Abstract
Studies have revealed a possible connection between orexin, narcolepsy, and obstructive sleep apnea (OSA). Orexin has an important role in the maintenance of arousal and wakefulness/sleeping states. To better understand the pathophysiological mechanism of OSA, we used a chronic intermittent hypoxia (CIH) model in mice to mimic OSA. In this way, we explored the effect of CIH on the locomotor activity and orexin system in the hypothalamus, cerebral cortex, and brainstem of mice. Male C57BL/6 J mice (8 weeks) in the CIH group were exposed in a hypoxia chamber for 8 h/day for 28 weeks. The re-oxygenation groups comprised the W2 group and W4 group, which were exposed to 28 weeks of CIH followed by 2 weeks and 4 weeks of re-oxygenation, respectively. The open field test was undertaken to observe locomotor activity. mRNA expression of orexin, orexin receptor type 1 (OX1R), and OX2R mRNA was evaluated by real-time reverse transcription-quantitative polymerase chain reaction. Mice subjected to long-term CIH exhibited significant anxiety-like behavior during the light period, and this behavior lasted until 4 weeks of re-oxygenation. mRNA expression of orexin was upregulated in the hypothalamus. mRNA expression of OX1R mRNA in the cerebral cortex and brainstem was downregulated by CIH. Two weeks and 4 weeks of re-oxygenation could not reverse these alternations. Long-term CIH may induce anxiety-like behavior and re-oxygenation cannot reverse these behavior. Moreover, OX1R has a significant role in the anxiety-related symptoms observed in long-term CIH.
Keywords: Chronic intermittent hypoxia, Obstructive sleep apnea, Orexin receptors, Orexin, Re-oxygenation
Introduction
Obstructive sleep apnea (OSA) is a dynamically developing breathing disease characterized by repetitive collapse of the upper airways during sleep [1]. Chronic intermittent hypoxia (CIH) and hypercapnia caused by ventilation reduction/absence during airway collapse leads to a series of pathologic changes in the body: cardiovascular disease, metabolic dysfunction, and neuronal dysfunction (including psychiatric disorders such as anxiety and depression) [1, 2]. In addition, repetitive obstructive respiratory events lead to arousal, sleep fragmentation, and excessive daytime sleepiness (EDS) [3]. One study showed EDS prevalence to be associated positively with OSA severity: 47% in patients with mild OSA and 58% in patients with severe OSA [4].
Narcolepsy is a chronic sleep disorder characterized by EDS and cataplexy. Narcolepsy can be accompanied by sleep paralysis, fragmented sleep as well as motor, cognitive, and autonomic disturbances. People suffering from narcolepsy and patients with OSA share common symptoms (particularly EDS). Coexistent OSA is a common phenomenon in patients with narcolepsy [5]. One study showed that among 132 patients with narcolepsy, 10 were diagnosed initially with OSA mainly because physicians ignored the cataplexy that was present before patients were first evaluated for EDS [6].
Orexin (also known as “hypocretin”) is a neurotransmitter. Orexin has important roles in the regulation of sleep and wakefulness. A growing body of research has shown that orexin is involved in regulating stress, energy homeostasis, and respiratory via binding to the G protein-coupled receptors orexin receptor type 1 (OX1R) and OX2R [7, 8]. Degeneration/dysfunction of orexin-containing neurons is associated with narcolepsy [9]. According to criteria set in the third edition of International Classification of Sleep Disorders, the levels of orexin-A in cerebrospinal fluid can be used as clinical diagnostic criteria for human narcolepsy [10]. Accordingly, there may be a connection between narcolepsy, orexin, and OSA.
Several studies have evaluated the plasma level of orexin in patients with OSA compared with that in healthy volunteers, but the results have been controversial [11–13]. The reason for this difference between results may be that the severity and diagnostic criteria of OSA differ between studies. Specifically, some studies have suggested that the plasma level of orexin is correlated negatively with OSA severity [11, 12]. Conversely, Igarashi et al. reported that the plasma orexin level was higher in patients with OSA [13]. Furthermore, a cross-sectional study by Busquets et al. showed no significant differences in the plasma orexin level between patients with severe OSA receiving continuous positive airway pressure (CPAP) treatment and those not receiving CPAP treatment [14]. Another study reported that the plasma level of orexin decreased after CPAP treatment [13]. Based on such contradictory findings, the physiologic relationship between orexin and OSA has not been well defined.
CIH and hypercapnia are the primary events associated with OSA. Recent studies have suggested that orexin-containing neurons are sensitive to hypoxia alone or a combination of hypoxia and hypercapnia. However, the effects of hypoxia or hypercapnia on orexin-containing neurons in animal studies have been controversial. Intermittent hypoxia (8 h/day for 1 week) led to low expression of prepro-orexin mRNA in the hypothalamus, whereas a longer duration of intermittent hypoxia revealed a contradictory result [15]. Yamaguchi et al. showed that intermittent hypoxia activated orexin-containing neurons in mice in a pattern-sensitive manner [16]. Sithirungson et al. reported a hypothetical model representing the association between orexin and OSA severity. They reported that in early-onset OSA or mild OSA, the orexin level increases to enhance the activity of the upper-airway dilator muscles. This phenomenon leads to sleep fragmentation, and long-term OSA or severe OSA can induce dysfunction of orexin-containing neurons and reduce mRNA expression of orexin [5].
Until now, investigation of the effect of long-term CIH on the orexin system has been lacking. We used a mouse model of long-term CIH and re-oxygenation to mimic OSA. In this way, we explored how OSA affects locomotory activity and function of the orexin system.
Materials and methods
Animal subgroups and experimental design of long-term CIH
The study protocol was approved by the Animal Care and Use Committee of Nanjing Medical University (Nanjing, China) and complied with the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health, Bethesda, MD, USA).
Thirty-six male C57BL/6 J mice (8 weeks) were used. Mice were kept in a departmental animal house on a 12-h light–dark cycle (light period = 7 am to 7 pm; dark period = 7 pm to 7 am). Mice had free access to water and chow.
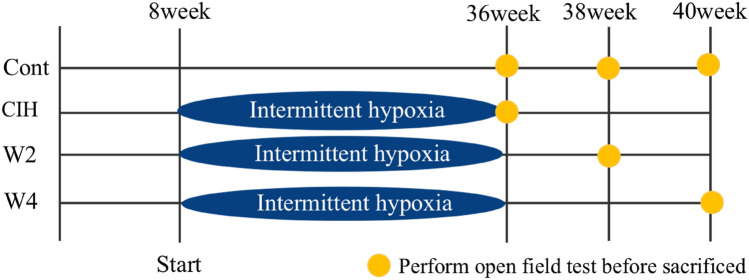
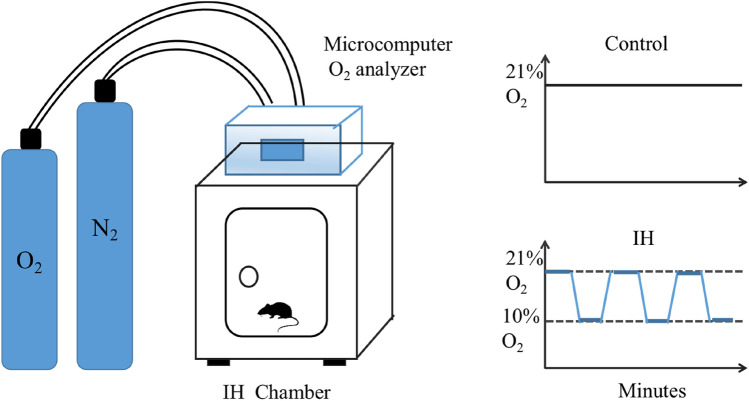
Mice were divided randomly into six groups of six. Three groups were exposed to a normal oxygen environment (control groups). One group was exposed to 28 weeks of CIH (CIH group). The final two groups were exposed to 28 weeks of CIH followed by re-oxygenation after placement into a normal oxygen environment for 2 weeks (W2 group) and 4 weeks (W4 group), respectively. A mouse model of CIH was established by a specific experimental procedure. Briefly, mice were exposed to CIH for 8 h/day from 09:00 every day for 28 weeks in a chamber. An oxygen controller monitored the change in O2 concentration continuously. The oxygen concentration in the chamber fluctuated between 21 and 10%, and the cycle time of hypoxia and re-oxygenation was 90 s. The timeline and experimental setup are shown in Figs. 1 and 2, respectively.
Fig. 1.
Timeline of the experimental protocol. Mice in the CIH group were exposed to 28 weeks of chronic intermittent hypoxia (CIH). Mice in the W2 group were exposed to 28 weeks of CIH followed by 2 weeks of re-oxygenation. Mice in the W4 group were exposed to 28 weeks of CIH followed by 4 weeks of re-oxygenation. Mice were sacrificed at age 36 weeks, 38 weeks, and 40 weeks respectively
Fig. 2.
Experimental setup (schematic). The O2 concentration was measured continuously by an O2 analyzer. Desired O2 concentrations were regulated by adding nitrogen (N2), which were controlled by a microcomputer. In the chronic intermittent hypoxia (CIH) model, the O2 concentration in the chamber fluctuated between 10 and 21% in a 90-s CIH cycle. Control mice were placed in normoxic room air (21% O2)
Open field test (OFT)
The OFT was conducted in a quiet room during the light period (between 1 and 5 pm). Mice were placed in a open field apparatus (55 cm × 55 cm × 55 cm) made of blue plastic (polyvinyl chloride) board for testing. The arena was divided into a center area (defined as a central square of dimension 20 cm × 20 cm) and a peripheral area. Before testing of the each mouse, 75% alcohol was used to remove odors and clean the apparatus. Tests were conducted after CIH at a mouse age of 36 weeks, 2 weeks of re-oxygenation at the age of 38 weeks, and 4 weeks of re-oxygenation at the age of 40 weeks. To start the test, each mouse was placed in a particular corner of the apparatus and allowed to explore freely for 5 min. Then, the mouse was placed in the center of the arena. Then, using a tracking system (EthoVision XT 11; Noldus, Wageningen, the Netherlands), we measured locomotor activity for 10 min and collected behavioral data: distance travelled, inactivity time, the number of times the four corners were entered, and the number of times the center of the arena was entered.
Extraction of brain tissue and RNA isolation
Animals were sacrificed by decapitation. The hypothalamus, cerebral cortex, and brainstem were collected at 8 pm during the dark period. These brain regions were dissected out on the pre-chilled stainless-steel block. The sample of cerebral cortex included the medial prefrontal cortex, such as cingulate cortex area, prelimbic and infralimbic cortices. The sample of brain stem included the pons and medulla oblongata.
Tissue was frozen immediately in liquid nitrogen and stored at − 80 °C until RNA processing. Total RNA was extracted from samples homogenized in TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA). According to manufacturer instructions, 0.2 mL of chloroform was added per 1 mL of TRIzol Reagent, and samples were centrifuged at 12,000 × g for 15 min at 4 °C. After centrifugation, the colorless upper aqueous phase was used. The concentration and purity of isolated RNA were measured using a spectrophotometer (Hitachi, Tokyo, Japan) according to the ratio of the optical density at 260 nm and 280 nm.
Real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was reverse-transcribed into complementary-DNA using a reverse transcription reagent kit (Takara Biotech, Otsu, Japan) in accordance with manufacturer protocols. A two-step RT-qPCR was undertaken using an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). After initial denaturation at 95 °C for 30 s, the thermal profile was 40 cycles at 95 °C for 5 s followed by 60 °C for 30 s. Relative quantification was based on the difference of the threshold cycle of orexin, OX1R, and OX2R compared with that of β-actin. Results were obtained using the comparative Ct method using formula 2–ΔΔCt.
Statistical analyses
SPSS 19 (IBM, Armonk, NY, USA) was used to carry out all statistical analyses. Data are the mean ± SD. The Shapiro–Wilk test was used to examine data distribution. The Student’s t-test was employed to compare data from the experimental group and control group. p < 0.05 was deemed significant.
Results
Changes of behavior in the OFT
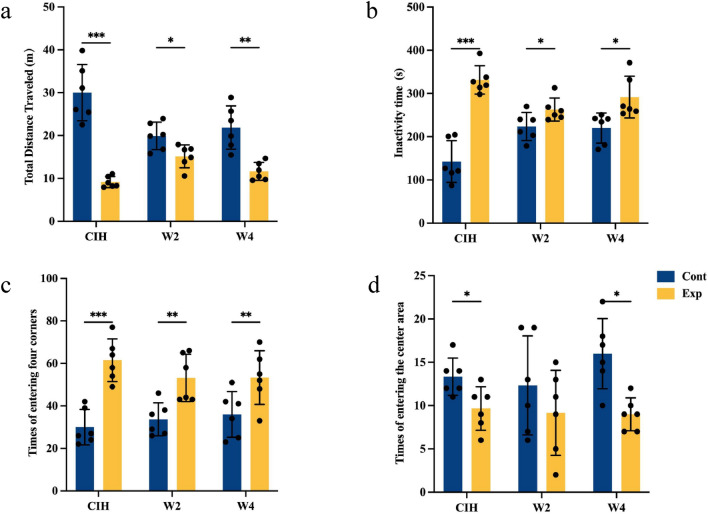
We carried out the OFT to investigate the effect of long-term CIH on locomotory activity. Overall, there was a general reduction in locomotory activity and increase in anxiety-like behavior in mice exposed to CIH. The number of times a mouse entered the center of the arena (common index of anxiety-like behavior) was reduced significantly, whereas the number of times a mouse entered the four corners was increased in the CIH group mice compared with the control group. In addition to the number of times a mouse entered the center of the arena, the distance traveled and inactivity time were measured. The inactivity time in the CIH group was longer, whereas the distance traveled was reduced significantly, in mice exposed to CIH. After 4 weeks of re-oxygenation, locomotor activity tended to recover, but did not revert to normal (Fig. 3).
Fig. 3.
Open field test. Mice subjected to 28 weeks of CIH showed significant anxiety-like behavior. This behavior lasted until four weeks of re-oxygenation was administered. Data are the mean ± standard deviation, n = 6 for each group (*p < 0.05, **p < 0.01, ***p < 0.005). a Total distance travelled. b Inactivity time. c Number of times the four corners was entered. d Number of times the center of the arena was entered
Long-term CIH upregulated mRNA expression of orexin in the hypothalamus and re-oxygenation reversed this upregulation
In the hypothalamus, mice exposed to long-term CIH showed higher mRNA expression of orexin than that in the control group. After 2 weeks and 4 weeks of re-oxygenation, upregulation of mRNA expression of orexin was reversed completely. Specifically, six CIH mice from the W2 group were exposed to a normal oxygen environment for 2 weeks, and there was no significant difference in mRNA expression of orexin between the W2 group and control group. Mice in the W4 group exposed to 28 weeks of CIH followed by 4 weeks of re-oxygenation showed mRNA expression of orexin similar to that of control groups subjected to 32 weeks of a normal oxygen environment (Fig. 4).
Fig. 4.
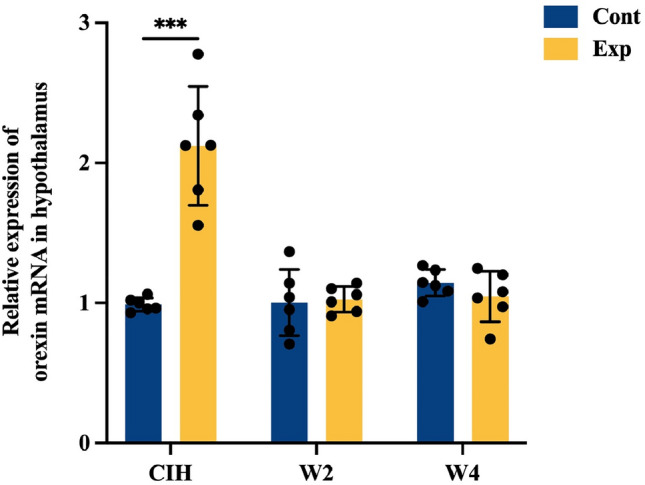
Effects of long-term chronic intermittent hypoxia (CIH) and re-oxygenation on mRNA expression of orexin in the hypothalamus of mice. Values are the mean ± standard deviation, n = 6 for each group (*p < 0.05, **p < 0.01, ***p < 0.005). β-actin was used as an internal control for each group. mRNA expression of orexin was upregulated after 28 weeks of CIH. Upregulated mRNA expression of orexin after 28 weeks of CIH was reversed by 2 weeks of re-oxygenation (W2 group) and 4 weeks of re-oxygenation (W4 group)
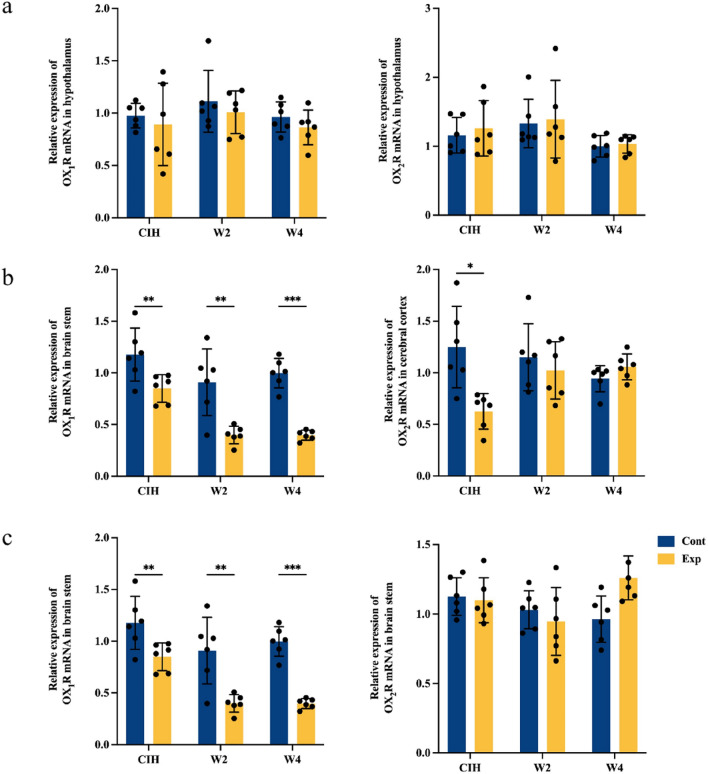
Effects of long-term CIH and re-oxygenation on expression of orexin receptors in the hypothalamus, cerebral cortex, and brainstem
Orexin is a small neuropeptide produced exclusively in neurons of the lateral hypothalamus, but orexin-containing nerve fibers are widespread from the hypothalamus to different brain regions. We measured expression of orexin receptors in the hypothalamus, cerebral cortex, and brainstem. Significant alteration in mRNA expression of OX1R or OX2R was not observed in the long-term CIH group or re-oxygenation group (W2 group and W4 group) compared with that in the control group in the hypothalamus (Fig. 5a). In the cerebral cortex, mRNA expression of OX1R and OX2R was reduced significantly in the presence of long-term CIH. After 2 weeks and 4 weeks of re-oxygenation, lower mRNA expression of OX1R continued to be observed in the W2 group and W4 group compared with that in the control group, whereas mRNA expression of OX2R reverted to normal; mice in W2 and W4 groups showed mRNA expression similar to that in control group (Fig. 5b).
Fig. 5.
Effects of long-term chronic intermittent hypoxia (CIH) and re-oxygenation on mRNA expression of OX1R and OX2R in the hypothalamus, cerebral cortex, and brainstem of mice. Values are the mean ± standard deviation, n = 6 for each group (*p < 0.05, **p < 0.01, ***p < 0.005). a There was no significant difference in mRNA expression of OX1R or OX2R in the hypothalamus in the CIH group or re-oxygenation group (W2 group and W4 group) compared with that in the control group. b 28 weeks of CIH downregulated mRNA expression of OX1R and OX2R significantly in the cerebral cortex. Lower expression of mRNA expression of OX1R after 2 weeks and 4 weeks of re-oxygenation compared with that in the control group was found. c mRNA expression of OX1R in the brainstem was reduced significantly by long-term CIH, and this effect could not be reversed by 2 weeks or 4 weeks of re-oxygenation
RT-qPCR revealed mRNA expression of OX1R in the brainstem to be reduced significantly by long-term CIH, and this effect could not be reversed by 2 weeks and 4 weeks of re-oxygenation. mRNA expression of the OX2R in the brainstem showed no significant difference during the experimental period (Fig. 5c).
Discussion
The main goal of our study was to determine if the long-term CIH and re-oxygenation affected the locomotory activity and function of the orexin system in mice. The OFT was developed originally by Calvin Hall in the 1930s, and was used to measure defection as an indicator of timidity in mice [17]. Nowadays, with the development of automatic-tracking systems, the time spent and number of times the center of an arena is entered are used as indices for anxiety [18]. However, mice exposed to long-term CIH expressed an anxiogenic-like phenotype in the OFT. The distance traveled was decreased significantly after undergoing 28 weeks of CIH, and the number of times the center of the arena was entered (a parameter often used to measure anxiety) was reduced in the CIH group compared with that in the control group.
Large cohort studies have shown that OSA is often associated with psychiatric disorders such as depression and anxiety [19]. Studies have suggested that patients with mild-to-moderate sleep apnea suffer undue anxiety, and that anxiety can induce sleep disorders and be involved in the progression of sleep apnea [20]. CIH is the main pathophysiologic feature of OSA. Similar experiments evaluating the effects of CIH on anxiety-like behavior have been undertaken. However, the effect of CIH on anxiety is dependent on the animal model of hypoxia used, including the partial pressure of oxygen and duration of exposure. Leconet et al. reported that repeat exposure to mild hypoxia (12 h/day for 3 times/week or 8% O2-equivalent during 6 weeks) attenuated anxiety-like behavior in mice [21]. Carissimi et al. showed that mice exposed to 1 week of intermittent hypoxia displayed less anxiety-like behavior than control group as perceived by a significant increase in the number of entries and total time spent in open arms [22]. Conversely, in accordance with our results, Puech et al. reported that after animals had been exposed for 16 weeks to intermittent hypoxia during daylight, an increase in anxiety-like behavior was exhibited [23]. Marrone showed that CPAP treatment had no effect on anxiety [24]. Our results suggest that, after 2 weeks and 4 weeks of re-oxygenation, the anxiety-like behavior of mice did not revert to normal. We showed, for the first time, that long-term mild CIH increased anxiety-like behavior, and that this behavior continued until 4 weeks of re-oxygenation in adult mice.
We also evaluated the effect of long-term CIH on the orexin system. Orexin is one of the most important neurotransmitters responsible for the regulation of sleep and arousal. It is also a key molecule in defense responses against stress, which can be activated by hypoxia in a pattern-sensitive manner [16]. The effect of CIH on the orexin system has been mired in controversy. Our results showed that long-term CIH induced mRNA expression of orexin in the hypothalamus and, after 2 weeks and 4 weeks of re-oxygenation, mRNA expression of orexin reverted to normal. However, significant differences in mRNA expression of OX1R and OX2R in the hypothalamus were not observed in the CIH group or re-oxygenation group compared with that in the control group in our study. These findings indicated that long-term CIH could upregulate orexin expression but not alter the function of orexin receptors in the hypothalamus. Conversely, one study showed that expression of OX1R and OX2R was increased by intermittent hypercapnic hypoxia (IHH) in a region-dependent manner. IHH increased OX1R expression in the dorsal medial nucleus, paraventricular nucleus, and retrochiasmatic area, and increased OX2R expression in the dorsal medial nucleus, perifornical area, paraventricular nucleus, ventral medial nucleus, and tuberal mammillary nucleus [25]. We did not focus on the nuclei of the hypothalamus, which may be one of the reasons for the different results obtained.
OX1R and OX2R are involved in the regulation of sleep and wakefulness according to a gene-knockout model in mice, but the OX2R-mediated pathway appears to be more important. Mice lacking OX2R show mildly narcoleptic cataplexy-like episodes and fragmentation of sleep/awake symptoms. OX1R knockout mice displayed only mild fragmentation of the sleep/wake cycle, increased anxiety-like behavior, and decreased locomotory activity, with no other overt signs of narcoleptic symptomatology [26]. OX1R-deficient mice show more anxiety in the elevated-plus maze test and depression-like behavior in the forced-swimming test and the tail-suspension test [27]. Furthermore, use of antagonists of orexin receptors to treat anxiety, panic, and depression, as well as the effects of treatment, seem to be related mainly to OX1R activity [28]. We showed that mRNA expression of OX1R in the cerebral cortex and brainstem was reduced significantly by long-term CIH, and that this effect could not be reversed by 2 weeks and 2 weeks re-oxygenation. Conversely, there was no significant difference of mRNA expression of Ox2R.
Orexin neurons innervate monoaminergic neurons such as noradrenergic neurons in locus coeruleus, and histaminergic neurons in tuberomammillary nucleus. OX1R is expressed in noradrenergic locus coeruleus neurons. It has been confirmed that CPAP therapy for OSA does not fully reserve wake impairments [29]. Neuroimaging studies of OSA patients identified gray matter lesions which support the irreversible impairments [30]. Previous studies showed that long-term hypoxia/reoxygenation in adult mice results in irreversible impairments and functionally significant injury in both noradrenergic locus ceruleus and dopaminergic ventral periaqueductal gray wake neurons. In contrast, cholinergic, histaminergic, orexinergic, and serotonergic neurons appeared unperturbed. Additionally, Six month exposure to hypoxia/reoxygenation resulted in a 40% loss of catecholaminergic neurons [31]. There is evidence of brain cortical neuronal cell apoptosis associated with chronic intermittent hypoxia in a mouse model of sleep apnea oxygenation [32]. The irreversible damage of these neurons may lead to the down-regulation of OX1R in the cerebral cortex and brainstem after reoxygenation.
Conclusions
Long-term CIH induced anxiety-like behavior in mice, and re-oxygenation did not reverse this behavior. Long-term CIH affected orexin and its receptors differently in different regions of the mouse brain. The mechanism for the anxiety-related behavior caused by long-term CIH may be involved in OX1R. Further investigation with long-term CIH and a greater range of molecular and behavioral evaluations is necessary to explore the internal mechanisms.
Author contributions
Conception and design of the work: HT, HS and WW. Creating figures and tables: ZJ and YH. Writing and preparing the original draft: HT and HS. Writing, reviewing, and editing the manuscript: HT, HS, WW and BY. Funding acquisition: WW and BY. All authors approved the final version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (81901067) and Key Research and Development Program of Jiangsu Province (BE2022795).
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Animal Care and Use Committee of Nanjing Medical University (Nanjing, China) and complied with the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health, Bethesda, MD, USA). All efforts were made to minimize animal suffering.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huan Tang and Huijie Shen have contributed equally to this work.
Contributor Information
Wei Wang, Email: king_8652@163.com.
Bin Yan, Email: yanb@njmu.edu.cn.
References
- 1.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51(4):313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (Auckl) 2016;4:99–108. doi: 10.2147/HP.S103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132(1):325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorvatn B, Lehmann S, Gulati S, et al. Prevalence of excessive sleepiness is higher whereas insomnia is lower with greater severity of obstructive sleep apnea. Sleep Breath. 2015;19:1387–1393. doi: 10.1007/s11325-015-1155-5. [DOI] [PubMed] [Google Scholar]
- 5.Sithirungson S, Sonsuwan N, Chattipakorn SC, et al. Functional roles of orexin in obstructive sleep apnea: from clinical observation to mechanistic insights. Sleep Med. 2023;101:44. doi: 10.1016/j.sleep.2022.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Sansa G, Iranzo A, Santamaria J. Obstructive sleep apnea in narcolepsy. Sleep Med. 2010;11(1):93–95. doi: 10.1016/j.sleep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 8.Ganjavi H, Shapiro CM. Hypocretin/orexin: a molecular link between sleep, energy regulation, and pleasure. J Neuropsychiatry Clin Neurosci. 2007;19(4):413–419. doi: 10.1176/jnp.2007.19.4.413. [DOI] [PubMed] [Google Scholar]
- 9.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/S0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 11.Aksu K, Guven SF, Aksu F, et al. Obstructive sleep apnoea, cigarette smoking and plasma orexin-A in a sleep clinic cohort. J Int Med Res. 2009;37(2):331–340. doi: 10.1177/147323000903700207. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai S, Nishijima T, Takahashi S, et al. Clinical significance of daytime plasma orexin-A-like immunoreactivity concentrations in patients with obstructive sleep apnea hypopnea syndrome. Respiration. 2004;71(4):380–384. doi: 10.1159/000079643. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi N, Tatsumi K, Nakamura A, et al. Plasma orexin-A levels in obstructive sleep apnea-hypopnea syndrome. Chest. 2003;124(4):1381–1385. doi: 10.1378/chest.124.4.1381. [DOI] [PubMed] [Google Scholar]
- 14.Busquets X, Barbe F, Barcelo A, et al. Decreased plasma levels of orexin-A in sleep apnea. Respiration. 2004;71(6):575–579. doi: 10.1159/000081757. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Jiang L, Zhu F, et al. Chronic intermittent hypoxia and the expression of orexin and its receptor in the brains of rats. Sleep Biol Rhythms. 2014;12:22–29. doi: 10.1111/sbr.12043. [DOI] [Google Scholar]
- 16.Yamaguchi K, Futatsuki T, Ushikai J, et al. Intermittent but not sustained hypoxia activates orexin-containing neruons in mice. Respir Physiol Neurobiol. 2015;206(15):11–14. doi: 10.1016/j.resp.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Walsh RN, Cummins RA. The open field test: a critical review. Psychological Bull. 1976;83:482–504. doi: 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- 18.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 19.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 20.Vanek J, Prasko J, Genzor S, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi: 10.1016/j.sleep.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Leconet C, Leger M, Boulouard M. Repeated mild hypoxic exposures decrease anxiety-like behavior in the adult mouse together with an increased brain adrenomedullin gene expression. Behav Brain Res. 2012;230(1):78–84. doi: 10.1016/j.bbr.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 22.Carissimi A, Martinez D, Kim LJ, et al. Intermittent hypoxia, brain glyoxalase-1 and glutathione reductase-1, and anxiety-like behavior in mice. Brazil J Psychiatry. 2018;40(4):376–381. doi: 10.1590/1516-4446-2017-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puech C, Badran M, Runion AR. Explicit memory, anxiety and depressive like behavior in mice exposed to chronic intermittent hypoxia, sleep fragmentation, or both during the daylight period. Neurobiol Sleep Circad Rhythms. 2022;11(13):100084. doi: 10.1016/j.nbscr.2022.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrone O. Mood after CPAP: fewer patients with depression, but not fewer with anxiety. Eclin Med. 2019;11:9–10. doi: 10.1016/j.eclinm.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt NJ, Waters KA, Machaalani R. Orexin receptors in the developing piglet hypothalamus, and effects of nicotine and intermittent hypercapnic hypoxia exposures. Brain Res. 2013;1508(1):73–82. doi: 10.1016/j.brainres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Tisdale RK, Kilduff TS. Hypocretin/orexin receptor pharmacology and sleep phases. Front Neurol Neurosci. 2021;45:22–37. doi: 10.1159/000514963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas MG, Shoji H, Soya S, et al. Comprehensive behavioral analysis of male Ox1R−/− mice showed implication of orexin receptor-1 in mood, anxiety, and social behavior. Front Behav Neurosci. 2015;9:324–334. doi: 10.3389/fnbeh.2015.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summers CH, Yaeger JDW, Staton CD, et al. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: potential for therapy. Brain Res. 2020;1731(15):146085. doi: 10.1016/j.brainres.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta analysis. Thorax. 2006;61:430–434. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 31.Y Zhu, P Fenik, G Zhan, et al (2007) Selective loss of catecholaminergic wake–active neurons in a murine sleep apnea model. The Journal of Neuroscience. l27(37):10060–10071. [DOI] [PMC free article] [PubMed]
- 32.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126(2):313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]