Abstract
Purpose of Review
Review updates for the association of HDL-cholesterol with atherosclerotic cardiovascular disease (ASCVD) and discuss the approach to incorporating HDL-cholesterol within risk assessment.
Recent Findings
There is a U-shaped relationship between HDL-cholesterol and ASCVD. Both low HDL-cholesterol (< 40 mg/dL in men, < 50 mg/dL in women) and very-high HDL-cholesterol (≥ 80 mg/dL in men) are associated with a higher risk of all-cause and ASCVD mortality, independent from traditional risk factors. There has been inconsistency for the association between very-high HDL-cholesterol and mortality outcomes in women. It is uncertain whether HDL-cholesterol is a causal ASCVD risk factor, especially due to mixed results from Mendelian randomization studies and the collinearity of HDL-cholesterol with established risk factors, lifestyle behaviors, and socioeconomic status.
Summary
HDL-cholesterol is a risk factor or risk enhancer in primary prevention and high-risk condition in secondary prevention when either low (men and women) or very-high (men). The contribution of HDL-cholesterol to ASCVD risk calculators should reflect its observed U-shaped association with all-cause and ASCVD mortality.
Keywords: HDL cholesterol, Lipids, Cardiovascular disease, Risk
Introduction
Measurement of high-density lipoprotein-cholesterol (HDL-C) level is important in primary and secondary atherosclerotic cardiovascular disease (ASCVD) risk assessment because reduced HDL-C is one of the most common lipid abnormalities in patients with clinical ASCVD [1]. Early observational studies have demonstrated a strong, independent, and inverse relationship between HDL-C and ASCVD risk [2, 3], as initial findings from the Framingham Heart Study found that each 5 mg/dL lower HDL-C below the median for men and women is associated with a 25% higher risk for myocardial infarction [4]. These data have supported the role of HDL-C in protection from atherosclerosis, predominantly mediated through the process of reverse cholesterol transport [5].
Despite the important role of HDL-C in reverse cholesterol transport, emerging evidence from randomized controlled clinical trials and more recent prospective cohort studies has suggested a more nuanced contribution of HDL-C to ASCVD risk. The intentional raising of HDL-C in primary and secondary prevention with niacin [6], fibrates [7], and/or cholesteryl ester transfer protein (CETP) inhibitors [8] has not resulted in reduced ASCVD risk, while contemporary observational cohort studies have found that very-high HDL-C (≥ 80 mg/dL in men, ≥ 100 mg/dL in women) may be associated with higher all-cause and ASCVD mortality [9••, 10••]. Taken together, these findings underline that our understanding of HDL-C in ASCVD is continuing to evolve and how to optimally incorporate HDL-C within routine clinical risk assessment is thus of great interest [11].
In this review, we aim to predominantly focus on the role of HDL-cholesterol in ASCVD risk by discussing (1) genomics involving HDL-C, (2) implications of HDL-C and very-high HDL-C in primary and secondary prevention, (3) potential mechanisms underlying the association of very-high HDL-C and adverse outcomes, and (4) propose an alternative approach for evaluating the contribution of HDL-C to current clinical ASCVD risk prediction.
HDL-C Genomics and Inherited Deficiencies
Genetic profile plays a critical role in HDL-C levels, supported by a high estimated heritability of 40–60% [12, 13]. While genome-wide association studies (GWAS) have identified many single nucleotide polymorphisms (SNPs) that can serve as robust genetic instrumental variables of HDL-C [14, 15], these SNPs account for relatively small variation in HDL levels [9••]. The most recent large-scale multi-ancestry GWAS that included ~ 1.6 million individuals have identified 562 genomic loci associated with HDL-C at a stringent genome-wide association level [16]. Multiple Mendelian randomization models have been widely used for causal effect estimation of HDL-C, including the inverse variance weighting, Egger, as well as the pleiotropy residual sum and outlier models [17–20].
A large Mendelian randomization study of over 20,000 myocardial infarction cases investigated the causal effect of HDL-C using the endothelial lipase gene LIPG and 14 common SNPs associated with HDL-C exclusively and suggested that genetic mechanisms that raise HDL-C may not necessarily lead to a decreased risk of myocardial infarction [21]. A study investigating the lecithin-cholesterol acyltransferase (LCAT) gene similarly found that genetically decreased HDL-C levels did not result in lower myocardial infarction rates [22]. In over 12,000 coronary heart disease (CHD) cases, although a causal effect of triglycerides was recognized, there was no clear evidence of causal effect for HDL-C [23]. Another multivariable Mendelian randomization study identified a small magnitude inverse association between HDL-C and coronary artery disease after adjustment for other lipids, concluding that interventions targeting HDL-C as compared to low-density lipoprotein cholesterol (LDL-C) levels may have a modest impact on CHD risk [19]. Overall, the current evidence regarding the causal effect of HDL-C on ASCVD demonstrates limited impact or non-causality.
Several inherited disorders that lead to major reductions in HDL-C are associated with a heightened risk factor for ASCVD. For example, familial hypoalphalipoproteinemia is an autosomal dominant disorder associated with premature ASCVD. These individuals have reduced levels of apolipoprotein A-1, the main protein component of HDL, due to a compromise in protein production, increased breakdown, or enzymatic alterations [24]. Similarly, mutations in the adenosine triphosphate-binding cassette transporter (ABCA1) gene encoding the cholesterol efflux regulatory protein contribute to the higher HDL catabolism found in familial HDL deficiency and Tangier disease which are also associated with premature CHD [25–27]. However, the association between mutations in ABCA1 and CHD is independent of serum HDL-C levels [28] and there appears to be notable heterogeneity among individuals with inherited causes of low HDL-C, such that not all develop premature atherosclerosis [27]. These observations may corroborate with Mendelian randomization studies and underline that a more nuanced approach beyond measurement of HDL-C itself may be beneficial to understanding its association with ASCVD.
HDL-C as a Protective Risk Marker for ASCVD
Many observational cohort studies have demonstrated that HDL-C shares a linear, inverse association with ASCVD. Among the most notable and initial findings were derived from nearly 3,000 men and women free of clinical ASCVD from the Framingham Heart Study. Here, investigators found that individuals with HDL-C > 65 mg/dL had an eight-fold lower incidence of ASCVD compared to those with HDL-C < 35 mg/dL over a median follow-up time of 4 years [3]. Similar findings were subsequently discovered across an independent collection of observational cohort studies, suggesting that each 10 mg/dL higher increment in HDL-C was associated with up to a 3% lower incidence of ASCVD [29]. Thereafter, the association of HDL-C with ASCVD was studied in larger and more diverse cohorts, including the ARIC (Atherosclerosis Risk in Communities), Cardiovascular Health, the CARDIA (Coronary Artery Risk Development in Young Adults), and the Framingham Original and Offspring Study cohorts, which contributed to the 2013 Pooled Cohort Equations (PCE) risk calculator [30]. The 2013 PCE calculator includes HDL-C as a negative risk factor in risk equations and remains as the guideline-based first-line tool for risk assessment to guide eligibility for statin therapy in primary prevention patients [30].
The protective association between higher HDL-C and ASCVD outcomes appears to be stronger among those with known CHD. For example, among nearly 2200 patients with stable CHD, those in the highest quintile of HDL-C values (≥ 48 mg/dL) have a one-third lower crude rate of myocardial infarction and all-cause mortality compared to patients in the lowest quintile of HDL-C (< 35 mg/dL) after adjusting for age, sex, body mass index, hypertension, diabetes, current cigarette smoking, LDL-C, and serum triglycerides [31]. Furthermore, meta-analysis of large randomized controlled trials involving statins has demonstrated that the inverse association between HDL-C and ASCVD outcomes is not attenuated or modified by statin therapy [32]. However, not all studies among patients with clinical CHD have identified an independent inverse association between HDL-C and ASCVD outcomes, especially among patients treated with high-intensity statins [33], and after adjusting for LDL-cholesterol particle concentration [34].
There are several possible explanations for a lack of association between low HDL-C and a higher risk of ASCVD after considering adjacent risk factors, lifestyle, demographics, and socioeconomic status. First, low HDL-C is often found among persons with metabolic syndrome [35], and/or type 2 diabetes [36]—which have also been identified to be independent risk factors for incident ASCVD. Second, low HDL-C is associated with both unhealthy (suboptimal physical activity) [37] and healthy lifestyle behaviors (low-fat, plant-based dietary pattern) [38]. Ethnicity also appears to modify the association between HDL-C and ASCVD, as low HDL-C has been recently observed to confer a 22% higher risk of ASCVD events in White, but not Black, adults [39•]. Lastly, low HDL-C has been associated with a lower median income [40]. While the inverse association between HDL-C and ASCVD risk is important within both primary and secondary prevention, clinicians and population health scientists should also consider the company that low HDL-C keeps, including accompanying metabolic disorders, lifestyle habits, and socioeconomic status.
Very-High HDL-C and ASCVD Risk
In the past decade, observational evidence has indicated that very-high HDL-C level is paradoxically associated with a higher risk for all-cause and ASCVD mortality in patients with and without clinical ASCVD [9••, 10••, 40–53]. In particular, the association between very-high HDL-C and adverse outcomes has been most notable in men compared to women. Despite this rapidly evolving evidence, no universal definition of very-high HDL-C exists, though spline analyses suggest that elevated risk begins at approximately 80 mg/dL in men and 100 mg/dL in women [46]. Using these clinical thresholds, initial estimates suggest that the prevalence of very-high HDL-C would range between 2 and 11% based on the sample studied [9••, 40, 49]. While very-high HDL-C may be associated with certain underlying genetic polymorphisms, alcohol consumption is also higher in this subset [9••, 50], which may help explain a certain proportion of the downstream risk attributable to very-high HDL-C.
In one of the most recent studies among 415,416 participants without clinical ASCVD enrolled in the UK Biobank, very-high HDL-C (≥ 80 mg/dL in men [2%], ≥ 100 mg/dL in women [11%]) conferred an 80% higher risk of all-cause and ASCVD mortality in men, but not women over nearly one decade of follow-up [9••]. The reference/comparator group in this study were men and women with HDL-C values between 40 and 60 mg/dL, and covariables adjusted for included traditional risk factors and an HDL-specific genetic risk score. This study underlines the U-shaped relationship between HDL-C and ASCVD mortality, especially among men, in a primary prevention sample (Fig. 1). Similar findings have been observed in large, independent cohort of individuals without known ASCVD, suggesting that the association of very-high HDL-C with all-cause mortality is more pronounced in men compared to women [40, 47, 50, 52]. For example, among a pooled cohort of nearly 43,000 individuals without clinical ASCVD, men with HDL-C ≥ 90 mg/dL had a 2.6-fold higher risk of ASCVD mortality after adjustment for traditional risk factors and current alcohol consumption, whereas no significant association was identified in women [50]. Given the high burden of hypertension in primary prevention, the association of very-high HDL-C has also been studied specifically among participants who have not had an ASCVD event but who have hypertension [41]. Here, men with HDL-C ≥ 80 mg/dL had a 35% higher risk of ASCVD events compared to those with HDL-C 40–79 mg/dL, and a similar magnitude risk was found for individuals with low HDL-C (< 40 mg/dL) [41]. While not all studies involving very-high HDL-C have observed sex differences [40, 49] or significant associations [48, 53] of very-high HDL-C with all-cause or ASCVD mortality, the general summary of current literature is that very-high HDL-C may be a marker of excess downstream risk, more strongly in men compared to women.
Fig. 1.
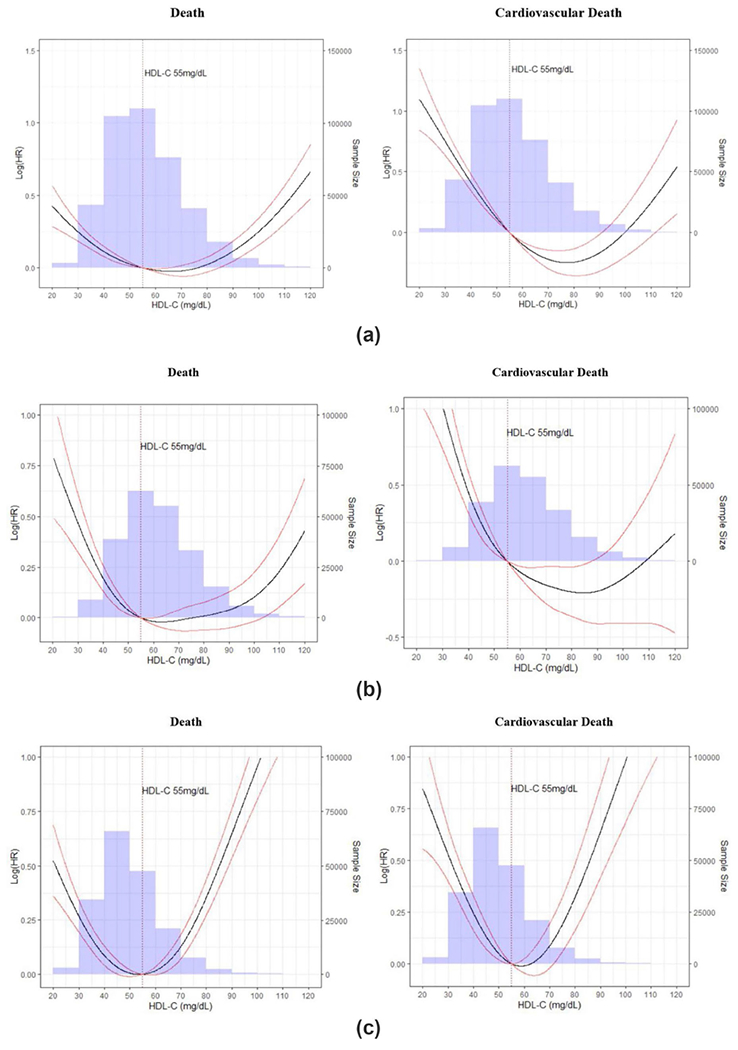
Association of very-high HDL-C with death and ASCVD mortality in the overall sample (a), women (b), and men (c). ASCVD, atherosclerotic cardiovascular disease; HDL-C, high density lipoprotein-cholesterol. (From Liu, C et al. Am J Cardiol. 2022;167(188):120–1, with permission from Elsevier) [9••]. ASCVD, atherosclerotic cardiovascular disease; HDL-C, high-density lipoprotein-cholesterol
In general, very-high HDL-C has most strongly conferred a higher risk for all-cause mortality compared to other ASCVD outcomes in analyses that have also included individuals with CHD. To our knowledge, in the only study performed exclusively among individuals with clinical CHD, those with HDL-C ≥ 80 mg/dL (2% of individuals) experienced a 96% and 71% higher risk for all-cause mortality and ASCVD mortality when compared to persons with HDL-C values between 40 and 60 mg/dL after adjustment for traditional risk factors, alcohol consumption, medications, kidney function, and genetic polymorphisms associated with higher HDL-C [10••]. In this study, individuals with HDL-C ≥ 30 mg/dL also had an adjusted 33% and 42% higher risk of all-cause and ASCVD mortality. In sensitivity analyses, the risk associated with very-high HDL-C was replicated and significant only for men and the outcome of all-cause mortality [10••]. Thus, there is evidence for very-high HDL-C as a high-risk condition and risk enhancer among individuals who have and have not experienced an index ASCVD event, respectively, which may have implications for routine risk assessment and current clinical risk calculators.
Potential Mechanisms Underlying Very-High HDL-C and Adverse Outcomes
There are several possible explanations for the observed associations between very-high HDL-C and adverse outcomes. With respect to atherosclerosis, very-high HDL-C may be associated with reduced cholesterol efflux. However, measurement of serum ApoA-I concentrations, the main HDL-C component that facilitates reverse cholesterol transport, does not seem to provide additional value beyond measurement of HDL-C itself as both ApoA-I and HDL-C have identical U-shaped associations with ASCVD outcomes [54].
Measurement of peripheral HDL-C is not a precise measure of HDL function, specifically cholesterol efflux capacity. Instead, current evidence suggests that a mass effect underlies cholesterol efflux capacity, such that both the number and size of HDL particles may be most important. Previous studies have shown that cholesterol efflux capacity is positively associated with large- and medium-sized HDL particles, while being inversely associated with small HDL particles [55, 56]. Independent of traditional risk factors, including HDL-C, individuals in the highest tertile of cholesterol efflux capacity have a 36% lower risk of CHD compared to individuals in the lowest tertile of cholesterol efflux capacity [57]. Similar findings have been replicated in independent cohorts including patients with and without clinical CHD. Cholesterol efflux assays thus may provide clinical value for individuals with very-high HDL-C as they measure the total efflux cholesterol from macrophages [56, 58]. In the first trial of its kind, the AEGIS-II trial is currently ongoing and will report on whether augmenting cholesterol efflux capacity with ApoA-I infusion in high-risk patients post myocardial infarction can reduce risk for recurrent CHD events [59]. Furthermore, while CETP inhibitors substantially increase HDL-C levels (as much as two-fold), there is an ongoing trial of CETP inhibition with obicetrapib in combination with ezetimibe for additional LDL-C and apolipoprotein-B (ApoB) lowering for patients who are already on high-intensity statin therapy [60].
Additionally, inflammation modifies the protective association between HDL-C and incident CHD, such that C-reactive protein and interleukin-6 are more strongly associated with CHD events among individual with lower (< 40 mg/dL) versus higher HDL-C (≥ 60 mg/dL) [61]. Thus, concomitant measurement of select inflammatory markers may be helpful to guide ASCVD risk assessment among individuals with very-high HDL-C; however, future studies in this research space are required.
As noted earlier, alcohol consumption is an important environmental factor potentially underlying the U-shaped relationship between HDL-C and adverse outcomes. Genetic epidemiology studies suggest that habitual alcohol consumption is linearly associated with both hypertension and CHD [62]. Given the dose-dependent association between alcohol consumption and HDL-C levels [63], the association between very-high HDL-C and mortality may in part be mediated through or explained by alcohol consumption patterns.
Contribution of HDL-C to ASCVD Risk Assessment and Prediction
In primary prevention, the PCE calculator to quantify 10-year ASCVD risk and guide statin therapy incorporates HDL-C as a strictly negative risk factor [30]. While it is certainly reasonable to consider low HDL-C as a risk factor for incident ASCVD events, this approach may be overly simplistic to guide ASCVD risk assessment for several reasons. First, ethnicity is known to modify the association between low HDL-C and ASCVD risk, as observational analyses from the REGARDS (REasons for Geographic and Racial Differences in Stroke) have found low HDL-C is associated with a higher risk of incident CHD in White but not Black men and women [39•]. Second, several other HDL metrics, including density, apolipoprotein content, particle number, and particle size also, contribute to function and may thus not be captured by simply measuring absolute HDL-C [11]. Lastly, carefully conducted observational evidence over the past decade has shown that very-high HDL-C may be a marker of elevated risk when compared to normal HDL-C values, more consistently in men versus women and patients with versus without clinical ASCVD [9••, 10••].
In the setting of such evidence, we propose to use HDL-C as both a traditional risk factor and a risk enhancer/high-risk condition in ASCVD risk assessment (Fig. 2). Here, low HDL-C (< 40 mg/dL in men, < 50 mg/dL in women) and very-high (≥ 80 mg/dL in men) would contribute as a risk-enhancing factor (risk enhancer) or high-risk condition (secondary prevention) to global risk assessment for the consideration of diagnostic (e.g., Lp(a) testing or coronary artery calcium scoring) and therapeutic (e.g., statin and aspirin therapy) decision-making in primary prevention, and inform treatment intensification (e.g., combination lipid-lowering therapy) in secondary prevention. In the situation when HDL-C values are between 40–80 mg/dL in men and 50–100 mg/dL in women, HDL-C would not necessarily contribute to higher or lower risk unless there was clinical suspicion for a functional HDL-C abnormality, which could prompt more in-depth assessments including measurement of HDL particle number and/or cholesterol efflux capacity assays. For women, while very-high HDL-C (≥ 100 mg/dL) has not consistently conferred an increased for ASCVD or all-cause mortality, there does not appear to be a lower ASCVD risk for HDL-C ≥ 100 versus 50–100 mg/dL. Importantly, a normal or high HDL-C should not be used as a rationale to discourage the use of statins in a patient who meets statin eligibility criteria based on calculated 10-year risk, LDL-C, ApoB levels, and/or presence of coronary artery calcium.
Fig. 2.
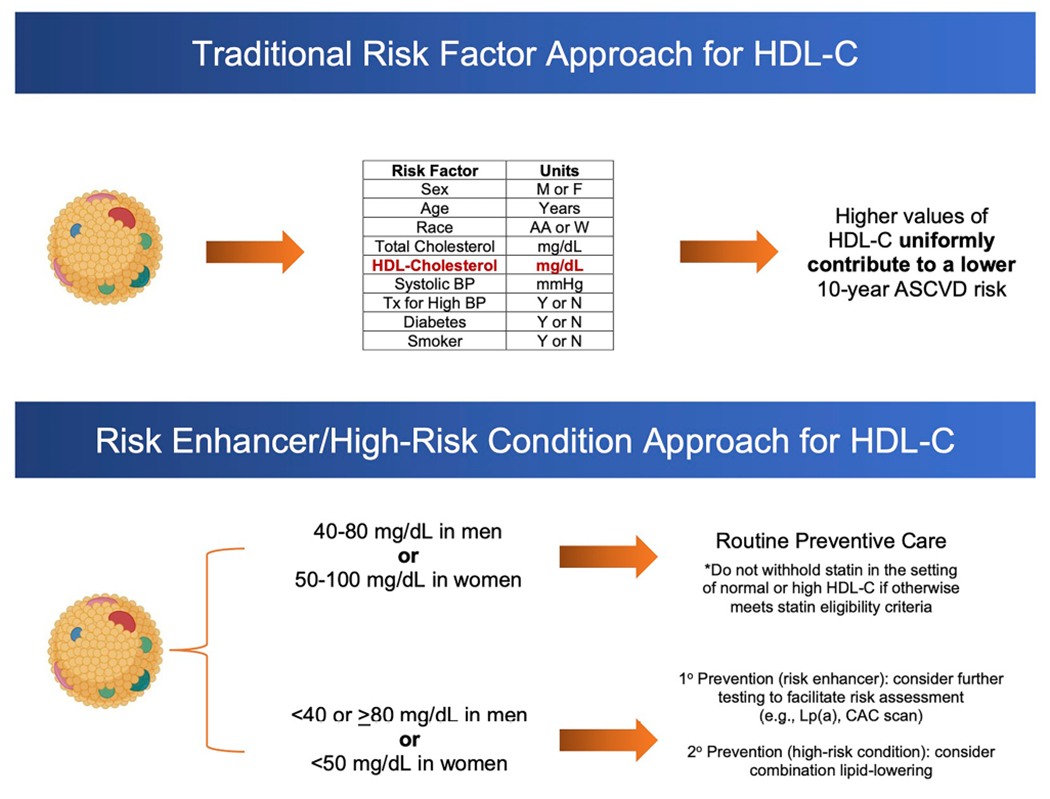
HDL-C as a traditional risk factor or risk enhancer in primary prevention and high-risk condition in secondary prevention. CAC, coronary artery calcium; HDL-C, high-density lipoprotein-cholesterol; Lp(a), lipoprotein(a)
Conclusion
The current summary of evidence indicates a U-shaped relationship between HDL-C values and ASCVD risk. Both low HDL-C (< 40 mg/dL in men, < 50 mg/dL in women) and very-high HDL-C (≥ 80 mg/dL in men) are associated with an increased risk of ASCVD and all-cause mortality, independent from traditional risk factors. For women, while very-high HDL-C (≥ 100 mg/dL) has not consistently conferred an increased risk for ASCVD or all-cause mortality, there does not appear to be added protection for HDL-C ≥ 100 versus 50–100 mg/dL. It is likely that HDL-C is not a causal risk factor for ASCVD based on the overwhelming evidence from Mendelian randomization studies and the collinearity of HDL-C with established ASCVD risk factors, lifestyle behaviors, and socioeconomic status. In the setting of these findings, it is important to continue evolving current risk assessment approaches that involve HDL-C to help facilitate more precise diagnostic and therapeutic decision-making in the primary and secondary prevention of ASCVD.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Rohatgi A, Westerterp M, von Eckardstein A, Remaley A, Rye KA. HDL in the 21st Century. Circulation. 2021;143(23):2293–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA - J Am Med Assoc. 2009;302(18):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med. 1977;62(5):707–14. [DOI] [PubMed] [Google Scholar]
- 4.Castelli WP. Cardiovascular disease and multifactorial risk: challenge of the 1980s. Am Heart J. 1983;106(5 PART 2). [DOI] [PubMed] [Google Scholar]
- 5.von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. 2022;39:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–12. [DOI] [PubMed] [Google Scholar]
- 7.ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR III, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brousseau ME, Schaefer EJ, Wolfe ML, Bloedon LT, Digenio AG, Clark RW, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350(15):1505–15. [DOI] [PubMed] [Google Scholar]
- 9.••.Liu C, Dhindsa D, Almuwaqqat Z, Sun YV, Quyyumi AA. Very high high-density lipoprotein cholesterol levels and cardiovascular mortality. Am J Cardiol. 2022;167(188):120–1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Among individuals without overt CVD, very-high HDL-C was independently associated with a higher risk of cardiovascular and all-cause mortality in men, but not women.
- 10.••.Liu C, Dhindsa D, Almuwaqqat Z, Ko YA, Mehta A, Alkhoder AA, et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. 2022;7(7):672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; Among individuals with coronary artery disease, very-high HDL-C was independently associated with a higher risk of cardiovascular and all-cause mortality.
- 11.Razavi A, Jain V, Grandhi G, Patel P, Karagiannis A, Patel N, et al. Does elevated high-density lipoprotein cholesterol protect against cardiovascular disease? J Clin Endocrinol Metab. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissglas-Volkov D, Pajukanta P. Genetic causes of high and low serum HDL-cholesterol. J Lipid Res. 2010;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qasim A, Rader D. Human genetics of variation in high-density lipoprotein cholesterol. Curr Atheroscler Rep. 2006;8. [DOI] [PubMed] [Google Scholar]
- 14.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, et al. Genetics of blood lipids among ~300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet. 2018;50(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham SE, Clarke SL, Wu KHH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600(7890). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J, Smith GD, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith GD, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S, Freitag DF, Khan H, Gorman DN, Thompson SG. Using multivariable Mendelian randomization to disentangle the causal effects of lipid fractions. PLoS One. 2014;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haase CL, Tybjærg-Hansen A, Ali Qayyum A, Schou J, Nordestgaard BG, Frikke-Schmidt R. LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J Clin Endocrinol Metab. 2012;97(2):248–56. [DOI] [PubMed] [Google Scholar]
- 23.Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genest J, Bard JM, Fruchart JC, Ordovas JM, Schaefer EJ. Familial hypoalphalipoproteinemia in premature coronary artery disease. Arterioscler Thromb. 1993;13(12). [DOI] [PubMed] [Google Scholar]
- 25.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, Van Dam M, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22(4). [DOI] [PubMed] [Google Scholar]
- 26.Marcil M, Brooks-Wilson A, Clee SM, Roomp K, Zhang LH, Yu L, et al. Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet. 1999;354(9187). [DOI] [PubMed] [Google Scholar]
- 27.Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994;107(1). [DOI] [PubMed] [Google Scholar]
- 28.Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis. 2010;208. [DOI] [PubMed] [Google Scholar]
- 29.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. [DOI] [PubMed] [Google Scholar]
- 30.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 31.Acharjee S, Boden WE, Hartigan PM, Teo KK, Maron DJ, Sedlis SP, et al. Low levels of high-density lipoprotein cholesterol and increased risk of cardiovascular events in stable ischemic heart disease patients: a post-hoc analysis from the COURAGE trial (clinical outcomes utilizing revascularization and aggressive drug evaluat. J Am Coll Cardiol. 2013;62(20):1826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jafri H, Alsheikh-Ali AA, Karas RH. Meta-analysis: Statin therapy does not alter the association between low levels of high-density lipoprotein cholesterol and increased cardiovascular risk. Ann Intern Med. 2010;153. [DOI] [PubMed] [Google Scholar]
- 33.Van De Woestijne AP, Van Der Graaf Y, Liem AH, Cramer MJM, Westerink J, Visseren FLJ. Low high-density lipoprotein cholesterol is not a risk factor for recurrent vascular events in patients with vascular disease on intensive lipid-lowering medication. J Am Coll Cardiol. 2013;62(20). [DOI] [PubMed] [Google Scholar]
- 34.MacKey RH, Greenland P, Goff DC, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2012;60(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson PWF, Grundy SM. The metabolic syndrome. A practical guide to origins and treatment: Part II. Circulation. 2003;108. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, et al. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28(8). [DOI] [PubMed] [Google Scholar]
- 37.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167. [DOI] [PubMed] [Google Scholar]
- 38.Kent L, Morton D, Rankin P, Ward E, Grant R, Gobble J, et al. The effect of a low-fat, plant-based lifestyle intervention (CHIP) on serum HDL levels and the implications for metabolic syndrome status - a cohort study. Nutr Metab. 2013;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.•.Zakai NA, Minnier J, Safford MM, Koh I, Irvin MR, Fazio S, et al. Race-dependent association of high-density lipoprotein cholesterol levels with incident coronary artery disease. J Am Coll Cardiol. 2022;80(22):2104–15. [DOI] [PubMed] [Google Scholar]; Low HDL-C (<40 mg/dL in men, <50 mg/dL in women) independently associated with a higher coronary heart disease in white but not black adults. High HDL-C (>60 mg/dL) was not independently associated with a lower coronary heart disease risk in either black or white adults.
- 40.Ko DT, Alter DA, Guo H, Koh M, Lau G, Austin PC, et al. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol. 2016;68(19). [DOI] [PubMed] [Google Scholar]
- 41.Trimarco V, Izzo R, Morisco C, Mone P, Virginia Manzi M, Falco A, et al. High HDL (high-density lipoprotein) cholesterol increases cardiovascular risk in hypertensive patients. Hypertension. 2022;79(10):2355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowe B, Xie Y, Xian H, Balasubramanian S, Zayed MA, Al-Aly Z. High density lipoprotein cholesterol and the risk of all-cause mortality among U.S. veterans. Clin J Am Soc Nephrol. 2016;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamer M, O’Donovan G, Stamatakis E. High-density lipoprotein cholesterol and mortality too much of a good thing? Arterioscler Thromb Vasc Biol. 2018;38(3). [DOI] [PubMed] [Google Scholar]
- 44.Li ZH, Lv YB, Zhong WF, Gao X, Byers Kraus V, Zou MC, et al. High-density lipoprotein cholesterol and all-cause and cause-specific mortality among the elderly. J Clin Endocrinol Metab. 2019;104(8). [DOI] [PubMed] [Google Scholar]
- 45.Li X, Guan B, Wang Y, Tse G, Zou F, Khalid BW, et al. Association between high-density lipoprotein cholesterol and all-cause mortality in the general population of northern China. Sci Rep. 2019;9(1):14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madsen CM, Varbo A, Nordestgaard BG. Novel insights from human studies on the role of high-density lipoprotein in mortality and noncardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41(1):128–40. [DOI] [PubMed] [Google Scholar]
- 47.Wilkins JT, Ning H, Stone NJ, Criqui MH, Zhao L, Greenland P, et al. Coronary heart disease risks associated with high levels of HDL cholesterol. J Am Heart Assoc. 2014;3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirata A, Okamura T, Sugiyama D, Kuwabara K, Kadota A, Fujiyoshi A, et al. The relationship between very high levels of serum high-density lipoprotein cholesterol and cause-specific mortality in a 20-year follow-up study of Japanese general population. J Atheroscler Thromb. 2016;23(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordestgaard BG, Madsen CM, Varbo A. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Atherosclerosis. 2017;263(38):2478–86. [DOI] [PubMed] [Google Scholar]
- 50.Hirata A, Sugiyama D, Watanabe M, Tamakoshi A, Iso H, Kotani K, et al. Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: the EPOCH–JAPAN study. J Clin Lipidol. 2018;12(3). [DOI] [PubMed] [Google Scholar]
- 51.Mazidi M, Mikhailidis DP, Banach M. Associations between risk of overall mortality, cause-specific mortality and level of inflammatory factors with extremely low and high high-density lipoprotein cholesterol levels among American adults. Int J Cardiol. 2019;276. [DOI] [PubMed] [Google Scholar]
- 52.Oh IH, Hur JK, Ryoo JH, Jung JY, Park SK, Yang HJ, et al. Very high high-density lipoprotein cholesterol is associated with increased all-cause mortality in South Koreans. Atherosclerosis. 2019;283. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi D, Noto H, Shimbo T, Ino T, Osugi Y, Takahashi O, et al. Repeated measures of extremely high levels of high-density lipoprotein cholesterol and subsequent all-cause mortality and cardiovascular events: a longitudinal study. Atherosclerosis. 2019;288. [DOI] [PubMed] [Google Scholar]
- 54.Faaborg-Andersen CC, Liu C, Subramaniyam V, Desai SR, Sun YV, Wilson PWF, et al. U-shaped relationship between apoli-poprotein A1 levels and mortality risk in men and women. Eur J Prev Cardiol [Internet]. 2022;30(4):293–304. Available from: 10.1093/eurjpc/zwac263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mutharasan RK, Thaxton CS, Berry J, Daviglus ML, Yuan C, Sun J, et al. HDL efflux capacity, HDL particle size, & high-risk carotid atherosclerosis in a cohort of asymptomatic older adults: the Chicago Healthy Aging Study. J Lipid Res. 2017;58(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(7):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibson CM, Kastelein JJP, Phillips AT, Aylward PE, Yee MK, Tendera M, et al. Rationale and design of ApoA-I Event Reducing in Ischemic Syndromes II (AEGIS-II): a phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to investigate the efficacy and safety of CSL112 in subjects after acute myocardial infarction. Am Heart J. 2021;231:121–7. [DOI] [PubMed] [Google Scholar]
- 60.Ballantyne CM, Ditmarsch M, Kastelein JJ, Nelson AJ, Kling D, Hsieh A, et al. Obicetrapib plus ezetimibe as an adjunct to high-intensity statin therapy: a randomized phase 2 trial. J Clin Lipidol. 2023. [DOI] [PubMed] [Google Scholar]
- 61.Tehrani DM, Gardin JM, Yanez D, Hirsch CH, Lloyd-Jones DM, Stein PK, et al. Impact of inflammatory biomarkers on relation of high density lipoprotein-cholesterol with incident coronary heart disease: Cardiovascular Health Study. Atherosclerosis. 2013;231(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biddinger KJ, Emdin CA, Haas ME, Wang M, Hindy G, Ellinor PT, et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Oliveira e Silva ER, Foster D, Harper MM, Seidman CE, Smith JD, Breslow JL, et al. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102(19 SUPPL.). [DOI] [PubMed] [Google Scholar]


