Abstract
The dioxin receptor is a ligand-regulated transcription factor that mediates signal transduction by dioxin and related environmental pollutants. The receptor belongs to the basic helix-loop-helix (bHLH)–Per-Arnt-Sim (PAS) family of factors, which, in addition to the bHLH motif, contain a PAS region of homology. Upon activation, the dioxin receptor dimerizes with the bHLH-PAS factor Arnt, enabling the receptor to recognize xenobiotic response elements in the vicinity of target genes. We have studied the role of the PAS domain in dimerization and DNA binding specificity of the dioxin receptor and Arnt by monitoring the abilities of the individual bHLH domains and different bHLH-PAS fragments to dimerize and bind DNA in vitro and recognize target genes in vivo. The minimal bHLH domain of the dioxin receptor formed homodimeric complexes, heterodimerized with full-length Arnt, and together with Arnt was sufficient for recognition of target DNA in vitro and in vivo. In a similar fashion, only the bHLH domain of Arnt was necessary for DNA binding specificity in the presence of the dioxin receptor bHLH domain. Moreover, the bHLH domain of the dioxin receptor displayed a broad dimerization potential, as manifested by complex formation with, e.g., the unrelated bHLH-Zip transcription factor USF. In contrast, a construct spanning the dioxin receptor bHLH domain and an N-terminal portion of the PAS domain failed to form homodimers and was capable of dimerizing only with Arnt. Thus, the PAS domain is essential to confer dimerization specificity of the dioxin receptor.
Members of the large family of basic helix-loop-helix (bHLH) transcription factors are often involved in regulation of cell type differentiation and proliferation and are characterized by the requirement of formation of homo- or heterodimeric complexes with bHLH partner factors for DNA binding activity (for reviews, see references 13, 28, and 51). In the case of a number of these factors, dimerization specificity has been shown to be determined by residues within the HLH domain itself, whereas structural studies have demonstrated that the basic region binds DNA (18; reviewed in reference 32). In addition, regions outside the bHLH motif, most notably the leucine zipper (Zip) domain in the bHLH-Zip subclass of transcription factors, have been shown to influence dimerization specificity and DNA binding activity of bHLH proteins. Zip domains form amphipathic coiled-coil structures (15, 38) and have the ability to interact with one another or various other dimerization interfaces in a highly specific manner. In the case of the transcription factor TFE3, which binds the symmetrical CACGTG E-box motif recognized by a distinct subclass of bHLH transcription factors (29), mutation of the Zip domain yields a protein that binds the E box with reduced affinity. Moreover, mutation of conserved amino acids in the HLH domain completely inhibits specific DNA binding activity (43).
The c-myc oncoprotein binds DNA poorly by itself and lacks the ability to form homodimers. The bHLH-Zip partner factor max, however, can homodimerize but, in the presence of myc, preferentially forms heterodimeric complexes (1). Heterodimer formation is largely regulated by the Zip domain (11, 41). This domain appears to be a critical determinant of dimerization specificity, since a mutant of max lacking the Zip domain is unable to heterodimerize with c-myc and subsequently to bind DNA. On the other hand, the Zip domain is dispensable for max-max homodimerization and DNA binding by max homodimeric complexes (41).
The dioxin (also termed aryl hydrocarbon) receptor (6, 16), the hypoxia-inducible factor HIF-1α (50) and the closely related endothelial cell-specific factor EPAS 1/HLF (17, 49), their common dimerization partner factor Arnt (25), Sim/NPAS proteins (10, 37, 59), and Clock (2, 30) contain a bHLH motif contiguous with a conserved region, Per-Arnt-Sim (PAS), that is also found in the circadian rhythm regulator Per (references 27, 46, and 48 and references therein). bHLH-PAS proteins thus represent a novel subclass of bHLH transcription factors. The PAS region contains two hydrophobic repeat motifs, A and B, and has been demonstrated to function as a dimerization interface in Per (27), Arnt (31, 42), and the dioxin receptor (20, 31). Per, which lacks a bHLH domain, appears to be a non-DNA-binding protein representing a dominant negative regulator of bHLH-PAS factors. For instance, it has the ability to dimerize with both the dioxin receptor and Arnt and to attenuate ligand-induced transcriptional activity of the dioxin receptor, probably by forming an abortive complex that does not bind target DNA (31).
In the absence of ligand, the dioxin receptor is present in the cytosolic compartment of the cell associated with a dimer of the heat shock protein hsp90 (56). hsp90 appears to be required for maintaining the receptor in a nonactivated, ligand binding conformation (40). Upon addition of ligand, the receptor is imported into the cell nucleus and acquires DNA binding activity following release of hsp90 (56) and dimerization with the bHLH-PAS factor Arnt (25, 52). The ligand-activated dioxin receptor-Arnt complex specifically recognizes an asymmetrical E-box motif present in xenobiotic response elements (XREs) of a battery of dioxin-inducible genes, including the cytochrome P4501A1 and glutathione-S-transferase (GST) Ya genes (reviewed in reference 23). Individually, neither Arnt nor the dioxin receptor binds the asymmetric XRE target sequence motif (52). In contrast, Arnt constitutively recognizes the symmetric CACGTG E-box motif, possibly as a homodimeric complex (3, 45, 47), whereas the ligand-free dioxin receptor-hsp90 complex does not display any detectable DNA binding activity (57). Arnt also dimerizes with HIF-1α and EPAS1. These complexes recognize an asymmetric E-box motif that is distinct from the XRE motif and is found in hypoxia response elements of, e.g., the erythropoietin and vascular endothelial growth factor genes (21, 49, 50). Consistent with the critical importance of Arnt for recognition of the hypoxia response element of the vascular endothelial growth factor gene, a null mutation of the Arnt gene in mice severely impairs angiogenesis, resulting in early embryonal lethality (34).
We have examined in vivo and in vitro dimerization and DNA binding properties of dioxin receptor and Arnt proteins spanning the bHLH domain alone or in combination with PAS regions of various lengths. In the absence of the PAS domain, the bHLH motifs of the dioxin receptor and Arnt were sufficient for XRE recognition. The bHLH domain of the dioxin receptor was able to both homodimerize and form heterodimeric complexes with Arnt. In contrast, the dioxin receptor failed to homodimerize when fused to PAS structures. Although all tested dioxin receptor bHLH-PAS fragments dimerized with Arnt, proteins lacking a critical region of the PAS domain did not show XRE binding activity, indicating conformational regulation of the bHLH domain by PAS structures. Our data further demonstrate that the bHLH domain of the dioxin receptor displays a rather broad dimerization capacity that is narrowly restricted by the adjacent PAS domain. These results illustrate the functional complexity of the PAS domain and indicate that an N-terminal portion of the PAS domain spanning the PAS-A motif is critical for dimerization specificity.
MATERIALS AND METHODS
Plasmid constructs.
pGEX/DR-1-82 was constructed by insertion of PCR fragments spanning amino acids 1 to 82 of the murine dioxin receptor (6, 16) into BamHI- and EcoRI-digested pGEX-4T3 (Pharmacia). pGEX/DR-1-287 was obtained by subcloning a CelII-XhoI fragment from pGEM/DR-1-287 into CelII-XhoI-digested pGEX/DR-1-82. pGEX/DR-1-165 and pGEX/DR-1-188 were constructed by digestion of pGEX/DR-1-287 (36) with XhoI together with EcoRI or NcoI, filling in the ends by use of the Klenow fragment of DNA polymerase I, and religation. Plasmids containing full-length Arnt (pArnt/GEM7 or Arnt pCMV [52]), dioxin receptor (pDR/ATG/BS [35]), USF (pdI2 [22]), and the Arnt bHLH swap mutant pDRbHLH/Arnt (4) have been described previously. pGEX-4T3 Arnt-1-140 was constructed by insertion of a PCR fragment into a BamHI-XhoI-digested pGEX-4T3 vector. pGEX-4T3 Arnt-1-407 and full-length Arnt cDNA were subcloned as NcoI-XhoI fragments into NcoI-XhoI-digested pGEX-4T3 Arnt-1-140 vector, generating pGEX Arnt constructs. Fidelity of PCR was verified by dideoxy sequencing. Mammalian expression vectors were constructed by inserting the different dioxin receptor fragments into pCMX mammalian expression vectors in frame with either the Gal4 DNA binding domain or the VP16 transactivation domain (39).
Bacterial expression of proteins.
The Escherichia coli strain BL-21(DE3)pLysS was transformed with the different dioxin receptor or Arnt pGEX-4T3 bacterial expression vectors or parental pGEX-4T3 (Pharmacia) containing the glutathione binding domain of Schistosoma japonicum GST. Bacteria were grown in the presence of 1 μg of chloramphenicol per ml and 100 μg of ampicillin per ml at 30°C for 12 h in 50 ml of Luria-Bertani medium (44) supplemented with 0.5% glucose. The cultures were then transferred to 250 ml of Terrific broth medium (44) and grown at 37°C for 1 h in the presence of 1 μg of chloramphenicol per ml and 100 μg of ampicillin per ml to an absorbance at 600 nm of 0.7 before induction with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Pharmacia) for 3 h at 37°C. IPTG-induced bacteria were pelleted by centrifugation and washed once in 10 ml of ice-cold Tris-EDTA buffer (44). The pellet was resuspended in 10 ml of Tris-EDTA buffer and incubated with 1% Triton X-100 (Sigma) and 1 mg of lysozyme per ml for 20 min before being snap frozen in liquid nitrogen. After thawing of the cells, DNase I and RNase A were added to final concentrations of 100 and 50 μg/ml, respectively, and incubated for an additional 20 min at room temperature. At this point, the lysate was supplemented with 1 mM phenylmethylsulfonyl fluoride, 0.5 mM pepstatin, and 1 mM β-mercaptoethanol and cleared by centrifugation. Purification of recombinant proteins by affinity chromatography on glutathione-Sepharose was performed according to the suggestions of the manufacturer (Pharmacia). Briefly, the bacterial lysate (300 ml of bacterial culture) was incubated with 2.0 ml of a 50% (vol/vol) slurry of glutathione-Sepharose in phosphate-buffered saline (PBS) buffer at 0 to 4°C for 1 h. The resin was transferred to a column and eluted by gravity flow. Flowthrough material was discarded. The column was washed with 200 ml of PBS buffer containing 1% Triton X-100, and GST fusion proteins were eluted from the resin by incubation with the same buffer containing 10 mM glutathione for 30 min at 0 to 4°C.
GST precipitation assay.
Purified recombinant protein was cleared from free glutathione by elution through a Sephadex PD10 column (Pharmacia). GST precipitation assays were performed by routinely incubating for 30 min at 30°C, in the presence of 1 mM ATP, 1 μg of recombinant protein with a 15-μl aliquot of protein expressed and labeled with [35S]methionine (Amersham) by in vitro translation in rabbit reticulocyte lysate (Promega). Proteins were precipitated by addition of 20 μl of a 50% (vol/vol) slurry of GST-Sepharose in PBS buffer, incubation at 0 to 4°C for 1 h, and subsequent centrifugation. Precipitated material was washed five times in PBS buffer containing 1% Triton X-100, and proteins were eluted in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (44).
Electrophoretic mobility shift assay.
DNA binding activities of bacterially expressed dioxin receptor and Arnt fragments (typically 0.1 μg of protein) were analyzed by a gel mobility shift assay performed essentially as described previously (52). Briefly, DNA binding reactions were assembled in the presence of 2 μg of poly(dI-dC) in 10 mM HEPES (pH 7.9), 5% (vol/vol) glycerol, 0.5 mM dithiothreitol, 2.5 mM MgCl2, 1 mM EDTA, and 80 mM NaCl. The total volume of the DNA binding reaction mixtures ranged between 30 and 50 μl. As radioactive probes 32P-3′-end-labeled, double-stranded oligonucleotides spanning either the wild-type XRE motif from the cytochrome P-4501A1 promoter (9) or the adenovirus major late E-box motif (3) were used. Protein-DNA complexes were resolved on 5% low-ionic-strength native polyacrylamide gels (acrylamide/bisacrylamide ratio of 29:1) at 30 mA at 0 to 4°C with a Tris-glycine-EDTA buffer (24). In some DNA binding experiments, polyclonal antibodies against GST, Arnt (35), or preimmune serum were added to the binding reaction mixtures to assess the identities of protein-DNA complexes.
Transient-transfection assays.
COS-7 cells were cotransfected with Lipofectamine (Life Technologies) in 3-cm-diameter plates with 0.5 μg of the pTXIXI reporter plasmid containing the luciferase gene under the control of tandem copies of the XRE element (21) together with up to 0.2 μg of a pCMV promoter-driven expression vector containing various regions of the dioxin receptor and/or Arnt. Cells were incubated with DNA and Lipofectamine overnight, and then the medium was removed by aspiration and replaced with fresh medium. The cells were allowed to grow for an additional 24 h before harvest, and luciferase activity was assayed as described previously (39).
RESULTS
Bacterial expression of dioxin receptor bHLH and bHLH-PAS fragments.
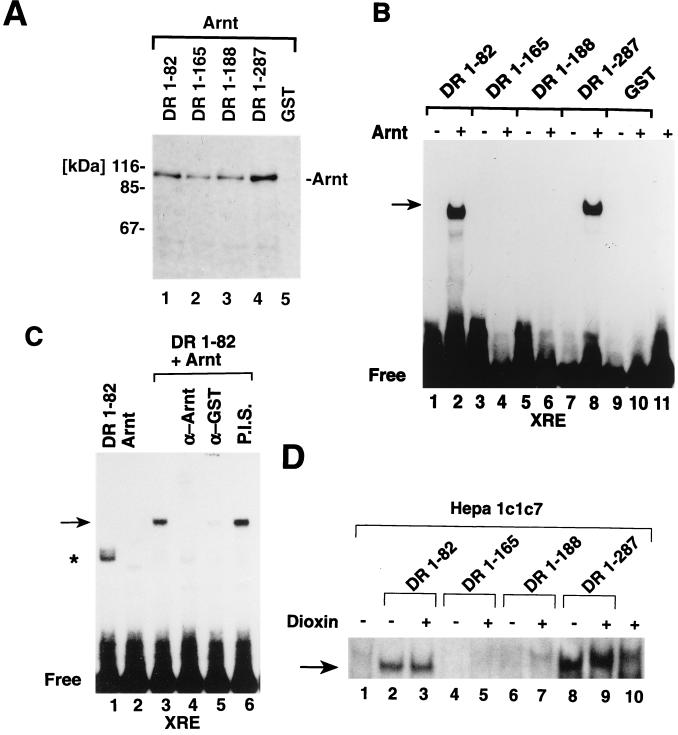
To study structural determinants that regulate dimerization and DNA binding activity of the dioxin receptor, we have expressed in E. coli four different dioxin receptor constructs as GST fusion proteins spanning either the bHLH domain or various bHLH-PAS fragments (schematically represented in Fig. 1A). The expressed proteins were purified to near homogeneity by affinity chromatography on glutathione-Sepharose and then analyzed by SDS-PAGE and silver staining. A typical result of such a purification is shown in Fig. 1B.
FIG. 1.
Bacterial expression and purification of dioxin receptor-GST fusion proteins. (A) Schematic representation of the dioxin receptor fragments that were expressed as GST fusion proteins. The wild-type mouse dioxin receptor (mDR) is presented for comparison. (B) Bacterial fusion proteins were affinity purified on glutathione-Sepharose, and 0.5 μg of protein was analyzed by SDS–10% PAGE and silver staining. Arrows indicate the various purified bHLH-PAS fragments.
Dioxin receptor bHLH-PAS fragments form either non-DNA-binding or DNA-binding complexes with the bHLH-PAS partner factor Arnt.
To test functional properties of the bacterially expressed dioxin receptor fusion proteins, we studied whether they were able to dimerize with Arnt. We performed GST precipitation experiments, taking advantage of the GST moiety of the fusion proteins. Full-length Arnt was expressed by in vitro translation in rabbit reticulocyte lysates in the presence of [35S]methionine. The labeled Arnt protein was then incubated with equal concentrations of the different bacterially expressed dioxin receptor fragments, and the fusion proteins were affinity precipitated with glutathione-Sepharose. Precipitated material was analyzed by SDS-PAGE and fluorography. These experiments demonstrated that the minimal dioxin receptor bHLH domain and the three different bHLH-PAS fragments all interacted with Arnt, resulting in coprecipitation of [35S]methionine-labeled Arnt with the receptor fusion proteins (Fig. 2A, lanes 1 to 4). In control reactions the GST moiety alone did not show any interaction with Arnt (Fig. 2A, lane 5). The minimal dioxin receptor bHLH domain, DR-1-82, and the two smallest dioxin receptor bHLH-PAS fragments, DR-1-165 and DR-1-188, all interacted with Arnt with similar affinities in vitro (Fig. 2A, lanes 1 to 3), whereas the largest dioxin receptor bHLH-PAS fragment, DR-1-287, extending to the N-terminal border of the PAS-B motif (Fig. 1A), showed about a two- to threefold increase in dimerization activity with Arnt (Fig. 2A, lanes 3 and 4). These results are consistent with our previous observations that a region of the dioxin receptor located between amino acids 230 and 421 and spanning the PAS-B motif is a strong dimerization interface in a cellular hybrid interaction assay (31). Thus, in addition to the HLH motif, C-terminal portions of the PAS domain of the dioxin receptor serve as a dimerization interface in vitro and in vivo.
FIG. 2.
Bacterially expressed dioxin receptor bHLH– and bHLH-PAS–GST fusion proteins interact with Arnt. (A) Full-length Arnt was in vitro translated in the presence of [35S]methionine and incubated with 0.1 μg of bacterially expressed receptor proteins, as indicated, or the GST protein as a negative control. The mixtures were precipitated by adsorption to glutathione-Sepharose and centrifugation. Eluted material was separated by SDS-PAGE and visualized by fluorography. (B) bHLH-PAS fragments of the dioxin receptor form non-DNA-binding or DNA-binding complexes with Arnt. The indicated bacterially expressed dioxin receptor fragments (0.1 μg) were incubated in the absence or presence of bacterially expressed Arnt, and XRE binding activity was assayed by gel mobility shift analysis. (C) XRE binding activity of the DR-1-82–Arnt complex was analyzed in the absence or presence of polyclonal antibodies against Arnt (α-Arnt) (lane 4), the GST domain of the recombinant protein (α-GST) (lane 5), or preimmune serum (P.I.S.) (lane 6). (D) Certain dioxin receptor bHLH-PAS fragments function as dominant negative regulators of XRE binding activity. A cytosolic extract from untreated Hepa 1c1c7 cells was treated with 10 nM dioxin (+) or with dimethyl sulfoxide vehicle alone (−) for 2 h at 30°C and incubated with 0.1 μg of purified dioxin receptor fragments for 30 min at 25°C. XRE binding activity was assayed by gel mobility shift analysis. The asterisk indicates nonspecific DNA binding activity occasionally observed with some DR-1-82 preparations. Specific dioxin receptor-Arnt DNA complexes are indicated by arrows.
In contrast to the strong Arnt heterodimerization activity of the various bacterially expressed N-terminal dioxin receptor fragments, only the GST fusion proteins spanning either the bHLH domain or the bHLH domain together with the longest N-terminal PAS fragment, DR-1-82 and DR-1-287, respectively, formed a complex with the XRE target sequence in the presence of bacterially expressed full-length Arnt in a gel mobility shift assay (Fig. 2B, lanes 2 and 8). Individually neither Arnt (52), DR-1-82, nor DR-1-287 recognized the XRE sequence motif. Interestingly, although the two GST fusion proteins spanning the dioxin receptor bHLH–PAS-A fragments DR-1-165 and DR-1-188 dimerized with Arnt (Fig. 2A), these complexes exhibited no detectable XRE binding activity in gel mobility shift experiments (Fig. 2B, lanes 3 to 6). The present experiments demonstrate that these two dioxin receptor bHLH-PAS fragments form abortive, non-DNA-binding complexes with the Arnt partner factor, suggesting that the DNA binding basic motif within the bHLH domain of either the receptor, Arnt, or both proteins is maintained in a conformation that prevents contact with DNA.
Strikingly, the minimal bHLH motif of the dioxin receptor could both dimerize with Arnt (Fig. 2A) and, in the presence of Arnt, bind the XRE sequence motif (Fig. 2B). We therefore examined the specificity of XRE complex formation by using polyclonal antibodies against either the GST moiety of the bacterially expressed GST-dioxin receptor fusion protein or Arnt. In the presence of these antibodies, XRE complex formation was abrogated, whereas addition of preimmune serum to the reaction did not affect the XRE binding activities of DR-1-82 and Arnt (Fig. 2C, lanes 3 to 6). In DNA binding competition experiments, an excess of the unlabeled XRE probe inhibited XRE complex formation by the DR-1-82–Arnt heterodimer. In contrast, an XRE motif, XM1, that contains a single point mutation and fails to bind the wild-type dioxin receptor–Arnt heterodimer (9) did not compete for DNA binding by the DR-1-82–Arnt complex (data not shown). In conclusion, in the absence of any PAS domain structures, the minimal bHLH motif of the dioxin receptor maintained its ability both to dimerize with Arnt and to recognize the asymmetric E-box core within the XRE target sequence. These data demonstrate that the PAS domain per se is not required for XRE recognition.
Since the minimal bHLH domain of the dioxin receptor and all of the bacterially expressed bHLH-PAS fragments maintained their ability to dimerize with Arnt, we next examined the effect of these proteins on ligand inducibility and XRE binding activity of the wild-type dioxin receptor–Arnt complex. To reconstitute ligand regulation of the wild-type receptor in vitro, we prepared a cytosolic extract from nontreated, dioxin-responsive mouse Hepa 1c1c7 hepatoma cells. Incubation of this extract with dioxin in vitro induces the XRE binding activity of the dioxin receptor-Arnt complex (9) (Fig. 2D, lanes 1 and 10). Addition of the minimal dioxin receptor bHLH fragment DR-1-82 to the extract generates a constitutive XRE complex that is not affected by addition of ligand (Fig. 2D, lanes 2 and 3). In a similar fashion, the largest bacterially expressed bHLH–PAS-A fragment, DR-1-287, produced a constitutive XRE complex (Fig. 2D, lanes 8 and 9). We interpret these results to indicate that these two proteins saturated the pool of Arnt available for dimerization with the dioxin receptor so that ligand-dependent activation of the wild-type dioxin receptor did not result in any net increase in XRE binding activity. In contrast, the two dioxin receptor bHLH–PAS-A fragments DR-1-165 and DR-1-188 efficiently inhibited dioxin-dependent induction of XRE binding activity by the wild-type dioxin receptor–Arnt complex (Fig. 2D, lanes 4 to 7), strongly supporting the notion that they can form abortive, non-DNA-binding complexes with Arnt.
The PAS domain is not required for XRE recognition by the dioxin receptor in vivo.
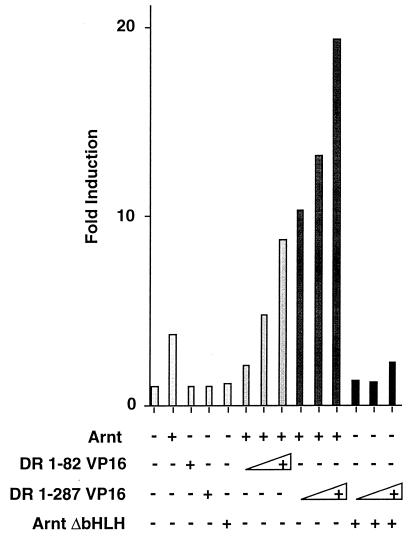
To examine the role of the PAS domain of the dioxin receptor in XRE recognition in vivo, we fused the bHLH domain of the receptor (amino acids 1 to 82) or a bHLH–PAS-A fragment (amino acids 1 to 287) to the potent transactivation domain of the herpes simplex virus protein VP16. In excellent agreement with the in vitro DNA binding experiments shown in Fig. 2B, the DR-1-82– and DR-1-287–VP16 fusion proteins did not show any activity on an XRE-dependent reporter gene in the absence of Arnt upon transient transfection of COS-7 cells. In the presence of full-length Arnt, however, both the DR-1-82– and DR-1-287–VP16 fusion proteins potently induced reporter gene activity (Fig. 3), further demonstrating that the PAS domain of the dioxin receptor is not required for recognition of the XRE motif. In control experiments, the dioxin receptor fusion proteins were coexpressed with a dominant negative Arnt mutant, ArntΔbHLH, that lacks the bHLH DNA binding region and generates non-DNA-binding complexes with the dioxin receptor (31). Under these conditions, the dioxin receptor-VP16 fusion proteins failed to induce reporter gene activity (Fig. 3), demonstrating that they strictly required Arnt for XRE recognition.
FIG. 3.
The bHLH domain of the dioxin receptor is sufficient for XRE recognition. COS-7 cells were cotransfected with 0.5 μg of the XRE-driven pTXIXI luciferase reporter gene, 0.2 μg of pCMV-Arnt, and increasing amounts (10 to 100 ng) of pCMX-VP16 expression vectors containing either the DR-1-82 or the DR-1-287 fragment fused to the VP16 transactivation domain and were assayed for luciferase activity. Results of a representative experiment are shown.
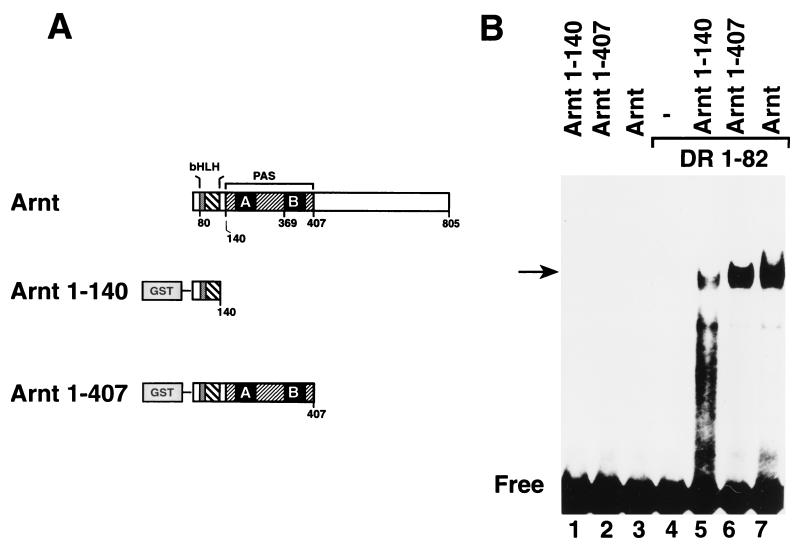
Given that the PAS domain was not necessary for XRE recognition in vitro and in vivo, we also tested the XRE binding activities of bacterially expressed Arnt fragments containing or lacking the PAS domain (Fig. 4A). Consistent with our data on the corresponding dioxin receptor proteins, these Arnt fragments and full-length Arnt did not recognize the XRE motif in the absence of the dioxin receptor bHLH domain (Fig. 4B, lanes 1 to 3) or in transient-transfection experiments in vivo (data not shown). In the presence of the bHLH domain of the dioxin receptor, however, Arnt bHLH-PAS proteins as well as Arnt-1-140, spanning only the bHLH motif, generated distinct complexes with the XRE target sequence (Fig. 4B, lanes 4 to 7). Thus, we conclude that the PAS domain is not necessary for XRE recognition by either the dioxin receptor or Arnt.
FIG. 4.
The bHLH domain of Arnt is sufficient to reconstitute XRE binding activity of the bHLH-PAS dioxin receptor. (A) Schematic representation of the different Arnt fragments expressed in E. coli. (B) Bacterially expressed dioxin receptor fragment DR-1-82 and Arnt-1-140 or Arnt-1-407 were coincubated for 30 min at 25°C. XRE binding activity was analyzed by gel mobility shift assay as described in the legend to Fig. 2B.
The PAS domain determines dimerization specificity of the dioxin receptor.
Since dimerization is a key event in regulation of bHLH transcription factor function (reviewed in references 28 and 29), we next investigated the dimerization specificity of the dioxin receptor and the role of the PAS domain in this process. Initially we examined whether the various bacterially expressed dioxin receptor bHLH and bHLH-PAS proteins could homodimerize with the full-length dioxin receptor. In these experiments full-length dioxin receptor was expressed and labeled with [35S]methionine by in vitro translation in reticulocyte lysate and finally incubated with dioxin. The ligand-activated, [35S]methionine-labeled receptor was affinity-precipitated with glutathione-Sepharose upon addition of the DR-1-82 fusion protein spanning only the bHLH domain of the dioxin receptor. In contrast, the three different GST–bHLH-PAS proteins and the GST moiety alone failed to interact with the ligand-activated dioxin receptor (Fig. 5A). Interestingly, interaction between DR-1-82 and in vitro-translated full-length dioxin receptor was not regulated by ligand treatment, since DR-1-82 showed very similar levels of precipitation of [35S]methionine-labeled dioxin receptor both in the absence and presence of dioxin (data not shown). Thus, the bHLH domain of the full-length receptor appeared to be constitutively available for homodimerization with DR-1-82. In summary, our results demonstrate that the bHLH domain of the dioxin receptor had the intrinsic ability to homodimerize. However, fusion of the bHLH domain to PAS domain structures dramatically restricted the ability of the bHLH domain of the dioxin receptor to form homodimeric complexes.
FIG. 5.
Homodimerization and XRE binding activities of the bHLH domain of the dioxin receptor. (A) In vitro-translated full-length dioxin receptor was labeled with [35S]methionine incubated with 0.1 μg of the indicated dioxin receptor fusion proteins, precipitated with glutathione-Sepharose, and analyzed by SDS-PAGE and fluorography. (B) Homodimerization activity of the minimal DR-1-82 receptor fragment was monitored in vivo by using a mammalian two-hybrid assay. COS-7 cells were cotransfected with 0.2 μg of pCMX DR-1-82–Gal4 containing the dioxin receptor bHLH domain fused to the Gal4 DNA binding domain and up to 100 ng of a DR-1-82–VP16 fusion construct together with 0.5 μg of a Gal4 luciferase reporter construct. The cells were assayed for luciferase activity, and results of a representative experiment are shown.
We next used a two-hybrid interaction assay to examine the ability of the bHLH domain of the dioxin receptor to homodimerize in vivo. In this assay, the bHLH domain of the dioxin receptor was fused to the heterologous Gal4 DNA binding domain and monitored in transient-transfection experiments with COS-7 cells for its ability to activate a Gal4-dependent luciferase reporter gene. In the presence of increasing concentrations of the DR-1-82–VP16 fusion protein, reporter gene activity was significantly induced (Fig. 5B), demonstrating homodimeric interaction between these two proteins.
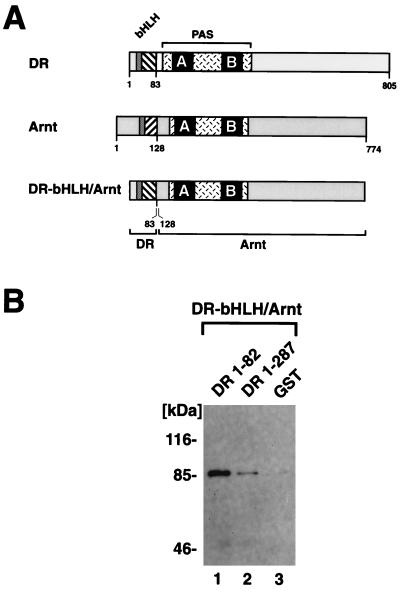
The observation that the minimal bHLH domain of the dioxin receptor mediated homodimerization prompted us to perform coprecipitation experiments with DR-1-82 and a swap mutant of Arnt, DRbHLH-Arnt (4), in which the bHLH domain of Arnt has been replaced by that of the dioxin receptor (schematically represented in Fig. 6A). In vitro-translated, [35S]methionine-labeled DRbHLH-Arnt was precipitated by the DR-1-82 fusion protein in GST precipitation assays, whereas in the presence of only the GST moiety, negligible levels of background precipitation of the labeled swap mutant were detected (Fig. 6B, lanes 1 and 3). At an identical concentration of the DR-1-287 fusion protein, however, low but significant levels of the labeled DRbHLH-Arnt protein were coprecipitated. These results support our earlier conclusion that DR-1-82 can homodimerize with the bHLH motif of the dioxin receptor and that this homodimerization activity is significantly reduced by the juxtaposed PAS-A motif. Using a hybrid protein interaction assay in mammalian host cells, we have previously observed that the PAS domains of the dioxin receptor and Arnt serve as dimerization interfaces in addition to the bHLH domains. In this assay the PAS domains could mediate hetero- but not homodimerization processes (31). In agreement with this model, we could detect low but significant levels of association of DR-1-287 with the DRbHLH-Arnt swap mutant, possibly reflecting heterodimerization between the PAS domains of Arnt and DR-1-287. In contrast, DR-1-287 did not interact with the dioxin-activated full-length dioxin receptor (Fig. 5A), showing neither bHLH- nor PAS domain-mediated homodimerization activity.
FIG. 6.
Constitutive dimerization of dioxin receptor bHLH and bHLH–PAS-A proteins with a dioxin receptor bHLH swap mutant. (A) Structural organizations of the dioxin receptor (DR), Arnt, and a bHLH swap mutant (DRbHLH/Arnt) in which the bHLH domain of Arnt has been replaced by that of the dioxin receptor. (B) DRbHLH/Arnt was expressed and labeled with [35S]methionine by in vitro translation in reticulocyte lysates and incubated with either DR-1-82, DR-1-287, or the purified GST domain, as indicated, prior to precipitation with glutathione-Sepharose. Precipitated material was analyzed by SDS-PAGE and fluorography.
The PAS-A domain restricts dimerization activity of the bHLH domain of the dioxin receptor.
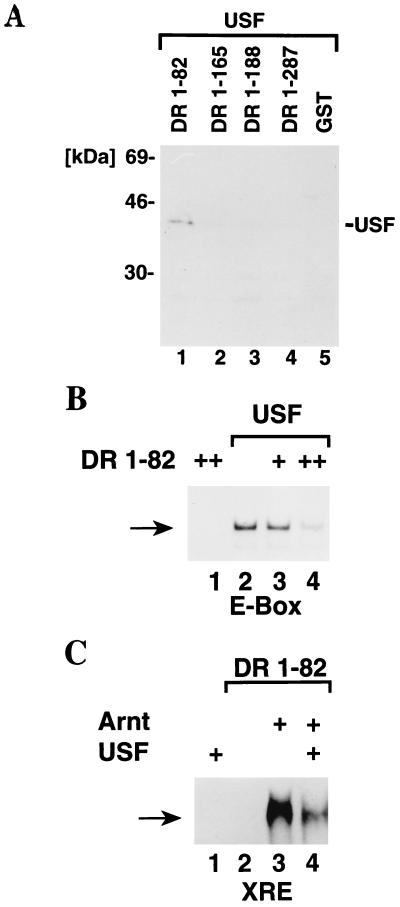
We next examined whether the bacterially expressed bHLH and bHLH–PAS-A fragments of the dioxin receptor could heterodimerize with an unrelated bHLH factor, i.e., the ubiquitous and constitutively active bHLH-Zip transcription factor USF that binds to the dyad symmetry E-box motif CACGTG (22). Ligand-activated, in vitro-translated full-length dioxin receptor did not dimerize with in vitro-translated full-length USF (data not shown), and in GST precipitation experiments, bacterially expressed DR-1-287 did not show any interaction with in vitro-translated, [35S]methionine-labeled USF. However, in these experiments the labeled USF protein was coprecipitated by DR-1-82 protein (Fig. 7A, lane 1). In conclusion, the dioxin receptor bHLH domain not only dimerized with Arnt but showed both homodimerization activity and interaction with the unrelated bHLH-Zip factor USF. The latter two dimerization activities were not observed with any of the dioxin receptor bHLH-PAS proteins, strongly suggesting that the PAS-A motif restricts the broad dimerization specificity of the bHLH domain of the dioxin receptor. Thus, identical concentrations of DR-1-165, DR-1-188, and DR-1-287 failed to show any association with USF (Fig. 7A, lanes 2 to 4). The N-terminal portion of the PAS-A domain extending to amino acid 165 was sufficient to inhibit the dimerization activity between the dioxin receptor bHLH motif and USF.
FIG. 7.
The PAS domain restricts a broad dimerization activity of the minimal bHLH domain of the dioxin receptor. (A) USF dimerizes with the dioxin receptor bHLH domain. Wild-type USF was in vitro translated in the presence of [35S]methionine and incubated with the bacterially expressed dioxin receptor proteins DR-1-82, DR-1-165, DR 1-188, and DR 1-287. GST precipitation experiments were performed and analyzed as described in the legend to Fig. 2A. (B and C) Alteration in DNA specificity upon formation of USF–DR-1-82 heterodimeric complexes. Purified, bacterially expressed His10-tagged USF was incubated with DR-1-82 in the absence (B) or presence (C) of Arnt and analyzed for DNA binding activity in gel mobility shift experiments using either a 32P-labeled E-box probe from the adenovirus major late promoter (B) or an XRE probe (C) as described in the legend to Fig. 2B.
The ability of the minimal dioxin receptor DNA binding fragment DR-1-82 to dimerize with USF prompted us to examine the DNA binding activity of the generated heterodimeric complexes. DR-1-82 did not recognize the CACGTG E-box motif of the adenovirus major late promoter in gel mobility shift experiments (Fig. 7B, lane 1). Moreover, in the presence of increasing concentrations of DR-1-82, the ability of USF to bind to this E-box motif was inhibited in a dose-dependent manner (Fig. 7B, lanes 2 to 4), indicating an altered DNA binding specificity upon formation of USF–DR-1-82 heterodimeric complexes. In a similar fashion, addition of USF to DNA binding reactions containing DR-1-82 and Arnt impaired binding of the DR-1-82–Arnt complex to the asymmetric E-box motif contained within the XRE sequence (Fig. 7C, lanes 3 and 4). Thus, USF could not substitute for Arnt in XRE recognition but showed an altered DNA binding specificity following dimerization with DR-1-82.
DISCUSSION
Regulation of XRE binding activity by the dioxin receptor-Arnt proteins.
The complexity of gene regulation is well illustrated by the fact that many transcription factors share similar dimerization and DNA binding motifs and bind to common or related target DNA sequence motifs. Although a principal determinant of gene regulation is the sequence specificity of DNA binding, dimerization specificity also plays a critical role in regulation of functional activities of, among others, bHLH factors (28, 29, 32).
In the present study, we have examined the role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor. We have previously identified the PAS domain of the dioxin receptor as a dimerization interface that can function independently of the bHLH domain, as demonstrated by a hybrid-protein interaction assay in mammalian cells (31). These functional properties of the PAS domain have also been demonstrated in studies on the circadian rhythm regulator Per (27). What is the role of the PAS domain when juxtaposed to a bHLH motif? The PAS domain of the dioxin receptor is different from that of, for instance, Arnt in that it harbors the ligand binding domain of the dioxin receptor. We have found that the minimal ligand binding domain of the mouse dioxin receptor is located between amino acids 230 and 421 (8, 53), a segment that includes within its borders the PAS-B motif (schematically shown in Fig. 1A). This domain, in turn, harbors a region stretching from amino acid 230 to 337 that was UV cross-linked with a radiolabeled photoaffinity ligand for the dioxin receptor (12).
The complex architecture of the PAS domain is further enhanced by the fact that the minimal ligand binding domain of the dioxin receptor coincides with a region that binds the molecular chaperone hsp90 (53). In vitro studies have indicated multiple roles of hsp90 in modulation of dioxin receptor function: (i) repression of dioxin receptor function, possibly by steric interference with Arnt dimerization (52) or with the interaction between the C-terminal transactivation domains and putative cofactors (54), and (ii) chaperoning of a ligand binding conformation of the ligand binding domain (4, 8, 40). The latter model is based on the observation that artificial disruption of the hsp90-dioxin receptor complex yields a dioxin receptor form that is not capable of binding ligand (40). In a similar fashion, the receptor fails to bind ligand upon expression in either wheat germ lysate or E. coli, which contain hsp90 homologs that do not associate with the ligand binding domain of the receptor (4, 8). In agreement with these observations, ligand responsiveness of the dioxin receptor is abrogated in a mutant strain showing low levels of hsp90 expression (7, 55).
In the present experiments we have expressed in E. coli GST fusion proteins spanning either the bHLH domain of the dioxin receptor (DR-1-82) or bHLH–PAS-A structures extending up to amino acid 287. In the presence of Arnt, the isolated dioxin receptor bHLH domain could specifically bind the XRE motif in vitro. Moreover, it not only dimerized with Arnt but also showed homodimerization activity and formed complexes with the bHLH-Zip factor USF. In contrast, all tested dioxin receptor bHLH–PAS-A fragments showed a strict dimerization specificity for Arnt and failed to homodimerize or interact with USF. In transient-transfection experiments the minimal bHLH domain of the dioxin receptor activated an XRE-driven luciferase reporter gene in the presence of Arnt, demonstrating the ability of this dioxin receptor domain to both dimerize in vivo with the Arnt partner protein and activate transcription. Thus, the bHLH motif of the dioxin receptor did not require the PAS domain for XRE recognition. Importantly, these results also clearly demonstrate that the PAS domain efficiently restricted the dimerization potential of the bHLH domain of the dioxin receptor determining very strict specificity for Arnt. In a less strict fashion, the Zip motif modulates dimerization specificities of a number of bHLH-Zip factors, including TFE-3, AP-4, c-myc, and max (5, 26).
In analogy to the case for the full-length dioxin receptor (52), both the bHLH domain and the bHLH–PAS-A fragment DR-1-287 required Arnt to recognize the XRE motif. Dioxin receptor bHLH–PAS-A fragments that were shorter than the DR-1-287 fragment dimerized with Arnt at levels that were simeilar to those produced by the bHLH domain. Strikingly, however, they produced non-DNA-binding complexes with Arnt, suggesting that the PAS-A structure may confer a conformational change on the bHLH domain that renders it unable to interact with DNA. It will now be interesting to investigate whether the DNA binding b domain or even larger portions of the receptor proteins are folded differently when fused to PAS structures of various lengths. To understand this issue, it will be critical to elucidate the three-dimensional structures of relevant bHLH-PAS fragments and to compare them to known structures of bHLH or bHLH-Zip domains (14, 18, 19, 33).
Our present experiments demonstrate that the PAS-A domain has an important function in determining the dimerization specificity of the dioxin receptor. The two Drosophila bHLH-PAS factors Trachealess (Trh) and Single-minded (Sim) have recently also been shown to dimerize with dArnt. In the case of these two Drosophila factors, the bHLH domains are highly similar, indicating similar if not identical DNA binding specificities. Yet these two proteins induce diverse cell fates during Drosophila development, suggesting that they activate nonoverlapping target genes (58). Recent genetic experiments have demonstrated that an exchange of the PAS domains between these two factors results in a corresponding exchange of developmental activity without apparently altering the ability of the resulting chimeric proteins to functionally interact with Arnt (58). It has therefore been proposed that the PAS domain determines recruitment of specific cofactors or coactivators to either Trh or Sim, determining the developmental specificities of these factors. These data are consistent with the ability of the PAS domain to serve as a potent protein-protein interaction interface (27, 31). In addition, our experiments demonstrate a novel function of the PAS domain: regulation of the dimerization activity of the bHLH domain. It will now be interesting to examine if the PAS domain shows a similar mode of regulation of the bHLH domain in other members of this rapidly growing family of biologically important proteins and to elucidate the structure of the PAS domain to understand its function.
ACKNOWLEDGMENTS
We thank Jacqueline McGuire for pGEM/DR-1-287 and stimulating discussions; Katarina Pettersson for the pCMX Gal4 and VP16 expression vectors, the Gal4 luciferase reporter, and valuable advice; and Hank Barnes for valuable advice regarding preparation of recombinant proteins.
This work was supported by grants from the Swedish Society for Medical Research and the Swedish Cancer Society.
REFERENCES
- 1.Amati B, Dalton S, Brooks M W, Littlewood T D, Evan G I, Land H. Transcriptional activation by the human c-Myc oncoprotein in yeast requires interaction with Max. Nature. 1992;359:423–426. doi: 10.1038/359423a0. [DOI] [PubMed] [Google Scholar]
- 2.Antoch M P, Song E J, Chang A M, Vitaterna M H, Zhao Y, Wilsbacher L D, Sangoram A M, King D P, Pinto L H, Takahashi J S. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonsson C, Arulampalam V, Whitelaw M L, Pettersson S, Poellinger L. Constitutive function of the basic helix-loop-helix PAS factor Arnt—regulation of target promoters via the E-box motif. J Biol Chem. 1995;270:13968–13972. doi: 10.1074/jbc.270.23.13968. [DOI] [PubMed] [Google Scholar]
- 4.Antonsson C, Whitelaw M L, McGuire J, Gustafsson J-Å, Poellinger L. Distinct roles of the molecular chaperone hsp90 in modulating dioxin receptor function via the basic helix-loop-helix and PAS domains. Mol Cell Biol. 1995;15:756–765. doi: 10.1128/mcb.15.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckmann H, Kadesch T. The leucine zipper of TFE3 dictates helix-loop-helix dimerization specificity. Genes Dev. 1991;5:1057–1066. doi: 10.1101/gad.5.6.1057. [DOI] [PubMed] [Google Scholar]
- 6.Burbach K M, Poland A, Bradfield C A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carver L A, Jackiw V, Bradfield C A. The 90-kda heat shock protein is essential for Ah receptor signaling in a yeast expression system. J Biol Chem. 1994;269:30109–30112. [PubMed] [Google Scholar]
- 8.Coumailleau P, Poellinger L, Gustafsson J-Å, Whitelaw M L. Definition of a minimal domain of the dioxin receptor that is associated with hsp90 and maintains wild type ligand binding affinity and specificity. J Biol Chem. 1995;270:25291–25300. doi: 10.1074/jbc.270.42.25291. [DOI] [PubMed] [Google Scholar]
- 9.Cuthill S, Wilhelmsson A, Poellinger L. Role of the ligand in intracellular receptor function: receptor affinity determines activation in vitro of the latent dioxin receptor to a DNA-binding form. Mol Cell Biol. 1991;11:401–411. doi: 10.1128/mcb.11.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahmane N, Charron G, Lopes C, Yaspo M-L, Manoury C, Decorte L, Sinet P-M, Bloch M, Delabar J-M. Down syndrome critical region contains a gene homologous to drosophila sim expressed during rat and human central nervous system development. Proc Natl Acad Sci USA. 1995;92:9191–9195. doi: 10.1073/pnas.92.20.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis L J, Halazonetis T D. Both the helix-loop-helix and the leucine zipper motifs of c-Myc contribute to its dimerization specificity with Max. Oncogene. 1993;8:125–132. [PubMed] [Google Scholar]
- 12.Dolwick K M, Swanson H I, Bradfield C A. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci USA. 1993;90:8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorshkind K. Transcriptional control points during lymphopoiesis. Cell. 1994;79:751–753. doi: 10.1016/0092-8674(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 14.Ellenberger T, Fass D, Arnaud M, Harrison S C. Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev. 1994;8:970–980. doi: 10.1101/gad.8.8.970. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberger T E, Brandl C J, Struhl K, Harrison S C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein-DNA complex. Cell. 1992;71:1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- 16.Ema M, Sogawa K, Watanabe N, Chujoh Y, Matsushita N, Gotoh O, Funae Y, Fujii K Y. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992;184:246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- 17.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferré-D’Amare A, Prendergast G C, Ziff E B, Burley S K. Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature. 1993;363:38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- 19.Ferré-D’Amare A R, Pognonec P, Roeder R G, Burley S K. Structure and function of the B/HLH/Z domain of USF. EMBO J. 1994;13:180–189. doi: 10.1002/j.1460-2075.1994.tb06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukunaga B N, Probst M R, Reiszporszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995;270:29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 21.Gradin K, McGuire J, Wenger R H, Kvietikova I, Whitelaw M L, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregor P D, Sawadogo M, Roeder R G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990;4:1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 24.Hapgood J, Cuthill S, Denis M, Poellinger L, Gustafsson J-Å. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci USA. 1989;86:60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman E C, Reyes H, Chu F F, Sander F, Conley L H, Brooks B A, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y F, Luscher B, Admon A, Mermod N, Tjian R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev. 1990;4:1741–1752. doi: 10.1101/gad.4.10.1741. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z J, Edery I, Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993;364:259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- 28.Jan Y N, Jan L Y. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993;75:827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- 29.Kadesch T. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ. 1993;4:49–55. [PubMed] [Google Scholar]
- 30.King D P, Zhao Y, Sangoram A M, Wilsbacher L D, Tanaka M, Antoch M P, Steeves T D, Vitaterna M H, Kornhauser J M, Lowrey P L, Turek F W, Takahashi J S. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindebro M C, Poellinger L, Whitelaw M L. Protein-protein interaction via Pas domains. Role of the Pas domain in positive and negative regulation of the bHLH/Pas dioxin receptor-Arnt transcription factor complex. EMBO J. 1995;14:3528–3539. doi: 10.1002/j.1460-2075.1995.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littlewood T D, Evan G I. Transcription factors 2: helix-loop-helix. Protein Profile. 1995;2:621–702. [PubMed] [Google Scholar]
- 33.Ma P C, Rould M A, Weintraub H, Pabo C O. Crystal structure of MyoD bHLH domain-DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell. 1994;77:451–459. doi: 10.1016/0092-8674(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 34.Maltepe E, Schmidt J V, Baunoch D, Bradfield C A, Simon M C. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 35.Mason G G F, Witte A M, Whitelaw M L, Antonsson C, Mcguire J, Wilhelmsson A, Poellinger L, Gustafsson J-Å. Purification of the DNA binding form of dioxin receptor—role of the Arnt cofactor in regulation of dioxin receptor function. J Biol Chem. 1994;269:4438–4449. [PubMed] [Google Scholar]
- 36.McGuire, J., and L. Poellinger. 1997. Unpublished data.
- 37.Nambu J R, Lewis J O, Wharton K A, Jr, Crews S T. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991;67:1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- 38.O’Shea E K, Klemm J D, Kim P S, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 39.Pettersson K, Grandien K, Kuiper G G, Gustafsson J-Å. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 40.Pongratz I, Mason G F, Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. J Biol Chem. 1992;267:13728–13734. [PubMed] [Google Scholar]
- 41.Reddy C D, Dasgupta P, Saikumar P, Dudek H, Rauscher F J D, Reddy E P. Mutational analysis of Max: role of basic, helix-loop-helix/leucine zipper domains in DNA binding, dimerization and regulation of Myc-mediated transcriptional activation. Oncogene. 1992;7:2085–2092. [PubMed] [Google Scholar]
- 42.Reisz-Porszasz S, Probst M R, Fukunaga B N, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor nuclear translocator protein (ARNT) Mol Cell Biol. 1994;14:6075–6086. doi: 10.1128/mcb.14.9.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roman C, Matera A G, Cooper C, Artandi S, Blain S, Ward D C, Calame K. mTFE3, an X-linked transcriptional activator containing basic helix-loop-helix and zipper domains, utilizes the zipper to stabilize both DNA binding and multimerization. Mol Cell Biol. 1992;12:817–827. doi: 10.1128/mcb.12.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbour Laboratory; 1989. [Google Scholar]
- 45.Sogawa K, Nakano R, Kobayashi A, Kikuchi Y, Ohe N, Matsushita N, Fujiikuriyama Y. Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation. Proc Natl Acad Sci USA. 1995;92:1936–1940. doi: 10.1073/pnas.92.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Z-S, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee C-C. RIGUI, a putative mammalian ortholog of the drosophila period gene. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- 47.Swanson H I, Chan W K, Bradfield C A. DNA binding specificities and pairing rules of the Ah Receptor, Arnt, and Sim proteins. J Biol Chem. 1995;270:26292–26302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- 48.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 49.Tian H, McKnight S L, Russell D W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 50.Wang G L, Jiang B H, Rue E A, Semenza G L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular O-2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- 52.Whitelaw M, Pongratz I, Wilhelmsson A, Gustafsson J-Å, Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993;13:2504–2514. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitelaw M L, Gottlicher M, Gustafsson J-Å, Poellinger L. Definition of a novel ligand binding domain of a nuclear bHLH receptor—co-localization of ligand and hsp90 binding activities within the regulable inactivation domain of the dioxin receptor. EMBO J. 1993;12:4169–4179. doi: 10.1002/j.1460-2075.1993.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitelaw M L, Gustafsson J-Å, Poellinger L. Identification of transactivation and repression functions of the dioxin receptor and its basic helix-loop-helix/PAS partner Factor Arnt: inducible versus constitutive modes of regulation. Mol Cell Biol. 1994;14:8343–8355. doi: 10.1128/mcb.14.12.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitelaw M L, McGuire J, Picard D, Gustafsson J-Å, Poellinger L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc Natl Acad Sci USA. 1995;92:4437–4441. doi: 10.1073/pnas.92.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilhelmsson A, Cuthill S, Denis M, Wikstrom A C, Gustafsson J-Å, Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990;9:69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilhelmsson A, Wikström A C, Poellinger L. Polyanionic-binding properties of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. A comparison with the glucocorticoid receptor. J Biol Chem. 1986;261:13456–13463. [PubMed] [Google Scholar]
- 58.Zelzer E, Wappner P, Shilo B-Z. The PAS domain confers target gene specificity of Drosophila bHLH/PAS proteins. Genes Dev. 1997;11:2079–2089. doi: 10.1101/gad.11.16.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y D, Barnard M, Tian H, Ring H Z, Francke U, Shelton J, Richarson J, Russell D W, McKnight S L. Molecular characterisation of two mammalian bHLH-PAS domain proteins selectively expressed in the central nervous system. Proc Natl Acad Sci USA. 1997;94:713–718. doi: 10.1073/pnas.94.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]