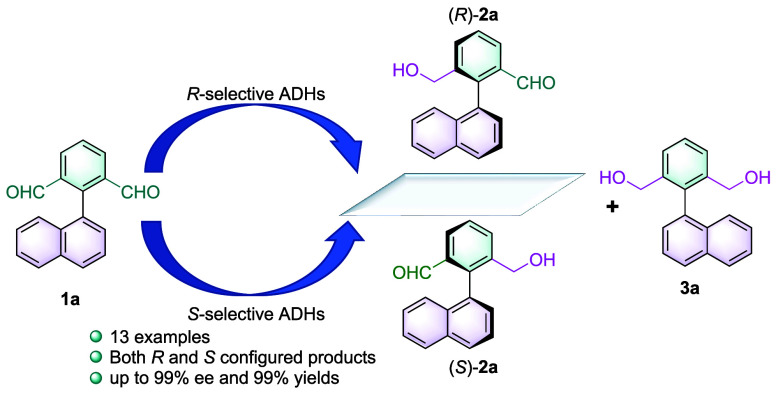
Abstract
Axially chiral aldehydes have emerged recently as a unique class of motifs for drug design. However, few biocatalytic strategies have been reported to construct structurally diverse atropisomeric aldehydes. Herein, we describe the characterization of alcohol dehydrogenases to catalyze atroposelective desymmetrization of the biaryl dialdehydes. Investigations into the interactions between the substrate and key residues of the enzymes revealed the distinct origin of atroposelectivity. A panel of 13 atropisomeric monoaldehydes was synthesized with moderate to high enantioselectivity (up to >99% ee) and yields (up to 99%). Further derivatization allows enhancement of the diversity and application potential of the atropisomeric compounds. This study effectively expands the scope of enzymatic synthesis of atropisomeric aldehydes and provides insights into the binding modes and recognition mechanisms of such molecules.
Keywords: atropisomers, axially chiral compounds, alcohol dehydrogenases, desymmetrization, biaryl aldehydes
Introduction
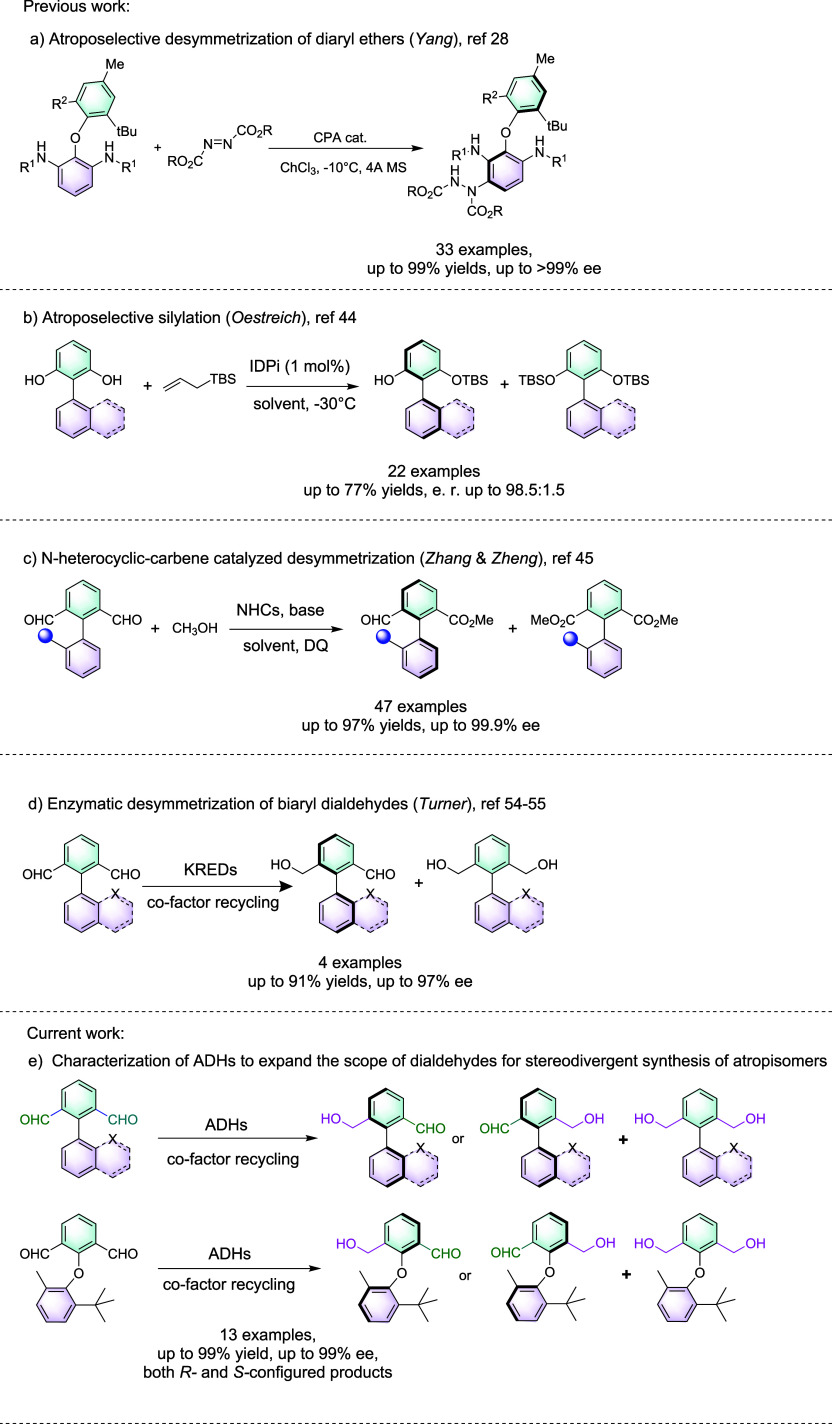
Atropisomerism refers to stereoisomerism caused by restricted bond rotation and was discovered by Christie and Kenner in 1922.1 It frequently constitutes vital structural elements for natural products,2−5 functional materials,6,7 molecular machines,8 and bioactive molecules.9−11 Various strategies have been developed for the construction of the stereogenic axis or asymmetric induction after formation of the atropisomeric bond, including desymmetrization, kinetic resolution (KR), and dynamic kinetic resolution (DKR).12−19 Various synthetic methodologies, such as organocatalysis20,21 utilizing N-heterocyclic carbene (NHC),22−27 chiral phosphoric acid (CPA),28−33 chiral secondary amine,34 thiourea,35,36 and peptide37 catalysts, have been reported in the past decade. Metal catalysis has also been applied in the atroposelective synthesis utilizing Cu,38 Pd,39−41 Ir,42 and Rh43 catalysts. Very recently, Yang and co-workers reported the CPA-catalyzed desymmetrization of diaryl ether amines with azodicarboxylates, which afforded diaryl ethers in up to 99% yields and >99% ee (Scheme 1a).28 Other desymmetrization strategies were reported by Zhu et al., who employed the imidodiphosphorimidate (IDPi)-catalyzed silylation of biaryl diols to enable the atroposelective kinetic resolution with up to 77% yields and 98.5:1.5 er (Scheme 1b).44 In addition, Wu et al. reported the NHC-catalyzed desymmetrization followed by kinetic resolution of biaryl dialdehydes to afford 47 value-added, structurally diverse aldehydes in up to 97% yields and >99% ee (Scheme 1c).45 However, requirements for directing groups and additional removal steps; difficulties in the synthesis of the complex chiral ligands, auxiliaries, or catalysts; and stoichiometric addition of selected catalysts or reagents are often encountered in chemical synthetic methods. Conversely, biocatalytic asymmetric synthesis of axially chiral compounds may offer distinct advantages, such as environmental benignity, mild conditions, and superior selectivity. Currently, only a handful of examples have been reported so far to synthesize biaryls in high atroposelectivity during the past decade utilizing biocatalysis.46,47 Enzymatic atroposelective coupling reactions by laccases,48 copper-dependent oxidase,49 and cytochrome P450 enzymes50−52 have been previously explored, albeit with very limited substrate scope and atroposelectivity. Recently, flavin-dependent halogenases (FDHs) have enabled highly stereoselective DKR of 3-aryl-4(3H)-quinazolinones; nevertheless, the substrate loadings were limited, thus preventing further application utility. Vanadium chloroperoxidase from Curvularia inaequalis (CiVCPO) can catalyze the tribromination of biaryls to transform the freely rotating biaryl axis to sterically hindered bond, but only in racemic forms.53 We and others have established that ketoreductases (KREDs) or alcohol dehydrogenases (ADHs) can be utilized in the desymmetrization, KR, and DKR of biaryl dialdehydes or diaryl ethers (Scheme 1d).54−56 However, the scope was limited to less than five substrates, the mechanisms of enzymatic atroposelective recognition for biaryls or diaryl ethers were not investigated, and only a limited number of commercial KREDs have been utilized. In this endeavor, we demonstrate that screening against a large panel of structurally diverse ADHs enables the synthesis of both (R)- and (S)-configured atropisomeric biaryls (Scheme 1e), and the differences in the interactions between the substrates and the enzymes have led to the stereodivergent desymmetrization of the biaryl and diaryl ether dialdehydes.
Scheme 1. Representative Atroposelective Desymmetrizations.
Results and Discussion
To first identify highly active and selective ADHs toward the biaryl substrates, we employed 1a as the model substrate to screen a panel of 92 ADHs (phylogenetic analysis of the ADHs is shown in Figure S1) from a variety of sources (full panel results are shown in Tables S3 and S4). To our delight, a number of wild-type ADHs displayed high activity toward 1a, which led to either (R)- or (S)-2a. The 10 best-performing ADHs that exhibited the highest activity and selectivity were selected (Table 1). The turnover number (TON, [product]final × [catalysts]−1) of ADH-R9 was up to 481 (the enzyme content was estimated as shown in Figure S2). ADH-R2 and ADH-R4 afforded the (S)-2a in ee > 99%, whereas ADH-R7, ADH-R9, and ADH-R10 afforded (R)-2a with ee > 99%. The yields of 2a were also high (>87%). A majority of short-chain dehydrogenases (SDRs) (except ADH-R7) displayed clear preference toward (S)-2a, and all medium-chain dehydrogenases (MDRs) displayed preference toward (R)-2a.
Table 1. Screening of ADHs Utilizing 1a as the Model Substratea.
| entry | enzyme | sources | types | 2a [%] | 3a [%] | ee [%] | configuration of 2a |
|---|---|---|---|---|---|---|---|
| 1 | ADH-R1 | Burkholderia gladioli | SDR | 94.3 ± 3.1 | 3.0 ± 0.8 | 80.4 ± 0.8 | S |
| 2 | ADH-R2 | Ralstonia sp. | SDR | 92.4 ± 2.5 | 1.9 ± 0.9 | 99.9 ± 0.1 | S |
| 3 | ADH-R3 | Lactobacillus kefiri | SDR | 47.7 ± 0.3 | 1.6 ± 0.3 | 70.1 ± 0.7 | S |
| 4 | ADH-R4 | Oenococcus alcoholitolerans | SDR | 87.5 ± 0.7 | 11.4 ± 1.8 | 99.4 ± 0.2 | S |
| 5 | ADH-R5 | Zymomonas mobilis | MDR | 87.9 ± 1.8 | 2.9 ± 0.4 | 88.3 ± 0.0 | R |
| 6 | ADH-R6 | Parageobacillus thermoglucosidasius | MDR | 90.6 ± 1.8 | 2.6 ± 0.4 | 92.5 ± 1.5 | R |
| 7 | ADH-R7 | Thermus thermophilus | SDR | 95.4 ± 0.3 | 3.7 ± 0.3 | 99.9 ± 0.1 | R |
| 8 | ADH-R8 | Rhodopseudomonas palustris | MDR | 93.5 ± 1.4 | 2.9 ± 0.6 | 95.5 ± 5.4 | R |
| 9 | ADH-R9 | Rhizobium etli | MDR | 96.7 ± 1.2 | 2.9 ± 1.0 | 99.4 ± 0.1 | R |
| 10 | ADH-R10 | Pseudomonas meliae | MDR | 95.5 ± 0.9 | 4.3 ± 0.9 | 99.9 ± 0.1 | R |
Reaction conditions: 1a (10 mM), NADP+ (1 mM), glucose (20 mM), glutamate dehydrogenase (GDH) (2.5 mg mL–1), ADHs (0.1 g mL–1 of whole cell with 6 units mL–1 of DNaseI and 1 mg mL–1 of lysozyme), DMSO (5% v/v) in NaPi buffer (pH 7.4, 50 mM), at 30 °C, 800 rpm. Total volume: 1 mL.
Subsequently, the substrate concentrations and the compositions of the cosolvent DMSO were optimized (Figures S4 and S5). Ten mM 1a in sodium phosphate buffer (NaPi buffer) with 5% DMSO was selected as the optimal reaction conditions. The temperature and pH of the reactions were employed with reference to previous reports.55 Time-course profiles of the desymmetrization of 1a by the 10 best-performing enzymes were investigated to examine the formation of 2a (Figures S6–S15). The over-reduced product 3a can also be observed in <5% conversions when catalyzed by a majority of the ADHs except ADH-R4 (11.4%, Table 1, entry 4). The ee for 2a reached a maximum in 3–6 h in all cases, but the yields of 2a exhibited different time-course profiles for various enzymes. For example, 2a was consumed rapidly after 3 h when the reaction was catalyzed by ADH-R1 and R2 compared with no consumption after reaching the maximum yield with ADH-R7-R9. We also performed the kinetic resolution of rac-2a employing selected ADHs, and indeed, the catalytic activities varied among different enzymes. As shown in Table S5, while ADH-R3, ADH-R5-6, and ADH-R8 are inactive toward rac-2a, the rest of enzymes are able to catalyze the kinetic resolution of 2a with moderate conversions. ADH-R2 exhibited the highest activity for kinetic resolution of 2a, and therefore, as shown in Figure S7, the composition of 2a rapidly declined after 3 h. The ee of 2a did not decrease since the desymmetrization formed (S)-2a, and the kinetic resolution consumed (R)-2a, and vice versa. The absolute configuration of the substrates was confirmed by comparison with reported analytical methodologies,55 and the rotation barriers of the monoaldehydes have also been reported previously.54
Having established the optimal reaction conditions, the generality of the ADH-catalyzed desymmetrization of biaryl dialdehydes was explored. First, the scope of the biaryl framework of the dialdehydes was examined (Scheme 2). A variety of substrates 1b–m were synthesized and characterized. The racemic standards for 2a–m were established by reducing the dialdehydes by boronic hydride reagents (Figures S16–S47). The electronic effects on the naphthalene ring of the dialdehydes were investigated by varying the functional motifs. Substrates bearing electron-withdrawing (F) and electron-donating (Me, OMe) substituents are well tolerated to afford desired products 2b–2d in good yields (up to 98%) and ee (up to 99% for R-configuration and up to 98% for S-configuration). Excellent ee could also be obtained for the phenanthrene-substituted product (S)-2f. Substrates bearing phenyl (1e), methyl (1h), vinyl (1i), halogen (1k), or alkyl (1l) substituents on phenyl rings of the dialdehydes react well to furnish the desired biaryl monoaldehyde atropisomers at up to 99% yields with access to both (R)- and (S)-configured products in up to 99% ee. (R)-2j with two substitutions has been obtained in excellent ee, but only moderate to good yields and ee have been obtained for (S)-2j. The diaryl ether product (R)-2m was obtained in good yields and excellent ee (up to 99%), but no enzyme showed (S)-selectivity for this substrate. ADH-R1, -R2, and -R4 catalyzed the formation of (S)-configured products for the substrates 1a, 1b, 1c, 1g, 1h, 1i, and 1l. Correspondingly, (R)-monoaldehydes can be obtained for the same range of substrates catalyzed by ADH-R7, ADH-R9, and ADH-R10. These substrates have relatively large or hydrophobic motifs at the phenyl ring and may have situated in the active sites of the enzymes in similar poses. However, when the size of the substituent increases to the anthracyl group, ADH-R1, -R9, and -R10 all catalyze the formation of (S)-2f, thereby indicating different binding modes compared with the model substrate. The selectivity for ADH-R7, -R9, and -R10 are reversed utilizing 1k as the substrate, and this is possibly due to change in the Cahn–Ingold–Prelog rule for 2k compared with 2a. This is not the case for 2k and 2h, which display reversed selectivity possibly due to different interactions between the substrates and the enzymes (Figure S3). Overall, there is no definitive correlation between the type of substituent and the selectivity. Further mechanisms of the enzymatic recognition of substrates have been analyzed to provide explanations at molecular levels.
Scheme 2. Substrate Scope for the ADH-Catalyzed Desymmetrization of Prochiral Dialdehydes.
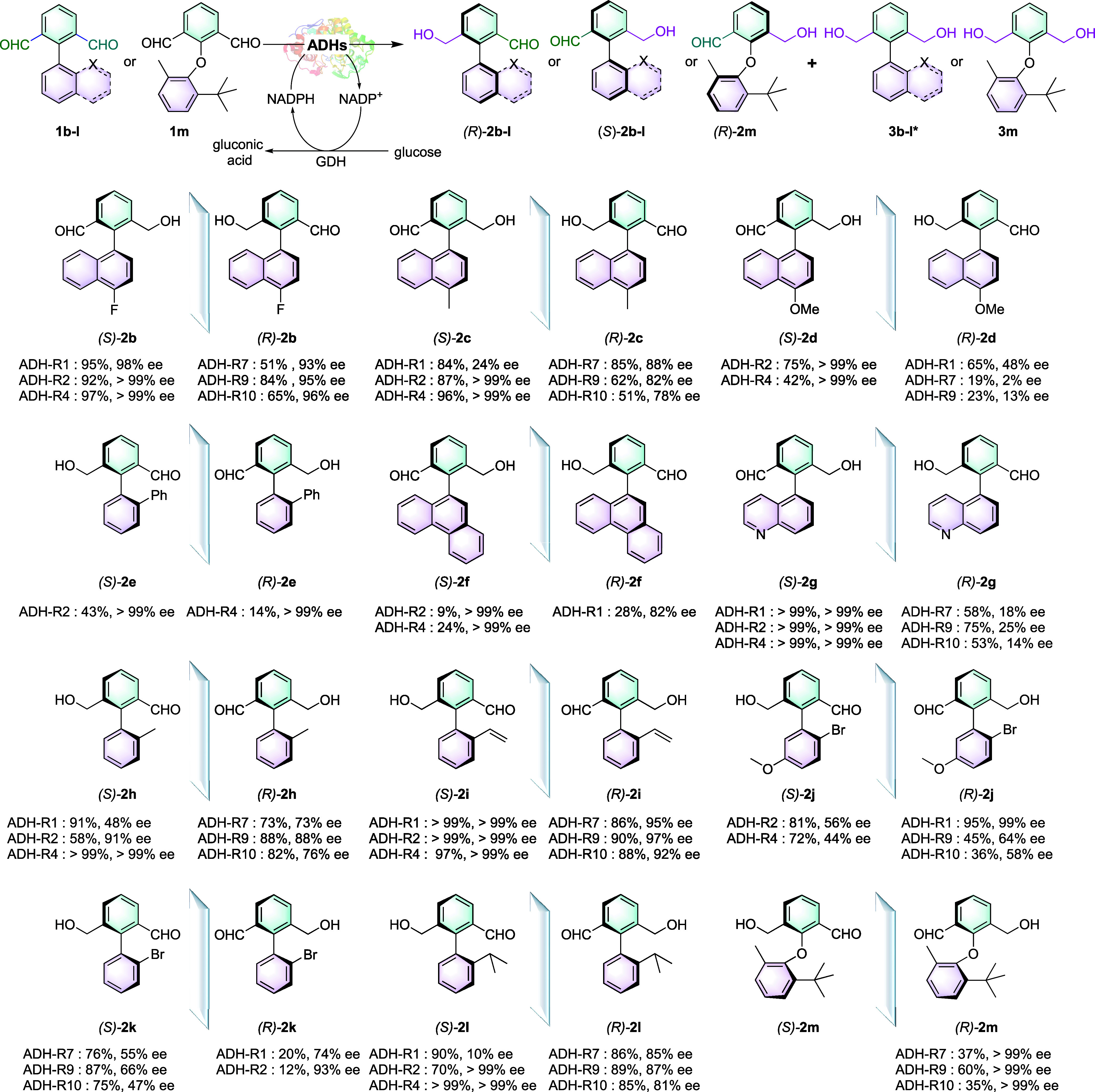
Reaction conditions: 1a (10 mM), NADP+ (1 mM), glucose (20 mM), GDH (2.5 mg mL–1), ADHs (0.1 g mL–1 of whole cell with 6 units mL–1 of DNaseI and 1 mg mL–1 of lysozyme), DMSO (5% v/v) in NaPi buffer (pH 7.4, 50 mM), at 30 °C, 3–24 h, 800 rpm. Total volume: 1 mL. *3b (5% by ADH-R2), 3c (3% by ADH-R1), 3i (3% by ADH-R7), 3k (16% by ADH-R1 and 53% by ADH-R2), 3l (2% by ADH-R1) were formed at the sampling time, and the diol products were not found for the rest of the substrates.
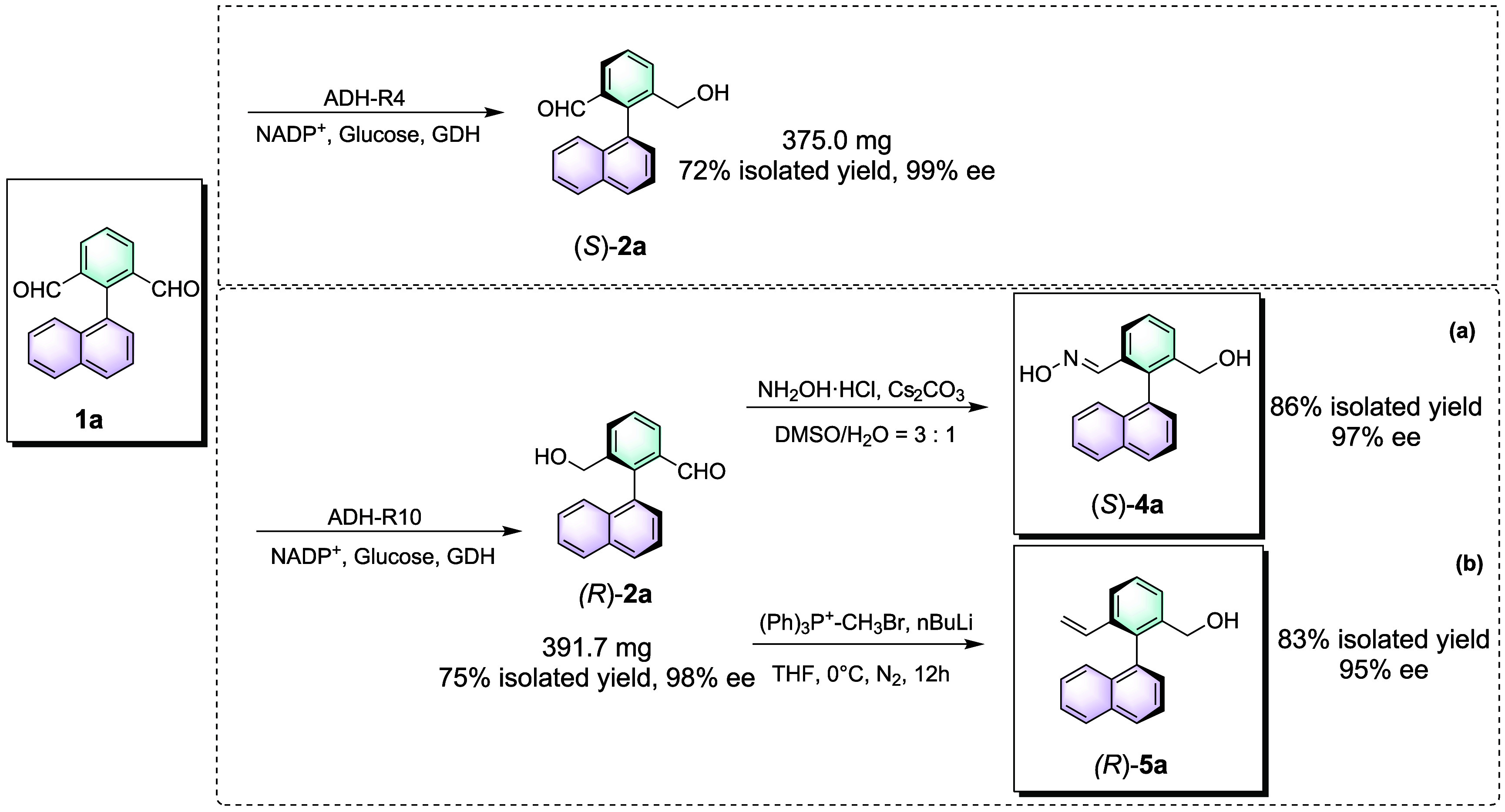
Subsequently, we demonstrated synthetic applications of the ADH-catalyzed atroposelective desymmetrization of biaryl dialdehydes by performing semipreparative scale reaction of 1a with ADH-R10 to afford (R)-2a and ADH-R4 to afford (S)-2a (Scheme 3). The isolated products were obtained, respectively in 375.0 and 391.7 mg amounts with almost perfect ee (98–99%) and good isolated yields (72–75%). Further derivatization of the atropisomeric (R)-2a is illustrated in Scheme 3. Condensation with hydroxylamine hydrochloride produced 4a in 86% isolated yield and 97% ee (Scheme 3a). (R)-2a could also undergo a Wittig reaction to afford 5a in 83% isolated yield and 95% ee (Scheme 3b).
Scheme 3. Semipreparative Scale Reactions and Synthetic Transformations of Atropisomeric Biaryls.
To gain further insights into the interactions between the substrates and key binding residues of the enzymes and the mechanisms underlying atroposelectivity, docking of 1a to binding sites of ADH-R4 and ADH-R10 were performed utilizing Autodock 4.2, respectively.57 ADH-R4 and ADH-R10 afforded either (S)-2a or (R)-2a with >99% ee, respectively. ADH-R4 is a SDR from Oenococcus alcoholitolerans (KGO31568.1), and ADH-R10 is an MDR from Pseudomonas meliae (WP_044345496.1). We predicted the structures using AlphaFold258 and identified the positions of the cofactor of ADH-R4 and ADH-R10 with reference to the previously reported crystal structures (PDB ID: 4RF2 and 3OX4). Subsequently, the interactions between the substrate and enzymes were analyzed (Figure 1a,b). The residues S144 and Y157 from ADH-R4 form hydrogen bonds with the substrate, which is the main driving force for 1a to situate at a position where the pro-S hydroxyl group points to the catalytic hydride transfer site of the cofactor NADPH. In addition, interactions to the naphthyl ring by surrounding residues can also be observed, which may also contribute to the binding pose of 1a. Similarly, hydrogen bonds between residue H266 and the hydroxyl group at 1a are the major contributing factor for the formation of (R)-2a. Therefore, 1a is situated in different positions at different binding pockets of ADHs, which leads to reversed atroposelectivity.
Figure 1.
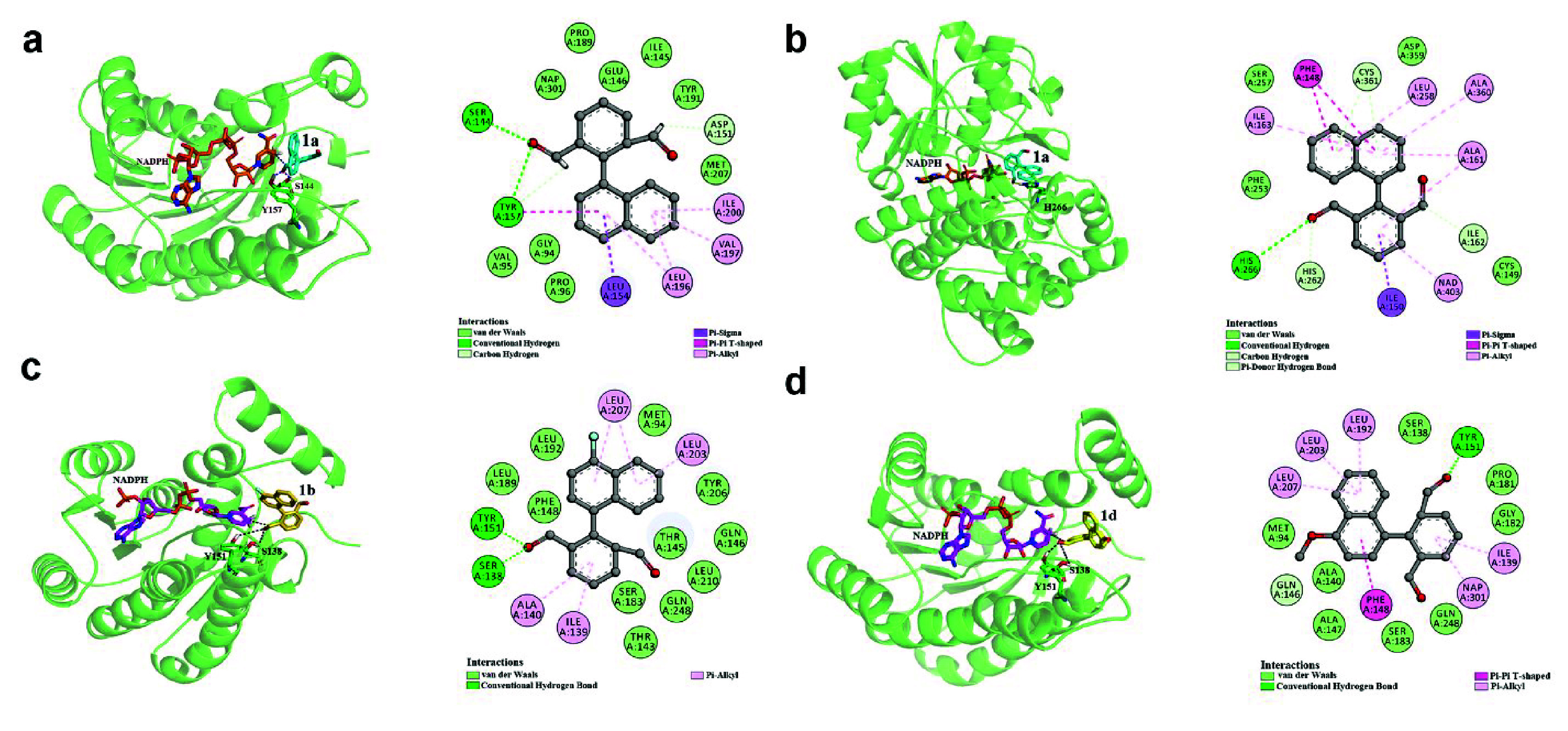
Investigations into the origin of atroposelectivity. (a) Docking model of 1a at ADH-R4. (b) Docking model of 1a at ADH-R10. (c) Docking model of 1b at ADH-R1. (d) Docking model of 1d at ADH-R1.
Furthermore, pro-atropisomeric 1b and 1d were converted to (S)-2b and (R)-2d by ADH-R1, respectively (Figure 1c,d). Both substrates were docked into ADH-R1 from Burkholderia gladioli (WP_013688875.1, structure predicted by AlphaFold2). The residues Y151 and S138 at ADH-R1 form hydrogen bonds with the carbonyl group at 1b, whereas only Y151 forms hydrogen bonds with 1d. This difference is possibly the origin of the lower atroposelectivity for ADH-R1 toward 1d than 1b and it correlates well with the experimental results. In addition, the naphthyl rings at 1b and 1d are positioned differently. π-Alkyl interactions are observed between residues L203 and L207 and the naphthyl ring of 1b, whereas additional π–π or π-alkyl interactions are formed between residues L192 and F148 and the naphthyl ring at 1d because of a different binding pose of 1d caused by steric hindrance of the methoxy substitutions at 1d. Therefore, with only the replacement of fluoro- to methoxy-substitution at the naphthyl rings of the substrates, the configuration of the products is reversed. In summary, the recognition of pro-atropisomeric substrates and origin of atroposelectivity of ADHs are distinct from the center-chiral substrates where the dihedral angle for the hydride transfer to the carbonyl groups on the substrates is the major contributing factor to the configuration of the products.59,60
Conclusions
In summary, we have developed the highly efficient asymmetric synthesis of axially chiral biaryls and diaryl ethers by ADH-catalyzed desymmetrization. Several ADHs were identified and characterized to catalyze the formation of a range of atropisomeric monoaldehydes in moderate to excellent ee and yields with both (R)- and (S)-configuration. The reactions are easy to scale up with good to high isolated yields and can be further derivatized to afford novel products. The current methodologies greatly expand the scope of enzymatic atroposelective reactions of biaryls and diaryl ethers. Future work may include engineering of the enzymes with the purpose of improving the selectivity and activity for the stereodivergent synthesis of axially chiral products to meet application requirements.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 32171462), the National Key Research and Development Program of China (No. 2019YFA0905100), Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (No. TSBICIP-CXRC-040), and the Natural Science Foundation Applying System of Tianjin (No. 21JCJQJC00110).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.3c00814.
General information, preparation of alcohol dehydrogenases, procedures for molecular docking simulations and alcohol dehydrogenases-catalyzed desymmetrization of biaryl dialdehydes, supplementary figures, synthesis of substrates, HPLC, and 1H and 13C NMR spectra (PDF)
Author Contributions
# These authors contributed equally. CRediT: Mengjing Ye data curation, validation; Congcong Li data curation, software; Dongguang Xiao conceptualization, supervision; Ge Qu conceptualization, supervision; Bo Yuan conceptualization, supervision, writing-original draft, writing-review & editing; Zhoutong Sun supervision.
The authors declare no competing financial interest.
Special Issue
Published as part of JACS Auvirtual special issue “Biocatalysis in Asia and Pacific”.
Supplementary Material
References
- Christie G. H.; Kenner J. The molecular configurations of polynuclear aromatic compounds. Part I. The resolution of γ-6 : 6′-dinitro- and 4 : 6 : 4′ : 6′-tetranitro-diphenic acids into optically active components. J. Chem. Soc. Trans. 1922, 121, 614–620. 10.1039/CT9222100614. [DOI] [Google Scholar]
- Bringmann G.; Gulder T.; Gulder T. A. M.; Breuning M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 2011, 111, 563–639. 10.1021/cr100155e. [DOI] [PubMed] [Google Scholar]
- Kozlowski M. C.; Morgan B. J.; Linton E. C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 2009, 38, 3193–3207. 10.1039/b821092f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg S. H.; Gao Y.; Walker A. S.; Helfrich E. J. N.; Clardy J.; Baran P. S. Total synthesis reveals atypical atropisomerism in a small-molecule natural product, tryptorubin A. Science 2020, 367, 458–463. 10.1126/science.aay9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldemir H.; Richarz R.; Gulder T. A. M. The biocatalytic repertoire of natural biaryl formation. Angew. Chem., Int. Ed. 2014, 53, 8286–8293. 10.1002/anie.201401075. [DOI] [PubMed] [Google Scholar]
- Pu L. Enantioselective fluorescent sensors: a tale of binol. Acc. Chem. Res. 2012, 45, 150–163. 10.1021/ar200048d. [DOI] [PubMed] [Google Scholar]
- Zhang D. W.; Li M.; Chen C. F. Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem. Soc. Rev. 2020, 49, 1331–1343. 10.1039/C9CS00680J. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Calupitan J. P.; Rojas T.; Tumbleson R.; Erbland G.; Kammerer C.; Ajayi T. M.; Wang S.; Curtiss L. A.; Ngo A. T.; Ulloa S. E.; Rapenne G.; Hla S. W. A chiral molecular propeller designed for unidirectional rotations on a surface. Nat. Commun. 2019, 10, 3742. 10.1038/s41467-019-11737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden J.; Moran W. J.; Edwards P. J.; LaPlante S. R. The challenge of atropisomerism in drug discovery. Angew. Chem., Int. Ed. 2009, 48, 6398–6401. 10.1002/anie.200901719. [DOI] [PubMed] [Google Scholar]
- LaPlante S. R.; Fader L. D.; Fandrick K. R.; Fandrick D. R.; Hucke O.; Kemper R.; Miller S. P. F.; Edwards P. J. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 2011, 54, 7005–7022. 10.1021/jm200584g. [DOI] [PubMed] [Google Scholar]
- Glunz P. W. Recent encounters with atropisomerism in drug discovery. Bioorg. Med. Chem. Lett. 2018, 28, 53–60. 10.1016/j.bmcl.2017.11.050. [DOI] [PubMed] [Google Scholar]
- Wencel-Delord J.; Panossian A.; Leroux F. R.; Colobert F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. 10.1039/C5CS00012B. [DOI] [PubMed] [Google Scholar]
- Cheng J. K.; Xiang S. H.; Li S.; Ye L.; Tan B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 2021, 121, 4805–4902. 10.1021/acs.chemrev.0c01306. [DOI] [PubMed] [Google Scholar]
- Kumarasamy E.; Raghunathan R.; Sibi M. P.; Sivaguru J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 2015, 115, 11239–11300. 10.1021/acs.chemrev.5b00136. [DOI] [PubMed] [Google Scholar]
- Mei G. J.; Koay W. L.; Guan C. Y.; Lu Y. Atropisomers beyond the C–C axial chirality: advances in catalytic asymmetric synthesis. Chem. 2022, 8, 1855–1893. 10.1016/j.chempr.2022.04.011. [DOI] [Google Scholar]
- Bai X. F.; Cui Y. M.; Cao J.; Xu L. W. Atropisomers with axial and point chirality: synthesis and applications. Acc. Chem. Res. 2022, 55, 2545–2561. 10.1021/acs.accounts.2c00417. [DOI] [PubMed] [Google Scholar]
- Carmona J. A.; Rodriguez-Franco C.; Fernandez R.; Hornillos V.; Lassaletta J. M. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021, 50, 2968–2983. 10.1039/D0CS00870B. [DOI] [PubMed] [Google Scholar]
- Di Iorio N.; Crotti S.; Bencivenni G. Organocatalytic desymmetrization reactions for the synthesis of axially chiral compounds. Chem. Rec. 2019, 19, 2095–2104. 10.1002/tcr.201800194. [DOI] [PubMed] [Google Scholar]
- Lassaletta J. M.Atropisomerism and Axial Chirality; World Scientific Publishing Co Pte Ltd: Singapore, 2019. [Google Scholar]
- Xiang S. H.; Tan B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 2020, 11, 3786. 10.1038/s41467-020-17580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. B.; Tan B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. 10.1021/acs.accounts.7b00602. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Guo D.; Munkerup K.; Huang K. W.; Li F.; Wang J. Enantioselective [3 + 3] atroposelective annulation catalyzed by N-heterocyclic carbenes. Nat. Commun. 2018, 9, 611. 10.1038/s41467-018-02952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K.; Li W.; Zhu S.; Zhu T. Atroposelective arene formation by carbene-catalyzed formal [4 + 2] cycloaddition. Angew. Chem., Int. Ed. 2019, 58, 17625–17630. 10.1002/anie.201910049. [DOI] [PubMed] [Google Scholar]
- Yang X.; Wei L.; Wu Y.; Zhou L.; Zhang X.; Chi Y. R. Atroposelective access to 1,3-oxazepine-containing bridged biaryls via carbene-catalyzed desymmetrization of imines. Angew. Chem., Int. Ed. 2023, 62, e202211977 10.1002/anie.202211977. [DOI] [PubMed] [Google Scholar]
- Lv Y.; Luo G.; Liu Q.; Jin Z.; Zhang X.; Chi Y. R. Catalytic atroposelective synthesis of axially chiral benzonitriles via chirality control during bond dissociation and CN group formation. Nat. Commun. 2022, 13, 36. 10.1038/s41467-021-27813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.; Guo D.; Meng D.; Wang J. NHC-catalyzed atropoenantioselective synthesis of axially chiral biaryl amino alcohols via a cooperative strategy. Nat. Commun. 2019, 10, 3062. 10.1038/s41467-019-10878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R.; Xie Y.; Jin Z.; Chi Y. R. Carbene-catalyzed asymmetric construction of atropisomers. Angew. Chem., Int. Ed. 2021, 60, 26026–26037. 10.1002/anie.202108630. [DOI] [PubMed] [Google Scholar]
- Bao H.; Chen Y.; Yang X. Catalytic asymmetric synthesis of axially chiral diaryl ethers through enantioselective desymmetrization. Angew. Chem., Int. Ed. 2023, 62, e202300481 10.1002/anie.202300481. [DOI] [PubMed] [Google Scholar]
- Dai L.; Liu Y.; Xu Q.; Wang M.; Zhu Q.; Yu P.; Zhong G.; Zeng X. A dynamic kinetic resolution approach to axially chiral diaryl ethers by catalytic atroposelective transfer hydrogenation. Angew. Chem., Int. Ed. 2023, 62, e202216534 10.1002/anie.202216534. [DOI] [PubMed] [Google Scholar]
- Vaidya S. D.; Toenjes S. T.; Yamamoto N.; Maddox S. M.; Gustafson J. L. Catalytic atroposelective synthesis of N-aryl quinoid compounds. J. Am. Chem. Soc. 2020, 142, 2198–2203. 10.1021/jacs.9b12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H. Y.; Tan F.X.; Liu T.Q.; Zhu G.D.; Tian J.M.; Ding T.M.; Chen Z.M.; Zhang S.Y. Highly atroposelective synthesis of nonbiaryl naphthalene-1,2-diamine N-C atropisomers through direct enantioselective C-H amination. Nat. Commun. 2019, 10, 3063. 10.1038/s41467-019-10858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. W.; Chen Z. H.; Yang S.; Wu S. F.; Zhang Y. C.; Shi F. Organocatalytic atroposelective synthesis of N–N axially chiral indoles and pyrroles by de novo ring formation. Angew. Chem., Int. Ed. 2022, 61, e2021168 10.1002/anie.202116829. [DOI] [PubMed] [Google Scholar]
- Mori K.; Ichikawa Y.; Kobayashi M.; Shibata Y.; Yamanaka M.; Akiyama T. Enantioselective synthesis of multisubstituted biaryl skeleton by chiral phosphoric acid catalyzed desymmetrization/kinetic resolution sequence. J. Am. Chem. Soc. 2013, 135, 3964–3970. 10.1021/ja311902f. [DOI] [PubMed] [Google Scholar]
- Zheng S. C.; Wu S.; Zhou Q.; Chung L. W.; Ye L.; Tan B. Organocatalytic atroposelective synthesis of axially chiral styrenes. Nat. Commun. 2017, 8, 15238. 10.1038/ncomms15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday E. S.; Grove M. A.; Feoktistova T.; Brueckner A. C.; Walden D. M.; Young C. M.; Slawin A. M. Z.; Campbell A. D.; Cheong P. H.; Smith A. D. Isothiourea-catalyzed atropselective acylation of biaryl phenols via sequential desymmetrization /Kinetic Resolution. Angew. Chem., Int. Ed. 2020, 59, 7897–7905. 10.1002/anie.201916480. [DOI] [PubMed] [Google Scholar]
- Yu C.; Huang H.; Li X.; Zhang Y.; Wang W. Dynamic kinetic resolution of biaryl lactones via a chiral bifunctional amine thiourea-catalyzed highly atropo-enantioselective transesterification. J. Am. Chem. Soc. 2016, 138, 6956–9. 10.1021/jacs.6b03609. [DOI] [PubMed] [Google Scholar]
- Gustafson J. L.; Lim D.; Miller S. J. Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination. Science 2010, 328, 1251–1255. 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen S.; Huang N.; Lian D.; Shen A.; Zhao M. X.; Zhang Z. Conformational enantiodiscrimination for asymmetric construction of atropisomers. Nat. Commun. 2022, 13, 4735. 10.1038/s41467-022-32432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Zhang T.; Wang L.; Liao G.; Yao Q. J.; Wu Y. J.; Zhan B. B.; Lan Y.; Lin X. F.; Shi B. F. Enantioselective synthesis of biaryl atropisomers by Pd-catalyzed C-H olefination using chiral spiro phosphoric acid ligands. Angew. Chem., Int. Ed. 2019, 58, 6708–6712. 10.1002/anie.201902126. [DOI] [PubMed] [Google Scholar]
- Yao W.; Lu C. J.; Zhan L. W.; Wu Y.; Feng J.; Liu R. R. Enantioselective synthesis of N-N atropisomers by palladium-catalyzed C-H functionalization of pyrroles. Angew. Chem., Int. Ed. 2023, 62, e202218871 10.1002/anie.202218871. [DOI] [PubMed] [Google Scholar]
- Gu X. W.; Sun Y. L.; Xie J. L.; Wang X. B.; Xu Z.; Yin G. W.; Li L.; Yang K. F.; Xu L. W. Stereospecific Si-C coupling and remote control of axial chirality by enantioselective palladium-catalyzed hydrosilylation of maleimides. Nat. Commun. 2020, 11, 2904. 10.1038/s41467-020-16716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Wang J. Atropoenantioselective redox-neutral amination of biaryl compounds through borrowing hydrogen and dynamic kinetic resolution. Angew. Chem., Int. Ed. 2018, 57, 465–469. 10.1002/anie.201711126. [DOI] [PubMed] [Google Scholar]
- Feng J.; Bi X.; Xue X.; Li N.; Shi L.; Gu Z. Catalytic asymmetric C-Si bond activation via torsional strain-promoted Rh-catalyzed aryl-Narasaka acylation. Nat. Commun. 2020, 11, 4449. 10.1038/s41467-020-18273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Jiang H. J.; Sharanov I.; Irran E.; Oestreich M. Atroposelective silylation of 1,1′-biaryl-2,6-diols by a chiral counteranion directed desymmetrization enhanced by a subsequent kinetic resolution. Angew. Chem., Int. Ed. 2023, 62, e202304475 10.1002/anie.202304475. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Li M.; Sun J.; Zheng G.; Zhang Q. Synthesis of axially chiral aldehydes by N-Heterocyclic-Carbene-Catalyzed desymmetrization followed by kinetic resolution. Angew. Chem., Int. Ed. 2022, 61, e202117340 10.1002/anie.202117340. [DOI] [PubMed] [Google Scholar]
- Watts O. F. B.; Berreur J.; Collins B. S. L.; Clayden J. Biocatalytic enantioselective synthesis of atropisomers. Acc. Chem. Res. 2022, 55, 3362–3375. 10.1021/acs.accounts.2c00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrobo B.; Rolfes J. D.; Deska J. Enzymatic approaches for the preparation of optically active non-centrochiral compounds. Tetrahedron 2016, 72, 1257–1275. 10.1016/j.tet.2016.01.026. [DOI] [Google Scholar]
- Obermaier S.; Thiele W.; Furtges L.; Muller M. Enantioselective phenol coupling by laccases in the biosynthesis of fungal dimeric naphthopyrones. Angew. Chem., Int. Ed. 2019, 58, 9125–9128. 10.1002/anie.201903759. [DOI] [PubMed] [Google Scholar]
- Guo H.; Sun N.; Guo J.; Zhou T. P.; Tang L.; Zhang W.; Deng Y.; Liao R. Z.; Wu Y.; Wu G.; Zhong F. Expanding the promiscuity of a copper-dependent oxidase for enantioselective cross-coupling of indoles. Angew. Chem., Int. Ed. 2023, 62, e202219034 10.1002/anie.202219034. [DOI] [PubMed] [Google Scholar]
- Zetzsche L. E.; Yazarians J. A.; Chakrabarty S.; Hinze M. E.; Murray L. A. M.; Lukowski A. L.; Joyce L. A.; Narayan A. R. H. Biocatalytic oxidative cross-coupling reactions for biaryl bond formation. Nature 2022, 603, 79–85. 10.1038/s41586-021-04365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girol C. G.; Fisch K. M.; Heinekamp T.; Guenther S.; Huettel W.; Piel J.; Brakhage A. A.; Mueller M. Regio-and stereoselective oxidative phenol coupling in Aspergillus niger. Angew. Chem., Int. Ed. 2012, 51, 9788–9791. 10.1002/anie.201203603. [DOI] [PubMed] [Google Scholar]
- Mazzaferro L. S.; Huettel W.; Fries A.; Mueller M. Cytochrome P450-catalyzed regio- and stereoselective phenol coupling of fungal natural products. J. Am. Chem. Soc. 2015, 137, 12289–12295. 10.1021/jacs.5b06776. [DOI] [PubMed] [Google Scholar]
- Huang W.; Huang S.; Sun Z.; Zhang W.; Zeng Z.; Yuan B. Chemoenzymatic synthesis of sterically hindered biaryls by Suzuki coupling and vanadium chloroperoxidase catalyzed halogenations. ChemBioChem. 2023, 24, e202200610 10.1002/cbic.202200610. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Page A.; Worrall C. P.; Escalettes F.; Willies S. C.; McDouall J. J. W.; Turner N. J.; Clayden J. Biocatalytic desymmetrization of an atropisomer with both an enantioselective oxidase and ketoreductases. Angew. Chem., Int. Ed. 2010, 49, 7010–7013. 10.1002/anie.201002580. [DOI] [PubMed] [Google Scholar]
- Staniland S.; Yuan B.; Gimenez-Agullo N.; Marcelli T.; Willies S. C.; Grainger D. M.; Turner N. J.; Clayden J. Enzymatic desymmetrising redox reactions for the asymmetric synthesis of biaryl atropisomers. Chem.—Eur. J. 2014, 20, 13084–13088. 10.1002/chem.201404509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniland S.; Adams R. W.; McDouall J. J. W.; Maffucci I.; Contini A.; Grainger D. M.; Turner N. J.; Clayden J. Biocatalytic dynamic kinetic resolution for the synthesis of atropisomeric biaryl N-oxide Lewis base catalysts. Angew. Chem., Int. Ed. 2016, 55, 10755–10759. 10.1002/anie.201605486. [DOI] [PubMed] [Google Scholar]
- Morris G. M.; Huey R.; Lindstrom W.; Sanner M. F.; Belew R. K.; Goodsell D. S.; Olson A. J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Zidek A.; Potapenko A.; Bridgland A.; Meyer C.; Kohl S. A. A.; Ballard A. J.; Cowie A.; Romera-Paredes B.; Nikolov S.; Jain R.; Adler J.; Back T.; Petersen S.; Reiman D.; Clancy E.; Zielinski M.; Steinegger M.; Pacholska M.; Berghammer T.; Bodenstein S.; Silver D.; Vinyals O.; Senior A. W.; Kavukcuoglu K.; Kohli P.; Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Zidek A.; Potapenko A.; Bridgland A.; Meyer C.; Kohl S. A. A.; Ballard A. J.; Cowie A.; Romera-Paredes B.; Nikolov S.; Jain R.; Adler J.; Back T.; Petersen S.; Reiman D.; Clancy E.; Zielinski M.; Steinegger M.; Pacholska M.; Berghammer T.; Bodenstein S.; Silver D.; Vinyals O.; Senior A. W.; Kavukcuoglu K.; Kohli P.; Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G.; Bi Y.; Liu B.; Li J.; Han X.; Liu W.; Jiang Y.; Qin Z.; Sun Z. Unlocking the stereoselectivity and substrate acceptance of enzymes: proline-induced loop engineering test. Angew. Chem., Int. Ed. 2022, 61, e202110793 10.1002/anie.202110793. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Qu G.; Sheng X.; Tong F.; Sun Z. Unraveling the mechanism of enantio-controlling switches of an alcohol dehydrogenase toward sterically small ketone. Catal. Sci. Technol. 2022, 12, 1777–1787. 10.1039/D2CY00031H. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.