Abstract
Background
Acute exacerbation (AE) is a life-threatening condition taking place not only in idiopathic pulmonary fibrosis (IPF) but also in interstitial lung diseases (ILD) other than IPF (non-IPF ILD). This study aims to compare the clinical manifestations between patients hospitalised with AE-IPF and AE-non-IPF ILD, and further analyse the risk factors related to in-hospital mortality.
Methods
Clinical data of 406 patients hospitalised with AE-IPF (93 cases) and AE-non-IPF ILD (313 cases) were retrospectively collected. Clinical features were compared between the two groups. Risk factors related to in-hospital mortality in patients with overall AE-ILD, AE-IPF and AE-non-IPF ILD were identified by multiple logistic regression analyses, respectively, and assessed by receiver operating characteristic curve.
Results
In addition to having more smokers and males, the AE-IPF group also had more respiratory failure on admission, comorbidities of pulmonary hypertension (PAH) or coronary artery disease/heart failure, a longer history of pre-existing ILD. Comorbidity of coronary heart disease/heart failure, respiratory failure at admission, neutrophil (N)%, serum hydroxybutyrate dehydrogenase (HBDH), lactate dehydrogenase (LDH) and low cholesterol levels were independent risk factors for patients with AE-ILD, while respiratory failure on admission, N%, serum HBDH, urea nitrogen, LDH and low albumin levels were risk factors for the AE-non-IPF ILD group, and fever, N% and PAH were the AE-IPF group’s. Among them, HBDH 0.758 (sensitivity 85.5%, specificity 56%, cut-off 237.5 U/L) for patients with AE-ILD; N% 0.838 (sensitivity 62.5%, specificity 91.18%, cut-off 83.55%) for the AE-IPF group and HBDH 0.779 (sensitivity 86.4%, specificity 55.1%, cut-off 243.5 U/L) for the AE-non-IPF ILD group were the risk factors with the highest area under the curve.
Conclusions
Clinical characteristics differ between patients with AE-IPF and AE-non-IPF ILD. HBDH outperformed LDH in predicting the prognosis for patients with AE-ILD and AE-non-IPF ILD. N% was an independent predictor of death in-hospital in all three groups, especially in the AE-IPF group.
Keywords: Interstitial Fibrosis, Critical Care, Inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC
The mortality for acute exacerbation interstitial lung diseases (AE-ILD) is high and no effective treatment is available.
WHAT THIS STUDY ADDS
Our study explored the clinical manifestations between patients hospitalised with AE of idiopathic pulmonary fibrosis (IPF) and non-IPF ILD, and further analysed the risk factors related to in-hospital mortality of patients with AE-ILD, AE-IPF and AE-non-IPF ILD, respectively.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The values of neutrophilia (N)%, serum hydroxybutyrate dehydrogenase and lactate dehydrogenase in predicting the in-hospital death of patients with AE-ILD should not be ignored.
Introduction
As a heterogeneous group of diseases, interstitial lung diseases (ILDs) are characterised by abnormalities of the alveolar wall and often involve the interstitial, alveoli or bronchioles of the lungs. As a special type of ILD, idiopathic pulmonary fibrosis (IPF) is marked by dyspnoea and progressive deterioration of lung function.1 Acute exacerbations (AEs) are critical clinical events associated with ILDs. The prognosis for AE-IPF is extremely poor,2 AE of ILD other than IPF, is referred to as AE-non-IPF ILD.3
The mortality rate of AE-ILD continues to be high as its incidence rises annually. It is estimated that 5%–15% of patients with IPF experience AE at 1 year.4 The yearly incidence of AE per 1000 patients with IPF in the USA was 130 cases, whereas in South Korea, the incidence was 14.2% and 20.7% in 1 year and 3 years, respectively, according to a 2016 report on AE-IPF by an International Working Group5 A 9.4% rate of AE-ILD was also recorded in Japanese.6 For AE-IPF, the in-hospital mortality rate exceeded 50%,7 8 while for AE-ILD, it was marginally lower but still lethal.9
The high-dose steroid proved helpful in the majority of AE-ILD cases, especially in AE-non-IPF ILD cases. The survival rate was increased when prednisolone levels above 1 mg/kg, but not in cases with AE-IPF.10 Although corticosteroids are thought to be the most successful treatment in many cases with AE-non-IPF ILD, they do not yet appear to have a precise curative effect on AE-IPF. The severity of AE-ILD and the subclass classification of ILD (eg, whether it is IPF) may influence the choice of therapeutic alternatives, such as steroid therapy. It is worthwhile to explore, therefore, how to accurately identify high-risk patients in AE-ILD by predicting the probability of death and if the subtype of ILD is IPF based on clinical features.
Methods
Study design
We retrospectively reviewed the data of adult patients who met the criteria of AE-ILD hospitalised in Beijing Chaoyang Hospital between January 2017 and June 2022. The medical records of the first episode of AE were collected for inpatients with AE-ILD.
Inclusion/exclusion criteria
The AE-ILD diagnosis was based on the AE-IPF diagnostic guidelines reported by an International Working Group Report in 20165 with the criteria including an acute worsening or development of dyspnoea with a duration of <1 month, new bilateral ground glass opacity and/or consolidation superimposed on fibrosis in chest high-resolution CT (HRCT) imaging, the deterioration not fully explained by fluid overload or cardiac failure and the previous existed ILD.
Those who treat the following diseases primarily were excluded: (1) heart failure or coronary artery disease; (2) pulmonary embolism; (3) sarcoidosis, occupational disease-related and other rare ILDs; (4) chest tumours; (5) other infected lung illnesses; (6) pleural effusion, pneumothorax and asthma; (7) lung transplantation and (8) illness in other systems.
Screening of cases
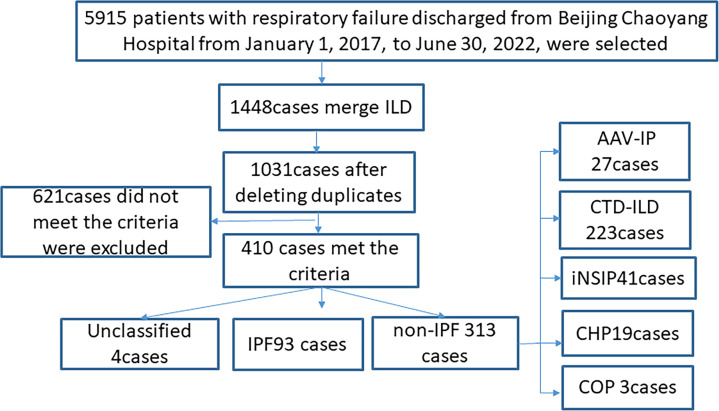
When the patients were chosen, the diagnosis of AE-ILD was reassessed by the two trained pulmonologists in our institution who reviewed the patient’s current and previous medical records, laboratory findings and the specific characteristics of chest HRCT thoroughly. Multidisciplinary discussion in our institution was carried out to reconfirm the diagnosis of each underlying ILD subtype in accordance with the ILD guidelines or consensus statement,11–16 at the time of patients enrolled. The detailed description of the case screening process is in figure 1.
Figure 1.
Flow diagram of patient screening and enrolment. AAV-IP, antineutrophil cytoplasmic antibody-associated vasculitis with interstitial pneumonia; CHP, chronic hypersensitivity pneumonia; COP, cryptogenic organising pneumonia; CTD-ILD, connective tissue disease associated with interstitial lung disease; iNSIP, idiopathic non-specific interstitial pneumonia; IPF, idiopathic pulmonary fibrosis.
Data collection
The following data should be gathered from patients who meet the criteria for AE-ILD: basic information such as age and gender, comorbidities such as arterial hypertension, diabetes, clinical symptoms such as cough and fever, physical examinations such as bilateral basal crackles and clubbing, pulmonary function test (PFT) (the data from this hospitalisation or the most recent examination within the last 12 months), laboratory data like blood routines, biochemistry and treatment such as steroid use, as well as the outcomes of disease.
Statistical analysis
SPSS V.25.0 and GraphPad Prisma V.8.0 were used for statistical analysis. Continuous values were shown as mean and SD (X±S) or median with IQR (M (Q1, Q3)) according to the distributions. Categorical variables were presented as frequencies and proportions. The t-test or Mann-Whitney U test or χ2 test was used to compare the two groups. The Spearman’s correlation test was used to assess the association between lactate dehydrogenase (LDH) and hydroxybutyrate dehydrogenase (HBDH). Binary logistic multiple regression analysis was used to screen the risk factors for in-hospital death among patients with AE-ILD, AE-IPF and AE-non-IPF ILD, respectively. The cut-off value for each risk factor be found using the receiver operating characteristic (ROC) curve, and the differences between ROC curves be compared. Statistical significance was set at p<0.05.
Results
Study population
406 patients were included (93 with IPF and 313 with non-IPF ILD) and we looked into all 410 patients with first-episode AE-ILD. Due to the inability to classify their ILDs, four patients were omitted. The non-IPF ILD group was composed of 223 patients with connective tissue disease-related ILD (CTD-ILD), 41 patients with idiopathic nonspecific interstitial pneumonia (iNSIP), 27 patients with antineutrophil cytoplasmic antibody-associated vasculitis-related interstitial pneumonia (AAV-IP), 19 patients with chronic hypersensitivity pneumonitis (CHP) and 3 patients with cryptogenic organising pneumonia (COP)).
Baseline characteristics, clinical presentation, laboratory data, PFT data: AE-IPF versus AE-non-IPF ILD
In contrast to the AE-non-IPF ILD group, the AE-IPF group had a significantly greater percentage of male patients (87.1% vs 54%, p<0.001), more former or ex-smokers (73.12% vs 46.33%, p<0.001), longer duration of pre-existing ILD (18 vs 4 months, p=0.001) and more patients with a former ILD diagnosis (90.32% vs 66.45%, p<0.001). There was no statistical difference in the age distribution (64 vs 66 years, p=0.813), body mass index (BMI) (23.78 vs 24.16 kg/m2, p=0.867) or days of exacerbation before to admission (15 vs 15, p=0.898) between patients with AE-IPF and AE-non-IPF ILD. As for comorbidities, the AE-IPF group exhibited a higher frequency of coronary artery disease/heart failure (49.5% vs 31.6%, p=0.002), pulmonary hypertension (PAH) (38.71% vs 24.92%, p=0.009) and famiy history of pulmonary fibrosis(6.59% vs 1.59%, p=0.010) in comparison to the AE-non-IPF ILD group. However, no statistically significant differences were observed in arterial hypertension, diabetes mellitus, lung cancer or tachyarrhythmia between the two groups (table 1).
Table 1.
Baseline characteristics of patients with AE-IPF and AE-non-IPF ILD
| Variables | IPF (N=93) | Non-IPF (N=313) | P value | ||
| Baseline characteristics | n/X/M | %/S/Q1–Q3 | n/X/M | %/S/Q1-Q3 | |
| Men | 81 | (87.1%) | 169 | (54%) | <0.001 |
| Age (year) | 64 | (61, 72) | 66 | (59, 74) | 0.813 |
| BMI (kg/m2) | 23.78 | (22.46, 25.95) | 24.16 | (21.78, 26.76) | 0.867 |
| Smoker (current/ex) | 68 | 73.12% | 145 | 46.33% | <0.001 |
| Duration of pre-existing ILD(m) | 18 | (2.5,48) | 4 | (0,36) | 0.001 |
| Exacerbation duration before admission(d) | 15 | (7,30) | 15 | (7.5,30) | 0.898 |
| No former ILD diagnosis | 9 | 9.68% | 105 | 33.55% | <0.001 |
| Arterial hypertension | 43 | 46.20% | 119 | 38.00% | 0.155 |
| Diabetes mellitus | 32 | 34.40% | 91 | 29.10% | 0.326 |
| Coronary artery disease and/or heart failure | 46 | 49.50% | 99 | 31.60% | 0.002 |
| PAH | 36 | 38.71% | 78 | 24.92% | 0.009 |
| Lung cancer | 3 | 3.23% | 5 | 1.60% | 0.321 |
| Tachyarrhythmia | 8 | 8.60% | 26 | 8.31% | 0.928 |
| Family history of pulmonary fibrosis | 6 | 6.59% | 5 | 1.59% | 0.010 |
AE, acute exacerbation; BMI, body mass index; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; PAH, pulmonary hypertension.
Patients with AE-IPF did not vary from those with AE-non-IPF ILD in terms of symptoms or physical findings at admission. In both groups, cough, expectoration (only about 15% purulent) and dyspnoea were the most common symptoms. Although clubbing was more frequent in AE-IPF group (23.66% vs 15.65%), it was not statistically significant. In patients with non-IPF ILD, laboratory data showed lower levels of haemoglobin (HGB) (p=0.002), serum albumin (p=0.001), serum creatinine (p=0.009) and peripheral blood platelet counts (p=0.001) and serum C reactive protein (CRP) level (p=0.027). The AE-IPF group had more patients with respiratory failure on admission (79.57% vs 69.01%, p=0.048) (table 2).
Table 2.
Clinical presentation and laboratory data of patients with AE-IPF and AE-non-IPF ILD
| Variables | IPF (N=93) | Non-IPF (N=313) | P value | ||
| n/X/M | %/S/Q1-Q3 | n/X/M | %/S/Q1-Q3 | ||
| Symptoms | |||||
| Cough | 87 | 93.55% | 288 | 92.01% | 0.624 |
| Expectoration | 76 | 80.46% | 242 | 75.30% | 0.365 |
| Non-purulent | 66 | 86.80% | 203 | 83.90% | |
| Purulent | 10 | 13.20% | 39 | 16.10% | 0.533 |
| Chest pain | 6 | 6.45% | 25 | 7.99% | 0.624 |
| Dyspnoea | 74 | 79.57% | 265 | 84.66% | 0.245 |
| Fever | 32 | 34.41% | 131 | 41.85% | 0.198 |
| Physical examination | |||||
| Respiratory rate | 20 | (20, 22) | 20 | (20, 22) | 0.693 |
| Heart rate | 92 | (81.5, 100.5) | 90 | (80, 100) | 0.505 |
| Bilateral basal crackles | 65 | 69.90% | 194 | 62.00% | 0.163 |
| Clubbing | 22 | 23.66% | 49 | 15.65% | 0.075 |
| Laboratory data | |||||
| WBC (×109/L) | 8.945 | (6.72, 11.32) | 8.975 | (6.84, 11.33) | 0.865 |
| N% | 70.1 | (63.75, 83.25) | 75.35 | (65.7, 84.05) | 0.111 |
| HGB (g/L) | 137.5 | (122, 149) | 128 | (116.25, 142) | 0.002 |
| PLT (×109/L) | 208.5 | (160, 266) | 243.5 | (192, 309) | 0.001 |
| ALB (g/L) | 36.6 | (33.4, 38.7) | 34.5 | (31.7, 37.1) | 0.001 |
| CHOL (mmol/L) | 4.01 | (3.47, 4.7) | 4.27 | (3.47, 5.01) | 0.144 |
| LDL (mmol/L) | 2.44 | (2.1, 3.4) | 2.7 | (2.1, 3.4) | 0.276 |
| CK (U/L) | 50.5 | (35, 63) | 46 | (31, 71.75) | 0.620. |
| LDH (U/L) | 278 | (235, 354) | 296 | (233.5, 383.5) | 0.370. |
| HBDH (U/L) | 235 | (193, 291.5) | 250 | (198.5, 317.5) | 0.270. |
| GGT (U/L) | 29 | (21.5, 45) | 33 | (20.25, 54) | 0.359 |
| Urea (mmol/L) | 5.9 | (4.35, 7.395) | 5.5 | (4.07, 7.51) | 0.439 |
| CREA (mmol/L) | 63.5 | (55.7, 76.18) | 59.05 | (50.25, 72.53) | 0.009 |
| CK-MB (ng/mL) | 1.05 | (0.4, 1.775) | 1 | (0.5, 1.9) | 0.963 |
| D-dimer (ug/L) | 730 | (365, 2473.67) | 890 | (415, 1862) | 0.845 |
| CRP (mg/dL) | 1.18 | (0.58, 7.27) | 2.4 | (0.865, 7.91) | 0.027 |
| ESR (mm/hour) | 28.43 | ±21.6 | 33.92 | ±24.28 | 0.062 |
| Respiratory failure on admission | 74 | 79.57% | 216 | 69.01% | 0.048 |
AE, acute exacerbation; ALB, albumin; CHOL, cholesterol; CK, creatine kinase; CK-MB, creatine kinase isoenzymes; CREA, serum creatinine; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; GGT, glutamyl transpeptidase; HBDH, hydroxybutyrate dehydrogenase; HGB, haemoglobin; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; N, neutrophil; PLT, platelet; Urea, urea nitrogen; WBC, white blood cell.
For 24 patients with AE-IPF and 88 patients with AE-non-IPF ILD, PFT data in stability before AE were available. Comparing the percent-predicted forced vital capacity (FVC%) of the two groups, they exhibited similar moderate restrictive respiratory function impairment (75.25%±23.72% vs 68.87%±19.78%, p=0.182). The ratio of forced expiratory volume in 1 s to FVC (FEV1/FVC) was within the normal range in both groups with no difference between them. Both groups displayed a severe decrease in diffusing capacity, and with a much lower percent-predicted diffusing capacity for carbon monoxide in a single breath (DLCOSB%) (33.08%±9.76%vs 42.99%±16.07%, p<0.001) in patients with AE-IPF (table 3).
Table 3.
Baseline lung function tests in patients with AE-IPF and AE-non-IPF ILD
| Variables | IPF (n=24) | Non-IPF (n=88) | P value | ||
| FVC% | 75.25 | ±23.72 | 68.87 | ±19.78 | 0.182 |
| FEV1/FVC | 81.80 | ±6.64 | 80.67 | ±8.95 | 0.557 |
| DLCOC SB% | 33.08 | ±9.76 | 42.99 | ±16.07 | <0.001 |
AE-ILD, acute exacerbation of interstitial lung disease; DLco SB%, percent-predicted diffusing capacity for carbon monoxide in a single breath; FEV1, forced expiratory volume in the 1 s; FVC%, percent-predicted forced vital capacity; IPF, idiopathic pulmonary fibrosis.
Treatment and outcomes
In the AE-non-IPF ILD group, more patients (76% vs 65.6%, p=0.045) received systemic steroids treatment during hospitalisation for AE. There were no significant differences between the two groups in terms of bronchoscopy performed, intensive care unit (ICU) admission, mechanical ventilation and usage of antibiotics. Hospital mortality was higher in the AE-IPF group (25.8% vs 18.8%, p=0.144) but not statistically significant. No differences in mortality were seen between the two groups when the patients were assigned steroid doses; however, as the steroid doses increased, the mortality increased and reached 85.71% and 46.15%, respectively, at large dosages (>2 mg/kg/day) for patients with AE-IPF and AE-non-IPF ILD (table 4).
Table 4.
Treatment and prognosis in patients with AE-IPF and AE-non-IPF ILD
| Variables | IPF (n=93) | Non-IPF (n=313) | P value | ||
| Treatment and prognosis | n/X/M | %/S/Q1-Q3 | n/X/M | %/S/Q1-Q3 | |
| ICU admission | 7 | 7.53% | 41 | 13.10% | 0.144 |
| Bronchoscopy performed | 18 | 19.35% | 77 | 24.60% | 0.294 |
| Mechanical ventilation | 28 | 30.11% | 84 | 26.84% | 0.056 |
| Steroids | 61 | 65.60% | 238 | 76.00% | 0.045 |
| Antibiotics | 75 | 80.65% | 248 | 79.23% | 0.767 |
| In-hospital death | 24 | 25.80% | 59 | 18.80% | 0.144 |
| Steroid dose and mortality | 0.146 | ||||
| None | 6 | 18.75% | 11 | 14.67% | 0.597 |
| <1 mg/kg/day | 6 | 17.14% | 13 | 10.08% | 0.247 |
| 1–2 mg/kg/day | 6 | 31.58% | 23 | 27.71% | 0.736 |
| >2 mg/kg/day | 6 | 85.71% | 12 | 46.15% | 0.062 |
| Hospitalisation length (day) | 12 | (8,16) | 13 | (8,18) | 0.180 |
AE, acute exacerbation; ICU, intensive care unit; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis.
Clinical characteristics: survivors versus non-survivors
As for the comparisons of overall patients with AE-ILD. The non-survivors had more proportion of males (p=0.046), shorter duration of AE before admission (p<0.001) and more frequencies to be accompanied by a history of coronary artery disease/heart failure (p<0.001), PAH (p=0.017) and tachyarrhythmia (p<0.001). For clinical presentation, the non-survivors demonstrated faster respiratory rate (p<0.001) and heart rate (p=0.003), lower frequency of bilateral basal cracks auscultated (p<0.001). Laboratory data displayed significantly higher white blood cell(WBC) (p=0.017), neutrophil% (N%) (p<0.001), LDH (p<0.001), HBDH (p<0.001), γ-glutamyl transpeptidase (GGT) (p=0.001), Urea nitrogen (p<0.001), creatine kinase isoenzymes (CK-MB) (p=0.041), CRP (p<0.001), D-dimer (p<0.001) and lower levels of ALB (p<0.001), cholesterol (CHOL) (p=0.048), low-density lipoprotein (p=0.022) in non-survivors. Significantly more patients had respiratory failure on admission (p=0.002), were admitted to ICU (p<0.001), experienced bronchoscopy (p=0.049), received therapies of mechanical ventilation(p<0.001), antibiotics (p=0.003) and systemic steroid with medium (p=0.021) and high (p<0.001) doses in non-survivors (online supplemental tables 1–3).
bmjresp-2023-001997supp001.pdf (136.8KB, pdf)
As far as the comparisons of the AE-IPF group. The non-survivors had more proportion of smokers (p=0.021), shorter duration of AE before admission (p=0.008) and accompanied with more PAH (p=0.001). For clinical presentation, the non-survivors had more fever (p=0.001), faster respiratory rate (p=0.019) and heart rate (p=0.023), lower frequency of bilateral basal cracks auscultated (p=0.014). Laboratory data displayed significantly higher WBC (p<0.001), N% (p<0.001), LDH (p=0.023), HBDH (p=0.023), urea nitrogen (p=0.001), CRP (p<0.001) levels and lower CHOL (p=0.037) in non-survivors. Moreover, more patients were admitted to ICU (p=0.012), received therapies of mechanical ventilation (p<0.001) and high steroid doses(p=0.001) in non-survivors.
For the comparisons of the AE-non-IPF ILD group. The non-survivors had shorter duration of AE before admission(p=0.003) and accompanied with more coronary artery disease/heart failure history (p=0.001) and tachyarrhythmia (p<0.001). For clinical presentation, the non-survivors demonstrated faster respiratory rate (p=0.001) and heart rate (p=0.034). Laboratory data displayed significantly higher N% (p<0.001), LDH (p<0.001), HBDH (p<0.001), GGT (p=0.001), urea nitrogen (p<0.001), CRP (p<0.001), D-dimer (p=0.016) levels and lower level of ALB (p<0.001) in non-survivors. Significantly more patients had respiratory failure on admission (p=0.001), were admitted to ICU (p<0.001), experienced bronchoscopy (p=0.012), received therapies of mechanical ventilation (p<0.001), antibiotics (p<0.001) and systemic steroid with medium (p=0.016) and high (p<0.001) doses in non-survivors.
Prognostic factors analysis
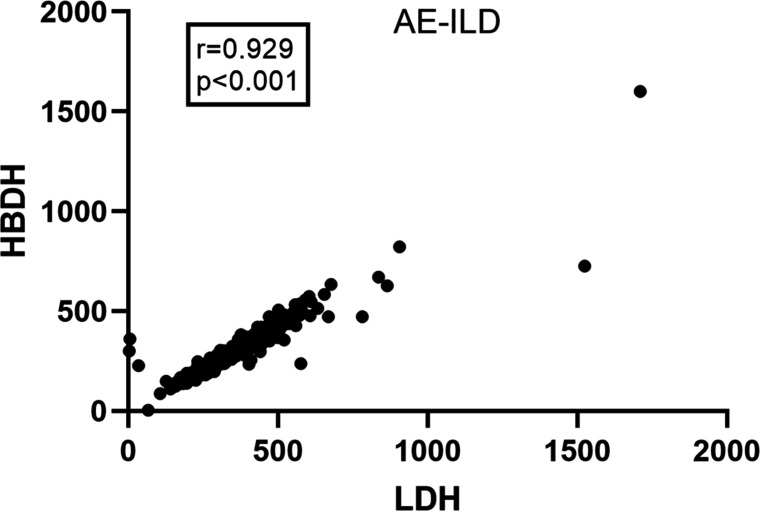
The prognostic factors related to in-hospital mortality were assessed by multivariate regression and ROC analysis. Since HBDH and LDH had a correlation coefficient of 0.929 (figure 2), they were added to separate screening models, respectively. Five predictive models in all were obtained: two for AE-ILD (model A/HBDH and B/LDH), one for AE-IPF (model C) and two for AE-non-IPF ILD (model D/HBDH and E/LDH) (table 5, figure 3, online supplemental table 4)
Figure 2.
The correlation between LDH and HBDH in patients with AE-ILD. AE-ILD, acute exacerbation of interstitial lung disease; HBDH, hydroxybutyrate dehydrogenase; LDH, lactate dehydrogenase; r, coefficient of correlation.
Table 5.
Multivariate regression analysis of risk factors related to in-hospital mortality and the cut-off values in the ROC curve
| Variables | B | P value | OR | 95% CI | Cut-off | |
| (A) AE-ILD (HBDH model) p=1/(1+e−(−7.188+0.869×coronary artery disease or heart failure+0.815×respiratory failure upon admission+0.047×N%−0.291×CHOL+0.008×HBDH)) | ||||||
| Coronary artery disease or heart failure | 0.869 | 0.003 | 2.385 | 1.344 | 4.231 | |
| Respiratory failure on admission | 0.815 | 0.031 | 2.259 | 1.075 | 4.747 | |
| N% | 0.047 | 0.001 | 1.049 | 1.019 | 1.079 | 77.75 |
| CHOL (mmol/L) | −0.291 | 0.027 | 0.748 | 0.578 | 0.968 | 4.45 |
| HBDH (U/L) | 0.008 | <0.001 | 1.008 | 1.005 | 1.011 | 237.5 |
| Constant | −7.188 | <0.001 | 0.001 | |||
| (B) AE-ILD (LDH model) p=1/(1+e−(−7.294+0.779×coronary artery disease or heart failure+0.855×respiratory failure upon admission+0.054×N%−0.258×CHOL+0.005×LDH)) | ||||||
| Coronary artery disease or heart failure | 0.779 | 0.006 | 2.179 | 1.245 | 3.814 | |
| Respiratory failure on admission | 0.855 | 0.022 | 2.35 | 1.133 | 4.874 | |
| N% | 0.054 | <0.001 | 1.056 | 1.026 | 1.086 | 77.75 |
| CHOL (mmol/L) | −0.258 | 0.045 | 0.773 | 0.6 | 0.994 | 4.45 |
| LDH (U/L) | 0.005 | <0.001 | 1.005 | 1.003 | 1.008 | 306 |
| Constant | −7.294 | <0.001 | 0.001 | |||
| (C) AE-IPF p=1/(1+e−(−12.287+2.047×PAH+1.462×fever+0.127×N%)) | ||||||
| PAH | 2.047 | 0.003 | 7.742 | 1.965 | 30.508 | |
| Fever | 1.462 | 0.031 | 4.317 | 1.145 | 16.268 | |
| N% | 0.127 | <0.001 | 1.135 | 1.059 | 1.217 | 83.55 |
| Constant | −12.29 | <0.001 | 0 | |||
| (D) AE-nonIPF (HBDH model) p=1/(1+e−(−1.938−0.082×ALB+0.009×HBDH+0.063×urea)) | ||||||
| ALB (g/L) | −0.082 | 0.005 | 0.921 | 0.869 | 0.976 | 32.45 |
| HBDH (U/L) | 0.009 | <0.001 | 1.009 | 1.006 | 1.012 | 243.5 |
| Urea(mmol/L) | 0.063 | 0.038 | 1.066 | 1.003 | 1.131 | 6.495 |
| Constant | −1.938 | 0.074 | 0.144 | |||
| (E) AE-non-IPF (LDH model) p=1/(1+e−(−8.193+1.064×respiratory failure on admission+0.044×N%+0.006×LDH+0.076×urea)) | ||||||
| Respiratory failure on admission | 1.064 | 0.015 | 2.898 | 1.23 | 6.829 | |
| N% | 0.044 | 0.007 | 1.045 | 1.012 | 1.079 | 77.75 |
| LDH (U/L) | 0.006 | <0.001 | 1.006 | 1.003 | 1.008 | 373.48 |
| Urea (mmol/L) | 0.076 | 0.036 | 1.079 | 1.005 | 1.157 | 6.495 |
| Constant | −8.193 | <0.001 | 0 | |||
AE-ILD, acute exacerbation of interstitial lung disease; ALB, albumin; CHOL, cholesterol; HBDH, Hydroxybutyrate dehydrogenase; IPF, idiopathic pulmonary fibrosis; LDH, Lactate dehydrogenase; N%, neutrophil%; PAH, pulmonary artery hypertension; ROC, receiver operating characteristic; Urea, urea nitrogen.
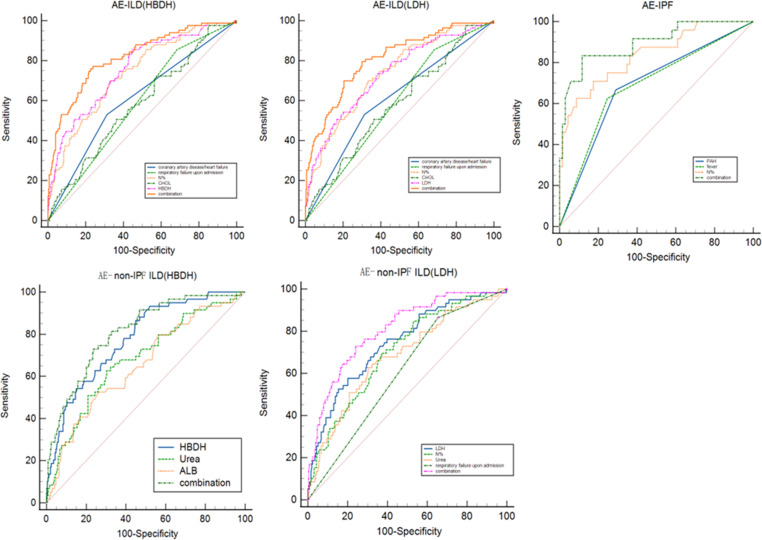
Figure 3.
The receiver operating characteristic curves of independent risk factors for prognosis in patients with AE-ILD, AE-IPF and AE-non-IPF. AE-ILD, acute exacerbation of interstitial lung disease; AE-IPF, AE of idiopathic pulmonary fibrosis; ALB, albumin; CHOL, cholesterol; HBDH, hydroxybutyrate dehydrogenase; LDH, Lactate dehydrogenase; N%, neutrophil%; PAH, pulmonary hypertension; Urea, urea nitrogen.
In all populations with AE-ILD, we identified that a history of coronary artery disease/heart failure, respiratory failure on admission and high N%, serum HBDH and LDH levels were significantly associated with in-hospital death; in contrast, a high level of serum CHOL was a favourable, independent prognostic factor related to in-hospital survival. Variables with an area under the ROC curve (AUC)>0.7 were: N% (AUC, 0.734; sensitivity, 69.9%; specificity, 66.3%; cut-off, 77.75%), HBDH (AUC,0.758; sensitivity, 85.5%; specificity, 56%; cut-off, 237.5 U/L) and LDH (AUC, 0.733; sensitivity, 72.3%; specificity, 62.2%; cut-off, 306 U/L). When the five predictors were considered together, the AUC was 0.823 (sensitivity, 77.1%; specificity, 76.8%) (model A) and 0.809 (sensitivity, 78.3%; specificity, 72.4%) (model B).
In AE-IPF group, we found that PAH, fever and high N% level were significantly linked to in-hospital mortality. Among these, N% (AUC, 0.838; sensitivity, 62.5%; specificity, 91.18%; cut-off, 83.55%) was the variable with an AUC>0.7. The AUC of the three predictors combined was 0.893 (sensitivity, 83.3%; specificity, 87%) (model C).
In AE-non-IPF ILD group, we detected that respiratory failure on admission, high N%, serum HBDH, urea and LDH levels were significantly related to in-hospital death; however, high serum ALB level was a favourable, independent predictive factor related to in-hospital survival. HBDH (AUC, 0.779; sensitivity, 86.4%; specificity, 55.1%; cut-off, 243.5 U/L), LDH (AUC, 0.744; sensitivity, 57.6%; specificity, 79.9%; cut-off, 373.48 U/L) and N% (AUC, 0.704; sensitivity, 69.5%; specificity, 63%; cut-off, 77.75%) were the variables with AUC>0.7 . The AUC was 0.81 (sensitivity, 72.9%; specificity, 76.4%) and 0.808 (sensitivity, 72.9%; specificity, 76%) for model D and model E, respectively, when the five predictors combined.
Discussion
This study included 406 cases of AE-ILD, among which 93 cases (22.91%) were IPF and 313 cases (77.09%) were non-IPF, of which 223 cases were CTD-ILD, accounting for the highest proportion, which was different from the highest proportion of IPF previously reported in other countries.7 17 18 IPF, iNSIP, AAV-IP, CHP and COP were the next most common non-IPF cases. Furthermore, our results might not match those of other studies that included sarcoidosis, occupational diseases and other rare ILD in the non-IPF group.17 In this study, the proportion of males and smokers in the IPF group was higher than the non-IPF group, but no significant difference was observed in age or BMI, which was line with previous studies.7 17 19 Smoking history decreased in-hospital mortality in patients with fibrotic ILD hospitalised for acute respiratory exacerbations,7 and smoking has been demonstrated to be a predictive protect factor for 90-day mortality after AE-ILD.19 According to this study, the proportion of smokers with AE-IPF was higher in survivors than in non-survivors (52.2% vs 25%, p=0.021), although it was not a reliable indicator of survival.
ILD is a collection of diffuse interstitial lesions that primarily damage the lungs’ interstitial and alveolar regions, causing dyspnea to worsen over time. Loss of alveolar-capillary function may be the cause of hypoxia, and persistent hypoxia over an extended period of time may aggravate PAH due to right ventricular enlargement, which can lead to heart failure. Hypoxia can also result in coronary artery disease. Consistent with earlier research, the results showed that the ILD duration was longer in the IPF group than in the non-IPF group.18 In this study, the IPF group had higher rates of respiratory failure on admission, history of coronary artery disease or heart failure, PAH, family history of pulmonary fibrosis and percentage of patients with previous ILD diagnosis than the non-IPF group. This indicates that patients with IPF may experience hypoxia for a longer period of time or have more severe pulmonary fibrosis. Further, PAH was an independent risk factor for AE-IPF (OR 7.742; 95% CI 1.965 to 30.508), which was consistent with the findings in previous studies that PAH is an independent prognostic factor for AE-ILD (OR 1.85; 95% CI 1.17 to 2.92),17 and secondary PAH increases the risk of AE-IPF.20 An independent prognostic risk factor for both AE-ILD and AE-non IPF ILD was respiratory failure at admission, a sign of a reasonably severe disease. Furthermore, among patients with AE-ILD, a history of heart failure or coronary artery disease was an independent predictive risk factor for in-hospital death. Furthermore, the degree and duration of hypoxia were related to the outcome of AE-ILD, although this has rarely been reported in previous studies. As previously reported, a lower FVC% in the 12 months preceding AE-IPF onset and a lower ratio of partial pressure of oxygen to the fraction of inspiratory oxygen at diagnosis were independent risk factors for death.21 In this study, the FVC% and FEV1/FVC were not significantly different between the IPF and non-IPF groups. Nevertheless, there was a significant decrease in DLCO SB% in both groups, with a greater difference observed in the IPF group. This suggests that patients with ILD often experience restrictive ventilatory dysfunction and diffusion dysfunction. Additionally, those with IPF had lower diffuse capacities. Therefore, hypoxia might be more serious in patients with IPF . However, because there were insufficient data for this investigation, prognostic analysis of PFT was not carried out.
Pulmonary infection often triggers AE-ILD. In the past, the name ‘AE-IPF’ was reserved for idiopathic illnesses; however, this concept was amended to exclude ‘idiopathic’ conditions in the 2016 AE-IPF diagnostic criteria. Infections such as tracheal or lung infections might cause AE-IPF. AE-IPF is distinguished from stable IPF by a neutrophil-driven inflammatory process as opposed to a fibrotic one.22 23 According to earlier research, CRP could be a biomarker for AE-IPF patients’ mortality.24 Moreover, N is a predictor of outcome for patients with AE-ILD .17 In patients with AE-IPF, alveolar macrophages produce ferritin, and elevated serum ferritin level is associated with a poor prognosis.25 Thus, it seems that pulmonary infection plays an important role in the determining the prognosis of patients with AE-ILD. In this study, there was no difference in the WBC, N% and body temperature between the IPF and non-IPF groups. However, the non-IPF group’s CRP was greater, suggesting that their pulmonary infection may more serious and more easily cause AE-ILD. In this study, N% was an independent prognostic factor in three groups: AE-ILD (OR 1.049 with model A/1.056 with model B), AE-IPF (OR 1.132) and AE-non-IPF ILD (OR 1.045) (all AUCs>0.7). The cut-off value for N% was 83.55% in the AE-IPF group and 77.75% in the AE-ILD and AE-non-IPF ILD groups. This suggests that N% for AE-ILD is a solid predictive factor. Additionally, fever was found to be an independent prognostic factor for AE-IPF in this study (OR 4.317), suggesting that the prognosis of AE-IPF may be influenced by immunological status and the severity of pulmonary infection. Moreover, D-dimer levels were higher in non-survivors than in survivors, indicating a potential influence of infection severity on the outcome of AE-ILD.
Often, organ damage is associated with a poor prognosis. Patients with AE-IPF who are underweight have a greater mortality rate than patients who are fat.26 Furthermore, it has been suggested that organ function and nutritional condition may have an impact on the prognosis of patients with AE-IPF. CHOL and LDH may be biomarkers for the prediction of mortality in these patients.24 The non-IPF group in this study had lower levels of HGB, ALB and CREA, suggesting a worse nutritional condition. With a cut-off value of 4.45 mmol/L, this study demonstrated that CHOL was an independent predictive protective factor in patients with AE-ILD. With a cut-off value of 32.45 g/L, ALB was an independent predictive protective factor in patients with AE-non-IPF ILD. These results suggest a relationship between nutritional condition and the prognosis of AE-ILD and AE-non-IPF ILD. LDH and HBDH show possible tissue or cell damage or inflammation. Increased levels of serum LDH or HBDH can signal the severity of the disease when different cells, including muscle, liver, red blood and cardiomyocytes, are destroyed. There is a positive correlation between HBDH and LDH, and HBDH is a biomarker of liver damage in patients with systemic lupus erythematosus.27 Furthermore, a poor prognosis is linked to elevated expression of HBDH in patients with lung cancer. HBDH is more sensitive than LDH, thus, it can potentially be used as an independent early biomarker for predicting survival.28 As biomarkers for disease severity and prognosis, LDH and HBDH can be used to evaluate patients with Pneumocystis carinii pneumonia associated with AIDS.29 Interestingly, the serum HBDH levels in patients with COVID-19 are independently associated with in-hospital mortality and disease severity.30 These studies indicate that HBDH has a prognostic value for patients suffering from lung disease. However, few studies have evaluated the prognosis value of HBDH in patients with ILD. In this study, a linear correlation was observed between LDH and HBDH (r=0.929), both HBDH and LDH were independent prognostic factors for AE-ILD and AE-non-IPF ILD, HBDH had a greater predictive value than LDH. With a cut-off of 6.495 mmol/L, the blood urea nitrogen level was an independent predictive predictor in patients with AE-non-IPF ILD in this study.
Patients with AE-IPF did not have a better outcome when corticosteroids were administered.4 31 The 2011 diagnostic guideline3 stated that systemic corticosteroids should only be administered to patients with AE-IPF in order to buy time before lung transplantation. The precise dosage and length of treatment were not stated.32 Usually, pulse therapy for 3 days followed by prednisolone maintenance therapy is administered. However, most patients have a poor prognosis. Steroids were not recommended for patients with AE-IPF according to the 2016 diagnostic guideline.5 In this study, the prevalence of steroid use was lower in the IPF group, which was in line with the guidelines. In previous studies on AE-ILD, no difference was observed between patients with IPF and those without IPF.7 18 However, other studies have found a difference:43% in the IPF group and 19% in the non-IPF group.17 Patients with AE-CTD-ILD had a better prognosis than those with AE-IPF,33 and the non-IPF group had a higher survival rate after AE than the IPF group.19 34 In this study, although the in-hospital mortality was not different between the two groups (25.8% vs 18.8%, p=0.144), the high-dose subgroup had the highest mortality, indicating that in-hospital mortality was related to the steroid dose, but not to IPF. This finding is consistent with that of a previous study that mortality increased after high-dose corticosteroid pulse therapy.7 However, this could be attributed to the more severe disease in non-survivors. Mortality in patients with IPF receiving mechanical ventilation has decreased significantly from 58.4% in 2006 to 49.3% in 2012.35 In this study, no difference in the frequency of bronchoscopy was observed between the IPF and non-IPF groups. However, the higher proportion of patients with mechanical ventilation, bronchoscopy and antibiotic use among non-survivors of AE-ILD may be due to more serious disease.
Because it was retrospective in nature, this study had certain limitations. This article did not cover patients with stable ILD, nor did it address the risk factors for adverse events in ILD. Furthermore, this study did not analyse the many subclasses of non-IPF further; rather, it focused solely on the distinctions between AE-IPF and AE-non-IPF ILD and their prognostic variables. Furthermore, a high number of missing values prevented several data—like DLco SB% and D-dimer—from being included in the analysis of prognostic variables, despite the fact that they may have been useful for prognosis. Future large-scale prospective studies are necessary to further verify and improve the findings and limitations of this investigation.
Conclusion
In conclusion, there is a greater risk of in-hospital mortality among patients with AE-ILD who have a history of coronary artery disease or heart failure, respiratory failure on admission, N%>77.75%, serum CHOL>4.45 mmol/L, HBDH>237.5 U/L and LDH>306 U/L. The same to those with PAH, fever and N%>83.55% in patients with AE-IPF; and those with respiratory failure on admission, ALB>32.45 g/L, HBDH>243.5 U/L, Urea>6.495 mmol/L, N%>77.75% and LDH>373.48 U/L in patients with AE-non-IPF ILD. Moreover, the nutritional state, liver and renal function, and other organ functions all have an impact on the prognosis of patients with AE-ILD.
Acknowledgments
We are grateful for the statistical guidance provided by Professors Lirong Liang and Jiachen Li from the Respiratory and Disease Research Center of Beijing Institute of Respiratory Medicine and Beijing Chao-yang Hospital.
Footnotes
Contributors: CB carried out the study design, data extraction, and statistical analysis, and drafted the manuscript. QF carried out the study design and revision of the paper. HW, CJ, XS and JJ participated in the revision of the paper. QF is the guarantor of this study. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was performed in accordance with the Declaration of Helsinki, which was revised in 1983. The study protocol was approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (2021-KE-295), and all patient information in this study was handled anonymously. The need for informed consent was waived by the Ethics Committee of Bei-Jing Chao-Yang Hospital, because of the retrospective nature of the study.
References
- 1. Asai N, Katsuda E, Hamanaka R, et al. “The ATS/ERS/JRS/ALAT statement "IPF by HRCT" could predict acute exacerbation of interstitial lung disease in non-small cell lung cancer”. Tumori 2017;103:60–5. 10.5301/tj.5000574 [DOI] [PubMed] [Google Scholar]
- 2. Azadeh N, Moua T, Baqir M, et al. Treatment of acute exacerbations of interstitial lung disease. Expert Rev Respir Med 2018;12:309–13. 10.1080/17476348.2018.1446831 [DOI] [PubMed] [Google Scholar]
- 3. Travis WD, Costabel U, Hansell DM, et al. An official American thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryerson CJ, Cottin V, Brown KK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J 2015;46:512–20. 10.1183/13993003.00419-2015 [DOI] [PubMed] [Google Scholar]
- 5. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. an international working group report. Am J Respir Crit Care Med 2016;194:265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 6. Tomiyama F, Watanabe R, Ishii T, et al. High prevalence of acute exacerbation of interstitial lung disease in Japanese patients with systemic sclerosis. Tohoku J Exp Med 2016;239:297–305. 10.1620/tjem.239.297 [DOI] [PubMed] [Google Scholar]
- 7. Moua T, Westerly BD, Dulohery MM, et al. Patients with fibrotic interstitial lung disease hospitalized for acute respiratory worsening: a large cohort analysis. Chest 2016;149:1205–14. 10.1016/j.chest.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki A, Kondoh Y, Brown KK, et al. Acute exacerbations of fibrotic interstitial lung diseases. Respirology 2020;25:525–34. 10.1111/resp.13682 [DOI] [PubMed] [Google Scholar]
- 9. Leuschner G, Behr J. Acute exacerbation in interstitial lung disease. Front Med (Lausanne) 2017;4:176. 10.3389/fmed.2017.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jang HJ, Yong SH, Leem AY, et al. Corticosteroid responsiveness in patients with acute exacerbation of interstitial lung disease admitted to the emergency department. Sci Rep 2021;11:5762. 10.1038/s41598-021-85539-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 12. Travis WD, Hunninghake G, King TE, et al. Idiopathic nonspecific interstitial pneumonia: report of an American thoracic society project. Am J Respir Crit Care Med 2008;177:1338–47. 10.1164/rccm.200611-1685OC [DOI] [PubMed] [Google Scholar]
- 13. Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of hypersensitivity pneumonitis in adults. an official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2020;202:e36–69. 10.1164/rccm.202005-2032ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koo SM, Kim SY, Choi SM, et al. Korean guidelines for diagnosis and management of interstitial lung diseases: part 5. connective tissue disease associated interstitial lung disease. Tuberc Respir Dis (Seoul) 2019;82:285–97. 10.4046/trd.2019.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King TE, Lee JS. Cryptogenic organizing pneumonia. N Engl J Med 2022;386:1058–69. 10.1056/NEJMra2116777 [DOI] [PubMed] [Google Scholar]
- 16. Dirikgil E, Tas SW, Rutgers A, et al. A Dutch consensus statement on the diagnosis and treatment of ANCA-associated vasculitis. Neth J Med 2020;78:71–82. [PubMed] [Google Scholar]
- 17. Faverio P, Stainer A, Conti S, et al. Differences between acute exacerbations of idiopathic pulmonary fibrosis and other interstitial lung diseases. Diagnostics (Basel) 2021;11:1623. 10.3390/diagnostics11091623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salonen J, Vähänikkilä H, Purokivi M, et al. Causes of acute respiratory hospitalizations predict survival in fibrosing interstitial lung diseases. PLoS One 2020;15:e0242860. 10.1371/journal.pone.0242860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyashita K, Kono M, Saito G, et al. Prognosis after acute exacerbation in patients with interstitial lung disease other than idiopathic pulmonary fibrosis. Clin Respir J 2021;15:336–44. 10.1111/crj.13304 [DOI] [PubMed] [Google Scholar]
- 20. Qiu M, Chen Y, Ye Q. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Clin Respir J 2018;12:1084–92. 10.1111/crj.12631 [DOI] [PubMed] [Google Scholar]
- 21. Suzuki T, Hozumi H, Miyashita K, et al. Prognostic classification in acute exacerbation of idiopathic pulmonary fibrosis: a multicentre retrospective cohort study. Sci Rep 2021;11:9120. 10.1038/s41598-021-88718-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen S, Zhang X, Yang C, et al. Essential role of IL-17 in acute exacerbation of pulmonary fibrosis induced by non-typeable haemophilus influenzae. Theranostics 2022;12:5125–37. 10.7150/thno.74809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kono M, Miyashita K, Hirama R, et al. Prognostic significance of bronchoalveolar lavage cellular analysis in patients with acute exacerbation of interstitial lung disease. Respir Med 2021;186:106534. 10.1016/j.rmed.2021.106534 [DOI] [PubMed] [Google Scholar]
- 24. Hachisu Y, Murata K, Takei K, et al. Possible serological markers to predict mortality in acute exacerbation of idiopathic pulmonary fibrosis. Medicina (Kaunas) 2019;55:132. 10.3390/medicina55050132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enomoto N, Oyama Y, Enomoto Y, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Respir J 2018;12:2378–89. 10.1111/crj.12918 [DOI] [PubMed] [Google Scholar]
- 26. Awano N, Jo T, Yasunaga H, et al. Body mass index and in-hospital mortality in patients with acute exacerbation of idiopathic pulmonary fibrosis. ERJ Open Res 2021;7:00037-2021. 10.1183/23120541.00037-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu H, Han H, Li J, et al. Alpha-hydroxybutyrate dehydrogenase as a biomarker for predicting systemic lupus erythematosus with liver injury. Int Immunopharmacol 2019;77:105922. 10.1016/j.intimp.2019.105922 [DOI] [PubMed] [Google Scholar]
- 28. Yuan ZM, Wang LH, Chen C. Prognostic value of serum Α-HBDH levels in patients with lung cancer. World J Surg Oncol 2023;21:78. 10.1186/s12957-023-02965-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun J, Su J, Xie Y, et al. Plasma IL-6/IL-10 ratio and IL-8, LDH, and HBDH level predict the severity and the risk of death in AIDS patients with pneumocystis pneumonia. J Immunol Res 2016;2016:1583951. 10.1155/2016/1583951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu H, Qu G, Yu H, et al. Features of Α-HBDH in COVID-19 patients: a cohort study. J Clin Lab Anal 2021;35:e23690. 10.1002/jcla.23690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrade J de, Schwarz M, Collard HR, et al. The idiopathic pulmonary fibrosis clinical research network (IPFnet): diagnostic and adjudication processes. Chest 2015;148:1034–42. 10.1378/chest.14-2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao M, Sheng J, Qiu X, et al. Acute exacerbations of fibrosing interstitial lung disease associated with connective tissue diseases: a population-based study. BMC Pulm Med 2019;19:215. 10.1186/s12890-019-0960-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salonen J, Purokivi M, Bloigu R, et al. Prognosis and causes of death of patients with acute exacerbation of fibrosing interstitial lung diseases. BMJ Open Respir Res 2020;7:e000563. 10.1136/bmjresp-2020-000563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rush B, Wiskar K, Berger L, et al. The use of mechanical ventilation in patients with idiopathic pulmonary fibrosis in the United States: a nationwide retrospective cohort analysis. Respir Med 2016;111:72–6. 10.1016/j.rmed.2015.12.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001997supp001.pdf (136.8KB, pdf)
Data Availability Statement
Data are available on reasonable request.