Graphical abstract:
Keywords: Roasted chicken, SAFE, GC−O-MS, ADEA, Recombination and omission test
Highlights
-
•
Twenty key odorants of roasted chicken were identified.
-
•
Hexanal and (E, E)-2, 4-decadienal were the most abundant aldehydes.
-
•
4-hydroxy-5-methyl-3(2H)-furanone gave the roasted chicken caramel aroma.
-
•
Pyrazines, such as 2,5-dimethylpyrazine, significantly affected the roasty aroma.
Abstract
Aroma compounds in the roasted breasts, thighs and skins of chicken were isolated by solvent-assisted flavor evaporation (SAFE), quantitated by gas chromatography–olfactometry-mass (GC-O-MS), analyzed by aroma extract dilution analysis (AEDA), and determined by recombination-omission tests and sensory evaluation. Forty-seven aroma compounds in total, including aldehydes, ketones, furans, pyrazines, and furanones, were selected by AEDA. Twenty-five compounds were selected as pivotal odorants (Odor Activity Value, OAV ≥ 1). Twenty aroma compounds significantly were identified by recombination and omission experiments. Anethole (fennel odor) was the highest OAV (> 1843). Hexanal (grassy) and (E, E)-2,4-decadienal (meaty) were the most abundant aldehydes identified in roasted chicken. 1-octen-3-ol (mushroom), methanethiol (cabbage) and dimethyl trisulfide (areca, sulfur) were considered the key compounds of the breast and thighs of roasted chicken. Notably, furanone and pyrazines, 4-hydroxy-5-methyl-3(2H)-furanone (caramel, sweet and burning odor), 3-ethyl-2,5-dimethylpyrazine (nutty, toasty) and 2,3-dimethyl-5-ethylpyrazine (nutty, toasty) had the most significant effect on roasted chicken odor, especially in the skin.
1. Introduction
The flavor is a critical indicator of judged quality attributes in meat products and affects the acceptance of consumers (Liu and Fang, 2022, Yu et al., 2021). Roasted chicken, renowned as a traditional Chinese culinary delicacy, garners favor among consumers due to its exceptional palatability and distinctive flavor profile (Zhang et al., 2022a). Roasted chicken comes in three varieties: soil oven-roasted, iron oven-roasted, and electric-roasted, depending on the cooking method. Soil and iron oven-roasted chicken use charcoal fire, while electric-roasted chicken relies on infrared technology. These methods create the chicken's unique flavor and appealing appearance (Zhang et al., 2022a). Aromatic and most characteristic odorants of meat are mainly contributed by volatile compounds produced by complex heat-induced reactions (Mottram, 1998). Meaty aroma precursors can be primarily classified into two categories, with the first comprising water-soluble compounds such as amino acids, reducing sugars, and thiamine (Jayasena et al., 2013). The second category encompasses triglycerides, free fatty acids, structural phospholipids, and other fat-soluble compounds (Bassam et al., 2021).
Solid phase microextraction (SPME), a wide range of extraction method, is applied in food flavor analysis (Bleicher et al., 2022, Brunton et al., 2000). Additionally, the Solvent-Assisted Flavor Evaporation (SAFE) method has proven effective for the identification of aroma compounds through Gas Chromatography-Mass Spectrometry (GC–MS), offering high accuracy and satisfactory limit of detection (Majcher and Jelen, 2009). Furthermore, a more comprehensive identification of aroma compounds can be achieved through the application of Gas Chromatography-Olfactometry (GC-O), Aroma Extract Dilution Analysis (AEDA), and Aroma Activity Value (OAV) (Sarhir et al., 2019). However, the research conclusions on the aroma compounds of roasted chicken are not uniform. Hsu and Chen et al. studied the effects of fan oven and superheated steam oven baking methods on roasted chicken quality (Hsu and Chen, 2020). Yu et al. used GC–MS to examine aroma compounds in chicken breasts cooked by different methods and identified 3-methyl butyraldehyde as the key aroma compound in roasted chicken (Yu et al., 2021). Feng et al. summarized 16 aroma compounds in a chicken broth had average OAVs greater than 1 using GC-O/MS and flavor dilution (FD), including (E, E)-2,4-decadienal, (E, E)-2,4-nonadienal, and (E)-2-nonenal (Feng et al., 2018). Over time, the preparation methods for roasted chicken have evolved, from traditional soil ovens to modern iron and electric ovens. Additionally, the key aroma compounds in roasted chicken have not been thoroughly analyzed using advanced techniques like SPME, SAFE with GC-O-MS, and AEDA.
The purposes of this study were to (i) analyze the aroma compounds of roasted chicken qualitatively by SPME, SAFE, and GC-O-MS, (ii) estimate the potency of flavor compounds by GC-O and ADEA, (iii) calculate OAVs and determine the key aroma compounds that have the dominant influence on the overall aroma through flavor recombination and omission experiments.
2. Materials and methods
2.1. Materials
The three kinds of roasted chicken were acquired from the two restaurants in Shandong and Beijing (Beijing Xiangfei Roasted Chicken Fast Food Restaurant, Pingdu Roasted Chicken shop in Shandong). Firstly, roasted chicken was processed into three groups of roasted chicken according to three roasting methods, and three chickens were chosen randomly from 20 roasted chickens in each group. The carcass weight and age of each roasted chicken was 800 ± 50 g and 18 months. The chicken of Pingdu Roasted Chicken shop in Shandong was local Sanhuang chicken, and the chicken of Beijing Xiangfei Roasted Chicken Fast Food Restaurant was white feather chicken. After visible fat and connective tissue had been removed, the skin, breast, and thigh of roasted chicken were chopped by a QSJ-B02X5 knife grinder. Then all samples were quickly frozen in liquid nitrogen in polyamide/polyethylene bags and preserved at −40℃.
2.2. Chemicals
The flavor standards, methanethiol (97 %), eucalyptol (99 %), 2,5-dimethyltetrahydrofuran (98 %), 2,5-dimethylpyrazine (98 %), hexanal (98 %), 2-ethyl-5-methylpyrazine (96 %), (E)-2-octenal (97 %), 3-ethyl-2,5-dimethylpyrazine (98 %), benzaldehyde (99.5 %), (Z)-2-octen-1-ol (97 %), 2-pentylfuran (98 %), carvone (96 %), methyleugenol (98 %), anethole (99 %), (E, E)-2,4-decadienal (97 %), 2,3,5-trimethylpyrazine (99 %), (Z)-3-phenylacrylaldehyde (97 %), p-cresol (99 %), 4-hydroxy-5-methyl-3(2H)-furanone (97 %), 2,3-dimethyl-5-ethylpyrazine (99 %), dimethyl trisulfide (98 %), 2(5H)-furanone (98 %), 1-octen-3-ol (98 %), 3-furaldehyde (97 %), and 2-furanmethanol (98 %) were commercially purchased from Sigma-Aldrich (Shanghai, China). And methyl pyrazine (98 %) and benzene acetaldehyde (98 %) were purchased from TCI (Shanghai, China). Meanwhile, 2-methyl-3-heptanone (99 %) from Dr. Ehrenstorfer (Beijing, China) as the internal standard and the n-alkanes external standard (C7-C40, ≥ 97 %) from o2si Smart Solutions (Shanghai, China) were purchased.
2.3. Headspace Solid-Phase microextraction (HS-SPME)
Briefly, 3.00 g of the crushed samples were put into a 20 mL extraction bottle, and 0.5 μL 2.00 μg/μL 2-methyl-3-heptanone (dissolved in methanol) was mixed as an internal standard. The piece was extracted by 100 μm PDMS at 40 °C for 54 min, and analyzed by GC–MS.(Liu et al., 2019).
2.4. Solvent-Assisted flavor evaporation (SAFE)
50 g of crushed samples and 50 mL of dichloromethane were put into a 1000 mL bottom flask, and 20 μL of 2.00 μg/μL 2-methyl-3-heptanone (dissolved in methanol) was mixed as the internal standard. After three hours extraction, the filtrate was filtered and repeated three times. The extract was combined and distilled by a SAFE device (42℃, pressure of about 10-3-10-4 Pa). The extraction obtained after distillation was removed moisture by anhydrous sodium sulfate (overnight). Those samples were concentrated to 2–3 mL (about 9 h, actually 1 h at 44℃, and 8 h at 48℃) by Vigreux column, then concentrated to 1 mL (about 10 min) by N2 (Gasior et al., 2021).
2.5. Gas Chromatography-Olfactometry-Mass Spectrometry (GC-O-MS) analysis using a DB-Wax column and a DB-5 ms column
Thermo Scientific TM ISQTM LT Single Quadrupole Gas-Mass System with DB-Wax and DB-5 ms (60 m × 0.25 mm i.d., 0.25 μm film thickness) columns for analysis at a helium flow rate of 1 mL/min. And the DB-Wax and DB-5 ms chromatographic columns procedure: initial temperature 40℃, maintained for 3 min, increased to 70℃ at 2℃/min, increased to 130℃ at 3℃/min, and then increased to 230℃ at 10℃/min, maintained for 10 min. Electron bombardment voltage 70 eV, ionization source temperature 230℃, quadrupole temperature 150℃, full scan mode, mass scan range 30–450 amu. The solvent delay time was 5 min with either DB-Wax or DB-5 ms. N-alkanes (C7-C40) were injected under the same procedure to determine the retention index.
In the formula, tn and tn+1 are the retention time of n-alkanes with carbon number n and n + 1, respectively, and it is the retention time of a compound (Liu et al., 2019).
2.6. Aroma extract dilution analysis (AEDA)
To confirm flavor dilution (FD) of aroma compounds, the concentrated SAFE distillate of the breast, thigh, and skin of roasted chicken was detected by five trained panelists. ADEA was detected by diluting with dichloromethane (2v volume). The dilutions were conducted: 3-, 9-, 27-, 81-, 243-, and 732-fold. The dilutions were determined by GC-O until the experimenters could not perceive any odor (Zhang et al., 2022b). Besides, the maximum dilution, in which the aroma compounds were determined, indicated FD factor of each odorant (Shi et al., 2021). The AEDA was conducted by four experimenters and the data were averaged.
2.7. Quantitation of volatile compounds.
The quantitation of aroma compounds was conducted on Thermo Scientific TM ISQTM LT Single Quadrupole Gas-Mass System. The concentrated SAFE extract was placed into the detector of the GC–MS system using the DB-Wax and DB-5 ms columns with the same programs and the essential aroma compounds of roasted chicken were quantitated according to the calibration curves. Besides, 2-methyl-3-heptanone was used as the internal standard. All aroma compounds selected on the basis of GC-O results (Table 2) on a concentration gradient of 1 mg/L to 1000 mg/L were added to methanol to detect by GC–MS. To further ensure the accuracy of flavor standards and eliminate distractions, three characteristic mass ion fragments of each aroma compound were selected in SIM mode.
Table 2.
Authentic standards, scanned ions, calibration equations, thresholds, and OAVs of determining aroma compounds in roasted chicken.
| No. | compound | ions(m/z) a | calibration equationsb | Concentration (μg/kg) | Threshold | OAV | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (R2) | T-P | T-R | T-T | T-P | T-R | T-T | |||||
| 1 | methanethiol | 47, 48, 45 | y = 0.0000099 x + 0.1884500 | 0.978 | 310.45 ± 10.05 | 227.90 ± 1.71 | 490.25 ± 5.67 | 0.2 | 1,548 | 1,143 | 2,425 |
| 2 | hexanal | 44, 56, 41 | y = 0.0006428 + 10.9591600 | 0.985 | 198.14 ± 6.91 | 5227.44 ± 2.19 | 8662.19 ± 38.88 | 5 | 40 | 1,046 | 1,724 |
| 3 | 2,5-dimethyltetrahydrofuran | 56, 41, 85 | y = 0.0000190 x-0.2312300 | 0.975 | 25.90 ± 0.97 | ND | ND | 9.5 | 3 | ND | ND |
| 4 | eucalyptol | 43, 81, 108 | y = 0.0000043 x-0.0695100 | 0.986 | 656.49 ± 18.31 | 123.00 ± 1.07 | 183.71 ± 1.58 | 1.1 | 603 | 112 | 167 |
| 5 | 2,5-dimethylpyrazine | 42, 108, 39 | y = 0.0000048 x-0.0652100 | 0.982 | 102.99 ± 2.43 | ND | 38.19 ± 0.86 | 1.75 | 58 | ND | 22 |
| 6 | 2-pentylfuran | 81, 82, 138 | y = 0.0000008 x + 0.0072100 | 0.987 | 43.01 ± 2.21 | 32.45 ± 0.55 | 92.15 ± 1.29 | 5.8 | 7 | 6 | 16 |
| 7 | dimethyl trisulfide | 126, 45, 79 | y = 0.0000158 x-0.2450500 | 0.986 | ND | 167.50 ± 0.57 | ND | 0.1 | ND | 1,672 | ND |
| 8 | 2-ethyl-5-methylpyrazine | 121, 122, 94 | y = 0.0000023 x + 0.0014500 | 0.999 | 44.83 ± 4.57 | ND | 17.52 ± 0.47 | 16 | 3 | ND | 1 |
| 9 | 1-octen-3-ol | 55, 57, 43 | y = 0.0000043 x + 0.0343800 | 0.990 | ND | 699.37 ± 1.35 | 1286.87 ± 2.56 | 1.5 | ND | 465 | 858 |
| 10 | (E)-2-octenal | 41, 55, 29 | y = 0.0000231 x + 0.9608000 | 0.999 | ND | 27.69 ± 1.58 | 115.80 ± 0.99 | 3 | ND | 10 | 38 |
| 11 | 3-ethyl-2,5-dimethylpyrazine | 135, 136, 42 | y = 0.0008600 + 11.1590700 | 0.970 | 152.89 ± 1.94 | ND | ND | 8.6 | 18 | ND | ND |
| 12 | benzaldehyde | 77, 106, 51 | y = 0.0000005 x-0.0033600 | 0.992 | 267.08 ± 7.41 | 173.87 ± 0.94 | 186.32 ± 1.63 | 750 | <1 | <1 | <1 |
| 13 | (Z)-2-octen-1-ol | 57, 41, 54 | y = 0.0000120 x + 0.0211100 | 0.990 | ND | 66.33 ± 0.34 | 121.39 ± 1.88 | 20 | ND | 3 | 6 |
| 14 | carvone | 82, 54, 39 | y = 0.0000327 x-0.5597600 | 0.985 | 729.92 ± 4.02 | 131.50 ± 0.88 | 134.22 ± 2.01 | 6.7 | 109 | 20 | 20 |
| 15 | methyleugenol | 178, 146, 163 | y = 0.0000004 x-0.0033200 | 0.988 | 686.30 ± 7.92 | 268.18 ± 0.75 | 158.59 ± 1.85 | 0.71 | 963 | 378 | 223 |
| 16 | anethole | 148, 147, 117 | y = 0.0006043 x + 3.2658500 | 0.997 | 46234.75 ± 257.04 | 28765.11 ± 188.58 | 27707.34 ± 222.07 | 15 | 3,073 | 1,911 | 1,843 |
| 17 | (E, E) −2,4-decadienal | 81, 41, 29 | y = 0.0002429 + 21.7471500 | 0.979 | ND | ND | 47.33 ± 0.89 | 0.027 | ND | ND | 1,766 |
| 18 | trimethylpyrazine | 42, 122, 39 | y = 0.0066700 x-3.1449800 | 0.999 | 204.04 ± 2.05 | ND | ND | 350 | 1 | ND | ND |
| 19 | benzeneacetaldehyde | 91, 92, 65 | y = 0.0000024 x-0.0179500 | 0.995 | ND | 29.06 ± 1.32 | 30.65 ± 1.06 | 6.3 | ND | 5 | 5 |
| 20 | (Z)-3-phenylacrylaldehyde | 131, 103, 32 | y = 0.0000013 x-0.0098600 | 0.997 | 1217.77 ± 3.53 | 346.67 ± 1.68 | 215.48 ± 4.73 | 1.1 | 1,108 | 314 | 198 |
| 21 | p-cresol | 107, 108, 77 | y = 0.0000110 x + 0.0974100 | 0.996 | ND | ND | 26.49 ± 0.48 | 2.7 | ND | ND | 10 |
| 22 | 4-hydroxy-5-methyl-3(2H)-furanone | 99, 71, 85 | y = 0.0000019 x-0.0260700 | 0.987 | 85.49 ± 0.81 | 116.09 ± 0.36 | 221.92 ± 1.93 | 9 | 9 | 13 | 25 |
| 23 | 2,3-dimethyl-5-ethylpyrazine | 135, 136, 53 | y = 0.0000030 x-0.0817000 | 0.971 | 4.97 ± 0.10 | ND | 8.63 ± 0.31 | 0.0031 | 1,610 | ND | 2,781 |
| 24 | methylpyrazine | 107, 108, 80 | y = 0.0000006 x-0.0085500 | 0.981 | 38.88 ± 2.19 | 10.39 ± 0.30 | 31.51 ± 0.78 | 30 | 1 | <1 | 1 |
| 25 | 2(5H)-furanone | 55, 84, 27 | y = 0.0000047 x + 0.0057200 | 0.999 | 115.97 ± 0.33 | ND | ND | 5 | 23 | ND | ND |
| 26 | 3-furaldehyde | 95, 96, 39 | y = 0.0000316 x + 0.0487400 | 0.996 | 12.56 ± 0.36 | ND | 8.73 ± 0.15 | 9.56 | 1 | ND | 1 |
| 27 | 2-furanmethanol | 98, 41, 97 | y = 0.0000438 x-0.7800000 | 0.983 | 281.89 ± 2.32 | ND | ND | 770 | <1 | ND | ND |
Monitored ions.
Variables: x is the peak area relative to that of the internal standard, 2-methyl-3-heptanone, and y is the concentration (ng/g) in the sample relative to that of the internal standard, 2-methyl-3-heptanone. ND means not identified.
2.8. Odor-Activity values (OAVs) analysis
To characterize the contribution of key aroma compounds to the overall aroma profile of roasted chicken, the OAVs were calculated. Moreover, the perception thresholds in water were applicable to reveal the odorants of roasted meat (Sohail et al., 2022). Specifically, the aroma compounds with OAVs ≥ 1 might have a significant contribution to roasted chicken, while compounds with OAVs < 1 indicated a minor contribution.
where Cα is the content of odorant α in the roasted chicken; Tα is the perception threshold of odorant α in water..(Liu et al., 2019)
2.9. Recombination and omission experiments
The specific method referenced Liu et al.(Liu et al., 2019). To assess whether these important odorants (OAVs > 1) could mimic the overall aroma profile in the samples, the recombination and omission experiment was performed by using authentic flavor standards in the same concentrations as observed in the sample. Three recombination models were prepared, which comprised skin (TP), thighs (TT), and breasts (TR) of roasted chicken with 17 (TP), 20 (TT), and 14 (TR) odorants (OAVs ≥ 1) (Bi et al., 2020). A group of twelve experienced panelists (eight females and four males aged 20–35) were recruited for a sensory evaluation. They were instructed to avoid eating for two hours prior to the experiment. The preparation method of roasted chicken matrix was to add a mixture of ether and n-pentane to the sample, shake the shaker for 8 h, filter and discard the organic solvent until the sample was tasteless. The tasteless sample residue was freeze-dried for 12 h. On this basis, the tasteless matrix of roasted chicken was constructed by tasteless residue and water.
To further describe, the sensory evaluation consisted of three parts. The first part: the sensory panel members discussed and confirmed the odor descriptors of roasted chicken (Shi et al., 2021), which included grassy, caramel, roasted, meaty, oily, and fennel odors. The second part: For the accuracy of the evaluation, all sensory panel members were trained to ensure that they reached an agreement on the description of each aroma. Specifically, the flavor standards references were conducted as followings (Dach and Schieberle, 2021, Jonas and Schieberle, 2021), earthy (3-ethyl-2,5-dimethylpyrazine, trimethyl pyrazine), fatty [(E, E)-2,4-decadienal] (Warner and Munafo, 2022), caramel-like (4-hydroxy-5-dimethylfuran-3(2H)-one), grassy [hexanal, (Z)-3-hexenal] (Dein and Munafo, 2022), fennel-like (anethole), flowery (phenylacetaldehyde), popcorn-like (2-acetyl-1-pyrroline) and the last part (Neugebauer et al., 2021, Zhai and Granvogl, 2020): All aroma compounds (OAVs ≥ 1) of the three models were separately mixed in water with the detected concentrations and placed in 20 mL headspace vials containing an artificial odorless matrix (Liu et al., 2019). The sensory panel members determined the flavor profiles on a 10-point (0 undetectable; 2 very weak; 4 weak; 6 middle; 8 strong; 10 very strong).
Omission experiments was slightly modified by referring to the method of Gorman et al. (2022). Missing models of TP, TR and TT and complete aroma simulation models were submitted to 12 panelists (n = 12). All the samples were estimated in triplicate by the above methods, and the obtained data was recorded as the average value. The results of sensory evaluation were published with the informed consent of 12 sensory evaluators.
2.10. Statistical analysis.
In this study, all experimental results were presented on the basis of triplicate values and expressed as the means ± standard deviations. The cluster heatmap and chord diagram of aroma compounds were created using Origin 2022 software (Origin Lab Corporation). One-way analysis of variance (ANOVA) and the quantitative analysis of aroma compounds were conducted using SPSS 23.0 (IBM Corporation).
3. Results and discussion
3.1. Comparison of aroma compounds among three kinds of roasted chicken
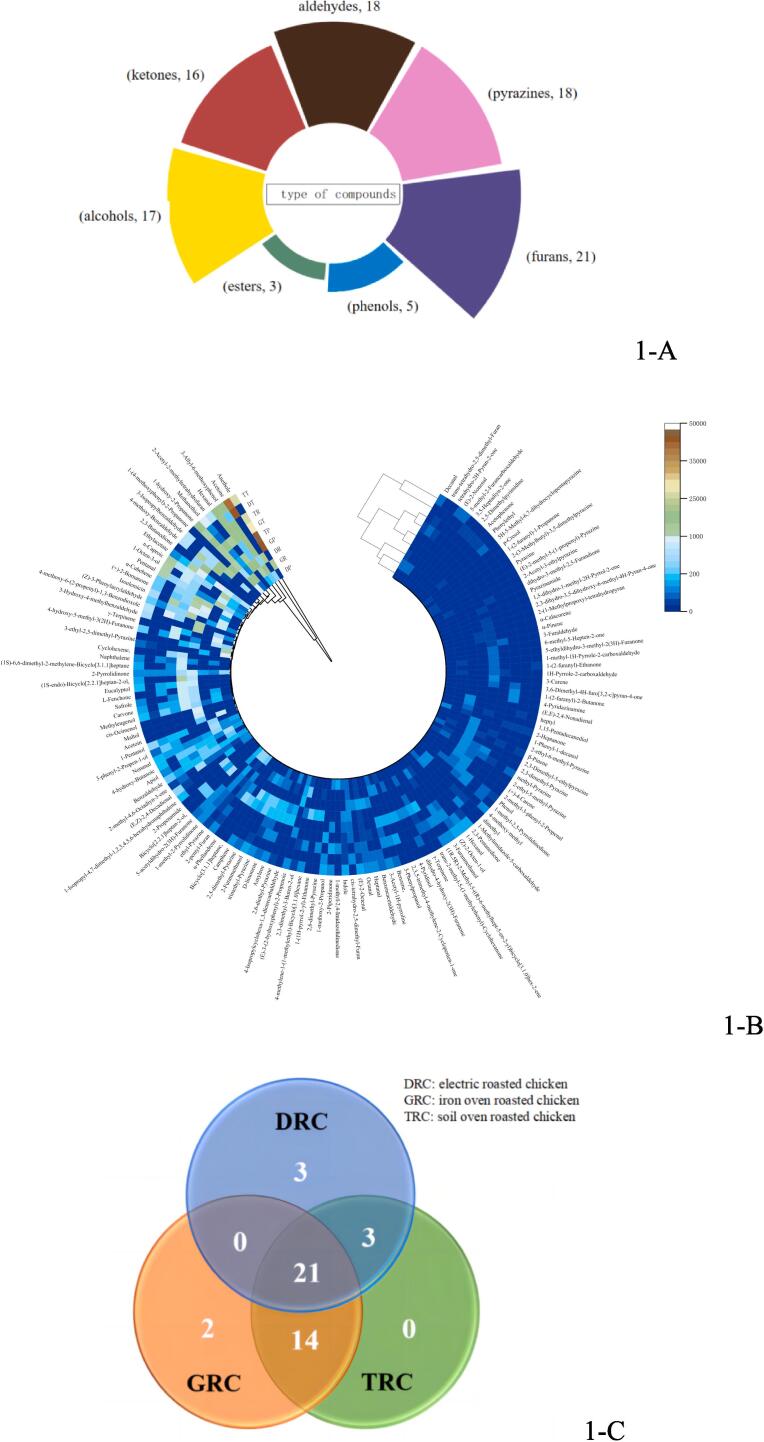
To compare the differential in the aroma compounds of the three roasted chickens, all the aroma components of roasted chicken were determined in the light of the internal standards by the semi-quantitative method. As shown in Fig. 1, using GC-O-MS by two kinds of extracting volatiles (SAFE, SPME) and two columns (DB-5 ms, DB-wax), a total of 141 aroma compounds were detected from roasted chicken, among which 73 aroma compounds were identified from electric roasted chicken (DRC), 104 for iron oven roasted chicken (GRC) and 118 for soil oven roasted chicken (TRC). To determine the better pretreatment modalities for analyzing the volatile flavor compounds of roasted chicken, two kinds of extracting volatile compounds (SAFE, SPME) and two columns (polar columns: DB-Wax, nonpolar columns: DB-5 ms) were compared separately. The results showed that the aldehydes, ketones and alcohols detected by DB-wax columns were more than those detected by DB-5 ms columns, and the lipids and aromatic flavor compounds were roughly the same. In addition, the types of aldehydes, esters and aromatic compounds extracted by SAFE were similar to those extracted by SPME, while the types of sulfur-containing and nitrogen-containing substances such as furan and pyrazine were higher than those extracted by SPME. Maillard reactions can produce several types of flavor compounds, some of which are volatile and semi-volatile in nature. These components include pyrazine, furan, thiophene, and pyridine. However, our results showed that the SAFE method could extract a wider range of pyrazines and furans than SPME, indicating that SAFE is more suitable for the extraction of polar flavors. The reason is that SPME extracts more of the low molecular weight and low boiling point volatiles. In addition, SAFE is a gentle and inexpensive sample treatment technique, whereas SPME is also a gentle treatment method. However, SAFE has the added advantage of directly obtaining aroma concentrates. These are suitable for subsequent GC-O analysis. Therefore, with a more comprehensive analysis of the volatile flavor compounds of roasted chicken, the method of SAFE combined with DB-wax column was selected.
Fig. 1.
The types and contents of flavor compounds in roasted chicken (1-A: the types of compounds; 1-B: the contents of compounds;1-C:three kinds of roasted chicken).
The aroma compounds were divided into seven classes, including aldehydes, ketones, alcohols, esters, phenols, pyrazines, and furans (Fig. 1-A). The content of aldehydes was the highest in DRC, while benzene derivatives containing oxygen, such as anethole, were found to be the highest content in GRC and TRC. One possible explanation for the differences is the roasting process. During roasting, chicken meat undergoes chemical reactions that produce aldehydes. Cooking temperature, time, and other factors influence these reactions. TRC production or ingredients may contain higher levels of benzene derivatives, which can be transferred to cooked chicken. Differences in aldehyde and oxygenated benzene derivative content in different types of roasted chicken may be due to cooking process, type of chicken, and ingredients used in preparation. However, further research is needed to understand these observations and underlying causes. Similar results were found that the content of aldehydes was nearly 50 % of aroma compounds from Beijing roasted duck (Liu et al., 2019). Moreover, aliphatic aldehydes contributed to producing the fatty odor of chicken meat (Jayasena et al., 2013). From three parts of the roasted chicken, the skin of the roasted chicken was identified as the most kinds of aroma compounds, and the second was the thigh, in which the kinds of pyrazines of skin were more than thigh and breast observably. The requirement for generating pyrazines was high temperature and low moisture (Jayasena et al., 2013).
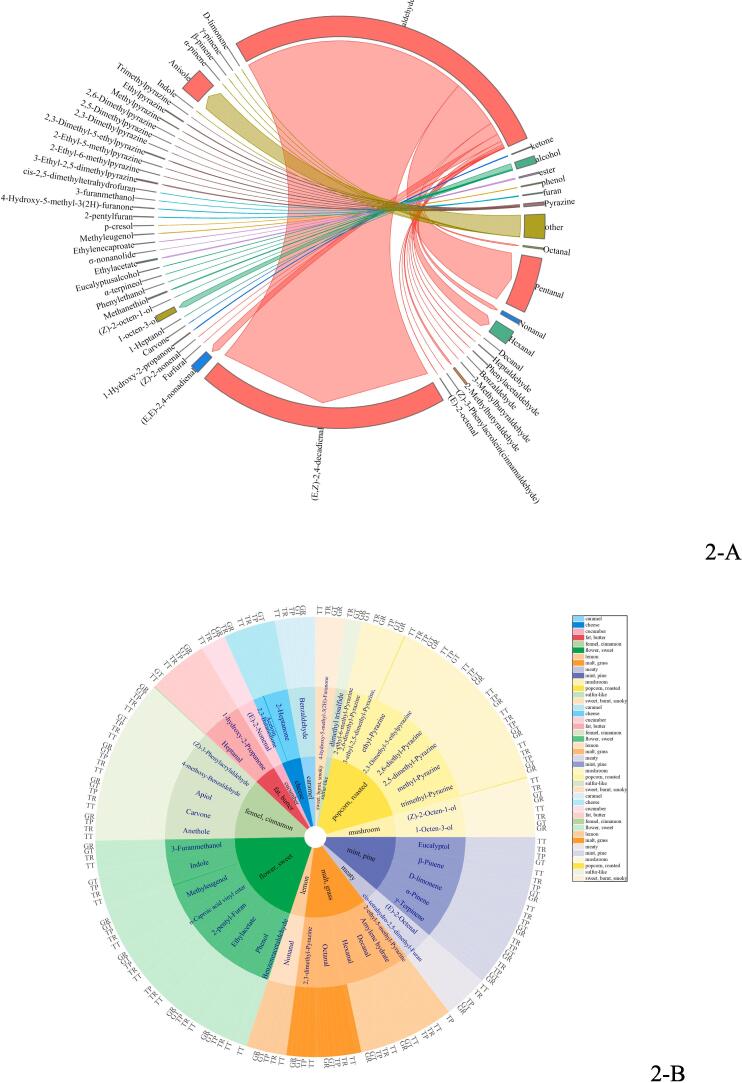
The critical aroma compounds shared by the three roasted chickens were composed of aliphatic aldehydes and nitrogen-containing compounds (Fig. 2-A), especially 3-ethyl-2,5-dimethylpyrazine, hexanal, 5-dimethyltetrahydrofuran, 2-ethyl-5-methylpyrazine, 2,3-dimethyl-5-ethylpyrazine, 2-methylbutanal, 1-hydroxy-2-acetone, (E, E)-2,4-decadienal, 1-octen-3-ol and methyl mercaptan. Fig. 2-A showed the chord diagram of the main aroma compounds content of the three roasted chickens, indicating the proportion of 7 types of aroma compounds in the total aroma compounds. In addition, it also showed the aroma compounds with a high proportion in each type of aroma compounds, such as the highest content of aldehydes in 7 types of aroma compounds. Comparing the number of aroma compounds of the three roasted chickens, it can be exhibited that the unique odorants of the soil oven-roasted chicken are 2-ethyl-3,5-dimethylpyrazine, pyrazinamide, σ-nonolactone, mainly roasted meat-flavored nitrogen-containing compounds, and fruit-flavored lipids. It might be due to the closed furnace during the soil oven roasting process and the loss of aroma compounds was relatively tiny. Secondly, the soil oven-roasted chicken was roasted by the fruit wood fire, in which produced some fruit-odorants during the burning process of the wood. And those fruit-odorants might be combined with the roasted chicken, therefore the fruit-odor in the soil oven-roasted chicken was more obvious than that of the other two kinds of roasted chicken. This cooking method might bring significant advantages in industrial applications and was continuing to explore (Taskiran et al., 2020).
Fig. 2.
Classification and of contents of main aroma substances in roasted chicken by GC-O (2-A: Chord diagram of the contents of main aroma compounds; 2-B: classification of of main aroma compounds).
3.2. Identification and quantitation of the Odor-Active compounds in roasted chicken with GC-O-MS
Sensory evaluation and GC-O-MS analysis were applied to the breast, thigh, and skin of the three roasted chickens. According to GC-O analysis, the fifty-seven compounds were classified into 12 aroma types (Fig. 2-B), such as roasty, fatty, meaty, fennel-like and caramel-like played the critical role in the aroma profile of roasted chicken, followed by grassy, mushroom-like, flower and butter odor, similar to the properties of roasted chicken odor mainly described as fatty, meaty, roasty and lemon-like, (Hashim et al., 1999) as reported in the literature. (Hashim et al., 1999, Niu et al., 2016) The results showed that the aroma compounds of popcorn and roast were 2-ethyl-6-methylpyrazine, methylpyrazine, 3-ethyl-2,5-dimethylpyrazine, 2,3-dimethyl-5-ethylpyrazine, 2,6-dimethylpyrazine, and 2,5-dimethylpyrazine.
According to the above results, the types of aroma compounds of soil oven roasted chicken (TRC) were more than those of the other two kinds of chicken (Fig. 1-C). Three roasted chickens shared the 21 same aroma compounds, and the main compounds were aldehydes, ketone, furans, and pyrazines. Comparing the influence of three cooking methods on the flavor compounds of roasted chicken, the DRC was roasted by infrared heat production, and the TRC and the GRC were roasted by fruit wood. The difference between the TRC and GRC was that the former used the soil to seal the furnace, while the latter was not sealed. To be specific, the hermetically sealed oven environment is conducive to the preservation of the flavor of roasted chicken. Moreover, the fruit charcoal roasting method imparts a more pronounced aroma to the roasted chicken compared to infrared heating.
The aroma compounds of thighs in roasted chicken were the most, and the breast is the least (Table 1). The three parts had the same twenty compounds. The number of detected aroma compounds in the soil oven roasted chicken exceeded that of the other two roasted chickens. Specifically, 37 aroma compounds were detected by GRC, 27 by DRC, and 38 by TRC, so TRC was more representative. TRC was selected further to analyze the detection frequency (DF) value to compare the flavor differences of breast, thighs, and skin of the roasted chicken. Application of aroma extract dilution analysis (AEDA) revealed 47 odor-active areas in the FD factor range between 3 and 729 (Table 1). The FD factor of methyl eugenol and anethole in TP (skin of soil-oven roasted chicken) was highest (7 2 9). A total of 47 aroma compounds were identified from TRC by DF, including 29 compounds in TP (skin of soil-oven roasted chicken), 27 compounds in TR (breast of soil-oven roasted chicken), 31 compounds in TT (thigh of soil oven-roasted chicken) (Table 1). Only ten compounds were detected in TP, including eucalyptol (81, flavor dilution), 2(5H)-furanone (81), 2,5-dimethyl tetrahydrofuran (9), 2-ethyl-6-methyl pyrazine (3), trimethyl pyrazine (9), 3-ethyl-2,5-dimethyl pyrazine (81), 2-furanmethanol (9), methyl pyrazine (2 4 3), 2,6-diethyl pyrazine (3), 2,3-dimethyl-5-ethyl pyrazine (81). And nine aroma compounds were detected in TT, including heptanal (3), octanal (3), unknown2 (27), n-caproic acid vinyl ester (9), p-cresol (9), unknown 7 (27), unknown 8 (81), unknown 9 (81). There are only two unique compounds, dimethyl trisulfide (9) and 3-furaldehyde (9), in chicken breast (TR).
Table 1.
The identified aroma compounds and their odor descriptors in roasted chicken using GC–MS/O.
| NO. | compound | LRI | FD | Odor descriptors | Identification | |||
|---|---|---|---|---|---|---|---|---|
| DB-wax | DB-5 | TP | TR | TT | ||||
| 1 | hexanal | 1024 | 650 | 3 | 27 | 27 | grass, fat | MSa, RI b, O c, S d |
| 2 | 2,5-dimethyltetrahydrofuran | 1093 | – | 9 | / | / | sweet, barbecue | MS, RI, O, S |
| 3 | heptanal | 1183 | 902 | / | / | 3 | fat fragrance, rancid smell | MS, RI, O, S |
| 4 | eucalyptol | 1222 | 1034 | 81 | 3 | 3 | mint, sweet | MS, RI, O, S |
| 5 | octanal | 1287 | 1002 | / | / | 3 | green grassy, lemon | MS, RI, O, S |
| 6 | 2,5-dimethylpyrazine | 1316 | 925 | 81 | / | 3 | roasted, popcorn | MS, RI, O, S |
| 7 | ethylpyrazine | 1325 | 924 | 9 | / | / | nutty | MS, RI, O, S |
| 8 | 2-pentylfuran | 1346 | 988 | 81 | 27 | 81 | floral, fruity, earthy | MS, RI, O, S |
| 9 | dimethyl trisulfide | 1354 | 505 | / | 9 | / | areca, sulfur | MS, RI, O, S |
| 10 | 2-ethyl-6-methylpyrazine | 1363 | 999 | 3 | / | / | roasted,earthy | MS, RI, O, S |
| 11 | 2-ethyl-5-methylpyrazine | 1383 | 1002 | 27 | / | 3 | green,grass,hot | MS, RI, O, S |
| 12 | 1-octen-3-ol | 1394 | 982 | / | 9 | 243 | mushroom | MS, RI, O, S |
| 13 | (E)-2-octenal | 1425 | 1426 | / | 243 | 243 | caramel, nutty, smoky | MS, RI, O, S |
| 14 | 3-ethyl-2,5-dimethylpyrazine | 1439 | 1100 | 81 | / | / | nutty, toasty | MS, RI, O, S |
| 15 | 2,6-diethylpyrazine | 1463 | 925 | 3 | / | / | roasted, popcorn | MS, RI, O, S |
| 16 | unknown1 | 1469 | – | 27 | 9 | 243 | fennel | O |
| 17 | unknown2 | 1505 | – | / | / | 27 | woody | O |
| 18 | benzaldehyde | 1538 | 944 | 243 | 9 | 81 | fragrance, sweet | MS, RI, O, S |
| 19 | unknow3 | 1545 | – | / | 81 | 27 | mint, sweet | O |
| 20 | nonanal | 1574 | 1107 | 1 | 3 | 1 | lemon | MS, RI, O, S |
| 21 | (Z)-2-octen-1-ol | 1605 | 1067 | / | 3 | 9 | cheese | MS, RI, O, S |
| 22 | carvone | 1657 | 1242 | 243 | 27 | / | mint, basil, fennel | MS, RI, O, S |
| 23 | methyleugenol | 1659 | 1401 | 729 | 27 | 81 | burned, woody | MS, RI, O, S |
| 24 | anethole | 1691 | 1282 | 729 | 81 | 243 | licorice, fennel | MS, RI, O, S |
| 25 | unknow4 | 1698 | – | 27 | 9 | / | woody | O |
| 26 | (E, E) −2,4-decadienal | 1710 | 1295 | / | 81 | 81 | fried, fat, cheesy | MS, RI, O, S |
| 27 | unknow5 | 1738 | – | / | 9 | 243 | fennel | MS, RI, O, S |
| 28 | apiol | 1773 | 1642 | 9 | 81 | 81 | liquorice | MS, RI, O, S |
| 29 | trimethylpyrazine | 1796 | 999 | 9 | / | / | burnt, nutty | MS, RI, O, S |
| 30 | benzeneacetaldehyde | 1857 | 1043 | / | 27 | 27 | floral, musk, rose | MS, RI, O, S |
| 31 | n-caproic acid vinyl ester | 1905 | 993 | / | / | 9 | fruity | MS, RI, O, S |
| 32 | (Z)-3-phenylacrylaldehyde | 2052 | 1273 | 27 | 27 | 81 | honey, cinnamon | MS, RI, O, S |
| 33 | unknow6 | 2217 | – | 243 | 243 | / | spicy, potato chips | MS, RI, O, S |
| 34 | p-cresol | 2348 | 1052 | / | / | 9 | drugs, phenol, smoke | MS, RI, O, S |
| 35 | unknow7 | 2622 | – | / | / | 27 | meaty | O |
| 36 | methanethiol | – | – | 3 | 3 | 9 | cabbage | MS, O, S |
| 37 | pentanal | – | – | / | 3 | 3 | malty | MS, O, S |
| 38 | 1-hydroxy-2-propanone | – | – | 27 | 9 | 27 | butter | MS, O, S |
| 39 | ethylacetate | – | – | 1 | 3 | / | floral, fruity | MS, O, S |
| 40 | 4-hydroxy-5-methyl-3(2H)-furanone | – | – | 81 | 9 | 3 | caramel, sweet, burning smell | MS, O, S |
| 41 | 2,3-dimethyl-5-ethylpyrazine | – | – | 81 | / | / | nutty, toasty | MS, O, S |
| 42 | methylpyrazine | – | – | 243 | / | / | nutty, toasty | MS, O, S |
| 43 | 3-furaldehyde | – | – | / | 9 | / | bread, caramel, baked | MS, O, S |
| 44 | 2(5H)-furanone | – | – | 81 | / | / | rice, burnt | MS, O, S |
| 45 | unknow8 | – | – | / | / | 81 | fatty | O |
| 46 | unknow9 | – | – | / | / | 81 | caramel, smoky | O |
| 47 | 2-furanmethanol | – | – | 9 | / | / | almond, sweet | MS, O, S |
MS mass spectrum.
RI retention index, calculated based on n-alkanes (C7-C40).
O olfactory confirmation, d S authentic aroma standards.
3.3. Quantitation of the key odorants and calculation of odor Activity values (OAVs)
The significance of odorants in roasted chicken was not only confirmed by the AEDA/GC-O experiment examines, but also odor activity values (OAVs) (Fan et al., 2018). Moreover, to verify the contribution of each aroma compound to the odor profiles in roasted chicken, the OAVs of selected odorants were calculated by dividing the contents by the OT (aroma threshold) measured in water from published references (Sohail et al., 2022). The standard curves for quantification of total twenty-seven compounds were listed (Table 2). In order to ensure the accuracy of quantification, the three ion fragments (m/z) of each odorant were selected by using the authentic flavor standards under the same program of GC–MS. Table 2 reported only the odorants of soil oven-roasted chicken (TRC), whose OAVs were greater than or equal to 1 (OAVs ≥ 1). The results of the GC-O experiments screened out 20 aroma compounds in the skin of the roasted chicken, 14 compounds in the breast, and 16 compounds in the thighs, for which the OAVs were greater than or equal to 1. The type of aroma compounds with a higher contribution rate was aldehydes. The highest OAV value was anethole. From the analysis of the production process of roasted chicken, there was a pickling process before roasting, in which the spices were transmitted to the interior of chicken through chicken skin, so the content of anethole in the skin of chicken was the highest. As carvone is oil-soluble, it can easily pass through the lipid bilayer of the cell membrane (Lowry et al., 2023). Moreover, the interaction between spices and chicken meat is significant because it facilitates chemical and physical reactions between the spices and the surface of the chicken meat, further enabling the penetration of carvone during the marination process. Additionally, the marination process typically involves heat and pressure steps, which aid in better absorption of carvone into the chicken.
In the three parts of soil oven-roasted chicken, the concentrations of anethole (46234.75 ng/g in skin, 28765.11 ng/g in breast, 27647.99 ng/g in thigh, No.1) were highest. Furthermore, Carvone (729.92 ng/g in skin, 131.50 ng/g in breast, 134.22 ng/g in thigh), Eucalyptol (656.49 ng/g in skin, 123.00 ng/g in breast, 183.71 ng/g in thigh), and methyl eugenol (686.30 ng/g in skin, 268.18 ng/g in breast, 158.59 ng/g in thigh) were detected, whose OAVs ranges from 20 to 963. The chicken was roasted after being marinated with a blend of cardamom, galangal, clove, and peppercorns. This marinade added a unique aroma that was both sweet and herbaceous, with hints of clove and anise. The marinade also helped to mask any unpleasant odors (Aprotosoaie et al., 2016, Huang et al., 2021). Moreover, Spices played an important role in the retention of aroma compounds in meat products. The concentrations of spices flavor compounds in the skin were higher than in the other two roasted chicken parts, possibly because the skin was exposed to more spices during the curing process.
Pyrazine compounds were one major class of heterocycles,(Shen et al., 2019) and formed by condensing two α-amino-carbonyls molecules, such as amino acids or fragments of amino sugars (Maillard reaction) (Wang et al., 2022, Resconi et al., 2013).A total of 11 pyrazines, such as 3-ethyl-2,5-dimethyl pyrazine, 2,5-dimethyln pyrazine, 2-ethyl-5-methyl pyrazine, were detected in roasted chicken. Those aroma compounds were identified as emanating roasty, popcorn-like, nutty-like odors by GC-O, and Liu and Fang et al. obtained a similar conclusion (Liu and Fang, 2022). And they all have high OAVs (OAVs range from 1 to 2781). Pyrazine was a characteristic aroma compound derived from the Maillard reaction, which was present generally in high-temperature processed foods and provided the baked-like, roasty, and nutty odors thermally (Mortzfeld et al., 2020). The pyrazine content in the skin of roasted chicken was significantly higher than that in the breast and thighs. In addition, by comparing the types of pyrazine compounds detected in three kinds of roasted chickens (TRC, GRC, DRC), the types and contents of pyrazine compounds in TRC were higher than those in the other two kinds of roasted chickens. The surface temperature of the roasted chicken was higher than the internal temperature during the roasting process. This elevated temperature environment effectively promotes the caramelization process, resulting in the browning and flavor development of the chicken skin. Consequently, the roasted chicken gains a more desirable flavor and appealing color. Similarly, the Maillard reaction is accelerated due to the higher surface temperature of the chicken skin. This chemical reaction occurs between amino acids and reducing sugars, leading to the formation of browning and the development of new flavors. In addition, roasty odors could not be generated when the microwaves was at temperatures below 200 °C (Yeo and Shibamoto, 1991).
4-Hydroxy-5-methyl-3-(2H)-furanone (HMFO), mainly caramel (Tonsbeek et al., 1968), sweet, burnt odor, also contributed significantly to the aroma of roasted chicken (OAV: 12.93 in the chicken breast, 24.63 in the chicken thighs). Furanone, a product of the Maillard reaction, often serves as a vital natural aromatic compound. HMFO primarily arises from the Maillard reaction between xylose and lysine. Moreover, it can be extracted not only from xylose but also from arabinose and ribose. Additionally, it's important to note that dicarbonyl compounds are recognized as the principal precursors for the formation of polymers derived from HMFO (Mikami et al., 2017). In addition to its role in roasted chicken aroma, HMFO has also been identified as a key component contributing to the development of beef flavor, imparting a distinct caramel-like character to various meat products (Tonsbeek et al., 1968). The presence of HMFO adds complexity and richness to the overall sensory profile, enhancing the perception of desirable flavors in meat products. Further research in this area could focus on exploring the influence of HMFO on other food matrices and its potential application in flavor modulation.
3.4. Determination of the important odorants in roasted chicken on the basis of recombination and omission tests
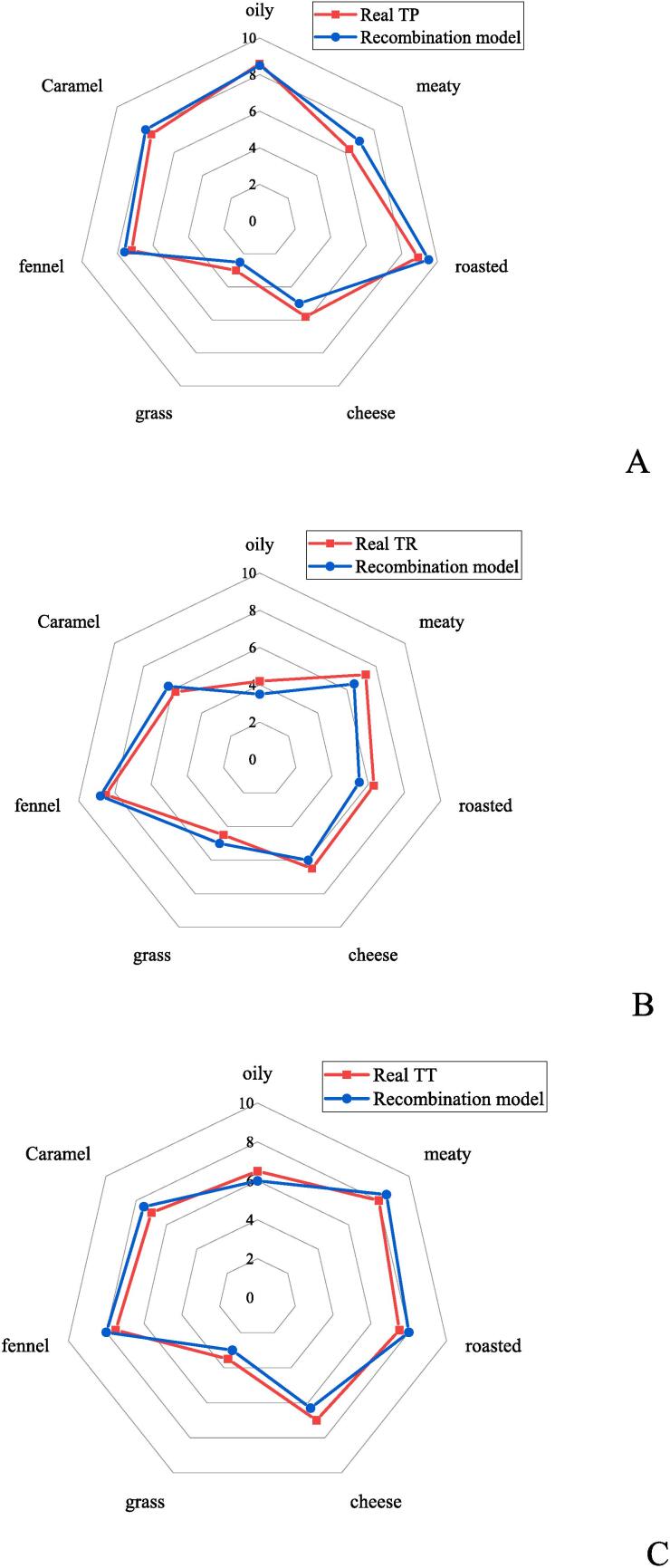
To identify the key aroma compounds of roasted chicken, aroma recombination experiments were conducted with twenty-five aroma compounds, selected from the quantitative results, which had OAVs greater than 1 at their original concentrations (Table 3). The above results screened out 17 aroma compounds in skin of the roasted chicken, 14 compounds in the breast, and 20 compounds in the thighs to conduct aroma recombination experiments. Fig. 3 exhibited the radar charts for differential analysis of the three recombined aroma models and the TP (skin of soil-oven roasted chicken), TR (breast of soil-oven roasted chicken), and TT (thigh of soil-oven roasted chicken) original models. As shown in Fig. 3, those recombinant models have a high degree of overlap with the original models for each odor, such as oily, meaty, roasty, cheese, grassy, fennel, and caramel-like.
Table 3.
Omission experiments for the roasted chicken models.
| No. | Omitted compounds | Correct number | Sig. | ||||
|---|---|---|---|---|---|---|---|
| TP | TR | TT | TP | TR | TT | ||
| 1 | methanethiol | 10/12 | 10/12 | 8/12 | / | * | * |
| 2 | hexanal | 8/12 | ND | 8/12 | * | ** | ** |
| 3 | 2,5-dimethyltetrahydrofuran | 6/12 | 2/12 | ND | ** | ND | ND |
| 4 | eucalyptol | 7/12 | 2/12 | 10/12 | * | / | / |
| 5 | 2,5-dimethylpyrazine | 3/12 | ND | 2/12 | *** | ND | *** |
| 6 | 2-pentylfuran | 8/12 | 2/12 | 4/12 | * | *** | *** |
| 7 | dimethyl trisulfide | ND | 11/12 | ND | ND | *** | ND |
| 8 | 2-ethyl-5-methylpyrazine | 8/12 | ND | 6/12 | * | ND | ** |
| 9 | 1-octen-3-ol | ND | 10/12 | 1/12 | ND | *** | *** |
| 10 | (E)-2-octenal | ND | 8/12 | 9/12 | ND | / | * |
| 11 | 3-ethyl-2,5-dimethylpyrazine | 3/12 | 8/12 | ND | *** | ND | ND |
| 12 | (Z)-2-octen-1-ol | ND | 5/12 | 10/12 | ND | / | / |
| 13 | carvone | 8/12 | ND | 8/12 | * | * | * |
| 14 | methyleugenol | 9/12 | ND | 8/12 | / | * | / |
| 15 | anethole | 6/12 | 12/12 | 5/12 | ** | ** | ** |
| 16 | (E, E) −2,4-decadienal | ND | 7/12 | 2/12 | ND | ND | *** |
| 17 | trimethylpyrazine | 8/12 | ND | ND | * | ND | ND |
| 18 | benzeneacetaldehyde | ND | 6/12 | 11/12 | ND | / | / |
| 19 | (Z)-3-phenylacrylaldehyde | ND | ND | 7/12 | ND | * | * |
| 20 | p-cresol | ND | ND | 10/12 | ND | ND | / |
| 21 | 4-hydroxy-5-methyl-3(2H)-furanone | 3/12 | ND | 5/12 | *** | ** | ** |
| 22 | 2,3-dimethyl-5-ethylpyrazine | 2/12 | ND | 6/12 | *** | ND | ** |
| 23 | methylpyrazine | 10/12 | 10/12 | 10/12 | / | ND | / |
| 24 | 2(5H)-furanone | 6/12 | ND | ND | ** | ND | ND |
| 25 | 3-furaldehyde | 10/12 | 2/12 | 10/12 | / | ND | / |
*, 5% significance level.
**, 1% significance level.
***, 0.1% significance level.
Fig. 3.
Odor profiles of the recombination skin of roasted chicken (TP) model and the real TP obtained using QDA (A); odor profiles of recombination breast of roasted chicken (TR) model and the real TR obtained using QDA (B); odor profiles of recombination thigh of roasted chicken (TT) model and the real TT obtained using QDA (C).
There were 17 groups, in which respectively one odorant was omitted from the TP sample (Table 3). As the Table 3 exhibited, thirteen odorants had a remarkable influence on the aroma profile of the recombination TP model. Particularly, hexanal, eucalyptol, 2-pentyl furan, 2-ethyl-5-methyl pyrazine, trimethyl pyrazine, and carvone considerably effected the TP odor (* p < 0.05). Besides, anethole, 2(5H)-furanone, and 2,5-dimethyl tetrahydrofuran had a significant impact on the TP odor (** p < 0.01). And 2,3-dimethyl-5-ethyl pyrazine, 4-hydroxy-5-methyl-3(2H)-furanone, 2,5-dimethyl pyrazine and 3-ethyl-2,5-dimethyl pyrazine also affected the TP odor (*** p < 0.001). The sensory panelists evaluated the intensity of various odorants, including oily, meaty, roasted, cheese, grassy, fennel, and caramel aromas. The resulting odor profiles are visually depicted in Fig. 3. Moreover, Fig. 3-A illustrates that the aroma profile of the recombination model exhibited a remarkable similarity of 92 % with the target product (TP) concerning odor attributes, with noticeable distinctions noted in the cheese and grassy notes (Fig. 3-A).
The TR model, including 14 compounds, was conducted with the same methods as the TP model (Table 3). As the results revealed, ten key odorants contributed observably to the overall odor notes for the recombination of TR (Table 3). Eucalyptol, (E)-2-octenal, (Z)-2-octen-1-ol, and benzeneacetaldehyde had no effect on TR odor. In addition to these aroma compounds, other compounds significantly influenced on TR odor. Furthermore, 2-pentylfuran, dimethyl trisulfide, and 1-octen-3-ol have the most significant effect (*** p<0.001) on the flavor of TR. Furthermore, there was a high degree of similarity (90 %) in odor properties between the recombination model and the TR sample. The main disparity was on the grassy and oily odors (Fig. 3-B).
Meanwhile, from the omitted TT model, 20 groups were contained. In the results of TT recombination, apart from eucalyptol, (Z)-2-octen-1-ol, benzeneacetaldehyde, methyleugenol, p-cresol, methylpyrazine, and 3-furaldehyde, 13 aroma compounds played a major role in the overall odor properties (Table 3). Notably, 2,5-dimethyl pyrazine, 2-pentyl furan, 1-octen-3-ol, and (E, E)-2,4-decadienal significantly affected the raw TT odor (* p < 0.05). And hexanal, 2-ethyl-5-methylpyrazine, anethole, 4-hydroxy-5-methyl-3(2H)-furanone and 2,3-dimethyl-5-ethyl pyrazine had main influences on the TT model of the overall odor property (** p < 0.01). Moreover, methanethiol, (E)-2-octenal, carvone and (Z)-3-phenylacrylaldehyde majorly affected the TT model (*** p < 0.001). Fig. 3-C showed that the TT recombination model had a high degree of similarity (91 %) with TT in odor property, and the significant distinction between the two was on the grassy odor.
Through a systematic elimination process, it was determined that the thigh contained 13 important aroma compounds, the breast had 10, and the chicken skin had 13. Interestingly, five essential aroma compounds were found to be common to all three parts of the roasted chicken: hexanal, 2-pentyl furan, anethole, 4-hydroxy-5-methyl-3(2H)-furanone, and carvone (Table 3). Upon comparing the TP sample with the TR and TT samples, specific key compounds unique to TP included 3-ethyl-2,5-dimethyl pyrazine, 2,5-dimethyl tetrahydrofuran, trimethyl pyrazine, 2(5H)-furanone, and eucalyptol. Notably, dimethyl trisulfide, which was exclusively detected in the TR sample, was identified as the critical aroma compound distinguishing TR, and it also plays a key role in the aroma of Beijing roasted duck (Liu et al., 2019). (E)-2-octenal and (E, E) −2,4-decadienal were found to be the distinct key compounds in the TT sample. Additionally, it's noteworthy that the chicken breast sample exhibited higher levels of organic acids compared to the thigh sample (Wang et al., 2018).
To replicate the aroma of roasted chicken, it may be essential to create aroma profiles by blending by-products in suitable proportions or combining volatile flavor extracts from various by-products (Wettasinghe et al., 2000). Consequently, we have identified the key aroma compounds in 20 different types of roasted chicken, as outlined in Table 3.
4. Conclusions
Overall, a total of twenty aroma compounds of roasted chicken significantly were identified by recombination and omission experiments. Hexanal and (E, E)-2,4-decadienal are the most abundant aldehydes identified in chicken flavor. 1-octen-3-ol was considered the key compound of breast and thighs of roasted chicken exhaling like-mushroom odor. Besides, sulfur-containing compounds, methanethiol, and dimethyl trisulfide, also were the key compounds, but they were not detected in the skin of roasted chicken. Notably, pyrazines and furanone, 2,5-dimethyltetrahydrofuran, 4-hydroxy-5-methyl-3(2H)-furanone, 2(5H)-furanone, 3-ethyl-2,5-dimethyl pyrazine, 2,5-dimethyl pyrazine, 2-ethyl-5-methyl pyrazine, 2,3-dimethyl-5-ethyl pyrazine, and trimethyl pyrazine, had the most significant effect on roasted chicken odor. This finding laid the foundation for the industrial production and keeping the aroma compounds of roasted chicken.
CRediT authorship contribution statement
Ruotong Nie: Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. Chunjiang Zhang: Investigation, Software, Visualization. Huan Liu: Formal analysis, Investigation, Resources. Xiangru Wei: Formal analysis, Resources. Rongmei Gao: Writing – review & editing. Haonan Shi: Methodology, Writing – review & editing. Dequan Zhang: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. Zhenyu Wang: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study has been financially supported by National Key R&D Program of China (2021YFD2100104), Beijing Innovation Consortium of Agriculture Research System (BAIC06-2023-BJJQ-G12), and National Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2023-IFST).
Data availability
Data will be made available on request.
References
- Aprotosoaie A.C., Irina-Iuliana C., Anca M. Anethole and its role in chronic diseases. Drug Discovery from Mother Nature. 2016;929:247–267. doi: 10.1007/978-3-319-41342-6_11. [DOI] [PubMed] [Google Scholar]
- Bassam S.M., Noleto-Dias C., Farag M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chemistry. 2021;371 doi: 10.1016/j.foodchem.2021.131139. [DOI] [PubMed] [Google Scholar]
- Bi S., Xu X., Luo D., Lao F., Pang X., Shen Q., Hu X., Wu J. Characterization of key aroma compounds in raw and roasted peas (Pisum sativum L.) by application of instrumental and sensory techniques. Journal of Agricultural and Food Chemistry. 2020;68(9):2718–2727. doi: 10.1021/acs.jafc.9b07711. [DOI] [PubMed] [Google Scholar]
- Bleicher J., Elmar E.E., Kathrine H.B. Formation and analysis of volatile and odor compounds in meat—a review. Molecules. 2022;27(19):6703. doi: 10.3390/molecules27196703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dach A., Schieberle P. Characterization of the key aroma compounds in a freshly prepared oat (Avena sativa L.) pastry by application of the sensomics approach. Journal of Agricultural and Food Chemistry. 2021;69(5):1578–1588. doi: 10.1021/acs.jafc.0c07498. [DOI] [PubMed] [Google Scholar]
- Dein M., Munafo J.P. Characterization of odorants in southern mountain mint, pycnanthemum pycnanthemoides. Journal of Agricultural and Food Chemistry. 2022;70:9722–9729. doi: 10.1021/acs.jafc.2c02860. [DOI] [PubMed] [Google Scholar]
- Fan M., Xiao Q., Xie J., Cheng J.B.G., Sun B., Du W., Wang Y., Wang T. Aroma compounds in chicken broths of beijing youji and commercial broilers. Journal of Agricultural and Food Chemistry. 2018;66(39):10242–10251. doi: 10.1021/acs.jafc.8b03297. [DOI] [PubMed] [Google Scholar]
- Feng Y., Cai Y., Fu X., Zheng L., Xiao Z., Zhao M. Comparison of aroma-active compounds in broiler broth and native chicken broth by aroma extract dilution analysis (AEDA), odor activity value (OAV) and omission experiment. Food Chemistry. 2018;265:274–280. doi: 10.1016/j.foodchem.2018.05.043. [DOI] [PubMed] [Google Scholar]
- Gasior R., Wojtycza K., Majcher M.A., Bielinska H., Odrzywolska A., Baczkowicz M., Migdal W. Key aroma compounds in roasted white koluda goose. Journal of Agricultural and Food Chemistry. 2021;69(21):5986–5996. doi: 10.1021/acs.jafc.1c01475. [DOI] [PubMed] [Google Scholar]
- Gorman C., Murray A.F., Dein M., Munafo J.P. Characterization of key odorants in cumberland rosemary, conradina verticillata. Journal of Agricultural and Food Chemistry. 2022;70(40):12916–12924. doi: 10.1021/acs.jafc.2c04872. [DOI] [PubMed] [Google Scholar]
- Hashim I.B., McWatters K.H., Hung Y. Marination method and honey level affect physical and sensory characteristics of roasted chicken. Journal of Food Science. 1999;64(1):163–166. [Google Scholar]
- Hsu K.Y., Chen B.H. Analysis and reduction of heterocyclic amines and cholesterol oxidation products in chicken by controlling flavorings and roasting condition. Food Research International. 2020;131 doi: 10.1016/j.foodres.2020.109004. [DOI] [PubMed] [Google Scholar]
- Huang L., Ho C., Wang Y. Biosynthetic pathways and metabolic engineering of spice flavors. Critical Reviews in Food Science and Nutrition. 2021;61(12):2047–2060. doi: 10.1080/10408398.2020.1769547. [DOI] [PubMed] [Google Scholar]
- Jayasena D., Ahn D., Nam K., Jo C. Flavour chemistry of chicken meat: a review. Asian-Australasian Journal of Animal Sciences. 2013;26(5):732–742. doi: 10.5713/ajas.2012.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas M., Schieberle P. Characterization of the key aroma compounds in fresh leaves of garden sage (Salvia officinalis L.) by means of the sensomics approach: influence of drying and storage and comparison with commercial dried sage. Journal of Agricultural and Food Chemistry. 2021;69(17):5113–5124. doi: 10.1021/acs.jafc.1c01275. [DOI] [PubMed] [Google Scholar]
- Liu H., Fang M. Characterization of aroma active volatile components in roasted mullet roe. Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132736. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang Z., Zhang D., Shen Q., Pan T., Hui T., Ma J. Characterization of key aroma compounds in beijing roasted duck by gas chromatography-olfactometry-mass spectrometry, odor-activity values, and aroma-recombination experiments. Journal of Agricultural and Food Chemistry. 2019;67:5847–5856. doi: 10.1021/acs.jafc.9b01564. [DOI] [PubMed] [Google Scholar]
- Lowry T.W., Kusi-Appiah A.E., Fadool D.A., Lenhert S. Odor discrimination by lipid membranes. Membranes. 2023;13(2):151. doi: 10.3390/membranes13020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majcher M., Jelen H. Comparison of suitability of SPME, SAFE and SDE methods for isolation of flavor compounds from extruded potato snacks. Journal of Food Composition and Analysis. 2009;22(6):606–612. [Google Scholar]
- Mikami Y., Nakamura M., Yamada S., Murata M. 4-hydroxy-5-methyl-3(2H)-furanone (HMFO) contributes to browning in the xylose-lysine maillard reaction system. Food Science and Technology Research. 2017;23(2):283–289. [Google Scholar]
- Mortzfeld F.B., Hashem C., Vranková K., Winkler M., Rudroff F. Pyrazines: synthesis and industrial application of these valuable flavor and fragrance compounds. Biotechnology Journal. 2020;15(11) [Google Scholar]
- Mottram D.S. Flavour formation in meat and meat products: a review. Food Chemstry. 1998;62(4):415–424. [Google Scholar]
- Neugebauer A., Schieberle P., Granvogl M. Characterization of the key odorants causing the musty and fusty/muddy sediment off-flavors in olive oils. Journal of Agricultural and Food Chemistry. 2021;69(49):14878–14892. doi: 10.1021/acs.jafc.1c02228. [DOI] [PubMed] [Google Scholar]
- Brunton N.P., Cronin D.A., Monahan F.J., Durcan R. A comparison of solid-phase microextraction (SPME) for measurement of hexanal and pentanal in cooked turkey. Food Chemistry. 2000;68(3):339–345. [Google Scholar]
- Niu Y., Wu M., Xiao Z.B., Chen F., Zhu J., Zhu G. Effect of fatty acids profile with thermal oxidation of chicken fat on characteristic aroma of chicken flavors assessed by gas chromatography mass spectrometry and descriptive sensory analysis. Food Science and Technology Research. 2016;22(2):245–254. [Google Scholar]
- Resconi V.C., Escudero A., Campo M.M. The development of aromas in ruminant meat. Molecules. 2013;18(6):6748–6781. doi: 10.3390/molecules18066748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhir S.T., Amanpour A., Selli S. Characterization of ayran aroma active compounds by solvent-assisted flavor evaporation (SAFE) with gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and aroma extract dilution analysis (AEDA) Analytical Letters. 2019;52(13):2077–2091. [Google Scholar]
- Shen H., Zhao M., Sun W. Effect of pH on the interaction of porcine myofibrillar proteins with pyrazine compounds. Food Chemistry. 2019;287:93–99. doi: 10.1016/j.foodchem.2019.02.060. [DOI] [PubMed] [Google Scholar]
- Shi J., Tong G., Yang Q., Huang M., Ye H., Liu Y., Wu J., Zhang J., Sun X., Zhao D. Characterization of key aroma compounds in tartary buckwheat (Fagopyrum tataricum Gaertn.) by means of sensory-directed flavor analysis. Journal of Agricultural and Food Chemistry. 2021;69(38):11361–11371. doi: 10.1021/acs.jafc.1c03708. [DOI] [PubMed] [Google Scholar]
- Sohail A., Al-Dalali S., Wang J., Xie J., Shakoor A., Asimi S., Shah H., Patil P. Aroma compounds identified in cooked meat: a review. Food Research International. 2022;157 doi: 10.1016/j.foodres.2022.111385. [DOI] [PubMed] [Google Scholar]
- Taskiran M., Olum E., Candogan K. Changes in chicken meat proteins during microwave and electric oven cooking. Journal of Food Processing and Preservation. 2020;44(2) [Google Scholar]
- Tonsbeek C.H.T., Plancken A.J., Weerdhof T.V.D. Components contributing to beef flavor. Isolation of 4-hydroxy-5-methyl-3(2H)-furanone and its 2,5-dimethyl homolog from beef broth. Journal of Agricultural and Food Chemistry. 1968;16(6):1016–1021. [Google Scholar]
- Wang L.-H., Qiao K.-N., Ding Q., Zhang Y.-Y., Sun B.-G., Chen H.-T. Effects of two cooking methods on the taste components of Sanhuang chicken and Black-bone silky fowl meat. Journal of Food Processing and Preservation. 2018;42(11) [Google Scholar]
- Wang Z., Cui H., Ma M., Hayat K., Zhang X., Ho C.-T. Controlled formation of pyrazines: inhibition by ellagic acid interaction with N-(1-Deoxy-d-xylulos-1-yl)-glycine and promotion through ellagic acid oxidation. Journal of Agricultural and Food Chemistry. 2022;70(5):1618–1628. doi: 10.1021/acs.jafc.1c07391. [DOI] [PubMed] [Google Scholar]
- Warner S., Munafo J.P. Characterization of key odorants in chardonnay seeds. Journal of Agricultural and Food Chemistry. 2022;70(51):16316–16322. doi: 10.1021/acs.jafc.2c06119. [DOI] [PubMed] [Google Scholar]
- Wettasinghe M., Vasanthan T., Temelli F., Swallow K. Volatiles from roasted byproducts of the poultry-processing industry. Journal of Agricultural and Food Chemistry. 2000;48(8):3485–3492. doi: 10.1021/jf000122a. [DOI] [PubMed] [Google Scholar]
- Yeo H.C.H., Shibamoto T. Chemical comparison of flavours in microwaved and conventionally heated foods. Trends in Food Science & Technology. 1991;2(C):329–332. [Google Scholar]
- Yu Y., Wang G., Yin X., Ge C., Liao G. Effects of different cooking methods on free fatty acid profile, water-soluble compounds and flavor compounds in Chinese Piao chicken meat. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110696. [DOI] [PubMed] [Google Scholar]
- Zhai X., Granvogl M. Key Odor-Active Compounds in Raw Green and Red Toona sinensis (A. Juss.) Roem. and Their Changes during Blanching. Journal of Agricultural and Food Chemistry. 2020;68(27):7169–7183. doi: 10.1021/acs.jafc.0c02012. [DOI] [PubMed] [Google Scholar]
- Zhang L., Badar I.H., Chen Q., Xia X., Liu Q., Kong B. Changes in flavor, heterocyclic aromatic amines, and quality characteristics of roasted chicken drumsticks at different processing stages. Food Control. 2022;139 [Google Scholar]
- Zhang Z.Y., Blank I., Wang B., Cao Y. Changes in odorants and flavor profile of heat-processed beef flavor during storage. Journal of Food Science. 2022;87(12):5208–5224. doi: 10.1111/1750-3841.16363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.