Abstract
Adult American horseshoe crabs, Limulus polyphemus, possess endogenous circadian and circatidal clocks controlling visual sensitivity and locomotion, respectively. The goal of this study was to determine the types of activity rhythms expressed by juvenile horseshoe crabs (n=24) when exposed to a 14:10 light:dark cycle (LD) for ten days, followed by ten days of constant darkness (DD). Horseshoe crab activity was recorded with a digital time-lapse video system that used an infrared sensitive camera so animals could be monitored at night. In LD, 15 animals expressed daily patterns of activity, six displayed a circatidal pattern, and the remaining three were arrhythmic. Of the 15 animals with daily patterns of locomotion, seven had a significant preference (P < 0.05) for diurnal activity and three for nocturnal activity; the remainder did not express a significant preference for day or night activity. In DD, 13 horseshoe crabs expressed circatidal rhythms and eight maintained an ~24 hour pattern. While these results suggest the presence of a circadian clock influencing circatidal patterns of locomotion, these apparent circadian rhythms are perhaps more likely to represent the expression of one of two circalunidian clocks. Overall, these results indicate that, like adults, juvenile horseshoe crabs express both daily and tidal patterns of activity and that at least one, and maybe both, of these patterns is driven by endogenous clocks.
Keywords: American horseshoe crabs, Limulus polyphemus, circadian, circatidal, biological clock, locomotion, circalunidian
Introduction
Animals inhabiting, or visiting, the intertidal zone often express either a daily pattern of activity (synchronized to the light cycle), a tidal pattern (synchronized to the tidal cycle), or some combination of the two. Three intertidal organisms whose activity patterns have been scrutinized in detail are the green crab (Carcinus maenas, Naylor, 1958), mole crab (Emerita talpoida, Forward et al., 2005), and fiddler crab (Uca spp., Bennett et al., 1957; Barnwell, 1966). All three exhibit a combination of tidal and daily patterns of activity and, within each species, there is wide individual variation in the patterns expressed. This variation has been attributed to varying selection pressures, native environments, reproductive status, and molt states (Bennett et al., 1957; Naylor, 1958; Barnwell, 1966; Forward et al., 2005). Importantly, when these three species are placed in the laboratory, under constant conditions, the rhythms persist, indicating that they are driven by endogenous biological clocks (Bennett et al., 1957; Naylor, 1958; Aschoff, 1960; Barnwell, 1966; Forward et al., 2005).
Another common species that inhabits and/or visits the intertidal zone is the American horseshoe crab, Limulus polyphemus. Adult horseshoe crabs are known to express both tidal and daily rhythms of locomotion (Chabot et al., 2011). They also have an endogenous circadian rhythm of visual sensitivity, enabling their eyes to become ~1,000,000 times more sensitive to light at night (Barlow et al., 1980; Barlow, 1983; Barlow and Powers, 2003). However, at least in adults, it appears that the circadian clock controlling eye sensitivity does not control patterns of locomotion (Watson et al., 2008). To date, in adult horseshoe crabs, only their tidal pattern of activity has been clearly demonstrated to be under the control of an endogenous clock (Chabot et al., 2004, 2007, 2008, 2011; Watson et al., 2008, 2009; Chabot and Watson, 2010; Watson and Chabot, 2010). At the present time, there are no data demonstrating the presence of a circadian clock controlling locomotion, even though many adult horseshoe crabs exposed to a LD cycle express a preference for either daytime, or nighttime, activity (Chabot et al., 2004, 2007, 2011). Thus, like the three aforementioned crabs, some horseshoe crabs express a complex pattern of activity that is a mixture of daily and tidal components.
Larval horseshoe crabs also express both tidal and daily activity rhythms. After they hatch from their egg cases, larvae spend up to six days in the water column before they settle to the bottom, and during this time they are positively phototactic and most active at night and during high tides (Rudloe, 1979, Shuster, 1982). When collected in the field and brought into the laboratory, larvae express a circatidal rhythm of activity in constant conditions, becoming active near the start of the expected ebb tide (Ehlinger and Tankersley, 2006). Moreover, it was demonstrated that they can be entrained to 12.4 h cycles of agitation and will continue to express a circatidal rhythm of activity when the agitation cycles are terminated (Ehlinger and Tankersley, 2006). Therefore, like adult horseshoe crabs, it is clear that larvae have a circatidal clock that controls the timing of their activity and, while they also appear to have a preference for nocturnal activity, and they can sense light (Errigo et al., 2001; Meadors et al., 2001), currently there is no evidence for an underlying circadian clock influencing their activity.
Juvenile horseshoe crabs also appear to be influenced by tidal cycles and daily changes in light levels. A simulated ebbing tide causes animals to follow the water down the beach and bury in the sand at the edge of the water. They only move when covered with water, and express a preference for slack tide (Meury and Gibson, 1990). Studies of the juvenile horseshoe crab, Tachypleus tridentatus, in Japan revealed that they increased their activity two hours before low tide; however, no data were collected during high tides (Chiu and Morton, 2004). Juvenile horseshoe crabs, in Florida also express a peak of activity two hours prior to low tide, during the ebb tide. However, when placed in subtidal enclosures in water deeper than where they are typically found, activity correlated to the light rather than the tidal cycle (Rudloe, 1981). In a laboratory study by Rudloe (1981), juvenile horseshoe crabs exposed to water of a constant depth, ambient light, and ambient temperature expressed diurnal activity. However, because the animals were not exposed to constant light conditions and some equipment malfunctions limited the amount of data obtained, it was not possible to determine if their daily rhythms were driven by an endogenous circadian clock.
Interestingly, two other laboratory studies determined that juvenile American horseshoe crabs tended to be nocturnal but, due to the low sample size and short duration of the studies, firm conclusions could not be drawn about the factors giving rise to this pattern of activity (Casterlin and Reynolds, 1979; Borst and Barlow, 2002). Further, the length of time and conditions under which they were held prior to testing were not described in these papers. This is important because tidal rhythms tend to degrade in the laboratory (Palmer, 1995b; Chabot et al., 2008) and this might explain why these animals were mainly nocturnal. The study by Borst and Barlow (2002) tested for endogenous rhythms, but the resolution of the data (by hour) was not fine enough to determine whether the patterns expressed were circatidal or circadian. Therefore, while the main conclusion of the study was that juvenile horseshoe crabs are nocturnal, there were also indications in their data suggesting that the juvenile horseshoe crabs in that study might have expressed circatidal or circadian patterns of locomotion, or a combination of the two.
The primary goal of this study was to determine the types of activity rhythms expressed by juvenile horseshoe crabs when exposed to a summer LD cycle and then placed in DD. In the first situation the data obtained enabled us to test the hypothesis that these animals have an endogenous clock that enables them to express a tidal rhythm of activity in the absence of any tidal cues. The constant darkness section of the experiment was designed to test the hypothesis that juvenile horseshoe crabs have an endogenous circadian clock that enables them to express an ~ 24 h rhythm in the absence of LD cues. Finally, the combination of the two phases of the experiment made it possible to determine if LD input might override an underlying tendency of some animals to express a tidal rhythm of activity.
In LD conditions, we found that most animals expressed a daily rhythm of activity and the majority of these animals preferred to be most active during the daytime. Eight of the 15 animals that expressed a daily rhythm in LD continued this pattern in DD. A number of animals expressed a tidal rhythm of activity both in LD and DD, clearly indicating the presence of a circatidal rhythm influencing their tendency to be active. However, more animals expressed tidal rhythms in DD than LD, suggesting that LD cycles may, at least partially, override the expression of endogenous tidal rhythms in these animals. These data, taken together, also suggest that the complex patterns of behavior expressed by juvenile horseshoe crabs, and perhaps some of the aforementioned marine invertebrates, are likely the result of the interactions between at least one endogenous clock and natural LD cycles.
Materials and Methods
Collection and care of animals
Juveniles of L. polyphemus (size range = 40 – 55 mm carapace width, ~3 – 4 years old, Sekiguchi et al., 1988) were collected from Little Sippewissett Marsh, Falmouth, MA on four different dates (6/9/2010, 7/2/2010, 8/22/2010, and 9/1/2010). This area experiences a semidiurnal tidal pattern, with two high and two low tides of approximately equal size within each lunar day. Day length at the collection location was within 1 h of the laboratory imposed light cycle (14:10) for all collection dates.
After collection, animals were transported in coolers to an indoor facility at University of New Hampshire, Durham, NH. Less than 24 h elapsed between collection, the placement of animals in individual chambers and initiation of recordings. The activity patterns expressed by 24 juveniles were recorded during four different trials, each lasting 20 days. In the trials that began on 6/9/2010 and 7/2/2010 five animals were used, and in the trials beginning on 8/22/2010 and 9/1/2010 seven animals were used.
Prior to the start of a trial, all animals were fed to satiation using frozen Spirulina brine shrimp (Hikari Bio-Pure, Hikari, Hayward, CA) thawed in saltwater. Satiation was determined by cessation of food consumption, based on the end of the stereotyped feeding movements of the legs, as described by Wyse and Dwyer (1973).
Monitoring animal activity
To increase the visibility of animals at night, a 1 cm2 piece of reflective tape (Nashua® Multi-purpose Foil Tape, Barry Plastics, Evansville, IN) was attached to the dorsal carapace of each horseshoe crab using cyanoacrylate-based glue (Krazy® glue, Columbus, OH). This did not cover any of their simple or complex eyes. Horseshoe crabs were then placed in individual 20-cm diameter plastic chambers filled with 3 cm of sand and 15 cm of 30 psu seawater held in an air-conditioned room that was slightly cooler than normal room temperature (20°C ± 0.004, n = 21,693, range 18.6 – 21.9°C, values obtained every minute). For the first ten days of the experiment, animals were subjected to a summer LD cycle (14:10) using a modified dawn/dusk simulator to gradually increase and decrease incandescent lights for 2 h at the beginning and end of each day (SunUp, Light Therapy Products, Plymouth, MA). Imposed laboratory light cycles were within ± 1 h of the ambient light cycle at the time of collection. Light levels were 163 ± 0.35 lux (mean ± S.E.M., n = 24,190) during the day and < 10 lux at night. Light levels were measured using a HOBO Pendant® Temperature/Light Data Logger (Onset Computer Corporation, Bourne, MA), which took one reading per minute. All samples from the time sunrise was initiated to the end of sunset were averaged to calculate the day light levels while light samples from the end of sunset until the start of sunrise were averaged to calculate the nighttime light levels. At the end of the initial ten-day period, animals were subjected to a ten-day period of constant darkness (DD). During DD, and the nights associated with LD, infrared lights illuminated the chambers. Infrared lights were chosen for illumination due to the limited ability of horseshoe crabs to see in this spectrum (Graham and Hartline, 1935, Chapman and Lall, 1967; reviewed in Battelle, 2006).
Activity was recorded using black and white, infrared sensitive camera(s) (PC-222, Supercircuits, Austin, TX). Camera outputs were digitized (Canopus® ADVC-110, Grass Valley, San Francisco, CA), time-stamped and recorded with a computer (Macintosh, OS 10.5.6) using video capture software (Gawker, v. 0.8.3, Seattle, WA), that captured one frame every 30 s. In trials 1 and 2, a single camera was used to simultaneously record the activity of the animals in all of the chambers. In trials 3 and 4, a multiplexer (Pelco Genex™ MX4009MD, 9 channel) was used to combine the output of four different cameras into a single video stream that was digitized as described above.
Videos were analyzed by eye in 5 min blocks. Any movement during a 5 min block was recorded as a “1” while no movement was recorded as a “0”. These data were then plotted and analyzed further as actograms and Lomb-Scargle periodograms (P < 0.001) using the program ClockLab® (MatLab, Actimetrics, Evanston, IL, v. R2009a), as described in Chabot et al. (2008). The maximum Lomb-Scargle periodogram peak in the circadian (periods of 22–26h) or circatidal (periods of 10.4–14.4h) range was used to determine if animals expressed circadian (~24 h) or circatidal (~12.4 h) activity patterns. Actograms were also visually inspected to confirm the results yielded by the periodogram analyses, with specific attention paid to the number of activity bouts each day. Significant differences in activity levels during the day and night were determined for animals that expressed a daily rhythm in LD. The percentage of each hour that animals were active during light hours and dark hours was calculated by calendar day and compared using a non-parametric Wilcoxon matched-pairs signed-ranks test using InStat® (GraphPad Software, La Jolla, CA, v. 3.1a 2009). To determine alpha, or the duration of the main bout of activity, best eye-fit lines were drawn through the “onset” and “offset” of active periods using a single-blind protocol.
Results
Of the 24 animals tested, 15 (63%) expressed a daily rhythm of activity when exposed to LD (tau (τ) = 23.90 ± 0.35 h; mean ± S.E.M.; P < 0.001; Figs. 1 and 2). Six of the original 24 animals (25%) expressed primarily circatidal rhythms of activity during LD (τ = 12.36 ± 0.20 h, Fig. 3). There was no clear correlation between the circatidal rhythms expressed in the laboratory and the natural tidal cycle of the collection location. Three of the 24 animals tested did not express a clear pattern of activity (arrhythmic).
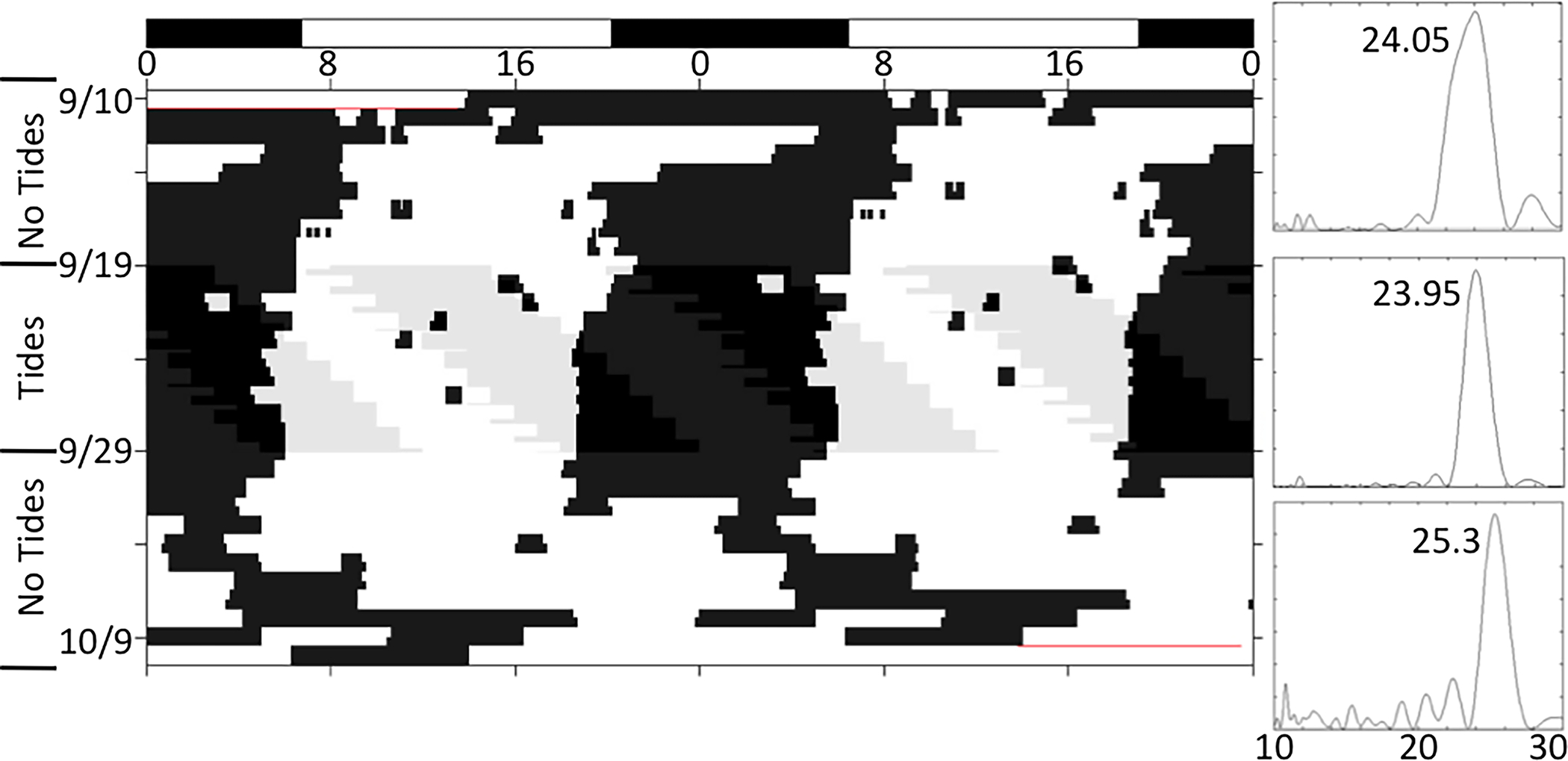
Figure 1.
Locomotor activity of a juvenile horseshoe crab expressing a daily pattern of activity during LD, and a circadian pattern in DD. Image on the left is a double-plotted actogram; activity is indicated by black tick marks. The imposed light:dark cycle (LD) is indicated at the top of the image. During the first ten days, horseshoe crabs were exposed to a 14:10 LD cycle, and during the second ten days they were in constant darkness (DD). Images on the right are Lomb-Scargle periodograms for the same animal during either the LD period (top) or the DD period (bottom). Vertical scale is the relative strength of rhythmicity (Q(p)); the horizontal axis shows the length of periods in hours (10 – 30); the largest peak above the horizontal line of significance (P < 0.001) is indicated by a numerical value.
Figure 2.
Locomotor activity of a horseshoe crab expressing a daily pattern of activity in LD and a combination of circadian and circatidal activity in DD. Refer to Figure 1 for a detailed description of figure components.
Figure 3.
Locomotor activity recorded from a juvenile horseshoe crab expressing circatidal activity in both LD and DD. Tidal activity increases when the animal was exposed to DD, indicating masking of the activity pattern during LD. Refer to Figure 1 for a detailed description of figure components.
Of the 15 animals expressing daily rhythms in LD, seven (47%) were significantly more active during the day and three (20%) during the night (Wilcoxon matched-pairs signed-ranks test, diurnal: 54 < W < 110, P < 0.0302; nocturnal: −52 < W < −78, P < 0.02). The remaining five animals did not express significantly different activity levels between day and night. When animals that were diurnal during LD were exposed to DD, three expressed a circadian rhythm of activity, while four switched to a circatidal pattern of activity. Of the three animals that were most active at night in LD, one maintained an ~24 h rhythm of activity in DD, while two switched to circatidal patterns of behavior.
When exposed to DD, seven of the 15 animals that expressed a daily rhythm in LD continued to express an ~24 h rhythm of activity (τ = 23.38 ± 0.30 h; Figs. 1 and 2), while eight switched to circatidal rhythms of activity (τ= 15.02 ± 1.83 h; Fig. 4). Five of the six animals that originally expressed a circatidal rhythm in LD continued this same pattern of behavior when subjected to DD (τ = 12.48 ± 0.13 h, Fig. 3). One animal switched from a circatidal pattern of behavior in LD to an ~24 h rhythm of activity in DD (τ = 23.45, data not shown). Thus, in DD, 13/24 exhibited circatidal rhythms, while 8/24 exhibited apparent circadian rhythms (~24 h rhythm of activity) with one peak of activity per 24 h. However, it should be noted that several of the animals that exhibited a unimodal, apparently circadian, pattern of activity also expressed secondary peaks of activity in the circatidal range (τ = 12.01 ± 0.24 h, Fig. 2). There was no clear correlation between the circatidal rhythms expressed under DD conditions in the laboratory and the natural tidal cycle of the collection location.
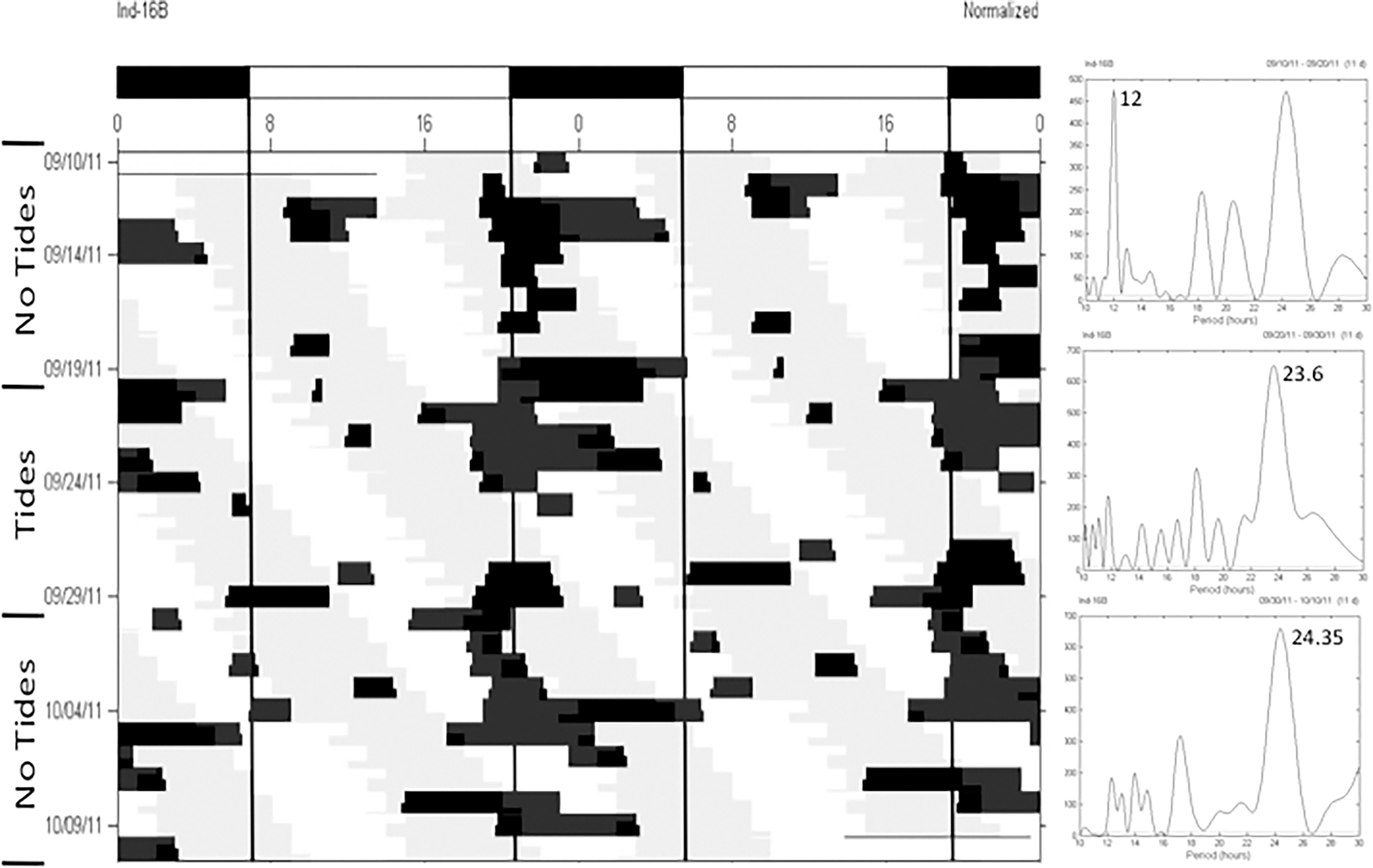
Figure 4.
Locomotor activity recorded from a juvenile horseshoe crab expressing a nocturnal pattern of activity in LD, and then switching to a circatidal pattern of activity DD. Initially during LD, there is activity present during both day and night. The activity during the day becomes ‘masked’ and is reduced in amount. Note that the nocturnal bout of activity in LD transitioned into one of the apparent tidal bouts of activity in DD. During DD, there were two bouts of activity each day and they each had a different duration. Refer to Figure 1 for a detailed description of figure components.
Activity duration (alpha) was calculated for 11 animals (the remaining animals did not have clear enough patterns to calculate alpha). During the LD portion of the experiment, nocturnal animals had bouts of activity lasting 9.32 ± 2.20 h (n = 6, D lasted 10 h in the 14:10 LD cycle). The one clear diurnal animal had an alpha of 16.4 h (L lasted 14 h), and the four circatidal animals expressed bouts of activity that were 7.39 ± 1.66 h long. During DD, alpha was 8.8 ± 0.69 h for animals expressing circadian rhythms (n = 7) and 5.57 ± 1.27 h (n = 4) for those expressing circatidal rhythms. The duration of activity for daily or circatidal patterns of activity was not significantly different between LD and DD conditions.
Discussion
This is the first study to document clear circatidal activity rhythms in juvenile American horseshoe crabs. While only 20% of the animals expressed a circatidal rhythm when exposed to LD, approximately half of them expressed a circatidal rhythm when in DD. Furthermore, many of the animals that expressed daily activity patterns in LD also exhibited subtle indications of a circatidal rhythm, which can also be seen in the previous studies by Casterlin and Reynolds (1979) and Borst and Barlow (2002). With the benefit of hindsight, it is possible that, with an extended time in DD, the secondary peak of activity reported in these previous studies would have become more prevalent, yielding a more obvious circatidal rhythm.
The activity patterns seen in the juveniles tested in this study are similar to previous results with adult horseshoe crabs. When adults are exposed to a LD cycle, different individuals express different types of rhythms: some express tidal rhythms while others express daily rhythms, and still others a mixture of the two (Chabot et al., 2008; Chabot and Watson, 2010). Moreover, when they are subsequently exposed to DD, they also tend to switch to a tidal pattern of activity (Chabot et al., 2004, 2008; Watson et al., 2008; reviewed in Chabot and Watson, 2010). These patterns of activity could be the result of two different mechanisms, both of which are described below.
While all life history stages of horseshoe crabs express some type of daily activity pattern, it is not known if this is the result of an endogenous circadian clock or a phenomenon called “masking”. Masking occurs when exogenous cues, such as light, influence the expression of a behavior, such as locomotion, in a manner that make it appear as if the behavior has a particular type of rhythm, or pattern. In this study, 15 of 24 animals expressed a daily pattern of activity in LD. It is well documented that adult horseshoe crabs possess an endogenous circadian clock that controls the sensitivity of their eyes (Barlow et al., 1980; Barlow, 1983; Barlow and Powers, 2003), but recent studies indicate that this particular clock does not appear to influence locomotion (Watson et al., 2008). An alternative explanation for the data presented is that patterns of locomotion expressed by juvenile and adult horseshoe crabs are the result of the interplay between an endogenous clock controlling circatidal rhythms and the masking influences of light and dark. For example, in some cases (Fig. 2), the nocturnal bout of activity expressed by animals in LD continued in DD, and then a second bout of activity emerged in DD during the subjective day. This second peak, apparently under the control of a clock controlling circatidal rhythms, may have been suppressed by light during the LD cycle. Masking of a circatidal activity pattern by a light cycle is not unusual; it has been shown in at least three other organisms, in addition to adult horseshoe crabs: Uca spp., C. maenas, and Sesarma reticulatum (Naylor, 1958; Honegger, 1973; Palmer, 1990; Chabot et al., 2008). We have also documented this preference for activity during only one of the two high tides in a given day in freely behaving adult horseshoe crabs in their natural habitat. This particular animal clearly preferred to be active only during the high tide that took place in the day (Watson, unpublished data). Therefore, while our data appear to provide evidence of a circadian clock influencing locomotion in juvenile horseshoe crabs, it is perhaps more likely that the patterns observed are the result of the influence of LD cycles on an underlying tendency to express a tidal rhythm of locomotion.
While the molecular mechanisms underlying circadian clocks are well established (Dunlap, 1999), it remains to be determined how ~12.4 h rhythms are created within the nervous system of many intertidal organisms. Our working hypothesis is that the circatidal activity rhythm in horseshoe crabs is created by combining two circalunidian clocks that are 180 degrees out of phase, with each clock controlling one of the two bouts of tidal activity that occur each day. This is the essence of the circalunidian hypothesis proposed by Palmer (1990, 1995a), and it would not require the evolution of a new network of molecular interactions capable of producing an ~ 12.4 h, vs an ~ 24 h, rhythm. Instead, two separate ~ 24 h clocks could be used, which is how fruit flies control their two daily bouts of activity at dawn and dusk (Taghert and Shafer, 2006). Of the four possible indicators of a circalunidian clock, the juveniles in this study exhibited three: 1) two rhythms with different periodicities (Fig. 5); 2) “splitting” of one activity bout into two (Figs. 4 and 6); and 3) activity bouts of different durations (Figs. 4 and 5). The variety of activity patterns expressed by the animals in this study is not unusual for intertidal organisms. Three different species of crabs (C. maenas, Emerita talpoida, and Uca spp.) also exhibit a wide variety of patterns, including tidal, daily, a combination of both, and arrhythmic (Bennett et al., 1957; Barnwell, 1966). In addition, adult horseshoe crabs express a wide range of activity patterns, both in the laboratory and in their natural habitat (Chabot and Watson, 2010). This variability is evident even when using only males of the same size, collected at the same location. Furthermore, horseshoe crabs have a terminal molt, so molt stage is likely not a factor influencing the type of behavioral rhythms that we have documented in adult animals. While molt stage could influence the results of this experiment, no horseshoe crabs molted during their trial, or for the two weeks following the trial, reducing the probability that molt stage was a factor. Therefore, it appears that horseshoe crabs, and probably many other marine intertidal animals, possess endogenous clocks that help them to synchronize to tidal rhythms, but allow environmental inputs to synchronize the behavioral patterns expressed under natural conditions.
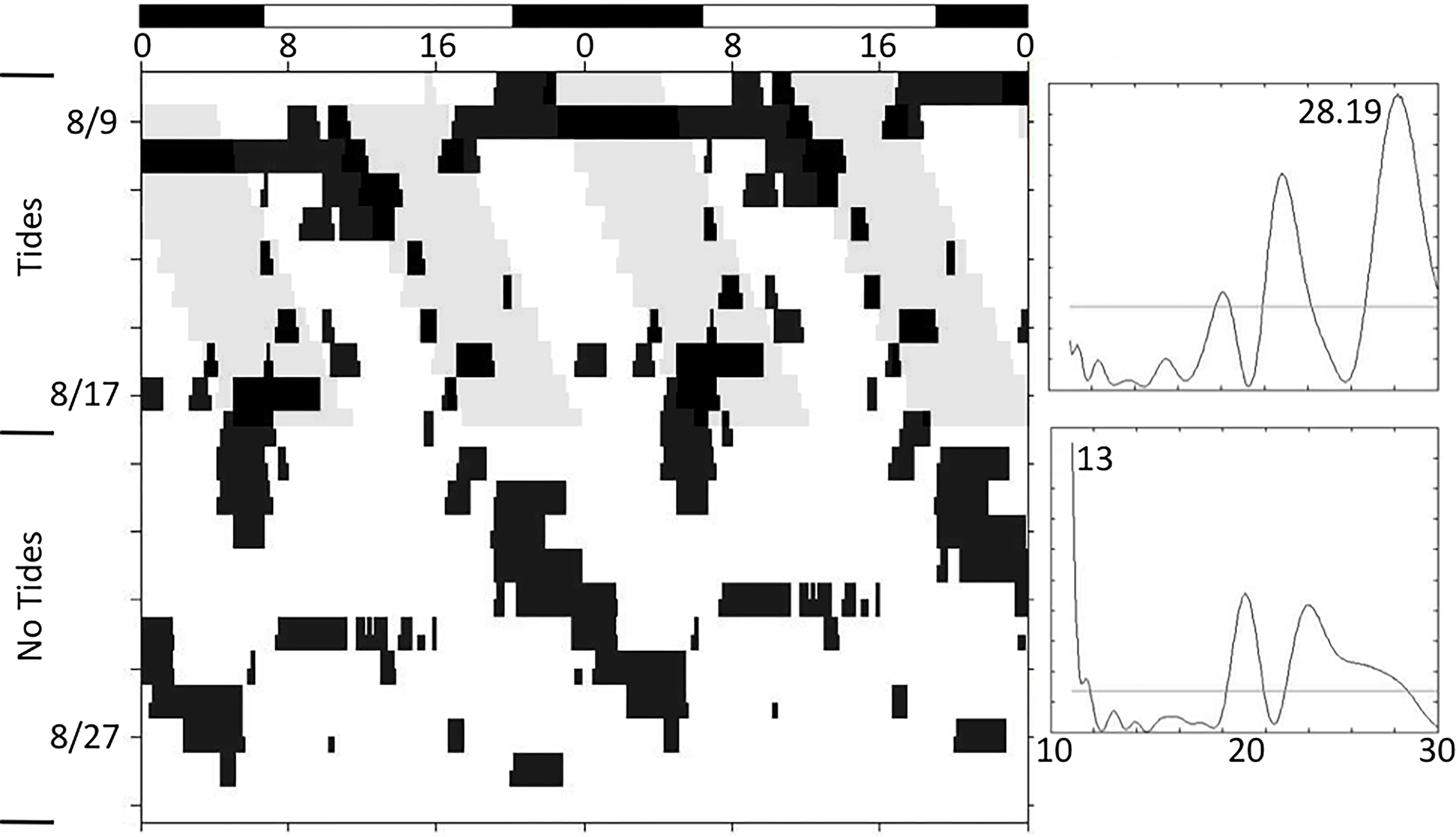
Figure 5.
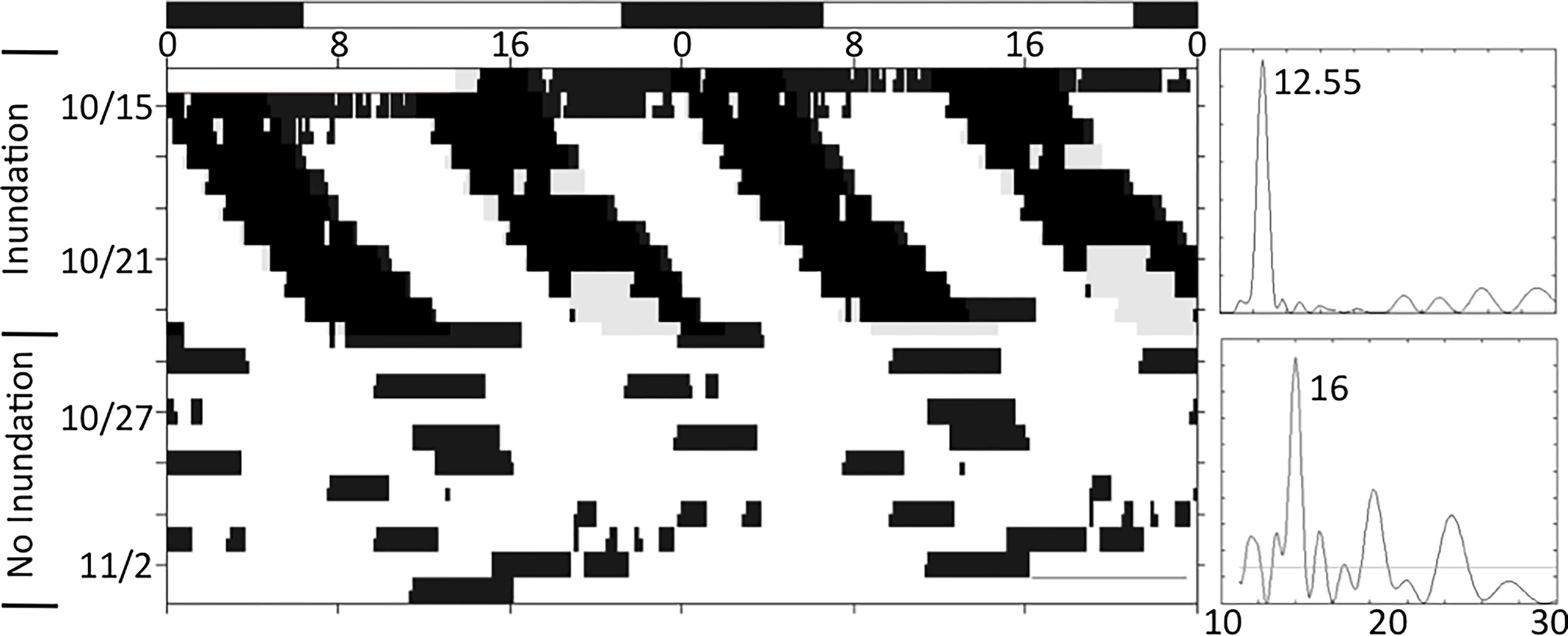
Apparent masking of the locomotor activity of a juvenile horseshoe crab exposed to LD, and then DD. Initially, this animal exhibited mixture of tidal and diurnal patterns of activity, showing two rhythms with different periods. These are also indicated in the periodogram, which shows two periods: one at 12 h and one at 24 h. When not exposed to LD cues, this animal reverted to four bouts of activity daily, or a circatidal rhythm. These bouts of activity were unequal in duration, which is one indicator of a circalunidian rhythm. Refer to Figure 1 for detailed description of figure components.
Figure 6.
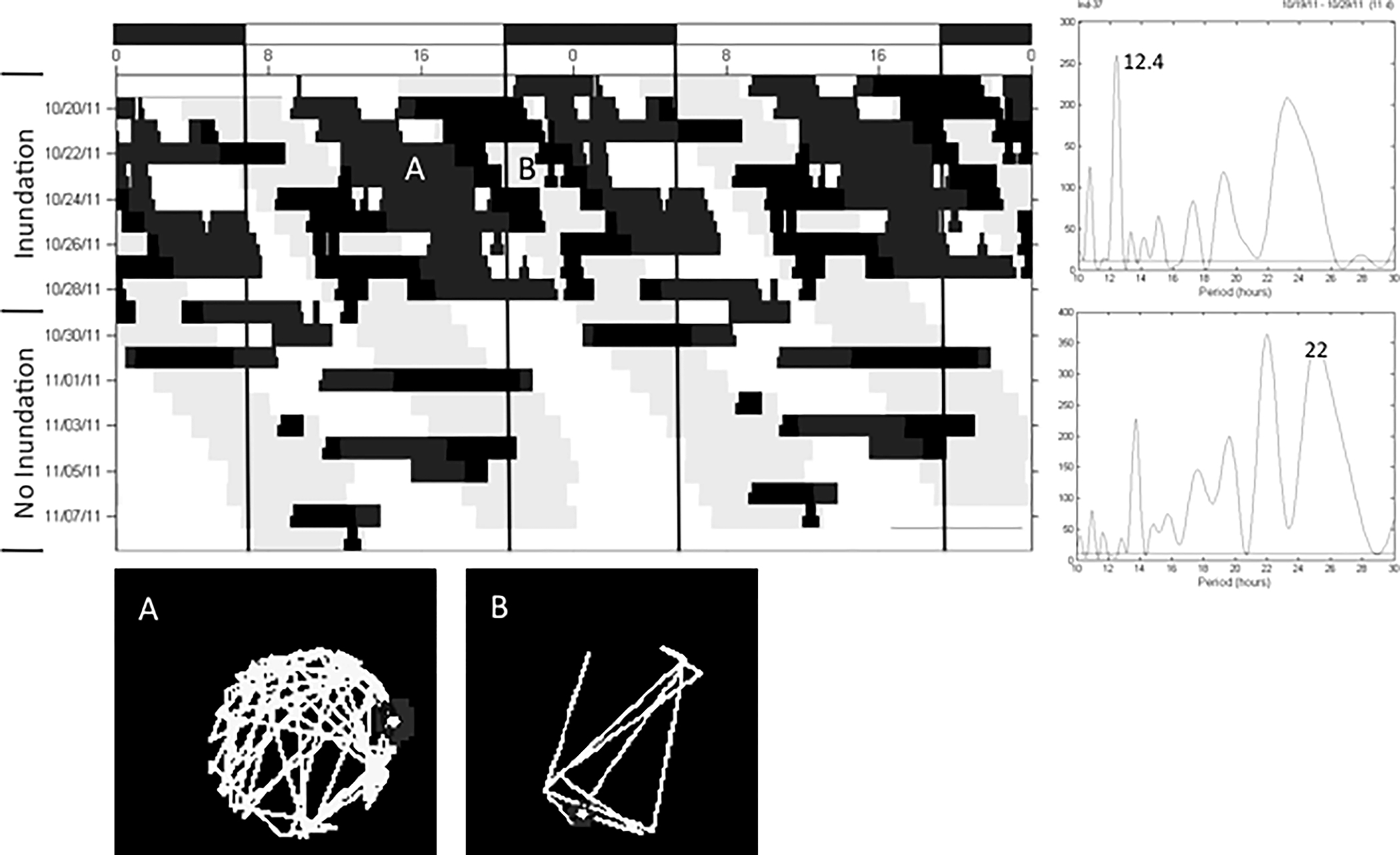
The locomotor activity of a juvenile horseshoe crab that expressed one bout of activity during LD that split into two bouts of activity per day in DD. Refer to Figure 1 for detailed description of figure components.
Acknowledgments
We are very grateful to Dr. Daniel Gibson (Worcester Polytechnic Institute) for helping us find, and collect, juvenile horseshoe crabs on Cape Cod, MA. He also provided many excellent suggestions that helped our study. We also thank Jason Goldstein, Tom Langley, and Tracy Pugh for many helpful discussions and assistance with the experimental design and data analyses. Finally, we are grateful for the help of many undergraduates with many aspects of the project, including Haley White, Suzanne Johnson, Suzanne LaChance, Alysia Campbell, Michael Lemmen, Mallory Chaput, and Kyle Jenks. This research was supported by UNH Graduate School grants to ED and NSF grant (NSF-IOS 090342) to CCC and WHW III.
Abbreviations:
- LD
Light:dark
- DD
constant darkness
Literature Cited
- Aschoff J 1960. Exogenous and endogenous components in circadian rhythms. Symp. Quant. Biol, 25: 11–28. [DOI] [PubMed] [Google Scholar]
- Barlow RB Jr. 1983. Circadian rhythms in the Limulus visual system. J. Neurobiol. 3: 856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB Jr., Chamberlain SC, and Levinson JZ. 1980. Limulus brain modulates the structure and function of the lateral eyes. Science. 210: 1037–1039. [DOI] [PubMed] [Google Scholar]
- Barlow RB and Powers MK. 2003. Seeing at night and finding mates: The role of vision. Pp. 83–102. in The American Horseshoe Crab, Shuster CN Jr., Barlow RB Jr., and Brockmann HJ eds. Harvard University Press, Cambridge, Massachusetts. [Google Scholar]
- Barnwell FH 1966. Daily and tidal patterns of activity in individual fiddler crab (genus Uca) from the Woods Hole region. Biol. Bull. 130: 1–17. [DOI] [PubMed] [Google Scholar]
- Battelle BA 2006. The eyes of Limulus polyphemus (Xiphosure, Chelicerata) and their afferent and efferent projections. Arthropod. Struct. Dev. 35: 261–274. [DOI] [PubMed] [Google Scholar]
- Bennett MF, Shriner J, and Brown RA. 1957. Persistent tidal cycles of spontaneous motor activity in the fiddler crab, Uca pugnax. Biol. Bull. 112: 267–275. [Google Scholar]
- Borst D and Barlow RB Jr. 2002. Circadian rhythms in locomotor activity of juvenile horseshoe crabs. Biol. Bull. 203: 227–228. [DOI] [PubMed] [Google Scholar]
- Casterlin ME and Reynolds WW. 1979. Diel locomotor-activity pattern of juvenile Limulus polyphemus (Linnaeus). Rev. Can. Biol. Exptl. 38: 43–44. [PubMed] [Google Scholar]
- Chabot CC, Betournay SH, Braley NR, and Watson WH. 2007. Endogenous rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. J. Exp. Mar. Biol. Ecol. 345: 79–89. [Google Scholar]
- Chabot CC, Kent J, and Watson WH. 2004. Circatidal and circadian rhythms of locomotion in Limulus polyphemus. Biol. Bull. 207: 72–75. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Skinner SJ, and Watson WH. 2008. Rhythms of locomotion expressed by Limulus polyphemus, the American horseshoe crab: I. Synchronization by artificial tides. Biol. Bull. 215: 34–45. [DOI] [PubMed] [Google Scholar]
- Chabot CC and Watson WH. 2010. Circatidal rhythms of locomotion in the American horseshoe crab Limulus polyphemus: underlying mechanisms and cues that influence them. Curr. Zool. 56: 499–517. [Google Scholar]
- Chabot CC, Yelle JF, O’Donnell CB, and Watson WH. 2011. The effects of water pressure, temperature, and current cycles on circatidal rhythms expressed by the American horseshoe crab, Limulus polyphemus. Mar. Freshw. Behav. Physiol. 44: 43–60. [Google Scholar]
- Chapman RM and Lall AB. 1967. Electroretinogram characteristics and the spectral mechanisms of the median ocellus and the lateral eye in Limulus polyphemus. J. Gen. Physiol. 50: 2267–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HMC and Morton B. 2004. The behaviour of juvenile horseshoe crabs, Tachypleus tridentatus (Xiphosura), on a nursery beach at Shui Hau Wan, Hong Kong. Hydrobiologia. 523: 29–35. [Google Scholar]
- Dunlap JC 1999. Molecular bases for circadian clocks. Cell. 96: 271 – 290. [DOI] [PubMed] [Google Scholar]
- Ehlinger GS and Tankersley RA. 2006. Endogenous rhythms and entrainment cues of larval activity in the horseshoe crab Limulus polyphemus. J. Exp. Mar. Biol. Ecol. 337: 205–214. [Google Scholar]
- Errigo M, McGuiness C, Meadors S, Mittmann B, Dodge F, and Barlow RB. 2001. Visually guided behavior of juvenile horseshoe crabs. Biol. Bull. 201: 271 – 272. [DOI] [PubMed] [Google Scholar]
- Forward RB Jr., Diaz H, and Cohen JH. 2005. The tidal rhythm in activity of the mole crab Emerita talpoida. J. Mar. Biol. Ass . U.K. 85: 895–901. [Google Scholar]
- Graham CH and Hartline HK. 1935. The response of single visual sense cells to lights of different wave lengths. J. Gen. Physiol. 18: 917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger HW 1973. Rhythmic motor activity responses of the California fiddler crab Uca crenulata to artificial light conditions. Mar. Biol. 18: 19–31. [Google Scholar]
- Meadors S, McGuiness C, Dodge FA, and Barlow RB. 2001. Growth, visual field, and resolution in the juvenile Limulus lateral eye. Biol. Bull. 201: 272–274. [DOI] [PubMed] [Google Scholar]
- Meury TW and Gibson D. G DG,. 1990. Force generation in juvenile Limulus polyphemus: effect on mobility in the intertidal environment. Bull. Mar. Sci. 47: 536–545. [Google Scholar]
- Naylor E 1958. Tidal and diurnal rhythms of locomotory activity in Carcinus maenas (L.). J. Exp. Biol. 35: 602–610. [Google Scholar]
- Palmer JD 1995b. The Biological Rhythms and Clocks of Intertidal Animals. Oxford University Press, New York, NY. [Google Scholar]
- Palmer JD 1995a. Review of the dual-clock control of tidal rhythms and the hypothesis that the same clock governs both circatidal and circadian rhythms. Chronobiol. Int. 12: 299–310. [Google Scholar]
- Palmer JD 1990. The rhythmic lives of crabs. BioScience 40: 352–358. [Google Scholar]
- Rudloe A 1981. Aspects of the biology of juvenile horseshoe crabs, Limulus polyphemus. Bull. Mar. Sci. 31: 125–133. [Google Scholar]
- Rudloe A 1979. Locomotor and light responses of larvae of the horseshoe crab, Limulus polyphemus (L.). Biol. Bull. 157: 494–505. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K, Seshimo H, and Sugita H. 1988. Post-embryonic development of the horseshoe crab. Biol. Bull. 174: 337 – 345. [Google Scholar]
- Shuster CN Jr. 1982. A pictorial review of the natural history and ecology of the horseshoe crab limulus polyphemus, with reference to other Limulidae. Pp. 1–52. in Physiology and Biology of Horseshoe Crabs: Studies on Normal and Environmentally Stressed Animals, Bonaventura J, Bonaventura C, Tesh S eds. Alan R. Liss, New York, NY. [PubMed] [Google Scholar]
- Taghert PH, and Shafer OT. 2006. Mechanisms of clock output in the Drosophila circadian pacemaker system. J. Biol. Rhythms 21: 445 – 457. [DOI] [PubMed] [Google Scholar]
- Watson WH, Bedford L, and Chabot CC. 2008. Rhythms of locomotion expressed by Limulus polyphemus, the American horseshoe crab: II. Relationship to circadian rhythms of visual sensitivity. Biol. Bull. 215: 46–56. [DOI] [PubMed] [Google Scholar]
- Watson WH, Schaller SY, and Chabot CC. 2009. The relationship between small- and large-scale movements of horseshoe crabs in the great bay estuary and Limulus behavior in the laboratory. Pp. 131–147. in Biology and Conservation of Horseshoe Crabs, Tanacredi JT, Botton ML, and Smith DR eds. Springer, New York. [Google Scholar]
- Watson WH and Chabot CC. 2010. High resolution tracking of adult horseshoe crabs Limulus polyphemus in a New Hampshire estuary using fixed array ultrasonic telemetry. Curr. Zool. 56: 599–610. [Google Scholar]
- Wyse GA and Dwyer NK. 1973. The neuromuscular basis of coxal feeding and locomotory movements in Limulus. Biol. Bull. 144: 567–579. [Google Scholar]


