Abstract
Background
The antitumor activity of natural killer (NK) cells can be enhanced by specific targeting with therapeutic antibodies that trigger antibody-dependent cell-mediated cytotoxicity (ADCC) or by genetic engineering to express chimeric antigen receptors (CARs). Despite antibody or CAR targeting, some tumors remain resistant towards NK cell attack. While the importance of ICAM-1/LFA-1 interaction for natural cytotoxicity of NK cells is known, its impact on ADCC induced by the ErbB2 (HER2)-specific antibody trastuzumab and ErbB2-CAR-mediated NK cell cytotoxicity against breast cancer cells has not been investigated.
Methods
Here we used NK-92 cells expressing high-affinity Fc receptor FcγRIIIa in combination with trastuzumab or ErbB2-CAR engineered NK-92 cells (NK-92/5.28.z) as well as primary human NK cells combined with trastuzumab or modified with the ErbB2-CAR and tested cytotoxicity against cancer cells varying in ICAM-1 expression or alternatively blocked LFA-1 on NK cells. Furthermore, we specifically stimulated Fc receptor, CAR and/or LFA-1 to study their crosstalk at the immunological synapse and their contribution to degranulation and intracellular signaling in antibody-targeted or CAR-targeted NK cells.
Results
Blockade of LFA-1 or absence of ICAM-1 significantly reduced cell killing and cytokine release during trastuzumab-mediated ADCC against ErbB2-positive breast cancer cells, but not so in CAR-targeted NK cells. Pretreatment with 5-aza-2'-deoxycytidine induced ICAM-1 upregulation and reversed NK cell resistance in ADCC. Trastuzumab alone did not sufficiently activate NK cells and required additional LFA-1 co-stimulation, while activation of the ErbB2-CAR in CAR-NK cells induced efficient degranulation independent of LFA-1. Total internal reflection fluorescence single molecule imaging revealed that CAR-NK cells formed an irregular immunological synapse with tumor cells that excluded ICAM-1, while trastuzumab formed typical peripheral supramolecular activation cluster (pSMAC) structures. Mechanistically, the absence of ICAM-1 did not affect cell–cell adhesion during ADCC, but rather resulted in decreased signaling via Pyk2 and ERK1/2, which was intrinsically provided by CAR-mediated targeting. Furthermore, while stimulation of the inhibitory NK cell checkpoint molecule NKG2A markedly reduced FcγRIIIa/LFA-1-mediated degranulation, retargeting by CAR was only marginally affected.
Conclusions
Downregulation of ICAM-1 on breast cancer cells is a critical escape mechanism from trastuzumab-triggered ADCC. In contrast, CAR-NK cells are able to overcome cancer cell resistance caused by ICAM-1 reduction, highlighting the potential of CAR-NK cells in cancer immunotherapy.
Keywords: Killer Cells, Natural; Receptors, Chimeric Antigen; Antibody-Dependent Cell Cytotoxicity; Trastuzumab; Intercellular Adhesion Molecule-1
WHAT IS ALREADY KNOWN ON THIS TOPIC
Redirecting natural killer (NK) cells by antibodies (antibody-dependent cell-mediated cytotoxicity (ADCC)) or chimeric antigen receptors (CARs) has been shown to be effective against a variety of otherwise NK-resistant cancer cells.
WHAT THIS STUDY ADDS
Here, we demonstrated that downregulation of the adhesion molecule ICAM-1 is a critical tumor escape mechanism from ErbB2-specific monoclonal antibodies trastuzumab-mediated NK cell cytotoxicity, whereas NK cells genetically engineered to express an ErbB2-specific CAR can overcome this resistance by bypassing LFA-1 signaling. This observation is new for CAR-NK cells and seems to distinguish them from what is known for CAR-T cells, which apparently still rely on additional LFA-1/ICAM-1 signals.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These data suggest that ADCC in patients with ICAM-1 low/negative cancers may be less effective. Strategies to upregulate ICAM-1 expression in tumors and/or the use of CAR engineered NK cells may represent a suitable strategy to leverage the NK cell response in patients with cancer.
Background
Natural killer (NK) cells are highly effective in recognizing and eliminating virus-infected or malignant cells. Because of their unique anticancer properties, NK cells are very attractive for the development of cell-based cancer immunotherapies.1 For adoptive transfer, NK cells can be isolated from peripheral and cord blood of healthy donors, or differentiated from hematopoietic or induced pluripotent stem cells.2–4 Alternatively, phenotypically stable NK cell lines are being considered as promising therapeutics, and in the case of NK-92 cells, have entered clinical trials.5 6
Natural cytotoxicity of NK cells is tightly orchestrated by cumulative signals from germline-encoded activating and inhibitory receptors. These signaling networks are naturally programmed to trigger cytotoxicity on encountering diseased cells while maintaining tolerance to healthy tissues.7 Although NK cells are in principle able to eliminate many types of transformed cells, certain tumors still evade NK cell-mediated killing, limiting the efficacy of NK cell-based immunotherapies. Different resistance mechanisms that enable cancer cells to escape from NK cell cytotoxicity have been described,8 including downregulation of activating and upregulation of inhibitory ligands on tumor cells, which results in insufficient triggering of cytotoxic signals.9–11
To enhance activating signals, monoclonal antibodies (mAbs) such as ErbB2 (HER2)-specific trastuzumab are infused into a patient, where they can opsonize tumor cells and bind via their Fc region to Fcγ receptor IIIa (FcγRIIIa, CD16) on endogenous NK cells, thereby triggering antibody-dependent cell-mediated cytotoxicity (ADCC). Alternatively, mAbs can be combined with adoptive transfer of donor-derived primary NK cells or clinically applicable NK cell lines such as high-affinity FcγRIIIa-modified NK-92 cells (haNK).12 Treatment regimens based on trastuzumab are the clinical standard of care for ErbB2-positive breast cancer. Nevertheless, not all patients benefit from this therapy, with treatment resistance developing despite continued presence of the target antigen.13 Although ADCC is considered an important effector mechanism of trastuzumab in breast cancer,14 parameters predicting ADCC efficacy are not well established.
In recent years, NK cells genetically engineered with chimeric antigen receptors (CARs) have been introduced as a novel approach to target NK cells specifically to tumors and enhance their antitumor activity.15 An initial clinical study demonstrated encouraging efficacy of CD19-specific CAR-NK cells in hematologic malignancies comparable to that reported for CD19-CAR T cells, but without observing major side effects typical for CAR-T cell therapies.2 In contrast to hematological malignancies, in clinical trials in solid tumor indications efficacy of CAR-T cells is still limited.16 Also CAR-NK cells are being investigated in early phase clinical trials for the treatment of solid tumors. In a current phase I trial (CAR2BRAIN; NCT03383978) patients with recurrent ErbB2-positive glioblastoma are treated with ErbB2-targeted CAR-NK-92 cells (NK-92/5.28.z). Data from the dose-escalation part of this study so far demonstrated safety and feasibility of intracranial injections of these cells, with evaluation of NK-92/5.28.z in combination with an anti-programmed cell death protein 1 (PD-1) immune checkpoint inhibitor ongoing.17 Similar to tumor-specific targeting of NK cells with antibodies and induction of ADCC, a better understanding of the signals required for CAR-NK cells to efficiently eliminate cancer cells of solid tumor origins is critical for the success of these cell-based advanced therapy medicinal products.
We previously showed that tumor-specific targeting of NK cells mediated either by combination with an antibody or expression of a CAR can provide the necessary signals to direct cytotoxic granules to the immunological synapse and enable secretion of the lytic enzymes toward otherwise NK-resistant cancer cells.18 However, it is not clear at present whether CAR-mediated or FcγRIIIa-mediated signals alone are sufficient to overcome NK-resistance, or whether synergistic mechanisms are required for efficient NK cell cytotoxicity. For example, adhesion molecules are known to play an important role for NK cell potency. Thereby, LFA-1 is essential for efficient natural cytotoxicity of NK cells, as it initiates the assembly of the immunological synapse and mediates firm adhesion to the target, which contributes to NK cell activation.19 Signaling through the cell adhesion molecule LFA-1 after binding to its natural ligand ICAM-1 on tumor cells is a prerequisite for targeted convergence of lytic granules toward the immunological synapse, allowing for maximum on-target efficiency with minimal collateral damage.20 21 Conversely, ICAM-1 can be downregulated or shed by tumor cells, leading to insufficient LFA-1 activation or decreased cell adhesion due to receptor blockade.22–24 While in hematological malignancies downregulation of ICAM-1 on acute myeloid leukemia (AML) cells led to escape from natural NK cell cytotoxicity,25 the role of ICAM-1 in solid tumors has not yet been investigated with respect to NK cell activation. Nevertheless, a dysregulated interferon (IFN)-γ pathway in CAR-T cells was recently shown to result in low ICAM-1 expression in solid tumors, leading to escape from CAR-T cell-mediated killing.26
While this suggests a critical role of cell adhesion molecules for CAR-T cell-based therapies, the relevance of ICAM-1 expression in NK cells for ADCC and CAR-mediated killing is presently unknown. In particular, it is unclear whether downregulation or cleavage of ICAM-1 on breast cancer cells can lead to escape from trastuzumab-mediated ADCC or CAR-induced cytotoxicity of NK cells. However, considering the limited efficacy of trastuzumab in some patients with breast cancer and the need to improve treatment approaches with CAR-engineered effector cells for solid tumor indications, a better understanding of such tumor escape mechanisms is of high clinical relevance.
Here, we investigated the role of ICAM-1/LFA-1 interaction and LFA-1-dependent stimulation in trastuzumab-induced ADCC of FcγRIIIa-expressing NK cells or CAR-mediated cytotoxicity of ErbB2-CAR-NK cells against ErbB2-positive breast cancer cells.
Materials and methods
Materials and methods are available in the online supplemental material.
jitc-2023-008155supp002.pdf (222.4KB, pdf)
Results
Co-stimulatory signals are required for effective ADCC
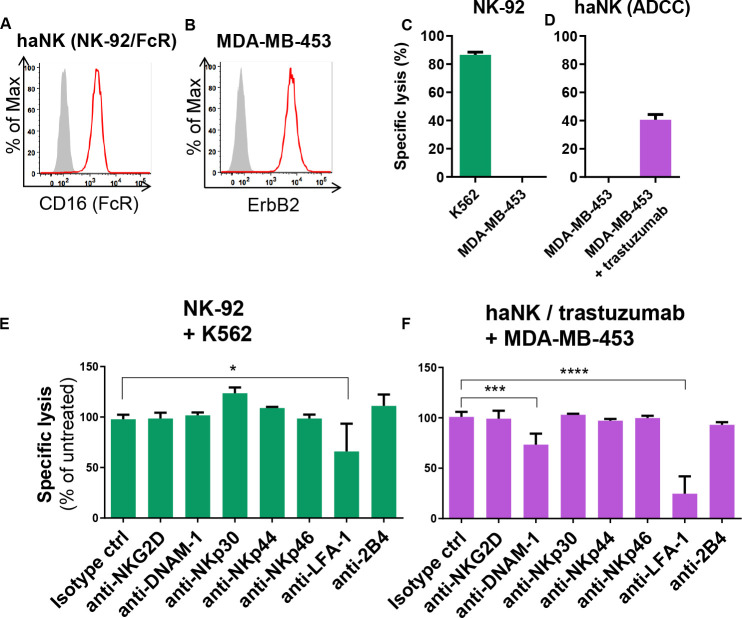
Previously, we demonstrated that ErbB2 (HER2)-specific antibody trastuzumab, when bound to FcγRIIIa (CD16) of NK cells, triggers their activation, granule polarization and subsequent cytotoxicity against ErbB2-expressing tumor cells.18 Nevertheless, it remained unclear to what extent additional signaling pathways contribute to efficient trastuzumab-mediated ADCC. Hence, we tested whether other NK cell activating or co-stimulatory receptors synergize with FcγRIIIa activation. We used haNK cells, an NK-92 cell line expressing high-affinity FcγRIIIa12 (figure 1A) in combination with the therapeutic antibody trastuzumab as a model. NK-92 cells are phenotypically and functionally similar to activated primary NK cells, except that NK-92 cells do not express KIRs that interact with HLA molecules.27 Therefore, the NK-92 model allows the study of ADCC in the absence of HLA-mediated NK inhibition. As target cells, we employed the ErbB2-expressing breast cancer cell line MDA-MB-453 (figure 1B). Consistent with previous studies,18 28 NK-92 cells were highly cytotoxic to NK-sensitive K562 cells, whereas MDA-MB-453 cells proved resistant to natural NK-92 cytotoxicity (figure 1C). Likewise, in agreement with previous study,12 in the absence of trastuzumab MDA-MB-453 cells were also resistant to haNK cells, but readily killed by haNK cells via ADCC in the presence of the ErbB2-specific IgG1 antibody (figure 1D). To test potential contribution of signals from other activating receptors or adhesion molecules to trastuzumab-mediated ADCC, we co-cultured MDA-MB-453 cells with haNK cells and trastuzumab in the presence of antibodies that block the NK cell activating receptors NKG2D, NKp30, NKp44, NKp46 or DNAM-1, co-stimulatory 2B4, or the adhesion molecule LFA-1. We found that blockade of LFA-1, and to a lesser extent DNAM-1, reduced trastuzumab-mediated ADCC (figure 1F). Natural cytotoxicity of NK-92 against K562 cells was only affected by blockade of LFA-1 (figure 1E). These data indicate that signaling via LFA-1 and DNAM-1 is required for effective trastuzumab-triggered cytotoxicity, whereas natural cytotoxicity of the NK cells depends in part on LFA-1.
Figure 1.
Blockade of LFA-1 alleviates natural cytotoxicity and ADCC of NK cells. (A, B) Histograms confirm FcγRIIIa (CD16) expression on haNK cells (A) and ErbB2 expression on MDA-MB-453 breast cancer cells (B). Filled gray areas indicate isotype controls. Representative data from at least three independent experiments are shown. (C, D) NK-92 (C) or haNK (D) cells were co-cultured with K562 or MDA-B-453 cells at an effector to target ratio of 10:1 for 2 hours, and specific cytotoxicity was measured using a Europium-based cytotoxicity assay. Trastuzumab was added where indicated at a concentration of 2 µg/mL. Mean values from three independent experiments±SEM are shown. (E, F) The indicated receptors on NK-92 (E) or haNK (F) cells were blocked with specific antibodies (20 µg/mL) for 60 min, followed by 2 hours co-culture with K562 or MDA-MB-453 cells. Isotype control IgG1 is shown (IgG2b was comparable). Trastuzumab (2 µg/mL) was added as indicated. Specific cytotoxicity was measured using a Europium-based cytotoxicity assay and is depicted as the percentage of cytotoxicity in the absence of blocking antibodies. Data were pooled from at least three independent experiments. Mean values±SEM are shown. ADCC, antibody-dependent cell-mediated cytotoxicity; haNK, high-affinity FcγRIIIa-modified NK-92 cells; NK, natural killer.
ICAM-1 downregulation facilitates escape from trastuzumab-mediated NK cell killing
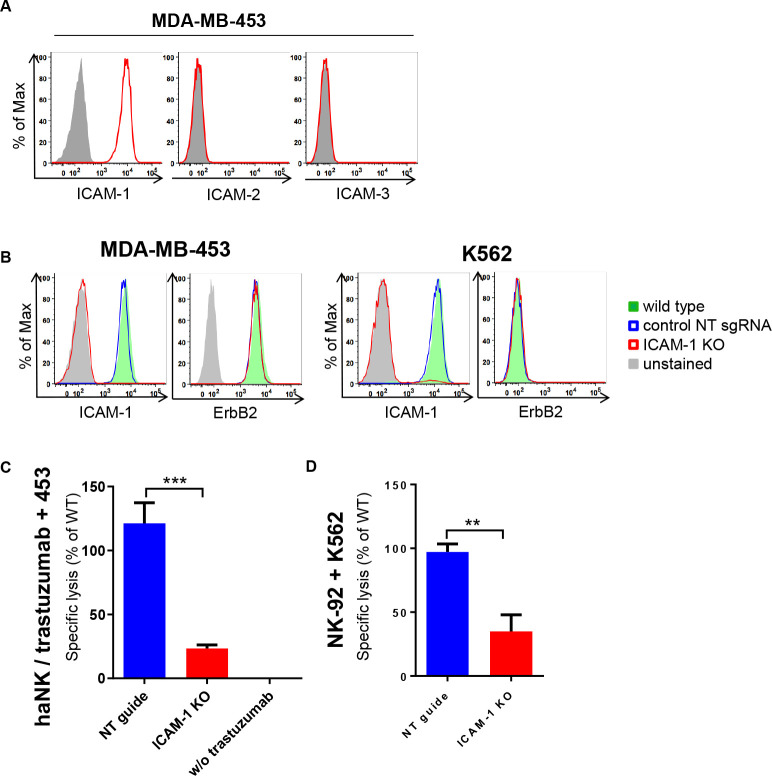
Next, we investigated which ligands on MDA-MB-453 cells stimulate LFA-1 on NK cells. Flow cytometric analysis of the most common LFA-1 ligands ICAM-1, ICAM-2 and ICAM-3 showed that only ICAM-1 is expressed on the surface of MDA-MB-453 cells (figure 2A). Thus, we hypothesized that decreased ICAM-1 expression may affect trastuzumab-mediated killing. We used CRISPR/Cas9 to generate ICAM-1 knockout (ICAM-1 KO) MDA-MB-453 and K562 cells, which in the case of MDA-MB-453 still displayed high ErbB2 expression (figure 2B). ICAM-1 and ErbB2 levels in cells edited with control non-targeting (NT) sgRNA did not differ from those of the parental wild type (WT) cells. Subsequently, we tested NK cytotoxicity toward these different target cells. Thereby, haNK cells in combination with trastuzumab showed high cell killing activity against MDA-MB-453 WT and MDA-MB-453 NT control cells, while cytotoxicity toward MDA-MB-453 ICAM-1 KO cells was markedly reduced (figure 2C). Similarly, natural cytotoxicity of NK-92 cells against K562 ICAM-1 KO cells was decreased when compared with K562 NT control cells (figure 2D). Accordingly, ICAM-1 downregulation can contribute to escape from natural and trastuzumab-mediated killing by NK cells.
Figure 2.
Downregulation of ICAM-1 results in escape from trastuzumab-mediated ADCC. (A) Flow cytometric analysis of ICAM-1, ICAM-2, and ICAM-3 expression on MDA-MB-453 cells. Filled gray areas indicate isotype controls. (B) ICAM-1 and ErbB2 expression on K562 and MDA-MB-453 ICAM-1 knock-out (KO) cells, and non-targeting sgRNA transduced (NT) or parental wild type (WT) control cells. Representative data from at least three independent experiments are shown. (C, D) haNK cells combined with trastuzumab (C) or NK-92 (D) cells were co-cultured for 2 hours with MDA-MB-453 or K562 ICAM-1 KO or NT and parental WT control cells at an effector to target ratio of 10:1 as indicated. Specific cytotoxicity was measured using a Europium-based cytotoxicity assay. Values for ICAM-1 KO and NT control cells are plotted relative to those obtained with parental WT MDA-MB-453 or K562 cells. To confirm trastuzumab-mediated ADCC against MDA-MB-453 cells, control samples without trastuzumab were included. Data were pooled from at least three independent experiments. Mean values±SEM are shown. ADCC, antibody-dependent cell-mediated cytotoxicity; haNK, high-affinity FcγRIIIa-modified natural killer-92 cells.
ErbB2-specific CAR-NK cells overcome immune escape caused by ICAM-1 downregulation
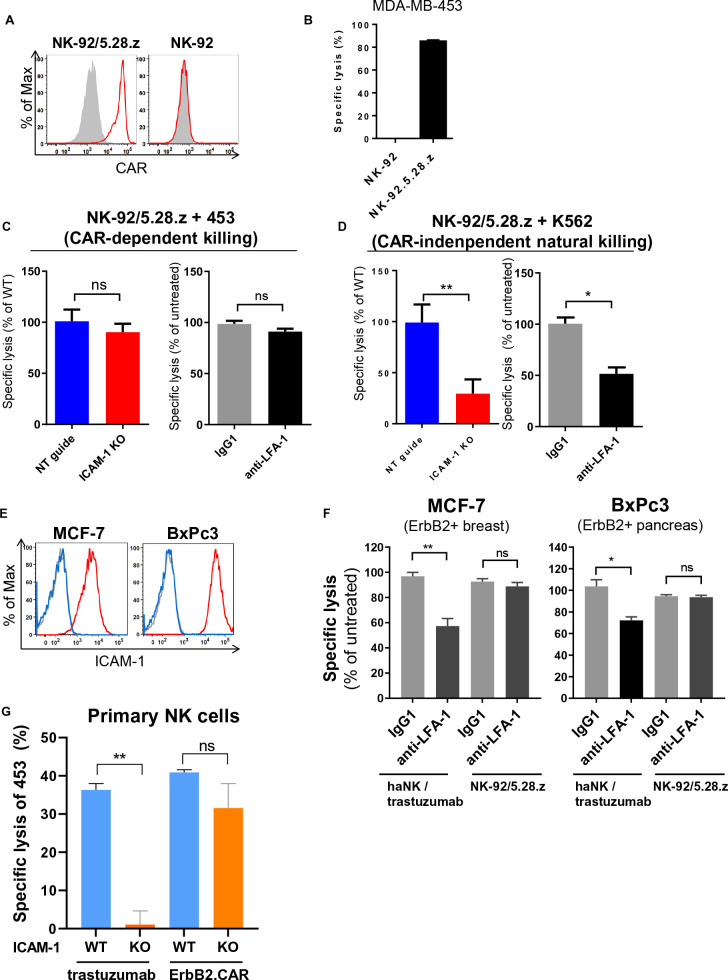
Similar to ADCC, CAR-expression in NK cells can trigger targeted cytotoxicity against otherwise NK-resistant cancer cells, but does not depend on antibody-mediated FcγRIIIa activation. To determine whether ICAM-1 downregulation in tumor cells can also affect CAR-mediated killing, we employed the clinically applied ErbB2-specific CAR NK-92 cell line NK-92/5.28.z (figure 3A).17 28 In contrast to parental NK-92, NK-92/5.28.z cells are highly cytotoxic against MDA-MB-453 cells (figure 3B). Interestingly, we did not observe a significant reduction in CAR-mediated cytotoxicity toward MDA-MB-453 ICAM-1 KO compared with NT control cells. Similarly, blockade of LFA-1 did not affect CAR-mediated cytotoxicity (figure 3C). To investigate the contribution of ICAM-1 to CAR-independent natural cytotoxicity of NK-92/5.28.z cells, we co-cultured them with ErbB2-negative but NK-sensitive K562 cells. As expected, NK-92/5.28.z killed K562 NT control cells, while cytotoxicity toward K562 ICAM-1 KO was markedly reduced, comparable to parental NK-92 cells. Similar results were obtained when LFA-1 on NK-92/5.28.z cells was blocked (figure 3D). These data indicate that in contrast to natural cytotoxicity, CAR-mediated killing by NK-92/5.28.z cells is ICAM-1 independent. To analyze whether this finding can be generalized, we also tested the ICAM-1-positive and ErbB2-positive breast and pancreatic cancer cell lines MCF-7 and BxPc3 (figure 3E). Similar to the results obtained with MDA-MB-453 breast cancer cells, in both cell lines blockade of LFA-1 reduced trastuzumab-mediated ADCC of haNK cells, while CAR-mediated cytotoxicity by NK-92/5.28.z cells remained unaffected (figure 3F). To test whether this is also the case with primary NK cells, we transduced primary human peripheral blood NK (pNK) cells with an ErbB2-specific CAR construct.28 pNK cells had a purity of 95±1%, and CAR expression was detected in 35±1% of the transduced cells (online supplemental figure S1 A and B). ADCC was tested by combining unmodified pNK cells with trastuzumab. As shown in figure 3G, both, pNK cells in combination with trastuzumab and CAR-modified pNK cells effectively killed MDA-MB-453 WT cells, while only CAR pNK cells were able to effectively eliminate MDA-MB-453 ICAM-1 KO cells, which were resistant to trastuzumab-mediated ADCC by pNK cells. Accordingly, CAR-mediated targeting of established NK-92 and primary NK cells can both overcome immune escape caused by ICAM-1 downregulation.
Figure 3.
CAR-mediated targeting overcomes immune escape caused by ICAM-1 downregulation. (A) CAR expression on NK-92/5.28.z cells (left) was detected by flow cytometry using human recombinant ErbB2-Fc protein followed by anti-Fc secondary antibody. Parental NK-92 cells (right) were included for comparison. Filled gray areas indicate negative controls only stained with secondary antibody. Representative data from at least three independent experiments are shown. (B) Specific cytotoxicity of NK-92/5.28.z against MDA-MB-453 cells was measured using a Europium-based assay after 2 hours of co-culture at an effector to target ratio of 10:1. (C) Specific CAR-mediated cytotoxicity of NK-92/5.28.z against MDA-MB-453 ICAM-1 KO, NT and WT cells (left) and against WT cells following incubation of NK-92/5.28.z cells with LFA-1 blocking antibody or control IgG for 60 min prior to co-culture (right). (D) Natural cytotoxicity of NK-92/5.28.z against K562 ICAM-1 KO, NT and WT cells (left) and against WT cells following incubation of NK-92/5.28.z cells with LFA-1 blocking antibody or control IgG for 60 min prior to co-culture (right). (E) Flow cytometric analysis of ICAM-1 expression on MCF-7 breast and BxPc3 pancreatic cancer cells. Blue lines indicate isotype controls and gray lines indicate unstained controls. (F) Specific cytotoxicity of haNK cells in combination with trastuzumab or CAR-engineered NK-92/5.28.z cells against MCF-7 and BxPc3 cells. NK cells were pretreated with LFA-1 blocking antibody or control IgG as indicated. Cytotoxicity data were pooled from at least three independent experiments. Mean values±SEM are shown. (G) Primary NK cells modified with an ErbB2-specific CAR or combined with trastuzumab were co-cultured with MDA-MB-453 ICAM-1 KO or WT cells for 2 hours and specific cytotoxicity was measured using a Europium-based assay. Representative data from three independent experiments using three different donors are depicted. Mean values±SEM are shown. CAR, chimeric antigen receptor; haNK, high-affinity FcγRIIIa-modified NK-92 cells; KO, knockout; NK, natural killer; NT, non-targeting; WT, wild type.
jitc-2023-008155supp003.pdf (89.3KB, pdf)
CAR-NK cells retain ICAM-1 independent cytotoxicity and cytokine production during long-term interaction with cancer cells
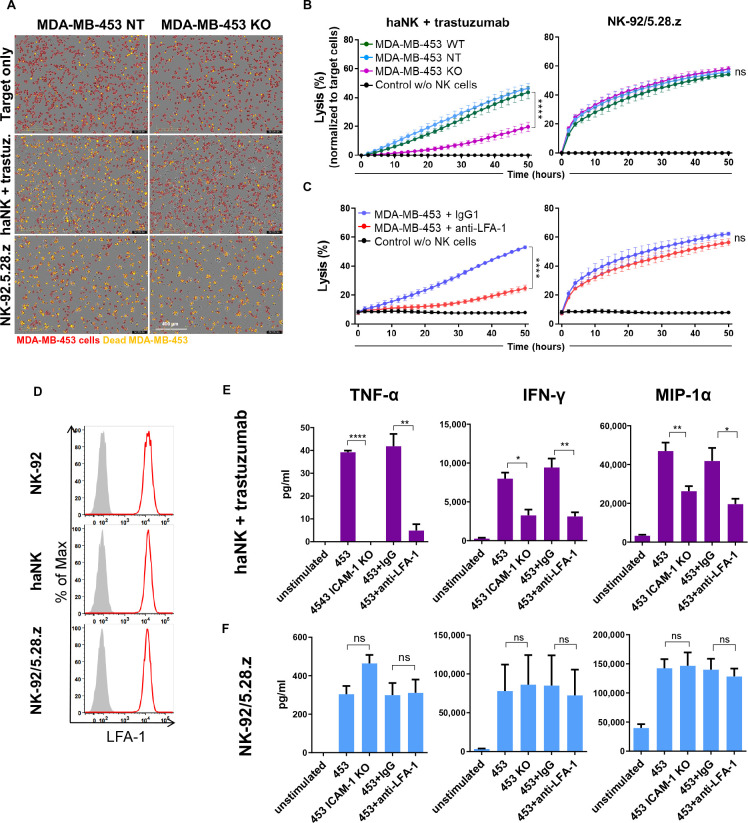
To challenge CAR-NK cells with ICAM-1 negative targets for a longer time period, we mixed CytoLightRed-labeled MDA-MB-453 ICAM-1 KO cells with NK-92/5.28.z cells, or haNK cells combined with trastuzumab at an effector to target ratio of 1:1, and co-cultured them for 50 hours (figure 4A). The co-cultures were monitored every 6 hours using the IncuCyte live-cell imaging system, and cytotoxicity was quantified based on the proportion of viability dye-positive cancer cells (figure 4B and C). In agreement with our data from short-term assays, both, the absence of ICAM-1 on cancer cells and LFA-1 blockade on NK cells resulted in a marked reduction of trastuzumab-induced ADCC of haNK cells against MDA-MB-453 target cells. Again, CAR-engineered NK-92/5.28.z cells were unaffected by the loss of ICAM-1 on tumor cells and able to kill MDA-MB-453 ICAM-1 KO cells as efficiently as the control targets. Likewise, LFA-1 blockade on NK-92/5.28.z cells did not affect CAR-mediated cytotoxicity. LFA-1 expression on NK-92, haNK and NK-92/5.28.z cells was comparable (figure 4D), ruling out potential differences in initial LFA-1 levels. In addition to direct target cell killing, the production of cytokines like IFN-γ, tumor necrosis factor alpha (TNF-α) or chemokines such as macrophage inflammatory protein 1-alpha (MIP-1α) is important for the antitumor activity of NK cells. MIP-1α has also been reported to be induced by LFA-1 stimulation.29 Hence, we tested whether cytokine production accompanying ADCC or CAR-mediated NK cell cytotoxicity is affected in the absence of ICAM-1/LFA-1 interaction. We co-cultured haNK cells in combination with trastuzumab or NK-92/5.28.z cells with MDA-MB-453 ICAM-1 KO or parental WT cells after blockade of LFA-1 on the NK cells, and measured cytokine release in the supernatants after 6 hours of co-culture. As expected, exposure to MDA-MB-453 WT cells resulted in the production of IFN-γ, TNF-α and MIP-1α by haNK cells combined with trastuzumab and NK-92/5.28.z cells (figure 4E and F). In contrast, co-culture with MDA-MB-453 ICAM-1 KO cells did not induce any measurable TNF-α and significantly reduced the amounts of IFN-γ and MIP-1α produced by haNK cells combined with trastuzumab, while cytokine production by NK-92/5.28.z cells was unaffected by the lack of ICAM-1. Consistent with these results, LFA-1 blockade only reduced cytokine production by haNK cells combined with trastuzumab, but not by CAR-engineered NK-92/5.28.z cells.
Figure 4.
CAR-NK cells retain ICAM-1-independent activity and cytokine production in long-term killing assays. (A–C) MDA-MB-453 ICAM-1 KO, NT and WT control cells were labeled with CytoLightRed and allowed to adhere to 96-well plates. NK-92/5.28.z CAR-NK cells or haNK cells in combination with trastuzumab were added at an E:T ratio of 1:1 in medium supplemented with CytoToxGreen viability dye, and cytotoxicity over time was analyzed using the IncuCyte live-cell imaging system. Where indicated, NK cells were treated with LFA-1 blocking antibody or control IgG1 prior to co-culture. (A) Microscopic images showing the co-cultures after 50 hours, with MDA-MB-453 cells labeled in red. Overlapping red and green signals (yellow) indicate dead MDA-MB-453 cells. (B, C) Graphs depicting cytotoxicity at the indicated time points calculated as the percentage of dead (double-positive) cancer cells out of total (red) cancer cells. Data were pooled from at least three independent experiments. Mean values±SEM are shown. Statistical analysis was performed using two-way analysis of variance. (D) Flow cytometric analysis of LFA-1 expression on NK-92, haNK and NK-92/5.28.z cells. Filled gray areas indicate isotype controls. Representative data out of at least three independent experiments are shown. (E, F) haNK cells combined with trastuzumab (E) or NK-92/5.28.z cells (F) were co-cultured with MDA-MB-453 ICAM-1 KO or WT cells at an E:T ratio of 1:1 for 6 hours in duplicates. Where indicated, NK cells were treated with LFA-1 blocking antibody or control IgG prior to co-culture. Supernatants from duplicate samples were pooled, and cytokine concentrations were measured using the ProcartaPlex Luminex system. Mean values±SEM calculated from three independent experiments are shown. CAR, chimeric antigen receptor; E:T, effector to target; haNK, high-affinity FcγRIIIa-modified NK-92 cells; IFN, interferon; KO, knockout; NK, natural killer; NT, non-targeting; MIP-1α, macrophage inflammatory protein 1-alpha; TNF-α, tumor necrosis factor alpha; WT, wild type.
Pharmacological intervention improves trastuzumab-mediated ADCC against ICAM-1low cancer cells
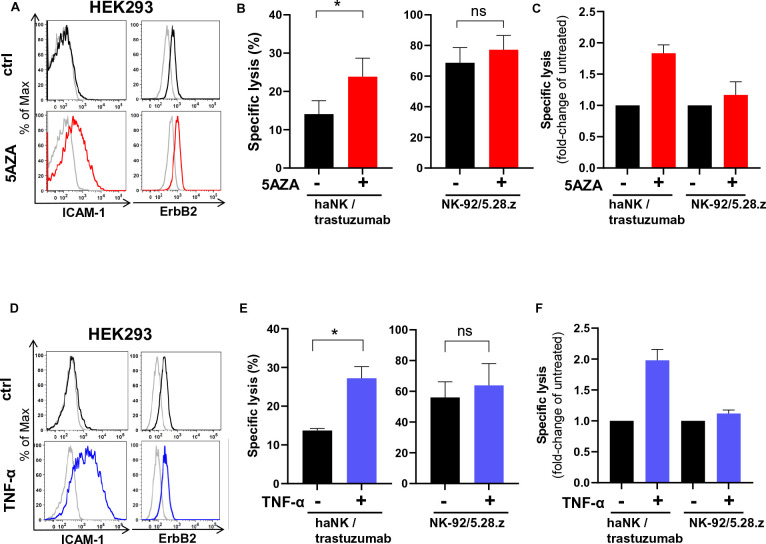
The data obtained in our models suggest that trastuzumab therapy in patients with cancers expressing low levels of ICAM-1 may result in low efficacy or treatment failure. To investigate whether pharmacological intervention to upregulate ICAM-1 can improve trastuzumab-mediated NK cell cytotoxicity, we applied the clinically used DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine (5AZA), previously shown to increase ICAM-1 expression in cancer cells.25 As a model, we employed HEK293 cells which are ICAM-1 low/negative but express moderate levels of ErbB2. Indeed, treatment with 5AZA increased ICAM-1 expression in HEK293 cells, while ErbB2 expression remained unchanged (figure 5A). Consistent with this, 5AZA treatment increased trastuzumab-mediated ADCC of haNK cells 1.8-fold compared with untreated control cells, while CAR-mediated cytotoxicity of NK-92/5.28.z against 5AZA-treated cells was comparable to that against untreated control targets (figure 5B and C). Also cytokines like TNF-α and IFN-γ have been reported to upregulate ICAM-1 in cancer cells.26 30 As shown above, high amounts of these cytokines are secreted by activated NK cells (see figure 4E and F). When we treated HEK293 cells with recombinant TNF-α, we observed upregulation of ICAM-1, but no influence of the cytokine on ErbB2 expression (figure 5D). Consequently, HEK293 treated with TNF-α were more sensitive to trastuzumab-mediated ADCC by haNK cells (figure 5E and F). In contrast, as in the case after 5AZA treatment, CAR-mediated cytotoxicity of NK-92/5.28.z remained unchanged. Next, we tested whether 5AZA and TNF-α can further enhance trastuzumab-mediated NK cell cytotoxicity against cancer cells with high levels of ICAM-1. In ICAM-1 high MDA-MB-453 cells, we observed further upregulation of ICAM-1 after treatment with TNF-α, but not after treatment with 5AZA (online supplemental figure S3A). As expected, in MDA-MB-453 ICAM-1 KO, treatment with 5AZA or TNF-α did not increase ICAM-1 expression (online supplemental figure S3C). In WT MDA-MB-453 cells, 5AZA did not increase trastuzumab-mediated NK cell cytotoxicity, whereas TNF-α moderately increased haNK/trastuzumab cytotoxicity (although not statistically significant), which correlated with increased ICAM-1 expression levels (online supplemental figure S3B). As expected, when ICAM-1 KO MDA-MB-453 cancer cells were subjected to 5AZA or TNF-α treatment, the cytotoxicity was not improved (online supplemental figure S3D), indicating that 5AZA and TNF-α confer sensitivity to NK-mediated killing exclusively by upregulating ICAM-1 in ICAM-1-negative/low cancer cells. Taken together, these data suggest that treatment with 5AZA may enhance the efficacy of trastuzumab in patients with cancer with tumors with low ICAM-1 expression. In addition, secretion of TNF-α by activated NK cells may further upregulate ICAM-1 expression on cancer cells, thereby functioning as a maintenance or amplification loop.
Figure 5.
Treatment with 5-aza-2'-deoxycytidine or TNF-α upregulates ICAM-1 expression and improves trastuzumab-mediated NK cell cytotoxicity. (A, D) HEK293 cells were treated with 1 µM 5-aza-2'-deoxycytidine (5AZA) for 72 hours (A) or with 100 ng/mL TNF-α for 48 hours (D). Expression of ICAM-1 and ErbB2 was analyzed by flow cytometry. Representative histograms are shown. (B, E) haNK cells combined with trastuzumab, or CAR-engineered NK-92/5.28.z cells were incubated for 2 hours with HEK293 cells pretreated with 5AZA (B) or TNF-α (E). Specific cytotoxicity was measured using a Europium-based cytotoxicity assay. (C, F) Specific lysis is depicted as fold-change in comparison to cytotoxicity of the NK cells against HEK293 control cells without 5AZA or TNF-α pretreatment. Data were pooled from three independent experiments. Mean values±SEM are shown. CAR, chimeric antigen receptors; haNK, high-affinity FcγRIIIa-modified NK-92 cells; NK, natural killer; TNF-α, tumor necrosis factor alpha.
jitc-2023-008155supp006.pdf (1.2MB, pdf)
CAR and LFA-1 do not interact at the immunological synapse of NK cells
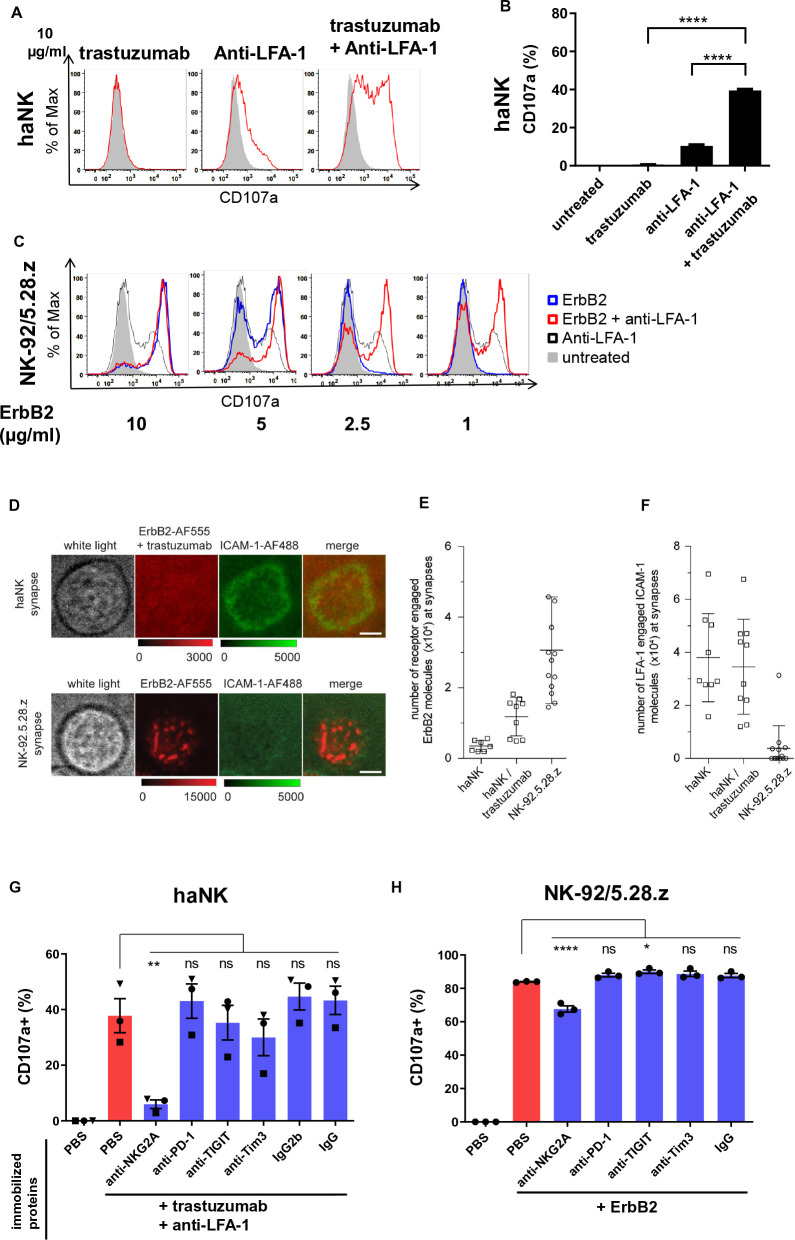
Close contact between cancer and NK cells takes place at the immunological synapse, where directed cytotoxicity is orchestrated by activating and inhibitory receptors and ligands. To narrow down which molecules are minimally required for trastuzumab-mediated ADCC and CAR-mediated cytotoxicity, we immobilized single activating ligands or their combinations on a microtiter plate and measured the degranulation marker CD107a as a readout for NK cell activation. In the case of haNK cells, immobilized trastuzumab alone did not induce any degranulation events, and immobilized LFA-1-specific antibody only induced a low level of degranulation. In contrast, in combination trastuzumab and anti-LFA-1 induced strong degranulation (figure 6A and B). Conversely, at moderate and high concentrations, immobilized recombinant ErbB2 protein alone already induced strong degranulation in CAR-engineered NK-92/5.28.z cells, which was not enhanced any further by additional immobilized anti-LFA-1. Only when immobilized ErbB2 protein became limiting at low concentrations, additional stimulation of LFA-1 synergized with CAR activation and increased the CD107a signal (figure 6C, online supplemental figure S2 A). These data suggest that the CAR does not require LFA-1 for degranulation if sufficient target antigen is present on the cancer cell.
Figure 6.
Chimeric antigen receptor-NK cells form an irregular immunological synapse, which is LFA-1 independent. (A) haNK cells were incubated with indicated plate-bound antibodies in the presence of labeled anti-CD107a antibody for 2 hours, followed by flow cytometric analysis of CD107a as a degranulation marker. Untreated NK cells are depicted in gray. (B) Quantification of CD107a expression. (C) NK-92/5.28.z cells were incubated with plate-bound ErbB2 protein in the presence of labeled anti-CD107a antibody for 2 hours, followed by flow cytometric analysis of CD107a. Representative data from three independent experiments are shown. (D) Total internal reflection fluorescence (TIRF) microscopy images displaying synaptic recruitment of fluorescently labeled ErbB2-AF555 and ICAM-1-AF488 molecules at the synapses of haNK cells in the presence of trastuzumab and NK-92.5.28.z cells. Scale bars: 5 µm. (E, F) Quantification of ErbB2-AF555 (E) and ICAM-1-AF488 (F) molecules recruited at the synapses of haNK and NK-92.5.28.z cells. Each data point represents the number of molecules recruited at the synapse of one cell. Mean values are indicated. Error bars represent±SD. (G, H) haNK cells were incubated with plate-bound trastuzumab and anti-LFA-1 (G) and NK-92/5.28.z with plate-bound ErbB2 protein (H) in each case combined with plate-bound antibodies targeting the indicated inhibitory receptors or immune checkpoint molecules, or respective isotype controls. Degranulation was measured by flow cytometric analysis of CD107a expression. Data were pooled from three independent experiments. Mean values±SEM are shown. haNK, high-affinity FcγRIIIa-modified NK-92 cells; NK, natural killer; PBS, phosphate-buffered saline; PD-1, programmed cell death protein 1; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TIM-3, T-cell immunoglobulin and mucin-domain containing-3.
jitc-2023-008155supp004.pdf (127.3KB, pdf)
To investigate indirect interaction of FcγRIIIa with ErbB2 via trastuzumab and direct interaction of the CAR with the antigen, and to follow recruitment of ICAM-1 by LFA-1 at the NK cell immunological synapse, we next employed single molecule resolution TIRF (total internal reflection fluorescence) microscopy. Thereby, precise quantitation of the number of antigens and co-stimulatory molecules at the interface of NK and target cell conjugates is challenging due to three-dimensional cellular movement and phototoxicity. To circumvent these limitations, we generated supported lipid layers (SLB) and loaded them with fluorescently labeled ErbB2 and ICAM-1 molecules as an artificial target cell surface. haNK cells combined with trastuzumab, and NK-92/5.28.z cells were seeded onto SLBs, and immunological synapses were imaged after 10 min in total internal reflection mode. We observed that both, haNK cells in the presence of trastuzumab, and NK-92/5.28.z cells displayed target antigen enrichment within their immunological synapses. At NK-92/5.28.z cell synapses, ErbB2 was recruited to discrete molecular clusters, whereas in haNK cells combined with trastuzumab the antigen was more homogeneously distributed (figure 6D). Next, we quantified the absolute numbers of ErbB2 and ICAM-1 molecules recruited to haNK and NK-92/5.28.z immunological synapses. We found that NK-92/5.28.z cells recruited approximately 2.6-times more ErbB2 molecules than haNK cells (figure 6E). In contrast, haNK cells recruited approximately 10-times more ICAM-1 than NK-92/5.28.z cells, and induced formation of distinct ICAM-1 rings (figure 6F). These data suggest that despite recruiting fewer ErbB2 molecules to their immunological synapse, haNK cells are more efficient in initiating LFA-1-mediated clustering of ICAM-1, while clustering of ICAM-1 at the NK-92/5.28.z immunological synapse appears to be dysregulated.
Subsequently, we investigated whether immune checkpoint molecules and inhibitory receptors can affect trastuzumab-mediated or CAR-mediated degranulation of NK cells. To enable maximum degranulation, haNK cells were activated with immobilized trastuzumab and anti-LFA-1, and NK-92/5.28.z cells with immobilized ErbB2. Additionally, we added immobilized antibodies specific for NKG2A, T cell immunoreceptor with Ig and ITIM domains (TIGIT), PD-1 and T-cell immunoglobulin and mucin-domain containing-3 (Tim-3) to initiate signal transduction from these receptors in the NK cells. We found that ADCC in haNK cells was inhibited by crosslinking of NKG2A, but not PD-1. Ligation of TIGIT or Tim-3 also reduced degranulation, but not to a statistically significant extent (figure 6G, online supplemental figure S2 B). Degranulation of NK-92/5.28.z cells in response to ErbB2 was moderately inhibited by NKG2A ligation, while the other tested immune checkpoint molecules had no effect (figure 6H,online supplemental figure S2 B). We next tested whether this finding could be translated to cytotoxicity against HLA-E positive cancer cells. For this purpose, we blocked haNK/trastuzumab or NK-92/5.28.z cells with anti-NKG2A antibody and co-cultured them with ErbB2/HLA-E/ICAM-1 triple positive ovarian SKOV-3 cells (natural HLA-E expression) and glioblastoma HT18584-HLA-E cells, engineered to overexpress HLA-E (online supplemental figures S4A and S5A). Interestingly, we found no improvement in the cytotoxicity of either haNK/trastuzumab or NK-92/5.28.z in both short-term and long-term killing assays (online supplemental figures S4B,C and S5B). Taken together, these data demonstrate that engagement of LFA-1 at the immunological synapse of the NK cells is critical for trastuzumab-mediated degranulation, while CAR-mediated degranulation of NK cells is LFA-1 independent. NKG2A engagement can inhibit degranulation signals from FcγRIIIa/LFA-1 and partially also from CAR in an isolated artificial system. Analysis of cytotoxicity against tumor cells suggests that physiological inhibition of NKG2A by HLA-E may not be sufficient to confer resistance to ADCC or CAR-mediated NK cell killing in the tested cancer cells and that additional unknown inhibitory signals may play a role.
jitc-2023-008155supp005.pdf (539.7KB, pdf)
jitc-2023-008155supp007.pdf (426KB, pdf)
CAR activation can substitute for LFA-1 signaling
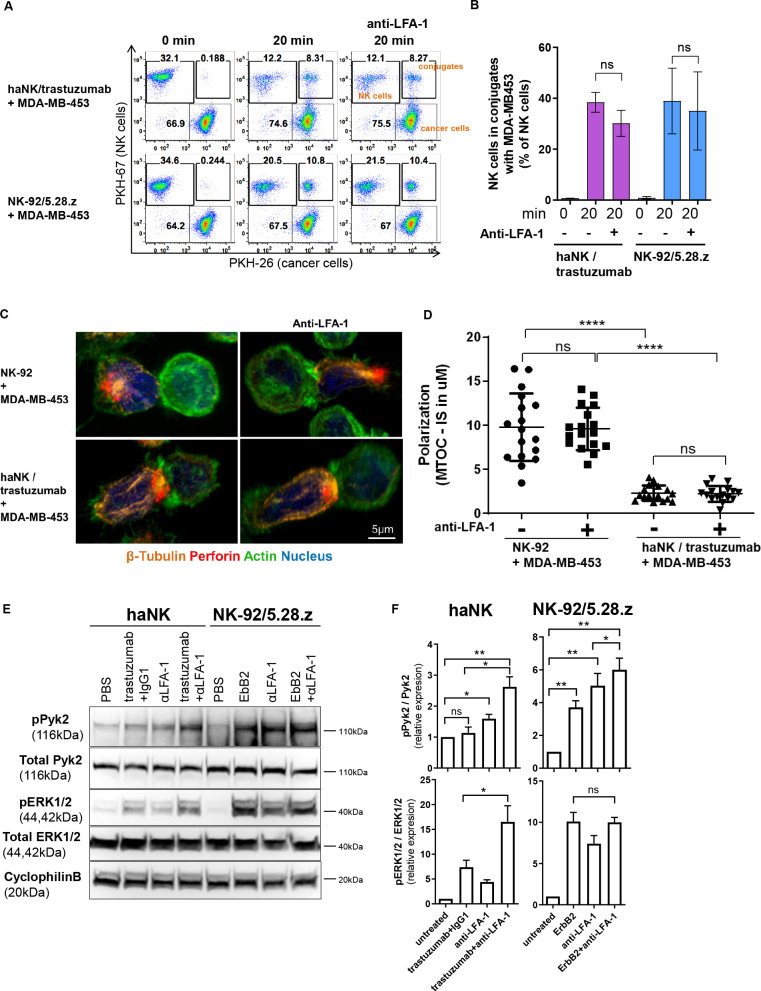
We further investigated how CAR signaling can overcome the dependence on LFA-1 for NK cell cytotoxicity. With the critical contribution of LFA-1 to trastuzumab-mediated degranulation already established, we took a step back and tested whether LFA-1 is required for initial conjugate formation between cancer cells and trastuzumab-armed or CAR-armed NK cells. We co-cultured labeled haNK cells combined with trastuzumab or NK-92/5.28.z cells with differentially labeled MDA-MB-453 cells in the presence or absence of LFA-1-blocking antibody. Both, haNK cells with trastuzumab and NK-92/5.28.z cells formed conjugates with MDA-MB-453 cells after 20 min of co-culture, without LFA-1 blockade resulting in a significant decrease in conjugation ability (figure 7A and B). Accordingly, we conclude that LFA-1 is not required for initial conjugate formation that precedes ADCC or CAR-mediated cell killing. We further tested whether granule polarization toward the immunological synapse in ADCC is affected by blockade of the LFA-1/ICAM-1 interaction. Our microscopy data showed that blockade of LFA-1 in haNK cells did not affect granule polarization toward the contact point with MDA-MB-453 cells in the presence of trastuzumab, suggesting that LFA-1 is rather required for the signaling leading to the subsequent release of cytotoxic granules (figure 7C and D). Hence, we further analyzed the signaling pathways downstream of LFA-1, FcγRIIIa and CAR. haNK cells were stimulated with immobilized trastuzumab, and NK-92/5.28.z cells with immobilized ErbB2, in each case in the presence or absence of immobilized anti-LFA-1. Then we measured phosphorylation of Pyk2 as a downstream signal after LFA-1 stimulation in NK cells,31 and phosphorylation of ERK as an indicator of effective ADCC and CAR-mediated cytotoxicity.18 As expected, stimulation of LFA-1 alone resulted in Pyk2 phosphorylation in both, haNK and NK-92/5.28.z cells (figure 7E and F). Trastuzumab alone did not induce Pyk2 signaling via FcγRIIIa in haNK cells, whereas ErbB2 alone was sufficient to stimulate Pyk2 phosphorylation in NK-92/5.28.z cells. Combining anti-LFA-1 with ErbB2 further increased Pyk2 phosphorylation in NK-92/5.28.z cells, although to a limited extent (19%). In contrast, combination of trastuzumab and anti-LFA-1 resulted in a 64% increase in haNK cells. Consistent with our degranulation data, phosphorylation of ERK was only slightly induced in haNK cells by trastuzumab alone, while strong phosphorylation was observed after exposure to trastuzumab in combination with anti-LFA-1. Conversely, stimulation of the CAR in NK-92/5.28.z cells by ErbB2 was already sufficient for strong ERK phosphorylation, and was not increased further by additional LFA-1 activation. Taken together, these data demonstrate that CAR activation can substitute for LFA-1 signaling, and thus overcome immune escape based on ICAM-1 downregulation.
Figure 7.
Chimeric antigen receptor activation can substitute for LFA-1 signaling by activating the Pyk2 pathway. (A) PKH-67-labeled haNK cells combined with trastuzumab or PKH-67-labeled NK-92/5.28.z cells were blocked with anti-LFA-1 antibody, and incubated with PKH-26-labeled MDA-MB-453 target cells for 20 min at 37°C as indicated. Control samples at 0 min were used to exclude unspecific binding or cross-staining. Cells were fixed and analyzed for conjugate formation by flow cytometry. (B) Quantification of conjugate formation. Pooled data from three independent experiments performed in triplicate are shown. Data represent mean values±SEM. (C) Confocal microscopy of NK-92 cells, and haNK cells combined with trastuzumab, incubated with MDA-MB-453 target cells. NK cells were blocked with anti-LFA-1 antibody prior to exposure to cancer cells as indicated. Representative images are shown. (D) Microtubule-organizing center (MTOC) polarization toward the immunological synapse (IS) was quantified from three independent experiments. Mean values±SEM are shown. (E) haNK or NK-92/5.28.z cells were stimulated with plate-bound trastuzumab, anti-LFA-1 antibody, ErbB2, or isotype control antibody as indicated. Cell lysates were prepared and analyzed by immunoblotting for phospho-Pyk2 (pPyk2; Tyr402) and phospho-ERK1/2 (pERK1/2; Thr202/Tyr204). As controls, total Pyk2 and ERK1/2 levels were analyzed. Cyclophilin B was used as a loading control. (F) Quantification of Pyk2 and ERK1/2 phosphorylation normalized to total protein levels. Data were pooled from three independent experiments. Mean values±SEM are shown. haNK, high-affinity FcγRIIIa-modified NK-92 cells; NK, natural killer; PBS, phosphate-buffered saline.
Discussion
NK cells are increasingly recognized as promising and potent effectors for cancer immunotherapy. To enhance and specifically direct their cytotoxicity to tumor cells, NK cells can be combined with therapeutic antibodies such as trastuzumab, or genetically engineered with CAR constructs.18 28 32 Treatment regimens based on trastuzumab are standard of care for ErbB2-positive breast cancers. Nevertheless, some patients with breast cancer fail antibody therapy despite continued ErbB2 expression in their tumors, with the underlying mechanisms not yet fully understood.14 NK-mediated ADCC is considered to be important for trastuzumab efficacy, suggesting that escape of cancer cells from antibody-mediated NK cell cytotoxicity could contribute to treatment resistance. In an initial clinical trial, adoptive transfer of CD19-targeted CAR-NK cells has shown encouraging efficacy against B-cell malignancies.2 Nevertheless, similar to CAR-T cells, CAR-NK cells employed for the treatment of solid tumors may face challenges such as inhomogeneous target antigen expression and an immunosuppressive tumor microenvironment. In addition, tumor cell-intrinsic resistance may affect ADCC and CAR-mediated cytotoxicity of NK cells despite sufficient target antigen expression. Hence, to fully exploit the potential of antibody-targeted and CAR-targeted NK cells for cancer immunotherapy, it is crucial to better understand escape of cancer cells from NK cell cytotoxicity, and to identify ways to overcome respective resistance mechanisms. In this study, we demonstrated for the first time that downregulation of ICAM-1 on breast and pancreatic cancer cells can abrogate trastuzumab-mediated NK cell cytotoxicity, while antitumor activity of CAR-NK cells remained unaffected.
ICAM-1 can be downregulated in cancer cells or cleaved by specific proteases.22–24 This has been shown to lead to escape from natural NK cell cytotoxicity,25 which is triggered by activating receptors such as natural cytotoxicity receptors and NKG2D in response to stress-induced ligands on the tumor cell surface.33 Accordingly, modulation of adhesion molecules constitutes a clinically relevant mechanism enabling cancer cells to evade attack by NK cells. Our data provide evidence that decrease or loss of ICAM-1 may also affect therapeutic efficacy of trastuzumab and possibly also other therapeutic antibodies. This is consistent with an earlier observation that cancer cells, which were experimentally kept under ADCC pressure, spontaneously downregulated ICAM-1.34 Here, we showed that cancer cells with low ICAM-1 expression do not stimulate LFA-1 on NK cells, resulting in insufficient trastuzumab-induced ADCC. In addition, shedding of soluble ICAM-1 could result in blockade of LFA-1 and prevent its binding to membrane-anchored ICAM-1 on cancer cells.22 Hence, our data may have strong clinical implications with respect to trastuzumab therapy, and may at least in part explain why some patients do not respond to treatment. In this respect, it appears warranted to include analysis of ICAM-1 expression on cancer cells as a diagnostic parameter to enable correlative studies that investigate the relationship between ICAM-1 levels and clinical trastuzumab efficacy.
In the present study, we showed that 5AZA upregulates ICAM-1 expression and thereby prevents escape from trastuzumab-mediated NK cell cytotoxicity. 5AZA is a clinically approved drug for the treatment of myelodysplastic syndrome and AML, which may aid the development of 5AZA combination therapies with trastuzumab. Furthermore, also TNF-α and IFN-γ can increase ICAM-1 expression.26 30 In our experiments, IFN-γ and TNF-α were readily secreted by NK cells after trastuzumab-induced activation, but not when ICAM-1 was downregulated. Hence, 5AZA may be suitable to initially upregulate ICAM-1 on cancer cells, which would in turn enhance activation of NK cells, resulting in the secretion of IFN-γ and TNF-α, and in a positive loop further amplifying ICAM-1 levels. Likewise, other demethylating agents or ICAM-1 upregulating reagents may be tested for their potential to enhance antibody-dependent cytotoxicity of NK cells.
Recent data from CAR-T cell studies showed that sufficient ICAM-1 expression is critical for efficient antitumor activity of CAR-T cells.26 To our surprize, we found that effective cell killing by CAR-NK cells targeting ErbB2 did not depend on ICAM-1, and was not affected by genetic KO of ICAM-1 in cancer cells or blockade of LFA-1 on the NK cells. Thereby, the CAR-NK cells could bypass ICAM-1-based escape in short-term and long-term cytotoxicity assays. Furthermore, in contrast to trastuzumab-stimulated NK cells, the secretion of critical cytokines and chemokines such as IFN-γ, TNF-α or MIP-1α by the CAR-NK cells was not affected after contact with ICAM-1-negative cancer cells. Accordingly, CAR-NK cells can provide robust anticancer functionality independent of stimulation by ICAM-1.
Our experiments with immobilized activating antibodies showed that trastuzumab alone is not able to stimulate degranulation of FcγRIIIa-positive NK cells, but requires additional stimulation of LFA-1. This is consistent with previous findings in insect cells using rabbit anti-serum that triggers the FcγRIIIa on human NK cells.21 In contrast, similar to our observations in cytotoxicity assays, CAR-activation by antigen was sufficient to induce degranulation without additional LFA-1 stimulation. Nevertheless, when the concentration of the CAR target antigen was decreased, ensuing suboptimal degranulation was rescued by additional LFA-1 activation. Accordingly, trastuzumab-mediated degranulation requires co-stimulation by LFA-1, while CAR-induced degranulation only requires additional signals if antigen concentration is low. In addition, degranulation induced by simultaneous FcγRIIIa and LFA-1 stimulation was still inhibited by NKG2A, whereas NKG2A had little effect on CAR-mediated NK cell activation. A previous report already described resistance of NK-92/5.28.z CAR-NK cells to inhibition by transforming growth factor-beta (TGF-β).35 This suggests that CAR signaling in the NK cells is particularly strong, and difficult to override by inhibitory signaling pathways. Interestingly, our cytotoxicity studies with two HLA-E positive cancer cell types did not show improved ADCC or CAR-mediated cytotoxicity after NKG2A blockade. This suggests that although NKG2A can inhibit FcγRIIIa/LFA-1 NK cell activation, the situation in cell–cell interaction is more complex and additional inhibitory mechanisms might co-suppress NK cell cytotoxicity. In fact, the therapeutic anti-NKG2A antibody monalizumab showed improved natural NK cell cytotoxicity in vitro and in vivo and is currently under clinical evaluation.36 37 Interestingly, and in agreement with our cytotoxicity data, the combination of monalizumab and trastuzumab did not induce objective responses in patients with heavily pretreated HER2-positive breast cancer in a small cohort, while ICAM-1 expression levels on cancer cells were not investigated.38 These data suggest that at least in some tumor types an additional mechanism may contribute to the inhibition of NK and T-cell responses. Further studies should address whether blockade of NKG2A can increase ADCC and thus therapeutic benefit. In fact, genetic KO of NKG2A expression has been shown to increase natural NK cell cytotoxicity against leukemic cells,39 but whether disruption of the HLA-E/NKG2A checkpoint axis will improve CAR-NK cell cytotoxicity remains unclear. Based on our findings, we propose screening patient tumors for ICAM-1 expression, as this may influence the ADCC response in combination with tumor-specific therapeutic antibodies.
In addition to our models with MDA-MB-453 breast cancer cells, the sensitivity of trastuzumab-triggered ADCC and resistance of CAR-mediated cytotoxicity to LFA-1 blockade was confirmed with other breast and pancreatic cancer cells. Likewise, unmodified and CAR-engineered primary human NK cells from peripheral blood demonstrated the same dependence on LFA-1 for effective trastuzumab-induced ADCC and LFA-1-independence on CAR-activation as respective haNK and NK-92/5.28.z cells. This demonstrates the robustness and general validity of our findings, which will likely be relevant also for therapeutic antibodies and CAR-NK cells targeting antigens other than ErbB2. However, it is important to note that this study is limited as it has been conducted in vitro only. Therefore next studies are required to further validate the role of the ICAM-1/LFA-1 axis in preclinical models and in primary tumors from patients responding and non-responding to trastuzumab treatment.
It would also be interesting to test whether other attempts to improve targeted NK cell therapy can synergize with our findings, such as targeting TGF-β signaling or improved metabolic activity by CIS (cytokine-inducible SH2-containing protein) KO, expression of transgenic cytokines such as interleukin-15, and many others.40–43
In recent years, different approaches have been developed to enhance NK cell cytotoxicity and facilitate targeted antitumor activity. NK cells have been modified with chimeric CD64/CD16A Fc receptors, or combined with bispecific or trispecific killer cell engagers such as bispecific antibodies cross-linking tumor antigens with FcγRIIIa (CD16) or NKG2D, or tetraspecific ANKET molecules.44–49 In addition, NK cells carrying a universal CAR have been developed, which can be targeted to a tumor antigen of choice by combining them with a respective bispecific adaptor molecule.50 51 Further research appears justified to investigate whether these therapeutic strategies like classical ADCC are affected by ICAM-1 downregulation, or can overcome this resistance mechanism similar to NK cells engineered with a conventional CAR. Recently, FcγRIIIa-expressing haNK cells have been further modified to also express a tumor-targeting CAR (t-haNK cells).52 In the context of our findings, this approach may benefit from both, the flexibility to induce ADCC by combination with different therapeutic antibodies, and the CAR-dependent resistance to inhibition by ICAM-1 downregulation.
High-resolution analysis of the immunological synapse with TIRF microscopy revealed that CAR-NK cells do not recruit ICAM-1 molecules to the immunological synapse. In contrast, NK cells combined with trastuzumab recruited ICAM-1, and formed typical pSMAC structures. Nevertheless, how ICAM-1 molecules are excluded from the immunological synapse of CAR-NK cells is unclear at present, and requires further investigation.
LFA-1 plays multiple roles in NK cells, including intercellular adhesion and signal transduction.19 Our data suggest that its contribution to cell–cell adhesion is not relevant for trastuzumab-mediated or CAR-mediated targeting of NK cells. Presumably, the interaction with target cells via trastuzumab and FcγRIIIa or the CAR is already strong enough, and LFA-1 does not provide any additional benefit. We also showed that the ICAM-1/LFA-1 interaction does not affect granule polarization toward the immunological synapse, suggesting that a different mechanism is involved in ICAM-1-based resistance of ICAM-1low cells to trastuzumab-mediated ADCC. Instead, our data demonstrate that LFA-1 is rather required to provide critical molecular signals for ADCC, which is consistent with earlier data obtained with insect target cells.31 53 In our case, activation of Pyk2 signaling via LFA-1 was essential for trastuzumab-mediated ADCC, with FcγRIIIa stimulation alone unable to activate this pathway. In contrast, CAR-activation resulted in Pyk2 phosphorylation in NK cells independent of LFA-1, providing a mechanistic explanation for the observation that functionality of CAR-NK cells was not affected by ICAM-1 downregulation on cancer cells. At the same time, we showed that during both, FcγRIIIa/LFA-1 and CAR-based signaling, Pyk2 phosphorylation correlated with the phosphorylation of ERK1/2, which is an important downstream NK cell signaling molecule leading to NK cell activation and cytotoxicity.54 It has been shown by others that ERK1/2 is one of the downstream signaling pathways of Pyk2, suggesting that FcγRIIIa/LFA-1 or CAR-mediated phosphorylation of Pyk2 contributes to downstream NK cell activation signals through ERK1/2-mediated signaling.55 56 Further research is needed to elucidate these complex molecular signaling pathways in antibody-targeted and CAR-targeted NK cells in order to improve NK cell-based therapies. Importantly, in our study we tested one of the most widely used CAR signaling molecules based on CD3z and CD28 stimulation, but recently novel CAR signaling molecules have been introduced to replace classical T cell-based signaling modules to enhance NK cell cytotoxicity,57 58 and it would be clinically relevant to test whether these novel CARs are dependent on ICAM-1 expression on cancer cells and which signaling pathways are employed.
Conclusion
Our study clearly demonstrates that ICAM-1 is an important checkpoint in trastuzumab-mediated NK cell cytotoxicity. Hence, cancer cells that downregulate or shed ICAM-1 are more likely to escape trastuzumab treatment. In sharp contrast, CAR-NK cells targeting the same tumor antigen remained unaffected by ICAM-1 downregulation or loss of LFA-1 stimulation. Mechanistically, the distinct signaling properties of FcγRIIIa and CAR can explain this difference. CAR-activation can substitute for LFA-1-mediated Pyk2 signaling and rescue NK cell degranulation, cytotoxicity and cytokine release that is otherwise abrogated on encounter of ICAM-1low or ICAM-1-negative cancer cells. Accordingly, for the treatment of ErbB2-positive but ICAM-1low breast cancers a combination of trastuzumab with ICAM-1 upregulating drugs like 5AZA, or application of ErbB2-specific CAR-NK cells may be warranted. Future studies will be aimed at investigating whether the same mechanisms apply to therapeutic antibodies and CAR-NK cells targeting tumor antigens other than ErbB2, as well as to alternative targeting approaches based on bispecific NK cell engagers and modular CAR systems. This is expected to provide more comprehensive insights in ICAM-1-based immune escape, which will be relevant for the development of further refined and more effective NK cell therapies.
jitc-2023-008155supp001.pdf (3.8MB, pdf)
Acknowledgments
The authors thank Erik Zenker and Madeleine Teichert for outstanding technical assistance, Silke Tulok for excellent support with light microscopy, and Professor Peter Fasching for helpful discussions. BioRender software was used to generate the illustrations in the graphical abstract.
Footnotes
JE and WR contributed equally.
Contributors: Designing of research studies: JE, TT. Conducting experiments: JE, WR, NW, VG, NMS, AS, PO-M, LRL, SM. Acquiring data: JE, WR, NW, VG, NMS, AS, CO, LRL. Analyzing data: JE, WR, VG, NMS, AS, LRL. Writing the manuscript: JE, TT, WSW, LB, HK, JBH, AF, MB, SRK, AT. Study supervision: JE, TT. All authors read and approved the final version of the manuscript.
Funding: This research was supported in part by the German Research Foundation (DFG) through grant EI1223/2-1 to JE; German Federal Ministry of Education and Research (Clusters4Future SaxoCell, 03ZU1111DA) and German Red Cross Blood Donation Service internal grants to TT; Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 116026 (T2EVOLVE) to VG and JBH.
Competing interests: TT and WSW are named as inventors on patents in the field of cancer immunotherapy owned by their respective institutions. HK and LB are employed by ImmunityBio, California, USA. Other authors declare that they have no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Further inquiries can be directed to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol 2021;18:85–100. 10.1038/s41571-020-0426-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced natural killer cells in Cd19-positive Lymphoid tumors. N Engl J Med 2020;382:545–53. 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu H, Lai YS, Li Y, et al. Concise review: human pluripotent stem cells to produce cell-based cancer immunotherapy. Stem Cells 2018;36:134–45. 10.1002/stem.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105:3051–7. 10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 5. Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013;15:1563–70. 10.1016/j.jcyt.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 6. Tonn T, Becker S, Esser R, et al. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res 2001;10:535–44. 10.1089/15258160152509145 [DOI] [PubMed] [Google Scholar]
- 7. Watzl C, Long EO. Signal transduction during activation and inhibition of natural killer cells. Curr Protoc Immunol 2010;11. 10.1002/0471142735.im1109bs90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Retecki K, Seweryn M, Graczyk-Jarzynka A, et al. The immune landscape of breast cancer: strategies for overcoming Immunotherapy resistance. Cancers (Basel) 2021;13:6012. 10.3390/cancers13236012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romanski A, Bug G, Becker S, et al. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Exp Hematol 2005;33:344–52. 10.1016/j.exphem.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 10. Maki G, Hayes GM, Naji A, et al. NK resistance of tumor cells from multiple myeloma and chronic lymphocytic leukemia patients: implication of HLA-G. Leukemia 2008;22:998–1006. 10.1038/leu.2008.15 [DOI] [PubMed] [Google Scholar]
- 11. Hasenkamp J, Borgerding A, Wulf G, et al. Resistance against natural killer cell cytotoxicity: analysis of mechanisms. Scand J Immunol 2006;64:444–9. 10.1111/j.1365-3083.2006.01803.x [DOI] [PubMed] [Google Scholar]
- 12. Jochems C, Hodge JW, Fantini M, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity Cd16 allele. Oncotarget 2016;7:86359–73. 10.18632/oncotarget.13411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maadi H, Soheilifar MH, Choi W-S, et al. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers (Basel) 2021;13:3540. 10.3390/cancers13143540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of Her2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism Br J Cancer 2006;94:259–67. 10.1038/sj.bjc.6602930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karvouni M, Vidal-Manrique M, Lundqvist A, et al. Engineered NK cells against cancer and their potential applications beyond. Front Immunol 2022;13. 10.3389/fimmu.2022.825979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finck AV, Blanchard T, Roselle CP, et al. Engineered cellular Immunotherapies in cancer and beyond. Nat Med 2022;28:678–89. 10.1038/s41591-022-01765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burger MC, Forster M-T, Romanski A, et al. Intracranial injection of NK cells engineered with a Her2-targeted chimeric antigen receptor in patients with recurrent glioblastoma. Neuro Oncol 2023;25:2058–71. 10.1093/neuonc/noad087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eitler J, Wotschel N, Miller N, et al. Inability of granule polarization by NK cells defines tumor resistance and can be overcome by CAR or ADCC mediated targeting. J Immunother Cancer 2021;9:e001334. 10.1136/jitc-2020-001334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Urlaub D, Höfer K, Müller M-L, et al. LFA-1 activation in NK cells and their subsets: influence of receptors, maturation, and cytokine stimulation. J Immunol 2017;198:1944–51. 10.4049/jimmunol.1601004 [DOI] [PubMed] [Google Scholar]
- 20. Mentlik AN, Sanborn KB, Holzbaur EL, et al. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell 2010;21:2241–56. 10.1091/mbc.e09-11-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu H-T, Mace EM, Carisey AF, et al. NK cells converge lytic granules to promote cytotoxicity and prevent bystander killing. J Cell Biol 2016;215:875–89. 10.1083/jcb.201604136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Becker JC, Dummer R, Hartmann AA, et al. Shedding of ICAM-1 from human melanoma cell lines induced by IFN-gamma and tumor necrosis factor-alpha. functional consequences on cell-mediated cytotoxicity. J Immunol 1991;147:4398–401. 10.4049/jimmunol.147.12.4398 [DOI] [PubMed] [Google Scholar]
- 23. Hamaï A, Meslin F, Benlalam H, et al. ICAM-1 has a critical role in the regulation of metastatic melanoma tumor susceptibility to CTL lysis by interfering with Pi3K/AKT pathway. Cancer Res 2008;68:9854–64. 10.1158/0008-5472.CAN-08-0719 [DOI] [PubMed] [Google Scholar]
- 24. Ueda R, Kohanbash G, Sasaki K, et al. Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc Natl Acad Sci U S A 2009;106:10746–51. 10.1073/pnas.0811817106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao Y, Chen J, Wang J, et al. Acute myeloid leukemia epigenetic immune escape from nature killer cells by ICAM-1. Front Oncol 2021;11. 10.3389/fonc.2021.751834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larson RC, Kann MC, Bailey SR, et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature 2022;604:563–70. 10.1038/s41586-022-04585-5 [DOI] [PubMed] [Google Scholar]
- 27. Maki G, Klingemann HG, Martinson JA, et al. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res 2001;10:369–83. 10.1089/152581601750288975 [DOI] [PubMed] [Google Scholar]
- 28. Schönfeld K, Sahm C, Zhang C, et al. Selective inhibition of tumor growth by clonal NK cells expressing an Erbb2/Her2-specific Chimeric antigen receptor. Mol Ther 2015;23:330–8. 10.1038/mt.2014.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy A, Long A, Volkov Y, et al. Cross-linking of LFA-1 induces secretion of macrophage inflammatory protein (MIP)-Lalpha and MIP-1Beta with consequent directed migration of activated lymphocytes. Eur J Immunol 2000;30:3006–11. [DOI] [PubMed] [Google Scholar]
- 30. Wu T, Shi J-X, Geng S, et al. The Mk2/Hur signaling pathway regulates TNF-Α-induced ICAM-1 expression by promoting the stabilization of ICAM-1 mRNA. BMC Pulm Med 2016;16:84. 10.1186/s12890-016-0247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang M, March ME, Lane WS, et al. A signaling network stimulated by beta2 integrin promotes the polarization of lytic granules in cytotoxic cells. Sci Signal 2014;7. 10.1126/scisignal.2005629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uherek C, Tonn T, Uherek B, et al. Retargeting of natural killer-cell cytolytic activity to erbb2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 2002;100:1265–73. [PubMed] [Google Scholar]
- 33. Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol 2008;9:503–10. 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 34. Aldeghaither DS, Zahavi DJ, Murray JC, et al. A mechanism of resistance to antibody-targeted immune attack. Cancer Immunol Res 2019;7:230–43. 10.1158/2326-6066.CIR-18-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Burger MC, Jennewein L, et al. Erbb2/Her2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst 2016;108. 10.1093/jnci/djv375 [DOI] [PubMed] [Google Scholar]
- 36. André P, Denis C, Soulas C, et al. Anti-Nkg2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 2018;175:1731–1743. 10.1016/j.cell.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herbst RS, Majem M, Barlesi F, et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non-small-cell lung cancer. JCO 2022;40:3383–93. 10.1200/JCO.22.00227 [DOI] [PubMed] [Google Scholar]
- 38. Geurts VCM, Voorwerk L, Balduzzi S, et al. Unleashing NK- and Cd8 T cells by combining monalizumab and trastuzumab for metastatic Her2-positive breast cancer: results of the MIMOSA trial. Breast 2023;70:76–81. 10.1016/j.breast.2023.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bexte T, Alzubi J, Reindl LM, et al. CRISPR-Cas9 based gene editing of the immune checkpoint Nkg2A enhances NK cell mediated cytotoxicity against multiple myeloma. Oncoimmunology 2022;11. 10.1080/2162402X.2022.2081415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delconte RB, Kolesnik TB, Dagley LF, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol 2016;17:816–24. 10.1038/ni.3470 [DOI] [PubMed] [Google Scholar]
- 41. Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a Cd19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32:520–31. 10.1038/leu.2017.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silvestre RN, Eitler J, de Azevedo JTC, et al. Engineering NK-CAR.19 cells with the IL-15/IL-15ralpha complex improved proliferation and anti-tumor effect in vivo. Front Immunol 2023;14. 10.3389/fimmu.2023.1226518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chaudhry K, Geiger A, Dowlati E, et al. Co-transducing B7H3 CAR-NK cells with the DNR preserves their cytolytic function against GBM in the presence of exogenous TGF-beta. Mol Ther Methods Clin Dev 2022;27:415–30. 10.1016/j.omtm.2022.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Snyder KM, Hullsiek R, Mishra HK, et al. Expression of a recombinant high affinity IgG FC receptor by engineered NK cells as a docking platform for therapeutic mAbs to target cancer cells. Front Immunol 2018;9. 10.3389/fimmu.2018.02873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Demaria O, Gauthier L, Vetizou M, et al. Antitumor immunity induced by antibody-based natural killer cell engager therapeutics armed with not-alpha IL-2 variant. Cell Rep Med 2022;3:100783. 10.1016/j.xcrm.2022.100783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wingert S, Reusch U, Knackmuss S, et al. Preclinical evaluation of Afm24, a novel Cd16A-specific innate immune cell engager targeting EGFR-positive tumors. MAbs 2021;13. 10.1080/19420862.2021.1950264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vallera DA, Oh F, Kodal B, et al. A Her2 tri-specific NK cell engager mediates efficient targeting of human ovarian cancer. Cancers (Basel) 2021;13. 10.3390/cancers13163994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pinto S, Pahl J, Schottelius A, et al. Reimagining antibody-dependent cellular cytotoxicity in cancer: the potential of natural killer cell engagers. Trends Immunol 2022;43:932–46. 10.1016/j.it.2022.09.007 [DOI] [PubMed] [Google Scholar]
- 49. Zhang C, Röder J, Scherer A, et al. Bispecific antibody-mediated Redirection of Nkg2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity. J Immunother Cancer 2021;9:10. 10.1136/jitc-2021-002980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mitwasi N, Feldmann A, Arndt C, et al. Unicar-modified off-the-shelf NK-92 cells for targeting of Gd2-expressing tumour cells. Sci Rep 2020;10:2141. 10.1038/s41598-020-59082-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pfeifer Serrahima J, Zhang C, Oberoi P, et al. Multivalent adaptor proteins specifically target NK cells carrying a universal chimeric antigen receptor to Erbb2 (Her2)-expressing cancers. Cancer Immunol Immunother 2023;72:2905–18. 10.1007/s00262-023-03374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fabian KP, Padget MR, Donahue RN, et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J Immunother Cancer 2020;8:e000450. 10.1136/jitc-2019-000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. March ME, Long EO. Beta2 integrin induces tcrzeta-syk-phospholipase C-gamma phosphorylation and paxillin-dependent granule polarization in human NK cells. J Immunol 2011;186:2998–3005. 10.4049/jimmunol.1002438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen X, Trivedi PP, Ge B, et al. Many NK cell receptors activate Erk2 and Jnk1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci USA 2007;104:6329–34. 10.1073/pnas.0611655104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Selitrennik M, Lev S. Pyk2 integrates growth factor and cytokine receptors signaling and potentiates breast cancer invasion via a positive feedback loop. Oncotarget 2015;6:22214–26. 10.18632/oncotarget.4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu X, Bao Y, Guo Y, et al. Proline-rich protein tyrosine kinase 2 in inflammation and cancer. Cancers (Basel) 2018;10:139. 10.3390/cancers10050139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li Y, Hermanson DL, Moriarity BS, et al. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 2018;23:181–92. 10.1016/j.stem.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Töpfer K, Cartellieri M, Michen S, et al. Dap12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol 2015;194:3201–12. 10.4049/jimmunol.1400330 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-008155supp002.pdf (222.4KB, pdf)
jitc-2023-008155supp003.pdf (89.3KB, pdf)
jitc-2023-008155supp006.pdf (1.2MB, pdf)
jitc-2023-008155supp004.pdf (127.3KB, pdf)
jitc-2023-008155supp005.pdf (539.7KB, pdf)
jitc-2023-008155supp007.pdf (426KB, pdf)
jitc-2023-008155supp001.pdf (3.8MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Further inquiries can be directed to the corresponding author.