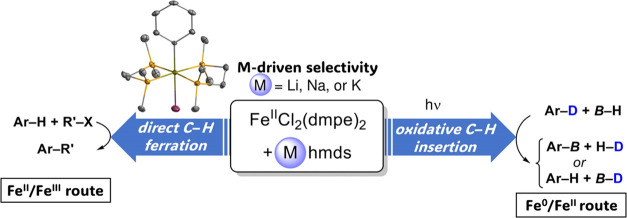
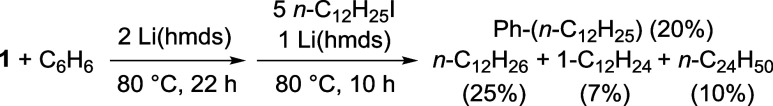Abstract
The association of the ferrous complex FeIICl2(dmpe)2 (1) with alkali bases M(hmds) (M = Li, Na, K) proves to be an efficient platform for the activation of Ar–H bonds. Two mechanisms can be observed, leading to either Ar–FeII species by deprotonative ferration or hydrido species Ar–FeII–H by oxidative addition of transient Fe0(dmpe)2 generated by reduction of 1. Importantly, the nature of the alkali cation in M(hmds) has a strong influence on the preferred path. Starting from the same iron precursor, diverse catalytic applications can be explored by a simple modulation of the MI cation. Possible strategies enabling cross-coupling using arenes as pro-nucleophiles, reductive dehydrocoupling, or deuteration of B–H bonds are discussed.
Keywords: iron, C–H activation, cross-coupling, dehydrocoupling, boranes, mechanisms
Introduction
Metalation of C–H bonds by transition metals (TMs) is a pivotal transformation in organometallic catalysis, leading to reactive organotransition species. Such compounds are key intermediates in numerous classic transformations such as cross-couplings, carbometalations, electrophilic additions, etc. Moreover, use of hydrocarbons as pro-nucleophiles in metalation processes is a more atom-economic alternative to main-group nucleophiles (e.g., RMgX, RZnX, RLi, etc.) used as transmetalation reagents.1
Catalytic processes relying on a C–H metalation step with a TM source usually require the C–H bond target to be turned as a metalated intermediate at each catalytic cycle. Among this variety of organometallic species, organoiron compounds featuring well-defined C–Fe bonds have kept drawing particular attention during the last few decades.2 Indeed, iron catalysts are appealing candidates for synthetic applications, since they are a sustainable and cheap alternative to noble metals.3 Formation of well-defined organoiron compounds is still particularly challenging due to the intrinsic instability of the C–Fe bond. For the same reason, a fine mechanistic analysis of the reactivity patterns followed by such species is also a delicate question.4 Indeed, depending on the iron oxidation state, C–Fe bonds can undergo fast homolytic cleavage,5 and two-electron reductive eliminations also easily occur from multiple coordinated Rn[FeII] complexes.6
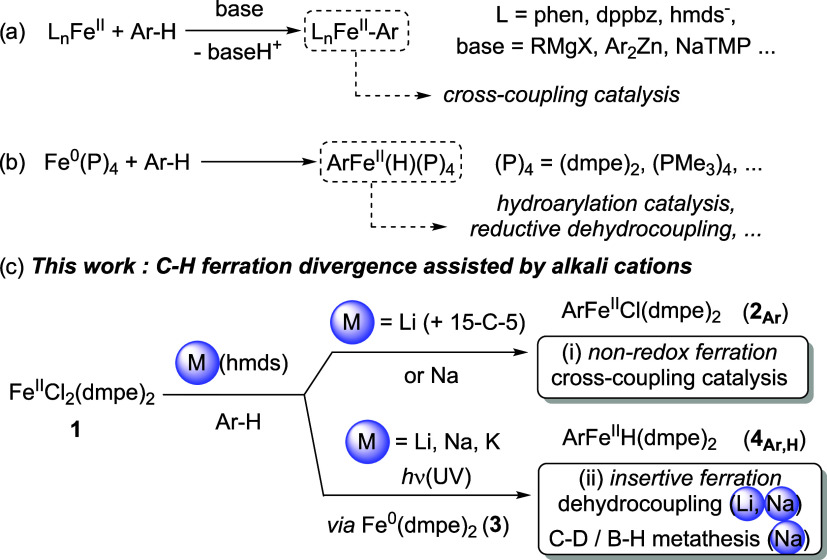
Two very distinct strategies can be adopted for the generation of C–Fe bonds from C–H substrates and are particularly applied to the activation of Ar–H bonds. The first one relies on a direct ferration of the C–H bond, which is deprotonated by a suitable base at a Lewis-acidic iron center (Scheme 1a). Classic directed deprotonative ferrations were for example reported by Knochel, using FeII(TMP)2·2MgCl2·4LiCl (TMP = 2,2,6,6-tetramethylpiperidine).7 Nakamura also reported an efficient aryl–aryl cross-coupling involving a directed CAr–H deprotonation mediated by PhMgBr in the presence of a ZnII source, the iron catalyst being associated with 1,10-phenanthroline;8a the same group also recently reported improved methods of C–H functionalizations relying on a key deprotonative ferration.8b−8d Similarly, Neidig and Ackermann demonstrated that directed CAr–H activation of triazole derivatives at an FeII intermediate followed by cross-coupling can be mediated by the Ar2Zn/dppbz combination (dppbz = 1,2-bis(diphenylphosphino)benzene).9 Hevia recently reported an example of nondirected ferration of unactivated arenes (including C6H6) using FeII(hmds)2 (hmds– = N(SiMe3)2–) and NaTMP relying on a cooperative effect between the FeII and NaI cations.10
Scheme 1. Classic Strategies for the Generation of C–Fe Bonds.
(a) Deprotonative ferration; (b) oxidative insertion; (c) this work: non-redox and oxidative ferrations unlocked by alkali additives.
A second strategy consists in the ferration of C–H bonds by oxidative insertion of a Fe0 complex, leading to the formation of heteroleptic hydrido-organoiron(II) species (Scheme 1b). Early reports by Field and Tolman showed that transient Fe0(dmpe)2 (dmpe = 1,2-bis(dimethylphosphino)ethane) generated by photolysis of the ferrous precursor FeII(R)(R′)(dmpe)2 (R,R′ = H, Me or 2-Np, H) easily underwent insertion into a variety of Csp2–H and Csp3–H bonds.11 Similar Fe0(P)4 systems have been reported ever since. It was also recently demonstrated that in situ generated Fe0(dmpe)2 was an efficient catalyst for the reductive dehydrocoupling of CAr–H and B–H bonds in order to access arylpinacolboranes Ar–B(pin),12a or for hydrogen isotopic exchange (HIE) between alkenes or arenes and CD3OD.12b In the latter case, Fe0(dmpe)2 is generated from 1 in the presence of pinacolborane (pinBH) and CD3OD under blue light irradiation. Fe0(PMe3)4 also proved to efficiently promote ortho-directed insertion into CAr–H bonds13 and was successfully used by several groups such as Kakiuchi’s and Ackermann’s as a catalyst for the addition of Ar–H bonds onto alkenes14 and allenes,15 or for the homoallylation of aryl ketones using methylenecyclopropanes.16
The two activation pathways of a CAr–H bond depicted in Scheme 1a,b lead to strongly different aryliron(II) intermediates. In the first case, the H atom coming from the activated CAr–H bond acts as a proton H+ trapped by a basic partner and is not transferred onto the final product (Scheme 1a). Conversely, in the second case, this H atom affords a Fe-bound hydride H–, the formal two-electron reduction of the CAr–H bond being ensured by the Fe0/FeII couple. This leads to possible catalytic transformations in which both the Ar group and the H atom are transferred onto a suitable target (Scheme 1b). The two aforementioned strategies (that is nonredox deprotonation, Scheme 1a, or two-electron oxidative insertion, Scheme 1b) usually require drastically different iron precursors and are extremely sensitive to ligand effects. Moreover, generation of efficient precursors of Fe0(dmpe)2 demands the prior multistep synthesis and purification of extremely air-sensitive complexes such as FeII(R)2(dmpe)2 (R = H, Me) or FeII(2-Np)(H)(dmpe)2, the latter being formed by reduction of FeCl2(dmpe)2 (1) by sodium naphthalenide (NaNp).11a
In this report, we demonstrate that the well-known, easily accessible complex FeCl2(dmpe)2 (1) can be associated with alkali amides M(hmds) (M = Li, Na, K) to perform nondirected CAr–H activations by either deprotonative or oxidative addition paths (Scheme 1c, paths i–ii). Ar–[FeII] compounds formed by deprotonation can be active in cross-coupling with organic iodides, a key demetalation of a dmpe ligand being ensured by Lewis-acidic cations such as LiI (as well as MgII) in the catalytic process. When Ar–[FeII]–H compounds are obtained, use of LiI salts can selectively promote the borylation of Ar–H into Ar–B(pin) by a Ar–H/(pin)B–H dehydrocoupling. On the other hand, use of NaI salts affords a near equimolar mixture of ArD–B(pin) borylation product along with hydrogen isotopic exchange (HIE) between C6D6 and (pin)B–H (formation of (pin)B–D).
This finding opens the door to particularly appealing synthetic strategies, since a simple ferrous precursor 1, readily synthesized from commercially available FeCl2 and dmpe, combined with the suitable alkali salt M(hmds), can be used as a single precursor for generating organoiron species active in either cross-coupling or in dehydrocoupling/HIE catalysis. The role of the alkali cation in the speciation of the iron-containing species formed by action of M(hmds) on the precursor 1 is discussed. Characterization of key CAr–H activated products featuring Ar–FeII bonds was probed by 31P and 1H NMR spectroscopies, X-ray diffraction, and Mössbauer spectroscopy.
Speciation of the Iron-Containing Species upon Treatment of 1 by M(hmds) (M = Li, Na, K)
We began by investigating the distribution of species, which can be formed under treatment of 1 by M(hmds) (M = Li, Na, K) in C6D6 at room temperature, under thermal activation (80 °C) or under UV irradiation (365 nm). Our main objective was to evidence the different C–D activation compounds that can be formed under those conditions. Treatment of 1 by M(hmds) in C6D6 can afford a distribution of species, which for some of them have been previously described in the literature (complexes 2–5, Table 1).17
Table 1. Speciation of Complexes 1–5 Obtained by Treatment of 1 by n equiv of M(hmds) (M = Li, Na, K) under Various Conditions.
| entry | M (n) | t (h) | T (°C) | solventa | light | 1 | 2C6D5 | 3′ | 3″ | 4C6D5,D | 5– |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Li (2) | 72 | 20 | C6D6 | no | 97 | 0 | 0 | 0 | 0 | 2 |
| 2 | Na (2) | 72 | 20 | C6D6 | no | 0 | 0 | 40 | 7 | 5 | 4 |
| 3 | K (2) | 72 | 20 | C6D6 | no | 5 | 0 | 10 | 20 | 4 | 7 |
| 4 | Li (2) | 72 | 20 | C6D6 | yes | 5 | 0 | 0 | 0 | 40b | 0 |
| 5 | Na (2) | 72 | 20 | C6D6 | yes | 0 | 0 | 0 | 0 | 48b | 2 |
| 6 | K (2) | 72 | 20 | C6D6 | yes | 5 | 0 | 0 | 0 | 45b | 5 |
| 7 | Li (10) | 22 | 20 | C6D6/THF | no | 97 | 0 | 0 | 0 | 0 | 2 |
| 8 | Na (10) | 22 | 20 | C6D6/THF | no | 0 | 7 | 16 | 8 | 5 | 3 |
| 9 | K (10) | 22 | 20 | C6D6/THF | no | 0 | 0 | 8 | 4 | 3 | 10 |
| 10 | Li (10) | 22 | 80 | C6D6/THF | no | 0c | 0c | 0c | 0c | 0c | 18c |
| 0d | 0d | 0d | 0d | 38b,d | 15b,d | ||||||
| 11 | Na (10) | 22 | 80 | C6D6/THF | no | 0 | 0 | 12 | 13 | 0 | 0 |
| 12 | K (10) | 22 | 80 | C6D6/THF | no | 0 | 0 | 7 | 5 | 0 | 2 |
Neat C6D6 or a 9:1 mixture of C6D6/THF were used.
Mixture of cis:trans-4C6D5,D in a ca. 4:3 ratio.
Spectra recorded after cooling down the samples at room temperature during 1 h.
Spectra recorded after 10 additional hours at room temperature.
In neat C6D6, 1 remained mostly unreacted upon action of 2 equiv of Li(hmds) after 72 h at room temperature (97%, Table 1, entry 1), and less than 5% of 5– were formed. On the other hand, action of 2 equiv of Na(hmds) under the same conditions led to an almost total conversion of 1. Reduced Fe0 complexes 3′ and 3″ were obtained (respectively 40 and 7%, entry 2), as well as the C–D activated complex 4C6D5,D (5%). Formation of traces of the latter suggests that transient Fe0(dmpe)2 (3) was obtained under those conditions, followed by oxidative insertion in the C–D bond of C6D6 (Scheme 1c, path (ii)). Use of K(hmds) (entry 3) afforded a similar distribution of 3′, 3″, and 4C6D5,D (respectively, 10, 20, and 4%). It is of note that the possibility to use K(hmds) as a reductant of ferrous complexes stabilized by noninnocent coordinating platforms has already been reported in the past.18a Similarly, Lei demonstrated that Na(hmds) was able to perform one-electron reduction of CuII salts into dicoordinated CuI(hmds)2–.18b Moreover, the sole detection of NH(SiMe3)2 as an organic byproduct by GC-MS also suggests that the alkali amides used in the reduction course of 1 described herein promote formation of the Fe0 stage by successive one-electron transfers. The lack of reactivity of Li(hmds), compared to that of Na(hmds) and K(hmds), can be explained by the kinetic inertia of 1 (low-spin d6 octahedral complex19) and the strong electrostatic lithium/amide interaction existing in Li(hmds), which will slow down any reaction of Li(hmds) with the ferrous ion. Given that alkali cations can lead to the formation of π-arene adducts in the presence of an aromatic solvent (C6D6 herein),20 the same experiments were carried out in the presence of a more donating solvent (C6D6/THF 9:1 mixture). No significant variations were obtained, meaning that reducing properties of Na(hmds) and K(hmds) remained unchanged under those conditions (Figures S15–S17). Treatment of 1 by 2 equiv of M(hmds) was then performed under UV irradiation (λ = 365 nm) during 72 h at room temperature. In all cases (respectively M = Li, Na, or K, entries 4–6), complex 4C6D5,D was detected in significant quantities (respectively, 40, 48, and 45%) and was the major species detected by NMR along with traces of 5– and unreacted 1. In other words, this means that a clean reduction of 1 by 2 equiv of M(hmds) into transient 3 can be triggered by UV irradiation, affording 4C6D5,D by oxidative insertion. Formation of 3 by photolytic evolution of reactive sensitive precursors such as FeII(R)(R′)(dmpe)2 (R,R′ = H, Me or 2-Np, H) has already been used and described in the past.11,12a However, accessing 3 by reduction of a stable, easily handled precursor such as 1 in the presence of reductants classically used in synthetic chemistry such as M(hmds) is an appealing alternative to the use of the aforementioned sensitive precursors. A previous example of in situ activation of FeCl2(dmpe)2 in 3 by tBuOK had been reported by the group of Thomas in 2022.12b
The action of an excess of M(hmds) on 1 at room temperature (10 equiv during 22 h) was then investigated (entries 7–9). In that case, a C6D6/THF 9:1 mixture was used, in order to circumvent solubility issues, which precluded the obtention of interpretable NMR spectra. Almost no conversion was observed with Li(hmds) (entry 7). Reduction by Na(hmds) (respectively K(hmds)) afforded ca. 25% of 3′ and 3″ (respectively 12%, entries 8–9) and 3% of complex 5– (respectively 10%). Traces of 4C6D5,D were observed for both Na(hmds) and K(hmds). Overall, the amount of NMR-detected species is smaller with 10 equiv of M(hmds) than with 2 equiv of the latter (M = Na, K; compare entries 8–9 and 2–3).21 However, in the case of Na(hmds) (entry 8), ca. 7% of complex (C6D5)FeII(Cl)(dmpe)2 (2C6D5) was also observed, with its diagnostic signal at δ = 67 ppm in 31P NMR.11b Detection of small amounts of 2C6D5 thus shows that Na(hmds) also enables C–D activation of C6D6 by nonredox deprotonation path i (Scheme 1c).
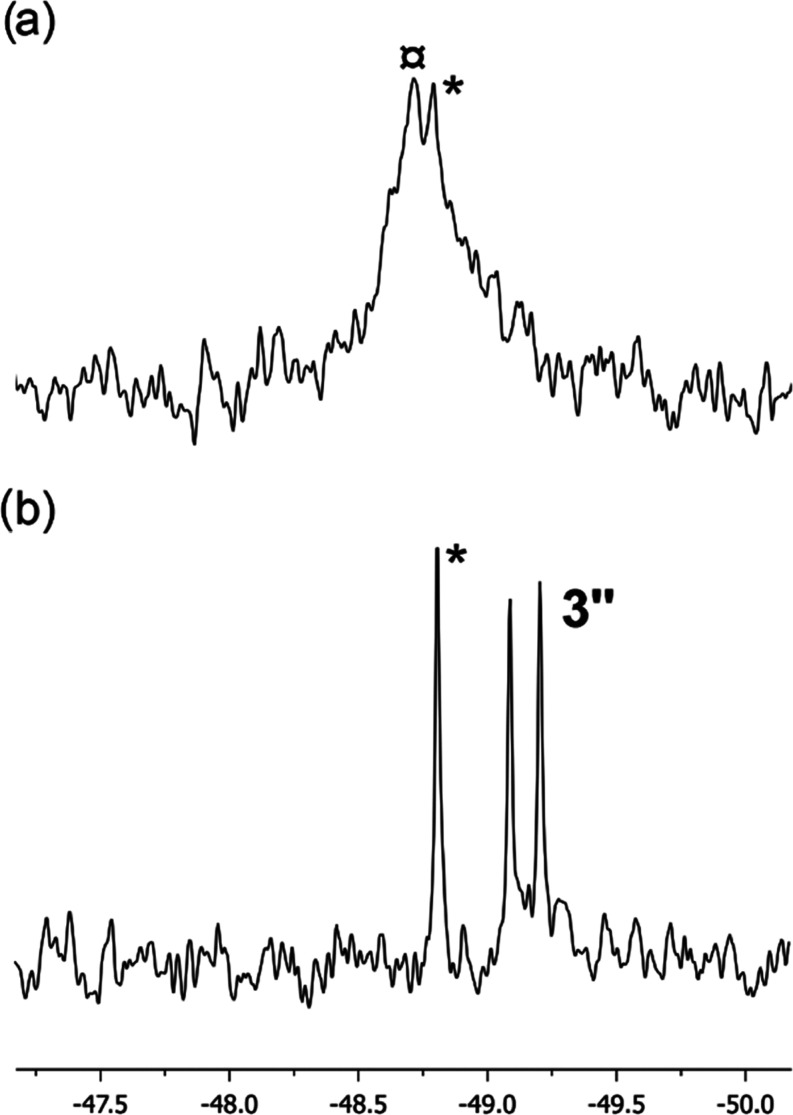
At a higher temperature (80 °C), reduction of 1 by 10 equiv of Na(hmds) (respectively K(hmds)) afforded a mostly NMR-silent distribution (entries 11–12), with reduced 3′, 3″ quantities roughly similar to what was obtained at room temperature. This distribution did not evolve after 10 h at room temperature. This means that the NMR-silent ill-defined reduced iron species obtained in that case are not able to promote the formation of 4C6D5,D. The situation was strongly different when Li(hmds) was used (10 equiv per mole of 1, at 80 °C during 22 h, entry 10). 5– (18%) is the sole species detected under these conditions and is almost undetected with Na(hmds) or K(hmds). This highlights the enhanced stability of 5– in the presence of LiI countercations, likely by the formation of a tight {5–;Li+} ion pair. However, the speciation progressively evolves at room temperature (Figure S33), and 4C6D5,D is detected after 10 additional hours at 20 °C (ca. 38%, cis:trans = 4:3, Figure 1a, and Table 1, entry 10, note d) additionally to 5–, which remains almost unreacted (15%). New species 3* (ca. 10%), which displays 31P resonances similar to that of Fe20(dmpe)5 (3′), is also detected (Figure 1a,b; a classic splitting of those signals upon use of 57Fe-labeled material is observed, confirming the presence of one Fe atom in 3*).22 Additionally, broad resonances in the 50–60 ppm range are also observed and do not display any particular additional splitting upon 57Fe-labeling experiments (Figure S33). Given that this area can correspond to diamagnetic Fe0-phosphine species,11,12 formation of ill-defined diamagnetic aggregates stabilized by dmpe ligands is not excluded. Overall, this means that reduced species obtained using Li(hmds) at 80 °C can progressively act as an efficient reservoir of 3, enabling the C–D activation of C6D6 affording 4C6D5,D, which is not the case when Na(hmds) and K(hmds) are used (entries 11 and 12).
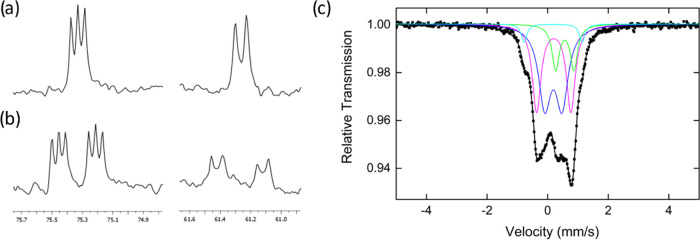
Figure 1.
31P NMR patterns of (a) 4C6D5,D; 3* and (b) 57Fe-4C6D5,D; 57Fe-3* obtained after thermolysis of respectively 1 and 57Fe-1 in C6D6 in the presence of 10 equiv of Li(hmds) during 22 h at 80 °C; spectra recorded after 10 additional hours at 20 °C, see Figure S33 for the full spectra; (c) 80 K 57Fe Mössbauer spectrum of a frozen solution corresponding to spectrum (b).
Since a significant mass of iron is not detected by NMR speciation, 57Fe Mössbauer spectroscopy of the reaction medium was also performed in the same conditions (treatment of 57Fe-1 by 10 equiv of Li(hmds) in C6D6/THF (9:1) at 80 °C during 22 h, followed by 10 h at 20 °C, Figure 1c). The resulting spectrum could be simulated taking into account the presence of four different iron species. Complex 5– was detected with Mössbauer parameters analogous to previous reports (δ = 0.57 mm/s, |ΔEQ|= 0.60 mm/s, green component), accounting for ca. 15% of the overall iron in solution in line with the NMR speciation (Table 1, entry 10).17b The major species observed by Mössbauer represents ca. 45% of the overall iron quantity (δ = 0.19 mm/s, |ΔEQ|= 0.57 mm/s, Figure 1c, blue line), which is in fair agreement with the amount of 4C6D5,D observed by 31P NMR speciation (38%). The nuclear parameters of this species are moreover also in agreement with what can be expected from low-spin FeII hydride species.23 The broadness of this signal might be due to the existence of both cis and trans isomers of 4C6D5,D, which overall lead to the superposition of two doublets with close parameters. Slight differences in Mössbauer parameters may indeed be expected for the signals of a cis/trans isomeric pair, as reported by Herber and Hayter.23c Moreover, a third, rather broad signal accounting for 35% of the iron speciation displays parameters consistent with low-spin, Fe0 species (δ = 0.20 mm/s, |ΔEQ| = 1.13 mm/s, magenta component).23a Formation of such a diamagnetic compound is also in line with the 31P NMR speciation given in Table 1 and Figure S33, which shows that several ill-defined P-ligated diamagnetic compounds as well as ca. 10% of 3* are formed. Thus, it is likely that several low-spin Fe0 species, including 3*, may all contribute to the same Mössbauer signal. Last minor unidentified species (δ = 0.18 mm/s, |ΔEQ| = 1.92 mm/s, 5% of the total Fe, cyan component) has also been included in order to obtain the best fit to the experimental spectrum. Overall, both Mössbauer and NMR speciations point toward the formation of 5–, 4C6D5,D, and a distribution of diamagnetic P-ligated Fe0 species upon treatment of 1 by Li(hmds) at 80 °C during 22 h, those species accounting for more than 90% of the iron quantity according to the Mössbauer simulation.
This section shows that C6D6 activation by oxidative insertion of in situ generated Fe0 species upon treatment of 1 by M(hmds) (M = Li, Na, K) is amenable under photolytic conditions and under thermal activation for M = Li.
Formation of Ar–FeII Bonds by Deprotonative Ferration
In addition to the detection of Fe0 complexes (which can yield 4C6D5,D) that has been described in the previous section, formation of a small amount of 2C6D5 (Table 1, entry 8) obtained by combination of 1, C6D6, and Na(hmds) also suggests that deprotonative ferration can be reached with the 1/M(hmds) system. Given that conditions discussed in Table 1 mostly led to easy reduction of 1 with Na(hmds) and K(hmds), the reactivity of Li(hmds) was more closely investigated. 1 was then subjected to treatment by 2 equiv of Li(hmds) in the presence of crown-ether 15-C-5.24 It was anticipated that quenching of the LiI cation by 15-C-5 would lead to an enhancement of the hmds anion basicity, thus favoring the C–D bond deprotonation.
When 1 is treated by 2 equiv of Li(hmds) associated with 2 equiv of 15-C-5 at 20 °C during 72 h, reduction into 3′ and 3″ is observed (25% overall, Table 2, entry 1). Deprotonation of the C6D5–D bond also occurs as a side pathway, and 10% 2C6D5 are detected. Under photolytic conditions (entry 2), 40% of 4C6D5,D are detected, and the quantity of 2C6D5 remains roughly unchanged (14%). In line with the results discussed in Table 1 (comparison between entries 2–3 and 5–6), 3′ and 3″ are not observed upon irradiation: it is not excluded that they are formed under photolytic conditions and that their evolution toward 3 and, ultimately, to 4C6D5,D is triggered by light. However, under thermal activation (80 °C, entry 3), 2C6D5 was detected (34%) along with unreacted 1 (60%), and small amounts of ill-defined P-ligated diamagnetic species were detected (ca. 5% Fex0(P,P)y, 50–60 ppm area in 31P NMR, see Figure S36). This means that deprotonation of the C6D5–D bond by Li(hmds) can be achieved while limiting the reduction in the presence of 15-C-5 under thermal conditions. In the absence of crown-ether at 80 °C (Table 2, entry 4), 90% conversion of 1 is observed. 2C6D5 is not detected, and reduced ill-defined diamagnetic Fex0(P,P)y species are formed (ca. 30% according to 31P NMR speciation, see Figure S37).25 In other words, it means that quenching the acidity of the LiI cation does not preclude formation of 2C6D5, but it seems to enhance its stability at 80 °C.
Table 2. Speciation of Complexes 1–5 Obtained by Treatment of 1 by 2 equiv of Li(hmds) in the Presence or the Absence of 2 equiv of 15-C-5 under Various Conditions.
| entry | M (n), additives | t (h) | T (°C) | solvent | light | 1 | 2C6D5 | 3′ | 3″ | Fex0(P,P)y | 4C6D5,D | 5– |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Li (2), 2 equiv 15-C-5 | 72 | 20 | C6D6 | no | 50 | 10 | 25% overalla | 0 | 0 | 5 | |
| 2 | 72 | 20 | C6D6 | yes | 40 | 14 | 0 | 0 | 0 | 40b | 0 | |
| 3 | 22 | 80 | C6D6 | no | 60 | 34 | 0 | 0 | 5 | 0 | traces | |
| 4 | Li (2), no 15-C-5 | 22 | 80 | C6D6 | no | 10 | 0 | 0 | 0 | 30 | 0 | traces |
8% 3* were observed.
Mixture of cis:trans-4C6D5,D in a ca. 4:3 ratio.
In order to further analyze the reactivity of 2C6D5, its brominated analogue PhFeII(Br)(dmpe)2 (2Ph′) was synthesized by anion metathesis between 1 and PhMgBr and isolated.2631P NMR analysis shows that signatures of 2Ph′ and 2C6D5 are close (δ = 67 ppm for both, see Figures 2a and S3–S4 for 31P and 1H NMR spectra of 2Ph′ and Figure S36 for 2C6D5). Use of labeled 57Fe-1 showed unambiguously that 57Fe-2Ph′ features a 57Fe-ligated phenyl anion C6H5–, characteristic 31P–57Fe and 1HAr–57Fe couplings being observed (J(31P–57Fe) = 42 Hz; J(1Hmeta–57Fe) = 4 Hz; J(1Hpara–57Fe) = 3 Hz; see Figure 2b).
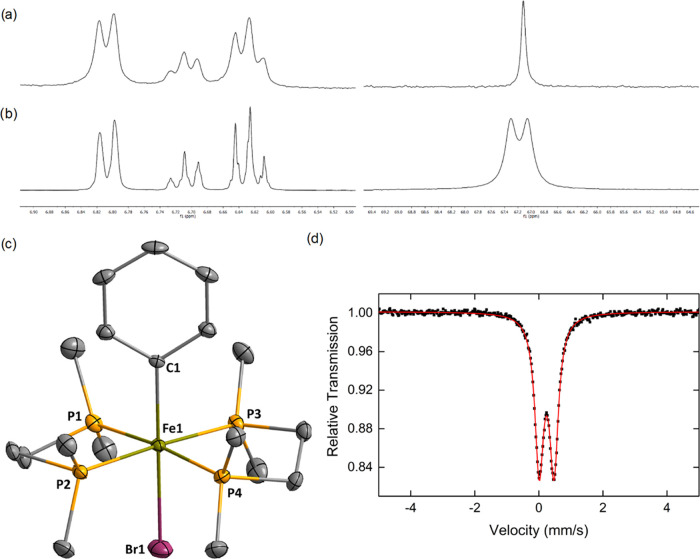
Figure 2.
1H (left) and 31P (right) NMR spectra of complexes 2Ph′ (a) and 57Fe-2Ph′ (b) generated by addition of 1 equiv of PhMgBr (1 M in THF) onto 1 and 57Fe-1; see Figures S3–S8 for full spectra; (c) crystal structure of 2Ph′ (hydrogen atoms omitted for clarity, ellipsoids drawn at the 30% probability level); (d) 80 K 57Fe Mössbauer spectrum of a frozen C6D6/THF (9:1) solution of 57Fe-2Ph′ formed by addition of PhMgBr onto 57Fe-1, with δ = 0.24 mm/s and |ΔEQ| = 0.46 mm/s.
Single crystals of 2Ph′ suitable for X-ray diffraction analysis were obtained by cooling down a C6H6/THF (9:1) solution layered with pentane at −35 °C during 16 h (Figure 2c). Characterization of this structure by X-ray diffraction completes the NMR description of 2Ph reported by Tolman and Ittel in 1979.11b2Ph′ is one of the rare examples of well-defined structurally characterized low-spin aryliron species in an octahedral environment, with a Cipso–Fe distance of 2.08 Å. Camadanli described the formation of low-spin hydrido aryliron(II) species by insertion of Fe0(PMe3)4 into the ortho C–H bond of an aromatic ketimine, where the C=N bond acts as a directing group.13 In those species, the Cipso–Fe bond is slightly shorter than in 2Ph′, probably due to the presence of the intramolecular N–Fe ligation. By comparison, the cyclometalated complex [(benzamide)(dppe)(Ph)FeII]− described as a dimer by Neidig and Ackermann and involving a nontethered phenyl anion features Ph–Fe bonds of 2.036/2.039 Å, closer to those of 2Ph′.9 The 57Fe Mössbauer spectrum of 57Fe-2Ph′ was also recorded in a frozen C6D6/THF (9:1) solution (Figure 2d). The observed Mössbauer parameters (δ = 0.24 mm/s and |ΔEQ| = 0.46 mm/s) are consistent with a low-spin FeII center (diamagnetic, S = 0). Similar parameters were reported by Neidig and Gutierrez for the formation of an octahedral aryliron(II) complex featuring depe ligands (depe = 1,2-bis-diethylphosphinoethane), although no X-ray structure was reported for this species.27
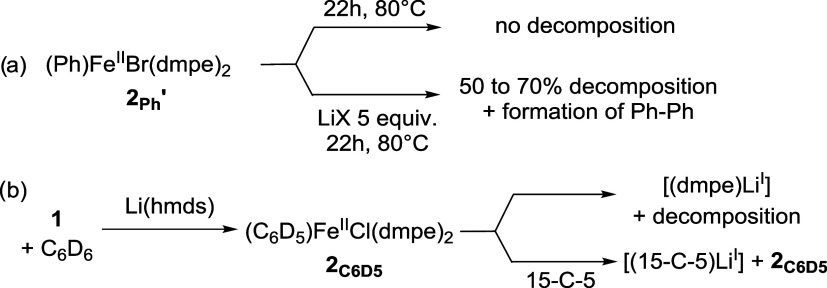
We then investigated the fate of 2Ph′ in the presence of LiI salts such as LiBr or LiOTf. When 2Ph′ was treated by 5 equiv of those salts in neat C6D6, degradation of respectively 70 and 50% of 2Ph′ was observed after 22 h at 80 °C, along with an insoluble dark precipitate. Concomitant formation of Ph–Ph was confirmed by GC-MS, attesting to a reduction of the FeII ion from 2Ph′. On the other hand, 2Ph′ remained unreacted in the absence of LiI salts under the same conditions (Scheme 2a). Those results demonstrate that 2Ph′ is thermally stable in the absence of a Lewis-acidic cation, explaining the persistence at 80 °C of its analogue 2C6D5 when LiI cation acidity is quenched in the presence of 15-C-5 (Table 2, entry 3).
Scheme 2. Reactivity of Isolated 2Ph′ (a) or In Situ Generated 2C6D5 (b) in the Presence of LiI Salts; X = Br, OTf.
Decomposition of 2Ph′ in the presence of LiI salts into unidentified reduced iron species points toward an interaction of the lithium cation with the iron coordination sphere prior to the reduction. When 1 is treated by Li(hmds) at 80 °C during 22 h (conditions of Table 1, entry 10), a broad distribution in the −49/–50 ppm area is also observed in 31P NMR (Figure 3a). This is diagnostic of the release of free dmpe in the reaction medium, along with various adducts formed between the latter and LiI salts, e.g., [dmpe·Li(Y)] (Y = Cl, hmds). σ-Ligation of LiI cations by phosphines indeed leads to a minor shift of the latter in 31P NMR, as reported by Lappert and Layh,28a or more recently by Reid for the Li(dmpe)3+ cation (δ(31P) = −45.9 ppm in toluene-d8).28b
Figure 3.
31P{1H} NMR spectrum (C6D6/THF (9:1), 20 °C, restricted to the free dmpe area) of a solution of 1 treated by 10 equiv of M(hmds) after 22 h at 80 °C; M = (a) Li; (b) Na; * = free dmpe, ¤ = [dmpe·LiI] adducts; see Figures S27–S32 for the full spectra.
The instability of 2Ph′ in the presence of LiI salts (Scheme 2a) and the observation of dmpe-lithium adducts upon treatment of 1 by Li(hmds) (Figure 3a) attest to the demetallating power of the LiI cation, able to decoordinate the dmpe from the iron. As summarized in Scheme 2b, consumption of 2C6D5 in the absence of 15-C-5 suggests that the former readily undergoes a demetalation by LiI cations, affording [dmpe·LiI] adducts along with NMR-silent species. Conversely, trapping the LiI cation by 15-C-5 prevents demetalation of dmpe from iron, enhancing stability of 2C6D5 even at 80 °C. No [dmpe·MI] adducts are observed using the Na/K hmds salts, in agreement with their weaker coordinating properties (Figure 3b). This observation is also in line with the strength of the NaI–P ligation described by Reid in the case of the 1,2-bis-dimethylphosphinobenzene (diphos) ligand, much weaker than that of the LiI–P analogues (discussion based on the comparison of the metric parameters of the X-ray structure of the [M(diphos)3]+ adducts; M = Li, Na).28b The interaction observed between the LiI cation and the dmpe ligand in the system discussed herein is also in line with other reports from the recent literature. This again demonstrates the crucial role of main-group cations in the evolution of a distribution of organotransition species.29 Bedford also reported a similar reactivity of ZnII cations in Negishi couplings catalyzed by various (P,P)FeII salts, where Zn-(P,P) ligation is a key step of the catalytic cycle.30
Association of 1 with alkali bases M(hmds) (M = Li, Na, or K) thus affords a simple platform, which unlocks the access to both deprotonative (nonredox) and oxidative ferrations (Scheme 1c, paths (i) and (ii)). The nature of the preferred path is impacted by the alkali countercation. Under thermal activation, K(hmds) promotes formation of reduced species, affording 4C6D5,D by oxidative insertion of 3 into C6D6, and Na(hmds) allows detection of both 2C6D5 and 4C6D5,D intermediates. While treatment of 1 by Na(hmds) and K(hmds) mostly affords silent species (probably reduced aggregates), use of Li(hmds) can lead to either deprotonative ferration (formation of 2C6D5) under thermal conditions or 4C6D5,D under harsher conditions. Reduction of 1 followed by C–D activation yielding 4C6D5,D is however observed for M = Li, Na and K under photolytic activation.
Possible catalytic applications of this divergent ferration system are discussed in the next section of this article, with a focus on the cross-coupling, reductive dehydrocoupling, and hydrogen isotopic exchange (HIE) chemistry.
Reactivity in Cross- and Dehydrogenative Coupling as well as in HIE Chemistry
Cross-Coupling of Arenes with Organic Electrophiles
The possible generation of Ar–FeII compounds such as 2C6D5 from 1 by deprotonation of an Ar–H pro-nucleophile with M(hmds) (M = Li, Na) invited us to investigate the possible catalytic applications of this system in cross-coupling transformations. As outlined in the introduction of this report, organoiron(II) species are indeed key intermediates in cross-coupling processes; they are moreover known for efficiently activating aliphatic electrophiles by single electron transfers (SETs).31 The cross-coupling of C6H6 with n-C12H25I in the presence of 0.1 equiv of 1 and M(hmds) (1 equiv vs n-C12H25I) has been chosen as a benchmark reaction (Table 3); the main byproduct obtained under those conditions was n-C12H26, formed by reduction of the starting C–I bond.
Table 3. Cross-Coupling between C6H6 and R-I Mediated by 1 in the Presence of M(hmds) (1 equiv); Yields Based on GC-MS Analysis.
| entry | M | R | T (°C) | t (h) | additives | Ph-R (%) | R-H (%) |
|---|---|---|---|---|---|---|---|
| 1 | Li | n-C12H25 | 40 | 72 | 2 | 3 | |
| 2 | Li | n-C12H25 | 80 | 72 | 25a | 10 | |
| 3 | Na | n-C12H25 | 80 | 72 | 7 | 17 | |
| 4 | K | n-C12H25 | 80 | 72 | 9 | 9 | |
| 5 | Li | n-C12H25 | 80 | 72 | 15-C-5 20 equiv | 1 | 2 |
| 6b | Li | n-C12H25 | 80 | 72 | 30a | 4 | |
| 7b | Li | cyclohexyl | 80 | 72 | 35a | n.d. | |
| 8c | Li | n-C12H25 | 80 | 72 | 4 | traces | |
| 9 | Li | n-C12H25 | 80 | 72 | dmpe 1 equiv | 3 | 5 |
| 10 | Li | n-C12H25 | 80 | 72 | dmpe 3 equiv | 2 | 3 |
| 11d | Li | n-C12H25 | 80 | 72 | 0 | 0 | |
| 12d | Li | n-C12H25 | 80 | 72 | dmpe 1 equiv | 0 | 0 |
Isolated yield.
0.1 equiv of Fe(hmds)2 + 1 equiv of dmpe vs Fe; other iron sources.
0.1 equiv of FeCl2
No iron source was used.
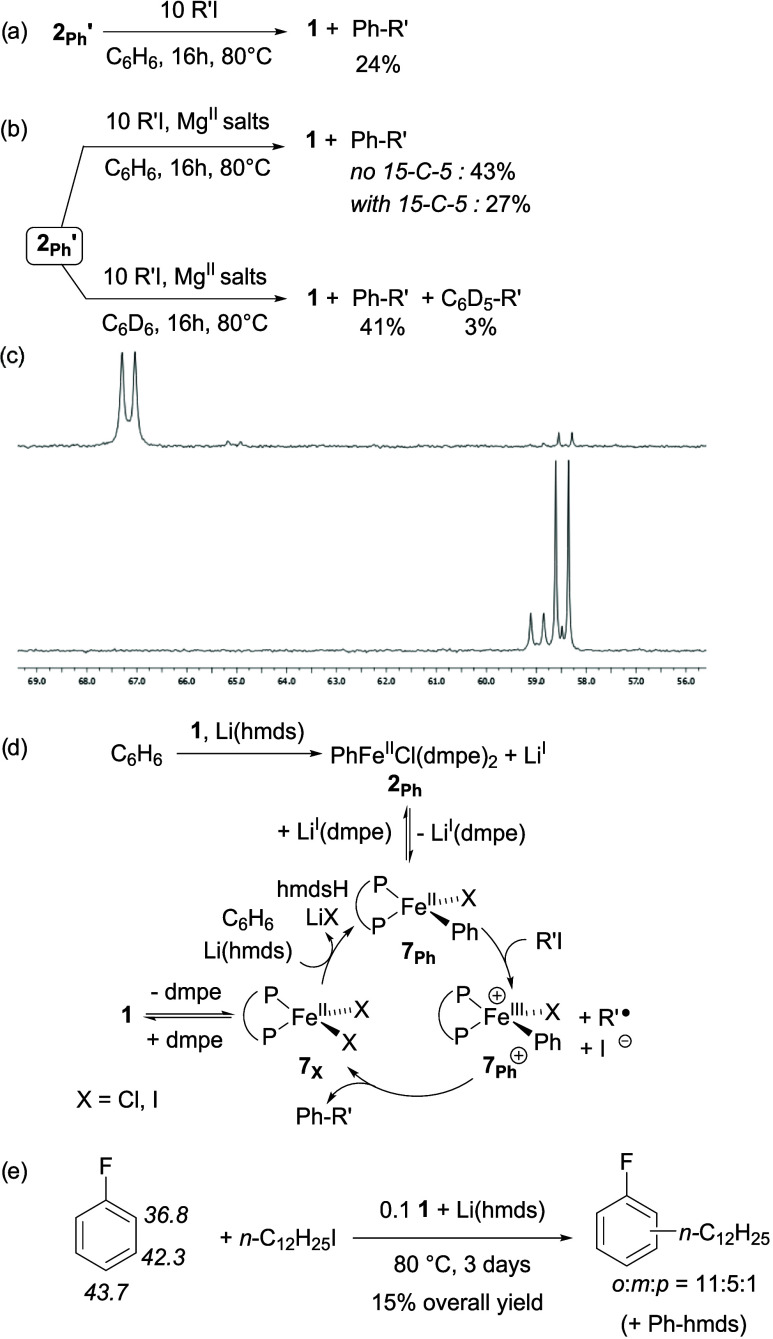
A first coupling attempt using Li(hmds) as a base at 40 °C during 72 h only led to traces of the coupling product (Table 3, entry 1). Increasing reaction temperature up to 80 °C however afforded a modest 25% coupling yield (entry 2). Owing to the competitive reduction of 1 into 3, 3′ and 3″, Na(hmds) was a much less efficient alkali base and afforded Ph-(n-C12H25) with a poor 7% yield (entry 3). In line with the formation of more reduced iron species in the reaction medium, reduction product n-C12H26 is obtained with a higher 17% yield. In a similar way, use of K(hmds) only afforded 9% of coupling product (entry 4). Addition of 15-C-5 to Li(hmds) (entry 5) led to a strong decrease of the coupling yield (1%). Since 15-C-5 proved to enhance the stabilization of 2C6D5 in the presence of LiI salts (Scheme 2b), this shows that the deprotonation of the Ph–H bond is not the more energetically demanding step of the coupling sequence. Conversely, this demonstrates that the electrophilicity of the LiI cation is requested in the coupling process. Moreover, the best coupling yield (30%, entry 6) is obtained when FeII(hmds)2 is associated with only 1 equiv of dmpe, evidencing the best performances of unsaturated, single-dmpe coordinated intermediates. Under the same conditions, a similar 35% yield was obtained when iodocyclohexane was used (entry 7). Traces of coupling product are also detected when FeCl2 is used in the absence of dmpe (entry 8). Use of an excess of dmpe is highly detrimental to the reaction outcome, since the coupling yield dropped to 3% (respectively 2%) when 1 equiv of dmpe (respectively 3 equiv) is added to 1 in the reaction medium (entries 9–10, compare with entry 2). Control experiments performed in the absence of iron confirm that the latter is mandatory in the coupling process, since no product is detected when Li(hmds) is used alone or in the presence of dmpe (entries 11–12). At this stage, two possible mechanisms can explain the formation of the C–H coupling product. Either the electrophile is activated by FeII species such as an arylated complex derived from 2Ph, or the formation of a reduced Fe0 complex (e.g., 3, 3′ or 3″) is required to do so. A stoichiometric experiment was performed, in which reduced iron species are formed by addition of 2 equiv of Li(hmds) on 1 at 80 °C (conditions of Table 2, entry 4), prior to reaction with n-C12H25I in the presence of 1 additional equivalent of Li(hmds) (Scheme 3).
Scheme 3. Stoichiometric Experiment for the Cross-Coupling between C6H6 and n-C12H25I.
In those conditions, 20% of cross-coupling product Ph-(n-C12H25) are formed, along with 25% n-C12H26, 7% of 1-dodecene, and 10% n-C24H50. Those three latter products are formed by reduction of the starting halide n-C12H25I. In particular, the n-C12H26/Ph-(n-C12H25) ratio is much higher in conditions of Scheme 3 (5:4) than in cross-coupling conditions (2:5, Table 3, entry 2). Moreover, 1-dodecene and n-C24H50 are solely detected as traces in cross-coupling conditions (Table 3, entry 2), whereas they represent 27% of the conversion of n-C12H25I in conditions of Scheme 3. This suggests that a sacrificial reduction of n-C12H25I occurs when reduced iron species are formed upon treatment of 1 with Li(hmds) and that those species do not contribute to the formation of the coupling product Ph-(n-C12H25). This thus points toward the possible implication of nonreduced FeII intermediates such as 2Ph or 5– in the coupling process. Given that the arylated complex 2Ph has been identified as an intermediate on the reduction route of 1 to the Fe0 stage, its reactivity as a coupling intermediate has been further investigated.
The brominated analogue 2Ph′ was used as an analytically pure compound, and its treatment by an excess of n-C12H25I at 80 °C during 16 h afforded the expected coupling product Ph-(n-C12H25) in a 24% yield (Scheme 4a), demonstrating that 2Ph′ is able to promote the coupling step. A similar experiment was performed using 2Ph′ generated in situ by transmetalation of PhMgBr with 1 (in conditions of Figure 2, affording a near-quantitative formation of 2Ph′ as evidenced by Mössbauer spectroscopy, Figure 2d). This led, after treatment by 10 equiv of n-C12H25I in C6H6, to 43% of coupling product, whereas the coupling yield dropped to 27% in the presence of 15-C-5 (1 equiv vs PhMgBr, Scheme 4b). First, this result importantly demonstrates that the ability of 2Ph′ to promote a cross-coupling-type reactivity with n-C12H25I is enhanced in the presence of Lewis-acidic cations such as MgII. It is important to note that the Ph-(n-C12H25) yield remains unchanged when thermolysis of 2Ph′ is performed in C6D6 instead of C6H6 and that only traces of the deuterated compound C6D5-(n-C12H25) are obtained (Scheme 4b). This confirms that the aromatic core contained in Ph-(n-C12H25) comes from the Ph– anion bound to the iron. Given that the coupling reactivity of 2Ph′ with n-C12H25I in the presence of MgII salts associated with crown-ether 15-C-5 is very close to that of the salt-free, pure complex 2Ph′ (respectively 27%, Scheme 4b, and 24%, Scheme 4a), this means that MgII cations play a key role in the implication of 2Ph′ in the catalytic cycle, which is quenched by the presence of the crown-ether. 31P NMR monitoring of reaction of a 57Fe-2Ph′ sample (Scheme 4c, top, δ = 67 ppm, J(31P–57Fe) = 42 Hz), generated in conditions of Figure 2, with 3 equiv of n-C12H25I at 80 °C during 4 h also shows that 57Fe-1 and its bromide analogue 57Fe-1Br are generated in addition to the coupling product Ph-(n-C12H25) (Scheme 4c, bottom, respectively δ = 59.0 ppm, J(31P–57Fe) = 42 Hz, and δ = 58.5 ppm, J(31P–57Fe) = 41 Hz). The stoichiometric reactivity of 2Ph′ in cross-coupling (Scheme 4a,b) shows without doubt that the presence of a Lewis-acidic cation (MgII in Scheme 4a,b) plays a beneficial role in the reaction outcome. In line with the observations of adducts such as [dmpe·Li(Y)] by 31P NMR (Figure 3a), it can be suggested that MgII salts enable the decoordination of one dmpe ligand from 2Ph′, unlocking the access to unsaturated ferrous species able to promote the cross-coupling. Despite the apparent HSAB mismatch between the hardness of MgII ions and softness of P-ligands, weak, labile interaction between PMe3 and MgII ions in Grignard reagents has already been probed.32a Moreover, Arnold also reported an example of structurally characterized MgII adduct with alkyl 1,2-diphosphines, demonstrating the possible interaction between alkyl phosphines and MgII salts.32b
Scheme 4. Stoichiometric Reactivity of 2Ph′.
Used either (a) pure or (b) generated by addition of 1 equiv of PhMgBr on 1 followed by 15 h at 20 °C with R′I (R′ = n-C12H25); (c) 31P NMR monitoring of reaction of 57Fe-2Ph′ (top) with 3 equiv of n-C12H25I (bottom) during 4 h at 80 °C; (d) plausible catalytic cycle for the cross-coupling of C6H6 with R′I involving 2Ph as a catalyst reservoir (X = Cl, I); (e) cross-coupling attempt performed on PhF (pKa of the C–H bonds italicized).
Since 2C6D5 is readily obtained by direct ferration of C6D6 mediated by Li(hmds) (Table 2, entry 3), this also means that 2C6D5 can act as an intermediate in the cross-coupling between C6D6 and n-C12H25I. LiI and MgII cations can thus act as a dmpe reservoir in a catalytic process: the main role of the dmpe therefore mostly seems to provide a coordination sphere able to stabilize transiently unsaturated Ph–FeII intermediates toward decomposition pathways, even if decoordination of the dmpe ligand from the iron center also seems to be mandatory in another step of the coupling cycle.
Those results unambiguously show that the coordination strength of the alkali countercation is a key parameter of the coupling discussed in Table 3. A likely explanation is that 2Ph, owing to its octahedral low-spin d6 configuration, also displays a certain inertia toward reaction with n-C12H25I. The LiI cation is then prompt to promote the decoordination of one dmpe ligand of 2Ph, affording a nonsaturated 14-VE complex PhFeII(dmpe)Cl (7Ph), which will react with the organic electrophile in a SET step to afford FeIII intermediate 7Ph+ (Scheme 4d). Similar trends were reported by Gutierrez and Neidig, who showed that saturated octahedral 18-VE (P,P)2FeII(Ar)X proved to be inert in coupling processes, whereas unsaturated tetrahedral 14-VE analogues (P,P)FeII(Ar)X displayed excellent performances.27 The requirement of a decoordination of one dmpe ligand during the process is moreover confirmed herein by the detrimental effect of the excess of dmpe on the reaction outcome (Table 3, entries 9–10, to be compared with entry 2). An outer-sphere radical recombination between 7Ph+ and n-C12H25• then affords the coupling product Ph-(n-C12H25) with 7X, as observed in classic aryl-alkyl Fe-catalyzed couplings (Scheme 4d).317X (in equilibrium with 1, as evidenced in Scheme 4c) finally re-enters the catalytic cycle after deprotonation of another equivalent of benzene. The proficiency of a SET/radical recombination sequence as key steps has been probed by the coupling of C6H6 with 1-bromohexene as a radical clock, which selectively afforded cyclization product PhCH2C5H9 (see Section S4b). It is also not excluded that a dmpe-free intermediate such as FeII(hmds)3– (5–, evidenced by 1H NMR and Mössbauer speciations, see Table 1, entry 10 and Figure 1c) also initiates a catalytic cycle with deprotonation of C6H6, leading to a dmpe-free Ph-FeII compound. The latter would react in a SET step with the electrophile, since traces of cross-coupling product are also observed when FeCl2 is used as an iron precursor in the absence of dmpe (Table 3, entry 8). This would be in line with the reducing activity of similar ferrate species such as [Me3FeII]−, which was reported by Uchiyama, those ate complexes proving to readily undergo single-electron oxidation by a variety of electrophiles.33 In our case, the poor amount of cross-coupling product obtained in the absence of dmpe however suggests that the latter is required to stabilize on-cycle, undercoordinated intermediates.
As detailed above, formation of 2Ph′ under coupling relevant conditions and ability of the latter to provide the expected cross-coupling product Ph-(n-C12H25) by reaction with n-C12H25I (Scheme 4a–c) suggests that metalation of the Ar–H bond occurs prior to formation of the radical n-C12H25•. An alternative mechanism would be the formation of n-C12H25• followed by addition of the latter onto C6H6 in a radical nucleophilic addition mechanism. In this alternative mechanism, a C–H bond then undergoes homolytic cleavage to restore the aromaticity. In order to decipher whether the coupling step may also proceed following this alternative radical-based mechanism, a cross-coupling attempt has been performed using fluorobenzene (PhF) as a C–H bond source in the conditions of Table 3, entry 2 (n-C12H25I treated by 0.1 equiv of 1 and 1 equiv of Li(hmds) in neat PhF at 80 °C during 3 days). PhF has been chosen as a mechanistic probe since it can be activated by both the deprotonative pathway and radical addition mechanism;34 it moreover displays three C–H bonds with very distinct acidities (pKa(ortho H) = 36.8, pKa(meta H) = 42.3, pKa(para H) = 43.7, Scheme 4e).34a Cross-coupling of PhF with n-C12H25I in the presence of Li(hmds) (1 equiv per mole of electrophile) led to a poor 15% yield in coupling products, significant quantities of Ph-N(SiMe3)2 being formed by defluorination of the starting material. A 11:5:1 distribution of the ortho/meta/para isomers of the cross-coupled product C6H4F(n-C12H25) is observed. This distribution can be indicative of the proficiency of a radical-based mechanism, since it is very similar to the 11.2:7.6:1 ratio reported in such conditions under Pd-catalyzed addition of alkyl radicals onto PhF.34b In a pure deprotonative mechanism, an almost exclusive metalation at the ortho position would be expected, in line with the ability of PhF to undergo this process.34c Therefore, taken together, those results demonstrate that some part of the coupling activity can also be attributed to a radical-based mechanism, in addition to the deprotonative way discussed earlier and involving the intermediate 2Ph. This would explain the detection of traces of C6D5-(n-C12H25) as a coupling product when C6D6 is used as a solvent in the thermolysis of 2Ph′ (Scheme 4b). 2Ph′ would thus undergo oxidation by SET with n-C12H25I, but C6D5-(n-C12H25) would be formed by addition of the n-C12H25• radical onto C6D6, bypassing its recombination with the iron-bound C6H5 group. In this radical mechanism, it is not excluded that nonarylated species such as FeII(hmds)3– (5–) also act as one-electron reductants. However, it is also possible that the nature of the preferred mechanism (deprotonative way or radical addition) also depends on the Ar–H target.
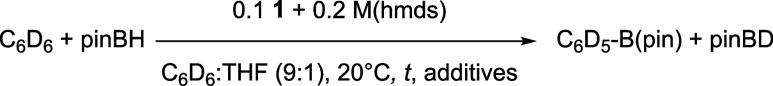
C–H/B–H Bond Metathesis: Reductive Dehydrocoupling
Given that formation of elusive species 3 proved to occur upon reduction of 1 with Li, Na, and K hmds salts under photochemical activation, leading to 3′, 3″, or 4C6D5,D (Table 1, entries 4−6), we also aimed at exploring potential catalytic applications of this system. As outlined in the introduction of this report, formation of hydrido aryliron species 4Ar,H (Scheme 1b) is a key step in various transformations such as reductive dehydrocoupling. It was for example reported in 2015 that borylation of CAr–H bonds could be performed by photolytic generation of 3 from FeII(R)2(dmpe)2 (R = H, Me) in good to moderate yields.12a Performances of 1 associated with M(hmds) (M = Li, Na, K) in the reductive dehydrocoupling of C6D6 and pinacolborane pinBH, leading to formation of perdeuterophenyl pinacolborane C6D5-B(pin), were then investigated (Table 4). The transformation was monitored at room temperature by multinuclear NMR analysis (31P, 11B, 1H). No borylation product C6D5-B(pin) was detected after 3 days when 1 was treated by 2 equiv of Na(hmds) in C6D6 in the presence of 10 equiv pinBH per mole of Fe (Table 4, entry 1). Under those conditions, no reduction of 1 and no further evolution to 4C6D5,D were observed. To circumvent this matter, 1 was treated with 2 equiv of Na(hmds) during 72 h prior to addition of pinBH, affording the expected distribution of 3, 3′, and 4C6D5,D species as detailed in the previous section (see the SI). After addition of pinBH, no evolution was observed after 7 days at 20 °C when the medium was kept in darkness (entry 2). However, upon irradiation at 365 nm, 11B NMR monitoring showed progressive formation of C6D5-B(pin) (δ = 31.3 ppm, see the SI), see entry 3. A 15% borylation yield (respectively 30%) was observed after 72 h (respectively 6 days). After 10 days, 35% of C6D5-B(pin) were formed, with an overall incomplete 80% pinBH conversion, suggesting a deactivation of the catalyst. Interestingly, substitution of Na(hmds) by Li(hmds) led to a much more efficient conversion, since 75% of C6D5–B(pin) are formed after 3 days (entry 4). Progressive consumption of pinBH leads to a 80% borylation yield after 4 days; no starting material is detected after 6 days, with a 87% C6D5–B(pin) yield (isolated yield: 60%). The isolated yield obtained using this method is moreover higher than that was reported using the more sensitive precursor FeII(Me)2(dmpe)2 (34% PhB(pin) after 3 days12a). K(hmds) led to a poor 15% borylation yield after 10 days and an incomplete 40% conversion, also suggesting a catalyst deactivation (entry 5).
Table 4. Borylation of C6D6 by pinBH and Deuteration of pinBH Mediated by 1 in the Presence of M(hmds) (M = Li, Na, K) under Photochemical Activation (in C6D6/THF 9:1)a.
| entry | M | t (days) | conditions | C6D5–B(pin) | pinBD | pinBH |
|---|---|---|---|---|---|---|
| 1 | Na | 3 | no light | 0 | 0 | 100 |
| 2 | Na | 7 | no lightb | 0 | 0 | 100 |
| 3 | Na | 3 | hνb | 15 | 17 | 66 |
| 6 | 30 | 30 | 29 | |||
| 10 | 35 | 35 | 21 | |||
| 4 | Li | 3 | hνb | 75 | 8 | 6 |
| 4 | 80 | 4 | 3 | |||
| 6 | 87 (60)c | 0 | 0 | |||
| 5 | K | 4 | hνb | 5 | <5 | 88 |
| 10 | 15 | 15 | 60 |
11B NMR yields are given.
Premix of M(hmds) with 1 (72 h) prior to addition of pinBH; see Figures S56–S58 for 11B NMR monitoring.
Isolated yield.
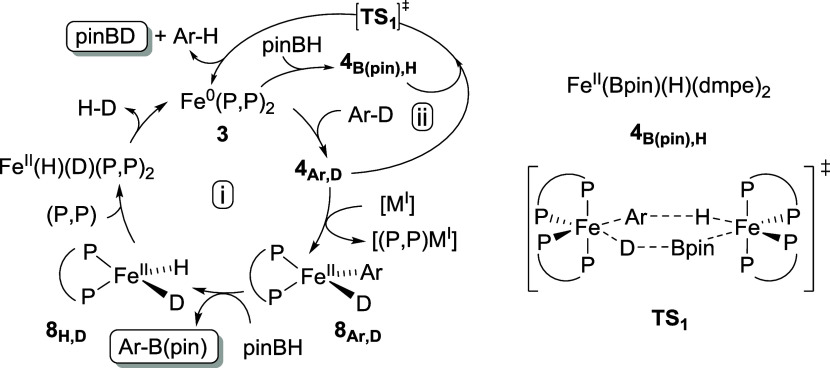
The slow but complete evolution of the pinBH conversion using Li(hmds) as a reductant of 1 under irradiation clearly demonstrates that in situ generated active species 3 does not decompose over time, whereas catalyst deactivation is observed with Na(hmds) and especially with K(hmds). A possible explanation is that Na(hmds) and K(hmds) have a too strong reducing power, leading to higher concentrations of Fe0 species (such as 3, 3′, and 3″) and hence to decomposition of the latter over time by formation of unreactive metallic aggregates, leading overall to poorer catalytic activities. In line with this remark, significant quantities of unreacted 1 are observed by 31P NMR monitoring (Figures S48, S51, S54) of the reaction medium during catalysis when Li(hmds) is used as a precursor; a smaller quantity of 1 is detected when Na(hmds) is used, and only traces of 1 are detected for K(hmds), in line with the increasing reducing ability of the M(hmds) salts in the Li/Na/K series. Owing to the less strong reducing power of Li(hmds), the dynamic concentration of transient Fe0 species in the reduction course of 1 with Li(hmds) would then remain low enough to prevent their aggregation. 31P NMR monitoring shows that complexes 4C6D5,D, FeII(H)2(dmpe)2 along with FeII(H)(D)(dmpe)2 (δ = 76.7 (triplet) and 67.1 (triplet) ppm,12a) are formed during the photolyses with Li, Na, and K hmds salts, which attests to the feasibility of the C–D and B–H bond activations by oxidative additions of 3 in this catalytic system. Formation of H2 and of its isotopologue H–D is also confirmed by 1H NMR (Figure S52), also attesting to the proficiency of the C–D and B–H bond activations in the dehydrocoupling process (Scheme 5, cycle (i)). Those species are not detected in the absence of irradiation. Moreover, pinBD is also detected by 11B NMR during the photolysis with a diagnostic signal at δ = 28.4 ppm (triplet, 1J(11B–2D) = 21 Hz), regardless of the nature of the M(hmds) cation (Table 4). This is also in line with the known reactivity of 3 in CAr–H and B–H bond activations.12a
Scheme 5. Plausible Borylation (i) and C–D/B–H σ-Bond Metathesis (ii) Cycles Mediated by In Situ Generated 3 in the Presence of M(hmds) (M = Li, Na); (P,P) = dmpe, Ar = C6D5.
In the recent literature, pinacolborane has been clasically associated with several nucleophilic partners such as tBuONa to efficiently reduce divalent transition-metal complexes L[MII] of the third row (L = P- or N-based pincers and M = Fe, Co).35 In those cases, tBuONa acts as a “masked reducing agent”, enabling formation of [pinB(OtBu)(H)]−;Na+, which itself allows the reduction of the [MII] complex. It is thus not excluded that this mechanism also contributes to the generation of the active Fe0 species in the case discussed herein, additionally to the direct reduction of 1 under irradiation as evidenced by speciation in Table 1. However, since no reduction of 1 by Na(hmds) was observed in the presence of pinBH (Table 4, entry 1), a reduction mechanism relying on boron hydrides seems unlikely in the case of the Na(hmds). More importantly, the outcome of the transformation is also strongly impacted by the nature of the M(hmds) cation. As discussed earlier, Li(hmds) indeed allows a full conversion of pinBH, yielding 87% of C6D5–B(pin) (Table 4, entry 4), whereas Na(hmds) affords a modest 35% yield (entry 3). In the former case, a fast C–D/B–H σ-bond metathesis is observed (Scheme 5, cycle (ii)). However, both pinBD and pinBH are consumed by the borylation path (entry 4, and Scheme 5, cycle (i)), leading to C6D5–B(pin). On the other hand, when Na(hmds) is used, a progressive increase of the pinBD yield is observed (up to 35%, entry 3). This suggests that the CAr–B(pin) bond formation path is less operative when Na(hmds) is used instead of Li(hmds), but that the C–D/B–H σ-bond metathesis path still operates efficiently with Na(hmds).
The nature of the alkali cation can thus govern the reactivity of 4C6D5,D, formed after oxidative insertion of 3 into the CAr–D bond (Scheme 5). It has been suggested previously that formation of unsaturated species by loss of at least one arm of a dmpe ligand was involved in the CAr–B bond formation step.12a When Li(hmds) is used, this step can be triggered by the demetallating power of LiI, leading to unsaturated complex 8C6D5,D (Scheme 5, cycle (i)). Demetalation of one dmpe is also probably triggered by the photoirradiation of the system, which usually leads to a ligand labilization in saturated, inert intermediates, such as low-spin octahedral ferrous complexes. 8C6D5,D affords the borylation product C6D5–B(pin), along with 8H,D. Reintroduction of dmpe in the iron coordination sphere followed by reductive elimination regenerates 3 and affords H–D, detected by 1H NMR (Scheme 5, cycle (i)). However, in the presence of Na(hmds), owing to the weak demetallating power of the NaI cation, cycle (i) in Scheme 5 is difficultly followed due to the slow formation of 8C6D5,D. Instead, a C–D/B–H σ-bond metathesis is observed, possibly by reaction between C–D and B–H activated intermediates 4C6D5,D and 4B(pin),H (Scheme 5, cycle (ii)). This ligand scrambling step between two saturated octahedral species might involve a bimetallic transition state (TS1), as suggested in Scheme 5. The use of Na(hmds), in this case, slows down the demetalation of a dmpe ligand from one of the FeII ions, enabling the σ-bond metathesis to occur between those two saturated complexes, overall leading to the formation of pinBD and C6D5H.
These preliminary results show that 1 can be used as an efficient precursor to induce the formation of Fe0 species such as 3 in a reducing medium leading to activation of CAr–D and B–H bonds under photochemical conditions. More importantly, the judicious choice of the alkali countercation of the reductant, herein M(hmds), can drive the formation of CAr–B bonds by reductive dehydrocoupling (M = Li, Na), or the deuteration of B–H bonds affording B–D species by σ-bond metathesis can be observed (M = Na). This opens the way to promising synthesis of deuteroboranes R2BD from boranes R2BH, using C6D6 as a mild deuterium source and 1 as an easily handled iron catalyst, thus making a sustainable alternative to the classic formation of B–D bonds by addition of borodeuterides such as NaBD4 or LiAlD4 on electrophilic halogenoboranes R2BX. Those methods indeed lead to stoichiometric amounts of salts as byproducts and rely on expensive deuteride sources (molar price of NaBD4 (respectively LiAlD4) being roughly 6 (respectively 30) times higher than that of C6D6).36
Conclusions
In this report, we demonstrated that alkali salts M(hmds) can be used as bases or reductants to promote C–Fe bond formations from C–H substrates, respectively, by deprotonative ferration at a FeII center or by oxidative insertion of transient Fe0 species. These striking differences clearly show that the reactivity of M(hmds) toward C6H6 and 1 is impacted by the nature of the alkali countercation M+. Depending on the latter, hmds– can act as a base and follow a nonredox ferration path affording Ar–FeII species able to react with organic halides in a cross-coupling process (M = Li). On the other hand, hmds– can also act as a two-electron reductant of 1, affording elusive species Fe0(dmpe)2 and promoting C–H insertion reactions leading to heteroleptic Ar–FeII–H intermediates (M = Li, Na, K). Under photolytic activation, this path can be efficiently used to promote either the catalytic borylation of arenes (the best yield and selectivity being afforded by M = Li) or the deuteration of boranes R2BH using C6D6 as a deuterium source by σ-bond metathesis (being observed for M = Na, along with borylation). In all cases, the coordinating properties of the alkali cation M+ can govern the evolution of the iron complex distribution, playing a crucial role in the control of the oxidation state of the latter species as well as of their coordination sphere. Those countercations are thus far from being mere spectators in the catalytic transformations discussed herein and strongly contribute to the generation of the key on-cycle species from their inert resting states. This report thus also provides another example of the crucial importance of alkali cations in organometallic catalysis, which have long proved to strongly contribute to the selectivity of a large variety of transformations.37
Methods
Materials and Reagents
All the samples were prepared in a Jacomex Campus glovebox under an argon atmosphere or vacuum Schlenk lines. Glassware equipped with a J. Young valve was dried overnight at 120 °C before use. Deuterated solvents purchased from Eurisotop were thoroughly degassed by freeze–thaw cycles and dried overnight on 4 Å molecular sieves. Nondeuterated solvents were dried over a Na/benzophenone mixture and distilled before use. NMR tubes equipped with a J. Young valve were used for all NMR experiments. Chemicals were obtained from commercial sources and used as purchased, unless specified otherwise or after drying when necessary. A Thorlabs’ M365LP1 LED (nominal wavelength = 365 nm, fwhm = 9 nm) mounted to the end of a 57 mm heat sink enabling heat dissipation has been used for the irradiation setup. The details of the synthetic procedures to prepare both iron complexes, Fe(Cl)2(dmpe)2 (1) and FeBr(C6H5)(dmpe)2 (2Ph′), are described in the Supporting Information.
General Procedure for Cross-Coupling Reactions
The reaction with Fe(Cl)2(dmpe)2 (1) and Li(hmds) at 80 °C is given as an example (Table 3, entry 2). In an argon-filled glovebox, a 12 mL vial equipped with a magnetic stir bar was loaded with Fe(Cl)2(dmpe)2 (3.5 mg, 8.4 μmol, 0.1 equiv), C6H6 (1.5 mL), and decane as an internal standard for GC-MS analysis. The mixture was placed under stirring, and n-C12H25I (20.2 μL, 82 μmol, 1 equiv) was added dropwise followed by Li(hmds) (15.0 mg, 82 μmol, 1 equiv). The color of the reaction medium gradually changed from emerald green to yellow/orange. The reaction mixture was stirred for 5 min and transferred into an NMR tube fitted with a J. Young valve. The tube was placed in an oil bath at 80 °C and left for 72 h. The color turned pale yellow. The tube was cooled to room temperature and quenched with 2 mL of H2O. The solution was extracted with 2 mL of MTBE and analyzed by GC-MS.
General Procedure for Dehydrocoupling Reactions
The reaction with Fe(Cl)2(dmpe)2 (1) and Li(hmds) is given as an example (Table 4, entry 4). In an argon-filled glovebox, a 12 mL vial equipped with a magnetic stir bar was loaded with Fe(Cl)2(dmpe)2 (3.7 mg, 8.7 μmol, 0.1 equiv), C6D6 (0.6 mL), and THF in a 9:1 ratio. The mixture was placed under stirring, and Li(hmds) (2.9 mg, 17.3 μmol, 0.2 equiv) was added. The color of the reaction medium gradually changed from emerald green to yellow/orange. The reaction mixture was stirred for 3 days at room temperature in order to afford the expected distribution of 3, 3′, and 4C6D5,D species, monitored by 31P NMR. Pinacolborane (12.6 μL, 87 μmol, 1 equiv) was added, and the mixture was transferred into an NMR tube fitted with a J. Young valve. The tube was irradiated, and the reaction was monitored by 11B, 31P{1H} and 1H NMR.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.3c00649.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
G.L. thanks the ERC (Project DoReMI StG, 852640) and the CNRS (Project IrMaCAR) for their financial support. The NMR shared facilities of Chimie ParisTech (Dr. M.-N. Rager) are thanked for technical support.
The authors declare no competing financial interest.
Supplementary Material
References
- Gensch T.; Hopkinson M. N.; Glorius F.; Wencel-Delord J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. 10.1039/C6CS00075D. [DOI] [PubMed] [Google Scholar]
- Shang R.; Ilies L.; Nakamura E. Iron-Catalyzed C–H Bond Activation. Chem. Rev. 2017, 117 (13), 9086–9139. 10.1021/acs.chemrev.6b00772. [DOI] [PubMed] [Google Scholar]
- a Bauer I.; Knölker H.-J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. 10.1021/cr500425u. [DOI] [PubMed] [Google Scholar]; b Bedford R. B.; Brenner P. B.. The Development of Iron Catalysts for Cross-coupling Reactions. Iron Catalysis II; Bauer E., Ed.; Springer International, 2015. [Google Scholar]
- a Carpenter S. H.; Neidig M. L. A Physical-Inorganic Approach for the Elucidation of Active Iron Species and Mechanism in Iron-Catalyzed Cross-Coupling. Isr. J. Chem. 2017, 57, 1106–1116. 10.1002/ijch.201700036. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Neidig M. L.; Carpenter S. H.; Curran D. J.; DeMuth J. C.; Fleischauer V. E.; Iannuzzi T. E.; Neate P. G. N.; Sears J. D.; Wolford N. J. Development and Evolution of Mechanistic Understanding in Iron-Catalyzed Cross-Coupling. Acc. Chem. Res. 2019, 52 (1), 140–150. 10.1021/acs.accounts.8b00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clémancey M.; Cantat T.; Blondin G.; Latour J.-M.; Dorlet P.; Lefèvre G. Structural Insights into the Nature of Fe0 and FeI Low-Valent Species Obtained upon the Reduction of Iron Salts by Aryl Grignard Reagents. Inorg. Chem. 2017, 56, 3834–3848. 10.1021/acs.inorgchem.6b02616. [DOI] [PubMed] [Google Scholar]
- For recent references by some of us on 2-electron reductive eliminations in Fe-catalyzed couplings, see; a Rousseau L.; Herrero C.; Clémancey M.; Imberdis A.; Blondin G.; Lefèvre G. Evolution of Ate-Organoiron(II) Species towards Lower Oxidation States: Role of the Steric and Electronic Factors. Chem. - Eur. J. 2020, 26, 2417–2428. 10.1002/chem.201904228. [DOI] [PubMed] [Google Scholar]; b Wowk V.; Rousseau L.; Lefèvre G. Importance of Two-Electron Processes in Fe-Catalyzed Aryl-(hetero)aryl Cross-Couplings: Evidence of Fe0/FeII Couple Implication. Organometallics 2021, 40 (19), 3253–3266. 10.1021/acs.organomet.1c00338. [DOI] [Google Scholar]; c Zhou E.; Chourreu P.; Lefèvre N.; Ahr M.; Rousseau L.; Herrero C.; Gayon E.; Cahiez G.; Lefèvre G. Mechanistic Facets of the Competition between Cross-Coupling and Homocoupling in Supporting Ligand-Free Iron-Mediated Aryl–Aryl Bond Formations. ACS Org. Inorg. Au 2022, 2 (4), 359–369. 10.1021/acsorginorgau.2c00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich S. H.; Knochel P. Preparation of Functionalized Aryl Iron(II) Compounds and a Nickel-Catalyzed Cross-Coupling with Alkyl Halides. Angew. Chem., Int. Ed. 2009, 48, 9717–9720. 10.1002/anie.200905196. [DOI] [PubMed] [Google Scholar]
- a Norinder J.; Matsumoto A.; Yoshikai N.; Nakamura E. Iron-Catalyzed Direct Arylation through Directed C–H Bond Activation. J. Am. Chem. Soc. 2008, 130 (18), 5858–5859. 10.1021/ja800818b. [DOI] [PubMed] [Google Scholar]; b Doba T.; Matsubara T.; Ilies L.; Shang R.; Nakamura E. Homocoupling-free iron-catalysed twofold C–H activation/cross-couplings of aromatics via transient connection of reactants. Nat. Catal. 2019, 2, 400–406. 10.1038/s41929-019-0245-3. [DOI] [Google Scholar]; c Doba T.; Ilies L.; Sato W.; Shang R.; Nakamura E. Iron-catalysed regioselective thienyl C–H/C–H coupling. Nat. Catal. 2021, 4, 631–638. 10.1038/s41929-021-00653-7. [DOI] [Google Scholar]; d Doba T.; Shang R.; Nakamura E. Iron-Catalyzed C–H Activation for Heterocoupling and Copolymerization of Thiophenes with Enamines. J. Am. Chem. Soc. 2022, 144 (47), 21692–21701. 10.1021/jacs.2c09470. [DOI] [PubMed] [Google Scholar]
- Boddie T. E.; Carpenter S. H.; Baker T. M.; DeMuth J. C.; Cera G.; Brennessel W. W.; Ackermann L.; Neidig M. L. Identification and Reactivity of Cyclometalated Iron(II) Intermediates in Triazole-Directed Iron-Catalyzed C–H Activation. J. Am. Chem. Soc. 2019, 141 (31), 12338–12345. 10.1021/jacs.9b05269. [DOI] [PubMed] [Google Scholar]
- Maddock L. C. H.; Mu M.; Kennedy A. R.; García-Melchor M.; Hevia E. Facilitating the Ferration of Aromatic Substrates through Intramolecular Sodium Mediation. Angew. Chem., Int. Ed. 2021, 60, 15296–15301. 10.1002/anie.202104275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Tolman C. A.; Ittel S. D.; English A. D.; Jesson J. P. The Chemistry of 2-Naphthyl Bis[bis(dimethylphosphino)ethane] Hydride Complexes of Fe, Ru, and Os. 1. Characterization and Reactions with H2 and Lewis Base Ligands. J. Am. Chem. Soc. 1978, 100, 4080–4089. 10.1021/ja00481a016. [DOI] [Google Scholar]; b Tolman C. A.; Ittel S. D.; English A. D.; Jesson J. P. Chemistry of 2-naphthyl bis[bis(dimethylphosphino)ethane] hydride complexes of iron, ruthenium, and osmium. 3. Cleavage of sp2 carbon-hydrogen bonds. J. Am. Chem. Soc. 1979, 101, 1742–1751. 10.1021/ja00501a017. [DOI] [Google Scholar]; c Baker M. V.; Field L. D. Reaction of sp2 carbon-hydrogen bonds in unactivated alkenes with bis(diphosphine) complexes of iron. J. Am. Chem. Soc. 1986, 108, 7433–7434. 10.1021/ja00283a062. [DOI] [Google Scholar]; d Baker M. V.; Field L. D. Reaction of carbon-hydrogen bonds in alkanes with bis(diphosphine) complexes of iron. J. Am. Chem. Soc. 1987, 109, 2825–2826. 10.1021/ja00243a046. [DOI] [Google Scholar]
- a Dombray T.; Werncke C. G.; Jiang S.; Grellier M.; Vendier L.; Bontemps S.; Sortais J.-B.; Sabo-Etienne S.; Darcel C. Iron-Catalyzed C–H Borylation of Arenes. J. Am. Chem. Soc. 2015, 137 (12), 4062–4065. 10.1021/jacs.5b00895. [DOI] [PubMed] [Google Scholar]; b Britton L.; Docherty J. H.; Sklyaruk J.; Cooney J.; Nichol G. S.; Dominey A. P.; Thomas S. P. Iron-catalysed alkene and heteroarene H/D exchange by reversible protonation of iron-hydride intermediates. Chem. Sci. 2022, 13, 10291–10298. 10.1039/D2SC03802A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camadanli S.; Beck R.; Flörke U.; Klein H.-F. C–H Activation of Imines by Trimethylphosphine-Supported Iron Complexes and Their Reactivities. Organometallics 2009, 28 (7), 2300–2310. 10.1021/om800828j. [DOI] [Google Scholar]
- Kimura N.; Kochi T.; Kakiuchi F. Iron-Catalyzed Regioselective Anti-Markovnikov Addition of C–H Bonds in Aromatic Ketones to Alkenes. J. Am. Chem. Soc. 2017, 139 (42), 14849–14852. 10.1021/jacs.7b08385. [DOI] [PubMed] [Google Scholar]
- Messinis A. M.; Finger L. H.; Hu L.; Ackermann L. Allenes for Versatile Iron-Catalyzed C–H Activation by Weak O-Coordination: Mechanistic Insights by Kinetics, Intermediate Isolation, and Computation. J. Am. Chem. Soc. 2020, 142, 13102–13111. 10.1021/jacs.0c04837. [DOI] [PubMed] [Google Scholar]
- Kimura N.; Katta S.; Kitazawa Y.; Kochi T.; Kakiuchi F. Iron-Catalyzed Ortho C–H Homoallylation of Aromatic Ketones with Methylenecyclopropanes. J. Am. Chem. Soc. 2021, 143 (12), 4543–4549. 10.1021/jacs.1c00237. [DOI] [PubMed] [Google Scholar]
- For the 31P/1H NMR characterizations of complexes 1, 3′, 3″, see ref (11a); for complexes 2Ph, 4C6D5,D, 4Ph,H, see ref (11b). For complex {5–;Na+} see; a Maddock L. C. H.; Cadenbach T.; Kennedy A. R.; Borilovic I.; Aromí G.; Hevia E. Accessing Sodium Ferrate Complexes Containing Neutral and Anionic N-Heterocyclic Carbene Ligands: Structural, Synthetic, and Magnetic Insights. Inorg. Chem. 2015, 54 (18), 9201–9210. (1H NMR spectroscopy) and 10.1021/acs.inorgchem.5b01638. [DOI] [PubMed] [Google Scholar]; b Eichhöfer A.; Lan Y.; Mereacre V.; Bodenstein T.; Weigend F. Slow Magnetic Relaxation in Trigonal-Planar Mononuclear Fe(II) and Co(II) Bis(trimethylsilyl)amido Complexes—A Comparative Study. Inorg. Chem. 2014, 53 (4), 1962–1974. (57Fe-Mössbauer spectroscopy) 10.1021/ic401677j. [DOI] [PubMed] [Google Scholar]
- a Salanouve E.; Bouzemame G.; Blanchard S.; Derat E.; Desage-El Murr M.; Fensterbank L. Tandem C–H Activation/Arylation Catalyzed by Low-Valent Iron Complexes with Bisiminopyridine Ligands. Chem. - Eur. J. 2014, 20, 4754–4761. 10.1002/chem.201304459. [DOI] [PubMed] [Google Scholar]; b Yang D.; Yi H.; Chen H.; Qi X.; Lan Y.; Pao C.-W.; Lee J.-F.; Zhang H.; Chen Y.-H.; Lei A. Revealing the reduction process of Cu(ii) by sodium bis(trimethylsilyl)amide. Faraday Discuss. 2019, 220, 105–112. 10.1039/C9FD00034H. [DOI] [PubMed] [Google Scholar]
- Crabtree R. H.The Organometallic Chemistry of the Transition Metals; John Wiley & Sons, Inc.: Hoboken, NJ, 2005; pp 1–39. [Google Scholar]
- Banerjee S.; Yang Y.-F.; Jenkins I. D.; Liang Y.; Toutov A. A.; Liu W.-B.; Schuman D. P.; Grubbs R. H.; Stoltz B. M.; Krenske E. H.; Houk K. N.; Zare R. N. Ionic and Neutral Mechanisms for C–H Bond Silylation of Aromatic Heterocycles Catalyzed by Potassium tert-Butoxide. J. Am. Chem. Soc. 2017, 139 (20), 6880–6887. 10.1021/jacs.6b13032. [DOI] [PubMed] [Google Scholar]
- Detection of smaller quantities of well-defined Fe0 species when an excess of Na or K(hmds) is used (compare entries 8–9 (10 equiv M(hmds)) with entries 2–3 (2 equiv M(hmds))) is probably due to the strong reducing power of the latter, leading to the formation of higher quantities of ill-defined aggregates during the reduction course of 1.
- 3*: δ(31P) = 61 ppm, d, J(31P-31P) = 12 Hz, to be compared with δ(31P) = 60.4 ppm and J(31P-31P) = 10 Hz for 3′; 9.1 ppm, m, (7.2 ppm for 3′). The nuclearity of 3* has further been probed by using 57Fe-labeled starting material (Figure 1b), and a diagnostic splitting of the 31P NMR signal of 57Fe-3* is observed (J(31P-57Fe) = 49 Hz, to be compared with J(31P-57Fe) = 39 Hz for 57Fe-4C6D5,D), confirming that 3* is a monometallic Fe0 well-defined complex, structurally close to 3′.
- a Gütlich P.; Bill E.; Trautwein A. X.. Mössbauer Spectroscopy and Transition Metal Chemistry: Fundamentals and Applications, 2011th ed.; Springer, 2010. [Google Scholar]; b The isomer shift observed for this species, slightly lower than that of 2Ph′ (0.19 mm/s vs 0.24 mm/s, Figure 1c and 2d), is also consistent with the substitution of one bromine ligand in 2Ph′ by a more electron-donating hydride ligand in 4C6D5,D;; c Herber R. H.; Hayter R. G. Mössbauer Effect in cis-trans Isomers. J. Am. Chem. Soc. 1964, 86 (2), 301–302. 10.1021/ja01056a053. [DOI] [Google Scholar]
- Use of 12-C-4 crown-ether, which displays a better affinity for LiI cations than 15-C-5, was first used, but significant amounts of 12-C-4 ring-opening were observed. 15-C-5 however also leads to well-defined lithium adducts, in which the LiI cation is coordinated by the five O atoms from the crown ether; seeBoulatov R.; Du B.; Meyers E. A.; Shore S. G. Two Novel Lithium-15-Crown-5 Complexes: An Extended LiCl Chain Stabilized by Crown Ether and a Dimeric Complex Stabilized by Hydrogen Bonding with Water. Inorg. Chem. 1999, 38, 4554–4558. 10.1021/ic9904790. [DOI] [PubMed] [Google Scholar]
- Since ferration of C6D6 is still observed when the Lewis acidity of LiI is quenched by 15-C-5 crown ether, it is not excluded that formation of the C-Fe bond proceeds within a concerted metalation mechanism in which acidity of C-D bond is triggered by complexation to the iron, as LiI cations can usually enhance the acidity of such bonds by formation of π-arene complexes, see reference (20).
- 2Ph′ was chosen as a synthetic target as no satisfying elemental analyses for the chlorinated analogue 2Ph were obtained (see SI for analytic data related to 2Ph′).
- Liu L.; Aguilera M. C.; Lee W.; Youshaw C. R.; Neidig M. L.; Gutierrez O. General method for iron-catalyzed multicomponent radical cascades–cross-couplings. Science 2021, 374, 432–439. 10.1126/science.abj6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bowen R. J.; Fernandes M. A.; Hitchcock P. B.; Lappert M. F.; Layh M. Synthesis and crystal structures of novel 1-aza-2-silacyclobut-3-enes. J. Chem. Soc., Dalton Trans. 2002, 3253–3259. 10.1039/b202629p. [DOI] [Google Scholar]; b Carravetta M.; Concistre M.; Levason W.; Reid G.; Zhang W. Unique Group 1 cations stabilised by homoleptic neutral phosphine coordination. Chem. Commun. 2015, 51, 9555–9558. 10.1039/C5CC03184B. [DOI] [PubMed] [Google Scholar]
- For a recent frontier article written by us on the role of such main-group cations in Fe-mediated cross-couplings, seeWowk V.; Lefèvre G. The crucial and multifaceted roles of main-group cations and their salts in iron-mediated cross-couplings. Dalton Trans. 2022, 51, 10674–10680. 10.1039/D2DT00871H. [DOI] [PubMed] [Google Scholar]
- Messinis A. M.; Luckham S. L. J.; Wells P. P.; Gianolio D.; Gibson E. K.; O’Brien H. M.; Sparkes H. A.; Davis S. A.; Callison J.; Elorriaga D.; Hernandez-Fajardo O.; Bedford R. B. The highly surprising behaviour of diphosphine ligands in iron-catalysed Negishi cross-coupling. Nat. Catal. 2019, 2, 123–133. 10.1038/s41929-018-0197-z. [DOI] [Google Scholar]
- Kyne S. H.; Lefèvre G.; Ollivier C.; Petit M.; Ramis Cladera V.-A.; Fensterbank L. Iron and cobalt catalysis: new perspectives in synthetic radical chemistry. Chem. Soc. Rev. 2020, 49, 8501–8542. 10.1039/D0CS00969E. [DOI] [PubMed] [Google Scholar]
- a Benn R.; Lehmkuhl H.; Mehler K.; Rufinska A. 25Mg-NMR: A Method for the Characterization of Organomagnesium Compounds, their Complexes, and Schlenk Equilibria. Angew. Chem., Int. Ed. 1984, 23, 534–535. 10.1002/anie.198405341. [DOI] [Google Scholar]; b Gindelberger D. E.; Arnold J. Preparation and Properties of Magnesium, Calcium, Strontium, and Barium Selenolates and Tellurolates. Inorg. Chem. 1994, 33, 6293–6299. 10.1021/ic00104a045. [DOI] [Google Scholar]
- Uchiyama M.; Matsumoto Y.; Nakamura S.; Ohwada T.; Kobayashi N.; Yamashita N.; Matsumiya A.; Sakamoto T. Development of a Catalytic Electron Transfer System Mediated by Transition Metal Ate Complexes: Applicability and Tunability of Electron-Releasing Potential for Organic Transformations. J. Am. Chem. Soc. 2004, 126 (28), 8755–8759. 10.1021/ja039674a. [DOI] [PubMed] [Google Scholar]
- a Shen K.; Fu Y.; Li J.-N.; Liu L.; Guo Q.-X. What are the pKa values of C–H bonds in aromatic heterocyclic compounds in DMSO?. Tetrahedron 2007, 63, 1568–1576. 10.1016/j.tet.2006.12.032. [DOI] [Google Scholar]; b Kim D.; Lee G. S.; Kim D.; Hong S. H. Direct C(sp2)–H alkylation of unactivated arenes enabled by photoinduced Pd catalysis. Nat. Commun. 2020, 11, 5266 10.1038/s41467-020-19038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bauer W.; von Ragué Schleyer P. Mechanistic Evidence for Ortho-Directed Lithiations from One- and Two-Dimensional NMR Spectroscopy and MNDO Calculations. J. Am. Chem. Soc. 1989, 111, 7191–7198. 10.1021/ja00200a044. [DOI] [Google Scholar]
- Docherty J. H.; Peng J.; Dominey A. P.; Thomas S. P. Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide. Nat. Chem. 2017, 9, 595–600. 10.1038/nchem.2697. [DOI] [PubMed] [Google Scholar]
- Kopf S.; Bourriquen F.; Li W.; Neumann H.; Junge K.; Beller M. Recent Developments for the Deuterium and Tritium Labeling of Organic Molecules. Chem. Rev. 2022, 122, 6634–6718. 10.1021/acs.chemrev.1c00795. [DOI] [PubMed] [Google Scholar]
- Gentner T. X.; Mulvey R. E. Alkali-Metal Mediation: Diversity of Applications in Main-Group Organometallic Chemistry. Angew. Chem., Int. Ed. 2021, 60, 9247–9262. 10.1002/anie.202010963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.