Abstract
Online poker gambling (OPG) involves various executive control processes and emotion regulation. In this context, we hypothesized that online poker players, accustomed to handling virtual cards, would show high performance on computerized decision‐making tasks such as the Iowa Gambling Task (IGT). Using press advertisements, we recruited a non‐gambler group (NG; n = 20) and an OPG group (n = 22). All participants performed the IGT while their cerebral activity was recorded by electroencephalography. Compared with the OPG group, the NG group showed significantly better progression in the IGT in the last trials. Recording of brain activity revealed the appearance of a temporal map between 150 and 175 ms specific to the gain condition in both groups. A second map was observed at 215–295 ms specifically in the NG group, and the generators were identified in the occipital regions. This activity is indicative of a high level of visual awareness; thus, it reflects additional processing of visual information, which can be assumed to be induced by the lower exposure of the NGs to online card games. We hypothesize that the absence of this activity in the OPG group might be due to their online habituation to virtual environments.
Keywords: anhedonia, decision‐making, Iowa Gambling Task, online poker gambling
Online poker playing involves various executive control and emotion regulation processes that could confer advantageous decision‐making over non‐gamblers. Here, we found that this was non‐gamblers that showed a better progression at the Iowa Gambling Task than online poker gamblers. EEG recording showed a reduced activation of some cerebral areas in late outcome processing for online poker gamblers. Compared to non‐gamblers, online poker gamblers showed a reduced activation of occipital regions, indicating a lower level of visual awareness.
1. INTRODUCTION
Poker represents a considerable and ever‐growing economic sector. In 2010, the total number of regular poker gamblers was estimated to be 44.5 million, largely due to the advent of online poker. 1 Almost 20 years after the poker boom, we have observed that its popularity remained stable, particularly in France. 2 Poker, whether offline or online, is categorized as strategic gambling. 3 Strategic dimension allows gamblers to use their skills and knowledge in an attempt to influence the game or predict outcomes. 4 Thus, poker activity involves different executive control processes, and its regular practice gives poker gamblers greater efficiency in exerting willpower during a gambling session.
Poker compels gamblers to choose between short‐ and long‐term consequences of an action. 5 In addition, the ability to adopt better decision‐making is affected by the individual's emotional self‐regulation. 6 Indeed, it is well known among poker gamblers that intense feelings of frustration and strong negative emotions following repeated losses lead to a decrease in strategic or calculated play and an increase in aggressive and reckless betting. This phenomenon is particularly well known among poker gamblers as ‘tilt’. 7 Decision‐making ability under conditions of uncertainty is usually evaluated using the Iowa Gambling Task (IGT). 8 In this task, participants are given a choice of four decks of cards and, via successive choices, learn to choose the advantageous decks. 8 Altered performance in this task in individuals with gambling disorder (GD) is well known. 9 Indeed, poker, like all other gambles, exposes gamblers to the risk of addiction and GD. 10 The IGT is often used in this population to study how their disorder may alter their decision‐making skills. There is a vast literature on the identification of the neural correlates of these decisional deficits at IGT in populations with addictions, including GD. Studies using functional Magnetic Resonance Imagery (fMRI) showed that impaired performance in the IGT is linked to a diminished activity of the ventromedial prefrontal cortex (vmPFC) 11 or with increased neural activity associated with reward processing. 5 Because the decision‐making process involves many brain structures in a short period of time, the recording of event‐related potentials (ERPs) using electroencephalography (EEG) allows an accurate assessment of the time course of neural activation in response to reward and punishment over the fMRI. 12 Most studies have focused on two ERPs, the feedback‐related negativity (FRN) and the P300. The FRN is a negative early ERP usually measured ~200–300 ms after feedback, 13 which reflects the early appraisal of binary reward/loss. 14 A higher post‐reward FRN was identified in GDs who failed IGT, corroborating the thesis of increased reward processing. 15 The P300 is a positive ERP characterized by a large amplitude that is measured ~300–400 ms after the presentation of stimuli in any sensory modality. 16 The P300 is associated with performance monitoring and behavioural adaptation 17 and is influenced by attention and working memory updating. 18 Hence, it plays an essential role in decision‐making. In line with what has been observed with fMRI, 5 many ERP studies showed that hypersensitivity to a reward undermines a person's ability to develop an optimal strategy. 15 , 19 More precisely, in a previous study, we found that participants with a blunted P300 showed better performance in the IGT, 19 while another group showed a positive correlation among all ERPs in IGT performance, except for the P300. 20 In addition, the P300 is related to the ability to discriminate relevant stimuli. 21 Thus, a blunted P300 in the IGT translates to less attention being paid to the immediate task reward because it is judged to be irrelevant due to a greater ability to filter distractors, in favour of an efficient long‐term strategy. 19 In view of these data, it seems to us that before proceeding with a population suffering from GD, it would be appropriate to identify whether poker gambling alone has an impact on decision‐making ability at the IGT and on neural activity.
The main objective of the present study was to evaluate the neural bases of decision‐making in online poker gambling (OPG) without GD compared to non‐gamblers (NGs). We know from previous studies that there are different behavioural performances at the IGT between gamblers with GD and NGs. Here, we want to explore the neural mechanisms related to this difference. Usually, studies of GD compare neural activity to a healthy, often NG group. However, we believe that a certain modification of neuronal activity could be induced by exposure to the environment and by regular practice of the game even without GD. Herewith, it seems necessary to us, before continuing research on OPG with GD, to clarify this aspect in order to not wrongly attribute differences to the disorder of the game when they could be solely due to online gambling exposure. The IGT and, more particularly, its computerized version 22 are particularly interesting because they are reminiscent of online poker, in terms of game structure with the management of uncertainty and because of their interface with the virtual card selection. 5 We hypothesized that the specific design of the IGT regarding its gambling‐like structure should confer an advantage to gamblers. Indeed, through a ripple effect, OPGs without GD can be expected to perform better than NGs. We chose to record neuronal activity during IGT execution with an EEG to obtain a dynamic view of the links between the reward and executive pathways necessary for successful task completion. 5 We tested the following hypotheses: (1) that OPGs would perform better in the IGT than NGs due to their habituation to the computerized environment and manipulation of virtual cards; (2) that OPGs would be able to regulate their emotional drive to a specific neuronal activity, with a distinct feedback processing from NGs; and (3) that source localization would identify a higher activity of cerebral structures involved in the ‘reflective’ system in OPGs.
2. METHODS
2.1. Participants and recruitment
Forty‐two male volunteers (mean age, 25.8 ± 5.8 years) were recruited through Besançon (France) local press to participate in the current study. The press release specified that we were looking for OPG and NG subjects, without any neurological or psychiatrist disorder, current or past. All participants were right‐handed (assessed with the Handedness Questionnaire of Oldfield 23 ), older than 18 years old and had normal or corrected‐to‐normal vision.
Participants were divided into two groups based on their gambling habits: an NG group (20 participants; mean age, 23.4 ± 4.2 years) and an OPG without GD group (22 participants; mean age, 28 ± 6.2 years). The NG group reported that they never did poker betting nor any other gambling activities (except some gambling experiences in the past), while the OPG group reported an active online poker activity (predominantly or exclusively online).
Each participant was interviewed to verify inclusion and exclusion criteria. None of them had any medical history of psychiatric disorders, substance use (except for tobacco and alcohol), neurological diseases, traumatic brain injury or stroke, and they were not taking any medication at the time of the study.
Prior to participating in the study, the participants received information regarding the aims and procedures of the experiment and gave their written informed consent to participate. All participants received €85 in compensation at the end of the experiment. All methods were performed in accordance with the relevant guidelines and regulations, and all methods were approved by the Ethics Committee of Besançon University Hospital (authorized by the General Health Administration [ANSM 2016‐A00870‐51]) and carried out in accordance with the protocol and with the principles enunciated in the Declaration of Helsinki.
2.2. Clinical and psychometric measures
Depressive symptoms were assessed using the Montgomery and Åsberg Depression Rating Scale (MADRS). 24 All participants with a score above 7 were excluded, and the existence of a GD was assessed in the OPG group with the administration of the DSM‐5 ‘GD’. Each participant performed several self‐reported questionnaires to ensure the comparability of the groups in terms of habits. To determine their gambling habits and risk profiles, especially for the OPG group, participants performed the Canadian Problem Gambler Index (CPGI). 25 As tobacco and alcohol consumption were not excluded, their level of consumption was verified by means of a questionnaire. The Alcohol Use Disorder Identification Test (AUDIT) identifies the alcohol consumption level and the existence of an alcohol use disorder. 26 The Fagerström Test is a screening instrument for physical nicotine dependence, 27 and the DSM‐IV‐TR Substance‐Dependence Adapted Scale (DAS) screens for video gaming disorder. 28 In addition, each participant performed self‐reported questionnaires to identify personality traits that can influence IGT performance: the Barratt Impulsiveness Scale (BIS‐10), which used to assess impulsivity and its subcomponents (cognitive, motor and non‐planning) 29 ; the Chapman Anhedonia Scale (CAS), which measures physical (CAS‐P) and social (CAS‐S) anhedonia 30 ; the Snaith‐Hamilton Pleasure Scale (SHAPS), 30 which assesses the hedonic response; and the ‘Behavioural Inhibition System’ and ‘Behavioural Approach System’ (BIS/BAS), which evaluates appetitive and aversive motivation. In particular, the BIS/BAS comprises three subcategories of the BAS: Drive (BAS‐D), Fun‐Seeking (BAS‐FS) and Reward Responsiveness (BAS‐RR). 31
2.3. Experimental task: the IGT
The virtual IGT used in this study is an electronic version of the IGT adapted for the study of ERPs and the analysis of brain activity sources. This adaptation have been previously validated in a previous study (see Giustiniani et al.). 12 All the changes made to the original IGT are designed to increase the number of trials required for ERP analysis and to limit artefacts caused by movements such as blinks. The aim of the task is to win as much money as possible by making successive selections among four decks.
Deck compositions, values and schedules of reward/punishment were predetermined identically to the original form of the IGT. While the back of each deck looked identical, they differed in composition. Decks A and B were the disadvantageous decks: they provided immediate rewards but, in the long run, yielded major economic losses. Decks C and D were the advantageous decks: they provided frequent small wins and smaller long‐term penalties, which resulted in a long‐term gain. The participants were not informed of the number of trials that they would be playing. To adapt the IGT for our French participants, the money used in the task was converted from US dollars to euros. At the beginning of the IGT, participants received a virtual loan of €2000. 8 , 12
Some changes had to be made to adapt the original IGT task to work with the EEG. Firstly, to extend the electrophysiological recording from the hunch phase, the number of trials was increased from 100 to 200, and the participants received no hints about the presence of advantageous or disadvantageous decks. Each deck contained 200 cards. Secondly, the design of the trial process was modified to minimize ocular artefacts. For each trial, participants were required to focus on a cross or a square while making their selection by pressing a key. After the selection, feedback on the deck chosen and the total amount of credit were displayed, followed by the amount of money involved in that trial. Then, a fixation point appeared in order to focus the eyes, followed by a green square if money was won or a red square if money was lost. Participants received instructions to focus on the square and not to blink until they had made their next selection. Before beginning the task, the participants were trained with a five‐trial short version of the game. 12
2.4. EEG recording
EEG signals were recorded using a 256‐channel Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR) during the IGT. Continuous recordings were performed at a sampling rate of 1000 Hz. All channels were referenced to the vertex (Cz) and collected with a high‐impedance amplifier (Net Amp 400, Electrical Geodesics) using Net Station 4.5 software (Electrical Geodesics). Participants were instructed to limit body movements, eye blinks and muscular contractions during task selection and reward feedback. 12
2.5. Data analysis
2.5.1. Behavioural data analysis
The 200 trials of the IGT were divided into 10 blocks of 20 trials. The individual net score was calculated by subtracting the number of disadvantageous decks from the number of advantageous decks obtained for each block.
2.5.2. EEG data analysis
ERP data analysis was performed using Cartool Software 3.551 (https://sites.google.com/site/cartoolcommunity/home). Raw EEG data were re‐referenced offline to a common average reference. Data were bandpass‐filtered between 1 and 30 Hz (Butterworth), and a notch filter fixed to 50 Hz was applied to remove environmental artefacts.
The main interval of interest in the IGT was after the reward screen. Epochs of 700 ms (100 ms prior to reward feedback to 600 ms after reward feedback) were extracted from the raw data and analysed, with a baseline correction applied from before to the onset of the feedback (100–0 ms). A semi‐automatic artefact rejection method was used, with a fixed criterion of ±100 μV. The remaining epochs were visually inspected for the manual removal of any blinks, eye movements, or other sources of transient noise from the analysis. Electrodes with an aberrant signal (e.g., excessive noise due to malfunctioning or a bad signal during data collection) were interpolated using a three‐dimensional spline algorithm.
Microstate analysis was also performed to determine whether the four conditions (win or loss in the IGT for both the OPG and NG groups) differed in terms of global electric fields. 32 Spatiotemporal segmentation was performed on the group‐averaged ERPs from the displayed result to 600 ms after for each condition. Changes in electric fields occur when the configuration of the underlying generator has changed and suggest the activation of different brain networks. A k‐means cluster analysis of topographic dissimilarities was applied to determine which topographic template (map) best explained the participants' ERP responses to each experimental condition.
Following the microstate procedure, two types of analyses were then performed:
A conventional analysis of ERPs in the topographic maps resulting from the group‐averaged data uncovering the latency of the FRN and P300. Based on previous literature on feedback processing, six central electrodes (Fpz, Fz, FCz, Cz, CPz, Pz) were chosen for the current analysis. 14 , 33 , 34
A fitting procedure comparing the group‐averaged data with the scalp topography of ERPs at the individual level. For each condition of each participant, we were then able to extract various parameters, such as the number of time frames (TFs), the global field power (GFP) and the global explained variance (GEV) for each map. Within our frequency range, the number of TFs represents the current length duration time of the map in milliseconds, while the GEV is the percentage of total variance explained by a given microstate that ‘reflects the relative time coverage of its underlying neural generators compared to others’. 35
Finally, a source localization procedure was also performed by using a distributed linear inverse solution based on a local auto‐regressive average (Loreta) model for the maps resulting from the segmentation analysis and showing differences between the OPG and NG groups. These source estimations were computed from the averages of ERPs at all 256 electrodes into a solution space represented by a three‐dimensional grid composed of 5018 nodes. These 5018 nodes were selected from a grid equally distributed over the grey matter of the average brain provided by the Montreal Neurological Institute.
2.6. Statistical analysis
Statistical analyses were performed with Statistica (StatSoft Europe, Hamburg, Germany) and R 3.4.1 (R Development Core Team) software. For the psychometric statistics, Bartlett's test was applied and a t‐test was used to test potential differences in psychometric scales between the two groups.
For the behavioural statistics, we assessed whether there were group differences in the net score by using a general linear model (GLM) analysis, including Group (OPG group versus NG group) × Block (1 to 10). A least significant difference post hoc correction was applied when necessary. To assess whether there was a relationship between decision‐making in the IGT and gambling habits, we performed a Pearson two‐tailed correlation between the net score during the conceptual phase and the CPGI score in the OPG group.
In the conventional analysis of ERPs, we assessed whether there were group differences in the mean signal amplitude in the map relative to the FRN and P300 by using a GLM analysis that included Group (OPG group versus NG group) × Result (win versus loss) × Electrode (FPz, Fz, Cz, CPz, Pz and Oz). In the fitting procedure, the topographic maps specific to either groups (OPG group versus NG group) or results were analysed with a second GLM including Group × Results. The dependent variables were the mean number of TFs, the mean GFP, the maximum GFP and the GEV. For both GLMs, a Bonferroni post hoc correction was applied when necessary.
3. RESULTS
3.1. Sociodemographic and psychometric data
No significant psychometric variations were observed between the two groups in the BIS‐10, BFI‐Fr, CAS‐S and SHAPS. However, compared with the NG group, the OPG group showed a higher level of physical anhedonia in the CAS‐P (t[42] = −2.3175, p = 0.0262) and a higher score in the BAS‐Drive in the BIS/BAS (t[42] = −2.3077, p = 0.02642). Sociodemographic data and psychometric are presented in Table 1.
TABLE 1.
Sociodemographic and psychometric characteristics of the study sample.
| Non‐gamblers | Online poker gamblers | p‐Value | |
|---|---|---|---|
| 20 (47.6%) | 22 (52.4%) | ||
| Mean (SD) | Mean (SD) | ||
| DAS (video gaming) | 0 | 1.18 (1.65) | 0.002987 |
| MADRS | 0.55 (1.67) | 0.409 (0.666) | 0.7273 |
| AUDIT | 5.1 (2.83) | 7 (3.85) | 0.078 |
| Fagerström | 0.15 (0.489) | 0.955 (1.43) | 0.022 |
| CAS, physical | 14 (4.27) | 17.9 (6.52) | 0.0262 |
| CAS, social | 7.15 (4.39) | 7.23 (4.44) | 0.9551 |
| BIS‐10 | 49.6 (12.3) | 46.6 (13.7) | 0.4717 |
|
16.2 (6.39) | 13.9 (6.08) | 0.2433 |
|
17.0 (5.51) | 15.5 (5.21) | 0.3699 |
|
16.4 (3.96) | 17.2 (6.91) | 0.6131 |
| BAS | 38.4 (5.90) | 39.0 (3.73) | 0.7213 |
|
8.8 (1.67) | 9.95 (1.56) | 0.02642 |
|
11.6 (1.73) | 12.0 (1.53) | 0.4288 |
|
16.9 (1.62) | 17.0 (1.76) | 0.7816 |
| BIS | 21.0 (2.96) | 20.3 (2.90) | 0.4244 |
| SHAPS | 1 (0.918) | 1.41 (1.37) | 0.2588 |
Note: Differences were determined using a t‐test.
Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; BAS, Behavioural Approach System; BIS, Behavioural Inhibition System; BIS‐10 CI, cognitive impulsiveness; BIS‐10 MI, motor impulsiveness; BIS‐10 NPI, non‐planning impulsiveness; BIS‐10, Barratt Impulsiveness Scale; CAS, Chapman Anhedonia Scale; I DAS, Dependence Adapted Scale; FS, Fun‐Seeking; MADRS, Montgomery and Åsberg Depression Rating Scale; RR, Reward Responsiveness; SHAPS, Snaith‐Hamilton Pleasure Scale.
We observed in the OPG group, a score range of 0 to 10 for the CPGI (median, 2), five volunteers had excessive gambling activity as defined by the CPGI (score ≥7). The administration of the DSM‐5 section ‘GD’ in the OPG group confirmed that they did not suffer from a GD (mean, 1.64 ± 1.79; median, 1).
3.2. Behavioural results in the IGT
A significant interaction was observed between Group and Block (F(9,360) = 2.02, p < 0.05, ƞ2 = 0.05). For both groups, a learning effect was observed during the task, as reflected by significant differences in the net score between the first and last blocks of the task (Figure 1). In the NG group, this difference was observed between the first three blocks of the tasks (i.e., trials 1–60) and the last five blocks (i.e., trials 101–200; p < 0.01 for all comparisons). In the OPG group, the difference was observed between the first three blocks and the last three blocks (i.e., trials 140–200; p < 0.05 for all comparisons).
FIGURE 1.
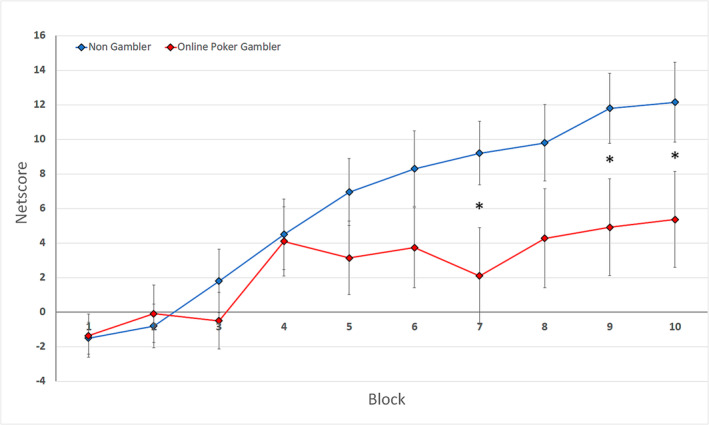
Behavioural performance in the Iowa Gambling Task. Evolution of the net score in each block for the non‐gambler (NG) group (in blue) and the online poker gambler (OPG) group (in red)
We also observed an increased performance in the IGT of the NG group compared to the OPG group during the last blocks of the task: p < 0.05 for trials 121–140, 161–180 and 181–200; p = 0.06 for trials 140–160. These trials were considered part of the conceptual phase. Interestingly, a negative relationship was found between the mean score in trials 120–200 in the IGT and the ICJE score in the OPG group (r = 0.45, p < 0.05).
3.3. EEG results
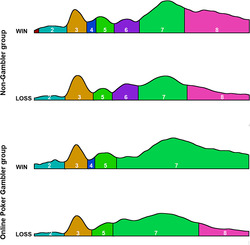
The result of the segmentation is shown in Figure 2. One topographic map was specific to the win condition and was observed between 150 and 175 ms after the outcome (time window 4 [TW4]). The latency was similar to the FRN ERP observed during conventional ERP analyses. 34 One other topographic map was specific to the NG group and was observed between 215 and 295 ms after the outcome (time window 6 [TW6]). The latency was partly similar to the beginning of the P300 in similar studies on ERPs. 18
FIGURE 2.
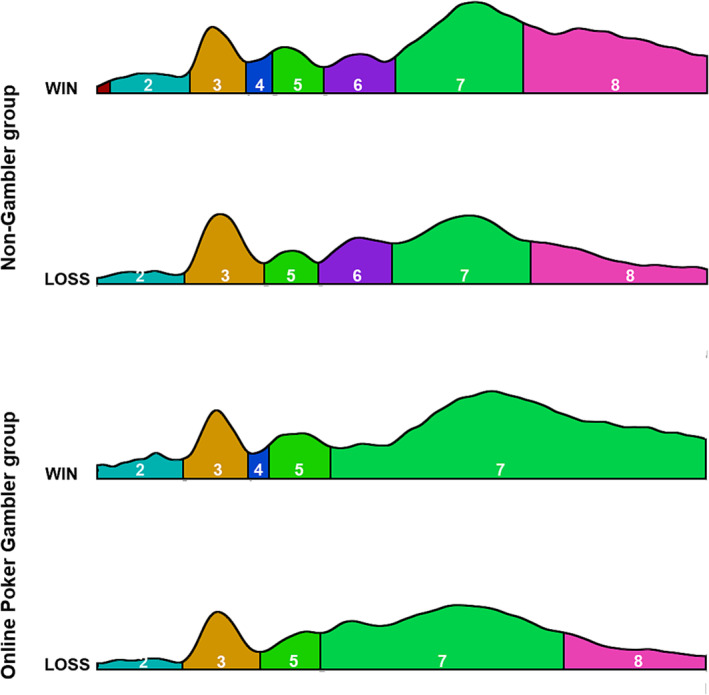
Results of segmentation after a win and a loss in the non‐gambler and online poker gambler groups
For TW4, the number of TFs, the GEV, the maximum GFP (maxGFP) and the mean GFP (meanGFP) were increased in case of a win compared to a loss (TF: F(1,40) = 20.1, p < 0.001, ƞ2 = 0.33; GEV: F(1,40) = 14.5, p < 0.001, ƞ2 = 0.27; max GFP: F(1,40) = 12.3, p < 0.01, ƞ2 = 0.24; and mean GFP: F(1,40) = 11.4, p < 0.01, ƞ2 = 0.22).
A conventional analysis in the same time period confirmed this difference, with an Outcome × Electrode effect (F(5,200) = 16.7, p < 0.00001, ƞ2 = 0.29). A more negative response was observed in parieto‐occipital electrodes after a loss (p < 0.001 for Pz and Oz).
Source localization for TW4 revealed that the topography originated from the temporal inferior and parietal regions (Figure 3).
FIGURE 3.
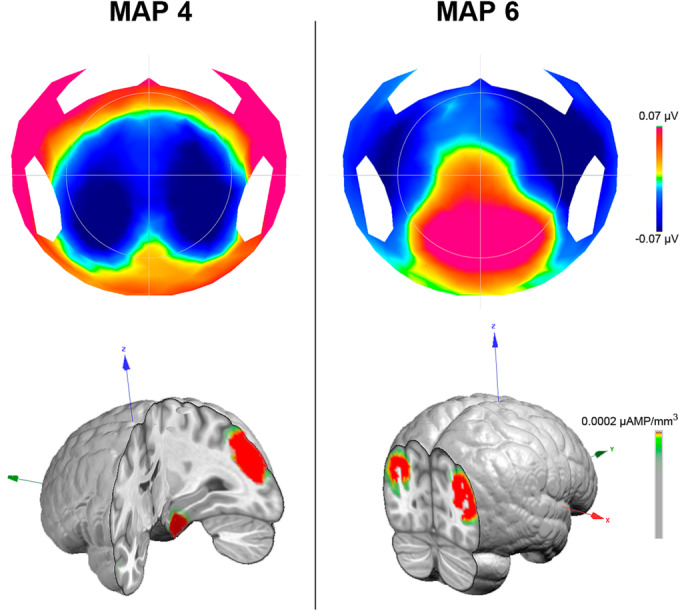
Topography and source localization of time window 4 (map 4) and time window 6 (map 6). Left: topography (top) and source localization (bottom) of map 4. Right: topography (top) and source localization (bottom) of map 6
For TW6, the number of TFs (F(1,40) = 7.1, p < 0.05, ƞ2 = 0.15) and the GEV (F(1,40) = 5.6, p < 0.05, ƞ2 = 0.12) were reduced for the OPG group compared to the NG group. Conventional analysis in the same time period revealed an interaction between Group and Electrode (F(5,200) = 2.49, p < 0.05, ƞ2 = 0.06). Post hoc Bonferroni tests did not reveal any significant difference, but LSD tests confirmed this difference in the FPz. Source localization for TW6 revealed that this map mainly originated from the occipital regions (Figure 3).
4. DISCUSSION
The present study aimed to determine if the practice of online poker impacted gambler's decision‐making ability in the virtual environment, as well as their neural activity. We hypothesized that, due to their previous training with virtual card games, OPGs would show a greater ability to make advantageous choices than NGs. Contrary to our hypothesis, the OPG group did not show better performance but showed an even worse performance than the NG group from the seventh block of the IGT. ERP analysis identified a supplementary process in the NG group compared with the OPG group. We observed in the segmentation analysis that the NG group exhibited an added process in late processing information that could be related to the absence of environmental habituation in this group. Indeed, the fact that the NG group was not familiar with virtual card games could explain why they had supplementary processing of information because this brain region handles environmental information. However, we observed that, in our OPG group, some gamblers had a risk of developing a GD, as determined by the CPGI, which could explain why they showed worse performance.
4.1. Behavioural interpretation: the IGT
Behavioural analysis revealed that the NG group learned and adopted the favourable strategy faster than the OPG group. Indeed, the difference in the net score for the NG group appeared between the first block and the last five blocks, whereas the difference appeared later for the OPG group (between the first three blocks and the last three). These results contradict our hypothesis, which stipulated that better decision‐making performance in the virtual environment of the OPG group would be witnessed. However, psychometric data showed that our OPG group was not exactly comparable to the NG group. Indeed, the OPG group showed a higher level of addictive risk. In addition, while our OPG group was not recruited from the addiction service but through communication and advertising in the local Besançon‐based press, we observed that some participants had a CPGI score that indicated risky gambling behaviour. The OPG group had an average score in the CPGI of 3.14 (3.12) and a median of 2, with a score higher than 3 suggesting at‐risk gambling behaviour and higher than 8 identifying excessive gambling. 36 Thus, the range of participants with a maximum score of 10 indicates that some subjects should have at least one risky gambling behaviour. The heterogeneity in our OPG group in terms of addictive risk could influence performance on the IGT, which is known to be lower for people with GDs. 37 , 38 , 39 Furthermore, in the OPG group, we observed a negative correlation between IGT performance on the last blocks (conceptual phase) and CPGI scores. From this observation, we can hypothesize that our OPG group has lower IGT performance because it lacks homogeneity.
4.2. Segmentation analysis and source localization
Independently of the IGT performance, both of our groups showed early outcome processing, with an ERP production by the brain for the win condition produced around 150–175 ms after the receipt of the reward. This finding was confirmed by microstate analysis in which the number of TFs, GEV and both the maximum and mean of the GFP were significantly higher for a loss than for a win. The mean GEV, which reflects the stability of the map, is in accordance with conventional ERP results showing brain responses in this time period that indicate the early processing of outcomes on the basis of a binary classification of ‘good’ or ‘bad’. 34 Source localization in this time window revealed that the topography originated from the temporal inferior and parietal regions. This is different from the FRN, a response associated with the prediction error signal triggered by phasic dopaminergic signals that is conveyed to the anterior cingulate cortex to adjust behaviour. 13 , 40 In our situation, the map originating between 150 and 175 ms was generated by the temporal lobe, previously identified as playing a leading role in the learning of visual material, which, given the visual nature of our task, appears coherent. 40 In addition, parietal activity is associated with working memory 41 and attention ability. 42 This could translate into a higher mobilization of attention processing and working memory induced by the instruction to not react immediately but after a few seconds.
One other topographic map was specific to the NG group and was observed between 215 and 295 ms after the outcome. This time window indicates additional neuronal activity in this group compared with the OPG group. In addition, source localization revealed that the occipital regions generated this activity. This TF typically corresponds to the beginning period of the P300, 18 an ERP implicated in result processing. 13 There is considerable literature concerning the P300, and it can be generated in various situations, such as for attention, working memory and conscious awareness. 13 , 18 In particular, it has been suggested that the P300 could correlate with conscious perception 43 and, along these lines, the occipital origin reflects visual awareness. 44 This cognitive sense particularly attracts our attention in the context of a single visual virtual task to which one group was not accustomed. Indeed, our NG group was naturally not familiar with virtual card games, which would naturally mobilize visual attention and awareness. We hypothesize that the absence of this time window in the OPG group reflects a lower level of visual awareness caused by their habituation to the virtual environment.
Brain activity measurements allow us to understand the impact of online poker activity on decision‐making behaviour. To reach firm conclusions, we need to continue the analyses by deriving two groups of players according to their CPGI score: a low‐risk group and a high‐risk group. Indeed, we have seen that our OPG group was not homogeneous in terms of GD risk (with some participants having a CPGI score >3). In addition, it will be necessary to clarify whether the additional time window observed in the NG group is due to a reduced virtual exposure to poker or more globally to virtual environments. Indeed, the significantly higher DAS score suggests that our OPG group was more exposed to the virtual environment, which is indicative of video game habits. At this point, it will be interesting to continue this research by comparing the OPGs with offline poker gamblers.
4.3. Limits
This study has several limitations, some of which concern the significantly older OPG group, with a higher proportion of lifestyle habits that could lead to a use disorder, such as video games, alcohol consumption or smoking, as suggested by the psychometric test (AUDIT, Fagerström and DAS). These data confirm that adopting behaviours at risk of addiction constitutes in itself a risk of manifesting another addiction. In the future, it would be interesting to continue the investigations by considering the time spent on the screen in general, and more specifically, on videogames to confirm that the differences observed are not due to this exposure. In addition, psychometric test revealed that groups showed some differences considering the hedonic perception. In our study, using the CAS‐P, we found higher physical anhedonia in the OPG group than in the NG group. In addition, we observed a higher score in the BAS‐D (Drive) in our OPG group. At this stage of our reflection, we can assume that the hedonic deficit in daily life associated with a compensatory higher level of motivation (BAS‐D) could be joint elements motivating gambling activity. 45 , 46 Moreover, these two joint elements can induce altered decision‐making.
4.4. Conclusion
NGs, who performed better in the IGT, showed additional neural activity indicating greater awareness of visual perception. We hypothesize that the absence of this activity in the OPG group might be due to their online habituation, without being able to state that this neural activity was not present before exposure. To definitively clarify the role of online support on neural activity, future studies should compare OPGs to offline poker gamblers and to video gamers. Future research should clarify the impact of screens on decision‐making before continuing on populations with screen‐mediated addiction.
ACKNOWLEDGEMENTS
The authors thank all volunteers for participating in the study. They also thank Nathan Galmès for his contribution to the data analysis during his internship.
Giustiniani J, Nicolier M, Diwoux A, et al. Impact of online poker gambling on behavioural and neurophysiological responses to a virtual gambling task. Addiction Biology. 2024;29 (2): e13373. doi: 10.1111/adb.13373.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. MacKay TL, Bard N, Bowling M, Hodgins DC. Do pokers players know how good they are? Accuracy of poker skill estimation in online and offline players. Comput Hum Behav. 2014;31:419‐424. doi: 10.1016/j.chb.2013.11.006 [DOI] [Google Scholar]
- 2. Eroukmanoff V. Tableau de Bord Des « Jeux d'argent et de Hasard » En France ‐ Données 2019 ‐ OFDT; Observatoire Français des Drogues et des Toxicomanies; 2021:1‐7. [Google Scholar]
- 3. Griffiths M. An exploratory qualitative study of online poker professional players. Social Psychological Review. 2012;14(2):13‐25. doi: 10.53841/bpsspr.2012.14.2.13 [DOI] [Google Scholar]
- 4. Grant JE, Odlaug BL, Chamberlain SR, Schreiber LRN. Neurocognitive dysfunction in strategic and non‐strategic gamblers. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):336‐340. doi: 10.1016/j.pnpbp.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brevers D, Noël X, He Q, Melrose JA, Bechara A. Increased ventral‐striatal activity during monetary decision making is a marker of problem poker gambling severity. Addict Biol. 2016;21(3):688‐699. doi: 10.1111/adb.12239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laakasuo M, Palomäki J, Salmela M. Emotional and social factors influence poker decision making accuracy. J Gambl Stud. 2015;31(3):933‐947. doi: 10.1007/s10899-014-9454-5 [DOI] [PubMed] [Google Scholar]
- 7. Moreau A, Sévigny S, Giroux I, Chauchard E. Ability to discriminate online poker tilt episodes: a new way to prevent excessive gambling? J Gambl Stud. 2020;36(2):699‐711. doi: 10.1007/s10899-019-09903-7 [DOI] [PubMed] [Google Scholar]
- 8. Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1‐3):7‐15. doi: 10.1016/0010-0277(94)90018-3 [DOI] [PubMed] [Google Scholar]
- 9. Linnet J, Røjskjaer S, Nygaard J, Maher BA. Episodic chasing in pathological gamblers using the Iowa gambling task. Scand J Psychol. 2006;47(1):43‐49. doi: 10.1111/j.1467-9450.2006.00491.x [DOI] [PubMed] [Google Scholar]
- 10. Moreau A, Chabrol H, Chauchard E. Psychopathology of online poker players: review of literature. J Behav Addict. 2016;5(2):155‐168. doi: 10.1556/2006.5.2016.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision‐making. Hum Brain Mapp. 2007;28(12):1276‐1286. doi: 10.1002/hbm.20344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giustiniani J, Gabriel D, Nicolier M, Monnin J, Haffen E. Neural correlates of successful and unsuccessful strategical mechanisms involved in uncertain decision‐making. PLoS ONE. 2015;10(6):e0130871. doi: 10.1371/journal.pone.0130871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal VA, Nusslock R. Beyond the FRN: broadening the time‐course of EEG and ERP components implicated in reward processing. Int J Psychophysiol. 2018;132(Pt B):184‐202. doi: 10.1016/j.ijpsycho.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 14. Cui J, Chen Y, Wang Y, Shum DHK, Chan RCK. Neural correlates of uncertain decision making: ERP evidence from the Iowa gambling task. Front Hum Neurosci. 2013;7:776. doi: 10.3389/fnhum.2013.00776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oberg SAK, Christie GJ, Tata MS. Problem gamblers exhibit reward hypersensitivity in medial frontal cortex during gambling. Neuropsychologia. 2011;49(13):3768‐3775. doi: 10.1016/j.neuropsychologia.2011.09.037 [DOI] [PubMed] [Google Scholar]
- 16. Sutton S, Braren M, Zubin J, John ER. Evoked‐potential correlates of stimulus uncertainty. Science. 1965;150(3700):1187‐1188. doi: 10.1126/science.150.3700.1187 [DOI] [PubMed] [Google Scholar]
- 17. Ferdinand NK, Kray J. Age‐related changes in processing positive and negative feedback: is there a positivity effect for older adults? Biol Psychol. 2013;94(2):235‐241. doi: 10.1016/j.biopsycho.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 18. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128‐2148. doi: 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giustiniani J, Nicolier M, Teti Mayer J, et al. Behavioral and neural arguments of motivational influence on decision making during uncertainty. Front Neurosci. 2020;14:583. doi: 10.3389/fnins.2020.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martínez‐Selva JM, Muñoz MA, Sánchez‐Navarro JP, Walteros C, Montoya P. Time course of the neural activity related to behavioral decision‐making as revealed by event‐related potentials. Front Behav Neurosci. 2019;13:191. doi: 10.3389/fnbeh.2019.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamp S‐M, Forester G, Vatheuer CC, Domes G. Stress effects on the oddball P300 and N2 in males and females. Biol Psychol. 2021;162:108095. doi: 10.1016/j.biopsycho.2021.108095 [DOI] [PubMed] [Google Scholar]
- 22. Dancy CL, Ritter FE. IGT‐open: an open‐source, computerized version of the Iowa gambling task. Behav Res Methods. 2017;49(3):972‐978. doi: 10.3758/s13428-016-0759-4 [DOI] [PubMed] [Google Scholar]
- 23. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97‐113. doi: 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- 24. Bondolfi G, Jermann F, Rouget BW, et al. Self‐ and clinician‐rated Montgomery‐Asberg depression rating scale: evaluation in clinical practice. J Affect Disord. 2010;121(3):268‐272. doi: 10.1016/j.jad.2009.06.037 [DOI] [PubMed] [Google Scholar]
- 25. Petry NM, Zajac K, Ginley MK. Behavioral addictions as mental disorders: to be or not to be? Annu Rev Clin Psychol. 2018;14(1):399‐423. doi: 10.1146/annurev-clinpsy-032816-045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐‐II. Addiction. 1993;88:791‐804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 27. Meneses‐Gaya IC d, Zuardi AW, Loureiro SR, Crippa JA d S. Psychometric properties of the Fagerström test for nicotine dependence. J Bras Pneumol. 2009;35(1):73‐82. doi: 10.1590/s1806-37132009000100011 [DOI] [PubMed] [Google Scholar]
- 28. Achab S, Nicolier M, Mauny F, et al. Massively multiplayer online role‐playing games: comparing characteristics of addict vs non‐addict online recruited gamers in a French adult population. BMC Psychiatry. 2011;11(1):144. doi: 10.1186/1471-244X-11-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bouvard M. Questionnaires et Échelles d'évaluation de La Personnalité. Elsevier Masson; 2009. [Google Scholar]
- 30. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85(4):374‐382. doi: 10.1037/0021-843X.85.4.374 [DOI] [PubMed] [Google Scholar]
- 31. Smillie LD, Jackson CJ, Dalgleish LI. Conceptual distinctions among carver and White's (1994) BAS scales: a reward‐reactivity versus trait impulsivity perspective. Personal Individ Differ. 2006;40(5):1039‐1050. doi: 10.1016/j.paid.2005.10.012 [DOI] [Google Scholar]
- 32. Michel CM, Murray MM. Towards the utilization of EEG as a brain imaging tool. Neuroimage. 2012;61(2):371‐385. doi: 10.1016/j.neuroimage.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 33. Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279‐2282. doi: 10.1126/science.1066893 [DOI] [PubMed] [Google Scholar]
- 34. Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Res. 2006;1105(1):93‐101. doi: 10.1016/j.brainres.2005.12.015 [DOI] [PubMed] [Google Scholar]
- 35. Khanna A, Pascual‐Leone A, Michel CM, Farzan F. Microstates in resting‐state EEG: current status and future directions. Neurosci Biobehav Rev. 2015;49:105‐113. doi: 10.1016/j.neubiorev.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ladouceur R, Sylvain C, Botin C, Doucet C. Le jeu excessif, comprendre et vaincre le gambling. De l'Homme; 2000. ISBN 10: 2761915801 [Google Scholar]
- 37. Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and Normal controls. Brain Res Cogn Brain Res. 2005;23(1):137‐151. doi: 10.1016/j.cogbrainres.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 38. Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Psychophysiological determinants and concomitants of deficient decision making in pathological gamblers. Drug Alcohol Depend. 2006;84(3):231‐239. doi: 10.1016/j.drugalcdep.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 39. Linnet J, Møller A, Peterson E, Gjedde A, Doudet D. Inverse association between dopaminergic neurotransmission and Iowa gambling task performance in pathological gamblers and healthy controls. Scand J Psychol. 2011;52(1):28‐34. doi: 10.1111/j.1467-9450.2010.00837.x [DOI] [PubMed] [Google Scholar]
- 40. De Pascalis V, Varriale V, D'Antuono L. Event‐related components of the punishment and reward sensitivity. Clin Neurophysiol. 2010;121(1):60‐76. doi: 10.1016/j.clinph.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 41. Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29(47):14980‐14986. doi: 10.1523/JNEUROSCI.3706-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berlucchi G, Vallar G. Chapter 1 ‐ The History of the Neurophysiology and Neurology of the Parietal Lobe. In: Vallar G, Coslett HB, eds. Handbook of clinical neurology. The Parietal Lobe. Vol.151. Elsevier; 2018:3‐30. doi: 10.1016/B978-0-444-63622-5.00001-2 [DOI] [PubMed] [Google Scholar]
- 43. Salti M, Bar‐Haim Y, Lamy D. The P3 component of the ERP reflects conscious perception, not confidence. Conscious Cogn. 2012;21(2):961‐968. doi: 10.1016/j.concog.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 44. Ye M, Lyu Y, Sclodnick B, Sun H‐J. The P3 reflects awareness and can be modulated by confidence. Front Neurosci. 2019;13:510. doi: 10.3389/fnins.2019.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sescousse G, Barbalat G, Domenech P, Dreher J‐C. Imbalance in the sensitivity to different types of rewards in pathological gambling. Brain. 2013;136(8):2527‐2538. doi: 10.1093/brain/awt126 [DOI] [PubMed] [Google Scholar]
- 46. Bakhshipour‐Rudsari A, Karimpour‐Vazifehkhorani A. The role of impulsivity and sensitivity to reward in dropout of addiction treatment in heroin addicts. Addict Health. 2021;13(1):45‐51. doi: 10.22122/ahj.v13i1.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


