Abstract
The cytoplasmic protein tyrosine kinase Syk has two amino-terminal SH2 domains that engage phosphorylated immunoreceptor tyrosine-based activation motifs in the signaling subunits of immunoreceptors. Syk, in conjunction with Src family kinases, has been implicated in immunoreceptor signaling in both lymphoid and myeloid cells. We have investigated the role of Syk in Fcγ receptor (FcγR)-dependent and -independent responses in bone marrow-derived macrophages and neutrophils by using mouse radiation chimeras reconstituted with fetal liver cells from Syk−/− embryos. Chimeric mice developed an abdominal hemorrhage starting 2 to 3 months after transplantation that was ultimately lethal. Syk-deficient neutrophils derived from the bone marrow were incapable of generating reactive oxygen intermediates in response to FcγR engagement but responded normally to tetradecanoyl phorbol acetate stimulation. Syk-deficient macrophages were defective in phagocytosis induced by FcγR but showed normal phagocytosis in response to complement. The tyrosine phosphorylation of multiple cellular polypeptides, including the FcγR γ chain, as well as Erk2 activation, was compromised in Syk−/− macrophages after FcγR stimulation. In contrast, the induction of nitric oxide synthase in macrophages stimulated with lipopolysaccharide and gamma interferon was not dependent on Syk. Surprisingly, Syk-deficient macrophages were impaired in the ability to survive or proliferate on plastic petri dishes. Taken together, these results suggest that Syk has specific physiological roles in signaling from FcγRs in neutrophils and macrophages and raise the possibility that in vivo, Syk is involved in signaling events other than those mediated by immunoreceptors.
The cytoplasmic tyrosine kinase Syk has been implicated in a variety of hematopoietic cell responses, including immunoreceptor (1, 3, 4, 12, 22, 29, 44) and integrin signaling (7, 15). Syk possesses two N-terminal SH2 domains that bind in tandem to closely spaced pTyr sites located within the immunoreceptor tyrosine-based activation motifs (ITAMs) of antigen receptor subunits, such as the α and β chains associated with surface immunoglobulin M (IgM) in B cells (30) or the γ and β subunits of FcɛRI in mast cells (27, 42).
Engagement of the B-cell antigen receptor (BCR) has been suggested to result in the activation of Src family kinases, followed by phosphorylation of ITAMs and, consequently, recruitment of the Syk SH2 domains. Syk is activated, either as a direct result of SH2 binding to the phospho-ITAM or through transphosphorylation by a Src family kinase (6, 31, 40, 43, 56). Activated Syk can phosphorylate downstream targets (38) and recruits additional SH2-containing proteins that bind to pTyr sites in its SH2-kinase linker region (33). The related tyrosine kinase ZAP-70 appears to play a similar role in signaling from the T-cell receptor (TCR) (2, 53).
Targeted mutagenesis of the Syk gene (5, 50) has revealed important functions for this kinase in B- and T-cell development, in BCR signaling, in macrophages, in platelets, and in mast cell degranulation (4, 8, 10, 34, 37, 49). Here, we have analyzed a possible role for Syk in macrophage and neutrophil responses to opsonized IgG. Opsonization allows the efficient elimination of foreign antigens, through recognition of the Fc portion of immunoglobulins by a family of Fcγ immunoreceptors (FcγRs). Receptor engagement in macrophages and neutrophils activates signaling pathways leading to cytoskeletal changes and the phagocytosis of IgG-coated particles, as well as to granule secretion. FcγR signaling also stimulates the production of cytotoxic reactive oxygen intermediates and nitric oxide and induces the expression of cytokines, chemokines, and cell surface proteins. In addition to the destruction of pathogens, the ingestion and subsequent presentation of pathogen-derived peptide determinants by macrophages enhances T-cell-mediated immune functions.
FcγRs belong to the immunoglobulin gene superfamily, and all share a highly homologous extracellular portion, which harbors the Fc binding domain. Three distinct classes of FcγRs have been identified. Class I and III receptors form multimeric complexes with disulfide-linked γ- or ζ-chain dimers, while class II receptors exist as monomers (22). Interestingly, FcγRIIB, which harbors a distinct phosphorylation motif, apparently transmits an inhibitory signal after receptor engagement. Signaling from FcγRs appears to proceed through a series of interactions similar to those described for antigen receptors in lymphoid cells. Clustering of FcγRs induces the activation of a Src family kinase, resulting in the phosphorylation of an ITAM within the receptor’s signaling subunit. Syk is recruited through its SH2 domains to the FcγR and subsequently undergoes autophosphorylation and induces the phosphorylation of multiple substrates, including other FcγR ITAMs and downstream effectors (17, 32).
Several lines of evidence suggest that Syk is a direct mediator of FcγR signaling. Upon transfection with human FcγRs, Cos-1 cells acquire phagocytic properties which, in the case of the FcγRI and FcγRIIIA isoforms, are dependent on an ITAM within the γ chain of the receptor (19, 20, 36). However, reconstitution of the receptor complex results in only marginal phagocytic activity, which can be significantly potentiated by cotransfection with Syk (23). Following FcγR engagement in monocytes/macrophages, Syk is associated with the γ chain, becomes phosphorylated on tyrosine, and is enzymatically activated. Introduction of a protein containing the two SH2 domains of Syk into permeabilized mast cells abolished degranulation and leukotriene production following FcɛRI activation (47). Furthermore, clustering of FcγRIII-Syk fusions in Cos-1 cells results in a phagocytic signal, which is dependent on an intact Syk kinase domain (18). Cross-linking of ectopically expressed FcγR fusion proteins in Syk-deficient lymphocytes failed to initiate cytoskeletal changes indicative of phagocytosis, while re-expression of Syk restored the response (9). In addition, treatment of monocytes with Syk antisense oligodeoxynucleotides has been reported to abrogate phagocytic activity (35). Targeted disruption of the Syk gene has demonstrated an essential role in murine development (5, 50). Syk-deficient mice show profound bleeding and edema at midgestation, commonly leading to death late during embryogenesis or shortly after birth. Adoptive transfer of Syk-deficient fetal liver into RAG−/− recipients revealed a block of B-cell development at the pre-B-cell stage consistent with the notion that Syk acts downstream of the pre-BCR (5). Syk also plays a unique role in the development of γ/δ T cells (34) and acts in early T cells in conjunction with ZAP-70 (4). Syk−/− mast cells fail to respond to IgE stimulation, in keeping with biochemical data indicating that Syk has a crucial role in FcɛRI signal transduction, notably through its association with the γ chain (8). Interestingly, although Syk has been shown to participate in collagen-mediated platelet responses (37), platelets derived from Syk mutant mice appear to respond normally to thrombin.
We have investigated the role of Syk in mediating FcγR-dependent and -independent signaling in macrophages and neutrophils by using bone marrow radiation chimeras reconstituted with wild-type or Syk−/− cells. We report essential functions for Syk in FcγR signal transduction.
MATERIALS AND METHODS
Mice and bone marrow transplantation.
CBA × C57BL6/J breeding pairs heterozygous for a deletion in the Syk locus (5) were selected for homozygosity at the gpi-1 locus (gpi-1aa). Timed pregnancies were terminated at midgestation (E13.5 to E15.5). Embryos were harvested and washed three times with cold phosphate-buffered saline (PBS). Single-cell suspensions of fetal liver were generated in 1 ml of Iscove’s modified Dulbecco’s medium (Gibco, Bethesda Research Laboratories [BRL]) supplemented with 2% fetal calf serum (FCS). The tissue was dispersed by five passages through a 22-gauge needle and three subsequent passages through a 25-gauge needle, and remaining clumps were allowed to sediment. Lethally irradiated (950 rads, Co) 6- to 12-week-old C57BL6/J females were injected intravenously with 200 μl of the fetal liver preparation. The remainder of the embryo was used to establish its genotype by Southern blotting and to confirm its glucose phosphate isomerase (GPI) isoenzyme type. Starting after 6 weeks, the transplant was monitored by assessing the GPI composition of the recipient’s peripheral blood.
Bone marrow preparation.
Transplant recipients were sacrificed, and the marrow-containing long bones (femur, tibia, and humerus) were harvested. Single-cell bone marrow suspensions were obtained by gentle passage of the marrow plug through a 25-gauge needle into Iscove’s modified Dulbecco’s medium supplemented with 5% FCS. Bone marrow colony assays were performed as described previously (26).
Culture of macrophages.
Bone marrow cells (6 × 106) were seeded into 10 ml of macrophage medium (Dulbecco modified Eagle medium [DMEM; Gibco, BRL]) supplemented with 15% L929 cell conditioned supernatant, 10% FCS, 2 mM glutamine, and 0.5 μM 2-mercaptoethanol in plastic petri dishes. After 4 days, 5 ml of fresh medium was added, and after 8 days, 10 ml of the culture medium was removed and replenished. At the end of the culture period, the macrophages were washed once with ice-cold PBS and incubated at 4°C in 10 ml of Versene buffer (0.8 mM EDTA, 120 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM glucose, pH 7.2). For Syk−/− cultures, special care was taken not to dislodge loosely attached cells. After 45 min of incubation, macrophages were gently flushed off the plate, collected, and resuspended in macrophage medium. For phagocytosis assays, 105 cells were seeded on glass coverslips, while for biochemical analysis, 106 cells were plated in tissue culture dishes.
Phagocytosis assays.
FcγR-mediated phagocytosis was assessed by internalization of IgG-coated erythrocytes (IgG-RBC). For this purpose, sheep erythrocytes (RBC; ICN, Aurora, Canada) were opsonized for 1 h at 37°C with rabbit anti-sheep antibodies (Cappel, West Chester, Pa.). After washing, a monolayer of IgG-RBC was applied to macrophages plated on glass coverslips and allowed to bind for 10 min on ice. Unbound IgG-RBC were removed by three washes with ice-cold PBS. Phagocytosis was initiated by addition of warm DMEM and incubation for 10 min at 37°C. Following incubation, noninternalized IgG-RBC were removed by a 20-s hypotonic shock (H2O), which was terminated by addition of 1/10 volume of 10× PBS and one wash with PBS. Coverslips were then mounted on glass microscope slides and photographed immediately with a Leica DMIRB microscope (20× objective). Phagocytosis was assessed as the percentage of cells that had internalized two or more IgG-RBC/cell. Background phagocytosis (0 min of incubation) was determined by hypotonic lysis of RBC immediately after binding on ice. The ability of both wild-type and Syk-deficient cells to bind IgG-RBC was determined by washing cells three times after the binding period on ice and omission of the hypotonic lysis.
To measure phagocytosis mediated by complement receptor, zymosan (Sigma, St. Louis, Mo.) particles were opsonized for 1 h at 37°C with fresh mouse plasma. Macrophages were overlaid on ice for 10 min with opsonized zymosan particles. After three washes with 1.0 ml of ice-cold PBS, cells were incubated for 10 min at 37°C in DMEM (Gibco, BRL) containing 2.0 mg of the fluorescent dye Lucifer Yellow (Molecular Probes, Eugene, Oreg.) per ml. Following phagocytosis, cells were washed three times in ice-cold PBS and fixed with 2% paraformaldehyde–PBS for 30 min at 22°C. Trapping of Lucifer Yellow in sealed phagosomes identified cells which had internalized zymosan particles (46). Sealed phagosomes were visualized by fluorescence microscopy with a Leica DMIRB microscope (40× objective).
Detection of tyrosine phosphorylation, immunoprecipitation, and immunoblotting.
For immunoprecipitation and immunoblotting experiments, 106 cells were plated in 30-mm-diameter plastic tissue culture dishes. Stimulation with IgG-RBC was carried out as described above. Briefly, cultures were washed once with ice-cold DMEM and incubated on ice for 10 min. After loading with IgG-RBC, cultures were incubated for 10 min on ice and washed three times with ice-cold DMEM. At that point, 0-min samples were lysed on ice in radioimmunoprecipitation assay (RIPA) lysis buffer (120 mM NaCl, 50 mM Tris [pH 8.0], 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 200 μM vanadate, 25 mM NaF, 10 mM pyrophosphate, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride). One milliliter of warm DMEM was added to the samples, and plates were incubated at 37°C. At the end of the incubation time, cells were immediately lysed on ice with RIPA lysis buffer. Lysates were sheared by three passages through a 25-gauge needle followed by five passages through a 30-gauge needle.
Phosphotyrosine-containing proteins were immunoprecipitated by using a mixture of PY20 (Transduction Laboratories, Lexington, Ky.) and 4G10 (Upstate Biochemicals, Lake Placid, N.Y.) antiphosphotyrosine antibodies. Immunocomplexes were harvested on anti-mouse IgG-agarose beads and washed five times in RIPA buffer.
For the induction of nitric oxide synthase 2 (NOS2), 10-μg/ml lipopolysaccharide (LPS; Sigma) and 2-U/ml gamma interferon (Sigma) were added to the cultures 16 h prior to cell lysis in ice-cold RIPA buffer. Lysates were homogenized by six passages through a 26-gauge needle.
For Western blotting, we used antibodies against phosphotyrosine RC20 (Transduction Laboratories), Syk (generously provided by Bruce Rowley), the FcγR γ chain (generously provided by Marie-Helene Jouvin), p42 Erk-2 (Upstate Biochemicals), and NOS2 (Santa Cruz Biotechnology, Santa Cruz, Calif.).
Isolation of neutrophils.
Single-cell bone marrow preparations (see above) were collected by centrifugation at 500 × g and 4°C for 5 min and resuspended in 4 ml of Hanks balanced salt solution (HBSS; without Ca2+; Gibco, BRL) supplemented with 0.38% sodium citrate. The crude marrow suspension was placed on top of a Percoll (Pharmacia, Uppsala, Sweden) step gradient (52, 65, and 75% Percoll diluted in 1× HBSS) in a 15-ml polypropylene tube. One hundred percent Percoll was defined as nine parts Percoll and one part 10× HBSS (Ca2+ free). The Percoll gradient was centrifuged at 1,500 × g for 30 min at 4°C in a swinging-bucket rotor (brake off). An enriched neutrophil preparation was recovered from the interface between the 65 and 75% Percoll and diluted with an equal volume of HBSS. Cells were sedimented by a 10-s centrifugation in a microcentrifuge, resuspended in 1 ml of RPMI medium, and counted with a Coulter counter.
Measurement of the neutrophil oxidative burst.
To assess the oxidative burst generated by neutrophils, production of H2O2 was measured as horseradish peroxidase-catalyzed oxidation of the fluorescent dye scopoletin (51). Briefly, 107 cells/ml were suspended in Na+-rich medium (140 mM NaCl, 5 mM KCl, 10 mM glucose, 1 mM MgCl2, 1 mM CaCl2, 10 mM Na-HEPES [pH 7.3]) containing IgG-opsonized zymosan (10 particles/cell). The suspension was briefly centrifuged to promote rapid and synchronous interaction of the opsonized zymosan with the cells (46). After binding, the pellet was resuspended in Na+-rich medium containing scopoletin (1 μM), horseradish peroxidase (2.4 U/ml), and sodium azide (0.1%) to inhibit cellular catalase and myeloperoxidase. Fluorescence was determined at an excitation wavelength of 365 nm and an emission wavelength of 473 nm (Hitachi F4000 fluorometer). Stimulation of the oxidative burst in the absence of zymosan was also performed by addition of tetradecanoyl phorbol acetate (TPA; 0.1 μM) directly to the cell suspension. Exogenously added H2O2 was used to generate a standard curve for each assay.
Peptide phosphorylation assays.
Phosphorylation assays were performed as described previously (32). Briefly, Lck and Myc-tagged versions of Syk and ZAP-70 (Myc-Syk and Myc-Zap) were expressed transiently in Cos-1 cells. The tyrosine kinases were then immunoprecipitated from 100 μg of cell lysate by using either anti-Myc (monoclonal antibody 9E10) or anti-Lck antibodies. Phosphorylation reactions were carried out in 20 mM Tris (pH 7.5)–10 mM MgCl2–10 mM MnCl2–2 μM nonradioactive ATP–25 μM [γ-32P]ATP (3,000 Ci/mmol) at room temperature for 15 min in the presence of 5 μg of synthetic peptide as a substrate. Phosphorylated products were separated on 16.5% Tricine gels and visualized by autoradiography. Synthetic peptides corresponding to the first ITAM of the TCR ζ chain (NQLYNELNLGRREEYDVLDK), the ITAM of the γ chain of FcγRs (DAVYTGLNTRSQETYETLKH), and a carboxy-terminal sequence motif of Syk (CAVELRLRNYYYDVVN) were obtained from commercial suppliers (phosphorylatable Tyr residues within each ITAM or the control sequence are underlined).
RESULTS
Syk-deficient bone marrow chimeras develop fatal abdominal hemorrhages.
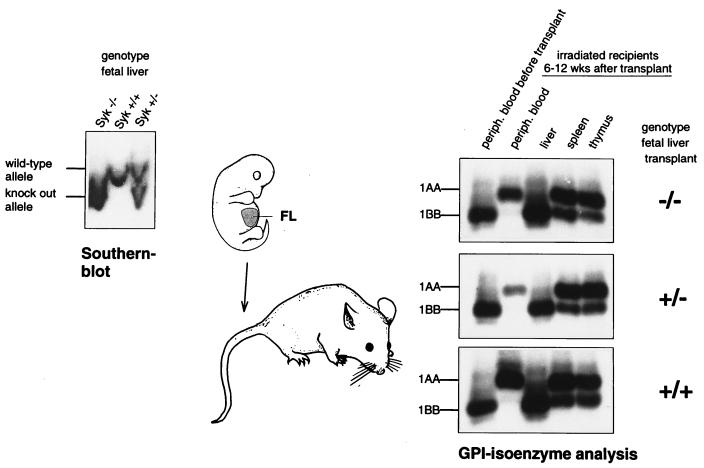
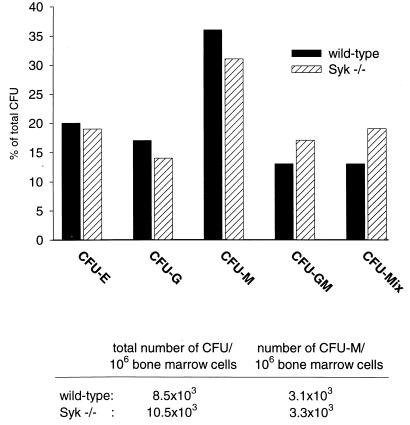
We studied the role of Syk in the development and function of bone marrow-derived macrophages by using a transplantation model. Pregnant females from crosses of mice heterozygous for a syk null allele (5) were sacrificed at midgestation, and fetal liver preparations from the recovered embryos were injected into lethally irradiated adult mice. We monitored the repopulation of bone marrow recipients by using a GPI isoenzyme marker (Fig. 1). GPI-1AA fetal liver cells efficiently repopulated irradiated GPI-1BB hosts, regardless of the syk alleles present in the donor cells, as demonstrated by the appearance of GPI-1AA hematopoietic cells in peripheral blood and the hematopoietic organs (bone marrow, spleen, and thymus). At the time of sacrifice, the bone marrow cellularity was comparable between mice that had been reconstituted with wild-type and Syk−/− fetal liver (+/+ and +/− phenotypes will be collectively referred to as the wild type). Analysis of wild-type and Syk−/− bone marrow cells for the ability to form colonies in semisolid medium did not reveal a significant difference in the frequency of various myeloid colony-forming progenitors (see Fig. 5). These findings indicate that the Syk tyrosine kinase is not essential for the function of the hematopoietic stem cell compartment.
FIG. 1.
Transplantation model for the generation of Syk−/− bone marrow macrophages. Timed intercrosses of mice heterozygous for a Syk null allele were sacrificed at midgestation. Fetal liver (FL) of recovered embryos was used to reconstitute lethally irradiated recipients. Part of the remaining embryos served to confirm the genotype at the Syk locus by Southern blotting. Starting at 6 weeks after the bone marrow graft, the extent of recipient repopulation was monitored from a drop of peripheral (periph.) blood by using the GPI isoenzyme marker. Donor embryos were of the isoenzyme type GPI-1AA, while recipients were of the GPI-1BB isoenzyme type. The peripheral blood GPI type of all recipients was completely converted to the donor type. A perfused liver sample taken at the same time served as a negative control. The mixed isoenzyme type of spleen and thymus is due to graft-derived functional precursors (GPI-1AA) and host-derived stromal components (GPI-1BB).
FIG. 5.
Colony assay of bone marrow derived from wild-type and Syk−/− fetal liver repopulated recipients. Single-cell bone marrow suspensions were seeded into semisolid medium in the presence of IL-1α, IL-3, IL-11, Kit ligand, and erythropoietin. After 10 days, colonies were typed and counted. CFU-E, erythrocyte burst-forming unit; CFU-G, granulocyte CFU; CFU-M, macrophage CFU; CFU-GM, granulocyte-macrophage CFU; CFU-Mix, multilineage CFU (colonies consist of erythrocytes and at least two additional myeloid lineages).
We noticed an increased number of deaths in recipients that had received Syk−/− rather than wild-type fetal liver, starting 3 months after engraftment. By 6 months after transplantation, all Syk−/− recipients had been lost. Analysis of the affected Syk−/− recipients revealed severe abdominal hemorrhage, ultimately accompanied by the development of hemorrhagic ascites and severe anemia. The intestinal walls of the affected animals showed distinct petechiae, but no gross ulceration was observed. Histological examination revealed diffuse bleeding into the lamina propria and, frequently, dramatic dilation of the central vessels in intestinal villi and edema (Fig. 2). This pathological alteration was observed along the entire intestine and was not confined to a particular area of the digestive tract.
FIG. 2.
Diffuse bleeding in the intestinal mucosa of bone marrow chimeras transplanted with Syk−/− fetal liver. Mice were reconstituted with Syk−/− (A and C) or wild-type (B and D) fetal liver. A and B are transverse sections through intestinal villi, while C and D show longitudinal sections through the lamina propria. Mice reconstituted with Syk-deficient bone marrow frequently show dilation of the central villus vessel (arrowheads in A) and display diffuse bleeding at discrete spots in the lamina propria (arrows in C). Pictures show eosin-hematoxilin-stained 5-μm sections; the bar corresponds to 250 μm.
Syk-deficient neutrophils fail to produce an oxidative burst in response to IgG-opsonized RBCs.
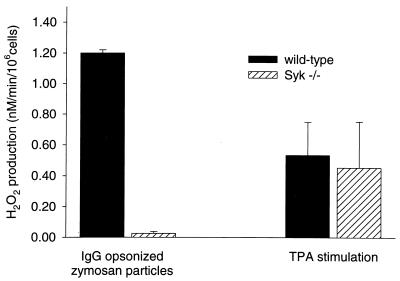
We used the radiation chimeras to test the dependence of specific FcγR-mediated responses in neutrophils and macrophages on the presence of Syk. In a first set of experiments, we investigated whether Syk is involved in the FcγR-mediated generation of an oxidative burst in neutrophils. Primary bone marrow-derived neutrophils were isolated by Percoll density centrifugation, and FcγRs were stimulated by exposure to IgG-opsonized zymosan particles. The generation of reactive oxygen intermediates was determined by quantification of H2O2 production, as measured by the horseradish peroxidase-catalyzed oxidation of the fluorescent dye scopoletin. Interestingly, H2O2 production and, hence, the IgG-dependent oxidative burst were completely absent in Syk-deficient neutrophils (Fig. 3). In contrast, the generation of reactive oxygen intermediates after exposure to the tumor promoter TPA, which is not FcγR dependent, was comparable in Syk-deficient and wild-type neutrophils. These results indicate that Syk−/− neutrophils have a selective block in FcγR signaling but are capable of generating reactive oxygen species in response to an FcγR-independent stimulus.
FIG. 3.
Quantitative analysis of H2O2 production by neutrophils during the oxidative burst following IgG or TPA stimulation. Primary bone marrow neutrophils were derived from either wild-type or Syk−/− bone marrow chimeras. Cells were incubated with IgG-opsonized zymosan particles, and H2O2 production was determined by using horseradish peroxidase-catalyzed oxidation of scopoletin. The ability to generate a response to an FcγR-independent signal was tested by exposure of an equivalent cell sample to 0.1 μM TPA. Each bar represents the mean and error range of two independent experiments.
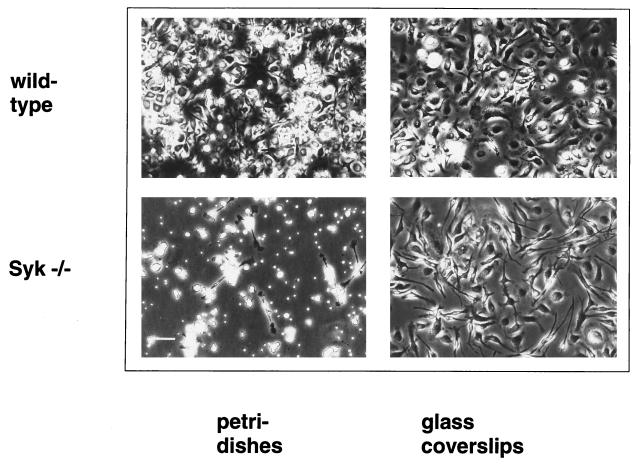
Reduced recovery of bone marrow-derived, Syk-deficient macrophages after in vitro culture.
To assess the contribution of Syk to FcγR-mediated responses in primary bone marrow-derived macrophages, we derived primary macrophage cultures. We followed a well-established protocol involving an 8- to 12-day period of expansion on plastic petri dishes in the presence of L929 cell-conditioned medium as a source of macrophage colony-stimulating factor (M-CSF) (13, 54). Surprisingly, we observed consistently reduced recovery of macrophages from Syk−/− marrow following this protocol. On average, the number of Syk−/− macrophages isolated was five times lower than the number of wild-type macrophages (Table 1). In comparison to wild-type cells, Syk−/− macrophages displayed a strongly reduced adherence to non-tissue culture plastic surfaces and the total cell number in Syk−/− macrophage cultures was significantly reduced (Fig. 4). Only a few Syk−/− macrophages were found in the culture supernatant, demonstrating that simple detachment of Syk−/− macrophages could not account for the reduced recovery (Fig. 4). This raises the possibility that Syk−/− macrophages have a decreased capacity to proliferate or an increased apoptosis rate under these culture conditions. Upon replating onto either glass or tissue culture plastic, the adherence defect was no longer apparent (Fig. 4). A possible explanation for the deficiency in macrophage production in plastic petri dishes is that the absence of Syk results in a reduced number of macrophage progenitors in Syk−/− bone marrow or in a block in macrophage development. Therefore, we determined the frequency of bone marrow precursors capable of forming monocyte-macrophage colonies in semisolid medium. Comparable progenitor numbers were detected in the bone marrow of wild-type and Syk−/− reconstituted mice (Fig. 5). Consistent with this result, equivalent numbers of macrophages were recovered from wild-type and Syk−/− bone marrow plated on tissue culture dishes. Our findings indicate that the development of the monocyte-macrophage lineage in Syk−/− bone marrow is not compromised but that there is a defect in Syk−/− macrophage production under a specific culture condition.
TABLE 1.
Reduced recovery of bone marrow-derived, Syk-deficient macrophages after culture on plastic petri dishesa
| Expt | No. of wild-type macrophages recovered/106 bone marrow cells | No. of Syk−/− macrophages recovered/106 bone marrow cells |
|---|---|---|
| 1 | 3.5 × 105 | 7.1 × 104 |
| 2 | 2.6 × 105 | 1.1 × 105 |
| 3 | 4.2 × 105 | 3.1 × 104 |
| 4 | 3.7 × 105 | 7.1 × 104 |
| 5 | 9.2 × 105 | 1.6 × 105 |
| Mean ± SD | 4.7 × 105 ± 2.6 × 105 | 8.8 × 104 ± 4.8 × 104 |
The values represent the recovery of macrophages after a 10- to 12-day culture period in the presence of M-CSF.
FIG. 4.
Syk-deficient macrophages plated on petri dishes display reduced adherence and proliferation. The pictures on the left are phase-contrast images of wild-type and Syk−/− bone marrow after a 10-day culture period in plastic petri dishes in the presence of M-CSF. Those on the right are phase-contrast micrographs of the same cultures after collection of the adherent fraction and reseeding onto glass coverslips. Bar, 500 μm.
Syk−/− macrophages are severely impaired in the ability to phagocytose IgG-presenting particles.
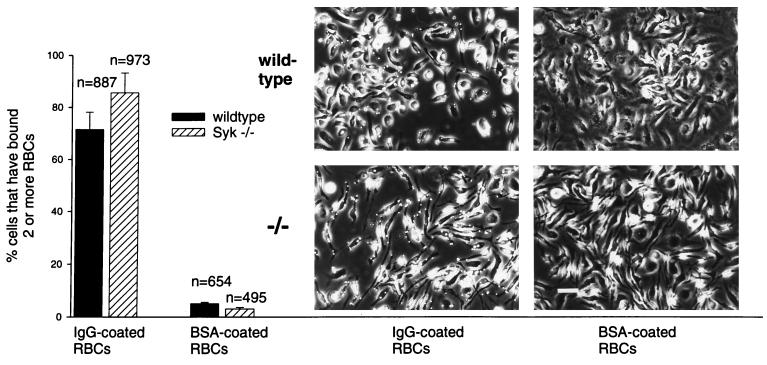
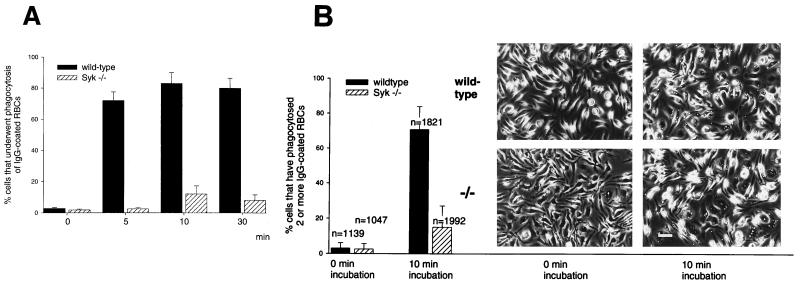
The availability of fully differentiated, Syk-deficient bone marrow macrophages allowed us to directly test the importance of this tyrosine kinase in IgG-mediated phagocytosis. The assay was performed with Syk−/− and wild-type macrophages that had been replated at equal densities on glass coverslips 24 to 48 h before the experiment. First, we assessed the binding of IgG-RBC to surface FcγRs during a 10-min incubation at 0°C. At the end of the incubation period, the macrophage cultures were washed to remove free IgG-RBC, and cells that retained opsonized particles were counted. Syk-deficient and wild-type macrophages bound IgG-RBC to comparable extents (Fig. 6), such that 86% of the Syk−/− macrophages and 72% of the wild-type macrophages had bound opsonized RBC after 10 min of incubation. The specificity of this interaction was demonstrated by the complete absence of binding of RBC that had been coated with bovine serum albumin (BSA). After attachment of the IgG-opsonized particles, phagocytosis was initiated by incubation of the cultures at 37°C and successful completion of the phagocytic process, taken as full enclosure of the phagocytosed IgG-RBC, was measured following a brief hypotonic shock to lyse unincorporated RBC. Phagocytosis by wild-type macrophages was optimal after 10 min of incubation at 37°C (Fig. 7A). Phagocytosis by Syk−/− macrophages also peaked at 10 min but was markedly reduced compared to that by wild-type cells. While practically all wild-type macrophages that had bound IgG-RBC successfully completed the phagocytic process after a 10-min incubation at 37°C, only 15% of the Syk-deficient macrophages were capable of completing phagocytosis (Fig. 7B). In the absence of a 37°C incubation period (0-min incubation, Fig. 7B), successful enclosure of IgG-RBC did not occur and only a small fraction (2 to 3%) of particles acquired resistance to hypotonic lysis. These findings directly demonstrate the importance of Syk in the phagocytosis of IgG-opsonized particles.
FIG. 6.
The capacity of Syk−/− macrophages to bind to IgG-RBC is not impaired. IgG-RBC were exposed to wild-type and Syk−/− macrophages on glass coverslips and allowed to bind at 0°C for 10 min. Free RBC were removed, and the percentage of cells that had bound two or more opsonized particles was determined. BSA-coated RBC served as a control for nonspecific binding. The corresponding phase micrographs are on the right. Bound IgG-RBC are visible as perfectly round, sharply demarcated, bright circles. The diagram represents the average of five independent experiments. n is the total number of cells evaluated. Bar, 500 μm.
FIG. 7.
Phagocytosis of IgG-RBC is selectively impaired in Syk-deficient macrophages. (A) Time course of phagocytosis of IgG-RBC by wild-type and Syk−/− macrophages. IgG-RBC were exposed to wild-type and Syk−/− macrophages on glass coverslips, and the percentage of cells that had taken up opsonized particles was determined after the indicated incubation times. Both wild-type and Syk-deficient macrophages showed maximal phagocytosis after 10 min; however, the extent of uptake was greatly reduced in Syk-deficient cells. (B) Quantitative analysis of the capacity of wild-type and Syk−/− macrophages to phagocytose IgG-RBC. IgG-RBC were allowed to bind to wild-type and Syk−/− macrophages, and after incubation at 37°C for the indicated intervals, incompletely phagocytosed RBC were lysed by hypotonic shock. The bars depict the percentage of macrophages that had taken up two or more opsonized particles. The photographs show the corresponding phase micrographs. n is the total number of evaluated cells. Bar, 500 μm.
Syk-deficient macrophages do not show a generalized defect in phagocytosis.
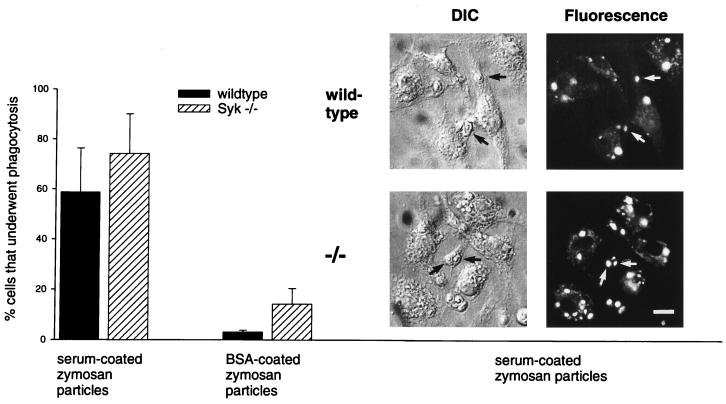
The observed phagocytic defect in Syk−/− macrophages is not necessarily a result of impaired FcγR signaling but might result from a block to the process of phagocytosis itself, for example, by preventing the formation of phagocytic vacuoles. To test this possibility, we examined the capacity of Syk-deficient macrophages to initiate phagocytosis in response to a surface receptor other than FcγR. The phagocytosis assay was therefore repeated by presenting serum-coated zymosan particles as a substrate for uptake. This experiment tested the ability of macrophages to carry out phagocytosis in response to stimulation of the complement receptor (Fig. 8). Under these conditions, 60% of wild-type macrophages successfully incorporated opsonized zymosan. Interestingly, 74% of Syk−/− macrophages showed successful phagocytic events, indicating that their ability to internalize complement-opsonized substrates was unimpaired. In four independent experiments, the rate of phagocytosis of Syk-deficient macrophages was consistently higher than that of wild-type macrophages. This also held true for the uptake of BSA-coated zymosan particles, which were used as a nonspecific control (Syk−/−, 14 ± 6%; wild type, 3 ± 1%). These experiments demonstrate that Syk−/− macrophages are, in principle, able to initiate and successfully complete the phagocytic process but have a specific block in phagocytosis induced by FcγR.
FIG. 8.
Syk−/− macrophages display normal phagocytosis of serum-coated particles. Wild-type and Syk−/− macrophages on glass coverslips were tested for the capacity to phagocytose opsonized zymosan particles in the presence of the fluorescent dye Lucifer Yellow. After 10 min of incubation, successful completion of the phagocytic process was determined by colocalization of zymosan and trapped Lucifer Yellow in sealed phagosomes. The percentage of phagocytosis-positive macrophages is depicted in the bar graph. The photographs show differential interference contrast (DIC) and corresponding fluorescence pictures. Arrows indicate phagocytic events. Bar, 500 μm.
Tyrosine phosphorylation of specific targets in response to FcγR is attenuated in Syk-deficient macrophages.
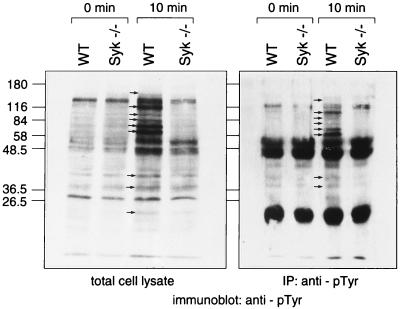
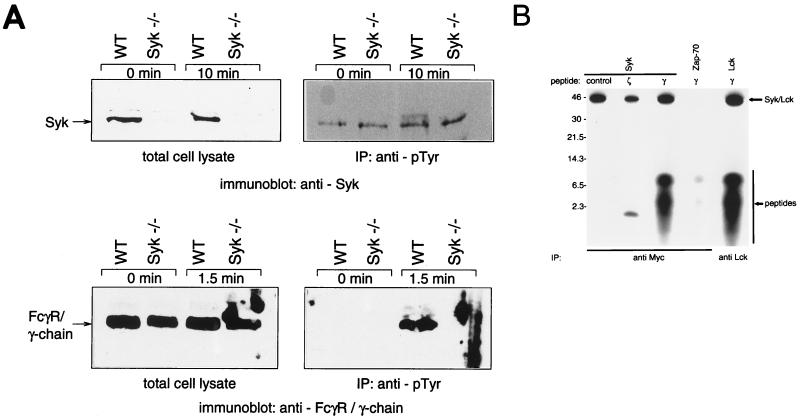
To investigate the molecular basis underlying the defect in FcγR signaling in Syk−/− macrophages, we analyzed the pattern of protein tyrosine phosphorylation after FcγR stimulation. Bone marrow-derived macrophages were plated at equal density on tissue culture plates, and FcγR binding was induced by incubation with IgG-RBC in the cold. Cells were then shifted to 37°C for various periods, and the incubation was stopped by cell lysis. A 10-min incubation at 37°C led to the appearance of several strongly tyrosine-phosphorylated bands in lysates prepared from wild-type macrophages, most of which were absent in Syk-deficient macrophages (Fig. 9). This result suggests that Syk is required for the tyrosine phosphorylation of intracellular targets after FcγR stimulation. Tyrosine-phosphorylated proteins with apparent molecular masses of 170, 125, 110, 95, 80, 70, 42, 38, and 25 kDa were readily detected in wild-type macrophages but not in Syk−/− macrophages (indicated by arrows in Fig. 9). In contrast, tyrosine phosphorylation of 49- and 55-kDa proteins was induced in both wild-type and Syk−/− macrophages (Fig. 9).
FIG. 9.
Syk-deficient macrophages fail to phosphorylate specific proteins on tyrosine in response to FcγR stimulation. Bone marrow-derived macrophages on tissue culture dishes were stimulated with IgG-RBC (see Fig. 6 and 7). After the indicated incubation times, cells were lysed and either analyzed directly by blotting with antiphosphotyrosine antibody (left panel) or immunoprecipitated (IP) with antiphosphotyrosine (pTyr) antibody prior to immunoblotting. WT, wild type. The values on the left are molecular masses in kilodaltons.
As expected, tyrosine phosphorylation of the Syk kinase itself was observed following FcγR stimulation of wild-type cells (Fig. 10A, top), whereas Syk protein was not detectable in mutant macrophages. In wild-type macrophages, IgG binding to FcγR rapidly (within 1.5 min) induced tyrosine phosphorylation of the receptor γ chain (Fig. 10A, bottom right, lane WT, 1.5 min). Phosphorylation of the γ chain was transient and no longer detectable after 10 min (data not shown). Interestingly, we could not detect significant phosphorylation of the FcγR γ chain on tyrosine in the absence of the Syk kinase (Fig. 10A, bottom right, lane Syk−/−, 1.5 min).
FIG. 10.
(A) FcγR γ-chain phosphorylation is not detectable in Syk-deficient macrophages. The presence of Syk (top row) and the FcγR γ chain (bottom row) and their tyrosine-phosphorylated forms in bone marrow macrophages stimulated with IgG-RBC was tested by Western blotting. FcγR γ-chain samples were separated on a 15% gel. IP, immunoprecipitation; WT, wild type. (B) Syk phosphorylates a peptide corresponding to the ITAM of the FcR γ chain efficiently in vitro. Lck- and Myc-tagged versions of Syk and ZAP-70 were expressed transiently in Cos-1 cells. The kinases were immunoprecipitated from cell lysates by using either anti-Myc (Syk, ZAP-70) or anti-Lck antibodies, and immune complex kinase reactions were performed in the presence of a peptide corresponding to the first ITAM of the TCR ζ chain or the ITAM of the FcR γ chain. A peptide derived from the carboxy-terminal sequence of Syk served as a control substrate. Reaction products were separated on a 16.5% Tricine gel and visualized by autoradiography. The values on the left are molecular masses in kilodaltons.
This absence of detectable γ-chain phosphorylation in Syk-deficient macrophages suggests that Syk might contribute to the phosphorylation of the γ-chain ITAM in vivo. We tested the ability of recombinantly expressed Syk kinase to phosphorylate a peptide corresponding to the FcγR γ chain in vitro. The γ-chain ITAM was efficiently phosphorylated by Syk (Fig. 10B). A control peptide derived from the C-terminal sequence of Syk containing three tyrosine residues was not phosphorylated by immunoprecipitated Syk. We also tested the ability of ZAP-70 and Lck to recognize the γ-chain peptide as a substrate. While only poor phosphorylation of the γ-chain peptide was detectable after incubation with ZAP-70, the γ-chain ITAM was an excellent substrate for Lck.
Finally, we probed for stimulation of the mitogen-activated protein kinase Erk-2 in response to FcγR activation by using a gel mobility shift assay which detects the activated threonine-tyrosine-phosphorylated form of the enzyme. We observed a background level of Erk-2 activity in wild-type macrophages in the absence of FcγR stimulation. While wild-type macrophages responded to IgG-RBC with elevated Erk-2 activity levels, this activation was absent in Syk-deficient macrophages (Fig. 11A). These results demonstrate the absence of specific signaling events downstream of FcγR in Syk−/− macrophages.
FIG. 11.
(A) Syk−/− macrophages display reduced phosphorylation of p42 Erk-2 after FcγR engagement. Activation of Erk-2 MAPK was visualized by the phosphorylation-induced mobility shift. Macrophages were allowed to bind to IgG-RBC, and after the indicated incubation times, cells were lysed and tested for the presence of doubly threonine-tyrosine-phosphorylated forms of Erk-2 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (B) Induction of NOS2 (iNOS) by LPS-gamma interferon is normal in Syk-deficient macrophages. Macrophage cultures were induced with 10-μg/ml LPS and 2-U/ml gamma interferon for 16 h. At the end of the incubation period, cells were lysed and probed for the presence of NOS2 by Western blotting. WT, wild type.
LPS-gamma interferon-induced induction of NOS2 is not affected in Syk−/− macrophages.
Upon exposure to LPS and gamma interferon, macrophages upregulate the expression of inflammatory cytokines and generate reactive oxygen intermediates. Inducible NOS2 is crucially involved in the formation of reactive oxygen species and contributes to the ability of macrophages to kill invading pathogens. When Syk−/− macrophages were stimulated with a combination of LPS and gamma interferon for 16 h, expression of NOS2 was clearly detectable by Western blotting. As shown in Fig. 11B, NOS2 induction was comparable between Syk−/− and wild-type macrophages. These data indicate that Syk has a selective role in macrophage signaling and is not required to link gamma interferon or LPS receptors to the control of gene expression.
DISCUSSION
The Syk tyrosine kinase is important in signaling downstream of immunoreceptors on T and B cells (3, 12, 29), as well as mast cells and macrophages (1, 22, 44). Furthermore, Syk has been implicated in pathways activated by integrins, G-coupled receptors, and cytokines (15, 37, 52, 55). We investigated the function of Syk in bone marrow-derived neutrophils and macrophages by using mouse radiation chimeras. Fetal liver cells derived from Syk−/− embryos were fully capable of engrafting lethally irradiated adult mice. Previous studies have indicated that the adherent bone marrow fraction contains the earliest hematopoietic stem cells and have suggested that integrins play an important role in stem cell homing and maintenance (16). Our results indicate that Syk is not essential for the functioning of the hematopoietic stem cell compartment. Studies using Syk-deficient lymphoid cells have demonstrated a block in B-cell development (5, 50) and shown a unique role for Syk in the development of γ/δ T cells (34). By contrast, we have not detected any difference in the progenitor number or differentiation ability of monocytes-macrophages and neutrophils and, hence, no developmental deficiency in these lineages in Syk−/− bone marrow. This result suggests that Syk function in myeloid lineages is restricted to immunoreceptor signaling in mature cells. It remains possible, though, that an earlier function of Syk in the development of myeloid lineages is masked in Syk-deficient cells by compensating kinases.
The failure of Syk−/− macrophages to thrive on plastic petri dishes might result from the absence of a specific survival signal.
We did observe a specific defect during our attempts to derive mature Syk−/− bone marrow macrophages by culture on nontreated plastic dishes. The effect was not apparent on glass or tissue culture plastic. The ability of Syk−/− macrophages to attach to plastic petri dishes was severely reduced, suggesting the absence of a contact-mediated growth or survival signal. It is tempting to speculate that an integrin signal is impaired in Syk−/− macrophages, leading to a process such as anoikis (14). However, integrin signaling may still be possible in Syk−/− macrophages, as shown by their unimpaired ability to signal through the complement receptor. The high percentage of cells undergoing apoptosis during the initial stages of the macrophage culture due to the presence of M-CSF as the sole cytokine essentially precludes direct measurement of apoptosis in macrophage progenitors. We are currently pursuing alternative approaches to the investigation of the basis of the inability of Syk−/− macrophages to flourish on petri dishes.
Abdominal hemorrhages in Syk−/− bone marrow chimeras and frequent dilation of the villus microvasculature.
Most surprisingly, we and others have observed the development of ultimately fatal abdominal hemorrhages in Syk−/− bone marrow chimeras (37). The onset of disease is slow. The first symptoms, including petechiae, diffuse bleeding into the lamina propria, and dilation of the villus microvasculature, can be detected 4 to 6 weeks after transplantation in otherwise apparently healthy animals. At this time point, repopulation of the bone marrow by the fetal liver graft is complete. We detected no signs of severe intestinal inflammation in the affected mice.
A straightforward explanation for this pathology is that it results from impaired platelet function. Indeed, activation of Syk after exposure of platelets to collagen is well established, and a novel direct mechanism of Syk activation by αIIb β3 integrin has recently been suggested (15). However, Syk−/− platelets appear to be functional. In particular, thrombin-mediated responses, such as aggregation and arachidonic acid and 5-hydroxytryptamine (5-HT) secretion, seem normal (37). Alternatively, a defect in the microvasculature itself is possible. This notion could explain the unusual dilation of vessels, suggestive of impaired vessel integrity. Also, the late onset of disease, which lags significantly behind the repopulation of the hematopoietic system, argues in favor of an endothelial defect. However, there is no substantial evidence for efficient repopulation of the endothelial compartment by bone marrow grafts. Finally, an alternative mechanism based on impaired macrophage function can be envisaged. There is evidence that contact with extracellular matrix components after extravasation causes macrophages to secrete proteases, allowing them to infiltrate the surrounding tissue (25, 41). If, as previously discussed, Syk−/− macrophages are defective in signaling by relevant integrins, they might fail to invade their target tissues yet be activated to secrete inflammatory components, ultimately leading to destruction of the integrity of adjacent vessels. We are presently testing these hypotheses.
Syk fulfills a crucial function in FcγR-mediated responses in bone marrow macrophages and neutrophils.
We tested the function of Syk in FcγR-mediated phagocytosis by monitoring the uptake of IgG-RBC by primary Syk−/− bone marrow macrophages. Binding of opsonized particles was not impaired, indicating expression of functional FcγR on the surface of Syk−/− macrophages. In contrast, we found a significant reduction in the ability of Syk−/− macrophages to complete the phagocytic process. Control experiments triggering complement-mediated phagocytosis indicated that the phagocytic machinery in Syk−/− macrophages is present and fully functional. Since the receptors for the complement components iC3b CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are integrins (39), the observation of unimpaired complement-stimulated phagocytosis suggests that intact integrin signaling may occur in Syk−/− macrophages. Furthermore, induction of NOS2 in response to LPS and gamma interferon was normal in mutant macrophages, arguing that Syk is not involved in these signaling pathways. Similar results were obtained with Syk−/− neutrophils, which showed a complete block in the generation of reactive oxygen intermediates in response to opsonin-IgG, whereas the generation of reactive oxygen species in response to TPA was not affected. Hence, in both the macrophage and neutrophil lineages, specific defects in signaling from the FcγR were observed but the cellular machinery was still responsive to alternative signals. While this report was in preparation, a complementary study was published by Crowley et al. (10), who used macrophages derived directly by in vitro culture from Syk−/− fetal liver and also described specific defects in FcγR-mediated phagocytosis and signaling. Crowley et al. reported a complete block in FcγR-mediated phagocytosis in Syk−/− macrophages, whereas we observed only a partial block. This apparent difference might be the result of the different protocols that were used to derive the macrophages under investigation. Mechanisms compensating for the absence of Syk might be activated during the homing, differentiation, and maturation of macrophage progenitors in the bone marrow, and additionally, a subpopulation of macrophages might be selected. Interestingly, after transfection into Cos-1 cells, neither FcγRIIb1 nor FcγRIIb2, both of which lack a cytoplasmic ITAM and contain a single YXXL motif only, is capable of eliciting a phagocytic signal (19, 21). In the mast cell line RBL-2H3, however, stably transfected FcγRIIb2 triggers a phagocytic response (11).
Syk is a principal tyrosine kinase activated after FcγR stimulation.
Comparison of the pattern of tyrosine-phosphorylated proteins after FcγR stimulation in the presence and absence of Syk suggests that Syk is required for tyrosine phosphorylation of most cellular targets of FcγR signaling. Furthermore, Syk−/− macrophages failed to activate p42 Erk2 after FcγR stimulation, indicating that Syk is responsible for the activation of upstream components of the mitogen activated protein kinase (MAPK) cascade. These findings are in agreement with those of previous studies in which the function of Syk was tested either in lymphoid cells lacking Syk or in nonlymphoid cells after introduction of immunoreceptor components. For example, in Syk-deficient B cells, phospholipase C-γ2 phosphorylation, inositol-1,4,5-triphosphate release, and Ca2+ mobilization after B-cell antigen receptor stimulation are dependent on the reintroduction and activation of the Syk tyrosine kinase (30). Stimulation of the ectopically expressed BCR in mouse pituitary cells fails to elicit tyrosine phosphorylation of downstream signaling elements. Coexpression of Syk restores this response, leading to phosphorylation of Shc and MAPK activation (38). Similarly, MAPK activation following cross-linking of an interleukin 2 (IL-2) receptor-FcɛRI γ-chain chimera in Cos-7 cells is dependent on the coexpression of Syk (48). In addition, Crowley et al. also reported the absence of MAPK activation in Syk-deficient fetal liver macrophages (10). Those authors demonstrated a more robust MAPK activation in wild-type macrophages after cross-linking of FcγRs with monoclonal antibodies than we observed after exposure of wild-type macrophages to IgG-RBC. The difference is most likely a consequence of the different activation protocols. Interestingly, we were unable to detect γ-chain phosphorylation in the absence of Syk. In mouse pituitary cells, which lack Syk and were engineered to express the BCR, phosphorylation of Ig-α and Ig-β after IgM stimulation has been reported (38). Furthermore, fetal liver mast cells derived from Syk-deficient mice respond to FcγRI stimulation with β- and γ-chain phosphorylation and hyperactivation of the Src kinase Lyn (8). Finally, in platelets derived from Syk-deficient mice, γ-chain phosphorylation is detected after collagen stimulation (37). While these data emphasize the importance of Src kinases in the initiation of immunoreceptor signaling, there is also evidence that once activated, Syk is able to directly phosphorylate ITAMs (32, 56). We observed efficient phosphorylation of a peptide corresponding to the FcR γ-chain ITAM by recombinant Syk in vitro. In the same assay, the γ-chain peptide was an excellent substrate for Lck, while it was only weakly recognized by recombinant ZAP-70. The latter finding may reflect a failure of ZAP-70 to become fully activated in the absence of Src kinases. Therefore, it is likely that Syk, in conjunction with Src family kinases, plays a significant physiological role in γ-chain phosphorylation. In mast cells, Lyn associates with the nonactivated FcɛRI β chain, which may facilitate efficient phosphorylation of the γ chain in the absence of Syk (24). In platelets, high Src expression levels may result in a similar effect. In macrophages, we only detected γ-chain phosphorylation immediately following receptor engagement, clearly before the peak of phagocytic activity. The fast and transient nature of γ-chain phosphorylation suggests that this signal is highly regulated. FcRs have been shown to associate with tyrosine phosphatases capable of direct dephosphorylation of ITAMs (28, 45). Binding of Syk SH2 domains to phosphorylated ITAMs may serve to protect the phosphotyrosine sites against the action of tyrosine phosphatases.
Taken together, our results demonstrate a crucial function of the cytoplasmic tyrosine kinase Syk in FcγR-mediated processes in neutrophils and macrophages. These data are consistent with the view that SH2 domain proteins play a crucial role in signaling from immunoreceptors. Furthermore, Syk is involved in additional cellular functions that are poorly understood. Among these, the bleeding disorders observed in Syk−/− embryos and bone marrow chimeras are particularly intriguing. Integrin-mediated signals are candidates that might explain these additional Syk functions, and it will be an interesting challenge to dissect their molecular basis.
ACKNOWLEDGMENTS
We thank Marie-Helen Jouvin and Bruce Rowley for generously providing γ-chain and Syk antibodies. We thank Matthias Clauss and Werner Risau, Bad Nauheim, Germany, for helpful discussions. We thank Ken Harpal for excellent help with histology.
N.A.-A. was supported by a Terry Fox postdoctoral fellowship from the National Cancer Institute of Canada. J.B. is the recipient of a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada. This work was supported by grants from Bristol-Meyers-Squibb, the Medical Research Council of Canada, and the National Cancer Institute of Canada to T.P. and from the Medical Research Council of Canada to S.G. T.P. and S.G. are International Research Scholars of the Howard Hughes Medical Institute. T.P. is a Terry Fox Cancer Research Scientist of the National Cancer Institute of Canada.
REFERENCES
- 1.Beaven M A, Baumgartner R A. Downstream signals initiated in mast cells by Fc epsilon RI and other receptors. Curr Opin Immunol. 1996;8:766–772. doi: 10.1016/s0952-7915(96)80002-1. [DOI] [PubMed] [Google Scholar]
- 2.Chan A C, Dalton M, Johnson R, Kong G H, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng A M, Chan A C. Protein tyrosine kinases in thymocyte development. Curr Opin Immunol. 1997;9:528–533. doi: 10.1016/s0952-7915(97)80106-9. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A M, Negishi I, Anderson S J, Chan A C, Bolen J, Loh D Y, Pawson T. The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc Natl Acad Sci USA. 1997;94:9797–9801. doi: 10.1073/pnas.94.18.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng A M, Rowley B, Pao W, Hayday A, Bolen J B, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 6.Chu D H, Spits H, Peyron J F, Rowley R B, Bolen J B, Weiss A. The Syk protein tyrosine kinase can function independently of CD45 or Lck in T cell antigen receptor signaling. EMBO J. 1996;15:6251–6261. [PMC free article] [PubMed] [Google Scholar]
- 7.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 8.Costello P S, Turner M, Walters A E, Cunningham C N, Bauer P H, Downward J, Tybulewicz V L. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- 9.Cox D, Chang P, Kurosaki T, Greenberg S. Syk tyrosine kinase is required for immunoreceptor tyrosine activation motif-dependent actin assembly. J Biol Chem. 1996;271:16597–16602. doi: 10.1074/jbc.271.28.16597. [DOI] [PubMed] [Google Scholar]
- 10.Crowley M T, Costello P S, Fitzer-Attas C J, Turner M, Meng F, Lowell C, Tybulewicz V L, DeFranco A L. A critical role for Syk in signal transduction and phagocytosis mediated by Fc gamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daeron M, Malbec O, Latour S, Bonnerot C, Segal D M, Fridman W H. Distinct intracytoplasmic sequences are required for endocytosis and phagocytosis via murine Fc gamma RII in mast cells. Int Immunol. 1993;5:1393–1401. doi: 10.1093/intimm/5.11.1393. [DOI] [PubMed] [Google Scholar]
- 12.DeFranco A L. Transmembrane signaling by antigen receptors of B and T lymphocytes. Curr Opin Cell Biol. 1995;7:163–175. doi: 10.1016/0955-0674(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 13.Fleit S A, Fleit H B, Zolla-Pazner S. Culture and recovery of macrophages and cell lines from tissue culture-treated and -untreated plastic dishes. J Immunol Methods. 1984;68:119–129. doi: 10.1016/0022-1759(84)90142-x. [DOI] [PubMed] [Google Scholar]
- 14.Frisch S M, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 15.Gao J, Zoller K E, Ginsberg M H, Brugge J S, Shattil S J. Regulation of the pp72syk protein tyrosine kinase by platelet integrin alpha IIb beta 3. EMBO J. 1997;16:6414–6425. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotoh A, Takahira H, Geahlen R L, Broxmeyer H E. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth Differ. 1997;8:721–729. [PubMed] [Google Scholar]
- 17.Greenberg S, Chang P, Silverstein S C. Tyrosine phosphorylation of the gamma subunit of Fc gamma receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J Biol Chem. 1994;269:3897–3902. [PubMed] [Google Scholar]
- 18.Greenberg S, Chang P, Wang D C, Xavier R, Seed B. Clustered syk tyrosine kinase domains trigger phagocytosis. Proc Natl Acad Sci USA. 1996;93:1103–1107. doi: 10.1073/pnas.93.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Indik Z, Kelly C, Chien P, Levinson A I, Schreiber A D. Human Fc gamma RII, in the absence of other Fc gamma receptors, mediates a phagocytic signal. J Clin Invest. 1991;88:1766–1771. doi: 10.1172/JCI115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indik Z K, Hunter S, Huang M M, Pan X Q, Chien P, Kelly C, Levinson A I, Kimberly R P, Schreiber A D. The high affinity Fc gamma receptor (CD64) induces phagocytosis in the absence of its cytoplasmic domain: the gamma subunit of Fc gamma RIIIA imparts phagocytic function to Fc gamma RI. Exp Hematol. 1994;22:599–606. [PubMed] [Google Scholar]
- 21.Indik Z K, Pan X Q, Huang M M, McKenzie S E, Levinson A I, Schreiber A D. Insertion of cytoplasmic tyrosine sequences into the nonphagocytic receptor Fc gamma RIIB establishes phagocytic function. Blood. 1994;83:2072–2080. [PubMed] [Google Scholar]
- 22.Indik Z K, Park J G, Hunter S, Schreiber A D. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86:4389–4399. [PubMed] [Google Scholar]
- 23.Indik Z K, Park J G, Pan X Q, Schreiber A D. Induction of phagocytosis by a protein tyrosine kinase. Blood. 1995;85:1175–1180. [PubMed] [Google Scholar]
- 24.Jouvin M H, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet J P. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- 25.Khan K F, Falcone D J. Role of laminin in matrix induction of macrophage urokinase-type plasminogen activator and 92-kDa metalloproteinase expression. J Biol Chem. 1997;272:8270–8275. doi: 10.1074/jbc.272.13.8270. [DOI] [PubMed] [Google Scholar]
- 26.Kiefer F, Wagner E F, Keller G. Fractionation of mouse bone marrow by adherence separates primitive hematopoietic stem cells from in vitro colony-forming cells and spleen colony-forming cells. Blood. 1991;78:2577–2582. [PubMed] [Google Scholar]
- 27.Kihara H, Siraganian R P. Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J Biol Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- 28.Kimura T, Zhang J, Sagawa K, Sakaguchi K, Appella E, Siraganian R P. Syk-independent tyrosine phosphorylation and association of the protein tyrosine phosphatases SHP-1 and SHP-2 with the high affinity IgE receptor. J Immunol. 1997;159:4426–4434. [PubMed] [Google Scholar]
- 29.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 30.Kurosaki T, Johnson S A, Pao L, Sada K, Yamamura H, Cambier J C. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latour S, Fournel M, Veillette A. Regulation of T-cell antigen receptor signalling by Syk tyrosine protein kinase. Mol Cell Biol. 1997;17:4434–4441. doi: 10.1128/mcb.17.8.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law C-L, Chandran K A, Sidorenko S P, Clark E A. Phospholipase C-γ1 interacts with conserved phosphotyrosyl residues in the linker region of Syk and is a substrate for Syk. Mol Cell Biol. 1996;16:1305–1315. doi: 10.1128/mcb.16.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallick-Wood C A, Pao W, Cheng A M, Lewis J M, Kulkarni S, Bolen J B, Rowley B, Tigelaar R E, Pawson T, Hayday A C. Disruption of epithelial gamma delta T cell repertoires by mutation of the Syk tyrosine kinase. Proc Natl Acad Sci USA. 1996;93:9704–9709. doi: 10.1073/pnas.93.18.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda M, Park J G, Wang D C, Hunter S, Chien P, Schreiber A D. Abrogation of the Fc gamma receptor IIA-mediated phagocytic signal by stem-loop Syk antisense oligonucleotides. Mol Biol Cell. 1996;7:1095–1106. doi: 10.1091/mbc.7.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J G, Murray R K, Chien P, Darby C, Schreiber A D. Conserved cytoplasmic tyrosine residues of the gamma subunit are required for a phagocytic signal mediated by Fc gamma RIIIA. J Clin Invest. 1993;92:2073–2079. doi: 10.1172/JCI116804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole A, Gibbins J M, Turner M, van Vugt M J, van de Winkel J G J, Saito T, Tybulewicz V L J, Watson S P. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards J D, Gold M R, Hourihane S L, DeFranco A L, Matsuuchi L. Reconstitution of B cell antigen receptor-induced signaling events in a nonlymphoid cell line by expressing the Syk protein-tyrosine kinase. J Biol Chem. 1996;271:6458–6466. doi: 10.1074/jbc.271.11.6458. [DOI] [PubMed] [Google Scholar]
- 39.Ross G D, Reed W, Dalzell J G, Becker S E, Hogg N. Macrophage cytoskeleton association with CR3 and CR4 regulates receptor mobility and phagocytosis of iC3b-opsonized erythrocytes. J Leukocyte Biol. 1992;51:109–117. doi: 10.1002/jlb.51.2.109. [DOI] [PubMed] [Google Scholar]
- 40.Rowley R B, Burkhardt A L, Chao H G, Matsueda G R, Bolen J B. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 41.Shaw L M, Messier J M, Mercurio A M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1 integrin. J Cell Biol. 1990;110:2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiue L, Green J, Green O M, Karas J L, Morgenstern J P, Ram M K, Taylor M K, Zoller M J, Zydowsky L D, Bolen J B, Brugge J S. Interaction of p72syk with the γ and β subunits of the high-affinity receptor for immunoglobulin E, FcɛRI. Mol Cell Biol. 1995;15:272–281. doi: 10.1128/mcb.15.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiue L, Zoller M J, Brugge J S. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem. 1995;270:10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- 44.Strzelecka A, Kwiatkowska K, Sobota A. Tyrosine phosphorylation and Fcgamma receptor-mediated phagocytosis. FEBS Lett. 1997;400:11–14. doi: 10.1016/s0014-5793(96)01359-2. [DOI] [PubMed] [Google Scholar]
- 45.Swieter M, Berenstein E H, Siraganian R P. Protein tyrosine phosphatase activity associates with the high affinity IgE receptor and dephosphorylates the receptor subunits, but not Lyn or Syk. J Immunol. 1995;155:5330–5336. [PubMed] [Google Scholar]
- 46.Tapper H, Grinstein S. Fc receptor-triggered insertion of secretory granules into the plasma membrane of human neutrophils: selective retrieval during phagocytosis. J Immunol. 1997;159:409–418. [PubMed] [Google Scholar]
- 47.Taylor J A, Karas J L, Ram M K, Green O M, Seidel-Dugan C. Activation of the high-affinity immunoglobulin E receptor FcɛRI in RBL-2H3 cells is inhibited by Syk SH2 domains. Mol Cell Biol. 1995;15:4149–4157. doi: 10.1128/mcb.15.8.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teramoto H, Salem P, Robbins K C, Bustelo X R, Gutkind J S. Tyrosine phosphorylation of the vav proto-oncogene product links Fc epsilon RI to the Rac1-JNK pathway. J Biol Chem. 1997;272:10751–10755. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- 49.Turner M, Gulbranson-Judge A, Quinn M E, Walters A E, MacLennan I M, Tybulewicz V J. Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J Exp Med. 1997;186:2013–2021. doi: 10.1084/jem.186.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner M, Mee P J, Costello P S, Williams O, Price A A, Duddy L P, Furlong M T, Geahlen R L, Tybulewicz V L. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 51.Waddell T K, Fialkow L, Chan C K, Kishimoto T K, Downey G P. Potentiation of the oxidative burst of human neutrophils. A signaling role for L-selectin. J Biol Chem. 1994;269:18485–18491. [PubMed] [Google Scholar]
- 52.Wan Y, Bence K, Hata A, Kurosaki T, Veillette A, Huang X Y. Genetic evidence for a tyrosine kinase cascade preceding the mitogen-activated protein kinase cascade in vertebrate G protein signaling. J Biol Chem. 1997;272:17209–17215. doi: 10.1074/jbc.272.27.17209. [DOI] [PubMed] [Google Scholar]
- 53.Wange R L, Guitian R, Isakov N, Watts J D, Aebersold R, Samelson L E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 54.Wood P R, Simes R J, Nelson D S. Activity of mouse macrophages purified by adherence to, and removal from, a plastic surface. J Immunol Methods. 1979;28:117–124. doi: 10.1016/0022-1759(79)90333-8. [DOI] [PubMed] [Google Scholar]
- 55.Yousefi S, Hoessli D C, Blaser K, Mills G B, Simon H U. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zoller K E, MacNeil I A, Brugge J S. Protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J Immunol. 1997;158:1650–1659. [PubMed] [Google Scholar]