Abstract
Microcystic adenocarcinoma is an uncommon histologic variant of prostate carcinoma. Despite its rarity, it has gained increasing recognition over the past decade for its diagnostic challenges and unclear prognostic significance.
Herein, we describe a rare case of metastatic microcystic prostate adenocarcinoma, presenting with discordance between imaging and histologic findings. This report highlights the diagnostic and therapeutic challenges of this pathological entity and the importance of multidisciplinary collaboration in the management of intermediate-risk prostate cancer.
1. Introduction
Microcystic adenocarcinoma is a rare histologic subtype of prostate cancer. Its benign-appearing morphology can be difficult to recognise on prostate biopsy, resulting in missed or delayed diagnoses.1 This report describes a case of metastatic microcystic prostatic adenocarcinoma, presenting with a diagnostic mismatch between histologic and radiological findings.
2. Case presentation
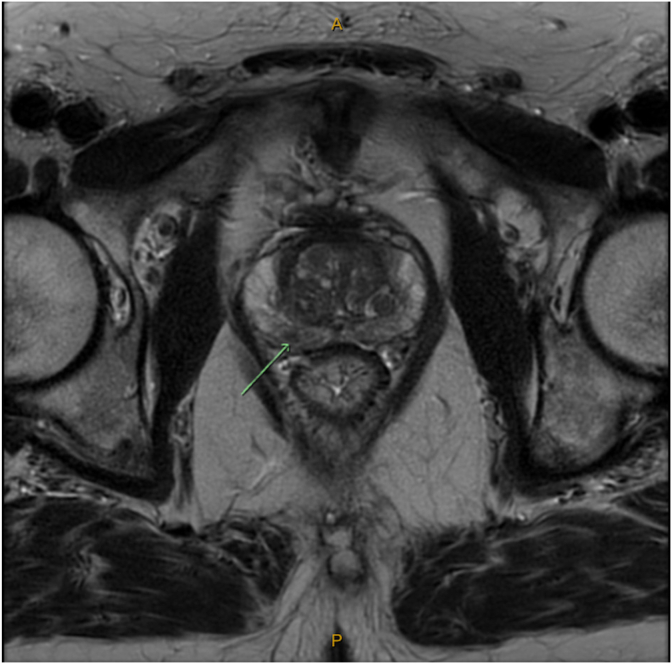
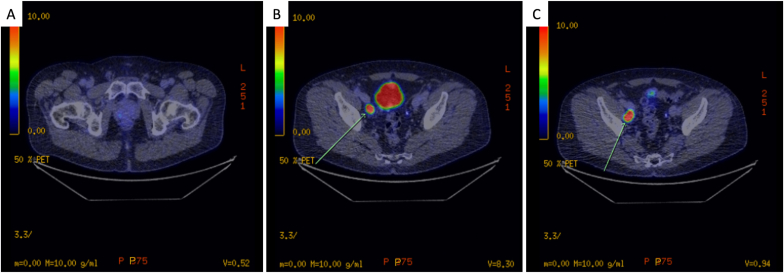
A 62-year-old male with no significant past medical history was referred for persistently elevated serum PSA levels (5.9ng/ml, 7.3ng/ml, and 5.1ng/ml over three successive months). Multiparametric-MRI (mp-MRI) demonstrated a PI-RADS 3 area in the right posterolateral peripheral zone with intact prostatic capsule and enlarged right-sided external iliac lymph nodes of unclear significance (Fig. 1). Trans-perineal biopsy was reported positive for Gleason Grade Group 2 acinar adenocarcinoma in the right apex and left base without extra-prostatic extension. PSMA-PET staging scan confirmed avid disease in the corresponding prostate regions and two right iliac lymph nodes without seminal vesicle involvement (Fig. 2).
Fig. 1.
Multiparametric-MRI. PI-RADS 3 lesion in the right posterolateral peripheral zone at the level of mid gland with intact capsule and margin.
Fig. 2.
PSMA-PET. Mild diffuse PSMA-avid disease (SUVmax 3.3) in the right posterior peripheral zone of the prostate (A) and two intensively avid right external nodes (SUVmax 19.4) (B,C).
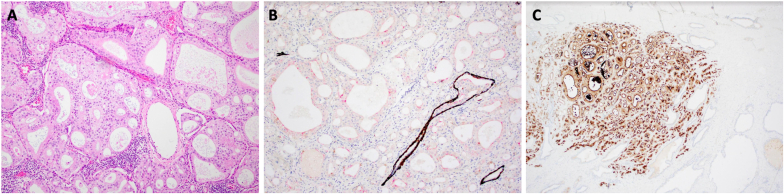
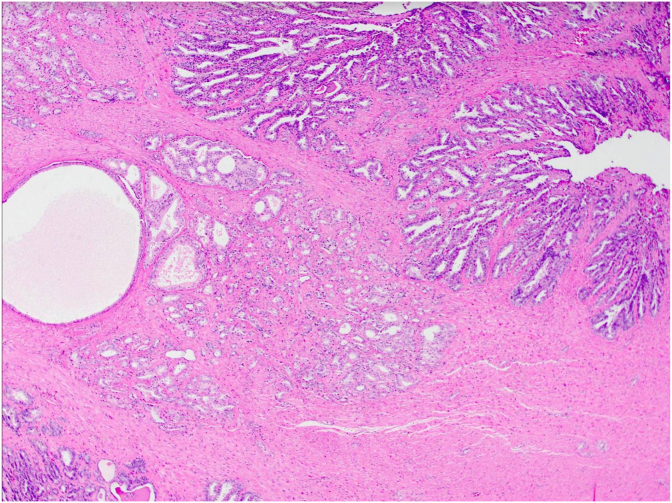
Following multidisciplinary team discussion, the patient proceeded to a robotic-assisted radical prostatectomy with bilateral lymph node dissection with diagnostic and therapeutic intent. Histopathology of the specimen revealed a highly distinct mix of usual pattern acinar adenocarcinoma and its unusual microcystic form, associated with areas of cribriform architecture (Fig. 3). Contrary to biopsy and scan results, there were focal, bilateral seminal vesicle invasion alongside lympho-vascular infiltration (Fig. 4). Metastatic deposits were detected in 3/20 of the pelvic lymph nodes (Fig. 5). The approximate total tumour volume is 0.49cm³. Clear surgical margins were achieved and post-prostatectomy PSA level at 6-month follow-up was 0.01 μg/L. Adjuvant treatment was not required.
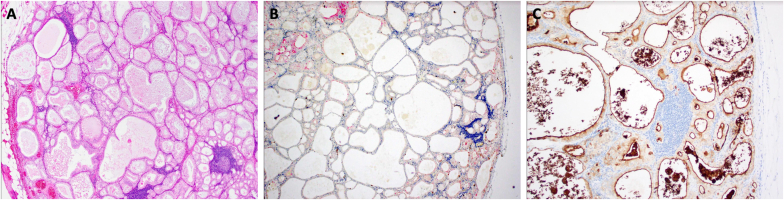
Fig. 3.
Haemoxylin and eosin (A), immunoperoxidase (B), and PSMA (C) staining of prostatectomy tissue demonstrating microcystic pattern carcinoma with loss of basal cells.
Fig. 4.
Haemoxylin and eosin staining of seminal vesicle demonstrating cancer invasion between benign glands.
Fig. 5.
Haemoxylin and eosin (A), immunoperoxidase (B), and PSMA (C) staining of lymph nodes demonstrating microcystic carcinoma with increased AMACR expression.
3. Discussion
The most common histologic subtype of prostate cancer is acinar adenocarcinoma. Recent years have also seen an increasing diagnostic awareness of its unusual variant, whose pathological attributes remain poorly understood. These variants, first defined in the 2016 World Health Organisation classification, include: signet ring-like cell, pleomorphic giant cell, mucinous, sarcomatoid, foamy, microcystic, pseudohyperplastic and atrophic.2 In particular, microcystic adenocarcinoma, as described in this report, is known for its deceptive morphology that mimics benign conditions of the prostate, contributing to an estimated 1–3% false negative rate on needle biopsy.1 Microscopically, it is characterised by cystic dilatation of the prostatic glands with intraluminal blue mucin and crystalloids, commonly admixed with the usual-type small acinar adenocarcinoma. In most cases, immunohistochemistry demonstrates Alpha-methylacyl-CoA racemase (AMACR) overexpression and absence of basal cells, both of which are useful clues in the diagnosis of malignancy. The incidence rate of microcystic adenocarcinoma is 11%, and periprostatic invasion, as confirmed in our patient's prostatectomy specimen, is extremely rare.1
In addition to accurate histologic diagnosis, timely staging of prostate cancer is equally important to guide individualised treatment. mpMRI is a commonly used imaging modality not only in the detection of intra-prostatic cancer foci but also locoregional disease. Arslan et al. reported a sensitivity of 56.2% and specificity of 82.6% in the use of mpMRI to detect extra-prostatic disease.3 Another emerging staging tool is PSMA-PET scan. In comparison to conventional imaging techniques such as CT and bone scans, PSMA-PET was superior in the primary detection of distant metastasis, with a higher sensitivity and specificity of 85% and 98%, respectively.4 When used in combination with mpMRI, PSMA-PET had an improved sensitivity (89% vs 76%, p < 0.01) and overall higher diagnostic accuracy than mpMRI alone in detecting clinically significant PI-RADS 3 lesions.5
Plausible reasons to explain an initial negative scan for seminal vesicle invasion in our patient relate to the intrinsically limited ability of mpMRI to visualise microscopic foci of extra-prostatic extension.6 The recognition of extra-prostatic extension on mpMRI hinges upon the detection of subjective findings secondary to macroscopic invasion of the tumours and its mechanical impacts, which vary based on the tumour's aggressivity.7 Previous studies have also shown that even when a prostate base tumour had extended beyond the capsule with concomitant loss of normal seminal vesicle architecture, their low sensitivity and inter-observer variability in the interpretation of MRI images were associated with highly heterogenous results in staging performance.6
Our study provides new insights into the elusive nature of microcystic adenocarcinoma, which was missed on the initial targeted prostate biopsy. Furthermore, despite the use of a combination of advanced imaging modalities, including mpMRI and PSMA-PET, its extra-prostatic extension was not entirely evident until radical prostatectomy had been performed.
4. Conclusion
Microcystic adenocarcinoma is a rare histologic variant of prostate cancer with unpredictable oncogenic progression. This report highlights the major diagnostic challenge of this pathological entity characterised by imaging-histologic discordance. It further exemplifies the need for a high index of suspicion for alternate diagnoses and atypical variants, as well as the importance of multidisciplinary collaboration in the management of intermediate-risk prostate cancer.
Consent
Informed written patient consent was attained to publish this case.
CRediT authorship contribution statement
Chun Khai Loh: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Juanita Noeline Chui: Writing – review & editing. Kevin Yinkit Zhuo: Writing – review & editing. Ashan Canagasingham: Writing – review & editing. Alexander Guminski: Writing – review & editing, Supervision, Conceptualization. Warick Delprado: Writing – review & editing, Supervision, Conceptualization. Thomas Eade: Writing – review & editing, Supervision, Conceptualization. Matthew Winter: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
Associate Professor Alexander Guminski has received unrestricted research support from Astra Zeneca and Sun Pharma for investigator-initiated clinical trials. The remaining authors declare that they have no competing interests.
References
- 1.Yaskiv O., Cao D., Humphrey P.A. Microcystic adenocarcinoma of the prostate: a variant of pseudohyperplastic and atrophic patterns. Am J Surg Pathol. 2010;34(4):556–561. doi: 10.1097/PAS.0b013e3181d2a549. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Arslan A., Karaarslan E., Güner A.L., Sağlıcan Y., Tuna M.B., Kural A.R. Comparing the diagnostic performance of multiparametric prostate MRI versus 68Ga-PSMA PET-CT in the evaluation lymph node involvement and extraprostatic extension. Acad Radiol. 2022;29(5):698–704. doi: 10.1016/j.acra.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Hofman M.S., Lawrentschuk N., Francis R.J., et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen M., Zhang Q., Zhang C., et al. Combination of (68)Ga-PSMA PET/CT and multiparametric MRI improves the detection of clinically significant prostate cancer: a lesion-by-lesion analysis. J Nucl Med. 2019;60(7):944–949. doi: 10.2967/jnumed.118.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung D.C., Lee H.J., Kim S.H., Choe G.Y., Lee S.E. Preoperative MR imaging in the evaluation of seminal vesicle invasion in prostate cancer: pattern analysis of seminal vesicle lesions. J Magn Reson Imag. 2008;28(1):144–150. doi: 10.1002/jmri.21422. [DOI] [PubMed] [Google Scholar]
- 7.Bakir B., Onay A., Vural M., Armutlu A., Yıldız S., Esen T. Can extraprostatic extension Be predicted by tumor-capsule contact length in prostate cancer? Relationship with international society of urological pathology grade groups. AJR Am J Roentgenol. 2020;214(3):588–596. doi: 10.2214/AJR.19.21828. [DOI] [PubMed] [Google Scholar]