Summary
Background
Open partial pancreatoduodenectomy (OPD) represents the current gold standard of surgical treatment of a wide range of diseases of the pancreatic head but is associated with morbidity in around 40% of cases. Robotic partial pancreatoduodenectomy (RPD) is being used increasingly, yet, no randomised controlled trials (RCTs) of RPD versus OPD have been published, leaving a low level of evidence to support this practice.
Methods
This investigator-initiated, exploratory RCT with two parallel study arms was conducted at a high-volume pancreatic centre in line with IDEAL recommendations (stage 2b). Patients scheduled for elective partial pancreatoduodenectomy (PD) for any indication were randomised (1:1) to RPD or OPD with a centralised web-based tool. The primary endpoint was postoperative cumulative morbidity within 90 days, assessed via the Comprehensive Complication Index (CCI). Biometricians were blinded to the intervention, but patients and surgeons were not. The trial was registered prospectively (DRKS00020407).
Findings
Between June 3, 2020 and February 14, 2022, 81 patients were randomly assigned to RPD (n = 41) or OPD (n = 40), of whom 62 patients (RPD: n = 29, OPD: n = 33) were analysed in the modified intention to treat analysis. Four patients in the OPD group were randomised, but did not undergo surgery in our department and one patient was excluded in the RPD group due to other reason. Nine patients in the RPD group and 3 patients in the OPD were excluded from the primary analysis because they did not undergo PD, but rather underwent other types of surgery. The CCI after 90 days was comparable between groups (RPD: 34.02 ± 23.48 versus OPD: 36.45 ± 27.65, difference in means [95% CI]: −2.42 [−15.55; 10.71], p = 0.713). The RPD group had a higher incidence of grade B/C pancreas-specific complications compared to the OPD group (17 (58.6%) versus 11 (33.3%); difference in rates [95% CI]: 25.3% [1.2%; 49.4%], p = 0.046). The only complication that occurred significantly more often in the RPD than in the OPD group was clinically relevant delayed gastric emptying. Procedure-related and overall hospital costs were significantly higher and duration of surgery was longer in the RPD group. Blood loss did not differ significantly between groups. The intraoperative conversion rate of RPD was 23%. Overall 90-day mortality was 4.8% without significant differences between RPD and OPD.
Interpretation
In the setting of a very high-volume centre, both RPD and OPD can be considered safe techniques. Further confirmatory multicentre RCTs are warranted to uncover potential advantages of RPD in terms of perioperative and long-term outcomes.
Funding
Federal Ministry of Education and Research (BMBF: 01KG2010).
Keywords: Pancreas, Open, Robotic, Partial pancreatoduodenectomy, Randomised controlled trial
Research in context.
Evidence before this study
We searched PubMed, Web of Science, and the Cochrane Central Register of Controlled Trials from inception to Aug 18, 2023. The patient-intervention-comparison-outcome (PICO) scheme was used with the keywords “robot” and “pancreatic surgery”, including mesh terms and synonyms. We searched the reference lists of the retrieved articles and the Evidence Map of Pancreatic Surgery for additional publications. No restrictions were applied to language or date of publication.
No randomised controlled trial comparing robotic pancreatoduodenectomy (RPD) versus open pancreatoduodenectomy (OPD) was identified. Since 2003, several small non-randomised and largely retrospective comparative single-centre studies and case series have investigated RPD compared to OPD yielding inconsistent results. Two meta-analyses of the limited data derived from few mainly observational studies available (Peng et al., 2017 and Zhao et al., 2018) suggested superiority of RPD regarding wound infection rate, margin positivity rate, length of hospital stay, intraoperative blood loss and overall morbidity. Study populations and the definition of endpoints were heterogeneous or were not sufficiently defined in previous studies. Thus, data might be affected by selection and attrition bias. Accordingly, there is only a low level of evidence for preferring one or the other approach.
Added value of this study
We found no difference in overall postoperative 90-day morbidity indicated by the Comprehensive Complication Index between robotic and open pancreatoduodenectomy. Clinically relevant delayed gastric emptying was the only complication that occurred significantly more often in the robotic approach. Moreover, duration of surgery was longer in the robotic than in the open group and RPD incurred higher procedure-related and overall hospital costs. No difference was observed regarding intraoperative blood loss, postoperative wound infection rate and length of hospital stay.
Implications of all the available evidence
This study is the first investigator-initiated, exploratory, randomised controlled trial comparing robotic and open pancreatoduodenectomy. We showed feasibility of patient recruitment and comparable overall postoperative morbidity for both approaches. The findings of some previous small non-randomised studies in favour of robotic resection concerning wound infection, blood loss and length of hospital stay did not hold true in the exploratory randomised setting. To establish potential benefits and clinical value of RPD over OPD, further large-scale confirmatory randomised controlled trials are imperative.
Introduction
Partial pancreatoduodenectomy (PD) is the indicated treatment option for localised pancreatic head cancer as well as a variety of benign and malignant conditions affecting the pancreatic head and periampullary region. Through advancements in surgical techniques and perioperative care, specialised high-volume centres have successfully reduced the perioperative mortality rate of pancreatic resection to below 3%.1 However, postoperative complications remain a significant concern, affecting approximately 40% of patients. Therefore, there is an urgent need to develop strategies aimed at reducing postoperative complications.
Minimally invasive surgery, initially introduced in the 1980s, has revolutionised surgery by reducing postoperative morbidity in a wide range of surgical procedures, including complex ones.2 In pancreatic surgery, minimally invasive distal pancreatectomy has already gained support from the 2019 Miami evidence-based guidelines,3 as less blood loss and faster or comparable functional recovery have been shown compared to the open approach in 3 RCTs and meta-analyses without significant drawbacks.4, 5, 6
Laparoscopic PD, with the first reported case dating back to 19947 has shown promising results in terms of shorter hospital stay or fewer complications in some studies.8, 9, 10, 11 However, other studies have reported higher morbidity and mortality rates associated with this approach.12,13 Furthermore, the learning curve for laparoscopic PD is quite long and can only be overcome in very high-volume centres,14 which has caused controversial opinions regarding this approach. In 2001, Giulianotti et al. pioneered robotic PD (RPD), capitalizing on the enhanced visualisation and three-dimensional dexterity of instruments of the robotic platform.15 RPD has a shorter learning curve compared to the laparoscopic technique16,17 and it has the potential to facilitate minimally-invasive PD, particularly during the challenging reconstruction phase.
To date, no RCTs have directly compared RPD to OPD or LPD. Existing evidence primarily relies on non-randomised, often retrospective and single-centre studies yielding inconsistent results.18, 19, 20, 21, 22, 23 Overall, based on available low-quality studies, RPD might be associated with comparable or superior surgical outcomes to OPD in terms of postoperative complications and 30-day mortality rates.24 However, for the efficacy of robotic PD to be established, RCTs with standardised reporting of well-defined and validated endpoints are necessary. Thus, the objective of this investigator-initiated exploratory RCT was to evaluate cumulative postoperative complications, safety, feasibility of recruitment, and costs associated with RPD compared to OPD.
Methods
Study design
EUROPA was an investigator-initiated, exploratory, open-label RCT with two parallel study groups. The trial was conducted at the Department of General, Visceral and Transplantation Surgery, University Hospital Heidelberg, Germany in accordance with §15 of the German Medical Association's professional code (Berufsordnung der Bundesärztekammer) and the ethical principles outlined in the Declaration of Helsinki. Prior to enrolment, written informed consent was obtained from all study participants. The trial has been approved by the independent ethics committee of the Medical Faculty of the University of Heidelberg (Ethikkommission Medizinische Fakultät Heidelberg; S-025/2020, 3 February 2020) and was registered with the German Clinical Trial Register (DRKS00020407, 9 March 2020; UTN U1111-1245-8931, 30 December 2019) before enrolling the first patient. The study protocol has been published,25 and reporting of this trial adheres to the recommendations outlined in the CONSORT guidelines. No formal protocol deviations or amendments were carried out during trial conduct. Some of the patients included in this trial have already been evaluated in a retrospective analysis from our center.26
Participants
The study included adult patients who were deemed eligible for elective PD for any indication, were able to understand the individual consequences of the clinical trial and judged suitable for both RPD and OPD by the treating pancreatic surgeon at our high-volume pancreatic surgery centre. The following exclusion criteria were defined: (1) borderline or unresectable carcinoma of the pancreatic head, as defined in the National Comprehensive Cancer Network guidelines,27 (2) presence of distant metastases, (3) American Society of Anesthesiologists (ASA) score >3, (4) participation in another interventional trial that could interfere with the intervention and outcome of this trial and (5) anticipated language difficulties or lack of compliance.
Randomisation and blinding
Eligible patients were randomly assigned (1:1) to receive either RPD or OPD. Randomisation (with block size of 4) was conducted using a centralised web-based tool (randomizer.at), provided by the Institute of Medical Informatics, Statistics and Documentation of the Medical University of Graz. Only authorised trial personnel, not involved in patient treatment, conducted the randomisation process. Due to the nature of the intervention, it was not feasible to blind participants, operating surgeons, data collectors, or outcome assessors to the treatment allocation. However, outcome assessment was conducted by trained study personnel who were independent of the surgical or ward team. To ensure unbiased data analysis, the biometricians were blinded to the study group assignment.
Procedures
Only surgeons who demonstrated sufficient proficiency were permitted to perform the surgical procedures within this trial. Proficiency in the open group was defined as having conducted ≥40 OPDs, while proficiency in the robotic group was defined as having performed ≥40 RPDs. The criterion of a minimum of 40 RPDs was determined based on research on learning curves for RPD.28, 29, 30 Two main surgeons of the trial performed both RPDs and OPDs, while the other surgeons (n = 13) exclusively performed the open procedures. The robotic surgeons had extensive experience, having completed each more than 500 pancreatic procedures. All surgeons were board-certified, specialised in HPB surgery and had a clinical experience of ≥8 years.
In both groups, after exclusion of hepatic and peritoneal metastases, PD was performed following conventional resection and reconstruction techniques, including pancreaticojejunostomy, hepaticojejunostomy, and duodenojejunostomy/gastrojejunostomy, in accordance with local standards.31 Decisions regarding the resection or preservation of the pylorus, the extent of lymphadenectomy, additional vascular resections, abdominal drain placement, and abdominal wall closure at the end of the procedure were left to the discretion of the operating surgeon based on individual patient characteristics. In general, drains were inserted in case of soft pancreatic tissue and a small pancreatic main duct diameter when a high risk for a pancreatic fistula was anticipated. Drain management was standardised, with removal scheduled no earlier than the second postoperative day, based on drain fluid volume and pancreatic enzyme values. The decision for or against vascular reconstruction depended on the individual patient. If vascular infiltration was unexpectedly assumed intraoperatively, vascular resection with reconstruction was performed if the surgeon deemed it necessary. Postoperative mobilisation and oral food intake followed institution's standard of care pathways.
OPD represents the current standard procedure of our institution, while RPD was introduced at the Department of General, Visceral, and Transplantation Surgery in November 2016, accompanied by a tutoring and proctoring program. The RPD procedure has been standardised and is performed as described in the Intuitive® surgical procedure guide, written by members of our department.32
To ensure consistency in peri- and postoperative care, the same standard operating procedures were implemented for both interventions. Additionally, patients in both study groups were accommodated in the same wards, confirming standardised postoperative care.
Outcomes
A comprehensive description of the assessed patient characteristics, study visits, and endpoints can be found in the published trial protocol.25 The primary endpoint of the EUROPA trial was cumulative morbidity within 90 days after PD, which was assessed by means of the CCI. The CCI is based on the well-established Clavien–Dindo classification of postoperative complications, enabling objective assessment of cumulative postoperative morbidity.
As a secondary outcome of the study feasibility of recruitment was assessed. Based on a conservative calculation of patient numbers for PD at our center, inclusion and exclusion criteria of this trial and study recruitment experiences from previous trials a recruitment of n = 80 patients within 18 months was defined for this endpoint. Further endpoints included duration of surgery, intraoperative blood loss, serious adverse intraoperative events, conversion rate in the robotic group, rate of microscopically complete margin clearance (>0.1 cm margin clearance, R0 (CRM-); ≤0.1 cm (R0 (CRM+)), rate of microscopic margin involvement (R1), total number of resected lymph nodes, and number of tumour-positive lymph nodes in patients with malignant tumours, mortality within 90 days, Quality of Recovery (QoR-15), time to functional recovery, total length of intensive care unit stay within 90 days, length of hospital stay for index operation, rate of superficial and deep surgical site infections (according to CDC) within 30 days, rate and severity of postoperative pancreas specific complications (according to ISGPS and ISGLS): pancreatic fistula (POPF), postpancreatectomy haemorrhage (PPH), delayed gastric emptying (DGE), biliary leak, chyle leak within 90 days, rate of non-surgical re-interventions within 90 days, rate of re-operations within 90 days from index operation, number of hospital re-admissions within 90 days, pain (Numeric Rating Scale) on POD 2 and 4, health-related quality of life measured by the SF-36, procedure-related costs and overall in-hospital costs without acquisition cost of the robotic system, evaluation of surgeons’ mental workload/stress.
These outcomes were assessed to evaluate various aspects of surgical outcomes, postoperative complications, recovery, quality of life, and economic factors related to the interventions.
Statistical analysis
The primary endpoint was the CCI within 90 days after the intervention. As a primary result, the 95% confidence interval (CI) for the difference in the means between the two groups based on the approximate normal distribution of the CCI is reported.
Given that EUROPA is an exploratory trial no formal sample size calculation was performed. However, a clinically relevant mean difference for the CCI of 10 and a standard deviation of 20 was assumed in the planning of this trial. A margin of 7.5 CCI points is tolerated as noninferior as this corresponds to the occurrence of less than one major complication and is judged as a clinically irrelevant difference. Including 64 patients (32 per group) in the analysis, this effect size could be estimated with a 95% CI of [0.00, 19.99]. It was envisioned that approximately eight patients per group will undergo neither RPD nor OPD due to inoperability, indication for another type of surgery (ascertained during the exploratory stage of the operation) or other reasons. Therefore, to reach the number of 64 patients to be analysed, recruitment of 80 patients was planned. An interim analysis was not performed in our study. Data analysis is based on three analysis sets. The modified intention-to-treat (mITT) set comprises all patients in the group to which they were randomised (converted patients remain in the RPD group) and serves as the primary analysis set. All data presented in the results section are based on the mITT set if not stated otherwise. Patients that did not undergo surgery at all or that received an operation other than PD or total pancreatectomy (explorative laparotomy, diagnostic laparoscopy, distal pancreatectomy etc.) were excluded from this and the other analysis sets.
The per-protocol (PP) set consists of all patients treated per protocol without major protocol violation and without conversions. No missing data was imputed in the PP set. In the PP set, certain patients were excluded to ensure a more rigorous analysis and to focus on patients who were treated with close adherence to the protocol. Patients with total pancreatectomy were included in the mITT set but were excluded from the PP set due to differences in postoperative complications associated with total pancreatectomy compared to PD. Furthermore, patients whose surgeons did not possess the required surgical expertise were excluded from the PP set. In the RPD group, patients who underwent conversion to open surgery or underwent open surgery contrary to randomisation were also excluded from the PP set.
In addition, the as-treated set was analysed, which considered the patients in the group in which they were finally treated (i.e. converted patients and patients with a primarily open procedure contrary to randomisation to RPD in the OPD group).
For the primary analysis of CCI in the mITT set, missing values were imputed by mean imputation and as sensitivity analyses worst- and best-case-scenarios in the sense of the study results were performed by imputing missing values with the lowest and the highest value of the corresponding treatment group, respectively.
The secondary endpoints were described as mean values along with standard deviations, median values, quartiles, minimum and maximum for continuous endpoints, and relative and absolute frequencies for categorical endpoints, stratified for treatment groups. For comparison of continuous endpoints an unpaired t-test was performed in case of normally distributed outcomes and a non-parametric Mann-Whitney-U test otherwise and for categorical endpoints a chi-squared test was performed. 95% confidence intervals for the difference in means and the difference in rates were reported for normal and binomial distributed endpoints, respectively. Quality of life scores were additionally presented as radar plots. The safety analysis included calculation of frequencies and rates of both minor (Clavien–Dindo class I and II) and major complications (Clavien–Dindo class III to V). All analyses were exploratory, having only descriptive character (including descriptive p values), and were conducted using SAS version 9.4. The trial was overseen by a data safety monitoring board.
Role of the funding source
The funder of the study had no involvement in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
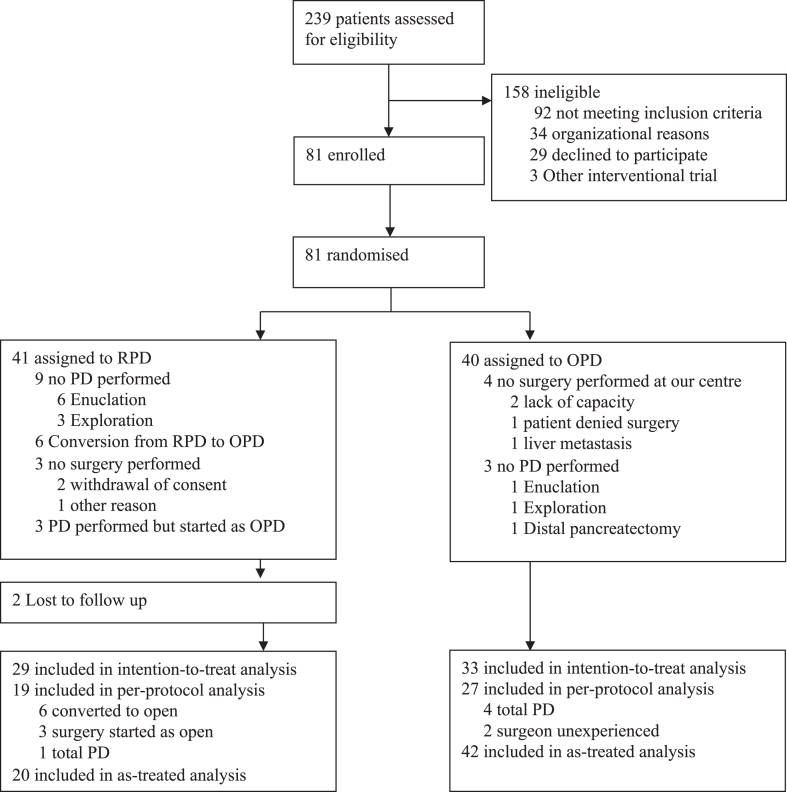
Between June 3, 2020, and February 14, 2022, 81 patients were randomly assigned to receive RPD (n = 41) or OPD (n = 40). Four patients in the OPD group were randomised at initial outpatient presentation to our pancreas department, but did ultimately not undergo surgery in our department (surgery in another hospital due to lack of capacity (n = 2), patient denied surgery (n = 1), liver metastasis diagnosed in preoperative imaging (n = 1)) and one patient was excluded in the RPD group due to other reason. Nine patients in the RPD group and 3 patients in the OPD were excluded from the primary analysis because they did not undergo PD, but rather underwent exploratory laparoscopy/laparotomy (RPD: n = 3; OPD: n = 1), enucleation (RPD: n = 6; OPD: n = 1) or distal pancreatectomy (OPD: n = 1) (Table 1). In three patients in the RPD group, surgery was started as open surgery contrary to randomisation due to organisational reasons (robotic system unavailable (n = 2), decision of surgeon (n = 1)) and 6 patients were converted to open surgery during the procedure, however, none of these conversions was necessary in an emergency setting (Reasons for conversion see Supplemental Table S1). In the RPD group two patients were lost to follow up before the primary endpoint could be evaluated (Fig. 1).
Table 1.
Baseline characteristics of the modified intention-to-treat population.
| RPD n = 29 | OPD n = 33 | |
|---|---|---|
| Age (years)a | 64.7 ± 9.8 | 62.6 ± 11.4 |
| Sex ratio (male:female) | 17:12 (58.6:41.4%) | 16:17 (48.5:51.5%) |
| BMIa | 26.9 ± 4.6 | 26.6 ± 6.1 |
| ASA score | ||
| II | 18 (62.1%) | 20 (60.6%) |
| III | 11 (37.9%) | 13 (39.4%) |
| Smoking | ||
| Present | 6 (20.7%) | 7 (21.2%) |
| Past | 9 (31.0%) | 12 (36.4%) |
| Never | 14 (48.3%) | 14 (42.4%) |
| Alcohol consumption | ||
| Yes, often | 2 (6.9%) | 1 (3.0%) |
| Yes, occasionally | 2 (6.9%) | 9 (27.3%) |
| Yes, rarely | 16 (55.2%) | 12 (36.4%) |
| Past | 3 (10.3%) | 6 (18.2%) |
| Never | 4 (13.8%) | 5 (15.2%) |
| Unknown | 2 (6.9%) | 0 (0.0%) |
| Previous abdominal surgeries | 17 (58.6%) | 18 (54.5%) |
| Preoperative biliary drainage | 9 (31.0%) | 7 (21.2%) |
| Updated charlson comorbidity index points (N)a | 1.7 ± 1.5 | 1.7 ± 1.4 |
Values in parentheses are percentages unless indicated otherwise.
Values in parentheses are percentages unless indicated otherwise.
Values are mean ± SD; ASA: American Society of Anaesthesiologists, BMI, body mass index.
Fig. 1.
Trial profile.
The baseline characteristics of the trial participants are presented in Table 1. Sex, age, body mass index, and comorbidities were well balanced between the groups.
The primary endpoint, cumulative postoperative complications according to the CCI within 90 days after the intervention, did not differ between RPD and OPD (34.02 ± 23.48 versus 36.45 ± 27.65, difference in means [95% CI]: −2.42 [−15.55; 10.71], p = 0.713, Table 2). Two patients randomised to the RPD group were lost to follow up (after 30 and 70 days). For these patients, the primary endpoint CCI was imputed. The sensitivity analyses (best- and worst-case scenario in the mITT set, per-protocol set and as-treated set) confirmed these results. The results of the PP set regarding the primary and secondary endpoints can be found in the Supplementary Material.
Table 2.
Postoperative course in the modified intention-to-treat population.
| RPD n = 29 | OPD n = 33 | Difference with 95% CI | p value | |
|---|---|---|---|---|
| CCI (mean imputation)j | 34.02 ± 23.48 | 36.45 ± 27.65 | −2.42 [−15.55; 10.71] | 0.713 |
| Mortality within 90 daysa | 0 (0.0%) | 3 (9.1%) | ||
| QoR-15 | ||||
| Preoperativelyb | 117 ± 33 | 107 ± 29 | 10 [−10; 30] | 0.312 |
| POD4c | 82 ± 29 | 92 ± 30 | −10 [−27; 6] | 0.217 |
| Change from preoperatively to POD 4d | −32 ± 30 | −19 ± 27 | −13 [−33; 7] | 0.183 |
| Time to functional recovery (days)e,k | 17 ± 15 | 13 ± 8 | 4 [−2; 11] | 0.163 |
| Length of stay in the ICU (days)f,k | 0 (0–4) | 0 (0–0) | 0.099 | |
| Length of hospital stay (days)j | 17 (11–27) | 13 (9–19) | 0.177 | |
| Superficial SSI within 30 days | 3 (10.3%) | 3 (9.4%) | 1.0% [−14.0%; 16.0%] | 0.899 |
| Number of patients with a least one pancreas specific complication | 18 (62.1%) | 15 (45.5%) | 16.6% [−7.9%; 41.1%] | 0.191 |
| Number of patients with at least one major (grade B/C) pancreas-specific complication | 17 (58.6%) | 11 (33.3%) | 25.3% [1.2%; 49.4%] | 0.046 |
| POPF grade B/C | 11 (37.9%) | 7 (21.2%) | 16.7% [−5.8%; 39.2%] | 0.148 |
| Biliary leak grade B/C | 5 (17.2%) | 3 (9.1%) | 8.2% [−8.7%; 25.0%] | 0.339 |
| PPH grade B/C | 4 (13.8%) | 1 (3.0%) | 10.8% [−3.1%; 24.6%] | 0.120 |
| DGE grade B/C | 10 (34.4%) | 2 (6.0%) | 28.4% [9.3%; 47.5%] | 0.005 |
| Chyle leak grade B/C | 2 (6.9%) | 1 (3.0%) | 3.9% [−7.1%; 14.8%] | 0.479 |
| Number of patients with non-surgical reintervention | 15 (51.7%) | 10 (30.3%) | 21.4% [−2.6%; 45.4%] | 0.086 |
| Type of reintervention | ||||
| CT guided drain placement | 20 (50.0%) | 11 (44.0%) | ||
| Angiography (with/without stent/coiling) | 4 (10.0%) | 0 (0.0%) | ||
| Endoscopy | 0 (0.0%) | 1 (4.0%) | ||
| Other | 7 (21.2%) | 2 (8.0%) | ||
| Number of patients with re-operation(s) | 4 (13.8%) | 5 (15.2%) | −1.4% [−18.9%; 16.2%] | 0.880 |
| Number of patients with readmission(s) | 5 (17.2%) | 5 (16.1%) | 1.1% [−17.8%; 20%] | 0.908 |
| Pain at restj | ||||
| POD2g | 1.3 ± 0.9 | 1.3 ± 0.9 | 0 [−0.5; 0.5] | 0.919 |
| POD4h | 1.1 ± 0.9 | 1.1 ± 1.6 | 0 [−0.8; 0.8] | 0.926 |
| Pain at movementj | ||||
| POD2g | 2.9 ± 1.3 | 2.9 ± 1.4 | 0 [−0.8; 0.8] | 0.974 |
| POD4h | 2.4 ± 1.4 | 2.6 ± 1.6 | −0.2 [−1.2; 0.8] | 0.684 |
| Procedure-related costs (Euro)j | 4744 ± 1254 | 866 ± 459 | 3878 [3410; 4347] | <0.001 |
| Overall inpatient hospital costs (Euro)i,j | 33,502 ± 22,314 | 21,429 ± 12,427 | 12,073 [2932; 21,213] | 0.011 |
Values in parentheses are percentages unless indicated otherwise.
Values in parentheses are percentages unless indicated otherwise.
90-day mortality: patients lost to FU were censored and counted as no death.
QoR-15 score preoperatively: n = 9 missing in RPD and n = 13 missing in OPD group.
QoR-15 score POD 4: n = 4 missing in RPD and n = 8 missing in OPD group.
QoR-15 score Change from preoperatively to POD 4: n = 10 missing in RPD and n = 17 missing in OPD group.
Time to functional recovery including 26 RPD and 31 OPD patients who achieved functional recovery.
Including 12 patients in the RPD and 8 patients in the RPD group with ICU stay and the other patients with lengths of intensive care unit stay of 0 days.
Pain at rest and at movement at POD 2: n = 6 missing in RPD and n = 5 missing in OPD group.
Pain at rest: n = 9 and at movement n = 10 in RPD and n = 10 missing at rest and at movement in OPD group.
Overall in-hospital costs n = 1 missing in OPD group.
Values are mean ± SD; Differences with p values < 0.05 were deemed statistically significant and these p values are presented in bold; CCI: Charlson comorbidity index, QoR-15: Quality of Recovery-15 score, ICU: intensive care unit, SSI: surgical site infection, POPF: postoperative pancreas fistula, POD: postoperative day, NASA: National Aeronautics and Space Administration.
Values are median (interquartile range).
The prespecified recruitment goal of n = 80 patients within 18 months was almost achieved, with a recruitment period of 19.5 months.
RPD was more expensive than OPD both regarding procedure-related costs and all inpatient hospital costs until postoperative day 90 (procedure related costs (Euro): 4744 ± 1254 versus 866 ± 459, difference in means [95% CI]: 3878 [3410; 4347], p < 0.001; overall inpatient hospital costs (Euro) 33,502 ± 22,314 versus 21,429 ± 12,427, difference in means [95% CI]: 12,073 [2932; 21,213], p = 0.011).
Characteristics of the surgical procedure are presented in Table 3. Duration of surgery was longer in the RPD compared to the OPD group (431 ± 103 versus 367 ± 106 min, difference in means [95% CI]: 64 [10; 118], p = 0.021). Blood loss and the number of patients with intraoperative blood transfusions did not differ relevantly between both groups (742 ± 512 versus 814 ± 685 ml, difference in means [95% CI]: −72 [−385; 240], p = 0.645; 3 (10.3%) versus 2 (6.1%); difference in rates [95% CI]: 4.3% [−9.5%; 18.0%], p = 0.536). Pylorus preserving PD was performed less frequent and pylorus resection more frequent in RPD (5 (17.2%) and 21 (72.4%)) compared to OPD (18 (54.5%) and 13 (39.4%)), p = 0.010). Extent of resection was comparable between groups regarding performance of Triangle procedure, arterial and venous resection, multivisceral resection, and number of resected lymph nodes (29 ± 14 versus 26 ± 9, difference in means [95% CI]: 3 [−6; 11] (p = 0.536)).
Table 3.
Intraoperative characteristics in the modified intention-to-treat population.
| RPD n = 29 | OPD n = 33 | Difference with 95% CI | p value | |
|---|---|---|---|---|
| Duration of surgery (minutes)a | 431 ± 103 | 367 ± 106 | 64 [10; 118] | 0.021 |
| Serious intraoperative complicationd | 0 (0.0%) | 1b (3.0%) | ||
| Blood loss (ml)a | 742 ± 512 | 814 ± 685 | −72 [−385; 240] | 0.645 |
| Blood transfusion | 3 (10.3%) | 2 (6.1%) | 4.3% [−9.5%; 18.0%] | 0.536 |
| Degree of stomach resection | 0.010 | |||
| Pylorus-preserving | 5 (17.2%) | 18 (54.5%) | ||
| Pylorus-resecting | 21 (72.4%) | 13 (39.4%) | ||
| Classicc | 2 (6.9%) | 0 (0.0%) | ||
| (Sub)total gastrectomy | 1 (3.4%) | 2 (6.1%) | ||
| Degree of SMA dissection | 0.170 | |||
| Inoue level 1 | 6 (20.7%) | 11 (33.3%) | ||
| Inoue level 2 | 20 (69.0%) | 15 (45.5%) | ||
| Inoue level 3 | 3 (10.3%) | 7 (21.2%) | ||
| Triangle procedure performed | 12 (41.4%) | 12 (36.4%) | 5.0% [−19.3%; 29.3%] | 0.686 |
| Arterial resection performed | 0 (0.0%) | 2 (6.1%) | ||
| Venous resection performed | 5 (17.2%) | 3 (9.1%) | 8.2% [−8.7%; 25.0%] | 0.339 |
| Number of resected lymph nodes (N)a | 29 ± 14 | 26 ± 9 | 3 [−6; 11] | 0.536 |
| Resection of additional organs performed beyond PD | 4 (13.8%) | 6 (18.2%) | −4.4% [−22.6%; 13.8%] | 0.639 |
| Texture of pancreas | 0.068 | |||
| Soft | 13 (44.8%) | 12 (36.4%) | ||
| Medium | 12 (41.4%) | 8 (24.2%) | ||
| Hard | 4 (13.8%) | 13 (39.4%) | ||
| Size of pancreatic duct at transection site | 0.719 | |||
| ≤3 mm | 11 (37.9%) | 14 (42.4%) | ||
| >3 mm | 18 (62.1%) | 19 (57.6%) | 4.5% [−19.9%; 28.9%] | |
| Drain insertion | 27 (93.1%) | 21 (63.6%) | 29.5% [10.6%; 48.3%] | 0.006 |
| NASA total scorea,d | 50.4 ± 13.7 | 49.7 ± 16.9 | 0.8 [−7.2; 8.7] | 0.845 |
Values in parentheses are percentages unless indicated otherwise.
Values are mean ± SD; Differences with p values < 0.05 were deemed statistically significant and these p values are presented in bold; in cases with zero count in one of the treatment groups no p values are presented.
Case with intraoperative venous gastric stasis.
1/3 Resection of stomach.
Calculation of NASA Total score was slightly modified as the weighting of the individual components of the score was not requested by the surgeons., SMA: superior mesenteric artery, PD: pancreatoduodenectomy.
Texture characteristic of the pancreas and size of pancreatic duct at transection site were similar between groups. Surgical drains were placed in more RPD than OPD procedures (27 (93.1%) versus 21 (63.3%); difference in rates [95% CI]: 29.5% [10.6%; 48.3%], p = 0.006).
Self-evaluation of the surgeon's mental workload/stress according to the modified National Aeronautics and Space Administration Task Load Index [27] at the end of surgery revealed no difference (50.4 ± 13 versus 49.7 ± 16.9, difference in means [95% CI]: 0.8 [−7.2; 8.7], p = 0.845).
Histopathological analysis of the surgical specimen revealed 34 patients (54.8%) with a malignant disease, 18 patients (29.0%) with a benign neoplastic lesion and 10 patients (16.1%) with a benign non-neoplastic lesion. The most common type of malignant disease observed was pancreatic ductal adenocarcinoma (PDAC) which was identified in 22 patients. Other types of malignancies observed included intraductal papillary-mucinous carcinoma (IPMN), ampullary cancer, intrapancreatic cholangiocarcinoma, and neuroendocrine tumour (NET, Table 4).
Table 4.
Histopathology in the modified intention-to-treat population.
| RPD n = 29 | OPD n = 33 | p value | |
|---|---|---|---|
| Malignant disease | 16 (55.2%) | 18 (54.5%) | |
| Benign neoplastic lesion | 9 (31.0%) | 9 (27.3%) | |
| Benign non-neoplastic lesion | 4 (13.8%) | 6 (18.2%) | |
| Pancreatic cancer subtype according to the current classification of the WHO | |||
| Pancreatic ductal adenocarcinoma | 12 (75.0%) | 10 (55.6%) | |
| Intraductal papillary-mucinous carcinoma | 0 (0.0%) | 3 (16.7%) | |
| Ampullary cancer | 1 (6.3%) | 2 (11.1%) | |
| Intrapancreatic cholangiocarcinoma | 1 (6.3%) | 2 (11.1%) | |
| Neuroendocrine tumour | 1 (6.3%) | 0 (0.0%) | |
| Other | 1 (6.3%) | 1 (6.7%) | |
| Resection | 0.154 | ||
| R0 CRM− | 7 (43.8%) | 9 (50.0%) | |
| R0 CRM+ | 6 (37.5%) | 9 (50.0%) | |
| R1 | 3 (18.8%) | 0 (0.0%) |
Values in parentheses are percentages unless indicated otherwise; CRM: Circumferential resection margin. No p values were calculated regarding histopathological results as these cannot be influenced by the trial intervention.
No clear difference was found between the RPD and OPD groups concerning the presence of clear resection margins. Three out of 16 patients with a malignant disease in the RPD group revealed an R1 resection in histopathology whereas no patient in the OPD group out of 18 with a malignant disease presented with tumour infiltration of the resection margin.
During the 90-day postoperative period, no deaths occurred in the RPD group while three patients in the OPD group died (0 (0.0%) versus 3 (9.1%)). The causes of death were as follows: One patient experienced a major cardiac event resulting in ischemic heart failure and died at the second postoperative day, another patient succumbed to surgical complications leading to septic shock and multiorgan failure on the 12th postoperative day, and the third patient died on the 83rd postoperative day due to recurrent disease with liver metastasis.
Quality of Recovery assessed via the quality of recovery questionnaire QoR-15 and time to functional recovery revealed no clear differences between groups. There was a trend towards longer intensive care unit and overall hospital stays in the RPD group compared to the OPD group (0 (0–4) versus 0 (0–0) and 17 (11–27) versus 13 (9–19), p = 0.177).
In terms of superficial SSI, there were comparable rates between the RPD and OPD groups (3 (10.3%) versus 3 (9.4%); difference in rates [95% CI]: 1.0% [−14.0%; 16.0%], p = 0.899). Similarly, there were no differences in the overall rates of pancreas-specific complications between the two groups (18 (62.1%) versus 15 (45.5%); difference in rates [95% CI]: 16.6% [−7.9%; 41.1%], p = 0.191). However, the RPD group had a higher incidence of grade B/C pancreas-specific complications compared to the OPD group (17 (58.6%) versus 11 (33.3%); difference in rates [95% CI]: 25.3% [1.2%; 49.4%], p = 0.046). Also, a higher number of RPD patients experienced a clinically relevant DGE compared to OPD patients (10 (34.4%) versus 2 (6.0%); difference in rates [95% CI]: 28.4% [9.3%; 47.5%], p = 0.005). POPF grade B or higher occurred in 11 (37.9%) RPD patients compared to 7 (21.2%) OPD patients (difference in rates [95% CI]: 16.7% [−5.8%; 39.2%], p = 0.148). Major biliary leak (grade B or C) was detected in 5 (17.2%) patients RPD and 3 (9.1%) patients in the OPD group, while PPH occurred in 4 (13.8%) patients with robotic surgery and in 1 (3.0%) patient with open surgery. Major chyle leak was observed in 2 (6.9%) RPD and 1 (3.0%) OPD patients.
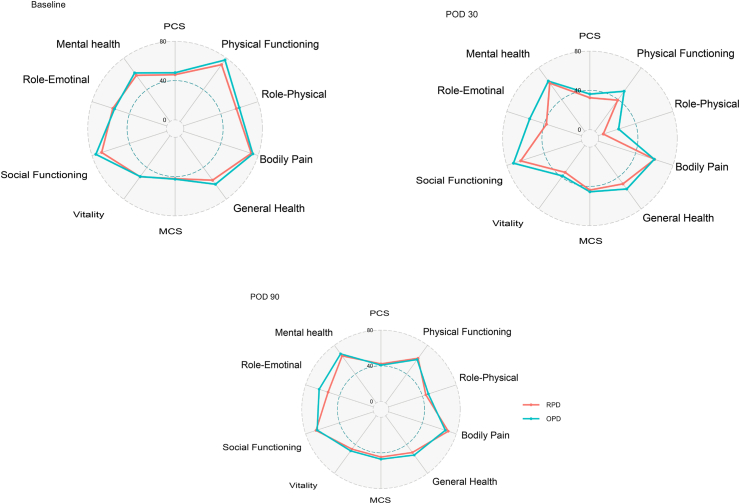
The rates of non-surgical reinterventions, re-operations, and readmissions were similar between the RPD and OPD groups. Pain levels at postoperative day 2 and 4 at rest and during movement were comparable. Furthermore, there was no difference observed in terms of quality of life between the RPD and OPD patients (Fig. 2).
Fig. 2.
Radar plots showing mean values of the two SF-36 sub-scores and the eight domains at baseline, POD30 and POD90. SF-36: short form 36 health survey questionnaire, PCS: physical component summary, MCS: mental component summary.
Discussion
The EUROPA trial is the first exploratory RCT to compare the robotic to the open approach for PD to treat a variety of diseases of the pancreatic head and periampullary region in a high-volume centre. Feasibility of recruitment was almost achieved, with a recruitment period of 19.5 instead of 18 months. The trial results indicate that RPD is comparable to OPD in terms of the primary endpoint, cumulative postoperative morbidity within 90 days. The incidence of individual complications did not show significant differences between the two groups, except for a higher rate of clinically relevant DGE in the RPD group. However, a number of pancreas-specific complications including POPF and biliary leak also showed a trend to occur more frequently in the RPD group. The EUROPA trial found that RPD was significantly more expensive than OPD, both in terms of procedure-related costs and overall inpatient hospital costs until postoperative day 90. The longer duration of surgery in the RPD group compared to the OPD group was also statistically significant. The study did not observe any significant differences between the two groups in terms patient-reported outcome measures (including quality of life and recovery), and surrogate oncological results such as resection margins and lymph node yield.
The IDEAL initiative has recommended a five-steps process towards the safe implementation of a new surgical innovation.33 Accordingly, after several mostly retrospective, non-randomised studies22,34,35 we performed this single-centre feasibility trial as an IDEAL stage 2b (exploration) study focusing on perioperative outcomes with adherence to standardised procedures performed by experienced surgeons, management, and outcome definitions.
The overall 90-day morbidity results in the OPD group of this trial were slightly higher compared to previously published data from other RCTs and non-randomised studies evaluating both OPD10 and RPD,21,36 which is most likely due to i) the rigorous complication recording by experienced study personnel in this single-centre trial with close study visits and monitoring, ii) the performance of not only standard PD, but also multivisceral resections, arterial or venous resections, divestment of the superior mesenteric and hepatic artery, and Triangle resections, and iii) a high risk pancreatic anastomosis due to a small pancreatic duct diameter and soft pancreatic tissue in many patients. Afterall, no difference was observed between the two groups, accordingly our study suggests RPD being equally safe as OPD.
The sole postoperative complication which occurred statistically significantly more often in RPD than OPD patients was clinically relevant DGE in our exploratory trial. This disparity might potentially be attributed to the utilisation of different techniques of gastro-/duodenojejunostomy: In the open approach, gastrojejunostomy was performed as a two-layered running suture anastomosis, while in the robotic approach, a stapler anastomosis was more often used. Based on the literature, the differences regarding pylorus-preservation between RPD and OPD do not explain the observed differences in rates of DGE.37
In our study, we observed a rather high rate of three cases of mortality within 90 postoperative days in the OPD group, while no death occurred in the RPD group. It is important to note, that only one of these deaths can be directly attributed to postoperative surgical complications, whereas death due to a cardiac event and due to very early recurrence with liver metastases are most likely not associated to the surgical approach, but rather associated with comorbidities of the patient and the biology and stage of the malignant tumour, respectively. However, in a previously published retrospective study from our centre, that included patients who were operated on before the surgeon's learning curve was completed, mortality was 6/99 (5.9%) in the RPD group.26 Therefore, in this aspect as well, robotic surgery seems to be on a par with open surgery.
The conversion rate was quite high with 23% in our study, however, no patient was converted in an emergency setting. In previous RCTs comparing laparoscopic PD to OPD, conversion rate was lower or at most the same (2–24%).8,10,11,13,38 Previous non-randomised studies from high-volume centres comparing RPD with OPD report even lower conversion rates of 3–10%. Accordingly, the rather high conversion rate suggests that the learning curve in our study was not yet fully completed.18,19,22 In addition to perioperative safety, costs are also a relevant aspect when comparing the two surgical approaches. While robotic-assisted surgery incurs higher initial costs due to the investment in robotic systems, associated equipment, and longer duration of surgery, it is important to consider the long-term economic impact. Currently, robotic PD is significantly more expensive than open surgery both regarding procedure related costs and overall in-hospital costs, as seen in this trial. However, contrary to our RCT previous studies have suggested that the use of robotic surgery can result in shorter hospital stays,35 which could potentially offset the initial investment by reducing overall healthcare costs in the long run. Afterall, with the progress of technology, cost-effectiveness might be equalised or at least aligned, with more data expected on this regard.
Regarding pathologic results, in this study, with >50% of cases with a malignant disease, no differences in numbers of lymph nodes retrieved, and R0 resections were found. Overall, the number of lymph nodes retrieved was good in both groups and the R1 resection rate in patients with malignant diseases was very low (<10%).39 While some previous studies found a higher number of harvested lymph nodes,22 other studies reported the contrary, with fewer harvested lymph nodes for RPD40 compared to OPD.
Indeed, it is important to acknowledge that the history of robotic surgery is relatively short compared to traditional open surgery. Despite rapid advancements in robotic surgery in recent years, it remains a relatively novel approach even for surgeons in high-volume centres. In smaller and medium-sized hospitals, RPD may not be used at all or only to a very limited extent. On the one hand, the use of robotic-assisted surgery in PD offers several potential advantages over the traditional open approach. Robotic technology provides enhanced visualisation, magnification, and improved maneuverability, which may contribute to better surgical precision. On the other hand, one important aspect to consider is the learning curve associated with the technically demanding RPD. Robotic-assisted surgery requires specific training and expertise, and proficiency in this technique may vary among surgeons. A clear definition of when a surgeon has passed the learning curve for RPD is still somewhat ambiguous, with reported numbers from 8 to 100 procedures for RPD, compared to 2–60 for open PD.16 In a recently published post-hoc multicentre study, the learning cut-offs for feasibility, proficiency, and mastery learning curves for RPD were defined at 15, 62, and 84 procedures after a structured training program.41 In our trial, all surgeons except two surpassed the recommended number of 40 RPDs and OPDs and underwent robotic training. The two cases operated by surgeons who had performed less than 40 PDs before the trial were included in the intention-to-treat but excluded in the per protocol set. Brescia guidelines on minimally invasive pancreatic surgery were not available at that time and should be followed when planning future trials on robotic pancreatic surgery.17 Ultimately, according to current literature even more than 40 procedures are most likely required to achieve full expertise in this operation. In future, with increasing experience and the widespread adoption of robotic technology, it is expected that the learning curve for RPD will continue to improve, making it a feasible option for more surgeons.
Thus, RPD is currently reserved for high-volume centres and individual experienced surgeons. However, as both surgeons, training at the console and robotic systems continue to improve it is to be expected that some major advances will lead to improved patient outcomes in the coming years. It is also possible that the advantage of robotics can be pronounced when a formal Enhanced Recovery after Surgery (ERAS) concept is applied, which was not the case in this study. Rather than settling for non-inferiority at the current state, technological innovation in robotic surgery should aim to enhance standards of care in the future.
Ultimately, the decision between RPD and OPD should be based on a comprehensive evaluation of various factors, including patient-specific characteristics, surgeon expertise, availability of resources, and individual preferences, in order to determine the most appropriate treatment approach for each patient. Until today, OPD can still be considered the standard of care. After all, further research is needed to determine the appropriate patient selection and the optimal settings for implementation with a multicenter RCT comparing RPD to OPD already on its way.42
This trial has several limitations: First, the monocentric design of our trial and the fact that more than one third of patients eligible to the trial could not be included due to missing informed consent or organisational reasons may impact the external generalizability of the findings. Since robotic surgery's success is closely linked to the procedure's frequency, which contributes to improved outcomes, and this trial was performed in a very high-volume centre, the trial results might currently be applicable to other high-volume centres, only. Secondly, due to the inherent nature of the different surgical approaches in robotic and open surgery, it was not feasible to blind the patients or the surgical team, which might introduce potential biases to some but most likely not to the well-defined, objective primary endpoint.
Besides, being an exploratory study, EUROPA aimed to generate initial insights and hypotheses rather than provide definitive conclusions. Further technical advances and even greater robotic experience of the operating surgeons until mastery is achieved might further improve robotic results. Finally, the follow-up period in our trial was relatively short, and long-term outcomes, such as long-term survival, were not assessed.
This is the first RCT evaluating RPD versus OPD in the setting of a very high-volume centre, showing comparable overall postoperative morbidity according to the CCI for both approaches. Further efforts and confirmatory larger-scale RCTs are urgently needed to prove benefits and the true clinical value of RPD over OPD.
Contributors
RK, ALM, MWB and TH conceived and designed the trial, supervised trial conduct, participated in data analysis and interpretation, and prepared and wrote the report. MJ and EK participated in data analysis and interpretation and prepared and wrote the manuscript. AS, CK and RB participated in trial design, data analysis, and data interpretation. YK, FN, PK, FP and MKD participated in patient recruitment, trial design, and trial conduct. All authors have proof-read the manuscript.
Data sharing statement
Deidentified data is available for other researchers through reasonable scientific request to the corresponding author with a signed data access agreement and after publication of this trial. The study protocol, statistical analysis plan and informed consent form can also be made available.
Declaration of interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
This study was funded by the Federal Ministry of Education and Research (BMBF: 01KG2010).
Besides, we thank the medical and nursing staff of the study centre and clinical partners who were not directly involved in the conduct of this trial but without whom successful completion of the trial would not have been possible.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100864.
Appendix A. Supplementary data
Tables S1–S5
References
- 1.Klotz R., Larmann J., Klose C., et al. Gastrointestinal complications after pancreatoduodenectomy with epidural vs patient-controlled intravenous analgesia: a randomized clinical trial. JAMA Surg. 2020;55(7) doi: 10.1001/jamasurg.2020.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariette C., Markar S.R., Dabakuyo-Yonli T.S., et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380(2):152–162. doi: 10.1056/NEJMoa1805101. [DOI] [PubMed] [Google Scholar]
- 3.Asbun H.J., Moekotte A.L., Vissers F.L., et al. The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg. 2020;271(1):1–14. doi: 10.1097/SLA.0000000000003590. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij T., van Hilst J., van Santvoort H., et al. Minimally invasive versus open distal pancreatectomy (leopard): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269(1):2–9. doi: 10.1097/SLA.0000000000002979. [DOI] [PubMed] [Google Scholar]
- 5.Bjornsson B., Larsson A.L., Hjalmarsson C., Gasslander T., Sandstrom P. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg. 2020;107(10):1281–1288. doi: 10.1002/bjs.11554. [DOI] [PubMed] [Google Scholar]
- 6.Korrel M., Jones L.R., van Hilst J., et al. Minimally invasive versus open distal pancreatectomy for resectable pancreatic cancer (DIPLOMA): an international randomised non-inferiority trial. Lancet Reg Health Eur. 2023;31 doi: 10.1016/j.lanepe.2023.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagner M., Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8(5):408–410. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 8.Wang M., Li D., Chen R., et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6(6):438–447. doi: 10.1016/S2468-1253(21)00054-6. [DOI] [PubMed] [Google Scholar]
- 9.Croome K.P., Farnell M.B., Que F.G., et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260(4):633–638. doi: 10.1097/SLA.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 10.Poves I., Burdío F., Morató O., et al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the PADULAP randomized controlled trial. Ann Surg. 2018;268(5):731–739. doi: 10.1097/SLA.0000000000002893. [DOI] [PubMed] [Google Scholar]
- 11.Palanivelu C., Senthilnathan P., Sabnis S.C., et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017;104(11):1443–1450. doi: 10.1002/bjs.10662. [DOI] [PubMed] [Google Scholar]
- 12.Adam M.A., Choudhury K., Dinan M.A., et al. Minimally invasive versus open pancreaticoduodenectomy for cancer: practice patterns and short-term outcomes among 7061 patients. Ann Surg. 2015;262(2):372–377. doi: 10.1097/SLA.0000000000001055. [DOI] [PubMed] [Google Scholar]
- 13.van Hilst J., de Rooij T., Bosscha K., et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4(3):199–207. doi: 10.1016/S2468-1253(19)30004-4. [DOI] [PubMed] [Google Scholar]
- 14.Adam M.A., Thomas S., Youngwirth L., Pappas T., Roman S.A., Sosa J.A. Defining a hospital volume threshold for minimally invasive pancreaticoduodenectomy in the United States. JAMA Surg. 2017;152(4):336–342. doi: 10.1001/jamasurg.2016.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giulianotti P.C., Coratti A., Angelini M., et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138(7):777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 16.Müller P.C., Kuemmerli C., Cizmic A., et al. Learning curves in open, laparoscopic, and robotic pancreatic surgery: a systematic review and proposal of a standardization. Ann Surg Open. 2022;3(1):e111. doi: 10.1097/AS9.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu Hilal M., van Ramshorst T.M.E., Boggi U., et al. The brescia internationally validated European guidelines on minimally invasive pancreatic surgery (EGUMIPS) Ann Surg. 2023;279(1):45–57. doi: 10.1097/SLA.0000000000006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zureikat A.H., Beane J.D., Zenati M.S., et al. 500 minimally invasive robotic pancreatoduodenectomies: one decade of optimizing performance. Ann Surg. 2021;273(5):966–972. doi: 10.1097/SLA.0000000000003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Oosten A.F., Ding D., Habib J.R., et al. Perioperative outcomes of robotic pancreaticoduodenectomy: a propensity-matched analysis to open and laparoscopic pancreaticoduodenectomy. J Gastrointest Surg. 2021;25(7):1795–1804. doi: 10.1007/s11605-020-04869-z. [DOI] [PubMed] [Google Scholar]
- 20.Baimas-George M., Watson M., Murphy K.J., et al. Robotic pancreaticoduodenectomy may offer improved oncologic outcomes over open surgery: a propensity-matched single-institution study. Surg Endosc. 2020;34(8):3644–3649. doi: 10.1007/s00464-020-07564-x. [DOI] [PubMed] [Google Scholar]
- 21.Kauffmann E.F., Napoli N., Menonna F., et al. A propensity score-matched analysis of robotic versus open pancreatoduodenectomy for pancreatic cancer based on margin status. Surg Endosc. 2019;33(1):234–242. doi: 10.1007/s00464-018-6301-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang S.E., Shyr B.U., Chen S.C., Shyr Y.M. Comparison between robotic and open pancreaticoduodenectomy with modified Blumgart pancreaticojejunostomy: a propensity score-matched study. Surgery. 2018;164(6):1162–1167. doi: 10.1016/j.surg.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W., Liu C., Li S., Geng D., Feng Y., Sun M. Safety and efficacy for robot-assisted versus open pancreaticoduodenectomy and distal pancreatectomy: a systematic review and meta-analysis. Surg Oncol. 2018;27(3):468–478. doi: 10.1016/j.suronc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Mantzavinou A., Uppara M., Chan J., Patel B. Robotic versus open pancreaticoduodenectomy, comparing therapeutic indexes; a systematic review. Int J Surg. 2022;101 doi: 10.1016/j.ijsu.2022.106633. [DOI] [PubMed] [Google Scholar]
- 25.Klotz R., Dorr-Harim C., Bruckner T., et al. Evaluation of robotic versus open partial pancreatoduodenectomy-study protocol for a randomised controlled pilot trial (EUROPA, DRKS00020407) Trials. 2021;22(1):40. doi: 10.1186/s13063-020-04933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickel F., Wise P., Muller P.C., et al. Short-term outcomes of robotic versus open pancreatoduodenectomy - propensity score-matched analysis. Ann Surg. 2023 doi: 10.1097/SLA.0000000000005981. [DOI] [PubMed] [Google Scholar]
- 27.Tempero M.A. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17(5.5):603–605. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 28.Chen S., Chen J.Z., Zhan Q., et al. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc. 2015;29(12):3698–3711. doi: 10.1007/s00464-015-4140-y. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T., Zhao Z.M., Gao Y.X., Lau W.Y., Liu R. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc. 2019;33(9):2927–2933. doi: 10.1007/s00464-018-6595-0. [DOI] [PubMed] [Google Scholar]
- 30.Napoli N., Kauffmann E.F., Palmeri M., et al. The learning curve in robotic pancreaticoduodenectomy. Dig Surg. 2016;33(4):299–307. doi: 10.1159/000445015. [DOI] [PubMed] [Google Scholar]
- 31.Hackert T., Werner J., Weitz J., Schmidt J., Buchler M.W. Uncinate process first--a novel approach for pancreatic head resection. Langenbecks Arch Surg. 2010;395(8):1161–1164. doi: 10.1007/s00423-010-0663-9. [DOI] [PubMed] [Google Scholar]
- 32.M R. Da Vinci pancreaticoduodenectomy procedure guide PN 1054487-EU Rev. A 01/19. 2018. [Google Scholar]
- 33.McCulloch P., Altman D.G., Campbell W.B., et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 34.Zureikat A.H., Moser A.J., Boone B.A., Bartlett D.L., Zenati M., Zeh H.J., 3rd 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258(4):554–559. doi: 10.1097/SLA.0b013e3182a4e87c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng L., Lin S., Li Y., Xiao W. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy. Surg Endosc. 2017;31(8):3085–3097. doi: 10.1007/s00464-016-5371-2. [DOI] [PubMed] [Google Scholar]
- 36.Kauffmann E.F., Napoli N., Menonna F., et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg. 2016;401(8):1111–1122. doi: 10.1007/s00423-016-1499-8. [DOI] [PubMed] [Google Scholar]
- 37.Klaiber U., Probst P., Strobel O., et al. Meta-analysis of delayed gastric emptying after pylorus-preserving versus pylorus-resecting pancreatoduodenectomy. Br J Surg. 2018;105(4):339–349. doi: 10.1002/bjs.10771. [DOI] [PubMed] [Google Scholar]
- 38.Wang M., Pan S., Qin T., et al. Short-term outcomes following laparoscopic vs open pancreaticoduodenectomy in patients with pancreatic ductal adenocarcinoma: a randomized clinical trial. JAMA Surg. 2023;158(12):1245–1253. doi: 10.1001/jamasurg.2023.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strobel O., Hank T., Hinz U., et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. 2017;265(3):565–573. doi: 10.1097/SLA.0000000000001731. [DOI] [PubMed] [Google Scholar]
- 40.Bao P.Q., Mazirka P.O., Watkins K.T. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg. 2014;18(4):682–689. doi: 10.1007/s11605-013-2410-3. [DOI] [PubMed] [Google Scholar]
- 41.Zwart M.J.W., van den Broek B., de Graaf N., et al. The feasibility, proficiency, and mastery learning curves in 635 robotic pancreatoduodenectomies following a multicenter training program: "standing on the shoulders of giants". Ann Surg. 2023;278(6):e1232–e1241. doi: 10.1097/SLA.0000000000005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Graaf N., Emmen A., Ramera M., et al. Minimally invasive versus open pancreatoduodenectomy for pancreatic and peri-ampullary neoplasm (DIPLOMA-2): study protocol for an international multicenter patient-blinded randomized controlled trial. Trials. 2023;24(1):665. doi: 10.1186/s13063-023-07657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5