Abstract
Introduction
A prognostic model to predict liver severity in people with metabolic dysfunction-associated steatotic liver disease (MASLD) is very important, but the accuracy of the most commonly used tools is not yet well established.
Objective
The meta-analysis aimed to assess the accuracy of different prognostic serological biomarkers in predicting liver fibrosis severity in people with MASLD.
Methods
Adults ≥18 years of age with MASLD were included, with the following: liver biopsy and aspartate aminotransferase-to-platelet ratio (APRI), fibrosis index-4 (FIB-4), non-alcoholic fatty liver disease fibrosis score (NFS), body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes score (BARD score), FibroMeter, FibroTest, enhanced liver fibrosis (ELF), Forns score, and Hepascore. Meta-analyses were performed using a random effects model based on the DerSimonian and Laird methods. The study’s risk of bias was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2.
Results
In total, 138 articles were included, of which 86 studies with 46,514 participants met the criteria for the meta-analysis. The results for the summary area under the receiver operating characteristic (sAUROC) curve, according to the prognostic models, were as follows: APRI: advanced fibrosis (AF): 0.78, any fibrosis (AnF): 0.76, significant fibrosis (SF): 0.76, cirrhosis: 0.72; FIB-4: cirrhosis: 0.83, AF: 0.81, AnF: 0.77, SF: 0.75; NFS: SF: 0.81, AF: 0.81, AnF: 0.71, cirrhosis: 0.69; BARD score: SF: 0.77, AF: 0.73; FibroMeter: SF: 0.88, AF: 0.84; FibroTest: SF: 0.86, AF: 0.78; and ELF: AF: 0.87.
Conclusion
The results of this meta-analysis suggest that, when comparing the scores of serological biomarkers with liver biopsies, the following models showed better diagnostic accuracy in predicting liver fibrosis severity in people with MASLD: FIB-4 for any fibrosis, FibroMeter for significant fibrosis, ELF for advanced fibrosis, and FIB-4 for cirrhosis.
Clinical trial registration: [https://clinicaltrials.gov/], identifier [CRD 42020180525].
Keywords: prognosis, liver biopsy, metabolic dysfunction-associated steatotic liver disease, non-invasive tests, meta-analysis
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is defined as the presence of hepatic steatosis along with at least one of five cardiometabolic risk factors that correspond to the components of metabolic syndrome (MetS) (1). The scenario of MASLD is evolving rapidly; according to the Global Burden of Disease study, MASLD increased considerably in both adolescents and adults between 1990 and 2019 (2, 3). In adolescents, the increase was from 3.73% in 1990 to 4.71% in 2019—an increase of 26.27% (2). In adults, the incidence of MASLD cases increased by 95.4% from 88,177 (95% uncertainty interval (95% UI): 62,304–128,319) in 1990 to 172,330 (95% UI: 125,775–243,640) in 2019. Deaths from MASLD increased by 80.2% from 93,758 (95% UI: 71,657–119,097) per 100,000 population in 1990 to 168,969 (95% UI: 130,575–211,295) per 100,000 population in 2019 (3).
Due to the burden of this disease, early diagnosis of MASLD is an important clinical strategy to prevent its rapid progression to the most severe stages of the disease. According to different international guidelines, liver biopsy is still considered the gold standard for diagnosing liver fibrosis in MASLD (4, 5). However, it is an invasive test that is not free of complications and is not recommended for monitoring disease severity (6). Therefore, the clinical practice guidelines for the management of MASLD recommend the use of non-invasive tests as a resource before the need for liver biopsy in order to stage the disease of fibrosis. These are non-invasive methods that make it feasible to assess disease progression (7).
Different studies have evaluated the diagnostic performance of prognostic models using biomarkers in MASLD (8–10). A meta-analysis of 64 studies published until 2017 compared the diagnostic performance of aspartate aminotransferase-to-platelet ratio index (APRI), fibrosis index-4 (FIB-4), fibrosis score for non-alcoholic fatty liver disease score (NFS), body mass index (BMI), aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (AST/ALT ratio), diabetes score (BARD score), FibroScan M probe, FibroScan XL probe, shear wave elastography (SWE), and magnetic resonance elastography (MRE) for staging significant fibrosis (SF), advanced fibrosis (AF), and cirrhosis in MASLD. This study concluded that MRE and SWE may provide better diagnostic accuracy for staging fibrosis in patients with MASLD, with the following results for the area under the receiver operating characteristic (AUROC) curve: SF: MRE: 0.88 and SEW: 0.89;: MRE: 0.93 and SEW: 0.91; and cirrhosis: MRE: 0.92 and SEW: 0.97 (8).
Similarly, a systematic review of 38 studies aimed to evaluate the common non-invasive tests, NFS, enhanced liver fibrosis (ELF), transient elastography, and MRE, in obese patients with SF, AF, and cirrhosis. Evidence showed better accuracy of complex biomarker panels: NFS: summary receiver operator characteristic (SROC): 0.79–0.81 vs. ELF: 0.96; however, the search focused only on studies published until 2016, in English, in four databases, and in individuals with obesity (9). Finally, a recent meta-analysis of 37 studies evaluated the individual diagnostic performance of liver stiffness measurement by vibration-controlled transient elastography (LSM-VCTE), FIB-4, and NFS to derive diagnostic strategies that could reduce the need for liver biopsies. The AUROC results of individual LSM-VCTE, FIB-4, and NFS for AF were 0.85, 0.76, and 0.73, respectively. However, only two invasive tests were included in just one stage of liver fibrosis (10).
Considering the growing body of evidence and lack of consensus on the diagnostic performance of clinical scores, this systematic review and meta-analysis aimed to assess the accuracy prognostic serological biomarkers (APRI, FIB-4, NFS, BARD score, FibroMeter, FibroTest, ELF, Forns score, and Hepascore) in predicting liver fibrosis severity in people with MASLD.
2. Materials and methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy Studies (PRISMA-DTA) guidelines (Supplementary Table S1) (11). The protocol for this meta-analysis was registered in the International Prospective Register of Systematic Reviews database (PROSPERO) under the number CRD42020180525.
2.1. Literature search strategy
This systematic review aimed to answer the following research questions: What is the diagnostic accuracy of the most clinically used serological biomarkers in predicting liver fibrosis severity in people with MASLD? The strategy was based on the participants, index tests, and target condition (PIT) criteria: P: adults ≥18 years with MASLD; I: APRI, FIB-4, NFS, BARD score, FibroMeter, FibroTest, ELF, Forns score, and Hepascore; and T: liver fibrosis. Liver biopsy was used as the reference standard.
We searched the following databases from their inception through December 2021: The Cochrane Hepato-Biliary Group Diagnostic Test Accuracy Studies Register; Medical Literature Analysis and Retrieval System Online (MEDLINE) [via Public/Publisher MEDLINE (PUBMED)]; Excerpt Medical dataBASE (EMBASE); Scientific Electronic Library Online (SciELO); Latin American and Caribbean Health Sciences Literature (LILACS); Cumulative Index to Nursing and Allied Health Literature (CINAHL); and Web of Science (WOS). The reference lists from eligible studies were manually searched to identify additional potentially relevant studies. In addition, we manually searched the abstracts of books from the American Association for the Study of Liver Diseases (AASLD) meetings and European Association for the Study of the Liver (EASL) meetings from the last 10 years. The MEDLINE search strategy was created and adapted for the other databases. There was no language or year of publication restrictions (Supplementary Text S1).
2.2. Eligibility criteria
The eligibility criteria were the PIT criteria described above. Studies were included if they defined liver fibrosis according to the histological classification of the Clinical Research Network (12), included at least 20 adult patients, and provided sensitivity (Sen), specificity (Spe), sample size, or enough information to obtain true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN).
Studies were excluded if participants had viral, autoimmune, or hepatic diseases and chronic hepatitis. Case series, experimental models, replies to letters, editorials, and duplicate publications were also excluded. Studies were considered duplicates if they belonged to the same study group and reported the same inclusion date and individual characteristics. In the case of duplicate studies, the one with the largest sample size was considered.
2.3. Selection of studies
Three review authors (SLT, PBR, and COA) independently selected the articles according to the eligibility criteria in two stages. The first selection stage consisted of screening the titles and abstracts of the articles identified through database searches. In the second stage, full-text articles were assessed using the same methodology. In the case of disagreement between the reviewers, a fourth reviewer (RM) assessed the articles according to the eligibility criteria to resolve any discrepancies.
2.4. Data extraction
Three authors (SLT, PBR, and COA) independently extracted the following data from the selected articles: first author; year of publication; type of paper; study design; study period; country; institution; number of participants; age (years); sex (percentage of males); race (percentages); BMI [kilograms (kg)/meters2 (m2)]; hypertension (percentage of participants); diabetes (percentage of participants); dyslipidemia (percentage of participants); MetS (percentage of participants); laboratory tests (AST, ALT, AST/ALT ratio, platelets, glycosylated hemoglobin (HbA1C), glycemia, triglycerides, and cholesterol); and score models (APRI, FIB-4, NFS, BARD score, FibroMeter, FibroTest, ELF, Forns score, and Hepascore). For diagnostic parameters, we considered cutoff values, AUROC, Sen, Spe, TP, FP, TN, and FN. When the authors did not describe TP, FP, TN, or FN, these were calculated based on the Sen and Spe and the number of participants in each study to obtain the values for each model.
2.5. Risk of bias assessment
Three authors (SLT, PBR, and COA) independently assessed the risk of bias in the primary studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) (13). QUADAS-2 is a tool for evaluating the quality of primary diagnostic studies by examining quality separately in terms of “risk of bias” and “concerns regarding applicability.” Risk of bias assessment items were organized into four domains: patient selection, index test, reference standard, and flow and timing. The applicability of a study was evaluated for the first three key domains and rated as “yes,” “no,” or “unclear,” where “yes” indicated a low risk of bias, “no” indicated a high risk of bias, and “unclear” indicated a lack of sufficient information (13). Disagreements were resolved by consulting a fourth reviewer (RM) to establish a consensus. The methodological quality of individual studies was visualized using the robvis web app, which depicts the plots obtained from these analyses (14).
2.6. Data synthesis and analysis
For inclusion in the meta-analysis, the score model should have been used in at least three studies in predicting liver fibrosis severity in people with MASLD. Diagnostic performance statistics were obtained for each study, including Sen, Spe, diagnostic odds ratio (DOR), positive likelihood ratio (LR+), and negative likelihood ratio (LR-), with their respective 95% confidence interval (95% CI). Then, for the DOR, LR+, and LR-, summarized meta-analytical estimates were obtained using a random effects model based on obtaining the variance between studies using the DerSimonian and Laird methods. Heterogeneity was evaluated using Cochran’s Q (Q) statistic and I2 statistic. The Cochran’s Q statistic of homogeneity was measured based on the null hypothesis that all eligible studies have the same underlying effect size. The I2 statistic, which represents the variability between studies, was 0–40%, 40–70%, and 70–100%, indicating low, moderate, and high variance, respectively (15, 16). In addition, summary area under the receiver operating characteristic (sAUROC) curve was obtained using a mixed linear model with known variance estimates according to Reitsma’s method. The area under curve (AUC) values were interpreted as follows: <0.5 indicated low accuracy, 0.6 to 0.79 indicated moderate accuracy, 0.8–0.90 showed good accuracy, and > 0.90 represented excellent accuracy (17). A sensitivity analysis was performed to assess whether the results changed when only studies that included the most frequently found scores, FIB-4, APRI, and NFS, and without any fibrosis severity (AF, SF and cirrhosis) were used. All calculations were performed with R version 4.1.3 and Rstudio version 2022.02.1 (Build 461) using the Meta-Analysis of Diagnostic Accuracy (MADA) version 0.5.10 package.1
The TP, FP, FN, and TN numbers were extracted to construct the 2×2 tables, and the values for each reported test cutoff were calculated. In some studies that did not have the numbers, the prevalence, sensitivity, specificity, and sample size were calculated.2
The diagnostic accuracy of the index tests was evaluated in the following dichotomized groups: any fibrosis (AnF) (F0 vs. F1-4), SF (F0-1 vs. F2-4), AF (F0-2 vs. F3-4), cirrhosis (F0-3 vs. F4).
3. Results
3.1. Identification and selection of studies
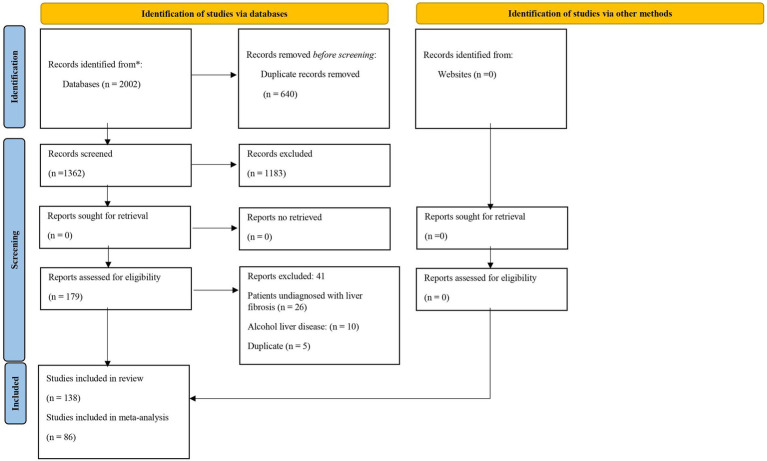
The search strategy identified 2002 articles. Of these, 640 articles were duplicates, leaving 1,362 for title and abstract assessment. At this stage, 1,183 articles were excluded: 353 on other populations with chronic hepatitis; 130 on patients on autoimmune medication; 74 on animal studies; 198 on alcoholic liver disease; and 428 that did not involve the evaluation or validation of model performance. One hundred and seventy-nine studies were read in full, of which 41 studies were excluded: 26 studies did not include patients diagnosed with hepatic fibrosis; 10 on alcoholic liver disease; and 5 duplicates. Thus, 138 articles were included in this systematic review, of which 86 were included in the meta-analysis and met the eligibility criteria in Figure 1.
Figure 1.
PRISMA 2020 flowchart of the study selection process.
3.2. Characteristics of the included studies
The characteristics of the studies included in the systematic review are described in Table 1. The articles were published between 2004 (123) and 2021 (29, 33, 153). The majority were cross-sectional (68%) (20–22, 27–29, 31–35, 40–44, 46–48, 50–55, 58, 59, 62, 65, 69, 70, 75, 78, 79, 83, 84, 86, 88, 90–93, 95, 97–100, 103–105, 108, 109, 111–114, 116, 117, 119–122, 124, 126–128, 130–136, 138, 140–143, 146, 147, 149–151, 156). Regarding the type of publication, 70.3% of the studies were full-text articles (18, 20, 22, 25, 26, 28–31, 33, 35, 37, 39–42, 44–50, 52, 53, 55, 57, 58, 60, 62, 63, 65, 66, 68, 70, 74, 75, 77–79, 82–88, 90–100, 103, 106, 108, 109, 111–122, 124, 126, 128, 130–135, 138, 140–143, 146, 147, 149–154), and the remaining 29.7% were conference abstracts (19, 21, 23, 24, 32, 34, 36, 38, 43, 51, 54, 56, 60, 61, 64, 67, 69, 71–73, 76, 80, 81, 89, 101, 102, 104, 105, 107, 110, 118, 123, 125, 127, 129, 137, 139, 144, 145, 155). Regarding the geographical origin of the studies, most studies were conducted in Europe (41%) (20, 25, 27, 28, 31, 35–37, 40–44, 47, 51–55, 58, 61, 62, 66, 69, 72, 73, 84–86, 88, 94, 97–100, 102, 103, 105–107, 110, 114, 117, 120, 122, 123, 125, 126, 138–141, 144, 153) and Asia (30%) (22, 29, 46, 59, 67, 70, 71, 74–76, 78, 79, 83, 91, 93, 95, 109, 111, 112, 118, 121, 130–132, 142, 143, 146–152, 154). The total study population consisted of 46,514 participants. The sample size ranged from 29 (46) to 3022 (28) patients. The mean age of the participants ranged from 30 to 67 years old. In 48% of the studies, the majority of participants were male (19, 20, 25, 27, 29–31, 35, 37, 40–42, 44, 46–48, 52–57, 61, 62, 66, 75, 78, 80, 83–86, 91, 93, 95, 97, 98, 103, 106, 107, 112, 114, 120–122, 124, 126, 132, 138, 140–143, 149–151, 153, 154, 156). The mean BMI ranged from 25 (46, 146, 151) to 52.9 (101) kg/m2.
Table 1.
Characteristics of studies included in the systematic review.
| References | Country/Region | Type of publication | No. patients | Age (SD) | Male % | BMI (SD) | Stage system | Fibrosis 0/1 | F1 | F0-F1-F2% | F2 | F2-F3-F4% | F3 | F3-F4% | F4 | Serological biomarkers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abe et al. (18) | Japan | Article | 289 | 54.8 ± 14 | 55 | 27.6 ± 4.7 | Brunt | 12.1 | 39.1 | 68.1 | 16.9 | 49.3 | 14.8 | 32.4 | 17.6 | FIB-4, APRI, NFS |
| Adams et al. (19) | Australia | Abstract | 119 | 48.7 ± 13 | 54 | ? | Kleiner and Brunt | 41.0 | ? | ? | ? | ? | ? | ? | ? | APRI, Hepa score, FibroTest |
| Adams et al. (20) | Australia/Italy | Article | 242 | 46.8 ± 12 | 60.3 | 30.2 ± 6 | Kleiner and Brunt | 35.9 | 23.9 | 78.0 | 18.1 | 40.1 | 12.3 | 22.0 | 9.5 | FIB-4, APRI, Hepa score, FibroTest, BARD score |
| Ahmed et al. (21) | United States | Abstract | 771 | ? | ? | ? | Batts Ludwig | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI |
| Aida et al. (22) | Japan | Article | 148 | 61 ± 12 | 36 | 26.9 ± 1.25 | Kleiner and Brunt | 18.9 | 34.4 | 71.5 | 18.2 | 46.4 | 16.8 | 28.2 | 11.4 | FIB-4, APRI |
| Alkhouri et al. (23) | United States | Abstract | 78 | 30 ± 9 | 32 | ? | ? | 35 | 42 | 80 | 13 | 23 | 10 | FIB-4, APRI, NFS | ||
| Anam et al. (24) | ? | Abstract | 40 | ? | ? | ? | Kleiner and Brunt | 40.9 | 27 | 80 | 12.1 | 32.1 | 10.7 | 20 | 9.3 | FIB-4, APRI, NFS, FibroMeter, BARD score |
| Angelidi et al. (25) | Greece | Article | 110 | 60.1 ± 9.5 | 52.7 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD score |
| Angulo et al. (26) | United States/United Kingdom/Italy/ Australia |
Article | 1,014 | 46.9 ± 0.4 | 58 | 31.3 ± 0.2 | Kleiner and Brunt | 34.6 | 24.7 | 73.2 | 13.9 | 40.5 | 15.8 | 26.6 | 10.8 | FIB-4, APRI, NFS, BARD score |
| Angulo et al. (27) | United States/United Kingdom/Italy/ Australia |
Article | 733 | 47.7 ± 13.2 | 52.2 | 32.3 ± 0. | Kleiner and Brunt | ? | 26.0 | 72.9 | 13.6 | 40.7 | 13.0 | 27.1 | 14.1 | NFS |
| Anstee et al. (28) | United States/Europe | Article | 3,202 | 57.5 ± 5.6 | 47 | ? | ? | 26 | 29 | 100 | 45 | 145 | 43 | 100 | 57 | FIB-4, NFS, ELF |
| Amernia et al. (29) | Iran | Article | 205 | 42.9 ± 10.9 | 70.2 | ? | ? | ? | 45.9 | 78.6 | 32.7 | 54.1 | 14.1 | 21.4 | 7.3 | FIB-4, APRI |
| Arora et al. (30) | United States | Article | 141 | 56 ± 4.3 | 65 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD score |
| Aykut et al. (31) | Turkey | Article | 88 | 46 ± 9 | 56 | 30.3 ± 4.6 | Kleiner and Brunt | 26.0 | 24.0 | 69.0 | 19.0 | 50.0 | 21.0 | 31.0 | 10.0 | NFS, FibroMeter |
| Balakrishnan et al. (32) | United States | Abstract | 122 | 47 ± 9 | 20 | 34 ± 7.5 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD score |
| Balakrishnan et al.(33) | United States | Article | 99 | 46.8 ± 11.5 | 26.3 | 32.4 ± 6.8 | Brunt | 46.3 | 38.3 | 90.7 | 44.4 | 63.6 | 8.1 | 19.2 | 11.1 | BARD score, FIB-4, APRI, NFS |
| Barritt et al. (34) | United States | Abstract | 859 | 57 ± 9 | 38 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | APRI, NFS |
| Boursier et al. (35) | France | Article | 588 | 55.9 ± 12 | 57.3 | 31.7 ± 5.8 | Kleiner and Brunt | 9 | 25.9 | 61.5 | 26.5 | 63.3 | 24.8 | 38.6 | 13.8 | FIB-4, APRI, NFS, FibroMeter, Hepa score, FibroTest, BARD score |
| Boursier et al. (36) | France | Abstract | 618 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | NFS, FibroMeter |
| Boursier et al. (37) | France | Article | 938 | 56.5 ± 12.1 | 58.5 | 31.8 ± 5.8 | ? | 9.5 | 22.8 | 69.2 | 26.9 | 57.7 | 27.4 | 30.8 | 13.4 | FIB-4, NFS, FibroTest, FibroMeter, Hepascore |
| Brandman et al. (38) | United States | Abstract | 1,483 | 50 ± 10 | 36 | ? | ? | ? | ? | ? | ? | ? | ? | 10 | ? | FIB-4, APRI, NFS, BARD score |
| Bril et al. (39) | United States | Article | 162 | 57 ± 9 | 82 | 34.7 ± 4.6 | Kleiner and Brunt | 25.1 | 41.7 | 83.5 | 16.5 | 33.1 | 12.5 | 16.5 | 3.9 | FibroTest |
| Broussier et al. (40) | France | Article | 283 | 56.5 ± 10 | 53.4 | 32.9 ± 6.6 | ? | ? | ? | ? | ? | ? | ? | 54.8 | ? | FIB-4, FibroMeter |
| Cales et al. (41) | France | Article | 235 | 51.1 ± 11 | 74.5 | 28.7 ± 4.9 | ? | 28.9 | 81.2 | 8.9 | 27.7 | 8.1 | 18.7 | 10.6 | APRI, NFS, FibroMeter | |
| Cales et al. (42) | France | Article | 226 | 50.9 ± 10.8 | 75.2 | 28.7 ± 4.9 | Kleiner and Brunt | 26.1 | 29.7 | 77.5 | 21.6 | 44.5 | 16.2 | 22.5 | 6.3 | NFS, FibroMeter |
| Cebreiros et al. (43) | Spain | Abstract | 55 | 43.9 ± 12 | 24.6 | 49.9 | Metavir | ? | ? | ? | ? | ? | ? | ? | ? | FibroMeter, ELF |
| Cengiz et al. (44) | Turkey | Article | 123 | 49 ± 11 | 56.1 | 29.5 ± 0.58 | Kleiner and Brunt | 64.2 | 86.2 | 22 | 35.8 | 8.9 | 13.8 | 4.9 | FIB-4, APRI | |
| Chan et al. (45) | Malaysia | Article | 147 | 50.5 ± 11 | 54.4 | 29.3 ± 4.5 | Kleiner and Brunt | 29.3 | 41.5 | 79 | 8.2 | 29.2 | 19 | 21 | 2 | NFS |
| Chowdhury et al. (46) | India | Article | 29 | 43 ± 4.9 | 75.8 | 25.1 ± 2.6 | Kleiner and Brunt | 41.3 | 20.6 | 77.5 | 10.3 | 37.9 | 6.8 | 27.5 | 20.6 | APRI |
| Cichoz-Lach et al. (47) | Poland | Article | 126 | 42.7 ± 13 | 57.9 | 28.5 ± 2.6 | Kleiner and Brunt | 26.1 | 35.7 | 78.5 | 16.6 | 38.0 | 19.0 | 21.0 | 2.3 | NFS, BARD score |
| Cui et al. (48) | United States | Article | 102 | 51.3 ± 14 | 58.8 | 31.7 ± 5.5 | Kleiner and Brunt | 47.1 | 25.5 | 81.4 | 8.8 | 21.5 | 12.7 | 18.6 | 5.9 | FIB-4, APRI, NFS, BARD score |
| de Carli et al. (49) | Brazil | Article | 324 | 38.7 ± 10.7 | 34.5 | 43.8 ± 4.8 | Kleiner and Brunt | ? | 40.8 | 91.1 | 4.3 | 13.2 | 8.6 | 8.9 | 0.3 | FIB-4, APRI, NFS, BARD Score |
| de Cleva et al. (50) | Brazil | Article | 131 | 45.8 ± 11 | ? | 47.8 ± 6.3 | Kleiner and Brunt | 56.5 | 29 | 92.3 | 6.8 | 14.4 | 3.8 | 7.6 | 3.8 | APRI |
| Demir et al. (51) | Germany | Abstract | 323 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | NFS, BARD score |
| Demir et al. (52) | Germany | Article | 165 | 44.8 ± 12 | 60 | 28.6 ± 4.3 | Kleiner and Brunt | 3.6 | 49.0 | 87.6 | 35.1 | 47.1 | 9.6 | 12.0 | 2.4 | FIB-4, NFS, BARD score |
| Dincses et al. (53) | Turkey | Article | 52 | 45 ± 9 | 57.6 | 30.8 ± 5.4 | Kleiner and Brunt | ? | ? | 81 | ? | 38 | ? | 19 | ? | NFS, FibroMeter |
| Drolz et al. (54) | Germany | Abstract | 101 | 54 ± 10 | 54 | 29 ± 1.8 | ? | ? | 25.7 | 45.5 | 19.8 | 53.4 | 13.8 | 33.6 | 19.8 | FIB-4, APRI, NFS, BARD Score |
| Dvorak et al. (55) | Czech Republic | Article | 56 | 44.1 ± 15 | 70 | 30 ± 3.7 | Matteoni | ? | 51.7 | 17.8 | 48.0 | 16 | 30.2 | 14.2 | FIB-4, APRI, NFS, ELF, BARD score | |
| Eddowes et al. (56) | ? | Abstract | 356 | 53 ± 12 | 57 | 34.4 ± 6.5 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, NFS, FibroMeter |
| Fagan et al. (57) | Australia | Article | 329 | 45.9 ± 11 | 64.1 | ? | Metavir | ? | ? | ? | ? | ? | ? | 23.7 | ? | ELF |
| Francque et al. (58) | Belgium | Article | 542 | 43.5 ± 12.7 | 28.6 | 38.2 ± 6.4 | Kleiner and Brunt | 64.2 | 16.3 | ? | 12.1 | ? | 7.0 | ? | 0.2 | FIB-4, APRI, NFS, Forns score, BARD score |
| Fujii et al. (59) | Japan | Article | 50 | 55.8 ± 15.2 | 26 | 27.1 ± 3.8 | Kleiner and Brunt | ? | 28.0 | 56.0 | 28.0 | 54.0 | 26.0 | 44.0 | 18.0 | APRI |
| Fujii et al. (60) | Japan | Abstract | 122 | 59 ± 15.3 | 39 | ? | Kleiner and Brunt | ? | ? | 55.0 | ? | ? | ? | 38.0 | ? | BARD score |
| Gallego-Duran et al. (61) | Spain | Abstract | 49 | 49 ± 13 | 61 | ? | Kleiner and Brunt | ? | ? | ? | ? | 79.0 | ? | ? | ? | NFS, FibroTest |
| Guha et al. (62) | United Kingdom | Article | 192 | 48.7 ± 12.5 | 64 | 32.4 ± 5.7 | Kleiner and Brunt | 16.1 | 19.0 | 77.0 | 17.0 | 40.0 | 13.0 | 23.0 | 10.0 | ELF |
| Guillaume et al. (63) | France | Article | 417 | 56.1 ± 1,211 | 59.2 | 33.3 ± 6.6 | Kleiner and Brunt | 29 | 23.5 | 67.4 | 27.3 | ? | 32.4 | 40.1 | 7.7 | FibroMeter, ELF |
| Guturu et al. (64) | United States | Abstract | 118 | ? | ? | ? | Batts Ludwig | ? | 39.8 | 75.3 | 19.4 | 43.9 | 8.4 | 24.5 | 16.1 | APRI, BARD score |
| Harrison et al. (65) | United States | Article | 827 | 49 ± 5.6 | 49 | 33 | Kleiner and Brunt | ? | 24.0 | ? | 80.8 | ? | ? | ? | ? | BARD score |
| Hagström et al. (66) | Sweden | Article | 646 | 50 ± 14.8 | 62 | 28 ± 3.7 | Kleiner | 65 | 40 | 88 | 23 | 35 | 9 | 11 | 3 | NFS, BARD score, APRI, FIB-4 |
| Huang et al. (67) | Singapore | Abstract | 161 | 60 ± 14 | ? | 26.8 ± 4.6 | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD score |
| Inadomi et al. (68) | Japan | Article | 200 | 595 ± 17 | 48 | 28.1 ± 6.8 | Kleiner and Brunt | ? | 37.5 | 76 | 22 | 58.5 | 32 | 36.5 | 4.5 | FIB-4, ELF |
| Isgro et al. (69) | Italy | Abstract | 74 | 44.3 ± 4.9 | ? | ? | ? | 8.1 | 45.8 | 93.2 | 39.2 | 46 | 5.4 | 6.8 | 1.4 | ELF |
| Itoh et al. (70) | Japan | Article | 400 | 56 ± 20 | 48.7 | 27.3 ± 9.8 | Kleiner and Brunt | 16.7 | 45.7 | 76.1 | 13.7 | 37.5 | 15.7 | 23.7 | 8 | ELF |
| Joo et al. (71) | Korea | Abstract | 315 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, NFS, BARD score |
| Joo et al. (72) | United Kingdom | Abstract | 116 | 54.3 ± 10.7 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4 |
| Jouness et al. (73) | Italian | Abstract | 254 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, NFS |
| Kao et al. (74) | Taiwan | Article | 73 | 35.2 ± 7.7 | 31.5 | 41.2 ± 5.6 | ? | ? | ? | ? | ? | 22.8 | ? | 11.4 | ? | FIB-4, APRI, NFS |
| Kawamur et al. (75) | Japan | Article | 90 | 51.2 ± 5.9 | 55.5 | 26, 1 | Kleiner and Brunt | ? | 47.7 | 61 | 13.3 | 52.1 | 33.3 | 38.8 | 5.5 | FIB-4, APRI |
| Kim et al. (76) | Korea | Abstract | 481 | ? | ? | ? | Metavir | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD score |
| Kim et al. (77) | United States | Article | 142 | 52.8 ± 12 | 26.8 | 36.3 ± 7.4 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD Score |
| Kobayashi et al. (78) | Japan | Article | 140 | 56 ± 6.8 | 54.3 | 27.1 ± 4 | Matteoni | 7.1 | 44.3 | 74.3 | 22.9 | 48.6 | 21.4 | 25.7 | 4.3 | FIB-4, APRI |
| Kolhe et al. (79) | India | Article | 100 | 47 ± 12.3 | 49 | ? | Metavir | ? | ? | 73 | ? | ? | ? | 27 | ? | FIB-4, APRI |
| Kosick et al. (80) | Canada | Abstract | 541 | 50.5 ± 13 | 56.5 | 32.3 ± 5.5 | ? | ? | ? | ? | ? | ? | ? | ? | 45.5 | FIB-4, APRI, NFS, BARD score |
| Kruger et al. (81) | United States | Abstract | 111 | ? | ? | ? | Kleiner and Brunt | 50.0 | ? | ? | ? | ? | ? | ? | ? | APRI, NFS |
| Kruger et al. (82) | South Africa | Article | 111 | 52 ± 10 | ? | ? | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | ? | 17.0 | APRI, NFS |
| Kumar et al. (83) | India | Article | 120 | 39.1 ± 12 | 75 | 26.1 ± 3.6 | Kleiner and Brunt | 26.6 | 28.3 | 77.4 | 22.5 | 44.8 | 14.1 | 22.3 | 8.3 | FIB-4, APRI, NFS, BARD score |
| Labenz et al. (84) | Germany | Article | 261 | 51 ± 18.5 | 52.5 | 30.9 ± 6.9 | Kleiner and Brunt | 15.5 | 43.6 | 84.3 | 40.9 | ? | ? | ? | 15.7 | FIB-4, APRI, NFS |
| Lambrecht et al. (85) | Germany | Article | 2088 | 54.5 ± 11.5 | 64.5 | 28.6 ± 5.2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI |
| Lang et al. (86) | Germany | Article | 96 | 57 ± 14.6 | 53 | 31 ± 6.9 | Kleiner and Brunt | ? | 30.8 | ? | 67.7 | 130.4 | 44.4 | 63.1 | 18.7 | FIB-4, NFS |
| Lardi et al. (87) | Brazil | Article | 73 | ? | 636 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | FibroTest |
| Lassailly et al. (88) | France | Article | 288 | 41.6 ± 12 | 33.6 | 48.6 ± 8 | Metavir | 59.0 | 34.0 | 97.5 | 4.5 | 6.9 | 0.7 | 2.4 | 1.7 | FibroTest |
| Le et al. (89) | ? | Abstract | 254 | 50.3 ± 10.5 | 35.4 | 34.2 ± 6 | Metavir | ? | ? | ? | ? | 44 | ? | 23 | ? | FIB-4, APRI, BARD Score |
| Lee et al. (90) | United States | Article | 107 | 48.9 ± 23 | 38.3 | 35.9 ± 3.7 | ? | 20.5 | 18.6 | 68.0 | 28.9 | 48.14 | 16.8 | 32.0 | 14.9 | FIB-4, NFS, FibroMeter, BARD Score |
| Liu et al. (91) | China | Article | 349 | 40.2 ± 12.5 | 76.5 | 26.8 ± 3.3 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4 |
| Loaeza-del-Castill et al. (92) | Mexico | Article | 30 | 43 ± 12 | 43 | ? | Metavir | 26.0 | 33.0 | 71.5 | 40.0 | 51.5 | 10.0 | 10.0 | 0.0 | APRI |
| Loong et al. (93) | China | Article | 215 | 52 ± 4 | 55.3 | 26.8 ± 1.3 | ? | ? | ? | ? | 40.9 | 27 | 80 | 12.1 | 32.1 | FibroMeter |
| Luger et al. (94) | Austria | Article | 46 | 42 ± 13 | 20 | 43.8 ± 4.3 | Kleiner and Brunt | ? | ? | ? | ? | 30 | ? | 13 | ? | FIB-4, NFS |
| Mahadeva et al. (95) | Malaysia | Article | 131 | 49.9 ± 12 | 52.7 | ? | Kleiner and Brunt | 40.8 | ? | ? | 35.1 | ? | 35.1 | ? | 6.1 | APRI, NFS |
| Marella et al. (96) | United States | Article | 907 | 46.7 ± 12 | 32.6 | 39.9 ± 6 9 | Kleiner and Brunt | 32.9 | 36.4 | 87.2 | 17.9 | 30.7 | 6.9 | 12.8 | 5.9 | FIB-4, APRI, NFS |
| McPherson et al. (97) | United Kingdom | Article | 145 | 51 ± 12 | 61 | 35 ± 5 | Kleiner and Brunt | 25.0 | 43.0 | 78.0 | 13.0 | 29.0 | 10.0 | 19.0 | 9.0 | FIB-4, APRI, NFS, BARD score |
| McPherson et al. (98) | United Kingdom/ Belgium/France |
Article | 634 | 49.8 | 54.8 | 34 ± 4.5 | Kleiner and Brunt | 37.4 | 23.2 | ? | 14.2 | ? | 17 | ? | 8.2 | FIB-4, APRI, NFS |
| McPherso et al. (99) | United Kingdom | Article | 305 | 51 ± 12 | 60 | 33.6 ± 4.7 | Kleiner and Brunt | ? | ? | 80.5 | ? | 37.5 | ? | 20.5 | ? | FIB-4, NFS |
| Meneses et al. (100) | Spain | Article | 50 | 49 ± 8 | 30 | 44.3 ± 5 | Kleiner and Brunt | 60 | 22 | 94 | 12 | 18 | 6 | 6 | 0 | FIB-4, APRI, NFS, Forns score, BARD score |
| Miao et al. (101) | United States | Abstract | 686 | ? | ? | 52.9 ± 9.7 | ? | ? | ? | ? | ? | 12.3 | ? | 3.1 | ? | FIB-4, NFS, BARD score |
| Miele et al. (102) | Italy | Abstract | 82 | 46 ± 12 | ? | ? | ? | 7.3 | 39 | 81.7 | 35.4 | 53.7 | ? | 18.3 | ? | ELF |
| Miele et al. (103) | Italy | Article | 82 | 46 ± 9 | 62 | 28 ± 22–38 | ? | 7.3 | 39 | 82.7 | 35.4 | 53.7 | 6.1 | 18.3 | 12.2 | ELF |
| Miller et al. (104) | United States | Abstract | 354 | 50 ± 13 | 42.7 | 33.9 ± 8.5 | ? | ? | ? | ? | 73.7 | ? | ? | 26.3 | FIB-4, APRI, NFS | |
| Miller et al. (105) | United Kingdom | Abstract | 42 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, NFS, BARD score |
| Munteanu et al. (106) | France/Italy/Brazil/ United Kingdom/Austria/Greece/ Spain |
Article | 600 | 53.2 ± 24 | 63.3 | 29.7 ± 0.25 | Kleiner and Brunt | 20.3 | 30.8 | ? | 23.3 | ? | 20.2 | ? | 5.5 | FIB-4, NFS, FibroTest, BARD score |
| Nascimben et al. (107) | France | Abstract | 884 | 55 ± 12 | 61 | 30 ± 5 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD score |
| Nassif et al. (108) | Brazil | Article | 298 | 40.1 ± 8 | 11.1 | 43.6 ± 10 | ? | ? | ? | ? | ? | ? | 7.3 | ? | ? | BARD score |
| Okajima et al. (109) | Japan | Article | 163 | 55.8 ± 14 | 49.5 | 27.2 ± 4.3 | ? | 38 | 34.4 | 86.5 | 14.1 | 26.5 | 8 | 12.5 | 5.5 | FIB-4, APRI |
| Pastor-Ramire et al. (110) | Spain | Abstract | 1,256 | 54.1 ± 14 | 46 | ? | ? | ? | ? | ? | 57.7 | ? | ? | ? | ? | FIB-4, APRI, NFS, BARD score |
| Pathik et al. (111) | India | Article | 110 | 42.3 ± 3.2 | ? | 29.1 | ? | ? | ? | ? | ? | ? | ? | 34.5 | ? | APRI, NFS |
| Peleg et al. (112) | Israel | Article | 153 | 51.8 ± 17 | 55.5 | 29.9 ± 1.6 | Metavir | ? | ? | 79.1 | ? | ? | ? | 20.9 | ? | FIB-4, APRI |
| Pérez-Gutiérrez et al. (113) | Mexico/Chile | Article | 228 | 48.6 ± 12 | 49 | ? | Kleiner and Brunt | 81.6 | 25.0 | 88.2 | 6.6 | 18.4 | 7.0 | 11.8 | 4.8 | FIB-4, APRI, NFS, BARD score |
| Petta et al. (114) | Italy | Article | 321 | 44.6 ± 12 | 67.5 | 29.3 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | 22.9 | ? | FIB-4, NFS |
| Petta et al. (115) | Italy. Hong Kong. France | Article | 741 | 50.9 ± 12.7 | 60.2 | 29.6 ± 4.9 | Kleiner and Brunt | ? | ? | ? | ? | 34.3 | ? | 30.9 | ? | FIB-4, NFS |
| Pimentel et al. (116) | Brazil | Article | 158 | 36 ± 10 | 22.7 | 41 ± 5 | ? | ? | 7.5 | 30.3 | 85.9 | 48.1 | 61.9 | 12.0 | 13.8 | NFS |
| Polyzos et al. (117) | Greece | Article | 31 | 53.3 ± 2.7 | 25.8 | 32.2 ± 1.4 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | 22.5 | APRI, NFS, ELF, FIB-4 | |
| Prasad et al. (118) | India | Abstract | 240 | 39.3 ± 10 | ? | ? | ? | ? | ? | ? | ? | ? | ? | 4 | ? | FIB-4, APRI, NFS |
| Qureshi et al. (119) | United States | Article | 401 | 40.5 ± 8.5 | 17 | 48.4 ± 7.2 | Kleiner and Brunt | 43.4 | 40.0 | 35.9 | 86.5 | 13.8 | 27.3 | 11.4 | 13.5 | NFS |
| Raszeja-Wyszomirska et al. (120) | Poland | Article | 104 | 48 ± 12 | 65.4 | 29.6 ± 3 | Kleiner and Brunt | ? | ? | 84.6 | ? | ? | ? | 14.4 | BARD score | |
| Rath et al. (121) | India | Article | 60 | 39.7 ± 9.6 | 85 | 26.4 ± 3.3 | Kleiner and Brunt | 31.6 | 28.3 | 96.7 | 36.6 | 66 | 3.3 | 3.3 | 0 | APRI, NFS, BARD score |
| Ratziu et al. (122) | France | Article | 267 | 50.75 ± 9.4 | 58 | > 27 | Kleiner and Brunt | 58.2 | 36.0 | 79.0 | 19.0 | 28.0 | 5.0 | 5.0 | 0 | FibroTest |
| Ratziu et al. (123) | France | Abstract | 89 | ? | ? | ? | Kleiner and Brunt | 36.0 | ? | ? | ? | 45, 0 | ? | 11, 0 | ? | FibroTest |
| Ruffillo et al. (124) | Argentina | Article | 138 | 49 ± 5.6 | 67 | 30, 3 | Kleiner and Brunt | 5.0 | 6.5 | 76.9 | 61.5 | 88.4 | 23.1 | 26.8 | 3.6 | NFS, BARD score |
| Saez et al. (125) | Spain | Abstract | 78 | 54.2 ± 11 | 39.7 | ? | ? | ? | ? | ? | ? | 55, 1 | ? | ? | ? | APRI, NFS, BARD score |
| Sebastiani et al. (126) | France/Italy | Article | 190 | 51.2 ± 13 | 74.7 | 28.9 ± 5 | Kleiner and Brunt | 49.0 | 36.3 | 74.7 | 26.3 | 51.6 | 11.6 | 25.3 | 13.7 | APRI, FibroTest |
| Seth et al. (127) | United States | Abstract | 137 | 47 ± 11 | 22 | 32 ± 6.7 | ? | ? | ? | ? | ? | ? | ? | 40 | ? | FIB-4, APRI, NFS, BARD score |
| Shah et al. (128) | United States | Article | 541 | 47.5 ± 12 | 40 | 34.7 ± 6.5 | Kleiner and Brunt | ? | ? | 76.8 | ? | ? | ? | 23.1 | ? | FIB-4 |
| Shaheen et al. (129) | Canada | Abstract | 44 | 51.5 ± 6.6 | ? | ? | ? | ? | ? | ? | ? | ? | ? | 32 | ? | FIB-4, APRI, NFS |
| Shima et al. (130) | Japan | Article | 278 | 57.8 ± 14.8 | 48.2 | 27.5 ± 4.7 | Kleiner and Brunt | 34.1 | 23.3 | 72.1 | 14.7 | 42.4 | 23 | 27.6 | 4.6 | FIB-4, APRI |
| Shoji et al. (131) | Japan | Article | 197 | 60 ± 14 | 45.1 | 27.5 ± 6.2 | Kleiner and Brunt | 40.6 | ? | 63.9 | 23.3 | 59.3 | 20.8 | 36 | 15.2 | FIB-4, APRI, NFS, BARD score |
| Shukla et al. (132) | India | Article | 51 | 50.4 ± 11 | 53 | ? | Kleiner and Brunt | ? | ? | 78.4 | ? | ? | ? | 21.6 | ? | FIB-4 |
| Siddiqui et al. (133) | United States | Article | 145 | 52.9 ± 11 | 37.7 | 35.8 ± 19 | Kleiner and Brunt | 29 | 29 | 64.9 | ? | ? | ? | 35.2 | 7.6 | FIB-4, APRI, NFS, FibroMeter, BARD score |
| Siddiqui et al. (134) | United States | Article | 1904 | 50.3 ± 12.2 | 47 | 34.4 ± 6.4 | Kleiner and Brunt | 24 | 28 | 72 | 20 | 48 | 20 | 28 | 8 | FIB-4, APRI, NFS |
| Simo et al. (135) | United States | Article | 225 | 43.2 ± 9.6 | 14.7 | 44.6 ± 5.4 | Kleiner and Brunt | ? | 58.2 | 21.8 | 93.4 | 13.3 | 19.9 | 6.2 | 6.6 | NFS |
| Singh et al. (136) | United States | Article | 1,157 | 51.1 ± 11.5 | 35.4 | 35.5 ± 8.1 | Kleiner and Brunt | ? | ? | 68.2 | ? | ? | ? | 38.1 | ? | FIB-4, APRI, NFS |
| Singh et al. (137) | ? | Abstract | 1969 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 7 | ? | FIB-4, APRI, NFS, BARD score |
| Sjowall et al. (138) | Sweden | Article | 82 | 59.8 ± 11 | 67 | 28.9 ± 4.4 | Kleiner and Brunt | ? | ? | ? | ? | ? | ? | 17 | ? | APRI, NFS, BARD score |
| Stauber et al. (139) | Austria | Abstract | 122 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 28 | ? | ELF |
| Staufer et al. (140) | Austria | Article | 186 | 52 ± 5.2 | 57 | 30.5 ± 2.7 | Kleiner and Brunt | ? | ? | 61.8 | 55 | 27 | ? | FIB-4, FibroMeter, ELF | ||
| Subasi et al. (141) | Turkey | Article | 142 | 45 ± 9 | 52.8 | 30.9 ± 5 | Kleiner and Brunt | 28.2 | 35.2 | 78.9 | 15.5 | 36.6 | 14.1 | 21.1 | 7 | FIB-4, APRI, NFS, FibroMeter, BARD Score |
| Sumida et al. (142) | Japan | Article | 576 | 52.3 ± 15 | 51 | 27.9 ± 4.9 | Kleiner and Brunt | 45.6 | 29.3 | ? | 13.8 | 24.9 | 7.8 | 11.1 | 3.2 | FIB-4, APRI, NFS, BARD score |
| Takeuchi et al. (143) | Japan | Article | 71 | 50.8 ± 15.7 | 64.8 | 29.1 ± 5.1 | Kleiner and Brunt | 8 | 17 | 39 | 14 | 46 | 27 | 32 | 5 | FIB-4 |
| Tanwar et al. (144) | United Kingdom | Abstract | 177 | ? | ? | ? | Kleiner and Brunt | 59.0 | 19.2 | 75.7 | 17.5 | 23.8 | 13.6 | 23.8 | 10.2 | FIB-4, APRI, NFS, ELF, BARD score |
| Thanapirom et al. (145) | ? | Abstract | 92 | 49.6 ± 13.7 | 44.9 | 27.4 ± 5.1 | ? | 97.8 | ? | 100 | 2, 2 | ? | ? | ? | ? | FIB-4, APRI |
| Tomeno et al. (146) | Japan | Article | 106 | 67 ± 7.8 | 41.5 | 25.8 ± 3.1 | ? | ? | 52.8 | 10.3 | 21.6 | 36.6 | 11.3 | 15 | 3.7 | FIB-4 |
| Treeprasertsuk et al. (147) | Thailand | Article | 139 | 40.9 ± 13 | 47 | 36.1 ± 14.7 | ? | ? | ? | 93.5 | ? | ? | ? | 6.4 | ? | FIB-4, NFS, BARD score |
| Uy et al. (148) | Philippines | Abstract | 61 | 46 ± 11 | 46 | 29.1 ± 4.3 | ? | ? | ? | ? | ? | ? | ? | 9, 8 | ? | FIB-4, APRI, BARD Score |
| Wong et al. (149) | China | Article | 246 | 51 ± 11 | 54.9 | 28 ± 4.5 | Kleiner and Brunt | 28.4 | 30.4 | 77.3 | 18.2 | 40.9 | 12.6 | 22.7 | 10.1 | FIB-4, APRI, NFS, BARD score |
| Xun et al. (150) | China | Article | 152 | 37.1 ± 9.7 | 79.6 | 26.1 ± 3.3 | Kleiner and Brunt | 31.6 | 33.5 | 84.0 | 19.1 | 34.9 | 13.8 | 15.8 | 1.9 | FIB-4, APRI, NFS, BARD score |
| Yang et al. (151) | China | Article | 453 | 36.5 ± 16.7 | 58.9 | 25.9 ± 3.6 | Kleiner and Brunt | ? | ? | 72, 2 | ? | ? | ? | 27.8 | ? | FIB-4, APRI, NFS, FibroMeter, Forns score, BARD score |
| Yoneda et al. (152) | Japan | Article | 235 | 59.9 ± 12 | ? | 26.9 ± 4 | Kleiner and Brunt | 38.7 | 27.6 | 83.8 | 17.4 | 33.6 | 8.9 | 16.2 | 7.2 | FIB-4, NFS, BARD Score |
| Younes et al. (153) | Italy, United Kingdom, and Spain | Article | 1,173 | 40 ± 14.1 | 64.7 | 29.4 ± 7.5 | Kleiner and Brunt | APRI, NFS, FIB-4, BARD score, Hepascore | ||||||||
| Zhou et al. (154) | China | Article | 207 | 41.8 | 73.4 | ? | ? | ? | 47.8 | 38.2 | 96.1 | 10.1 | 14 | 3.9 | 3.9 | FIB-4, APRI, NFS, BARD score |
| Zou et al. (155) | China | Abstract | 107 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 28 | FIB-4, APRI, NFS, BARD score |
APRI, aspartate aminotransferase-to-platelet ratio index; ELF, enhanced liver fibrosis; FIB-4, fibrosis index-4; LR+, positive likelihood ratio; LR-, negative likelihood ratio; NFS, non-alcoholic fatty liver disease score; SD, standard deviation;?, not responded.
3.3. Serological biomarkers
The 138 included studies evaluated the nine serological biomarkers (FIB-4; FibroMeter; ELF; NFS; BARD; Hepascore; APRI; FibroTest; Forns score) for liver fibrosis. The most described was the FIB-4, in 89 studies (20–26, 28, 29, 32, 33, 35, 37, 38, 40, 44, 48, 49, 52, 54–56, 58, 66, 67, 71–75, 77–80, 83–86, 89–91, 94, 97–101, 104–107, 109, 110, 112–115, 118, 127–134, 136, 137, 140, 141, 143–146, 148–154), followed by the NFS score in 87 studies (22–27, 30–38, 41, 42, 47–49, 51–56, 58, 61, 66, 67, 71, 73, 74, 76, 77, 80–84, 86, 90, 95, 97–101, 104–107, 110, 111, 113–119, 121, 124, 125, 127, 129, 131, 133–138, 141, 144, 147, 149–154) and the APRI in 80 studies (19–21, 23–26, 29, 30, 32–35, 37, 38, 41, 42, 44, 46, 48, 49, 54, 55, 58, 59, 64, 66, 67, 74, 75, 77, 79–85, 89, 92, 95, 97, 100, 104, 105, 107, 110–113, 118, 121, 125–127, 129–131, 133, 134, 136–138, 141, 144, 145, 148–151, 153, 154). The least used were the ELF in 14 studies (46, 58, 60, 65, 66, 71–73, 105, 106, 120, 142, 143, 147), the Forns score in three studies (58, 100, 151), and the Hepascore in four studies (19, 20, 35, 153). The stage system used to perform the biopsy in most studies was the Kleiner and Brunt system in 55% of the studies (19, 20, 22, 24, 26, 27, 31, 32, 35, 39, 42, 44–50, 52, 53, 56, 58–63, 65, 68, 70, 75, 76, 81–84, 86, 91, 94–100, 106, 107, 113, 114, 117, 119–124, 126, 128, 130–136, 138, 140–144, 149–153). Regarding the severity of fibrosis, AF was the most diagnosed, with 182 studies (20–26, 28, 29, 32, 33, 35, 37, 38, 40, 44, 48, 49, 52, 54–56, 58, 66, 67, 71–75, 77–80, 83–86, 89–91, 94, 97–101, 104–107, 109, 110, 112–115, 118, 127–134, 136, 137, 140, 141, 143–154), followed by SF, with 140 studies (22–25, 30, 35, 36, 38, 41, 42, 47–49, 51, 52, 54, 55, 58, 61, 71, 73, 74, 76, 77, 81–84, 86, 90, 95, 98–101, 105–107, 110, 113, 115, 116, 119, 121, 124, 127, 129, 131, 134–136, 138, 144, 147, 150–152), then by any type of liver fibrosis (107, 112, 114, 120–122, 124, 126, 132, 138, 140–143, 149–151, 153, 154) and cirrhosis (8, 18, 19, 22, 26, 40, 47, 52, 92, 105, 111, 113, 116, 119, 128, 131, 154) in 18 and 16 studies, respectively (Supplementary Text S2 and Supplementary Tables S2, S3). The serological biomarker cutoff values for each severity level have been described in more detail in (Supplementary Table S4).
Table 1. Characteristics of the studies included in the systematic review.
3.4. Analysis of the quality and risk of bias in the included studies
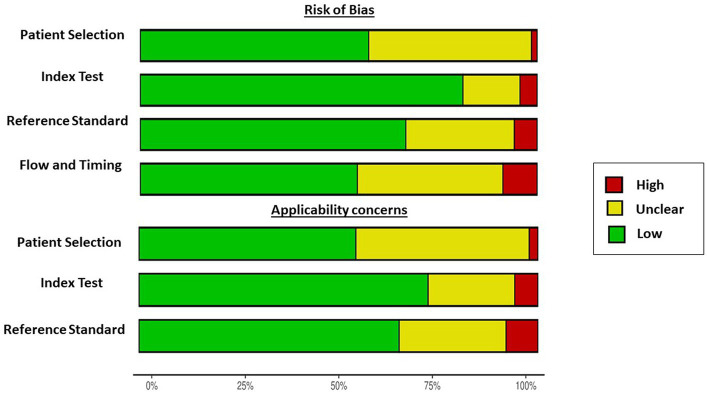
The quality assessment was performed using the QUADAS-2 tool as shown in Figure 2. Studies with patients with MASLD and other morbid conditions were considered a high applicability concern due to the consecutive or random sample of patients enrolled, a case–control design, and inappropriate inclusions such as populations with diabetes, obesity, high levels of transaminases, and selected age.
Figure 2.
Graphical summary of the risk of bias of the included studies using the QUADAS-2 tool.
The risk of bias was unclear in 41% of the studies regarding patient selection (18, 19, 21, 22, 24, 31, 36–38, 51, 56, 59, 61, 72, 73, 76, 83, 85, 88–90, 94, 105, 107, 111, 119, 120, 125, 139, 142, 148, 152, 155, 157). Concerning the reference standard of the studies, several studies did not describe whether all patients received the reference standard and whether all patients were included in the studies, and therefore, 27% of the studies were unclear about the risk of bias (20, 25, 30, 34, 35, 46, 49, 57, 59, 66, 74, 79, 80, 86, 91, 93, 98, 100, 106–109, 115, 116, 118, 123, 129, 130, 138, 142–145, 149, 150, 154, 158). Most of the studies described the pre-specified thresholds (Supplementary Tables S5, S6).
3.5. Meta-analysis results
For inclusion in the meta-analysis, the score model should have been used in at least three studies in predicting liver fibrosis severity in people with MASLD. Only seven scores (APRI, FIB-4, NFS, BARD score, FibroMeter, FibroTest, and ELF) were used in at least three studies to evaluate the four degrees of liver fibrosis severity (AnF, SF, AF, and cirrhosis) and were therefore meta-analyzed (Supplementary Figure S1).
3.6. APRI
The APRI serological biomarker was evaluated for diagnostic accuracy in detecting AnF (> F1) (3 studies), SF (≥ F2–F4) (14 studies), AF (≥ F3) (33 studies), and cirrhosis (F4) (3 studies) (Supplementary Table S7).
3.6.1. Diagnosis of AnF (F0 vs. F1–F4)
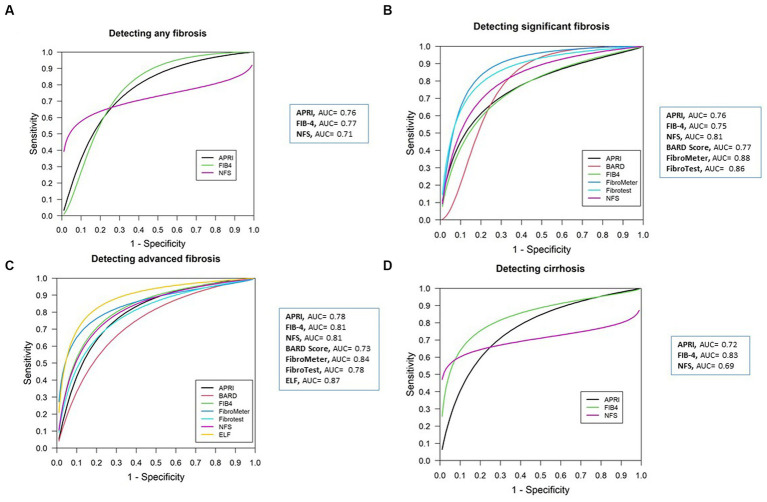
The DOR of the APRI in the diagnosis of AnF was 5.61 (95% CI 4.61–6.82), the LR+ was 2.18 (95% CI 1.63–2.91), the LR- was 0.35 (95% CI 0.22–0.56), and moderate heterogeneity was detected (Q = 1.04, p = 0.59, I2 = 64.35%) (Table 2; Supplementary Figures S2, S3). The sAUROC had a moderate diagnostic accuracy of 0.76, Sen of 77% (95% CI 61–88%), and Spe of 64% (95% CI 48–78%) (Figure 3A, Supplementary Table S7, and Supplementary Figure S1).
Table 2.
Comparison of serological biomarkers in predicting liver fibrosis severity in people with MASLD: DOR; LR+, and LR−.
| DOR | (95% CI) | Cochran’s Q | p | I 2 | LR+ | (95% CI) | LR- | (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| APRI | |||||||||
| Any fibrosis | 5.61 | (4.61–6.82) | 1.04 | 0.59 | 64.35 | 2.18 | (1.63–2.91) | 0.35 | (0.22–0.56) |
| Significant fibrosis | 6.29 | (4.47–8.92) | 16.13 | 0.24 | 19.4 | 2.69 | (2.23–3.23) | 0.48 | (0.40–0.58) |
| Advanced fibrosis | 6.45 | (4.83–8.60) | 42.78 | 0.009 | 25.21 | 2.96 | (2.49–3.52) | 0.50 | (0.43–0.57) |
| Cirrhosis | 6.21 | (4.34–8.89) | 1.71 | 0.42 | 0 | 3.11 | (2.15–4.50) | 0.53 | (0.31–0.89) |
| FIB-4 | |||||||||
| Any fibrosis | 6.57 | (4.56–9.48) | 5.35 | 0.25 | 25.24 | 2.32 | (1.94–2.77) | 0.38 | (0.29–0.49) |
| Significant fibrosis | 5.75 | (4.11–8.05) | 18.26 | 0.19 | 23.33 | 2.51 | (2.07–3.05) | 0.50 | (0.43–0.59) |
| Advanced fibrosis | 10.43 | (7.25–15.02) | 33.1 | 0.83 | 0 | 4.09 | (3.33–5.02) | 0.45 | (0.39–0.52) |
| Cirrhosis | 14.95 | (9.96–22.44) | 4.16 | 0.24 | 27.88 | 4.66 | (2.41–9.02) | 0.38 | (0.19–0.78) |
| NFS | |||||||||
| Any fibrosis | 4.85 | (3.32–7.09) | 6.63 | 0.15 | 39.66 | 2.27 | (1.86–2.78) | 0.49 | (0.42–0.57) |
| Significant fibrosis | 9.45 | (5.17–17.5) | 13.53 | 0.40 | 3.91 | 3.35 | (2.42–4.63) | 0.42 | (0.33–0.54) |
| Advanced fibrosis | 9.74 | (6.69–14.17) | 37.99 | 0.64 | 0 | 3.56 | (2.93–4.32) | 0.44 | (0.38–0.51) |
| Cirrhosis | 9.13 | (4.25–19.62) | 1.72 | 0.42 | 0 | 3.88 | (2.35–6.39) | 0.43 | (0.32–0.58) |
| BARD score | |||||||||
| Significant fibrosis | 5.98 | (2.62–13.66) | 4.11 | 0.53 | 0 | 2.49 | (1.72–3.61) | 0.46 | (0.30–0.70) |
| Advanced fibrosis | 4.34 | (3.40–5.55) | 26.11 | 0.16 | 23.4 | 1.88 | (1.65–2.14) | 0.48 | (0.41–0.56) |
| FibroMeter | |||||||||
| Significant fibrosis | 17.82 | (4.91–64.7) | 2.69 | 0.44 | 0 | 6.00 | (2.72–13.23) | 0.35 | (0.18–0.67) |
| Advanced fibrosis | 13.72 | (7.51–25.07) | 9.42 | 0.58 | 0 | 4.16 | (2.89–5.99) | 0.31 | (0.24–0.40) |
| FibroTest | |||||||||
| Significant fibrosis | 5.19 | (1.77–15.18) | 12.21 | 0.007 | 75.42 | 2.10 | (1.36–3.25) | 0.56 | (0.36–0.85) |
| Advanced fibrosis | 7.45 | (5.15–10.77) | 4.48 | 0.48 | 0 | 3.81 | (2.18–6.64) | 0.58 | (0.43–0.79) |
| ELF | |||||||||
| Advanced fibrosis | 18.82 | (9.52–37.18) | 7.05 | 0.21 | 29.08 | 4.42 | (3.12–6.25) | 0.29 | (0.23–0.38) |
APRI, aspartate aminotransferase-to-platelet ratio index; CI, confidence interval; DOR, diagnostic odds ratio; ELF, enhanced liver fibrosis; FIB-4, fibrosis index-4; I2, heterogeneity; LR+, positive likelihood ratio; LR-, negative likelihood ratio; NFS, non-alcoholic fatty liver disease fibrosis score; p, statistically significant value, MASLD, metabolic dysfunction-associated steatotic liver disease.
Figure 3.
Summary AUROC plot of tests. (A) APRI, FIB-4, and NFS in detecting any fibrosis. (B) APRI, FIB-4, NFS, BARD score, FibroMeter, and FibroTest in detecting significant fibrosis. (C) APRI, FIB-4, NFS, BARD score, FibroMeter, FibroTest, and ELF in detecting advanced fibrosis. (D) APRI, FIB-4, and NFS in detecting cirrhosis.
3.6.2. Diagnosis of SF (F0–F1 vs. F2–F4)
The DOR of the APRI in the diagnosis of SF was 6.29 (95% CI 4.47–8.92), the LR+ was 2.69 (95% CI 2.23–3.23), the LR- was 0.48 (95% CI 0.40–0.58), and low heterogeneity was detected (Q = 16.13, p = 0.24, I2 = 19.40%) (Table 2; Supplementary Figures S4, S5). The sAUROC had a moderate diagnostic accuracy of 0.76, Sen of 63% (95% CI 53–72%), and Spe of 79% (95% CI 69–86%) (Figure 3B, Supplementary Table S7, and Supplementary Figure S1).
3.6.3. Diagnosis of AF (F0–F2 vs. F3–F4)
The DOR of the APRI in the diagnosis of AF was 6.45 (95% CI 4.83–8.60), the LR+ was 2.96 (95% CI 2.49–3.52), the LR- was 0.50 (95% CI 0.43–0.57), and low heterogeneity was detected (Q = 42.78, p = 0.009, I2 = 19.40%) (Table 2; Supplementary Figures S6, S7). The sAUROC had a moderate diagnostic accuracy of 0.78, Sen of 60% (95% CI 50–69%), and Spe of 82% (95% CI 76–87%) (Figure 3C, Supplementary Table S7, and Supplementary Figure S1).
3.6.4. Diagnosis of cirrhosis (F0–F3 vs. F4)
The DOR of the APRI in the diagnosis of cirrhosis was 6.21 (95% CI 4.34–8.89), the LR+ was 3.11 (95% CI 2.15–4.50), the LR- was 0.53 (95% CI 0.43–0.57), and no heterogeneity was detected (Q = 1.71, p = 0.42, I2 = 0%) (Table 2; Supplementary Figures S8, S9). The sAUROC had a moderate diagnostic accuracy of 0.72, Sen of 47% (95% CI 3–84%), and Spe of 87% (95% CI 50–98%) (Figure 3D, Supplementary Table S7, and Supplementary Figure S1).
3.7. FIB-4
The FIB-4 serological biomarker was evaluated for diagnostic accuracy in detecting AnF (> F1) (5 studies), SF (≥ F2–F4) (15 studies), AF (≥ F3) (43 studies), and cirrhosis (F4) (4 studies) (Supplementary Table S7).
3.7.1. Diagnosis of AnF (F0 vs. F1–F4)
The DOR of the FIB-4 in the diagnosis of AnF was 6.57 (95% CI 4.56–9.48), the LR+ was 2.32 (95% CI 1.94–2.77), the LR- was 0.38 (95% CI 0.29–0.49), and low heterogeneity was detected (Q = 5.35, p = 0.25, I2 = 25.24%) (Table 2; Supplementary Figures S10, S11). The sAUROC had a moderate diagnostic accuracy of 0.77, Sen of 77% (95% CI 61–87%), and Spe of 68% (95% CI 57–78%) (Figure 3A, Supplementary Table S7, and Supplementary Figure S1).
3.7.2. Diagnosis of SF (F0–F1 vs. F2–F4)
The DOR of the FIB-4 in the diagnosis of SF was 5.75 (95% CI 4.11–8.05), the LR+ was 2.51 (95% CI 2.07–3.05), the LR- was 0.50 (95% CI 0.43–0.59), and low heterogeneity was detected (Q = 18.26, p = 0.19, I2 = 23.33%) (Table 2; Supplementary Figures S12, S13). The sAUROC had a moderate diagnostic accuracy of 0.75, Sen of 64% (95% CI 52–74%), and Spe of 76% (95% CI 66–84%) (Figure 3B, Supplementary Table S7, and Supplementary Figure S1).
3.7.3. Diagnosis of AF (F0–F2 vs. F3–F4)
The DOR of the FIB-4 in the diagnosis of AF was 10.43 (95% CI 7.25–15.02), the LR+ was 4.09 (95% CI 3.33–5.02), the LR- was 0.45 (95% CI 0.39–0.52), and no heterogeneity was detected (Q = 33.1, p = 0.83, I2 = 0%) (Table 2; Supplementary Figures 14, S15). The sAUROC had a good diagnostic accuracy of 0.81, Sen of 60% (95% CI 52–68%), and Spe of 87% (95% CI 82–91%) (Figure 3C, Supplementary Table S7, and Supplementary Figure S1).
3.7.4. Diagnosis of cirrhosis (F0–F3 vs. F4)
The DOR of the FIB-4 in the diagnosis of cirrhosis was 14.95 (95% CI 9.96–22.44), the LR+ was 4.66 (95% CI 2.41–9.02), the LR- was 0.38 (95% CI 0.19–0.78), and low heterogeneity was detected (Q = 4.16, p = 0.24, I2 = 27.88%) (Table 2; Supplementary Figures S16, S17). The sAUROC had a good diagnostic accuracy of 0.83, Sen of 69% (95% CI 43–86%), and Spe of 87% (95% CI 57–97%) (Figure 3D, Supplementary Table 7, and Supplementary Figure S1).
3.8. NFS
The NFS serological biomarker was evaluated for diagnostic accuracy in detecting AnF (> F1) (5 studies), SF (≥ F2–F4) (14 studies), AF (≥ F3) (43 studies), and cirrhosis (F4) (3 studies) (Supplementary Table S7).
3.8.1. Diagnosis of AnF (F0 vs. F1–F4)
The DOR of the NFS in the diagnosis of AnF was 4.85 (95% CI 3.32–7.09), the LR+ was 2.27 (95% CI 1.86–2.78), the LR- was 0.49 (95% CI 0.42–0.57), and moderate heterogeneity was detected (Q = 6.63, p = 0.15, I2 = 39.66%) (Table 2; Supplementary Figures 18, 19). The sAUROC had a moderate diagnostic accuracy of 0.71, Sen of 66% (95% CI 62–70%), and Spe of 73% (95% CI 64–81%) (Figure 3A and Supplementary Table S7, and Supplementary Figure S1).
3.8.2. Diagnosis of SF (F0–F1 vs. F2–F4)
The DOR of the NFS in the diagnosis of SF was 9.45 (95% CI 5.17–17.5), the LR+ was 3.35 (95% CI 2.42–4.63), the LR- was 0.42 (95% CI 0.33–0.54), and low heterogeneity was detected (Q = 13.53, p = 0.40, I2 = 3.91%) (Table 2; Supplementary Figures S20, S21). The sAUROC had a good diagnostic accuracy of 0.81, Sen of 69% (95% CI 56–79%), and Spe of 80% (95% CI 71–88%) (Figure 3B, Supplementary Table S7, and Supplementary Figure S1).
3.8.3. Diagnosis of AF (F0–F2 vs. F3–F4)
The DOR of the NFS in the diagnosis of AF was 9.74 (95% CI 6.69–14.17), the LR+ was 3.56 (95% CI 2.93–4.32), the LR- was 0.44 (95% CI 0.38–0.51), and no heterogeneity was detected (Q = 37.99, p = 0.64, I2 = 0%) (Table 2; Supplementary Figures S22, S23). The sAUROC had a good diagnostic accuracy of 0.81, Sen of 62% (95% CI 53–70%), and Spe of 85% (95% CI 79–90%) (Figure 3C, Supplementary Table S7, and Supplementary Figure S1).
3.8.4. Diagnosis of cirrhosis (F0–F3 vs. F4)
The DOR of the NFS in the diagnosis of cirrhosis was 9.13 (95% CI 4.25–19.62), the LR+ was 3.88 (95% CI 2.35–6.39), the LR- was 0.43 (95% CI 0.32–0.58), and no heterogeneity was detected (Q = 1.72, p = 0.42, I2 = 0%) (Table 2; Supplementary Figures S24, S25). The sAUROC had a moderate diagnostic accuracy of 0.69, Sen of 63% (95% CI 58–68%), and Spe of 84% (95% CI 73–91%) (Figure 3D, Supplementary Table S7, and Supplementary Figure S1).
3.9. BARD score
The BARD score serological biomarker was evaluated for diagnostic accuracy in detecting SF (≥ F2–F4) (6 studies) and AF (≥ F3) (21 studies) (Supplementary Table S6).
3.9.1. Diagnosis of SF (F0–F1 vs. F2–F4)
The DOR of the BARD score in the diagnosis of SF was 5.98 (95% CI 2.62–13.66), the LR+ was 2.49 (95% CI 1.72–3.61), the LR- was 0.46 (95% CI 0.30–0.70), and no heterogeneity was detected (Q = 4.11, p = 0.53, I2 = 0%) (Table 2; Supplementary Figures S26, S27). The sAUROC had a moderate diagnostic accuracy of 0.76, Sen of 63% (95% CI 45–82%), and Spe of 79% (95% CI 65–83%) (Figure 3B, Supplementary Table S7, and Supplementary Figure S1).
3.9.2. Diagnosis of AF (F0–F2 vs. F3–F4)
The DOR of the BARD score in the diagnosis of AF was 4.34 (95% CI 3.40–5.55), the LR+ was 1.88 (95% CI 1.65–2.14), the LR- was 0.48 (95% CI 0.41–0.56), and low heterogeneity was detected (Q = 26.11, p = 0.16, I2 = 23.4%) (Table 2; Supplementary Figures S28, S29). The sAUROC had a moderate diagnostic accuracy of 0.73, Sen of 72% (95% CI 64–79%), and Spe of 63% (95% CI 54–71%) (Figure 3C, Supplementary Table S7, and Supplementary Figure S1).
3.10. FibroMeter
The FibroMeter serological biomarker was evaluated for diagnostic accuracy in detecting SF (≥ F2–F4) (4 studies) and AF (≥ F3) (12 studies) (Supplementary Table S7).
3.10.1. Diagnosis of SF (F0–F1 vs. F2–F4)
The DOR of the FibroMeter in the diagnosis of SF was 17.82 (95% CI 4.91–64.7), the LR+ was 6.00 (95% CI 2.07–3.05), the LR- was 0.35 (95% CI 0.18–0.67), and no heterogeneity was detected (Q = 2.69, p = 0.44, I2 = 0%) (Table 2; Supplementary Figures S30, S31). The sAUROC had a good diagnostic accuracy of 0.88, Sen of 68% (95% CI 48–82%), and Spe of 89% (95% CI 80–95%) (Figure 3B, Supplementary Table 7, and Supplementary Figure S1).
3.10.2. Diagnosis of AF (F0–F2 vs. F3–F4)
The DOR of the FibroMeter in the diagnosis of AF was 13.72 (95% CI 7.51–25.07), the LR+ was 4.16 (95% CI 2.89–5.99), the LR- was 0.31 (95% CI 0.24–0.40), and no heterogeneity was detected (Q = 9.42, p = 0.58, I2 = 0%) (Table 2; Supplementary Figures 32, 33). The sAUROC had a good diagnostic accuracy of 0.84, Sen of 74% (95% CI 68–79%), and Spe of 82% (95% CI 76–87%) (Figure 3C, Supplementary Table S7, and Supplementary Figure S1).
3.11. FibroTest
The FibroTest serological biomarker was evaluated for diagnostic accuracy in detecting SF (≥ F2–F4) (4 studies) and AF (≥ F3) (6 studies) (Supplementary Table S7).
3.11.1. Diagnosis of SF (F0–F1 vs. F2–F4)
The DOR of the FibroTest in the diagnosis of SF was 5.19 (95% CI 1.77–15.18), the LR+ was 2.10 (95% CI 1.36–3.25), the LR- was 0.56 (95% CI 0.36–0.85), and high heterogeneity was detected (Q = 12.21, p = 0.007, I2 = 75.42%) (Table 2; Supplementary Figures 34, S35). The sAUROC had a good diagnostic accuracy of 0.86, Sen of 72% (95% CI 28–94%), and Spe of 85% (95% CI 45–98%) (Figure 3B, Supplementary Table S7, and Supplementary Figure S1).
3.11.2. Diagnosis of AF (F0–F2 vs. F3–F4)
The DOR of the FibroTest in the diagnosis of AF was 7.45 (95% CI 5.15–10.77), the LR+ was 3.81 (95% CI 2.18–6.64), the LR- was 0.58 (95% CI 0.43–0.79), and no heterogeneity was detected (Q = 4.48, p = 0.48, I2 = 0%) (Table 2; Supplementary Figures S36, S37). The sAUROC had a moderate diagnostic accuracy of 0.78, Sen of 40% (95% CI 15–72%), and Spe of 93% (95% CI 73–99%) (Figure 3C, Supplementary Table S7, and Supplementary Figure S1).
3.12. ELF
The ELF serological biomarker was evaluated for diagnostic accuracy in detecting AF (≥ F3) (6 studies) (Supplementary Table S7).
3.12.1. Diagnosis of AF (F0–F2 vs. F3–F4)
The DOR of the ELF in the diagnosis of AF was 18.82 (95% CI 9.52–37.18), the LR+ was 4.42 (95% CI 3.12–6.25), the LR- was 0.29 (95% CI 0.23–0.38), and low heterogeneity was detected (Q = 7.05, p = 0.21, I2 = 29.08%) (Table 2; Supplementary Figures S38, S39). The sAUROC had a good diagnostic accuracy of 0.87, Sen of 79% (95% CI 68–87%), and Spe of 84% (95% CI 75–90%) (Figure 3C, Supplementary Table S7, and Supplementary Figure S1).
3.13. Sensitivity analysis
The sensitivity analysis showed that there were no changes in the results when only tests with more than 40% of participants (APRI, FIB-4, NFS, and BARD score) and severities (SF, AF, and cirrhosis) were included (Supplementary Figures S40–S58; Supplementary Table S8).
4. Discussion
This systematic review and meta-analysis aimed to assess the accuracy of different prognostic serological biomarkers in predicting liver fibrosis severity in people with MASLD. The serological biomarkers varied according to the different degrees of severity of liver fibrosis. For any type of fibrosis, all the models had moderate precision. For significant fibrosis, the FibroMeter, FibroTest, and NFS models had high precision, and APRI, FIB-4, and BARD score had moderate precision. For advanced fibrosis, the ELF, FibroMeter, FIB-4, and NFS models had high precision, and BARD score, FibroTest, and APRI presented moderate precision. Finally, for cirrhosis, only FIB-4 showed high precision, while APRI and NFS had moderate diagnostic precision in the evaluation of this severity.
The APRI showed moderate diagnostic accuracy across all degrees of liver fibrosis severity, from AnF to cirrhosis, the results that are consistent with previous meta-analyses reporting moderate accuracy in assessing AF with this prognostic model. In addition, different studies have reported inconsistencies in predicting liver fibrosis using this score (8, 96). Therefore, due to conflicting results regarding the effectiveness of the APRI score, the MASLD practice guideline of the AASLD, American College of Gastroenterology, and American Gastroenterological Association recommends using the FIB-4 or NFS score to identify patients with MASLD with stage 3 or 4 fibrosis (6). Our results support this recommendation as FIB-4 and NFS showed good diagnostic accuracy in the assessment of liver fibrosis severity, for SF and AF, and AF and cirrhosis, respectively.
As science has advanced, several serum tests have been developed using either direct biomarkers (reflecting the pathophysiology of hepatic fibrogenesis) or indirect biomarkers (reflecting functional changes in the liver) alone or in combination (57). Complex panels (such as FibroMeter and ELF) have been shown to be more accurate and reproducible for detecting AF than simple panels (159). Our results support these findings, suggesting that both models have good diagnostic accuracy for AF, whereas simple panels such as APRI and BARD score, although cheaper, easier to calculate, and widely available, are not as accurate as complex panels (159).
Different studies have consistently reported that the ELF model provides good results in the assessment of AF, including the 2021 National Institute of Health and Care Excellence guidelines, which established that for the assessment and treatment of people with MASLD, the ELF score is considered “the most cost-effective and appropriate test for AF in adults with MASLD” (160). However, the reality of clinical practice is different as the ELF score is not accessible to frontline health professionals, which may represent a barrier to the detection of liver fibrosis (9, 57).
The FibroTest also showed good diagnostic performance for the assessment of SF in this review. FibroTest and FibroMeter are models that include the analysis of extracellular matrix substances directly involved in the progression of fibrosis and have better Sen and Spe, suggesting that the inclusion of a direct marker of liver fibrosis in a non-invasive test can improve its diagnostic accuracy (8, 9).
Another relevant result was that only three models detected AnF: APRI, FIB-4, and NFS. These models are considered simple scores, that is, none of the complex models analyzed in this review identified this severity. Therefore, there is still a lack of studies evaluating any of these models in the assessment of AnF as most scores have focused on the importance of histological determinants of severe fibrosis and its relevance in the development of future disease. However, the identification of AnF in community settings will allow for the implementation of early lifestyle interventions and consequently inform the decision to refer to secondary care in severe cases (62, 134).
MASLD is also strongly correlated with MetS. Of the 138 included studies, 54.6% reported at least some component of this syndrome. Two recent reviews have suggested that MASLD is both a cause and a consequence of MetS (161, 162). This is because liver fat is presented as a marker of metabolic abnormalities that characterize MetS, and the possibility of MASLD should be considered in all patients diagnosed with MetS with any of the different sets of criteria (161, 162). In the present review, the mean values for both transaminases were above normal, indicating that the studies were conducted in populations with at least some alteration in the serological tests of the liver. In people with MASLD with normal transaminase levels, 16–24% of them may have AF, with the sAUROC for the BARD score, FIB-4, and NFS ranging from 0.71 to 0.85 (99, 152).
In this review, we found a mean BMI of 32.8 kg/m2 in the total study population, which is considered grade-I obesity. The findings of a meta-analysis suggest that there is evidence of a high predictive value of abdominal obesity as an indicator of increased risk of metabolic disorders and cardiovascular disease, as well as evidence supporting the cause-and-effect relationship between abdominal obesity and MASLD (163). A recent review showed that there is less evidence when evaluating the tests in populations of patients with obesity, and non-invasive tests tend to be less favorable in these populations due to differences in terms of BMI and alanine aminotransferase levels, which may mean that serum-based scores derived from the liver clinical setting in groups with different hepatic risk profiles do not adequately reflect the accuracy of these tests in the obese population (9).
Conversely, the present results of prognostic models showing moderate diagnostic accuracy may also be related to the fact that this meta-analysis included a larger number of studies, heterogeneous populations and their variables, and all degrees of fibrosis severity compared to previous meta-analyses (9, 10). Although the objective of non-invasive models is not to replace the biopsy, our results highlight the importance of using these models in the evaluation of MALSD patients with suspected liver fibrosis, which determines the prognosis of the disease, as well as the usefulness and feasibility of performing these tests, given the lack of other methods in primary care for these patients (159).
5. Limitations
However, our meta-analysis has limitations. First of all, there was no stratification of the different models by age, race, weight, and morbidities, only by stages of fibrosis, since few studies were conducted in clinical trials to compare homogeneous populations. Another limitation of the present study is the non-inclusion of imaging biomarkers such as MRE. The decision not to include these biomarkers was made to focus on the serological biomarkers recommended by the guidelines to provide a more comprehensive assessment of their performance. However, this is a study with a large sample of participants, with low heterogeneity between the different studies, which aims to contribute to the generalization of results based on possible limitations in health services.
6. Conclusion
The findings of this meta-analysis suggest that when comparing the scores of serological biomarkers with liver biopsies for predicting liver fibrosis severity in people with MASLD, the FIB-4 has good predictive diagnostic accuracy for any fibrosis, the FibroMeter has good predictive diagnostic accuracy for significant fibrosis, the ELF has good predictive diagnostic accuracy for advanced fibrosis, and the FIB-4 has good diagnostic accuracy for cirrhosis. These non-invasive serological biomarkers can thus be considered as an alternative to determine the prognosis of this disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SL: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CA: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. PR: Data curation, Writing – original draft. CC: Writing – review & editing, Writing – original draft. MW: Formal analysis, Software, Writing – review & editing, Writing – original draft. AP: Conceptualization, Writing – review & editing, Funding acquisition, Writing – original draft. RM: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Acknowledgments
The authors are grateful to the Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), the National Research Council of Brazil (CNPq).
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance Code 001).
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1284509/full#supplementary-material
Glossary
| 95% CI | 95% confidence interval |
| 95% UI | 95% uncertainty interval |
| AASLD | American Association for the Study of Liver Diseases |
| AF | Advanced fibrosis |
| ALT | Alanine transaminase |
| AnF | Any fibrosis |
| APRI | Aspartate aminotransferase-to-platelet ratio |
| AST | Aspartate aminotransferase |
| AST/ALT ratio | Aspartate aminotransferase/alanine aminotransferase ratio |
| AUC | Area under curve |
| AUROC | Area under the receiver operating characteristic |
| BARD score | Body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes score |
| BMI | Body mass index |
| CINAHL | Cumulative Index to Nursing and Allied Health Literature |
| DOR | Diagnostic odds ratio |
| EASL | European Association for the Study of the Liver |
| ELF | Enhanced liver fibrosis |
| EMBASE | Excerpt Medical dataBASE |
| FN | False negatives |
| FP | False positives |
| HbA1C | Glycosylated hemoglobin |
| kg | Kilograms |
| LILACS | Latin American and Caribbean Health Sciences Literature |
| LR- | Negative likelihood ratio |
| LR+ | Positive likelihood ratio |
| LSM-VCTE | Liver stiffness measurement by vibration-controlled transient elastography |
| m2 | Meters2 |
| MADA | Meta-analysis of diagnostic accuracy |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| MetS | Metabolic syndrome |
| MRE | Magnetic resonance elastography |
| NFS | Non-alcoholic fatty liver disease fibrosis score |
| PIT | Participants, index tests, and target condition |
| PRISMA-DTA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy Studies |
| PROSPERO | International Prospective Register of Systematic Reviews database |
| PUBMED | Public/Publisher MEDLINE |
| Q | Cochran’s Q |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies-2 |
| sAUROC | Summary area under the receiver operating characteristic |
| SciELO | Scientific Electronic Library Online |
| Sen | Sensitivity |
| SF | Significant fibrosis |
| Spe | Specificity |
| SROC | Summary receiver operator characteristic |
| SWE | Shear wave elastography |
| TN | True negatives |
| TP | True positives |
| WOS | Web of Science |
References
- 1.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. (2023) 78:1966–86. doi: 10.1097/hep.0000000000000520, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartmann P, Zhang X, Loomba R, Schnabl B. Global and national prevalence of nonalcoholic fatty liver disease in adolescents: an analysis of the global burden of disease study 2019. Hepatology. (2023) 78:1168–81. doi: 10.1097/HEP.0000000000000383, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian H, Zhang K, Hui Z, Ren F, Ma Y, Han F, et al. Global burden of non-alcoholic fatty liver disease in 204 countries and territories from 1990 to 2019. Clin Res Hepatol Gastroenterol. (2023) 47:102068. doi: 10.1016/j.clinre.2022.102068, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Ando Y, Jou JH. Nonalcoholic fatty liver disease and recent guideline updates. Clin Liver Dis. (2021) 17:23–8. doi: 10.1002/cld.1045, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang JZ, Cai JJ, Yu Y, She ZG, Li H. Nonalcoholic fatty liver disease: an update on the diagnosis. Gene Expr J Liver Res. (2019) 19:187–98. doi: 10.3727/105221619X15553433838609, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. (2012) 55:2005–23. doi: 10.1002/hep.25762, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht J, Verhulst S, Mannaerts I, Reynaert H, van Grunsven LA. Prospects in non-invasive assessment of liver fibrosis: liquid biopsy as the future gold standard? Biochim Biophys Acta Mol basis Dis. (2018) 1864:1024–36. doi: 10.1016/j.bbadis.2018.01.009, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. (2017) 66:1486–501. doi: 10.1002/hep.29302 [DOI] [PubMed] [Google Scholar]
- 9.Ooi GJ, Mgaieth S, Eslick GD, Burton PR, Kemp WW, Roberts SK, et al. Systematic review and meta-analysis: non-invasive detection of non-alcoholic fatty liver disease related fibrosis in the obese. Obes Rev. (2018) 19:281–94. doi: 10.1111/obr.12628, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. (2021) 71:1006–19. doi: 10.1136/gutjnl-2021-324243, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and Meta-analysis of diagnostic test accuracy studies the PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. (2005) 41:1313–21. doi: 10.1002/hep.20701, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Reitsma JB, Leeflang MMG, Sterne JAC, Bossuyt PMM, Whiting PF, Rutjes AWSS, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 14.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2021) 12:55–61. doi: 10.1002/jrsm.1411, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr Int J Paediatr. (2007) 96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. (2014) 50:776–84. doi: 10.1007/s00535-014-1007-2, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Adams LA, George J, Rossi E, van der Poorten D, Kench J, DeBoer B, et al. Non-invasive prediction of liver fibrosis in nonalcoholic fatty liver disease. Hepatology. (2008) 48:506–608A. [Google Scholar]
- 20.Adams LA, George J, Bugianesi E, Rossi E, De Boer WB, van der Poorten D, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2011) 26:1536–43. doi: 10.1111/j.1440-1746.2011.06774.x, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Ahmed Z, Ren J, Martin D, Walayat S, Moole H, Yong S, et al. The development and validation of a novel serological index to predict cirrhosis. Gastroenterology. (2016) 150:S338. doi: 10.1016/s0016-5085(16)31187-8 [DOI] [Google Scholar]
- 22.Aida Y, Abe H, Tomita Y, Nagano T, Seki N, Sugita T, et al. Serum Immunoreactive collagen IV detected by monoclonal antibodies as a marker of severe fibrosis in patients with non- alcoholic fatty liver disease. J Gastrointest Liver Dis. (2015) 24:61–8. doi: 10.15403/jgld.2014.1121.yad, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Alkhouri N, Allende D, Guirguis J, Shaker M, Yeriaj L, Lopez R, et al. Commonly used hepatic fibrosis scores have poor performance in Young adult with nonalcoholic fatty liver disease. Am J Gastroenterol. (2015) 110:S847–8. doi: 10.1038/ajg.2015.277 [DOI] [PubMed] [Google Scholar]
- 24.Anam MK, Alam S, Ahmad N. Validation of the BARD (BMI, AST/ALT ratio, DMt2) scoring system for detection of fibrosis in patients with nonalcoholic fatty liver disease. Hepatol Int. (2017) 11:1–1093. doi: 10.1007/s12072-016-9783-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelidi A, Angelidi M, Papazafiropoulou A, Anagnostopoulou K, Vagena E, Velissaris V, et al. Evaluation of different scores to predict nonalcoholic fatty liver disease in overweight or obese patients with type 2 diabetes. Obes Facts. (2017) 10:1–274. doi: 10.1159/000468958, 24th European Congress on Obesity (ECO2017), Porto, Portugal, May 17-20, 2017: Abstracts, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angulo P, George J, Day CP, Vanni E, Russell L, De la Cruz AC, et al. Serum ferritin levels lack diagnostic accuracy for liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2014) 12:1163–1169.e1. doi: 10.1016/j.cgh.2013.11.035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. (2007) 45:846–54. doi: 10.1002/hep.21496, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Anstee QM, Lawitz EJ, Alkhouri N, Wong VW, Romero-Gomez M, Okanoue T, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. (2019) 70:1521–30. doi: 10.1002/hep.30842, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Amernia B, Moosavy SH, Banookh F, Zoghi G. FIB-4, APRI, and AST/ALT ratio compared to FibroScan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar Abbas. Iran BMC Gastroenterol. (2021) 21:453. doi: 10.1186/s12876-021-02038-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora S, Young S, Singal A. Comparison of fibrosis scoring tools in predicting liver fibrosis in nonalcoholic fatty liver disease. Hepatology. (2016) 64:361–601A. [Google Scholar]
- 31.Aykut UE, Akyuz U, Yesil A, Eren F, Gerin F, Ergelen R, et al. A comparison of FibroMeter™ NAFLD score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Scand J Gastroenterol. (2014) 49:1343–8. doi: 10.3109/00365521.2014.958099, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Balakrishnan M, Gaba R, Jain S, Thrift AP. Clinical fibrosis prediction scores perform poorly among Mexican/central American patients with NAFLD. Hepatology. (2018) 68:184–1353. doi: 10.1002/hep.30257 [DOI] [Google Scholar]
- 33.Balakrishnan M, Seth A, Cortes-Santiago N, Jain S, Sood GK, El-Serag HB, et al. External validation of four point-of-care noninvasive scores for predicting advanced hepatic fibrosis in a predominantly Hispanic NAFLD population. Dig Dis Sci. (2021) 66:2387–93. doi: 10.1007/s10620-020-06501-1, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Barrit AS, Lok AS, Reddy KR, Weiss LM, Firpi RJ, Thuluvath PJ, et al. Routinely avaliable noninvasive tests performs well in identifying patients with advanced fibrosis due to NASH: data from the target-Nash observational cohort. Hepatology. (2019) 70:188–1382. doi: 10.1002/hep.30941 [DOI] [Google Scholar]
- 35.Boursier J, Vergniol J, Guillet A, Hiriart J-B, Lannes A, Le Bail B, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. (2016) 65:570–8. doi: 10.1016/j.jhep.2016.04.023, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Boursier J, Vergniol J, Lannes A, Hiriart J-B, Oberti F, Le Bail B, et al. The combination of Fibroscan with blood markers in the fibrometerVCTE significantly reduces the use of liver biopsy for the assessment of advanced fibrosis in non-alcoholic fatty liver disease. J Hepatol. (2017) 66:S161–2. doi: 10.1016/S0168-8278(17)30597-4 [DOI] [Google Scholar]
- 37.Boursier J, Guillaume M, Leroy V, Irlès M, Roux M, Lannes A, et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol. (2019) 71:389–96. doi: 10.1016/j.jhep.2019.04.020, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Brandman D, Boyle M, McPherson S, Van Natta M, Sanyal A, Kowdley K, et al. Comparison of clinical prediction rules for detection of cirrhosis in non-alcoholic fatty liver disease: a multicenter, international, collaborative study- NASH CRN (USA) and Newcastle (UK) cohort. J Hepatol. (2017) 66:S69–70. doi: 10.1016/s0168-8278(17)30400-2 [DOI] [Google Scholar]
- 39.Bril F, McPhaul MJ, Caulfield MP, Clark VC, Soldevilla-Pico C, Firpi-Morell RJ, et al. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care. (2020) 43:290–7. doi: 10.2337/dc19-1071, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Broussier T, Lannes A, Zuberbuhler F, Oberti F, Fouchard I, Hunault G, et al. Simple blood fibrosis tests reduce unnecessary referrals for specialized evaluations of liver fibrosis in NAFLD and ALD patients. Clin Res Hepatol Gastroenterol. (2020) 44:349–55. doi: 10.1016/j.clinre.2019.07.010, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Calès P, Lainé F, Boursier J, Deugnier Y, Moal V, Oberti F, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol. (2009) 50:165–73. doi: 10.1016/j.jhep.2008.07.035 [DOI] [PubMed] [Google Scholar]
- 42.Calès P, Boursier J, Chaigneau J, Lainé F, Sandrini J, Michalak S, et al. Diagnosis of different liver fibrosis characteristics by blood tests in non-alcoholic fatty liver disease. Liver Int. (2010) 30:1346–54. doi: 10.1111/j.1478-3231.2010.02314.x [DOI] [PubMed] [Google Scholar]
- 43.Cebreiros IL, Guzmán FA, Velasco J, Ruiz CR, Villanueva MM, Elízaga I De M, et al. Clinical usefulness of ELF index in the assessment of non alcoholic fatty liver disease. Clin Chem Lab Med. (2014) 52:205–379. doi: 10.1515/cclm-2014-089023898022 [DOI] [Google Scholar]
- 44.Cengiz M, Ozenirler S. Comparative diagnostic accuracy of red cell distribution width-to-platelet ratio versus noninvasive fibrosis scores for the diagnosis of liver fibrosis in biopsy-proven nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. (2015) 27:1293–9. doi: 10.1097/MEG.0000000000000445, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Chan W-K, Nik Mustapha NR, Mahadeva S. A novel 2-step approach combining the NAFLD fibrosis score and liver stiffness measurement for predicting advanced fibrosis. Hepatol Int. (2014) 9:594–602. doi: 10.1007/s12072-014-9596-7, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Chowdhury SD, Ramakrishna B, Eapen E, Goel A, Zachariah UG, Pugazendhi S, et al. Fibrosis in non-alcoholic fatty liver disease: correlation with simple blood indices and association with tumor necrosis factor-alpha polymorphisms. Trop Gastroenterol. (2013) 34:31–5. doi: 10.7869/tg.2012.88 [DOI] [PubMed] [Google Scholar]
- 47.Cichoż-Lach H, Celiński K, Prozorow-Król B, Swatek J, Słomka M, Lach T. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease. Med Sci Monit. (2012) 18:CR735–40. doi: 10.12659/MSM.883601, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. (2015) 41:1271–80. doi: 10.1111/apt.13196, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Carli MAL, de Carli LA, Correa MB, Junqueira G, Tovo CV, Coral GP. Performance of noninvasive scores for the diagnosis of advanced liver fibrosis in morbidly obese with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. (2020) 32:420–5. doi: 10.1097/MEG.0000000000001519, PMID: [DOI] [PubMed] [Google Scholar]
- 50.de Cleva R, Duarte LF, Crenitte MRF, de Oliveira CPM, Pajecki D, Santo MA. Use of noninvasive markers to predict advanced fibrosis/cirrhosis in severe obesity. Surg Obes Relat Dis. (2016) 12:862–7. doi: 10.1016/j.soard.2015.11.011, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Demir M, Lang S, Schulte S, Quasdorff M, Drebber U, Hardt A, et al. Prediction of fibrosis in Nafld – comparison of different scoring systems using routine laboratory parameters. J Hepatol. (2011) 54:S332. doi: 10.1016/s0168-8278(11)60831-3 [DOI] [Google Scholar]
- 52.Demir M, Lang S, Nierhoff D, Drebber U, Hardt A, Wedemeyer I, et al. Stepwise combination of simple noninvasive fibrosis scoring systems increases diagnostic accuracy in nonalcoholic fatty liver disease. J Clin Gastroenterol. (2013) 47:719–26. doi: 10.1097/MCG.0b013e3182819a89, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Dincses E, Yilmaz Y. Diagnostic usefulness of FibroMeter VCTE for hepatic fibrosis in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. (2015) 27:1149–53. doi: 10.1097/MEG.0000000000000409, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Drolz A, Wehmeyer M, Diedrich T, Zur Wiesch JS, Lohse AW, Kluwe J. Validation of non-invasive fibrosis assessments in biopsy-proven non-alcoholic fatty liver disease. J Hepatol. (2017) 66:S150–1. doi: 10.1016/s0168-8278(17)30573-1 [DOI] [Google Scholar]
- 55.Dvorak K, Stritesky J, Petrtyl J, Vitek L, Sroubkova R, Lenicek M, et al. Use of non-invasive parameters of non-alcoholic steatohepatitis and liver fibrosis in daily practice - an exploratory case-control study. PLoS One. (2014) 9:e111551. doi: 10.1371/journal.pone.0111551, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eddowes P, Allison M, Tsochatzis E, Anstee Q, Sheridan D, Guha IN, et al. Combination of FibroScan and FibroMeter (FibroMeter VCTE) improves identification of patients with advanced fibrosis in patients with NAFLD. J Hepatol. (2019) 70:e766–7. doi: 10.1016/s0618-8278(19)31525-7 [DOI] [Google Scholar]
- 57.Fagan KJ, Pretorius CJ, Horsfall LU, Irvine KM, Wilgen U, Choi K, et al. ELF score ≥9.8 indicates advanced hepatic fibrosis and is influenced by age, steatosis and histological activity. Liver Int. (2015) 35:1673–81. doi: 10.1111/liv.12760, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Francque SMA, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, et al. Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin Gastroenterol Hepatol. (2012) 10:1162–8. doi: 10.1016/j.cgh.2012.06.019 [DOI] [PubMed] [Google Scholar]
- 59.Fujii H, Enomoto M, Fukushima W, Ohfuji S, Mori M, Kobayashi S, et al. Noninvasive laboratory tests proposed for predicting cirrhosis in patients with chronic hepatitis C are also useful in patients with non-alcoholic steatohepatitis. J Gastroenterol. (2009) 44:608–14. doi: 10.1007/s00535-009-0046-6, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Fujii H, Enomoto M, Fukushima W, Tamori A, Sakaguchi H, Kawada N. Applicability of BARD score to Japanese patients with NAFLD. Gut. (2009) 58:1566–7. doi: 10.1136/gut.2009, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Gallego-Durán R, Pareja MJ, Ranchal I, Ampuero J, Camacho IM, Chaves P, et al. Validation of FibroMax and NAFLDscore in a cohort of NAFLD Spanish patients. Hepatology. (2012) 56:191–1144. [Google Scholar]
- 62.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology. (2008) 47:455–60. doi: 10.1002/hep.21984, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Guillaume M, Moal V, Delabaudiere C, Zuberbuhler F, Robic MA, Lannes A, et al. Direct comparison of the specialised blood fibrosis tests FibroMeterV2G and enhanced liver fibrosis score in patients with non-alcoholic fatty liver disease from tertiary care centres. Aliment Pharmacol Ther. (2019) 50:1214–22. doi: 10.1111/apt.15529, PMID: [DOI] [PubMed] [Google Scholar]
- 64.Guturu P, Steffer K, Petersen JR, Snyder N. A new risk index for the estimation of fibrosis in non alcoholic fatty liver disease (NAFLD): comparison with the mayo score and the ast platelet ration index (APRI). Hepatology. (2008) 48:506–608A. [Google Scholar]
- 65.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. (2008) 57:1441–7. doi: 10.1136/gut.2007.146019, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Hagström H, Nasr P, Ekstedt M, Stål P, Hultcrantz R, Kechagias S. Accuracy of noninvasive scoring Systems in Assessing Risk of death and liver-related endpoints in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2019) 17:1148–1156.e4. doi: 10.1016/j.cgh.2018.11.030, PMID: [DOI] [PubMed] [Google Scholar]
- 67.Huang CYW, Seah JJ, Kam JW, Ang TL, Fock KM, Teo EK, et al. For prediction of non-alcoholic fatty liver disease (NAFLD) induced liver cirrhosis, modified AST to platelet ratio index (m- APRI) performs better than other non-invasive scores. Hepatol Int. (2019) 13:S1–S266. doi: 10.1007/s12072-019-09936-5 [DOI] [Google Scholar]
- 68.Inadomi C, Takahashi H, Ogawa Y, Oeda S, Imajo K, Kubotsu Y, et al. Accuracy of the enhanced liver fibrosis test, and combination of the enhanced liver fibrosis and non-invasive tests for the diagnosis of advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol Res. (2020) 50:682–92. doi: 10.1111/hepr.13495, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Isgro M, Miele L, Cefalo C, Giannace A, Morlacchi C, Rapaccini G, et al. ELF test as a new non invasive diagnostic tool staging liver fibrosis: validation in a cohort of patients with nonalcoholic fatty liver diasease. Clin Chem Lab Med. (2014) 52:S1–S1760. doi: 10.1515/cclm-2014-4041 [DOI] [Google Scholar]
- 70.Itoh Y, Seko Y, Shima T, Nakajima T, Mizuno K, Kawamura Y, et al. Accuracy of non-invasive scoring systems for diagnosing non-alcoholic steatohepatitis-related fibrosis: multicenter validation study. Hepatol Res. (2018) 48:1099–107. doi: 10.1111/hepr.13226, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Joo SK, Kim W, Jung YJ. Obesity and steatosis severity affect diagnostic performances of noninvasive fibrosis tests in nonalcoholic fatty liver disease. J Hepatol. (2017) 66:S590–1. doi: 10.1016/s0168-8278(17)31607-0 [DOI] [PubMed] [Google Scholar]
- 72.Joo SK, Kim JH, Oh S, Kim BG, Lee KL, Kim HY, et al. Prospective comparison of noninvasive fibrosis assessment to predict advanced fibrosis or cirrhosis in Asian patients with hepatitis C. J Clin Gastroenterol. (2015) 49:697–704. doi: 10.1097/MCG.0000000000000215 [DOI] [PubMed] [Google Scholar]
- 73.Jouness RIK, Rosso C, Petta S, Cucco M, Marietti M, Caviglia GP, et al. The combination of index of NASH score and liver stiff- ness improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatology. (2016) 64:361–601A. [Google Scholar]
- 74.Kao WY, Chang IW, Chen CL, Su CW, Fang SU, Tang JH, et al. Fibroscan-based score to predict significant liver fibrosis in morbidly obese patients with nonalcoholic fatty liver disease. Obes Surg. (2020) 30:1249–57. doi: 10.1007/s11695-019-04192-w, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Kawamura Y, Ikeda K, Arase Y, Sorin Y, Fukushima T, Kunimoto H, et al. New discriminant score to predict the fibrotic stage of non-alcoholic steatohepatitis in Japan. Hepatol Int. (2015) 9:269–77. doi: 10.1007/s12072-014-9605-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim D, Yu S, Chung G, Lee J, Kim W, Kim Y, et al. Comparison of non-invasive markers of fibrosis in the Asian non-alcoholic fatty liver Disease’S population with low prevalence of advanced fibrosis. J Hepatol. (2011) 54:S338–9. doi: 10.1016/s0168-8278(11)60850-7 [DOI] [Google Scholar]
- 77.Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR Elastography. Radiology. (2013) 268:411–9. doi: 10.1148/radiol.13121193, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobayashi N, Kumada T, Toyoda H, Tada T, Ito T, Kage M, et al. Ability of Cytokeratin-18 fragments and FIB-4 index to diagnose overall and mild fibrosis nonalcoholic steatohepatitis in Japanese nonalcoholic fatty liver disease patients. Dig Dis. (2017) 35:521–30. doi: 10.1159/000480142, PMID: [DOI] [PubMed] [Google Scholar]
- 79.Kolhe KM, Amarapurkar A, Parikh P, Chaubal A, Chauhan S, Khairnar H, et al. Aspartate transaminase to platelet ratio index (APRI) but not FIB-5 or FIB-4 is accurate in ruling out significant fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) in an urban slum-dwelling population. BMJ Open Gastroenterol. (2019) 6:e000288–6. doi: 10.1136/bmjgast-2019-000288, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kosick H, Cerocchi O, Sebastiani G, Patel K. Non-invasive prediction of advanced fibrosis in NAFLD—A stepwise, algorithmic approach. Can Liver J. (2019) 2:123–124. [Google Scholar]
- 81.Kruger F, Daniels C, Kidd M, Swart G, Brundyn K, van Rensburg C, et al. APRI, a non-invasive for advanced fibrosis in NASH, and new proposed algorithm for the detection of advanced fibrosis. SAMJ. (2008) 98:633–48. [Google Scholar]
- 82.Kruger FC, Daniels CR, Kidd M, Swart G, Brundyn K, van Rensburg C, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. South African Med J. (2011) 101:477–80. [PubMed] [Google Scholar]
- 83.Kumar R, Rastogi A, Sharma MK, Bhatia V, Tyagi P, Sharma P, et al. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig Dis Sci. (2013) 58:265–74. doi: 10.1007/s10620-012-2306-1, PMID: [DOI] [PubMed] [Google Scholar]
- 84.Labenz C, Huber Y, Kalliga E, Nagel M, Ruckes C, Straub BK, et al. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther. (2018) 48:1109–16. doi: 10.1111/apt.14976, PMID: [DOI] [PubMed] [Google Scholar]
- 85.Lambrecht J, Verhulst S, Reynaert H, van Grunsven LA. The miRFIB-score: a serological miRNA-based scoring algorithm for the diagnosis of significant liver fibrosis. Cell. (2019) 8:1003. doi: 10.3390/cells8091003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lang S, Farowski F, Martin A, Wisplinghoff H, Vehreschild MJGT, Krawczyk M, et al. Prediction of advanced fibrosis in non-alcoholic fatty liver disease using gut microbiota-based approaches compared with simple non-invasive tools. Sci Rep. (2020) 10:9385–9. doi: 10.1038/s41598-020-66241-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lardi L, Lul R, Port G, Coral G, Peres A, Dornelles G, et al. Fibromax and inflamatory markers cannot replace liver biopsy in the evaluation of non-alcoholic fatty liver disease. Minerva Gastroenterol. (2020) 68:85–90. doi: 10.23736/s2724-5985.20.02746-4, PMID: [DOI] [PubMed] [Google Scholar]
- 88.Lassailly G, Caiazzo R, Hollebecque A, Buob D, Leteurtre E, Arnalsteen L, et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastroenterol Hepatol. (2011) 23:499–506. doi: 10.1097/MEG.0b013e3283464111, PMID: [DOI] [PubMed] [Google Scholar]
- 89.Le P, Yu P-C, Singh A, Nguyen C, Singh T, McCullough A, et al. Validation of noninvasive fibrosis scores in Prediabetic patients with nonalcoholic fatty liver disease. Gastroenterology. (2018) 154:S-1169–70. doi: 10.1016/s0016-5085(18)33874-5 [DOI] [Google Scholar]
- 90.Lee TH, Han SH, Yang JD, Kim D, Ahmed M. Prediction of advanced fibrosis in nonalcoholic fatty liver disease: an enhanced model of BARD score. Gut Liver. (2013) 7:323–8. doi: 10.5009/gnl.2014.8.2.228, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu WY, Zheng KI, Pan XY, Ma HL, Zhu PW, Wu XX, et al. Effect of PNPLA3 polymorphism on diagnostic performance of various noninvasive markers for diagnosing and staging nonalcoholic fatty liver disease. J Gastroenterol Hepatol. (2020) 35:1057–64. doi: 10.1111/jgh.14894, PMID: [DOI] [PubMed] [Google Scholar]
- 92.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, Sánchez-Ávila F, Vargas-Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. (2008) 7:350–7. doi: 10.1016/s1665-2681(19)31836-8 [DOI] [PubMed] [Google Scholar]
- 93.Loong TCW, Wei JL, Leung JCF, Wong GLH, Shu SST, Chim AML, et al. Application of the combined FibroMeter vibration-controlled transient elastography algorithm in Chinese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2017) 32:1363–9. doi: 10.1111/jgh.13671, PMID: [DOI] [PubMed] [Google Scholar]
- 94.Luger M, Kruschitz R, Kienbacher C, Traussnigg S, Langer FB, Schindler K, et al. Prevalence of liver fibrosis and its association with non-invasive fibrosis and metabolic markers in morbidly obese patients with vitamin D deficiency. Obes Surg. (2016) 26:2425–32. doi: 10.1007/s11695-016-2123-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahadeva S, Mahfudz AS, Vijayanathan A, Goh KL, Kulenthran A, Cheah PL. Performance of transient elastography (TE) and factors associated with discordance in non-alcoholic fatty liver disease. J Dig Dis. (2013) 14:604–10. doi: 10.1111/1751-2980.12088, PMID: [DOI] [PubMed] [Google Scholar]
- 96.Marella HK, Reddy YK, Jiang Y, Ganguli S, Podila PSB, Snell PD, et al. Accuracy of noninvasive fibrosis scoring systems in african american and white patients with nonalcoholic fatty liver disease. Clin Transl Gastroenterol. (2020) 11:1–12. doi: 10.14309/ctg.0000000000000165, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. (2010) 59:1265–9. doi: 10.1136/gut.2010.216077, PMID: [DOI] [PubMed] [Google Scholar]
- 98.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. (2017) 112:740–51. doi: 10.1038/ajg.2016.453, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McPherson S, Anstee QM, Henderson E, Day CP, Burt AD. Are simple noninvasive scoring systems for fibrosis reliable in patients with NAFLD and normal ALT levels? Eur J Gastroenterol Hepatol. (2013) 25:652–8. doi: 10.1097/MEG.0b013e32835d72cf, PMID: [DOI] [PubMed] [Google Scholar]
- 100.Meneses D, Olveira A, Corripio R, del Carmen MM, Romero M, Calvo-Viñuelas I, et al. Performance of noninvasive liver fibrosis scores in the morbid obese patient, same scores but different thresholds. Obes Surg. (2020) 30:2538–46. doi: 10.1007/s11695-020-04509-0, PMID: [DOI] [PubMed] [Google Scholar]
- 101.Miao CL, Pineda-Bonilla JJ, Smith RE, Zhanna V, Vladimir Proudan Proudan V, Pogrebnaya ZV, et al. Independent predictors and non-invansive markers of nonalcoholic steatohepatitis and fibrosis in morbidly obese patients. Hepatology. (2010) 52:627–724. [Google Scholar]
- 102.Miele L, De Michele T, Isgrò M, Marrone G, Cefalo C, Biolato M, et al. ELF test is a reliable non invasive test for fibrosis in NAFLD subjects. J Hepatol. (2015) 62:S736. doi: 10.1016/S0168-8278(15)31235-6 [DOI] [Google Scholar]
- 103.Miele L, De Michele T, Marrone G, Antonietta Isgrò M, Basile U, Cefalo C, et al. Enhanced liver fibrosis test as a reliable tool for assessing fibrosis in nonalcoholic fatty liver disease in a clinical setting. Int J Biol Markers. (2017) 32:e397–402. doi: 10.5301/ijbm.5000292, PMID: [DOI] [PubMed] [Google Scholar]
- 104.Miller A, Li N, Hinton A, Mumtaz K. Performance of lab-based scoring tests for the assessment ofHepatic fibrosis compared to the liver biopsy among patients with non-alcoholic fatty liver disease. Am J Gastroenterol. (2019) 114:S585–6. doi: 10.14309/01.ajg.0000593612.37731.af [DOI] [Google Scholar]
- 105.Miller M, Jafferbhoy H, Walsh S, Dillon JF. Performance of simple algorithm tests for the detection of fibrosis in Nalfd. J Hepatol. (2010) 52:S150–82. doi: 10.1016/s0168-8278(10)60363-7 [DOI] [Google Scholar]
- 106.Munteanu M, Tiniakos D, Anstee Q, Charlotte F, Marchesini G, Bugianesi E, et al. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment Pharmacol Ther. (2016) 44:877–89. doi: 10.1111/apt.13770, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nascimbeni F, Petta S, Romero-Gomez M, Marchesini G, Buagianesi E, Bellantani S, et al. Substantial variability of the diagnostic accuracy of serum fibrosis markers across multiple cohorts of Nash patients in various centers: the case for the negative predictive value. Hepatology. (2015) 62:1262A–1263A. [Google Scholar]
- 108.Nassif AT, Nagano TA, Okayama S, Nassif LS, Branco Filho A, Sampaio NJ. Performance of the Bard scoring system in bariatric surgery patients with nonalcoholic fatty liver disease. Obes Surg. (2017) 27:394–8. doi: 10.1007/s11695-016-2284-z, PMID: [DOI] [PubMed] [Google Scholar]
- 109.Okajima A, Sumida Y, Taketani H, Hara T, Seko Y, Ishiba H, et al. Liver stiffness measurement to platelet ratio index predicts the stage of liver fibrosis in non-alcoholic fatty liver disease. Hepatol Res. (2017) 47:721–30. doi: 10.1111/hepr.12793, PMID: [DOI] [PubMed] [Google Scholar]
- 110.Pastor-Ramírez H, Aller R, Gallego-Durán R, Bañales J, Arias-Lotes M, García-Monzón C, et al. Validation of non-invasive methods for advanced fibrosis detection in NAFLD patients. Inflamm Intest Dis. (2017) 2:1–92. doi: 10.1159/000478719 [DOI] [Google Scholar]
- 111.Pathik P, Ravindra S, Ajay C, Prasad B, Jatin P, Prabha S. Fibroscan versus simple noninvasive screening tools in predicting fibrosis in high-risk nonalcoholic fatty liver disease patients from western India. Ann Gastroenterol. (2015) 28:281–6. [PMC free article] [PubMed] [Google Scholar]
- 112.Peleg N, Issachar A, Sneh-Arbib O, Shlomai A. AST to platelet ratio index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis. (2017) 49:1133–8. doi: 10.1016/j.dld.2017.05.002, PMID: [DOI] [PubMed] [Google Scholar]
- 113.Pérez-Gutiérrez OZ, Hernández-Rocha C, Candia-Balboa RA, Arrese MA, Benítez C, Brizuela-Alcántara DC, et al. Validation study of systems for noninvasive diagnosis of fibrosis in nonalcoholic fatty liver disease in Latin population. Ann Hepatol. (2013) 12:416–24. doi: 10.1016/s1665-2681(19)31004-x, PMID: [DOI] [PubMed] [Google Scholar]
- 114.Petta S, Vanni E, Bugianesi E, Di Marco V, Cammà C, Cabibi D, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. (2015) 35:1566–73. doi: 10.1111/liv.12584, PMID: [DOI] [PubMed] [Google Scholar]
- 115.Petta S, Wong VWS, Cammà C, Hiriart JB, Wong GLH, Vergniol J, et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther. (2017) 46:617–27. doi: 10.1111/apt.14219, PMID: [DOI] [PubMed] [Google Scholar]
- 116.Pimentel SK, Strobel R, Gonçalves CG, Sakamoto DG, Ivano FH, Coelho CU. Evaluation of the nonalcoholic fat liver diasease fibrosis score for patients undergoing bariatric surgery. Arq Gastroenterol. (2010) 47:170–3. doi: 10.1590/S0004-28032010000200010, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Polyzos SA, Slavakis A, Koumerkeridis G, Katsinelos P, Kountouras J. Noninvasive liver fibrosis tests in patients with nonalcoholic fatty liver disease: an external validation cohort. Horm Metab Res. (2019) 51:134–40. doi: 10.1055/a-0713-1330, PMID: [DOI] [PubMed] [Google Scholar]
- 118.Prasad SS, Ranjan KC, Kanta SS, Dinesh M, Gautam N, Resu K, et al. Utility of noninvasive scoring systems of fibrosis for detecting advanced fibrosis in patients with leaner nonalcoholic fatty liver disease (NAFLD). Hepatol Int. (2020) 14:S1–S470. doi: 10.1007/s12072-020-10030-4 [DOI] [Google Scholar]
- 119.Qureshi K, Clements RH, Abrams GA. The utility of the “NAFLD fibrosis score” in morbidly obese subjects with NAFLD. Obes Surg. (2008) 18:264–70. doi: 10.1007/s11695-007-9295-8, PMID: [DOI] [PubMed] [Google Scholar]
- 120.Raszeja-Wyszomirska J, Szymanik B, Ławniczak M, Kajor M, Chwist A, Milkiewicz P, et al. Validation of the BARD scoring system in polish patients with nonalcoholic fatty liver disease (NAFLD). BMC Gastroenterol. (2010) 10:67. doi: 10.1186/1471-230X-10-67, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rath MM, Panigrahi MK, Pattnaik K, Bhuyan P, Kar SK, Misra B, et al. Histological evaluation of non-alcoholic fatty liver disease and its correlation with different noninvasive scoring systems with special reference to fibrosis: a single center experience. J Clin Exp Hepatol. (2016) 6:291–6. doi: 10.1016/j.jceh.2016.08.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, et al. Diagnostic value of biochemical markers (fibro test-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. (2006) 6:1–13. doi: 10.1186/1471-230X-6-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ratziu V, Le Calves S, Messous D, Charlotte F, Bonhay L, Munteanu M, et al. Diagnostic value of biochemical markers (FIBROTEST) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol. (2004) 40:175–597. doi: 10.1016/S0168-8278(04)90596-X [DOI] [Google Scholar]
- 124.Ruffillo G, Fassio E, Alvarez E, Landeira G, Longo C, Domínguez N, et al. Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. J Hepatol. (2011) 54:160–3. doi: 10.1016/j.jhep.2010.06.028, PMID: [DOI] [PubMed] [Google Scholar]
- 125.Saez E, Conde I, Blazquez T, Garcia-Morales N, Perez J, Tenias JM, et al. Performance of the fibroscan and other noninvasive scales for detecting hepatic fibrosis in patients with nonalcoholic fatty liver disease. J Hepatol. (2017) 66:S156. doi: 10.1016/s0168-8278(17)30585-8 [DOI] [Google Scholar]
- 126.Sebastiani G, Castera L, Halfon P, Pol S, Mangia A, Di Marco V, et al. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: an international study of 2411 cases. Aliment Pharmacol Ther. (2011) 34:1202–16. doi: 10.1111/j.1365-2036.2011.04861.x, PMID: [DOI] [PubMed] [Google Scholar]
- 127.Seth A, Balakrishnan M, Thrift A, Sood GK. Utility of four non-invasive scores in predicting advanced fibrosis in a predominantly hispanic population. Am J Gastroenterol. (2016) 111:S374. doi: 10.1038/ajg.2016.359 [DOI] [Google Scholar]
- 128.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2009) 7:1104–12. doi: 10.1016/j.cgh.2009.05.033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shaheen A, Rye P, Urbanski S, Swain M, Jayakumar S. Sequential algorithm of non-invasive tools to assess severe fibrosis in non alcoholic fatty liver disease. Can J Gastroenterol Hepatol. (2016) 2016:1–204. doi: 10.1155/2016/4792898, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shima T, Sakai K, Oya H, Katayama T, Mitsumoto Y, Mizuno M, et al. Diagnostic accuracy of combined biomarker measurements and vibration-controlled transient elastography (VCTE) for predicting fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. (2019) 55:100–12. doi: 10.1007/s00535-019-01626-1, PMID: [DOI] [PubMed] [Google Scholar]
- 131.Shoji H, Yoshio S, Mano Y, Kumagai E, Sugiyama M, Korenaga M, et al. Interleukin-34 as a fibroblast-derived marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep. (2016) 6:1–11. doi: 10.1038/srep28814, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shukla A, Kapileswar S, Gogtay N, Joshi A, Dhore P, Shah C, et al. Simple biochemical parameters and a novel score correlate with absence of fibrosis in patients with nonalcoholic fatty liver disease. Indian J Gastroenterol. (2015) 34:281–5. doi: 10.1007/s12664-015-0580-5, PMID: [DOI] [PubMed] [Google Scholar]
- 133.Siddiqui MS, Patidar KR, Boyett S, Luketic VA, Puri P, Sanyal AJ. Performance of non-invasive models of fibrosis in predicting mild to moderate fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. (2016) 36:572–9. doi: 10.1111/liv.13054, PMID: [DOI] [PubMed] [Google Scholar]
- 134.Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. (2019) 17:1877–1885.e5. doi: 10.1016/j.cgh.2018.12.031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Simo KA, McKillop IH, McMillan MT, Ahrens WA, Walters AL, Thompson KJ, et al. Does a calculated “nAFLD fibrosis score” reliably negate the need for liver biopsy in patients undergoing bariatric surgery? Obes Surg. (2014) 24:15–21. doi: 10.1007/s11695-013-1044-6, PMID: [DOI] [PubMed] [Google Scholar]
- 136.Singh A, Gosai F, Siddiqui MT, Gupta M, Lopez R, Lawitz E, et al. Accuracy of noninvasive fibrosis scores to detect advanced fibrosis in patients with type-2 diabetes with biopsy-proven nonalcoholic fatty liver disease. J Clin Gastroenterol. (2020) 54:891–7. doi: 10.1097/MCG.0000000000001339, PMID: [DOI] [PubMed] [Google Scholar]
- 137.Singh T, Frakes CM, Lopez R, McCullough A. Comparison of prediction models of advanced fibrosis in morbidly and non-morbidly obese patients with nonalcoholic fatty liver disease. Gastroenterology. (2018) 154:S–1359. doi: 10.1016/s0016-5085(18)34445-7 [DOI] [Google Scholar]
- 138.Sjöwall C, Martinsson K, Cardell K, Ekstedt M, Kechagias S. Soluble urokinase plasminogen activator receptor levels are associated with severity of fibrosis in nonalcoholic fatty liver disease. Transl Res. (2015) 165:658–66. doi: 10.1016/j.trsl.2014.09.007, PMID: [DOI] [PubMed] [Google Scholar]
- 139.Stauber RE, Staufer K, Stift J, Marculescu R, Obermayer-Pietsch B, Trauner M, et al. Enhanced liver fibrosis (ELF) score accurately detects advanced fibrosis in nonalcoholic fatty liver disease (NAFLD). J Hepatol. (2018) 68:S563. doi: 10.1016/s0168-8278(18)31383-7 [DOI] [Google Scholar]
- 140.Staufer K, Halilbasic E, Spindelboeck W, Eilenberg M, Prager G, Stadlbauer V, et al. Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United Eur Gastroenterol J. (2019) 7:1113–1123. doi: 10.1177/2050640619865133, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Subasi CF, Aykut UE, Yilmaz Y. Comparison of noninvasive scores for the detection of advanced fibrosis in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. (2015) 27:137–41. doi: 10.1097/MEG.0000000000000255, PMID: [DOI] [PubMed] [Google Scholar]
- 142.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. (2012) 12:1–13. doi: 10.1186/1471-230X-12-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takeuchi H, Sugimoto K, Oshiro H, Iwatsuka K, Kono S, Yoshimasu Y, et al. Liver fibrosis: noninvasive assessment using supersonic shear imaging and FIB4 index in patients with non-alcoholic fatty liver disease. J Med Ultrason. (2018) 45:243–9. doi: 10.1007/s10396-017-0840-3, PMID: [DOI] [PubMed] [Google Scholar]
- 144.Tanwar S, Trembilng P, Thorbun D, Guha I, Parkes J, Kaye P, et al. Direct serum markers are more accurate than simple marker panels for the detection of fibrosis in non-alcoholic fatty liver disease (NAFLG). Gut. (2012) 61:A414.1–A414. doi: 10.1136/gutjnl-2012-302514d.287 [DOI] [Google Scholar]
- 145.Thanapirom K, Treeprasertsuk S, Chaopathomkul B, Tanpowpong N, Suksawatamnuay S, Thaimai P, et al. Correlation of magnetic resonance Elastography, Fibroscan, shear wave Elastography, APRI and FIB-4 for staging of liver fibrosis. Gastroenterology. (2017) 152:S1107–8. doi: 10.1016/s0016-5085(17)33731-9 [DOI] [Google Scholar]
- 146.Tomeno W, Imajo K, Kuwada Y, Ogawa Y, Kikuchi M, Honda Y, et al. Distribution of liver stiffness in non-alcoholic fatty liver disease with higher fibrosis-4 index than low cut-off index. J Gastroenterol Hepatol. (2019) 34:1411–6. doi: 10.1111/jgh.14559, PMID: [DOI] [PubMed] [Google Scholar]
- 147.Treeprasertsuk S, Piyachaturawat P, Soontornmanokul T, Wisedopas-Klaikaew N, Komolmit P, Tangkijavanich P. Accuracy of noninvasive scoring systems to assess advanced liver fibrosis in Thai patients with nonalcoholic fatty liver disease. Asian Biomed. (2016) 10:S49–55. doi: 10.5372/1905-7415.1000.521 [DOI] [Google Scholar]
- 148.Uy D, Cua I, Bocobo J, Cervantes J, Edano J. FIB-4: more accurate non-invasive assessment for advanced fi brosis among patients with NAFLD. J Gastroenterol Hepatol. (2011) 26:16–288. [Google Scholar]
- 149.Wong VWS, Vergniol J, Wong GLH, Foucher J, Chan HLY, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. (2010) 51:454–62. doi: 10.1002/hep.23312 [DOI] [PubMed] [Google Scholar]
- 150.Xun YH, Fan JG, Zang GQ, Liu H, Jiang YM, Xiang J, et al. Suboptimal performance of simple noninvasive tests for advanced fibrosis in Chinese patients with nonalcoholic fatty liver disease. J Dig Dis. (2012) 13:588–95. doi: 10.1111/j.1751-2980.2012.00631.x, PMID: [DOI] [PubMed] [Google Scholar]
- 151.Yang M, Jiang L, Wang Y, Li X, Zhou G, Zou Z, et al. Step layered combination of noninvasive fibrosis models improves diagnostic accuracy of advanced fibrosis in nonalcoholic fatty liver disease. J Gastrointest Liver Dis. (2019) 28:289–96. doi: 10.15403/jgld-420, PMID: [DOI] [PubMed] [Google Scholar]
- 152.Yoneda M, Imajo K, Eguchi Y, Fujii H, Sumida Y, Hyogo H, et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal alanine aminotransferase levels. J Gastroenterol. (2013) 48:1051–60. doi: 10.1007/s00535-012-0704-y, PMID: [DOI] [PubMed] [Google Scholar]
- 153.Younes R, Caviglia GP, Govaere O, Rosso C, Armandi A, Sanavia T, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. (2021) 75:786–94. doi: 10.1016/j.jhep.2021.05.008, PMID: [DOI] [PubMed] [Google Scholar]
- 154.Zhou YJ, Ye FZ, Li YY, Pan XY, Chen YX, Wu XX, et al. Individualized risk prediction of significant fibrosis in non-alcoholic fatty liver disease using a novel nomogram. United Eur Gastroenterol J. (2019) 7:1124–34. doi: 10.1177/2050640619868352, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zou C, Wang Q, Ou X, Zhao X, Wang M, Wang P, et al. A noninvasive diagnostic system for cirrhosis in patients with non-alcoholic fatty liver disease. Hepatol Int. (2019) 13:766–76. doi: 10.1007/s12072-019-09982-z [DOI] [PubMed] [Google Scholar]
- 156.Bril F, McPhaul MJ, Caulfield MP, Castille J-M, Poynard T, Soldevila-Pico C, et al. Performance of the SteatoTest, ActiTest, NashTest and FibroTest in a multiethnic cohort of patients with type 2 diabetes mellitus. J Investig Med. (2019) 67:303–11. doi: 10.1136/jim-2018-000864, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang C, Seah JJ, Tan CK, Kam JW, Tan J, Teo EK, et al. Modified AST to platelet ratio index improves APRI and better predicts advanced fibrosis and liver cirrhosis in patients with non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. (2021) 45:101528. doi: 10.1016/j.clinre.2020.08.006, PMID: [DOI] [PubMed] [Google Scholar]
- 158.Fujii H, Enomoto M, Fukumoto S, Kimura T, Nadatani Y, Takashima S, et al. Validation of a two-step approach combining serum biomarkers and liver stiffness measurement to predict advanced fibrosis. J Gastroenterol Hepatol. (2021) 5:801–8. doi: 10.1002/jgh3.12590, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Van Dijk A, Vali Y, Mak AL, Lee J, Tushuizen ME, Zafarmand MH, et al. Systematic review with Meta-analyses: diagnostic accuracy of FibroMeter tests in patients with non-alcoholic fatty liver disease. J Clin Med. (2021) 10:2910. doi: 10.3390/jcm10132910, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.National Institute for health and care excellence. Assessment and Management of non-alcoholic Fatty Liver Disease. Singapore Fam Physician. (2021) 47:1–17. doi: 10.33591/sfp.47.1.u5 [DOI] [PubMed] [Google Scholar]
- 161.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. (2014) 2:901–10. doi: 10.1016/S2213-8587(14)70032-4, PMID: [DOI] [PubMed] [Google Scholar]
- 162.Zarghamravanbakhsh P, Frenkel M, Poretsky L. Metabolic causes and consequences of nonalcoholic fatty liver disease (NAFLD). Metab Open. (2021) 12:100149. doi: 10.1016/j.metop.2021.100149, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. (2017) 46:85–95. doi: 10.1111/apt.14112, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.