Abstract
In the year 2020, Hong Kong experienced four COVID-19 epidemic waves. The present study aimed to examine the transition of sleep disturbances and explore its associated factors across the later three epidemic waves. Among the 1138 respondents who participated in an online survey at the second wave (T1, April 2020), 338 and 378 participants also completed a follow-up at the third (T2, August 2020) and fourth waves (T3, December 2020), respectively. Participants completed the Insomnia Severity Index and an investigator-designed questionnaire regarding potential factors associated with sleep change such as perceived risk of being infected, economic stress, and confidence in the government and health care professional. Sample of this study were mainly female (67.7%), married (50.3%), young adults (54.2%) with tertiary education (81.6%). Maintaining normal sleep was the most prevalent trajectory of sleep of all three waves (50.5%), followed by persistent insomnia (17.2%) and remitted insomnia (9.0%). Besides female, older-age and lower education level, the results showed that increment in worry about family being infected (adjusted risk ratio, RR = 1.28), perceived interference of daily lives (adjusted RR = 1.19), and economic distress (adjusted RR = 1.24) were significantly associated with the development of clinical insomnia during the three epidemic waves. These factors were also associated with worsening of other sleep parameters. Insomnia being persistent across the three waves of COVID-19 outbreaks was common. Increasing economic distress, daily interference, and worry about family members being infected were associated with an increasing risk of clinical insomnia across the three COVID-19 outbreaks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41105-023-00486-w.
Keywords: Sleep disturbance, Insomnia, Epidemic, Pandemic, Trajectory
Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic, which is a stressful life event to many people [1, 2]. During times of stress, major psychologic distress and symptoms may arise, including poor sleep quality, for which existing sleep disturbances may be exacerbated and new ones may emerge [2, 3]. A recent meta-analysis of sleep problems during COVID-19 included 44 studies comprising 54,231 participants from 13 countries, indicating that the global pooled prevalence rate of sleep problems is 40% while the pre-pandemic prevalence of sleep disturbance was about 10–25% in adult population [4, 5].
Previous sleep research during COVID-19 in the general population was mostly of a cross-sectional nature. Indeed, sleep disturbance throughout the pandemic may vary with periodic epidemic waves and confinement measures. Each time the pandemic surged in regions, governments and citizens responded by restricting social gatherings, border control, large-scale event cancelations, school closures, active case findings, lengthening quarantines, and isolating as many cases as possible. Transmissions might fall within weeks, allowing daily life to return to a semblance of normalcy. As social distancing measures are relaxed, a rebound in cases may occur. As a result, cycles of periodic social distancing measures lead to interruptions of people’s lifestyles and daily routine. People’s psychological symptoms are affected by these periodic changes. A national, longitudinal probability-based sample of UK adults (n = 10,918) indicated that the prevalence of clinically significant psychological distress increased from pre-pandemic levels of 20.8% in 2019 to 29.5% in April 2020 and then declined significantly to pre-pandemic levels in September 2020 (20.8%) [6]. This recovery may be explained by the relaxation of restrictions at the latter time point. Regarding changes in sleep, a longitudinal study conducted in the UK demonstrated that more people reported a positive change in sleep than those reporting a negative change in sleep as lockdown restrictions initially started to ease compared with the first national lockdown [7]. However, the sleep measurement was limited to the self-reported number of hours spent sleeping, but a validated sleep questionnaire was lacking. Another longitudinal study (n = 2013) including two time points at two contagion peaks in Italy (25 March to 7 April 2020; 28 November to 11 December 2020) found no significant change on overall sleep quality using the Pittsburgh Sleep Quality Index (PSQI) but a significant reduction in insomnia severity by the Insomnia Severity Index (ISI) [8].
The above studies showed that evidence on people sleep and psychological disturbances during the pandemic remains dynamic. More research on the change in sleep is warranted, particularly among people of different cultural backgrounds.
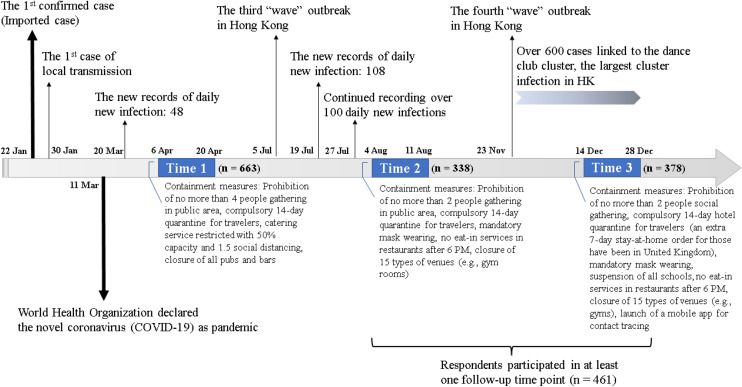
In 2020, Hong Kong experienced four COVID-19 epidemic waves (i.e., February, April, August, and December; Fig. 1). Targeting the local epidemic waves, we conducted a survey on the impact of the second wave on sleep with 1138 citizens in April 2020 [9], among which 339 respondents participated in the follow-up immediately after the third wave in August 2020 [10]. The overall prevalence of insomnia was found to be similar in the second and third waves of the outbreak (33.4% vs. 33.6%). In December 2020, we conducted another follow-up regarding the fourth wave. To date, several longitudinal studies on the sleep changes during the COVID-19 pandemic have been conducted in various countries, such as the USA [11, 12], Canada [13, 14], Australia [12], Italy [15, 16], and China [17–19]. Their observations on the sleep changes were mixed. Some studies suggested a decline in insomnia overtime [16, 17], while some found the insomnia problems were persistent [13, 18] or even deteriorated [11, 19]. Given that the Hong Kong peoples’ reaction to the COVID outbreaks can be different from other regions and may change across time, this research sought to (1) examine the transition [7] of sleep across three epidemic waves and (2) explore the factors associated with changes in sleep quantity and incidence of clinical insomnia.
Fig. 1.
Survey timeline
Methods
Study design and participants
This longitudinal online survey included three time points: the first cross-sectional survey (Time 1, 6–20 April 2020, T1) and the 4-month (Time 2, 4–11 August 2020, T2) and 8-month follow-up surveys (Time 3, 14–28 December 2020, T3) (Fig. 1). A convenient sample of 1138 Hong Kong adults who were capable of reading Chinese were recruited via Facebook and instant messaging application (WhatsApp) at T1 [9], and 663 subjects agreed to be followed up. Participants received reminder messages with an anonymous survey link generated from the online survey platform at both T2 and T3. A final sample of 461 respondents completed at least one follow-up survey. Eventually, 338 and 378 participants provided sufficient data and were included in analysis at T2 and T3 (51.0 and 57.0%, respectively, of those consented to participate in the follow-up survey at T1). Informed consents were obtained prior to data collection. The present study was approved by the institutional ethic review committees (ref: HSEARS20200226003). Study design and report were in accordance with the STROBE guidelines [20].
Measures
Sociodemographic data
Individuals’ sociodemographic information was collected, including age, gender, marital status, employment status, burden of child rearing by the youngest child in the family, educational attainment, social media usage, and any chronic condition.
Sleep outcomes
Subjects were also asked to report their average sleep–wake parameters in the recent 2 weeks with the items adopted from the Brief Insomnia Questionnaire, which was validated for telephone-based screening of sleep disturbance in Chinese adults [21, 22]. Sleep onset latency (SOL), wake after sleep onset (WASO), early morning awakening (EMA), total sleep time (TST), and bed and rise times were inquired across three waves.
Insomnia symptoms and related daytime impairments in the past 2 weeks were measured at T1, T2, and T3 by the validated Chinese version ISI (r = 0.79, Cronbach alpha = 0.83) [23–25]. Higher score of ISI indicates more severe insomnia symptoms and larger impairments of daytime functioning. Respondents were classified as having clinical insomnia using the cutoff of ISI ≥ 10 (specificity: 87.7%; sensitivity: 86.1%).
Risk factors
Participants were inquired about their perceived risk of themselves and their family being infected with COVID-19 at all three time points (T1, T2, and T3) with the responses ranging from “Not at all” to “Very much worried”. The confidence levels toward health professionals and the government curing and suppressing the local transmission of COVID-19 were measured in a 5-point rating (1 = No at all; 5 = Very much confident). Participants rated the intensity of their reactions to the COVID-19 pandemic at three time points, such as the perceived economic stress, depressed mood, and level of interference toward daily lives, using a 5-point Likert-like scale (“Not at all”, “a little bit”, “somewhat”, “much” and “Very much”).
Statistical analysis
Data were double entered and analyzed with SPSS 26.0. Sociodemographic data, sleep outcomes, and perceived risk at baseline (T1) between the completers and non-completers of the follow-up survey were compared using independent t-test or chi-square test. ISI scores were coded as binary (with and without clinical insomnia) using the cutoff of 10 [26]. The prevalence of clinical insomnia was weighted according to the 2016 Hong Kong population by-census distribution of sex and age for describing the trajectory of clinical insomnia.
Generalized estimating equation (GEE) was performed for describing the progression of sleep parameters and clinical insomnia over time due to better adjustment of within-subject variance and robust parameter estimation for small sampled longitudinal data [27]. The present study included SOL, WASO, and TST as sleep outcomes in the longitudinal analyses, while these sleep parameters were log-transformed prior to further analysis. To analyze both the associated factors of the development of insomnia and poorer sleep across three time points as well as the associations of changes between sleep and time-varying variables, a list of explanatory variables was prespecified (shown in Supplementary Table 1), e.g., subjects’ demographic characteristics, presence of chronic disease, perceived risks of infection, and responses of the pandemic in different domains.
Longitudinal associations of the pre-designed variables with the prevalence of insomnia by ISI (dichotomous) and the self-reported sleep parameters, namely, SOL, WASO, and TST (continuous), were first explored in the crude model. The associated factors identified in univariate analysis, with a statistical significance of p < 0.05, were examined in the final model of multivariate analysis. Results from the GEE were presented in the forms of relative risk (RR) for SOL, WASO, and TST whereas odds ratio (OR) for the prevalence of clinical insomnia with 95% confidence interval (CI).
Results
Subject characteristics
Table 1 presents the demographic data of all the respondents as well as the comparison between participants only contributed data at T1 and participants completed at least once follow-up. For those who had participated in the follow-up were more likely to be older, with tertiary education, economically inactive, longer early morning awakening, and higher confidence in health professionals (All P < 0.05).
Table 1.
Sociodemographic and clinical characteristics of respondents
| Variables | All respondents (n = 663) |
Participated follow-up (n = 461) | Did not participate in follow-up (n = 202) |
p-value |
|---|---|---|---|---|
| Female | 442 (66.7) | 312 (67.7) | 130 (64.4) | 0.46 |
| Age (years) | 0.004 | |||
| 18–39 | 346 (52.2) | 250 (54.2) | 96 (47.5) | |
| 40–59 | 260 (39.2) | 164 (35.6) | 96 (47.5) | |
| 60 or above | 57 (8.6) | 47 (10.2) | 10 (5.0) | |
| Married | 342 (51.6) | 232 (50.3) | 110 (54.5) | 0.55 |
| Burden of child rearing (the youngest child in household) | 0.24 | |||
| Without children | 503 (75.9) | 356 (77.2) | 147 (72.8) | |
| Secondary school students | 29 (4.4) | 16 (3.5) | 13 (6.4) | |
| Primary school students | 48 (7.2) | 35 (7.6) | 13 (6.4) | |
| Kindergartener | 83 (12.5) | 54 (11.7) | 29 (14.4) | |
| Co-residence | 616 (92.9) | 432 (93.7) | 184 (91.1) | 0.30 |
| With tertiary education | 527 (79.5) | 376 (81.6) | 151 (74.8) | 0.06 |
| Employment status at T1 | 0.002 | |||
| Employed | 492 (74.2) | 327 (70.9) | 165 (81.7) | |
| Unemployed | 29 (4.4) | 18 (3.9) | 11 (5.5) | |
| Economically inactive | 142 (21.4) | 116 (25.2) | 26 (12.9) | |
| With chronic diseasea | 55 (16.3) | 55 (16.3) | NR | – |
| With psychiatric diagnosisa | 15 (4.4) | 15 (4.4) | NR | – |
| Time spent on social media per day | 0.08 | |||
| 2 h or less | 285 (43.0) | 209 (45.3) | 76 (37.6) | |
| More than 2 h | 378 (57.0) | 252 (54.7) | 126 (62.4) | |
| Sleep parameters | ||||
| SOL, minutes (n = 658) | 27.65 ± 35.13 | 27.42 ± 32.28 | 28.19 ± 41.00 | 0.81 |
| WASO, minutes (n = 650) | 21.60 ± 42.38 | 21.42 ± 40.24 | 22.00 ± 47.07 | 0.88 |
| EMA, minutes (n = 655) | 24.01 ± 40.88 | 23.4 ± 41.79 | 25.41 ± 38.81 | 0.55 |
| TST, hours (n = 657) | 6.81 ± 1.30 | 6.84 ± 1.33 | 6.74 ± 1.24 | 0.38 |
| SE, % (n = 599)b | 87.36 ± 12.83 | 87.47 ± 13.08 | 87.10 ± 12.27 | 0.74 |
| ISI, ranged 0–28 | 7.57 ± 5.15 | 7.49 ± 5.17 | 7.77 ± 5.12 | 0.51 |
| Clinical insomnia (ISI ≥ 10)c | 224 (33.8) | 156 (33.8) | 68 (33.7) | 1.00 |
| Risk factors, ranged 1–5 | ||||
| Interfered with daily life due to COVID-19 | 3.72 ± 1.06 | 3.70 ± 1.06 | 3.76 ± 1.08 | 0.55 |
| Experiencing depressed mood | 2.31 ± 0.94 | 2.27 ± 0.95 | 2.42 ± 0.92 | 0.05 |
| Experiencing economic stress | 2.41 ± 1.23 | 2.36 ± 1.24 | 2.52 ± 1.19 | 0.11 |
| Worrying about own self being infected | 2.81 ± 0.97 | 2.78 ± 0.96 | 2.88 ± 1.00 | 0.21 |
| Worrying about family members being infected | 3.06 ± 1.08 | 3.03 ± 1.08 | 3.15 ± 1.07 | 0.16 |
| Confidence in health professional against COVID-19 | 3.50 ± 0.94 | 3.51 ± 0.94 | 3.49 ± 0.94 | 0.80 |
| Confidence in the government against COVID-19 | 1.89 ± 1.10 | 1.90 ± 1.10 | 1.86 ± 1.12 | 0.68 |
Date presented as mean ± standard deviation or number (percentage)
T1 Time point 1, NR Not reported, SOL Sleep onset latency, WASO Wake after sleep onset, EMA early morning awakening, TST Total sleep time, SE Sleep efficiency, ISI Insomnia severity index, COVID-19 Coronavirus Disease 2019
a Not included at T1. Data only available in 338 subjects
b SE was estimated with the TST divided by total time in bed and expressed as a percentage
c Scored at least 10 points of ISI was used as a cutoff as suffering from clinical insomnia
Sleep changes across T1, T2, and T3
In general, maintaining normal sleep was the most prevalent trajectory of sleep of all three waves (50.5%), followed by persistent insomnia (having insomnia at all three time points) (17.2%) and having insomnia at T1 and then without insomnia in the subsequent time points (9.0%) (Table 2). Of those who reported normal sleep at T1 (n = 164), 78.1% were good sleepers whose sleep remained normal at all three time points, followed by 10.6% having transient insomnia (insomnia at T2 but became normal sleep at T3) and 6.4% experienced sleep deterioration after T1. By contrast, among respondents who reported clinical insomnia (n = 90), persistent insomnia (48.7%) was the most found pattern, followed by remitted insomnia (without insomnia in one or all of the subsequent time points) (25.5 and 16.3%).
Table 2.
Trajectories for sleep status of respondents across three time points
| Sleep status at T1–T2–T3 | Weighted % |
|---|---|
| The Top 3 Trajectories (N = 254)a | |
| Normal–Normal–Normal | 50.5 |
| Insomnia–Insomnia–Insomnia | 17.2 |
| Insomnia–Normal–Normal | 9.0 |
| Normal Sleeper at T1 (N = 164) | |
| Normal–Normal–Normal | 78.1 |
| Normal–Insomnia–Normal | 10.6 |
| Normal–Insomnia–Insomnia | 6.4 |
| With Clinical Insomnia at T1 (N = 90) | |
| Insomnia–Insomnia–Insomnia | 48.7 |
| Insomnia–Normal–Normal | 25.5 |
| Insomnia–Insomnia–Normal | 16.3 |
Percentages are adjusted for the Hong Kong 2016 Population of sex and age distribution
T1 Time point 1, T2 Time point 2, T3 Time point 3
aThere were only 254 respondents with available data for all three time points
As shown in Table 3, similar pattern was observed that there were slightly higher but no significant time point effect on the occurrence of clinical insomnia at T2 and T3 as compared to T1 (All p > 0.05). The probability of reporting longer SOL at T2 and T3 were also indifferent from T1 (All p > 0.05). However, compared to T1, respondents at T2 and T3 were 1.25 (p = 0.004) and 1.26 times (p = 0.02) higher in reporting increased WASO, respectively. Individuals were at continuously reduced odds of having longer sleep length at subsequent time points compared to T1 (adjusted RR at T2 = 0.98, p = 0.046 and T3 = 0.95, p < 0.001).
Table 3.
The progression of the insomnia and sleep parameters during covid-19 pandemic using multivariate generalized estimating equation (GEE)
| Variables | Worsened insomnia symptomsa | Increased SOL | Increased WASO | Longer TST | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Adjusted RR | 95% CI | Adjusted RR | 95% CI | Adjusted RR | 95% CI | |
| Time point | ||||||||
| T3 | −0.30 | −0.75 to 0.16 | 0.99 | 0.91–1.08 | 1.24 | 0.94–1.33 | 0.95*** | 0.93–0.97 |
| T2 | 0.22 | −0.21 to 0.66 | 1.05 | 0.96–1.15 | 1.24* | 1.05–1.48 | 0.98 | 0.97–1.00 |
| T1 (reference group) | ||||||||
The sleep onset latency, wake after sleep onset and total sleep time were log-transformed for analysis
The time point effect was adjusted with the identified factors associated with the changes in insomnia prevalence and sleep changes
ISI Insomnia Severity Index, SOL Sleep onset latency, WSAO Wake after sleep onset, TST Total sleep time, RR Relative Risk, CI Confidence interval, T1 Time point 1, T2 Time point 2, T3 Time point 3
aAssessed by Insomnia Severity Index. Higher scores indicate increased severity of insomnia symptoms
*p < 0.05, **p < 0.01, ***p < 0.001
Multivariate analyses of the predictors of sleep changes
Prevalence of clinical insomnia
The multivariate GEE model revealed that the longitudinal development of clinical insomnia was associated with lower education (Table 4, adjusted OR = 1.45, 95% CI [1.09, 1.93], p = 0.01). The significant change in the likelihood of having insomnia among three epidemic waves was significantly associated with the increased in worry about family members being infected (adjusted OR = 1.26, 95% CI [1.08, 1.47], p = 0.004), perceived interference of their daily lives (adjusted OR = 1.19, 95% CI [1.07, 1.33], p = 0.002), and economic distress (adjusted OR = 1.24, 95% CI [1.13, 1.37], p < 0.001).
Table 4.
Predictors of the prevalence of insomnia and sleep changes over time using multivariate generalized estimating equation (GEE)
| Variables | Worsened insomnia symptomsa | Increased SOL | Increased WASO | Longer TST | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Adjusted RR | 95% CI | Adjusted RR | 95% CI | Adjusted RR | 95% CI | |
| Gender: female (male as reference) | 0.79* | 0.05–1.52 | 1.21* | 1.04–1.41 | 1.65*** | 1.26–2.16 | – | – |
| Age | ||||||||
| 60 or above | 2.20*** | 0.91–3.48 | – | – | 3.92*** | 2.54–6.04 | 0.85*** | 0.79–0.92 |
| 40–59 | 0.82* | 0.03–1.61 | – | – | 1.74*** | 1.32–2.31 | 0.92*** | 0.89–0.95 |
| Aged 18–39 (reference group) | – | – | – | – | – | – | – | – |
| Heavy user of social mediab (normal user as reference) | 0.32 | −0.43 to 1.07 | 1.17* | 1.00–1.37 | – | – | – | – |
| Risk factors across three timepoints | ||||||||
| Worry about selfc | 0.13 | −0.43 to 0.70 | 1.00 | 0.89–1.12 | – | – | 0.98* | 0.96–1.00 |
| Worry about family membersc | 0.45 | −0.9 to 0.99 | 1.05 | 0.94–1.17 | – | – | – | – |
| Daily interferenced | 0.41* | 0.03–0.79 | 1.01 | 0.93–1.09 | 1.13 | 1.00–1.29 | – | – |
| Depressed moode | 1.79*** | 1.37–2.21 | 1.29*** | 1.18–1.41 | 1.36*** | 1.19–1.56 | 0.97*** | 0.95–0.99 |
The sleep onset latency, wake after sleep onset and total sleep time were log-transformed for analysis
Model was adjusted with the progression of insomnia and sleep parameters over time
SOL Sleep onset latency, WSAO Wake after sleep onset, TST Total sleep time, RR Relative Risk, CI Confidence interval
a Assessed by Insomnia Severity Index. Higher scores indicate increased severity of insomnia symptoms
b Reported at least 2 h of daily social media usage was categorized as heavy social media user
c Higher scores indicated higher level of worry
dHigher scores indicated greater distress
eHigher scores indicated more depressed mood in response to the outbreak of COVID-19
*p < 0.05; **p < 0.01; ***p < 0.001
Sleep onset latency
Female gender (Table 4, adjusted RR = 1.18, 95% CI [1.06, 1.32], p = 0.003), older age (adjusted RR = 1.32, 95% CI [1.05, 1.66], p = 0.02), and lower education (adjusted RR = 1.16, 95% CI [1.01, 1.34], p = 0.04) were associated with an increase in SOL between two consecutive point times. In addition, increasing worry about family members being infected (adjusted RR = 1.06, 95% CI [1.00, 1.12], p = 0.04), worsening of poor confidence in the government in combating COVID-19 (adjusted RR = 1.10, 95% CI [1.04, 1.15], p < 0.001), higher perceived daily life interference (adjusted RR = 1.06, 95% CI [1.02, 1.11], p = 0.008), and greater economic stress (adjusted RR = 1.11, 95% CI [1.07, 1.16], p < 0.001) were significantly associated with longer SOL over time.
Wake after sleep onset
Female gender (Table 4, adjusted RR = 1.68, 95% CI [1.23, 2.30], p = 0.001) and older age (adjusted RR = 2.54, 95% CI [1.34, 4.82], p = 0.004) were identified as risk factor of developing a longer WASO. Moreover, increment in the level of daily life being interfered was correlated with greater likelihood of having higher WASO across time (adjusted RR = 1.16, 95% CI [1.04, 1.29], p = 0.009).
Total sleep time
Older- (adjusted RR = 0.91, 95% CI [0.84, 0.97], p = 0.005) and middle-age (adjusted RR = 0.93, 95% CI [0.89, 0.97], p < 0.001) were less likely associated with having a longer TST (Table 4). Moreover, increasing worry about family members being infected was less likely to associate with the increase in TST over time (adjusted RR = 0.98, 95% CI [0.96, 0.99], p = 0.009).
Discussion
This study is the first longitudinal survey on sleep and its associated factors across three peaks of the COVID-19 outbreaks. The results revealed that maintaining normal sleep was the most prevalent trajectory of sleep the three outbreaks. However, insomnia was likely persistent for those who reported clinical insomnia at the second wave of outbreak (T1). Besides female gender and older age, which have long been recognized as associated factors of poor sleep, the present study found that exacerbating economic distress, perceived incremental daily interference, decreasing confidence in the government in controlling the epidemic, and increasing worry about family members being infected were associated with increasing risk of insomnia and/or worse sleep parameters across the three COVID-19 outbreaks.
In this study, the probability of reporting increased wakefulness during sleep and reduced sleep length were higher at the third wave (T2) and fourth waves (T3) compared with those in the second (T1). Notably, the incidence rates of COVID-19 were much higher during at T2 (74 confirmed cases on average per day between 4 and 11 August 2020) and T3 (75 confirmed cases on average per day between 14 and 28 December 2020) of outbreak in Hong Kong than in the T1 (9 confirmed cases per day on average between 6 and 20 April 2020). Our results were contrast to the findings of a longitudinal study in Italy that a reduction in ISI score was observed at the second peak even though the incidence rates at the second peak were much higher (18,591 daily confirmed cases on average between 28 November and 11 December 2020 vs. 4644 confirmed cases on average per day between 25 March and 7 April 2020) [8]. However, our observation was that the RR at T3 was similar to T2, which implied that the people may not have psychologically adapted to the burden of the outbreak, especially the largest COVID-19 cluster was reported at T3 in Hong Kong. Our findings will inform public health efforts to improve negative sequelae during the course of the pandemic.
Previous data from longitudinal studies on the course of insomnia over 1–5 years demonstrated that the most frequent course of insomnia was persistence, with rates (insomnia at baseline and at follow-up) ranging from 44.4 to 86% [22, 28–32]. In the present study, the analysis of the transition of insomnia status provided further evidence about the persistent course of insomnia, with almost half (48.7%) of the participants with clinical insomnia at T1 having clinical insomnia at the subsequent two time points (4 and 8 months), and more than one-third (41.8%) of the participants resumed as normal sleepers at T2 and T3. Our study found that persistent insomnia was the most common pattern, but the rate of persistence appeared to be at the lower end of prevalence reported in the literature [28–32]. A plausible explanation to the observed lower rates of prevalence of insomnia persistence is that the insomnia experienced by some participants in the present study is of acute onset or worsening triggered by a local COVID-19 outbreak, which may be less persistent.
Our study found that being female, older age, lower education, increasing daily interference by COVID-19 and increasing economic stress were associated with a higher risk of having clinical insomnia and/or sleep disturbances such as longer SOL and WASO; the results are consistent with previous research conducted both before [8] and during the COVID-19 pandemic. Specifically, being female [13, 18], experiencing stress [13, 16], economic stress [33], and being impacted by COVID-19 [34] have been identified as factors associated with insomnia. Due to the prolonged maintenance of community precautionary measures such as the closure of community centers and social distancing orders, the subsequent shift towards virtual social contacts significantly compromises their social lives, resulting in higher loneliness and reduced connectedness in older adults. This claim was in line with the higher loneliness and social isolation observed in older adults during the pandemic and subsequent impacts on their vulnerability of developing mental disorders [35, 36]. Older adults are known to have a higher prevalence of insomnia meanwhile the increased mental burden from a bulk of psychosocial factors (i.e., social isolation, circadian rhythm changes) may have more deleterious consequences in older adults than in other age groups [37, 38]. Moreover, a recent longitudinal study in Italy demonstrated that facing economic hardships and having lower education levels predict sleep disturbances over time, reflecting that these groups may have an underlying vulnerability to worsened sleep during the pandemic. In addition, decreasing confidence in the government combating COVID-19 was found to be associated with a longer SOL in the present study. It is consistent with a study in Taiwan which found that a lower perceived confidence in COVID-19 management by the regional government was associated with a higher level of worry [39] which may precipitate their sleep problems.
This study found that depression was linked to an increase in insomnia symptoms. This association had been seen in previous research conducted before the COVID-19 outbreak [40, 41]. Indeed, studies found that during the COVID-19 pandemic, both depression and insomnia were prevalent and severe among certain populations like healthcare professionals [42] and university students [43]. It is important to develop strategies tailored to the specific population to address these issues during infectious disease outbreaks.
Our research found that there was a link between longer sleep duration and depression. Previous studies have shown that the connection between depression and sleep duration is not linear, but rather has a U-shaped relationship [44, 45]. A dose–response meta-analysis revealed that both shorter and longer sleep duration, compared to a 7-h night's sleep, may increase the likelihood of developing depression [46]. However, there is still controversy about the direct negative effects of long sleep duration on overall health [47, 48] as other confounding factors such as poor health and low physical activity levels may also play a significant role [49]. Therefore, further research is necessary to understand the mechanisms behind the negative effects of long sleep duration.
The major strength of the current cohort study is the utilization of validated measures of sleep and psychological disturbances across three epidemic peaks. However, the study had some limitations. First, the studied outcomes were measured using self-reported measures, which may have led to self-report bias as individuals with insomnia usually overestimate their onset latency but underestimate their TST. Second, the sample size was small, and females predominantly constituted the sample. This limits the generalization of results to the larger population.
In summary, our findings showed that a common trajectory for individuals who reported experiencing clinical insomnia during the initial stages of the COVID pandemic tend to continue struggling with insomnia during subsequent outbreaks. Increasing economic distress, daily interference, and worry about family members being infected and decreasing confidence in the government in controlling the epidemic were associated with increasing risk of insomnia and/or worse sleep parameters across the three COVID-19 outbreaks.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the participants for participating in this study.
Funding
This study received no funding.
Declarations
Conflict of interest
All the authors declare that there are no potential conflict of interests.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Footnotes
Denise Shuk Ting Cheung and Branda Yee-Man Yu are considered as co-first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bueno-Notivol J, Gracia-García P, Olaya B, et al. Prevalence of depression during the COVID-19 outbreak: A meta-analysis of community-based studies. Int J Clin Health Psychol. 2021;21(1):100196–100211. doi: 10.1016/j.ijchp.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezaei N, Grandner MA. Changes in sleep duration, timing, and variability during the COVID-19 pandemic: Large-scale Fitbit data from 6 major US cities. Sleep Health. 2021;7(3):303–313. doi: 10.1016/j.sleh.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID-19 outbreak: Practical recommendations from a task force of the European CBT-I Academy. J Sleep Res. 2020;29(4):e13052. doi: 10.1111/jsr.13052. [DOI] [PubMed] [Google Scholar]
- 4.Jahrami H, BaHammam AS, Bragazzi NL, et al. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(2):299–313. doi: 10.5664/jcsm.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morin CM, Jarrin DC. Epidemiology of insomnia. Sleep Med Clin. 2013;8(3):281–297. doi: 10.1016/j.jsmc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Daly M, Robinson E. Longitudinal changes in psychological distress in the UK from 2019 to September 2020 during the COVID-19 pandemic: Evidence from a large nationally representative study. Psychiatry Res. 2021;300:113920. doi: 10.1016/j.psychres.2021.113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen X, Fleming L, Kirk A, et al. Changes in physical activity, sitting and sleep across the COVID-19 national lockdown period in Scotland. Int J Environ Res Public Health. 2020;17(24):9362. doi: 10.3390/ijerph17249362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salfi F, D'Atri A, Tempesta D, Ferrara M. Sleeping under the waves: A longitudinal study across the contagion peaks of the COVID-19 pandemic in Italy. J Sleep Res. 2021;30:e13313. doi: 10.1111/jsr.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu BY-M, Yeung WF, Lam JCS, et al. Prevalence of sleep disturbances during covid-19 outbreak in an urban Chinese population: A cross-sectional study. Sleep Med. 2020;74:18–24. doi: 10.1016/j.sleep.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam CS, Yu BY-M, Cheung DST, et al. Sleep and mood disturbances during the covid-19 outbreak in an urban Chinese population in Hong Kong: A longitudinal study of the second and third waves of the outbreak. Int J Environ Res Public Health. 2021;18(16):8444. doi: 10.3390/ijerph18168444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingerson MJ, Baugh CM, Provance AJ, et al. Change in quality of life, sleep, and physical activity during COVID-19: A longitudinal study of adolescent athletes. J Athl Train. 2023 doi: 10.4085/1062-6050-0529.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meaklim H, Saunders WJ, Byrne ML, et al. Insomnia is a key risk factor for persistent anxiety and depressive symptoms: A 12-month longitudinal cohort study during the COVID-19 pandemic. J Affect Disord. 2023;322:52–62. doi: 10.1016/j.jad.2022.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Gong K, Garneau J, Grenier S, et al. Insomnia symptoms among older adults during the first year of the COVID-19 pandemic: A longitudinal study. Sleep Health. 2023 doi: 10.1016/j.sleh.2023.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matovic S, Grenier S, Jauvin F, et al. Trajectories of psychological distress during the COVID-19 pandemic among community-dwelling older adults in Quebec: A longitudinal study. Int J Geriatr Psychiatry. 2023;38(1):e5879. doi: 10.1002/gps.5879. [DOI] [PubMed] [Google Scholar]
- 15.Riva E, Terraneo M, Lucchini M, Gerosa T. The prevalence of insomnia in different COVID-19 policy phases: Longitudinal evidence from ITA.LI - Italian Lives. BMC Public Health. 2022;22(1):1657. doi: 10.1186/s12889-022-14048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salfi F, Amicucci G, Corigliano D, et al. Two years after lockdown: Longitudinal trajectories of sleep disturbances and mental health over the COVID-19 pandemic, and the effects of age, gender and chronotype. J Sleep Res. 2023;32(3):e13767. doi: 10.1111/jsr.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J, Zhao Y, Wang J, et al. The associations among the stress symptoms, depressive symptoms, anxiety symptoms and insomnia symptoms in depressed patients after the first COVID-19 outbreak was initially controlled in China: a prospective cohort study. J Affect Disord. 2022;314:253–258. doi: 10.1016/j.jad.2022.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang DF, Zhao JB, Zhai SY, et al. Longitudinal trajectories of insomnia symptoms among college students during the COVID-19 lockdown in China. J Psychosom Res. 2022;157:110795. doi: 10.1016/j.jpsychores.2022.110795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang K, Mi Z, Parks-Stamm EJ, et al. Adaptability protects university students from anxiety, depression, and insomnia during remote learning: A three-wave longitudinal study from China. Front Psychiatry. 2022;13:868072. doi: 10.3389/fpsyt.2022.868072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Chung K-F, Yeung W-F, Ho FY-Y, et al. Comparison of scoring methods for the Brief Insomnia Questionnaire in a general population sample. J Psychosom Res. 2015;78(1):34–38. doi: 10.1016/j.jpsychores.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Chung KF, Yeung WF, Yu YM, Ho FY. A population-based 2-year longitudinal study of insomnia disorder in a Chinese population in Hong Kong. Psychol Health Med. 2018;23(5):505–510. doi: 10.1080/13548506.2017.1363397. [DOI] [PubMed] [Google Scholar]
- 23.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 24.Chung K-F, Kan KK-K, Yeung W-F. Assessing insomnia in adolescents: Comparison of insomnia severity index, Athens insomnia scale and sleep quality index. Sleep Med. 2011;12(5):463–470. doi: 10.1016/j.sleep.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76–81. doi: 10.1016/j.sleep.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muth C, Bales KL, Hinde K, et al. Alternative models for small samples in psychological research: Applying linear mixed effects models and generalized estimating equations to repeated measures data. Educ Psychol Meas. 2016;76(1):64–87. doi: 10.1177/0013164415580432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansson-Fröjmark M, Linton SJ. The course of insomnia over one year: a longitudinal study in the general population in Sweden. Sleep. 2008;31(6):881–886. doi: 10.1093/sleep/31.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 30.Fok M, Stewart R, Besset A, et al. Incidence and persistence of sleep complaints in a community older population. Int J Geriatr Psychiatry. 2010;25(1):37–45. doi: 10.1002/gps.2295. [DOI] [PubMed] [Google Scholar]
- 31.Perlis M, Gehrman P, Ellis J. The natural history of insomnia: What we know, don’t know, and need to know. Sleep Med Res. 2011;2(3):79–88. doi: 10.17241/smr.2011.2.3.79. [DOI] [Google Scholar]
- 32.Morin CM, Jarrin DC, Ivers H, et al. Incidence, persistence, and remission rates of insomnia over 5 years. JAMA Netw Open. 2020;3(11):e2018782. doi: 10.1001/jamanetworkopen.2020.18782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morin CM, Vezina-Im LA, Ivers H, et al. Prevalent, incident, and persistent insomnia in a population-based cohort tested before (2018) and during the first-wave of COVID-19 pandemic (2020) Sleep. 2022;45(1):zsab258. doi: 10.1093/sleep/zsab258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heron PN, Henderson LM, Crosland S, et al. Sleep health among people with severe mental ill health during the COVID-19 pandemic: Results from a linked UK population cohort study. Front Psychiatry. 2022;13:975593. doi: 10.3389/fpsyt.2022.975593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cueto, M.a., Mental health and psychosocial considerations during the COVID-19 outbreak, 18 March 2020. 2020(WHO/2019-nCoV/MentalHealth/2020.1).
- 36.Wong SYS, Zhang D, Sit RWS, et al. Impact of COVID-19 on loneliness, mental health, and health service utilisation: A prospective cohort study of older adults with multimorbidity in primary care. Br J Gen Pract. 2020;70(700):817–824. doi: 10.3399/bjgp20X713021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ancoli-Israel S. Insomnia in the elderly: A review for the primary care practitioner. Sleep. 2000;23(1):S23–S30. [PubMed] [Google Scholar]
- 38.Cardinali DP, Brown GM, Reiter RJ, Pandi-Perumal SR. Elderly as a high-risk group during COVID-19 pandemic: Effect of circadian misalignment, sleep dysregulation and melatonin administration. Sleep Vigil. 2020;4(2):81–87. doi: 10.1007/s41782-020-00111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu W-H, Ko N-Y, Chang Y-P, et al. The coronavirus disease 2019 pandemic in Taiwan: An online survey on worry and anxiety and associated factors. Int J Environ Res Public Health. 2020;17(21):1–13. doi: 10.3390/ijerph17217974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaneita Y, Ohida T, Uchiyama M, et al. The relationship between depression and sleep disturbances: A Japanese nationwide general population survey. J Clin Psychiatry. 2006;67(2):196–203. doi: 10.4088/JCP.v67n0204. [DOI] [PubMed] [Google Scholar]
- 41.Li WZ, Ruan WY, Peng Y, et al. Associations of socioeconomic status and sleep disorder with depression among US adults. J Affect Disord. 2021;295:21–27. doi: 10.1016/j.jad.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Hasen AA, Seid AA, Mohammed AA. Depression and insomnia among healthcare professionals during COVID-19 pandemic in Ethiopia: a systematic review and meta-analysis. Peerj. 2023;11:e15039. doi: 10.7717/peerj.15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan K, Zheng YB, Wang YJ, et al. A systematic review and meta-analysis on prevalence of and risk factors associated with depression, anxiety and insomnia in infectious diseases, including COVID-19: a call to action. Mol Psychiatry. 2022;27(8):3214–3222. doi: 10.1038/s41380-022-01638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu BP, Wang XT, Liu ZZ, et al. Depressive symptoms are associated with short and long sleep duration: A longitudinal study of Chinese adolescents. J Affect Disord. 2020;263:267–273. doi: 10.1016/j.jad.2019.11.113. [DOI] [PubMed] [Google Scholar]
- 45.Zhu GW, Cassidy S, Hiden H, et al. Exploration of sleep as a specific risk factor for poor metabolic and mental health: A UK biobank study of 84,404 participants. Nat Sci Sleep. 2021;13:1903–1912. doi: 10.2147/NSS.S323160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li XL, Wei JY, Zhang XY, et al. Relationship between night-sleep duration and risk for depression among middle-aged and older people: A dose-response meta-analysis. Front Physiol. 2023;14:1085091. doi: 10.3389/fphys.2023.1085091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson KL, Turek FW. The U-shaped association between sleep and health: The 2 peaks do not mean the same thing - Comment on Patel SR; Malhotra A; Gottlieb DJ et al. correlates of long sleep duration. SLEEP 2006;9(7): 881-889. Sleep. 2006;29(7):878–879. doi: 10.1093/sleep/29.7.878. [DOI] [PubMed] [Google Scholar]
- 48.Liu TZ, Xu C, Rota M, et al. Sleep duration and risk of all-cause mortality: A flexible, non-linear, meta-regression of 40 prospective cohort studies. Sleep Med Rev. 2017;32:28–36. doi: 10.1016/j.smrv.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Kakizaki M, Kuriyama S, Nakaya N, et al. Long sleep duration and cause-specific mortality according to physical function and self-rated health: The Ohsaki Cohort Study. J Sleep Res. 2013;22(2):209–216. doi: 10.1111/j.1365-2869.2012.01053.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.