Abstract
Background
Poly(adenosine diphosphate-ribose) polymerase inhibitors (PARPi) improve progression free survival among patients with HER2 negative (HER2-ve) advanced breast cancer (ABC) and a BRCA1 or BRCA2 mutation compared to chemotherapy (CT). The objective of this prospective study was to evaluate the clinical benefit of PARPi treatment in terms of response, outcomes and survival by breast cancer type and treatment in a Latin-American population.
Methods
From September 2019 to April 2023, we analyzed the data of patients with HER2-ve ABC with germline and/or somatic mutation of BRCA1 or BRCA2, or in the homologous recombination repair genes, treated with olaparib or talazoparib in daily clinical practice by oncologist from Argentina and México. real-world objective response rate (rwORR), best response rate, real-world progression-free survival (rwPFS) and real-world overall survival (rwOS) were analysed with R software and RStudio version 14.0.
Results
After a median follow-up of 18.07 months (95% CI 10.53–30.07), 51 patients were treated with PARPi. Mean age at starting treatment was 47.08 years. 62.7% had ER + ve/HER2-ve and 35.3% had triple negative breast cancer (TNBC). 62.7% and 37.3% of patients received talazoparib and olaparib, respectively. BRCA 1 and 2 germline mutations were the most common alterations found in 96% of patients. 37.5% of patients received platinum-based CT in the (neo)adjuvant/metastatic setting. At the time to starting PARPi treatment, 57.5% had visceral metastasis, the median number of metastatic sites was 2 (range 1–4), the median number of lines was 2 (range 0–8), and 23.5% and 31.4% received PARPi in the 1st line and 2nd line, respectively.
The rwORR was 47.0%, and the median real-world progression-free survival-1 (rwPFS1) was 7.77 months (95% CI 5.67–14.7). There was a tendency of better rwPFS1 in patients with versus without previous CT in the advance setting, 6.37 months (95% CI 5.03–8.73) and 14.30 months (95% CI 6.47–NR), respectively (p 0.084). The median rwOS was 26.97 months (95% CI 13.50–NR) and higher in the subgroup of patients naïve for CT versus previous exposure to CT in the advance setting, the median rwOS was 32.1 months (95% CI 27.0–NR) versus 13.0 (95% CI10.1–NR), respectively (p 0.022). The medium real-world progression-free survival-2 (next treatment after PARPi failure) was 4.00 months (95% CI 3.43–7.13). Treatment was discontinued due to adverse events in 4.0% of patients.
Conclusion
This is the first evidence in a Latin-American population that replicates the data already published in randomised clinical trials and other scanty real-world evidence studies in this field, showing positive results in rwORR and rwPFS, and encouraging data in rwOS. Notably, there was a high proportion of patients with visceral progression even with visceral crisis and need for CT. Interestingly, there were similar rwOS results among subgroups (TNBC versus ER + ve/HER2-ve, talazoparib versus olaparib, etc).
Introduction
Approximately 5%–10% of breast cancer (BC) patients carry germline mutations in BRCA1 or BRCA2 genes (gBRCA1/2m), tumor suppressor genes that synthetise proteins that are essential for the recognition and subsequent high-fidelity repair of DNA double-strand breaks by homologous recombination repair machinery (HRR) [1].
The detection of homologous recombination deficiency (HRD) tumors lies in the potential benefit from DNA-damage repair (DDR) targeted agents, however, their efficacy outside the context of BRCA1 and BRCA2 mutations is still under investigation. Approximately 35% of triple negative (TN) tumors exhibit HRD, which makes them particularly sensitive to drugs that act through DNA damaging [2].
Over the past few years, many studies have been focused on developing companion molecular tests that detect and quantify HRD, hence, predict tumor response to platinum agents and/or HRD-targeted treatment, but to date, they have not yet been validated [3].
Deficiency in BRCA1/2 results in HRD, and this confers a particularly sensitivity to poly(adenosine diphosphate-ribose) polymerase inhibitors (PARPi) in early and advanced disease. Based on EMBRACA and OlympiAD studies, two PARPi: talazoparib and olaparib, respectively, obtained U.S. Food and Drug Administration approval for the treatment of gBRCA1/2m HER2 negative (HER2-ve) advanced breast cancer (ABC) [4, 5].
To date, germline pathogenic or likely-pathogenic mutations of BRCA1/2 genes are the only predictive genetic biomarkers of response to PARPi [6]. Little is known about somatic genetic biomarkers (from the broad family of genes related to DDR) predictors of response or resistance originating in biopsy samples of metastasis or circulating tumor DNA [7]. Limited data are available worldwide on the effectiveness and patient predisposition of PARPi in daily practice.
The aim of this collaborative prospective real-world evidence study is to evaluate the effectiveness, patient´s profile characterisation, patient predisposition description, treatment sequence of PARPi, and treatment beyond PARPi progression in patients with HER2-ve ABC, who have germline and/or somatic mutation in BRCA1/2 genes, or HRR genes treated with olaparib or talazoparib. The study recruited patients principally from an Argentina and México collaboration.
Patients and methods
Being a real-life, observational, prospective, non-comparative, non-intervention study, as many patients as possible were included consecutively as long as they met the inclusion criteria.
A confidential list was maintained which allowed the association of the coding assigned to the patient and the identified data. We maintained the rules of the International Conference on Harmonization Good Clinical Practice and the applicable local regulations. The trial protocol was approved by ethics review committees at all participating institutions/countries.
Patients included had to comply with the following inclusion criteria: a) to be aged ≥18 years, b) histological and immunohistochemical diagnosis of hormone-receptor positive and HER2-ve BC or hormone-receptor negative (<1%) and HER2-ve BC triple negative breast cancer (TNBC), at primary or metastatic site; c) presence of pathogenic or probably pathogenic germline and/or somatic mutation in BRCA1 and/or BRCA2 genes, or in genes linked to DNA repair by homologous recombination listed here: PALB2, CHEK2, ATM, ATR, NBN, BARDO, BRIP1, RAD50, RAD51C RAD51D, MRE11, PTEN, FANCA, FANCC, FANCD2, FANCE, FANCF, FANCG and FANCL; d) have metastatic or locally advanced unresectable disease; and e) have completed at least one course (28 days) of treatment with olaparib or talazoparib approved by local agencies.
The following were considered as exclusion criteria: a) insufficient data in the clinical chart for prospective evaluation, b) not having completed at least one cycle (28 days) of treatment with a PARPi, c) HER2-receptor positive BC, and d) absence of germline or somatic mutations in predefined genes. Patients were not required to be receiving PARPi therapy for any minimum period of time.
Tumour assessments were conducted in accordance with local practice and standard of care at each patient visit.
Study design and population
LuciA-15 is a collaborative prospective, observational, non-comparative (one cohort) study for patients treated with olaparib or talazoparib with HER2-ve ABC, who carry pathogenic or likely-pathogenic germline and/or somatic mutations in BRCA1 and/or BRCA2 genes, or in genes related to HRR mechanism.
The standard and basic determination to indicate olaparib or talazoparib in daily practice is the presence of pathogenic or likely-pathogenic germline mutation of the BRCA1/2 genes by the Next Generation Sequencing (NGS) technique. The genetic tests for each patient were indicated at the discretion of the treating physician and/or genetic counsellor, and covered out of pocket by the patient, their insurance or access programs offered by the pharmaceutical industry.
Considering this, there were differences in the number of genes, panels and/or germline or somatic alterations found that did not allow further subgroup analysis. The aim of this study was to evaluate the effectiveness and identify patient´s profile to improve the management and understanding of PARPi use in ABC in daily clinical practice.
The primary objectives of this study: real-world objective response rate (rwORR), real-world progression-free survival (rwPFS) on PARPi treatment real-world progression-free survival-1 (rwPFS1) and real-world overall survival (rwOS). The secondary objectives were: rwPFS after PARPi progression real-world progression-free survival-2 (rwPFS2), progression-free and survival rates (SRs) at 6, 12, 18, 24 and 30-months, patient predisposition, patterns of progression, and first treatment beyond progression.
Based on the number of patients included, subgroup analysis was considered, the outcomes depended on BC subtype, previous exposition or not to platinum-based CT (PbCT) and general chemotherapy (CT), etc.
Data source and extraction
Prospective collection of patient´s data loaded in the electronic or paper clinical chart, and reports of imaging studies and data were provided by the treating oncologists. National collaborations were requested to improve recruitment, considering the infrequent indication of PARPi in ABC. Demographic, epidemiological, clinical-pathological and patient predisposition data were collected and detailed in Table 1.
Table 1. Endpoint definition.
| Real-world objective response rate | Definition/variables |
|---|---|
| CR | No clinical or by images evidence of disease after start PARPi treatment (at any time, no 24-week minimum) |
| PR | Partial reduction in tumor burden in some or all areas without any areas of increasing disease. rwPR captures a decrease in disease burden though disease is still present |
| Stable disease ≥24 weeks (SD≥24 w) | Patients remained on PARPi for a minimum of 24 weeks without complete or PR, death, treatment switch, or progression |
| Stable disease <24 weeks (SD<24w) | Stable disease recorded for initial response, with a subsequent progression recorded after <24 weeks or treatment switch for reason other than progression after <24 weeks, or death without recorded progression after <24 weeks |
| rwORR | Proportion of patients achieving a complete or PR as assessed by the physician and reported in the patient records |
| Clinical benefit rate ≥24 weeks (CBR≥24w) | Proportion of patients who achieved a complete, PR and had stable disease for ≥24 weeks as assessed by the physician |
| Progressive disease <24 weeks (PD<24w) | PD recorded from start of PARPi treatment to <24 weeks. Progression could be radiological (e.g., RECIST), symptomatic, or clear progression of non-measurable disease, as long as progression could be documented from start of PARPi treatment to <24 weeks. |
| Progressive disease (PD) | PD recorded from start of PARPi treatment to last visit, death or lost of follow-up. |
| Clinical outcomes | |
| rwPFS1 | Time from start of PARPi treatment to date of progression, death for any cause, or date of start next treatment or last visit reported in the medical chart if the patient had not progressed or died. |
| rwPFS2 | Time from start next treatment after PARPi to death for any cause, clinical progression, loss of follow-up o date of start next treatment, only for those patients with disease progression on PARPi |
| rwOS | Time from start of PARPi treatment to death for any cause or loss of follow-up or alive at last visit. |
| PFR | Proportion of patients with no evidence of progression or death at 6, 12, 18, and 24 months |
| SR | Proportion of patients alive at 6, 12, 18, and 24 months |
Genetic data collected: name of gene (BRCA1, BRCA2, PALB2, CHEK2, ATM, ATR, NBN, BARD, BRIP1, RAD50, RAD51C, RAD51D, MRE11, PTEN, FANCA, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCL, others), type of mutation (pathogenic or likely-pathogenic, others), and genetic tests requested (germline and/or somatic, individual genes, panels or platforms like FoundationOneCDx).
Data collected for the effectiveness analysis were rwORR, the best responses achieved in the first 6 months of treatment based on RECIST version 1.1 (https://recist.eortc.org/recist-1-1-2/) adapted by each oncologist decision; rwPFS1, rwPFS2, rwOS, progression-free rate (PFR) and SR at 6, 12, 18, 24 and 30 months. No data regarding side effects were collected. Definitions of all clinical outcome variables are presented in Table 2.
Table 2. Baseline characteristics.
| Patient characteristics | n: 51 p | % |
|---|---|---|
| Female Male |
50 1 |
98.0 2.0 |
| Mean age (years) | 47.08 | 23–83 |
| Premenopausal | 26 | 52.0 |
| Meta or synchronic bilateral BCa | 4 | 8.0 |
| BC subtype | n: 51 p | % |
| ER+/HER2- TN ER+/HER2+ |
32 18 1 |
62.7 35.3 2.0 |
| Disease-free intervalb | n: 47 p | % |
| De novo stage IV or ≤12-months Relapse >12-months |
17 30 |
36.2 63.8 |
| sPIK3-CA statusc | n: 26 p | % |
| Wild-type Mutated |
19 7 |
73.1 26.9 |
| Previous treatment | n: 51 p | % |
| Neoadjuvant and/or adjuvant CT Anthracyclines Taxanes Anthracyclines + taxanes CT + platinum-based CT + capecitabine Others |
30 3 2 15 4 2 4 |
75.0 10.0 6.7 50.0 13.3 6.7 13.3 |
| Adjuvant endocrine therapy | n: 19 p | % |
| Tamoxifen Aromatase inhibitors |
14 5 |
73.7 26.3 |
| Advanced CT lines | n: 50 p | % |
| 0 lines 1 line 2 lines 3 lines Median lines of ABC CT |
28 16 4 2 0 |
56.0 32.0 8.0 4.0 0–3 |
| Advanced endocrine therapy lines | n: 35 p | % |
| 0 lines 1 line 2 lines 3 lines Median Lines of ABC ET |
6 17 11 1 1 |
17.1 48.6 31.4 2.9 0–3 |
| ET + CDK4/6 inhibitors | 28 | 82.4 |
| ET + mTOR inhibitors | 7 | 20.6 |
| Alpelisib | 2 | 6.1 |
| Fulvestrant | 15 | 44.1 |
| ICP inhibitors | 1 | 5.0 |
| Number of lines before PARP inhibitors | ||
| 0 lines 1 line 2 lines 3 lines ≥4 lines Median lines before PARPi |
12 16 15 2 6 2 |
23.5 31.4 29.4 3.9 11.8 0–8 |
| PARP inhibitor indicated | ||
| Talazoparib Olaparib |
32 19 |
62.7 37.3 |
| PbCT in (neo)adjuvant or advance setting | 18 | 37.5 |
| PbCT in the advance setting pre-PARPi | 13 | 25.5 |
| PARP inhibitors before or after CT in ABC | ||
| PARP inhibitors before CT PARP inhibitors after CT |
25 22 |
53.2 46.8 |
| Platinum-free interval | ||
| ≤6 months >6 to 12 months >12 months |
9 5 4 |
50.0 27.8 22.2 |
| Metastatic sites | ||
| Bone Visceral More than two Soft tissue CNS w/wo others |
7 27 8 5 1 |
14.9 57.4 17.0 10.6 6.7 |
| Number of metastatic sites | ||
| 1 2 3 4 Median number of metastatic sites |
17 19 14 1 2 |
33.3 37.3 27.5 2.0 1–4 |
| ECOG performance status | ||
| 0 1 ≥2 |
31 18 2 |
60.8 35.3 4.0 |
| Concomitant RT with PARP inhibitors | 9 | 17.6 |
3 p with billateral BC with different phenotypes
6 p unknown
3 p unknown
ICP, immune-check points inhibitors. CT, chemotherapy. ET, endocrine therapy. ABC, advanced breast cancer. RT, radiation therapy
A periodic review of health records by physicians was carried out to ensure data quality control. The study was closed (March, 2023) at the discretion of the research team, after a plateau of no new patients included.
Ethical considerations and data safety
The present study has no impact on patient safety from a clinical point of view. The data obtained in this study was strictly confidential, no initial or subsequent contact with patients was performed. The study was developed under strict supervision of the ethical principles outlined in the Declaration of Helsinki, rules of Good Clinical Practices and applicable legislation. The final protocol of the study was approved by the Ethics Committee of the Instituto Alexander Fleming, Buenos Aires, Argentina.
Statistical analysis
The present study included descriptive analyses, in which means, medians, SDs, percentiles and ranges were used. Categorical variables were described by proportions. Where appropriate, the 95% confidence interval was presented at both ends. The data will be censored at the last follow-up if the patient is alive. Logistic regression and Kaplan-Meier models were used for survival analyses. Statistical analyses will be performed with R software and RStudio version 14.0.
Results
Between September 2019 and April 2023, 51 patients were included for analysis (50 patients from Argentina and 1 patient from Mexico). At the time to start PARPi the median number of metastatic sites was 2 (range 1–4), most of the patients had exclusive visceral disease (n: 27, 57.0%), followed by bone only disease (n: 7, 14.0%) and ≥2 metastatic sites (n: 8, 17.0%). The ECOG-PS was 0 and 1 in 60.8% (n: 31) and 35.3% (n: 18), respectively. All the detailed data is summarised in Table 2.
Regarding genetic tests performed, based on oncologist personal decision and/or genetic counseling, from 50 patients´ data, 96% of alterations found were germline mutation (one patient with somatic mutation and one patient with concurrent germline and somatic pathogenic mutation), and gBRCA2 follow by gBRCA1 mutations were the most common mutations encountered, 52% and 42%, respectively. One patient tested by somatic comprehensive gene panel evidenced concomitant pathogenic alteration in sATM, sRAD51c and sBIR-I genes. The most common genetic assays performed were 2-genes by NGS with or without MLPA (germline BRCA1/2) follow by 26, 30 or 33-genes panel, and three patients were tested by FoundationOneCDx.
Treatment patterns
At the time of final analysis (March 2023), PARPi treatment was ongoing in 16 patients (31.4%). Talazoparib was the first PARPi approved for commercialisation in Argentina in 2019, followed by Olaparib in 2020. Talazoparib was indicated in 62.7% (n: 32) and olaparib in 37.3% (n: 19) of patients, only one patient with a TNBC treated with olaparib in second line was rechallenged in 6th line with talazoparib.
The median number of lines for advanced setting before PARPi indication was 2 (range 0–8). In the general population the most frequent indication of PARPi was in early lines of ABC, first line 23.5% (n: 12), second line 31.4% (n: 16), third line 29.4% (n: 15), and four lines and beyond 15.7% (n: 8). Similar rate of use of PARPi before or after CT for ABC, 53.2% and 46.8%, respectively.
The most common starting dose was the recommended daily full dose for talazoparib 1 mg and olaparib 600 mg (n = 48; 94.1%), only three patients (5.9%) started with the first reduction dose. At least one dose adjustment was seen in 14 patients (29.2%), most frequently reduction from 1 to 0.75 mg for talazoparib (n = 9) and 600–450 mg for olaparib (n:4). At least one dose interruption was seen in 16 patients (33.3%), most frequently with talazoparib (n: 14) than olaparib (n: 2). Treatment was discontinued due adverse events in 2 patients (4.0 %).
First treatment beyond PARPi progression
In the first 6 months of treatment, 23.5% (n: 12) of patients progressed under talazoparib or olaparib treatment, and at the time of final analysis 76.5% (n: 39) of patients progressed. Visceral disease (n: 11, 32.4%) and ≥2 metastatic sites (n:9, 26.5%) were the most frequent sites of progression leading to CT (mono or in combination) as the treatment of choice (n:21, 55.3%), and one out of four with platinum-based schemes (n: 5). Furthermore, visceral crisis by oncologist criteria as progression event was common, data available from 36 patients showed 27.8% (n: 10) of cases.
Outcomes
On March 2023, after a median follow-up of (IQR): 18.0 months (95% CI 10.5–30.0), 51 patients were treated with PARPi, 23.5% of them still on treatment with PARPi (n: 12), and 76.5% of patients progressed (n: 39), 39.2% (n: 20) had died, and 9.8% were lost on follow-up (n: 5). The rwORR, calculated based on the physician’s assessments as recorded in patient charts, were achieved in 47.0% of patients (n: 24), including complete response (CR) in 7.8% (n: 4) and partial response (PR) in 39.2% (n: 20). The CBR≥24w was 76.4% (n: 39) including those patients with SD≥24w of 29.4% (n: 15), and one out of four of patients progressed on PARPi during the first 6 months of treatment (n:12, 23.5%). One patient with a TNBC who carries the somatic concurrent mutation in ATM, RAD51c and MYH by FoundationOneCDx, progressed at 5.0 months with olaparib treatment in fifth line. Another patient with ER+ve/HER2-ve gPALB2m progressed at 5.7 months with talazoparib in second line. And finally, one patient with ER + ve/HER2-ve sBRCA2m was treated with talazoparib in the first line and progressed at 6.5 months.
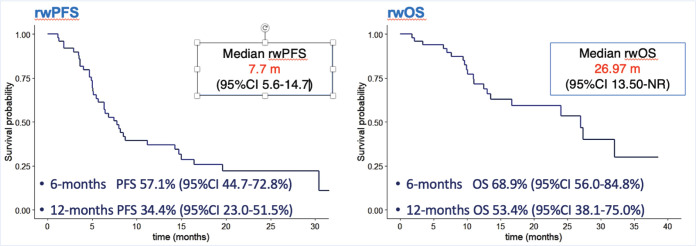
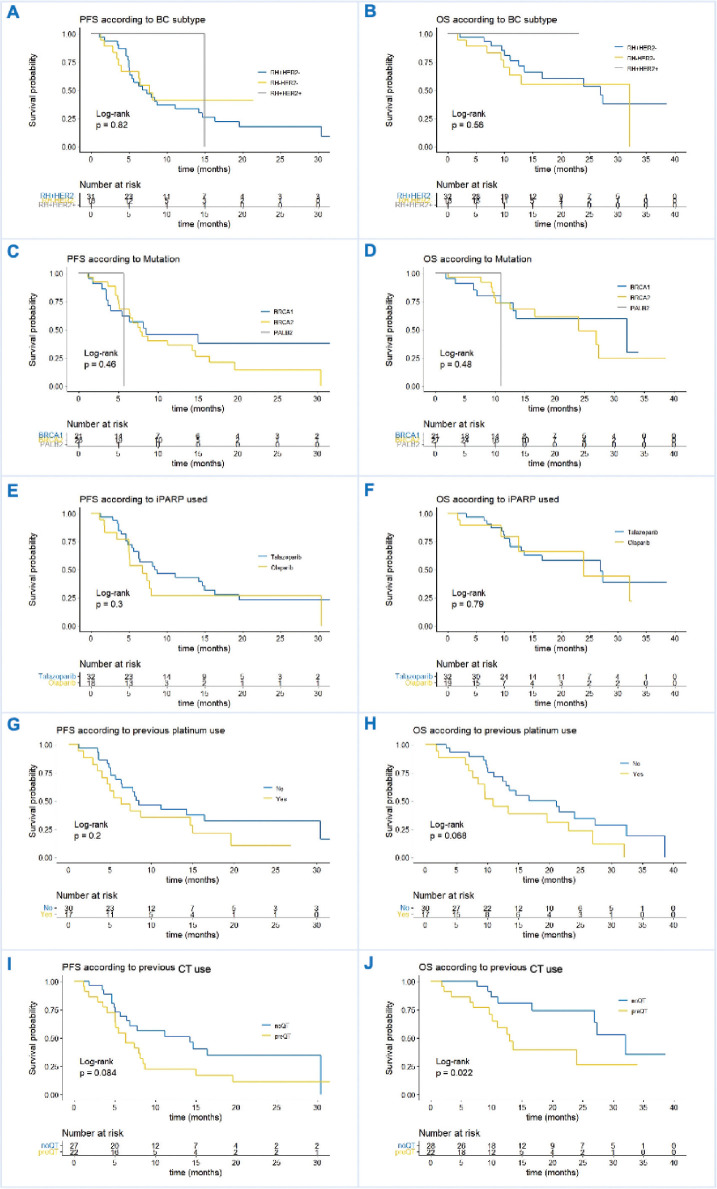
Based on real-world evidence studies definitions we performed ways of progression and survival analysis. Two of the primary objectives of the study were analysed the rwPFS1 and rwOS in the general population. Due to the limited number of patients included, subgroup analysis was insufficient for conclusions, despite that, we present Kaplan-Meier curves comparing outcomes between BC subtypes. The median rwPFS1 was 7.7 months (95% CI 5.6–14.7) and the rwPFS at 6 and 12 months were 57.1% (95% CI 44.7%–72.8%) and 34.4% (95% CI 23.0%–51.5%), respectively. Regarding rwOS in the whole population, the median rwOS was 26.9 months (95% CI 13.5–NR) and the rwOS at 6 and 12 months were 68.9% (95% CI 56.0%–84.8%) and 53.4% (95% CI 38.1%–75.0%), respectively (Figure 1). The statistical analysis showed no differences between BC subtypes, menopausal status, type of gene mutations, previous exposure to PbCT, and type of PARPi utilised. There was a tendency of better rwPFS in patients without versus previous CT in the advance setting, the rwPFS was 14.3 months (95% CI 6.4–NR) versus 6.3 months (95% CI 5.0–8.7), respectively with a p value of 0.084. The positive result was found in the subgroup of patients naïve versus previous exposure to CT in the advance setting, the median rwOS was 32.1 months (95% CI 27.0–NR) versus 13.0 (95% CI 10.1–NR), respectively, with a p value of 0.022. For other subgroup analysis, there were no differences in rwOS as in rwPFS. (Figure 2).
Figure 1. Kaplan–Meier curves in general population. rwPFS, Real-world progression-free survival and rwOS, real-world overall survival. 95% CI, 95% Confidence interval.
Figure 2. (a and b): rwPFS and rwOS in BC subtype ER + ve/HER2-ve versus TN. (c and d): rwPFS and rwOS in BC based on gene mutation: BRCA1 versus BRCA2 versus PALB2. (e and f): rwPFS and rwOS based on PARPi subtype: talazoparib versus olaparib. (g and h): rwPFS and rwOS based on previous PbCT use yes or no. (i and j): rwPFS and rwOS based on previous CT use yes or no.
Analysing in isolation the effect of the use or not of PbCT prior to the start of PARPi had a negative trend in rwOS. When adjusted this effect in a bivariable analysis including the effect of the general CT use for advance setting before PARPi treatment, showed its value is lost. (PbCT HR 1.5, 95% CI 0.6–3.8, p 0.36 and overall CT HR 2.5, 95% CI 0.9–6.5, p 0.05).
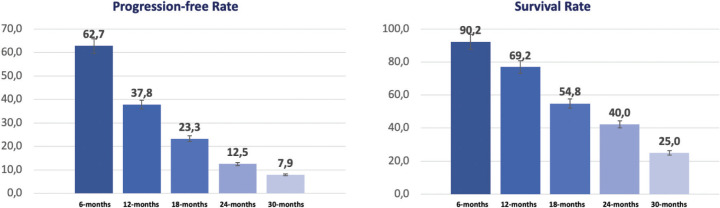
One of the secondary objectives of the study was evaluating the proportion of patients alive without progression at 6, 12, 18, 24 and 30 months, the progression-free rate; and for the same periods of time the proportion of patients alive, the SR (Figure 3).
Figure 3. Progression-free and SR at 6, 12, 18, 24 and 30 months.
Regarding PFR, coinciding with the median PFS extracted in the Kaplan-Meier curves (57.1% at 6 months), 62.7% of patients were alive without evidence of disease progression, descending to 23.3% at 18 months of follow-up. Remarkably, close to 70% of patients were alive at 1-year, and one out of four (25.0%) was alive at 30-months of follow up.
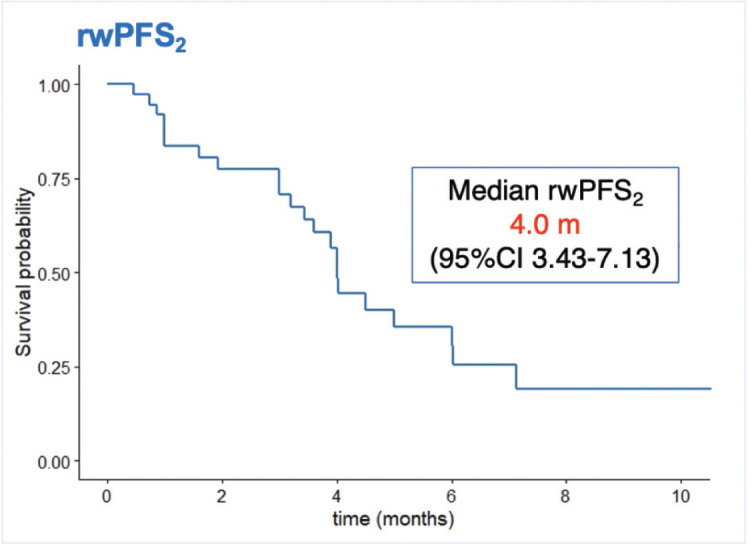
Another secondary objective analysis was to evaluate the rwPFS with the first or next treatment after PARPi failure, defined as rwPFS2. Based on disease distribution, systemic treatment resistance criteria, previous exposure to CT and PbCT, the scenario beyond PARPi progression in our population had a poor prognosis. Notably, 23.5% of patients progressed on PARPi during the first 6 months of treatment, 32.4% with visceral compromise, 27.8% with visceral crisis, and 26.5% with ≥2 metastatic sites. CT alone or in combination was the treatment of choice in most patients (55.3%), and one out of four included CT with platinum-based schemes. Considering all of this, the medium rwPFS2 was 4.0 months (95% CI 3.4–7.1) (Figure 4).
Figure 4. rwPFS2, rwPFS with de first or next treatment after PARPi failure.
Discussion
LuciA-15 trial is the first prospective trial to evaluate the clinical effectiveness of PARPi in patients with BRCA and non-BRCA mutations, HER2-ve ABC in a setting that closely reflects real-life clinical practice. Only three patients were included with non-gBRCAm to reach any conclusion about effectiveness in this subgroup. Not many trials in this field were published, only three real-world studies, two phase II trials, and two major phase III randomised clinical trials (RCTs) that support our current treatment decisions, summarised in Table 3. The LUCY trial is a pragmatic, open-label, single-arm trial of olaparib, in HER2-ve BRCAm ABC. At the interim analysis, 54.4% received olaparib in 1 L, the median investigator assessed PFS was 8.1 months (95% CI 6.9–8.6), the clinical response rate was 48.6%, the median duration of clinical response was 6.6 months (IQ range, 4.2–10.8), and median time to first subsequent treatment or death was 9.6 months (95% CI 8.6–11.1) [8].
Table 3. Available studies in ABC with PARPi.
| Study | n | PARPi | Subgroups | Line | ORR (%) | PFS (Median in months, 95%IC) |
OS (Median in months, 95% IC) |
|---|---|---|---|---|---|---|---|
| LUCY [8] | 252 | Olaparib | g/sBRCA1/2m | 54% 1 L | 48.6 | 8.1 (6.9–8.6) | NA |
| Dawood [9] | 586 | Olaparib talazoparib others |
g/sBRCA1/2m | 31% 1 L 51% 2 L 30% ≥3 L |
NA | NA | 23.0 (NA) |
| Batalini et al [10] | 62 | NA | No gBRCAm gBRCAm |
NA | NA | 7.7 (NA) 5.5 (NA) |
15.0 (NA) 11.5 (NA) |
| TBCRC-048 [11] | 20 | Olaparib | g/sBRCA1/2m g/sHRRm |
≥3 L | 33.0 | 13.3 (12–NA) | NA |
| TBB [12] | 13 | Talazoparib | g/sHRRm | NA | 31.0 | NA | NA |
| OlympiAD [13] | 302 | Olaparib | gBRCA1/2m | 29% 1 L | 59.9 | 7.4 (NA) | 19.3 (NA) |
| EMBRACA [14] | 431 | Talazoparib | gBRCA1/2m | 39% 1 L 37% 2 L 24% ≥3 L |
62.6 | 8.6 (7.2–9.3) | 19.3 (16.6–22.5) |
| LuciA-15 | 51 | Olaparib talazoparib |
g/sBRCA1/2m g/sHRRm |
23.5% 1 L 31.4% 2 L 45.1% ≥3 L |
47.0 | 7.7 (5.6–14.7) | 26.67 (13.5–NR) |
PARPi, PARP inhibitor; g/sBRCA1/2, germline/somatic BRCA 1 or 2 mutations; g/sHRR, germline/somatic Homologous Recombination Repair genes mutation; ORR, objective response rate; PFS, pprogression-free survival; NA, not available or not specified; NR, not reached
Dawood [9] published one of the few studies available in real-world evidence based on retrospective data extracted from US TriNetX Research Platform, 586 patients with HER2-ve ABC treated with PARPi. Median OS was 23 months, 18.0 months in TNBC and 22.0 months in ER + ve/HER2-ve (p 0.37) [9]. Another interesting study was published by Batalini et al [10] data were extracted from Flatiron Health – Foundation Medicine Clinico-Genomic Database, assessed outcomes for patients with gBRCAm compared to patients with either sBRCAm or other HRD treated with PARPi. Neither median rwPFS (7.0 versus 5.5 months) nor rwOS (15.0 versus 11.5 months) were significantly different between the non-gBRCA and gBRCA cohorts. For 9 patients with sBRCAm, median rwPFS was 7.1 mos (range 1.4–12.4) and all patients had progressed by data cut off [10]. These findings are consistent with the results from TBCRC -048, a phase II single-arm, two-stage design trial that explored the effectivity of olaparib in ABC with pathogenic mutation in HRR genes, excluding gBRCA1/2m tumors, platinum-refractory disease or >2 L of CT for ABC. In the germline mutated cohort (n: 27) the primary endpoint ORR was 33% (all PR) and the CBR18w was 67%. Notably, all PR were reached in gPALB2 (ORR, 82%) with CBR18w of 100% and a median PFS of 13.3 months (90% CI 12–NA). In the somatic mutated cohort (n: 26) the ORR was 31% and the CBR18w of 44%. All PR were achieved in sBRCA1/2m carriers (ORR, 50%) and the median PFS of 6.3 months (90% CI 4.4–NA). No responses were observed with ATM or CHEK2 mutations alone [11]. The talazoparib beyond BRCA (TBB) is an open-label phase II trial, to evaluate talazoparib in patients with pretreated HER2-ve ABC (n = 13) or other solid tumors (n = 7) with mutations in HRR genes other than BRCA1 and BRCA2. In patients with BC the ORR was 31% and the CBR≥6m was 54%. All patients with gPALB2m had treatment-associated tumor regression [12].
In the randomised phase III OlympiAD trial, olaparib monotherapy significantly increased PFS compared with CT treatment of physician’s choice in patients with gBRCAm, HER2-ve ABC. The median PFS was 7.4 months versus 4.2 months with a HR 0.58 (p < 0.001). The phase III EMBRACA trial compared talazoparib to the physician’s choice of CT in gBRCAm patients with unresectable or ABC. The median PFS was 8.6 months for those treated with talazoparib compared with 5.6 months in those treated with CT (HR 0.54, p < 0.0001) with an ORR of 62.6% with talazoparib compared with 27.2% with CT [14].
LuciA-15 is the first prospective real-world evidence in a specific subgroup of patient that treatment decision-making is based on specific biomarkers. This is the first evidence in Latin-American population, with appropriate follow-up and good quality and complete data collected. A limited number of patients included in our trial and few number of patients with non-BRCAm tumors did not allow subgroup analysis with statistical value.
Conclusion
In conclusion, LuciA-15 adds important and valuable evidence on real life PARPi management and patient profile in ABC, and reinforces the hard data already published in RCTs. More evidence is necessary to expand the indication in germline or somatic non-BRCAm tumors, in the meantime case by case discussion with a multidisciplinary team and molecular tumor board is mandatory.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
This work received no specific grant from any funding agency.
References
- 1.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361(15):1475e85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 2.Castroviejo-Bermejo M, Cruz C, Llop-Guevara A, et al. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol Med. 2018;10(12):e9172. doi: 10.15252/emmm.201809172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knijnenburg TA, Wang L, Zimmermann MT, et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep. 2018;23(1):239–254.e236. doi: 10.1016/j.celrep.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurvitz SA, Gonçalves A, Rugo HS, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncologist. 2019;24:1–12. doi: 10.1634/theoncologist.2019-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turk A, Wisinski KB. PARP inhibition in BRCA-mutant breast cancer. Cancer. 2018;124(12):2498–2506. doi: 10.1002/cncr.31307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley D, Alumkal JJ, Wyatt AW, et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017;7(9):999–1005. doi: 10.1158/2159-8290.CD-17-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelmon KA, Fasching PA, Couch FJ, et al. Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: phase IIIb LUCY interim analysis. Eur J Cancer. 2021;152:68–77. doi: 10.1016/j.ejca.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Dawood S. ESMO 2021 Eposter Display. Dubai: Mediclinic City Hospital, Mohammed Bin Rashid University of Medicine and Health Sciences; Use of PARPi among patients with advanced breast cancer: results from a real world database; p. 313. [Google Scholar]
- 10.Batalini F, Madison R, Pavlick DC, et al. Analysis of real-world (RW) data for metastatic breast cancer (mBC) patients (pts) with somatic BRCA1/2 (sBRCA) or other homologous recombination (HR)-pathway gene mutations (muts) treated with PARP inhibitors (PARPi) J Clin Oncol. 2021;39(15_suppl):10512–10512. doi: 10.1200/JCO.2021.39.15_suppl.10512. [DOI] [Google Scholar]
- 11.Tung NM, Robson ME, Ventz S, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 12.Gruber JJ, Afghahi A, Timms K, et al. A phase II study of talazoparib monotherapy in patients with wild-type BRCA1 and BRCA2 with a mutation in other homologous recombination genes. Nat Cancer. 2022;3(10):1181–1191. doi: 10.1038/s43018-022-00439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523e33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 14.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753e63. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]