Abstract
Anaplastic lymphoma kinase (ALK)-fusion sarcomas are rare part of the emerging theoretically targetable tyrosine kinase RAS::MAPK pathway fusion myopericytic-ovoid sarcomas. We report our clinicopathologic and treatment experience with an ALK fusion sarcoma. A novel ELKS/RAB6-interacting/CAST family member 1–unaligned ALK fusion infiltrative nonmetastatic low-grade sarcoma of the right hand of a 15-month-old male was treated with crizotinib, an ALK tyrosine kinase inhibitor as oral monotherapy, inducing complete radiographic and clinical resolution by 10 months and sustained response now over 12 months after elective discontinuation. Crizotinib can successfully be used to treat unresectable novel ALK fusion sarcomas.
Key Words: ALK fusion, crizotinib, unresectable pediatric sarcoma, molecular targets
Albeit rare overall, emerging anaplastic lymphoma kinase (ALK)-fusion myofibroblastic/infantile-fibrosarcoma–like spindled-to-ovoid sarcomas with tyrosine kinase RAS::MAPK pathway fusions,1,2 are increasingly identified using next-generation sequencing and other advanced molecular studies for diagnosis, prognosis, and targeted therapy. Crizotinib treatment of ALK gene mutation tumors, including carcinomas, lymphoma, and neuroblastoma, has been reported.3 Experience with targeted therapy in ALK fusion sarcomas has mostly included inflammatory myofibroblastic tumor,4 yet treatment of emerging novel ALK fusions has not previously been described in detail. Crizotinib, an ALK tyrosine kinase inhibitor, is a first-line agent used for ALK mutations.3 We demonstrate successful treatment of a pediatric unique ELKS/RAB6-Interacting/CAST family member 1 (ERC1)-ALK fusion sarcoma interdigitating the right hand that avoided distal forearm amputation by use of neoadjuvant crizotinib.
CASE PRESENTATION
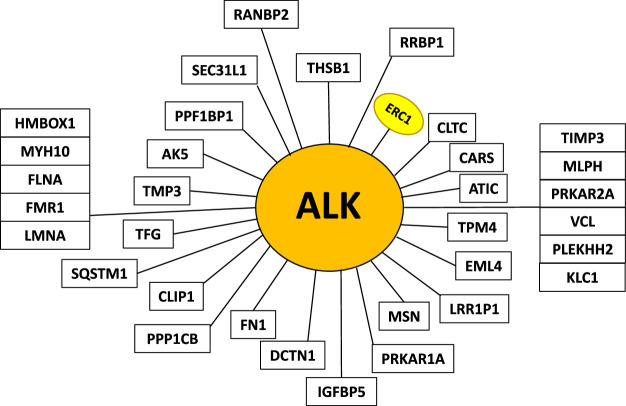
A 15-month-old previously healthy male presented with a slowly enlarging, firm right-hand mass, first noticed at around 6 months of age. After visits to multiple specialists across many institutions, he was diagnosed by biopsy with low-grade myopericytoid-ovoid sarcoma infiltrating around digits, revealing a novel ERC1-unaligned ALK fusion. ALK fusion sarcoma fusion partners known to date are depicted in Figure 1. Histologically, the tumor had a vascular staghorn and bland, up to moderately cellular lipofibromatosis-like infiltrative appearance into skeletal muscle and adipose tissue (Fig. 2) and was focally positive for S100 protein, negative for CD34 and SOX10. Metastatic workup, including chest computed tomography and full-body magnetic resonance imaging (MRI), which revealed a 3-mm nonspecific stable pulmonary nodule, was considered negative. Other than increasingly restricted range of motion and painless stiffness due to tumor bulk, the patient was asymptomatic and met developmental and growth milestones.
FIGURE 1.
Newest update of ALK fusion sarcomas including our case of ERC1::ALK.
FIGURE 2.
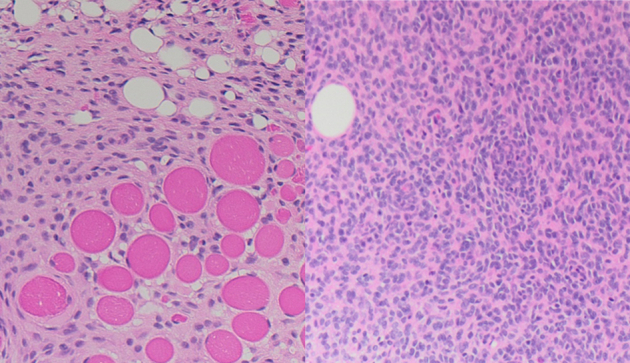
ERC1::ALK fusion sarcoma of interdigitating hand in 15-month-old male infiltrates as blander ovoid myopericytoid cells into adipose tissue with smaller fat cells (lipofibromatosis-like) and skeletal muscle and has focal moderate cellularity, perivascular whorling, similar to that described in infantile fibrosarcoma or myopericytic tumors.
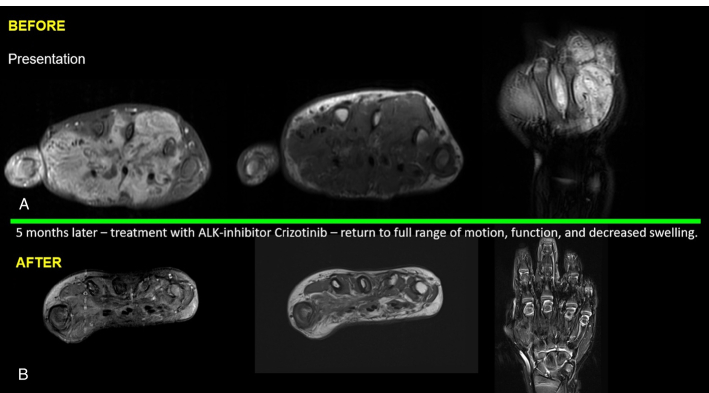
Initial MRI imaging of the right hand revealed a homogenous enhancing mass 5.6×4×5.1 cm within the hand at the level of the metacarpal bones, interdigitating around and remodeling adjacent bones without destruction (Fig. 3A). The radiologic presentation was initially considered a vascular malformation, due to the prominent vessels that correspond to the myopericytic staghorn vascular appearance by morphology. Surgical resection, likely curative, would require distal forearm amputation based on orthopedic and plastic surgical expertise. The consensus was reached across multiple disciplines and institutions to trial enteral neoadjuvant crizotinib and determine tumor responsiveness while limiting long-term morbidity. Due to our patient’s age and size, emergency use authorization was granted by the Food and Drug Administration through Pfizer and approved by our institutional review board for single patient use to access the oral crizotinib liquid formulation being employed as part of the Children’s Oncology Group ANBL 1531 trial. Due to taste intolerance, a gastrostomy tube was placed for drug administration. Initial MRI was obtained approximately 5 months before treatment initiation with repeat baseline imaging 5 days after stating crizotinib. Treatment response was monitored via MRI at 3, 7, 10, and 16 months after drug initiation. Marked tumor regression and return to normal use and function were noted at the 3- and 7-month checkpoints, with complete radiographic resolution at 10 and 16 months of treatment.
FIGURE 3.
A ERC1::ALK fusion sarcoma before treatment forming interdigitating mass, unresectable. B, No residual sarcoma by radiologic or clinical findings post-treatment with tyrosine kinase inhibitor crizotinib.
Although the targeted dosing for crizotinib is 215 mg/m2/dose bid, our patient’s chronic mild neutropenia limited dosing to ~140 mg/m2/dose bid. Since gastrostomy tube insertion, he tolerated all doses as scheduled except for a brief hold and dose readjustment for one early episode of grade IV neutropenia and individual doses held due to sedation for MRIs. Side effects were otherwise limited to intermittent mild neutropenia and occasional constipation. Toxicity was monitored with routine ophthalmology evaluations and serial EKGs to track QT/QTc intervals. Crizotinib was electively discontinued after 16 months, 6 months after his complete response. Tumor resection was not attempted at the completion of therapy because there was no remaining radiologic or clinical tumor (Fig. 3B). Radiographic monitoring at 1, 6, and 8 months after discontinuation of crizotinib demonstrated complete radiologic resolution of the mass.
DISCUSSION
Novel ERC1::ALK fusion sarcoma can be successfully treated with oral crizotinib, in this case sparing distal forearm amputation in a pediatric patient.
The oncogenesis of ALK fusion–positive sarcomas involves promoter interruption with subsequent dysregulated tyrosine kinase activity. In nonmesenchymal tumors, the discovery of ALK mutations in 3% to 5% of patients with non–small cell lung cancer4,5 drove early-phase clinical studies of crizotinib, a first-in-class dual ALK/MET/ ROS1 small molecular tyrosine kinase inhibitor6,7 with good oral bioavailability that targets (adenosine triphosphate [ATP])-induced catalytic capacity of anaplastic lymphoma kinase (ALK) kinases, inducing apoptosis of tumor cells at the G1-S phase checkpoint.8 The dramatic response rates in non–small cell lung cancer validated ALK as a therapeutic target and led to expedited Food and Drug Administration approval of crizotinib in August 2011 for use in patients with ALK-rearranged lung cancer.9,10
Use of targeted therapy for ALK fusion sarcoma/mesenchymal tumors, including inflammatory myofibroblastic tumor has been demonstrated11 and is still being described with some of the newer fusions. The tyrosine kinase RAS::MAPK pathway fusion sarcomas, including NTRK, BRAF, RAF1, RET, FGFR1, and ABL1, are still evolving. These pathway sarcomas were first noted as infantile fibrosarcoma in infants with an ETV6:NTRK3 fusion.12 An identical fusion was subsequently noted in morphologically similar congenital mesoblastic nephroma.13 In addition to other NTRK1, NTRK2, and NTRK3 fusions, these have been reported as an emerging entity of NTRK-rearranged spindle cell neoplasm in the latest World Health Organization Bone and Soft Tissue Tumors Classification.14 Most of these tumors appear low grade with myopericytic spindled-to-ovoid cells that infiltrate into muscle and fat in a lipofibromatosis-like pattern.1,2,15 These low-grade fusion sarcomas often have focal CD34 and/or S100 protein and are negative for SOX1016,17 excluding the possibility of a nerve sheath tumor. A second fusion sarcoma morphology of a high-grade spindled pleomorphic tumor has been reported, observed only with certain NTRK fusion partners including TPR and KANK1.1 These tumors can be superficial dermal and subcutaneous18 or deep and intramuscular/intraosseous.1,19 While the low-grade behavior of these ovoid-spindled tumors generally allow for complete surgical excision, those with deep involvement, metastasis, or in our case the interdigitating infiltration of the tumor that would have required a distal forearm amputation, are best treated with crizotinib. The correct decision to pursue single-agent monotherapy may guide future management if nonresectable and medical agents aimed at molecular targets demonstrates promise in reducing long-term morbidity. Serious adverse events include cytopenias, visual disturbances, and gastrointestinal upset, which may result in skipped doses or dose reduction, as in our patient.
Novel ALK fusion sarcomas may respond to molecular-targeting agents, such as crizotinib and next-generation ALK inhibitors. In particular, the ERC1::ALK fusion sarcoma in our patient, representing a RAS::MAPK tyrosine kinase pathway tumor, was targeted and treated successfully by a single oral agent, crizotinib, precluding amputation and resulting in complete radiographic and clinical remission.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Megan L. Wood, Email: megwood1225@gmail.com.
Julie C. Fanburg-Smith, Email: jcfsmd@gmail.com.
James M. Brian, Email: jbrian@pennstatehealth.psu.edu.
Jason C. White, Email: jason.white@towerhealth.org.
Jonathan L. Powell, Email: Jonathan.Powell@nemours.org.
Andrew S. Freiberg, Email: afreiberg@pennstatehealth.psu.edu.
REFERENCES
- 1. Chen T, Wang Y, Goetz L, et al. Novel fusion sarcomas including targetable NTRK and ALK. Ann Diagn Pathol. 2021;54:1–12. [DOI] [PubMed] [Google Scholar]
- 2. Davis JL, Al-Ibraheemi A, Rudzinski ER, et al. Mesenchymal neoplasms with NTRK and other kinase gene alterations. Histopathology. 2022;80:4–18. [DOI] [PubMed] [Google Scholar]
- 3. Foster JH, Voss SD, Hall D, et al. Activity of crizotinib in patients with ALK-aberrant relapsed/refractory neuroblastoma: a Children’s Oncology Group Study (ADVL0912). Clin Cancer Res. 2021;27:3543–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu J, Hu Y, Abdihamid O, et al. Crizotinib in sarcomatous malignancies harboring ALK fusion with a definitive partner(s): response and efficacy. Front Oncol. 2021;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. [DOI] [PubMed] [Google Scholar]
- 6. Crystal AS, Shaw AT. New targets in advanced NSCLC: EML4-ALK. Clin Adv Hematol Oncol. 2011;9:207–214. [PubMed] [Google Scholar]
- 7. Shaw AT, Yeap BY, Solomon BJ, et al. Eff ect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet. 2011;12:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaw AT, Solomon BJ, Mino-Knudson M. Crizotinib and testing for ALK. JNCCN. 2011;9:1335–1341. [DOI] [PubMed] [Google Scholar]
- 9. Chabner BA. Early accelerated approval for highly targeted cancer drugs. N Engl J Med. 2011;364:1087–1089. [DOI] [PubMed] [Google Scholar]
- 10. Nwizu T, Kanteti R, Kawada I, et al. Crizotinib (PF02341066) as a ALK/MET inhibitor—special emphasis as a therapeutic drug against lung cancer. Drugs Futur. 2011;36:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baranov E, Winsnes K, O’Brien M, et al. Histologic characterization of paediatric mesenchymal neoplasms treated with kinase-targeted therapy. Histopathology. 2022;81:215–227. [DOI] [PubMed] [Google Scholar]
- 12. Knezevich SR, McFadden DE, Tao W, et al. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–187. [DOI] [PubMed] [Google Scholar]
- 13. Rubin BP, Chen CJ, Morgan TW, et al. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: Cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am J Pathol. 1998;153:1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suurmeijer AJ, Antonescu CR. World Health Organization Classification of Soft Tissue and Bone Tumours, 5th ed. Fletcher D. IARC Press; 2020. [Google Scholar]
- 15. Kao YC, Suurmeijer AJH, Argani P, et al. Soft tissue tumors characterized by a wide spectrum of kinase fusions share a lipofibromatosis-like neural tumor pattern. Genes Chromosom Cancer. 2020;59:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abs D, Landman S, Osio A, et al. Spindle cell tumor with CD34 and S100 co-expression and distinctive stromal and perivascular hyalinization showing EML4-ALK fusion. J Cutan Pathol. 2021;48:896–901. [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Nunez O, Surrey LF, Alaggio R, et al. Novel PPP1CB-ALK fusion in spindle cell tumor defined by S100 and CD34 coexpression and distinctive stromal and perivascular hyalinization. Genes Chromosom Cancer. 2020;59:495–499. [DOI] [PubMed] [Google Scholar]
- 18. Dermawan JK, Azzato EM, Goldblum JR, et al. Superficial ALK-rearranged myxoid spindle cell neoplasm: a cutaneous soft tissue tumor with distinctive morphology and immunophenotypic profile. Mod Pathol. 2021;34:1710–1718. [DOI] [PubMed] [Google Scholar]
- 19. Dermawan JK, DiNapoli SE, Mullaney KA, et al. ALK-rearranged Mesenchymal Neoplasms: a Report of 9 cases Further Expanding the Clinicopathologic Spectrum of Emerging Kinase Fusion Positive Group of Tumors. Genes Chromosom Cancer. 2022;62:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]