ABSTRACT
Background:
Polyetheretherketone (PEEK) has favorable properties that make it able to be used as a denture base material, but it is also susceptible to the adhesion of microorganisms. In this study, we applied Octafluoropentyl (meth) acrylate (OFPA) coating on the PEEK polymer surface by using plasma spray and investigated the functional groups present on the surface, changes in the surface energy and Candida albicans adhesion.
Materials and Methods:
In this experimental study, the samples were placed in a control group without surface preparation and three experimental groups that were subjected to plasma spray for 10, 30, and 60 s and then impregnated with degassed Octa fluoropentyl (meth) acrylate (Sigma-Aldrich, USA) monomer. Fourier transform infrared spectroscopy (FTIR) was used to identify the functional groups and new chemical bonds between PEEK and OFPA, and Sessile Drop Method was used to evaluate the surface’s wettability. The surface morphology was checked using a LEXT OLS4000 (Olympus®-Japan) microscope, and the inhibition of C. albicans adhesion was also checked by counting the colonies in terms of colony forming unit/mL (CFU/mL). Kurskal–Wallis analysis was conducted to assess Candida adhesion, while wettability was evaluated using analysis of variance and post hoc analyses. The level of statistical significance was set at P < 0.05.
Results:
FTIR analysis confirmed that a chemical between OFPA and PEEK was established. The samples showed a significant increase in the contact angle after 30 s of plasma application (CA = 88.2 ± 7.3). The contact angle decreased again by increasing the surface modification to 60 s (CA = 64.33 ± 5.5). Examining the surface morphology of the samples shows an increase in surface roughness with increasing plasma time up to 60 s. The number of adherent colonies was the lowest in 30 s group, but it was not statistically significant (P = 0.658).
Conclusion:
No statistically significant difference in C. albicans CFU/mL count was found between groups. The contact angle of the 30 s group was significantly higher than the control group.
Keywords: Bacterial adhesion, Candida albicans, plasma gases, polyetheretherketone, surface properties
INTRODUCTION
Denture stomatitis is a chronic inflammatory infection that affects complete denture wearers.[1] Although many microorganisms can cause denture stomatitis, a strong connection between Candida albicans and this inflammatory condition has been reported in the literature.[2] The current evidence has found the topical use of Nystatin to be effective in the treatment of stomatitis caused by C. albicans, but this is considered a temporary treatment because this drug does not play a role in preventing Candida from attaching to the denture bases and only treats the symptoms of the disease. Hence, the chance of re-infection in them will remain high.[3] Full or partial dentures used in the treatment of edentulous patients are usually made of Polymethylmethacrylate (PMMA). This material has advantages such as reasonable price, sufficient beauty, and the ability to reline and repair and it is known as the most common material for making denture bases. However, it also has disadvantages such as high porosity, tendency to accumulate biofilm, and poor mechanical properties.[4,5]
According to the studies, it has been determined that polyetheretherketone (PEEK) has more flexural strength and hardness, and its water absorption is less than PMMA.[5] High resistance to dissolution and high biocompatibility are other features of this material.[6] Therefore, PEEK material has favorable characteristics that make it possible to be used as a denture base. Despite the benefits mentioned for PEEK, several articles stated that this material is susceptible to the adhesion of microorganisms.[7,8] So far, several solutions such as PEEK sulfonation,[9] adding bioactive agents to the PEEK matrix,[10] covering its surface with agents such as titanium oxide or polydimethylsiloxane,[11] and also plasma spraying the PEEK surface[12] have been proposed to reduce the adhesion of bacteria or increase the contact of osteoblastic cells to the surface of implants made of PEEK. The application of plasma treatment has received much attention and acceptance as one of the surface modification methods due to its simplicity and stability, as well as the limited changes to the surface layer and without a negative impact on the mass properties of the material.[13] Octafluoropentyl methacrylate (OFPA) is one of the covering materials that has been used as an antibiofilm and antibacterial compound to cover the surfaces of contact and intraocular lenses, catheters, and vascular stents.[14,15] Therefore, in this study, we applied OFPA coating on the PEEK polymer surface and evaluated the changes in surface energy, functional groups, and C. albicans adhesion on the surface.
MATERIALS AND METHODS
The present research is an experimental study and the protocol was approved by the Research Ethics Committee of the Research Institute of Dental Sciences, Shahid Beheshti University of Medical Science (IR.SBMU.DRC.REC.1401.025).
Preparation of samples
PEEK sheets (Goodfellow, Cambridge Ltd., England) with 1-mm thickness were prepared. These samples were cut with dimensions of 10 mm × 10 mm and then cleaned with the help of ethanol and an ultrasonic device. Then, they were dried in an oven at 60°C for 24 h.[16] Surface preparation was done in a PE-25JW (Plasma Etch, USA) plasma chamber with a gas mixture of 25% oxygen and 75% argon under a pressure of 20 pascals, a frequency of 40 KHz and a flow rate of 20 sccm (standard cubic centimeters per minute). The samples were subjected to plasma spray in three groups of 10, 30, and 60 s. Then, the prepared sheets were coated entirely with degassed OFPA (Sigma-Aldrich, USA) monomer and subjected to a temperature of 100°C for 2 h. Since surface stability is essential, after creating a surface coating to remove unreacted free monomers, the samples were placed in an ultrasonic bath containing ethanol at 40°C for 1 h.
Investigation of functional groups and new chemical bonds
Fourier transform infrared spectroscopy (FTIR) analysis was used to identify the surface and functional groups and new chemical bonds between OFPA and PEEK. The basis of FTIR analysis is the examination of infrared rays absorbed by the analyzed samples. The samples were divided into four groups (control, 10, 30, and 60 s), and five samples were placed in each group. FTIR analysis was done at the room temperature and in Nicolet iS5 (Thermo Fisher Scientific Inc., Germany) device in total reflection mode of 500–4000 cm − 1 and a resolution of 4 cm − 1.
Investigation of surface wettability
The sessile drop method was used to evaluate the wettability of the surface. In this test, a drop of water with a size of 3 μL is placed on the sample. After 10 s, a high-precision camera (Dino-Lite Digital Microscope-USA) takes a picture of the contact angle of the droplet at the point of contact with the surface.[17] One sample from each group was examined ten times.
Investigation of surface morphology
The surface morphology was investigated using a LEXT OLS4000 (Olympus®-Japan) microscope. Using a three-dimensional (3D) laser measuring microscope, surface morphology and thickness of thin layers can be measured with high accuracy in noncontact mode. Surface texture parameters are acquired through 3D surface texture data information instead of the conventional 2D contour profile curves used in profile method measurement. The thickness of the coating is measured by detecting changes in refractive index that the high sensitivity light detectors (photo multipliers) use in a laser microscope.[18]
Assaying the inhibition of Candida albicans adhesion
C. albicans biofilm adhesion was checked by counting colonies in colony-forming unit/mL (CFU/mL).[19] The C. albicans oral yeast strain with code ATCC: 10231 was purchased from Iran’s Industrial Microbes Collection Center as a vial. Then in the microbiology laboratory of the dental biomaterials department of Shahid Beheshti Faculty of Dentistry, under aseptic conditions, the contents of the vial were poured into the tube containing Brain Heart Infusion Broth (BHI) liquid medium and cultured for 24 h in the incubator (memment-Germany) at 37°C and under aerobic conditions. Then to prepare a single colony of yeast, it was passaged from the liquid culture medium to the solid culture medium with BHI Agar (Merck-Germany) by the streak method and incubated for 24 h at a temperature of 37°C. Then to prepare a cell suspension, several yeast colonies were removed from the BHI Agar medium and mixed well in the BHI Broth culture medium to obtain a cell suspension. After that, using a spectrophotometer (Unico-Canada) that was set at a wavelength of 600 nm, the amount of optical absorption (optical density) was adjusted in the range of 0.08–0.1 to obtain a suspension equivalent to 0.5 of McFarland (equal to 1.5 × 108 CFU/mL). Furthermore, a cuvette-containing medium without C. albicans was used as a blank. To simulate the intraoral environment and use intraoral pellicles, 2 mL of saliva was collected from 4 nonsmokers (two men and two women) without active caries who were also systemically healthy, fasting in falcon tubes, and were immediately taken to the laboratory. Then, the saliva samples were centrifuged (Eppendorf-Germany) at a temperature of 4°C and force of 3000 g for 15 min to settle the debris and cells. Then the saliva supernatant solution was slowly collected, and four saliva samples were mixed and sterilized via a 0.2-μm filter (MS sterile syringe filter). After this step, 100 μL of saliva were poured into each well of the 24-well plates, and in each well, one sample of the PEEK sheets treated with UV waves (each side 20 min) under a class II laminar flow hood (Aster II-Iran). Finally, another 100 μL of filtered saliva were added to each sample. In the next step, the plates were incubated in a shaking incubator (HYSC-Korea) for 30 min at 37°C and 70 rpm. Then, the samples were taken out of the wells under a Class II laminar hood under sterile conditions, and each side of them was washed with 5 mL of sterilized normal saline. Then, each sample was placed in the bottom of the well of a new 24-well plate. In the next step, we diluted the suspension equivalent to half of McFarland at a ratio of 1/10 using BHI Broth culture medium to which 1% sucrose was added (cell count equal to 107 CFU/mL), and we added 500 μL of this suspension to all the wells except the negative control well. After sealing the microplates with paraffin, they were incubated for 24 h in a shaking incubator (HYSC-Korea) at 37°c and 70 rpm. After 24 h, the plate was removed from the incubator, and first, the positive and negative controls were examined, and it was ensured that both of them answered correctly. Then, in the sterile conditions, the samples were removed from the plate and, to remove planktonic and nonadherent yeast cells, each side of them was washed with 5 mL of normal saline. In the next step, we transferred each sample to microtubes containing 1 mL of phosphate buffer and sonicated in an ultrasonic bath (cristofoli-China) with a voltage of 180 W for 360 s. At this time, with the help of ultrasonic waves, the attached yeast cells were separated from the samples’ surfaces and suspended inside the saline phosphate buffer. Then, each microtube was vortexed for 30 s, and 40 μL of it were added to 360 μL of the culture medium to reach a dilution of 1/10. After that, serial dilution continued at the ratio of 1/100, 1/1000–1/100000. Finally, 100 μL were removed from each dilution was removed and cultured on sterile Sabouraud dextrose agar (Merck-Germany) plates by spread method. Then, the plates were incubated at 37°C for 24 h. After incubation time, a plate containing between 20 and 100 colonies was selected, and the number of colonies per milliliter was calculated and reported.
Statistic analysis
Data analysis was done using SPSS 28(IBM-NY-USA 2021) software with a significance level of 0.05. After calculating the mean, standard deviation, and normality of the data in each group, Kurskal–Wallis analysis was conducted to assess C. albicans adhesion, while wettability was evaluated using one-way analysis of variance and post hoc analyses.
RESULTS
Investigation of functional groups and new chemical bonds
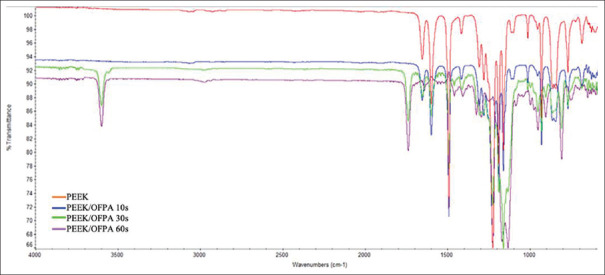
According to Figure 1, wavelengths of 1160, 1220, 1500, 1600, and 1650 cm − 1 are observed in both PEEK and PEEK-OFPA. These peaks are related to diphenyl ether groups, phenolic ring, and aromatic ring hydrogens.[20,21]
Figure 1.
Fourier transform infrared spectroscopy analysis of the samples that were exposed to plasma spray for 10, 30 and 60 s compared to the sample without preparation.
In the PEEK-OFPA group, in addition to the mentioned peaks, new peaks were also observed at 1140 and 1750 cm−1. These peaks are related to (C = O) and (C-F) carbonyl groups. The OH transition vibrational peak in the region of 3600 cm − 1 is also observed in the FTIR spectrum.
Investigation of surface wettability
Tables 1 and 2 show that the contact angle was measured before and after surface modification. The results indicated that the 30-second group had a significantly higher contact angle than the control group (P < 0.05). Furthermore, the 60-second group had a significantly lower contact angle than the 10 and 30-second groups (P < 0.05).
Table 1.
Mean and standard deviation of contact angles (°) measured in the groups under investigation
| Group | Mean±SD (°) | 95% CI for mean (°) | Minimum (°) | Maximum (°) | One-way ANOVA | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Lower bound | Upper bound | Source of variation | df | F | P | ||||
| Control | 72.75±2.75 | 68.37 | 77.13 | 70.00 | 76.00 | Between groups | 3 | 11.046 | 0.001 |
| 10 s | 81.60±6.88 | 73.06 | 90.14 | 72.00 | 90.00 | Within groups | 13 | ||
| 30 s | 88.20±7.39 | 79.02 | 97.38 | 78.00 | 98.00 | Total | 16 | ||
| 60 s | 64.33±5.51 | 50.65 | 78.01 | 58.00 | 68.00 | ||||
SD: Standard deviation; CI: Confidence interval
Table 2.
The results of the post hoc test to find the statistical differences between groups
| Group (A) | Group (B) (s) | Mean difference (A−B) | P |
|---|---|---|---|
| Control | 10 | −8.85 | 0.190 |
| 30 | −15.45 | 0.011 | |
| 60 | 8.42 | 0.320 | |
| 10 s | 30 | −6.60 | 0.364 |
| 60 | 17.27 | 0.010 | |
| 30 s | 60 | 23.87 | 0.001 |
Investigation of surface morphology
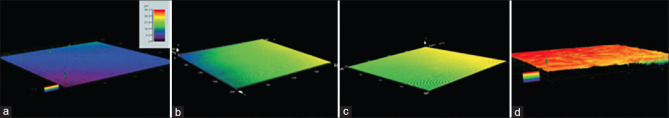
The surface morphology of the samples using a 3D laser microscope shows an increase in surface roughness with an increase in plasma spray for up to 60 s. The colors of the sample provide an estimate of the coating thickness. The color scale bar refers to height in microns. The thickness of the OFPA layer was measured as 10–20 μ after 10 s of plasma application, 20–30 μ after 30 s, and 36–46 μ after 60 s [Figure 2].
Figure 2.
Color-coded representation of the surface morphology and thickness of the Octafluoropentyl (meth) acrylate (OFPA) layer on the samples using a three dimensional laser microscope: The color scale bar refers to height in microns. There is a strong resemblance between the topographic profiles, the fine structure has lower amplitude. Color-coded, red indicates the crests. PEEK (a), PEEK/OFPA 10s (b), PEEK/OFPA 30s (c), PEEK/OFPA60s (d).
Assaying the inhibition of Candida albicans adhesion
According to Table 3, the group subjected to 30 s of plasma spray showed the lowest amount of fungal adhesion. However, this result was not statistically significant (P = 0.658).
Table 3.
Comparison of the average number of colonies attached to polyetheretherketone samples in terms of log10 colony-forming unit/mL
| Group Log10 CFU/mL | n | Mean±SD | P |
|---|---|---|---|
| Control | 10 | 5.29±0.50 | 0.658 |
| 10 s | 10 | 5.15±0.51 | |
| 30 s | 10 | 5.07±0.51 | |
| 60 s | 10 | 5.38±0.16 |
SD: Standard deviation; CFU: Colony-forming unit
DISCUSSION
Despite the availability of antimicrobial drugs to treat stomatitis caused by C. albicans, the recurrence of this disease has made Candida-related stomatitis a big challenge in treating patients using removable prostheses.[22] Chandra et al. found that C. albicans became resistant to several antifungal agents that are currently available.[23] Therefore, researchers tried to solve this clinical challenge by using different strategies, such as including antimicrobial substances or different drugs in methacrylate or using antimicrobial copolymers as surface coating on the denture base material.[24]
Surface characteristics have an influential role in biofilm formation, bacteria absorption, and prostheses’ final performance. For this reason, many studies tried to create surface changes without adverse effects on the mass properties of the material. One of these solutions is the use of surface coatings that are used for various purposes. Compared to other methods, the use of plasma for surface activation has advantages such as limited changes to surface layers without interfering with the main chain, which makes the resulting polymer have favorable surface properties while maintaining mechanical strength.[25] PEEK has less water absorption and shrinkage and greater bending strength than PMMA.[5] However, some studies stated that this polymer is prone to the adhesion of microorganisms.[7] When PEEK is exposed to plasma, two critical chemical processes are expected on its surface. One is chain scission, in which plasma ions bombard polymer chains, and the other is the placement of oxygen-nitrogen functional groups on the surface, resulting from interaction with plasma radicals. These functional groups on the PEEK surface have shown the ability to change the surface adhesion properties.[26] The type of gas used in plasma preparation and controlling the duration of its application are two essential parameters in achieving ideal results and creating a stable coating with anti-microbial properties. The study’s results by Zhan et al. show that the samples modified with Argon plasma have higher bond strength than N2 or O2 plasma due to forming more surface polar components and increased surface energy.[26] In the present study, the appearance of new transmission vibration peaks at 1750 cm − 1 and 1140 cm − 1 was observed in the FTIR spectrum of OFPA-coated samples, which confirms the success of the bonding process. These peaks are related to C = O and C-F groups, respectively.
The wettability of a liquid on a solid surface can be studied by measuring the contact angle between them. The lower the contact angle, the better the tendency to wet the surface.[27] In the present study, the average contact angle for the 10-second group was measured as 81.60, which showed an increase in contact angle compared to the control group.[28,29] This increase can be attributed to the hydrophobic nature of OFPA. The contact angle of the samples increased again after 30 s of plasma application due to the more uniform and thicker coating of OFPA on the PEEK surface. Nevertheless, with the increase of the surface modification time to 60 s, the contact angle decreased again. Examining the surface morphology of the samples using a 3D laser microscope showed an increase in surface roughness with increasing plasma time within 60 s. This finding was consistent with the study of Dupuis et al. They found that by increasing the plasma preparation time, surface roughness in the form of nanometer peaks and valleys increases on the material’s surface.[13] Another parameter affecting the surface roughness is the type of gas used in the plasma spray process. Dupuis et al. found surface roughness is higher when using air plasma than argon or nitrogen gas alone.[13] Fricke et al. also found that Argon and oxygen plasma cause less surface roughness than argon gas alone.[30]
From the C. albicans adhesion test, it was understood that although the average number of colonies counted in the groups subjected to plasma spray was less than the control group, and the best results were obtained for the 30-second group, this difference was not statistically significant. The limitation in the number of tested samples in each group can be considered one of the reasons for the lack of statistical significance of the results. Another reason that can be mentioned is that we counted the fungal colonies after 24 h, and due to the exponential growth of microorganisms, the difference in the number of colonies between the groups may have increased with the increase of the incubation period. Another point of the obtained results was that the 60-second group had more Candida adhesion than the 30-second group. This result could be the increase in the surface roughness of the samples in the 60-second group. In fact, with the increase in preparation time, the surface roughness also increases, and as a result, the microorganisms can be trapped in the pores created.
CONCLUSION
Compared to other methods, the use of plasma spray in surface activation has the advantage of limiting the changes to the surface layer and maintaining the mechanical properties of the materials
Surface modification of PEEK with OFPA anti-biofilm polymer is possible by plasma spray technique
Compared to the control group, the 30-s group showed a significant increase in the contact angle
Samples subjected to plasma spray for extended period show higher surface roughness
In terms of C. albicans adhesion, no statistically significant difference could be discerned between the groups exposed to different duration of plasma spray.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.AlMojel N, AbdulAzees PA, Lamb EM, Amaechi BT. Determining growth inhibition of Candida albicans biofilm on denture materials after application of an organoselenium-containing dental sealant. J Prosthet Dent. 2023;129:205–12. doi: 10.1016/j.prosdent.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Polke M, Hube B, Jacobsen ID. Candida survival strategies. Adv Appl Microbiol. 2015;91:139–235. doi: 10.1016/bs.aambs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Park SE, Blissett R, Susarla SM, Weber HP. Candida albicans adherence to surface-modified denture resin surfaces. J Prosthodont. 2008;17:365–9. doi: 10.1111/j.1532-849X.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 4.Gad MM, Al-Thobity AM, Shahin SY, Alsaqer BT, Ali AA. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: A new approach for denture stomatitis prevention. Int J Nanomedicine. 2017;12:5409–19. doi: 10.2147/IJN.S142857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shrivastava SP, Dable R, Raj AP, Mutneja P, Srivastava SB, Haque M. Comparison of mechanical properties of PEEK and PMMA: An in vitro study. J Contemp Dent Pract. 2021;22:179–83. [PubMed] [Google Scholar]
- 6.Sobieraj MC, Rimnac CM. Fracture, Fatigue, and Notch Behavior of PEEK, PEEK Biomaterials Handbook. William Andrew, Norwich, NY: Elsevier; 2019. pp. 61–73. [Google Scholar]
- 7.Rochford ET, Poulsson AH, Salavarrieta Varela J, Lezuo P, Richards RG, Moriarty TF. Bacterial adhesion to orthopaedic implant materials and a novel oxygen plasma modified PEEK surface. Colloids Surf B Biointerfaces. 2014;113:213–22. doi: 10.1016/j.colsurfb.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Gorth DJ, Puckett S, Ercan B, Webster TJ, Rahaman M, Bal BS. Decreasedbbacteria activity on Si(3)N(4) surfaces compared with PEEK or titanium. Int J Nanomed. 2012;7:4829–40. doi: 10.2147/IJN.S35190. doi:10.2147/IJN. S35190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anusavice KJ, Shen C, Rawls HR. Phillips'Science of Dental Materials. 12th ed. St. Louis, Mo: Elsevier/Saunders: Elsevier Health Sciences; 2013. [Google Scholar]
- 10.Díez-Pascual AM, Díez-Vicente AL. Development of nanocomposites reinforced with carboxylated poly (ether ether ketone) grafted to zinc oxide with superior antibacterial properties. ACS Appl Mater Interfaces. 2014;6:3729–41. doi: 10.1021/am500171x. [DOI] [PubMed] [Google Scholar]
- 11.Tran N, Kelley MN, Tran PA, Garcia DR, Jarrell JD, Hayda RA, et al. Silver doped titanium oxide-PDMS hybrid coating inhibits Staphylococcus aureus and Staphylococcus epidermidis growth on PEEK. Mater Sci Eng C Mater Biol Appl. 2015;49:201–9. doi: 10.1016/j.msec.2014.12.072. [DOI] [PubMed] [Google Scholar]
- 12.Lu T, Li J, Qian S, Cao H, Ning C, Liu X. Enhanced osteogenic and selective antibacterial activities on micro-/nano-structured carbon fiber reinforced polyetheretherketone. J Mater Chem B. 2016;4:2944–53. doi: 10.1039/c6tb00268d. [DOI] [PubMed] [Google Scholar]
- 13.Dupuis A, Ho TH, Fahs A, Lafabrier A, Louarn G, Bacharouche J, et al. Improving adhesion of powder coating on PEEK composite: Influence of atmospheric plasma parameters. Appl Surf Sci. 2015;357:1196–204. [Google Scholar]
- 14.Fang F, Szleifer I. Effect of molecular structure on the adsorption of protein on surfaces with grafted polymers. Langmuir. 2002;18:5497–5510. [Google Scholar]
- 15.Wang Z, Zuilhof H. Self-healing superhydrophobic fluoropolymer brushes as highly protein-repellent coatings. Langmuir. 2016;32:6310–8. doi: 10.1021/acs.langmuir.6b01318. [DOI] [PubMed] [Google Scholar]
- 16.Briem D, Strametz S, Schröder K, Meenen NM, Lehmann W, Linhart W, et al. Response of primary fibroblasts and osteoblasts to plasma treated polyetheretherketone (PEEK) surfaces. J Mater Sci Mater Med. 2005;16:671–7. doi: 10.1007/s10856-005-2539-z. [DOI] [PubMed] [Google Scholar]
- 17.Velasco-Ortega E, Alfonso-Rodríguez CA, Monsalve-Guil L, España-López A, Jiménez-Guerra A, Garzón I, et al. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater Sci Eng C Mater Biol Appl. 2016;64:1–10. doi: 10.1016/j.msec.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y, Guo H, Chu L, He Z, Wang M, Zhang S, et al. Promoting osteoblasts responses in vitro and improving osteointegration in vivo through bioactive coating of nanosilicon nitride on polyetheretherketone. J Orthop Translat. 2020;24:198–208. doi: 10.1016/j.jot.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshizaki S, Saito M, Shinozaki Kuwahara N, Asano T, Kurita Ochiai T, Komiyama O. Evaluation of bending properties and adherence of Candida albicans to antibacterial glass- added polyetheretherketone as a denture base material. Int J Oral Med Sci. 2020;18:317–24. [Google Scholar]
- 20.Kyomoto M, Ishihara K. Self-initiated surface graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on poly (ether ether ketone) by photoirradiation. ACS Appl Mater Interfaces. 2009;1:537–42. doi: 10.1021/am800260t. [DOI] [PubMed] [Google Scholar]
- 21.He D, Susanto H, Ulbricht M. Photo-irradiation for preparation, modification and stimulation of polymeric membranes. Prog Polym Sci. 2009;34:62–98. [Google Scholar]
- 22.Panagoda GJ, Ellepola AN, Samaranayake LP. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses. 2001;44:29–35. doi: 10.1046/j.1439-0507.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- 23.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–94. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An S, Evans JL, Hamlet S, Love RM. Incorporation of antimicrobial agents in denture base resin: A systematic review. J Prosthet Dent. 2021;126:188–95. doi: 10.1016/j.prosdent.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Learn G, Lai E, von Recum H. Nonthermal plasma treatment improves uniformity and adherence of cyclodextrin-based coatings on hydrophobic polymer substrates. Coatings. 2020;10:1056. [Google Scholar]
- 26.Zhang S, Awaja F, James N, McKenzie D, Ruys A. Autohesion of plasma treated semi-crystalline PEEK: Comparative study of argon, nitrogen and oxygen treatments. Colloid Surf A Physicochem. 2010;374:88–95. [Google Scholar]
- 27.Agrawal G, Negi YS, Pradhan S, Dash M, Samal SK. In: Characterization of Polymeric Biomaterials. Amsterdam, The Netherlands: Elsevier Ltd; 2017. Wettability and contact angle of polymeric biomaterials; pp. 57–81. [Google Scholar]
- 28.Ramanna PK. Wettability of three denture base materials to human saliva, saliva substitute, and distilled water: A comparative in vitro study. J Indian Prosthodont Soc. 2018;18:248–56. doi: 10.4103/jips.jips_301_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fricke K, Reuter S, Schroder D, Schulz-von der Gathen V, Weltmann KD, von Woedtke T. In: IEEE Transactions on Plasma Science. Vol. 40. Institute of Electrical and Electronics Engineers, The IEEE is headquartered in New York City; 2012. Nvestigation of surface etching of poly (ether ether ketone) by atmospheric-pressure plasmas; pp. 2900–11. [Google Scholar]
- 30.Mozaffari A, Parvinzadeh Gashti M, Mirjalili M, Parsania M. Argon and argon-oxygen plasma surface modification of gelatin nanofibers for tissue engineering applications. Membranes (Basel) 2021;11:31. doi: 10.3390/membranes11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]