Abstract
Background
Dietary factors have consistently been associated with breast cancer risk. However, there is limited evidence regarding their associations in women with different genetic susceptibility to breast cancer, and their interaction with alcohol consumption is also not well understood.
Methods
We analyzed data from 261,853 female participants in the UK Biobank. Multivariable adjusted Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for associations between dietary factors and breast cancer risk. Additionally, we assessed the interaction of dietary factors with alcohol consumption and polygenic risk score (PRS) for breast cancer.
Results
A moderately higher risk of breast cancer was associated with the consumption of processed meat (HR = 1.10, 95% CI 1.03, 1.18, p-trend = 0.016). Higher intake of raw vegetables and fresh fruits, and adherence to a healthy dietary pattern were inversely associated with breast cancer risk [HR (95% CI):0.93 (0.88–0.99), 0.87 (0.81, 0.93) and 0.93 (0.86–1.00), p for trend: 0.025, < 0.001, and 0.041, respectively]. Furthermore, a borderline significant interaction was found between alcohol consumption and the intake of processed meat with regard to breast cancer risk (P for interaction = 0.065). No multiplicative interaction was observed between dietary factors and PRS.
Conclusion
Processed meat was positively associated with breast cancer risk, and vegetables, fruits, and healthy dietary patterns were negatively associated with breast cancer risk. We found no strong interaction of dietary factors with alcohol consumption and genetic predisposition for risk of breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-023-03269-8.
Keywords: Dietary factors, Alcohol, Polygenic risk score, Breast cancer
Introduction
Breast cancer is the most prevalent cancer in women worldwide and the second most common cause of cancer mortality [1, 2]. With the growing incidence of breast cancer around the world [3], it is crucial to identify lifestyle risk factors that may help reduce the risk of breast cancer, including physical activity, breastfeeding, and consumption of healthy food.
Population-based studies have reported several dietary patterns that are associated with the risk of breast cancer [4], including Healthy/Mediterranean patterns or based on dietary guidelines (e.g., the Healthy Eating Score) [5], while specific foods such as oily fish, fruits, and vegetables may help reduce the risk of developing breast cancer [6]. However, previous evidence regarding the associations between different types of meat consumption and breast cancer risk has been inconclusive [7, 8], which could be attributed to differences in study design or the use of specific subgroups of women. Moreover, despite the known association between alcohol intake and breast cancer [9, 10], no study to date has assessed their synergistic effect with food on the risk of breast cancer, which could result in targeted interventions for women and increase the cost-effectiveness of interventions.
Besides the interaction with alcohol intake, the association between dietary factors and breast cancer could also be influenced by genetic factors. Recent GWAS studies have revealed 313 SNPs associated with breast cancer risk that could be used as a tool for risk stratification [11]. However, it is still unclear whether the associations with dietary factors differ according to different genetic predispositions to breast cancer, which is important for personalized prevention.
The aim of this study was to evaluate the association between dietary factors and the risk of breast cancer and to investigate whether the association was influenced by alcohol consumption, particularly when taken with meals. We also examined the association between dietary factors and breast cancer risk taking into account genetic susceptibility measured by polygenic risk score.
Methods
Study population, exposure, and outcome
Between 2006 and 2010, a total of 503,317 participants agreed to participate in the baseline assessment of the UK Biobank study, of which 273,382 were women [12]. Participants were followed up from the date of enrollment until the date of diagnosis of breast cancer, withdrawal from the study, death, loss of follow-up, or the end of follow-up (December 31, 2019), whichever occurred first. Information on breast cancer diagnosis was obtained by linking the cohort to the National Health Service (NHS) Digital for England and Wales and NHS Scotland, using unique personal identification numbers. The ICD-10 code C50 and ICD-9 code 174 were used to identify breast cancer diagnoses in the cancer register. Women with breast cancer before participating in the UK Biobank were excluded from the analysis. The date of death was retrieved from death certificates held by the NHS Information Center and the NHS Central Register. The study was approved by The National Information Governance Board for Health and Social Care and the NHS North West Multicentre Research Ethics Committee (06/MRE08/65). The participants gave informed consent at the baseline and agreed to be followed up via data linkage.
Diet group classification
Dietary intake data were collected at recruitment using a self-reported touchscreen questionnaire (http://biobank.ctsu.ox.ac.uk/showcase/showcase/docs/Touchscreen QuestionsMainFinal.pdf). The frequency of processed meat, beef, lamb, pork, poultry, fish (oily/non-oily), and cheese consumption was coded into three categories: never, less than once a week, and more than once a week. In addition, for vegetables (cooked/raw) and fruit (fresh/dried), consumption was coded into four categories (< 2 servings/d, 2.0–2.9 servings/d, 3.0–3.9 servings/d, ≥ 4 servings/d) as suggested by previous studies [13]. Participants who had missing information or responded with "prefer not to answer" or "do not know" were categorized as "missing". A healthy diet was defined according to the Healthy Eating Score (HDS), which was calculated based on: consuming at least four tablespoons of vegetables each day; consuming at least three pieces of fruit each day; consuming fish at least twice each week; consuming red meat (beef, lamb, and pork) no more than twice each week; consuming processed meat no more than twice each week. One point was awarded for each advantageous dietary factor, with the total diet score ranging from 0 to 5 [14]. Participants were grouped into poor dietary patterns (score 0 or 1), medium dietary patterns (score 2 or 3), and ideal dietary patterns (score 4 or 5) [15].
The frequency of alcohol consumption was collected through a questionnaire, and we dichotomized it into whether or not had daily alcohol drinking. Participants reported different types of alcohol, including beer/cider, white wine/champagne, fortified wine, red wine, spirits, and “other”. According to official UKB statistics, a pint or can of beer/lager/cider = 2 units, a 25 ml single shot of spirits = 1 unit, and a standard glass of wine (175 ml) = 2 units were used to estimate alcohol content. When the alcohol consumption was reported monthly, we divided the intake by 4.3. Then, the weekly intake divided by 7 is the daily unit of consumption. The World Health Organization recommends no more than two "standard intakes of alcohol" per day, and alcohol consumption was therefore dichotomized accordingly to investigate their potential interaction with dietary factors. In the questionnaire, the participants also provided information on whether the alcohol was usually taken together with meals.
Polygenic risk score
Blood samples were collected from the participants upon enrollment, and genotyped using the UK Biobank Axiom array. A brief description of the procedures for genotype calling, array design, sample handling, quality control, and imputation of the UK Biobank samples has been provided elsewhere [16]. To determine whether the influence of dietary factors varied based on genetic susceptibility to breast cancer, significant SNPs from a recent meta-analysis of breast cancer GWAS were selected to create polygenic risk scores for breast cancer overall and by estrogen receptor (ER) status [11]. For all individuals, the weighted polygenic risk score (PRS) can be used to calculate the PRS, which is the sum of the products of the logarithmic odds ratio (OR) per allele and the allele dose for each SNP associated with breast cancer. Overall, ER+, and ER− PRS were categorized into quartiles, respectively. Detailed information on PRS score generation is provided in Supplementary Table 1.
Statistical analysis
Cox proportional hazards models were used to assess the associations between dietary factors and breast cancer risk, adjusting for various factors, including smoking status (never, previous, current), ethnicity (grouped into five categories where possible: White, Mixed other, Asian or Asian British, Black or Black British, and unknown), physical activity level (measured in metabolic equivalents task units and categorized into quartiles), Townsend deprivation index (categorized into quintiles), frequency of alcohol intake (≥ once/day or < once/day), employment status (in paid employment, pension, not in paid employment), educational qualifications (college or university degree/vocational qualification; national examination at ages 17–18 years; national examination at age 16 years; other qualifications were treated as missing), body mass index (BMI, categorized as < 18.5, 18.5 to < 25, 25 to < 30, or ≥ 30 kg/m2), 22 UKB centers, number of births (categorized as 0, 1, 2, or ≥ 3), age at menarche (categorized as < 13, 13–15, 16 to < 30 years), menopausal status (categorized as no, yes, not sure—had a hysterectomy, or not sure—other reason), age at first birth (categorized as < 23, 23–27, > 27 years, nulliparous/ missing), ever use of oral contraceptive pill (categorized as no or yes), ever use of hormone replacement therapy (categorized as no or yes), and family history of breast cancer (categorized as no or yes). Missingness in the covariates was categorized as a separate category.
To test the multiplicative interaction between the dietary factors and alcohol consumption, as well as between the dietary factors and PRS, an interaction term was included in the regression models and tested using the likelihood ratio (LR) test. Stratified analyses were also conducted according to alcohol intake frequency, whether alcohol was consumed with a meal and quartiles of PRS. We further estimated interaction in the additive scale for dietary factors and PRS using relative excess risk due to interaction (RERI), and a bootstrap approach was used to estimate the confidence interval and the p values.
Besides, sensitivity analyses were also performed by stratifying the analyses by menopausal status, Townsend deprivation index, and educational level. Likelihood ratio (LR) tests were used to examine the potential interaction by these variables. In this sensitivity analysis, menopausal status was divided into pre- and post-menopause. The Townsend deprivation index was dichotomized by median, and education was divided into a college degree and others.
All statistical analyses were performed using Stata 15.1. All P values were two-sided, and a P value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Among all 273,382 women in the UK Biobank, 58 withdrew their consent and were dropped, and 11,471 women were excluded due to a breast cancer diagnosis before baseline, leaving 261,853 participants in our study with 9069 incident breast cancer cases (Supplementary Fig. 1). The median follow-up time was 10.8 years and the incidence rate was 327.457/100000 person-year in the cohort.
Table 1 shows the characteristics of all participants. Breast cancer cases were more likely to be White ethnicity, less physically active, living in more affluent areas (measured by Townsend score), paid employed, holding a university/college degree/NVQ, consuming more alcohol, smoking, having two or more children, experiencing menarche from 13 to 15 years old, being postmenopausal, having their first birth at an age over 23 years old, taking oral contraceptives or hormone replacement therapy, having no family history of breast cancer, and having a higher BMI.
Table 1.
Baseline characteristics in the UK Biobank, by frequency of breast cancer (N = 261,853)
| Characteristic | All participants | Participants who developed |
|---|---|---|
| (n = 261,853) | Breast cancer (n = 9069) | |
| Means of age at recruitment | 59.64 ± 1.53 | |
| Ethnicity, n (%) | ||
| White | 236,135 (90.18) | 8253 (91.00) |
| Mixed other | 9273 (3.54) | 309 (3.41) |
| Asian or British Asian | 11,238 (4.29) | 374 (4.12) |
| Black or Black British | 1462 (0.56) | 32 (0.35) |
| Unknown | 2519 (0.96) | 67 (0.74) |
| Missing | 1226 (0.47) | 34 (0.37) |
| Physical activity level, n (%) | ||
| Quartile 1 | 50,535 (19.30) | 1834 (20.22) |
| Quartile 2 | 50,499 (19.29) | 1831 (20.19) |
| Quartile 3 | 50,472 (19.27) | 1711 (18.87) |
| Quartile 4 | 50,492 (19.28) | 1584 (17.47) |
| Unknown | 59,855 (22.86) | 2109 (23.26) |
| Townsend deprivation index, n (%) | ||
| Quartile 1 | 52,405 (20.01) | 1891 (20.85) |
| Quartile 2 | 52,214 (19.94) | 1799 (19.84) |
| Quartile 3 | 52,312 (19.98) | 1803 (19.88) |
| Quartile 4 | 52,305 (19.97) | 1889 (20.83) |
| Quartile 5 | 52,305 (19.97) | 1683 (18.56) |
| Unknown | 312 (0.12) | 4 (0.04) |
| Employment, n (%) | ||
| In paid employment | 144,683 (55.25) | 4778 (52.68) |
| Retired | 89,602 (34.22) | 3450 (38.04) |
| Not in paid employment | 26,100 (9.97) | 795 (8.77) |
| Unknown | 1468 (0.56) | 46 (0.51) |
| Qualification, n (%) | ||
| College/university degree/NVQ | 113,318 (43.28) | 3855 (42.51) |
| National examination at ages 17–18 | 36,889 (14.09) | 1282 (14.14) |
| National examination at age 16 | 93,191 (35.59) | 3257 (35.91) |
| Others/unknown | 18,455 (7.05) | 675 (7.44) |
| Body mass index(kg/m2), n (%) | ||
| < 18.5 | 1997 (0.76) | 57 (0.63) |
| 18.5 to < 25 | 101,395 (38.72) | 3251 (35.85) |
| 25 to < 30 | 95,394 (36.43) | 3396 (37.45) |
| ≥ 30 | 61,665 (23.55) | 2317 (25.55) |
| Unknown | 1402 (0.54) | 48 (0.53) |
| Alcohol intake frequency, n (%) | ||
| ≥ once/day | 41,853 (15.98) | 1634 (18.02) |
| < once/day | 219,291 (83.75) | 7418 (81.80) |
| Unknown | 709 (0.27) | 17 (0.19) |
| Smoking, n (%) | ||
| Never | 155,651 (59.44) | 5213 (57.48) |
| Previous | 81,280 (31.04) | 2966 (32.70) |
| Current | 23,476 (8.97) | 852 (9.39) |
| Unknown | 1446 (0.55) | 38 (0.42) |
| Number of births, n (%) | ||
| 0 | 48,862 (18.66) | 1787 (19.70) |
| 1 | 34,895 (13.33) | 1252 (13.81) |
| 2 | 114,079 (43.57) | 3948 (43.53) |
| ≥ 3 | 63,216 (24.14) | 2060 (22.71) |
| Unknown | 801 (0.31) | 22 (0.24) |
| Age at menarche—years, n (%) | ||
| < 13 | 98,504 (37.62) | 3562 (39.28) |
| 13–15 | 139,793 (53.39) | 4724 (52.09) |
| 16 to < 30 | 15,034 (5.74) | 526 (5.80) |
| Unknown | 8522 (3.25) | 257 (2.83) |
| Menopausal status, n (%) | ||
| No | 63,520 (24.26) | 1892 (20.86) |
| Yes | 156,335 (59.7) | 5709 (62.95) |
| Not sure—had a hysterectomy | 29,912 (11.42) | 1063 (11.72) |
| Not sure—other reason | 11,107 (4.24) | 379 (4.18) |
| Unknown | 979 (0.37) | 26 (0.29) |
| Age at frst birth—years, n (%) | ||
| < 23 | 51,487 (19.66) | 1696 (18.70) |
| 23–27 | 71,581 (27.34) | 2387 (26.32) |
| > 27 | 54,226 (20.71) | 1925 (21.23) |
| Unknown | 84,559 (32.29) | 3061 (33.75) |
| Oral contraceptive pill use, n (%) | ||
| No | 48,881 (18.67) | 1759 (19.40) |
| Yes | 211,593 (80.81) | 7275 (80.22) |
| Unknown | 1379 (0.53) | 35 (0.39) |
| Hormone replacement therapy, n (%) | ||
| No | 160,979 (61.48) | 5222 (57.58) |
| Yes | 99,350 (37.94) | 3800 (41.90) |
| Unknown | 1524 (0.58) | 47 (0.52) |
| Family history, n (%) | ||
| No | 216,149 (82.55) | 7062 (77.87) |
| Yes | 26,537 (10.13) | 1365 (15.05) |
| Unknown | 19,167 (7.32) | 642 (7.08) |
NVQ national vocational qualification
Diet, alcohol consumption, and risk of breast cancer
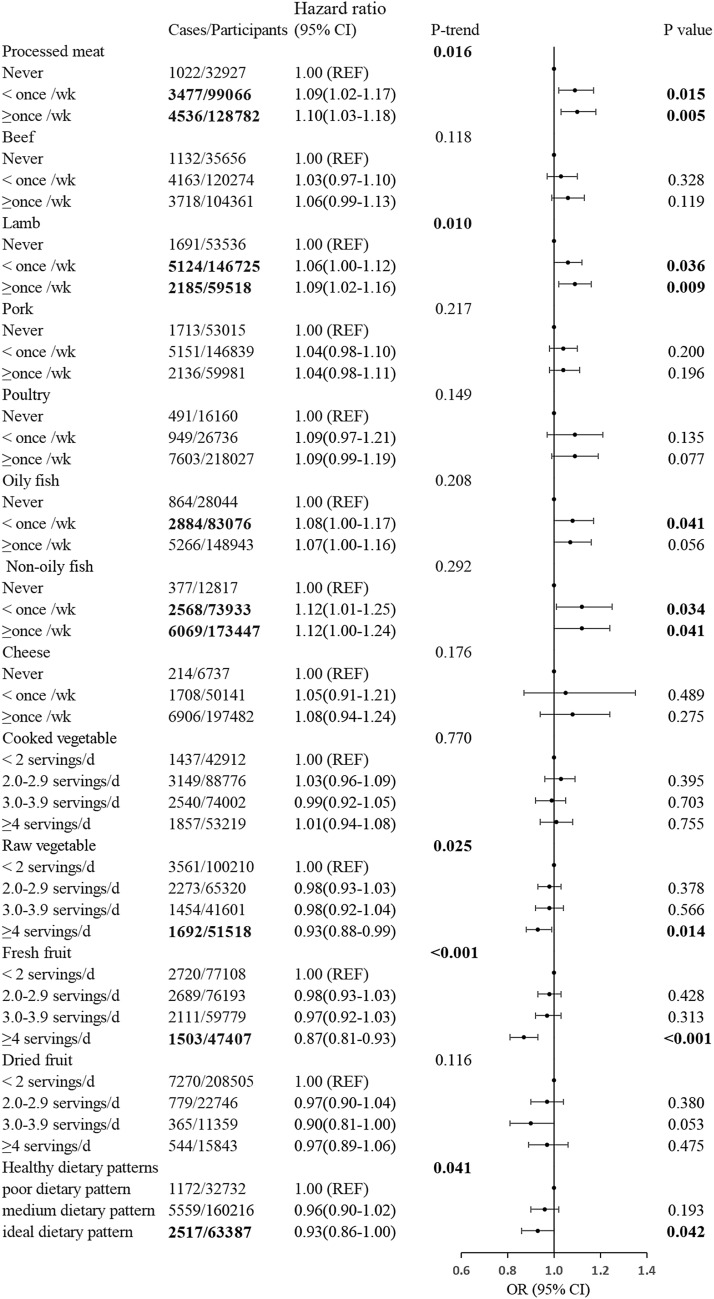
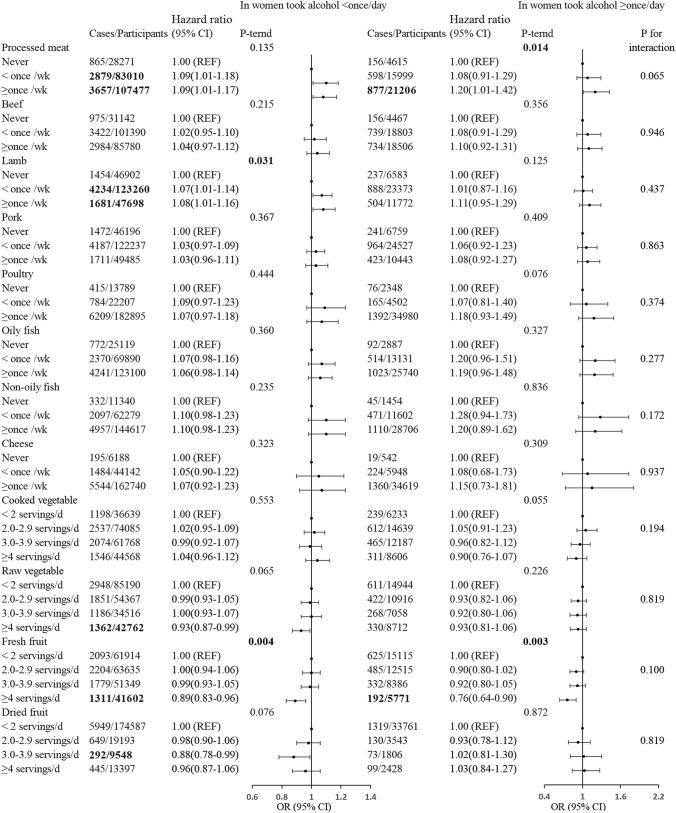
Figure 1 shows the HRs and 95% CIs for breast cancer by consumption level of each food item, using the lowest consumption category as the reference. It is noteworthy that an increase in the risk of breast cancer was observed with the frequent consumption of processed and red meat (HR for processed meat ≥ once/week = 1.10, 95% CI 1.03, 1.18, P-trend = 0.016; HR for lamb ≥ once/week = 1.09, 95% CI 1.02–1.16, P-trend = 0.010). However, the intake of beef, pork, poultry, cooked vegetables, and cheese was not significantly associated with the risk of breast cancer. Conversely, women with high levels of raw vegetables, fresh fruits, and a healthy dietary pattern may have a reduced risk of breast cancer, with corresponding HRs (95% CIs) of 0.93 (0.88, 0.99), 0.87 (0.81, 0.93), and 0.93 (0.86, 1.00), P-trend of 0.025, < 0.001, and 0.041, respectively. Among women who consumed alcohol ≥ once/day, processed meat intake was positively associated with the risk of breast cancer [HR (95% CI)1.20 (1.01, 1.29), p-trend = 0.014], while fresh fruit intake was negatively associated with the risk [HR (95% CI) 0.76 (0.64, 0.90), p-trend = 0.003] (Fig. 2). Moreover, a borderline significant interaction with alcohol consumption was also observed for processed meat (P for interaction = 0.065). To further investigate the synergistic effect between food and alcohol, we stratified the analysis by timing of alcohol consumption (Table 2 and Supplementary Table 2). In women who usually took alcohol together with the meal, the p for interaction between processed meat and alcohol consumption was 0.18. In these women, fresh fruit intake was negatively associated with the risk of breast cancer [HR (95% CI):0.73 (0.59–0.91), p-trend < 0.01], although the interaction with alcohol consumption was not statistically significant.
Fig. 1.
HRs (95% CIs) for the associations between dietary and breast cancer in UK Biobank participants, by frequency of alcohol consumption. Multivariable Cox regression model adjusted for age at recruitment, smoking, ethnicity, physical activity level, Townsend deprivation index, alcohol intake frequency, employment status, educational qualifications, BMI, 22 UKB centers, number of births, age at menarche, menopausal status, age at first birth, ever use of oral contraceptive pill, ever use of hormone replacement therapy, family history of breast cancer, stratified by alcohol intake frequency. The age at the end of the follow-up (i.e., attained age) was used as the underlying time scale. REF, reference
Fig. 2.
Associations between diet and any breast cancer, by frequency of alcohol consumption (in women took alcohol < 1/d or in women took alcohol ≥ 1/d). Multivariable Cox regression model adjusted for age at recruitment, smoking, ethnicity, physical activity level, Townsend deprivation index, alcohol intake frequency, employment status, educational qualifications, BMI, 22 UKB centers, number of births, age at menarche, menopausal status, age at first birth, ever use of oral contraceptive pill, ever use of hormone replacement therapy, family history of breast cancer, stratified by alcohol intake frequency. The age at the end of the follow-up (i.e., attained age) was used as the underlying time scale. REF,reference
Table 2.
The interaction between meat and alcohol consumption on breast cancer risk, by the timing of alcohol consumption
| Alcohol usually taken with meals (Yes) | Alcohol usually taken with meals (No) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no | No. of cases | Haz ratio (95% CI) | P value | P-trend | Total no | No. of cases | Haz ratio (95% CI) | P value | P-trend | |
| Overall | ||||||||||
| °Processed meat | 0.29 | 0.14 | ||||||||
| Never | 11,297 | 375 | 1.00 (REF) | 9845 | 299 | 1.00 (REF) | ||||
| < once/wk | 38,670 | 1390 | 1.05 (0.94–1.18) | 0.41 | 33,443 | 1152 | 1.09 (0.96–1.24) | 0.19 | ||
| ≥ once/wk | 46,121 | 1679 | 1.07 (0.95–1.20) | 0.26 | 48,814 | 1702 | 1.11 (0.98–1.26) | 0.09 | ||
| In women took alcohol < once/day | ||||||||||
| °Processed meat | 0.96 | 0.35 | ||||||||
| Never | 8733 | 285 | 1.00 (REF) | 7794 | 233 | 1.00 (REF) | ||||
| < once/wk | 29,491 | 1043 | 1.04 (0.91–1.19) | 0.53 | 26,625 | 901 | 1.09 (0.95–1.27) | 0.22 | ||
| ≥ once/wk | 34,961 | 1207 | 1.02 (0.90–1.17) | 0.73 | 38,773 | 1297 | 1.10 (0.95–1.26) | 0.20 | ||
| In women took alcohol ≥ once/day | ||||||||||
| °Processed meat | 0.05 | 0.15 | ||||||||
| Never | 2564 | 90 | 1.00 (REF) | 2051 | 66 | 1.00 (REF) | ||||
| < once/wk | 9179 | 347 | 1.07 (0.84–1.35) | 0.59 | 6818 | 251 | 1.09 (0.83–1.43) | 0.55 | ||
| ≥ once/wk | 11,160 | 472 | 1.20 (0.95–1.51) | 0.12 | 10,041 | 405 | 1.18 (0.91–1.54) | 0.22 | ||
| P for interaction | 0.18 | 0.51 | ||||||||
| Overall | ||||||||||
| °Beef | 0.66 | 0.32 | ||||||||
| Never | 10,988 | 377 | 1.00 (REF) | 11,296 | 356 | 1.00 (REF) | ||||
| < once/wk | 44,747 | 1595 | 0.99 (0.88–1.11) | 0.83 | 42,929 | 1453 | 1.03 (0.92–1.16) | 0.64 | ||
| ≥ once/wk | 40,253 | 1467 | 1.01 (0.90–1.13) | 0.85 | 37,698 | 1336 | 1.06 (0.94–1.19) | 0.36 | ||
| In women took alcohol < once/day | ||||||||||
| °Beef | 0.92 | 0.45 | ||||||||
| Never | 8676 | 287 | 1.00 (REF) | 9141 | 290 | 1.00 (REF) | ||||
| < once/wk | 34,425 | 1193 | 0.99 (0.87–1.13) | 0.93 | 34,449 | 1116 | 0.98 (0.86–1.12) | 0.79 | ||
| ≥ once/wk | 30,005 | 1050 | 1.00 (0.88–1.14) | 0.98 | 29,445 | 1019 | 1.03 (0.90–1.17) | 0.69 | ||
| In women took alcohol ≥ once/day | ||||||||||
| °Beef | 0.50 | 0.58 | ||||||||
| Never | 2312 | 90 | 1.00 (REF) | 2155 | 66 | 1.00 (REF) | ||||
| < once/wk | 10,322 | 402 | 0.96 (0.76–1.21) | 0.73 | 8480 | 337 | 1.23 (0.95–1.61) | 0.12 | ||
| ≥ once/wk | 10,248 | 417 | 1.03 (0.82–1.30) | 0.80 | 8253 | 317 | 1.18 (0.90–1.54) | 0.24 | ||
| P for interaction | 0.92 | 0.42 | ||||||||
| Overall | ||||||||||
| °Lamb | 0.15 | 0.19 | ||||||||
| Never | 15,461 | 518 | 1.00 (REF) | 19,015 | 592 | 1.00 (REF) | ||||
| < once/wk | 55,820 | 1967 | 1.01 (0.91–1.11) | 0.87 | 53,097 | 1841 | 1.07 (0.98–1.18) | 0.13 | ||
| ≥ once/wk | 24,604 | 949 | 1.07 (0.96–1.19) | 0.21 | 19,658 | 710 | 1.08 (0.97–1.21) | 0.17 | ||
| In women took alcohol < once/day | ||||||||||
| °Lamb | 0.34 | 0.30 | ||||||||
| Never | 12,294 | 400 | 1.00 (REF) | 15,600 | 473 | 1.00 (REF) | ||||
| < once/wk | 43,138 | 1482 | 1.02 (0.91–1.14) | 0.75 | 42,407 | 1438 | 1.09 (0.98–1.21) | 0.12 | ||
| ≥ once/wk | 17,596 | 643 | 1.06 (0.93–1.20) | 0.37 | 14,899 | 512 | 1.07 (0.94–1.22) | 0.29 | ||
| In women took alcohol ≥ once/day | ||||||||||
| °Lamb | 0.23 | 0.40 | ||||||||
| Never | 3167 | 118 | 1.00 (REF) | 3415 | 119 | 1.00 (REF) | ||||
| < once/wk | 12,682 | 485 | 0.97 (0.79–1.19) | 0.80 | 10,690 | 403 | 1.03 (0.83–1.26) | 0.80 | ||
| ≥ once/wk | 7008 | 306 | 1.09 (0.88–1.36) | 0.42 | 4759 | 198 | 1.10 (0.87–1.39) | 0.42 | ||
| P for interaction | 0.51 | 0.64 | ||||||||
| Overall | ||||||||||
| °Pork | 0.36 | 0.79 | ||||||||
| Never | 16,176 | 545 | 1.00 (REF) | 16,990 | 561 | 1.00 (REF) | ||||
| < once/wk | 56,762 | 2042 | 1.02 (0.93–1.13) | 0.62 | 53,281 | 1823 | 1.00 (0.91–1.10) | 0.98 | ||
| ≥ once/wk | 22,936 | 851 | 1.05 (0.94–1.17) | 0.37 | 21,491 | 760 | 1.01 (0.91–1.13) | 0.80 | ||
| In women took alcohol < once/day | ||||||||||
| °Pork | 0.52 | 0.94 | ||||||||
| Never | 12,734 | 415 | 1.00 (REF) | 13,673 | 450 | 1.00 (REF) | ||||
| < once/wk | 43,228 | 1510 | 1.03 (0.93–1.15) | 0.56 | 42,289 | 1391 | 0.97 (0.87–1.08) | 0.54 | ||
| ≥ once/wk | 17,055 | 604 | 1.04 (0.92–1.19) | 0.50 | 16,935 | 584 | 1.00 (0.88–1.13) | 0.99 | ||
| In women took alcohol ≥ once/day | ||||||||||
| °Pork | 0.47 | 0.71 | ||||||||
| Never | 3442 | 130 | 1.00 (REF) | 3317 | 111 | 1.00 (REF) | ||||
| < once/wk | 13,534 | 532 | 1.00 (0.82–1.21) | 0.98 | 10,992 | 432 | 1.14 (0.92–1.40) | 0.23 | ||
| ≥ once/wk | 5881 | 247 | 1.07 (0.86–1.32) | 0.56 | 4556 | 176 | 1.08 (0.84–1.37) | 0.55 | ||
| P for interaction | 0.86 | 0.56 | ||||||||
Multivariable Cox regression model adjusted for age at recruitment, smoking, ethnicity, physical activity level, Townsend deprivation index, alcohol intake frequency, employment status, educational qualifications, BMI, 22 UKB centers, number of births, age at menarche, menopausal status, age at first birth, ever use of oral contraceptive pill, ever use of hormone replacement therapy, family history of breast cancer, stratified by alcohol intake frequency, alcohol, and meal
Stronger associations between fresh fruit, processed meat, and the risk of breast cancer were also observed in women who consumed alcohol ≥ 2 units per day. However, their interactions with a dosage of alcohol consumption were not statistically significant (Supplementary Table 3).
For sensitivity analysis, when stratifying the analysis by menopausal status, processed meat, beef, and lamb were associated with the risk of breast cancer in postmenopausal women, while statistically significant association only persisted in pre-menopausal women who took processed meat ≥ once/week and alcohol ≥ once/day (Supplementary Table 4). Stratification by Townsend deprivation index and education levels suggested no interaction between socio-economic status and those dietary factors identified to be associated with breast cancer in the main analysis, although the point estimates changed slightly (Supplementary Tables 5, 6).
Diet, genetic predisposition, and risk of breast cancer
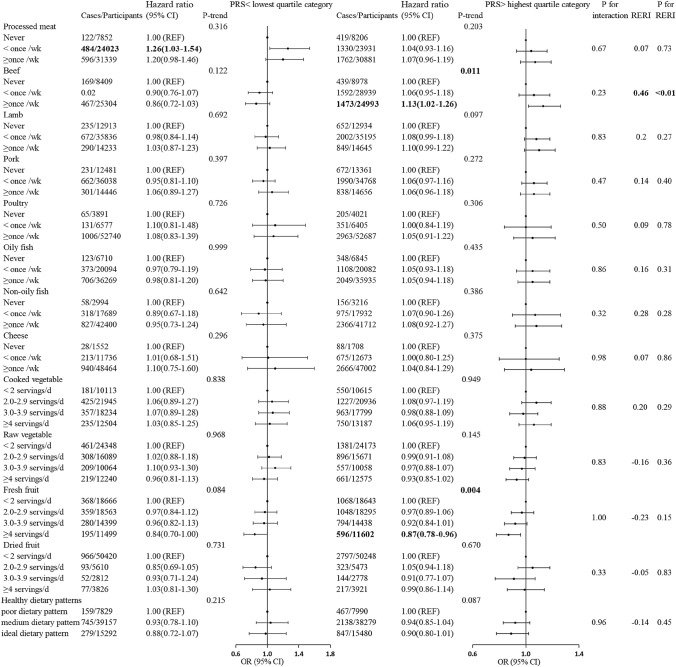
The association between dietary factors and breast cancer risk was additionally compared among women with the highest and the lowest quartile of PRS (Fig. 3). In women with the highest quartile of PRS, the HRs (95% CIs) for breast cancer were 1.13 (1.02–1.26) for the highest levels of beef consumption compared with the lowest levels. Although multiplicative interaction was not observed, the RERI of beef consumption in women with the highest quartile of PRS was 0.46, and the p value of RERI was < 0.01, which was statistically significant. This significant interaction was still observed when stratified by genetic susceptibility to breast cancer using ER+ PRS and ER− PRS in quartiles (Supplementary Table 7). No interaction was observed for other food.
Fig. 3.
Associations between diet and breast cancer for individuals with different breast cancer PRSs. Multivariable Cox regression model adjusted for age at recruitment, smoking, ethnicity, physical activity level, Townsend deprivation index, alcohol intake frequency, employment status, educational qualifications, BMI, 22 UKB centers, number of births, age at menarche, menopausal status, age at first birth, ever use of oral contraceptive pill, ever use of hormone replacement therapy, family history of breast cancer, stratified by alcohol intake frequency. The age at the end of the follow-up (i.e., attained age) was used as the underlying time scale. REF, reference
Discussion
Key results
We found a significant association between processed meat, vegetables, fruits, and HDS and the risk of breast cancer, particularly in women with a high frequency of alcohol consumption. Only a borderline significant interaction between processed meat and alcohol consumption was observed among women. We have also discovered a stronger association between beef consumption and breast cancer in women who have a strong genetic predisposition to the disease. No interaction was observed for other dietary factors.
The significant association between processed meat intake and breast cancer risk in our study is supported by several previous findings [8, 17]. Processed meat contains carcinogenic components that can directly damage DNA, such as heterocyclic aromatic amines (HAA) and polycyclic aromatic hydrocarbons (PAHs) resulting from meat processing or preparation, including high-temperature cooking. Nitrites, used as additives, can also induce the formation of n-nitroso compounds (NOCs) in the digestive tract, as confirmed in both animal and human biomonitoring studies [18]. These compounds can also play a carcinogenic role through other mechanisms, such as the estrogenic properties of pseudo-hot isostatic pressing (PhIP) [19, 20].
Furthermore, we found a penitential interaction between intake of processed meat and alcohol consumption on the risk of developing breast cancer. Recent large-scale prospective studies have shown that alcohol intake may increase the risk of breast cancer [21–23], likely due to hormonal influences [24–26]. Meanwhile, alcohol has also been suggested to have toxic effects and these effects were mediated by DNA damage and carcinogenic effects of alcohol and through mutagenesis by acetaldehyde and by induction of oxidative damage [27–29]. One plausible mechanism for the synergistic effect between processed meat and alcohol is that alcohol may enhance the penetration of carcinogenic compounds in processed meat as a solvent [30]. For instance, the concurrent consumption of processed meat and alcohol may result in an increased expression of CYP2E1, leading to elevated levels of oxidative stress and DNA damage. Consequently, CYP2E1 reinforced the activation of Reactive Oxygen Species (ROS) that cause DNA damage through ethanol and ROS production. This activation further stimulated PhIP through an oxidation process, which could trigger or sustain tumor growth [26]. Despite these, the synergic effect between processed meat and alcohol still requires more studies to verify.
Consistent with previous studies [6, 9], our findings support the view that an overall healthy dietary pattern was negatively associated with the risk of breast cancer, and this appears to be due to high consumption of vegetables and fruits [31]. Vegetables and fruits are abundant in potentially anti-carcinogenic nutrients including fiber, vitamins C and E, carotenoids, and other bioactive substances [32–34], which may lessen the risk of developing cancer.
In the current study, we observed an association between beef consumption and the risk of breast cancer in women who had the highest quartile of breast cancer PRS. Several studies have also observed additive interactions between lifestyle factors [35], and the genetic predisposition to breast cancer risk [36–38]. Moreover, the slightly higher risk of breast cancer among women with high beef consumption and ER+ PRS compared to the overall PRS may suggest a stronger association with ER-positive disease [39, 40]. Our finding of the additive interaction between ER+ PRS and beef further suggested the role of genetic testing in individualized dietary intervention for breast cancer.
The main strength of our study is its large sample size and population-based cohort design. Other strengths include the UK Biobank cohort’s abundant lifestyle and genetic data, which allowed us to explore the gene and lifestyle interactions for the associations investigated. However, our study has several limitations. On one hand, there is no real measurement of alcohol consumption, but only frequency of alcohol consumption. On the other hand, the data from the touchscreen dietary questionnaire might be affected by recall bias. Besides, as we have performed a lot of interaction tests and subgroup analyses, findings with nominal P values for these analyses should be interpreted with caution, considering the multiple testing issues. Further studies are, therefore, needed to validate our findings. Finally, given the observational nature of this study, it is possible that there are still unmeasured confounding factors or residual confounding in our analysis.
In conclusion, our findings support the view that processed meat, vegetable, fresh fruits, and HDS can affect the risk of breast cancer, suggesting that a combined intervention consisting of a well-balanced diet that includes lower processed meat consumption and an increase in vegetable and fresh fruit intake may contribute to preventing breast cancer in women at high risk. However, no strong interaction of dietary factors with alcohol consumption and genetic predisposition was observed for the risk of breast cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
PZ, YZ, and HY had full access to all data, and took responsibility for the integrity of the data and the accuracy of the analysis. HY and ML conceived and designed the study. All authors acquired, analyzed, or interpreted the data. PZ and QC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. PZ performed the statistical analysis. HY obtained the funding. All authors read and approved the final manuscript.
Funding
Open access funding provided by Karolinska Institute. This work was supported by the Natural Science Foundation of China [Grant No: 82204132], the Natural Science Foundation of Fujian Province [Grant No: 2021J01721], the Startup Fund for High-level Talents of Fujian Medical University [Grant No: XRCZX2020007], and Startup Fund for Scientific Research, Fujian Medical University [Grant No: 2019QH1002]. The funders had no role in the study design, data collection, analyses, data interpretation, writing the manuscript, or in the decision to submit the manuscript for publication.
Data availability
Data from the UK Biobank (http://www.ukbiobank.ac.uk/) are available to researchers upon application. This research was conducted using the UK Biobank Resource under Application 61083.
Declarations
Competing interests
The authors declare no competing interest.
Ethics approval and consent to participate
The UK Biobank was approved by the National Information Governance Board for Health and Social Care and the National Health Service North West Centre for Research Ethics Committee (Ref: 11/NW/0382, 17 June 2011). All participants gave informed consent to participate and be followed up.
Footnotes
Pingxiu Zhu, Yanyu Zhang and Qianni Chen shared the first authorship of the work and each made an equal contribution.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Pawitan Y, Fang F, Czene K, Ye W. Biomarkers and disease trajectories influencing women's health: results from the UK Biobank Cohort. Phenomics (Cham, Switzerland) 2022;2(3):184–193. doi: 10.1007/s43657-022-00054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima SM, Kehm RD, Terry MB. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine. 2021;38:100985. doi: 10.1016/j.eclinm.2021.100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu XO, Huang YB, Gao Y, Chen C, Yan Y, Dai HJ, Song FJ, Wang YG, Wang PS, Chen KX. Association between dietary factors and breast cancer risk among Chinese females: systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15(3):1291–1298. doi: 10.7314/apjcp.2014.15.3.1291. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Espin C, Bonet C, Crous-Bou M, Katzke V, Le Cornet C, Jannasch F, Schulze MB, Olsen A, Tjonneland A, Dahm CC, Antoniussen CS, Sanchez MJ, Amiano P, Chirlaque MD, Guevara M, Agnoli C, Tumino R, Sacerdote C, De Magistris MS, Sund M, Boden S, Jensen TE, Olsen KS, Skeie G, Gunter MJ, Rinaldi S, Gonzalez-Gil EM, Weiderpass E, Christakoudi S, Heath AK, Dossus L, Agudo A. Dietary patterns related to biological mechanisms and survival after breast cancer diagnosis: results from a cohort study. Br J Cancer. 2023;128(7):1301–1310. doi: 10.1038/s41416-023-02169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farvid MS, Chen WY, Rosner BA, Tamimi RM, Willett WC, Eliassen AH. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int J Cancer. 2019;144(7):1496–1510. doi: 10.1002/ijc.31653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farvid MS, Stern MC, Norat T, Sasazuki S, Vineis P, Weijenberg MP, Wolk A, Wu K, Stewart BW, Cho E. Consumption of red and processed meat and breast cancer incidence: a systematic review and meta-analysis of prospective studies. Int J Cancer. 2018;143(11):2787–2799. doi: 10.1002/ijc.31848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JJ, Darwis NDM, Mackay DF, Celis-Morales CA, Lyall DM, Sattar N, Gill JMR, Pell JP. Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur J Cancer. 2018;90:73–82. doi: 10.1016/j.ejca.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Arthur RS, Wang T, Xue X, Kamensky V, Rohan TE. Genetic factors, adherence to healthy lifestyle behavior, and risk of invasive breast cancer among women in the UK Biobank. J Natl Cancer Inst. 2020;112(9):893–901. doi: 10.1093/jnci/djz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauchi JP, Camilleri L, Scerri C. Environmental and lifestyle risk factors of breast cancer in Malta—a retrospective case-control study. EPMA J. 2016;7(1):20. doi: 10.1186/s13167-016-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, Tyrer JP, Chen TH, Wang Q, Bolla MK, Yang X, Adank MA, Ahearn T, Aittomaki K, Allen J, Andrulis IL, Anton-Culver H, Antonenkova NN, Arndt V, Aronson KJ, Auer PL, Auvinen P, Barrdahl M, Beane Freeman LE, Beckmann MW, Behrens S, Benitez J, Bermisheva M, Bernstein L, Blomqvist C, Bogdanova NV, Bojesen SE, Bonanni B, Borresen-Dale AL, Brauch H, Bremer M, Brenner H, Brentnall A, Brock IW, Brooks-Wilson A, Brucker SY, Bruning T, Burwinkel B, Campa D, Carter BD, Castelao JE, Chanock SJ, Chlebowski R, Christiansen H, Clarke CL, Collee JM, Cordina-Duverger E, Cornelissen S, Couch FJ, Cox A, Cross SS, Czene K, Daly MB, Devilee P, Dork T, Dos-Santos-Silva I, Dumont M, Durcan L, Dwek M, Eccles DM, Ekici AB, Eliassen AH, Ellberg C, Engel C, Eriksson M, Evans DG, Fasching PA, Figueroa J, Fletcher O, Flyger H, Forsti A, Fritschi L, Gabrielson M, Gago-Dominguez M, Gapstur SM, Garcia-Saenz JA, Gaudet MM, Georgoulias V, Giles GG, Gilyazova IR, Glendon G, Goldberg MS, Goldgar DE, Gonzalez-Neira A, Grenaker Alnaes GI, Grip M, Gronwald J, Grundy A, Guenel P, Haeberle L, Hahnen E, Haiman CA, Hakansson N, Hamann U, Hankinson SE, Harkness EF, Hart SN, He W, Hein A, Heyworth J, Hillemanns P, Hollestelle A, Hooning MJ, Hoover RN, Hopper JL, Howell A, Huang G, Humphreys K, Hunter DJ, Jakimovska M, Jakubowska A, Janni W, John EM, Johnson N, Jones ME, Jukkola-Vuorinen A, Jung A, Kaaks R, Kaczmarek K, Kataja V, Keeman R, Kerin MJ, Khusnutdinova E, Kiiski JI, Knight JA, Ko YD, Kosma VM, Koutros S, Kristensen VN, Kruger U, Kuhl T, Lambrechts D, Le Marchand L, Lee E, Lejbkowicz F, Lilyquist J, Lindblom A, Lindstrom S, Lissowska J, Lo WY, Loibl S, Long J, Lubinski J, Lux MP, MacInnis RJ, Maishman T, Makalic E, Maleva Kostovska I, Mannermaa A, Manoukian S, Margolin S, Martens JWM, Martinez ME, Mavroudis D, McLean C, Meindl A, Menon U, Middha P, Miller N, Moreno F, Mulligan AM, Mulot C, Munoz-Garzon VM, Neuhausen SL, Nevanlinna H, Neven P, Newman WG, Nielsen SF, Nordestgaard BG, Norman A, Offit K, Olson JE, Olsson H, Orr N, Pankratz VS, Park-Simon TW, Perez JIA, Perez-Barrios C, Peterlongo P, Peto J, Pinchev M, Plaseska-Karanfilska D, Polley EC, Prentice R, Presneau N, Prokofyeva D, Purrington K, Pylkas K, Rack B, Radice P, Rau-Murthy R, Rennert G, Rennert HS, Rhenius V, Robson M, Romero A, Ruddy KJ, Ruebner M, Saloustros E, Sandler DP, Sawyer EJ, Schmidt DF, Schmutzler RK, Schneeweiss A, Schoemaker MJ, Schumacher F, Schurmann P, Schwentner L, Scott C, Scott RJ, Seynaeve C, Shah M, Sherman ME, Shrubsole MJ, Shu XO, Slager S, Smeets A, Sohn C, Soucy P, Southey MC, Spinelli JJ, Stegmaier C, Stone J, Swerdlow AJ, Tamimi RM, Tapper WJ, Taylor JA, Terry MB, Thone K, Tollenaar R, Tomlinson I, Truong T, Tzardi M, Ulmer HU, Untch M, Vachon CM, van Veen EM, Vijai J, Weinberg CR, Wendt C, Whittemore AS, Wildiers H, Willett W, Winqvist R, Wolk A, Yang XR, Yannoukakos D, Zhang Y, Zheng W, Ziogas A, Investigators A. kConFab AI. Collaborators N. Dunning AM, Thompson DJ, Chenevix-Trench G, Chang-Claude J, Schmidt MK, Hall P, Milne RL, Pharoah PDP, Antoniou AC, Chatterjee N, Kraft P, Garcia-Closas M, Simard J, Easton DF. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104(1):21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Zeng Y, Yang H, Hu Y, Hu Y, Chen W, Ying Z, Sun Y, Qu Y, Li Q, Valdimarsdottir UA, Song H. Familial factors, diet, and risk of cardiovascular disease: a cohort analysis of the UK Biobank. Am J Clin Nutr. 2021;114(5):1837–1846. doi: 10.1093/ajcn/nqab261. [DOI] [PubMed] [Google Scholar]
- 14.Pazoki R, Dehghan A, Evangelou E, Warren H, Gao H, Caulfield M, Elliott P, Tzoulaki I. Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood pressure levels and cardiovascular events. Circulation. 2018;137(7):653–661. doi: 10.1161/CIRCULATIONAHA.117.030898. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task F, Statistics C Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 16.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O'Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J, Wei W, Zhan L. Red and processed meat intake and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2015;151(1):191–198. doi: 10.1007/s10549-015-3380-9. [DOI] [PubMed] [Google Scholar]
- 18.Pouchieu C, Deschasaux M, Hercberg S, Druesne-Pecollo N, Latino-Martel P, Touvier M. Prospective association between red and processed meat intakes and breast cancer risk: modulation by an antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Int J Epidemiol. 2014;43(5):1583–1592. doi: 10.1093/ije/dyu134. [DOI] [PubMed] [Google Scholar]
- 19.Steck SE, Gaudet MM, Eng SM, Britton JA, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD. Cooked meat and risk of breast cancer–lifetime versus recent dietary intake. Epidemiology. 2007;18(3):373–382. doi: 10.1097/01.ede.0000259968.11151.06. [DOI] [PubMed] [Google Scholar]
- 20.Egeberg R, Olsen A, Autrup H, Christensen J, Stripp C, Tetens I, Overvad K, Tjonneland A. Meat consumption, N-acetyl transferase 1 and 2 polymorphism and risk of breast cancer in Danish postmenopausal women. Eur J Cancer Prev. 2008;17(1):39–47. doi: 10.1097/CEJ.0b013e32809b4cdd. [DOI] [PubMed] [Google Scholar]
- 21.Lew JQ, Freedman ND, Leitzmann MF, Brinton LA, Hoover RN, Hollenbeck AR, Schatzkin A, Park Y. Alcohol and risk of breast cancer by histologic type and hormone receptor status in postmenopausal women: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;170(3):308–317. doi: 10.1093/aje/kwp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA J Am Med Assoc. 2011;306(17):1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J, Million Women Study C Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez SV. Estrogen, alcohol consumption, and breast cancer. Alcohol Clin Exp Res. 2011;35(3):389–391. doi: 10.1111/j.1530-0277.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 25.Gill J. The effects of moderate alcohol consumption on female hormone levels and reproductive function. Alcohol Alcohol. 2000;35(5):417–423. doi: 10.1093/alcalc/35.5.417. [DOI] [PubMed] [Google Scholar]
- 26.Endogenous H, Breast Cancer Collaborative G. Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, Rollison DE, Dorgan JF, Brinton LA, Overvad K, Kaaks R, Trichopoulou A, Clavel-Chapelon F, Panico S, Duell EJ, Peeters PH, Rinaldi S, Fentiman IS, Dowsett M, Manjer J, Lenner P, Hallmans G, Baglietto L, English DR, Giles GG, Hopper JL, Severi G, Morris HA, Hankinson SE, Tworoger SS, Koenig K, Zeleniuch-Jacquotte A, Arslan AA, Toniolo P, Shore RE, Krogh V, Micheli A, Berrino F, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Lui LY, Cummings SR, Gunter MJ, Rohan TE, Strickler HD. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105(5):709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis of epidemiological studies. Int J Cancer. 2008;122(8):1832–1841. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- 28.Ristow H, Seyfarth A, Lochmann ER. Chromosomal damages by ethanol and acetaldehyde in Saccharomyces cerevisiae as studied by pulsed field gel electrophoresis. Mutat Res. 1995;326(2):165–170. doi: 10.1016/0027-5107(94)00165-2. [DOI] [PubMed] [Google Scholar]
- 29.Dumitrescu RG, Shields PG. The etiology of alcohol-induced breast cancer. Alcohol. 2005;35(3):213–225. doi: 10.1016/j.alcohol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Lin S, Wang X, Huang C, Liu X, Zhao J, Yu IT, Christiani DC. Consumption of salted meat and its interactions with alcohol drinking and tobacco smoking on esophageal squamous-cell carcinoma. Int J Cancer. 2015;137(3):582–589. doi: 10.1002/ijc.29406. [DOI] [PubMed] [Google Scholar]
- 31.Hirko KA, Willett WC, Hankinson SE, Rosner BA, Beck AH, Tamimi RM, Eliassen AH. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat. 2016;155(3):579–588. doi: 10.1007/s10549-016-3706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 33.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, Hallmans G, Helzlsouer KJ, Hoffman-Bolton J, Hulten K, Sesso HD, Sowell AL, Tamimi RM, Toniolo P, Wilkens LR, Winkvist A, Zeleniuch-Jacquotte A, Zheng W, Hankinson SE. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104(24):1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farvid MS, Eliassen AH, Cho E, Liao X, Chen WY, Willett WC. Dietary fiber intake in young adults and breast cancer risk. Pediatrics. 2016;137(3):e20151226. doi: 10.1542/peds.2015-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nickels S, Truong T, Hein R, Stevens K, Buck K, Behrens S, Eilber U, Schmidt M, Haberle L, Vrieling A, Gaudet M, Figueroa J, Schoof N, Spurdle AB, Rudolph A, Fasching PA, Hopper JL, Makalic E, Schmidt DF, Southey MC, Beckmann MW, Ekici AB, Fletcher O, Gibson L, Silva Idos S, Peto J, Humphreys MK, Wang J, Cordina-Duverger E, Menegaux F, Nordestgaard BG, Bojesen SE, Lanng C, Anton-Culver H, Ziogas A, Bernstein L, Clarke CA, Brenner H, Muller H, Arndt V, Stegmaier C, Brauch H, Bruning T, Harth V, Genica N, Mannermaa A, Kataja V, Kosma VM, Hartikainen JM, kConFab. Group AM. Lambrechts D, Smeets D, Neven P, Paridaens R, Flesch-Janys D, Obi N, Wang-Gohrke S, Couch FJ, Olson JE, Vachon CM, Giles GG, Severi G, Baglietto L, Offit K, John EM, Miron A, Andrulis IL, Knight JA, Glendon G, Mulligan AM, Chanock SJ, Lissowska J, Liu J, Cox A, Cramp H, Connley D, Balasubramanian S, Dunning AM, Shah M, Trentham-Dietz A, Newcomb P, Titus L, Egan K, Cahoon EK, Rajaraman P, Sigurdson AJ, Doody MM, Guenel P, Pharoah PD, Schmidt MK, Hall P, Easton DF, Garcia-Closas M, Milne RL, Chang-Claude J. Evidence of gene-environment interactions between common breast cancer susceptibility loci and established environmental risk factors. PLoS Genet. 2013;9(3):e1003284. doi: 10.1371/journal.pgen.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nkondjock A, Robidoux A, Paredes Y, Narod SA, Ghadirian P. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res Treat. 2006;98(3):285–294. doi: 10.1007/s10549-006-9161-8. [DOI] [PubMed] [Google Scholar]
- 38.Bissonauth V, Shatenstein B, Fafard E, Maugard C, Robidoux A, Narod S, Ghadirian P. Weight history, smoking, physical activity and breast cancer risk among French-Canadian women non-carriers of more frequent BRCA1/2 mutations. J Cancer Epidemiol. 2009;2009:748367. doi: 10.1155/2009/748367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holm J, Li J, Darabi H, Eklund M, Eriksson M, Humphreys K, Hall P, Czene K. Associations of breast cancer risk prediction tools with tumor characteristics and metastasis. J Clin Oncol. 2016;34(3):251–258. doi: 10.1200/JCO.2015.63.0624. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Holm J, Bergh J, Eriksson M, Darabi H, Lindstrom LS, Tornberg S, Hall P, Czene K. Breast cancer genetic risk profile is differentially associated with interval and screen-detected breast cancers. Ann Oncol. 2015;26(3):517–522. doi: 10.1093/annonc/mdu565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the UK Biobank (http://www.ukbiobank.ac.uk/) are available to researchers upon application. This research was conducted using the UK Biobank Resource under Application 61083.