Abstract
Introduction:
The most dreaded complication during laparoscopic cholecystectomy still remains to be injury to the common bile duct. The primary cause for bile duct injury during LC is misinterpretation of the biliary anatomy. Intra-operative cholangiography was introduced as a means of reducing the chances of biliary injury, done using Fluoroscopic imaging or Near-infrared fluorescence imaging method. NIRF is one of the most popular imaging methods in biomedical sciences. Indocyanine Green is sterile and water soluble which completely binds to albumin and is excreted in bile.
Patients and Methods:
This prospective study was conducted among 70 patients between July 2020 and December 2021. Subjects were administered 5mg of ICG dye pre-operatively and procedure performed using Karl Storz HD image S1 system with a D-light P light source for NIRF imaging.
Results:
The average duration of surgery was 58.10 minutes. After calot’s dissection, the CBD was visualized in 88.71 % patients, with a mean time to visualization at 26.33 minutes. The cystic duct was visualized in 87.3% cases with a mean time of visualization of 32.10 minutes. The hepatic duct was visualized in 28.57% and the hepatic duct-CBD confluence was visualized in 34.28% patients.
Conclusion:
Near infrared imaging based intra-operative cholangiography, using Indocyanine Green dye, during Lap. Cholecystectomy is an easy, useful and inexpensive method of visualizing the biliary ductal anatomy.
Keywords: Biliary anatomy, indocyanine green, laparoscopic cholecystectomy, near-infrared imaging
INTRODUCTION
Laparoscopic cholecystectomy (LC) was first described in 1983 by a Russian, Lukichev, but only printed in the Russian literature. The credit for the first actual LC is given to German Prof. Dr. Erich Muhe, who performed the procedure in 1985.[1] The first LC in India was performed by Dr. Udwadia, at JJ Hospital, Mumbai, in 1990.[2] LC is still one of the most common laparoscopic procedures done worldwide. Indications listed by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) for LC include symptomatic cholelithiasis, biliary dyskinesia, acute cholecystitis and biliary pancreatitis.[3] Furthermore, some additional indications include gall bladder mass/polyp, empyema gall bladder and complicated cholecystitis, including patients with frozen Calot’s triangle, a history of previous abdominal surgery or with Mirizzi syndrome.[4,5]
The most dreaded complication during LC still remains to be an injury to the common bile duct (CBD); the incidence of which ranges from 0.3% to 0.6%.[6,7] It is a well-known fact that the primary cause for bile duct injury is the misinterpretation of biliary anatomy intraoperatively, happening in 71%–97% of all such cases.[8,9] There is great variation in the normal biliary ductal anatomy [Figure 1].[10]
Figure 1.
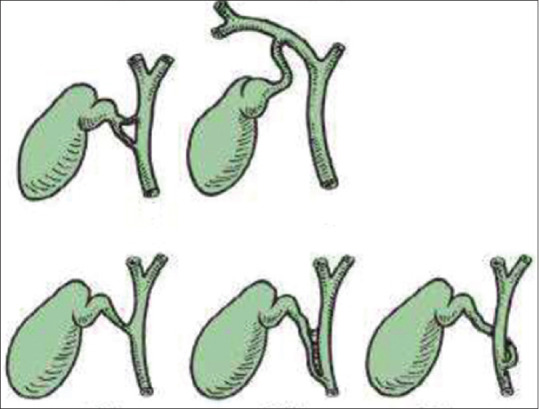
Variations in cystic duct anatomy and their union with the common bile duct
Strasberg defined his concept of error traps, which can lead to misperception of the biliary anatomy intraoperatively.[11,12,13,14,15] These include:
The infundibular view error trap [Figure 2]: dangerous anatomic variants of the right posterior hepatic duct draining into the following: (A) cystic duct (CD); (B) gall bladder neck and (C) common hepatic duct (CHD)
Fundus-down cholecystectomy [Figure 3]: the perceived, safe operative plane coming down the medial wall of the gall bladder is, in such cases, obliterated by an inflammatory reaction, which incorporates the right-sided porta hepatis and the CBD. Thus, this injury is commonly associated with major biliary and vascular injury, at times requiring liver resection for the ischaemic injury. Failure to perceive the presence of an aberrant or posterior right hepatic duct on cholangiography (intraoperative cholangiography [IOC])
Injury to the CBD in case of a parallel union CD.
Figure 2.
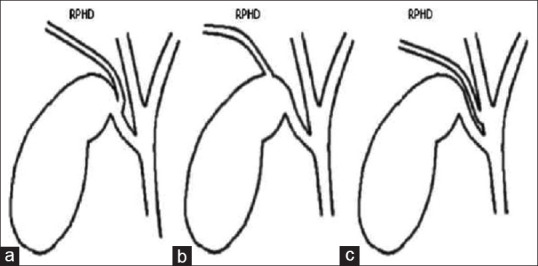
Infundibular view error trap. Right posterior hepatic duct (RPHD) draining into (a): Cystic duct, (b): gall bladder neck and (c): Common hepatic duct
Figure 3.
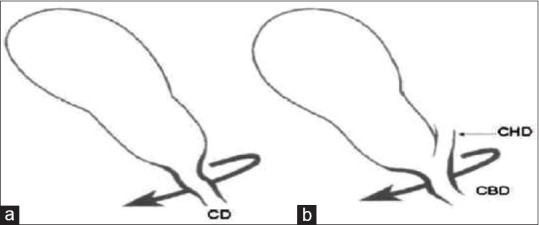
Error trap of fundus-down cholecystectomy. (a) The usual anatomy when the infundibular technique is used; note the flaring of the cystic duct-common bile duct (CBD) union which has a characteristic funnel shape (heavy black line). (b): Anatomical situation in some cases of classical injuries. Flaring (heavy black line) occurs when the CBD is followed up to an inflammatory mass within which the cystic duct is hidden. As a result, the CBD is mistakenly identified as the cystic duct. Note position of common hepatic duct (CHD)
To overcome this, the concept of safe LC had been introduced. The main components of which include:
30° or 45° high-definition laparoscope
Cephalad traction on the dome of the gall bladder and lateral traction on the infundibulum
Staying on the gall bladder wall during dissection. Dissecting from above and down to the neck
Widely opening the cholecystohepatic triangle
Waving the flag: moving the infundibulum back and forth repeatedly to look at both sides of the gall bladder
Dividing the CD as close to the gall bladder as possible
Achieving the critical view of safety before clipping the CD
Never divide the CD with an energy device because if it turns out to be the CBD, the ischaemic injury will lessen the chances of a good repair at a later stage.[16]
The SAGES expert Delhi consensus has deemed the critical view of safety to be the most critical factor for overall safety during LC.
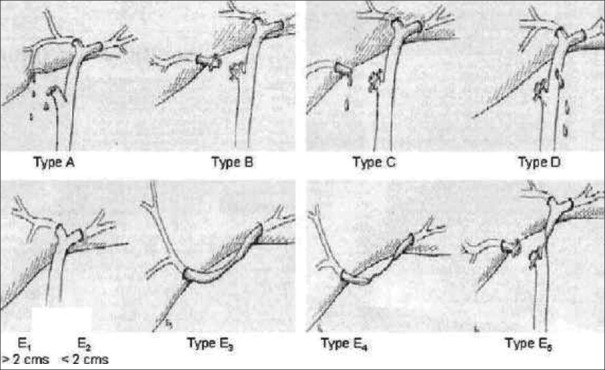
The most commonly used classification system for biliary injuries is the one described by Strasberg [Figure 4]:
Figure 4.
Strasberg’s classification of common bile duct injury
Type A – Bile leak from a minor duct retaining continuity with the CBD
Types B and C – Creation of discontinuity for part of the biliary tree
Ductal occlusion injuries are designated as type B and transections without occlusion as type C
Type D – Lateral injury to the major bile duct. These injuries are partial (i.e. 50%)
Type E – Circumferential injury to the major bile duct. These injuries are further divided into five types (E1–E5). Types E1–E4 are based on the level of injury, whereas E5 is a combination of common hepatic and right sectoral duct injury.
IOC was introduced as a means of reducing the chances of biliary injury, done using fluoroscopic imaging or near-infrared fluorescence (NIRF) imaging method. The SAGES recommends IOC be done in every case of LC,[17] while certain other reviews recommend selective use of IOC. Some of the indications for selective IOC include pain on the day of operation, abnormal hepatic function panel, anomalous or confusing biliary anatomy and alteration in anatomy that precludes the ability to perform ERCP after cholecystectomy, such as Roux-en-Y gastric bypass, dilated biliary tree or any pre-operative suspicion of choledocholithiasis. Studies suggest that near-infrared imaging with indocyanine green (ICG) provides a good overall visualisation of the cystic, common bile, CHDs and the CD junction with CBD before and following the dissection of Calot’s triangle as proven by Vlek et al., in their study, in 2016.[18]
NIRF is one of the most popular imaging methods in biomedical sciences; as it provides a high contrast, i.e. a good signal-to-noise ratio, tissues do not show any autofluorescence in this spectrum, high sensitivity, is cheap and easy to use.[19,20] ICG is sterile, tricarbocyanine, water-soluble, that completely binds to albumin, is taken up by hepatocytes and then excreted in bile. It does not undergo enterohepatic circulation and stays in the blood for only 8 min after injection. It is non-toxic and non-ionising. NIRF with ICG avoids radiation exposure to the patient and the operating team.[20,21] The risk of anaphylaxis with ICG is 0.003% at doses exceeding 0.5 mg/kg.[22] It has also been noted that the use of ICG was associated with a significant reduction in the rate of conversion from laparoscopic to open procedure.[23]
During IOC, the fluoroscopic images should be assessed for the length of the CD and its junction with the CBD, diameter of the CBD, any luminal filling defects within the CBD, free flow of contrast into the duodenum and anatomy of the intrahepatic biliary tree [Figures 5 and 6].[7]
Figure 5.
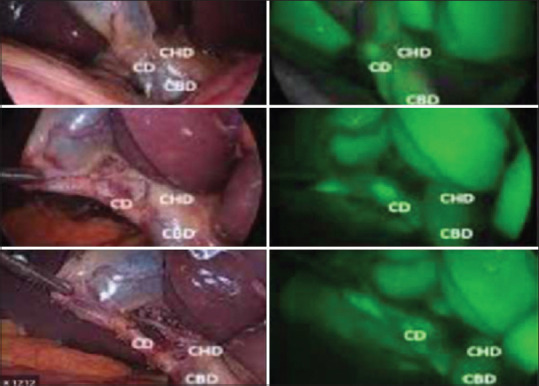
Anatomy of ducts as seen during ICG-IOC. ICG-IOC: Indocyanine green intraoperative cholangiography, CBD: Common bile duct, CHD: Common hepatic duct, CD: Cystic duct
Figure 6.
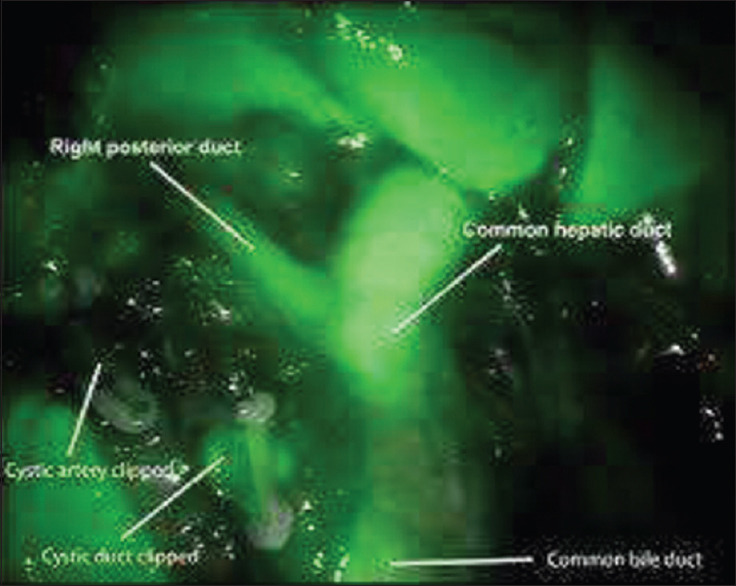
Right posterior hepatic duct draining into common hepatic duct
The timing of injecting ICG to the patient can be variable, ranging anywhere from 12 h before surgery to just at induction of anaesthesia with the dose of ICG given varying between 0.05 and 0.25 mg/kg.[18,23]
In a systematic review by Serban et al., ICG-NIRC was deemed to be a promising tool to increase safety in LC.[24]
PATIENTS AND METHODS
This prospective study was conducted amongst 70 patients admitted to the general surgery department of DMC and H, Ludhiana, between July 2020 and December 2021, after obtaining informed consent from all the patients. The data were collected through a pre-designed pro forma for each patient. The inclusion criteria for the patients included any symptomatic gall bladder disease requiring surgery, including acute cholecystitis, symptomatic gallstone disease, empyema gall bladder, gangrenous cholecystitis, acute mild pancreatitis and mucocele gall bladder. The pathology was confirmed preoperatively using imaging through USG or MRCP.
One patient was excluded from the study due to a previously documented iodine allergy. Other exclusion criteria included a previous history of cholecystostomy or comorbidities in a patient that precludes surgery. Patient-related factors recorded were age, gender, presence or absence of diabetes, acute or chronic duration of the disease, history of pancreatitis and pre-operative ERCP. Patients with jaundice preoperatively or suspicion of CBD stone were evaluated with MRCP.
The patients were injected preoperatively with 5 mg/1 mL of ICG, diluted in 5 mL of distilled water. Of the 70 patients, seven were administered the dye 15 min before surgery, 62 were administered 30 min before surgery and one patient was injected with the dye 1 h before surgery. The procedure was performed using the Karl Storz HD Image S1 system with a D-light P-light source for NIRF imaging with Aurogreen (ICG) injection given preoperatively. The cost of Aurogreen available in our institution was approximately Rs. 2000, and a single vial of the product can be used for up to five cases on the same day, once opened. After Calot’s dissection, visualisation of the CBD, hepatic duct, CD and the hepatic duct-CBD confluence was noted under NIRF imaging. After the surgery, the majority of the patients were discharged within 3 days (52.1%). Thirty-one patients were discharged within 7 days (43.7%), and only three patients were required to be hospitalised for more than 7 days.
RESULTS
In the present study, the mean age of the patient population was 48.20 years, with 80.3% of patients between the fourth and seventh decades of life. The population had a female preponderance with a male-to-female ratio of 47:24 (66.2% of females). The most common indication for undergoing LC in the present study was symptomatic gallstone disease, in 47.9% (34/71) patients; of which seven patients had concomitant CBD stones (9.9%). Two (2.8%) patients underwent LC during their index admission for acute mild biliary pancreatitis, while two were follow-up cases of severe biliary pancreatitis. Six (8.5%) patients in the study had jaundice before surgery.
All the patients were evaluated preoperatively using USG of the abdomen, and nine patients further underwent MRCP, primarily for suspected CBD stones [Table 1].
Table 1.
Pre-operative imaging findings
| Imaging findings | Number of cases (%) |
|---|---|
| Acute calculous cholecystitis | 5 (7.0) |
| Acute gangrenous cholecystitis | 2 (2.8) |
| Acute gangrenous cholecystitis with intrahepatic perforation | 1 (1.4) |
| Chronic calculous cholecystitis | 23 (32.4) |
| Empyema GB | 1 (1.4) |
| Gallstones | 19 (26.8) |
| Gallstones with acute pancreatitis with peripancreatic fluid | 2 (2.8) |
| Gallstones with CBD stones | 7 (9.9) |
| Gallstones with sludge | 8 (11.3) |
| Mucocele GB | 3 (4.2) |
| Total | 71 (100.0) |
GB: Gall bladder, CBD: Common bile duct
The average duration of surgery was 58.10 min, with a majority of cases being performed within an hour (55 of 71). Sixty-two out of 70 patients were administered the ICG dye 30 min before surgery, seven patients were given the dye 15 min before surgery and one patient was given 1 h before surgery. There was no significant difference in the quality of visualisation of the biliary anatomy between the three different groups according to time on ICG administration. One patient was not administered the dye due to a prior history of allergy to iodine. Two patients developed a reaction to the ICG dye administered, one had a skin rash, while another patient developed an arrhythmia intraoperatively, which were managed accordingly.
After Calot’s dissection, the CBD was visualised in 63 of the 70 patients, with a mean time to visualisation at 26.33 min from the beginning of surgery, ranging between 15 and 50 min [Figure 7]. Of the cases in which CBD was not visualised, 3 (4.2%) patients had acute gangrenous cholecystitis, 1 (1.4%) had empyema GB, 1 (1.4%) had acute calculous cholecystitis and 3 (4.2%) had mucocele GB.
Figure 7.
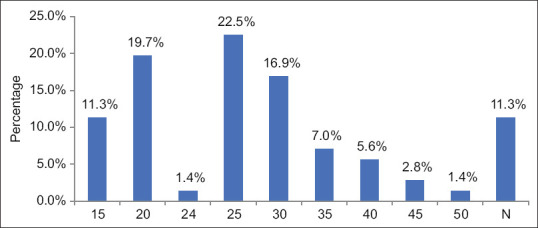
Time to common bile duct visualisation
The CD was visualised in 62 of 71 cases using ICG with a mean time of visualisation of 32.10 min, ranging between 20 and 50 min [Figure 8].
Figure 8.
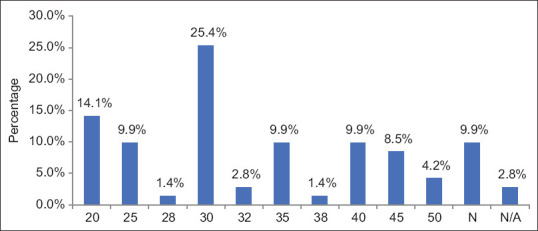
Time to cystic duct visualisation
The hepatic duct was visualised in 20 of the 70 (28.57%) patients, and the hepatic duct-CBD confluence was visualised in 24/70 (34.28%) patients. One patient in the study had a CBD injury, which was identified intraoperatively. The injury was class E2 as per the Strasberg classification. The procedure was converted to open, and CBD was repaired over the stent.
The following are intra-operative pictures of the IOC findings in our cases [Figures 9-14].
Figure 9.
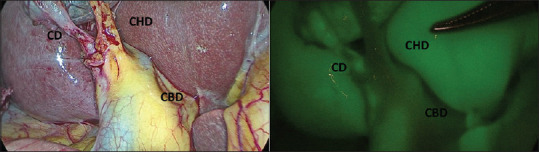
Normal biliary anatomy as visualised after Calot’s dissection on real-time imaging and near-infrared indocyanine green intraoperative cholangiography. CD: Cystic duct, CBD: Common bile duct, CHD: Common hepatic duct
Figure 14.
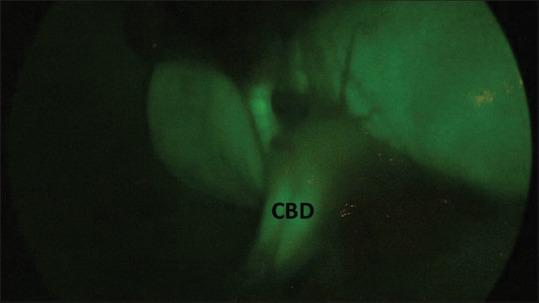
Dilated common bile duct
Figure 10.
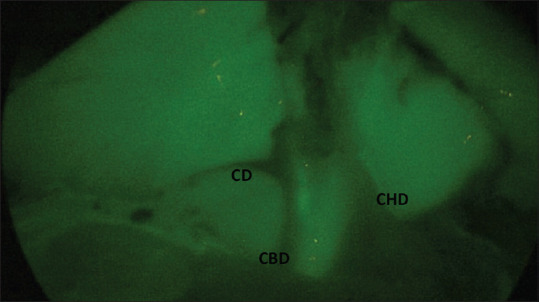
Parallel union of the CD with CBD. Visualization of CD, CHD and CBD. CD: Cystic duct, CBD: Common bile duct, CHD: Common hepatic duct
Figure 11.
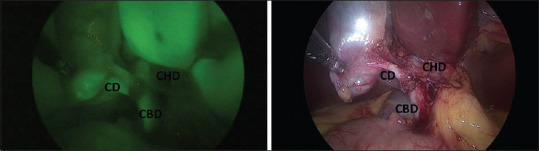
Visualization of the CD, CHD and CBD with the CHD-CBD confluence. CD: Cystic duct, CBD: Common bile duct, CHD: Common hepatic duct
Figure 12.
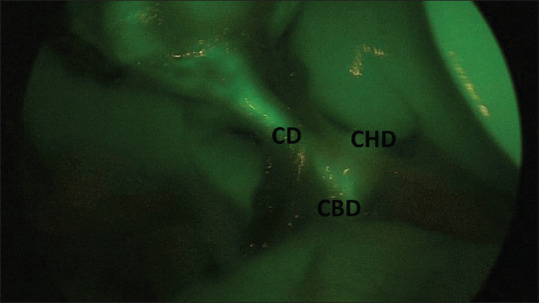
Low medial insertion of CD into CBD. Visualization of CD, CBD and CHD. CD: Cystic duct, CBD: Common bile duct, CHD: Common hepatic duct
Figure 13.
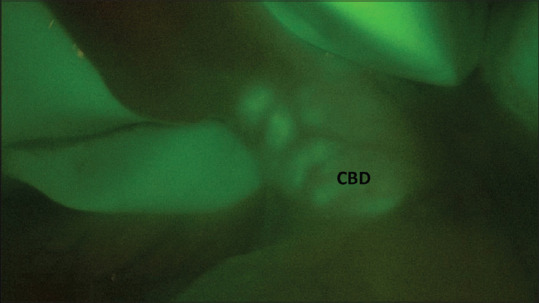
Common bile duct stones as seen on intraoperative cholangiography
DISCUSSION
In the present study, most patients underwent surgery for gallstone disease, in 34 of 71 patients (47.9%); of which seven had concomitant CBD stones 44 (9.9%). Twenty-three patients were operated for chronic cholecystitis (32.4%). Eight patients had acute cholecystitis (11.2%). In the study by Ambe et al., 58 (82.9%) patients underwent surgery for symptomatic gallstone disease, while 12 (17.1%) had acute cholecystitis.[22]
In the study by Pesce et al., 50 patients underwent LC with ICG-IOC, of which 84% of patients underwent the procedure for symptomatic gallstone disease, while 8% of patients underwent the same for chronic cholecystitis.[25]
In the study by Ishizawa et al., 52 patients were evaluated; amongst which six patients could not be studied with ICG IOC in view of iodine allergy in five and one being a known case of CRF.[26]
In a study done on laparoscopic cholecystectomy and its complications during the same duration in DMC and H, Ludhiana, the average duration of the surgery was 94.438 min, ranging between 25 and 225 min. In the study by Hiwatashi et al. on 65 patients, amongst 61 patients wherein CBD was identified using ICG, the mean duration of surgery was 130.62 min, while in four patients, where CBD was not visualised, the mean duration of surgery was 165.75 min.[21]
In our study, all the patients were injected with 5 mg of ICG preoperatively, and the majority (62/70) of the patients in this study were administered the dye about 30 min before surgery (88.6%), while seven patients were administered the dye 15 min before surgery (10%) and one patient was given the dye 1 h before surgery (1.4%). In the study by Zarrinpar et al., ICG was administered intravenously by the study coordinator or the anaesthesiologist at the following doses: 0.02, 0.04, 0.08 and 0.25 mg/kg. The timing of the injection also systematically varied: 10 ± 3 min, 45 ± 15 min and 3 ± 1 h before anticipated visualisation, and the operating surgeon was blinded to the dose and timing of ICG administration. They concluded that a dose as low as 0.02 mg/kg was enough for visualisation of the biliary anatomy given enough time.[23] In the study by Tsutsui et al., 72 patients were randomised into groups that were administered 25 mg of ICG dye immediately before surgery or at 3, 6, 9, 12, 15, 18 and 24 h before surgery. They concluded that the proportion of cases in which two or more evaluators classified the visibility of the gall bladder and bile ducts as Grade A, first increased with the 12-h group, reached a peak in the 15-h group and decreased thereafter, and also based on contrast to background ratios, concluded that ICG is best administered at 15 h before surgery.[27]
In the study by Pesce et al., the CD was identified by FC in 43 (86%) and 45 of 50 patients (90%) before and after Calot’s dissection, respectively. The CHD and CBD were successfully identified in 12 (24%) and 33 (66%) patients before Calot’s dissection, respectively, and in 26 (52%) and 47 (94%) patients after complete Calot’s dissection, respectively[9] In the study by Hiwatashi et al., they visualised CD in 54 of 65 patients undergoing LC, and factors associated with non-visualisation of the CD were higher CRP, higher WBC, higher conversion rates to open and higher rates of acute cholecystitis. Fluorescence imaging systems showed 61 (93.8%) patients in the identified CBD group and 4 (6.2%) patients in the not identified CBD group.[21]
In the study by Patankar et al., the CD was visualised in 86.6% of patients, the common bile and hepatic ducts were visualised in 34.4% of patients and the junction between common bile and hepatic ducts was visualised in 11.1% of patients before completing dissection at Calot’s triangle; whereas after completing dissection, the CD was visualised in 87.7% of patients, the common bile and hepatic ducts were visualised in 94.4% of patients, the junction between common bile and hepatic ducts was visualised in 80% of patients and the right and left hepatic ducts were visualised in 14.4% of patients.[28] In the present study, the CBD was visualised well in 87.3% of cases, the CD in 88.7% of cases and the hepatic duct in 28.57% of cases.
CONCLUSION
Through our study, we conclude that near-infrared imaging-based IOC using ICG dye during laparoscopic cholecystectomy is an easy, useful and inexpensive method of visualising the biliary ductal anatomy and its anatomical variations during the surgery and, thus, helpful to prevent injury to the CBD and can be done in any patient without a previous history of allergy to iodine, without significantly adding to the duration of surgery. The overall visualisation of the biliary anatomy was decreased in patients as the severity of inflammation around the Calot’s triangle increased, like in cases of acute biliary pancreatitis, empyema GB or some cases of acute cholecystitis. Although, the number of such patients was less compared to the patients in which the biliary anatomy was visualised with adequate clarity. The dye can be administered conveniently as an intravenous injection at a dose of 5 mg injected 15–30 min before the induction of anaesthesia, without any significant risks associated. We also observed that in cases with cirrhosis, alcoholic or NASH, the biliary anatomy was not clearly visualised, likely due to delayed excretion of ICG from the liver.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Polychronidis A, Laftsidis P, Bounovas A, Simopoulos C. Twenty years of laparoscopic cholecystectomy: Philippe Mouret –March 17, 1987. JSLS. 2008;12:109–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Udwadia TE. Laparoscopy in India –A personal perspective. J Minim Access Surg. 2005;1:51–2. doi: 10.4103/0972-9941.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overby DW, Apelgren KN, Richardson W, Fanelli R Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc. 2010;24:2368–86. doi: 10.1007/s00464-010-1268-7. [DOI] [PubMed] [Google Scholar]
- 4.Kuldip S, Ashish O. Difficult laparoscopic cholecystectomy: A large series from North India. Indian J Surg. 2006;68:205–8. [Google Scholar]
- 5.Reynolds W., Jr The first laparoscopic cholecystectomy. JSLS. 2001;5:89–94. [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra PK, Saluja SS, Nayeem M, Sharma BC, Patil N. Bile duct injury-from injury to repair: An analysis of management and outcome. Indian J Surg. 2015;77:536–42. doi: 10.1007/s12262-013-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly CM, Doyle MB. Technique of cholecystectomy: Open and minimally invasive. In: Jarnagin WR, Belghiti J, Blumgart LH, editors. Blumgart's Surgery of the Liver, Biliary Tract, and Pancreas. 6th ed. Philadelphia: Elsevier Saunders; 2012. pp. 569–84. [Google Scholar]
- 8.Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639–44. doi: 10.1001/jama.289.13.1639. [DOI] [PubMed] [Google Scholar]
- 9.Pesce A, Portale TR, Minutolo V, Scilletta R, Li Destri G, Puleo S. Bile duct injury during laparoscopic cholecystectomy without intraoperative cholangiography: A retrospective study on 1,100 selected patients. Dig Surg. 2012;29:310–4. doi: 10.1159/000341660. [DOI] [PubMed] [Google Scholar]
- 10.Blumgart L, Lawrence H, DeMatteo R. Surgical and radiologic anatomy of the liver, biliary tract, and pancreas. In: Jarnagin WR, Belghiti J, Blumgart LH, editors. Blumgart's Surgery of the Liver, Biliary Tract, and Pancreas. 6th ed. Philadelphia: Elsevier Saunders; 2012. p. 48. [Google Scholar]
- 11.Strasberg SM. Error traps and vasculo-biliary injury in laparoscopic and open cholecystectomy. J Hepatobiliary Pancreat Surg. 2008;15:284–92. doi: 10.1007/s00534-007-1267-9. [DOI] [PubMed] [Google Scholar]
- 12.Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 2010;211:132–8. doi: 10.1016/j.jamcollsurg.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Strasberg SM, Eagon CJ, Drebin JA. The “hidden cystic duct” syndrome and the infundibular technique of laparoscopic cholecystectomy – The danger of the false infundibulum. J Am Coll Surg. 2000;191:661–7. doi: 10.1016/s1072-7515(00)00717-1. [DOI] [PubMed] [Google Scholar]
- 14.Strasberg SM, Gouma DJ. 'Extreme'vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford) 2012;14:1–8. doi: 10.1111/j.1477-2574.2011.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strasberg SM, Helton WS. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford) 2011;13:1–14. doi: 10.1111/j.1477-2574.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peitzman AB, Watson GA, Marsh JW. Acute cholecystitis: When to operate and how to do it safely. J Trauma Acute Care Surg. 2015;78:1–12. doi: 10.1097/TA.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 17.Soper NJ, Scott-Conner CE, editors. The SAGES Manual: Volume 1 Basic Laparoscopy and Endoscopy. 3rd ed. Springer Science and Business Media; 2012. p. 273. [Google Scholar]
- 18.Vlek SL, van Dam DA, Rubinstein SM, de Lange-de Klerk ES, Schoonmade LJ, Tuynman JB, et al. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: Results of a systematic review. Surg Endosc. 2017;31:2731–42. doi: 10.1007/s00464-016-5318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizawa T, Bandai Y, Kokudo N. Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: An initial experience. Arch Surg. 2009;144:381–2. doi: 10.1001/archsurg.2009.9. [DOI] [PubMed] [Google Scholar]
- 20.Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, et al. Areview of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012. 2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiwatashi K, Okumura H, Setoyama T, Ando K, Ogura Y, Aridome K, et al. Evaluation of laparoscopic cholecystectomy using Indocyanine green cholangiography including cholecystitis: A retrospective study. Medicine (Baltimore) 2018;97:e11654. doi: 10.1097/MD.0000000000011654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambe PC, Plambeck J, Fernandez-Jesberg V, Zarras K. The role of indocyanine green fluoroscopy for intraoperative bile duct visualization during laparoscopic cholecystectomy: An observational cohort study in 70 patients. Patient Saf Surg. 2019;13:2. doi: 10.1186/s13037-019-0182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarrinpar A, Dutson EP, Mobley C, Busuttil RW, Lewis CE, Tillou A, et al. Intraoperative laparoscopic near-infrared fluorescence cholangiography to facilitate anatomical identification: When to give indocyanine green and how much. Surg Innov. 2016;23:360–5. doi: 10.1177/1553350616637671. [DOI] [PubMed] [Google Scholar]
- 24.Serban D, Badiu DC, Davitoiu D, Tanasescu C, Tudosie MS, Sabau AD, et al. Systematic review of the role of indocyanine green near-infrared fluorescence in safe laparoscopic cholecystectomy (Review) Exp Ther Med. 2022;23:187. doi: 10.3892/etm.2021.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pesce A, Latteri S, Barchitta M, Portale TR, Di Stefano B, Agodi A, et al. Near-infrared fluorescent cholangiography –Real-time visualization of the biliary tree during elective laparoscopic cholecystectomy. HPB (Oxford) 2018;20:538–45. doi: 10.1016/j.hpb.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369–77. doi: 10.1002/bjs.7125. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsui N, Yoshida M, Nakagawa H, Ito E, Iwase R, Suzuki N, et al. Optimal timing of preoperative indocyanine green administration for fluorescent cholangiography during laparoscopic cholecystectomy using the PINPOINT®endoscopic fluorescence imaging system. Asian J Endosc Surg. 2018;11:199–205. doi: 10.1111/ases.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patankar R, Mishra RK, Bindal V, Kothari CP, Rahate P, Patnaik S, et al. Efficacy of near-infrared fluorescence cholangiography using indocyanine green in laparoscopic cholecystectomy: A retrospective study. J Minim Access Surg. 2023;19:57–61. doi: 10.4103/jmas.jmas_369_21. [DOI] [PMC free article] [PubMed] [Google Scholar]