Abstract
Background
Platinum-etoposide chemotherapy combined with immune checkpoint inhibitors (ICIs) has been recommended as the first-line standard treatment for extensive-stage small-cell lung cancer (ES-SCLC). However, the effect of thoracic radiotherapy (TRT) on these patients is still unknown. This study aimed to evaluate the efficacy and safety of TRT for ES-SCLC patients who responded to first-line ICIs and chemotherapy (CHT).
Methods
Patients who received 4 to 6 cycles of ICIs and CHT as first-line therapy at three hospitals between 2018 and 2022 were included in the analysis. All patients were divided into two groups based on whether they received TRT as first-line treatment, and propensity score matching (PSM) was performed to ensure that the characteristics of two groups were well-balanced. The primary endpoints were overall survival (OS) and progression-free survival (PFS), and the secondary endpoint was toxic effects.
Results
A total of 276 patients were included, and the median follow-up time was 22.3 (range, 4.0-53.73) months. After PSM, 197 patients were further analysed, and 99 of whom received TRT. The baseline characteristics were well-balanced between patients in the TRT and non-TRT groups. There were significant differences in PFS between the TRT and non-TRT groups, with the median PFS of 10.76 and 7.63 months, respectively (P = 0.014). Significantly improved OS was observed in the TRT group (21.67 vs. 16.6 months, P = 0.009). In addition, the use of TRT was an independent prognostic factor for PFS and OS of ES-SCLC patients receiving ICIs plus CHT. In terms of safety, no significant increase of any grades adverse event (AE) (P = 0.874) and G3-4 AE (P = 0.909) was observed for patients receiving TRT. Radiation esophagitis, gastrointestinal and hematologic toxicities were the most common AEs in TRT group, which were tolerable. And high-dose radiotherapy was associated with higher incidence of pneumonitis.
Conclusion
Addition of TRT showed significant survival benefits and well tolerability in ES-SCLC patients receiving platinum-etoposide CHT and ICIs, which could be a feasible first-line treatment strategy for ES-SCLC patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13014-024-02420-x.
Keywords: Extensive-stage small-cell lung cancer, Immune checkpoint inhibitors, Thoracic radiotherapy, Immunotherapy, Chemotherapy, Real-world data
Introduction
Small-cell lung cancer (SCLC) accounts for 13–17% of lung cancer and is characterized by rapid proliferation, aggressive growth, and early widespread metastasis [1, 2]. Approximately two-thirds of patients with SCLC are classified as extensive-stage SCLC (ES-SCLC) at initial diagnosis. Four to six cycles of platinum-based chemotherapy (CHT) are the cornerstone of the treatment for ES-SCLC. However, the survival of ES-SCLC patients is poor, with the median progression-free survival (PFS) of less than 5 months and overall survival (OS) of less than 9 months [3]. Several studies investigating other approaches for ES-SCLC treatment, such as radiotherapy, targeted drugs, and immunotherapy, have been performed [4–14].
The notable improvement in survival from atezolizumab was showed based on the IMpower133 trial [4], with the median PFS of 5.2 months and OS of 12.3 months. Survival benefits from immune checkpoint inhibitors (ICIs) were also observed in the CASPIAN, CAPSTONE-1 and ASTRUM-005 trials [5–7]. Thus, the combination of ICIs and CHT has been recommended as the standard first-line treatment strategy for ES-SCLC.
Radiotherapy (RT) plays an important role in all stages of disease presentation, especially in intrathoracic tumour control after first-line treatment. Previous studies have shown that the combination of thoracic radiotherapy (TRT) with CHT could decrease local recurrence-free survival rates and prolong OS in ES-SCLC patients compared to CHT alone [15–17]. The CREST trial showed 10% improvement in 2-year survival rate in patients submitted to TRT after responding to first-line CHT [18]. Moreover, several retrospective studies have indicated that TRT combined with CHT is related to long-term survival [14, 17, 19–21]. Thus, TRT is recommended for patients with ES-SCLC in NCCN and ASTRO guidelines [22, 23].
In the era of immunotherapy, RT has been proven to remodulate the immune microenvironment and to have synergistic effects with ICIs [24, 25]. Adding radiotherapy to pembrolizumab immunotherapy has been found to significantly increase responses and outcomes in patients with metastatic non-small cell lung cancer (NSCLC) [26]. A real-world study has demonstrated favorable survival and good tolerability of the combination of PD-1/PD‐L1 inhibitors plus palliative radiotherapy in metastatic NSCLC patients [27]. However, the effect of applying TRT to ES-SCLC patients who receiving ICIs is unclear. Whether the combined TRT with ICIs and CHT can further improve the treatment efficacy without significantly increasing toxicity is worth further investigation.
This multicentre retrospective analysis is carried out with the intention of evaluating the efficacy and safety of TRT for ES-SCLC patients who responded to first-line ICIs and CHT.
Materials and methods
Patients
This is a retrospective cohort study. Patients who were histologically confirmed ES-SCLC at three hospitals from July 2018 to October 2022 were included in this study. All participants meeting the following criteria were eligible for this study: (1) pathologically confirmed SCLC; (2) radiological confirmed extensive-stage SCLC according to the AJCC TNM staging system(stage IV [T any, N any, M1a or M1b]) [28], or Veterans Administration Lung Study Group(VALG) staging system (T3-4 due to multiple lung nodules that are too extensive or tumour/nodal volume that is too large to be encompassed in a tolerable radiation plan) [29]; (3) receiving at least 4 cycles of immunotherapy plus CHT as the first-line treatment; (4) without progression after 4 treatment cycles; and (5) have accurate clinical follow-up data. The exclusion criteria as follows: (1) patients with limited SCLC disease; (2) patients who progressed after first-line therapy; (3) previously received TRT. All enrolled patients were divided into two groups according to whether they received TRT during the first-line treatment.
Treatment strategy
All patients received CHT combined with ICIs. The CHT regimens consisted primarily of platinum and etoposide, while the ICIs included PD-1/L1 inhibitors. Patients who received TRT after 4 cycles of CHT and ICI treatment were assigned to the TRT group. The radiotherapy treatment plan was either 3-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). The gross tumour volume (GTV) included the residual primary tumour after CHT-ICIs treatment and the positive lymph nodes before CHT-ICIs treatment. The clinical target volume (CTV) was defined as the gross tumour volume (GTV) with a margin of 5 mm and positive lymph node drainage areas. The planning target volume (PTV) was expanded from the CTV with a margin of 5 to 8 mm. Given the different dose fractionations regimens of radiotherapy, we used the biological effective dose (BED) formula: BED = nd×[1 + d/(α/β)] [30–32], where n is the fraction of radiotherapy, d represents the dose per fraction, and α/β is the ratio of radiosensitivity coefficients.
Data records and assessment
Clinical characteristics for analysis included patients age, gender, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), histological type, tumour stage, baseline metastasis sites, immunotherapy and chemotherapy regimens, thoracic radiotherapy data, and survival data. Clinical assessments were performed by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [33]. Response assessment was performed every other cycle during immunotherapy, and every 6–8 weeks post-treatment until disease progression. PFS was defined as the time from initial treatment to disease progression or death or last follow-up, and OS defined as the time from initial treatment to death of any cause or last follow-up. The best overall response was defined as the best response during the first-line treatment setting. AEs were assessed and graded by the senior doctors according to the Common Terminology Criteria for Adverse Events version 5.0 [34]. Receiver operating characteristic (ROC) curve was performed to evaluate the predictive effect of radiotherapy dose (BED) and volumes on the AEs. And Square test or Fisher’s exact test was performed to explore the associations between the radiation dose and volume and AEs.
Statistical analysis
Propensity score matching (PSM) (1:1) was performed to ensure that the patients characteristics between the TRT and non-TRT groups were well-balanced. Square test or Fisher’s exact test was performed to compare the baseline characteristics between different groups. Kaplan-Meier method and log-rank tests were used for survival analysis. The hazard ratios (HR) and 95% confidence interval (95% CI) for OS and PFS were estimated by stratified Cox regression model. All statistical analyses were two-tailed tests and P value < 0.05 was considered statistically significant. The statistical analysis was carried out with IBM SPSS 26.0.
Results
Patient characteristics
From July 2018 to October 2022, a total of 276 patients from three hospitals were enrolled in analysis. The characteristics of the study population are summarized in Table 1. The median age was 62 (range, 41-80) years old. Most patients were men (82%), and 159 (58%) patients had a history of smoking. A total of 187 patients (68%) received PD-L1 inhibitors (atezolizumab and durvalumab), while the other 89 patients (32%) received PD-1 inhibitor s (serplulimab, tislelizumab, etc.). 117 (42%) of them received TRT in the first-line treatment, whereas 159 (58%) did not. After PSM, 197 patients in total were further analyzed, and 99 of whom received TRT. Before receiving TRT, 67.7% of patients had a partial response (PR) or complete response (CR) in the TRT group. The ORR was 70.4% in the non-TRT group after chemotherapy and ICIs. In addition, 32.3% and 29.6% patients showed stable disease (SD) in the TRT and non-TRT groups, respectively. Baseline characteristics were well balanced between patients receiving TRT or not.
Table 1.
Baseline characteristics of ES-SCLC patients before and after PSM
| Characteristic | Before matching | Characteristic | After matching | ||||
|---|---|---|---|---|---|---|---|
| TRT Group (n = 117) |
non-TRT Group (n = 159) |
P value | TRT Group (n = 99) |
non-TRT Group (n = 98) |
P value | ||
| Age, years | 0.363 | Age, years | 0.939 | ||||
| < 60 | 55(47.0%) | 66(41.5%) | < 60 | 46(46.5%) | 45(45.9%) | ||
| ≥60 | 62(53.0%) | 93(58.5%) | ≥60 | 53(53.5%) | 53(54.1%) | ||
| Gender | 0.135 | Gender | 0.492 | ||||
| Male | 92(78.6%) | 136(85.5%) | Male | 78(78.8%) | 81(82.7%) | ||
| Female | 25(21.4%) | 23(14.5%) | Female | 21(21.2%) | 17(17.3%) | ||
| Smoking history | 0.486 | Smoking history | 0.821 | ||||
| Yes | 65(55.6%) | 95(59.7%) | Yes | 56(56.6%) | 57(58.2%) | ||
| No | 52(44.4%) | 64(40.3%) | No | 43(43.4%) | 41(41.8%) | ||
| ECOG PS | 0.08 | ECOG PS | 0.418 | ||||
| 0–1 | 92(78.6%) | 110(69.2%) | 0–1 | 74(74.7%) | 78(79.6%) | ||
| 2 | 25(21.4%) | 49(30.8%) | 2 | 25(25.3%) | 20(20.4%) | ||
| T stage | 0.909 | T stage | 0.832 | ||||
| T1-T2 | 61(52.1%) | 84(52.8%) | T1-T2 | 48(48.5%) | 49(50.0%) | ||
| T3-T4 | 56(47.9%) | 75(47.2%) | T3-T4 | 51(51.5%) | 49(50.0%) | ||
| N stage | 0.387 | N stage | 0.434 | ||||
| N0-N2 | 61(52.1%) | 73(45.9%) | N0-N2 | 53(53.5%) | 47(48.0%) | ||
| N3 | 56(47.9%) | 86(54.1%) | N3 | 46(46.5%) | 51(52.0%) | ||
| M stage | 0.002 | M stage | 0.665 | ||||
| M1a | 24 (20.5%) | 21 (13.2%) | M1a | 19 (19.2%) | 17 (17.4%) | ||
| M1b | 72 (61.5%) | 80 (50.3%) | M1b | 60 (60.6%) | 56 (57.1%) | ||
| M1c | 21 (18.0%) | 58 (36.5%) | M1c | 20 (20.2%) | 25 (25.5%) | ||
| Type of ICIs | 0.652 | Type of ICIs | 0.793 | ||||
| PD-1 | 36(30.8%) | 53(33.3%) | PD-1 | 31(31.3%) | 29(29.6%) | ||
| PD-L1 | 81(69.2%) | 106(66.7%) | PD-L1 | 68(68.7%) | 69(70.4%) | ||
| NO. of metastatic sites | 0.004 | NO. of metastatic sites | 0.837 | ||||
| ≤ 2 | 106(90.6%) | 123(77.4%) | ≤ 2 | 88(88.9%) | 88(89.8%) | ||
| > 2 | 11(9.4%) | 36(22.6%) | > 2 | 11(11.1%) | 10(10.2%) | ||
| Brain metastases | 0.295 | Brain metastases | 0.725 | ||||
| Yes | 40(34.2%) | 45(28.3%) | Yes | 33(33.3%) | 35(35.7%) | ||
| No | 77(65.8%) | 114(71.7%) | No | 66(66.7%) | 63(64.3%) | ||
| Liver metastases | < 0.001 | Liver metastases | 0.899 | ||||
| Yes | 24(20.5%) | 66 (41.5%) | Yes | 24(24.2%) | 23(23.5%) | ||
| No | 93(79.5%) | 93(58.5%) | No | 75(75.8%) | 75(76.5%) | ||
| Bone metastases | 0.039 | Bone metastases | 0.596 | ||||
| Yes | 27(23.1%) | 55(34.6%) | Yes | 24(24.2%) | 27(27.6%) | ||
| No | 90(76.9%) | 104(65.4%) | No | 75(75.8%) | 71(72.4%) | ||
| Response evaluation* | 0.794 | Response evaluation* | 0.758 | ||||
| CR/PR | 81(69.2%) | 107(67.3%) | CR/PR | 67(67.7%) | 69(70.4%) | ||
| SD | 36(30.8%) | 52(32.7%) | SD | 32(32.3%) | 29(29.6%) | ||
PSM, propensity score matching; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICIs, immune checkpoint inhibitors; NO., number; TRT, thoracic radiotherapy
*Response evaluation before TRT or immunotherapy maintenance
The total dose of radiation ranges from 30 to 66 Gy, with a median dose of 50 Gy and median BED of 60 Gy. The median PTV volume was 197.3 cm3. Out of 99 patients who received TRT, 58 received conventional fractionated TRT (44-66 Gy/1.8-2.1 Gy/22-30f, BED = 36.0-79.2 Gy), 29 received hypofractionated TRT (36-60 Gy/2.5-3 Gy/12-24f, BED = 39.0-62.5 Gy), and the rest 12 patients received hyperfractionated TRT (30-60 Gy/1.5 Gy/20-40f, BED = 34.5-69.0 Gy) (Supplementary Table 1). Besides, there were 8.1% patients receiving PCI.
Survival outcomes and treatment response
The median follow-up time was 22.3 (range, 4.0-53.7) months at the time of data cut-off. A total of 132 patients (67%) experienced disease progression, and 119 patients (60.4%) died from any cause. The median PFS and OS were 9.17 and 17.70 months, respectively, in the whole population.
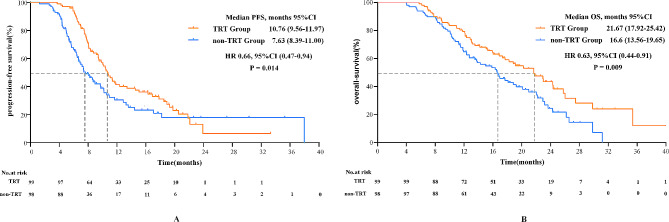
Survival analysis indicated that patients who received TRT in the first-line setting had better PFS than patients who did not receive TRT (median PFS: 10.76 months vs. 7.63 months; P = 0.014) after matching (Fig. 1A). The estimated 1-year PFS rate was 41.9% versus 30.6% in the TRT group and non-TRT group, respectively (Table 2).
Fig. 1.
Kaplan–Meier curves of (A) PFS and (B) OS between ES-SCLC patients in TRT or non-TRT groups. PFS, progression-free survival; OS, overall survival; TRT, thoracic radiotherapy; CI, confidence interval; HR, hazard ratio
Table 2.
Survival estimate of patients in the TRT and non-TRT group
| Survival estimated | TRT Group (n = 99) |
non-TRT Group (n = 98) |
P value |
|---|---|---|---|
| 1-year PFS, % | 41.9 | 30.6 | 0.030 |
| 1-year OS, % | 78.2 | 64.8 | 0.033 |
| 2-year OS, % | 41.5 | 24.5 | 0.019 |
PFS, progression-free survival; OS, overall survival; TRT, thoracic radiotherapy
Prolonged survival was observed in TRT group with the median OS of 21.67 months compared to 16.60 months in non-TRT group (HR, 0.63; 95% CI, 0.44-0.91; P = 0.009) (Fig. 1B). And the 1-year survival rate was 78.2% versus 64.8%, and 2-year survival rate was 41.5% versus 24.5% in the TRT group and non-TRT group, respectively.
In total, 73.7% patients had a partial response (PR) in TRT group, 57.1% in non-TRT group. Additionally, 25.3% vs. 40% patients showed stable disease (SD) in TRT group and non-TRT group, respectively. The objective response rate (ORR) was 74.7% in TRT group, which was considerably higher than that in non-TRT group (59.2%, P = 0.048) (Fig. 2).
Fig. 2.
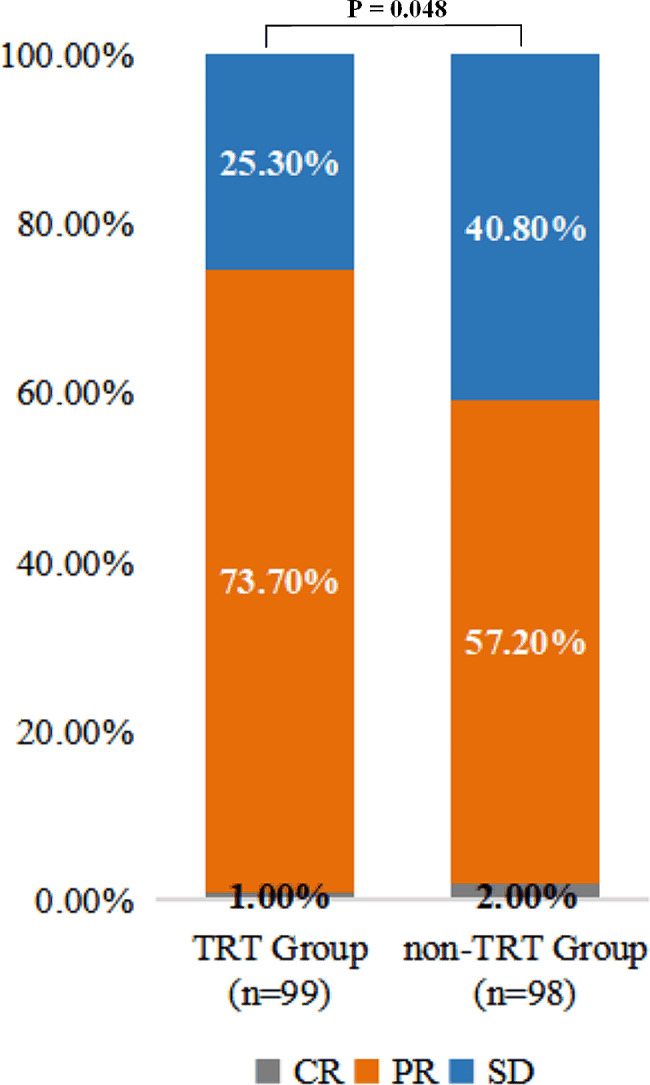
The comparison of best overall response between patients in TRT and non-TRT group. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; TRT, thoracic radiotherapy
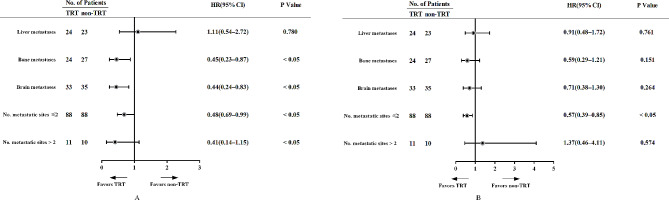
Subgroup analysis of survival outcomes
Subgroup analysis was performed based on the number of metastatic sites and site of metastases, and the results are shown in Fig. 3. The addition of TRT was beneficial for both PFS and OS in ES-SCLC patients with ≤ 2 metastatic sites but not in patients with > 2 metastatic sites. Besides, patients with liver metastasis did not achieve clinical benefit from TRT. The superiority of TRT in PFS but not OS was shown in patients with bone or brain metastases.
Fig. 3.
Subgroup analysis of survival outcomes in TRT and non-TRT group. TRT, thoracic radiotherapy; No., number; HR, hazard ratio; CI, confidence interval
Patients with bone metastases, brain metastases, or distant metastases who received TRT could reduce the risk of disease progression (Fig. 3A). Patients with liver metastases, bone metastases, brain metastases, or metastatic sites ≤ 2 who received TRT had a decreased risk of death (Fig. 3B). However, only those metastatic sites ≤ 2 patients received TRT with significant differences.
Univariate and multivariate cox analyses of PFS and OS
Univariate Cox analysis revealed that male, T3-T4 stage, N3 stage, M1c stage, PD-L1 inhibitors, and not receiving TRT were significantly related to shorter PFS. Multivariate analysis demonstrated that male, T3-T4 stage, M1c stage, PD-L1 inhibitors, and not receiving TRT were independent factors for worse PFS (Table 3). In terms of OS, univariate analysis revealed that age ≥ 60 years, M1b stage, M1c stage, liver metastases, metastases sites > 2, and not receiving TRT were associated with poor OS. Multivariate analysis showed that age ≥ 60, M1b stage, M1c stage, liver metastases, and not receiving TRT were independent poor prognostic factors of poor OS (Table 4).
Table 3.
Univariate and multivariate cox analyses of PFS for ES-SCLC patients receiving ICIs
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | ||||
| Male | ||||
| Female | 0.608 (0.390–0.946) | 0.027* | 0.586 (0.374–0.917) | 0.019* |
| Age | ||||
| ≥ 60 | ||||
| < 60 | 0.999 (0.707–1.410) | 0.994 | ||
| Smoking History | ||||
| Yes | ||||
| No | 0.785 (0.552–1.116) | 0.177 | ||
| ECOG PS | ||||
| 2 | ||||
| ≥ 2 | 1.266 (0.845–1.898) | 0.253 | ||
| T stage | ||||
| ≤ 2 | ||||
| > 2 | 1.603 (1.132–2.272) | 0.008* | 1.778 (1.240–2.550) | 0.002* |
| N stage | ||||
| ≤ 2 | ||||
| > 2 | 1.596 (1.121–2.270) | 0.009* | 1.436 (0.998–2.066) | 0.051 |
| M stage | ||||
| M1a | ||||
| M1b | 1.096 (0.681–1.764) | 0.705 | ||
| M1c | 1.866 (1.706–3.293) | 0.026* | 1.956 (1.117–3.424) | 0.019* |
| Type of ICIs | ||||
| PD-1 | ||||
| PD-L1 | 1.718 (1.156–2.554) | 0.007* | 1.684 (1.120–2.533) | 0.012* |
| Receiving TRT | ||||
| No | ||||
| Yes | 0.651 (0.460–0.920) | 0.015* | 0.599(0.420–0.854) | 0.005* |
| NO. of metastatic sites | ||||
| < 3 | ||||
| ≥ 3 | 1.605 (0.917–2.808) | 0.098 | ||
| Liver metastases | ||||
| No | ||||
| Yes | 1.192 (0.798–1.780) | 0.391 | ||
| Bone metastases | ||||
| No | ||||
| Yes | 1.409 (0.964–2.060) | 0.077 | ||
| Brain metastases | ||||
| No | ||||
| Yes | 1.039 (0.719–1.502) | 0.838 | ||
PFS, progression-free survival; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICIs, immune checkpoint inhibitors; TRT, thoracic radiotherapy; NO., number; HR, hazard ratio; CI, confidence interval; *P < 0.05
Table 4.
Univariate and multivariate cox analyses of OS for ES-SCLC patients receiving ICIs
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | ||||
| Male | ||||
| Female | 0.802 (0.507–1.266) | 0.343 | ||
| Age | ||||
| ≥ 60 | ||||
| < 60 | 0.661 (0.457–0.956) | 0.028* | 0.593 (0.408–0.862) | 0.006* |
| Smoking History | ||||
| Yes | ||||
| No | 0.835 (0.580–1.202) | 0.332 | ||
| ECOG PS | ||||
| < 2 | ||||
| ≥ 2 | 1.208 (0.791–1.845) | 0.382 | ||
| T stage | ||||
| ≤ 2 | ||||
| > 2 | 1.335 (0.929–1.920) | 0.118 | ||
| N stage | ||||
| ≤ 2 | ||||
| > 2 | 1.249 (0.869–1.794) | 0.230 | ||
| M stage | ||||
| M1a | ||||
| M1b | 1.994 (1.153–3.449) | 0.014 | 2.051 (1.163–3.620) | 0.013* |
| M1c | 3.516 (1.878–6.583) | < 0.001* | 2.818 (1.437–5.527) | 0.003* |
| Type of ICIs | ||||
| PD-1 | ||||
| PD-L1 | 0.996 (0.676–1.466) | 0.983 | ||
| Receiving TRT | ||||
| No | ||||
| Yes | 0.618 (0.428–0.892) | 0.010* | 0.670 (0.464–0.966) | 0.032* |
| NO. of metastatic sites | ||||
| ≤ 2 | ||||
| > 2 | 1.883 (1.070–3.314) | 0.028* | 1.836 (0.939–3.592) | 0.076 |
| Liver metastases | ||||
| No | ||||
| Yes | 2.007 (1.364–2.953) | < 0.001 | 1.722 (1.160–2.558) | 0.007* |
| Bone metastases | ||||
| No | ||||
| Yes | 1.094 (0.726–1.648) | 0.669 | ||
| Brain metastases | ||||
| No | ||||
| Yes | 1.247 (0.849–1.829) | 0.260 | ||
OS, overall survival; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICIs, immune checkpoint inhibitors; TRT, thoracic radiotherapy; NO., number; HR, hazard ratio; CI, confidence interval; *P < 0.05
Safety
The toxicity profiles of the combination of TRT and CHT plus immunotherapy are shown in Tables 5 and 6. Most of adverse events (AEs) were tolerable and self-limiting, which were easily handled and managed. Leucopenia/white-cell count decreased was the most common G1-2 AEs (44.4%). Radiation esophagitis was the second common G1-2 AEs (38.4%) in patients receiving TRT, and the third G1-2 AEs was nausea (27.3%). Neutropenia (8.1%) and pneumonitis (6.1%) were the most common and second common G3-4 AEs, respectively. G3-4 radiation esophagitis developed 4% of patients. Only one patient developed grade 4 pneumonitis leading to radiotherapy withdrawal. No grade 5 adverse events occurred.
Table 5.
Comparison of G1-2 adverse events between TRT and non-TRT group
| Adverse Events | TRT Group (n = 99) |
non-TRT Group (n = 98) |
P value |
|---|---|---|---|
| Any Grades | 82 (82.8%) | 82 (83.7%) | 0.874 |
| G1-2 Totally | 78 (78.8%) | 78 (79.6%) | 0.889 |
|
Leucopenia /White-cell count decreased |
44 (44.4%) | 34 (34.7%) | 0.190 |
|
Neutropenia /Neutrophil count decreased |
23 (23.2%) | 21 (21.4%) | 0.864 |
|
Thrombocytopenia /Platelet count decreased |
7 (7.1%) | 2 (2.0%) | 0.170 |
| Anaemia | 5 (5.1%) | 13 (13.3%) | 0.051 |
| Nausea | 27 (27.3%) | 25 (25.5%) | 0.872 |
| Decreased appetite | 17 (17.2%) | 28 (28.6%) | 0.063 |
| Constipation | 9 (9.1%) | 14 (14.3%) | 0.276 |
| Diarrhea | 6 (6.1%) | 7 (7.1%) | 0.783 |
| Radiation esophagitis | 38 (38.4%) | / | / |
| Pneumonitis | 16 (16.2%) | 9 (9.2%) | 0.199 |
| Myocarditis | 0 (0%) | 0 (0%) | / |
| Hypothyroid | 1 (1.0%) | 1 (1.0%) | 1.000 |
| Atrial fibrillation | 0 (0%) | 0 (0%) | / |
G1-2: grade 1 to 2
Table 6.
Comparison of G3-4 adverse events between TRT and non-TRT group
| Adverse Events | TRT Group (n = 99) |
non-TRT Group (n = 98) |
P value |
|---|---|---|---|
| Any Grades | 81 (81.8%) | 82 (83.7%) | 0.874 |
| G3-4 Totally | 28 (28.3%) | 27 (27.6%) | 0.909 |
|
Leucopenia /White-cell count decreased |
4 (4.0%) | 2 (2.0%) | 0.683 |
|
Neutropenia /Neutrophil count decreased |
8 (8.1%) | 12(12.2%) | 0.356 |
|
Thrombocytopenia /Platelet count decreased |
1 (1.0%) | 2 (2.0%) | 0.621 |
| Anaemia | 1 (1.0%) | 2 (2.0%) | 0.621 |
| Nausea | 4 (4.0%) | 7 (7.1%) | 0.373 |
| Decreased appetite | 1 (1.0%) | 3 (3.1%) | 0.369 |
| Constipation | 0 (0%) | 0 (0%) | / |
| Diarrhea | 0 (0%) | 2 (2.0%) | 0.246 |
| Radiation esophagitis | 4 (4.0%) | / | / |
| Pneumonitis | 6 (6.1%) | 3 (3.1%) | 0.498 |
| Myocarditis | 3 (2.0%) | 0 (0%) | 0.497 |
| Hypothyroid | 1 (1.0%) | 0 (0%) | 1.000 |
| Atrial fibrillation | 0 (0%) | 1 (1.0%) | 0.497 |
G3-4: grade 3 to 4
Compared with the non-TRT group, there was no significantly increased any grades AEs (P = 0. 874) and G3-4 AEs (P = 0.909) for patients receiving TRT. The incidence of G1-2 pneumonitis was proportionally higher in the TRT group (16.2%) compared to non-TRT group (9.2%), but the difference was not statistically significant (P = 0.199). And G3-4 pneumonitis was 6.1% versus 3.1% in two groups, respectively (P = 0.498).
Receiver operating characteristic (ROC) curves were plotted to determine the relationship between BED, PTV volume and incidence of AE in the TRT group, with a cut-off of 62.45 Gy and 209 cm3. Patients were classified into low-dose (BED ≤ 62.45 Gy) and high-dose (BED > 62.45 Gy), based on a predetermined cut-off value by ROC. Significantly higher pneumonitis was observed for patients high-dose group compared to low-dose group (34.2% vs. 14.8%, P = 0.028). Radiation esophagitis, and hematologic toxicities were more common in the high-dose group but no statistical significance was observed. (Supplementary Table 2). There were no significant differences on high grade toxicities between patients in high-dose and low-dose group (Supplementary Table 2). Besides, higher PTV volume was associated with increased incidence of radiation esophagitis and hematologic toxicities, but not pneumonitis and gastrointestinal toxicities (Supplementary Table 3). And, the volume of PTV was not associated with the incidence of grade 3-4 AEs.
Discussion
Nowadays, ICIs combined with CHT have been recommended as the standard first-line treatment option for ES-SCLC patients [35]. Although ES-SCLC patients always experience a high objective response to first-line systemic therapy, most patients progress or die rapidly from recurrence, metastasis, and drug-resistance [5–7, 36]. Thus, we intended to seek treatment modalities to improve efficacy and prolong survival of ES-SCLC patients. The results of the present study indicated prolonged survival and acceptable AEs with the addition of TRT in the first-line treatment of patients with ES-SCLC receiving ICIs plus CHT.
CHT could stimulate tumour antigen expression, priming the tumour for response to ICIs. In addition, pre-clinical evidence has demonstrated that synergistic immune stimulation against cancer cells from incorporating RT and immunotherapy [37]. RT can increase antigen presentation, promote T cell infiltration, and favorably modulate the tumour microenvironment [24, 38, 39], which would amplify immune response and improve efficacy when combined with ICIs [40]. Given the evidence of preclinical data and the benefit of RT in local control of lung cancer, the combination treatment of RT and ICIs is recommended in clinical practice [23]. Previous reports have suggested the clinical benefit from combination treatment in local advanced and metastatic NSCLC patients [26, 41–44]. However, the efficacy and safety of this treatment strategy are largely unknown in ES-SCLC. Diamond et al. performed a single-arm retrospective study enrolling 20 patients and found favorable safety profile and improved OS (median OS) with the use of TRT and ICIs plus CHT in the management of ES-SCLC [45]. Another single-arm retrospective analysis included 36 patients also suggested that combined with TRT in the first-line treatment was safe and had ample survival benefits [46].
To our knowledge, two ongoing randomized trials (RAPTOR trial and TREASURE study) evaluating the safety and efficacy of the addition of TRT for ES-SCLC patients receiving first-line ICIs plus chemotherapy, and no results have been reported yet. Our investigation is the first multicentre, head-to-head study to compare the efficacy and safety of ES-SCLC patients treated with TRT and ICIs-CHT in the first-line setting. The results of this study showed the 5-and 11-month improvement of PFS and OS from the addition of TRT respectively, which indicated efficacy from the addition of TRT. And the subgroup analysis indicated the obvious benefit from the TRT in oligometastatic ES-SCLC patients. However, the addition of TRT was not recommended for patients with liver metastasis.
In terms of safety, no significantly increase in AEs was observed for patients receiving TRT in this study. A single-arm phase II trial of 21 patients cross China showed that the combination of low-dose radiotherapy was tolerable in patients with ES-SCLC (NCT04622228) [47]. Additionally, a phase I trial (NCT02402920) conducted at MD Anderson, which included 38 patients, demonstrated that TRT-pembrolizumab was well tolerated, with low grade 3 and greater toxic events [48].
Esophagitis, gastrointestinal and hematologic toxicities were the common AEs associated with the addition of TRT for ES-SCLC patients receiving ICI and CHT. In the CREST trial, the frequency of G3-4 esophagitis was 1.6% [18], which is lower than our study (4%) and a real-world retrospective study (8.3%) [14]. The possible reason for this result might to be related to the differences in the dose of TRT, with a segment of patients in our research received a dose of 50-60 Gy compared to 30 Gy in the CREST trial. Another real-world retrospective investigation included ES-SCLC patients receiving CHT and TRT with a dose of 40-60 Gy, and the results indicated that 8.3% of the patients developed G3-4 esophagitis [14].
Pneumonitis as an adverse event with high mortality requires great attention. The PACIFIC study indicated that no significantly increased risk of pneumonitis observed with the combination of TRT and ICIs plus CHT (33.9% vs. 24.8%) for NSCLC patients [41]. Notably, the real-world studies have indicated that the incidence of pneumonitis is 7 -19% for NSCLC patients receiving ICIs and CHT [49–55], which is higher than that reported in clinical trials. The higher incidence of pneumonitis in real-world studies was also observed for NSCLC patients receiving TRT and ICIs plus CHT [50, 56]. The heterogeneity of general conditions and ethnic groups in real-world settings versus clinical trials may contribute to the different incidences of pneumonitis [57–59]. In our study, the incidence of pneumonitis in ICIs plus CHT group is 12.2%, which was higher than the results of the clinical trial (4-8.2%) [5–7]. Moreover, there was no statistically significant increase in the incidence of pneumonitis with the addition of TRT (22.2% vs. 12.2%, respectively, P = 0.089) in the present study. It should be noted that the radiation dose was significantly associated with the incidence of pneumonitis. In summary, these data suggest a favorable safety profile of adding TRT to ICIs plus CHT in first-line treatment of ES-SCLC patients. And high-dose TRT was associated with the higher incidence of pneumonitis.
In this study, there were several limitations. First, as a retrospective cohort analysis, there was potential selection bias. Though we performed a multicentre analysis to minimize the impact of possible confounding factors, the study findings need to be interpreted cautiously. Second, due to the retrospective nature of the study, some heterogeneities emerged in the study population, such as different cycles of CHT plus ICIs, TRT techniques and doses et. As a result of these flaws, further large phase III prospective studies are needed to validate the results of our findings.
Conclusion
In conclusion, the addition of TRT showed significant survival benefits and well tolerability in ES-SCLC patients receiving platinum-etoposide chemotherapy and ICIs. The results suggest that TRT plus CHT and ICIs could be a feasible first-line treatment strategy for ES-SCLC patients but should be investigated in further studies, such as the ongoing RAPTOR trial and TREASURE study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the authors of the primary studies.
Abbreviations
- ICIs
immune checkpoint inhibitors
- ES-SCLC
extensive-stage small-cell lung cancer
- TRT
thoracic radiotherapy
- CHT
chemotherapy
- OS
overall survival
- PFS
progression-free survival
- AE
adverse event
- 3D-CRT
3-dimensional conformal radiotherapy
- IMRT
intensity-modulated radiotherapy
- BED
biological effective dose
- PTV
planning target volume
- GTV
gross tumour volume
- CTV
clinical target volume
- ROC
receiver operating characteristic
Author contributions
YY: Data Curation, Formal analysis, Methodology, Writing– Original Draft. BL: Data Curation, Formal analysis, Methodology, Writing– Review & Editing. RS, LY, and BZ: Data Curation, Investigation, Methodology. LW: Methodology, Investigation, Project Administration, Supervision, Writing– Review & Editing.All authors read and approved the final version of the manuscript.
Funding
This research was supported by National Natural Science Foundation of China (Grant number 82172865 and 82203198), Natural Science Foundation of Shandong Province (Grant number ZR2022QH023), Start-up fund of Shandong Cancer Hospital (Grant number 2020-B14), Clinical Research Special Fund of Wu Jieping Medical Foundation (Grant number 320.6750.2021-02-51 and 320.6750.2021-17-13).
Data availability
The data that support the findings of this study are available from the corresponding author, [LL.W], upon reasonable request.
Declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute (NO. SDTHEC2021003186) and individual consent for this retrospective analysis was waived.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yueyuan Yao and Butuo Li authors are contributed equally.
References
- 1.Oronsky B, Reid TR, Oronsky A, Carter CA. What’s New in SCLC? A review. Neoplasia. 2017;19(10):842–7. doi: 10.1016/j.neo.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7(1):1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans WK, Shepherd FA, Feld R, Osoba D, Dang P, Deboer G. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol. 1985;3(11):1471–7. doi: 10.1200/JCO.1985.3.11.1471. [DOI] [PubMed] [Google Scholar]
- 4.Mansfield AS, Kazarnowicz A, Karaseva N, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol. 2020;31(2):310–7. doi: 10.1016/j.annonc.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–47. doi: 10.1016/S1470-2045(22)00224-8. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Han L, Wu L, et al. Effect of First-Line Serplulimab vs Placebo added to Chemotherapy on Survival in patients with extensive-stage small cell Lung Cancer: the ASTRUM-005 Randomized Clinical Trial. JAMA. 2022;328(12):1223–32. doi: 10.1001/jama.2022.16464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line therapy for extensive-stage small-cell Lung Cancer: Randomized, Double-Blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–79. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leal T, Wang Y, Dowlati A, et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. J Clin Oncol. 2020;38(15suppl):9000–0. doi: 10.1200/JCO.2020.38.15_suppl.9000. [DOI] [Google Scholar]
- 10.Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in extensive-stage small-cell Lung Cancer. J Clin Oncol. 2016;34(31):3740–8. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 11.Owonikoko TK, Dahlberg SE, Sica GL, et al. Randomized Phase II Trial of Cisplatin and Etoposide in Combination with Veliparib or Placebo for extensive-stage small-cell Lung Cancer: ECOG-ACRIN 2511 study. J Clin Oncol. 2019;37(3):222–9. doi: 10.1200/JCO.18.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng P, Yang H, Chen C, et al. The efficacy and safety profile of anlotinib with etoposide plus cisplatin/carboplatin in treatment-naive extensive-stage small cell lung cancer(SCLC) patients: results from a phase II single-arm trial. J Clin Oncol. 2020;38(15suppl):9066–6. doi: 10.1200/JCO.2020.38.15_suppl.9066. [DOI] [Google Scholar]
- 13.Tiseo M, Boni L, Ambrosio F, Italian M, Phase III, et al. Randomized study of Cisplatin Plus Etoposide with or without Bevacizumab as First-Line treatment in extensive-disease small-cell Lung Cancer: the GOIRC-AIFA FARM6PMFJM Trial. J Clin Oncol. 2017;35(12):1281–7. doi: 10.1200/JCO.2016.69.4844. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Zhou Z, Wang Y, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer. 2011;117(23):5423–31. doi: 10.1002/cncr.26206. [DOI] [PubMed] [Google Scholar]
- 15.Jeremic B, Casas F, Wang L, Perin B. Radiochemotherapy in extensive disease small cell lung cancer ED-SCLC. Front Radiat Ther Oncol. 2010;42:180–6. doi: 10.1159/000262474. [DOI] [PubMed] [Google Scholar]
- 16.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol. 1999;17(7):2092–9. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 17.Yee D, Butts C, Reiman A, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol. 2012;102(2):234–8. doi: 10.1016/j.radonc.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 19.Giuliani ME, Atallah S, Sun A, et al. Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy. Clin Lung Cancer. 2011;12(6):375–9. doi: 10.1016/j.cllc.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Wang T, Wang J, Qu B, Wang H, Hu Y. Effect of radiotherapy on the treatment of patients with extensive stage small cell lung cancer. Genet Mol Res. 2014;13(4):8577–85. doi: 10.4238/2014.January.24.7. [DOI] [PubMed] [Google Scholar]
- 21.Tian S, Zhang X, Jiang R, et al. Survival outcomes with thoracic radiotherapy in extensive-stage small-cell lung Cancer: a propensity score-matched analysis of the National Cancer Database. Clin Lung Cancer. 2019;20(6):484–493e486. doi: 10.1016/j.cllc.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger DS, Cox JD, Ginsberg RJ, et al. NCCN Non-small-cell Lung Cancer Practice guidelines. The National Comprehensive Cancer Network. Oncol (Williston Park) 1996;10(11 Suppl):81–111. [PubMed] [Google Scholar]
- 23.Simone CB, II, Bogart JA, Cabrera AR, et al. Radiation Therapy for Small Cell Lung Cancer: an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2020;10(3):158–73. doi: 10.1016/j.prro.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in Immunotherapy. Trends Cancer. 2016;2(6):286–94. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon H, Ramapriyan R, Cushman TR, et al. Role of Radiation Therapy in Modulation of the Tumor Stroma and Microenvironment. Front Immunol. 2019;10:193. doi: 10.3389/fimmu.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theelen W, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467–75. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 27.Zhou ZC, Chen KY, Li N, et al. Real-world utilization of PD-1/PD-L1 inhibitors with palliative radiotherapy in patients with metastatic non-small cell lung cancer. Thorac Cancer. 2022;13(16):2291–300. doi: 10.1111/1759-7714.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin MB, Edge SB, Greene FL et al. AJCC cancer staging manual Vol 1024: Springer; 2017.
- 29.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the study of Lung Cancer—what limits limited disease? Lung Cancer. 2002;37(3):271–6. doi: 10.1016/S0169-5002(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 30.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–94. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 31.Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DA. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol) 2001;13(2):71–81. doi: 10.1053/clon.2001.9221. [DOI] [PubMed] [Google Scholar]
- 32.Guerrero M, Li XA, Earl MA, Sarfaraz M, Kiggundu E. Simultaneous integrated boost for breast cancer using IMRT: a radiobiological and treatment planning study. Int J Radiat Oncol Biol Phys. 2004;59(5):1513–22. doi: 10.1016/j.ijrobp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Shah S. Common terminology criteria for adverse events. 2022.
- 35.Ganti AKP, Loo BW, Bassetti M, et al. Small cell Lung Cancer, Version 2.2022, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(12):1441–64. doi: 10.6004/jnccn.2021.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell Lung Cancer. N Engl J Med. 2018;379(23):2220–9. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 37.Eckert F, Schilbach K, Klumpp L, et al. Potential role of CXCR4 targeting in the context of Radiotherapy and Immunotherapy of Cancer. Front Immunol. 2018;9:3018. doi: 10.3389/fimmu.2018.03018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–5. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demaria S, Golden EB, Formenti SC. Role of local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325–32. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 40.Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy-immunotherapy combinations - perspectives and challenges. Mol Oncol. 2020;14(7):1529–37. doi: 10.1002/1878-0261.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-small-cell Lung Cancer. N Engl J Med. 2017;377(20):1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 42.Lin SH, Lin Y, Yao L, et al. Phase II trial of Concurrent Atezolizumab with Chemoradiation for Unresectable NSCLC. J Thorac Oncol. 2020;15(2):248–57. doi: 10.1016/j.jtho.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC Trial: Durvalumab after Chemoradiotherapy in Stage III Non-small-cell Lung Cancer. J Clin Oncol. 2022;40(12):1301–11. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh J, Menon H, Chen D et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer 2020;8(2). [DOI] [PMC free article] [PubMed]
- 45.Diamond BH, Verma N, Shukla UC, Park HS, Koffer PP. Consolidative thoracic Radiation Therapy after First-Line chemotherapy and immunotherapy in extensive-stage small cell Lung Cancer: a multi-institutional Case Series. Adv Radiat Oncol. 2022;7(2):100883. doi: 10.1016/j.adro.2021.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Yang D, Min Y, et al. First-line atezolizumab/durvalumab plus platinum-etoposide combined with radiotherapy in extensive-stage small-cell lung cancer. BMC Cancer. 2023;23(1):318. doi: 10.1186/s12885-023-10784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, Sun J, Xie C, et al. Efficacy and safety of low-dose radiotherapy (LDRT) concurrent atezolizumab plus chemotherapy as first-line therapy for ES-SCLC: interim analysis of phase II MATCH trial. J Clin Oncol. 2022;40(16suppl):e20611–1. doi: 10.1200/JCO.2022.40.16_suppl.e20611. [DOI] [Google Scholar]
- 48.Welsh JW, Heymach JV, Chen D, et al. Phase I trial of Pembrolizumab and Radiation Therapy after induction chemotherapy for extensive-stage small cell Lung Cancer. J Thorac Oncol. 2020;15(2):266–73. doi: 10.1016/j.jtho.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with Anti-programmed Death-1/Programmed death Ligand 1 therapy. J Clin Oncol. 2017;35(7):709–17. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-small Cell Lung Cancer patients receiving Immune Checkpoint Immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–9. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 51.Galant-Swafford J, Troesch A, Tran L, Weaver A, Doherty TA, Patel SP. Landscape of Immune-related pneumonitis in Cancer patients with Asthma being treated with Immune Checkpoint Blockade. Oncology. 2020;98(2):123–30. doi: 10.1159/000503566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–6. doi: 10.1016/j.lungcan.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Suresh K, Psoter KJ, Voong KR, et al. Impact of checkpoint inhibitor pneumonitis on Survival in NSCLC patients receiving Immune Checkpoint Immunotherapy. J Thorac Oncol. 2019;14(3):494–502. doi: 10.1016/j.jtho.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 54.Cui P, Huang D, Wu Z, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol. 2020;12:1758835920922033. doi: 10.1177/1758835920922033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukihara J, Sakamoto K, Koyama J, et al. Prognostic impact and risk factors of Immune-related pneumonitis in patients with non-small-cell Lung Cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20(6):442–450e444. doi: 10.1016/j.cllc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Fujimoto D, Yoshioka H, Kataoka Y, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung Cancer. 2018;119:14–20. doi: 10.1016/j.lungcan.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 57.Kato T, Masuda N, Nakanishi Y, et al. Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer. 2017;104:111–8. doi: 10.1016/j.lungcan.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Suh CH, Park HS, Kim KW, Pyo J, Hatabu H, Nishino M. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients: Meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer. 2018;123:60–9. doi: 10.1016/j.lungcan.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Zhang T, Huang Y, et al. Real-world safety and efficacy of consolidation Durvalumab after Chemoradiation Therapy for Stage III Non-small Cell Lung Cancer: a systematic review and Meta-analysis. Int J Radiat Oncol Biol Phys. 2022;112(5):1154–64. doi: 10.1016/j.ijrobp.2021.12.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [LL.W], upon reasonable request.