Abstract
Objectives
To determine the most epidemiologically effective and cost-effective school-based SARS-CoV-2 antigen-detection rapid diagnostic test (Ag-RDT) self-testing strategies among teachers and students.
Design
Mathematical modelling and economic evaluation.
Setting and participants
Simulated school and community populations were parameterised to Brazil, Georgia and Zambia, with SARS-CoV-2 self-testing strategies targeted to teachers and students in primary and secondary schools under varying epidemic conditions.
Interventions
SARS-CoV-2 Ag-RDT self-testing strategies for only teachers or teachers and students—only symptomatically or symptomatically and asymptomatically at 5%, 10%, 40% or 100% of schools at varying frequencies.
Outcome measures
Outcomes were assessed in terms of total infections and symptomatic days among teachers and students, as well as total infections and deaths within the community under the intervention compared with baseline. The incremental cost-effectiveness ratios (ICERs) were calculated for infections prevented among teachers and students.
Results
With respect to both the reduction in infections and total cost, symptomatic testing of all teachers and students appears to be the most cost-effective strategy. Symptomatic testing can prevent up to 69·3%, 64·5% and 75·5% of school infections in Brazil, Georgia and Zambia, respectively, depending on the epidemic conditions, with additional reductions in community infections. ICERs for symptomatic testing range from US$2 to US$19 per additional school infection averted as compared with symptomatic testing of teachers alone.
Conclusions
Symptomatic testing of teachers and students has the potential to cost-effectively reduce a substantial number of school and community infections.
Keywords: COVID-19, Health economics, Health policy
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This analysis uses an agent-based simulation to provide specific population outcomes under the impact of SARS-CoV-2 self-testing strategies in schools.
The use of a viral load trajectory by day of infection and the associated antigen-detection rapid diagnostic test sensitivity in lieu of a broad sensitivity estimate provides more accurate testing outcomes.
The model was parameterised to three country contexts, under various epidemic conditions, broadening the generalisability of the results.
The model assumes perfect compliance of the school-going population with testing and self-isolation, so the benefits may overestimate the effect of a school-based testing strategy in practice.
The school-based testing strategies in this model were analysed in the absence of any additional interventions.
Introduction
The COVID-19 pandemic continues to evolve. Increasing levels of population immunity through both vaccines and infections has resulted in diminished cases of severe disease in many settings.1 2 In this context, testing remains a critical component to mitigate transmission by enabling the rapid identification of infectious cases for self-isolation; moreover, testing can support prompt clinical management and maintain visibility on virus circulation and evolution.3
Access to COVID-19 diagnostic testing has not been equal globally. High income countries have reported testing rates 10–100 times than that of low- and middle-income countries (LMICs), partially attributable to constrained infrastructure, which has severely limited our knowledge of disease burden in many limited resource settings.4 5 As SARS-CoV-2 antigen-detection rapid diagnostic tests (Ag-RDTs) have become more widely available, there is potential to scale up and meet future testing demand within LMICs creating a more equitable pandemic response landscape.6 Access to testing can be expanded even further through the implementation of Ag-RDT self-testing. Self-tests have been proven effective for personal use, reducing the need for healthcare-related infrastructure and staff, thus lowering the barriers to access.7
Strategic deployment of SARS-CoV-2 Ag-RDTs to the sectors of society with increased transmission potential may reduce the forward transmission of SARS-CoV-2 and the overall burden of COVID-19 within communities. Providing convenient access to self-testing in these settings, with no additional cost to the user, is also likely to increase uptake, as users would not have to diverge from their daily routines to seek out testing. Within a specific setting, such as primary and secondary schools, self-testing could not only limit transmission, but reduce the amount of time students and teachers are absent from the classroom, with additional benefits for the broader community.
Other studies have modelled the effects of school-based testing strategies on in-school SARS-CoV-2 transmissions but have yet to fully evaluate the impact of school testing on community transmission or its potential for cost-effectiveness.8–14 To address this gap, we used an agent-based model, parameterised to three countries—Brazil, Georgia and Zambia—to evaluate the impact of different self-testing strategies in schools on broader community transmission. We then performed an economic analysis to determine which school-based testing strategies would be the most cost-effective.
Methods
To evaluate self-testing strategies in schools, we used a previously developed agent-based stochastic simulation model, termed Propelling Action for Test And Treat.15–17 With the model parameterised to Brazil, Georgia and Zambia, we analysed 11 different self-testing strategies within the school-going population (teachers and students) at three testing frequencies and under 24 combinations of epidemic conditions, comprising a total of 648 scenarios per country. Each scenario was simulated five times to capture parameter variability. For each country and epidemic context, we performed a cost-effectiveness analysis to identify the optimal COVID-19 self-testing strategies. All data analysed in this study was simulated using the defined parameters and is available in the online supplemental data file. Statistical analyses were conducted in Microsoft Excel (V.16.65). Cost-effectiveness analysis and figure generation were performed in RStudio (V.2022.7.02).
bmjopen-2023-078674supp001.xlsx (1.8MB, xlsx)
Simulated population
COVID-19 self-testing strategies were modelled in three different demographic contexts—Brazil, Georgia and Zambia. The school structure and school-going population in each country differs (table 1). The model was simulated for a population of one million and results were extrapolated to the total population of each country. Epidemic context considerations included the effective reproductive number (Rt; 0.9, 1.2, 1.5, 2.0), vaccination coverage (10%, 50%, 80%) and vaccine effectiveness (30% protective against infection and 70% protective against severe disease, or 70% protective against infection and 90% protective against severe disease).18–20
Table 1.
School-going and population level parameters (further detailed in online supplemental table S1)
| Parameter | Brazil | Georgia | Zambia |
| Total population size26 | 212 600 000 | 3 990 000 | 18 380 000 |
| Students | 39 100 000 | 567 000 | 4 100 000 |
| Teachers | 2 100 000 | 73 000 | 111 000 |
| Per cent of population in schools | 19.4% | 16.1% | 22.8% |
| Student–teacher ratio | 17–20 | 8 | 42 |
| Class size | 20–26 | 20 | 37 |
| Number of schools | 383 | 985 | 310 |
| Average school size (number of students) | 400–500 | 135 | 700 |
bmjopen-2023-078674supp002.pdf (1.2MB, pdf)
Modelling scenarios
The agent-based model and relevant population parameters are detailed in online supplemental table S1.15–17 The underlying population-level symptomatic COVID-19 testing rate was parameterised to that of each country—30 tests per 100 000 population per day in Brazil, 50 in Georgia and 10 in Zambia (June 2022).5 When community members in the simulation test positive, 50% enter self-isolation, of which 86% complete a full 7 days.15–17 We assumed 100% compliance with testing and self-isolation within the school-going population for those who test positive, as a member of the school-going population who tests positive cannot return before 7 days. Individuals with a true infection who falsely test negative do not enter self-isolation and continue to contribute to SARS-CoV-2 transmission within schools and the community. No other intervention measures aside from testing and self-isolation were considered in the model. We assessed the impact in terms of the total true, absolute number of infections, deaths and symptomatic days within the school population and overall community. The absolute number of infections and symptomatic days were simulated in each scenario, while the number of deaths were calculated as 7% of severe infections—defined as infections requiring hospitalisation.21 The probability of severe infection was age dependent and is further detailed in online supplemental file 2.
In addition to a baseline level of symptomatic testing in the community, we simulated:
Symptomatic testing of all teachers.
Symptomatic testing of all teachers and students.
Then, in addition to symptomatic testing of teachers or teachers and students:
-
Asymptomatic testing of teachers at 5%, 20%, 40% or 100% of schools.
One time per 2 weeks, one time per week or two times per week.
-
Asymptomatic testing of teachers and students at 5%, 20%, 40% or 100% of schools.
One time per 2 weeks, one time per week or two times per week.
Asymptomatic testing of contacts of positive cases.
Ag-RDT sensitivity
A viral load trajectory associated with SARS-CoV-2 PCR cycle threshold (Ct) values was used to determine Ag-RDT sensitivity by day of infection.22 Ag-RDT sensitivity varied from 20.9% when the Ct is above 30, up to 96.5% when the Ct value is below 20 (online supplemental table S1).23
Cost-effectiveness analysis
The simulation model allowed for the analysis of the relative performance, with respect to infections prevented, of the school-based self-testing strategies, across a range of epidemic scenarios, which were then assessed for their cost-effectiveness in an economic evaluation. The total cost to distribute one self-test kit in a school, from the funder perspective, was assumed to be US$2.50. This includes a purchasing cost of US$1.00 per test kit, which comprises 40% of the total distribution cost.24 25 The funder is assumed to be the implementer of the self-testing strategy. The total number of true school infections averted, under the self-testing strategies, was used as the effect measure in the analysis. This measure is most relevant to the school-going population, as the self-testing strategies can directly influence the incidence of school infections, reducing absenteeism. Other outcomes, such as deaths averted, were incurred by agents in the community outside of the school-going population and were not used in the cost-effectiveness analysis. The total cost and incremental cost-effectiveness ratio (ICER) were calculated for each scenario, under every epidemic context, equalling 648 scenarios per country. The ICER in this analysis represents the cost to prevent one additional infection by comparison to the next least-costly scenario. This can be interpreted as a measure of efficiency, as scenarios with lower ICERs are more efficient at preventing additional infections than scenarios with higher ICERs.
Patient and public involvement
This modelling analysis aims to expand access to COVID-19 testing for the public through influencing global policy. However, as a mathematical modelling analysis, this study did not involve patients and the public was not involved in the design, conduct or dissemination of the research.
Results
For the 3 different country contexts, we analysed 11 SARS-CoV-2 Ag-RDT self-testing strategies in the school-going population, at 3 frequencies, across 24 different epidemic conditions. Self-testing strategies in schools are more effective at reducing the number of infections within the school population than in the broader community. The impact varies depending on the epidemic conditions and country. Targeting testing strategies to both students and teachers, versus only teachers, is more effective at reducing the total number of infections in both the school and community populations. When analysing the cost-effectiveness, 5 out of 11 strategies consistently appeared on the cost-effectiveness frontier (indicating the potential to be cost-effective) across country and epidemic contexts.
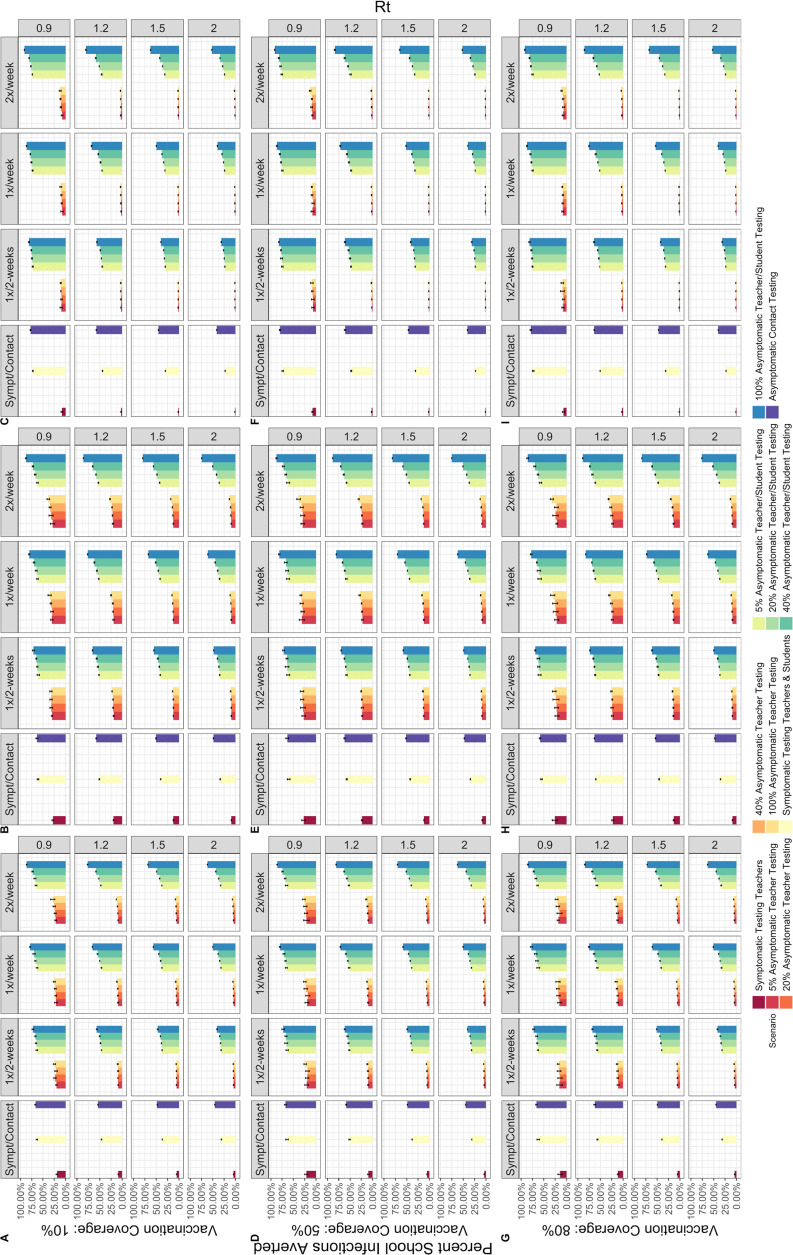
Symptomatic testing is most effective when targeted to both teachers and students and can prevent 30.9%–69.3% of school infections in Brazil, 33.7%–64.5% in Georgia and 22.8%–75.5% in Zambia, across all epidemic scenarios, over a 90-day period (figure 1). Additionally, symptomatic testing of all teachers and students can prevent anywhere between 4000 and 77 200 symptomatic days per 100 000 teachers and students in Brazil, 2030–80 900 in Georgia and 15 800–1 07 800 in Zambia, depending on the epidemic conditions. Extending to the community, this strategy can prevent up to 46.7% of community infections and 47.6% of deaths in Brazil, 31.0% of community infections and 34.6% of deaths in Georgia and 55.7% of community infections and 57.8% of deaths in Zambia. The percent of community infections averted is shown in figure 2, while the percent of deaths averted can be found in online supplemental figures S1 and S2.
Figure 1.
Per cent of infections averted among students and teachers over a 90-day period in Brazil (A,D,G), Georgia (B,E,H) and Zambia (C,F,I). Varied by effective reproductive number (Rt) (right), frequency of testing (top) and vaccination coverage (left), when vaccine effectiveness is 30%/70%.
Figure 2.
Per cent of community infections averted over a 90-day period in Brazil (A,D,G), Georgia (B,E,H) and Zambia (CFI). Varied by effective reproductive number (Rt) (right), frequency of testing (top) and vaccination coverage (left), when vaccine effectiveness is 30%/70%.
Asymptomatic testing is most effective when targeted to teachers and students at 100% of schools and can prevent 40.5%–90.7% of infections within the school population in Brazil, 47.8%–91.2% in Georgia and 31.2%–92.5% in Zambia across all epidemic conditions and testing frequencies (figure 1). This strategy can prevent 5300–154 200 symptomatic days per 100 000 teachers and students over 90 days in Brazil, 3000–135 700 in Georgia and 33 000–196 700 in Zambia, depending on the epidemic conditions. At the community level, up to 62.8% of community infections and 65.7% of deaths in Brazil, 55.4% of community infections and 56.5% of deaths in Georgia and 76.6% of community infections and 78.6% of deaths in Zambia can be prevented (figure 2).
Symptomatic and asymptomatic testing of students and teachers prevents the greatest proportion of both school and community infections when transmission levels are low to moderate (Rt=0.9, 1.2), and vaccination coverage is high (50%–80%). Conversely, testing prevents the smallest proportion of infections when transmission levels are high (Rt=2.0). When transmission is high, though testing may prevent more absolute infections than when transmission is low, this represents a smaller proportion of the overall total infections. Testing does not always prevent community infections or deaths under every epidemic scenario. When vaccines are 70% effective at preventing infection and 90% effective at preventing severe disease, the impact of testing both teachers and students on community level infections is improved at higher levels of transmission (online supplemental figure S3). Across all testing strategies, the greatest effect was observed in Zambia.
Within the asymptomatic testing strategies, testing frequency has a smaller impact than increasing the proportion of schools undergoing testing, but this impact depends on the epidemic and vaccination conditions. Testing at a biweekly frequency prevents fewer infections than weekly or two times per week testing, and the magnitude of this difference increases as the level of community transmission increases (figures 1–2).
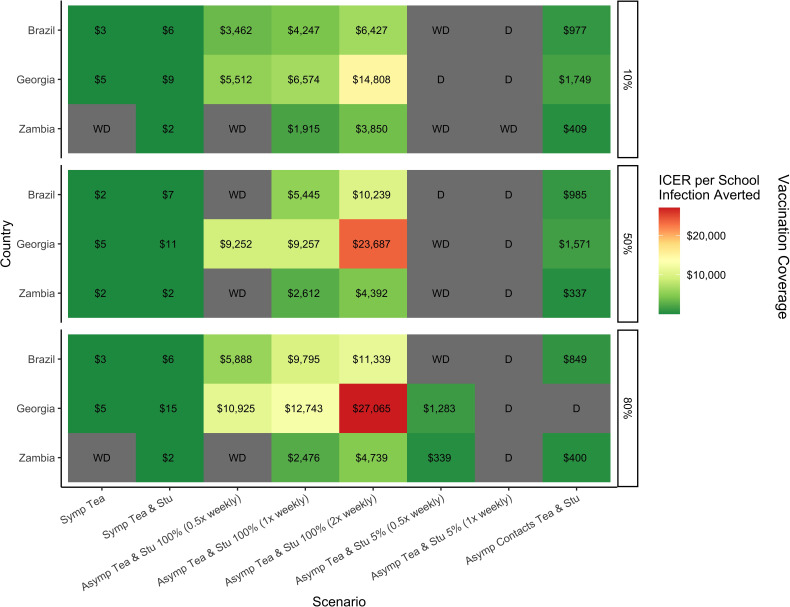
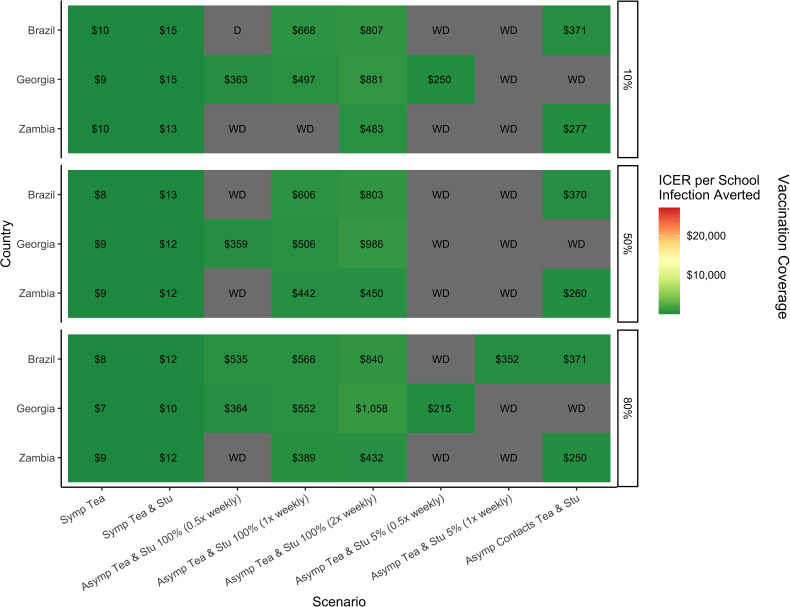
Symptomatic testing of only teachers, of teachers and students, asymptomatic testing of teachers and students at 5% and 100% of schools (biweekly frequency) and asymptomatic contact testing were the scenarios that appeared on the cost-effectiveness frontier most frequently. ICERs for all three countries are shown Rt 0.9 and 1.5 in figures 3 and 4, while Rt 1.2 and 2.0 are shown in online supplemental figures S4 and S5. Total costs of these strategies are shown by country in online supplemental table S2. Symptomatic testing of teachers and students, as well as asymptomatic testing two times per week of teachers and students at 100% of schools, were found to be on the cost-effectiveness frontier for every country and epidemic context. Asymptomatic testing at 100% of schools once-weekly was on the frontier for every context in Georgia but was less effective at high levels of Rt in Brazil and Zambia; it appeared on the frontier for 21 out of 24 contexts in Brazil, and for 20 in Zambia. Biweekly asymptomatic testing at 100% of schools was generally less cost-effective across contexts. Testing of asymptomatic contacts on top of symptomatic testing for teachers and students was always cost-effective in Brazil and Zambia, but for only half of the scenarios in Georgia. Of testing strategies on the frontier, asymptomatic testing of teachers and students at 5% of schools (biweekly) appeared the most infrequently, predominantly in the Georgian context, and was less cost-effective than asymptomatic contact testing, with a greater overall cost. Symptomatic testing of only teachers had the lowest ICERs and overall cost, but prevented the fewest number of infections.
Figure 3.
Incremental cost-effective ratios of COVID-19 self-testing strategies in the school population that appeared on the cost-effectiveness frontier most frequently by country at an Rt of 0.9 and vaccine effectiveness of 30%/70%. D is dominated and WD is weakly dominated, representing scenarios not on the cost-effectiveness frontier. ICER, incremental cost-effectiveness ratio. All ICERs reported in US$.
Figure 4.
Incremental cost-effective ratios of COVID-19 self-testing strategies in the school population that appeared on the cost-effectiveness frontier most frequently by country at an Rt of 1.5 and vaccine effectiveness of 30%/70%. ICER, incremental cost-effectiveness ratio.
Discussion
Ag-RDT self-testing is an important component of the pandemic response and may help to mitigate the continued spread and potential emergence of novel SARS-CoV-2 variants. In this analysis, we have shown the potential benefit of offering school-based testing strategies within the school environment itself and for the broader community to reduce transmission. Symptomatic testing of both teachers and students, without the addition of asymptomatic screening, is the most cost-effective strategy.
Restricting routine asymptomatic testing to a smaller proportion of schools (5%–40%) in addition to symptomatic testing at all schools resulted in marginal gains in additional infections averted compared with symptomatic testing alone—but came at significantly increased costs. Alternatively, asymptomatic testing can be improved by targeting the exposed contacts of known cases, increasing the probability of identifying a positive case. However, adding any level of asymptomatic screening on top of symptomatic testing alone incurs significant cost, limiting feasibility for implementation in many settings.
While asymptomatic testing for teachers and students at 100% of schools consistently appeared on the cost-effectiveness frontier, it is important to consider not only the cost of the ICER, but also the total cost of the programme. The ICER represents the cost to prevent one additional infection by comparison to the next least-costly scenario. The ICERs for 100% asymptomatic testing range from US$278 to US$39 813 across the different epidemic conditions and countries. Symptomatic testing has ICERs ranging from US$2.00 to US$19.03, 10–1000 times less than that of the asymptomatic testing. In between these two ranges were the ICERs for testing of asymptomatic contacts (US$197–US$1749). The total cost of these testing strategies is relative to the size of the school population within each country. Symptomatic testing of teachers and students costs 30–100 times less than 100% asymptomatic testing or 10 times less than asymptomatic contact testing. Although asymptomatic self-testing strategies prevent more infections than symptomatic testing alone, the high ICERs indicate an inefficient use of funding, regardless of available resources. In all settings, but particularly in the context of limited resource settings, symptomatic testing for teachers and students would be the most efficient and feasible school-based testing strategy to reduce COVID-19 infections. The implementation of such a strategy, however, will be ultimately determined by the funders and their interpretation of the available evidence.
Epidemic conditions and country contexts had the greatest influence on the impact of school self-testing strategies. At both the community and school level, Zambia had the greatest reduction in infections, peaking at low levels of transmission (Rt=0.9). Zambia has a younger population distribution, with a higher proportion of the population in school, as well as larger household, school and classroom sizes. Therefore, when infections are prevented in the school-going population, the potential for onward transmission within the community is reduced. These results indicate school-based self-testing could be most effective if implemented prior to the start of an epidemic wave in settings with a large school-going population, highlighting the need for routine surveillance at the community level and preparedness plans with defined triggers for action.
Beyond improving the health and well-being of students, teachers and community members, self-testing strategies reduce the number of symptomatic days experienced by the school-going population, limiting the time lost from the classroom after nearly 2 years of disrupted learning. Symptomatic testing alone can prevent thousands of absences among students and teachers during the height of an epidemic wave. Indeed, schools may therefore be a prime focal point within communities for the distribution of COVID-19 self-tests to improve access and increase testing uptake. In the future, rapid diagnostic technologies with combined detection of COVID-19, influenza and respiratory syncytial virus that have satisfactory performance and are quality assured may be useful in schools for symptomatic screening especially in countries that experience seasonal respiratory illness, at the start of the season to mitigate the effects of respiratory illnesses more broadly.
To the best of our knowledge, this study is the first of its kind to simultaneously conduct a modelling simulation and economic analysis of COVID-19 self-testing strategies in schools across multiple country contexts. Some studies have modelled COVID-19 screening strategies in schools, but without analysing the impact on the broader community, while others have evaluated school-based screening strategies implemented in the real world.8–14 Asgary et al developed an agent-based model to evaluate school-based COVID-19 testing strategies with the ability to change the number of classes and class sizes for the simulation of one school.13 They predicted a greater impact on the number of infections prevented when the number of tests per class and the frequency of testing is increased. Torneri et al developed an individual-based simulation model to assess the impact of symptomatic screening, reactive screening (of an entire class with a symptomatic individual) and repetitive screening of the entire school once per week.14 In their simulation, repetitive screening performed the best, reducing the attack rate more than reactive screening or symptomatic testing, which was the least effective. However, Torneri et al assumed 80% of infections to be asymptomatic, making symptomatic testing quite ineffective in their model. Our model assumes an asymptomatic rate of 10%–50%, depending on age. While our modelling results agree that more frequent asymptomatic testing prevents a greater number of infections, we conclude symptomatic testing to be a more cost-effective testing strategy under a wide range of epidemic conditions.
Our analysis comes with several important limitations. The model was parameterised to the original Omicron variant (BA.1), so may not capture the behaviour of future variants with respect to their transmissibility or vaccine responsiveness. To address this, we varied vaccination coverage and effectiveness, which is representative of overall population immunity, to increase the robustness of the modelling results. True underlying population immunity may vary more widely, waning with time, but then again increasing following an epidemic wave or vaccination campaign. The minimum age of vaccination in the simulated populations is 18 years, but many countries have authorised vaccines for age groups younger than 18 years. The model may therefore overestimate how readily the virus would spread in the school setting. We did not consider other mitigation strategies, such as masking or social distancing, as these measures have been widely discontinued. We did assume 100% compliance with testing and self-isolation (from school) for the school-going population, indicating the maximal potential impact, and may be overestimating the benefit of a programme in the real world. The transmissibility represented in the model hinges largely on density and contact rate assumptions, as well as compliance with self-isolation in the community more broadly, making it context dependent. Additionally, the total cost to offer a COVID-19 self-test in schools was estimated to be US$2.50, but depending on the purchasing cost or distribution modality, this could vary by country. Varying the cost of the test may change the magnitude of the ICER but not the magnitude of difference between scenarios, so our conclusions are unlikely to change with a different self-test kit cost. Finally, the impact of school-based testing is highly dependent on context—represented here by the defined parameters—and the true feasibility of such a programme would be decided by funders and implementers.
In conclusion, symptomatic testing for teachers and students in primary and secondary schools can be a cost-effective mechanism to reduce COVID-19 infections under a variety of conditions both within schools and in the community—in addition to underlying community-based symptomatic testing. School-based self-testing could be more impactful—in terms of infections prevented—in settings with a younger population distribution to reach a larger proportion of the population. Our results show that schools may be one cost-effective distribution point for COVID-19 self-tests that could be combined with other community focal points—such as workplaces, health centres or mass gatherings—to systematically increase testing access and reduce the community spread of SARS-CoV-2. Pre-emptively distributing tests to central points in communities for more convenient access to testing coupled with messaging to promote prompt testing, especially when symptomatic prior to epidemic waves, would be an effective strategy as we look for new ways to prevent the spread of seasonal respiratory infections and remain prepared for future pandemics.
Supplementary Material
Footnotes
Twitter: @ccasejohn, @brookenichols
Contributors: JMC, AH, HJ, CJ, JAS, CAR and BEN designed the study. AH built the original agent-based model used in the study and ran the model simulations. Methods for data analysis were developed by JMC, MH, EK and HP. Data analysis was conducted by JMC and reviewed by CJ, JAS and BEN. AdN developed R code for cost-effectiveness analysis. TO, SG and NAL informed costing. JMC, AH, MH, EK, HP, HJ and BEN had full access to all data. JMC, AH and BEN verified the data. The manuscript was written by JMC. CJ, JAS, BEN, HJ, SK and CAR critically evaluated the study, while all authors aided in revising the manuscript, provided feedback and approved for publication submission. BEN acts as guarantor for this study.
Funding: This study was funded by the German Federal Ministry of Education and Research and the Government of Switzerland.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The data for this study was generated using an agent-based simulation model with underlying assumptions from publicly available data. Model parameters and assumptions are provided in the supplement. Model output, with an accompanying data dictionary, will be provided as an Excel file in the online supplemental appendix.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-Cov-2 variants in the United States: prospective observational study. BMJ 2022;376:e069761. 10.1136/bmj-2021-069761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aleem A, Akbar Samad AB, Slenker AK. Statpearls. In: Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 3. World Health Organization . WHO policy brief: COVID-19 testing. 2022. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-Policy_Brief-Testing-2022.1 [Accessed 1 Dec 2022].
- 4. Duma Z, Chuturgoon AA, Ramsuran V, et al. The challenges of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) testing in low-middle income countries and possible cost-effective measures in resource-limited settings. Global Health 2022;18:5. 10.1186/s12992-022-00796-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. FIND . SARS-Cov-2 test Tracker. 2022. Available: https://www.finddx.org/tools-and-resources/dxconnect/test-directories/covid-19-test-tracker/ [Accessed 22 Sep 2022].
- 6. Batista C, Hotez P, Amor YB, et al. The silent and dangerous inequity around access to COVID-19 testing: a call to action. EClinicalMedicine 2022;43:101230. 10.1016/j.eclinm.2021.101230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Use of SARS-Cov-2 antigen-detection rapid diagnostic tests for COVID-19 self-testing. 2022. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-Ag-RDTs-Self_testing-2022.1 [Accessed 1 Dec 2022].
- 8. Lee RC, Soto DW, Deva S, et al. Evaluation of a COVID-19 rapid antigen testing program in a supervised community distance learning setting for K-8 students. J Sch Health 2022;92:445–51. 10.1111/josh.13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young BC, Eyre DW, Kendrick S, et al. Daily testing for contacts of individuals with SARS-Cov-2 infection and attendance and SARS-Cov-2 transmission in English secondary schools and colleges: an open-label, cluster-randomised trial. Lancet 2021;398:1217–29. 10.1016/S0140-6736(21)01908-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leng T, Hill EM, Holmes A, et al. Quantifying pupil-to-pupil SARS-Cov-2 transmission and the impact of lateral flow testing in English secondary schools. Nat Commun 2022;13:1106. 10.1038/s41467-022-28731-9 Available: 10.1038/s41467-022-28731-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanchard AC, Desforges M, Labbé A-C, et al. Evaluation of real-life use of point-of-care rapid antigen testing for SARS-Cov-2 in schools (EPOCRATES): a cohort study. CMAJ Open 2022;10:E1027–33. 10.9778/cmajo.20210327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rennert L, McMahan C, Kalbaugh CA, et al. Surveillance-based informative testing for detection and containment of SARS-Cov-2 outbreaks on a public University campus: an observational and modelling study. Lancet Child Adolesc Health 2021;5:428–36. 10.1016/S2352-4642(21)00060-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asgary A, Cojocaru MG, Najafabadi MM, et al. Simulating preventative testing of SARS-Cov-2 in schools: policy implications. BMC Public Health 2021;21:125. 10.1186/s12889-020-10153-1 Available: https://doi-org.ezproxy.bu.edu/10.1186/s12889-020-10153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torneri A, Willem L, Colizza V, et al. Controlling SARS-Cov-2 in schools using repetitive testing strategies. Elife 2022;11:e75593. 10.7554/eLife.75593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han AX, Girdwood SJ, Khan S, et al. Strategies for using antigen rapid diagnostic tests to reduce transmission of severe acute respiratory syndrome coronavirus 2 in low- and middle-income countries: a mathematical modelling study applied to Zambia [Accepted]. Clin Infect Dis 2023;76:620–30. 10.1093/cid/ciac814 Available: 10.1093/cid/ciac814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han AX, Hannay E, Carmona S, et al. Estimating the potential need and impact of SARS-Cov-2 test-and-treat programs with oral antivirals in low-and-middle-income countries. medRxiv 2022;2022. 10.1101/2022.10.05.22280727 [DOI] [Google Scholar]
- 17. Han AX, Toporowski A, Sacks JA, et al. SARS-Cov-2 diagnostic testing rates determine the sensitivity of genomic surveillance programs. Nat Genet 2023;55:26–33. 10.1038/s41588-022-01267-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Institute for Health Metrics and Evaluation (IHME) . COVID-19 results briefing: Brazil. Seattle, USA: IHME, University of Washington; 2022. Available: https://www.healthdata.org/covid/updates [Accessed 9 Jan 2023]. [Google Scholar]
- 19. Institute for Health Metrics and Evaluation (IHME) . COVID-19 results briefing: Georgia. Seattle, USA IHME, University of Washington; 2022. Available: https://www.healthdata.org/covid/updates [Accessed 9 Jan 2023]. [Google Scholar]
- 20. Institute for Health Metrics and Evaluation (IHME) . COVID-19 results briefing: Zambia. Seattle, USA: IHME, University of Washington; 2022. Available: https://www.healthdata.org/covid/updates [Accessed 9 Jan 2023]. [Google Scholar]
- 21. Portmann L, de Kraker MEA, Fröhlich G, et al. Hospital outcomes of community-acquired SARS-Cov-2 Omicron variant infection compared with influenza infection in Switzerland. JAMA Netw Open 2023;6:e2255599. 10.1001/jamanetworkopen.2022.55599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hay JA, Kissler SM, Fauver JR, et al. Quantifying the impact of immune history and variant on SARS-Cov-2 viral kinetics and infection rebound: a retrospective cohort study. Elife 2022;11. 10.7554/eLife.81849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brümmer LE, Katzenschlager S, Gaeddert M, et al. Accuracy of novel antigen rapid diagnostics for SARS-Cov-2: a living systematic review and meta-analysis [published correction appears in Plos MED. PLoS Med 2021;18:e1003735. 10.1371/journal.pmed.1003735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FIND . Support package including US$7 million investment accelerates availability of affordable COVID-19 self-tests in Low- and middle-income countries. 2022. Available: https://www.finddx.org/publications-and-statements/support-package-including-us7-million-investment-accelerates-availability-of-affordable-covid-19-self-tests-in-low-and-middle-income-countries/ [Accessed 1 Nov 2022].
- 25. Sande LA, Matsimela K, Mwenge L, et al. Costs of integrating HIV self-testing in public health facilities in Malawi, South Africa, Zambia, and Zimbabwe. BMJ Glob Health 2021;6:1–9. 10.1136/bmjgh-2021-005191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Worldometer.info . World population. 2022. Available: https://www.worldometers.info/world-population/ [Accessed 1 Nov 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078674supp001.xlsx (1.8MB, xlsx)
bmjopen-2023-078674supp002.pdf (1.2MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The data for this study was generated using an agent-based simulation model with underlying assumptions from publicly available data. Model parameters and assumptions are provided in the supplement. Model output, with an accompanying data dictionary, will be provided as an Excel file in the online supplemental appendix.