Abstract
Background:
Comprehensive assessment of morbidity after allogeneic BMT performed in childhood remains understudied.
Methods:
Seven hundred and eighty-nine allogeneic BMT recipients who had survived ≥2y after BMT performed between 1974 and 2014 at age <22y, and 690 siblings completed a 255-item survey, self-reporting sociodemographics and chronic health conditions. A severity score (grade 3 [severe], 4 [life-threatening] or 5 [fatal]) was assigned to the conditions using CTCAE, v5.0. For the BMT cohort, the cumulative incidence of chronic health conditions was calculated as a function of time from BMT. Proportional subdistribution hazards models were used to determine predictors of grade 3–5 conditions. Logistic regression was used for estimating the risk of grade 3–4 conditions in BMT recipients alive at study compared to siblings.
Results:
Median age at transplantation was 11.3y (range: 0.4–22.0); median length of follow-up was 11.7y (2.0–45.3). The most prevalent primary diagnoses were acute lymphoblastic leukemia (30.7%), and acute myeloid leukemia/myelodysplastic syndrome (26.9%). At age 35y, the cumulative incidence of a grade 3–4 condition was 53.8% (95%CI; 46.7–60.3). The adjusted odds ratio of a grade 3–4 condition was 15.1 in survivors (95%CI=9.5–24.0) compared to siblings. The risk of a grade 3–5 condition increased with age at BMT (hazard ratio [HR]=1.03, 95%CI=1.01–1.05) and was higher among females (HR=1.27, 95%CI; 1.02–1.59), patients receiving TBI (HR=1.71, 95%CI; 1.27–2.31), and those reporting chronic GVHD (HR=1.38, 95%CI; 1.09–1.74).
Conclusion:
Two-year survivors of allogeneic BMT in childhood have an increased risk of grade 3–4 chronic health conditions compared to siblings, suggesting the need for long-term follow-up.
Keywords: hematopoietic stem cell transplantation, morbidity, neoplasms, pediatrics
Condensed abstract:
Two-year survivors of allogeneic BMT in childhood have a substantially increased risk of chronic health conditions compared to siblings.
By the age of 35 years, more than half of BMT recipients have a severe or life-threatening chronic health condition.
INTRODUCTION
Allogeneic blood or marrow transplantation (BMT) is used with curative intent for children with several malignant and non-malignant disorders. Survivors of childhood cancer treated in a non-transplant setting are at increased risk of late morbidity that is directly related to therapeutic exposures.1, 2 The additional therapeutic exposures in the transplant setting potentially further increases the burden of late morbidity. Previous studies have reported a high burden of long-term morbidity borne by allogeneic BMT survivors; however, these studies either focused exclusively on adults or on cohorts that consisted largely of adults.3–10 Studies focusing exclusively on survivors of BMT performed in childhood are limited by small samples.11–18 Armenian et al compared morbidity in survivors of childhood cancer undergoing BMT with those treated with conventional therapy and found that BMT recipients had a higher burden of adverse health.19 However, this study included only 105 allogeneic BMT recipients, all transplanted before 1998. Gaps in knowledge remain regarding the risk of specific chronic health conditions in survivors of allogeneic BMT performed in childhood and whether the burden of morbidity has changed with modifications in transplant practice over the past four decades. We addressed these gaps by using the resources offered by the BMT Survivor Study (BMTSS).
METHODS
BMTSS is a retrospective cohort study examining long-term outcomes in individuals who survived two or more years after BMT performed between 1974 and 2014 at the University of Minnesota, City of Hope, or University of Alabama at Birmingham. To be included in the present analysis, patients had to have received allogeneic BMT before the age of 22y and survived at least 2y after transplantation, regardless of disease status at cohort entry or vital status after cohort entry. Patients who received BMT for an inborn error of metabolism were not included, nor were patients who received a second BMT. A comparison cohort of siblings without a history of BMT was selected from the sibling cohort of BMTSS (Supplemental Material 1).
Of the 1,322 patients eligible for study participation, 1,039 (79%) were successfully contacted, and 789 (60%) participated. Participants were older at BMT than non-participants (mean age, 11.3 vs. 10.3 years, P=0.002). Females (45.3% vs. 37.7%, P=0.007), non-Hispanic whites (74.8% vs. 60.4%, P<0.001), and patients transplanted before year 2000 (58.3% vs. 29.8%, P<0.001), were more likely to participate (Supplemental Table 1).
Information on primary diagnosis, age at BMT, pre-BMT treatment, conditioning intensity (myeloablative; non-myeloablative/reduced intensity),20 type of donor (related; unrelated), stem cell source (bone marrow; peripheral blood; cord blood), chronic graft vs. host disease (cGvHD) and exposure to total body irradiation (TBI) was obtained on all participating BMT survivors from the institutional transplant databases. Survivors and siblings (or parents of participants <18y) completed a 255-item BMTSS survey that covers the following areas: diagnosis by a healthcare provider of chronic health conditions (including age at diagnosis), healthcare utilization and sociodemographic characteristics (race/ethnicity, sex, education, household income and insurance). The reliability and validity of the BMTSS survey was tested, and responses regarding chronic health conditions demonstrate a high level of agreement with the medical records.21
Chronic health conditions diagnosed after BMT were graded using the Common Terminology Criteria for Adverse Events (CTCAE), v5.0, distinguishing grades 1 through 5 based on the severity of each event (grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening/disabling; grade 5, death from chronic health conditions).22 The same scoring system was applied to responses from the sibling comparison group. A detailed description of the questions asked in the BMTSS survey, the corresponding chronic health condition categories created from the responses and the scoring of these conditions are presented in Supplemental Table 2. For participants with more than one chronic health condition, the condition with a maximum grade was used. For the deceased patients, we used the cause of death information from the National Death Index (NDI) Plus; a grade 5 was assigned to the chronic health conditions stated as the cause of death in the NDI Plus files. Deaths from primary disease, accident, or suicide were not graded. The University of Alabama at Birmingham institutional review board (IRB) served as the single IRB of record. Informed consent was obtained in accordance with the Declaration of Helsinki.
Statistical analysis
Standard parametric and nonparametric techniques were used for comparison of demographic and clinical characteristics and the prevalence of specific chronic health conditions between BMT survivors alive at study participation and siblings, as well as within the whole cohort of BMT recipients (alive and deceased) overall, and by treatment era.
For the comparison between BMT recipients (alive at study participation) and siblings, the cumulative incidence of chronic health conditions as a function of attained age was calculated. Logistic regression was used for estimating the odds of grade 3–4 conditions in BMT survivors compared to siblings, adjusting for sex, race/ethnicity, education, household income and health insurance.
For the BMT cohort, the cumulative incidence of chronic health conditions was calculated as a function of time from BMT, using death from other causes as competing risk. When analyzing predictors of grade 3–5 conditions in BMT recipients, proportional subdistribution hazards models (Fine-Gray) were used, treating death from primary disease or accident/homicide/suicide as competing risk. We examined the following variables: sex, race/ethnicity, age at BMT, year of BMT, primary disease, source of stem cells, donor type, pre-BMT treatment, conditioning intensity, TBI, history of cGvHD, treating institution, education, household income and health insurance. BMT recipients with missing pre-BMT treatment data were treated as a separate group. Variables with statistical significance at p<0.1 in the multivariable model were subsequently included in a parsimonious model. Mediation analysis was done by adding individual risk factors to the model examining the association between transplant era (<1990; 1990–1999; ≥2000) and risk of grade 3–5 conditions to examine if the hazard ratios changed.
Data were analyzed using SAS Version 9.4 (SAS Institute). All statistical tests were 2-sided, and P <0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics of BMT recipients
The demographic and clinical characteristics of the BMT recipients overall and by primary disease are shown in Table 1. In this cohort, 789 patients had received allogeneic BMT at age <22y and survived ≥2y after BMT (540 alive at study participation; 249 deceased after surviving ≥2y). The median age at transplantation was 11.3y (range: 0.4–22.0); median length of follow-up was 11.7y (2.0–45.3); 432 (54.8%) patients were male, 590 (74.8%) were non-Hispanic white, and 329 (41.7%) had undergone BMT in the year 2000 or later. The three most common indications for BMT were acute lymphoblastic leukemia (ALL; 30.7%), acute myeloid leukemia or myelodysplastic syndrome (AML/MDS; 26.9%) and severe aplastic anemia (SAA; 12.9%). Four hundred and thirty-eight patients (55.5%) had received stem cells from a related donor, and bone marrow was the major source of stem cells for 609 patients (77.2%). TBI was used for conditioning in 572 patients (72.5%), and 243 patients (31.0%) received reduced intensity/non-myeloablative conditioning; 262 (33.2%) patients had a history of cGvHD.
Table 1.
Demographic and clinical characteristics of recipients of allogeneic BMT before the age of 22y
| Variable | All patients (N=789) | ALL (N=242) | AML/MDS (N=212) | SAA (N=102) | BMF syndrome (N=72) | CML (N=54) | Immunodeficieny (N=40) | Lymphoma* (N=23) | Other** (N=44) |
|---|---|---|---|---|---|---|---|---|---|
| Age at BMT in years | |||||||||
| Median (Range) | 11.3 (0.4–22.0) | 12.3 (0.5–22.0) | 13.7 (0.5–22.0) | 13.1 (0.5–21.5) | 9.0 (1.4–22.0) | 14.7 (1.1–21.9) | 2.5 (0.4–14.8) | 17.2 (5.7–21.9) | 4.1 (0.6–20.4) |
| Follow-up in years | |||||||||
| Median (Range) | 11.7 (2.0–45.3) | 8.9 (2.0–36.8) | 12.9 (2.0–43.0) | 14.6 (2.0–45.3) | 9.9 (2.2–23.0) | 12.3 (2.1–32.5) | 13.3 (2.2–38.1) | 10.7 (2.5–40.5) | 10.8 (2.2–28.5) |
| Age at BMT in years, n (%) | |||||||||
| 0–4 | 166 (21.0%) | 37 (15.3%) | 40 (18.9%) | 15 (14.7%) | 14 (19.4%) | 6 (11.1%) | 29 (72.5%) | 0 (0%) | 25 (56.8%) |
| 5–9 | 190 (24.1%) | 66 (27.3%) | 37 (17.5%) | 24 (23.5%) | 31 (43.1%) | 14 (25.9%) | 6 (15.0%) | 4 (17.4%) | 8 (18.2%) |
| 10–14 | 156 (19.8%) | 49 (20.3%) | 46 (21.7%) | 21 (20.6%) | 16 (22.2%) | 8 (14.8%) | 5 (12.5%) | 3 (13.0%) | 8 (18.2%) |
| 15–21 | 277 (35.1%) | 90 (37.2%) | 89 (42.0%) | 42 (41.2%) | 11 (15.3%) | 26 (48.1%) | 0 (0%) | 16 (69.6%) | 3 (6.8%) |
| Sex, n (%) | |||||||||
| Female | 357 (45.2%) | 97 (40.1%) | 103 (48.6%) | 57 (55.9%) | 34 (47.2%) | 32 (59.3%) | 10 (25.0%) | 7 (30.4%) | 17 (38.6%) |
| Race/ethnicity, n (%) | |||||||||
| Non-hispanic white | 590 (74.8%) | 172 (71.1%) | 164 (77.4%) | 75 (73.5%) | 55 (76.4%) | 42 (77.8%) | 33 (82.5%) | 13 (56.5%) | 36 (81.8%) |
| Hispanic | 109 (13.8%) | 51 (21.1%) | 28 (13.2%) | 15 (14.7%) | 6 (8.3%) | 3 (5.6%) | 0 (0%) | 5 (21.7%) | 1 (2.3%) |
| Black | 33 (4.2%) | 5 (2.1%) | 4 (1.9%) | 5 (4.9%) | 4 (5.6%) | 3 (5.6%) | 5 (12.5%) | 2 (8.7%) | 5 (11.4%) |
| Asian | 38 (4.8%) | 12 (5.0%) | 7 (3.3%) | 4 (3.9%) | 6 (8.3%) | 4 (7.4%) | 1 (2.5%) | 3 (13.0%) | 1 (2.3%) |
| Mixed/Other/Unknown | 19 (2.4%) | 2 (0.8%) | 9 (4.2%) | 3 (2.9%) | 1 (1.4%) | 2 (3.7%) | 1 (2.5%) | 0 (0%) | 1 (2.3%) |
| Year of BMT, n (%) | |||||||||
| <1990 | 230 (29.2%) | 66 (27.3%) | 74 (34.9%) | 42 (41.2%) | 1 (1.4%) | 19 (35.2%) | 12 (30.0%) | 10 (43.5%) | 6 (13.6%) |
| 1990–1999 | 230 (29.2%) | 79 (32.6%) | 67 (31.6%) | 26 (25.5%) | 10 (13.9%) | 16 (29.6%) | 16 (40.0%) | 1 (4.3%) | 15 (34.1%) |
| ≥2000 | 329 (41.7%) | 97 (40.1%) | 71 (33.5%) | 34 (33.3%) | 61 (84.7%) | 19 (35.2%) | 12 (30.0%) | 12 (52.2%) | 23 (52.3%) |
| Donor type, n (%) | |||||||||
| Related | 438 (55.5%) | 150 (62.0%) | 136 (64.2%) | 71 (69.6%) | 15 (20.8%) | 23 (42.6%) | 7 (17.5%) | 14 (60.9%) | 22 (50.0%) |
| Unrelated | 351 (44.5%) | 92 (38.0%) | 76 (35.8%) | 31 (30.4%) | 57 (79.2%) | 31 (57.4%) | 33 (82.5%) | 9 (39.1%) | 22 (50.0%) |
| Stem cell source, n (%) | |||||||||
| Cord blood | 103(13.1%) | 32 (13.2%) | 31 (14.6%) | 3 (2.9%) | 19 (26.4%) | 3 (5.6%) | 3 (7.5%) | 4 (17.4%) | 8 (18.2%) |
| Peripheral blood stem cells | 77 (9.8%) | 35 (14.5%) | 27 (12.7%) | 4 (3.9%) | 0(0%) | 4 (7.4%) | 2 (5.0%) | 3 (13.0%) | 2 (4.5%) |
| Bone marrow | 609 (77.2%) | 175 (72.3%) | 154 (72.6%) | 95 (93.1%) | 53 (73.6%) | 47 (87.0%) | 35 (87.5%) | 16 (69.6%) | 34 (77.3%) |
| Total body irradiation, n (%) | |||||||||
| Yes | 572 (72.5%) | 233 (96.3%) | 158(74.5%) | 32 (31.4%) | 58 (80.6%) | 46 (85.2%) | 8 (20.0%) | 22 (95.7%) | 15 (34.1%) |
| Conditioning intensity***, 20, n (%) | |||||||||
| Myelooablative | 542 (69.0%) | 225 (93.0%) | 145 (68.4%) | 71 (71.0%) | 9 (12.5%) | 48 (88.9%) | 9 (23.7%) | 15 (65.2%) | 20 (45.5%) |
| NMA/Reduced intensity | 243 (31.0%) | 17 (7.0%) | 67 (31.6%) | 29 (28.4%) | 63 (87.5%) | 6 (11.1%) | 29 (72.5%) | 8 (24.8%) | 24 (54.5%) |
| cGVHD status, n (%) | |||||||||
| No | 505 (64.0%) | 142 (58.7%) | 124 (58.5%) | 69 (67.7%) | 64 (88.9%) | 26 (48.2%) | 30 (75.0%) | 13 (56.5%) | 37 (84.1%) |
| Yes | 262 (33.2%) | 94 (38.8%) | 81 (38.2%) | 30 (29.4%) | 7 (9.7%) | 27 (50.0%) | 8 (20.0%) | 9 (39.1%) | 6 (13.6%) |
| Missing | 22 (2.8%) | 6 (2.5%) | 7 (3.3%) | 3 (2.9%) | 1 (1.4%) | 1 (1.9%) | 2 (5.0%) | 1 (4.3%) | 1 (2.3%) |
| Pre-BMT treatment, n (% of patients with pre-BMT data available) # | |||||||||
| Alkylators | 124 (33.4%) | 88 (61.5%) | 10 (8.2%) | 0 (0%) | 0 (0%) | 8 (17.8%) | 2 (66.7%) | 14 (77.8%) | 2 (28.6%) |
| Anthracyclines | 267 (72.0%) | 130 (90.9%) | 116 (95.1%) | 0 (0%) | 0 (0%) | 4 (8.9%) | 2 (66.7%) | 14 (77.8%) | 1 (14.3%) |
| Antimetabolites | 285 (76.8%) | 140 (97.9%) | 116 (95.1%) | 0 (0%) | 0 (0%) | 9 (20.0%) | 2 (66.7%) | 14 (77.8%) | 4 (57.1%) |
| Topoisomerase inhibitors | 126 (34.0%) | 53 (37.1%) | 60 (49.2%) | 0 (0%) | 0 (0%) | 1 (2.2%) | 2 (66.7%) | 6 (33.3%) | 4 (57.1%) |
| Plant alkaoids | 176 (47.4%) | 139 (97.2%) | 15 (12.3%) | 0 (0%) | 0 (0%) | 3 (6.7%) | 2 (66.7%) | 15 (83.3%) | 2 (28.6%) |
| Radiotherapy | 63 (16.9%) | 49 (34.0%) | 6 (4.9%) | 0 (0%) | 0 (0%) | 1 (2.2%) | 1 (33.3%) | 5 (27.8%) | 1 (14.3%) |
| Deceased, n (%) | |||||||||
| Yes | 249 (31.6%) | 92 (38.0%) | 69 (32.5%) | 19 (18.6%) | 19 (26.4%) | 19 (35.2%) | 6 (15.0%) | 6 (26.1%) | 19 (43.2%) |
Lymphoma includes non-Hodgkin lymphoma (N=20) and Hodgkin lymphoma (N=3);
Other include hemophagocytic lymphohistiocytosis (N=10), neuroblastoma (N=8), recessive dystrophic epidermolysis bullosa (N=9), sickle cell disease (N=5), Langerhans cell histiocytosis (N=3), thalassemia (N=2), other leukemia (N=5), other (N=2);
Information on conditioning missing for 2 patients with SAA and 2 patients with immunodeficiency;
Pre-BMT treatment data available for 372 of 789 BMT recipients (47%); of the 540 BMT recipients alive at study participation, 304 (56%) had pre-BMT data available. ALL=acute lymphoblastic leukemia, AML/MDS=acute myeloid leukemia/myelodysplastic syndrome, SAA=severe aplastic leukemia, BMF=bone marrow failure, CML=chronic myeloid leukemia, NMA=non-myeloablative, cGVHD=chronic graft vs. host disease
Risk of severe or life-threatening chronic health conditions in BMT survivors compared with siblings
The sibling comparison cohort consisted of 690 individuals. A comparison of the demographic characteristics between siblings and BMT survivors alive at study participation is shown in Supplemental Table 3. The cumulative incidence of a grade 3–4 chronic health condition by age 35 among BMT survivors was significantly higher than among siblings (53.8%, 95%CI, 46.7–60.3 vs. 6.8%, 95%CI, 4.7–8.9, p<0.0001) (Figure 1). The adjusted odds of developing a grade 3–4 chronic health condition were 15.1-fold higher in BMT survivors (95%CI, 9.5–24.0). The prevalence of specific types of grades 3–4 chronic health conditions are shown in Table 2. As illustrated in Figure 2, higher odds were observed for developing cataracts (OR=38.0; 95%CI 15.1–95.2), cardiovascular disease (OR=12.7, 95%CI, 4.7–34.2), diabetes (OR=9.7; 95%CI, 3.6–26.2), thyroid nodules (OR=9.0, 95%CI, 3.6–22.2), joint replacement (OR=5.3, 85%CI, 2.2–12.7) and sensorineural disorders (OR=3.5, 95%CI, 1.8–6.8) among BMT survivors as compared with the sibling comparison group.
Figure 1.
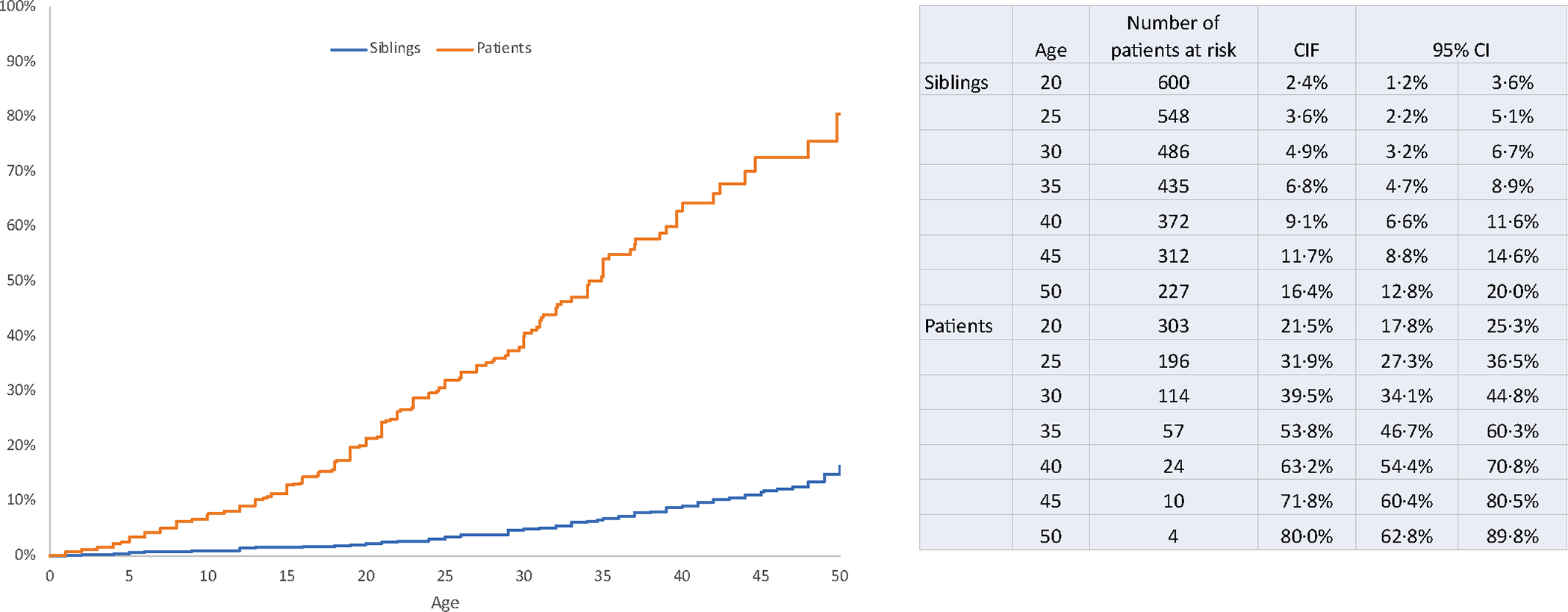
Cumulative incidence of a grade 3–4 chronic health condition among allogeneic BMT recipients alive at follow-up and siblings, respectively.
Table 2.
Prevalence of a grade 3–4 chronic health conditions in survivors of allogeneic BMT in childhood alive at follow-up and sibling comparison cohort
| Variable | BMT survivors (N=540) | Siblings (N=690) | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Any grade 3–4 chronic health condition | 192 | 35.6 | 88 | 12.8 | <0.0001 |
| Specific chronic health conditions | |||||
| Subsequent malignant neoplasm | 80 | 14.8 | 2 | 0.3 | <0.0001 |
| Cataract | 47 | 8.7 | 8 | 1.2 | <0.0001 |
| Cardiovascular disease* | 27 | 5.0 | 7 | 1.0 | <0.0001 |
| Gastrointestinal disorder** | 19 | 3.5 | 5 | 0.7 | 0.0004 |
| Sensorineural disorder*** | 37 | 6.9 | 27 | 3.9 | 0.02 |
| Diabetes | 26 | 4.8 | 7 | 1.0 | <0.0001 |
| Joint replacement | 23 | 4.3 | 14 | 2.0 | 0.02 |
| Thyroid nodules | 22 | 4.1 | 11 | 1.6 | 0.008 |
| Blood clot | 12 | 2.2 | 15 | 2.2 | 1.0 |
| Lung fibrosis/lung transplant | 4 | 0.7 | 0 | 0.0 | 0.02 |
| Dialysis | 0 | 0.0 | 3 | 0.4 | 0.1 |
Includes heart attack, congestive heart failure, stroke, stiff or leaky valve, arrhythmia;
Includes liver disease, rectal disorders, or intestinal obstruction;
Includes hearing loss, balance/vertigo, legally blind
Figure 2.
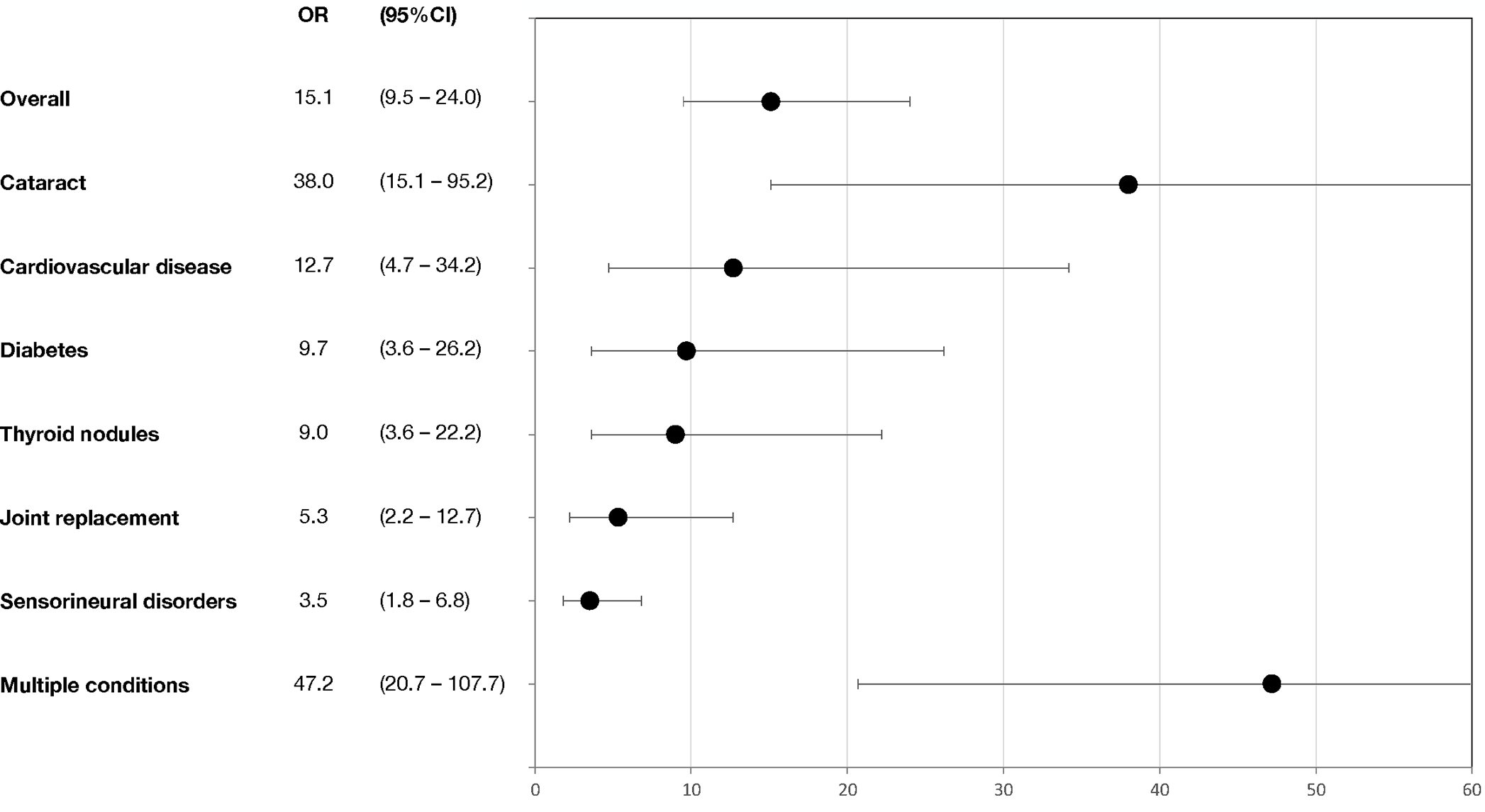
The adjusted odds of developing a grade 3–4 chronic health condition among recipients of allogeneic BMT, overall and specific chronic health conditions.
Cardiovascular disease includes heart attack, congestive heart failure, stroke, stiff or leaky valve, and arrhythmia; gastrointestinal disease includes liver disease, rectal disorders, and intestinal obstruction; and sensorineural disorders includes hearing loss, balance/vertigo and legally blind.
A larger proportion of BMT survivors (13.5%) had multiple (≥2) grade 3–4 chronic health conditions compared to the siblings (1.5%), P<0.0001, yielding an adjusted odds ratio of 47.2 (95%CI 20.7–107.7) for multiple grade 3–4 conditions among BMT survivors.
Risk of multiple severe or life-threatening chronic health conditions among BMT recipients
Among the BMT survivors, the risk of a multiple grade 3–4 chronic health condition was higher among females (HR=1.89, 95%CI 1.20–2.94). Furthermore, the risk of multiple grade 3–4 conditions was increased among those receiving an unrelated donor transplant (HR=1.77, 95%CI 1.01–3.09), and TBI (HR=4.82, 95%CI 2.07–11.25). Compared to patients receiving myeloablative conditioning, those receiving reduced intensity conditioning (HR=2.65, 95%CI 1.51–4.63), as well as non-myeloablative conditioning (HR=4.09, 95%CI 1.64–10.19), had an increased risk for multiple grade 3–4 chronic health conditions. Patients transplanted 1990–1999 and ≥2000 had a lower risk of multiple grade 3–4 conditions compared to those transplanted <1990 (HR=0.48, 95%CI 0.27–0.86 and HR=0.18, 95%CI 0.08–0.40, respectively).
Risk of severe, life-threatening or fatal chronic health conditions among BMT recipients
Of the 789 patients, 562 (71.2%) developed any grade 1–5 condition and 326 (41.3%) a grade 3–5 condition (Supplemental Table 4). The risk of a grade 3–5 chronic health condition increased with increasing age at BMT (HR_per_y_increase_in_age=1.03, 95%CI, 1.01–1.05) and was higher among females (HR=1.26, 95%CI 1.02–1.58) (Table 3). Furthermore, the risk was increased among those receiving an unrelated donor transplant (HR=1.30, 95%CI 1.03–1.64), those exposed to TBI (HR=1.71, 95%CI 1.27–2.31), as well as those with cGvHD (HR=1.38, 95%CI 1.09–1.74). The full multivariable model is shown in Supplemental Table 5. The most pertinent negative findings included the lack of association between conditioning intensity and chronic health conditions, as well as between treatment era and chronic health conditions. In the comparison of the prevalence of specific chronic health conditions between the sexes, females had a higher prevalence of thyroid nodules (4.5% vs. 1.4%, P=0.009) (Supplemental Table 6). Compared to patients without cGvHD, those with cGvHD had a higher prevalence of joint replacement (5.3% vs. 1.6%, P=0.003), and lung fibrosis/lung transplant (2.3% vs. 0.4%, P=0.01) (Supplemental Table 7). Given that pre-BMT exposures were not available for 53% of the patients, we conducted a sensitivity analysis of risk factors for a grade 3–5 chronic health condition excluding pre-BMT data and found no major difference in the HRs shown in Table 3 and Supplemental Table 5.
Table 3.
Hazard ratio of any grade 3–5 conditions among 789 recipients of allogeneic BMT in childhood
| Variable | Parsimonious model | ||
|---|---|---|---|
| HR | 95%CI | P-value | |
| Age at BMT in years | |||
| Per year increase in age | 1.03 | 1.01–1.05 | 0.006 |
| Sex | |||
| Male | REF | ||
| Female | 1.27 | 1.02–1.59 | 0.029 |
| Donor type | |||
| Related | REF | ||
| Unrelated | 1.30 | 1.03–1.64 | 0.028 |
| Total body irradiation | |||
| No | REF | ||
| Yes | 1.71 | 1.27–2.31 | 0.0004 |
| Chronic GVHD | |||
| No | REF | ||
| Yes | 1.38 | 1.09–1.74 | 0.007 |
| Pre-BMT treatment with alkylators | |||
| No | REF | ||
| Yes | 0.72 | 0.52–0.99 | 0.046 |
The 10y post-BMT cumulative incidence of any grade 3–5 chronic health condition was 29.4% (SD 1.7%). The 10y cumulative incidence for specific grades 3–5 chronic health conditions was as follows: cataract: 7.0% (SD 1.2%), second malignant neoplasms (SMNs): 6.6% (SD 0.9%), sensorineural disorder: 4.3% (0.8%), cardiovascular disease: 3.1% (SD 0.6%), diabetes: 1.9% (SD 0.5%), and gastrointestinal disorders: 1.8% (SD 0.5%) (Figure 3).
Figure 3.
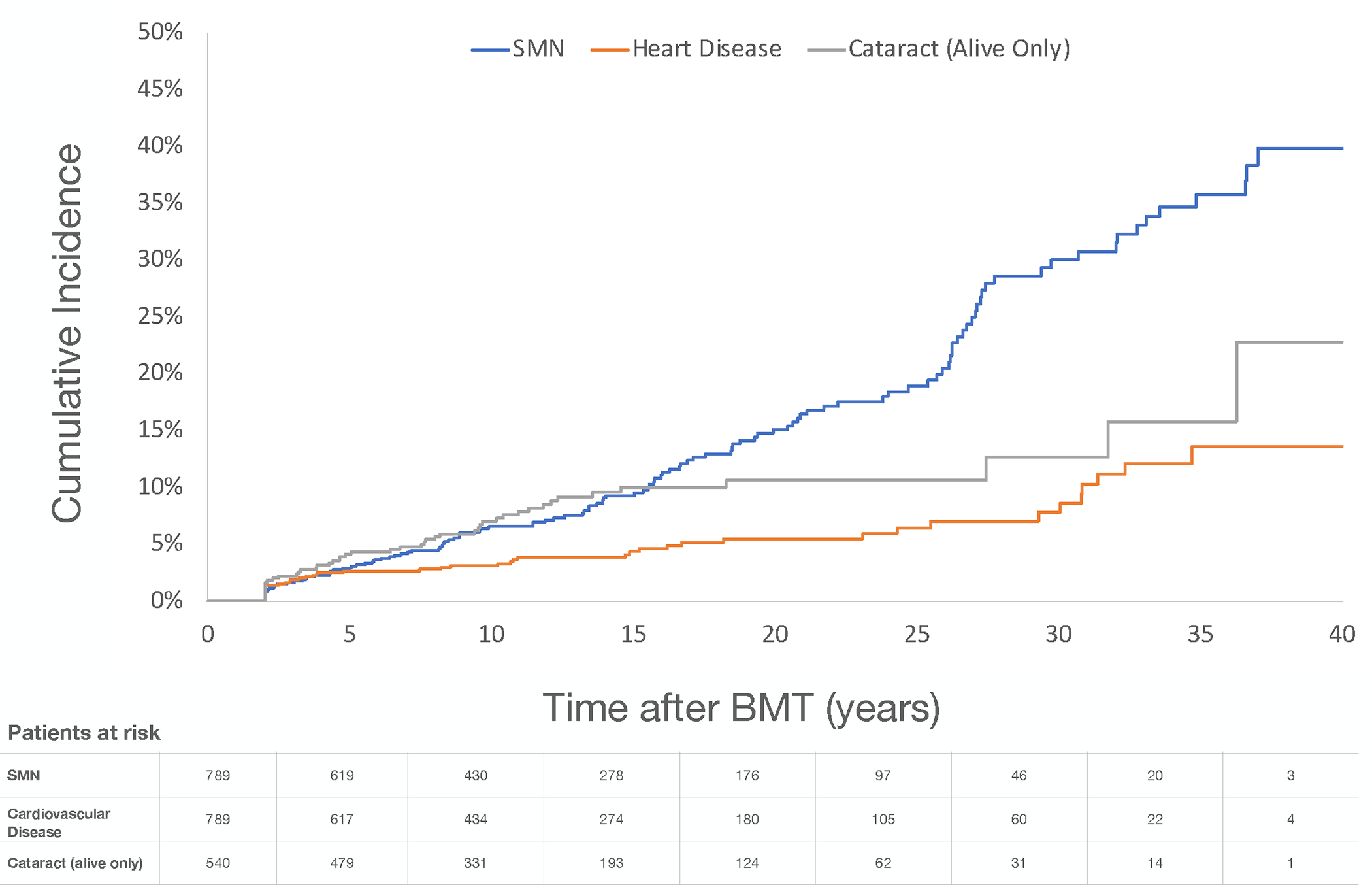
Cumulative incidence of selected grade 3–5 chronic health conditions among recipients of allogeneic BMT
The risk of a grade 3–5 SMN was increased among patients with AML/MDS (HR=1.88, 95%CI 1.09–3.25) and bone marrow failure syndrome (HR=6.68, 95%CI 3.08–14.51), compared to those with ALL (Supplemental Table 8). Patients transplanted 1990–1999 had an increased risk of a grade 3–5 SMN (HR=1.57, 95%CI 1.00–2.44) compared to those transplanted before 1990. For those transplanted ≥2000, the risk was not significantly increased compared to <1990. Furthermore, patients exposed to TBI had an increased risk (HR=2.08, 95%CI 1.09–3.99). The SMNs included solid SMNs (55.1%), skin cancer (27.1%), lymphoma (9.3%) and leukemia (8.5%) (Supplemental Table 9).
Chronic health conditions in BMT recipients by treatment era
The demographic and clinical characteristics and the prevalence of chronic health conditions by treatment era are detailed in Supplemental Table 10. Noteworthy differences in the three eras (<1990, 1990–1999 and ≥2000) were an increase in prevalence of unrelated donor transplants (11.7%→48.7%→64.4%), transplantation for bone marrow failure syndromes (0.4%→4.3→18.5%), and the use of cord blood (0%→7.4%→26.1%) and peripheral blood (0.4%→2.2%→21.6%) as sources of stem cells. The proportion of patients receiving myeloablative conditioning regimens declined (87.6%→79.1%→49.2%).
The 10y cumulative incidence of a grade 3–5 chronic health condition was higher among those transplanted in the most recent transplant era (2000–2014: 35.0%), compared to those transplanted before (<1990: 26.1%; 1990–1999: 25.1%, P=0.001) (Supplemental Figure 1). The unadjusted hazard of any grade 3–5 chronic health condition was increased for those transplanted ≥2000 compared to <1990 (HR=1.46, 95%CI 1.07–1.99). Inclusion of stem cell source in the model mitigated the difference in risk of grades 3–5 chronic health conditions in the most recent era (Supplemental Table 11). The unadjusted hazard of any grade 3–5 chronic health condition was increased for those receiving peripheral blood stem cells (HR=1.77, 95%CI 1.23–2.53) and cord blood (HR=1.41, 95%CI 1.01–1.97) as stem cell source compared to bone marrow.
DISCUSSION
Two-year survivors of allogeneic BMT performed in childhood have a 15-fold higher risk of a severe or life-threatening chronic health condition compared to a sibling comparison cohort. By age 35, more than half of the BMT recipients had developed a severe or life-threatening condition. The 10y cumulative incidence of a severe, life-threatening or fatal condition was highest among those transplanted in the most recent transplant era, and the more recent use of peripheral blood stem cells or cord blood explained this difference.
The prevalence of a grade 3–4 condition in BMT survivors after a median follow-up of 7y in a cohort of 162 2y-survivors after allogeneic BMT performed before the age of 18y seen in a late effects clinic in the Netherlands was 25%,13 which aligns well with a prevalence of 36% in our study with a longer median length of follow-up of 11y. The cumulative incidence of a grade 3–4 condition by age 35 was 54% among BMT survivors, compared to only 7% in siblings. The present study adds to the existing knowledge of the burden of morbidity among childhood BMT recipients by including a comparison with siblings, showing that childhood BMT recipients have a 15-fold increase in risk of a grade 3–4 condition. Higher adjusted odds were observed for a wide range of chronic health conditions; cataracts, diabetes, cardiovascular disease, thyroid nodules, joint replacement, and sensorineural disorders when compared to the siblings; the adjusted odds for multiple grade 3–4 chronic health condition were remarkably high at 47.
In the current study, the prevalence of a grade 3–4 cardiovascular disease among BMT recipients was 5.0% and the BMT survivors were at 12.7-fold higher odds of developing a cardiovascular disease compared to siblings. Our results are in line with those reported from the Center for International Blood and Marrow Transplant Research (CIBMTR) where the prevalence of cardiovascular disease was 4.2% after a median follow-up of 8y after pediatric allogeneic BMT.12 In the CIBMTR study, cardiovascular risk factors were prevalent, increasing the risk for cardiovascular disease with time. Diabetes was diagnosed in 7% of the patients in the CIBMTR study, a prevalence close to that of 4.8% in the present cohort. An increase in both cardiovascular disease and cardiovascular risk factors, as well as an increase in risk with time, was also reported in cohorts of allogeneic BMT recipients in childhood and adults.5, 6, 23 These results, together with those of Uderzo et al describing a decline in pulmonary and cardiac function in children as early as 5y after allogeneic BMT,15 calls for close follow-up from the time of transplantation, continuing through life.
In our cohort, the 10y cumulative incidence of SMN classified as grade 3–5 chronic health condition was 6.6%. In a study by Ferry et al, 1y survivors of pediatric allogeneic BMT for a hematological malignancy performed between 1985 and 2000 had a 10y cumulative incidence of second malignancy of 7%.11 The corresponding cumulative incidence among those transplanted between 1990 and 1999 in our study is 7.2%. The reported 10y cumulative incidence is higher than that of earlier studies of solid SMNs following allogeneic BMT in children and adults ranging from 2% to 6 %.10, 24–27
The present study demonstrates that older age at BMT and female sex are important risk factors for the development of a grade 3–5 chronic health condition. Our finding of older age at BMT as a risk factor confirms that of the few, much smaller earlier studies examining the influence of age at BMT on the development of chronic health conditions,11, 13 and could possibly be explained by the higher risk for developing cGvHD among older children. In contrast to the study by Bresters et al,13 we found female sex to be a risk factor for a grade 3–5 chronic health condition, which was mainly explained by the higher incidence of thyroid nodules among females compared to males in our study. A larger cohort with longer follow-up, may have influenced the differences in results. To the best of our knowledge, there are no other studies of allogeneic BMT performed in childhood examining the impact of sex on the risk of chronic health conditions. The current study confirms TBI being an important risk factor for late effects, identified in previous studies of pediatric BMT recipients.11, 13, 16, 19, 28 Our study also identified cGvHD as a risk factor for grade 3–5 conditions; joint replacement and lung fibrosis/lung transplant were more prevalent among patients with cGvHD, both chronic health conditions with known associations with cGvHD.29
In the present study, the unadjusted 10y cumulative incidence of a grade 3–5 chronic health condition was highest among those transplanted in the most recent era (35.0%). However, there was a lack of association between the treatment era and chronic health conditions in the multivariable model. Notably, of the demographic and transplant-related factors included in the mediation analysis, only stem cell source was shown to explain the increase in grade 3–5 chronic health conditions among the most recently transplanted survivors. The share of cord blood transplants increased from 7.4% to 26.1% and that of peripheral blood stem cells from 2.2% to 21.6% from the era 1990–1999 to ≥2000. The present findings call for close risk-based monitoring of also those receiving modern transplantations, to detect early signs of chronic health conditions in these patients in order to minimize their negative effects, but also to further increase our knowledge of transplant-related risk factors for these late effects.
We were unable to assess the risk of chronic health conditions associated with changes in supportive care practices, due to the lack of this information. Additional limitations of the present study are the lack of detailed information on cGvHD and its treatment, as well as on cancer predisposition syndromes. Additionally, a potential limitation is the use of self-reported data, although our survey has been validated and demonstrated a high level of sensitivity and specificity. These limitations notwithstanding, the current investigation describes the burden of morbidity in a large cohort of patients undergoing allogeneic BMT in childhood followed for a median of 11y. Furthermore, detailed clinical data, including conditioning regimen, was available for all patients. Finally, inclusion of patients transplanted over four decades, allowed us to evaluate trends in chronic health conditions for patients transplanted over a period of four decades. The findings of the present study provide evidence for long-term anticipatory risk-based follow-up of survivors of BMT performed in childhood, from the time of transplantation and continuing throughout life.
Supplementary Material
Acknowledgments
This study was supported in parts by grants from the National Cancer Institute U01CA213140 (Bhatia), R01CA078938 (Bhatia) and the Leukemia Lymphoma Society (Bhatia).
Footnotes
The authors have no financial conflicts to declare.
References
- 1.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. The New England journal of medicine. Oct 12 2006;355(15):1572–82. doi:355/15/1572 [pii] 10.1056/NEJMsa060185 [DOI] [PubMed] [Google Scholar]
- 2.de Fine Licht S, Rugbjerg K, Gudmundsdottir T, et al. Long-term inpatient disease burden in the Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study: A cohort study of 21,297 childhood cancer survivors. PLoS Med. May 2017;14(5):e1002296. doi: 10.1371/journal.pmed.1002296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. Oct 28 2010;116(17):3129–39; quiz 3377. doi: 10.1182/blood-2009-06-229369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker KS, Leisenring WM, Goodman PJ, et al. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood. Jun 27 2019;133(26):2790–2799. doi: 10.1182/blood.2018874115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. Aug 2008;93(8):1203–10. doi: 10.3324/haematol.12949 [DOI] [PubMed] [Google Scholar]
- 6.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. Nov 29 2012;120(23):4505–12. doi: 10.1182/blood-2012-06-437178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi M, Sun CL, Kurian S, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer. Oct 1 2008;113(7):1580–7. doi: 10.1002/cncr.23773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. Jan 2009;43(1):49–54. doi: 10.1038/bmt.2008.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Mourad YR, Lau BC, Barnett MJ, et al. Long-term outcome after allo-SCT: close follow-up on a large cohort treated with myeloablative regimens. Bone Marrow Transplant. Feb 2010;45(2):295–302. doi: 10.1038/bmt.2009.128 [DOI] [PubMed] [Google Scholar]
- 10.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. Jan 29 2009;113(5):1175–83. doi: 10.1182/blood-2008-05-158782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferry C, Gemayel G, Rocha V, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. Aug 2007;40(3):219–24. doi: 10.1038/sj.bmt.1705710 [DOI] [PubMed] [Google Scholar]
- 12.Duncan CN, Brazauskas R, Huang J, et al. Late cardiovascular morbidity and mortality following pediatric allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. Oct 2018;53(10):1278–1287. doi: 10.1038/s41409-018-0155-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bresters D, van Gils IC, Kollen WJ, et al. High burden of late effects after haematopoietic stem cell transplantation in childhood: a single-centre study. Bone Marrow Transplant. Jan 2010;45(1):79–85. doi: 10.1038/bmt.2009.92 [DOI] [PubMed] [Google Scholar]
- 14.Friedman DN, Hilden P, Moskowitz CS, et al. Cardiovascular Risk Factors in Survivors of Childhood Hematopoietic Cell Transplantation Treated with Total Body Irradiation: A Longitudinal Analysis. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. Mar 2017;23(3):475–482. doi: 10.1016/j.bbmt.2016.12.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uderzo C, Pillon M, Corti P, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. Jun 2007;39(11):667–75. doi: 10.1038/sj.bmt.1705652 [DOI] [PubMed] [Google Scholar]
- 16.Wilhelmsson M, Vatanen A, Borgstrom B, et al. Adverse health events and late mortality after pediatric allogeneic hematopoietic SCT-two decades of longitudinal follow-up. Bone Marrow Transplant. Jun 2015;50(6):850–7. doi: 10.1038/bmt.2015.43 [DOI] [PubMed] [Google Scholar]
- 17.Wilhelmsson M, Jahnukainen K, Winiarski J, et al. Hospitalizations in long-term survivors of childhood AML treated with allogeneic HCT-An Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study. American journal of hematology. Mar 1 2021;96(3):E74–E77. doi: 10.1002/ajh.26071 [DOI] [PubMed] [Google Scholar]
- 18.Eissa HM, Lu L, Baassiri M, et al. Chronic disease burden and frailty in survivors of childhood HSCT: a report from the St. Jude Lifetime Cohort Study. Blood Adv. Nov 14 2017;1(24):2243–2246. doi: 10.1182/bloodadvances.2017010280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. Aug 4 2011;118(5):1413–20. doi: 10.1182/blood-2011-01-331835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. Jul 17 2014;124(3):344–53. doi: 10.1182/blood-2014-02-514778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. Jun 2000;25(11):1191–6. doi: 10.1038/sj.bmt.1702419 [DOI] [PubMed] [Google Scholar]
- 22.Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Accessed October 5, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 [Google Scholar]
- 23.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Annals of internal medicine. Jul 5 2011;155(1):21–32. doi: 10.7326/0003-4819-155-1-201107050-00004 [DOI] [PubMed] [Google Scholar]
- 24.Friedman DL, Rovo A, Leisenring W, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. Jan 15 2008;111(2):939–44. doi: 10.1182/blood-2007-07-099283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. Jan 15 2001;19(2):464–71. doi: 10.1200/JCO.2001.19.2.464 [DOI] [PubMed] [Google Scholar]
- 26.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. Apr 1 2003;21(7):1352–8. doi: 10.1200/JCO.2003.05.108 [DOI] [PubMed] [Google Scholar]
- 27.Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. Jan 1 2007;109(1):84–92. doi: 10.1002/cncr.22375 [DOI] [PubMed] [Google Scholar]
- 28.Bernard F, Auquier P, Herrmann I, et al. Health status of childhood leukemia survivors who received hematopoietic cell transplantation after BU or TBI: an LEA study. Bone Marrow Transplant. May 2014;49(5):709–16. doi: 10.1038/bmt.2014.3 [DOI] [PubMed] [Google Scholar]
- 29.Li X, Brazauskas R, Wang Z, et al. Avascular necrosis of bone after allogeneic hematopoietic cell transplantation in children and adolescents. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. Apr 2014;20(4):587–92. doi: 10.1016/j.bbmt.2013.12.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


