Abstract
Introduction
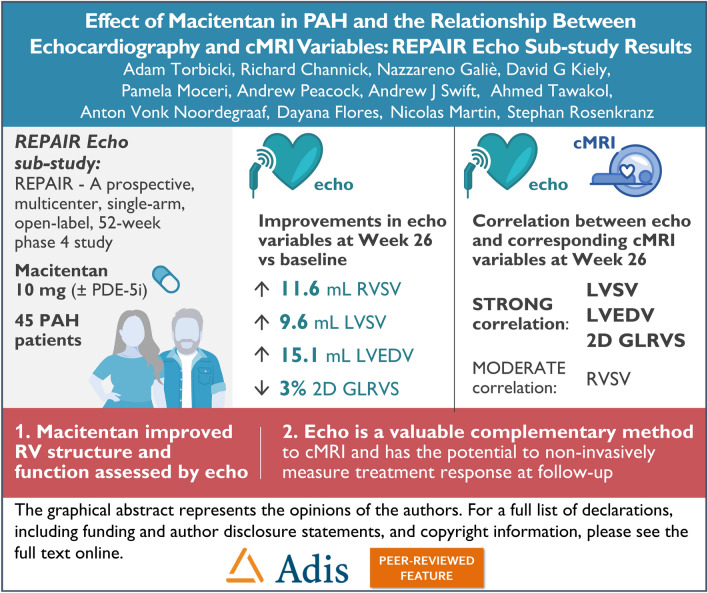
The aim of this sub-study was to evaluate the relationship between echocardiography (echo) and cardiac magnetic resonance imaging (cMRI) variables and to utilize echo to assess the effect of macitentan on right ventricle (RV) structure and function.
Methods
REPAIR (NCT02310672) was a prospective, multicenter, single-arm, open-label, 52-week, phase 4 study in pulmonary arterial hypertension (PAH) patients, which investigated the effect of macitentan 10 mg as monotherapy, or in combination with a phosphodiesterase 5 inhibitor, on RV structure, function, and hemodynamics using cMRI and right heart catheterization. In this sub-study, patients were also assessed by echo at screening and at weeks 26 and/or 52. Post hoc correlation analyses between echo and cMRI variables were performed using Pearson’s correlation coefficient, Spearman's correlation coefficient, and Bland–Altman analyses.
Results
The Echo sub-study included 45 patients. Improvements in echo-assessed RV stroke volume (RVSV), left ventricular SV (LVSV), LV end-diastolic volume (LVEDV), RV fractional area change (RVFAC), tricuspid annular plane systolic excursion (TAPSE), and in 2D global longitudinal RV strain (2D GLRVS) were observed at weeks 26 and 52 compared to baseline. There was a strong correlation between echo (LVSV, 2D GLRVS, and LVEDV) and cMRI variables, with a moderate correlation for RVSV. Bland–Altman analyses showed a good agreement for LVSV measured by echo versus cMRI, whereas an overestimation in echo-assessed RVSV was observed compared to cMRI (bias of − 15 mL). Hemodynamic and functional variables, as well as safety, were comparable between the Echo sub-study and REPAIR.
Conclusions
A good relationship between relevant echo and cMRI parameters was shown. Improvements in RV structure and function with macitentan treatment was observed by echo, consistent with results observed by cMRI in the primary analysis of the REPAIR study. Echo is a valuable complementary method to cMRI, with the potential to non-invasively monitor treatment response at follow-up.
Trial registration number
REPAIR NCT02310672.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40119-023-00345-2.
Keywords: Echocardiography, Pulmonary arterial hypertension, Cardiac magnetic resonance imaging, Macitentan, Agreement, Correlation
Key Summary Points
| Why carry out this study? | |
| In the phase 4 REPAIR (NCT02310672) study, macitentan treatment in pulmonary arterial hypertension (PAH) patients, as monotherapy or in combination with a phosphodiesterase-5 inhibitor, led to clinically relevant improvements in right ventricle (RV) structure and function assessed by cardiac magnetic resonance imaging (cMRI) and right heart catheterization. | |
| While cMRI is the gold standard non-invasive technique for RV assessment, echocardiography (echo) may be more appropriate to obtain serial measurements to assess treatment responses over time, due to the more demanding logistics of cMRI. | |
| The aim of this post hoc subgroup analysis is to evaluate the correlation and agreement between comparable echo and cMRI variables and to use echo to assess the effect of macitentan on RV structure and function. | |
| What was learned from the study? | |
| A good relationship between relevant echo and cMRI parameters was observed and macitentan improved RV structure and function in PAH patients as assessed by echo. | |
| Echo is a valuable complementary method to cMRI, with the potential to non-invasively monitor treatment response at follow-up. |
Digital Features
This article is published with digital features, including a Graphical Abstract, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.24723714.
Introduction
The hallmark of pulmonary arterial hypertension (PAH) is a progressive increase in pulmonary vascular resistance (PVR) as a result of pathological pulmonary vascular remodeling [1]. Subsequently, changes to the structure of the right ventricle (RV) occur, leading to a decline in function, with RV failure constituting the primary cause of death [1]. Formal diagnosis of PAH requires an invasive right heart catheterization (RHC) procedure. However, complementary non-invasive procedures including cardiac magnetic resonance imaging (cMRI) and echocardiography (echo) can provide prognostic information on RV structure and function and are currently recommended as part of the multistep diagnostic approach for suspected pulmonary hypertension in the 2022 European Society of Cardiology/European Respiratory Society guidelines [2, 3].
Recent advances in non-invasive imaging modalities have led to increased use of these techniques in the diagnosis and management of patients with PAH [4, 5], and can aid evaluation of the long-term effects of PAH therapies on risk status, prognosis and RV remodeling [2–4, 6–9]. The usefulness of each technique varies depending on the parameters being assessed. While cMRI is the gold standard and most reliable non-invasive tool for assessing RV structure and function, and one which demonstrates high repeatability [4, 5], it may not be suitable in specific situations (e.g., in patients with implanted metallic devices) [5, 10]. Moreover, the associated higher cost and lower availability of cMRI limit its accessibility to all patients [10]. On the other hand, echo is a widely available imaging modality providing complementary comprehensive information to cMRI in patients with PAH [8], and may be more appropriate to obtain serial measurements to assess treatment responses over time [11], given the more demanding logistics of cMRI [1, 12]. Furthermore, echo is the method of choice for the assessment of valvular disease and is an essential screening test in symptomatic patients at risk for PAH [1, 13]. Official recommendations published by the American Society of Echocardiography and the European Association of Cardiovascular Imaging aim to standardize the methodology for echo measurements [14]. The value of imaging parameters as surrogate endpoints in PAH clinical trials still requires further investigation, and more evidence is needed to validate and standardize their applicability.
In the REPAIR study, patients diagnosed with PAH initiated macitentan 10 mg as monotherapy, or as part of combination therapy with a phosphodiesterase 5 inhibitor (PDE-5i) [15]. At final analysis (N = 71), PVR assessed by RHC decreased by 38%, and mean RV stroke volume (RVSV) assessed by cMRI increased by 12.0 mL from baseline to week 26, suggesting that macitentan contributes to beneficial remodeling of the RV in PAH patients [15]. In addition to the primary endpoints of PVR and RVSV, secondary and exploratory RHC-derived and cMRI-assessed endpoints were analyzed in the REPAIR study [15]. For this study, additional analyses using echo variables were also performed and are considered post hoc. We report here data from echo-assessed variables, in addition to the RHC-derived hemodynamics and non-invasive cMRI variables assessed during the REPAIR study. These data provide a unique opportunity to evaluate the relationship between echo and cMRI-derived measures, in relation to cardiac hemodynamics.
The aim of this post hoc subgroup analysis is to evaluate the correlation and agreement between comparable echo and cMRI variables and to assess the effect of macitentan on RV structure and function by echo.
Methods
Data Sharing Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
REPAIR Study Design
REPAIR (NCT02310672) was a prospective, multicenter, single-arm, open-label, 52-week phase 4 study (Supplementary Material Fig. S1) in patients with PAH, investigating the effect of macitentan 10 mg either as monotherapy, or in combination with a PDE-5i, on RV function and hemodynamics as assessed by cMRI and RHC, respectively. The study design has been described in detail previously [15]. Briefly, patients aged 18–74 years with idiopathic or heritable PAH, PAH related to connective tissue disease, drug use or toxin exposure, or simple congenital systemic-to-pulmonary shunts at least 2 years after repair were eligible. The main exclusion criteria were body weight < 40 kg, body mass index > 35 kg/m2, or any contraindication to macitentan treatment or cMRI. At screening, patients were required to be PAH treatment-naïve or receiving a stable background PDE-5i for at least 3 months, have a 6-min walk distance (6MWD) of ≥ 150 m, and be in World Health Organization functional class (WHO FC) I-III. All patients eligible for the REPAIR study were given the option to participate in the Echo sub-study, and this was conducted at selected centers.
Clinical Assessments
Participants in the REPAIR study had cMRI and assessments of 6MWD, WHO FC and N-terminal pro-brain natriuretic peptide (NTproBNP) performed at screening and at weeks 26 and 52, while RHC was performed at screening and at week 26. Analysis of plasma NTproBNP was performed at a central laboratory. For patients that also participated in the Echo sub-study, all echo assessments (including 2D, M mode, Doppler) were performed at screening and at weeks 26 and/or 52. All echocardiograms were centrally assessed by reviewers blinded to patient identity and the date/sequence of image acquisition. All images of a patient were assessed at the same time by the same reviewer. For the echocardiogram, patients were positioned in left lateral recumbent position or in a position that permitted optimal imaging. Images were acquired during quiet respiration. At least five cardiac cycles were required for spectral pulsed-wave and continuous-wave Doppler.
Treatment-emergent adverse events (AEs) were defined as any AE with onset date from study drug initiation until 30 days after study drug discontinuation.
Outcome Measures
All echo variables, cMRI variables and 6MWD were assessed by change from baseline to weeks 26 and 52 least squares (LS) mean (95% confidence limits [CL]). For RHC variables, change from baseline to week 26 LS mean (95% CL) was measured. Change from baseline to weeks 26 and 52 geometric mean (95% CL) was assessed for NTproBNP and change from baseline to weeks 26 and 52 was assessed for WHO FC.
Statistical Methodology
The REPAIR safety set comprised all screened patients who received ≥ 1 dose of macitentan. The Echo subgroup comprised all screened patients who received ≥ 1 dose of macitentan and who were included in the Echo sub-study. Changes in echo and cMRI variables were analyzed using an analysis of covariance (ANCOVA) model with a factor for PAH-targeted therapy (macitentan initiated alone in treatment-naïve patients, on top of stable background PDE-5i, or as initial combination with a PDE-5i) and a covariate for baseline parameter value. The relationship between the following echo-derived and cMRI-derived variables was evaluated using linear regression analysis with Pearson’s correlation coefficient and the non-parametric Spearman's correlation coefficient: (1) RVSV by echo (by pulmonic valve Doppler and pulmonary artery annulus) versus RVSV by cMRI (determined from pulmonary artery flow); (2) left ventricular (LV) stroke volume (LVSV) by echo (by Doppler) versus LVSV by cMRI (determined from aortic flow); (3) 2D global longitudinal RV strain (2D GLRVS) by echo versus RV ejection fraction (RVEF) by cMRI (by volume); and (4) LV end-diastolic volume (LVEDV) by echo (biplane mod) versus LVEDV by cMRI. Additionally, Bland–Altman analysis was performed to assess agreement between the comparable echo and cMRI measured variables for RVSV and LVSV in terms of bias and 95% limits of agreement. The correlation and agreement analyses are based on week 26 data. All analyses were performed using SAS version 9.4.
Monitoring and Ethics Statement
The study was conducted in compliance with the Declaration of Helsinki and ethical approval was obtained from the independent ethics committee/institutional review board of all participating centers (Supplementary Materials Table S1). All patients provided written informed consent, and all echo and cMRI results were centrally assessed by a blinded imaging committee.
Results
Patient Characteristics
A total of 112 patients were screened across 29 sites in 11 countries. Patient disposition is shown in Supplementary Material Fig. S2; 87 patients received at least one dose of macitentan (safety set) [15]. From the safety set, 46 patients were enrolled into the Echo sub-study; 45 patients had a baseline echo assessment (one patient withdrew consent prior to the baseline assessment) and 43 and 38 patients had weeks 26 and 52 echo assessments, respectively. Patient demographics and baseline disease characteristics for the Echo subgroup (N = 45) are shown in Table 1, and are generally comparable to those reported in the REPAIR study [15]. Briefly, 82.2% of patients were female with a median (interquartile range [Q1, Q3]) age of 45 (35, 57) years. Most patients had a diagnosis of idiopathic PAH (66.7%) and were in either WHO FC II (31.1%) or III (68.9%). The median (Q1, Q3) 6MWD was 387 (324, 453) m. Macitentan was mostly initiated in treatment-naïve patients as monotherapy (26.7%) or in combination with a PDE-5i (44.4%); 28.9% of patients were receiving stable background PDE-5i prior to macitentan initiation.
Table 1.
Patient demographics and baseline characteristics
| Echo subgroup (N = 45) | |
|---|---|
| Female sex, n (%) | 37 (82.2) |
| Age, median (Q1, Q3), years | 45 (35, 57) |
| BMI, mean (SD), kg/m2 | 25.4 (4.7) |
| PAH etiology, n (%) | |
| Idiopathic | 30 (66.7) |
| Heritable | 2 (4.4) |
| Drug- or toxin-induced | 1 (2.2) |
| Associated with | |
| Congenital heart diseasea | 2 (4.4) |
| Connective tissue disease | 10 (22.2) |
| WHO FC, n (%) | |
| I | 0 |
| II | 14 (31.1) |
| III | 31 (68.9) |
| 6MWD, median (Q1, Q3), m | 387 (324, 453) |
| PAH treatment strategy, n (%) | |
| Macitentan initiated in treatment-naïve patients as initial combination therapy with a PDE-5i | 20 (44.4) |
| Macitentan initiated alone: | |
| In treatment-naïve patients | 12 (26.7) |
| In patients receiving stable background PDE-5i | 13 (28.9) |
aOnly simple congenital systemic to pulmonary shunts at least 2 years post-surgical repair. 6MWD 6-min walk distance, BMI body mass index, echo echocardiography, PAH pulmonary arterial hypertension, PDE-5i phosphodiesterase 5 inhibitors, Q1, Q3 interquartile range, SD standard deviation, WHO FC World Health Organization functional class
Echo Variables
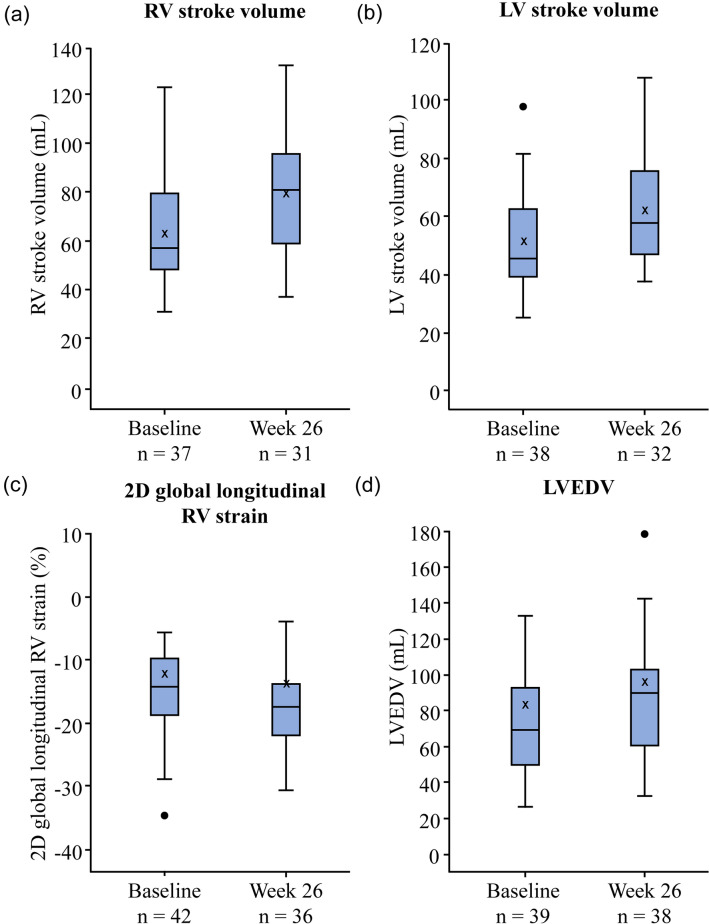
Changes in echo variables from baseline to weeks 26 and 52 for the Echo subgroup are shown in Table 2 and Supplementary Material Table S2. At week 26, the LS mean increased from baseline for 20 variables including RVSV, LVSV, LVEDV, RV fractional area change (RVFAC) and tricuspid annular plane systolic excursion (TAPSE), and decreased for 14 variables including 2D GLRVS. At week 26, RVSV increased by 11.6 mL, LVSV increased by 9.6 mL, 2D GLRVS decreased by 3%, and LVEDV increased by 15.1 mL, compared to baseline (Table 2). RVFAC increased by 7.5% and TAPSE increased by 2.5 mm at week 26 compared to baseline (Table 2). The absolute values of echo variables RVSV, LVSV, 2D GLRVS and LVEDV at baseline and week 26 are shown in Fig. 1. The direction of the LS mean change from baseline observed at week 52 was the same as at week 26 for these variables.
Table 2.
Change in echo variables from baseline to weeks 26 and 52 (Echo subgroup, N = 45)
| Echo variables | n | Baseline Mean (SD) | Change from baseline to week 26 LS meana (95% CL) | n | Change from baseline to week 52 LS meana (95% CL) |
|---|---|---|---|---|---|
| RV fractional area change, % | 42 | 25.9 (11.9) | 7.5 (4.4, 10.7) | 38 | 7.1 (4.3, 9.9) |
| TAPSE, mm | 39 | 16.4 (3.5) | 2.5 (1.4, 3.6) | 35 | 3.2 (2.2, 4.2) |
| 2D global longitudinal RV strain, % | 36 | − 13.9 (5.7) | − 3.0 (− 4.7, − 1.4) | 33 | − 3.5 (− 4.9, − 2.1) |
| RV stroke volumeb, mL | 28 | 68.4 (22.6) | 11.6 (3.2, 20.0) | 25 | 15.5 (4.6, 26.5) |
| RV stroke volume index, mL/m2 | 28 | 39.1 (12.8) | 5.9 (1.7, 10.1) | 25 | 8.7 (2.2, 15.2) |
| RV cardiac index, L/min/m2 | 28 | 2.9 (1.0) | 0.23 (− 0.10, 0.57) | 25 | 0.46 (− 0.03, 0.94) |
| RV cardiac output, L/min | 28 | 5.1 (1.9) | 0.47 (− 0.12, 1.06) | 25 | 0.81 (0.02, 1.60) |
| LV stroke volumec, mL | 29 | 51.5 (18.2) | 9.6 (3.8, 15.5) | 25 | 11.9 (5.1, 18.6) |
| RV myocardial performance indexc | 29 | 0.69 (0.38) | − 0.20 (− 0.26, − 0.15) | 25 | − 0.30 (− 0.40, − 0.20) |
| Mitral E/A ratio | 35 | 0.95 (0.35) | 0.19 (0.06, 0.32) | 31 | 0.30 (0.16, 0.44) |
| RV end-diastolic diameter, mm | 43 | 45.3 (7.1) | − 2.8 (− 4.7, − 0.8) | 38 | − 3.2 (− 5.1, − 1.4) |
| LV end-diastolic volume, mL | 32 | 70.6 (27.6) | 15.1 (8.8, 21.4) | 31 | 16.5 (8.9, 24.2) |
| Tricuspid regurgitation PJV, m/s | 21 | 4.3 (0.74) | − 0.36 (− 0.70, − 0.03) | 19 | − 0.29 (− 0.63, 0.05) |
| Heart rate, bpm | 43 | 78.3 (14.6) | − 7.0 (− 10.2, − 3.8) | 38 | − 8.2 (− 12.0, − 4.4) |
aAnalyzed using ANCOVA with a factor for PAH background therapy and a covariate for baseline parameter value
bDetermined from pulmonic valve Doppler and pulmonary artery annulus dimension
cBy pulsed-wave Doppler
ANCOVA analysis of covariance, bpm beats per minute, CL confidence limit, echo echocardiography, LS least squares, LV left ventricular, PAH pulmonary arterial hypertension, PJV peak jet velocity, RV right ventricular, SD standard deviation, TAPSE tricuspid annular plane systolic excursion
Fig. 1.
Boxplots of echo variables a RV stroke volume, b LV stroke volume, c 2D global longitudinal RV strain, and d LVEDV, at baseline and at week 26 (Echo subgroup, N = 45). The box plots show the median (line), mean (X), and the first and third quartiles (outer box borders). The upper and lower whiskers represent the location of the maximum and minimum within the first and third quartiles ± 1.5 times the interquartile range. The circle represents outliers. Echo echocardiography, LV left ventricular, LVEDV LV end-diastolic volume, RV right ventricular
cMRI Variables
Change in cMRI variables from baseline to week 26 and 52 for the Echo subgroup and safety set (N = 87) are shown in Table 3 and Supplementary Material Table S3 respectively. At week 26 in the Echo subgroup, the LS mean increased from baseline for eight variables, including RVSV (by flow and by volume), RVEF (by flow and by volume), LVSV (by flow and by volume), and LVEDV, and decreased for four variables, including RV mass. All parameters showed the same direction of change at week 26 and 52. For the majority of variables, the LS mean change from baseline to weeks 26 and 52 were comparable between the Echo subgroup and safety set.
Table 3.
Change in cMRI variables from baseline to weeks 26 and 52 (Echo subgroup, N = 45)
| cMRI variables | n | Baseline Mean (SD) | Change from baseline to week 26a LS mean (95% CL) | n | Baseline Mean (SD) | Change from baseline to week 52a LS mean (95% CL) |
|---|---|---|---|---|---|---|
| RV stroke volume by flowb, mL | 38 | 48.9 (18.5) | 14.0 (9.4, 18.6) | 35 | 48.1 (19.1) | 17.3 (12.7, 21.8) |
| RV stroke volume by volumec, mL | 40 | 55.9 (19.1) | 15.2 (10.6, 19.7) | 35 | 56.0 (19.5) | 15.8 (11.1, 20.5) |
| RVEDV, mL | 40 | 149.0 (48.2) | 0.9 (− 7.3, 9.0) | 35 | 147.1 (44.1) | 3.1 (− 5.9, 12.0) |
| RVESV, mL | 40 | 93.1 (43.9) | − 15.2 (− 21.8, − 8.6) | 35 | 91.1 (39.0) | − 13.7 (− 22.4, − 5.0) |
| RV ejection fraction by flowb, % | 37 | 35.9 (16.1) | 9.3 (6.2, 12.4) | 34 | 34.9 (15.7) | 12.0 (7.7, 16.3) |
| RV ejection fraction by volumec, % | 40 | 39.7 (13.3) | 9.8 (7.1, 12.4) | 35 | 39.8 (12.8) | 10.5 (6.7, 14.3) |
| RV mass, g | 40 | 102.8 (40.7) | − 6.5 (− 12.2, − 0.9) | 35 | 103.8 (42.5) | − 2.7 (− 9.5, 4.1) |
| LV stroke volume by flowd, mL | 38 | 46.9 (17.2) | 16.1 (12.1, 20.2) | 34 | 46.2 (17.8) | 16.7 (12.5, 20.9) |
| LV stroke volume by volumec, mL | 40 | 53.3 (19.6) | 19.9 (15.6, 24.3) | 35 | 53.3 (20.6) | 17.5 (13.7, 21.3) |
| LVEDV, mL | 40 | 87.8 (28.9) | 21.3 (15.2, 27.5) | 35 | 88.1 (30.2) | 19.0 (13.6, 24.4) |
| LVESV, mL | 40 | 34.5 (17.0) | 1.3 (− 2.4, 4.9) | 35 | 34.8 (17.0) | 1.4 (− 2.4, 5.3) |
| LV ejection fraction by flowd, % | 37 | 53.7 (11.9) | 4.0 (0.9, 7.1) | 34 | 53.1 (11.9) | 7.3 (3.5, 11.1) |
| LV ejection fraction by volumec, % | 40 | 61.5 (12.9) | 6.2 (4.0, 8.4) | 35 | 61.2 (12.8) | 6.1 (3.8, 8.4) |
| LV mass, g | 40 | 102.7 (24.3) | 4.1 (0.8, 7.4) | 35 | 103.2 (25.5) | 5.3 (1.4, 9.2) |
| RVEDV/LVEDVe | 40 | 0.53 (0.32) | − 0.22 (− 0.29, − 0.16) | 35 | 0.52 (0.31) | − 0.19 (− 0.26, − 0.12) |
| RVESV/LVESVe | 40 | 1.00 (0.48) | − 0.23 (− 0.34, − 0.13) | 35 | 0.99 (0.49) | − 0.23 (− 0.37, − 0.09) |
aAnalyzed using an ANCOVA with a factor for PAH background therapy and a covariate for baseline value
bDetermined from pulmonary artery flow
cDetermined from volume
dDetermined from aortic flow
eLog transformed
ANCOVA analysis of covariance, CL confidence limit, cMRI cardiac magnetic resonance imaging, echo echocardiography, LS least squares, LV left ventricular, LVEDV LV end-diastolic volume, LVESV LV end-systolic volume, PAH pulmonary arterial hypertension, RV right ventricular, RVEDV RV end-diastolic volume, RVESV RV end-systolic volume, SD standard deviation
RHC Variables and Functional Parameters
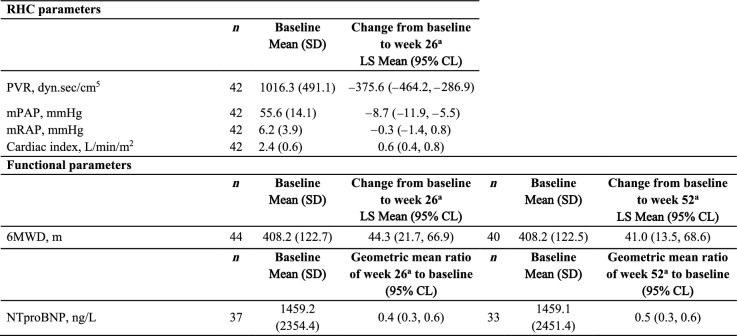
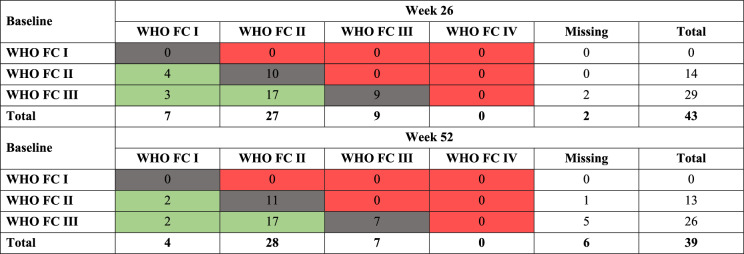
The change in RHC variables and functional parameters are shown for the Echo subgroup in Table 4. From baseline to week 26, the LS mean decreased for PVR, mean pulmonary arterial pressure and mean right atrial pressure; the cardiac index LS mean increased. Similar results were observed for the safety set (Supplementary Material Table S4). The change in 6MWD LS mean increased from baseline to week 26 by 44 m and this was maintained at week 52 (41 m). NTproBNP decreased by 60% and 50% from baseline to weeks 26 and 52, respectively. The mean change in functional parameters were similar for the Echo subgroup and safety set (Supplementary Material Table S5). At weeks 26 and 52, 24 out of 43 patients with available post-baseline assessment (56%) and 21 out of 39 patients (54%), respectively, had an improved WHO FC; no patients worsened from baseline at either timepoint (Table 5).
Table 4.
Changes in RHC variables and functional parameters from baseline to week 26 and week 52 (Echo subgroup, N = 45)
aAnalyzed using an ANCOVA with a factor for PAH background therapy and a covariate for baseline value
6MWD 6-minute walk distance, ANCOVA analysis of covariance, CL confidence limit, echo echocardiography, LS least squares, mPAP mean pulmonary arterial pressure, mRAP mean right atrial pressure, NTproBNP N-terminal pro-brain natriuretic peptide, PAH pulmonary arterial hypertension, PVR pulmonary vascular resistance, RHC right heart catheterization, SD standard deviation
Table 5.
Changes in WHO FC from baseline to week 26 and week 52 (Echo subgroup, N = 45)
Subjects displayed under column ‘Missing’ are those with no available post-baseline assessment. Shading indicates change in WHO FC category (green: improved; grey: no change; red: worsened)
Echo echocardiography, WHO FC World Health Organization functional class
Correlation/Agreement Between Echo and cMRI Variables
The relationship between comparable echo and cMRI variables was assessed by correlation and agreement analyses for the Echo subgroup. Linear regression analysis by Pearson’s correlation coefficient and Spearman’s correlation coefficient resulted in a moderate correlation between RVSV measured by echo and cMRI (r = 0.47 and r = 0.38, respectively) at week 26; strong correlations were observed between LVSV by echo and cMRI (r = 0.71 and r = 0.62, respectively), 2D GLRVS by echo and RVEF by cMRI (r = − 0.70 and r = − 0.70, respectively), and LVEDV by echo and cMRI (r = 0.80 and r = 0.74, respectively), at week 26 (Table 6, Supplementary Material Fig. S3).
Table 6.
Correlation between echo and cMRI variables at week 26 (Echo subgroup, N = 45)
| Echo variable | cMRI variable | Pearson’s correlation coefficient (r) | Spearman’s correlation coefficient (r) | Level of correlation |
|---|---|---|---|---|
| RV stroke volumea | RV stroke volumeb | 0.47 | 0.38 | Moderate |
| LV stroke volumec | LV stroke volumed | 0.71 | 0.62 | Strong |
| 2D GLRVS | RV ejection fractione | − 0.70 | − 0.70 | Strong |
| LVEDV | LVEDV | 0.80 | 0.74 | Strong |
Level of correlation reported according to Cohen guidelines; r < 0.3 = weak, r ≥ 0.3 to < 0.5 = moderate, and r ≥ 0.5 = strong [32]
aDetermined from pulmonic valve Doppler and pulmonary artery annulus dimension
bDetermined from pulmonary artery flow
cDetermined from pulsed-wave Doppler
dDetermined from aortic flow
eDetermined from volume
2D GLRVS 2D global longitudinal RV strain, cMRI cardiac magnetic resonance imaging, echo echocardiography, LV left ventricular, LVEDV LV end-diastolic volume, RV right ventricular
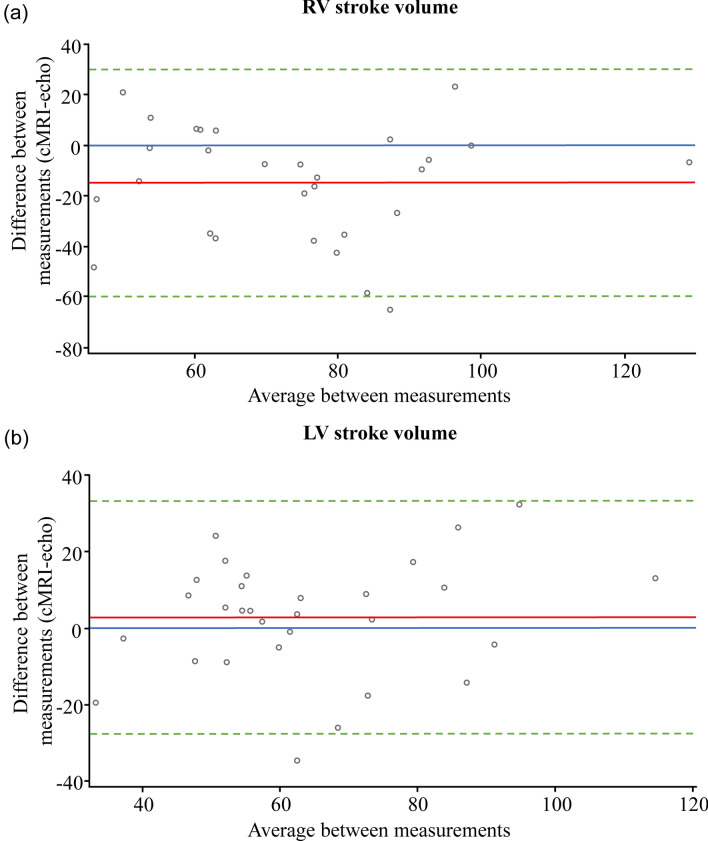
Bland–Altman analysis was performed to assess agreement between the echo and cMRI corresponding variables for RVSV and LVSV. The bias at week 26 for RVSV measured by echo compared to cMRI was − 15 mL, reflecting an overestimation of RVSV by echo (Fig. 2a). For LVSV measured by echo and cMRI, the Bland–Altman analysis bias was close to zero, indicating good agreement between the two methods at week 26 (Fig. 2b). For both RVSV and LVSV, 95% of the observations lied within 2 standard deviations of the mean change at week 26.
Fig. 2.
Bland–Altman agreement analysis of a RV stroke volume (N = 29), and b LV stroke volume (N = 30), measured by echo and cMRI at week 26 (Echo subgroup, N = 45). The red line indicates the bias (mean difference). The dashed green lines indicate the mean difference ± 2 standard deviations. The blue line indicates zero bias. cMRI cardiac magnetic resonance imaging, echo echocardiography, LV left ventricular, RV right ventricular
Safety and Tolerability
Safety data for the REPAIR study have been previously reported [15]. The exposure and safety data for the Echo subgroup are shown in Supplementary Material Table S4. The median (min, max) macitentan exposure time was 51.9 (5.4, 54.7) weeks. Thirty-nine (86.7%) patients reported at least one treatment-emergent AE and five (11.1%) reported at least one treatment-emergent serious AE (SAE), with no fatal treatment-emergent SAEs. The most frequent AEs (≥ 10% of patients) included peripheral edema, headache, and dizziness. There were five (11.1%) patients that discontinued macitentan treatment due to at least one AE.
Discussion
In this REPAIR Echo sub-study, correlation and agreement analyses both demonstrated a good relationship between selected echo and cMRI variables, and showed that echo is a valuable complementary method for the assessment of macitentan treatment effect on RV structure and function in patients with PAH. Macitentan treatment led to improvement in RV structure and function as assessed by echo and cMRI, and improved hemodynamics and functional variables in patients with PAH. Results from this post hoc analysis were consistent with those observed in the REPAIR primary analysis that demonstrated improvements in RV functional and hemodynamic parameters following macitentan treatment assessment by cMRI and RHC, respectively [15].
Most echo variables of cardiac structure and function in this sub-study improved after treatment with macitentan, including those with known prognostic value in PAH, such as RVSV, LVSV, LVEDV, RVFAC, TAPSE, and 2D GLRVS [16–22]. Of note, the change in RVSV measured by echo surpassed the threshold of a minimally important difference (10 mL) [23], with an increase of 11.6 mL at week 26 for the Echo subgroup after treatment with macitentan. This was consistent with the change in RVSV as assessed by cMRI previously reported in the REPAIR study [15]. Improvements were also observed for LVSV and LVEDV, with increases of 9.6 mL and 15.1 mL respectively, at week 26 compared to baseline. In this analysis, macitentan also improved the key prognostic parameters RVFAC and TAPSE [19–21], with RVFAC increasing by 7.5 and 7.1% at weeks 26 and 52, respectively, compared to baseline. The mean increase in TAPSE above 17 mm observed at weeks 26 and 52 is associated with better systolic function and survival [2, 3].
Measures of 2D GLRVS are particularly useful for the evaluation of the function of the right heart and a marker of subclinical worsening, as it shows changes before other more conventional parameters deteriorate [24]. The results from our analysis suggest macitentan improved 2D GLRVS, as a worse 2D GLRVS (≥ − 15.5%) has been shown to independently predict adverse clinical events and death in patients with PAH [22].
Mitral flow (E/A ratio) improved at week 26, suggesting that improvements in RV function are associated with normalization of LV filling patterns, likely as a result of reduced leftward septal bowing [25]. These data indicate that echo can be sensitive enough to show clinically relevant treatment effects in these parameters.
Given the unique RV anatomy, the estimation of 2D echo parameters relying on volume modelling by simple geometrical assumptions can be challenging [26, 27]. These challenges highlight the importance of central assessments of echo parameters if used as endpoints in clinical trials, as this has been shown to reduce the variability of the measurements obtained [28]. In this study, assessment of imaging data for each individual patient was performed batch-wise at the same time by the same reviewer.
Assessment of the structure and function of the heart plays an essential role in the follow-up of patients with PAH. Although cMRI is considered the gold standard technique for assessing RV structure and function, it is not always feasible and available. In its absence, echo can help clinicians to assess response to therapy at clinical follow-up. In a prospective study of 27 patients with PAH, 3D reconstruction of 2D transthoracic echo images to measure RVSV, RV end-diastolic volume (RVEDV), RV end-systolic volume (RVESV) and RVEF correlated well with cMRI assessed using Pearson’s correlation coefficient [12]. Similar observations were seen in a prospective study of 30 patients with PAH, which showed a strong correlation between 2D GLRVS by echo and RVEF by cMRI (r = 0.69) [29].
Our analysis found a strong correlation in three of the four variables measured by echo and cMRI, including LVSV by echo and cMRI, LVEDV by echo and cMRI, and 2D GLRVS by echo and corresponding RVEF by cMRI in patients with PAH. However, only a moderate correlation was observed for RVSV assessed by echo and cMRI for the patients in our study. The difference in the level of correlation for RVSV by echo and cMRI between this analysis and the aforementioned study by Bhave et al. [12], could be due to their use of 3D reconstruction of 2D transthoracic images, in comparison to 2D echo used in this study.
In our study, the Bland–Altman analysis showed a good agreement for LVSV measured by echo and cMRI. RVSV reflected an overestimation compared to cMRI, as shown by the mean RVSV at baseline of 68.4 mL measured by echo and 48.9 mL by cMRI. The overestimation of RVSV by echo could be explained by the underlying pathophysiology of this patient population and the method of choice to assess the RVSV. In this study, RVSV by echo was assessed using pulmonic valve Doppler and pulmonary artery annulus. This method offers an indirect way of measuring the RVSV as it relies on the assumption that the cross-sectional area of the pulmonary artery annulus remains constant during the cardiac cycle, which may not always be the case in patients with PAH. In contrast, cMRI provides a direct measurement of the RVSV using 3D images without the need for geometric assumptions [30]. However, cMRI can underestimate the RVSV by flow, for example, by the occurrence of off plane orientation of the slice as well as turbulent flow leading to further underestimation [31]. Yet, it is difficult to deduce with any degree of certainty what the true reasons are for this overestimation.
The most frequent AEs reported for the Echo subgroup; peripheral edema, headache, and dizziness, were comparable to those previously reported in the REPAIR study [15]. Altogether, these results add to the evidence supporting the beneficial effects of macitentan either initiated as monotherapy or as part of an initial or sequential combination therapy regimen, and also demonstrate the value of echo measures to capture such improvements.
Due to the post hoc nature of this study, some potential biases exist; no adjustments were made for multiplicity, patients may not have had complete data for all variables, and there was limited interpretation of an increase and/or decrease in specific echo variables due to a lack of published validated thresholds in PAH. Furthermore, very few patients in the REPAIR study experienced disease progression and therefore no assessment could be made on the use of echo and cMRI variables in measuring PAH worsening. A number of echo variables had a considerable level of missing data, mainly attributed to absent calibrations for M-mode and Doppler images, absent calibrations for time-based measurements, and poor resolution/quality which made some images unmeasurable. Lastly, the Echo subgroup sample size was small (however, observed cMRI, RHC, and functional results were consistent with the larger REPAIR safety set), and some echo-assessed variables, including tricuspid regurgitation peak jet velocity, were only reported for a small number of participants.
Conclusions
In this Echo sub-study, a good relationship between relevant echo and cMRI parameters was shown. Improvements in RV structure and function with macitentan treatment were observed by echo, consistent with results observed by cMRI in the primary analysis of the REPAIR study. These data show that echo is a valuable complementary method for the assessment of macitentan treatment effect on RV structure and function and has the potential to non-invasively monitor treatment response at follow-up.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Medical Writing Assistance.
Medical writing assistance was provided by Emma Connolly (EluSCIdate Ltd, Meggen, Switzerland), funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
Author Contributions
Adam Torbicki, Richard Channick, Nazzareno Galiè, David G Kiely, Pamela Moceri, Andrew Peacock, Andrew J Swift, Ahmed Tawakol, Anton Vonk Noordegraaf, Dayana Flores, and Stephan Rosenkranz contributed to the conceptualization, writing—original draft and writing—reviewing and editing. Nicolas Martin contributed to the data curation, formal analysis, validation, writing—original draft and writing—reviewing and editing.
Funding
Sponsorship of the REPAIR study and the journal’s Rapid Service Fee were funded by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland), a Janssen Pharmaceutical Company of Johnson & Johnson.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Adam Torbicki receives consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Arena Pharmaceuticals Ltd, Bayer, MSD, Pfizer, United Therapeutics, and Janssen, in addition to grant/research support from Janssen Pharmaceutical Companies of Johnson & Johnson and Speaker’s bureau fees from Janssen Pharmaceutical Companies of Johnson & Johnson, AOP, Bayer, MSD and Pfizer; advisory board member for Janssen Pharmaceutical Companies of Johnson & Johnson. Richard Channick serves as a steering committee member, served on an advisory board, and received research grants/support, and speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson; has served on an advisory board for Bayer; has received consultancy and speaker fees from Bayer and Arena Pharmaceuticals; and has received research grants from United Therapeutics. Nazzareno Galiè is a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received grant support, personal fees, and non-financial support from Janssen Pharmaceutical Companies of Johnson & Johnson, and has received grant support and personal fees from Bayer Healthcare, Pfizer and GSK. David G Kiely is a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson, receives grant/research support from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, and GSK, in addition to other financial or material support, including consultancy and speaker fees, from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, GSK, Ferrer and MSD. Pamela Moceri has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson and MSD. Andrew Peacock receives grant/research support from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, and GSK, in addition to other financial or material support from Arena Pharmaceuticals Ltd and MSD. Andrew J Swift has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson and General Electric Ltd, as well as grant support from GSK. Ahmed Tawakol receives consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson and Esperion, as well as grant/research support from Genentech. Anton Vonk Noordegraaf receives ongoing grant/research support from Janssen Pharmaceutical Companies of Johnson & Johnson, GSK and MSD and Speaker’s bureau payments from Janssen Pharmaceutical Companies of Johnson & Johnson. Dayana Flores and Nicolas Martin are employees of Janssen Pharmaceutical Companies of Johnson & Johnson. Stephan Rosenkranz has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants from AstraZeneca, Bayer, Janssen Pharmaceutical Companies of Johnson & Johnson and Novartis; has received speaker and/or consulting fees from and/or been an advisory board member for Abbott, Acceleron, Actelion, Aerovate, Altavant, AOP, Bayer, BMS, Boehringer-Ingelheim, Edwards, Ferrer, Gossamer, Janssen Pharmaceutical Companies of Johnson & Johnson, MSD, Novartis, Pfizer, UT and Vifor.
Ethical Approval
The study was conducted in compliance with the Declaration of Helsinki and ethical approval was obtained from the independent ethics committee/institutional review board of all participating centers. All patients provided written informed consent, and all echo and cMRI results were centrally assessed by a blinded imaging committee.
Footnotes
Prior Presentation: A version of the data was previously presented at the virtual CHEST 2020 Annual Meeting, October 18–21.
References
- 1.Vonk Noordegraaf A, Galiè N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev. 2011;20:243–253. doi: 10.1183/09059180.00006511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61:2200879. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RA, Johns CS, Cogliano M, Capener D, Tubman E, Elliot CA, et al. Identification of cardiac magnetic resonance imaging thresholds for risk stratification in pulmonary arterial hypertension. Am J Resp Crit Care. 2020;201:458–468. doi: 10.1164/rccm.201909-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benza R, Biederman R, Murali S, Gupta H. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;52:1683–1692. doi: 10.1016/j.jacc.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Peacock AJ, Crawley S, McLure L, Blyth KG, Vizza CD, Poscia R, et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging. 2014;7:107–114. doi: 10.1161/CIRCIMAGING.113.000629. [DOI] [PubMed] [Google Scholar]
- 7.Hassoun PM, Zamanian RT, Damico R, Lechtzin N, Khair R, Kolb TM, et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. Am J Resp Crit Care. 2015;192:1102–1110. doi: 10.1164/rccm.201507-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossone E, Ferrara F, Grünig E. Echocardiography in pulmonary hypertension. Curr Opin Cardiol. 2015;30:574–586. doi: 10.1097/HCO.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 9.Goh ZM, Balasubramanian N, Alabed S, Dwivedi K, Shahin Y, Rothman AMK, et al. Right ventricular remodelling in pulmonary arterial hypertension predicts treatment response. Heart. 2022;108:1392–1400. doi: 10.1136/heartjnl-2021-320733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badano LP, Ginghina C, Easaw J, Muraru D, Grillo MT, Lancellotti P, et al. Right ventricle in pulmonary arterial hypertension: haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur J Echocardiogr. 2010;11:27–37. doi: 10.1093/ejechocard/jep152. [DOI] [PubMed] [Google Scholar]
- 11.Galiè N, Hinderliter AL, Torbicki A, Fourme T, Simonneau G, Pulido T, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and Doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:1380–1386. doi: 10.1016/S0735-1097(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 12.Bhave NM, Patel AR, Weinert L, Yamat M, Freed BH, Mor-Avi V, et al. Three-dimensional modeling of the right ventricle from two-dimensional transthoracic echocardiographic images: utility of knowledge-based reconstruction in pulmonary arterial hypertension. J Am Soc Echocardiogr. 2013;26:860–867. doi: 10.1016/j.echo.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Cordina RL, Playford D, Lang I, Celermajer DS. State-of-the-art review: echocardiography in pulmonary hypertension. Heart Lung Circ. 2019;28:1351–1364. doi: 10.1016/j.hlc.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 15.Vonk Noordegraaf A, Channick R, Cottreel E, Kiely DG, Marcus JT, Martin N, et al. The REPAIR study: effects of Macitentan on RV structure and function in pulmonary arterial hypertension. JACC Cardiovasc Imaging. 2022;15:240–253. doi: 10.1016/j.jcmg.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 16.van Wolferen SA, Marcus JT, Boonstra A, Marques KMJ, Bronzwaer JGF, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 17.Alabed S, Shahin Y, Garg P, Alandejani F, Johns CS, Lewis RA, et al. Cardiac-MRI predicts clinical worsening and mortality in pulmonary arterial hypertension: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brewis MJ, Bellofiore A, Vanderpool RR, Chesler NC, Johnson MK, Naeije R, Peacock AJ. Imaging right ventricular function to predict outcome in pulmonary arterial hypertension. Int J Cardiol. 2016;218:206–211. doi: 10.1016/j.ijcard.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard LS, Grapsa J, Dawson D, Bellamy M, Chambers JB, Masani ND, et al. Echocardiographic assessment of pulmonary hypertension: standard operating procedure. Eur Respir Rev. 2012;21:239–248. doi: 10.1183/09059180.00003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghio S, Klersy C, Magrini G, D'Armini AM, Scelsi L, Raineri C, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140:272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 21.Hulshof HG, Eijsvogels TMH, Kleinnibbelink G, van Dijk AP, George KP, Oxborough DL, Thijssen DHJ. Prognostic value of right ventricular longitudinal strain in patients with pulmonary hypertension: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2019;20:475–484. doi: 10.1093/ehjci/jey120. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Park MM, Farha S, Sharp J, Lundgrin E, Comhair S, et al. Impaired global right ventricular longitudinal strain predicts long-term adverse outcomes in patients with pulmonary arterial hypertension. J Cardiovasc Ultrasound. 2015;23:91–99. doi: 10.4250/jcu.2015.23.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, Jacobs W, Marcus JT, Marques KM, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011;139:1003–1009. doi: 10.1378/chest.10-1066. [DOI] [PubMed] [Google Scholar]
- 24.Farmakis IT, Demerouti E, Karyofyllis P, Karatasakis G, Stratinaki M, Tsiapras D, et al. Echocardiography in pulmonary arterial hypertension: is it time to reconsider its prognostic utility? J Clin Med. 2021;10:2826. doi: 10.3390/jcm10132826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 26.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 27.Brugger N, Lichtblau M, Maeder M, Muller H, Pellaton C, Yerly P, Swiss SFPHS Two-dimensional transthoracic echocardiography at rest for the diagnosis, screening and management of pulmonary hypertension. Swiss Med Wkly. 2021;151:w20486. doi: 10.4414/smw.2021.20486. [DOI] [PubMed] [Google Scholar]
- 28.Hinderliter AL, Willis PW, Barst RJ, Rich S, Rubin LJ, Badesch DB, et al. Effects of long-term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Primary Pulmonary Hypertension Study Group. Circulation. 1997;95:1479–1486. doi: 10.1161/01.cir.95.6.1479. [DOI] [PubMed] [Google Scholar]
- 29.Freed BH, Tsang W, Bhave NM, Patel AR, Weinert L, Yamat M, et al. Right ventricular strain in pulmonary arterial hypertension: a 2D echocardiography and cardiac magnetic resonance study. Echocardiography. 2015;32:257–263. doi: 10.1111/echo.12662. [DOI] [PubMed] [Google Scholar]
- 30.Gopal AS, Chukwu EO, Iwuchukwu CJ, Katz AS, Toole RS, Schapiro W, Reichek N. Normal values of right ventricular size and function by real-time 3-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2007;20:445–455. doi: 10.1016/j.echo.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Mauritz GJ, Marcus JT, Boonstra A, Postmus PE, Westerhof N, Vonk-Noordegraaf A. Non-invasive stroke volume assessment in patients with pulmonary arterial hypertension: left-sided data mandatory. J Cardiovasc Magn Reson. 2008;10:51. doi: 10.1186/1532-429X-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.