Abstract
Respiratory viral infections are a major public health problem, with much of their morbidity and mortality due to post-viral lung diseases that progress and persist after the active infection is cleared. This paradigm is implicated in the most common forms of chronic lung disease, such as asthma and COPD, as well as other virus-linked diseases including progressive and long-term coronavirus disease 2019. Despite the impact of these diseases, there is a lack of small-molecule drugs available that can precisely modify this type of disease process. Here we will review current progress in understanding the pathogenesis of post-viral and related lung disease with characteristic remodelling phenotypes. We will also develop how this data leads to mitogen-activated protein kinase (MAPK) in general and MAPK13 in particular as key druggable targets in this pathway. We will also explore recent advances and predict the future breakthroughs in structure-based drug design that will provide new MAPK inhibitors as drug candidates for clinical applications. Each of these developments point to a more effective approach to treating the distinct epithelial and immune cell based mechanisms, which better account for the morbidity and mortality of post-viral and related types of lung disease. This progress is vital given the growing prevalence of respiratory viruses and other inhaled agents that trigger stereotyped progression to acute illness and chronic disease.
Shareable abstract
This review covers progress on pathogenesis and consequent MAPK-guided treatment for post-viral and related types of lung disease, including asthma, COPD, and COVID-19. The advances refine a paradigm that features MAPK13 to control the disease process. https://bit.ly/49nzgRl
Introduction to post-viral lung disease
Respiratory viral infections are perhaps the most common reason for individuals seeking medical attention for illnesses, particularly during pandemic conditions such as outbreaks of influenza virus or coronavirus infections [1, 2]. Further, even after clearance of an infectious virus, the acute illness can progress to respiratory failure in the intermediate term and chronic respiratory disease in the long term [3, 4]. Indeed, chronic lung disease has emerged as the third leading cause of death from disease in the US and the fifth leading cause worldwide [5, 6], with morbidity and mortality linked to lung inflammation and mucus production [7] that is often triggered by respiratory viral infection [8]. As developed below, these disease phenotypes also overlap with long-term coronavirus disease 2019 (COVID-19) as the latest manifestation of post-viral lung disease. Despite the magnitude of these public health problems, there are still no precisely designed small molecules that serve as drugs to modify post-viral and related lung disease. In this context, we will present both the background and then the progress made towards a new type of small-molecule kinase inhibitor aimed at modifying lung diseases that otherwise might develop in response to present and future types of respiratory viral infections and related types of lung injury.
A new approach to post-viral and related lung disease
A distinct paradigm for pathogenesis
A major obstacle to discovery of a drug to control chronic lung disease is the need to better define the pathogenesis of this type of disease process. In that regard, common respiratory diseases exhibit a fundamental susceptibility to persistent inflammation in response to inhaled environmental stimuli that are classically thought to involve exposure to allergen (for asthma) and smoke from tobacco or biomass fuel (for COPD). However, respiratory viral infection could also be critical to the disease process alone or in synergy with other stimuli in asthma [8–10] and COPD [11–21]. Our pursuit of this idea provides a paradigm that depends on viral activation of immune cells [22–25] and reprogramming of epithelial stem cells (ESCs), particularly basal-ESCs [26, 27]. In the case of basal-ESCs, this learning process includes epigenetic instructions to grow and sometimes migrate, activate the type-2 immune response, and differentiate into mucous cells (figure 1) [22–36]. Key cell and molecular steps include the dual role of nuclear interleukin (IL)-33 as a switch point for basal cell growth and secreted IL-33 as a signature of immune activation. The activation of downstream immune cells (including innate lymphoid cells and macrophages) is key to feed-forward IL-13 production. This paradigm is derived primarily from the study of the natural pathogen Sendai virus in mice [22–24, 26, 27] but similar responses develop after infection with influenza A virus, enterovirus-D68, human rhinovirus, respiratory syncytial virus and coronavirus in experimental and clinical settings [33, 34, 37–40].
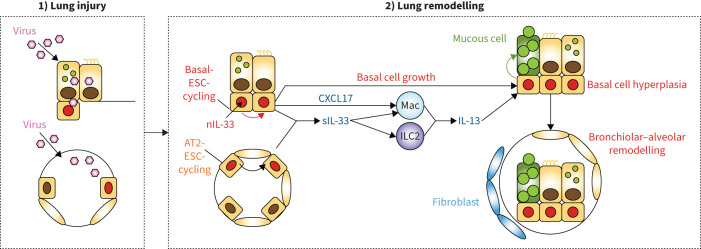
FIGURE 1.
Scheme for acute lung injury and chronic lung remodelling after viral infection. Key steps include 1) initial lung injury and infectious illness with damage to bronchiolar and alveolar epithelial cells and 2) transition to long-term lung remodelling disease with basal-epithelial stem cell (ESC) growth linked to a nuclear IL-33 (nIL-33) checkpoint. Concomitant immune activation of epithelial cells for expression and secretion of C-X-C motif ligand 17 (CXCL17) and interleukin (IL)-33 (sIL-33) that can drive tissue macrophage (Mac) and innate lymphoid cell 2 (ILC2) production of IL-13 for type 2 inflammation and mucinous differentiation. Together, these events result in local epithelial cell hyperplasia at bronchiolar sites and, in turn, basal-ESC migration and further growth and differentiation at bronchiolar–alveolar remodelling sites that also include post-inflammatory fibrosis. These events are based on the mouse model, recognising that AT2 cells are not a significant source of IL-33 in humans, where intracellular and extracellular functions of IL-33 might be combined in basal cells. Reproduced and modified from [27].
The result is stereotyped tissue remodelling characterised by metaplastic, inflammatory, hypersecretory and fibrotic phenotypes. In turn, the paradigm offers IL-13 blockade as a druggable end-point for correcting downstream effectors, but importantly might not correct the upstream drivers. Nonetheless, this scheme addresses the basis for the increasingly common issue of respiratory viral infections that can initiate, exacerbate and accelerate long-term respiratory disease [4, 8, 41–48]. The reprogramming nature of the process also explains the chronicity of similar phenotypes found in asthma, COPD and even fibrotic lung diseases that persist long after the infectious illness is resolved. This paradigm also provides a roadmap for addressing the challenge of developing a new therapeutic approach. This challenge includes the special need to select targets in this pathway that can be precisely drugged and feasibly monitored for the purpose of modifying the disease and not just temporarily dampening it.
In that context, we focused on the abnormality of excess mucus production as an unmet treatment end-point that (as introduced above) is closely linked to morbidity and mortality across chronic lung diseases. In the case of COPD, this connection derives from histopathology [7, 49], but has been reinforced more recently from newer imaging technologies [50]. In any case, the mucus-correction strategy led to cell-culture and clinical-sample screens for activated kinase targets that correlated with chloride channel calcium-activated 1-dependent and IL-13-stimulated mucus production in human airway epithelial cells [32]. These approaches led to the identification of mitogen-activated protein kinase (MAPK) 13 as a relatively orphan target of currently undefined significance. Indeed, the assignment of MAPK13 function posed an additional challenge, given the overlapping network of MAPK activators, MAPK themselves, MAPK substrates and consequent cellular responses (figure 2). This issue is particularly relevant to MAPK13 given the possible intersection with closely related MAPK12 expression, activation and function at some tissue sites [51].
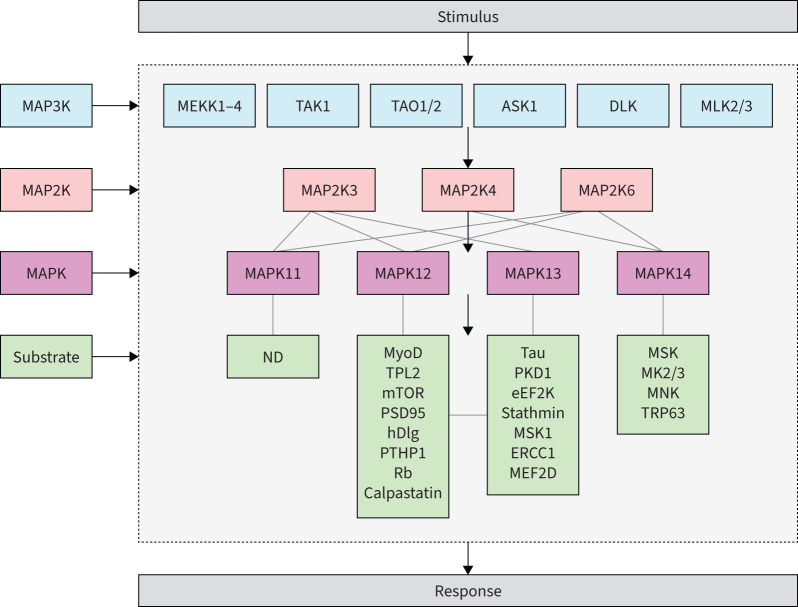
FIGURE 2.
Scheme for mitogen-activated protein kinase (MAPK) signalling pathways. The scheme depicts the pathway from initial signal to final response through sequential activation of MAP3K, MAP2K and MAPK. For each of the four MAPKs (MAPK11–14), there are predicted to be hundreds of downstream substrates that in turn result in a range of cellular responses. There is also overlap between MAPK12 and MAPK13 substrates and functions based on high homology between these two kinases. ASK1: apoptosis signal-regulating kinase-1; eEF2K: eukaryotic elongation factor 2 kinase; ERCC1: excision repair cross-complementing 1; hDlg: human disc large; MEF2D: myocyte enhancer factor 2D; MEKK: MAPK/extracellular signal-regulated kinase; MK: MAPK-activated protein kinase; MLK: mixed-lineage kinases; MNK: MAPK-interacting protein kinase; MSK: mitogen and stress-activated kinase; mTOR: mammalian target of rapamycin; MyoD: myoblast determination protein; ND: not determined; PKD1: protein kinase D1; PSD95: post-synapse density 95; PTPH1: protein tyrosine phosphatase H1; Rb: retinoblastoma protein; TAK1: transforming growth factor-β-activated kinase 1; TAO: thousand-and-one amino acid; TPL2: tumour progression locus 2; TRP63: transformation-related protein 63. For details, see references [93–96].
Despite these predictions, MAPK13 has proven to play an unexpectedly distinct role in controlling fundamental features of lung biology. Thus, initial work using gene knockdown and relatively nonspecific kinase inhibitors suggested that MAPK13 inhibition alone provided effective control of mucus production in human airway epithelial cell culture models stimulated with IL-13 [32]. In addition, activated MAPK13 was also increased in lung tissue samples from patients with COPD [32]. These findings raised the possibility that MAPK13 might regulate the pathway to mucus production, particularly in the context of type 2 inflammation in the lung and perhaps other sites of epithelial injury and repair. Further, these studies identified MAPK13 as a drug target that was missed in favour of the conventional focus on MAPK14 [32, 52–63]. This existing strategy was derived at least in part from the MAPK14 connection to IL-1β and tumour necrosis factor-α (TNF-α) signals in chronic lung disease, perhaps ignoring the disease impact of type 2 immune signals such as IL-13. In fact (10 years later), it appears that at least a subset of COPD patients is responsive to anti-IL-13-receptor antibody blockade [64], whereas MAPK14 inhibitors might be relatively less effective in the same types of patients [56]. This issue continues to be studied, but current data indicate the need for the development of the first MAPK13 inhibitor or perhaps a combined MAPK13-14 inhibitor for disease modification in chronic lung disease.
Corresponding need for a small-molecule drug
As introduced above, the data from experimental models and corresponding clinical samples supported the development of a small-molecule kinase inhibitor with potent MAPK13 blocking activity. However, any drug discovery approach also needs to assess the practical rationale for introducing another agent targeting type 2 inflammation, particularly given the success of monoclonal antibodies directed against cytokines and cytokine receptors in the same immune pathway [65–73]. In that regard, even in the face of considerable investment in newer therapeutic technologies, there remains significant advantages of conventional small-molecule drugs. Thus, in comparison to antibody therapeutics, the benefits of small-molecules include decreases in manufacturing costs, inconveniences and complications of injections, side-effects such as Ig-related reactions, and restrictions for use in paediatric populations. Moreover, there is an inherent advantage in localised delivery to the disease site and, at least to date, only small-molecule drugs are amenable to inhaled dosing. Indeed, the combination of intravenous and inhaled dosing offers a tailored treatment approach that caters to the full spectrum of disease severity.
There also remains some uncertainty around the safety of biologics given near the time of disease exacerbation, particularly due to viral infection, when treatment is most critically needed in some patients. Furthermore, existing monoclonal antibody products (against IgE, IL-5/5R, IL-4RA, IL-33 and thymic stromal lymphopoietin targets alone or in combination) still do not fully control exacerbations in adults or teenagers. These reasons, along with others, continue to drive small-molecule efforts in this field. These approaches include reducing agents, muscarinic antagonists, glucocorticoids, phosphodiesterase-4 inhibitors, purinergic receptor antagonists, macrolide antibiotics and kinase inhibitors [74–80]. Similarly, there are ongoing and new applications of nonpharmacologic therapies including respiratory therapy, pulmonary rehabilitation, cough suppressants and mucolytics [81]. However, as introduced above, evidence that any of these strategies can provide specific, direct, potent, safe and long-lasting modification of the key disease phenotypes still needs to be determined. In particular, none of the existing therapies address the complete paradigm for stem-cell and immune-cell reprogramming that would likely be required for modification of the disease drivers.
Progress in generating a new MAPK inhibitor
In response to these issues, our research group focused on the need for a more successful small-molecule MAPK inhibitor. However, we recognised at the outset that previous kinase-inhibitor screening approaches (and perhaps other unpublished drug development efforts) did not identify a potent MAPK13 inhibitor, leaving this target as undrugged and potentially in the category of the undruggable [82]. Therefore, we built upon our knowledge of the MAPK13 structure (derived from X-ray crystallography) [32] and initial albeit limited success with compounds derived from modifying a parent compound known as BIRB-796 (NuP-43 in our chemical series) that was developed as a selective MAPK14 inhibitor but had weak activity against MAPK13 as well. In that initial work, modifications were made to eliminate the naphthalene moiety and thereby enhance active site access and MAPK13 inhibition and avoid idiosyncratic hepatotoxicity [83]. These efforts resulted in the development of first-generation compounds demonstrating modest potency for MAPK 13 blockade but fell short of providing a suitable solution as an effective inhibitor.
Subsequently, we used additional structure-based drug-design technologies to better block MAPK13 but not (as yet) eliminate the possible benefit of MAPK14 blocking activity. These modifications targeted each of the major MAPK domains (left-hand hinge-binding, active site and allosteric pocket) (figure 3a), as recently reported [84]. Screening of candidate compounds resulted in the discovery of a potent MAPK13-14 inhibitor (designated NuP-3) that gained MAPK13 blocking activity likely based on additional hinge-binding interactions (figure 3b). Subsequent characterisation of NuP-3 demonstrated favourable drug characteristics and proof-of-concept results in human cell and animal models. In particular, NuP-3 was shown to effectively attenuate IL-13-stimulated mucus production in human airway epithelial cells in culture, even in the context of marked IL-13 induction of MAPK13 expression [84]. In addition, given orally, this compound prevented airway inflammation and mucus production in new minipig models driven by type-2 cytokine-challenge (using IL-13) or respiratory viral infection (using natural pathogen Sendai virus). Moreover, NuP-3 treatment did not influence tissue virus levels or clinical signs of acute illness, thereby suggesting the safety of the treatment during active infection. The data thereby provide an effective MAPK13-14 inhibitor and, in combination with previous data, yield a scheme for MAPK-guided control of short-term respiratory inflammation and mucus production by actions on epithelial and immune cells (figure 4).
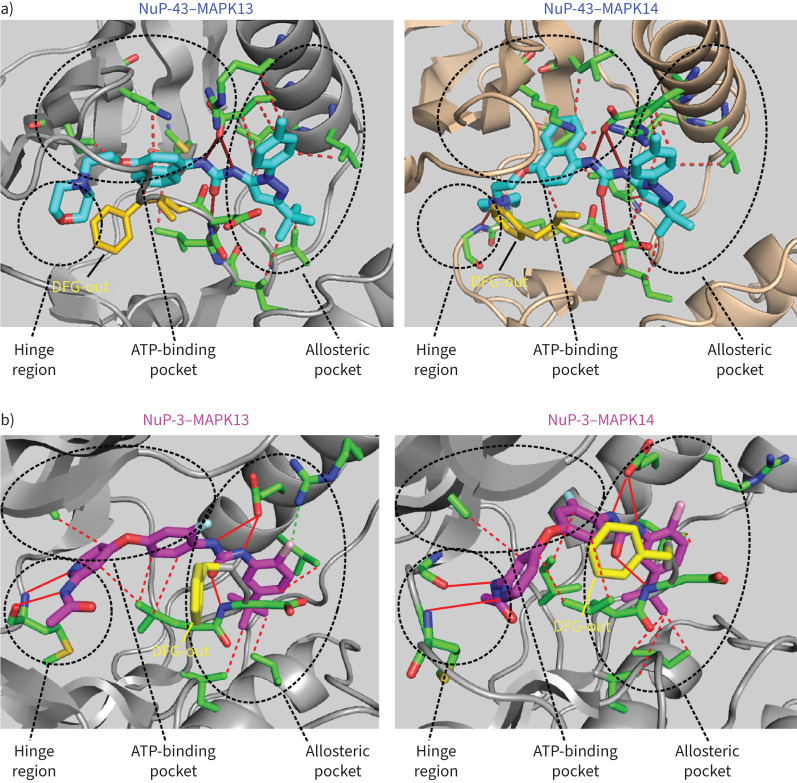
FIGURE 3.
Mitogen-activated protein kinase (MAPK)13-14-guided development of a new small-molecule kinase inhibitor. a) Structure for parent compound NuP-43 (BIRB-796) docking to MAPK13 (model) and MAPK14 (co-crystal) that illustrates functional targets, hydrogen-bond (solid red lines) and hydrophobic (dashed lines) interactions, and DFG-out binding mode (yellow structure). b) Structure for NuP-3 bound to MAPK13 based on X-ray crystallography and comparable docking to MAPK14 with features labelled as in a) for potential hydrogen-bond interactions for pyridine lone-pair and acetamide-NH with M110/M109/H107 in the hinge region, bidentate urea-NH with E72/E71, urea-O with D168 and chlorine with R68 in the allosteric pocket, and seven sets of hydrophobic contacts. Reproduced and modified from reference [84].
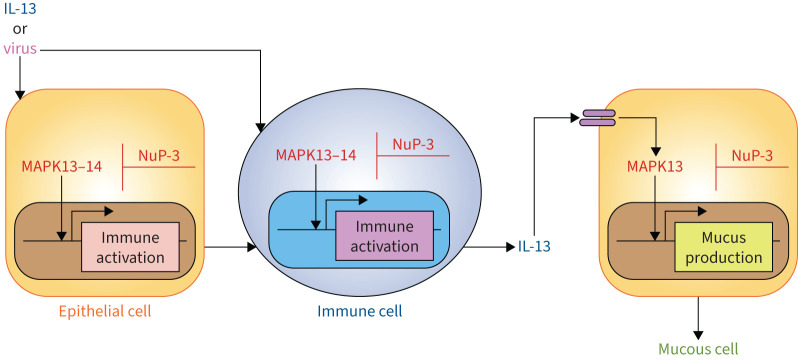
FIGURE 4.
Scheme for mitogen-activated protein kinase (MAPK)-guided control of short-term respiratory inflammation and mucus production from minipig models. The scheme depicts key events leading from cytokine challenge (using interleukin (IL)-13) or respiratory viral infection (using Sendai virus) to immune activation of epithelial cells (including basal epithelial cells) and immune cells (including innate myeloid and lymphoid cells). This activation pathway could include epithelial and immune cell production of immune activators, including immune-cell derived IL-13 after viral infection. Subsequent IL-13R activation on epithelial progenitor cells results in mucinous differentiation (marked by chloride channel accessory 1, mucin 5AC, oligomeric mucus/gel-forming and mucin 5B, oligomeric mucus/gel-forming expression) and mucus production. Each of these steps for cellular activation could be sensitive to blockade by a small-molecule kinase inhibitor such as NuP-3. Reproduced from [84].
Next steps towards reaching the clinic
The discovery of NuP-3 provides a significant advance towards developing an MAPK inhibitor for post-viral and related lung disease. However, this progress also raises the next set of questions and tasks required to understand pathogenesis and reach therapeutic application.
Role of MAPK13
An initial question to consider is the role of MAPK13 versus related kinases (including MAPK14) in lung biology and disease. As introduced above (figure 2), there could be considerable overlap and interaction among MAPK signalling pathways and consequent functions. Nonetheless, MAPK14 was awarded a canonical role in inflammatory signalling and disease [52–56, 58–62, 85, 86] given its linkage to specific cytokines (notably TNF-α and IL-1β). However, even the most advanced versions of these compounds have not proved very effective in clinical trials of patients with COPD [56, 87]. Nonetheless, this approach remains a target for immune-based lung disease, including asthma, COPD and COVID-19 [88, 89]. Similarly, NuP-3 was designed to maintain MAPK14-blocking activity. Thus, the present data suggest that attacking MAPK13-14 together might achieve an unprecedented therapeutic benefit for a broad range of cytokine-signalling and viral-infection conditions. However, additional studies are needed to address the role of MAPK13 alone in post-viral lung disease and these related conditions. In that regard, NuP-3 was more effective than the parent compound, despite similar potencies for MAPK14 inhibition, and our earlier MAPK gene knockdown work showed no significant effect of MAPK14 blockade on mucus production [32]. Additional studies of targeted gene knockout and kinase structure function, as well as more selective inhibitors, will be needed to fully define MAPK13 function alone and in combination with MAPK14. Indeed, the insights from NuP-3 are already proving useful for these next studies to better define the priority of MAPK13 as a drug target.
Role of the type-2 immune response
More generally, there remains the broader question of the role of the type 2 immune response for host defence and inflammatory disease. As introduced above (figure 1), we identified basal-ESC growth, immune activation and mucinous differentiation as requirements for long-term lung remodelling disease after viral infection in mice [27]. In that case, however, we observed basal-ESC hyperplasia and metaplasia at bronchiolar–alveolar sites in the setting of a more severe and widespread infection. Here we find the same markers of basal-epithelial cell activation, but lung disease is limited to an airway site in the context of milder and localised infection and injury. Further basal-epithelial cell markers for immune activation are often shared with interacting immune cells. In fact, the rapid time course of illness in the minipig model suggests that epithelial and immune cells might rely on MAPK13 signalling (figure 4). Moreover, epithelial–immune cell interactions are constructed for feed-forward and feed-back amplification. Thus, additional work is also needed to assign specific cellular and molecular functions. Of particular interest is whether animal models with more severe viral infection could be used to better define these issues and thereby better establish the relative role of ESC reprogramming as already seems to be the case in COVID-19 patients [40]. Our current perspective is that the epithelial and immune components of the type 2 immune response represent a primordial system for repair, regeneration and defence in response to barrier injury, but it can become disease-producing based on host genetics, viral type, viral severity, age, sex, biological clock and other factors. In these cases, the excessive response needs at least partial attenuation for the host's benefit, potentially via down-regulation of MAPK13 function.
Timing: acute versus chronic post-viral disease
An additional question in terms of mechanism and practical application concerns the time course of cell and molecular events in the development of post-viral and related lung disease. In that regard, respiratory viral infection generally proceeds as a top-down infection that starts in the upper and then lower airways and extends distally to bronchiolar–alveolar and in some cases alveolar sites. Relatedly, acute immune activation (including TNF-α, IL-1β and type 1 and type 2 cytokine actions) can transition to chronic activation (including type 2 cytokines such as IL-13). This paradigm is consistent with our observations in experimental models and clinical samples, but here again further work will be needed to establish this pattern and define functional consequences. The present data suggests that MAPK blockade that includes MAPK13 inhibition is beneficial even for relatively short-term airway inflammation and mucus production. However, the timing of MAPK expression and activation is critical to establishing and tracking it as a drug target in vivo, particularly in patients with chronic lung disease. This remains a challenge, since most clinical samples are derived from stable patients versus those with proximity to recent infection and disease exacerbation. Nonetheless, strategies to establish long-term MAPK (particularly MAPK13) function are well underway. In particular, basal-ESC reprogramming is required for post-viral lung disease [26, 27]. Thus, control of a renewable basal-ESC population could provide long-term disease modification versus transient blockade of downstream cytokine production. Accordingly, MAPK13 control of basal-ESC reprogramming could offer the potential for long-term disease modification not achieved with current therapies. Indeed, studies of MAPK13-14 inhibitors in this reprogramming process are ongoing in human cell models (particularly basal-ESC conventional and organoid cultures) and animal models (particularly Sendai virus infected mice).
Drug development process
Another relevant consideration for MAPK-guided inhibitors (or any new therapeutic) relates to the pre-clinical and clinical processes of discovering and developing small-molecule drugs. Thus, the work on NuP-3 brings us significantly closer to clinical application by providing a roadmap for MAPK13 inhibitors that are more potent than their predecessors [32, 90]. However, this advance leads to next steps in pre-clinical studies. Important goals include pharmacology to establish optimal dosing level, route, and timing in relation to disease prevention and reversal. In addition, safety pharmacology and toxicology studies in preclinical species must establish therapeutic index and adverse effect level. Indeed, the chemical modifications used to discover NuP-3 were designed to eliminate the problematic toxicity in previous candidates [83, 91]. Further, it will be key to define biomarkers for target status and engagement to select and monitor the subset of patients that benefit from this type of treatment. In concert with this process, there must be ongoing analysis of newly identified MAPK13–inhibitor interactions to guide further development of the MAPK-targeted drug pipeline. As introduced above, this approach includes the goal of more selective kinase blockade. Each of these issues is also a subject of active and already encouraging research studies.
Summary
We provide an update on the progress made towards meeting a practical need for more successful therapy for post-viral lung disease, including its progression to chronic lung diseases in the form of asthma, COPD, and long-term COVID-19. Key advances include a distinct paradigm for this type of disease and how this scheme provides a substrate for MAPK control of ESCs and immune cells [32]. We describe the recent report of a potent MAPK13-14 inhibitor that can prevent respiratory inflammation and mucus production based on studies of human cell and minipig models of short-term airway disease [84]. We also present the next set of questions that must be addressed for further progress towards therapeutic application to practice and a better understanding of pathogenesis of lung remodelling disease. Overall, the present insights represent significant progress in developing a safe and effective small-molecule drug for respiratory disease and other diseases that feature expression and activation of MAPKs in general and MAPK13 in particular, as well as related kinases. Precision medicine will require stratification and monitoring of patients using biomarkers of the MAPK13-14 target in airway epithelial cells (particularly basal-lineage cells) and type 2 response in immune cells (particularly monocyte-derived dendritic cells and macrophages) as previously validated in experimental models and clinical samples [22-27, 32, 40, 92]. The combination of data provides renewed optimism towards meeting the goal of developing a small-molecule drug with the capacity to precisely modify and thereby correct the epithelial and immune cell activation that drives long-term post-viral and related types of lung disease.
Acknowledgements
Outstanding contributions to the cited work were provided by E. Agapov, Y. Alevy, S.L. Brody, D.E. Byers, M. Li, D. Mao, C.A. Iberg, S.P. Keeler, W.T. Roswit and J. Yantis (Washington University, St. Louis, MO, USA).
Provenance: Commissioned article, peer reviewed.
Conflict of interest: M.J. Holtzman is the Founder of NuPeak Therapeutics, Inc. and inventor along with K. Wu and A.G. Romero on a patent for MAPK13 inhibitor composition and use thereof. M.J. Holtzman also reports membership on a data safety monitoring board for AstraZeneca, outside the submitted work. Y. Zhang has nothing to disclose.
Support statement: Funding was provided by grants from the National Institutes of Health (National Heart, Lung, and Blood Institute UH2-HL123429, R35-HL145242, and STTR R41-HL149523, and National Institute of Allergy and Infectious Diseases R01 AI130591), US Department of Defense (TTDA W81XWH2010603 and W81XWH2210281), Harrington Discovery Institute, and the Hardy Research Fund. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362: 1708–1719. doi: 10.1056/NEJMra1000449 [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of clinically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtzman MJ, Byers DE, Alexander-Brett J, et al. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol 2014; 14: 686–698. doi: 10.1038/nri3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Data and Statistics. Date last accessed: 12 February 2024. Date last updated: 11 July 2022. www.cdc.gov/copd/data.html
- 6.Centers for Disease Control and Prevention . Data, Statistics, and Surveillance. Date last accessed: 12 February 2024. Date last updated: 29 March 2023. www.cdc.gov/asthma/asthmadata.htm
- 7.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 8.Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest 2012; 122: 2741–2748. doi: 10.1172/JCI60325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol 2017; 140: 895–906. doi: 10.1016/j.jaci.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan KS, Lim RL, Liu J, et al. Respiratory viral infections in exacerbation of chronic airway inflammatory diseases: novel mechanisms and insights from the upper airway epithelium. Front Cell Dev Biol 2020; 8: 99. doi: 10.3389/fcell.2020.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang M-J, Lee CG, Lee J-Y, et al. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 2008; 118: 2771–2784. doi: 10.1172/JCI32709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst JR, Vestibo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 13.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest 2012; 122: 2749–2755. doi: 10.1172/JCI60324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 1618–1623. doi: 10.1164/ajrccm.164.9.2105011 [DOI] [PubMed] [Google Scholar]
- 15.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case–control study. Thorax 2003; 58: 37–42. doi: 10.1136/thorax.58.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron RJ, de Wit D, Welsh TN, et al. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med 2006; 32: 1022–1029. doi: 10.1007/s00134-006-0202-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins CR, Celli B, Anderson JA, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J 2012; 39: 38–45. doi: 10.1183/09031936.00194610 [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos G, Lerikou M, Tsiodras S, et al. Viral epidemiology of acute exacerbations of chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2012; 25: 12–18. doi: 10.1016/j.pupt.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 2011; 183: 734–742. doi: 10.1164/rccm.201006-0833OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallia P, Footitt J, Sotero R, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 1117–1124. doi: 10.1164/rccm.201205-0806OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter MJ, Morton JD, Kajiwara N, et al. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest 2002; 110: 165–175. doi: 10.1172/JCI0214345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med 2008; 14: 633–640. doi: 10.1038/nm1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Byers DE, Jin X, et al. TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J Exp Med 2015; 212: 681–697. doi: 10.1084/jem.20141732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu K, Wang X, Keeler SP, et al. Group 2 innate lymphoid cells must partner with the myeloid-macrophage lineage for long-term postviral lung disease. J Immunol 2020; 205: 1084–1101. doi: 10.4049/jimmunol.2000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Wu K, Keeler SP, et al. TLR3-activated monocyte-derived dendritic cells trigger progression from acute viral infection to chronic disease in the lung. J Immunol 2021; 206: 1297–1314. doi: 10.4049/jimmunol.2000965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013; 123: 3967–3982. doi: 10.1172/JCI65570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu K, Kamimoto K, Zhang Y, et al. Basal-epithelial stem cells cross an alarmin checkpoint for post-viral lung disease. J Clin Invest 2021; 131: e149336. doi: 10.1172/JCI149336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyner JW, Kim EY, Ide K, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2006; 116: 309–321. doi: 10.1172/JCI25167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel AC, Morton JD, Kim EY, et al. Genetic segregation of airway disease traits despite redundancy of chloride channel calcium-activated (CLCA) family members. Physiol Genomics 2006; 25: 502–513. doi: 10.1152/physiolgenomics.00321.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grayson MH, Cheung D, Rohlfing MM, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med 2007; 204: 2759–2769. doi: 10.1084/jem.20070360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agapov E, Battaile JT, Tidwell R, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol 2009; 41: 379–384. doi: 10.1165/2009-0122R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alevy Y, Patel AC, Romero AG, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest 2012; 122: 4555–4568. doi: 10.1172/JCI64896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keeler SP, Agapov EV, Hinojosa ME, et al. Influenza A virus infection causes chronic lung disease linked to sites of active viral RNA remnants. J Immunol 2018; 201: 2354–2368. doi: 10.4049/jimmunol.1800671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Mao D, Keeler SP, et al. Respiratory enterovirus (like parainfluenza virus) can cause chronic lung disease if protection by airway epithelial STAT1 is lost. J Immunol 2019; 202: 2332–2347. doi: 10.4049/jimmunol.1801491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keeler SP, Yantis J, Youkilis S, et al. Chloride channel accessory 1 gene deficiency causes selective loss of mucus production in a new pig model. Am J Physiol Lung Cell Mol Physiol 2022; 322: L842–LL52. doi: 10.1152/ajplung.00443.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin RA, Keeler SP, Wu K, et al. An alternative mechanism for skeletal muscle dysfunction in long-term post-viral lung disease. bioRxiv 2022; preprint [ 10.1101/2022.10.07.511313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson DJ, Makrinioti H, Rana BMJ, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 2014; 190: 1373–1382. doi: 10.1164/rccm.201406-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung Y, Hong JY, Lei J, et al. Rhinovirus infection induces interleukin-13 production from CD11b-positive, M2-polarized exudative macrophages. Am J Respir Cell Mol Biol 2015; 52: 205–216. doi: 10.1165/rcmb.2014-0068OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stier MT, Bloodworth MH, Toki S, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoic cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 2016; 138: 814–824. doi: 10.1016/j.jaci.2016.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu K, Zhang Y, Yin Declue H, et al. Lung remodeling regions in long-term coronavirus disease 2019 feature basal epithelial cell reprogramming. Am J Pathol 2023; 193: 680–689. doi: 10.1016/j.ajpath.2023.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Wu J, Hao S, et al. Long term outcomes in survivors of epidemic influenza A (H7N9) virus infection. Sci Rep 2017; 7: 17275. doi: 10.1038/s41598-017-17497-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020; 324: 1723–1724. doi: 10.1001/jama.2020.19719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021; 594: 259–264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 44.Blomberg B, Greve-Isdahl Mohn K, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27: 1607–1613. doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdallah SJ, Voduc N, Corrales-Medina VF, et al. Symptoms, pulmonary function and functional capacity four months after COVID-19. Ann Am Thorac Soc 2021; 18: 1912–1917. doi: 10.1513/AnnalsATS.202012-1489RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19. JAMA Network Open 2021; 4: e2111417. doi: 10.1001/jamanetworkopen.2021.11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanichkachom G, Newcomb R, Cowl CT, et al. Post-COVID-19 syndrome (long haul syndrome): description of a multidisciplinary clinic at Mayo Clinic and characteristics of the initial patient cohort. Mayo Clin Proc 2021; 96: 1782–1791. doi: 10.1016/j.mayocp.2021.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frija-Masson J, Debray M-P, Boussouar S, et al. Residual ground glass opacities three months after Covid-19 pneumonia correlate to alteration of respiratory function: the post Covid M3 study. Respir Med 2021; 184: 106435. doi: 10.1016/j.rmed.2021.106435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogg JC, Chu FS, Tan WC, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007; 176: 454–459. doi: 10.1164/rccm.200612-1772OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaz AA, Orejas JL, Grumley S, et al. Airway-occluding mucus plugs and mortality in patients with chronic obstructive pulmonary disease. JAMA 2023; 329: 1832–1839. doi: 10.1001/jama.2023.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomas-Loba A, Manieri E, Gonzalez-Teran B, et al. p38γ is essential for cell cycle progression and liver tumoriogenesis. Nature 2019; 568: 557–560. doi: 10.1038/s41586-019-1112-8 [DOI] [PubMed] [Google Scholar]
- 52.Underwood DC, Osborn RR, Kotzer CJ. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J Pharmacol Exp Ther 2000; 293: 281–288. [PubMed] [Google Scholar]
- 53.Duan W, Chan JH, McKay K, et al. Inhaled p38α mitogen-activated protein kinase antisense oligonucleotide attenuates asthma in mice. Am J Respir Crit Care Med 2005; 171: 571–578. doi: 10.1164/rccm.200408-1006OC [DOI] [PubMed] [Google Scholar]
- 54.Medicherla S, Fitzgerald MF, Spicer D, et al. p38α-selective mitogen-activated protein kinase inhibitor SD-282 reduces inflammation in a subchronic model of tobacco smoke-induced airway inflammation. J Pharmacol Exp Ther 2008; 324: 921–929. doi: 10.1124/jpet.107.127092 [DOI] [PubMed] [Google Scholar]
- 55.Renda T, Baraldo S, Pelaia G, et al. Increased activation of p38 MAPK in COPD. Eur Respir J 2008; 31: 62–69. doi: 10.1183/09031936.00036707 [DOI] [PubMed] [Google Scholar]
- 56.MacNee W, Allan RJ, Jones I, et al. Efficacy and safety of the oral p38 inhibitor PH-797804 in chronic obstructive pulmonary disease: a randomised clinical trial. Thorax 2013; 68: 738–745. doi: 10.1136/thoraxjnl-2012-202744 [DOI] [PubMed] [Google Scholar]
- 57.Lora JM, Zhang DM, Liao SM, et al. Tumor necrosis factor-α triggers mucus production in airway epithelium through an IκB kinase β-dependent mechanism. J Biol Chem 2005; 280: 36510–36517. doi: 10.1074/jbc.M507977200 [DOI] [PubMed] [Google Scholar]
- 58.Ha U, Lim JH, Jono H, et al. A novel role for IκB kinase (IKK) α and IKKβ in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J Immunol 2007; 178: 1736–1747. doi: 10.4049/jimmunol.178.3.1736 [DOI] [PubMed] [Google Scholar]
- 59.Fujisawa T, Velichko S, Thai P, et al. Regulation of airway MUC5AC expression by IL-1β and IL-17A; the NF-κB paradigm. J Immunol 2009; 183: 6236–6243. doi: 10.4049/jimmunol.0900614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Na HG, Kim Y-D, Bae CH, et al. High concentration of insulin induces MUC5AC expression via phosphoinositide 3 kinase/AKT and mitogen-activated protein kinase signaling pathways in human airway epithelial cells. Am J Rhinol Allergy 2018; 32: 350–358. doi: 10.1177/1945892418782223 [DOI] [PubMed] [Google Scholar]
- 61.Xu H, Sun Q, Lu L, et al. MicroRNA-218 acts by repressing TNFR1-mediated activation of NF-κB, which is involved in MUC5AC hyper-production and inflammation in smoking-induced bronchiolitis of COPD. Toxicol Lett 2017; 280: 171–180. doi: 10.1016/j.toxlet.2017.08.079 [DOI] [PubMed] [Google Scholar]
- 62.Wu S, Li H, Yu L, et al. IL-1b upregulates Muc5ac expression via NF-κB-induced HIF-1α in asthma. Immunol Lett 2017; 192: 20–26. doi: 10.1016/j.imlet.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 63.Patel NR, Cunoosamy DM, Fageras M, et al. The development of AZD7624 for prevention of exacerbations in COPD: a randomized controlled trial. Int J COPD 2018; 13: 1009–1019. doi: 10.2147/COPD.S150576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med 2023; 389: 205–214. doi: 10.1056/NEJMoa2303951 [DOI] [PubMed] [Google Scholar]
- 65.Wenzel S, Ford L, Pearlman D, et al. Duplimab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013; 368: 2455–2466. doi: 10.1056/NEJMoa1304048 [DOI] [PubMed] [Google Scholar]
- 66.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 67.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 68.Wechsler ME, Ruddy MK, Pavord ID, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med 2021; 385: 1656–1668. doi: 10.1056/NEJMoa2024257 [DOI] [PubMed] [Google Scholar]
- 69.Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381: 1023–1034. doi: 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 70.Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384: 1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 71.Rabe KF, Celli BR, Wechsler ME, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021; 9: 1288–1298. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 72.Jeffery MM, Inselman JW, Maddux JT, et al. Asthma patients who stop asthma biologics have a similar risk of asthma exacerbations as those who continue asthma biologics. J Allergy Clin Immunol Pract 2021; 9: 2742–2750. doi: 10.1016/j.jaip.2021.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore WC, Kornmann O, Humbert M, et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur Respir J 2022; 59: 2100396. doi: 10.1183/13993003.00396-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodruff PG, Wolff M, Hohlfeld JM, et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 181: 438–445. doi: 10.1164/rccm.200909-1415OC [DOI] [PubMed] [Google Scholar]
- 75.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365: 689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanabe T, Kanoh S, Tsushima K, et al. Clarithromycin inhibits interleukin-13-induced goblet cell hyperplasia in human airway cells. Am J Respir Cell Mol Biol 2011; 45: 1075–1083. doi: 10.1165/rcmb.2010-0327OC [DOI] [PubMed] [Google Scholar]
- 77.Tse HN, Raiteri L, Wong KY, et al. High-dose N-acetylcysteine in stable COPD. The 1-year, double-blind, randomized placebo-controlled HIACE study. Chest 2013; 144: 106–118. doi: 10.1378/chest.12-2357 [DOI] [PubMed] [Google Scholar]
- 78.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659–668. doi: 10.1016/S0140-6736(17)31281-3 [DOI] [PubMed] [Google Scholar]
- 79.Lachowicz-Scroggins ME, Finkbeiner WE, Gordon ED, et al. Corticosteroid and long-acting β-agonist therapy reduces epithelial goblet cell metaplasia. Cin Exp Allergy 2017; 47: 1534–1545. doi: 10.1111/cea.13015 [DOI] [PubMed] [Google Scholar]
- 80.McGarvey LP, Birring SS, Morice AH, et al. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parrallel-group, placebo-controlled, phase 3 trials. Lancet 2022; 399: 909–923. doi: 10.1016/S0140-6736(21)02348-5 [DOI] [PubMed] [Google Scholar]
- 81.Mulhall P, Criner G. Non-pharmacological treatments for COPD. Respirology 2016; 21: 791–809. doi: 10.1111/resp.12782 [DOI] [PubMed] [Google Scholar]
- 82.Dang CV, Premkumar Reddy E, Shokat KM, et al. Drugging the “undruggable” cancer targets. Nat Rev Cancer 2017; 17: 502–508. doi: 10.1038/nrc.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iwano S, Asaoka Y, Akiyama H, et al. A possible mechanism for hepatotoxicity induced by BIRB-796, an orally active p38 mitogen-activated protein kinase inhibitor. J Appl Toxicol 2010; 31: 671–677. doi: 10.1002/jat.1622 [DOI] [PubMed] [Google Scholar]
- 84.Keeler SP, Wu K, Zhang Y, et al. A potent MAPK13-14 inhibitor prevents airway inflammation and mucus production. Am J Physiol Lung Cell Mol Physiol 2023; 325: L726–L740. doi: 10.1152/ajplung.00183.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol 2005; 17: 338–344. doi: 10.1016/j.coi.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 86.Haller V, Nahidino P, Forster M, et al. An updated patent review of p38 MAPK kinase inhibitors (2014–2019). Expert Opin Ther Pat 2020; 30: 453–466. doi: 10.1080/13543776.2020.1749263 [DOI] [PubMed] [Google Scholar]
- 87.Selness SR, Devraj RV, Devadas B, et al. Discovery of PH-797804, a highly selective and potent inhibitor of p38 MAP kinase. Bioorg Med Chem Lett 2011; 21: 4066–4071. doi: 10.1016/j.bmcl.2011.04.121 [DOI] [PubMed] [Google Scholar]
- 88.Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020; 395: 1407–1409. doi: 10.1016/S0140-6736(20)30858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kato T, Asakura T, Edwards CE, et al. Prevalence and mechanisms of mucus accumulation in COVID-19 lung disease. Am J Respir Crit Care Med 2022; 206: 1336–1352. doi: 10.1164/rccm.202111-2606OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuma Y, Sabio G, Bain J, et al. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem 2005; 280: 19472–19479. doi: 10.1074/jbc.M414221200 [DOI] [PubMed] [Google Scholar]
- 91.Robinson C, Xia K, Russell P, et al. A 12 week, randomized, double-blind, placebo controlled study of the efficacy of RV568 (JNJ49095397), a narrow spectrum kinase inhibitor, in COPD patients. Am J Respir Crit Care Med 2016; 193: A6843. doi: 10.1074/jbc.M414221200 [DOI] [Google Scholar]
- 92.Byers DE, Wu K, Dang-Vu G, et al. Triggering receptor expressed on myeloid cells-2 (TREM-2) expression tracks with M2-like macrophage activity and disease severity in COPD. Chest 2018; 153: 77–86. doi: 10.1016/j.chest.2017.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O'Callaghan C, Fanning LJ, Barry OP. p38δ MAPK: emerging roles of a neglected isoform. Int J Cell Biol 2014; 2014: 272689. doi: 10.1155/2014/272689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Remy G, Risco A, Inesta-Vaquera F, et al. Differential activation of p38MAPK isoforms by MKK6 and MKK3. Cellular Signaling 2010; 22: 660–667. doi: 10.1016/j.cellsig.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 95.Escos A, Risco A, Alsina-Beauchamp D, et al. p38γ and p38δ mitogen-activated protein kinases (MAPKs), new stars in the MAPK galaxy. Front Cell Dev Biol 2016; 4: 31. doi: 10.3389/fcell.2016.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Juyoux P, Galdadas J, Gobbo D, et al. Architecture of the MKK6-p38α complex defines the basis of MAPK specificity and activation. Science 2023; 381: 1217–1225. doi: 10.1126/science.add7859 [DOI] [PMC free article] [PubMed] [Google Scholar]