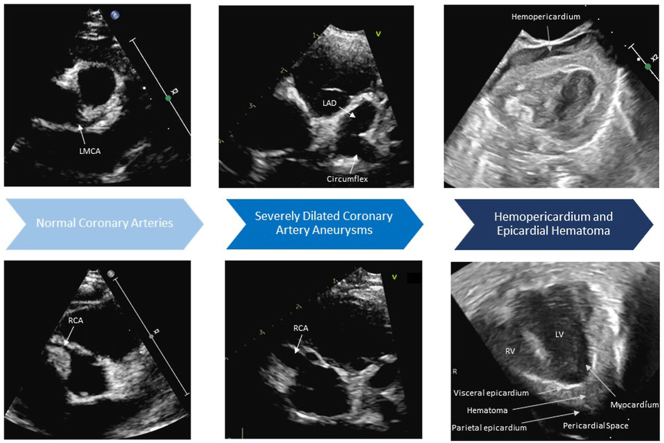
Graphical abstract
Keywords: Coronary artery aneurysm, Rupture, Kawasaki disease, SARS-COV-2, Hemopericardium
Highlights
-
•
CAA rupture is a rare, life-threatening complication of KD.
-
•
SARS-CoV-2 and KD may have a synergistic effect resulting in severe inflammation.
-
•
Recognizing specific echocardiogram findings is necessary to diagnosis CAA rupture.
Introduction
Rupture of a coronary artery aneurysm (CAA) is rare and is the most serious complication of acute Kawasaki disease (KD) with giant CAAs. Giant aneurysms that rapidly dilate and continue to increase in size are more susceptible to rupture. We describe and present echocardiographic imaging from a unique case of KD associated with coincidental SARS-CoV-2 infection that progressed to coronary artery rupture despite aggressive treatment.
Case Presentation
A 2-month-old previously healthy boy developed rhinorrhea, cough, and fever and was diagnosed with SARS-CoV-2 infection. Their symptoms resolved after a few days, but fevers reoccurred after 7 days of being afebrile and they were hospitalized 12 days later due to 6 days of high fever. On physical examination, the patient had a polymorphous rash, erythematous and cracked lips, pedal edema, and recent bilateral conjunctival injection meeting the criteria for KD. Lab abnormalities at admission included a sodium level of 131 mmol/L, white blood cell count of 15,100 cells/mm3 with a neutrophil percentage of 76%, hemoglobin level of 8.7 g/dL, C-reactive protein (CRP) level of 11.94 mg/dL, pro-brain natriuretic peptide N-terminal level of 1,837 pg/mL, D-dimer level of 3,145 ng/mL, fibrinogen level of 188 mg/dL, and albumin level 2.5 g/dL. SARS CoV-2 polymer chain reaction was positive with an otherwise negative extended viral panel. Transthoracic echocardiogram (TTE) at admission demonstrated normal biventricular size and function, normal coronary artery sizes (Figures 1A and 2A, Videos 1 and 2), and no pericardial effusion. Although multisystem inflammatory syndrome in children (MIS-C) was considered—given the close timeline from acute infection (6 days after initial COVID symptoms and testing), patient age, and meeting all of the classic features of KD—a diagnosis of SARS-CoV-2-triggered KD was favored.
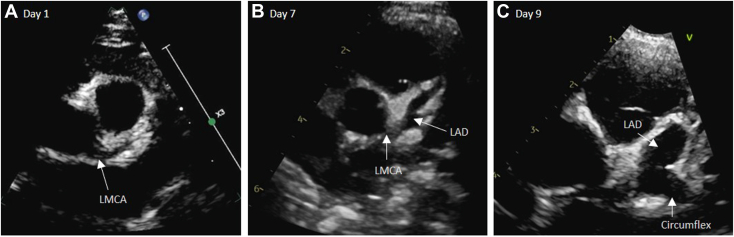
Figure 1.
Two-dimensional TTE, basal parasternal short-axis view in diastole, demonstrates (A) the normal LMCA upon hospital admission, (B) progressive dilation of the LMCA (3.5 mm, Z score +5.1) and LAD (3.6 mm, Z score +12.2) on day 7, and (C) rapid formation of the giant LAD aneurysm (5.7 mm, Z score +22) on day 9.
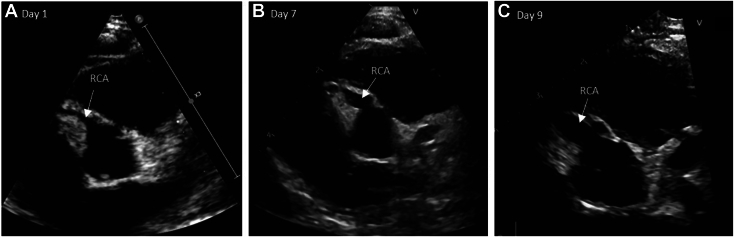
Figure 2.
Two-dimensional TTE, basal parasternal short-axis view in diastole, demonstrates (A) the normal RCA upon hospital admission, (B) progressive dilation of the RCA (3.7 mm, Z score +7.1) on day 7, and (C) rapid formation of the giant RCA aneurysm (4.2 mm, Z score +8.6) on day 9.
The patient was admitted to the general pediatrics service and started on treatment for KD with aspirin 40 mg/kg/day and intravenous immunoglobin (IVIG) 2 g/kg. After the initial dose of IVIG, there was improvement in clinical exam (resolving rash) and the CRP trend within 24 hours. They remained afebrile for 48 hours, but due to fever recurrence on hospital day 4 they were initiated on treatment for refractory KD with methylprednisolone 2 mg/kg and a second dose of IVIG. A repeat TTE on hospital day 5 demonstrated normal coronary arteries without aneurysms. Despite treatment, they had evidence of ongoing systemic inflammation, and the CRP doubled over the next 2 days, although they remained afebrile. Repeat TTE on hospital day 7 showed normal ventricular function and new CAAs of the left main coronary artery (LMCA), left anterior descending (LAD), and right coronary artery (RCA) with LMCA 3.5 mm (Z score +5.1), LAD 3.6 mm (Z score +12.2), and RCA 3.7 mm (Z score +7.1; Figures 1B and 2B, Videos 3 and 4). Given steroid-refractive KD and the development of new CAA, they were started on infliximab, high-dose methylprednisolone, and low-molecular-weight heparin for coronary thrombosis prophylaxis. Inflammatory markers, including the CRP, trended down to <1 mg/dL within 48 hours and remained low throughout the remainder of the hospital course. On hospital day 9, repeat TTE continued to show progression of coronary artery dilation with a giant LAD aneurysm of 5.7 mm (Z score +22) and RCA 4.2 mm (Z score +8.6; Figures 1C and 2C, Videos 5 and 6). Ventricular function remained normal. They were started on amlodipine for strict blood pressure control, clopidogrel in addition to low-molecular-weight heparin, and aspirin for coronary artery thrombosis prevention and continued on telemetry with daily electrocardiograms to closely monitor for signs of coronary artery ischemia. On hospital day 11, TTE demonstrated further worsening coronary artery dilation with 2 giant CAAs with LAD of 7.3 mm (Z score +30.6) and RCA 6.4 mm (Z score +15.4), as well as a large circumflex aneurysm. On hospital day 12, they received cyclophosphamide for rapid progression of CAA and were started on carvedilol for additional blood pressure control with good response. They were clinically well, remaining afebrile with improvements in rash, mucositis, irritability, and appetite. However, on hospital day 14 they acutely became hypoxic and bradycardic, rapidly developing into a cardiac arrest. They received cardiopulmonary resuscitation while being placed on extracorporeal membrane oxygenation (ECMO). Despite multiple attempts at ECMO cannulation, cannula repositioning, and blood product administration, adequate ECMO flows could not be achieved. Transthoracic echocardiogram showed no cardiac activity, as well as a global epicardial hematoma and hemopericardium, consistent with CAA rupture as the etiology of the cardiac arrest (Figure 3A and B, Videos 7 and 8). Given the absence of cardiac activity for over 90 minutes, further interventions (such as sternotomy exploration) were not pursued, and the patient died after resuscitation efforts were stopped.
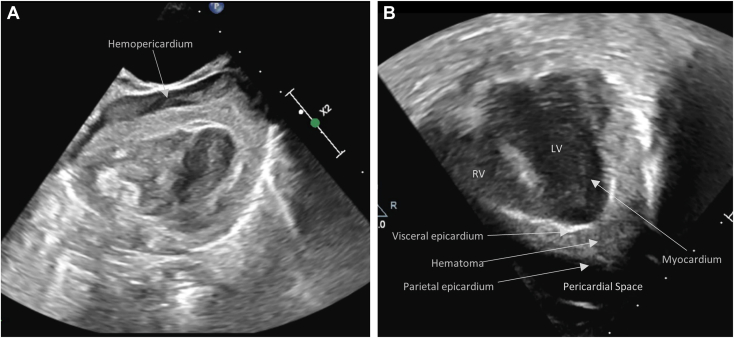
Figure 3.
(A) Two-dimensional TTE, subcostal and (B) apical 4-chamber views, demonstrates fluid in the pericardial space and echogenic material between the thickened visceral epicardial layer and separate parietal epicardial layer consistent with a hemopericardium and epicardial hematoma, respectively. LV, Left ventricle; RV, right ventricle.
Discussion
Kawasaki disease is an acute vasculitis and inflammatory syndrome of childhood with coronary artery involvement representing the most significant complication, ranging from coronary artery dilation to giant CAA.1 In the era of IVIG therapy, about 5% of patients will have CAA, with about 1% to 2.6% developing giant CAA (absolute coronary artery dimension ≥8 mm or Z score ≥10).2 Most patients that develop CAA will have coronary artery dilation seen by echocardiogram in the first 10 days of illness; however, our patient had normal coronary artery measurements until day 13 of illness prior to rapid dilation. Regression of CAA occurs in up to 75% of patients, although giant CAA usually persists, with regression occurring in <20% of those with giant CAA at diagnosis. Giant CAA puts patients at risk for coronary artery thrombosis, stenosis, myocardial infarction, and, rarely, aneurysm rupture.3
In this case, TTE was indicated to diagnose and closely follow the progressive coronary artery dilation1 and was necessary to ultimately diagnose an extensive epicardial hematoma associated with hemopericardium by imaging performed during the resuscitation to guide ECMO cannulation. Specific echocardiographic findings for epicardial hematoma include extensive thickening of the visceral epicardial layer with material of a different echogenicity than the blood pool or normal pericardial fluid and separate from the compacted myocardium (Figure 3B, Video 8). The parietal epicardial layer should also appear intact so that a distinction between the epicardial space and pericardial space is clear. Similarly, imaging of an acute hemopericardium demonstrates fluid with increased echogenicity relative to a chronic or transudative effusion (Figure 3A, Video 7); this increased echogenicity may often result in misdiagnosis if clinical suspicion is not high. The combination of the epicardial hematoma and hemopericardium for this patient is consistent with rupture of a giant CAA leading to epicardial hematoma that itself ruptured into the pericardial sac, producing acute myocardial ischemia, tamponade, and unrecoverable cardiac arrest.
Although CAA rupture is an extremely rare event, it is a catastrophic complication. A review performed in 2014 identified only 11 known patients with CAA rupture, with 4 of these patients 6 months old or less.4 Some of these patients were similar to our patient and developed giant CAA despite adequate treatment with IVIG. Mortality is high as CAA rupture results in hemopericardium and cardiac tamponade. Only 3 cases have reported survival of patients with ruptured CAA, all significantly older than the currently reported patient. One case described a 3-year-old with rupture of a CAA who underwent emergent ECMO cannulation and pericardiocentesis as a bridge to sternotomy for drainage of hemopericardium and suture repair of the ruptured portion of the left coronary artery, ultimately proceeding to coronary artery bypass graft (CABG).5 However, this patient suffered severe hypoxic brain injury. Another case described a 3-year-old with CAA rupture of the LMCA who underwent sternotomy and decompression of the hemopericardium, emergent CABG, and oversewing of the aneurysm but who ultimately required heart transplantation.6 Lastly, a 5-year-old with rupture of a right CAA underwent pericardiocentesis and bedside pericardial window and required an emergent CABG ultimately leading to survival.7 For our case, unfortunately, despite 90 minutes of CPR and multiple attempts at ECMO cannulation, there was no return of cardiac activity so further interventions were not pursued.
Notably, our patient was diagnosed with SARS-CoV-2 12 days prior to hospital presentation. With the clinical symptoms and inflammatory lab findings, they met the Centers for Disease Control and Prevention definition of MIS-C. SARS-CoV-2-associated MIS-C typically occurs 3 to 6 weeks after SARS-CoV-2 infection and has significant overlap with KD causing diffuse inflammation, cytokine storm, and multiorgan involvement.8 Cardiac complications including coronary artery involvement in MIS-C are common, with up to 24% of patients developing coronary artery dilation or aneurysms.9, 10, 11 Widespread inflammation on pathologic specimens has been found in the epicardium and coronary arteries in both KD and MIS-C.12 Although dilation is usually mild, giant CAA has been reported in some patients with MIS-C.13,14 One case describes a 16-month-old infant with a recent COVID-19 infection who met the criteria for KD and was diagnosed with a KD/MIS-C overlap syndrome that developed rapid coronary dilation and a giant CAA, sharing some similarities with our patient.14 There are no other known prior reported cases of CAA rupture in patients with SARS-CoV-2 or MIS-C. In our case, we hypothesize that the recent SARS-CoV-2 infection prior to the development of KD may have had a synergistic effect resulting in treatment-resistant inflammation and rapid coronary artery dilation, ultimately resulting in CAA rupture and death despite aggressive treatment.
Conclusion
Rupture of a CAA is a rare but life-threatening complication of KD. Giant aneurysms that dilate rapidly despite treatment are more susceptible to rupture. In our case, a recent SARS-CoV-2 infection prior to the development of KD may have had a synergistic effect resulting in treatment-resistant inflammation and rapid coronary artery dilation. A high index of suspicion and knowledge of echocardiographic findings specific for epicardial hematoma and hemopericardium are necessary to promptly diagnose CAA rupture. Rapid diagnosis is critical given the high rate of mortality associated with CAA rupture.
Consent Statement
The authors declare that informed patient consent was not provided for the following reason: The authors declare that informed patient consent was not provided for the following reason.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Acknowledgments
We thank Ebonee Gabbidon for acquisition of images.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2023.11.007.
Supplementary Data
Two-dimensional TTE, basal parasternal short-axis view of the left coronary arteries, demonstrates normal measurements upon hospital admission.
Two-dimensional TTE, basal parasternal short-axis view, demonstrates the normal RCA upon hospital admission.
Two-dimensional TTE, basal parasternal short-axis view of the left coronary arteries, demonstrates progressive dilation of the LMCA (3.5 mm, Z score +5.1) and LAD (3.6 mm, Z score +12.2) on day 7.
Two-dimensional TTE, basal parasternal short-axis view, demonstrates progressive dilation of the RCA (3.7 mm, Z score +7.1) on day 7.
Two-dimensional TTE, basal parasternal short-axis view of the left coronary arteries, demonstrates rapid giant CAA formation of the LAD (5.7 mm, Z score +22) on day 9.
Two-dimensional TTE, basal parasternal short-axis view, demonstrates rapid formation of the giant RCA aneurysm (4.2 mm, Z score +8.6) on day 9.
Two-dimensional TTE, subcostal 4-chamber view, demonstrates fluid in the pericardial space with increased echogenicity consistent with hemopericardium.
Two-dimensional TTE, apical 4-chamber view, demonstrates echogenic material between the thickened visceral epicardial layer and separate parietal epicardial layer consistent with epicardial hematoma.
References
- 1.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young. Council on Cardiovascular and Stroke Nursing. Council on Cardiovascular Surgery and Anesthesia. Council on Epidemiology and prevention Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Ogata S., Tremoulet A.H., Sato Y., Ueda K., Shimizu C., Sun X., et al. Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol. 2013;168:3825–3828. doi: 10.1016/j.ijcard.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman K.G., Gauvreau K., Hamaoka-Okamoto A., Tang A., Berry E., Tremoulet A.H., et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5:e003289. doi: 10.1161/JAHA.116.003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto T., Ikeda K., Ishii Y., Kobayashi T. Rupture of a coronary artery aneurysm in Kawasaki disease: a rare case and review of the literature for the past 15 years. J Thorac Cardiovasc Surg. 2014;147:e67–e69. doi: 10.1016/j.jtcvs.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H., Kuroko Y., Kotani Y., Sakoda N., Kasahara S. A ruptured coronary artery aneurysm secondary to Kawasaki disease. JACC Case Rep. 2022;4:790–793. doi: 10.1016/j.jaccas.2022.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutlas T.C., Wernovsky G., Bridges N.D., Suh E.J., Godinez R.I., Nicolson S.C., et al. Orthotopic heart transplantation for Kawasaki disease after rupture of a giant coronary artery aneurysm. J Thorac Cardiovasc Surg. 1997;113:217–218. doi: 10.1016/S0022-5223(97)70421-5. [DOI] [PubMed] [Google Scholar]
- 7.Mok G.C., Sung R.Y., Yam M.C., Arifi A.A., Lam W.W., Fok T.F. A child with Kawasaki disease who survived after rupture of a coronary artery aneurysm. Eur J Pediatr. 2003;162:634–636. doi: 10.1007/s00431-003-1265-0. [DOI] [PubMed] [Google Scholar]
- 8.Chin S.E., Bhavsar S.M., Corson A., Ghersin Z.J., Kim H.S. Cardiac complications associated with COVID-19, MIS-C, and mRNA COVID-19 vaccination. Pediatr Cardiol. 2022;43:483–488. doi: 10.1007/s00246-022-02851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son M.B.F., Murray N., Friedman K., Young C.C., Newhams M.M., Feldstein L.R., et al. Overcoming COVID-19 Investigators Multisystem inflammatory syndrome in children–initial therapy and outcomes. N Engl J Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J., et al. COVID-19-Associated multisystem inflammatory syndrome in children–United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperotto F., Friedman K.G., Son M.B.F., VanderPluym C.J., Newburger J.W., Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180:307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giryes S., McGonagle D. Immune and non-immune mechanisms that determine vasculitis and coronary artery aneurysm topography in Kawasaki disease and MIS-C. Autoimmun Rev. 2022;22 doi: 10.1016/j.autrev.2022.103240. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. J Am Med Assoc. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navaeifar M.R., Shahbaznejad L., Sadeghi Lotfabadi A., Rezai M.S. COVID-19-associated multisystem inflammatory syndrome complicated with giant coronary artery aneurysm. Case Rep Pediatr. 2021;2021 doi: 10.1155/2021/8836403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, basal parasternal short-axis view of the left coronary arteries, demonstrates normal measurements upon hospital admission.
Two-dimensional TTE, basal parasternal short-axis view, demonstrates the normal RCA upon hospital admission.
Two-dimensional TTE, basal parasternal short-axis view of the left coronary arteries, demonstrates progressive dilation of the LMCA (3.5 mm, Z score +5.1) and LAD (3.6 mm, Z score +12.2) on day 7.
Two-dimensional TTE, basal parasternal short-axis view, demonstrates progressive dilation of the RCA (3.7 mm, Z score +7.1) on day 7.
Two-dimensional TTE, basal parasternal short-axis view of the left coronary arteries, demonstrates rapid giant CAA formation of the LAD (5.7 mm, Z score +22) on day 9.
Two-dimensional TTE, basal parasternal short-axis view, demonstrates rapid formation of the giant RCA aneurysm (4.2 mm, Z score +8.6) on day 9.
Two-dimensional TTE, subcostal 4-chamber view, demonstrates fluid in the pericardial space with increased echogenicity consistent with hemopericardium.
Two-dimensional TTE, apical 4-chamber view, demonstrates echogenic material between the thickened visceral epicardial layer and separate parietal epicardial layer consistent with epicardial hematoma.