Graphical abstract
Keywords: Duck meat, Sensory evaluation, HS-GC-IMS, ROAV, Characteristic odorous smell compounds
Highlights
-
•
HS-GC-IMS was used to detect volatile flavor compounds in duck meat.
-
•
The odorous smell of reheated duck meat after storage for 7 days was stronger.
-
•
Differences are in volatile flavor compounds content rather than kinds.
-
•
9 volatile compounds were screened as potential characteristic fishy compounds.
Abstract
To clarify the characteristic odor of compounds present in duck meat, especially reheating after storage, the effect of duck breast cooked at three temperatures (90 °C, 100 °C, 105 °C) and reheating after 7 days of storage was studied. Electronic nose analysis and sensory evaluation revealed a significant increase (p < 0.05) in reheated duck meat odor after 7 days of storage. The 90 °C treatment group had the heaviest odor, which increased by 12.19 % after seven days of storage. Using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS), 60 volatile flavor compounds were identified across various groups. Although the volatile compounds were consistent among different groups, their relative contents varied. By combining the sensory evaluation results with the Relative Odor Activity Value (ROAV) of these flavor compounds, chemometric orthogonal partial least squares discriminant analysis (OPLS-DA) was used to identify the following 9 characteristic volatile compounds: 2-methylbutanal, pentanal, octanal, heptanal, hexanal, (E)-2-octenal, (E)-2-nonenal, and 2-pentyl furan
1. Introduction
China is a major producer, consumer, as well as exporter of duck meat (DM). DM is popular with consumers because it contains high content of polyunsaturated fatty acids (PUFAs), vitamins, essential amino acids, minerals, and low levels of cholesterol, which benefit human health (Liu, Pan, Ye, & Cao, 2013). The increased demand for cooked DM can be attributed to the current living standards and fast-paced life. Over the past decade, the number of DM products has increased substantially, particularly based on the demand for pre-made dishes. However, cooked DM produces an odor after storage and reheating, which has a direct impact on the food quality. Therefore, it is essential to explore the compounds responsible for the characteristic odor of compounds present in DM to improve its flavor.
It is known that precooked meat and meat products reheated after brief refrigeration possess an unpleasant off-flavor, referred to as warmed-over flavor (WOF) (Tims & Watts, 1958). WOF was discovered by Tims et al. in cooked DM. It was probably formed via the auto-oxidation of phospholipids present in the meat cell membranes, where the metal ions are known to play a critical catalytic role. The level of fatty oxidation products (linear fatty aldehydes, alcohols, ketones, hydrocarbons, and pentylfuran) has been shown to increase with an increase in the WOF in the volatile flavor components of the samples (Shi et al., 2020). WOF developed primarily due to the increased production of these substances exerts a positive effect on meat flavor at low concentrations and a negative effect at elevated concentrations (O’Sullivan, Byrne, Jensen, Andersen, & Vestergaard, 2003). These substances, including pentanal, hexanal, heptanal, octanal, nonanal, pentanol, 1-octen-3-ol, 2,3-octanedione, 2-pentylfuran and 2-heptanone, can be used to determine the degree of WOF formation (Wu & Sheldon, 2006). Soncin, Chiesa, Cantoni, & Biondi, 2007 analyzed the levels of volatile compounds found in raw goose, raw pork, and raw DM and found that the main product of lipid peroxidation had a maximum contribution to DM flavor. Liu, Xu, Ouyang, & Zhou, 2010 also reported that the primary volatile compounds of DM were degradation products of fatty acids. They were also primarily responsible for the odor associated with DM.
Currently, the research on odorous smell is mainly focused on animal sources, parts, meat byproducts, processing, storage, etc. Some researchers have investigated the odor of raw meat, heat treatment, and reheating of meat after storage. They found that the combination of the volatile small molecules generated during the thermal degradation reaction lipid oxidation, or Maillard reaction during heat treatment as well as after reheating, have made the study of odorous smell more complicated (Jose Beriain, Teresa Murillo-Arbizu, Insausti, Victoria Sarries, & Gomez, 2020). In recent years, Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) has emerged as an advanced technology for the rapid visual detection of volatile flavor molecules. The electronic nose can mimic human nose in recognizing odors, and can qualitatively detect volatile odors. Therefore, the bionic determination of the electronic nose, combined with HS-GC-IMS, can directly analyze the volatile flavor compounds of DM.
Duck breeders have determined that DM produced at 90 °C, 100 °C, and 105 °C develops a foul odor when it reaches the consumer after seven days (Khan et al., 2014; Liu et al., 2007). Here, the HS-GC-IMS technique was used to identify the volatile compounds present in the reheated DM after cooking at three different temperatures (90 °C, 100 °C, 105 °C) and storing for 0 days and 7 days. This study uses sensory evaluation, relative odor activity value (ROAV) and variable importance projection (VIP) methods to screen characteristic odorous compounds. The screening of the characteristic odorous compounds could provide reference standard for the targeted reduction of DM odor and broaden the processing channels of DM.
2. Materials and methods
2.1. Materials and chemicals
The study used Cherry Valley duck breasts that were procured from New Hope Liuhe Co., Ltd. (Guantao, China). The raw duck breasts were transported under cold chain conditions and stored at −40 °C until further use. The study used analytical-grade chemicals that were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The experimental procedures were conducted over approximately 9 days.
2.2. Sample preparation
The excess fat and connective tissue were removed from the duck breasts and were cut into uniform sections (2 × 2 × 2 cm). The samples were separately cooked at 90 °C, 100 °C, and 105 °C for 30 min, using a 1:2 ratio of sample: water. After cooling, the samples were quickly sealed in vacuum bags and stored in a 4 ± 1 °C freezer for 7 days. The treated DM on day 0 was used as the respective control group, and the reheated samples (75 °C for 10 min) were used as the respective experimental groups.
2.3. Sensory evaluation
The postgraduates who were majoring in meat underwent sensory evaluation training. Of these, ten sensory evaluators (male: 5, female: 5, aged 20–26 years old) formed the sensory evaluation group to assess the smell of DM samples. An appropriate amount of chopped DM samples was weighed and placed in 10 mL (n = 10) volumetric cups for rapid sniffing. The samples were scored on a 10-point scale based on their smell (0 < absent ≤ 2, 2 < very weak ≤ 4, 4 < moderate ≤ 6, 6 < strong ≤ 8, 8 < very strong ≤ 10).
2.4. Electronic nose (E-nose) analysis
An electronic nose system (PEN3, Airsense Analytics, Mecklenburg, Germany) containing ten metal oxide gas sensors was used to evaluate the DM. Briefly, 8.0 g of DM was equilibrated in a 50 mL headspace vial for 5 min at 45 °C. The injection flow rate was 300 mL/min, and response data were recorded at 1-s intervals for 75 s. Each sample determination was done four times.
2.5. Lipid oxidation
2.5.1. Analysis of peroxide value (POV)
The minced DM (2.0 g) was mixed with 40 mL chloroform/methanol (CHCl3/CH3OH) solvents (2:1, v/v), homogenized for 60 s at 4000 rpm (Germany Eppendorf Co. Ltd., Germany), followed by the addition of 0.7 % NaCl (8 mL) after 1 h. The bottom layer of the solution was achieved using a rotary evaporator (Rongsheng Biochemical Instrument Ltd., Shanghai, China) and was used to extract the lipids (Jin et al., 2015). Next, the lipid sample (1.0 g) was mixed with 30 mL of an organic solvent mixture (2:3, chloroform (CHCl3): acetic acid (CH3COOH)) and mixed vigorously. Next, 0.5 mL of saturated potassium iodide (KI) solution was added to the mixture and kept in the dark for 5 min. Then, 75 mL of distilled water was added, followed by stirring. An indicator, 0.5 mL of a 1 % starch solution (w/v), was added to the solution. The released iodine from KI was titrated using a standard 0.01 mol/L sodium thiosulfate (Na2S2O3) solution to obtain the POV values (g/100g of lipid).
2.5.2. Thiobarbituric acid reactive substances (TBARS) analysis
First, 50 mL of 7.5 % trichloroacetic acid (in 0.1 % EDTA) was used to dissolve 5.0 g of minced DM before mixing for 30 min. The supernatant was filtered using a double layer of filter paper. The filtrate (5 mL) was added to 5 mL of 0.02 M 2-thiobarbituric acid and reacted for 40 min at 80 °C in a water bath. Next, 2 mL of chloroform solution was added, followed by thorough mixing, and layered once the solution reached room temperature. The absorbance was determined at 532 nm and 600 nm for the supernatant, respectively, using the formula below (Xia et al., 2021):
A532 and A600 represent the absorbance values at 532 nm and 600 nm, respectively. V denotes the total volume of the reaction solution in mL. V1 means the volume of malondialdehyde extraction solution in the reaction solution in mL. V2 represents the total extraction solution volume in mL. The molar extinction coefficient of malondialdehyde was 155,000 L/(mol•cm). The molecular weight of malondialdehyde was 72.06 g/mol. m represents the mass of the sample in grams (g).
2.6. HS-GC-IMS
The volatile flavor compounds in DM samples were analyzed using HS-GC-IMS (FlavourSpec®, G.A.S., Dortmund, Germany). For each sample, 4.0 g of meat puree was placed in a 20 mL sample bottle for GC-IMS analysis. The following analysis parameters were used: (1) Headspace Injection: carrier gas was high-purity N2 (≥99.99 % purity). The headspace incubation was done at 75 °C for 15 min, at 500 rpm. The needle temperature was 85 °C. (2) Gas Chromatography (GC): carrier gas was high-purity N2 (≥99.99 % purity). The column temperature was 60 °C, and the analysis time was 20 min. The flow rate was initially 2 mL/min for 2 min, followed by 10 mL/min, and finally to 150 mL/min. (3) Ion Mobility Spectrometry (IMS): drift gas was high-purity N2 (≥99.99 % purity). The MXT-5 (15 m × 0.53 mm) column was used, with a column temperature of 60 °C and an analysis period of 20 min. The IMS temperature was 45 °C.
2.7. Calculation of ROAV
ROAV was used to determine the role of specific odor components in overall fragrance using the following formula. The odor threshold values for these compounds were obtained using the Flavour-Base 10th Edition (http://www.leffingwell.com/flavbase.htm).
Ci and Ti indicate the relative content (%) and sensory threshold (μg/kg), respectively, of a specific volatile compound. Cstan and Tstan indicate the relative content (%) and sensory threshold (μg/kg) of the volatile compounds with the highest contribution to the overall volatile compound.
2.8. Statistical analysis
Experimental procedures were conducted in triplicate. The one-way analysis was performed among the temperature groups and paired t-tests between 0 and 7 days. The results have been shown as the mean ± standard error. 'M' or 'D' represent the monomer and dimer, respectively. Microsoft Excel 2018 and Origin 2018 were used to prepare the figures. Statistical analysis was performed using SPSS 21.0 software (SPSS Inc., Chicago, USA). SIMCA 14.1 (Umetrics, Malmö, Scania, Sweden) was used to conduct the orthogonal partial least squares discriminant analysis (OPLS-DA).
3. Results and discussion
3.1. Sensory evaluation
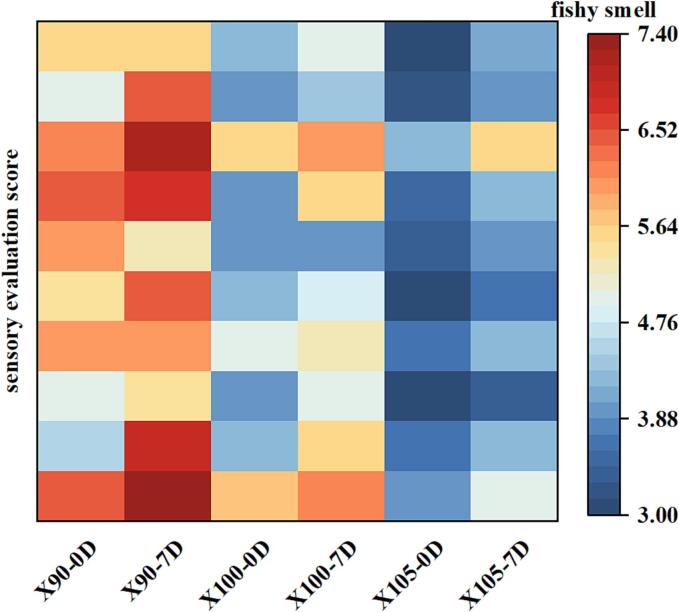
Fig. 1 shows the sensory evaluation of DM across different treatment groups. Among the three treatment groups, the odorous smell was strongest in the 90 °C group both on day 0 and day 7, while the 105 °C group exhibited the weakest odor. This difference in odor was attributed to the fact that the melanin-like and pre-melanin produced by the non-enzymatic Maillard reaction possess antioxidant properties that have the potential to inhibit odor production with an increase in temperature (Bailey, Shin-Lee, Dupuy, Angelo, & Vercellotti, 1987). When reheated after 7 days of storage, the sensory evaluation scores of three groups increased, indicating the odorous smell was stronger compared with that without storage. This result was consistent with that reported by Mukojima et al., 2022, who studied the effects of reheating after storage on the odor of yellowtail fish muscles. They found that the intensity of muscle odor increased with an increase in the number of storage days.
Fig. 1.
Sensory evaluation score of duck meat with different temperatures and storage time. X90-0, X90-7D, X100-0D, X100-7D, X105-0D and X105-7D represent duck breast meat treated at 90 °C, 100 °C and 105 °C for 0 and 7 days, respectively.
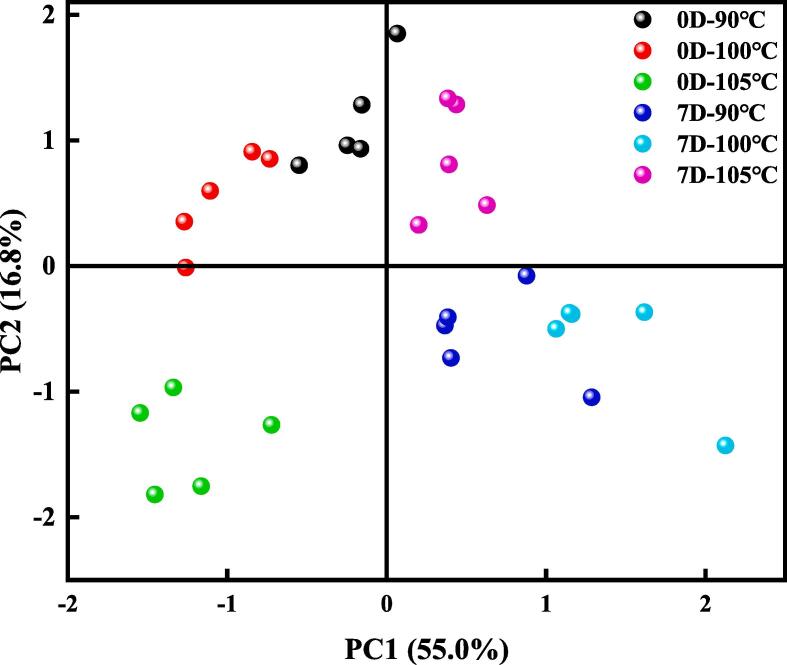
3.2. Principal components analysis (PCA) of E-nose data
PCA was used to investigate the response signals of the DM samples (Fig. 2). The combination of PC1 and PC2 contributed to 71.8 % of the total variance (55.0 % for PC1 and 16.8 % for PC2), which indicated that the sample information was comprehensive. The sample point distribution indicated that the odor of DM treated at 105 °C significantly varied compared with the ones treated at 90 °C and 100 °C on both days 0 and 7. On day 7, all three temperature-treated samples were present at the far end of the coordinate axis. This observation indicated that post-storage reheating significantly affected the odor development, which is consistent with the sensory evaluation results.
Fig. 2.
Principal component analysis score plot of volatile compounds of reheated duck meat after cooking at three temperatures and storage time.
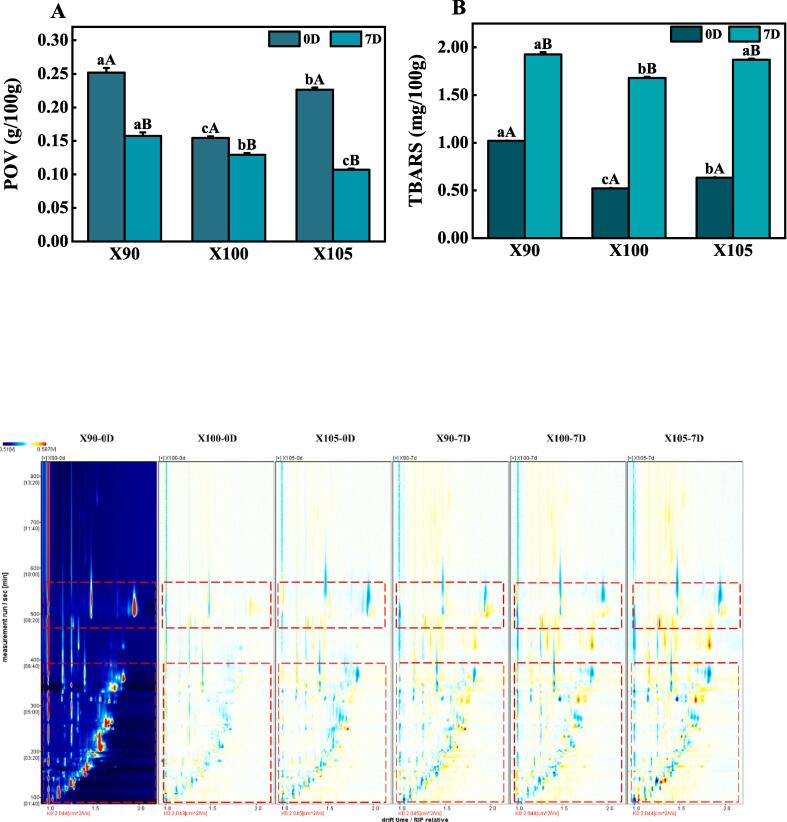
3.3. Lipid oxidation
Lipid oxidation is important in producing flavor in meat products (Xia et al., 2021). Although transient in nature, hydroperoxides are the major products of lipid oxidation. POV can be determined from the ratio of formation and degradation of hydroperoxide. Fig. 3 shows that when the samples were treated at 90 °C, 100 °C, and 105 °C, there was an initial decrease in the POV and TBARS values, followed by an increase in value. Mild heating of meats at roughly 90 °C causes muscle membrane rupture, enhancing the interaction of lipid oxidation catalysts with unsaturated fatty acids, which results in free radical formation and increased lipid oxidation (Pearson, Love, & Shorland, 1977). However, at higher temperatures (>100 °C), hydroperoxides decomposed into unsaturated aldehydes, binary aldehydes, etc., which underwent Maillard reaction, causing the loss of MDA and other carbonyl compounds that can react with TBA. This reduces the POV and TBARS (Xie et al., 2022). With a further increase in temperature, lipid oxidation is accelerated, resulting in the accumulation of primary and secondary products of lipid oxidation, which in turn increases POV and TBARS.
Fig. 3.
Peroxide values (A) and thiobarbituric acid reactive substances values (B) of duck meat with different temperatures and storage times. Differential spectrogram of volatile compounds in duck meat with different temperatures and storage time (C). X90, X100, and X105 represent duck breast meat treated at 90 °C, 100 °C, and 105 °C respectively. The lowercase letters a, b, and c represent the significant differences among the three temperatures (Duncan’s test, p < 0.05), and the uppercase letters A and B represent the significant difference between the storage days in the same temperature (Duncan’s test, p < 0.05). Each point on both sides of the RIP peak represents a flavor compound, with colors indicating concentration levels. White represents equal concentration, blue signifies low concentration, and red indicates high concentration. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Compared with the treatment groups on day 0, there was a significant decrease in the POV value of the reheated DM samples after storage for 7 days (p < 0.05), while there was a significant increase in the TBARS values (p < 0.05). This can be attributed to the lipid oxidation occurring during storage. Some hydroperoxides degraded to obtain thiobarbituric acid reactive substances, causing an increase in aldehydes and alcohols. This could also explain the increase in DM odor in samples after 7 days of storage (Huang, Li, Huang, Li, & Sun, 2014).
3.4. HS-GC-IMS analysis
3.4.1. Spectral analysis of volatile flavor compounds
The spectrum of DM processed at 90 °C for 0 days was chosen as the reference. If the two flavor compounds are the same, the backdrop after deduction is white. In that case, red and blue indicate that the substance concentration is higher and lower than the reference, respectively (Feng et al., 2018). Fig. 3C shows that some volatile compounds were present in significantly elevated concentrations in the 105 °C treatment groups compared with those in the 90 °C and 100 °C treatment groups. Also, some volatile compounds in DM samples reheated after 7 days of storage were higher. Thus, the changes in the amounts of the volatile compounds may cause the aggravation of odorous smell.
3.4.2. Qualitative analysis of volatile flavor compounds
N-ketones C4-C9 (Sinopharm Chemical Reagent Beijing Co., Ltd, China) were used as external standards to measure the retention index (RI) of volatile compounds to evaluate the alterations in these compounds (Jia et al., 2019). Volatile compounds were identified by comparing sample mass spectra to the ones from databases, such as the NIST 2014 (National Institute of Standards and Technology, Gaithersburg, MD, USA) and IMS mass spectra databases. 60 compounds were identified, which comprised 29 aldehydes, 9 ketones, 7 alcohols, 5 esters, 3 furans, 1 pyrrole, 1 benzene, and 5 unknown compounds (Table 2). The volatile compounds identified in the different treatment groups included aldehydes, ketones, alcohols, esters, and furans. Aldehydes have the largest percentage, followed by esters and furans. Aldehydes primarily result from lipid oxidation and amino acid degradation (Wu & Wang, 2019). Their low odor thresholds facilitate their role in the flavor of meat products (Song et al., 2021). Typically, ketones are formed in meat products due to lipid auto-oxidation or fermentation (Arief, Afiyah, Wulandari, & Budiman, 2016). However, they have limited contribution to flavor due to a higher odor threshold. Alcohols from fat oxidation significantly contribute to the desirable flavor and aroma of DM. Esters are synthesized either via the esterification of free fatty acids and alcohols or via the transesterification of fatty acids in triglycerides and ethanol (Liu, Zuo, Wang, & Xu, 2020). The esters formed from short-chain fatty acids have pleasant fruity aromas, while those formed from long-chain fatty acids possess greasy flavors. The primary flavor components present in meat products are known to be furans, which result from the Maillard reactions. For instance, 2-Pentylfuran is a non-carboxyl molecule synthesized from linoleic acid and other w-6 fatty acids, which have a relatively lower threshold and plant odor (Wall, Kerth, Miller, & Alvarado, 2019).
Table 2.
Threshold and ROAV of volatile flavor compounds in duck meat with different temperatures and storage time. X90-0, X90-7D, X100-0D, X100-7D, X105-0D, and X105-7D represent duck breast meat treated at 90 °C, 100 °C and 105 °C for 0 and 7 days, respectively. N.A., not found about the relevant sensory threshold. “-” indicates not found about the flavor description words of volatile flavor compounds. And the relevant thresholds of some important volatile flavor compounds were not found, so they were not analyzed.
| Number | Volatile compounds | Threshold (μg/kg) | Odor characteristics | ROAV |
|||||
|---|---|---|---|---|---|---|---|---|---|
| X90-0D | X100-0D | X105-0D | X90-7D | X100-7D | X105-7D | ||||
| 1 | Nonanal | 1 | Fat flavor, delicate fragrance, oils, fishy smell; citrus flavor | 386.45 | 390.85 | 348.89 | 412.16 | 404.85 | 442.36 |
| 2 | Octanal | 0.7 | Oily flavour, grass smell | 135.78 | 133.37 | 110.69 | 127.99 | 127.78 | 131.80 |
| 3 | Heptanal | 3 | Fishy smell, oily flavor | 13.18 | 12.93 | 12.61 | 13.75 | 13.13 | 15.41 |
| 4 | Decanal | 0.1 | Fat flavor, grass smell, cucumber taste | 108.29 | 97.36 | 104.61 | 127.99 | 127.78 | 151.20 |
| 5 | Butanal | 11 | Penetrating odor | 0.55 | 0.46 | 0.51 | 0.59 | 0.73 | 0.96 |
| 6 | Pentanal | 12 | Fruit drops, bread fragrance | 6.45 | 6.20 | 5.73 | 6.33 | 6.34 | 6.93 |
| 7 | Hexanal | 4.5 | Grass smell, fishy smell, fat flavor | 15.72 | 15.91 | 14.99 | 16.20 | 17.09 | 19.03 |
| 8 | 3-Octenal | N.A. | – | N.A.. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 9 | (E)-2-octenal | 3 | Fat flavor, delicate fragrance, grass smell | 12.75 | 12.38 | 13.33 | 17.79 | 20.17 | 24.31 |
| 10 | (E)-hept-2-enal | 13 | Strong fragrance, fat grease | 0.87 | 0.93 | 0.94 | 1.03 | 1.04 | 1.15 |
| 11 | (E)-2-nonenal | 0.19 | Dust, cucumber taste | 54.10 | 39.40 | 72.52 | 84.29 | 51.68 | 72.38 |
| 12 | (E)-2-pentenal | N.A. | – | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 13 | 2-Methyl-2-pentenal | N.A. | – | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 14 | 2-methylbutanal | 1 | Apple fragrance | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| 15 | 3-methylbutanal | 2 | Apple fragrance | 12.34 | 12.28 | 11.53 | 11.78 | 11.84 | 15.15 |
| 16 | Benzaldehyde | 350 | Bitter almond, nut flavor | 0.10 | 0.10 | 0.10 | 0.12 | 0.13 | 0.15 |
| 17 | 2-Methyl-3-heptanone | N.A. | – | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 18 | Methyl isobutyl ketone | 1500 ∼ 5000 | – | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 19 | oct-1-en-3-ol | 1 | Oily flavor, soily, mushroomy | 26.88 | 25.91 | 25.25 | 26.37 | 25.47 | 26.94 |
| 20 | pent-1-en-3-ol | 400 | mushroomy, grass smell | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 21 | n-Hexanol | 250 | Fruit drops | 0.07 | 0.06 | 0.07 | 0.08 | 0.08 | 0.09 |
| 22 | Ethyl Acetate | 5 | Sweet fruit aroma | 8.02 | 8.15 | 7.90 | 8.02 | 7.85 | 11.55 |
| 23 | Butyl acetate | 66 | Fruit drops | 0.83 | 0.87 | 0.87 | 1.03 | 1.08 | 1.19 |
| 24 | 2-pentyl furan | 6 | Grass smell, roast, fishy smell | 3.44 | 3.21 | 3.80 | 3.87 | 3.78 | 4.14 |
| 25 | Tetrahydrofurane | N.A. | – | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
Supplementary Fig. 1 shows the proportion of volatile compounds in different treatment groups. Compared with day 0, there was no significant difference in the relative contents of aldehydes, ketones, and alcohols in DM treated at 90 °C (p > 0.05). However, there was a decrease in the esters increased and furans (p < 0.05). For the samples treated at 100 °C, there were no significant differences in the relative contents of alcohols, esters, and furans in DM treated at (p > 0.05). However, the aldehydes decreased significantly, while the ketones increased significantly (p < 0.05). For the DM treated at 105 °C, there was no significant difference in the relative content of aldehydes between day 0 and day 7. However, the ketones and esters increased significantly, while the alcohols and furans decreased significantly (p < 0.05). Thus, the results showed that there was no substantial difference in the primary volatile flavor compounds in the samples from different treatment groups. However, the overall relative content of the main volatile compounds was significantly different, implying that the odor was not caused by specific volatile compounds but was rather related to the change in the relative content of some volatile compounds. The interactions between these volatile small molecular compounds caused the odor. Therefore, it was critical to evaluate the relative concentrations of specific volatile compounds and compare the differences in volatile compounds between different samples to identify the primary characteristic compounds causing odor in DM.
The sensory evaluation results showed that the odorous smell was the lowest in the DM samples on day 0 in the 105 °C treatment group. However, it increased on reheating after 7 days of storage. Therefore, the odorous smell was enhanced by the synthesis of novel volatile compounds or the enhancement in the relative content of certain volatile compounds. Supplementary Fig. 2 and Table 1 suggested that in the 90 °C treatment group, the significantly elevated volatile substances in the reheated samples after 7 days of storage included decanal, nonanal-D, (E)-2-octenal, 3-octenal, benzaldehyde (dimer), (E) −2-heptenal, (E) −2-pentenal, (E) −2-nonenal, 2-methyl-2-pentenal, pentanal-D, 2-methylbutanal-M, 3-methylbutanal-M, 2-methyl-3-heptanone, 4-methyl-2-pentanone, n-hexanol-D, 1-penten-3-ol, ethyl acetate-M, butyl acetate, 2-pentylfuran, tetrahydrofuran, etc. (p < 0.05). On day 7, the 100 °C treatment of DM with additional butyraldehyde was significantly increased (p < 0.05). The volatile substances at 0 d and at 7 d in the 105 °C treatment group differed from the ones in the 90 °C treatment group. The 3-octenal-M, (E)-2-nonenal, and 2-pentylfuran levels in the DM treated at 105 °C for 7 d were substantially reduced, while the content of butyraldehyde and 2-pentanone levels were significantly enhanced (p < 0.05). Besides, on day 0, the relative content of volatile substances in the 105 °C treatment group was significantly higher than the 90 °C treatment group. They included nonanal, octanal, benzaldehyde-M, pentanal-M, 2-methylbutanal-M, 3-methylbutanal-M, butyraldehyde, 2-butanone-D, 1-penten-3-ol, ethyl acetate-M, 2-n-butylfuran, tetrahydrofuran, etc. (p < 0.05). In the 100 °C treatment group, the substances with a significant increase in the relative content of volatile substances on day 0 included nonanal-D, octanal, hexanal-M, pentanal-M, 2-n-butylfuran, etc. (p < 0.05). In the 90 °C and 100 °C treatment groups, the substances with a significant increase in the relative content of volatile substances on day 7 were similar (p < 0.05). The common substances included decanal, nonanal-D, (E)-2-octenal, octanal-M, benzaldehyde-D, (E)-2-pentenal, pentanal-D, 2-methylbutanal-M, 3-methylbutanal-M, ethyl acetate-M, butyl acetate, tetrahydrofuran, etc. Moreover, in the 90 °C treatment group, n-hexanol-D and 1-penten-3-ol increased significantly (p < 0.05) on day 7. In contrast, 2-methyl-2-pentenal, butyraldehyde, 2-methyl-3-heptanone, and 4-methyl-2-pentanone increased significantly on day 7 in the 100 °C treatment group (p < 0.05).
Table 1.
Qualitative analysis of volatile compounds of duck meat with different temperatures and storage time. X90-0, X90-7D, X100-0D, X100-7D, X105-0D and X105-7D represent duck breast meat treated at 90 °C, 100 °C and 105 °C for 0 and 7 days, respectively. The data are expressed as the means ± standard deviations (n = 3). The lowercase letters a, b, and c represent the significant differences among the three temperatures (Duncan’s test, p < 0.05), and the uppercase letters A and B represent the significant difference between the storage days in the same temperature (Duncan’s test, p < 0.05). “–”: not detected in the database and not reported in the relevant literature. The “M” and “D” represent the monomer, and dimer, respectively. MW: molecular weight, RI: retention index, RT: retention time, DT: drift time.
| Volatile compound | CAS | Molecular formula | Molecular weight | Retention index RI | Retention time/s | drift time/ms | relative content/% |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X90-0D | X100-0D | X105-0D | X90-7D | X100-7D | X105-7D | |||||||
| Aldehydes | 70.19 | 70.81 | 69.56 | 70.10 | 70.25 | 69.74 | ||||||
| Decanal | C112312 | C10H20O | 156.3 | 1278.9 | 751.99 | 1.54015 | 0.78 ± 0.01aA | 0.71 ± 0.02bA | 0.79 ± 0.05aA | 0.86 ± 0.05A | 0.86 ± 0.06B | 0.90 ± 0.00A |
| Nonanal-M | C124196 | C9H18O | 142.2 | 1109.9 | 509 | 1.47552 | 9.89 ± 0.28aA | 9.39 ± 0.02bA | 9.37 ± 0.19bA | 8.79 ± 0.04B | 8.69 ± 0.09B | 8.66 ± 0.10B |
| Nonanal-D | C124196 | C9H18O | 142.2 | 1109.4 | 508.28 | 1.95562 | 17.81 ± 0.08bA | 19.05 ± 0.10aA | 17.13 ± 0.23cA | 18.78 ± 0.13aB | 18.43 ± 0.32aB | 17.71 ± 0.20bA |
| (E)-2-octenal-M | C2548870 | C8H14O | 126.2 | 1056.4 | 432.04 | 1.33703 | 1.93 ± 0.04bA | 1.91 ± 0.05bA | 2.13 ± 0.07aA | 2.33 ± 0.03cB | 2.55 ± 0.02bB | 2.64 ± 0.06aB |
| (E)-2-octenal-D | C2548870 | C8H14O | 126.2 | 1055.9 | 431.33 | 1.83006 | 0.81 ± 0.09A | 0.80 ± 0.02A | 0.91 ± 0.05A | 1.24 ± 0.03cB | 1.50 ± 0.05bB | 1.71 ± 0.11aB |
| Octanal-M | C124130 | C8H16O | 128.2 | 1014.8 | 372.18 | 1.40166 | 5.55 ± 0.16aA | 5.59 ± 0.03aA | 4.73 ± 0.08bA | 5.02 ± 0.08aB | 4.89 ± 0.07aB | 4.46 ± 0.14bB |
| Octanal-D | C124130 | C8H16O | 128.2 | 1005.3 | 358.64 | 1.8319 | 1.27 ± 0.07aA | 1.21 ± 0.04abA | 1.16 ± 0.04bA | 1.01 ± 0.04B | 1.04 ± 0.02B | 1.04 ± 0.03B |
| 3-Octenal-M | R286265 | C8H14O | 126.2 | 990.5 | 340.83 | 1.39243 | 2.80 ± 0.02cA | 2.87 ± 0.04bA | 3.11 ± 0.02aA | 2.97 ± 0.03bB | 2.96 ± 0.04bA | 3.07 ± 0.01aB |
| 3-Octenal-D | R286265 | C8H14O | 126.2 | 988.6 | 339.16 | 1.80296 | 0.98 ± 0.02bA | 0.97 ± 0.00bA | 1.17 ± 0.02aA | 1.17 ± 0.02B | 1.14 ± 0.04B | 1.22 ± 0.04A |
| Benzaldehyde-M | C100527 | C7H6O | 106.1 | 958.8 | 313.77 | 1.15278 | 0.72 ± 0.01aA | 0.66 ± 0.01bA | 0.69 ± 0.02bA | 0.59 ± 0.01aB | 0.45 ± 0.03bB | 0.35 ± 0.01cB |
| Benzaldehyde-D | C100527 | C7H6O | 106.1 | 958.8 | 313.77 | 1.47689 | 1.90 ± 0.09A | 1.90 ± 0.05A | 1.92 ± 0.04A | 2.22 ± 0.02cB | 2.49 ± 0.11bB | 2.88 ± 0.09aB |
| (E)-2-heptenal | C18829555 | C7H12O | 112.2 | 953.3 | 309.09 | 1.67619 | 0.81 ± 0.03bA | 0.88 ± 0.02aA | 0.93 ± 0.04aA | 0.90 ± 0.01B | 0.90 ± 0.02B | 0.89 ± 0.02A |
| Heptanal-M | C111717 | C7H14O | 114.2 | 900.6 | 264.15 | 1.3299 | 2.28 ± 0.06A | 2.26 ± 0.04A | 2.28 ± 0.01A | 2.25 ± 0.03aA | 2.12 ± 0.03bA | 2.24 ± 0.02aA |
| Heptanal-D | C111717 | C7H14O | 114.2 | 900.6 | 264.15 | 1.70866 | 0.55 ± 0.02bA | 0.57 ± 0.01bA | 0.59 ± 0.01aA | 0.51 ± 0.02B | 0.52 ± 0.01B | 0.52 ± 0.01B |
| Hexanal-M | C66251 | C6H12O | 100.2 | 796.5 | 205.65 | 1.25544 | 4.51 ± 0.05bA | 4.67 ± 0.06aA | 4.55 ± 0.09abA | 4.36 ± 0.06bA | 4.61 ± 0.00aA | 4.59 ± 0.02aA |
| Hexanal-D | C66251 | C6H12O | 100.2 | 800.3 | 207.68 | 1.56878 | 0.56 ± 0.03A | 0.54 ± 0.02A | 0.58 ± 0.00A | 0.51 ± 0.01A | 0.54 ± 0.00A | 0.52 ± 0.02B |
| (E)-2-pentenal-M | C1576870 | C5H8O | 84.1 | 748.8 | 184.69 | 1.10702 | 0.28 ± 0.02A | 0.31 ± 0.01A | 0.29 ± 0.01A | 0.35 ± 0.01cB | 0.43 ± 0.01bB | 0.49 ± 0.02aB |
| (E)-2-pentenal-D | C1576870 | C5H8O | 84.1 | 747.5 | 184.16 | 1.36378 | 0.76 ± 0.05A | 0.78 ± 0.00A | 0.81 ± 0.03A | 1.02 ± 0.01cB | 1.15 ± 0.03bB | 1.28 ± 0.04aB |
| (E)-2-hexenal | C6728263 | C6H10O | 98.1 | 847.7 | 233.09 | 1.52121 | 0.07 ± 0.01cA | 0.09 ± 0.00bA | 0.10 ± 0.00aA | 0.07 ± 0.01bA | 0.07 ± 0.00bA | 0.10 ± 0.01aA |
| (E)-2-nonenal | C18829566 | C9H16O | 140.2 | 1188.7 | 622.29 | 1.41457 | 0.74 ± 0.05bA | 0.54 ± 0.04cA | 1.05 ± 0.07aA | 1.07 ± 0.03aB | 0.66 ± 0.02cB | 0.82 ± 0.05bB |
| 3-Methyl-2-butenal | C107868 | C5H8O | 84.1 | 776.8 | 196.07 | 1.36179 | 0.11 ± 0.00A | 0.10 ± 0.00A | 0.11 ± 0.00A | 0.10 ± 0.01A | 0.10 ± 0.00A | 0.10 ± 0.01A |
| 2-Methyl-2-pentenal | C623369 | C6H10O | 98.1 | 833.1 | 225.28 | 1.50454 | 0.17 ± 0.01A | 0.17 ± 0.00A | 0.18 ± 0.01A | 0.19 ± 0.00bB | 0.21 ± 0.01aB | 0.21 ± 0.01aB |
| Pentanal-M | C110623 | C5H10O | 86.1 | 697.5 | 163.88 | 1.18541 | 5.34 ± 0.13aA | 5.20 ± 0.13abA | 5.01 ± 0.05bA | 4.85 ± 0.04aB | 4.85 ± 0.03aB | 4.72 ± 0.04bB |
| Pentanal-D | C110623 | C5H10O | 86.1 | 694.1 | 162.51 | 1.42947 | 0.21 ± 0.01A | 0.21 ± 0.01A | 0.22 ± 0.01A | 0.24 ± 0.00bB | 0.25 ± 0.01aB | 0.24 ± 0.00bB |
| 2-methylbutanal-M | C96173 | C5H10O | 86.1 | 663.8 | 153.6 | 1.16481 | 0.39 ± 0.02aA | 0.36 ± 0.01bA | 0.38 ± 0.01abA | 0.44 ± 0.02aB | 0.43 ± 0.01aB | 0.38 ± 0.01bA |
| 2-methylbutanal-D | C96173 | C5H10O | 86.1 | 661.2 | 152.91 | 1.39755 | 6.78 ± 0.02bA | 6.92 ± 0.11bA | 7.21 ± 0.01aA | 6.25 ± 0.03aB | 6.27 ± 0.09aB | 5.58 ± 0.09bB |
| 3-methylbutanal-M | C590863 | C5H10O | 86.1 | 642.6 | 147.89 | 1.17614 | 0.31 ± 0.00aA | 0.27 ± 0.01bA | 0.27 ± 0.01bA | 0.32 ± 0.00cB | 0.33 ± 0.00bB | 0.45 ± 0.00aB |
| 3-methylbutanal-D | C590863 | C5H10O | 86.1 | 643.5 | 148.12 | 1.41711 | 1.46 ± 0.01A | 1.52 ± 0.02A | 1.48 ± 0.05A | 1.25 ± 0.01bB | 1.26 ± 0.06bB | 1.35 ± 0.01aB |
| Butanal | C123728 | C4H8O | 72.1 | 551.1 | 123.21 | 1.28633 | 0.44 ± 0.01aA | 0.37 ± 0.01bA | 0.42 ± 0.02aA | 0.43 ± 0.01cA | 0.54 ± 0.02bB | 0.63 ± 0.03aB |
| Ketone | 4.87 | 5.08 | 5.35 | 5.21 | 5.30 | 5.45 | ||||||
| 2-Methyl-3-heptanone | C13019200 | C8H16O | 128.2 | 1089.1 | 479.07 | 1.28348 | 1.06 ± 0.25bA | 1.45 ± 0.04aA | 1.58 ± 0.09aA | 1.65 ± 0.03bA | 1.78 ± 0.05bB | 2.10 ± 0.10aB |
| 3-hydroxybutan-2-one | C513860 | C4H8O2 | 88.1 | 712.7 | 170.05 | 1.05772 | 1.25 ± 0.01bA | 1.23 ± 0.02bA | 1.30 ± 0.01aA | 1.23 ± 0.01aA | 1.23 ± 0.02aA | 1.15 ± 0.02bB |
| 2-Butanone-D | C78933 | C4H8O | 72.1 | 576.5 | 130.07 | 1.25029 | 1.19 ± 0.02aA | 1.03 ± 0.07bA | 1.01 ± 0.04bA | 0.94 ± 0.04aB | 0.91 ± 0.01aA | 0.79 ± 0.04bB |
| acetone | C67641 | C3H6O | 58.1 | 485 | 105.39 | 1.12259 | 0.35 ± 0.02A | 0.33 ± 0.01A | 0.35 ± 0.01A | 0.31 ± 0.01aB | 0.31 ± 0.01aB | 0.26 ± 0.01bB |
| 2-heptanone | C110430 | C7H14O | 114.2 | 893.3 | 257.87 | 1.63907 | 0.24 ± 0.01A | 0.23 ± 0.01A | 0.24 ± 0.01A | 0.22 ± 0.01B | 0.21 ± 0.01B | 0.23 ± 0.01B |
| Methyl isobutyl ketone | C108101 | C6H12O | 100.2 | 731.3 | 177.62 | 1.48394 | 0.41 ± 0.01bA | 0.40 ± 0.01bA | 0.46 ± 0.01aA | 0.48 ± 0.01B | 0.48 ± 0.00B | 0.49 ± 0.00B |
| methyl-5-hepten-2-one | C110930 | C8H14O | 126.2 | 990.3 | 340.66 | 1.18289 | 0.23 ± 0.01bA | 0.26 ± 0.00aA | 0.28 ± 0.01aA | 0.24 ± 0.00bA | 0.25 ± 0.01bA | 0.28 ± 0.00aA |
| (E)-3-penten-2-one | C3102338 | C5H8O | 84.1 | 734.7 | 178.99 | 1.35345 | 0.04 ± 0.00bA | 0.05 ± 0.01aA | 0.04 ± 0.00bA | 0.03 ± 0.00B | 0.03 ± 0.00B | 0.03 ± 0.00B |
| 2-Pentanone | C107879 | C5H10O | 86.1 | 691.7 | 161.55 | 1.37708 | 0.10 ± 0.00A | 0.10 ± 0.01A | 0.10 ± 0.00A | 0.10 ± 0.01bA | 0.11 ± 0.01abA | 0.12 ± 0.00aB |
| Alcohols | 4.44 | 4.36 | 4.55 | 4.49 | 4.34 | 4.32 | ||||||
| oct-1-en-3-ol-M | C3391864 | C8H16O | 128.2 | 984.7 | 335.84 | 1.16161 | 0.90 ± 0.04A | 0.84 ± 0.02A | 0.85 ± 0.02A | 0.77 ± 0.01aB | 0.68 ± 0.03bB | 0.60 ± 0.01cB |
| oct-1-en-3-ol-D | C3391864 | C8H16O | 128.2 | 979.5 | 331.41 | 1.60353 | 1.03 ± 0.05A | 1.04 ± 0.02A | 1.06 ± 0.02A | 1.00 ± 0.01A | 1.02 ± 0.03A | 1.01 ± 0.00B |
| pentan-1-ol-M | C71410 | C5H12O | 88.1 | 764.1 | 190.92 | 1.2555 | 0.99 ± 0.02bA | 1.05 ± 0.04abA | 1.07 ± 0.02aA | 1.10 ± 0.02A | 1.09 ± 0.00A | 1.11 ± 0.02A |
| pentan-1-ol-D | C71410 | C5H12O | 88.1 | 763.5 | 190.68 | 1.51638 | 0.15 ± 0.00A | 0.15 ± 0.00A | 0.15 ± 0.00A | 0.13 ± 0.00B | 0.14 ± 0.01B | 0.13 ± 0.00B |
| n-Hexanol-M | C111273 | C6H14O | 102.2 | 872.9 | 246.61 | 1.32537 | 0.05 ± 0.00A | 0.05 ± 0.00A | 0.05 ± 0.01A | 0.05 ± 0.00A | 0.05 ± 0.01A | 0.06 ± 0.00B |
| n-Hexanol-D | C111273 | C6H14O | 102.2 | 868.4 | 244.2 | 1.64449 | 1.19 ± 0.01bA | 1.12 ± 0.00cA | 1.25 ± 0.01aA | 1.31 ± 0.01aB | 1.23 ± 0.01bB | 1.29 ± 0.02aA |
| pent-1-en-3-ol | C616251 | C5H10O | 86.1 | 673.1 | 156.12 | 1.35342 | 0.12 ± 0.00aA | 0.10 ± 0.01bA | 0.11 ± 0.00bA | 0.13 ± 0.00B | 0.12 ± 0.01A | 0.13 ± 0.01B |
| Esters | 7.15 | 7.48 | 7.71 | 7.55 | 7.71 | 8.47 | ||||||
| Ethyl Acetate-M | C141786 | C4H8O2 | 88.1 | 610.4 | 139.21 | 1.09891 | 0.42 ± 0.02aA | 0.33 ± 0.03bA | 0.34 ± 0.02bA | 0.50 ± 0.01bB | 0.48 ± 0.02bB | 1.09 ± 0.03aB |
| Ethyl Acetate-D | C141786 | C4H8O2 | 88.1 | 608.7 | 138.75 | 1.34091 | 2.45 ± 0.01bA | 2.63 ± 0.05aA | 2.66 ± 0.04aA | 2.19 ± 0.01bB | 2.15 ± 0.02bB | 2.35 ± 0.05aB |
| n-Propyl acetate | C109604 | C5H10O2 | 102.1 | 707.1 | 167.78 | 1.48202 | 0.15 ± 0.01A | 0.14 ± 0.01A | 0.15 ± 0.00A | 0.14 ± 0.00aA | 0.13 ± 0.00bA | 0.14 ± 0.00aB |
| Butyl acetate | C123864 | C6H12O2 | 116.2 | 818.8 | 217.62 | 1.62002 | 3.93 ± 0.06cA | 4.19 ± 0.05bA | 4.35 ± 0.04aA | 4.55 ± 0.02bB | 4.77 ± 0.04aB | 4.70 ± 0.08aB |
| 2-Methylbutanol acetate | C624419 | C7H14O2 | 130.2 | 879 | 249.86 | 1.71157 | 0.20 ± 0.01bA | 0.18 ± 0.00cA | 0.21 ± 0.00aA | 0.18 ± 0.01B | 0.18 ± 0.00A | 0.19 ± 0.00B |
| Furans | 3.69 | 3.34 | 3.65 | 3.50 | 3.45 | 3.38 | ||||||
| 2-n-Butylfuran | C4466244 | C8H12O | 124.2 | 892.7 | 257.33 | 1.18274 | 1.74 ± 0.02aA | 1.64 ± 0.03abA | 1.53 ± 0.11bA | 1.36 ± 0.04B | 1.47 ± 0.09B | 1.47 ± 0.04A |
| 2-pentyl furan | C3777693 | C9H14O | 138.2 | 995.8 | 345.3 | 1.25571 | 1.53 ± 0.11bA | 1.40 ± 0.04cA | 1.73 ± 0.01aA | 1.55 ± 0.03B | 1.52 ± 0.06A | 1.48 ± 0.05B |
| Tetrahydrofuran | C109999 | C4H8O | 72.1 | 628.2 | 144 | 1.06493 | 0.47 ± 0.02aA | 0.29 ± 0.01cA | 0.39 ± 0.00bA | 0.58 ± 0.02aB | 0.46 ± 0.01bB | 0.43 ± 0.01cB |
| Others | 0.99 | 1.01 | 1.10 | 0.87 | 0.93 | 1.06 | ||||||
| Pyrrole | C109977 | C4H5N | 67.1 | 738 | 180.33 | 0.96607 | 0.64 ± 0.04A | 0.62 ± 0.02A | 0.63 ± 0.04A | 0.54 ± 0.02A | 0.56 ± 0.07A | 0.59 ± 0.02A |
| p-xylene | C106423 | C8H10 | 106.2 | 864.1 | 241.88 | 1.06786 | 0.35 ± 0.00cA | 0.39 ± 0.02bA | 0.47 ± 0.02aA | 0.33 ± 0.00cB | 0.37 ± 0.01bA | 0.47 ± 0.01aA |
| Unknown | 8.68 | 7.93 | 8.07 | 8.28 | 8.02 | 7.58 | ||||||
| 1 | – | – | – | 782.6 | 198.39 | 1.48726 | 0.16 ± 0.00A | 0.17 ± 0.00A | 0.17 ± 0.00A | 0.16 ± 0.00cA | 0.18 ± 0.00bB | 0.19 ± 0.00aB |
| 2 | – | – | – | 537.6 | 119.56 | 1.20498 | 7.60 ± 0.19aA | 6.87 ± 0.14bA | 6.85 ± 0.19bA | 7.03 ± 0.41aA | 6.81 ± 0.07abA | 6.35 ± 0.25bB |
| 3 | – | – | – | 1027.4 | 390.31 | 1.69661 | 0.41 ± 0.02bA | 0.41 ± 0.02bA | 0.50 ± 0.04aA | 0.56 ± 0.00aB | 0.52 ± 0.03bA | 0.52 ± 0.01bA |
| 4 | – | – | – | 952.5 | 308.39 | 1.73355 | 0.48 ± 0.01bA | 0.46 ± 0.00cA | 0.53 ± 0.01aA | 0.50 ± 0.01B | 0.49 ± 0.00B | 0.50 ± 0.00B |
| 5 | – | – | – | 733.4 | 178.44 | 1.11021 | 0.03 ± 0.00aA | 0.02 ± 0.00bA | 0.03 ± 0.00aA | 0.03 ± 0.00aA | 0.02 ± 0.00bA | 0.02 ± 0.00bA |
Furthermore, previous studies on the meat odor have revealed that substances, such as heptanal, hexanal, pentanal, and 1-octene-3-ol significantly contribute to the formation of the odorous smell in meat(Guo, Kong, Hu, Zhou, & Shen, 2019). The oxidation of linoleic acid and arachidonic acid in ω-6 polyunsaturated fatty acids are the primary sources of heptanal and hexanal (Watanabe et al., 2015). Heptanal possesses a greasy taste, while hexanal possesses a grassy, fishy taste (Banaszak, Kuźniacka, Biesek, Maiorano, & Adamski, 2020). The latter is a characteristic substance for the fishy taste of meat. This smell has also been defined as an odorous duck smell in some studies (Azarbad & Jeleń, 2015). Pentanal, generated from oleic acid oxidation, is reported to produce almond flavor (Xie et al., 2022). 1-octen-3-ol is produced by lipase-catalyzed reactions and oxidative breakdown of unsaturated polyunsaturated fatty acids (such as arachidonic acid). Because of the low threshold of 1-octen-3-ol, it is one of the factors producing a unique odor (Iglesias et al., 2009). Here, the relative levels of these compounds had a decreasing trend, probably due to their reactions with the degradation products produced by the DM after high-temperature heating (Xie et al., 2022). Esters are commonly occurring compounds with low odor threshold values. They were synthesized via the esterification reaction and could enhance fruit flavor or mask putrid stench (Zhang et al., 2020). Particularly, ethyl acetate possesses a fresh, fruity, and winey smell and positively impacts the odorous smell of DM. Therefore, it is not one of the characteristic substances responsible for the odorous smell of DM.
3.5. Relative odor activity value (ROAV)
Based on the relative levels of the volatile flavor compounds, it could not be effectively determined if they played a vital role in the overall flavor of DM. Therefore, it was critical to further analyze them based on the combination of their flavor thresholds. Currently, the ROAV method is used to examine essential flavor compounds in multiple meat products (Zhang et al., 2019). Here, the ROAV of compounds was determined by normalization to 2-methylbutanal. Volatile compounds with ROAV ≥ 1 were considered essential odor compounds, while compounds with 0.1 ≤ ROAV ≤ 1 modified the odor of the DM (Wang et al., 2020). The common essential odor compounds with ROAV ≥ 1 in different treatment groups were nonanal, octanal, heptanal, decanal, pentanal, hexanal, (E)-2-octenal, (E)-2-nonenal, 2-methylbutanal, 3-methylbutanal, 1-octen-3-ol, ethyl acetate, and 2-pentylfuran, respectively (Table 1). Additionally, the ROAV of (E)-2-heptenal in DM samples that were treated at three different temperatures in the day 0 group was approximately 1. After storage and reheating, the ROAV was determined to be 0.1–1 (including 0.1), which was a critical contributor to the overall odor of DM. However, ethyl acetate positively affected the odorous smell of DM. Thus, combined with the sensory evaluation and ROAV, it was concluded that the following 13 volatile flavor substances contributed to the odorous smell of DM: nonanal, octanal, heptanal, decanal, pentanal, hexanal, (E)-2-octenal, (E)-2-nonenal, 2-methylbutanal, 3-methylbutanal, 1-octen-3-ol, (E)-2-heptenal, and 2-pentylfuran.
3.6. Screening of key volatile flavor compounds based on OPLS-DA
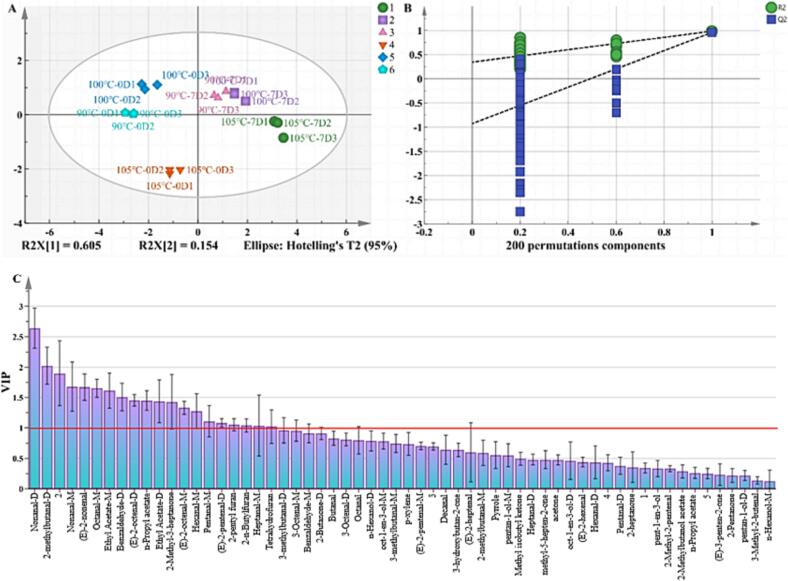
The OPLS-DA method is highly effective in conducting sample categorization and discriminant modeling (Du et al., 2021). The OPLS-DA model’s degree of fit (R2) and prediction capacity (Q2) were used to distinguish DM samples with significant differences (Bhumireddy, Rocchetti, Pallerla, Lucini, & Sripadi, 2021). Higher values closer to 1 indicated better predictability and interpretability (Kandasamy et al., 2020). Here, the parameters of the models R2X, R2Y, and Q2 were 0.971, 0.969, and 0.911, respectively (Fig. 4A). Furthermore, there was an intersection between the Q2 regression line and the horizontal axis with a negative intercept (Fig. 4B), supporting the reliability of the model. These results showed a good model fit and acceptable predictable accuracy. The DM samples treated at 90 °C and 100 °C on day 0 had similar results; both were present in the second quadrant, away from those in the 105 °C group, which were present in the lower end of the third quadrant (Fig. 4A). The samples in the 7-day treatment groups were present on the right side of the coordinate axis, with the DM samples treated at 90 °C and 100 °C in the first quadrant- Both of them were further away from the ones present in the 105 °C treatment groups. Thus, the volatile characteristic flavor compounds in the DM could completely differentiate between the DM samples from different treatment groups. These results were consistent with the results of the E-nose analysis.
Fig. 4.
OPLS-DA score and replacement test of duck meat with different temperatures and storage time (A, B). VIP distribution map of characteristic odorous smell compounds in duck meat (C). Permutation test scores (n = 200) based on paired OPLS- DA models. R2 and Q2 are indicators of model fit and predictive capability, respectively. R2X and R2Y express the proportion of information in the X and Y matrices and are commonly employed to assess model goodness of fit and reliability. The “M” and “D” represent the monomer, and dimer, respectively.
The VIP value is used to analyze the variable contributions in the model (Arendse, Fawole, Magwaza, Nieuwoudt, & Opara, 2018). The variables with a VIP > 1 are known to be the most influential (Song et al., 2021). An elevated VIP value indicates a more significant contribution to the model. In our OPLS-DA model, VIP values > 1 were screened to determine potential flavor components in the DM. There were 16 volatile compounds with VIP > 1, including nonanal, 2-methylbutyraldehyde-D, (E)-2-nonenal, octanal-M, ethyl acetate, benzaldehyde-D, (E)-2-octenal, butyl acetate, 2-methyl-3-heptanone, hexanal-M, glutaraldehyde-M, (E)-2-pentenal-D, 2-pentylfuran, 2-n-butylfuran, heptanal-M, tetrahydrofuran, etc. (Fig. 4C). Also, combined with the previous ROAV calculation and sensory evaluation results, it was finally determined that nonanal, 2-methylbutanal, pentanal, octanal, heptanal, hexanal, (E)-2-octenal, (E)-2-nonenal, and 2-pentyl furan played a critical role in the odorous smell of DM, and could be used as the critical volatile flavor compounds for the later study of duck odorous smell. Also, the VIP value graph was studied based on the relative levels of volatile compounds. The threshold value of volatile molecules needed to be combined to cause the odorous smell. The low threshold value of some volatile compounds with VIP < 1 also had an essential impact on odorous smell, which requires further analysis.
4. Conclusions
The study results showed that different processing temperatures influenced lipid oxidation and odorous characteristics in DM. When samples were treated at 90 °C, elevated levels of lipid oxidation and a stronger odor were observed. Notably, the apparent odor of DM treated at three different temperatures for 7 days was greater than that of the 0-day samples. The volatile compounds identified in the DM samples of different treatment groups were of the same type, and the difference in content was probably related to the smell of DM. The results of sensory evaluation and ROAV identified the following 13 critical volatile compounds: nonanal, octanal, heptanal, decanal, pentanal, hexanal, (E)-2-octenal, (E)-2-nonenal, 2-methylbutanal, 3-methylbutanal, 1-octen-3-ol, (E)-2-heptenal, and 2-pentylfuran. Of these, nine critical volatile flavor compounds were further screened based on VIP > 1, including nonanal, 2-methylbutanal, pentanal, octanal, heptanal, hexanal, (E)-2-octenal, (E)-2-nonenal, and 2-pentyl furan. These compounds were mainly produced by the oxidative degradation of linoleic acid, arachidonic acid, and oleic acid. Therefore, the subsequent purpose of removing the odorous smell of DM could be achieved by inhibiting their oxidative degradation.
CRediT authorship contribution statement
Gaiming Zhao: Conceptualization, Data curation, Formal analysis, Funding acquisition, Resources. Jiali Zhang: Software, Writing – original draft, Writing – review & editing. Sen Wang: Formal analysis, Software, Supervision, Validation. Xiaoling Yu: Conceptualization, Formal analysis, Methodology, Resources. Qiuhui Zhang: Formal analysis, Resources, Supervision. Chaozhi Zhu: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (NO. 31701626). The authors acknowledge the cooperation and assistance received from the technicians and staff of Liuhe Corporation.
Ethics declaration
All experiments involving human sensory perception were conducted according to the protocol approved by the Research Ethics Committee of Henan Agricultural University (authorization No. IACUC-henau-20210830). Before conducting the sensory evaluation, written informed consent was obtained from all participants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101242.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Arendse E., Fawole O.A., Magwaza L.S., Nieuwoudt H., Opara U.L. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Scientia Horticulturae. 2018;232:240–249. doi: 10.1016/j.scienta.2018.01.022. [DOI] [Google Scholar]
- Arief I.I., Afiyah D.N., Wulandari Z., Budiman C. Physicochemical properties, fatty acid profiles, and sensory characteristics of fermented beef sausage by probiotics Lactobacillus plantarum IIA-2C12 or Lactobacillus acidophilus IIA-2B4. Journal of Food Science. 2016;81(10–12):2761–2769. doi: 10.1111/1750-3841.13509. [DOI] [PubMed] [Google Scholar]
- Azarbad M.H., Jeleń H. Determination of hexanal—an indicator of lipid oxidation by static headspace gas chromatography (SHS-GC) in fat-rich food matrices. Food Analytical Methods. 2015;8(7):1727–1733. doi: 10.1007/S12161-014-0043-0. [DOI] [Google Scholar]
- Bailey M.E., Shin-Lee S.Y., Dupuy H.P., Angelo A.J.S., Vercellotti J.R. In: Warmed-over Flavor of Meat. Angelo A.J.S., Bailey M.E., editors. Academic Press; 1987. Inhibition of warmed-over flavor by Maillard reaction products; pp. 237–266. [Google Scholar]
- Banaszak M., Kuźniacka J., Biesek J., Maiorano G., Adamski M. Meat quality traits and fatty acid composition of breast muscles from ducks fed with yellow lupin. Animal. 2020;14(9):1969–1975. doi: 10.1017/S1751731120000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumireddy S.R., Rocchetti G., Pallerla P., Lucini L., Sripadi P. A combined targeted/untargeted screening based on GC/MS to detect low-molecular-weight compounds in different milk samples of different species and as affected by processing. International Dairy Journal. 2021;118 doi: 10.1016/j.idairyj.2021.105045. [DOI] [Google Scholar]
- Du H., Chen W.L., Lei Y.T., Li F.C., Li H.M., Deng W., Jiang G.H. Discrimination of authenticity of Fritillariae Cirrhosae Bulbus based on terahertz spectroscopy and chemometric analysis. Microchemical Journal. 2021;168 doi: 10.1016/j.microc.2021.106440. [DOI] [Google Scholar]
- Feng Y., Cai Y., Fu X., Zheng L., Xiao Z., Zhao M. Comparison of aroma-active compounds in broiler broth and native chicken broth by aroma extract dilution analysis (AEDA), odor activity value (OAV) and omission experiment. Food Chemistry. 2018;265:274–280. doi: 10.1016/j.foodchem.2018.05.043. [DOI] [PubMed] [Google Scholar]
- Guo Q., Kong X., Hu C., Zhou B., Shen Q.W. Fatty Acid Content, Flavor compounds, and sensory quality of pork loin as affected by dietary supplementation with l-rginine and glutamic acid. Journal of Food Science. 2019;84(12):3445–3453. doi: 10.1111/1750-3841.14959. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li H., Huang T., Li F., Sun J. Lipolysis and lipid oxidation during processing of Chinese traditional smoke-cured bacon. Food Chemistry. 2014;149:31–39. doi: 10.1016/j.foodchem.2013.10.081. [DOI] [PubMed] [Google Scholar]
- Iglesias J., Medina I., Bianchi F., Careri M., Mangia A., Musci M. Study of the volatile compounds useful for the characterisation of fresh and frozen-thawed cultured gilthead sea bream fish by solid-phase microextraction gas chromatography–mass spectrometry. Food Chemistry. 2009;115(4):1473–1478. doi: 10.1016/j.foodchem.2009.01.076. [DOI] [Google Scholar]
- Jia S., Li Y., Zhuang S., Sun X., Zhang L., Shi J.…Luo Y. Biochemical changes induced by dominant bacteria in chill-stored silver carp (Hypophthalmichthys molitrix) and GC-IMS identification of volatile organic compounds. Food Microbiology. 2019;84 doi: 10.1016/j.fm.2019.103248. [DOI] [PubMed] [Google Scholar]
- Jin G., He L., Li C., Zhao Y., Chen C., Zhang Y.…Ma M. Effect of pulsed pressure-assisted brining on lipid oxidation and volatiles development in pork bacon during salting and drying-ripening. LWT. 2015;64(2):1099–1106. doi: 10.1016/j.lwt.2015.07.016. [DOI] [Google Scholar]
- Jose Beriain M., Teresa Murillo-Arbizu M., Insausti K., Victoria Sarries M., Gomez I. Raw-cured Spanish traditional meat product “chistorra de Navarra”: Sensory and composition quality standards. Foods. 2020;9(8) doi: 10.3390/foods9081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy S., Yoo J., Yun J., Kang H.B., Seol K.H., Ham J.S. 1H HRMAS-NMR based metabolic fingerprints for discrimination of cheeses based on sensory qualities. Saudi Journal of Biological Sciences. 2020;27(6):1446–1461. doi: 10.1016/j.sjbs.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Ali S., Abid M., Cao J., Jabbar S., Tume R.K., Zhou G. Improved duck meat quality by application of high pressure and heat: A study of water mobility and compartmentalization, protein denaturation and textural properties. Food Research International. 2014;62:926–933. doi: 10.1016/j.foodres.2014.04.006. [DOI] [Google Scholar]
- Liu C., Pan D., Ye Y., Cao J. H NMR and multivariate data analysis of the relationship between the age and quality of duck meat. Food Chemistry. 2013;141(2):1281–1286. doi: 10.1016/j.foodchem.2013.03.102. [DOI] [PubMed] [Google Scholar]
- Liu L., Zuo Z.T., Wang Y.Z., Xu F.R. A fast multi-source information fusion strategy based on FTIR spectroscopy for geographical authentication of wild Gentiana rigescens. Microchemical Journal. 2020;159 doi: 10.1016/j.microc.2020.105360. [DOI] [Google Scholar]
- Liu Y., Xu X.L., Zhou G.H. Changes in taste compounds of duck during processing. Food Chemistry. 2007;102(1):22–26. doi: 10.1016/j.foodchem.2006.03.034. [DOI] [Google Scholar]
- Liu Y., Xu X.L., Ouyang G.F., Zhou G.H. Changes in volatile compounds of traditional Chinese nanjing water-boiled salted duck during processing. Journal of Food Science. 2010;71(4):S371–S377. doi: 10.1111/j.1750-3841.2006.00020.x. [DOI] [Google Scholar]
- Mukojima K., Yoshii M., Tone A., Mabuchi R., Furuta A., Tanimoto S. Effect of storage after heating on odor of muscles of yellowtail (Seriola quinqueradiata) Bioscience Biotechnology and Biochemistry. 2022;86(7):902–915. doi: 10.1093/bbb/zbac059. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M.G., Byrne D.V., Jensen M.T., Andersen H.J., Vestergaard J. A comparison of warmed-over flavour in pork by sensory analysis, GC/MS and the electronic nose. Meat Science. 2003;65(3):1125–1138. doi: 10.1016/S0309-1740(02)00342-X. [DOI] [PubMed] [Google Scholar]
- Pearson A.M., Love J.D., Shorland F.B. “Warmed-over” flavor in meat, poultry, and fish. Advances in Food Research. 1977;23(08):1–74. doi: 10.1016/S0065-2628(08)60326-2. [DOI] [Google Scholar]
- Shi J., Nian Y., Da D., Xu X., Zhou G., Zhao D., Li C. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography–mass spectrometry and electronic nose. LWT. 2020;124 doi: 10.1016/j.lwt.2020.109182. [DOI] [Google Scholar]
- Soncin S., Chiesa L.M., Cantoni C., Biondi P.A. Preliminary study of the volatile fraction in the raw meat of pork, duck and goose. Journal of Food Composition and Analysis. 2007;20(5):436–439. doi: 10.1016/j.jfca.2006.09.001. [DOI] [Google Scholar]
- Song J., Shao Y., Yan Y., Li X., Peng J., Guo L. Characterization of volatile profiles of three colored quinoas based on GC-IMS and PCA. LWT. 2021;146 doi: 10.1016/j.lwt.2021.111292. [DOI] [Google Scholar]
- Tims M.J., Watts B.M. Protection of cooked meats with phosphates. Food Technology. 1958;12(5):240–243. [Google Scholar]
- Wall K.R., Kerth C.R., Miller R.K., Alvarado C. Grilling temperature effects on tenderness, juiciness, flavor and volatile aroma compounds of aged ribeye, strip loin, and top sirloin steaks. Meat Science. 2019;150:141–148. doi: 10.1016/j.meatsci.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Wang M.Z., Zhang J., Chen J., Jing B.Y., Zhang L.Y., Yu X.Z. Characterization of differences in flavor in virgin rapeseed oils by using gas chromatography-mass spectrometry, electronic nose, and sensory analysis. European Journal of Lipid Science and Technology. 2020;122(3) doi: 10.1002/ejlt.201900205. [DOI] [Google Scholar]
- Watanabe A., Kamada G., Imanari M., Shiba N., Yonai M., Muramoto T. Effect of aging on volatile compounds in cooked beef. Meat Science. 2015;107:12–19. doi: 10.1016/j.meatsci.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Wu N., Wang X.C. Identification of important odorants derived from phosphatidylethanolamine species in steamed male Eriocheir sinensis hepatopancreas in model systems. Food Chemistry. 2019;286:491–499. doi: 10.1016/j.foodchem.2019.01.201. [DOI] [PubMed] [Google Scholar]
- Wu T., Sheldon B. Flavor components and factors associated with the development of off-flavors in Cooked Turkey Rolls. Journal of Food Science. 2006;53:49–54. doi: 10.1111/j.1365-2621.1988.tb10176.x. [DOI] [Google Scholar]
- Xia C., He Y., Cheng S., He J., Pan D., Cao J., Sun Y. Free fatty acids responsible for characteristic aroma in various sauced-ducks. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128493. [DOI] [PubMed] [Google Scholar]
- Xie Q., Xu B., Xu Y., Yao Z., Zhu B., Li X., Sun Y. Effects of different thermal treatment temperatures on volatile flavour compounds of water-boiled salted duck after packaging. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112625. [DOI] [Google Scholar]
- Zhang Q., Ding Y., Gu S., Zhu S., Zhou X., Ding Y. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109339. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang S., Fan W., Duan M., Han Y., Li H. Identification of volatile compounds and odour activity values in quinoa porridge by gas chromatography-mass spectrometry. Journal of the Science of Food and Agriculture. 2019;99(8):3957–3966. doi: 10.1002/jsfa.9621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.