Abstract
The Fifth Artificial Pancreas Workshop: Enabling Fully Automation, Access, and Adoption was held at the National Institutes of Health (NIH) Campus in Bethesda, Maryland on May 1 to 2, 2023. The organizing Committee included representatives of NIH, the US Food and Drug Administration (FDA), Diabetes Technology Society, Juvenile Diabetes Research Foundation (JDRF), and the Leona M. and Harry B. Helmsley Charitable Trust. In previous years, the NIH Division of Diabetes, Endocrinology, and Metabolic Diseases along with other diabetes organizations had organized periodic workshops, and it had been seven years since the NIH hosted the Fourth Artificial Pancreas in July 2016. Since then, significant improvements in insulin delivery have occurred. Several automated insulin delivery (AID) systems are now commercially available. The workshop featured sessions on: (1) Lessons Learned from Recent Advanced Clinical Trials and Real-World Data Analysis, (2) Interoperability, Data Management, Integration of Systems, and Cybersecurity, Challenges and Regulatory Considerations, (3) Adaptation of Systems Through the Lifespan and Special Populations: Are Specific Algorithms Needed, (4) Development of Adaptive Algorithms for Insulin Only and for Multihormonal Systems or Combination with Adjuvant Therapies and Drugs: Clinical Expected Outcomes and Public Health Impact, (5) Novel Artificial Intelligence Strategies to Develop Smarter, More Automated, Personalized Diabetes Management Systems, (6) Novel Sensing Strategies, Hormone Formulations and Delivery to Optimize Close-loop Systems, (7) Special Topic: Clinical and Real-world Viability of IP-IP Systems. “Fully automated closed-loop insulin delivery using the IP route,” (8) Round-table Panel: Closed-loop performance: What to Expect and What are the Best Metrics to Assess it, and (9) Round-table Discussion: What is Needed for More Adaptable, Accessible, and Usable Future Generation of Systems? How to Promote Equitable Innovation? This article summarizes the discussions of the Workshop.
Keywords: continuous glucose monitoring, glucose, hospital, inpatient, insulin, metrics
Introduction
The conference was held on May 1 and 2, 2023 at the National Institutes of Health (NIH) campus in Bethesda, Maryland and was attended by 180 professionals in-person and 145 by video conference. The previous conference in this series had focused on early trials and potential. Now, with devices in clinical use and late-stage trials, this conference focused on real-world data, information technology (IT) issues, applicability to various populations, adaptive algorithms, use of artificial intelligence (AI), novel strategies for sensing, hormones and delivery, intraperitoneal (IP) delivery, and concluded with a round-table discussion of closed-loop systems performance and expectations regarding future systems.
Real-world data indicate a positive impact on glycemic control that occurs quickly after initiation of an automated insulin delivery (AID). There is greater impact for those with worse baseline control. Other common outcomes have been improved sleep quality, normalized effort toward diabetes management, and decreased perceived stigma. Key barriers limiting widespread adoption of AID systems in real-world populations have been limitations in access, coverage, affordability, and training and racial and ethnic minority groups have been particularly affected by some of these problems. Insufficient provider skills, patient selection and onerous training have limited the usefulness of AID systems. To translate the powerful impact of AID to real-world populations, the diabetes community must continue to advocate for broadened access and insurance coverage. The diabetes community can continue to use multicenter real-world data and best practices to inform quality improvement efforts to improve access to critical diabetes technology and reduce health inequities.
Open standards and interoperability are the key to success of complex devices, but the AID industry has been slow to adopt these ideas. Like other complex systems, such as the electronic health record (EHR), AID systems need standard terminologies, ontologies, data models, and metadata. This allows advanced analytics, such as AI and innovative applications. Cybersecurity is critical and IEEE 2621, a standard completed and recognized by the Food and Drug Administration (FDA) provides an opportunity for diabetes manufacturers to demonstrate sound cybersecurity both to give patients confidence and to acquire a competitive advantage. The 2022 iCoDE-1 Report with collaboration from 130 individuals, 60 organizations, has 54 recommendations with data standards and workflows. Clinical implementation guides like iCoDE are the first steps in driving this form of system integration.
Besides adults with type 1 diabetes (T1D), automated insulin deliveries have recently been tested in special T1D populations, such as infants and the elderly, patients with gastroparesis or cystic fibrosis (CF), pregnancy and insulin-treated type 2 diabetes (T2D). Automated insulin deliveries appear to be especially beneficial in people with challenges related to marked glycemic extremes, especially recurrent hypoglycemia. In high-burden conditions, such as pregnancy, end-stage renal disease (ESRD) and CF, AIDs seem to lower the burden. During pregnancy, the use of a customized home-AID system during the second and third trimesters appears to provide maternal as well as fetal benefits. For patients that transitioned from open-loop therapy to AIDs, a greater improvement in time-in-range (TIR) is seen in individuals with higher hemoglobin A1c (HbA1c) concentrations and lower TIR at entry. Meal and exercise announcements remain necessary in patients using hybrid AIDs.
The performance of AID systems is challenged by slow absorption of insulin, lagging glucose values, and inaccuracies of reported food and exercise, but learning algorithms systems using AI are starting to emerge. These systems, which are currently being tested in silico, use physiologic principles, data from previous patients and analysis of performance of the AID to function within a model predictive control (MPC) paradigm.
AID systems may be optimized by better sensing of glucose and other metabolites. Implantable sensors have extended lifetimes and may eventually measure lactate and ketones, which are additional indicators of the metabolic state and may better inform novel adaptive algorithms. More rapid-acting insulins may be possible with co-polymers that stabilize insulin and allow co-formulation with amylin analogs. Skin changes due to chronic insulin pump usage may influence insulin absorption. A new glucose-responsive insulin analog can be locked and unlocked by conformational changes.
IP insulin delivery is more physiologic than subcutaneous (SC) infusion, delivering faster, dose-dependent insulin absorption with a higher therapeutic index and less hyperinsulinism. Intraperitoneal insulin also restores the glucagon secretion response to hypoglycemia. Recent clinical trials suggest that IP insulin may be superior to SC insulin in AID systems. Delivery of IP insulin can be done by an implantable insulin pump or an IP port. Studies of new models of both types of systems are underway.
Performance metrics are critical to understanding the quality of current AID systems and developing better systems. The Glycemic Risk Index (GRI) is a consensus metric derivative of five separate glycemic range results, mean glucose, and glycemic variability, designed to reflect the hypoglycemic and hyperglycemic risks in percentiles that can be further sorted into quintiles. Time-in-range metrics are not necessarily indicative of the quality of glycemia, because they do not account for severe hypoglycemia and severe hyperglycemia suggesting that TIR may not be a good single parameter to evaluate AID systems and perhaps two metrics are needed, one for hypoglycemia exposure and one for hyperglycemia exposure. These numeric parameters for the quality of glycemia may be best for performance evaluation, but other patient centric metrics, such as simplicity, usability, health impact, and effects on activities of daily living may also be important.
The conference ended with a round-table discussion of AID promotion and innovation. Important aspects that covered stakeholder engagement, accessibility, simplicity and usability, training, and follow-up. Other considerations discussed included psychosocial, ethical, and cost considerations.
Session 1: Lessons Learned From Recent Advanced Clinical Trials and Real-World Data Analysis
Moderators
Jessica Castle, MD
Oregon Health and Science University
Viral Shah, MD
Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus
Speakers
Sue Brown, MD
University of Virginia
Lessons Learned through the Development of Advanced AID Systems
Steven Russell, MD, PhD
Massachusetts General Hospital/BetaBionics
Generalizability of Trial Results to Diverse Populations: Lessons from the Bionic Pancreas Pivotal Trial
John Lum, MS
JAEB Center for Health Research
Real-World Testing of Open Source AID systems
Roman Hovorka, PhD
University of Cambridge
Using CamAPS FX Across All Groups: RCTs and Real-World Settings
Roy Beck, MD, PhD
JAEB Center for Health Research
Control-IQ Outcomes from Age 2 to 72
Nelly Mauras, MD
Nemours Children’s Health
Disparities in the Access to Advanced Technologies
Ohad Cohen, MD
Sheba Medical Center/Medtronic
Lessons Learned from the Real-World Data of Automatic Insulin Delivery Systems
Osagie Ebekozien, MD, MPH
T1D Exchange
Improving Access to Hybrid Closed Systems: Insights from the T1D Exchange Clinic Network
Session 1 Summary Written By
Tejaswi Kompala, MD
University of Utah, Salt Lake City, UT, USA
In this session, real-world evidence (RWE) for AID systems was reviewed, with an emphasis on performance and clinical outcomes across demographic groups stratified by age, race/ethnicity, education, and income. A common theme throughout the several distinct AID systems reviewed was the positive impact on glycemic control that occurs quickly after initiation and the typically stronger impact on glycemic outcomes for those with worse baseline control. Outcomes for race and ethnicity groups are more variable: while some studies identified comparable impact, other real-world data suggest an ongoing performance gap in whites vs minority populations. Biologically, one would expect AID systems to work the same across race and ethnic groups. Because of social determinants of health, racial, and ethnic minority groups may be at higher risk of hyperglycemia. Multiple presenters urged the diabetes research community to intentionally recruit for study cohorts more representative of the real world. 1 Randomized controlled trials routinely under-enroll vulnerable populations (eg, worse baseline control, racial, and ethnic minority groups lower educational/income status) who may actually benefit the most from these technologies. Challenges to conducting randomized control trials (RCTs) for AID systems are presented in Figure 1.
Figure 1.
Challenges of randomized controlled trials.
Source: Figure courtesy of Ohad Cohen.
Alongside glycemic outcomes, patient-reported outcomes (PROs) are also critically important in evaluation of AID systems. Some differences were noted in various age groups, but common themes included improvements in sleep quality, normalizing effort toward diabetes management, and decreased perceived stigma.
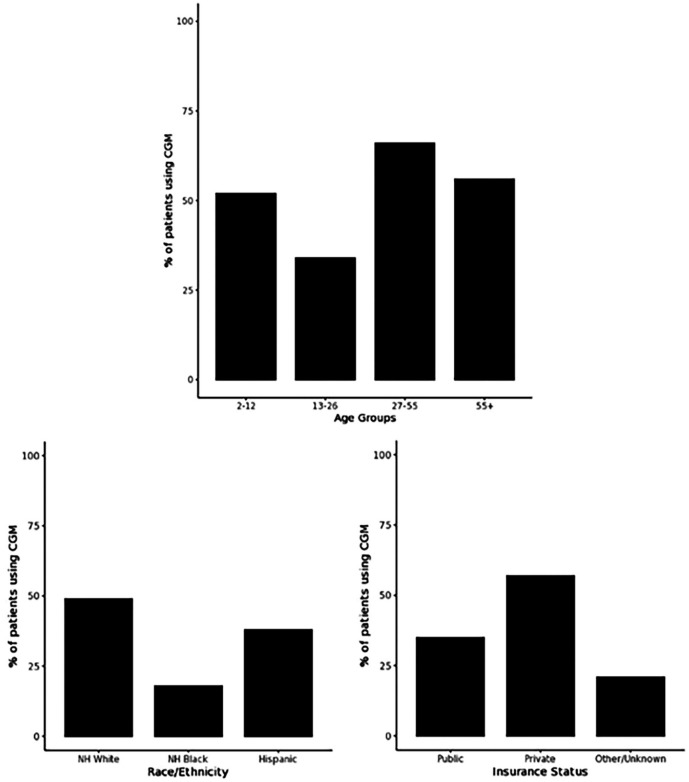
Limitations in access, coverage, affordability, and training are key barriers limiting widespread adoption of AID in real-world populations. According to EHR data from the T1DX-Q1 Collaborative during 2017 to 2019, continuous glucose monitor (CGM) use has not been equally distributed according to patient age, race/ethnicity, and insurance coverage as presented in Figure 2. 2 Multiple speakers noted implicit bias and provider bias that limit people with diabetes’ (PWD) access to technologies. Speakers urged the clinical community to move past the previous approach that required users to prove themselves capable by being in good baseline control, attending onerous training classes, and mastering carb counting, and so on. Improvements in the technologies have eased user burden, such that, many PWD stand to benefit, including groups with lower health literacy or significant life chaos who may have been previously overlooked.
Figure 2.
Patient demographics of CGM users.
Source: Figure reproduced from DeSalvo et al. 2
Abbreviations: CGM, continuous glucose monitor; NH, non-Hispanic.
Gaps in provider knowledge and/or comfort were also highlighted as a challenge limiting real-world uptake of AID technologies. This is particularly true when considering that the majority of adults with T1D receive diabetes care exclusively from a primary care professional. Training and supporting health care professionals are required to overcome the clinical inertia of adopting new technologies. Improvements in AID technology might decrease the burden of learning for professionals as more decision-making is shifted autonomously to the device, which may help to improve access to diabetes technology beyond mainly subspecialty diabetes care.
To translate the powerful impact of AID to real-world populations, the diabetes community must continue to advocate for broadened access and insurance coverage. Locally within one’s own clinic or health system, evaluating pain points in the routine workflow can improve access. Other health systems have leveraged insurance navigators to help the most vulnerable groups advocate for access. As we increasingly use AIDs, open access to real-world data promote a shared, collaborative learning experience. Supported by ongoing work of the T1D Exchange QI Network, the diabetes community can have access to multicenter real-world data and best practices information to inform quality improvement efforts for improving access to critical diabetes technology and reduce health inequities.
Session 2: Interoperability, Data Management, Integration of Systems and Cybersecurity; Challenges and Regulatory Considerations
Moderators
Jessica Flynn, PhD
US Food and Drug Administration
Juan Espinoza, MD
Northwestern University, Lurie Children’s Hospital
Speakers
Jessica Flynn, PhD
US Food and Drug Administration
Streamlining Regulatory Processes to Facilitate Innovations for Automated Insulin Dosing Systems
Howard Look, BSCE
Tidepool
Bridging the Gap Between DIY/Open-Source Systems and Regulated Systems
Lane Desborough, MSc
Nudge BG, Inc.
Composing Secure AID Systems from Interoperable Components: Challenges and Opportunities
David Klonoff, MD
Mills-Peninsula Medical Center
The IEEE 2621 Cybersecurity Standard for Connected Diabetes Devices
Juan Espinoza, MD
Northwestern University, Lurie Children’s Hospital
Data Considerations to Enable Advanced Analytics and Applications
Amy Criego, MD
International Diabetes Center
Device Integration into the EHR: Challenges and Opportunities
Session 2 Summary Written By
Wei-An (Andy) Lee, DO
Los Angeles County and University of Southern California Medical Center, Los Angeles, CA, USA
The goal of creating a broadly accessible AID system faces significant challenges with interoperability, data management, cybersecurity, and integration of systems. The key to addressing these issues lies in the development and adoption of open standards.
AID interoperability, the ability to exchange, and interpret information between devices and software systems, has been limited because of a closed ecosystem of proprietary protocols leading to a stagnant marketplace with limited options for PWD. The FDA has a regulatory classification, framework, and pathway as presented in Table 1, with the goal of interoperability to support patient preference and product innovation for AID systems. AID companies have been slow to adopt these standards citing barriers with (1) FDA approvals, (2) cybersecurity, and (3) business risk. Countering this perspective, Tidepool Loop’s recent FDA clearance as the first truly interoperable automated glycemic controller demonstrates a patient-led DIY model leveraging open device protocols and open cloud application programming interfaces (APIs) with rapid product iteration cycles that can (1) navigate through the FDA regulatory pathway, (2) reduce cybersecurity risks, and (3) ignite business opportunities that further expand the consumer marketplace from AID-inspired technologies.
Table 1.
Regulatory Categories for Interoperable Diabetes Devices.
| Device | De Novo classification | Code of federal regulations |
|---|---|---|
| Integrated continuous glucose monitor (iCGM) | DEN170088, March 27, 2018 | 21 CFR 862.1355 |
| Alternate controller-enabled (ACE) pump | DEN180058, February 15, 2019 | 21 CFR 880.5730 |
| Interoperable automated glycemic controller (iAGC) | DEN190034, December 13, 2019 | 21 CFR 862.1356 |
Abbreviations: ACE, alternate controller-enabled; CFR, code of federal regulations; DEN, De Novo; iAGC, interoperable automated glycemic controller; iCGM, integrated continuous glucose monitor.
It has never been more difficult to launch an insulin pump, CGM, or AID system in the United States. Technical, commercial, and regulatory constraints affect the development and FDA review of AID systems, which much be developed for people from all socioeconomic groups. Cost, risk, and uncertainty of the commercial-regulatory pathway make it very difficult for innovators to participate. They lack access to venture capital and pump or CGM partners. Interoperable AID system development will benefit from an updated, harmonized and transparent commercial-regulatory pathway. Institute of Electrical and Electronics Engineers (IEEE) 11073 and Bluetooth secure plug-and-play interoperability standards will foster true “competitive compatibility.” Alternative evidence generation methods, such as simulation, post-market feedback loops, and FDA acceptance of PRO and RWE studies, would accelerate access to the benefits of AID.
Interoperability requires data management standards for seamless communication and analytics. Diabetes, a highly quantifiable disease rich with data for clinical assessment and intervention, needs standard terminologies, ontologies, data models, and metadata. Furthermore, data APIs and explainable algorithms with clear documentation are also necessary for advanced analytics, such as AI and innovative applications. Most importantly, data standards will reduce the digital divide in non-research, underfunded health care institutions serving marginalized populations, such as many Federally Qualified Health Centers (FQHC) minimizing the need for major departments of informatics.
Cybersecurity that is the protection of data and command information transmitted between connected medical devices from unauthorized disclosure, modification, and loss of function has emerged as a major issue for AIDs. To address the basic principles of connected diabetes device cybersecurity, IEEE 2621, a standard completed and recognized by FDA in 2022, (1) defines a framework for a connected electronic product security evaluation program for diabetes devices and (2) provides grounds for confidence that connected electronic products deliver the security protections claimed by their developers and deemed necessary by stakeholders.
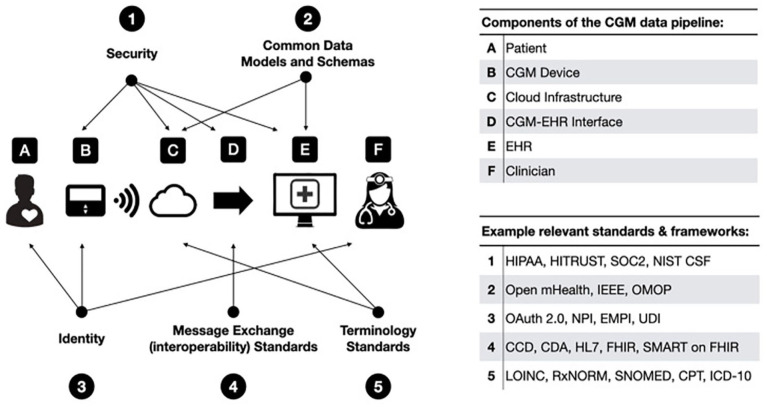
As interoperability, data management, and cybersecurity become more complex, integration of these systems can also be a major barrier. Figure 3 presents best practices for components of the AID data pipeline, so that, data can be viewable through the EHR in a secure, accessible, and meaningful way allowing for single sign-on visualization of consolidated trended data by clinicians and patients. The 2022 iCoDE-1 Report with collaboration from 130 individuals and 60 organizations, contains 54 recommendations with technical standards and considerations and clinical implementation guide. iCoDE is the first step in driving this form of system integration. 3
Figure 3.
Standards and best practices for systems integration.
Source: Figure reproduced from Xu et al. 3
Abbreviations: CCD, continuity of care documents; CDA, clinical document architecture; CGM, continuous glucose monitor; CPT, current procedural terminology; EHR, electronic health record; EMPI, enterprise master patient index; FHIR, fast health care interoperability resources; HIPAA, Health Insurance Portability and Accountability Act; HL7, health level 7; ICD-10, international classification of diseases 10th revision; IEEE, Institute of Electrical and Electronics Engineers; LOINC, logical observation identifiers names and codes; NIST CSF, National Institute of Standards and Technology Cybersecurity Framework; NPI, National Provider Identifier; OMOP, observational medical outcomes partnership; SMART, substitutable medical applications, reusable technologies; SNOMED, systemized nomenclature of medicine; SOC2, system and organization controls type 2—trust services criteria; UDI, unique device identifier; HITRUST, the health information trust alliance; RxNORM, medical prescription medical prescription.
Open standards development and adoption by stakeholders in the AID ecosystem can foster and expedite simpler and more accessible devices for diabetes patients. Future emphasis will be needed in (1) FDA advocacy for prioritized centers of excellence for technical regulatory expertise, (2) augmented versus human experimentation data for accelerated validation of models, and (3) AID post-marketing feedback loop as a way to further refine and improve devices with continuous monitoring.
Session 3: Adaptation of Systems Through the Life Span and Special Populations: Are Specific Algorithms Needed?
Moderators
Andrew Bremer, MD, PhD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
National Institute of Health
Eda Cengiz, MD, MHS
University of California, San Francisco
Speakers
R. Paul Wadwa, MD
Barbara David Center for Diabetes, University of Colorado Anschutz Medical Campus
Findings from an RCT of Automated Insulin Delivery in Young Children with Type 1 Diabetes
Carol Levy, MD
Carl Icahn School of Medicine at Mount Sinai
At-Home Use of a Pregnancy-Specific Hybrid Closed-Loop System for Pregnancies Complicated by Type 1 Diabetes: A Multicenter U.S. Clinical Study
Ananda Basu, MD
University of Virginia & University of Alabama at Birmingham (Current)
AP Research in T1DM with Severe Gastroparesis
Boris Kovatchev, PhD
University of Virginia
Automated Insulin Delivery Across the Lifespan: A Comparative Analysis of a Large Real-World Database
Robert Vigersky, MD
Medtronic Diabetes
Can One Algorithm Fit All Ages of People with Type 1 Diabetes?
Connie Rhee, MD
University of California, Irvine
The Role of Automated Diabetes Management Systems in Patients with Diabetes and Kidney Disease
Melissa Putman, MD
Massachusetts General Hospital/Harvard University
Artificial Pancreas Technology in the Management of Cystic Fibrosis-Related Diabetes
Charlotte Boughton, MD, PhD
University of Cambridge
Closed-Loop in Type 2 Diabetes
Session 3 Summary Written By
David Kerr, MD
Diabetes Technology Society
As the number of commercially available AID systems has increased over recent years, this has been paralleled by a growth in the indications for using these systems beyond T1D in adults. These include diabetes at the extremes of life, diabetes associated with complications (gastroparesis), pregnancy, and insulin-treated T2D.
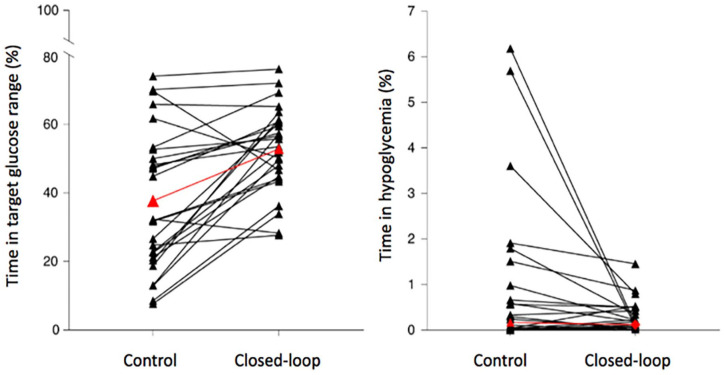
AID systems appear to be especially beneficial in situations where PWD are experiencing challenges related to high HbA1c levels and/or marked glycemic variability and where recurrent hypoglycemia may be especially problematic. There is a need for further research on exploring modified artificial pancreas (AP) algorithms to improve post prandial glucose control in those with diabetic gastroparesis. It remains an unmet need and a continuing challenge for AP therapy and hence for such patients with T1D. Furthermore, in other clinical situations, where the disease burden associated with diabetes is high (eg, ESRD, CF), early results from clinical trials suggest that the use of AID systems has important benefits beyond glucose, including a reduction in the personal burden experienced by individuals dealing with these serious conditions each day and night as presented in Figure 4.
Figure 4.
Changes in glycemic control in 26 outpatients with type 2 diabetes and end-stage renal disease requiring dialysis who switched from SC insulin therapy to a fully closed-loop automated insulin delivery system. Before vs after metrics were: TIR, (100-180 mg/dL) 38% vs 53%; time in hyperglycemia 57% vs 43%; mean glucose, 209 mg/dL vs 182 mg/dL; and time in hypoglycemia, 0.2% vs 0.1%. Subjects using AIDs achieved 3.5 additional hours each day with glucose in target range.
Source: Figure reproduced from Boughton et al. 4 under the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0, https://creativecommons.org/licenses/by/4.0/). Abbreviations: ESRD, end-stage renal disease; SC, subcutaneous; TIR, time-in-range.
Real-world data across the age continuum demonstrate that one algorithm could accommodate PWD. Examples from a large data set of over 4000 patients using the MiniMedTM 780G system in ages ≤ 15, 15 to 55, and ≥ 56 years using the recommended settings of a glucose target of 100 mg/dL and Active Insulin Time (AIT) (not a physiologic parameter but a lever to set the aggressiveness of the algorithm) of two hours. The TIR was 77%, 80% and 82%, respectively, with TBR 2.3%, 1.8%, and 1.7%. Disparities in glycemic outcomes between children and adults are likely related to behavioral differences reduced by an algorithm that delivers basal insulin and autocorrections up to every five minutes. Elimination of the remaining disparities will require algorithms that incorporate other data, such as hand gestures captured on a smart watch (Klue app), which seamlessly announces meal detection and micro-bolus insulin delivery.
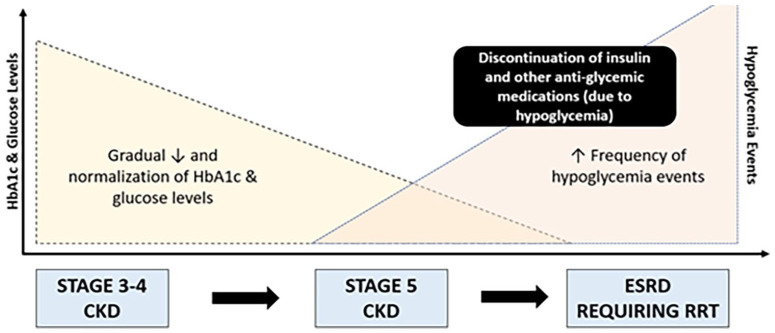
Patients with ESRD are prone to have hyperglycemic episodes early in the disease and hypoglycemic episodes later in the disease as presented in Figure 5. Automated insulin delivery for these patients can decrease the frequency of both types of out of range episodes. In situations where tight glycemic control is a priority, such as during pregnancy, the use of a home-AID system customized to the glycemic goals of pregnancy during the second and third trimesters appears to have maternal as well as fetal benefits. In many situations, training in AID systems can be delivered virtually with success.
Figure 5.
Glycemic status trajectory from chronic kidney disease (CKD) to end-stage renal disease (ESRD).
Source: Figure reproduced from Rhee et al. 5 Changes in TIR after transitioning from an open to hybrid closed-loop system.
Abbreviations: CKD, chronic kidney disease; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c; NDD-CKD, non-dialysis-dependent chronic kidney disease; NIH, National Institutes of Health; RRT, renal replacement therapy.
In a comparative analysis of a large real-world database of over 20 000 adults and children (including very young children) with T1D and a smaller number with insulin-treated T2D, who transitioned from open-loop therapy to AID systems, a greater improvement in TIR was seen in individuals with lower TIR (than higher) at entry and this was noted especially for older people irrespective of their gender over the initial three months. It was also noteworthy that user-initiated boluses decreased over time across all age groups. Around one in three used the system as in a fully closed mode without meal or correction bolusing for four to five days each month that was not associated with deterioration in their TIR.
For most currently available hybrid AID systems, persistent challenges related to meal-time announcement and dealing with exercise remain although these are likely to be ameliorated with input from additional (ie, peripheral) wearable devices. In conclusion, among subgroups of individuals with more challenging diabetes, AID systems appear to be beneficial across the lifespan, and in the future, additional clinical benefits are likely to be achieved by “tuning” of existing algorithms rather than developing new ones.
Session 4: Development of Adaptive Algorithms for Insulin Only and for Multihormonal Systems or Combination With Adjuvant Therapies and Drugs: Clinical Expected Outcomes and Public Health Impact
Moderators
Eyal Dassau, PhD
Harvard University/Eli Lilly and Company
Claudio Cobelli, PhD
University of Padova
Speakers
Marc Breton, PhD
University of Virginia
Design and Early Clinical Validation of an Insulin Only Automated Insulin Delivery Capable of Full Closed Loop
Edward Damiano, PhD
Boston University/BetaBionics
Autonomous Adaptation of the Single -and Dual -Hormone iLet Bionic Pancreas in Type 1 Diabetes
Ahmad Haidar, PhD
McGill University
Insulin and Pramlintide Fully Closed-Loop System
Patricio Colmegna, PhD
University of Virginia
Enabling Full Closed Control Using a Multi-Stage Model Predictive Control Approach
Peter G. Jacobs, PhD
Oregon Health and Science University
Utilizing AI, Metabolic Modeling, and Multiple Hormones to Enable Automated Responses to Meals and Exercise in Closed-Loop Systems
Greg Forlenza, MD
Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus
Islet Preservation in Children with New Onset T1D: Results from the CLVer Trial
Session 4 Summary Written By
Patricio Colmegna, PhD
Center for Diabetes Technology
University of Virginia, Charlottesville, VA, USA
A 21 000 PWD data set was used to design an insulin-only AID algorithm for a full closed-loop (FCL) system. With bolus automation, AID systems allow for a reduction in the number of manual correction and user-initiated correction boluses. Among this data set, PWD on average used four to five boluses per day to achieve 70% TIR. Better glycemic control was achieved with fewer boluses than this average number of daily boluses, while significantly, more boluses were associated with worse glycemic control. Real-world data show that AID systems are used in hybrid closed-loop (HCL) or FCL mode interchangeably. This fact denotes the undeniable need for more flexible AID designs that can better mitigate postprandial glycemic excursions without relying on appropriately sized premeal manual boluses. The iLet Bionic Pancreas (BP) is initialized solely with user’s body weight and then adapts itself to ever-changing insulin needs without depending on quantitative input by the user or health care professional. The insulin-only iLet BP has been evaluated in various studies,6,7 showing its effectiveness when compared with standard of care.
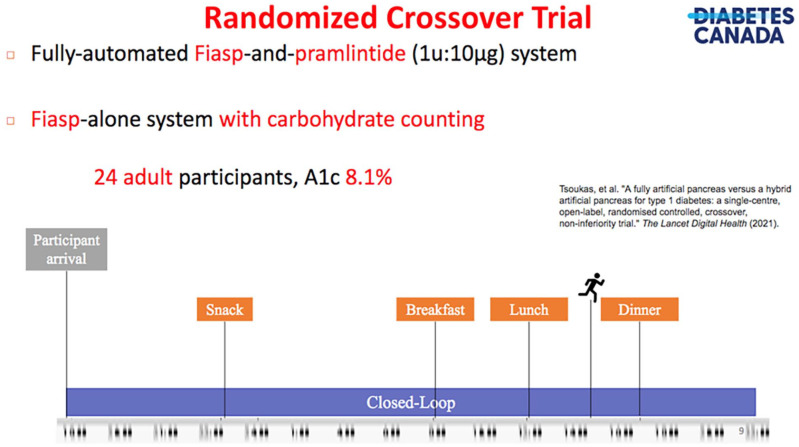
The use of adjunctive therapies represents an appealing means to overcome the inherent design limitations imposed by current insulin analogs. One promising option is pramlintide, an amylin analog, that helps reduce postprandial hyperglycemia by slowing gastric emptying and suppressing glucagon secretion. This naturally leads to the question of whether pramlintide can facilitate the performance of FCL systems. A positive answer can be found in an RCT conducted by Tsoukas et al 8 where authors showed that a Fiasp plus pramlintide system in FCL was not non-inferior to a Fiasp-alone system in HCL. Time in range (TIR; 70-180 mg/dL) was achieved by 78.1% in HCL mode and 74.3% in FCL mode (p=0.28).The study design for this trial is presented in Figure 6. On the downside, common side effects of pramlintide include nausea, vomiting, and increased risks of early postprandial hypoglycemia and late hyperglycemia. Co-formulations will not only facilitate its administration, but also enable new optimal control algorithms for co-formulation delivery that will be potentially superior to current independent delivery strategies.
Figure 6.
Randomized controlled, crossover, non-inferiority trial study design, comparing Fiasp-alone in a HCL system versus Fiasp-and-pramlintide in a FCL system.
Source: Figure courtesy of Ahmad Haidar. 8
The University of Virginia (UVA) AID algorithm combines classical automatic control designs and novel data-driven approaches for interchangeable HCL/FCL use, guaranteeing safe front-loading of insulin when the need arises. Performance of this strategy was assessed in silico 9 and confirmed through a sequence of supervised outpatient clinical trials. 10
Disturbance anticipation can represent another step forward toward effective FCL. In this context, patterns of glycemic disturbances can be extracted from historical records and informed to the controller to anticipate their impact. In practical terms, instead of having a single controller, multiple copies are created, one per distinct behavioral pattern. In a study conducted by Colmegna et al, 11 authors show how the UVA AID algorithm can be re-arranged in a multi-stage framework where reactive and anticipatory modes are smoothly emphasized/de-emphasized to further tighten postprandial glucose control. It is worth remarking that anticipation did not increase the risk of hypoglycemia even when meals were delayed, therefore, this approach represents a safe approach for algorithmic acceleration of insulin action.
It is also important to consider that the existence of large intra-patient and inter-patient physiologic variability demands adaptive algorithms in the pursuit of effective FCL solutions. Mosquera-Lopez et al 12 have evaluated a single-hormone robust artificial pancreas (RAP) with automated meal detection using machine learning in a randomized, single-center, crossover trial. Results indicated that RAP significantly and safely reduced the time above 180 mg/dL when compared with an MPC algorithm in HCL. The study was conducted using the Oregon Health & Science University (OHSU) iPancreas platform that ran the RAP within an Android app and aggregated CGM, insulin, and heart rate data from wearable fitness sensors on an Amazon Web Services cloud server. 13 Further studies are in progress to evaluate the performance of RAP on a larger sample size and for a longer study duration. Dr Jacobs has also created a digital twin program to simulate an FCL system in a digital environment. The model used insulin kinetics, glucose infusion, glucose absorption, and heart rate. The data used to build the model were in silico data from the open source OHSU metabolic simulator. 14 The model was evaluated on real-world data from the T1D in exercise initiative data set (T1Dexi). A decision-based algorithm using real-world data can be used to detect exercise or meals and generate promptly automated responses confirming duration and type of exercise (aerobic or anaerobic) to provide optimal glucose management recommendations.
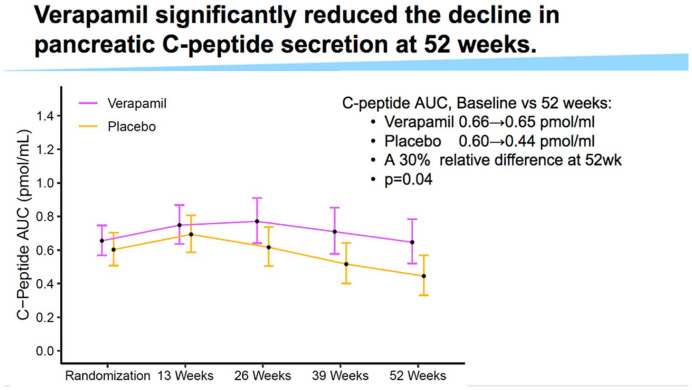
While a cure for diabetes is the ultimate goal, maintenance of at least residual beta cell function is desired to reduce diabetes-related risks. Verapamil, a calcium channel blocker, was found to reduce the expression of thioredoxin interacting protein (TXNIP), which is involved in glucotoxicity and beta cell death. A double-blind, randomized clinical trial was presented where the effect of Verapamil on pancreatic beta cell function was evaluated in children and adolescents (7-18 years old) with newly diagnosed T1D. 15 Results revealed that Verapamil helped preserved the C-peptide area under the curve at 52 weeks from diagnosis compared with placebo as presented in Figure 7. Similar rates of adverse effects between Verapamil and placebo groups were observed. There was a prominent concern regarding electrocardiogram (EKG) abnormalities among adolescents, including Wenckebach second-degree AV block. However, no treatment interventions are recommended for children presenting with asymptotic Wenckebach. This effect of Verapamil could be an example of the benefit of initiating biological defense mechanisms to supplement the effects of diabetes technology.
Figure 7.
Use of Verapamil reduces the decline of C-peptide secretion at 52 weeks from diagnosis of type 1 diabetes.
Source: Figure courtesy of Gregory Forlenza.
Abbreviation: AUC, area under the curve.
Special Lecture: AID for Restoration of Impaired Awareness of Hypoglycemia
Speaker
Michael Rickels, MD
University of Pennsylvania
Special Lecture Summary Written By
Rachel Aaron, BA
Diabetes Technology Society
Dr Michael R. Rickels, from the University of Pennsylvania, discussed his recent work regarding how AID may improve physiologic defenses against hypoglycemia in individuals with impaired awareness of hypoglycemia (IAH). 16 Impaired awareness of hypoglycemia occurs when a PWD may be dangerously unaware of the onset of hypoglycemia and may predispose PWDs to severe episodes of hypoglycemia. 17 In people without diabetes, the typical hypoglycemic physiological defense begins with the islet beta cells turning off insulin production and increasing glucagon secretion from the alpha cells. Increased glucagon promotes glucose production by the liver to raise blood glucose levels. With beta cell loss in people with T1D, this defense may be lost and the body will turn to secondary autonomic responses, including the secretion of epinephrine from the adrenal medulla to promote the conversion of glycogen into glucose.
Studies have shown that avoidance of hypoglycemia with meticulous glycemic control could preserve both an individual’s epinephrine secretion and autonomic response; however, in the long term, PWD eventually maintain only autonomic responses. These findings suggest that disease duration seems to impact an individual’s ability to recover their physiological defense against hypoglycemia. Dr Rickels has sought to investigate how the use of AID within a HCL system for hypoglycemia avoidance could improve autonomic responses to defend against hypoglycemia in persons with T1D complicated by IAH.
The study he presented included ten individuals with T1D who have lived over 30 years with a diagnosis of diabetes, had a normal body mass index (BMI) and a HbA1c of less than 7%. Impaired awareness of hypoglycemia was defined in this study by a Clarke score of 4 or more, a validated measure for identifying individuals with an abnormal autonomic symptom response to insulin-induced hypoglycemia. 18 Patients used the Medtronic 670G system or Tandem t:slim system and were observed for 18 months.
Patients using automated suspension of insulin from a HCL system had fewer episodes of sleep and awake-associated hypoglycemia and spent significantly less TBR than prior to intervention. To observe sleep, actigraphy data were used to see how using a HCL system affected sleep-associated hypoglycemia.
Observing individuals’ epinephrine responses was important to distinguish between IAH and intact awareness of hypoglycemia. Epinephrine levels were measured prior to intervention, at 6 and 18 months. There was an increase in epinephrine secretion over this time interval, suggesting restoration of secondary sympathetic autonomic responses, as also observed for autonomic symptoms like palpitation, sweat, and tremors. Parasympathetic activation was observed by measuring levels of pancreatic polypeptide that also increased over the 18 months. Despite the improvement in epinephrine secretion in response to insulin-induced hypoglycemia, there was no difference in endogenous glucose production by the liver, but there was a decrease in peripheral glucose utilization, suggesting the increased epinephrine decreased use of glucose in peripheral tissues, such as in muscle and adipose tissue. Thus, avoiding hypoglycemia can improve physiological epinephrine secretion and autonomic symptom generation, as well as decrease peripheral glucose utilization and improve the overall physiological defense mechanisms against hypoglycemia. It is important to highlight that the duration of T1D can predispose a cycle of worsening IAH through hypoglycemia-associated autonomic failure (HAAF) that may be more difficult to reverse by hypoglycemia avoidance in individuals with longer disease duration. In addition, it appears that substantial hypoglycemia avoidance (defined as at least < 2% TBR), may be necessary to restore the epinephrine response to insulin-induced hypoglycemia and ultimately reverse the syndrome of HAAF in affected individuals with IAH.
Session 5: Novel AI Strategies to Develop Smarter, More Automated, Personalized Diabetes Management Systems
Moderators
Boris Kovatchev, PhD
University of Virginia
Ahmad Haidar, PhD
McGill University
Speakers
Jose Garcia Tirado, PhD
Inselspital–University Hospital Bern & University of Bern
Making Fully Automated Insulin Delivery a Reality—Strategies from the Engineer’s Toolbox
Clara Mosquera-Lopez, PhD
Oregon Health and Science University
Enabling Fully Automated Insulin Delivery in Type 1 Diabetes Using AI: From Task-Specific Predictive Models to AI-Based Digital Twins
Mudassir Rashid, PhD
Illinois Institute of Technology
Transforming Diabetes Treatment with Artificial Intelligence: Fully Automated Multivariable Artificial Pancreas to Mitigate the Effects of Meals, Exercise, and Stress
Laya Ekhlaspour, MD
University of California, San Francisco
Meal Composition and Postprandial Glucose Excursion in Closed-Loop Systems
Michael C. Riddell, PhD
York University
Physical Activity and Current Closed-Loop Control in Type 1 Diabetes
Session 5 Summary Written By
Cynthia Huang, BA
Diabetes Technology Society, Burlingame, California, USA
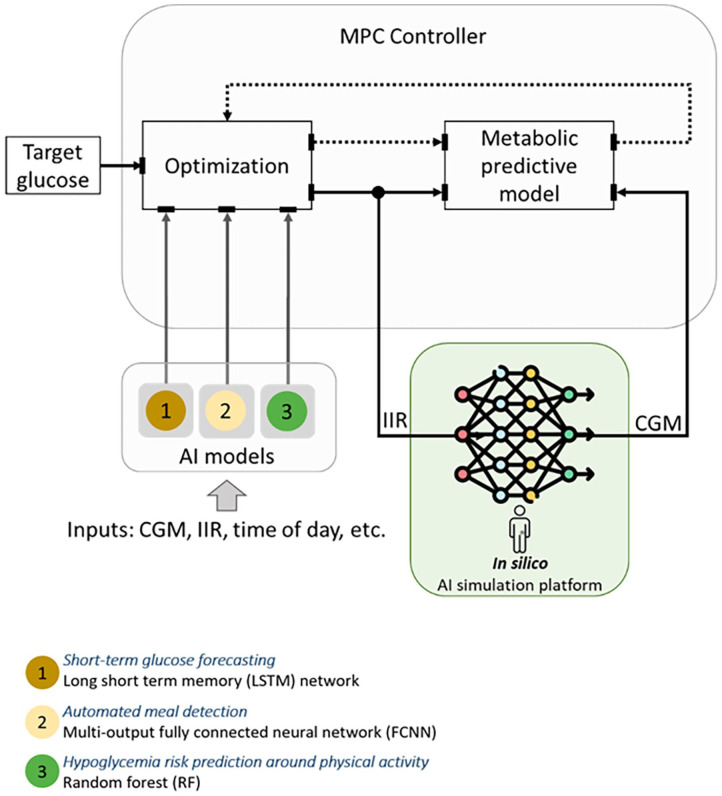
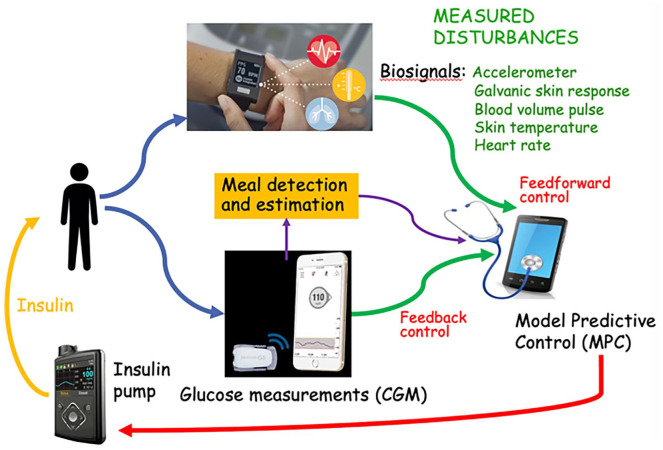
The last two decades have seen tremendous progress in novel technologies and drugs for managing T1D. For instance, hybrid closed-loop systems for AID have shown promise in improving glucose control, particularly at night when individuals are not eating or exercising. Current hybrid AID systems still require user input for insulin meal boluses and physical activity (PA)-related insulin adjustments. Artificial intelligence can be leveraged to develop algorithms for detection and classification of meal and exercise events to inform control decisions within an AID system. An AI-augmented AID system, presented in Figure 8, has been developed by OHSU to (1) inform control decisions around PA and meal intake, (2) prevent occurrence of low glucose events, and (3) optimize insulin delivery algorithms for individuals living with T1D.
Figure 8.
Physiology-guided AI models for replicating glucose dynamics of people living with T1D.
Source: Figure courtesy of Clara Mosquera-Lopez.
Abbreviations: CGM, continuous glucose monitor; IIR, insulin infusion rate; MPC, model predictive control.
Although several algorithms for detecting meals and dosing meal insulin have been proposed meal detectors that rely only on CGM data have shown delays, which might limit their impact in improving post prandial glucose control given additional insulin action delays. In the future, the availability of faster insulins has the potential to improve post prandial glucose control following automated detection of meal events. However, the use of new insulin preparations will require further refining and testing of AID systems. The use of extended boluses in a hybrid AID system can also be beneficial for handling the effects of specific meal compositions on postprandial hyperglycemia. In addition, retrospective analysis of meals with known carbohydrate, protein, and fat contents, combined with CGM and insulin values in closed-loop systems has shown that models for insulin requirements should take into consideration meals producing variable patterns of postprandial glycemia.
Exercise causes significant changes in glucose turnover making glycemic control difficult for individuals on insulin therapy, even if an AID system is used. While TIR tends to be higher and TBR tends to be lower in active individuals on AID systems, exercise-associated hypoglycemia and hyperglycemia still occur. Better user education, activity integration to the ambulatory glucose profile, faster on/off insulins, PA monitors, various prediction models, and adjuvants to enhance the glucagon-to-insulin ratio for aerobic exercise, such as sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists can be implemented to improve glycemic control around physical activity.
As presented in Figure 9, fully automated multivariable artificial pancreas (mvAP) systems have been developed that incorporate wearable sensors with novel AI techniques to accurately predict future blood glucose concentration trends and mitigate glycemic disturbances, such as what occurs with unplanned meals, physical activity (PA), acute psychological stress (APS), and sleep irregularities, without requiring manual inputs from users. At Illinois Institute of Technology, novel AI techniques have been integrated with mvAP systems for minimal user burden in achieving tight control of glucose levels despite the many complex glycemic disturbances occurring in free-living conditions. A fully automated mvAP system can accurately detect and handle subject-specific glycemic disturbances and identify historical patterns and daily activity behaviors, resulting in improved insulin dosing decisions and tighter glycemic control in people with T1D.19,20
Figure 9.
A fully automated multivariable artificial pancreas capable of detecting in real time occurrences of meals, physical activity, and acute psychological stress that disrupt euglycemia.
Source: Figure courtesy of Mudassir Rashid.
Abbreviations: CGM, continuous glucose monitor; MPC, model predictive control.
Session 6: Novel Sensing Strategies, Hormone Formulations and Delivery to Optimize CL Systems
Moderators
Barry H. Ginsberg, MD, PhD
Diabetes Technology Society
Jennifer Sherr, MD, PhD
Yale University School of Medicine
Speakers
Mukul Jain, PhD, MBA
Senseonics
Innovative Multi-Sensing Techniques Using Long-Term Implantable CGM
Alfonso Galderisi, MD, PhD
Yale University
Beyond the Glucose-Centric Diabetes Management: The Path to Multiple Sensing
Ketan Dhatariya, MBBS MSc MD MS FRCP PhD
Norfolk and Norwich University Hospitals
How Continuous Ketone Monitoring Can Augment an Automated Insulin Delivery System
Michael Weiss, MD, PhD, MBA
Indiana University
Design and Validation of a Mechanism-Based Glucose-Responsive Insulin
Eric A. Appel, PhD
Stanford University
Ultrafast Insulin and Insulin-Pramlintide Formulations to Improve Closed-Loop Control
Jeffrey Joseph, DO
Jefferson University
Why Are Insulin Pharmacokinetics So Variable . . .The Clinical Need for Consistent Insulin Absorption
Irl B. Hirsch, MD
University of Washington Medicine Diabetes Institute
The Impact of Long-Term Insulin Pump Use on the Skin: Results of the DERMIS Study
Session 6 Summary Written By
Carlos E. Mendez, MD, FACP
Medical College of Wisconsin, Milwaukee, MI, USA
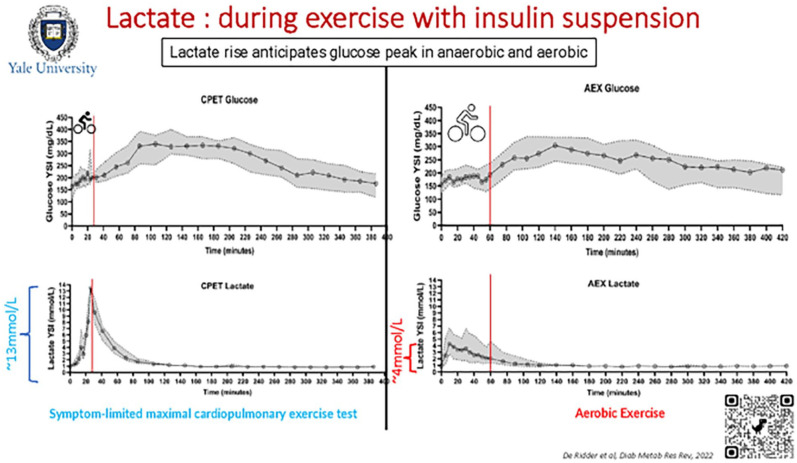
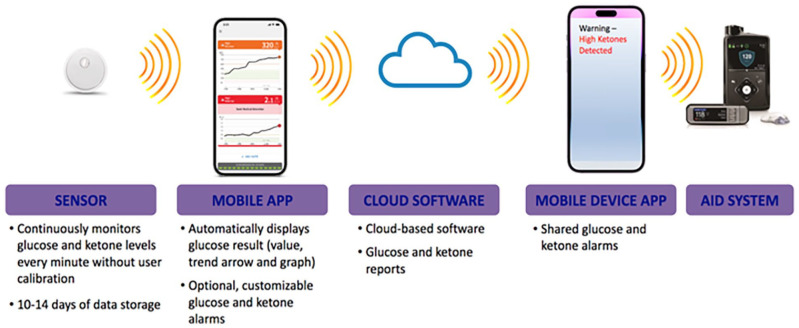
This session included presentations that highlighted innovations extending from glucose and other metabolites sensing technologies to the development of novel insulin formulations and the intricacies of local skin reactions that influence insulin absorption. The session started with the introduction of Senseonics’ next-generation sensor, which through redundant optical measurements offers the potential for measuring oxidation levels resulting in longer calibration intervals. The company is working toward the goal of eliminating the need of an external transmitter and the potential measurement of other metabolites, such as lactate and ketones. The concept of continuous lactate monitoring during exercise was subsequently discussed. It was highlighted how lactate levels are known to rise before glucose during exercise, and how by detecting increased lactate levels, a device could inform insulin delivery systems of impending hyperglycemia as presented in Figure 10. The potential benefits and challenges of continuous ketone monitoring in augmenting AID systems were also reviewed. Figure 11 presents how a combined CGM and ketone monitor could integrate with an AID. Benefits of ketone monitoring include the early warnings that could enable proactive actions to prevent diabetic ketoacidosis (DKA) in patients with T1D as well as those with T2D at high risk of DKA, such as those on SGLT-2 inhibitors or with ketosis prone diabetes.
Figure 10.
Lactate concentration during aerobic and anaerobic exercise with insulin suspension.
Source: Figure reproduced from De Ridder et al. 21
Abbreviations: AEX, aerobic exercise; CPET, cardiopulmonary exercise testing; YSI, yellow spring instruments.
Figure 11.
A theoretical example of an integrated continuous glucose and ketone monitoring system with an automated insulin delivery system.
Source: Figure courtesy of Ketan Dhatariya.
Abbreviation: AID, automated insulin delivery.
The session then proceeded to review advancements on insulin formulations. The development of a new glucose-responsive insulin based on the native mechanism of insulin binding and receptor activation was presented. This insulin analog that can be locked and unlocked in response to glucose levels functions by recapitulating as a complex choreography of transmitted conformational changes. 22 In addition, “MoNi,” a new co-polymer excipient that stabilizes insulin enabled the development of an ultra-fast absorbing insulin formulation. This new co-polymer excipient also allows for co-formulation of insulin with amylin analogs for dual hormone therapy, aiming to overcome the burden of separate injections. The session then went on to discuss the variability in insulin absorption caused by the local inflammatory reactions seen in the insertion sites of different insulin pump infusion sets. Histological evidence showing the thickening of connective tissue and the role of adipose cells, capillaries, and lymphatics in insulin absorption was discussed, highlighting the significant variability that exists in bolus delivery. Finally, results of a study aimed to characterize changes in pump insertion sites of patients with T1D using traditional histopathology and a form of noninvasive skin imaging called optical coherence tomography (OCT) were presented. The study involved comparing skin changes in control, current, and three-day previous sites using OCT, biopsies, and metabolomic analyses. The findings showed increased blood flow and inflammation at the current and previous pump site compared with the control site, but no difference between the three-day prior and the current site. To the surprise of the investigators, histology results also demonstrated eosinophilic infiltration that was significantly more prominent in patients with less than ten years compared with those with greater than 20 years of pump use.
Session 7: Special Topic: Clinical and Real-World Viability of IP-IP Systems. “Fully Automated Closed-Loop Insulin Delivery Using the IP Route”?
Moderators
Anna Casu, MD
AdventHealth Research Institute, Orlando, Florida, USA
Peter Lord, BS
Physiologic Devices Inc., Chatsworth, California, USA
Speakers
Eric Renard, MD, PhD
Montpellier University Hospital, Montpellier, France
Experience of Closed-Loop Insulin Delivery Using the Peritoneal Route and Perspectives
Rayhan A. Lal, MD
Stanford University, Stanford, California, USA
The Magic of Intraperitoneal Insulin Delivery
Chris Hanson, MS
Perikinetics, San Francisco, California, USA
Technical challenges of Intraperitoneal sensing and delivery
Claudio Cobelli, PhD
University of Padova, Padova, Italy
The European Project FORGETDIABETES: a fully implantable, fully automated, pancreas with IP insulin delivery and IP glucose sensing
Session 7 Summary Written By
Andrea Yeung, BA
Diabetes Technology Society, Burlingame, California, USA
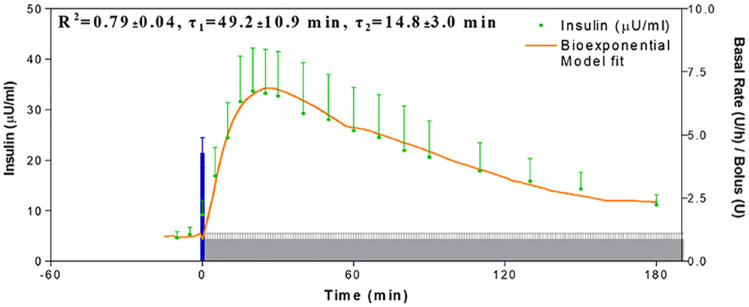
In normal physiology, insulin is secreted into the portal venous system, a route of delivery that offers numerous potential benefits. Intraperitoneal insulin delivery has been shown to be closer to physiology than SC infusion in both animal and human studies. Intraperitoneal insulin delivery offers a short peak and disappearance of insulin activity in the blood, whereas insulin remains in the blood for a sustained period of time with SC insulin delivery. Micossi demonstrated that bolusing with IP resulted in faster, dose-dependent insulin absorption, with SC being much slower. 23 The peritoneal route of insulin delivery describes the direct diffusion of insulin from the peritoneal cavity into the hepatic venous portal system resulting in a positive portal-systemic gradient of insulin concentration after IP injection. While SC insulin delivery is the standard of care, it has a narrow therapeutic window, requires systemic hyperinsulinism, and favors the overserved who have time for intensive diabetes management. Intraperitoneal insulin delivery has a wide therapeutic window, reduces hyperinsulinism, and offers rapid correction. IP insulin delivery leads to quicker and more reproducible insulin absorption as presented in Figure 12, preferential hepatic portal distribution, and restoration of a glucagon secretion response to hypoglycemia.
Figure 12.
Intraperitoneal insulin shows quick absorption with high reproducibility of insulin delivery from implanted pumps in people with T1D.
Source: Figure courtesy of Eric Renard.
The first trials for using IP insulin infusion for closed-loop glucose control in people with T1D combined IP insulin delivery with intravenous (IV) glucose sensing in a fully implantable system using a proportional-integral-derivative (PID) algorithm with no meal announcement. In 48-hour experiments, time in the 80 to 240 mg/dL glucose range reached 84%, including 91% in out-of-meal periods and 76% in two-hour post-meal periods. A delay in post-meal control was due to an internal delay of the IV-implanted sensor because the sensor was submitted to the sheer stress of the central blood flow in the heart. Thus, following 48-hour experiments of combined IP insulin delivery with SC glucose sensing, using a PID algorithm, 30% of meal boluses were delivered within 15 minutes before meals. Time in the 80 to 120 mg/dL glucose range reached 39% vs 28% with open-loop IP insulin delivery. A more recent trial compared 48-hour closed-loop insulin delivery with IP insulin infusion vs SC insulin infusion combined with SC glucose sensing using a MPC algorithm with no meal announcement. Time in 70 to 180 mg/dL glucose range was 66% with IP insulin vs 44% with SC insulin while time below 70 mg/dL was 2.5% with IP insulin vs 4.1% with SC insulin.
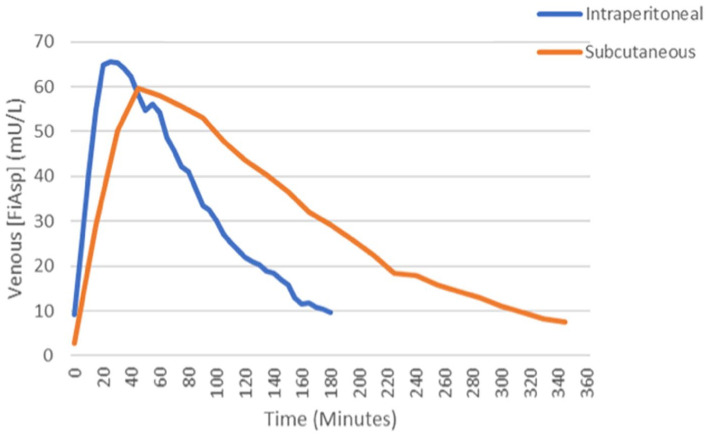
While the pharmacokinetics of portal insulin is relatively well-established and modeled, the frequently reported hypoglycemia prevention has not been simulated. It was hypothesized that the pharmacokinetics of IP insulin will be faster and lead to a greater counter-regulatory glucagon response than SC insulin. In one study, IP insulin was injected during visits 1 and 2, and SC insulin was injected during visit 3 with the goal of inducing one hypoglycemic event. The primary outcome was the glucagon response to hypoglycemia, and the secondary outcome was deducing pharmacokinetics. Overall, a very high dose of IP insulin is necessary to induce hypoglycemia. Around 40% of SC total daily dose (between 0.2 and 0.3 units/kg) given IP was typically necessary to induce one hypoglycemic event < 50 mg/dL; a matched SC dose averages four hypoglycemic episodes under similar conditions. Tmax is about half the time for IP, and the time to get down to half the value after the peak (T½max) is much shorter for IP. Preliminary pharmacokinetic data are presented in Figure 13.
Figure 13.
Preliminary pharmacokinetic data comparing intraperitoneal and subcutaneous of delivery of Fiasp insulin.
Source: Figure courtesy of Rayhan Lal.
Abbreviations: AUC, area under the curve; PK, Pharmacokinetic.
The FORGETDIABETES project, supported by the European Union within the Future Emerging Technology initiative, was described. The aim of this project is the development of a fully implanted, fully automated APs with IP insulin delivery and IP glucose sensing. FORGETDIABETES began in October 2020 and will last 54 months. An insulin capsule refills the reservoir of insulin for the pump. The structure and material of the insulin capsule are designed to withstand gastrointestinal (GI) fluids and allow for stable insulin structure. The capsule travels through the GI tract. When it reaches the implantable system, it is recognized by a sensor, and it is magnetically docked to two switchable magnets; this allows the capsule to stay stable. Insulin is aspirated and moves either to the reservoir or to the peritoneum. The following challenges are important to consider when developing a fully implantable, autonomous system: (1) decreased sizing of the actual implant with pumping mechanism, (2) temperature stability and highly concentrated insulin, (3) lifespan of glucose sensors in IP cavity, (4) cost of development, and (5) regulatory requirements.
First-generation IP insulin delivery has clinical proof but comes with many problems. The Roche Accu-Chek Diaport requires an ostomy and has a high infection risk, and the Medtronic Implantable MiniMed system results in occlusions and pump malfunctions. Therefore, expectations for the development of IP closed-loop insulin delivery come from forthcoming new models of miniaturized implantable pumps able to be connected to SC or IP sensing and using new specific MPC algorithms. For example, the Phoenix Pump by Medtronic and the ThinPump by PhysioLogic are pumps designed to be smaller and more discrete than their original versions. Because a fully implantable, autonomous IP system will take a considerable amount of time and resources to get to market, the team at Perikinetics is focusing on a less capitally intensive and less stringent regulatory approach to deliver a product more quickly to market, which will be important to demonstrate the key advantages of IP insulin delivery. The Perikinetics Insulin Delivery Conduit (IDC) features an implantable port in the IP space and an infusion set that is flush to the skin. The Perikinetics IDC tolerates skin laxity up to one inch in each direction to preserve needle integrity, increases adhesive patch distances to reduce adhesive irritation, and minimizes the reservoir size of the port to clear insulin out of the system.
Session 8: Round-table Panel: Closed-Loop Performance: What to Expect and What are the Best Metrics to Assess it
Moderators
Roy Beck, MD, PhD
JAEB Center for Health Research
Stu Weinzimer, MD
Yale University
Round Panel Members:
Roy Beck MD, PhD
JAEB Center for Health Research
Richard Bergenstal, MD
International Diabetes Center
David Klonoff, MD
Mills Peninsula Medical Center
Boris Kovatchev, PhD
University of Virginia
Anne Peters, MD
Keck School of Medicine
Moshe Phillip, MD
Schneider Medical Center
David Rodbard, MD
Biomedical Informatics
Robert Vigersky, MD
Medtronic Diabetes
Session 8 Summary Written By
Douglas B. Muchmore, MD
Kinexum, Harpers Ferry, WV, USA
The closed-loop performance panel discussion, moderated was divided into two subsections: (1) Performance Metrics and (2) Problems and Solutions.
Performance Metrics
The discussions of Performance Metrics can be divided into two categories: (1) Theoretical (ie, benefits and limitations of various metrics with respect to describing closed-loop system performance) and (2) Practical (ie, how the various metrics can be used to enhance patient care at the individual person level). With some overlap between presenters, the general flow of the presentations began with the Theoretical and moved progressively toward the Practical.
Opening the session was David Klonoff, who gave a brief history of the development of a new continuous glucose monitoring index metric dubbed the GRI. 24 This single-parameter assessment of overall glycemic control is a derivative of five separate glycemic range results along with mean glucose and glycemic variability taken from the commonly used Ambulatory Glycemic Profile (AGP).
The GRI weights these various input parameters based on rankings provided by 330 experienced diabetes clinicians who reviewed 225 AGPs and is reported as scalar result of percentile ranking. Low percentile values represent lower risk profiles, and higher values connote higher risk. For convenience in comparing results, Dr Klonoff has split the results into quintile percentage brackets. By applying the ranking system, the GRI weights time in mild hypoglycemia to a lesser degree than time in more profound hypoglycemic range, and, similarly, it weights time in mild hyperglycemia to a lesser extent than more serious hyperglycemic excursions. The GRI can be followed over time with multiple results from the same patient plotted on the same grid as presented in Figure 14.
Figure 14.
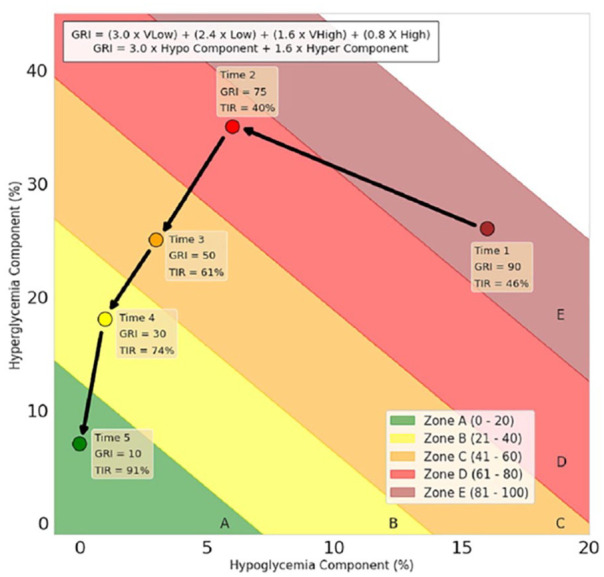
An example of synthetic patient Glycemia Risk Index (GRI) data plotted at five different times on the same grid. The box at the top of the figure contains the formula for calculating the GRI.
Source: Figure reproduced from Klonoff et al. 24
Abbreviations: GRI, Glycemia Risk Index; TIR, time-in-range.
The GRI has been applied to CGM tracings obtained from individuals using HCL AID systems, with the finding that a mean GRI result of approximately 20th percentile is observed this population. Dr Klonoff would like to see further research to validate the clinical utility of the GRI as a single metric assessment of diabetes care systems. This may be particularly germane in the comparison of different interventions within a population. He also suggests that the combined nature of the GRI may provide clinically meaningful input in the individual patient care setting and GRI data be combined with other personal data. 25 Glycemia Risk Index might eventually prove to correlate better with relevant outcomes than other single-parameter metrics, such as TIR.
David Rodbard then gave a brief review of correlations between various TIR intervals to mean glucose values, noting that time above range (TAR) values have a good linear relationship with mean glucose assessment. However, TIR has a nonlinear relationship to mean glucose, especially as mean glucose falls toward hypoglycemic range. This finding underscores the potential limitation of using TIR as a single-parameter metric for closed-loop systems that may bring mean glucose into normal ranges. The solution to this is to avoid using TIR as a single parameter to assess closed-loop system performance. Dr Rodbard then referenced various methods to add a weighting factor (ie, hypoglycemia risk) to single parameter to metrics (eg, GRI discussed above) or to add various additional metrics to system assessment (eg, TBR, etc).
Boris Kovatchev started his presentation with the statement that single-parameter metrics are insufficient to describe system performance, and that at least two parameters are required. He briefly reviewed various approaches to two parameter metrics, giving as an example use of TBR and time at or above range.
Rich Bergenstal concurred that two or more metrics are necessary for performance assessment. He then turned his attention beyond system performance assessment and focused on the question, “Which metrics are useful in driving clinical decision-making.” He suggests that TIR and TBR provide easily understandable, clinically relevant measures that can both describe systems, and importantly, provide clinical input at the individual patient level.
Roy Beck compared the results of both TIR and time in tight range (TITR) for both T1D and T2D. By plotting TITR on the x-axis against TIR on the y-axis, there is a relatively linear relationship between these until TITR exceeds about 60% to 70%, at which point, there is a diminishing return for TIR as this value approaches 100%. Dr Beck thus suggested that TITR may be a better metric as closed-loop systems continue to improve. However, applying this metric clinically may have negative psychological impact on individual persons with diabetes since being at “target” TIR 90% of the time would correspond to TITR of 70%, seemingly a step backwards.
Anne Peters focused her discussion on metrics that are relevant to individual persons with diabetes, especially those in her inner-city practice. “Lower tech” AID systems are needed to be more broadly prescribed. New metrics are also needed to show that these systems do help even if traditional measures of improved glycemia are not necessarily achieved. Three such metrics for assessing the use of AID systems in an under-resourced population are listed in Table 2. Rather than dealing with arcane concepts of glycemic control she wants to see more effort put into metrics that impact patient satisfaction and life needs, such as simplicity of training and system operation, usability, impact on adverse health outcomes (amputation, microvascular and macrovascular outcomes, etc), hospitalizations, time off work, and so forth.
Table 2.
Metrics for Using Automated Insulin Delivery Systems in an Under-Resourced Population.
| 1. | Population-specific PROs |
| 2. | Reductions in hospitalizations for DKA/severe hypoglycemia/infections/amputations |
| 3. | Incremental improvements in TIR and TBR |
Source: Table courtesy of Anne Peters.
Abbreviations: DKA, diabetic ketoacidosis; PRO, patient-reported outcome; TBR, time below range; TIR, time-in-range.
Q and A
The first questioner implored the panel to avoid using system performance descriptors using pejorative language (eg, “good,” or “bad”) as these may have profound impact on individual patients. Dr Vigersky commented that the former gold standard to diabetes control (A1C) has never been intuitively understandable to most patients, and the keep things simple, metrics should focus on intuitively understandable concepts, for example, TIR, TBR, and so on. More complex concepts are great for research, but simple is necessary for the clinic. Dr Heller suggested that the use of TBR is not sufficiently clinically relevant, and that efforts to characterize hypoglycemia should focus on means of identifying clinical events rather than simply dividing CGM results into time intervals corresponding to arbitrary ranges.
This sentiment was echoed by another questioner.
Problems and Solutions
Time was short for this second part of the session, and it was thus abbreviated.
Rich Bergenstal described what he calls the “feet on the ground” hyperglycemia that occurs upon arising from bed and that he feels affects about 50% of AID patients. He cautions that more attention needs to be drawn to the phenomenon for appropriate refinement on insulin dosing algorithms.
Boris Kovatchev reviewed the limitations of SC insulin absorption and action, noting that even “ultrafast” insulins fail to mimic the pharmacokinetics of endogenous insulin secretion. He also briefly noted that subcutaneous insulin has a non-physiologic distribution, perhaps alluding to the relative paucity of insulin exposure of the liver to peripherally administered insulin. Potential solutions to the subcutaneous insulin limitation could include (1) amylin congeners, (2) GLP-1 receptor agonists, (3) glucagon for pharmacologic counter-regulation, and (4) SGLT-2 inhibitors to provide an alternative route for glucose disposal.
Q and A
Dr Beck noted that Afrezza, an inhaled insulin, had not been mentioned, but included that it is an ultra-rapid profile that has been successfully deployed by some persons with T1D. Another questioner referenced a program in development for a newer, faster SC ultra-rapid insulin has been tested in porcine models.
Session 9: Round-table Discussion: What is Needed for More Adaptable, Accessible, and Usable Future Generation of Systems? How to Promote Equitable Innovation?
Moderators
Lori Laffel, MD, MPH
Joslin Diabetes Center, Harvard Medical School
David Kerr, MD
Diabetes Technology Society
Discussants
Simon Heller, MD
University of Sheffield
Desmond Schatz, MD
University of Florida
Speakers
Kellee Miller, PhD, MPH
T1D Exchange
Patients’ Expectations and Experience When Using AID Systems
Anne Peters, MD
Keck School of Medicine
Implementing Care and Research with Technologies in Under-Resourced Settings
Remi Rabasa-Lhoret, MD, PhD
Montreal Clinical Research Institute & Université de Montreal
Ethical Issues Associated with The Artificial Pancreas
Session 9 Summary Written By
Amisha Wallia, MD
Northwestern University Feinberg School of Medicine
Stakeholder engagement is a key component in any coordinated effort to bridge evidence to practice. Specifically for automated and personalized diabetes management systems, understanding stakeholder preferences and perspectives will be critical to ensure accessible, adaptable, and usable systems. Accessibility and use of technology, such as automated diabetes management systems in under-resourced areas may require a new framework for utilization and implementation. In research settings, funders and institutions are pivotal in the planning process, so that, community partners and other advocates can be involved at all pivotal junctures. From the clinician perspective, patient understanding and knowledge, including the consent process (risk/benefit) and simplification of training and onboarding materials may be needed; intensive monitoring of the technology itself along with patient assessments of current diabetes skills and knowledge are also needed. The current workforce is inadequate—both for children and adults for greater technology uptake. The situation is getting worse. There is a critical need to train more specialists in Pediatrics and Adults and bring in more racial and ethnic minority groups into the field.
A prime example of successful adaptation for low-income patients with Type 1 diabetes was the JDRF Artificial pancreas project 26 that successfully demonstrated the viability of CGM to be utilized in a low income public clinic; 27 however, while a majority of this population desired to continue on CGM, many were unable to clinically gain access for many (+15) years. Such input from PWD as qualitative assessments (focus groups), understanding the patient journey (narratives), PROs, familial and community participation, and budgetary considerations supporting such expansion work may be critical moving forward.
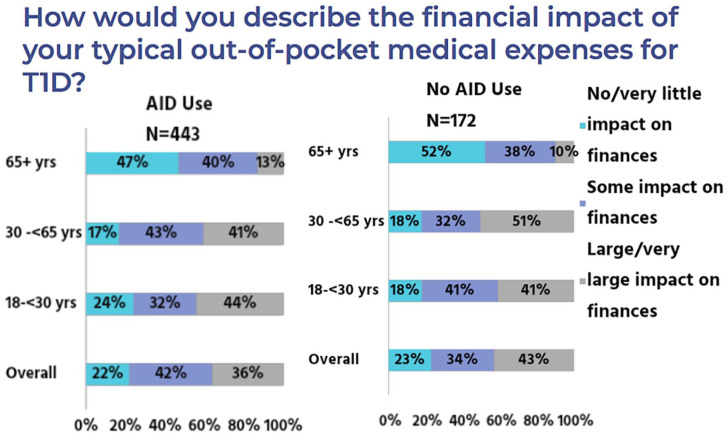
Psychosocial, ethical, and patient considerations must also be considered to ensure equitable adoption and access of such technology. Five key potential areas for ethical consideration when approaching technology use and delivery are listed in Table 3. 28 A survey of 123 patients with T1D noted that while a majority would trust an AP and would prefer one over current treatment, a majority would also like to be able to ignore or modify the AP decisions. 29 Patient narratives from prospective AP users note ethical considerations. Additional research from the T1D exchange also reported marked differences between AID system users and non-users. Financial burden is a considerable concern regardless of AID system use, and understanding and worry about insurance benefits and coverage was noted in both groups as presented in Figure 15. Clear differences in clinicians offering technology to patients and patient perceptions about the technology itself was also noted.
Table 3.
Five Types of Ethical Issues Related to the Use of an Automated Insulin Delivery System.
| 1 | Confidentiality and safety |
| 2 | Coverage of costs |
| 3 | Patient coaching and support |
| 4 | Patient selection in allocation decision |
| 5 | Personal identity and agency |
Source: Table courtesy of Remi Rabasa-Lhoret. 28
Figure 15.
Financial impact of out of pocket medical expenditures.
Source: Figure courtesy of Kellee Miller.
Abbreviations: AID, automated insulin delivery; T1D, type 1 diabetes.
From evidence to practice, clear opportunities for improvement have been identified for all stakeholders to engage and pursue. A pathway to continued progress can include user centered design, user technology, and usability standards and requirements for testing and trial cohort diversification. Health literacy standards for patient facing materials, especially related to technology use/onboarding and/or understanding of technology-related outcomes (eg, glucose analytics) may be critical. Regulatory considerations can potentially hinder and/or accelerate progress; therefore, we may need to understand these effects at the individual level. Clinical trial considerations, such as budget expansion for embedded psychologists, recruitment needs for participants with additional social health needs, and stakeholder engagement are especially critical. Actively addressing the current implementation gap may include proactively confronting current diabetes-related workforce shortages and need for staffing diversification, evaluating and addressing bias, and patient and clinical reported needs. Payor perspectives to help address disparities in access will also be essential; future and/or current research may include evaluation of cost and cost effectiveness and deleterious outcomes, such as hospitalization rates and health care utilization. Primary care will play a critical role in technology utilization, as will creation of new workflows (via quality or other frameworks) and tackling patient-specific technology needs (eg, technology navigators). Open-source data, social impact investing models, and specific supplements and grants for entry and continuation in the field for cross-disciplinary work (engineering, learning sciences, sociology, and anthropology) could accelerate accessibility, adoption, and use.
Conclusion
Automated insulin delivery is now a well-established therapy for PWD. Although used mostly for T1D, it has also been used successfully in T2D. The JDRF proposed a roadmap for development of the “artificial pancreas” almost 15 years ago. 30 The project has six steps, we are currently on step 4, the HCL. Step 5 is a fully automated closed loop and step 6 a multihormone closed-loop system. This conference focused on expanding step 4 and moving onto steps 5 and 6.
Currently only HCL systems are commercially available. 31 They will become smaller, less expensive, more easily used, and more available. We need to focus on special high-burden populations: pregnancy, CF, ESRD, and others with severe diabetes complications. Sight impairment should not be a barrier in the future. We need to be sure that literacy or economics do not prevent people from obtaining this important technology.
The future looks bright. 32 Better CGMs are coming, smaller pumps are entering the market, faster insulins are in late-stage testing, multifactorial sensors are being tested and far better algorithms many with AI are becoming available. The peritoneum is a far better site for insulin delivery with faster insulin absorption and lower peripheral insulin levels. Systems for IP insulin delivery by IP ports or implantable systems are coming.
No single commercial entity provides the best of everything, so that, the effort by the FDA to develop interoperability standards is important. 33 More companies need to develop interoperable systems. Importantly, we are in need of new metrics to be able to better assess how well these systems perform in diverse settings and populations.
We look forward to the Sixth Artificial Pancreas Workshop.
Acknowledgments
The authors thank Annamarie Sucher-Jones for her editorial assistance. The organizers of the Fifth Artificial Pancreas Workshop would also to thank Jessica Castle, MD, Viral Shah, MD, Sue Brown, MD, Steven Russell, MD, PhD, John Lum, MS, Roman Hovorka, PhD, Roy Beck, MD, PhD, Nelly Mauras, MD, Ohad Cohen, MD, Osagie Ebekozien, MD, MPH, Jessica Flynn, PhD, Howard Look, BSCE, Lane Desborough, MSc, David Klonoff, MD, Juan Espinoza, MD, Amy Criego, MD, Andrew Bremer, MD, PhD, Eda Cengiz, MD, R. Paul Wadwa, MD, Carol Levy, MD, Ananda Basu, MD, Boris Kovatchev, PhD, Robert Vigersky, MD, Connie Rhee, MD, Melissa Putman, MD, Charlotte Boughton, MD, PHD, Eyal Dassau, PhD, Claudio Cobelli, PhD, Marc Breton, PhD, Edward Damiano, PhD, Ahmad Haidar, PhD, Patricio Colmegna, PhD, Peter G. Jacobs, PhD, Greg Forlenza, MD, Michael Rickels, MD, Jose Garcia Tirado, PhD, Clara Mosquera-Lopez, PhD, Mudassir Rashid, PhD, Laya Ekhlaspour, MD, Michael C. Riddell, PhD, Barry H. Ginsberg, MD, PhD, Jennifer Sherr, MD, PhD, Mukul Jain, PhD, MBA, Ketan Dhatariya, MBBS MSc MD MS FRCP PhD, Michael Weiss, MD, PhD, MBA, Eric A. Appel, PhD, Jeffrey Joseph, DO, Irl B. Hirsch, MD, Anna Casu, MD, Peter Lord, BS, Eric Renard, MD, PhD, Rayhan A. Lal, MD, Chris Hanson, MS, Claudio Cobelli, PhD, Stu Weinzimer, MD, Richard Bergenstal, MD, Anne Peters, MD, Moshe Phillip, MD, David Rodbard, MD, Lori Laffel, MD, MPH, David Kerr, MD, Simon Heller, MD, Desmond Schatz, MD, Kellee Miller, PhD, MPH, Remi Rabasa-Lhoret, MD, PhD and Alfonso Galderisi, MD, PhD for their participation in the workshop.
Footnotes
Abbreviations: ACE, alternate controller enabled; AGP, ambulatory glucose profile; AI, artificial intelligence; AID, automated insulin delivery; AIT, active insulin time; AP, artificial pancreas; API, application programming interface; APS, acute psychological stress; AUC, area under the curve; BMI, body mass index; BP, bionic pancreas; CCD, Continuity of Care Documents; CDA, Clinical Document Architecture; CF, cystic fibrosis; CFR, Code of Federal Regulations; CGM, continuous glucose monitor; CKD, chronic kidney disease; CPT, Current Procedural Terminology; DEN, De Novo; DKA, diabetic ketoacidosis; EHR, Electronic Health Record; EKG, electrocardiogram; EMPI, Enterprise Master Index; ESRD, end-stage renal disease; FCL, fully closed loop; FDA, US Food and Drug Administration; FQHC, Federally Qualified Health Centers; GLP-1, glucagon-like peptide-1; GRI, Glycemic Risk Index; HAAF, hypoglycemia-associated autonomic failure; HbA1c, Hemoglobin A1c; HCL, hybrid-closed loop; HIPAA, Health Insurance Portability and Accountability Act; HL7, Health Level 7; iAGC, Interoperable Automated Glycemic Controller; IAH, impaired awareness of hypoglycemia; ICD-10, International Classification of Diseases 10th Revision; iCGM, integrated continuous glucose monitor; IDC, insulin delivery conduit; IEEE, Institute of Electrical and Electronics Engineers; IP, intraperitoneal; IT, information technology; IV, intravenous; JDRF, Juvenile Diabetes Research Foundation; LOINC, Logical Observation Identifiers Names and Codes; MPC, model predictive control; mvAP, multivariable artificial pancreas; NDD-CKDR, Non-Dialysis-Dependent Chronic Kidney Disease; NH, non-Hispanic; NIH, National Institutes of Health; NIST CSF, National Institute of Standards and Technology Cybersecurity Framework; NPI, National Provider Identifier; OCT, Optical Coherence Tomography; OHSU, Oregon Health & Science University; OMOP, Observational Medical Outcomes Partnership; PA, physical activity; PID, proportional-integral-derivative; PRO, patient-reported outcomes; PWD, people with diabetes; RAP, robust artificial pancreas; RCT, randomized control trial; RRT, renal replacement therapy; RWE, real-world evidence; SC, subcutaneous; SGLT-2, sodium-glucose cotransporter-2; SMART, Substitutable Medical Applications, Reusable Technologies; SNOMED, Systemized Nomenclature of Medicine; SOC2, System and Organization Controls type 2—Trust Services Criteria; T1D, type 1 diabetes; T2D, type 2 diabetes; TAR, time above range; TBR, time below range; TIDexi, Type 1 Diabetes in Exercise Initiative Data Set; TIR, time-in-range; TITR, time in tight range; TXNIP, thioredoxin interacting protein; UDI, Unique Device Identifier; UVA, University of Virginia; HITRUST, the health information trust alliance; RxNORM, medical prescription medical prescription.
Disclosures: The contents of this meeting report represent the authors’ views and do not constitute an official position of the National Institutes of Health or the United States Government.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DCK is a consultant for Better Therapeutics, EOFlow, Integrity, Lifecare, Nevro, Novo, Sanofi, and Thirdwayv. DK has received research support from Abbott Diabetes Care. AW receives research salary support from UnitedHealth Group. REA, TT, AMY, JH, CEM, WAL have no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This Fifth Artificial Pancreas Workshop was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Institutes of Health (NIH).
ORCID iDs: Rachel E. Aaron  https://orcid.org/0009-0005-5120-2264
https://orcid.org/0009-0005-5120-2264
Tiffany Tian  https://orcid.org/0009-0003-1417-6445
https://orcid.org/0009-0003-1417-6445
Andrea M. Yeung  https://orcid.org/0000-0002-5592-453X
https://orcid.org/0000-0002-5592-453X
Jingtong Huang  https://orcid.org/0000-0002-3119-9361
https://orcid.org/0000-0002-3119-9361
Guillermo A. Arreaza-Rubín  https://orcid.org/0009-0006-9632-1539
https://orcid.org/0009-0006-9632-1539
Barry H. Ginsberg  https://orcid.org/0000-0001-5404-826X
https://orcid.org/0000-0001-5404-826X
Tejaswi Kompala  https://orcid.org/0000-0002-4492-5035
https://orcid.org/0000-0002-4492-5035
Wei-An (Andy) Lee  https://orcid.org/0000-0002-2928-7338
https://orcid.org/0000-0002-2928-7338
David Kerr  https://orcid.org/0000-0003-1335-1857
https://orcid.org/0000-0003-1335-1857
Patricio Colmegna  https://orcid.org/0000-0001-9074-8634
https://orcid.org/0000-0001-9074-8634
Carlos E. Mendez  https://orcid.org/0000-0003-1875-6124
https://orcid.org/0000-0003-1875-6124
Douglas B. Muchmore  https://orcid.org/0000-0002-9300-3862
https://orcid.org/0000-0002-9300-3862
Amisha Wallia  https://orcid.org/0000-0002-3183-4062
https://orcid.org/0000-0002-3183-4062
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1. Castellanos LE, Russell SJ, Damiano ER, et al. The insulin-only bionic pancreas improves glycemic control in non-Hispanic white and minority adults and children with type 1 diabetes. Diabetes Care. 2023;46(6):1185-1190. doi: 10.2337/dc22-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeSalvo DJ, Noor N, Xie C, et al. Patient demographics and clinical outcomes among type 1 diabetes patients using continuous glucose monitors: data from T1D exchange real-world observational study. J Diabetes Sci Technol. 2023;17(2):322-328. doi: 10.1177/19322968211049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu NY, Nguyen KT, DuBord AY, et al. The launch of the iCoDE standard project. J Diabetes Sci Technol. 2022;16(4):887-895. doi: 10.1177/19322968221093662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boughton CK, Tripyla A, Hartnell S, et al. Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial. Nat Med. 2021;27(8):1471-1476. doi: 10.1038/s41591-021-01453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhee CM, Kovesdy CP, Kalantar-Zadeh K. Glucose homeostasis, hypoglycemia, and the burnt-out diabetes phenomenon in kidney disease. Semin Nephrol. 2021;41(2):96-103. doi: 10.1016/j.semnephrol.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lynch J, Kanapka LG, Russell SJ, et al. ; Bionic Pancreas Research Group. The insulin-only bionic pancreas pivotal trial extension study: a multi-center single-arm evaluation of the insulin-only configuration of the bionic pancreas in adults and youth with type 1 diabetes. Diabetes Technol Ther. 2022;24(10):726-736. doi: 10.1089/dia.2022.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck RW, Russell SJ, Damiano ER, et al. ; Bionic Pancreas Research Group. A multicenter randomized trial evaluating fast-acting insulin aspart in the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther. 2022;24(10):681-696. doi: 10.1089/dia.2022.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsoukas MA, Majdpour D, Yale JF, et al. A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: a single-centre, open-label, randomised controlled, crossover, non-inferiority trial. Lancet Digit Health. 2021;3(11):e723-e732. doi: 10.1016/S2589-7500(21)00139-4. [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Tirado J, Lv D, Corbett JP, Colmegna P, Breton MD. Advanced hybrid artificial pancreas system improves on unannounced meal response—in silico comparison to currently available system. Comput Methods Programs Biomed. 2021;211:106401. doi: 10.1016/j.cmpb.2021.106401. [DOI] [PubMed] [Google Scholar]
- 10. Garcia- Tirado J, Diaz JL, Esquivel-Zuniga R, et al. Advanced closed-loop control system improves postprandial glycemic control compared with a hybrid closed-loop system following unannounced meal. Diabetes Care. 2021;44(10):2379-2387. doi: 10.2337/dc21-0932. [DOI] [PubMed] [Google Scholar]
- 11. Colmegna P, Diaz JL, Garcia-Tirado J, Breton MD. Enabling anticipatory response in multi-stage MPC formulation for fully automated artificial pancreas system. In: 2022 IEEE 61st Conference on Decision and Control (CDC). Cancun, Mexico: IEEE; 2022:2566-2571. doi: 10.1109/CDC51059.2022.9993157. [DOI] [Google Scholar]
- 12. Mosquera-Lopez C, Wilson LM, El Youssef J, et al. Enabling fully automated insulin delivery through meal detection and size estimation using artificial intelligence. Npj Digit Med. 2023;6(1):39. doi: 10.1038/s41746-023-00783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs PG, Resalat N, Hilts W, et al. Integrating metabolic expenditure information from wearable fitness sensors into an AI-augmented automated insulin delivery system: a randomised clinical trial. Lancet Digit Health 2023;5:E607-E617. doi: 10.1016/S2589-7500(23)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Resalat N, El Youssef J, Tyler N, Castle J, Jacobs PG. A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. PLoS ONE. 2019;14(7):e0217301. doi: 10.1371/journal.pone.0217301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forlenza GP, McVean J, Beck RW, et al. Effect of Verapamil on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA. 2023;329(12):990. doi: 10.1001/jama.2023.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flatt AJ, Peleckis AJ, Dalton-Bakes C, et al. Automated insulin delivery for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes with hypoglycemia unawareness. Diabetes Technol Ther. 2023;25(5):302-314. doi: 10.1089/dia.2022.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type 1 diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17(7):697-703. doi: 10.2337/diacare.17.7.697. [DOI] [PubMed] [Google Scholar]
- 18. Flatt AJ, Chen E, Peleckis AJ, et al. Evaluation of clinical metrics for identifying defective physiologic responses to hypoglycemia in long-standing type 1 diabetes. Diabetes Technol Ther. 2022;24(10):737-748. doi: 10.1089/dia.2022.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rashid M, Samadi S, Sevil M, et al. Simulation software for assessment of nonlinear and adaptive multivariable control algorithms: glucose—insulin dynamics in type 1 diabetes. Comput Chem Eng. 2019;130:106565. doi: 10.1016/j.compchemeng.2019.106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sevil M, Rashid M, Hajizadeh I, Park M, Quinn L, Cinar A. Physical activity and psychological stress detection and assessment of their effects on glucose concentration predictions in diabetes management. IEEE Trans Biomed Eng. 2021;68(7):2251-2260. doi: 10.1109/TBME.2020.3049109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Ridder F, Ledeganck KJ, De Winter B, et al. Trends of glucose, lactate and ketones during anaerobic and aerobic exercise in subjects with type 1 diabetes: the ACTION-1 study. Diabetes Metab Res Rev. 2022;38(6):e3537. doi: 10.1002/dmrr.3537. [DOI] [PubMed] [Google Scholar]
- 22. Chen YS, Gleaton J, Yang Y, et al. Insertion of a synthetic switch into insulin provides metabolite-dependent regulation of hormone-receptor activation. Proc Natl Acad Sci USA. 2021;118(30):e2103518118. doi: 10.1073/pnas.2103518118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Micossi P, Cristallo M, Librenti MC, et al. Free-insulin profiles after intraperitoneal, intramuscular, and subcutaneous insulin administration. Diabetes Care. 1986;9(6):575-578. doi: 10.2337/diacare.9.6.575. [DOI] [PubMed] [Google Scholar]
- 24. Klonoff DC, Wang J, Rodbard D, et al. A Glycemia Risk Index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol. 2023;17(5):1226-1242. doi: 10.1177/19322968221085273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klonoff DC. Personalized medicine for diabetes. J Diabetes Sci Technol. 2008;2(3):335-341. doi: 10.1177/193229680800200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamborlane WV, Beck RW, Bode BW, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464-1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 27. Sequeira PA, Montoya L, Ruelas V, et al. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol Ther. 2013;15(10):855-858. doi: 10.1089/dia.2013.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quintal A, Messier V, Rabasa-Lhoret R, Racine E. A critical review and analysis of ethical issues associated with the artificial pancreas. Diabetes Metab. 2019;45(1):1-10. doi: 10.1016/j.diabet.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taleb N, Quintal A, Rakheja R, et al. Perceptions and expectations of adults with type 1 diabetes for the use of artificial pancreas systems with and without glucagon addition: results of an online survey. Nutr Metab Cardiovasc Dis. 2021;31(2):658-665. doi: 10.1016/j.numecd.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care. 2015;38(6):1036-1043. doi: 10.2337/dc15-0364. [DOI] [PubMed] [Google Scholar]
- 31. Peacock S, Frizelle I, Hussain S. A systematic review of commercial hybrid closed-loop automated insulin delivery systems. Diabetes Ther. 2023;14(5):839-855. doi: 10.1007/s13300-023-01394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoskins M, Tenderich A. “Artificial pancreas” aka automated insulin delivery: what you should know. Healthline. March 31, 2020. https://www.healthline.com/diabetesmine/artificial-pancreas-what-you-should-know. Accessed July 26, 2023.
- 33. Renard E. Certified interoperability allows a more secure move to the artificial pancreas through a new concept: “make-it-yourself.” J Diabetes Sci Technol. 2020;14(2):195-197. doi: 10.1177/1932296820901612. [DOI] [PMC free article] [PubMed] [Google Scholar]