Abstract
Background
Patellofemoral pain syndrome (PFPS) is a common knee problem, which particularly affects adolescents and young adults. PFPS, which is characterised by retropatellar (behind the kneecap) or peripatellar (around the kneecap) pain, is often referred to as anterior knee pain. The pain mostly occurs when load is put on the knee extensor mechanism when climbing stairs, squatting, running, cycling or sitting with flexed knees. Exercise therapy is often prescribed for this condition.
Objectives
To assess the effects (benefits and harms) of exercise therapy aimed at reducing knee pain and improving knee function for people with patellofemoral pain syndrome.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (May 2014), the Cochrane Central Register of Controlled Trials (2014, Issue 4), MEDLINE (1946 to May 2014), EMBASE (1980 to 2014 Week 20), PEDro (to June 2014), CINAHL (1982 to May 2014) and AMED (1985 to May 2014), trial registers (to June 2014) and conference abstracts.
Selection criteria
Randomised and quasi‐randomised trials evaluating the effect of exercise therapy on pain, function and recovery in adolescents and adults with patellofemoral pain syndrome. We included comparisons of exercise therapy versus control (e.g. no treatment) or versus another non‐surgical therapy; or of different exercises or exercise programmes.
Data collection and analysis
Two review authors independently selected trials based on pre‐defined inclusion criteria, extracted data and assessed risk of bias. Where appropriate, we pooled data using either fixed‐effect or random‐effects methods. We selected the following seven outcomes for summarising the available evidence: pain during activity (short‐term: ≤ 3 months); usual pain (short‐term); pain during activity (long‐term: > 3 months); usual pain (long‐term); functional ability (short‐term); functional ability (long‐term); and recovery (long‐term).
Main results
In total, 31 heterogeneous trials including 1690 participants with patellofemoral pain are included in this review. There was considerable between‐study variation in patient characteristics (e.g. activity level) and diagnostic criteria for study inclusion (e.g. minimum duration of symptoms) and exercise therapy. Eight trials, six of which were quasi‐randomised, were at high risk of selection bias. We assessed most trials as being at high risk of performance bias and detection bias, which resulted from lack of blinding.
The included studies, some of which contributed to more than one comparison, provided evidence for the following comparisons: exercise therapy versus control (10 trials); exercise therapy versus other conservative interventions (e.g. taping; eight trials evaluating different interventions); and different exercises or exercise programmes. The latter group comprised: supervised versus home exercises (two trials); closed kinetic chain (KC) versus open KC exercises (four trials); variants of closed KC exercises (two trials making different comparisons); other comparisons of other types of KC or miscellaneous exercises (five trials evaluating different interventions); hip and knee versus knee exercises (seven trials); hip versus knee exercises (two studies); and high‐ versus low‐intensity exercises (one study). There were no trials testing exercise medium (land versus water) or duration of exercises. Where available, the evidence for each of seven main outcomes for all comparisons was of very low quality, generally due to serious flaws in design and small numbers of participants. This means that we are very unsure about the estimates. The evidence for the two largest comparisons is summarised here.
Exercise versus control. Pooled data from five studies (375 participants) for pain during activity (short‐term) favoured exercise therapy: mean difference (MD) ‐1.46, 95% confidence interval (CI) ‐2.39 to ‐0.54. The CI included the minimal clinically important difference (MCID) of 1.3 (scale 0 to 10), indicating the possibility of a clinically important reduction in pain. The same finding applied for usual pain (short‐term; two studies, 41 participants), pain during activity (long‐term; two studies, 180 participants) and usual pain (long‐term; one study, 94 participants). Pooled data from seven studies (483 participants) for functional ability (short‐term) also favoured exercise therapy; standardised mean difference (SMD) 1.10, 95% CI 0.58 to 1.63. Re‐expressed in terms of the Anterior Knee Pain Score (AKPS; 0 to 100), this result (estimated MD 12.21 higher, 95% CI 6.44 to 18.09 higher) included the MCID of 10.0, indicating the possibility of a clinically important improvement in function. The same finding applied for functional ability (long‐term; three studies, 274 participants). Pooled data (two studies, 166 participants) indicated that, based on the 'recovery' of 250 per 1000 in the control group, 88 more (95% CI 2 fewer to 210 more) participants per 1000 recovered in the long term (12 months) as a result of exercise therapy.
Hip plus knee versus knee exercises. Pooled data from three studies (104 participants) for pain during activity (short‐term) favoured hip and knee exercise: MD ‐2.20, 95% CI ‐3.80 to ‐0.60; the CI included a clinically important effect. The same applied for usual pain (short‐term; two studies, 46 participants). One study (49 participants) found a clinically important reduction in pain during activity (long‐term) for hip and knee exercise. Although tending to favour hip and knee exercises, the evidence for functional ability (short‐term; four studies, 174 participants; and long‐term; two studies, 78 participants) and recovery (one study, 29 participants) did not show that either approach was superior.
Authors' conclusions
This review has found very low quality but consistent evidence that exercise therapy for PFPS may result in clinically important reduction in pain and improvement in functional ability, as well as enhancing long‐term recovery. However, there is insufficient evidence to determine the best form of exercise therapy and it is unknown whether this result would apply to all people with PFPS. There is some very low quality evidence that hip plus knee exercises may be more effective in reducing pain than knee exercise alone.
Further randomised trials are warranted but in order to optimise research effort and engender the large multicentre randomised trials that are required to inform practice, these should be preceded by research that aims to identify priority questions and attain agreement and, where practical, standardisation regarding diagnostic criteria and measurement of outcome.
Keywords: Adult, Humans, Exercise Therapy, Exercise Therapy/methods, Patellofemoral Pain Syndrome, Patellofemoral Pain Syndrome/therapy, Randomized Controlled Trials as Topic, Selection Bias
Plain language summary
Exercise therapy for adolescents and adults with pain behind or around the kneecap (patellofemoral pain)
Introduction
Patellofemoral pain syndrome (PFPS) is a common knee problem, which particularly affects adolescents and young adults. PFPS is characterised by retropatellar (behind the kneecap) or peripatellar (around the kneecap) pain. It is often referred to as anterior knee pain. The pain mostly occurs when load is put on the muscles that extend the leg when climbing stairs, squatting, running, cycling or sitting with bent knees. Exercise therapy is often prescribed for this condition.
Results of the search and description of studies
We searched the medical literature until May 2014 and found 31 relevant studies involving 1690 participants with patellofemoral pain. The studies varied a lot in the characteristics of their study populations (e.g. activity levels and duration of their symptoms) and type of exercises. We assessed most trials as being at high risk of bias because the people, often the trial participants, who assessed outcome knew what treatment group they were in.
The included studies, some of which contributed to more than one comparison, provided evidence for the following comparisons: exercise therapy versus control (10 trials); exercise therapy versus other conservative interventions (e.g. applying adhesive tape over the knee; eight trials evaluating different interventions); and different exercises or exercise programmes. The latter group comprised: supervised versus home exercises (two trials); foot fixed (closed kinetic chain) versus foot free (open kinetic chain) exercises (four trials); variants of closed kinetic chain exercises (two trials making different comparisons; other comparisons of other types of kinetic chain or miscellaneous exercises (five trials evaluating different interventions); hip and knee versus knee exercises (seven trials); hip versus knee exercises (two studies); and high‐ versus low‐intensity exercises (one study). There were no trials testing the exercise medium (land versus water) or duration of exercises.
Quality of the evidence
The evidence, where available, for each of seven main outcomes for all comparisons was of very low quality. This means that we are very unsure about the reliability of these results.
Results of the two largest comparisons
The evidence for the comparison of exercise therapy versus control (e.g. no treatment) showed that exercise therapy may provide a clinically important reduction in pain during activity and usual pain in the short term (three months or less) and in the long term (more than three months). The review also found evidence that exercise therapy may provide a clinically important improvement in functional ability in both the short and long term, as well as resulting in greater numbers reporting recovery from their symptoms in the long term.
The review found evidence that hip plus knee exercises may provide a clinically important reduction in pain during activity and usual pain in the short term and pain during activity in the long term, when compared with knee exercises only. There was inconclusive evidence to say whether functional ability or recovery was better in either group.
Conclusions
This review has found very low quality but consistent evidence that exercise therapy for PFPS may result in clinically important reduction in pain and improvement in functional ability, as well as enhancing long‐term recovery. However, we cannot say what is the best form of exercise therapy nor whether this result would apply to all people with patellofemoral pain. There is some very low quality evidence that hip plus knee exercises may be more effective in reducing pain than knee exercise alone.
Before further studies are done, research is needed to identify priority questions and achieve better consensus on diagnostic criteria and measurement of outcome.
Summary of findings
Summary of findings for the main comparison. Exercise therapy compared with a control strategy (no treatment, placebo or waiting list controls) for patellofemoral pain syndrome.
| Exercise therapy versus control for patellofemoral pain syndrome | ||||||
| Patient or population: patients with patellofemoral pain syndrome (symptoms > 3 weeks (1 study); symptoms > 1 month (3 studies); symptoms > 2 months (2 studies); symptoms > 3 months (2 studies; symptoms > 6 months (1 study). (Data from a study including participants with patella malalignment are not included here.) Settings: various: orthopaedic clinics, rheumatology consultants, general practices, rehabilitation service, physiotherapy practices, sports medical practices, chiropractor practices Intervention: exercise therapy (various descriptions in the included trials, including knee exercises, hip and knee exercises, home exercises, supervised exercises, closed kinetic chain, open kinetic chain) Comparison: control (no treatment, waiting list, health educational material) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control strategy | Exercise therapy | |||||
| Pain during activity (short‐term) Scale (0 to 10; higher scores mean worse pain)1 Follow‐up range: 4 weeks to 3 months | The mean pain in the control group ranged from 2.1 to 6.0 points2 | The mean pain during activity (short‐term) in the exercise group was 1.46 lower (2.39 to 0.54 lower) | MD ‐1.46 (‐2.39 to ‐0.54) | 375 (5 studies) | ⊕⊝⊝⊝ very low3 | The confidence interval includes the MCID of 1.34 in favour of exercises. Thus this includes the possibility of a clinically important effect of exercises on pain during activity (short‐term) |
| Usual pain (short‐term) Scale (0 to 10; higher scores mean worse pain)5 Follow‐up: 4 or 8 weeks | The mean difference in usual pain (short‐term) in the exercise group was 0.93 standard deviations lower (1.60 to 0.25 lower) |
SMD ‐0.93 (‐1.60 to ‐0.25) |
41 (2 studies) | ⊕⊝⊝⊝ very low6 | In order to interpret these results in terms of the VAS (0 to 10), the SMD was multiplied by the median SD of VAS usual pain (1.55) The mean usual pain (short‐term) in the exercises group was an estimated 1.44 lower (2.48 to 0.39 lower) The confidence interval includes the MCID of 2.07 in favour of exercises. Thus this includes the possibility of a clinically important effect on usual pain (short‐term) of exercises |
|
|
Pain during activity (long‐term) Scale (0 to 10; higher scores mean worse pain)8 Follow‐up: 12 months |
The mean pain in the control group ranged from 2.6 to 3.9 points2 | The mean pain during activity (long‐term) in the exercise group was 1.07 lower (1.93 to 0.21 lower) | MD ‐1.07 (‐1.93 to ‐0.21) | 180 (2 studies) | ⊕⊝⊝⊝ very low6 | The confidence interval includes the MCID of 1.34 in favour of exercises. Thus this includes the possibility of the effect of exercises on usual pain (long‐term) not being clinically important as well as the possibility of a clinically important effect |
| Usual pain (long‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 16 weeks | The mean pain in the control group was 6.6 points2 | The mean usual pain (long‐term) in the exercise group was 4.32 lower (7.75 to 0.89 lower) | MD ‐4.32 (‐7.75 to ‐0.89) | 94 (1 study) | ⊕⊝⊝⊝ very low9 | The confidence interval includes the MCID of 2.07 in favour of exercises. Thus this includes the possibility of a clinically important effect of exercises on pain during activity (long‐term) |
| Functional ability (short‐term) Scale (0 to 100; higher scores mean better function)10 Follow‐up range: 4 weeks to 3 months | The mean difference in functional ability (short‐term) in the exercise group was 1.10 standard deviations higher (0.58 to 1.63 higher) |
SMD 1.10 (0.58 to 1.63) |
483 (7 studies) | ⊕⊝⊝⊝ very low11 | In order to interpret these results in terms of the AKPS, values were scaled to 0 to 100 and the SMD was multiplied by the median SD of the AKPS (11.1) The mean functional ability (short‐term) in the exercises group was an estimated 12.21 higher (6.44 to 18.09 higher) The confidence interval includes the MCID of 10.012 in favour of exercises. Thus this includes the possibility of a clinically important effect on functional ability (short‐term) of exercises |
|
| Functional ability (long‐term) Scale (0 to 100; higher scores mean better function)13 Follow‐up range: 16 weeks to 12 months | The mean difference in functional ability (long‐term) in the exercise group was 1.62 standard deviations higher (0.31 to 2.94 higher) |
SMD 1.62 (0.31 to 2.94) |
274 (3 studies) | ⊕⊝⊝⊝ very low14 | In order to interpret these results in terms of the AKPS, values were scaled to 0 to 100 and the SMD was multiplied by the median SD of the AKPS (11.1) The mean functional ability (long‐term) in the exercises group was an estimated 17.98 higher (3.44 to 32.63 higher) The confidence interval includes the MCID of 10.012 in favour of exercises. Thus this includes the possibility of a clinically important effect on functional ability (long‐term) of exercises |
|
| Recovery (long‐term) Number of patients who had recovered or number of patients no longer troubled by symptoms Follow‐up: 12 months | 250 per 100015 | 338 per 1000 (248 to 460) | RR 1.35 (0.99 to 1.84) | 166 (2 studies) | ⊕⊝⊝⊝ very low16 | These data equate to 88 more (95% CI 2 fewer to 210 more) participants per 1000 who would recover in the long term as a result of exercise therapy |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKPS: Anterior Knee Pain Score; CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; NPRS: numerical pain rating scale; RR: risk ratio; SMD: standardised mean difference; VAS: visual analogue scale/score | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Data were from VAS (0 to 10), NPRS (0 to 10) and VAS (0 to 200). Values were scaled to 0 to 10 (higher is worse). These measures are comparable and thus we calculated MDs.
2The basis for the assumed risk is the range of the control group risk of the studies.
3In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (primarily relating to lack of assessor blinding), one level for imprecision (wide confidence intervals and small sample size) and one level for serious inconsistency (heterogeneity: P value = 0.0003, I2 = 74%).
4The minimal clinically important difference for VAS pain during activity was set at 1.3 points (Crossley 2004).
5Data were from VAS (0 to 10) and the McGill pain questionnaire (0 to 10).
6In our assessment of the quality of the evidence for this outcome, we downgraded two levels for serious risk of bias (relating to lack of allocation concealment and lack of assessor blinding) and one level for imprecision (small sample size).
7The minimal clinically important difference for VAS usual pain was set at 2.0 points (Crossley 2004).
8Data were from VAS (0 to 10) and VAS (0 to 200). Values were scaled to 0 to 10 (higher is worse). These measures are comparable and thus we calculated MDs.
9In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (primarily relating to lack of assessor blinding) and two levels for serious imprecision (wide confidence intervals and small sample size).
10Data were from the AKPS (0 to 100), Lysholm (0 to 100), Function Scale (0 to 53) and WOMAC Osteoarthritis Index (0 to 96). We rescaled data from the Function Scale and WOMAC to 0 to 100; we inverted those from WOMAC first.
11In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (primarily relating to lack of assessor blinding) and two levels for serious inconsistency (P value < 0.00001, I2 = 83%).
12The minimal clinically important difference for the AKPS was set at 10.0 points (Crossley 2004).
13Data were from the AKPS (0 to 100) and WOMAC Osteoarthritis Index (0 to 96). We inverted data from WOMAC and rescaled data to 0 to 100.
14In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (primarily relating to lack of assessor blinding), one level for imprecision (small sample size) and one level for serious inconsistency (heterogeneity: P value < 0.00001, I2 = 94%).
15The basis for the assumed risk is the median control group risk of the studies.
16In our assessment of the quality of the evidence for this outcome, we downgraded two levels for serious risk of bias (relating to lack of allocation concealment and lack of assessor blinding) and one level for imprecision (small sample size).
Summary of findings 2. Supervised exercises compared with home exercises for patellofemoral pain syndrome.
| Supervised exercises versus home exercises for patellofemoral pain syndrome | ||||||
| Patient or population: patients with patellofemoral pain syndrome (symptoms > 2 months (1 study); not stated (1 study)) Settings: orthopaedic clinics, general practices Intervention: supervised exercises Comparison: home exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Home exercises | Supervised exercises | |||||
| Pain during activity (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in either of the 2 studies for this comparison |
| Usual pain (short‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 8 weeks or 3 months | The mean pain in the home exercises group ranged from 1.7 to 2.0 points1 | The mean usual pain (short‐term) in the supervised exercises group was 0.22 lower (1.22 lower to 0.77 higher) |
MD ‐0.22 (‐1.22 to 0.77) |
59 (2 studies) | ⊕⊝⊝⊝ very low2 | The confidence interval excludes the MCID for usual pain of 2.0 points3 |
| Pain during activity (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in either of the 2 studies for this comparison |
| Usual pain (long‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 12 months | The mean pain in the home exercises group was 1.3 points1 | The mean usual pain (long‐term) in the supervised exercises group was 0.43 lower (1.84 lower to 0.98 higher) |
MD ‐0.43 (‐1.84 to 0.98) |
31 (1 study) | ⊕⊝⊝⊝ very low2 | The confidence interval excludes the MCID for usual pain of 2.0 points3 |
| Functional ability (short‐term) AKPS (0 to 100; higher scores mean better function) Follow‐up: 8 weeks (1 month) | The mean AKPS score in the home exercises group was 86.6 points1 | The mean functional ability (short‐term) in the supervised exercises group was 2.30 lower (11.33 lower to 6.73 higher) |
MD ‐2.30 (‐11.33 to 6.73) |
18 (1 study) | ⊕⊝⊝⊝ very low4 | The confidence interval includes the MCID of 10.05 in favour of home exercises. Thus this includes the fairly small possibility of a clinically important effect on functional ability (short‐term) of home exercises. The confidence interval also includes the possibility of a non‐clinically important effect in favour of supervised exercises The other study making this comparison (28 participants) found a greater number of people in the home exercises group with high (13 to 16) FIQ scores indicating best function6: RR 0.46, 95% CI 0.21 to 1.01; very low quality evidence7 |
| Functional ability (long‐term) FIQ (number of patients in top (best function) category 13 to 16)6 Follow‐up: 12 months | 632 per 10001 | 847 per 1000 (563 to 1000) | RR 1.34 (0.89 to 2.03) | 31 (1 study) | ⊕⊝⊝⊝ very low7 | These data equate to 215 more (95% CI 69 fewer to 468 more) participants per 1000 who would have best function in the long term as a result of supervised exercise |
| Recovery (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in either of the 2 studies for this comparison |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKPS: Anterior Knee Pain Score; CI: confidence interval; FIQ: Functional Index Questionnaire; MCID: minimal clinically important difference; MD: mean difference; RR: risk ratio; VAS: visual analogue scale/score | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The basis for the assumed risk is the range of the control group risk of the studies.
2In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding) and two levels for serious imprecision (small sample size).
3The minimal clinically important difference for VAS usual pain was set at 2.0 points (Crossley 2004).
4In our assessment of the quality of the evidence for this outcome, we downgraded two levels for serious risk of bias (relating to lack of allocation concealment and lack of assessor blinding) and one level for imprecision (small sample size).
5The minimal clinically important difference for the AKPS was set at 10.0 points (Crossley 2004).
6This trial presented the numbers of participants with scores split into four FIQ categories (0 to 4, 5 to 8, 9 to 12, 13 to 16). We present the data for those in the top (13 to 16, best function) category.
7In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding), one level of indirectness (reflecting the inadequateness of the outcome) and one level for imprecision (small sample size).
Summary of findings 3. Closed kinetic chain exercises compared with open kinetic chain exercises for patellofemoral pain syndrome.
| Closed kinetic chain exercises versus open kinetic chain exercises for patellofemoral pain syndrome | ||||||
| Patient or population: patients with patellofemoral pain syndrome (symptoms > 4 weeks (1 study); symptoms > 6 weeks (1 study); symptoms > 8 weeks (1 study); not stated (1 study)) Settings: orthopaedic clinics, physiotherapy practices Intervention: closed kinetic chain exercises Comparison: open kinetic chain exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open kinetic chain (OKC) exercises | Closed kinetic chain (CKC) exercises | |||||
| Pain during activity (short‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 6 weeks or 3 months | The mean pain in the OKC exercises group ranged from 0.9 to 2.7 points1 | The mean pain during activity (short‐term) in the CKC group was 0.03 higher (0.63 lower to 0.70 higher) |
MD 0.03 (‐0.63 to 0.70) |
90 (2 studies) | ⊕⊝⊝⊝ very low2 | The confidence interval excludes the MCID of pain during activity of 1.3 points3 |
| Usual pain (short‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up range: 4 weeks to 3 months | The mean pain in the OKC exercises group ranged from 1.8 to 4.87 points1 | The mean usual pain (short‐term) in the CKC group was 0.20 higher (0.37 lower to 0.76 higher) | MD 0.20 (‐0.37 to 0.76) | 122 (3 studies) | ⊕⊝⊝⊝ very low4 | The confidence interval excludes the MCID of usual pain of 2.0 points5 |
| Pain during activity (long‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 5 years | The mean pain in the OKC exercises group was 0.7 points1 | The mean pain during activity (long‐term) in the CKC group was 2.10 higher (1.08 to 3.12 higher) |
MD 2.10 (1.08 to 3.12) |
49 (1 study) | ⊕⊝⊝⊝ very low4 | The confidence interval includes the MCID of 1.33 in favour of OKC exercises. Thus this includes the possibility of a clinically important effect of OKC exercises on pain during activity (long‐term) |
| Usual pain (long‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 5 years | The mean pain in the OKC exercises group was 1.0 points1 | The mean usual pain (long‐term) in the CKC group was 0.80 higher (0.07 to 1.53 higher) |
MD 0.80 (0.07 to 1.53) |
49 (1 study) | ⊕⊝⊝⊝ very low4 | The confidence interval excludes the MCID for usual pain of 2.0 points5 |
| Functional ability (short‐term) AKPS (0 to 100; higher scores mean better function) Follow‐up: 6 weeks or 3 months | The mean AKPS score in the OKC exercises group ranged from 89.1 to 91.7 points1 | The mean functional ability (short‐term) in the CKC group was 3.51 lower (7.84 lower to 0.82 higher) | MD ‐3.51 (‐7.84 to 0.82) | 90 (2 studies) | ⊕⊝⊝⊝ very low6 | The confidence interval excludes the MCID for the AKPS of 10.0 points7 |
| Functional ability (long‐term) AKPS (0 to 100; higher scores mean better function) Follow‐up: 5 years | The mean AKPS score in the OKC exercises group was 90 points1 | The mean functional ability (long‐term) in the CKC group was 8.30 lower (12.95 to 3.65 lower) | MD ‐8.30 (‐12.95 to ‐3.65) | 49 (1 study) | ⊕⊝⊝⊝ very low4 | The confidence interval includes the MCID of 10.07 in favour of OKC exercises. Thus this includes the possibility of a clinically important effect on functional ability (long‐term) of OKC exercises |
| Recovery (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any of the 4 studies making this comparison |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKPS: Anterior Knee Pain Score; CKC: closed kinetic chain; CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; OKC: open kinetic chain; VAS: visual analogue scale/score | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The basis for the assumed risk is the range of the control group risk of the studies.
2In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding), one level for imprecision (small sample size) and one level for inconsistency (heterogeneity: P value = 0.08; I2 = 67%).
3The minimal clinically important difference for VAS pain during activity was set at 1.3 points (Crossley 2004).
4In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding) and two levels for serious imprecision (small sample size).
5The minimal clinically important difference for VAS usual pain was set at 2.0 points (Crossley 2004)
6In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding), one level for imprecision (small sample size) and one for inconsistency (heterogeneity: P value = 0.06; I2 = 71%).
7The minimal clinically important difference for the AKPS was set at 10.0 points (Crossley 2004).
Summary of findings 4. Target of exercise: hip + knee versus knee exercises for treating patellofemoral pain syndrome.
| Target of exercise: hip + knee versus knee exercises for treating patellofemoral pain syndrome | ||||||
| Patient or population: patients with patellofemoral pain syndrome (symptoms > 1 month (3 studies); symptoms > 2 months (1 study); symptoms > 3 months (2 studies); not stated (1 study)) Settings: various: orthopaedic clinics, rehabilitation service, physiotherapy practices/clinics Intervention: hip + knee exercises Comparison: knee exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Knee exercises | Hip + knee exercises | |||||
|
Pain during activity (short‐term) Scale (0 to 10; higher scores mean worse pain)1 Follow‐up range: 4 weeks to 3 months |
The mean pain in the knee exercises group ranged from 2.0 to 5.0 points2 | The mean pain during activity in the hip + knee exercise group was 2.02 lower (3.80 lower to 0.60 higher) |
MD ‐2.02 (‐3.80 to ‐0.60) |
104 (3 studies) | ⊕⊝⊝⊝ very low3 | The confidence interval includes the MCID of 1.34 in favour of hip + knee exercises. Thus this includes the possibility of a clinically important effect of hip + knee exercises on pain during activity (short‐term). However, the confidence interval also crossed the line of no effect resulting in the potential for a small non‐clinically important effect in favour of knee exercises |
| Usual pain (short‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 4 to 6 weeks | The mean pain in the knee exercises group ranged from 4.0 to 4.8 points2 | The mean usual pain in the hip + knee exercise group was 1.77 lower (2.78 to 0.76 lower) |
MD ‐1.77 (‐2.78 to ‐0.76) |
46 (2 studies) | ⊕⊝⊝⊝ very low5 | The confidence interval includes the MCID of 2.06 in favour of hip + knee exercises. Thus this includes the possibility of a clinically important effect of hip + knee exercises on usual pain (short‐term) |
| Pain during activity (long‐term) NPRS (0 to 10; higher scores mean worse pain) Follow‐up: 12 months | The mean pain in the knee exercises group was 6.4 points2 | The mean pain during activity in the knee + hip exercise group was 3.90 lower (4.46 to 3.34 lower) | MD ‐3.90 (‐4.46 to ‐3.34) | 49 (1 study) | ⊕⊝⊝⊝ very low7 | This confidence interval is fully outside the MCID of 1.3 points.4 This points to a clinically important difference in pain during activity (long‐term) in the hip + knee exercises group |
| Usual pain (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any of the 7 studies making this comparison |
| Functional ability (short‐term) Scale (0 to 100; higher scores mean better function)8 Follow‐up range: 4 weeks to 3 months | The mean difference in functional ability (short‐term) in the hip + knee exercise group was 0.61 standard deviations higher (0.39 lower to 1.61 higher) | SMD 0.61 (‐0.39 to 1.61) | 174 (4 studies) | ⊕⊝⊝⊝ very low9 | In order to interpret these results in terms of the AKPS, we scaled values to 0 to 100 and multiplied the SMD by the median SD of the AKPS (11.1) The mean functional ability (short‐term) in the hip + knee exercises group was an estimated 6.77 higher (4.33 lower to 17.87 higher) The confidence interval includes the MCID of 10.010 in favour of hip + knee exercises. Thus this includes the possibility of a clinically important effect on functional ability (short‐term) of hip and knee exercises. Since resulting the confidence interval also crossed the line of no effect, there is also the possibility of a smaller non‐clinically important effect in favour of knee exercises |
|
| Functional ability (long‐term) Scale (0 to 100; higher scores mean better function)11 Follow‐up range: 5 to 12 months | The mean difference in functional ability (long‐term) in the hip and knee exercise group was 1.49 standard deviations higher (0.17 lower to 3.15 higher) | SMD 1.49 (‐0.17 to 3.15) | 78 (2 studies) | ⊕⊝⊝⊝ very low12 | In order to interpret these results in terms of the AKPS, we scaled values to 0 to 100 and multiplied the SMD by the median SD of the AKPS (11.1) The mean functional ability (short‐term) in the hip + knee exercises group was an estimated 16.54 higher (1.89 lower to 34.97 higher) The confidence interval includes the MCID of 10.010 in favour of hip + knee exercises. Thus this includes the possibility of a clinically important effect on functional ability (long‐term) of hip and knee exercises. Since the resulting confidence interval also crossed the line of no effect, there is also the possibility of a smaller non‐clinically important effect in favour of knee exercises |
|
| Recovery long‐term Number of patients at least moderately better Follow‐up: 5 months | 688 per 10002 | 922 per 1000 (640 to 1000) | RR 1.34 (0.93 to 1.94) | 29 (1 study) | ⊕⊝⊝⊝ very low13 | These data equate to 234 more (95% CI 48 fewer to 312 more) participants per 1000 who would have recovered in the long term as a result of hip and knee exercise |
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKPS: Anterior Knee Pain Score; CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; NPRS: numerical pain rating score; RR: risk ratio; SMD: standardised mean difference; VAS: visual analogue scale/score | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Data were from VAS (0 to 10) and NPRS (0 to 10). We scaled values to 0 to 10 (higher is worse). These measures are comparable and thus we calculated MDs.
2The basis for the assumed risk is the range of the control group risk of the studies.
3In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding), one level for imprecision (wide confidence intervals and small sample size) and one level for serious inconsistency (heterogeneity: P value = 0.004, I2 = 82%).
4The minimal clinically important difference for VAS pain during activity was set at 1.3 points (Crossley 2004).
5In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding) and two levels for serious imprecision (wide confidence intervals and small sample size).
6The minimal clinically important difference for VAS usual pain was set at 2.0 points (Crossley 2004)
7In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding) and two levels for serious imprecision.
8Data were from the lower extremity function scale (LEFS) score (0 to 80) in one study, AKPS (0 to 100) in two studies and Lysholm (0 to 100) in one study. We rescaled data from the LEFS to 0 to 100.
9In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding), one level for imprecision (wide confidence intervals and small sample size) and one level for serious inconsistency (heterogeneity: P value < 0.00001, I2 = 90%).
10The minimal clinically important difference for the AKPS was set at 10.0 points (Crossley 2004).
11Data were from the lower extremity function scale (LEFS) score (0 to 80) in one study and AKPS (0 to 100) in the second study. We rescaled data from the LEFS to 0 to 100.
12In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding), one level for imprecision and one level for serious inconsistency (P value = 0.002, I2 = 90%).
13In our assessment of the quality of the evidence for this outcome, we downgraded one level for risk of bias (relating to lack of assessor blinding) and two levels for serious imprecision.
Summary of findings 5. Target of exercise: hip versus knee exercises for treating patellofemoral pain syndrome.
| Target of exercise: hip versus knee exercises for treating patellofemoral pain syndrome | ||||||
| Patient or population: patients with patellofemoral pain syndrome (symptoms > 1 month (1 study); symptoms > 6 months (1 study)) Settings: athletic trainer, physician (not‐specified) Intervention: hip exercises Comparison: knee exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Knee exercises | Hip exercises | |||||
| Pain during activity (short‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 8 weeks | The mean pain in the knee exercises group was 3.27 points1 | The mean pain in the hip exercises group was 1.16 lower (2.41 lower to 0.09 higher) | MD ‐1.16 (‐2.41 to 0.09) | 36 (1 study) | ⊕⊝⊝⊝ very low2 | The confidence interval includes the MCID of 1.33 in favour of hip exercises. Thus this includes the possibility of the effect of hip exercises on pain during activity (short‐term) being clinically important. The confidence interval also includes the potential for a small and non clinically important effect in favour of knee exercises. |
| Usual pain (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in either of the 2 studies for this comparison |
| Pain during activity (long‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 6 months | The mean pain in the knee exercises group was 4.0 points1 | The mean pain in the hip exercises group was 2.00 lower (3.45 to 0.55 lower) | MD ‐2.00 (‐3.45 to ‐0.55) | 36 (1 study) | ⊕⊝⊝⊝ very low2 | The confidence interval includes the MCID of 1.33 in favour of hip exercises. Thus this includes the possibility of a clinically important effect of hip exercises on pain during activity (short‐term) |
| Usual pain (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in either of the 2 studies for this comparison |
| Functional ability (short‐term) Scale (0 to 100; higher scores mean better function)4 Follow‐up: 8 weeks or 3 months | The mean difference in functional ability (short‐term) in the hip exercises group was 0.85 standard deviations higher (0.30 to 1.40 higher) | SMD 0.85 (0.30 to 1.40) | 58 (2 studies) | ⊕⊝⊝⊝ very low2,5 | In order to interpret these results in terms of the AKPS, we scaled values to 0 to 100 and multiplied the SMD by the median SD of AKPS (11.1) The mean functional ability (short‐term) in the hip exercises group was an estimated 9.44 higher (3.33 to 15.54 higher) The confidence interval includes the MCID of 10.06 in favour of hip exercises. Thus this includes the possibility of a clinically important effect of hip exercises on function (short‐term) |
|
| Functional ability (long‐term) WOMAC (0 to 96; inverted scores so that higher scores mean better function) Follow‐up: 6 months | The mean WOMAC score in the knee exercises group was 72.84 points1,7 | The mean functional ability continuous long‐term in the intervention groups was 16.22 higher (9.17 to 23.27 higher) | MD 16.22 (9.17 to 23.27) | 36 (1 study) | ⊕⊝⊝⊝ very low2 | The confidence interval includes the MCID of 15.08 in favour of hip exercises. Thus this includes the possibility of a clinically important effect of hip exercises on function (long‐term) |
| Recovery (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in either of the 2 studies for this comparison |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKPS: Anterior Knee Pain Score; CI: confidence interval; MCID: minimal clinically important difference; MD: mean difference; SMD: standardised mean difference; VAS: visual analogue scale/score | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The basis for the assumed risk is the control group risk of the study.
2In our assessment of the quality of the evidence for this comparison, we downgraded two levels for serious risk of bias (relating to lack of allocation concealment and/or lack of assessor blinding) and one or two levels for serious imprecision (wide confidence intervals and small sample size).
3The minimal clinically important difference for VAS pain during activity was set at 1.3 points (Crossley 2004).
4Data were from the lower extremity function scale (LEFS) score (0 to 80) in one study and WOMAC Osteoarthritis Index (0 to 96) in the other study. We rescaled data from both scales to 0 to 100; we inverted those from WOMAC first.
5We also downgraded the quality of the evidence for this outcome for inconsistency due to heterogeneity (heterogeneity: P value = 0.08; I2 = 68%).
6The minimal clinically important difference for the AKPS was set at 10.0 points (Crossley 2004).
7We inverted the data for the WOMAC score (subtracted from 96) so that higher scores = better outcome.
8The minimal clinically important difference for WOMAC was set at 15.0 points (Escobar 2006).
Summary of findings 6. High‐intensity versus low‐intensity exercise programmes for patellofemoral pain syndrome.
| High‐intensity versus low‐intensity exercise programmes for patellofemoral pain syndrome | ||||||
| Patient or population: patients with patellofemoral pain syndrome (untreated PFPS of over 2 months in duration) Settings: general practice or orthopaedic clinics Intervention: high‐intensity exercise programme Comparison: low‐intensity exercise programme | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low‐intensity exercise | High‐intensity exercise | |||||
| Pain during activity (short‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the single study testing this comparison |
| Usual pain (short‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 3 months | The mean pain in the low‐intensity exercise group was 2.6 points | The mean pain in the high‐intensity exercise group was 1.90 lower (2.85 to 0.95 lower) | MD ‐1.90 (‐2.85 to ‐0.95) | 40 (1 study) | ⊕⊝⊝⊝ very low1 | The confidence interval includes the MCID of 2.0 points2 in favour of high‐intensity exercise. This thus includes the possibility of a clinically important effect of high‐intensity exercise on usual pain (short‐term) |
| Pain during activity long‐term | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the single study testing this comparison |
| Usual pain (long‐term) VAS (0 to 10; higher scores mean worse pain) Follow‐up: 12 months | The mean pain in the low‐intensity exercise group was 3.5 points | The mean pain in the high‐intensity exercise group was 3.20 lower (4.05 to 2.35 lower) | MD ‐3.20 (‐4.05 to ‐2.35) | 28 (1 study) | ⊕⊝⊝⊝ very low1 | The confidence interval is fully outside the MCID of 2.0 points.2 This points to a clinically important difference in usual pain (long‐term) favouring high‐intensity exercise |
| Functional ability (short‐term) FIQ modified (0 to 16; higher scores mean better function) Follow‐up: 3 months | The mean FIQ score in the low‐intensity exercise group was 9.8 points | The mean FIQ score in the high‐intensity exercise groups was 3.70 higher (1.59 to 5.81 higher) |
MD 3.70 (1.59 to 5.81) |
40 (1 study) | ⊕⊝⊝⊝ very low1 | The confidence interval includes the MCID of 2.0 points3 in favour of high‐intensity exercise. This thus includes the possibility of a clinically important effect of high‐intensity exercise on functional ability (short‐term) |
| Functional ability (long‐term) FIQ modified (0 to 16; higher scores mean better function) Follow‐up: 12 months | The mean FIQ score in the low‐intensity exercise group was 10.2 points | The mean functional ability continuous long‐term in the intervention groups was 3.90 higher (1.72 to 6.08 higher) |
MD 3.90 (1.72 to 6.08) |
28 (1 study) | ⊕⊝⊝⊝ very low1 | The confidence interval includes the MCID of 2.0 points3 in favour of high‐intensity exercise. This thus includes the possibility of a clinically important effect of high‐intensity exercise on functional ability (long‐term) |
| Recovery (long‐term) | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the single study testing this comparison |
| *The basis for the assumed risk is the control group risk of the study. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FIQ: Functional Index Questionnaire; MCID: minimal clinically important difference; MD: mean difference; VAS: visual analogue scale/score | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1In our assessment of the quality of the evidence for this comparison, we downgraded one level for risk of bias (relating to lack of assessor blinding) and two levels for imprecision (wide confidence intervals and small sample size).
2The minimal clinically important difference for VAS usual pain was set at 2.0 points (Crossley 2004)
3The minimal clinically important difference for the modified FIQ was set at 2.0 points (Crossley 2004)
Background
Description of the condition
Patellofemoral pain syndrome (PFPS) is a common knee problem, which particularly affects adolescents and young adults (Rathleff 2013). Synonyms for patellofemoral pain syndrome are 'anterior knee pain syndrome', 'patellar dysfunction', 'chondromalacia patellae' or 'chondropathy'. Its incidence varies from 22 new cases per 1000 persons/year in highly active populations to five to six new cases per 1000 in general practice (Boling 2009; Van der Linden 2004). PFPS is characterised by retropatellar pain (behind the kneecap) or peripatellar pain (around the kneecap), mostly occurring when load is put on the knee extensor mechanism such as when climbing stairs, squatting, running, cycling or sitting with flexed knees (Davis 2010; Lankhorst 2012). The diagnosis is based on these symptoms after excluding other distinct knee pathologies, which potentially cause anterior knee pain, such as Hoffa's syndrome, Osgood Schlatter syndrome, Sinding‐Larsen‐Johansson syndrome, iliotibial band friction syndrome, tendinitis, neuromas, intra‐articular pathology including osteoarthritis, rheumatoid arthritis, traumatic injuries (such as injured ligaments, meniscal tears, patellar fractures and patellar luxation), plica syndromes and more rarely occurring pathologies. Physical tests, for example the Clarke's compression test, are used to diagnose PFPS, but the sensitivity and specificity of these tests are debated (Doberstein 2008; Post 1999).
Several factors have been implicated in the aetiology of PFPS. These include local factors (contribution of patellofemoral joint mechanics and surrounding tissues to patellofemoral pain), distal factors (contribution of foot and ankle mechanics) and proximal factors (contribution of hip, pelvis and trunk mechanics) (Davis 2010). However, the aetiology of the condition is still unclear, as is the origin of the pain. Other factors that have recently been described as factors associated with PFPS are a lower knee extension strength, a lower hip extension strength and decreased flexibility of the lower extremity muscles (Lankhorst 2012)
Description of the intervention
The majority of people with PFPS are treated conservatively (non‐surgically). Physically‐based conservative interventions include knee orthoses, foot orthoses (Hossain 2011), patellar taping (Callaghan 2012) and exercise therapy.
Most exercise therapy programmes for PFPS have focused on strengthening the quadriceps muscles, which was seen as the most promising conservative treatment method for patellofemoral pain syndrome (Heintjes 2003; Powers 1998; Thomeé 1999). More recently, studies have focused on hip muscle dysfunction as a possible contributor to patellofemoral pain (Souza 2009a; Souza 2009b; Willson 2008).
Exercise therapy comprises a broad range of possible variations and accompanying terms. Activity of the quadriceps muscles ‐ and other muscles involved in knee function ‐ can either be concentric, eccentric or isometric. During concentric activities the muscles shorten, whereas during eccentric activities the muscles lengthen in an actively controlled manner. During isometric activity the muscle length remains the same. Exercises can either be static or dynamic. Exercises are referred to as static if the position of the knee does not change. If the position of the knee does change, the exercise is called dynamic. In cases where the lower leg moves at a predetermined, constant speed, which requires an isokinetic dynamometer to control the velocity, the dynamic exercise is also called isokinetic. Exercises where the foot is in contact with a fixed surface are referred to 'closed kinetic chain exercises', as opposed to 'open kinetic chain' exercises where the foot is not in contact with a fixed surface.
Thus, exercises can be arranged in three ways: the type of muscle activity (concentric, eccentric, isotonic), joint movement (dynamic versus static) and the presence of reaction forces caused by contact of the foot with a fixed surface (closed versus open kinetic chain) (Witvrouw 2000; Witvrouw 2004). Combinations of the above apply to every type of exercise, and the terminology used for exercise programmes reflects the emphasis intended by the therapist or researcher. Emphasis during exercise therapy may be put on the co‐ordinated contraction of the medial and lateral parts of the quadriceps muscle, and also on the co‐ordinated contraction of hip adductor, hip abductor and gluteal muscles (Mellor 2005).
In addition, there are other differences such as in the delivery of exercise, for example, supervised exercise versus home exercise; or in the duration or intensity of exercise.
How the intervention might work
A recent published review on factors associated with PFPS concluded that people with PFPS have lower knee extension strength, lower hip extension strength and decreased flexibility of the lower extremity muscles compared with people without PFPS (Lankhorst 2012). Exercise programmes that comprise static and dynamic muscular exercises for both quadriceps and hip muscles aim to improve the strength of these muscles and consequently reduce pain by decreasing the load on the patellofemoral joint and improve function by normalising the kinematics.
Why it is important to do this review
Patellofemoral pain syndrome (PFPS) is a common knee problem, particularly affecting adolescents and young adults and exercise therapy to strengthen the quadriceps is often prescribed. However, the aetiology of the condition, including the structures causing the pain, and treatment methods are all debated and consensus has not been reached so far. This review updates and supercedes a former Cochrane review (Heintjes 2003).
Objectives
To assess the effects (benefits and harms) of exercise therapy aimed at reducing knee pain and improving knee function for people with patellofemoral pain syndrome.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised (using a method of allocating participants to a treatment or control condition by a method that is not strictly random, e.g. by hospital number) controlled clinical trials that evaluate exercise therapy for patellofemoral pain syndrome.
Types of participants
Adolescents and adults with patellofemoral pain (or a synonym of this) as defined by trial authors.
We excluded studies focusing on other named knee pathologies such as Hoffa's syndrome, Osgood Schlatter syndrome, Sinding‐Larsen‐Johansson syndrome, iliotibial band friction syndrome, tendinitis, neuromas, intra‐articular pathology including osteoarthritis, rheumatoid arthritis, traumatic injuries (such as injured ligaments, meniscal tears, patellar fractures and patellar luxation), plica syndromes and more rarely occurring pathologies (Nissen 1998; Thomeé 1999).
Types of interventions
We included studies evaluating exercise therapy for patellofemoral pain syndrome. Exercises could be applied on their own or in combination with other non‐surgical interventions, provided the same other intervention was applied to the whole population in the comparison. Exercises could be performed at home or under supervision of a therapist.
Comparisons
Exercise therapy versus control (no treatment, placebo or waiting list controls). This also includes 'exercise therapy + another intervention (e.g. taping) versus the other intervention alone (e.g. taping)'
-
Exercise therapy versus different conservative interventions (e.g. taping)
Exercise therapy versus unimodal conservative interventions
Exercise therapy versus multimodal conservative interventions
Comparisons of different exercises or exercise therapy programmes:
Delivery of exercises or exercise programmes (e.g. supervised versus home exercise; group versus individual supervision)
Medium of exercises or exercise programmes (water‐ versus land‐based exercise)
Types of exercises or exercise programmes (e.g. closed versus open kinetic chain exercises; dynamic versus static)
Target of exercises or exercise programmes (strengthening of hip or abdominal muscles versus quadriceps muscles)
Duration of exercises or exercise programmes (e.g. long duration (more than three months) versus shorter duration (three months or less))
Intensity of exercises or exercise programmes (e.g. high‐intensity (several times per week) versus low‐intensity (once weekly))
We defined the intervention group for comparisons of different exercises as the most novel, intensive or resource‐dependent intervention. For instance, the intervention was supervised exercise and the control was home exercise in the first comparison (3a). We also gave consideration to consistency in the choice of control groups.
For comparison 3c, types of exercises, we implemented a secondary categorisation based on the type of kinetic chain involved. These were closed versus open kinetic chain exercises; variants of closed kinetic chain exercise; and open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action (isometric, isotonic (concentric or eccentric) or isokinetic). We presented separately any exceptions that did not fit in.
In terms of the 'exercise therapy' group, combined interventions or treatment packages including exercise were not tested in this review, with the exception of exercises provided with instructions or advice, where exercise was the predominant intervention.
Types of outcome measures
Primary outcomes
Knee pain measured by validated self reporting methods (visual analogue scale (VAS), numerical rating scale (NRS) or McGill Pain questionnaire (Melzack 1987)). If multiple pain scales were reported in one study, we only included pain in daily life (usual pain, worst pain and pain at activities (e.g. sports, pain during descending stairs) (Crossley 2004)) in the analyses. We selected pain at descending for pooling on 'pain at activities' as this outcome measure was present in most studies eligible for pooling of pain at activity.
Secondary outcomes
Functional ability (i.e. knee function in activities of daily living) measured by questionnaires focusing on knee function (such as Functional Index Questionnaire (FIQ) (Chesworth 1989), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (McConnell 2001), Kujala Patellofemoral Function Scale or Anterior Knee Pain Score (AKPS) (Kujala 1993) and Lysholm scale (Lysholm 1982)). If multiple scales for functional ability were measured including the AKPS, we used the latter for pooling.
Functional performance tests, including squatting and hopping on one leg (Loudon 2002).
Subjective perception of recovery. Recovery from patellofemoral pain syndrome is an outcome measure inconsistently reported in studies and different methods are used to describe recovery. In this review, we gave preference to 'number of patients no longer troubled by symptoms' or 'perceived recovery' measured on a Likert scale (Van Linschoten 2009a).
Adverse events: we considered knee swelling or substantially increasing pain levels as a direct effect of treatment.
Based on Crossley 2004, we chose the following minimal clinically important differences for pain and function: 1.3 points on a VAS (0 to 10) for pain during activity; 2.0 points on a VAS (0 to 10) for usual and worst pain; 10 points for the AKPS (0 to 100) and 2 points for the FIQ (0 to 16).
Changes in knee function measured on impairment level only (e.g. range of motion, muscle strength) do not directly represent changes in the symptoms of patellofemoral pain or the resulting disability, and we therefore did not consider them clinically relevant outcome measures in this review (Dursun 2001; Gobelet 1992).
Timing of outcome measurement
We considered outcomes measured within three months after the baseline measurement short‐term outcomes of exercise therapy, and we considered measurements more than three months after the baseline measurement long‐term outcomes. If multiple short‐term outcomes were measured in one trial, we used the time point closest to three months for pooling.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (23 May 2014), the Cochrane Central Register of Controlled Trials (2014, Issue 4), MEDLINE (1946 to May Week 2 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (22 May 2014), EMBASE (1980 to 2014 Week 20), PEDro ‐ The Physiotherapy Evidence Database (to 26 June 2014), CINAHL (1982 to 23 May 2014) and AMED (1985 to May 2014). We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform and Current Controlled Trials for ongoing and recently completed trials (30 June 2014).
In MEDLINE (Ovid Online), we combined a subject‐specific strategy with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). Search strategies for MEDLINE, the Cochrane Central Register of Controlled Trials, EMBASE, CINAHL and AMED are shown in Appendix 1.
We did not apply any language restrictions.
Searching other resources
We checked reference lists of included studies and other relevant articles, including a previous Cochrane review (Heintjes 2003), for additional trials. We contacted institutions and experts in the field in order to identify unpublished studies. We searched conference abstracts from the International Patellofemoral Pain Research Retreat (Davis 2010).
Data collection and analysis
The intended methodology for data collection and analysis was described in our published protocol (van der Heijden 2013), which was based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (RAH and NEL) selected potentially eligible articles by reviewing the title and abstract of each citation. After obtaining full articles, both authors independently performed study selection. In cases of disagreement, we reached a consensus through discussion.
Data extraction and management
Two review authors (RAH and NEL) independently extracted the data within included trials using a piloted data collection form. We resolved any disagreements by consensus. Where data were missing or incompletely reported, we contacted authors of trials. Where pooling was possible, and if necessary, we converted pain scores (VAS, NRS) to a 0 to 10 scale and function scores to a 0 to 100 scale.
Assessment of risk of bias in included studies
Two review authors (RAH and NEL) independently assessed the risk of bias of the included trials using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). We assessed the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias. Other sources of bias included bias from major imbalance in baseline characteristics and performance bias such as from lack of comparability in clinicians' experience with the interventions under test, differences in care other than the interventions under test or compliance with the intervention.
We explicitly judged each of these criteria using: low risk of bias; high risk of bias; and unclear risk of bias (where 'unclear' relates to a lack of information or uncertainty over the potential for bias). Disagreements between review authors regarding the risk of bias for domains were resolved by consensus.
Measures of treatment effect
We calculated risk ratios with 95% confidence intervals for dichotomous outcomes. We calculated mean differences with 95% confidence intervals for continuous outcomes as appropriate. When two or more studies presented their data derived from the same instrument of evaluation (with the same units of measurement), we pooled data as a mean difference (MD). Conversely, we used the standardised mean difference (SMD) when primary studies express the same variables through clearly different instruments (and different units of measurement). In case of pooling of different units of measurements, we scaled values to 0 to 10 (lower is better) for pain and 0 to 100 (higher is better) for functional ability. In order to re‐express SMDs in VAS (0 to 10) and AKPS (0 to 100), we multiplied SMDs and 95% CIs by an estimate (the median of all control and intervention standard deviations (SDs)) of the SD of VAS or AKPS respectively.
Unit of analysis issues
The unit of randomisation in the studies likely to be included in this review is usually the individual participant. Exceptionally, as in the case of trials including people with bilateral complaints, data for trials could be evaluated for knees, instead of individual patients. Where such unit of analysis issues arose and appropriate corrections had not been made, we proposed to present data for such trials only where the disparity between the units of analysis and randomisation was small. Where data were pooled, we aimed to perform a sensitivity analysis to examine the effects of pooling these incorrectly analysed trials with the other correctly analysed trials. However, all the outcome measures, except functional performance, presented their outcome data based on the individual participant. For functional performance, studies including participants with bilateral complaints used the most painful side for analysis. So, no unit of analysis issues occurred.
For multi‐comparison studies, we attempted to combine data where two or more of the groups tested interventions in the same category. When combining was not appropriate but the data presented for the difference comparisons were presented in the same analysis, we divided the number of participants in the shared comparison (e.g. halved where this intervention appears twice) in order to avoid the 'double‐counting' of participants for the 'shared comparison' in the meta‐analyses. For cross‐over trials, we proposed to present data collected prior to the cross‐over of the intervention, but there were no cross‐over trials included.
Dealing with missing data
We contacted trial authors where further details of methodology or data were required for trial inclusion.
Where possible we performed intention‐to‐treat analyses to include all people randomised. However, where dropouts were identified, we used the actual numbers of participants contributing data at the relevant outcome assessment. We were alert to the potential mislabelling or non‐identification of standard errors and standard deviations (SDs). Unless missing standard deviations could be derived from confidence intervals or standard errors, we planned to consider whether it was appropriate to estimate values based on comparable data included in this review in order to present these in the analyses. We imputed no data in the review. Should we impute data in future, we will make clear for which trials imputed data have been used (e.g. footnotes in the forest plots).
Should data have been presented as the median (inter‐quartile range), we would not have transformed these to achieve normality or to estimate the mean and SD.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plot (analysis) along with consideration of the Chi² test for heterogeneity and the I² statistic (Higgins 2011). We considered heterogeneity statistically significant if the I² statistic was 70% or more or the P value < 0.1 for the Chi² test. We also examined studies for methodological and clinical heterogeneity, particularly if significant statistical heterogeneity was identified.
Assessment of reporting biases
For future updates of the review, we will explore the possibility of publication bias using a funnel plot if there are data from at least 10 trials available for pooling (Higgins 2011).
Data synthesis
When considered appropriate, we pooled results of comparable groups of trials using both fixed‐effect and random‐effects models. The choice of the model to report was guided by a careful consideration of the extent of heterogeneity and whether it could be explained, in addition to other factors such as the number and size of studies that were included. The fixed‐effect model was the standard. We used a random‐effects model in case of statistically significant heterogeneity.
Subgroup analysis and investigation of heterogeneity
Where data permitted, we proposed to perform the following subgroup analyses:
Gender
Duration of complaints (acute (less than three months) versus chronic)
Sport participation (athletes and/or military recruits versus the general population)
We intended to inspect the overlap of confidence intervals and perform the test for subgroup differences available in RevMan to test whether subgroups were statistically significantly different from one another. However, subgroup analysis to determine the effects of gender, duration of complaints and sports participation on the outcomes of interest was not possible due to the small number of participants in the studies and the inconsistent reporting of baseline characteristics.
Sensitivity analysis
Where appropriate, we performed sensitivity analyses investigating the effects of risks of bias by excluding trials with high or unclear risk of bias (such as selection bias for trials with lack of allocation concealment and lack of random sequence generation) and trials reported in abstracts only. We explored the effects of using different models (fixed‐effect versus random‐effects) for pooling data where there was substantial heterogeneity and retained the more conservative result (random‐effects) but also explored the effects on the results of removing single trials (outliers) in analyses where there were three trials or more. We did not need to perform sensitivity analyses to explore the effects of included trials with imputed data (e.g. SDs) for this version of the review.
'Summary of findings' tables
Where there were sufficient data, we summarised the results for the main comparisons described in the Types of interventions in 'Summary of findings' tables. We used the GRADE approach for systematic reviews (GRADE guideline 5; GRADE guideline 6; GRADE guideline 7; GRADE guideline 8) to assess the quality of evidence related to seven outcomes (pain during activity (short‐term; ≤ 3 months); usual pain (short‐term); pain during activity (long‐term; > 3 months); usual pain (long‐term); functional ability (short‐term); functional ability (long‐term); recovery (long‐term); see Types of outcome measures) (Higgins 2011; see section 12.2).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies; Table 7.
1. Summary of characteristics of included studies.
| Study | Recruitment setting Country | Number of participants included | % female gender | Age | % bilateral complaints | Activity level | BMI |
| Abd Elhafz 2011 | Physiotherapy clinic, Egypt | 30 | 30 | 35.8 | 0 | Not reported | Not reported |
| Abrahams 2003 | Orthopaedic, UK | 78 | 50 | 29.0 | 0 | Not reported | 24.8 |
| Avraham 2007 | Orthopaedic, Israel | 30 | Not reported | 35 | Not reported | Not reported | Not reported |
| Bakhtiary 2008 | Not reported, Iran | 32 | 100 | 22.1 | Not reported | Not reported | Not reported |
| Balci 2009 | Orthopaedic,Turkey | 40 | 100 | 37.6 | 0 | Not reported | 25.5 |
| Clark 2000 | Orthopaedic, rheumatology consultants or general practice, Australia | 81 | 44 | 27.8 | 55 | Not reported | 25.0 |
| Colón 1988 | Not reported, USA | 29 | 34 | Range: 15 to 24 | Not reported | Active1 | Not reported |
| De Marche 2014 | Physical therapy clinic, Brazil | 31 | 100 | 22 | Not reported | Active2 | 21.5 |
| Dolak 2011 | Athletic trainer, USA | 33 | 100 | 25.4 | 48 | Not reported | 25.5 |
| Eburne 1996 | Outpatient physiotherapy department, UK | 75 | Not reported | Not reported | Not reported | Not reported | Not reported |
| Fukuda 2010 | Rehabilitation service | 70 | 100 | 24.6 | 0 | Less active3 | 22.0 |
| Fukuda 2012 | Rehabilitation service | 54 | 100 | 22.5 | 0 | Less active3 | 24.0 |
| Gaffney 1992 | Department of community health and institute of sport, Australia | 72 | 35 | 33.9 | 50 | Not reported | 23.3 |
| Gobelet 1992 | Not reported, Switzerland | 94 | 53 | 20.7 | Not reported | Not reported | Not reported |
| Hafez 2012 | Orthopaedic, Egypt | 40 | 100 | 18 | Not reported | Not reported | Not reported |
| Harrison 1999 | General practice and orthopaedic, Canada | 112 | 60 | 22.2 | 54 | Not reported | Not reported |
| Herrington 2007 | Orthopaedic, Saudi Arabia | 45 | 0 | 26.9 | Not reported | Not reported | Not reported |
| Khayambashi 2012 | Physician, specialty not reported, Iran | 28 | 100 | 29.7 | 100 | Less active4 | 24.3 |
| Khayambashi 2014 | Physicians, specialty not reported, Iran | 36 | 50 | 27.8 | 61 | Less active4 | 23.2 |
| Loudon 2004 | Primary care, USA | 29 | 76 | 24.7 | 0 | Active5 | 26.9 |
| Lun 2005 | General practice or orthopaedic or via bulletin board posters and word of mouth, Canada | 98 | 58 | 34.8 | 44 | Not reported | 24.4 |
| Moyano 2013 | Physiotherapy clinic, Spain | 61 | 43 | 39.9 | Not reported | Less active6 | 24.6 |
| Nakagawa 2008 | Physiotherapy clinic, Brazil | 14 | 71 | 23.6 | Not reported | Not reported | Not reported |
| Razeghi 2010 | Screening of all female students at the physiotherapy clinic affiliated to the rehabilitation faculty, Iran | 33 | 100 | 22.6 | 62.5 | Not reported | Not reported |
| Schneider 2001 | Not reported, Germany | 40 | 70 | Not reported | Not reported | Active7 | Not reported |
| Song 2009 | Orthopaedic, Taiwan | 89 | 87 | 40.9 | Not reported | Less active8 | 22.6 |
| Taylor 2003 | Chiropractic clinic and poster advertisements in public places, UK | 12 | 33.3 | 30.2 | Not reported | Not reported | Not reported |
| Thomee 1997 | Orthopaedic, Sweden | 40 | 100 | 20.2 | 68 | Not reported | Not reported |
| Van Linschoten 2009 | General practices and sports medical centres, The Netherlands | 131 | 64.1 | 23.9 | 60.3 | Not reported | 23.1 |
| Witvrouw 2000 | Not reported, Belgium | 60 | 66.7 | 20.3 | 45 | Not reported | Not reported |
| Østeråsa 2013 | General practice and orthopaedics, Norway | 40 | 80 | 30.0 | 70 | Not reported | Not reported |
1Recreational athletes.
2Athletes with a minimum sport participation of 30 minutes, 3 times a week.
3Sedentary: not practised physical activity any day of the week, both aerobic and strengthening exercises, for at least the past six months.
4Patients were not physically active and did not participate in recreational sport activities or exercise beyond that of activities of daily living.
5Active in sports for at least 120 minutes per week.
6No engagement in regular sporting activities.
7Active amateur athletes.
8No engagement in regular sporting activities.
Results of the search
We found 1398 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (49 records); Cochrane Central Register of Controlled Trials (135), MEDLINE (326 records), EMBASE (491 records), AMED (178 records), CINAHL (146 records), PEDro (11 records), the WHO International Clinical Trials Registry Platform (42) and Current Controlled Trials (20). Furthermore, we identified 13 potentially eligible studies from the previous review of Heintjes 2003.
The search identified 107 potentially eligible studies of which 60 were clearly not eligible upon the retrieval of full‐text articles. Of those remaining, 31 studies (two with data published in two reports) were included in the review. We excluded 12 studies and there is one ongoing study. One study is reported in Turkish and has been placed in Characteristics of studies awaiting classification pending translation (Erel 2011).
A flow diagram summarising the study selection process is shown in Figure 1.
1.
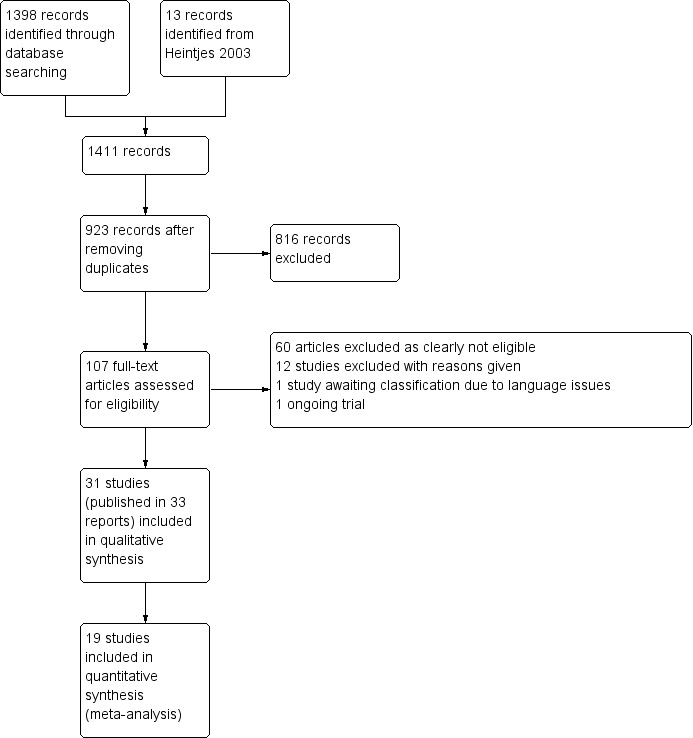
Study flow diagram
Included studies
Full details of the trials can be found in the Characteristics of included studies. A summary of key patient characteristics is presented in Table 7; and in the text below.
Design
We included 25 randomised controlled trials (Abd Elhafz 2011; Abrahams 2003; Avraham 2007; Bakhtiary 2008; Balci 2009; Clark 2000; De Marche 2014; Dolak 2011; Fukuda 2010; Fukuda 2012; Gaffney 1992; Gobelet 1992; Hafez 2012; Harrison 1999; Herrington 2007; Lun 2005; Moyano 2013; Nakagawa 2008; Razeghi 2010; Schneider 2001; Song 2009; Taylor 2003; Van Linschoten 2009; Witvrouw 2000; Østeråsa 2013) and six quasi‐randomised trials (Colón 1988; Eburne 1996; Khayambashi 2012; Khayambashi 2014; Loudon 2004; Thomee 1997).
We extracted data for one comparison from 21 trials and for two comparisons from 10 trials (Abrahams 2003; Clark 2000; Fukuda 2010; Gobelet 1992; Harrison 1999; Herrington 2007; Loudon 2004; Lun 2005; Moyano 2013; Song 2009).
Sample sizes
In total, 1690 participants from 31 trials were included in this review. The number of participants in the intervention groups in the individual studies ranged from six (Taylor 2003) to 65 (Van Linschoten 2009).
Recruitment setting
Participants were recruited from the following settings: orthopaedic clinics (Abrahams 2003; Avraham 2007; Balci 2009; Clark 2000; Hafez 2012; Harrison 1999; Herrington 2007; Lun 2005; Song 2009; Thomee 1997; Østeråsa 2013), general practices (Clark 2000; Harrison 1999; Loudon 2004; Lun 2005; Østeråsa 2013; Van Linschoten 2009), physiotherapy practices (Abd Elhafz 2011; De Marche 2014; Eburne 1996; Moyano 2013; Nakagawa 2008), chiropractic practices (Taylor 2003), rehabilitation services (Fukuda 2010; Fukuda 2012), athletic trainer practices (Dolak 2011), sports medicine practices (Van Linschoten 2009), rheumatology department (Clark 2000), department of community health (Gaffney 1992), institute of sports (Gaffney 1992), poster advertisements in public places (Taylor 2003), screening of all female students at the physiotherapy clinic affiliated to the rehabilitation faculty (Razeghi 2010), or via bulletin board posters and word of mouth (Lun 2005) (seeTable 7). Seven trials recruited from more than one setting (Clark 2000; Gaffney 1992; Harrison 1999; Lun 2005; Østeråsa 2013; Taylor 2003; Van Linschoten 2009). Seven trials did not report their recruitment setting (Bakhtiary 2008; Colón 1988; Gobelet 1992; Khayambashi 2012; Khayambashi 2014; Schneider 2001; Witvrouw 2000).
Trials were undertaken in 18 different countries (Australia (two trials); Belgium (one); Brazil (four); Canada (two); Egypt (two); Germany (one); Iran (four); Israel (one); Norway (one); Saudi Arabia (one); Spain (one); Sweden (one); Switzerland (one); Taiwan (one); The Netherlands (one); Turkey (one); UK (three); and USA (three) (seeTable 7).
Participants
All participants were diagnosed with patellofemoral pain syndrome based on clinical symptoms and, occasionally, radiological examination (Table 8). Exceptionally, in Abrahams 2003, malalignment also had to be diagnosed by X‐ray. The trials varied quite markedly in their inclusion criteria, such as the explicit mention of a minimum duration of symptoms and, if mentioned, the minimum required; this ranged from three weeks (Lun 2005) to eight months (Abrahams 2003). Five trials provided no details of pain provoking activities or pain provoking functional or clinical tests used for determining eligibility (Clark 2000; Eburne 1996; Gobelet 1992; Hafez 2012; Schneider 2001) (see Table 8). Trials consisted of populations with different levels of activity. Six trials reported that they included a less active population (Fukuda 2010; Fukuda 2012; Khayambashi 2012; Khayambashi 2014; Moyano 2013; Song 2009) and four trials an active population (Colón 1988; De Marche 2014; Loudon 2004; Schneider 2001). Eighteen trials included both male and female participants (Abd Elhafz 2011; Abrahams 2003; Clark 2000; Colón 1988; Gaffney 1992; Gobelet 1992; Harrison 1999; Khayambashi 2014; Loudon 2004; Lun 2005; Moyano 2013; Nakagawa 2008; Østeråsa 2013; Schneider 2001; Song 2009; Taylor 2003; Van Linschoten 2009; Witvrouw 2000). Ten studies involved only female participants (Bakhtiary 2008; Balci 2009; De Marche 2014; Dolak 2011; Fukuda 2010; Fukuda 2012; Hafez 2012; Khayambashi 2012; Razeghi 2010; Thomee 1997) and one included only male participants (Herrington 2007). Two studies did not report the number of females and males (Avraham 2007; Eburne 1996). The age of participants ranged from 10 to 65 years. The mean age of the participants reported in 28 trials ranged from 18 to 40.9 years. The mean body mass index (BMI), only reported in 15 trials, ranged from 21.5 to 26.9 (seeTable 7).
2. Summary of diagnostic inclusion criteria.
| Inclusion criterion | |||||||
| Study ID | Symptom | Symptom duration | Pain provoking functional activities | Pain provoking functional tests | Pain provoking clinical tests | Other clinical tests | Imaging tests |
| Abd Elhafz 2011 | Diffuse, unilateral anterior knee pain | At least 8 weeks | Exacerbated by activity | — | Exacerbated by isometric quadriceps contraction | — | — |
| Abrahams 2003 | Unilateral PFPS; retropatellar or anterior knee pain | 8 to 18 months | Pain on squatting | — | Positive direct patellofemoral grind test | — | Malalignment as diagnosed by X‐ray |
| Avraham 2007 | Anterior knee pain | — | Pain related to prolonged sitting, climbing stairs and descending stairs | — | Positive sign in patellofemoral gliding test; negative McMurray test | Full knee range of motion | No relevant patellofemoral degenerative changes on imaging |
| Bakhtiary 2008 | Chondromalacia patella | — | Pain during climbing up and down stairs and pain after sitting for a long time with the knee flexed and problem with knee extension after sitting for a long time with the knee flexed and giving away during walking | — | Positive Clark test | — | — |
| Balci 2009 | Patellofemoral pain | At least 2 months | Between at least 2 activities like long time sitting, stair/slope climbing and descending, crouching, running, bouncing and jumping | — | — | — | — |
| Clark 2000 | Anterior knee pain | > 3 months | — | — | — | — | — |
| Colón 1988 | Patellofemoral chondrosis | 2 out of the following 6 criteria: persistent aching in the knees while at rest, pain in the knees after sitting with the knees in a flexed position for more than 10 to 20 minutes, occurrence or exaggeration of pain on walking up or down stairs, crepitation in the knees with movement, snapping sensations in the knees upon extension or flexion, locking of the knees, inability to squat down without pain | — | Crepitation and compression sign during physical examination | — | — | |
| De Marche 2014 | Anterior or retropatellar knee pain of 3 or greater on the 10 cm VAS scale | Minimum of 8 weeks | Pain during at least 3 of the following activities: ascending/descending stairs, squatting, running, kneeling, jumping and prolonged sitting | — | — | — | — |
| Dolak 2011 | Anterior‐ or retropatellar knee | More than 1 month | Pain during at least 2 of the following activities: stair climbing, hopping, running, squatting, kneeling and prolonged sitting | — | Pain with compression of the patella: pain on palpation of patellar facets | — | — |
| Eburne 1996 | Anterior knee pain | — | — | — | — | — | — |
| Fukuda 2010 | Anterior knee pain | At least the past 3 months | Pain in 2 or more: ascending and descending stairs, squatting, kneeling, jumping, long sitting, isometric knee extension contraction at 60° of knee flexion, and pain on palpation of the medial and/or lateral facet of the patella | Pain on palpation of the medial and/or lateral facet of the patella | — | — | |
| Fukuda 2012 | Anterior knee pain | At least the past 3 months | Pain in 2 or more: ascending and descending stairs, squatting, kneeling, jumping, long sitting, isometric knee extension contraction at 60° of knee flexion, and pain on palpation of the medial and/or lateral facet of the patella | Pain on palpation of the medial and/or lateral facet of the patella | — | — | |
| Gaffney 1992 | Patellofemoral knee pain, usually retropatellar or medially | — | Pain during 1 of the following activities: ascending or descending stairs, squatting or rising from a squat or sitting with the knee bent at 90 degrees | — | No sign of ligament damage as determined by valgus and varus stress tests, Lachman's test and the anterior drawer of the knee in neutral, internal and external rotation no sign of meniscal involvement as determined by the McMurray and Steinmann test; no involvement of structures around the patella. Patients who had tenderness around the patella either on its margins or chondral surface were included | — | — |
| Gobelet 1992 | Retro‐patellar chondropathy | — | — | — | — | — | Without radiological lesion; with or without Wyberg dysplasia 1 or 2 |
| Hafez 2012 | Chondromalacia patellae | — | — | — | — | — | — |
| Harrison 1999 | Diagnosed with PFPS | — | — | — | 2 of the following criteria: patellar pain with manual compression of the patella against the femur, patellar tenderness with palpation of the posterior‐medial and postero‐lateral borders of the patella, patellar pain during resisted dynamic knee extensions or patellar pain with manual compression of the patella against the femur during isometric knee extensor contraction (Clarke's compression test) | — | — |
| Herrington 2007 | Anterior knee pain | At least 1 month | Anterior or retropatellar knee pain on at least 2 of the following activities: prolonged sitting, climbing stairs, squatting, running, kneeling and hopping/jumping | Average pain level of 3 or more on a 10 cm visual analogue scale during stepping up and down a 25 cm height | Presence of 2 of the following clinical criteria on assessment: pain during apprehension test, pain during the patellar compression test and crepitation during the compression test | — | — |
| Khayambashi 2012 | Diagnosis of bilateral PFP based on the location of symptoms (peripatellar and/or retropatellar | At least 6 months | Pain with activities commonly association with this condition, such as stair descent, squatting, kneeling and prolonged sitting | — | — | — | — |
| Khayambashi 2014 | Diagnosis of PFP based on the location of symptoms (peripatellar and/or retropatellar | At least 6 months | Pain with activities commonly associated with this condition, such as stair descent, squatting, kneeling and prolonged sitting | — | — | — | — |
| Loudon 2004 | Diagnosis of unilateral PFPS based on pain around or under the patella | At least a 2‐month duration | 3 of the 4 criteria: pain in the patellofemoral joint during or after activity, sitting, stair climbing squatting | — | — | — | — |
| Lun 2005 | Atraumatic unilateral and/or bilateral peripatellar or retropatellar knee pain | Pain for at least 3 weeks but no greater than 2 years | Patellofemoral knee pain with and/or after activity; inactivity patellofemoral pain and/or stiffness, especially with sitting with knees in a flexed position | — | Peripatellar tenderness ± mild inferior patellar pole tenderness | — | — |
| Moyano 2013 | Diagnosis of PFP | Pain history more than 6 months | — | — | Positive tests: patellofemoral grinding test and patellofemoral compression test | — | — |
| Nakagawa 2008 | Anterior or retropatellar knee pain | Pain persistent for at least 4 weeks | Pain during at least 3 of the following activities: ascending/descending stairs, squatting, running, kneeling, hopping/jumping and prolonged sitting | Pain on stepping down from a 25 cm step, or during a double‐legged squat | Pain on palpation of the patellar facets | — | — |
| Razeghi 2010 | Retro‐ or peripatellar pain | Insidious onset of pain without a history of trauma persisting for at least 4 weeks | Pain from at least 2 of the following activities: squatting, prolonged sitting, stair climbing, running, kneeling | — | Pain during patellar compression test, patellar grind test or medial/lateral patellar facet tenderness; negative patellar apprehension sign | — | — |
| Schneider 2001 | Unilateral retropatellar pain | More than 6 months | — | — | — | — | — |
| Song 2009 | Anterior or retropatellar knee pain | For more than 1 month | Pain after performing at least 2 of the following activities: prolonged sitting, stair climbing, squatting, running, kneeling, hopping and jumping and deep knee flexing | — | 2 of the following positive signs of anterior knee pain during the initial physical examination: patellar crepitus, pain following isometric quadriceps femoris muscle contraction against suprapatellar resistance with the knee in slight flexion (Clarke's sign), pain following compression of the patella against the femoral condyles with the knee in full extension (patellar grind test), tenderness upon palpation of the posterior surface of the patella or surrounding structures and pain following resisted knee extension | — | — |
| Taylor 2003 | Localised peri or retropatellar pain originating from the peripatellar tissue or the patellofemoral joint | At least 1 month | Pain during 2 of the following: squatting, running, ascending and/or descending stairs, isometric quadriceps femoris muscle contraction or after sitting for a prolonged period of time with the knee flexed | — | — | — | — |
| Thomee 1997 | Pain from the Patellofemoral joint | For a minimum of 6 months | 3 of the following 4 inclusion criteria were fulfilled: pain from the patellofemoral joint during or after activity, during or after sitting, during stair climbing, during squatting | — | — | — | — |
| Van Linschoten 2009 | Patellofemoral pain | Pain > 2 months and < 2 year | At least 3 of the following symptoms: pain when walking up or down stairs; pain when squatting; pain when running; pain when cycling; pain when sitting with knees flexed for a prolonged period of time; (grinding of the patella) | — | A positive clinical patellar test (such as Clarke's test or patellar femoral grinding test) | — | — |
| Witvrouw 2000 | Anterior knee pain | For more than 6 weeks | — | — | 2 of the following criteria on initial assessment: pain on direct compression of the patella against the femoral condyles with the knee in full extension, tenderness on palpation of the posterior surface of the patella, pain on resisted knee extension and pain with isometric quadriceps muscle contraction against suprapatellar resistance with the knee in slight flexion | — | — |
| Østeråsa 2013 | Anterior or retropatellar pain | For more than 2 months | Anterior or retropatellar pain from at least 2 of the following activities – prolonged sitting, climbing stairs, squatting, running, kneeling and hopping/jumping | — | Pain on palpation of the patellar facets or positive physical tests on grinding of the patella, Clarke's test or patellar crepitus | — | — |
PFP: patellofemoral pain PFPS: patellofemoral pain syndrome VAS: visual analogue scale
The duration of complaints ranged from four weeks (Nakagawa 2008) to nine years (Thomee 1997). Eleven trials included both participants with unilateral‐ or bilateral complaints (Clark 2000; Dolak 2011; Gaffney 1992; Harrison 1999; Khayambashi 2014; Lun 2005; Østeråsa 2013; Razeghi 2010; Thomee 1997; Van Linschoten 2009; Witvrouw 2000). Seven trials included only participants with unilateral complaints (Abd Elhafz 2011; Abrahams 2003; Balci 2009; Fukuda 2010; Fukuda 2012; Loudon 2004) and one trial included only patients with bilateral complaints (Khayambashi 2012). The remaining 13 studies did not mention the proportion of unilateral and bilateral complaints. A total of six trials excluded participants who had prior exercise therapy (Clark 2000; Herrington 2007; Khayambashi 2012; Lun 2005; Østeråsa 2013; Van Linschoten 2009).
Interventions
A range of exercise therapy interventions were evaluated in the included trials. We distinguished three comparisons:
1. Exercise therapy versus control (no treatment, placebo or waiting list controls) 2. Exercise therapy versus different conservative interventions:
Exercise therapy versus unimodal conservative interventions
Exercise therapy versus multimodal conservative interventions
3. Different types of exercise therapy
Delivery of exercises or exercise programmes (e.g. supervised versus home exercise; group versus individual supervision)
Medium of exercises or exercise programmes (water‐ versus land‐based exercise)
Types of exercises or exercise programmes (with the primary categorisation being by the type of kinetic chain involved)
Target of exercises or exercise programmes (strengthening of hip and knee muscles versus knee muscles)
Duration of exercises or exercise programmes (e.g. long duration (more than three months) versus shorter duration (three months or less))
Intensity of exercises or exercise programmes (e.g. high‐intensity (several times per week) versus low‐intensity (once weekly)
The intervention period ranged from three weeks (Bakhtiary 2008) to four months (Moyano 2013) and participants exercised on average three times per week.
Exercise therapy versus control (no treatment, placebo or waiting list)
For further details, see Appendix 2.
Ten trials compared exercise therapy with a control strategy (no treatment, placebo or waiting list controls) (Abrahams 2003; Clark 2000; Fukuda 2010; Herrington 2007; Loudon 2004; Lun 2005; Moyano 2013; Song 2009; Taylor 2003; Van Linschoten 2009). Clark 2000 compared exercise therapy and education versus education alone. Abrahams 2003 compared both a traditional exercise protocol and an exercise protocol with thigh adduction and tibia medial rotation during eccentric squat with waiting list. This study was not pooled due to clinical heterogeneity (participants in this study had to be diagnosed with malalignment and PFPS). Taylor 2003 compared exercise and patella mobilisation/manipulation with patella mobilisation/manipulation alone. A supervised exercise programme and a home exercise programme were both compared with a control intervention (information leaflet) by Loudon 2004. Lun 2005 compared a home exercise programme with brace versus brace alone. Herrington 2007 compared both weightbearing exercises (CKC) and non weightbearing exercises (OKC) with a control group without treatment. Knee exercises and knee and hip exercises were both compared with no intervention by Song 2009. Van Linschoten 2009 compared exercise therapy with usual care ('wait and see policy'). Moyano 2013 compared classic stretching and quadriceps exercises with education and proprioceptive neuromuscular facilitation stretching (including aerobic exercise) with education. Finally, Fukuda 2010 compared both a knee exercise group and a knee and hip exercise group with a group that received no treatment.
Exercise therapy versus different conservative treatments
For further details, see Appendix 3.
Exercise therapy versus unimodal conservative interventions
Four trials compared exercise therapy with different unimodal conservative interventions (Clark 2000; Gobelet 1992; Khayambashi 2012; Lun 2005). Gobelet 1992 compared both an isokinetic exercise programme and an isometric exercise programme with a muscle electrostimulation group. In Clark 2000, the data comparing exercise therapy versus tape were used. In Lun 2005, data from a structured home exercise programme were compared with a brace group. Khayambashi 2012 compared hip exercises with 1000 mg of Omega‐3 and 400 mg of calcium daily.
Exercise therapy versus multimodal conservative interventions
Four trials compared exercise therapy with different multimodal conservative interventions including exercises (Eburne 1996; Gaffney 1992; Harrison 1999; Schneider 2001). Harrison 1999 compared both a supervised exercise programme and a home exercise programme versus a vastus medialis‐specific supervised exercise programme including taping. Eburne 1996 compared isometric quadriceps exercise versus the multimodal McConnell regimen comprising different types of exercises and taping. Gaffney 1992 compared concentric exercises versus a multimodal intervention comprising excentric exercises and taping. Schneider 2001 compared physiotherapeutic exercises based on proprioceptive neuromuscular facilitation versus a special knee resistance‐controlled knee splint combined with a special exercise programme.
Different exercises or exercise programmes
For further details, see Appendix 4.
Delivery of exercises or exercise programmes
Two studies compared supervised exercise programmes with home exercise programmes (Harrison 1999; Loudon 2004). Harrison 1999 compared a supervised exercise programme with a home exercise programme. Loudon 2004 compared a supervised exercise programme and additional home exercises with home exercises and five physiotherapy sessions. A supervised exercise programme was regarded as the intervention group.
Medium of exercises or exercise programmes
There were no trials eligible for this comparison.
Types of exercise or exercise programmes
Eleven studies compared types of exercises or exercise programmes with each other (Abd Elhafz 2011; Abrahams 2003; Bakhtiary 2008; Balci 2009; Colón 1988; Gobelet 1992; Hafez 2012; Herrington 2007; Moyano 2013; Thomee 1997; Witvrouw 2000). Of these, four studies compared closed kinetic chain exercises with open kinetic chain exercises (Abd Elhafz 2011; Bakhtiary 2008; Herrington 2007; Witvrouw 2000). Closed kinetic chain (CKC) exercise was regarded as the intervention group. Two studies tested variants of closed kinetic chain exercises (Abrahams 2003; Balci 2009). The first listed CKC variant was regarded as the intervention group. Abrahams 2003 compared an exercise protocol with thigh adduction and tibia medial rotation during eccentric squat versus a traditional exercise protocol. This study was not pooled due to clinical heterogeneity (participants also had to be diagnosed with malalignment). Balci 2009 compared closed kinetic chain exercises with internally rotated hip versus closed kinetic chain exercises with externally rotated hip. Four studies studied open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action (Colón 1988; Gobelet 1992; Hafez 2012; Thomee 1997). The first listed kinetic chain exercise group was regarded as the intervention group. Hafez 2012 compared eccentric exercises versus concentric exercises. One study compared eccentric exercises versus isometric exercises (Thomee 1997). One study compared isokinetic exercises versus isometric exercises (Gobelet 1992). One study compared combined isotonic and isometric exercises (pogo stick) versus isometric exercises (Colón 1988).
One study (Moyano 2013), which is presented separately in Effects of interventions, compared proprioceptive neuromuscular facilitation stretching and aerobic exercise with classic stretching and quadriceps exercises.
Target of exercise or exercise programmes
Nine trials compared different targets of exercises or exercises programmes with each other (Avraham 2007; De Marche 2014; Dolak 2011; Fukuda 2010; Fukuda 2012; Khayambashi 2014; Nakagawa 2008; Razeghi 2010; Song 2009). Seven trials compared exercises for the knee and hip with exercises for the knee (Avraham 2007; De Marche 2014; Fukuda 2010; Fukuda 2012; Nakagawa 2008; Razeghi 2010; Song 2009). Two trials compared exercises for the knee with exercises for the hip (Dolak 2011; Khayambashi 2014). Since studies investigated similar exercises (i.e. quadriceps exercises or knee exercises) but named them differently, we defined them all as knee exercises. An exercise programme including hip exercises was regarded as the intervention group.
Duration of exercises or exercise programmes
There were no trials eligible for this comparison.
Intensity of exercises of exercise programmes
Østeråsa 2013 was the only trial that compared high‐dose, high‐repetition medical exercise therapy (MET) with low‐dose, low‐repetition exercises. The high‐intensity group was regarded as the intervention group.
Outcomes
Pain was measured by a visual analogue scale (VAS) or numerical (pain) rating scale (N(P)RS), the McGill pain score (Melzack 1987) and as number of patients experiencing pain. A higher score on VAS, N(P)RS or McGill means worse pain. Pain was scored in various ways: during activity, usual, worst, at rest, after exposure, least, one hour after sport activity, following 30 minutes of sitting with knees flexed, experienced at four different positions of the knee, during isometric knee extension, during triple jump test, during walking, ascending stairs, during running, during jumping, during sports, during squatting, during prolonged sitting, during the night and during isokinetic test. If multiple pain scales were reported only pain in daily life (usual pain), worst pain and pain at activities (e.g. sports, pain during descending stairs) are presented in Effects of interventions. We selected pain at descending for pooling on 'pain at activities' as this outcome measure was present in most studies eligible for pooling of pain at activity.
Functional ability was scored with the Anterior Knee Pain Scale (AKPS) (Kujala 1993), (Modified) Functional Index Questionnaire ((M)FIQ) ((Chesworth 1989; Selfe 2001), Arpège function scale, Lower Extremity Function Scale (LEFS) (Binkley 1999), (modified) function scale (Werner 1993), patient specific function score, patellofemoral scale, Bessette and Hunter score (Bessette 1988), WOMAC Osteoarthritis Index (McConnell 2001), Patellofemoral Joint Evaluation Scale (Shea 1992), Lysholm score (Lysholm 1982)) and dichotomously as the number of patients improved in function. If multiple scales for functional ability were measured including the AKPS, we used the latter for pooling. A higher score means better function, except for WOMAC. For consistency, we have inverted the WOMAC scale, in order that a higher score means better function.
Functional performance was scored with, for example, the single‐leg triple hop test, step (down) test, single‐limb hop test, bilateral and unilateral squat, anteromedial lunge, step‐down dips, leg press, balance and reach and vertical jump test. Studies including participants with bilateral complaints used the most painful side for analysis; thus avoiding unit of analysis issues.
Recovery was measured with eight different measures: a Likert scale (Van Linschoten 2009), number of patients no longer troubled by symptoms (Clark 2000), number of patients with more than 50% improved on pain scale (Colón 1988), improvement percentage (Eburne 1996), patients' impression of change (ordinal scale of three) (Harrison 1999), subjective success (yes or no) (Gaffney 1992), number of patients participating in sports with or without pain (Thomee 1997), and the global rating of change on a 15‐point scale (De Marche 2014).
Four trials reported adverse events (Colón 1988; Dolak 2011; Khayambashi 2012; Taylor 2003). Two trials reported that they actively recorded adverse events (Colón 1988; Dolak 2011).
Most trials measured the outcomes post‐intervention; however, a few studies reported on a longer term follow‐up period ranging from five months (De Marche 2014) to a maximum of five years (Witvrouw 2000).
Excluded studies
We discussed and excluded 12 potentially eligible studies after consensus (Collins 2008; Crossley 2002; Dursun 2001; Mason 2011; McMullen 1990; Roush 2000; Stiene 1996; Syme 2009; Timm 1998; Tunay 2003; Wiener‐Ogilvie 2004; Wijnen 1996; see the Characteristics of excluded studies).
Two studies were neither randomised nor quasi‐randomised (McMullen 1990; Stiene 1996). Two trials also included patients with osteoarthritis (Mason 2011; Wiener‐Ogilvie 2004) and Roush 2000 also included participants with patellofemoral osteoarthritis, plica syndrome, patellar tendinitis, quadriceps tendinitis and Osgood–Schlatter's disease. Dursun 2001 studied the effect of electromyographic (EMG) feedback rather than our interventions of interest; and the other trials studied a combination of interventions and we were unable to extract the effect of exercise alone (Collins 2008; Crossley 2002; Syme 2009; Timm 1998; Tunay 2003; Wijnen 1996).
Ongoing studies
There is one ongoing study that investigates the effect of lumbo‐pelvic stabilisation training in women with patellofemoral pain (RBR‐2cxrpp). This study includes women from 18 to 30 years with patellofemoral pain. The women allocated to the experimental group carry out strengthening exercises for the lumbo‐pelvic muscles as well as functional training to correct any dynamic lower limb misalignment. The control group receives a conventional treatment focusing on quadriceps strengthening and stretching of the lower limb muscles. Both groups perform the activities three times a week for eight consecutive weeks.
Studies awaiting classification
Erel 2011 is reported in Turkish and is awaiting classification pending translation.
Risk of bias in included studies
We explicitly judged all criteria using: low risk of bias; high risk of bias; and unclear risk of bias (where 'unclear' relates to a lack of information or uncertainty over the potential for bias). Full details of the risk of bias for the 31 trials are provided in Figure 2 and Figure 3.
2.
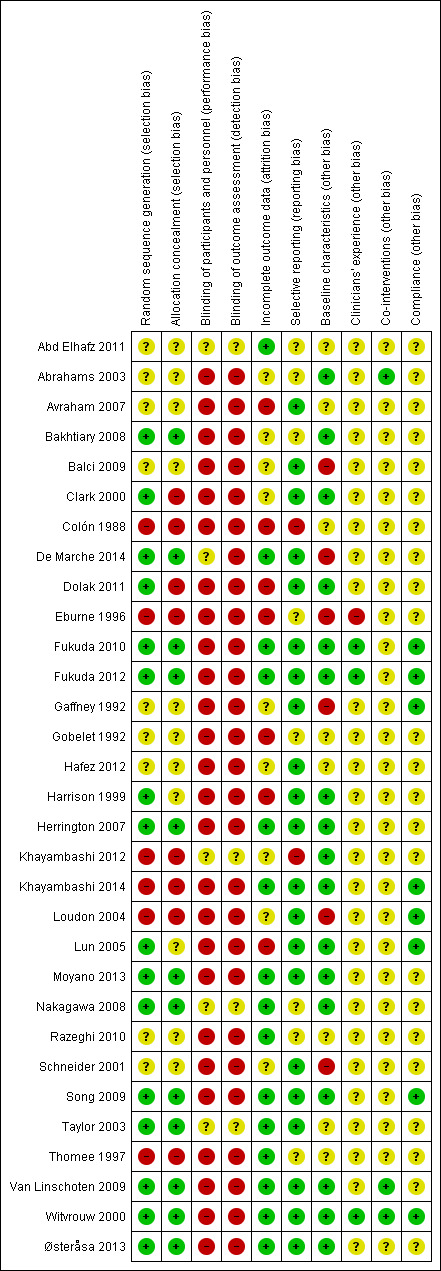
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
3.
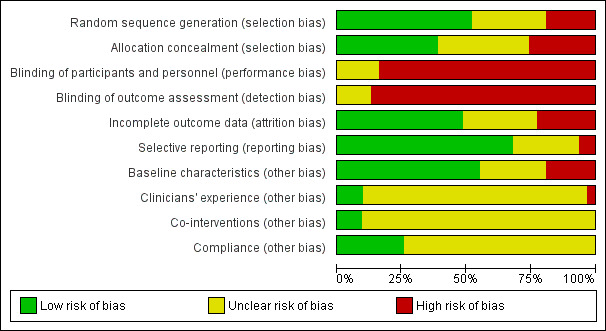
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation was applied in 16 out of 31 trials and was mainly done by computer‐generated lists (Bakhtiary 2008; Clark 2000; De Marche 2014; Dolak 2011; Fukuda 2010; Fukuda 2012; Harrison 1999; Herrington 2007; Lun 2005; Moyano 2013; Nakagawa 2008; Østeråsa 2013; Song 2009; Taylor 2003; Van Linschoten 2009; Witvrouw 2000). Six trials were quasi‐randomised (Colón 1988; Eburne 1996; Khayambashi 2012; Khayambashi 2014; Loudon 2004; Thomee 1997). Allocation of the participants was concealed in 12 out of 31 trials mainly by using sealed and opaque envelopes (Bakhtiary 2008; De Marche 2014; Fukuda 2010; Fukuda 2012; Herrington 2007; Moyano 2013; Nakagawa 2008; Østeråsa 2013; Song 2009; Taylor 2003; Van Linschoten 2009; Witvrouw 2000). Eight trials were at high risk of allocation bias (Clark 2000; Colón 1988; Dolak 2011; Eburne 1996; Khayambashi 2012; Khayambashi 2014; Loudon 2004; Thomee 1997), because of matching, because the randomisation was done by the physiotherapist/investigator or because allocation concealment was highly unlikely in quasi‐randomised trials. In the remaining 11 trials the process of allocation was not specified or unclear.
Blinding
Blinding of personnel was impractical due to the nature of the intervention, and while standardisation of interactions between personnel and patients (i.e. use of standardised scripts) would have been possible, none of the included studies took this approach. Five studies attempted to address performance bias by means of blinding the patients. Abd Elhafz 2011 stated that patients were unaware about the number of groups, randomisation technique or interventions for each group. De Marche 2014 and Nakagawa 2008 reported that patients were blinded to group allocation. In Khayambashi 2012, participants were aware of an alternative treatment group in the study but had no knowledge of intervention details. In Taylor 2003, participants were aware that they were receiving what was believed to be 'real' treatments, but were not aware of which treatment was considered better by those delivering the treatments or collecting data. As the success of these measures was uncertain, we rated all as unclear for performance bias. We rated the other studies as high risk on this criterion.
The risk of detection bias is inevitably high for studies where patients who have not been blinded to interventions self report on outcomes; but we rated the risk as unclear in four of the five studies when patient blinding had been attempted (Abd Elhafz 2011; Khayambashi 2012; Nakagawa 2008; Taylor 2003). We rated the other study reporting patient blinding at high risk because assessor blinding was not done for functional performance (De Marche 2014).
Incomplete outcome data
We judged incomplete outcome data on three items. We considered a dropout rate greater than 20% in the short‐term or greater than 30% on follow‐up at 12 months or longer, cross‐over or dropout due to adverse events to be high risk criteria if no reliable intention‐to‐treat analysis was carried out. We rated 15 trials low risk since they reported no cross‐overs and low dropout rates (Abd Elhafz 2011; De Marche 2014; Fukuda 2010; Fukuda 2012; Herrington 2007; Khayambashi 2014; Moyano 2013; Nakagawa 2008; Østeråsa 2013; Razeghi 2010; Song 2009; Taylor 2003; Thomee 1997; Van Linschoten 2009; Witvrouw 2000). We rated six trials high risk as they reported a high dropout rate, cross‐overs or dropouts due to adverse events and did not report a intention‐to‐treat‐analysis (Avraham 2007; Colón 1988; Eburne 1996; Gobelet 1992; Harrison 1999; Lun 2005). Avraham 2007 reported 29% dropout in the short‐term and no intention‐to‐treat analysis. In Colón 1988, a patient dropped out due to increased pain after the intervention, and no intention‐to‐treat analysis was reported. Eburne 1996 reported 29% dropout in the short‐term and no intention‐to‐treat analysis. Gobelet 1992 reported 22% dropout, not equally distributed among groups: 12 patients stopped because of ineffectiveness of treatment and no intention‐to‐treat analysis was reported. Harrison 1999 reported a 33% dropout in the short‐term, 48% dropout at 12 months and no intention‐to‐treat analysis. Lun 2005 reported that two participants crossed over to another treatment group before three months. These were considered to be withdrawals from the study and no intention‐to‐treat analysis was reported. We rated one trial high risk because they reported an 18% dropout rate in the short‐term, a withdrawal by the investigators for increased pain and an unreliable imputation method (Dolak 2011). They carried out the last available measure moved forward method, which is generally considered conservative, but there are more reliable methods such as multiple imputation (Jørgensen 2014). We rated the remaining nine trials unclear as no further details were reported.
Selective reporting
None of the trials, except Van Linschoten 2009, published a study protocol. We considered any outcomes of pain and functional ability to be expected outcomes and they had to be reported at all time points in order to get a low risk rating. One study did not report any of these expected outcomes and we therefore rated it high risk (Colón 1988). Khayambashi 2012 did not provide long‐term (six months) results on pain or functional ability for the comparator group and we also rated it high risk. We rated eight studies unclear risk (Abd Elhafz 2011; Abrahams 2003; Bakhtiary 2008; Eburne 1996; Gobelet 1992; Nakagawa 2008; Razeghi 2010; Thomee 1997). Two studies did not report pain data (Abrahams 2003; Gobelet 1992) and six studies did not report functional ability data (Abd Elhafz 2011; Bakhtiary 2008; Eburne 1996; Nakagawa 2008; Razeghi 2010; Thomee 1997). The remaining 21 trials did report pain and functional ability data at all time points listed in their methods and we therefore rated them low risk.
Other potential sources of bias
We judged all studies on four potential other sources of bias: difference in baseline characteristics, comparability in clinician's experience with the interventions under test, differences in care other than the interventions and compliance with therapy.
We rated a total of 17 trials low risk. Twelve trials reported no significant statistical difference in demographic variables and outcome variables (Bakhtiary 2008; Clark 2000; Fukuda 2010; Fukuda 2012; Herrington 2007; Khayambashi 2012; Khayambashi 2014; Moyano 2013; Nakagawa 2008; Østeråsa 2013; Song 2009; Witvrouw 2000). Five trials reported no statistical significant difference in demographic variables, but did not statistically test the difference in outcome variables (Abrahams 2003; Dolak 2011; Harrison 1999; Lun 2005; Van Linschoten 2009). Their outcome values seemed similar and therefore we also rated them low risk. We rated six trials high risk since demographics or outcome variables were statistically different or did not seem to be similar (Balci 2009; De Marche 2014; Eburne 1996; Gaffney 1992; Loudon 2004; Schneider 2001). In Balci 2009, the groups differed in height. BMI was not statistically tested, but the difference between groups was 2.3 points. Gaffney 1992 reported a significant difference in BMI attributed to the fact that there were slightly more females and some 11 to 13 years old in the concentric group. Eburne 1996 reported a significant difference between groups for age. The duration of complaints between groups in the study of De Marche 2014 seemed to be rather different with a remarkably higher duration of complaints in the stabilisation group. The VAS in the physiotherapy group was higher compared with the other two groups in the study of Loudon 2004. In Schneider 2001, there was a difference in VAS at rest across groups. Hafez 2012 did report comparable baseline outcome data, but did not report demographics and we rated it unclear. The remaining seven trials did not report on demographics or outcome variables and we therefore rated them unclear.
Only Fukuda 2010, Fukuda 2012 and Witvrouw 2000 reported that the therapists were trained and we therefore rated them low risk. We rated Eburne 1996 high risk as there were two changes of therapist in the McConnell and three in the isometric quadriceps group. The remaining trials did not report comparability of clinician's experience with the interventions under test.
We rated three studies low risk as they reported on co‐interventions and the comparability across groups in individual studies. Abrahams 2003 excluded participants who started a co‐intervention. Van Linschoten 2009 reported that other interventions, like the use of bandages or braces, insoles or ice application, or consumption of medication other than simple analgesics, were allowed in both groups (despite from exercise therapy in the control group) and equally used. Witvrouw 2000 reported that no medication was prescribed as part of their treatment. No brace or tape was used by any patient in this study. We rated the remaining trials unclear.
Compliance was adequately reported in eight trials and we rated these low risk (Fukuda 2010; Fukuda 2012; Gaffney 1992; Khayambashi 2014; Loudon 2004; Lun 2005; Song 2009; Witvrouw 2000). Gaffney 1992 reported a self reported compliance of 86% in eccentric and 88% in concentric programmes. Fukuda 2010 and Fukuda 2012 excluded patients if they missed treatment sessions. In Khayambashi 2014, all participants were required to complete at least 19 out of the 24 treatment sessions (= 80%) to remain in the study. In addition, if a patient missed three consecutive treatment sessions, their participation in the study was terminated. All participants completed the required number of treatment sessions. Loudon 2004 asked participants to keep a diary and excluded those who did not complete 90% of the exercise programme. Lun 2005 asked participants to document in a journal when the exercises were done and/or when the brace or sleeve was worn. These journals were submitted to the second research assistant on a monthly basis. Overall, the compliance was very good and similar among all treatment groups. Song 2009 reported that all exercise intervention participants except one attended all scheduled exercise sessions. One participant in the knee exercises only group completed only half of the intervention and subsequently dropped out of the study due to work commitments. Witvrouw 2000 reported that every patient followed the exercise programme for the required period of five weeks. Four trials reported a method for aiding compliance but did not report the actual compliance at the end of the intervention (Bakhtiary 2008; Clark 2000; Dolak 2011; Van Linschoten 2009). The remaining nine trials did not report on compliance.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Exercise therapy versus control (no treatment, placebo or waiting list controls)
Ten studies compared exercise therapy with a control strategy (no treatment, placebo or waiting list controls) (Abrahams 2003; Clark 2000; Fukuda 2010; Herrington 2007; Loudon 2004; Lun 2005; Moyano 2013; Song 2009; Taylor 2003; Van Linschoten 2009). In the analyses, these are subgrouped according to the main characteristic of exercise therapy. Although, with the exception of Abrahams 2003, we have pooled the results of these heterogeneous studies, the pooled result should be taken as illustrative, especially where the heterogeneity is statistically significant. We presented Abrahams 2003 in a separate analysis (malalignment group) because of clear clinical heterogeneity since participants also had to be diagnosed with malalignment. Where a trial tested two separate exercise interventions and one control group, we split the data in the control group so that the individual results of the each intervention could be presented while avoiding double counting of those in the control group (Fukuda 2010; Herrington 2007; Song 2009). We extracted standard deviations for pain and function (Herrington 2007) from error bars, which we interpreted to be standard deviations (SDs), in graphs presented in the publications of this trial.
Knee pain in the short term
During activity (0 to 10 scale; higher scores mean worse pain)
Pooled data from five studies (Clark 2000; Fukuda 2010; Herrington 2007; Lun 2005; Van Linschoten 2009; 375 participants) showed a mean difference (MD) of ‐1.46 favouring exercise therapy, 95% confidence interval (CI) ‐2.39 to ‐0.54, P value = 0.002, random‐effects model used due to statistical heterogeneity (P value = 0.0003; I² = 74%); very low quality evidence due to risk of bias, imprecision and inconsistency; seeAnalysis 1.1 and Figure 4. The results were homogeneous (P value = 0.55 and I2 = 0%) upon removal of Herrington 2007, but with a reduced effect size (MD ‐0.76, 95% CI ‐1.26 to ‐0.25, P value = 0.003).
1.1. Analysis.
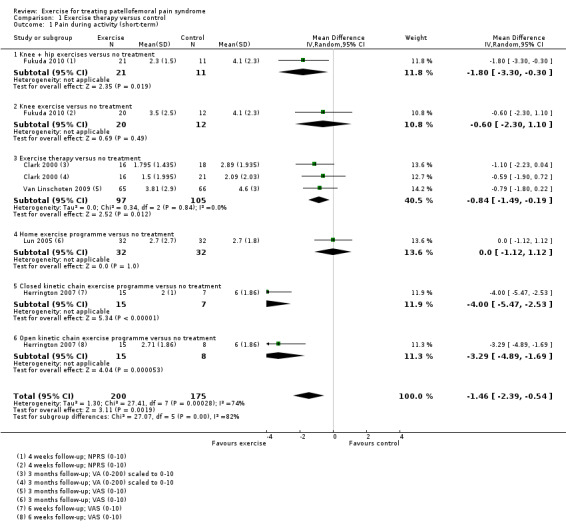
Comparison 1 Exercise therapy versus control, Outcome 1 Pain during activity (short‐term).
4.
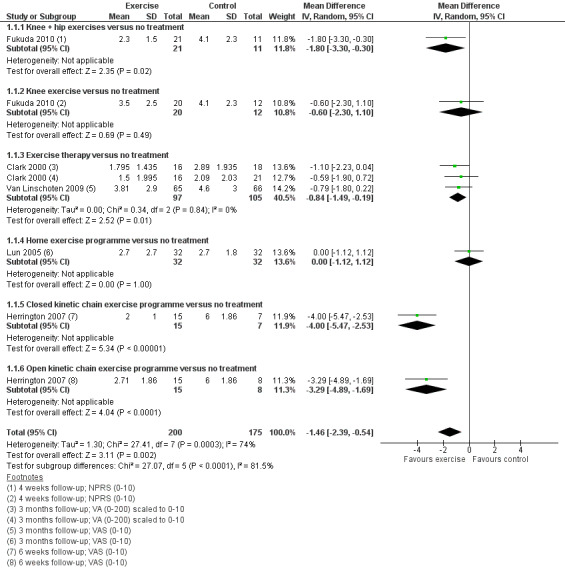
Forest plot of comparison: 1 1: Exercise therapy versus control, outcome: 1.1 Sum: pain during activity continuous short‐term
Usual pain (0 to 10 scale; higher scores mean worse pain)
Pooled data from two studies (Loudon 2004; Taylor 2003; 41 participants) showed a standardised mean difference (SMD) of ‐0.93 favouring exercise therapy, 95% CI ‐1.60 to ‐0.25, P value = 0.007; very low quality evidence due to serious risk of bias and imprecision; seeAnalysis 1.2.
1.2. Analysis.
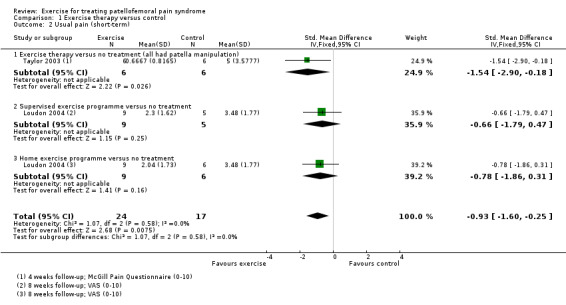
Comparison 1 Exercise therapy versus control, Outcome 2 Usual pain (short‐term).
Worst pain (0 to 10 scale; higher scores mean worse pain)
Pooled data from two studies (Song 2009; Taylor 2003; 91 participants) resulted in a MD of ‐2.28 favouring exercise therapy, 95% CI ‐3.33 to ‐1.23, P value < 0.0001; low quality evidence due to risk of bias and imprecision; seeAnalysis 1.3.
1.3. Analysis.
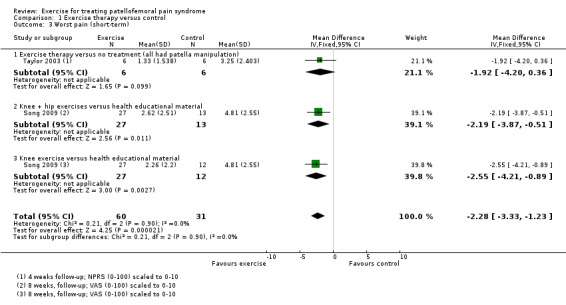
Comparison 1 Exercise therapy versus control, Outcome 3 Worst pain (short‐term).
Knee pain in the long term
During activity (0 to 10 scale; higher scores mean worse pain)
Pooled data from two studies (Clark 2000;Van Linschoten 2009; 180 participants) resulted in a MD of ‐1.07 favouring exercise therapy, 95% CI ‐1.93 to ‐0.21, P value = 0.01; very low quality evidence due to serious risk of bias and imprecision; seeAnalysis 1.4).
1.4. Analysis.
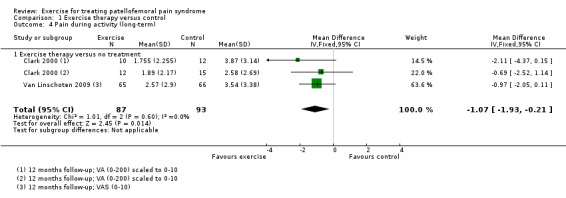
Comparison 1 Exercise therapy versus control, Outcome 4 Pain during activity (long‐term).
Usual pain (visual analogue scale (VAS) 0 to 10; higher scores mean worse pain)
Pooled data from two exercise interventions tested by one study (Moyano 2013; 94 participants) showed a MD of ‐4.32 favouring exercise therapy, 95% CI ‐7.75 to ‐0.89, P value < 0.00001; random‐effects model used due to statistical heterogeneity (heterogeneity P value < 0.00001, I² = 97%); very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 1.5.
1.5. Analysis.
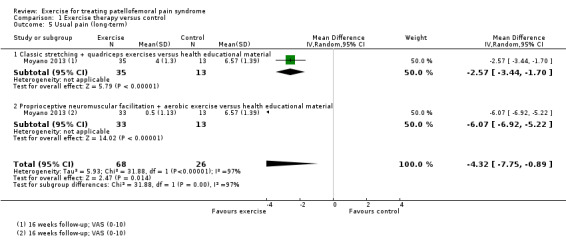
Comparison 1 Exercise therapy versus control, Outcome 5 Usual pain (long‐term).
Functional ability in the short term (0 to 100 scale; modified Functional Index Questionnaire (MFIQ) 0 to 16; higher scores mean better function)
Based on a 0 to 100 scale (higher scores mean better function), pooled data from seven studies (Clark 2000; Fukuda 2010; Herrington 2007; Loudon 2004; Lun 2005; Song 2009; Van Linschoten 2009; 483 participants) showed a SMD of 1.10 favouring exercise therapy, 95% CI 0.58 to 1.63, P value < 0.0001, random‐effects model used due to statistical heterogeneity (P value < 0.00001, I² = 83%); very low quality evidence due to risk of bias and serious inconsistency; seeAnalysis 1.6 and Figure 5. The results did not became homogeneous after excluding any single study.
1.6. Analysis.
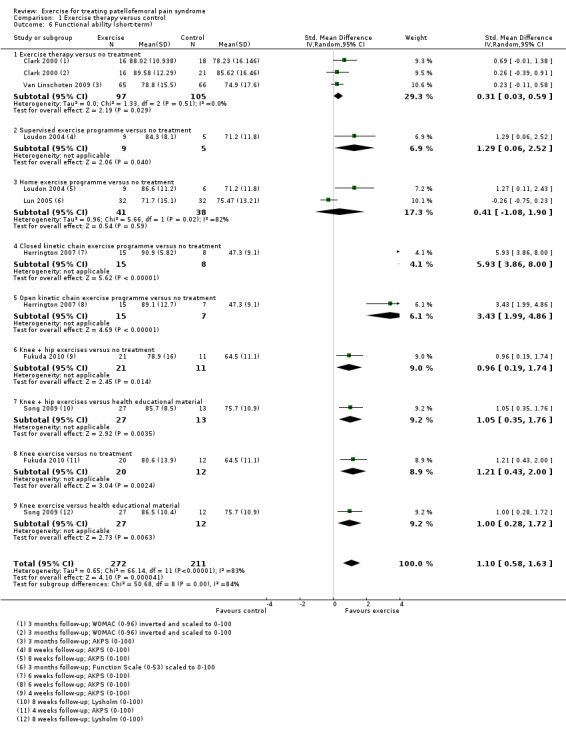
Comparison 1 Exercise therapy versus control, Outcome 6 Functional ability (short‐term).
5.
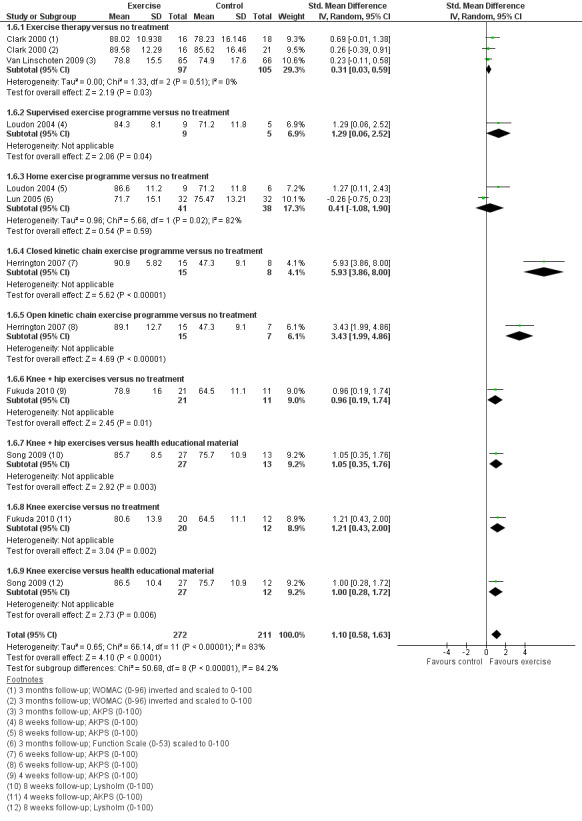
Forest plot of comparison: 1 1: Exercise therapy versus control, outcome: 1.5 Sum: functional ability continuous short‐term
Based on the MFIQ (0 to 16), Abrahams 2003 (78 participants) reported a MD of ‐1.90, favouring a control strategy, 95% CI ‐3.24 to ‐0.56, P value = 0.005; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 1.7.
1.7. Analysis.
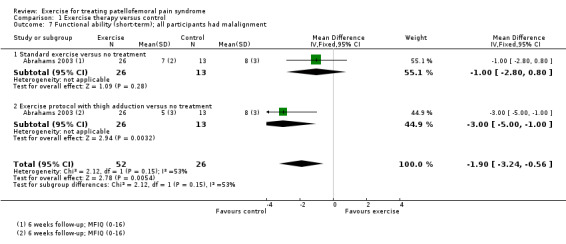
Comparison 1 Exercise therapy versus control, Outcome 7 Functional ability (short‐term); all participants had malalignment.
Functional ability in the long term (0 to 100 scale; patient specific function scale; higher scores mean better function)
Pooled data from three studies (Clark 2000; Moyano 2013; Van Linschoten 2009; 274 participants) resulted in a SMD of 1.62, favouring exercise therapy, 95% CI 0.31 to 2.94, P value = 0.02; random‐effects model used due to statistical heterogeneity (heterogeneity P value < 0.00001, I² = 94%); very low quality evidence due to risk of bias, imprecision and inconsistency; seeAnalysis 1.8. The results were homogeneous (I² = 0%) upon removal of Moyano 2013, but smaller in effect size (SMD 0.27, 95% CI ‐0.02 to 0.56, P value = 0.07).
1.8. Analysis.
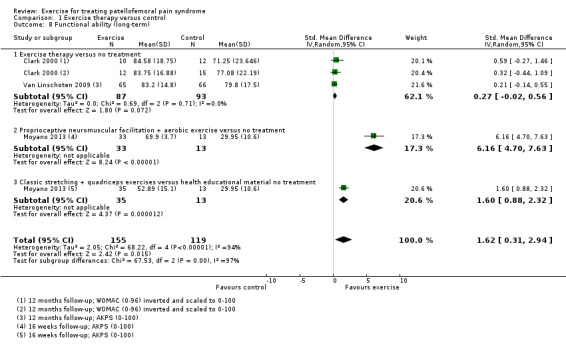
Comparison 1 Exercise therapy versus control, Outcome 8 Functional ability (long‐term).
Taylor 2003 (12 participants) reported that there were no statistically significant differences between groups for patient specific function scale scores for three different activities.
Functional performance in the short term (single‐limb hop test; bilateral squat)
Fukuda 2010 (64 participants) reported for the single‐limb hop test a MD of 8.73 cm favouring exercise therapy, 95% CI ‐3.35 to 20.80, P value = 0.16; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 1.9.
1.9. Analysis.
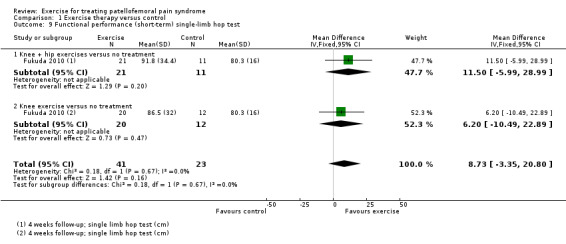
Comparison 1 Exercise therapy versus control, Outcome 9 Functional performance (short‐term) single‐limb hop test.
Loudon 2004 (29 participants) reported for the bilateral squat test (number completed in 30 seconds) a MD of 1.08 favouring exercise therapy, 95% CI ‐1.68 to 3.84, P value = 0.44; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 1.10.
1.10. Analysis.
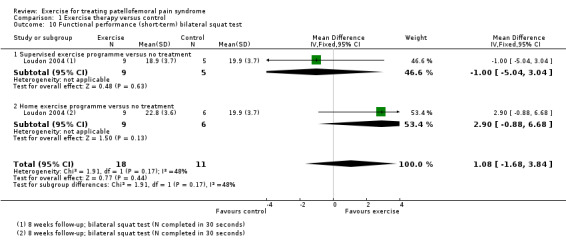
Comparison 1 Exercise therapy versus control, Outcome 10 Functional performance (short‐term) bilateral squat test.
Full data were not available for the four other functional performance tests, based on limb symmetry index, measured by Loudon 2004 (29 participants): anteromedial lunge, step‐down dip, leg press, and balance and reach.
Recovery in the short term (number of participants no longer troubled by symptoms)
Van Linschoten 2009 (122 participants) reported that 26/62 participants in the exercise group versus 21/60 participants in the tape group were no longer troubled by pain at three months; risk ratio (RR) 1.20 favouring exercise therapy, 95% CI 0.76 to 1.88, P value = 0.43; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 1.11.
1.11. Analysis.
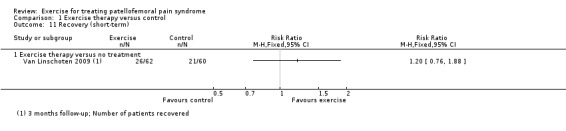
Comparison 1 Exercise therapy versus control, Outcome 11 Recovery (short‐term).
Recovery in the long term (number of patients recovered and number of patients no longer troubled by symptoms)
Pooled data from two studies (Clark 2000; Van Linschoten 2009; 166 participants) reported that 45/80 participants in the exercise group versus 35/86 participants in the tape group were no longer troubled by pain at 12 months; RR 1.35 favouring exercise therapy, 95% CI 0.99 to 1.84, P value = 0.06; very low quality evidence due to serious risk of bias and imprecision; seeAnalysis 1.12.
1.12. Analysis.
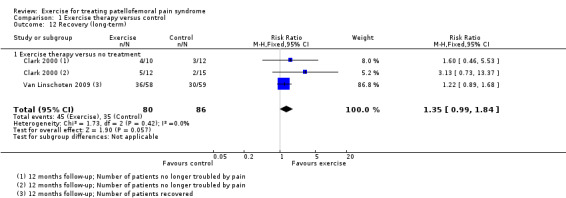
Comparison 1 Exercise therapy versus control, Outcome 12 Recovery (long‐term).
Adverse events
Taylor 2003 reported no harmful side effects.
Exercise therapy versus different conservative treatments: exercise therapy versus unimodal conservative interventions
For convenience, the available data for five different comparisons, tested within four trials (Clark 2000; Gobelet 1992; Khayambashi 2012; Lun 2005), are presented together in Analyses 2.1 to 2.5 but without pooling. The five comparisons are presented in turn below. None of the four trials reported on functional performance or adverse events.
Hip exercises versus 1000 mg of Omega‐3 and 400 mg of calcium
One study evaluated this comparison (Khayambashi 2012). It did not report on functional performance or aspects of recovery and did not provide long‐term (six months) results on pain or functional ability for the comparator group.
Knee pain in the short term
During activity (VAS 0 to 10; higher scores mean worse pain)
Khayambashi 2012 (28 participants) reported a MD of ‐5.30 favouring hip exercises, 95% CI ‐6.90 to ‐3.70, P value < 0.00001; very low quality evidence due to serious risk of bias and serous imprecision; seeAnalysis 2.1.
2.1. Analysis.
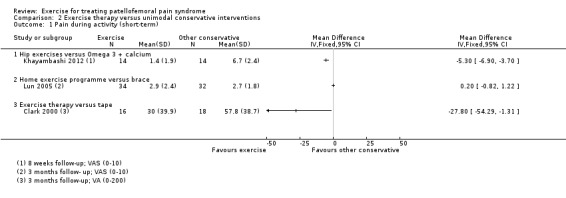
Comparison 2 Exercise therapy versus unimodal conservative interventions, Outcome 1 Pain during activity (short‐term).
Functional ability in the short term (WOMAC 0 to 96) (inverted score; higher scores mean better function)
Khayambashi 2012 (28 participants) reported a MD of 49.20 favouring hip exercises, 95% CI 38.49 to 59.91, P value < 0.00001; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.3.
2.3. Analysis.
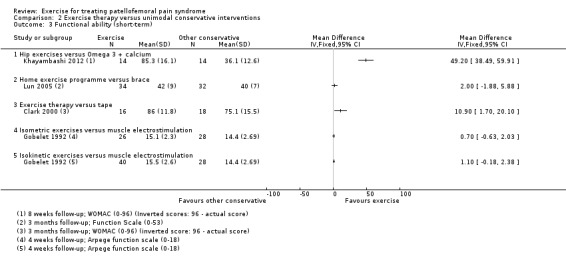
Comparison 2 Exercise therapy versus unimodal conservative interventions, Outcome 3 Functional ability (short‐term).
Adverse events
Khayambashi 2012 stated that no adverse effects were reported.
Home exercise programme versus brace
The one study making this comparison did not report on long‐term outcome, functional performance, aspects of recovery or adverse events (Lun 2005).
Knee pain in the short term
During activity (VAS 0 to 10; higher scores mean worse pain)
Lun 2005 (66 participants) reported a MD of 0.20 favouring bracing, 95% CI ‐0.82 to 1.22, P value = 0.70; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 2.1.
Functional ability in the short term (function scale 0 to 53; higher scores mean better function)
Lun 2005 (66 participants) reported a MD of 2.00 favouring a home exercise programme, 95% CI ‐1.88 to 5.88, P value = 0.31; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 2.3).
Exercise therapy versus tape
One study made this comparison (Clark 2000). It did not report on functional performance or adverse events.
Knee pain in the short term
During activity (VAS 0 to 200; higher scores mean worse pain)
Clark 2000 (34 participants) reported a MD of ‐27.80 favouring exercise therapy, 95% CI ‐54.29 to ‐1.31, P value = 0.04; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.1.
Knee pain in the long term
During activity (VAS 0 to 200; higher scores mean worse pain)
Clark 2000 (24 participants) reported a MD of ‐39.50 favouring exercise therapy, 95% CI ‐82.69 to 3.69, P value = 0.07; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.2.
2.2. Analysis.
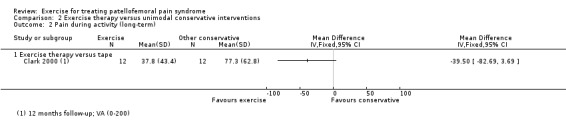
Comparison 2 Exercise therapy versus unimodal conservative interventions, Outcome 2 Pain during activity (long‐term).
Functional ability in the short term (WOMAC 0 to 96) (inverted score; higher scores mean better function)
Clark 2000 (34 participants) reported a MD of 10.90 favouring exercise therapy, 95% CI 1.70 to 20.10, P value = 0.02; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.3.
Functional ability in the long term (WOMAC 0 to 96) (inverted scores; higher scores mean better function)
Clark 2000 (24 participants) reported a MD of 12.00 favouring exercise therapy, 95% CI ‐3.78 to 27.78, P value = 0.14; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.4.
2.4. Analysis.
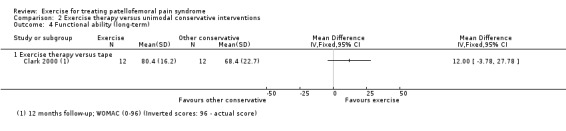
Comparison 2 Exercise therapy versus unimodal conservative interventions, Outcome 4 Functional ability (long‐term).
Recovery (number of participants no longer troubled by symptoms)
Clark 2000 reported that 5/12 participants in the exercise group versus 3/12 participants in the tape group were no longer troubled by pain at 12 months; RR 1.6 favouring exercise therapy, 95% CI 0.51 to 5.46, P value = 0.40; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.5).
2.5. Analysis.
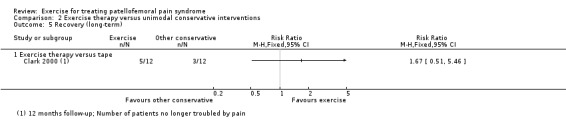
Comparison 2 Exercise therapy versus unimodal conservative interventions, Outcome 5 Recovery (long‐term).
Isometric exercises versus muscle electrostimulation
The one study making this comparison did not report on long‐term outcome, knee pain (during activity, usual or worse), functional performance, aspects of recovery or adverse events (Gobelet 1992).
Functional ability in the short term (Arpège function scale 0 to 18; higher scores mean better function)
Gobelet 1992 (54 participants) reported a MD of 0.70 favouring isometric exercises, 95% CI ‐0.63 to 2.03, P value = 0.30; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.3).
Isokinetic exercises versus muscle electrostimulation
The one study making this comparison did not report on long‐term outcome, knee pain (during activity, usual or worse), functional performance, aspects of recovery or adverse events (Gobelet 1992).
Functional ability in the short term (Arpège function scale 0 to 18; higher scores mean better function)
Gobelet 1992 (68 participants) reported a MD of 1.10 favouring isokinetic exercises, 95% CI ‐0.18 to 2.38, P value = 0.09; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 2.3).
Exercise therapy versus different conservative treatments: exercise therapy versus multimodal conservative interventions
For convenience, the available data for five different comparisons, tested within four trials (Eburne 1996; Gaffney 1992; Harrison 1999; Schneider 2001), are presented together in Analyses 3.1 to 3.5 but without pooling. The five comparisons are presented in turn below. None of the four trials reported on functional performance. Only Eburne 1996 reported on adverse events but did not report on denominators. Harrison 1999 presented functional ability via a Functional Index Questionnaire (FIQ) modified score and a non‐validated patellofemoral scale. Therefore the FIQ is presented.
Isometric quadriceps exercises versus McConnell regimen including exercises and tape
One study made this comparison (Eburne 1996). It did not report on long‐term outcome, knee pain during activity, usual pain or worse pain, functional ability or functional performance.
Knee pain in the short term
Pain experienced at four different positions of the knee
Eburne 1996 (53 participants) reported that a positive McConnell critical test (pain experienced at four different positions of the knee) was "abolished" in 25% of participants in the isometric exercises group and 30% in the McConnell regimen group; very low quality evidence due to serious risk of bias and imprecision.
Recovery in the short term
Eburne 1996 concluded that there was improvement in 50% of each group; very low quality evidence due to serious risk of bias, indirectness and imprecision.
Adverse events
Eburne 1996 (75 participants) did not report the numbers assigned or followed up in each group. However, one participant was withdrawn from the trial for surgery (group not stated) and "three due to severe allergy to the strapping" (presumably in the McConnell regimen group); very low quality evidence due to serious risk of bias and imprecision.
Supervised exercise programme versus vastus medius specific exercise programme plus taping
The one study making this comparison did not report on adverse events (Harrison 1999).
Knee pain in the short term
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (40 participants) reported a MD of ‐0.01 favouring supervised exercise, 95% CI ‐1.08 to 1.06, P value = 0.99; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.1.
3.1. Analysis.
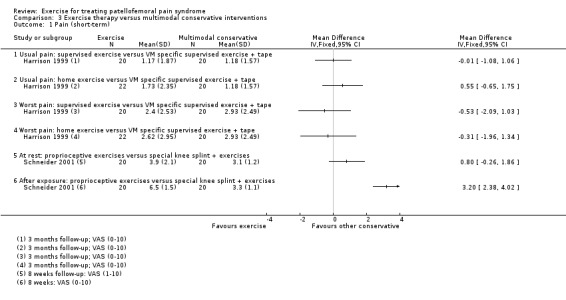
Comparison 3 Exercise therapy versus multimodal conservative interventions, Outcome 1 Pain (short‐term).
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (40 participants) reported a MD of ‐0.53 favouring supervised exercise, 95% CI ‐2.09 to 1.03, P value = 0.50; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.1.
Knee pain in the long term
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (31 participants) reported a MD of 0.24 favouring vastus medius specific supervised exercise plus tape, 95% CI ‐0.88 to 1.36, P value = 0.68; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.2.
3.2. Analysis.
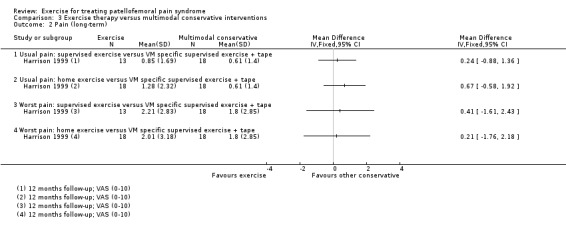
Comparison 3 Exercise therapy versus multimodal conservative interventions, Outcome 2 Pain (long‐term).
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (31 participants) reported a MD of 0.41 favouring vastus medius specific supervised exercise plus tape, 95% CI ‐1.61 to 2.43, P value = 0.69; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.2.
Functional ability in the short term (FIQ modified 0 to 16 scale; higher scores mean better function)
Harrison 1999 (54 participants) presented the numbers of participants with scores split into four FIQ categories (0 to 4, 5 to 8, 9 to 12, 13 to 16). Although we present the data for those in the top (13 to 16, best function) category, the ordinal nature of the data and extent of the loss to follow‐up in both groups raises serious questions as to the validity of these results (6/24 versus 17/28; RR 0.41 favouring a vastus medius specific exercise programme plus taping, 95% CI 0.19 to 0.88, P value = 0.02; very low quality evidence due to risk of bias, indirectness and serious imprecision; seeAnalysis 3.3).
3.3. Analysis.
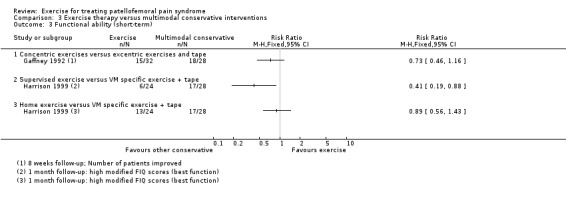
Comparison 3 Exercise therapy versus multimodal conservative interventions, Outcome 3 Functional ability (short‐term).
Functional ability in the long term (FIQ modified 0 to 16 scale; higher scores mean better function)
As described above, Harrison 1999 (33 participants) presented modified FIQ data split into four categories. The results for participants in the best function category (13 to 16) were: 11/13 versus 14/20; RR 1.21 favouring a supervised exercise programme, 95% CI 0.84 to 1.75, P value = 0.31; very low quality evidence due to risk of bias, indirectness and serious imprecision; seeAnalysis 3.4).
3.4. Analysis.
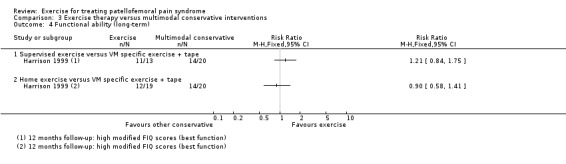
Comparison 3 Exercise therapy versus multimodal conservative interventions, Outcome 4 Functional ability (long‐term).
Functional performance in the short term (step test)
Harrison 1999 (44 participants) performed a step test (time until pain) and reported a MD of 0.00 seconds favouring neither intervention, 95% CI ‐60.72 to 60.72, P value = 1.00; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.6.
3.6. Analysis.
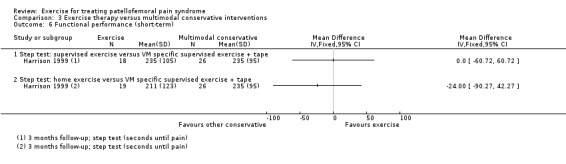
Comparison 3 Exercise therapy versus multimodal conservative interventions, Outcome 6 Functional performance (short‐term).
Functional performance in the long term (step test)
Harrison 1999 (34 participants) performed a step test (time until pain) and reported a MD of ‐5.00 seconds favouring a vastus medius specific exercise programme plus taping, 95% CI ‐70.14 to 60.14, P value = 0.88; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.7.
3.7. Analysis.
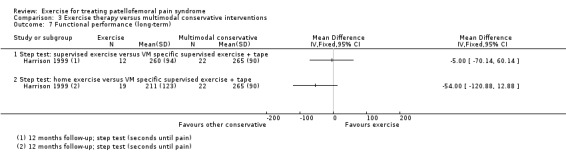
Comparison 3 Exercise therapy versus multimodal conservative interventions, Outcome 7 Functional performance (long‐term).
Recovery in the short term
Harrison 1999 (54 participants) reported that 6/29 participants in the supervised exercise programme versus 17/25 participants in the vastus medius specific exercise programme plus taping reported significant improvement; RR 0.30 favouring the vastus medius specific exercise programme plus taping, 95% CI 0.14 to 0.65, P value = 0.002; very low quality evidence due to serious risk of bias, indirectness and imprecision; seeAnalysis 3.5.
3.5. Analysis.
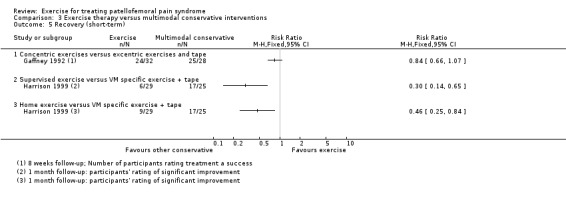
Comparison 3 Exercise therapy versus multimodal conservative interventions, Outcome 5 Recovery (short‐term).
Home exercise programme versus vastus medius specific exercise programme plus taping
The one study making this comparison did not report on adverse events (Harrison 1999).
Knee pain in the short term
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (42 participants) reported a MD of 0.55 favouring vastus medius specific supervised exercise plus tape, 95% CI ‐0.65 to 1.75, P value = 0.37; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.1.
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (42 participants) reported a MD of ‐0.31 favouring home exercise, 95% CI ‐1.96 to 1.34, P value = 0.71; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.1.
Knee pain in the long term
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (36 participants) reported a MD of 0.67 favouring vastus medius specific supervised exercise plus tape, 95% CI ‐0.58 to 1.92, P value = 0.29; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.2.
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (36 participants) reported a MD of 0.21 favouring vastus medius specific supervised exercise plus tape, 95% CI ‐1.76 to 2.18, P value 0.83; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.2.
Functional ability in the short term (FIQ modified 0 to 16 scale; higher scores mean better function)
Harrison 1999 (52 participants) presented the numbers of participants with scores split into four FIQ categories (0 to 4, 5 to 8, 9 to 12, 13 to 16). Although we present the data for those in the top (13 to 16, best function) category, the ordinal nature of the data and extent of the loss to follow‐up in both groups raises serious questions as to the validity of these results (13/24 versus 17/28; RR 0.89 favouring the vastus medius specific exercise programme plus taping, 95% CI 0.56 to 1.43, P value = 0.64; very low quality evidence due to risk of bias, indirectness and serious imprecision; seeAnalysis 3.3).
Functional ability in the long term (FIQ modified 0 to 16 scale; higher scores mean better function)
As described above, Harrison 1999 (39 participants) presented modified FIQ data split into four categories. The results for participants in the best function category (13 to 16) were: 12/19 versus 14/20; RR 0.90 favouring the vastus medius specific exercise programme plus taping, 95% CI 0.58 to 1.41, P value = 0.65; very low quality evidence due to risk of bias, indirectness and serious imprecision; seeAnalysis 3.4).
Functional performance in the short term (step test)
Harrison 1999 (45 participants) performed a step test (time until pain) and reported a MD of ‐24.00 seconds favouring the vastus medius specific exercise programme plus taping, 95% CI ‐90.27 to 42.27, P value = 0.48; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.6.
Functional performance in the long term (step test)
Harrison 1999 (31 participants) performed a step test (time until pain) and reported a MD of ‐54.00 seconds favouring the vastus medius specific exercise programme plus taping, 95% CI ‐120.88 to 12.88, P value = 0.11; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 3.7.
Recovery in the short term
Harrison 1999 (54 participants) reported that 9/29 participants in the home exercise programme versus 17/25 participants in the vastus medius specific exercise programme plus taping reported significant improvement; RR 0.46 favouring the vastus medius specific exercise programme plus taping, 95% CI 0.25 to 0.84, P value = 0.001; very low quality evidence due to serious risk of bias, indirectness and imprecision; seeAnalysis 3.5.
Concentric exercises versus eccentric exercises and tape
One study made this comparison (Gaffney 1992). It did not report on long‐term outcome, functional performance or adverse events.
Knee pain in the short term
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Gaffney 1992 (60 participants) reported no significant between‐group difference in mean maximum pain values (concentric 2.64 versus eccentric 2.86); very low quality evidence due to serious risk of bias and imprecision.
Functional ability in the short term (number of patients improved)
Gaffney 1992 (60 participants) reported that 15/32 in the concentric exercises and 18/28 in the eccentric plus tape group had improved function; RR 0.73 favouring the eccentric plus tape group, 95% CI 0.46 to 1.16, P value = 0.18; very low quality evidence due to serious risk of bias and imprecision; seeAnalysis 3.3.
Recovery in the short term (participant‐rated success)
Gaffney 1992 (60 participants) reported that 24/32 in the concentric exercises and 25/28 in the eccentric plus tape group rated their outcome as a success; RR 0.84 favouring the eccentric plus tape group, 95% CI 0.66 to 1.07, P value = 0.15; very low quality evidence due to serious risk of bias, indirectness and imprecision; seeAnalysis 3.3.
Physiotherapeutic exercises based on proprioceptive neuromuscular facilitation versus special knee splint combined with exercises
One study (40 participants) made this comparison (Schneider 2001). It did not report on long‐term outcome, knee pain during activity, usual pain or worse pain, functional performance, aspects of recovery or adverse events.
Knee pain in the short term
Pain at rest and pain after exposure (VAS 0 to 10; higher scores mean worse pain)
Schneider 2001 (40 participants) reported on knee pain at rest and "after exposure" to some muscle tests. Schneider 2001 reported a MD of 0.80 favouring special knee splint and exercises for pain at rest, 95% CI ‐0.26 to 1.86, P value = 0.83; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 3.1. For pain after exposure, Schneider 2001 reported a MD of 3.20 favouring special knee splint and exercises for pain at rest, 95% CI 2.38 to 4.02, P value < 0.00001; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 3.1.
Functional ability in the short term (Bessette and Hunter score: 0 to 100; higher scores mean better function)
Schneider 2001 (40 participants) reported significant improvements in both groups from 53 to 69 points in the physiotherapeutic exercises based on proprioceptive neuromuscular facilitation group and from 53 to 72 points in the group receiving a special knee splint combined with exercises. However, Schneider 2001 did not report SDs for the Bessette and Hunter score; very low quality evidence due to serious risk of bias and lack of data.
Different modes of delivery of exercises or exercise programmes
Supervised versus home exercise programmes
Two studies compared supervised with home exercise programmes (Harrison 1999; Loudon 2004). Harrison 1999 reported functional ability using a modified FIQ and a non‐validated patellofemoral scale; only the modified FIQ is presented below. Neither study reported on adverse events. We obtained missing standard deviations for pain and function for Loudon 2004.
Knee pain in the short term
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Pooled data from two studies (Harrison 1999; Loudon 2004; 59 participants) showed a MD of ‐0.22 favouring a supervised exercise programme, 95% CI ‐1.22 to 0.77, P value = 0.66; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 4.1.
4.1. Analysis.
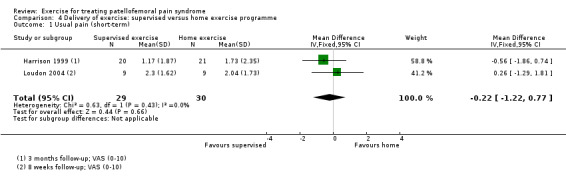
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 1 Usual pain (short‐term).
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (42 participants) reported a MD of ‐0.22 favouring a supervised exercise programme, 95% CI ‐1.88 to 1.44, P value = 0.79; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 4.2.
4.2. Analysis.
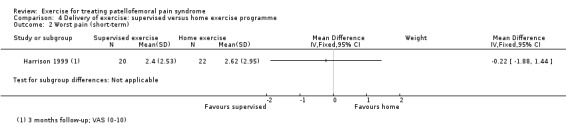
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 2 Worst pain (short‐term).
Knee pain in the long term
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (31 participants) reported a MD of ‐0.43 favouring a supervised exercise programme, 95% CI ‐1.84 to 0.98, P value = 0.55; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 4.3.
4.3. Analysis.
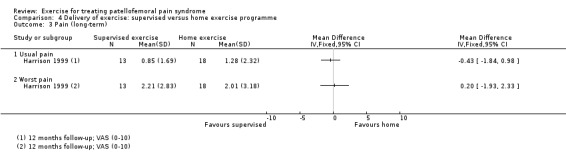
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 3 Pain (long‐term).
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Harrison 1999 (31 participants) reported a MD of 0.20 favouring a home exercise programme, 95% CI ‐1.93 to 2.33, P value = 0.85; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 4.3.
Functional ability in the short term (Anterior Knee Pain Score (AKPS) 0 to 100; modified FIQ 0 to 16; higher scores mean better function)
Loudon 2004 (18 participants) measured the AKPS (higher scores mean better function) and reported a MD of ‐2.30 favouring a home exercise programme, 95% CI ‐11.33 to 6.73, P value = 0.62; very low quality evidence due to serious risk of bias and imprecision; seeAnalysis 4.4.
4.4. Analysis.
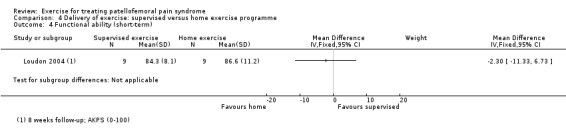
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 4 Functional ability (short‐term).
Harrison 1999 (48 participants) presented the numbers of participants with scores split into four FIQ categories (0 to 4, 5 to 8, 9 to 12, 13 to 16). Although we present the data for those in the top (13 to 16, best function) category, the ordinal nature of the data and extent of the loss to follow‐up in both groups raises serious questions as to the validity of these results (6/24 versus 13/24; RR 0.46 favouring the home exercise group, 95% CI 0.21 to 1.01, P value = 0.05; very low quality evidence due to risk of bias, indirectness and serious imprecision; seeAnalysis 4.5).
4.5. Analysis.
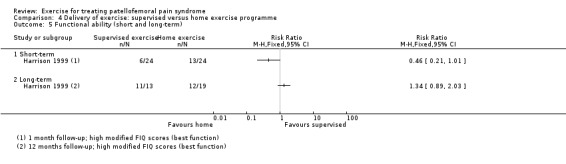
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 5 Functional ability (short and long‐term).
Functional ability in the long term (modified FIQ 0 to 16; higher scores mean better function)
As described above, Harrison 1999 presented modified FIQ data split into four categories. They reported a significant improvement in function scores for both groups but for even fewer participants at 12 months follow‐up. The results for participants in the best function category (13 to 16) were: 11/13 versus 12/19; RR 1.34, 95% CI 0.89 to 2.03, P value = 0.17; very low quality evidence due to risk of bias, indirectness and serious imprecision; seeAnalysis 4.5).
Functional performance in the short term (step test, bilateral squat)
Harrison 1999 (46 participants) performed a step test (time until pain) and reported a MD of 47.00 seconds favouring a supervised exercise programme, 95% CI ‐19.04 to 113.04, P value = 0.16; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 4.6.
4.6. Analysis.
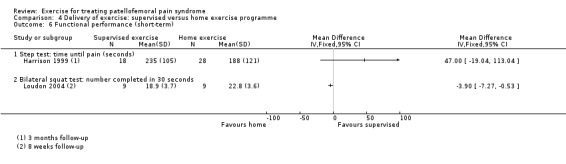
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 6 Functional performance (short‐term).
Loudon 2004 (18 participants) performed the bilateral squat test (number completed in 30 seconds) and reported a MD of ‐3.90 favouring a home exercise programme, 95% CI ‐7.27 to ‐0.53, P value = 0.02; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 4.6.
Full data were not available for the four other functional performance tests, based on limb symmetry index, measured by Loudon 2004 (18 participants): anteromedial lunge, step‐down dip, leg press, and balance and reach.
Functional performance in the long term (step test: time until pain)
Harrison 1999 (31 participants) reported a MD of 49.00 seconds favouring a supervised exercise programme, 95% CI ‐27.73 to 125.73 seconds, P value = 0.21; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 4.7.
4.7. Analysis.
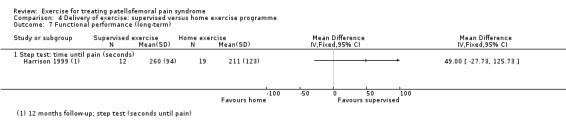
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 7 Functional performance (long‐term).
Recovery in the short term
Harrison 1999 (58 participants) reported that 9/29 participants in the home exercise programme versus 6/29 participants in the supervised exercise programme reported significant improvement; RR 0.67 favouring a home exercise programme, 95% CI 0.27 to 1.63, P value = 0.37; very low quality evidence due to serious risk of bias, indirectness and imprecision; seeAnalysis 4.8.
4.8. Analysis.
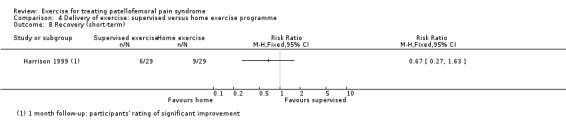
Comparison 4 Delivery of exercise: supervised versus home exercise programme, Outcome 8 Recovery (short‐term).
Medium of exercises or exercise programmes
There were no trials evaluating this comparison, i.e. water‐ versus land‐based exercise.
Different types of exercise or exercise programmes
Eleven studies compared different types of exercises or exercise programmes (Abd Elhafz 2011; Abrahams 2003; Bakhtiary 2008; Balci 2009; Colón 1988; Gobelet 1992; Hafez 2012; Herrington 2007; Moyano 2013; Thomee 1997; Witvrouw 2000). We grouped the seven different comparisons into three groups defined according to type of kinetic chain exercise: closed kinetic chain exercises versus open kinetic chain exercises; variants of closed kinetic chain exercises; and open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action. For convenience, these are presented subgrouped in the same forest plots, but without overall pooling. A comparison of proprioceptive neuromuscular facilitation stretching and aerobic exercise versus classic stretching and quadriceps exercises is presented separately (Moyano 2013). Recovery was not reported in any study making these comparisons.
Closed kinetic chain exercises versus open kinetic chain exercises
Four studies compared closed kinetic chain exercises versus open kinetic chain exercises (Abd Elhafz 2011; Bakhtiary 2008; Herrington 2007; Witvrouw 2000). None of the four studies reported on aspects of recovery or adverse events. We extracted standard deviations for pain and function (Herrington 2007) and function (Witvrouw 2000) from error bars, which we interpreted to be SDs, in graphs presented in the publications of these two trials.
Knee pain in the short term
Pain during activity (VAS 0 to 10; higher scores mean worse pain)
Pooled data from two studies (Herrington 2007; Witvrouw 2000; 90 participants) showed a MD of 0.03 favouring open kinetic chain exercises, 95% CI ‐0.63 to 0.70, P value = 0.92; very low quality evidence due to risk of bias, inconsistency and serious imprecision; seeAnalysis 5.1.
5.1. Analysis.
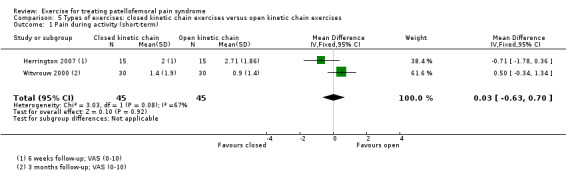
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 1 Pain during activity (short‐term).
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Pooled data from three studies (Abd Elhafz 2011; Bakhtiary 2008; Witvrouw 2000; 122 participants) showed a MD of 0.20 favouring open kinetic chain exercises, 95% CI ‐0.37 to 0.76, P value = 0.38; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.2.
5.2. Analysis.
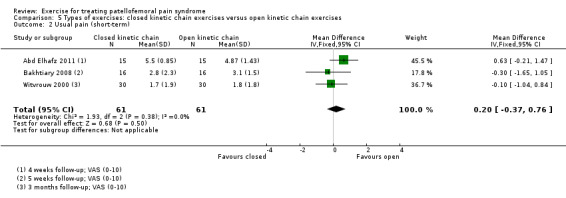
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 2 Usual pain (short‐term).
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Witvrouw 2000 (60 participants) reported a MD of ‐0.10 favouring closed kinetic chain exercises, 95% CI ‐1.21 to 1.01, P value = 0.86; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.3.
5.3. Analysis.
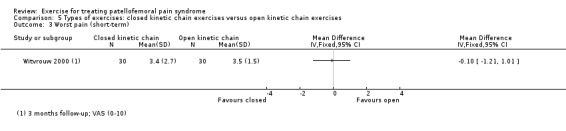
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 3 Worst pain (short‐term).
Knee pain in the long term (five years follow‐up)
Pain during activity (VAS 0 to 10; higher scores mean worse pain)
Witvrouw 2000 (49 participants) showed a MD of 2.10 favouring open kinetic chain exercises, 95% CI 1.08 to 3.12, P value < 0.0001; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.4.
5.4. Analysis.
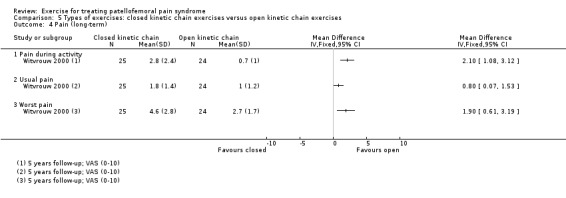
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 4 Pain (long‐term).
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Witvrouw 2000 (49 participants) reported a MD of 0.80 favouring open kinetic chain exercises, 95% CI 0.07 to 1.53, P value 0.03; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.4.
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Witvrouw 2000 (49 participants) reported a MD 1.90 favouring open kinetic chain exercises, 95% CI 0.61 to 3.19, P value 0.004; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.4.
Functional ability in the short term (AKPS 0 to 100; higher scores mean better function)
Pooled data from two studies (Herrington 2007; Witvrouw 2000; 90 participants) showed a MD of ‐3.51 favouring open kinetic chain exercises, 95% CI ‐7.84 to 0.82, P value = 0.11; very low quality evidence due to risk of bias, imprecision and inconsistency; seeAnalysis 5.5.
5.5. Analysis.
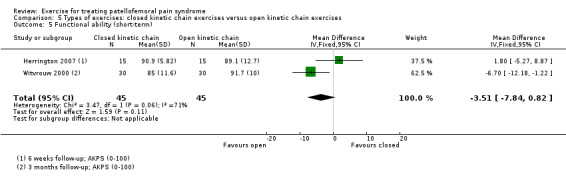
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 5 Functional ability (short‐term).
Functional ability in the long term (AKPS 0 to 100; higher scores mean better function)
Data from Witvrouw 2000 (49 participants) showed a MD of ‐8.30 favouring open kinetic chain exercises, 95% CI ‐12.95 to ‐3.65, P value = 0.0005; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.6.
5.6. Analysis.
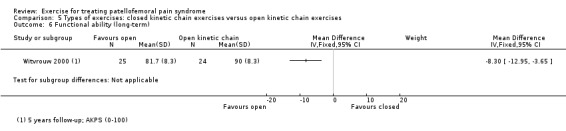
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 6 Functional ability (long‐term).
Functional performance in the short term (step‐up, step‐down, unilateral squat)
Witvrouw 2000 (60 participants) reported that 22/30 participants in each group were without symptoms during the step‐up test; RR 1.00 favouring neither intervention, 95% CI 0.32 to 3.14, P value = 1.00; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.7.
5.7. Analysis.
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 7 Functional performance (short‐term).
Witvrouw 2000 (60 participants) reported that 23/30 participants in the closed kinetic chain exercise group and 20/30 participants in the open kinetic chain exercise group were without symptoms during the step‐down test; RR of 1.15 favouring closed kinetic chain exercises, 95% CI 0.83 to 1.59, P value = 0.39; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.7.
Witvrouw 2000 (60 participants) reported that 17/30 participants in the closed kinetic chain exercise group and 16/30 participants in the open kinetic chain exercise group were without symptoms during the unilateral squat test; RR 1.06 favouring closed kinetic chain exercises, 95% CI 0.67 to 1.68, P value = 0.80; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.7.
Witvrouw 2000 also reported there were no significant differences between treatment groups for the triple jump test but did not provide supporting data.
Functional performance in the long term (triple jump test (cm), step‐up (N of patients without symptoms) and step‐down (N of patients without symptoms))
Witvrouw 2000 (49 participants) reported that 20/25 participants in the closed kinetic chain exercise group and 17/24 participants in the open kinetic chain exercise group were without symptoms during the step‐down test; RR 1.13, favouring closed kinetic chain exercises, 95% CI 0.82 to 1.56, P value = 0.46; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.8.
5.8. Analysis.
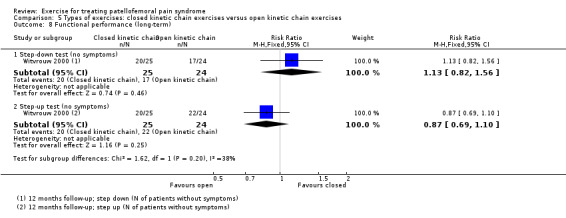
Comparison 5 Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises, Outcome 8 Functional performance (long‐term).
Witvrouw 2000 (49 participants) reported that 20/25 participants in the closed kinetic chain exercise group and 22/24 participants in the open kinetic chain exercise group were without symptoms during the step‐up test; RR 0.87, favouring open kinetic chain exercises, 95% CI 0.69 to 1.10, P value = 0.25; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 5.8.
Witvrouw 2000 also reported that there were no significant differences between treatment groups for the triple jump test but did not provide supporting data.
Variants of closed kinetic chain exercises
Two studies tested variants of closed kinetic chain exercises. Abrahams 2003 compared an exercise protocol with thigh adduction and tibia medial rotation during eccentric squat versus a traditional exercise protocol. Balci 2009 compared closed kinetic chain exercises with internally rotated hip versus closed kinetic chain exercises with externally rotated hip. For convenience, these two heterogeneous studies are presented subgrouped in the same forest plots, but without overall pooling. Neither trial reported on long‐term outcomes, functional performance, aspects of recovery or adverse events.
Knee pain in the short term
This outcome was not reported in Abrahams 2003.
Pain during activity (VAS 0 to 10; higher scores mean worse pain)
Balci 2009 (40 participants) showed a MD of ‐0.30 favouring closed kinetic chain exercises with internal hip rotation, 95% CI ‐1.46 to 0.86, P value = 0.61; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 6.1.
6.1. Analysis.
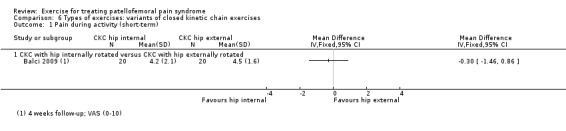
Comparison 6 Types of exercises: variants of closed kinetic chain exercises, Outcome 1 Pain during activity (short‐term).
Functional ability in the short term (MFIQ 0 to 16, AKPS 0 to 100; higher scores mean better function)
Based on the MFIQ (0 to 16) score, Abrahams 2003 (52 participants) reported a MD of ‐2.00 favouring the novel exercise protocol, 95% CI ‐3.39 to ‐0.61, P value = 0.005; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 6.2.
6.2. Analysis.
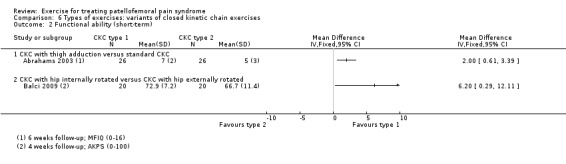
Comparison 6 Types of exercises: variants of closed kinetic chain exercises, Outcome 2 Functional ability (short‐term).
Based on the AKPS 0 to 100 score, Balci 2009 (40 participants) showed a MD of 6.20 favouring closed kinetic chain exercises with internal hip rotation, 95% CI 0.29 to 12.11, P value = 0.04; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 6.2.
Open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action
The comparisons undertaken by four studies fell into this category. One study compared eccentric exercises versus concentric exercises (Hafez 2012). One study compared eccentric exercises versus isometric exercises (Thomee 1997). One study compared isokinetic exercises versus isometric exercises (Gobelet 1992). One study compared combined isotonic and isometric exercises (pogo stick) versus isometric exercises (Colón 1988).
Knee pain in the short term
This was not reported in Colón 1988 or Gobelet 1992.
Pain during activity (number of patients with pain)
Thomee 1997 (40 participants) reported that 9/20 participants in the eccentric exercise group and 12/20 participants in the isometric exercise group had pain during jogging; RR of 0.75 favouring eccentric exercises, 95% CI 0.41 to 1.37, P value = 0.35; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 7.1.
7.1. Analysis.
Comparison 7 Types of exercises: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action, Outcome 1 Pain during activity (short‐term).
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Hafez 2012 (40 participants) reported a MD of ‐1.30 favouring eccentric exercise, 95% CI ‐1.97 to ‐0.63, P value = 0.0002; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 7.2.
7.2. Analysis.
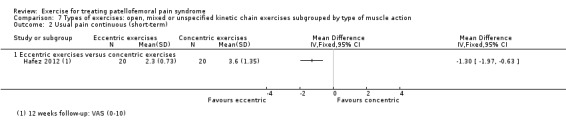
Comparison 7 Types of exercises: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action, Outcome 2 Usual pain continuous (short‐term).
Knee pain in the long term
This was not reported in Colón 1988, Gobelet 1992 or Hafez 2012.
Pain during activity (number of patients with pain)
Thomee 1997 (40 participants) reported that 4/20 participants in the eccentric exercise group and 6/20 participants in the isometric exercise group had pain during jogging; RR of 0.67 favouring eccentric exercises, 95% CI 0.22 to 2.01, P value = 0.47; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 7.3.
7.3. Analysis.
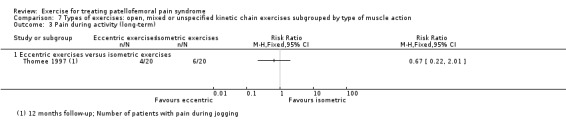
Comparison 7 Types of exercises: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action, Outcome 3 Pain during activity (long‐term).
Functional ability in the short term (WOMAC 0 to 96 (inverted scores; higher scores mean better function), Arpège function scale 0 to 18; higher scores mean better function)
This was not reported in Colón 1988 or Thomee 1997.
Based on the WOMAC (0 to 96) score, Hafez 2012 (40 participants) reported a MD of 11.65 favouring eccentric exercises, 95% CI 5.15 to 18.15, P value = 0.0004; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 7.4.
7.4. Analysis.
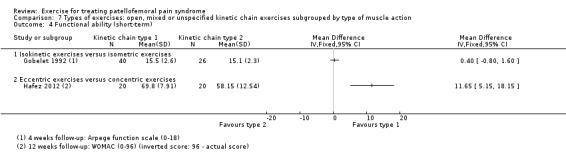
Comparison 7 Types of exercises: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action, Outcome 4 Functional ability (short‐term).
Based on the Arpège scale (0 to 18), Gobelet 1992 (66 participants) reported a MD of 0.40 favouring isometric exercises, 95% CI ‐0.80 to 1.60, P value = 0.51; very low quality evidence due to serious risk of bias and imprecision; seeAnalysis 7.4.
Functional ability in the long term
This was not reported in any of the four trials.
Functional performance in the short term (vertical jump test)
Only Thomee 1997 reported on functional performance, using the vertical jump test; however, only the overall data for the trial population were provided.
Recovery in the short and long term
Colón 1988 reported that 13/14 participants in the isotonic and isokinetic group versus 9/11 participants in the isometric exercise group had 50% or higher pain relief at eight weeks follow‐up; RR 1.13 favouring isotonic and isokinetic exercises, 95% CI 0.83 to 1.55, P value = 0.43; very low quality evidence due to serious risk of bias, indirectness and imprecision; seeAnalysis 7.5.
7.5. Analysis.
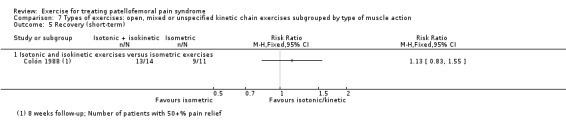
Comparison 7 Types of exercises: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action, Outcome 5 Recovery (short‐term).
Thomee 1997 (40 participants) reported that all participant except one (group not identified) rated their knee function as excellent at 12 months; the exception rated her knee function as improved although still poor; very low quality evidence due to serious risk of bias, indirectness and imprecision. Two participants, one in each group, had chosen to undergo surgery at nine months.
Adverse events (number of patients with increased pain)
Colón 1988 reported that 1/16 participants in the isotonic and isokinetic group versus 0/11 participants in the isometric exercise group had an adverse event; RR 2.12 favouring isometric exercises, 95% CI 0.09 to 47.68, P value = 0.64; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 7.6.
7.6. Analysis.
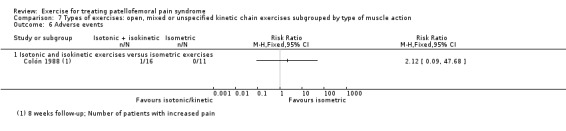
Comparison 7 Types of exercises: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action, Outcome 6 Adverse events.
Proprioceptive neuromuscular facilitation stretching and aerobic exercise versus classic stretching and quadriceps exercises
The one study making this comparison (Moyano 2013; 68 participants) reported on long‐term (16 weeks) pain and function only.
Knee pain in the long term
Usual pain (VAS 0 to 10)
Moyano 2013 reported a MD of ‐3.50, favouring proprioceptive neuromuscular facilitation stretching and aerobic exercise, 95% CI ‐4.08 to ‐2.92, P value < 0.00001; very low quality evidence due to risk of bias and serious imprecision; see Analysis 8.1.
8.1. Analysis.

Comparison 8 Types of exercises: proprioceptive neuromuscular facilitation + aerobic exercise versus classic stretching + quadriceps exercises, Outcome 1 Usual pain (long‐term).
Functional ability in the long term (0 to 100 AKPS scale; higher scores mean better function)
Moyano 2013 reported a MD of 17.01, favouring proprioceptive neuromuscular facilitation stretching and aerobic exercise, 95% CI 11.85 to 22.17, P value < 0.00001; very low quality evidence due to risk of bias and serious imprecision; see Analysis 8.2.
8.2. Analysis.

Comparison 8 Types of exercises: proprioceptive neuromuscular facilitation + aerobic exercise versus classic stretching + quadriceps exercises, Outcome 2 Functional ability (long‐term).
Target of exercises or exercise programmes
Knee and hip exercises versus knee exercises alone
Seven studies compared knee and hip exercises versus knee exercises alone (Avraham 2007; De Marche 2014; Fukuda 2010; Fukuda 2012; Nakagawa 2008; Razeghi 2010; Song 2009). Only De Marche 2014 reported on aspects of recovery, which was assessed via a global rating of improvement (15‐point scale). None of the trials reported on adverse events. Avraham 2007, which provided very low quality evidence reflecting very serious risk of bias and imprecision, only presented P values in a graph for the comparisons of three groups of which two were knee and hip exercises and one was knee exercises.
Knee pain in the short term
Pain during activity (0 to 10 scale; higher scores mean worse pain)
Pooled data from three studies (Fukuda 2010; Fukuda 2012; Nakagawa 2008; 104 participants) showed a MD of ‐2.02 favouring knee and hip exercises, 95% CI ‐3.80 to ‐0.60, P value = 0.007; very low quality evidence due to risk of bias, serious inconsistency and imprecision (significant heterogeneity: P value = 0.004, I2 = 82%); seeAnalysis 9.1. The results were homogeneous (P value = 0.66 and I2 = 0%) upon removal of Fukuda 2012, but smaller in effect size (MD ‐1.37, 95% CI ‐2.40 to ‐0.33, P value = 0.010).
9.1. Analysis.
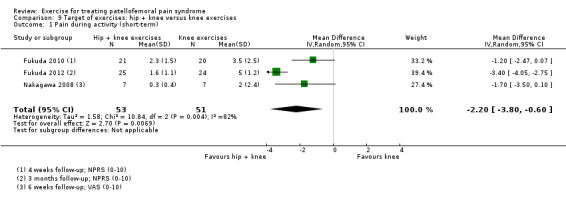
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 1 Pain during activity (short‐term).
Usual pain (VAS 0 to 10; higher scores mean worse pain)
Pooled data from two studies (Nakagawa 2008; Razeghi 2010; 46 participants) showed a MD of ‐1.77 favouring knee and hip exercises, 95% CI ‐2.78 to ‐0.76, P value = 0.0006; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 9.2.
9.2. Analysis.
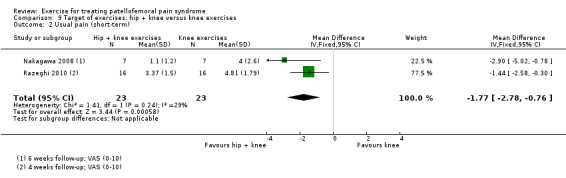
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 2 Usual pain (short‐term).
Avraham 2007 (30 participants) reported that no significant between‐group differences were found for pain (reported P value = 0.11 and P value = 0.72, P values extracted from graph).
Worst pain (0 to 10 scale; higher scores mean worse pain)
Pooled data from three studies (De Marche 2014; Nakagawa 2008; Song 2009; 98 participants) showed a MD of ‐0.79 favouring knee and hip exercises, 95% CI ‐1.66 to 0.09, P value = 0.08; very low quality evidence due to risk of bias, inconsistency and imprecision; seeAnalysis 9.3.
9.3. Analysis.
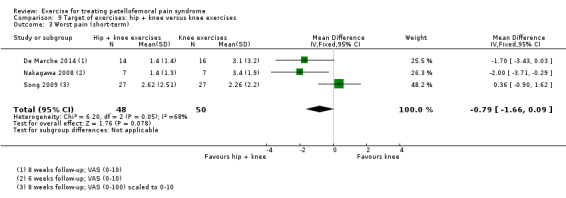
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 3 Worst pain (short‐term).
Knee pain in the long term
Pain during activity (numerical pain rating scale (NPRS) 0 to 10; higher scores mean worse pain)
Fukuda 2012 (49 participants) reported a MD of ‐3.90 favouring knee and hip exercises, 95% CI ‐4.46 to ‐3.34, P value < 0.00001; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 9.4.
9.4. Analysis.
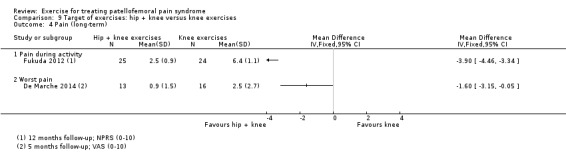
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 4 Pain (long‐term).
Worst pain (VAS 0 to 10; higher scores mean worse pain)
De Marche 2014 (29 participants) reported a MD of ‐1.60 favouring knee and hip exercises, 95% CI ‐3.15 to ‐0.05, P value = 0.04; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 9.4.
Functional ability in the short term (0 to 100 scale; higher scores mean better function)
Pooled data from four studies (De Marche 2014; Fukuda 2010; Fukuda 2012; Song 2009; 174 participants) showed a SMD of 0.61 favouring knee and hip exercises, 95% CI ‐0.39 to 1.61, P value = 0.23; very low quality evidence due to risk of bias, imprecision and serious inconsistency (significant heterogeneity: P value < 0.00001, I2 = 90%); seeAnalysis 9.5. Upon removal of Fukuda 2012, the results were homogeneous (P value = 0.33 and I² = 11%) with little difference between the two groups (SMD 0.06, 95% CI ‐0.32 to 0.43, P value = 0.76).
9.5. Analysis.
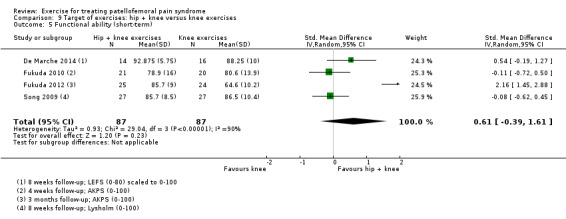
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 5 Functional ability (short‐term).
Avraham 2007 (20 participants) reported no significant between‐group differences were found for function assessed using the patellofemoral joint evaluation scale (0 to 100) (reported P value = 0.74 and P value = 0.70; P values extracted from graph).
Functional ability in the long term (0 to 100 scale; higher scores mean better function)
Pooled data from two studies (De Marche 2014; Fukuda 2012; 78 participants) showed a SMD of 1.49 favouring knee and hip exercises, 95% CI ‐0.17 to 3.15, P value = 0.08; very low quality evidence due to risk of bias, imprecision and serious inconsistency (significant heterogeneity: P value = 0.002, I² = 90%); seeAnalysis 9.6.
9.6. Analysis.
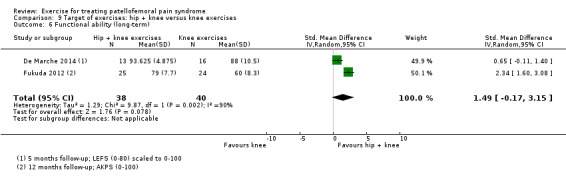
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 6 Functional ability (long‐term).
Functional performance in the short term (single‐limb hop test)
Pooled data from two trials (Fukuda 2010; Fukuda 2012) (90 participants) reporting the single‐limb hop test showed a MD of 13.89 cm favouring knee and hip exercises, 95% CI 5.21 to 22.56, P value = 0.002; low quality evidence due to risk of bias and imprecision; seeAnalysis 9.7.
9.7. Analysis.
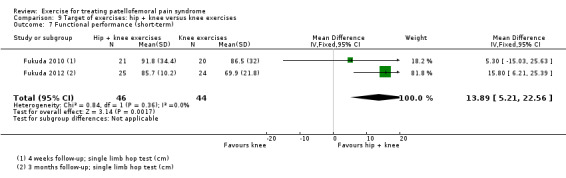
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 7 Functional performance (short‐term).
Functional performance in the long term (single‐leg triple hop test and single‐limb hop test)
De Marche 2014 (29 participants) reported for the single‐leg triple hop test a MD of 45.20 cm favouring knee and hip exercises, 95% CI 1.03 to 89.37, P value = 0.04; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 9.8.
9.8. Analysis.
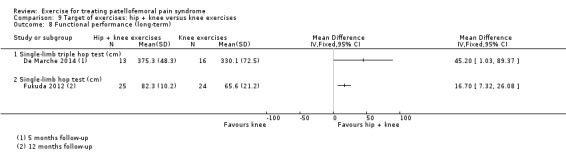
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 8 Functional performance (long‐term).
Fukuda 2012 (49 participants) reported for the single‐limb hop test a MD of 16.70 cm favouring knee and hip exercises, 95% CI 7.32 to 26.08, P value = 0.001; low quality evidence due to risk of bias and imprecision; seeAnalysis 9.8.
Recovery in the short and long term (number of participants at least moderately better)
De Marche 2014 (30 participants in the short term, 29 participants in the long term) reported on the number of participants who perceived themselves as at least moderately better in the short term (14/14 versus 12/16, RR 1.31 favouring hip and knee exercises, 95% CI 0.97 to 1.78, P value = 0.07; very low quality evidence due to risk of bias, indirectness and serious imprecision) and in the long term (12/13 versus 11/16, RR 1.34 favouring hip and knee exercises, 95% CI 0.93 to 1.94, P value = 0.11; very low quality evidence due to risk of bias, indirectness and serious imprecision), seeAnalysis 9.9.
9.9. Analysis.
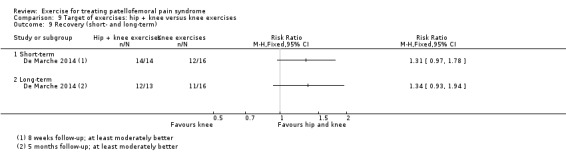
Comparison 9 Target of exercises: hip + knee versus knee exercises, Outcome 9 Recovery (short‐ and long‐term).
Target of exercises or exercise programmes
Hip exercises versus knee exercises
Two studies compared hip versus knee exercises (Dolak 2011; Khayambashi 2014). Dolak 2011 did not report on long‐term outcome. Neither study reported on aspects of recovery.
Knee pain in the short term
During activity (VAS 0 to 10; higher scores mean worse pain)
Khayambashi 2014 (36 participants) reported a MD of ‐1.16 favouring hip exercises, 95% CI ‐2.41 to 0.09, P value = 0.07; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 10.1.
10.1. Analysis.
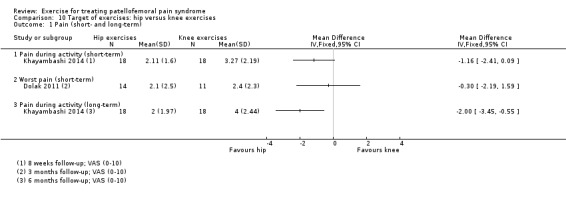
Comparison 10 Target of exercises: hip versus knee exercises, Outcome 1 Pain (short‐ and long‐term).
Worst pain (VAS 0 to 10; higher scores mean worse pain)
Dolak 2011 (25 participants) reported a MD of ‐0.30 favouring hip exercises, 95% CI ‐2.19 to 1.59, P value = 0.76; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 10.1.
Knee pain in the long term
During activity (VAS 0 to 10; higher scores mean worse pain)
Khayambashi 2014 (36 participants) reported a MD of ‐2.00 favouring hip exercises, 95% CI ‐3.45 to ‐0.55, P value = 0.007; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 10.1.
Functional ability in the short term (0 to 100 scale; higher scores mean better function)
Pooled data from two studies (Dolak 2011; Khayambashi 2014; 58 participants) showed a SMD of 0.85 favouring hip exercises, 95% CI 0.30 to 1.40, P value = 0.002, which was statistically heterogeneous (P value = 0.08; I2 = 68%); very low quality evidence due to serious risk of bias, imprecision and inconsistency; seeAnalysis 10.2.
10.2. Analysis.
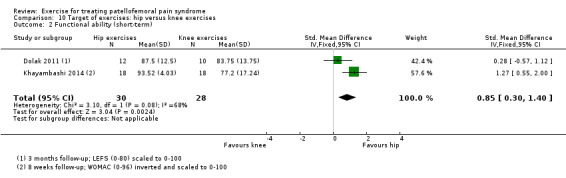
Comparison 10 Target of exercises: hip versus knee exercises, Outcome 2 Functional ability (short‐term).
Functional ability in the long term (WOMAC 0 to 96, score inverted so that higher scores mean better function)
Khayambashi 2014 (36 participants) reported a MD of 16.22 favouring hip exercises, 95% CI 9.17 to 23.27, P value < 0.00001; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 10.3.
10.3. Analysis.
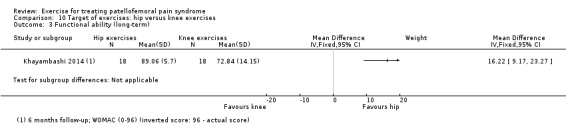
Comparison 10 Target of exercises: hip versus knee exercises, Outcome 3 Functional ability (long‐term).
Functional performance in the short term (step‐down test (N of repetitions in 30 seconds))
Dolak 2011 (27 participants) performed the step‐down test (number of repetitions in 30 seconds) and reported a MD of ‐1.00 favouring quadriceps exercises, 95% CI ‐5.18 to 3.18, P value = 0.64; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 10.4.
10.4. Analysis.
Comparison 10 Target of exercises: hip versus knee exercises, Outcome 4 Functional performance (short‐term).
Adverse events
Dolak 2011 (31 participants) reported that 0/17 participants in the hip exercise group versus 1/16 participants in the knee exercise group had an adverse event; RR of 0.31 favouring hip exercises, 95% CI 0.01 to 7.21, P value = 0.47; very low quality evidence due to serious risk of bias and serious imprecision; seeAnalysis 10.5.
10.5. Analysis.
Comparison 10 Target of exercises: hip versus knee exercises, Outcome 5 Adverse events.
Duration of exercises or exercise programmes
There were no trials testing duration of exercise therapy.
Intensity of exercises or exercise programmes
High‐ versus low‐intensity exercise programme
One study compared high‐dose, high‐repetition medical exercise therapy (MET) with low‐dose, low‐repetition exercises (Østeråsa 2013). Østeråsa 2013 did not report on aspects of recovery or adverse events.
Knee pain in the short term
Usual pain (0 to 10 scale; higher scores mean worse pain)
Østeråsa 2013 (40 participants) reported a MD of ‐1.90 favouring a high‐intensity programme, 95% CI ‐2.85 to ‐0.95, P value < 0.0001; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 11.1.
11.1. Analysis.
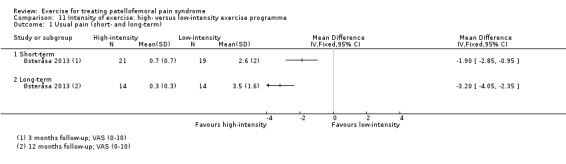
Comparison 11 Intensity of exercise: high‐ versus low‐intensity exercise programme, Outcome 1 Usual pain (short‐ and long‐term).
Knee pain in the long term
Usual pain (0 to 10 scale; higher scores mean worse pain)
Østeråsa 2013 (28 participants) reported a MD of ‐3.20 favouring a high‐intensity programme, 95% CI ‐4.05 to ‐2.35, P value < 0.00001; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 11.1.
Functional ability in the short term (FIQ 0 to 16 scale; higher scores mean better function)
Østeråsa 2013 (40 participants) reported a MD of 3.70 favouring a high‐intensity programme, 95% CI 1.59 to 5.81, P value = 0.0006; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 11.2.
11.2. Analysis.
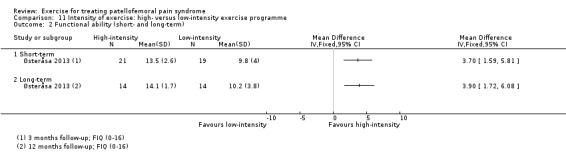
Comparison 11 Intensity of exercise: high‐ versus low‐intensity exercise programme, Outcome 2 Functional ability (short‐ and long‐term).
Functional ability in the long term (FIQ 0 to 16 scale; higher scores mean better function)
Østeråsa 2013 (28 participants) reported a MD of 3.90 favouring a high‐intensity programme, 95% CI 1.72 to 6.08, P value = 0.0005; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 11.2).
Functional performance in the short term (step‐down test)
Østeråsa 2013 (40 participants) performed the step‐down test (number of repetitions in 30 seconds) and reported a MD 9.40 favouring a high‐intensity programme, 95% CI 4.24 to 14.56, P value = 0.0004; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 11.3.
11.3. Analysis.
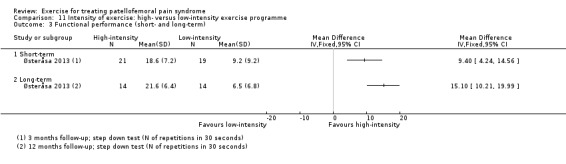
Comparison 11 Intensity of exercise: high‐ versus low‐intensity exercise programme, Outcome 3 Functional performance (short‐ and long‐term).
Functional performance in the long term (step‐down test)
Østeråsa 2013 (28 participants) performed the step‐down test (number of repetitions in 30 seconds) and reported a MD of 15.10 favouring a high‐intensity programme, 95% CI 10.21 to 19.99, P value < 0.00001; very low quality evidence due to risk of bias and serious imprecision; seeAnalysis 11.3.
Subgroup analyses for patient characteristics
We did not perform subgroup analyses to determine the effects of patient characteristics (gender, duration of complaints and sports participation) on outcome. This reflected the lack of data and the inconsistent and incomplete reporting of baseline characteristics.
Sensitivity analysis excluding trials at high risk of selection bias
The results of pooled studies were robust when excluding trials with a high risk of bias of selection bias: Clark 2000; Colón 1988; Dolak 2011; Eburne 1996; Khayambashi 2012; Khayambashi 2014; Loudon 2004; Thomee 1997 (results not shown).
Discussion
Summary of main results
This systematic review assessed the effects (benefits and harms) of exercise therapy aimed at reducing knee pain and improving knee function for people with patellofemoral pain syndrome. This review comprises 31 heterogeneous trials including 1690 participants with a diagnosis of patellofemoral pain syndrome. As well as variation in the patient characteristics and diagnostic criteria for study inclusion, the exercise interventions tested in the trials varied considerably. We assessed the evidence as being very low quality (seeQuality of the evidence). We based our assessment of clinical relevance on the following minimal clinically important differences: 1.3 points on a visual analogue scale (VAS) for pain during activity; 2.0 points on a VAS for usual and worst pain; 10.0 points on the Anterior Knee Pain Score (AKPS) and 2.0 points on the modified Functional Index Questionnaire (FIQ) (0 to 16) (Crossley 2004); and 15.0 points for the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Escobar 2006). In our summary of the main results for each comparison, we restrict our report to seven outcomes (pain during activity (short‐term: ≤ 3 months); usual pain (short‐term); pain during activity (long‐term: > 3 months); usual pain (long‐term); functional ability (short‐term); functional ability (long‐term); and recovery (long‐term)).
Exercise therapy versus control (no treatment, placebo or health educational material)
Although 10 studies compared exercise therapy versus control, we do not discuss the findings from Abrahams 2003 here because this trial also required participants to have patella malalignment and was thus presented separately in Effects of interventions. All nine trials stipulated a minimum duration of symptoms; this ranged from three weeks to six months. We assessed the quality of the available evidence as being of very low quality for each outcome (seeTable 1). Pooled data from five studies (375 participants) for pain during activity in the short term (four weeks to three months) favoured exercise therapy; the confidence interval, which did not cross the line of no effect, included the minimal clinically important difference pointing to the possibility of a clinically important effect. The same finding applied for pooled data from two studies (41 participants) for usual pain in the short term (four to eight weeks); for pooled data from two studies (180 participants) for pain during activity in the long term (12 months) and for data from a single study (94 participants) for usual pain in the long term (16 weeks). Pooled data from seven studies (483 participants) for functional ability in the short term (four weeks to three months) also favoured exercise therapy. In order to interpret the standardised mean difference results, we converted these to AKPS; the resulting confidence interval, which did not cross the line of no effect, included the minimal clinically important difference pointing to the possibility of a clinically important effect. The same finding applied to pooled data from three studies (274 participants) for functional ability in the long term (16 weeks to 12 months). Pooled data from two studies (166 participants) indicated that, based on the recovery of 250 per 1000 in the control group, 88 more (95% confidence interval (CI) 2 fewer to 210 more) participants per 1000 recovered in the long term (12 months) as a result of exercise therapy. It is important to note the very significant heterogeneity in the contributing trials and in the results for pain during activity and functional ability in the short term. However, sensitivity analyses did retain the positive findings for both of these outcomes, although the effect sizes were reduced.
Exercise therapy versus different unimodal or multimodal conservative interventions
All comparisons in this category are represented by single trials only, with no pooling undertaken because of the heterogeneity in the control groups (other conservative intervention).
Exercise therapy versus different unimodal interventions
Four trials provided very low quality and incomplete evidence for five comparisons of exercise therapy versus different unimodal conservative interventions.
One study (28 less active female participants; bilateral symptoms of at least six months duration) comparing hip exercises versus 1000 mg of Omega‐3 and 400 mg of calcium daily found a clinically important and highly statistically significant difference favouring the hip exercises group for pain during activity and functional ability in the short term (eight weeks).
One study (66 participants; symptoms of at least three weeks duration) comparing home exercises versus brace reporting on short‐term (three months) results found slightly lower pain during activity in the brace group and better functional ability in the exercises group. However, the confidence interval for pain during activity crossed the line of no effect and did not include the minimal clinically important difference. The confidence interval for functional ability also crossed the line of no effect but may have included a clinically important effect for exercise as well as a non‐clinically important effect for bracing.
One study (24 participants with symptoms of at least three months) comparing exercise therapy versus tape found lower pain during activity in the short term (three months) in the exercises group; the confidence interval, which did not cross the line of no effect, included a clinically important effect. A similar finding applied to pain during activity in the long term (12 months); however the confidence interval also crossed the line of no effect and a small but clinically irrelevant effect in favour of tape cannot be ruled out. The same pattern, in favour of exercise, applied to functional ability at short‐ and long‐term follow‐up. Slightly more participants in the exercise group had recovered by 12 months; the confidence interval crossed the line of no effect and thus a result in favour of taping cannot be ruled out.
One study (54 participants) comparing isometric exercises versus muscle electrostimulation found better functional ability in the short term (four weeks) in the exercise group; the confidence interval included a clinically important effect but also crossed the line of no effect and thus included a non‐clinically important effect in favour of muscle electrostimulation. The same observation applies to short‐term functional ability results from the comparison of isokinetic exercises versus muscle electrostimulation made in the same trial (68 participants).
Exercise therapy versus multimodal conservative interventions
Four trials provided very low quality and incomplete evidence for five comparisons of exercise therapy versus different multimodal conservative interventions.
One quasi‐randomised study (53 participants), which compared isometric quadriceps exercise versus the multimodal McConnell regimen comprising different types of exercises and taping, provided no usable quantitative data. It concluded that there was improvement in 50% of each group in the short term (three months). It also reported that three participants withdrew because of "severe allergy to the strapping" (presumably in the McConnell regimen group).
One study, which compared a supervised exercise programme versus a vastus medialis‐specific supervised exercise programme including taping found no clinically important difference between the two groups in usual pain in the short term (three months; 40 participants) or long term (12 months; 31 participants). In both cases the confidence intervals crossed the line of no effect and did not include the minimal clinically important difference. This study found over twice as many participants in the multimodal group had best function in the short term (52 participants overall). Conversely, the result at 12 months (33 participants) favoured the exercise group; however, the confidence intervals crossed the line of no effect.
The same study as above also compared a home exercise programme versus a vastus medialis‐specific supervised exercise programme including taping. For usual pain and functional ability at both short (42 and 52 participants respectively) and long‐term follow‐up (36 and 39 participants respectively), the confidence intervals crossed the line of no effect and, for usual pain, did not include the minimal clinically important difference.
One study (60 participants), which compared concentric exercises versus a multimodal intervention comprising excentric exercises and taping, found better functional ability (expressed in terms of the number of participants with improved function) and recovery in the short term (eight weeks follow‐up) in the multimodal group. In both cases, the confidence intervals crossed the line of no effect and thus a greater benefit from concentric exercises alone cannot be ruled out.
One study (40 active participants with symptoms for at least six months), which compared physiotherapeutic exercises based on proprioceptive neuromuscular facilitation versus a special knee resistance‐controlled knee splint combined with a special exercise programme, provided no data on the selected pain measures and incomplete data for functional ability at short‐term (eight weeks) follow‐up. It did not find a statistically or clinically significant difference between the two groups in pain at rest or functional ability.
Different exercises or exercise programmes
Delivery of exercises or exercise programmes: supervised versus home exercise
Two trials, one of which stipulated a minimum duration of symptoms of two months, provided very low quality evidence for this comparison (seeTable 2). Pooled data (59 participants) for usual pain in the short term (eight weeks or three months) marginally favoured supervised exercises but the confidence interval crossed the line of no effect and did not include the minimal clinically important difference for usual pain. The same observation applied to data from one study (31 participants) for usual pain in the long term (12 months). One study (18 active participants) found functional ability in the short term (eight weeks) slightly favoured home exercise; however, although the confidence interval included the minimal clinically important difference, it also crossed the line of no effect. The other trial (31 participants) reported higher numbers of participants with best function in the home group in the short term (one month; 48 participants) but the converse in the long term (12 months). In both cases, the confidence intervals crossed the line of no effect and thus a benefit from supervised exercises in the short term and home exercises in the long term cannot be ruled out.
Types of exercises or exercise programmes: closed kinetic chain exercises versus open kinetic chain exercises
This comparison was tested in four trials; the three providing quantitative data stipulated a minimum duration of symptoms (four, six and eight weeks respectively). We assessed all evidence for this comparison as being of very low quality (seeTable 3). Recovery was not reported. Although pooled data from two studies (90 participants) for pain during activity in the short term (six weeks or three months) marginally favoured open kinetic exercises, the confidence interval crossed the line of no effect and did not include the minimal clinically important difference. The same observation applied to pooled data from three studies (122 participants) for usual pain in the short term (four weeks to three months). In the long term (five years), one study (49 participants) found less pain during activity and usual pain in the open kinetic chain group; the confidence interval included a clinically important effect for the first outcome but not the second. Although pooled data from two studies (90 participants) for functional ability in the short term (six weeks or three months) marginally favoured open kinetic exercises, the confidence interval crossed the line of no effect and did not include the minimal clinically important difference. In the long term (five years), one study (49 participants) found better function in the open kinetic chain group; the confidence interval included a clinically important effect. It is important to note that data for long‐term effect were from one trial only and that data for functional ability were extracted from graphs for both trials reporting these data.
Types of exercises or exercise programmes: variants of closed kinetic chain exercises
Two trials provided very low quality and incomplete evidence for two different comparisons of variants of closed kinetic chain exercises. Neither trial reported on long‐term outcomes or recovery.
One trial (52 participants with a minimum duration of symptoms of eight months plus patella malalignment) comparing an exercise protocol with thigh adduction and tibia medial rotation during eccentric squat versus a traditional exercise protocol found better functional ability in the short term (six weeks) in the first intervention group; the confidence interval, which did not cross the line of no effect, included a clinically important effect.
One trial (40 female participants with symptoms for at least two months) comparing closed kinetic chain exercises with internally rotated hip versus closed kinetic chain exercises with externally rotated hip reported less pain during activity in the short term (four weeks) in the internally rotated group; the confidence interval included a clinically important effect but also crossed the line of no effect and included a non‐clinically important effect in favour of the externally rotated group. This trial reported better functional ability in the short term in the internally rotated group; the confidence interval, which did not cross the line of no effect, included a clinically important effect.
Types of exercises or exercise programmes: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action
Four trials provided very low quality and incomplete evidence for four different comparisons.
One study (40 female participants) comparing eccentric exercises versus concentric exercises found lower usual pain in the short term (12 weeks) for eccentric exercises; however, the confidence interval, which did not cross the line of no effect, excluded a clinically important effect. This study found better WOMAC scores in the short term for eccentric exercises; in this case the confidence interval, which did not cross the line of no effect, included a clinically important effect.
One study (40 female participants; symptoms for a minimum of six months) comparing eccentric exercises versus isometric exercises reported slightly fewer participants in the eccentric exercise group had pain during activity (jogging) in the short term (three months) and long term (12 months); the confidence intervals crossed the line of no effect and thus included the potential for an effect in favour of isometric exercises. All participants except one (group not identified) rated their knee function as excellent at 12 months.
One study (66 participants) comparing isokinetic exercises versus isometric exercises found a small and clinically non‐relevant between‐group difference in favour of isometric exercises in functional ability in the short term (four weeks). The confidence interval crossed the line of no effect and thus included the possibility of a better but probably not clinically important result after isokinetic exercises.
One study comparing combined isotonic and isometric exercises (pogo stick) versus isometric exercises reported only on recovery (more in the first group reported 50% or higher pain relief at eight weeks; 25 active participants) and adverse events (one person in the first group had increased pain; 27 active participants). Although favouring isotonic and isokinetic exercises, the confidence interval for recovery crossed the line of no effect and thus also included the possibility of a better result after isometric exercises.
Types of exercises or exercise programmes: proprioceptive neuromuscular facilitation stretching and aerobic exercise versus classic stretching and quadriceps exercises
Very low quality evidence from one trial (68 less active participants with a minimum duration of pain of six months) that reported only on usual pain and functional ability in the long term (16 weeks) showed a strong clinically important effect on both outcomes in favour of proprioceptive neuromuscular facilitation stretching and aerobic exercise compared with classic stretching and quadriceps exercises. The confidence intervals for both outcomes were located beyond the minimal clinically important differences.
Target of exercises or exercise programmes: hip and knee exercises compared with knee exercises
This comparison was tested in seven trials; the six providing quantitative data stipulated a minimum duration of symptoms (one month (three studies), two months (one study), three months (two studies)) (seeTable 4). Very low quality evidence pooled from three studies (104 participants) showed lower pain during activity in the short term (four weeks to three months) in the hip and knee exercise group compared with the knee exercises group; the confidence interval, which did not cross the line of no effect, included a clinically important effect. Very low quality evidence pooled from two studies (46 participants) showed lower usual pain in the short term (four or six weeks) in the hip and knee exercise group; the confidence interval, which did not cross the line of no effect, included a clinically important effect. Very low quality evidence pooled from one study (49 less active female participants) showed lower pain during activity in the long term (12 months) in the hip and knee exercise group compared with the knee exercise group; the confidence interval was located beyond the minimal clinically important difference of 1.3 points on a 0 to 10 scale. No study reported on usual pain in the long term. Very low quality evidence for functional ability in both the short term (four weeks to three months; four studies, 174 participants) and long term (5 or 12 months; two studies, 78 participants) was in favour of hip and knee exercises. However, both confidence intervals crossed the line of no effect and while including a clinically important effect in favour of hip and knee exercises there was also the potential for a non‐clinically important effect in favour of knee exercises. Very low quality evidence from one trial (29 active female participants) showed that long‐term (five months) recovery was greater in the hip and knee exercises group; however, the confidence interval also included the possibility of better recovery in the knee exercises group.
Target of exercises or exercise programmes: hip exercises compared with knee exercises
This comparison was tested in two studies, both of which stipulated a minimum duration of symptoms (one and six months respectively). Neither trial reported on usual pain or recovery (seeTable 5). Very low quality evidence from one quasi‐randomised trial (36 less active participants) showed that hip exercises may reduce pain during activity to a greater extent compared with knee exercise in the short term (eight weeks) and long term (six months); the confidence intervals at both time points included a clinically important effect. The short‐term result also included the potential for a small clinically non‐relevant difference in favour of knee exercises, whilst the confidence interval for the long‐term result did not cross the line of no effect. Very low quality evidence from two studies (58 participants) showed that hip exercises may improve functional ability in the short term (eight weeks or three months) compared with knee exercises; the confidence interval, which did not cross the line of no effect, included a clinically important effect. Very low quality evidence from one quasi‐randomised trial (36 less active participants) showed that hip exercises may improve functional ability in the long term (six months) compared with knee exercises; the confidence interval, which did not cross the line of no effect, included a clinically important effect.
Intensity of exercises
There is very low quality evidence from one trial (40 participants with untreated patellofemoral pain syndrome (PFPS) of over two months in duration) that a 12‐week long high‐intensity exercise programme is more effective than a 12‐week long low‐intensity exercise programme in reducing usual pain and improving functional ability in the short term (three months) and the long term (12 months) (seeTable 6). However, the confidence intervals for usual pain (short‐term) and functional ability (short‐ and long‐term), which did not cross the line of no effect, included both a non‐clinically important effect and a clinically important effect. The confidence interval for usual pain (long‐term) was located beyond the minimal clinically important difference of 2.0 points on a 0 to 10 scale. Pain during activity and recovery were not reported.
Overall completeness and applicability of evidence
This multi‐comparison review comprised 31 heterogeneous trials including 1690 participants with a diagnosis of patellofemoral pain syndrome. The largest comparison (exercise versus control (no exercise)) was tested in 10 trials but the largest analysis in this review, which was for this comparison, included data from only 483 participants (Analysis 1.6). There were no trials testing the medium of exercise or duration of exercises. Many other comparisons, notably those comparing exercise with other conservative interventions and different intensities of exercise were tested in small single trials only.
The inclusion criteria of the included trials were diverse. In the majority of trials, the diagnosis of PFPS was based on a set of clinical criteria and most trials excluded other knee pathologies (seeTable 8). The clinical diagnosis was made by a variety of clinical practitioner disciplines and together with the absence of a gold standard diagnostic test, differences in examination and judgements of suitability for inclusion are inevitable. Nonetheless, we judged that it was very likely that there was sufficient similarity in the underlying condition (i.e. all had PFPS) in participants recruited into all trials to warrant pooling where data were available. A notable exception was Abrahams 2003, since participants of this trial also had to be diagnosed with malalignment. We presented data for this trial separately. Otherwise, we made the decision to pool data despite the heterogeneity in the characteristics of the trial populations. Most trials studied the general population, but some focused on specific populations, such as sedentary individuals (Fukuda 2010; Fukuda 2012; Khayambashi 2012), and people who did not engage in regular sports activity (Moyano 2013; Song 2009), compared with more active patients who participated in sports for at least 120 minutes/week (Loudon 2004) and recreational athletes (Colón 1988; De Marche 2014; Schneider 2001). Some studies included only males or females or people who had not undergone previous physiotherapy. The minimum duration of the compliant or symptoms was specified as an inclusion criterion in the majority of trials but varied from a few weeks to several months. This diversity in baseline characteristics of the trial participants hampers the applicability of the results but the main assumption that these trials were testing the effects of exercise for the same underlying condition is key to consideration of applicability.
The variety of the exercises tested by different trials for the same comparison is shown by an inspection of Analysis 1.1, where six different types of exercise, tested in five trials, were compared with no treatment. The heterogeneity in the types of exercise together with the lack of or insufficient data available for direct comparisons of different types of exercise means that the interpretation of the applicability of the results should be levelled at generic exercise and not at specific types of exercise.
Outcome measures
Although there was also considerable heterogeneity in outcome measurement, most trials reported scores for pain during activity, usual pain (pain in daily life) and worst pain. We selected 'pain during descending' when pooling pain during activities because this again was frequently reported. Most studies reported functional ability with the Anterior Knee Pain Score (AKPS), (modified) FIQ or Lower Extremity Function Scale (LEFS). If multiple measures were reported, including the AKPS score, we used the latter for pooling as this score is reliable, valid and responsive when measuring the effect of therapy for PFPS (Crossley 2004). Some studies reported function with scores initially designed for other purposes, such as knee instability (Lysholm score) or osteoarthritis (WOMAC). When assessing the quality of the evidence from these different measures of functional ability, whether presented alone or pooled in a meta‐analysis, we did not downgrade the evidence for indirectness because all of these measures, when presented as continuous outcomes, can be considered to be directly related to functional ability for people with PFPS. This is in contrast to recovery, which was assessed in different ways by the eight studies that reported on recovery. Notably, Van Linschoten 2009 found the effects of exercise on pain and function scores were not reflected in the effect on self reported recovery between groups. Van Linschoten 2009 commented on the difficulties in "understanding what exactly comprises recovery from the patient's point of view". Furthermore, incomplete recovery might reflect the true nature of PFPS (Blond 1998; Kannus 1999; Nimon 1998). Hence, self reported recovery can give additional insights on the natural course of PFPS or the effects of therapeutic interventions, since it cannot be fully understood by pain and function outcomes alone. Functional performance tests might also contribute in assessing a patient's 'recovery', as the ultimate goal of rehabilitation is return to the highest functional level. These tests are widely used in other sport‐related injuries (Loudon 2002) and could be of use in patellofemoral pain research. However, standardisation is needed since the studies that performed these tests could not be pooled because they did not perform similar tests.
Applicability
The implications of pooling data from trials with different inclusion criteria and different exercise therapies, in particular for the comparison of exercise therapy versus control, means that only a general interpretation should be made in terms of the population (people diagnosed with PFPS) and the intervention (exercise therapy). This does not rule out that some subgroups of patients may benefit from a certain intervention while others may not (Witvrouw 2014), nor that some exercise interventions may be more effective or, indeed, that some may not be effective. Direct comparisons of different exercise interventions should help inform this issue but, although several trials have compared different exercises, the current evidence is very poor quality and does not provide definitive answers.
The studies on exercise therapy reflect the changing opinions through the years concerning preferred treatment strategy. For example, in the late 1970s and mid 1980s questions arose about the effect and possible side effects of open and closed kinetic chain exercises for PFPS. The very low quality evidence available in this review generally favoured open kinetic exercise but did not establish there being a clinically important difference between these two approaches. Around the turn of the 21st century there was increased interest in the delivery of exercises, in particular supervised versus home exercises. The very low quality evidence available on this comparison did not establish a difference between these two approaches. In the last decade, attention has shifted to hip exercises with or without knee exercises. Again there is only very low quality evidence to inform on the choice of hip plus knee versus knee only exercises or hip versus knee exercises. The available evidence tends to favour hip plus knee exercises or hip exercises with the potential for a clinically important effect on pain and function; but again is not definitive. Lastly, although one study provides evidence that a high‐intensity exercise programme is more effective than a low‐intensity exercise programme for patients with untreated PFPS of over two months in duration (Østeråsa 2013), such a finding needs verification by further research and in a more general population.
Besides exercise, many other interventions are used for PFPS. Only very poor quality and generally incomplete evidence from single trials was available for comparisons of exercise therapy versus different unimodal or multimodal conservative treatment strategies. In terms of applicability, the focus should be on conservative treatment strategies in common use; the evidence base for such treatments, such as taping, also needs consideration (Callaghan 2012). This review did not aim to investigate the additional value of other strategies when they are combined with exercise therapy.
Quality of the evidence
In the previous systematic review by Heintjes 2003, the authors pointed to the need for higher quality in study methodology and reporting. This need continues as several of the newly included studies were at high or unclear risk of bias for multiple domains (Figure 2), including selection bias reflecting the use of quasi‐randomisation methods in two recently published trials. We assessed most trials as being at high risk of performance bias and detection bias; although blinding is generally impractical for exercise trials, some measures such as standardisation of interactions between personnel and patients can still be taken to reduce bias.
Overall, the quality of the evidence, expressed using GRADE terminology, varies between 'low quality' ("Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate") and 'very low quality' ("We are very uncertain about the estimate"). All the evidence for the outcomes presented in our 'Summary of findings' tables was very low quality. In our assessment of the quality of the evidence according to the GRADE guidelines, downgrading resulted from risk of bias (primarily relating to sequence generation, allocation concealment and assessor blinding), imprecision (wide confidence intervals and small sample size), inconsistency (significant heterogeneity) and indirectness (here this was used only for inadequate outcome measures). In some cases we downgraded our assessment of the quality of the evidence by two levels for serious risk of bias, serious imprecision and/or serious inconsistency. In assessing imprecision, we planned to downgrade one level where there were fewer than 400 cases for continuous data or fewer than 300 cases for dichotomous data. More often, however, downgrading was based on an assessment of the spread of the 95% confidence interval or that the evidence was available solely from one small study, often with a large effect size.
We did not downgrade for indirectness relating to patient characteristics because the results are 'direct' when the focus is on patients with PFPS. We avoided the problem of indirectness associated with Abrahams 2003, which focused on a different population by including only patients with a diagnosed malalignment, by not pooling this study with other studies comparing exercise versus a control strategy. Some studies focused on different predefined activity‐based populations (less active or active) or included only males or females or patients without previous physiotherapy. Where studies included a more specific population, we took this into consideration by stating the specific population in the case of single studies and checking for heterogeneity in the case of pooled studies.
Potential biases in the review process
With some exceptions, as detailed in Differences between protocol and review, we conducted this review in accordance with our previously published protocol (van der Heijden 2013). Although the changes to the protocol were often prompted by our review of the evidence (for example, the division of the comparison 'exercise therapy versus different conservative interventions' into two separate comparisons), we strived to avoid bias by establishing the new rules and methods prior to our interpretation of the evidence. Although we conducted a comprehensive literature search and were systematic and over‐inclusive in our screening process, it is likely that we failed to identify some, particularly unpublished, small single‐centre trials. It is not possible to determine the bias resulting from this but it is notable that we have found only one ongoing trial; another small trial awaits classification pending translation.
Agreements and disagreements with other studies or reviews
We have found four recently published systematic reviews investigating the effects of exercise therapy for PFPS (Bolgla 2011; Collins 2012; Frye 2012; Harvie 2011). The scopes and inclusion criteria of all four reviews differed substantively from our review. For example, Bolgla 2011 and Frye 2012 also included cohort and case‐control studies. Harvie 2011 set out to examine the "parameters of exercise programs reported in primary research", and thus excluded randomised controlled trials (RCTs) that did not show an effect of exercise therapy. Collins 2012 included RCTs comparing all types of non‐surgical interventions, including acupuncture, electromyography and taping.
Checks of the RCTs included in the four reviews did not reveal any that were missing from our review. Moreover, our review includes more trials, which also reflects our more up‐to‐date search. All four reviews assessed the quality of their included studies with a quality scale. Frye 2012 and Harvie 2011 used the PEDro scale. Collins 2012 used a modified version of the PEDro scale, and Bolgla 2011 used the Strength of Recommended Taxonomy (Ebell 2004). However, the use of quality scales is not recommended, because these scales are inconsistent and unpredictable (Higgins 2011). Other choices, such as pooling and presentation of the results and transparency of the reporting (for instance, it was unclear which studies were pooled in Frye 2012) also differed amongst the four reviews and with our review. Inspection of all four reviews mainly revealed the diversity in the approaches taken by the investigators and did not yield additional insights relating to exercise therapy.
Authors' conclusions
Implications for practice.
This review has found very low quality but consistent evidence that exercise therapy for patellofemoral pain syndrome (PFPS) may result in clinically important reduction in pain and improvement in functional ability, as well as enhancing long‐term recovery. However, the best form of exercise therapy and whether this result would apply to all people with PFPS are unknown.
There is insufficient evidence to draw conclusions about the relative effects of exercise versus other conservative interventions, either unimodal (e.g. taping) or multimodal (combinations of interventions that may include different exercises to the exercise intervention). The very low quality evidence for each comparison examined by the included trials was from small single trials only.
The very low quality evidence available for comparisons of different exercises was insufficient to draw conclusions on the relative effects of supervised versus home exercises; closed versus open kinetic chain exercises; different variants of closed kinetic chain exercises; other comparisons of other types of kinetic chain exercises; proprioceptive neuromuscular facilitation stretching and aerobic exercise versus classic stretching and quadriceps exercises; hip versus knee exercises; and high‐ versus low‐intensity exercises. There is some very low quality evidence that hip plus knee exercises may be more effective in reducing pain than knee exercise alone, but the relative effect of these two exercise types on functional ability is uncertain. There is a lack of evidence from randomised controlled trials on exercise medium (land versus water) and duration of exercises.
Implications for research.
Further randomised trials, which conform to international standards in their design, conduct and reporting, are needed. However, to optimise research effort and underpin the large multicentre randomised trials that are required to inform practice, it is preferable to precede this with research that aims to identify priority questions and attain agreement on these and, where practical, standardisation regarding diagnostic criteria and measurement of outcome. The selection of priority areas for research should take into account the current coverage of the evidence, current practice and differences in practice, and should involve consultation with patients as to their preferences and values. Achieving professional consensus on treatment uncertainties should facilitate sufficient centre recruitment into multicentre trials and also implementation of their findings.
Although the identification of priority topics requires input from others, we make a few suggestions drawing from the evidence in this review. First, although we accept that the underpinning evidence for the effectiveness of exercise therapy, while consistent in effect direction, is of very poor quality, we suggest that research should be directed at comparisons of different exercises rather than comparisons of exercise therapy versus control. In our perception, recent trends in clinical practice for patellofemoral pain syndrome are moving towards protocols featuring combined knee and hip exercise programmes and high‐intensity exercise programmes. Both trends are insufficiently evidenced and thus further evaluation by randomised trials on these seems warranted. Linked with this is the need to determine whether there are important differences in the effectiveness of exercise or different types of exercise in different patient populations. This points to the need for clear definitions of patient characteristics and pre‐specified subgroups in trials, such as by pre‐PFPS activity level, which can help to inform on potential variation in the effects of exercise therapy.
In terms of outcomes, we suggest that consideration is given to standardising pain during a patient‐nominated activity and, until a better instrument is developed, using the Anterior Knee Pain Score (AKPS) (Kujala 1993) to assess functional ability in future studies. The natural course of patellofemoral pain syndrome varies considerably and more research is needed to identify the risk factors for prolonged pain and functional deficit, and the potential association with degenerative joint disease. As evidenced in this review, not all patients show full recovery and thus the development of a validated outcome measure that captures patient‐rated recovery seems warranted.
Acknowledgements
We thank the editors, Nigel Hanchard and Helen Handoll, for their extensive support and feedback on the review and the external referees, Michael Callaghan and James Selfe, for their helpful comments. We thank Joanne Elliott for her help with the search strategies and for providing search updates, and both Joanne and Lindsey Elstub for their helpful feedback and support on the review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (Wiley Online Library)
#1 MeSH descriptor: [Patellofemoral Pain Syndrome] this term only (68) #2 MeSH descriptor: [Patella] this term only (243) #3 MeSH descriptor: [Knee Joint] explode all trees (2304) #4 MeSH descriptor: [Knee] this term only (573) #5 #2 or #3 or #4 (2957) #6 MeSH descriptor: [Arthralgia] this term only (466) #7 MeSH descriptor: [Pain] explode all trees (32936) #8 #6 or #7 (32936) #9 #5 and #8 (710) #10 anterior knee pain:ti,ab,kw (353) #11 (patell* or femoropatell* or femoro‐patell* or retropatell*) near/2 (pain or syndrome or dysfunction):ti,ab,kw (284) #12 ((lateral compression or lateral facet or lateral pressure or odd facet) near/2 syndrome):ti,ab,kw (0) #13 (chondromalac* or chondropath* or chondrosis) near/2 (knee* or patell* or femoropatell* or femoro‐patell* or retropatell*):ti,ab,kw (31) #14 MeSH descriptor: [Chondromalacia Patellae] this term only (5) #15 #1 or #9 or #10 or #11 or #12 or #13 or #14 (1185) #16 MeSH descriptor: [Exercise Therapy] explode all trees (7116) #17 MeSH descriptor: [Exercise] explode all trees (13885) #18 exercis* or strengthen* or stretch* or train* or physiotherapy or physical therap*:ti,ab,kw (70701) #19 #16 or #17 or #18 (71833) #20 #9 and #15 and #19 in Trials (148)
MEDLINE (Ovid Online)
1 Patellofemoral Pain Syndrome/ (453) 2 Patella/ or exp Knee Joint/ or Knee/ (56364) 3 Arthralgia/ or Pain/ (112939) 4 2 and 3 (3290) 5 anterior knee pain.tw. (1003) 6 ((patell* or femoropatell* or femoro‐patell* or retropatell*) adj2 (pain or syndrome or dysfunction)).tw. (1766) 7 ((lateral compression or lateral facet or lateral pressure or odd facet) adj2 syndrome).tw. (20) 8 ((chondromalac* or chondropath* or chondrosis) adj2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*)).tw. (513) 9 Chondromalacia Patellae/ (59) 10 or/1,4‐9 (5753) 11 exp Exercise Therapy/ or exp Exercise/ (140226) 12 (exercis* or strengthen* or stretch* or train* or physiotherapy or physical therap*).tw. (595688) 13 or/11‐12 (655179) 14 Randomized controlled trial.pt. (373732) 15 Controlled clinical trial.pt. (88369) 16 randomized.ab. (293610) 17 placebo.ab. (153908) 18 Drug therapy.fs. (1698370) 19 randomly.ab. (212608) 20 trial.ab. (304899) 21 groups.ab. (1353578) 22 or/14‐21 (3335964) 23 exp Animals/ not Humans/ (3938734) 24 22 not 23 (2860785) 25 and/10,13,24 (343)
EMBASE (Ovid Online)
1 Patellofemoral Pain Syndrome/ (678) 2 Patella/ or Patellofamoral Joint/ (6639) 3 Arthralgia/ or Pain/ (229980) 4 2 and 3 (518) 5 Knee Pain/ (7720) 6 anterior knee pain.tw. (1178) 7 ((patell* or femoropatell* or femoro‐patell* or retropatell*) adj2 (pain or syndrome or dysfunction)).tw. (2017) 8 ((lateral compression or lateral facet or lateral pressure or odd facet) adj2 syndrome).tw. (25) 9 ((chondromalac* or chondropath* or chondrosis) adj2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*)).tw. (601) 10 Patella Chondromalacia/ (581) 11 or/1,4‐10 (11083) 12 exp Exercise/ or exp Kinesiotherapy/ (228968) 13 (exercis* or strengthen* or stretch* or train* or physiotherapy or physical therap*).tw. (700876) 14 12 or 13 (777358) 15 exp Randomized Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ (384984) 16 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. (1230960) 17 15 or 16 (1303210) 18 (exp Animal/ or Animal.hw. or Nonhuman/) not (exp Human/ or Human Cell/ or (human or humans).ti.) (5041638) 19 17 not 18 (1144157) 20 11 and 14 and 19 (471)
CINAHL (EBSCO)
S1 (MH "Patellofemoral Pain Syndrome") (915) S2 (MH "Patella") OR (MH "Knee") OR (MH "Knee Joint") (15,082) S3 (MH "Arthralgia") and (MH "Pain") (60) S4 S2 AND S3 (10) S5 TX anterior knee pain (436) S6 TX ((patell* or femoropatell* or femoro‐patell* or retropatell*) n2 (pain or syndrome or dysfunction)) (1,263) S7 TX ((lateral compression or lateral facet or lateral pressure or odd facet) n2 syndrome) (7) S8 TX ((chondromalac* or chondropath* or chondrosis) n2 (knee* or patell* or femoropatell* or femoro‐patell* or retropatell*)) (107) S9 (MH "Chondromalacia Patella") (61) S10 S1 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 (1,626) S11 (MH "Therapeutic Exercise+") and (MH "Exercise+") (18,366) S12 (exercis* or strengthen* or stretch* or train* or physiotherapy or physical therap*) (249,334) S13 S11 OR S12 (249,543) S14 PT clinical trial (75,963) S15 (MH "Clinical Trials+") (174,859) S16 TI clinical trial* OR AB clinical trial* (41,307) S17 TI ( (single blind* or double blind*) ) OR AB ( (single blind* or double blind*) ) (19,881) S18 TI random* OR AB random* (136,297) S19 S14 OR S15 OR S16 OR S17 OR S18 (255,533) S20 S10 AND S13 AND S19 (147)
AMED (Ovid Online)
1 Patellofemoral pain syndrome/ (58) 2 Patella/ or Knee/ or Knee Joint/ (4479) 3 Pain/ or Arthralgia/ (10265) 4 2 and 3 (631) 5 anterior knee pain.tw. (128) 6 ((patell* or femoropatell* or femoro‐patell* or retropatell*) adj2 (pain or syndrome or dysfunction)).tw. (449) 7 ((lateral compression or lateral facet or lateral pressure or odd facet) adj2 syndrome).tw. (1) 8 ((chondromalac* or chondropath* or chondrosis) adj2 (knee*1 or patell* or femoropatell* or femoro‐patell* or retropatell*)).tw. (29) 9 or/1,4‐8 (905) 10 Randomized controlled trial.pt. (2931) 11 Controlled clinical trial.pt. (70) 12 Randomized Controlled Trials/ (1658) 13 Random Allocation/ (311) 14 Double‐Blind Method/ (506) 15 or/10‐14 (5218) 16 exp Animals/ not Humans/ (7553) 17 15 not 16 (5189) 18 Clinical trial.pt. (1160) 19 exp Clinical trials/ (3368) 20 (clinic* adj25 trial*).tw. (5872) 21 ((singl* or doubl* or trebl* or tripl*) adj (mask* or blind*)).tw. (2343) 22 Placebos/ (547) 23 placebo*.tw. (2655) 24 random*.tw. (14183) 25 exp Research design/ (17924) 26 (latin adj square).tw. (24) 27 or/18‐26 (31604) 28 27 not 16 (31059) 29 28 not 17 (26011) 30 9 and 29 (174)
Appendix 2. Exercise therapy versus control (no treatment, 'placebo' or waiting list)
| Study ID (arranged by date) | Exercise therapy | Control group | Notes |
| Clark 2000 | Supervised exercise programme + home exercises | No treatment | Additional intervention in both groups: education |
| Clark 2000 | Supervised exercise programme + home exercises | No treatment | Additional intervention in both groups: tape |
| Abrahams 2003 | Traditional exercise protocol | Waiting list | Inclusion criteria: malalignment as diagnosed by X‐ray |
| Abrahams 2003 | Exercise protocol with thigh adduction and tibia medial rotation during eccentric squat | Waiting list | Inclusion criteria: malalignment as diagnosed by X‐ray |
| Taylor 2003 | Isometric + eccentric exercises | No treatment | Additional intervention in both groups: patella mobilisation/manipulation |
| Loudon 2004 | Supervised exercises | No treatment | — |
| Loudon 2004 | Home exercises | No treatment | — |
| Lun 2005 | Home exercise programme | No treatment | Additional intervention in both groups: brace |
| Herrington 2007 | Closed kinetic chain exercises | No treatment | — |
| Herrington 2007 | Open kinetic chain exercises | No treatment | — |
| Song 2009 | Quadriceps exercises | Health educational material | — |
| Song 2009 | Knee + hip exercises | Health educational material | — |
| Van Linschoten 2009 | Supervised exercise programme + home exercises | No treatment | Additional intervention in both groups: written information about patellofemoral pain syndrome and general instructions for home exercises |
| Fukuda 2010 | Knee exercises | No treatment | — |
| Fukuda 2010 | Knee + hip exercises | No treatment | — |
| Moyano 2013 | Classic stretching and quadriceps exercises | Health educational material | — |
| Moyano 2013 | Proprioceptive neuromuscular facilitation stretching and aerobic exercise | Health educational material | — |
Appendix 3. Exercise therapy versus different conservative interventions
| Study ID (arranged by date) | Exercise therapy | Control group | Notes |
| 2a. Exercise therapy versus unimodal conservative interventions | |||
| Gobelet 1992 | Isokinetic exercise programme | Quadriceps electrostimulation | — |
| Gobelet 1992 | Isometric exercise programme | Quadriceps electrostimulation | — |
| Clark 2000 | Exercise therapy | Tape | Additional intervention in both groups: education |
| Lun 2005 | Home exercise programme | Brace | — |
| Khayambashi 2012 | Hip exercises | 1000 mg of Omega‐3 and 400 mg of calcium | — |
| 2b. Exercise therapy versus multimodal conservative interventions | |||
| Gaffney 1992 | Concentric exercises | Excentric exercises and tape | — |
| Eburne 1996 | Isometric quadriceps exercises | McConnell regimen: different types of exercises and tape | — |
| Harrison 1999 | Supervised exercise programme | Vastus medius specific exercise programme + taping | — |
| Harrison 1999 | Home exercise programme | Vastus medius specific exercise programme + taping | — |
| Schneider 2001 | Physiotherapeutic exercises based on proprioceptive neuromuscular facilitation | Special knee splint combined with exercises | — |
Appendix 4. Comparison of different exercises or exercise programmes
|
Study ID (arranged by date) |
Exercise protocol | Control group | Notes |
| 3a. Delivery of exercises or exercise programmes | |||
| Harrison 1999 | Supervised exercise programme | Home exercise programme | — |
| Loudon 2004 | Supervised exercises + home exercises | Home exercises + five physiotherapy sessions | — |
| 3b. Medium of exercises or exercise programmes | |||
| — | — | — | — |
| 3c. Types of exercises or exercise programmes | |||
| Gobelet 1992 | Isokinetic exercise programme | Isometric exercise programme | — |
| Thomee 1997 | Eccentric exercises | Isometric exercises | — |
| Colón 1988 | Isotonic exercises (pogo stick) | Isometric exercises | — |
| Witvrouw 2000 | Closed kinetic chain exercises | Open kinetic chain exercises | — |
| Abrahams 2003 | Exercise protocol with thigh adduction and tibia medial rotation during eccentric squat | Traditional exercise protocol | — |
| Herrington 2007 | Weight‐bearing exercises = closed kinetic chain | Non weight‐bearing exercises = open kinetic chain | — |
| Bakhtiary 2008 | Closed kinetic chain exercise programme | Open kinetic chain exercise programme | — |
| Balci 2009 | Closed kinetic chain exercises with internally rotated hip | Closed kinetic chain exercises with externally rotated hip | — |
| Abd Elhafz 2011 | Closed kinetic chain exercises | Open kinetic chain exercises | Additional intervention both groups:medial patellar taping |
| Hafez 2012 | Eccentric exercises | Concentric exercises | — |
| 3d. Target of exercises or exercise programmes | |||
| Avraham 2007 | Knee + hip exercises | Knee exercises | Additional intervention both groups: TENS No data available |
| Nakagawa 2008 | Quadriceps + hip exercises | Quadriceps exercises | Additional intervention both groups: patellar mobilisation |
| Song 2009 | Knee + hip exercises | Knee exercises | — |
| Razeghi 2010 | Knee + hip exercises | Knee exercises | — |
| Fukuda 2010 | Knee + hip exercises | Knee exercises | — |
| Dolak 2011 | Hip exercises | Quadriceps exercises | — |
| Fukuda 2012 | Knee + hip exercises | Knee exercises | — |
| De Marche 2014 | Knee + hip exercises | Quadriceps exercises | — |
| Khayambashi 2014 | Hip exercises | Quadriceps exercises | — |
| 3e. Duration of exercises or exercise programmes | |||
| — | — | — | — |
| 3f. Intensity of exercises or exercise programmes | |||
| Østeråsa 2013 | High‐dose, high‐repetition medical exercise therapy (MET) | Low‐dose, low‐repetition exercise programme | — |
Data and analyses
Comparison 1. Exercise therapy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during activity (short‐term) | 5 | 375 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐2.39, ‐0.54] |
| 1.1 Knee + hip exercises versus no treatment | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.30, ‐0.30] |
| 1.2 Knee exercise versus no treatment | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.30, 1.10] |
| 1.3 Exercise therapy versus no treatment | 2 | 202 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.49, ‐0.19] |
| 1.4 Home exercise programme versus no treatment | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.12, 1.12] |
| 1.5 Closed kinetic chain exercise programme versus no treatment | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐5.47, ‐2.53] |
| 1.6 Open kinetic chain exercise programme versus no treatment | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐3.29 [‐4.89, ‐1.69] |
| 2 Usual pain (short‐term) | 2 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.60, ‐0.25] |
| 2.1 Exercise therapy versus no treatment (all had patella manipulation) | 1 | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.54 [‐2.90, ‐0.18] |
| 2.2 Supervised exercise programme versus no treatment | 1 | 14 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.79, 0.47] |
| 2.3 Home exercise programme versus no treatment | 1 | 15 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.86, 0.31] |
| 3 Worst pain (short‐term) | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐2.28 [‐3.33, ‐1.23] |
| 3.1 Exercise therapy versus no treatment (all had patella manipulation) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.92 [‐4.20, 0.36] |
| 3.2 Knee + hip exercises versus health educational material | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐3.87, ‐0.51] |
| 3.3 Knee exercise versus health educational material | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐4.21, ‐0.89] |
| 4 Pain during activity (long‐term) | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐1.93, ‐0.21] |
| 4.1 Exercise therapy versus no treatment | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐1.93, ‐0.21] |
| 5 Usual pain (long‐term) | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐4.32 [‐7.75, ‐0.89] |
| 5.1 Classic stretching + quadriceps exercises versus health educational material | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐3.44, ‐1.70] |
| 5.2 Proprioceptive neuromuscular facilitation + aerobic exercise versus health educational material | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐6.07 [‐6.92, ‐5.22] |
| 6 Functional ability (short‐term) | 7 | 483 | Std. Mean Difference (IV, Random, 95% CI) | 1.10 [0.58, 1.63] |
| 6.1 Exercise therapy versus no treatment | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.03, 0.59] |
| 6.2 Supervised exercise programme versus no treatment | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 1.29 [0.06, 2.52] |
| 6.3 Home exercise programme versus no treatment | 2 | 79 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐1.08, 1.90] |
| 6.4 Closed kinetic chain exercise programme versus no treatment | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | 5.93 [3.86, 8.00] |
| 6.5 Open kinetic chain exercise programme versus no treatment | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 3.43 [1.99, 4.86] |
| 6.6 Knee + hip exercises versus no treatment | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.96 [0.19, 1.74] |
| 6.7 Knee + hip exercises versus health educational material | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.05 [0.35, 1.76] |
| 6.8 Knee exercise versus no treatment | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 1.21 [0.43, 2.00] |
| 6.9 Knee exercise versus health educational material | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [0.28, 1.72] |
| 7 Functional ability (short‐term); all participants had malalignment | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.24, ‐0.56] |
| 7.1 Standard exercise versus no treatment | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.80, 0.80] |
| 7.2 Exercise protocol with thigh adduction versus no treatment | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐3.00, ‐1.00] |
| 8 Functional ability (long‐term) | 3 | 274 | Std. Mean Difference (IV, Random, 95% CI) | 1.62 [0.31, 2.94] |
| 8.1 Exercise therapy versus no treatment | 2 | 180 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.02, 0.56] |
| 8.2 Proprioceptive neuromuscular facilitation + aerobic exercise versus no treatment | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | 6.16 [4.70, 7.63] |
| 8.3 Classic stretching + quadriceps exercises versus health educational material no treatment | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 1.60 [0.88, 2.32] |
| 9 Functional performance (short‐term) single‐limb hop test | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.73 [‐3.35, 20.80] |
| 9.1 Knee + hip exercises versus no treatment | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 11.5 [‐5.99, 28.99] |
| 9.2 Knee exercise versus no treatment | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐10.49, 22.89] |
| 10 Functional performance (short‐term) bilateral squat test | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [‐1.68, 3.84] |
| 10.1 Supervised exercise programme versus no treatment | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.04, 3.04] |
| 10.2 Home exercise programme versus no treatment | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐0.88, 6.68] |
| 11 Recovery (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Exercise therapy versus no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Recovery (long‐term) | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.84] |
| 12.1 Exercise therapy versus no treatment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.84] |
Comparison 2. Exercise therapy versus unimodal conservative interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during activity (short‐term) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Hip exercises versus Omega 3 + calcium | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Home exercise programme versus brace | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Exercise therapy versus tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain during activity (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Exercise therapy versus tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Functional ability (short‐term) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Hip exercises versus Omega 3 + calcium | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Home exercise programme versus brace | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Exercise therapy versus tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Isometric exercises versus muscle electrostimulation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Isokinetic exercises versus muscle electrostimulation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Functional ability (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Exercise therapy versus tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Recovery (long‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Exercise therapy versus tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Exercise therapy versus multimodal conservative interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (short‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Usual pain: supervised exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Usual pain: home exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Worst pain: supervised exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Worst pain: home exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 At rest: proprioceptive exercises versus special knee splint + exercises | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 After exposure: proprioceptive exercises versus special knee splint + exercises | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Usual pain: supervised exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Usual pain: home exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Worst pain: supervised exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Worst pain: home exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Functional ability (short‐term) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Concentric exercises versus excentric exercises and tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Supervised exercise versus VM specific exercise + tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Home exercise versus VM specific exercise + tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Functional ability (long‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Supervised exercise versus VM specific exercise + tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Home exercise versus VM specific exercise + tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Recovery (short‐term) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Concentric exercises versus excentric exercises and tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Supervised exercise versus VM specific exercise + tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Home exercise versus VM specific exercise + tape | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Functional performance (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Step test: supervised exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Step test: home exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Functional performance (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Step test: supervised exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Step test: home exercise versus VM specific supervised exercise + tape | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Delivery of exercise: supervised versus home exercise programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Usual pain (short‐term) | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐1.22, 0.77] |
| 2 Worst pain (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Pain (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Usual pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Worst pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Functional ability (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Functional ability (short and long‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Short‐term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Long‐term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Functional performance (short‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Step test: time until pain (seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Bilateral squat test: number completed in 30 seconds | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Functional performance (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Step test: time until pain (seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Recovery (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Types of exercises: closed kinetic chain exercises versus open kinetic chain exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during activity (short‐term) | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.63, 0.70] |
| 2 Usual pain (short‐term) | 3 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.37, 0.76] |
| 3 Worst pain (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Pain during activity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Usual pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Worst pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Functional ability (short‐term) | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐3.51 [‐7.84, 0.82] |
| 6 Functional ability (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Functional performance (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Step‐down test (no symptoms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Step‐up test (no symptoms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Unilateral squat (no symptoms) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Functional performance (long‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Step‐down test (no symptoms) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.56] |
| 8.2 Step‐up test (no symptoms) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.69, 1.10] |
Comparison 6. Types of exercises: variants of closed kinetic chain exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during activity (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 CKC with hip internally rotated versus CKC with hip externally rotated | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional ability (short‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 CKC with thigh adduction versus standard CKC | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 CKC with hip internally rotated versus CKC with hip externally rotated | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Types of exercises: open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during activity (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Eccentric exercises versus isometric exercises | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Usual pain continuous (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Eccentric exercises versus concentric exercises | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pain during activity (long‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Eccentric exercises versus isometric exercises | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Functional ability (short‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Isokinetic exercises versus isometric exercises | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Eccentric exercises versus concentric exercises | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Recovery (short‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Isotonic and isokinetic exercises versus isometric exercises | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Isotonic and isokinetic exercises versus isometric exercises | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Types of exercises: proprioceptive neuromuscular facilitation + aerobic exercise versus classic stretching + quadriceps exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Usual pain (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Functional ability (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 9. Target of exercises: hip + knee versus knee exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during activity (short‐term) | 3 | 104 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐3.80, ‐0.60] |
| 2 Usual pain (short‐term) | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐2.78, ‐0.76] |
| 3 Worst pain (short‐term) | 3 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.66, 0.09] |
| 4 Pain (long‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Pain during activity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Worst pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Functional ability (short‐term) | 4 | 174 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.39, 1.61] |
| 6 Functional ability (long‐term) | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 1.49 [‐0.17, 3.15] |
| 7 Functional performance (short‐term) | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 13.89 [5.21, 22.56] |
| 8 Functional performance (long‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Single‐limb triple hop test (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Single‐limb hop test (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Recovery (short‐ and long‐term) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Short‐term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Long‐term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Target of exercises: hip versus knee exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (short‐ and long‐term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain during activity (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Worst pain (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pain during activity (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional ability (short‐term) | 2 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.30, 1.40] |
| 3 Functional ability (long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Functional performance (short‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 11. Intensity of exercise: high‐ versus low‐intensity exercise programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Usual pain (short‐ and long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Short‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Long‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional ability (short‐ and long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Short‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Long‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Functional performance (short‐ and long‐term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Short‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Long‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd Elhafz 2011.
| Methods | Design: RCT, randomisation method not reported Objectives: to compare the combined effect(s) of taping and open kinetic chain (OKC) versus taping and closed kinetic chain (CKC) exercises in patients with patellofemoral pain syndrome (PFPS) |
|
| Participants | Data collection period: not reported Recruitment setting: selected from patients' files of physiotherapy clinic; Egypt Inclusion: diffuse, unilateral anterior knee pain for at least 8 weeks, exacerbated by activity and isometric quadriceps contraction Exclusion: history of lower limb surgery, deformities or patellar fractures or dislocations 30 patients, 30% female, mean age 35.83 years (± 5.36), BMI not reported, duration of complaints not reported, all unilateral complaints 1) n = 15 2) n = 15 |
|
| Interventions | Setting of intervention: physiotherapy clinic Duration: 4 weeks, 3 times per week Supervisor of the interventions: not reported 1) open kinetic chain exercises: flexion, straight leg raise from supine, isometric exercise of the quadriceps from supine, short arc knee extension from sitting position, 30 degrees flexion to full extension 2) closed kinetic chain exercises: leg press machine, mini squats, squat‐to‐stand and stand‐to‐squat tasks, forward step‐up exercise on stairs Additional intervention both groups: medial patellar taping |
|
| Outcomes | Baseline, 4 weeks Pain: VAS (0 to 10), usual Adverse events: not actively sought |
|
| Notes | Types of exercises or exercise programmes: 2 = experimental, 1 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Impossible for exercise therapy; patients were blinded; unaware about number of groups, randomisation technique, or interventions for each group; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout; no cross‐over |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no functional ability data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Abrahams 2003.
| Methods | Design: RCT, randomisation method not reported Objectives: to investigate the effects of a functional semi‐squat, utilising medial rotation of the tibia and adduction of the thigh in patients with patellofemoral joint pain compared to current standard physiotherapy exercises (neutral semi squat) |
|
| Participants | Data collection period: 1999 to 2002 Recruitment setting: referred by an experienced orthopaedic knee surgeon; United Kingdom Inclusion: unilateral PFPS for 8 to 18 months; retropatellar or anterior knee pain; pain on squatting; positive direct patellofemoral grind test; malalignment as diagnosed by X‐ray Exclusion: previous trauma or surgery of the knee; history of (sub) luxation, rheumatologic neurologic or intra‐articular pathology of the knee 78 patients, 50% female, duration of complaints not reported, all unilateral complaints 1) n = 26, mean age 30.3 (± 13.95), mean BMI 22.59 (± not reported) 2) n = 26, mean age 26.1 (± 14.53), mean BMI 25.89 (± not reported) 3) n = 26, mean age 30.5 (± 12.49), mean, BMI 25.78 (± not reported) |
|
| Interventions | Setting of intervention: not reported Duration: 6 weeks Supervisor of the interventions: not reported 1) Traditional exercise protocol: semi squat in neutral to 30 degrees knee flexion held for 2 seconds with subsequent straightening of the knee and rising: 15 repetitions, 3 times daily 2) Same exercise protocol with thigh adduction and tibia medial rotation during eccentric squat: 15 repetitions, 3 times daily 3) Waiting list |
|
| Outcomes | Baseline, 3 weeks, 6 weeks Function: MFIQ (0 to 16) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 1 = experimental versus 3 = control Exercise therapy versus control: 2 = experimental versus 3 = control Types of exercises or exercise programmes: 2 = experimental versus 1 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar, but waiting list intervention clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropout; cross‐over not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no pain data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables; outcome variables seemed to be similar |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Low risk | Subjects who started a co‐intervention were excluded |
| Compliance (other bias) | Unclear risk | Not reported |
Avraham 2007.
| Methods | Design: RCT, randomisation method not reported Objectives: to objectively evaluate 3 different PFPS rehabilitation programmes |
|
| Participants | Data collection period: not reported Recruitment setting: diagnosed by an orthopaedic surgeon; Israel Inclusion: positive sign in patellofemoral gliding test; negative McMurray test; full knee range of motion; anterior knee pain related to prolonged sitting, climbing stairs and descending stairs Exclusion: patellofemoral degenerative changes on imaging; history of knee trauma 42 patients, % female not reported, mean age 35 (± not reported), BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 10 2) n = 10 3) n = 10 |
|
| Interventions | Setting of intervention: physical therapy institute Duration: 3 weeks, 2 times a week + 4 home self treatments Supervisor of the interventions: physical therapist 1) 7.5 minutes straight leg raise, 7.5 minutes single‐leg squats 2) Knee and hip exercises (3 minutes iliotibial band stretching, 3 minutes hamstring stretching, 9 minutes hip external rotators strengthening) 3) Knee and hip exercises (3 minutes straight leg raises, 3 minutes single‐leg squats, 3 minutes iliotibial band stretching, 3 minutes hamstring stretching, 3 minutes hip external rotators strengthening) Additional intervention in all groups: 15 minutes TENS |
|
| Outcomes | Baseline, 3 weeks Pain: VAS (0 to 10), usual Function: Patellofemoral Joint Evaluation Scale (0 to 100) Adverse events: not actively sought |
|
| Notes | Target of exercises or exercise programmes: knee + hip versus knee: 2 = experimental versus 1 = control Target of exercises or exercise programmes: knee + hip versus knee: 3 = experimental versus 1 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29% dropout in the short term, equal among groups due to inconsistency in the programme; cross‐over not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Bakhtiary 2008.
| Methods | Setting: RCT, computer‐generated random sequence, in sealed, numbered envelopes given to physiotherapist Objectives: to compare the effect of open kinetic chain exercises and closed kinetic chain exercises on the treatment of patella chondromalacia |
|
| Participants | Data collection period: not reported Recruitment setting: not reported; Iran Inclusion: patellar chondromalacia based on 4 criteria (pain during climbing up and down stairs, pain after sitting for a long time with the knee flexed, knee extension after sitting for a long time with the knee flexed, giving away during walking) and positive Clark test Exclusion: no history of neuromuscular or musculoskeletal disorders or deformity in the knee or ankle joint 32 patients, all female, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 16, mean age 22.3 (± 1.7) 2) n = 16, mean age 21.8 (± 0.6) |
|
| Interventions | Setting of intervention: not reported Duration: 3 weeks, twice daily 20 to 70 repetitions Supervisor of the interventions: physical therapist 1) Open kinetic chain exercise programme including straight leg raises 2) Closed kinetic chain exercise programme including semi squat |
|
| Outcomes | Baseline, 3 weeks; follow‐up 5 weeks Pain: VAS (0 to 10), usual Adverse events: not actively sought |
|
| Notes | Types of exercises or exercise programmes: 2 = experimental, 1 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | In sealed, numbered envelopes given to physiotherapist |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout and cross‐over not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | A timetable had to be filled in after exercises; compliance not reported |
Balci 2009.
| Methods | Design: RCT, randomisation method not reported Objectives: the effects of 2 different closed kinetic chain exercises were compared in patients with patellofemoral pain syndrome (PFPS) |
|
| Participants | Data collection period: not reported Recruitment setting: diagnosed by 1 orthopaedist; Turkey Inclusion: female patients with patellofemoral pain for at least 2 months and between at least 2 activities like longtime sitting, stair/slope climbing and descending, crouching, running, bouncing and jumping Exclusion: history of meniscus and ligament lesions, patellofemoral osteoarthritis, patellofemoral dislocation and/or subluxation history, bone anomaly and surgical knee history 40 patients, 100% female, all unilateral 1) n = 20, mean age 39.1 (± 8.0), mean BMI 26.6 (± 5.3), mean duration of complaints 35.8 (± 29.3) months 2) n = 20, mean age 36.1 (± 8.7), mean BMI 24.3 (± 3.9), mean duration of complaints 27.8 (± 31.7) months |
|
| Interventions | Setting of intervention: not reported Duration: 4 weeks, 20 sessions in total Supervisor of the interventions: 1 physiotherapist 1) CKC exercises with hip internally rotated: functional squat exercise in the rehabilitation mode of the Monitored Rehab Systems – Functional Squat System at 45° internal rotation of the hip and 0° to 45° flexion interval of knee 2) CKC exercises with hip externally rotated: functional squat exercise in the rehabilitation mode of the Monitored Rehab Systems – Functional Squat System at 45° external rotation of the hip and 0° to 45° flexion interval of knee |
|
| Outcomes | Baseline, 4 weeks Pain: VAS (0 to 10) during physical activity (stair and slope climbing and descending) Function: AKPS (0 to 100) Adverse events: not actively sought |
|
| Notes | Types of exercises or exercise programmes: 1 = experimental, 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients who gave informed consent were divided into 2 groups by method of random selection |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropout; cross‐over not reported; intention‐to‐treat analysis unclear |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | High risk | Significant difference in mean height; BMI not statistically tested |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Clark 2000.
| Methods | Design: RCT, computer‐generated randomisation by physiotherapist Objectives: to determine the efficacy of the individual components of physiotherapy in participants with anterior knee pain |
|
| Participants | Data collection period: September 1995 to February 1998 Recruitment setting: diagnosed by orthopaedic, rheumatology consultants or general practitioner; Australia Inclusion: 16 to 40 years; anterior knee pain > 3 months Exclusion: history of true locking, patella dislocation, arthritis; any knee radiograph abnormality; ligament laxity; malignancy; infection or previous knee physiotherapy 81 patients, 44% female, duration of complaints on average > 12 months, 55% bilateral complaints 1) n = 20, 50% females, mean age 26.0 (± 7.4), mean BMI 24.8 (± 5.7), 35% bilateral complaints 2) n = 20, 40% females, mean age 29.5 (± 6.2), mean BMI 24.9 (± 4.2), 35% bilateral complaints 3) n = 19, 47% females, mean age 29.3 (± 6.8), mean BMI 25.0 (± 3.9), 58% bilateral complaints 4) n = 22, 41% females, mean age 27.1 (± 7.2), mean BMI 25.2 (± 4.2), 45% bilateral complaints |
|
| Interventions | Setting of intervention: physical therapy department Duration: 3 months Supervisor of the interventions: not reported 1) Exercise + tape: 6 sessions and daily training at home 2) Exercise: 6 sessions and daily training at home 3) Tape: 6 sessions and daily at home 4) No treatment Additional intervention in all groups: education Exercise included wall squat, sit to stand, proprioceptive balance, specific exercises for gluteus medius and maximus, progressive step‐down exercises |
|
| Outcomes | Baseline, 3 months; follow‐up 12 months Pain: VA during walking and stair climbing (0 to 200) Function: WOMAC (0 to 96) Recovery: number of patients no longer troubled by pain Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 2 = experimental versus 4 = control Exercise therapy versus control: 1 = experimental versus 3 = control Exercise therapy versus different unimodal conservative interventions: 2 = experimental versus 3 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | High risk | Randomisation done by the physiotherapist him/herself |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients were aware that of the 4 types of treatment 1 group would receive advice only; patients' awareness of expected effects not reported; no protocol for provider/patient interactions reported; interventions clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12% dropout in the short term; 39% dropout at 12 months follow‐up; cross‐over not reported; intention‐to‐treat analysis done, imputation method unknown |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | A diary sheet was supplied to help compliance; actual compliance not reported |
Colón 1988.
| Methods | Design: RCT, quasi‐randomised (matched for age, physical findings and disability) Objectives: to compare the value of a straight leg raising programme with a pogo stick rehabilitation programme in patients with patellofemoral chondrosis |
|
| Participants | Data collection period: not reported Recruitment setting: not reported; USA Inclusion: patients with patellofemoral chondrosis; 2 out of the following 6 criteria: persistent aching in the knees while at rest, pain in the knees after sitting with the knees in a flexed position for more than 10 to 20 minutes, occurrence or exaggeration of pain on walking up or down stairs, crepitation in the knees with movement, snapping sensations in the knees upon extension or flexion, locking of the knees, inability to squat down without pain. Crepitation and compression sign during physical examination Exclusion: not reported 29 recreational athletes, 34% female, age range 15 to 24 years mean and SD not reported, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 16, 31% females 2) n = 13, 38% females |
|
| Interventions | Setting of intervention: not reported Duration: 6 to 8 weeks Supervisor of the interventions: the pogo stick group was under direct staff supervision, for the control group not reported 1) Isotonic exercises including pogo stick bounces, incremental increase in repetitions: twice daily from 250 up to 700 to 1000 bounces 2) Isometric exercises including straight leg raises with increasing weights: twice daily 3 sets of 10 repetitions |
|
| Outcomes | Baseline, 6 to 8 weeks Recovery: number of patients with more than 50% improved on pain scale Adverse events actively sought: number of patients with increased pain |
|
| Notes | Types of exercises or exercise programmes: 1 = experimental, 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised (matched for age, physical findings and disability) |
| Allocation concealment (selection bias) | High risk | No concealment, due to matching |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14% dropout in the short term; 1 female participant was lost to the pogo stick group because of pain; cross‐over not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | High risk | No study protocol; no pain and functional ability data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
De Marche 2014.
| Methods | Design: RCT, randomisation was performed in blocks of 4, consecutively numbered, opaque envelopes, randomly assigned by a computer‐generated table of random numbers. A person blinded to the information about the information performed the randomisation Objectives: to compare the effects of functional stabilisation training versus standard training on knee pain and function, lower‐limb and trunk kinematics, trunk muscle endurance, and eccentric hip and knee muscle strength with patellofemoral pain |
|
| Participants | Data collection period: March to November 2012 Recruitment setting: recruitment through flyers posted in the university physical therapy clinic; Brazil Inclusion: female; athlete (minimum 30 minutes 3 times a week sport participation) anterior knee pain of 3 or greater on the 10 cm VAS; anterior or retropatellar knee pain during at least 3 of the following activities: ascending/descending stairs, squatting, running, kneeling, jumping and prolonged sitting; insidious onset of symptoms unrelated to trauma Exclusions: intra‐articular pathology; involvement of cruciate or collateral ligaments; patellar instability; Osgood‐Schlatter or Sinding‐Larsen‐Johansson syndrome; hip pain; knee joint effusion; previous surgery in the lower limb or if palpation of the patellar tendon, iliotibial band or pes anserinus tendons reproduced the pain 31 recreational athletes, all female, % bilateral complaints not reported (the affected limb was used for analysis of functional performance) 1) n = 15, mean age 22.7 (± 3.2), mean BMI 20.6 (± 2.0), mean duration of complaints 27 (± not reported) months 2) n = 16, mean age 21.3 (± 2.6), mean BMI 22.3 (± 2.5), mean duration of complaints 60 (± not reported) months |
|
| Interventions | Setting of intervention: laboratory of intervention and assessment in Orthopaedics and Traumatology Duration: 8 weeks, 3 times a week Supervisor of the interventions: 1 physical therapist 1) Functional stabilisation training including hip and knee exercises: quadruped and prone, sitting on Swiss ball, lateral bridge, ventral bridge, trunk extension on Swiss ball, isometric hip abduction/lateral rotation in standing, hip abduction/lateral rotation/extension in side lying, hip lateral rotation in closed kinetic chain, single‐leg dead lift, single‐leg squat, forward lunge, prone knee flexion, seated knee extension, single‐leg standing on unstable platform 2) Standard training including quadriceps exercises: straight leg raise in supine position, seated knee extensions, leg press, wall squat, step‐up, step‐down, single‐leg standing on unstable platform |
|
| Outcomes | Baseline, 8 weeks; follow‐up 5 months Pain: VAS (0 to 10), worst Function: LEFS (0 to 80), single‐leg triple hop test (cm) Recovery: global rating of improvement (15‐point scale) Adverse events: not actively sought |
|
| Notes | Target of exercises or exercise programmes: knee + hip versus knee: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list, blocks of 4 |
| Allocation concealment (selection bias) | Low risk | "A person blinded to information about the patients performed the randomization and provided the group assignment to the treating physical therapist." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Impossible for exercise therapy; patients were blinded to group allocation; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unclear for patient‐reported outcome; no blinding for functional performance tests |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% dropout in the short term; cross‐over not reported; intention‐to‐treat analysis using the multiple‐imputation method to impute values for all missing data |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | High risk | Seemed not to be similar for duration of complaints |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Dolak 2011.
| Methods | Design: RCT, random number generator Objectives: to determine if females with patellofemoral pain syndrome (PFPS) who perform hip strengthening prior to functional exercises demonstrate greater improvements than females who perform quadriceps strengthening prior to the same functional exercises |
|
| Participants | Data collection period: not reported Recruitment setting: diagnosed by a certified athletic trainer; USA Inclusion: anterior or retropatellar knee pain during at least 2 activities: stair climbing, hopping, running, squatting, kneeling and prolonged sitting. An insidious onset of symptoms not related to trauma; pain with compression of the patella: pain on palpation of patellar facets Exclusion: symptoms present for less than 1 month; self reported other knee pathology, such as cartilage injury or ligamentous tear; a history of knee surgery within the last year, or patella dislocations or subluxations, and any other concurrent significant injury affecting the lower extremity 33 patients, all female, age range 16 to 35 years, 48% bilateral complaints (the most painful limb was used for analysis of functional performance) 1) n = 17, mean age 25 (± 5), mean BMI 24 (± 4), mean duration of complaints 36 (± 34) months, 53% bilateral complaints 2) n = 16, mean age 26 (± 6), mean BMI 27 (± 6), mean duration of complaints 27 (± 34) months, 44% bilateral complaints |
|
| Interventions | Setting of intervention: not reported Duration: 4 weeks, weekly supervised session + 2 times weekly at home Supervisor of the interventions: not reported 1) Hip exercises 2) Quadriceps exercises |
|
| Outcomes | Baseline, 4 weeks; after 4 weeks both groups started the same weightbearing exercise for 4 weeks Pain: VAS (0 to 10), worst Function: LEFS (0 to 80) and step‐down test (N of repetitions completed in 30 seconds) Adverse events actively sought: number of patients with increased pain |
|
| Notes | Target of exercises or exercise programmes: hip versus knee: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generator |
| Allocation concealment (selection bias) | High risk | Computer‐generated randomisation, done by the investigator him/herself |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome; blinding for functional performance tests only during initial testing session |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% dropout in the short term, 1 withdrawn by investigators for increased pain; cross‐overs not reported; intention‐to‐treat analysis done with the last available measure moved forward |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different with respect to demographic variables; outcome variables seemed to be similar |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Log to document medication use; comparability and other interventions not reported |
| Compliance (other bias) | Unclear risk | Exercise log to document home exercise compliance; actual compliance not reported |
Eburne 1996.
| Methods | Design: RCT, quasi‐randomised by odd or even birth month Objectives: to compare the McConnell regimen with isometric quadriceps exercises assessing pain, function and subjective and objective testing before and after treatment |
|
| Participants | Data collection period: not reported Recruitment setting: outpatient physiotherapy department; United Kingdom Inclusion: patients with anterior knee pain; 10 to 35 years; no previous back or lower extremity surgery; good general health; no pathological or infectious disease; and normal ligamentous and meniscal test Exclusion: not reported 75 patients, % female not reported, age not reported, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = not reported 2) n = not reported |
|
| Interventions | Setting of intervention: not reported Duration: monthly until pain free, or for 3 months Supervisor of the interventions: there were 2 changes of therapist in the McConnell and 3 in the isometric quadriceps group 1) Isometric quadriceps group: static quadriceps exercises and straight leg raising with re‐education of function and steps, running and walking in a gymnasium programme 2) McConnell regimen: taping of the patella, training of the VMO at different degrees (0, 30, 60, 90 and 120) at knee flexion, with the leg externally rotated and adducted, whilst performing an isometric contraction of the VMO. This progressed to include eccentric muscle action and subconscious activity. Eccentric action included weight bearing, knee flexion and extension with the foot supinated, and stairs forwards and backwards, with weight on the affected lower limb. Subconscious activity included running, squatting, jumping and walking, with directional changes |
|
| Outcomes | Baseline, 1 week, 3 months Pain: McConnell critical test in different knee angles (on analogue scale) Recovery: improvement (percentage) Adverse events: not actively sought (report of allergy to tape: "strapping") |
|
| Notes | Exercise therapy versus different multimodal conservative interventions: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised by odd or even birth month |
| Allocation concealment (selection bias) | High risk | Unlikely in the case of randomisation by odd or even birth month |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; interventions outwardly not similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29% dropout in the short term; cross‐overs not reported; intention‐to‐treat analysis not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no functional ability data reported |
| Baseline characteristics (other bias) | High risk | Treatment groups were comparable for all admission variables, except the mean age, which was 5 years older (P value = 0.003) in the McConnell group |
| Clinicians' experience (other bias) | High risk | There were 2 changes of therapist in the McConnell and 3 in the isometric quadriceps group |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Fukuda 2010.
| Methods | Design: RCT, randomisation method not reported; opaque, sealed envelopes; independent person Objectives: to investigate the influence of strengthening the hip abductor and lateral rotator musculature on pain and function of females with patellofemoral pain syndrome |
|
| Participants | Data collection period: not reported Recruitment setting: recruited from the Rehabilitation Service by a single physical therapist with more than 10 years of clinical experience in knee rehabilitation; Brazil Inclusion: 20 to 40 years; history of anterior knee pain for at least the past 3 months and reported pain in 2 or more: ascending and descending stairs, squatting, kneeling, jumping, long sitting, isometric knee extension contraction at 60° of knee flexion, and pain on palpation of the medial and/or lateral facet of the patella Exclusion: pregnant; neurological disorders; hip or ankle injuries; low back or sacroiliac joint pain; rheumatoid arthritis; used corticosteroids and/or antiinflammatory drugs; a heart condition that precluded performing the exercises; or previous surgery involving the lower extremities; other knee pathologies such as patellar instability, patellofemoral dysplasia, meniscal or ligament tears, osteoarthritis, tendinopathies and epiphysitis 70 sedentary patients, all female, mean age 25 (± 0.7), duration of complaints not reported, all unilateral complaints 1) n = 25, mean age 24 (± 7), mean BMI 22.6 (± not reported) 2) n = 22, mean age 25 (± 6), mean BMI 21.2 (± not reported) 3) n = 23, mean age 25 (± 7), mean BMI 23.4 (± not reported) |
|
| Interventions | Setting of intervention: not reported Duration: 3 treatment sessions per week for 4 weeks Supervisor of the interventions: 2 trained therapists 1) No treatment 2) Knee exercises including iliopsoas strengthening in non‐weight bearing, seated knee extension 90°‐45°, leg press 0°‐45°, squatting 0°‐45° 3) Knee and hip exercises including iliopsoas strengthening in non‐weight bearing, seated knee extension 90°‐45°, leg press 0°‐45°, squatting 0°‐45°, hip abduction against elastic band (standing), hip abduction with weights (side lying), hip external rotation against elastic band (sitting), side‐stepping against elastic band, 3 x 1 minute lateral rotator muscles |
|
| Outcomes | Baseline, 4 weeks Pain: NPRS (0 to 10) ascending and descending (during activity) Function: LEFS (0 to 80) and AKPS (0 to 100), single‐limb single hop test (cm) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 2 = experimental versus 1 = control Exercise therapy versus control: 3 = experimental versus 1 = control Target of exercises or exercise programmes: knee + hip versus knee: 3 = experimental versus 2 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly with envelopes |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes; independent person |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar, no treatment intervention clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome; for functional performance tests the examiner was blind to the group assignment of the patients and did not participate in the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% dropout in the short term; cross‐over not reported; intention‐to‐treat analysis done based on the imputation of the group mean to each missing value for each of the 3 groups |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Low risk | 2 trained therapists supervised the intervention |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Low risk | Patients excluded after missing treatments |
Fukuda 2012.
| Methods | Design: RCT, randomisation method not reported; opaque, sealed envelopes; independent person Objectives: to determine if adding hip‐strengthening exercises to a conventional knee exercise programme produces better long‐term outcomes than conventional knee exercises alone in women with patellofemoral pain syndrome |
|
| Participants | Data collection period: not reported Recruitment setting: recruited from the Rehabilitation Service by a single physical therapist with more than 10 years of clinical experience in knee rehabilitation; Brazil Inclusion: 20 to 40 years; history of anterior knee pain for at least the past 3 months and reported pain in 2 or more of the following: ascending and descending stairs, squatting, kneeling, jumping, long sitting, isometric knee extension contraction at 60° of knee flexion, and pain on palpation of the medial and/or lateral facet of the patella Exclusion: pregnant; neurological disorders; hip or ankle injuries; low back or sacroiliac joint pain; rheumatoid arthritis; used corticosteroids and/or antiinflammatory drugs; a heart condition that precluded performing the exercises; or previous surgery involving the lower extremities; other knee pathologies such as patellar instability, patellofemoral dysplasia, meniscal or ligament tears, osteoarthritis, tendinopathies and epiphysitis 54 sedentary patients, all female, all unilateral complaints 1) n = 26, mean age 23 (± 3.0), mean BMI 24.5 (± 3.0), mean duration of complaints 21.0 (± 17.7) months 2) n = 28, mean age 22 (± 3.0), mean BMI 23.6 (± 2.7), mean duration of complaints 23.2 (± 19.0) months |
|
| Interventions | Setting of intervention: not reported Duration: 3 treatment sessions per week for 4 weeks, 3 x 10 repetitions Supervisor of the interventions: 3 trained therapists 1) Knee exercises including seated knee extension from 90° to 45°, leg press from 0° to 45°, squatting from 0° to 45°, single‐leg calf raises, 3 sets of 10 repetitions, prone knee flexion 2) Knee and hip exercises including hip abduction with weights (side lying), hip abduction against elastic band (standing), hip lateral rotation against elastic band (sitting), hip extension (machine) |
|
| Outcomes | Baseline; follow‐up 3, 6 and 12 months Pain: NPRS ascending and descending (0 to 10), during activity Function: LEFS (0 to 80) and AKPS (0 to 100), single‐limb single hop test (cm) Adverse events: not actively sought |
|
| Notes | Target of exercises or exercise programmes: knee + hip versus knee: 2 = experimental versus 1 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly with envelopes |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes; independent person |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar, no treatment intervention clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome; for functional performance tests the examiner was blind to the group assignment of the patients and did not participate in the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% dropout in the short term; cross‐over not reported; intention‐to‐treat analysis done using the last value carried forward method to impute values for all missing data |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Low risk | 3 trained therapists supervised the intervention |
| Co‐interventions (other bias) | Unclear risk | No patient reported the use of anti‐inflammatory or analgesic drugs during this period; other interventions not reported |
| Compliance (other bias) | Low risk | Patients excluded after missing treatments |
Gaffney 1992.
| Methods | Design: RCT, randomisation method not reported Objectives: to compare the efficacy of 2 exercise programmes, 'concentric' and 'eccentric' |
|
| Participants | Data collection period: not reported Recruitment setting: diagnosis by 1 doctor at the department of community health and 1 doctor at the institute of sport; Australia Inclusion: a typical history and examination findings of PF joint pain; history of knee pain, usually retropatellar or medially, which was present on one of the following activities: ascending or descending stairs, squatting or rising from a squat, or sitting with the knee bent at 90 degrees. If the patients had normal test: no sign of ligament damage as determined by valgus and varus stress tests, Lachman's test and the anterior drawer of the knee in neutral, internal and external rotation no sign of meniscal involvement as determined by the McMurray and Steinmann test; no involvement of structures around the patella Exclusion: certain pathology; knee pain referred from the back or hip; a systemic rheumatic condition such as rheumatoid arthritis or gout 72 patients, 35% female, mean age 33.9 range 11 to 65, BMI not reported, mean duration of complaints 40.7 months, 50% bilateral complaints 1) n = 36, 36% female, mean age 31.9 (± not reported), mean BMI 22.2 (± not reported), mean duration of symptoms 39.0 (± not reported) 2) n = 36, 33.3% female, mean age 35.9 (± not reported), mean BMI 24.4 (± not reported), mean duration of symptoms 42.1 (± not reported) |
|
| Interventions | Setting of intervention: department of community health and institute of sport Duration: 6 weeks Supervisor of the interventions: 2 therapists per institution 1) Concentric programme: concentric quadriceps contractions; straight leg raises to 45 degrees elevation in the sitting position, with 3 sets of 10 in the first week and 6 sets of 10 in the second week of treatment. Straight leg raises were continued throughout the treatment programme with a minimum of 60 repetitions daily and with weight added progressively to the ankle 2) Eccentric programme + taping: isometric and eccentric quadriceps contraction with taping of the patella to approximate normal alignment; isometric self resisted quadriceps (using the opposite leg) at any knee angle where pain was reproduced when not taped; squats involving both legs; step‐ups performed with the affect led on the step and step‐downs with the affected leg remaining on the step |
|
| Outcomes | Baseline, 4 weeks, 8 weeks Pain: maximal pain score (worst pain, scale unknown) Function: number of patients improved Recovery: subjective success (yes or no) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus different multimodal conservative interventions: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly not similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17% dropout in the short term; cross‐over not reported; intention‐to‐treat analysis not reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | High risk | Significant difference in BMI attributed to the fact that there were slightly more females and some 11 to 13 years old in the concentric group |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Low risk | Self reported compliance revealed 86% in eccentric and 88% in concentric programmes |
Gobelet 1992.
| Methods | Design: RCT, randomisation method not reported Objectives: to study the efficacy of 3 different muscle training programmes (electrostimulation of the muscle, isokinetic training and isometric training) in patients with patellar chondromalacia |
|
| Participants | Data collection period: not reported Recruitment setting: not reported; Switzerland Inclusion: retro‐patellar chondropathy with or without Wyberg dysplasia 1 or 2 Exclusion: trauma; radiological lesion; Wiberg dysplasia 3 120 patients, 53% female, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 28, 61% females, mean age 27.6 (± 12.4) range 14 to 63 2) n = 40, 45% females, mean age 24.6 (± 8.5) range 15 to 40 3) n = 26, 58% females, mean age 27.9 (± 13.3) range 13 to 45 |
|
| Interventions | Setting of intervention: not reported Duration: 4 weeks Supervisor of the interventions: not reported 1) Electro stimulation of quadriceps: 4 hours a day at home 2) Isokinetic exercise programme including flexion/extension on Cybex: 3 times a week 3) Isometric exercise programme: 3 times a week |
|
| Outcomes | Baseline, 4 weeks Function: Arpège function scale (0 to 18) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus different unimodal conservative interventions: 2 experimental versus 1 control Exercise therapy versus different unimodal conservative interventions: 3 experimental versus 1 control Types of exercises or exercise programmes: 2 = experimental versus 3 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar, but control group clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% dropout not equal among groups, 12 stopped because of ineffectiveness of treatment; cross‐overs not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no pain data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Hafez 2012.
| Methods | Design: RCT, randomisation method not reported Objectives: to compare between eccentric contraction and concentric contraction exercises in the management of chondromalacia patellae patients |
|
| Participants | Data collection period: not reported Recruitment setting: all patients were listed at outpatient clinic of orthopaedic departments; Egypt Inclusion: chondromalacia patellae Exclusion: not reported 40 patients, all female, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 20, mean age 17.25 (± 1.46) 2) n = 20, mean age 18.75 (± 1.64) |
|
| Interventions | Setting of intervention: not reported Duration: 3 sessions per week for 3 months, 3 x 10 repetitions Supervisor of the interventions: 1 physical therapist 1) Eccentric exercises 2) Concentric exercises Additional intervention both groups: ultrasonic therapy |
|
| Outcomes | Baseline, 12 weeks Pain: VAS (0 to 10), usual Function: WOMAC (0 to 96) Adverse events: not actively sought |
|
| Notes | Types of exercises or exercise programmes: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout and cross‐over not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported; outcome variables seemed to be similar |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Harrison 1999.
| Methods | Design: RCT, random number table method Objectives: to evaluate the efficacy of 3 treatment approaches for PFPS |
|
| Participants | Data collection period: not reported Recruitment setting: referred by GPs and orthopaedic surgeons; Canada Inclusion: diagnosed with PFPS; 12 to 35 years; 2 of the following criteria: patellar pain with manual compression of the patella against the femur, patellar tenderness with palpation of the posterior‐medial and postero‐lateral borders of the patella, patellar pain during resisted dynamic knee extensions, or patellar pain with manual compression of the patella against the femur during isometric knee extensor contraction (Clarke's compression test) Exclusion: other musculoskeletal conditions of the knee; previous or pending knee surgery; gross knee effusion; knee pain referred from the hip or spine; upper of lower motor neuron lesions and previous steroid injections to the knee; major pathology on radiograph 112 patients, 60% female, mean age: 22.2 (± 8.2), duration of complaints not reported, 54% bilateral complaints (most painful side was used for analysis of functional performance), BMI not reported 1) n = 42 2) n = 34 3) n = 36 |
|
| Interventions | Setting of intervention: not reported Duration 4 weeks Supervisor of the interventions: physical therapist 1) Home exercise programme including straight leg raises, hip adduction, step‐down: daily training 2) Supervised exercise programme including straight leg raises, hip adduction, step‐down: 3 times weekly supervised, daily at home 3) Supervised exercise programme including vastus medialis‐specific exercises, like stride standing, standing with foot supination, step‐downs, plié squats with control of foot supination and wall squats (hip adduction if necessary) combined with patellar taping and biofeedback: 3 times weekly supervised, daily at home |
|
| Outcomes | Baseline; follow‐up 1, 3, 6, 12 months Pain: VAS (0 to 10) 3 days average of worst pain Function: FIQ modified (0 to 16), patellofemoral scale (0 to 100), step test (seconds until pain) Recovery: patient's impression of change (ordinal scale of 3) Adverse events: not actively sought |
|
| Notes | Delivery of exercises or exercise programmes: 2 = experimental versus 1 = control Exercise therapy versus different multimodal conservative interventions: 3 = experimental versus 1 = control Exercise therapy versus different multimodal conservative interventions: 3 = experimental versus 2 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome; functional performance tests done by physical therapists who were blind to participant grouping |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33% dropout in the short term; 48% dropout at 12 months; cross‐over not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different with respect to demographic variables, outcome variables seemed to be similar |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Herrington 2007.
| Methods | Design: RCT, randomisation method not reported, sealed and numbered envelopes Objectives: to compare the efficacy of non–weight‐bearing single‐joint quadriceps exercise (SJNWBE) versus weight‐bearing multiple‐joint quadriceps exercise (MJWBE) for individuals with patellofemoral pain syndrome |
|
| Participants | Data collection period: not reported Recruitment setting: referred by orthopaedic surgeon; Saudi Arabia Inclusion: symptoms of anterior knee pain for at least 1 month; average pain level of 3 or more on a 10 cm visual analogue scale during stepping up and down a 25 cm height; anterior or retropatellar knee pain on at least 2 of the following activities: prolonged sitting, climbing stairs, squatting, running, kneeling and hopping/jumping; presence of 2 of the following clinical criteria on assessment: pain during apprehension test, pain during the patellar compression test and crepitation during the compression test Exclusion: previous knee surgery or arthritis; history of patellar dislocation or subluxation, malalignment, or ligament laxity; patellar tendon pathology or chondral damage; spinal referred pain; history of other abnormalities such as leg length inequalities (2 cm); medication as a part of the treatment; previous physical therapy or acupuncture treatment for the knee within the previous 30 days 45 patients, all male, mean age 26.9 (± 5.6) range 18 to 29, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 15 2) n = 15 3) n = 15 |
|
| Interventions | Setting of intervention: Physical Therapy Department at Riyadh Armed Forces Hospital Duration: 6 weeks, 3 times per week Supervisor of the interventions: physical therapist 1) Single Joint Non‐Weight Bearing (= OKC) including knee extension exercises in a seated position from 90° of knee flexion to full extension 2) Multi Joint Weight Bearing (= CKC) including leg press exercise in a seated position from 90° of knee flexion to full extension 3) No treatment |
|
| Outcomes | Baseline, 6 weeks Pain: VAS (0 to 10) with stepping up and down (during activity), during isometric knee extension Function: AKPS (0 to 100) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 1 = experimental versus 3 = control Exercise therapy versus control: 2 = experimental versus 3 = control Type of exercises or exercise programmes: 2 = experimental versus 1 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly with envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout; no cross‐over |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Khayambashi 2012.
| Methods | Design: RCT, quasi‐randomised by sequentially assigning in an alternating fashion Objectives: to examine the effectiveness of isolated hip abductor and external rotator strengthening on pain, health status and hip strength in females with patellofemoral pain |
|
| Participants | Data collection period: not reported Recruitment setting: diagnosed by single physician, specialty not reported; Iran Inclusion: diagnosis of bilateral PFP lasting at least 6 months; peripatellar and/or retropatellar pain with activities commonly association with this condition, such as stair descent, squatting, kneeling and prolonged sitting Exclusion: ligamentous laxity; meniscal injury; pes anserine bursitis; iliotibial band syndrome; patellar tendinitis; history of previous patella dislocation, patellar fracture or knee surgery; previously received physical therapy 28 sedentary patients, all female; duration of complaints not reported, all bilateral complaints 1) n = 14, mean age 28.9 (± 5.8), mean BMI 24.3 (± not reported) 2) n = 14, mean age 30.5 (± 4.8), mean BMI 24.2 (± not reported) |
|
| Interventions | Setting of intervention: not reported Duration: 8 weeks Supervisor of the interventions: not reported 1) Supervised hip exercises including hip abduction and hip external rotation strengthening exercises: 3 times per week 2) 1000 mg of Omega‐3 and 400 mg of calcium: daily |
|
| Outcomes | Baseline, 8 weeks; follow‐up 6 months Pain: VAS (0 to 10), average pain of both knees while performing activities that aggravated symptoms (during activity) Function: WOMAC (0 to 96) Adverse events: not actively sought, no adverse effects were reported |
|
| Notes | Exercise therapy versus different unimodal conservative interventions: 1 = intervention versus 2 = control Follow‐up only available for exercise group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequentially assigned in an alternating fashion |
| Allocation concealment (selection bias) | High risk | Unlikely in the case of sequential assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Impossible for exercise therapy; participants were aware of an alternative treatment group in the study but had no knowledge of intervention details; no protocol for provider/patient interactions reported; interventions clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropout; cross‐over not reported; intention‐to‐treat analysis unclear |
| Selective reporting (reporting bias) | High risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Patients were asked to refrain from (additional) exercise and were allowed to take over‐the‐counter pain and/or anti‐inflammatory medication as needed; not reported if patients did refrain and if the use of over‐the‐counter medication was equal among groups |
| Compliance (other bias) | Unclear risk | Not reported |
Khayambashi 2014.
| Methods | Design: comparative controlled trial; quasi‐randomised by sequentially assigning in an alternating fashion Objectives: to compare the efficacy of posterolateral hip muscle strengthening versus quadriceps strengthening in reducing pain and improving health status in persons with patellofemoral pain |
|
| Participants | Data collection period: not reported Recruitment setting: screening for specific inclusion and exclusion criteria was performed by 2 physicians, specialty not reported; Iran Inclusion criteria: peripatellar and/or retropatellar knee pain and reproduction of pain with activities commonly associated with PFP (e.g. stair decent, squatting, kneeling, prolonged sitting) Exclusion criteria: ligamentous laxity, meniscal injury, pes anserine bursitis, iliotibial band syndrome and patella tendinitis; a history of patella dislocation, patella fracture, knee surgery; previous physical therapy; symptoms that had been present for < 6 months 36 patients who were not physically active and did not participate in recreational sport activities or exercise beyond that of activities of daily living, 50% female, duration of complaints not reported, 61% bilateral complaints 1) n = 18, 50% female, mean age 28.2 (± 7.9), mean BMI 23.6 (± 2.4) 1) n = 18, 50% female, mean age 27.3 (± 6.7), mean BMI 22.7 (± 3.6) |
|
| Interventions | Setting of intervention: not reported Duration: 8 weeks, 3 times a week Supervisor of the interventions: physical therapist 1) Hip exercises including hip abduction and external rotation 2) Quadriceps exercises including knee extension and partial squat |
|
| Outcomes | Baseline, 8 weeks; follow‐up: 6 months Pain: VAS (0 to 10) during activity Function: WOMAC (0 to 96) Adverse events: not actively sought |
|
| Notes | Target of exercises or exercise programmes: hip versus knee: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequentially assigned in an alternating fashion |
| Allocation concealment (selection bias) | High risk | Unlikely in case of sequential assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout; no cross‐over |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Patients were allowed to take over‐the‐counter pain and/or anti‐inflammatory medication as needed; not reported if the use of over‐the‐counter medication was equal among groups |
| Compliance (other bias) | Low risk | All participants were required to complete at least 19 out of the 24 treatment sessions (= 80%) to remain in the study. In addition, if a patient missed 3 consecutive treatment sessions, their participation in the study was terminated; all participants completed the required number of treatment sessions over the 8‐week intervention period |
Loudon 2004.
| Methods | Design: RCT, quasi‐randomised in order of referral Objectives: to determine the effect of exercise on patients with patellofemoral pain syndrome |
|
| Participants | Data collection period: not reported Recruitment setting: diagnosed by primary care physician; USA Inclusion: diagnosis of unilateral PFPS of at least a 2‐month duration based on pain around or under the patella and 3 of the 4 criteria: pain in the patellofemoral joint during or after activity, sitting, stair climbing, squatting Exclusion: history of patella trauma, subluxation or dislocation; confirmed ligamentous, meniscal or fat‐pad damage; evidence of tendinitis, bursitis or chronic effusion; surgery in the lower extremity; osteochondral or chondral fractures; upper or lower motor‐neuron lesions; radiographic evidence of ostearthritis in the patellofemoral or tibiofemoral joint; difficulty understanding English; open physeal growth plate and use of intra‐articular injections or glycosaminoglycans polysulphate 32 patients active in sports at least 120 minutes/week, 76% female, age range 21 to 35 years; duration of complaints not reported, all unilateral 1) n = 11, 73% female, mean age 27.9 (± 6.0), mean BMI 27.8 (± not reported) 2) n = 9, 78% female, mean age 25.9 (± 4.7), mean BMI 27.2 (± not reported) 3) n = 9, 78% female, mean age 27.7 (± 8.5), mean BMI 34.6 (± not reported) (seems that the height is not correct) |
|
| Interventions | Setting of intervention: not reported Duration: 8 weeks Supervisor of the interventions: physical therapist 1) No treatment 2) Home exercises + 5 physical therapy visits 3) Supervised exercises twice a week for 4 weeks, plus 1 physical therapy visit at 6 weeks and 1 at 8 weeks; and additional home exercises Exercises included quadriceps exercises starting with isometrics followed by straight leg raises followed by closed kinetic chain, such as leg press, mini squat, step‐up, lunge and balance and reach |
|
| Outcomes | Baseline, 8 weeks Pain: VAS 0 to 10, usual Function: AKPS (0 to 100), bilateral squat (number completed in 30 seconds), anteromedial lunge, step‐down dips, leg press, balance and reach Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 2 = experimental versus 1= control Exercise therapy versus control: 3 = experimental versus 1= control Delivery of exercises or exercise programmes: 3 = experimental versus 2 = control SDs for pain, AKPS and bilateral squat received from Janice Loudon (21 December 2013) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Order of referral |
| Allocation concealment (selection bias) | High risk | Unlikely in the case of randomisation by order of referral |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; interventions different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome; no blinding for functional performance tests |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% dropout; cross‐over not reported; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | High risk | Not significantly different for demographic variables; VAS in physiotherapy group seemed higher than the other 2 groups |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | NSAIDs allowed, tape/orthotic within the programme if necessary; comparability across groups unclear |
| Compliance (other bias) | Low risk | Data from participants completing 90% or more of the exercise programme were included in the statistics |
Lun 2005.
| Methods | Design: RCT, random number generator with block design Objectives: to determine the effectiveness of patellar bracing for treatment of patellofemoral pain syndrome |
|
| Participants | Data collection period: not reported Recruitment setting: referred to the primary care physicians and orthopaedic sport medicine physicians or diagnosed by family physician or recruited via bulletin board posters and word of mouth; Canada Inclusion: at least 18 years; atraumatic unilateral and/or bilateral peripatellar or retropatellar knee pain for at least 3 weeks but no greater than 2 years; patellofemoral knee pain with and/or after activity; inactivity patellofemoral pain and/or stiffness, especially with sitting with knees in a flexed position; peripatellar tenderness ± mild inferior patellar pole tenderness Exclusion: history of any significant knee injury (patellar subluxations/dislocations/fractures and ligament or meniscal injuries, and so forth) or knee surgery; significant joint line tenderness; articular or soft‐tissue periarticular effusion or bursitis; intra‐articular ligamentous instability; previous treatment with physiotherapy; any bony abnormalities on X‐ray including bony fracture, osteochondritis dissecans, bipartite patella or osteoarthritis 129 patients, 58% female, mean age 35 (± not reported) range 18 to 60 years, 44% bilateral 1) n = 32, mean age 35 (± 11), mean BMI 24.2 (± not reported), mean duration of complaints 10 (± 7) months, 41% bilateral complaints 2) n = 34, mean age 35 (± 11), mean BMI 24.7 (± not reported), mean duration of complaints 11 (± 8) months, 47% bilateral complaints 3) n = 32, mean age 34 (± 11), mean BMI 24.9 (± not reported), mean duration of complaints 8 (± 6) months, 47% bilateral complaints 4) n = 31, mean age 35 (± 9), mean BMI 23.6 (± not reported), mean duration of complaints 7 (± 5) months, 42% bilateral complaints |
|
| Interventions | Setting of intervention: at home Duration: 12 weeks Supervisor of the interventions: 1 research assistant 1) Home exercise programme and brace: daily 2) Home exercise programme: daily 3) Brace 4) Home exercise programme and a knee sleeve: daily Exercise started with 2‐leg eccentric drop squats and progressed to 1‐ leg eccentric drop squats, to single‐leg lunges, to single‐leg squats |
|
| Outcomes | Baseline, 3, 6 and 12 weeks Pain: VAS (0 to 10) during sport activity, 1 hour after sport activity, following 30 minutes of sitting with knees flexed Function: function scale 0 to 53 Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 1 = experimental versus 3 = control Exercise therapy versus different unimodal conservative interventions: 2 = experimental versus 3 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator with block design |
| Allocation concealment (selection bias) | Unclear risk | A second research assistant, not certain if independent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; interventions clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16% dropout in the short term; 2 participants crossed over to another treatment group before 3 months and were considered to be withdrawals from the study; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different with respect to demographic variables; outcome variables seemed quite similar |
| Clinicians' experience (other bias) | Unclear risk | 1 research assistant, experience unclear |
| Co‐interventions (other bias) | Unclear risk | No additional lower limb‐strengthening exercise was permitted; not reported if participants obeyed, not reported about other co‐interventions |
| Compliance (other bias) | Low risk | Participants were given a journal to document when the exercises were done and/or when the brace or sleeve was worn. These journals were submitted to the second research assistant on a monthly basis |
Moyano 2013.
| Methods | Design: RCT, computer‐generated list, blocks of 8 with no stratification; the randomisation sequence was drawn up and kept off‐site by an independent body Objectives: to compare the effectiveness of proprioceptive neuromuscular facilitation combined with exercise, classic stretching physiotherapy intervention and educational intervention at improving patient function and pain in patients with patellofemoral pain syndrome |
|
| Participants | Data collection period: February to October 2011 Recruitment setting: referred to a physiotherapy clinic with a medial diagnosis of patellofemoral pain syndrome; Spain Inclusion: diagnosis of PFP, pain history of more than 6 months, with no previous history of apophysitis or osteoarthritis and with positive results in the physical examination tests: patellofemoral grinding test and patellofemoral compression test Exclusion: ‐ 94 patients, no engagement in regular sporting activities, 43% female, duration of complaints not reported, % bilateral complaints not reported 1) n = 26, 20% female, mean age 39.36 (± 3.5), mean BMI 24.55 (± 6.21) 2) n = 35, 37.1% female, mean age 40.26 (± 3.72), mean BMI 24.8 (± 5.1) 3) n = 33, 42.9% female, mean age 40.13 (± 2.84), mean BMI 25.2 (± 6.54) |
|
| Interventions | Setting of intervention: not reported Duration: 16 weeks Supervisor of the interventions: physical therapist 1) Health educational materials 2) 'Classic stretching protocol' (stretching exercises for hip and knee muscles) and quadriceps strengthening exercises: 3 times per week 3) Proprioceptive neuromuscular facilitation stretching applied to hamstrings and quadriceps and, after the 4th week, aerobic exercise: 3 times per week |
|
| Outcomes | Baseline, 16 weeks Pain: VAS (0 to 10), usual Function: AKPS (0 to 100) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 2 = experimental versus 1 = control Exercise therapy versus control: 3 = experimental versus 1 = control Types of exercises or exercise programmes: 3 = experimental versus 2= control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list, blocks of 8 with no stratification |
| Allocation concealment (selection bias) | Low risk | Sequence kept by an independent body |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar, health educational group clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.7% dropout in the short term; no cross‐over; intention‐to‐treat analysis unclear |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different concerning any of the demographic variables of study outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Nakagawa 2008.
| Methods | Design: RCT, preprinted cards in sealed, opaque envelopes Objectives: to study the effect of additional strengthening of hip abductor and lateral rotator muscles in a strengthening quadriceps exercise rehabilitation programme for patients with the patellofemoral pain syndrome. |
|
| Participants | Data collection period: not reported Recruitment setting: diagnosed with patellofemoral pain syndrome and referred for physical therapy treatment; Brazil Inclusion: anterior or retropatellar knee pain during at least 3 of the following activities: ascending/descending stairs, squatting, running, kneeling, hopping/jumping and prolonged sitting; the insidious onset of these symptoms being unrelated to a traumatic incident and persistent for at least 4 weeks; and the presence of pain on palpation of the patellar facets, on stepping down from a 25 cm step, or during a double‐legged squat Exclusion: signs or symptoms of any of the following: meniscal or other intra‐articular pathologic conditions; cruciate or collateral ligament involvement; tenderness over the patellar tendon, iliotibial band, or pes anserinus tendons; sign of patellar apprehension; Osgood–Schlatter or Sinding–Larsen–Johansson syndromes; hip or lumbar referred pain; a history of patellar dislocation; evidence of knee joint effusion; or previous surgery on the patellofemoral joint 14 patients, 71% female, mean age 23.6 (± 5.9) range 17 to 40, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 7 2) n = 7 |
|
| Interventions | Setting of intervention: clinical with home programme Duration: 6 weeks, 5 times a week Supervisor of the interventions: the principal investigator 1) Quadriceps and hip exercises including open and closed kinetic chain exercises for quadriceps strengthening and strengthening and functional training exercises focused on the transversus abdominis muscle, hip abductors and lateral rotator muscles 2) Quadriceps exercises including open and closed kinetic chain exercises for quadriceps strengthening Additional intervention all groups: patellar mobilisation |
|
| Outcomes | Baseline, 6 weeks Pain: VAS (0 to 10) usual, worst Adverse events: not actively sought |
|
| Notes | Target of exercises or exercise programmes: knee + hip versus knee: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Preprinted cards in sealed, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | The principal investigator remained blind to treatment allocation until all baseline assessment had been completed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Impossible for exercise therapy; participants were blind to treatment allocation; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout; no cross‐over |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables and outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Razeghi 2010.
| Methods | Design: RCT, randomisation method not reported Objectives: to evaluate whether a non‐operative treatment programme emphasising hip and knee strengthening exercise results in decreased patellofemoral pain |
|
| Participants | Data collection period: not reported Recruitment setting: screening of all female students at the physiotherapy clinic affiliated to the rehabilitation faculty; Iran Inclusion: retro‐ or peripatellar pain from at least 2 of the following activities: squatting, prolonged sitting, stair climbing, running, kneeling; insidious onset of pain without a history of trauma persisting for at least 4 weeks; pain during patellar compression test, patellar grind test or medial/lateral patellar facet tenderness Exclusion: professional sports activity; meniscal injury; cruciate or collateral ligament involvement; tenderness over iliotibial band; patellar or pes anserinus tendon; a positive history of patellar dislocation; a positive patellar apprehension sign; knee surgery in the past 2 years; diagnosis of peri‐patellar bursitis; Sinding‐Larsen‐Johansson and Osgood‐Schlatter disease; referral pain from the lumbar or hip region; pes planus or cavus; leg length discrepancy; lower limb malignancy; pregnancy; a positive history of being on a steroidal or nonsteroidal medication during the previous 6 months 33 patients, all female, mean age 22.62 (± 2.67) range 18 to 30 years, BMI not reported, duration of complaints not reported, 62.5% bilateral complaints n = 17 n = 16 |
|
| Interventions | Setting of intervention: physiotherapy clinic affiliated to the rehabilitation faculty Duration: 4 weeks Supervisor of the interventions: not reported 1) Quadriceps + hip exercises including progressive resistive exercises for the hip and knee extension and mini squat 2) Quadriceps exercises including knee extension and mini squat |
|
| Outcomes | Baseline, 4 weeks Pain: VAS (0 to 10), usual Adverse events: not actively sought |
|
| Notes | Target of exercises or exercise programmes: knee + hip versus knee: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Systemic random allocation strategy |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% dropout in the short term; no cross‐over; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no functional ability data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Schneider 2001.
| Methods | Design: RCT, randomisation method not reported Objectives: to evaluate the therapeutic benefit of the knee splint with integrated resistance‐controlled torque versus physiotherapeutic exercises by proprioceptive neuromuscular facilitation (PNF) in patients with chronic PFS |
|
| Participants | Data collection period: not reported Recruitment setting: not reported; Germany Inclusion: persistence of unilateral retropatellar pain for more than 6 months; unsuccessful conservative therapy; use of anti‐inflammatory and analgesic agents; electrotherapy and physiotherapeutic exercises without PNF; and patient age between 16 and 40 years Exclusion: meniscopathy and damage to their cruciate ligaments; chronic inflammatory processes and "femoropatellar arthrosis greater than I°" as evaluated according to Fairbank (1948) 40 patients, active amateur athletes, 70% female, age not reported, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 20, 75% female 2) n = 20, 65% female |
|
| Interventions | Setting of intervention: not reported Duration: 8 weeks Supervisor of the interventions: not reported 1) 16 rounds of physiotherapeutic exercises based on proprioceptive neuromuscular facilitation combined with extension of the tractus iliotibialis and quadriceps femoris muscles. Patients were treated by 3 therapists on an outpatient basis in 2 1‐hour sessions per week. 2) Unsupported use of a special knee splint (Protonics®, ORMED a Company of EMPI Inc., USA) for 15 minutes 3 times daily combined with exercises performed according to instructions, along with knee flexion in both knees, to reach an individually preset torque. Exercises were carried out in seated and standing positions to strengthen the ischiocrural musculature |
|
| Outcomes | Baseline, 4 weeks, 8 weeks Pain: VAS (0 to 10) at rest and after exposure Function: score of Bessette and Hunter (0 to 100) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus different multimodal conservative interventions: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly not similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout not reported; cross‐over not reported; intention‐to‐treat analysis not reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | High risk | Not significantly different for demographic variables; VAS at rest at baseline seems not similar |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Song 2009.
| Methods | Design: RCT, random blocks of 9, numbered opaque envelopes, independent person (Stratified allocation was carried out with regard to the number of affected sides (unilateral or bilateral) and symptom severity (Lysholm scale scores 65 or 65)) Objectives: to determine the surplus effect of hip adduction on the VMO |
|
| Participants | Data collection period: not reported Recruitment setting: referred by orthopaedic surgeon; Taiwan Inclusion: anterior or retropatellar knee pain after performing at least 2 of the following activities: prolonged sitting, stair climbing, squatting, running, kneeling, hopping and jumping, and deep knee flexing; insidious onset of symptoms unrelated to traumatic accident; presence of pain for more than 1 month; and age of 50 years and under; 2 of the following positive signs of anterior knee pain during the initial physical examination: patellar crepitus, pain following isometric quadriceps femoris muscle contraction against suprapatellar resistance with the knee in slight flexion (Clarke's sign), pain following compression of the patella against the femoral condyles with the knee in full extension (patellar grind test), tenderness upon palpation of the posterior surface of the patella or surrounding structures, and pain following resisted knee extension. Exclusion: self reported clinical evidence of other knee pathology; patellar tendinitis or knee plica; a history of knee surgery; central or peripheral neurological pathology; knee radiographic abnormalities (e.g. knee osteoarthritis) or lower extremity malalignment (e.g. foot pronation); severe knee pain (VAS score or received nonsteroidal anti‐inflammatory drugs, injections or physical therapy intervention in preceding 3 months 89 patients, no engagement in regular sporting activities, 87% female, % bilateral complaints not reported 1) n = 29, 72% female, mean age 38.6 (± 10.8), mean BMI 22.2 (± 3.2), mean duration of complaints 41.8 (± 36.1) months 2) n = 30, 73% female, mean age 40.2 (± 9.9), mean BMI 23.0 (± 3.0), mean duration of complaints 38.3 (± 34.2) months 3) n = 30, 87% female, mean age 43.9 (± 9.8), mean BMI 22.5 (± 2.1), mean duration of complaints 27.7 (± 41.0) months |
|
| Interventions | Setting of intervention: a kinesiology laboratory Duration: 8 weeks Supervisor of the interventions: single physical therapist 1) Hip adduction combined with leg‐press exercise (knee + hip): 3 times a week 2) Leg‐press exercise only (knee): 3 times a week 3) Health educational material |
|
| Outcomes | Baseline, 8 weeks Pain: VAS (0 to 100), worst Function: Lysholm (0 to 100) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 1 = experimental versus 3 = control Exercise therapy versus control: 2 = experimental versus 3 = control Target of exercises or exercise programmes: knee + hip versus knee: 1 = experimental versus 2 = control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random blocks of 9; numbered, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Independent person |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | A single physical therapist, unaware of the purpose of the study, was responsible for randomisation and interventions; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% dropout in the short term; no cross‐over; intention‐to‐treat analysis done, method unclear |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables or outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Low risk | Measured whether the participants attended all scheduled sessions |
Taylor 2003.
| Methods | Design: RCT, random selection of a sealed envelope, concealed allocation not clear Objectives: to evaluate the effect of exercise combined with patella mobilisation/manipulation in the treatment of patellofemoral pain syndrome |
|
| Participants | Data collection period: not reported Recruitment setting: recruited by poster advertisements and selected from patients presenting with knee pain at the chiropractic clinic; United Kingdom Inclusion: localised peri or retropatellar pain originating from the peripatellar tissue or the patellofemoral joint for at least 1 month during 2 of the following: squatting, running, ascending and/or descending stairs, isometric quadriceps femoris muscle contraction or after sitting for a prolonged period of time with the knee flexed. Exclusion: any previous surgery of the lower extremities; history of traumatic patellar dislocation; known damage to the articular cartilage; major muscle, ligament or tendon strain; sprain or ruptures in the lower extremities; any neurological involvement that influences their gait. 12 patients, 33.3% female, mean age: 30.17 (± not reported) range 19 to 54 years, BMI not reported, duration of complaints not reported, % bilateral complaints not reported 1) n = 6 2) n = 6 |
|
| Interventions | Setting: chiropractic clinic Duration: 4 weeks, 2 times a week Supervisor of the interventions: chiropractor 1) Patella mobilisation/manipulation 2) Patella mobilisation/manipulation + daily exercises including isometric muscle contractions (straight leg raises, short‐arc‐type quadriceps exercises) and eccentric muscle contractions (standing one leg squats) |
|
| Outcomes | Baseline, 4 weeks; no follow‐up Pain: McGill Pain Questionnaire (0 to 10), NPRS (0 to 100) worst pain, least pain Function: patient‐specific function score (for 3 separate activities) Adverse events: not actively sought; number of patients with side effects reported |
|
| Notes | Exercise therapy versus control: 2 = experimental versus 1 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection of a sealed envelope |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Impossible for exercise therapy; participants were aware that they were receiving what are believed to be 'real' treatments, but were not aware of which treatment was considered better by those delivering the treatments or collecting data; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout; no cross‐over |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Medication not allowed; not reported if medication or other co‐interventions were used |
| Compliance (other bias) | Unclear risk | Not reported |
Thomee 1997.
| Methods | Design: RCT, quasi‐randomised, numbered consecutively Objectives: the purposes of this study were (1) to evaluate a comprehensive treatment approach for patients with patellofemoral pain syndrome and (2) to compare a training programme using isometric muscle contractions with a training programme using eccentric muscle contractions. |
|
| Participants | Data collection period: not reported Recruitment setting: referred by orthopaedic surgeons; Sweden Inclusion: unsuccessful periods of no treatment and no physical activity, as well as some form of therapy, after contacts with orthopaedic surgeons, school physicians, school nurses or physical therapists for a minimum of 6 months; 3 of the following 4 inclusion criteria were fulfilled: pain from the patellofemoral joint during or after activity, during or after sitting, during stair climbing, during squatting Exclusion: history of any recurrent patellar subluxation or dislocation; history of intermittent or persistent knee swelling in the previous year; other injuries to the knee joint such as any tears of the menisci, ligaments or joint capsule; known damage to the articular cartilage 40 patients, all female, mean age: 20.2 (± 3.2) range 15 to 28, BMI not reported, mean duration of symptoms 43 months (± 31.2), 68% bilateral complaints (symptomatic leg was used for analysis of functional performance) 1) n = 20 2) n = 20 |
|
| Interventions | Setting of intervention: not reported Duration: 12 weeks, 3 times a week during week 1 and 2, thereafter 2 times a week Supervisor of the interventions: not reported 1) Isometric exercises including straight leg raises, leg pulls, toe raises 2) Eccentric exercises including leg raises using eccentric contractions, knee extension using eccentric contractions, step‐down, one‐legged squat, toe raises |
|
| Outcomes | Baseline, 12 weeks; follow‐up 12 months Pain: number of patients experiencing pain during jogging, during heavy loading Function: vertical jump test (cm) Recovery: number of patients participating in sports with or without pain Adverse events: not actively sought |
|
| Notes | Types of exercises or exercise programmes: 2 = experimental versus 1= control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised (numbered consecutively) |
| Allocation concealment (selection bias) | High risk | Unlikely in case of randomisation based on consecutive numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; exercise interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout; no cross‐over |
| Selective reporting (reporting bias) | Unclear risk | No study protocol; no functional ability data reported |
| Baseline characteristics (other bias) | Unclear risk | Not reported |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
Van Linschoten 2009.
| Methods | Design: RCT, computer‐generated list, blocks of 8, concealed allocation by independent researcher Objectives: to assess the effectiveness of supervised exercise therapy compared with usual care with respect to recovery, pain and function in patients with patellofemoral pain syndrome |
|
| Participants | Data collection period: April 2005 to April 2007 Recruitment setting: recruited from general practices and sports medical centres; the Netherlands Inclusion: patients with patellofemoral pain syndrome; 14 to 40 years; presence of pain > 2 months and < 2 year; at least 3 of the following symptoms: pain when walking up or down stairs; pain when squatting; pain when running; pain when cycling; pain when sitting with knees flexed for a prolonged period of time; grinding of the patella; and a positive clinical patellar test (such as Clarke's test or patellar femoral grinding test) Exclusion: knee osteoarthritis, patellar tendinopathy, Osgood‐Schlatter disease or other defined pathological conditions of the knee, or had previous knee injuries or surgery, already treated with supervised exercise therapy 131 patients, 64.1% female, duration of complaints 67.9% between 2 to 6 months, 60.3% bilateral complaints 1) n = 65, 64.6% female, mean age 24.7 (± 8.6), mean BMI 23.2 (± 3.9), 55.4% bilateral complaints 2) n = 66, 63.6% female, mean age 23.2 (± 7.8), mean BMI 23.0 (± 3.4), 65.2% bilateral complaints |
|
| Interventions | Setting of intervention: not reported Duration: 12 weeks Supervisor of the interventions: physical therapist 1) Exercise therapy including static and dynamic exercises for quadriceps, adductor and gluteal muscles: 9 times in 6 weeks + daily at home 2) Usual care, which comprised a "wait and see" approach Additional intervention in both groups: written information about patellofemoral pain syndrome and general instructions for home exercises |
|
| Outcomes | Baseline, 3 months; follow‐up: 12 months Pain: NRS (0 to 10) on activity Function: AKPS (0 to 100) Recovery: number of patients recovered (patients were deemed to have recovered if they rated themselves as "fully recovered" or "strongly recovered" on a 7‐point Likert scale) Adverse events: not actively sought |
|
| Notes | Exercise therapy versus control: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list, blocks of 8 |
| Allocation concealment (selection bias) | Low risk | Independent person |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of intervention groups and expected effects not reported; no protocol for provider/patient interactions reported; interventions clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% dropout in the short term; no cross‐over; intention‐to‐treat analysis done, method not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported according to the previously published study protocol |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables; outcome measures seemed similar |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Low risk | Allowed in both groups (despite from exercise therapy in the control group) and equally used |
| Compliance (other bias) | Unclear risk | To enhance compliance, patients received a tutorial with photographs, a text explaining the exercises and a diary to register the amount of exercising; actual compliance not reported |
Witvrouw 2000.
| Methods | Design: RCT, randomisation using sealed envelopes, concealed allocation unclear Objectives: to investigate, in a randomised, prospective study, the efficacy of open versus closed kinetic chain exercises |
|
| Participants | Data collection period: November 1995 to May 1997 Recruitment setting: not reported; Belgium Inclusion: anterior knee pain for more than 6 weeks and had to exhibit 2 of the following criteria on initial assessment: pain on direct compression of the patella against the femoral condyles with the knee in full extension, tenderness on palpation of the posterior surface of the patella, pain on resisted knee extension, and pain with isometric quadriceps muscle contraction against suprapatellar resistance with the knee in slight flexion Exclusion: knee problems other than patellofemoral pain, a history of a knee operation 60 patients, 66.7% female, mean age 20.3 (± not reported) range 14 to 33, BMI not reported, duration of complaints 15.1 months (± not reported), 45% bilateral complaints (most painful knee used was used for analysis of functional performance) 1) n = 30, 66.7% female 2) n = 30, 66.7% female |
|
| Interventions | Setting of intervention: physical therapy department Duration: 5 weeks, 3 times per week Supervisor of the interventions: a trained physical therapist experienced in knee rehabilitation 1) Open kinetic chain exercise: maximal static quadriceps muscle contractions in full extension, straight leg raises in supine position, short arc terminal knee extensions, leg adductions in lateral decubitus position 2) Closed kinetic chain exercise: seated leg presses, one‐third knee bends on one and both legs, stationary bicycling, rowing‐machine exercises, step‐up and step‐down, progressive jumping |
|
| Outcomes | Baseline, 5 weeks; follow ‐up: 3 months, 5 years Pain: VAS (0 to 100 reported as 0 to 10) during descending stairs (during activity), worst pain, in daily life (usual), during triple jump test, during walking, ascending stairs, during running, during jumping, during sports, during squatting, during prolonged sitting, during the night, during isokinetic test Function: AKPS (0 to 100), triple jump test (cm), N without symptoms during: unilateral squat test (unknown for 5‐year follow‐up), step‐up, step‐down Adverse events: not actively sought |
|
| Notes | Types of exercises or exercise programmes: 2 = experimental, 1 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection of a sealed envelope |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy; patients' awareness of groups and intervention effects not reported; no protocol for provider/patient interactions reported; interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome and functional performance tests |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout in the short term; 15% dropout at 5 years; no cross‐over; no intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for any of the evaluated variables |
| Clinicians' experience (other bias) | Low risk | Trained physical therapist experienced in knee rehabilitation |
| Co‐interventions (other bias) | Low risk | No medication was prescribed as part of their treatment. No brace or tape was used by any patient in this study |
| Compliance (other bias) | Low risk | Every patient followed the exercise programme for the required period of 5 weeks |
Østeråsa 2013.
| Methods | Design: RCT, block randomisation with concealed envelopes Objectives: to evaluate 2 different therapeutic exercise regimens in patients with patellofemoral pain syndrome |
|
| Participants | Data collection period: not reported Recruitment setting: referred by general practitioners and orthopaedics; Norway Inclusion: 16 to 50 years, with untreated PFPS and symptoms lasting for more than 2 months; anterior or retropatellar pain from at least 2 of the following activities – prolonged sitting, climbing stairs, squatting, running, kneeling and hopping/jumping; insidious onset of symptoms unrelated to a traumatic incident; and presence of pain on palpation of the patellar facets or positive physical tests on grinding of the patella, Clarke's test or patellar crepitus Exclusion: knee osteoarthrosis/arthritis; previous knee injury or knee surgery; patellar tendinopathy; Osgood–Schlatter's disease or other defined pathological conditions of the knee 40 patients, 80% female, BMI not reported, 70% bilateral complaints (most affected knee was used for analysis of functional performance) 1) n = 21, 71.4% female, mean age 33 (± 12.3), mean duration of complaints 3.6 (± 2.7) months, 71.4% bilateral complaints 2) n = 19, 89.5% female, mean age 26.8 (± 10.5), mean duration of complaints 2.9 (± 3.1) months, 68.4% bilateral complaints |
|
| Interventions | Setting of intervention: 3 primary healthcare physiotherapy clinics Duration: 12 weeks, 3 times a week Supervisor of the interventions: physical therapist 1) High‐dose, high‐repetition medical exercise therapy (MET) including deloaded step‐up, seated deloaded knee extension, deloaded squat, deloaded step‐down, seated deloaded knee extension, seated loaded knee extension 2) Low‐dose, low‐repetition exercise programme including step‐up, seated knee extension, squat, step‐down |
|
| Outcomes | Baseline, 12 weeks; follow‐up 12 months Pain: VAS (0 to 10), usual Function: step‐down test, FIQ modified (0 to 16) Adverse events: not actively sought |
|
| Notes | Intensity of exercises or exercise programmes: 1 = experimental versus 2 = control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible for exercise therapy, allocation was only concealed for the patients and physiotherapists until the first treatment; patients' awareness of intervention effects not reported; no protocol for provider/patient interactions reported; interventions outwardly similar |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Highly unlikely for patient‐reported outcome; functional performance testing was done by an unblinded physiotherapist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% dropout in the short term; 30% dropout at 12 months; cross‐over not reported; no intention‐to‐treat analysis done in the short term as the 2 dropouts did not influence the group sizes significantly |
| Selective reporting (reporting bias) | Low risk | No study protocol; pain and functional ability data reported |
| Baseline characteristics (other bias) | Low risk | Not significantly different for demographic variables or outcome variables |
| Clinicians' experience (other bias) | Unclear risk | Not reported |
| Co‐interventions (other bias) | Unclear risk | Not reported |
| Compliance (other bias) | Unclear risk | Not reported |
ABBREVIATIONS AND ACRONYMS
AKP: anterior knee pain AKPS: Anterior Knee Pain Scale BMI: body mass index CKC: closed kinetic chain GP: general practitioner LEFS: Lower Extremity Function Scale (M)FIQ: (modified) Functional Index Questionnaire N(P)RS: numerical (pain) rating scale N: number NSAID: non‐steroidal anti‐inflammatory drug OKC: open kinetic chain P(S)FS: patient (specific) function scale PF: patellofemoral PFP: patellofemoral pain PFPS: patellofemoral pain syndrome PNF: proprioceptive neuromuscular facilitation RCT: randomised controlled trial SD: standard deviation TENS: transcutaneous electrical nerve stimulation VA(S): visual analogue (scale) VMO: vastus medialis obliquus WOMAC: osteoarthritis index, measuring pain, disability and stiffness of the knee or hip
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Collins 2008 | Combined interventions: unable to extract the effect of exercise alone |
| Crossley 2002 | Combined intervention: unable to extract the effect of exercise alone |
| Dursun 2001 | EMG feedback is the intervention. Exercise is the same for both groups |
| Mason 2011 | Did not exclude patients with patellofemoral osteoarthritis |
| McMullen 1990 | Not a randomised controlled trial |
| Roush 2000 | Did not exclude patients with patellofemoral osteoarthritis, plica syndrome, patellar tendinitis, quadriceps tendinitis and Osgood–Schlatter's disease |
| Stiene 1996 | Not a randomised controlled trial |
| Syme 2009 | Combined interventions: unable to extract the effect of exercise alone |
| Timm 1998 | Combined interventions: unable to extract the effect of exercise alone |
| Tunay 2003 | Combined interventions: unable to extract the effect of exercise alone |
| Wiener‐Ogilvie 2004 | Did not exclude patients with patellofemoral osteoarthritis |
| Wijnen 1996 | Combined interventions: unable to extract the effect of exercise alone |
EMG: electromyographic
Characteristics of studies awaiting assessment [ordered by study ID]
Erel 2011.
| Methods | RCT Randomisation method? |
| Participants | Diagnosis: no information available Inclusion: no information available Exclusion: no information available 54 patients, % female not reported, duration of complaints not reported, unilateral 1) 27 mean age 37.8 2) 27 mean age 38.3 |
| Interventions | Duration 8 weeks 1) Closed kinetic chain (CKC) 2) Open kinetic chain (OKC) |
| Outcomes | Baseline, 8 weeks Pain: VAS (0 to 10) Function: WOMAC |
| Notes | Types of exercises or exercise programmes: CKC = experimental, OKC = control |
CKC: closed kinetic chain OKC: open kinetic chain RCT: randomised controlled trial WOMAC: osteoarthritis index, measuring pain, disability and stiffness of the knee or hip
Characteristics of ongoing studies [ordered by study ID]
RBR‐2cxrpp.
| Trial name or title | Effect of two kinds of therapy on women with patellofemoral pain syndrome |
| Methods | Randomised clinical trial, 2 arms |
| Participants | Women aged between 18 and 30 years old, anterior or retropatellar knee pain during at least 2 of the following activities: ascending/descending stairs, squatting, running, jumping and prolonged sitting, insidious onset of the symptoms being unrelated to a traumatic incident and persistent for at least 8 weeks, presence of pain on palpation of the patellar facets, usual pain in the last week of at least 3 cm on a visual analogue scale (VAS) of 10 cm |
| Interventions | 1) Strengthening exercises for the lumbo‐pelvic muscles as well as functional training to correct any dynamic lower limb misalignment. 2) Conventional treatment for patellofemoral pain syndrome focusing on quadriceps strengthening and stretching of the lower limb muscles. Both groups performed the activities 3 times per week for 8 consecutive weeks |
| Outcomes | Worst patellofemoral pain in the last week evaluated with a 10 cm visual analogue scale Functional performance will be evaluated through the triple hop test Functional limitation will be evaluated using the anterior knee pain scale and the lower extremity functional scale. The eccentric knee extensor, knee flexor, hip abductor, hip adductor, hip medial rotator and hip lateral rotator isokinetic peak torques will be studied using the isokinetic dynamometer. 3‐dimensional kinematics will be assessed during the single‐leg squat and the step‐down task Trunk muscles resistance will be defined as the time duration the participants will be able to maintain the body in a determined static position |
| Starting date | 4 June 2012 |
| Contact information | Fabio Serrao, Universidade Federal de São Carlos Rodovia Washington Luis, Km 235 13.565‐905 Sao Carlos Brazil +55(16)33518754 fserrao@ufscar.br |
| Notes | — |
Differences between protocol and review
Differences between protocol and review reflected refinement of the categorisation of interventions and processing and presentation of outcome data.
Types of interventions
We further divided the comparison 'exercise therapy versus different conservative interventions' into: 'exercise therapy versus different unimodal conservative interventions' and 'exercise therapy versus multimodal conservative interventions'.
Where appropriate, we grouped the comparison '3c. Types of exercises or exercise programmes' into three groups according to type of kinetic chain exercise: closed kinetic chain exercises versus open kinetic chain exercises; variants of closed kinetic chain exercises; and open, mixed or unspecified kinetic chain exercises subgrouped by type of muscle action. For convenience, these are presented subgrouped in the same forest plots, but without overall pooling. The one comparison that did not fit in was listed as a separate group.
Processing and presentation of outcome data
We stipulated that if multiple short‐term outcomes were measured in one trial, the time point closest to three months was used for pooling. (We considered measurements more than three months after the baseline measurement long‐term outcomes.)
We selected 'pain during descending' for pooling on 'pain at activities' because this outcome measure was present in most studies eligible for pooling of pain at activities.
When pooling different units of measurements, we scaled values to 0 to 10 (lower is better) for pain and 0 to 100 (higher is better) for functional ability.
If multiple pain scales were reported in one study, we only included pain in daily life (usual pain, worst pain and pain at activities (e.g. sports, pain during descending stairs) (Crossley 2004)) in the analyses.
If multiple scales for functional ability were measured, including the AKPS (Kujala), we used the latter for pooling.
We presented all measures of recovery rather than the preferred outcome measure listed in the protocol (Van Linschoten 2009)
The WOMAC score was the only functional outcome measure for which a lower score is better. Hence, we inverted (subtracted from 96) the WOMAC score, and rescaled it to 0 to 100 when pooling these data with other functional scores.
In order to re‐express SMDs in VAS (0 to 10) and AKPS (0 to 100), we multiplied SMDs and 95% CIs by an estimate (the median of all control and intervention SDs) of the SD of VAS or AKPS respectively.
We selected seven outcomes for presentation in the 'Summary of findings' tables
Contributions of authors
RAH: revised the protocol, performed data extraction and data analysis, drafted and revised the text of the review. NEL: revised the protocol, performed data extraction and approved the final version. RL: conceived the review, drafted the protocol and approved the final version. SB‐Z: conceived the review, drafted the protocol and is the guarantor of the review. MM: conceived the review, drafted and revised the protocol, co‐ordinated the review and approved the final version.
Sources of support
Internal sources
Erasmus Medical Center, Netherlands.
External sources
National Institute for Health Research, UK.
Declarations of interest
Study selection, data collection and risk of bias assessment of Van Linschoten 2009 were conducted by review authors who were not study investigators of this trial.
Rianne A van der Heijden: none declared. Nienke E Lankhorst: none declared. Robbart van Linschoten: none declared. Sita MA Bierma‐Zeinstra: none declared. Marienke van Middelkoop: none declared.
New
References
References to studies included in this review
Abd Elhafz 2011 {published data only}
- Abd Elhafz YN, Abd El Salam MS, Abd Elkader SM. Taping and OKC exercises versus taping and CKC exercises in treating patients with patellofemoral pain syndrome. Indian Journal of Physiotherapy and Occupational Therapy 2011;5(1):103‐6. [101392232] [Google Scholar]
Abrahams 2003 {published data only}
- Abrahams S, Guilliford D, Korkia P, Prince J. The influence of leg positioning in exercise programmes for patellofemoral joint pain. Journal of Orthopaedic Medicine 2003;25(3):107‐13. [Google Scholar]
Avraham 2007 {published data only (unpublished sought but not used)}
- Avraham F, Aviv S, Ya'akobi P, Faran H, Fisher Z, Goldman Y, et al. The efficacy of treatment of different intervention programs for patellofemoral pain syndrome ‐ a single blinded randomized clinical trial. Pilot study. Scientific World Journal 2007;7:1256‐62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakhtiary 2008 {published data only}
- Bakhtiary AH, Fatemi E. Open versus closed kinetic chain exercises for patellar chondromalacia. British Journal of Sports Medicine 2008;42:99‐102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Balci 2009 {published data only}
- Balci P, Tunay VB, Baltaci G, Atay AO. The effects of two different closed kinetic chain exercises on muscle strength and proprioception in patients with patellofemoral pain syndrome [Turkish]. Acta Orthopaedica et Traumatologica Turcica 2009;43(5):419‐25. [DOI] [PubMed] [Google Scholar]
Clark 2000 {published data only}
- Clark DI, Downing N, Mitchell J, Coulson L, Syzpryt EP, Doherty M. Physiotherapy for anterior knee pain: a randomised controlled trial. Annals of the Rheumatic Diseases 2000;59(9):700–4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colón 1988 {published data only}
- Colón VF, Mangine R, McKnight C, Kues J. The pogo stick in rehabilitating patients with patellofemoral chondrosis. Journal of Rehabilitation 1988;54(1):73–7. [Google Scholar]
De Marche 2014 {published data only}
- Marche Baldon R, Serrao F, Silva R, Piva S. Effects of functional stabilization training on pain, function, and lower extremity biomechanics in females with patellofemoral pain: a randomized clinical trial. Journal of Orthopaedic and Sports Physical Therapy 2014;44(4):240‐51. [DOI] [PubMed] [Google Scholar]
Dolak 2011 {published data only}
- Dolak KL, Silkman C, Medina McKeon J, Hosey RG, Lattermann C, Uhl TL. Hip strengthening prior to functional exercises reduces pain sooner than quadriceps strengthening in females with patellofemoral pain syndrome: a randomized clinical trial. Journal of Orthopaedic and Sports Physical Therapy 2011;41(8):560‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eburne 1996 {published data only (unpublished sought but not used)}
- Eburne J, Bannister G. The McConnell regimen versus isometric quadriceps exercises in the management of anterior knee pain. A randomised prospective controlled trial. Knee 1996;3:151–3. [Google Scholar]
Fukuda 2010 {published data only}
- Fukuda TY, Rossetto FM, Magalhaes E, Bryk FF, Lucareli PR, Almeida Aparecida Carvalho N. Short‐term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. Journal of Orthopaedic and Sports Physical Therapy 2010;40(11):736‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fukuda 2012 {published data only}
- Fukuda TY, Melo WP, Zaffalon BM, Rossette FM, Magalhaes E, Bryk FF, et al. Hip posterolateral musculature strengthening in sedentary women with patellofemoral pain syndrome: a randomized controlled clinical trial with 1‐year follow‐up. Journal of Orthopaedic & Sports Physical Therapy 2012;42(10):823‐30. [DOI] [PubMed] [Google Scholar]
Gaffney 1992 {published data only (unpublished sought but not used)}
- Gaffney K, Fricker P, Dwyer T, Barrett E, Skibinski K, Coutts R. Patellofemoral joint pain: a comparison of two treatment programmes. Excel 1992;8:179–89. [Google Scholar]
Gobelet 1992 {published data only}
- Gobelet C, Frey M, Bonard A. Muscle training techniques and retropatellar chondropathy [Techniques de musculation et chondropathie rétro–patellaire]. Revue du Rhumatisme et des Maladies Osteo‐Articulaires 1992;59(1):23–7. [MEDLINE: ] [PubMed] [Google Scholar]
Hafez 2012 {published data only}
- Hafez AR, Zakaria A, Buragadda S. Eccentric versus concentric contraction of quadriceps muscles in treatment of chondromalacia patellae. World Journal of Medical Sciences 2007;7(3):197‐203. [EMBASE: 2013016376] [Google Scholar]
Harrison 1999 {published data only}
- Harrison EL, Sheppard MS, McQuarrie AM. A randomized controlled trial of physical therapy treatment programs in patellofemoral pain syndrome. Physiotherapy Canada 1999;51(2):93‐100, 106. [Google Scholar]
Herrington 2007 {published data only (unpublished sought but not used)}
- Herrington L, Al‐Sherhi A. A controlled trial of weight‐bearing versus non‐weight‐bearing exercises for patellofemoral pain. Journal of Orthopaedic & Sports Physical Therapy 2007;37(4):155‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khayambashi 2012 {published data only}
- Khayambashi K, Mohammadkhani Z, Ghaznavi K, Lyle MA, Powers CM. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and hip strength in females with patellofemoral pain: a randomized controlled trial. Journal of Orthopaedic and Sports Physical Therapy 2012;42(1):22‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khayambashi 2014 {published data only}
- Khayambashi K, Fallah A, Movahedi A, Bagwell J, Powers C. Posterolateral hip muscle strengthening versus quadriceps strengthening for patellofemoral pain: a comparative control trial. Archives of Physical Medicine and Rehabilitation 2014;95(5):900‐7. [DOI] [PubMed] [Google Scholar]
Loudon 2004 {published and unpublished data}
- Loudon J. Personal communication (email) 21 December 2013.
- Loudon JD, Gajewski B, Goist‐Foley HL, Loudon KL. The effectiveness of exercise in treating patellofemoral‐pain syndrome. Journal of Sport Rehabilitation 2004;13:323‐42. [Google Scholar]
Lun 2005 {published data only (unpublished sought but not used)}
- Lun VMY, Wiley JP, Meeuwisse WH, Yanagawa TL. Effectiveness of patellar bracing for treatment of patellofemoral pain syndrome. Clinical Journal of Sport Medicine 2005;15(4):235‐40. [DOI] [PubMed] [Google Scholar]
Moyano 2013 {published data only}
- Moyano F, Valenza MC, Martin L, Caballero Y, Gonzalez‐Jimenez E, Demet GV. Effectiveness of different exercises and stretching physiotherapy on pain and movement in patellofemoral pain syndrome: a randomized controlled trial. Clinical Rehabilitation 2013;27(5):409‐17. [DOI] [PubMed] [Google Scholar]
Nakagawa 2008 {published data only}
- Nakagawa TH, Muniz TB, Baldon R De M, Dias Maciel C, Menezes Reiff RB, Serraro FV. The effect of additional strengthening of hip abductor and lateral rotator muscles in patellofemoral pain syndrome: a randomized controlled pilot study. Clinical Rehabilitation 2008;22(12):1051‐60. [DOI] [PubMed] [Google Scholar]
Razeghi 2010 {published data only}
- Razeghi M, Etemadi Y, Taghizadeh Sh, Ghaem H. Could hip and knee muscle strengthening alter the pain intensity in patellofemoral pain syndrome?. Iranian Red Crescent Medical Journal 2010;12(2):104‐10. [Google Scholar]
Schneider 2001 {published data only}
- Schneider F, Labs K, Wagner S. Chronic patellofemoral pain syndrome: alternatives for cases of therapy resistance. Knee Surgery, Sports Traumatology, Arthroscopy 2001;9:290‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song CY, Lin YF, Wei TC, Lin DH, Yen TY, Jan MH. Surplus value of hip adduction in leg‐press exercise in patients with patellofemoral pain syndrome: a randomized controlled trial. Physical Therapy 2009;89(5):409‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor KE, Brantingham JW. An investigation into the effect of exercise combined with patella mobilization/manipulation in the treatment of patellofemoral pain syndrome: a randomized, assessor‐blinded, controlled clinical pilot trial. European Journal of Chiropractic 2003;51(1):5‐17. [Google Scholar]
Thomee 1997 {published data only (unpublished sought but not used)}
- Thomee R. A comprehensive treatment approach for patellofemoral pain syndrome in young women. Physical Therapy 1997;77(12):1690–703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Linschoten 2009 {published data only}
- Linschoten R, Middelkoop M, Berger MY, Heintjes EM, Verhaar JA, Willemsen SP, et al. Supervised exercise therapy versus usual care for patellofemoral pain syndrome: an open label randomised controlled trial. BMJ 2009;339(7728):1010‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Witvrouw 2000 {published data only (unpublished sought but not used)}
- Witvrouw E, Danneels L, Tiggelen D, Willems TW, Cambier D. Open versus closed kinetic chain exercises in patellofemoral pain. A 5‐year prospective randomized study. American Journal of Sports Medicine 2004;32(5):1122‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Witvrouw E, Lysens R, Bellemans J, Peers K, Vanderstraeten G. Open versus closed kinetic chain exercises for patellofemoral pain. A prospective, randomised study. American Journal of Sports Medicine 2000;28(5):687–94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Østeråsa 2013 {published data only}
- Østeråsa B, Østeråsa H, Torstensen TA. Long‐term effects of medical exercise therapy in patients with patellofemoral pain syndrome: results from a single‐blinded randomised controlled trial with 12 months follow‐up. Physiotherapy 2013;99(4):311‐6. [DOI] [PubMed] [Google Scholar]
- Østeråsa B, Østeråsa H, Torstensen TA, Vasseljen O. Dose‐response effects of medical exercise therapy in patients with patellofemoral pain syndrome: a randomised controlled clinical trial. Physiotherapy 2013;99(2):126‐31. [S0031‐9406(12)00057‐0 [pii]10.1016/j.physio.2012.05.009] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Collins 2008 {published data only}
- Collins N, Crossley K, Beller E, Darnell R, McPoil T, Vicenzino B. Foot orthoses and physiotherapy in the treatment of patellofemoral pain syndrome: randomised clinical trial. British Journal of Sports Medicine 2008;24(337):1034‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crossley 2002 {published data only}
- Crossley K, Bennell K, Green S, Cowan S, McConnell J. Physical therapy for patellofemoral pain: a randomized, double‐blinded, placebo‐controlled trial. American Journal of Sports Medicine 2002;30(6):857–65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dursun 2001 {published data only}
- Dursun N, Dursun E, Kilic Z. Electromyographic biofeedback controlled exercise versus conservative care for patellofemoral pain syndrome. Archives of Physical Medicine and Rehabilitation 2001;82(12):1692–5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mason 2011 {published data only}
- Mason M, Keays SL, Newcombe PA. The effect of taping, quadriceps strengthening and stretching prescribed separately or combined on patellofemoral pain. Physiotherapy Research International 2011;16(2):109‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McMullen 1990 {published data only}
- McMullen W, Roncarati A, Koval P. Static and isokinetic treatments of chondromalacia patella: a comparative investigation. Journal of Orthopaedic and Sports Physical Therapy 1990;12(6):256–66. [DOI] [PubMed] [Google Scholar]
Roush 2000 {published data only}
- Roush MB, Sevier TL, Wilson JK, Jenkinson DM, Helfst RH, Gehlsen GM, et al. Anterior knee pain: a clinical comparison of rehabilitation methods. Clinical Journal of Sport Medicine 2000;10(1):22–8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stiene 1996 {published data only}
- Stiene HA, Brosky T, Reinking MF, Nyland J, Mason MB. A comparison of closed kinetic chain and isokinetic joint isolation exercise in patients with patellofemoral dysfunction. Journal of Orthopaedic and Sports Physical Therapy 1996;24(3):136–41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Syme 2009 {published data only}
- Syme G, Rowe P, Martin D, Daly G. Disability in patients with chronic patellofemoral pain syndrome: a randomised controlled trial of VMO selective training versus general quadriceps strengthening. Manual Therapy 2009;14(3):252‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Timm 1998 {published data only}
- Timm KE. Randomized controlled trial of Protonics on patellar pain, position, and function. Medicine and Science in Sports and Exercise 1998;30(5):665–70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tunay 2003 {published data only}
- Tunay VB, Baltaci G, Tunay S, Ergun N. A comparison of different treatment approaches to patellofemoral pain syndrome. Pain Clinic 2003;15:179‐84. [Google Scholar]
Wiener‐Ogilvie 2004 {published data only}
- Wiener‐Ogilvie S, Jones RB. A randomised trial of exercise therapy and foot orthoses as treatment for knee pain in primary care. British Journal of Podiatry 2004;7(2):43‐9. [Google Scholar]
Wijnen 1996 {published data only}
- Wijnen LCAM, Lenssen AF, Kuys‐Wouters YMS, Bie RA, Borghouts JAJ, Bulstra SK. McConnell therapy versus Coumans bandage for patellofemoral pain ‐ a randomised pilot study [McConnell–therapie versus Coumans–bandage bij patellofemoralepijnklachten – een gerandomiseerde pilotstudie]. NederlandsTijdschrift voor Fysiotherapie 1996;Sept (Special):12–7. [Google Scholar]
References to studies awaiting assessment
Erel 2011 {published data only}
- Erel S, Ozkan H. A comparison of the effects of closed and open kinetic chain exercises on functional status in patellofemoral pain syndrome [Turkish]. Fizyoterapi Rehabilitasyon 2011;22(3):217‐23. [Google Scholar]
References to ongoing studies
RBR‐2cxrpp {published data only}
- Serrao FV. Effects of lumbo‐pelvic stabilization training on women with patellofemoral pain syndrome. http://www.ensaiosclinicos.gov.br/rg/RBR‐2cxrpp/ (accessed 30 June 2014).
Additional references
Bessette 1988
- Bessette GC, Hunter RE. The Maquet procedure. A retrospective review. Clinical Orthopaedics and Related Research 1988;232:159‐67. [PubMed] [Google Scholar]
Binkley 1999
- Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Physical Therapy 1999;79(4):371‐83. [PubMed] [Google Scholar]
Blond 1998
- Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7‐year retrospective follow‐up study of 250 athletes. Acta Orthopaedica Belgica 1998;64(4):393‐400. [PubMed] [Google Scholar]
Bolgla 2011
- Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. International Journal of Sports Physical Therapy 2011;6(2):122‐5. [PMC free article] [PubMed] [Google Scholar]
Boling 2009
- Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scandinavian Journal of Medicine & Science in Sports 2009;20(5):725‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Callaghan 2012
- Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006717.pub2] [DOI] [PubMed] [Google Scholar]
Chesworth 1989
- Chesworth BM, Culham EG, Tata GE, Peat M. Validation of outcome measures in patients with patellofemoral syndrome. Journal of Orthopaedic and Sports Physical Therapy 1989;10(8):302‐8. [DOI] [PubMed] [Google Scholar]
Collins 2012
- Collins NJ, Bisset LM, Crossley KM, Vicenzio B. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta‐analysis of randomized trials. Sports Medicine 2012;42(1):31‐49. [DOI] [PubMed] [Google Scholar]
Crossley 2004
- Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid?. Archives of Physical Medicine & Rehabilitation 2004;85(5):815‐22. [DOI] [PubMed] [Google Scholar]
Davis 2010
- Davis IS, Powers CM. Patellofemoral pain syndrome: proximal, distal, and local factors, an international retreat, April 30‐May 2, 2009, Fells Point, Baltimore, MD. Journal of Orthopaedic and Sports Physical Therapy 2010;40(3):A1‐16. [DOI: 10.2519/jospt.2010.0302] [DOI] [PubMed] [Google Scholar]
Doberstein 2008
- Doberstein ST, Romeyn RL, Reineke DM. The diagnostic value of the Clarke sign in assessing chondromalacia patella. Journal of Athletic Training 2008;43(2):190‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebell 2004
- Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): a patient centered approach to grading evidence in the medical literature. American Family Physician 2004;69(3):548‐56. [PubMed] [Google Scholar]
Escobar 2006
- Escobar A, Quintana JM, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF‐36 after total knee replacement. Osteoarthritis and Cartilage 2007;3:273‐80. [DOI] [PubMed] [Google Scholar]
Frye 2012
- Frye JL, Ramey LN, Hart JM. The effects of exercise on decreasing pain and increasing function in patients with patellofemoral pain syndrome: a systematic review. Sports Health 2012;4(3):205‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE guideline 5
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [DOI] [PubMed] [Google Scholar]
GRADE guideline 6
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
GRADE guideline 7
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [DOI] [PubMed] [Google Scholar]
GRADE guideline 8
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [DOI] [PubMed] [Google Scholar]
Harvie 2011
- Harvie D, O'Leary T, Kumar S. A systematic review of randomized controlled trials on exercise parameters in the treatment of patellofemoral pain: what works?. Journal of Multidisciplinary Healthcare 2011;4:383‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heintjes 2003
- Heintjes EM, Berger M, Bierma‐Zeinstra SMA, Bernsen RMD, Verhaar JAN, Koes BW. Exercise therapy for patellofemoral pain syndrome. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003472] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hossain 2011
- Hossain M, Alexander P, Burls A, Jobanputra PC. Foot orthoses for patellofemoral pain in adults. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008402.pub2] [DOI] [PubMed] [Google Scholar]
Jørgensen 2014
- Jørgensen AW, Lundstrøm LH, Wetterslev J, Astrup A, Gøtzsche PC. Comparison of results from different imputation techniques for missing data from an anti‐obesity drug trial. PLoS One 2014;9(11):e111964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kannus 1999
- Kannus P, Natri A, Paakkala T, Järvinen M. An outcome study of chronic patellofemoral pain syndrome. Seven‐year follow‐up of patients in a randomized, controlled trial. Journal of Bone and Joint Surgery. American Volume 1999;81(3):355‐63. [DOI] [PubMed] [Google Scholar]
Kujala 1993
- Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy 1993;9(2):159‐63. [DOI] [PubMed] [Google Scholar]
Lankhorst 2012
- Lankhorst NE, Bierma‐Zeinstra SM, Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. British Journal of Sports Medicine 2012;42(2):81‐94. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Loudon 2002
- Loudon J, Wiesner D, Goist‐Foley H, Asjes C, Loudon K. Intrarater reliability of functional performance tests for subjects with patellofemoral pain syndrome. Journal of Athletic Training 2002;37(3):256‐61. [PMC free article] [PubMed] [Google Scholar]
Lysholm 1982
- Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. American Journal of Sports Medicine 1982;10(3):150–4. [DOI] [PubMed] [Google Scholar]
McConnell 2001
- McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis and Rheumatism 2001;45(5):453‐61. [DOI] [PubMed] [Google Scholar]
Mellor 2005
- Mellor R, Hodges PW. Motor unit synchronization is reduced in anterior knee pain. Journal of Pain 2005;6(8):550‐8. [DOI] [PubMed] [Google Scholar]
Melzack 1987
- Melzack R. The short‐form McGill Pain Questionnaire. Pain 1987;30(2):191‐7. [DOI] [PubMed] [Google Scholar]
Nimon 1998
- Nimon G, Murray D, Sandow M, Goodfellow J. Natural history of anterior knee pain: a 14‐ to 20‐year follow‐up of nonoperative management. Journal of Pediatric Orthopaedics 1998;18(1):118‐22. [PubMed] [Google Scholar]
Nissen 1998
- Nissen CW, Cullen MC, Hewett TE, Noyes FR. Physical and arthroscopic examination techniques of the patellofemoral joint. Journal of Orthopaedic and Sports Physical Therapy 1998;28(5):277‐85. [DOI] [PubMed] [Google Scholar]
Post 1999
- Post WR. Clinical evaluation of patients with patellofemoral disorders. Arthroscopy 1999;15:841‐51. [DOI] [PubMed] [Google Scholar]
Powers 1998
- Powers CM. Rehabilitation of patellofemoral joint disorders: a critical review. Journal of Orthopaedic & Sports Physical Therapy 1998;28(5):345‐54. [DOI] [PubMed] [Google Scholar]
Rathleff 2013
- Rathleff CR, Baird WN, Olesen JL, Roos EM, Rasmussen S, Rathleff MS. Hip and knee strength is not affected in 12‐16 year old adolescents with patellofemoral pain‐‐a cross‐sectional population‐based study. PLoS One 2013;8(11):e79153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selfe 2001
- Selfe J, Harper L, Perdersen I, Breen‐Turner J, Waring J. Four outcome measures for patellofemoral joint problems: Part 1. Development and validity. Physiotherapy 2001;87(10):507‐15. [Google Scholar]
Shea 1992
- Shea KP, Fulkerson JP. Preoperative computed tomography scanning and arthroscopy in predicting outcome after lateral retinacular release. Arthroscopy 1992;8(3):327‐34. [DOI] [PubMed] [Google Scholar]
Souza 2009a
- Souza RB, Powers CM. Predictors of hip internal rotation during running: an evaluation of hip strength and femoral structure in women with and without patellofemoral pain. American Journal of Sports Medicine 2009;37(3):579‐87. [DOI] [PubMed] [Google Scholar]
Souza 2009b
- Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. Journal of Orthopaedic and Sports Physical Therapy 2009;39(1):12‐9. [DOI] [PubMed] [Google Scholar]
Thomeé 1999
- Thomeé R, Augustsson J, Karlsson J. Patellofemoral pain syndrome: a review of current issues. Sports Medicine 1999;28(4):245‐62. [DOI] [PubMed] [Google Scholar]
Van der Linden 2004
- Linden FG, Westert M, Bakker DG, Schellevis D. Tweede nationale studie naar ziekten en verrichtingen in de huisartsenpraktijk. Klachten en aandoeningen in de bevolking en in de huisartsenpraktijk. Utrecht/Bilthoven: NIVEL/RIVM, 2004. [Google Scholar]
Van Linschoten 2009a
- Linschoten R, Middelkoop M, Berger MY, Heintjes EM, Verhaar JA, Willemsen SP, et al. Supervised exercise therapy versus usual care for patellofemoral pain syndrome: an open label randomised controlled trial. British Journal of Sports Medicine 2009;339(7728):1010‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Werner 1993
- Werner S, Arvidsson H, Arvidsson I, Eriksson E. Electrical stimulation of vastus medialis and stretching of lateral thigh muscles in patients with patello‐femoral symptoms. Knee Surgery, Sports Traumatology, Arthroscopy 1993;1(2):85‐92. [DOI] [PubMed] [Google Scholar]
Willson 2008
- Willson JD, Davis IS. Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clinical Biomechanics 2008;23(2):203‐11. [DOI] [PubMed] [Google Scholar]
Witvrouw 2004
- Witvrouw E, Danneels L, Tiggelen D, Willems TM, Cambier D. Open versus closed kinetic chain exercises in patellofemoral pain: a 5‐year prospective randomized study. American Journal of Sports Medicine 2004;32(5):1122‐30. [DOI] [PubMed] [Google Scholar]
Witvrouw 2014
- Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett‐Jones DM, Willson JD, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. British Journal of Sports Medicine 2014;48(6):411‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van der Heijden 2013
- Heijden RA, Lankhorst NE, Linschoten R, Bierma‐Zeinstra SMA, Middelkoop M. Exercise for treating patellofemoral pain syndrome. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010387] [DOI] [PMC free article] [PubMed] [Google Scholar]


