks. Design: parallel. Location: n.i.. Setting: inpatients. | |
| Participants | Diagnosis: acute exacerbation of schizophrenia (DSM‐III‐R) and symptoms of anxiety (Psychotic Anxiety Scale > 34). N = 41. Gender: 24 M, 17 F. Age: mean 34.05 years. History: duration stable ‐ n.i., duration ill ‐ 3 participants < 1 year; 4 participants 1‐3 years; 33 participants > 3 years, number of previous hospitalisations ‐ n.i., age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose n.i.. | |
| Interventions | 1. Haloperidol ‐ flexible doses. Allowed dose range: 4 to 12 mg/day, mean dose 7.6 mg/day. N = 20.
2. Methotrimeprazine (levomepromazine) ‐ flexible doses. Allowed dose range: 50 to 150 mg/day, mean dose 100 mg/day. N = 21. Other medication: no other antipsychotics allowed except diazepam for extremely disturbed behavioUr; benzodiazepines, biperiden for EPS, heptaminol hydrochloride. |
|
| Outcomes | Response to treatment: at least 20% reduction in PANSS total score. Mental state: PANSS, BPRS. Global state: CGI. Leaving the study early. Adverse events: Extrapyramidal Symptom Rating Scale, movement disorders (tremor), adverse effects other (asthenia, constipation, dizziness, dry mouth, headache, hypotension, micturition disturbances, orthostatic symptoms, palpitations, repercussions on sexual life, somnolence, sleep disturbances, sweating). Unable to use: Mental state: PANSS anxiety reduction scores (incomplete data, no SD). Mental state: Psychotic Anxienty Scale (incomplete data, no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% of the participants in the haloperidol group left the study early, as compared to 38% in the levomepromazine group. The number was clearly higher in the low‐potency group and the reasons differed. Data were analysed on a LOCF method, which is an imperfect method. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No clear other bias. |
Borison 1989.
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double, no further details. Duration: 6 weeks. Design: parallel. Location: n.i., Setting: n.i., | |
| Participants | Diagnosis: schizophrenia (DSM‐III). N = 10. Gender: n.i., Age: range 18‐60 years. History: duration stable ‐ n.i., duration ill ‐ n.i., nr. of prev. hospitalisations ‐ n.i., age at onset ‐ n.i., severity of illness ‐ min. 35 on BPRS, baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 15 to 75 mg/t.i.d., mean dose n.i.. N = 8. 2. Thioridazine: flexible dose, allowed dose range 150 to 750 mg/t.i.d., mean dose n.i.. N = 8. Other medication: chloral hydrate. | |
| Outcomes | Response to treatment: BPRS (no definition).
Mental state: BPRS. Relapse. Leaving the study early. Adverse events : movement disorders (akathisia, dystonia, rigor, use of antiparkinson medication), other adverse effects (hypotension, sedation). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 out of 8 participants from the thioridazine group (13%) left the study early due to inefficacy of treatment. 3 out of 8 participants from the haloperidol group (38%) left the study early to due improvement and administrative reasons. Drop‐outs were not included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Clark 1969.
| Methods | Randomisation: n.i. but double‐blind. Allocation: procedure not described. Blinding: double, no further details. Duration: 12 weeks. Design: parallel. Location: n.i., Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (clinical diagnosis). N = 28. Gender: 11 M, 17 F. Age: range 22‐61 years. History: duration stable ‐ n.i., duration ill ‐ n.i., number of previous hospitalisations ‐ n.i., age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 3 to 15 mg/day, mean dose n.i.. N = 14. 2. Chlorpromazine: flexible dose, allowed dose range 200 to 1600 mg/day, mean dose n.i.. N = 14. Other medication: n.i.. | |
| Outcomes | Leaving the study early. Adverse effects: at least one adverse effect, movement disorders (use of antiparkinson medication). Unable to use: Mental state: BPRS (incomplete data, no mean, no SD). Global state: CGI (incomplete data, no mean, no SD). Behaviour: NOSIE (incomplete data, no mean, no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. but double‐blind. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21% of participants from the chlorpromazine group left the study early. A completer analysis was applied. |
| Selective reporting (reporting bias) | High risk | CGI, BPRS, no mean and no SD. |
| Other bias | Unclear risk | There is insufficient information to permit judgment about other bias. |
Dufresne 1993.
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double, identical capsules. Duration: 6 weeks. Design: parallel. Location: n.i., Setting: n.i., | |
| Participants | Diagnosis: schizophrenia (DSM‐III). N = 30. Gender: 20 F, 10 M. Age: mean 34 years. History: duration stable ‐ n.i., duration ill ‐ mean 13 years, nr. of prev. hospitalisations ‐ 7, age at onset ‐ mean 21 years, severity of illness ‐ BPRS at baseline 59.9 (SD12.3), baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 5 to 40 mg/day, mean dose n.i.. N = 16. 2. Thioridazine: flexible dose, allowed dose range 100 to 800 mg/day, mean dose N = 14. Other medication: amantadine (antiparkinson medication), chloral hydrate. | |
| Outcomes | Response to treatment: at least 20% reduction in BPRS total score. Leaving the study early. Adverse effects: at least one adverse effect, movement disorders (use of antiparkinson medication), other adverse effects (confusion, dry mouth, hypotension). Unable to use: Mental state: BPRS (incomplete data, no SD). Depression: HAM‐D (incomplete data, no SD). Global state: CGI (incomplete data, no SD). Adverse effects: weight (incomplete data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, identical capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double, identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% of haloperidol participants left the study early. A completers‐only method was used in the study. |
| Selective reporting (reporting bias) | High risk | No complete data for BPRS and HAM‐D, SDs are missing. |
| Other bias | Low risk | No clear other bias. |
Fox 1964.
| Methods | Randomisation: randomly selected, no further details. Allocation: procedure not described. Blinding: double, no further details. Duration: 1 month. Design: parallel. Location: single centre. Setting: inpatient. | |
| Participants | Diagnosis: chronic schizophrenic reaction (clinical diagnosis). N = 30. Gender: 45 F. Age: mean 43.1 years. History: duration stable ‐ n.i., duration ill ‐ n.i., number of previous hospitalisations ‐ years mean 14.3, age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 2 to 16 mg/day, mean dose 13 mg/day. N = 15. 2. Chlorpromazine: flexible dose, allowed dose range 100 to 800 mg/day, mean dose 593 mg/day. N = 15. Other medication: Cogentin. | |
| Outcomes | Response to treatment: clinical judgement. Unable to use: Mental state: BPRS (incomplete data, no mean). Global state: CGI (incomplete data, no mean). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly selected, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence for incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Incomplete BPRS results (no SDs). |
| Other bias | Low risk | No clear other bias. |
Gallant 1967.
| Methods | Randomisation: randomly assigned, no further details. Allocation: procedure not described. Blinding: double, all drugs were supplied in identical capsules and dispensed from individual medication bottles, which were prepared and coded prior to the study. Duration: 4 weeks. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: acute schizophrenia patients, clinical diagnosis. N = 28. Gender: 30 M, 28 F. Age: mean 33.4 years. History: duration stable ‐ n.i., duration ill ‐ n.i., nr. of prev. hospitalisations ‐ n.i., age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range max. 16 mg/day, mean dose n.i.. N = 19. 2. Chlorpromazine: flexible dose, allowed dose range max. 800 mg/day, mean dose n.i.. N = 19. Other medication: antiparkinson medication. | |
| Outcomes | Response to treatment: clinical judgement. Adverse effects: movement disorders (at least one movement disorder, akathisia, dyskinesia), other adverse effects (blurring of vision, dry mouth, hypotension, rash, somnolence). Unable to use: Mental: Beckomberga Rating Scale (incomplete data, no SD). Behaviour: MACC Behavioral Adjustment Scale (incomplete data, no SD). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, all drugs were supplied in identical capsules and dispensed from individual medication bottles, which were prepared and coded prior to the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double, all drugs were supplied in identical capsules and dispensed from individual medication bottles, which were prepared and coded prior to the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence for incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | SDs were not reported for any of the outcome scales. |
| Other bias | Low risk | No evidence for other bias. |
Klimke 1993.
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double, patients and psychiatrists were blind to treatment conditions. Duration: 24 days. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: acute schizophrenia (ICD‐9). N = 50. Gender: 21 M, 29 F. Age: mean 36.6 years. History: duration stable ‐ n.i., duration ill ‐ mean 6.7 years, number of previous hospitalisations ‐ mean 2.7, age at onset ‐ mean 29.9 years, severity of illness ‐ BPRS score mean 47.8 SD 14.1, baseline antipsychotic dose ‐ 15 mg/day haloperidol i.v.. |
|
| Interventions | 1. Haloperidol: fixed dose, mean dose 15 mg/day. N = 25. 2. Perazine: fixed dose, mean dose 300 mg/day. N = 25. Other medication: 2 mg flunitrazepam (sleep disturbance), biperiden. | |
| Outcomes | Relapse. Leaving the study early. Unable to use: Response to treatment: BPRS (no definition, results analysed by early responders and non‐responders). Mental state: BPRS (incomplete data, no SD). Global state: global judgment of therapeutic efficacy (incomplete data, no SD). Adverse effects (no usable data, results analysed by early responders and non‐responders). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, patients and psychiatrists were blind to treatment conditions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double, patients and psychiatrists were blind to treatment conditions. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 out of 25 participants from the haloperidol group (52%) and 8 out of 25 participants from the perazine group (32%) left the study early. A LOCF methods was applied. |
| Selective reporting (reporting bias) | High risk | BPRS results only available for subgroups or independent of treatment condition. |
| Other bias | Low risk | No evidence for other bias. |
McCreadie 1977.
| Methods | Randomisation: randomly assigned, no further details. Allocation: procedure not described. Blinding: double, no further details, but tested at the end of the study before blind was broken. Psychiatrists guessed correctly in 8 participants, wrongly in 6. Duration: 12 weeks. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (Schneiderian first rank symptoms). N = 20. Gender: all male. Age: mean 52 years. History: duration stable ‐ n.i., duration ill ‐ n.i., number of previous hospitalisations ‐ mean length 20 years, age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 15 to 100 mg/day, mean dose n.i.. N = 10.
2. Chlorpromazine: flexible dose, allowed dose range 100 to 600 mg/day, mean dose n.i.. N = 10. Other medication: antiparkinson medication. |
|
| Outcomes | Response to treatment: clinical judgement. Relapse. Leaving the study early. Adverse effects: movement disorders (akathisia, dystonia, use of antiparkinson medication), other adverse effects (photosensitivity, somnolence, weight gain). Unable to use: Mental state: Lorr Rating Scale modification ‐ Hamilton scale (unpublished rating scale). Behaviour: NOSIE (incomplete data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double, no further details, but tested at the end of the study before blind was broken. Psychiatrists guessed correctly in 8 participants, wrongly in 6. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Double, no further details, but tested at the end of the study before blind was broken. Psychiatrists guessed correctly in 8 participants, wrongly in 6. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 out of 10 (20%) participants in the CPZ group left the study early due to adverse effects and inefficacy and were not included in the final analysis (completers only). |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No evidence for other bias. |
Nishizono 1994.
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: n.i., probably open. Duration: 4 weeks. Design: n.i., Location: multicentre. Setting: n.i., | |
| Participants | Diagnosis: schizophrenia (ICD‐10). N = 109. Gender: n.i., Age: n.i., History: duration stable ‐ n.i., duration ill ‐ n.i., number of previous hospitalisations ‐ n.i., age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range n.i., dose max. 21 mg/day. N = 57. 2. Chlorpromazine: flexible dose, allowed dose range n.i., dose max. 450 mg/day. N = 52. Other medication: n.i.. | |
| Outcomes | Response to treatment: at least 50% reduction in BPRS total score. Unable to use: Mental state: BPRS (incomplete data, no SD). Global state: Global Assessment Scale (incomplete data, no SD). Leaving the study early (no data available). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | n.i., probably open. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | n.i., probably open. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not enough information to permit judgement about incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement about selective reporting. |
| Other bias | Unclear risk | Insufficient information about other sources of bias. |
Prasad 1966.
| Methods | Randomisation: not described, but double‐blind. Allocation: procedure not described. Blinding: double, drugs were supplied in identical bottles marked by serial number without disclosing identity. Duration: 12 weeks. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia symptoms (clinical diagnosis). N = 40. Gender: 3 males, 37 females. Age: mean 51.8 years. History: duration stable ‐ n.i., duration ill ‐ mean 15.07 years, nr. of prev. hospitalisations ‐ n.i., age at onset ‐ mean 36.7 years, severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 2 to 24 mg/day, mean dose 16.1 mg/day. N = 20. 2. Thioridazine: flexible dose, allowed dose range 60 to 720 mg/day, mean dose 518.6 mg/day. N = 20. Other medication: trihexyphenicyl, benztropine. | |
| Outcomes | Response to treatment: clinical judgement. Leaving the study early. Adverse effects: at least one adverse effect, other adverse effects (hypotension, weight gain, weight loss). Unable to use: Mental state: Lorr Rating Scale (incomplete data, no mean, no SD). Adverse effects: diminished vision, corneal haziness, nasal congestion, agitation, akathisia, rigidity, drowsiness, drooling, flushed face, nictitation, tremor, nervous, dysphagia, sluggishness, confusion (all number of incidences, not number of participants). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not described, but double‐blind. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, drugs were supplied in identical bottles marked by serial number without disclosing identity. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double, drugs were supplied in identical bottles marked by serial number without disclosing identity. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence for incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | Lorr Scale no means and SDs. |
| Other bias | Low risk | No clear other bias. |
Rompel 1978.
| Methods | Randomisation: random, no further details. Allocation: procedure not described. Blinding: double, tablets were in ordinary commercial form and in numbered bottles, issued by senior nursing staff, who were strictly forbidden to tell, in order to maintain investigator blind. Duration: 8 weeks. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: firmly diagnosed as schizophrenics (clinical diagnosis). N = 24. Gender: 15 M, 10 F. Age: mean 43.3 years. History: duration stable ‐ n.i., duration ill ‐ n.i., number of previous hospitalisations ‐ years mean 11.8, age at onset ‐ n.i., severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 5 to 30 mg/day, mean dose n.i.. N = 12. 2. Chlorpromazine: flexible dose, allowed dose range 50 to 600 mg/day, mean dose n.i.. N = 12. Other medication: antiparkinson medication (Kemadrin). | |
| Outcomes | Response to treatment: clinical judgement. Adverse effects: at least one adverse effect, movement disorders (at least one MD). Unable to use: Leaving the study early (no data available). Mental state: assessment on 18‐symptom criteria by unpublished rating scale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double, tablets were in ordinary commercial form and in numbered bottles, issued by senior nursing staff, who were strictly forbidden to tell, in order to maintain investigator blind. This means that at least part of the personnel was not blind which can have led to performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double, tablets were in ordinary commercial form and in numbered bottles, issued by senior nursing staff, who were strictly forbidden to tell, in order to maintain investigator blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 out of 13 participants from the chlorpromazine group left the study early but were replaced with others to keep the total up to 25 participants, thus having a total of 28 participants. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
Schmidt 1982.
| Methods | Randomisation: randomised, no further details. Allocation: procedure not described. Blinding: double, no further details. Duration: 4 weeks. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: acute paranoid hallucinatory psychosis (ICD 9). N = 32. Gender: 32 M. Age: mean 31.3 years. History: duration stable ‐ n.i., duration ill ‐ mean 3.8 years, number of previous hospitalisations ‐ mean 3, age at onset ‐ mean 27.5, severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 15 to 45 mg/day, mean dose 27.68 mg/day. N = 15. 2. Perazine: flexible dose, allowed dose range 300 to 900 mg/day, mean dose 612 mg/day. N = 17. Other medication: biperiden. | |
| Outcomes | Response to treatment: CGI Scale (no definition). Relapse. Leaving the study early. Adverse effects: at least one adverse effect, movement disorders (dystonia, use of antiparkinson medication). Unable to use: Behaviour: weekly records of ward behaviour (incomplete data, no SD). Mental state: subjective well‐being EWL‐K Jahnke & Debus 1977 (incomplete data, no Sd). Global state: global clinical impression (incomplete data, no mean, no SD). Adverse effects: gait, drooling (number of incidence, not number of participants). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 out of 17 participants from the perazine group (12%) left the study early due to inefficacy and had to be switched to haloperidol. |
| Selective reporting (reporting bias) | High risk | Outcomes of interest reported incompletely (Simpson Angus Scale ‐ only sum scores, CGI ‐ no data, EWL ‐ no data, fine motor skills ‐ no data). |
| Other bias | Low risk | No clear other bias. |
Serafetinides 1972.
| Methods | Randomisation: randomly assigned, no further details. Allocation: procedure not described. Blinding: double, all medications prepared in identically appearing capsules. Duration: 12 weeks. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: chronic schizophrenia, clinical diagnosis. N = 28. Gender: 25 M, 32 F. Age: mean 41.5 years. History: duration stable ‐ n.i., duration ill ‐ mean 15 years, number of previous hospitalisations ‐ 10.5, age at onset ‐ mean 26.5 years, severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range n.i., mean dose 12.3 mg/day. N = 14.
2. Chlorpromazine: flexible dose, allowed dose range n.i., mean dose 830 mg/day. N = 14. Other medication: concomitant medications for Parkinsonism, bedtime sedation. |
|
| Outcomes | Response to treatment: CGI Scale (no definition). Relapse. Leaving the study early. Adverse effects: at least one movement disorder, adverse effects ‐ other (ankle oedema, constipation, excitement, photosensitivity, sedation, weight gain, weight loss). Unable to use: Mental state: BPRS (incomplete data). Global state: CGI scale for Severity of Illness and Severity of Improvement (incomplete data). Behaviour: NOSIE, OBRS (all analysed by single items, no total score). Cognitive: Purdue Pegboard Test, Digit Symbol test (incomplete data) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, all medications prepared in identically appearing capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double, all medications prepared in identically appearing capsules. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 out of 14 participants in the CPZ group (21%) left the study early due to worsening and were not included in the final analysis (completers only). No participant from the HAL group left the study early. |
| Selective reporting (reporting bias) | High risk | Incomplete data for BPRS, NOSIE and OBRS (no SD´s). |
| Other bias | Low risk | No evidence for other bias. |
Shalev 1993.
| Methods | Randomisation: randomly assigned, no further details. Allocation: treating psychiatrists were blind to the sequence in which the drugs were to be given to the patient. Blinding: not described, probably open. Duration: 4 weeks. Design: parallel (second part cross‐over). Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: chronic or subchronic schizophrenia (DSM‐III). N = 39. Gender: 35 F, 25 M. Age: mean 33 years. History: duration stable ‐ n.i., duration ill ‐ mean 4.8 years, number of previous hospitalisations ‐ mean 4.2, age at onset ‐ mean 28.2 years, severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range n.i., mean dose 29.3 mg/day (SD 19.9). N = 18. 2. Levomepromazine: flexible dose, allowed dose range n.i., mean dose 379 mg/day (SD 128). N = 21. Other medication: antiparkinson medication. | |
| Outcomes | Response to treatment: clinical judgement. Unable to use: Mental state: BPRS (analysed by responders and non‐responders, not by medication). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | "Treating psychiatrists were blind to the sequence in which the drugs were to be given to the patient." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, probably open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described, probably open |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 participants left the study early but it is not mentioned how many from which drug group |
| Selective reporting (reporting bias) | Low risk | No clear selective outcome reporting. |
| Other bias | Low risk | No evidence for other bias. |
Weston 1973.
| Methods | Randomisation: randomly assigned, no further details. Allocation: procedure not described. Blinding: double ‐ drugs supplied as tablets of identical colour and form, each patient being provided with a coded drug container. Duration: 12 weeks. Design: parallel. Location: single centre. Setting: inpatients. | |
| Participants | Diagnosis: chronic schizophrenic patients (clinical diagnosis). N = 86. Gender: 45 M, 41 F. Age: mean 49.9 years. History: duration stable ‐ n.i., duration ill ‐ mean 21 years, number of previous hospitalisations ‐ 2.1, age at onset ‐ mean 27.8 years, severity of illness ‐ n.i., baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 4.5 to 9 mg/day, mean dose 5.25 mg/day. N = 42. 2. Thioridazine: flexible dose, allowed dose range 300 to 600 mg/day, mean dose 331.08 mg/day. N = 44. Other medication: antiparkinson medication. | |
| Outcomes | Response to treatment: clinical judgement. Leaving the study early. Adverse effects: movement disorders (akathisia, dystonia, tremor), adverse effects ‐ other (allergic reactions, amenorrhoea, blurring of vision, constipation, dizziness, dry mouth, drooling, headache, hypotension, loss of associated movement, nausea, oculogyric symptoms, photosensitivity, somnolence, syncope, tongue changes, urinary retention). Unable to use: Mental state: Psychiatric Reaction Profile, IMPS (all analysed by sex and single items). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double ‐ drugs supplied as tablets of identical colour and form, each patient being provided with a coded drug container. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double ‐ drugs supplied as tablets of identical colour and form, each patient being provided with a coded drug container. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two participants in the haloperidol group are missing from global assessment, yet there were no drop‐outs according to the authors. It is not clear what happened with these two participants. |
| Selective reporting (reporting bias) | Unclear risk | No evidence for selective reporting. |
| Other bias | Low risk | No clear other bias. |
White 1981.
| Methods | Randomisation: not mentioned, but double‐blinded. Allocation: procedure not described. Blinding: double, no further information. Duration: 4 weeks. Design: parallel. Location: n.i., Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (Feighner criteria). N = 39. Gender: 18 M, 21 F. Age: mean 25.3 years. History: duration stable ‐ n.i., duration ill ‐ n.i., number of previous hospitalisations ‐ n.i., age at onset ‐ n.i., severity of illness ‐ mean BPRS 43.36 (SD 7.46), mean CGI 3.18 (SD 0.96), baseline antipsychotic dose ‐ n.i., |
|
| Interventions | 1. Haloperidol: flexible dose, allowed dose range 2 to 100 mg/day, mean dose 28 mg/day. N = 21. 2. Mesoridazine: flexible dose, allowed dose range 100 to 800 mg/day, mean dose 421 mg/day. N = 18. Other medication: antiparkinson medication. | |
| Outcomes | Mental state: BPRS (no definition). Global state: CGI scale for Severity of Illness and Severity of Improvement. Adverse effects: movement disorders (at least one movement disorder, dystonia, use of antiparkinson medication), other adverse effects (somnolence). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned, but double‐blind. |
| Allocation concealment (selection bias) | Unclear risk | Procedure not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double, no further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant left the study early. No evidence for other incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence for selective reporting. |
| Other bias | Unclear risk | No clear evidence for other bias, the study had a short duration. |
General Abbreviations i.v. ‐ intravenous n.i. ‐ not indicated mg ‐ milligram max ‐ maximum SD ‐ standard deviation LOCF ‐ Last‐observation‐carried‐forward EPS ‐ extrapyramidal symptoms DSM ‐ Diagnostic and Statistical Manual of Mental Disorders ICD ‐ International Statistical Classification of Diseases and Related Health Problems CPZ ‐ chlorpromazine HAL ‐ haloperidol
Rating scales CGI ‐ Clinical Global Impression PANSS ‐ Positive and Negative Syndrome Scale BPRS ‐ Brief Psychiatric Rating Scale IMPS ‐ Inpatient Multidimensional Psychiatric Rating Scale MACC ‐ Behavioral Adjustment Scale RDC ‐ Research Diagnostic Criteria HAM ‐ D ‐ Hamilton Rating Scale for Depression CCMD ‐ Chinese classification of mental disorders NOSIE ‐ Nurses Observation Scale for Inpatient Evaluation OBRS ‐ Oklahoma Behavior Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Azima 1960 | Allocation: not randomised. |
| Bagne 1992 | Allocation: randomised. Participants: chronic schizophrenic patients. Intervention: haloperidol, thioridazine. Outcome: no relevant outcome, testing of benefit/harm scores. |
| Blum 1969 | Allocation: not randomised. |
| Cassano 1975 | Method: not randomised, but double‐blind. Participants: chronic schizophrenic patients. Intervention: haloperidol versus sulpiride, which is not a low‐potency antipsychotic because it has similar properties as amisulpride and is not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation at all. |
| Cosar 1999 | Allocation:randomised. Participants people with schizophrenia. Intervention: haloperidol versus chlorpromazine. Outcomes: no usable data. |
| Crow 1986 | Allocation: randomised. Participants: first episode schizophrenia. Intervention: haloperidol, flupenthixol, trifluoperazine, pimozide, chlorpromazine versus placebo. Outcomes: no usable data, results of drugs pooled, no data for single drugs. |
| Davies 2007 | Allocation: randomised. Participants: schizophrenia, schizoaffective disorder or delusional disorder. Intervention: first‐ generation (e.g. chlorpromazine, loxapine, sulpiride, haloperidol) and second‐generation antipsychotics (risperidone, olanzapine, amisulpride, quetiapine). Outcome: no usable data, no efficacy of treatment. |
| de Lima 2005 | Allocation: randomised. Participants: schizophrenia. Intervention: first‐ generation (chlorpromazine, haloperidol, trifluoperazine) versus olazapine, data not available for single FGAs separately. |
| Dubin 1985 | Allocation: randomised. Participants: mostly bipolar disorder, manic, etiology unknown, not schizophrenia or schizophrenia‐like illnesses. |
| Eitan 1992 | Allocation: not randomised. |
| Fux 1991 | Allocation:randomised. Participants: people with chronic schizophrenia. Intervention: haloperidol versus chlorprothixene (low‐potency). Outcomes: no usable data, results only available for combined cross‐over phase, no results for first part separately. |
| Garry 1962 | Allocation: randomised. Participants: schizophrenia. Intervention: haloperidol versus placebo. |
| Gerlach 1978 | Allocation: not randomised. |
| Gillis 1977 | Allocation: not randomised. |
| Giordana 1984 | Allocation: randomised. Participants: chronic delusional psychosis or schizophrenic psychosis. Intervention: haloperidol versus pipotiazine, no low‐potency antipsychotic. |
| Gonier 1970 | Method: randomised, but randomisation did not work. |
| Guazzelli 1995 | Allocation: randomised. Participants: chronic schizophrenic patients. Intervention: haloperidol versus sulpiride, which is not a low‐potency antipsychotic because it has similar properties as amisulpride and is not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation at all. |
| Harris 1992 | Allocation: randomised. Participants: ambulatory psychiatric patients, not exclusively psychosis, also dementia. |
| Hayano 1989 | Allocation: not randomised. |
| Hogan 1992 | Allocation:randomised. Participants people with schizophrenia. Intervention: haloperidol versus chlorpromazine (low‐potency). Outcomes: no usable data, results analysed by dysphoric and non‐dysphoric patients, not by drugs. |
| Horodnicki 1985 | Allocation: not randomised. |
| Lempérière 1962 | Allocation: randomised. Participants people with paranoid schizophrenia. Intervention: haloperidol versus chlorpromazine (low‐potency). Outcomes: no usable data, only abstract available without data. |
| Liu 1996 | Allocation: not randomised. |
| Marjerrison 1971 | Allocation: randomised. Participants people with acute schizophrenia. Intervention: haloperidol versus chlorprothixene (low‐potency), placebo. Outcomes: no usable data, no data for haloperidol versus chlorprothixene, just versus placebo. |
| Mechri 2006 | Allocation: not randomised. |
| Minami 1990 | Allocation: not randomised. |
| Mori 1989 | Method: probably randomised, double‐blind. Participants: schizophrenia. Intervention: haloperidol versus sulpiride, which is not a low‐potency antipsychotic because it has similar properties as amisulpride and is not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation at all. |
| Mori 1990 | Allocation: not randomised. |
| Munk‐Andersen 1984 | Method: randomised. Participants: hebephrenic and paranoid schizophrenic participants. Intervention: haloperidol versus sulpiride, which is not a low‐potency antipsychotic because it has similar properties as amisulpride and is not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation at all. |
| Nahunek 1982 | Method: controlled trial, but double‐blind. Participants: schizophrenia. Intervention: haloperidol, oxyprothepine (not low‐potency antipsychotic), placebo. |
| Okuda 1979 | Method: not randomised, but double‐blind. Participants: schizophrenia. Intervention: haloperidol versus sulpiride, which is not a low‐potency antipsychotic because it has similar properties as amisulpride and is not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation at all. |
| Palma 1997 | Allocation: randomised. Participants: schizophrenia. Intervention: chlorpromazine, haloperidol, thioridazine, fluphenazine decanoate. Outcomes: no usable data, data not available for drugs separately. |
| Rama 1981 | Allocation: randomised. Participants: chronic schizophrenic patients. Intervention: haloperidol versus sulpiride, which is not a low‐potency antipsychotic because it has similar properties as amisulpride and is not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation at all. |
| Ropert 1989 | Method: randomisation not mentioned, but double‐blind. Participants: acutely psychotic patients. Intervention: haloperidol versus sulpiride, which is not a low‐potency antipsychotic because it has similar properties as amisulpride and is not within the scope of the review. Moreover, sulpiride does not cause a lot of sedation at all. |
| Shvartsburd 1984 | Allocation: randomised. Participants: schizophrenia, schizoaffective disorder or delusional disorder. Intervention: haloperidol and thioridazine. Outcome: no usable outcome, only data on plasma and levels of antipsychotics. |
| Singh 1975 | Allocation: randomised. Participants: schizophrenia. Intervention: haloperidol and chlorpromazine versus haloperidol and chlorpromazine in combination with benztropine. |
| Smith 1985 | Allocation: randomised. Participants: chronic schizophrenic patients. Intervention: only haloperidol, no comparator drug, comparison of haloperidol dosages (7.5 mg, 10, mg, 25 mg, 40mg). |
| Teja 1975 | Allocation: randomised. Participants people with chronic schizophrenia. Intervention: haloperidol versus chlorpromazine (low‐potency), placebo, amitriptyline. Outcomes: no usable data, no data available for haloperidol versus chlorpromazine. |
| Terminska 1989 | Allocation: not randomised. |
| Wang 2000 | Allocation: not randomised. |
| Zuoning 1999 | Allocation: not randomised. |
FGAs ‐ first‐generation antipsychotics
Differences between protocol and review
We decided post‐hoc to include all outcomes reported by a study, not only the predefined outcomes in the methods section. A randomised sample of 25% of the newly extracted outcomes was independently extracted by a second review author (MH).
Contributions of authors
Magdolna Tardy ‐ protocol development, trial selection, data exctraction, report writing. Maximilian Huhn ‐ protocol development, trial selection, data exctraction, report writing. Werner Kissling ‐ protocol development, advice. Rolf Engel ‐ protocol development, advice. Stefan Leucht ‐ protocol development, report writing.
Sources of support
Internal sources
Freistaat Bayern, Germany.
External sources
Bundesministerium für Bildung und Forschung Grant number 01KG09228816532, Germany.
Declarations of interest
Magdolna Tardy ‐ none to declare. Maximilian Huhn ‐ none to declare. Werner Kissling ‐ has received speaker and/or advisory board/consultancy honoraria from Janssen, Sanofi‐Aventis, Johnson and Johnson, Pfizer, Bayer, BMS, Astra Zeneca, Lundbeck, Novartis and EliLilly. Rolf Engel ‐ none to declare. Stefan Leucht ‐ has received honoraria for lectures from Abbvie, Astra Zeneca, BristolMyersSquibb, ICON, EliLilly, Janssen, Johnson & Johnson, Roche, SanofiAventis, Lundbeck and Pfizer; honoraria for consulting/advisory boards from Roche, EliLilly, Medavante, BristolMyersSquibb, Alkermes, Janssen, Johnson & Johnson and Lundbeck. EliLilly has provided medication for a study with SL as primary investigator.
New
References
References to studies included in this review
Bi 1994 {published data only}
- Bi Y. A relation study of the extrapyramidal symptoms, serum prolactin levels and clinical response during treatment of antipsychotics. Chinese Journal of Nervous and Mental Diseases 1994;20(2):70‐3. [Google Scholar]
Blin 1996 {published data only}
- Blin O, Azorin JM, Bouhours P. Antipsychotic and anxiolytic properties of risperidone, haloperidol, and methotrimeprazine in schizophrenic patients. Journal of Clinical Psychopharmacology 1996; Vol. 16, issue 1:38‐44. [DOI] [PubMed]
- Blin O, Azorin JM, Bouhours P. Anxiolytic profiles of levomepromazine, haloperidol and risperidone in 62 schizophrenic patients. Clinical Pharmacology and Therapeutics. France, 1992; Vol. 51, issue 2:189.
- Blin O, Azorin JM, Bouhours P. Anxiolytic profiles of levomepromazine, haloperidol, and risperidone in 62 schizophrenic patients. Psychopharmacology 1992:31‐2.
- Blin O, Azorin JM, Bouhours P. Comparison of anxiolytic properties of levomepromazine, haloperidol and risperidone in schizophrenic patients. Biological Psychiatry. France, 1991; Vol. 29:388.
- Blin O, Azorin JM, Bouhours P, Fondarai J. Anxiolytic profiles of risperidone, haloperidol, and levomepromazine in schizophrenia: a factorial and regression analysis. Proceedings of the 9th World Congress of Psychiatry; 1993 Jun 6‐12; Rio de Janeiro, Brazil. France, 1993:24.
- Blin O, Azorin JM, Bouhours Ph, Fondarai J. Comparison of risperidone, haloperidol and levomepromazine in schizophrenia: a factorial analysis and discriminant function analysis. Clinical Neuropharmacology 1992; Vol. 15, issue Suppl 1. Pt B:169B.
Borison 1989 {published data only}
- Borison RL, Sinha D, Haverstock S, McLarnon MC, Diamond BI. Efficacy and safety of tiospirone vs. haloperidol and thioridazine in a double‐blind, placebo‐controlled trial. Psychopharmacology Bulletin 1989; Vol. 25, issue 2:190‐3. [PubMed]
Clark 1969 {published data only}
- Clark ML. Haloperidol versus chlorpromazine versus placebo. Psychopharmacology Bulletin 1969; Vol. 5, issue 3:57‐9.
Dufresne 1993 {published data only}
- Dufresne RL, Valentino D, Kass DJ. Thioridazine improves affective symptoms in schizophrenic patients. Psychopharmacology Bulletin 1993; Vol. 29, issue 2:249‐55. [PubMed]
Fox 1964 {published data only}
- Fox W, Gobble IF, Clos M. A clinical comparison of trifluperidol, haloperidol and chlorpromazine. Current Therapeutic Research 1964;6(6):409‐15. [PubMed] [Google Scholar]
Gallant 1967 {published data only}
- Gallant DM, Bishop M, Guerrero‐Figueroa R. Effects of two butyrophenone compounds on acute schizophrenic patients: speculation on the neurophysiologic sites of action. International Journal of Neuropsychiatry 1967;3(1):53‐60. [PubMed] [Google Scholar]
Klimke 1993 {published data only}
- Klimke A, Klieser E, Lehmann E, Miele L. Initial improvement as a criterion for drug choice in acute schizophrenia. Pharmacopsychiatry 1993;26:25‐9. [DOI] [PubMed] [Google Scholar]
McCreadie 1977 {published data only}
Nishizono 1994 {published data only}
- Nishizono M. A comparative trial of zotepine, chlorpromazine and haloperidol in schizophrenic patients. Neuropsychopharmacology 1994;10:30. [Google Scholar]
Prasad 1966 {published data only}
- Prasad L, Townley MC. Haloperidol and thioridazine in treatment of chronic schizophrenics. Diseases of the Nervous System 1966;27(11):722‐6. [PubMed] [Google Scholar]
Rompel 1978 {published data only}
Schmidt 1982 {published data only}
- Schmidt LG, Schüssler G, Kappes CV, Müller‐Oerlinghausen B. Comparison of higher‐dose haloperidol therapy with perazine standard therapy in acute schizophrenic patients [Vergleich einer höher dosierten Haloperidol‐Therapie mt einer Perazin‐Standard‐Therapie bei akut‐schizophrenen Patienten]. Nervenarzt 1982;53(9):530‐6. [PubMed] [Google Scholar]
Serafetinides 1972 {published data only}
- Serafetinides EA, Clark ML. Psychological effects of single dose antipsychotic medication. Biological Psychiatry 1973; Vol. 7, issue 3:263‐7. [PubMed]
- Serafetinides EA, Collins S, Clark ML. Haloperidol, clopenthixol, and chlorpromazine in chronic schizophrenia. Chemically unrelated antipsychotics as therapeutic alternatives. Journal of Nervous and Mental Disease 1972; Vol. 154, issue 1:31‐42. [DOI] [PubMed]
Shalev 1993 {published data only}
Weston 1973 {published data only}
White 1981 {published data only}
References to studies excluded from this review
Azima 1960 {published data only}
Bagne 1992 {published data only}
Blum 1969 {published data only}
- Blum RA, Livingston PB, Shader RI. Changes in cognition, attention and language in acute schizophrenia. Diseases of the Nervous System 1969; Vol. 30, issue 1:31‐6. [PubMed]
Cassano 1975 {published data only}
- Cassano GB, Castrogiovanni P, Conti L. The computer diagnosis in a multicenter study of psychoactive agents. Psychopharmacology Bulletin 1976; Vol. 12, issue 2:22‐4. [PubMed]
- Cassano GB, Castrogiovanni P, Conti L, Bonollo L. Sulpiride versus haloperidol in schizophrenia: a double‐blind comparative trial. Current Therapeutic Research 1975; Vol. 17, issue 2:189‐201. [PubMed]
- Castrogiovanni P, Cassano GB, Conti L, Maggini C, Bonollo L, Sarteschi P. An automated diagnostic process (PDA) in clinical psychopharmacology. An exemplification of its use in a sulpiride versus haloperidol comparative trial. International Pharmacopsychiatry 1976; Vol. 11, issue 2:74‐83. [DOI] [PubMed]
Cosar 1999 {published data only}
- Cosar B, Candansayar S, Taner E, Isik E. Comparison of efficacy of clozapine, sulpiride, chlorpromazine and haloperidol in chronic schizophrenic patients therapy. European Neuropsychopharmacology 1999; Vol. 9:S287.
Crow 1986 {published data only}
- Crow TJ, MacMillan JF, Johnson AL, Johnstone EC. A randomised controlled trial of prophylactic neuroleptic treatment. British Journal of Psychiatry 1986; Vol. 148, issue 2:120‐7. [MEDLINE: ] [DOI] [PubMed]
- Johnstone EC, Crow J, Johnson AL, MacMillan JF. The northwick park study of first episodes of schizophrenia. British Journal of Psychiatry 1986;148:115‐20. [DOI] [PubMed] [Google Scholar]
Davies 2007 {published data only}
- Davies LM, Lewis S, Jones PB, Barnes TR, Gaughran F, Hayhurst K, et al. CUtLASS team. Cost‐effectiveness of first‐ versus second‐generation antipsychotic drugs: results from a randomised controlled trial in schizophrenia responding poorly to previous therapy. British Journal of Psychiatry. United Kingdom, 2007; Vol. 191, issue July:14‐22. [DOI] [PubMed]
- Jones PB, Batnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, et al. Randomized controlled trial of the effect on quality of life of second‐ vs first‐generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Archives of General Psychiatry. United States, 2006; Vol. 63, issue 10:1079‐87. [DOI] [PubMed]
de Lima 2005 {published data only}
- Lima MS, Jesus MJ, Breier A, Costa AM, Sena EP, Hotopf M. Quality of life in schizophrenia: a multicenter, randomized, naturalistic, controlled trial comparing olanzapine to first‐generation antipsychotics. Journal of Clinical Psychiatry. United States, 2005; Vol. 66, issue 7:831‐8. [PubMed]
Dubin 1985 {published data only}
- Dubin WR, Waxman HM, Weiss KJ, Ramchandani D, Tavani‐Petrone C. Rapid tranquilization: the efficacy of oral concentrate. Journal of Clinical Psychiatry 1985; Vol. 46, issue 11:475‐8. [PubMed]
Eitan 1992 {published data only}
Fux 1991 {published data only}
- Fux M, Belmaker RH. A controlled comparative study of chlorprothixene vs. haloperidol in chronic schizophrenia. Israel Journal of Psychiatry and Related Sciences 1991; Vol. 28, issue 1:37‐40. [PubMed]
Garry 1962 {published data only}
Gerlach 1978 {published data only}
Gillis 1977 {published data only}
- Gillis JS. The effects of selected antipsychotic drugs of human judgment. Current Therapeutic Research, Clinical and Experimental 1977; Vol. 21, issue 2:224‐32. [PubMed]
Giordana 1984 {published data only}
- Giordana JY, Frenay S. Comparative study of pipotiazine (in its non‐esterified form) and of haloperidol in acute or subacute psychotic states [Etude comparative de la pipothiazine (dans sa forme non esterifiee) et de l'haloperidol au cours d'etats psychotiques aigus et subaigus]. Psychologie Medicale 1984;16(10):1803‐15. [Google Scholar]
Gonier 1970 {published data only}
- Gonier T, Schiele BC, Vestre ND. A comparison of haloperidol and thioridazine HC1 in chronic treatment‐resistant schizophrenics. Behavioral Neuropsychiatry 1970;2(3):47‐50. [PubMed] [Google Scholar]
Guazzelli 1995 {published data only}
- Guazzelli M, Barracchia E, Bertolino A, Bertolino lA, Casacchia M, Ciapparelli A, et al. Clinical effects of l‐sulpiride vs haloperidol in acutely relapsed chronic schizophrenics: therapeutic efficacy on "disorganization"?. Proceedings of the 8th European College of Neuropsychopharmacology Congress; 1995 Sep 30 ‐ Oct 4; Venice, Italy 1995.
Harris 1992 {published data only}
- Harris MJ, Panton D, Caligiuri MP, Krull AJ, Tran Johnson TK, Jeste DV. High incidence of tardive dyskinesia in older outpatients on low doses of neuroleptics. Psychopharmacology Bulletin 1992; Vol. 28, issue 1:87‐92. [PubMed]
Hayano 1989 {published data only}
- Hayano T, Kadomae S, Kikuoka M, Imade T, Ikoma Y, Nakatani Y, et al. Pharmacometrics of double‐blind test of two drugs, haloperidol and sultopride (barnetil(R); MS‐5024), on the schizophrenic patients. Journal of the Wakayama Medical Society 1989; Vol. 40, issue 4:607‐20.
Hogan 1992 {published data only}
Horodnicki 1985 {published data only}
- Horodnicki JM, Czekalski S, Jarema M, Kubasiewicz A, Pobocha J, Wdowiak J. Comparison of the effects of chlorprothixene and haloperidol on hypothalamic function in patients with paranoid schizophrenia [Porownanie wplywu chloroprotyksenu i haloperidolu na czynnosc podwzgorza u chorych na schizofrenie paranoidalna]. Psychiatria Polska 1985; Vol. 19, issue 3:181‐7. [PubMed]
Lempérière 1962 {published data only}
- Lempérière T, Delay J, Pichot P, Piret J. A comparison of the effects of four major antipsychotic drugs (chlorpromazine, thioproperazine, prochlorpremazine and haloperidol) for paraniod schizophrenia [Comparaison de l'action de quatre neuroleptiques majeurs (chlorpromazine, thiopropérazine, prochlorprémazine ethalopéridol) dans les formes paranoides de la schizophrénie]. Neuro‐Psychopharmacology 1962;3:89‐93. [Google Scholar]
Liu 1996 {published data only}
- Liu K, LLung FW. Difference in prolaction response of schizophrenic patients to equivalent doses of haloperidol, remoxipride and sulpiride. Kao Hsiung I Hsueh Ko Hsueh Tsa Chih Kaohsiung (Journal of Medical Sciences) 1996; Vol. 12, issue 12:685‐90. [PubMed]
Marjerrison 1971 {published data only}
Mechri 2006 {published data only}
- Mechri A, Fendri C, Ben Othman L, Mekhinini A, Kerkeni A, Gaha L. Effect of first generation antipsychotic treatment on oxidative stress markers in schizophrenia. European Neuropsychopharmacology 2006; Vol. 16, issue Suppl 4:S438.
Minami 1990 {published data only}
- Minami H, Miyahara A, Murasaki O, Nakanishi T, Ogawa H, Ohkubo T, et al. Prediction of response to chlorpromazine and haloperidol after test dose. Proceedings of the 17th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1990 Sep 10‐14; Kyoto, Japan 1990:215.
Mori 1989 {published data only}
- Mori A, Kazamatsuri H, Kaneno S, Kamijima K, Kariya T, Murasaki M, et al. A double‐blind comparison of a new benzamide compound YM 09151 with haloperidol in the treatment of schizophrenia. Rinsho Hyoka (Clinical Evaluation) 1989; Vol. 17, issue 3‐4:349‐77.
Mori 1990 {published data only}
- Mori A, Kazamatsuri H, Kaneno S, Kamijima K, Kariya T, Murasaki M, et al. A double‐blind comparison of emonapride with haloperidol in the treatment of schizophrenia. Proceedings of the 17th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1990 Sep 10‐14; Kyoto, Japan 1990:296.
Munk‐Andersen 1984 {published data only}
- Gerlach J, Behnke K, Heltberg J, Munk‐Anderson E, Nielsen H. Sulpiride and haloperidol in schizophrenia: a double‐blind cross‐over study of therapeutic effect, side effects and plasma concentrations. British Journal of Psychiatry 1985; Vol. 147:283‐8. [DOI] [PubMed]
- Munk‐Andersen E, Behnke K, Heltberg J, Nielsen H. Sulpiride versus haloperidol in the treatment of schizophrenia. A short preliminary report [Sulpirid versus haloperidol i behandling af skizofreni. En kort, praeliminaer rapport]. Nordisk Psykiatrisk Tidsskrift 1984; Vol. 38, issue 4:223‐8.
- Munk‐Andersen EM, Behnke K, Heltberg J, Nielsen H, Gerlach J. Sulpiride versus haloperidol, a clinical trial in schizophrenia. A preliminary report. Acta Psychiatrica Scandinavica Supplementum 1984; Vol. 311:31‐41. [DOI] [PubMed]
Nahunek 1982 {published data only}
- Nahunek K, Svestka J, Ceskova E, Rysanek R. Blind comparison of oxyprothepin and haloperidol in six‐week treatment periods in schizophrenia. Activitas Nervosa Superior 1982; Vol. 24, issue 4:219‐20.
Okuda 1979 {published data only}
- Okuda O, Akaue Y, Okishio Y. The efficacy of sulpiride in the treatment of schizophrenia by the double‐blind method. Yakuri To Chiryo 1979; Vol. 7, issue 2:439‐58.
Palma 1997 {published data only}
- Palma Wenzel MI, Parada R, Osorio C, Dorr A, Bauer S. Flupentixol decanoate versus other neuroleptics in chronic schizophrenia [Decanoato de flupentixol versus otros neurolepticos en esquizofrenicos cronicos]. Revista Chilena de Neuropsiquiatria 1997; Vol. 35, issue 1:29‐35.
Rama 1981 {published data only}
Ropert 1989 {published data only}
- Ropert R, Payan C, Allard S. Sultopride versus haloperidol for the treatment of acute psychosis. Results of a multicenter double‐blind controlled trial [Sultopride versus haloperidol dans le traitement des etats psychotiques aigus]. Annales de Psychiatrie 1989; Vol. 4, issue 1:92‐8.
Shvartsburd 1984 {published data only}
- Shvartsburd A, Sajadi C, Morton V, Mirabi M, Gordon J, Smith RC. Blood levels of haloperidol and thioridazine during maintenance neuroleptic treatment of schizophrenic outpatients. Journal of Clinical Psychopharmacology 1984; Vol. 4, issue 4:194‐8. [PubMed]
Singh 1975 {published data only}
- Singh MM. Dysphoric response to neuroleptic treatment in schizophrenia and its prognostic significance. Diseases of the Nervous System 1976; Vol. 37:191‐6. [PubMed]
- Singh MM, Kay SR. A comparative study of haloperidol and chlorpromazine in terms of clinical effects and therapeutic reversal with benztropine in schizophrenia. Theoretical implications for potency differences among neuroleptics. Psychopharmacology 1975; Vol. 43, issue 2:103‐13. [DOI] [PubMed]
- Singh MM, Kay SR. A longitudinal therapeutic comparison between two prototypic neuroleptics (haloperidol and chlorpromazine) in matched groups of schizophrenics. Nontherapeutic interactions with trihexyphenidyl. Theoretical implications for potency differences. Psychopharmacology 1975; Vol. 43, issue 2:115‐21. [DOI] [PubMed]
- Singh MM, Kay SR. Therapeutic reversal with benztropine in schizophrenics: practical and theoretical significance. Journal of Nervous and Mental Disease 1975; Vol. 160, issue 4:258‐66. [DOI] [PubMed]
Smith 1985 {published data only}
- Smith RC, Baumgartner R, Burd A, Ravichandran GK, Mauldin M. Haloperidol and thioridazine drug levels and clinical response in schizophrenia: comparison of gas‐liquid chromatography and adioreceptor drug level assays. Psychopharmacology Bulletin. United States, 1985; Vol. 21, issue 1:52‐8. [PubMed]
Teja 1975 {published data only}
- Teja JS, Grey WH, Clum JM, Warren C. Tranquilizers or anti‐depressanrs for chronic schizophrenics: a long term study. Australian and New Zealand Journal of Psychiatry 1975;9:241‐7. [DOI] [PubMed] [Google Scholar]
Terminska 1989 {published data only}
- Terminska K, Mrowiec W. Comparison of influence of perazine, fluphenazine, trifluoroperazine, chloropromazine and haloperidol on primary and deficit schizophrenic symptoms in patients first hospitalized because of paranoid schizophrenia [Badanie porownawcze wplywu perazyny, flufenazyny, trifluoroperazyny, chloropromazny i haloperydolu na objawy pierwotne i deficytowe pierwszego zachorowania na schizofrenie paranoidalna]. Psychiatria Polska 1989; Vol. 23, issue 1:24‐30. [PubMed]
Wang 2000 {published data only}
- Wang YC, Cuo JH, Chen JS. The relation of qeeg with the therapeutic effect of antipsychotic drugs. Modern Rehabilitation 2000; Vol. 4, issue 11:12‐3.
Zuoning 1999 {published data only}
- Zuoning J, Huang Shu Zheng, Qin Ying Fu, Yamamoto M. Comparative clinical study of nemonapride, a benzamide compound with dopamine D‐2‐like receptor blocking activity, and haloperidol in schizophrenic patients. Research Communications in Biological Psychology and Psychiatry 1999; Vol. 24, issue 1‐2:35‐46.
Additional references
Adams 2013
- Adams Clive E, Bergman H, Irving Claire B, Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2013, issue 11. [DOI: 10.1002/14651858.CD003082.pub3; CD003082] [DOI] [PMC free article] [PubMed]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 2010
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic drug equivalents and dose‐years: A standardized method for comparing exposure to different drugs. Biological Psychiatry 2010;67:255‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM III). Washington DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd edition‐ Revised (DSM‐III‐R). Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
Asberg 1970
- Asberg M, Cronholm B, Sjöckvist F, Tuck D. Correlation of subjective side effects with plasma concentrations of nortriptyline. British Medical Journal 1970;4:18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2003
- Berger M. Psychische Erkrankungen. Klinik und Therapie. 2nd Edition. München: Urban & Fischer, 2003. [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. [DOI] [PubMed] [Google Scholar]
Chouinard 1980
- Chouinard G, Ross‐Chouinard A, Annable L, Jones BD. Extrapyramidal Symptom Rating Scale. Canadian Journal of Neurological Sciences 1980;7:233‐9. [Google Scholar]
Davis 1974
- Davis JM. Overview: maintenance therapy in psychiatry: I. Schizophrenia. American Journal of Psychiatry 1975;132(12):1237‐45. [DOI] [PubMed] [Google Scholar]
Davis 1989
- Davis JM, Barter JT, Kane JM. Antipsychotic drugs. Comprehensive Textbook of Psychiatry. Williams and Wilkins, 1989. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. Cape Town, 2000.
Der‐Simonian 1986
- Der‐Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
DiMascio 1976
- DiMascio A, Bernardo DC. A controlled trial of amantadine in drug‐induced extrapyramidal disorders. Archives of General Psychiatry 1976;33:599‐602. [DOI] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Dold 2012
- Dold M, Li C, Tardy M, Leucht S. Haloperidol versus first generation antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009831] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donnelly 2013
- Donnelly L, Rathbone J, Adams Clive E. Haloperidol dose for the acute phase of schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001951.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duggan 2005
- Duggan L, Fenton M, Rathbone J, Dardennes R, El‐Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001359.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Falkai 2005
- Falkai P, Wobrock T, Lieberman J. World Federation of Societies of Biological Psychiatry (WFSBP) ‐ Guidelines for biological treatment of schizophrenia, part 1: Acute treatment of schizophrenia. World Journal of Biological Psychiatry 2005;6:132‐91. [DOI] [PubMed] [Google Scholar]
Feighner 1972
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26:57‐63. [DOI] [PubMed] [Google Scholar]
Fenton 2007
- Fenton M, Rathbone J, Reilly J, Sultana A. Thioridazine for schizophrenia. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001944.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Furukawa 2011
- Furukawa TA, Akechi T, Wagenpfeil S, Leucht S. Relative indices of treatment effect may be constant across different definitions of response in schizophrenia trials. Schizophrenia Research 2011;126:212‐9. [DOI] [PubMed] [Google Scholar]
Gaebel 2006
- Gaebel W, Falkai P, Weinmann S. Behandlungsleitlinie Schizophrenie. Darmstadt: Steinkopff, 2006. [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions Scale. ECDEU Assessment Manual for Psychopharmacology (DOTES: Dosage Record and Treatment Emergent Symptom Scale). National Institute of Mental Health, 1976. [Google Scholar]
Haase 1983
- Haase HJ. Dosierung der Neuroleptika. Ein Leitfaden für Klinik und Praxis unter besonderer Berücksichtigung psychotisch Kranker. Erlangen: Perimed fachbuch‐verlagsgesellschaft, 1983. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Jones 2006
- Jones PB, Barnes TRE, Davies L. Randomized controlled trial of the effect on quality of life of second‐ vs first‐generation antipsychotic drugs in schizophrenia ‐ cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1). Archives of General Psychiatry 2006;63:1079‐86. [DOI] [PubMed] [Google Scholar]
Kane 1996
- Kane JM. Schizophrenia. New England Journal of Medicine 1996;334:34‐41. [DOI] [PubMed] [Google Scholar]
Kane 2011
- Kane JM, Mackle M, Snow‐Adami L, Zhao J, Szegedi A, Panagides J. A randomized placebo‐controlled trial of asenapine for the prevention of relapse of schizophrenia after long‐term treatment. Journal of Clinical Psychiatry 2011;72(3):349‐55. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda (NY): Multi‐Health Systems, 1986. [Google Scholar]
Kaye 2003
- Kaye JA, Bradbury BD, Jick H. Changes in antipsychotic drug prescribing by general practitioners in the United Kingdom from 1991 to 2000: a population‐based observational study. British Journal of Clinical Pharmacology 2003;56:569‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 1969
- Klein DF, Davis JM. Diagnosis and Drug Treatment of Psychiatric Disorders. Baltimore: Williams and Wilkins, 1969. [Google Scholar]
Lehman 2004
- Lehman AF, Lieberman JA, Dixon LB. Practice guideline for the treatment of patients with schizophrenia, second edition. American Journal of Psychiatry 2004;161:1‐56. [PubMed] [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang‐Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59:1001‐5. [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [DOI] [PubMed] [Google Scholar]
Leucht 2009a
- Leucht S, Corves C, Arbter D, Engel R, Li C, Davis JM. A meta‐analysis comparing second‐generation and first‐generation antipsychotics for schizophrenia. Lancet 2009;373:31‐41. [DOI] [PubMed] [Google Scholar]
Leucht 2009b
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second‐generation versus first‐generation antipsychotic drugs for schizophrenia: a meta‐analysis. Lancet 2009;373(9657):31‐41. [DOI] [PubMed] [Google Scholar]
Lieberman 2005
- Lieberman JA, Stroup TS, McEvoy JP. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353:1209‐23. [DOI] [PubMed] [Google Scholar]
Lohse 2005
- Lohse MJ, Lorenzen A, Müller‐Oerlinghausen B. Psychotropic drugs [Psychopharmaka]. Arzneimittel Verordnungs Report 2005:820‐64.
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Marvaha 2004
- Marwaha S, Johnson S. Schizophrenia and employment ? a review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
NIMH 1985
- National Institute of Mental Health. Special feature: rating scales and assessment instruments for use in pediatric psychopharmacology research. Psychopharmacological Bulletin 1985;21(4):714‐1124. [PubMed] [Google Scholar]
Omori 2009
- Omori Ichiro M, Wang J, Soares B, Fenton M. Sulpiride versus other antipsychotics for schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008126] [DOI] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐12. [Google Scholar]
Paton 2003
- Paton C, Lelliott P, Harrington M. Patterns of antipsychotic and anticholinergic prescribing for hospital inpatients. Journal of Psychopharmacology 2003;17:223‐9. [DOI] [PubMed] [Google Scholar]
Schneider 1959
- Schneider K. Clinical Psychopathology. New York: Grune and Stratton, 1959. [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Seeman 1975
- Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 1975;188:1217‐9. [DOI] [PubMed] [Google Scholar]
Simpson 1970
- Simpson EN, Angus JWF. A rating scale for extrapyramidal side‐effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. [DOI] [PubMed] [Google Scholar]
Soares 1999
- Soares Bernardo GO, Fenton M, Chue P. Sulpiride for schizophrenia. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 1978
- Spitzer RL, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35(6):773‐82. [DOI] [PubMed] [Google Scholar]
Tardy 2011b
- Tardy M, Leucht S, Potapov A, Engel R, Huhn M, Komossa K. Perphenazine versus low‐potency first generation antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tardy 2011c
- Tardy M, Leucht S, Potapov A, Engel R, Huhn M, Komossa K. Fluphenazine versus low‐potency first generation antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tardy 2011d
- Tardy M, Leucht S, Potapov A, Engel R, Dold M, Kissling W. Trifluoperazine versus low‐potency first generation antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009396] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tardy 2011e
- Tardy M, Leucht S, Potapov A, Engel R, Dold M, Kissling W. Flupenthixol versus low‐potency first generation antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trikalinos 2004
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, Ioannidis JP, EU‐PSI project. Effect sizes in cumulative meta‐analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology 2004;57(11):1124‐30. [DOI] [PubMed] [Google Scholar]
Tsuang 1978
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35:153‐5. [DOI] [PubMed] [Google Scholar]
Whitehead 2002
- Whitehead C, Moss S, Cardno A, Lewis G, Furtado VA. Antidepressants for people with both schizophrenia and depression. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002305] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1978
- World Health Organization. The Ninth Revision of the International Classification of Diseases and Related Health Problems (ICD‐9). Geneva: World Health Organization, 1978. [Google Scholar]
WHO 1992
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10). Geneva: World Health Organization, 1992. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]


