Abstract
Cardiovascular disease is the primary cause of pregnancy-related mortality and morbidity in the United States, and maternal mortality has increased over the last decade. Pregnancy and the postpartum period are associated with significant vascular, metabolic, and physiologic adaptations that can unmask new heart failure or exacerbate heart failure symptoms in women with known underlying cardiomyopathy. There are unique management considerations for heart failure in women throughout pregnancy, and it is imperative that clinicians caring for pregnant women understand these important principles. Early involvement of multidisciplinary cardio-obstetrics teams is key to optimizing maternal and fetal outcomes. In this review, we discuss the unique challenges and opportunities in the diagnosis of heart failure in pregnancy, management principles along the continuum of pregnancy, and the safety of heart failure therapies during and after pregnancy.
Keywords: Pregnancy, Heart failure, Cardiomyopathy, Cardio-obstetrics, Peripartum cardiomyopathy
Key Summary Points
| Cardiomyopathy in pregnancy contributes to maternal mortality rates in the United States and is associated with adverse maternal and fetal events. |
| Pregnancy is associated with significant hemodynamic adaptations that can worsen underlying heart failure or unmask de novo heart failure in women throughout the continuum of pregnancy. |
| There are key treatment considerations in heart failure management in pregnant women that must balance the maternal benefits of the therapy against the potential fetal risks associated with the therapy. |
| Counseling and shared decision-making is the cornerstone in the management of heart failure in pregnancy. |
| A multidisciplinary cardio-obstetrics team should be involved in the care of high-risk birthing individuals early in and throughout pregnancy to optimize maternal and fetal outcomes. |
Introduction
Cardiovascular disease (CVD) is the leading cause of pregnancy-related morbidity and mortality in the United States (US) and globally, and the prevalence of CVD has gradually increased over time [1–4], in part due to advanced maternal age and an increasing comorbidity burden [5]. Cardiomyopathy accounts for over 11% of maternal deaths in the US [6]. Pregnancy is widely recognized as a time of significant hemodynamic adaptation, and undiagnosed cardiovascular disease in at-risk women or heart failure (HF) in women with known underlying cardiomyopathy can be unmasked during this time. Pregnancy is a critical period in a woman’s life, warranting close observation and management, especially in those at high risk for cardiovascular decompensation. The peripartum period is also a unique opportunity for early detection and risk mitigation of future cardiovascular disease [3]. In this review, we discuss some common HF syndromes in pregnancy, key diagnostic and treatment considerations in the management of HF in pregnant women. In particular, we review the unique challenges and opportunities in the diagnosis of HF in pregnancy, evidence-based management principles along the continuum of pregnancy, and the safety of HF medications and therapies during pregnancy and the lactation period. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Heart Failure in Pregnancy
Common HF Syndromes in Pregnancy
Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a rare but serious cardiac complication of pregnancy and is diagnosed when new onset cardiomyopathy (defined as a left ventricular ejection fraction [LVEF] < 45%) develops toward the end of pregnancy or in the months following delivery [7]. It is a diagnosis of exclusion and considered when no other cause of cardiomyopathy is apparent. Several risk factors have been associated with PPCM and include African ancestry, older maternal age, hypertensive disorders of pregnancy, and multi-gestational pregnancies [8]. Of the various prognostic factors that have been studied, LVEF at the time of PPCM diagnosis is the most reliable predictor of adverse events or long-term recovery. The IPAC (Investigations of Pregnancy Associated Cardiomyopathy) study followed 100 women in North America with PPCM for 1 year after delivery and found that LVEF < 30% was associated with lower rates of myocardial recovery and an increased risk of adverse events [9]. Severe left ventricular (LV) systolic dysfunction at PPCM presentation has also been shown to be associated with the presence of a genetic variant causal for dilated cardiomyopathy (DCM) [10]. Additional predictors of worse outcome include LV dilation (LV end diastolic diameter > 6 cm), LV thrombus, right ventricular systolic dysfunction, obesity, and African ancestry [8]. Subgroup analyses of the IPAC cohort further demonstrated an association between baseline LVEF and LVEF at 1 year only in Black women and that Black race was independently associated with lower LVEF at 1 year.
Medical management of PPCM is similar to general HF management in pregnant women and includes guideline-directed medical therapy (GDMT), which is discussed later in this review. In addition, there are ongoing clinical trials of the use of bromocriptine, a dopamine agonist that inhibits the release of prolactin, in PPCM. In small studies of European and African participants, bromocriptine in addition to standard therapy was associated with a higher rate of LVEF recovery [11, 12], though no significant difference in overall rates of recovery was seen in subsequent trials [12, 13]. The 2018 European Society of Cardiology (ESC) guidelines include a weak recommendation (class IIb, level of evidence: B) for the use of bromocriptine in PPCM [5]. A randomized double-blind, placebo-controlled study (REBIRTH [Randomized Evaluation of Bromocriptine In Myocardial Recovery Therapy]) to investigate the effect of bromocriptine on myocardial recovery and clinical outcome in 200 women with PPCM in the United States and Canada is currently enrolling. Due to its association with thrombotic complications, therapeutic anticoagulation is recommended if bromocriptine is used. Women with a history of PPCM carry a risk of relapse with subsequent pregnancies, and dedicated counseling and monitoring are essential. The risks associated with a subsequent pregnancy depend primarily upon recovery of myocardial function, and the pre-pregnancy LVEF is the strongest predictor of outcomes.
Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) refers to a large group of heterogeneous myocardial disorders characterized by depressed LV systolic function and LV dilation [14]. DCM can be caused by different myocardial processes, including ischemic heart disease, inflammatory insults such as myocarditis, genetic cardiomyopathies, and direct cardiotoxins such as alcohol or chemotherapy. Up to half of all cases of DCM are considered idiopathic [15]. Women with underlying DCM who become pregnant are at the highest risk for adverse maternal or fetal outcomes in the setting of moderate to severe LV dysfunction (LVEF < 45%) or a poor functional class prior to pregnancy (New York Heart Association [NYHA] III/IV) [16]. The hemodynamic challenge of pregnancy, labor, and delivery may result in further LV dilation and clinical decompensation. Furthermore, women who become pregnant appropriately interrupt their use of evidence-based HF medications that are known to be teratogenic, such as angiotensin-converting enzyme (ACE) inhibitors, which could delay the remodeling benefits or result in worsening HF. The long-term impact of pregnancy in women with DCM remains unclear [17].
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is diagnosed in the setting of asymmetric LV hypertrophy (> 15 mm) in the absence of another cause. Hypertrophy can be present with or without left ventricular outflow tract (LVOT) obstruction, which can become especially significant in pregnancy. During pregnancy, the increased plasma volume and stroke volume are well tolerated and can, in fact, lower a dynamic LVOT gradient. However, the tachycardia, decreased afterload, and increased LV contractility of pregnancy can worsen obstructive physiology [18]. Beta blockers, the first-line therapy for symptomatic obstructive HCM, are generally safe during pregnancy and should be continued in pregnant women with HCM. Any associated arrhythmias, such as atrial fibrillation, may be poorly tolerated, and non-pharmacologic interventions such as cardioversions can still be considered. Most women with HCM will experience pregnancy and labor with minimal risk [19]. As with other cardiomyopathies, peripartum risk is highest in those with advanced symptoms, severe LV systolic dysfunction, and severe LVOT obstruction [17]. The risk of experiencing an adverse cardiac event is proportional to the degree of symptoms and/or the degree of LVOT obstruction prior to pregnancy. Limited retrospective and observational data have shown that the incidence of maternal death in individuals with HCM was low at 0.2%, with a similarly low occurrence of neonatal death (0.2%) [20]. Family planning and pre-pregnancy counseling are especially important for women with HCM, as 40–60% of individuals have an identifiable genetic etiology of disease that is largely inherited in an autosomal dominant pattern. Pre-implantation genetic testing can be offered to individuals with HCM who are considering expanding their families. Women should also be prospectively counseled about the risks of future pregnancy [15].
Diagnosis
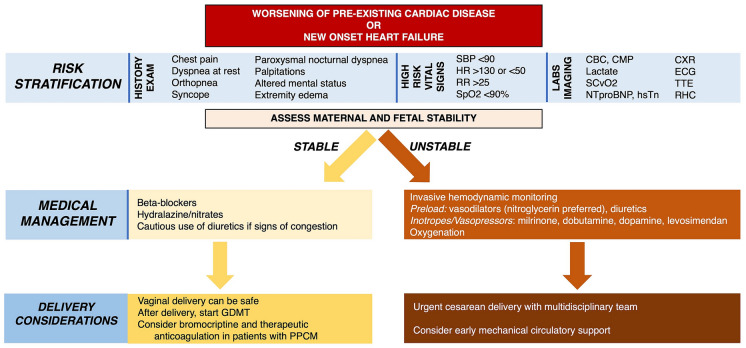
There is substantial overlap between symptoms that commonly accompany normal pregnancy and those that signal early manifestations of cardiovascular disease, such as dyspnea, anemia, weight gain, and edema [21], and this can delay the detection and timely management of HF in pregnancy. However, there are important signs and symptoms that should prompt a review by a cardiologist, including presentation with chest pain, dyspnea at rest or with minimal exertion (especially when sudden in onset), orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, syncope, sustained palpitations, new systolic or diastolic murmurs (excluding flow murmurs), persistent tachycardia > 100 beats per minute, cyanosis or clubbing, an S4, pulmonary rales, and/or elevated jugular venous pressure (Fig. 1). Pregnant women with suspected HF should undergo guideline-recommended imaging and laboratory testing for further evaluation [22].
Fig. 1.
Assessment of worsening symptoms or acute heart failure in pregnancy. CBC complete blood count, CMP comprehensive metabolic panel, CXR chest X-ray, ECG electrocardiogram, GDMT guideline-directed medical therapy, HR heart rate, NTproBNP N-terminal-pro B-type natriuretic peptide, hsTn high-sensitivity troponin, PPCM peripartum cardiomyopathy, RHC right heart catheterization, RR respiratory rate, SBP systolic blood pressure, SCvO2 central mixed venous oxygen saturation, SpO2 oxygen saturation, TTE transthoracic echocardiography
Role of Imaging
Echocardiography remains the first-line imaging modality for the initial evaluation of pregnant women with suspected cardiovascular disease because of its availability, cost-effectiveness, and safety. Computed tomography and cardiac catheterization may be considered where indicated, but the risks of teratogenicity with ionizing radiation must be weighed, and shared decision making and informed consent must be obtained. If used, radiation must be kept ‘as low as reasonably achievable,’ and doses should be kept as low as possible (preferably < 50 mGy) [5]. Magnetic resonance imaging is advised if other noninvasive tests are not sufficient for definitive diagnosis and is preferred over ionizing-radiation-based imaging modalities [5]. Evidence regarding gadolinium-based contrast in pregnancy is controversial; its use should be avoided if possible, especially in the first trimester. Excretion of gadolinium-based agents into breast milk is limited, and breastfeeding after gadolinium administration is generally felt to be safe.
Role of Biomarkers
N-terminal pro–brain-type natriuretic peptide (NT-proBNP) levels can be elevated in normal pregnancy. The median NT-pro BNP level in normal pregnant woman is about twice that in non-pregnant women, rising early in pregnancy and remaining high throughout gestation [23, 24] until about 72 h after delivery [25]. The best use of NT-proBNP in pregnancy is in its negative predictive value. In one series, the negative predictive value of NT-proBNP < 128 pg/mL at 20 weeks’ gestation was 96.9% [26]. Adverse maternal cardiac events have been associated with high concentrations (> 128 pg/mL) [26], and NT-proBNP levels > 200 pg/mL should raise concern for HF and/or pre-eclampsia [27]. Cardiac troponin (cTn) assays using high-sensitivity methods can also provide a key role in the diagnosis of acute or chronic cardiac injury in pregnant women with cardiomyopathy, and in some cases may signal early cardiac injury prior to detection on imaging [28]. An elevated troponin level is never normal in pregnancy and should prompt further evaluation for myocardial injury [27, 29–31]. The combined measurement of natriuretic peptides and cTn may provide complementary information in patients with cardiovascular diseases. Outside of obstetrics, prior meta-analyses have shown that individuals with no HF symptoms but who had elevated cTn levels are at greater risk for future cardiovascular events and also for progression to HF [32]. Furthermore, the combination of NT-proBNP with cTn improves risk stratification in non-ST elevation acute myocardial infarctions [33]. Further work is needed to further elucidate the complementary roles of cardiac biomarkers in pregnant populations.
Management
There are unique management considerations for women with pre-existing cardiomyopathy as well as for those women with de novo cardiomyopathy with planned or ongoing pregnancies. When at-risk women of reproductive age desire conception, there should be an expert assessment of cardiovascular and obstetrical risk, counseling and shared decision making regarding the safety of pregnancy, and safe and effective contraception should be offered if pregnancy is contraindicated. Additional considerations include lactation goals and risk mitigation for future cardiovascular disease. A comprehensive assessment should include a complete history, including relevant family and social history, and a physical examination with electrocardiography, echocardiography, and other testing, including natriuretic peptide assessment if there is suspicion of or known CVD. If CVD is present or suspected, functional assessment (e.g., exercise echocardiography) can be considered.
Pre-conception Assessment in Women with Heart Failure
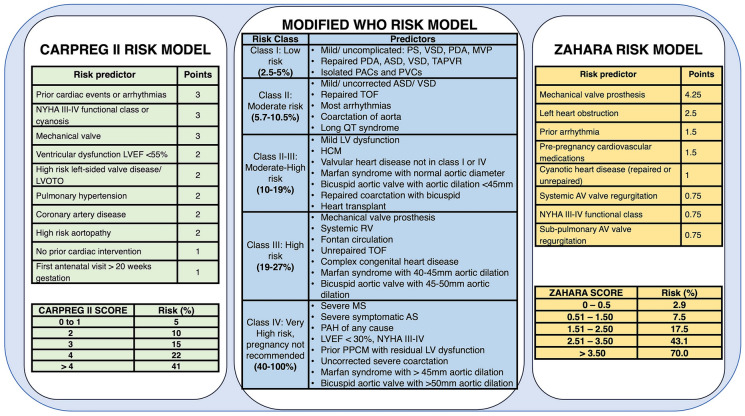
There are various risk scores that are used to risk stratify women at the pre-conception stage, the most commonly used being the CARPREG (Cardiac Disease in Pregnancy Study) II [34, 35], modified WHO (World Health Organization) [36, 37], and ZAHARA II (Zwangerschap bij Aangeboren HARtAfwijkingen [Pregnancy in Women with Congenital Heart Disease]) [38] scores (Fig. 2). Notably, all incorporate cardiac lesions that require echocardiography. Available risk scores are only applicable to women with known CVD and do not aid in the risk stratification of women without pre-existing CVD. The CARPREG II score was derived from a single-center cohort of > 1,900 pregnancies and assigns points based on risk predictors shown in Fig. 2. Women with CARPREG II scores > 4 are generally considered at high risk, with a risk for maternal complications of up to 41% [34]. The modified WHO (mWHO) risk assessment system assigns a class ranging from I to IV to women. Women who are assessed to be class I are expected to have a risk of maternal adverse cardiac events similar to that of the general population. Those who are assigned class IV risk are at significantly elevated risk for complications (40–100%), and for these individuals, pregnancy is contraindicated [36]. Cardiac assessment should be performed regularly during pregnancy on the basis of mWHO class. Patients in class II should be evaluated every trimester, those in class III monthly or bimonthly, and those in class IV monthly [39]. Class IV patients should be offered the option of termination, recognizing that these recommendations need to be considered in the context of health care systems, resources, and policies. The ZAHARA assigns points to various risk predictors, with the highest score for women with mechanical valves. Scores > 3.50 are considered to be indicative of the highest risk (70%) for adverse outcome with pregnancy [38].
Fig. 2.
Risk stratification tools and models for the assessment of maternal cardiac morbidity and mortality during pregnancy: Cardiac Disease in Pregnancy Study (CARPREG) II [33], modified World Health Organization (WHO) [34], and ZAHARA (Zwangerschap bij Aangeboren HARtAfwijkingen [Pregnancy in Women with Congenital Heart Disease]) [35]. AS aortic stenosis, ASD atrial septal defect, AV atrioventricular, HCM hypertrophic cardiomyopathy, LV left ventricle, LVEF left ventricular ejection fraction, LVOTO left ventricular outflow tract obstruction, MS mitral stenosis, MVP mitral valve prolapse, NYHA New York Heart Association, PAC premature atrial contraction, PAH pulmonary arterial hypertension, PDA patent ductus arteriosus, PPCM peripartum cardiomyopathy, PS pulmonic stenosis, PVC premature ventricular contraction, RV right ventricle, TAPVR total anomalous pulmonary vein return, TOF tetralogy of Fallot, VSD ventricular septal defect
Counseling
Preconception counseling requires that intentions surrounding pregnancy are solicited during all routine cardiovascular visits and ensures that a plan for reliable contraception is in place. Tools such as the One Key Question® of “Would you like to become pregnant in the next year?” [40] can be incorporated into all encounters for women of reproductive potential. This simple screening question provides an opportunity to further explore pregnancy risk. Women with high-risk cardiovascular lesions should be referred for evaluation and counseling to a center with multidisciplinary cardio-obstetric expertise [41]. However, even among women at the highest risk for adverse maternal cardiac events, data suggest that the prevalence of preconception counseling is low, highlighting critical care gaps for this population [42].
Contraception
For patients not desiring conception or those for whom pregnancy is a prohibitive risk, contraception should be addressed. Progestin-releasing intrauterine devices are the preferred contraception option for women with high-risk cardiac conditions [43]. Combined hormonal contraceptives (CHCs) are generally avoided, especially in women in mWHO risk classes III and IV, as well as in women with left ventricular assist devices (LVADs) and heart transplants, given the increased risk of hypertension, venous thromboembolism, and fluid retention [36, 44]. If alternative methods of contraception are not possible, shared decision making must be conducted, and the risks of an unplanned pregnancy must be weighed against potential risks of CHCs. CHCs may be used in lower-risk pregnant women with close monitoring. It is generally accepted that barrier methods alone have high failure rates and are not recommended in any patient as a sole method of contraception. Tubal ligation and vasectomies should only be pursued after thorough counseling and shared decision making with the patient. With regard to emergency contraception, the copper intrauterine device and emergency contraceptive pills such as ulipristal acetate and progestin-only levonorgestrel may be safely used [45]. As with CHCs, combined estrogen and progestin for emergency contraception should be used cautiously.
Treatment Considerations During Pregnancy and Breastfeeding
Pregnant women with HF should be treated with GDMT that balances the maternal benefits against the potential fetal risks. For women who are planning to conceive or pregnant, medications should be reviewed to minimize exposure to any potential teratogens. Drug dosages should be adjusted to account for pregnancy-associated physiological changes that could alter drug pharmacokinetics and pharmacodynamics [5, 46]. The following sections discuss drugs commonly used in stable HF, acute HF, and refractory HF in pregnant and lactating women, and these drugs are also summarized in Table 1.
Table 1.
Safety of medications commonly used for the treatment of heart failure in pregnant and lactating women
| Medication class | Safe during pregnancy? | Safe while breastfeeding? | Comments |
|---|---|---|---|
| Beta blockers [45, 47–50] | |||
| – Metoprolol | Yes | Yes | Higher doses of beta blockers are associated with low fetal birth weight and hypoglycemia |
| – Bisoprolol | Yes | Yes | |
| – Carvedilol | Unknown | Unknown | |
| – Atenolol | No | No | |
| ACEi/ARB/ARNi [46, 51] | No | Captopril, benazepril, and enalapril considered safe | Teratogenic, with risks for oligohydramnios and skeletal, cranial, and fetal renal malformations |
| MR antagonists [5, 46, 52] | |||
| – Spironolactone | No | Yes | – Spironolactone is associated with antiandrogenic effects on fetus |
| – Eplerenone | Yes | Yes | – Eplerenone is associated with post-implantation losses at the highest administered doses in rabbits |
| SGLT2i [45, 53] | |||
| – Dapagliflozin | No | No | Insufficient data for pregnant or breastfeeding humans; renal harm noted in fetuses of rats |
| – Empagliflozin | No | No | |
| Loop diuretics [45, 46] | Yes | Yes, but can suppress lactation at high doses | More data with furosemide than with torsemide, bumetanide, and metolazone. Can be associated with oligohydramnios; close monitoring is warranted |
| Digoxin [46, 55, 56] | Yes | Yes | No adverse effects on the mother or fetus have been observed [72]. Digoxin intoxication has, however, been associated with miscarriage and fetal death [80, 81], so periodic drug monitoring is encouraged, especially given the altered pharmacokinetics with physiologic pregnancy changes |
| HCN channel blocker [97, 98] | |||
| – Ivabradine | No | No | Ivabradine is associated with embryonic bradycardia, hypoxia, malformations, and death in animal studies |
| sGC stimulator [99] | |||
| – Vericiguat | No | No | Vericiguat is associated with fetal harm in animal studies. No data on excretion in breastmilk |
| Inotropes [8, 45, 68, 69] | Used similarly in non-pregnant patients. Some suggestion of increased harm with beta-agonists in severe PPCM | ||
| – Dopamine | Yes | Yes | |
| – Dobutamine | Yes | Yes | |
| – Milrinone | Yes | Yes | |
| – Levosimendan | Yes | Yes | |
| Vasodilators [71, 72] | Nitroglycerin preferred over nitroprusside due to toxic fetal cyanide levels with latter | ||
| – Nitrates | Yes (except nitroprusside) | Yes | |
| – Calcium channel blockers | Yes | Yes | |
| – Hydralazine | Yes | Yes | |
| – Methyldopa | Yes | Yes | |
| Anticoagulants [5, 46, 57–59, 61–64, 100] | |||
| – VKA | Only at low doses in high risk scenarios | Yes | VKAs only to be considered for mechanical valves and LVADs and at doses of less than 5 mg/day |
| – LMWH | Yes | Yes | Close monitoring of factor Xa levels for LMWH |
| – DOACs | No | No | Limited data on DOAC use in pregnancy |
| Immunosuppressive agents [89] | |||
| – Corticosteroids | Yes | Yes | Cleft palate at high steroid doses |
| – Calcineurin inhibitors | Yes | Yes | Close monitoring and dose adjustments of calcineurin inhibitors needed in pregnancy |
| – mTOR inhibitors | Insufficient data | Insufficient data | Evaluate mTOR use on a case-by-case basis and discontinue use 6–12 weeks before conception if able |
| – Mycophenolate | No | No | Mycophenolate is teratogenic; associated with a high rate of spontaneous abortions and congenital malformations |
| – Azathioprine | Yes | Yes | Azathioprine with no evidence of teratogenic effects |
ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, ARNi angiotensin-receptor neprilysin inhibitor, MR mineralocorticoid receptor, SGLT2i sodium glucose cotransporter 2 inhibitor, HCN hyperpolarization-activated cyclic nucleotide-gated, sGC soluble guanylyl cyclase, VKA vitamin K antagonist, LMWH low molecular weight heparin, LVAD left ventricular assist device, DOAC direct oral anticoagulant, mTOR mammalian target of rapamycin, PPCM peripartum cardiomyopathy
Stable Heart Failure
Beta Blockers
Beta-adrenergic blocking agents are generally safe in pregnancy but may be associated with hypoglycemia and increased rates of fetal growth restriction [47, 48]. However, the overall magnitude of these neonatal complications is not thought to outweigh the maternal benefits of therapy. Beta blockers should be cautiously used in pregnancy. Beta-1-selective drugs such as metoprolol and bisoprolol are preferred [47], as they are less likely to affect uterine contraction and peripheral vasodilation and are associated with lower rates of fetal growth restriction [48]. Nonselective beta blockers such as atenolol have been associated with higher rates of fetal growth restriction and so are generally avoided in pregnancy [49]. Among the alpha/beta blockers, labetalol is the drug of choice for hypertension in pregnancy [50]. Data for carvedilol use in pregnancy are limited, though in a recently published small study with 13 patients with HF receiving this drug, there was no association with fetal growth restriction [48]. The use of metoprolol, bisoprolol, and labetalol in lactating women is generally considered safe. Beta blockers should be continued in patients on an existing beta-blocker therapy, cautiously started in those with new cardiomyopathies, and offered to HCM patients with signs of LVOT obstruction or arrhythmias.
ACE Inhibitors, Angiotensin-Receptor Blockers, Angiotensin-Receptor Neprilysin Inhibitors
Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin-receptor blockers (ARBs) are teratogenic and contraindicated during pregnancy [46]. Renal or tubular dysplasia, renal failure, oligohydramnios, growth retardation, ossification disorders of the skull, lung hypoplasia, contractures, large joints, anemia, and intrauterine fetal death have been described. In a systematic review, 48% of 118 fetuses exposed to ACEi and 87% of fetuses exposed to ARBs had complications related to their use [46]. These risks also apply to the angiotensin-receptor neprilysin inhibitor (ARNI) since it contains an ARB. ACEi can be used during lactation; in particular, enalapril and captopril appear safe as long as fetal weight is monitored every 4 weeks. ARB use during lactation, however, is not well described, and their use postpartum is cautiously advised with breastfeeding [51].
Aldosterone Antagonists
Aldosterone antagonist use is not advised in pregnancy [46]. Spironolactone has been associated with feminization of developing male rats [52], raising concern about anti-androgenic effects during the first trimester. Eplerenone has been associated with post-implantation losses at the highest administered doses in rabbits [5], so caution is advised when used in pregnant humans. Both spironolactone and eplerenone may be used safely during breastfeeding.
Sodium Glucose Cotransporter 2 Inhibitors
There are limited data regarding the use of sodium glucose cotransporter 2 (SGLT2) inhibitors. Prior animal studies have suggested that that SGLT2 inhibition may affect fetal renal development and maturation [53], so their use is not recommended during pregnancy or with breastfeeding [45].
Diuretics
Loop diuretics can be safely used in pregnancy but can result in oligohydramnios and increase the risk of electrolyte imbalance in the fetus [46]. Diuretics have also been suggested to suppress lactation at high doses [54]. Given these important physiologic concerns, diuretic use should be judicious and mainly limited to patients with clinical signs of congestion. More data exist on the use of furosemide than torsemide, bumetanide, or metolazone in pregnancy, so the former is usually the first-line therapy [45].
Digoxin
Digoxin is generally used in pregnant women with persistent HF symptoms who are on beta blockers, nitrate/hydralazine, and diuretics. Digoxin crosses the placenta readily during later pregnancy, but no adverse maternal or fetal effects have been observed [46]. Digoxin intoxication has, however, been associated with miscarriage and fetal death [55, 56], so periodic drug monitoring is encouraged, especially given the altered pharmacokinetics in pregnancy.
Anticoagulants
The most widely used anticoagulants in pregnancy are vitamin K antagonists (VKAs), unfractionated heparin (UFH), and low molecular weight heparins (LMWH); timing and indication are key to their safety. VKAs such as warfarin cross the placenta and can result in embryopathy (limb defects and nasal hypoplasia) in a dose-dependent manner in 0.6–10% of cases when used in the first trimester [57, 58]. Warfarin also carries a 0.7–2% risk of fetopathy, such as ocular and central nervous system abnormalities and intracranial hemorrhage beyond the first trimester [57, 59], and so it is generally avoided throughout pregnancy. However, in high-risk scenarios such as the presence of prosthetic mechanical valves and LVADs, warfarin may be used throughout pregnancy at doses of less than 5 mg/day, though counseling is still required [5]. In general, LMWH are the preferred anticoagulant for use throughout pregnancy as they have not been associated with embryopathy or fetopathy [5]. Close monitoring of factor Xa levels is recommended when LMWH are used. Special consideration is needed regarding the use of anticoagulation around delivery given the importance of epidural anesthesia during labor and for uterine hemostasis after delivery [60]. LMWH should be switched to UFH at least 36 h before the induction of labor or cesarean delivery to minimize the bleeding risk during delivery [5]. Factor Xa inhibitors are considered when there is an allergy or adverse response to heparin [61]; however, data on their safe use in pregnancy are sparse. One study showed minor transplacental passage of fondaparinux [62], and more work is required to assess the risk of congenital malformations. Direct oral anticoagulants (DOACs) have been shown to be harmful to the fetus in animal studies, and data in humans are limited [46]. They should be avoided in pregnant patients. A systematic review of 137 pregnancies on rivaroxaban revealed a miscarriage rate of 23% (n = 31), elective terminations in 29% (n = 39) of cases, and possible embryopathy in 2.2% (n = 3) of cases [63]. Thrombolytics are considered to be relatively contraindicated and should only be used in high-risk patients with severe hypotension or shock [64]. Maternal hemorrhagic complications can occur with all regimens [59].
Cardiac Implantable Electronic Devices
Pregnant women with advanced HF may require cardiac implantable electronic devices (CIEDs), including permanent pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy devices. Data on the use and safety of these devices in pregnancy are limited. In two small cohorts of pregnant women with CIEDs, the live birth rate was 70–95% [65, 66]. Between the two cohorts, three of 39 pregnancies were complicated by ventricular arrhythmias, and in two cases, women received appropriate ICD shocks.
For women with ICDs in place at the time of cesarean delivery, a magnet should be placed to prevent electric interference with electrocautery [39, 45]. Transcutaneous pads can be applied for added safety at the time of delivery. Data on ICD implantation in pregnant women with de novo systolic dysfunction is limited. In the case of primary prevention, implantation should be ideally deferred until after delivery, and after at least 3 months of GDMT if LV function remains depressed. If an ICD is indicated more expeditiously for secondary prevention, fluoroscopy during implantation should be minimized as much as possible. In some cases, a subcutaneous defibrillator may be necessary to avoid excessive fluoroscopy exposure [45]. The data on the benefit of a wearable cardioverter defibrillator (WCD) in the general population are controversial [67], and such data in pregnancy are sparse. In general, these devices should be considered for women with HF who do not meet the duration of time on GDMT required for primary prevention ICD implantation or in whom LVEF recovery is anticipated [45].
Acute Severe Heart Failure
Inotropes
In cardiogenic shock, decision making regarding the use of inotropes and vasodilators in the pregnant patient should follow the same considerations as for the non-pregnant patient. If inotropic support is required during pregnancy, milrinone, dobutamine, dopamine, and levosimendan are generally considered safe. There is some concern about excessive vasodilation with milrinone in the setting of already decreased systemic vascular resistance in pregnancy [45], though this is not prohibitive. Dopamine may also reduce breastmilk production [68], though this is not rigorously established and does not limit its use. Possible adverse effects have been reported with dobutamine in patients with severe PPCM [69]. Unlike epinephrine (which is generally avoided in pregnancy), levosimendan, an inodilator and calcium sensitizer, is not known to increase myocardial oxygen demand, and is recommended by the ESC as the preferred inotrope for use in pregnant women with cardiogenic shock; however, this is not currently approved by the Food and Drug Administration (FDA) for use in the United States [70].
Vasodilators
If vasodilator therapy is required, nitroglycerin is preferred over nitroprusside due to potential concerns related to toxic fetal cyanide levels in animal models exposed to nitroprusside [71], though this finding has not been replicated in humans. Calcium channel blockers such as intravenous nicardipine do not seem to pose a teratogenic risk to the fetus and are safe for use in pregnancy [72]. If continuous infusions are not needed, oral alternatives such as oral nitrates, methyldopa, and hydralazine are also safe for use in pregnancy and in the postpartum period [71].
Refractory Heart Failure
Mechanical Circulatory Support
Mechanical circulatory support (MCS) should be considered when severe heart failure persists despite maximal pharmacological support. Early initiation of MCS can mitigate the hemometabolic consequences of cardiogenic shock (CS) [73]. Data from the National Inpatient Sample have shown that pregnant women who developed CS and were treated with MCS within 6 days of the diagnosis of CS had lower mortality compared with those who were placed on MCS later [74]. However, there are no prospective studies investigating the optimal use of MCS in the peripartum period. Though the intraaortic balloon pump (IABP) only provides 0.5–1.0 L/min of cardiac output, it can be quickly and easily placed at the bedside and may not mandate anticoagulation [71]. IABPs have been successfully used for hemodynamic support in pregnant patients who have undergone surgery requiring cardiopulmonary bypass [75, 76] and during cesarean section prior to percutaneous coronary intervention or coronary bypass surgery [77]. IABP use has also been successful in patients with severe PPCM [78]. The Impella (Abiomed, Danvers, MA, USA) device can provide up to 5.0 L/min of cardiac output and has also successfully been used in cases of severe PPCM [79, 80] as well as in cardiac arrest in pregnancy [81, 82]. If additional hemodynamic support is required, extracorporeal membrane oxygenation (ECMO) may be considered and has been successfully used in pregnancy [81, 83]. However, as with Impella, ECMO carries both bleeding and thrombosis risks [8, 80], so both temporary MCS platforms require adherence to specialized anticoagulation protocols. Another unique challenge is that prolactin levels have been shown to increase in pregnant patients supported on ECMO, and elevated prolactin levels are associated with poor outcomes [84]. The ESC recommends the suppression of prolactin while on ECMO with doses of bromocriptine of up to 10 mg twice daily [70].
For pregnant patients with persistent LV systolic dysfunction, durable MCS may be an option as a bridge to recovery, a bridge to heart transplantation, or as destination therapy. In one of the largest observational studies on this topic, an Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis identified 99 women with PPCM who underwent surgically implanted left, right, or biventricular assist devices between June 2006 and March 2012 and compared their outcomes with those who did not have PPCM. Women with PPCM had improved survival, with a 2-year survival of 83%. Recovery occurred at a frequency of 6% and 2% in the PPCM and non-PPCM groups, respectively. Adverse event rates were similar in patients with and without PPCM except for higher cardiac arrhythmias and respiratory failure in the non-PPCM group in the first 3 months post-implant [85].
For women with pre-existing durable MCS devices, pregnancy is contraindicated, given the risk of worsening biventricular failure from volume expansion, increased thrombotic risk, teratogenicity of HF medications, complications associated with anticoagulation, and risk of RV failure related to impingement of the enlarging uterus on LVAD components. Nevertheless, unplanned pregnancies still occur, and successful births have been reported [45]. As hemodynamics evolve during pregnancy and postpartum, changes in LVAD speed may be required to provide increased cardiac output and maintain optimal unloading conditions [45]. At delivery, invasive hemodynamic monitoring should be considered. Vaginal delivery is the generally preferred mode of delivery; however, if cesarean delivery is needed, there should be careful coordination with LVAD experts on device and hemodynamic management. With respect to anticoagulation, there are no specific guideline recommendations for patients with LVAD in pregnancy. Extrapolating the recommendations used for the management of prosthetic mechanical valves, patients on less than 5 mg/day of warfarin are maintained on this throughout pregnancy for international normalized ratio (INR) goal 2–3, though patients need to be counseled on the risk of warfarin embryopathy [86]. Patients requiring doses larger than 5 mg/day before pregnancy (and those who, after counseling, decline the use of any warfarin during pregnancy) should be transitioned to LMWH during the first trimester, with close monitoring of anti-Xa levels. Pregnancy in patients supported by durable LVADs requires early involvement of a multidisciplinary cardio-obstetrics team in a center with LVAD expertise [45].
Heart Transplantation
The majority of data on heart transplantation in pregnant women comes from the PPCM literature. Many women with PPCM have LVEF recovery, but a small subset develop persistently severe cardiomyopathy requiring advanced therapy. In the IPAC study, 72% of women achieved LVEF ≥ 50% by 1 year postpartum, whereas 13% had a persistent LVEF < 30% and required LVAD or underwent cardiac transplantation at 1 year [9]. Heart transplantation may be the superior treatment option for those with significant RV dysfunction, malignant arrhythmias, a desire for future pregnancy (although generally not encouraged), or contraindications to anticoagulation. Importantly, the higher incidence of allograft rejection and lower allograft survival in patients with PPCM are potential risks that must be expectantly managed [87]. Once transplanted, patients with a history of PPCM have poorer survival outcomes compared with all transplant recipients. A review of data from 485 patients with PPCM transplanted between 1987 and 2010 demonstrated both a higher incidence of allograft rejection within the first year post-transplant and higher rate of mortality. It is posited that the poor outcomes in the PPCM population were partly related to their demographics: younger age, female sex, history of pregnancy, and increased pre-transplant panel reactive antibodies that may predispose to higher allograft rejection rates [87]. Black race was also an independent risk factor for worse post-transplant survival, although that has changed somewhat in recent years [88].
There are several unique considerations for heart transplant recipients considering pregnancy. To ensure the stability of graft function and immunosuppression regimens, pregnancy is discouraged in the first year after the transplant [89, 90]. Contraception counseling and planning should be discussed early given the possible rapid return of fertility after transplantation [91]. Contraindications to pregnancy in the post-transplant population include reduced graft function, non-adherence, donor-specific antibodies, significant allograft vasculopathy, poorly controlled hypertension, diabetes, renal dysfunction, and active infection [89]. In patients with a history of antibody-mediated rejection, pregnancy may not be advisable given the risk for sensitization from the fetus [89]. Allograft assessment should include echocardiography, evaluation for cardiac allograft vasculopathy, and checking for donor-specific antibodies [89]. Post-transplantation outcomes including rejection, graft survival, and age-adjusted survival are worse in patients with PPCM compared with other recipients [87]; and this should be considered as part of the pre-conception risk assessment and counseling in that population. If conception is being considered, immunosuppression regimens should be adjusted to minimize the risk for fetal injury. Calcineurin inhibitors and corticosteroids are typically considered safe [89]. Mycophenolate mofetil and mycophenolic acid are teratogenic and are discontinued at least 6 weeks prior to conception or as soon as pregnancy is confirmed if otherwise unplanned [89]. Data on mammalian target of rapamycin (mTOR) inhibitors are limited, and they should be discontinued prior to conception but may be continued on a case-by-case basis [89].
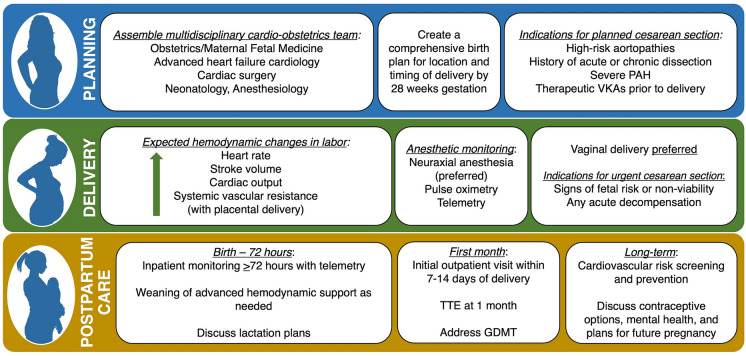
Treatment and Monitoring Considerations During Delivery
Labor is associated with significant hemodynamic changes, including increases in heart rate, systolic blood pressure, and cardiac output, which become greater due to pain and anxiety as women progress through the stages of labor [92]. Additional important hemodynamic changes can happen at birth, when increases in heart rate, stroke volume, and cardiac output occur due to sudden decompression of the inferior vena cava, when stroke volume is augmented by auto-transfusion from the contracting evacuated uterus, and when systemic vascular resistance increases with delivery of the placenta. Delivery is therefore a critical period in pregnancy and requires advance planning in high-risk women. A detailed, individualized delivery plan involving clinicians from multiple specialists should be created early [92]. The plan should be easily accessible to all health care professionals involved and should include recommendations regarding the location, timing, and mode of delivery; intrapartum monitoring; management of complications and necessary resources; and a plan for postpartum surveillance [92]. For pregnant patients with signs of fetal risk or non-viability, urgent pre-term or early term delivery is recommended [93]. In general, vaginal delivery is recommended for most pregnant women with HF if there are no obstetrical contraindications, as it is associated with a shortened hospital stay and a reduced risk of sudden death, peripartum infections, and hemorrhage [92, 94]. However, cesarean section may be needed in cases of acute decompensation as well as in women with high-risk aortopathies, a history of acute or chronic aortic dissection, or severe pulmonary arterial hypertension (PAH), or in those who receive therapeutic anticoagulation with VKAs, which place the fetus at risk for intracranial hemorrhage at the time of vaginal delivery [45, 92].
Appropriate postpartum monitoring is critically important in women with HF and high-risk cardiomyopathies. In most cases, cardiovascular imaging is not indicated in the immediate postpartum period. Women at high risk for hemodynamically significant arrhythmias should be placed on telemetry. In women on MCS, invasive hemodynamic monitoring tools are advisable. Postpartum length of stay varies depending on the specific condition, but women at the highest risk for postpartum complications should be monitored for ≥ 72 h. Postpartum hemodynamic changes may pose a significant risk to women with HF, PAH, or valvular disease, thus warranting extended monitoring [92].
Treatment and Monitoring Considerations During the Postpartum Period
Women with preexisting HF or de novo HF in pregnancy require close postpartum follow-up. The American College of Obstetrics and Gynecology currently recommends that women with CVD have an initial postpartum visit with an internist or cardiologist within 7–14 days of delivery [95]. Follow-up echocardiography a month after delivery is generally recommended [8]. Women with HF should be followed by a cardio-obstetrics team with input from advanced HF physicians. After delivery, medications should be adjusted accordingly, as fluid status and drug levels may change. Lactation goals should be reviewed and medication adjustments made in breastfeeding women. Recent ESC guidelines recommend against breastfeeding in women with severe HF (NYHA III/IV) (class IIb) in order to reduce the associated high metabolic demand and enable early initiation of optimal HF therapy [5], but this remains controversial [96]. Women should be counseled again regarding options for contraception [39, 45], and particular attention should be paid to mental health assessments and supportive psychotherapy during this time [92] (Fig. 3). Women with a history of HF carry a risk of decompensation with future pregnancies, though this risk is not as well characterized in all HF syndromes, with the exception of PPCM, where the risks associated with a subsequent pregnancy depend primarily upon recovery of myocardial function [9]. Dedicated counseling and monitoring are essential for women desiring future pregnancies.
Fig. 3.
Key principles in the management of women with heart failure across the continuum of pregnancy. GDMT guideline-directed medical therapy, PAH pulmonary arterial hypertension, TTE transthoracic echocardiography, VKAs vitamin K antagonists
Conclusions
Pregnancy is a high-risk period for a pregnant woman with underlying cardiomyopathy and HF. Early patient-centered discussions about the safety of pregnancy are critical before conception, and these conversations and family planning should continue in the postpartum period. Attention should be paid to symptoms suggestive of decompensated HF during pregnancy, and early diagnosis and management should be instituted with input from multidisciplinary cardio-obstetrics teams. While HF therapies can largely be universally implemented, there are unique considerations in pregnant women that should balance maternal benefit and fetal risks of GDMT in the broader context of clinical acuity and patient goals. Future areas of study include how pregnancy can change the natural history of cardiomyopathy and how to improve systems of care to support pregnant women with cardiomyopathy and HF along the continuum of pregnancy.
Author Contribution
All authors contributed to the review article. The first draft of the manuscript was written by Henrietta Afari and Nosheen Reza. Henrietta Afari, Megan Sheehan, and Nosheen Reza commented on all subsequent versions of the manuscript. All authors read and approved the final manuscript.
Funding
Nosheen Reza is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under award number K23HL166961. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No funding or sponsorship was received for this study or the publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Nosheen Reza reports speaking honoraria from Zoll, Inc. and consulting fees from Roche Diagnostics. Henrietta Afari and Megan Sheehan declare that they have no competing interests.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet Lond Engl. 2006;367(9516):1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Collier ARY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. NeoReviews. 2019;20(10):e561–e574. doi: 10.1542/neo.20-10-e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–62. [DOI] [PMC free article] [PubMed]
- 4.Heemelaar S, Petrus A, Knight M, van den Akker T. Maternal mortality due to cardiac disease in low- and middle-income countries. Trop Med Int Health. 2020;25(6):673–86. [DOI] [PMC free article] [PubMed]
- 5.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Kardiol Pol. 2019;77(3):245–326. [DOI] [PubMed]
- 6.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116(6):1302–1309. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 7.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 8.Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(2):207–221. doi: 10.1016/j.jacc.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 9.McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66(8):905–14. [DOI] [PMC free article] [PubMed]
- 10.Reza N, Packard E, Goli R, Chowns JL, Owens AT, Arany Z, et al. Clinical predictors of referral for and yield of genetic testing in peripartum cardiomyopathy. JACC Heart Fail. 2023;11(9):1278–1280. doi: 10.1016/j.jchf.2023.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121(13):1465–1473. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 12.Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108(4):366. doi: 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, Franke A, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 2017;38(35):2671–2679. doi: 10.1093/eurheartj/ehx355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Writing Committee Members, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):e240-327. [DOI] [PubMed]
- 15.Lewey J, Haythe J. Cardiomyopathy in pregnancy. Semin Perinatol. 2014;38(5):309–317. doi: 10.1053/j.semperi.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Grewal J, Siu SC, Ross HJ, Mason J, Balint OH, Sermer M, et al. Pregnancy outcomes in women with dilated cardiomyopathy. J Am Coll Cardiol. 2009;55(1):45–52. doi: 10.1016/j.jacc.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Stergiopoulos K, Shiang E, Bench T. Pregnancy in patients with pre-existing cardiomyopathies. J Am Coll Cardiol. 2011;58(4):337–350. doi: 10.1016/j.jacc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Lloji A, Panza JA. The challenge of pregnancy in women with hypertrophic cardiomyopathy. Cardiol Rev. 2022;30(5):258–262. doi: 10.1097/CRD.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 19.Autore C, Conte MR, Piccininno M, Bernabò P, Bonfiglio G, Bruzzi P, et al. Risk associated with pregnancy in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40(10):1864–1869. doi: 10.1016/s0735-1097(02)02495-6. [DOI] [PubMed] [Google Scholar]
- 20.Moolla M, Mathew A, John K, Yogasundaram H, Alhumaid W, Campbell S, et al. Outcomes of pregnancy in women with hypertrophic cardiomyopathy: a systematic review. Int J Cardiol. 2022;15(359):54–60. doi: 10.1016/j.ijcard.2022.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135(8):e50–87. doi: 10.1161/CIR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 22.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 23.Hameed AB, Chan K, Ghamsary M, Elkayam U. Longitudinal changes in the B-type natriuretic peptide levels in normal pregnancy and postpartum. Clin Cardiol. 2009;32(8):E60–62. doi: 10.1002/clc.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanous D, Siu SC, Mason J, Greutmann M, Wald RM, Parker JD, et al. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol. 2010;56(15):1247–1253. doi: 10.1016/j.jacc.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 25.Resnik JL, Hong C, Resnik R, Kazanegra R, Beede J, Bhalla V, et al. Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am J Obstet Gynecol. 2005;193(2):450–454. doi: 10.1016/j.ajog.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Kampman MAM, Balci A, van Veldhuisen DJ, van Dijk APJ, Roos-Hesselink JW, Sollie-Szarynska KM, et al. N-terminal pro-B-type natriuretic peptide predicts cardiovascular complications in pregnant women with congenital heart disease. Eur Heart J. 2014;35(11):708–715. doi: 10.1093/eurheartj/eht526. [DOI] [PubMed] [Google Scholar]
- 27.Chang SA, Khakh P, Janzen M, Lee T, Kiess M, Rychel V, et al. Trending cardiac biomarkers during pregnancy in women with cardiovascular disease. Circ Heart Fail. 2022;15(8):e009018. doi: 10.1161/CIRCHEARTFAILURE.121.009018. [DOI] [PubMed] [Google Scholar]
- 28.Clerico A, Zaninotto M, Aimo A, Cardinale DM, Dittadi R, Sandri MT, et al. Variability of cardiac troponin levels in normal subjects and in patients with cardiovascular diseases: analytical considerations and clinical relevance. Clin Chem Lab Med. 2023;61(7):1209–1229. doi: 10.1515/cclm-2022-1285. [DOI] [PubMed] [Google Scholar]
- 29.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 30.Dockree S, Brook J, Shine B, James T, Green L, Vatish M. Cardiac-specific troponins in uncomplicated pregnancy and pre-eclampsia: A systematic review. PLoS ONE. 2021;16(2):e0247946. doi: 10.1371/journal.pone.0247946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furenäs E, Eriksson P, Wennerholm UB, Dellborg M. Pregnancy in a healthy population: dynamics of NTproBNP and hs-cTroponin T. Open Heart. 2020;7(2):e001293. doi: 10.1136/openhrt-2020-001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrone MA, Zaninotto M, Masotti S, Musetti V, Padoan A, Prontera C, et al. The combined measurement of high-sensitivity cardiac troponins and natriuretic peptides: a useful tool for clinicians? J Cardiovasc Med (Hagerstown). 2020;21(12):953–63. [DOI] [PubMed]
- 33.Thygesen K, Mair J, Mueller C, Huber K, Weber M, Plebani M, et al. Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J. 2012;33(16):2001–2006. doi: 10.1093/eurheartj/ehq509. [DOI] [PubMed] [Google Scholar]
- 34.Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71(21):2419–30. [DOI] [PubMed]
- 35.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(5):515–521. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 36.Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart Br Card Soc. 2006;92(10):1520–1525. doi: 10.1136/hrt.2006.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hagen IM, Boersma E, Johnson MR, Thorne SA, Parsonage WA, Escribano Subías P, et al. Global cardiac risk assessment in the registry of pregnancy and cardiac disease: results of a registry from the European Society of Cardiology. Eur J Heart Fail. 2016;18(5):523–533. doi: 10.1002/ejhf.501. [DOI] [PubMed] [Google Scholar]
- 38.Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJM, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31(17):2124–2132. doi: 10.1093/eurheartj/ehq200. [DOI] [PubMed] [Google Scholar]
- 39.DeFilippis EM, Haythe JH, Walsh MN, Kittleson MM. Intersection of heart failure and pregnancy: beyond peripartum cardiomyopathy. Circ Heart Fail. 2021;14(5):e008223. doi: 10.1161/CIRCHEARTFAILURE.120.008223. [DOI] [PubMed] [Google Scholar]
- 40.Power to Decide. One Key Question online. Available from: https://powertodecide.org/one-key-question. Accessed 27 oct 2023.
- 41.Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, et al. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation. 2020;141(23):e884–903. doi: 10.1161/CIR.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 42.Cauldwell M, Ghonim S, Uebing A, Swan L, Steer PJ, Gatzoulis M, et al. Preconception counseling, predicting risk and outcomes in women with mWHO 3 and 4 heart disease. Int J Cardiol. 2017;1(234):76–80. doi: 10.1016/j.ijcard.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 43.American College of Obstetricians and Gynecologists’ Committee on Adolescent Health Care. Gynecologic considerations for adolescents and young women with cardiac conditions: ACOG Committee Opinion, number 819. Obstet Gynecol. 136(5):e90–9. [DOI] [PubMed]
- 44.Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 206. Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133(2):e128–50. [DOI] [PubMed]
- 45.DeFilippis EM, Bhagra C, Casale J, Ging P, Macera F, Punnoose L, et al. Cardio-obstetrics and heart failure: JACC: heart failure state-of-the-art review. JACC Heart Fail. 2023;11(9):1165–1180. doi: 10.1016/j.jchf.2023.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Pieper PG. Use of medication for cardiovascular disease during pregnancy. Nat Rev Cardiol. 2015;12(12):718–729. doi: 10.1038/nrcardio.2015.172. [DOI] [PubMed] [Google Scholar]
- 47.Garg J, Palaniswamy C, Lanier GM. Peripartum cardiomyopathy: definition, incidence, etiopathogenesis, diagnosis, and management. Cardiol Rev. 2015;23(2):69–78. doi: 10.1097/CRD.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka K, Tanaka H, Kamiya C, Katsuragi S, Sawada M, Tsuritani M, et al. Beta-blockers and fetal growth restriction in pregnant women with cardiovascular disease. Circ J. 2016;80(10):2221–6. [DOI] [PubMed]
- 49.Lip GY, Beevers M, Churchill D, Shaffer LM, Beevers DG. Effect of atenolol on birth weight. Am J Cardiol. 1997;79(10):1436–1438. doi: 10.1016/s0002-9149(97)00163-x. [DOI] [PubMed] [Google Scholar]
- 50.Shekhar S, Gupta N, Kirubakaran R, Pareek P. Oral nifedipine versus intravenous labetalol for severe hypertension during pregnancy: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol. 2016;123(1):40–47. doi: 10.1111/1471-0528.13463. [DOI] [PubMed] [Google Scholar]
- 51.Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol. 2015;58(4):868–884. doi: 10.1097/GRF.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 52.Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh) 1980;95(4):540–545. doi: 10.1530/acta.0.0950540. [DOI] [PubMed] [Google Scholar]
- 53.Mosley JF, Smith L, Everton E, Fellner C. Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. P T Peer-Rev J Formul Manag. 2015;40(7):451–462. [PMC free article] [PubMed] [Google Scholar]
- 54.Cominos DC, van der Walt A, van Rooyen AJ. Suppression of postpartum lactation with furosemide. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 1976;50(8):251–252. [PubMed] [Google Scholar]
- 55.Chow T, Galvin J, McGovern B. Antiarrhythmic drug therapy in pregnancy and lactation. Am J Cardiol. 1998;82(4A):58I–62I. doi: 10.1016/s0002-9149(98)00473-1. [DOI] [PubMed] [Google Scholar]
- 56.Potondi A. Congenital rhabdomyoma of the heart and intrauterine digitalis poisoning. J Forensic Sci. 1966;11(1):81–88. [PubMed] [Google Scholar]
- 57.Sillesen M, Hjortdal V, Vejlstrup N, Sørensen K. Pregnancy with prosthetic heart valves—30 years’ nationwide experience in Denmark. Eur J Cardio-Thorac Surg. 2011;40(2):448–454. doi: 10.1016/j.ejcts.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med. 2000;160(2):191–196. doi: 10.1001/archinte.160.2.191. [DOI] [PubMed] [Google Scholar]
- 59.Xu Z, Fan J, Luo X, Zhang WB, Ma J, Lin YB, et al. Anticoagulation regimens during pregnancy in patients with mechanical heart valves: a systematic review and meta-analysis. Can J Cardiol. 2016;32(10):1248.e1–1248.e9. doi: 10.1016/j.cjca.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Fogerty AE. Challenges of anticoagulation therapy in pregnancy. Curr Treat Options Cardiovasc Med. 2017;19(10):76. doi: 10.1007/s11936-017-0575-x. [DOI] [PubMed] [Google Scholar]
- 61.De Carolis S, di Pasquo E, Rossi E, Del Sordo G, Buonomo A, Schiavino D, et al. Fondaparinux in pregnancy: could it be a safe option? A review of the literature. Thromb Res. 2015;135(6):1049–1051. doi: 10.1016/j.thromres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Dempfle CEH. Minor transplacental passage of fondaparinux in vivo. N Engl J Med. 2004;350(18):1914–1915. doi: 10.1056/NEJM200404293501825. [DOI] [PubMed] [Google Scholar]
- 63.Beyer-Westendorf J, Michalski F, Tittl L, Middeldorp S, Cohen H, Abdul Kadir R, et al. Pregnancy outcome in patients exposed to direct oral anticoagulants—and the challenge of event reporting. Thromb Haemost. 2016;116(4):651–658. doi: 10.1160/TH16-04-0305. [DOI] [PubMed] [Google Scholar]
- 64.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–69. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 65.Boulé S, Ovart L, Marquié C, Botcherby E, Klug D, Kouakam C, et al. Pregnancy in women with an implantable cardioverter-defibrillator: is it safe? Europace. 2014;16(11):1587–94. [DOI] [PubMed]
- 66.Schuler PK, Herrey A, Wade A, Brooks R, Peebles D, Lambiase P, et al. Pregnancy outcome and management of women with an implantable cardioverter defibrillator: a single centre experience. Europace. 2012;14(12):1740–5. [DOI] [PubMed]
- 67.Adler A, Halkin A, Viskin S. Wearable cardioverter-defibrillators. Circulation. 2013;127(7):854–860. doi: 10.1161/CIRCULATIONAHA.112.146530. [DOI] [PubMed] [Google Scholar]
- 68.Leblanc H, Lachelin GC, Abu-Fadil S, Yen SS. Effects of dopamine infusion on pituitary hormone secretion in humans. J Clin Endocrinol Metab. 1976;43(3):668–674. doi: 10.1210/jcem-43-3-668. [DOI] [PubMed] [Google Scholar]
- 69.Stapel B, Kohlhaas M, Ricke-Hoch M, Haghikia A, Erschow S, Knuuti J, et al. Low STAT3 expression sensitizes to toxic effects of β-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J. 2017;38(5):349–361. doi: 10.1093/eurheartj/ehw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauersachs J, Arrigo M, Hilfiker-Kleiner D, Veltmann C, Coats AJS, Crespo-Leiro MG, et al. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy. Eur J Heart Fail. 2016;18(9):1096–105. [DOI] [PubMed]
- 71.Sharma S, Thomas SS. Management of heart failure and cardiogenic shock in pregnancy. Curr Treat Options Cardiovasc Med. 2019;21(12):83. doi: 10.1007/s11936-019-0797-1. [DOI] [PubMed] [Google Scholar]
- 72.Carbonne B, Jannet D, Touboul C, Khelifati Y, Milliez J. Nicardipine treatment of hypertension during pregnancy. Obstet Gynecol. 1993;81(6):908–914. [PubMed] [Google Scholar]
- 73.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology–Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015;65(19):e7-26. [DOI] [PubMed]
- 74.Banayan J, Rana S, Mueller A, Tung A, Ramadan H, Arany Z, et al. Cardiogenic shock in pregnancy: analysis from the National Inpatient Sample. Hypertens Pregnancy. 2017;36(2):117–123. doi: 10.1080/10641955.2016.1242606. [DOI] [PubMed] [Google Scholar]
- 75.Willcox TW, Stone P, Milsom FP, Connell H. Cardiopulmonary bypass in pregnancy: possible new role for the intra-aortic balloon pump. J Extra Corpor Technol. 2005;37(2):189–191. [PubMed] [Google Scholar]
- 76.Lin TY, Chiu KM, Shieh JS, Chu SH. Emergency redo mitral valve replacement in a pregnant woman at third trimester: case report and literature review. Circ J. 2008;72(10):1715–7. [DOI] [PubMed]
- 77.Allen JN, Wewers MD. Acute myocardial infarction with cardiogenic shock during pregnancy: treatment with intra-aortic balloon counterpulsation. Crit Care Med. 1990;18(8):888–889. doi: 10.1097/00003246-199008000-00020. [DOI] [PubMed] [Google Scholar]
- 78.Tapaskar N, Tremblay-Gravel M, Khush KK. Contemporary management of cardiogenic shock during pregnancy. J Card Fail. 2023;29(2):193–209. doi: 10.1016/j.cardfail.2022.09.014. [DOI] [PubMed] [Google Scholar]
- 79.Elkayam U, Schäfer A, Chieffo A, Lansky A, Hall S, Arany Z, et al. Use of Impella heart pump for management of women with peripartum cardiogenic shock. Clin Cardiol. 2019;42(10):974–981. doi: 10.1002/clc.23249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schroeter MR, Unsöld B, Holke K, Schillinger W. Pro-thrombotic condition in a woman with peripartum cardiomyopathy treated with bromocriptine and an Impella LP 2.5 heart pump. Clin Res Cardiol. 2013;102(2):155–7. [DOI] [PMC free article] [PubMed]
- 81.Golzarian H, Mariam A, Shah SR, Pasley BA, Haq SH, Edgerton AR, et al. Amniotic fluid embolism-induced cardiopulmonary collapse successfully treated with combination VA-ECMO and Impella CP. ESC Heart Fail. 2023;10(2):1440–1444. doi: 10.1002/ehf2.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desai N, Chaudhry K, Aji J. Impella left ventricular assist device in cardiac arrest after spinal anaesthesia for caesarean section. BMJ Case Rep. 2015;2015:2bcr015211958. doi: 10.1136/bcr-2015-211958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pacheco LD, Saade GR, Hankins GDV. Extracorporeal membrane oxygenation (ECMO) during pregnancy and postpartum. Semin Perinatol. 2018;42(1):21–25. doi: 10.1053/j.semperi.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Neumann A, Hilfiker-Kleiner D, Kühn C, Fegbeutel C, Hilfiker A, Haverich A, et al. Prolactin—a new marker for ECMO-related mortality. J Heart Lung Transplant. 2013;32(4):S225. [Google Scholar]
- 85.Loyaga-Rendon RY, Pamboukian SV, Tallaj JA, Acharya D, Cantor R, Starling RC, et al. Outcomes of patients with peripartum cardiomyopathy who received mechanical circulatory support. Data from the Interagency Registry for Mechanically Assisted Circulatory Support. Circ Heart Fail. 2014;7(2):300–9. doi: 10.1161/CIRCHEARTFAILURE.113.000721. [DOI] [PubMed] [Google Scholar]
- 86.Pacheco LD, Saad AF, Lick SD, Iturrizaga JC, Saade GR. Care and monitoring of pregnant patients with left ventricular assist devices. Obstet Gynecol. 2023;142(5):1029–1035. doi: 10.1097/AOG.0000000000005351. [DOI] [PubMed] [Google Scholar]
- 87.Rasmusson K, Brunisholz K, Budge D, Horne BD, Alharethi R, Folsom J, et al. Peripartum cardiomyopathy: post-transplant outcomes from the United Network for Organ Sharing Database. J Heart Lung Transplant. 2012;31(2):180–6. [DOI] [PubMed]
- 88.Kwon JH, Tedford RJ, Ramu B, Witer LJ, Pope NH, Houston BA, et al. Heart transplantation for peripartum cardiomyopathy: outcomes over 3 decades. Ann Thorac Surg. 2022;114(3):650–658. doi: 10.1016/j.athoracsur.2021.12.059. [DOI] [PubMed] [Google Scholar]
- 89.Kittleson MM, DeFilippis EM, Bhagra CJ, Casale JP, Cauldwell M, Coscia LA, et al. Reproductive health after thoracic transplantation: an ISHLT expert consensus statement. J Heart Lung Transplant. 2023;42(3):e1–42. doi: 10.1016/j.healun.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 90.Mckay DB, Josephson MA, Armenti VT, August P, Coscia LA, Davis CL, et al. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant. 2005;5(7):1592–9. [DOI] [PubMed]
- 91.French VA, Davis JS, Sayles HS, Wu SS. Contraception and fertility awareness among women with solid organ transplants. Obstet Gynecol. 2013;122(4):809–814. doi: 10.1097/AOG.0b013e3182a5eda9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis MB, Arendt K, Bello NA, Brown H, Briller J, Epps K, et al. Team-based care of women with cardiovascular disease from pre-conception through pregnancy and postpartum: JACC focus seminar 1/5. J Am Coll Cardiol. 2021;77(14):1763–1777. doi: 10.1016/j.jacc.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Committee on Obstetric Practice. ACOG Committee Opinion no. 765: avoidance of nonmedically indicated early-term deliveries and associated neonatal morbidities. Obstet Gynecol. 2019;133(2):e156–63. [DOI] [PubMed]
- 94.Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS, et al. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176(4):455–60. [DOI] [PMC free article] [PubMed]
- 95.American College of Obstetricians and Gynecologists’ Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin no. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133(5):e320–56. [DOI] [PubMed]
- 96.Arany Z, Feldman AM. To breastfeed or not to breastfeed with peripartum cardiomyopathy. JACC Basic Transl Sci. 2019;4(3):301–303. doi: 10.1016/j.jacbts.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ritchie HE, Telenius C, Gustaffson E, Webster WS. The effects of nifedipine and ivabradine on the functionality of the early rat embryonic heart. Are these drugs a risk in early human pregnancy? Birth Defects Res. 2019;111(5):281–8. [DOI] [PubMed]
- 98.Kearney L, Wright P, Fhadil S, Thomas M. Postpartum cardiomyopathy and considerations for breastfeeding. Card Fail Rev. 2018;4(2):112–118. doi: 10.15420/cfr.2018.21.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Merck Sharp & Dohme LLC. Verquvo (vericiguat) [prescribing information]. Rahway: Merck Sharp & Dohme LLC; 2023. Available from: https://www.merckconnect.com/verquvo/coverage/?safety_overlay&cid=PPC-accountype:MICROSOFT-campaign:Verquvo+HCP_2023+Generic_BRND_NA_ENGM_PHRS_TEXT_NA-searchterm:Vericiguat-adgroup:Generic+Core-keywordid:p76545871180&utm_source=bing&utm_medium=cpc&utm_campaign=Verquvo%20HCP_2023%20Generic_BRND_NA_ENGM_PHRS_TEXT_NA&utm_term=Vericiguat&utm_content=Generic%20Core&gclid=8f4f4acd541318362c972fd5148bf551&gclsrc=3p.ds&msclkid=8f4f4acd541318362c972fd5148bf551. Cited 30 Sep 2023.
- 100.D’Souza R, Ostro J, Shah PS, Silversides CK, Malinowski A, Murphy KE, et al. Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J. 2017;38(19):1509–1516. doi: 10.1093/eurheartj/ehx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.