Abstract
Gab1 has structural similarities with Drosophila DOS (daughter of sevenless), which is a substrate of the protein tyrosine phosphatase Corkscrew. Both Gab1 and DOS have a pleckstrin homology domain and tyrosine residues, potential binding sites for various SH2 domain-containing adapter molecules when they are phosphorylated. We found that Gab1 was tyrosine phosphorylated in response to various cytokines, such as interleukin-6 (IL-6), IL-3, alpha interferon (IFN-α), and IFN-γ. Upon the stimulation of IL-6 or IL-3, Gab1 was found to form a complex with phosphatidylinositol (PI)-3 kinase and SHP-2, a homolog of Corkscrew. Mutational analysis of gp130, the common subunit of IL-6 family cytokine receptors, revealed that neither tyrosine residues of gp130 nor its carboxy terminus was required for tyrosine phosphorylation of Gab1. Expression of Gab1 enhanced gp130-dependent mitogen-activated protein (MAP) kinase ERK2 activation. A mutation of tyrosine 759, the SHP-2 binding site of gp130, abrogated the interactions of Gab1 with SHP-2 and PI-3 kinase as well as ERK2 activation. Furthermore, ERK2 activation was inhibited by a dominant negative p85 PI-3 kinase, wortmannin, or a dominant negative Ras. These observations suggest that Gab1 acts as an adapter molecule in transmitting signals to ERK MAP kinase for the cytokine receptor gp130 and that SHP-2, PI-3 kinase, and Ras are involved in Gab1-mediated ERK activation.
Cytokine receptors, such as receptors for interleukins, colony-stimulating factors (CSFs), hormones, and interferons, utilize Janus tyrosine kinases (JAKs) for transmitting signals downstream. The JAKs associate with the juxtamembrane domains, called box 1 and box 2, of cytokine receptors. Upon ligand binding to receptors, the receptors dimerize and the receptor-associated JAKs are thought to undergo transphosphorylation as well as phosphorylation of the tyrosine residues in the cytoplasmic domain of the receptors involved. The tyrosine-phosphorylated receptors recruit various SH2 domain-containing adapter molecules such as STATs (signal transducers and activators of transcription) and SHPs (protein tyrosine phosphatases), resulting in the activation of downstream pathways (reviewed in references 9, 23, and 24).
gp130 is the common subunit of receptors for the interleukin-6 (IL-6) family of cytokines (leukemia-inhibitory factor, ciliary neurotropic factor, oncostatin M, IL-11, and CT-1) (reviewed in references 18 and 20). It associates with JAK1, JAK2, and TYK2, and its tyrosine residues are phosphorylated upon stimulation (42). Among the six tyrosine residues in the cytoplasmic domain of gp130, tyrosine 759 (the second tyrosine from the membrane) was shown to be necessary for the recruitment of SHP-2 on gp130 and its tyrosine phosphorylation (16, 43). The four tyrosines in the carboxy terminus have a glutamine at position +3 of tyrosines (YXXQ) and were shown to be required for tyrosine phosphorylation and activation of STAT3 (43, 50). STAT3 was shown to be involved in the cell cycle arrest and macrophage differentiation of a mouse leukemia cell line, M1 (32, 50), and in an antiapoptotic signal for gp130-mediated cell proliferation (16). The mutation of tyrosine 759 to phenylalanine attenuated activation of ERK mitogen-activated protein (MAP) kinases and abolished the transition to G2/M in the cell cycle, correlating with loss of SHP-2 tyrosine phosphorylation (16). These observations indicate a possible involvement of SHP-2 in the activation of MAP kinase pathway. However, the biochemical mechanisms by which SHP-2 regulates downstream signals have not yet been elucidated clearly.
SHP-2 is a protein tyrosine phosphatase bearing two SH2 domains in the amino-terminal region and a phosphatase domain in the carboxy-terminal region (1). SHP-2 was reported to regulate signaling through the receptor tyrosine kinases such as receptors for epidermal growth factor (EGF), fibroblast growth factor (FGF), and insulin (4, 34, 45) and cytokine receptors such as receptors for prolactin, alpha/beta interferon (IFN-α/β), and granulocyte-macrophage CSF (2, 10, 25). SHP-2 was shown to associate with Grb2, which links to Ras pathway through the GDP-GTP exchanger Sos, upon the stimulation of platelet-derived growth factor receptor, EGF receptor, and cytokine receptors (3, 16, 29, 49). Expression of inactive phosphatase mutants suppresses EGF, FGF, or insulin-dependent MAP kinase activation (4, 34, 45, 49). These data indicate that there are two signaling pathways to MAP kinases through SHP-2; one depends on tyrosine phosphorylation of SHP-2, and the other depends on tyrosine phosphatase activity of SHP-2.
Corkscrew (CSW) is a Drosophila homolog of SHP-2 and reported to act downstream of the receptor tyrosine kinases Torso and Sevenless and Drosophila EGF and FGF receptors (DER and Breathless) (36, 37). Biochemical and genetic analysis of Drosophila eye development revealed that Daughter of Sevenless (DOS) is a substrate for CSW and is required for Sevenless signaling, possibly by activating the Ras pathway (17, 38). DOS has structural homologies with mammalian adapter molecules IRS-1, IRS-2, and Gab1. Gab1 is a 115-kDa molecule originally identified as a Grb2-associated docking protein (21). Gab1 was shown to be tyrosine phosphorylated in response to EGF, insulin, nerve growth factor, and c-Met stimulation, and it was shown to bind phospholipase C-γ, phosphatidylinositol (PI)-3 kinase, and SHP-2 in addition to Grb2 (14, 21, 22, 33).
Here we report that Gab1 acts as an adapter molecule in transmitting signals to the ERK MAP kinase for the cytokine receptor gp130.
MATERIALS AND METHODS
Cell culture, transfection, and biological reagents.
HepG2 and 293T cells were maintained as described previously (44). Hep3B cells were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). TF-1 cells were cultured in RPMI medium supplemented with 10% fetal calf serum, human recombinant IL-3 (5 ng/ml; Gibco BRL), penicillin (100 U/ml), and streptomycin (100 μg/ml). To establish 293T stable transfectant cells expressing the chimeric receptors, 293T cells in a 10-cm-diameter dish (approximately 106 cells) were transfected with 20 μg of the expression vectors for the granulocyte CSF receptor (G-CSFR)–gp130 chimeric receptors (16) and 2 μg of pMIK-HygB by a standard calcium phosphate precipitation method. Transfectants were selected with hygromycin (200 μg/ml), and expression of the chimeric receptors was detected by immunoblotting with an anti-G-CSFR antibody. For transient transfection, 1 μg of each expression plasmid (except for the assay represented in Fig. 6b) was transfected in a 6-cm-diameter dish (approximately 5 × 105 cells) of 293T or HepG2 cells, and cells were harvested 20 h after the transfection for further analysis. For the stimulation of cells with cytokines, cells were starved of serum (or IL-3 in the case of TF-1 cells) for 12 h and stimulated with cytokines as indicated in the figure legends.
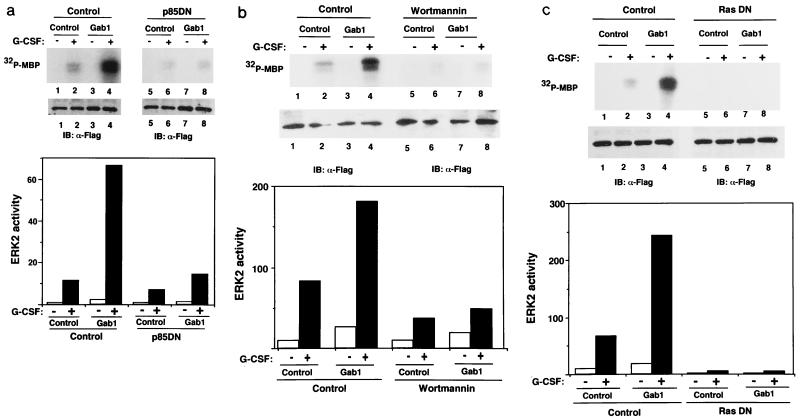
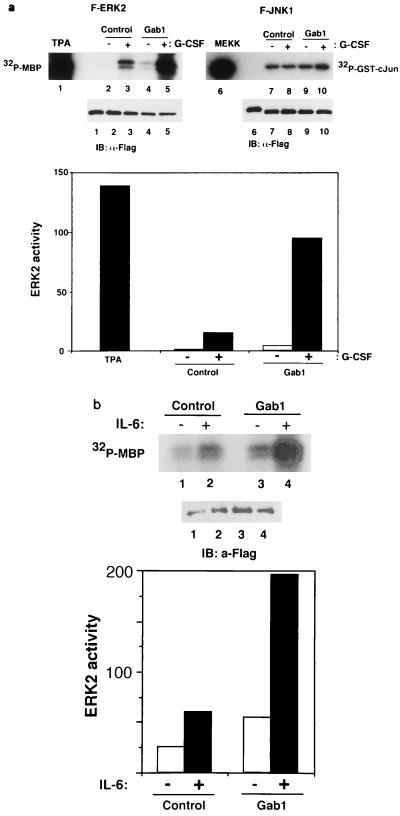
FIG. 6.
PI-3 kinase and Ras are involved in the Gab1-mediated ERK activation. (a) p85DN inhibits ERK2 activation. 293T-G277 cells were transfected with expression vectors for F-ERK2 (lanes 1 and 2), F-ERK2 and H-Gab1 (lanes 3 and 4), F-ERK2 and dominant negative p85DN (lanes 5 and 6), or F-ERK2, H-Gab1, and p85DN (lanes 7 and 8). The amounts of expression vectors were normalized by addition of a mock control vector, pcDNA3. Cells were stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or left unstimulated (−; lanes 1, 3, 5, and 7). ERK2 activities and expression were determined as described for Fig. 5. ERK2 activities in the graph are percentages of that in unstimulated cells (lane 1). (b) Wortmannin inhibits the Gab1-mediated ERK2 activation. 293T-G277 cells were transfected with expression vectors for F-ERK2 (lanes 1, 2, 5, and 6) or F-ERK2 and H-Gab1 (lanes 3, 4, 7, and 8). Cells were incubated in 100 nM wortmannin for 1 h before and during stimulation (lanes 5 to 8) and then stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or left unstimulated (lanes 1, 3, 5, and 7). ERK2 activities were determined by in vitro kinase assay. (c) RasN17 inhibits ERK2 activation. 293T-G277 cells were transfected with expression vectors for F-ERK2 (lanes 1 and 2), F-ERK2 and H-Gab1 (lanes 3 and 4), F-ERK2 and RasN17 (lanes 5 and 6), or F-ERK2, H-Gab1, and RasN17 (lanes 7 and 8). Cells were stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or left unstimulated (−; lanes 1, 3, 5, and 7). ERK2 activities and expression were determined.
Plasmid construction.
Human Gab1 cDNA was amplified from a human bone marrow cDNA library (Clontech) by PCR using primers based on the published sequence (21). The amino acid 472–694 region of human Gab1 was amplified by PCR and subcloned into the EcoRI and HindIII sites of pGEX-KG to generate glutathione S-transferase (GST)–Gab1(472/694) protein for immunization. To construct the expression vector for Gab1, a three-tandem repeat of hemagglutinin (HA) or one Flag epitope was fused to the amino terminus of Gab1 in pBluescript SK+ (Stratagene), and the HindIII fragments containing epitope-tagged Gab1 were subcloned into pcDNA3 (Invitrogen). To construct an expression vector for HA-tagged SHP-2 (H-SHP-2), an NcoI site was generated on the translation initiation codon of SHP-2 by PCR using SRα-PTP1D (a gift from T. Matozaki and M. Kasuga) (19), the NcoI-EcoRI fragments were subcloned in pBluescript 3xHA (44), and the HindIII-EcoRI fragment of H-SHP-2 was subcloned into pcDNA3 (Invitrogen). To construct expression vectors for Myc-tagged p85α (M-p85α) PI-3 kinase and dominant negative p85 (p85DN), Myc epitopes were attached to the carboxy termini of the p85α subunits of PI-3 kinase (wild type and dominant negative; gifts from W. Ogawa, M. Kasuga, and M. D. Waterfield) (39) by PCR as described previously (41) and the EcoRI-ApaI M-p85α fragments were subcloned into pcDNA3. The expression vector for human JAK1 (pEF-JAK1) was previously described (44). The expression vector for Flag epitope-tagged ERK2 (F-ERK2) was constructed by the insertion of NcoI-BamHI sites of ERK2 in SRαHA-ERK2 (30) into the HindIII site of pcDNA3 with a Flag-encoding fragment. The expression vector for F-JNK1 was described previously (12, 13). The dominant negative Ras (RasN17) (a gift from T. Deng) was previously described (11). For all plasmids, we generated restriction sites by PCR using KOD polymerase (Toyobo) to subclone cDNAs in either epitope-tagged vectors or a GST fusion protein expression vector in frame. Primers used for PCR are available on request. Fragments obtained by PCR and subcloning were confirmed by DNA sequencing using an automated ALF sequencer (Pharmacia). GST fusion proteins were prepared as described previously (40).
Immunoprecipitation, immunoblotting, and antibody preparation.
After stimulation, cells were lysed in lysis buffer (20 mM Tris HCl [pH 7.4], 150 mM NaCl, 1% Nonidet P-40, 500 μM sodium vanadate, 1 mM dithiothreitol, aprotinin [5 μg/ml], leupeptin [5 μg/ml] 1 mM phenylmethylsulfonyl fluoride). The lysates were incubated at 4°C for 30 min and were cleared by centrifugation at 10,000 × g for 30 min. The cleared lysates were incubated with 2 μg of monoclonal antibodies (anti-HA, Flag, and Myc antibodies) or 5 μl of antiserum (anti-Gab1 antibody) or polyclonal antibodies and with 10 μl of protein A-Sepharose (Pharmacia). After 10 h of incubation at 4°C, immunoprecipitates were washed with 1 ml of lysis buffer without the protease inhibitors five times. Proteins were eluted with Laemmli’s sodium dodecyl sulfate (SDS) loading buffer, separated on an SDS–4 to 20% gradient polyacrylamide gel (Dai-ich Kagaku), and electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore). The membranes were blocked with TBST (20 mM Tris HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20) containing 1% gelatin and incubated with the primary antibodies (1 μg of monoclonal antibodies or 2,000-times-diluted antiserum or polyclonal antibodies per ml) for 1 h at room temperature. The membranes were washed with TBST for 5 min three times and incubated with 5,000-times-diluted peroxidase-conjugated goat anti-mouse (for monoclonal antibodies) or rabbit (for serum and polyclonal antibodies) immunoglobulin antibodies (Zymed) at room temperature. Then membranes were washed with TBST three times and TBS (20 mM Tris HCl [pH 7.4], 150 mM NaCl) once. The immune complexes were visualized by a chemiluminescence system (Renaissance; Dupont NEN Products). Anti-Gab1 antibody was raised by immunizing a rabbit with purified GST-Gab1(472/694). Anti-HA (12CA5), anti-Myc (9E10), and anti-Flag (M2) antibodies were purchased from Boehringer Mannheim, Genosys Biotechnologies Inc., Kodak, and UBI Corporation, respectively. Anti-SHP-2 (sc 280) and anti-Grb2 (sc 255) antibodies were purchased from Santa Cruz Biotechnology Co. Antiphosphotyrosine (4G10) and anti-p85 PI-3 kinase (06-195) antibodies were purchased from UBI.
PI-3 kinase assay.
PI-3 kinase activity was assayed essentially as described previously (48). Immunoprecipitates from lysates of 106 HepG2 cells were washed once with Dulbecco’s phosphate-buffered saline, twice with 0.5 M LiCl in 100 mM Tris HCl (pH 7.4), and twice with 10 mM Tris HCl (pH 7.4)–100 mM NaCl–1 mM EDTA. Immunoprecipitates were then incubated with 35 μl of final wash buffer containing 20 mM MgCl2 and 0.2 mg of sonicated PI per ml on ice for 20 min. After 10 min of incubation at 25°C, 10 μl of 50 μM ATP and 10 μCi of [γ-32P]ATP were added to the reaction mixture, which was incubated for 20 min at 25°C; the reaction was stopped with 250 μl of 1 N HCl. After extraction with 80 μl of chloroform-methanol (2:1), the mixture was separated on a Silica Gel 60 thin-layer chromatography plate (Merck) in the atmosphere saturated with chloroform–methanol–4 M NH4OH (9:7:2) for 2 h. Labeled PI-3 phosphate was visualized by autoradiography and quantitated by using a BAS1000 image analyzer (Fuji Photo Film Co.).
In vitro MAP kinase assay.
Twenty hours after transfection, Flag epitope-tagged ERK2, JNK1, and p38 MAP kinases were immunoprecipitated with anti-Flag antibody M2 from cell lysates. Immunoprecipitates were washed with lysis buffer without protease inhibitors three times and with kinase buffer (20 mM Tris HCl [pH 7.4], 20 mM MgCl2, 2 mM dithiothreitol). The kinase reaction was performed by incubation with 20 μl of kinase buffer containing 10 μM ATP, [γ-32P]ATP (5 μCi/reaction), and 1 μg of substrate (myelin basic protein [MBP] for ERK2, GST–c-Jun for JNK1, and GST-ATF2 for p38) at 30°C for 10 min. The reaction was stopped by the addition of 3× Laemmli’s SDS loading buffer. The phosphorylated substrates were separated by SDS-polyacrylamide gel electrophoresis, and incorporated 32P was quantitated with a BAS1000 image analyzer.
RESULTS
SHP-2 and PI-3 kinase associate with a tyrosine-phosphorylated Gab1 in response to IL-6.
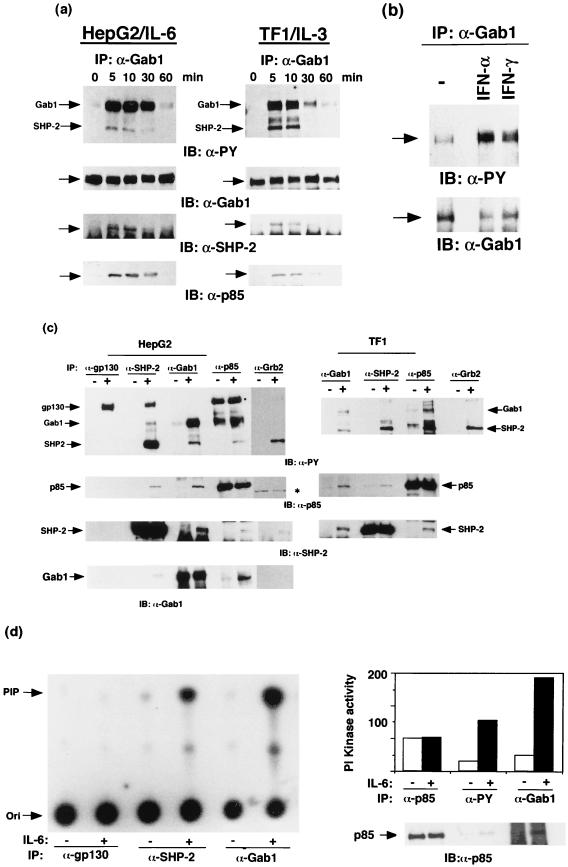
To reveal the roles of SHP-2 and PI-3 kinase in cytokine signaling, we examined the nature of the molecules associating with SHP-2 and the p85 subunit of PI-3 kinase following stimulation. When HepG2 cells were stimulated with IL-6, gp130, SHP-2 (70 kDa), and an unknown 110-kDa molecule were detected in the SHP-2 immunoprecipitates on the antiphosphotyrosine blots (Fig. 1c). We also detected tyrosine-phosphorylated 110- and 70-kDa molecules, identical to SHP-2 as described below, in the p85 immunoprecipitates from the stimulated cells, although the p85 PI-3 kinase itself was not tyrosine phosphorylated. These observations raised the possibility that both SHP-2 and PI-3 kinase form a complex with the tyrosine-phosphorylated 110-kDa molecule (pp110) in response to IL-6.
FIG. 1.
Tyrosine phosphorylation of Gab1 and its association with SHP-2 and the p85 subunit of PI-3 kinase. (a) IL-6 and IL-3 induce tyrosine phosphorylation of Gab1. HepG2 and TF-1 cells (106) were stimulated with IL-6 (100 ng/ml) and IL-3 (10 ng/ml), respectively, for the indicated period. Gab1 was immunoprecipitated (IP) with an anti-Gab1 antibody (α-Gab1), transferred to a membrane, and immunoblotted (IB) with antiphosphotyrosine (α-PY), anti-Gab1, anti-SHP-2, or anti-p85 antibodies. The arrows indicate the locations of Gab1, SHP-2, and p85. Abbreviations apply to all figures. (b) IFN-α and -γ induce tyrosine phosphorylation of Gab1. Hep3B cells were stimulated with IFN-α or IFN-γ (100 ng/ml) for 15 min or not stimulated (−). The Gab1 immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine (upper panel) or anti-Gab1 (lower panel) antibodies. Locations of Gab1 are indicated by arrows. (c) Gab1 associates with SHP-2 and PI-3 kinase in response to IL-6 or IL-3. HepG2 and TF-1 cells were stimulated with IL-6 and IL-3, respectively. gp130, SHP-2, Gab1, the p85 subunit of PI-3 kinase, or Grb2 was immunoprecipitated with anti-gp130, anti-SHP-2, anti-Gab1, anti-p85, or anti-Grb2 antibodies, respectively, and blotted with antiphosphotyrosine, p85, SHP-2, or Gab1 antibodies as indicated. The 160-kDa bands (marked by a dot) in the p85 immunoprecipitates on antiphosphotyrosine blotting were nonspecific, since they were not recognized by anti-gp130 antibody (data not shown), and the 80-kDa bands in the Grb2-immunoprecipitates (marked by an asterisk) were also nonspecific since they migrated differently from p85. Note that a relatively large amount of Gab1 was detected in the p85 immunoprecipitates from the unstimulated HepG2 cells. This is likely due to a low level of tyrosine phosphorylation of Gab1 in the unstimulated cells and the fact that phosphorylated Gab1 was concentrated by the associated p85. (d) PI-3 kinase activity associates with Gab1 and SHP-2. HepG2 cells (106) were stimulated with IL-6 (100 ng/ml) for 10 min (+) or not stimulated (−). Lysates were immunoprecipitated with anti-gp130, anti-Gab1, anti-SHP-2, anti-p85, or antiphosphotyrosine antibodies (near-saturation amount), and their associated PI-3 kinase activities were determined by in vitro kinase reaction using PI as a substrate. PI phosphate (PIP) was separated by thin-layer chromatography as described in Materials and Methods and analyzed by autoradiography. The incorporated radioactivity was quantitated by an image analyzer. Two independent data sets obtained from separate experiments are represented by an autoradiograph (left panel) and a bar graph (right panel). The amounts of p85 in anti-p85, antiphosphotyrosine, or anti-Gab1 antibody immunoprecipitates were analyzed by immunoblotting with the anti-p85 antibody (right panel, bottom). Arrows on the left indicate locations of origin (Ori) and PIP. Numbers on the graph are arbitrary units of PI kinase activity obtained from the image analyzer.
Gab1 is a possible candidate for pp110, since it was shown to bind SHP-2 and p85 in vivo (21) and the molecular mass of Gab1 was ∼115 kDa. To examine whether pp110 is Gab1, we raised a polyclonal antibody that specifically recognizes the Gab1 molecule. The 110-kDa molecule was immunoprecipitated with the anti-Gab1 antibody from HepG2 and TF-1 (human IL-3-dependent leukemia cell line) cells. Gab1 was found to be tyrosine phosphorylated within 5 min following stimulation with either IL-6 or IL-3 (Fig. 1a). In correlation with the Gab1 tyrosine phosphorylation, SHP-2 and p85 were detected in the Gab1 immunoprecipitates (Fig. 1a and c). Gab1 was detected in both the SHP-2 and p85 immunoprecipitates from the stimulated cells (in the case of TF-1 cells, Gab1 was not detected in the SHP-2 immunoprecipitates due to the low amount of SHP-2-associated Gab1, but SHP-2 was detected in the Gab1 immunoprecipitates). Furthermore, the immunoprecipitates of SHP-2 and p85 from the stimulated cells contained p85 and SHP-2, respectively (Fig. 1c). The data indicated that Gab1 forms a complex with SHP-2 and p85 in response to IL-6 and IL-3. Tyrosine phosphorylation of Gab1 was also induced in Hep3B cells in response to IFN-α and -γ (Fig. 1b). A low level of tyrosine phosphorylation of Gab1 was detected in unstimulated cells in certain conditions (Fig. 1b and c; see also Fig. 3b and 5a). This may reflect the fact that these cells secrete low amounts of soluble factors, such as EGF, hepatocyte growth factor, and insulin, inducing tyrosine phosphorylation of Gab1. Tyrosine-phosphorylated gp130 was detected in the SHP-2 immunoprecipitates from the stimulated cells but not in the Gab1 or p85 immunoprecipitates (Fig. 1c, left panel; SHP-2 was not detected in the gp130 immunoprecipitates due to the stoichiometric interaction between SHP-2 and gp130). Consistent with these data, we detected PI kinase activities in both the SHP-2 and Gab1 immunoprecipitates from the stimulated cells but not the gp130 immunoprecipitates (Fig. 1d). These results suggest that the Gab1–SHP-2–PI-3 kinase complex is distinct from the SHP-2–gp130 complex. Furthermore, the PI kinase activities associated with Gab1 were comparable to those in immunoprecipitates of the anti-p85 and antiphosphotyrosine antibodies (Fig. 1d, right panel). We could not simply compare the PI kinase activities in the immunoprecipitates of those antibodies since they have different affinities for their own antigens. However, given that Gab1 was the major tyrosine-phosphorylated protein in the p85 immunoprecipitates (Fig. 1c), it is likely that Gab1 was the major component interacting with the active PI-3 kinase in IL-6-stimulated HepG2 cells.
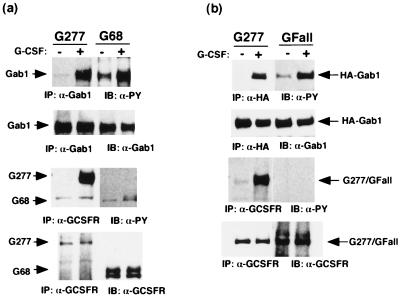
FIG. 3.
Tyrosine phosphorylation or the carboxy-terminal region of gp130 is not necessary for tyrosine phosphorylation of Gab1. (a) The carboxy-terminal region of gp130 is not necessary for tyrosine phosphorylation of Gab1. 293T cells stably expressing the chimeric receptor G277 (containing the entire cytoplasmic domain of gp130) or G68 (containing 68 amino acid residues from the membrane) were stimulated with G-CSF (100 ng/ml) for 10 min (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti-Gab1 or anti-G-CSFR antibodies and immunoblotted with antiphosphotyrosine, anti-Gab1, or anti-G-CSFR antibodies, as indicated. The arrows indicate the locations of Gab1 and the chimeric receptors. (b) Tyrosine phosphorylation of gp130 is not necessary for Gab1 tyrosine phosphorylation. 293T cells were transiently transfected with the expression vectors for G-CSFR–gp130 chimeric receptors, G277 and G-Fall, in which all six tyrosines in the cytoplasmic domain of gp130 were mutated to phenylalanines. Cells were stimulated with G-CSF (+) or left unstimulated (−). Gab1 or the chimeric receptors were immunoprecipitated and blotted with antiphosphotyrosine, anti-Gab1, or anti-G-CSFR antibodies. Arrows indicate the locations of Gab1 and the chimeric receptors.
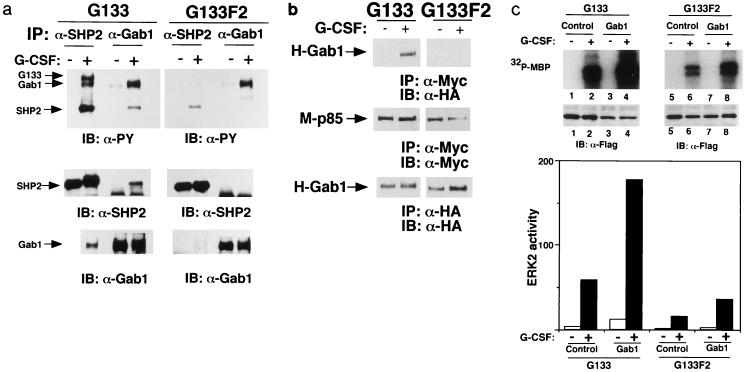
FIG. 5.
Tyrosine 759, the SHP-2 binding site of gp130, is essential for the interactions between Gab1 and SHP-2, or Gab1 and PI-3 kinase, and Gab1-mediated ERK activation. (a) Interaction between Gab1 and SHP-2 depends on tyrosine 759. 293T cells stably expressing G133 (293T-G133) or G133F2 (293T-G133F2), in which tyrosine 759 was mutated to phenylalanine, were stimulated with G-CSF or left unstimulated. SHP-2 and Gab1 were immunoprecipitated with the specific antibodies and blotted with antiphosphotyrosine, anti-SHP-2, or anti-Gab1 antibodies. The locations of SHP-2, Gab1, and G133 (the chimeric receptor) are indicated by arrows. (b) Interaction between Gab1 and PI-3 kinase depends on tyrosine 759. 293T-G133 and 293T-133F2 cells were transfected with expression vectors for M-p85 and H-Gab1. Cells were stimulated with G-CSF or left unstimulated. Lysates were immunoprecipitated with anti-Myc (for p85) or anti-HA (Gab1) antibodies and analyzed by immunoblotting. Essentially the same results were obtained for the interaction between endogenous p85 and Gab1 (data not shown). (c) Tyrosine 759 of gp130 is necessary for Gab1-dependent ERK2 activation. 293T-G133 and 293T-G133F2 cells were transfected with expression vectors for F-ERK2 (lanes 1, 2, 5, and 6) or F-ERK2 and H-Gab1 (lanes 3, 4, 7, and 8). Cells were stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or not stimulated (−; lanes 1, 3, 5, and 7). ERK2 kinase activities were determined by in vitro kinase assay and are illustrated by an autoradiograph and a bar graph as described for Fig. 6. The expression of ERK2 was analyzed by immunoblotting with anti-Flag antibody. ERK2 activities in the graph are percentages of MBP-incorporated 32P for ERK2 obtained from unstimulated G133F2-expressing cells (lane 5).
PI-3 kinase associates with SHP-2 indirectly through Gab1.
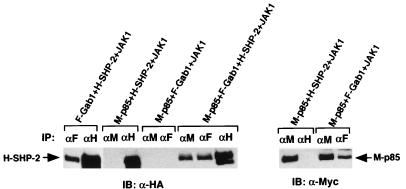
To characterize the Gab1–SHP-2–PI-3 kinase complex, we reconstituted the complex in 293T cells (Fig. 2). Expression vectors were constructed to express Gab1, SHP-2, and p85 tagged by Flag, HA, and Myc epitopes, respectively, and transfected together with a JAK1 expression vector into 293T cells in various combinations. Gab1 and SHP-2 were tyrosine phosphorylated when JAK1 was highly expressed (Fig. 4b and data not shown). When Gab1 and SHP-2, or Gab1 and p85, were expressed, SHP-2 and p85 were detected in the Gab1 immunoprecipitates, indicating interactions between Gab1 and SHP-2 or between Gab1 and p85. When p85 and SHP-2 were expressed, neither SHP-2 nor p85 was detected in the p85 or SHP-2 immunoprecipitates, respectively. However, when p85 and SHP-2 were expressed together with Gab1, SHP-2 was detected in both the p85 and Gab1 immunoprecipitates, indicating that the interaction between p85 and SHP-2 is not direct but mediated by Gab1.
FIG. 2.
Interaction between Gab1 and PI-3 kinase, Gab1 and SHP-2, or SHP-2 and PI-3 kinase. PI-3 kinase associates with Gab1 directly and with SHP-2 through Gab1. 293T cells were transfected with the expression vectors for F-Gab1, H-SHP-2, and M-p85 with a JAK1 expression vector as indicated. Cell lysates were immunoprecipitated with anti-Flag (F), -HA (H), or -Myc (M) antibodies, and SHP-2 and p85 protein were blotted with anti-HA (left) and anti-Myc (right) antibodies.
FIG. 4.
Expression of Gab1 enhances gp130-mediated ERK2 MAP kinase activation. (a) Expression of Gab1 and the stimulation of gp130 synergistically induce kinase activity of ERK2. For the analysis of ERK2 activation, 293T cells expressing the G277 chimeric receptor (293T-G277) were transiently transfected with expression vectors for F-ERK2 alone (lane 1), F-ERK2 and a mock control (pcDNA3; lanes 2 and 3), or F-ERK2 and H-Gab1 (lanes 4 and 5). Cells were stimulated with 100 μM tetradecanoyl phorbol acetate (lane 1) or G-CSF (100 ng/ml) for 30 min (+; lanes 3 and 5) or left unstimulated (−; lanes 4 and 6). ERK2 was immunoprecipitated with anti-Flag antibody, and its activity was determined by in vitro kinase assay using MBP as a substrate. Phosphorylated MBP was separated on an SDS-polyacrylamide gel and analyzed by autoradiography. For the analysis of JNK1 activation, 293T-G277 cells were transfected with expression vectors for F-JNK1 and MEKK1 (a positive control; lane 6), F-JNK1 and a mock control (pcDNA3; lanes 7 and 8), or F-JNK1 and H-Gab1 (lanes 9 and 10). Cells were stimulated with G-CSF (+; lanes 8 and 10) or left unstimulated (−; lanes 7 and 9). JNK1 was immunoprecipitated with anti-Flag antibody, and its activity was determined by in vitro kinase assay using GST–c-Jun (1/79) as a substrate. ERK2 and JNK1 expression was detected by immunoblotting with anti-Flag antibodies. Incorporated 32P in MBP was quantified by an imaging analyzer, and the results are shown in a bar graph (lower panel). ERK2 activities in the graph are percentages of MBP-incorporated 32P for ERK2 obtained from unstimulated cells (lane 2). (b) Gab1 activates IL-6-dependent ERK2 activation. HepG2 cells were transiently transfected with expression vectors for F-ERK2 and a mock control (lanes 1 and 2) or F-ERK2 and H-Gab1 (lanes 3 and 4). Cells were stimulated with IL-6 (100 ng/ml) for 30 min (+; lanes 2 and 4) or left unstimulated (−; lanes 1 and 3). Kinase activities and expression levels of F-ERK2 were determined as described above.
Tyrosine phosphorylation or carboxy-terminal region of gp130 is not required for Gab1 tyrosine phosphorylation.
Tyrosine phosphorylation of SHP-2 and STAT3 strictly depends on phosphorylation of tyrosine residues on gp130. The IL-4-induced tyrosine phosphorylation of IRS-1, a structural homolog of Gab1, strictly depends on phosphorylation of tyrosine 497 of the IL-4 receptor α chain (27). We examined whether tyrosine phosphorylation of Gab1 requires tyrosine phosphorylation of gp130. We used 293T cells stably expressing the G-CSFR–gp130 chimeric receptor (293T-G277 cells) and its mutants. Endogenous Gab1 was tyrosine phosphorylated upon stimulation of 293T cells expressing the chimeric receptor G277, which contains the entire cytoplasmic domain of gp130. This observation was similar to that for cells expressing G68, which contains only the 68 amino acid residues of the cytoplasmic domain and was not tyrosine phosphorylated upon stimulation (Fig. 3a). To completely rule out the involvement of tyrosine residues of gp130, we transiently transfected the expression vector for the chimeric receptor G277 and the mutant G-Fall, in which all six tyrosine residues of the cytoplasmic gp130 were mutated to phenylalanines, together with H-Gab1. Gab1 was tyrosine phosphorylated in 293T cells expressing G-Fall as much as in the cells expressing G277 (Fig. 3b), revealing that tyrosine residues were not necessary for the Gab1 phosphorylation.
Gab1 acts upstream of ERK MAP kinases in gp130 signaling.
To identify the downstream signaling molecules from Gab1, we determined the effect of Gab1 expression on gp130-dependent MAP kinase activation. 293T cells expressing the G277 chimeric receptor were transfected with expression vectors for Gab1 with Flag epitope-tagged MAP kinase ERK2, JNK1, or p38a. The activities of immunoprecipitated MAP kinases were determined by an in vitro kinase reaction. Expression of Gab1 enhanced the basal ERK2 activity and further increased gp130-mediated ERK2 activation (Fig. 4a). The activity of JNK1 or p38a was not affected by the expression of Gab1 in either unstimulated or stimulated cells (Fig. 4a and data not shown). Furthermore, enhancement of ERK2 activities by Gab1 expression was also observed in the IL-6-stimulated HepG2 cells (Fig. 4b). The data indicate that Gab1 acts specifically upstream of ERK MAP kinase in gp130 signaling.
SHP-2 and PI-3 kinase are involved in the Gab1-dependent ERK activation.
To determine the role of SHP-2 in Gab1-mediated ERK activation, we used 293T cells expressing the G133 chimeric receptor (293T-G133) and its mutant G133F2 (293T-G133F2), in which tyrosine 759, the SHP-2 binding site of gp130, was mutated to phenylalanine. We observed the interaction between Gab1 and SHP-2 in 293T-G133 cells upon stimulation, but the interaction was diminished in 293T-G133F2 cells in correlation with a reduction of tyrosine phosphorylation of SHP-2 (Fig. 5a). The interaction between Gab1 and p85 was also observed in the stimulated 293T-G133 cells but was abolished in 293T-G133F2 cells (Fig. 5b). ERK2 was activated upon stimulation in 293T-G133 cells, but its activation was strongly diminished in 293T-G133F2 cells (Fig. 5c). Although the expression of Gab1 enhanced ERK2 activation by the G133 chimeric receptor, it did not do so efficiently in 293T-G133F2 cells (Fig. 5c). The remaining activity of ERK2 and tyrosine phosphorylation of SHP-2 in the stimulated 293T-G133F2 cells were observed (Fig. 5a and c). These might be caused by the direct interaction between JAK and SHP-2 as reported previously (51). The correlation among the Gab1–SHP-2 interaction, the Gab1–PI-3 kinase interaction, and ERK2 activation suggests that the complex formation of Gab1 with SHP-2 and PI-3 kinase is required for the Gab1-mediated ERK2 activation.
To further confirm the role of PI-3 kinase in Gab1-mediated ERK2 activation, p85DN, which lacks the inter-SH2 domain, the binding region for the p110α catalytic subunit of PI-3 kinase (39), was expressed with Gab1 in 293T-G277 cells. The activation of ERK2 was strongly inhibited by the expression of p85DN but not the control vector (Fig. 6a). The inhibition of ERK by p85DN was also observed in 293T-G133 cells (data not shown), indicating that its effect did not depend on the carboxy terminus of gp130. Gab1-dependent ERK2 activation was also inhibited by incubation with 100 nM wortmannin (Fig. 6b). These data indicate that PI-3 kinase is involved in Gab1-mediated ERK2 activation.
Moreover, we examined whether Ras is involved in Gab1-mediated ERK activation. A dominant negative Ras (RasN17) was expressed with Gab1 in 293T-G277 cells and inhibited the ERK2 activation induced by gp130 stimulation and Gab1 expression (Fig. 6c), revealing that Ras is essential for gp130-mediated and Gab1-mediated ERK2 activation.
DISCUSSION
Gab1 is an adapter for the cytokine receptor.
Recent reports have shown the interaction of SHP-2 with a tyrosine-phosphorylated molecule with a molecular mass of 90 to 120 kDa in signaling of receptor tyrosine kinases such as EGF, insulin, and macrophage CSF as well as IL-3 receptor (7, 8, 15, 28). These include the recently identified SHPS-1/SIR family transmembrane proteins (15, 28) and other proteins (8, 28). It was also reported that NGF, BDNF, and IL-3 induce the interaction between SHP-2 and PI-3 kinase in certain cells (8, 35, 47). Ciliary neurotropic factor, leukemia-inhibitory factor, and IL-6 were reported to induce the interaction between p85 and a tyrosine-phosphorylated 110-kDa molecule in the Ewing’s sarcoma cell line (5). We found that SHP-2 interacted with p85 and that the interaction was mediated by the 110-kDa tyrosine-phosphorylated Gab1. These reports and our present findings suggest that the 110-kDa molecule interacting with SHP-2 or p85 in response to various stimuli is Gab1. Gab1 acts as an adapter for certain cytokine receptors and possibly receptor tyrosine kinases. Our data do not exclude the possibility that other 90- to 120-kDa molecules such as other unidentified DOS homologs are involved in the formation of the complex.
Gab1 is a direct substrate for JAKs.
Like Gab1, IRS-1 was shown to be tyrosine phosphorylated in response to IL-2, IL-4, IL-7, IL-15, oncostatin M, and interferons in addition to insulin (6, 26). Tyrosine phosphorylation of IRS-1 and the interaction of IRS-1 with the L-4 receptor α chain in response to IL-4 depends on the phosphorylation of tyrosine 497 of IL-4 receptor α chain (27). The interaction is mediated by the phosphotyrosine binding domain of IRS-1. Gab1 also has a phosphotyrosine binding domain, named MBD (Met binding domain), which bind the specific tyrosine residues of the hepatocyte growth factor receptor c-Met (46). In the case of gp130 signaling, our data revealed that tyrosine phosphorylation of Gab1 does not require tyrosine residues of gp130 and that the membrane-proximal region of gp130 containing only 68 amino acid residues is sufficient to induce tyrosine phosphorylation of Gab1 (Fig. 3). This region of gp130 contains the JAK binding sites and is sufficient to activate JAKs (data not shown). Receptors for IL-3, IFN-α, and IFN-γ, which associate with JAKs but do not share tyrosine-based motifs on their receptors with gp130, all could induce tyrosine phosphorylation of Gab1 (Fig. 1a and b). Taken together, our findings suggest that Gab1 is a substrate of JAKs and that its phosphorylation does not depend on tyrosine phosphorylation of the cytokine receptors. IRS-1 and IRS-2 were shown to associate with JAK1 and JAK3 (26). Gab1 may interact with JAKs through the MBD of Gab1 and phosphotyrosines of JAKs, subjected to tyrosine phosphorylation by JAKs. Biochemical analysis of the interaction between the MBD of Gab1 and JAKs is necessary to elucidate this point.
Roles of Gab1 in signal transduction of cytokine receptors.
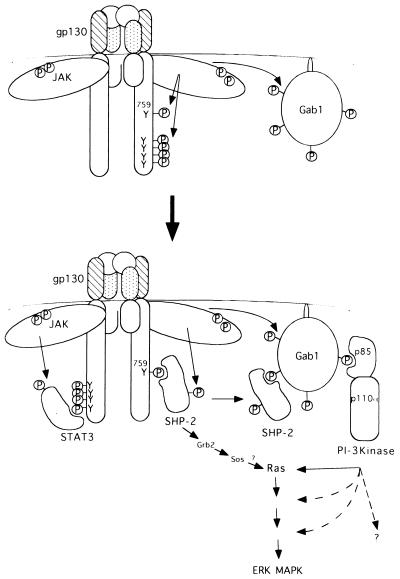
The high expression of Gab1 induced kinase activity of ERK2 in 293T cells, consistent with the finding that expression of Gab1 in MDCK cells induced a mobility shift of ERK MAP kinase (46). Further, the expression of Gab1 enhanced the gp130-dependent ERK2 activation, suggesting a role of Gab1 in transmitting signals to Ras. Gab1 was shown to bind Grb2 (21), but we could not detect an interaction between Gab1 and Grb2 in HepG2 or TF-1 cells, indicating the possible utilization of some other pathway in MAP kinase activation in these cells. The mutation of tyrosine 759 of gp130 diminished the ERK2 activation induced by gp130 stimulation and Gab1 expression, in correlation with a reduction of interactions of Gab1 with SHP-2 and PI-3 kinase (Fig. 5a and b), suggesting roles of SHP-2 and PI-3 kinase in ERK activation. The inhibition of ERK2 activation by the p85DN PI-3 kinase or wortmannin further supports the role of PI-3 kinase in ERK activation (Fig. 6a and b). It is likely that Gab1 mediates signaling through PI-3 kinase to Ras (Fig. 7), since the dominant negative Ras also inhibited the ERK2 activation elicited by the gp130 stimulation and Gab1 expression (Fig. 6c). However, the present study did not exclude the possibility that PI-3 kinase acts in parallel to the Ras pathway, and both Ras and PI-3 kinase activation may be required for gp130-mediated ERK activation (Fig. 7). The SHP-2–Grb2–Sos pathway previously described (16) may cooperate with the Gab1-mediated PI-3 kinase pathway to activate the ERK MAP kinase.
FIG. 7.
Model of roles of Gab1 in gp130 signaling. For details, see Discussion. MAPK, MAP kinase.
The mechanism by which SHP-2 mediates signals to the ERK MAP kinases has been largely unknown. Intriguingly, we found that Gab1 did not interact with SHP-2 or PI-3 kinase, both containing SH2 domains, in cells expressing G133F2 although Gab1 was tyrosine phosphorylated (Fig. 5a and b). These data indicate that the interactions of Gab1 with SHP-2 and PI-3 kinase are not simply mediated by interactions between phosphotyrosines and SH2 domains. The Gab1–SHP-2 interaction may be mediated by the MBD of Gab1 and phosphotyrosines of SHP-2, which depends on phosphorylation of tyrosine 759 of gp130. The interaction of Gab1 with SHP-2 may modify the conformation or phosphorylation status of Gab1, allowing Gab1 to interact with PI-3 kinase and activate downstream signaling pathways. Further mutational analysis of Gab1 and SHP-2 will clarify this point. In any case, Gab1 plays an important role in transmitting signals to ERK MAP kinases through SHP-2 and PI-3 kinase in gp130 signaling.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank T. Matozaki, W. Ogawa, M. Kasuga, M. D. Waterfield, K. Matsuoka, and T. Deng for various reagents. We also thank E. Barsoumian for comments on the manuscript and R. Masuda and T. Kimura for excellent secretarial assistance.
This work was supported in part by a Grant-Aid for COE Research from the Ministry and Education, Science, Sports, and Culture in Japan, the Yamanouchi Foundation for Research on Metabolic Disorders, and the Osaka Foundation for Promotion of Clinical Immunology.
REFERENCES
- 1.Adachi M, Fischer E H, Ihle J, Imai K, Jirik F, Neel B, Pawson T, Shen S-H, Thomas M, Ullrich A, Zhao Z. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85:15. doi: 10.1016/s0092-8674(00)81077-6. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Chen Z, Lebrun J-J, Vogel W, Kharitonenkov A, Kelly P A, Ullrich A. PTP1D is a positive regulator of the prolactin signal leading to beta-casein promoter activation. EMBO J. 1996;15:135–142. [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett A M, Tang T L, Sugimoto S, Walsh C T, Neel B G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett A M, Housdorff S F, O’Reilly A M, Freeman R O, Jr, Neel B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulton T G, Stahl N, Yancopoulos G D. Ciliary neurotropic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269:11648–11655. [PubMed] [Google Scholar]
- 6.Burfoot M S, Rogers N C, Watling D, Smith J M, Pons S, Ciliberto G, Pellegrini S, White M F, Kerr I M. Janus kinase-dependent activation of insulin receptor substrate 1 in response to IL-4, oncostatin M and the interferons. J Biol Chem. 1997;272:24183–24190. doi: 10.1074/jbc.272.39.24183. [DOI] [PubMed] [Google Scholar]
- 7.Carlberg K, Rohrschneider L R. Characterization of a novel tyrosine phosphorylated 100kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J Biol Chem. 1997;272:15943–15950. doi: 10.1074/jbc.272.25.15943. [DOI] [PubMed] [Google Scholar]
- 8.Craddock B L, Welham M J. Interleukin-3 induces association of the protein-tyrosine phosphatase SHP2 and phosphatidylinositol 3-kinase with a 100-kDa tyrosine-phosphorylated protein in hematopoietic cells. J Biol Chem. 1997;272:29281–29289. doi: 10.1074/jbc.272.46.29281. [DOI] [PubMed] [Google Scholar]
- 9.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 10.David M, Zhou G, Pine R, Dixon J E, Larner A C. The SH2 domain-containing tyrosine phosphatase PTP1D is required for interferon alpha/beta-induced gene expression. J Biol Chem. 1996;271:15862–15865. doi: 10.1074/jbc.271.27.15862. [DOI] [PubMed] [Google Scholar]
- 11.Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371:171–175. doi: 10.1038/371171a0. [DOI] [PubMed] [Google Scholar]
- 12.Dérijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 13.Dérijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 14.Fixman E D, Holgado-Madruga M, Nguyen L, Kamikura D M, Fournier T M, Wong A J, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cb1 and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- 15.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 17.Herbst R, Carroll R M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of Sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during Sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 18.Hibi M, Nakajima K, Hirano T. IL-6 cytokine family and signal transduction: a model of the cytokine system. J Mol Med. 1996;74:1–12. doi: 10.1007/BF00202068. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi R, Krummel B, Saiki R K. A general method of in vitro preparation and specific mutagenesis of DNA fragments stably of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 21.Holgado-Madruga M, Emlet D R, Mosacatello D K, Godwin A K, Wang A J. Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;319:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 22.Holgado-Madruga M, Moscatello D K, Emlet D R, Dieterich R, Wong A J. Grb2-associated binder-1 mediates phosphatidyl inositol 3-kinase activation and promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ihle J N. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 24.Ihle J N. Stats: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T, Muto A, Watanabe S, Miyajima A, Yokota T, Arai K. Granulocyte-macrophage stimulating factor provokes RAS activation and transcription of c-fos through different modes of signaling. J Biol Chem. 1996;271:7587–7592. doi: 10.1074/jbc.271.13.7587. [DOI] [PubMed] [Google Scholar]
- 26.Johnston J A, Wang L M, Hanson E P, Sun X J, White M F, Oakes S A, Pierce J H, O’Shea J J. Interleukin 2, 4, 7, and 15 stimulate tyrosine phosphorylation of insulin receptor substrates 1 and 2 in T cells. Potential role of JAK kinases. J Biol Chem. 1995;270:28527–28530. doi: 10.1074/jbc.270.48.28527. [DOI] [PubMed] [Google Scholar]
- 27.Keegan A D, Nelms K, White M, Wang L-M, Pierce J H, Paul W E. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76:811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 28.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibits signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J H, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis R J, Johnson G C, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 31.Miura O, Nakamura N, Ihle J N, Aoki N. Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J Biol Chem. 1994;269:614–620. [PubMed] [Google Scholar]
- 32.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen L, Holgado-Maruga M, Maroun C, Fixman E D, Kamikura D, Fournier T, Charest A, Tremblay M L, Wong A, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada N, Wada K, Goldsmith B A, Koizumi S. SHP-2 is involved in neurotrophin signaling. Biochem Biophys Res Commun. 1996;229:607–611. doi: 10.1006/bbrc.1996.1851. [DOI] [PubMed] [Google Scholar]
- 36.Perkins L A, Larsen I, Perrimon N. Corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70:225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- 37.Perkins L A, Johnson M R, Melnick M B, Perrimon N. The nonreceptor protein tyrosine phosphatase corkscrew functions in multiple receptor tyrosine kinase pathway in Drosophila. Dev Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- 38.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maröy P, Hafen H. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between Sevenless and Ras1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 39.Sakae H, Hara K, Noguchi T, Matozaki T, Kotani K, Ogawa W, Yonezawa K, Waterfield M D, Kasuga M. Ras-independent and wortmannin-sensitive activation of glycogen synthase by insulin in Chinese hamster ovary cells. J Biol Chem. 1995;270:11304–11309. doi: 10.1074/jbc.270.19.11304. [DOI] [PubMed] [Google Scholar]
- 40.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1990;67:37–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 41.Squinto S P, Aldrich T H, Lindsay R M, Morrissey D M, Panayotatos N, Bianco S M, Furth M E, Yancopoulos G D. Identification of functional receptors for ciliary neurotrophic factor on neuronal cell lines and primary neurons. Neuron. 1990;5:757–766. doi: 10.1016/0896-6273(90)90334-c. [DOI] [PubMed] [Google Scholar]
- 42.Stahl N, Boulton T G, Farruggella T, Ip N Y, Davis S, Witthuhn B A, Quelle F W, Silvennoinen O, Barbieri G, Pellegrini S, Ihle J N, Yancopoulos G D. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 43.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J J, Yancopoulos G D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi-Tezuka M, Hibi M, Fujitani Y, Fukada T, Yamaguchi T, Hirano T. Tec tyrosine kinase links the cytokine receptors to PI-3 kinase probably through JAK. Oncogene. 1997;14:2273–2282. doi: 10.1038/sj.onc.1201071. [DOI] [PubMed] [Google Scholar]
- 45.Tang T L, Freeman R M, Jr, O’Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 46.Weidner K M, Di-Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1997;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 47.Welham M J, Dechert U, Leslie K B, Jirik F, Schrader J S. Interleukin (IL)-3 and granulocyte/macrophage colony stimulating factor, but not IL-4, induce tyrosine phosphorylation, activation, association of SHPTP2 with Grb2 and phosphatidylinositol 3′-kinase. J Biol Chem. 1994;269:23764–23768. [PubMed] [Google Scholar]
- 48.Whitman M, Kaplan D R, Schaffhausen B, Cantley L, Roberts T M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 49.Wong L, Johnson G R. Epidermal growth factor induces coupling of protein-tyrosine phosphatase 1D to GRB2 via the COOH-terminal SH3 domain of GRB2. J Biol Chem. 1996;271:20981–20984. doi: 10.1074/jbc.271.35.20981. [DOI] [PubMed] [Google Scholar]
- 50.Yamanaka Y, Nakajima K, Fukada T, Hibi M, Hirano T. Differentiation and growth arrest signals generate through the cytoplasmic region of gp130 that is essential for Stat3 activation. EMBO J. 1996;15:1557–1565. [PMC free article] [PubMed] [Google Scholar]
- 51.Yin T, Shen R, Feng G S, Yang Y C. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J Biol Chem. 1997;272:1032–1037. doi: 10.1074/jbc.272.2.1032. [DOI] [PubMed] [Google Scholar]