Abstract
The pre-mRNA splicing factor U2AF (U2 small nuclear ribonucleoprotein particle [snRNP] auxiliary factor) plays a critical role in 3′ splice site selection. U2AF binds site specifically to the intron pyrimidine tract between the branchpoint and the 3′ splice site and targets U2 snRNP to the branch site at an early step in spliceosome assembly. Human U2AF is a heterodimer composed of large (hU2AF65) and small (hU2AF35) subunits. hU2AF65 contains an arginine-serine-rich (RS) domain and three RNA recognition motifs (RRMs). hU2AF35 has a degenerate RRM and a carboxyl-terminal RS domain. Genetic studies have recently shown that the RS domains on the Drosophila U2AF subunit homologs are each inessential and might have redundant functions in vivo. The site-specific pyrimidine tract binding activity of the U2AF heterodimer has previously been assigned to hU2AF65. While the requirement for the three RRMs on hU2AF65 is firmly established, a role for the large-subunit RS domain in RNA binding remains unresolved. We have analyzed the RNA binding activity of the U2AF heterodimer in vitro. When the Drosophila small-subunit homolog (dU2AF38) was complexed with the large-subunit (dU2AF50) pyrimidine tract, RNA binding activity increased 20-fold over that of free dU2AF50. We detected a similar increase in RNA binding activity when we compared the human U2AF heterodimer and hU2AF65. Surprisingly, the RS domain on dU2AF38 was necessary for the increased binding activity of the dU2AF heterodimer. In addition, removal of the RS domain from the Drosophila large-subunit monomer (dU2AF50ΔRS) severely impaired its binding activity. However, if the dU2AF38 RS domain was supplied in a complex with dU2AF50ΔRS, high-affinity binding was restored. These results suggest that the presence of one RS domain of U2AF, on either the large or small subunit, promotes high-affinity pyrimidine tract RNA binding activity, consistent with redundant roles for the U2AF RS domains in vivo.
The generation of functional mRNA in eukaryotes requires the accurate removal of noncoding regions (introns) from pre-mRNA by a process termed pre-mRNA splicing (14, 25). Splicing takes place in the spliceosome, a dynamic RNA-protein complex that assembles in a stepwise manner on the pre-mRNA (10, 14). The spliceosome is composed of small nuclear ribonucleoprotein particles (snRNPs) and extrinsic (non-snRNP) factors. The recognition of exon/intron boundaries, the splice sites, by the splicing apparatus is a critical step in processing of both constitutively and alternatively spliced pre-mRNAs. U1 snRNP defines the 5′ splice site, and U2 snRNP defines the branchpoint sequence (3, 10, 14, 17). Since in most cases the first AG dinucleotide downstream of the branchpoint is used as the 3′ splice site, by defining the branchpoint, U2 snRNP defines the 3′ splice site (18, 27). Targeting of U2 snRNP to the branch site requires the extrinsic splicing factor U2 snRNP auxiliary factor (U2AF). U2AF binds site specifically to the intron pyrimidine tract between the branchpoint sequence and 3′ splice site at an early step in spliceosome assembly and recruits U2 snRNP to the branch site (22, 29, 42). Regulation of 3′ splice site choice, both positive and negative, can be realized by influencing the pyrimidine tract binding of U2AF (33, 35, 45). Because U2AF is a major determinant in 3′ splice site selection, it has been the subject of extensive biochemical and genetic investigation.
Human U2AF is a heterodimer composed of a 65-kDa large subunit (hU2AF65) and a 35-kDa small subunit (hU2AF35) (41). Both subunits are conserved in other organisms (40), and U2AF homologs have been identified in Drosophila melanogaster (9, 21), Schizosaccharomyces pombe (16, 36), and Caenorhabditis elegans (3a, 39). The Drosophila U2AF large (dU2AF50)- and small (dU2AF38)-subunit homologs are 50 and 38 kDa, respectively (9, 21). The U2AF large subunit contains three RNA recognition motifs (RRMs) and an amino-terminal arginine-serine-rich (RS) domain (42). The small subunit contains a highly degenerate RRM (pseudo-RRM) (2), two Zn2+ binding motifs (37), and a carboxyl-terminal RS domain and glycine-rich region (43).
Both U2AF subunits are involved in recognition of the intron pyrimidine tract. The large subunit (hU2AF65 and dU2AF50) is required for site-specific pyrimidine tract binding (9, 42). The small subunit acts as a cofactor to stabilize the large subunit on the pyrimidine tract, apparently through protein-protein interactions with constitutive and alternative splicing factors (38, 45). While it has been firmly established that all three RRMs on hU2AF65 are necessary for high-affinity RNA binding, a role for the large-subunit RS domain in RNA binding remains unresolved (11, 42). In one study removal of the hU2AF65 RS domain had a modest effect on RNA binding (42). In a second study, the RS domain was found to be absolutely required for RNA binding (11).
In vitro splicing assays using U2AF-depleted extracts prepared by two independent methods have identified independent and essential roles for the two U2AF RS domains: the large-subunit RS domain is required to target U2 snRNP to the branch site (34, 42), and in the immunodepleted extracts under certain conditions, the small-subunit RS domain is apparently necessary for protein-protein interactions with constitutive and alternative splicing factors to stabilize hU2AF65 on the pyrimidine tract (38, 45). In contrast to the essential roles assigned to the two U2AF RS domains in vitro, molecular genetic analysis of the Drosophila U2AF RS domains indicates that either one of the RS domains is dispensable in vivo (19). Importantly, at least one RS domain on U2AF is essential for viability (19).
The observation that the dU2AF38 RS domain is not essential in vivo (19) refocused our attention on domains present in the U2AF small subunit that are phylogenetically conserved. In an exhaustive database search for proteins containing RRMs, some of the signature sequences of this motif were identified in hU2AF35 (2). These sequences are also present in the Drosophila and S. pombe small-subunit homologs (21, 36). Although some of the most conserved residues in the RNA recognition motif are present in the U2AF small subunits, the RNP-1 octamer is highly degenerate and the RNP-2 hexamer is absent. Since these defining submotifs and other conserved residues are not present in the U2AF small-subunit RRM, it was termed a degenerate RRM or pseudo-RRM (2). Degenerate RRMs have been identified in a collection of RNA binding proteins, including several of the SR proteins, the pyrimidine tract binding protein, and the large subunit of U2AF (10). The degenerate RRMs in SRp30a (ASF/SF2) (4, 46), pyrimidine tract-binding protein (15), and hU2AF65 (42) were all found to be required for high-affinity RNA binding.
Two putative Zn2+ binding domains, one on either side of the pseudo-RRM, were recently identified in hU2AF35 in a database search (37). These Cys3His Zn2+ binding motifs are conserved in all three small subunit homologs. Though sequence-specific RNA binding has not been described for proteins that contain this type of Zn2+ binding motif, several proteins that have this domain are involved in RNA metabolism (37). The evolutionary conservation of the pseudo-RRM and the two Zn2+ binding motifs in all three U2AF small subunit homologs suggested to us that these domains are important for function.
The lack of requirement for the dU2AF38 RS domain in vivo prompted us to search for novel biochemical activities associated with the small subunit. The phylogenetically conserved, degenerate RRM (2) and two Zn2+ binding motifs (37) in the small subunit suggested that it might participate in RNA binding. While we detected weak RNA binding activity for the Drosophila small subunit on its own, we found that when complexed with the large subunit, dU2AF pyrimidine tract binding affinity increased 20-fold. This increase in RNA binding activity was not specific to Drosophila U2AF; the human U2AF heterodimer bound RNA with 15-fold-higher affinity than hU2AF65. Surprisingly, removal of the dU2AF38 RS domain abolished the increase in binding activity of the dU2AF heterodimer, indicating that the RS domain is necessary for high-affinity binding. Deletion of the dU2AF50 RS domain (dU2AF50ΔRS) dramatically reduced RNA binding activity of the large-subunit monomer. High-affinity binding was restored when the dU2AF38 RS domain was supplied in trans to dU2AF50ΔRS. These data suggest that high-affinity RNA binding activity requires at least one RS domain on U2AF, which is consistent with the requirement for at least one RS domain in vivo.
MATERIALS AND METHODS
Recombinant proteins.
Expression plasmids used to purify His6-dU2AF50, His6-dU2AF50/dU2AF38, His6-dU2AF50ΔRS/dU2AF38, hU2AF65/His6-hU2AF35, and His6-hU2AF65 were as described previously (9, 20). His6-dU2AF50/dU2AF38ΔRS was made by insertion of an oligonucleotide linker (top strand, 5′CTCTACTAATAGCTGCA3′; bottom strand, 5′GCTATTAGTAGAGGTAC3′) between KpnI and PstI sites in the dU2AF38 coding sequence in pdr154 (20) to create pdr234.
All recombinant proteins were purified as described previously (20). Briefly, all U2AF subunits were expressed or coexpressed in Escherichia coli BL21(DE3)pLysS, except His6-dU2AF50 and dU2AF38ΔRS, which were coexpressed in E. coli BL21(DE3)pLysE. Cells were grown in LB at 30°C to an optical density at 600 nm of 0.4, induced by the addition of isopropyl-β-d-thiogalactopyranoside to 0.5 mM, and harvested after 3 to 4 h. All subsequent manipulations were carried out at 4°C. Cells were harvested by centrifugation and resuspended in buffer I (50 mM Tris-HCl [pH 8.0], 1 M NaCl, 5 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride). A crude extract was prepared by freeze-thawing of the cells, followed by sonication and centrifugation at 30,000 rpm for 30 min. The soluble extract was loaded on an HR5/5 (Pharmacia) Ni2+-nitrilotriacetic acid (NTA)-agarose (Qiagen) column equilibrated with buffer I containing 10% glycerol. The column was washed with buffer I containing 10% glycerol and 20 mM imidazole, and bound protein was eluted in buffer I containing 10% glycerol and 200 mM imidazole. Peak fractions were pooled, frozen in liquid nitrogen, and stored at −80°C or diluted to 350 mM NaCl with buffer H (20 mM HEPES-NaOH [pH 7.6], 1 mM EDTA, 0.5 mM dithiothreitol [DTT], 10% glycerol) and further purified on an HR5/5 MonoS column (Pharmacia) equilibrated with buffer H containing 350 mM NaCl. Bound protein was eluted with a linear NaCl gradient from 350 mM to 1.5 M NaCl. dU2AF50 monomer eluted at ∼550 mM NaCl, and dU2AF heterodimer eluted at ∼900 mM NaCl. His6-dU2AF50ΔRS was purified on an HR5/5 MonoQ column (Pharmacia) equilibrated with 50 mM Tris-HCl (pH 8.0)–100 mM NaCl–0.5 mM DTT–10% glycerol. His6-dU2AF50/dU2AF38ΔRS flowed through the MonoS column in 350 mM NaCl, while His6-dU2AF50 eluted at ∼550 mM NaCl. The His6-dU2AF50/dU2AF38ΔRS flowthrough and the His6-dU2AF50 peak fractions were separately concentrated on a 200-μl Ni2+-NTA-agarose (Qiagen) column. The dU2AF50/dU2AF38ΔRS fractions were further concentrated by using Aquacide I (Calbiochem) according to the manufacturer’s specifications.
Protein-RNA binding analysis.
Apparent dissociation constants (Kds) for interaction of U2AF large subunits and heterodimers with RNA were determined by use of native gel electrophoresis. Binding reactions were performed in a volume of 10 μl containing the indicated concentrations of proteins, 0.1 nM 32P-labeled oligonucleotide, 20 mM HEPES-KOH (pH 7.6), 100 mM KCl, 0.2 mM EDTA, 10% glycerol, 0.5 mM DTT, 50 μg of bovine serum albumin per ml, and 10 mg of heparin per ml. Incubations were continued for 1 h at 4°C. One half of the reaction mixtures were subjected to electrophoresis through 4% polyacrylamide gels (60:1; 0.5× Tris-borate-EDTA [pH 8.3]) at 4°C for 100 min at 20 V/cm. All binding experiments were repeated a minimum of two times; most were performed four times. In some binding assays, a fraction of the U2AF heterodimer-RNA complex failed to enter the gel (see, for example, Fig. 3). However, in independent runs of the identical experiment, none of the complex was retained in the well. Importantly, the binding affinities were the same in both cases. RNA binding was quantitated with the use of the Fuji phosphorimager, and Kd values were obtained by fitting binding curves to the data obtained from the native gels. A simple two-state binding reaction was assumed; using DeltaGraph Pro, the data were fitted to the following equation: y = 1/[1 + (Kd/x)], in which y represents the fraction of RNA bound by protein and x equals the protein concentration. The variation in Kds obtained from independent experiments was between 10 and 25%.
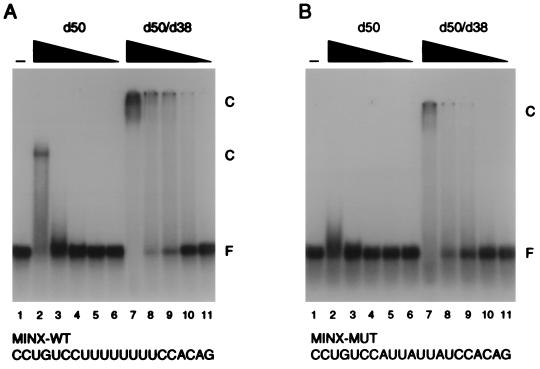
FIG. 3.
The dU2AF heterodimer binds pyrimidine tract RNA with higher affinity than the dU2AF50 monomer. (A) Electrophoretic mobility shift analysis of dU2AF heterodimer and dU2AF50 monomer interaction with MINX-WT pyrimidine tract RNA. dU2AF50 monomer protein concentrations were 5,000, 1,000, 200, 40, and 8 nM (lanes 2 to 6). dU2AF heterodimer concentrations were 5,000, 1,000, 200, 40, and 8 nM (lanes 7 to 11). Proteins were incubated with 100 pM 32P-labeled RNA oligonucleotide. Protein-RNA complexes (C) and unbound RNA (F) were separated by electrophoresis through a native polyacrylamide gel and visualized by autoradiography. Occasionally, the dU2AF50/dU2AF38 heterodimer-RNA complex appeared to be retained in the sample well. However, this effect was variable. The sequence of the MINX-WT pyrimidine tract oligonucleotide is displayed below the autoradiogram. The apparent Kd for the dU2AF50 monomer was ∼2.2 × 10−6 M. The apparent Kd for the dU2AF heterodimer was ∼1.0 × 10−7 M. (B) Electrophoretic mobility shift analysis of dU2AF heterodimer and dU2AF50 monomer interaction with MINX-MUT pyrimidine tract RNA. Protein concentrations were identical to those in panel A. The sequence of the MINX-MUT pyrimidine tract oligonucleotide is displayed below the autoradiogram. The apparent Kd for the dU2AF50 monomer could not be determined. The apparent Kd for the U2AF heterodimer was ∼1.1 × 10−6 M. Although it may appear that U2AF binding is cooperative, this is due to the broad titration of protein used in this experiment. When a finer titration is used, we detect no evidence of cooperative binding (Fig. 5B and data not shown) (9).
RESULTS
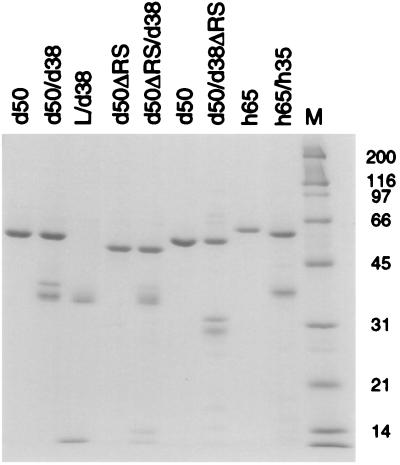
To examine whether the small subunit could bind RNA or contribute to the binding activity of the large subunit, we purified recombinant U2AF heterodimers (dU2AF and hU2AF), large-subunit monomers (dU2AF50 and hU2AF65), and the Drosophila small-subunit monomer (dU2AF38) from E. coli. To purify recombinant U2AF heterodimers, both subunits were coexpressed in the same E. coli cells (20). One of the two subunits was His6 tagged (dU2AF50 in dU2AF and hU2AF35 in hU2AF), and recombinant heterodimers were purified by affinity chromatography using Ni2+-NTA-agarose followed by ion-exchange chromatography. Recombinant large subunits His6-dU2AF50 and His6-hU2AF65) were expressed and purified similarly. However, His6-dU2AF38 was completely insoluble when expressed independently in E. coli. To avoid solubilization by denaturants, we coexpressed dU2AF38 with the His6-tagged interaction domain from dU2AF50 (His6-linker) (see Materials and Methods). The interaction domain contains 28 amino acids of dU2AF50 followed by 15 unrelated residues that result from a frameshift mutation introduced after the interaction domain (20). Soluble dU2AF38, complexed with the His6-tagged dU2AF50 interaction domain (linker/dU2AF38), was purified under the same conditions as the recombinant heterodimers. Samples of the final preparations were analyzed by electrophoresis through a sodium dodecyl sulfate (SDS)-polyacrylamide gel (Fig. 1). The heterogeneity observed in recombinant dU2AF38 was due to proteolysis at its carboxyl terminus. We detected a similar heterogeneity when the amino-terminal His6-tagged dU2AF38 was purified separately (data not shown).
FIG. 1.
SDS-polyacrylamide gel electrophoresis of purified recombinant U2AF proteins. Samples from the final preparations of U2AF monomers and heterodimers were run on an SDS–12% polyacrylamide gel and stained with Coomassie blue. All individually purified monomers are His6 tagged. dU2AF50 is His6 tagged in the dU2AF heterodimers, and hU2AF35 is His6 tagged in the hU2AF heterodimer. Marker sizes are indicated in kilodaltons.
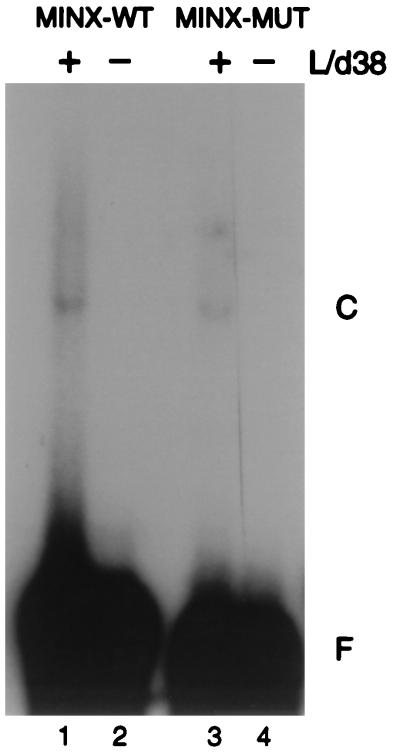
To determine whether the soluble form of the small subunit could bind RNA on its own, we performed electrophoretic mobility shift analyses. The purified, recombinant small subunit (Linker/dU2AF38) was incubated for 1 h with a 32P-labeled pyrimidine tract RNA oligonucleotide derived from the adenovirus L1-L2 intron (MINX-WT) and a mutant derivative (MINX-MUT). The sequences of MINX-WT and MINX-MUT pyrimidine tracts are shown in Fig. 3. Complex formation was assessed by native gel electrophoresis. dU2AF38 exhibited very weak but reproducible RNA binding activity (Fig. 2, lanes 2 and 4). Complex formation was detected in high salt (600 mM KCl) and with excess nonspecific competitor (heparin [10 mg/ml] or heparin [5 mg/ml] and tRNA [100 μg/ml]). There was some preference for U-rich tracts, as dU2AF38 bound better to MINX-WT than to MINX-MUT (Fig. 2; compare lanes 2 and 4). A similar preference of dU2AF38 RNA binding was observed in assays using the non-sex-specific, tra pyrimidine tract RNA and a tra mutant that disrupted the U-rich tract (reference 6 and data not shown).
FIG. 2.
dU2AF38 interacts weakly with pyrimidine tract RNA. For electrophoretic mobility shift analysis of dU2AF38 with MINX (WT and MUT) pyrimidine tract RNA, 200 pM 32P-labeled RNA oligonucleotide was incubated in the presence (+) or absence (−) of 5 μM dU2AF38 complexed with the His6-dU2AF50 interaction domain (L/d38). Protein-RNA complexes (C) and unbound RNA (F) were separated by electrophoresis through a native polyacrylamide gel and visualized by autoradiography.
Encouraged by the weak RNA binding activity of dU2AF38, we examined whether association of dU2AF38 with dU2AF50 could influence the RNA binding activity of dU2AF50. To rule out the possibility that any difference in activity between dU2AF50 and the dU2AF heterodimer was due to differences occurring during the preparation of the recombinant proteins, we purified the dU2AF heterodimer and free dU2AF50 monomer from the same E. coli cells. To do this, we took advantage of a second coexpression plasmid from which expression of the two subunits is not stoichiometric (20). Expression of His6-dU2AF50 was ∼10-fold higher than that of dU2AF38 in this expression plasmid. Heterodimer and His6-dU2AF50 monomer were first copurified on Ni2+-NTA-agarose and then resolved from each other by cation-exchange chromatography (see Materials and Methods). The binding activities of these proteins were determined by gel mobility shift analysis using the MINX-WT pyrimidine tract RNA (Fig. 3A). Consistent with the increased size of the dU2AF50/dU2AF38 complex, the dU2AF heterodimer retarded the mobility of the MINX-WT RNA oligonucleotide to a greater extent than the dU2AF50 monomer (Fig. 3A; compare lanes 2 and 7). The apparent Kd of the dU2AF50 monomer for MINX-WT was ∼2.2 × 10−6 M (Table 1), in good agreement with previous analysis (6). Significantly, the dU2AF heterodimer had a 20-fold-higher affinity for RNA than uncomplexed dU2AF50 (apparent Kd of ∼1.0 × 10−7 M [Table 1]). The increased RNA binding was unaffected by high salt (350 mM KCl) or nonspecific competitor (10 mg of heparin or 450 μg of tRNA per ml). Thus, dU2AF50 monomer can bind the pyrimidine tract RNA on its own, but the heterodimer binds with increased affinity.
TABLE 1.
Affinities of U2AF proteins for pyrimidine tract RNA
| Protein | Apparent Kda (M)
|
|
|---|---|---|
| MINX-WT RNA | MINX-MUT RNA | |
| dU2AF50b | 2.2 × 10−6 | Not detected |
| dU2AF50/dU2AF38 | 1.0 × 10−7 | 1.1 × 10−6 |
| dU2AF50ΔRS | NDc | ND |
| dU2AF50ΔRS/dU2AF38 | 1.7 × 10−7 | ND |
| dU2AF50b | 8.0 × 10−7 | ND |
| dU2AF50/dU2AF38ΔRS | 3.0 × 10−7 | ND |
| hU2AF65 | 3.8 × 10−8 | 2.6 × 10−7 |
| hU2AF65/hU2AF35 | 2.5 × 10−9 | 6.1 × 10−9 |
Determined by phosphorimager quantitation of 32P RNA signals (see Materials and Methods).
Monomer and heterodimer were purified from the same E. coli cells to allow direct comparison of RNA binding activities.
ND, not determined.
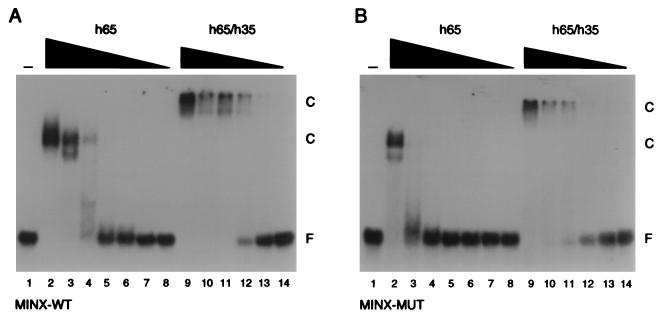
We analyzed human U2AF heterodimer and large-subunit monomer for RNA binding activity to determine whether this increase in RNA binding activity was specific to Drosophila U2AF (Fig. 4). The apparent Kd of the hU2AF65 monomer for MINX-WT RNA was ∼3.8 × 10−8 M (Table 1). This value is ∼8-fold lower than previously reported (9, 42). We have no explanation for the increased binding activity of our recombinant protein. By comparison, the human U2AF heterodimer bound RNA with 15-fold-higher affinity (apparent Kd of ∼2.5 × 10−9 M [Table 1]) than reported previously for the hU2AF65 monomer. Taken together, these data indicate there is a similar increase in RNA binding affinity for both the human and Drosophila U2AF heterodimers over free large-subunit monomers and suggest that a role for the small subunit in RNA binding is a common feature of U2AF.
FIG. 4.
The hU2AF heterodimer binds pyrimidine tract RNA with higher affinity than the hU2AF65 monomer. (A) Electrophoretic mobility shift analysis of hU2AF heterodimer and hU2AF65 monomer interaction with MINX-WT pyrimidine tract RNA. hU2AF65 monomer protein concentrations were 2,000, 400, 80, 16, 3.2, 0.64, and 0.128 nM (lanes 2 to 8). hU2AF heterodimer concentrations were 400, 80, 16, 3.2, 0.64, and 0.128 nM (lanes 9 to 14). Proteins were incubated with 100 pM 32P-labeled RNA oligonucleotide. Protein-RNA complexes (C) and unbound RNA (F) were separated by electrophoresis through a native polyacrylamide gel and visualized by autoradiography. The apparent Kd for hU2AF65 was ∼3.8 × 10−8 M. The apparent Kd for hU2AF heterodimer was ∼2.5 × 10−9 M. (B) Electrophoretic mobility shift analysis of hU2AF heterodimer and hU2AF65 monomer interaction with MINX-MUT pyrimidine tract RNA. Protein concentrations were identical to those in panel A. The apparent Kd for hU2AF65 was ∼2.6 × 10−7 M. The apparent Kd for the hU2AF heterodimer was ∼6.1 × 10−9 M.
To determine whether the specificity of U2AF for pyrimidine tracts was maintained in the recombinant U2AF heterodimers, we analyzed RNA binding by using the MINX pyrimidine tract mutant (MINX-MUT). As previously reported, binding of both dU2AF50 and hU2AF65 to MINX-MUT was significantly reduced (compare lanes 2 in Fig. 3A and B and lanes 3 in Fig. 4A and B). dU2AF50 binding to MINX-MUT was undetectable, and the affinity of hU2AF65 was ∼7-fold lower (apparent Kd of ∼2.6 × 10−7 M [Table 1]). Similarly, the U2AF heterodimers bound the MINX mutant with reduced affinity. The dU2AF heterodimer bound MINX-MUT with ∼10-fold-lower affinity (apparent Kd of ∼1.1 × 10−6 M [Table 1]), and the hU2AF heterodimer binding affinity was reduced ∼2.5-fold (apparent Kd of ∼6.1 × 10−9 M [Table 1]). Taken together, these data indicate that the U2AF heterodimer retains the specificity for pyrimidine tract RNA demonstrated by the large-subunit monomer.
The dU2AF50 monomer and the dU2AF heterodimer lacking the small subunit RS domain (dU2AF50/dU2AF38ΔRS) were analyzed for RNA binding activity to assess whether the RS domain on dU2AF38 was responsible for the increased binding activity of the U2AF heterodimer. The dU2AF38 RS domain deletion was identical to the deletion mutation used in the in vivo analysis of dU2AF38 (20). This deletion removed the glycine-rich carboxyl terminus and the entire RS domain (see Materials and Methods). To minimize differences in the preparation of the proteins, we purified monomer and heterodimer from the same E. coli cells (see above and Materials and Methods). dU2AF50 and dU2AF50/dU2AF38ΔRS were analyzed for RNA binding activity by using MINX-WT (Fig. 5B). Surprisingly, removal of the RS domain on dU2AF38 reduced the RNA binding activity of the heterodimer to within threefold of the activity of the dU2AF50 monomer (Fig. 5B). Phosphorimager quantitation analysis of bound and free radiolabeled RNA indicates a 2.5-fold-higher binding affinity of the heterodimer (dU2AF50/dU2AF38ΔRS) compared to the dU2AF50 monomer (apparent Kd of dU2AF50, ∼8.0 × 10−7 M; apparent Kd of dU2AF50/dU2AF38ΔRS, ∼3.0 × 10−7 M [Table 1]). We note that this dU2AF50 protein preparation binds RNA with higher affinity than our previous preparations (∼3-fold) (Table 1). However, since the dU2AF50 monomer and the dU2AF50/dU2AF38ΔRS heterodimer were purified from the same E. coli cells, their binding activities can be compared directly. Because of the variation in the protein preparations, comparison of the RNA binding activity of the dU2AF50/dU2AF38ΔRS heterodimer to the activity of the independent dU2AF50 (or dU2AF50/dU2AF38 heterodimer) preparation described above would be misleading. We conclude that the RS domain on dU2AF38 is necessary for the enhanced RNA binding activity of the dU2AF heterodimer and that the pseudo-RRM and two Zn2+ binding motifs make only a modest contribution to this binding activity.
FIG. 5.
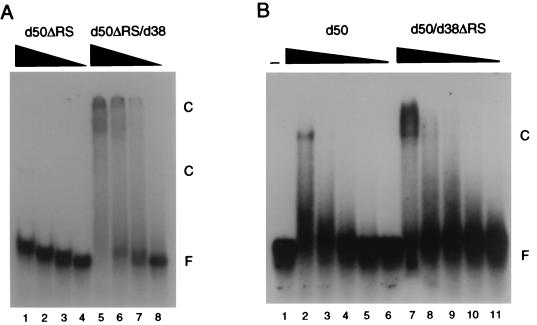
At least one RS domain on U2AF is required for high-affinity RNA binding. (A) Electrophoretic mobility shift analysis of dU2AF50ΔRS monomer and dU2AF50ΔRS/dU2AF38 heterodimer interaction with MINX-WT pyrimidine tract RNA. dU2AF50ΔRS monomer protein concentrations were 5,000, 1,000, 200, and 40 nM (lanes 1 to 4). dU2AF50ΔRS/dU2AF38 heterodimer concentrations were 5,000, 1,000, 200, and 40 nM (lanes 5 to 8). Proteins were incubated with 100 pM 32P-labeled RNA oligonucleotide. Protein-RNA complexes (C) and unbound RNA (F) were separated by electrophoresis through a native polyacrylamide gel and visualized by autoradiography. The apparent Kd for dU2AF50ΔRS could not be determined. The apparent Kd for dU2AF50ΔRS/dU2AF38 was ∼1.7 × 10−7 M. (B) Electrophoretic mobility shift analysis of dU2AF50 and dU2AF50/dU2AF38ΔRS heterodimer interaction with MINX-WT pyrimidine tract RNA. dU2AF50 monomer protein concentrations were 4,000, 1,600, 640, 256, and 102 nM (lanes 2 to 6). dU2AF50/dU2AF38ΔRS heterodimer protein concentrations were 4,000, 1,600, 640, 256, and 102 nM (lanes 7 to 11). The apparent Kd for dU2AF50 was ∼8.0 × 10−7 M. The apparent Kd for the dU2AF50/dU2AF38ΔRS heterodimer was ∼3.0 × 10−7 M.
To determine whether the dU2AF50 RS domain also plays a role in RNA binding in our assay, we analyzed dU2AF50ΔRS and dU2AF50ΔRS/dU2AF38 for binding to the MINX-WT RNA pyrimidine tract. Interestingly, removal of the dU2AF50 RS domain severely reduced RNA binding activity of dU2AF50 (compare lane 1 in Fig. 5A and lane 3 in Fig. 3A). Significantly, when an RS domain was supplied to dU2AF50ΔRS in trans (from dU2AF38), binding activity was restored (Fig. 5A). The RNA binding activity of dU2AF50ΔRS/dU2AF38 was determined to be only ∼1.7-fold lower than that of the wild-type dU2AF heterodimer (apparent Kd of ∼1.7 × 10−7 M [Table 1]). Taken together, these data indicate that the presence of at least one RS domain on U2AF, in addition to the three RRMs of the large subunit, is necessary for high-affinity RNA binding activity in vitro.
DISCUSSION
Although the known biochemical activities associated with the U2AF small subunit require the C-terminal RS domain and glycine-rich region, we have shown that these domains are dispensable in vivo (20). We therefore examined the amino-terminal 189 amino acids of dU2AF38 for other biochemical activities. This region is the most conserved part of the U2AF small subunit and contains a degenerate RRM and two putative Zn2+ binding domains (21). Since pseudo-RRMs and related Zn2+ binding domains have been implicated in RNA binding of other proteins (10, 37), we analyzed the small subunit for RNA binding activity. We found that a soluble form of dU2AF38 bound RNA weakly on its own and when complexed with the large subunit increased the pyrimidine tract binding affinity of dU2AF50 20-fold. The contribution of the U2AF small subunit to pyrimidine tract RNA binding was not specific to Drosophila; the human U2AF heterodimer also bound RNA with a 15-fold-higher affinity than the hU2AF65 monomer. Like the large subunits, both human and Drosophila heterodimers bound with reduced affinity to mutant pyrimidine tract RNAs, indicating that binding specificity was retained. Surprisingly, removal of the RS domain and glycine-rich region from dU2AF38 abolished virtually all of the increased binding activity of the dU2AF heterodimer. Although this result does not exclude a role for the degenerate RRM and Zn2+ binding motifs in RNA binding, it suggests that the RS domain on dU2AF38 is responsible for the increased binding activity of the dU2AF heterodimer. Finally, we have found that in our binding assays, the RS domain on dU2AF50 is required for RNA binding and that the dU2AF38 RS domain supplied by association with the large subunit will restore high-affinity binding activity to dU2AF50ΔRS. These results suggest that at least one of the RS domains on dU2AF is required for high-affinity RNA binding.
What is the role of the U2AF RS domain in RNA binding?
It has been shown previously that when hU2AF65 is bound to an intron pyrimidine tract RNA, its RS domain can be UV cross-linked to the branchpoint adenosine. This result indicates that the large-subunit RS domain is in close proximity to the RNA and suggests that the positively charged residues in the hU2AF65 RS domain are in a position to stabilize the base-pairing interaction between U2 snRNA and the branch site (34). The small-subunit U2AF RS domains could similarly stabilize the large-subunit RRMs on the pyrimidine tract through nonspecific interactions with the phosphodiester backbone of the pre-mRNA. However, the RNA binding activity of the heterodimer (and dU2AF38) was resistant to high salt, suggesting that it is unlikely that these interactions are purely electrostatic in nature.
It is not clear whether the U2AF small subunit is in direct contact with RNA or if the small subunit stabilizes or positions the large-subunit RRMs or RS domain on the RNA. There is precedent for heterodimer formation enhancing the affinity of an RNA binding protein for its substrate RNA (1, 23, 24, 30, 31). The best-characterized example is the requirement for the U2 snRNP-specific protein, U2A′, for the RNA binding activity of U2B" (1, 23, 24). In this case, U2A′ interacts with an RRM on U2B" that is required for U2 snRNA binding. This finding is consistent with a role for U2A′ in positioning or stabilizing the U2B" RRM on the RNA rather than in directly contacting the U2 snRNA (23, 24). The weak interaction between dU2AF38 and pyrimidine tract RNA observed by gel mobility shift analysis (Fig. 2) suggests that dU2AF38 is capable of direct contact with RNA. However, the inability to detect interaction between hU2AF35 and RNA by UV cross-linking in nuclear extracts (28) suggests that any interaction between RNA and the small subunit must be either weak or transient. Alternatively, in the context of the wild-type U2AF heterodimer, it is possible that the RS domain on hU2AF65 instead of hU2AF35 is in direct contact with the RNA (see above). In the absence of the large subunit (linker/dU2AF38) or the large-subunit RS domain (dU2AF50ΔRS/dU2AF38), direct contact between dU2AF38 and RNA might be observed as suggested by our gel mobility shift analysis of soluble, nondenatured small subunit (Fig. 2).
It has been shown that proteins with RS domains are phosphorylated on serine residues (6, 12). In fact, for the SR protein ASF/SF-2, phosphorylation has been shown to affect RNA binding specificity (32, 39). While it has been shown that U2AF65 is a substrate for both the SRPK-1 and Clk/Sty protein kinases in vitro (5), the phosphorylation state and sites of modification in vivo have not been determined. It is possible that the U2AF RS domains are modified by phosphorylation in vivo; however, the functional consequences of this modification(s) have not yet been addressed.
Requirement for the large-subunit RS domain for RNA binding.
Our biochemical analysis of the U2AF heterodimer and the requirements for an RS domain for high-affinity RNA binding differ from previous studies of U2AF (11, 42). The first biochemical analysis of hU2AF65 (42) detected a very modest decrease in RNA binding activity when the hU2AF65 RS domain was deleted. In our analysis, dU2AF50ΔRS was severely impaired in RNA binding activity (Fig. 5A). There are several possible explanations for this difference. In the analysis of the hU2AF65 RS domain, the RS deletion removed the entire RS domain but an adjacent region containing several positively charged residues was retained (42). If the large-subunit RS domain stabilizes U2AF on the pyrimidine tract RNA by interaction with the phosphodiester backbone (see above), this basic region retained in the original hU2AF65ΔRS deletion mutant might be sufficient for improved RNA binding activity. Significantly, this deletion derivative was originally determined to have no splicing activity; however, it was recently found that the positively charged region retained in the mutant could partially reactivate a U2AF-depleted extract (34). hU2AF65 splicing activity was completely abolished only when this basic region was also deleted. The dU2AF50 RS domain deletion used in the present study is similar to the previously characterized, more extensive hU2AF65 RS domain deletion (dU2AF50ΔRS retains only three basic residues).
A second possible explanation for the finding of differential requirements for the large subunit RS domain is the difference in recombinant proteins analyzed. The hU2AF65ΔRS used in the earlier study was a glutathione S-transferase (GST) fusion protein. Since the GST moiety is capable of homodimerization (13), the GST-hU2AF65ΔRS fusion protein might have six RRMs to contact the pyrimidine tract rather than three. Consistent with the proposed dimerization of this fusion protein, the sequence preference of GST-hU2AF65ΔRS, determined by iterative in vitro genetic selection, contained tandem repeats (26). Since the His6 tag used in the present study will not dimerize, the His6-dU2AF50ΔRS fusion only had three RRMs to contact RNA. Curiously, in a second study (11), using the identical hU2AF65 GST fusion proteins, the hU2AF65 RS domain was found to be essential for high-affinity RNA binding, consistent with our analysis of dU2AF50. We have no explanation for the discrepancy between the first and second studies, although it is noteworthy that the RNA substrate used in the later study lacked a well-defined pyrimidine tract (11). Finally, it is also possible that the different requirements for the large subunit RS domain reflect an intrinsic difference in U2AF from the two species.
We have found that the RNA binding activity of dU2AF50 (or hU2AF65) is augmented 15- to 20-fold when complexed with dU2AF38 (or hU2AF35). In the second study described above (11), hU2AF35 had no effect on RNA binding activity of hU2AF65. We believe that this discrepancy can be explained by the difference in preparation of the proteins analyzed. In the previous study, hU2AF35 and hU2AF65 were separated by reverse-phase chromatography, lyophilized, and resuspended in buffer containing 6 M guanidine hydrochloride. Under these conditions, it is possible that hU2AF35 was inactivated or lost the ability to interact with hU2AF65. Previously, we have found that separately purified recombinant dU2AF50 and dU2AF38 will not reassociate even after denaturation and step renaturation (20). The dU2AF heterodimer and monomer used in the present study were purified from the same E. coli cells, and complex formation was demanded by the purification procedure.
The lack of requirement for the dU2AF38 RS domain in vivo (20) and the presence of a pseudo-RRM on the small subunit prompted our analysis of the RNA binding activity of the U2AF heterodimer. Although an increase in RNA binding was observed when dU2AF38 was complexed with dU2AF50, surprisingly, the increased activity could be attributed to the dU2AF38 RS domain. Although this result appears at odds with the molecular genetic analysis of the dU2AF38 RS domain, resolution of these disparate results comes from the analysis of the RS domain on dU2AF50. In the absence of the dU2AF50 RS domain, dU2AF50 bound pyrimidine tracts with low affinity. If dU2AF38 containing an RS domain was complexed with dU2AF50ΔRS, high-affinity binding was restored. Thus, our data suggest that at least one RS domain is required for high-affinity binding of U2AF, which is consistent with the in vivo requirement for at least one dU2AF RS domain. A role for the dU2AF38 RS domain in RNA binding might also explain results in which hU2AF35 was required for pre-mRNA splicing in vitro (45) and why in some assays an excess of free hU2AF65 might suffice (7, 34).
ACKNOWLEDGMENTS
We thank members of the Rio and Cline labs for encouragement and support; K. Collins for critical reading of the manuscript; A. Rudner, R. Hampton, and C. Lee for useful discussions; and J. Lieber, C. Shea, and Aryeh for a place to complete the writing of the manuscript.
This work was initially supported by grant DB112 from the American Cancer Society and has more recently been supported by the NIH.
REFERENCES
- 1.Bentley R C, Keene J D. Recognition of U1 and U2 small nuclear RNAs can be altered by a 5-amino-acid segment in the U2 small nuclear ribonucleoprotein particle (snRNP) B" protein and through interactions with U2 snRNP-A′ protein. Mol Cell Biol. 1991;11:1829–1839. doi: 10.1128/mcb.11.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birney E, Kumar S, Krainer A R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black D L. Finding splice sites within a wilderness of RNA. RNA. 1995;1:763–771. [PMC free article] [PubMed] [Google Scholar]
- 3a.Blumenthal, T. Personal communication.
- 4.Caceres J F, Krainer A R. Functional analysis of pre-mRNA splicing factor SF2/ASF structural domains. EMBO J. 1993;12:4715–4726. doi: 10.1002/j.1460-2075.1993.tb06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colwill K, Feng L L, Yeakley J M, Gish G D, Caceres J F, Pawson T, Fu X D. SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors. J Biol Chem. 1996;271:24569–24575. doi: 10.1074/jbc.271.40.24569. [DOI] [PubMed] [Google Scholar]
- 6.Fu X D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 7.Gama-Carvalho M, Krauss R D, Chiang L, Valcarcel J, Green M R, Carmo-Fonseca M. Targeting of U2AF65 to sites of active splicing in the nucleus. J Cell Biol. 1997;137:975–987. doi: 10.1083/jcb.137.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanaar R, Lee A L, Rudner D Z, Wemmer D E, Rio D C. Interaction of the Sex-lethal RNA binding domains with RNA. EMBO J. 1995;14:4530–4539. doi: 10.1002/j.1460-2075.1995.tb00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanaar R, Roche S E, Beall E L, Green M R, Rio D C. The conserved pre-mRNA splicing factor U2AF from Drosophila: requirement for viability. Science. 1993;262:569–573. doi: 10.1126/science.7692602. [DOI] [PubMed] [Google Scholar]
- 10.Kramer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- 11.Lee C-G, Zamore P D, Green M R, Hurwitz J. RNA annealing activity is intrinsically associated with U2AF. J Biol Chem. 1993;268:13472–13478. [PubMed] [Google Scholar]
- 12.Manley J L, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 13.Maru Y, Afar D E, Witte O N, Shibuya M. The dimerization property of glutathione S-transferase partially reactivates Bcr-Abl lacking the oligomerization domain. J Biol Chem. 1996;271:15353–15357. doi: 10.1074/jbc.271.26.15353. [DOI] [PubMed] [Google Scholar]
- 14.Moore M J, Query C C, Sharp P A. Splicing of precursors to mRNAs by the spliceosome. In: Gesteland R F, Atkins J F, editors. The RNA world. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1993. pp. 303–357. [Google Scholar]
- 15.Patton J G, Mayer S A, Tempst P, Nadal-Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev. 1991;5:1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- 16.Potashkin J, Naik K, Wentz-Hunter K. U2AF homolog required for splicing in vivo. Science. 1993;262:573–575. doi: 10.1126/science.8211184. [DOI] [PubMed] [Google Scholar]
- 17.Reed R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr Opin Genet Dev. 1996;6:215–220. doi: 10.1016/s0959-437x(96)80053-0. [DOI] [PubMed] [Google Scholar]
- 18.Reed R. The organization of 3′ splice-site sequences in mammalian introns. Genes Dev. 1989;3:2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- 19.Rudner D Z, Breger K S, Rio D C. Molecular genetic analysis of the heterodimeric splicing factor U2AF: the RS domain on either the large or small Drosophila subunit is dispensable in vivo. Genes Dev. 1998;12:1010–1021. doi: 10.1101/gad.12.7.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudner D Z, Kanaar R, Breger K S, Rio D C. Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol Cell Biol. 1998;18:1765–1773. doi: 10.1128/mcb.18.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudner D Z, Kanaar R, Breger K S, Rio D C. Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects. Proc Natl Acad Sci USA. 1996;93:10333–10337. doi: 10.1073/pnas.93.19.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruskin B, Zamore P D, Green M R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988;52:207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- 23.Scherly D, Boelens W, Dathan N A, van Venrooij W J, Mattaj I W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B" and their cognate RNAs. Nature. 1990;345:502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- 24.Scherly D, Dathan N A, Boelens W, van Venrooij W J, Mattaj I W. The U2B" RNP motif as a site of protein-protein interaction. EMBO J. 1990;9:3675–3681. doi: 10.1002/j.1460-2075.1990.tb07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp P A. Split genes and RNA splicing. Cell. 1994;77:805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 26.Singh R, Valcárcel J, Green M R. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 27.Smith C W J, Patton J G, Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- 28.Staknis D, Reed R. Direct interactions between pre-mRNA and six U2 small nuclear ribonucleoproteins during spliceosome assembly. Mol Cell Biol. 1994;14:2994–3005. doi: 10.1128/mcb.14.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staknis D, Reed R. SR proteins promote the first specific recognition of pre-mRNA and are present together with the U1 small nuclear ribonucleoprotein particle in a general splicing enhancer complex. Mol Cell Biol. 1994;14:7670–7682. doi: 10.1128/mcb.14.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern S, Powers T, Changchien L M, Noller H F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989;244:783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- 31.Strub K, Moss J, Walter P. Binding sites of the 9- and 14-kilodalton heterodimeric protein subunit of the signal recognition particle (SRP) are contained exclusively in the Alu domain of SRP RNA and contain a sequence motif that is conserved in evolution. Mol Cell Biol. 1991;11:3949–3959. doi: 10.1128/mcb.11.8.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tacke R, Chen Y, Manley J L. Sequence-specific RNA binding by an SR protein requires RS domain phosphorylation: creation of an SRp40-specific splicing enhancer. Proc Natl Acad Sci USA. 1997;94:1148–1153. doi: 10.1073/pnas.94.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian M, Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- 34.Valcárcel J, Gaur R K, Singh R, Green M R. Interaction of U2AF65 RS region with pre-mRNA of branch point and promotion base pairing with U2 snRNA. Science. 1996;273:1706–1709. doi: 10.1126/science.273.5282.1706. [DOI] [PubMed] [Google Scholar]
- 35.Valcárcel J, Singh R, Zamore P D, Green M R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature. 1993;362:171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- 36.Wentz-Hunter K, Potashkin J. The small subunit of the splicing factor U2AF is conserved in fission yeast. Nucleic Acids Res. 1996;24:1849–1854. doi: 10.1093/nar/24.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worthington M T, Amann B T, Nathans D, Berg J M. Metal binding properties and secondary structure of the zinc-binding domain of Nup475. Proc Natl Acad Sci USA. 1996;93:13754–13759. doi: 10.1073/pnas.93.24.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- 39.Xiao S H, Manley J L. Phosphorylation of the ASF/SF2 RS domain affects both protein-protein and protein-RNA interactions and is necessary for splicing. Genes Dev. 1997;11:334–344. doi: 10.1101/gad.11.3.334. [DOI] [PubMed] [Google Scholar]
- 40.Zamore P D, Green M R. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991;10:207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamore P D, Green M R. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci USA. 1989;86:9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamore P D, Patton J G, Green M R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355:609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Zamore P D, Carmo-Fonseca M, Lamond A I, Green M R. Cloning and intracellular localization of the U2 small nuclear ribonucleoprotein auxiliary factor small subunit. Proc Natl Acad Sci USA. 1992;89:8769–8773. doi: 10.1073/pnas.89.18.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zorio A R, Lea K, Blumenthal T. Cloning of Caenorhabditis U2AF65: an alternatively spliced RNA containing a novel exon. Mol Cell Biol. 1997;17:946–953. doi: 10.1128/mcb.17.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuo P, Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]
- 46.Zuo P, Manley J L. Functional domains of the human splicing factor ASF/SF2. EMBO J. 1993;12:4727–4737. doi: 10.1002/j.1460-2075.1993.tb06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]