Abstract
Background
Tumor immunity plays an important role in assessing the tumor progression. The purpose of this study was to investigate the prognostic value of combined systemic inflammation response index (SIRI) and platelet–lymphocyte ratio (PLR) of gastroesophageal junction cancer (AEG) and upper gastric cancer (UGC) patients.
Methods
In this retrospective study, patients from 2003 to 2014 were divided into training and validation sets. The prognostic accuracy of each variable was compared using time-independent ROC analysis. The scoring system was calculated by cut-off values of SIRI and PLR in 5-year. Kaplan-Meier and Log-rank tests were used to analyze overall survival (OS). Chi-square test was used to analyze the association between clinical characteristics and the scoring system. Univariate and multivariate analyses based on the competitive risk regression model were used to analyze independent predictors of death due to AGC and UGC. R software was used to construct the Nomogram model of risk assessment.
Results
Patients with SIRI–PLR = 2 had worse survival time than those with 0 and 1 (P < 0.001) and more suitable for postoperative adjuvant chemotherapy (P = 0.002). High PLR patients were more suitable for proximal gastrectomy (P = 0.049). SIRI-PLR were independent predictors in training set (P < 0.001), which could be combined with age, pTNM stage and postoperative chemotherapy to construct Nomogram for predicting OS.
Conclusions
Preoperative SIRI–PLR score was an independent predictor for patients with AEG and UGC. The Nomogram model constructed by age, SIRI-PLR, pTNM stage and postoperative chemotherapy can correctly predict the prognosis of patients.
Keywords: Gastric cancer, Adenocarcinoma of esophagogastric junction, Upper gastric cancer, Systemic infammation response index, Platelet–lymphocyte ratio, Prognosis
1. Introduction
Gastric cancer (GC) is still the third leading cause of cancer mortality worldwide [1]. The incidence of gastroesophageal junction cancer (AEG) and upper gastric cancer (UGC) has increased annually, especially in Japan, where it has increased from 2.3% to 10% in the past 40 years [2]. For AEG, according to Siewert type, >66% of Siewert type II and 90% of Siewert type III patients are mainly treated by radical gastrectomy [[3], [4], [5], [6]]. AEG and UGC have the clinical features of strong invasion, poor prognosis, advanced clinical stage and high rate of postoperative recurrence [7,8]. Regarding the surgical method, there is debate about total gastrectomy (TG) and proximal gastrectomy (PG). Golematis et al. [9] found that TG can ensure a sufficient distal margin and extended lymph node dissection, which can brings survival benefit. However, Harrison et al. found that patients who underwent TG and PG had no difference in overall survival (OS) rate, although PG led to better postoperative nutritional status [10]. Therefore, it is important to find suitable clinical prognostic factors to help surgeons choose a suitable method of gastrectomy as well as evaluate prognosis after surgery.
Researchers have proposed use of the American Joint Committee on Cancer (AJCC) eighth edition for treatment guidelines for AEG. If the tumor invades the gastroesophageal junction and its center is located beyond 2 cm below the gastroesophageal junction, or if the tumor center is within 2 cm below the gastroesophageal junction but does not invade the gastroesophageal junction, it should be classified according to the gastric cancer stage [11]. We found that the current clinical guidelines are only based on assessment of the tumor's general progression and do not consider the immune response caused by tumor cells. In 2014, Galon et al. [12] first proposed pTNM-I, which combines the immune response in the tumor microenvironment and pTNM stage. In 2018 [13], pTNM-I staging was used to guide postoperative chemotherapy in patients with colon cancer. However, the high degree of heterogeneity makes it difficult for pathologists to assess the immune status of patients individually. Reichert et al. [14] found that changes in the peripheral immune microenvironment of the tumor can be reflected by circulating immune-related cells such as neutrophils (N), platelets (P), monocytes (M) and lymphocytes (L). Systemic immune inflammation index (SII) [[15], [16], [17]], neutrophil–lymphocyte ratio (NLR) [18,19], platelet–lymphocyte ratio (PLR) [20], lymphocyte–monocyte ratio (LMR) [21], and scoring systems that combine with inflammation index, such as C-reactive protein (CRP)–NLR [22], NLR–PLR [23], Fibrinogen (F)–NLR [24] and SIRI–PLR [25] have been confirmed to evaluate accurately the prognosis of GC and other malignant tumors. Therefore, as a new comprehensive inflammatory index scoring system combining lymphocytes, neutrophils, monocytes and platelets, the systemic inflammatory response index (SIRI) and platelet-lymphocyte ratio (PLR) encompass more comprehensive clinical immune factors, which are expected to predict the prognosis of GC more accurately and even help clinical doctors choose appropriate surgical methods for AEG and UGC.
In this study, 371 patients with AEG and UGC who underwent radical surgery at the Cancer Hospital of Harbin Medical University were selected consecutively. The relationship between SIRI–PLR and pathological factors was investigated by cohort study to explore the clinical significance of SIRI–PLR scoring.
2. Materials and methods
2.1. Patients
Tumors that were mainly located in the upper third of the stomach were designated UGC. Tumors that were mainly located at 2–5 cm from the gastroesophageal junction were designated Siewert type Ⅲ GC [26], as well as AEG. This study retrospectively analyzed 371 patients underwent radical surgery at the Harbin Medical University Cancer Hospital from 2003 to 2014. Inclusion criteria were: (a) patients diagnosed with gastric adenocarcinoma by experienced pathologists, (b) tumors mainly located in the upper third of stomach or 2–5 cm from the gastroesophageal junction, (c) patients without neoadjuvant chemotherapy, (d) complete follow-up records (including missed visits and end-point event occurrence) and (e) complete clinical and pathological data. Exclusion criteria were: (a) history of blood transfusion in the last 2 months, (b) thyroid diseases, (c) intravascular coagulation, (d) history of heparin treatment in the last 1 month, (e) connective tissue diseases and (f) active bleeding.
Total patients were divided into two independent cohorts according to admission time, 194 patients from 2003 to 2010 were the training set, and 177 patients from 2011 to 2014 were the validation set. Over the all known died patients, 186 patients died due to GC and 21 patients (11 patients in the training set, 10 patients in the validation set) died from other causes (7 deaths because of heart disease, 4 patients died naturally, 2 patients died from accidents and 8 deaths from unknown causes).
Operation methods and postoperative chemotherapy standards are based on The Japanese gastric cancer treatment guidelines [27]. The range of gastrectomy is determined according to the patient's clinical stage and tumor location. TG is for clinically node-positive (cN+) or T2-T4a. PG is for the patients with acceptable proximal resection margin (>5 cm or frozen section examination of the resection line is desirable). The invasion of the pancreas by tumors requiring pancreaticosplenectomy must also be performed by TG, regardless of the location of the tumor and distance of the surgical margin. TG includes all gastric tissues, including pylorus and cardia, combined with D2 lymph node dissection range (No. 1–7 and No. 8a, 9, 10, 11p, 11d, 12a). Digestive tract reconstruction methods are Roux-en-Y esophagojejunostomy, Jejunal interposition and Double tract. PG involves proximal 2/3 of the gastric tissues, with gastroesophageal junction and pylorus retained, in combination with the D1+ lymph node dissection area (No. 1, 2, 3a, 4sa, 4sb, 7 and No. 8a, 9, 11p, and No.110 dependent on the need for surgery), and the methods of digestive tract reconstruction include Esophagogastrostomy, Jejunal interposition and Double tract method. There were 38 patients with T4b, of which 7 patients had intragastric resection of adjacent organs (2 patients with partial liver resection, 1 with transverse colon, 2 with spleen, and 2 with pancreatic tail). In addition, oxaliplatin + capecitabine (XELOX) or oxaliplatin + S-1 (SOX) are the main treatment options for patients with postoperative pathological stages II-III, which were 336 patients in the study. In order to ensure the accuracy of this study, we included all patients in our institution with a total of 117 patients. The remaining 219 patients were not included in the postoperative chemotherapy patient group. This is because these patients did not complete all postoperative chemotherapy regimens in our institution. Most of the patients returned to the local hospital for treatment after surgery and did not have complete chemotherapy records.
Patients’ clinicopathological data were saved in the Gastric Cancer Information Management System v1.2 of Harbin Medical University Cancer Hospital (Copyright No. 2013SR087424, http://www.sgihmu.com). All patients were re-examined by checking tumor markers or radiological examination [computed tomography (CT), ultrasound and gastroscopy] every 3–6 months, and positron emission tomography-CT were performed as needed.
2.2. Inflammation index
Blood samples were collected from patients in fasting condition 1 week before surgery. Neutrophil, lymphocyte, monocyte and platelet counts were obtained by hematological examination. For inflammation index, systemic immune inflammation index (SII) = N × P/L [28], neutrophil–lymphocyte ratio (NLR) = N/L, platelet–lymphocyte ratio (PLR) = P/L and systemic inflammation response index (SIRI) = N × M/L [29] (N = Neutrophil count, L = Lymphocyte count, M = Monocyte count and P=Platelets).
2.3. Statistical analysis
Overall survival (OS) was calculated as the time from surgery to death from any cause. If patients were alive at the last follow-up, they were censored. Patient's survival time in each group were shown as mean [95% confidence interval (CI)]. We used R software version 3.6.1 and the ‘survivalROC’ package to investigate the prognostic or predictive accuracy of each variable by time-dependent receiver operating characteristic (ROC) analysis. An optimal cutoff value was defined to classify the patients into two groups (high vs low) for each variable with use of the receiver operating characteristic curve for survival in 5-year, and the maximum value of ‘Youden index (Sensitivity + Specificity - 1)’ is the best cutoff value. Delong nonparametric method was used to estimate the AUC confidence interval. Kaplan–Meier method and Log–rank test were used to analysis survival curves. Median follow up was calculated by the reverse Kaplan–Meier analysis. Yates' correction is selected for data with a theoretical frequency between 1 and 5 and a total sample size greater than or equal to 40; Chisq test is used for data with a theoretical frequency greater than or equal to 5 and a total sample size greater than or equal to 40; For data with normal distribution and homogeneity of variance, T-test was used for comparison between the two groups; For data that does not meet the normal distribution, Wilcoxon test was used for comparison between two groups. Univariate and multivariate analyses based on the competitive risk regression model were used to analyze independent predictors for high risk of death due to AGC and UGC. The R software was used to construct the Nomogram model of risk assessment by ‘SvyNom’ and ‘rms’ packages. Standardized Hazard Ratio and 95% CIs were estimated for each factor. SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) was used for analysis and two-tailed P values < 0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
Patient characteristics including sex, age, tumor size, tumor location, pTNM stage, blood cell count, histological type, vascular infiltration and adjuvant chemotherapy are shown in Supplementary Table I. The training set comprised 194 patients [166 (85.57%) male and 28 (14.43%) female]. The 5-year survival rate was 39.7%. The validation set comprised 177 patients [144 (81.36%) male and 33 (18.64%)]. The 5-year survival rate was 46.3%. The flowchart of this study is shown in Fig. 1.
Fig. 1.
The flowchart of this study.
3.2. SIRI–PLR
The time-dependent ROC curve shows that these variables were continuously keep satisfactory significance (Fig. 2A). For the SIRI and PLR, 0.82 and 134.62 were the cutoff value. The area under the curve (AUC) was 0.677 (95% CI: 0.602–0.752) and 0.678 (95% CI: 0.602–0.754). Similarly, the optimal cutoff values of SII and NLR were 464.23 and 2.46 by ROC curve analysis (Fig. 2B–D). And patients with SIRI ≤0.82 and PLR ≤134.62 were in the score 0 group, patients with SIRI ≤0.82 and PLR >134.62 and SIRI >0.82 and PLR ≤134.62 were in the score 1 group, and patients with SIRI >0.82 and PLR >134.62 were in the score 2 group.
Fig. 2.
(A): Time dependent ROC curve of SIRI, PLR, SII and NLR. (B–D): Receiver operating characteristic curve of SIRI, PLR, SII and NLR in 1, 3 and 5-year. (E): Receiver operating characteristic curve of inflammation index.
3.3. ROC analysis of inflammation index
In the training set patients, we compared the SIRI–PLR, NLR and SII by ROC. The AUC of the SIRI–PLR was 0.729 [95% CI: 0.658–0.800], the sensitivity was 47.5% and specificity was 86.3%. The AUC of NLR was 0.658 [95% CI: 0.581–0.735], the sensitivity was 50.5% and specificity was 81.1%. The AUC of SII was 0.669 [95% CI: 0.593–0.746], the sensitivity was 70.7% and specificity was 63.2%. (Fig. 2E) (Table 1).
Table 1.
Relevant results of inflammation index.
| Inflammation index | AUC | SE | P value | 95% CI | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| SIRI–PLR | 0.729 | 0.036 | <0.001 | (0.658–0.800) | 47.5 | 86.3 |
| NLR | 0.658 | 0.039 | <0.001 | (0.581–0.735) | 50.5 | 81.1 |
| SII | 0.669 | 0.039 | <0.001 | (0.593–0.746) | 70.7 | 63.2 |
AUC the area under the curve, SE Standard Error, CI confidence interval.
3.4. The relationship between SIRI–PLR and patient characteristics
The relationship between SIRI–PLR score and clinical and pathological factors is shown in Table 2. In the training set, SIRI–PLR score had a significant association with tumor size, surgical procedure, NLR, SII and pTNM stage (P = 0.004, P = 0.014, P = 0.002, P < 0.001 and P = 0.037). In the validation set, SIRI–PLR had a significant association with tumor size, NLR and SII (P = 0.003, P < 0.001 and P < 0.001).
Table 2.
Connection between SIRI–PLR score and clinicopathologic factors of AEG and UGC patients in training, validation set.
| Clinicopathologic factors | Training set |
Validation set |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | X^2 | P value | Score 0 | Score 1 | Score 2 | X^2 | P value | |
| Sex | 5.959 | 0.051 | 0.374 | 0.829 | ||||||
| Male | 46 | 66 | 54 | 43 | 64 | 37 | ||||
| Female | 4 | 18 | 6 | 10 | 13 | 10 | ||||
| Age (years) | 3.545 | 0.170 | 1.122 | 0.571 | ||||||
| ≤60 | 29 | 41 | 24 | 26 | 43 | 22 | ||||
| >60 | 21 | 43 | 36 | 27 | 34 | 25 | ||||
| Tumor size (mm) | 10.900 | 0.004 | 11.725 | 0.003 | ||||||
| ≤50 | 35 | 54 | 25 | 29 | 42 | 12 | ||||
| >50 | 15 | 30 | 35 | 24 | 35 | 35 | ||||
| Tumor location | 4.538 | 0.360 | 3.301 | 0.509 | ||||||
| UE | 12 | 14 | 7 | 13 | 12 | 7 | ||||
| U | 31 | 55 | 38 | 29 | 50 | 27 | ||||
| UM/EUM/UML | 7 | 15 | 15 | 11 | 15 | 13 | ||||
| Surgical procedure | 8.501 | 0.014 | 2.072 | 0.355 | ||||||
| Proximal gastrectomy | 35 | 59 | 29 | 28 | 32 | 19 | ||||
| Total gastrectomy | 15 | 25 | 31 | 25 | 45 | 28 | ||||
| NLR | 12.479 | 0.002 | 43.526 | < 0.001 | ||||||
| ≤2.46 | 50 | 63 | 13 | 52 | 62 | 20 | ||||
| >2.46 | 0 | 21 | 47 | 1 | 15 | 27 | ||||
| SII | 82.294 | < 0.001 | 75.729 | < 0.001 | ||||||
| ≤464.23 | 48 | 35 | 6 | 49 | 46 | 3 | ||||
| >464.23 | 2 | 49 | 54 | 4 | 31 | 44 | ||||
| T stage | 8.101 | 0.424 | 8.029 | 0.431 | ||||||
| T1 | 2 | 3 | 2 | 6 | 4 | 1 | ||||
| T2 | 4 | 3 | 3 | 6 | 11 | 4 | ||||
| T3 | 4 | 7 | 1 | 18 | 29 | 14 | ||||
| T4a | 32 | 62 | 40 | 22 | 29 | 26 | ||||
| T4b | 8 | 9 | 14 | 1 | 4 | 2 | ||||
| N stage | 15.288 | 0.054 | 8.993 | 0.343 | ||||||
| N0 | 20 | 23 | 13 | 25 | 32 | 13 | ||||
| N1 | 17 | 18 | 11 | 9 | 18 | 11 | ||||
| N2 | 7 | 26 | 19 | 11 | 16 | 9 | ||||
| N3a | 6 | 13 | 13 | 7 | 7 | 9 | ||||
| N3b | 0 | 4 | 4 | 1 | 4 | 5 | ||||
| pTNM stage | 10.194 | 0.037 | 6.033 | 0.197 | ||||||
| Ⅰ | 5 | 3 | 4 | 9 | 11 | 3 | ||||
| Ⅱ | 16 | 24 | 7 | 22 | 33 | 15 | ||||
| Ⅲ | 29 | 57 | 49 | 22 | 33 | 29 | ||||
| Histological type | 5.958 | 0.202 | 6.225 | 0.183 | ||||||
| Well and Moderately differentiated adenocarcinoma | 20 | 41 | 19 | 26 | 32 | 19 | ||||
| Poorly differentiated adenocarcinoma | 23 | 38 | 34 | 18 | 40 | 21 | ||||
| Others | 7 | 5 | 7 | 9 | 5 | 7 | ||||
SII Systemic immune inflammation index, NLR neutrophil–lymphocyte ratio, SIRI systemic inflammation response index, UE esophagus and upper stomach, U upper, UM upper and middle, EUM esophagus and upper middle of stomach, UML total stomach.
Histological type, T stage, N stage and pTNM stage are according to the eighth edition of the AJCC Cancer Staging Manual. Tumor location and vascular infiltration were according to the postoperative pathology report. Statistically significant P values are in bold (P < 0.05).
3.5. SIRI–PLR and patient survival
In the training and validation sets, patients with SIRI–PLR score 2 had worse survival than patients with score 0 and 1 (All P < 0.001) (Fig. 3A–C). In the training set, patients with SIRI–PLR score 0, 1, and 2 had the survival times of 54.43 (95% CI: 51.085–57.767), 41.23 (95% CI: 36.590–45.865) and 26.83 (95% CI: 21.710–31.956) months, respectively, and 5-year survival rates of 55.9%, 41.6% and 15.2%, respectively. In the validation set, the survival times were 51.01 (95% CI: 46.764–55.248), 45.44 (95% CI: 40.895–49.985) and 29.51 (95% CI: 23.624–35.386) months, respectively, and 5-year survival rates were 56.0%, 44.0%, and 20.0%, respectively. In the total patients, the survival times were 52.08 (95% CI: 49.377–54.791), 41.42 (95% CI: 38.199–44.635) and 25.34 (95% CI: 21.028–29.641) months, respectively, and 5-year survival rates were 52.8%, 57.1% and 21.3%, respectively.
Fig. 3.
Survival curves of patients with AEG and UGC according to SIRI–PLR score. (A): OS of patients with SIRI– PLR score 0, 1, and 2 in training set (n = 194, P < 0.001), (B–C): validation set (n = 177, P < 0.001) and all patients (n = 371, P < 0.001).
3.6. SIRI–PLR and pTNM
In all training set patients. Patients in stage Ⅰ/Ⅱ and Ⅲ with SIRI–PLR score 2 had a worse survival rate than score 0 and 1. Patients in stage Ⅰ/Ⅱ with SIRI–PLR score 0, 1, and 2 had the survival times were 54.11 (95% CI: 49.290–58.929), 49.66 (95% CI: 42.935–56.379) and 32.68 (95% CI: 20.243–45.115) months, respectively. The 5-year survival rates were 76.2%, 66.7% and 27.3%, respectively (P = 0.002) (Fig. 4A). Patients in stage Ⅲ with SIRI–PLR score 0, 1, and 2 had the survival times were 54.74 (95% CI: 50.088–59.392), 37.09 (95% CI: 31.321–42.857) and 25.51 (95% CI: 19.964–31.056) months, respectively. The 5-year survival rates were 41.4%, 33.3% and 18.4%, respectively (P < 0.001) (Fig. 4B).
Fig. 4.
Survival curves based on SIRI–PLR of AEG and UGC patients (TNM stage I–III). (A): Survival curves of patients with TNM stage I/II with SIRI–PLR score 0, 1 and 2 (P < 0.001). (B): Survival curves of patients with TNM stage III with SIRI–PLR score 0, 1 and 2 (P < 0.001).
3.7. SIRI–PLR and postoperative chemotherapy and surgical method
In patients with stage Ⅱ and Ⅲ GC. There was no significant difference between patients with and without postoperative chemotherapy in the score 0 and 1 groups (P = 0.875), and patients without postoperative chemotherapy had shorter survival than patients with postoperative chemotherapy in the score 2 group (P = 0.002) (Fig. 5A and B). In the score 0 and 1 groups, patients with and without postoperative chemotherapy had the survival times of 45.91 (95% CI: 41.687–50.132) and 44.83 (95% CI: 41.608–48.044) months, respectively, and 5-year survival rates were 53.1% and 53.3%, respectively. In the score 2 group, the survival times were 38.75 (95% CI: 31.908–45.601) and 23.211 (95% CI: 18.527–27.795) months, respectively, and 5-year survival rates were 36.7% and 14.6%, respectively.
Fig. 5.
Relationship between SIRI–PLR score and benefit from postoperative chemotherapy in total patents with SIRI–PLR score 0,1 (A) and 2 (B). Relationship between SIRI, PLR and benefit from surgical method in patents with low PLR (C), high PLR (D), low SIRI (E) and high SIRI (F).
Although SIRI–PLR cannot guide the surgical method and there was also no significant difference between patients with PG and TG in the low PLR, low SIRI and high SIRI patients (P = 0.271, P = 0.271 and P = 0.260), in the high PLR group, patients with TG had shorter survival than patients with PG (P = 0.049) (Fig. 5C–F). In the high PLR group, patients with PG and TG had the survival times of 37.31 (95% CI: 32.855–41.763) and 29.875 (95% CI: 25.525–34.224) months, respectively, and 5-year survival rates were 36.4% and 25.0%, respectively.
3.8. Univariate and multivariate regression analyses
To identify the independent predictors for OS of patients with AGC and UGC, univariate and multivariate analyses based on competitive risk regression model in the training set. According to univariate analysis, age (P = 0.001), NLR (P < 0.001), SII (P < 0.001), SIRI-PLR (P < 0.001), pTNM stage (P = 0.048) and postoperative chemotherapy (P = 0.016) were significant. According to multivariate analyses, age (P = 0.033), SIRI-PLR (P < 0.001), pTNM stage (P = 0.005) and postoperative chemotherapy (P = 0.001) were independent predictors for high risk of death due to AGC and UGC (Table 3).
Table 3.
Risk factors of patients with AEG and UGC by univariate and multivariate based on competitive risk regression model.
| Characteristics | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| SHR | SE | P value | SHR | 95 % CI | P value | |
| Sex | – | – | – | |||
| Male | 1 | |||||
| Female | 0.611 | 0.330 | 0.140 | |||
| Age (years) | 1.033 | 0.010 | 0.001 | 1.023 | (1.002–1.045) | 0.033 |
| Tumor location | – | – | – | |||
| UE | 1 | |||||
| U | 1.065 | 0.270 | 0.820 | |||
| UM/EUM/UML | 1.351 | 0.343 | 0.380 | |||
| Surgical procedure | – | – | – | |||
| Proximal gastrectomy | 1 | |||||
| Total gastrectomy | 1.300 | 0.210 | 0.210 | |||
| NLR | 1.211 | 0.049 | <0.001 | 1.027 | (0.850–1.240) | 0.780 |
| SII | 1.001 | 0.0001 | <0.001 | 1.000 | (1.000–1.001) | 0.480 |
| SIRI-PLR | 2.558 | (1.816–3.603) | <0.001 | |||
| 0 | 1 | |||||
| 1 | 2.990 | 0.291 | <0.001 | |||
| 2 | 7.431 | 0.301 | <0.001 | |||
| pTNM stage | 1.798 | (1.489–2.172) | 0.005 | |||
| Ⅰ | 1 | |||||
| Ⅱ | 1.632 | 0.615 | 0.430 | |||
| Ⅲ | 3.156 | 0.582 | 0.048 | |||
| Histological type | – | – | – | |||
| Well-differentiated and Moderately differentiated adenocarcinoma | 1 | |||||
| Poorly differentiated adenocarcinoma | 1.066 | 0.219 | 0.770 | |||
| Others | 1.523 | 0.278 | 0.130 | |||
| Postoperative chemotherapy | 0.426 | (0.417–0.435) | 0.001 | |||
| No | 1 | |||||
| Yes | 0.581 | 0.225 | 0.016 | |||
NLR neutrophil–lymphocyte ratio, PLR platelet–lymphocyte ratio, SIRI systemic inflammation response index, SHR subdistribution hazard ratio, SE Standard Error, CI confidence interval, UE esophagus and upper stomach, U upper, UM upper and middle, EUM esophagus and upper middle of stomach, UML total stomach.
Histological type, T stage, N stage and pTNM stage are according to the eighth edition of the AJCC Cancer Staging Manual. Tumor location and vascular infiltration were according to the postoperative pathology report. Statistically significant P values are in bold (P < 0.05).
3.9. Prognostic nomogram for OS
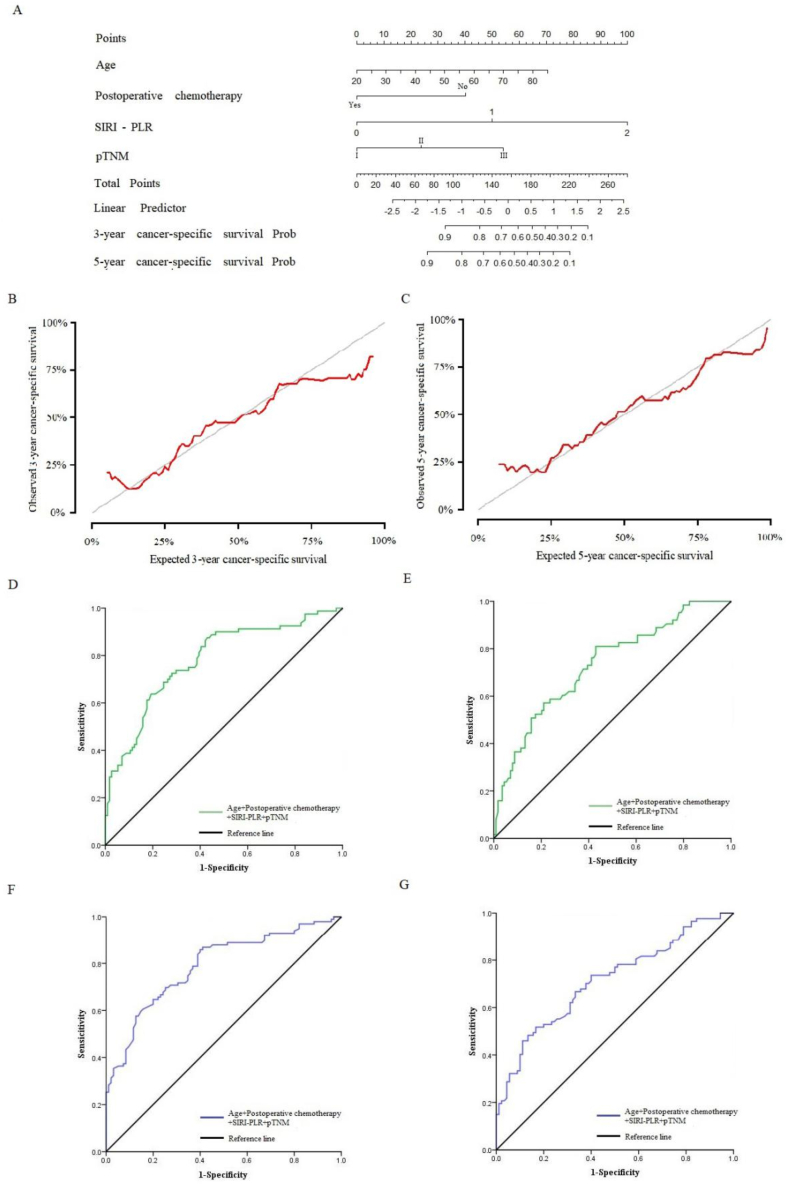
Because age, SIRI, PLR and pTNM stage are independent predictors for patients with AGC and UGC in the training set, we first combined these clinical features in the training set and constructed a Nomogram model of continuous variable data to predict the 3-year and 5-year prognosis (Fig. 6A). Concordance was 0.739 and standard error was 0.017 (Fig. 6B and C). Calibration curves for predicting survival at 3 and 5-year were performed. The AUC were 0.781 [95% CI: 0.714–0.847] (Fig. 6D) and 0.790 [95% CI: 0.727–0.853], respectively (Fig. 6F). The sensitivity were 87.5% and 85.9%, respectively, and the specificity were 57.0% and 60.0%, respectively.
Fig. 6.
(A): Nomogram model predicting 3-year and 5-year prognosis of patients in the training set. (B–C): The calibration curve for predicting patient survival in 3-year and 5-year in the training set. (D): ROC curve of Nomogram model predicting 3-year prognosis of patients in the training set. (E): ROC curve of Nomogram model predicting 3-year prognosis of patients in the validation set. (F): ROC curve of Nomogram model predicting 5-year prognosis of patients in the training set. (G): ROC curve of Nomogram model predicting 5-year prognosis of patients in the validation set.
We validate the Nomogram model in the validation set. The AUC related to 3-year prognosis was 0.730 [95% CI: 0.653–0.807], the sensitivity was 81.0%, and the specificity was 57.0% (Fig. 6E). The AUC related to 5-year prognosis was 0.713 [95% CI: 0.637–0.788], the sensitivity was 51.7%, and the specificity was 83.3% (Fig. 6G).
4. Discussion
More than 70% patients with AEG and UGC are diagnosed with advanced GC [30], which make patients who had undergone radical surgery still have a high risk of recurrence and metastasis, and suitable clinical prognostic factors are still needed to help surgeons identify high-risk patients. With recognition of the role of systemic inflammation in promoting tumor growth, progression and metastasis of malignant tumors, C-reactive protein, cytokines, inflammatory proteins and immune cells in the systemic inflammatory response are also considered as potential clinical markers to guide treatment and evaluate the prognosis [[31], [32], [33]]. Recently, the prognostic importance of SIRI for patients with renal, pancreatic and nasopharyngeal cancers has been confirmed [25,[34], [35], [36], [37], [38]]. Patients with high SIRI not only represent worse disease-free survival and OS, but also are more suitable for thoracoscopy in lung cancer patients and mFOLFIRINOX chemotherapy in pancreatic cancer patients. Besides, studies of upper tract urothelial carcinoma also have shown that combination of SIRI and PLR scoring evaluates prognosis more effectively.
In this study, the AUC of SIRI and PLR curve are 0.677 and 0.678, respectively, and the cut-off values are 0.82 and 134.62, respectively, which is similar to the research by Zheng et al. [25]. But we found the AUC of PLR in our study is higher than that reported by Zhang et al. [39]. According to chi-square analysis between SIRI–PLR with clinical and pathological features it could be found that it was correlated with tumor size, NLR and SII. Many previous studies confirmed that NLR and SII could be used as inflammatory indexes for evaluating prognosis of GC patients, which means that SIRI–PLR is expected to become a more significant clinical biomarker. In our study, ROC analysis of SIRI–PLR, NLR and SII showed that the AUC of SIRI–PLR was not only larger than that of NLR and SII, but also had better specificity. We can conclude that the SIRI–PLR is better to other inflammatory indexes in predicting the prognosis, which was consistent with the study by Li et al. [40] and Huang et al. [41].
The tumor immune microenvironment of GC patients can be reflected by postoperative pathological tissue sections or immune indexes. Postoperative pathological tissue sections can be used to analyze the condition of the tumor immune microenvironment by immunohistochemistry, which had high specificity. However, the randomness of tissue site selection and development of preoperative adjuvant therapy reduce the accuracy of the test. On the other hand, the immune indexes constructed by circulating immune cells were calculated based on preoperative hematological tests by clinical data, which had high accuracy, fast and convenient features. So the immune indexes were easy for clinical application [42,43].
The SIRI–PLR scoring system comprehensively covered the circulating immune cells for calculation of the inflammatory index, which could evaluate more comprehensively the physical inflammatory status of patients than NLR and SII did. The tumor immunity plays an important role in measuring tumor progression. GC cells can induce enrichment of neutrophils in tumors, especially GC positive for programmed death ligand 1 and correlative with Epstein–Barr virus [[44], [45], [46]]. Formation of neutrophils and secretion of interferon-γ and tumor necrosis factor-α can inhibit proliferation of lymphocytes and biological activity of CD4+ and CD8+ T cells, lead to immune escape of cancer cells and distant micro-metastasis of tumor cells. Nie et al. [47] showed that expression of a large number of monocytes can inhibit the immune response of T cells near the tumor and promote immune escape of cancer cells by increasing cyclo-oxygenase-2 expression. Senescence-associated secretory phenotype interaction between platelets also plays an important role in metastasis and invasion of cancer cells [48]. Tumor occurrence, development and metastasis lead to changes in immune cells and inflammation in the tumor microenvironment, which would affect the circulating immune cells with disease progression.
As we all know, choosing appropriate individualized treatment for each patient can greatly improve the quality of life even survival time. Surgery is still generally the first choice intervention in patients with GC [49]. At present, clinicians mainly choose TG and PG methods for gastrectomy according to tumor size, tumor location, clinical stage and surgical experience. However, there is still a part of GC which is consistent with both surgical indications of TG and PG. Moreover, the different surgical methods of gastrectomy affect the quality of life and survival time after operation. PG had high postoperative body mass index, albumin, nutrition index and high risk of gastroesophageal reflux, which may be related to the high rate of tumor recurrence. TG can ensure a sufficient distal surgical margin, which can reduce the risk of recurrence and prolong survival [9,50,51]. However Pu et al. [52] found that there was no difference in survival time between patients with PG and TG. This has led to a controversy about how to choose the operation method for such patient. Therefore, this study suggests that in addition to the conventional surgical guidelines, surgeons can also use an additional inflammatory index to help choose the method of gastrectomy. We found that for patients with high PLR patients. By comparing the postoperative survival of patients who received TG and PG, patients who underwent PG had better prognosis than those who underwent TG. These two methods can effectively remove tumor lesions and reduce the tumor burden, but Rashid et al. [53] showed that effective preservation of lesion-peripheral CD4+ and CD8+ T cells could retain tumor immunity potential after surgery, prolong survival, and reduce postoperative recurrence rate. This might explain the difference in postoperative survival between TG and PG patients.
In addition, researchers worldwide have developed abundant preoperative and postoperative treatments for GC, such as neoadjuvant chemotherapy, postoperative chemotherapy, postoperative radiotherapy and targeted therapy. However, it was difficult to assess the sensitivity of patients to different treatment methods. Yuka et al. found that preoperative PLR can evaluate the sensitivity of postoperative chemotherapy [54]. This study also found that patients with SIRI–PLR score 2 had better prognosis than those who did not receive postoperative chemotherapy. Although patients with high SIRI score are reported not to be suitable for chemotherapy [33], we focused on the scoring system that combined SIRI with PLR, and on GC patients who also had AGC and UGC, which might lead to different results.
Previous studies using serum immune index alone to predict the prognosis of patients or guide therapy have unsatisfactory sensitivity and specificity, which is difficult to individualize evaluation. With the development of Real World Study and Big Data for Cancer Research, the mode combining clinicopathological features and serum immunity markers to predicting the prognosis of GC is widely used in clinical [55,56]. For tumors, many studies currently focus on constructing prognostic models based on molecular expression combined with clinical pathological features. However, it is difficult to achieve unified standards for detecting molecular expression levels, resulting in no standard for evaluating prognosis applied in clinical practice except for the TNM staging system currently. We believe that expanding the sample size and regressing to basic clinical features to construct predictive models is more valuable for studying the real world of tumors. Especially for gastric cancer with high heterogeneity, including tumor volume size, Borrmann classification, Laurent classification, differentiation degree, and combination of serum immune indicators, will be assisted in constructing predictive models in the context of massive data and multicenter retrospective analysis. In this study, we found SIRI–PLR, age, pTNM stage and postoperative chemotherapy were independent predictors for high risk of death due to AGC and UGC in the training set. Age and postoperative chemotherapy had been proved to be related to the prognosis of patients. The older patients, or the patients who didn't receive postoperative chemotherapy will had a significantly increased risk of postoperative recurrence [57,58]. Then, a Nomogram model was constructed in the training set to predict the prognosis for 3 and 5-year. Through ROC analysis, it was found that AUC was 0.781 and 0.790, sensitivity was 87.5% and 85.9%, and specificity was 57.0% and 60.0%. And the constructed Nomogram model also can be used well in the verification set. Although this result may be limited by the small number of patients and the cohort grouped by time of admission, it can still indicate that for the patients with AEG and UGC, the prediction model established by age, SIRI–PLR and pTNM stage and postoperative chemotherapy is worth further clinical verification and application.
5. Limitations
This retrospective study still had some limitations. First, the prevalence of AGC and UGC in non-Asian populations needs further exploration. Second, it was difficult to determine whether preoperative gastritis and Helicobacter pylori infection affected circulatory immune cells in patients with GC, and this also needs further exploration. Third, there are only 35 patients in stage I, it is not enough to analyze separately. Therefore, whether the constructed Nomogram model has the same clinical significance in stage I should be further studied. Lastly, molecular characteristics, such as HER-2 should be included in future research.
6. Conclusion
SIRI–PLR score is an independent predictor of survival in patients who undergo curative surgery for AEG and UGC. It can further subgroup patients with stage Ⅰ/Ⅱ and Ⅲ to supplement the eighth edition of the AJCC guidelines. Patients with SIRI–PLR score 2 with postoperative chemotherapy have better survival than patients with SIRI–PLR score 0 or 1. Patients with high PLR with PG have better survival than patients with TG. SIRI–PLR may help clinicians to decide upon individualized treatment for patients with AEG or UGC. The Nomogram with combination of SIRI-PLR, age, pTNM stage and postoperative chemotherapy can predict postoperative survival.
Declarations
The informed consent was obtained from all patients for the publication of all their data. This study was approved by the Ethics Committee of the Harbin Medical University Cancer Hospital (Approval Number: SHGC-1029). All experiments were performed in accordance with established ethical guidelines, and informed consent was obtained from the patients to confirm their voluntary participation.
Patient consent statement
All patients signed informed consent when their clinical information was collected.
Funding information
This work was supported by Nn10 program of Harbin Medical University Cancer Hospital from Yingwei Xue (No. Nn10 PY 2017-03), Haiyan Research Fund of Harbin Medical University Cancer Hospital from Tianyi Fang (No. JJQN2021-06) and Fundamental Research Funds for the Provincial Universities from Tianyi Fang (No. 2022-KYYWF-0286).
Data availability statement
The datasets used in this study are available from the corresponding author on reasonable request. More information can also be obtained from the Gastric Cancer Information Management System v1.2 of Harbin Medical University Cancer Hospital (Copyright No. 2013SR087424, http://www.sgihmu.com/).
CRediT authorship contribution statement
Tianyi Fang: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft. Xin Yin: Conceptualization, Methodology, Formal analysis, Writing – original draft. Yufei Wang: Conceptualization, Formal analysis, Methodology. Lei Zhang: Formal analysis, Methodology. Shuo Yang: Data curation, Formal analysis. Xinju Jiang: Data curation. Yingwei Xue: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26176.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Global Burden of Disease Cancer C., Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusano C., Gotoda T., Khor C.J., Katai H., Kato H., Taniguchi H., et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J. Gastroenterol. Hepatol. 2008;23:1662–1665. doi: 10.1111/j.1440-1746.2008.05572.x. [DOI] [PubMed] [Google Scholar]
- 3.Haverkamp L., Seesing M.F., Ruurda J.P., Boone J., R Vh Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis. Esophagus. 2017;30:1–7. doi: 10.1111/dote.12480. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa S., Yoshikawa T., Rino Y., Oshima T., Aoyama T., Hayashi T., et al. Priority of lymph node dissection for Siewert type II/III adenocarcinoma of the esophagogastric junction. Ann. Surg Oncol. 2013;20:4252–4259. doi: 10.1245/s10434-013-3036-0. [DOI] [PubMed] [Google Scholar]
- 5.Mazer L.M., Poultsides G.A. What is the best operation for proximal gastric cancer and distal esophageal cancer? Surg. Clin. 2019;99:457–469. doi: 10.1016/j.suc.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Rice T.W., Gress D.M., Patil D.T., Hofstetter W.L., Kelsen D.P., Blackstone E.H. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017;67:304–317. doi: 10.3322/caac.21399. [DOI] [PubMed] [Google Scholar]
- 7.Huang Q., Shi J., Sun Q., Gold J.S., Chen J., Wu H., et al. Clinicopathological characterisation of small (2 cm or less) proximal and distal gastric carcinomas in a Chinese population. Pathology. 2015;47:526–532. doi: 10.1097/PAT.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Liu F., Li Y., Tang S., Zhang Y., Chen Y., et al. Comparison on clinicopathological features, treatments and prognosis between proximal gastric cancer and distal gastric cancer: a national cancer data base analysis. J. Cancer. 2019;10:3145–3153. doi: 10.7150/jca.30371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golematis B., Misitzis J., Yiannitsiotis A., Papachristou D.N., Delicaris P. Total colectomy with resection of rectal mucosa and ileo-anal implantation for ulcerative colitis. S. Afr. J. Surg. 1982;20:221–226. [PubMed] [Google Scholar]
- 10.Harrison L.E., Karpeh M.S., Brennan M.F. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. 1998;123:127–130. [PubMed] [Google Scholar]
- 11.The AJCC Cancer Staging Manual eighth ed.. Published by Springer Nuture. PP.203-220.
- 12.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pages C., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 13.Pages F., Mlecnik B., Marliot F., Bindea G., Ou F.S., Bifulco C., et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 14.Reichert T.E., Rabinowich H., Johnson J.T., Whiteside T.L. Mechanisms responsible for signaling and functional defects. J. Immunother. 1998;21:295–306. doi: 10.1097/00002371-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Chen L., Yan Y., Zhu L., Cong X., Li S., Song S., et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag. Res. 2017;9:849–867. doi: 10.2147/CMAR.S151026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q., Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J. Gastrointest. Oncol. 2019;10:965–978. doi: 10.21037/jgo.2019.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H., Jiang Y., Cao H., Zhu H., Chen B., Ji W. Nomogram based on systemic immune-inflammation index to predict overall survival in gastric cancer patients. Dis. Markers. 2018;2018 doi: 10.1155/2018/1787424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto R., Inagawa S., Sano N., Tadano S., Adachi S., et al. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018;44:607–612. doi: 10.1016/j.ejso.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Wang C., Xu M., Kong C., Qu A., Zhang M., et al. Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer. J. Cancer Res. Clin. Oncol. 2017;143:861–871. doi: 10.1007/s00432-016-2330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Bai N., Hu X., OuYang X.W., Yao L., Tao Y., et al. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7 doi: 10.7717/peerj.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong H., Chen J., Cheng S., Chen S., Shen R., Shi Q., et al. Prognostic nomogram incorporating inflammatory cytokines for overall survival in patients with aggressive non-Hodgkin's lymphoma. EBioMedicine. 2019;41:167–174. doi: 10.1016/j.ebiom.2019.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J., Chen S., Chen Y., Li S., Xu D. Combination of CRP and NLR: a better predictor of postoperative survival in patients with gastric cancer. Cancer Manag. Res. 2018;10:315–321. doi: 10.2147/CMAR.S156071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X., Liu X., Liu J., Chen S., Xu D., Li W., et al. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I-II gastric cancer. Chin. J. Cancer. 2016;35:57. doi: 10.1186/s40880-016-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cong X., Li S., Zhang Y., Zhu Z., Wang Y., Song S., et al. The combination of preoperative fibrinogen and neutrophil-lymphocyte ratio is a predictive prognostic factor in esophagogastric junction and upper gastric cancer. J. Cancer. 2019;10:5518–5526. doi: 10.7150/jca.31162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y., Chen Y., Chen J., Chen W., Pan Y., Bao L., et al. Combination of systemic inflammation response index and platelet-to-lymphocyte ratio as a novel prognostic marker of upper tract urothelial carcinoma after radical nephroureterectomy. Front. Oncol. 2019;9:914. doi: 10.3389/fonc.2019.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siewert J.R. Adenocarcinoma of the esophago-gastric junction. Gastric Cancer. 1999;2:87–88. doi: 10.1007/s101200050028. [DOI] [PubMed] [Google Scholar]
- 27.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B., Yang X.R., Xu Y., Sun Y.F., Sun C., Guo W., et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014 Dec 01;20(23) doi: 10.1158/1078-0432.CCR-14-0442. 6212-6122. [DOI] [PubMed] [Google Scholar]
- 29.Qi Q., Zhuang L., Shen Y., Geng Y., Yu S., Chen H., et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016 07 15;122(14):2158–2167. doi: 10.1002/cncr.30057. [DOI] [PubMed] [Google Scholar]
- 30.Baba H., Kakeji Y., Oki E., Tokunaga E., Ushiro S., Watanabe M., et al. [Chemotherapy for gastric cancer] Gan To Kagaku Ryoho. 2003;30:1881–1888. [PubMed] [Google Scholar]
- 31.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Diakos C.I., Charles K.A., McMillan D.C., Clarke S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 33.Hu J.J., Urbanic J.J., Case L.D., Takita C., Wright J.L., Brown D.R., et al. Association between inflammatory biomarker C-reactive protein and radiotherapy-induced early adverse skin reactions in a multiracial/ethnic breast cancer population. J. Clin. Oncol. 2018;36:2473–2482. doi: 10.1200/JCO.2017.77.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacheco-Barcia V., Mondejar Solis R., France T., Asselah J., Donnay O., Zogopoulos G., et al. A systemic inflammation response index (SIRI) correlates with survival and predicts oncological outcome for mFOLFIRINOX therapy in metastatic pancreatic cancer. Pancreatology. 2020;20:254–264. doi: 10.1016/j.pan.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Li S., Xu H., Wang W., Gao H., Li H., Zhang S., et al. The systemic inflammation response index predicts survival and recurrence in patients with resectable pancreatic ductal adenocarcinoma. Cancer Manag. Res. 2019;11:3327–3337. doi: 10.2147/CMAR.S197911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z., Wang K., Lu H., Xue D., Fan M., Zhuang Q., et al. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: a propensity score-matched analysis. Cancer Manag. Res. 2019;11:909–919. doi: 10.2147/CMAR.S186976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y., Jiang W., Xi D., Chen J., Xu G., Yin W., et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J. Invest. Med. 2019;67:691–698. doi: 10.1136/jim-2018-000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S., Yang Z., Du H., Zhang W., Che G., Liu L. Novel systemic inflammation response index to predict prognosis after thoracoscopic lung cancer surgery: a propensity score-matching study. ANZ J. Surg. 2019;89:E507–E513. doi: 10.1111/ans.15480. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L.X., Wei Z.J., Xu A.M., Zang J.H. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int. J. Surg. 2018;56:320–327. doi: 10.1016/j.ijsu.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Lan X., Gao H., Li Z., Chen L., Wang W., et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J. Cancer Res. Clin. Oncol. 2017;143:2455–2468. doi: 10.1007/s00432-017-2506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Z., Liu Y., Yang C., Li X., Pan C., Rao J., et al. Combined neutrophil/platelet/lymphocyte/differentiation score predicts chemosensitivity in advanced gastric cancer. BMC Cancer. 2018;18:515. doi: 10.1186/s12885-018-4414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renshaw A.A., Young M.L., Jiroutek M.R. How many cases need to be reviewed to compare performance in surgical pathology? Am. J. Clin. Pathol. 2003;119:388–391. doi: 10.1309/qyyb3k0bhpcegqg3. [DOI] [PubMed] [Google Scholar]
- 43.Renshaw A.A., Gould E.W. Correlation of workload with disagreement and amendment rates in surgical pathology and nongynecologic cytology. Am. J. Clin. Pathol. 2006;125:820–822. doi: 10.1309/4G41-TXC0-6902-MWCK. [DOI] [PubMed] [Google Scholar]
- 44.Wang T.T., Zhao Y.L., Peng L.S., Chen N., Chen W., Lv Y.P., et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900–1911. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang D., Zhou J., Tang D., Zhou L., Chou L., Chou K.Y., et al. Neutrophil infiltration mediated by CXCL5 accumulation in the laryngeal squamous cell carcinoma microenvironment: a mechanism by which tumour cells escape immune surveillance. Clin. Immunol. 2017;175:34–40. doi: 10.1016/j.clim.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki S., Nishikawa J., Sakai K., Iizasa H., Yoshiyama H., Yanagihara M., et al. EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer. 2019;22:486–496. doi: 10.1007/s10120-018-0880-4. [DOI] [PubMed] [Google Scholar]
- 47.Nie W., Yu T., Sang Y., Gao X. Tumor-promoting effect of IL-23 in mammary cancer mediated by infiltration of M2 macrophages and neutrophils in tumor microenvironment. Biochem. Biophys. Res. Commun. 2017;482:1400–1406. doi: 10.1016/j.bbrc.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 48.Valenzuela C.A., Quintanilla R., Olate-Briones A., Venturini W., Mancilla D., Cayo A., et al. SASP-dependent interactions between senescent cells and platelets modulate migration and invasion of cancer cells. Int. J. Mol. Sci. 2019 Oct 24;(21):20. doi: 10.3390/ijms20215292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Cutsem E., Sagaert X., Topal B., Haustermans K., Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 50.Karanicolas P.J., Graham D., Gonen M., Strong V.E., Brennan M.F., Coit D.G. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann. Surg. 2013;257:1039–1046. doi: 10.1097/SLA.0b013e31828c4a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosa F., Quero G., Fiorillo C., Bissolati M., Cipollari C., Rausei S., et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG) Gastric Cancer. 2018;21:845–852. doi: 10.1007/s10120-018-0804-3. [DOI] [PubMed] [Google Scholar]
- 52.Pu Y.W., Gong W., Wu Y.Y., Chen Q., He T.F., Xing C.G. Proximal gastrectomy versus total gastrectomy for proximal gastric carcinoma. A meta-analysis on postoperative complications, 5-year survival, and recurrence rate. Saudi Med. J. 2013;34:1223–1228. [PubMed] [Google Scholar]
- 53.Rashid O.M., Nagahashi M., Ramachandran S., Graham L., Yamada A., Spiegel S., et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. 2013;153:771–778. doi: 10.1016/j.surg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohe Y., Fushida S., Yamaguchi T., Kinoshita J., Saito H., Okamoto K., et al. Peripheral blood platelet-lymphocyte ratio is good predictor of chemosensitivity and prognosis in gastric cancer patients. Cancer Manag. Res. 2020;12:1303–1311. doi: 10.2147/CMAR.S241069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fanotto V., Cordio S., Pasquini G., Fontanella C., Rimassa L., Leone F., et al. Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer. 2017;20:825–833. doi: 10.1007/s10120-016-0681-6. [DOI] [PubMed] [Google Scholar]
- 56.Huang C., Liu Z., Xiao L., Xia Y., Huang J., Luo H., et al. Clinical significance of serum CA125, CA19-9, CA72-4, and fibrinogen-to-lymphocyte ratio in gastric cancer with peritoneal dissemination. Front. Oncol. 2019;9:1159. doi: 10.3389/fonc.2019.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi T., Yoshikawa T., Aoyama T., Hasegawa S., Yamada T., Tsuchida K., et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer. 2015 Apr;18(2):368–374. doi: 10.1007/s10120-014-0361-3. [DOI] [PubMed] [Google Scholar]
- 58.Hejna M., Wöhrer S., Schmidinger M., Raderer M. Postoperative chemotherapy for gastric cancer. Oncol. 2006 Feb;11(2):136–145. doi: 10.1634/theoncologist.11-2-136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study are available from the corresponding author on reasonable request. More information can also be obtained from the Gastric Cancer Information Management System v1.2 of Harbin Medical University Cancer Hospital (Copyright No. 2013SR087424, http://www.sgihmu.com/).