ABSTRACT
Background:
This review aims to perform a complete evaluation of the impact of photobiomodulation (PMB) on postoperative endodontic pain.
Materials and Methods:
The PRISMA checklist was used to perform this systematic review. The electronic databases were searched, including Google Scholar, PubMed, and Embase. Sixty-three papers were obtained through a main electronic search and a hand search. Nine trials met the criteria after screening the titles, abstracts, and/or full texts.
Results:
Seven out of nine studies showed that PMB has a significant impact on relieving postoperative endodontic pain, with no statistically significant difference in the severity of pain between the laser and control groups in the two remaining studies. In addition, eight studies showed no adverse effects, indicating that we can remove the adverse effects of drugs such as nonsteroidal anti-inflammatory drugs. However, one study showed evidence of the consequences of PMB application on teeth with symptomatic irreversible pulpitis. Therefore, it can be concluded that PMB should not be used in teeth with pain because of irreversible pulpitis.
Conclusion:
Although there is some understanding from a cellular viewpoint of the effects of PMB, there is still some uncertainty about whether these cell-level modifications impact reducing the postendodontic pain.
Keywords: Clinical trial, low-level light therapy, pain, review, root canal therapy
INTRODUCTION
The goal of endodontic treatment is complete debridement and cleaning of the root canals of an infected tooth so that the root canal space can be shaped and prepared for obturation with a neutral substance to prevent or limit any possibilities of reinfection.[1]
The common factors that can be ascribed to endodontic failure are the persistence of bacteria (intracanal and extracanal), insufficient filling of the canal, overextension of root filling materials, incorrect coronal seal, and untreated canals. Furthermore, iatrogenic procedural errors due to poor access cavity design and instrumentation complications (ledges, perforations, or separated instruments) could be considered as other etiologies for endodontic failure.
One of the most well-known complications of endodontic treatment is postoperative pain. Not only does it bring distress to patients but also it can lead to visiting emergency rooms. Pain symptomatology may exist in around 40% of the instances after endodontic treatment, regardless of the instrumentation approach utilized.[2,3] This may also influence patients’ lifestyles after endodontic treatment.[4,5] One of the predominant reasons for behavioral changes is odontogenic pain because it impacts the mood and capability of performing daily routines, for example, working, doing household chores, sleeping, eating, and even speaking.[6 7,8] The pain following endodontic treatment can be classified into two specific groups: Common postoperative pain and flare-up. Flare-up can be seen as swelling and/or torment within a couple of hours or days after treatment, which is often more severe than the common postoperative pain.
The postoperative pain due to root canal treatment is associated with an inflammatory reaction within periapical tissues.[9] This reaction activates nociceptors through inflammatory mediators, for example, serotonin, bradykinin, leukotrienes, and prostaglandins, resulting in pain after the procedure. Chemical, mechanical, and microbial elements are the main links to periapical inflammation following the root canal treatment, indeed, when the root canal preparation does not surpass the apical foramen throughout the treatment. Extrusion of tooth debris, intracanal medicaments, root canal irrigation solutions, and microorganisms may still happen, accompanied by sudden inflammation and pain.[10,11]
Research must evaluate the factors mediating postoperative pain after root canal treatment. Preoperative pain is extensively recommended to be linked significantly to postoperative pain severity. Law et al. showed that pain interfered with daily activities and intensified by tension. A diagnosis of symptomatic apical periodontitis can be considered an independent risk factor for postoperative pain severity.[12,13,14] The frequency and severity of postoperative pain can be influenced by distinct instrumentation strategies for root canal preparation, for example, reducing the occlusion, working length determination methods, cold lateral compaction irrigation system, and shuttering procedures.[15,16,17,18]
Many treatment procedures have been suggested to control postendodontic pain; most of them are pharmaceutical remedies, prescribed as prophylactic medicines and postoperative analgesics.[15] The pharmaceutical remedies include the prescription of nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, antihistamines, steroidal anti-inflammatory drugs, long-lasting anesthesia, narcotic analgesics, and intracanal medicine.[15,19,20,21] Nonpharmacological techniques contain protocols for decreasing nervousness, laser treatment,[22] and cryotherapy.[23]
Photobiomodulation (PMB) is commonly used in clinical practice and is defined by various parameters: (1) Laser power (10-3 – 10-1 W); (2) wavelength (300–10,600 nm); (3) pulse rate (0–5000 Hz); (4) intensity (10-2 – 102 J/cm2); and (5) electromagnetic spectrum (630–980 nm from visible red to nearly visible red). The use of PMB has been shown to be effective in pain relief, wounds, and nerve damage.[24] Due to its ability to facilitate wound healing, function in root canal disinfection, pain relief, and the lack of adverse events, PMB was applied to endodontic therapy.[25,26] Although the basic components of pain reduction through PMB have not been completely understood, theories have hypothesized that PMB may diminish pain through biochemical components based on increased adenosine triphosphates (ATPs) and decreased oxidative stress. The generally accepted mechanism of action is that absorbed light from the laser on the target tissue creates reactive oxygen species which leads to gene transcription and cellular healing. Mitochondria are very receptive to this process and the near-infrared light is absorbed and the energy is converted into ATP for cellular functions.[27,28]
Reducing pain after endodontic treatment has invariably been a popular area of research.[15,16,17,18,29] Nonetheless, just a few papers have systematically reviewed the impacts of PMB on postoperative endodontic pain.[30] Thus, this review aimed to perform a complete evaluation of the impact of PMB on postoperative endodontic pain.
MATERIALS AND METHODS
The PRISMA checklist was used as a guideline to perform this comprehensive review.[18]
Eligibility criteria
The inclusion criteria consisted of the following: Studies assessing the effectiveness of lasers in relieving postendodontic pain, randomized clinical trials (RCTs), or controlled clinical trials, and studies providing relevant information; participants: Systemically healthy patients but in need of endodontic treatment; intervention type: Subjects allocated to experimental or control/placebo groups depending on whether they received the laser treatment; outcome variables: The intensity of pain, the prevalence of pain, or the need for analgesics.
Exclusion criteria consisted of the following: Reviews and primary studies, including cohort studies, case-control studies, case series, case reports, descriptive studies, opinion articles, and abstracts, repeated publications, animal experiments, patients with systemic diseases or on any medication influencing the postoperative endodontic pain, application of a high-level laser, and studies not written in English.
Information sources, search strategy, and study selection
The searched electronic databases included Google Scholar, Scopous, PubMed, and Embase from January 1, 2015 to December 30, 2020, using the following keywords: Endodontic, root canal therapy, pain, postoperative, low-level laser, PMB and diode laser. The keywords were validated by Medical subject headings dictionary. The keywords were combined through Boolean operators to improve the search: “Pain” OR “postoperative pain” AND “endodontic” OR “root canal therapy” OR “low-level laser” OR “PMB” OR “diode laser.” Endnote X8 was used for electronic management of the literature. The primary outcome included pain intensity and secondary outcomes were hyperesthesia, hyperemia, and touch pain. For this study, 63 articles were selected concerning the title. Afterward, 22 duplicate studies were eliminated. By reviewing the abstracts, some of them were excluded: 12 nonrelevant papers, one book reference, one thesis reference, 14 review articles, and one case due to the lack of access to the full text. Subsequently, the methods and materials of all the papers were reviewed, and 12 referrals were selected. Then, the full texts of all the references were studied and analyzed to find articles strongly relevant to the subject matter assessed, referring to the eligibility criteria. As a result, three articles that did not follow the inclusion criteria were excluded from this comprehensive review.
Data items and collection
A specialized data extraction form was developed. First, we extracted the general information of the studies, including authors, year of publication, the research designs, country, the number of participants along with their age and gender, type of teeth, endodontic diagnoses, endodontic treatment, pain evaluation method, laser parameters, method of laser application, and outcomes.
RESULTS
In this study, due to the heterogeneity of the data, meta-analysis was not used for the statistical analysis of results.
Search results
By a main electronic search and a hand search, 63 papers were obtained. Nine trials met the inclusion criteria after screening the titles, abstracts, and/or full texts. The flow diagram of the study inclusion of the comprehensive review is shown in Figure 1. Nine randomized clinical trials[15,16,17,18,28,31,32,33,34] were finally included. Table 1 shows the specifications of the included studies.
Figure 1.
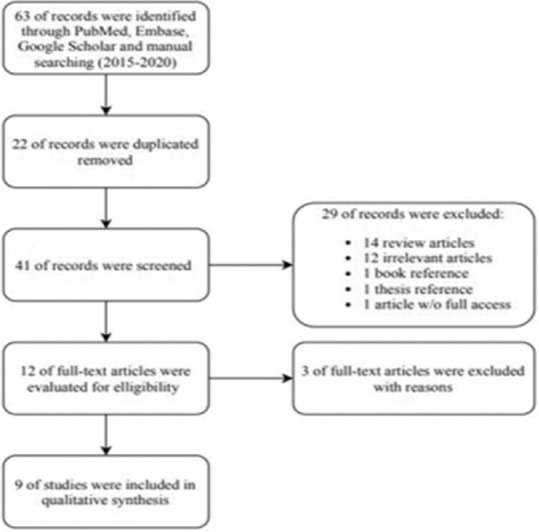
Flow diagram of the study.
Table 1.
The specifications of the included studies
| Study ID | Study design | Number of participants/age/gender | Tooth type | Endodontic diagnoses | Endodontic treatment | Pain evaluation method | Pain evaluation interval |
|---|---|---|---|---|---|---|---|
| Nabi et al.[18] | RCT (Randomized clinical trial) | 120 participants | Permanent teeth with fully formed apex | Symptomatic irreversible pulpitis with no periapical radiolucency | RCT (Root canal therapy), single visit | Heft and parker pain rating scale | 4, 8, 12, 24, and 48 h of posttreatment |
| 68 males and 52 females | |||||||
| 18–64 years | |||||||
| Morsy et al. (2018)[29] | Parallel RCT (Randomized clinical trial) | 56 participants | Anterior teeth (maxillary central incisors permanent teeth) | Necrotic teeth with chronic periapical lesions | RCT (Root canal therapy), two-visit by a single endodontist | NRS | Pretreatment, and 6, 12, 24, 48 h and 7 after treatment |
| 24 males and 32 females | |||||||
| 18–35 years | |||||||
| Doğanay Yıldız and Arslan[16] | Randomized placebo-controlled clinical trial | 42 participants | Mandibular molar | Symptomatic apical periodontitis | RCT (Root canal therapy), single visit by a single endodontist | VAS | 1, 3, 5, 7, and 30 days of posttreatment |
| 19 males and 23 females | |||||||
| 18–64 years | |||||||
| Ramalho et al. (2016)[32] | Randomized placebo-blind clinical trial | 60 participants | Irreversible pulpitis | VAS | Immediately and 15 min after treatment | ||
| 21 males and 39 females | |||||||
| Mean age | |||||||
| Control group: 33.7±11.6 | |||||||
| Placebo group: 31.1±13.8 | |||||||
| Laser at 4 J/cm2 group: 34.6±13.04 | |||||||
| Laser at 40 J/cm2 group: 30.3±11.8 | |||||||
| Arslan et al.[15] | Preliminary placebo-controlled, triple-blind, RCT (Randomized clinical trial) | 36 participants, whom 3 participants dropped out 21 males and 12 females | Mandibular molars | Teeth with periapical lesions and pain VAS <50 and a percussion pain VAS <50 | RCT (Root canal therapy), two-visit | VAS | 1, 2, 3, 4, 5, 6, and 7 days of posttherapy |
| Mean age | |||||||
| Placebo group: 25.76±8.14 | |||||||
| PMB group: 32.62±9.27 | |||||||
| Coelho et al. (2019)[33] | Prospective, randomized, double-blind clinical trial | 60 participants | Single-rooted teeth with fully developed apices and healthy periodontal tissue | Asymptomatic necrotic pulp | RCT (Root canal therapy), single visit | VAS | 24 h, 72 h, and 1-week after treatment |
| 22 males and 38 females | |||||||
| Mean age | |||||||
| Control group: 46.97±13.23 | |||||||
| PDT group: 46.73±16.30 | |||||||
| Lopes et al., (2019)[17] | Randomized, controlled, clinical study | 60 participants | Mandibular first or second molar | Irreversible pulpitis | RCT (Root canal therapy), single visit | VRS, NRS | 6, 12, and 24 h after treatment |
| 23 males and 37 females | |||||||
| 18–60 years | |||||||
| Barciela et al. (2019)[34] | Prospective RCT (Randomized clinical trial) | 40 participants | Single-root | Pulp necrosis, showing apical periodontitis visible on periapical radiography, without spontaneous painful symptoms | RCT (Root canal therapy), single visit by a single experienced endodontist | VAS | 24 h, 72 h, and 1 week after treatment |
| 18–76 years |
RCT: Randomized clinical trial; RCT: Root canal therapy; NRS: Numerical Rating Scale; VAS: Visual Analog Scale; PMB: Photobiomodulation; PDT: Photo Dynamic Therapy
In addition, summary details of laser parameters are shown in Table 2.
Table 2.
Laser parameters and pain intervals
| Study ID | Type of laser | Mode | Wavelength (nm) | Energy density (J/cm2) | Power output | Exposure time | Total dose per point (tooth) | Method of application |
|---|---|---|---|---|---|---|---|---|
| Nabi et al.[18] | Pulse wave | 905 | 12–16 mW | 180 s | 1.08–1.44 J/point, 2.16–2.88 J/tooth | In contact mode perpendicular to the periapical region of the teeth both buccally as well as lingually (two points/tooth) | ||
| Morsy et al. (2018)[29] | Diode laser coupled with optical fiber 200 µm | Pulse wave | 980 | 1.2 W | 20 s (a 5 s irradiation followed by a 10 s pause, four times for each tooth) | The tip was positioned 1 mm short of the apex, slowly dragged at a speed of approximately 2 mm/s in a way that the root canals were irradiated from the apical to the coronal portion in a helicoidal movement touching the canal walls | ||
| Doğanay Yıldız and Arslan[16] | Diode laser coupled with optical fiber 200 µm | 970±15 | 85.8 J/cm2 | 0.5 W | 60 s total The tissue around the apex of the mesial root apex 30 s The tissue around the apex of the distal root 30 s | 15 J/point, 30 J/tooth | At a distance of approximately 10 mm from the tissue around the apex of the mesial and distal root (two points/tooth) | |
| Ramalho et al. (2016)[32] | Diode laser | 780 | 4 J/cm2 and 40 J/cm2 | 40 mW | 8 s (4 s/point) and 80 (40 s/point) | 0.16 J/point, 0.32 J/tooth and 1.6 J/point, 3.2 J/tooth | Two points of irradiation; the first perpendicular to the tooth in the middle third of the crown, the second perpendicularly to the periapex | |
| Arslan et al.[15] | Diode laser coupled with optical fiber 200 µm | 970±15 | 85.8 J/cm2 | 0.5 W | 60 s the tissue around the apex of the mesial root apex 30 s the tissue around the apex of the distal root 30 s | 15 J/point, 30 J/tooth | At a distance of approximately 10 mm from the tissue around the mesial and distal root apexes (two points/tooth) | |
| Coelho et al. (2019)[33] | 660 | 600 J/cm2 | 100 mW | 180 s | 18 J/tooth | The tip was moved in a gentle vertical motion | ||
| Lopes et al., (2019)[17] | Indium- gallium- aluminum laser | 808 | 90 J/cm2 | 0.1 W | 100 s (25 s/point) | 2.5 J/point, 10 J/tooth | Perpendicular in contact with the gingiva on two points of the buccal and lingual side (4 points/tooth) | |
| Barciela et al. (2019)[34] | Low-intensity red laser | 660 | 320 J/cm2 | 90 s |
Table 3 shows the results of all thse included studies.
Table 3.
The outcomes of the included studies
| Study ID | Outcomes | P | Significance |
|---|---|---|---|
| Nabi et al.[18] | PMB can be an effective alternative to the conventional use of NSAIDs in controlling postendodontic pain | 0.044 | Significant |
| Morsy et al.[31] | Intracanal diode laser irradiation can decrease the postoperative pain after conventional RCT in cases of necrotic teeth with periapical lesions | <0.001 | Significant |
| Doğanay Yıldız and Arslan[16] | PMB can be beneficial in reducing postoperative pain in endodontics | 0.037 | Significant |
| Ramalho et al.[32] | The application of 780-nm diode laser irradiation at 4 and 40 J/cm2 showed no effect in reducing pain. The influence of 4 J/cm2 showed a negative effect on local anesthetics, resulting in a significant increase in complimentary local anesthesia | 0.056 | NS |
| Arslan et al.[15] | PMB may reduce postoperative pain after RCT of mandibular molar (on first 4 days) | <0.05 | Significant |
| Coelho et al.[33] | Photodynamic therapy was efficient in reducing postoperative pain in single-visit RCT of teeth with necrotic pulps | 0.036 | Significant |
| Lopes et al., (2019)[17] | The PBM therapy reduces the prevalence of postoperative pain | 0.04 | Significant |
| Barciela et al.[34] | The pain between the control and laser groups was equivalent | 0.28 | NS |
NSAIDs: Nonsteroidal anti-inflammatory drugs; RCT: Root canal therapy; PMB: Photobiomodulation; NS: Not significant
Main outcomes of the studies
Nabi et al.[18] showed no statistically significant differences in age and gender between the groups. Morsy et al.[31] demonstrated no significant differences in demographic data, age, and gender. Furthermore, they showed that the bacterial counts of both aerobic and anaerobic bacteria in the laser group were significantly less than in the control group. As a result, a 980-nm diode laser can be a good supplement to the routine endodontic treatment of necrotic cases with chronic periapical lesions concerning postoperative pain and root canal disinfection.
Doğanay Yıldız and Arslan[16] reported no significant differences between the groups in demographic data, age, sex, pulpal status, tooth number (first or second molar), preoperative palpation, an extra root canal, and postoperative percussion pain levels.
In a study by Ramalho et al.,[32] the 4-J/cm2 irradiation significantly increased the failure of local anesthetics in lower jaw teeth due to the PMB, which can improve local circulation and vasodilatation, increasing local anesthetic agent absorption. Therefore, they concluded that PMB should be avoided in teeth with pain because of irreversible pulpitis.
Arslan et al.[15] showed no statistically significant differences between the groups in gender, tooth number, and postoperative pain on percussion. The participants in the PMB group exhibited less pain compared to patients in the placebo group in the first 4 days, but no significant differences were detected between the groups after 5 and 7 days.
According to Doğanay Yıldız et al.,[28] there were no significant differences between the groups in age and sex. In addition, postoperative percussion level was significantly lower in the laser group than in the placebo group. Furthermore, the whole postoperative amount of substance P was significantly higher than the preoperative amounts in the laser group; therefore, the low-level laser can heal wounds through the substance P pathway.
Number of patients in need of analgesics or ibuprofen
Nabi et al.[18] showed that taking Ibuprofen 1 h before the endodontic treatment reduced postendodontic pain significantly at 4–8-h postoperative intervals. However, the combination of PMB after the treatment and ibuprofen before the treatment demonstrated the best outcomes in reducing postoperative pain. As a result, PMB can be a successful alternative to the routine use of NSAIDs in handling postendodontic pain without the adverse effects of these drugs.
In the study by Doğanay Yıldız and Arslan,[16] only one patient among 14 patients required postoperative analgesics in the PMB group. Therefore, they concluded that PMB could reduce postendodontic pain.
In the study by Arslan et al.,[15] the number of patients requiring analgesics was lower in the PMB group compared to the placebo group. Doğanay Yıldız et al.[28] could not find any significant differences between the placebo and laser groups in terms of the need for analgesics.
Adverse effects
None of these eight studies showed any adverse effects; in addition, they showed that we can eliminate the adverse effects of drugs such as NSAIDs using PMB.[18] However, we should notice that Ramalho et al.[32] showed evidence of the consequences of PMB application on teeth with symptomatic irreversible pulpitis. As a result, we can conclude that PMB should not be used in teeth with pain because of irreversible pulpitis.
DISCUSSION
As mentioned previously, one of the most well-known complications of endodontic treatment is postoperative pain, and there are different factors resulting in this pain. Based on several clinical trials, endodontic treatment using the reciprocating approach is correlated with postoperative pain as this symptom is related to increased C-type nerve fiber neuropeptides, likely induced by the extrusion of infected debris into the peri-apex.[4,35] Caviedes-Bucheli et al.[36] reported that the expression of these neuropeptides and consequent pain is more related to the cross-sectional structure of the tools rather than to their kinematics. Furthermore, Türker et al.,[37] when examining the number of bacteria apically expelled by rotating and reciprocating systems, discovered that the single reciprocating instrument caused much less bacterial extrusion. There was no difference in postoperative pain in a clinical trial by Relvas et al.,[38] when comparing the rotary system to the reciprocating system.
Histologically, Scarparo et al.[39] showed that the extrusion of root canal filling material results in inflammatory cellular elements, elevated fibrous concentration, and the creation of micro-abscesses. In contact with nerve cells, Ruparel et al.[40] tested the direct impact of different endodontic-luting agents in vitro and suggested that all cement blocks somehow boosted neuropeptide expression of nerve fibers; they also prompt nociceptors and may enhance neurogenic inflammation. The authors of this clinical trial observed that unintentional cement extravasation into the periapical region were a high risk for postoperative pain in both groups. In a systematic review of Rosen et al.’s case reports,[41] the extrusion of obturating materials worsened postoperative pain induced by nerve cell damage as soon as the substances came into contact. They found out that in both groups, accidental extrusion of cement into the periapical area posed a high risk of postoperative pain.
Nitzan et al.[42] identified several instances such that the extrusion of root canal filling material should be avoided as this event brings about inessential chemical and mechanical annoyance, hampering the regeneration of periapical tissues. As a result, how endodontists carry out root canal therapy is a really critical factor that can impact the severity of postendodontic pain.
Six out of nine articles reviewed in this study[16,17,18,28,33,34] carried out single-visit root canal therapy, two studies[15,31] performed two-visit endodontic treatments, and Ramalho et al.[32] did an emergency endodontic treatment. The number of treatment sessions may influence postoperative pain. Thus, this element should also be included in future studies. In addition, some of the studies included in this review used laser after root canal treatment,[16,17,18,41] and some used it before root canal obturation[15,31,33,42] or during endodontic emergency treatment.[32]
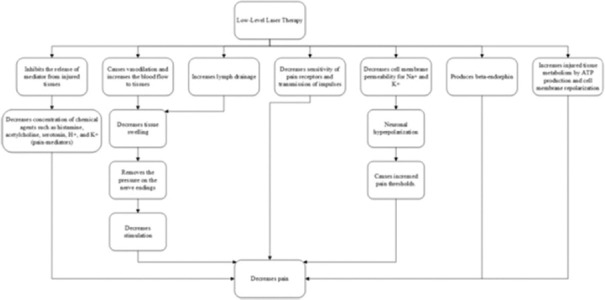
PMB can decrease the amount of pain through different biological pathways, as shown in Figure 2.[43]
Figure 2.
Impact paths of photobiomodulation concerning pain.
As with other fields (e.g., orthodontics), PMB has been recognized as an efficient and noninvasive tool for managing pain in orthodontic patients. In addition, PMB has been widely used to relieve pain in different fields of dentistry.[29]
Laser parameters can have an impact on the pain outcome
One of the factors preventing a quantitative study is the extensive heterogeneity of the laser parameters among the covered studies, which may play a critical role in the pain outcome. The outcome of PMB can be influenced by laser type, emission mode, energy density, energy output, wavelength, and laser exposure time. Except for three studies that failed to describe the exact type of laser used, all lasers used in the included studies were diode laser, with one study describing it to be indium-gallium-aluminum diode laser.[44]
Over time, different laser types have been developed and used in various dentistry fields.[45] Diode lasers are the most commonly used among various lasers. The 980-nm diode laser active medium is a solid-state semiconductor made up of gallium, indium, and arsenide. Lasers with diode have many benefits such as extreme compactness, operation simplicity, affordability, convenient setting-up, flexibility, and small size.[46] The wavelengths related to diode lasers are highly soluble in melanin and hemoglobin and poorly absorbed in dental hard tissues. They are also absorbed by water,[47] providing the laser with the benefit of acting selectively and accurately.[46]
Radiation of diode laser has some bactericidal impact by modifying the bacterial cell wall. Microbiologists discuss permanent cell membrane loss, usually in conjunction with direct heat.[48] The diode laser applies a photothermal impact on the bacteria and a photo-disruptive impact on the unreachable bacteria.[49]
Gutknecht et al.[50] showed that diode laser light could penetrate the dentin by up to >1000 μm. Therefore, it can be an efficient way to clean the root canals along with traditional biomechanical procedures, penetrating areas that were previously unattainable. In addition to traditional methods of cleaning and forming, the application of appropriate wavelengths can efficiently sterilize the dentin, root canals, and periapical areas and reduce bacterial recolonization. Therefore, based on Morsy et al.’s study,[31] it can be inferred that the 980-nm diode laser may be utilized as an alternative to traditional endodontic therapy.
Age and gender
In six studies,[16,17,18,28,31,33] there was no significant relationship between the age of the patient and postoperative pain. This observation supports the results of studies by Ng et al.[51] and Polycarpou et al.[52] However, these studies disagree with Ali et al.,[53] who believed that in older age groups, postoperative pain would be more severe in comparison with younger ones. Ali et al.[53] categorized patients into two groups and found that postoperative pain was higher in the older age group (41–65 years old) than the younger age group (15–40 years old). In Arslan’s study,[15] the age range was 18–46 years, which may explain the differences between study results. The simple randomization technique used in this study resulted in an unequal distribution of age among the groups, and the difference was statistically significant. However, the mean age of the patients in the two groups was 25.76 and 32.62 years; this category is typically considered one group. Therefore, this age difference does not have any clinical significance.
Although some studies concluded that postoperative pain in females was more prevalent than in males,[20,21,22,23] five studies have concluded that sex did not have a significant effect.[15,16,18,31,41] Also, in Lopes’s study,[17] stratifying by sex showed that differences between genders persisted after 24 h.
In a clinical study, Ostrom et al.[54] showed that women had higher sensitivity to pain than men, measured as follows: Sensitivity to pressure and mechanical and thermal pain. Wiesenfeld-Hallin,[55] however, attributed the difference in sensitivity between men and women to the innate biological systems and thereby suggested that sex hormones affect pain tolerance and threshold. This literature disparity can be clarified because women show a higher incidence of pain than men.[56] Nevertheless, the inference of Lopes et al.[17] could be inaccurate since it relies on the pain stimulus. Women appear to experience more pain than men in brief and intense stimuli, but it has been found that they feel greater pain adaptation to persistent painful stimuli.[57]
Photobiomodulation in comparison with nonsteroidal anti-inflammatory drugs
This research suggests that pain substantially decreased postoperatively in all treatment groups. Ibuprofen demonstrated significant pain relief at 4–8-h intervals compared to the control group. In this research, a prophylactic dose of 400 mg was used.[18] These findings are consistent with those of Dionne and Cooper[58] Lately, Arslan et al.[59] also found that ibuprofen is more efficient in postendodontic pain reduction at a 6-h interval. Prophylactic ibuprofen administration before RCT (Root canal therapy) blocks the Cox pathway, and the sense of pain can be blocked by this procedure.[18]
In addition, using a placebo as a control, one of the included studies also assessed the discrepancies between the effectiveness of PMB and ibuprofen, a type of NSAID.[18] In this analysis, PMB indicated significant effectiveness in pain control at intervals of 4–8 h and showed a significant pain intensity reduction after RCT (Root canal therapy) at 12-, 24-, and 48-h intervals compared to the ibuprofen group. PMB paired with preoperative NSAIDs led to a greater pain reduction than using NSAIDs or PMB alone. NSAIDs are commonly used in pain management after endodontic treatment, relieving pain by decreasing chemical inflammatory mediators in peripheral nociceptors and thus triggering the associated subsequent events.[60] In addition, NSAIDs are noted for their side effects, i.e., wound healing delay, which is undesirable for restoring postendodontic tissues.[61] PMB has a significant effect, along with the ability to mediate tissue repair; it could be an alternative to the traditional treatment choice based on NSAIDs in pain control after RCT (Root canal therapy).[44]
Of the nine studies included in the systematic analysis, seven studies[15,16,17,18,28,31,33] indicated that PMB could efficiently alleviate postoperative endodontic pain, and two studies did not indicate it.[32,34] Ramalho suggested a high standard deviation in the results since pain is a subjective experience. The application of laser irradiation of 780-nm diode at 4–40 J/cm2 indicated no impact on pain reduction. This outcome may be attributed to variations in radiation parameters and the limited number of examined patients in each group, just 15, and it can also impact the study outcome.
Barciela et al.[34] indicated no significance in postendodontic pain between the control and laser groups. These findings may be explained by differences in radiation parameters.
Given that this systematic review conformed to standard procedures, a few restrictions remained. First, although a comprehensive literature search was conducted, the survey included only nine studies. Second, the qualitative description of this study might have been affected by the methodological variability and the lack of compatibility of the key results. Since different tools for assessment of pain in the different studies have been used, the comparison between them was difficult. Third, some studies have not gathered the details necessary for the article; however, the authors attempted to reach out to the researchers for further information, with no responses. Fourth, the language constraint in the literature search may have caused bias in this study. Although we attempted to contact the researchers, there was no response. Fifth, limited number of databases and limited number of key words, which were searched, was one of the other limitations of this study.
CONCLUSION
Although there is some level of understanding from a cellular viewpoint of the effects of PMB, there is still some uncertainty about whether these cellular-level modifications have any impact on reducing postendodontic pain. The findings are incomplete and cannot be generalized to the whole population; as a result, more well-structured studies are necessary to eliminate bias to obtain a clearer understanding of the PMB impact on postendodontic pain.
Ethical consideration
The study was approved by the Ethical Committee of Urmia University of Medical Sciences and conducted in accordance with the ethical guidelines of the Declaration of Helsinki (IR.UMSU.REC.1399.177).
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
Acknowledgment
The authors wish to thank the Research Vice Chancellor of Urmia University of Medical Sciences.
REFERENCES
- 1.Siqueira JF., Jr Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int Endod J. 2001;34:1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Pasqualini D, Mollo L, Scotti N, Cantatore G, Castellucci A, Migliaretti G, et al. Postoperative pain after manual and mechanical glide path: A randomized clinical trial. J Endod. 2012;38:32–6. doi: 10.1016/j.joen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Pak JG, White SN. Pain prevalence and severity before, during, and after root canal treatment: A systematic review. J Endod. 2011;37:429–38. doi: 10.1016/j.joen.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Pasqualini D, Corbella S, Alovisi M, Taschieri S, Del Fabbro M, Migliaretti G, et al. Postoperative quality of life following single-visit root canal treatment performed by rotary or reciprocating instrumentation: A randomized clinical trial. Int Endod J. 2016;49:1030–9. doi: 10.1111/iej.12563. [DOI] [PubMed] [Google Scholar]
- 5.Vena DA, Collie D, Wu H, Gibbs JL, Broder HL, Curro FA, et al. Prevalence of persistent pain 3 to 5 years post primary root canal therapy and its impact on oral health-related quality of life: PEARL network findings. J Endod. 2014;40:1917–21. doi: 10.1016/j.joen.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Batista MJ, Perianes LB, Hilgert JB, Hugo FN, Sousa Mda L. The impacts of oral health on quality of life in working adults. Braz Oral Res. 2014;28:1–6. doi: 10.1590/1807-3107bor-2014.vol28.0040. [DOI] [PubMed] [Google Scholar]
- 7.Cavalheiro CH, Abegg C, Fontanive VN, Davoglio RS. Dental pain, use of dental services and oral health-related quality of life in Southern Brazil. Braz Oral Res. 2016;30:e39. doi: 10.1590/1807-3107BOR-2016.vol30.0039. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LA, Harris SL, Bonito AJ, Manski RJ, Macek MD, Edwards RR, et al. Coping with toothache pain: A qualitative study of low-income persons and minorities. J Public Health Dent. 2007;67:28–35. doi: 10.1111/j.1752-7325.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- 9.Sathorn C, Parashos P, Messer H. The prevalence of postoperative pain and flare-up in single- and multiple-visit endodontic treatment: A systematic review. Int Endod J. 2008;41:91–9. doi: 10.1111/j.1365-2591.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 10.Trope M. Flare-up rate of single-visit endodontics. Int Endod J. 1991;24:24–6. doi: 10.1111/j.1365-2591.1991.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 11.Imura N, Zuolo ML. Factors associated with endodontic flare-ups: A prospective study. Int Endod J. 1995;28:261–5. doi: 10.1111/j.1365-2591.1995.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 12.Law AS, Nixdorf DR, Aguirre AM, Reams GJ, Tortomasi AJ, Manne BD, et al. Predicting severe pain after root canal therapy in the national dental PBRN. J Dent Res. 2015;94:37S–43S. doi: 10.1177/0022034514555144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadaf D, Ahmad MZ. Factors associated with postoperative pain in endodontic therapy. Int J Biomed Sci. 2014;10:243–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Alí A, Olivieri JG, Duran-Sindreu F, Abella F, Roig M, García-Font M. Influence of preoperative pain intensity on postoperative pain after root canal treatment: A prospective clinical study. J Dent. 2016;45:39–42. doi: 10.1016/j.jdent.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Arslan H, Doğanay E, Karataş E, Ünlü MA, Ahmed HM. Effect of low-level laser therapy on postoperative pain after root canal retreatment: A preliminary placebo-controlled, triple-blind, randomized clinical trial. J Endod. 2017;43:1765–9. doi: 10.1016/j.joen.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Doğanay Yıldız E, Arslan H. Effect of low-level laser therapy on postoperative pain in molars with symptomatic apical periodontitis: A randomized placebo-controlled clinical trial. J Endod. 2018;44:1610–5. doi: 10.1016/j.joen.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Lopes LP, Herkrath FJ, Vianna EC, Gualberto Júnior EC, Marques AA, Sponchiado Júnior EC. Effect of photobiomodulation therapy on postoperative pain after endodontic treatment: A randomized, controlled, clinical study. Clin Oral Investig. 2019;23:285–92. doi: 10.1007/s00784-018-2435-9. [DOI] [PubMed] [Google Scholar]
- 18.Nabi S, Amin K, Masoodi A, Farooq R, Purra AR, Ahangar FA. Effect of preoperative ibuprofen in controlling postendodontic pain with and without low-level laser therapy in single visit endodontics: A randomized clinical study. Indian J Dent Res. 2018;29:46–50. doi: 10.4103/ijdr.IJDR_327_15. [DOI] [PubMed] [Google Scholar]
- 19.Torabinejad M, Dorn SO, Eleazer PD, Frankson M, Jouhari B, Mullin RK, et al. Effectiveness of various medications on postoperative pain following root canal obturation. J Endod. 1994;20:427–31. doi: 10.1016/S0099-2399(06)80031-2. [DOI] [PubMed] [Google Scholar]
- 20.Arslan H, Gündoğdu EC, Sümbüllü M. The effect of preoperative administration of antihistamine, analgesic and placebo on postoperative pain in teeth with symptomatic apical periodontitis: A randomized controlled trial. Eur Endod J. 2016;1:1–5. doi: 10.5152/eej.2016.16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan JL, Jureidini B, Hodges JS, Baisden M, Swift JQ, Bowles WR. Gender differences in analgesia for endodontic pain. J Endod. 2008;34:552–6. doi: 10.1016/j.joen.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Koba K, Kimura Y, Matsumoto K, Gomyoh H, Komi S, Harada S, et al. Aclinical study on the effects of pulsed Nd: YAG laser irradiation at root canals immediately after pulpectomy and shaping. J Clin Laser Med Surg. 1999;17:53–6. doi: 10.1089/clm.1999.17.53. [DOI] [PubMed] [Google Scholar]
- 23.Keskin C, Özdemir Ö, Uzun İ, Güler B. Effect of intracanal cryotherapy on pain after single-visit root canal treatment. Aust Endod J. 2017;43:83–8. doi: 10.1111/aej.12175. [DOI] [PubMed] [Google Scholar]
- 24.Baghizadeh Fini M, Olyaee P, Homayouni A. The effect of low-level laser therapy on the acceleration of orthodontic tooth movement. J Lasers Med Sci. 2020;11:204–11. doi: 10.34172/jlms.2020.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solmaz H, Dervisoglu S, Gulsoy M, Ulgen Y. Laser biostimulation of wound healing: Bioimpedance measurements support histology. Lasers Med Sci. 2016;31:1547–54. doi: 10.1007/s10103-016-2013-9. [DOI] [PubMed] [Google Scholar]
- 26.Asnaashari M, Godiny M, Azari-Marhabi S, Tabatabaei FS, Barati M. Comparison of the antibacterial effect of 810 nm diode laser and photodynamic therapy in reducing the microbial flora of root canal in endodontic retreatment in patients with periradicular lesions. J Lasers Med Sci. 2016;7:99–104. doi: 10.15171/jlms.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll JD, Milward MR, Cooper PR, Hadis M, Palin WM. Developments in low level light therapy (LLLT) for dentistry. Dent Mater. 2014;30:465–75. doi: 10.1016/j.dental.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Doğanay Yıldız E, Arslan H, Köseoğlu S, Arabacı T, Yıldız DA, Savran L. The effect of photobiomodulation on total amount of substancePin gingival crevicular fluid: Placebo-controlled randomized clinical trial. Lasers Med Sci. 2019;34:517–23. doi: 10.1007/s10103-018-2625-3. [DOI] [PubMed] [Google Scholar]
- 29.Asnaashari M, Ashraf H, Daghayeghi AH, Mojahedi SM, Azari-Marhabi S. Management of post endodontic retreatment pain with low level laser therapy. J Lasers Med Sci. 2017;8:128–31. doi: 10.15171/jlms.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anagnostaki E, Mylona V, Parker S, Lynch E, Grootveld M. Systematic review on the role of lasers in endodontic therapy: Valuable adjunct treatment? Dent J (Basel) 2020;8:63. doi: 10.3390/dj8030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morsy DA, Negm M, Diab A, Ahmed G. Postoperative pain and antibacterial effect of 980 nm diode laser versus conventional endodontic treatment in necrotic teeth with chronic periapical lesions: A randomized control trial. F1000Res. 2018;7:1795. doi: 10.12688/f1000research.16794.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramalho KM, de Souza LM, Tortamano IP, Adde CA, Rocha RG, de Paula Eduardo C. A randomized placebo-blind study of the effect of low power laser on pain caused by irreversible pulpitis. Lasers Med Sci. 2016;31:1899–905. doi: 10.1007/s10103-016-2068-7. [DOI] [PubMed] [Google Scholar]
- 33.Coelho MS, Vilas-Boas L, Tawil PZ. The effects of photodynamic therapy on postoperative pain in teeth with necrotic pulps. Photodiagnosis Photodyn Ther. 2019;27:396–401. doi: 10.1016/j.pdpdt.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Barciela B, da Silva Limoeiro AG, Bueno CE, Fernandes SL, Mandarini DR, Boer NC, et al. In vivo evaluation of painful symptomatology after endodontic treatment with or without the use of photodynamic therapy. J Conserv Dent. 2019;22:332–5. doi: 10.4103/JCD.JCD_39_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nekoofar MH, Sheykhrezae MS, Meraji N, Jamee A, Shirvani A, Jamee J, et al. Comparison of the effect of root canal preparation by using WaveOne and ProTaper on postoperative pain: A randomized clinical trial. J Endod. 2015;41:575–8. doi: 10.1016/j.joen.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 36.Caviedes-Bucheli J, Moreno JO, Carreño CP, Delgado R, Garcia DJ, Solano J, et al. The effect of single-file reciprocating systems on substancePand calcitonin gene-related peptide expression in human periodontal ligament. Int Endod J. 2013;46:419–26. doi: 10.1111/iej.12005. [DOI] [PubMed] [Google Scholar]
- 37.Türker SA, Uzunoğlu E, Aslan MH. Evaluation of apically extruded bacteria associated with different nickel-titanium systems. J Endod. 2015;41:953–5. doi: 10.1016/j.joen.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Relvas JB, Bastos MM, Marques AA, Garrido AD, Sponchiado EC., Jr Assessment of postoperative pain after reciprocating or rotary NiTi instrumentation of root canals: A randomized, controlled clinical trial. Clin Oral Investig. 2016;20:1987–93. doi: 10.1007/s00784-015-1692-0. [DOI] [PubMed] [Google Scholar]
- 39.Scarparo RK, Grecca FS, Fachin EV. Analysis of tissue reactions to methacrylate resin-based, epoxy resin-based, and zinc oxide-eugenol endodontic sealers. J Endod. 2009;35:229–32. doi: 10.1016/j.joen.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Ruparel NB, Ruparel SB, Chen PB, Ishikawa B, Diogenes A. Direct effect of endodontic sealers on trigeminal neuronal activity. J Endod. 2014;40:683–7. doi: 10.1016/j.joen.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Rosen E, Goldberger T, Taschieri S, Del Fabbro M, Corbella S, Tsesis I. The prognosis of altered sensation after extrusion of root canal filling materials: A systematic review of the literature. J Endod. 2016;42:873–9. doi: 10.1016/j.joen.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Nitzan DW, Stabholz A, Azaz B. Concepts of accidental overfilling and overinstrumentation in the mandibular canal during root canal treatment. J Endod. 1983;9:81–5. doi: 10.1016/S0099-2399(83)80081-8. [DOI] [PubMed] [Google Scholar]
- 43.Khalighi HR, Anbari F, Beygom Taheri J, Bakhtiari S, Namazi Z, Pouralibaba F. Effect of low-power laser on treatment of orofacial pain. J Dent Res Dent Clin Dent Prospects. 2010;4:75–8. doi: 10.5681/joddd.2010.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Chen XL, Zou XL, Chen SZ, Zou J, Wang Y. Efficacy of low-level laser therapy in pain management after root canal treatment or retreatment: A systematic review. Lasers Med Sci. 2019;34:1305–16. doi: 10.1007/s10103-019-02793-6. [DOI] [PubMed] [Google Scholar]
- 45.Pirnat S. Versatility of an 810 nm diode laser in dentistry: An overview. J Laser Health Acad. 2007;4:1–9. [Google Scholar]
- 46.Maturo P, Perugia C, Docimo R. Versatility of an 810 nm diode laser in pediatric dentistry. Int J Clin Dent. 2013;6:161–72. [Google Scholar]
- 47.Shirani AM, Gutknecht N, Taghizadeh M, Mir M. Low-level laser therapy and myofacial pain dysfunction syndrome: A randomized controlled clinical trial. Lasers Med Sci. 2009;24:715–20. doi: 10.1007/s10103-008-0624-5. [DOI] [PubMed] [Google Scholar]
- 48.Dai S, Xiao G, Dong N, Liu F, He S, Guo Q. Bactericidal effect of a diode laser on Enterococcus faecalis in human primary teeth-an in vitro study. BMC Oral Health. 2018;18:154. doi: 10.1186/s12903-018-0611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmeduddin M, Nagesh B, Reddy KN, Raj KS. An assessment of bactericidal effect of two different types of lasers on Enterococcus faecalis: An in vitro study. J Dent Lasers. 2012;6:2. [Google Scholar]
- 50.Gutknecht N, Franzen R, Schippers M, Lampert F. Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth. J Clin Laser Med Surg. 2004;22:9–13. doi: 10.1089/104454704773660912. [DOI] [PubMed] [Google Scholar]
- 51.Ng YL, Glennon JP, Setchell DJ, Gulabivala K. Prevalence of and factors affecting post-obturation pain in patients undergoing root canal treatment. Int Endod J. 2004;37:381–91. doi: 10.1111/j.1365-2591.2004.00820.x. [DOI] [PubMed] [Google Scholar]
- 52.Polycarpou N, Ng YL, Canavan D, Moles DR, Gulabivala K. Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographic healing. Int Endod J. 2005;38:169–78. doi: 10.1111/j.1365-2591.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 53.Ali SG, Mulay S, Palekar A, Sejpal D, Joshi A, Gufran H. Prevalence of and factors affecting post-obturation pain following single visit root canal treatment in Indian population: A prospective, randomized clinical trial. Contemp Clin Dent. 2012;3:459–63. doi: 10.4103/0976-237X.107440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostrom C, Bair E, Maixner W, Dubner R, Fillingim RB, Ohrbach R, et al. Demographic predictors of pain sensitivity: Results from the OPPERA study. J Pain. 2017;18:295–307. doi: 10.1016/j.jpain.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–45. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 56.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashmi JA, Davis KD. Deconstructing sex differences in pain sensitivity. Pain. 2014;155:10–3. doi: 10.1016/j.pain.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 58.Dionne RA, Cooper SA. Evaluation of preoperative ibuprofen for postoperative pain after removal of third molars. Oral Surg Oral Med Oral Pathol. 1978;45:851–6. doi: 10.1016/s0030-4220(78)80004-8. [DOI] [PubMed] [Google Scholar]
- 59.Arslan H, Topcuoglu HS, Aladag H. Effectiveness of tenoxicam and ibuprofen for pain prevention following endodontic therapy in comparison to placebo: A randomized double-blind clinical trial. J Oral Sci. 2011;53:157–61. doi: 10.2334/josnusd.53.157. [DOI] [PubMed] [Google Scholar]
- 60.Holstein A, Hargreaves K, Niederman R. Evaluation of NSAIDs for treating post-endodontic pain. Endod Top. 2002;3:3–13. [Google Scholar]
- 61.Iwamoto S, Koga T, Ohba M, Okuno T, Koike M, Murakami A, et al. Non-steroidal anti-inflammatory drug delays corneal wound healing by reducing production of 12-hydroxyheptadecatrienoic acid, a ligand for leukotriene B(4) receptor 2. Sci Rep. 2017;7:13267. doi: 10.1038/s41598-017-13122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]