Abstract
Glycan binding properties of respiratory viruses have been difficult to probe due to a lack of biologically relevant glycans for binding studies. Here, a stop-and-go chemoenzymatic methodology is presented that gave access to a panel of 32 asymmetrical biantennary N-glycans having various numbers of N-acetyl lactosamine (LacNAc) repeating units capped by α2,3- or α2,6-sialosides resembling structures found in airway tissues. It exploits that the branching enzymes MGAT1 and MGAT2 can utilize unnatural UDP-2-deoxy-2-trifluoro-N-acetamido-glucose (UDP-GlcNTFA) as donor. The TFA moiety of the resulting glycans can be hydrolyzed to give GlcNH2 at one of the antennae, which temporarily blocks extension by glycosyl transferases. The N-glycans were printed as a microarray that was probed for receptor binding specificities of the evolutionary distinct human A(H3N2) and A(H1N1)pdm09 viruses. It was found that not only the sialoside type but also the length of the LacNAc chain and presentation at the α1,3-antenna of N-glycans are critical for binding. Early A(H3N2) viruses bound to 2,6-sialosides at a single LacNAc moiety at the α1,3-antenna whereas later viruses required the sialoside to be presented at a tri-LacNAc moiety. Surprisingly, most of the A(H3N2) viruses that appeared after 2021 regained binding capacity to sialosides presented at a di-LacNAc moiety. As a result, these viruses again agglutinate erythrocytes, commonly employed for antigenic characterization of influenza viruses. Human A(H1N1)pdm09 viruses have similar receptor binding properties as recent A(H3N2) viruses. The data indicate that an asymmetric N-glycan having 2,6-sialoside at a di-LacNAc moiety is a commonly employed receptor by human influenza A viruses.
Keywords: glycosyl transferases, N-glycans, chemoenzymatic, hemagglutination, antigenic drift
Introduction
Respiratory viruses, which cause enormous disease burden,1,2 often employ glycans as receptor for cell attachment and/or entry. The relentless pressure of viral infections at the mucosal interface has driven the evolution of host and pathogen.1 It has shaped the glycomes of the host, and even closely related species can express substantially different collections of glycans.3 In turn, pathogens evolved glycan receptor specificities that determine the host range and tissue tropism. Furthermore, immunogenic pressure can cause substitutions in receptor binding domains, which in turn can influence receptor specificities. Despite the importance, glycan binding properties of respiratory viruses have been difficult to probe due to a lack of panels of biologically relevant glycans for structure-binding studies.
Glycomic analyses of respiratory tissues of several animal species and humans have shown the abundant presence of biantennary N-glycans having poly-N-acetyl-lactosamine (poly-LacNAc) extensions that can be modified by terminal α2,3- or α2,6-sialosides.4,5 In human lung tissue, α2,3-linked sialosides are mainly presented on elongated LacNAc chains whereas α2,6-sialosides are more often found on structures that have a single LacNAc moiety.6−8 Lectin staining has demonstrated that upper airway tissues of humans are rich in α2,6-linked sialosides, whereas duck enteric and chicken upper respiratory tract tissues display ample quantities of α2,3-linked sialosides.3−5 These differences in expression of sialosides represent a species barrier, because human influenza A viruses (IAVs) recognize sialosides that are α2,6-linked to galactoside (Gal), whereas ancestorial avian IAVs prefer α2,3-linked isomers. A notion is emerging that α2,3- vs α2,6-selectivity of avian and human IAVs is an oversimplification and further structural elements of glycans can determine receptor specificity.5,9−16 Comprehensive panels of biological relevant glycans are needed to adequately uncover such binding properties.17,18
We report here a stop-and-go strategy that made it possible to conveniently prepare a large panel of asymmetrical biantennary N-glycans having various numbers of LacNAc repeating units capped by α2,3- or α2,6-sialosides. The resulting collection of glycans, which resemble structures found in airway tissues, was printed as a microarray that was probed for binding of evolutionary distinct A(H3N2) viruses ranging from 1968 to 2023. Also, receptor binding properties were examined for recent human A(H1N1)pdm09 viruses and an avian virus that can infect but not transmit between humans. It was found that for human viruses, not only the sialoside type but also the length of the LacNAc chain and presentation at a specific antenna determine receptor binding properties. Initially, A(H3N2) viruses evolved to have restricted binding patterns and recognize only 2,6-sialosides presented on a tri-LacNAc moiety on the α1,3-antenna of N-glycans. However, recently this trend reversed and most strains that appeared after 2021 also exhibit some affinity for 2,6-sialosides presented on a di-LacNAc moiety. We hypothesize that this binding pattern relates to a balancing act between antigenic divergence and glycan binding properties to the more abundant of 2,6-sialylated di-LacNAc structures.19 Our data show that this is a property not only of A(H3N2) but also of A(H1N1)pdm09 viruses.
The new glycan microarray provides an attractive platform to assess the receptor binding properties of influenza viruses. It has provided new insight into the evolution of viruses during epidemics, where there is an interplay between virus–receptor interactions on the one hand and virus–antibody interactions on the other hand. Mutations that affect one of these properties may also affect the other.
Results and Discussion
Chemoenzymatic Synthesis of Asymmetrical Biantennary Glycans
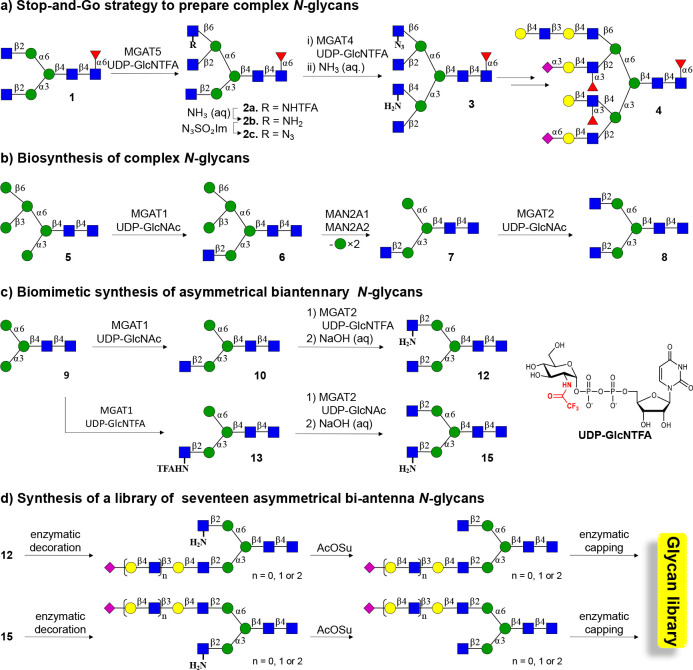
Recently, we introduced chemoenzymatic methodologies that were coined “Chemoenzymatic Glycosylations by a Stop-and-Go Strategy”.20 It employs symmetric biantennary glycan 1 as the key intermediate (Scheme. 1a) that could readily be prepared from a sialoglycopeptide isolated from egg yolk powder.21 Next, we took advantage of recombinant α-1,3-mannosyl-glycoprotein 4-β-N-acetyl-glucosaminyltransferase (MGAT4), β-1,6-mannosylglycoprotein 6-β-N-acetyl-glucosaminyltransferase (MGAT5), and unnatural UDP-2-deoxy-2-trifluoro-N-acetamido-glucose (UDP-GlcNTFA) to transform 1 into tetra-antennary glycan 3 in only four steps. A key strategic principle is that after the installation of a GlcNTFA moiety, the trifluoro-N-acetamido (TFA) moiety can be removed under mild basic conditions to give glucosamine (GlcNH2) that can be further transformed into 2-deoxy-2-azido-glucose (GlcN3). GlcNH2 and GlcN3 are inert (stop) to modifications by our panel of mammalian glycosyltransferases. Selective elaboration of the natural GlcNAc residues at the MGAT1 or 2 antenna was possible by exploiting the inherent branch selectivities of ST6Gal1 and Escherichia coli galactosidase for the α3-antenna. At the next stage of synthesis, the GlcNH2 and GlcN3 can be sequentially “unmasked” (go) to give natural GlcNAc termini for selective enzymatic elaboration into complex appendages to give compounds such as 4. Intermediates such as 2b were also employed for the preparation of triantennary glycans.
Scheme 1. Stop-and-Go Strategy for the Synthesis of Asymmetrical Biantennary N-Glycans.
a) Modification of symmetrical glycan 1, which was derived from egg yolk powder, with MAGT4 and MAGT5 using the unnatural donor UDP-GlcNTFA, followed by removal of the TFA moiety and chemical functionalization of the resulting amines gave 3, which is an appropriate starting material to prepare asymmetrical glycans such as 4. b) The biosynthesis of N-glycan involves trimming of high mannose N-glycans to 5 that can be modified by MGAT1 to provide 6 that after further mannoside trimming provides a substrate MGAT2. c) Compound 9, which is assessable from 1, was expected to be an appropriate substrate for MGAT1 and 2 and in combination with the natural and unnatural donor UDP-GlcNAc and UDP-GlcNTFA should give access to asymmetrical glycans 12 and 15. d) Asymmetrical glycans 12 and 15 were expected to be appropriate starting materials for the preparation of asymmetrical biantennary N-glycans having extended LacNAc moieties typical of the respiratory glycome.
The stop-and-go strategy exploits the preference of ST6Gal122 and E. coli galactosidase23 for the α3-antenna of a G2 structure to selectively elaborate the α3- and α6-antenna with specific appendages. The reaction conditions need careful controlling to achieve selectivity, and even when controlled, a regioisomer (∼10%) is formed that needs to be removed by time-consuming HPLC purification.
In the biosynthesis of N-glycans, the α3- and α6-antenna are introduced by the branching enzymes α-1,3-mannosyl-glycoprotein 2-β-N-acetylglucosaminyltransferase (MGAT1) and 2-β-N-acetylglucosaminyltransferase (MGAT2), respectively. To achieve full control over the extension of the α3- and α6-antennae, we explored whether the stop-and-go strategy can be adapted to MGAT1 and MGAT2 to install a GlcNH2 moiety at one of these antennae to temporarily block extension by galactosyl transferases. MGAT1 and MGAT2 act early in biosynthetic pathway, and MGAT1 utilizes a Man5 structure as substrate (5) to produce hybrid Man5GlcNAc1 (6, Scheme 1b).24 Further processing of this compound by mannosidases creates Man3GlcNAc1 (7) that can be modified by MGAT2 to install a β1,2GlcNAc to the α1,6Man antenna to give Man3GlcNAc2 (8). It is, however, known that MGAT1 can also modify Man325 thereby providing a substrate that potentially is adaptable to a stop-and-go strategy to prepare, in a controlled manner, asymmetrical biantennary N-glycans. It was found that treatment of Man3 (9) (Scheme 1c), which was readily obtained from a sialoglycopeptide isolated from egg yolk powder,21,26 can be modified by MGAT1 in the presence of UDP-GlcNAc or UDP-GlcNTFA to give glycans 10 and 13. The latter two compounds could be further modified by MGAT2 in the presence of UDP-GlcNTFA or UDP-GlcNAc to give, after treatment with a base, asymmetrical glycans 12 and 15. The MGAT1 and MGAT2 antenna of these compounds can then be selectively extended by various numbers of N-acetyl lactosamine moieties, which in turn can be capped by 2,3- and 2,6-linked sialosides to give structurally diverse asymmetrical N-glycans.
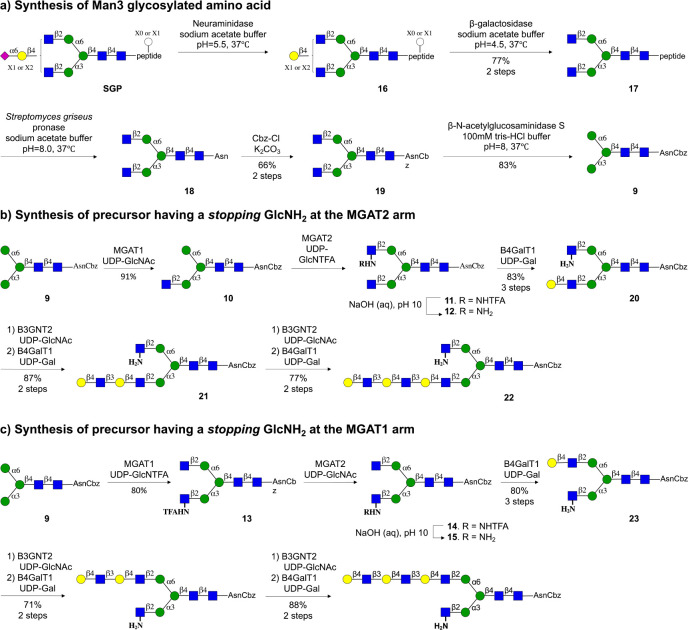
Man3 glycosylated amino acid 9, which at its anomeric center has a benzyloxycarbamante (Cbz) protected asparagine, could readily be prepared from a sialoglycopeptide (SGP) isolated from egg yolk powder (Scheme 2a).21,26 Thus, SGP was subsequently treated with a neuraminidase from Clostridium perfringens (→16) and galactosidase from Aspergillus niger to remove the sialosides and galactosides to give glycopeptide 17 having a G0 structure. Next, the peptide moiety was hydrolyzed by Pronase to leave a single Asn residue to afford 18. The α-amine of Asn provides a convenient handle for immobilization on microarray slides having N-hydroxysuccinimide (NHS). It was, however, important to temporarily protect this residue because, in the subsequent synthesis, GlcNH2 residues are introduced at the MGAT1 or MGAT2 antenna, and it was critical to differentiate the amines of GlcN and Asn. The latter could easily be accomplished by treatment of 18 with benzyloxycarbonyl chloride (CbzCl) in the presence of K2CO3 to give benzyloxycarbamate (Cbz) protected 19. Finally, the terminal GlcNAc residues of 19 were removed by treatment with β-N-acetylglucosaminidase S from Streptococcus pneumoniae to give targeted M3 glycosylated amino acid 9, which was purified by size-exclusion and Hypercarb solid phase extraction (SPE) column chromatography and fully characterized by NMR and MS.
Scheme 2. Chemoenzymatic Synthesis of Asymmetrical N-Glycans Having an Extended LacNAc Moiety at the MGAT1 or MGAT2 Antenna.
a) Preparation of tri-mannoside 9 from a sialoglycopeptide isolated from egg yok powder. b) Preparation of asymmetrical N-glycans having an extended LacNAc moiety at the MGAT1 antenna. c) Preparation of asymmetrical N-glycans having an extended LacNAc moiety at the MGAT2 antenna.
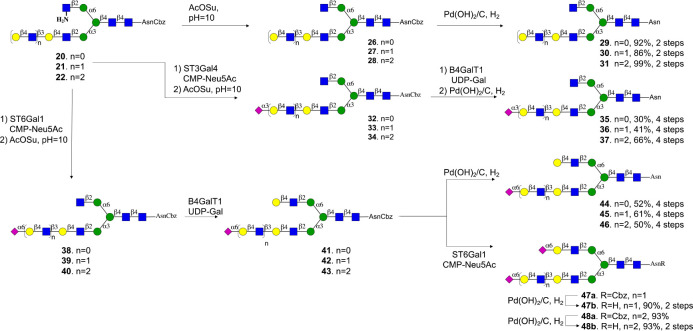
Next, attention was focused on the preparation of asymmetrical glycan 12, which has a natural GlcNAc and an unnatural GlcNH2 moiety at the MGAT1 and MGAT2 antennae, respectively (Scheme 2b). Thus, treatment of Man3 derivative 9 with UDP-GlcNAc in the presence of recombinant MGAT127 resulted in the formation of 10, which was further treated with UDP-GlcNTFA in the presence recombinant MGAT2 to afford 11, which was subjected aqueous sodium hydroxide (pH = 10) to provide target compound 12. These results highlight that MGAT2 can utilize an unnatural donor such as UDP-GlcNTFA, which agrees with the finding that metabolic labeling of cells with diazirine modified GlcNAc results in low incorporation of the modified GlcN moiety at the MGAT2 position of N-glycans.28 The GlcNH2 residue of 12 is not a substrate for B4GalT1, thereby temporarily stopping all of the enzymatic modifications at this antenna. It allowed the MGAT1 antenna to be selectively extended by various LacNAc moieties by employing recombinant B4GalT1 and B3GnT2. Thus, treatment of 12 with B4GalT1 in the presence of UDP-GlcNAc introduced a Gal residue to give LacNAc-containing derivative 20. Additional LacNAc moieties could be introduced by one or two cycles of B3GnT2 and GalT1 catalyzed extensions to give compounds 21 and 22, respectively. The intermediate and final compounds were purified by solid phase extraction using porous graphitized carbon, which was followed by P-2 Biogel size exclusion column chromatography. All compounds were fully characterized by nuclear magnetic resonance (NMR) including Homonuclear Correlation Spectroscopy (COSY) and Heteronuclear Single Quantum Coherence (HSQC) experiments. Further confirmation of structural identity and purity came from analysis by liquid chromatography–mass spectrometry (LC-MS) using hydrophilic interaction liquid chromatography (Waters XBridge BEH, Amide column) (see the Supporting Information for details).
We found that MGAT1 can also accept unnatural glycosyl donors, and treatment of 9 with UDP-GlcNTFA in the presence of MGAT1 resulted in a quantitative conversion into 13 (Scheme 3). Despite the presence of the NTFA moiety, the latter compound is a proper acceptor for MGAT2 and could readily be converted into 14, which after treatment with aqueous sodium hydroxide (pH = 10), gave the target derivative 15. In this case, the GlcNH2 at the MGAT1 stops temporary modification by glycosyl transferases, and thus it was possible to selectively extend the MGAT2 antenna by various LacNAc moieties to give access to compounds 23, 24, and 25 having a mono-, di-, or tri-LacNAc moiety.
Scheme 3. Selective Modification of Termini of the MGAT1 and MGAT2 Antennae of Glycans 20–22.
Compounds 20–25 are ideally suited to prepare a panel of asymmetrical N-glycans having different patterns of sialosides at LacNAc moieties of different length. For example, the terminal galactoside of compounds 20–22 could be modified by an α2,6-sialoside by ST6Gal1 in the presence of CMP-Neu5Ac to give, after acylation of the amine (→38–40) and galactosylation of the resulting terminal GlcNAc moiety with B4GalT1, compounds 41–43 (Scheme 3). The Cbz protecting group at the α-amine of the asparagine residue of the latter compounds was removed by hydrogenation over Pd/C to give sialosides 44–46. The terminal Gal moiety of 41–43 could be further sialylated with ST6Gal1 (→47a, 48a) to give, after hydrogenation, disialosides 47b and 48b. Alternatively, the terminal galactoside of 20–22 could be modified by a 2,3-sialoside by treatment with ST3Gal4 to provide compounds 32–34, which were subjected to hydrogenation to give target compounds 35–37. Reference compounds 29–31 were prepared by acylation of the amine of 20–22 to give 26–28 followed by hydrogenation over Pd/C.
Compounds 23–25 were subjected to a similar sequence of enzymatic and chemical modifications to give a complementary panel of 11 asymmetrical glycans (52–60, 67–71, Scheme S1) that have an extended LacNAc moiety at the MGAT2 antenna. N-Glycans have been observed in which the MGAT1 is extended by various numbers of LacNAc moieties and lack a GlcNAc moiety at the MGAT2 antenna.29 Such compounds can also be prepared by the methodology described here by employing compound 10 as the starting material, which can be galactosylated by B4GalT1 to install a LacNAc moiety. Various cycles of modification by B3GnT2 and B4GalT1 could install additional LacNAc moieties that could be capped by sialosides by using an appropriate sialyl transferase to give compounds 75, 77, 79, 82, 87, and 89 (Scheme S2).
Receptor Specificities of Influenza A Viruses
A(H3N2) and A(H1N1)pdm09 influenza virus subtypes that originate from the pandemics of 1968 and 2009, respectively, are major causes of the current seasonal influenza.30 Due to immunity caused by natural infection and vaccination, influenza A viruses continuously evolve to escape antibody-mediated neutralization. Protective antibody responses are mainly directed to the globular head of the hemagglutinin protein where binding occurs with sialic acid receptors of host cells.31 These mutational changes results in antigenic divergence from employed vaccine strains and circulating epidemic viruses, resulting in immune escape by the virus and poor protection.32 It can also alter receptor binding properties by orchestrated mutations of several amino acids in the globular head that allow immune evasion.14 The set of asymmetrical N-glycans developed here offers unique opportunities to investigate receptor binding determinants of evolutionary distinct influenza A viruses. It can determine the importance of valency and sialoside-linkage type, the length of a LacNAc chain, and placement of the binding epitope at the α1,3- (MGAT1) or α1,6- (MGAT2) antenna of biantennary N-glycans.
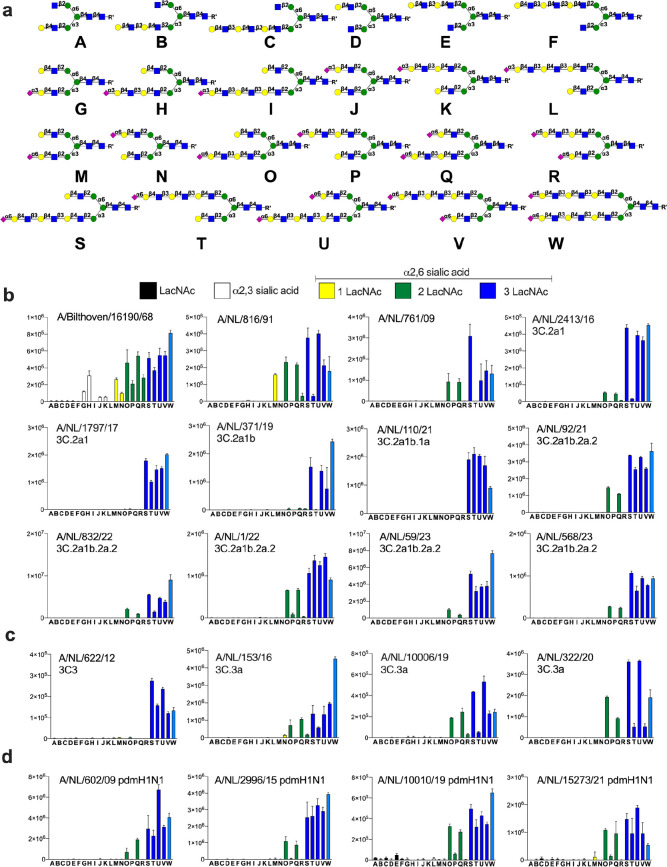
The glycans, which have an α-amine at the anomeric asparagine moiety, were printed on amine reactive NHS activated glass slides using a noncontact microarray printer (S3, Scienion, Inc.). Quality control was performed using biotin-labeled plant lectins such as SNA, ECA, and MAL. SNA binds 2,6-linked sialosides, and as expected, all compounds having such a moiety (Figure 1, M–W) were recognized by approximate equal intensity (Figure S40). ECA, which binds to terminal LacNAc moieties, bound to all compound having such a residue (Figure 1, A–P, S, and T) and as expected only the disialosides (Figure 1, Q, R, U, V, and W) did not show responsiveness. As expected, the MAL proteins specifically bound to 2,3-linked sialosides (Figure 1, G–L).
Figure 1.
Probing receptor binding specificities of A(H3N2) and A(H1N1)pdm09. a) Collection of glycans printed on succinimide reactive microarray slides. Glycan binding data of b) early A(H3N2) and A(H3N2) 3C.2 viruses; c) A (H3N2) 3C.3 viruses; and d) A(H1N1)pdm09 viruses. Whole viruses were exposed to a glycan microarray, and binding was visualized antistalk antibodies. Bars represent the average relative fluorescence units (RFU) of four replicates ± SD.
Next, receptor binding properties were examined of evolutionary distinct A/H3N2 viruses. These viruses entered the human population during the 1968 pandemic and split into different antigenic clades such as those designated as 3C.2 and 3C.3 earlier this century, which further evolved into subclades. We analyzed receptor preferences of viruses isolated from the pandemic period to current epidemics, including the most recent 3C.2 and 3C.3 clades (Figure 1). Also, receptor binding properties of several recent A(H1N1)pdm09 viruses and an avian A/H5N1 virus that can infect humans were examined. Viral isolates were applied to the array and detection of binding was accomplished using an appropriate anti-stalk antibody and a goat anti-human IgG antibody labeled with AlexaFluor-647.16 The array studies were performed in the presence of a neuramidase inhibitor to avoid interference with this protein.
An A(H3N2) virus that was isolated during the pandemic (A/Bilthoven/68) showed promiscuous binding behavior and bound to 2,3- and 2,6-linked sialosides (Figure 1a). Interestingly, very little binding was observed when the 2,3-linked sialoside is presented on a tri-LacNAc moiety (I and L), whereas proper recognition is observed when such a sialoside is placed on a di-LacNAc structure (G and H). An A(H3N2) virus isolated in 1991 (A/NL/861/91), lost all binding to 2,3-linked sialosides and only recognized 2,6-linked sialosides. It displays a strong preference for the presentation of this epitope at the α1,3-antenna (MGAT1 extension). For example, compound M, in which the 2,6-sialoside is presented at a single LacNAc moiety at the α1,3-antenna, was bound strongly by the virus, whereas this was not the case for isomeric glycan N that has the epitope at the α1,6-antenna (MGAT2 extension). N-Glycans that have extended 2,6-sialyl-LacNAc moieties were also recognized and in these cases presentation of the epitope at the α1,3-antenna was also strongly preferred (O vs P, Q vs R, and S vs T). A virus isolated in 2009 (A/NL/761/NL09) became even more selective, recognizing fewer glycans and did not bind to structures having α2,6-sialosides presented at a mono-LacNAc moiety (M and N). These viruses required a 2,6-sialoside to be presented at a di- or longer LacNAc moiety (O and Q) at the α1,3-antenna. Isomeric compounds in which these epitopes are presented at the α1,6-antennae (MGAT2 extension) (P and T) were bound poorly. A/NL/2413/16, which belongs to the 3C.2a clade, showed similar structure-binding properties; however, in this case the tri-LacNAc-containing compounds bound much more robustly (O vs S and Q vs U). The further evolved A/NL/1797/17, A/NL/371/19, and A/NL/110/21 viruses (3C.2a clade) showed the most restrictive binding pattern and had an obligatory requirement for presentation of the α2,6-sialoside at a tri-LacNAc structure. A/NL/371/19 exhibited an antenna preference and for monosialosides, the sialyl-tri-LacNAc epitope needs to be displayed at the α1,3-arm (S vs T), whereas this is not the case for the bis-sialosides (Q, U, and V). A/NL/1797/17 and A/NL/110/21 did not exhibit such an antenna preference, and compounds S and T were strongly bound. Subsequent 3C.2a1b.2a.2 viruses (A/NL/92/21, A/NL/832/22, A/NL/1/22, A/NL/59/23, A/NL/568/23) exhibited broader receptor binding properties compared to early 3C.2a viruses and also bound to O and Q that have the 2,6-sialoside at a di-LacNAc moiety at the α1,3-antenna only.
An early 3C3 viruses (A/NL/622/12) required a tri-LacNAc moiety modified by a 2,6-sialoside (S–V) for binding, whereas later viruses also recognized compounds having the sialoside presented at a di-LacNAc moiety (O and Q) and here asymmetry is also noted (S vs T and U vs V (Figure 1c). Interestingly, several A(H1N1)pdm09 subtype viruses that re-entered the human population during the 2009 pandemic, showed a preference for 2,6-linked sialosides at di-LacNAc moieties presented at the α1,3-antenna (Figure 1d) (O vs P). In the cases of tri-LacNAc derivatives, this antenna preference was not observed, and compounds S–V bound robustly.
Previously, it was proposed that A(H3N2) viruses bind host N-glycans through a bidentate binding mode in which N-glycan having two sialic acid moieties bind to a protomer of the same HA trimer thereby increasing the binding avidity.13 Modeling studies indicated that the sialosides need to be displayed at a tri-LacNAc moiety to bind to two HA protomers. We observed that compound W, which has 2,6-sialylated tri-LacNAc moieties at the α1,3- and α1,6-antenna, showed similar responsiveness compared to compound S that only has one such moiety at the α1,3-antennae. Thus, it is unlikely that these viruses establish a bidentate binding mode, and it is probable that the extended LacNAc moiety makes interactions with the HA protein to compensate for reduced contacts with sialic acid caused by substitutions in the receptor-binding site arisen from antigenic pressure.31
Computational studies have indicated that A(H3N2) 3C2a viruses have an obligatory requirement for a 2,6-sialylated tri-LacNAc moiety. This was acquired with mutational changes distal to the receptor binding domain that reoriented the Y159 side chain, resulting in an extended receptor binding site that can accommodate the tri-LacNAc moiety.16 Sequence alignment showed that contemporary 3C.2a1b.2a.2 viruses that can bind sialylated di-LacNAc-containing structures have aspartic acid at position 159 (Table S3). Such a mutation was also observed in 3C3 viruses that adapted to binding to the 2,6-sialylated di-LacNAc moiety. Moreover, the adjacent T160I abrogates an N-glycosylation site that may affect receptor binding properties.33 Importantly, the 190-helix, which can make interactions with the LacNAc moiety, has been under heavy antigenic pressure as several positions have changed including G186D, D190N, F193S, and Y196F. Positions 186,34 190,35 and 19336 are hallmark mutations for different receptor binding properties in A/H3 and other subtypes; however, their individual contributions to receptor binding specificity and antigenicity remain to be determined. Thus, it appears that recent A(H3N2) viruses have made compensatory mutations for the loss of interactions with Y159. Furthermore, for most of the recent subtypes, it has resulted in some ability to bind di-LacNAc-containing sialosides.
Receptor binding properties were also investigated of an avian A/H5N1 virus that can infect but not transmit between humans (A/Indonesia/05/05). In this case, all 2,3-linked sialosides were bound without differentiation of presentation of the epitope on the α1,3- or α1,6-antenna and, for example, isomeric compounds G and J gave similar responsiveness (Figure S40).
Hemagglutination Properties of Contemporary A(H3N2) Viruses
For antigenic surveillance and vaccine strain selection of influenza A viruses, the hemagglutination inhibition (HI) assay is used, in which the ability of serum antibodies to prevent virus-mediated agglutination of erythrocytes is measured.37 HI titers provide a correlate of protection and make it possible to select virus strains that are antigenically representative of circulating viruses for vaccine development.38 3C2a viruses, which have an obligatory requirement for a 2,6-sialylated tri-LacNAc moiety, lost the ability to agglutinate commonly employed chicken and turkey erythrocytes.14 Glycomic analysis of the latter erythrocytes have shown these erythrocytes do not express N-glycans having such residues thereby providing a rationale for a lack.16 The microarray studies presented here demonstrate that recent A(H3N2) strains of C2 and C3 clades regained the ability to bind di-LacNAc-containing sialosides. Turkey erythrocytes express low levels of these glycans, and thus we were compelled to investigate whether the recent C2 and C3 strains can agglutinate turkey erythrocytes. Previously, we introduced a glycan remodeling approach for turkey erythrocytes to elongate the LacNAc moieties at which sialosides are presented.16 It is based on treatment with a sialidase to remove sialosides to reveal terminal galactosides that can be extended by additional LacNAc moieties by treatment with B4GalT1 and B3GnT2 followed by sialylation of terminal galactosides by the sialyltransferase ST6Gal1. Agglutination properties of these glycan remodeled erythrocytes (2,6-Sia-poly-LN cells) were also examined. To investigate whether an increase in the abundance of α2,6-sialosides on mono- and di-LacNAc-containing structures can improve agglutination, control erythrocytes were prepared by treatment with neuraminidase followed by resialylation with ST6Gal1(2,6-Sia cells). In this way, 2,3-sialosides and terminal galactosides are converted into 2,6-sialosides resulting in a higher receptor density of human viruses.
Table 1 summarizes hemagglutination titers for the A(H3N2) viruses, for which the receptor binding properties were investigated using the new glycan microarray. Regular as well as glycan remodeled turkey erythrocytes (2,6-Sia- and 2,6-Sia-poly-LN cells) were employed. The early viruses (1968 and 1991), which require for binding a single LacNAc moiety, could readily agglutinate the three erythrocyte types. A/NL/761/09 did not agglutinate regular erythrocytes while the 2,6-Sia and 2,6-Sia-poly-LN cells were robustly agglutinated with similar titers. The glycan array data for this virus showed it can bind di- and tri-LacNAc-containing sialoglycans with similar affinities (Figure 1b), and thus, for this virus, increasing the abundance of α2,6-linked sialosides having two consecutive LacNAc repeating units is sufficient to induce agglutination. The further evolved A/NL/2413/16 did bind 2,6-sialosides presented on a di-LacNAc moiety but with substantial lower responsiveness compared the tri-LacNAc-containing counterparts. The hemagglutination properties agree with this binding pattern and no agglutination was observed for regular erythrocytes, while the 2,6-Sia and 2,6-Sia-poly-LN cells exhibited agglutination with the latter giving a substantial higher titer. The 3C.2 viruses that appeared in 2017 and 2019 have a strict requirement for a 2,6-linked sialoside to be displayed at a tri-LacNAc moiety, and as expected, they did not agglutinate the regular and 2,6-Sia erythrocytes and required the N-glycans to be extended with additional LacNAc moieties (2,6-Sia-poly-LN cells) to achieve agglutination.
Table 1. Hemagglutination Titers of A(H3N2) Viruses for Wild Type (WT) and Glyco-Remodeled (Mod) Turkey Erythrocytes and the Minimal Requirement of the Length of the LacNAc Moiety for Sialoglycan Binding.
| name | GISAID | HA (WT) titer | HA (2,6-Sia) titera | HA (2,6-Sia-poly-LN) titer | min. epitope (# LacNAc) |
|---|---|---|---|---|---|
| A/H3N2 3C.2 viruses (Figure 1b) | |||||
| A/Bilthoven/16190/68 | EPI_ISL_4253 | 64 | 48 | 64 | 1 |
| A/NL/816/91 | EPI_ISL_4286 | 64 | 128 | 128 | 1 |
| A/NL/761/09 | EPI_ISL_110727 | 0 | 64 | 64 | 2 |
| A/NL/2413/16 | EPI_ISL_242904 | 3 | 24 | 128 | 2 |
| A/NL/1797/17 | EPI_ISL_526242 | 0 | 0 | 32 | 3 |
| A/NL/371/19 | EPI_ISL_343216 | 0 | 0 | 32 | 3 |
| A/NL/110/21 | EPI_ISL_9027259 | 0 | 0 | 128 | 3 |
| A/NL/92/21 | EPI_ISL_7892106 | 48 | NA | 128 | 2 |
| A/NL/832/22 | EPI_ISL_12555385 | 48 | NA | 128 | 2 |
| A/NL/1/22 | EPI_ISL_8710616 | 24 | NA | 256 | 2 |
| A/NL/59/23 | EPI_ISL_16585498 | 24 | NA | 96 | 2 |
| A/NL/568/23 | EPI_ISL_16955806 | 3 | NA | 24 | 2 |
| A/H3N2 3C.3 viruses (Figure 1c) | |||||
| A/NL/622/12 | EPI_ISL_166348 | 0 | 0 | 12 | 3 |
| A/NL/153/16 | EPI_ISL_233371 | 12 | 24 | 128 | 2 |
| A/NL/10006/19 | EPI_ISL_336268 | 8 | 12 | 64 | 2 |
| A/NL/322/20 | EPI_ISL_415717 | 48 | NA | 64 | 2 |
NA not available.
Most of the 3C.2 viruses that appeared after 2021 regained the ability to agglutinate regular erythrocytes, and importantly these viruses reverted to bind to di-LacNAc-containing structures (Figure 1b,c). Unlike A/NL/2413/16, these viruses do not require an increase in receptor density to achieve agglutination. This indicates that in addition to the length of the LacNAc chain, other factors such as avidity of binding and the balance between HA/NA activity may contribute to the ability to agglutinate regular erythrocytes. These recent viruses did give higher agglutination titers for the 2,6-Sia-poly-LN erythrocytes, which agrees with the glycan array data that showed substantially higher responsiveness for tri-LacNAc-containing structures.
A/NL/110/2021 is a recent 3.C2 virus that could not agglutinate regular erythrocytes. Interestingly, it has an obligatory requirement for a sialylated tri-LacNAc-containing N-glycans (Figure 1b) thereby providing a rationale for this behavior. A/Netherlands/568/23 has some binding capacity to bind di-LacNAc-containing sialosides. However, it agglutinated regular erythrocytes with a very low titer, and its behavior is more akin to A/NL/2413/16. Hemagglutination titers of a broader range of A/H3N2 viruses isolated between 2021 and 2023 were determined and almost all gave good titers for regular erythrocytes (Table S2).
The agglutination properties of the 2C.2 viruses agreed with the glycan microarray data, and a virus that only bound tri-LacNAc-containing compounds only agglutinated the 2,6-Sia-poly-LN erythrocytes.
Conclusion
Glycan-binding proteins often recognize relatively small oligosaccharide motifs found at termini of complex glycans and glycoconjugates.1,39,40 There are, however, indications that the topology of a complex glycan can modulate recognition of terminal glycan epitopes.41−43 This can be due to an extended binding site, unfavorable interactions by a glycan moiety at which a minimal epitope is appended, conformational changes of larger glycan moieties, and multivalency. Panels of well-defined glycans are needed to probe the importance of glycan topology to glycan binding properties. Here, we describe a synthetic methodology to prepare asymmetrical biantennary glycans that have extended LacNAc moieties at the α1,3- (MGAT1) or α1,6-antenna (MGAT2). It exploits the finding that MGAT1 and MGAT2 can utilize the unnatural sugar donor UDP-GlcNTFA. The TFA moiety of the resulting glycans can be selectively hydrolyzed to give compounds having a GlcNH2 moiety at one of the antennae to temporarily block extension by glycosyl transferases. It made it possible to conveniently prepare a library of asymmetrical N-glycans that resemble structures found in the respiratory tract.4,5 The compounds were printed as a glycan microarray that was used to examine receptor binding properties of evolutionaryly distinct A(H3N2) influenza viruses ranging from a pandemic strain of 1968 to recent isolates. Earlier this century, A(H3N2) viruses split into two different antigenic clades designated as 3C.2 and 3C.3, which further evolved in subclades. Binding studies with the collection of compounds described here revealed that not only the length of the LacNAc chain but also presentation on a specific antenna is critical for receptor binding. For many of the examined viruses, a preference for the presentation of the epitope on the α1,3-antenna was observed. The data also reveals that a single sialylated LacNAc moiety is sufficient for binding and thus it is unlikely that these viruses engage in a previously proposed bident binding mode.44
3C.2 viruses isolated between 2017 and 2019 displayed the most restricted binding pattern and only recognized glycans having a 2,6-linked sialoside presented at a tri-LacNAc structure on the α1,3-antenna (Figure 1b). This specificity was also observed for a 3C.2a1b.1a virus isolated in 2021. However, most virus isolates at that time until now belong to the 2a.2 subclade and most of these viruses regained some binding capacity to sialylated di-LacNAc structures.45 These di-LacNAc-containing structures need to be displayed on the mannose of the α3-antenna for recognition (O vs P and Q vs R). This observation was further strengthened by analyzing A(H3N2) viruses of the 3C.3 antigenic clade that already in 2016 regained binding of these di-LacNAc-containing structures (Figure 1c).
ST6Gal1, which is the only sialyl transferase that installs terminal 2,6-sialosides at terminal LacNAc moieties, preferentially modifies the LacNAc moiety displayed at the α1,3-antenna,44 and thus early H3 viruses such as NL91 likely evolved to bind such glycans (Figure 1b). The requirement for presentation of an extended sialylated LacNAc moiety on the MGAT1 antenna may be due to a similar antenna preference of ST6Gal1. It is also possible that due to steric reasons, the α1,3-antenna is less assessable for binding. The molecular mechanism for α1,3-antenna preference needs further examination.
A(H3N2) influenza viruses that appeared after the turn of the centenary lost the ability to agglutinate commonly employed fowl erythrocytes.46 This greatly complicates antigenic surveillance and vaccine strain selection, which relies on the hemagglutination inhibition assay, in which the ability of serum antibodies to block receptor binding by the influenza virus HA protein is quantified. We found that all viruses that lost the ability to agglutinate regular and 2,6-resiaylated erythrocytes require as a minimal epitope a 2,6-sialoside presented at a tri-LacNAc moiety. Surprisingly, most of the A(H3N2) that appeared after 2021 regained an ability to agglutinate common erythrocytes, and these viruses had reverted to the use of a sialoside presented a di-LacNAc-containing structures as a minimal epitope. Earlier A(H3N2) viruses, such as A/NL/761/09 and A/NL/2413/16, employ similar structures as a minimal epitope; however, these viruses cannot agglutinate regular erythrocytes and require a higher density of receptors as found on the 2,6-Sia cells. This indicates that properties such as the avidity of binding and HA/NA balance contribute to agglutination properties of these viruses.
We also analyzed receptor binding properties of A(H1N1)pdm09 viruses that were reintroduced during the 2009 pandemic (Figure 1d). All viruses tested had receptor binding properties similar to those of contemporary A(H3N2) viruses. Thus, a 2,6-sialoside at a di-LacNAc moiety presented at the α1,3-antenna appears to be a commonly employed receptor for human influenza A viruses. It is likely that the di-LacNAc moiety allows for additional interactions with HA for sufficient high affinity of binding to compensate for reduced binding of sialic acid due to mutational changes caused by antigenic pressure.19
Methods
General Methods of Enzymatic Synthesis
All enzymatic reactions were performed in aqueous buffers at the appropriate pH for each enzyme. Recombinant human glycosyl transferases including α-1,3-mannosyl-glycoprotein 2-β-N-acetylglucosaminyltransferase (MGAT1), α-1,6-mannosyl-glycoprotein 2-β-N-acetylglucosaminyltransferase (MGAT2), β-1,3-N-acetylglucosaminyltransferase 2 (B3GNT2), β-1,4-galactosyltransferase 1 (B4GalT 1), β-galactoside-α-2,6-sialyltransferase 1 (ST6Gal1), and β-galactoside-α-2, 3-sialyltransferase 4 (ST3Gal4) were provided by Dr. K. W. Moremen (Complex Carbohydrate Research Center, Athens, GA, USA) which were expressed according to published protocols.22,27,47 Alkaline phosphatase from calf intestine (CIAP) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich. Uridine 5′-diphospho-N-acetylglucosamine (UDP-GlcNAc) was purchased from Sigma-Aldrich. Uridine 5′-diphosphogalactose diphosphate galactose (UDP-Gal) and cytidine 5′monophospho-N-acetylneuraminc acid (CMP-Neu5Ac) were both purchased from Roche. Uridine 5′-diphospho-N-trifluoroglucosamine (UDP-GlcNTFA) was synthesized utilizing a one-pot three-enzyme combination as previously reported.48 The progress of enzymatic reactions was monitored using a Shimadzu 20AD UFLC LCMS-IT-TOF. To facilitate isolation and purification of products, enzymatic reactions were driven to completion by adding additional glycosyl transferase until all starting material was consumed. Nuclear magnetic resonance (NMR) spectra were acquired on a 600 MHz Varian Inova operating at 25 °C. Samples were dissolved in 99.96% D2O and chemical shifts were referenced to the residual HDO signal at 4.79 ppm. Data were collected using standard pulse programs.
General Procedure for the Purification of Synthesized N-Glycans
Reaction mixtures were heated for 10 min at 80 °C to precipitate the enzyme. The suspension was centrifugated and the decanted solution was lyophilized to yield a powder. To purify base-sensitive products having a TFA moiety, a Hypercarb SPE column was used using 50 mM buffered ammonium formate as eluant (pH = 7, including 0%–50% (v/v) of acetonitrile). Fractions containing product were collected and lyophilized. The lyophilized product was dissolved and loaded on a P-2 Biogel size-exclusion column, which was eluted with a 5% (v/v) n-butanol/water, and the product fractions were collected and lyophilized to give the desired product as a fluffy white powder. To purify the other products, the same procedures was applied but different buffered elution solutions were used. The 50 mM ammonium bicarbonate buffer was replaced with 50 mM ammonium formate buffer for eluting the SPE column, and the 0.1 M ammonium bicarbonate buffer was used for eluting the P-2 Biogel column rather than the 5% (v/v) n-butanol/water solution. Residual sugar nucleotides were eluted using 0%–10% (v/v) acetonitrile/salt solution and the different N-glycan were eluted and collected using 11%–18% (v/v) acetonitrile/salt solution.
General Procedure for the Installation of β1,2-GlcNAc or β1,2-GlcNTFA Using MGAT1
Compound 9 (1.0 equiv) and UDP-GlcNAc or UDP-GlcNTFA (1.5 equiv) were dissolved in a HEPES buffer solution (100 mM, pH 7.0) containing MnCl2 (2 mM) and BSA (1% total volume, stock solution = 10 mg/mL) keeping a final acceptor concentration of 10 mM. Calf intestine alkaline phosphatase (CIAP, 1% total volume, stock solution = 1 kU/mL) and recombinant MGAT1 (50 μg/μmol acceptor) were successively added, and the reaction mixture was incubated overnight at 37 °C with shaking. The product was purified by Hypercarb SPE followed by P-2 Biogel size-exclusion column chromatography according to the general protocol.
General Procedure for the Installation of β1,2-GlcNAc or β1,2-GlcNTFA Using MGAT2
Acceptor (1.0 equiv) and UDP-GlcNAc or UDP-GlcNTFA (1.5 equiv) were dissolved in a HEPES buffer solution (100 mM, pH 7.0) containing MnCl2 (2 mM), and BSA (1% total volume, stock solution = 10 mg/mL) to provide a final acceptor concentration of 10 mM. CIAP (1% total volume, stock solution = 1 kU/mL) and MGAT2 (50 μg/μmol acceptor) were successively added, and the mixture solution was incubated overnight at 37 °C with shaking. The product was purified by Hypercarb SPE followed P-2 Biogel size-exclusion column chromatography according to the general protocol.
General Procedure for the Installation of β1,4 Gal Using B4GALT1
Acceptor (1.0 equiv) and UDP-Gal (1.5 equiv) were dissolved in a Tris-HCl buffer solution (100 mM, pH 7.5) containing MnCl2 (2 mM), and BSA (1% total volume, stock solution = 10 mg/mL) to provide a final acceptor concentration of 5 mM. CIAP (1% total volume, stock solution = 1 kU/mL) and B4GalT 1 (1% wt/wt relative to acceptor) were successively added, and the mixture solution was incubated overnight at 37 °C with shaking. The product was purified by Hypercarb SPE followed P-2 Biogel size-exclusion column chromatography according to the general protocol
General Procedure for the Installation of β1,3 GlcNAc Using B3GNT2
Acceptor (1.0 equiv) and UDP-GlcNAc (1.5 equiv) were dissolved at a final acceptor concentration of 10 mM in a HEPES buffer solution (100 mM, pH 7.3) containing MnCl2 (2 mM), KCl (25 mM), MgCl2 (2 mM), DTT (1 mM) and BSA (1% total volume, stock solution = 10 mg/mL). CIAP (1% total volume, stock solution = 1 kU/mL) and B3GNT2 (1% wt/wt relative to acceptor) were successively added, and the reaction mixture was incubated overnight at 37 °C with shaking. The product was purified by Hypercarb SPE followed P-2 Biogel size-exclusion column chromatography according to the general protocol.
General Procedure for the Installation of Terminal α2,6-Neu5Ac Using ST6GAL1
Acceptor (1 equiv) and CMP-Neu5Ac (1.5 equiv) were dissolved at a final acceptor concentration of 5 mM in a sodium cacodylate buffer (100 mM, pH 6.5) containing BSA (1% volume total, stock solution = 10 mg/mL). CIAP (1% volume total, stock solution = 1 kU/mL) and ST6GAL1 (1% wt/wt relative to acceptor) were added. The reaction mixture was incubated with gentle shaking overnight at 37 °C. The product was purified by Hypercarb SPE followed P-2 Biogel size-exclusion column chromatography according to the general protocol.
General Procedure for the Installation of Terminal α2,3-Neu5Ac Using ST3GAL4
Acceptor substrate (1 equiv) and CMP-Neu5Ac (1.5 equiv) were dissolved at a final acceptor concentration of 5 mM in a sodium cacodylate buffer (100 mM, pH 7.2) containing BSA (1% volume total, stock solution = 10 mg/mL). CIAP (1% volume total, stock solution = 1 kU/mL) and ST3GAL4 (1% wt/wt relative to acceptor) were added. The reaction mixture was incubated with gentle shaking overnight at 37 °C. The product was purified by Hypercarb SPE followed by P-2 Biogel size-exclusion column chromatography according to the general protocol.
General Procedure for Removal of TFA Group of Synthesized N-Glycans
The substrate was dissolved in deionized water, providing a final substrate concentration of 10 mM. The pH of the reaction solution was adjusted to 10 using NaOH (1 M), and the mixture solution was incubated for 1–2 h at 37 °C with shaking. When complete, the reaction was neutralized by aqueous acetic acid (1 M). The product was purified by Hypercarb SPE followed P-2 Biogel size-exclusion column chromatography according to the general protocol.
General Procedure for Amine Acetylation of Synthesized N-Glycans
The amine containing N-glycans were dissolved (1 equiv) in 50 mM of sodium acetate (NaOAc) buffered solution (pH = 8.5) with a final substrate concentration of 10 mM. Solids of AcOSu (10 equiv) were added to convert the NH2 moiety into NHAc moiety and the reaction mixture was vigorously vortexed until all solids were dissolved. The reaction mixture was incubated with gentle shaking for 1–2 h at 37 °C. The reaction progress was monitored by LC-ESI-IT-TOF MS and additional AcOSu was added if the starting material was remaining. The product was purified by P-2 Biogel size-exclusion column chromatography and lyophilized to yield the desired product.
General Procedure for Removal of Cbz Group of Synthesized N-Glycans
The Cbz containing starting material was dissolved in a 10% t-butanol/water solution (1 mg/mL substrate concentration). To the solution was added a palladium hydroxide on carbon (20% wt suspended in 100 μL of t-butanol/water solution) with 1 mg/mL final concentration of 20% Pd(OH)2/C solid. The reaction was stirred vigorously under an atmosphere of hydrogen (1 atm) and monitored by LC-ESI-IT-TOF MS. Once the reaction had gone to completion, it was filtered through a Whatman syringe filter (0.2 μm) to remove the catalyst, and the filtrate was lyophilized to provide the final product.
General Procedure for LC-MS of Analysis of Final Compounds
LC-MS was performed on a Shimadzu LC-ESI-IT-TOF with a Waters XBridge BEH, amide column, 2.5 μm, 130 Å, 2.1 × 150 mm2 at a flow rate of 0.15 mL/min. Mobile phase A was 10 mM ammonium formate in water (pH 4.5); mobile phase B was acetonitrile (LC-MS grade). The final compounds were purified using a linear gradient with the following conditions (1 and/or 2):
| Condition 1 | Condition 2 | |
| Time (min) | B (%) | B (%) |
| 0 | 65 | 85 |
| 18 | 50 | 60 |
| 20 | 25 | 25 |
| 24 | 25 | 25 |
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01 AI165692 (to G.J.B) and R01AI165818 (to R.A.M.F). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.P.d.V is supported by the European Commission (802780). S. van Leeuwen, K. de Haan, C. Russell, and Z. Felix-Garza (Amsterdam UMC, The Netherlands) have contributed to the production of A(H1N1)pdm09 viruses. M. Pronk and P. Lexmond (Erasmus MC, The Netherlands) assisted with hemagglutination studies. R. Liang (Utrecht University, The Netherlands) prepared the glycan remodeled erythrocytes.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.3c00695.
Synthetic protocols, compound characterization, experimental procedure for microarray screening, NMR spectra (PDF)
Author Contributions
CRediT: Shengzhou Ma conceptualization, data curation, methodology, writing-original draft, writing-review & editing; Lin Liu conceptualization, data curation, methodology, writing-review & editing; Dirk Eggink methodology, writing-review & editing; Sander Herfst methodology, writing-review & editing; Ron A. M. Fouchier methodology, resources, writing-review & editing; Robert P. de Vries data curation, methodology, resources, writing-review & editing; Geert-Jan Boons conceptualization, methodology, resources, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Thompson A. J.; de Vries R. P.; Paulson J. C. Virus recognition of glycan receptors. Curr. Opin. Virol. 2019, 34, 117–129. 10.1016/j.coviro.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The top 10 causes of death. World Health Organization Newsroom, 2020, https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Le Pendu J.; Nystrom K.; Ruvoen-Clouet N. Host-pathogen co-evolution and glycan interactions. Curr. Opin. Virol. 2014, 7, 88–94. 10.1016/j.coviro.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Walther T.; Karamanska R.; Chan R. W.; Chan M. C.; Jia N.; Air G.; Hopton C.; Wong M. P.; Dell A.; Malik Peiris J. S.; Haslam S. M.; Nicholls J. M. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013, 9, e1003223 10.1371/journal.ppat.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N.; Byrd-Leotis L.; Matsumoto Y.; Gao C.; Wein A. N.; Lobby J. L.; Kohlmeier J. E.; Steinhauer D. A.; Cummings R. D. The human lung glycome reveals novel glycan ligands for influenza A virus. Sci. Rep. 2020, 10, 5320. 10.1038/s41598-020-62074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N.; Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983, 127, 361–373. 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Shinya K.; Ebina M.; Yamada S.; Ono M.; Kasai N.; Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature 2006, 440, 435–436. 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Long J. S.; Mistry B.; Haslam S. M.; Barclay W. S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81. 10.1038/s41579-018-0115-z. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran A.; Srinivasan A.; Raman R.; Viswanathan K.; Raguram S.; Tumpey T. M.; Sasisekharan V.; Sasisekharan R. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat. Biotechnol. 2008, 26, 107–113. 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]
- Gambaryan A. S.; Tuzikov A. B.; Pazynina G. V.; Desheva J. A.; Bovin N. V.; Matrosovich M. N.; Klimov A. I. 6-Sulfo sialyl Lewis X is the common receptor determinant recognized by H5, H6, H7 and H9 influenza viruses of terrestrial poultry. Virol. J. 2008, 5, 85. 10.1186/1743-422X-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Chinoy Z. S.; Ambre S. G.; Peng W.; McBride R.; de Vries R. P.; Glushka J.; Paulson J. C.; Boons G. J. A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science 2013, 341, 379–383. 10.1126/science.1236231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Carney P. J.; Chang J. C.; Guo Z.; Villanueva J. M.; Stevens J. Structure and receptor binding preferences of recombinant human A(H3N2) virus hemagglutinins. Virology 2015, 477, 18–31. 10.1016/j.virol.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.; de Vries R. P.; Grant O. C.; Thompson A. J.; McBride R.; Tsogtbaatar B.; Lee P. S.; Razi N.; Wilson I. A.; Woods R. J.; Paulson J. C. Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity. Cell Host Microbe 2017, 21, 23–34. 10.1016/j.chom.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. D.; Ross T. M. H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation. Hum. Vaccin Immunother. 2018, 14, 1840–1847. 10.1080/21645515.2018.1462639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd-Leotis L.; Gao C.; Jia N.; Mehta A. Y.; Trost J.; Cummings S. F.; Heimburg-Molinaro J.; Cummings R. D.; Steinhauer D. A. Antigenic pressure on H3N2 influenza virus drift strains imposes constraints on binding to sialylated receptors but not phosphorylated glycans. J. Virol. 2019, 93, JVI.01178-19. 10.1128/JVI.01178-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broszeit F.; van Beek R. J.; Unione L.; Bestebroer T. M.; Chapla D.; Yang J. Y.; Moremen K. W.; Herfst S.; Fouchier R. A. M.; de Vries R. P.; Boons G. J. Glycan remodeled erythrocytes facilitate antigenic characterization of recent A/H3N2 influenza viruses. Nat. Commun. 2021, 12, 5449. 10.1038/s41467-021-25713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.; Blixt O.; Paulson J. C.; Wilson I. A. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nat. Rev. Microbiol. 2006, 4, 857–864. 10.1038/nrmicro1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. L.; Fisher C. J.; Godula K. Glycomaterials for probing host-pathogen interactions and the immune response. Exp. Biol. Med. 2016, 241, 1042–1053. 10.1177/1535370216647811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruit C. M.; Sweet I. R.; Maliepaard J. C. L.; Bestebroer T.; Lexmond P.; Qiu B.; Damen M. J. A.; Fouchier R. A. M.; Reiding K. R.; Snijder J.; Herfst S.; Boons G. J.; de Vries R. P. Contemporary human H3N2 influenza a viruses require a low threshold of suitable glycan receptors for efficient infection. Glycobiology 2023, 33, 784–800. 10.1093/glycob/cwad060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Prudden A. R.; Capicciotti C. J.; Bosman G. P.; Yang J. Y.; Chapla D. G.; Moremen K. W.; Boons G. J. Streamlining the chemoenzymatic synthesis of complex N-glycans by a stop and go strategy. Nat. Chem. 2019, 11, 161–169. 10.1038/s41557-018-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Prudden A. R.; Bosman G. P.; Boons G. J. Improved isolation and characterization procedure of sialylglycopeptide from egg yolk powder. Carbohydr. Res. 2017, 452, 122–128. 10.1016/j.carres.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L.; Forouhar F.; Thieker D.; Gao Z.; Ramiah A.; Moniz H.; Xiang Y.; Seetharaman J.; Milaninia S.; Su M.; Bridger R.; Veillon L.; Azadi P.; Kornhaber G.; Wells L.; Montelione G. T.; Woods R. J.; Tong L.; Moremen K. W. Enzymatic basis for N-glycan sialylation: structure of rat alpha2,6-sialyltransferase (ST6GAL1) reveals conserved and unique features for glycan sialylation. J. Biol. Chem. 2013, 288, 34680–34698. 10.1074/jbc.M113.519041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Eijnden D. H.; Blanken W. M.; van Vliet A. Branch specificity of beta-D-galactosidase from Eschericha coli. Carbohydr. Res. 1986, 151, 329–335. 10.1016/S0008-6215(00)90352-5. [DOI] [PubMed] [Google Scholar]
- Moremen K. W.; Tiemeyer M.; Nairn A. V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K. J.; Hindsgaul O. A simple synthesis of octyl 3,6-di-O-(alpha-D-mannopyranosyl)-beta-D-mannopyranoside and its use as an acceptor for the assay of N-acetylglucosaminyltransferase-I activity. Glycoconj. J. 1991, 8, 90–94. 10.1007/BF00731017. [DOI] [PubMed] [Google Scholar]
- Seko A.; Koketsu M.; Nishizono M.; Enoki Y.; Ibrahim H. R.; Juneja L. R.; Kim M.; Yamamoto T. Occurence of a sialylglycopeptide and free sialylglycans in hen’s egg yolk. Biochim. Biophys. Acta 1997, 1335, 23–32. 10.1016/S0304-4165(96)00118-3. [DOI] [PubMed] [Google Scholar]
- Kadirvelraj R.; Yang J. Y.; Sanders J. H.; Liu L.; Ramiah A.; Prabhakar P. K.; Boons G. J.; Wood Z. A.; Moremen K. W. Human N-acetylglucosaminyltransferase II substrate recognition uses a modular architecture that includes a convergent exosite. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 4637–4642. 10.1073/pnas.1716988115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Shajahan A.; Yang J. Y.; Capota E.; Wands A. M.; Arthur C. M.; Stowell S. R.; Moremen K. W.; Azadi P.; Kohler J. J. A photo-cross-linking GlcNAc analog enables covalent capture of N-linked glycoprotein-binding partners on the cell surface. Cell Chem. Biol. 2022, 29, 84–97.e8. 10.1016/j.chembiol.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Madunić K.; Zhang T.; Lageveen-Kammeijer G. S. M.; Wuhrer M. Profound diversity of the N-glycome from microdissected regions of colorectal cancer, stroma, and normal colon mucosa. Engineering 2023, 26, 32–43. 10.1016/j.eng.2022.08.016. [DOI] [Google Scholar]
- Harrington W. N.; Kackos C. M.; Webby R. J. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 2021, 53, 737–749. 10.1038/s12276-021-00603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koel B. F.; Burke D. F.; Bestebroer T. M.; van der Vliet S.; Zondag G. C.; Vervaet G.; Skepner E.; Lewis N. S.; Spronken M. I.; Russell C. A.; Eropkin M. Y.; Hurt A. C.; Barr I. G.; de Jong J. C.; Rimmelzwaan G. F.; Osterhaus A. D.; Fouchier R. A.; Smith D. J. Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science 2013, 342, 976–979. 10.1126/science.1244730. [DOI] [PubMed] [Google Scholar]
- Belongia E. A.; McLean H. Q. Influenza vaccine effectiveness: Defining the H3N2 problem. Clin. Infect. Dis. 2019, 69, 1817–1823. 10.1093/cid/ciz411. [DOI] [PubMed] [Google Scholar]
- Bolton M. J.; Ort J. T.; McBride R.; Swanson N. J.; Wilson J.; Awofolaju M.; Furey C.; Greenplate A. R.; Drapeau E. M.; Pekosz A.; Paulson J. C.; Hensley S. E. Antigenic and virological properties of an H3N2 variant that continues to dominate the 2021–22 Northern Hemisphere influenza season. Cell Rep. 2022, 39, 110897 10.1016/j.celrep.2022.110897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N. C.; Lv H.; Thompson A. J.; Wu D. C.; Ng W. W. S.; Kadam R. U.; Lin C. W.; Nycholat C. M.; McBride R.; Liang W.; et al. Preventing an antigenically disruptive mutation in egg-based H3N2 seasonal influenza vaccines by mutational incompatibility. Cell Host Microbe 2019, 25, 836–844.e5. 10.1016/j.chom.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N. C.; Thompson A. J.; Xie J.; Lin C. W.; Nycholat C. M.; Zhu X.; Lerner R. A.; Paulson J. C.; Wilson I. A. A complex epistatic network limits the mutational reversibility in the influenza hemagglutinin receptor-binding site. Nat. Commun. 2018, 9, 1264. 10.1038/s41467-018-03663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries R. P.; Peng W.; Grant O. C.; Thompson A. J.; Zhu X.; Bouwman K. M.; de la Pena A. T. T.; van Breemen M. J.; Ambepitiya Wickramasinghe I. N.; de Haan C. A. M.; Yu W.; McBride R.; Sanders R. W.; Woods R. J.; Verheije M. H.; Wilson I. A.; Paulson J. C. Three mutations switch H7N9 influenza to human-type receptor specificity. PLoS Pathog. 2017, 13, e1006390 10.1371/journal.ppat.1006390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Global Influenza Surveillance Network . Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization, 2011.
- Coudeville L.; Bailleux F.; Riche B.; Megas F.; Andre P.; Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med. Res. Methodol. 2010, 10, 18. 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D.; Pierce J. M. The challenge and promise of glycomics. Chem. Biol. 2014, 21, 1–15. 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. E.; Drickamer K. Mammalian sugar-binding receptors: known functions and unexplored roles. FEBS J. 2019, 286, 1800–1814. 10.1111/febs.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.; Varki A. The sialome--far more than the sum of its parts. OMICS 2010, 14, 455–464. 10.1089/omi.2009.0148. [DOI] [PubMed] [Google Scholar]
- Lowary T. L. Context and complexity: The next big thing in synthetic glycobiology. Curr. Opin. Chem. Biol. 2013, 17, 990–996. 10.1016/j.cbpa.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Haji-Ghassemi O.; Blackler R. J.; Martin Young N.; Evans S. V. Antibody recognition of carbohydrate epitopesdagger. Glycobiology 2015, 25, 920–952. 10.1093/glycob/cwv037. [DOI] [PubMed] [Google Scholar]
- Barb A. W.; Prestegard J. H. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat. Chem. Biol. 2011, 7, 147–153. 10.1038/nchembio.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Neher R.; Bedford T.. Real-time tracking of influenza A/H3N2 evolution. https://nextstrain.org/flu/seasonal/h3n2/ha/2y. [DOI] [PMC free article] [PubMed]
- Gulati S.; Smith D. F.; Cummings R. D.; Couch R. B.; Griesemer S. B.; St George K.; Webster R. G.; Air G. M. Human H3N2 influenza viruses isolated from 1968 to 2012 show varying preference for receptor substructures with no apparent consequences for disease or spread. PLoS One 2013, 8, e66325 10.1371/journal.pone.0066325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudden A. R.; Liu L.; Capicciotti C. J.; Wolfert M. A.; Wang S.; Gao Z.; Meng L.; Moremen K. W.; Boons G. J. Synthesis of asymmetrical multiantennary human milk oligosaccharides. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 6954–6959. 10.1073/pnas.1701785114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Thon V.; Li Y.; Yu H.; Ding L.; Lau K.; Qu J.; Hie L.; Chen X. One-pot three-enzyme synthesis of UDP-GlcNAc derivatives. Chem. Commun. 2011, 47, 10815–10817. 10.1039/c1cc14034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.