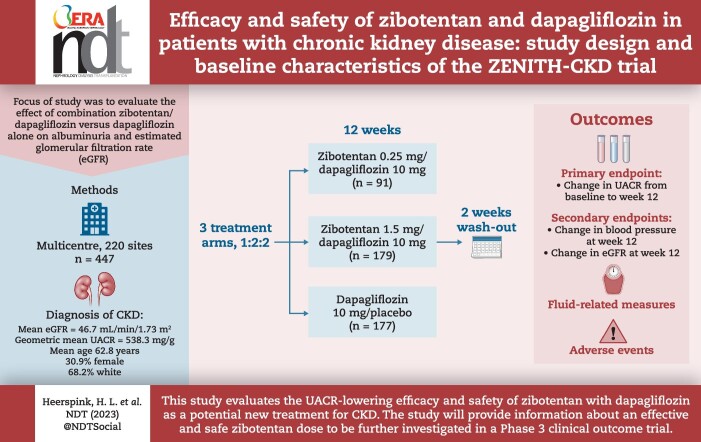
ABSTRACT
Background
Sodium–glucose co-transporter 2 inhibitors (SGLT2is) are part of the standard of care for patients with chronic kidney disease (CKD), both with and without type 2 diabetes. Endothelin A (ETA) receptor antagonists have also been shown to slow progression of CKD. Differing mechanisms of action of SGLT2 and ETA receptor antagonists may enhance efficacy. We outline a study to evaluate the effect of combination zibotentan/dapagliflozin versus dapagliflozin alone on albuminuria and estimated glomerular filtration rate (eGFR).
Methods
We are conducting a double-blind, active-controlled, Phase 2b study to evaluate the efficacy and safety of ETA receptor antagonist zibotentan and SGLT2i dapagliflozin in a planned 415 adults with CKD (Zibotentan and Dapagliflozin for the Treatment of CKD; ZENITH-CKD). Participants are being randomized (1:2:2) to zibotentan 0.25 mg/dapagliflozin 10 mg once daily (QD), zibotentan 1.5 mg/dapagliflozin 10 mg QD and dapagliflozin 10 mg QD alone, for 12 weeks followed by a 2-week off-treatment wash-out period. The primary endpoint is the change in log-transformed urinary albumin-to-creatinine ratio (UACR) from baseline to Week 12. Other outcomes include change in blood pressure from baseline to Week 12 and change in eGFR the study. The incidence of adverse events will be monitored. Study protocol–defined events of special interest include changes in fluid-related measures (weight gain or B-type natriuretic peptide).
Results
A total of 447 patients were randomized and received treatment in placebo/dapagliflozin (n = 177), zibotentan 0.25 mg/dapagliflozin (n = 91) and zibotentan 1.5 mg/dapagliflozin (n = 179). The mean age was 62.8 years, 30.9% were female and 68.2% were white. At baseline, the mean eGFR of the enrolled population was 46.7 mL/min/1.73 m2 and the geometric mean UACR was 538.3 mg/g.
Conclusion
This study evaluates the UACR-lowering efficacy and safety of zibotentan with dapagliflozin as a potential new treatment for CKD. The study will provide information about an effective and safe zibotentan dose to be further investigated in a Phase 3 clinical outcome trial.
Clinical Trial Registration Number
Keywords: chronic kidney disease, dapagliflozin, efficacy, safety, zibotentan
Graphical Abstract
Graphical Abstract.
 Watch the video of this contribution at https://academic.oup.com/ndt/pages/author_videos
Watch the video of this contribution at https://academic.oup.com/ndt/pages/author_videos
KEY LEARNING POINTS.
What was known:
Sodium–glucose co-transporter 2 inhibitors (SGLT2is), including dapagliflozin, are used as an add-on therapy to angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers for patients with chronic kidney disease (CKD).
SGLT2is are part of the standard of care for patients with CKD and type 2 diabetes, and can exert diuretic effects.
Zibotentan is an endothelin A receptor antagonist that can reduce albuminuria in patients with CKD and type 2 diabetes, and is also associated with an increased risk of fluid retention at high dose.
This study adds:
A combination of dapagliflozin and zibotentan may have a beneficially enhanced effect due to different mechanisms of action, and therefore could be a novel treatment option for CKD.
The primary objective of the Zibotentan and Dapagliflozin for the Treatment of CKD (ZENITH-CKD) study is to evaluate the ability of combination zibotentan/dapagliflozin to reduce urinary albumin-to-creatinine ratio, compared with dapagliflozin alone.
In this report, we describe the design of the Phase 2b ZENITH-CKD study and summarize the demographic and baseline characteristics of participants.
Potential impact:
The combination of zibotentan and dapagliflozin may offer greater therapeutic benefit for patients with CKD, compared with dapagliflozin alone.
INTRODUCTION
Diabetes and hypertension are the main risk factors for the development of chronic kidney disease (CKD) [1]. Kidney failure is the most obvious adverse outcome of CKD, but the disease is also associated with an increased risk of cardiovascular events [2, 3].
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) have been used as standard of care (SoC) for CKD for many years; however, these drugs offer incomplete protection, and so there is an unmet need for new treatment options [4].
Sodium–glucose co-transporter 2 inhibitors (SGLT2is) have recently been included as an add-on to optimized SoC with ACE inhibitors and ARBs in treatment guidelines for patients with CKD [5] and are now part of the SoC for patients with CKD with type 2 diabetes [6]. These agents decrease proximal tubule glucose and sodium reabsorption, leading to natriuresis, osmotic diuresis and a reduction in intra-glomerular pressure, which is associated with a reduction in albuminuria [7]. This favourable effect in part explains the reduction in the risk of CKD progression and comorbidities observed in recent kidney outcome trials [8–10]. Dapagliflozin is a potent, selective and reversible SGLT2i that reduces kidney failure and heart failure hospitalizations and prolongs survival in patients with CKD with and without type 2 diabetes [11–13].
Endothelial dysfunction, including an increased production of the potent vasoconstrictor endothelin-1 (ET-1), contributes to cardiorenal risk in patients with CKD [14], and therefore is a potential therapeutic target. ET-1 signals through two receptors: endothelin A (ETA) and endothelin B (ETB) [15]. Selective ETA antagonists are attractive, since the ETB receptor works to clear circulating ET-1 via the lungs, whereas antagonizing the ETA receptor can lower blood pressure, reduce fibrosis and decrease renal inflammation [16–18]. ETA receptor antagonists have also shown efficacy for reducing albuminuria and the risk of kidney outcomes in patients with type 2 diabetes and CKD [17, 19], yet they are also associated with fluid retention [20, 21], and high doses of relatively unselective ETA receptor antagonists increase the risk of heart failure in patients with diabetes and CKD [22, 23].
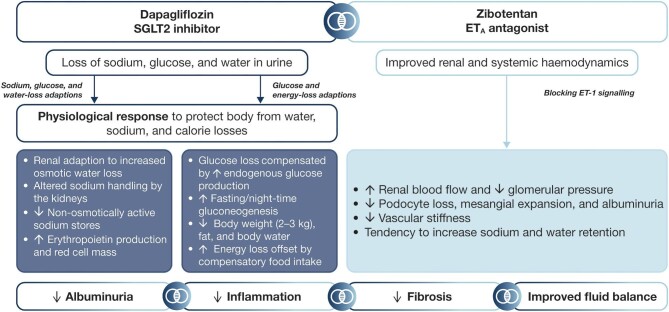
As SGLT2is exert diuretic effects, and ETA receptor antagonists can increase fluid retention, the diuretic properties of SGLT2 inhibition could potentially abrogate fluid retention induced by ETA blockade, whereas the albuminuria-lowering effects could be enhanced, owing to the different mechanisms of action (Fig. 1) [24]. A small post hoc analysis of study of diabetic nephropathy with atrasentan (SONAR) analysed combined treatment with the ETA receptor antagonist atrasentan and an SGLT2i, which demonstrated no increases in body weight—as a surrogate for fluid retention—and enhanced albuminuria reduction after 6 weeks of treatment [24].
Figure 1:
Effect of SGLT2is and ETA receptor antagonists in combination.
The selective ETA receptor antagonist zibotentan was previously assessed as a treatment option for prostate cancer [25] and is now under investigation as a treatment for CKD. The combination of zibotentan and dapagliflozin represents a novel therapeutic option for CKD, owing to different and potentially complementary mechanisms of action. In an experimental rat study, we demonstrated that zibotentan-induced fluid retention was mitigated with combined zibotentan/dapagliflozin treatment which suggests that adding dapagliflozin to zibotentan may be an effective strategy to reduce fluid retention and maximize clinical utility of zibotentan [26]. Here, we report the design and baseline characteristics of the Zibotentan and Dapagliflozin for the Treatment of CKD (ZENITH-CKD) trial. This trial evaluates combination zibotentan/dapagliflozin versus dapagliflozin alone, to assess the contribution of both components to the clinical benefit on kidney function and inform the appropriate zibotentan dose for further Phase 3 investigation.
MATERIALS AND METHODS
Study participants
Eligible participants are males and females of non-childbearing potential aged 18 years or older, with a diagnosis of CKD defined by an estimated glomerular filtration rate (eGFR) ≥20 mL/min/1.73 m2 and urinary albumin-to-creatinine ratio (UACR) ≥150 and ≤5000 mg albumin/g creatinine. Participants had no current or prior (within 1 month of enrolment) treatment with an SGLT2i or any fixed-dose combination with SGLT2i. All participants were required to be receiving a stable dose of an ACE inhibitor or ARB for at least 4 weeks before screening. However, participants with documented ACE inhibitor or ARB intolerance were allowed to participate. Key exclusion criteria included autosomal dominant or autosomal recessive polycystic kidney disease, acute coronary syndrome events within 3 months prior to screening, type 1 diabetes, unstable heart failure requiring hospitalization or B-type natriuretic peptide (BNP) ≥200 pg/mL or N-terminal-proBNP ≥600 pg/mL (BNP ≥400 pg/mL or NT-proBNP ≥1200 pg/mL, respectively, if associated with atrial fibrillation). Full inclusion and exclusion criteria are listed in Table 1.
Table 1:
Inclusion and exclusion criteria in ZENITH-CKD.
| Inclusion criteria |
| Aged 18 years or older |
| Diagnosis of CKD, defined as: |
| eGFR ≥20 mL/min/1.73 m2 |
| UACR ≥150 and ≤5000 mg albumin/g creatinine based on a single first morning void spot urine sample at screening |
| No current or prior medical treatment with: |
| an SGLT2i, or any current fixed dose combination with SGLT2i (within 1 month of screening) |
| cytotoxic/immunosuppressive therapy or other immunotherapy (within 6 months of screening) |
| Body mass index ≤40 kg/m2 |
| Male or female of non-childbearing potential |
| Capable of giving signed informed consent |
| Exclusion criteria |
| Evidence of medical conditions including: |
| unstable, rapidly progressing renal disease or autosomal polycystic kidney disease |
| acute coronary syndrome events within 3 months prior to screening |
| unstable heart failure requiring hospitalization |
| BNP ≥200 pg/mL or NT-proBNP ≥600 pg/mL (BNP ≥400 pg/mL or NT-proBNP) |
| ≥1200 pg/mL, respectively, if associated with atrial fibrillation) measured by local laboratory at screening |
| heart failure owing to cardiomyopathies that would require other specific treatments |
| uncontrolled diabetes (HbA1c >12%) |
| type 1 diabetes |
| hyponatraemia, defined as serum Na+ <135 mmol/L at screening |
| prolonged QT interval (QTcF >470 ms) on ECG at screening or randomization |
| cardiac surgery planned or within 3 months prior to screening |
| heart transplantation |
| kidney transplantation |
| history of allergy/hypersensitivity to SGLT2is |
| stroke, transient ischaemic attack, carotid surgery or carotid angioplasty within 3 months prior to screening |
| active malignancy |
| severe hepatic impairment at screening |
| drug or alcohol abuse, current or within 12 months of screening |
| positive hepatitis C antibody, hepatitis B virus surface antigen or HIV test |
| confirmed COVID-19 infection by positive SARS-CoV-2 test at screening |
| Participation in another clinical study with an investigational product administered in the last 3 months |
| Involvement in the planning of the study |
| Plasma donation within 1 month of the clinic visit, or any blood donation/loss >500 mL during the 3 months prior to any visit at the clinic |
COVID-19: coronavirus disease 2019; ECG, electrocardiogram; HbA1c, glycated haemoglobin; HIV, human immunodeficiency virus; Na+, sodium; QT, QT interval; QTcF, QT interval corrected using Fridericia's formula; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Study design
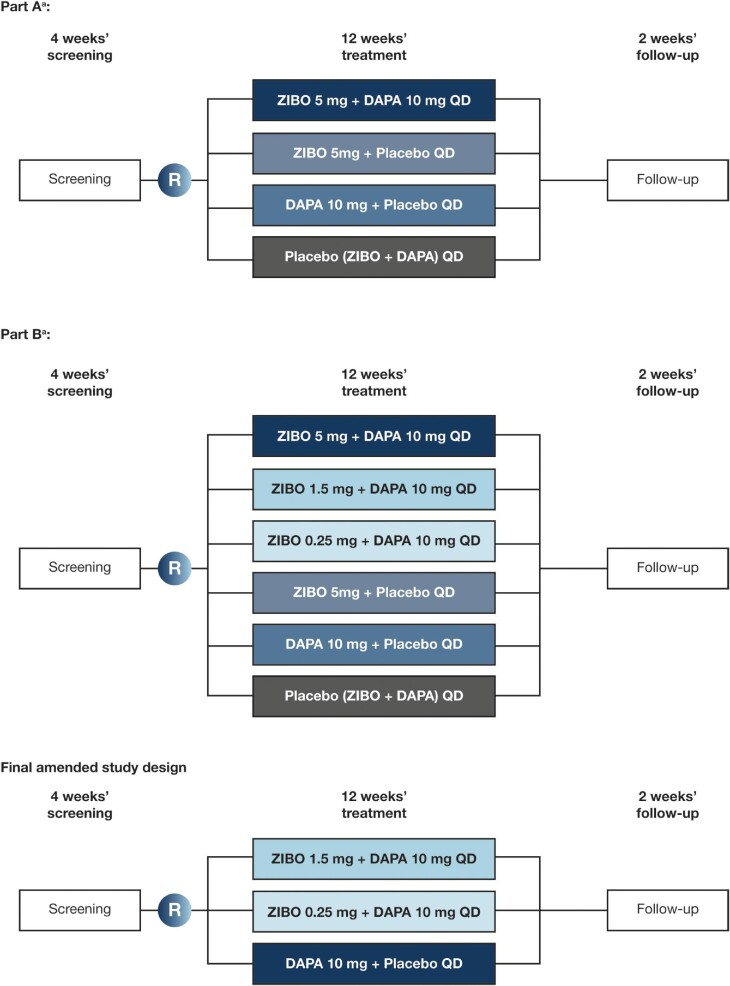
We are conducting a Phase 2b, multicentre, randomized, double-blind, active-controlled, parallel-group dose-ranging study to assess the efficacy, safety and tolerability of zibotentan and dapagliflozin in participants with CKD (ZENITH-CKD). The study is taking place across approximately 220 sites in North America, South America, Africa, Asia/Pacific and Europe. Under the original study design, participants who met the eligibility criteria were randomized to either Part A or Part B. In Part A, participants were randomized into four treatment arms: zibotentan 5 mg/dapagliflozin 10 mg daily (QD), zibotentan 5 mg QD, dapagliflozin 10 mg QD or placebo QD. In Part B, an additional cohort of eligible participants were randomized into six treatment arms: the same four treatment arms as Part A, and two additional treatment arms of zibotentan 0.25 mg/dapagliflozin 10 mg QD or zibotentan 1.5 mg/dapagliflozin 10 mg QD. However, following an ad hoc safety review, a protocol amendment was implemented on 5 April 2022. Owing to the rate of fluid-retention events in the zibotentan 5 mg QD and the zibotentan 5 mg/dapagliflozin 10 mg QD groups, randomization to these groups was closed. Additionally, recent clinical guidelines establishing SGLT2is as SoC in the management of CKD led to the closure of the placebo group [6]. As a result, dapagliflozin 10 mg QD is used as the active comparator. A planned 495 participants are being randomized into this study, including 106 participants randomized under the earlier study design before the most recent amendment; 415 are being randomized in a 1:2:2 allocation to zibotentan 0.25 mg/dapagliflozin 10 mg QD (n = 83), zibotentan 1.5 mg/dapagliflozin 10 mg QD (n = 166) and dapagliflozin 10 mg QD (n = 166).
Participants meeting the eligibility criteria are randomized to 12 weeks of treatment and 2 weeks of follow-up assessments (Fig. 2). The total duration of the study is approximately 17–19 weeks for each participant, including a screening period of 4 weeks. All participants are centrally assigned to randomized, blinded study intervention on a background of SoC, and treatments are administered in the clinic at scheduled visits, or at home. Participants have no current or prior (within 1 month of treatment) treatment with an SGLT2i or any fixed-dose combination with SGLT2i. Any prescribed SoC treatments for CKD (ACE inhibitors, ARBs, mineralocorticoid receptor agonists) should have been stable for at least 4 weeks prior to screening and remain stable for the duration of the study. At randomization, participants are stratified by diabetes status and baseline estimated glomerular filtration rate (eGFR) (≤45 versus >45 mL/min/1.73 m2). Randomization in each stratum is monitored to ensure the non-diabetic CKD subpopulation is a minimum of 30% and a maximum of 50% of the total number of participants randomized.
Figure 2:
ZENITH-CKD study design. aFollowing an ad hoc safety review, a protocol amendment was implemented on 5 April 2022. Owing to the rate of fluid-retention events in the zibotentan 5 mg QD and the zibotentan 5 mg/dapagliflozin 10 mg QD groups, randomization to these groups was closed. DAPA, dapagliflozin; R, randomization; ZIBO, zibotentan.
To ensure blinding to treatment and zibotentan dose, daily dosing for all participants consists of two dose units, one dapagliflozin tablet, containing dapagliflozin 10 mg, and one capsule containing zibotentan 0.25 mg, zibotentan 1.5 mg or placebo. When 50% of participants have completed 6 weeks of treatment, a pre-specified interim analysis will be performed; a second interim analysis will be performed when 100% of participants have completed 6 weeks of treatment or at a time selected by the sponsor/percentage of participants with a planned Week 6 visit before data cut selected by the sponsor.
Objectives, endpoints and assessments
The primary objective is to evaluate the effect of zibotentan 1.5 mg/dapagliflozin 10 mg versus dapagliflozin alone on UACR, and the primary endpoint is the change in log-transformed UACR from baseline to Week 12. Urine samples to determine albumin and creatinine levels, and subsequently calculate UACR, are collected at multiple timepoints over the course of the study.
Secondary endpoints include the change from baseline to Week 12 in log-transformed UACR for the zibotentan 0.25 mg/dapagliflozin 10 mg group versus dapagliflozin alone comparison, and the change from baseline to Week 12 in office systolic and diastolic blood pressure, the change from baseline in eGFR at Weeks 1, 12 and 14, and from Week 1 to Week 12 in the zibotentan 0.25 mg/dapagliflozin 10 mg and zibotentan 1.5 mg/dapagliflozin 10 mg groups versus dapagliflozin alone. Blood pressure is measured at every clinic visit over the course of the study. eGFR is calculated based on serum creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration equation [27].
The exploratory endpoints include change in body weight up to Visit 8, changes in cardiovascular biomarkers at all visits, changes in total body water, extracellular water and intracellular water volumes using bioimpedance spectroscopy (ImpediMed SOZO Body Composition Analyser) at all visits from baseline, and plasma concentrations of zibotentan metabolites at Visit 4. Plasma samples for pharmacokinetic analysis are collected at Weeks 1, 3, 6, 9 and 12. Participants measure their body weight at home each morning, using provided digital scales, and plasma or serum samples are collected to assess the effect of zibotentan and dapagliflozin on cardiovascular biomarkers. Quantifications of ET-1, C-terminal pro-endothelin 1 (CT-proET-1) and endothelin-like domain peptide (ELDP) are assessed at baseline and during follow-up. To monitor body fluid volumes, bioimpedance spectroscopy is performed at all visits from baseline to follow-up [28].
Safety analyses include assessment of adverse events (AEs), vital signs, clinical laboratory tests, 12-lead electrocardiogram, and other events of special interest such as changes in fluid-related measures (body weight or BNP) (Table 2). AEs are collected from the first dose of study drug, throughout the interventional period including follow-up, and serious AEs are collected from the time of informed consent. Any AEs that are unresolved at the last AE assessment or other assessment/visit in the study are followed up by the investigator for as long as medically indicated. Laboratory safety variables (Table 3) are assessed at screening, throughout the interventional period and at follow-up. As zibotentan is associated with an increased risk of fluid retention [25], any participant who experiences a >3% increase in body weight (≥2.5% as total body water measured by bioimpedance) from the start of treatment or who has BNP increase >100% from baseline and is >200 pg/mL without atrial fibrillation, or BNP increase >100% from baseline and is >400 pg/mL with atrial fibrillation, will be flagged as a participant with an event of special interest.
Table 2:
Collection of data during ZENITH-CKD.
| Screening | Dosing | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| Week: | –4 | 0 | 1 | 3 | 6 | 9 | 12 | 14 |
| Procedures | ||||||||
| Informed consent, demography | ✓ | |||||||
| Inclusion and exclusion criteria | ✓ | ✓ | ||||||
| Screening in IRT/RTSM | ✓ | |||||||
| Randomization in IRT/RTSM | ✓ | |||||||
| Physical exam | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Medical and surgical history | ✓ | |||||||
| Serology | ✓ | |||||||
| FSH/LH | ✓ | |||||||
| SARS-CoV-2 local test | ✓ | |||||||
| Concomitant medication | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study intervention dispensed | ✓ | ✓ | ✓ | ✓ | ||||
| Study intervention account | ✓ | ✓ | ✓ | ✓ | ||||
| Study intervention intake at the clinic | ✓ | ✓ | ✓ | |||||
| Assessments | ||||||||
| Spot urine from first morning void: albumin and creatinine | ✓ | |||||||
| Spot urine from first morning void over three consecutive days: albumin and creatinine | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Spot urine from first morning void: Na+, K+, uric acid, urea, glucose, creatinine, osmolality and cortisol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Body weight | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Echocardiography | ✓ | ✓ | ||||||
| Bioimpedance spectroscopy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Daily digital body weight measurement (home-based) | Daily from (2 days before) randomization to end of follow-up | |||||||
| Plasma/serum K+, Na+, uric acid, BUN, fasting plasma glucose, cystatin C, haematocrit, haemoglobin, ET-1, ELDP, CT-proET-1, copeptin, NT-proBNP and BNP | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Adverse event review | ✓a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Vital signs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Digital 12-lead safety ECG | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Clinical chemistry and haematology | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Urinalysis | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| HbA1c, cholesterol and lipids | ✓ | ✓ | ✓ | ✓ | ||||
| Post-dose PK plasma sample | ✓ | ✓ | ✓ | |||||
| 4-h PK blood sample profile | ✓ | |||||||
| Pre-dose PK sample | ✓ | |||||||
| Exploratory metabolite evaluation | ✓ | |||||||
| Collect and store serum, plasma, and urine samples for exploratory assessment of biomarkers | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Optional genetic sampling (blood) | ✓ | |||||||
aSerious adverse events only.
BUN, blood urea nitrogen; ECG, electrocardiogram; FSH, follicle-stimulating hormone; HbA1c, glycated haemoglobin; IRT, interactive response technology; K+, potassium; LH, luteinizing hormone; Na+, sodium; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PK, pharmacokinetic; RTSM, randomization and trial supply management; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 3:
Clinical laboratory tests performed in ZENITH-CKD.
| Haematology | Clinical chemistry | Urinalysis | Serology | Other |
|---|---|---|---|---|
| • White blood cell count | • Serum sodium | • Glucose | • Hepatitis B virus surface antigen | • Cystatin C |
| • Red blood cell count• Haemoglobin• Haematocrit | • Serum potassium• Serum urea• BUN | • Erythrocytes• Protein• Albumin | • Hepatitis C antibodies• HIV | • Test for SARS-CoV-2 (at screening) |
| • FSH and LH (women only, at screening) | ||||
| • Serum pregnancy test (women only, at screening) | ||||
| • Neutrophils (absolute) | • Serum creatinine | • Creatinine | ||
| • Lymphocytes (absolute) | • eGFR | |||
| • Monocytes (absolute) | • Uric acid | |||
| • Eosinophils (absolute) | • Albumin | |||
| • Basophils (absolute) | • Calcium | |||
| • Platelets | • Phosphate | |||
| • International normalised ratio | • Alkaline phosphatase | |||
| • Alanine aminotransferase | ||||
| • Aspartate aminotransferase | ||||
| • Total bilirubin | ||||
| • Creatine kinase | ||||
| • Chloride | ||||
| • Magnesium | ||||
| • Fasting glucose | ||||
| • Fasting HbA1c | ||||
| • Fasting cholesterol and lipids |
BUN, blood urea nitrogen; FSH, follicle-stimulating hormone; HbA1c, glycated haemoglobin; HIV, human immunodeficiency virus; LH, luteinizing hormone; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Rationale for treatment assignment
Zibotentan dose selection was informed by an exposure UACR-response model of clinical data from another ETA receptor antagonist, atrasentan [29, 30]. Zibotentan was studied at a dose of 10 mg in the previous oncology programme, but as zibotentan exposure is known to increase with decreasing renal function (in severe renal impairment, the area under the curve is 2.17-fold higher than in healthy controls) [31], the studied zibotentan range did not extend above 5 mg. Zibotentan 5 mg was predicted to achieve a close-to-maximal UACR response. Zibotentan 1.5 mg was predicted to give a clinically meaningful UACR response, and zibotentan 0.25 mg was predicted to lie at the lower end of the exposure–response curve.
Concomitant therapy
All concomitant medications taken during the study are recorded along with indication for use. Participants taking SGLT2is, direct renin inhibitors, ciclosporin/tacrolimus, strong or moderate cytochrome P450 3A4 inhibitors or inducers, or cytotoxic/immunosuppressive therapies are excluded from the study. Medications that can induce hypoglycaemia, including insulin and sulfonylurea, are permitted, but participants may need to reduce the amount administered.
Statistical considerations
Statistical hypotheses
The primary hypothesis tested in this study is that zibotentan 1.5 mg/dapagliflozin 10 mg will reduce UACR compared with dapagliflozin alone. The secondary hypothesis tested is that zibotentan 0.25 mg/dapagliflozin 10 mg will reduce UACR compared with dapagliflozin alone.
Sample size
With a one-sided type I error rate of 5%, assuming a 10% drop-out rate, 150 evaluable participants in the zibotentan 1.5 mg/dapagliflozin 10 mg group and dapagliflozin 10 mg group (300 participants in total) will have approximately 80% power to detect a dapagliflozin-corrected UACR reduction of ≥25% assuming a standard deviation of 1.0 on the natural log-scale, whereas in participants with CKD and diabetes, which is expected to be about 70% of the total population, the power to detect the same reduction is estimated to be 67%. For the dose–response models, a sample size of 150 evaluable participants in the zibotentan 1.5 mg/dapagliflozin 10 mg and dapagliflozin 10 mg groups, and 77 evaluable participants in the zibotentan 0.25 mg/dapagliflozin 10 mg group will have at least 78% power across multiple dose–response models to detect dose–response significance. This assumes a one-sided type I error of 5% and a maximum UACR reduction of 25% for zibotentan 1.5 mg/dapagliflozin 10 mg relative to dapagliflozin alone. Participants are considered evaluable if they have received at least one dose of study intervention, have a baseline UACR and have at least one post-treatment UACR result available.
Statistical analyses
Demographic baseline characteristics and efficacy data are analysed in the full analysis set (FAS), defined as all participants who are randomized and receive any study intervention. Participants in the FAS will be evaluated according to the arm to which they are randomized. Safety data are analyzed in the safety analysis set (SAS), defined as all participants who are randomized and receive any study intervention. Participants in the SAS will be evaluated according to the actual treatment they received. To assess the primary endpoint, UACR will be log-transformed, as it is assumed to follow a log-normal distribution. Analysis will be via a mixed-model repeated-measures (MMRM) method, with values back-transformed onto the original scale to give the geometric mean relative change from baseline to Week 12. The analysis model will include the fixed categorical effects of stratification factor, Study protocol version (Amendment 2 versus pre-Amendment 2), treatment, visit and treatment-by-visit interaction, plus the continuous covariates of baseline log(UACR) and baseline log(UACR)-by-visit interaction. An unstructured covariance structure will be used for the within-participant errors. No imputation for missing UACR values will be performed for the primary efficacy analysis.
A similar MMRM model will be utilized for the secondary endpoint of change from baseline to Week 12 in UACR for zibotentan 0.25 mg/dapagliflozin 10 mg versus dapagliflozin alone. The dose–response relationship between different doses of zibotentan in combination with dapagliflozin 10 mg and UACR reduction will be characterized by assessing the UACR reduction analyzed in the primary objective and first secondary objective. To select the dose for future studies, additional dose– and exposure–response models, and modelling of safety events will be performed based on subject-level data. To analyse changes in blood pressure and eGFR, an analysis of covariance will be used, adjusting for stratification factors, treatment arm and baseline.
For the exploratory endpoints of body weight changes in response to different doses of zibotentan/dapagliflozin versus dapagliflozin alone and changes in body fluid volume, summaries by treatment arm will be produced to show distribution over time. Safety analyses will also be summarized descriptively and presented by treatment arm. The dose–response for the reduction in UACR, as a function of zibotentan dose on top of dapagliflozin, will be modelled using the mixed models for repeated measures (DR-MMRM) method as described by Wellhagen et al. [32].
Ethics and dissemination
Ethics
ZENITH-CKD is conducted in accordance with ethical principles derived from the Declaration of Helsinki and Council for International Organisations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation Good Publication Practice Guidelines, and applicable laws and regulations. Before the study was initiated, the protocol, protocol amendments, informed consent and other forms were reviewed and approved by a local independent review board/ethics committee. All study participants provide written informed consent before undergoing any study-specific procedures, and any who are re-screened will be required to sign a new informed consent form. All participants are informed that their participation in the study is voluntary, and they may withdraw their consent at any time.
Dissemination
The study is reported on ClinicalTrials.gov (NCT04724837). A description of the study is available at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Search and http://www.clinicaltrials.gov. A summary of the main results will also be included when available. The study and summary of main findings may also be made available on other websites according to the regulations of the countries in which the main study is conducted.
RESULTS
Baseline characteristics of participants
Between 28 April 2021 and 17 January 2023, 1492 participants were assessed for eligibility of whom 525 were randomized. Overall, 76 participants were randomized in Part A or discontinued arms to zibotentan 5 mg, zibotentan 5 mg/dapagliflozin, placebo/dapagliflozin or placebo. A further 447 participants were randomized and received treatment in zibotentan 0.25 mg/dapagliflozin (n = 91), zibotentan 1.5 mg/dapagliflozin (n = 179) or placebo/dapagliflozin (n = 177). The baseline characteristics of these 447 participants are shown in Table 4.
Table 4:
Baseline demographic and disease characteristics.
| Characteristic | Total (N=447) |
|---|---|
| Age, years; mean (SD) | 62.8 (12.1) |
| Female sex; n (%) | 138 (30.9) |
| Race; n (%) | |
| Asian | 70 (15.7) |
| Black or African American | 46 (10.3) |
| Native Hawaiian or Other Pacific Islander | 2 (0.4) |
| White | 305 (68.2) |
| Other | 24 (5.4) |
| Weight, kg; mean (SD) | 85.3 (17.3) |
| BMI, kg/m2; mean (SD) | 30.0 (5.16) |
| Current nicotine user; n (%) | 59 (13.2) |
| Blood pressure, mmHg; mean (SD) | |
| Systolic | 136.9 (17.0) |
| Diastolic | 79.5 (9.8) |
| eGFR, mL/min/1.73 m2; mean (SD) | 46.7 (22.4) |
| eGFR ≥60 mL/min/1·73 m2 – n (%) | 99 (22.1) |
| eGFR 45 to <60 mL/min/1·73 m2 – n (%) | 87 (19.5) |
| eGFR 30 to <45 mL/min/1·73 m2 – n (%) | 151 (33.8) |
| eGFR <30 mL/min/1·73 m2 – n (%) | 110 (24.6) |
| Median UACR (Q1–Q3) | 565.5 (243.0-1212.6) |
| UACR >1000 mg/g; n (%) | 145 (32.4) |
| Type 2 diabetes diagnosis; n (%) | 261 (58.4) |
| CKD aetiology; n (%) | |
| Cystic kidney disease | 4 (0.9) |
| Type 2 diabetes and CKD | 225 (50.4) |
| Ischaemic/Hypertensive nephropathy | 82 (18.4) |
| Chronic glomerulonephritis | 55 (12.3) |
| IgA | 19 (4.3) |
| Others | 36 (8.1) |
| Unknown | 43 (9.6) |
| Other | 37 (8.3) |
| Family history of cardiovascular disease; n (%) | 88 (19.7) |
| Heart failure, n (%) | 31 (6.9) |
| Prior medication; n (%) | |
| ACE inhibitor | 147 (32.9) |
| ARB | 242 (54.1) |
| Diuretic | 172 (38.5) |
| Calcium Channel Blocker | 226 (50.6) |
| β-blocker | 164 (36.7) |
| Statin | 315 (70.5) |
Data are from the FAS.
aUACR inclusion criteria for the study is 150–5000 mg/g at screening; a participant could be outside this at baseline and still be allowed to participate.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FAS, full analysis set; MRA, mineralocorticoid receptor antagonists; Q1, 1st quartile; Q3, 3rd quartile; SD, standard deviation; UACR, urinary albumin-to-creatinine ratio.
The mean age at baseline was 62.8 years, 138 (30.9%) were female and 305 (68.2%) were white. The most common aetiology of CKD was type 2 diabetes and CKD [n = 225 (50.4%)] followed by ischaemic/hypertensive nephrosclerosis [n = 82 (18.4%)] and chronic glomerulonephritis [n = 55 (12.3%)]. The mean eGFR was 46.7 mL/min/1.73 m2, geometric mean UACR was 538.3 mg/g and mean systolic/diastolic blood pressure was 136.9/79.5 mmHg.
DISCUSSION
ZENITH-CKD is the first clinical trial to evaluate the efficacy of the ETA receptor antagonist zibotentan in combination with the SGLT2i dapagliflozin in participants with CKD. Both ETA receptor antagonists and SGLT2is, including dapagliflozin, are effective for CKD, as demonstrated in dedicated kidney outcome trials [8, 11]. Owing to their different mechanisms of action targeting both key pathophysiological pathways involved in CKD progression, combining both drug classes may confer enhanced effects.
Dapagliflozin acts on the proximal convoluted tubule and thus facilitates glucosuria, which is associated with diuretic-like effects demonstrated by reductions in 24-h blood pressure, body weight, GFR and plasma volume [7, 33–35]. Despite its potent albuminuria-lowering effect, residual severe albuminuria remains present in a substantial number of people following SGLT2 inhibition, which is independently associated with a higher risk of kidney failure and cardiovascular outcomes [36]. Consequently, zibotentan may add to the nephroprotective effects offered by dapagliflozin, as ETA receptor antagonists can significantly reduce albuminuria when given in combination with, or on top of, an SGLT2i [24, 37]. On the other hand, high doses of the relatively non-selective ETA receptor antagonist avosentan resulted in severe fluid retention, which was associated with congestive heart failure and cardiovascular death in a previous trial in patients with diabetic kidney disease [22]. This trial highlighted the importance of careful dose selection, not only focusing on optimal albuminuria reduction, but also considering drug-related adverse outcomes, in particular sodium retention and oedema. Dose selection, considering both efficacy and safety outcomes, therefore, is a key element of the ZENITH-CKD study. Importantly, a previous study with atrasentan indicated that the dose–response curve of fluid retention may be shifted to the right compared with the UACR dose–response, suggesting that it is possible to select a dose with high UACR efficacy and minimal fluid retention [38]. A further strategy to minimize the risk of sodium retention and adverse outcomes is to use a selective ETA receptor antagonist. Zibotentan is a selective ETA receptor antagonist demonstrating a >100 000-fold greater selectivity for ETA than ETB and, therefore, is more selective for ETA than avosentan [39]. In addition, owing to the mild diuretic effects of dapagliflozin, ZENITH-CKD will assess combined treatment of zibotentan with dapagliflozin to further reduce potential risks of sodium retention and oedema, and maximize clinical applicability. Indeed, an experimental study in a rat model demonstrated that adding dapagliflozin to high-dose zibotentan reduced zibotentan-induced fluid retention [26].
In the current study, the efficacy of zibotentan and dapagliflozin will be assessed by changes in UACR, which is a key marker of kidney health and predictor of kidney disease outcomes, previously established as a potential surrogate endpoint for kidney outcomes in clinical trials [40]. A pre-specified analysis from the SONAR trial with the ETA receptor antagonist atrasentan reported that larger reductions in albuminuria during treatment with atrasentan were associated with a lower risk of kidney failure [41]. These data support a role for albuminuria as a surrogate endpoint for kidney failure during treatment with ETA receptor antagonists.
The ZENITH-CKD trial enrolled participants at high risk of CKD progression as reflected by the low eGFR and high UACR levels. The majority of participants had diabetic nephropathy, followed by ischaemic/hypertensive nephropathy and chronic glomerulonephritis. Compared with the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial, which assessed the efficacy and safety of dapagliflozin in patients with CKD with and without type 2 diabetes [11, 42], participants in the ZENITH trial were more likely to have other types of CKD than diabetic nephropathy. In contrast to the SONAR trial, which assessed the efficacy and safety of the ERA atrasentan in participants with type 2 diabetes and CKD, ZENITH-CKD enrolled a broader cohort of patients with various CKD aetiologies. Moreover, all participants in the SONAR trial had macroalbuminuria whereas in our study participants also had microalbuminuria [19]. The study of the effect and safety of sparsentan in the treatment of patients with IgA nephropathy (PROTECT) trial demonstrated the albuminuria-lowering effect of the dual endothelin angiotensin receptor inhibitor sparsentan in patients with immunoglobulin A nephropathy [43]. ZENITH enrolled a more heterogeneous population which allows exploration of the efficacy and safety of dapagliflozin/zibotentan in various aetiologies of CKD.
The strengths of this study include the larger sample size than previous dose-finding studies with ETA antagonists in kidney disease [17, 44]. Furthermore, the range of doses of zibotentan studied affords the opportunity to optimize the efficacy of albuminuria reduction with the risk of fluid retention and related complications. A limitation of this study is the lack of true GFR measurements to assess haemodynamic effects of zibotentan alone and in combination; however, this will be explored in a dedicated mechanistic study (NCT05570305). Other limitations include the relatively short duration, which means that long-term efficacy and safety cannot be addressed, although the follow-up period is sufficient to capture UACR and fluid-retention effects.
Overall, this study evaluates zibotentan as a treatment for CKD through combination with dapagliflozin. This study will provide information about the safety as combination treatment and identify an effective zibotentan dose to progress to a full Phase 3 trial to evaluate if zibotentan is efficacious. The design of the ZENITH-CKD study will identify whether there is any greater therapeutic benefit for CKD patients of treatment with zibotentan in combination with dapagliflozin rather than treatment with dapagliflozin alone.
ACKNOWLEDGEMENTS
This study was sponsored by AstraZeneca. Medical writing support, under the guidance of the authors, was provided by Megan Melody, MSc, CMC Connect, IPG Health Medical Communications, and was funded by AstraZeneca.
Contributor Information
Hiddo J L Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; The George Institute for Global Health, Sydney, New South Wales, Australia.
Peter J Greasley, Research and Early Development, Cardiovascular, Renal and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Christine Ahlström, DMPK, Research and Early Development Cardiovascular, Renal and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Magnus Althage, Translational Science & Experimental Medicine, Research and Early Development Cardiovascular, Renal, and Metabolism, Biopharmaceutical R&D, AstraZeneca, Gaithersburg, MD, USA.
Jamie P Dwyer, Division of Nephrology/Hypertension, University of Utah Health, Salt Lake City, UT, USA.
Gordon Law, Early Biometrics & Statistical Innovation, Data Science and Artificial Intelligence, R&D, AstraZeneca, Gaithersburg, MD, USA.
Emma Wijkmark, Biometrics Late Development, Cardiovascular, Renal and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Min Lin, Biometrics Late Development, Cardiovascular, Renal and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, MD, USA.
Anne-Kristina Mercier, Clinical Pharmacology and Quantitative Pharmacology, Clinical Pharmacology and Safety Sciences, R&D, AstraZeneca, Gothenburg, Sweden.
Mikael Sunnåker, Clinical Pharmacology and Quantitative Pharmacology, Clinical Pharmacology and Safety Sciences, R&D, AstraZeneca, Gothenburg, Sweden.
Michelle Turton, Biopharma Clinical Operations, Early CVRM, AstraZeneca, Cambridge, UK.
David C Wheeler, Department of Renal Medicine, University College London, London, UK.
Philip Ambery, Clinical Late Development, Cardiovascular, Renal and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
FUNDING
This study was sponsored by AstraZeneca.
AUTHORS’ CONTRIBUTIONS
H.J.L.H., P.J.G., C.A., M.A., J.P.D., G.L., A.-K.M., M.S., M.T., D.C.W. and P.A. contributed to the study design. H.J.L.H., P.J.G., J.P.D., M.T. and P.A. carried out the study. P.J.G., J.P.D., G.L., E.W., A.K.M., M.S., D.C.W. and P.A. analysed and interpreted the data. H.J.L.H., P.J.G., C.A., M.A., J.P.D., G.L., E.W., A.-K.M., M.S., D.C.W. and P.A. drafted, revised and provided final approval of the manuscript. M.L. was involved in the statistical analyses.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
CONFLICT OF INTEREST STATEMENT
H.J.L.H. reports grant funding and honoraria for consultancy as a member of the steering committee of the DAPA-CKD trial paid to their institution from AstraZeneca; research grants paid to his employer from AstraZeneca, Boehringer Ingelheim, Janssen and Novo Nordisk for clinical trials; consulting fees, paid to his employer, from AbbVie, Boehringer Ingelheim, Travere Pharmaceuticals and Novo Nordisk; fees for steering committee membership, paid to his employer, from Bayer, Chinook, CSL Pharma, Dimerix, Gilead and Janssen; honoraria for lectures from AstraZeneca, Bayer, Mitsubishi Tanabe and Novo Nordisk; and honoraria for advisory board participation for Merck (paid to his employer), Mitsubishi Tanabe and Mundipharma. P.J.G. is an employee and shareholder of AstraZeneca. C.A. is an employee and shareholder of AstraZeneca. M.A. is an employee and shareholder of AstraZeneca. J.P.D. reports grant funding from AstraZeneca and Cincor, Inc.; received consulting fees from Akebia, Inc., AstraZeneca, Bayer AG, Boehringer Ingelheim, Caladrius Biosciences, CSL Behring, Eli Lilly, Fibrogen, Inc., GlaxoSmithKline, LLC., Inversago, Novo Nordisk, ProKidney, LLC., Reata Inc., RenalytixAI, Inc., Sanofi and Tricida Inc.; has participated on a data safety monitoring board or advisory board for Eli Lilly and Novo Nordisk; has held a leadership or fiduciary role for BioRasi; and holds stock in Biorasi and Venostent. G.L. is an employee and shareholder of AstraZeneca. E.W. is an employee and shareholder of AstraZeneca. A.-K.M. is an employee of AstraZeneca. M.S. is an employee and shareholder of AstraZeneca. M.T. is an employee and shareholder of AstraZeneca. D.C.W. reports honoraria and/or consultancy fees from Amgen, AstraZeneca (ongoing), Astellas, Bayer, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Mundipharma, Merck Sharp, Napp, and Dohme, Takeda, Vifor Fresenius, and Zydus. P.A. is an employee and shareholder of AstraZeneca.
REFERENCES
- 1. Xie Y, Bowe B, Mokdad AH et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018;94:567–81. 10.1016/j.kint.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 2. Currie CJ, Berni ER, Berni TR et al. Major adverse cardiovascular events in people with chronic kidney disease in relation to disease severity and diabetes status. PLoS One 2019;14:e0221044. 10.1371/journal.pone.0221044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ali I, Chinnadurai R, Ibrahim ST et al. Adverse outcomes associated with rapid linear and non-linear patterns of chronic kidney disease progression. BMC Nephrol 2021;22:82. 10.1186/s12882-021-02282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans M, Lewis RD, Morgan AR et al. A narrative review of chronic kidney disease in clinical practice: current challenges and future perspectives. Adv Ther 2022;39:33–43. 10.1007/s12325-021-01927-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence . Dapagliflozin for Treating Chronic Kidney Disease. Available from: www.nice.org.uk/guidance/ta775 (23 May 2022, date last accessed). [Google Scholar]
- 6. Kidney Disease: Improving Global Outcomes Diabetes Work Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022;102:S1–127. 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 7. Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 2021;83:503–28. 10.1146/annurev-physiol-031620-095920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perkovic V, Jardine MJ, Neal B et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 9. Jongs N, Greene T, Chertow GM et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9:755–66. 10.1016/S2213-8587(21)00243-6 [DOI] [PubMed] [Google Scholar]
- 10. Heerspink HJ, Perkins BA, Fitchett DH et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–72. 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 11. Heerspink HJL, Stefansson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 12. Wheeler DC, Stefansson BV, Jongs N et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9:22–31. 10.1016/S2213-8587(20)30369-7 [DOI] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Sjostrom CD, Jongs N et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J 2021;42:1216–27. 10.1093/eurheartj/ehab094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li YH, Sheu WH, Lee WJ et al. Synergistic effect of renalase and chronic kidney disease on endothelin-1 in patients with coronary artery disease a cross-sectional study. Sci Rep 2018;8:7378. 10.1038/s41598-018-25763-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kowalczyk A, Kleniewska P, Kolodziejczyk M et al. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz) 2015;63:41–52. 10.1007/s00005-014-0310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goddard J, Johnston NR, Hand MF et al. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: a comparison of selective and combined endothelin receptor blockade. Circulation 2004;109:1186–93. 10.1161/01.CIR.0000118499.69469.51 [DOI] [PubMed] [Google Scholar]
- 17. de Zeeuw D, Coll B, Andress D et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 2014;25:1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cosenzi A, Bernobich E, Trevisan R et al. Nephroprotective effect of bosentan in diabetic rats. J Cardiovasc Pharmacol 2003;42:752–6. 10.1097/00005344-200312000-00009 [DOI] [PubMed] [Google Scholar]
- 19. Heerspink HJL, Parving HH, Andress DL et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019;393:1937–47. 10.1016/S0140-6736(19)30772-X [DOI] [PubMed] [Google Scholar]
- 20. Hoekman J, Lambers Heerspink HJ, Viberti G et al. Predictors of congestive heart failure after treatment with an endothelin receptor antagonist. Clin J Am Soc Nephrol 2014;9:490–8. 10.2215/CJN.07040713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smeijer JD, Koomen J, Kohan DE et al. Increase in BNP in response to endothelin-receptor antagonist atrasentan is associated with incident heart failure. JACC Heart Fail 2022;10:498–507. 10.1016/j.jchf.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 22. Mann JF, Green D, Jamerson K et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 2010;21:527–35. 10.1681/ASN.2009060593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlaich MP, Bellet M, Weber MA et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet 2022;400:1927–37. 10.1016/S0140-6736(22)02034-7 [DOI] [PubMed] [Google Scholar]
- 24. Heerspink HJL, Kohan DE, de Zeeuw D. New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction. Kidney Int 2021;99:346–9. 10.1016/j.kint.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 25. Miller K, Moul JW, Gleave M et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2013;16:187–92. 10.1038/pcan.2013.2 [DOI] [PubMed] [Google Scholar]
- 26. Veenit V, Heerspink HJL, Ahlstrom C et al. The sodium glucose co-transporter 2 inhibitor dapagliflozin ameliorates the fluid-retaining effect of the endothelin A receptor antagonist zibotentan. Nephrol Dial Transplant 2023;gfad078. 10.1093/ndt/gfad078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stupin DD, Kuzina EA, Abelit AA et al. Bioimpedance spectroscopy: basics and applications. ACS Biomater Sci Eng 2021;7:1962–86. 10.1021/acsbiomaterials.0c01570 [DOI] [PubMed] [Google Scholar]
- 29. Lin CW, Mostafa NM, Andress DL et al. Relationship between atrasentan concentrations and urinary albumin to creatinine ratio in Western and Japanese patients with diabetic nephropathy. Clin Ther 2018;40:242–51. 10.1016/j.clinthera.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 30. Koomen JV, Stevens J, Bakris G et al. Inter-individual variability in atrasentan exposure partly explains variability in kidney protection and fluid retention responses: a post hoc analysis of the SONAR trial. Diabetes Obes Metab 2021;23:561–8. 10.1111/dom.14252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomkinson H, Kemp J, Oliver S et al. Pharmacokinetics and tolerability of zibotentan (ZD4054) in subjects with hepatic or renal impairment: two open-label comparative studies. BMC Clin Pharmacol 2011;11:3. 10.1186/1472-6904-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wellhagen GJ, Hamren B, Kjellsson MC et al. Dose-response mixed models for repeated measures - a new method for assessment of dose-response. Pharm Res 2020;37:157. 10.1007/s11095-020-02882-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lambers Heerspink HJ, de Zeeuw D, Wie L et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013;15:853–62. 10.1111/dom.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scholtes RA, Muskiet MHA, van Baar MJB et al. Natriuretic effect of two weeks of dapagliflozin treatment in patients with type 2 diabetes and preserved kidney function during standardized sodium intake: results of the DAPASALT trial. Diabetes Care 2021;44:440–7. 10.2337/dc20-2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Komoroski B, Vachharajani N, Feng Y et al. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009;85:513–9. 10.1038/clpt.2008.250 [DOI] [PubMed] [Google Scholar]
- 36. Oshima M, Neuen BL, Li J et al. Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE trial. J Am Soc Nephrol 2020;31:2925–36. 10.1681/ASN.2020050723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heerspink HJL, Andress DL, Bakris G et al. Baseline characteristics and enrichment results from the SONAR trial. Diabetes Obes Metab 2018;20:1829–35. 10.1111/dom.13315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koomen JV, Stevens J, Mostafa NM et al. Determining the optimal dose of atrasentan by evaluating the exposure-response relationships of albuminuria and bodyweight. Diabetes Obes Metab 2018;20:2019–22. 10.1111/dom.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davenport AP, Hyndman KA, Dhaun N et al. Endothelin. Pharmacol Rev 2016;68:357–418. 10.1124/pr.115.011833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heerspink HJL, Greene T, Tighiouart H et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 2019;7:128–39. 10.1016/S2213-8587(18)30314-0 [DOI] [PubMed] [Google Scholar]
- 41. Heerspink HJL, Xie D, Bakris G et al. Early response in albuminuria and long-term kidney protection during treatment with an endothelin receptor antagonist: a prespecified analysis from the SONAR trial. J Am Soc Nephrol 2021;32:2900–11. 10.1681/ASN.2021030391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wheeler DC, Stefansson BV, Batiushin M et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020;35:1700–11. 10.1093/ndt/gfaa234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heerspink HJL, Radhakrishnan J, Alpers CE et al. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet 2023;401:1584–94. 10.1016/S0140-6736(23)00569-X [DOI] [PubMed] [Google Scholar]
- 44. Wenzel RR, Littke T, Kuranoff S et al. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 2009;20:655–64. 10.1681/ASN.2008050482 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.