Table 1.
Comparing irreversible analogues of 1–10 against GACKIX N627C.[a]
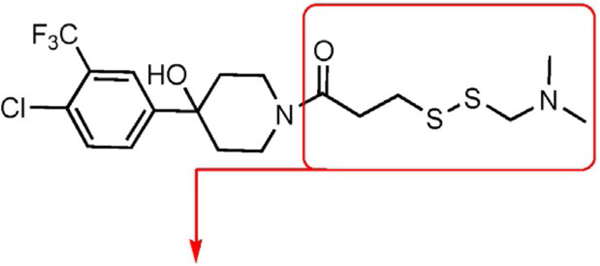
| ||||
|---|---|---|---|---|
| Compound | Dose-response | Fold inhibition | ||
| [mM] | MLL | pKID | ||
|
| ||||
| 1–10a |
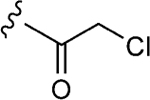
|
25 | 12±1 | 0.9±0.1 |
| 1–10b |
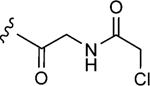
|
150 | 13±1 | 1.4±0.2 |
| 1–10c |
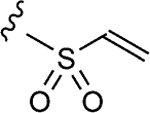
|
6.8 | 17±2 | 1.5±0.2 |
| 1–d |
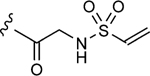
|
4.6 | 17±2 | 0.65±0.08 |
| 1–e |
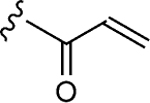
|
>500 | 14±2 | 0.88±0.09 |
| 1–f |
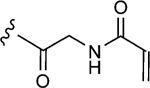
|
>500 | 5.9±0.6 | 0.66±0.07 |
The values were assessed by measuring the extent of GACKIX N627C labeled by means of Q-TOF LC-MS at various concentrations of compound. The fold inhibition values were obtained by comparing the dissociation constants () for the unlabeled (DMSO) KIX N627C construct with the values for the labeled KIX N627C–1–10 alkylator complex for both the MLL and pKID tracers. The values were measured from fluorescence polarization (FP) experiments that were performed in triplicate and errors reflect the standard deviation (SD) error.
