Highlights
-
•
Flavor properties of hemp seed oil were determined using e-sensors and GC–MS/O.
-
•
The sweetness of hempseed oil tends to increase with roasting.
-
•
Main volatile compounds and odor active compounds were Maillard reaction products.
-
•
Roasting conditions influenced bitterness and grassy odor in hemp seed oil.
-
•
The results can be used as basic data on hemp seed oil for an edible oil industry.
Keywords: Hemp seed oil, Maillard reaction, Chemosensory properties, Multivariate analysis, Odor active compounds
Abstract
This study analyzed the flavor of six types of hemp seed oil (HSO) extracted with roasted hemp seed (RHS) under various conditions (Raw, 140 °C_9 min, 140 °C_12 min, 160 °C_12 min, 180 °C_6 min). Electronic tongue (E-tongue), electronic nose (E-nose), GC–MS (gas chromatography–mass spectrometry), and GC-O (gas chromatography–olfactometry) were used for HSO flavor analysis. As a result of the E-tongue analysis, the sweetness tends to increase in most samples as roasting. A total of 89 and 77 volatile compounds were detected through E-nose and GC–MS, and the main volatile compounds were identified as Maillard reaction products. A total of 16 odor active compounds were detected in the GC-O analysis, and in the case of 160 ℃_12 min and 180 ℃_6 min, the scent of Roasted hemp seed oil was more dominant than other aroma profiles. The results of this study are basic data on the flavor characteristics of HSO.
Introduction
Hempseed (HS) is a peeled seed of hemp, an annual hemp plant, mainly grown in Central Asia (Jang, Park, & Nam, 2018). It has a savory and soft texture, so it is eaten raw or is widely used as a raw material for food and beverages such as cereal, rice cake, bread, and milk. Hemp seeds have a high fat content of 25–35 % and are also widely used as a resource for oil (Jang et al., 2018). HSO has a higher fat-soluble provitamin A, vitamin E, minerals, phosphorus, and calcium content than other plant oils such as soybean oil and canola oil (Xu et al., 2021). HSO is characterized by a high proportion of polyunsaturated fatty acids, especially linoleic acid and α-linolenic acid accounting for about 80 % of the total fatty acids (Xu et al., 2021, Cerino et al., 2021). In addition, the ω-3 and ω-6 ratios, which are considered optimal for nutrition, are 1:3, and γ-linoleic acid is widely used as a component of cosmetics because it exists at a higher ratio than other vegetable oils (Da Porto, Decorti, & Tubaro, 2012). These HSOs are also used as raw materials for medicines for the treatment of glaucoma and cancer, and are also reported to lower cholesterol levels in human blood and control high blood pressure, making them nutritionally valuable foods (Dunford, 2015).
HSO is distinguished from light green to dark green and is known for its nut taste, accompanied by a slightly bitter aftertaste (Xu et al., 2021, Cerino et al., 2021). However, HSO extracted after heat treatment such as roasting on HS is known to help improve the quality of oil, such as showing better smell and taste, and to show beneficial changes in oil, such as increasing oil extraction yield (Oomah et al., 2002, Durmaz and Gökmen, 2010). Roasting is a major step in improving aroma, color, or texture by changing the chemical composition and physical properties of seeds and nuts, which causes pyrolysis of other materials such as Maillard reactions, Strecker degradation, caramelization, and lipid oxidation, providing savory aroma and baked taste through the formation of some volatile and colored compounds (Durmaz and Gökmen, 2010, Yin et al., 2022). In addition, such roasting improves antioxidant activity, total phenol and flavonoid content, and increases the concentration of major minerals such as potassium and calcium (Ahmed et al., 2020). Therefore, the roasting process is very important for improving sensory properties and is considered essential for the production of oil with enhanced flavor (Zhang, Li, Lu, Sun, & Wang, 2021).
Over the past few years, the food industry has been making efforts to analyze the flavor characteristics of food. Among them, E-tongue, E-nose, and GC–MS have widely used analysis techniques (Jeong et al., 2023). E-tongue is an analysis system that derives relative values for five tastes using electronic sensors, and provides analysis of individual taste compounds and patterns of overall taste compounds (Jeong et al., 2023). The E-nose is a sensor that analyzes volatile compounds and volatile compounds in many foods in a short time to derive results, and E-tongue and E-nose are widely used for convenience (Jeong et al., 2023). GC–MS is commonly used to identify different substances in liquid or volatile samples, and the headspace method using solid-phase microextraction (SPME) is widely used as a method of adsorption and extraction of volatile compounds(Boo, Hong, Lee, Park, & Shin, 2020). In addition, an analysis method that identifies odor active compounds of food using GC-O has recently been widely used to compensate for the shortcomings of GC–MS, which is an effective analysis technology for identifying volatile compounds but cannot determine the characteristics of volatile compounds (Song & Liu, 2018). Through these analysis techniques, many studies have been conducted on roasted seeds (Hou, Zhang, & Wang, 2019) and analyzing the physicochemical properties and volatile compounds of oil extracted from various seeds (Iseppi et al., 2019).
Currently, there is a great deal of research being conducted on the physicochemical and flavor properties of various seed oils. However, there is still a lack of research on the sensory properties of oils extracted from seeds with different roasting conditions. In this study, we analyzed the changes in taste components and volatile compounds of hempseed oil extracted from hempseeds treated with roasting conditions {(raw (0 min), 140℃_9 min, 140℃_12 min, 160℃_6 min, 160℃_12 min, 180℃_6 min)} selected from previous studies to improve the flavor of existing hempseed oil, which is slightly bitter taste, and to confirm its potential as a flavored oil. This data is expected to provide a basis for future studies on the flavor of hemp seed oil.
Materials and methods
Experimental materials
The HS used in this experiment was purchased at an online store(DGFARM, Gyeongiu, Gyeongsangbuk-do, Republic of Korea), and was used after freezing at −18 °C before being used in the experiment.
Roasting and oil extraction of hemp seed
Using the light-wave oven function of the multi-light oven(EON-C200F, SK Magic, Seoul, Republic of Korea (EON-C200F, SK Magic, Seoul, Republic of Korea), 30 g of HS was roasted. Roasting was performed 140 °C_9 min, 140 °C_12 min, 160 °C_6 min, 160 °C_12 min, and 180 °C_6 min, and then the RHS was pulverized to the same size using a grinder. Roasting conditions were set as the most suitable conditions for extracting oil through experiments on chemosensory characteristics such as taste, aroma, and color of RHS through preliminary experiments. Subsequently, a total of 150 g of pulverized RHS was extracted from 750 mL of hexane for about 24 hr, and a total of six types were used for the experiment.
E-tongue analysis for taste compounds
E-tongue systems (ASTREE Ⅱ, Alpha MOS, Toulouse, France) were used to analyze the taste compounds of the extracted HSO. Sensors attached to the E-tongue system are sourness, saltiness, umami, sweetness, and bitterness (SRS-sourness, STS-saltiness, UMS-umami, SWS-sweetness, and BRS-bitterness), consisting of sensors related to five tastes felt by humans and two sensors (GPS-metallic, SPS-spiciness). For the sample used for E-tongue analysis, 10 mL of sample and 90 mL of purified water were stirred at 300 rpm for 30 min at 60 °C for elution of taste compounds. The upper oil part of the stirred sample was removed and used for E-tongue analysis. The prepared sample solution was mounted on a sampler of the E-tongue, and then the sensor was immersed in the sample solution for 2 min to measure the strength of individual taste components through contact. In order to reduce contamination and errors between samples during the analysis process, after each analysis, the washing process was performed using purified water, and six times per sample was repeated. The taste component pattern was confirmed using multivariate analysis (Jeong et al., 2023).
E-nose analysis for volatile compounds
An E-nose system (HERACLES Neo, Alpha MOS, Toulouse, France) was used to analyze the volatile compounds of the extracted HSO, and an MXT-5 column was used as the analysis column. For the electronic nose analysis, 2 mL of extracted HSO was taken and placed in a headspace vial (22.5 × 75 mm, PTEE/silicone septum, aluminum cap) for E-nose analysis, and stirred at 500 rpm at 50 °C for 20 min to saturate volatile compounds inside the vial. Volatile compounds were collected through an automatic sample collector attached to the E-nose, and volatile compounds of 2000 μL of the collected gas were taken using a syringe and injected into the gas chromatography injection port mounted on the E-nose. The analysis conditions were 1 mL/min of hydrogen gas flow rate, acquisition time was 110 sec, trap absorption temperature was 40 ℃, and trap desorption temperature was 250 ℃. The oven temperature was maintained at 40 °C for 5 sec, and then increased to 270 °C at a ratio of 4 °C/s and maintained at 270 °C for 30 sec. Retention index based on carbon number was based on Kovat's index library, and peak components separated by using AcroChemBase (Alpha MOS) of the electronic nose were identified. The electronic nose analysis system was based on 3 repetitions per sample, and the scent component pattern was confirmed using multivariate analysis (Jeong et al., 2023).
GC–MS coupled with GC-O for volatile odor compounds
In order to collect volatile compounds contained in HSO, a headspace analysis method using SPME (Supelco Inc., Bellefonte, PA, USA) coated with 50/30,m, DVB/CAR/PDMS was used. 3 g Sample was placed in a headspace vial and sealed with an aluminum cap, and then the vial was heated for 20 min at 60 ℃ to reach its equilib-rium. After the equilibration, the SPME (solid-phase microextraction) fiber was injected into the vial and the volatile compounds were absorbed by the fiber for 30 min at 60 °C. Volatile compounds in HSOs were analyzed using GC–MS (Agilent 7890A & 5975C, Agilent Technologies, Santa Clara, CA, USA) and HP-5MS column (30 m x 0.25 mm i.d., 0.25 um film thick-ness). For the GC–MS analysis conditions, the oven temperature was at 40 ℃ for 5 min and then accelerated to 200 ℃ at a rate of 5 ℃/hr. The injector temperature was 220 ℃, the flow rate of helium was 1.0 mL/min, and the splitless. The identification of volatile compounds separated by a total ionization chromatogram was conducted by using a mass spectrum library (NIST version 12) and literature. Each volatile compound in the sample was calculated as μg/g using pentadecane as an internal standard. Odor active compounds were measured using GC–MS coupled with GC-O (olfactometry detection port III (ODP-III), Gerstel Co., Linthicum, MD, USA). Identification of odor active compounds in HSOs were performed 20 min (5–25 min) in odor to solvent elution time (5 min) and general detected time of odor active compounds (OACs). The intensity of odor active compound was expressed as four levels ranged from 1 to 4, and a higher number indicates a higher odor level(Boo et al., 2020). Also, the OAV and odor contribution (OC) were calculated to determine the contribution of odor active compounds in HSO. OAV is the concentration of each volatile aroma substance analysed by GC–MS divided by the odor threshold value of the substance, with compounds with an OAV greater than 1 considered to be important odor active compounds. Odor threshold values were those reported in the literature (Culleré et al., 2007, Costa et al., 2016, Hua et al., 2022, Pino and Quijano, 2012, Pino, 2012, Ueno et al., 2006, Xiao et al., 2019, Yin et al., 2023, Zhu and Xiao, 2018, Zhu et al., 2018, Zhu et al., 2018, Zhu and Xiao, 2018). OC expressed the OAV of each volatile fragrance component as a relative ratio (%) to the total OAV.
Statistical analysis
Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were applied for multivariate analysis using XLSTAT software ver. 9.2 (Addinsoft, New York, NY, USA) to identify how six types HSOs were located in the pattern of chemical sensory properties. PCA creates a new orthogonal axis or set of variables from the original variable, known as the principal component (PC). Each PC is defined as a vector known as an eigenvector of the variance–covariance matrix, and the variance that follows the vector is called an eigenvalue. Based on Kaiser rules, the PCA displays the variables and samples mapped through the loading and scoring of dimensional spaces determined by the PC with eigenvalues greater than 1.0 based on Kaiser’s rule (Shin, Craft, Pegg, Phillips, & Eitenmiller, 2010a). HCA was performed to identify relative dissimilarity among samples and reported as a dendrogram. Each dissimilarity in the dendrogram was achieved based on the Euclidean distance between samples using Ward’s algorithm as the agglomerative method. The hierarchical algorithm constructs the nested grouping of patterns and similarity levels at which groupings change (Shin, Pegg, Phillips, & Eitenmiller, 2010b).
Results and discussion
E-tongue analysis results
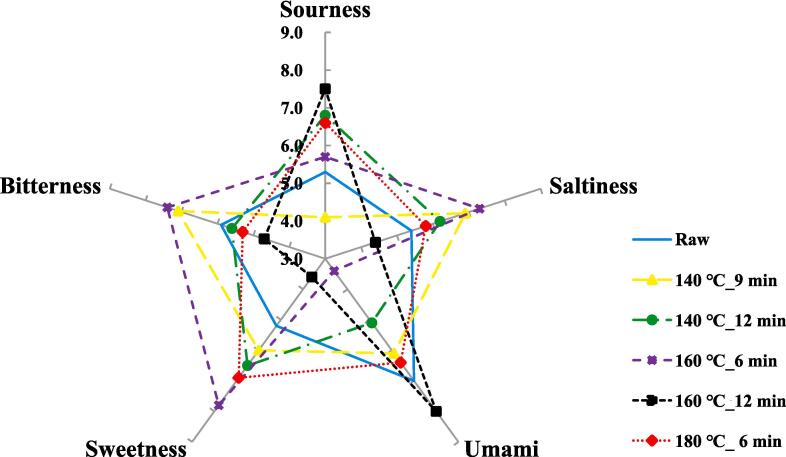
The taste compound results for the six types of HSO are shown in Fig. 1. In the case of raw, UMS had the highest sensor value of 7.0 among the five taste compounds, and SWS was 5.2 which was the lowest sensor value. In the case of 140 ℃_9 min, the BRS showed the highest sensor value at 7.1, and the SRS showed the lowest sensor value at 4.1. In the case of 140 ℃_12 min, SRS showed the highest sensor value at 6.8, and UMS showed the lowest sensor value at 5.1. In the case of 160 ℃_6 min, SWS was the highest sensor value at 7.8, and UMS was the lowest sensor value at 3.4. In the case of 160 ℃_12 min, the UMS was 8.0, the highest sensor value, and the SWS was 3.6, the lowest sensor value. In the case of 180 ℃_6 min, SWS was the highest sensor value at 6.9, and BRS was the lowest sensor value at 5.3. In this study, except for 160 ℃_12 min, most samples tended to increase SWS as the roasting temperature and time increased, and in the case of BRS, which is considered the original taste of HSO, tended to be relatively lower than raw as the roasting time increased.
Fig. 1.
Taste intensities of raw and roasted hemp seed oil by electronic tongue. Raw: hemp seed oil extracted with raw hemp seed, 140 ℃_9 min: hemp seed oil extracted with roasted hemp se ed at 140 ℃_9min, 140 ℃_12 min: hemp seed oil extracted with roasted hemp seed at 140 ℃_12 min, 1 60 ℃_6 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_6 min, 160 ℃_12 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_12 min, 180 ℃_6 min: hemp seed oil extracted with roast ed hemp seed at 180 ℃_6 min.
HSO is known to have a similar taste to nuts and a slight bit of bitterness (Cerino et al., 2021) and in the case of roasted HSO, it is known to have a better taste and aroma (Durmaz & Gökmen, 2010). According to Navicha et al., while roasting reduces the unpleasant taste of beans, it has shown sweet flavor characteristics by increasing sweetness and baked taste (Navicha, Hua, Masamba, Kong, & Zhang, 2018). In addition, it is known that even when hazelnuts belonging to nuts are used, such as HS, they go through a roasting process to increase sweetness (Bagheri, 2020). In this study, all samples except 160 ℃_12 min showed a tendency to increase SWS compared to raw as roasting temperature and time increased. In addition, a little bit of BRS, one of the characteristics of HSO's taste compounds, tended to increase more than raw when roasting for a short time, but the BRS tended to decrease as the roasting time increased. According to Zhang et al. (2021), the variety of oil seeds, roasting temperature, and roasting time have a significant impact on oil products (Zhang et al., 2021). Therefore, the change in the taste compound of roasted HSO in this study is also judged to be the difference according to the roasting temperature and time, and the seed characteristics of the oil.
E-tongue multivariate analysis results
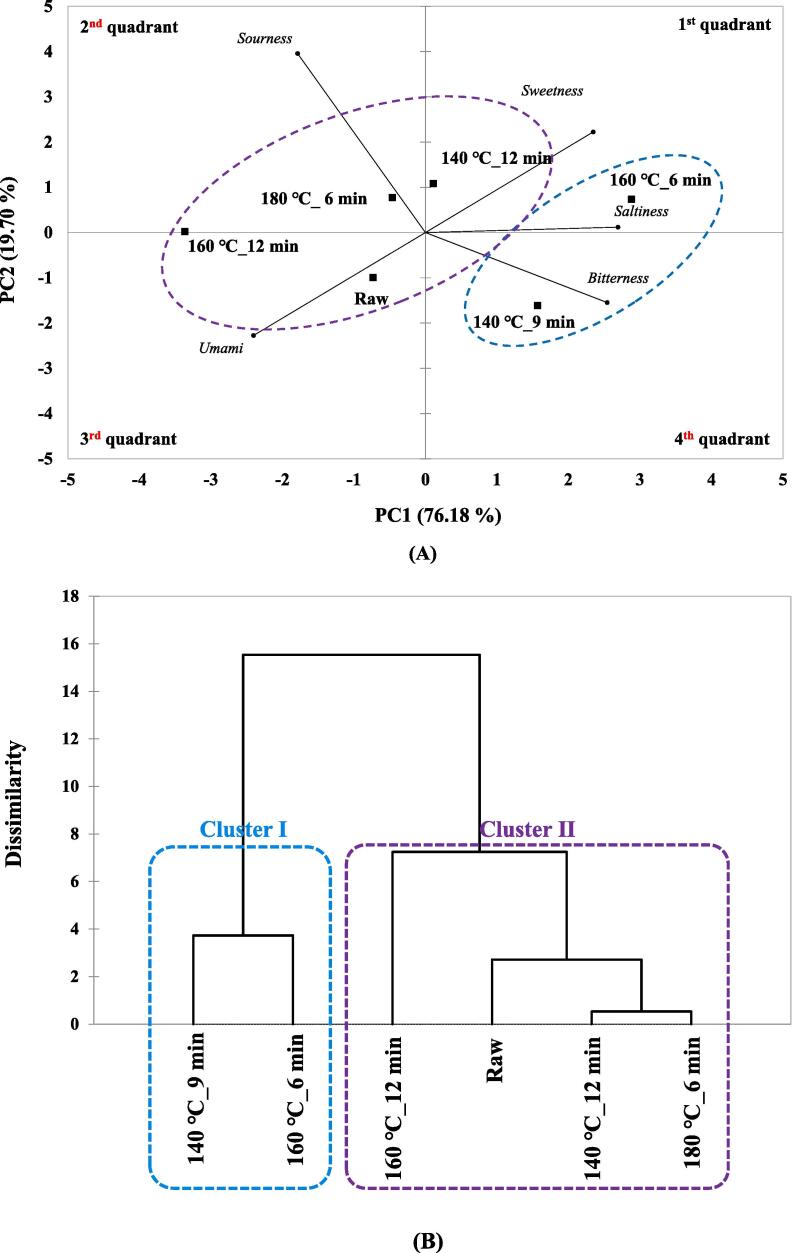
The results of the taste components of the six types of HSO analyzed using the E-tongue were shown through PCA and HCA, and are shown in Fig. 2 (A) and (B), respectively. Fig. 2(A) shows the results of confirming the pattern of HSO's taste compounds using PCA, PC1 showed 76.18 % variation, and PC2 showed 19.70 % variation, showing a total of 95.88 % variation. In the case of raw, due to the influence of UMS, it was located in a negative direction on PC1 and PC2. In the case of 140 ℃_9 min, it was located in four quadrants, positive to PC1 and negative to PC2, affected by the BRS. In the case of 140 ℃_12 min and 180 ℃_6 min, it was located in a positive direction to PC2 due to the SRS, and 160 ℃_6 min was located in a positive direction to PC1 and PC2 due to the SWS, STS, and BRS. In addition, 140 ℃_12 min showed relatively low separation in PC1 compared to other samples. In the case of 160 ℃_12 min, it was located in a negative direction in PC1 due to the influence of UMS but showed a low degree of separation in PC2. In comprehensive, 140℃_9 min and 160℃_6 min, which were affected by bitterness due to roasting, were positive for PC1, while the remaining conditions were affected by other taste components besides bitterness and were in the negative direction for PC1.
Fig. 2.
(A) Principal component analysis of raw and roasted hemp seed oil by electronic tongue; (B) Hierarchical cluster analysis of raw and roasted hemp seed oil by electronic tongue. Raw: hemp seed oil extracted with raw hemp seed, 140 ℃_9 min: hemp seed oil extracted with roasted hemp s eed at 140 ℃_9 min, 140 ℃_12 min: hemp seed oil extracted with roasted hemp seed at 140 ℃_12 m in, 160 ℃_6 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_6 min, 160 ℃_12 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_12 min, 180 ℃_6 min: hemp seed oil extracted with ro asted hemp seed at 180 ℃_6 min.
The HCA results for the taste compounds of the six types of HSO are shown in Fig. 2(B). As a result of HCA, a total of two clusters were identified. 140 ℃_9 min and 160 ℃_6 min are clusterⅠ, cluster Ⅱ is raw, 140 ℃_12 min, 160 ℃_12 min, and 180 ℃_6 min. Multivariate analysis showed a high degree of separation based on bitterness. Currently, many studies are being conducted to confirm the separation pattern between samples through multivariate analysis, such as the effect of roasting time on the aroma change of sunflower seeds (Guo, Na, & Ge, 2019) and study on the sensory characteristics of coffee according to roasting (Jeong et al., 2023).
E-nose analysis results
Table 1 shows the results of the volatile compounds analysis of six types of HSO using the E-nose. There were 6 terpenes, 9 furans, 2 pyrroles, 8 pyrazines, 8 acids and esters, 13 alcohols, 3 aldehydes, 14 heterocyclic compounds, 16 hydrocarbons, 5 ketones, and 5 sulfur-containing compounds. Therefore, a total of 89 volatile compounds were detected. In the case of raw, a total of 30 volatile compounds were detected, and the peak area of terpenes was higher than that of other samples. In the case of 140 ℃_9 min, 27 volatile compounds were detected, and many terpenes were detected among samples such as α-pinene, sabinene, α-phellandrene, and linalool. In addition, aldehydes were not detected, and hydrocarbons showed the highest peak area. In the case of 140 ℃_12 min, a total of 28 volatile compounds were detected, and α-pinene and β-pinene were detected among terpenes. In addition, furans showed the highest peak area, and hydrocarbons also showed a high volatile compounds peak area. In the case of 160 ℃_6 min, a total of 27 volatile compounds were detected, and α-pinene and 1,3,8-p-menthatriene were detected among terpenes. Among the peak areas of the detected volatile compounds, furans were detected the highest, and the peak area was higher in acid than other samples. In the case of 160 ℃_12 min, a total of 30 volatile compounds were detected, the peak area of furans was detected high among the detected volatile compounds, and the sulfur-containing compounds were detected as the lowest peak area among the samples. In the case of 180 ℃_6 min, a total of 28 volatile compounds were detected, and the peak area of pyrazines was the highest among the samples. In addition, peak areas of alcohols, aldehydes, heterocyclic compounds, and sulfur-containing compounds were detected as high, and abundant volatile compounds were detected compared to other samples.
Table 1.
Volatile compounds of hemp seed oil under six roasting conditions using electronic nose.
| (peak area x 103) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Volatile compounds | RT1(RI2) | Sensory description | Raw | 140 ℃_9 min | 140 ℃_12 min | 160 ℃_6 min | 160 ℃_12 min | 180 ℃_6 min |
| Terpenes (6) | ||||||||
| α-Pinene | 55.75(9 2 4) | Terpenic | 0.55 ± 0.32 | 0.13 ± 0.07 | 1.61 ± 1.32 | 1.66 ± 1.64 | 1.19 ± 0.32 | 0.71 ± 0.28 |
| Sabinene | 59.13(9 6 3) | Citrus | ND3 | 0.50 ± 0.42 | ND | ND | ND | ND |
| β-Pinene | 59.17(9 6 4) | Fresh | 4.56 ± 1.99 | ND | 1.18 ± 1.25 | ND | ND | ND |
| α-Phellandrene | 63.03(1,011) | Citrus | ND | 2.47 ± 0.50 | ND | ND | ND | ND |
| Linalool | 69.85(1,111) | Oily | ND | 0.14 ± 0.05 | ND | ND | ND | ND |
| 1,3,8-p-Menthatriene | 69.99(1,113) | Terpenic | ND | ND | ND | 0.19 ± 0.07 | ND | ND |
| Furans (9) | ||||||||
| Furan | 17.50(4 9 0) | – | ND | ND | ND | ND | 8.22 ± 5.45 | ND |
| 2-Methylfuran | 25.48(6 3 4) | Burnt | ND | ND | 430.69 ± 11.79 | 366.31 ± 7.69 | 382.26 ± 1.91 | 369.36 ± 12.02 |
| 2-Ethylfuran | 31.73(7 0 2) | Burnt | ND | 42.18 ± 22.19 | 56.29 ± 39.58 | ND | ND | ND |
| 2-Furanmethanol | 47.55(8 4 1) | Sweet | ND | ND | 1.76 ± 1.60 | ND | 0.37 ± 0.20 | ND |
| Furfural | 45.75(8 2 3) | Sweet | ND | ND | ND | ND | 1.18 ± 0.30 | 1.53 ± 0.33 |
| 2-Methyl-3-furanthiol | 49.57(8 6 0) | Nutty | ND | 0.56 ± 0.74 | ND | 1.43 ± 1.70 | ND | 3.40 ± 1.42 |
| 2-Butylfuran | 50.97(8 7 3) | Sweet | ND | ND | 1.05 ± 1.05 | ND | ND | ND |
| 2-Furanone | 53.72(9 0 0) | Butter | ND | ND | ND | ND | 1.90 ± 0.62 | 4.41 ± 1.51 |
| 5-Methylfurfural | 59.11(9 6 3) | Almond | ND | ND | ND | ND | ND | 3.59 ± 1.56 |
| Pyrroles (2) | ||||||||
| Pyrrole | 39.57(7 6 8) | Nutty | ND | ND | ND | ND | ND | 1.82 ± 1.64 |
| 2-Acetly-1-pyrroline | 56.64(9 3 4) | Sweet | ND | ND | 3.04 ± 3.54 | ND | 1.09 ± 0.88 | ND |
| Pyrazines(8) | ||||||||
| Pyrazine | 37.88(7 5 4) | Nutty | ND | ND | ND | 13.83 ± 9.21 | 5.46 ± 3.06 | 23.58 ± 7.86 |
| 2,5-Dimethylpyrazine | 53.07(8 9 4) | Roasted | ND | 1.57 ± 1.54 | ND | 4.11 ± 3.32 | ND | 7.83 ± 2.73 |
| Ethylpyrazine | 55.31(9 1 8) | Nutty | ND | 0.71 ± 0.64 | ND | 2.15 ± 1.62 | ND | ND |
| 2,3-Dimethylpyrazine | 55.72(9 2 3) | Sweet | ND | ND | 0.27 ± 0.23 | ND | 1.24 ± 0.39 | ND |
| 2-Ethyl-3-methylpyrazine | 61.89(9 9 6) | Nutty | ND | ND | ND | 3.55 ± 1.67 | ND | 5.78 ± 1.09 |
| Acethylpyrazine | 62.52(1,004) | Nutty | ND | ND | ND | ND | 4.09 ± 0.60 | ND |
| 2,3-Diethylpyrazine | 67.13(1,070) | Nutty | ND | ND | ND | ND | ND | 0.53 ± 0.15 |
| Ethenyl-dimethylpyrazine | 69.95(1,112) | – | ND | ND | 0.15 ± 0.05 | ND | ND | ND |
| Acids and esters (8) | ||||||||
| Ethyl propanoate | 32.05(7 0 5) | Fruity | ND | ND | ND | 68.92 ± 37.90 | 33.43 ± 13.52 | ND |
| Methyl 2-methylbutanoate | 39.50(7 6 8) | Oil | 1.81 ± 0.56 | ND | ND | ND | ND | ND |
| Methyl crotonate | 40.58(7 7 7) | Fruity | ND | ND | ND | ND | 12.30 ± 9.86 | ND |
| Ethyl 2-methylbutyrate | 47.61(8 4 1) | Sweet | ND | ND | ND | 1.92 ± 1.92 | ND | 2.54 ± 0.95 |
| 3-Methylbutanoic acid | 48.27(8 4 8) | Sweet | ND | ND | 1.15 ± 1.04 | ND | 0.25 ± 0.07 | ND |
| 2-Methylbutanoic acid | 50.93(8 7 3) | Sweet | ND | 0.77 ± 0.53 | ND | ND | ND | ND |
| Pentanoic acid | 53.69(9 0 0) | Sweet | ND | 2.30 ± 1.62 | ND | ND | ND | ND |
| Propyl 2-butenoate | 57.87(9 4 9) | – | ND | ND | ND | 0.27 ± 0.29 | ND | ND |
| Alcohols (13) | ||||||||
| Ethanol | 14.53(4 2 0) | Sweet | 35.56 ± 10.18 | ND | ND | ND | ND | ND |
| 3-Pentanol | 32.16(7 0 6) | Nutty | ND | ND | ND | ND | ND | 109.35 ± 34.81 |
| Pentanol | 37.91(7 5 4) | Sweet | ND | 7.95 ± 5.63 | ND | ND | ND | ND |
| 3-Hexanol | 42.95(7 9 7) | – | 49.94 ± 6.28 | 23.72 ± 6.22 | 21.15 ± 10.43 | 25.20 ± 10.31 | 16.66 ± 2.84 | ND |
| 3-Hexen-1-ol | 45.68(8 2 3) | Fresh | 1.13 ± 0.60 | ND | ND | ND | ND | ND |
| 4-Methylpentanol | 47.49(8 4 0) | Nutty | ND | 0.91 ± 0.97 | ND | ND | ND | ND |
| 2-Hexen-1-ol | 49.64(8 6 1) | Caramelized | ND | ND | ND | ND | 0.43 ± 0.19 | ND |
| 1-Hexanol | 50.87(8 7 2) | Sweet | ND | ND | ND | ND | ND | 2.76 ± 1.23 |
| 5-Methyl-1-hexanol | 55.30(9 1 8) | – | 3.02 ± 1.48 | ND | ND | ND | ND | ND |
| 4-Cresol | 67.16(1,071) | Phenolic | 0.27 ± 0.15 | ND | ND | ND | ND | ND |
| 1-Octanol | 67.25(1,072) | Burnt | ND | 0.13 ± 0.02 | ND | ND | ND | ND |
| Fenchol | 69.84(1,111) | Sweet | ND | ND | ND | ND | 0.42 ± 0.05 | ND |
| 2-Undecanol | 80.68(1,300) | Fresh | 0.23 ± 0.09 | ND | ND | ND | ND | ND |
| Aldehydes (3) | ||||||||
| Propenal | 15.17(4 3 8) | Almond | ND | ND | 24.37 ± 20.97 | ND | 10.76 ± 6.23 | ND |
| Propanal | 17.52(4 9 0) | Nutty | 37.62 ± 10.11 | ND | ND | 23.43 ± 16.91 | ND | 41.89 ± 17.60 |
| 2,4-Octadienal | 69.98(1,113) | Oil | 0.24 ± 0.11 | ND | ND | ND | ND | ND |
| Heterocyclic compounds (14) | ||||||||
| Trimethylamine | 13.83(4 0 8) | Almond | 47.49 ± 13.79 | 8.95 ± 5.09 | 10.95 ± 8.86 | 11.67 ± 9.14 | 8.42 ± 4.67 | 39.90 ± 16.97 |
| Methyl formate | 13.86(4 0 9) | Fruity | 18.71 ± 5.67 | ND | ND | ND | 4.75 ± 2.33 | 21.90 ± 9.40 |
| Methyl isobutyrate | 28.61(6 6 8) | Sweet | ND | ND | ND | 78.40 ± 2.48 | ND | 81.32 ± 3.23 |
| Ethyl isobutyrate | 37.92(7 5 4) | Sweet | 21.62 ± 5.24 | ND | 11.32 ± 9.25 | ND | ND | ND |
| Ethyl isovalerate | 45.97(8 2 5) | Sweet | ND | ND | 0.81 ± 0.75 | 1.21 ± 1.18 | ND | ND |
| Isoamyl acetate | 50.91(8 7 3) | Sweet | 2.29 ± 0.95 | ND | ND | 1.34 ± 1.43 | ND | ND |
| Dimethyl fumarate | 63.13(1,013) | – | ND | ND | 3.29 ± 0.90 | ND | ND | ND |
| Indole | 79.93(1,286) | Burnt | ND | 0.21 ± 0.15 | 0.19 ± 0.06 | 0.15 ± 0.01 | ND | 0.12 ± 0.01 |
| Ethyl nonanoate | 80.92(1,304) | Fruity | ND | ND | ND | ND | 0.30 ± 0.01 | ND |
| Methyl cinnamate | 85.54(1,398) | Fruity | 0.18 ± 0.08 | ND | ND | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.03 |
| Myristicin | 91.16(1,521) | Balsamic | ND | ND | ND | 0.21 ± 0.01 | 0.23 ± 0.01 | ND |
| Propyl cinnamate | 94.55(1,598) | – | ND | 0.15 ± 0.04 | ND | ND | ND | ND |
| Octyl caprylate | 101.87(1,763) | Oil | ND | ND | 0.17 ± 0.03 | ND | ND | ND |
| Ambroxide | 104.64(1,826) | Sweet | 1.26 ± 0.22 | ND | 1.16 ± 0.09 | 1.16 ± 0.05 | 1.21 ± 0.02 | 1.03 ± 0.13 |
| Hydrocarbons (16) | ||||||||
| 2-Methylbutane | 16.39(4 6 5) | – | ND | ND | 0.86 ± 0.54 | ND | ND | ND |
| Cyclopentane | 20.79(5 6 3) | Sweet | ND | 34.89 ± 2.44 | ND | 29.00 ± 1.56 | ND | ND |
| 2-Methylpentane | 20.80(5 6 3) | – | 43.16 ± 1.03 | ND | 32.01 ± 2.44 | ND | 27.51 ± 0.50 | 30.60 ± 1.72 |
| Methylcyclopentane | 25.45(6 3 3) | – | 330.91 ± 3.88 | 448.52 ± 21.17 | ND | ND | ND | ND |
| Cyclohexane | 28.52(6 6 7) | – | ND | 91.65 ± 4.61 | ND | ND | ND | ND |
| 3-Methylhexane | 28.53(6 6 8) | – | 61.55 ± 2.35 | ND | 91.63 ± 5.99 | ND | 80.32 ± 0.49 | ND |
| Fluorobenzene | 30.32(6 8 7) | – | ND | 5.95 ± 1.36 | ND | ND | ND | ND |
| Trichloethylene | 32.09(7 0 5) | Sweet | 98.39 ± 21.64 | ND | ND | ND | ND | ND |
| Methylcyclohexane | 35.59(7 3 5) | Sweet | ND | 35.06 ± 24.27 | ND | ND | ND | ND |
| Ethylcyclopentane | 35.62(7 3 5) | – | 94.57 ± 21.37 | ND | ND | 60.79 ± 39.53 | 23.34 ± 14.29 | 102.41 ± 35.02 |
| 1-Octene | 40.63(7 7 7) | – | ND | ND | 25.78 ± 22.77 | ND | ND | ND |
| 4-Octene | 42.45(7 9 3) | – | ND | ND | ND | ND | ND | 34.76 ± 8.99 |
| Chlorobenzene | 49.88(8 6 3) | Almond | 2.69 ± 1.39 | ND | ND | ND | ND | ND |
| 4-Ethyl-octane | 59.12(9 6 3) | – | 2.74 ± 1.18 | ND | ND | ND | ND | ND |
| Tetradecane | 85.59(1,399) | Sweet | ND | 0.17 ± 0.02 | ND | ND | ND | ND |
| 4-Methylheptadecane | 101.63(1,758) | – | ND | 0.10 ± 0.04 | ND | ND | ND | ND |
| Ketones (5) | ||||||||
| 1-Hydroxy-2-propanone | 26.97(6 5 0) | Sweet | 13.70 ± 2.31 | ND | ND | ND | ND | 14.93 ± 3.22 |
| 3-Pentanone | 30.36(6 8 8) | Acetone | 8.62 ± 2.45 | ND | 6.53 ± 2.06 | 6.84 ± 2.51 | 4.74 ± 0.75 | 9.96 ± 3.49 |
| 2-Cyclopenten-1-one | 47.54(8 4 0) | – | 3.30 ± 1.29 | ND | ND | ND | ND | ND |
| 3-Methylcyclopentanone | 48.25(8 4 7) | Roasted | ND | 0.67 ± 0.65 | ND | ND | ND | ND |
| Acetophenone | 67.15(1,071) | Sweet | ND | ND | ND | 0.22 ± 0.13 | ND | ND |
| Sulfur-containing compounds (5) | ||||||||
| Butanethiol | 33.31(7 1 6) | Sulfurous | 123.40 ± 30.23 | 39.34 ± 29.99 | 58.15 ± 53.83 | 76.62 ± 52.39 | 26.00 ± 18.67 | 132.90 ± 48.45 |
| Dimethyl disulfide | 35.66(7 3 5) | Sulfurous | ND | ND | 48.59 ± 41.21 | ND | ND | ND |
| 2-Methylthiophene | 39.79(7 7 0) | Sulfurous | 53.01 ± 11.64 | 17.67 ± 14.03 | 1.16 ± 1.13 | 34.56 ± 21.36 | 1.06 ± 0.92 | 57.69 ± 18.30 |
| Methional | 53.71(9 0 0) | Creamy | 5.76 ± 2.67 | ND | 3.63 ± 2.89 | ND | ND | ND |
| 2,4-Dimethyl-5-ethylthiazole | 66.94(1,068) | Nutty | ND | ND | ND | ND | 0.37 ± 0.06 | ND |
Data represent the mean ± SD in triplicate.
RT: retention time.
RI: retention index.
ND: not detected.
The main volatile compound of HS is terpenes, and the main volatile compound of HSO also includes terpenes such as α-pinene, β-pinene, myrcene, terpinolene, trans-caryophyllene, and α-humulene. They mainly exhibit fresh, herb, and lemon-like aroma, and are also known as dried grass aroma (Shen et al., 2020, Li et al., 2023). In addition, various terpenes such as α-pinene, β-pinene, myrcene, terpinolene, α-humulene, and caryophyllene oxide were also detected as major volatile compounds extracted from hemp inflorescences (Cannabis sativa L) in addition to HS (Da Porto et al., 2014, Iseppi et al., 2019). In this study, α-pinene was detected in all samples, and in the case of 140 ℃_12 min, terpenes, which are major volatile compounds of HSO such as α-pinene, sabinene, α-phellandrene, and linalool, were detected the most. In addition, in the case of roasted HSO, a lot of pyrazines, pyrroles, and furans that represent sweet and roasted aromas were detected, the volatile compounds are products of the Maillard reaction produced by the pyrolysis of d-glucose and polysaccharides, which are volatile compounds that undergo significant changes during roasting and are known as important compounds in processed foods and food flavors (Caporaso et al., 2014, Diez-Simon et al., 2020) In this study, the peak area of volatile compounds, which represent the original aroma of HSO, known as the aroma of grass and herbs, was reduced through the roasting process, and volatile compounds showing sweet and savory aroma were detected in all samples except raw. These results show that the roasting temperature and roasting time give a variety of volatile compounds of HSO.
E-nose multivariate analysis results
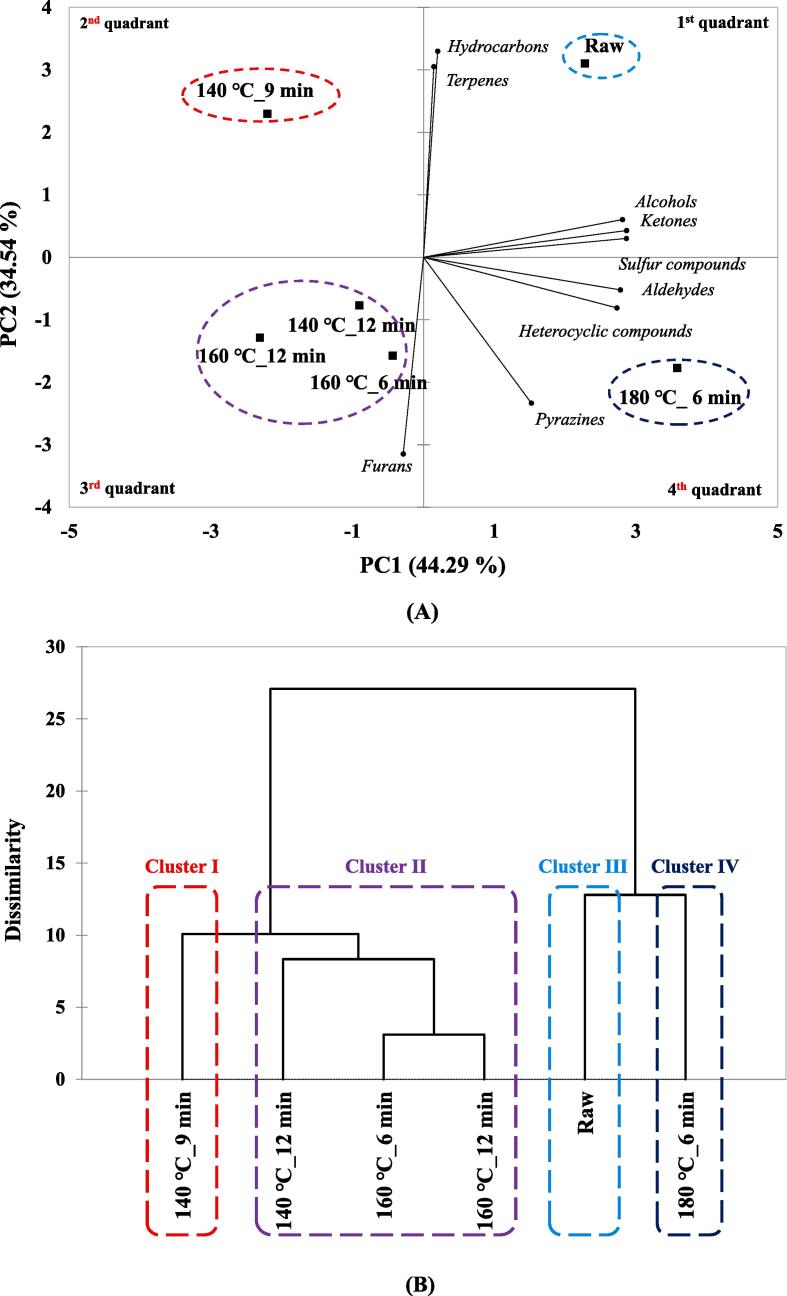
The results of volatile compounds for six types of HSO analyzed using an E-nose were shown through PCA and HCA, respectively, and are shown in Fig. 3(A) and (B). Fig. 3(A) shows the results of confirming the pattern of HSO volatile compounds using PCA, PC1 showed 44.29 % variation, and PC2 showed 34.54 % variation, showing a total of 78.83 % variation. In the case of raw, it was located in the first quadrant, which is a positive direction for PC1 and PC2, under the influence of terpenes and hydrocarbons. In the case of 140 ℃_12 min, 160 ℃_6 min, and 160 ℃_12 min, it was located in a negative direction for PC1 and PC2, and in the case of 140 ℃_9 min, it was located in a negative direction for PC1 and positive direction for PC2. In the case of 180 ℃_6 min, it was located in the fourth quadrant, the direction positive to PC1 and negative to PC2, affected by most of the volatile compounds detected except hydrocarbons, terpenes, and furans.
Fig. 3.
(A) Principal component analysis of raw and roasted hemp seed oil by electronic nose; (B) Hierarchical cluster analysis of raw and roasted hemp seed oil by electronic nose. Raw: hemp seed oil extracted with raw hemp seed, 140 ℃_9 min: hemp seed oil extracted with roasted hemp se ed at 140 ℃_9 min, 140 ℃_12 min: hemp seed oil extracted with roasted hemp seed at 140 ℃_12 min, 1 60 ℃_6 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_6 min, 160 ℃_12 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_12 min, 180 ℃_6 min: hemp seed oil extracted with roast ed hemp seed at 180 ℃_6 min.
The HCA results for the volatile compounds of the six types of HSO are shown in Fig. 3(B). As a result of HCA, a total of four clusters were identified. 140 ℃_9 min included cluster Ⅰ, 140 ℃_12 min, 160 ℃_6 min, and 160 ℃_12 min included cluster Ⅱ, cluster Ⅲ included raw, and cluster Ⅳ included 180 ℃_6 min. As a result of the multivariate analysis of this study, Maillard reaction product produced during the roasting process was detected 180 ℃_6 min, indicating a high degree of separation from the other samples. Many studies are still being conducted to confirm the separation pattern between samples through this multivariate analysis (Caporaso et al., 2014, Boo et al., 2020).
GC–MS analysis results
Table 2 shows the results of volatile compounds analysis for six types of HSO analyzed using GC–MS. There were 9 terpenes, 8 furans, 6 pyrroles, 5 pyridines, 8 pyrazines, 2 esters, 5 alcohols, 6 aldehydes, 7 heterocyclic compounds, 16 hydrocarbons, and 5 ketones. Therefore, a total of 77 volatile compounds were detected. In the case of raw, a total of 12 volatile compounds were detected, and β-myrcene, β-cymene, limonene, and β-caryophyllene were detected among the terpenes. Except for 140 ℃_9 min, the content of terpenes was measured to be higher than that of other samples. In the case of 140 ℃_9 min, a total of 12 volatile compounds were detected, and the terpenes content was the highest among the samples. Among the terpenes detected were δ-3-carene, trans-sabinene hydrate, β-phellandrene, α-fenchone, and β-caryophyllene. In the case of 140 ℃_12 min, a total of 14 volatile compounds were detected, and limonene and β-caryophyllene were detected as terpenes. In the case of 160 ℃_6, a total of 17 volatile compounds were detected, and trans-β-ocimene, β-cymene, limonene, and β-caryophyllene were detected as terpenes. In addition, the content of furans was measured higher than that of other samples. In the case of 160 ℃_12, a total of 38 volatile compounds were detected, and the contents of pyrroles, pyridines, and pyrazines were measured higher than those of other samples. In the case of 180 ℃_6, a total of 19 volatile compounds were detected. Compared to other samples, the content of the terpene was the lowest, and many volatile compounds of the pyrazines were detected.
Table 2.
Volatile compounds in hemp seed oil under six roasting conditions using chromatography-mass spectrometry (μg/kg).
| Volatile compounds | RT1 | RI2 | I.D.3 | Raw | 140 ℃_9 min | 140 ℃_12 min | 160 ℃_6 min | 160 ℃_12 min | 180 ℃_6 min |
|---|---|---|---|---|---|---|---|---|---|
| (min) | |||||||||
| Terpenes (9) | |||||||||
| β-Myrcene | 14.253 | 995 | MS/RI | 188.17 ± 0.30 | ND4 | ND | ND | ND | ND |
| δ-3-Carene | 14.579 | 1,005 | MS/RI | ND | 467.54 ± 0.01 | ND | ND | ND | ND |
| trans-β-Ocimene | 14.95 | 1,018 | MS | ND | ND | ND | 18.26 ± 0.01 | ND | ND |
| p-Cymene | 15.345 | 1,031 | MS/RI | 28.96 ± 13.00 | ND | ND | 8.19 ± 2.53 | ND | ND |
| trans-Sabinene hydrate | 15.528 | 1,037 | MS | ND | 48.45 ± 0.01 | ND | ND | ND | ND |
| β-Phellandrene | 15.532 | 1,037 | MS | ND | 37.36 ± 0.01 | ND | ND | ND | ND |
| Limonene | 15.469 | 1,035 | MS/RI | ND | ND | 37.23 ± 2.77 | 48.88 ± 21.22 | 48.58 ± 0.01 | 32.26 ± 21.13 |
| α-Fenchone | 17.411 | 1,095 | MS | ND | 22.96 ± 5.94 | ND | ND | ND | ND |
| β-Caryophyllene | 26.523 | 1,428 | MS | 15.48 ± 2.23 | 9.74 ± 1.90 | 9.89 ± 1.09 | 12.16 ± 0.26 | 17.23 ± 0.01 | 10.85 ± 0.19 |
| Furans (8) | |||||||||
| Tetrahydro-3-methylfuran | 4.434 | <800 | MS | ND | ND | ND | 189.31 ± 0.01 | ND | ND |
| 3-Furaldehyde | 9.109 | 846 | MS | ND | ND | ND | ND | 9.52 ± 0.01 | ND |
| 2,5-Dimethylfuran | 9.156 | 847 | MS | ND | ND | ND | ND | 21.02 ± 0.01 | ND |
| Thiofuran | 11.066 | 899 | MS | ND | ND | ND | ND | 16.58 ± 0.01 | ND |
| 3-Acetyldibenzofuran | 13.137 | 964 | MS | 15.13 ± 0.01 | ND | ND | ND | ND | ND |
| 5-Methyl-2-furfural | 13.43 | 973 | MS | ND | ND | ND | ND | 22.47 ± 0.01 | ND |
| 2-pentyl- Furan | 14.321 | 997 | MS | ND | ND | ND | 44.49 ± 0.01 | ND | ND |
| 2-Methyltetrahydrofuran | 16.299 | 1,062 | MS | ND | ND | 14.77 ± 0.01 | ND | ND | ND |
| Pyrroles(6) | |||||||||
| 1-Butylpyrrole | 12.883 | 957 | MS | ND | ND | ND | ND | 46.68 ± 34.00 | ND |
| Amylpyrrole | 16.289 | 1,061 | MS | ND | ND | ND | ND | 131.81 ± 2.73 | 26.87 ± 0.01 |
| 1-Pyrrole | 16.317 | 1,062 | MS | ND | ND | ND | ND | ND | 30.51 ± 2.67 |
| Isoamylpyrrole | 16.412 | 1,065 | MS | ND | ND | ND | ND | 144.47 ± 0.01 | ND |
| 1-Formylpyrrolidine | 17.096 | 1,086 | MS | ND | ND | ND | ND | 209.45 ± 0.01 | ND |
| Acetylpyrrolidine | 19.624 | 1,171 | MS | ND | ND | ND | ND | 12.35 ± 0.01 | ND |
| Pyridines (5) | |||||||||
| 2-Ethylpyridine | 11.452 | 912 | MS | ND | ND | ND | ND | 14.56 ± 0.01 | ND |
| 2-Methyl-3-pyridinamine | 11.701 | 920 | MS | ND | ND | 31.61 ± 1.74 | ND | ND | ND |
| 5-Methyl-2-pyridinamine | 11.829 | 924 | MS | ND | ND | ND | ND | 4.06 ± 0.01 | ND |
| 4,5-Dimethylpyrimidine | 11.866 | 926 | MS | ND | ND | ND | ND | 5.45 ± 0.01 | 5.58 ± 0.01 |
| 2-Amino-6-ethyl-3-methylpyridine | 17.213 | 1,089 | MS | ND | ND | ND | ND | 26.56 ± 0.01 | ND |
| Pyrazines (8) | |||||||||
| 2,5-Dimethylpyrazine | 11.654 | 919 | MS | ND | ND | 24.76 ± 0.01 | ND | 402.54 ± 75.67 | 171.58 ± 16.48 |
| 2-Isobutyl-3-methylpyrazine | 11.848 | 925 | MS | ND | ND | ND | ND | 2.71 ± 0.01 | 19.57 ± 0.01 |
| Trimethylpyrazine | 14.64 | 1,007 | MS | ND | ND | 46.60 ± 0.01 | ND | ND | 156.68 ± 40.56 |
| 2-Ethyl-3,5-dimethylpyrazine | 17.045 | 1,084 | MS | ND | ND | 27.52 ± 0.01 | 8.80 ± 0.47 | 194.27 ± 103.52 | 93.71 ± 30.11 |
| 3-Ethyl-2,5-dimethylpyrazine | 17.065 | 1,085 | MS | ND | ND | ND | ND | ND | 14.27 ± 0.01 |
| 2,6-Diethylpyrazine | 17.072 | 1,085 | MS | ND | ND | 23.75 ± 0.01 | ND | ND | ND |
| 2-Ethyl-3,6-dimethylpyrazine | 17.209 | 1,089 | MS | ND | ND | ND | ND | 69.63 ± 0.01 | ND |
| 2,5-Diethyl-3-methylpyrazine | 19.398 | 1,164 | MS | ND | ND | ND | ND | 13.39 ± 0.01 | ND |
| Esters (2) | |||||||||
| Linalyl acetate | 14.305 | 996 | MS | ND | ND | ND | 35.88 ± 0.01 | ND | ND |
| Oxalic acid, 6-ethyloct-3-yl isobutyl ester | 16.406 | 1,065 | MS | ND | ND | ND | 13.38 ± 0.01 | ND | ND |
| Alcohols (5) | |||||||||
| 2-Methyl-2-propyl-1,3-propanediol | 8.075 | 813 | MS | ND | ND | ND | 6.96 ± 0.01 | ND | ND |
| 1-Dodecanol | 13.245 | 967 | MS | ND | 18.37 ± 0.01 | ND | ND | ND | ND |
| 1-Octen-3-ol | 13.915 | 986 | MS | ND | ND | ND | 36.03 ± 0.01 | 367.66 ± 0.01 | ND |
| 9-Hexadecyn-1-ol | 15.654 | 1,041 | MS | ND | ND | 21.18 ± 0.01 | ND | ND | ND |
| 5-Phenol | 17.093 | 1,086 | MS | ND | ND | ND | ND | 129.29 ± 0.01 | ND |
| Aldehydes (6) | |||||||||
| 2-Propenal | 10.377 | 882 | MS | ND | 37.15 ± 0.01 | ND | ND | ND | ND |
| 2-Heptenal | 13.185 | 966 | MS | ND | 11.18 ± 0.01 | 25.23 ± 0.01 | 27.80 ± 0.01 | 185.00 ± 0.01 | ND |
| Heptenal | 13.194 | 966 | MS | ND | ND | 19.64 ± 0.01 | 23.21 ± 0.01 | 82.10 ± 0.01 | ND |
| Benzaldehyde | 13.299 | 969 | MS | ND | ND | ND | ND | 55.58 ± 22.61 | 9.45 ± 0.01 |
| 2,4-Heptadienal | 14.923 | 1,017 | MS/RI | ND | ND | ND | ND | 109.46 ± 72.72 | ND |
| Nonanal | 17.811 | 1,108 | MS/RI | ND | ND | ND | ND | 42.71 ± 33.33 | ND |
| Heterocyclic compounds (7) | |||||||||
| Isobutyl carbonate | 7.867 | 805 | MS | ND | ND | ND | ND | 19.45 ± 0.01 | ND |
| Ethyl-1,3-dithioisoindoline | 8.794 | 836 | MS | ND | ND | ND | ND | 86.45 ± 0.01 | ND |
| 1,5-Dimethyl-1-imidazole | 9.165 | 847 | MS | ND | ND | ND | ND | 10.24 ± 0.01 | ND |
| 2,4-Dimethylimidazole | 9.206 | 849 | MS | ND | ND | ND | ND | 28.01 ± 0.01 | ND |
| Methoxy-phenyl-oxime | 11.296 | 907 | MS | 19.79 ± 0.01 | ND | ND | ND | ND | ND |
| 1,4-Benzenediamine | 11.843 | 925 | MS | ND | ND | ND | ND | ND | 12.67 ± 0.01 |
| Formylpiperidine | 18.859 | 1,145 | MS | ND | ND | ND | ND | 42.14 ± 0.01 | ND |
| Hydrocarbons (16) | |||||||||
| 3-Methylpentane | 4.308 | <800 | MS | ND | ND | ND | 415.29 ± 0.01 | ND | 240.64 ± 0.01 |
| Methyl-cyclopentane | 4.334 | <800 | MS | 471.27 ± 353.87 | 75.38 ± 0.01 | 119.09 ± 0.01 | ND | ND | 390.63 ± 174.01 |
| 2-Methylhexane | 4.411 | <800 | MS | ND | 84.73 ± 0.01 | ND | ND | ND | ND |
| Hexahydrobenzene | 4.416 | <800 | MS | 1020.59 ± 0.01 | ND | ND | ND | ND | 376.84 ± 0.01 |
| 2-Methyl-1-pentene | 4.459 | <800 | MS | ND | ND | ND | ND | ND | 31.61 ± 0.01 |
| 3,4-Dimethyloctane | 4.726 | <800 | MS | ND | ND | ND | 36.66 ± 0.01 | ND | ND |
| Methylbenzene | 7.017 | <800 | MS | 26.84 ± 32.48 | ND | ND | ND | ND | ND |
| 1,2-Decane | 7.83 | <800 | MS | ND | ND | ND | ND | 54.82 ± 0.01 | ND |
| 1,3-Dimethylbenzene | 10.224 | 878 | MS | 48.01 ± 0.01 | ND | ND | ND | ND | ND |
| 2-Methyl-3-decene | 10.337 | 881 | MS | ND | ND | ND | 36.07 ± 0.01 | ND | ND |
| 1-Methyl-2-cyclopropane | 10.343 | 881 | MS | ND | 31.24 ± 0.01 | ND | ND | ND | ND |
| 1,1-Difluorodecane | 16.415 | 1,065 | MS | ND | ND | 16.06 ± 0.40 | ND | ND | ND |
| 2,4-Dimethylhexane | 16.551 | 1,069 | MS | ND | ND | ND | ND | 29.95 ± 0.01 | ND |
| Decane | 17.694 | 1,104 | MS | 7.97 ± 0.01 | ND | ND | 7.56 ± 0.01 | ND | 5.33 ± 0.01 |
| Tridecane | 17.74 | 1,106 | MS | ND | ND | ND | ND | 14.44 ± 0.01 | ND |
| Dodecane | 20.52 | 1,200 | MS/RI | 15.69 ± 0.01 | ND | ND | ND | ND | ND |
| Ketones(5) | |||||||||
| 2-Methylcyclobutanone | 4.46 | <800 | MS | ND | ND | ND | ND | ND | 206.57 ± 0.01 |
| 3-Methyl-2-pentanone | 7.721 | 800 | MS | ND | ND | ND | ND | 7.61 ± 0.01 | ND |
| Bicyclooctan-2-one | 12.875 | 957 | MS | ND | ND | ND | ND | 26.56 ± 0.01 | ND |
| Menth-1-en-3-one | 13.129 | 964 | MS | 17.66 ± 0.01 | ND | ND | ND | ND | ND |
| 2-Octanone | 14.311 | 997 | MS | ND | 96.27 ± 22.69 | 134.56 ± 14.31 | ND | 209.18 ± 189.51 | 140.89 ± 0.01 |
Data represent the mean ± SD in duplicate.
RT: retention time.
RI: retention index.
I.D.: indentification.
ND: not detected.
The terpenes detected in this study are main volatile compounds such as β-myrcene, δ-3-carene, trans-ocimene, β-cymene, trans-sabinenehydrate, β-cellandrene, limonene, α-fenchone, and β-caryophyllene, which are considered main volatile compounds of HSO (Shen et al., 2020). Hemp, where various terpenes are considered to be the main volatile compound, is known to contain very high β-myrcene and limonene (Cerino et al., 2021). In this study, β-myrcene was detected only in raw, and limonene was detected in all samples except raw and 140 ℃_9 min. These volatile compounds are commonly found in essential oils of food and have been widely used in cosmetics and food (Bonamin et al., 2014; Erasto and Viljoen et al., 2008). In addition, β-caryophyllene detected in all samples except 140 ℃_12 min is one of the terpenes found in hemp and is known as a common component of essential oils in numerous edible plants (Cerino et al., 2021, Kuwahata et al., 2012). As a result of the GC–MS analysis, various Maillard reaction products such as furans, pyrroles, pyridines, and pyrazines were detected after roasting, and volatile compounds indicating roasted aromas such as 2,5-dimethylpyrazine and 2-ethyl-3,5-dimethylpyrazine were also detected (Guo et al., 2019). In particular, 160 ℃_12 min and 180 ℃_6 min detected more types of Maillard reaction products than other samples. Therefore, in this study, various types of terpenes known as HSO aroma was detected, and odor active compounds were analyzed through GC-O to comfirm how volatile compounds produced through roasting change the perception of HSO aroma known as grass.
Analysis of odor active compounds by GC–MS and GC-O
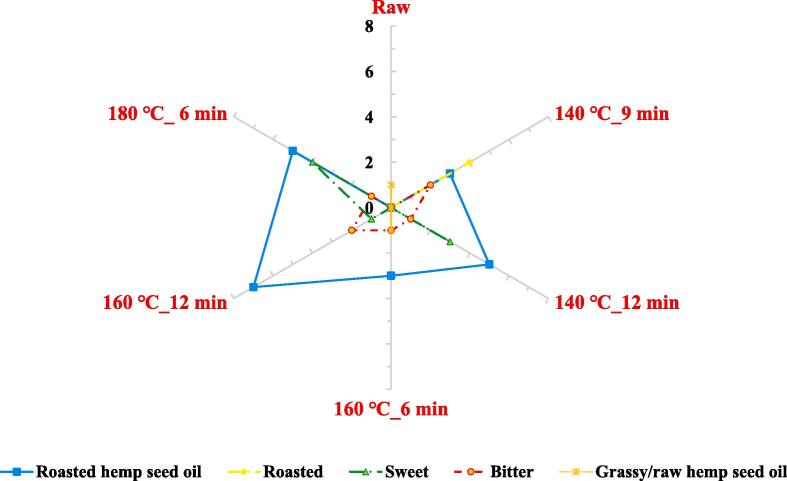
GC–MS and GC-O were used to analyze the odor active compounds of the six types of HSO. The OAVs, OCs and perceived aroma profile of the odor active compounds analyzed by GC–MS/O are shown in Table 3 and Fig. 4. The OAV of each odor active compound analyzed in this study is the ratio of the compound concentration divided by a threshold suggested in the literature, where a value greater than or equal to 1 is considered to be significantly and strongly involved in the flavor of the food. In this study, 5 terpenes, 7 pyrazines, 2 alcohols, aldehyde, and ketone were detected, and a total of 16 odor active compounds were identified. The OAV of the identified odor active compounds in all samples was measured to be greater than 1. In addition, the recognition of the detected odor active compounds was divided into five groups (Roasted hemp seed oil, Roasted, Sweet, Bitter, Grassy/raw hemp seed oil). The odor active compounds identified as the scent of Roasted hemp seed oil included 2,5-dimethylpyrazine, (E)-2-heptenal, trans-sabinene hydrate, limonene, 2-ethyl-3,5-dimethylpyrazine, and 2,5-diethyl-3-methylpyrazine.
Table 3.
Odor active value and odor contribution (%) of odor active compounds in hemp seed oil under six roasting conditions using gas chromatography-mass spectrometry and gas chromatography–olfactometry.
| Volatile compounds | Odor note | RT1 | RI2 | threshold | Raw | 140℃_9 min | 140℃_12 min | 160℃_6 min | 160℃_12 min | 180℃_6 min | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (min) | (μg/kg) | OAV3 | OC4 | OAV | OC | OAV | OC | OAV | OC | OAV | OC | OAV | OC | |||
| 2,5-Dimethylpyrazine | Rosted hemp seed oil | 11.654 | 919 | 8 | ND5 | ND | ND | ND | 3.10 | 0.37 | ND | ND | 50.32 | 0.94 | 21.45 | 0.71 |
| (E)-2-Heptenal | 13.185 | 966 | 4.6 | ND | ND | 2.43 | 0.52 | 5.48 | 0.65 | 6.04 | 2.26 | 40.22 | 0.75 | ND | ND | |
| trans-Sabinenehydrate | 15.528 | 1,037 | 9.59 | ND | ND | 5.05 | 1.08 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Limonene | 15.469 | 1,035 | 10 | ND | ND | ND | ND | 3.27 | 0.39 | 4.29 | 1.60 | 4.26 | 0.08 | 2.83 | 0.09 | |
| 2-Ethyl-3,5-dimethylpyrazine | 17.045 | 1,084 | 0.04 | ND | ND | ND | ND | 688.00 | 81.94 | 220.00 | 82.19 | 4856.75 | 90.85 | 2342.75 | 78.00 | |
| 2,5-Diethyl-3-methylpyrazine | 19.398 | 1,164 | 8.6 | ND | ND | ND | ND | ND | ND | ND | ND | 1.56 | 0.03 | ND | ND | |
| δ-3-Carene | Roasted | 14.579 | 1,005 | 5 | ND | ND | 93.51 | 19.99 | ND | ND | ND | ND | ND | ND | ND | ND |
| β-Phellandrene | 15.532 | 1,037 | 36 | ND | ND | 1.04 | 0.22 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 1-Dodecanol | 13.245 | 967 | 0.066 | ND | ND | 278.33 | 59.49 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Isobutyl-3-methylpyrazine | Sweet | 11.848 | 925 | 0.13 | ND | ND | ND | ND | ND | ND | ND | ND | 20.85 | 0.39 | 150.54 | 5.01 |
| Trimethylpyrazine | 14.64 | 1,007 | 0.35 | ND | ND | ND | ND | 133.14 | 15.86 | ND | ND | ND | ND | 447.66 | 14.90 | |
| 3-Ethyl-2,5-dimethylpyrazine | 17.065 | 1,085 | 0.4 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 35.68 | 1.19 | |
| 2,6-Diethylpyrazine | 17.072 | 1,085 | 6 | ND | ND | ND | ND | 3.96 | 0.47 | ND | ND | ND | ND | ND | ND | |
| 1-Octen-3-ol | Bitter | 13.915 | 986 | 1 | ND | ND | ND | ND | ND | ND | 36.03 | 13.46 | 367.66 | 6.88 | ND | ND |
| 2-Octanone | 14.311 | 997 | 50 | ND | ND | 1.93 | 0.50 | 2.69 | 0.32 | ND | ND | 4.18 | 0.08 | 2.82 | 0.09 | |
| p-Cymene | Grassy/ raw hemp seed oil | 15.345 | 1,031 | 6.2 | 4.67 | 100.00 | ND | ND | ND | ND | 1.32 | 0.49 | ND | ND | ND | ND |
RT: retention time.
RI: retention index.
OAV: odor active value.
OC: odor contribution.
ND: not detected.
Fig. 4.
Aroma profile of the odor active compounds analyzed by gas chromatography–mass spectrometry and gas chromatography–olfactometry. Raw: hemp seed oil extracted with raw hemp seed, 140 ℃_9 min: hemp seed oil extracted with roas ted hemp seed at 140 ℃ _9 min, 140 ℃_12 min: hemp seed oil extracted with roasted hemp seed at 14 0 ℃_12 min, 160 ℃_6 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_6 min, 160 ℃_1 2 min: hemp seed oil extracted with roasted hemp seed at 160 ℃_12 min, 180 ℃_6 min: hemp seed oil ext racted with roasted hemp seed at 180 ℃_6 min.
2-Ethyl-3,5-dimethylpyrazine was identified as the odor active compound with the highest OAV of 220.00–4,856.75 in all samples except raw and 140 °C_9 min. OC was also measured to be high at 78.00–90.85 %, suggesting that it is the most important odor active compound in those conditions. The odor active compounds identified for the Roasted included δ-3-carene, β-phellandrene, and 1-dodecanol, with 1-dodecanol showing the highest OAV of 278.33 at 140 °C_9 min, and a high OC of 59.49 %, indicating a high contribution. Among the odor active compounds identified as Sweet, trimethylpyrazine showed a high OAV of 133.14–447.66 at 140 ℃_12 min and 180 ℃_6 min, with an OC of 15.86–14.90 %, which was found to be above 1. The p-cymene, which was identified as the scent of Grassy/raw hemp seed oil, was only detected in raw and 160 ℃_6 min. In particular, the OAV and OC in the raw were found to be 4.67 and 100 %, respectively, the odor active compound was identified as the odor active compound responsible for the overall aroma of the raw.
The pyrazines detected in this study, including 2,5-dimethylpyrazine, 2,5-diethyl-3-methylpyrazine, and 2-ethyl-3,5-dimethylpyrazine, are known to be the aromas detected in roasted sunflower seeds, pumpkin seeds, and almonds(Mansouri et al., 2023). In particular, 2-ethyl-3,5-dimethylpyrazine exhibited values above the threshold and is believed to be the aroma of roasted hemp seeds(Mansouri et al., 2023), also exhibited the highest odor activity in this study and is believed to be the major odor active compound. The p-cymene, which was found to be responsible for the overall scent of the raw, is known to be one of the volatile compounds of the important terpenes found in Cannabis(Srivastava & Singh, 2019). GC–MS/O analysis results, the raw, only the scent of Grassy/raw hemp seed oil was perceived, and the perception of the scent of Roasted, Sweet, and Roasted hemp seed oil increased as the roasting progressed. In particular, the perceived intensity of Roasted hemp seed oil was stronger at 160 ℃_12 min and 180 ℃_6 min.
Conclusion
In this study, the sensory characteristics of six types of HSOs extracted using the E-tongue, E-nose, GC–MS, and GC-O were evaluated. As a result of E-tongue analysis, as the roasting temperature and the time increased, most of the samples tended to increase in sweetness compared to raw. As a result of the E-nose analysis, various types of Maillard reaction products such as pyridine, pyrazines, and furans were detected and terpenes, which represent the original grassy scent decreased during the roasting process. GC–MS and GC-O analysis showed an increase in the content of Maillard reaction products and total of 16 odor active compounds detected. In addition, GC-O analysis showed that the scent of Roasted hemp seed oil was superior to other aroma profiles for 160 ℃_12 min and 180 ℃_6 min. This study will provide data on changes in flavor characteristics of hemp seed oil extracted from roasted hemp seed under various conditions.
CRediT authorship contribution statement
Hyangyeon Jeong: Writing – original draft, Methodology, Conceptualization. Sojeong Yoon: Writing – original draft, Formal analysis, Conceptualization. Seong Min Jo: Conceptualization, Formal analysis, Writing – original draft. Seong Jun Hong: Writing – original draft, Formal analysis, Conceptualization. Younglan Ban: Methodology, Formal analysis. Hyeonjin Park: Methodology, Formal analysis. Moon Yeon Youn: . Eui-Cheol Shin: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2022R1I1A3066192).
Data availability
Data will be made available on request.
References
- Ahmed I.A.M., Al Juhaimi F.Y., Osman M.A., Al Maiman S.A., Hassan A.B., Alqah H.A., Babiker E.E., Ghafoor K. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals, and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT. 2020;131 doi: 10.1016/j.lwt.2020.109825. [DOI] [Google Scholar]
- Bagheri H. Application of infrared heating for roasting nuts. Journal of Food Quality. 2020;1–10 doi: 10.1155/2020/8813047. [DOI] [Google Scholar]
- Bonamin F., Moraes T.M., Dos Santos R.C., Kushima H., Faria F.M., Silva M.A., Junior I.V., Nogueira L., Bauab T.M., Souza Brito A.R.M., da Rocha L.R.M., Hiruma-Lima C.A. The effect of a minor constituent of essential oil from Citrus aurantium: The role of β-myrcene in preventing peptic ulcer disease. Chemico-biological interactions. 2014;212:11–19. doi: 10.1016/j.cbi.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Boo C.G., Hong S.J., Lee Y., Park S.S., Shin E.C. Quality characteristics of wintering radishes produced in Jeju island using E-Nose, E-Tongue, and GC-MSD Approach. Journal of the Korean Society of Food Science and Nutrition. 2020;49(12):1407–1415. doi: 10.3746/jkfn.2020.49.12.1407. [DOI] [Google Scholar]
- Caporaso N., Genovese A., Canela M.D., Civitella A., Sacchi R. Neapolitan Coffee Brew Chemical Analysis in Comparison to Espresso, Moka, and American Brews. Food Research International. 2014;61:152–160. doi: 10.1016/j.foodres.2014.01.020. [DOI] [Google Scholar]
- Cerino P., Buonerba C., Cannazza G., D'Auria J., Ottoni E., Fulgione A., Di Stasio A., Pierri B., Gallo A. A review of hemp as food and nutritional supplement. Cannabis and cannabinoid research. 2021;6(1):19–27. doi: 10.1089/can.2020.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P., Velasco C.V., Loureiro J.M., Rodrigues A.E. Effect of cosmetic matrices on the release and odour profiles of the supercritical CO 2 extract of Origanum majorana L. International Journal of Cosmetic Science. 2016;38(4):364–374. doi: 10.1111/ics.12297. [DOI] [PubMed] [Google Scholar]
- Culleré L., Cacho J., Ferreira V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. Journal of Agricultural and Food chemistry. 2007;55(3):876–881. doi: 10.1021/jf062432k. [DOI] [PubMed] [Google Scholar]
- Da Porto C., Decorti D., Natolino A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Industrial Crops and Products. 2014;58:99–103. doi: 10.1016/j.indcrop.2014.03.042. [DOI] [Google Scholar]
- Da Porto C., Decorti D., Tubaro F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Industrial Crops and Products. 2012;36(1):401–404. doi: 10.1016/j.indcrop.2011.09.015. [DOI] [Google Scholar]
- Diez-Simon C., Ammerlaan B., van den Berg M., van Duynhoven J., Jacobs D., Mumm R., Hall R.D. Comparison of volatile trapping techniques for the comprehensive analysis of food flavourings by Gas Chromatography-Mass Spectrometry. Journal of Chromatography A. 2020;1624 doi: 10.1016/j.chroma.2020.461191. [DOI] [PubMed] [Google Scholar]
- Dunford N.T. Hemp and flaxseed oil: properties and applications for use in food. In Specialty oils and fats in food and nutrition. 2015;39–63 doi: 10.1016/B978-1-78242-376-8.00002-8. [DOI] [Google Scholar]
- Durmaz G., Gökmen V. Impacts of roasting oily seeds and nuts on their extracted oils. Lipid Technology. 2010;22(8):179–182. doi: 10.1002/lite.201000042. [DOI] [Google Scholar]
- Guo S., Na Jom K., Ge Y. Influence of roasting condition on flavor profile of sunflower seeds: A flavoromics approach. Scientific Reports. 2019;9(1):11295. doi: 10.1038/s41598-019-47811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Zhang Y., Wang X. Characterization of the volatile compounds and taste attributes of sesame pastes processed at different temperatures. Journal of Oleo Science. 2019;68(6):551–558. doi: 10.5650/jos.ess19014. [DOI] [PubMed] [Google Scholar]
- Hua J., Li J., Ouyang W., Wang J., Yuan H., Jiang Y. Effect of Strobilanthes tonkinensis Lindau addition on black tea flavor quality and volatile metabolite content. Foods. 2022;11(12):1678. doi: 10.3390/foods11121678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseppi R., Brighenti V., Licata M., Lambertini A., Sabia C., Messi P., Pellati F., Benvenuti S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp) Molecules. 2019;24(12):2302. doi: 10.3390/molecules24122302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H.L., Park S.Y., Nam J.S. The effects of heat treatment on the nutritional composition and antioxidant properties of hempseed (Cannabis sativa L.) Journal of the Korean Society of Food Science and Nutrition. 2018;47(9):885–894. doi: 10.3746/jkfn.2018.47.9.885. [DOI] [Google Scholar]
- Jeong, H., Yoon, S., Jo, S. M., Hong, S. J., Kim, Y. J., Kim, J. K., & Shin, E. C. (2023). Chemical sensory investigation in green and roasted beans Coffea arabica L.(cv. Yellow Bourbon) by various brewing methods using electronic sensors. Journal of Food Science, 88(3), 1033-1047. 10.1111/1750-3841.16470. [DOI] [PubMed]
- Kuwahata H., Katsuyama S., Komatsu T., Nakamura H., Corasaniti M.T., Bagetta G., Sakurada S., Sakurada T., Takahama K. Local peripheral effects of β-Caryophyllene through CB 2 receptors in neuropathic pain in mice. Pharmacology & Pharmacy. 2012;3(04):397–403. doi: 10.4236/pp.2012.34053. [DOI] [Google Scholar]
- Li Y., Jiang S., Zhu Y., Shi W., Zhang Y., Liu Y. Effect of different drying methods on the taste and volatile compounds, sensory characteristics of Takifugu obscurus. Food Science and Human Wellness. 2023;12(1):223–232. doi: 10.1016/j.fshw.2022.07.012. [DOI] [Google Scholar]
- Navicha W., Hua Y., Masamba K.G., Kong X., Zhang C. Effect of soybean roasting on soymilk sensory properties. British Food Journal. 2018;120(12):2832–2842. doi: 10.1108/BFJ-11-2017-0646. [DOI] [Google Scholar]
- Mansouri F., Allay A., Moumen A.B., Benkirane C., Taaifi Y., Belhaj K., Addi M., Hano C., Fauconnier M.L., Caid H.S., Elamrani A. Laboratory-Scale Optimization of Hemp Seed Roasting Temperature and Time for Producing a High-Quality Pressed Oil. Journal of Food Processing and Preservation. 2023;2023:16. doi: 10.1155/2023/8261279. [DOI] [Google Scholar]
- Oomah B.D., Busson M., Godfrey D.V., Drover J.C. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chemistry. 2002;76(1):33–43. doi: 10.1016/S0308-8146(01)00245-X. [DOI] [Google Scholar]
- Pino J.A. Odour-active compounds in mango (Mangifera indica L. cv. Corazón). International journal of. Food Science & Technology. 2012;47(9):1944–1950. doi: 10.1111/j.1365-2621.2012.03054.x. [DOI] [Google Scholar]
- Pino J.A., Quijano C.E. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Food Science and Technology. 2012;32:76–83. doi: 10.1590/S0101-20612012005000006. [DOI] [Google Scholar]
- Shen P., Gao Z., Xu M., Ohm J.B., Rao J., Chen B. The impact of hempseed dehulling on chemical composition, structure properties and aromatic profile of hemp protein isolate. Food Hydrocolloids. 2020;106 doi: 10.1016/j.foodhyd.2020.105889. [DOI] [Google Scholar]
- Song H., Liu J. GC-O-MS technique and its applications in food flavor analysis. Food Research International. 2018;114:187–198. doi: 10.1016/j.foodres.2018.07.037. [DOI] [PubMed] [Google Scholar]
- Srivastava A.K., Singh V.K. Biological action of essential oils (terpenes) International Journal of Biological and Medical Research. 2019;10(3):6854–6859. [Google Scholar]
- Ueno T., Kiyohara S., Ho C.T., Masuda H. Potent inhibitory effects of black tea theaflavins on off-odor formation from citral. Journal of Agricultural and Food Chemistry. 2006;54(8):3055–3061. doi: 10.1021/jf052803h. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Xiang P., Zhu J., Zhu Q., Liu Y., Niu Y. Evaluation of the perceptual interaction among sulfur compounds in mango by Feller’s additive model, odor activity value, and vector model. Journal of Agricultural and Food Chemistry. 2019;67(32):8926–8937. doi: 10.1021/acs.jafc.9b03156. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li J., Zhao J., Wang W., Griffin J., Li Y., Bean S., Tilley M., Wang D. Hempseed as a nutritious and healthy human food or animal feed source: A review. International Journal of Food Science & Technology. 2021;56(2):530–543. doi: 10.1111/ijfs.14755. [DOI] [Google Scholar]
- Yin W.T., Maradza W., Xu Y.F., Ma X.T., Shi R., Zhao R.Y., Wang X.D. Comparison of key aroma-active composition and aroma perception of cold-pressed and roasted peanut oils. International Journal of Food Science & Technology. 2022;57(5):2968–2979. doi: 10.1111/ijfs.15615. [DOI] [Google Scholar]
- Yin X., Wei Y., Li T., Zhang J., Zou L., Cui Q., Lu C., Ning J. Heterocyclic compounds formation in large-leaf yellow tea induced by the Maillard reaction at different roasting temperatures. LWT. 2023;182 doi: 10.1016/j.lwt.2023.114856. [DOI] [Google Scholar]
- Zhang Y., Li X., Lu X., Sun H., Wang F. Effect of oilseed roasting on the quality, flavor and safety of oil: A comprehensive review. Food Research International. 2021;150 doi: 10.1016/j.foodres.2021.110791. [DOI] [PubMed] [Google Scholar]
- Zhu J., Wang L., Xiao Z., Niu Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography–olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC–MS) and flame photometric detection (FPD) Food Chemistry. 2018;245:775–785. doi: 10.1016/j.foodchem.2017.11.112. [DOI] [PubMed] [Google Scholar]
- Zhu J., Xiao Z. Characterization of the major odor-active compounds in dry jujube cultivars by application of gas chromatography–olfactometry and odor activity value. Journal of Agricultural and Food Chemistry. 2018;66(29):7722–7734. doi: 10.1021/acs.jafc.8b01366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.