ABSTRACT
Background
There are no consensus definitions for evaluating kidney function recovery after acute kidney injury (AKI) and acute kidney disease (AKD), nor is it clear how recovery varies across populations and clinical subsets. We present a federated analysis of four population-based cohorts from Canada, Denmark and Scotland, 2011–18.
Methods
We identified incident AKD defined by serum creatinine changes within 48 h, 7 days and 90 days based on KDIGO AKI and AKD criteria. Separately, we applied changes up to 365 days to address widely used e-alert implementations that extend beyond the KDIGO AKI and AKD timeframes. Kidney recovery was based on resolution of AKD and a subsequent creatinine measurement below 1.2× baseline. We evaluated transitions between non-recovery, recovery and death up to 1 year; within age, sex and comorbidity subgroups; between subset AKD definitions; and across cohorts.
Results
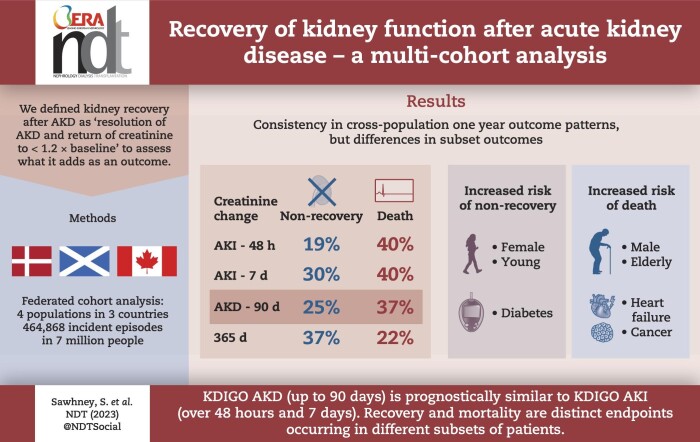
There were 464 868 incident cases, median age 67–75 years. At 1 year, results were consistent across cohorts, with pooled mortalities for creatinine changes within 48 h, 7 days, 90 days and 365 days (and 95% confidence interval) of 40% (34%–45%), 40% (34%–46%), 37% (31%–42%) and 22% (16%–29%) respectively, and non-recovery of kidney function of 19% (15%–23%), 30% (24%–35%), 25% (21%–29%) and 37% (30%–43%), respectively. Recovery by 14 and 90 days was frequently not sustained at 1 year. Older males and those with heart failure or cancer were more likely to die than to experience sustained non-recovery, whereas the converse was true for younger females and those with diabetes.
Conclusion
Consistently across multiple cohorts, based on 1-year mortality and non-recovery, KDIGO AKD (up to 90 days) is at least prognostically similar to KDIGO AKI (7 days), and covers more people. Outcomes associated with AKD vary by age, sex and comorbidities such that older males are more likely to die, and younger females are less likely to recover.
Keywords: AKI, CKD, epidemiology, prognosis, recovery
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
There are no consensus definitions for evaluating kidney function recovery after KDIGO acute kidney injury (AKI) and acute kidney disease (AKD), nor is it clear what recovery assessment adds as an outcome for clinical evaluation.
Implementation of AKI and AKD is variable in clinical practice and research, and widespread ‘AKI’ e-alerts are pragmatically extended beyond both the AKI (7 days) and AKD (90 days) timeframes.
KDIGO have called for large-scale population-level studies to reconcile the use of these definitions and characterize kidney recovery to address this knowledge gap.
This study adds:
This multi-cohort study rigorously applied KDIGO AKI, AKD and e-alert implementations across a population of 7 million to identify 464 868 incident cases. It characterized both recovery and mortality outcomes across cohorts, clinical subsets and definitions (which rarely overlapped).
Non-recovery and mortality were both common, but occurred in different clinical subgroups such that older males and those with heart failure or cancer were more likely to die than experience sustained non-recovery, whereas the converse was true for younger females and those with diabetes.
In every population and subset, the KDIGO definition of AKD was at least as prognostically important as KDIGO AKI for both mortality and recovery, so long as it as strictly interpreted as creatinine changes up to and not beyond 90 days.
Potential impact:
With consistency across populations and clinical subsets, this analysis supports the notion of KDIGO AKD being a condition at least as prognostically important as KDIGO AKI and that would not be served by a focus on AKI alone.
E-alert implementations should consider aligning with KDIGO AKD by restricting to creatinine changes with 90 days.
Mortality and non-recovery typically happen in different subsets of patients. The recovery endpoint introduced and evaluated in this study should be considered in future clinical outcome evaluations.
INTRODUCTION
Over 20 years since introduction of the term acute kidney injury (AKI), and 10 years since the KDIGO AKI clinical practice guidelines were published [1], clinical research has consistently demonstrated associations between AKI and adverse outcomes, including mortality, development and progression of chronic kidney disease (CKD), and cardiovascular events [2]. In many jurisdictions, AKI is now identified in clinical settings using e-alerts to facilitate and monitor improvement work [3, 4]. The 2012 KDIGO guideline also described AKI as a condition within a broader group of disorders termed acute kidney diseases and disorders (AKD). As reaffirmed by KDIGO in 2022, AKD includes not only AKI episodes of longer duration up to 90 days [5], but more broadly encompasses changes in serum creatinine identified within a period of 90 days, with AKI being a disorder nested within AKD, and AKD occurring even in the absence of AKI [6]. However, as noted in a recent KDIGO consensus conference, further work has been called for to reconcile the use of these definitions for clinicians and researchers, and to provide a common understanding of disease definitions for comparisons of the burden and outcomes of AKI and AKD across time, patient subgroups and clinical settings. In particular, little research has characterized the prognosis for recovery of kidney function after AKI and AKD, even though this may represent an important and common outcome for survivors.
Federated analyses, using shared code and harmonized curation of study populations, provide an opportunity to evaluate the consistency of measures of disease burden and outcomes [7]. While the concepts of kidney recovery and non-recovery may seem clinically intuitive, examination of the consistency of these measurements at the population level is important to ensure meaningful comparisons of outcomes across groups, for evaluating interventions to optimize kidney recovery and to recognize people who may be more vulnerable to adverse outcomes [8, 9].
In this study we applied a common analytical approach to data from four cohorts with complete population laboratory test capture to evaluate kidney function recovery trajectories over the first year after AKD. We used this approach to determine the timing, extent and persistence of recovery over the first year after AKD, and the consistency across subset AKD definitions, population cohorts, and demographic and disease subgroups. Our purpose was to; (i) characterize kidney function recovery according to contemporary definitions of AKD, (ii) determine whether recovery profiles were consistent across geographically distinct clinical populations and (iii) identify potential differences in recovery according to age, sex or comorbidities.
MATERIALS AND METHODS
Data sources
Complete population community and hospital laboratory data were extracted from 2009 to 2019 from four regions with a combined population of 7 million inhabitants: Alberta (Canada), North and Central Denmark, Grampian (UK) and Tayside (UK) [7, 10–18]. These populations, served by universal healthcare systems, were selected for their ability to provide integrated data on isotope-dilution mass spectrometry–calibrated creatinine measurements for all residents within their source population, irrespective of clinical setting (hospital inpatient, outpatient specialty, community). Ethical and other approvals for use of unconsented routine health data were provided by research ethics boards and/or other relevant authorities for each region as summarized in the Supplementary data.
Data processing and harmonization
Datasets for each region were prepared using a common analytical protocol and statistical code for both data preparation and analysis (the code and instructions for use are provided in the Supplementary data to allow replication of these methods in other cohorts). All creatinine results for each individual within each cohort were used for analyses. Creatinine values that were recorded as a non-value (e.g. ‘sample inadequate’, ‘sample error’), or were outside the limits for detection of the analyser were excluded. To avoid privacy risks associated with movement of individual-level patient data between regions, the analytical code was designed for each centre to produce output files of aggregated data only, which were then sent to the coordinating centre (University of Aberdeen) for pooling and final reporting.
Study population
All adult (age ≥18 years) residents within each population region with at least one serum creatinine test during 2009–18 were included. Creatinine tests taken after initiation of long-term kidney replacement therapy (KRT) (dialysis or transplant) for established kidney failure were excluded, as established by KRT registry data for each site and performed previously [7].
The first instance of AKD occurring between 2011 and 2018 was identified for each participant based on KDIGO serum creatinine criteria. Those meeting criteria for AKD in 2009 and 2010 were excluded to ensure only patients with incident AKD were included and to avoid inclusion of prevalent/recurrent episodes (prevalent pool effect).
Exposure—AKD subsets
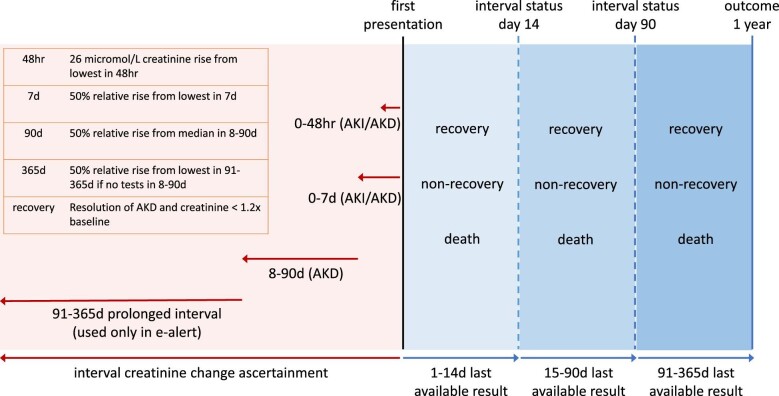
Four definition subsets based on serum creatinine change were evaluated (Fig. 1). The 48-h and 7-day subsets followed the existing KDIGO AKI criteria, and an 8- to 90-day subset followed the KDIGO AKD criteria. A separate final group covered those with creatinine changes up to 365 days if no blood tests were available within 8–90 days. This was to understand the implications of using longer creatinine intervals beyond 90 days, as adopted in existing e-alert systems [19]. Additional detail is available in our previous work [7], with accompanying code in the Supplementary data.
Figure 1:
Visual overview of the study. Red shading represents study definitions of AKD subsets up to 90 days and the extension to address e-alert implementations beyond 90 days (adapted from Sawhney et al. [7]). Blue shading represents follow-up of the clinical course with status updated in three periods up to 1 year based on the most recently available clinical information.
Because the subset definitions of AKD can co-occur, occur in isolation or occur sequentially in a patient, in the main analysis we assessed the extent to which individual patients ‘overlapped’ in the presenting subsets of an AKD episode if they met multiple subset criteria within 1 week of first AKD onset. In a secondary analysis this definition of overlap was restricted to co-presentation of subsets only if they occurred on the first day of AKD onset in a given patient.
For analyses of characteristics and outcomes in the main analysis, each of the four AKD subsets were reported separately, while in a secondary analysis only the characteristics of those who presented with one subset exclusively (e.g. 48-h subset without being in the 7-, 90- or 365-day subsets) were reported. In this secondary analysis, outcomes for the exclusive 90-day subset can be understood to represent outcomes of those who have ‘AKD without AKI’.
Covariates
Additional variables collected included age, sex, comorbidities, hospital context (whether the participant was in hospital at time of AKD onset) and baseline level of kidney function. Baseline kidney function was determined from the reference creatinine measurement that served as baseline for the AKD episode (Fig. 1), which was used to calculate estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation excluding the coefficient for race (2009 version) in the main analysis, and the CKD-EPI equation 2021 version in a secondary analysis [20, 21]. The coefficient for race was excluded in line with current clinical practice in each of the populations of study.
Comorbidities, including diabetes, cancer, coronary heart disease, heart failure, stroke and peripheral arterial disease, were identified using validated coding approaches [International Classification of Diseases (ICD)-10 codes] applied to hospital discharge abstract records within 2 years prior to AKD onset date. In the Alberta cohort, comorbidities were also extracted from physicians’ claims from hospital and community settings, using ICD-9-CM codes (see Supplementary data) [18, 22, 23].
Outcomes
Subsequent kidney function trajectories were characterized based on absolute and relative changes (vs baseline) moving forward from the date of AKD onset to the peak serum creatinine (and the corresponding eGFR) within 7 days, and the latest recorded subsequent measures at 14, 90 and 365 days following AKD (subset definition) onset (Fig. 1).
Kidney function recovery was operationalized by a subsequent return of serum creatinine to within 1.2× of the baseline value for all participants meeting any AKD subset criteria [11, 24]. Of note, those meeting a 48-h absolute creatinine change of 0.3 mg/dL (26 µmol/L) as per the KDIGO AKI definition may not exceed the threshold of a 1.2× increase from baseline. Accordingly, for consistency, our operationalized definition of recovery required both a fall in creatinine to within 1.2× baseline and resolution of the serum creatinine based AKD/AKI criteria.
Mortality and date of death were determined by linkage to national or regional vital statistics for each region as in previous work [7].
Statistical analyses
Descriptive statistics and outcomes were reported for each cohort separately according to participants meeting each AKD subset definition, and also with pooling of the 1-year outcomes across regions using random effects proportional meta-analysis. One-year outcomes were also reported within subgroups by age (</≥70 years), sex and comorbidities (cancer, diabetes and heart failure).
The proportions of participants who were identified according to each combination of AKD subset definition met within 1 week of AKD onset (to capture patterns of overlap of individuals who met multiple subset definitions but on different days during the same episode) (primary analysis), as well as those identified only on the day of first AKD onset (secondary analysis), were reported using Euler diagrams to illustrate the degree of overlap of patients co-presenting with multiple AKD subset definitions.
For those who survived 1 year after AKD onset, distributions of serum creatinine and eGFR at baseline, peak within 0–7 days of AKD onset, and during follow-up to 14, 90 and 365 days were determined. In addition, for all people, trajectories of kidney function and recovery were reported using Sankey plots to visualize the flow over time in the proportion of participants with statuses of kidney function recovery, non-recovery and death at 14, 90 and 365 days. Because blood testing in routine practice is non-protocolized, follow-up was considered an ‘informative observation’ and missing data on a given day ‘missing not at random’ (i.e. fewer tests occur among patients who have become stable). Accordingly, multiple imputation was deemed inappropriate. In the main analysis, the most recent available result for participants was carried forward when a creatinine measurement was missing from any follow-up interval. A secondary analysis was also performed that categorized those missing a measurement within each time period in a separate ‘untested’ group. Cohort preparation was conducted in Stata SE 16, with Sankey and Euler plots produced in R [25, 26].
RESULTS
Cohort characteristics
There were 464 868 patients with incident AKD from the four cohorts, with median age ranging from 67 to 75 years and 50%–54% females across the cohorts (Table 1). The proportions of patients presenting with each AKD subset criterion were similar across cohorts, with the greatest number of patients presenting with AKD in the 90-day subset. Across the cohorts, 93%–96% of patients in the 48-h subset were identified in a hospital setting, whereas 47%–58% of patients with 365-day subset were identified in a community setting. Comorbidities of diabetes, cancer, heart failure and cardiovascular diseases were most common among those in the 48-h subset, and least common among those in the 90- and 365-day subsets. When the cohort was restricted to patients meeting only one of the AKD subset criteria exclusively, similar differences in baseline characteristics were observed between the groups (Supplementary data, Table S1).
Table 1:
Description of AKD subsets for each cohort.
| Alberta | Denmark | Grampian | Tayside | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | |
| N | 100 278 | 101 075 | 136 465 | 93 640 | 57 659 | 56 043 | 77 254 | 37 240 | 18 620 | 18 524 | 22 767 | 13 252 | 24 932 | 24 441 | 31 696 | 18 011 |
| Age, median (IQR) | 72 (60–83) | 69 (56–81) | 69 (55–81) | 63 (46–78) | 75 (65–83) | 72 (63–81) | 72 (62–81) | 72 (59–81) | 75 (64–83) | 73 (62–82) | 73 (61–82) | 72 (57–82) | 75 (68–85) | 76 (65–84) | 76 (65–84) | 74 (61–83) |
| Female % | 42.8 | 51.5 | 53.1 | 59.5 | 51.4 | 48.7 | 49.6 | 54.4 | 45.4 | 52.6 | 53.4 | 58.3 | 47.7 | 55 | 54 | 57.7 |
| Inpatient % | 96.0 | 91.7 | 74.0 | 58.2 | 96.7 | 91.6 | 71.3 | 53.4 | 93.8 | 88.7 | 66.7 | 46.5 | 96.4 | 93.2 | 74.2 | 55.2 |
| Reference eGFR, median (IQR) | 63 (40–89) | 89 (62–106) | 84 (59–102) | 93 (70–111) | 62 (40–87) | 86 (60–101) | 83 (59–98) | 86 (64–101) | 66 (43–90) | 88 (63–102) | 84 (60–100) | 88 (66–104) | 59 (38–85) | 85 (58–99) | 80 (56–96) | 84 (61–100) |
| Reference Cr, median (IQR) | 97 (73–139) | 70 (51–98) | 76 (59–100) | 69 (54–89) | 99 (74–140) | 73 (54–100) | 77 (61–101) | 73 (58–93) | 93 (70–131) | 69 (52–95) | 74 (58–97) | 71 (55–90) | 99 (73–142) | 72 (53–99) | 77 (60–101) | 74 (57–94) |
| Comorbidities (%) | ||||||||||||||||
| Diabetes | 16.3 | 14.9 | 13.5 | 12.2 | 18.7 | 16.6 | 16.8 | 13.7 | 22.5 | 20.3 | 20.5 | 17.0 | 23.5 | 20.7 | 19.7 | 15.3 |
| Cancer | 18.3 | 18.7 | 16.8 | 9.6 | 25.8 | 28.7 | 27.5 | 10.3 | 23.8 | 25.6 | 24.1 | 11.0 | 22.2 | 24.4 | 22.2 | 9.3 |
| Coronary heart disease | 26.1 | 23.0 | 19.1 | 12.8 | 23.6 | 20.5 | 17.9 | 13.0 | 33.0 | 29.2 | 27.0 | 21.5 | 27.1 | 24.2 | 21.9 | 17.3 |
| Heart failure | 20.6 | 18.4 | 14.8 | 8.7 | 16.0 | 13.8 | 12.3 | 8.4 | 17.9 | 15.1 | 14.0 | 8.9 | 18.0 | 15.2 | 13.4 | 8.5 |
| Stroke | 15.8 | 15.4 | 13.6 | 9.9 | 13.6 | 13.5 | 12.2 | 10.9 | 12.3 | 12.3 | 10.2 | 8.6 | 11.4 | 11.6 | 9.8 | 7.9 |
| Peripheral arterial disease | 12.1 | 11.2 | 9.7 | 6.6 | 11.8 | 10.9 | 9.6 | 7.1 | 12.3 | 11.0 | 9.6 | 7.0 | 10.3 | 9.5 | 7.8 | 5.6 |
Cr, creatinine; IQR, inter-quartile range.
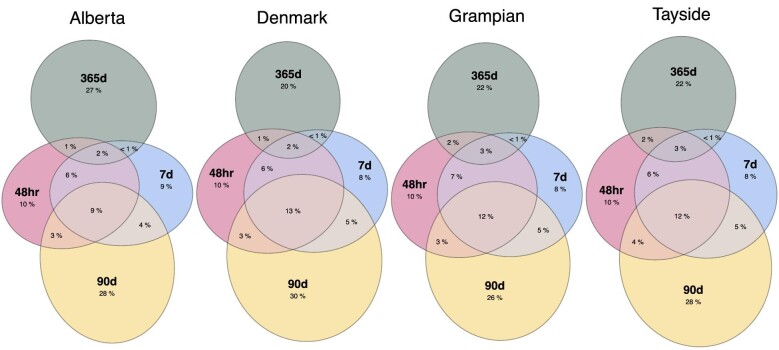
Frequency and overlap according to AKD subset criteria
The scaled proportions and overlap of people based on all combinations of subset criteria met within 1 week of AKD onset are illustrated in Fig. 2, and combinations of co-presentation on the same day of first AKD onset are provided in Supplementary data, Fig. S1. Overall, 71% (330 305/464 868) of people met only one of the AKD subset criteria during their episode, and 80% (370 545/464 868) of people met only one of the AKD subset criteria if co-presentation was restricted to the same day of first onset.
Figure 2:
Proportions and overlap of people meeting each combination of AKD criteria in each cohort (co-presenting within 1 week of first AKD onset).
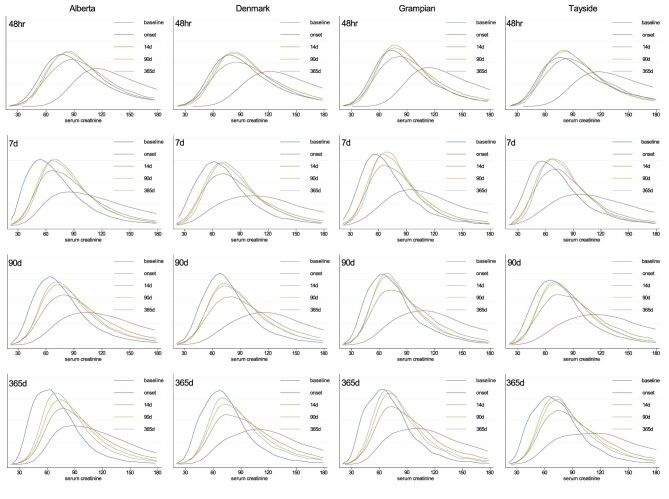
Trajectories of kidney function
Among people surviving 1 year, the distributions of serum creatinine at baseline, AKD onset, and 14, 90 and 365 days after onset of AKD, according to each AKD subset criterion are illustrated in Fig. 3 and the clinical course of creatinine, ratio vs baseline, and eGFR are elaborated in Supplementary data, Table S2. The patterns were similar across the four cohorts, and illustrate a positive (right) shift of distributions from baseline to peak creatinine within the first 7 days of AKD onset. Patients identified based on changes within 48 h had a larger positive shift in distribution of serum creatinine at the onset of AKI, with the distributions returning closer to that at baseline by 14 days and beyond. In contrast, the distributions of serum creatinine for those identified based on other definitions showed positive shifts that did not return as close to the baseline by 14, 90 or 365 days. Similar findings were observed when kidney function was evaluated based on eGFR or based on the ratio of creatinine concentration at each time point relative to baseline (Supplementary data, Table S2). These differences in the pattern of distribution were even more apparent when restricted to those exclusively meeting each subset criterion in isolation (e.g. those with AKD based on interval changes within 90 days but in no other subset) (Supplementary data, Fig. S2).
Figure 3:
Distribution of creatinine over the course of 1 year according to each AKD subset definition and cohort.
Mortality and recovery of kidney function
Overall, at 1 year, patients meeting the 48-h, 7-day and 90-day AKD criteria, and 365-day (i.e. e-alert) interval changes had pooled mortalities (95% confidence intervals) of 40% (34%–45%), 40% (34%–46%), 37% (31%–42%) and 22% (16%–29%), respectively, and non-recovery of kidney function of 19% (15%–23%), 30% (24%–35%), 25% (21%–29%) and 37% (30%–43%), respectively. This pattern of lower mortality for people with 365-day interval changes, and more recovery among those within a 48-h change was consistent across all cohorts (Table 2); and across age, sex and disease subgroups (Table 3) although notably those of male sex, older age and with cancer had higher mortality, whereas female sex, young age and diabetes more frequently experienced non-recovery. Further sensitivity analysis identified consistent findings when patients without creatinine test results in a follow-up window were included in a separate ‘untested’ category, consistent when the analysis was restricted to those meeting exclusively one subset definition in isolation (Supplementary data, Table S3) and when further broken down by combinations of AKD subset definitions met during the AKD episode (Supplementary data, Table S4).
Table 2:
One-year outcome percentages of AKD subsets for each cohort.
| Alberta | Denmark | Grampian | Tayside | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | |
| N | 100 278 | 101 075 | 136 465 | 93 640 | 57 659 | 56 043 | 77 254 | 37 240 | 18 620 | 18 524 | 22 767 | 13 252 | 24 932 | 24 441 | 31 696 | 18 011 |
| Dead | 33.7 | 33.4 | 29.9 | 15.4 | 42.0 | 41.9 | 37.0 | 23.8 | 39.3 | 39.8 | 38.1 | 23.4 | 44.2 | 44.9 | 41.8 | 27.4 |
| (95% CI) | (33.4–34.0) | (33.1–33.7) | (29.7–30.1) | (15.2–15.6) | (41.6–42.4) | (41.5–42.3) | (36.7–37.3) | (23.4–24.2) | (38.6–40.0) | (39.1–40.5) | (37.5–38.7) | (22.7–24.1) | (43.6–44.8) | (44.3–45.5) | (41.3–42.3) | (26.7–28.1) |
| Non-recovery | 23.6 | 36.6 | 30.3 | 45.0 | 19.4 | 29.8 | 26.0 | 36.8 | 17.5 | 27.0 | 22.0 | 33.1 | 15.7 | 25.4 | 21.8 | 32.0 |
| (95% CI) | (23.3–23.9) | (36.3–36.9) | (30.1–30.5) | (44.7–45.3) | (19.1–19.7) | (29.4–30.2) | (25.7–26.3) | (36.3–37.3) | (16.9–18.1) | (26.4–276) | (21.5–22.5) | (32.3–33.9) | (15.2–16.2) | (24.9–26.0) | (21.3–22.3) | (31.3–32.7) |
| Recovery | 42.7 | 30.0 | 39.8 | 39.6 | 38.6 | 28.3 | 37.0 | 39.3 | 43.3 | 33.2 | 29.9 | 43.5 | 40.1 | 29.6 | 36.4 | 40.5 |
| (95% CI) | (42.4–43.0) | (29.7–30.3) | (39.5–40.1) | (39.3–40.0) | (38.2–39.0) | (27.9–28.7) | (36.7–37.3) | (38.8–39.8) | (42.6–44.0) | (32.5–33.9) | (29.3–30.5) | (42.7–44.4) | (39.5–40.7) | (29.0–30.2) | (35.9–36.9) | (39.8–41.2) |
Percentages may not add to exactly 100% due to rounding.
CI, confidence interval.
Table 3:
One-year outcome percentages of AKD subsets for each cohort across age, sex and morbidity subgroups.
| Alberta | Denmark | Grampian | Tayside | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | 48 h | 7 days | 90 days | 365 days | |
| N | 100 278 | 101 075 | 136 465 | 93 640 | 57 659 | 56 043 | 77 254 | 37 240 | 18 620 | 18 524 | 22 767 | 13 252 | 24 932 | 24 441 | 31 696 | 18 011 |
| Diabetes (N) | 15 849 | 13 523 | 18 668 | 10 026 | 12 019 | 10 380 | 14 863 | 6181 | 4195 | 3761 | 4666 | 2255 | 5853 | 5052 | 6235 | 2757 |
| Dead | 36.3 | 38.5 | 34.3 | 23.0 | 41.6 | 43.2 | 35.7 | 24.1 | 40.0 | 41.7 | 39.5 | 26.5 | 43.6 | 46.7 | 42.8 | 30.3 |
| Non-recovery | 24.3 | 32.1 | 27.0 | 39.1 | 19.6 | 27.0 | 24.8 | 36.1 | 17.3 | 23.2 | 19.1 | 28.2 | 15.4 | 22.8 | 20.1 | 28.8 |
| Recovery | 39.4 | 29.4 | 38.7 | 37.9 | 38.8 | 29.8 | 39.5 | 39.9 | 42.7 | 35.2 | 41.4 | 45.4 | 41.0 | 30.6 | 37.1 | 40.9 |
| Cancer (N) | 6879 | 6734 | 9399 | 4203 | 17 535 | 18 494 | 24 644 | 5591 | 4428 | 4738 | 5497 | 1452 | 5525 | 5972 | 7048 | 1670 |
| Dead | 48.1 | 50.6 | 47.1 | 29.9 | 54.1 | 54.9 | 52.3 | 35.3 | 56.0 | 59.1 | 58.0 | 39.7 | 62.3 | 64.4 | 62.9 | 48.0 |
| Non-recovery | 17.9 | 26.8 | 21.0 | 33.0 | 16.3 | 24.6 | 20.7 | 32.6 | 15.1 | 20.9 | 16.9 | 27.2 | 12.8 | 19.4 | 17.0 | 27.0 |
| Recovery | 34.0 | 22.6 | 31.9 | 37.1 | 29.6 | 20.5 | 27.0 | 32.1 | 29.0 | 20.0 | 25.1 | 33.1 | 24.9 | 16.2 | 20.1 | 25.0 |
| Heart failure (N) | 7227 | 5976 | 7894 | 3258 | 11 026 | 9158 | 11 556 | 4120 | 3329 | 2801 | 3181 | 1186 | 4489 | 3723 | 4244 | 1523 |
| Dead | 48.4 | 50.9 | 48.1 | 39.0 | 51.1 | 52.1 | 45.8 | 38.4 | 55.4 | 57.8 | 56.0 | 45.4 | 60.5 | 62.3 | 58.2 | 45.1 |
| Non-recovery | 18.1 | 23.8 | 19.7 | 29.1 | 16.9 | 23.5 | 23.4 | 29.7 | 12.6 | 17.1 | 14.4 | 22.3 | 12.1 | 16.9 | 16.0 | 23.7 |
| Recovery | 33.5 | 25.3 | 32.2 | 31.9 | 32.0 | 24.4 | 30.8 | 31.9 | 32.0 | 25.1 | 29.6 | 32.3 | 27.4 | 20.8 | 25.8 | 31.2 |
| Female (N) | 42 872 | 52 053 | 72 409 | 55 693 | 23 858 | 27 300 | 38 320 | 20 266 | 8448 | 9750 | 12 165 | 7723 | 11 885 | 13 440 | 17 121 | 10 397 |
| Dead | 34.7 | 31.2 | 26.8 | 13.0 | 43.2 | 40.4 | 34.8 | 22.3 | 38.8 | 37.1 | 35.0 | 20.6 | 43.7 | 42.1 | 38.9 | 25.2 |
| Non-recovery | 24.0 | 39.9 | 33.6 | 50.0 | 19.9 | 33.1 | 28.7 | 40.4 | 17.4 | 29.2 | 24.4 | 36.7 | 16.3 | 28.3 | 24.2 | 35.6 |
| Recovery | 41.2 | 28.9 | 39.5 | 37.0 | 36.9 | 26.5 | 36.5 | 37.3 | 43.7 | 33.7 | 40.7 | 42.8 | 40.1 | 29.6 | 36.8 | 39.2 |
| Male (N) | 57 406 | 49 022 | 64 056 | 37 947 | 33 801 | 28 743 | 38 934 | 16 974 | 10 172 | 8774 | 10 602 | 5529 | 13 047 | 11 001 | 14 575 | 7614 |
| Dead | 33.0 | 35.8 | 33.3 | 19.0 | 41.1 | 43.4 | 39.2 | 25.7 | 39.6 | 42.8 | 41.7 | 27.3 | 44.6 | 48.4 | 45.2 | 30.5 |
| Non-recovery | 23.3 | 33.0 | 26.6 | 37.5 | 19.0 | 26.6 | 23.4 | 32.5 | 17.5 | 24.6 | 19.2 | 28.1 | 15.3 | 22.0 | 18.9 | 27.2 |
| Recovery | 43.7 | 31.1 | 40.1 | 43.5 | 39.9 | 30.0 | 37.4 | 41.8 | 42.9 | 32.6 | 39.1 | 44.6 | 40.1 | 29.6 | 35.9 | 42.4 |
| Age ≥70 years (N) | 54 779 | 49 157 | 64 007 | 35 090 | 36 997 | 32 659 | 45 036 | 20 618 | 11 762 | 10 774 | 13 161 | 7135 | 17 528 | 15 973 | 20 256 | 10 784 |
| Dead | 42.3 | 44.4 | 42.3 | 30.0 | 49.2 | 50.6 | 46.0 | 34.7 | 46.3 | 48.2 | 47.7 | 34.4 | 49.3 | 51.4 | 49.3 | 37.1 |
| Non-recovery | 20.2 | 29.2 | 22.3 | 32.5 | 17.1 | 25.0 | 21.6 | 30.9 | 14.4 | 20.8 | 16.0 | 23.5 | 13.9 | 21.0 | 16.9 | 24.0 |
| Recovery | 37.5 | 26.5 | 35.4 | 37.5 | 33.7 | 24.5 | 32.4 | 34.4 | 39.3 | 30.9 | 36.2 | 42.1 | 36.8 | 27.6 | 33.8 | 38.9 |
| Age <70 years (N) | 45 499 | 51 918 | 72 458 | 58 550 | 22 221 | 24 991 | 34 354 | 17 507 | 6858 | 7750 | 9606 | 6117 | 7404 | 8468 | 11 440 | 7227 |
| Dead | 23.4 | 23.1 | 18.9 | 6.7 | 29.6 | 30.5 | 25.2 | 10.8 | 27.2 | 28.1 | 24.9 | 10.5 | 31.9 | 32.7 | 28.6 | 13.0 |
| Non-recovery | 27.8 | 43.5 | 37.4 | 52.4 | 23.3 | 36.1 | 31.8 | 43.7 | 22.7 | 35.6 | 30.1 | 44.2 | 20.1 | 33.9 | 30.3 | 44.0 |
| Recovery | 48.9 | 33.3 | 43.7 | 40.9 | 47.1 | 33.4 | 43.0 | 45.5 | 50.1 | 36.3 | 45.0 | 45.3 | 48.0 | 33.4 | 41.1 | 43.0 |
Percentages may not add to exactly 100% due to rounding.
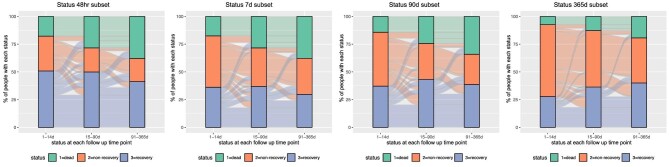
The proportions of people with recovery of kidney function, non-recovery and death at 14, 90 and 365 days after AKD, according to AKD subset definitions, are shown in Fig. 4. The proportion of people with non-recovery decreased over time from 14 to 365 days, with the largest proportion with recovery among those within the 48-h subset. Across all subsets, a substantial proportion of people with evidence of recovery at 14 and 90 days subsequently deteriorated to a state of non-recovery or death. Overall, non-recovery deteriorations occurred in 44% and 30% of people who had apparent recovery at 14 and 90 days, respectively. These findings were similar when earlier creatinine values were not carried forward for those without tests in each time period, with the exception of those with 365-day interval change where a larger proportion of people did not have available repeat creatinine tests (Table 2, Supplementary data, Fig. S3). When the cohort was restricted to patients meeting only one of the AKD subset criteria, a similar pattern of differences in recovery between subset definitions was observed (Supplementary data, Table S2).
Figure 4:
Proportions of patients with kidney function recovery status over the first year according to each AKD subset definition and cohort.
DISCUSSION
This study used a harmonized analytical approach to measure kidney recovery after AKD across four population-based cohorts from three high-income countries with universal health coverage. There were consistent findings across all cohorts, age, sex and comorbidity subgroups underlining the transportability and reproducibility both of AKD as an exposure, and kidney recovery (defined as a resolution of AKD and a fall in creatinine to within 1.2× baseline) as a reliable outcome measurement. Using this replicable method, there were two key findings. First, both non-recovery and mortality at 1 year were common outcomes for people within each AKD subset encompassing interval creatinine changes within but not beyond 90 days, with changes over longer intervals than 90 days (i.e. not AKD) associated with lower mortality. This is consistent with the current scope of AKD encompassing all creatinine changes within 90 days whether with or without AKI, and indeed 90-day creatinine change intervals were as serious for both kidney non-recovery and mortality prognosis as AKI identified by shorter creatinine change intervals. In contrast, these findings do not reconcile with the design of existing e-alert systems, suggesting consideration should be given to limiting the algorithms that underpin such systems to 90 days where they currently span longer intervals. Secondly, across all populations, we found consistent patterns of the balance between mortality and non-recovery across subsets, including higher mortality among males and at older ages, and higher rates of non-recovery among females and at younger ages. This underlines the clinical importance to consider both mortality and non-recovery as separate outcomes experienced by different people for whom priorities may also differ: for instance younger individuals may benefit from greater focus on strategies to maximize kidney recovery after AKD, whereas elderly individuals may benefit more from strategies to minimize the risk of recurrent acute illnesses and to ensure advance care plans are accurately updated.
It is notable that kidney recovery was more frequent and rapid when creatinine changes were over a short interval (48 h), and persistent non-recovery was more common when creatinine changes were over longer intervals. This is clinically intuitive and likely reflects an arbitrary distinction between AKI, AKD and CKD across the spectrum of progression of kidney diseases over time. Also notably, the 48-h subset had a lower eGFR at baseline. Possible explanations could either be a later presentation of AKI, or a tendency for transient absolute creatinine rises to occur more frequently among those with CKD. As recovery was more frequent and rapid in this subset, we favour the latter explanation. Nevertheless, as mortality was high across all three AKD subsets (48 h, 7 days, 90 days; ∼40% at 1 year), this analysis suggests that all forms of AKD merit clinical attention. Collectively, these findings suggest that AKD (either with or without AKI) cannot be viewed conceptually as a ‘milder’ form of AKI, but as a syndrome of similar prognostic importance with respect to both mortality and non-recovery. Moreover, those who had AKD without AKI more commonly presented in the community and therefore could be less visible within the health system despite the clinical importance and potential urgency.
A final consideration relates to the transition between states of kidney recovery, non-recovery and death over the course of 1 year after AKD. Among people in our analysis who initially appeared to recover kidney function within 14 or 90 days after onset, it was commonplace to subsequently deteriorate. Thus, while current KDIGO AKI guidelines suggest following people until 90 days for assessment of recovery or de novo CKD, future guidelines should consider that this needs to be tailored to each individual, approached with caution and with the assurance of safety nets, such as a clarity on the responsibility and frequency of primary care (or non-specialist) surveillance and measures to avoid recurrence/relapse. In addition, differences in the relative frequencies of non-recovery and mortality outcomes for different subgroups (i.e. males, old age, cancer and heart failure had higher frequencies of death, and females, young age and diabetes had higher frequencies of non-recovery) indicate that the priorities and considerations within follow-up also require individualization beyond a single assessment for de novo CKD.
A limited number of prior studies have assessed recovery of kidney function following either AKI or AKD, although variable populations, definitions and timeframes for identification of kidney recovery makes comparisons between studies challenging [27]. A systematic review found that transient AKI (occurring and recovering within 48 h) was associated with lower mortality than AKI that persisted for >7 days, and that AKI that persisted at hospital discharge carried the poorest long-term prognosis [28]. Heung et al. reported increasing time to recovery up to 10 days from AKI onset was associated with greater risk of developing CKD 1 year later [29]. Among patients hospitalized with AKI in Canada, age, sex, AKI stage, prehospitalization serum creatinine level, albuminuria and discharge serum creatinine were identified as predictors for developing de novo advanced CKD stage G4 or greater [30]. More recently, a population-based study by Wang et al. [24] reported complete recovery in 35% of patients at 7 days after AKI onset and 49% of patient at 90 days, with risk factors for lack of recovery within 7 days including greater AKI severity, pre-existing cancer or heart failure, and recent use of loop diuretics. Our study extends this knowledge about kidney recovery by assessing differences in kidney recovery, persistence of recovery and mortality across AKI/AKD subset criteria, populations and subgroups, and provides tools to allow replication of these methods in a consistent manner in other cohorts.
Strengths of this study include the use of four large population-based cohorts from three different countries that capture all blood tests for all residents, accompanied by the consistency of findings across these cohorts. There are also important limitations. First, in this analysis we restricted the definition of AKD to functional creatinine change criteria within 90 days. Structural changes such as proteinuria were not assessed. Secondly, our study was dependent on the complete capture of blood test data within four populations from high-income countries, both for initial identification of AKD, and for following the outcomes of non-recovery and death. Decision making is dependent on good quality data, but unfortunately such completeness is not possible in countries where access to blood tests to identify AKD is limited, care is not integrated across clinical locations, or surveillance systems and infrastructure are insufficient. Thirdly, in this analysis we focused on new (incident) presentations of AKD. Elsewhere we have shown that 20% of people with AKI have had prior events within the past year and have more vascular morbidities than those presenting for the first time [22]. This association is plausible across all subsets of AKI/AKD discussed here and may influence kidney recovery. Future work should evaluate how these recurrent presentations differ with respect to recovery and how this interacts with underlying cause. Finally, we did not have granular information on detailed attributed causative factors for each presentation, but we did find that recovery differed with the presence of comorbidities of cancer, diabetes and heart failure. Further steps are also now warranted to apply these operationalized definitions of AKD, subsets and kidney recovery, to examine the prognostic implications of combining them with other clinical and biological information to predict patient outcomes or develop clinical phenotypes that warrant different clinical approaches.
In summary, this study applied and shared the tools to replicate a harmonized approach to study AKD across geographically distinct populations and operationalize kidney recovery as an outcome. It demonstrated, consistently across populations, that while the case-mix and setting may vary between subsets of AKD (over intervals of 48 h, 7 days and 90 days), all subsets of AKD confer a high mortality and non-recovery at 1 year. The relative balance between mortality and non-recovery rates differs according to age and case-mix, which reinforces the need for a personalized approach to post-AKI care. Irrespectively, across populations, age, sex and comorbidity subgroups, AKD covering an interval up to but not beyond 90 days represents a clinical syndrome of at least similar prognostic importance to AKI with respect to both mortality and sustained non-recovery.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the support of the Grampian Data Safe Haven (DaSH) facility within the Aberdeen Centre for Health Data Science and the associated financial support of the University of Aberdeen, and NHS Research Scotland (through NHS Grampian investment in DaSH). For more information, visit the DaSH website: http://www.abdn.ac.uk/iahs/facilities/grampian-data-safe-haven.php.
M.T.J. is supported by a Canadian Institutes of Health Research Foundation Grant. C.B. is supported by Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and the Wellcome Trust. W.B. is supported by The Health Foundation Networked Data Lab grant (FR-000002362). C.F.C. is supported by the Independent Research Fund Denmark (grant number 0134-00407B).
Contributor Information
Simon Sawhney, Aberdeen Centre for Health Data Science, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK; Department of Renal Medicine, NHS Grampian, Aberdeen, UK.
William Ball, Aberdeen Centre for Health Data Science, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK.
Samira Bell, Division of Population Health and Genomics, University of Dundee, Dundee, UK.
Corri Black, Aberdeen Centre for Health Data Science, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK; Department of Renal Medicine, NHS Grampian, Aberdeen, UK.
Christian F Christiansen, Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark.
Uffe Heide-Jørgensen, Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark.
Simon K Jensen, Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark.
Emilie Lambourg, Division of Population Health and Genomics, University of Dundee, Dundee, UK.
Paul E Ronksley, Department of Community Health Sciences, O'Brien Institute for Public Health, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Zhi Tan, Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Marcello Tonelli, Department of Community Health Sciences, O'Brien Institute for Public Health, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Matthew T James, Department of Community Health Sciences, O'Brien Institute for Public Health, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
FUNDING
S.S. was supported by a Starter Grant for Clinical Lecturers from the Academy of Medical Sciences, Wellcome Trust, Medical Research Council, British Heart Foundation, Arthritis Research UK, the Royal College of Physicians and Diabetes UK (SGL020\1076).
DATA AVAILABILITY STATEMENT
Datasets cannot be made available to other researchers due to contractual arrangements with government agencies who are the data custodian. Information on how researchers may make requests to obtain similar datasets from health research dataset custodians may be provided upon request.
CONFLICT OF INTEREST STATEMENT
This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta, nor Alberta Health or Alberta Health Services express any opinion in relation to this study. All authors declared no competing interests.
REFERENCES
- 1. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17(1):204. 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. See EJ, Jayasinghe K, Glassford N et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 2019;95:160–72. 10.1016/j.kint.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 3. NHS England . Patient safety alert on standardising the early identification of acute kidney injury. https://www.england.nhs.uk/patientsafety/wp-content/uploads/sites/32/2014/06/psa-aki2.pdf (28 August 2023, date last accessed). [DOI] [PubMed] [Google Scholar]
- 4. Ivica J, Sanmugalingham G, Selvaratnam R. Alerting to acute kidney injury—challenges, benefits, and strategies. Pract Lab Med 2022;30:e00270. 10.1016/j.plabm.2022.e00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chawla LS, Bellomo R, Bihorac A et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13:241–57. 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
- 6. Lameire NH, Levin A, Kellum JA et al. Harmonizing acute and chronic kidney disease definition and classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Kidney Int 2021;100:516–26. 10.1016/j.kint.2021.06.028 [DOI] [PubMed] [Google Scholar]
- 7. Sawhney S, Bell S, Black C et al. Harmonization of epidemiology of acute kidney injury and acute kidney disease produces comparable findings across four geographic populations. Kidney Int 2022;101:1271–81. 10.1016/j.kint.2022.02.033 [DOI] [PubMed] [Google Scholar]
- 8. James MT, Pannu N. Can acute kidney injury be considered a clinical quality measure? Nephron 2015;131:237–41. 10.1159/000441426 [DOI] [PubMed] [Google Scholar]
- 9. Sawhney S, Fraser SD. Epidemiology of AKI: utilizing large databases to determine the burden of AKI. Adv Chronic Kidney Dis 2017;24:194–204. 10.1053/j.ackd.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hemmelgarn BR, Clement F, Manns BJ et al. Overview of the Alberta kidney disease network. BMC Nephrol 2009;10:30. 10.1186/1471-2369-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vestergaard SV, Christiansen CF, Thomsen RW et al. Identification of patients with CKD in medical databases: a comparison of different algorithms. Clin J Am Soc Nephrol 2021;16:543–51. 10.2215/CJN.15691020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graversen HV, Norgaard M, Nitsch D et al. Preadmission kidney function and risk of acute kidney injury in patients hospitalized with acute pyelonephritis: a Danish population-based cohort study. PLoS One 2021;16:e0247687. 10.1371/journal.pone.0247687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slagelse C, Gammelager H, Iversen LH et al. Acute kidney injury and 1-year mortality after colorectal cancer surgery: a population-based cohort study. BMJ Open 2019;9:e024817. 10.1136/bmjopen-2018-024817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Logan R, Davey P, De Souza N et al. Assessing the accuracy of ICD-10 coding for measuring rates of and mortality from acute kidney injury and the impact of electronic alerts: an observational cohort study. Clin Kidney J 2019;13:1083–90. 10.1093/ckj/sfz117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bell S, James MT, Farmer CKT et al. Development and external validation of an acute kidney injury risk score for use in the general population. Clin Kidney J 2020;13:402–12. 10.1093/ckj/sfaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sawhney S, Robinson HA, van der Veer SN. et al. Acute kidney injury in the UK: a replication cohort study of the variation across three regional populations. BMJ Open 2018;8:e019435. 10.1136/bmjopen-2017-019435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawhney S, Tan Z, Black C et al. Validation of risk prediction models to inform clinical decisions after acute kidney injury. Am J Kidney Dis 2021;78:28–37. 10.1053/j.ajkd.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mclean A, Nath M, Sawhney S. Population epidemiology of hyperkalemia: cardiac and kidney long-term health outcomes. Am J Kidney Dis 2022;79(4):527–38. 10.1053/j.ajkd.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 19. NHS England . Acute kidney injury (AKI) programme. 2014. https://www.england.nhs.uk/akiprogramme/ (28 August 2023, date last accessed). [Google Scholar]
- 20. Inker LA, Eneanya ND, Coresh J et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sawhney S, Marks A, Fluck N et al. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017;69:18–28. 10.1053/j.ajkd.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Interdisciplinary Chronic Disease Collaboration . Database programming resources. 2022. https://cumming.ucalgary.ca/research/icdc/health-tools/codes (28 August 2023, date last accessed). [Google Scholar]
- 24. Wang H, Lambourg E, Guthrie B et al. Patient outcomes following AKI and AKD: a population-based cohort study. BMC Med 2022;20:229–8. 10.1186/s12916-022-02428-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. StataCorp . Stata statistical software: Release 16. College Station, TX: StataCorp LLC, 2019. [Google Scholar]
- 26. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R foundation for statistical; computing, 2016. https://www.R-project.org/ (28 August 2023, date last accessed). [Google Scholar]
- 27. Guthrie G, Guthrie B, Walker H et al. Developing an AKI consensus definition for database research: findings from a scoping review and expert opinion using a Delphi process. Am J Kidney Dis 2022;79:488–96.e1. [DOI] [PubMed] [Google Scholar]
- 28. Mehta S, Chauhan K, Patel A et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol 2018;19:91–7. 10.1186/s12882-018-0876-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heung M, Steffick DE, Zivin K et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis 2016;67:742–52. 10.1053/j.ajkd.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. James MT, Pannu N, Hemmelgarn BR et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA 2017;318:1787–97. 10.1001/jama.2017.16326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets cannot be made available to other researchers due to contractual arrangements with government agencies who are the data custodian. Information on how researchers may make requests to obtain similar datasets from health research dataset custodians may be provided upon request.