Abstract
Rheumatoid Arthritis (RA) is an autoimmune disorder that hinders the normal functioning of bones and joints and reduces the quality of human life. Every year, millions of people are diagnosed with RA worldwide, particularly among elderly individuals and women. Therefore, there is a global need to develop new biomaterials, medicines and therapeutic methods for treating RA. This will improve the Healthcare Access and Quality Index and also relieve administrative and financial burdens on healthcare service providers at a global scale. Hydrogels are soft and cross-linked polymeric materials that can store a chunk of fluids, drugs and biomolecules for hydration and therapeutic applications. Hydrogels are biocompatible and exhibit excellent mechanical properties, such as providing elastic cushions to articulating joints by mimicking the natural synovial fluid. Hence, hydrogels create a natural biological environment within the synovial cavity to reduce autoimmune reactions and friction. Hydrogels also lubricate the articulating joint surfaces to prevent degradation of synovial surfaces of bones and cartilage, thus exhibiting high potential for treating RA. This work reviews the progress in injectable and implantable hydrogels, synthesis methods, types of drugs, advantages and challenges. Additionally, it discusses the role of hydrogels in targeted drug delivery, mechanistic behaviour and tribological performance for RA treatment.
Keywords: Rheumatoid arthritis, Orthopeadic joints, Hydrogel, Therapeutics, Mechanical properties, Medicine
Graphical abstract
1. Introduction
Rheumatoid arthritis (RA) is a chronic disorder that damages the human joints due to an autoimmune abnormality [1,2]. The immune system starts destroying its cells, and due to immune auto-response, some cytokines are released, such as interleukins (IL 6, IL 7, etc.), leukotrienes, and other inflammatory mediators like tumor necrosis factors (TNF-α), which lead to the destruction of biological and physiochemical structures. The average incidence rate of RA is 0.5% to 1% for adults and between 5 and 50 new cases per 100,000 among elderly or female individuals are reported every year among certain populations [3].
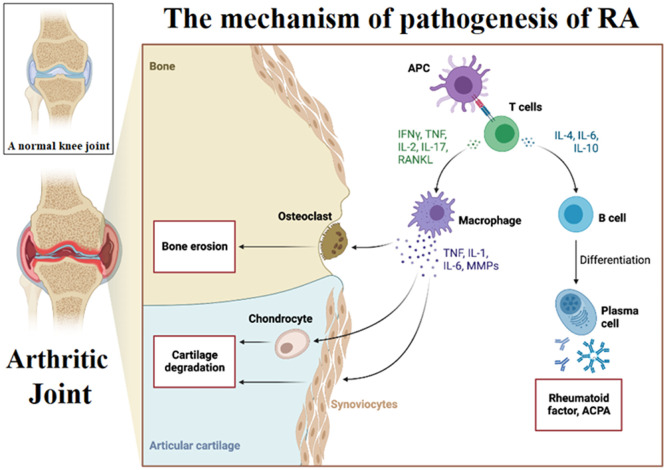
RA is characterized by continual synovitis, angiogenesis and immunological reactions which gradually damage joints, bones and cartilage. Fig. 1 presents a systematic illustration of the pathogenesis and associated inflammatory mechanisms in RA joint compared to a normal joint [4,5]. Autoantibodies can be detected within the affected joints and serum of patients to assess rheumatoid factors and citrullinated peptides [3,6]. Up to a 50% genetic risk factor is attributed to RA development [7], while smoking is another prominent risk that contributes to RA in well-developed countries [8]. This disease proportionally exists more in women groups than in men and elderly groups [3,9]. Active RA is often uncontrollable which makes patients suffer from painful chronic swelling, tissue damage, instability and deformity [10,11].
Fig. 1.
The mechanism of pathogenesis in a rheumatoid arthritis joint and the associated inflammatory changes compared to a healthy joint. The figure has been reproduced from a template of an open-source platform of “biorender”.
RA being a chronic inflammatory disorder usually affects the patient's synovial joints and bone tissue [12,13]. Immunological reactions in RA patients may lead to persistent synovitis, systemic inflammatory reactions, and the presence of autoantibodies in serum samples [14,15]. The RA treatment requires preventing discomfort and inflammation, recovering the synovial fluids [16,17], and slowing down the autoimmune-driven gradual damage in joints [18,19]. Hydrogels may exhibit mechanical biomimicry of synovial fluids to protect the joints [20] to sustain normal functioning of joints. In this context, the last decade has witnessed numerous advancements in biomedical materials and polymer technology, and multiple studies have demonstrated the potential application of biomedical hydrogels in RA treatment. Thus, this work reviews the progress made in the synthesis and performance of hydrogels being used for RA treatment in recent years.
1.1. Synergistic behaviours of synovial fluids and biomedical hydrogels
Biomedical hydrogels have demonstrated a good record of bio-mimicking the synovial fluids for mechanistic, therapeutics and lubrication purposes which are described below.
1.1.1. Mechanical behaviour of hydrogels for biomedical applications
Hydrogels have been continuously applied to fabricate lenses and drug delivery vehicles for medical purposes and tissue engineering [21,22]. Physically, hydrogels are soft, porous and elastic materials in which a polymer matrix encapsulates large amounts of water, drugs or other biological fluids without destroying the unique polymer matrix structure [23,24]. The hydrogel reaction mixture solidified into an elastic cushion-like structure after being injected into the synovial cavity of the synovial joints. The cushion-like structure is capable of absorbing mechanical stresses and presents high elastic recovery to restore its original structure from mechanistic strains and elastic deformations. The self-healing feature of hydrogels makes them suitable candidates for RA treatment [25,26] which are capable of protecting articulating bone surfaces from degradation during movements.
1.1.2. Biomimetic properties of hydrogels
Hydrogels are produced by hydrophilic polymers forming a three-dimensional network with crosslinking structures. Biomolecules and drugs can be incorporated into a this network for therapy or biochemical analysis [27,28]. In addition, the biocompatibility of hydrogels can be controlled by the permeability of oxygen, nutrients and other water-soluble metabolites, which promotes the growth, restoration and regeneration of damaged tissues. Hydrogels also possess free radical scavenging properties that are necessary for biomedical applications in treating various diseases including RA [29], [30], [31].
1.1.3. Biotribology and lubrication performance of hydrogels for arthritic joints
Biotribology investigates the friction and degradation of articulating joint surfaces due to the frictional effect subject to load/weight. The wear of joint materials is subject-specific and appears as a critical factor for load-carrying joints such as hip and knee joints. Friction increases and joint degradation accelerates when joint surfaces articulate under dry conditions i.e., when synovial fluid dimmishes in a synovial cavity. In this case, the role of hydrogel role becomes significant as it can effectively decrease the friction between the articulating surfaces.
Since hydrogels hold a large amount of fluids, they are tailored for a controlled-release mechanism and jelly-like lubricant for articulating joints [32], reducing the friction and degradation between the surfaces of moving bones and cartilage [33,34]. Furthermore, the hydrogels exhibit intrinsic properties such as swelling, de-swelling, re-swelling and self-healing, which make them usable for an extended period to protect the joints.
1.2. Application of biomedical hydrogels for RA treament
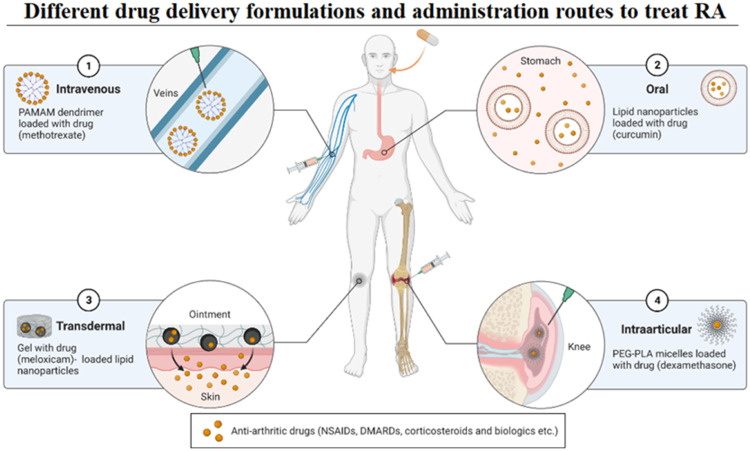
The medicated hydrogels are reported to exhibit a stimuli-responsive, temperature-sensitive, site-specific and pH-responsive controlled release of antirheumatic drugs to the joints [35]. Therefore, they can be directly applied to the target joints to relieve pain, limit inflammation, mitigate surface deterioration, and cushion the joints to sustain normal joint functioning [36,37]. Injectable hydrogel with intra-articular injection has been introduced to advance the distribution of drug loads to address the aforementioned challenges [38,39]. Hence, sustained release and precise permeability of therapeutic drugs to the target site becomes more advantageous than rapid exposure to free drugs which may trigger adverse effects [40]. Fig. 2 illustrates the therapeutic mechanism of this methodology [41]. Based on available literature reports, it is conceived that biomedical hydrogels are a high-potential candidate for the therapeutic needs of RA patients [42,43]. Therefore, the following sections discuss formulations of medicated hydrogels for potential use in RA treatment in detail. The mechanical and pharmacological manifestation of hydrogels, as well as their pros and cons, are elaborated for their use in RA treatment.
Fig. 2.
Illustration of different formulations to treat RA. The delivery carriers can transport a variety of drugs to treat RA, such as DMARDs (MTX), natural drugs (curcumin), NSAIDs (meloxicam), steroidal drugs (dexamethasone), etc. Among different formulations, hydrogels effectively provide a cushion between articulating joints to alleviate cartilage damage through immunomodulation. Hydrogels decrease friction, swellings, inflammations and pain, promoting bone/cartilage repair, recovery and regeneration. Moreover, hydrogels may carry nanoparticles, drugs, biomolecules and therapeutic modalities for targeted and prolonged delivery to the articulating joints. The figure has been reproduced from a template of an open-source platform of “biorender”.
1.3. Intra-articular injections of drug-loaded hydrogels
The intra-articular injection is the localized administration of the hydrogel reaction mixture into the soft tissues around a joint, cartilage and synovial cavity. This technique has certain advantages over the direct administration of free drugs into the bloodstream. Therefore, this targeted approach can avoid high-risk systemic toxicity of antirheumatic and anti-arthritic drugs, or any negative impact on biocompatibility, distribution, and pharmacokinetic profiles, due to poor systemic stability caused by factors like metabolism or excretion. Moreover, intra-articular injections bypass the physiological barriers such as hard tissues which hinder the accessibility of drug molecules to the synovial cavity at the arthritic site. However, the intra-articular injection technique is still struggling against certain challenges such as swelling, allergic reactions to drugs or tissue damage after localized and prolonged drug retention. It is expected that the ongoing research and technological advancements will overcome the challenges of prolonged drug-release rates. Overall intra-articular injections prove advantageous for minimal invasive surgery and protection of critical body organs against toxic drugs used for RA treatment.
2. Overview of methodologies for RA treatment
2.1. Current practices for RA treatment
The current options for RA treatment predominantly rely on medications, which primarily consist of painkillers or occasionally steroidal drugs for adverse cases [44]. These medications include but are not limited to analgesics e.g., non-steroidal anti-inflammatory drugs (NSAIDs) such as piroxicam, ibuprofen, and naproxen. [45]. They can also be immune-suppressants and disease-modifying antirheumatic drugs (DMARDs) such as methotrexate (MTX), glucocorticoids/steroids like prednisolone, antioxidants and other immuno-modifiers [46].
2.2. Anti-inflammatory drugs for RA treatment
Pain-relieving medications can be categorized into three types. The first type is NSAIDs. The second type is called steroidal drugs such as corticosteroids and glucocorticoids which also possess anti-inflammatory activity. The third type is monoclonal antibodies that can target immune cells to block the synthesis of immune modulators, preventing the body's immune system from damaging its cells to treat autoimmune diseases like RA.
2.2.1. NSAIDs for RA treatment
NSAIDs are known as pain-relieving medications such as piroxicam, naproxen, ketoprofen and aceclofenac. These drugs inhibit the cyclooxygenase (Cox) enzyme to limit the synthesis of inflammatory eicosanoids. Thus, the conversion of arachidonic acid into prostaglandins, thromboxane and prostacyclin is blocked by NSAIDs. The inflammation of the joints can be relieved by inhibiting pain mediators. Thus, NSAIDs are effective in relieving pain and alleviating joint inflammation, redness, swelling and fever, and they also can slow down the progression of RA symptoms and preserve the structure and function of the joints.
2.2.2. Steroids-based anti-inflammatory drugs for RA treatment
The oral administration of steroids, including both corticosteroids and glucocorticoids, can suppress the immune system. Therefore, corticosteroids such as prednisolone and prednisone can quickly relieve inflammation, stiffness, tenderness and pain caused by RA within minutes which lasts up to a few hours. However, the intake of artificial steroids decreases the release of natural steroids produced by the body, and doses may need to be increased over time to achieve the same level of therapeutic response. Prolonged intake of steroids may have many adverse effects on protein and fat metabolism in the body including adverse effects on mineralocorticoids, salt-ion balance and homeostasis, leading to bone desorption. Thus, prolonged use or higher doses of steroids are not advised in medical practice. Most of the time, physicians prescribe low doses for a maximum of 2–3 months. If a high dose of prednisone is required, then the dosage should be changed from 10 mg once per day to 5 mg twice per day.
2.2.3. DMARDs for RA treatment
DMARDs are a diverse group of drugs with many sub-types available in the market to treat RA. MTX, also known as a cytotoxic drug and immunosuppressant, is one type of DMARD used to treat RA. Other types may include drugs that are not cytotoxic but still inhibit the immune system through various mechanisms. The newest class of DMARDs recently approved by the FDA is known as Janus kinase inhibitors, which can inhibit JAK 1, 2, 3, and even TYK2 to disrupt the intracellular pathway of JAK-STAT. The inhibition of the JAK/STAT pathway will block the release of inflammatory cytokines to decrease inflammation. Other types of DMARDs include monoclonal antibodies inhibiting interleukins (IL-6 and IL-7) like tocilizumab and sarilumab, as well as inhibitors of tumor necrosis factor (TNF) such as certolizumab and golimumab, etc.
2.3. Surgical interventions for RA treatment
In addition to drugs, surgery is another ongoing treatment practice. However, it is considered as the ultimate solution according to the latest clinical guidelines [47]. The associated risk includes a higher chance of trauma and subject-specific suitability of the patient to withstand repeated surgical procedures conducted within a short period [48]. Nevertheless, post-surgical interventions still require the use of drugs for RA therapy, that will aggravate the patient's condition, complicate healing, and may bring tremendous adverse effects [49].
2.4. Major challenges in RA treatment
Surgery is not feasible for most patients due to subject-specific medical profiles, while none of the aforementioned drugs used for RA treatment adequately address the treatment specifications and requirements [50,51]. The major drawback includes inadequate therapeutic effects, which can not be achieved completely and permanently by using oral drugs due to poor performance and lack of targeted dosage to intended sites [52]. Furthermore, many antirheumatic drugs have lower accessibility at drug absorption sites and have high clearance and metabolic rates including the first-pass effect which refers to the metabolism of drugs by the liver [53,54]. Some drugs bind with the plasma proteins such as albumin, which slows down the drug metabolism and increases their half-life and toxicity to the non-targeted tissues [55,56]. Non-specific pharmacokinetic biodistribution and toxic metabolites may sometimes aggravate drug-disease, drug-food and drug-drug interactions [38,57,58]. Furthermore, inadequate elimination or excretion of antirheumatic drugs leads to frequent complications in patients [37,59]. Therefore, antirheumatic agents raise noticeable and adverse incidents due to such reasons. Hence, the major approach to address these unsatisfactory outcomes is achieving specific distribution of antirheumatic drugs to the targeted sites [60,61].
3. Hydrogel for RA treatment
3.1. Scope of hydrogels for RA treatment
As discussed above, painful surgical interventions [63] and antirheumatic drugs pose adverse effects [62], have slower recovery, and may encounter super-infections by resistant microbes [64]. Recently, the research and development scope has focused on formulating safe medication target approaches for RA treatment [65]. The hydrogel can be directly injected into intended sites (joints) or targeted after implantation [66,67]. Thus, intra-articular implantation of the hydrogels (or post-injection) can provide a mechanically elastic, sustainable and biocompatible cushion for the joints [68,69]. It may protect against damage or mechanical fragmentation of cartilage or bone layers and joint surfaces [70]. The hydrogel-protected joints are expected to undergo osteogenesis and re-mineralization of joints with a possibility of recovery from RA [1,71]. Therefore, this work also covers the synthesis of hydrogels and corresponding applications for RA treatment. The principle behind the combination of hydrogel with medications is also described.
3.2. Requirements of biomedical hydrogels for RA treatment
There are some general properties required by hydrogel for their successful application in RA treatment [72]. Firstly, the hydrogel scaffold intended for RA treatment needs to be highly biocompatible and immuno-modulatory, and should not initiate hypersensitivity or allergic reactions, which is essential and fundamental for RA treatment [73]. The second requirement of hydrogel is to possess excellent mechanical properties within the articular joints, including porosity, self-healing, elastic recovery and biodegradation [74] by design. The third requirement is suitable tribological performance which provides sufficient lubrication to minimize frictional effect, material degradation caused by wear, and effective heat transfer [75] at a local scale. Finally, the fourth important requirement of biomedical hydrogel is the capability to control and sustainably release the loaded drugs [76]. Recent studies show that there are two main types of hydrogels suitable for RA treatment, including intra-articular injection hydrogel and transdermal hydrogel [77], [78], [79]. Injectable hydrogel shows excellent performance in substance delivery, which needs to be degradable for drug-release purposes [80].
3.3. Development of medicated hydrogels for biomedical purposes
The injectable hydrogels can be crosslinked for gelation within the target site after injection [81]. Light can penetrate target sites like intra-articular joint cavities, and initiate the conversion of a liquid monomer or macromer by the cross-linking reaction to form a hydrogel through free radical polymerization, even under controlled ambient or physiological conditions [77]. Nevertheless, photopolymerization for in vivo implementations is challenging [75]. However, it has limited applicability in certain disease conditions and narrow ranges of body temperatures where pH may also vary at the site of infection [42]. The treatment goal further involves avoiding toxic materials like organic solvents and most monomers at the injection site [82].
3.4. Drug loading in hydrogels for RA treatment
Various types of injectable hydrogels, as well as transdermal hydrogels, have been reported for delivering NSAIDs, steroidal anti-inflammatory drugs and DMARDs. Examples are summarized in Table 1. The corresponding mechanisms of steroidal, non-steroidal drugs and DMARDs to relieve RA symptoms have been explained in earlier Sections 2.2 and 2.3.
Table 1.
A summary of drug-loaded hydrogel scaffolds for rheumatoid arthritis treatment.
| Drug name | Technique and method | Advantages | Ref |
|---|---|---|---|
| Injectable hydrogels loaded with DMARDs | |||
| MTX | MTX-loaded HA-PLGA-DOTA complex nanoparticle-incorporated hydrogels were synthesized. | A bi-modal MTX treatment mechanism was achieved using Lutetium177. | [112] |
| Iguratimod | Hyaluronic acid (HA) was loaded with the biodegradable polyvinyl alcohol micelles encapsulating IGUR and was gelated in situ after injection. | The hydrogel provided sustained control of drug release with excellent mechanical properties. | [74] |
| MTX | A click-chemistry reaction to cross-link novel tetrazine-modified HA and trans-cyclooctene-modified HA. | An excellent drug-release profile was achieved. | [113] |
| MTX | The gelation was carried out in situ, and it contained AuNPs. | Cytocompatibility was enhanced with improved mechanical properties of the hydrogel achieving controllable drug release. | [114] |
| MTX | They integrated black phosphorus nanosheet material with a thermosensitive chitosan hydrogel rich in platelet plasma. | The hydrogel exhibited thermosensitive properties and precision for controlled drug release. | [75] |
| MTX | A chitosan-polyolefin was complexed with the Fe3O4 nanocomposite and was loaded with MXT and growth factor β1 to attain a multifunctional hydrogel. | The mechanical as well as anti-inflammatory properties were excellent, and it promoted the regeneration of cartilage. | [115] |
| MTX | A self-assembled supramolecular hydrogel was synthesized using GDFDFDY. | The synoviocytes exhibited controlled migration and proliferation using this hydrogel scaffold showing its potential to treat RA. | [65] |
| MTX | The polyelectrolyte complexes were synthesized by combining oligo-chitosan and hypromellose phthalates. | This product provided precise control over drug release with thermosensitive properties. | [103] |
| Transdermal hydrogels loaded with DMARDs | |||
| MTX | The PCL- PEG-PCL triblock copolymers were synthesized with MTX-loaded nano-micelles. | The hydrogels were loaded with nano-micelles releasing the MTX drug in a controlled manner across the skin layers. | [108] |
| MTX | An MTX-loaded reservoir patch was linked to the hydrogel-based formation of microneedle arrays (HFMN). | The hydrogels released the MTX drug in a controlled manner across the barrier layers of the skin. | [107] |
| MTX | Mesoporous-silica NPs-laden eutectic hydrogel. | Excellent transdermal therapeutic effects. | [60] |
| Injectable hydrogels loaded with various drug combinations (DMARDs/NSAIDs/siRNA) | |||
| IND and MTX (and siRNA) | A nanoparticles-laden hydrogel delivering MTX and Indomethacin (IND) with or without siRNA. | Thermosensitive and controlled drug release. | [116,38] |
| Injectable hydrogels loaded with monoclonal antibodies-based anti-inflammatory drugs | |||
| Infliximab | Combining thermo-responsive hydrogel with microparticles by combining the pluronic F127/HA with poly (γ-glutamic acid) (PGA). | Inhibited inflammatory cytokines (IL-6, IL-7, IL-1β) and TNF-α, reduced auto-immune damage to joints and enhanced repairing. | [76] |
| Injectable hydrogels loaded with NSAIDs | |||
| Ketoprofen | Anti-inflammatory action for RA. | Sustained release of ketoprofen. | [117] |
| Transdermal hydrogels loaded with NSAIDs | |||
| Ibuprofen | The NPs made up of Eudragit L100 polymer were loaded with ibuprofen and were complexed with the hydrogel matrix. | The drug was controlled release and crossed the skin barrier. | [118] |
| Aceclofenac | A NSAID containing transdermal hydrogels. | Sustained release of the drug was achieved. The drug was delivered across skin layers towards the target site. | [119,120] |
4. Hydrogel synthesis through photopolymerization
Photopolymerization is an established technique to overcome treatment obstacles, as it offers mild polymerization which is safe for cells and tissues [75,83]. The process involves the use of photosensitizers for photo-initiations to start crosslinking reactions that are capable of being activated at lower light intensities [77,84]. This technique possesses a short irradiation time, physiological temperature, and lower levels of organic solvents, ultimately providing a viable option to develop polymeric hydrogels within in vivo environments [24].
4.1. Development of photo-crosslinked polymerized hydrogels
Photopolymerization produces mechanically elastic and crosslinked structures of the polymers [85]. A crosslinked polymer matrix is essential for obtaining the required mechanical elasticity for diverse applications [24]. The photopolymerization technique has been extensively demonstrated in different fields, such as material production, microfluidics, membrane design, surface modifications and coatings for biomedical engineering [86]. A key feature of this technology is its ability to control polymerization in terms of space and time. This technique exhibits high curing rates at a range of temperatures without excessive heat generation, and also the reaction time is significantly quick which spans from a few seconds to several minutes [87].
4.2. Methods to produce biomedical hydrogel scaffolds for RA treament
Since photopolymerization is the most reported method to synthesize hydrogel, biomedical hydrogels are designed to undergo photopolymerization in both in vitro and in vivo environments, facilitated by photo-initiators using ultraviolet (UV), visible light or relatively safe low-power infrared (IR) light which involves numerous reactions and pathways. Hence, they have higher suitability to be tailored for biomedical applications including RA treatment [88]. The development of targetable technologies for RA treatment involves injecting a mixture of reactants into the targeted area of the body including affected RA joints. The short irradiation of light initiates cross-linking reactions and transforms the reaction mixture into a hydrogel. The properties and compositions of hydrogel matrix can be tuned in situ to form intended shapes and structures, and to induce desired mechanical properties [89]. This swift and manipulatable photopolymerization process has made it a valuable technique for various biomedical applications, such as preventing thrombosis, reducing post-operative trauma, targeted/sustained drug delivery, biosensor coatings and cell transplantation [90].
4.3. Role of photo-initiators for free radical photopolymerization to develop injectable hydrogels
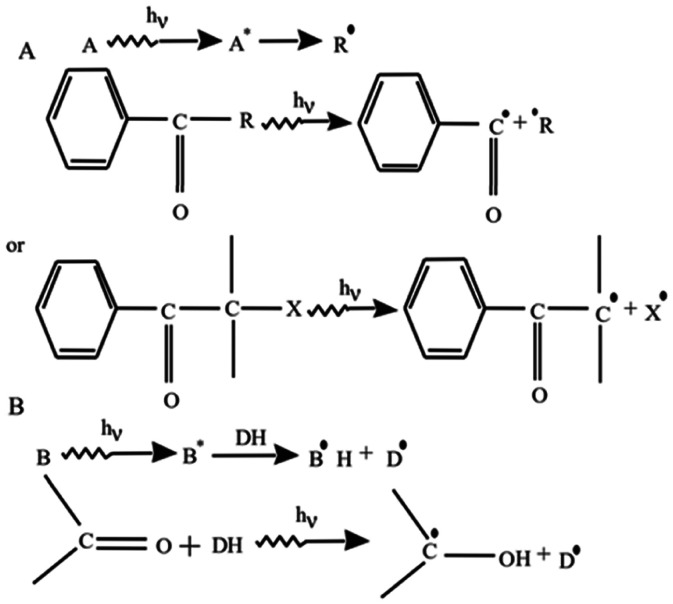
Various aromatic carbonyl compounds are used as photo-initiators which can be photocleaved with UV irradiation to form free radicals as illustrated in Fig. 3A [24]. Thus, photocleavage promotes the polymerization of a variety of aromatic compounds including benzoin derivatives, benzil ketals, benzil dimethyl ketals, hydroxy‑alkyl-phenones, acetophenones, etc. [24,91,92]. Acetophenone derivatives such as 2,2-dimethoxy-2-phenyl acetophenone with acrylic groups work efficiently as a photo-initiator. This type of photo-initiator is used to formulate hydrogels derived from acrylate polyethylene glycol (PEG) derivatives [93], [94], [95]. Another type of photo-initiator acts as a proton (H) donor molecule. It is oxidized after photo-cleavage by eliminating hydrogen and promoting a reduction reaction as illustrated in Fig. 3B [24]. These photo-initiators not only act as H-donors but may also contain aromatic ketones to form ketyl radicals. After the initial reaction of the proton release, ketyl-radicals undergo a coupling reaction to form a growing macromolecular chain.
Fig. 3.
Schematic of a free radical generation using photo-initiators to promote photopolymerizations. (A) A photo-initiator undergoes photocleavage to form free radicals after being exposed to UV light to promote the photopolymerization reaction. (B) Some photo-initiators generate free radicals by eliminating hydrogen (proton) from the H-donor to promote photopolymerization. (Reproduced with permission from [24]. Copyright 2002 Elsevier).
4.4. Photopolymerization advantages in developing hydrogels for treatment
Polymer cross-linking and hydrogel fabrication for in situ treatment purposes is an aspiration for biomedical applications [96]. This technique enables the formation of intricate shapes of hydrogel scaffolds that have good compliance with tissue structures and well adhere to them, while providing great versatility and functionality [97]. The matrix composed of crosslinked polymers entraps water and drugs within its cavities to form flexible biomedical hydrogels [91]. The cross-linking reaction is carried out and the free radical formations are initiated using photo-responsive compounds that are sensitive to UV, visible or IR light [98]. A few researchers have safely carried out reactions in situ (and in vivo) from natural and aqueous precursors. Photopolymerization is minimally invasive and offers major advantages for hydrogel applications [99]. For instance, the use of hydrogel also showed noteworthy benefits when made with laparoscopy techniques, catheterization or subcutaneous injections with or without the aid of transdermal illumination [89].
4.5. Biomimicry of hydrogels to natural synovial fluid
This section highlights the biomimicry of hydrogels to the natural synovial fluid based on self-healing and mechanistic behaviours.
4.5.1. Self-healing properties
The self-healing characteristics of hydrogels are crucial to withstand contact stresses and frictional effects between the articulating bones and cartilage surfaces. The self-healing property of hydrogel has been improved lately by using light-sensitive compounds such as irgacure that can enhance the photopolymerization of host-guest complexes of beta-cyclodextrin derivatives as a host molecule and adamantane derivatives as guest polymolecules to form a highly crosslinked hydrogel.
4.5.2. Mechanistic behaviours
Mechanical toughness, resilience, elasticity, elastic recovery and fatigue strength are important criteria for hydrogels to withstand stress and strain distributions at the interface of articulating bone surfaces. Lubrication is another essential element for establishing biomimicry in the synovial fluids as discussed in Section 1.3.
5. Functionalization of hydrogels for RA treatment
Hydrogel scaffolds can be used as drug delivery vehicles and also to carry bifunctional molecules to develop a variety of approaches for RA treatment [20,100]. Some recent approaches are described in this section.
5.1. Hydrogel functionalization to deliver DMARDs
DMARDs are the recommended medications to treat RA [56]. MTX is the most frequently used DMARDs due to its outstanding performance in improving the functionality of joints [101]. However, RA patients often overdose on MTX in their daily intake, taking 15–25 mg per week which is significantly higher than the official amount of 10 mg per week [102,103]. The treatment even continues for four to eight weeks or longer, reaching and surpassing the maximum tolerable dosage [102]. MTX is highly toxic to normal body cells if administered orally (or parentally) and is likely to raise biocompatibility concerns such as poor solubility, short plasma half-life and less permeability [104]. Moreover, MTX is an immunosuppressant that can slow down RA auto-immune damage, and at the same time weaken the body's immune system against major pathogens such as bacteria and viruses. To solve these problems, targeted localized delivery of hydrogels can overcome MTX toxicity and even improve its low biocompatibility after oral administration [105,106]. The hydrogels loaded with DMARDs (MTX) are expected to slow down RA, restore joint functioning, and reduce joint damage [107]. These medicated hydrogels can effectively reduce joint swelling, friction, pain and acute phases of inflammatory reactions [108].
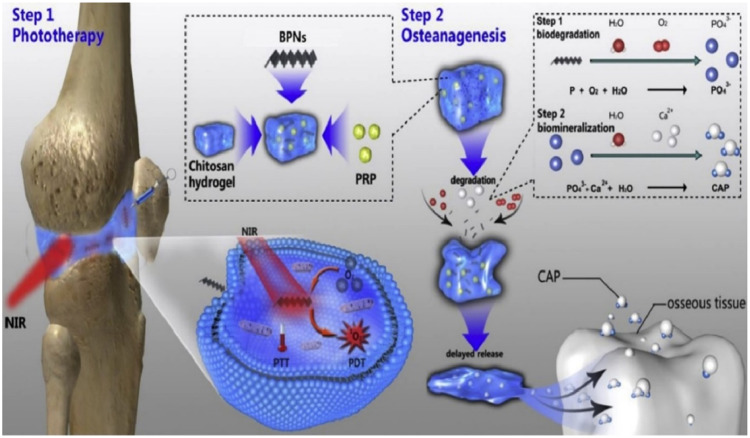
5.2. Types of injectable thermo-responsive hydrogel scaffolds
Injectable hydrogels play a significant role by injecting liquid reactants into joints that are crosslinked in situ under IR irradiation as illustrated in Fig. 4. Therefore, a novel thermo-responsive hydrogel is reported by researchers that integrate platelet-rich plasma (PRP) to chitosan and black phosphorus nanosheets (BPNs) and have demonstrated encouraging outcomes to treat RA and osteogenesis [75]. This thermos-sensitive as well as photo-activable hydrogel not only holds the potential to reduce inflammation but also promote osteogenesis within the joints.
Fig. 4.
Schematic of injectable PRP-chitosan hydrogels as a thermo-responsive scaffold biomaterial composite with BPNs as a photo-eradiation approach for RA therapy. (Reproduced from [75] Copyright 2020 Elsevier Ltd.).
The application of BPNs within the hydrogel generates localized heat when exposed to IR and releases reactive oxygen species (ROS) within the inflamed parts. It helps to eliminate the hyperplastic synovial tissue in the joint [75]. Moreover, the chitosan hydrogel is improved by adding PRP which enhances mesenchymal stem cell capacity and adhesion. In an experiment conducted with mice, the joint surface treated with this hydrogel was smooth and showed excellent lubrication, which reduced joint surface damage [75]. This thermo-responsive hydrogel has demonstrated the unique ability of drug release to promote the adhesion of mesenchymal stem cells. Thus, the BPNs/Chitosan/PRP thermo-responsive hydrogel has shown significant potential for RA treatment through anti-inflammatory and osteogenesis mechanisms [75].
5.3. Novel in vivo polymerized AuNPs-based composite hydrogel
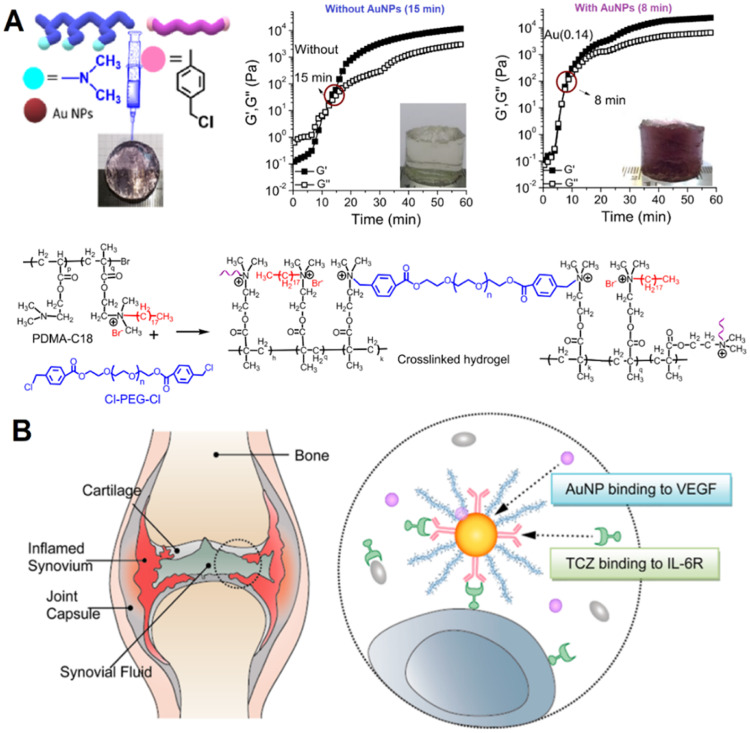
An in vivo study has reported a novel nanocomposite of gold nanoparticles (AuNPs) with hydrogel containing MTX drug as illustrated in Fig. 5A [109]. After administration into the joint, it could rapidly crosslinked into the gel at the targeted location within the body and could be injected into the intra-articular cavity of joints. Besides, this hydrogel was quickly produced within 8 min and exhibited enhanced elastic modulus, cytocompatibility and cell adhesion. Whereas, the hydrogels without AuNPs took a longer time of 15 min to solidify [109]. The superior mechanical and biological properties of this hydrogel were also attributed to the incorporation of AuNPs. Another similar study has investigated the composite of hyaluronate with or without the incorporation of AuNPs and tocilizumab [110]. It was observed that the efficacy of treating RA was improved with AuNPs incorporation when compared with the standard formulation of the drug (without AuNPs) as shown in Fig. 5B [110].
Fig. 5.
Gold nanoparticles incorporated formulations as a potential rheumatoid arthritis treatment (A) Hydrogel with AuNPs has showen better cross-linking, mechanical properties, and sustained release of MTX. (Reproduced from [109]. Copyright 2020 American Chemical Society). (B) Hyaluronate-AuNPs-toclizumab complex exhibited better efficacy in treating rheumatoid arthritis than without incorporating AuNPs. (Reproduced from [110]. Copyright 2014 American Chemical Society).
5.4. Self-assembled injectable hydrogel for RA treatment
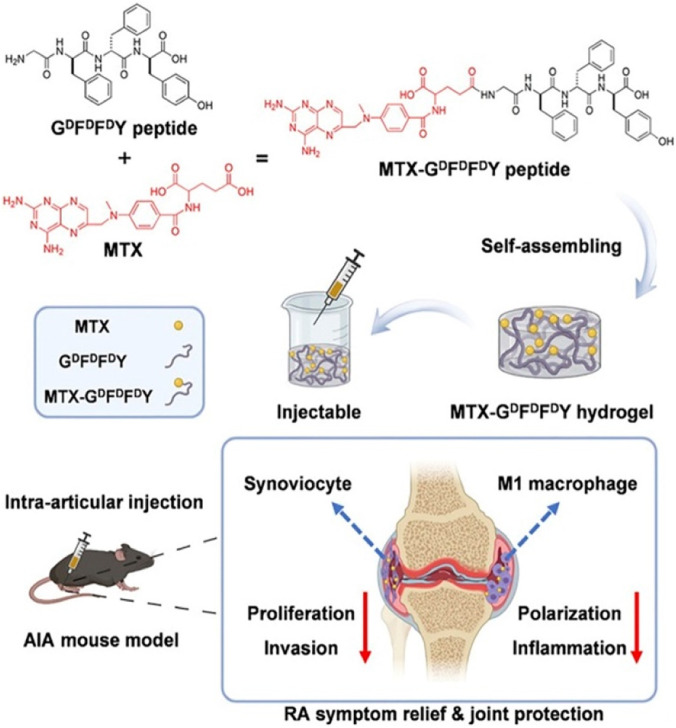
A group of scientists have explored a self-assembling mechanism for drug delivery through hydrogel with GDFDFDY peptide and MTX [65]. A conjugation was developed due to the interaction between carboxyl groups within MTX and the aminated glycine in peptides as illustrated in Fig. 6 [65]. The peptides were stuck through π-π interactions and successfully produced an injectable hydrogel upon intra-articular injection. The hydrogels released MTX at a consistent rate for a prolonged duration for passive biodegradation after administration. After the MTX-GDFDFDY peptide was administered via intra-articular injection, it formed a gel in the knee joints of adjuvant-induced arthritis mice (AIA mice). That hydrogel inhibited proliferation, synoviocyte invasion and polarization of pro-inflammatory M1-type macrophages. It led to an effective therapy for RA by reducing joint inflammation and degradation. Also, the performed experiments turned out to give excellent results in targeted drug delivery. It selectively increases the MTX toxicity for RA synoviocytes only while decreasing its toxicity to other normal cells to minimize the adverse effects on the body. This injectable hydrogel significantly alleviated joint swelling and fever in adjuvant-induced arthritis during mice experiments. Hence, this study successfully established the potential of MTX- GDFDFDY hydrogels as a novel drug delivery system for RA treatment.
Fig. 6.
Illustration of the synthesis and administration of injectable hydrogels composed of GDFDFDY peptide and MTX for rheumatoid arthritis treatment. (Reproduced from [65]. Copyright 2022 The Authors. Published by Elsevier Ltd).
6. Discussion and summary
6.1. Advantages of photopolymerized injectable hydrogels over implantable hydrogels
The application of injectable hydrogel for RA treatment can benefit patients with subject-specific compliance or adherence toward treatment and improved therapeutic outcomes. The drug effects are boosted by their specific distribution to inflammatory parts. A precise and targeted drug delivery to the desired site not only protects drug diffusion to the bloodstream but also the core body organs such as the kidney and liver from damage caused by the toxic metabolites of drugs. Furthermore, the human body can regulate the degradation of the injected hydrogel scaffolds according to the constituents and physiological situation within the diseased tissue. A properly cross-linked hydrogel can be converted by the body tissues into less toxic or even non-toxic intermediates, which are then eliminated from the body in a prolonged and biocompatible manner. Additionally, due to their softness and elasticity, hydrogel acts as an excellent mechanical cushion for the mechanical support of the joints and bone articulating surfaces, reducing friction between the internal lining of the joints, and protecting the joints comprehensively.
6.2. Disadvantages of photopolymerized injectable hydrogels over implantable hydrogels
Although hydrogel injection can provide several advantages to patients, some challenges still need to be addressed if photo-initiations and the cross-linking reaction are not executed properly. In this situation, the in vivo polymerization reactions may remain incomplete due to undesired synthesis, and the produced hydrogels could be mechanically unstable due to poor crosslinking or improper chemical reactions. This will influence the biological environments and biomolecules of the target organs as well. Also, ineffective crosslinking and chemical reactions may lead to systemic exposure to toxic organic compounds. A mechanically loose network of polymers may lead to rapid cytotoxic drug release to the unintended tissues or even the targeted tissue, potentially resulting in serious damage to core body organs. Hypersensitive reactions at the administration sites may also occur, further aggravating the biocompatibility issues. To overcome these drawbacks, highly cross-linked and biomimetic hydrogels with self-healing and mechanically elastic properties have been introduced recently, especially those made from host-guest type of inclusion complexes derived from freshly synthesized derivatives of β-cyclodextrin and adamantane. These hydrogels can withstand outstanding numbers of stress-strain cycles and mechanical stresses and can hold a certain amount of water, drugs and biomolecules against articulating bone/cartilage surfaces during their use for RA treatment.
7. Conclusion
Conclusively, RA is a chronic autoimmune disease in which autoantibodies are formed which continuously cause synovitis and systematic inflammation. The current treatment primarily involves drug therapy, which carries a risk of toxicity, adverse effects and complications due to uncontrolled and generic distribution of drugs. This work has reviewed different approaches for synthesizing and modifying hydrogels for biomedical applications along with targeted drug delivery to alleviate RA. Moreover, the advantages and challenges of applying hydrogels to treat RA are discussed. Hydrogel is a promising drug delivery system for treating RA because it has increased precision in drug delivery, drug distribution and release rates, and also reduced adverse effects on patients. The essential properties required by hydrogels for effective RA treatment include suitable self-healing, biodegradability design, and excellent tribological properties. Moreover, excellent mechanical properties and targeted biocompatibility are also crucial parameters. Based on recent reports, studies have shown that injectable hydrogels and transdermal hydrogels are two major types of hydrogels that are suitable for RA treatment [111]. The injectable hydrogel can deliver drugs such as MTX as well as platelet-rich plasma. The hydrogel delivery systems offer numerous benefits, including sustained drug effects and mechanical cushioning for joints. However, improper administration of hydrogels may also lead to the mechanical instability of hydrogels and hypersensitive reactions at administration sites. Overall, hydrogels have the great potential to revolutionize the treatment of RA by providing more targeted, safeand effective drug delivery systems.
Conflicts of interest
No competing interests including any financial or personal nature.
Acknowledgments
Authors' contributions
Mirza Muhammad Faran Ashraf Baig: Major re-writing, Conceptualization, Editing, review, and grammar revision. Lee Ki Wong: Initial draft writing, Investigation, Validation. Abdul Wasy Zia: Grammar, Editing, Revision. Hongkai Wu: Supervision, Review, Editing.
Acknowledgement
The work was supported by grant # SZ-SZSTI2010 by the Shenzhen Science and Technology Innovation Committee (SZSTI), Guang Dong Basic and Applied Basic Research Foundation (2022B1515130010), Hong Kong Research Grant Council (RGC) funding projects (GRF#16308818, GRF#16309920, and GRF#16309421), and Hong Kong Innovation and Technology Commission (HKITC) funding project (MHP/003/19).
Contributor Information
Mirza Muhammad Faran Ashraf Baig, Email: faran@ust.hk.
Hongkai Wu, Email: chhkwu@ust.hk.
References
- 1.Chen H., Tian B., Fang X., Bai J., Ma Q., Zhang Y., et al. Injectable erythrocyte gel loaded with bulleyaconitine A for the treatment of rheumatoid arthritis. ACS Biomater Sci Eng. 2021;7:5706–5716. doi: 10.1021/acsbiomaterials.1c01175. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y.Y., Li X.F., Wu S., Niu X.N., Yin S.Q., Huang C., et al. Role of the S100Protein family in rheumatoid arthritis. Arthritis Res Ther. 2022;24:35. doi: 10.1186/s13075-022-02727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott D.L., Wolfe F., Huizinga T.W.J. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 4.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 5.Smolen J.S., Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 6.Auréal M., Machuca-Gayet I., Coury F. Rheumatoid arthritis in the view of osteoimmunology. Biomolecules. 2021;11:1–18. doi: 10.3390/biom11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padyukov L. Genetics of rheumatoid arthritis. Semin immunopathol. 2022;44:47–62. doi: 10.1007/s00281-022-00912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullock J., Rizvi S.A.A., Saleh A.M., Ahmed S.S., Do D.P., Ansari R.A., et al. Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract. 2019;27:501–507. doi: 10.1159/000493390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaiser F., Ibrahim M., Mazhar R., Sohail F. Nanotechnology: a new hope for the cure of osteoarthritis, osteoporosis and rheumatoid arthritis. Glob Pharm Sci Rev. 2020;v-i:25–39. [Google Scholar]
- 10.Alivernini S., Firestein G.S., McInnes I.B. The pathogenesis of rheumatoid arthritis. Immunity. 2022;55:2255–2270. doi: 10.1016/j.immuni.2022.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Zewail M., Nafee N., Helmy M.W., Boraie N. Synergistic and receptor-mediated targeting of arthritic joints via intra-articular injectable smart hydrogels containing leflunomide-loaded lipid nanocarriers. Drug Deliv Transl Res. 2021;11:2496–2519. doi: 10.1007/s13346-021-00992-9. [DOI] [PubMed] [Google Scholar]
- 12.Scherer H.U., Häupl T., Burmester G.R. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110 doi: 10.1016/j.jaut.2019.102400. [DOI] [PubMed] [Google Scholar]
- 13.Paul A.K., Paul A., Jahan R., Jannat K., Bondhon T.A., Hasan A., et al. Probiotics and amelioration of rheumatoid arthritis: significant roles of lactobacillus ccasei and lactobacillus acidophilus. Microorganisms. 2021;9(5):1070. doi: 10.3390/microorganisms9051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derksen V.F.A.M., Huizinga T.W.J., van D.W.D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol. 2017;39:437–446. doi: 10.1007/s00281-017-0627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Li R., Zhang J. On-demand drug delivery of triptolide and celastrol by poly(lactic-co-glycolic acid) nanoparticle/triglycerol monostearate-18 hydrogel composite for rheumatoid arthritis treatment. Adv Compos Hybrid Mater. 2022;5:2921–2935. [Google Scholar]
- 16.Vemula P.K., Boilard E., Syed A., Campbell N.R., Muluneh M., Weitz D.A., et al. On-demand drug delivery from self-assembled nanofibrous gels: a new approach for treatment of proteolytic disease. J Biomed Mater Res - Part A. 2011;97A:103–110. doi: 10.1002/jbm.a.33020. [DOI] [PubMed] [Google Scholar]
- 17.Keshtiara P., Mirahmadi-Zare S.Z., Hadadzadeh H., Safaeinejad Z., Farrokhpour H., Askari Z. Simultaneous immunomodulation and tissue protection on the rheumatoid arthritis models using a tragacanth frankincense-based core-shell nanostructure. ACS Appl Nano Mater. 2023;6(4):2741–2754. [Google Scholar]
- 18.Wang K., Yin C., Ye X., Chen Q., Wu J., Chen Y., et al. A metabolic driven bio-responsive hydrogel loading psoralen for therapy of rheumatoid arthritis. Small. 2023;19(21) doi: 10.1002/smll.202207319. [DOI] [PubMed] [Google Scholar]
- 19.Pandolfi F., Franza L., Carusi V., Altamura S., Andriollo G., Nucera E. Interleukin-6 in rheumatoid arthritis. Int J Mol Sci. 2020;21:1–12. doi: 10.3390/ijms21155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H., Wu X., Liu R., Zhao Y., Sun L. ECM-inspired hydrogels with ADSCs encapsulation for rheumatoid arthritis treatment. Adv Sci. 2023;10(9) doi: 10.1002/advs.202206253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu H., Jing X., Lin J., Wang L., Jiang H., Yu B., et al. Knowledge domain and hotspots analysis concerning applications of two-photon polymerization in biomedical field: a bibliometric and visualized study. Front Bioeng Biotechnol. 2022;10:1–13. doi: 10.3389/fbioe.2022.1030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehghan-Baniani D., Mehrjou B., Wang D., Bagheri R., Solouk A., Chu P.K., et al. A dual functional chondro-inductive chitosan thermogel with high shear modulus and sustained drug release for cartilage tissue engineering. Int J Biol Macromol. 2022;205:638–650. doi: 10.1016/j.ijbiomac.2022.02.115. [DOI] [PubMed] [Google Scholar]
- 23.Zayed M., Tourne-Peteilh C., Ramonda M., Rethore G., Weiss P., Martinez J., et al. Microgels of silylated HPMC as a multimodal system for drug co-encapsulation. Int J Pharm. 2017;532:790–801. doi: 10.1016/j.ijpharm.2017.07.074. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen K.T., West J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z., Ren Y., Zhu Y., Hao L., Chen Y., An G., et al. A rapidly self-Healing Host-guest supramolecular hydrogel with high mechanical strength and excellent biocompatibility. Angew Chemie. 2018;130:9146–9150. doi: 10.1002/anie.201804400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z., An G., Zhu Y., Liu X., Chen Y., Wu H., et al. 3D-printable self-healing and mechanically reinforced hydrogels with host-guest non-covalent interactions integrated into covalently linked networks. Mater Horizons. 2019;6:733–742. doi: 10.1039/C8MH01208C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bobula T., Buffa R., Hermannová M., Kohutová L., Procházková P., Vágnerová H., et al. A novel photopolymerizable derivative of hyaluronan for designed hydrogel formation. Carbohydr Polym. 2017;161:277–285. doi: 10.1016/j.carbpol.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Bi Y., Du X., He P., Wang C., Liu C., Guo W. Smart bilayer polyacrylamide/DNA hybrid hydrogel film actuators exhibiting programmable responsive and reversible macroscopic shape deformations. Small. 2020;16(42) doi: 10.1002/smll.201906998. [DOI] [PubMed] [Google Scholar]
- 29.Reis A.V., Guilherme M.R., Mattoso L.H.C., Rubira A.F., Tambourgi E.B., Muniz E.C. Nanometer- and submicrometer-sized hollow spheres of chondroitin sulfate as a potential formulation strategy for anti-inflammatory encapsulation. Pharm Res. 2009;26:438–444. doi: 10.1007/s11095-008-9732-y. [DOI] [PubMed] [Google Scholar]
- 30.Xu L., Pan J., Chen Q., Yu Q., Chen H., Xu H., et al. In vivo evaluation of the safety of triptolide-loaded hydrogel-thickened microemulsion. Food Chem Toxicol. 2008;46:3792–3799. doi: 10.1016/j.fct.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y., Nie Y., Han Z., Huang H., Liao X., Wang X., et al. Au@Polydopamine nanoparticles/tocilizumab composite as efficient scavengers of oxygen free radicals for improving the treatment of rheumatoid arthritis. Mater Sci Eng C. 2021;118 doi: 10.1016/j.msec.2020.111434. [DOI] [PubMed] [Google Scholar]
- 32.Fan Z., Li J., Liu J., Jiao H., Liu B. Anti-inflammation and joint lubrication dual effects of a novel hyaluronic acid/curcumin nanomicelle improve the efficacy of rheumatoid arthritis therapy. ACS Appl Mater Interfaces. 2018;10:23595–23604. doi: 10.1021/acsami.8b06236. [DOI] [PubMed] [Google Scholar]
- 33.Wu W., Liu J., Lin X., He Z., Zhang H., Ji L., et al. Dual-functional MOFs-based hybrid microgel advances aqueous lubrication and anti-inflammation. J Colloid Interface Sci. 2023;644:200–210. doi: 10.1016/j.jcis.2023.04.071. [DOI] [PubMed] [Google Scholar]
- 34.Dehghan-Baniani D., Chen Y., Wang D., Bagheri R., Solouk A., Wu H. Injectable in situ forming Kartogenin-loaded chitosan hydrogel with tunable rheological properties for cartilage tissue engineering. Colloids Surfaces B Biointerfaces. 2020;192 doi: 10.1016/j.colsurfb.2020.111059. [DOI] [PubMed] [Google Scholar]
- 35.Suhail M., Chiu I.H., Hung M.C., Vu Q.L., Lin I.L., Wu P.C. In vitro evaluation of smart and pH-sensitive chondroitin sulfate/sodium polystyrene sulfonate hydrogels for controlled drug delivery. Gels. 2022;8(7):406. doi: 10.3390/gels8070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang N., Ma J., Song W., Zhao C. An injectable hydrogel to disrupt neutrophil extracellular traps for treating rheumatoid arthritis. Drug Deliv. 2023;30 doi: 10.1080/10717544.2023.2173332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira I.M., Gonçalves C., Shin M.E., Lee S., Reis R.L., Khang G., et al. Anti-inflammatory properties of injectable betamethasone-loaded tyramine-modified gellan gum/silk fibroin hydrogels. Biomolecules. 2020;10:1–17. doi: 10.3390/biom10101456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin N., Guo X., Sun R., Liu H., Tang L., Gou J., et al. Intra-articular injection of indomethacin-methotrexate: in situ hydrogel for the synergistic treatment of rheumatoid arthritis. J Mater Chem B. 2020;8:993–1007. doi: 10.1039/c9tb01795j. [DOI] [PubMed] [Google Scholar]
- 39.Collins K.H., Pferdehirt L., Saleh L.S., Savadipour A., Springer L.E., Lenz K.L., et al. Hydrogel encapsulation of genome-engineered stem cells for long-term self-regulating anti-cytokine therapy. Gels. 2023;9(2):169. doi: 10.3390/gels9020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira I.M., Gonçalves C., Shin M.E., Lee S., Reis R.L., Khang G., et al. Enzymatically crosslinked tyramine-gellan gum hydrogels as drug delivery system for rheumatoid arthritis treatment. Drug Deliv Transl Res. 2021;11:1288–1300. doi: 10.1007/s13346-020-00855-9. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A., Lee J., Ghosh T., Nguyen V.Q., Dey A., Yoon B., et al. Polymeric hydrogels for controlled drug delivery to treat arthritis. Pharmaceutics. 2022;14(3):540. doi: 10.3390/pharmaceutics14030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai H., Zhao Y., Wang C., Wang Z., Wang J., Liu H., et al. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics. 2020;10:4779–4794. doi: 10.7150/thno.43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim T., Suh J., Kim W.J. Polymeric aggregate-embodied hybrid nitric-oxide-scavenging and sequential drug-releasing hydrogel for combinatorial treatment of rheumatoid arthritis. Adv Mater. 2021;33(34) doi: 10.1002/adma.202008793. [DOI] [PubMed] [Google Scholar]
- 44.Radu A.F., Bungau S.G. Management of rheumatoid arthritis: an overview. Cells. 2021;10(11):2857. doi: 10.3390/cells10112857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babasahib S.K., Born R.W., Raghavendra N.M. Trans ethosomal hybrid composites of naproxen-sulfapyridine in hydrogel carrier: anti-inflammatory response in complete Freund's adjuvant induced arthritis rats. Artif Cells Nanomed Biotechnol. 2022;50(1):59–70. doi: 10.1080/21691401.2022.2047712. [DOI] [PubMed] [Google Scholar]
- 46.Mucke J., Krusche M., Burmester G.R. A broad look into the future of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2022;14:1–8. doi: 10.1177/1759720X221076211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadota Y., Nishida K., Hashizume K., Nasu Y., Nakahara R., Kanazawa T., et al. Risk factors for surgical site infection and delayed wound healing after orthopedic surgery in rheumatoid arthritis patients. Mod Rheumatol. 2016;26:68–74. doi: 10.3109/14397595.2015.1073133. [DOI] [PubMed] [Google Scholar]
- 48.Ramos-Peterson L., Nester C.J., Reinoso-Cobo A., Nieto-Gil P., Ortega-Avila A.B., Gijon-Nogueron G. A systematic review to identify the effects of biologics in the feet of patients with rheumatoid arthritis. Medicina. 2021;57(1):23. doi: 10.3390/medicina57010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishida Y.K.K., Hashizume K., Nasu Y., Nakahara R., Kanazawa T., Ozawa M., et al. Risk factors for surgical site infection and delayed wound healing after orthopedic surgery in rheumatoid arthritis patients. Mod Rheumatol. 2016;26(1):68–74. doi: 10.3109/14397595.2015.1073133. [DOI] [PubMed] [Google Scholar]
- 50.Ito H., Kojima M., Nishida K., Matsushita I., Kojima T., Nakayama T., et al. Postoperative complications in patients with rheumatoid arthritis using a biological agent - a systematic review and meta-analysis. Mod Rheumatol. 2015;25:672–678. doi: 10.3109/14397595.2015.1014302. [DOI] [PubMed] [Google Scholar]
- 51.Ito H., Tsuji S., Nakayama M., Mochida Y., Nishida K., Ishikawa H., et al. Does abatacept increase postoperative adverse events in rheumatoid arthritis compared with conventional synthetic disease-modifying drugs? J Rheumatol. 2020;47:502–509. doi: 10.3899/jrheum.181100. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka Y. Rheumatoid arthritis. Inflamm Regen. 2020;40:20. doi: 10.1186/s41232-020-00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pradal J., Jordan O., Allémann E. Intra-articular drug delivery for arthritis diseases: the value of extended release and targeting strategies. J Drug Deliv Sci Technol. 2012;22:409–419. [Google Scholar]
- 54.Akhtar M.F., Raza S.A., Saleem A., Hamid I., Baig M.M.F.A., Sharif A., et al. Appraisal of anti-arthritic and anti-inflammatory potential of folkloric medicinal plant Peganum harmala. Endocr Metab Immune Disord Drug Targets. 2021;22(1):49–63. doi: 10.2174/1871530321666210208211310. [DOI] [PubMed] [Google Scholar]
- 55.Rajitha P., Biswas R., Sabitha M., Jayakumar R. Methotrexate in the treatment of psoriasis and rheumatoid arthritis: mechanistic insights, current issues and novel delivery approaches. Curr Pharm Des. 2017;23(24):3550–3566. doi: 10.2174/1381612823666170601105439. [DOI] [PubMed] [Google Scholar]
- 56.Zhang F., Du Y., Zheng J., Cai Z., Ding T., Zhuang P., et al. Oral administration of multistage albumin nanomedicine depots (MANDs) for targeted efficient alleviation of chronic inflammatory diseases. Adv Funct Mater. 2023;33(9) [Google Scholar]
- 57.Ghosh S., Mukherjee B., Chaudhuri S., Roy T., Mukherjee A., Sengupta S. Methotrexate aspasomes against rheumatoid arthritis: optimized hydrogel loaded liposomal formulation with in vivo evaluation in Wistar rats. AAPS PharmSciTech. 2018;19:1320–1336. doi: 10.1208/s12249-017-0939-2. [DOI] [PubMed] [Google Scholar]
- 58.Kurakula M., Srinivas C., Kasturi N., Diwan P.V. Formulation and evaluation of prednisolon proliposomal gel for effective topical pharmacotherapy. Int J Pharm Sci Drug Res. 2012;4:35–43. [Google Scholar]
- 59.Chen G., Hao B., Ju D., Liu M., Zhao H., Du Z., et al. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch Under microneedles on rats with collagen-induced arthritis. Acta Pharm Sin B. 2015;5:569–576. doi: 10.1016/j.apsb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li M., Cui H., Cao Y., Lin Y., Yang Y., Gao M., et al. Deep eutectic solvents—hydrogels for the topical management of rheumatoid arthritis. J Control Release. 2023;354:664–679. doi: 10.1016/j.jconrel.2023.01.050. [DOI] [PubMed] [Google Scholar]
- 61.Xue X., Hu Y., Deng Y., Su J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv Funct Mater. 2021;31(19) [Google Scholar]
- 62.Ren S., Liu H., Wang X., Bi J., Lu S., Zhu C., et al. Acupoint nanocomposite hydrogel for simulation of acupuncture and targeted delivery of triptolide against rheumatoid arthritis. J Nanobiotechnology. 2021;19:409. doi: 10.1186/s12951-021-01157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tada M., Inui K., Sugioka Y., Mamoto K., Okano T., Kinoshita T., et al. Delayed wound healing and postoperative surgical site infections in patients with rheumatoid arthritis treated with or without biological disease-modifying antirheumatic drugs. Clin Rheumatol. 2016;35:1475–1481. doi: 10.1007/s10067-016-3274-1. [DOI] [PubMed] [Google Scholar]
- 64.Ito H., Murata K., Sobue Y., Kojima T., Nishida K., Matsushita I., et al. Comprehensive risk analysis of postoperative complications in patients with rheumatoid arthritis for the 2020 update of the Japan college of rheumatology clinical practice guidelines for the management of rheumatoid arthritis. Mod Rheumatol. 2021;28(32):296–306. doi: 10.1080/14397595.2021.1913824. [DOI] [PubMed] [Google Scholar]
- 65.Ma S., Gu S., Zhang J., Qi W., Lin Z., Zhai W., et al. Robust drug bioavailability and safety for rheumatoid arthritis therapy using d-amino acids-based supramolecular hydrogels. Mater Today Bio. 2022;15 doi: 10.1016/j.mtbio.2022.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim T., Suh J., Kim W.J. Polymeric aggregate-embodied hybrid nitric-oxide-scavenging and sequential drug-releasing hydrogel for combinatorial treatment of rheumatoid arthritis. Adv Mater. 2021;33(34) doi: 10.1002/adma.202008793. [DOI] [PubMed] [Google Scholar]
- 67.Wang Q.S., Xu B.X., Fan K.J., Li Y.W., Wu J., Wang T.Y. Dexamethasone-loaded thermosensitive hydrogel suppresses inflammation and pain in collagen-induced arthritis rats. Drug Des Devel Ther. 2020;14:4101–4113. doi: 10.2147/DDDT.S256850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cokelaere S.M., Plomp S.G.M., de Boef E., de Leeuw M., Bool S., van de Lest C.H.A., et al. Sustained intra-articular release of celecoxib in an equine repeated LPS synovitis model. Eur J Pharm Biopharm. 2018;128:327–336. doi: 10.1016/j.ejpb.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Kim K.S., Park S.J., Yang J.A., Jeon J.H., Bhang S.H., Kim B.S., et al. Injectable hyaluronic acid-tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater. 2011;7:666–674. doi: 10.1016/j.actbio.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Du Y., Li C., Zhang Y., Xiong W., Wang F., Wang J., et al. In situ-activated phospholipid-mimic artemisinin prodrug via injectable hydrogel nano/microsphere for rheumatoid arthritis therapy. Research. 2022;2022:0003. doi: 10.34133/research.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seo J., Park S.H., Kim M.J., Ju H.J., Yin X.Y., Min B.H., et al. Injectable click-crosslinked hyaluronic acid depot to prolong therapeutic activity in articular joints affected by rheumatoid arthritis. ACS Appl Mater Interfaces. 2019;11:24984–24998. doi: 10.1021/acsami.9b04979. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y., Song S., Wang D., Liu H., Zhang J., Li Z., et al. Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nat Commun. 2022;13:6758. doi: 10.1038/s41467-022-34481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanazawa T., Tamano K., Sogabe K., Endo T., Ibaraki H., Takashima Y., et al. Intra-articular retention and anti-arthritic effects in collagen-induced arthritis model mice by injectable small interfering RNA containing hydrogel. Biol Pharm Bull. 2017;40:1929–1933. doi: 10.1248/bpb.b17-00481. [DOI] [PubMed] [Google Scholar]
- 74.Ma Z., Tao C., Sun L., Qi S., Le Y., Wang J., et al. In situ forming injectable hydrogel for encapsulation of nanoiguratimod and sustained release of therapeutics. Int J Nanomedicine. 2019;14:8725–8738. doi: 10.2147/IJN.S214507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan W., Dai C., Li Y., Yin Y., Gong L., Machuki J.O., et al. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials. 2020;239 doi: 10.1016/j.biomaterials.2020.119851. [DOI] [PubMed] [Google Scholar]
- 76.Chen W., Li Z., Wang Z., Gao H., Ding J., He Z. Intraarticular injection of infliximab-loaded thermosensitive hydrogel alleviates pain and protects cartilage in rheumatoid arthritis. J Pain Res. 2020;13:3315–3329. doi: 10.2147/JPR.S283518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiang C.W., Hsiao Y.C., Jheng P.R., Chen C.H., Manga Y.B., Lekha R., et al. Strontium ranelate-laden near-infrared photothermal-inspired methylcellulose hydrogel for arthritis treatment. Mater Sci Eng C. 2021;123 doi: 10.1016/j.msec.2021.111980. [DOI] [PubMed] [Google Scholar]
- 78.Djekic L., Martinovic M., Primorac M. Microemulsion hydrogels - properties and current applications in drug delivery. In: Torres T, editor. Microemulsions: Systems, properties and application. Nova Science Publishers, Inc;2016,p.1–36.
- 79.Donnelly R.F., Singh T.R.R., Garland M.J., Migalska K., Majithiya R., McCrudden C.M., et al. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22:4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li C., Du Y., Lv H., Zhang J., Zhuang P., Yang W., et al. Injectable amphipathic artesunate prodrug-hydrogel microsphere as gene/drug nano-microplex for rheumatoid arthritis therapy. Adv Funct Mater. 2022;32(44) [Google Scholar]
- 81.Haloi P., Chawla S., Konkimalla V.B. Thermosensitive smart hydrogel of PEITC ameliorates the therapeutic efficacy in rheumatoid arthritis. Eur J Pharm Sci. 2023;181 doi: 10.1016/j.ejps.2022.106367. [DOI] [PubMed] [Google Scholar]
- 82.Chen Z., Xing L., Fan Q., Cheetham A.G., Lin R., Holt B., et al. Drug-bearing supramolecular filament hydrogels as anti-inflammatory agents. Theranostics. 2017;7:2003–2014. doi: 10.7150/thno.19404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan Z., Senapati D., Khan S.A., Singh A.K., Hamme A., Yust B., et al. Popcorn-shaped magnetic core-plasmonic shell multifunctional nanoparticles for the targeted magnetic separation and enrichment, label-free SERS imaging, and photothermal destruction of multidrug-resistant bacteria. Chem - A Eur J. 2013;19:2839–2847. doi: 10.1002/chem.201202948. [DOI] [PubMed] [Google Scholar]
- 84.Kim H.J., Lee S.M., Park K.H., Mun C.H., Park Y.B., Yoo K.H. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95–102. doi: 10.1016/j.biomaterials.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Liu V.A., Bhatia S.N. Three-dimensional photopatterning of hydrogels containing living cells. Biomed Microdevices. 2002;4:257–266. [Google Scholar]
- 86.Younes H.M. In: Polymer photopolymerization of polymeric composites in drug delivery, tissue engineering, and other biomedical applications.nanocomposites in biomedical engineering. Sadasivuni K, Ponnamma D, Rajan M, Ahmed B, Al-Maadeed M, editors. Springer Cham; 2019. pp. 271–297. editors. Lecture notes in bioengineering. [Google Scholar]
- 87.Qin X.H., Ovsianikov A., Stampfl J., Liska R. Additive manufacturing of photosensitive hydrogels for tissue engineering applications. BioNanoMaterials. 2014;15:49–70. [Google Scholar]
- 88.Zhang M., Wan T., Fan P., Shi K., Chen X., Yang H., et al. Photopolymerizable chitosan hydrogels with improved strength and 3D printability. Int J Biol Macromol. 2021;193:109–116. doi: 10.1016/j.ijbiomac.2021.10.137. [DOI] [PubMed] [Google Scholar]
- 89.Park J., Elmlund H., Ercius P., Yuk J.M., Limmer D.T., Chen Q., et al. 3D structure of individual nanocrystals in solution by electron microscopy. Science. 2015;349(80):290–295. doi: 10.1126/science.aab1343. [DOI] [PubMed] [Google Scholar]
- 90.Yu C., Schimelman J., Wang P., Miller K.L., Ma X., You S., et al. Photopolymerizable biomaterials and light-based 3D printing strategies for biomedical applications. Chem Rev. 2020;120:10695–10743. doi: 10.1021/acs.chemrev.9b00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guvendiren M., Purcell B., Burdick J.A. 9.22 - Photopolymerizable systems. In: Matyjaszewski K, Möller M, editors. Polymer science: A comprehensive reference. Elsevier; 2012,p. 413–3.
- 92.Tiwari A., Grailer J.J., Pilla S., Steeber D.A., Gong S. Biodegradable hydrogels Based on novel photopolymerizable guar gum-methacrylate macromonomers for in situ fabrication of tissue Engineering scaffolds. Acta Biomater. 2009;5:3441–3452. doi: 10.1016/j.actbio.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Mann B.K., Gobin A.S., Tsai A.T., Schmedlen R.H., West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 94.Hill-West J.L., Chowdhury S.M., Sawhney A.S., Pathak C.P., Dunn R.C., Hubbell J.A. Prevention of postoperative adhesions in the rat by in situ photopolymerization of bioresorbable hydrogel barriers. Obstet Gynecol. 1994;83:59–64. [PubMed] [Google Scholar]
- 95.Hill-West J.L., Chowdhury S.M., Dunn R.C., Hubbell J.A. Efficacy of a resorbable hydrogel barrier, oxidized regenerated cellulose, and hyaluronic acid in the prevention of ovarian adhesions in a rabbit model. Fertil Steril. 1994;62:630–634. doi: 10.1016/s0015-0282(16)56956-8. [DOI] [PubMed] [Google Scholar]
- 96.Anseth K.S., Burdick J.A. New directions in photopolymerizable biomaterials. MRS Bull. 2002;27:130–136. [Google Scholar]
- 97.Joshi P., Breaux S., Naro J., Wang Y., Ahmed M.S.U., Vig K., et al. Synthesis and characterization of photopolymerizable hydrogels based on poly (ethylene glycol) for biomedical applications. J Appl Polym Sci. 2021;138:50489. [Google Scholar]
- 98.Arakawa C., Ng R., Tan S., Kim S., Wu B., Lee M. Photopolymerizable chitosan–collagen hydrogels for bone tissue engineering. J Tissue Eng Regen Med. 2017;11:164–174. doi: 10.1002/term.1896. [DOI] [PubMed] [Google Scholar]
- 99.Meng W., Gao L., Venkatesan J.K., Wang G., Madry H., Cucchiarini M. Translational applications of photopolymerizable hydrogels for cartilage repair. J Exp Orthop. 2019;6:47. doi: 10.1186/s40634-019-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao J., Su J., An M., Yang Y., Zhang Y., Zuo J., et al. Novel DEK-targeting aptamer delivered by a hydrogel microneedle attenuates collagen-induced arthritis. Mol Pharm. 2021;18:305–316. doi: 10.1021/acs.molpharmaceut.0c00954. [DOI] [PubMed] [Google Scholar]
- 101.Haloi P., Lokesh B.S., Chawla S., Konkimalla V.B. Formulation of a dual drug-loaded nanoparticulate co-delivery hydrogel system and its validation in rheumatoid arthritis animal model. Drug Deliv. 2023;30(1) doi: 10.1080/10717544.2023.2184307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smolen J.S., Aletaha D., Machold K.P. Therapeutic strategies in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2005;19:163–177. doi: 10.1016/j.berh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 103.Agostini S.B.N., Malta I.H.S., Rodrigues R.F., Freitas J.T.J., Lino M.E.D.S., Santos R.S.D., et al. Preclinical evaluation of methotrexate-loaded polyelectrolyte complexes and thermosensitive hydrogels as treatment for rheumatoid arthritis. Eur J Pharm Sci. 2021;163:1–9. doi: 10.1016/j.ejps.2021.105856. [DOI] [PubMed] [Google Scholar]
- 104.Herráez D.L., Martínez-Bueno M., Riba L., De La Torre I.G., Sacnún M., Goñi M., et al. Rheumatoid arthritis in Latin Americans enriched for Amerindian ancestry is associated With loci in chromosomes 1, 12, and 13, and the HLA class II region. Arthritis Rheum. 2013;65:1457–1467. doi: 10.1002/art.37923. [DOI] [PubMed] [Google Scholar]
- 105.Lu S., Neoh K.G., Huang C., Shi Z., Kang E.T. Polyacrylamide hybrid nanogels for targeted cancer chemotherapy via co-delivery of gold nanoparticles and MTX. J Colloid Interface Sci. 2013;412:46–55. doi: 10.1016/j.jcis.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 106.Garg N.K., Singh B., Tyagi R.K., Sharma G., Katare O.P. Effective transdermal delivery of methotrexate through nanostructured lipid carriers in an experimentally induced arthritis model. Colloids Surfaces B Biointerfaces. 2016;147:17–24. doi: 10.1016/j.colsurfb.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 107.Tekko I.A., Chen G., Domínguez-Robles J., Thakur R.R.S., Hamdan I.M.N., Vora L., et al. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int J Pharm. 2020;586 doi: 10.1016/j.ijpharm.2020.119580. [DOI] [PubMed] [Google Scholar]
- 108.Qindeel M., Khan D., Ahmed N., Khan S., Ur Rehman Asim. Surfactant-free, self-assembled nanomicelles-based transdermal hydrogel for safe and targeted delivery of methotrexate against rheumatoid arthritis. ACS Nano. 2020;14:4662–4681. doi: 10.1021/acsnano.0c00364. [DOI] [PubMed] [Google Scholar]
- 109.Nutan B., Chandel A.K.S., Biswas A., Kumar A., Yadav A., Maiti P., et al. Gold nanoparticle promoted formation and biological properties of injectable hydrogels. Biomacromolecules. 2020;21:3782–3794. doi: 10.1021/acs.biomac.0c00889. [DOI] [PubMed] [Google Scholar]
- 110.Lee H., Lee M.Y., Bhang S.H., Kim B.S., Kim Y.S., Ju J.H., et al. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano. 2014;8:4790–4798. doi: 10.1021/nn500685h. [DOI] [PubMed] [Google Scholar]
- 111.Xiao S., Wang L., Han W., Gu L., Cui X., Wang C. Novel deep eutectic solvent–hydrogel systems for synergistic transdermal delivery of Chinese herb medicine and local treatments for rheumatoid arthritis. Pharm Res. 2022;39:2431–2446. doi: 10.1007/s11095-022-03239-5. [DOI] [PubMed] [Google Scholar]
- 112.Trujillo-Nolasco R.M., Morales-Avila E., Ocampo-García B.E., Ferro-Flores G., Gibbens-Bandala B.V., Escudero-Castellanos A., et al. Preparation and in vitro evaluation of radiolabeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Mater Sci Eng C. 2019;103 doi: 10.1016/j.msec.2019.109766. [DOI] [PubMed] [Google Scholar]
- 113.Park S.H., Park J.Y., Ji Y.B., Ju H.J., Min B.H., Kim M.S. An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020;117:108–120. doi: 10.1016/j.actbio.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 114.Nunes D., Andrade S., Ramalho M.J., Loureiro J.A., Pereira M.C. Polymeric nanoparticles-loaded hydrogels for biomedical applications: a systematic review on in vivo findings. Polymers. 2022;14:1010. doi: 10.3390/polym14051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gang F., Zhang Q., Jiang L., Xiao Y., Xu N., Wang Y., et al. Thermochemotherapy meets tissue engineering for rheumatoid arthritis treatment. Adv Funct Mater. 2021;31 [Google Scholar]
- 116.Yin N., Tan X., Liu H., He F., Ding N., Gou J., et al. A novel indomethacin/methotrexate/MMP-9 siRNA: in situ hydrogel with dual effects of anti-inflammatory activity and reversal of cartilage disruption for the synergistic treatment of rheumatoid arthritis. Nanoscale. 2020;12:8546–8562. doi: 10.1039/d0nr00454e. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y., Gao Z., Chao S., Lu W., Zhang P. Transdermal delivery of inflammatory factors regulated drugs for rheumatoid arthritis. Drug Deliv. 2022;29:1934–1950. doi: 10.1080/10717544.2022.2089295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khan D., Qindeel M., Ahmed N., Asad M.I., Ullah S.K., Rehman A.U. Development of an intelligent, stimuli-responsive transdermal system for efficient delivery of ibuprofen against rheumatoid arthritis. Int J Pharm. 2021;610 doi: 10.1016/j.ijpharm.2021.121242. [DOI] [PubMed] [Google Scholar]
- 119.Singh V., Chaubey N. Design and evaluation of topical hydrogel formulation of aceclofenac for improved therapy. J Drug Deliv Ther. 2019;9:118–122. [Google Scholar]
- 120.Garg N.K., Tandel N., Bhadada S.K., Tyagi R.K. Nanostructured lipid carrier–mediated transdermal delivery of aceclofenac hydrogel present an effective therapeutic approach for inflammatory diseases. Front Pharmacol. 2021;12:713616. doi: 10.3389/fphar.2021.713616. [DOI] [PMC free article] [PubMed] [Google Scholar]