Abstract
Background
Rapid decreases in activated CD4+ and CD8+ (HLA-DR + and CD38+ co-expressed) T-lymphocytes have been described within 1–2 weeks of initiating direct-acting antiviral (DAA) therapy among chronic Hepatitis C (CHC) patients. However, it is not known whether these changes are maintained past sustained virologic response (SVR), particularly in those who are HIV/HCV-coinfected.
Methods
We investigated the changes in immune parameters of T-lymphocytes from pre-DAA therapy to post-SVR among HIV negative and HIV positive patients with CHC. Repeated measurements of activated CD4+ and CD8+ T cells were analyzed by flow cytometry at pre-DAA therapy, DAA therapy, end of treatment, SVR, and post-SVR. A general linear model for repeated measurements was used to estimate the mean outcome at each timepoint and change between timepoints.
Results
HCV-monoinfected (n = 161) and HIV/HCV-coinfected (n = 59) patients who achieved SVR with DAA therapy were predominately middle aged, male, black, and non-cirrhotic. At pre-DAA therapy, HCV-monoinfected patients had significantly higher CD4+ T cells and CD4+:CD8+ T-cell ratio, while significantly lower CD8+ and activated CD4+ and CD8+ T cells compared to HIV/HCV-coinfected patients (p < 0.0001). HCV-monoinfected and HIV/HCV-coinfected patients had a significant mean decrease from pre-DAA therapy to post-SVR year 1 for activated CD4+ (HCV-monoinfected: 4.8–3.9%, p < 0.0001; HIV/HCV-coinfected: 6.6–4.5%, p < 0.0001) and activated CD8+ T cells (HCV-monoinfected V: 13.8–11.8%, p = 0.0002; HIV/HCV-coinfected: 18.0–12.4%, p < 0.0001).
Conclusion
This longitudinal study showed CHC patients treated with DAA therapy had continued decrease of T-lymphocytes from start of DAA therapy to after achievement of SVR suggesting improvement as HCV clearance normalizes activated T-cell phenotype.
Keywords: HCV, HIV, Direct-acting antiviral therapy, T cell, Immune activation
Introduction
Hepatitis C virus (HCV) coinfection with HIV is common due to shared transmission routes, with an estimated 25% of HIV-infected patients being HIV/HCV-coinfected in the United States (US) [1, 2]. HIV infection is known to accelerate the natural history of HCV among HIV/HCV-coinfected patients resulting in faster rates of fibrosis progression, development of cirrhosis, hepatocellular carcinoma, decompensated liver disease, and death compared to HCV-monoinfected patients [3–6]. Loss of CD4+ T cells likely results in impaired HCV-specific immune response, which is blunted in HCV-monoinfected patients and exhausted in HIV/HCV-coinfected patients [7–10]. Numerous studies have shown that HIV/HCV-coinfected patients have higher levels of activated CD4+ and CD8+ T cells (HLA-DR+ and CD38+ co-expression on CD4+ and CD8+ T cells, respectively) as compared to patients with HCV-monoinfected, HIV-monoinfected, and healthy controls [7, 11–15].
HCV treatment has progressed from interferon (IFN)-based regimens with low HCV clearance to IFN-free regimens of direct-acting antiviral (DAA) drugs with high rates of successful HCV clearance known as sustained virologic response (SVR) [16]. Changes in immune parameters remain important to understand how the host resets the immune homeostasis after HCV clearance with DAA therapy following decades of chronicity. The state of T-cell activation has not been well understood in both IFN-based and IFN-free therapy. Najafi Fard et al. found patients with HCV clearance after IFN therapy had a decrease in immune activation as determined by CD38+ expression in CD4+ and CD8+ T cells. Among HCV-monoinfected (n = 18) and HIV/HCV-coinfected (n = 17) patients treated with DAA therapy, there was no significant change from pre-DAA therapy to SVR for activated CD4+ and CD8+ T cells and no difference between HCV-monoinfected and HIV/HCV-coinfected patients for all levels of activation markers (CD38+ and/or HLA-DR on CD4+ and CD8+ T cell) [15]. In a larger sample of HCV-monoinfected patients (n = 95) treated with DAA therapy with follow-up until SVR, Meissner et al. [17] showed that activated CD4+ and CD8+ T-lymphocytes decreased rapidly within 1–2 weeks of initiating DAA therapy. A decrease in activated T-cell post-DAA therapy would highlight positive outcome of DAA therapy in the recovery and normalization of the immune system without HCV infection. Few studies have analyzed HCV-specific T-cell function [18, 19]. These studies have shown an enhancement of both CD4+ and CD8+ T-cell responses with DAA therapy; however, no such studies have analyzed in HIV-coinfected patients nor timepoints beyond SVR. To date, the evaluation of these measures has not been possible for longer term immune status post-SVR, which is important with HCV clearance in the DAA therapy era. Today, with the availability of long-term follow-up in HCV-monoinfected and HIV/HCV-coinfected patients treated with DAA therapy in the US that achieved SVR, this study investigated the distribution of change, pre-DAA therapy to post-SVR, in T-lymphocyte immunophenotype.
Materials and methods
Study design
This is a retrospective, observational cohort constructed by combining HCV-monoinfected and HIV/HCV-coinfected patients from four clinical trials of DAA therapy conducted at the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) known as SPARE (www.clinicaltrials.gov #NCT01441180), SYN-ERGY (NCT01805882), ERADICATE (NCT01878799), and CONQUER (NCT02124044) [20–27]. Patient recruitment and enrollment for all four clinical trials was from the same HCV clinics in Washington DC (District of Columbia) [20–27]. Baseline demographics and clinical characteristics were previously collected in these clinical trials with repeated laboratory measurement for immune parameters from pre-DAA therapy to post-SVR. Additional post-SVR laboratory data were collected from patients enrolled after the clinical trials into NIH longitudinal study known as HEPPRO (NCT01350648). The clinical trial’s inclusion and exclusion criteria and the DAA regimen and duration have been previously described [20–27]. Briefly, the inclusion criteria were: male or female, at least 18 years of age, documented HCV genotype 1 (1a or 1b) and fibrosis staging, and confirmed CHC prior to enrollment. Plasma HCV RNA levels were measured using the real-time HCV assay [Abbott], with a lower limit of quantification LLOQ of 12 IU/mL. This assay was used to measure HCV RNA levels in all participants at all-timepoints [20–27]. The exclusion criteria were: pregnancy or breastfeeding, abnormal hematological and biochemical parameters at screening, and clinically significant illness other than HCV or a major medical disorder that may interfere with treatment and compliance, including any chronic liver disease or hepatocellular carcinoma [20–27]. For this study, CHC patients without HIV coinfection are referred to as HCV-coinfected, while CHC patients with HIV coinfection are referred to as HIV/HCV-coinfected.
Study population
The study population consisted of HCV-monoinfected and HCV/HIV-coinfected patients previously treated in one of the four DAA clinical trials and eligible if: they had achieved SVR, if HIV-coinfected on antiretroviral therapy (ART) prior to starting DAA therapy (stable, protocol-approved, ART for at least 8 weeks prior to starting DAA therapy with HIV RNA values of 50 copies/mL or fewer with a CD4 T-lymphocyte count of 100 cells/μL or greater), and at least one pre-DAA therapy and one post-SVR measurement (through May 5, 2017) for each immune outcome.
Immune measurements
Flow cytometry was conducted in the NIH Clinical Center laboratory on the day of collection and has been previously described [17]. Briefly, cells were stained with combinations of monoclonal antibodies and then lysed after staining with Optilyse C (Beckman Coulter, Hialeah, FL), washed twice, and resuspended in 500 μL of phosphate-buffered saline (Cambrex, Walkersville,MD). The samples were then analyzed immediately on a Becton–Dickinson FacsCanto flow cytometer (BD Biosciences, San Jose, CA). The four-color antibody panels used for cellular identification and enumeration are shown in Supplemental Table 1.
The primary immune outcomes were activated CD4+ and activated CD8+ T cells defined as a percentage of HLA-DR+ and CD38+ co-expression on CD4+ and CD8+, respectively. The secondary immune outcomes were CD4+, CD8+, and CD4+:CD8+ ratio. Both CD4+ and CD8+ T cells were reported as absolute cell count (cells/μL) and percentage, while CD4+:CD8+ ratio is the ratio between CD4+ and CD8+ (cells/μL) T cells. Exploratory immune parameters included: CD3+, CD3+CD25+, CD3+HLA-DR+, CD3+CD38+, activated CD3+, CD4+CD25+, CD4+HLA-DR+, CD4+CD38+, CD8+CD25+, CD8+HLA-DR+, CD8+CD38+, CD3+:CD8+ ratio, and CD3+:CD4+ ratio T cells.
Statistical analysis
Repeated measurements of immune outcomes were separated into five clinical timepoints: pre-DAA therapy, DAA therapy, end of treatment (EOT), SVR, and post-SVR. Pre-DAA was the single, closest measurement prior to start of any DAA therapy, yet no longer than 6 months prior to the start of any DAA therapy. Repeated measurements for each patient during DAA therapy (4–24 week time period depending on DAA duration which leads to SVR) and EOT (12 week time period before SVR). The SVR timepoint was the single measurement at SVR or the first available measurement within 6 months of SVR. The post-SVR timepoint was repeated measurements after SVR for each patient as available and categorized into year 1 (SVR until 1.5 years) and year 2 (1.5–2.5 years). A general linear model for repeated measurements was used for the clinical timepoints (pre-DAA therapy, DAA therapy, EOT, SVR, and post-SVR [year 1–2]) with unstructured variance/covariance matrices to generate restricted maximum likelihood estimates. The unadjusted and adjusted immune outcome mean estimates (95% confidence interval [CI]) for each timepoint were reported for HCV-monoinfected and HIV/HCV-coinfected patients (adjusted for age, sex, race, ART, and fibrosis). The mean change of outcomes was assessed between pre-DAA therapy and post-SVR year 1. All analyses were conducted in SAS version 9.4 (Gary, NC).
Results
HCV-monoinfected (n = 161) and HIV/HCV-coinfected (n = 59) patients who achieved SVR with DAA therapy were predominately middle aged (median 56 years), male (66%), black (79%), and non-cirrhotic (92%). The baseline demographics and clinical characteristics of patients were similar among HCV-monoinfected and HIV/HCV-coinfected patients (Table 1). Among HIV/HCV-coinfected patients, 19% (n = 11) had ART duration of 1–4 years and 81% (n = 48) had > 4 years of ART duration prior to starting DAA therapy. There was no significant difference in the immune parameters between the two ART duration categories for HIV/HCV-coinfected patients.
Table 1.
Baseline characteristics of HCV-monoinfected and HIV/ HCV-coinfected, treated with DAAs and who achieved SVR
| Characteristic | HCV (n = 161) | HIV/HCV (n = 59) |
|---|---|---|
|
| ||
| Median age (IQR) (years) | 56 (52–60) | 57 (50–62) |
| Male [n (%)] | 103 (69) | 43 (73) |
| Race [n (%)] | ||
| White | 31 (19) | 13 (22) |
| Black | 128 (80) | 46 (78) |
| Other | 2 (1) | 0 (0) |
| Hispanic [n (%)]a | 3 (2) | 5 (21) |
| Median body mass index (IQR) (kg/m2) | 28 (25–32) | 27 (23–30) |
| Fibrosis stage [n (%)]b | ||
| 0 | 20 (12) | 13 (22) |
| 1 | 64 (40) | 27 (47) |
| 2 | 11 (7) | 3 (5) |
| 3 | 51 (32) | 12 (21) |
| 4 | 15 (9) | 3 (5) |
| Cirrhosis [n (%)]b | 15 (9) | 3 (5) |
| Median HCV RNA log10 IU/mL (IQR) | 6.2 (5.7–6.5) | 5.9 (5.4–6.5) |
| HCV treatment naïve [n (%)] | 174 (90) | 51 (82) |
| HCV genotype 1a [n (%)] | 112 (70) | 40 (68) |
| Retreated with DAA therapy [n (%)] | 15 (9) | 0 (0) |
| DAA regimen for SVR [n (%)] | ||
| ASV/DCV | 0 (0) | 7 (12) |
| ASV/DCV/BCV | 0 (0) | 17 (29) |
| LDV/SOF | 35 (22) | 35 (59) |
| LDV/SOF/GS-9451 | 65 (40) | 0 (0) |
| LDV/SOF/GS-9669 | 19 (12) | 0 (0) |
| LDV/SOF/GS-9669/GS-9451 | 4 (2) | 0 (0) |
| SOF/RBV | 38 (24) | 0 (0) |
| DAA duration for SVR [n (%)] | ||
| 4 weeks | 12 (7) | 0 (0) |
| 6 weeks | 76 (47) | 0 (0) |
| 12 weeks | 35 (22) | 52 (88) |
| 24 weeks | 38 (24) | 7 (12) |
| ART [n (%)] | N/A | 59 (100) |
| ART regimen [n (%)] | ||
| ABC/3TC + RAL | 10 (17) | |
| TDF/FTC + RAL | 24 (41) | |
| TDF/FTC/EFV | 14 (24) | |
| TDF/FTC/EFV + RAL | 1 (2) | |
| TDF/FTC/RPV | 7 (12) | |
| TDF/FTC/RPV + RAL | 3 (5) | |
DAA direct-acting antiviral, ASV asunaprevir, DCV daclatasvir, BCV beclabuvir, LDV ledipasvir, SOF sofosbuvir, RBV ribavirin, ART antiretroviral therapy, ABC abacavir, 3TC lamivudine, RAL raltegravir, TDF tenofovir, FTC emtricitabine, EFV efavirenz, RPV rilpivirine
Missing for 38 HCV and 35 HIV/HCV patients
Missing for 1 HIV/HCV patient
At baseline, pre-DAA therapy, HCV-monoinfected patients had a significantly higher CD4+ T cells (cells/μL and %) and CD4+:CD8+ ratio, while significantly lower CD8+ T cells (cells/μL and %), activated CD4+, and activated CD8+ T cells compared to HIV/HCV-coinfected patients (Table 2). Among the exploratory immune parameters, HIV/HCV-coinfected patients had significantly higher CD3+HLA-DR+, CD3+CD38+, activated CD3+, CD3:CD4+ ratio, CD4+HLA-DR+, CD4+CD38+, and CD8+HLA-DR+T cells and lower CD3+CD25+ and CD3+:CD8+ ratio T cells compared to HCV-monoinfected patients (Supplemental Table 2).
Table 2.
Pre-DAA therapy immune outcome (median [interquartile range]) among HCV-monoinfected and HIV/HCV-coinfected treated with DAAs and who achieved SVR
| HCV | HIV/HCV |
||
|---|---|---|---|
| (n = 161) | (n = 59) | p value* | |
|
| |||
| CD4+ (cells/μL) | 951 (754–1203) | 629 (453–835) | < 0.0001 |
| CD4+(%) | 49 (44–53) | 34 (25–42) | < 0.0001 |
| CD8+ (cells/μL) | 425(314–616) | 731 (465–1010) | < 0.0001 |
| CD8+(%) | 23(19–28) | 39 (32–46) | < 0.0001 |
| CD4+:CD8 ratio | 2.1 (1.6–2.8) | 0.9 (0.6–1.3) | < 0.0001 |
| Activated CD4+ (%) | 4.0 (3.0–5.0) | 6.0 (4.0–8.0) | < 0.0001 |
| Activated CD8+ (%) | 11.0 (9.0–17.0) | 18.0 (12.0–23.0) | < 0.0001 |
p value from non-parametric, two-tailed Wilcoxon rank sum test
T-cell activation
The mean activated CD4+ and activated CD8+ T cells, from pre-DAA therapy to post-SVR among HCV-monoinfected and HIV/HCV-coinfected patients is shown in Fig. 1. The analysis was reported until post-SVR year 1 (since all patients had measurement at the post-SVR year 1 timepoint compared to only 29% in post-SVR year 2 timepoint) with the crude outcome as the adjusted analysis was no different.
Fig. 1.
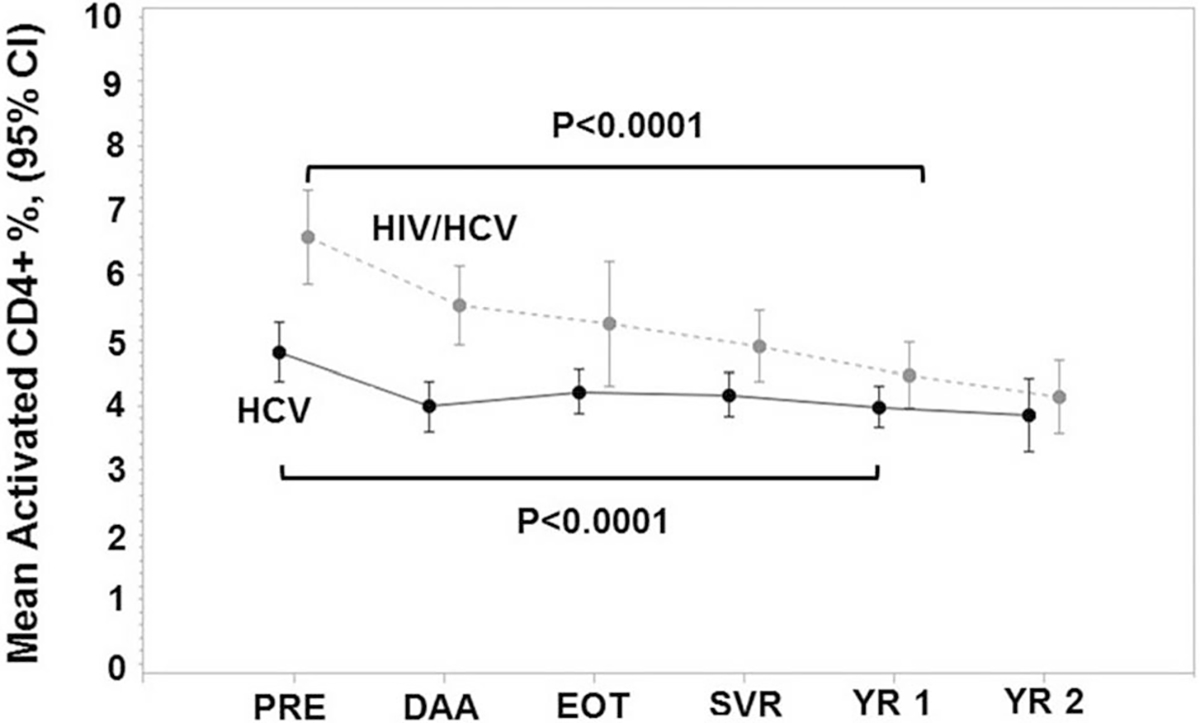
Mean ± 95% confidence interval (CI) for percentage of CD4+ T cells with an activated HLA-DR+ and CD38+ phenotype at pre-DAA therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1–2 (YR 1–2) among HCV-monoinfected (n = 161) and HIV/HCV-coinfected (n = 59) patients treated with DAA therapy and achieved SVR
The mean activated CD4+ T cells decreased from pre-DAA therapy to post-SVR year 1 in HCV-monoinfected (4.8–3.9%, p < 0.0001) and HIV/HCV-monoinfected (6.6–4.5%, p < 0.0001) patients (Fig. 1). There was a significant difference by HIV-coinfection status at each timepoint, which disappears at post-SVR year 1 (Table 3). The mean change in activated CD4+ T cells (pre-DAA therapy to post-SVR year 1) between HCV-monoinfected and HIV/HCV-coinfected patients was significantly different (p < 0.0001).
Table 3.
Mean ± 95% confidence interval (CI) for percentage of CD4+ and CD8+ T cells with an activated HLA-DR+ and CD38 + phenotype at pre-DAA therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1–2 (YR 1–2) among HCV-monoinfected (n = 160) and HIV/HCV-coinfected (n = 58) patients treated with DAAtherapy and achieved SVR
| HCV (n = 161) |
HIV/HCV (n = 59) |
p value* | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
|
| |||||||
| Activated CD4+ (%) | |||||||
| Pre-DAA therapy | 4.8 | 5.3 | 4.4 | 6.6 | 7.3 | 5.9 | < 0.0001 |
| DAA therapy | 4.0 | 4.4 | 3.6 | 5.5 | 6.2 | 4.9 | < 0.0001 |
| End of treatment | 4.2 | 4.6 | 3.9 | 5.3 | 6.2 | 4.3 | 0.05 |
| SVR | 4.2 | 4.5 | 3.8 | 4.9 | 5.5 | 4.4 | 0.02 |
| Post-SVR Year 1 | 4.0 | 4.3 | 3.7 | 4.5 | 5.0 | 4.0 | 0.11 |
| Post-SVR year 2 | 3.8 | 4.4 | 3.3 | 4.1 | 4.7 | 3.6 | 0.50 |
| Activated CD8+ (%) | |||||||
| Pre-DAA therapy | 13.8 | 15.1 | 12.4 | 18.0 | 20.2 | 15.8 | 0.001 |
| DAA therapy | 12.1 | 13.3 | 10.8 | 15.3 | 17.3 | 13.4 | 0.01 |
| End of treatment | 12.7 | 13.9 | 11.4 | 14.0 | 17.0 | 11.0 | 0.42 |
| SVR | 12.3 | 13.6 | 11.1 | 13.3 | 15.3 | 11.4 | 0.38 |
| Post-SVR year 1 | 11.8 | 13.0 | 10.7 | 12.4 | 14.2 | 10.6 | 0.61 |
| Post-SVR year 2 | 10.9 | 12.7 | 9.1 | 13.3 | 15.2 | 11.3 | 0.08 |
p value for the mean difference between HCV-monoinfected and HIV/HCV-coinfected patients
The mean activated CD8+ T cells decreased from pre-DAA therapy to post-SVR year 1 in HCV-monoinfected (13.8–11.8%, p = 0.0002) and HIV/HCV-coinfected (18.0–12.4%, p < 0.0001) patients (Fig. 2). Mean activated CD8+ was different by HIV-coinfection status at pre-DAA therapy (p ≤ 0.0001) and DAA therapy (p = 0.01), but disappears at EOT, SVR, and post-SVR year 1 (Table 3). The mean change in activated CD8+ T cells (pre-DAA therapy to post-SVR year 1) between HCV-monoinfected and HIV/HCV-coinfected patients was significantly different (p = 0.0002).
Fig. 2.

Mean activated ± 95% confidence interval (CI) for percentage of CD8+ T cells with an activated HLA-DR+ and CD38+ phenotype at pre-DAA therapy (PRE), DAA therapy (DAA), end of treatment (EOT), sustained virologic response (SVR), and post-SVR year 1–2 (YR 1–2) among HCV (n = 161) and HIV/HCV (n = 69) patients treated with DAA therapy and achieved SVR
Secondary outcomes
The mean CD4+, CD8+, and CD4+:CD8+ T cells among HCV-monoinfected and HIV/HCV-coinfected patients are shown in Supplemental Fig. 1 and Table 3 from pre-DAA therapy to post-SVR.
Discussion
Chronic Hepatitis C patients in this study had improved immune activation in T cells with SVR following DAA therapy, which persisted up to after SVR. CD4+ and CD8+ T cells with activated phenotype (HLA-DR+ and CD38+) decreased from pre-DAA therapy to post-SVR in HIV/HCV-coinfected and HCV-monoinfected patients, suggesting that HCV clearance normalizes activated T-cell phenotype. With DAA therapy, this study illustrated continued improvements in immune activation in T cells after SVR, likely due to a combination of three factors: first, recovery of the immune system after HCV elimination leads to normalization of T-cell phenotypes, second, improved liver function aiding immune recovery and redistribution of T cells, and third, these effects are blunted in the presence of HIV coinfection, which is most likely the result of persistent immune activation despite ongoing ART. Elimination of HCV results in recovery of immune function in many components of immune system [18]. Elimination of HCV with a non-immune based therapy removes the chronic antigenic stimulation of immune cells. In addition, alanine transaminase (ALT) and aspartate aminotransferase (AST) demonstrated immediate improvement during DAA therapy in this study cohort of HCV-monoinfected and HIV/HCV-coinfected patients and is consistent with a quiescent immune system post-SVR regardless of HIV-coinfection status. Immune recovery could result in liver improvements as measured by fibrosis stage. Patients with greater liver improvement could have different immune recovery compared to patients with minimal liver improvements, as demonstrated by fibrosis stage at start of DAA therapy. If accurate, this would indicate immune recovery is likely due to reduced migration of lymphocytes to the inflamed liver resulting in regression of fibrosis over time and not SVR, a surrogate marker for the elimination of HCV. In this study, the distribution of fibrosis staging between HCV-monoinfected and HIV/HCV-coinfected patients was similar before the start of DAA therapy; however, staging after achievement of SVR was not available at the time of analysis. The current literature on DAA therapy has not explored the impact of liver improvements with immune parameters. Potential challenges include long-term post-SVR liver fibrosis data and that fibrosis staging (F0–F4) is not linear. Future studies focusing on organ systems outside the liver should address how regression of liver fibrosis may impact their study outcomes beyond achievement of SVR.
With DAA therapy, both Najafi Fard et al. and Meissner et al. showed different results for change in activated CD4+ and CD8+ T cell [15, 17]. The difference could be due to the study population (Rome, Italy vs. District of Columbia, USA), small sample size (18 HCV-monoinfected and 17 HIV/HCV-coinfected vs. 95 HCV-coinfected), and different follow-up timepoints [15, 17]. Najafi Fard et al. also reported no difference in the level of activation of different T-cell subsets between HCV-monoinfected and HIV/HCV-coinfected patients, which is surprising since this study and others have shown HIV/HCV-coinfected patients have higher levels of activated CD4+ and CD8+ T cells compared to patients with HCV-monoinfected, HIV-monoinfected, and healthy controls [7, 11–15]. While both the previous studies did not have post-SVR follow-up, our study had a larger sample size with post-SVR follow-up analysis including the same patients used by Meissner et al. [17] yet with longer follow-up after SVR.
The study had limitations as a result of using secondary data from clinical trials. The clinical trial inclusion criteria could have led to potential selection bias in an observational cohort. There was no difference in demographic and clinical characteristics among patients who had longer follow-up data in HEPPRO than those who did not, except more HIV-coinfected patients likely as a result of staying in follow-up because many of them were already connected to the research clinic for their HIV. However, since our inferences are related to an underlying biological mechanism, it is unlikely that the response observed in our study would differ among HCV- and HIV-infected patients of similar disease progression who did not enroll in the trials from which our study population arose. Second, while there were different DAA drugs and duration, patients were selected on SVR achievement and there is no evidence in the literature of a direct biological effect of DAA drugs or duration of treatments with immune parameters. Finally, fibrosis staging after achievement of SVR was not available at the time of analysis. Repeated laboratory measurements were at non-uniform time periods; however, the use of general linear model for repeated measurements with Proc MIXED allowed for analyzing unbalanced repeated measurements.
The study has strengths and innovations including the enrollment of an at risk urban study population; the use of a longitudinal study design; and the evaluation of immune outcomes. The study population included a large African-American cohort with both HCV-monoinfected and HIV/HCV-coinfected patients treated with DAA therapy in the US. The homogenous population in the community-based clinics of Washington DC strengthens our external validity to similar urban cohorts in the US. This study used an established infrastructure of four clinical trials to construct an observational, retrospective cohort with repeated immune data among HCV-monoinfected and HIV/HCV-coinfected patients who achieved SVR with DAA therapy and with longer post-SVR follow-up visits than prior studies.
For the first time, we have a disease model in which chronic HCV viral infection that persisted for over 30 years is suddenly eliminated in 12 weeks with DAA therapy. Prior to this, recovery of an immune system under continuous assault with HCV chronic replication has not been studied and was poorly understood. The results from this study provide a preliminary step in our understanding of viral–host interactions immediately after HCV elimination, but more importantly providing long-term, post-SVR follow-up, which has not been assessed in the current literature. This longitudinal study showed HCV-monoinfected and HIV/HCV-coinfected patients treated with DAA therapy had continued improvements in immunological recovery in T-cell activation during DAA therapy and after achievement of SVR, highlighting a novel long-term clinical benefit of HCV clearance on morbidity.
Supplementary Material
Footnotes
Compliance with ethical standards
Conflict of interest B. Emmanuel, S.S. El-Kamary, L.S. Magder, K.A. Stafford, M.E. Charurat, B. Poonia, C. Chairez, M. McLaughlin, C. Hadigan, H. Masur, and S. Kottilil have no conflict of interest.
Ethical standard The primary study was approved by the NIAID IRB and conducted in compliance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and regulatory requirements.
Informed consent All patients provided written informed consent and all protocols were approved by the NIH/NIAID Institutional Review Board.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12072-019-09941-8) contains supplementary material, which is available to authorized users.
References
- 1.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res 2010;85:303–315 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Viral Hepatitis Surveillance United States, 2014 [Internet]. 2014. http://www.cdc.gov/hepatitis/statistics/2014surveillance/index.htm
- 3.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of Hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001;33:562–569 [DOI] [PubMed] [Google Scholar]
- 4.Hernandez MD, Sherman KE. HIV/HCV coinfection natural history and disease progression, a review of the most recent literature. Curr Opin HIV AIDS 2011;6:478–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotman Y, Liang TJ. Coinfection with Hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J Virol 2009;83:7366–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaegel-Faucher O, Bregigeon S, Cano CE, Obry-Roguet V, Nicolino-Brunet C, Tamalet C, et al. Impact of Hepatitis C virus coinfection on T-cell dynamics in long-term HIV-suppressors under combined antiretroviral therapy. AIDS 2015;29:1505–1510 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez VD, Falconer K, Blom KG, Reichard O, Mørn B, Laursen AL, et al. High levels of chronic immune activation in the T-cell compartments of patients coinfected with Hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol 2009;83:11407–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claassen MA, Janssen HL, Boonstra A. Role of T cell immunity in hepatitis C virus infections. Curr Opin Virol 2013;3:461–467 [DOI] [PubMed] [Google Scholar]
- 9.Harcourt G, Gomperts E, Donfield S, Klenerman P. Diminished frequency of hepatitis C virus specific interferon γ secreting CD4+ T cells in human immunodeficiency virus/hepatitis C virus coinfected patients. Gut 2006;55:1484–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim AY, Lauer GM, Ouchi K, Addo MM, Lucas M, Zur Wiesch JS, et al. The magnitude and breadth of hepatitis C virus-specific CD8+ T cells depend on absolute CD4+ T-cell count in individuals coinfected with HIV-1. Blood.2005;105:1170–1178 [DOI] [PubMed] [Google Scholar]
- 11.Feuth T, Arends JE, Fransen JH, Nanlohy NM, van Erpecum KJ, Siersema PD, et al. Complementary role of HCV and HIV in T-Cell activation and exhaustion in HIV/HCV coinfection. PLoS ONE [Internet] 2013. [cited 2015 Oct 15];8. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3598709/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs A, Al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8+ T cell activation in women coinfected with human immunodeficiency virus type 1 and Hepatitis C virus. J Infect Dis 2008;197:1402–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandberg JK, Falconer K, Gonzalez VD. Chronic immune activation in the T cell compartment of HCV/HIV-1 co-infected patients. Virulence 2010;1:177–179 [DOI] [PubMed] [Google Scholar]
- 14.Hodowanec AC, Brady KE, Gao W, Kincaid SL, Plants J, Bahk M, et al. Characterization of CD4+ T-cell immune activation and interleukin 10 levels among HIV, Hepatitis C virus, and HIV/HCV-coinfected patients. JAIDS J Acquir Immune Defic Syndr 2013;64:232–240 [DOI] [PubMed] [Google Scholar]
- 15.Najafi Fard S, Schietroma I, Corano Scheri G, Giustini N, Serafino S, Cavallari EN, et al. Direct-acting antiviral therapy enhances total CD4+ and CD8+ T-cells responses, but does not alter T-cells activation among HCV mono-infected, and HCV/HIV-1 co-infected patients. Clin Res Hepatol Gastroenterol [Internet] 2017. [cited 2018 Jan 23]. http://www.sciencedirect.com/science/article/pii/S2210740117302620 [DOI] [PubMed] [Google Scholar]
- 16.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA 2014;312:631–640 [DOI] [PubMed] [Google Scholar]
- 17.Meissner EG, Kohli A, Higgins J, Lee Y-J, Prokunina O, Wu D, et al. Rapid changes in peripheral lymphocyte concentrations during interferon-free treatment of chronic hepatitis C virus infection. Hepatol Commun 2017;1:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett L, Shivasabesan G, Wang C, Osinusi A, Kohli A, Meissner EG, et al. 1 altered HCV specific T cell immunity very early in interferon free HCV DAA therapy. J Hepatol 2013;58(Supplement 1):S1 [Google Scholar]
- 19.Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol 2014;61:538–543 [DOI] [PubMed] [Google Scholar]
- 20.Osinusi A, Meissner EG, Lee Y-J, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for Hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA 2013;310:804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohli A, Osinusi A, Sims Z, Nelson A, Meissner EG, Barrett LL, et al. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet 2015;385:1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson EM, Kattakuzhy S, Sidharthan S, Sims Z, Tang L, McLaughlin M, et al. Successful retreatment of chronic HCV genotype-1 infection with ledipasvir and sofosbuvir after initial short course therapy with direct-acting antiviral regimens. Clin Infect Dis 2016;62:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osinusi A, Kohli A, Marti MM, Nelson A, Zhang X, Meissner EG, et al. Re-treatment of chronic Hepatitis C virus genotype 1 infection after relapse: an open-label pilot Study. Ann Intern Med 2014;161:634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kattakuzhy S, Wilson E, Sidharthan S, Sims Z, McLaughlin M, Price A, et al. Moderate sustained virologic response rates with 6-week combination directly acting anti-Hepatitis C virus therapy in patients with advanced liver disease. Clin Infect Dis 2016;62:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohli A, Kattakuzhy S, Sidharthan S, Nelson A, McLaughlin M, Seamon C, et al. Four-week direct-acting antiviral regimens in noncirrhotic patients with Hepatitis C virus genotype 1 infection: an open-label, nonrandomized trial anti-HCV regimens in noncirrhotic patients with genotype 1 infection. Ann Intern Med 2015;163:899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osinusi A, Townsend K, Kohli A, Nelson A, Seamon C, Meissner EG, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA 2015;313:1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenthal ES, Howard L, Purdy J, McLaughlin M, Kattakuzhy S, Kohli A, et al. Virologic response following asunaprevir/daclatasvir with or without beclabuvir for treatment of HCV genotype 1 in patients co-infected with HIV. J Hepatol 2016;64:S760–S761 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


