Abstract
Exposure to polyphenols is relevant throughout critical windows of infant development, including the breastfeeding phase. However, the quantitative assessment of polyphenols in human breast milk has received limited attention so far, though polyphenols may positively influence infant health. Therefore, a targeted LC–MS/MS assay was developed to investigate 86 analytes representing different polyphenol classes in human breast milk. The sample preparation consisted of liquid extraction, salting out, freeze-out, and a dilution step. Overall, nearly 70% of the chemically diverse polyphenols fulfilled all strict validation criteria for full quantitative assessment. The remaining analytes did not fulfill all criteria at every concentration level, but can still provide useful semi-quantitative insights into nutritional and biomedical research questions. The limits of detection for all analyzed polyphenols were in the range of 0.0041–87 ng*mL−1, with a median of 0.17 ng*mL−1. Moreover, the mean recovery was determined to be 82% and the mean signal suppression and enhancement effect was 117%. The developed assay was applied in a proof-of-principle study to investigate polyphenols in breast milk samples provided by twelve Nigerian mothers at three distinct time points post-delivery. In total, 50 polyphenol analytes were detected with almost half being phenolic acids. Phase II metabolites, including genistein-7-β-D-glucuronide, genistein-7-sulfate, and daidzein-7-β-D-glucuronide, were also detected in several samples. In conclusion, the developed method was demonstrated to be fit-for-purpose to simultaneously (semi-) quantify a wide variety of polyphenols in breast milk. It also demonstrated that various polyphenols including their biotransformation products were present in breast milk and therefore likely transferred to infants where they might impact microbiome development and infant health.
Graphical abstract
Supplementary information
The online version contains supplementary material available at 10.1007/s00216-024-05179-y.
Keywords: Polyphenols, Breast milk, Tandem mass spectrometry, Human biomonitoring, Exposome research
Introduction
Polyphenols are secondary plant metabolites that contain a minimum of one aromatic ring substituted with at least one hydroxyl group [1, 2]. In general, they can be split into two major groups, flavonoids and non-flavonoids, that can be further divided into several classes (see Fig. S1). Examples of flavonoids are flavanones, flavones, flavonols, isoflavones, and proanthocyanidins, whereas non-flavonoids contain stilbenes, lignans, and phenolic acids such as hydroxybenzoic acids, hydroxycinnamic acids, and hydroxyphenylacetic acids. As polyphenols typically have functional groups, namely hydroxyl groups, they are frequently and abundantly conjugated by xenobiotic metabolizing enzymes in the human body. The resulting conjugates, mostly glucuronides and sulfates, are commonly found in human biofluids, especially in urine. The biotransformation of xenobiotics, including polyphenols, impacts their chemical properties and bioavailability [3].
Polyphenols are widely studied due to various health benefits, including antibacterial, anti-inflammatory, and antioxidant properties [4–7]. Current research indicates that polyphenols may contribute to a reduced risk of noncommunicable diseases such as cancer [8], cardiovascular disease [9], and neurodegenerative disorders [10, 11]. Besides potentially beneficial effects, polyphenols may also exhibit adverse properties that depend on various factors, e.g., dosage and environmental interactions [12]. These adverse human effects of polyphenols include reducing iron absorption [13–15], interactions with drugs and other xenobiotics [16–19], inhibiting of digestive enzymes [12], and affecting the hormonal balance [20, 21]. For example, combinatory effects between polyphenols and mycotoxins may contribute to increased estrogenic effects of both the polyphenols and the mycotoxins [22]. In addition to their bioactive properties, polyphenols are of great interest as they are a class of molecules prevalent in numerous plant-based foods including fruits, vegetables, grains, tea, cocoa, and coffee [23–25].
Due to the health-promoting effects of polyphenols and their prevalence in foodstuff, significant research interest exists in evaluating uptake, distribution, metabolism, and excretion of polyphenols in humans. In particular, the question arises if polyphenols are present in breast milk and follow lactational transfer to infants, and if so, whether they have a positive or negative influence on infant health, such as aiding in preventing the development of chronic diseases [26] or modulating microbiome development [27]. In general, breast milk is considered the ideal food for infants. The World Health Organization (WHO) recommends exclusively breastfeeding infants for the first 6 months of life and to continue breastfeeding following the introduction of complementary foods for up to 2 years or longer [28, 29]. To determine the potential impact of polyphenols on infant development and health during this critical window of susceptibility, reliable quantification in breast milk is needed. This would allow investigation of the transfer and biotransformation of ingested polyphenols from the diet of the mothers to their breast milk and subsequently their infants. Moreover, this information would yield new insights to pediatricians and mothers, potentially allowing a tailored adjustment of their diet to positively impact their infant’s health. For example, it could be investigated if the consumption of a polyphenol-rich diet may be an alternative to antibiotics for either treating or preventing (mild) urinary tract infections in susceptible neonates and infants.
Since polyphenols are an extensive family of diverse molecules containing many different classes, it is advantageous to quantify individual polyphenols rather than simply the total polyphenol content [30]. Therefore, a suitable sample preparation approach and a sensitive and specific analytical method are required for the comprehensive quantification of polyphenols. An essential technique in modern human biomonitoring is liquid chromatography coupled to triple quadrupole mass spectrometry (LC–MS/MS) using multiple reaction monitoring (MRM) mode [31]. Targeted LC–MS/MS allows to selectively detect and quantify specific analytes with a high sensitivity, specificity, and accuracy.
Therefore, the aim of this study was to develop and in-house validate a targeted LC–MS/MS method to quantify a comprehensive selection of analytes representing all main polyphenol classes in human breast milk. This involved transferring a previously published method for polyphenols in other human specimens (urine, serum, and plasma) [32] to the highly complex breast milk matrix. A sample preparation method was developed to extract 86 polyphenols representing 15 different chemical classes for broad coverage. After optimization, the method was validated and applied in a pilot study to prove its suitability and fit-for-purpose.
Materials and methods
Chemicals, reagents, and solvents
Information on the reference standards, reagents, and solvents used during method development, validation, and pilot study are available in Table S1 in the supplementary information (SI). Single standard stock solutions were prepared by dissolving the solid polyphenol standards in methanol (MeOH), as described by Oesterle et al. [32]. For optimization of the sample preparation and the method validation, individual stock solutions were mixed at different concentrations and diluted with MeOH to prepare multiple working solutions with concentrations between 0.2 and 130,000 ng*mL−1. All working and individual standard solutions were stored at − 20 °C.
Sample preparation
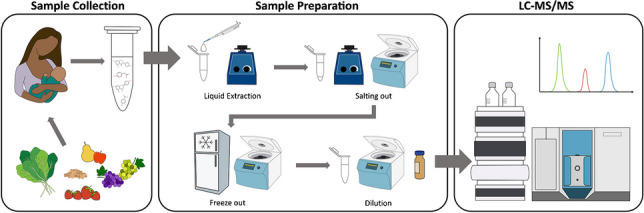
As breast milk is a highly complex biological matrix, different sample preparation approaches were tested and optimized, including solid phase extraction (SPE) with Waters Oasis cartridges. The final optimized sample preparation protocol was established as follows: to an aliquot of 200 µL of human breast milk, 400 µL of acetonitrile (ACN) acidified with 1% v/v formic acid (FA) was added and thoroughly vortexed for 3 min. Subsequently, 80 mg anhydrous magnesium sulfate and 20 mg sodium chloride were added, and the sample was again vortexed for 3 min. The sample was then centrifuged for 10 min (2000 × g, 4 °C), and the supernatant was chilled for 2 h at − 20 °C. Following the freeze-out step, the sample was centrifuged for 2 min (18,000 × g, 4 °C) and the supernatant diluted 1:1 with acidified water (1% v/v FA). The sample was then centrifuged for 5 min (18,000 × g, 4 °C) and the supernatant was transferred to an amber LC glass vial. Enzymatic deconjugation was not performed as several conjugated reference standards were included in the method for direct determination and because deconjugation enzymes are typically contaminated with a high number of xenobiotics, especially polyphenols [33].
LC–MS/MS instrumentation
The UHPLC-ESI-QTrap-MS/MS system used was composed of a 1290 Infinity II LC (Agilent) connected to a QTrap 7500 MS (Sciex), equipped with a heated electrospray ionization source (ESI). Data was acquired in scheduled multiple reaction monitoring (sMRM) mode using fast polarity switching. An optimized LC–MS/MS method that was previously developed for the measurement of polyphenols in other complex biological matrices, i.e., urine, serum, and plasma [32], was transferred from a QTrap 6500+ to a QTrap 7500 system and used as the basis for the breast milk assay described here. The majority of the LC and MS parameters remained the same; however, some parameters, such as retention times, retention time windows, and declustering potential, were adjusted accordingly (Table S2). A VanGuard precolumn (1.8 μm, Waters) attached to an Acquity UPLC HSS T3 column (1.8 μm, 2.1 × 100 mm, Waters) was used to achieve chromatographic separation. The temperature of the column compartment was set to 30 °C and of the autosampler to 7 °C. The mobile phases used were 0.1% v/v FA in H2O (eluent A) and 0.1% v/v FA in ACN (eluent B). The injection volume was 3 µL and the flow rate was set to 0.6 mL*min−1. The gradient (Table S3) started with 5% eluent B and was held for 2 min. Afterwards, eluent B was raised linearly to 64% within 10 min and then increased to 95% for a 2 min hold. Eluent B was then immediately decreased to 5% for a final 2 min re-equilibration step. The following ESI parameters were used: curtain gas 35 arb, sheath gas 90 arb, drying gas 90 arb, collision gas set to medium, source temperature 550 °C, and entrance potential at 10 V in positive and − 10 V in negative mode. The voltage of the ion capillary was set to 5500 V in positive and − 4500 V in negative mode.
Validation experiments
The method was validated in-house following the guidelines set by Eurachem [34] and the EU Commission decision 2002/657/EC [35]. Analytical figures of merit including selectivity, repeatability (RSDr), intermediate precision (RSDR), regression coefficient (R2), recovery (RE), and signal suppression or enhancement (SSE) were evaluated at three concentration levels.
Due to a lack of matrix-matched reference material, multiple breast milk samples were pooled and used as “blank” breast milk for spiking experiments and for the matrix-matched calibration curves [36]. For spiking and creation of the calibration curves, a multi-standard working solution was prepared from the individual polyphenol stock solutions. This working solution was then serially diluted to create five additional multi-standard working solutions. With these six working solutions, a six-point neat solvent (ACN:H2O:FA, 49.5:49.5:1) and a matrix-matched calibration curve (calibration ranges are reported in Table 1) were prepared. During method optimization, a multi-standard solution was measured to estimate the LOQs of the analytes. Based on these values, the calibration points for each analyte were set as 0.33, 1, 3, 10, 30, and 100 times their respective estimated LOQ. Matrix-matched samples were spiked at three different concentration levels: low, middle, and high (Table S4) before the sample preparation procedure (pre-spiked samples). For each validation experiment, triplicates of the pre-spiked samples were prepared at each spiking level. Overall, three individual validation experiments were performed over the course of 3 months, and one of the validation experiments included two additional re-measurements of the acquisition sequence on the same day to determine the intraday stability (RSDr) of the method. To ensure the selectivity of the method, solvent and matrix-matched blanks and spiked samples were examined for any potential interfering signals throughout the validation procedure.
Table 1.
Range of the calibration curve, regression coefficient (R2), signal suppression and enhancement (SSE), limit of detection (LOD), limit of quantification (LOQ), and the mean recovery (RE) of the three spiking levels for each analyte as evaluated during in-house validation. Parameters that could not be determined are listed as n.d
| Analyte | CAS number | Calibration range (ng*mL−1) | R2 | SSE (%) | LOD (ng*mL−1) | LOQ (ng*mL−1) | RE (%) |
|---|---|---|---|---|---|---|---|
| Dihydrochalcones | |||||||
| Phloretin | 60–82-2 | 0.015–7.2 | 0.991 | 114 | 0.017 | 0.034 | 96 |
| Hydroxybenzoic acids | |||||||
| 3,5-Dihydroxybenzoic acid | 99–10-5 | 0.22–29a | 0.903 | 120 | 0.41 | 0.82 | 44 |
| 3-Hydroxybenzoic acid | 99–06-9 | 1.2–590 | 0.989 | 109 | 0.84 | 1.7 | 96 |
| 4-Hydroxybenzoic acid | 99–96-7 | 0.08–35 | 0.988 | 109 | 0.19 | 0.38 | 88 |
| Benzoic acid | 65–85-0 | 3.5–1600 | 0.976 | 107 | 46b | 92b | 84 |
| Ellagic acid | 476–66-4 | 1.74–78c | 0.991 | 163 | 8.9b | 18b | 8 |
| Ethyl gallate | 831–61-8 | 0.004–2.4 | 0.992 | 113 | 0.0024 | 0.0048 | 88 |
| Gallic acid | 149–91-7 | 0.023–3.0a | 0.905 | 129 | 0.028 | 0.056 | 33 |
| Protocatechuic acid | 99–50-3 | 0.015–6.6 | 0.873 | 112 | 0.059 | 0.12 | 42 |
| Salicylic acid | 69–72-7 | 0.2–27a | 0.994 | 124 | 0.48 | 0.96 | 86 |
| Syringic acid | 530–57-4 | 0.022–11 | 0.992 | 112 | 0.068 | 0.14 | 95 |
| Vanillic acid | 121–34-6 | 0.16–70 | 0.988 | 111 | 0.17 | 0.33 | 94 |
| Hydroxycinnamic acids | |||||||
| Caffeic acid | 501–16-6 | 0.3–130 | 0.993 | 110 | 0.55 | 1.1 | 88 |
| Caffeic acid-3-β-D-glucuronide | 1093679–73-2 | 0.014–6.8 | 0.991 | 107 | 0.0085 | 0.017 | 69 |
| Chlorogenic acid | 327–97-9 | 0.29–38a | 0.996 | 112 | 0.32 | 0.65 | 60 |
| Cinnamic acid | 621–82-9 | 1.5–650 | 0.994 | 113 | 2.1 | 4.3 | 90 |
| Dihydrocaffeic acid | 1078–61-1 | 0.082–36 | 0.996 | 110 | 0.16 | 0.33 | 88 |
| Dihydroferulic acid | 1135–23-5 | 0.11–49 | 0.989 | 114 | 0.35 | 0.71 | 100 |
| Ferulic acid/Isoferulic acid | 537–98-4/537–76-5 | 0.058–26 | 0.994 | 110 | 0.096 | 0.19 | 89 |
| p-Coumaric acid | 501–98-4 | 0.044–19 | 0.990 | 109 | 0.046 | 0.092 | 94 |
| Sinapic acid | 530–59-6 | 0.022–11 | 0.993 | 110 | 0.087 | 0.17 | 96 |
| trans-m-Coumaric acid | 588–30-7 | 0.25–110 | 0.992 | 107 | 0.69 | 1.4 | 86 |
| trans-o-Coumaric acid | 583–17-5 | 0.13–64 | 0.994 | 107 | 0.2 | 0.4 | 94 |
| Hydroxyphenylacetic acids | |||||||
| 3-(3-Hydroxyphenyl)propionic acid | 621–54-5 | 0.09–37 | 0.990 | 108 | 0.38 | 0.76 | 95 |
| 3-Hydroxyphenylacetic acid | 621–37-4 | 2–890 | 0.990 | 107 | 4.5 | 9 | 98 |
| 4-Hydroxyphenylacetic acid | 156–38-7 | 2.6–1100 | 0.991 | 104 | 5.9 | 12 | 94 |
| Homoprotocatechuic acid | 102–32-9 | 0.4–182 | 0.992 | 107 | 1.7 | 3.4 | 93 |
| Homovanillic acid | 306–08-1 | 0.73–331 | 0.991 | 109 | 0.64 | 1.3 | 97 |
| Lignans | |||||||
| Enterodiol | 80226–00-2 | 0.005–2.1 | 0.987 | 109 | 0.017 | 0.034 | 88 |
| Enterolactone | 78473–71-9 | 0.014–6.8 | 0.992 | 117 | 0.018 | 0.036 | 89 |
| Others | |||||||
| 2,6-Dimethoxyphenol | 91–10-1 | 0.05–21 | 0.986 | 106 | 0.061 | 0.12 | 98 |
| 3,5-Dimethoxy-4-hydroxyphenylacetic acid | 4385–56-2 | 0.31–140 | 0.993 | 109 | 0.43 | 0.86 | 97 |
| 3-Methylcatechol | 488–17-5 | 0.15–67 | 0.992 | 109 | 0.16 | 0.32 | 94 |
| 4-Methylcatechol | 452–86-8 | 0.33–150 | 0.993 | 110 | 0.24 | 0.48 | 94 |
| Catechol | 120–80-9 | 3.3–130a | 0.759 | 110 | 5 | 10 | 57 |
| Eugenol | 97–53-0 | 2.2–990 | 0.985 | 103 | 4.7 | 9.4 | 91 |
| Hydroxytyrosol | 90–05-1 | 0.041–19 | 0.992 | 112 | 0.034 | 0.068 | 90 |
| Pyrogallol | 10597–60-1 | 0.42–190 | 0.985 | 127 | 1.4 | 2.8 | 91 |
| Thymol | 89–83-8 | 0.67–300 | 0.937 | 99 | 69b | 140b | 76 |
| Urolithin A | 1143–70-0 | 0.007–3.6 | 0.992 | 111 | 0.014 | 0.028 | 90 |
| Stilbenes | |||||||
| Dihydroresveratrol | 58,436–28-5 | 0.035–16 | 0.991 | 109 | 0.054 | 0.11 | 91 |
| Polydatin | 65914–17-2 | 0.012–5 | 0.988 | 106 | 0.061 | 0.12 | 99 |
| Pterostilbene | 537–42-8 | 0.016–7 | 0.994 | 121 | 0.034 | 0.068 | 94 |
| Resveratrol | 501–36-0 | 0.03–14 | 0.992 | 113 | 0.043 | 0.086 | 89 |
| Anthocyanins | |||||||
| Cyanidin | 87725–42-6 | 4.4–2000a | 0.975 | 115 | 39b | 78b | 46 |
| Cyanidin-3-O-glucoside | 47705–70-4 | 0.06–8.1a | 0.995 | 140 | 0.18b | 0.36b | 15 |
| Cyanidin-3-O-rutinoside | 28338–59-2 | 0.052–6.9a | 0.995 | 136 | 0.26b | 0.52b | 21 |
| Cyanidin-3-O-sambubioside | 63535–17-1 | 0.1–15a | 0.996 | 138 | 0.29b | 0.58b | 7 |
| Delphinidin | 528–53-0 | 5.3–703a | 0.959 | 178 | 28b | 56b | 23 |
| Delphinidin-3-O-glucoside | 50986–17-9 | 2.1–300a | 0.981 | 160 | 11b | 22b | 10 |
| Catechins | |||||||
| (-)-Epicatechin | 490–46-0 | 0.2–90 | 0.992 | 107 | 0.24 | 0.48 | 90 |
| (-)-Epicatechin gallate | 1257–08-5 | 0.08–36 | 0.989 | 117 | 0.13 | 0.26 | 74 |
| (-)-Epigallocatechin | 970–74-1 | 1.4–620 | 0.968 | 123 | 5.3 | 11 | 92 |
| (-)-Epigallocatechin gallate | 989–51-5 | 1–440 | 0.970 | 135 | 4.2 | 8.4 | 74 |
| (-)-Gallocatechin | 3371–27-5 | 1.4–620 | 0.983 | 250 | 4.9 | 9.8 | 86 |
| ( +)-Catechin | 154–23-4 | 0.12–53 | 0.993 | 113 | 0.17 | 0.34 | 83 |
| Flavanones | |||||||
| (+/-)-Naringenin | 153–18-4 | 0.008–1.1a | 0.992 | 121 | 0.036 | 0.072 | 86 |
| 8-Prenylnaringenin | 53846–50-7 | 0.02–8.8 | 0.992 | 114 | 0.016 | 0.032 | 93 |
| Hesperetin | 520–33-2 | 0.009–3.8 | 0.993 | 114 | 0.013 | 0.026 | 96 |
| Hesperidin | 520–26-3 | 0.006–2.6 | 0.991 | 120 | 0.0094 | 0.019 | 93 |
| Isoxanthohumol | 521–48-2 | 0.004–1.8 | 0.994 | 115 | 0.0054 | 0.011 | 93 |
| Naringin | 10236–47-2 | 0.23–100 | 0.989 | 112 | 0.56 | 1.1 | 84 |
| Neohesperidin | 13241–33-3 | 0.3–140 | 0.992 | 110 | 0.6 | 1.2 | 85 |
| Neohesperidin dihydrochalcone | 20702–77-6 | 0.006–2.6 | 0.994 | 109 | 0.0085 | 0.017 | 85 |
| Xanthohumol | 6754–58-1 | 0.012–5.3 | 0.992 | 109 | 0.017 | 0.034 | 91 |
| Flavones | |||||||
| Apigenin | 520–36-5 | 0.009–3.9 | 0.988 | 117 | 0.0047 | 0.0094 | 89 |
| Diosmetin | 520–34-3 | 0.005–2.9 | 0.990 | 112 | 0.015 | 0.03 | 93 |
| Diosmin | 520–27-4 | 0.024–11 | 0.982 | 119 | 0.069 | 0.14 | 100 |
| Flavonols | |||||||
| ( +)-Rutin | 480–41-1 | 0.03–13 | 0.988 | 106 | 0.031 | 0.062 | 67 |
| Isorhamnetin | 480–19-3 | 0.006–2.6 | 0.993 | 119 | 0.0089 | 0.018 | 79 |
| Kaempferol | 520–18-3 | 0.12–60 | 0.990 | 121 | 0.17 | 0.34 | 90 |
| Kaempferol-3-O-glucuronide | 22688–78-4 | 0.008–3.5 | 0.992 | 111 | 0.013 | 0.026 | 87 |
| Quercetin | 117–39-5 | 0.052–7.1a | 0.991 | 129 | 0.12 | 0.24 | 63 |
| Quercetin-7-O-β-D-glucuronide | 38934–20-2 | 0.031–14 | 0.989 | 121 | 0.066 | 0.13 | 62 |
| Isoflavones | |||||||
| Biochanin A | 491–80-5 | 0.009–3.9 | 0.994 | 117 | 0.014 | 0.028 | 90 |
| Daidzein | 486–66-8 | 0.01–4.8 | 0.992 | 109 | 0.034 | 0.068 | 89 |
| Daidzein-7-β-D-glucuronide | 38482–80-3 | 0.032–14 | 0.993 | 107 | 0.063 | 0.13 | 88 |
| Genistein | 446–72-0 | 0.01–4.3 | 0.977 | 119 | 0.0047 | 0.0094 | 79 |
| Genistein-7-β-D-glucuronide | 38482–81-4 | 0.04–16 | 0.993 | 108 | 0.054 | 0.11 | 92 |
| Genistein-7-sulfate | 182322–62-9 | 0.27–13d | 0.930 | 105 | 0.18 | 0.36 | 59 |
| S-Equol | 531–95-3 | 0.42–190 | 0.986 | 106 | 2.2 | 4.4 | 88 |
| Proanthocyanidins | |||||||
| Procyanidin A2 | 41743–41-3 | 0.07–31 | 0.983 | 108 | 0.24 | 0.48 | 82 |
| Procyanidin B1 | 20315–25-7 | 1.1–500 | 0.994 | 106 | 1.1 | 2.2 | 67 |
| Procyanidin B2 | 29106–49-8 | 0.3–130 | 0.950 | 108 | 16b | 33b | 69 |
| Procyanidin C1 | 37064–30-5 | 0.21–93 | 0.986 | 113 | 0.62 | 1.2 | 60 |
aThe maximum concentrations of the calibration curve exceeded the range of linearity, thus the highest calibration point was excluded
bNo chromatographic peak at the lowest spiking level thus, the standard deviation of the next highest spiking level with a chromatographic peak was used to calculate LOD and LOQ
cThe two highest concentrations of the calibration curve exceeded the range of linearity, thus they were excluded
dThe concentrations of the calibration curve were chosen too high, and the limit of linearity was reached. Therefore, the three highest calibration points were excluded
The recovery was calculated by dividing the measured concentration of the pre-spiked samples by the theoretical concentration spiked at each of the three different levels. For each spiking level, the overall recovery was calculated as the mean of all measurements (n = 9). Limit of detection (LOD) was evaluated by dividing the standard deviation of the measured concentration of the pre-spiked samples (low level) by the square root of the number of replicates of all measurements (n = 9) and multiplying it by three. The limit of quantification (LOQ) was defined as two times the LOD. Intermediate precision and repeatability were evaluated at each spiking level. The intermediate precision was defined as the relative standard deviation of the measured concentration of the nine pre-spiked samples from the three separate validation experiments, measured on different days. Intraday repeatability was defined as the relative standard deviation of the measured concentration of the nine pre-spiked samples from the validation experiment that was measured three times on the same day. The regression coefficient from each matrix-matched calibration curve was calculated. Signal suppression and enhancement (SSE) effect was calculated by dividing the slope of the matrix-matched calibration curve by the slope of the solvent calibration curve and expressed as percentage. Therefore, a SSE value below 100% indicates signal suppression, while a SSE value greater than 100% indicates signal enhancement [37]. The mean of the regression coefficients and the signal suppression and enhancement effect over the three validation experiments were calculated and reported. Ensuring the evaluation of these validation figures of merit for each analyte, the following criteria for validation requirements were used: a recovery between 50 and 120%, a regression coefficient of at least 0.95, and repeatability and intermediate precision below 45%, 30%, and 25% for low, middle, and high spiking levels, respectively. The repeatability and intermediate precision criteria were determined with the Horwitz equation [34].
Data analysis, peak integration, and concentration calculations were evaluated with SCIEX OS (v3.0). All chromatographic peaks were smoothed with a low-grade filter. A 1/x weighting was applied to all calibration curves. Standard addition was applied to the calibration curves of analytes in which a signal was detected in the non-spiked matrix-matched samples. Calculations of the standard addition and the other validation figures of merit were performed in Excel 16.0.
Biological samples
The pooled breast milk used for method development and validation was kindly provided by the Semmelweis Women’s Clinic in Vienna [36, 38]. The proof-of-principle experiments included aliquots of breast milk samples from a previous study conducted by Ayeni et al. [39] that explored mycotoxin exposure patterns in different biological matrices and a potential impact on gut microbiome development. Details of sample collection are reported in Ayeni et al. [39]. In brief, breast milk samples were collected from twelve Nigerian mothers from Ilishan-Remo, Ogun state. The mothers’ age ranged between 25 and 40 years, and their diet consisted of various cereal-based foods (e.g., bread, rice, ogi), tubers (yam, cassava), legumes (e.g., beans), vegetables (e.g., okra, onion), fruits (e.g., tomatoes, oranges, apples, bananas), fish, and meat. The breast milk was expressed manually by the mothers and stored in a fridge overnight until they were collected by trained study personnel and stored at − 20 °C. The samples were transported on dry ice to the laboratory in Vienna for mass spectrometric analysis. Ethical approval was obtained from the Ethical Committee of Babcock University (BUHREC421/21R, BUHREC466/23). Prior to their inclusion in the studies, all mothers were informed and provided written consent.
For the positive identification of the polyphenol analytes in the biological samples, stringent criteria were defined. Analytes with a retention time deviation greater than 0.05 min compared to their respective matrix-matched calibration curve were excluded. Additionally, only analytes that had both the quantifier and qualifier ions present, with an ion ratio deviation of less than 20% compared to their respective matrix-matched calibration curve, were considered. For analytes that showed a chromatographic signal near the LOD, an ion ratio deviation of up to 50% was considered acceptable, since the background noise has a strong influence on the ion ratios at these low concentrations. For all positively identified analytes, the concentration was determined using the matrix-matched calibration curve and corrected with the recoveries calculated during method validation.
Results and discussion
Method optimization
Extracting a wide range of analytes from a complex biological matrix such as breast milk is a challenging task. In several studies, a QuEChERS approach (quick, easy, cheap, effective, rugged, and safe) has been successfully utilized to extract analytes such as pesticides [38, 40, 41] and other xenobiotics [42–44] from foods with a high lipid content. Few studies [45, 46] investigated the quantification of polyphenols in breast milk, but these did not include as many analytes from multiple polyphenol classes. Moreover, the method presented here includes phase II metabolites of polyphenols such as sulfates and glucuronides, whereas previous studies from Song et al. [45] and Lu et al. [46] used β-glucuronidase/sulfatase treatment to deconjugate potential phase II metabolites. As a starting point, a method established for quantifying mycotoxins in breast milk was selected [36, 47]. This method combined a QuEChERS approach with a freeze-out step, a SPE cleanup, and an evaporation step. Here, in the first step, the procedure was scaled down in order to use a reduced volume of breast milk (200 µL instead of 1 mL) and improve the high-throughput feasibility. However, the results showed low recoveries and severe matrix interferences. Therefore, various extraction solvents, including ACN, MeOH, and hexane, both pure and acidified with up to 3% v/v FA, were tested at different extraction ratios (solvent to breast milk), e.g., 1:1 v/v, 2:1 v/v, and 3:1 v/v, for lipid removal. Different approaches were also tested to optimize the SPE step, including acidifying the ACN with up to 3% FA used to elute the analytes from the C18 SPE cartridges (Oasis HLB Prime, 1 cc, 30 mg, Waters). Protocols with and without the SPE step as well as procedures with and without the drying step with a vacuum concentrator were additionally tested.
The final, optimized sample preparation procedure, which yielded the overall best analyte recoveries with the least signal suppression/enhancement effects, is described in “Sample preparation.” In brief, the procedure contained a liquid extraction step with acidified ACN, a salting-out step with anhydrous magnesium sulfate and sodium chloride, a freeze-out step, and finally a dilution step with acidified H2O.
Validation experiments
Overall, the in-house validation was successful with 59 out of 86 (69%) of the polyphenol analytes fulfilling all validation criteria at all three spiking levels. An additional ten polyphenols (11%) passed all validation criteria for the medium and high spiking levels. A summary of the validation results is listed in Table 1 and the detailed results are reported in Tables S4–S5. In comprehensive multi-analyte human biomonitoring assays, pragmatic compromises are essential to keep a fine balance between covering as many analytes as possible while ensuring high sensitivity and minimal matrix interferences [48]. Consequently, it was not expected that all 86 analytes will perform ideally applying this method. For the polyphenols that did not fulfill all the strict validation figures of merit, semi-quantification is still possible and can be helpful in comprehensive exposome studies as well as for answering biological and nutrition-related questions. The selectivity of the method was evaluated by comparing the matrix-matched samples enriched with standards to the matrix-matched “blank” and solvent samples enriched with standards. No interferences were detected for the majority of the analytes. Due to a lack of available reference material, the biological matrix used was not a true “blank”, thus, several analytes, e.g., (+/-)-naringenin, had a chromatographic peak present in the matrix-matched “blank” (Table S4). Consequently, standard addition was applied for these analytes. Moreover, despite having individual standards for the isomers ferulic acid and isoferulic acid, these two analytes co-eluted and were acquired as a sum parameter because the same MRM transitions were observed during MS parameter optimization.
The recovery, intermediate precisions, and repeatability of the method are listed in Table S4, with the mean recoveries also reported in Table 1. For 70% of all analytes, the mean recoveries, calculated from the three spiking levels of each analyte, were in the range of 80–120%. The intermediate precision of the low, middle, and high spiking levels was in the ranges of 5–61%, 4–56%, and 7–62%, respectively, and the repeatability for the three spiking levels was in the ranges of 4–87%, 3–59%, and 2–71%, respectively. These results demonstrate the overall stability of the workflow for most analytes, both intraday and interday when taking into account that the higher values were typically derived from very few analytes for which full quantitative assessment was not intended by design. The LOD and LOQ values, calibration range, regression coefficient, and SSE are reported in Table 1. The linear calibration curves of each analyte from one validation sequence are depicted in Table S7. It was observed that the regression coefficients for all analytes were between 0.76 and 0.996, with a median R2 of 0.991. Moreover, 93% of all analytes had a regression coefficient greater than 0.95. For some analytes, the maximum concentrations chosen for calibration were too high and exceeded the linear range of the detector; thus, the highest points of the calibrations were removed (Table S4). As expected, the limits of detection varied greatly between the different analytes and the polyphenol classes. The LODs for all analytes ranged between 0.0041 and 87 ng*mL−1, with a median LOD of 0.17 ng*mL−1. Many of the included polyphenol classes showed very low LODs, such as flavanones, flavonols, hydroxycinnamic acids, isoflavones, and stilbenes with LODs ranging from 0.0069 to 0.48 ng*mL−1, 0.015–0.15 ng*mL−1, 0.014–2.5 ng*mL−1, 0.0041–1.9 ng*mL−1, and 0.039–0.069 ng*mL−1, respectively. The SSE was evaluated throughout the validation procedure by comparing the slope of the matrix-matched calibration curve with that of the solvent calibration curve. The SSE was calculated in a manner that a value of 100% indicates that there is no effect of the biological matrix on the ionization efficiency, while a value above 100% would indicate an enhanced signal and a value below 100% that the signal is decreased. Overall, the SSE for all the analytes was in the range of 99% (thymol) to 250% ((-)-gallocatechin). Furthermore, 91% of all analytes had a SSE between 99 and 130%. The two polyphenol classes that showed the highest average SSE were anthocyanins and catechins which were 145% and 141%, respectively. The signal enhancement of these two classes may be attributed to their structure, as e.g. anthocyanins have a positive charge unlike other polyphenol classes. Although breast milk is an extremely complex matrix, the optimized sample preparation resulted in minimal SSE, a high sensitivity, and decent recoveries for most analytes.
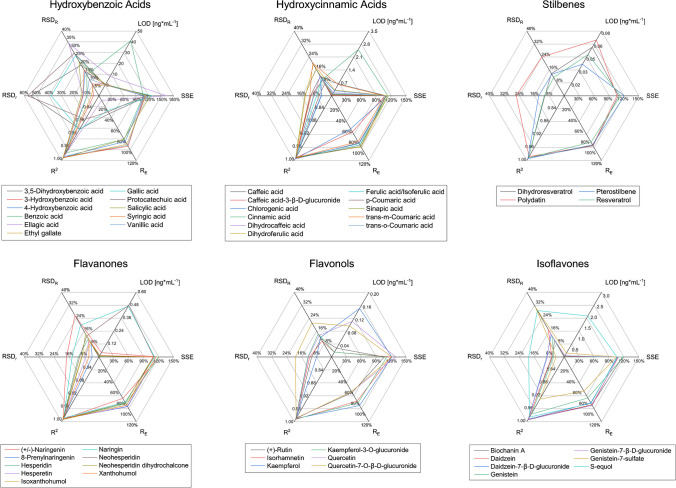
Since the presented assay is a comprehensive multi-analyte method, it was expected that some polyphenol classes performed better than others based on the accepted compromises during sample preparation, chromatographic separation, and mass spectrometric detection. However, the classes without superb performance were not excluded, to give a more holistic overview. The overall validation results and specific figures of merit that did not meet the validation criteria are shown in Table S5. For example, the anthocyanins did not fulfill all validation criteria. This could be attributed to their structure with a positive charge, which makes anthocyanins more polar than other polyphenols. Therefore, during sample preparation, anthocyanins may remain in the aqueous phase during the liquid–liquid extraction step with an organic solvent, leading to their lower recoveries. Moreover, carry-over was observed for anthocyanins in the LC–MS/MS method; thus, for a successful validation, different or more acidic chromatographic conditions would be needed [49, 50]. Also, several catechins, proanthocyanidins, and hydroxybenzoic acids were not successfully validated as some of these analytes showed carry-over. In addition, for the two hydroxybenzoic acids, benzoic acid and ellagic acid, only one MRM transition was available. On the contrary, for dihydrochalcones, flavanones, flavones, flavonols, hydroxycinnamic acids, isoflavones, lignans, and stilbenes, more than 70% of the included analytes fulfilled all stringent validation criteria. The analytical figures of merit evaluated during the method validation for all analytes, separated by polyphenol class, are displayed in Fig. 1 and Fig. S2. It can be observed that polyphenols from the same chemical class typically behave in a similar manner, as they show comparable recoveries, SSEs, intermediate precisions, and repeatability.
Fig. 1.
Analytical figures of merit evaluated during method validation for six selected polyphenol classes (three flavonoid and three non-flavonoid classes). Detailed results for all analytes are reported in Table 1, S4, and S5. The recovery (RE), intermediate precision (RSDR), and repeatability (RSDr) are displayed as the mean of the three spiking levels (low, middle, high). The limit of detection (LOD), calculated from the standard deviation of the lowest spiking level, and signal suppression and enhancement effect (SSE), calculated from the slopes of the calibration curves, are also displayed. For graphical representations of the remaining polyphenol classes, the interested reader is referred to the SI (Fig. S2)
Comparing this novel workflow with previously published methods is challenging as only a limited number of methods have been published that were designed specifically for polyphenols in human breast milk. Many biomonitoring methods investigating xenobiotics in breast milk focused on toxicants, including mycotoxins [36, 47, 51], heavy metals [52, 53], persistent organic pollutants [54, 55], volatile organic compounds [56], phthalates [57], and perfluorinated compounds [58], to study their transfer and potential adverse health impact on infants. The methods that quantify polyphenols in breast milk commonly focus on a fraction of the number of analytes that were included in the method developed here and do not comprehensively investigate all the main polyphenol classes [38, 45, 46, 59]. A method published by Song et al. [45] measured eight flavonoids and several carotenoids in breast milk and reported LODs that were higher than those established in the present study for the majority of the analytes common between both methods. For example, the LODs determined for epicatechin gallate, hesperetin, and quercetin (2.7 ng*mL−1, 6.7 ng*mL−1, and 2.5 ng*mL−1, respectively) were approximately 21, 516, and 21 times, respectively, higher than the LODs determined herein. The next-generation biomonitoring method developed by Jamnik et al. [38] for a wide range of xenobiotics in different biofluids showed LODs that were overall in a similar range as reported here for breast milk, e.g., for the analytes 8-prenylnaringenin, isoxanthohumol, and resveratrol, Jamnik et al. [38] reported LODs of 0.0075 ng*mL−1, 0.0048 ng*mL−1, and 0.15 ng*mL−1, respectively, whereas the LODs reported here were at 0.016 ng*mL−1, 0.0054 ng*mL−1, and 0.043 ng*mL−1 respectively. However, unlike in this work, the included polyphenols, 8-prenylnaringenin, daidzein, enterodiol, enterolactone, genistein, isoxanthohumol, resveratrol, and xanthumol did not fulfill their defined validation criteria. Finally, Lu et al. [46] analyzed twelve polyphenols (six flavonoids and six non-flavonoids) in breast milk. Lu et al. [46] reported mainly higher LODs than the values achieved with the method presented here. For instance, their reported LODs for kampferol, quercetin, and daidzein (2.2 ng*mL−1, 1.2 ng*mL−1, and 0.5 ng*mL−1, respectively) were approximately 15, 11, and 19 times, respectively, higher than the LODs determined with the workflow presented here. Considering the large quantity of positively validated analytes and their relatively low LODs, it can be concluded that, despite its broad chemical coverage and the quite generic sample preparation, the method performs favorably.
Application of the developed method to human breast milk samples
To show its applicability in real-life samples, the validated method was applied in a pilot study to comprehensively assess the polyphenol profiles in 30 breast milk samples from twelve Nigerian mothers obtained at months one, six, and twelve post-delivery. Since some mothers dropped out of the study, and others did not breastfeed until the twelfth month, not all samples were available for every time point.
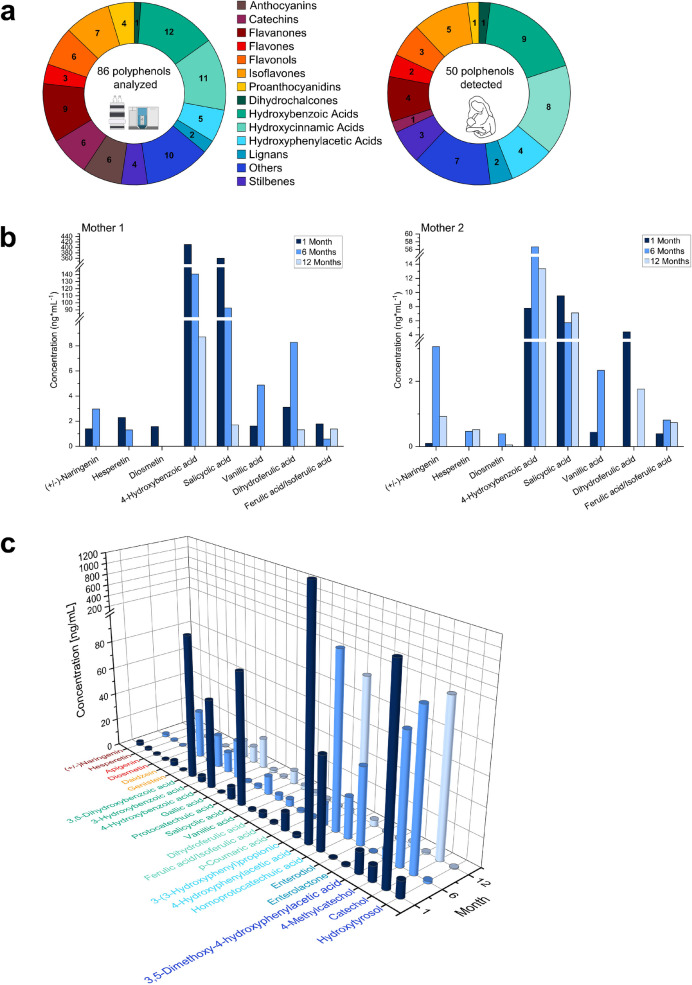
From the 86 polyphenol analytes included in the method, a total of 50 polyphenols, including some metabolic products, were identified in the breast milk samples (Fig. 2a, Table 2). The majority of the detected polyphenols were phenolic acids, a class that includes numerous biotransformation products of larger polyphenols, such as proanthocyanidins [60–62]. Several analytes were detected in a high number of the samples including salicylic acid (found in all 30 samples), an abundant plant metabolite, (+/-)-naringenin (27 samples), a biomarker for citrus fruit consumption [63, 64], and protocatechuic acid (17 samples), a hydroxybenzoic acid present in many vegetables and fruits, and one of the main metabolites of anthocyanins and procyanidins [65, 66]. The polyphenol contents in breast milk can be significantly influenced by several factors. These include dietary habits and the metabolism of the mothers, as well as the polyphenol content of the consumed food, which can be influenced by geographic location and climatic conditions [67]. Examples of chromatographic peaks for polyphenols identified in the pilot study for selected analytes are illustrated in Fig. 2d and e. The quantification of polyphenols present in breast milk provides only a brief insight on the breast milks’ current composition, and it is difficult to compare between different mothers and time points, especially as the sample size is relatively small.
Fig. 2.
a Pie charts showing the number of polyphenol analytes included in the method (left) and the number of polyphenol analytes detected in the pilot study (right) separated by their polyphenol class. b Boxplots of the concentrations for selected analytes at the three different sampling time points for two Nigerian mothers. Only analytes detected with concentrations over the LOQ are displayed. c A 3D boxplot of the average concentration between the twelve mothers for each analyte detected, separated by time points. Only the analytes that were detected at least once per time point and had a concentration over the LOQ are shown
Table 2.
Minimum (min), maximum (max), and mean concentratione of the 50 detected polyphenols in the pilot study of breast milk samples from Nigerian mothers. In addition, the number of samples (n) in which the analyte was positively detected, out of 30 total samples, is listed. The limit of quantification (LOQ) for each detected polyphenol is also given
| Analyte | LOQ (ng*mL−1) | Min (ng*mL−1) | Max (ng*mL−1) | Mean ± standard deviation (ng*mL−1) | n |
|---|---|---|---|---|---|
| Dihydrochalcones | |||||
| Phloretin | 0.034 | < LOQ | < LOQ | - | 2 |
| Hydroxybenzoic acids | |||||
| 3,5-Dihydroxybenzoic acid | 0.82 | < LOQ | 400 | 49 ± 110 | 16 |
| 3-Hydroxybenzoic acid | 1.7 | < LOQ | 43 | 12 ± 12 | 21 |
| 4-Hydroxybenzoic acid | 0.38 | 6.7 | 410 | 38 ± 76 | 30 |
| Ethyl gallate | 0.0048 | 0.05 | 0.05 | 0.05 | 1 |
| Gallic acid | 0.056 | 0.098 | 3.2 | 0.98 ± 1.2 | 6 |
| Protocatechuic acid | 0.12 | < LOQ | 32 | 3.9 ± 9 | 17 |
| Salicylic acid | 0.96 | 1.4 | 360 | 41 ± 93 | 30 |
| Syringic acid | 0.14 | < LOQ | 9.8 | 2.8 ± 4 | 7 |
| Vanillic acid | 0.32 | 0.44 | 4.9 | 2 ± 1.5 | 12 |
| Hydroxycinnamic acids | |||||
| Caffeic acid | 1.1 | 2.7 | 2.8 | 2.8 ± 0.05 | 2 |
| Chlorogenic acid | 0.65 | < LOQ | 8.1 | 4.2 ± 3.3 | 4 |
| Cinnamic acid | 4.3 | 16 | 24 | 20 ± 5.7 | 2 |
| Dihydrocaffeic acid | 0.33 | < LOQ | 290 | 88 ± 120 | 6 |
| Dihydroferulic acid | 0.71 | 1.2 | 8.3 | 3.4 ± 2.3 | 9 |
| Ferulic acid/Isoferulic acid | 0.19 | < LOQ | 5 | 1.3 ± 1.2 | 29 |
| p-Coumaric acid | 0.092 | < LOQ | 23 | 5.6 ± 7.8 | 17 |
| Sinapic acid | 0.17 | < LOQ | 2 | 1.2 ± 1.2 | 6 |
| Hydroxyphenylacetic acids | |||||
| 3-(3-Hydroxyphenyl)propionic acid | 0.76 | < LOQ | 77 | 8.5 ± 20 | 18 |
| 4-Hydroxyphenylacetic acid | 12 | < LOQ | 12000 | 970 ± 2800 | 23 |
| Homovanillic acid | 3.4 | < LOQ | 14 | 6.9 ± 4.2 | 11 |
| Homoprotocatechuic acid | 1.3 | 4.4 | 65 | 27 ± 28 | 5 |
| Lignans | |||||
| Enterodiol | 0.034 | 0.14 | 110 | 22 ± 42 | 6 |
| Enterolactone | 0.038 | 0.21 | 1.9 | 0.54 ± 0.5 | 11 |
| Others | |||||
| 2,6-Dimethoxyphenol | 0.12 | 0.33 | 0.39 | 0.36 ± 0.04 | 2 |
| 3,5-Dimethoxy-4-hydroxyphenylacetic acid | 0.86 | 2.9 | 24 | 8 ± 7.6 | 7 |
| 4-Methylcatechol | 0.48 | 0.96 | 170 | 41 ± 72 | 5 |
| Catechol | 10 | < LOQ | 2100 | 410 ± 550 | 24 |
| Hydroxytyrosol | 0.068 | 0.16 | 23 | 4.3 ± 8.6 | 7 |
| Pyrogallol | 2.8 | 3.6 | 5.4 | 4.4 ± 0.84 | 5 |
| Urolithin A | 0.028 | 0.15 | 0.39 | 0.27 ± 0.17 | 2 |
| Stilbenes | |||||
| Dihydroresveratrol | 0.11 | < LOQ | < LOQ | - | 2 |
| Polydatin | 0.12 | 1 | 1 | 1 | 1 |
| Pterostilbene | 0.068 | 1.4 | 1.4 | 1.4 | 1 |
| Catechins | |||||
| (-)-Epicatechin | 0.48 | 1.2 | 3.5 | 2.4 ± 1.7 | 2 |
| Flavanones | |||||
| (+/-)-Naringenin | 0.072 | < LOQ | 13 | 2.1 ± 2.9 | 27 |
| 8-Prenylnaringenin | 0.032 | 1.3 | 1.3 | 1.3 | 1 |
| Hesperetin | 0.026 | 0.4 | 2.3 | 0.96 ± 0.7 | 6 |
| Xanthohumol | 0.034 | < LOQ | < LOQ | - | 1 |
| Flavones | |||||
| Apigenin | 0.0094 | 0.047 | 1.8 | 0.38 ± 0.59 | 8 |
| Diosmetin | 0.03 | < LOQ | 1.6 | 0.27 ± 0.41 | 20 |
| Flavonols | |||||
| Isorhamnetin | 0.018 | < LOQ | 0.34 | 0.1 ± 0.12 | 10 |
| Kaempferol | 0.34 | < LOQ | 0.64 | 0.6 ± 0.06 | 5 |
| Kaempferol-3-O-glucuronide | 0.026 | 0.21 | 0.82 | 0.51 ± 0.43 | 2 |
| Isoflavones | |||||
| Daidzein | 0.068 | < LOQ | 67 | 16 ± 25 | 15 |
| Daidzein-7-β-D-glucuronide | 0.13 | 0.13 | 0.59 | 0.42 ± 0.22 | 5 |
| Genistein | 0.0094 | 0.08 | 1.1 | 0.35 ± 0.4 | 10 |
| Genistein-7-β-D-glucuronide | 0.11 | < LOQ | 1.9 | 1.1 ± 0.89 | 5 |
| Genistein-7-sulfate | 0.36 | < LOQ | < LOQ | - | 11 |
| Proanthocyanidins | |||||
| Procyanidin C1 | 1.2 | < LOQ | < LOQ | - | 6 |
eThe concentrations were calculated using the matrix-matched calibration curve and corrected with the recovery determined during the method validation
As previously mentioned, comparing the polyphenol concentrations to other studies is not straightforward since only a few published reports focused on polyphenols in human breast milk. A previous study by Jamnik et al. [38] investigated xenobiotics in breast milk from one individual over the first 211 days after birth, including several polyphenols. In that study, 8-prenylnargingenin, daidzein, enterodiol, and enterolactone were quantified at mean concentrations of 0.11 ng*mL−1, 0.032 ng*mL−1, 0.013 ng*mL−1, and < LOQ, respectively, which was lower than the values of 1.3 ng*mL−1, 16 ng*mL−1, 22 ng*mL−1, and 0.54 ng*mL−1, respectively, reported for the same analytes in the present study. Song et al. [45] investigated the phytochemical content in breast milk samples, collected at three different time points, from 17 mothers donated by the Cincinnati Children’s Hospital Medical Center and reported epicatechin, (+/-)-naringenin, hesperetin, and kaempferol at higher average concentrations (42 ng*mL−1, 60 ng*mL−1, 120 ng*mL−1, and 7 ng*mL−1, respectively) compared to the values reported here (2.4 ng*mL−1, 2.1 ng*mL−1, 0.96 ng*mL−1, and 0.6 ng*mL−1, respectively). Furthermore, Song et al. [45] detected epigallocatechin, epigallocatechin gallate, and quercetin, which were not detected in the Nigerian samples. Lu et al. [46] detected twelve different polyphenols in 89 breast milk samples from Hong Kong women. Higher mean concentrations were reported for quercetin, (+/-)-naringenin, caffeic acid, and protocatechuic acid (41 ng*mL−1, 110 ng*mL−1, 30 ng*mL−1, and 112 ng*mL−1, respectively) compared to the values of 2.1 ng*mL−1, 2.8 ng*mL−1, and 3.9 ng*mL−1 for (+/-)-naringenin, caffeic acid, and protocatechuic acid, respectively, in the present study. However, chlorogenic acid, (-)-epicatechin, and daidzein had similar average concentrations of 2 ng*mL−1, 9 ng*mL−1, and 15 ng*mL−1, respectively, compared to the present study. An increased consumption of e.g. tea, which is rich in flavanols, can lead to an increased quercetin concentration, which could explain the amount of quercetin found in Lu et al. [46], whereas an increased intake of legumes and seeds can lead to an increased enterodiol and enterlactone concentrations, as their parent molecule, matairesinol, is prevalent in legumes and seeds [68, 69]. The disparities in the type and concentrations of polyphenols found in the various studies can be attributed to several factors such as different diets of the mothers, differences in analytical sensitivities, and sample size, as well as seasonal and growth-related differences in polyphenol contents [67].
Polyphenols readily undergo phase II biotransformation in the small intestine and liver; hence, a higher concentration of glucuronidated, compared to unconjugated, metabolites are typically detected in urine [60, 70, 71]. Phase II conjugated metabolites, including daidzein-7-β-D-glucuronide and genistein-7-β-D-glucuronide, were detected in several breast milk samples albeit at low concentrations (0.42 ng*mL−1 and 1.1 ng/mL−1, respectively). Overall, genistein and daidzein were present in more breast milk samples than their respective glucuronides. Interestingly, when a sample contained both, the parent compound and the glucuronidated compound, the glucuronide concentration was usually higher than the parent compound (Fig. 2e). It must be noted that as polyphenols have several hydroxyl groups, different positional isomers are possible and only one isomer was included in this method. Thus, different positional isomers of conjugated metabolites could be missed, especially as the LODs for most of the conjugated metabolites were similar or lower than their respective parent compound, e.g., caffeic acid-3-β-D-glucuronide had an LOD of 0.0085 ng*mL−1 and caffeic acid had 0.55 ng*mL−1. To get a more complete picture of polyphenol biotransformation, additional analyses, for example, by untargeted workflows, would be beneficial [72]. Previous studies have also shown that phase II metabolites of other xenobiotics, such as plasticizers, pesticides, and phytoestrogens, can be found in breast milk [33, 41, 57]. Further research is needed to study the pathways and presence of polyphenols and their biotransformation products in human milk.
A rough estimation of the exposure levels of infants to polyphenols was conducted. In order to exclude other possible polyphenol sources, e.g., from complementary foods, only breast milk sampled at month one after birth was used for this estimation. Analytes that were detected below the LOQ value were considered positive and the corresponding LOQ value was applied (i.e., upper bound scenario). An average infant body weight of 4 kg [73] and a daily consumption of 500 mL breast milk were assumed. Based on this estimation (individual, median, and mean daily intakes are reported in Table S6), it was derived that the approximate daily intake per polyphenol detected was in the lower microgram per kilogram body weight range, with the median analyte concentration ranging from 0.0044 µg*kg−1 body weight per day (phloretin) to 31 µg*kg−1 body weight per day (catechol). The most common analytes detected in the breast milk samples were 4-hydroxybenzoic acid, diosmetin, salicylic acid, ferulic acid, and (+/-)-naringenin, and had an estimated median daily intake of 1.9 µg*kg−1, 0.022 µg* kg−1, 0.54 µg* kg−1, 0.094 µg* kg−1, and 0.14 µg* kg−1 of body weight, respectively. Though numerous known polyphenols have not been included in this method, the sum of the investigated polyphenols detected yielded an estimated median daily intake of 57 µg*kg−1. It must be noted that these estimations were calculated for only one sampling time point and should be interpreted with caution. However, the estimates provide rough insights into the exposure of infants towards a large panel of polyphenols. Therefore, to better ascertain the daily polyphenol exposure, further studies are needed that include a larger sample size and information on the polyphenol content of the food consumed by the mothers on the day of sampling (Fig. 3).
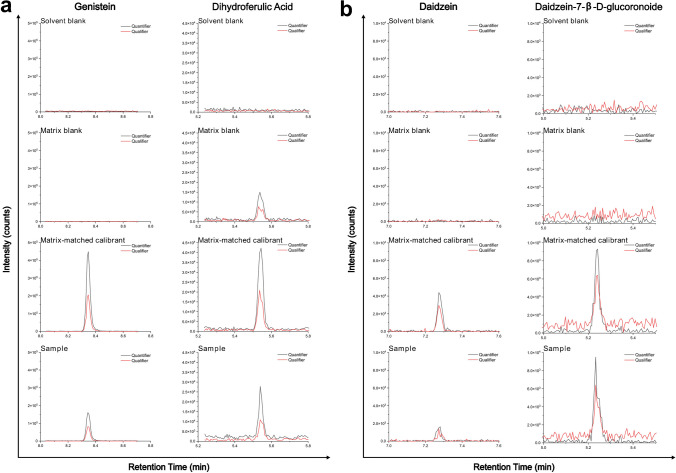
Fig. 3.
a MRM chromatograms (quantifier and qualifier ions) of a solvent blank, a non-spiked breast milk “blank,” a matrix-matched calibrant (0.43 ng*mL−1 for genistein and 1.5 ng*mL−1 for dihydroferulic acid), and a breast milk sample obtained from a Nigerian mother. b MRM chromatograms (quantifier and qualifier ions) of daidzein and daizein-7-β-D-glucuronide from the same mother and same timepoint, with the MRM chromatograms of a solvent blank, a matrix-matched breast milk “blank,” and a matrix-matched calibrant (0.037 ng*mL−1 for daidzein and 0.11 ng*mL−1 for daizein-7-β-D-glucuronide)
Conclusion and outlook
In conclusion, the successful optimization and in-house validation of an LC–MS/MS method targeting 86 polyphenols that are representatives of all major polyphenol classes in human breast milk are presented. Despite low sample volumes, a high-throughput sample preparation, and a wide variety of analytes, this approach demonstrated high sensitivity while retaining high recoveries and low signal suppression and enhancement effects. Moreover, the application of the method in a pilot study demonstrated its feasibility to be readily used in large cohort studies. Thus, it can be applied to investigate and better comprehend the transfer of ingested dietary polyphenols to breast milk, which would be beneficial in further nutritional intervention and prevention studies. Moreover, it can also be used to investigate human metabolism in vivo. Its application in large cohorts would also aid the advanced investigation of the impact of polyphenols in nutritional intervention studies. Finally, this method can also be applied, to better understand the transfer of polyphenols to newborns. Especially in the era of exposome-type research, it may reveal new insights on potential health benefits and polyphenol impact on microbiome development and of co-exposure and mixture of toxicological effects with other xenobiotics that infants are exposed to via their diet and environment.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to sincerely acknowledge all the participating mothers for generously providing milk samples, the Semmelweis Women’s Clinic in Vienna for providing pooled milk samples, and the health personnel involved in the sample processing. Moreover, we thank all team members of the Warth and Rompel labs for their feedback and support during the method optimization and validation process. The Mass Spectrometry Center of the Faculty of Chemistry, University of Vienna is acknowledged for technical support and Sciex for providing mass spectrometric instrumentation and technical advice. For open access purposes, the authors have applied a CC BY public copyright license to any author-accepted manuscript version arising from this submission. Furthermore, the authors would like to thank the University of Vienna for its support via the Exposome Austria Research Infrastructure.
Funding
Open access funding provided by University of Vienna. This research was funded in part by the Austrian Science Fund (FWF) [P32326; A.R.] and the University of Vienna.
Declarations
Ethics approval
Ethical approval was obtained from the Ethical Committee of Babcock University (BUHREC421/21R, BUHREC466/23). Prior to their inclusion in the studies, all mothers were informed and provided written consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singla RK, Dubey AK, Garg A, Sharma RK, Fiorino M, Ameen SM, Haddad MA, Al-Hiary M. Natural polyphenols: chemical classification, definition of classes, subcategories, and structures. J AOAC Int. 2019;102:1397–1400. doi: 10.1093/jaoac/102.5.1397. [DOI] [PubMed] [Google Scholar]
- 2.Dini I, Grumetto L. Recent advances in natural polyphenol research. Molecules. 2022;27:8777. doi: 10.3390/molecules27248777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and human health: the role of bioavailability. Nutrients. 2021;13:273. doi: 10.3390/nu13010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey K, Rizvi S. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi EJ, Kim GH. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol Med Rep. 2014;9:328–332. doi: 10.3892/mmr.2013.1752. [DOI] [PubMed] [Google Scholar]
- 6.Shahidi F, Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. Int J Mol Sci. 2018;19:1573. doi: 10.3390/ijms19061573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scalbert A, Manach C, Morand C, Rémésy C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 9.Michalska M, Gluba A, Mikhailidis D, Nowak P, Bielecka-Dabrowa A, Rysz J, Banach M. The role of polyphenols in cardiovascular disease. Med Sci Monit. 2010;16:110–119. [PubMed] [Google Scholar]
- 10.Bhullar KS, Rupasinghe HPV. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid Med Cell Longev. 2013;2013:891748. doi: 10.1155/2013/891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moradi SZ, Jalili F, Farhadian N, Joshi T, Wang M, Zou L, Cao H, Farzaei MH, Xiao J. Polyphenols and neurodegenerative diseases: focus on neuronal regeneration. Crit Rev Food Sci Nutr. 2022;62:3421–3436. doi: 10.1080/10408398.2020.1865870. [DOI] [PubMed] [Google Scholar]
- 12.Duda-Chodak A, Tarko T. Possible side effects of polyphenols and their interactions with medicines. Molecules. 2023;28:2536. doi: 10.3390/molecules28062536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasa F, Abera T. Factors affecting iron absorption and mitigation mechanisms: a review. Int J Agric Sci Food Technol. 2018:024–030. 10.17352/2455-815X.000033.
- 14.Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81:289–295. doi: 10.1017/S0007114599000537. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Li Y, Han L, Li J, Liu C, Sun C. Role of flavonoids in the treatment of iron overload. Front Cell Dev Biol. 2021;9:685364. doi: 10.3389/fcell.2021.685364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerbin-Koczorowska M, Waszyk-Nowaczyk M, Bakun P, Goslinski T, Koczorowski T. Current view on green tea catechins formulations, their interactions with selected drugs, and prospective applications for various health conditions. Appl Sci. 2021;11:4905. doi: 10.3390/app11114905. [DOI] [Google Scholar]
- 17.Mohos V, Bencsik T, Boda G, Fliszár-Nyúl E, Lemli B, Kunsági-Máté S, Poór M. Interactions of casticin, ipriflavone, and resveratrol with serum albumin and their inhibitory effects on CYP2C9 and CYP3A4 enzymes. Biomed Pharmacother. 2018;107:777–784. doi: 10.1016/j.biopha.2018.08.068. [DOI] [PubMed] [Google Scholar]
- 18.Steuck M, Hellhake S, Schebb NH. Food polyphenol apigenin inhibits the cytochrome P450 monoxygenase branch of the arachidonic acid cascade. J Agric Food Chem. 2016;64:8973–8976. doi: 10.1021/acs.jafc.6b04501. [DOI] [PubMed] [Google Scholar]
- 19.Warth B, Raffeiner P, Granados A, Huan T, Fang M, Forsberg EM, Benton HP, Goetz L, Johnson CH, Siuzdak G. Metabolomics reveals that dietary Xenoestrogens alter cellular metabolism induced by Palbociclib/Letrozole combination cancer therapy. Cell Chem Biol. 2018;25:291–300. doi: 10.1016/j.chembiol.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miadoková E. Isoflavonoids — an overview of their biological activities and potential health benefits. Interdiscip Toxicol. 2009;2:211–218. doi: 10.2478/v10102-009-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchins AM, McIver IE, Johnston CS. Hypertensive crisis associated with high dose soy isoflavone supplementation in a post-menopausal woman: a case report [ISRCTN98074661] BMC Womens Health. 2005;5:9. doi: 10.1186/1472-6874-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vejdovszky K, Schmidt V, Warth B, Marko D. Combinatory estrogenic effects between the isoflavone genistein and the mycotoxins zearalenone and alternariol in vitro. Mol Nutr Food Res. 2017;61:1600526. doi: 10.1002/mnfr.201600526. [DOI] [PubMed] [Google Scholar]
- 23.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies2. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 24.Silva RFM, Pogačnik L. Polyphenols from food and natural products: neuroprotection and safety. Antioxidants. 2020;9:61. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 26.Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Slechta DC, Thompson C, Hanson M. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/en.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25:428–438. doi: 10.1111/pai.12232. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Infant and young child feeding. 2021. https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding. Accessed 7 Nov 2023.
- 29.Poniedziałek B, Rzymski P, Pięt M, Gąsecka M, Stroińska A, Niedzielski P, Mleczek M, Rzymski P, Wilczak M. Relation between polyphenols, malondialdehyde, antioxidant capacity, lactate dehydrogenase and toxic elements in human colostrum milk. Chemosphere. 2018;191:548–554. doi: 10.1016/j.chemosphere.2017.10.098. [DOI] [PubMed] [Google Scholar]
- 30.Vázquez CV, Rojas MGV, Ramírez CA, Chávez-Servín JL, García-Gasca T, Ferriz Martínez RA, García OP, Rosado JL, López-Sabater CM, Castellote AI, Montemayor HMA, De La Torre CK. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin-Ciocalteu method. Food Chem. 2015;176:480–486. doi: 10.1016/j.foodchem.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 31.Grace van der Gugten J. Tandem mass spectrometry in the clinical laboratory: a tutorial overview. Clin Mass Spectrom. 2020;15:36–43. doi: 10.1016/j.clinms.2019.09.002. [DOI] [Google Scholar]
- 32.Oesterle I, Braun D, Rompel A, Warth B. Quantifying up to 90 polyphenols simultaneously in human bio-fluids by LC-MS/MS. Anal Chim Acta. 2022;1216:339977. doi: 10.1016/j.aca.2022.339977. [DOI] [PubMed] [Google Scholar]
- 33.Fareed Y, Braun D, Flasch M, Globisch D, Warth B. A broad, exposome-type evaluation of xenobiotic phase II biotransformation in human biofluids by LC-MS/MS. Exposome. 2022;2:osac008. doi: 10.1093/exposome/osac008. [DOI] [Google Scholar]
- 34.Magnusson B, Örnemark U. Eurachem Guide: The fitness for purpose of analytical methods – a laboratory guide to method validation and related topics, 2014; 2nd ed.
- 35.Commission European. 2002/657/EC Commission decision of 12 august 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. J Eur Commun. 2002;221:8–36. [Google Scholar]
- 36.Braun D, Schernhammer E, Marko D, Warth B. Longitudinal assessment of mycotoxin co-exposures in exclusively breastfed infants. Environ Int. 2020;142:105845. doi: 10.1016/j.envint.2020.105845. [DOI] [PubMed] [Google Scholar]
- 37.Gosetti F, Mazzucco E, Zampieri D, Gennaro MC. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2010;1217:3929–3937. doi: 10.1016/j.chroma.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 38.Jamnik T, Flasch M, Braun D, Fareed Y, Wasinger D, Seki D, Berry D, Berger A, Wisgrill L, Warth B. Next-generation biomonitoring of the early-life chemical exposome in neonatal and infant development. Nat Commun. 2022;13:2653. doi: 10.1038/s41467-022-30204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayeni KI, Seki D, Pjevac P, Hausmann B, Krausová M, Braun D, Wisgrill L, Berry D, Warth B, Ezekiel CN Biomonitoring of dietary mycotoxin exposure and associated impact on the gut microbiome in Nigerian infants. Environ Sci Technol acs.est. 2024;3c07786. 10.1021/acs.est.3c07786. [DOI] [PMC free article] [PubMed]
- 40.Wilkowska A, Biziuk M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011;125:803–812. doi: 10.1016/j.foodchem.2010.09.094. [DOI] [Google Scholar]
- 41.Payá P, Anastassiades M, Mack D, Sigalova I, Tasdelen B, Oliva J, Barba A. Analysis of pesticide residues using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal Bioanal Chem. 2007;389:1697–1714. doi: 10.1007/s00216-007-1610-7. [DOI] [PubMed] [Google Scholar]
- 42.Yang Q, Ai X, Dong J, Liu Y, Zhou S, Yang Y, Xu N. A QuEChERS-HPLC-MS/MS method with matrix matching calibration strategy for determination of imidacloprid and its metabolites in Procambarus clarkii (Crayfish) tissues. Molecules. 2021;26:274. doi: 10.3390/molecules26020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia CV, Gotah A. Application of QuEChERS for determining xenobiotics in foods of animal origin. J Anal Methods Chem. 2017;2017:1–13. doi: 10.1155/2017/2603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuzimski T, Sherma J. Determination of target xenobiotics and unknown compound residues in food, environmental, and biological samples. CRC Press; 2018. [Google Scholar]
- 45.Song BJ, Jouni ZE, Ferruzzi MG. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition. 2013;29:195–202. doi: 10.1016/j.nut.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Lu Z, Chan Y-T, Lo KK-H, Wong VW-S, Ng Y-F, Li S-Y, Ho W-W, Wong M-S, Zhao D. Levels of polyphenols and phenolic metabolites in breast milk and their association with plant-based food intake in Hong Kong lactating women. Food Funct. 2021;12:12683–12695. doi: 10.1039/D1FO02529E. [DOI] [PubMed] [Google Scholar]
- 47.Braun D, Ezekiel CN, Marko D, Warth B. Exposure to mycotoxin-mixtures via breast milk: an ultra-sensitive LC-MS/MS biomonitoring approach. Front Chem. 2020;8:423. doi: 10.3389/fchem.2020.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Y, Peach JT, Warth B. Sample preparation strategies for mass spectrometry analysis in human exposome research: current status and future perspectives. TrAC Trends Anal Chem. 2023;166:117151. doi: 10.1016/j.trac.2023.117151. [DOI] [Google Scholar]
- 49.Grace MH, Xiong J, Esposito D, Ehlenfeldt M, Lila MA. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019;277:336–346. doi: 10.1016/j.foodchem.2018.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuzuak S, Ma Q, Lu Y, Xie D-Y. HPLC-MS (n) applications in the analysis of anthocyanins in fruits. In: Núñez O, Sentellas S, Granados M, Saurina J (eds) High performance liquid chromatography - recent advances and applications. IntechOpen; 2023.
- 51.Muñoz K, Campos V, Blaszkewicz M, Vega M, Alvarez A, Neira J, Degen GH. Exposure of neonates to ochratoxin A: first biomonitoring results in human milk (colostrum) from Chile. Mycotoxin Res. 2010;26:59–67. doi: 10.1007/s12550-009-0040-0. [DOI] [PubMed] [Google Scholar]
- 52.Motas M, Jiménez S, Oliva J, Cámara MÁ, Pérez-Cárceles MD. Heavy metals and trace elements in human breast milk from industrial/mining and agricultural zones of Southeastern Spain. Int J Environ Res Public Health. 2021;18:9289. doi: 10.3390/ijerph18179289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Philip-Slaboh TP, Eleke C, Ezejiofor AN. Comparison of toxic heavy metals in the breast milk of diabetic and non-diabetic postpartum mothers in Yenagoa. Nigeria PLOS ONE. 2023;18:e0264658. doi: 10.1371/journal.pone.0264658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hassan HF, Elaridi J, Kharma JA, Abiad MG, Bassil M. Persistent organic pollutants in human milk: exposure levels and determinants among lactating mothers in Lebanon. J Food Prot. 2022;85:384–389. doi: 10.4315/JFP-21-325. [DOI] [PubMed] [Google Scholar]
- 55.Wasser J, Berman T, Lerner-Geva L, Grotto I, Rubin L. Biological monitoring of persistent organic pollutants in human milk in Israel. Chemosphere. 2015;137:185–191. doi: 10.1016/j.chemosphere.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 56.Kim SR, Halden RU, Buckley TJ. Volatile organic compounds in human milk: methods and measurements. Environ Sci Technol. 2007;41:1662–1667. doi: 10.1021/es062362y. [DOI] [PubMed] [Google Scholar]
- 57.Calafat AM, Slakman AR, Silva MJ, Herbert AR, Needham LL. Automated solid phase extraction and quantitative analysis of human milk for 13 phthalate metabolites. J Chromatogr B. 2004;805:49–56. doi: 10.1016/j.jchromb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Tao L, Ma J, Kunisue T, Libelo EL, Tanabe S, Kannan K. Perfluorinated compounds in human breast milk from several Asian countries, and in infant formula and dairy milk from the United States. Environ Sci Technol. 2008;42:8597–8602. doi: 10.1021/es801875v. [DOI] [PubMed] [Google Scholar]
- 59.Franke AA, Custer LJ. Daidzein and genistein concentrations in human milk after soy consumption. Clin Chem. 1996;42:955–964. doi: 10.1093/clinchem/42.6.955. [DOI] [PubMed] [Google Scholar]
- 60.Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33:220–235. doi: 10.1016/S0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 61.Déprez S, Mila I, Lapierre C, Brezillon C, Rabot S, Philippe C, Scalbert A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr. 2000;130:2733–2738. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- 62.Yang P, Yuan C, Wang H, Han F, Liu Y, Wang L, Liu Y. Stability of anthocyanins and their degradation products from cabernet sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules. 2018;23:354. doi: 10.3390/molecules23020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saenger T, Hübner F, Lindemann V, Ganswind K, Humpf H. Urinary biomarkers for orange juice consumption. Mol Nutr Food Res. 2021;65:2000781. doi: 10.1002/mnfr.202000781. [DOI] [PubMed] [Google Scholar]
- 64.Salehi B, Fokou P, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, Sharifi-Rad J. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12:11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:1–9. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masella R, Santangelo C, D’Archivio M, LiVolti G, Giovannini C, Galvano F. Protocatechuic acid and human disease prevention: biological activities and molecular mechanisms. Curr Med Chem. 2012;19:2901–2917. doi: 10.2174/092986712800672102. [DOI] [PubMed] [Google Scholar]
- 67.Uddin N, Muhammad N, Nisar M, Aisha AN, Ullah R, Ali EA, Khan AA, Rahman IU, Khan A, Zeb A. Distribution of polyphenolic compounds, antioxidant potential, and free amino acids in Ziziphus fruits extract; a study for determining the influence of wider geography. Food Sci Nutr. 2022;10:1414–1430. doi: 10.1002/fsn3.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durazzo A, Turfani V, Azzini E, Maiani G, Carcea M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013;140:666–671. doi: 10.1016/j.foodchem.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 69.Milder IEJ, Arts ICW, Putte BVD, Venema DP, Hollman PCH. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr. 2005;93:393–402. doi: 10.1079/BJN20051371. [DOI] [PubMed] [Google Scholar]
- 70.Spencer JPE, Abd El Mohsen MM, Minihane A-M, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22. doi: 10.1017/S0007114507798938. [DOI] [PubMed] [Google Scholar]
- 71.Van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, Van Velzen EJJ, Gross G, Roger LC, Possemiers S, Smilde AK, Doré J, Westerhuis JA, Van De Wiele T. Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci. 2011;108:4531–4538. doi: 10.1073/pnas.1000098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oesterle I, Pristner M, Berger S, Wang M, Verri Hernandes V, Rompel A, Warth B. Exposomic biomonitoring of polyphenols by non-targeted analysis and suspect screening. Anal Chem. 2023;95:10686–10694. doi: 10.1021/acs.analchem.3c01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. Child growth standards - Weight for age. https://www.who.int/tools/child-growth-standards/standards/weight-for-age. Accessed 7 Nov 2023. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.