Abstract
Introduction
Atrial fibrillation is highly prevalent in patients on chronic dialysis. It is unclear whether anticoagulant therapy for stroke prevention is beneficial in these patients. Vitamin K-antagonists (VKA) remain the predominant anticoagulant choice. Importantly, anticoagulation remains inconsistently used and a possible benefit remains untested in randomised clinical trials comparing oral anticoagulation with no treatment in patients on chronic dialysis. The Danish Warfarin-Dialysis (DANWARD) trial aims to investigate the safety and efficacy of VKAs in patients with atrial fibrillation on chronic dialysis. The hypothesis is that VKA treatment compared with no treatment is associated with stroke risk reduction and overall benefit.
Methods and analysis
The DANWARD trial is an investigator-initiated trial at 13 Danish dialysis centres. In an open-label randomised clinical trial study design, a total of 718 patients with atrial fibrillation on chronic dialysis will be randomised in a 1:1 ratio to receive either standard dose VKA targeting an international normalised ratio of 2.0–3.0 or no oral anticoagulation. Principal analyses will compare the risk of a primary efficacy endpoint, stroke or transient ischaemic attack and a primary safety endpoint, major bleeding, in patients allocated to VKA treatment and no treatment, respectively. The first patient was randomised in October 2019. Patients will be followed until 1 year after the inclusion of the last patient.
Ethics and dissemination
The study protocol was approved by the Regional Research Ethics Committee (journal number H-18050839) and the Danish Medicines Agency (case number 2018101877). The trial is conducted in accordance with the Helsinki declaration and standards of Good Clinical Practice. Study results will be disseminated to participating sites, at research conferences and in peer-reviewed journals.
Trial registration numbers
NCT03862859, EUDRA-CT 2018-000484-86 and CTIS ID 2022-502500-75-00.
Keywords: Dialysis, End stage renal failure, Thromboembolism, Clinical Trial, Anticoagulation
Strengths and limitations of this study.
A national, multicentre, investigator-initiated, open-label randomised clinical trial congruent with general clinical practice.
Adequate power to investigate the harm and benefit of warfarin treatment on the risk of bleeding, thromboembolic outcomes and death.
Pragmatic study design permitting broad inclusion of patients on chronic dialysis with both incident and prevalent atrial fibrillation.
Open-label design enabling non-protocolised international normalised ratio-dependent dose adjustments and continuous evaluation of requirements for dialysis-related anticoagulation and antiplatelet treatment.
Trial limited to allocation of warfarin versus no treatment due to non-approval of direct-acting oral anticoagulants in chronic dialysis by the European Medicines Agency.
Introduction
End-stage kidney disease (ESKD) is associated with a substantial risk of cardiovascular disease1 accounting for >50% of deaths in the population.2
Atrial fibrillation (AF) is the most common sustained arrhythmia in the general population with a global prevalence of approximately 0.5%3 and is associated with a fivefold increase in risk of ischaemic stroke.4 The prevalence of AF is correlated with kidney dysfunction5 6 and is found in >20% of patients with ESKD.7–10
The net benefit of oral anticoagulation remains debated in ESKD. In patients on chronic dialysis, the risk of stroke is increased twofold to threefold compared with the general population.7 However, chronic dialysis is also associated with an increased risk of bleeding attributable to impaired platelet function and platelet-endothelial interactions,11 12 a high prevalence of antiplatelet drugs13 and recurring exposure to low-molecular-weight heparin to minimise clotting of dialysis filters during haemodialysis treatment. Furthermore, the benefit is dependent on expected survival, given an annual mortality >20%,14 potential treatment-associated risk of accelerated vascular calcification through inhibition by carboxylation of Matrix Gla protein,15 16 and induction of calciphylaxis.17
The benefit-to-risk ratio of vitamin K-antagonists (VKA) compared with no treatment remains untested in prospective trials in patients with advanced kidney disease, due to the exclusion of patients on chronic dialysis from all six existing randomised controlled trials demonstrating the benefit of VKA for stroke prevention in patients with AF.18–23 Furthermore, data from retrospective studies remains inconclusive,7 13 24–31 and although direct-acting oral anticoagulants (DOAC) are advocated as first-line treatment in patients with preserved kidney function,32 33 the role of DOAC remains debated in ESKD,34 with inconclusive results reported in recent prospective trials comparing DOACs and VKAs.35–37 Consequently, guideline recommendations for anticoagulation in patients on chronic dialysis are ambiguous and based on low-level evidence,32 33 38 and anticoagulation remains inconsistently prescribed with <50% of patients with incident AF initiating treatment.26 28 39
A prospective evaluation of the safety and efficacy of oral anticoagulation with warfarin in these patients is warranted.
Aims and objectives
The main objectives of the Danish Warfarin-Dialysis (DANWARD) trial are to investigate the benefit, tolerability and safety of warfarin compared with no oral anticoagulation in patients with AF on chronic dialysis. The main hypothesis is that anticoagulation based on warfarin dosing targeting an international normalised ratio (INR) of 2.0–3.0 in patients with AF on chronic dialysis reduces the risk of stroke compared with no oral anticoagulation. Although the risk of major bleeding is increased in patients on chronic dialysis, we also hypothesise that warfarin is not associated with an increased risk of major bleeding as defined by the International Society on Thrombosis and Haemostasis compared with no oral anticoagulation.
Methods and analysis
Study design
The DANWARD trial is an investigator-initiated, prospective, open-label, parallel-group, randomised clinical trial. Patients ≥18 years with AF on chronic haemodialysis or peritoneal dialysis will be randomised to either oral anticoagulation with warfarin or no oral anticoagulation. From 1 October 2019 to 31 January 2026, participants will be included from 13 centres across Denmark (online supplemental table S1) by site investigators who will obtain written informed consent. As warfarin requires continued monitoring of INR, the study will be conducted open-label, that is, with no placebo study medication.
bmjopen-2023-081961supp001.pdf (1.4MB, pdf)
An overview of the study design, randomisation of allocated treatment and scheduled visits and examinations is provided in figure 1. Details of inclusion and exclusion criteria are provided in box 1. We used the Standard Protocol Items: Recommendations for Interventional Trials checklist when writing our report.40 Study protocol and patient information material are provided in online supplemental appendix 1 and 2.
Figure 1.
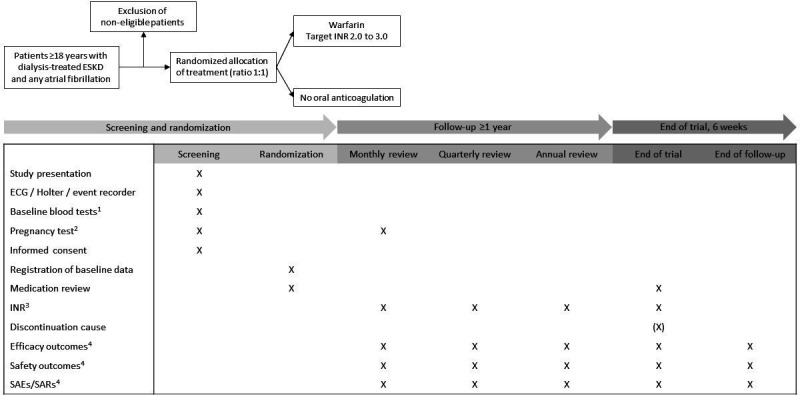
Study design. Eligibility is assessed in accordance with the inclusion and exclusion criteria. 1Plasma-haemoglobin, platelet count, albumin, phosphate, ionised calcium, parathyroid hormone, C reactive protein, urea nitrogen and creatinine. 2Required in all women of childbearing potential at inclusion and monthly throughout the trial. 3Last recorded INR 4SAEs/SARs will be recorded continuously with reporting of SAEs/SARs to the study sponsor within 24 hours of identification for assessment. Efficacy and safety outcomes will be registered every month in an electronic database. ESKD, end-stage kidney disease; INR, international normalised ratio; SAE, serious adverse event; SAR, serious adverse reaction.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Patients ≥18 years on chronic dialysis.
Any non-valvular paroxysmal, persistent or permanent atrial fibrillation or flutter documented by an ECG, episode of ≥30 s on Holter monitor or episode of ≥6 min on event recorder or any other recording device.
Competence to understand the study rationale, including potential risks and benefits associated with treatment, necessary for written informed consent.
Exclusion criteria
CHA2DS2-VASc score * ≤1.
Other indications for oral anticoagulation treatment (pulmonary embolism <6 months, deep vein thrombosis <3 months, mechanical heart valve prosthesis)—irrespective of whether treatment is implemented.
Ongoing dual antiplatelet treatment.
Malignancy (with exception of non-melanoma skin cancer) with recent (<1 year), ongoing or planned curative or palliative chemotherapy, radiation therapy and/or surgical therapy.
Endoscopy with gastrointestinal ulcer <1 month.
Oesophageal varices.
Autoimmune or genetic coagulation disorders.
Congenital alactasia, Lapp lactase deficiency or glucose-galactose malabsorption.
Pending spinal tap.
Cerebrovascular malformations.
Arterial aneurysms.
Ulcers or wounds (Wagner grade >1).
Bacterial endocarditis <3 months.
Active bleeding contraindicating anticoagulation.
Any non-elective and/or non-ambulant surgery <7 days.
Cerebral haemorrhage <4 weeks.
Thrombocytopenia (platelet count <100×109/L) <30 days.
Severe liver insufficiency (spontaneous international normalised ratio >1.5) <30 days.
Known intolerance to warfarin.
Use of hypericum perforatum/St. John’s Wort.
Uncontrolled hypertension (repeated blood pressure >180/110 mm Hg) <30 days.
Uncontrolled hyperthyroidism (thyroid-stimulating hormone <0.1 μIU/mL) <30 days.
Pregnancy or lactation.
Participation in other ongoing intervention trials adjudged to influence study outcomes.
* CHA2DS2-VASc score: congestive heart failure (1 point), hypertension (1 point), age ≥75 years (2 points), diabetes mellitus (1 point), stroke (2 points), vascular disease (1 point), age 65-74 years (1 point), female sex (1 point).
Randomisation
In accordance with a computer-generated allocation, participants are randomised 1:1 to either treatment with warfarin or no treatment via a secure web application. Allocation is stratified by centre using permuted blocks of random sizes, with block size and allocation ratio concealed. Participants not receiving anticoagulation at inclusion are randomised to either initiation of oral anticoagulation or to continued non-treatment. Participants treated with anticoagulation at inclusion are randomised to either continued anticoagulation with warfarin or discontinuation of treatment.
The date of randomisation defines treatment initiation and the beginning of follow-up. All study participants are treated in accordance with the randomisation for at least 12 months. Participants included early in the trial remain under-allocated treatment throughout the study until 1 year after the inclusion of the last participant. In accordance with the pragmatic nature of the trial, no specific recommendation regarding other medication, including dialysis-related anticoagulation and antiplatelet agents, is provided in the protocol. Except from INR measurements, patients in both allocation groups are monitored equally.
Allocation to treatment with warfarin
Warfarin, a coumarin anticoagulant, inhibits vitamin K-reductase, leading to depletion of vitamin K-hydroquinone. This causes a reduction in gamma-carboxylation and subsequent activation of vitamin K-dependent proteins involved in the synthesis of the vitamin K-dependent coagulation factors II, VII, IX and X, and the anticoagulant proteins C and S. Reduced availability of coagulation factors II, VII, IX and X results in reductions in prothrombin and thrombin levels leading to decreased clot formation.
Monitoring of warfarin treatment will be done in accordance with the local standard of care at the individual trial site following standard international guidelines for anticoagulation therapy,32 33 with dose adjustments based on prothrombin time targeting an INR of 2.0–3.0. All study medication will be provided free of charge.
Allocation to no treatment
Participants randomised to no treatment will be monitored in accordance with the predefined monitoring plan (figure 1) at monthly intervals.
Departure from allocated treatment
Patients’ departure from allocated treatment is registered throughout the trial to ensure correct identification of cross-over.
End of trial
The end of trial is defined by either the date of withdrawal of informed consent or the date of trial termination, which is set by the Trial Steering Committee at least 12 months after the inclusion of the last recruited patient (figure 1).
End of follow-up
The end of follow-up for each participant is defined as 6 weeks after the end of the trial (figure 1).
Study outcomes
Primary outcomes
Efficacy endpoint: Any fatal or non-fatal transient ischaemic attack, ischaemic stroke or unspecified stroke (box 2).
Safety endpoint: Major bleeding as defined by the International Society on Thrombosis and Haemostasis, that is, major intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, intramuscular with compartment syndrome or gastrointestinal bleeding in non-surgical patients (box 2).
Box 2. Primary, secondary and tertiary outcomes.
Primary efficacy outcome
Fatal or non-fatal transient ischaemic attack, ischaemic stroke or unspecified stroke.
Primary safety outcome
Fatal or non-fatal major bleeding.*
Secondary outcomes
Fatal or non-fatal ischaemic or unspecified stroke.†
Fatal or non-fatal ischaemic stroke.†
Fatal or non-fatal haemorrhagic stroke.†
Fatal or non-fatal ischaemic or haemorrhagic stroke.†
All-cause mortality.
Combination of any non-fatal stroke and all-cause mortality.
Combination of any non-fatal stroke, any non-fatal major bleeding and all-cause mortality.
Tertiary outcomes
Discontinuation of allocated randomised therapy.
Calciphylaxis/calcific uraemic arteriolopathy.
Fatal or non-fatal acute myocardial infarction.
Hospitalisation due to left-sided heart failure.
Peripheral artery disease.
Thrombosis of arteriovenous fistula.
Osteoporotic fractures including low-energy fractures of the proximal femur, distal radius, humerus, pelvis and vertebrae.
*As defined by the International Society on Thrombosis and Haemostasis.
†For patients who meet a primary outcome.
Secondary and tertiary outcomes
Box 2 displays outcomes.
Adjudication of outcomes
Outcomes will be identified based on clinical registration in an electronic database and on registration of diagnosis codes in the comprehensive national administrative health registers41–43 with subsequent adjudication of outcomes by an event committee.
Statistical analysis
Treatment with warfarin will be compared with no treatment for all outcomes. Principal analyses will be performed based on the intention-to-treat population comparing the cumulative risk of the primary efficacy endpoint in patients allocated to warfarin treatment with no treatment, while accounting for competing risks. Grey’s test will be employed. The null hypothesis is that warfarin is associated with an equivalent risk of stroke or transient ischaemic attack compared with no treatment. Hence, the alternative hypothesis is that warfarin is associated with a reduced risk of stroke or transient ischaemic attack compared with no treatment.
Secondary analyses will evaluate the safety of warfarin compared with no treatment on the risk of major bleeding based on a secondary hypothesis that although the bleeding risk is increased in patients on chronic dialysis, warfarin is not associated with increased risk of major bleeding as defined by the International Society on Thrombosis and Haemostasis compared with no treatment.
Tertiary analyses will include (1) univariate and multiple cause-specific Cox regression models with stratification of treatment effect on predefined variables including, age, sex, comorbidity, trial site, dialysis modality (haemodialysis or peritoneal dialysis), AF class (prevalent or incident), prior anticoagulation treatment (no treatment or ongoing treatment) and concomitant platelet inhibitor treatment; (2) as-treated time-updated cause-specific Cox proportional hazards models permitting time-dependent assessment of treatment including time in therapeutic range, dialysis modality and risk covariates; and (3) on-treatment analyses with censoring of patients deviating from the allocated randomised treatment.
Subgroup analyses are planned for strata of age (<75 years and ≥75 years), sex (male/female), antiplatelet treatment (yes/no), dialysis modality (haemodialysis/peritoneal dialysis), previous stroke (yes/no), previous bleeding (yes/no), and CHA2DS2-VASc score (2, 3-4, ≥5) calculated as congestive heart failure (1 point), hypertension (1 point), age ≥75 years (2 points), diabetes mellitus (1 point), stroke (2 points), vascular disease (1 point), age 65-74 years (1 point), and female sex (1 point).
For all analyses, statistical significance will be determined by a p value≤0.05. Determination of superiority will be based on the primary efficacy endpoint. A finalised statistical analysis plan will be prepared by the trial statistician and approved by the Trial Steering Committee before the conclusion of randomisation and initiation of data analyses.
Sample size calculations
Prior to compiling this protocol, a retrospective study on Danish patients on chronic dialysis with incident AF diagnosed between 2002 and 2012 was made. During the study period, the incidence of AF increased from 4.0 (95% CI 3.8 to 4.2) per 100 person-years to 7.8 (95% CI 6.8 to 9.0) per 100 person-years.44 Standardised 1-year risks of stroke and major bleeding, respectively, were calculated as the average treatment effect using G-computation based on cause-specific Cox regression models.45 This gave an absolute 1-year risk of stroke of 1.7% (95% CI 0.1% to 8.9%) and 6.1% (95% CI 4.2% to 8.0%) in patients treated with and without warfarin, respectively (online supplemental figure S1A). The absolute 1-year risk of major bleeding was 9.2% (95% CI 0.1% to 20.8%) and 8.1% (95% CI 6.0% to 10.0%) in patients with and without warfarin treatment, respectively (online supplemental figure S1B). Based on these results and the assumption of the absence of a period effect, a sample size of n=299 per group is required to achieve a power of 1-β=0.80 for a two-sided t-test with α=0.05. To compensate for ≤20% drop-out, 359 patients will be included in each group. A plot depicting sample size calculation is provided in online supplemental figure S2. Other trials investigating oral anticoagulation for patients with advanced chronic kidney disease and AF using a two-group study design include the RENAL-AF trial (Renal Hemodialysis Patients Allocated Apixaban vs Warfarin in Atrial Fibrillation), the AXADIA-AFNET8 trial (Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation an End-Stage Kidney Disease), the AVKDIAL trial (Oral Anticoagulation in Hemodialysis Patients) and the SACK trial (Stroke Prophylaxis with Apixaban in CKD5 Patients with Atrial Fibrillation). Planned enrolment in these trials was 762, 222, 855 and 1000–1400, respectively.
Monitoring
Data Safety Monitoring Committee
An independent Data Safety and Monitoring Committee has been established with the aim of safeguarding the interests of enrolled patients, assessing the safety and efficacy of the allocated treatment during the trial and monitoring the overall conduct of the trial. The responsibilities of the Data Safety and Monitoring Committee have been described in a separate charter.
Safety management
Serious adverse events (SAEs), serious adverse reactions (SARs) and suspected unexpected serious adverse reactions (SUSARs) will be reported to the sponsor in accordance with the standards of Good Clinical Practice.46 Harm will be adjudged based on the predefined safety endpoints pertaining to fatal or non-fatal major bleeding. Safety monitoring will be accomplished by tracing of SAEs, SARs and SUSARs, safety and efficacy endpoints and mortality. Registration of SAEs, SARs and SUSARs for evaluation of potential late side effects will be continued until 6 weeks following the end of trial irrespective of the cause of discontinuation.
Premature trial termination
The Data and Safety Monitoring Committee will have exclusive unblinded access to all data and may recommend discontinuation of the study to the Trial Steering Committee based on reviews of safety events. Throughout inclusion, interim analyses will be supplied in strict confidence for overview to the Data Safety Monitoring Committee. If results from the interim analyses provide sufficient evidence as to the harm or benefit of the allocated treatments, the Data Safety Monitoring Committee will advise the Trial Steering Committee, thus enabling possible trial modifications or trial discontinuation.
Trial auditing
Auditing of trial conduct will be performed by independent monitors from the regional Good Clinical Practice units.
Patient and public involvement
The design and conduction of this study were performed without public or patient involvement. Conclusions from the study will be communicated as a newsletter in relevant media.
Ethics and dissemination
The trial is to be conducted in accordance with the Helsinki declaration and standards of Good Clinical Practice.47 48 The trial is registered at www.clinicaltrials.gov, EudraCT and CTIS. The study protocol was approved by the Regional Research Ethics Committee on 21 December 2018 (journal number H-18050839), and by the Danish Medicines Agency on 14 February 2019 (case number 2018101877). The sponsor will release protocol amendments to the site investigators. The trial registration data set is provided in online supplemental table S2.
A Trial Steering Committee (online supplemental table S3) has been involved in the study design and monitors the conduction of the study, analysis of study data and publication of study results. The Trial Steering Committee will have full access to all study data on trial completion. The Vancouver recommendations will be used to access authorship eligibility. Data collection and management will be performed in accordance with the General Data Protection Regulations and all study-related material will be stored securely at the study site.
Dissemination of results
Study results will be disseminated to participating sites, at research conferences and in peer-reviewed journals.
Supplementary Material
Footnotes
Contributors: Study conceptualisation and design: A-LK, CNFB, CT-P, DH, ELFB, ELG, GG, JDJ, JBO, LK, MH, MR, MS, NC, PVC, TL. Statistical analysis plan: CT-P, NC, TL. Acquisition, analysis and interpretation of data: ASN, CDP, D-CN, DHK, ELFB, FHM, FTN, IB, INT, JMBB, JDK, JKB, JMR, KL, LB, MCB, ML, NC, RB. Drafting of manuscript: ELFB, NC. Critical revision of manuscript and approval of final version: All authors.
Funding: This work was supported by the Danish Heart Foundation (23015) and the Augustinus Foundation (19-2397).
Competing interests: CDP has received speaker honoraria or consultancy fees from AstraZeneca and Astellas. Support for attending meetings and travel from Boehringer Ingelheim. CDP also has an ongoing collaboration with Vifor Pharma including donation of a research grant unrelated to this study. DH has received speaker and consultancy fees from AstraZeneca, GlaxoSmithKline and UCB Nordic. ELFB has received a donation of a research grant from AstraZeneca unrelated to this study. ELG has received speaker honoraria or consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Novo Nordisk, MSD, Lundbeck Pharma and Organon. He is an investigator in clinical studies sponsored by AstraZeneca, Idorsia or Bayer and has received unrestricted research grants from Boehringer Ingelheim. IB has received speaker honoraria from Amgen and Bayer. JBO has received speaker honoraria or consultancy fees from Bayer, Bristol-Myers Squibb, Organon and Pfizer. LB has received consultancy fees from AstraZeneca, Astellas, Vifor Pharma, Pfizer and Novartis. LK has received speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis and Novo Nordisk. MH has received speaker and consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Vifor and GSK within the last 3 years. ML has received speaker and consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk and GlaxoSmithKline. NC has received speaker honoraria from AstraZeneca and Bristol-Myers Squibb. RB has received speaker honoraria or consultancy fees from AstraZeneca, Bayer and Boehringer Ingelheim. She is an investigator in clinical studies sponsored by Boehringer Ingelheim, AstraZeneca or Bayer and has received unrestricted research grants from Boehringer Ingelheim.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Roberts MA, Polkinghorne KR, McDonald SP, et al. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis 2011;58:64–72. 10.1053/j.ajkd.2011.01.024 [DOI] [PubMed] [Google Scholar]
- 2. Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2018;71:A7. 10.1053/j.ajkd.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 2021;16:217–21. 10.1177/1747493019897870 [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 5. Iguchi Y, Kimura K, Kobayashi K, et al. Relation of atrial fibrillation to glomerular filtration rate. Am J Cardiol 2008;102:1056–9. 10.1016/j.amjcard.2008.06.018 [DOI] [PubMed] [Google Scholar]
- 6. Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis risk in communities (ARIC) study. Circulation 2011;123:2946–53. 10.1161/CIRCULATIONAHA.111.020982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zimmerman D, Sood MM, Rigatto C, et al. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant 2012;27:3816–22. 10.1093/ndt/gfs416 [DOI] [PubMed] [Google Scholar]
- 8. Liao J-N, Chao T-F, Liu C-J, et al. Incidence and risk factors for new-onset atrial fibrillation among patients with end-stage renal disease undergoing renal replacement therapy. Kidney Int 2015;87:1209–15. 10.1038/ki.2014.393 [DOI] [PubMed] [Google Scholar]
- 9. Genovesi S, Pogliani D, Faini A, et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis 2005;46:897–902. 10.1053/j.ajkd.2005.07.044 [DOI] [PubMed] [Google Scholar]
- 10. Goldstein BA, Arce CM, Hlatky MA, et al. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation 2012;126:2293–301. 10.1161/CIRCULATIONAHA.112.099606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sohal AS, Gangji AS, Crowther MA, et al. Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res 2006;118:417–22. 10.1016/j.thromres.2005.03.032 [DOI] [PubMed] [Google Scholar]
- 12. Weigert AL, Schafer AI. Uremic bleeding: pathogenesis and therapy. Am J Med Sci 1998;316:94–104. 10.1097/00000441-199808000-00005 [DOI] [PubMed] [Google Scholar]
- 13. Olesen JB, Lip GYH, Kamper A-L, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–35. 10.1056/NEJMoa1105594 [DOI] [PubMed] [Google Scholar]
- 14. Collins AJ, Foley RN, Herzog C, et al. United States renal data system 2008 annual data report abstract. Am J Kidney Dis 2009;53:S1–374. 10.1053/j.ajkd.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 15. Schurgers LJ, Joosen IA, Laufer EM, et al. Vitamin K-antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS One 2012;7:e43229. 10.1371/journal.pone.0043229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997;386:78–81. 10.1038/386078a0 [DOI] [PubMed] [Google Scholar]
- 17. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015;66:133–46. 10.1053/j.ajkd.2015.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. Lancet 1989;1:175–9. 10.1016/s0140-6736(89)91200-2 [DOI] [PubMed] [Google Scholar]
- 19. Stroke prevention in atrial fibrillation study. Final results. Circulation 1991;84:527–39. 10.1161/01.CIR.84.2.527 [DOI] [PubMed] [Google Scholar]
- 20. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators, Singer DE, Hughes RA, et al. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med 1990;323:1505–11. 10.1056/NEJM199011293232201 [DOI] [PubMed] [Google Scholar]
- 21. Connolly SJ, Laupacis A, Gent M, et al. Canadian atrial fibrillation anticoagulation (CAFA) study. J Am Coll Cardiol 1991;18:349–55. 10.1016/0735-1097(91)90585-w [DOI] [PubMed] [Google Scholar]
- 22. Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. N Engl J Med 1992;327:1406–12. 10.1056/NEJM199211123272002 [DOI] [PubMed] [Google Scholar]
- 23. EAFT (European Atrial Fibrillation Trial) Study Group . Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342:1255–62. 10.1016/0140-6736(93)92358-Z [DOI] [PubMed] [Google Scholar]
- 24. Chan KE, Lazarus JM, Thadhani R, et al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 2009;20:2223–33. 10.1681/ASN.2009030319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen JI, Montez-Rath ME, Lenihan CR, et al. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis 2015;66:677–88. 10.1053/j.ajkd.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014;129:1196–203. 10.1161/CIRCULATIONAHA.113.004777 [DOI] [PubMed] [Google Scholar]
- 27. Genovesi S, Rossi E, Gallieni M, et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 2015;30:491–8. 10.1093/ndt/gfu334 [DOI] [PubMed] [Google Scholar]
- 28. Bonde AN, Lip GYH, Kamper A-L, et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014;64:2471–82. 10.1016/j.jacc.2014.09.051 [DOI] [PubMed] [Google Scholar]
- 29. Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int 2010;77:1098–106. 10.1038/ki.2009.477 [DOI] [PubMed] [Google Scholar]
- 30. Wiesholzer M, Harm F, Tomasec G, et al. Incidence of stroke among chronic Hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol 2001;21:35–9. 10.1159/000046216 [DOI] [PubMed] [Google Scholar]
- 31. Lai HM, Aronow WS, Kalen P, et al. Incidence of thromboembolic stroke and of major bleeding in patients with atrial fibrillation and chronic kidney disease treated with and without warfarin. Int J Nephrol Renovasc Dis 2009;2:33–7. 10.2147/ijnrd.s7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:546–7. 10.1093/eurheartj/ehaa945 [DOI] [PubMed] [Google Scholar]
- 33. January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart R. Circulation 2019;140:e125–51. 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 34. Klil-Drori AJ, Tagalakis V. Direct oral anticoagulants in end-stage renal disease. Semin Thromb Hemost 2018;44:353–63. 10.1055/s-0037-1621715 [DOI] [PubMed] [Google Scholar]
- 35. De Vriese AS, Caluwé R, Van Der Meersch H, et al. Safety and efficacy of vitamin K antagonists versus Rivaroxaban in Hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol 2021;32:1474–83. 10.1681/ASN.2020111566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pokorney SD, Chertow GM, Al-Khalidi HR, et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation 2022;146:1735–45. 10.1161/CIRCULATIONAHA.121.054990 [DOI] [PubMed] [Google Scholar]
- 37. Reinecke H, Engelbertz C, Bauersachs R, et al. A randomized controlled trial comparing apixaban to the vitamin K-antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation 2023;147:296–309. 10.1161/CIRCULATIONAHA.122.062779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wanner C, Herzog CA, Turakhia MP, et al. Chronic kidney disease and arrhythmias: highlights from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 2018;94:231–4. 10.1016/j.kint.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 39. Winkelmayer WC, Liu J, Setoguchi S, et al. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol 2011;6:2662–8. 10.2215/CJN.04550511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39:38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 42. Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish national Registry of patients. BMC Med Res Methodol 2011;11:83. 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rasmussen L, Valentin J, Gesser KM, et al. Validity of the prescriber information in the Danish national prescription registry. Basic Clin Pharmacol Toxicol 2016;119:376–80. 10.1111/bcpt.12610 [DOI] [PubMed] [Google Scholar]
- 44. Carlson N, Gislason G, Torp-Pedersen C. Use of warfarin in end-stage renal disease and atrial fibrillation: risk of stroke and bleeding - a nationwide cohort study. Nephrol Dial Transplant 2018;33:i3. 10.1093/ndt/gfy104.FO006 [DOI] [Google Scholar]
- 45. Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health 2006;60:578–86. 10.1136/jech.2004.029496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. European Commission . Clinical investigations: serious adverse event reporting under directives 90/385/EEC and 93/42/EEC. MEDDEV 2.7 (Revision 3). Guidel Med Devices 2015. [Google Scholar]
- 47. Association WM. World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 48. ICH-GCP . International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. n.d. Available: https://www.ich.org/ [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081961supp001.pdf (1.4MB, pdf)


